User login
In this column I have previously discussed the microbiome and its importance to health, especially as it relates to infections in children. Given the appreciated connection between microbiome and immunity, my group in Rochester, N.Y., recently undertook a study of the effect of antibiotic usage on the immune response to routine early childhood vaccines. In mouse models, it was previously shown that antibiotic exposure induced a reduction in the abundance and diversity of gut microbiota that in turn negatively affected the generation and maintenance of vaccine-induced immunity.1,2 A study from Stanford University was the first experimental human trial of antibiotic effects on vaccine responses. Adult volunteers were given an antibiotic or not before seasonal influenza vaccination and the researchers identified specific bacteria in the gut that were reduced by the antibiotics given. Those normal bacteria in the gut microbiome were shown to provide positive immunity signals to the systemic immune system that potentiated vaccine responses.3
My group conducted the first-ever study in children to explore whether an association existed between antibiotic use and vaccine-induced antibody levels. In the May issue of Pediatrics we report results from 560 children studied.4 From these children, 11,888 serum antibody levels to vaccine antigens were measured. Vaccine-induced antibody levels were determined at various time points after primary vaccination at child age 2, 4, and 6 months and boosters at age 12-18 months for 10 antigens included in four vaccines: DTaP, Hib, IPV, and PCV. The antibody levels to vaccine components were measured to DTaP (diphtheria toxoid, pertussis toxoid, tetanus toxoid, pertactin, and filamentous hemagglutinin), Hib conjugate (polyribosylribitol phosphate), IPV (polio 2), and PCV (serotypes 6B, 14, and 23F). A total of 342 children with 1,678 antibiotic courses prescribed were compared with 218 children with no antibiotic exposures. The predominant antibiotics prescribed were amoxicillin, cefdinir, amoxicillin/clavulanate, and ceftriaxone, since most treatments were for acute otitis media.
Of possible high clinical relevance, we found that from 9 to 24 months of age, children with antibiotic exposure had a higher frequency of vaccine-induced antibody levels below protection compared with children with no antibiotic use, placing them at risk of contracting a vaccine-preventable infection for DTaP antigens DT, TT, and PT and for PCV serotype 14.
For time points where antibody levels were determined within 30 days of completion of a course of antibiotics (recent antibiotic use), individual antibiotics were analyzed for effect on antibody levels below protective levels. Across all vaccine antigens measured, we found that all antibiotics had a negative effect on antibody levels and percentage of children achieving the protective antibody level threshold. Amoxicillin use had a lower association with lower antibody levels than the broader spectrum antibiotics, amoxicillin clavulanate (Augmentin), cefdinir, and ceftriaxone. For children receiving amoxicillin/clavulanate prescriptions, it was possible to compare the effect of shorter versus longer courses and we found that a 5-day course was associated with subprotective antibody levels similar to 10 days of amoxicillin, whereas 10-day amoxicillin/clavulanate was associated with higher frequency of children having subprotective antibody levels (Figure).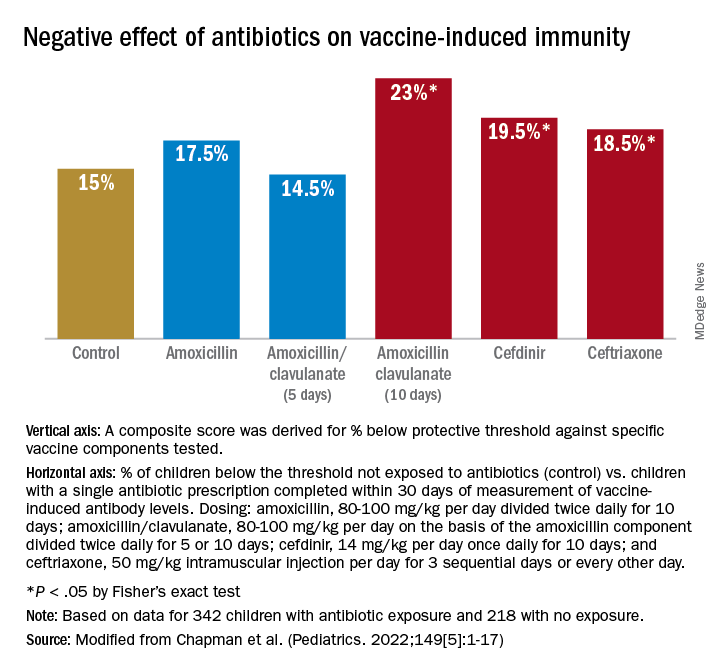
We examined whether accumulation of antibiotic courses in the first year of life had an association with subsequent vaccine-induced antibody levels and found that each antibiotic prescription was associated with a reduction in the median antibody level. For DTaP, each prescription was associated with 5.8% drop in antibody level to the vaccine components. For Hib the drop was 6.8%, IPV was 11.3%, and PCV was 10.4% – all statistically significant. To determine if booster vaccination influenced this association, a second analysis was performed using antibiotic prescriptions up to 15 months of age. We found each antibiotic prescription was associated with a reduction in median vaccine-induced antibody levels for DTaP by 18%, Hib by 21%, IPV by 19%, and PCV by 12% – all statistically significant.
Our study is the first in young children during the early age window where vaccine-induced immunity is established. Antibiotic use was associated with increased frequency of subprotective antibody levels for several vaccines used in children up to 2 years of age. The lower antibody levels could leave children vulnerable to vaccine preventable diseases. Perhaps outbreaks of vaccine-preventable diseases, such as pertussis, may be a consequence of multiple courses of antibiotics suppressing vaccine-induced immunity.
A goal of this study was to explore potential acute and long-term effects of antibiotic exposure on vaccine-induced antibody levels. Accumulated antibiotic courses up to booster immunization was associated with decreased vaccine antibody levels both before and after booster, suggesting that booster immunization was not sufficient to change the negative association with antibiotic exposure. The results were similar for all vaccines tested, suggesting that the specific vaccine formulation was not a factor.
The study has several limitations. The antibiotic prescription data and measurements of vaccine-induced antibody levels were recorded and measured prospectively; however, our analysis was done retrospectively. The group of study children was derived from my private practice in Rochester, N.Y., and may not be broadly representative of all children. The number of vaccine antibody measurements was limited by serum availability at some sampling time points in some children; and sometimes, the serum samples were collected far apart, which weakened our ability to perform longitudinal analyses. We did not collect stool samples from the children so we could not directly study the effect of antibiotic courses on the gut microbiome.
Our study adds new reasons to be cautious about overprescribing antibiotics on an individual child basis because an adverse effect extends to reduction in vaccine responses. This should be explained to parents requesting unnecessary antibiotics for colds and coughs. When antibiotics are necessary, the judicious choice of a narrow-spectrum antibiotic or a shorter duration of a broader spectrum antibiotic may reduce adverse effects on vaccine-induced immunity.
References
1. Valdez Y et al. Influence of the microbiota on vaccine effectiveness. Trends Immunol. 2014;35(11):526-37.
2. Lynn MA et al. Early-life antibiotic-driven dysbiosis leads to dysregulated vaccine immune responses in mice. Cell Host Microbe. 2018;23(5):653-60.e5.
3. Hagan T et al. Antibiotics-driven gut microbiome perturbation alters immunity to vaccines in humans. Cell. 2019;178(6):1313-28.e13.
4. Chapman T et al. Antibiotic use and vaccine antibody levels. Pediatrics. 2022;149(5);1-17. doi: 10.1542/peds.2021-052061.
In this column I have previously discussed the microbiome and its importance to health, especially as it relates to infections in children. Given the appreciated connection between microbiome and immunity, my group in Rochester, N.Y., recently undertook a study of the effect of antibiotic usage on the immune response to routine early childhood vaccines. In mouse models, it was previously shown that antibiotic exposure induced a reduction in the abundance and diversity of gut microbiota that in turn negatively affected the generation and maintenance of vaccine-induced immunity.1,2 A study from Stanford University was the first experimental human trial of antibiotic effects on vaccine responses. Adult volunteers were given an antibiotic or not before seasonal influenza vaccination and the researchers identified specific bacteria in the gut that were reduced by the antibiotics given. Those normal bacteria in the gut microbiome were shown to provide positive immunity signals to the systemic immune system that potentiated vaccine responses.3
My group conducted the first-ever study in children to explore whether an association existed between antibiotic use and vaccine-induced antibody levels. In the May issue of Pediatrics we report results from 560 children studied.4 From these children, 11,888 serum antibody levels to vaccine antigens were measured. Vaccine-induced antibody levels were determined at various time points after primary vaccination at child age 2, 4, and 6 months and boosters at age 12-18 months for 10 antigens included in four vaccines: DTaP, Hib, IPV, and PCV. The antibody levels to vaccine components were measured to DTaP (diphtheria toxoid, pertussis toxoid, tetanus toxoid, pertactin, and filamentous hemagglutinin), Hib conjugate (polyribosylribitol phosphate), IPV (polio 2), and PCV (serotypes 6B, 14, and 23F). A total of 342 children with 1,678 antibiotic courses prescribed were compared with 218 children with no antibiotic exposures. The predominant antibiotics prescribed were amoxicillin, cefdinir, amoxicillin/clavulanate, and ceftriaxone, since most treatments were for acute otitis media.
Of possible high clinical relevance, we found that from 9 to 24 months of age, children with antibiotic exposure had a higher frequency of vaccine-induced antibody levels below protection compared with children with no antibiotic use, placing them at risk of contracting a vaccine-preventable infection for DTaP antigens DT, TT, and PT and for PCV serotype 14.
For time points where antibody levels were determined within 30 days of completion of a course of antibiotics (recent antibiotic use), individual antibiotics were analyzed for effect on antibody levels below protective levels. Across all vaccine antigens measured, we found that all antibiotics had a negative effect on antibody levels and percentage of children achieving the protective antibody level threshold. Amoxicillin use had a lower association with lower antibody levels than the broader spectrum antibiotics, amoxicillin clavulanate (Augmentin), cefdinir, and ceftriaxone. For children receiving amoxicillin/clavulanate prescriptions, it was possible to compare the effect of shorter versus longer courses and we found that a 5-day course was associated with subprotective antibody levels similar to 10 days of amoxicillin, whereas 10-day amoxicillin/clavulanate was associated with higher frequency of children having subprotective antibody levels (Figure).
We examined whether accumulation of antibiotic courses in the first year of life had an association with subsequent vaccine-induced antibody levels and found that each antibiotic prescription was associated with a reduction in the median antibody level. For DTaP, each prescription was associated with 5.8% drop in antibody level to the vaccine components. For Hib the drop was 6.8%, IPV was 11.3%, and PCV was 10.4% – all statistically significant. To determine if booster vaccination influenced this association, a second analysis was performed using antibiotic prescriptions up to 15 months of age. We found each antibiotic prescription was associated with a reduction in median vaccine-induced antibody levels for DTaP by 18%, Hib by 21%, IPV by 19%, and PCV by 12% – all statistically significant.
Our study is the first in young children during the early age window where vaccine-induced immunity is established. Antibiotic use was associated with increased frequency of subprotective antibody levels for several vaccines used in children up to 2 years of age. The lower antibody levels could leave children vulnerable to vaccine preventable diseases. Perhaps outbreaks of vaccine-preventable diseases, such as pertussis, may be a consequence of multiple courses of antibiotics suppressing vaccine-induced immunity.
A goal of this study was to explore potential acute and long-term effects of antibiotic exposure on vaccine-induced antibody levels. Accumulated antibiotic courses up to booster immunization was associated with decreased vaccine antibody levels both before and after booster, suggesting that booster immunization was not sufficient to change the negative association with antibiotic exposure. The results were similar for all vaccines tested, suggesting that the specific vaccine formulation was not a factor.
The study has several limitations. The antibiotic prescription data and measurements of vaccine-induced antibody levels were recorded and measured prospectively; however, our analysis was done retrospectively. The group of study children was derived from my private practice in Rochester, N.Y., and may not be broadly representative of all children. The number of vaccine antibody measurements was limited by serum availability at some sampling time points in some children; and sometimes, the serum samples were collected far apart, which weakened our ability to perform longitudinal analyses. We did not collect stool samples from the children so we could not directly study the effect of antibiotic courses on the gut microbiome.
Our study adds new reasons to be cautious about overprescribing antibiotics on an individual child basis because an adverse effect extends to reduction in vaccine responses. This should be explained to parents requesting unnecessary antibiotics for colds and coughs. When antibiotics are necessary, the judicious choice of a narrow-spectrum antibiotic or a shorter duration of a broader spectrum antibiotic may reduce adverse effects on vaccine-induced immunity.
References
1. Valdez Y et al. Influence of the microbiota on vaccine effectiveness. Trends Immunol. 2014;35(11):526-37.
2. Lynn MA et al. Early-life antibiotic-driven dysbiosis leads to dysregulated vaccine immune responses in mice. Cell Host Microbe. 2018;23(5):653-60.e5.
3. Hagan T et al. Antibiotics-driven gut microbiome perturbation alters immunity to vaccines in humans. Cell. 2019;178(6):1313-28.e13.
4. Chapman T et al. Antibiotic use and vaccine antibody levels. Pediatrics. 2022;149(5);1-17. doi: 10.1542/peds.2021-052061.
In this column I have previously discussed the microbiome and its importance to health, especially as it relates to infections in children. Given the appreciated connection between microbiome and immunity, my group in Rochester, N.Y., recently undertook a study of the effect of antibiotic usage on the immune response to routine early childhood vaccines. In mouse models, it was previously shown that antibiotic exposure induced a reduction in the abundance and diversity of gut microbiota that in turn negatively affected the generation and maintenance of vaccine-induced immunity.1,2 A study from Stanford University was the first experimental human trial of antibiotic effects on vaccine responses. Adult volunteers were given an antibiotic or not before seasonal influenza vaccination and the researchers identified specific bacteria in the gut that were reduced by the antibiotics given. Those normal bacteria in the gut microbiome were shown to provide positive immunity signals to the systemic immune system that potentiated vaccine responses.3
My group conducted the first-ever study in children to explore whether an association existed between antibiotic use and vaccine-induced antibody levels. In the May issue of Pediatrics we report results from 560 children studied.4 From these children, 11,888 serum antibody levels to vaccine antigens were measured. Vaccine-induced antibody levels were determined at various time points after primary vaccination at child age 2, 4, and 6 months and boosters at age 12-18 months for 10 antigens included in four vaccines: DTaP, Hib, IPV, and PCV. The antibody levels to vaccine components were measured to DTaP (diphtheria toxoid, pertussis toxoid, tetanus toxoid, pertactin, and filamentous hemagglutinin), Hib conjugate (polyribosylribitol phosphate), IPV (polio 2), and PCV (serotypes 6B, 14, and 23F). A total of 342 children with 1,678 antibiotic courses prescribed were compared with 218 children with no antibiotic exposures. The predominant antibiotics prescribed were amoxicillin, cefdinir, amoxicillin/clavulanate, and ceftriaxone, since most treatments were for acute otitis media.
Of possible high clinical relevance, we found that from 9 to 24 months of age, children with antibiotic exposure had a higher frequency of vaccine-induced antibody levels below protection compared with children with no antibiotic use, placing them at risk of contracting a vaccine-preventable infection for DTaP antigens DT, TT, and PT and for PCV serotype 14.
For time points where antibody levels were determined within 30 days of completion of a course of antibiotics (recent antibiotic use), individual antibiotics were analyzed for effect on antibody levels below protective levels. Across all vaccine antigens measured, we found that all antibiotics had a negative effect on antibody levels and percentage of children achieving the protective antibody level threshold. Amoxicillin use had a lower association with lower antibody levels than the broader spectrum antibiotics, amoxicillin clavulanate (Augmentin), cefdinir, and ceftriaxone. For children receiving amoxicillin/clavulanate prescriptions, it was possible to compare the effect of shorter versus longer courses and we found that a 5-day course was associated with subprotective antibody levels similar to 10 days of amoxicillin, whereas 10-day amoxicillin/clavulanate was associated with higher frequency of children having subprotective antibody levels (Figure).
We examined whether accumulation of antibiotic courses in the first year of life had an association with subsequent vaccine-induced antibody levels and found that each antibiotic prescription was associated with a reduction in the median antibody level. For DTaP, each prescription was associated with 5.8% drop in antibody level to the vaccine components. For Hib the drop was 6.8%, IPV was 11.3%, and PCV was 10.4% – all statistically significant. To determine if booster vaccination influenced this association, a second analysis was performed using antibiotic prescriptions up to 15 months of age. We found each antibiotic prescription was associated with a reduction in median vaccine-induced antibody levels for DTaP by 18%, Hib by 21%, IPV by 19%, and PCV by 12% – all statistically significant.
Our study is the first in young children during the early age window where vaccine-induced immunity is established. Antibiotic use was associated with increased frequency of subprotective antibody levels for several vaccines used in children up to 2 years of age. The lower antibody levels could leave children vulnerable to vaccine preventable diseases. Perhaps outbreaks of vaccine-preventable diseases, such as pertussis, may be a consequence of multiple courses of antibiotics suppressing vaccine-induced immunity.
A goal of this study was to explore potential acute and long-term effects of antibiotic exposure on vaccine-induced antibody levels. Accumulated antibiotic courses up to booster immunization was associated with decreased vaccine antibody levels both before and after booster, suggesting that booster immunization was not sufficient to change the negative association with antibiotic exposure. The results were similar for all vaccines tested, suggesting that the specific vaccine formulation was not a factor.
The study has several limitations. The antibiotic prescription data and measurements of vaccine-induced antibody levels were recorded and measured prospectively; however, our analysis was done retrospectively. The group of study children was derived from my private practice in Rochester, N.Y., and may not be broadly representative of all children. The number of vaccine antibody measurements was limited by serum availability at some sampling time points in some children; and sometimes, the serum samples were collected far apart, which weakened our ability to perform longitudinal analyses. We did not collect stool samples from the children so we could not directly study the effect of antibiotic courses on the gut microbiome.
Our study adds new reasons to be cautious about overprescribing antibiotics on an individual child basis because an adverse effect extends to reduction in vaccine responses. This should be explained to parents requesting unnecessary antibiotics for colds and coughs. When antibiotics are necessary, the judicious choice of a narrow-spectrum antibiotic or a shorter duration of a broader spectrum antibiotic may reduce adverse effects on vaccine-induced immunity.
References
1. Valdez Y et al. Influence of the microbiota on vaccine effectiveness. Trends Immunol. 2014;35(11):526-37.
2. Lynn MA et al. Early-life antibiotic-driven dysbiosis leads to dysregulated vaccine immune responses in mice. Cell Host Microbe. 2018;23(5):653-60.e5.
3. Hagan T et al. Antibiotics-driven gut microbiome perturbation alters immunity to vaccines in humans. Cell. 2019;178(6):1313-28.e13.
4. Chapman T et al. Antibiotic use and vaccine antibody levels. Pediatrics. 2022;149(5);1-17. doi: 10.1542/peds.2021-052061.

