User login
Myth busting: SARS-CoV-2 vaccine
MYTH: I shouldn’t get the vaccine because of potential long-term side effects
We know that 68 million people in the United States and 244 million people worldwide have already received messenger RNA (mRNA) SARS-CoV-2 vaccines (Pfizer/BioNTech and Moderna). So for the short-term side effects we already know more than we would know about most vaccines.
What about the long-term side effects? There are myths that these vaccines somehow could cause autoimmunity. This came from three publications where the possibility of mRNA vaccines to produce autoimmunity was brought up as a discussion point.1-3 There was no evidence given in these publications, it was raised only as a hypothetical possibility.
There’s no evidence that mRNA or replication-defective DNA vaccines (AstraZeneca/Oxford and Johnson & Johnson) produce autoimmunity. Moreover, the mRNA and replication-defective DNA, once it’s inside of the muscle cell, is gone within a few days. What’s left after ribosome processing is the spike (S) protein as an immunogen. We’ve been vaccinating with proteins for 50 years and we haven’t seen autoimmunity.
MYTH: The vaccines aren’t safe because they were developed so quickly
These vaccines were developed at “warp speed” – that doesn’t mean they were developed without all the same safety safeguards that the Food and Drug Administration requires. The reason it happened so fast is because the seriousness of the pandemic allowed us, as a community, to enroll the patients into the studies fast. In a matter of months, we had all the studies filled. In a normal circumstance, that might take 2 or 3 years. And all of the regulatory agencies – the National Institutes of Health, the FDA, the Centers for Disease Control and Prevention – were ready to take the information and put a panel of specialists together and immediately review the data. No safety steps were missed. The same process that’s always required of phase 1, of phase 2, and then at phase 3 were accomplished.
The novelty of these vaccines was that they could be made so quickly. Messenger RNA vaccines can be made in a matter of days and then manufactured in a matter of 2 months. The DNA vaccines has a similar timeline trajectory.
MYTH: There’s no point in getting the vaccines because we still have to wear masks
Right now, out of an abundance of caution, until it’s proven that we don’t have to wear masks, it’s being recommended that we do so for the safety of others. Early data suggest that this will be temporary. In time, I suspect it will be shown that, after we receive the vaccine, it will be shown that we are not contagious to others and we’ll be able to get rid of our masks.
MYTH: I already had COVID-19 so I don’t need the vaccine
Some people have already caught the SARS-CoV-2 virus that causes this infection and so they feel that they’re immune and they don’t need to get the vaccine. Time will tell if that’s the case. Right now, we don’t know for sure. Early data suggest that a single dose of vaccine in persons who have had the infection may be sufficient. Over time, what happens in the vaccine field is we measure the immunity from the vaccine, and from people who’ve gotten the infection, and we find that there’s a measurement in the blood that correlates with protection. Right now, we don’t know that correlate of protection level. So, out of an abundance of caution, it’s being recommended that, even if you had the disease, maybe you didn’t develop enough immunity, and it’s better to get the vaccine than to get the illness a second time.
MYTH: The vaccines can give me SARS-CoV-2 infection
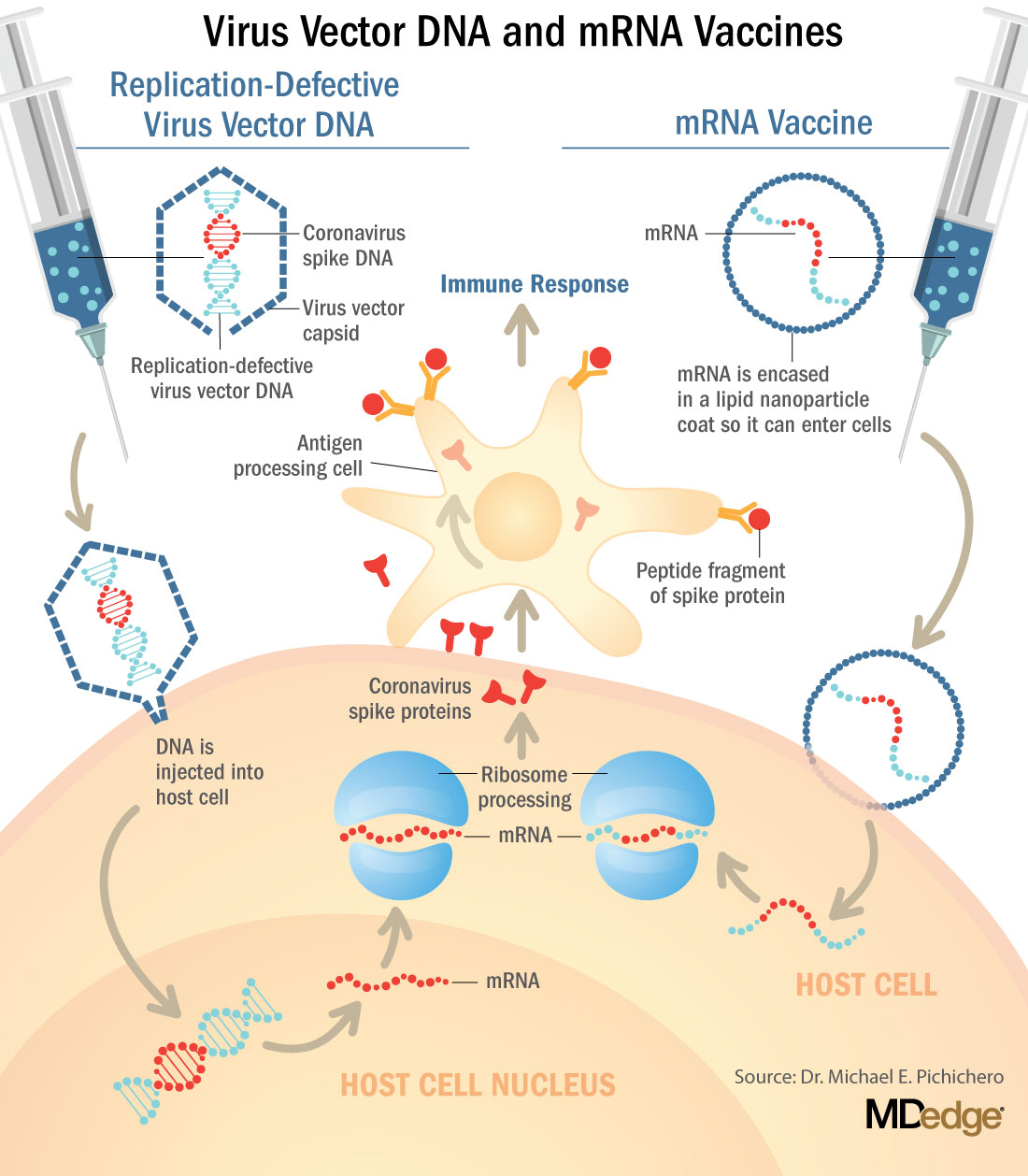
The new vaccines for COVID-19, released under emergency use Authorization, are mRNA and DNA vaccines. They are a blueprint for the Spike (S) protein of the virus. In order to become a protein, the mRNA, once it’s inside the cell, is processed by ribosomes. The product of the ribosome processing is a protein that cannot possibly cause harm as a virus. It’s a little piece of mRNA inside of a lipid nanoparticle, which is just a casing to protect the mRNA from breaking down until it’s injected in the body. The replication defective DNA vaccines (AstraZeneca/Oxford and Johnson & Johnson) are packaged inside of virus cells (adenoviruses). The DNA vaccines involve a three-step process:
- 1. The adenovirus, containing replication-defective DNA that encodes mRNA for the Spike (S) protein, is taken up by the host cells where it must make its way to the nucleus of the muscle cell.
- 2. The DNA is injected into the host cell nucleus and in the nucleus the DNA is decoded to an mRNA.
- 3. The mRNA is released from the nucleus and transported to the cell cytoplasm where the ribosomes process the mRNA in an identical manner as mRNA vaccines.
MYTH: The COVID-19 vaccines can alter my DNA
The mRNA and replication-defective DNA vaccines never interact with your DNA. mRNA vaccines never enter the nucleus. Replication-defective DNA vaccines cannot replicate and do not interact with host DNA. The vaccines can’t change your DNA.
Here is a link to YouTube videos I made on this topic: https://youtube.com/playlist?list=PLve-0UW04UMRKHfFbXyEpLY8GCm2WyJHD.
Here is a photo of me receiving my first SARS-CoV-2 shot (Moderna) in January 2021. I received my second shot in February. I am a lot less anxious. I hope my vaccine card will be a ticket to travel in the future.
Dr. Pichichero is a specialist in pediatric infectious diseases and director of the Research Institute at Rochester (N.Y.) General Hospital. He has no conflicts of interest to report.
References
1. Peck KM and Lauring AS. J Virol. 2018. doi: 10.1128/JVI.01031-17.
2. Pepini T et al. J Immunol. 2017 May 15. doi: 10.4049/jimmunol.1601877.
3. Theofilopoulos AN et al. Annu Rev Immunol. 2005. doi: 10.1146/annurev.immunol.23.021704.115843.
MYTH: I shouldn’t get the vaccine because of potential long-term side effects
We know that 68 million people in the United States and 244 million people worldwide have already received messenger RNA (mRNA) SARS-CoV-2 vaccines (Pfizer/BioNTech and Moderna). So for the short-term side effects we already know more than we would know about most vaccines.
What about the long-term side effects? There are myths that these vaccines somehow could cause autoimmunity. This came from three publications where the possibility of mRNA vaccines to produce autoimmunity was brought up as a discussion point.1-3 There was no evidence given in these publications, it was raised only as a hypothetical possibility.
There’s no evidence that mRNA or replication-defective DNA vaccines (AstraZeneca/Oxford and Johnson & Johnson) produce autoimmunity. Moreover, the mRNA and replication-defective DNA, once it’s inside of the muscle cell, is gone within a few days. What’s left after ribosome processing is the spike (S) protein as an immunogen. We’ve been vaccinating with proteins for 50 years and we haven’t seen autoimmunity.
MYTH: The vaccines aren’t safe because they were developed so quickly
These vaccines were developed at “warp speed” – that doesn’t mean they were developed without all the same safety safeguards that the Food and Drug Administration requires. The reason it happened so fast is because the seriousness of the pandemic allowed us, as a community, to enroll the patients into the studies fast. In a matter of months, we had all the studies filled. In a normal circumstance, that might take 2 or 3 years. And all of the regulatory agencies – the National Institutes of Health, the FDA, the Centers for Disease Control and Prevention – were ready to take the information and put a panel of specialists together and immediately review the data. No safety steps were missed. The same process that’s always required of phase 1, of phase 2, and then at phase 3 were accomplished.
The novelty of these vaccines was that they could be made so quickly. Messenger RNA vaccines can be made in a matter of days and then manufactured in a matter of 2 months. The DNA vaccines has a similar timeline trajectory.
MYTH: There’s no point in getting the vaccines because we still have to wear masks
Right now, out of an abundance of caution, until it’s proven that we don’t have to wear masks, it’s being recommended that we do so for the safety of others. Early data suggest that this will be temporary. In time, I suspect it will be shown that, after we receive the vaccine, it will be shown that we are not contagious to others and we’ll be able to get rid of our masks.
MYTH: I already had COVID-19 so I don’t need the vaccine
Some people have already caught the SARS-CoV-2 virus that causes this infection and so they feel that they’re immune and they don’t need to get the vaccine. Time will tell if that’s the case. Right now, we don’t know for sure. Early data suggest that a single dose of vaccine in persons who have had the infection may be sufficient. Over time, what happens in the vaccine field is we measure the immunity from the vaccine, and from people who’ve gotten the infection, and we find that there’s a measurement in the blood that correlates with protection. Right now, we don’t know that correlate of protection level. So, out of an abundance of caution, it’s being recommended that, even if you had the disease, maybe you didn’t develop enough immunity, and it’s better to get the vaccine than to get the illness a second time.
MYTH: The vaccines can give me SARS-CoV-2 infection
The new vaccines for COVID-19, released under emergency use Authorization, are mRNA and DNA vaccines. They are a blueprint for the Spike (S) protein of the virus. In order to become a protein, the mRNA, once it’s inside the cell, is processed by ribosomes. The product of the ribosome processing is a protein that cannot possibly cause harm as a virus. It’s a little piece of mRNA inside of a lipid nanoparticle, which is just a casing to protect the mRNA from breaking down until it’s injected in the body. The replication defective DNA vaccines (AstraZeneca/Oxford and Johnson & Johnson) are packaged inside of virus cells (adenoviruses). The DNA vaccines involve a three-step process:
- 1. The adenovirus, containing replication-defective DNA that encodes mRNA for the Spike (S) protein, is taken up by the host cells where it must make its way to the nucleus of the muscle cell.
- 2. The DNA is injected into the host cell nucleus and in the nucleus the DNA is decoded to an mRNA.
- 3. The mRNA is released from the nucleus and transported to the cell cytoplasm where the ribosomes process the mRNA in an identical manner as mRNA vaccines.
MYTH: The COVID-19 vaccines can alter my DNA
The mRNA and replication-defective DNA vaccines never interact with your DNA. mRNA vaccines never enter the nucleus. Replication-defective DNA vaccines cannot replicate and do not interact with host DNA. The vaccines can’t change your DNA.
Here is a link to YouTube videos I made on this topic: https://youtube.com/playlist?list=PLve-0UW04UMRKHfFbXyEpLY8GCm2WyJHD.
Here is a photo of me receiving my first SARS-CoV-2 shot (Moderna) in January 2021. I received my second shot in February. I am a lot less anxious. I hope my vaccine card will be a ticket to travel in the future.
Dr. Pichichero is a specialist in pediatric infectious diseases and director of the Research Institute at Rochester (N.Y.) General Hospital. He has no conflicts of interest to report.
References
1. Peck KM and Lauring AS. J Virol. 2018. doi: 10.1128/JVI.01031-17.
2. Pepini T et al. J Immunol. 2017 May 15. doi: 10.4049/jimmunol.1601877.
3. Theofilopoulos AN et al. Annu Rev Immunol. 2005. doi: 10.1146/annurev.immunol.23.021704.115843.
MYTH: I shouldn’t get the vaccine because of potential long-term side effects
We know that 68 million people in the United States and 244 million people worldwide have already received messenger RNA (mRNA) SARS-CoV-2 vaccines (Pfizer/BioNTech and Moderna). So for the short-term side effects we already know more than we would know about most vaccines.
What about the long-term side effects? There are myths that these vaccines somehow could cause autoimmunity. This came from three publications where the possibility of mRNA vaccines to produce autoimmunity was brought up as a discussion point.1-3 There was no evidence given in these publications, it was raised only as a hypothetical possibility.
There’s no evidence that mRNA or replication-defective DNA vaccines (AstraZeneca/Oxford and Johnson & Johnson) produce autoimmunity. Moreover, the mRNA and replication-defective DNA, once it’s inside of the muscle cell, is gone within a few days. What’s left after ribosome processing is the spike (S) protein as an immunogen. We’ve been vaccinating with proteins for 50 years and we haven’t seen autoimmunity.
MYTH: The vaccines aren’t safe because they were developed so quickly
These vaccines were developed at “warp speed” – that doesn’t mean they were developed without all the same safety safeguards that the Food and Drug Administration requires. The reason it happened so fast is because the seriousness of the pandemic allowed us, as a community, to enroll the patients into the studies fast. In a matter of months, we had all the studies filled. In a normal circumstance, that might take 2 or 3 years. And all of the regulatory agencies – the National Institutes of Health, the FDA, the Centers for Disease Control and Prevention – were ready to take the information and put a panel of specialists together and immediately review the data. No safety steps were missed. The same process that’s always required of phase 1, of phase 2, and then at phase 3 were accomplished.
The novelty of these vaccines was that they could be made so quickly. Messenger RNA vaccines can be made in a matter of days and then manufactured in a matter of 2 months. The DNA vaccines has a similar timeline trajectory.
MYTH: There’s no point in getting the vaccines because we still have to wear masks
Right now, out of an abundance of caution, until it’s proven that we don’t have to wear masks, it’s being recommended that we do so for the safety of others. Early data suggest that this will be temporary. In time, I suspect it will be shown that, after we receive the vaccine, it will be shown that we are not contagious to others and we’ll be able to get rid of our masks.
MYTH: I already had COVID-19 so I don’t need the vaccine
Some people have already caught the SARS-CoV-2 virus that causes this infection and so they feel that they’re immune and they don’t need to get the vaccine. Time will tell if that’s the case. Right now, we don’t know for sure. Early data suggest that a single dose of vaccine in persons who have had the infection may be sufficient. Over time, what happens in the vaccine field is we measure the immunity from the vaccine, and from people who’ve gotten the infection, and we find that there’s a measurement in the blood that correlates with protection. Right now, we don’t know that correlate of protection level. So, out of an abundance of caution, it’s being recommended that, even if you had the disease, maybe you didn’t develop enough immunity, and it’s better to get the vaccine than to get the illness a second time.
MYTH: The vaccines can give me SARS-CoV-2 infection
The new vaccines for COVID-19, released under emergency use Authorization, are mRNA and DNA vaccines. They are a blueprint for the Spike (S) protein of the virus. In order to become a protein, the mRNA, once it’s inside the cell, is processed by ribosomes. The product of the ribosome processing is a protein that cannot possibly cause harm as a virus. It’s a little piece of mRNA inside of a lipid nanoparticle, which is just a casing to protect the mRNA from breaking down until it’s injected in the body. The replication defective DNA vaccines (AstraZeneca/Oxford and Johnson & Johnson) are packaged inside of virus cells (adenoviruses). The DNA vaccines involve a three-step process:
- 1. The adenovirus, containing replication-defective DNA that encodes mRNA for the Spike (S) protein, is taken up by the host cells where it must make its way to the nucleus of the muscle cell.
- 2. The DNA is injected into the host cell nucleus and in the nucleus the DNA is decoded to an mRNA.
- 3. The mRNA is released from the nucleus and transported to the cell cytoplasm where the ribosomes process the mRNA in an identical manner as mRNA vaccines.
MYTH: The COVID-19 vaccines can alter my DNA
The mRNA and replication-defective DNA vaccines never interact with your DNA. mRNA vaccines never enter the nucleus. Replication-defective DNA vaccines cannot replicate and do not interact with host DNA. The vaccines can’t change your DNA.
Here is a link to YouTube videos I made on this topic: https://youtube.com/playlist?list=PLve-0UW04UMRKHfFbXyEpLY8GCm2WyJHD.
Here is a photo of me receiving my first SARS-CoV-2 shot (Moderna) in January 2021. I received my second shot in February. I am a lot less anxious. I hope my vaccine card will be a ticket to travel in the future.
Dr. Pichichero is a specialist in pediatric infectious diseases and director of the Research Institute at Rochester (N.Y.) General Hospital. He has no conflicts of interest to report.
References
1. Peck KM and Lauring AS. J Virol. 2018. doi: 10.1128/JVI.01031-17.
2. Pepini T et al. J Immunol. 2017 May 15. doi: 10.4049/jimmunol.1601877.
3. Theofilopoulos AN et al. Annu Rev Immunol. 2005. doi: 10.1146/annurev.immunol.23.021704.115843.
Understanding messenger RNA and other SARS-CoV-2 vaccines
In mid-November, Pfizer/BioNTech were the first with surprising positive protection interim data for their coronavirus vaccine, BNT162b2. A week later, Moderna released interim efficacy results showing its coronavirus vaccine, mRNA-1273, also protected patients from developing SARS-CoV-2 infections. Both studies included mostly healthy adults. A diverse ethnic and racial vaccinated population was included. A reasonable number of persons aged over 65 years, and persons with stable compromising medical conditions were included. Adolescents aged 16 years and over were included. Younger adolescents have been vaccinated or such studies are in the planning or early implementation stage as 2020 came to a close.
These are new and revolutionary vaccines, although the ability to inject mRNA into animals dates back to 1990, technological advances today make it a reality.1 Traditional vaccines typically involve injection with antigens such as purified proteins or polysaccharides or inactivated/attenuated viruses. In the case of Pfizer’s and Moderna’s vaccines, the mRNA provides the genetic information to synthesize the spike protein that the SARS-CoV-2 virus uses to attach to and infect human cells. Each type of vaccine is packaged in proprietary lipid nanoparticles to protect the mRNA from rapid degradation, and the nanoparticles serve as an adjuvant to attract immune cells to the site of injection. (The properties of the respective lipid nanoparticle packaging may be the factor that impacts storage requirements discussed below.) When injected into muscle (myocyte), the lipid nanoparticles containing the mRNA inside are taken into muscle cells, where the cytoplasmic ribosomes detect and decode the mRNA resulting in the production of the spike protein antigen. It should be noted that the mRNA does not enter the nucleus, where the genetic information (DNA) of a cell is located, and can’t be reproduced or integrated into the DNA. The antigen is exported to the myocyte cell surface where the immune system’s antigen presenting cells detect the protein, ingest it, and take it to regional lymph nodes where interactions with T cells and B cells results in antibodies, T cell–mediated immunity, and generation of immune memory T cells and B cells. A particular subset of T cells – cytotoxic or killer T cells – destroy cells that have been infected by a pathogen. The SARS-CoV-2 mRNA vaccine from Pfizer was reported to induce powerful cytotoxic T-cell responses. Results for Moderna’s vaccine had not been reported at the time this column was prepared, but I anticipate the same positive results.
The revolutionary aspect of mRNA vaccines is the speed at which they can be designed and produced. This is why they lead the pack among the SARS-CoV-2 vaccine candidates and why the National Institute of Allergy and Infectious Diseases provided financial, technical, and/or clinical support. Indeed, once the amino acid sequence of a protein can be determined (a relatively easy task these days) it’s straightforward to synthesize mRNA in the lab – and it can be done incredibly fast. It is reported that the mRNA code for the vaccine by Moderna was made in 2 days and production development was completed in about 2 months.2
A 2007 World Health Organization report noted that infectious diseases are emerging at “the historically unprecedented rate of one per year.”3 Severe acute respiratory syndrome (SARS), Zika, Ebola, and avian and swine flu are recent examples. For most vaccines against emerging diseases, the challenge is about speed: developing and manufacturing a vaccine and getting it to persons who need it as quickly as possible. The current seasonal flu vaccine takes about 6 months to develop; it takes years for most of the traditional vaccines. That’s why once the infrastructure is in place, mRNA vaccines may prove to offer a big advantage as vaccines against emerging pathogens.
Early efficacy results have been surprising
Both vaccines were reported to produce about 95% efficacy in the final analysis. That was unexpectedly high because most vaccines for respiratory illness achieve efficacy of 60%-80%, e.g., flu vaccines. However, the efficacy rate may drop as time goes by because stimulation of short-term immunity would be in the earliest reported results.
Preventing SARS-CoV-2 cases is an important aspect of a coronavirus vaccine, but preventing severe illness is especially important considering that severe cases can result in prolonged intubation/artificial ventilation, prolonged disability and death. Pfizer/BioNTech had not released any data on the breakdown of severe cases as this column was finalized. In Moderna’s clinical trial, a secondary endpoint analyzed severe cases of COVID-19 and included 30 severe cases (as defined in the study protocol) in this analysis. All 30 cases occurred in the placebo group and none in the mRNA-1273–vaccinated group. In the Pfizer/BioNTech trial there were too few cases of severe illness to calculate efficacy.
Duration of immunity and need to revaccinate after initial primary vaccination are unknowns. Study of induction of B- and T-cell memory and levels of long-term protection have not been reported thus far.
Could mRNA COVID-19 vaccines be dangerous in the long term?
These will be the first-ever mRNA vaccines brought to market for humans. In order to receive Food and Drug Administration approval, the companies had to prove there were no immediate or short-term negative adverse effects from the vaccines. The companies reported that their independent data-monitoring committees hadn’t “reported any serious safety concerns.” However, fairly significant local reactions at the site of injection, fever, malaise, and fatigue occur with modest frequency following vaccinations with these products, reportedly in 10%-15% of vaccinees. Overall, the immediate reaction profile appears to be more severe than what occurs following seasonal influenza vaccination. When mass inoculations with these completely new and revolutionary vaccines begins, we will know virtually nothing about their long-term side effects. The possibility of systemic inflammatory responses that could lead to autoimmune conditions, persistence of the induced immunogen expression, development of autoreactive antibodies, and toxic effects of delivery components have been raised as theoretical concerns.4-6 None of these theoretical risks have been observed to date and postmarketing phase 4 safety monitoring studies are in place from the Centers for Disease Control and Prevention and the companies that produce the vaccines. This is a risk public health authorities are willing to take because the risk to benefit calculation strongly favors taking theoretical risks, compared with clear benefits in preventing severe illnesses and death.
What about availability?
Pfizer/BioNTech expects to be able to produce up to 50 million vaccine doses in 2020 and up to 1.3 billion doses in 2021. Moderna expects to produce 20 million doses by the end of 2020, and 500 million to 1 billion doses in 2021. Storage requirements are inherent to the composition of the vaccines with their differing lipid nanoparticle delivery systems. Pfizer/BioNTech’s BNT162b2 has to be stored and transported at –80° C, which requires specialized freezers, which most doctors’ offices and pharmacies are unlikely to have on site, or dry ice containers. Once the vaccine is thawed, it can only remain in the refrigerator for 24 hours. Moderna’s mRNA-1273 will be much easier to distribute. The vaccine is stable in a standard freezer at –20° C for up to 6 months, in a refrigerator for up to 30 days within that 6-month shelf life, and at room temperature for up to 12 hours.
Timelines and testing other vaccines
Strong efficacy data from the two leading SARS-CoV-2 vaccines and emergency-use authorization Food and Drug Administration approval suggest the window for testing additional vaccine candidates in the United States could soon start to close. Of the more than 200 vaccines in development for SARS-CoV-2, at least 7 have a chance of gathering pivotal data before the front-runners become broadly available.
Testing diverse vaccine candidates, based on different technologies, is important for ensuring sufficient supply and could lead to products with tolerability and safety profiles that make them better suited, or more attractive, to subsets of the population. Different vaccine antigens and technologies also may yield different durations of protection, a question that will not be answered until long after the first products are on the market.
AstraZeneca enrolled about 23,000 subjects into its two phase 3 trials of AZD1222 (ChAdOx1 nCoV-19): a 40,000-subject U.S. trial and a 10,000-subject study in Brazil. AstraZeneca’s AZD1222, developed with the University of Oxford (England), uses a replication defective simian adenovirus vector called ChAdOx1.AZD1222 which encodes the SARS-CoV-2 spike protein. After injection, the viral vector delivers recombinant DNA that is decoded to mRNA, followed by mRNA decoding to become a protein. A serendipitous manufacturing error for the first 3,000 doses resulted in a half dose for those subjects before the error was discovered. Full doses were given to those subjects on second injections and those subjects showed 90% efficacy. Subjects who received 2 full doses showed 62% efficacy. A vaccine cannot be licensed based on 3,000 subjects so AstraZeneca has started a new phase 3 trial involving many more subjects to receive the combination lower dose followed by the full dose.
Johnson and Johnson (J&J) started its phase 3 trial evaluating a single dose of JNJ-78436735 in September. Phase 3 data may be reported by the end of2020. In November, J&J announced it was starting a second phase 3 trial to test two doses of the candidate. J&J’s JNJ-78436735 encodes the SARS-CoV-2 spike protein in an adenovirus serotype 26 (Ad26) vector, which is one of the two adenovirus vectors used in Sputnik V, the Russian vaccine reported to have 90% efficacy at an early interim analysis.
Sanofi and Novavax are both developing protein-based vaccines, a proven modality. Sanofi, in partnership with GlaxoSmithKline started a phase 1/2 clinical trial in the Fall 2020 with plans to commence a phase 3 trial in late December. Sanofi developed the protein ingredients and GlaxoSmithKline added one of their novel adjuvants. Novavax expects data from a U.K. phase 3 trial of NVX-CoV2373 in early 2021 and began a U.S. phase 3 study in late November. NVX-CoV2373 was created using Novavax’ recombinant nanoparticle technology to generate antigen derived from the coronavirus spike protein and contains Novavax’s patented saponin-based Matrix-M adjuvant.
Inovio Pharmaceuticals was gearing up to start a U.S. phase 2/3 trial of DNA vaccine INO-4800 by the end of 2020.
After Moderna and Pfizer-BioNTech, CureVac has the next most advanced mRNA vaccine. It was planned that a phase 2b/3 trial of CVnCoV would be conducted in Europe, Latin America, Africa, and Asia. Sanofi is also developing a mRNA vaccine as a second product in addition to its protein vaccine.
Vaxxinity planned to begin phase 3 testing of UB-612, a multitope peptide–based vaccine, in Brazil by the end of 2020.
However, emergency-use authorizations for the Pfizer and Moderna vaccines could hinder trial recruitment in at least two ways. Given the gravity of the pandemic, some stakeholders believe it would be ethical to unblind ongoing trials to give subjects the opportunity to switch to a vaccine proven to be effective. Even if unblinding doesn’t occur, as the two authorized vaccines start to become widely available, volunteering for clinical trials may become less attractive.
Dr. Pichichero is a specialist in pediatric infectious diseases, and director of the Research Institute at Rochester (N.Y.) General Hospital. He said he has no relevant financial disclosures. Email Dr. Pichichero at pdnews@mdedge.com.
References
1. Wolff JA et al. Science. 1990 Mar 23. doi: 10.1126/science.1690918.
2. Jackson LA et al. N Engl J Med. 2020 Nov 12. doi: 10.1056/NEJMoa2022483.
3. Prentice T and Reinders LT. The world health report 2007. (Geneva Switzerland: World Health Organization, 2007).
4. Peck KM and Lauring AS. J Virol. 2018. doi: 10.1128/JVI.01031-17.
5. Pepini T et al. J Immunol. 2017 May 15. doi: 10.4049/jimmunol.1601877.
6. Theofilopoulos AN et al. Annu Rev Immunol. 2005. doi: 10.1146/annurev.immunol.23.021704.115843.
In mid-November, Pfizer/BioNTech were the first with surprising positive protection interim data for their coronavirus vaccine, BNT162b2. A week later, Moderna released interim efficacy results showing its coronavirus vaccine, mRNA-1273, also protected patients from developing SARS-CoV-2 infections. Both studies included mostly healthy adults. A diverse ethnic and racial vaccinated population was included. A reasonable number of persons aged over 65 years, and persons with stable compromising medical conditions were included. Adolescents aged 16 years and over were included. Younger adolescents have been vaccinated or such studies are in the planning or early implementation stage as 2020 came to a close.
These are new and revolutionary vaccines, although the ability to inject mRNA into animals dates back to 1990, technological advances today make it a reality.1 Traditional vaccines typically involve injection with antigens such as purified proteins or polysaccharides or inactivated/attenuated viruses. In the case of Pfizer’s and Moderna’s vaccines, the mRNA provides the genetic information to synthesize the spike protein that the SARS-CoV-2 virus uses to attach to and infect human cells. Each type of vaccine is packaged in proprietary lipid nanoparticles to protect the mRNA from rapid degradation, and the nanoparticles serve as an adjuvant to attract immune cells to the site of injection. (The properties of the respective lipid nanoparticle packaging may be the factor that impacts storage requirements discussed below.) When injected into muscle (myocyte), the lipid nanoparticles containing the mRNA inside are taken into muscle cells, where the cytoplasmic ribosomes detect and decode the mRNA resulting in the production of the spike protein antigen. It should be noted that the mRNA does not enter the nucleus, where the genetic information (DNA) of a cell is located, and can’t be reproduced or integrated into the DNA. The antigen is exported to the myocyte cell surface where the immune system’s antigen presenting cells detect the protein, ingest it, and take it to regional lymph nodes where interactions with T cells and B cells results in antibodies, T cell–mediated immunity, and generation of immune memory T cells and B cells. A particular subset of T cells – cytotoxic or killer T cells – destroy cells that have been infected by a pathogen. The SARS-CoV-2 mRNA vaccine from Pfizer was reported to induce powerful cytotoxic T-cell responses. Results for Moderna’s vaccine had not been reported at the time this column was prepared, but I anticipate the same positive results.
The revolutionary aspect of mRNA vaccines is the speed at which they can be designed and produced. This is why they lead the pack among the SARS-CoV-2 vaccine candidates and why the National Institute of Allergy and Infectious Diseases provided financial, technical, and/or clinical support. Indeed, once the amino acid sequence of a protein can be determined (a relatively easy task these days) it’s straightforward to synthesize mRNA in the lab – and it can be done incredibly fast. It is reported that the mRNA code for the vaccine by Moderna was made in 2 days and production development was completed in about 2 months.2
A 2007 World Health Organization report noted that infectious diseases are emerging at “the historically unprecedented rate of one per year.”3 Severe acute respiratory syndrome (SARS), Zika, Ebola, and avian and swine flu are recent examples. For most vaccines against emerging diseases, the challenge is about speed: developing and manufacturing a vaccine and getting it to persons who need it as quickly as possible. The current seasonal flu vaccine takes about 6 months to develop; it takes years for most of the traditional vaccines. That’s why once the infrastructure is in place, mRNA vaccines may prove to offer a big advantage as vaccines against emerging pathogens.
Early efficacy results have been surprising
Both vaccines were reported to produce about 95% efficacy in the final analysis. That was unexpectedly high because most vaccines for respiratory illness achieve efficacy of 60%-80%, e.g., flu vaccines. However, the efficacy rate may drop as time goes by because stimulation of short-term immunity would be in the earliest reported results.
Preventing SARS-CoV-2 cases is an important aspect of a coronavirus vaccine, but preventing severe illness is especially important considering that severe cases can result in prolonged intubation/artificial ventilation, prolonged disability and death. Pfizer/BioNTech had not released any data on the breakdown of severe cases as this column was finalized. In Moderna’s clinical trial, a secondary endpoint analyzed severe cases of COVID-19 and included 30 severe cases (as defined in the study protocol) in this analysis. All 30 cases occurred in the placebo group and none in the mRNA-1273–vaccinated group. In the Pfizer/BioNTech trial there were too few cases of severe illness to calculate efficacy.
Duration of immunity and need to revaccinate after initial primary vaccination are unknowns. Study of induction of B- and T-cell memory and levels of long-term protection have not been reported thus far.
Could mRNA COVID-19 vaccines be dangerous in the long term?
These will be the first-ever mRNA vaccines brought to market for humans. In order to receive Food and Drug Administration approval, the companies had to prove there were no immediate or short-term negative adverse effects from the vaccines. The companies reported that their independent data-monitoring committees hadn’t “reported any serious safety concerns.” However, fairly significant local reactions at the site of injection, fever, malaise, and fatigue occur with modest frequency following vaccinations with these products, reportedly in 10%-15% of vaccinees. Overall, the immediate reaction profile appears to be more severe than what occurs following seasonal influenza vaccination. When mass inoculations with these completely new and revolutionary vaccines begins, we will know virtually nothing about their long-term side effects. The possibility of systemic inflammatory responses that could lead to autoimmune conditions, persistence of the induced immunogen expression, development of autoreactive antibodies, and toxic effects of delivery components have been raised as theoretical concerns.4-6 None of these theoretical risks have been observed to date and postmarketing phase 4 safety monitoring studies are in place from the Centers for Disease Control and Prevention and the companies that produce the vaccines. This is a risk public health authorities are willing to take because the risk to benefit calculation strongly favors taking theoretical risks, compared with clear benefits in preventing severe illnesses and death.
What about availability?
Pfizer/BioNTech expects to be able to produce up to 50 million vaccine doses in 2020 and up to 1.3 billion doses in 2021. Moderna expects to produce 20 million doses by the end of 2020, and 500 million to 1 billion doses in 2021. Storage requirements are inherent to the composition of the vaccines with their differing lipid nanoparticle delivery systems. Pfizer/BioNTech’s BNT162b2 has to be stored and transported at –80° C, which requires specialized freezers, which most doctors’ offices and pharmacies are unlikely to have on site, or dry ice containers. Once the vaccine is thawed, it can only remain in the refrigerator for 24 hours. Moderna’s mRNA-1273 will be much easier to distribute. The vaccine is stable in a standard freezer at –20° C for up to 6 months, in a refrigerator for up to 30 days within that 6-month shelf life, and at room temperature for up to 12 hours.
Timelines and testing other vaccines
Strong efficacy data from the two leading SARS-CoV-2 vaccines and emergency-use authorization Food and Drug Administration approval suggest the window for testing additional vaccine candidates in the United States could soon start to close. Of the more than 200 vaccines in development for SARS-CoV-2, at least 7 have a chance of gathering pivotal data before the front-runners become broadly available.
Testing diverse vaccine candidates, based on different technologies, is important for ensuring sufficient supply and could lead to products with tolerability and safety profiles that make them better suited, or more attractive, to subsets of the population. Different vaccine antigens and technologies also may yield different durations of protection, a question that will not be answered until long after the first products are on the market.
AstraZeneca enrolled about 23,000 subjects into its two phase 3 trials of AZD1222 (ChAdOx1 nCoV-19): a 40,000-subject U.S. trial and a 10,000-subject study in Brazil. AstraZeneca’s AZD1222, developed with the University of Oxford (England), uses a replication defective simian adenovirus vector called ChAdOx1.AZD1222 which encodes the SARS-CoV-2 spike protein. After injection, the viral vector delivers recombinant DNA that is decoded to mRNA, followed by mRNA decoding to become a protein. A serendipitous manufacturing error for the first 3,000 doses resulted in a half dose for those subjects before the error was discovered. Full doses were given to those subjects on second injections and those subjects showed 90% efficacy. Subjects who received 2 full doses showed 62% efficacy. A vaccine cannot be licensed based on 3,000 subjects so AstraZeneca has started a new phase 3 trial involving many more subjects to receive the combination lower dose followed by the full dose.
Johnson and Johnson (J&J) started its phase 3 trial evaluating a single dose of JNJ-78436735 in September. Phase 3 data may be reported by the end of2020. In November, J&J announced it was starting a second phase 3 trial to test two doses of the candidate. J&J’s JNJ-78436735 encodes the SARS-CoV-2 spike protein in an adenovirus serotype 26 (Ad26) vector, which is one of the two adenovirus vectors used in Sputnik V, the Russian vaccine reported to have 90% efficacy at an early interim analysis.
Sanofi and Novavax are both developing protein-based vaccines, a proven modality. Sanofi, in partnership with GlaxoSmithKline started a phase 1/2 clinical trial in the Fall 2020 with plans to commence a phase 3 trial in late December. Sanofi developed the protein ingredients and GlaxoSmithKline added one of their novel adjuvants. Novavax expects data from a U.K. phase 3 trial of NVX-CoV2373 in early 2021 and began a U.S. phase 3 study in late November. NVX-CoV2373 was created using Novavax’ recombinant nanoparticle technology to generate antigen derived from the coronavirus spike protein and contains Novavax’s patented saponin-based Matrix-M adjuvant.
Inovio Pharmaceuticals was gearing up to start a U.S. phase 2/3 trial of DNA vaccine INO-4800 by the end of 2020.
After Moderna and Pfizer-BioNTech, CureVac has the next most advanced mRNA vaccine. It was planned that a phase 2b/3 trial of CVnCoV would be conducted in Europe, Latin America, Africa, and Asia. Sanofi is also developing a mRNA vaccine as a second product in addition to its protein vaccine.
Vaxxinity planned to begin phase 3 testing of UB-612, a multitope peptide–based vaccine, in Brazil by the end of 2020.
However, emergency-use authorizations for the Pfizer and Moderna vaccines could hinder trial recruitment in at least two ways. Given the gravity of the pandemic, some stakeholders believe it would be ethical to unblind ongoing trials to give subjects the opportunity to switch to a vaccine proven to be effective. Even if unblinding doesn’t occur, as the two authorized vaccines start to become widely available, volunteering for clinical trials may become less attractive.
Dr. Pichichero is a specialist in pediatric infectious diseases, and director of the Research Institute at Rochester (N.Y.) General Hospital. He said he has no relevant financial disclosures. Email Dr. Pichichero at pdnews@mdedge.com.
References
1. Wolff JA et al. Science. 1990 Mar 23. doi: 10.1126/science.1690918.
2. Jackson LA et al. N Engl J Med. 2020 Nov 12. doi: 10.1056/NEJMoa2022483.
3. Prentice T and Reinders LT. The world health report 2007. (Geneva Switzerland: World Health Organization, 2007).
4. Peck KM and Lauring AS. J Virol. 2018. doi: 10.1128/JVI.01031-17.
5. Pepini T et al. J Immunol. 2017 May 15. doi: 10.4049/jimmunol.1601877.
6. Theofilopoulos AN et al. Annu Rev Immunol. 2005. doi: 10.1146/annurev.immunol.23.021704.115843.
In mid-November, Pfizer/BioNTech were the first with surprising positive protection interim data for their coronavirus vaccine, BNT162b2. A week later, Moderna released interim efficacy results showing its coronavirus vaccine, mRNA-1273, also protected patients from developing SARS-CoV-2 infections. Both studies included mostly healthy adults. A diverse ethnic and racial vaccinated population was included. A reasonable number of persons aged over 65 years, and persons with stable compromising medical conditions were included. Adolescents aged 16 years and over were included. Younger adolescents have been vaccinated or such studies are in the planning or early implementation stage as 2020 came to a close.
These are new and revolutionary vaccines, although the ability to inject mRNA into animals dates back to 1990, technological advances today make it a reality.1 Traditional vaccines typically involve injection with antigens such as purified proteins or polysaccharides or inactivated/attenuated viruses. In the case of Pfizer’s and Moderna’s vaccines, the mRNA provides the genetic information to synthesize the spike protein that the SARS-CoV-2 virus uses to attach to and infect human cells. Each type of vaccine is packaged in proprietary lipid nanoparticles to protect the mRNA from rapid degradation, and the nanoparticles serve as an adjuvant to attract immune cells to the site of injection. (The properties of the respective lipid nanoparticle packaging may be the factor that impacts storage requirements discussed below.) When injected into muscle (myocyte), the lipid nanoparticles containing the mRNA inside are taken into muscle cells, where the cytoplasmic ribosomes detect and decode the mRNA resulting in the production of the spike protein antigen. It should be noted that the mRNA does not enter the nucleus, where the genetic information (DNA) of a cell is located, and can’t be reproduced or integrated into the DNA. The antigen is exported to the myocyte cell surface where the immune system’s antigen presenting cells detect the protein, ingest it, and take it to regional lymph nodes where interactions with T cells and B cells results in antibodies, T cell–mediated immunity, and generation of immune memory T cells and B cells. A particular subset of T cells – cytotoxic or killer T cells – destroy cells that have been infected by a pathogen. The SARS-CoV-2 mRNA vaccine from Pfizer was reported to induce powerful cytotoxic T-cell responses. Results for Moderna’s vaccine had not been reported at the time this column was prepared, but I anticipate the same positive results.
The revolutionary aspect of mRNA vaccines is the speed at which they can be designed and produced. This is why they lead the pack among the SARS-CoV-2 vaccine candidates and why the National Institute of Allergy and Infectious Diseases provided financial, technical, and/or clinical support. Indeed, once the amino acid sequence of a protein can be determined (a relatively easy task these days) it’s straightforward to synthesize mRNA in the lab – and it can be done incredibly fast. It is reported that the mRNA code for the vaccine by Moderna was made in 2 days and production development was completed in about 2 months.2
A 2007 World Health Organization report noted that infectious diseases are emerging at “the historically unprecedented rate of one per year.”3 Severe acute respiratory syndrome (SARS), Zika, Ebola, and avian and swine flu are recent examples. For most vaccines against emerging diseases, the challenge is about speed: developing and manufacturing a vaccine and getting it to persons who need it as quickly as possible. The current seasonal flu vaccine takes about 6 months to develop; it takes years for most of the traditional vaccines. That’s why once the infrastructure is in place, mRNA vaccines may prove to offer a big advantage as vaccines against emerging pathogens.
Early efficacy results have been surprising
Both vaccines were reported to produce about 95% efficacy in the final analysis. That was unexpectedly high because most vaccines for respiratory illness achieve efficacy of 60%-80%, e.g., flu vaccines. However, the efficacy rate may drop as time goes by because stimulation of short-term immunity would be in the earliest reported results.
Preventing SARS-CoV-2 cases is an important aspect of a coronavirus vaccine, but preventing severe illness is especially important considering that severe cases can result in prolonged intubation/artificial ventilation, prolonged disability and death. Pfizer/BioNTech had not released any data on the breakdown of severe cases as this column was finalized. In Moderna’s clinical trial, a secondary endpoint analyzed severe cases of COVID-19 and included 30 severe cases (as defined in the study protocol) in this analysis. All 30 cases occurred in the placebo group and none in the mRNA-1273–vaccinated group. In the Pfizer/BioNTech trial there were too few cases of severe illness to calculate efficacy.
Duration of immunity and need to revaccinate after initial primary vaccination are unknowns. Study of induction of B- and T-cell memory and levels of long-term protection have not been reported thus far.
Could mRNA COVID-19 vaccines be dangerous in the long term?
These will be the first-ever mRNA vaccines brought to market for humans. In order to receive Food and Drug Administration approval, the companies had to prove there were no immediate or short-term negative adverse effects from the vaccines. The companies reported that their independent data-monitoring committees hadn’t “reported any serious safety concerns.” However, fairly significant local reactions at the site of injection, fever, malaise, and fatigue occur with modest frequency following vaccinations with these products, reportedly in 10%-15% of vaccinees. Overall, the immediate reaction profile appears to be more severe than what occurs following seasonal influenza vaccination. When mass inoculations with these completely new and revolutionary vaccines begins, we will know virtually nothing about their long-term side effects. The possibility of systemic inflammatory responses that could lead to autoimmune conditions, persistence of the induced immunogen expression, development of autoreactive antibodies, and toxic effects of delivery components have been raised as theoretical concerns.4-6 None of these theoretical risks have been observed to date and postmarketing phase 4 safety monitoring studies are in place from the Centers for Disease Control and Prevention and the companies that produce the vaccines. This is a risk public health authorities are willing to take because the risk to benefit calculation strongly favors taking theoretical risks, compared with clear benefits in preventing severe illnesses and death.
What about availability?
Pfizer/BioNTech expects to be able to produce up to 50 million vaccine doses in 2020 and up to 1.3 billion doses in 2021. Moderna expects to produce 20 million doses by the end of 2020, and 500 million to 1 billion doses in 2021. Storage requirements are inherent to the composition of the vaccines with their differing lipid nanoparticle delivery systems. Pfizer/BioNTech’s BNT162b2 has to be stored and transported at –80° C, which requires specialized freezers, which most doctors’ offices and pharmacies are unlikely to have on site, or dry ice containers. Once the vaccine is thawed, it can only remain in the refrigerator for 24 hours. Moderna’s mRNA-1273 will be much easier to distribute. The vaccine is stable in a standard freezer at –20° C for up to 6 months, in a refrigerator for up to 30 days within that 6-month shelf life, and at room temperature for up to 12 hours.
Timelines and testing other vaccines
Strong efficacy data from the two leading SARS-CoV-2 vaccines and emergency-use authorization Food and Drug Administration approval suggest the window for testing additional vaccine candidates in the United States could soon start to close. Of the more than 200 vaccines in development for SARS-CoV-2, at least 7 have a chance of gathering pivotal data before the front-runners become broadly available.
Testing diverse vaccine candidates, based on different technologies, is important for ensuring sufficient supply and could lead to products with tolerability and safety profiles that make them better suited, or more attractive, to subsets of the population. Different vaccine antigens and technologies also may yield different durations of protection, a question that will not be answered until long after the first products are on the market.
AstraZeneca enrolled about 23,000 subjects into its two phase 3 trials of AZD1222 (ChAdOx1 nCoV-19): a 40,000-subject U.S. trial and a 10,000-subject study in Brazil. AstraZeneca’s AZD1222, developed with the University of Oxford (England), uses a replication defective simian adenovirus vector called ChAdOx1.AZD1222 which encodes the SARS-CoV-2 spike protein. After injection, the viral vector delivers recombinant DNA that is decoded to mRNA, followed by mRNA decoding to become a protein. A serendipitous manufacturing error for the first 3,000 doses resulted in a half dose for those subjects before the error was discovered. Full doses were given to those subjects on second injections and those subjects showed 90% efficacy. Subjects who received 2 full doses showed 62% efficacy. A vaccine cannot be licensed based on 3,000 subjects so AstraZeneca has started a new phase 3 trial involving many more subjects to receive the combination lower dose followed by the full dose.
Johnson and Johnson (J&J) started its phase 3 trial evaluating a single dose of JNJ-78436735 in September. Phase 3 data may be reported by the end of2020. In November, J&J announced it was starting a second phase 3 trial to test two doses of the candidate. J&J’s JNJ-78436735 encodes the SARS-CoV-2 spike protein in an adenovirus serotype 26 (Ad26) vector, which is one of the two adenovirus vectors used in Sputnik V, the Russian vaccine reported to have 90% efficacy at an early interim analysis.
Sanofi and Novavax are both developing protein-based vaccines, a proven modality. Sanofi, in partnership with GlaxoSmithKline started a phase 1/2 clinical trial in the Fall 2020 with plans to commence a phase 3 trial in late December. Sanofi developed the protein ingredients and GlaxoSmithKline added one of their novel adjuvants. Novavax expects data from a U.K. phase 3 trial of NVX-CoV2373 in early 2021 and began a U.S. phase 3 study in late November. NVX-CoV2373 was created using Novavax’ recombinant nanoparticle technology to generate antigen derived from the coronavirus spike protein and contains Novavax’s patented saponin-based Matrix-M adjuvant.
Inovio Pharmaceuticals was gearing up to start a U.S. phase 2/3 trial of DNA vaccine INO-4800 by the end of 2020.
After Moderna and Pfizer-BioNTech, CureVac has the next most advanced mRNA vaccine. It was planned that a phase 2b/3 trial of CVnCoV would be conducted in Europe, Latin America, Africa, and Asia. Sanofi is also developing a mRNA vaccine as a second product in addition to its protein vaccine.
Vaxxinity planned to begin phase 3 testing of UB-612, a multitope peptide–based vaccine, in Brazil by the end of 2020.
However, emergency-use authorizations for the Pfizer and Moderna vaccines could hinder trial recruitment in at least two ways. Given the gravity of the pandemic, some stakeholders believe it would be ethical to unblind ongoing trials to give subjects the opportunity to switch to a vaccine proven to be effective. Even if unblinding doesn’t occur, as the two authorized vaccines start to become widely available, volunteering for clinical trials may become less attractive.
Dr. Pichichero is a specialist in pediatric infectious diseases, and director of the Research Institute at Rochester (N.Y.) General Hospital. He said he has no relevant financial disclosures. Email Dr. Pichichero at pdnews@mdedge.com.
References
1. Wolff JA et al. Science. 1990 Mar 23. doi: 10.1126/science.1690918.
2. Jackson LA et al. N Engl J Med. 2020 Nov 12. doi: 10.1056/NEJMoa2022483.
3. Prentice T and Reinders LT. The world health report 2007. (Geneva Switzerland: World Health Organization, 2007).
4. Peck KM and Lauring AS. J Virol. 2018. doi: 10.1128/JVI.01031-17.
5. Pepini T et al. J Immunol. 2017 May 15. doi: 10.4049/jimmunol.1601877.
6. Theofilopoulos AN et al. Annu Rev Immunol. 2005. doi: 10.1146/annurev.immunol.23.021704.115843.
COVID-19: A primary care perspective
With the COVID-19 pandemic, we are experiencing a once-in-a-100-year event. Dr. Steven A. Schulz, who is serving children on the front line in upstate New York, and I outline some of the challenges primary care pediatricians have been facing and solutions that have succeeded.
Reduction in direct patient care and its consequences
Because of the unknowns of COVID-19, many parents have not wanted to bring their children to a medical office because of fear of contracting SARS-CoV-2. At the same time, pediatricians have restricted in-person visits to prevent spread of SARS-CoV-2 and to help flatten the curve of infection. Use of pediatric medical professional services, compared with last year, dropped by 52% in March 2020 and by 58% in April, according to FAIR Health, a nonprofit organization that manages a database of 31 million claims. This is resulting in decreased immunization rates, which increases concern for secondary spikes of other preventable illnesses; for example, data from the Centers for Disease Control and Prevention showed that, from mid-March to mid-April 2020, physicians in the Vaccines for Children program ordered 2.5 million fewer doses of vaccines and 250,000 fewer doses of measles-containing vaccines, compared with the same period in 2019. Fewer children are being seen for well visits, which means opportunities are lost for adequate monitoring of growth, development, physical wellness, and social determinants of health.
This is occurring at a time when families have been experiencing increased stress in terms of finances, social isolation, finding adequate child care, and serving as parent, teacher, and breadwinner. An increase in injuries is occurring because of inadequate parental supervision because many parents have been distracted while working from home. An increase in cases of severe abuse is occurring because schools, child care providers, physicians, and other mandated reporters in the community have decreased interaction with children. Children’s Hospital Colorado in Colorado Springs saw a 118% increase in the number of trauma cases in its ED between January and April 2020. Some of these were accidental injuries caused by falls or bicycle accidents, but there was a 200% increase in nonaccidental trauma, which was associated with a steep fall in calls to the state’s child abuse hotline. Academic gains are being lost, and there has been worry for a prolonged “summer slide” risk, especially for children living in poverty and children with developmental disabilities.
The COVID-19 pandemic also is affecting physicians and staff. As frontline personnel, we are at risk to contract the virus, and news media reminds us of severe illness and deaths among health care workers. The pandemic is affecting financial viability; estimated revenue of pediatric offices fell by 45% in March 2020 and 48% in April, compared with the previous year, according to FAIR Health. Nurses and staff have been furloughed. Practices have had to apply for grants and Paycheck Protection Program funds while extending credit lines.
Limited testing capability for SARS-CoV-2
Testing for SARS-CoV-2 has been variably available. There have been problems with false positive and especially false negative results (BMJ. 2020 May 12. doi: 10.1136/bmj.m1808).The best specimen collection method has yet to be determined. Blood testing for antibody has been touted, but it remains unclear if there is clinical benefit because a positive result offers no guarantee of immunity, and immunity may quickly wane. Perhaps widespread primary care office–based testing will be in place by the fall, with hope for future reliable point of care results.
Evolving knowledge regarding SARS-CoV-2 and MIS-C
It initially was thought that children were relatively spared from serious illness caused by COVID-19. Then reports of cases of newly identified multisystem inflammatory syndrome of children occurred. It has been unclear how children contribute to the spread of COVID-19 illness, although emerging evidence indicates it is lower than adult transmission. What will happen when children return to school and daycare in the fall?
The challenges have led to creative solutions for how to deliver care.
Adapting to telehealth to provide care
At least for the short term, HIPAA regulations have been relaxed to allow for video visits using platforms such as FaceTime, Skype, Zoom, Doximity, and Doxy.me. Some of these platforms are HIPAA compliant and will be long-term solutions; however, electronic medical record portals allowing for video visits are the more secure option, according to HIPAA.
It has been a learning experience to see what can be accomplished with a video visit. Taking a history and visual examination of injuries and rashes has been possible. Addressing mental health concerns through the video exchange generally has been effective.
However, video visits change the provider-patient interpersonal dynamic and offer only visual exam capabilities, compared with an in-person visit. We cannot look in ears, palpate a liver and spleen, touch and examine a joint or bone, or feel a rash. Video visits also are dependent on the quality of patient Internet access, sufficient data plans, and mutual capabilities to address the inevitable technological glitches on the provider’s end as well. Expanding information technology infrastructure ability and added licensure costs have occurred. Practices and health systems have been working with insurance companies to ensure telephone and video visits are reimbursed on a comparable level to in-office visits.
A new type of office visit and developing appropriate safety plans
Patients must be universally screened prior to arrival during appointment scheduling for well and illness visits. Patients aged older than 2 years and caregivers must wear masks on entering the facility. In many practices, patients are scheduled during specific sick or well visit time slots throughout the day. Waiting rooms chairs need to be spaced for 6-foot social distancing, and cars in the parking lot often serve as waiting rooms until staff can meet patients at the door and take them to the exam room. Alternate entrances, car-side exams, and drive-by and/or tent testing facilities often have become part of the new normal everyday practice. Creating virtual visit time blocks in provider’s schedules has allowed for decreased office congestion. Patients often are checked out from their room, as opposed to waiting in a line at a check out desk. Nurse triage protocols also have been adapted and enhanced to meet needs and concerns.
With the need for summer physicals and many regions opening up, a gradual return toward baseline has been evolving, although some of the twists of a “new normal” will stay in place. The new normal has been for providers and staff to wear surgical masks and face shields; sometimes N95 masks, gloves, and gowns have been needed. Cleaning rooms and equipment between patient visits has become a major, new time-consuming task. Acquiring and maintaining adequate supplies has been a challenge.
Summary
The American Academy of Pediatrics, CDC, and state and local health departments have been providing informative and regular updates, webinars, and best practices guidelines. Pediatricians, community organizations, schools, and mental health professionals have been collaborating, overcoming hurdles, and working together to help mitigate the effects of the pandemic on children, their families, and our communities. Continued education, cooperation, and adaptation will be needed in the months ahead. If there is a silver lining to this pandemic experience, it may be that families have grown closer together as they sheltered in place (and we have grown closer to our own families as well). One day perhaps a child who lived through this pandemic might be asked what it was like, and their recollection might be that it was a wonderful time because their parents stayed home all the time, took care of them, taught them their school work, and took lots of long family walks.
Dr. Schulz is pediatric medical director, Rochester (N.Y.) Regional Health. Dr. Pichichero is a specialist in pediatric infectious diseases and director of the Research Institute at Rochester (N.Y.) General Hospital. Dr. Schulz and Dr. Pichichero said they have no relevant financial disclosures. Email them at pdnews@mdedge.com.
This article was updated 7/16/2020.
With the COVID-19 pandemic, we are experiencing a once-in-a-100-year event. Dr. Steven A. Schulz, who is serving children on the front line in upstate New York, and I outline some of the challenges primary care pediatricians have been facing and solutions that have succeeded.
Reduction in direct patient care and its consequences
Because of the unknowns of COVID-19, many parents have not wanted to bring their children to a medical office because of fear of contracting SARS-CoV-2. At the same time, pediatricians have restricted in-person visits to prevent spread of SARS-CoV-2 and to help flatten the curve of infection. Use of pediatric medical professional services, compared with last year, dropped by 52% in March 2020 and by 58% in April, according to FAIR Health, a nonprofit organization that manages a database of 31 million claims. This is resulting in decreased immunization rates, which increases concern for secondary spikes of other preventable illnesses; for example, data from the Centers for Disease Control and Prevention showed that, from mid-March to mid-April 2020, physicians in the Vaccines for Children program ordered 2.5 million fewer doses of vaccines and 250,000 fewer doses of measles-containing vaccines, compared with the same period in 2019. Fewer children are being seen for well visits, which means opportunities are lost for adequate monitoring of growth, development, physical wellness, and social determinants of health.
This is occurring at a time when families have been experiencing increased stress in terms of finances, social isolation, finding adequate child care, and serving as parent, teacher, and breadwinner. An increase in injuries is occurring because of inadequate parental supervision because many parents have been distracted while working from home. An increase in cases of severe abuse is occurring because schools, child care providers, physicians, and other mandated reporters in the community have decreased interaction with children. Children’s Hospital Colorado in Colorado Springs saw a 118% increase in the number of trauma cases in its ED between January and April 2020. Some of these were accidental injuries caused by falls or bicycle accidents, but there was a 200% increase in nonaccidental trauma, which was associated with a steep fall in calls to the state’s child abuse hotline. Academic gains are being lost, and there has been worry for a prolonged “summer slide” risk, especially for children living in poverty and children with developmental disabilities.
The COVID-19 pandemic also is affecting physicians and staff. As frontline personnel, we are at risk to contract the virus, and news media reminds us of severe illness and deaths among health care workers. The pandemic is affecting financial viability; estimated revenue of pediatric offices fell by 45% in March 2020 and 48% in April, compared with the previous year, according to FAIR Health. Nurses and staff have been furloughed. Practices have had to apply for grants and Paycheck Protection Program funds while extending credit lines.
Limited testing capability for SARS-CoV-2
Testing for SARS-CoV-2 has been variably available. There have been problems with false positive and especially false negative results (BMJ. 2020 May 12. doi: 10.1136/bmj.m1808).The best specimen collection method has yet to be determined. Blood testing for antibody has been touted, but it remains unclear if there is clinical benefit because a positive result offers no guarantee of immunity, and immunity may quickly wane. Perhaps widespread primary care office–based testing will be in place by the fall, with hope for future reliable point of care results.
Evolving knowledge regarding SARS-CoV-2 and MIS-C
It initially was thought that children were relatively spared from serious illness caused by COVID-19. Then reports of cases of newly identified multisystem inflammatory syndrome of children occurred. It has been unclear how children contribute to the spread of COVID-19 illness, although emerging evidence indicates it is lower than adult transmission. What will happen when children return to school and daycare in the fall?
The challenges have led to creative solutions for how to deliver care.
Adapting to telehealth to provide care
At least for the short term, HIPAA regulations have been relaxed to allow for video visits using platforms such as FaceTime, Skype, Zoom, Doximity, and Doxy.me. Some of these platforms are HIPAA compliant and will be long-term solutions; however, electronic medical record portals allowing for video visits are the more secure option, according to HIPAA.
It has been a learning experience to see what can be accomplished with a video visit. Taking a history and visual examination of injuries and rashes has been possible. Addressing mental health concerns through the video exchange generally has been effective.
However, video visits change the provider-patient interpersonal dynamic and offer only visual exam capabilities, compared with an in-person visit. We cannot look in ears, palpate a liver and spleen, touch and examine a joint or bone, or feel a rash. Video visits also are dependent on the quality of patient Internet access, sufficient data plans, and mutual capabilities to address the inevitable technological glitches on the provider’s end as well. Expanding information technology infrastructure ability and added licensure costs have occurred. Practices and health systems have been working with insurance companies to ensure telephone and video visits are reimbursed on a comparable level to in-office visits.
A new type of office visit and developing appropriate safety plans
Patients must be universally screened prior to arrival during appointment scheduling for well and illness visits. Patients aged older than 2 years and caregivers must wear masks on entering the facility. In many practices, patients are scheduled during specific sick or well visit time slots throughout the day. Waiting rooms chairs need to be spaced for 6-foot social distancing, and cars in the parking lot often serve as waiting rooms until staff can meet patients at the door and take them to the exam room. Alternate entrances, car-side exams, and drive-by and/or tent testing facilities often have become part of the new normal everyday practice. Creating virtual visit time blocks in provider’s schedules has allowed for decreased office congestion. Patients often are checked out from their room, as opposed to waiting in a line at a check out desk. Nurse triage protocols also have been adapted and enhanced to meet needs and concerns.
With the need for summer physicals and many regions opening up, a gradual return toward baseline has been evolving, although some of the twists of a “new normal” will stay in place. The new normal has been for providers and staff to wear surgical masks and face shields; sometimes N95 masks, gloves, and gowns have been needed. Cleaning rooms and equipment between patient visits has become a major, new time-consuming task. Acquiring and maintaining adequate supplies has been a challenge.
Summary
The American Academy of Pediatrics, CDC, and state and local health departments have been providing informative and regular updates, webinars, and best practices guidelines. Pediatricians, community organizations, schools, and mental health professionals have been collaborating, overcoming hurdles, and working together to help mitigate the effects of the pandemic on children, their families, and our communities. Continued education, cooperation, and adaptation will be needed in the months ahead. If there is a silver lining to this pandemic experience, it may be that families have grown closer together as they sheltered in place (and we have grown closer to our own families as well). One day perhaps a child who lived through this pandemic might be asked what it was like, and their recollection might be that it was a wonderful time because their parents stayed home all the time, took care of them, taught them their school work, and took lots of long family walks.
Dr. Schulz is pediatric medical director, Rochester (N.Y.) Regional Health. Dr. Pichichero is a specialist in pediatric infectious diseases and director of the Research Institute at Rochester (N.Y.) General Hospital. Dr. Schulz and Dr. Pichichero said they have no relevant financial disclosures. Email them at pdnews@mdedge.com.
This article was updated 7/16/2020.
With the COVID-19 pandemic, we are experiencing a once-in-a-100-year event. Dr. Steven A. Schulz, who is serving children on the front line in upstate New York, and I outline some of the challenges primary care pediatricians have been facing and solutions that have succeeded.
Reduction in direct patient care and its consequences
Because of the unknowns of COVID-19, many parents have not wanted to bring their children to a medical office because of fear of contracting SARS-CoV-2. At the same time, pediatricians have restricted in-person visits to prevent spread of SARS-CoV-2 and to help flatten the curve of infection. Use of pediatric medical professional services, compared with last year, dropped by 52% in March 2020 and by 58% in April, according to FAIR Health, a nonprofit organization that manages a database of 31 million claims. This is resulting in decreased immunization rates, which increases concern for secondary spikes of other preventable illnesses; for example, data from the Centers for Disease Control and Prevention showed that, from mid-March to mid-April 2020, physicians in the Vaccines for Children program ordered 2.5 million fewer doses of vaccines and 250,000 fewer doses of measles-containing vaccines, compared with the same period in 2019. Fewer children are being seen for well visits, which means opportunities are lost for adequate monitoring of growth, development, physical wellness, and social determinants of health.
This is occurring at a time when families have been experiencing increased stress in terms of finances, social isolation, finding adequate child care, and serving as parent, teacher, and breadwinner. An increase in injuries is occurring because of inadequate parental supervision because many parents have been distracted while working from home. An increase in cases of severe abuse is occurring because schools, child care providers, physicians, and other mandated reporters in the community have decreased interaction with children. Children’s Hospital Colorado in Colorado Springs saw a 118% increase in the number of trauma cases in its ED between January and April 2020. Some of these were accidental injuries caused by falls or bicycle accidents, but there was a 200% increase in nonaccidental trauma, which was associated with a steep fall in calls to the state’s child abuse hotline. Academic gains are being lost, and there has been worry for a prolonged “summer slide” risk, especially for children living in poverty and children with developmental disabilities.
The COVID-19 pandemic also is affecting physicians and staff. As frontline personnel, we are at risk to contract the virus, and news media reminds us of severe illness and deaths among health care workers. The pandemic is affecting financial viability; estimated revenue of pediatric offices fell by 45% in March 2020 and 48% in April, compared with the previous year, according to FAIR Health. Nurses and staff have been furloughed. Practices have had to apply for grants and Paycheck Protection Program funds while extending credit lines.
Limited testing capability for SARS-CoV-2
Testing for SARS-CoV-2 has been variably available. There have been problems with false positive and especially false negative results (BMJ. 2020 May 12. doi: 10.1136/bmj.m1808).The best specimen collection method has yet to be determined. Blood testing for antibody has been touted, but it remains unclear if there is clinical benefit because a positive result offers no guarantee of immunity, and immunity may quickly wane. Perhaps widespread primary care office–based testing will be in place by the fall, with hope for future reliable point of care results.
Evolving knowledge regarding SARS-CoV-2 and MIS-C
It initially was thought that children were relatively spared from serious illness caused by COVID-19. Then reports of cases of newly identified multisystem inflammatory syndrome of children occurred. It has been unclear how children contribute to the spread of COVID-19 illness, although emerging evidence indicates it is lower than adult transmission. What will happen when children return to school and daycare in the fall?
The challenges have led to creative solutions for how to deliver care.
Adapting to telehealth to provide care
At least for the short term, HIPAA regulations have been relaxed to allow for video visits using platforms such as FaceTime, Skype, Zoom, Doximity, and Doxy.me. Some of these platforms are HIPAA compliant and will be long-term solutions; however, electronic medical record portals allowing for video visits are the more secure option, according to HIPAA.
It has been a learning experience to see what can be accomplished with a video visit. Taking a history and visual examination of injuries and rashes has been possible. Addressing mental health concerns through the video exchange generally has been effective.
However, video visits change the provider-patient interpersonal dynamic and offer only visual exam capabilities, compared with an in-person visit. We cannot look in ears, palpate a liver and spleen, touch and examine a joint or bone, or feel a rash. Video visits also are dependent on the quality of patient Internet access, sufficient data plans, and mutual capabilities to address the inevitable technological glitches on the provider’s end as well. Expanding information technology infrastructure ability and added licensure costs have occurred. Practices and health systems have been working with insurance companies to ensure telephone and video visits are reimbursed on a comparable level to in-office visits.
A new type of office visit and developing appropriate safety plans
Patients must be universally screened prior to arrival during appointment scheduling for well and illness visits. Patients aged older than 2 years and caregivers must wear masks on entering the facility. In many practices, patients are scheduled during specific sick or well visit time slots throughout the day. Waiting rooms chairs need to be spaced for 6-foot social distancing, and cars in the parking lot often serve as waiting rooms until staff can meet patients at the door and take them to the exam room. Alternate entrances, car-side exams, and drive-by and/or tent testing facilities often have become part of the new normal everyday practice. Creating virtual visit time blocks in provider’s schedules has allowed for decreased office congestion. Patients often are checked out from their room, as opposed to waiting in a line at a check out desk. Nurse triage protocols also have been adapted and enhanced to meet needs and concerns.
With the need for summer physicals and many regions opening up, a gradual return toward baseline has been evolving, although some of the twists of a “new normal” will stay in place. The new normal has been for providers and staff to wear surgical masks and face shields; sometimes N95 masks, gloves, and gowns have been needed. Cleaning rooms and equipment between patient visits has become a major, new time-consuming task. Acquiring and maintaining adequate supplies has been a challenge.
Summary
The American Academy of Pediatrics, CDC, and state and local health departments have been providing informative and regular updates, webinars, and best practices guidelines. Pediatricians, community organizations, schools, and mental health professionals have been collaborating, overcoming hurdles, and working together to help mitigate the effects of the pandemic on children, their families, and our communities. Continued education, cooperation, and adaptation will be needed in the months ahead. If there is a silver lining to this pandemic experience, it may be that families have grown closer together as they sheltered in place (and we have grown closer to our own families as well). One day perhaps a child who lived through this pandemic might be asked what it was like, and their recollection might be that it was a wonderful time because their parents stayed home all the time, took care of them, taught them their school work, and took lots of long family walks.
Dr. Schulz is pediatric medical director, Rochester (N.Y.) Regional Health. Dr. Pichichero is a specialist in pediatric infectious diseases and director of the Research Institute at Rochester (N.Y.) General Hospital. Dr. Schulz and Dr. Pichichero said they have no relevant financial disclosures. Email them at pdnews@mdedge.com.
This article was updated 7/16/2020.
Why is AOM frequency decreasing in the pneumococcal conjugate vaccine era?
In 2000, pneumococcal conjugate vaccine 7 (PCV7) was introduced in the United States, and in 2010, PCV13 was introduced. When each of those vaccines were used, they reduced acute otitis media (AOM) incidence caused by the pneumococcal types included in the vaccines. In the time frame of those vaccine introductions, about one-third of AOM cases occurred because of pneumococci and half of those cases occurred because of strains expressing the serotypes in the two formulations of the vaccines. Efficacy is about 70% for AOM prevention for PCVs. The math matches clinical trial results that have shown about an 11%-12% reduction of all AOM attributable to PCVs. However, our group continues to do tympanocentesis to track the etiology of AOM, and we have reported that elimination of strains of pneumococci expressing capsular types included in the PCVs has been followed by emergence of replacement strains of pneumococci that express non-PCV capsules. We also have shown that Haemophilus influenzae has increased proportionally as a cause of AOM and is the most frequent cause of recurrent AOM. So what else is going on?
My colleague, Stephen I. Pelton, MD, – another ID Consult columnist – is a coauthor of a paper along with Ron Dagan, MD; Lauren Bakaletz, PhD; and Robert Cohen, MD, (all major figures in pneumococcal disease or AOM) that was published in Lancet Infectious Diseases (Dagan R et al. Lancet Infect Dis. 2016 Apr;16[4]:480-92.). They gathered evidence suggesting that prevention of early AOM episodes caused by pneumococci expressing PCV serotypes resulted in a reduction of subsequent complex cases caused by nonvaccine serotypes and other otopathogens. Thus, PCVs may have an impact on AOM indirectly attributable to vaccination.
However, the American Academy of Pediatrics made several recommendations in the 2004 and 2013 guidelines for diagnosis and management of AOM that had a remarkable impact in reducing the frequency that this infection is diagnosed and treated as well. The recommendations included:
- Stricter diagnostic criteria in 2004 that became more strict in 2013 requiring bulging of the eardrum.
- Introduction of “watchful waiting” as an option in management that possibly led to no antibiotic treatment.
- Introduction of delayed prescription of antibiotic when diagnosis was uncertain that possibly led to no antibiotic treatment.
- Endorsement of specific antibiotics with the greatest anticipated efficacy taking into consideration spectrum of activity, safety, and costs.
In the same general time frame, a second development occurred: The Centers for Disease Control and Prevention launched a national campaign to reduce unnecessary and inappropriate antibiotic use in an effort to reduce rising antibiotic resistance among bacteria. The public media and professional communication campaign emphasized that antibiotic treatment carried with it risks that should be considered by patients and clinicians.
Because of the AAP and CDC recommendations, clinicians diagnosed AOM less frequently, and they treated it less frequently. Parents of children took note of the fact that their children with viral upper respiratory infections suspected to have AOM were diagnosed with AOM less often; even when a diagnosis was made, an antibiotic was prescribed less often. Therefore, parents brought their children to clinicians less often when their child had a viral upper respiratory infections or when they suspected AOM.
In addition, guidelines endorsed specific antibiotics that had better efficacy in treatment of AOM. Therefore, when clinicians did treat the infection with antibiotics, they used more effective drugs resulting in fewer treatment failures. This gives the impression of less-frequent AOM as well.
Both universal PCV use and universal influenza vaccine use have been endorsed in recent years, and uptake of that recommendation has increased over time. Clinical trials have shown that influenza is a common virus associated with secondary bacterial AOM.
Lastly, returning to antibiotic use, we now increasingly appreciate the adverse effect on the natural microbiome of the nasopharynx and gut when antibiotics are given. Natural resistance provided by commensals is disrupted when antibiotics are given. This may allow otopathogens to colonize the nasopharynx more readily, an effect that may last for months after a single antibiotic course. We also appreciate more that the microbiome modulates our immune system favorably, so antibiotics that disrupt the microbiome may have an adverse effect on innate or adaptive immunity as well. These adverse consequences of antibiotic use on microbiome and immunity are reduced when less antibiotics are given to children, as has been occurring over the past 2 decades.
Dr. Pichichero is a specialist in pediatric infectious diseases and director of the Research Institute at Rochester (N.Y.) General Hospital. He said he had no relevent financial disclosures. Email him at pdnews@mdedge.com.
In 2000, pneumococcal conjugate vaccine 7 (PCV7) was introduced in the United States, and in 2010, PCV13 was introduced. When each of those vaccines were used, they reduced acute otitis media (AOM) incidence caused by the pneumococcal types included in the vaccines. In the time frame of those vaccine introductions, about one-third of AOM cases occurred because of pneumococci and half of those cases occurred because of strains expressing the serotypes in the two formulations of the vaccines. Efficacy is about 70% for AOM prevention for PCVs. The math matches clinical trial results that have shown about an 11%-12% reduction of all AOM attributable to PCVs. However, our group continues to do tympanocentesis to track the etiology of AOM, and we have reported that elimination of strains of pneumococci expressing capsular types included in the PCVs has been followed by emergence of replacement strains of pneumococci that express non-PCV capsules. We also have shown that Haemophilus influenzae has increased proportionally as a cause of AOM and is the most frequent cause of recurrent AOM. So what else is going on?
My colleague, Stephen I. Pelton, MD, – another ID Consult columnist – is a coauthor of a paper along with Ron Dagan, MD; Lauren Bakaletz, PhD; and Robert Cohen, MD, (all major figures in pneumococcal disease or AOM) that was published in Lancet Infectious Diseases (Dagan R et al. Lancet Infect Dis. 2016 Apr;16[4]:480-92.). They gathered evidence suggesting that prevention of early AOM episodes caused by pneumococci expressing PCV serotypes resulted in a reduction of subsequent complex cases caused by nonvaccine serotypes and other otopathogens. Thus, PCVs may have an impact on AOM indirectly attributable to vaccination.
However, the American Academy of Pediatrics made several recommendations in the 2004 and 2013 guidelines for diagnosis and management of AOM that had a remarkable impact in reducing the frequency that this infection is diagnosed and treated as well. The recommendations included:
- Stricter diagnostic criteria in 2004 that became more strict in 2013 requiring bulging of the eardrum.
- Introduction of “watchful waiting” as an option in management that possibly led to no antibiotic treatment.
- Introduction of delayed prescription of antibiotic when diagnosis was uncertain that possibly led to no antibiotic treatment.
- Endorsement of specific antibiotics with the greatest anticipated efficacy taking into consideration spectrum of activity, safety, and costs.
In the same general time frame, a second development occurred: The Centers for Disease Control and Prevention launched a national campaign to reduce unnecessary and inappropriate antibiotic use in an effort to reduce rising antibiotic resistance among bacteria. The public media and professional communication campaign emphasized that antibiotic treatment carried with it risks that should be considered by patients and clinicians.
Because of the AAP and CDC recommendations, clinicians diagnosed AOM less frequently, and they treated it less frequently. Parents of children took note of the fact that their children with viral upper respiratory infections suspected to have AOM were diagnosed with AOM less often; even when a diagnosis was made, an antibiotic was prescribed less often. Therefore, parents brought their children to clinicians less often when their child had a viral upper respiratory infections or when they suspected AOM.
In addition, guidelines endorsed specific antibiotics that had better efficacy in treatment of AOM. Therefore, when clinicians did treat the infection with antibiotics, they used more effective drugs resulting in fewer treatment failures. This gives the impression of less-frequent AOM as well.
Both universal PCV use and universal influenza vaccine use have been endorsed in recent years, and uptake of that recommendation has increased over time. Clinical trials have shown that influenza is a common virus associated with secondary bacterial AOM.
Lastly, returning to antibiotic use, we now increasingly appreciate the adverse effect on the natural microbiome of the nasopharynx and gut when antibiotics are given. Natural resistance provided by commensals is disrupted when antibiotics are given. This may allow otopathogens to colonize the nasopharynx more readily, an effect that may last for months after a single antibiotic course. We also appreciate more that the microbiome modulates our immune system favorably, so antibiotics that disrupt the microbiome may have an adverse effect on innate or adaptive immunity as well. These adverse consequences of antibiotic use on microbiome and immunity are reduced when less antibiotics are given to children, as has been occurring over the past 2 decades.
Dr. Pichichero is a specialist in pediatric infectious diseases and director of the Research Institute at Rochester (N.Y.) General Hospital. He said he had no relevent financial disclosures. Email him at pdnews@mdedge.com.
In 2000, pneumococcal conjugate vaccine 7 (PCV7) was introduced in the United States, and in 2010, PCV13 was introduced. When each of those vaccines were used, they reduced acute otitis media (AOM) incidence caused by the pneumococcal types included in the vaccines. In the time frame of those vaccine introductions, about one-third of AOM cases occurred because of pneumococci and half of those cases occurred because of strains expressing the serotypes in the two formulations of the vaccines. Efficacy is about 70% for AOM prevention for PCVs. The math matches clinical trial results that have shown about an 11%-12% reduction of all AOM attributable to PCVs. However, our group continues to do tympanocentesis to track the etiology of AOM, and we have reported that elimination of strains of pneumococci expressing capsular types included in the PCVs has been followed by emergence of replacement strains of pneumococci that express non-PCV capsules. We also have shown that Haemophilus influenzae has increased proportionally as a cause of AOM and is the most frequent cause of recurrent AOM. So what else is going on?
My colleague, Stephen I. Pelton, MD, – another ID Consult columnist – is a coauthor of a paper along with Ron Dagan, MD; Lauren Bakaletz, PhD; and Robert Cohen, MD, (all major figures in pneumococcal disease or AOM) that was published in Lancet Infectious Diseases (Dagan R et al. Lancet Infect Dis. 2016 Apr;16[4]:480-92.). They gathered evidence suggesting that prevention of early AOM episodes caused by pneumococci expressing PCV serotypes resulted in a reduction of subsequent complex cases caused by nonvaccine serotypes and other otopathogens. Thus, PCVs may have an impact on AOM indirectly attributable to vaccination.
However, the American Academy of Pediatrics made several recommendations in the 2004 and 2013 guidelines for diagnosis and management of AOM that had a remarkable impact in reducing the frequency that this infection is diagnosed and treated as well. The recommendations included:
- Stricter diagnostic criteria in 2004 that became more strict in 2013 requiring bulging of the eardrum.
- Introduction of “watchful waiting” as an option in management that possibly led to no antibiotic treatment.
- Introduction of delayed prescription of antibiotic when diagnosis was uncertain that possibly led to no antibiotic treatment.
- Endorsement of specific antibiotics with the greatest anticipated efficacy taking into consideration spectrum of activity, safety, and costs.
In the same general time frame, a second development occurred: The Centers for Disease Control and Prevention launched a national campaign to reduce unnecessary and inappropriate antibiotic use in an effort to reduce rising antibiotic resistance among bacteria. The public media and professional communication campaign emphasized that antibiotic treatment carried with it risks that should be considered by patients and clinicians.
Because of the AAP and CDC recommendations, clinicians diagnosed AOM less frequently, and they treated it less frequently. Parents of children took note of the fact that their children with viral upper respiratory infections suspected to have AOM were diagnosed with AOM less often; even when a diagnosis was made, an antibiotic was prescribed less often. Therefore, parents brought their children to clinicians less often when their child had a viral upper respiratory infections or when they suspected AOM.
In addition, guidelines endorsed specific antibiotics that had better efficacy in treatment of AOM. Therefore, when clinicians did treat the infection with antibiotics, they used more effective drugs resulting in fewer treatment failures. This gives the impression of less-frequent AOM as well.
Both universal PCV use and universal influenza vaccine use have been endorsed in recent years, and uptake of that recommendation has increased over time. Clinical trials have shown that influenza is a common virus associated with secondary bacterial AOM.
Lastly, returning to antibiotic use, we now increasingly appreciate the adverse effect on the natural microbiome of the nasopharynx and gut when antibiotics are given. Natural resistance provided by commensals is disrupted when antibiotics are given. This may allow otopathogens to colonize the nasopharynx more readily, an effect that may last for months after a single antibiotic course. We also appreciate more that the microbiome modulates our immune system favorably, so antibiotics that disrupt the microbiome may have an adverse effect on innate or adaptive immunity as well. These adverse consequences of antibiotic use on microbiome and immunity are reduced when less antibiotics are given to children, as has been occurring over the past 2 decades.
Dr. Pichichero is a specialist in pediatric infectious diseases and director of the Research Institute at Rochester (N.Y.) General Hospital. He said he had no relevent financial disclosures. Email him at pdnews@mdedge.com.
New research in otitis media
New research was presented at the International Society for Otitis Media meeting in June 2019, which I attended. I would like to share a selection of new findings from the many presentations.
Transtympanic antibiotic delivery
Topical therapy has been used to treat only otitis externa and acute otitis media (AOM) with ear discharge. Giving antibiotics through the tympanic membrane could mitigate many of the concerns about antibiotic use driving antibiotic resistance of bacteria among children. Up to now, using antibiotics in the ear canal to treat AOM has not been considered because the tympanic membrane is highly impermeable to the transtympanic diffusion of any drugs. However, in recent years, a number of different drug delivery systems have been developed, and in some cases, animal studies have shown that noninvasive transtympanic delivery is possible so that drugs can reach high concentrations in the middle ear without damage. Nanovesicles and nanoliposomes that contain antibiotics and are small enough to pass through the eardrum have been developed and tested in animal models; these show promise. Ototopical administration of a drug called vinpocetine that was repurposed has been tested in mice and shown to reduce inflammation and mucus production in the middle ear during otitis media.
Biofilms
Antibiotic treatment failure can occur in AOM for several reasons. The treatment of choice, amoxicillin, for example may fail to achieve an adequate concentration because of poor absorption in the gastrointestinal tract or poor penetration into the middle ear. Or, the antibiotic chosen may not be effective because of resistance of the strain causing the infection. Another explanation, especially in recurrent AOM and chronic AOM, could be the presence of biofilms. Biofilms are multicellular bacterial communities incorporated in a polymeric, plasticlike matrix in which pathogens are protected from antibiotic activity. The biofilm provides a physical barrier to antibiotic penetration, and bacteria can persist in the middle ear and periodically cause a new AOM. If AOM persists or becomes a more chronic otitis media with effusion, the “glue ear” causes an environment in the middle ear that is low in oxygen. A low-oxygen environment is favorable to biofilms. Also one might expect that middle ear pus would have a low pH, but actual measurements show the pH is highly alkaline. Species of Haemophilus influenzae have been identified as more virulent when in an alkaline pH or the alkaline pH makes the H. influenzae persist better in the middle ear, perhaps in a biofilm. To eliminate biofilms and improve antibiotic efficacy, a vaccine against a protein expressed by H. influenzae has been developed. Antibodies against this protein have been shown to disrupt and prevent the formation of biofilms in an animal model.
Probiotics
The normal bacteria that live in the nasopharynx of children with recurrent AOM is now known to differ from that of children who experience infrequent AOM or remain AOM-free throughout childhood. The use of oral pre- and probiotics for AOM prophylaxis remains debated because the results of studies are conflicting and frequently show no effect. So the idea of using prebiotics or probiotics to create a favorable “microbiome” of the nose is under investigation. Two species of bacteria that are gathering the most attention are Corynebacterium species (a few types in particular) and a bacteria called Dolosigranulum pigrum. Delivery of the commensal species would be as a nose spray.
Vaccines
The use of pneumococcal conjugate vaccines (PCVs) has reduced the frequency of AOM caused by Streptococcus pneumoniae. PCVs are not as effective against AOM as they are against invasive pneumococcal disease, but they still help a lot. However, because there are now at least 96 different serotypes of the pneumococcus based on different capsular types, we see a pattern of replacement of disease-causing strains by new strains within a few years of introduction of a new formulation. We started with 7 serotypes (Prevnar 7) in year 2000, and it was replaced by the current formulation with 13 serotypes (Prevnar 13) in 2010. Replacements have occurred again so vaccine companies are making new formulations for the future that include more serotypes, up to 20 serotypes. But, technically and feasibility-wise there is a limit to making such vaccines. A vaccine based on killed unencapsulated bacteria has been tested for safety and immunogenicity in young children. There is no test so far for prevention of AOM. Another type of vaccine based on proteins expressed by the pneumococcus that could be vaccine targets was tested in American Navajo children, and it failed to be as efficacious as hoped.
Biomarkers.
Due to recurrent AOM or persistent otitis media with effusion, about 15% of children in the United States receive tympanostomy tubes. Among those who receive tubes, about 20% go on to receive a second set of tubes, often with adenotonsillectomy. To find a biomarker that could identify children likely to require a second set of tubes, the fluid in the middle ear was tested when a first set of tubes were inserted. If bacteria were detected by polymerase chain reaction (PCR) testing or if a profile of specific inflammatory cytokines was measured, those results could be used to predict a high likelihood for a second set of tubes.
Overdiagnosis
Diagnosis of AOM is challenging in young children, in whom it most frequently occurs. The ear canal is typically about 3 mm wide, the child struggles during the examination, and diagnostic skills are not taught in training, resulting in a high overdiagnosis rate. I presented data that suggest too many children who are not truly otitis prone have been classified as otitis prone based on incorrect clinical diagnosis. My colleagues and I found that 30% of children reach the threshold of three episodes of AOM in 6 months or four within a year when diagnosed by community pediatricians, similar to many other studies. Validated otoscopists (trained by experts with diagnosis definitively proven as at least 85% accurate using tympanocentesis) classify 15% of children as otitis prone – half as many. If tympanocentesis is used to prove middle ear fluid has bacterial pathogens (about 95% yield a bacterial otopathogen using culture and PCR), then about 10% of children are classified as otitis prone – one-third as many. This suggests that children clinically diagnosed by community-based pediatricians are overdiagnosed with AOM, perhaps three times more often than true. And that leads to overuse of antibiotics and referrals for tympanostomy tube surgery more often than should occur. So we need to improve diagnostic methods beyond otoscopy. New types of imaging for the eardrum and middle ear using novel technologies are in early clinical trials.
Immunity
The notion that young children get AOM because of Eustachian tube dysfunction in their early years of life (horizontal anatomy) may be true, but there is more to the story. After 10 years of work, the scientists in my research group have shown that children in the first 3 years of life can have an immune system that is suppressed – it is poorly responsive to pathogens and routine pediatric vaccines. Many features resemble a neonatal immune system, beginning life with a suppressed immune system or being in cytokine storm from birth. We introduced the term “prolonged neonatal-like immune profile (PNIP)” to give a general description of the immune responses we have found in otitis-prone children. They outgrow this. So the immune maturation is delayed but not permanent. It is mostly resolved by age 3 years. We found problems in both innate and adaptive immunity. It may be that the main explanation for recurrent AOM in the first years of life is PNIP. Scientists from Australia also reported immunity problems in Aboriginal children and they are very otitis prone, often progressing to chronic suppurative otitis media. Animal model studies of AOM show inadequate innate and adaptive immunity importantly contribute to the infection as well.
Dr. Pichichero is a specialist in pediatric infectious diseases and director of the Research Institute at Rochester (N.Y.) General Hospital. He has no conflicts to declare. Email him at pdnews@mdedge.com.
New research was presented at the International Society for Otitis Media meeting in June 2019, which I attended. I would like to share a selection of new findings from the many presentations.
Transtympanic antibiotic delivery
Topical therapy has been used to treat only otitis externa and acute otitis media (AOM) with ear discharge. Giving antibiotics through the tympanic membrane could mitigate many of the concerns about antibiotic use driving antibiotic resistance of bacteria among children. Up to now, using antibiotics in the ear canal to treat AOM has not been considered because the tympanic membrane is highly impermeable to the transtympanic diffusion of any drugs. However, in recent years, a number of different drug delivery systems have been developed, and in some cases, animal studies have shown that noninvasive transtympanic delivery is possible so that drugs can reach high concentrations in the middle ear without damage. Nanovesicles and nanoliposomes that contain antibiotics and are small enough to pass through the eardrum have been developed and tested in animal models; these show promise. Ototopical administration of a drug called vinpocetine that was repurposed has been tested in mice and shown to reduce inflammation and mucus production in the middle ear during otitis media.
Biofilms
Antibiotic treatment failure can occur in AOM for several reasons. The treatment of choice, amoxicillin, for example may fail to achieve an adequate concentration because of poor absorption in the gastrointestinal tract or poor penetration into the middle ear. Or, the antibiotic chosen may not be effective because of resistance of the strain causing the infection. Another explanation, especially in recurrent AOM and chronic AOM, could be the presence of biofilms. Biofilms are multicellular bacterial communities incorporated in a polymeric, plasticlike matrix in which pathogens are protected from antibiotic activity. The biofilm provides a physical barrier to antibiotic penetration, and bacteria can persist in the middle ear and periodically cause a new AOM. If AOM persists or becomes a more chronic otitis media with effusion, the “glue ear” causes an environment in the middle ear that is low in oxygen. A low-oxygen environment is favorable to biofilms. Also one might expect that middle ear pus would have a low pH, but actual measurements show the pH is highly alkaline. Species of Haemophilus influenzae have been identified as more virulent when in an alkaline pH or the alkaline pH makes the H. influenzae persist better in the middle ear, perhaps in a biofilm. To eliminate biofilms and improve antibiotic efficacy, a vaccine against a protein expressed by H. influenzae has been developed. Antibodies against this protein have been shown to disrupt and prevent the formation of biofilms in an animal model.
Probiotics
The normal bacteria that live in the nasopharynx of children with recurrent AOM is now known to differ from that of children who experience infrequent AOM or remain AOM-free throughout childhood. The use of oral pre- and probiotics for AOM prophylaxis remains debated because the results of studies are conflicting and frequently show no effect. So the idea of using prebiotics or probiotics to create a favorable “microbiome” of the nose is under investigation. Two species of bacteria that are gathering the most attention are Corynebacterium species (a few types in particular) and a bacteria called Dolosigranulum pigrum. Delivery of the commensal species would be as a nose spray.
Vaccines
The use of pneumococcal conjugate vaccines (PCVs) has reduced the frequency of AOM caused by Streptococcus pneumoniae. PCVs are not as effective against AOM as they are against invasive pneumococcal disease, but they still help a lot. However, because there are now at least 96 different serotypes of the pneumococcus based on different capsular types, we see a pattern of replacement of disease-causing strains by new strains within a few years of introduction of a new formulation. We started with 7 serotypes (Prevnar 7) in year 2000, and it was replaced by the current formulation with 13 serotypes (Prevnar 13) in 2010. Replacements have occurred again so vaccine companies are making new formulations for the future that include more serotypes, up to 20 serotypes. But, technically and feasibility-wise there is a limit to making such vaccines. A vaccine based on killed unencapsulated bacteria has been tested for safety and immunogenicity in young children. There is no test so far for prevention of AOM. Another type of vaccine based on proteins expressed by the pneumococcus that could be vaccine targets was tested in American Navajo children, and it failed to be as efficacious as hoped.
Biomarkers.
Due to recurrent AOM or persistent otitis media with effusion, about 15% of children in the United States receive tympanostomy tubes. Among those who receive tubes, about 20% go on to receive a second set of tubes, often with adenotonsillectomy. To find a biomarker that could identify children likely to require a second set of tubes, the fluid in the middle ear was tested when a first set of tubes were inserted. If bacteria were detected by polymerase chain reaction (PCR) testing or if a profile of specific inflammatory cytokines was measured, those results could be used to predict a high likelihood for a second set of tubes.
Overdiagnosis
Diagnosis of AOM is challenging in young children, in whom it most frequently occurs. The ear canal is typically about 3 mm wide, the child struggles during the examination, and diagnostic skills are not taught in training, resulting in a high overdiagnosis rate. I presented data that suggest too many children who are not truly otitis prone have been classified as otitis prone based on incorrect clinical diagnosis. My colleagues and I found that 30% of children reach the threshold of three episodes of AOM in 6 months or four within a year when diagnosed by community pediatricians, similar to many other studies. Validated otoscopists (trained by experts with diagnosis definitively proven as at least 85% accurate using tympanocentesis) classify 15% of children as otitis prone – half as many. If tympanocentesis is used to prove middle ear fluid has bacterial pathogens (about 95% yield a bacterial otopathogen using culture and PCR), then about 10% of children are classified as otitis prone – one-third as many. This suggests that children clinically diagnosed by community-based pediatricians are overdiagnosed with AOM, perhaps three times more often than true. And that leads to overuse of antibiotics and referrals for tympanostomy tube surgery more often than should occur. So we need to improve diagnostic methods beyond otoscopy. New types of imaging for the eardrum and middle ear using novel technologies are in early clinical trials.
Immunity
The notion that young children get AOM because of Eustachian tube dysfunction in their early years of life (horizontal anatomy) may be true, but there is more to the story. After 10 years of work, the scientists in my research group have shown that children in the first 3 years of life can have an immune system that is suppressed – it is poorly responsive to pathogens and routine pediatric vaccines. Many features resemble a neonatal immune system, beginning life with a suppressed immune system or being in cytokine storm from birth. We introduced the term “prolonged neonatal-like immune profile (PNIP)” to give a general description of the immune responses we have found in otitis-prone children. They outgrow this. So the immune maturation is delayed but not permanent. It is mostly resolved by age 3 years. We found problems in both innate and adaptive immunity. It may be that the main explanation for recurrent AOM in the first years of life is PNIP. Scientists from Australia also reported immunity problems in Aboriginal children and they are very otitis prone, often progressing to chronic suppurative otitis media. Animal model studies of AOM show inadequate innate and adaptive immunity importantly contribute to the infection as well.
Dr. Pichichero is a specialist in pediatric infectious diseases and director of the Research Institute at Rochester (N.Y.) General Hospital. He has no conflicts to declare. Email him at pdnews@mdedge.com.
New research was presented at the International Society for Otitis Media meeting in June 2019, which I attended. I would like to share a selection of new findings from the many presentations.
Transtympanic antibiotic delivery
Topical therapy has been used to treat only otitis externa and acute otitis media (AOM) with ear discharge. Giving antibiotics through the tympanic membrane could mitigate many of the concerns about antibiotic use driving antibiotic resistance of bacteria among children. Up to now, using antibiotics in the ear canal to treat AOM has not been considered because the tympanic membrane is highly impermeable to the transtympanic diffusion of any drugs. However, in recent years, a number of different drug delivery systems have been developed, and in some cases, animal studies have shown that noninvasive transtympanic delivery is possible so that drugs can reach high concentrations in the middle ear without damage. Nanovesicles and nanoliposomes that contain antibiotics and are small enough to pass through the eardrum have been developed and tested in animal models; these show promise. Ototopical administration of a drug called vinpocetine that was repurposed has been tested in mice and shown to reduce inflammation and mucus production in the middle ear during otitis media.
Biofilms
Antibiotic treatment failure can occur in AOM for several reasons. The treatment of choice, amoxicillin, for example may fail to achieve an adequate concentration because of poor absorption in the gastrointestinal tract or poor penetration into the middle ear. Or, the antibiotic chosen may not be effective because of resistance of the strain causing the infection. Another explanation, especially in recurrent AOM and chronic AOM, could be the presence of biofilms. Biofilms are multicellular bacterial communities incorporated in a polymeric, plasticlike matrix in which pathogens are protected from antibiotic activity. The biofilm provides a physical barrier to antibiotic penetration, and bacteria can persist in the middle ear and periodically cause a new AOM. If AOM persists or becomes a more chronic otitis media with effusion, the “glue ear” causes an environment in the middle ear that is low in oxygen. A low-oxygen environment is favorable to biofilms. Also one might expect that middle ear pus would have a low pH, but actual measurements show the pH is highly alkaline. Species of Haemophilus influenzae have been identified as more virulent when in an alkaline pH or the alkaline pH makes the H. influenzae persist better in the middle ear, perhaps in a biofilm. To eliminate biofilms and improve antibiotic efficacy, a vaccine against a protein expressed by H. influenzae has been developed. Antibodies against this protein have been shown to disrupt and prevent the formation of biofilms in an animal model.
Probiotics
The normal bacteria that live in the nasopharynx of children with recurrent AOM is now known to differ from that of children who experience infrequent AOM or remain AOM-free throughout childhood. The use of oral pre- and probiotics for AOM prophylaxis remains debated because the results of studies are conflicting and frequently show no effect. So the idea of using prebiotics or probiotics to create a favorable “microbiome” of the nose is under investigation. Two species of bacteria that are gathering the most attention are Corynebacterium species (a few types in particular) and a bacteria called Dolosigranulum pigrum. Delivery of the commensal species would be as a nose spray.
Vaccines
The use of pneumococcal conjugate vaccines (PCVs) has reduced the frequency of AOM caused by Streptococcus pneumoniae. PCVs are not as effective against AOM as they are against invasive pneumococcal disease, but they still help a lot. However, because there are now at least 96 different serotypes of the pneumococcus based on different capsular types, we see a pattern of replacement of disease-causing strains by new strains within a few years of introduction of a new formulation. We started with 7 serotypes (Prevnar 7) in year 2000, and it was replaced by the current formulation with 13 serotypes (Prevnar 13) in 2010. Replacements have occurred again so vaccine companies are making new formulations for the future that include more serotypes, up to 20 serotypes. But, technically and feasibility-wise there is a limit to making such vaccines. A vaccine based on killed unencapsulated bacteria has been tested for safety and immunogenicity in young children. There is no test so far for prevention of AOM. Another type of vaccine based on proteins expressed by the pneumococcus that could be vaccine targets was tested in American Navajo children, and it failed to be as efficacious as hoped.
Biomarkers.
Due to recurrent AOM or persistent otitis media with effusion, about 15% of children in the United States receive tympanostomy tubes. Among those who receive tubes, about 20% go on to receive a second set of tubes, often with adenotonsillectomy. To find a biomarker that could identify children likely to require a second set of tubes, the fluid in the middle ear was tested when a first set of tubes were inserted. If bacteria were detected by polymerase chain reaction (PCR) testing or if a profile of specific inflammatory cytokines was measured, those results could be used to predict a high likelihood for a second set of tubes.
Overdiagnosis
Diagnosis of AOM is challenging in young children, in whom it most frequently occurs. The ear canal is typically about 3 mm wide, the child struggles during the examination, and diagnostic skills are not taught in training, resulting in a high overdiagnosis rate. I presented data that suggest too many children who are not truly otitis prone have been classified as otitis prone based on incorrect clinical diagnosis. My colleagues and I found that 30% of children reach the threshold of three episodes of AOM in 6 months or four within a year when diagnosed by community pediatricians, similar to many other studies. Validated otoscopists (trained by experts with diagnosis definitively proven as at least 85% accurate using tympanocentesis) classify 15% of children as otitis prone – half as many. If tympanocentesis is used to prove middle ear fluid has bacterial pathogens (about 95% yield a bacterial otopathogen using culture and PCR), then about 10% of children are classified as otitis prone – one-third as many. This suggests that children clinically diagnosed by community-based pediatricians are overdiagnosed with AOM, perhaps three times more often than true. And that leads to overuse of antibiotics and referrals for tympanostomy tube surgery more often than should occur. So we need to improve diagnostic methods beyond otoscopy. New types of imaging for the eardrum and middle ear using novel technologies are in early clinical trials.
Immunity
The notion that young children get AOM because of Eustachian tube dysfunction in their early years of life (horizontal anatomy) may be true, but there is more to the story. After 10 years of work, the scientists in my research group have shown that children in the first 3 years of life can have an immune system that is suppressed – it is poorly responsive to pathogens and routine pediatric vaccines. Many features resemble a neonatal immune system, beginning life with a suppressed immune system or being in cytokine storm from birth. We introduced the term “prolonged neonatal-like immune profile (PNIP)” to give a general description of the immune responses we have found in otitis-prone children. They outgrow this. So the immune maturation is delayed but not permanent. It is mostly resolved by age 3 years. We found problems in both innate and adaptive immunity. It may be that the main explanation for recurrent AOM in the first years of life is PNIP. Scientists from Australia also reported immunity problems in Aboriginal children and they are very otitis prone, often progressing to chronic suppurative otitis media. Animal model studies of AOM show inadequate innate and adaptive immunity importantly contribute to the infection as well.
Dr. Pichichero is a specialist in pediatric infectious diseases and director of the Research Institute at Rochester (N.Y.) General Hospital. He has no conflicts to declare. Email him at pdnews@mdedge.com.
Children who are coughing: Is it flu or bacterial pneumonia?
We are in the middle of flu season, and many of our patients are coughing. Is it the flu or might the child have a secondary bacterial pneumonia? Let’s start with the history for a tip off. The course of flu and respiratory viral infections in general involves a typical pattern of timing for fever and cough.
A late-developing fever or fever that subsides then recurs should raise concern. A prolonged cough or cough that subsides then recurs also should raise concern. The respiratory rate and chest retractions are key physical findings that can aid in distinguishing children with bacterial pneumonia. Rales and decreased breath sounds in lung segments are best heard with deep breaths.
What diagnostic laboratory and imaging tests should be used
Fortunately, rapid tests to detect influenza are available, and many providers have added those to their laboratory evaluation. A complete blood count and differential may be helpful. If a pulse oximeter is available, checking oxygen saturation might be helpful. The American Academy of Pediatrics community pneumonia guideline states that routine chest radiographs are not necessary for the confirmation of suspected community-acquired pneumonia (CAP) in patients well enough to be treated in the outpatient setting (Clin Inf Dis. 2011 Oct;53[7]:e25–e76). Blood cultures should not be performed routinely in nontoxic, fully immunized children with CAP managed in the outpatient setting.
What antibiotic should be used
Antimicrobial therapy is not routinely required for preschool-aged children with cough, even cough caused by CAP, because viral pathogens are responsible for the great majority of clinical disease. If the diagnosis of CAP is made, the AAP endorses amoxicillin as first-line therapy for previously healthy, appropriately immunized infants and preschool children with mild to moderate CAP suspected to be of bacterial origin. For previously healthy, appropriately immunized school-aged children and adolescents with mild to moderate CAP, amoxicillin is recommended for treatment of Streptococcus pneumoniae, the most prominent invasive bacterial pathogen.
However, the treatment paradigm is complicated because Mycoplasma pneumoniae also should be considered in management decisions. Children with signs and symptoms suspicious for M. pneumoniae should be tested to help guide antibiotic selection. This may be a simple bedside cold agglutinin test. The highest incidence of Mycoplasma pneumonia is in 5- to 20-year-olds (51% in 5- to 9-year-olds, 74% in 9- to 15-year-olds, and 3%-18% in adults with pneumonia), but 9% of CAP occurs in patients younger than 5 years old. The clinical features of Mycoplasma pneumonia resemble influenza: The patient has gradual onset of headache, malaise, fever, sore throat, and cough. Mycoplasma pneumonia has a similar incidence of productive cough, rales, and diarrhea as pneumococcal CAP, but with more frequent upper respiratory symptoms and a normal leukocyte count. Mycoplasma bronchopneumonia occurs 30 times more frequently than Mycoplasma lobar pneumonia. The radiologic features of Mycoplasma is typical of a bronchopneumonia, usually involving a single lobe, subsegmental atelectasis, peribronchial thickening, and streaky interstitial densities. While Mycoplasma pneumonia is usually self-limited, the duration of illness is shortened by oral treatment with doxycycline, erythromycin, clarithromycin, or azithromycin.
What is the appropriate duration of antimicrobial therapy
Recommendations by the AAP for CAP note that treatment courses of 10 days have been best studied, although shorter courses may be just as effective, particularly for mild disease managed on an outpatient basis.
When should children be hospitalized
Dr. Pichichero is a specialist in pediatric infectious diseases and director of the Research Institute at Rochester (N.Y.) General Hospital. He had no conflicts to declare. Email him at pdnews@mdedge.com.
We are in the middle of flu season, and many of our patients are coughing. Is it the flu or might the child have a secondary bacterial pneumonia? Let’s start with the history for a tip off. The course of flu and respiratory viral infections in general involves a typical pattern of timing for fever and cough.
A late-developing fever or fever that subsides then recurs should raise concern. A prolonged cough or cough that subsides then recurs also should raise concern. The respiratory rate and chest retractions are key physical findings that can aid in distinguishing children with bacterial pneumonia. Rales and decreased breath sounds in lung segments are best heard with deep breaths.
What diagnostic laboratory and imaging tests should be used
Fortunately, rapid tests to detect influenza are available, and many providers have added those to their laboratory evaluation. A complete blood count and differential may be helpful. If a pulse oximeter is available, checking oxygen saturation might be helpful. The American Academy of Pediatrics community pneumonia guideline states that routine chest radiographs are not necessary for the confirmation of suspected community-acquired pneumonia (CAP) in patients well enough to be treated in the outpatient setting (Clin Inf Dis. 2011 Oct;53[7]:e25–e76). Blood cultures should not be performed routinely in nontoxic, fully immunized children with CAP managed in the outpatient setting.
What antibiotic should be used
Antimicrobial therapy is not routinely required for preschool-aged children with cough, even cough caused by CAP, because viral pathogens are responsible for the great majority of clinical disease. If the diagnosis of CAP is made, the AAP endorses amoxicillin as first-line therapy for previously healthy, appropriately immunized infants and preschool children with mild to moderate CAP suspected to be of bacterial origin. For previously healthy, appropriately immunized school-aged children and adolescents with mild to moderate CAP, amoxicillin is recommended for treatment of Streptococcus pneumoniae, the most prominent invasive bacterial pathogen.
However, the treatment paradigm is complicated because Mycoplasma pneumoniae also should be considered in management decisions. Children with signs and symptoms suspicious for M. pneumoniae should be tested to help guide antibiotic selection. This may be a simple bedside cold agglutinin test. The highest incidence of Mycoplasma pneumonia is in 5- to 20-year-olds (51% in 5- to 9-year-olds, 74% in 9- to 15-year-olds, and 3%-18% in adults with pneumonia), but 9% of CAP occurs in patients younger than 5 years old. The clinical features of Mycoplasma pneumonia resemble influenza: The patient has gradual onset of headache, malaise, fever, sore throat, and cough. Mycoplasma pneumonia has a similar incidence of productive cough, rales, and diarrhea as pneumococcal CAP, but with more frequent upper respiratory symptoms and a normal leukocyte count. Mycoplasma bronchopneumonia occurs 30 times more frequently than Mycoplasma lobar pneumonia. The radiologic features of Mycoplasma is typical of a bronchopneumonia, usually involving a single lobe, subsegmental atelectasis, peribronchial thickening, and streaky interstitial densities. While Mycoplasma pneumonia is usually self-limited, the duration of illness is shortened by oral treatment with doxycycline, erythromycin, clarithromycin, or azithromycin.
What is the appropriate duration of antimicrobial therapy
Recommendations by the AAP for CAP note that treatment courses of 10 days have been best studied, although shorter courses may be just as effective, particularly for mild disease managed on an outpatient basis.
When should children be hospitalized
Dr. Pichichero is a specialist in pediatric infectious diseases and director of the Research Institute at Rochester (N.Y.) General Hospital. He had no conflicts to declare. Email him at pdnews@mdedge.com.
We are in the middle of flu season, and many of our patients are coughing. Is it the flu or might the child have a secondary bacterial pneumonia? Let’s start with the history for a tip off. The course of flu and respiratory viral infections in general involves a typical pattern of timing for fever and cough.
A late-developing fever or fever that subsides then recurs should raise concern. A prolonged cough or cough that subsides then recurs also should raise concern. The respiratory rate and chest retractions are key physical findings that can aid in distinguishing children with bacterial pneumonia. Rales and decreased breath sounds in lung segments are best heard with deep breaths.
What diagnostic laboratory and imaging tests should be used
Fortunately, rapid tests to detect influenza are available, and many providers have added those to their laboratory evaluation. A complete blood count and differential may be helpful. If a pulse oximeter is available, checking oxygen saturation might be helpful. The American Academy of Pediatrics community pneumonia guideline states that routine chest radiographs are not necessary for the confirmation of suspected community-acquired pneumonia (CAP) in patients well enough to be treated in the outpatient setting (Clin Inf Dis. 2011 Oct;53[7]:e25–e76). Blood cultures should not be performed routinely in nontoxic, fully immunized children with CAP managed in the outpatient setting.
What antibiotic should be used
Antimicrobial therapy is not routinely required for preschool-aged children with cough, even cough caused by CAP, because viral pathogens are responsible for the great majority of clinical disease. If the diagnosis of CAP is made, the AAP endorses amoxicillin as first-line therapy for previously healthy, appropriately immunized infants and preschool children with mild to moderate CAP suspected to be of bacterial origin. For previously healthy, appropriately immunized school-aged children and adolescents with mild to moderate CAP, amoxicillin is recommended for treatment of Streptococcus pneumoniae, the most prominent invasive bacterial pathogen.
However, the treatment paradigm is complicated because Mycoplasma pneumoniae also should be considered in management decisions. Children with signs and symptoms suspicious for M. pneumoniae should be tested to help guide antibiotic selection. This may be a simple bedside cold agglutinin test. The highest incidence of Mycoplasma pneumonia is in 5- to 20-year-olds (51% in 5- to 9-year-olds, 74% in 9- to 15-year-olds, and 3%-18% in adults with pneumonia), but 9% of CAP occurs in patients younger than 5 years old. The clinical features of Mycoplasma pneumonia resemble influenza: The patient has gradual onset of headache, malaise, fever, sore throat, and cough. Mycoplasma pneumonia has a similar incidence of productive cough, rales, and diarrhea as pneumococcal CAP, but with more frequent upper respiratory symptoms and a normal leukocyte count. Mycoplasma bronchopneumonia occurs 30 times more frequently than Mycoplasma lobar pneumonia. The radiologic features of Mycoplasma is typical of a bronchopneumonia, usually involving a single lobe, subsegmental atelectasis, peribronchial thickening, and streaky interstitial densities. While Mycoplasma pneumonia is usually self-limited, the duration of illness is shortened by oral treatment with doxycycline, erythromycin, clarithromycin, or azithromycin.
What is the appropriate duration of antimicrobial therapy
Recommendations by the AAP for CAP note that treatment courses of 10 days have been best studied, although shorter courses may be just as effective, particularly for mild disease managed on an outpatient basis.
When should children be hospitalized
Dr. Pichichero is a specialist in pediatric infectious diseases and director of the Research Institute at Rochester (N.Y.) General Hospital. He had no conflicts to declare. Email him at pdnews@mdedge.com.
The powerful virus inflammatory response
Inflammation is a double-edged sword. Controlled and modest proinflammatory responses can enhance host immunity against viruses and decrease bacterial colonization and infection, whereas excessive uncontrolled proinflammatory responses may increase the susceptibility to bacterial colonization and secondary infection to facilitate disease pathogenesis. The immune system produces both proinflammatory and anti-inflammatory cytokines and chemokines. It is a balanced response that is key to maintaining good health.
Viral upper respiratory tract infections (URIs) are caused by rhinoviruses, coronaviruses, enteroviruses, respiratory syncytial viruses, influenza A and B viruses, parainfluenza viruses, adenoviruses, and human metapneumoviruses. Viruses are powerful. In the nose, they induce hypersecretion of mucus, slow cilia beating, up-regulate nasal epithelial cell receptors to facilitate bacterial attachment, suppress neutrophil function, and cause increased release of proinflammatory cytokines and chemokines. All these actions by respiratory viruses promote bacterial overgrowth in the nasopharynx and thereby facilitate bacterial superinfections. In fact, progression in pathogenesis of the common bacterial respiratory infections – acute otitis media, acute sinusitis, acute conjunctivitis, and pneumonia – almost always is preceded by a viral URI. Viruses activate multiple target cells in the upper respiratory tract to produce an array of proinflammatory cytokines and chemokines. The symptoms of a viral URI resolve coinciding with an anti-inflammatory response and adaptive immunity.
In recent work, we found a higher frequency of viral URIs in children who experienced more frequent acute otitis media (AOM). We sought to understand why this might occur by comparing levels of inflammatory cytokines/chemokines in the nose during viral URI that did not precipitate AOM versus when a viral URI precipitated an AOM episode. When a child had a viral URI but did not go on to experience an AOM, the child had higher proinflammatory responses than when the viral URI precipitated an AOM. When differences of levels of proinflammatory cytokines/chemokines were compared in otitis-prone and non–otitis-prone children, lower nasal responses were associated with higher otitis-prone classification frequency (Clin Infect Dis. 2018. doi: 10.1093/cid/ciy750).
The powerful virus and the inflammatory response it can induce also play a major role in allergy and asthma. Viral URIs enhance allergic sensitization to respiratory viruses, such as influenza and respiratory syncytial virus, cause cytopathic damage to airway epithelium, promote excessive proinflammatory cytokine/chemokine production, and increase the exposure of allergens and irritants to antigen-presenting cells. Viral infections also may induce the release of epithelial mediators and cytokines that may propagate eosinophilia. Viral URIs, particularly with respiratory syncytial virus and rhinovirus, are the most common causes of wheezing in children, and they have important influences on the development of asthma. Studies have shown that viral infections trigger up to 85% of asthma exacerbations in school-aged children.
Because this column is being published during the winter, a brief discussion of influenza as a powerful virus is appropriate. Influenza occurs in winter outbreaks of varying extent every year. The severity of the influenza season reflects the changing nature of the antigenic properties of influenza viruses, and their spread depends on susceptibility of the population. Influenza outbreaks typically peak over a 2-3 week period and last for 2-3 months. Most outbreaks have attack rates of 10%-20% in children. There may be variations in disease severity caused by different influenza virus types. The symptoms are caused by excessive proinflammatory cytokine/chemokine production in the nose and lung.
Influenza and other viruses can precipitate the systemic inflammatory response syndrome (SIRS), a manifestation of extreme immune dysregulation resulting in organ dysfunction that clinically resembles bacterial sepsis. In this syndrome, tissues remote from the original insult display the cardinal signs of inflammation, including vasodilation, increased microvascular permeability, and leukocyte accumulation. SIRS is another example of the double-edged sword of inflammation.
The onset and progression of SIRS occurs because of dysregulation of the normal inflammatory response, usually with an increase in both proinflammatory and anti-inflammatory cytokines and chemokines, initiating a chain of events that leads to organ failure.
Dr. Pichichero is a specialist in pediatric infectious diseases and director of the Research Institute at Rochester (N.Y.) General Hospital. He reported having no conflicts of interest. Email him at pdnews@mdedge.com.
Inflammation is a double-edged sword. Controlled and modest proinflammatory responses can enhance host immunity against viruses and decrease bacterial colonization and infection, whereas excessive uncontrolled proinflammatory responses may increase the susceptibility to bacterial colonization and secondary infection to facilitate disease pathogenesis. The immune system produces both proinflammatory and anti-inflammatory cytokines and chemokines. It is a balanced response that is key to maintaining good health.
Viral upper respiratory tract infections (URIs) are caused by rhinoviruses, coronaviruses, enteroviruses, respiratory syncytial viruses, influenza A and B viruses, parainfluenza viruses, adenoviruses, and human metapneumoviruses. Viruses are powerful. In the nose, they induce hypersecretion of mucus, slow cilia beating, up-regulate nasal epithelial cell receptors to facilitate bacterial attachment, suppress neutrophil function, and cause increased release of proinflammatory cytokines and chemokines. All these actions by respiratory viruses promote bacterial overgrowth in the nasopharynx and thereby facilitate bacterial superinfections. In fact, progression in pathogenesis of the common bacterial respiratory infections – acute otitis media, acute sinusitis, acute conjunctivitis, and pneumonia – almost always is preceded by a viral URI. Viruses activate multiple target cells in the upper respiratory tract to produce an array of proinflammatory cytokines and chemokines. The symptoms of a viral URI resolve coinciding with an anti-inflammatory response and adaptive immunity.
In recent work, we found a higher frequency of viral URIs in children who experienced more frequent acute otitis media (AOM). We sought to understand why this might occur by comparing levels of inflammatory cytokines/chemokines in the nose during viral URI that did not precipitate AOM versus when a viral URI precipitated an AOM episode. When a child had a viral URI but did not go on to experience an AOM, the child had higher proinflammatory responses than when the viral URI precipitated an AOM. When differences of levels of proinflammatory cytokines/chemokines were compared in otitis-prone and non–otitis-prone children, lower nasal responses were associated with higher otitis-prone classification frequency (Clin Infect Dis. 2018. doi: 10.1093/cid/ciy750).
The powerful virus and the inflammatory response it can induce also play a major role in allergy and asthma. Viral URIs enhance allergic sensitization to respiratory viruses, such as influenza and respiratory syncytial virus, cause cytopathic damage to airway epithelium, promote excessive proinflammatory cytokine/chemokine production, and increase the exposure of allergens and irritants to antigen-presenting cells. Viral infections also may induce the release of epithelial mediators and cytokines that may propagate eosinophilia. Viral URIs, particularly with respiratory syncytial virus and rhinovirus, are the most common causes of wheezing in children, and they have important influences on the development of asthma. Studies have shown that viral infections trigger up to 85% of asthma exacerbations in school-aged children.
Because this column is being published during the winter, a brief discussion of influenza as a powerful virus is appropriate. Influenza occurs in winter outbreaks of varying extent every year. The severity of the influenza season reflects the changing nature of the antigenic properties of influenza viruses, and their spread depends on susceptibility of the population. Influenza outbreaks typically peak over a 2-3 week period and last for 2-3 months. Most outbreaks have attack rates of 10%-20% in children. There may be variations in disease severity caused by different influenza virus types. The symptoms are caused by excessive proinflammatory cytokine/chemokine production in the nose and lung.
Influenza and other viruses can precipitate the systemic inflammatory response syndrome (SIRS), a manifestation of extreme immune dysregulation resulting in organ dysfunction that clinically resembles bacterial sepsis. In this syndrome, tissues remote from the original insult display the cardinal signs of inflammation, including vasodilation, increased microvascular permeability, and leukocyte accumulation. SIRS is another example of the double-edged sword of inflammation.
The onset and progression of SIRS occurs because of dysregulation of the normal inflammatory response, usually with an increase in both proinflammatory and anti-inflammatory cytokines and chemokines, initiating a chain of events that leads to organ failure.
Dr. Pichichero is a specialist in pediatric infectious diseases and director of the Research Institute at Rochester (N.Y.) General Hospital. He reported having no conflicts of interest. Email him at pdnews@mdedge.com.
Inflammation is a double-edged sword. Controlled and modest proinflammatory responses can enhance host immunity against viruses and decrease bacterial colonization and infection, whereas excessive uncontrolled proinflammatory responses may increase the susceptibility to bacterial colonization and secondary infection to facilitate disease pathogenesis. The immune system produces both proinflammatory and anti-inflammatory cytokines and chemokines. It is a balanced response that is key to maintaining good health.
Viral upper respiratory tract infections (URIs) are caused by rhinoviruses, coronaviruses, enteroviruses, respiratory syncytial viruses, influenza A and B viruses, parainfluenza viruses, adenoviruses, and human metapneumoviruses. Viruses are powerful. In the nose, they induce hypersecretion of mucus, slow cilia beating, up-regulate nasal epithelial cell receptors to facilitate bacterial attachment, suppress neutrophil function, and cause increased release of proinflammatory cytokines and chemokines. All these actions by respiratory viruses promote bacterial overgrowth in the nasopharynx and thereby facilitate bacterial superinfections. In fact, progression in pathogenesis of the common bacterial respiratory infections – acute otitis media, acute sinusitis, acute conjunctivitis, and pneumonia – almost always is preceded by a viral URI. Viruses activate multiple target cells in the upper respiratory tract to produce an array of proinflammatory cytokines and chemokines. The symptoms of a viral URI resolve coinciding with an anti-inflammatory response and adaptive immunity.
In recent work, we found a higher frequency of viral URIs in children who experienced more frequent acute otitis media (AOM). We sought to understand why this might occur by comparing levels of inflammatory cytokines/chemokines in the nose during viral URI that did not precipitate AOM versus when a viral URI precipitated an AOM episode. When a child had a viral URI but did not go on to experience an AOM, the child had higher proinflammatory responses than when the viral URI precipitated an AOM. When differences of levels of proinflammatory cytokines/chemokines were compared in otitis-prone and non–otitis-prone children, lower nasal responses were associated with higher otitis-prone classification frequency (Clin Infect Dis. 2018. doi: 10.1093/cid/ciy750).
The powerful virus and the inflammatory response it can induce also play a major role in allergy and asthma. Viral URIs enhance allergic sensitization to respiratory viruses, such as influenza and respiratory syncytial virus, cause cytopathic damage to airway epithelium, promote excessive proinflammatory cytokine/chemokine production, and increase the exposure of allergens and irritants to antigen-presenting cells. Viral infections also may induce the release of epithelial mediators and cytokines that may propagate eosinophilia. Viral URIs, particularly with respiratory syncytial virus and rhinovirus, are the most common causes of wheezing in children, and they have important influences on the development of asthma. Studies have shown that viral infections trigger up to 85% of asthma exacerbations in school-aged children.
Because this column is being published during the winter, a brief discussion of influenza as a powerful virus is appropriate. Influenza occurs in winter outbreaks of varying extent every year. The severity of the influenza season reflects the changing nature of the antigenic properties of influenza viruses, and their spread depends on susceptibility of the population. Influenza outbreaks typically peak over a 2-3 week period and last for 2-3 months. Most outbreaks have attack rates of 10%-20% in children. There may be variations in disease severity caused by different influenza virus types. The symptoms are caused by excessive proinflammatory cytokine/chemokine production in the nose and lung.
Influenza and other viruses can precipitate the systemic inflammatory response syndrome (SIRS), a manifestation of extreme immune dysregulation resulting in organ dysfunction that clinically resembles bacterial sepsis. In this syndrome, tissues remote from the original insult display the cardinal signs of inflammation, including vasodilation, increased microvascular permeability, and leukocyte accumulation. SIRS is another example of the double-edged sword of inflammation.
The onset and progression of SIRS occurs because of dysregulation of the normal inflammatory response, usually with an increase in both proinflammatory and anti-inflammatory cytokines and chemokines, initiating a chain of events that leads to organ failure.
Dr. Pichichero is a specialist in pediatric infectious diseases and director of the Research Institute at Rochester (N.Y.) General Hospital. He reported having no conflicts of interest. Email him at pdnews@mdedge.com.
Summer colds
Enteroviruses cause most summer colds. The enteroviruses include echoviruses, coxsackieviruses, numbered enteroviruses, and the polioviruses. Most summer colds seen in private practice are self limited, presenting with fever alone or clinically distinctive pictures such as hand-foot-and-mouth disease (HFMD), herpangina, or pleurodynia. However, enteroviruses also cause serious illnesses such as meningitis, myocarditis, encephalitis, and neonatal sepsis. Enterovirus infections often are confused with bacterial infections and treated unnecessarily with antibiotics.
Enteroviral infections spread predominantly by the fecal-oral route. Contaminated swimming pools also may serve as a source of transmission. Enteroviruses colonize the respiratory and the gastrointestinal tract. The infection spreads to the lymph nodes, where the virus replicates and an initial viremia occurs on approximately the third postexposure day. The viremia results in subsequent spread to the throat (herpangina), and/or hands and feet (HFMD), lungs (pleurodynia), heart (myocarditis) or meninges (viral meningitis). Infection at the secondary sites corresponds to the onset of clinical symptoms 4-6 days after exposure. The clinical manifestations of enteroviral infections result from the damage caused by the virus at the secondary sites of infection.
Enterovirus pharyngitis starts abruptly and often is accompanied by fever. Younger children may present with increased drooling, hands in the mouth, and refusal to eat. Older children complain of sore throat as well as headache, myalgias, and malaise. Mild vomiting and diarrhea commonly accompany the respiratory symptoms. Herpangina is a specific syndrome of enterovirus pharyngitis; children with this syndrome have fever and characteristic papulovesicular lesions on the anterior tonsillar pillars, soft palate, uvula, tonsils, and pharyngeal wall. The lesions are discrete and average five per patient. They do not appear in the anterior part of the mouth.
Hand-foot-and-mouth disease is well recognized by clinicians who care for young children. The child presents with fever and papulovesicular lesions within the mouth that quickly become ulcerated and papulovesicular lesions on the palms and soles. The palms and soles often are puffy and red, and the child may act as though her hands and feet hurt, refusing to use her hands or walk. The fever accompanying herpangina and HFMD usually lasts 3 or 4 days, but fever that persists for a week is not uncommon. The pharyngitis follows a pattern similar to the fever.
Pleurodynia has a sudden onset of pain in the chest or upper abdomen. The pain appears to be muscular in origin; its intensity varies. It can be excruciatingly severe and accompanied by sweating and pallor. Older children describe the pain as sharp and stabbing. It occurs in spasms that can last for a few minutes to a few hours. During spasms, the patient has rapid, shallow respirations that suggest pneumonia. The symptoms usually last 1 or 2 days, but the illness can be biphasic, with symptoms resolving only to reappear a few days later.
Gastrointestinal manifestations are almost universal in enterovirus infections. The most common symptoms are anorexia, nausea, vomiting, and diarrhea. They usually are not severe and often occur in combination with other symptoms, such as fever and sore throat. Abdominal pain may be the only manifestation of infection; when severe, it can mimic appendicitis.
Enterovirus infections once were thought to be mild diseases that lasted 2-3 days. But a study of 380 children aged 4-18 years during July to October from private pediatric practices found that illness is prolonged in many patients (Pediatrics. 1998 Nov;102[5]:1126-34). The mean duration of illness was found to be 10 days for myalgia-malaise syndrome, 7 days for herpangina, and 7 days for HFMD.
Spread of enteroviral infections within a household was common. More than 50% of children studied had a family member with enterovirus illness. Half of siblings and 25% of adults within the household of the index case contracted an enteroviral infection. Some had the same presentation as the index patient, but it was not uncommon for other household members to have quite different presentations. For example, the first child seen might present with hand-foot-and-mouth disease, and a few days later a sibling might be brought for care with myalgia-malaise, and the parent might appear ill and complain of pleurodynia.
Summer colds can be costly to families. The duration of the illness and the multitude of nonspecific symptoms sometimes leads to concern about a possible bacterial cause, which prompts a diagnostic workup, including laboratory tests and empiric treatment with antibiotics. The direct costs vary with the syndrome; stomatitis and HFMD are the least expensive to treat because the clinical picture is diagnostic with a single office visit, but a severe manifestation such as aseptic meningitis are expensive to treat with associated emergency department visits, spinal tap, and sometimes hospitalization.
Dr. Pichichero is a specialist in pediatric infectious diseases and director of the Research Institute at Rochester (N.Y.) General Hospital. He reported having no conflicts of interest. Email him at pdnews@mdedge.com.
Enteroviruses cause most summer colds. The enteroviruses include echoviruses, coxsackieviruses, numbered enteroviruses, and the polioviruses. Most summer colds seen in private practice are self limited, presenting with fever alone or clinically distinctive pictures such as hand-foot-and-mouth disease (HFMD), herpangina, or pleurodynia. However, enteroviruses also cause serious illnesses such as meningitis, myocarditis, encephalitis, and neonatal sepsis. Enterovirus infections often are confused with bacterial infections and treated unnecessarily with antibiotics.
Enteroviral infections spread predominantly by the fecal-oral route. Contaminated swimming pools also may serve as a source of transmission. Enteroviruses colonize the respiratory and the gastrointestinal tract. The infection spreads to the lymph nodes, where the virus replicates and an initial viremia occurs on approximately the third postexposure day. The viremia results in subsequent spread to the throat (herpangina), and/or hands and feet (HFMD), lungs (pleurodynia), heart (myocarditis) or meninges (viral meningitis). Infection at the secondary sites corresponds to the onset of clinical symptoms 4-6 days after exposure. The clinical manifestations of enteroviral infections result from the damage caused by the virus at the secondary sites of infection.
Enterovirus pharyngitis starts abruptly and often is accompanied by fever. Younger children may present with increased drooling, hands in the mouth, and refusal to eat. Older children complain of sore throat as well as headache, myalgias, and malaise. Mild vomiting and diarrhea commonly accompany the respiratory symptoms. Herpangina is a specific syndrome of enterovirus pharyngitis; children with this syndrome have fever and characteristic papulovesicular lesions on the anterior tonsillar pillars, soft palate, uvula, tonsils, and pharyngeal wall. The lesions are discrete and average five per patient. They do not appear in the anterior part of the mouth.
Hand-foot-and-mouth disease is well recognized by clinicians who care for young children. The child presents with fever and papulovesicular lesions within the mouth that quickly become ulcerated and papulovesicular lesions on the palms and soles. The palms and soles often are puffy and red, and the child may act as though her hands and feet hurt, refusing to use her hands or walk. The fever accompanying herpangina and HFMD usually lasts 3 or 4 days, but fever that persists for a week is not uncommon. The pharyngitis follows a pattern similar to the fever.
Pleurodynia has a sudden onset of pain in the chest or upper abdomen. The pain appears to be muscular in origin; its intensity varies. It can be excruciatingly severe and accompanied by sweating and pallor. Older children describe the pain as sharp and stabbing. It occurs in spasms that can last for a few minutes to a few hours. During spasms, the patient has rapid, shallow respirations that suggest pneumonia. The symptoms usually last 1 or 2 days, but the illness can be biphasic, with symptoms resolving only to reappear a few days later.
Gastrointestinal manifestations are almost universal in enterovirus infections. The most common symptoms are anorexia, nausea, vomiting, and diarrhea. They usually are not severe and often occur in combination with other symptoms, such as fever and sore throat. Abdominal pain may be the only manifestation of infection; when severe, it can mimic appendicitis.
Enterovirus infections once were thought to be mild diseases that lasted 2-3 days. But a study of 380 children aged 4-18 years during July to October from private pediatric practices found that illness is prolonged in many patients (Pediatrics. 1998 Nov;102[5]:1126-34). The mean duration of illness was found to be 10 days for myalgia-malaise syndrome, 7 days for herpangina, and 7 days for HFMD.
Spread of enteroviral infections within a household was common. More than 50% of children studied had a family member with enterovirus illness. Half of siblings and 25% of adults within the household of the index case contracted an enteroviral infection. Some had the same presentation as the index patient, but it was not uncommon for other household members to have quite different presentations. For example, the first child seen might present with hand-foot-and-mouth disease, and a few days later a sibling might be brought for care with myalgia-malaise, and the parent might appear ill and complain of pleurodynia.
Summer colds can be costly to families. The duration of the illness and the multitude of nonspecific symptoms sometimes leads to concern about a possible bacterial cause, which prompts a diagnostic workup, including laboratory tests and empiric treatment with antibiotics. The direct costs vary with the syndrome; stomatitis and HFMD are the least expensive to treat because the clinical picture is diagnostic with a single office visit, but a severe manifestation such as aseptic meningitis are expensive to treat with associated emergency department visits, spinal tap, and sometimes hospitalization.
Dr. Pichichero is a specialist in pediatric infectious diseases and director of the Research Institute at Rochester (N.Y.) General Hospital. He reported having no conflicts of interest. Email him at pdnews@mdedge.com.
Enteroviruses cause most summer colds. The enteroviruses include echoviruses, coxsackieviruses, numbered enteroviruses, and the polioviruses. Most summer colds seen in private practice are self limited, presenting with fever alone or clinically distinctive pictures such as hand-foot-and-mouth disease (HFMD), herpangina, or pleurodynia. However, enteroviruses also cause serious illnesses such as meningitis, myocarditis, encephalitis, and neonatal sepsis. Enterovirus infections often are confused with bacterial infections and treated unnecessarily with antibiotics.
Enteroviral infections spread predominantly by the fecal-oral route. Contaminated swimming pools also may serve as a source of transmission. Enteroviruses colonize the respiratory and the gastrointestinal tract. The infection spreads to the lymph nodes, where the virus replicates and an initial viremia occurs on approximately the third postexposure day. The viremia results in subsequent spread to the throat (herpangina), and/or hands and feet (HFMD), lungs (pleurodynia), heart (myocarditis) or meninges (viral meningitis). Infection at the secondary sites corresponds to the onset of clinical symptoms 4-6 days after exposure. The clinical manifestations of enteroviral infections result from the damage caused by the virus at the secondary sites of infection.
Enterovirus pharyngitis starts abruptly and often is accompanied by fever. Younger children may present with increased drooling, hands in the mouth, and refusal to eat. Older children complain of sore throat as well as headache, myalgias, and malaise. Mild vomiting and diarrhea commonly accompany the respiratory symptoms. Herpangina is a specific syndrome of enterovirus pharyngitis; children with this syndrome have fever and characteristic papulovesicular lesions on the anterior tonsillar pillars, soft palate, uvula, tonsils, and pharyngeal wall. The lesions are discrete and average five per patient. They do not appear in the anterior part of the mouth.
Hand-foot-and-mouth disease is well recognized by clinicians who care for young children. The child presents with fever and papulovesicular lesions within the mouth that quickly become ulcerated and papulovesicular lesions on the palms and soles. The palms and soles often are puffy and red, and the child may act as though her hands and feet hurt, refusing to use her hands or walk. The fever accompanying herpangina and HFMD usually lasts 3 or 4 days, but fever that persists for a week is not uncommon. The pharyngitis follows a pattern similar to the fever.
Pleurodynia has a sudden onset of pain in the chest or upper abdomen. The pain appears to be muscular in origin; its intensity varies. It can be excruciatingly severe and accompanied by sweating and pallor. Older children describe the pain as sharp and stabbing. It occurs in spasms that can last for a few minutes to a few hours. During spasms, the patient has rapid, shallow respirations that suggest pneumonia. The symptoms usually last 1 or 2 days, but the illness can be biphasic, with symptoms resolving only to reappear a few days later.
Gastrointestinal manifestations are almost universal in enterovirus infections. The most common symptoms are anorexia, nausea, vomiting, and diarrhea. They usually are not severe and often occur in combination with other symptoms, such as fever and sore throat. Abdominal pain may be the only manifestation of infection; when severe, it can mimic appendicitis.
Enterovirus infections once were thought to be mild diseases that lasted 2-3 days. But a study of 380 children aged 4-18 years during July to October from private pediatric practices found that illness is prolonged in many patients (Pediatrics. 1998 Nov;102[5]:1126-34). The mean duration of illness was found to be 10 days for myalgia-malaise syndrome, 7 days for herpangina, and 7 days for HFMD.
Spread of enteroviral infections within a household was common. More than 50% of children studied had a family member with enterovirus illness. Half of siblings and 25% of adults within the household of the index case contracted an enteroviral infection. Some had the same presentation as the index patient, but it was not uncommon for other household members to have quite different presentations. For example, the first child seen might present with hand-foot-and-mouth disease, and a few days later a sibling might be brought for care with myalgia-malaise, and the parent might appear ill and complain of pleurodynia.
Summer colds can be costly to families. The duration of the illness and the multitude of nonspecific symptoms sometimes leads to concern about a possible bacterial cause, which prompts a diagnostic workup, including laboratory tests and empiric treatment with antibiotics. The direct costs vary with the syndrome; stomatitis and HFMD are the least expensive to treat because the clinical picture is diagnostic with a single office visit, but a severe manifestation such as aseptic meningitis are expensive to treat with associated emergency department visits, spinal tap, and sometimes hospitalization.
Dr. Pichichero is a specialist in pediatric infectious diseases and director of the Research Institute at Rochester (N.Y.) General Hospital. He reported having no conflicts of interest. Email him at pdnews@mdedge.com.
Antibiotic choice for acute otitis media 2018
It’s a new year and a new respiratory season so my thoughts turn to the most common infection in pediatrics where an antibiotic might appropriately be prescribed – acute otitis media (AOM). The guidelines of the American Academy of Pediatrics were finalized in 2012 and published in 2013 and based on data that the AAP subcommittee considered. A recommendation emerged for amoxicillin to remain the treatment of choice if an antibiotic was to be prescribed at all, leaving the observation option as a continued consideration under defined clinical circumstances. The oral alternative antibiotics recommended were amoxicillin/clavulanate and cefdinir (Pediatrics. 2013. doi: 10.1542/peds.2012-3488).
Since the AAP subcommittee deliberated, changes have occurred in AOM etiology and the frequency of antibiotic resistance among the common bacteria that cause the infection. Our group in Rochester (N.Y.) continues to be the only site in the United States conducting a prospective assessment of AOM; we hope our data are generalizable to the entire country, but that is not certain. In Rochester, we saw an overall drop in AOM incidence after introduction of Prevnar 7 of about 10%-15% overall and that corresponded reasonably well with the frequency of AOM caused by Streptococcus pneumoniae involving the seven serotypes in the PCV7 vaccine. We then had a rebound in AOM infections, largely caused by serotype 19A, such that the overall incidence of AOM returned back to levels nearly the same as before PCV7 by 2010. With the introduction of Prevnar 13, and the dramatic reduction of serotype 19A nasal colonization – a necessary precursor of AOM – the incidence of AOM overall fell again, and compared with the pre-PCV7 era, I estimate that we are seeing about 20%-25% less AOM today.
In late 2017, we published an article describing the epidemiology of AOM in the PCV era (Pediatrics. 2017 Aug. doi: 10.1542/peds.2017-0181), in which we described changes in otopathogen distribution over time from 1996 through 2016. It showed that by end of 2016, the predominant bacteria causing AOM were Haemophilus influenzae, accounting for 60% of all AOM (52% detected by culture from tympanocentesis and another 8% detected by polymerase chain reaction). Among the H. influenzae from middle ear fluid, beta-lactamase production occurred in 45%. Therefore, according to principles of infectious disease antibiotic efficacy predictions, use of amoxicillin in standard dose or high dose would not eradicate about half of the H. influenzae causing AOM. In the table included in this column, I show calculations of predicted outcomes from amoxicillin, amoxicillin/clavulanate, and cefdinir treatment based on the projected otopathogen mix and resistance frequencies of 2016. Added to the data on H. influenzae I have included results of S. pneumoniae high nonsusceptibility at 5% of strains and beta-lactamase production by Moraxella catarrhalis at 100% of strains. 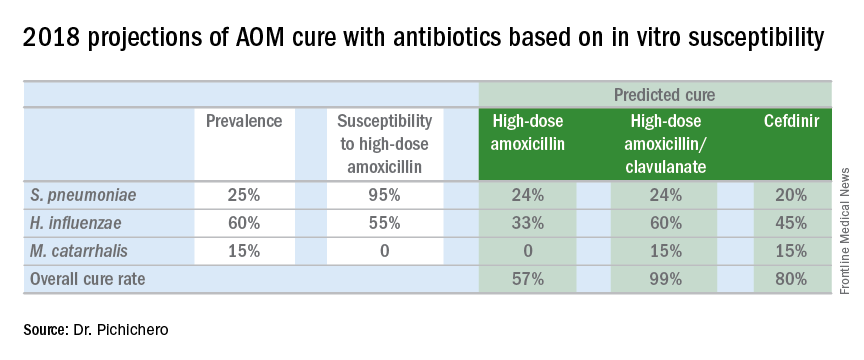
Strictly based on in vitro susceptibility and the known otopathogen mix, the calculations show that amoxicillin could result in a maximum cure of 57%, amoxicillin/clavulanate of 99%, and cefdinir of 80% of treated children.
In vitro susceptibility has its limitations. Pharmacodynamic calculations would drop the predicted success of all three antibiotics because suboptimal absorption after oral dosing occurs with amoxicillin and amoxicillin/clavulanate more so than with cefdinir, thereby resulting in lower than predicted levels of antibiotic at the site of infection within the middle ear, whereas the achievable level of cefdinir with recommended dosing sometimes is below the desired in vitro cut point.
To balance that lowered predicted efficacy, each of the otopathogens has an associated “spontaneous cure rate” that is often quoted as being 20% for S. pneumoniae, 50% for H. influenzae, and 80% for M. catarrhalis. However, to be clear, those rates were derived largely from assessments about 5 days after antibiotic treatment was started with ineffective drugs or with placebos and do not account for the true spontaneous clinical cure rate of AOM if assessed in the first few days after onset (when pain and fever are at their peak) nor if assessed 14-30 days later when almost all children have been cured by their immune systems.
The calculations also do not account for overdiagnosis in clinical practice. Indeed, if the child does not have AOM, then the child will have a cure regardless of which antibiotic is selected. Rates of overdiagnosis of AOM have been assessed with various methods and are subject to limitations. But overall the data and most experts agree that overdiagnosis by pediatricians, family physicians, urgent care physicians, nurse practitioners, and physician assistants is in the range of 30%-50%.
Before the reader leaps to the conclusion that I am endorsing any particular antibiotic strictly based on predicted in vitro efficacy, I would state that many considerations must be given to whether to use an antibiotic for AOM, and which antibiotic to use, at what dose, and for what duration. This column is just pointing out a few key up-to-date facts for your consideration.
It’s a new year and a new respiratory season so my thoughts turn to the most common infection in pediatrics where an antibiotic might appropriately be prescribed – acute otitis media (AOM). The guidelines of the American Academy of Pediatrics were finalized in 2012 and published in 2013 and based on data that the AAP subcommittee considered. A recommendation emerged for amoxicillin to remain the treatment of choice if an antibiotic was to be prescribed at all, leaving the observation option as a continued consideration under defined clinical circumstances. The oral alternative antibiotics recommended were amoxicillin/clavulanate and cefdinir (Pediatrics. 2013. doi: 10.1542/peds.2012-3488).
Since the AAP subcommittee deliberated, changes have occurred in AOM etiology and the frequency of antibiotic resistance among the common bacteria that cause the infection. Our group in Rochester (N.Y.) continues to be the only site in the United States conducting a prospective assessment of AOM; we hope our data are generalizable to the entire country, but that is not certain. In Rochester, we saw an overall drop in AOM incidence after introduction of Prevnar 7 of about 10%-15% overall and that corresponded reasonably well with the frequency of AOM caused by Streptococcus pneumoniae involving the seven serotypes in the PCV7 vaccine. We then had a rebound in AOM infections, largely caused by serotype 19A, such that the overall incidence of AOM returned back to levels nearly the same as before PCV7 by 2010. With the introduction of Prevnar 13, and the dramatic reduction of serotype 19A nasal colonization – a necessary precursor of AOM – the incidence of AOM overall fell again, and compared with the pre-PCV7 era, I estimate that we are seeing about 20%-25% less AOM today.
In late 2017, we published an article describing the epidemiology of AOM in the PCV era (Pediatrics. 2017 Aug. doi: 10.1542/peds.2017-0181), in which we described changes in otopathogen distribution over time from 1996 through 2016. It showed that by end of 2016, the predominant bacteria causing AOM were Haemophilus influenzae, accounting for 60% of all AOM (52% detected by culture from tympanocentesis and another 8% detected by polymerase chain reaction). Among the H. influenzae from middle ear fluid, beta-lactamase production occurred in 45%. Therefore, according to principles of infectious disease antibiotic efficacy predictions, use of amoxicillin in standard dose or high dose would not eradicate about half of the H. influenzae causing AOM. In the table included in this column, I show calculations of predicted outcomes from amoxicillin, amoxicillin/clavulanate, and cefdinir treatment based on the projected otopathogen mix and resistance frequencies of 2016. Added to the data on H. influenzae I have included results of S. pneumoniae high nonsusceptibility at 5% of strains and beta-lactamase production by Moraxella catarrhalis at 100% of strains. 
Strictly based on in vitro susceptibility and the known otopathogen mix, the calculations show that amoxicillin could result in a maximum cure of 57%, amoxicillin/clavulanate of 99%, and cefdinir of 80% of treated children.
In vitro susceptibility has its limitations. Pharmacodynamic calculations would drop the predicted success of all three antibiotics because suboptimal absorption after oral dosing occurs with amoxicillin and amoxicillin/clavulanate more so than with cefdinir, thereby resulting in lower than predicted levels of antibiotic at the site of infection within the middle ear, whereas the achievable level of cefdinir with recommended dosing sometimes is below the desired in vitro cut point.
To balance that lowered predicted efficacy, each of the otopathogens has an associated “spontaneous cure rate” that is often quoted as being 20% for S. pneumoniae, 50% for H. influenzae, and 80% for M. catarrhalis. However, to be clear, those rates were derived largely from assessments about 5 days after antibiotic treatment was started with ineffective drugs or with placebos and do not account for the true spontaneous clinical cure rate of AOM if assessed in the first few days after onset (when pain and fever are at their peak) nor if assessed 14-30 days later when almost all children have been cured by their immune systems.
The calculations also do not account for overdiagnosis in clinical practice. Indeed, if the child does not have AOM, then the child will have a cure regardless of which antibiotic is selected. Rates of overdiagnosis of AOM have been assessed with various methods and are subject to limitations. But overall the data and most experts agree that overdiagnosis by pediatricians, family physicians, urgent care physicians, nurse practitioners, and physician assistants is in the range of 30%-50%.
Before the reader leaps to the conclusion that I am endorsing any particular antibiotic strictly based on predicted in vitro efficacy, I would state that many considerations must be given to whether to use an antibiotic for AOM, and which antibiotic to use, at what dose, and for what duration. This column is just pointing out a few key up-to-date facts for your consideration.
It’s a new year and a new respiratory season so my thoughts turn to the most common infection in pediatrics where an antibiotic might appropriately be prescribed – acute otitis media (AOM). The guidelines of the American Academy of Pediatrics were finalized in 2012 and published in 2013 and based on data that the AAP subcommittee considered. A recommendation emerged for amoxicillin to remain the treatment of choice if an antibiotic was to be prescribed at all, leaving the observation option as a continued consideration under defined clinical circumstances. The oral alternative antibiotics recommended were amoxicillin/clavulanate and cefdinir (Pediatrics. 2013. doi: 10.1542/peds.2012-3488).
Since the AAP subcommittee deliberated, changes have occurred in AOM etiology and the frequency of antibiotic resistance among the common bacteria that cause the infection. Our group in Rochester (N.Y.) continues to be the only site in the United States conducting a prospective assessment of AOM; we hope our data are generalizable to the entire country, but that is not certain. In Rochester, we saw an overall drop in AOM incidence after introduction of Prevnar 7 of about 10%-15% overall and that corresponded reasonably well with the frequency of AOM caused by Streptococcus pneumoniae involving the seven serotypes in the PCV7 vaccine. We then had a rebound in AOM infections, largely caused by serotype 19A, such that the overall incidence of AOM returned back to levels nearly the same as before PCV7 by 2010. With the introduction of Prevnar 13, and the dramatic reduction of serotype 19A nasal colonization – a necessary precursor of AOM – the incidence of AOM overall fell again, and compared with the pre-PCV7 era, I estimate that we are seeing about 20%-25% less AOM today.
In late 2017, we published an article describing the epidemiology of AOM in the PCV era (Pediatrics. 2017 Aug. doi: 10.1542/peds.2017-0181), in which we described changes in otopathogen distribution over time from 1996 through 2016. It showed that by end of 2016, the predominant bacteria causing AOM were Haemophilus influenzae, accounting for 60% of all AOM (52% detected by culture from tympanocentesis and another 8% detected by polymerase chain reaction). Among the H. influenzae from middle ear fluid, beta-lactamase production occurred in 45%. Therefore, according to principles of infectious disease antibiotic efficacy predictions, use of amoxicillin in standard dose or high dose would not eradicate about half of the H. influenzae causing AOM. In the table included in this column, I show calculations of predicted outcomes from amoxicillin, amoxicillin/clavulanate, and cefdinir treatment based on the projected otopathogen mix and resistance frequencies of 2016. Added to the data on H. influenzae I have included results of S. pneumoniae high nonsusceptibility at 5% of strains and beta-lactamase production by Moraxella catarrhalis at 100% of strains. 
Strictly based on in vitro susceptibility and the known otopathogen mix, the calculations show that amoxicillin could result in a maximum cure of 57%, amoxicillin/clavulanate of 99%, and cefdinir of 80% of treated children.
In vitro susceptibility has its limitations. Pharmacodynamic calculations would drop the predicted success of all three antibiotics because suboptimal absorption after oral dosing occurs with amoxicillin and amoxicillin/clavulanate more so than with cefdinir, thereby resulting in lower than predicted levels of antibiotic at the site of infection within the middle ear, whereas the achievable level of cefdinir with recommended dosing sometimes is below the desired in vitro cut point.
To balance that lowered predicted efficacy, each of the otopathogens has an associated “spontaneous cure rate” that is often quoted as being 20% for S. pneumoniae, 50% for H. influenzae, and 80% for M. catarrhalis. However, to be clear, those rates were derived largely from assessments about 5 days after antibiotic treatment was started with ineffective drugs or with placebos and do not account for the true spontaneous clinical cure rate of AOM if assessed in the first few days after onset (when pain and fever are at their peak) nor if assessed 14-30 days later when almost all children have been cured by their immune systems.
The calculations also do not account for overdiagnosis in clinical practice. Indeed, if the child does not have AOM, then the child will have a cure regardless of which antibiotic is selected. Rates of overdiagnosis of AOM have been assessed with various methods and are subject to limitations. But overall the data and most experts agree that overdiagnosis by pediatricians, family physicians, urgent care physicians, nurse practitioners, and physician assistants is in the range of 30%-50%.
Before the reader leaps to the conclusion that I am endorsing any particular antibiotic strictly based on predicted in vitro efficacy, I would state that many considerations must be given to whether to use an antibiotic for AOM, and which antibiotic to use, at what dose, and for what duration. This column is just pointing out a few key up-to-date facts for your consideration.
Systems biology – A primer
Systems biology is relatively new. It is an interdisciplinary field that focuses on complex interactions within biological systems using a holistic approach in the pursuit of scientific discovery.
The systems biology approach seeks to integrate biological knowledge to understand how cells and molecules interact with one another. A key component is computational and mathematical modeling. The ever-increasing amount of biological data, and the judgment that this data cannot be understood by simply drawing lines between interacting cells and molecules, explains the demand for a systematic approach.
Prominent examples for biological systems are the immune system and the nervous system, which already have the word ”system” included. Although the idea of system-level understanding is not new, the growing interest in applying the systems approach has been driven by breakthrough advances in molecular biology and bioinformatics.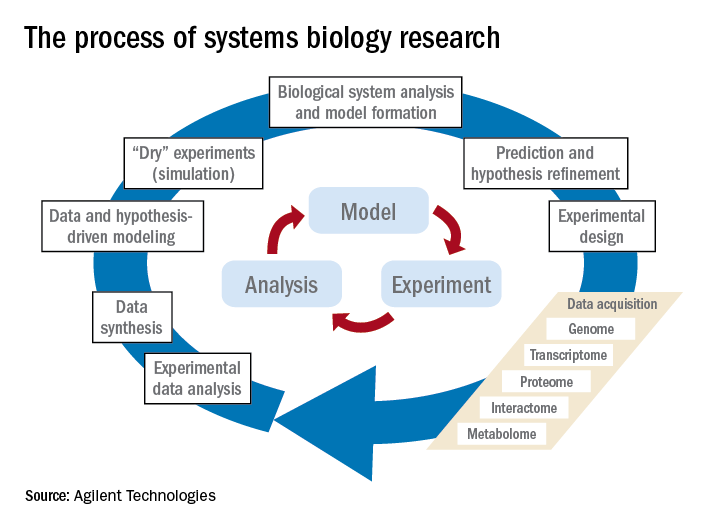
Over the past 10 years, our group has identified highly significant differences in immune functioning between the 10% of children who frequently develop acute otitis media (i.e., those who are “otitis prone”) and the children who develop AOM infrequently (60% of children) or not at all (30% of children). We also have identified a cohort of about 10% of children who fail to respond to infant vaccinations (low vaccine responders), compared with children who respond with protective immunity and establishment of immune memory. The differences in children who are prone to AOM vs. those who are not and in low vaccine responders vs. normal vaccine responders include differences in cytokine molecules in blood (providing biosignatures), reduced antibodies, immune memory, and aberrant intercellular signaling networks after otopathogen exposure (AOM prone vs. non–AOM prone) and routine pediatric vaccination (low vs. normal vaccine responders).
Dr. Pichichero, a specialist in pediatric infectious diseases, is director of the Research Institute at Rochester (N.Y.) General Hospital. He is also a pediatrician at Legacy Pediatrics in Rochester. He has no relevant financial disclosures. Email him at pdnews@frontlinemedcom.com.
Systems biology is relatively new. It is an interdisciplinary field that focuses on complex interactions within biological systems using a holistic approach in the pursuit of scientific discovery.
The systems biology approach seeks to integrate biological knowledge to understand how cells and molecules interact with one another. A key component is computational and mathematical modeling. The ever-increasing amount of biological data, and the judgment that this data cannot be understood by simply drawing lines between interacting cells and molecules, explains the demand for a systematic approach.
Prominent examples for biological systems are the immune system and the nervous system, which already have the word ”system” included. Although the idea of system-level understanding is not new, the growing interest in applying the systems approach has been driven by breakthrough advances in molecular biology and bioinformatics.
Over the past 10 years, our group has identified highly significant differences in immune functioning between the 10% of children who frequently develop acute otitis media (i.e., those who are “otitis prone”) and the children who develop AOM infrequently (60% of children) or not at all (30% of children). We also have identified a cohort of about 10% of children who fail to respond to infant vaccinations (low vaccine responders), compared with children who respond with protective immunity and establishment of immune memory. The differences in children who are prone to AOM vs. those who are not and in low vaccine responders vs. normal vaccine responders include differences in cytokine molecules in blood (providing biosignatures), reduced antibodies, immune memory, and aberrant intercellular signaling networks after otopathogen exposure (AOM prone vs. non–AOM prone) and routine pediatric vaccination (low vs. normal vaccine responders).
Dr. Pichichero, a specialist in pediatric infectious diseases, is director of the Research Institute at Rochester (N.Y.) General Hospital. He is also a pediatrician at Legacy Pediatrics in Rochester. He has no relevant financial disclosures. Email him at pdnews@frontlinemedcom.com.
Systems biology is relatively new. It is an interdisciplinary field that focuses on complex interactions within biological systems using a holistic approach in the pursuit of scientific discovery.
The systems biology approach seeks to integrate biological knowledge to understand how cells and molecules interact with one another. A key component is computational and mathematical modeling. The ever-increasing amount of biological data, and the judgment that this data cannot be understood by simply drawing lines between interacting cells and molecules, explains the demand for a systematic approach.
Prominent examples for biological systems are the immune system and the nervous system, which already have the word ”system” included. Although the idea of system-level understanding is not new, the growing interest in applying the systems approach has been driven by breakthrough advances in molecular biology and bioinformatics.
Over the past 10 years, our group has identified highly significant differences in immune functioning between the 10% of children who frequently develop acute otitis media (i.e., those who are “otitis prone”) and the children who develop AOM infrequently (60% of children) or not at all (30% of children). We also have identified a cohort of about 10% of children who fail to respond to infant vaccinations (low vaccine responders), compared with children who respond with protective immunity and establishment of immune memory. The differences in children who are prone to AOM vs. those who are not and in low vaccine responders vs. normal vaccine responders include differences in cytokine molecules in blood (providing biosignatures), reduced antibodies, immune memory, and aberrant intercellular signaling networks after otopathogen exposure (AOM prone vs. non–AOM prone) and routine pediatric vaccination (low vs. normal vaccine responders).
Dr. Pichichero, a specialist in pediatric infectious diseases, is director of the Research Institute at Rochester (N.Y.) General Hospital. He is also a pediatrician at Legacy Pediatrics in Rochester. He has no relevant financial disclosures. Email him at pdnews@frontlinemedcom.com.