User login
While our understanding of the mechanisms of psychosis continues to evolve beyond the dopamine hypothesis, the key role of dopamine in psychosis and its treatment has not faded.1 Over time, the dopamine hypothesis of schizophrenia has evolved from focusing on dopamine hyperactivity to specifying the regional abnormalities in the brain with subcortical hyperdopaminergia and prefrontal hypodopaminergia.2 Despite this divergence in dopaminergic function, antipsychotic medications that block dopamine D2 receptors (D2R) remain central to treating psychotic symptoms and preventing relapse.3,4 Notably, antipsychotics block both presynaptic and postsynaptic receptors affecting the regulation of dopamine synthesis and release in the brain.5,6
Chronic dopamine D2R blockade with antipsychotics induces adaptive changes that can contribute to both acute and chronic adverse effects. In this article, we discuss these changes, and steps clinicians can take to minimize their occurrence.
Dopamine D2R: A primer
There are 5 types of dopamine receptors, numbered D1 through D5, but there are only 2 families of dopamine receptors: the D1 family (D1 and D5), and the D2 family (D2, D3, and D4). All dopamine receptors are G protein–coupled, but the D2 family of receptors generally increases protein kinase A (PKA) as the second messenger, whereas the D1 family increases cyclic adenosine monophosphate (cAMP) as the second messenger.5 There are 2 distinct variants of the D2R of 2 different lengths made from the same gene (DRD2) via posttranslational modification. The long isoform of D2R (D2L) has an additional 29 amino acids compared to the short isoform (D2S).7 Additional evidence points to a third splice variant called D2Longer that arises from aberrant RNA splicing and contains 2 more amino acids than D2L; its relevance is not known.8
The D2L isoform is the primary postsynaptic receptor, expressed more in the striatum and nucleus accumbens (NAc) targeted by dopaminergic afferents. The D2S isoform, however, is predominantly presynaptic, more densely expressed on cell bodies and projection axons of the dopaminergic neurons of the midbrain and hypothalamus.9 Each isoform contributes differentially to the therapeutic and adverse effects of antipsychotics, and evidence from animal studies suggests that D2L is the main variant responsible for drug-induced parkinsonism.10 The D2S acts as the principal autoreceptor for the dopaminergic system.5,11,12
Autoreceptors regulate dopamine transmission. Dopamine itself and D2R agonists are reported to have higher affinity and potency with D2S. Activation of these autoreceptors is a negative feedback mechanism that decreases dopamine release. Similarly, when they are blocked (such as with use of an antipsychotic), there is an increase in dopamine release. Additionally, these autoreceptors modulate several key processes:
- neuronal firing rate by activating potassium conductance
- dopamine synthesis by downregulating the expression of tyrosine hydroxylase (TH) enzyme (the rate-limiting step)
- exocytotic release of dopamine and other neurotransmitters
- dopamine reuptake via increasing the activity of the dopamine transporter (DAT).12
Consequences of antipsychotic D2R blockade
Most antipsychotics begin to produce a therapeutic antipsychotic effect at 65% to 75% occupancy of the D2Rs.3 This level also produces an optimal balance between clinical efficacy and a lower incidence of adverse effects.3 A higher D2R occupancy by both first-generation (FGA) and second-generation (SGA) pure antagonist antipsychotics can lead to parkinsonism.
Parkinsonism is associated with the subsequent appearance of one of the most distressing consequences of long-term antipsychotic treatment, tardive dyskinesia (TD).13 TD is an iatrogenic, usually late-onset syndrome consisting of persistent, involuntary, and repetitive movements. It classically involves the highly innervated striated muscles of the tongue, mouth, face, and fingers, though it can also involve the trunk and extremities.14 It occurs secondary to chronic exposure to dopamine receptor–blocking agents, including dopaminergic antiemetics.15 The prevalence of TD is higher in patients treated long-term with FGAs (30.0% to 32.4%) than in those treated with SGAs (13.1% to 20.7%) due to serotonin 5HT2A blockade that results in increased dopamine release in the basal ganglia.16
Continue to: Dopamine supersenstivity psychosis...
Dopamine supersensitivity psychosis (DSP) is a term that describes the clinical iatrogenic phenomenon that might be observed with long-term antipsychotic treatment. DSP is suggested to be strongly associated with treatment failure/resistance in schizophrenia.17,18 Manifestations of DSP include development of antipsychotic drug tolerance that undermines treatment efficacy, rebound psychosis during or after treatment discontinuation, and the presence of TD. Like TD, it may be reversed temporarily by increasing the dose of the antipsychotic.18
DSP and (more extensively) TD are commonly hypothesized to result from the postsynaptic dopamine receptor supersensitivity that develops because of chronic D2Rs blockade by antipsychotics. Neostriatal dopamine receptor supersensitivity is believed to lead to TD, while mesolimbic supersensitivity leads to DSP.19 Supersensitivity has traditionally been believed to be due to upregulation of postsynaptic D2R number and sensitivity.20,21 However, both TD and DSP are more likely a consequence of a host of compensatory neurobiological adaptations across the synapse that include:
- postsynaptic increase in the number of D2Rs that amplifies the dopamine signal
- an increased number of synapses, dendritic spines, and perforated synapses (seen in animal models), all of which lead to a potentiated dopamine signal
- presynaptic changes with higher levels of dopamine released into the synapse via an increase in quantal size as postsynaptic D2Rs blockade results in more dopamine becoming available in the synapse for recycling via the dopamine transporter
- increased dopamine turnover due to presynaptic D2S autoreceptor blockade.22
So if giving a D2R blocking agent for a long time increases the dopamine signal, at least in some patients, what can the clinician do to treat the psychosis, and not cause changes in the brain that could lead to TD or DSP?
Partial agonist antipsychotics and biased agonism of D2Rs
One approach to try to avoid the compensatory changes to dopamine blockade might be to use a D2R partial agonist.18,23 For example, aripiprazole is a partial agonist at the D2R commonly used to manage schizophrenia and bipolar disorder. It possesses greater affinity at the D2R compared with the serotonin 2A (5-hydroxytryptamine, 5HT2A) serotonin receptor. Unlike full antagonists, aripiprazole requires exceptionally high D2 receptor occupancy (approximately 90%) to be at a clinically effective antipsychotic dose.24,25 This is a general requirement for all D2R partial agonists.26
A partial agonist generally has to possess greater affinity to the receptor than the neurotransmitter with which it is competing. Aripiprazole has more than twice the affinity to D2R than dopamine. Other partial agonists have similarly high, or higher, D2R affinity. Effective antipsychotic partial agonists stimulate the D2Rs at approximately 30% ± 10% the maximal signal achieved with dopamine. This is essentially equivalent to having approximately 70% receptor occupancy with a full antagonist, except it is built into how the molecule works. Having this low-grade partial activation of D2Rs creates multiple receptor-mediated actions:
- reduction of cAMP accumulation
- antagonism to guanosine 5’-0-(3-thio) triphosphate (GTPgamma S) binding with relatively less recruitment of beta-arrestin 2 (these diverging effects on G protein are the definition of biased agonism)
- antagonism of G protein activation of K+ channels (GIRK) activity
- agonism for the inhibition of TH.
Continue to: Additionally, aripiprazole was found...
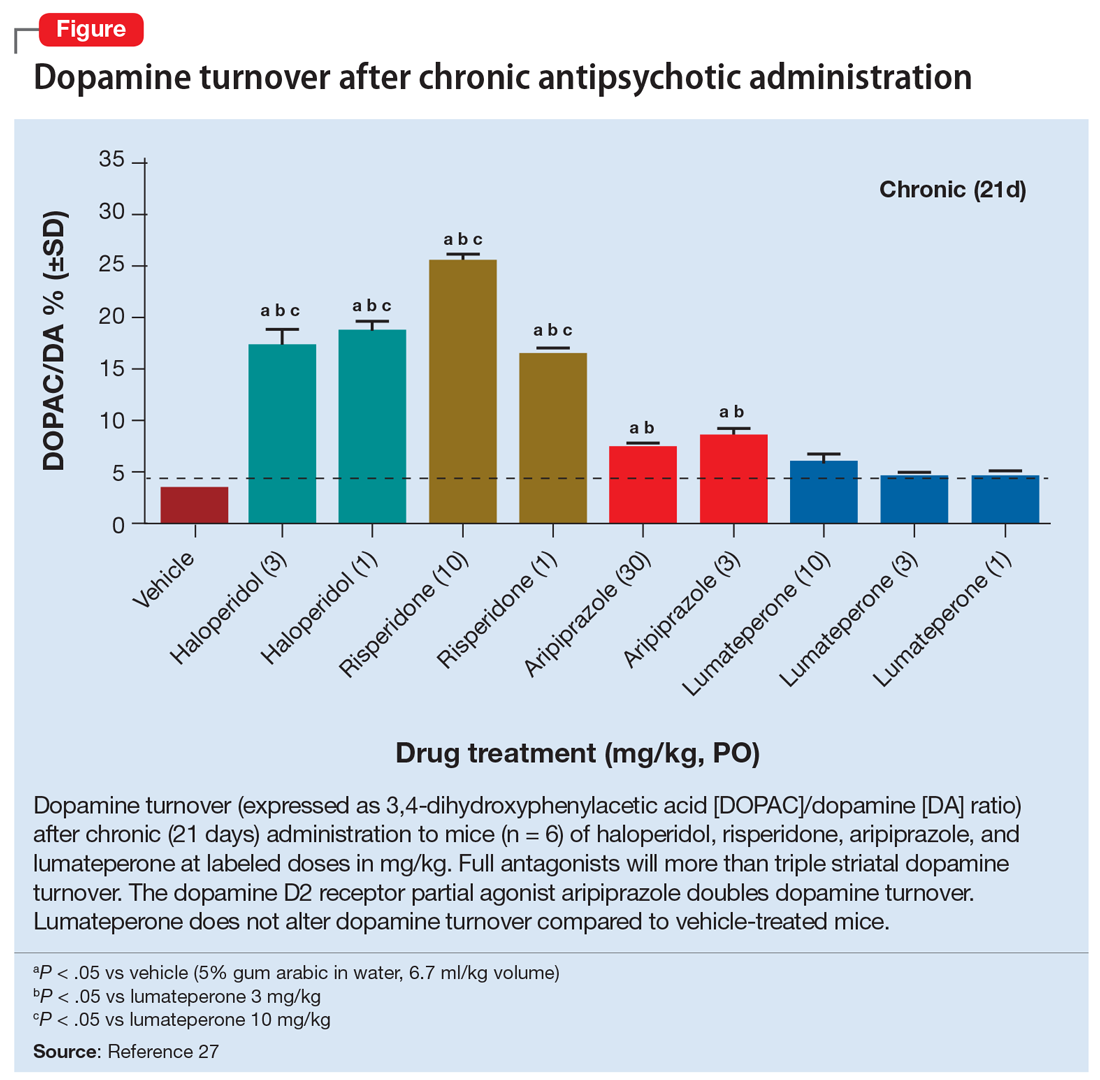
Additionally, aripiprazole was found to be associated with a lesser increase in dopamine turnover than full antagonist antipsychotics (Figure27) and decreased DAT binding density in NAc and the ventral tegmental area (VTA). The distinctive pharmacologic profile and biased agonism of this drug could be attributed to its ability to activate presynaptic D2 autoreceptors, which, as previously mentioned, regulate dopamine release via negative feedback mechanism.5,25 Cariprazine, another D2R partial agonist, has similar doubling of dopamine turnover.28
Activation of presynaptic D2S receptors ultimately leads to decreased dopamine synthesis and release, which combats or prevents the brain adaptations regarding dopamine supersensitivity and D2Rs upregulation. While TD can still occur occasionally with aripiprazole or other partial agonists,29,30 animal studies show that administration of methamphetamine significantly lowers locomotor response and the density of striatal D2Rs in a group treated with aripiprazole compared to a group treated with haloperidol.31 Aripiprazole also improved the supersensitivity parameters induced by chronic treatment with haloperidol, which suggests that it is associated with reduced dopamine supersensitivity.31 Similarly, in human studies, partial agonists appear to have a lower rate of parkinsonism and TD.32,33 One study reported that aripiprazole was associated with a significant improvement of TD in more than 50% of patients after 24 weeks of treatment.34
Lumateperone’s unique pharmacologic profile
Lumateperone is a newer antipsychotic that was FDA-approved in December 2019 for the treatment of adults with schizophrenia35 and more recently for the treatment of bipolar depression.36 It possesses a unique combination of pharmacologic properties; it is a postsynaptic D2R antagonist and a presynaptic D2R partial agonist.27
Interestingly, lumateperone has regional selectivity. It increases dopamine release in the medial prefrontal cortex (where D2R is rare) but not in the nigrostriatal pathways.27,37 It does not increase TH phosphorylation (which would increase dopamine concentration) or dopamine turnover in the striatum (Figure27). In a preclinical functional activity assay of lumateperone, the lack of change of dopamine turnover with lumateperone resembles placebo and is even less than that observed with aripiprazole (Figure27). This effect is consistent with partial agonism at the presynaptic D2S, where the stimulation of that receptor prevents the concomitant increase in dopamine synthesis and release that occurs when that receptor is blocked.
It is believed that the lack of increase in dopamine turnover is one of the reasons that lumateperone postsynaptic D2R occupancy is exceptionally low at clinically effective doses. In a positron emission tomography study analyzing posttreatment scans after approximately 2 weeks of a 60 mg/d dose, the mean peak striatal D2R occupancy was approximately 40%,38 which is remarkably lower than the 65% to 75% blockade needed for purely antagonist D2R antipsychotics.3 This low receptor occupancy appears to mediate the low incidence of parkinsonism and prolactin release seen with lumateperone.
Continue to: Take-home points
Take-home points
Adaptive upregulation of dopamine neurotransmission underlies acute adverse effects such as parkinsonism and is also key for delayed consequences such as TD, and possibly the development of treatment resistance. Adaptive upregulation results from an increase in postsynaptic dopamine receptors, numbers of synapses, and dopamine release. The latter has been demonstrated to be greatest with full antagonists, less with partial agonists, and not present with lumateperone, which is a postsynaptic antagonist but a presynaptic partial agonist (Figure27). Reducing adaptive upregulation can reduce both acute and long-term consequences of dopamine blockade. Early use of agents that minimize these adaptive changes, such as a postsynaptic partial agonist (aripiprazole, brexpiprazole, or cariprazine) or a presynaptic partial agonist (lumateperone), appears to be a reasonable clinical option.
Bottom Line
Chronic dopamine D2 receptor blockade with antipsychotics induces adaptive changes that can contribute to both acute and chronic adverse effects. The most severe of these are tardive dyskinesia (TD) and dopamine supersensitivity psychosis (DSP). The use of agents that mitigate these changes, such as the partial D2 agonists aripiprazole, brexpiprazole, and cariprazine and the postsynaptic antagonist/presynaptic partial agonist lumateperone, can potentially reduce these adaptive changes and reduce the likelihood of TD and DSP.
Related Resources
- Citrome L. Aripiprazole, brexpiprazole, and cariprazine: not all the same. Current Psychiatry. 2018;17(4):24-33,43.
- Meyer JM. Lumateperone for schizophrenia. Current Psychiatry. 2020;19(2):33-39.
Drug Brand Names
Aripiprazole • Abilify
Brexpiprazole • Rexulti
Cariprazine • Vraylar
Haloperidol • Haldol
Lumateperone • Caplyta
Methamphetamine • Desoxyn
Risperidone • Risperdal
1. Stahl SM. Beyond the dopamine hypothesis of schizophrenia to three neural networks of psychosis: dopamine, serotonin, and glutamate. CNS Spectr. 2018;23(3):187-191.
2. Howes OD, Kapur S. The dopamine hypothesis of schizophrenia: version III--the final common pathway. Schizophr Bull. 2009;35(3):549-562.
3. Ginovart N, Kapur S. Role of dopamine D2 receptors for antipsychotic activity. Handb Exp Pharmacol. 2012;(212):27-52.
4. Madras BK. History of the discovery of the antipsychotic dopamine D2 receptor: a basis for the dopamine hypothesis of schizophrenia. J Hist Neurosci. 2013;22(1):62-78.
5. Beaulieu JM, Gainetdinov RR. The physiology, signaling, and pharmacology of dopamine receptors. Pharmacol Rev. 201;63(1):182-217.
6. Martel JC, Gatti McArthur S. Dopamine receptor subtypes, physiology and pharmacology: new ligands and concepts in schizophrenia. Front Pharmacol. 2020;11:1003.
7. Monsma FJ Jr, McVittie LD, Gerfen CR, et al. Multiple D2 dopamine receptors produced by alternative RNA splicing. Nature. 1989;342(6252):926-929.
8. Seeman P, Nam D, Ulpian C, et al. New dopamine receptor, D2(Longer), with unique TG splice site, in human brain. Brain Res Mol Brain Res. 2000;76(1):132-141.
9. Khan ZU, Mrzljak L, Gutierrez A, et al. Prominence of the dopamine D2 short isoform in dopaminergic pathways. Proc Natl Acad Sci U S A. 1998;95(13):7731-7736.
10. Xu R, Hranilovic D, Fetsko LA, et al. Dopamine D2S and D2L receptors may differentially contribute to the actions of antipsychotic and psychotic agents in mice. Mol Psychiatry. 2002;7(10):1075-1082.
11. Anzalone A, Lizardi-Ortiz JE, Ramos M, et al. Dual control of dopamine synthesis and release by presynaptic and postsynaptic dopamine D2 receptors. J Neurosci. 2012;32(26):9023-9034.
12. Ford CP. The role of D2-autoreceptors in regulating dopamine neuron activity and transmission. Neuroscience. 2014;282:13-22.
13. Stroup TS, Gray N. Management of common adverse effects of antipsychotic medications. World Psychiatry. 2018;17(3):341-356.
14. El-Mallakh RS, Pant B, Caudill R, et al. Does peripheral neuropathy allow for the clinical expression of tardive dyskinesia by unmasking central nervous system changes? Med Hypotheses. 2001;57:210-215.
15. Citrome L, Saklad SR. Revisiting tardive dyskinesia: focusing on the basics of identification and treatment. J Clin Psychiatry. 2020;81(2):TV18059AH3C.
16. Carbon M, Kane JM, Leucht S, et al. Tardive dyskinesia risk with first- and second-generation antipsychotics in comparative randomized controlled trials: a meta-analysis. World Psychiatry. 2018;17(3):330-340.
17. Samaha AN, Seeman P, Stewart J, et al. “Breakthrough” dopamine supersensitivity during ongoing antipsychotic treatment leads to treatment failure over time. J Neurosci. 2007;27(11):2979-2986.
18. Yin J, Barr AM, Ramos-Miguel A, et al. Antipsychotic induced dopamine supersensitivity psychosis: a comprehensive review. Curr Neuropharmacol. 2017;15(1):174-183.
19. Chouinard G, Jones BD, Annable L. Neuroleptic-induced supersensitivity psychosis. Am J Psychiatry. 1978;135(11):1409-1410.
20. Burt DR, Creese I, Snyder SH. Antischizophrenic drugs: chronic treatment elevates dopamine receptor binding in brain. Science. 1977;196(4287):326-328.
21. Silvestri S, Seeman MV, Negrete JC, et al. Increased dopamine D2 receptor binding after long-term treatment with antipsychotics in humans: a clinical PET study. Psychopharmacology (Berl). 2000;152(2):174-180.
22. Ali Z, Roque A, El-Mallakh RS. A unifying theory for the pathoetiologic mechanism of tardive dyskinesia. Med Hypotheses. 2020;140:109682.
23. Lieberman JA. Dopamine partial agonists: a new class of antipsychotic. CNS Drugs. 2004;18(4):251-267.
24. Mailman RB, Murthy V. Third generation antipsychotic drugs: partial agonism or receptor functional selectivity? Curr Pharm Des. 2010;16(5):488-501.
25. Tuplin EW, Holahan MR. Aripiprazole, a drug that displays partial agonism and functional selectivity. Curr Neuropharmacol. 2017;15(8):1192-1207.
26. Hart XM, Schmitz CN, Gründer G. Molecular imaging of dopamine partial agonists in humans: implications for clinical practice. Front Psychiatry. 2022;13:832209.
27. Snyder GL, Vanover KE, Zhu H, et al. Functional profile of a novel modulator of serotonin, dopamine, and glutamate neurotransmission. Psychopharmacology (Berl). 2015;232(3):605-621.
28. Kiss B, Horváth A, Némethy Z, et al. Cariprazine (RGH-188), a dopamine D(3) receptor-preferring, D(3)/D(2) dopamine receptor antagonist-partial agonist antipsychotic candidate: in vitro and neurochemical profile. J Pharmacol Exp Ther. 2010;333(1):328-340.
29. Abbasian C, Power P. A case of aripiprazole and tardive dyskinesia. J Psychopharmacol. 2009;23(2):214-215.
30. Peña MS, Yaltho TC, Jankovic J. Tardive dyskinesia and other movement disorders secondary to aripiprazole. Mov Disord. 2011;26(1):147-152.
31. Tadokoro S, Okamura N, Sekine Y, et al. Chronic treatment with aripiprazole prevents development of dopamine supersensitivity and potentially supersensitivity psychosis. Schizophr Bull. 2012;38(5):1012-1020.
32. Kang NR, Kim MD. Tardive dyskinesia: treatment with aripiprazole. Clin Psychopharmacol Neurosci. 2011;9(1):1-8.
33. Frankel JS, Schwartz TL. Brexpiprazole and cariprazine: distinguishing two new atypical antipsychotics from the original dopamine stabilizer aripiprazole. Ther Adv Psychopharmacol. 2017;7(1):29-41.
34. Chan CH, Chan HY, Chen YC. Switching antipsychotic treatment to aripiprazole in psychotic patients with neuroleptic-induced tardive dyskinesia: a 24-week follow-up study. Int Clin Psychopharmacol. 2018;33(3):155-162.
35. Blair HA. Lumateperone: first approval. Drugs. 2020;80(4):417-423.
36. Calabrese JR, Durgam S, Satlin A, et al. Efficacy and safety of Lumateperone for major depressive episodes associated with bipolar I or bipolar II disorder: a phase 3 randomized placebo-controlled trial. Am J Psychiatry. 2021;178(12):1098-1106.
37. Nakai S, Hirose T, Uwahodo Y, et al. Diminished catalepsy and dopamine metabolism distinguish aripiprazole from haloperidol or risperidone. Eur J Pharmacol. 2003;472(12):89-97.
38. Vanover KE, Davis RE, Zhou Y, et al. Dopamine D2 receptor occupancy of lumateperone (ITI-007): a positron emission tomography study in patients with schizophrenia. Neuropsychopharmacology. 2019;44(3):598-605.
While our understanding of the mechanisms of psychosis continues to evolve beyond the dopamine hypothesis, the key role of dopamine in psychosis and its treatment has not faded.1 Over time, the dopamine hypothesis of schizophrenia has evolved from focusing on dopamine hyperactivity to specifying the regional abnormalities in the brain with subcortical hyperdopaminergia and prefrontal hypodopaminergia.2 Despite this divergence in dopaminergic function, antipsychotic medications that block dopamine D2 receptors (D2R) remain central to treating psychotic symptoms and preventing relapse.3,4 Notably, antipsychotics block both presynaptic and postsynaptic receptors affecting the regulation of dopamine synthesis and release in the brain.5,6
Chronic dopamine D2R blockade with antipsychotics induces adaptive changes that can contribute to both acute and chronic adverse effects. In this article, we discuss these changes, and steps clinicians can take to minimize their occurrence.
Dopamine D2R: A primer
There are 5 types of dopamine receptors, numbered D1 through D5, but there are only 2 families of dopamine receptors: the D1 family (D1 and D5), and the D2 family (D2, D3, and D4). All dopamine receptors are G protein–coupled, but the D2 family of receptors generally increases protein kinase A (PKA) as the second messenger, whereas the D1 family increases cyclic adenosine monophosphate (cAMP) as the second messenger.5 There are 2 distinct variants of the D2R of 2 different lengths made from the same gene (DRD2) via posttranslational modification. The long isoform of D2R (D2L) has an additional 29 amino acids compared to the short isoform (D2S).7 Additional evidence points to a third splice variant called D2Longer that arises from aberrant RNA splicing and contains 2 more amino acids than D2L; its relevance is not known.8
The D2L isoform is the primary postsynaptic receptor, expressed more in the striatum and nucleus accumbens (NAc) targeted by dopaminergic afferents. The D2S isoform, however, is predominantly presynaptic, more densely expressed on cell bodies and projection axons of the dopaminergic neurons of the midbrain and hypothalamus.9 Each isoform contributes differentially to the therapeutic and adverse effects of antipsychotics, and evidence from animal studies suggests that D2L is the main variant responsible for drug-induced parkinsonism.10 The D2S acts as the principal autoreceptor for the dopaminergic system.5,11,12
Autoreceptors regulate dopamine transmission. Dopamine itself and D2R agonists are reported to have higher affinity and potency with D2S. Activation of these autoreceptors is a negative feedback mechanism that decreases dopamine release. Similarly, when they are blocked (such as with use of an antipsychotic), there is an increase in dopamine release. Additionally, these autoreceptors modulate several key processes:
- neuronal firing rate by activating potassium conductance
- dopamine synthesis by downregulating the expression of tyrosine hydroxylase (TH) enzyme (the rate-limiting step)
- exocytotic release of dopamine and other neurotransmitters
- dopamine reuptake via increasing the activity of the dopamine transporter (DAT).12
Consequences of antipsychotic D2R blockade
Most antipsychotics begin to produce a therapeutic antipsychotic effect at 65% to 75% occupancy of the D2Rs.3 This level also produces an optimal balance between clinical efficacy and a lower incidence of adverse effects.3 A higher D2R occupancy by both first-generation (FGA) and second-generation (SGA) pure antagonist antipsychotics can lead to parkinsonism.
Parkinsonism is associated with the subsequent appearance of one of the most distressing consequences of long-term antipsychotic treatment, tardive dyskinesia (TD).13 TD is an iatrogenic, usually late-onset syndrome consisting of persistent, involuntary, and repetitive movements. It classically involves the highly innervated striated muscles of the tongue, mouth, face, and fingers, though it can also involve the trunk and extremities.14 It occurs secondary to chronic exposure to dopamine receptor–blocking agents, including dopaminergic antiemetics.15 The prevalence of TD is higher in patients treated long-term with FGAs (30.0% to 32.4%) than in those treated with SGAs (13.1% to 20.7%) due to serotonin 5HT2A blockade that results in increased dopamine release in the basal ganglia.16
Continue to: Dopamine supersenstivity psychosis...
Dopamine supersensitivity psychosis (DSP) is a term that describes the clinical iatrogenic phenomenon that might be observed with long-term antipsychotic treatment. DSP is suggested to be strongly associated with treatment failure/resistance in schizophrenia.17,18 Manifestations of DSP include development of antipsychotic drug tolerance that undermines treatment efficacy, rebound psychosis during or after treatment discontinuation, and the presence of TD. Like TD, it may be reversed temporarily by increasing the dose of the antipsychotic.18
DSP and (more extensively) TD are commonly hypothesized to result from the postsynaptic dopamine receptor supersensitivity that develops because of chronic D2Rs blockade by antipsychotics. Neostriatal dopamine receptor supersensitivity is believed to lead to TD, while mesolimbic supersensitivity leads to DSP.19 Supersensitivity has traditionally been believed to be due to upregulation of postsynaptic D2R number and sensitivity.20,21 However, both TD and DSP are more likely a consequence of a host of compensatory neurobiological adaptations across the synapse that include:
- postsynaptic increase in the number of D2Rs that amplifies the dopamine signal
- an increased number of synapses, dendritic spines, and perforated synapses (seen in animal models), all of which lead to a potentiated dopamine signal
- presynaptic changes with higher levels of dopamine released into the synapse via an increase in quantal size as postsynaptic D2Rs blockade results in more dopamine becoming available in the synapse for recycling via the dopamine transporter
- increased dopamine turnover due to presynaptic D2S autoreceptor blockade.22
So if giving a D2R blocking agent for a long time increases the dopamine signal, at least in some patients, what can the clinician do to treat the psychosis, and not cause changes in the brain that could lead to TD or DSP?
Partial agonist antipsychotics and biased agonism of D2Rs
One approach to try to avoid the compensatory changes to dopamine blockade might be to use a D2R partial agonist.18,23 For example, aripiprazole is a partial agonist at the D2R commonly used to manage schizophrenia and bipolar disorder. It possesses greater affinity at the D2R compared with the serotonin 2A (5-hydroxytryptamine, 5HT2A) serotonin receptor. Unlike full antagonists, aripiprazole requires exceptionally high D2 receptor occupancy (approximately 90%) to be at a clinically effective antipsychotic dose.24,25 This is a general requirement for all D2R partial agonists.26
A partial agonist generally has to possess greater affinity to the receptor than the neurotransmitter with which it is competing. Aripiprazole has more than twice the affinity to D2R than dopamine. Other partial agonists have similarly high, or higher, D2R affinity. Effective antipsychotic partial agonists stimulate the D2Rs at approximately 30% ± 10% the maximal signal achieved with dopamine. This is essentially equivalent to having approximately 70% receptor occupancy with a full antagonist, except it is built into how the molecule works. Having this low-grade partial activation of D2Rs creates multiple receptor-mediated actions:
- reduction of cAMP accumulation
- antagonism to guanosine 5’-0-(3-thio) triphosphate (GTPgamma S) binding with relatively less recruitment of beta-arrestin 2 (these diverging effects on G protein are the definition of biased agonism)
- antagonism of G protein activation of K+ channels (GIRK) activity
- agonism for the inhibition of TH.
Continue to: Additionally, aripiprazole was found...
Additionally, aripiprazole was found to be associated with a lesser increase in dopamine turnover than full antagonist antipsychotics (Figure27) and decreased DAT binding density in NAc and the ventral tegmental area (VTA). The distinctive pharmacologic profile and biased agonism of this drug could be attributed to its ability to activate presynaptic D2 autoreceptors, which, as previously mentioned, regulate dopamine release via negative feedback mechanism.5,25 Cariprazine, another D2R partial agonist, has similar doubling of dopamine turnover.28
Activation of presynaptic D2S receptors ultimately leads to decreased dopamine synthesis and release, which combats or prevents the brain adaptations regarding dopamine supersensitivity and D2Rs upregulation. While TD can still occur occasionally with aripiprazole or other partial agonists,29,30 animal studies show that administration of methamphetamine significantly lowers locomotor response and the density of striatal D2Rs in a group treated with aripiprazole compared to a group treated with haloperidol.31 Aripiprazole also improved the supersensitivity parameters induced by chronic treatment with haloperidol, which suggests that it is associated with reduced dopamine supersensitivity.31 Similarly, in human studies, partial agonists appear to have a lower rate of parkinsonism and TD.32,33 One study reported that aripiprazole was associated with a significant improvement of TD in more than 50% of patients after 24 weeks of treatment.34
Lumateperone’s unique pharmacologic profile
Lumateperone is a newer antipsychotic that was FDA-approved in December 2019 for the treatment of adults with schizophrenia35 and more recently for the treatment of bipolar depression.36 It possesses a unique combination of pharmacologic properties; it is a postsynaptic D2R antagonist and a presynaptic D2R partial agonist.27
Interestingly, lumateperone has regional selectivity. It increases dopamine release in the medial prefrontal cortex (where D2R is rare) but not in the nigrostriatal pathways.27,37 It does not increase TH phosphorylation (which would increase dopamine concentration) or dopamine turnover in the striatum (Figure27). In a preclinical functional activity assay of lumateperone, the lack of change of dopamine turnover with lumateperone resembles placebo and is even less than that observed with aripiprazole (Figure27). This effect is consistent with partial agonism at the presynaptic D2S, where the stimulation of that receptor prevents the concomitant increase in dopamine synthesis and release that occurs when that receptor is blocked.
It is believed that the lack of increase in dopamine turnover is one of the reasons that lumateperone postsynaptic D2R occupancy is exceptionally low at clinically effective doses. In a positron emission tomography study analyzing posttreatment scans after approximately 2 weeks of a 60 mg/d dose, the mean peak striatal D2R occupancy was approximately 40%,38 which is remarkably lower than the 65% to 75% blockade needed for purely antagonist D2R antipsychotics.3 This low receptor occupancy appears to mediate the low incidence of parkinsonism and prolactin release seen with lumateperone.
Continue to: Take-home points
Take-home points
Adaptive upregulation of dopamine neurotransmission underlies acute adverse effects such as parkinsonism and is also key for delayed consequences such as TD, and possibly the development of treatment resistance. Adaptive upregulation results from an increase in postsynaptic dopamine receptors, numbers of synapses, and dopamine release. The latter has been demonstrated to be greatest with full antagonists, less with partial agonists, and not present with lumateperone, which is a postsynaptic antagonist but a presynaptic partial agonist (Figure27). Reducing adaptive upregulation can reduce both acute and long-term consequences of dopamine blockade. Early use of agents that minimize these adaptive changes, such as a postsynaptic partial agonist (aripiprazole, brexpiprazole, or cariprazine) or a presynaptic partial agonist (lumateperone), appears to be a reasonable clinical option.
Bottom Line
Chronic dopamine D2 receptor blockade with antipsychotics induces adaptive changes that can contribute to both acute and chronic adverse effects. The most severe of these are tardive dyskinesia (TD) and dopamine supersensitivity psychosis (DSP). The use of agents that mitigate these changes, such as the partial D2 agonists aripiprazole, brexpiprazole, and cariprazine and the postsynaptic antagonist/presynaptic partial agonist lumateperone, can potentially reduce these adaptive changes and reduce the likelihood of TD and DSP.
Related Resources
- Citrome L. Aripiprazole, brexpiprazole, and cariprazine: not all the same. Current Psychiatry. 2018;17(4):24-33,43.
- Meyer JM. Lumateperone for schizophrenia. Current Psychiatry. 2020;19(2):33-39.
Drug Brand Names
Aripiprazole • Abilify
Brexpiprazole • Rexulti
Cariprazine • Vraylar
Haloperidol • Haldol
Lumateperone • Caplyta
Methamphetamine • Desoxyn
Risperidone • Risperdal
While our understanding of the mechanisms of psychosis continues to evolve beyond the dopamine hypothesis, the key role of dopamine in psychosis and its treatment has not faded.1 Over time, the dopamine hypothesis of schizophrenia has evolved from focusing on dopamine hyperactivity to specifying the regional abnormalities in the brain with subcortical hyperdopaminergia and prefrontal hypodopaminergia.2 Despite this divergence in dopaminergic function, antipsychotic medications that block dopamine D2 receptors (D2R) remain central to treating psychotic symptoms and preventing relapse.3,4 Notably, antipsychotics block both presynaptic and postsynaptic receptors affecting the regulation of dopamine synthesis and release in the brain.5,6
Chronic dopamine D2R blockade with antipsychotics induces adaptive changes that can contribute to both acute and chronic adverse effects. In this article, we discuss these changes, and steps clinicians can take to minimize their occurrence.
Dopamine D2R: A primer
There are 5 types of dopamine receptors, numbered D1 through D5, but there are only 2 families of dopamine receptors: the D1 family (D1 and D5), and the D2 family (D2, D3, and D4). All dopamine receptors are G protein–coupled, but the D2 family of receptors generally increases protein kinase A (PKA) as the second messenger, whereas the D1 family increases cyclic adenosine monophosphate (cAMP) as the second messenger.5 There are 2 distinct variants of the D2R of 2 different lengths made from the same gene (DRD2) via posttranslational modification. The long isoform of D2R (D2L) has an additional 29 amino acids compared to the short isoform (D2S).7 Additional evidence points to a third splice variant called D2Longer that arises from aberrant RNA splicing and contains 2 more amino acids than D2L; its relevance is not known.8
The D2L isoform is the primary postsynaptic receptor, expressed more in the striatum and nucleus accumbens (NAc) targeted by dopaminergic afferents. The D2S isoform, however, is predominantly presynaptic, more densely expressed on cell bodies and projection axons of the dopaminergic neurons of the midbrain and hypothalamus.9 Each isoform contributes differentially to the therapeutic and adverse effects of antipsychotics, and evidence from animal studies suggests that D2L is the main variant responsible for drug-induced parkinsonism.10 The D2S acts as the principal autoreceptor for the dopaminergic system.5,11,12
Autoreceptors regulate dopamine transmission. Dopamine itself and D2R agonists are reported to have higher affinity and potency with D2S. Activation of these autoreceptors is a negative feedback mechanism that decreases dopamine release. Similarly, when they are blocked (such as with use of an antipsychotic), there is an increase in dopamine release. Additionally, these autoreceptors modulate several key processes:
- neuronal firing rate by activating potassium conductance
- dopamine synthesis by downregulating the expression of tyrosine hydroxylase (TH) enzyme (the rate-limiting step)
- exocytotic release of dopamine and other neurotransmitters
- dopamine reuptake via increasing the activity of the dopamine transporter (DAT).12
Consequences of antipsychotic D2R blockade
Most antipsychotics begin to produce a therapeutic antipsychotic effect at 65% to 75% occupancy of the D2Rs.3 This level also produces an optimal balance between clinical efficacy and a lower incidence of adverse effects.3 A higher D2R occupancy by both first-generation (FGA) and second-generation (SGA) pure antagonist antipsychotics can lead to parkinsonism.
Parkinsonism is associated with the subsequent appearance of one of the most distressing consequences of long-term antipsychotic treatment, tardive dyskinesia (TD).13 TD is an iatrogenic, usually late-onset syndrome consisting of persistent, involuntary, and repetitive movements. It classically involves the highly innervated striated muscles of the tongue, mouth, face, and fingers, though it can also involve the trunk and extremities.14 It occurs secondary to chronic exposure to dopamine receptor–blocking agents, including dopaminergic antiemetics.15 The prevalence of TD is higher in patients treated long-term with FGAs (30.0% to 32.4%) than in those treated with SGAs (13.1% to 20.7%) due to serotonin 5HT2A blockade that results in increased dopamine release in the basal ganglia.16
Continue to: Dopamine supersenstivity psychosis...
Dopamine supersensitivity psychosis (DSP) is a term that describes the clinical iatrogenic phenomenon that might be observed with long-term antipsychotic treatment. DSP is suggested to be strongly associated with treatment failure/resistance in schizophrenia.17,18 Manifestations of DSP include development of antipsychotic drug tolerance that undermines treatment efficacy, rebound psychosis during or after treatment discontinuation, and the presence of TD. Like TD, it may be reversed temporarily by increasing the dose of the antipsychotic.18
DSP and (more extensively) TD are commonly hypothesized to result from the postsynaptic dopamine receptor supersensitivity that develops because of chronic D2Rs blockade by antipsychotics. Neostriatal dopamine receptor supersensitivity is believed to lead to TD, while mesolimbic supersensitivity leads to DSP.19 Supersensitivity has traditionally been believed to be due to upregulation of postsynaptic D2R number and sensitivity.20,21 However, both TD and DSP are more likely a consequence of a host of compensatory neurobiological adaptations across the synapse that include:
- postsynaptic increase in the number of D2Rs that amplifies the dopamine signal
- an increased number of synapses, dendritic spines, and perforated synapses (seen in animal models), all of which lead to a potentiated dopamine signal
- presynaptic changes with higher levels of dopamine released into the synapse via an increase in quantal size as postsynaptic D2Rs blockade results in more dopamine becoming available in the synapse for recycling via the dopamine transporter
- increased dopamine turnover due to presynaptic D2S autoreceptor blockade.22
So if giving a D2R blocking agent for a long time increases the dopamine signal, at least in some patients, what can the clinician do to treat the psychosis, and not cause changes in the brain that could lead to TD or DSP?
Partial agonist antipsychotics and biased agonism of D2Rs
One approach to try to avoid the compensatory changes to dopamine blockade might be to use a D2R partial agonist.18,23 For example, aripiprazole is a partial agonist at the D2R commonly used to manage schizophrenia and bipolar disorder. It possesses greater affinity at the D2R compared with the serotonin 2A (5-hydroxytryptamine, 5HT2A) serotonin receptor. Unlike full antagonists, aripiprazole requires exceptionally high D2 receptor occupancy (approximately 90%) to be at a clinically effective antipsychotic dose.24,25 This is a general requirement for all D2R partial agonists.26
A partial agonist generally has to possess greater affinity to the receptor than the neurotransmitter with which it is competing. Aripiprazole has more than twice the affinity to D2R than dopamine. Other partial agonists have similarly high, or higher, D2R affinity. Effective antipsychotic partial agonists stimulate the D2Rs at approximately 30% ± 10% the maximal signal achieved with dopamine. This is essentially equivalent to having approximately 70% receptor occupancy with a full antagonist, except it is built into how the molecule works. Having this low-grade partial activation of D2Rs creates multiple receptor-mediated actions:
- reduction of cAMP accumulation
- antagonism to guanosine 5’-0-(3-thio) triphosphate (GTPgamma S) binding with relatively less recruitment of beta-arrestin 2 (these diverging effects on G protein are the definition of biased agonism)
- antagonism of G protein activation of K+ channels (GIRK) activity
- agonism for the inhibition of TH.
Continue to: Additionally, aripiprazole was found...
Additionally, aripiprazole was found to be associated with a lesser increase in dopamine turnover than full antagonist antipsychotics (Figure27) and decreased DAT binding density in NAc and the ventral tegmental area (VTA). The distinctive pharmacologic profile and biased agonism of this drug could be attributed to its ability to activate presynaptic D2 autoreceptors, which, as previously mentioned, regulate dopamine release via negative feedback mechanism.5,25 Cariprazine, another D2R partial agonist, has similar doubling of dopamine turnover.28
Activation of presynaptic D2S receptors ultimately leads to decreased dopamine synthesis and release, which combats or prevents the brain adaptations regarding dopamine supersensitivity and D2Rs upregulation. While TD can still occur occasionally with aripiprazole or other partial agonists,29,30 animal studies show that administration of methamphetamine significantly lowers locomotor response and the density of striatal D2Rs in a group treated with aripiprazole compared to a group treated with haloperidol.31 Aripiprazole also improved the supersensitivity parameters induced by chronic treatment with haloperidol, which suggests that it is associated with reduced dopamine supersensitivity.31 Similarly, in human studies, partial agonists appear to have a lower rate of parkinsonism and TD.32,33 One study reported that aripiprazole was associated with a significant improvement of TD in more than 50% of patients after 24 weeks of treatment.34
Lumateperone’s unique pharmacologic profile
Lumateperone is a newer antipsychotic that was FDA-approved in December 2019 for the treatment of adults with schizophrenia35 and more recently for the treatment of bipolar depression.36 It possesses a unique combination of pharmacologic properties; it is a postsynaptic D2R antagonist and a presynaptic D2R partial agonist.27
Interestingly, lumateperone has regional selectivity. It increases dopamine release in the medial prefrontal cortex (where D2R is rare) but not in the nigrostriatal pathways.27,37 It does not increase TH phosphorylation (which would increase dopamine concentration) or dopamine turnover in the striatum (Figure27). In a preclinical functional activity assay of lumateperone, the lack of change of dopamine turnover with lumateperone resembles placebo and is even less than that observed with aripiprazole (Figure27). This effect is consistent with partial agonism at the presynaptic D2S, where the stimulation of that receptor prevents the concomitant increase in dopamine synthesis and release that occurs when that receptor is blocked.
It is believed that the lack of increase in dopamine turnover is one of the reasons that lumateperone postsynaptic D2R occupancy is exceptionally low at clinically effective doses. In a positron emission tomography study analyzing posttreatment scans after approximately 2 weeks of a 60 mg/d dose, the mean peak striatal D2R occupancy was approximately 40%,38 which is remarkably lower than the 65% to 75% blockade needed for purely antagonist D2R antipsychotics.3 This low receptor occupancy appears to mediate the low incidence of parkinsonism and prolactin release seen with lumateperone.
Continue to: Take-home points
Take-home points
Adaptive upregulation of dopamine neurotransmission underlies acute adverse effects such as parkinsonism and is also key for delayed consequences such as TD, and possibly the development of treatment resistance. Adaptive upregulation results from an increase in postsynaptic dopamine receptors, numbers of synapses, and dopamine release. The latter has been demonstrated to be greatest with full antagonists, less with partial agonists, and not present with lumateperone, which is a postsynaptic antagonist but a presynaptic partial agonist (Figure27). Reducing adaptive upregulation can reduce both acute and long-term consequences of dopamine blockade. Early use of agents that minimize these adaptive changes, such as a postsynaptic partial agonist (aripiprazole, brexpiprazole, or cariprazine) or a presynaptic partial agonist (lumateperone), appears to be a reasonable clinical option.
Bottom Line
Chronic dopamine D2 receptor blockade with antipsychotics induces adaptive changes that can contribute to both acute and chronic adverse effects. The most severe of these are tardive dyskinesia (TD) and dopamine supersensitivity psychosis (DSP). The use of agents that mitigate these changes, such as the partial D2 agonists aripiprazole, brexpiprazole, and cariprazine and the postsynaptic antagonist/presynaptic partial agonist lumateperone, can potentially reduce these adaptive changes and reduce the likelihood of TD and DSP.
Related Resources
- Citrome L. Aripiprazole, brexpiprazole, and cariprazine: not all the same. Current Psychiatry. 2018;17(4):24-33,43.
- Meyer JM. Lumateperone for schizophrenia. Current Psychiatry. 2020;19(2):33-39.
Drug Brand Names
Aripiprazole • Abilify
Brexpiprazole • Rexulti
Cariprazine • Vraylar
Haloperidol • Haldol
Lumateperone • Caplyta
Methamphetamine • Desoxyn
Risperidone • Risperdal
1. Stahl SM. Beyond the dopamine hypothesis of schizophrenia to three neural networks of psychosis: dopamine, serotonin, and glutamate. CNS Spectr. 2018;23(3):187-191.
2. Howes OD, Kapur S. The dopamine hypothesis of schizophrenia: version III--the final common pathway. Schizophr Bull. 2009;35(3):549-562.
3. Ginovart N, Kapur S. Role of dopamine D2 receptors for antipsychotic activity. Handb Exp Pharmacol. 2012;(212):27-52.
4. Madras BK. History of the discovery of the antipsychotic dopamine D2 receptor: a basis for the dopamine hypothesis of schizophrenia. J Hist Neurosci. 2013;22(1):62-78.
5. Beaulieu JM, Gainetdinov RR. The physiology, signaling, and pharmacology of dopamine receptors. Pharmacol Rev. 201;63(1):182-217.
6. Martel JC, Gatti McArthur S. Dopamine receptor subtypes, physiology and pharmacology: new ligands and concepts in schizophrenia. Front Pharmacol. 2020;11:1003.
7. Monsma FJ Jr, McVittie LD, Gerfen CR, et al. Multiple D2 dopamine receptors produced by alternative RNA splicing. Nature. 1989;342(6252):926-929.
8. Seeman P, Nam D, Ulpian C, et al. New dopamine receptor, D2(Longer), with unique TG splice site, in human brain. Brain Res Mol Brain Res. 2000;76(1):132-141.
9. Khan ZU, Mrzljak L, Gutierrez A, et al. Prominence of the dopamine D2 short isoform in dopaminergic pathways. Proc Natl Acad Sci U S A. 1998;95(13):7731-7736.
10. Xu R, Hranilovic D, Fetsko LA, et al. Dopamine D2S and D2L receptors may differentially contribute to the actions of antipsychotic and psychotic agents in mice. Mol Psychiatry. 2002;7(10):1075-1082.
11. Anzalone A, Lizardi-Ortiz JE, Ramos M, et al. Dual control of dopamine synthesis and release by presynaptic and postsynaptic dopamine D2 receptors. J Neurosci. 2012;32(26):9023-9034.
12. Ford CP. The role of D2-autoreceptors in regulating dopamine neuron activity and transmission. Neuroscience. 2014;282:13-22.
13. Stroup TS, Gray N. Management of common adverse effects of antipsychotic medications. World Psychiatry. 2018;17(3):341-356.
14. El-Mallakh RS, Pant B, Caudill R, et al. Does peripheral neuropathy allow for the clinical expression of tardive dyskinesia by unmasking central nervous system changes? Med Hypotheses. 2001;57:210-215.
15. Citrome L, Saklad SR. Revisiting tardive dyskinesia: focusing on the basics of identification and treatment. J Clin Psychiatry. 2020;81(2):TV18059AH3C.
16. Carbon M, Kane JM, Leucht S, et al. Tardive dyskinesia risk with first- and second-generation antipsychotics in comparative randomized controlled trials: a meta-analysis. World Psychiatry. 2018;17(3):330-340.
17. Samaha AN, Seeman P, Stewart J, et al. “Breakthrough” dopamine supersensitivity during ongoing antipsychotic treatment leads to treatment failure over time. J Neurosci. 2007;27(11):2979-2986.
18. Yin J, Barr AM, Ramos-Miguel A, et al. Antipsychotic induced dopamine supersensitivity psychosis: a comprehensive review. Curr Neuropharmacol. 2017;15(1):174-183.
19. Chouinard G, Jones BD, Annable L. Neuroleptic-induced supersensitivity psychosis. Am J Psychiatry. 1978;135(11):1409-1410.
20. Burt DR, Creese I, Snyder SH. Antischizophrenic drugs: chronic treatment elevates dopamine receptor binding in brain. Science. 1977;196(4287):326-328.
21. Silvestri S, Seeman MV, Negrete JC, et al. Increased dopamine D2 receptor binding after long-term treatment with antipsychotics in humans: a clinical PET study. Psychopharmacology (Berl). 2000;152(2):174-180.
22. Ali Z, Roque A, El-Mallakh RS. A unifying theory for the pathoetiologic mechanism of tardive dyskinesia. Med Hypotheses. 2020;140:109682.
23. Lieberman JA. Dopamine partial agonists: a new class of antipsychotic. CNS Drugs. 2004;18(4):251-267.
24. Mailman RB, Murthy V. Third generation antipsychotic drugs: partial agonism or receptor functional selectivity? Curr Pharm Des. 2010;16(5):488-501.
25. Tuplin EW, Holahan MR. Aripiprazole, a drug that displays partial agonism and functional selectivity. Curr Neuropharmacol. 2017;15(8):1192-1207.
26. Hart XM, Schmitz CN, Gründer G. Molecular imaging of dopamine partial agonists in humans: implications for clinical practice. Front Psychiatry. 2022;13:832209.
27. Snyder GL, Vanover KE, Zhu H, et al. Functional profile of a novel modulator of serotonin, dopamine, and glutamate neurotransmission. Psychopharmacology (Berl). 2015;232(3):605-621.
28. Kiss B, Horváth A, Némethy Z, et al. Cariprazine (RGH-188), a dopamine D(3) receptor-preferring, D(3)/D(2) dopamine receptor antagonist-partial agonist antipsychotic candidate: in vitro and neurochemical profile. J Pharmacol Exp Ther. 2010;333(1):328-340.
29. Abbasian C, Power P. A case of aripiprazole and tardive dyskinesia. J Psychopharmacol. 2009;23(2):214-215.
30. Peña MS, Yaltho TC, Jankovic J. Tardive dyskinesia and other movement disorders secondary to aripiprazole. Mov Disord. 2011;26(1):147-152.
31. Tadokoro S, Okamura N, Sekine Y, et al. Chronic treatment with aripiprazole prevents development of dopamine supersensitivity and potentially supersensitivity psychosis. Schizophr Bull. 2012;38(5):1012-1020.
32. Kang NR, Kim MD. Tardive dyskinesia: treatment with aripiprazole. Clin Psychopharmacol Neurosci. 2011;9(1):1-8.
33. Frankel JS, Schwartz TL. Brexpiprazole and cariprazine: distinguishing two new atypical antipsychotics from the original dopamine stabilizer aripiprazole. Ther Adv Psychopharmacol. 2017;7(1):29-41.
34. Chan CH, Chan HY, Chen YC. Switching antipsychotic treatment to aripiprazole in psychotic patients with neuroleptic-induced tardive dyskinesia: a 24-week follow-up study. Int Clin Psychopharmacol. 2018;33(3):155-162.
35. Blair HA. Lumateperone: first approval. Drugs. 2020;80(4):417-423.
36. Calabrese JR, Durgam S, Satlin A, et al. Efficacy and safety of Lumateperone for major depressive episodes associated with bipolar I or bipolar II disorder: a phase 3 randomized placebo-controlled trial. Am J Psychiatry. 2021;178(12):1098-1106.
37. Nakai S, Hirose T, Uwahodo Y, et al. Diminished catalepsy and dopamine metabolism distinguish aripiprazole from haloperidol or risperidone. Eur J Pharmacol. 2003;472(12):89-97.
38. Vanover KE, Davis RE, Zhou Y, et al. Dopamine D2 receptor occupancy of lumateperone (ITI-007): a positron emission tomography study in patients with schizophrenia. Neuropsychopharmacology. 2019;44(3):598-605.
1. Stahl SM. Beyond the dopamine hypothesis of schizophrenia to three neural networks of psychosis: dopamine, serotonin, and glutamate. CNS Spectr. 2018;23(3):187-191.
2. Howes OD, Kapur S. The dopamine hypothesis of schizophrenia: version III--the final common pathway. Schizophr Bull. 2009;35(3):549-562.
3. Ginovart N, Kapur S. Role of dopamine D2 receptors for antipsychotic activity. Handb Exp Pharmacol. 2012;(212):27-52.
4. Madras BK. History of the discovery of the antipsychotic dopamine D2 receptor: a basis for the dopamine hypothesis of schizophrenia. J Hist Neurosci. 2013;22(1):62-78.
5. Beaulieu JM, Gainetdinov RR. The physiology, signaling, and pharmacology of dopamine receptors. Pharmacol Rev. 201;63(1):182-217.
6. Martel JC, Gatti McArthur S. Dopamine receptor subtypes, physiology and pharmacology: new ligands and concepts in schizophrenia. Front Pharmacol. 2020;11:1003.
7. Monsma FJ Jr, McVittie LD, Gerfen CR, et al. Multiple D2 dopamine receptors produced by alternative RNA splicing. Nature. 1989;342(6252):926-929.
8. Seeman P, Nam D, Ulpian C, et al. New dopamine receptor, D2(Longer), with unique TG splice site, in human brain. Brain Res Mol Brain Res. 2000;76(1):132-141.
9. Khan ZU, Mrzljak L, Gutierrez A, et al. Prominence of the dopamine D2 short isoform in dopaminergic pathways. Proc Natl Acad Sci U S A. 1998;95(13):7731-7736.
10. Xu R, Hranilovic D, Fetsko LA, et al. Dopamine D2S and D2L receptors may differentially contribute to the actions of antipsychotic and psychotic agents in mice. Mol Psychiatry. 2002;7(10):1075-1082.
11. Anzalone A, Lizardi-Ortiz JE, Ramos M, et al. Dual control of dopamine synthesis and release by presynaptic and postsynaptic dopamine D2 receptors. J Neurosci. 2012;32(26):9023-9034.
12. Ford CP. The role of D2-autoreceptors in regulating dopamine neuron activity and transmission. Neuroscience. 2014;282:13-22.
13. Stroup TS, Gray N. Management of common adverse effects of antipsychotic medications. World Psychiatry. 2018;17(3):341-356.
14. El-Mallakh RS, Pant B, Caudill R, et al. Does peripheral neuropathy allow for the clinical expression of tardive dyskinesia by unmasking central nervous system changes? Med Hypotheses. 2001;57:210-215.
15. Citrome L, Saklad SR. Revisiting tardive dyskinesia: focusing on the basics of identification and treatment. J Clin Psychiatry. 2020;81(2):TV18059AH3C.
16. Carbon M, Kane JM, Leucht S, et al. Tardive dyskinesia risk with first- and second-generation antipsychotics in comparative randomized controlled trials: a meta-analysis. World Psychiatry. 2018;17(3):330-340.
17. Samaha AN, Seeman P, Stewart J, et al. “Breakthrough” dopamine supersensitivity during ongoing antipsychotic treatment leads to treatment failure over time. J Neurosci. 2007;27(11):2979-2986.
18. Yin J, Barr AM, Ramos-Miguel A, et al. Antipsychotic induced dopamine supersensitivity psychosis: a comprehensive review. Curr Neuropharmacol. 2017;15(1):174-183.
19. Chouinard G, Jones BD, Annable L. Neuroleptic-induced supersensitivity psychosis. Am J Psychiatry. 1978;135(11):1409-1410.
20. Burt DR, Creese I, Snyder SH. Antischizophrenic drugs: chronic treatment elevates dopamine receptor binding in brain. Science. 1977;196(4287):326-328.
21. Silvestri S, Seeman MV, Negrete JC, et al. Increased dopamine D2 receptor binding after long-term treatment with antipsychotics in humans: a clinical PET study. Psychopharmacology (Berl). 2000;152(2):174-180.
22. Ali Z, Roque A, El-Mallakh RS. A unifying theory for the pathoetiologic mechanism of tardive dyskinesia. Med Hypotheses. 2020;140:109682.
23. Lieberman JA. Dopamine partial agonists: a new class of antipsychotic. CNS Drugs. 2004;18(4):251-267.
24. Mailman RB, Murthy V. Third generation antipsychotic drugs: partial agonism or receptor functional selectivity? Curr Pharm Des. 2010;16(5):488-501.
25. Tuplin EW, Holahan MR. Aripiprazole, a drug that displays partial agonism and functional selectivity. Curr Neuropharmacol. 2017;15(8):1192-1207.
26. Hart XM, Schmitz CN, Gründer G. Molecular imaging of dopamine partial agonists in humans: implications for clinical practice. Front Psychiatry. 2022;13:832209.
27. Snyder GL, Vanover KE, Zhu H, et al. Functional profile of a novel modulator of serotonin, dopamine, and glutamate neurotransmission. Psychopharmacology (Berl). 2015;232(3):605-621.
28. Kiss B, Horváth A, Némethy Z, et al. Cariprazine (RGH-188), a dopamine D(3) receptor-preferring, D(3)/D(2) dopamine receptor antagonist-partial agonist antipsychotic candidate: in vitro and neurochemical profile. J Pharmacol Exp Ther. 2010;333(1):328-340.
29. Abbasian C, Power P. A case of aripiprazole and tardive dyskinesia. J Psychopharmacol. 2009;23(2):214-215.
30. Peña MS, Yaltho TC, Jankovic J. Tardive dyskinesia and other movement disorders secondary to aripiprazole. Mov Disord. 2011;26(1):147-152.
31. Tadokoro S, Okamura N, Sekine Y, et al. Chronic treatment with aripiprazole prevents development of dopamine supersensitivity and potentially supersensitivity psychosis. Schizophr Bull. 2012;38(5):1012-1020.
32. Kang NR, Kim MD. Tardive dyskinesia: treatment with aripiprazole. Clin Psychopharmacol Neurosci. 2011;9(1):1-8.
33. Frankel JS, Schwartz TL. Brexpiprazole and cariprazine: distinguishing two new atypical antipsychotics from the original dopamine stabilizer aripiprazole. Ther Adv Psychopharmacol. 2017;7(1):29-41.
34. Chan CH, Chan HY, Chen YC. Switching antipsychotic treatment to aripiprazole in psychotic patients with neuroleptic-induced tardive dyskinesia: a 24-week follow-up study. Int Clin Psychopharmacol. 2018;33(3):155-162.
35. Blair HA. Lumateperone: first approval. Drugs. 2020;80(4):417-423.
36. Calabrese JR, Durgam S, Satlin A, et al. Efficacy and safety of Lumateperone for major depressive episodes associated with bipolar I or bipolar II disorder: a phase 3 randomized placebo-controlled trial. Am J Psychiatry. 2021;178(12):1098-1106.
37. Nakai S, Hirose T, Uwahodo Y, et al. Diminished catalepsy and dopamine metabolism distinguish aripiprazole from haloperidol or risperidone. Eur J Pharmacol. 2003;472(12):89-97.
38. Vanover KE, Davis RE, Zhou Y, et al. Dopamine D2 receptor occupancy of lumateperone (ITI-007): a positron emission tomography study in patients with schizophrenia. Neuropsychopharmacology. 2019;44(3):598-605.