User login
Interventional psychiatry (Part 2)
While most psychiatric treatments have traditionally consisted of pharmacotherapy with oral medications, a better understanding of the pathophysiology underlying many mental illnesses has led to the recent increased use of treatments that require specialized administration and the creation of a subspecialty called interventional psychiatry. In Part 1 of this 2-part article (“Interventional psychiatry [Part 1],"
Neuromodulation treatments
Neuromodulation—the alteration of nerve activity through targeted delivery of a stimulus, such as electrical stimulation, to specific neurologic sites—is an increasingly common approach to treating a variety of psychiatric conditions. The use of some form of neuromodulation as a medical treatment has a long history (Box1-6). Modern electric neuromodulation began in the 1930s with electroconvulsive therapy (ECT). The 1960s saw the introduction of deep brain stimulation (DBS), spinal cord stimulation, and later, vagus nerve stimulation (VNS). Target-specific noninvasive brain stimulation became possible with transcranial magnetic stimulation (TMS). These approaches are used for treating major depressive disorder (MDD), obsessive-compulsive disorder (OCD), anxiety disorders, and insomnia. Nearly all these neuromodulatory approaches require clinicians to undergo special training and patients to participate in an invasive procedure. These factors also increase cost. Nonetheless, the high rates of success of some of these approaches have led to relatively rapid and widespread acceptance.
Box
The depth and breadth of human anatomical knowledge has evolved over millennia. The time frame “thousands of years” may appear to be an overstatement, but evidence exists for successful therapeutic limb amputation as early as 31,000 years ago.1 This suggests that human knowledge of bone, muscle, and blood supply was developed much earlier than initially believed. Early Homo sapiens were altering the body—regulating or adjusting it— to serve a purpose; in this case, the purpose was survival.
In 46 AD, electrical modulation was introduced by Scribonius Largus, a physician in court of the emperor Tiberius, who used “torpedoes” (most likely electric eels) to treat headaches and pain from arthritis. Loosely, these early clinicians were modulating human function.
In the late 1800s, electrotherapeutics was a growing branch of medicine, with its own national organization—the American ElectroTherapeutic Association.2 In that era, electricity was novel, powerful, and seen as “the future.” Because such novel therapeutics were offered by both mainstream and dubious sources,3 “many of these products were marketed with the promise of curing everything from cancer to headaches.”4
Modern electric neuromodulation began in the 1930s with electroconvulsive therapy,5 followed by deep brain stimulation and spinal cord stimulation in the 1960s. Target-specific noninvasive brain stimulation became possible when Anthony Barker’s team developed the first device that permitted transcranial magnetic stimulation in 1985.6
Electroconvulsive therapy
In ECT, electric current is applied to the brain to induce a self-limiting seizure. It is the oldest and best-known interventional psychiatric treatment. ECT can also be considered one of the first treatments specifically developed to address pathophysiologic changes. In 1934, Ladislas J. Meduna, who had observed in neuropathologic studies that microglia were more numerous in patients with epilepsy compared with patients with schizophrenia, injected a patient who had been hospitalized with catatonia for 4 years with camphor, a proconvulsant.7 After 5 seizures, the patient began to recover. The therapeutic use of electricity was subsequently developed and optimized in animal models, and first used on human patients in Italy in 1939 and in the United States in 1940.8 The link between psychiatric illness and microglia, which was initially observed nearly a century ago, is making a comeback, as excessive microglial activation has been demonstrated in animal and human models of depression.9
Administering ECT requires specialized equipment, anesthesia, physician training, and nursing observation. ECT also has a negative public image.10 All of these factors conspire to reduce the availability of ECT. Despite this, approximately 100,000 patients in the United States and >1 million worldwide receive ECT each year.10 Patients generally require 6 to 12 ECT treatments11 to achieve sufficient response and may require additional maintenance treatments.12
Although ECT is used to treat psychiatric illnesses ranging from mood disorders to psychotic disorders and catatonia, it is mainly employed to treat people with severe treatment-resistant depression (TRD).13 ECT is associated with significant improvements in depressive symptoms and improvements in quality of life.14 It is superior to other treatments for TRD, such as ketamine,15 though a recent study did not show IV ketamine inferiority.16 ECT is also used to treat other neuropsychiatric disorders, such as Parkinson disease.17
Clinicians have explored alternate methods of inducing therapeutic seizures. Magnetic seizure therapy (MST) utilizes a modified magnetic stimulation device to deliver a higher energy in such a way to induce a generalized seizure under anesthesia.18 While patients receiving MST generally experience fewer adverse effects than with ECT, the procedure may be equal to19 or less effective than ECT.20
Transcranial magnetic stimulation
In neuroimaging research, certain aberrant brain circuits have been implicated in the pathogenesis of depression.21 Specifically, anatomical and functional imaging suggests connections in the prefrontal cortex are involved in the depression process. In TMS, a series of magnetic pulses are administered via the scalp to stimulate neurons in areas of the brain associated with MDD. Early case reports on using TMS to stimulate the prefrontal cortex found significant improvement of symptoms in patients with depression.22 These promising results spurred great interest in the procedure. Over time, the dose and duration of stimulation has increased, along with FDA-approved indications. TMS was first FDA-approved for TRD.23 Although the primary endpoint of the initial clinical trial did not meet criteria for FDA approval, TMS did result in improvement across multiple other measures of depression.23 After the FDA approved the first TMS device, numerous companies began to produce TMS technology. Most of these companies manufacture devices with the figure-of-eight coil, with 1 company producing the Hesed-coil helmet.24
Continue to: An unintended outcome...
An unintended outcome of the increased interest in TMS has been an increased understanding of brain regions involved in psychiatric illness. TMS was able to bring knowledge of mental health from synapses to circuits.25 Work in this area has further stratified the circuits involved in the manifestation of symptom clusters in depression.26 The exact taxonomy of these brain circuits has not been fully realized, but the default mode, salience, attention, cognitive control, and other circuits have been shown to be involved in specific symptom presentations.26,27 These circuits can be hyperactive, hypoactive, hyperconnected, or hypoconnected, with the aberrancies compared to normal controls resulting in symptoms of psychiatric illness.28
This enhanced understanding of brain function has led to further research and development of protocols and subsequent FDA approval of TMS for OCD, anxious depression, and smoking cessation.29 In addition, it has allowed for a proliferation of off-label uses for TMS, including (but not limited to) tinnitus, pain, migraines, and various substance use disorders.30 TMS treatment for these conditions involves stimulation of specific anatomical brain regions that are thought to play a role in the pathology of the target disorder. For example, subthreshold stimulation of the motor cortex has shown some utility in managing symptoms of pain disorders and movement disorders,31,32 the ventromedial prefrontal cortex has been implicated in disorders in the OCD spectrum,33 stimulation of the frontal poles may help treat substance use disorders,34 and the auditory cortex has been a target for treating tinnitus and auditory hallucinations.35
The location of stimulation for treating depression has evolved. The Talairach-Tournoux coordinate system has been used to determine the location of the dorsolateral prefrontal cortex (DLPFC) in relation to the motor cortex. This was measured to be 5 cm from the motor hotspot and subsequently became “the 5.5 cm rule,” taking skull convexity into account. The treatment paradigm for the Hesed coil also uses a measurement from the motor hotspot. Another commonly used methodology for coil placement involves using the 10 to 20 EEG coordinate system to individualize scalp landmarks. In this method, the F3 location corresponds most accurately to the DLPFC target. More recently, using fMRI-guided navigation for coil placement has been shown to lead to a significant reduction in depressive symptoms.36
For depression, the initial recommended course of treatment is 6 weeks, but most improvement is seen in the first 2 to 3 weeks.14 Therefore, many clinicians administer an initial course of 3 weeks unless the response is inadequate, in which case a 6-week course is administered. Many patients require ongoing maintenance treatment, which can be weekly or monthly based on response.37
Research to determine the optimal TMS dose for treating neuropsychiatric symptoms is ongoing. Location, intensity of stimulation, and pulse are the components of stimulation. The pulse can be subdivided into frequency, pattern (single pulse, standard, burst), train (numbers of pulse groups), interval between trains, and total number of pulses per session. The Clinical TMS Society has published TMS protocols.38 The standard intensity of stimulation is 120% of the motor threshold (MT), which is defined as the amount of stimulation over the motor cortex required to produce movement in the extensor hallucis longus. Although treatment for depression traditionally utilizes rapid TMS (3,000 pulses delivered per session at a frequency of 10 Hz in 4-second trains), in controlled studies, accelerated protocols such as intermittent theta burst stimulation (iTBS; standard stimulation parameters: triplet 50 Hz bursts at 5 Hz, with an interval of 8 seconds for 600 pulses per session) have shown noninferiority.36,39
Recent research has explored fMRI-guided iTBS in an even more accelerated format. The Stanford Neuromodulation Therapy trial involved 1,800 pulses per session for 10 sessions a day for 5 days at 90% MT.36 This treatment paradigm was shown to be more effective than standard protocols and was FDA-approved in 2022. Although this specific iTBS protocol exhibited encouraging results, the need for fMRI for adequate delivery might limit its use.
Continue to: Transcranial direct current stimulation
Transcranial direct current stimulation
Therapeutic noninvasive brain stimulation technology is plausible due to the relative lack of adverse effects and ease of administration. In transcranial direct current stimulation (tDCS), a low-intensity, constant electric current is delivered to stimulate the brain via electrodes attached to the scalp. tDCS modulates spontaneous neuronal network activity40,41 and induces polarization of resting membrane potential at the neuronal level,42 though the exact mechanism is yet to be proven. N-methyl-
tDCS has been suggested as a treatment for various psychiatric and medical conditions. However, the small sample sizes and experimental design of published studies have limited tDCS from being clinically recommended.30 No recommendation of Level A (definite efficacy) for its use was found for any indication. Level B recommendation (probable efficacy) was proposed for fibromyalgia, MDD episode without drug resistance, and addiction/craving. Level C recommendation (possible efficacy) is proposed for chronic lower limb neuropathic pain secondary to spinal cord lesion. tDCS was found to be probably ineffective as a treatment for tinnitus and drug-resistant MDD.30 Some research has suggested that tDCS targeting the DLPFC is associated with cognitive improvements in healthy individuals as well as those with schizophrenia.44 tDCS treatment remains experimental and investigational.
Deep brain stimulation
DBS is a neurosurgical procedure that uses electrical current to directly modulate specific areas of the CNS. In terms of accurate, site-specific anatomical targeting, there can be little doubt of the superiority of DBS. DBS involves the placement of leads into the brain parenchyma. Image guidance techniques are used for accurate placement. DBS is a mainstay for the symptomatic treatment of treatment-resistant movement disorders such as Parkinson disease, essential tremor, and some dystonic disorders. It also has been studied as a potential treatment for chronic pain, cluster headache, Huntington disease, and Tourette syndrome.
For treating depression, researched targets include the subgenual cingulate gyrus (SCG), ventral striatum, nucleus accumbens, inferior thalamic peduncle, medial forebrain bundle, and the red nucleus.45 In systematic reviews, improvement of depression is greatest when DBS targets the subgenual cingulate cortex and the medial forebrain bundle.46
The major limitation of DBS for treating depression is the invasive nature of the procedure. Deep TMS can achieve noninvasive stimulation of the SCG and may be associated with fewer risks, fewer adverse events, and less collateral damage. However, given the evolving concept of abnormal neurologic circuits in depression, as our understanding of circuitry in pathological psychiatric processes increases, DBS may be an attractive option for personalized targeting of symptoms in some patients.
DBS may also be beneficial for severe, treatment-resistant OCD. Electrode implantation in the region of the internal capsule/ventral striatum, including the nucleus accumbens, is used47; there is little difference in placement as a treatment for OCD vs for movement disorders.48
Continue to: A critical review of 23 trials...
A critical review of 23 trials and case reports of DBS as a treatment for OCD demonstrated a 47.7% mean reduction in score on the Yale-Brown Obsessive-Compulsive Scale (Y-BOCS) and a mean response percentage (minimum 35% Y-BOCS reduction) of 58.2%.49 Most patients regained a normal quality of life after DBS.49 A more rigorous review of 15 meta-analyses of DBS found that conclusions about its efficacy or comparative effectiveness cannot be drawn.50 Because of the nature of neurosurgery, DBS has many potential complications, including cognitive changes, headache, infection, seizures, stroke, and hardware failure.
Vagus nerve stimulation
VNS, in which an implanted device stimulates the left vagus nerve with electrical impulses, was FDA-approved for treating chronic TRD in 2005.51 It had been approved for treatment-resistant epilepsy in 1997. In patients with epilepsy, VNS was shown to improve mood independent of seizure control.52 VNS requires a battery-powered pacemaker device to be implanted under the skin over the anterior chest wall, and a wire tunneled to an electrode is wrapped around the left vagus nerve in the neck.53 The pacemaker is then programmed, monitored, and reprogrammed to optimize response.
VNS is believed to stimulate deep brain nuclei that may play a role in depression.54 The onset of improvement is slow (it may take many months) but in carefully selected patients VNS can provide significant control of TRD. In addition to rare surgery-related complications such as a trauma to the vagal nerve and surrounding tissues (vocal cord paralysis, implant site infection, left facial nerve paralysis and Horner syndrome), VNS may cause hoarseness, dyspnea, and cough related to the intensity of the current output.51 Hypomania and mania were also reported; no suicidal behavior has been associated with VNS.51
Noninvasive vagus nerve stimulationIn noninvasive vagus nerve stimulation (nVNS) or transcutaneous VNS, an external handheld device is applied to the neck overlying the course of the vagus nerve to deliver a sinusoidal alternating current.55 nVNS is currently FDA-approved for treating migraine headaches.55,56 It has demonstrated actions on neurophysiology57 and inflammation in patients with MDD.58 Exploratory research has found a small beneficial effect in patients with depression.59,60 A lack of adequate reproducibility prevents this treatment from being more widely recommended, although attempts to standardize the field are evolving.61
Cranial electrical stimulation
Cranial electrical stimulation (CES) is an older form of electric stimulation developed in the 1970s. In CES, mild electrical pulses are delivered to the ear lobes bilaterally in an episodic fashion (usually 20 to 60 minutes once or twice daily). While CES can be considered a form of neuromodulation, it is not strictly interventional. Patients self-administer CES. The procedure has minimal effects on improving sleep, anxiety, and mood.62-66 Potential adverse effects include a tingling sensation in the ear lobes, lightheadedness, and fogginess. A review and meta-analysis of CES for treating addiction by Kirsch67 showed a wide range of symptoms responding positively to CES treatment, although this study was not peer-reviewed. Because of the low quality of nearly all research that evaluated CES, this form of electric stimulation cannot be viewed as an accepted treatment for any of its listed indications.
Continue to: Other neuromodulation techniques
Other
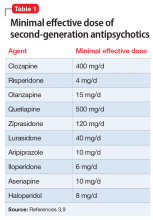
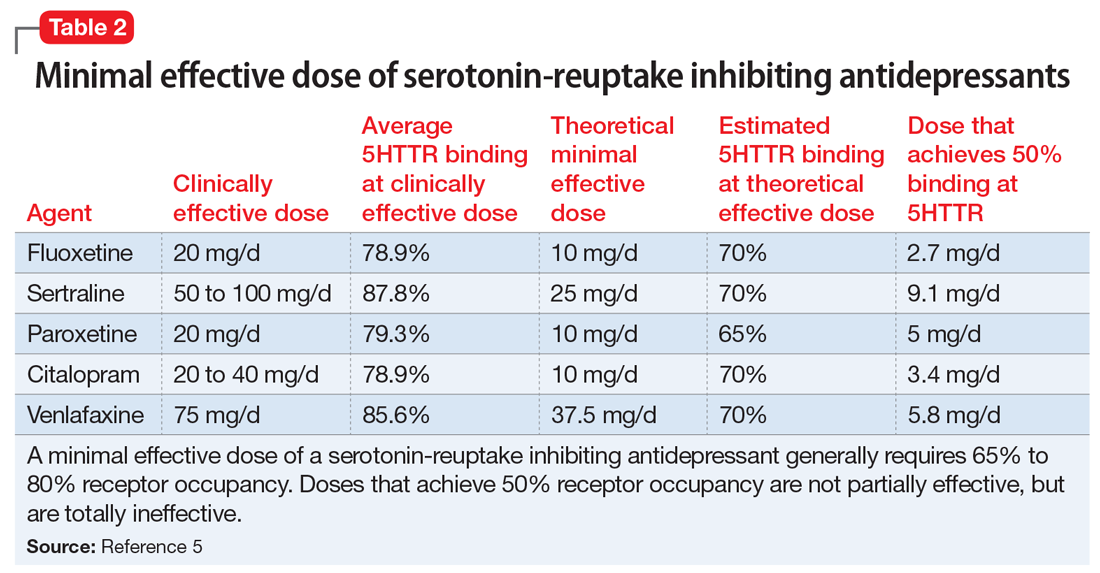
In addition to the forms of neuromodulation we have already described, there are many other techniques. Several are promising but not yet ready for clinical use. Table 1 and Table 2 summarize the neuromodulation techniques described in this article as well as several that are under development.

Acupuncture
Acupuncture is a Chinese form of medical treatment that began >3,000 years ago; there are written descriptions of it from >2,000 years ago.68 It is based on the belief that there are channels within the body through which the Qi (vital energy or life force) flow, and that inserting fine needles into these channels via the skin can rebalance Qi.68 Modern mechanistic hypotheses invoke involvement of inflammatory or pain pathways.69 Acupuncture frequently uses electric stimulation (electro-acupuncture) to increase the potency of the procedure. Alternatively, in a related procedure (acupressure), pressure can replace the needle. Accreditation in acupuncture generally requires a master’s degree in traditional Chinese medicine but does not require any specific medical training. Acupuncture training courses for physicians are widely available.
All forms of acupuncture are experimental for a wide variety of mental and medical conditions. A meta-analysis found that most research of the utility of acupuncture for depression suffered from various forms of potential bias and was considered low quality.70 Nonetheless, active acupuncture was shown to be minimally superior to placebo acupuncture.70 A meta-analysis of acupuncture for preoperative anxiety71,72 and poststroke insomnia73 reported a similar low study quality. A study of 72 patients with primary insomnia revealed that acupuncture was more effective than sham acupuncture for most sleep measures.74
Psychiatry is increasingly integrating medical tools in addition to psychological tools. Pharmacology remains a cornerstone of biological psychiatry and this will not soon change. However, nonpharmacologic psychiatric treatments such as therapeutic neuromodulation are rapidly emerging. These and novel methods of medication administration may present a challenge to psychiatrists who do not have access to medical personnel or may have forgotten general medical skills.
Our 2-part article has highlighted several interventional psychiatry tools—old and new—that may interest clinicians and benefit patients. As a rule, such treatments are reserved for the most treatment-resistant, challenging psychiatric patients, those with hard-to-treat chronic conditions, and patients who are not helped by more commonly used treatments. An additional complication is that such treatments are frequently not appropriately researched, vetted, or FDA-approved, and therefore are higher risk. Appropriate clinical judgment is always necessary, and potential benefits must be thoroughly weighed against possible adverse effects.
Bottom Line
Several forms of neuromodulation, including electroconvulsive therapy, transcranial magnetic stimulation, transcranial direct current stimulation, deep brain stimulation, and vagus nerve stimulation, may be beneficial for patients with certain treatment-resistant psychiatric disorders, including major depressive disorder and obsessive-compulsive disorder.
Related Resources
- Janicak PG. What’s new in transcranial magnetic stimulation. Current Psychiatry. 2019;18(3):10-16.
- Sharma MS, Ang-Rabanes M, Selek S, et al. Neuromodulatory options for treatment-resistant depression. Current Psychiatry. 2018;17(3):26-28,33-37.
1. Maloney TR, Dilkes-Hall IE, Vlok M, et al. Surgical amputation of a limb 31,000 years ago in Borneo. Nature. 2022;609(7927):547-551. doi:10.1038/s41586-022-05160-8
2. The American Electro-Therapeutic Association. JAMA. 1893;21(14):500. doi:10.1001/jama.1893.02420660030004
3. The American Electro-Therapeutic Association. JAMA. 1894;23(15):590-591. doi:10.1001/jama.1894.02421200024006
4. Wexler A. The medical battery in the United States (1870-1920): electrotherapy at home and in the clinic. J Hist Med Allied Sci. 2017;72(2):166-192. doi:10.1093/jhmas/jrx001
5. Gazdag G, Ungvari GS. Electroconvulsive therapy: 80 years old and still going strong. World J Psychiatry. 2019;9(1):1-6. doi:10.5498/wjp.v9.i1.1
6. Barker AT, Jalinous R, Freeston IL. Non-invasive magnetic stimulation of human motor cortex. Lancet. 1985;1(8437):1106-1107. doi:10.1016/s0140-6736(85)92413-4
7. Fink M. Historical article: autobiography of L. J. Meduna. Convuls Ther. 1985;1(1):43-57.
8. Suleman R. A brief history of electroconvulsive therapy. Am J Psychiatry. 2020;16(1):6. doi:10.1176/appi.ajp-rj.2020.160103
9. Ménard C, Hodes GE, Russo SJ. Pathogenesis of depression: insights from human and rodent studies. Neuroscience. 2016;321:138-162. doi:10.1016/j.neuroscience.2015.05.053
10. Payne NA, Prudic J. Electroconvulsive therapy: part II: a biopsychosocial perspective. J Psychiatr Pract. 2009;15(5):369-390. doi:10.1097/01.pra.0000361278.73092.85
11. Tirmizi O, Raza A, Trevino K, et al. Electroconvulsive therapy: how modern techniques improve patient outcomes. Current Psychiatry. 2012;11(10):24-46.
12. Kolar D. Current status of electroconvulsive therapy for mood disorders: a clinical review. Evid Based Ment Health. 2017;20(1):12-14. doi:10.1136/eb-2016-102498
13. Andrade C. Active placebo, the parachute meta-analysis, the Nobel Prize, and the efficacy of electroconvulsive therapy. J Clin Psychiatry. 2021;82(2):21f13992. doi:10.4088/JCP.21f13992
14. Giacobbe P, Rakita U, Penner-Goeke K, et al. Improvements in health-related quality of life with electroconvulsive therapy: a meta-analysis. J ECT. 2018;34(2):87-94. doi:10.1097/YCT.0000000000000486
15. Rhee TG, Shim SR, Forester BP, et al. Efficacy and safety of ketamine vs electroconvulsive therapy among patients with major depressive episode: a systematic review and meta-analysis. JAMA Psychiatry. 2022;79(12):1162-1172. doi:10.1001/jamapsychiatry.2022.3352
16. Anand A, Mathew SJ, Sanacora G, et al. Ketamine versus ECT for nonpsychotic treatment-resistant major depression. N Engl J Med. 2023. doi: 10.1056/NEJMoa2302399
17. Takamiya A, Seki M, Kudo S, et al. Electroconvulsive therapy for Parkinson’s disease: a systematic review and meta-analysis. Mov Disord. 2021;36(1):50-58. doi:10.1002/mds.28335
18. Singh R, Sharma R, Prakash J, et al. Magnetic seizure therapy. Ind Psychiatry J. 2021;30(Suppl 1):S320-S321. doi:10.4103/0972-6748.328841
19. Chen M, Yang X, Liu C, et al. Comparative efficacy and cognitive function of magnetic seizure therapy vs. electroconvulsive therapy for major depressive disorder: a systematic review and meta-analysis. Transl Psychiatry. 2021;11(1):437. doi:10.1038/s41398-021-01560-y
20. Cretaz E, Brunoni AR, Lafer B. Magnetic seizure therapy for unipolar and bipolar depression: a systematic review. Neural Plast. 2015;2015:521398. doi:10.1155/2015/521398
21. George MS, Ketter TA, Post RM. Prefrontal cortex dysfunction in clinical depression. In: Nemeroff CB, Weiss JM, Schatzberg AF, et al, eds. Depression. 2nd ed. Wiley Online Library; 1994:59-72. https://doi.org/10.1002/depr.3050020202
22. George MS, Wassermann EM, Williams WA, et al. Daily repetitive transcranial magnetic stimulation (rTMS) improves mood in depression. Neuroreport. 1995;6(14):1853-1856.
23. O’Reardon JP, Solvason HB, Janicak PG, et al. Efficacy and safety of transcranial magnetic stimulation in the acute treatment of major depression: a multisite randomized controlled trial. Biol Psychiatry. 2007;62(11):1208-1216.
24. Clinical TMS Society. TMS devices. Accessed January 2, 2023. https://www.clinicaltmssociety.org/devices
25. Goldstein-Piekarski AN, Ball TM, Samara Z, et al. Mapping neural circuit biotypes to symptoms and behavioral dimensions of depression and anxiety. Biol Psychiatry. 2022;91(6):561-571. doi:10.1016/j.biopsych.2021.06.024
26. Siddiqi SH, Taylor SF, Cooke D, et al. Distinct symptom-specific treatment targets for circuit-based neuromodulation. Am J Psychiatry. 2020;177(5):435-446. doi:10.1176/appi.ajp.2019.19090915
27. Williams LM. Defining biotypes for depression and anxiety based on large-scale circuit dysfunction: a theoretical review of the evidence and future directions for clinical translation. Depress Anxiety. 2017;34(1):9-24. doi:10.1002/da.22556
28. Drysdale AT, Grosenick L, Downar J, et al. Resting-state connectivity biomarkers define neurophysiological subtypes of depression. Nat Med. 2017;23(1):28-38. doi:10.1038/nm.4246
29. Cohen SL, Bikson M, Badran BW, et al. A visual and narrative timeline of US FDA milestones for transcranial magnetic stimulation (TMS) devices. Brain Stimul. 2022;15(1):73-75. doi:10.1016/j.brs.2021.11.010
30. Lefaucheur JP, Antal A, Ayache SS, et al. Evidence-based guidelines on the therapeutic use of transcranial direct current stimulation (tDCS). Clin Neurophysiol. 2017;128(1):56-92. doi:10.1016/j.clinph.2016.10.087
31. Li R, He Y, Qin W, et al. Effects of repetitive transcranial magnetic stimulation on motor symptoms in Parkinson’s disease: a meta-analysis. Neurorehabil Neural Repair. 2022;36(7):395-404. doi:10.1177/15459683221095034
32. Leung A, Shirvalkar P, Chen R, et al. Transcranial magnetic stimulation for pain, headache, and comorbid depression: INS-NANS expert consensus panel review and recommendation. Neuromodulation. 2020;23(3):267-290. doi:10.1111/ner.13094
33. Carmi L, Tendler A, Bystritsky A, et al. Efficacy and safety of deep transcranial magnetic stimulation for obsessive-compulsive disorder: a prospective multicenter randomized double-blind placebo-controlled trial. Am J Psychiatry. 2019;176(11):931-938. doi:10.1176/appi.ajp.2019.18101180
34. Harel M, Perini I, Kämpe R, et al. Repetitive transcranial magnetic stimulation in alcohol dependence: a randomized, double-blind, sham-controlled proof-of-concept trial targeting the medial prefrontal and anterior cingulate cortices. Biol Psychiatry. 2022;91(12):1061-1069. doi:10.1016/j.biopsych.2021.11.020
35. Folmer RL, Theodoroff SM, Casiana L, et al. Repetitive transcranial magnetic stimulation treatment for chronic tinnitus: a randomized clinical trial. JAMA Otolaryngol Head Neck Surg. 2015;141(8):716-722. doi:10.1001/jamaoto.2015.1219
36. Cole EJ, Phillips AL, Bentzley BS, et al. Stanford Neuromodulation Therapy (SNT): a double-blind randomized controlled trial. Am J Psychiatry. 2022;179(2):132-141. doi:10.1176/appi.ajp.2021.20101429
37. Wilson S, Croarkin PE, Aaronson ST, et al. Systematic review of preservation TMS that includes continuation, maintenance, relapse-prevention, and rescue TMS. J Affect Disord. 2022;296:79-88. doi:10.1016/j.jad.2021.09.040
38. Perera T, George MS, Grammer G, et al. The Clinical TMS Society consensus review and treatment recommendations for TMS therapy for major depressive disorder. Brain Stimul. 2016;9(3):336-346. doi:10.1016/j.brs.2016.03.010
39. Blumberger DM, Vila-Rodriguez F, Thorpe KE, et al. Effectiveness of theta burst versus high-frequency repetitive transcranial magnetic stimulation in patients with depression (THREE-D): a randomized non-inferiority trial. Lancet. 2018;391(10131):1683-1692. doi:10.1016/S0140-6736(18)30295-2
40. Nitsche MA, Cohen LG, Wassermann EM, et al. Transcranial direct current stimulation: state of the art 2008. Brain Stimul. 2008;1(3):206-223. doi:10.1016/j.brs.2008.06.004
41. Priori A, Hallett M, Rothwell JC. Repetitive transcranial magnetic stimulation or transcranial direct current stimulation? Brain Stimul. 2009;2(4):241-245.
42. Priori A, Berardelli A, Rona S, et al. Polarization of the human motor cortex through the scalp. Neuroreport. 1998;9(10):2257-2260. doi:10.1097/00001756-199807130-00020
43. Nitsche MA, Liebetanz D, Antal A, et al. Modulation of cortical excitability by weak direct current stimulation-- technical, safety and functional aspects. Suppl Clin Neurophysiol. 2003;56:255-276. doi:10.1016/s1567-424x(09)70230-2
44. Agarwal SM, Venkataram Shivakumar V, et al. Transcranial direct current stimulation in schizophrenia. Clin Psychopharmacol Neurosci. 2013;11(3):118-125.
45. Drobisz D, Damborská A. Deep brain stimulation targets for treating depression. Behav Brain Res. 2019;359:266-273. doi:10.1016/j.bbr.2018.11.004
46. Kisely S, Li A, Warren N, et al. A systematic review and meta-analysis of deep brain stimulation for depression. Depress Anxiety. 2018;35(5):468-480. doi:10.1002/da.22746
47. Blomstedt P, Sjöberg RL, Hansson M, et al. Deep brain stimulation in the treatment of obsessive-compulsive disorder. World Neurosurg. 2013;80(6):e245-e253. doi:10.1016/j.wneu.2012.10.006
48. Denys D, Mantione M, Figee M, et al. Deep brain stimulation of the nucleus accumbens for treatment-refractory obsessive-compulsive disorder. Arch Gen Psychiatry. 2010;67(10):1061-1068. doi:10.1001/archgenpsychiatry.2010.122
49. van Westen M, Rietveld E, Figee M, et al. Clinical outcome and mechanisms of deep brain stimulation for obsessive-compulsive disorder. Curr Behav Neurosci Rep. 2015;2(2):41-48. doi:10.1007/s40473-015-0036-3
50. Papageorgiou PN, Deschner J, Papageorgiou SN. Effectiveness and adverse effects of deep brain stimulation: umbrella review of meta-analyses. J Neurol Surg A Cent Eur Neurosurg. 2017;78(2):180-190. doi:10.1055/s-0036-1592158
51. O’Reardon JP, Cristancho P, Peshek AD. Vagus nerve stimulation (VNS) and treatment of depression: to the brainstem and beyond. Psychiatry (Edgmont). 2006;3(5):54-63.
52. Harden CL, Pulver MC, Ravdin LD, et al. A pilot study of mood in epilepsy patients treated with vagus nerve stimulation. Epilepsy Behav. 2000;1(2):93-99. doi:10.1006/ebeh.2000.0046
53. Giordano F, Zicca A, Barba C, et al. Vagus nerve stimulation: surgical technique of implantation and revision and related morbidity. Epilepsia. 2017;58(S1):85-90. doi:10.1111/epi.13687
54. George MS, Nahas Z, Bohning DE, et al. Mechanisms of action of vagus nerve stimulation (VNS). Clin Neurosci Res. 2004;4(1-2):71-79.
55. Nesbitt AD, Marin JCA, Tompkins E, et al. Initial use of a novel noninvasive vagus nerve stimulator for cluster headache treatment. Neurology. 2015;84:1249-1253. doi:10.1212/WNL.0000000000001394
56. Goadsby PJ, Grosberg BM, Mauskop A, et al. Effect of noninvasive vagus nerve stimulation on acute migraine: an open-label pilot study. Cephalalgia. 2014;34:986-993. doi:10.1177/0333102414524494
57. Fang J, Rong P, Hong Y, et al. Transcutaneous vagus nerve stimulation modulates default mode network in major depressive disorder. Biol Psychiatry. 2016;79(4):266-273. doi:10.1016/j.biopsych.2015.03.025
58. Liu CH, Yang MH, Zhang GZ, et al. Neural networks and the anti-inflammatory effect of transcutaneous auricular vagus nerve stimulation in depression. J Neuroinflammation. 2020;17(1):54. doi:10.1186/s12974-020-01732-5
59. Hein E, Nowak M, Kiess O, et al. Auricular transcutaneous electrical nerve stimulation in depressed patients: a randomized controlled pilot study. J Neural Transm (Vienna). 2013;120(5):821-827. doi:10.1007/s00702-012-0908-6
60. Rong P, Liu J, Wang L, et al. Effect of transcutaneous auricular vagus nerve stimulation on major depressive disorder: a nonrandomized controlled pilot study. J Affect Disord. 2016;195:172-179. doi:10.1016/j.jad.2016.02.031
61. Farmer AD, Strzelczyk A, Finisguerra A, et al. International consensus based review and recommendations for minimum reporting standards in research on transcutaneous vagus nerve stimulation (Version 2020). Front Hum Neurosci. 2021;14:568051. doi:10.3389/fnhum.2020.568051
62. Amr M, El-Wasify M, Elmaadawi AZ, et al. Cranial electrotherapy stimulation for the treatment of chronically symptomatic bipolar patients. J ECT. 2013;29(2):e31-e32. doi:10.1097/YCT.0b013e31828a344d
63. Kirsch DL, Nichols F. Cranial electrotherapy stimulation for treatment of anxiety, depression, and insomnia. Psychiatr Clin North Am. 2013;36(1):169-176. doi:10.1016/j.psc.2013.01.006
64. Lande RG, Gragnani C. Efficacy of cranial electric stimulation for the treatment of insomnia: a randomized pilot study. Complement Ther Med. 2013;21(1):8-13. doi:10.1016/j.ctim.2012.11.007
65. Ou Y, Li, C. Sertraline combined alpha-stim clinical observations on the treatment of 30 cases of generalized anxiety disorder. Chinese Journal of Ethnomedicine and Ethnopharmacy. 2015;24(17):73-75.
66. Price L, Briley J, Haltiwanger S, et al. A meta-analysis of cranial electrotherapy stimulation in the treatment of depression. J Psychiatr Res. 2021;135:119-134. doi:10.1016/j.jpsychires.2020.12.043
67. Kirsch D, Gilula M. CES in the treatment of addictions: a review and meta-analysis. Pract Pain Manag. 2007;7(9).
68. Hao JJ, Mittelman M. Acupuncture: past, present, and future. Glob Adv Health Med. 2014;3(4):6-8. doi:10.7453/gahmj.2014.042
69. Napadow V, Ahn A, Longhurst J, et al. The status and future of acupuncture mechanism research. J Altern Complement Med. 2008;14(7):861-869. doi:10.1089/acm.2008.SAR-3
70. Smith CA, Armour M, Lee MS, et al. Acupuncture for depression. Cochrane Database Syst Rev. 2018;3(3):CD004046. doi:10.1002/14651858.CD004046.pub4
71. Tong QY, Liu R, Zhang K, et al. Can acupuncture therapy reduce preoperative anxiety? A systematic review and meta-analysis. J Integr Med. 2021;19(1):20-28. doi:10.1016/j.joim.2020.10.007
72. Usichenko TI, Hua K, Cummings M, et al. Auricular stimulation for preoperative anxiety – a systematic review and meta-analysis of randomized controlled clinical trials. J Clin Anesth. 2022;76:110581. doi:10.1016/j.jclinane.2021.110581
73. Zhou L, Hu X, Yu Z, et al. Efficacy and safety of acupuncture in the treatment of poststroke insomnia: a systematic review and meta-analysis of twenty-six randomized controlled trials. Evid Based Complement Alternat Med. 2022;2022:5188311. doi:10.1155/2022/5188311
74. Yin X, Gou M, Xu J, et al. Efficacy and safety of acupuncture treatment on primary insomnia: a randomized controlled trial. Sleep Med. 2017;37:193-200. doi:10.1016/j.sleep.2017.02.012
While most psychiatric treatments have traditionally consisted of pharmacotherapy with oral medications, a better understanding of the pathophysiology underlying many mental illnesses has led to the recent increased use of treatments that require specialized administration and the creation of a subspecialty called interventional psychiatry. In Part 1 of this 2-part article (“Interventional psychiatry [Part 1],"
Neuromodulation treatments
Neuromodulation—the alteration of nerve activity through targeted delivery of a stimulus, such as electrical stimulation, to specific neurologic sites—is an increasingly common approach to treating a variety of psychiatric conditions. The use of some form of neuromodulation as a medical treatment has a long history (Box1-6). Modern electric neuromodulation began in the 1930s with electroconvulsive therapy (ECT). The 1960s saw the introduction of deep brain stimulation (DBS), spinal cord stimulation, and later, vagus nerve stimulation (VNS). Target-specific noninvasive brain stimulation became possible with transcranial magnetic stimulation (TMS). These approaches are used for treating major depressive disorder (MDD), obsessive-compulsive disorder (OCD), anxiety disorders, and insomnia. Nearly all these neuromodulatory approaches require clinicians to undergo special training and patients to participate in an invasive procedure. These factors also increase cost. Nonetheless, the high rates of success of some of these approaches have led to relatively rapid and widespread acceptance.
Box
The depth and breadth of human anatomical knowledge has evolved over millennia. The time frame “thousands of years” may appear to be an overstatement, but evidence exists for successful therapeutic limb amputation as early as 31,000 years ago.1 This suggests that human knowledge of bone, muscle, and blood supply was developed much earlier than initially believed. Early Homo sapiens were altering the body—regulating or adjusting it— to serve a purpose; in this case, the purpose was survival.
In 46 AD, electrical modulation was introduced by Scribonius Largus, a physician in court of the emperor Tiberius, who used “torpedoes” (most likely electric eels) to treat headaches and pain from arthritis. Loosely, these early clinicians were modulating human function.
In the late 1800s, electrotherapeutics was a growing branch of medicine, with its own national organization—the American ElectroTherapeutic Association.2 In that era, electricity was novel, powerful, and seen as “the future.” Because such novel therapeutics were offered by both mainstream and dubious sources,3 “many of these products were marketed with the promise of curing everything from cancer to headaches.”4
Modern electric neuromodulation began in the 1930s with electroconvulsive therapy,5 followed by deep brain stimulation and spinal cord stimulation in the 1960s. Target-specific noninvasive brain stimulation became possible when Anthony Barker’s team developed the first device that permitted transcranial magnetic stimulation in 1985.6
Electroconvulsive therapy
In ECT, electric current is applied to the brain to induce a self-limiting seizure. It is the oldest and best-known interventional psychiatric treatment. ECT can also be considered one of the first treatments specifically developed to address pathophysiologic changes. In 1934, Ladislas J. Meduna, who had observed in neuropathologic studies that microglia were more numerous in patients with epilepsy compared with patients with schizophrenia, injected a patient who had been hospitalized with catatonia for 4 years with camphor, a proconvulsant.7 After 5 seizures, the patient began to recover. The therapeutic use of electricity was subsequently developed and optimized in animal models, and first used on human patients in Italy in 1939 and in the United States in 1940.8 The link between psychiatric illness and microglia, which was initially observed nearly a century ago, is making a comeback, as excessive microglial activation has been demonstrated in animal and human models of depression.9
Administering ECT requires specialized equipment, anesthesia, physician training, and nursing observation. ECT also has a negative public image.10 All of these factors conspire to reduce the availability of ECT. Despite this, approximately 100,000 patients in the United States and >1 million worldwide receive ECT each year.10 Patients generally require 6 to 12 ECT treatments11 to achieve sufficient response and may require additional maintenance treatments.12
Although ECT is used to treat psychiatric illnesses ranging from mood disorders to psychotic disorders and catatonia, it is mainly employed to treat people with severe treatment-resistant depression (TRD).13 ECT is associated with significant improvements in depressive symptoms and improvements in quality of life.14 It is superior to other treatments for TRD, such as ketamine,15 though a recent study did not show IV ketamine inferiority.16 ECT is also used to treat other neuropsychiatric disorders, such as Parkinson disease.17
Clinicians have explored alternate methods of inducing therapeutic seizures. Magnetic seizure therapy (MST) utilizes a modified magnetic stimulation device to deliver a higher energy in such a way to induce a generalized seizure under anesthesia.18 While patients receiving MST generally experience fewer adverse effects than with ECT, the procedure may be equal to19 or less effective than ECT.20
Transcranial magnetic stimulation
In neuroimaging research, certain aberrant brain circuits have been implicated in the pathogenesis of depression.21 Specifically, anatomical and functional imaging suggests connections in the prefrontal cortex are involved in the depression process. In TMS, a series of magnetic pulses are administered via the scalp to stimulate neurons in areas of the brain associated with MDD. Early case reports on using TMS to stimulate the prefrontal cortex found significant improvement of symptoms in patients with depression.22 These promising results spurred great interest in the procedure. Over time, the dose and duration of stimulation has increased, along with FDA-approved indications. TMS was first FDA-approved for TRD.23 Although the primary endpoint of the initial clinical trial did not meet criteria for FDA approval, TMS did result in improvement across multiple other measures of depression.23 After the FDA approved the first TMS device, numerous companies began to produce TMS technology. Most of these companies manufacture devices with the figure-of-eight coil, with 1 company producing the Hesed-coil helmet.24
Continue to: An unintended outcome...
An unintended outcome of the increased interest in TMS has been an increased understanding of brain regions involved in psychiatric illness. TMS was able to bring knowledge of mental health from synapses to circuits.25 Work in this area has further stratified the circuits involved in the manifestation of symptom clusters in depression.26 The exact taxonomy of these brain circuits has not been fully realized, but the default mode, salience, attention, cognitive control, and other circuits have been shown to be involved in specific symptom presentations.26,27 These circuits can be hyperactive, hypoactive, hyperconnected, or hypoconnected, with the aberrancies compared to normal controls resulting in symptoms of psychiatric illness.28
This enhanced understanding of brain function has led to further research and development of protocols and subsequent FDA approval of TMS for OCD, anxious depression, and smoking cessation.29 In addition, it has allowed for a proliferation of off-label uses for TMS, including (but not limited to) tinnitus, pain, migraines, and various substance use disorders.30 TMS treatment for these conditions involves stimulation of specific anatomical brain regions that are thought to play a role in the pathology of the target disorder. For example, subthreshold stimulation of the motor cortex has shown some utility in managing symptoms of pain disorders and movement disorders,31,32 the ventromedial prefrontal cortex has been implicated in disorders in the OCD spectrum,33 stimulation of the frontal poles may help treat substance use disorders,34 and the auditory cortex has been a target for treating tinnitus and auditory hallucinations.35
The location of stimulation for treating depression has evolved. The Talairach-Tournoux coordinate system has been used to determine the location of the dorsolateral prefrontal cortex (DLPFC) in relation to the motor cortex. This was measured to be 5 cm from the motor hotspot and subsequently became “the 5.5 cm rule,” taking skull convexity into account. The treatment paradigm for the Hesed coil also uses a measurement from the motor hotspot. Another commonly used methodology for coil placement involves using the 10 to 20 EEG coordinate system to individualize scalp landmarks. In this method, the F3 location corresponds most accurately to the DLPFC target. More recently, using fMRI-guided navigation for coil placement has been shown to lead to a significant reduction in depressive symptoms.36
For depression, the initial recommended course of treatment is 6 weeks, but most improvement is seen in the first 2 to 3 weeks.14 Therefore, many clinicians administer an initial course of 3 weeks unless the response is inadequate, in which case a 6-week course is administered. Many patients require ongoing maintenance treatment, which can be weekly or monthly based on response.37
Research to determine the optimal TMS dose for treating neuropsychiatric symptoms is ongoing. Location, intensity of stimulation, and pulse are the components of stimulation. The pulse can be subdivided into frequency, pattern (single pulse, standard, burst), train (numbers of pulse groups), interval between trains, and total number of pulses per session. The Clinical TMS Society has published TMS protocols.38 The standard intensity of stimulation is 120% of the motor threshold (MT), which is defined as the amount of stimulation over the motor cortex required to produce movement in the extensor hallucis longus. Although treatment for depression traditionally utilizes rapid TMS (3,000 pulses delivered per session at a frequency of 10 Hz in 4-second trains), in controlled studies, accelerated protocols such as intermittent theta burst stimulation (iTBS; standard stimulation parameters: triplet 50 Hz bursts at 5 Hz, with an interval of 8 seconds for 600 pulses per session) have shown noninferiority.36,39
Recent research has explored fMRI-guided iTBS in an even more accelerated format. The Stanford Neuromodulation Therapy trial involved 1,800 pulses per session for 10 sessions a day for 5 days at 90% MT.36 This treatment paradigm was shown to be more effective than standard protocols and was FDA-approved in 2022. Although this specific iTBS protocol exhibited encouraging results, the need for fMRI for adequate delivery might limit its use.
Continue to: Transcranial direct current stimulation
Transcranial direct current stimulation
Therapeutic noninvasive brain stimulation technology is plausible due to the relative lack of adverse effects and ease of administration. In transcranial direct current stimulation (tDCS), a low-intensity, constant electric current is delivered to stimulate the brain via electrodes attached to the scalp. tDCS modulates spontaneous neuronal network activity40,41 and induces polarization of resting membrane potential at the neuronal level,42 though the exact mechanism is yet to be proven. N-methyl-
tDCS has been suggested as a treatment for various psychiatric and medical conditions. However, the small sample sizes and experimental design of published studies have limited tDCS from being clinically recommended.30 No recommendation of Level A (definite efficacy) for its use was found for any indication. Level B recommendation (probable efficacy) was proposed for fibromyalgia, MDD episode without drug resistance, and addiction/craving. Level C recommendation (possible efficacy) is proposed for chronic lower limb neuropathic pain secondary to spinal cord lesion. tDCS was found to be probably ineffective as a treatment for tinnitus and drug-resistant MDD.30 Some research has suggested that tDCS targeting the DLPFC is associated with cognitive improvements in healthy individuals as well as those with schizophrenia.44 tDCS treatment remains experimental and investigational.
Deep brain stimulation
DBS is a neurosurgical procedure that uses electrical current to directly modulate specific areas of the CNS. In terms of accurate, site-specific anatomical targeting, there can be little doubt of the superiority of DBS. DBS involves the placement of leads into the brain parenchyma. Image guidance techniques are used for accurate placement. DBS is a mainstay for the symptomatic treatment of treatment-resistant movement disorders such as Parkinson disease, essential tremor, and some dystonic disorders. It also has been studied as a potential treatment for chronic pain, cluster headache, Huntington disease, and Tourette syndrome.
For treating depression, researched targets include the subgenual cingulate gyrus (SCG), ventral striatum, nucleus accumbens, inferior thalamic peduncle, medial forebrain bundle, and the red nucleus.45 In systematic reviews, improvement of depression is greatest when DBS targets the subgenual cingulate cortex and the medial forebrain bundle.46
The major limitation of DBS for treating depression is the invasive nature of the procedure. Deep TMS can achieve noninvasive stimulation of the SCG and may be associated with fewer risks, fewer adverse events, and less collateral damage. However, given the evolving concept of abnormal neurologic circuits in depression, as our understanding of circuitry in pathological psychiatric processes increases, DBS may be an attractive option for personalized targeting of symptoms in some patients.
DBS may also be beneficial for severe, treatment-resistant OCD. Electrode implantation in the region of the internal capsule/ventral striatum, including the nucleus accumbens, is used47; there is little difference in placement as a treatment for OCD vs for movement disorders.48
Continue to: A critical review of 23 trials...
A critical review of 23 trials and case reports of DBS as a treatment for OCD demonstrated a 47.7% mean reduction in score on the Yale-Brown Obsessive-Compulsive Scale (Y-BOCS) and a mean response percentage (minimum 35% Y-BOCS reduction) of 58.2%.49 Most patients regained a normal quality of life after DBS.49 A more rigorous review of 15 meta-analyses of DBS found that conclusions about its efficacy or comparative effectiveness cannot be drawn.50 Because of the nature of neurosurgery, DBS has many potential complications, including cognitive changes, headache, infection, seizures, stroke, and hardware failure.
Vagus nerve stimulation
VNS, in which an implanted device stimulates the left vagus nerve with electrical impulses, was FDA-approved for treating chronic TRD in 2005.51 It had been approved for treatment-resistant epilepsy in 1997. In patients with epilepsy, VNS was shown to improve mood independent of seizure control.52 VNS requires a battery-powered pacemaker device to be implanted under the skin over the anterior chest wall, and a wire tunneled to an electrode is wrapped around the left vagus nerve in the neck.53 The pacemaker is then programmed, monitored, and reprogrammed to optimize response.
VNS is believed to stimulate deep brain nuclei that may play a role in depression.54 The onset of improvement is slow (it may take many months) but in carefully selected patients VNS can provide significant control of TRD. In addition to rare surgery-related complications such as a trauma to the vagal nerve and surrounding tissues (vocal cord paralysis, implant site infection, left facial nerve paralysis and Horner syndrome), VNS may cause hoarseness, dyspnea, and cough related to the intensity of the current output.51 Hypomania and mania were also reported; no suicidal behavior has been associated with VNS.51
Noninvasive vagus nerve stimulationIn noninvasive vagus nerve stimulation (nVNS) or transcutaneous VNS, an external handheld device is applied to the neck overlying the course of the vagus nerve to deliver a sinusoidal alternating current.55 nVNS is currently FDA-approved for treating migraine headaches.55,56 It has demonstrated actions on neurophysiology57 and inflammation in patients with MDD.58 Exploratory research has found a small beneficial effect in patients with depression.59,60 A lack of adequate reproducibility prevents this treatment from being more widely recommended, although attempts to standardize the field are evolving.61
Cranial electrical stimulation
Cranial electrical stimulation (CES) is an older form of electric stimulation developed in the 1970s. In CES, mild electrical pulses are delivered to the ear lobes bilaterally in an episodic fashion (usually 20 to 60 minutes once or twice daily). While CES can be considered a form of neuromodulation, it is not strictly interventional. Patients self-administer CES. The procedure has minimal effects on improving sleep, anxiety, and mood.62-66 Potential adverse effects include a tingling sensation in the ear lobes, lightheadedness, and fogginess. A review and meta-analysis of CES for treating addiction by Kirsch67 showed a wide range of symptoms responding positively to CES treatment, although this study was not peer-reviewed. Because of the low quality of nearly all research that evaluated CES, this form of electric stimulation cannot be viewed as an accepted treatment for any of its listed indications.
Continue to: Other neuromodulation techniques
Other
In addition to the forms of neuromodulation we have already described, there are many other techniques. Several are promising but not yet ready for clinical use. Table 1 and Table 2 summarize the neuromodulation techniques described in this article as well as several that are under development.


Acupuncture
Acupuncture is a Chinese form of medical treatment that began >3,000 years ago; there are written descriptions of it from >2,000 years ago.68 It is based on the belief that there are channels within the body through which the Qi (vital energy or life force) flow, and that inserting fine needles into these channels via the skin can rebalance Qi.68 Modern mechanistic hypotheses invoke involvement of inflammatory or pain pathways.69 Acupuncture frequently uses electric stimulation (electro-acupuncture) to increase the potency of the procedure. Alternatively, in a related procedure (acupressure), pressure can replace the needle. Accreditation in acupuncture generally requires a master’s degree in traditional Chinese medicine but does not require any specific medical training. Acupuncture training courses for physicians are widely available.
All forms of acupuncture are experimental for a wide variety of mental and medical conditions. A meta-analysis found that most research of the utility of acupuncture for depression suffered from various forms of potential bias and was considered low quality.70 Nonetheless, active acupuncture was shown to be minimally superior to placebo acupuncture.70 A meta-analysis of acupuncture for preoperative anxiety71,72 and poststroke insomnia73 reported a similar low study quality. A study of 72 patients with primary insomnia revealed that acupuncture was more effective than sham acupuncture for most sleep measures.74
Psychiatry is increasingly integrating medical tools in addition to psychological tools. Pharmacology remains a cornerstone of biological psychiatry and this will not soon change. However, nonpharmacologic psychiatric treatments such as therapeutic neuromodulation are rapidly emerging. These and novel methods of medication administration may present a challenge to psychiatrists who do not have access to medical personnel or may have forgotten general medical skills.
Our 2-part article has highlighted several interventional psychiatry tools—old and new—that may interest clinicians and benefit patients. As a rule, such treatments are reserved for the most treatment-resistant, challenging psychiatric patients, those with hard-to-treat chronic conditions, and patients who are not helped by more commonly used treatments. An additional complication is that such treatments are frequently not appropriately researched, vetted, or FDA-approved, and therefore are higher risk. Appropriate clinical judgment is always necessary, and potential benefits must be thoroughly weighed against possible adverse effects.
Bottom Line
Several forms of neuromodulation, including electroconvulsive therapy, transcranial magnetic stimulation, transcranial direct current stimulation, deep brain stimulation, and vagus nerve stimulation, may be beneficial for patients with certain treatment-resistant psychiatric disorders, including major depressive disorder and obsessive-compulsive disorder.
Related Resources
- Janicak PG. What’s new in transcranial magnetic stimulation. Current Psychiatry. 2019;18(3):10-16.
- Sharma MS, Ang-Rabanes M, Selek S, et al. Neuromodulatory options for treatment-resistant depression. Current Psychiatry. 2018;17(3):26-28,33-37.
While most psychiatric treatments have traditionally consisted of pharmacotherapy with oral medications, a better understanding of the pathophysiology underlying many mental illnesses has led to the recent increased use of treatments that require specialized administration and the creation of a subspecialty called interventional psychiatry. In Part 1 of this 2-part article (“Interventional psychiatry [Part 1],"
Neuromodulation treatments
Neuromodulation—the alteration of nerve activity through targeted delivery of a stimulus, such as electrical stimulation, to specific neurologic sites—is an increasingly common approach to treating a variety of psychiatric conditions. The use of some form of neuromodulation as a medical treatment has a long history (Box1-6). Modern electric neuromodulation began in the 1930s with electroconvulsive therapy (ECT). The 1960s saw the introduction of deep brain stimulation (DBS), spinal cord stimulation, and later, vagus nerve stimulation (VNS). Target-specific noninvasive brain stimulation became possible with transcranial magnetic stimulation (TMS). These approaches are used for treating major depressive disorder (MDD), obsessive-compulsive disorder (OCD), anxiety disorders, and insomnia. Nearly all these neuromodulatory approaches require clinicians to undergo special training and patients to participate in an invasive procedure. These factors also increase cost. Nonetheless, the high rates of success of some of these approaches have led to relatively rapid and widespread acceptance.
Box
The depth and breadth of human anatomical knowledge has evolved over millennia. The time frame “thousands of years” may appear to be an overstatement, but evidence exists for successful therapeutic limb amputation as early as 31,000 years ago.1 This suggests that human knowledge of bone, muscle, and blood supply was developed much earlier than initially believed. Early Homo sapiens were altering the body—regulating or adjusting it— to serve a purpose; in this case, the purpose was survival.
In 46 AD, electrical modulation was introduced by Scribonius Largus, a physician in court of the emperor Tiberius, who used “torpedoes” (most likely electric eels) to treat headaches and pain from arthritis. Loosely, these early clinicians were modulating human function.
In the late 1800s, electrotherapeutics was a growing branch of medicine, with its own national organization—the American ElectroTherapeutic Association.2 In that era, electricity was novel, powerful, and seen as “the future.” Because such novel therapeutics were offered by both mainstream and dubious sources,3 “many of these products were marketed with the promise of curing everything from cancer to headaches.”4
Modern electric neuromodulation began in the 1930s with electroconvulsive therapy,5 followed by deep brain stimulation and spinal cord stimulation in the 1960s. Target-specific noninvasive brain stimulation became possible when Anthony Barker’s team developed the first device that permitted transcranial magnetic stimulation in 1985.6
Electroconvulsive therapy
In ECT, electric current is applied to the brain to induce a self-limiting seizure. It is the oldest and best-known interventional psychiatric treatment. ECT can also be considered one of the first treatments specifically developed to address pathophysiologic changes. In 1934, Ladislas J. Meduna, who had observed in neuropathologic studies that microglia were more numerous in patients with epilepsy compared with patients with schizophrenia, injected a patient who had been hospitalized with catatonia for 4 years with camphor, a proconvulsant.7 After 5 seizures, the patient began to recover. The therapeutic use of electricity was subsequently developed and optimized in animal models, and first used on human patients in Italy in 1939 and in the United States in 1940.8 The link between psychiatric illness and microglia, which was initially observed nearly a century ago, is making a comeback, as excessive microglial activation has been demonstrated in animal and human models of depression.9
Administering ECT requires specialized equipment, anesthesia, physician training, and nursing observation. ECT also has a negative public image.10 All of these factors conspire to reduce the availability of ECT. Despite this, approximately 100,000 patients in the United States and >1 million worldwide receive ECT each year.10 Patients generally require 6 to 12 ECT treatments11 to achieve sufficient response and may require additional maintenance treatments.12
Although ECT is used to treat psychiatric illnesses ranging from mood disorders to psychotic disorders and catatonia, it is mainly employed to treat people with severe treatment-resistant depression (TRD).13 ECT is associated with significant improvements in depressive symptoms and improvements in quality of life.14 It is superior to other treatments for TRD, such as ketamine,15 though a recent study did not show IV ketamine inferiority.16 ECT is also used to treat other neuropsychiatric disorders, such as Parkinson disease.17
Clinicians have explored alternate methods of inducing therapeutic seizures. Magnetic seizure therapy (MST) utilizes a modified magnetic stimulation device to deliver a higher energy in such a way to induce a generalized seizure under anesthesia.18 While patients receiving MST generally experience fewer adverse effects than with ECT, the procedure may be equal to19 or less effective than ECT.20
Transcranial magnetic stimulation
In neuroimaging research, certain aberrant brain circuits have been implicated in the pathogenesis of depression.21 Specifically, anatomical and functional imaging suggests connections in the prefrontal cortex are involved in the depression process. In TMS, a series of magnetic pulses are administered via the scalp to stimulate neurons in areas of the brain associated with MDD. Early case reports on using TMS to stimulate the prefrontal cortex found significant improvement of symptoms in patients with depression.22 These promising results spurred great interest in the procedure. Over time, the dose and duration of stimulation has increased, along with FDA-approved indications. TMS was first FDA-approved for TRD.23 Although the primary endpoint of the initial clinical trial did not meet criteria for FDA approval, TMS did result in improvement across multiple other measures of depression.23 After the FDA approved the first TMS device, numerous companies began to produce TMS technology. Most of these companies manufacture devices with the figure-of-eight coil, with 1 company producing the Hesed-coil helmet.24
Continue to: An unintended outcome...
An unintended outcome of the increased interest in TMS has been an increased understanding of brain regions involved in psychiatric illness. TMS was able to bring knowledge of mental health from synapses to circuits.25 Work in this area has further stratified the circuits involved in the manifestation of symptom clusters in depression.26 The exact taxonomy of these brain circuits has not been fully realized, but the default mode, salience, attention, cognitive control, and other circuits have been shown to be involved in specific symptom presentations.26,27 These circuits can be hyperactive, hypoactive, hyperconnected, or hypoconnected, with the aberrancies compared to normal controls resulting in symptoms of psychiatric illness.28
This enhanced understanding of brain function has led to further research and development of protocols and subsequent FDA approval of TMS for OCD, anxious depression, and smoking cessation.29 In addition, it has allowed for a proliferation of off-label uses for TMS, including (but not limited to) tinnitus, pain, migraines, and various substance use disorders.30 TMS treatment for these conditions involves stimulation of specific anatomical brain regions that are thought to play a role in the pathology of the target disorder. For example, subthreshold stimulation of the motor cortex has shown some utility in managing symptoms of pain disorders and movement disorders,31,32 the ventromedial prefrontal cortex has been implicated in disorders in the OCD spectrum,33 stimulation of the frontal poles may help treat substance use disorders,34 and the auditory cortex has been a target for treating tinnitus and auditory hallucinations.35
The location of stimulation for treating depression has evolved. The Talairach-Tournoux coordinate system has been used to determine the location of the dorsolateral prefrontal cortex (DLPFC) in relation to the motor cortex. This was measured to be 5 cm from the motor hotspot and subsequently became “the 5.5 cm rule,” taking skull convexity into account. The treatment paradigm for the Hesed coil also uses a measurement from the motor hotspot. Another commonly used methodology for coil placement involves using the 10 to 20 EEG coordinate system to individualize scalp landmarks. In this method, the F3 location corresponds most accurately to the DLPFC target. More recently, using fMRI-guided navigation for coil placement has been shown to lead to a significant reduction in depressive symptoms.36
For depression, the initial recommended course of treatment is 6 weeks, but most improvement is seen in the first 2 to 3 weeks.14 Therefore, many clinicians administer an initial course of 3 weeks unless the response is inadequate, in which case a 6-week course is administered. Many patients require ongoing maintenance treatment, which can be weekly or monthly based on response.37
Research to determine the optimal TMS dose for treating neuropsychiatric symptoms is ongoing. Location, intensity of stimulation, and pulse are the components of stimulation. The pulse can be subdivided into frequency, pattern (single pulse, standard, burst), train (numbers of pulse groups), interval between trains, and total number of pulses per session. The Clinical TMS Society has published TMS protocols.38 The standard intensity of stimulation is 120% of the motor threshold (MT), which is defined as the amount of stimulation over the motor cortex required to produce movement in the extensor hallucis longus. Although treatment for depression traditionally utilizes rapid TMS (3,000 pulses delivered per session at a frequency of 10 Hz in 4-second trains), in controlled studies, accelerated protocols such as intermittent theta burst stimulation (iTBS; standard stimulation parameters: triplet 50 Hz bursts at 5 Hz, with an interval of 8 seconds for 600 pulses per session) have shown noninferiority.36,39
Recent research has explored fMRI-guided iTBS in an even more accelerated format. The Stanford Neuromodulation Therapy trial involved 1,800 pulses per session for 10 sessions a day for 5 days at 90% MT.36 This treatment paradigm was shown to be more effective than standard protocols and was FDA-approved in 2022. Although this specific iTBS protocol exhibited encouraging results, the need for fMRI for adequate delivery might limit its use.
Continue to: Transcranial direct current stimulation
Transcranial direct current stimulation
Therapeutic noninvasive brain stimulation technology is plausible due to the relative lack of adverse effects and ease of administration. In transcranial direct current stimulation (tDCS), a low-intensity, constant electric current is delivered to stimulate the brain via electrodes attached to the scalp. tDCS modulates spontaneous neuronal network activity40,41 and induces polarization of resting membrane potential at the neuronal level,42 though the exact mechanism is yet to be proven. N-methyl-
tDCS has been suggested as a treatment for various psychiatric and medical conditions. However, the small sample sizes and experimental design of published studies have limited tDCS from being clinically recommended.30 No recommendation of Level A (definite efficacy) for its use was found for any indication. Level B recommendation (probable efficacy) was proposed for fibromyalgia, MDD episode without drug resistance, and addiction/craving. Level C recommendation (possible efficacy) is proposed for chronic lower limb neuropathic pain secondary to spinal cord lesion. tDCS was found to be probably ineffective as a treatment for tinnitus and drug-resistant MDD.30 Some research has suggested that tDCS targeting the DLPFC is associated with cognitive improvements in healthy individuals as well as those with schizophrenia.44 tDCS treatment remains experimental and investigational.
Deep brain stimulation
DBS is a neurosurgical procedure that uses electrical current to directly modulate specific areas of the CNS. In terms of accurate, site-specific anatomical targeting, there can be little doubt of the superiority of DBS. DBS involves the placement of leads into the brain parenchyma. Image guidance techniques are used for accurate placement. DBS is a mainstay for the symptomatic treatment of treatment-resistant movement disorders such as Parkinson disease, essential tremor, and some dystonic disorders. It also has been studied as a potential treatment for chronic pain, cluster headache, Huntington disease, and Tourette syndrome.
For treating depression, researched targets include the subgenual cingulate gyrus (SCG), ventral striatum, nucleus accumbens, inferior thalamic peduncle, medial forebrain bundle, and the red nucleus.45 In systematic reviews, improvement of depression is greatest when DBS targets the subgenual cingulate cortex and the medial forebrain bundle.46
The major limitation of DBS for treating depression is the invasive nature of the procedure. Deep TMS can achieve noninvasive stimulation of the SCG and may be associated with fewer risks, fewer adverse events, and less collateral damage. However, given the evolving concept of abnormal neurologic circuits in depression, as our understanding of circuitry in pathological psychiatric processes increases, DBS may be an attractive option for personalized targeting of symptoms in some patients.
DBS may also be beneficial for severe, treatment-resistant OCD. Electrode implantation in the region of the internal capsule/ventral striatum, including the nucleus accumbens, is used47; there is little difference in placement as a treatment for OCD vs for movement disorders.48
Continue to: A critical review of 23 trials...
A critical review of 23 trials and case reports of DBS as a treatment for OCD demonstrated a 47.7% mean reduction in score on the Yale-Brown Obsessive-Compulsive Scale (Y-BOCS) and a mean response percentage (minimum 35% Y-BOCS reduction) of 58.2%.49 Most patients regained a normal quality of life after DBS.49 A more rigorous review of 15 meta-analyses of DBS found that conclusions about its efficacy or comparative effectiveness cannot be drawn.50 Because of the nature of neurosurgery, DBS has many potential complications, including cognitive changes, headache, infection, seizures, stroke, and hardware failure.
Vagus nerve stimulation
VNS, in which an implanted device stimulates the left vagus nerve with electrical impulses, was FDA-approved for treating chronic TRD in 2005.51 It had been approved for treatment-resistant epilepsy in 1997. In patients with epilepsy, VNS was shown to improve mood independent of seizure control.52 VNS requires a battery-powered pacemaker device to be implanted under the skin over the anterior chest wall, and a wire tunneled to an electrode is wrapped around the left vagus nerve in the neck.53 The pacemaker is then programmed, monitored, and reprogrammed to optimize response.
VNS is believed to stimulate deep brain nuclei that may play a role in depression.54 The onset of improvement is slow (it may take many months) but in carefully selected patients VNS can provide significant control of TRD. In addition to rare surgery-related complications such as a trauma to the vagal nerve and surrounding tissues (vocal cord paralysis, implant site infection, left facial nerve paralysis and Horner syndrome), VNS may cause hoarseness, dyspnea, and cough related to the intensity of the current output.51 Hypomania and mania were also reported; no suicidal behavior has been associated with VNS.51
Noninvasive vagus nerve stimulationIn noninvasive vagus nerve stimulation (nVNS) or transcutaneous VNS, an external handheld device is applied to the neck overlying the course of the vagus nerve to deliver a sinusoidal alternating current.55 nVNS is currently FDA-approved for treating migraine headaches.55,56 It has demonstrated actions on neurophysiology57 and inflammation in patients with MDD.58 Exploratory research has found a small beneficial effect in patients with depression.59,60 A lack of adequate reproducibility prevents this treatment from being more widely recommended, although attempts to standardize the field are evolving.61
Cranial electrical stimulation
Cranial electrical stimulation (CES) is an older form of electric stimulation developed in the 1970s. In CES, mild electrical pulses are delivered to the ear lobes bilaterally in an episodic fashion (usually 20 to 60 minutes once or twice daily). While CES can be considered a form of neuromodulation, it is not strictly interventional. Patients self-administer CES. The procedure has minimal effects on improving sleep, anxiety, and mood.62-66 Potential adverse effects include a tingling sensation in the ear lobes, lightheadedness, and fogginess. A review and meta-analysis of CES for treating addiction by Kirsch67 showed a wide range of symptoms responding positively to CES treatment, although this study was not peer-reviewed. Because of the low quality of nearly all research that evaluated CES, this form of electric stimulation cannot be viewed as an accepted treatment for any of its listed indications.
Continue to: Other neuromodulation techniques
Other
In addition to the forms of neuromodulation we have already described, there are many other techniques. Several are promising but not yet ready for clinical use. Table 1 and Table 2 summarize the neuromodulation techniques described in this article as well as several that are under development.


Acupuncture
Acupuncture is a Chinese form of medical treatment that began >3,000 years ago; there are written descriptions of it from >2,000 years ago.68 It is based on the belief that there are channels within the body through which the Qi (vital energy or life force) flow, and that inserting fine needles into these channels via the skin can rebalance Qi.68 Modern mechanistic hypotheses invoke involvement of inflammatory or pain pathways.69 Acupuncture frequently uses electric stimulation (electro-acupuncture) to increase the potency of the procedure. Alternatively, in a related procedure (acupressure), pressure can replace the needle. Accreditation in acupuncture generally requires a master’s degree in traditional Chinese medicine but does not require any specific medical training. Acupuncture training courses for physicians are widely available.
All forms of acupuncture are experimental for a wide variety of mental and medical conditions. A meta-analysis found that most research of the utility of acupuncture for depression suffered from various forms of potential bias and was considered low quality.70 Nonetheless, active acupuncture was shown to be minimally superior to placebo acupuncture.70 A meta-analysis of acupuncture for preoperative anxiety71,72 and poststroke insomnia73 reported a similar low study quality. A study of 72 patients with primary insomnia revealed that acupuncture was more effective than sham acupuncture for most sleep measures.74
Psychiatry is increasingly integrating medical tools in addition to psychological tools. Pharmacology remains a cornerstone of biological psychiatry and this will not soon change. However, nonpharmacologic psychiatric treatments such as therapeutic neuromodulation are rapidly emerging. These and novel methods of medication administration may present a challenge to psychiatrists who do not have access to medical personnel or may have forgotten general medical skills.
Our 2-part article has highlighted several interventional psychiatry tools—old and new—that may interest clinicians and benefit patients. As a rule, such treatments are reserved for the most treatment-resistant, challenging psychiatric patients, those with hard-to-treat chronic conditions, and patients who are not helped by more commonly used treatments. An additional complication is that such treatments are frequently not appropriately researched, vetted, or FDA-approved, and therefore are higher risk. Appropriate clinical judgment is always necessary, and potential benefits must be thoroughly weighed against possible adverse effects.
Bottom Line
Several forms of neuromodulation, including electroconvulsive therapy, transcranial magnetic stimulation, transcranial direct current stimulation, deep brain stimulation, and vagus nerve stimulation, may be beneficial for patients with certain treatment-resistant psychiatric disorders, including major depressive disorder and obsessive-compulsive disorder.
Related Resources
- Janicak PG. What’s new in transcranial magnetic stimulation. Current Psychiatry. 2019;18(3):10-16.
- Sharma MS, Ang-Rabanes M, Selek S, et al. Neuromodulatory options for treatment-resistant depression. Current Psychiatry. 2018;17(3):26-28,33-37.
1. Maloney TR, Dilkes-Hall IE, Vlok M, et al. Surgical amputation of a limb 31,000 years ago in Borneo. Nature. 2022;609(7927):547-551. doi:10.1038/s41586-022-05160-8
2. The American Electro-Therapeutic Association. JAMA. 1893;21(14):500. doi:10.1001/jama.1893.02420660030004
3. The American Electro-Therapeutic Association. JAMA. 1894;23(15):590-591. doi:10.1001/jama.1894.02421200024006
4. Wexler A. The medical battery in the United States (1870-1920): electrotherapy at home and in the clinic. J Hist Med Allied Sci. 2017;72(2):166-192. doi:10.1093/jhmas/jrx001
5. Gazdag G, Ungvari GS. Electroconvulsive therapy: 80 years old and still going strong. World J Psychiatry. 2019;9(1):1-6. doi:10.5498/wjp.v9.i1.1
6. Barker AT, Jalinous R, Freeston IL. Non-invasive magnetic stimulation of human motor cortex. Lancet. 1985;1(8437):1106-1107. doi:10.1016/s0140-6736(85)92413-4
7. Fink M. Historical article: autobiography of L. J. Meduna. Convuls Ther. 1985;1(1):43-57.
8. Suleman R. A brief history of electroconvulsive therapy. Am J Psychiatry. 2020;16(1):6. doi:10.1176/appi.ajp-rj.2020.160103
9. Ménard C, Hodes GE, Russo SJ. Pathogenesis of depression: insights from human and rodent studies. Neuroscience. 2016;321:138-162. doi:10.1016/j.neuroscience.2015.05.053
10. Payne NA, Prudic J. Electroconvulsive therapy: part II: a biopsychosocial perspective. J Psychiatr Pract. 2009;15(5):369-390. doi:10.1097/01.pra.0000361278.73092.85
11. Tirmizi O, Raza A, Trevino K, et al. Electroconvulsive therapy: how modern techniques improve patient outcomes. Current Psychiatry. 2012;11(10):24-46.
12. Kolar D. Current status of electroconvulsive therapy for mood disorders: a clinical review. Evid Based Ment Health. 2017;20(1):12-14. doi:10.1136/eb-2016-102498
13. Andrade C. Active placebo, the parachute meta-analysis, the Nobel Prize, and the efficacy of electroconvulsive therapy. J Clin Psychiatry. 2021;82(2):21f13992. doi:10.4088/JCP.21f13992
14. Giacobbe P, Rakita U, Penner-Goeke K, et al. Improvements in health-related quality of life with electroconvulsive therapy: a meta-analysis. J ECT. 2018;34(2):87-94. doi:10.1097/YCT.0000000000000486
15. Rhee TG, Shim SR, Forester BP, et al. Efficacy and safety of ketamine vs electroconvulsive therapy among patients with major depressive episode: a systematic review and meta-analysis. JAMA Psychiatry. 2022;79(12):1162-1172. doi:10.1001/jamapsychiatry.2022.3352
16. Anand A, Mathew SJ, Sanacora G, et al. Ketamine versus ECT for nonpsychotic treatment-resistant major depression. N Engl J Med. 2023. doi: 10.1056/NEJMoa2302399
17. Takamiya A, Seki M, Kudo S, et al. Electroconvulsive therapy for Parkinson’s disease: a systematic review and meta-analysis. Mov Disord. 2021;36(1):50-58. doi:10.1002/mds.28335
18. Singh R, Sharma R, Prakash J, et al. Magnetic seizure therapy. Ind Psychiatry J. 2021;30(Suppl 1):S320-S321. doi:10.4103/0972-6748.328841
19. Chen M, Yang X, Liu C, et al. Comparative efficacy and cognitive function of magnetic seizure therapy vs. electroconvulsive therapy for major depressive disorder: a systematic review and meta-analysis. Transl Psychiatry. 2021;11(1):437. doi:10.1038/s41398-021-01560-y
20. Cretaz E, Brunoni AR, Lafer B. Magnetic seizure therapy for unipolar and bipolar depression: a systematic review. Neural Plast. 2015;2015:521398. doi:10.1155/2015/521398
21. George MS, Ketter TA, Post RM. Prefrontal cortex dysfunction in clinical depression. In: Nemeroff CB, Weiss JM, Schatzberg AF, et al, eds. Depression. 2nd ed. Wiley Online Library; 1994:59-72. https://doi.org/10.1002/depr.3050020202
22. George MS, Wassermann EM, Williams WA, et al. Daily repetitive transcranial magnetic stimulation (rTMS) improves mood in depression. Neuroreport. 1995;6(14):1853-1856.
23. O’Reardon JP, Solvason HB, Janicak PG, et al. Efficacy and safety of transcranial magnetic stimulation in the acute treatment of major depression: a multisite randomized controlled trial. Biol Psychiatry. 2007;62(11):1208-1216.
24. Clinical TMS Society. TMS devices. Accessed January 2, 2023. https://www.clinicaltmssociety.org/devices
25. Goldstein-Piekarski AN, Ball TM, Samara Z, et al. Mapping neural circuit biotypes to symptoms and behavioral dimensions of depression and anxiety. Biol Psychiatry. 2022;91(6):561-571. doi:10.1016/j.biopsych.2021.06.024
26. Siddiqi SH, Taylor SF, Cooke D, et al. Distinct symptom-specific treatment targets for circuit-based neuromodulation. Am J Psychiatry. 2020;177(5):435-446. doi:10.1176/appi.ajp.2019.19090915
27. Williams LM. Defining biotypes for depression and anxiety based on large-scale circuit dysfunction: a theoretical review of the evidence and future directions for clinical translation. Depress Anxiety. 2017;34(1):9-24. doi:10.1002/da.22556
28. Drysdale AT, Grosenick L, Downar J, et al. Resting-state connectivity biomarkers define neurophysiological subtypes of depression. Nat Med. 2017;23(1):28-38. doi:10.1038/nm.4246
29. Cohen SL, Bikson M, Badran BW, et al. A visual and narrative timeline of US FDA milestones for transcranial magnetic stimulation (TMS) devices. Brain Stimul. 2022;15(1):73-75. doi:10.1016/j.brs.2021.11.010
30. Lefaucheur JP, Antal A, Ayache SS, et al. Evidence-based guidelines on the therapeutic use of transcranial direct current stimulation (tDCS). Clin Neurophysiol. 2017;128(1):56-92. doi:10.1016/j.clinph.2016.10.087
31. Li R, He Y, Qin W, et al. Effects of repetitive transcranial magnetic stimulation on motor symptoms in Parkinson’s disease: a meta-analysis. Neurorehabil Neural Repair. 2022;36(7):395-404. doi:10.1177/15459683221095034
32. Leung A, Shirvalkar P, Chen R, et al. Transcranial magnetic stimulation for pain, headache, and comorbid depression: INS-NANS expert consensus panel review and recommendation. Neuromodulation. 2020;23(3):267-290. doi:10.1111/ner.13094
33. Carmi L, Tendler A, Bystritsky A, et al. Efficacy and safety of deep transcranial magnetic stimulation for obsessive-compulsive disorder: a prospective multicenter randomized double-blind placebo-controlled trial. Am J Psychiatry. 2019;176(11):931-938. doi:10.1176/appi.ajp.2019.18101180
34. Harel M, Perini I, Kämpe R, et al. Repetitive transcranial magnetic stimulation in alcohol dependence: a randomized, double-blind, sham-controlled proof-of-concept trial targeting the medial prefrontal and anterior cingulate cortices. Biol Psychiatry. 2022;91(12):1061-1069. doi:10.1016/j.biopsych.2021.11.020
35. Folmer RL, Theodoroff SM, Casiana L, et al. Repetitive transcranial magnetic stimulation treatment for chronic tinnitus: a randomized clinical trial. JAMA Otolaryngol Head Neck Surg. 2015;141(8):716-722. doi:10.1001/jamaoto.2015.1219
36. Cole EJ, Phillips AL, Bentzley BS, et al. Stanford Neuromodulation Therapy (SNT): a double-blind randomized controlled trial. Am J Psychiatry. 2022;179(2):132-141. doi:10.1176/appi.ajp.2021.20101429
37. Wilson S, Croarkin PE, Aaronson ST, et al. Systematic review of preservation TMS that includes continuation, maintenance, relapse-prevention, and rescue TMS. J Affect Disord. 2022;296:79-88. doi:10.1016/j.jad.2021.09.040
38. Perera T, George MS, Grammer G, et al. The Clinical TMS Society consensus review and treatment recommendations for TMS therapy for major depressive disorder. Brain Stimul. 2016;9(3):336-346. doi:10.1016/j.brs.2016.03.010
39. Blumberger DM, Vila-Rodriguez F, Thorpe KE, et al. Effectiveness of theta burst versus high-frequency repetitive transcranial magnetic stimulation in patients with depression (THREE-D): a randomized non-inferiority trial. Lancet. 2018;391(10131):1683-1692. doi:10.1016/S0140-6736(18)30295-2
40. Nitsche MA, Cohen LG, Wassermann EM, et al. Transcranial direct current stimulation: state of the art 2008. Brain Stimul. 2008;1(3):206-223. doi:10.1016/j.brs.2008.06.004
41. Priori A, Hallett M, Rothwell JC. Repetitive transcranial magnetic stimulation or transcranial direct current stimulation? Brain Stimul. 2009;2(4):241-245.
42. Priori A, Berardelli A, Rona S, et al. Polarization of the human motor cortex through the scalp. Neuroreport. 1998;9(10):2257-2260. doi:10.1097/00001756-199807130-00020
43. Nitsche MA, Liebetanz D, Antal A, et al. Modulation of cortical excitability by weak direct current stimulation-- technical, safety and functional aspects. Suppl Clin Neurophysiol. 2003;56:255-276. doi:10.1016/s1567-424x(09)70230-2
44. Agarwal SM, Venkataram Shivakumar V, et al. Transcranial direct current stimulation in schizophrenia. Clin Psychopharmacol Neurosci. 2013;11(3):118-125.
45. Drobisz D, Damborská A. Deep brain stimulation targets for treating depression. Behav Brain Res. 2019;359:266-273. doi:10.1016/j.bbr.2018.11.004
46. Kisely S, Li A, Warren N, et al. A systematic review and meta-analysis of deep brain stimulation for depression. Depress Anxiety. 2018;35(5):468-480. doi:10.1002/da.22746
47. Blomstedt P, Sjöberg RL, Hansson M, et al. Deep brain stimulation in the treatment of obsessive-compulsive disorder. World Neurosurg. 2013;80(6):e245-e253. doi:10.1016/j.wneu.2012.10.006
48. Denys D, Mantione M, Figee M, et al. Deep brain stimulation of the nucleus accumbens for treatment-refractory obsessive-compulsive disorder. Arch Gen Psychiatry. 2010;67(10):1061-1068. doi:10.1001/archgenpsychiatry.2010.122
49. van Westen M, Rietveld E, Figee M, et al. Clinical outcome and mechanisms of deep brain stimulation for obsessive-compulsive disorder. Curr Behav Neurosci Rep. 2015;2(2):41-48. doi:10.1007/s40473-015-0036-3
50. Papageorgiou PN, Deschner J, Papageorgiou SN. Effectiveness and adverse effects of deep brain stimulation: umbrella review of meta-analyses. J Neurol Surg A Cent Eur Neurosurg. 2017;78(2):180-190. doi:10.1055/s-0036-1592158
51. O’Reardon JP, Cristancho P, Peshek AD. Vagus nerve stimulation (VNS) and treatment of depression: to the brainstem and beyond. Psychiatry (Edgmont). 2006;3(5):54-63.
52. Harden CL, Pulver MC, Ravdin LD, et al. A pilot study of mood in epilepsy patients treated with vagus nerve stimulation. Epilepsy Behav. 2000;1(2):93-99. doi:10.1006/ebeh.2000.0046
53. Giordano F, Zicca A, Barba C, et al. Vagus nerve stimulation: surgical technique of implantation and revision and related morbidity. Epilepsia. 2017;58(S1):85-90. doi:10.1111/epi.13687
54. George MS, Nahas Z, Bohning DE, et al. Mechanisms of action of vagus nerve stimulation (VNS). Clin Neurosci Res. 2004;4(1-2):71-79.
55. Nesbitt AD, Marin JCA, Tompkins E, et al. Initial use of a novel noninvasive vagus nerve stimulator for cluster headache treatment. Neurology. 2015;84:1249-1253. doi:10.1212/WNL.0000000000001394
56. Goadsby PJ, Grosberg BM, Mauskop A, et al. Effect of noninvasive vagus nerve stimulation on acute migraine: an open-label pilot study. Cephalalgia. 2014;34:986-993. doi:10.1177/0333102414524494
57. Fang J, Rong P, Hong Y, et al. Transcutaneous vagus nerve stimulation modulates default mode network in major depressive disorder. Biol Psychiatry. 2016;79(4):266-273. doi:10.1016/j.biopsych.2015.03.025
58. Liu CH, Yang MH, Zhang GZ, et al. Neural networks and the anti-inflammatory effect of transcutaneous auricular vagus nerve stimulation in depression. J Neuroinflammation. 2020;17(1):54. doi:10.1186/s12974-020-01732-5
59. Hein E, Nowak M, Kiess O, et al. Auricular transcutaneous electrical nerve stimulation in depressed patients: a randomized controlled pilot study. J Neural Transm (Vienna). 2013;120(5):821-827. doi:10.1007/s00702-012-0908-6
60. Rong P, Liu J, Wang L, et al. Effect of transcutaneous auricular vagus nerve stimulation on major depressive disorder: a nonrandomized controlled pilot study. J Affect Disord. 2016;195:172-179. doi:10.1016/j.jad.2016.02.031
61. Farmer AD, Strzelczyk A, Finisguerra A, et al. International consensus based review and recommendations for minimum reporting standards in research on transcutaneous vagus nerve stimulation (Version 2020). Front Hum Neurosci. 2021;14:568051. doi:10.3389/fnhum.2020.568051
62. Amr M, El-Wasify M, Elmaadawi AZ, et al. Cranial electrotherapy stimulation for the treatment of chronically symptomatic bipolar patients. J ECT. 2013;29(2):e31-e32. doi:10.1097/YCT.0b013e31828a344d
63. Kirsch DL, Nichols F. Cranial electrotherapy stimulation for treatment of anxiety, depression, and insomnia. Psychiatr Clin North Am. 2013;36(1):169-176. doi:10.1016/j.psc.2013.01.006
64. Lande RG, Gragnani C. Efficacy of cranial electric stimulation for the treatment of insomnia: a randomized pilot study. Complement Ther Med. 2013;21(1):8-13. doi:10.1016/j.ctim.2012.11.007
65. Ou Y, Li, C. Sertraline combined alpha-stim clinical observations on the treatment of 30 cases of generalized anxiety disorder. Chinese Journal of Ethnomedicine and Ethnopharmacy. 2015;24(17):73-75.
66. Price L, Briley J, Haltiwanger S, et al. A meta-analysis of cranial electrotherapy stimulation in the treatment of depression. J Psychiatr Res. 2021;135:119-134. doi:10.1016/j.jpsychires.2020.12.043
67. Kirsch D, Gilula M. CES in the treatment of addictions: a review and meta-analysis. Pract Pain Manag. 2007;7(9).
68. Hao JJ, Mittelman M. Acupuncture: past, present, and future. Glob Adv Health Med. 2014;3(4):6-8. doi:10.7453/gahmj.2014.042
69. Napadow V, Ahn A, Longhurst J, et al. The status and future of acupuncture mechanism research. J Altern Complement Med. 2008;14(7):861-869. doi:10.1089/acm.2008.SAR-3
70. Smith CA, Armour M, Lee MS, et al. Acupuncture for depression. Cochrane Database Syst Rev. 2018;3(3):CD004046. doi:10.1002/14651858.CD004046.pub4
71. Tong QY, Liu R, Zhang K, et al. Can acupuncture therapy reduce preoperative anxiety? A systematic review and meta-analysis. J Integr Med. 2021;19(1):20-28. doi:10.1016/j.joim.2020.10.007
72. Usichenko TI, Hua K, Cummings M, et al. Auricular stimulation for preoperative anxiety – a systematic review and meta-analysis of randomized controlled clinical trials. J Clin Anesth. 2022;76:110581. doi:10.1016/j.jclinane.2021.110581
73. Zhou L, Hu X, Yu Z, et al. Efficacy and safety of acupuncture in the treatment of poststroke insomnia: a systematic review and meta-analysis of twenty-six randomized controlled trials. Evid Based Complement Alternat Med. 2022;2022:5188311. doi:10.1155/2022/5188311
74. Yin X, Gou M, Xu J, et al. Efficacy and safety of acupuncture treatment on primary insomnia: a randomized controlled trial. Sleep Med. 2017;37:193-200. doi:10.1016/j.sleep.2017.02.012
1. Maloney TR, Dilkes-Hall IE, Vlok M, et al. Surgical amputation of a limb 31,000 years ago in Borneo. Nature. 2022;609(7927):547-551. doi:10.1038/s41586-022-05160-8
2. The American Electro-Therapeutic Association. JAMA. 1893;21(14):500. doi:10.1001/jama.1893.02420660030004
3. The American Electro-Therapeutic Association. JAMA. 1894;23(15):590-591. doi:10.1001/jama.1894.02421200024006
4. Wexler A. The medical battery in the United States (1870-1920): electrotherapy at home and in the clinic. J Hist Med Allied Sci. 2017;72(2):166-192. doi:10.1093/jhmas/jrx001
5. Gazdag G, Ungvari GS. Electroconvulsive therapy: 80 years old and still going strong. World J Psychiatry. 2019;9(1):1-6. doi:10.5498/wjp.v9.i1.1
6. Barker AT, Jalinous R, Freeston IL. Non-invasive magnetic stimulation of human motor cortex. Lancet. 1985;1(8437):1106-1107. doi:10.1016/s0140-6736(85)92413-4
7. Fink M. Historical article: autobiography of L. J. Meduna. Convuls Ther. 1985;1(1):43-57.
8. Suleman R. A brief history of electroconvulsive therapy. Am J Psychiatry. 2020;16(1):6. doi:10.1176/appi.ajp-rj.2020.160103
9. Ménard C, Hodes GE, Russo SJ. Pathogenesis of depression: insights from human and rodent studies. Neuroscience. 2016;321:138-162. doi:10.1016/j.neuroscience.2015.05.053
10. Payne NA, Prudic J. Electroconvulsive therapy: part II: a biopsychosocial perspective. J Psychiatr Pract. 2009;15(5):369-390. doi:10.1097/01.pra.0000361278.73092.85
11. Tirmizi O, Raza A, Trevino K, et al. Electroconvulsive therapy: how modern techniques improve patient outcomes. Current Psychiatry. 2012;11(10):24-46.
12. Kolar D. Current status of electroconvulsive therapy for mood disorders: a clinical review. Evid Based Ment Health. 2017;20(1):12-14. doi:10.1136/eb-2016-102498
13. Andrade C. Active placebo, the parachute meta-analysis, the Nobel Prize, and the efficacy of electroconvulsive therapy. J Clin Psychiatry. 2021;82(2):21f13992. doi:10.4088/JCP.21f13992
14. Giacobbe P, Rakita U, Penner-Goeke K, et al. Improvements in health-related quality of life with electroconvulsive therapy: a meta-analysis. J ECT. 2018;34(2):87-94. doi:10.1097/YCT.0000000000000486
15. Rhee TG, Shim SR, Forester BP, et al. Efficacy and safety of ketamine vs electroconvulsive therapy among patients with major depressive episode: a systematic review and meta-analysis. JAMA Psychiatry. 2022;79(12):1162-1172. doi:10.1001/jamapsychiatry.2022.3352
16. Anand A, Mathew SJ, Sanacora G, et al. Ketamine versus ECT for nonpsychotic treatment-resistant major depression. N Engl J Med. 2023. doi: 10.1056/NEJMoa2302399
17. Takamiya A, Seki M, Kudo S, et al. Electroconvulsive therapy for Parkinson’s disease: a systematic review and meta-analysis. Mov Disord. 2021;36(1):50-58. doi:10.1002/mds.28335
18. Singh R, Sharma R, Prakash J, et al. Magnetic seizure therapy. Ind Psychiatry J. 2021;30(Suppl 1):S320-S321. doi:10.4103/0972-6748.328841
19. Chen M, Yang X, Liu C, et al. Comparative efficacy and cognitive function of magnetic seizure therapy vs. electroconvulsive therapy for major depressive disorder: a systematic review and meta-analysis. Transl Psychiatry. 2021;11(1):437. doi:10.1038/s41398-021-01560-y
20. Cretaz E, Brunoni AR, Lafer B. Magnetic seizure therapy for unipolar and bipolar depression: a systematic review. Neural Plast. 2015;2015:521398. doi:10.1155/2015/521398
21. George MS, Ketter TA, Post RM. Prefrontal cortex dysfunction in clinical depression. In: Nemeroff CB, Weiss JM, Schatzberg AF, et al, eds. Depression. 2nd ed. Wiley Online Library; 1994:59-72. https://doi.org/10.1002/depr.3050020202
22. George MS, Wassermann EM, Williams WA, et al. Daily repetitive transcranial magnetic stimulation (rTMS) improves mood in depression. Neuroreport. 1995;6(14):1853-1856.
23. O’Reardon JP, Solvason HB, Janicak PG, et al. Efficacy and safety of transcranial magnetic stimulation in the acute treatment of major depression: a multisite randomized controlled trial. Biol Psychiatry. 2007;62(11):1208-1216.
24. Clinical TMS Society. TMS devices. Accessed January 2, 2023. https://www.clinicaltmssociety.org/devices
25. Goldstein-Piekarski AN, Ball TM, Samara Z, et al. Mapping neural circuit biotypes to symptoms and behavioral dimensions of depression and anxiety. Biol Psychiatry. 2022;91(6):561-571. doi:10.1016/j.biopsych.2021.06.024
26. Siddiqi SH, Taylor SF, Cooke D, et al. Distinct symptom-specific treatment targets for circuit-based neuromodulation. Am J Psychiatry. 2020;177(5):435-446. doi:10.1176/appi.ajp.2019.19090915
27. Williams LM. Defining biotypes for depression and anxiety based on large-scale circuit dysfunction: a theoretical review of the evidence and future directions for clinical translation. Depress Anxiety. 2017;34(1):9-24. doi:10.1002/da.22556
28. Drysdale AT, Grosenick L, Downar J, et al. Resting-state connectivity biomarkers define neurophysiological subtypes of depression. Nat Med. 2017;23(1):28-38. doi:10.1038/nm.4246
29. Cohen SL, Bikson M, Badran BW, et al. A visual and narrative timeline of US FDA milestones for transcranial magnetic stimulation (TMS) devices. Brain Stimul. 2022;15(1):73-75. doi:10.1016/j.brs.2021.11.010
30. Lefaucheur JP, Antal A, Ayache SS, et al. Evidence-based guidelines on the therapeutic use of transcranial direct current stimulation (tDCS). Clin Neurophysiol. 2017;128(1):56-92. doi:10.1016/j.clinph.2016.10.087
31. Li R, He Y, Qin W, et al. Effects of repetitive transcranial magnetic stimulation on motor symptoms in Parkinson’s disease: a meta-analysis. Neurorehabil Neural Repair. 2022;36(7):395-404. doi:10.1177/15459683221095034
32. Leung A, Shirvalkar P, Chen R, et al. Transcranial magnetic stimulation for pain, headache, and comorbid depression: INS-NANS expert consensus panel review and recommendation. Neuromodulation. 2020;23(3):267-290. doi:10.1111/ner.13094
33. Carmi L, Tendler A, Bystritsky A, et al. Efficacy and safety of deep transcranial magnetic stimulation for obsessive-compulsive disorder: a prospective multicenter randomized double-blind placebo-controlled trial. Am J Psychiatry. 2019;176(11):931-938. doi:10.1176/appi.ajp.2019.18101180
34. Harel M, Perini I, Kämpe R, et al. Repetitive transcranial magnetic stimulation in alcohol dependence: a randomized, double-blind, sham-controlled proof-of-concept trial targeting the medial prefrontal and anterior cingulate cortices. Biol Psychiatry. 2022;91(12):1061-1069. doi:10.1016/j.biopsych.2021.11.020
35. Folmer RL, Theodoroff SM, Casiana L, et al. Repetitive transcranial magnetic stimulation treatment for chronic tinnitus: a randomized clinical trial. JAMA Otolaryngol Head Neck Surg. 2015;141(8):716-722. doi:10.1001/jamaoto.2015.1219
36. Cole EJ, Phillips AL, Bentzley BS, et al. Stanford Neuromodulation Therapy (SNT): a double-blind randomized controlled trial. Am J Psychiatry. 2022;179(2):132-141. doi:10.1176/appi.ajp.2021.20101429
37. Wilson S, Croarkin PE, Aaronson ST, et al. Systematic review of preservation TMS that includes continuation, maintenance, relapse-prevention, and rescue TMS. J Affect Disord. 2022;296:79-88. doi:10.1016/j.jad.2021.09.040
38. Perera T, George MS, Grammer G, et al. The Clinical TMS Society consensus review and treatment recommendations for TMS therapy for major depressive disorder. Brain Stimul. 2016;9(3):336-346. doi:10.1016/j.brs.2016.03.010
39. Blumberger DM, Vila-Rodriguez F, Thorpe KE, et al. Effectiveness of theta burst versus high-frequency repetitive transcranial magnetic stimulation in patients with depression (THREE-D): a randomized non-inferiority trial. Lancet. 2018;391(10131):1683-1692. doi:10.1016/S0140-6736(18)30295-2
40. Nitsche MA, Cohen LG, Wassermann EM, et al. Transcranial direct current stimulation: state of the art 2008. Brain Stimul. 2008;1(3):206-223. doi:10.1016/j.brs.2008.06.004
41. Priori A, Hallett M, Rothwell JC. Repetitive transcranial magnetic stimulation or transcranial direct current stimulation? Brain Stimul. 2009;2(4):241-245.
42. Priori A, Berardelli A, Rona S, et al. Polarization of the human motor cortex through the scalp. Neuroreport. 1998;9(10):2257-2260. doi:10.1097/00001756-199807130-00020
43. Nitsche MA, Liebetanz D, Antal A, et al. Modulation of cortical excitability by weak direct current stimulation-- technical, safety and functional aspects. Suppl Clin Neurophysiol. 2003;56:255-276. doi:10.1016/s1567-424x(09)70230-2
44. Agarwal SM, Venkataram Shivakumar V, et al. Transcranial direct current stimulation in schizophrenia. Clin Psychopharmacol Neurosci. 2013;11(3):118-125.
45. Drobisz D, Damborská A. Deep brain stimulation targets for treating depression. Behav Brain Res. 2019;359:266-273. doi:10.1016/j.bbr.2018.11.004
46. Kisely S, Li A, Warren N, et al. A systematic review and meta-analysis of deep brain stimulation for depression. Depress Anxiety. 2018;35(5):468-480. doi:10.1002/da.22746
47. Blomstedt P, Sjöberg RL, Hansson M, et al. Deep brain stimulation in the treatment of obsessive-compulsive disorder. World Neurosurg. 2013;80(6):e245-e253. doi:10.1016/j.wneu.2012.10.006
48. Denys D, Mantione M, Figee M, et al. Deep brain stimulation of the nucleus accumbens for treatment-refractory obsessive-compulsive disorder. Arch Gen Psychiatry. 2010;67(10):1061-1068. doi:10.1001/archgenpsychiatry.2010.122
49. van Westen M, Rietveld E, Figee M, et al. Clinical outcome and mechanisms of deep brain stimulation for obsessive-compulsive disorder. Curr Behav Neurosci Rep. 2015;2(2):41-48. doi:10.1007/s40473-015-0036-3
50. Papageorgiou PN, Deschner J, Papageorgiou SN. Effectiveness and adverse effects of deep brain stimulation: umbrella review of meta-analyses. J Neurol Surg A Cent Eur Neurosurg. 2017;78(2):180-190. doi:10.1055/s-0036-1592158
51. O’Reardon JP, Cristancho P, Peshek AD. Vagus nerve stimulation (VNS) and treatment of depression: to the brainstem and beyond. Psychiatry (Edgmont). 2006;3(5):54-63.
52. Harden CL, Pulver MC, Ravdin LD, et al. A pilot study of mood in epilepsy patients treated with vagus nerve stimulation. Epilepsy Behav. 2000;1(2):93-99. doi:10.1006/ebeh.2000.0046
53. Giordano F, Zicca A, Barba C, et al. Vagus nerve stimulation: surgical technique of implantation and revision and related morbidity. Epilepsia. 2017;58(S1):85-90. doi:10.1111/epi.13687
54. George MS, Nahas Z, Bohning DE, et al. Mechanisms of action of vagus nerve stimulation (VNS). Clin Neurosci Res. 2004;4(1-2):71-79.
55. Nesbitt AD, Marin JCA, Tompkins E, et al. Initial use of a novel noninvasive vagus nerve stimulator for cluster headache treatment. Neurology. 2015;84:1249-1253. doi:10.1212/WNL.0000000000001394
56. Goadsby PJ, Grosberg BM, Mauskop A, et al. Effect of noninvasive vagus nerve stimulation on acute migraine: an open-label pilot study. Cephalalgia. 2014;34:986-993. doi:10.1177/0333102414524494
57. Fang J, Rong P, Hong Y, et al. Transcutaneous vagus nerve stimulation modulates default mode network in major depressive disorder. Biol Psychiatry. 2016;79(4):266-273. doi:10.1016/j.biopsych.2015.03.025
58. Liu CH, Yang MH, Zhang GZ, et al. Neural networks and the anti-inflammatory effect of transcutaneous auricular vagus nerve stimulation in depression. J Neuroinflammation. 2020;17(1):54. doi:10.1186/s12974-020-01732-5
59. Hein E, Nowak M, Kiess O, et al. Auricular transcutaneous electrical nerve stimulation in depressed patients: a randomized controlled pilot study. J Neural Transm (Vienna). 2013;120(5):821-827. doi:10.1007/s00702-012-0908-6
60. Rong P, Liu J, Wang L, et al. Effect of transcutaneous auricular vagus nerve stimulation on major depressive disorder: a nonrandomized controlled pilot study. J Affect Disord. 2016;195:172-179. doi:10.1016/j.jad.2016.02.031
61. Farmer AD, Strzelczyk A, Finisguerra A, et al. International consensus based review and recommendations for minimum reporting standards in research on transcutaneous vagus nerve stimulation (Version 2020). Front Hum Neurosci. 2021;14:568051. doi:10.3389/fnhum.2020.568051
62. Amr M, El-Wasify M, Elmaadawi AZ, et al. Cranial electrotherapy stimulation for the treatment of chronically symptomatic bipolar patients. J ECT. 2013;29(2):e31-e32. doi:10.1097/YCT.0b013e31828a344d
63. Kirsch DL, Nichols F. Cranial electrotherapy stimulation for treatment of anxiety, depression, and insomnia. Psychiatr Clin North Am. 2013;36(1):169-176. doi:10.1016/j.psc.2013.01.006
64. Lande RG, Gragnani C. Efficacy of cranial electric stimulation for the treatment of insomnia: a randomized pilot study. Complement Ther Med. 2013;21(1):8-13. doi:10.1016/j.ctim.2012.11.007
65. Ou Y, Li, C. Sertraline combined alpha-stim clinical observations on the treatment of 30 cases of generalized anxiety disorder. Chinese Journal of Ethnomedicine and Ethnopharmacy. 2015;24(17):73-75.
66. Price L, Briley J, Haltiwanger S, et al. A meta-analysis of cranial electrotherapy stimulation in the treatment of depression. J Psychiatr Res. 2021;135:119-134. doi:10.1016/j.jpsychires.2020.12.043
67. Kirsch D, Gilula M. CES in the treatment of addictions: a review and meta-analysis. Pract Pain Manag. 2007;7(9).
68. Hao JJ, Mittelman M. Acupuncture: past, present, and future. Glob Adv Health Med. 2014;3(4):6-8. doi:10.7453/gahmj.2014.042
69. Napadow V, Ahn A, Longhurst J, et al. The status and future of acupuncture mechanism research. J Altern Complement Med. 2008;14(7):861-869. doi:10.1089/acm.2008.SAR-3
70. Smith CA, Armour M, Lee MS, et al. Acupuncture for depression. Cochrane Database Syst Rev. 2018;3(3):CD004046. doi:10.1002/14651858.CD004046.pub4
71. Tong QY, Liu R, Zhang K, et al. Can acupuncture therapy reduce preoperative anxiety? A systematic review and meta-analysis. J Integr Med. 2021;19(1):20-28. doi:10.1016/j.joim.2020.10.007
72. Usichenko TI, Hua K, Cummings M, et al. Auricular stimulation for preoperative anxiety – a systematic review and meta-analysis of randomized controlled clinical trials. J Clin Anesth. 2022;76:110581. doi:10.1016/j.jclinane.2021.110581
73. Zhou L, Hu X, Yu Z, et al. Efficacy and safety of acupuncture in the treatment of poststroke insomnia: a systematic review and meta-analysis of twenty-six randomized controlled trials. Evid Based Complement Alternat Med. 2022;2022:5188311. doi:10.1155/2022/5188311
74. Yin X, Gou M, Xu J, et al. Efficacy and safety of acupuncture treatment on primary insomnia: a randomized controlled trial. Sleep Med. 2017;37:193-200. doi:10.1016/j.sleep.2017.02.012
Interventional psychiatry: What are the next steps?
The explosion of interest in interventional psychiatry is highlighted by 2 recent reviews published in
Psychiatry’s failure to address these changes would be a dire error, as psychiatrists could lose control of our field’s advances and growth. But this creates an even larger question: what are the next steps we need to take? We believe interventional psychiatry must be recognized as its own psychiatric subspeciality, receive greater emphasis in psychiatry residency training, and be subject to standardization by professional organizations.
Psychiatry has incorporated procedures into patient care for almost 100 years, starting with electroconvulsive therapy (ECT) and insulin shock therapy in the 1930s.3,4 However, in the last 10 years, the rapid expansion of FDA approvals of neuromodulation procedures to treat psychiatric conditions (including vagus nerve stimulation in 2005, transcranial magnetic stimulation [TMS] in 2008, and the device exception granted for the use of deep brain stimulation in 2009) has produced the moniker “interventional psychiatry” for this unofficial psychiatric subspeciality.5,6
If we are to establish interventional psychiatry as a recognized subspeciality, it is important to create a universally accepted definition. We propose the term refer to therapeutic techniques or processes that may or may not be invasive but require special training to perform. Additionally, interventional psychiatry should include even minimally invasive procedures, such as ketamine infusions, medication implants, long-acting injectable (LAI) medications, and processes that require a Risk Evaluation and Mitigation Strategy (REMS), such as those utilized with clozapine, esketamine, or olanzapine for extended-release injectable suspension7 (see “Risk Evaluation and Mitigation Strategy programs: How they can be improved”). The proportions of clinicians who prescribe clozapine (7%)8 or LAIs (32.1% to 77.7%, depending on the patient population being treated)9,10 is evidence that the interventional nature of these treatments creates obstacles to their use.
This vacuum of adequate training among psychiatrists has caused interventional psychiatry to grow beyond the confines of the psychiatric field. In most metropolitan areas of the United States, there are clinicians who focus on a specific interventional treatment, such as ketamine infusions or TMS administration. The creation of these specialized clinics has frequently been pioneered by nonpsychiatrists, such as anesthesiologists. This may be attributed to these clinicians’ level of comfort with procedures, or because they possess an infrastructure within their practice that facilitates delivery of the services. In certain states with independent-practice laws, midlevel clinicians are granted permission to open these clinics. However, having nonpsychiatrists provide these treatments to patients with complex psychiatric disorders without psychiatrist involvement makes it less likely that the appropriateness of treatment will be determined, or that the treatment will be incorporated into the patient’s overall biopsychosocial treatment plan.
A gap in training
There is evidence the growth of interventional psychiatry has exceeded the capacity of the current training infrastructure to provide trainees with adequate exposure to these procedures. The Accreditation Council for Graduate Medical Education requires that psychiatry residents be trained in the indications for and use of ECT and neuromodulation therapies but does not provide any specifics about how this training should occur,11 and the Psychiatry Milestones do not indicate how competency in these therapies can be achieved.12 Most trainees have exposure to some interventional treatments, such as ECT or clozapine administration, during residency. However, in 1 survey, only 63% of residents had prescribed clozapine, and 83% indicated they wanted additional experience.13 In a survey of 91 training programs, 75% stated that ECT was required of residents, but 37% estimated that a typical resident would participate in <10 treatments.14 Even more surprising, 27% estimated that the typical resident would care for <5 patients receiving ECT.14
Addressing the changing role of interventional practices in our field must occur on multiple levels, starting with a core curriculum during residency training, expanded learning opportunities for residents with a specific interest in interventional psychiatry, and, most important, a formal interventional psychiatry fellowship leading to certification from the American Board of Medical Specialties.5,6 There are growing numbers of 1-year fellowship programs that offer extensive experiences in neuromodulation and novel pharmacologic treatment and may produce the next generation of leaders in this field. However, training in interventional psychiatry techniques for practicing psychiatrists wishing to expand their treatment offerings is generally quite limited.
Oversight of interventional psychiatry training should be performed by peers. Therefore, creation of an interventional psychiatry society, or a work group within a larger organization, is necessary. While much of this already exists, it is fragmented into associations focused on unique aspects of interventional psychiatry, such as just ECT (eg, International Society for ECT and Neurostimulation), just TMS (eg, Clinical TMS Society), or just ketamine (eg, the American Society of Ketamine Physicians). Despite disparate foci, the goal would be for all to unite into a parent interventional organization that can face these challenges. These organizations have already united a core of individual interventional psychiatrists who can lead psychiatry into the future. They can provide input into guidelines, minimal standards, procedures, protocols, and outcome measures. They also can address any ethical issues that may arise with the use of more invasive treatments.
Change, especially the monumental changes in practice that accompany interventional psychiatry, is both exciting and intimidating. However, certain “growing pains” along the way require urgent consideration. Ultimately, as a field, we either adapt to change or get left behind.
1. Arbuck D, Farooqui A, El-Mallakh RS. Interventional psychiatry (Part 1). Current Psychiatry. 2023;22(5):25-35. doi:10.12788/cp.0356
2. Arbuck D, Farooqui A, El-Mallakh RS. Interventional psychiatry (Part 2). Current Psychiatry. 2023;22(7):27-35. doi:10.12788/cp.0364
3. Jones K. Insulin coma therapy in schizophrenia. J R Soc Med. 2000;93(3):147-149. doi:10.1177/014107680009300313
4. Gazdag G, Ungvari GS. Electroconvulsive therapy: 80 years old and still going strong. World J Psychiatry. 2019;9(1):1-6. doi:10.5498/wjp.v9.i1.1
5. Williams NR, Taylor JJ, Snipes JM, et al. Interventional psychiatry: how should psychiatric educators incorporate neuromodulation into training? Acad Psychiatry. 2014;38(2):168-176. doi:10.1007/s40596-014-0050-x
6. Trapp NT, Williams NR. The future of training and practice in neuromodulation: an interventional psychiatry perspective. Front Psychiatry. 2021;12:734487. doi:10.3389/fpsyt.2021.734487
7. Vincent KM, Ryan M, Palmer E, et al. Interventional psychiatry. Postgrad Med. 2020;132(7):573-574. doi:10.1080/00325481.2020.1727671
8. Tang Y, Horvitz-Lennon M, Gellad WF, et al. Prescribing of clozapine and antipsychotic polypharmacy for schizophrenia in a large Medicaid program. Psychiatr Serv. 2017;68(6):579-586. doi:10.1176/appi.ps.201600041
9. Zhdanava M, Starr HL, Lefebvre P, et al. Understanding the health system conditions affecting the use of long-acting injectable antipsychotics in the treatment of schizophrenia in clinical practice: a US healthcare provider survey. Neuropsychiatr Dis Treat. 2022;18:1479-1493. doi:10.2147/NDT.S369494
10. Bunting SR, Chalmers K, Yohanna D, et al. Prescription of long-acting injectable antipsychotic medications among outpatient mental health care service providers. Psychiatr Serv. 2023:appips20220586. doi:10.1176/appi.ps.20220586
11. Accreditation Council for Graduate Medical Education. Common program requirements. July 2022. Accessed June 6, 2023. https://www.acgme.org/programs-and-institutions/programs/common-program-requirements
12. Kinzie JM, DeJong SM, Edgar L, et al. Psychiatry Milestones 2.0: using the supplemental guide to create a shared model of the development of professional identity and expertise. Acad Psychiatry. 2021;45(4):500-505. doi:10.1007/s40596-021-01455-6
13. Singh B, Hughes AJ, Roerig JL. Comfort level and barriers to the appropriate use of clozapine: a preliminary survey of US psychiatric residents. Acad Psychiatry. 2020;44(1):53-58 doi:10.1007/s40596-019-01134-7
14. Dinwiddie SH, Spitz D. Resident education in electroconvulsive therapy. J ECT. 2010;26(4):310-316. doi:10.1097/YCT.0b013e3181cb5f78
The explosion of interest in interventional psychiatry is highlighted by 2 recent reviews published in
Psychiatry’s failure to address these changes would be a dire error, as psychiatrists could lose control of our field’s advances and growth. But this creates an even larger question: what are the next steps we need to take? We believe interventional psychiatry must be recognized as its own psychiatric subspeciality, receive greater emphasis in psychiatry residency training, and be subject to standardization by professional organizations.
Psychiatry has incorporated procedures into patient care for almost 100 years, starting with electroconvulsive therapy (ECT) and insulin shock therapy in the 1930s.3,4 However, in the last 10 years, the rapid expansion of FDA approvals of neuromodulation procedures to treat psychiatric conditions (including vagus nerve stimulation in 2005, transcranial magnetic stimulation [TMS] in 2008, and the device exception granted for the use of deep brain stimulation in 2009) has produced the moniker “interventional psychiatry” for this unofficial psychiatric subspeciality.5,6
If we are to establish interventional psychiatry as a recognized subspeciality, it is important to create a universally accepted definition. We propose the term refer to therapeutic techniques or processes that may or may not be invasive but require special training to perform. Additionally, interventional psychiatry should include even minimally invasive procedures, such as ketamine infusions, medication implants, long-acting injectable (LAI) medications, and processes that require a Risk Evaluation and Mitigation Strategy (REMS), such as those utilized with clozapine, esketamine, or olanzapine for extended-release injectable suspension7 (see “Risk Evaluation and Mitigation Strategy programs: How they can be improved”). The proportions of clinicians who prescribe clozapine (7%)8 or LAIs (32.1% to 77.7%, depending on the patient population being treated)9,10 is evidence that the interventional nature of these treatments creates obstacles to their use.
This vacuum of adequate training among psychiatrists has caused interventional psychiatry to grow beyond the confines of the psychiatric field. In most metropolitan areas of the United States, there are clinicians who focus on a specific interventional treatment, such as ketamine infusions or TMS administration. The creation of these specialized clinics has frequently been pioneered by nonpsychiatrists, such as anesthesiologists. This may be attributed to these clinicians’ level of comfort with procedures, or because they possess an infrastructure within their practice that facilitates delivery of the services. In certain states with independent-practice laws, midlevel clinicians are granted permission to open these clinics. However, having nonpsychiatrists provide these treatments to patients with complex psychiatric disorders without psychiatrist involvement makes it less likely that the appropriateness of treatment will be determined, or that the treatment will be incorporated into the patient’s overall biopsychosocial treatment plan.
A gap in training
There is evidence the growth of interventional psychiatry has exceeded the capacity of the current training infrastructure to provide trainees with adequate exposure to these procedures. The Accreditation Council for Graduate Medical Education requires that psychiatry residents be trained in the indications for and use of ECT and neuromodulation therapies but does not provide any specifics about how this training should occur,11 and the Psychiatry Milestones do not indicate how competency in these therapies can be achieved.12 Most trainees have exposure to some interventional treatments, such as ECT or clozapine administration, during residency. However, in 1 survey, only 63% of residents had prescribed clozapine, and 83% indicated they wanted additional experience.13 In a survey of 91 training programs, 75% stated that ECT was required of residents, but 37% estimated that a typical resident would participate in <10 treatments.14 Even more surprising, 27% estimated that the typical resident would care for <5 patients receiving ECT.14
Addressing the changing role of interventional practices in our field must occur on multiple levels, starting with a core curriculum during residency training, expanded learning opportunities for residents with a specific interest in interventional psychiatry, and, most important, a formal interventional psychiatry fellowship leading to certification from the American Board of Medical Specialties.5,6 There are growing numbers of 1-year fellowship programs that offer extensive experiences in neuromodulation and novel pharmacologic treatment and may produce the next generation of leaders in this field. However, training in interventional psychiatry techniques for practicing psychiatrists wishing to expand their treatment offerings is generally quite limited.
Oversight of interventional psychiatry training should be performed by peers. Therefore, creation of an interventional psychiatry society, or a work group within a larger organization, is necessary. While much of this already exists, it is fragmented into associations focused on unique aspects of interventional psychiatry, such as just ECT (eg, International Society for ECT and Neurostimulation), just TMS (eg, Clinical TMS Society), or just ketamine (eg, the American Society of Ketamine Physicians). Despite disparate foci, the goal would be for all to unite into a parent interventional organization that can face these challenges. These organizations have already united a core of individual interventional psychiatrists who can lead psychiatry into the future. They can provide input into guidelines, minimal standards, procedures, protocols, and outcome measures. They also can address any ethical issues that may arise with the use of more invasive treatments.
Change, especially the monumental changes in practice that accompany interventional psychiatry, is both exciting and intimidating. However, certain “growing pains” along the way require urgent consideration. Ultimately, as a field, we either adapt to change or get left behind.
The explosion of interest in interventional psychiatry is highlighted by 2 recent reviews published in
Psychiatry’s failure to address these changes would be a dire error, as psychiatrists could lose control of our field’s advances and growth. But this creates an even larger question: what are the next steps we need to take? We believe interventional psychiatry must be recognized as its own psychiatric subspeciality, receive greater emphasis in psychiatry residency training, and be subject to standardization by professional organizations.
Psychiatry has incorporated procedures into patient care for almost 100 years, starting with electroconvulsive therapy (ECT) and insulin shock therapy in the 1930s.3,4 However, in the last 10 years, the rapid expansion of FDA approvals of neuromodulation procedures to treat psychiatric conditions (including vagus nerve stimulation in 2005, transcranial magnetic stimulation [TMS] in 2008, and the device exception granted for the use of deep brain stimulation in 2009) has produced the moniker “interventional psychiatry” for this unofficial psychiatric subspeciality.5,6
If we are to establish interventional psychiatry as a recognized subspeciality, it is important to create a universally accepted definition. We propose the term refer to therapeutic techniques or processes that may or may not be invasive but require special training to perform. Additionally, interventional psychiatry should include even minimally invasive procedures, such as ketamine infusions, medication implants, long-acting injectable (LAI) medications, and processes that require a Risk Evaluation and Mitigation Strategy (REMS), such as those utilized with clozapine, esketamine, or olanzapine for extended-release injectable suspension7 (see “Risk Evaluation and Mitigation Strategy programs: How they can be improved”). The proportions of clinicians who prescribe clozapine (7%)8 or LAIs (32.1% to 77.7%, depending on the patient population being treated)9,10 is evidence that the interventional nature of these treatments creates obstacles to their use.
This vacuum of adequate training among psychiatrists has caused interventional psychiatry to grow beyond the confines of the psychiatric field. In most metropolitan areas of the United States, there are clinicians who focus on a specific interventional treatment, such as ketamine infusions or TMS administration. The creation of these specialized clinics has frequently been pioneered by nonpsychiatrists, such as anesthesiologists. This may be attributed to these clinicians’ level of comfort with procedures, or because they possess an infrastructure within their practice that facilitates delivery of the services. In certain states with independent-practice laws, midlevel clinicians are granted permission to open these clinics. However, having nonpsychiatrists provide these treatments to patients with complex psychiatric disorders without psychiatrist involvement makes it less likely that the appropriateness of treatment will be determined, or that the treatment will be incorporated into the patient’s overall biopsychosocial treatment plan.
A gap in training
There is evidence the growth of interventional psychiatry has exceeded the capacity of the current training infrastructure to provide trainees with adequate exposure to these procedures. The Accreditation Council for Graduate Medical Education requires that psychiatry residents be trained in the indications for and use of ECT and neuromodulation therapies but does not provide any specifics about how this training should occur,11 and the Psychiatry Milestones do not indicate how competency in these therapies can be achieved.12 Most trainees have exposure to some interventional treatments, such as ECT or clozapine administration, during residency. However, in 1 survey, only 63% of residents had prescribed clozapine, and 83% indicated they wanted additional experience.13 In a survey of 91 training programs, 75% stated that ECT was required of residents, but 37% estimated that a typical resident would participate in <10 treatments.14 Even more surprising, 27% estimated that the typical resident would care for <5 patients receiving ECT.14
Addressing the changing role of interventional practices in our field must occur on multiple levels, starting with a core curriculum during residency training, expanded learning opportunities for residents with a specific interest in interventional psychiatry, and, most important, a formal interventional psychiatry fellowship leading to certification from the American Board of Medical Specialties.5,6 There are growing numbers of 1-year fellowship programs that offer extensive experiences in neuromodulation and novel pharmacologic treatment and may produce the next generation of leaders in this field. However, training in interventional psychiatry techniques for practicing psychiatrists wishing to expand their treatment offerings is generally quite limited.
Oversight of interventional psychiatry training should be performed by peers. Therefore, creation of an interventional psychiatry society, or a work group within a larger organization, is necessary. While much of this already exists, it is fragmented into associations focused on unique aspects of interventional psychiatry, such as just ECT (eg, International Society for ECT and Neurostimulation), just TMS (eg, Clinical TMS Society), or just ketamine (eg, the American Society of Ketamine Physicians). Despite disparate foci, the goal would be for all to unite into a parent interventional organization that can face these challenges. These organizations have already united a core of individual interventional psychiatrists who can lead psychiatry into the future. They can provide input into guidelines, minimal standards, procedures, protocols, and outcome measures. They also can address any ethical issues that may arise with the use of more invasive treatments.
Change, especially the monumental changes in practice that accompany interventional psychiatry, is both exciting and intimidating. However, certain “growing pains” along the way require urgent consideration. Ultimately, as a field, we either adapt to change or get left behind.
1. Arbuck D, Farooqui A, El-Mallakh RS. Interventional psychiatry (Part 1). Current Psychiatry. 2023;22(5):25-35. doi:10.12788/cp.0356
2. Arbuck D, Farooqui A, El-Mallakh RS. Interventional psychiatry (Part 2). Current Psychiatry. 2023;22(7):27-35. doi:10.12788/cp.0364
3. Jones K. Insulin coma therapy in schizophrenia. J R Soc Med. 2000;93(3):147-149. doi:10.1177/014107680009300313
4. Gazdag G, Ungvari GS. Electroconvulsive therapy: 80 years old and still going strong. World J Psychiatry. 2019;9(1):1-6. doi:10.5498/wjp.v9.i1.1
5. Williams NR, Taylor JJ, Snipes JM, et al. Interventional psychiatry: how should psychiatric educators incorporate neuromodulation into training? Acad Psychiatry. 2014;38(2):168-176. doi:10.1007/s40596-014-0050-x
6. Trapp NT, Williams NR. The future of training and practice in neuromodulation: an interventional psychiatry perspective. Front Psychiatry. 2021;12:734487. doi:10.3389/fpsyt.2021.734487
7. Vincent KM, Ryan M, Palmer E, et al. Interventional psychiatry. Postgrad Med. 2020;132(7):573-574. doi:10.1080/00325481.2020.1727671
8. Tang Y, Horvitz-Lennon M, Gellad WF, et al. Prescribing of clozapine and antipsychotic polypharmacy for schizophrenia in a large Medicaid program. Psychiatr Serv. 2017;68(6):579-586. doi:10.1176/appi.ps.201600041
9. Zhdanava M, Starr HL, Lefebvre P, et al. Understanding the health system conditions affecting the use of long-acting injectable antipsychotics in the treatment of schizophrenia in clinical practice: a US healthcare provider survey. Neuropsychiatr Dis Treat. 2022;18:1479-1493. doi:10.2147/NDT.S369494
10. Bunting SR, Chalmers K, Yohanna D, et al. Prescription of long-acting injectable antipsychotic medications among outpatient mental health care service providers. Psychiatr Serv. 2023:appips20220586. doi:10.1176/appi.ps.20220586
11. Accreditation Council for Graduate Medical Education. Common program requirements. July 2022. Accessed June 6, 2023. https://www.acgme.org/programs-and-institutions/programs/common-program-requirements
12. Kinzie JM, DeJong SM, Edgar L, et al. Psychiatry Milestones 2.0: using the supplemental guide to create a shared model of the development of professional identity and expertise. Acad Psychiatry. 2021;45(4):500-505. doi:10.1007/s40596-021-01455-6
13. Singh B, Hughes AJ, Roerig JL. Comfort level and barriers to the appropriate use of clozapine: a preliminary survey of US psychiatric residents. Acad Psychiatry. 2020;44(1):53-58 doi:10.1007/s40596-019-01134-7
14. Dinwiddie SH, Spitz D. Resident education in electroconvulsive therapy. J ECT. 2010;26(4):310-316. doi:10.1097/YCT.0b013e3181cb5f78
1. Arbuck D, Farooqui A, El-Mallakh RS. Interventional psychiatry (Part 1). Current Psychiatry. 2023;22(5):25-35. doi:10.12788/cp.0356
2. Arbuck D, Farooqui A, El-Mallakh RS. Interventional psychiatry (Part 2). Current Psychiatry. 2023;22(7):27-35. doi:10.12788/cp.0364
3. Jones K. Insulin coma therapy in schizophrenia. J R Soc Med. 2000;93(3):147-149. doi:10.1177/014107680009300313
4. Gazdag G, Ungvari GS. Electroconvulsive therapy: 80 years old and still going strong. World J Psychiatry. 2019;9(1):1-6. doi:10.5498/wjp.v9.i1.1
5. Williams NR, Taylor JJ, Snipes JM, et al. Interventional psychiatry: how should psychiatric educators incorporate neuromodulation into training? Acad Psychiatry. 2014;38(2):168-176. doi:10.1007/s40596-014-0050-x
6. Trapp NT, Williams NR. The future of training and practice in neuromodulation: an interventional psychiatry perspective. Front Psychiatry. 2021;12:734487. doi:10.3389/fpsyt.2021.734487
7. Vincent KM, Ryan M, Palmer E, et al. Interventional psychiatry. Postgrad Med. 2020;132(7):573-574. doi:10.1080/00325481.2020.1727671
8. Tang Y, Horvitz-Lennon M, Gellad WF, et al. Prescribing of clozapine and antipsychotic polypharmacy for schizophrenia in a large Medicaid program. Psychiatr Serv. 2017;68(6):579-586. doi:10.1176/appi.ps.201600041
9. Zhdanava M, Starr HL, Lefebvre P, et al. Understanding the health system conditions affecting the use of long-acting injectable antipsychotics in the treatment of schizophrenia in clinical practice: a US healthcare provider survey. Neuropsychiatr Dis Treat. 2022;18:1479-1493. doi:10.2147/NDT.S369494
10. Bunting SR, Chalmers K, Yohanna D, et al. Prescription of long-acting injectable antipsychotic medications among outpatient mental health care service providers. Psychiatr Serv. 2023:appips20220586. doi:10.1176/appi.ps.20220586
11. Accreditation Council for Graduate Medical Education. Common program requirements. July 2022. Accessed June 6, 2023. https://www.acgme.org/programs-and-institutions/programs/common-program-requirements
12. Kinzie JM, DeJong SM, Edgar L, et al. Psychiatry Milestones 2.0: using the supplemental guide to create a shared model of the development of professional identity and expertise. Acad Psychiatry. 2021;45(4):500-505. doi:10.1007/s40596-021-01455-6
13. Singh B, Hughes AJ, Roerig JL. Comfort level and barriers to the appropriate use of clozapine: a preliminary survey of US psychiatric residents. Acad Psychiatry. 2020;44(1):53-58 doi:10.1007/s40596-019-01134-7
14. Dinwiddie SH, Spitz D. Resident education in electroconvulsive therapy. J ECT. 2010;26(4):310-316. doi:10.1097/YCT.0b013e3181cb5f78
Interventional psychiatry (Part 1)
Advances in the understanding of neurobiological and neuropsychiatric pathophysiology have opened new avenues of treatment for psychiatric patients. Historically, with a few exceptions, most psychiatric medications have been administered orally. However, many of the newer treatments require some form of specialized administration because they cannot be taken orally due to their chemical property (such as aducanumab); because there is the need to produce stable blood levels of the medication (such as brexanolone); because oral administration greatly diminished efficacy (such as oral vs IV magnesium or scopolamine), or because the treatment is focused on specific brain structures. This need for specialized administration has created a subspecialty called interventional psychiatry.
Part 1 of this 2-part article provides an overview of 1 type of interventional psychiatry: parenterally administered medications, including those administered via IV. We also describe 3 other interventional approaches to treatment: stellate ganglion blocks, glabellar botulinum toxin (BT) injections, and trigger point injections. In Part 2 we will review interventional approaches that involve neuromodulation.
Parenteral medications in psychiatry
In general, IV and IM medications can be more potent that oral medications due to their overall faster onset of action and higher blood concentrations. These more invasive forms of administration can have significant limitations, such as a risk of infection at the injection site, the need to be administered in a medical setting, additional time, and patient discomfort.
Table 1 lists short-acting injectable medications used in psychiatry, and Table 2 lists long-acting injectable medications. Parenteral administration of antipsychotics is performed to alleviate acute agitation or for chronic symptom control. These medications generally are not considered interventional treatments, but could be classified as such due to their invasive nature.1 Furthermore, inhalable loxapine—which is indicated for managing acute agitation—requires a Risk Evaluation and Mitigation Strategy program consisting of 2 hours observation and monitoring of respiratory status.2,3 Other indications for parenteral treatments include IM naltrexone extended release4 and subcutaneous injections of buprenorphine extended release5 and risperidone.6
IV administration
Most IV treatments described in this article are not FDA-approved for psychiatric treatment. Despite this, many interventional psychiatric treatments are part of clinical practice. IV infusion of ketamine is the most widely known and most researched of these. Table 3 lists other IV treatments that could be used as psychiatric treatment.
Ketamine
Since the early 1960s, ketamine has been used as a surgical anesthetic for animals. In the United States, it was approved for human surgical anesthesia in 1970. It was widely used during the Vietnam War due to its lack of inhibition of respiratory drive; medical staff first noticed an improvement in depressive symptoms and the resolution of suicidal ideation in patients who received ketamine. This led to further research on ketamine, particularly to determine its application in treatment-resistant depression (TRD) and other conditions.7 IV ketamine administration is most widely researched, but IM injections, intranasal sprays, and lozenges are also available. The dissociative properties of ketamine have led to its recreational use.8
Hypotheses for the mechanism of action of ketamine as an antidepressant include direct synaptic or extrasynaptic (GluN2B-selective), N-methyl-
Continue to: Ketamine is a schedule...
Ketamine is a schedule III medication with addictive properties. Delirium, panic attacks, hallucinations, nightmares, dysphoria, and paranoia may occur during and after use.13 Premedication with benzodiazepines, most notably lorazepam, is occasionally used to minimize ketamine’s adverse effects, but this generally is not recommended because doing so reduces ketamine’s antidepressant effects.14 Driving and operating heavy machinery is contraindicated after IV infusion. The usual protocol involves an IV infusion of ketamine 0.4 mg/kg to 1 mg/kg dosing over 1 hour. Doses between 0.4 mg/kg and 0.6 mg/kg are most common. Ketamine has a therapeutic window; doses >0.5 mg/kg are progressively less effective.15 Unlike the recommendation after esketamine administration, after receiving ketamine, patients remain in the care of their treatment team for <2 hours.
Esketamine, the S enantiomer of ketamine, was FDA-approved for TRD as an intranasal formulation. Esketamine is more commonly used than IV ketamine because it is FDA-approved and typically covered by insurance, but it may not be as effective.16 An economic analysis by Brendle et al17 suggested insurance companies would lower costs if they covered ketamine infusions vs intranasal esketamine.
Aducanumab and lecanemab
The most recent FDA-approved interventional agents are aducanumab and lecanemab, which are indicated for treating Alzheimer disease.18,19 Both are human monoclonal antibodies that bind selectively and with high affinity to amyloid beta plaque aggregates and promote their removal by Fc receptor–mediated phagocytosis.20
FDA approval of aducanumab and lecanemab was controversial. Initially, aducanumab’s safety monitoring board performed a futility analysis that suggested aducanumab was unlikely to separate from placebo, and the research was stopped.21 The manufacturer petitioned the FDA to consider the medication for accelerated approval on the basis of biomarker data showing that amyloid beta plaque aggregates become smaller. Current FDA approval is temporary to allow patients access to this potentially beneficial agent, but the manufacturer must supply clinical evidence that the reduction of amyloid beta plaques is associated with desirable changes in the course of Alzheimer disease, or risk losing the approval.
Lecanemab is also a human monoclonal antibody intended to remove amyloid beta plaques that was FDA-approved under the accelerated approval pathway.22 Unlike aducanumab, lecanemab demonstrated a statistically significant (although clinically imperceptible) reduction in the rate of cognitive decline; it did not show cognitive improvement.23 Lecanemab also significantly reduced amyloid beta plaques.23
Continue to: Aducanumab and lecanemab are generally...
Aducanumab and lecanemab are generally not covered by insurance and typically cost >$26,000 annually. Both are administered by IV infusion once a month. More monoclonal antibody medications for treating early Alzheimer disease are in the late stages of development, most notably donanebab.24 Observations during clinical trials found that in the later stages of Alzheimer disease, forceful removal of plaques by the autoimmune process damages neurons, while in less dense deposits of early dementia such removal is not harmful to the cells and prevents amyloid buildup.
Brexanolone is an aqueous formulation of allopregnanolone, a major metabolite of progesterone and a positive allosteric modulator of GABA-A receptors.25 Its levels are maximal at the end of the third trimester of pregnancy and fall rapidly following delivery. Research showed a 3-day infusion was rapidly and significantly effective for treating postpartum depression26 and brexanolone received FDA approval for this indication in March 2019.27 However, various administrative, economic, insurance, and other hurdles make it difficult for patients to access this treatment. Despite its rapid onset of action (usually 48 hours), brexanolone takes an average of 15 days to go through the prior authorization process.28 In addition to the need for prior authorization, the main impediment to the use of brexanolone is the 3-day infusion schedule, which greatly magnifies the cost but is partially circumvented by the availability of dedicated outpatient centers.
Magnesium
Magnesium is on the World Health Organization’s Model List of Essential Medicines.29 There has been extensive research on the use of magnesium sulfate for psychiatric indications, especially for depression.30 Magnesium functions similarly to calcium channel blockers by competitively blocking intracellular calcium channels, decreasing calcium availability, and inhibiting smooth muscle contractility.31 It also competes with calcium at the motor end plate, reducing excitation by inhibiting the release of acetylcholine.32 This property is used for high-dose IV magnesium treatment of impending preterm labor in obstetrics. Magnesium sulfate is the drug of choice in treating eclamptic seizures and preventing seizures in severe preeclampsia or gestational hypertension with severe features.33 It is also used to treat torsade de pointes, severe asthma exacerbations, constipation, and barium poisoning.34 Beneficial use in asthma treatment35 and the treatment of migraine36 have also been reported.
IV magnesium in myocardial infarction may be harmful,37 though outside of acute cardiac events, magnesium is found to be safe.38
Oral magnesium sulfate is a common over-the-counter anxiety remedy. As a general cell stabilizer (mediated by the reduction of intracellular calcium), magnesium is potentially beneficial outside of its muscle-relaxing properties, although muscle relaxing can benefit anxious patients. It is used to treat anxiety,39 alcohol withdrawal,40 and fear.41 Low magnesium blood levels are found in patients with depression, schizophrenia,42 and attention-deficit/hyperactivity disorder.43 However, it is important to note that the therapeutic effect of magnesium when treating anxiety and headache is independent of preinfusion magnesium blood levels.43
Continue to: The efficacy of oral magnesium...
The efficacy of oral magnesium is not robust. However, IV administration has a pronounced beneficial effect as an abortive and preventative treatment in many patients with anxiety.44
IV administration of magnesium can produce adverse effects, including flushing, sweating, hypotension, depressed reflexes, flaccid paralysis, hypothermia, circulatory collapse, and cardiac and CNS depression. These complications are very rare and dose-dependent.45 Magnesium is excreted by the kidneys, and dosing must be decreased in patients with kidney failure. The most common adverse effect is local burning along the vein upon infusion; small doses of IV lidocaine can remedy this. Hot flashes are also common.45
Various dosing strategies are available. In patients with anxiety, application dosing is based on the recommended preeclampsia IV dose of 4 g diluted in 250 mL of 5% dextrose. Much higher doses may be used in obstetrics. Unlike in obstetrics, for psychiatric indications, magnesium is administered over 60 to 90 minutes. Heart monitoring is recommended.
Scopolamine
Scopolamine is primarily used to relieve nausea, vomiting, and dizziness associated with motion sickness and recovery from anesthesia. It is also used in ophthalmology and in patients with excessive sweating. In off-label and experimental applications, scopolamine has been used in patients with TRD.46
Scopolamine is an anticholinergic medication. It is a nonselective antagonist at muscarinic receptors.47 Tricyclic antidepressants (TCAs) possess strong anticholinergic function. Newer generations of antidepressants were designed specifically not to have this function because it was believed to be an unwanted and potentially dangerous adverse effect. However, data suggest that anticholinergic action is important in decreasing depressive symptoms. Several hypotheses of anticholinergic effects on depression have been published since the 1970s. They include the cholinergic-adrenergic hypothesis,48 acetylcholine predominance relative to adrenergic action hypothesis,49 and insecticide poisoning observations.50 Centrally acting physostigmine (but not peripherally acting neostigmine) was reported to control mania.48,51 A genetic connection between the M2acetylcholine receptor in patients with major depressive disorder (MDD) and alcohol use disorder is also suggestive.52
Continue to: Multiple animal studies show...
Multiple animal studies show direct improvement in mobility and a decrease in despair upon introducing anticholinergic substances.53-55 The cholinergic theory of depression has been studied in several controlled clinical human studies.56,57 Use of a short-acting anticholinergic glycopyrrolate during electroconvulsive therapy (ECT) may contribute to the procedure’s efficacy.
Human research shows scopolamine has a higher efficacy as an antidepressant and anti-anxiety medication in women than in men,58 possibly because estrogen increases the activity of choline acetyltransferase and release of acetylcholine.59,60 M2receptors mediate estrogen influence on the NMDAR, which may explain the anticholinergic effects of depression treatments in women.61
Another proposed mechanism of action of scopolamine is a potent inhibition of the NMDAR.62 Rapid treatments of depression may be based on this mechanism. Examples of such treatments include IV ketamine and sleep deprivation.63 IV scopolamine shows potency in treating MDD and bipolar depression. This treatment should be reserved for patients who do not respond to or are not candidates for other usual treatment modalities of MDD and for the most severe cases. Scopolamine is 30 times more potent than amitriptyline in anticholinergic effect and reportedly produces sustained improvement in MDD.64
Scopolamine has no black-box warnings. It has not been studied in pregnant women and is not recommended for use during pregnancy. Due to possible negative cardiovascular effects, a normal electrocardiogram is required before the start of treatment. Exercise caution in patients with glaucoma, benign prostatic enlargement, gastroparesis, unstable cardiovascular status, or severe renal impairment.
Treatment with scopolamine is not indicated for patients with myasthenia gravis, psychosis, or seizures. Patients must be off potassium for 3 days before beginning scopolamine treatment. Patients should consult with their cardiologist before having a scopolamine infusion. Adverse reactions may include psychosis, tachycardia, seizures, paralytic ileus, and glaucoma exacerbation. The most common adverse effects of scopolamine infusion treatment include drowsiness, dry mouth, blurred vision, lightheadedness, and dizziness. Due to possible drowsiness, operating motor vehicles or heavy machinery must be avoided on the day of treatment.65 Overall, the adverse effects of scopolamine are preventable and manageable, and its antidepressant efficacy is noteworthy.66
Continue to: Treatment typically consists of 3 consecutive infusions...
Treatment typically consists of 3 consecutive infusions of 4 mcg/kg separated by 3 to 5 days.56 It is possible to have a longer treatment course if the patient experiences only partial improvement. Repeated courses or maintenance treatment (similar to ECT maintenance) are utilized in some patients if indicated. Cardiac monitoring is mandatory.
Clomipramine
Clomipramine, a TCA, acts as a preferential inhibitor of 5-hydroxytryptamine uptake and has proven effective in managing depression, TRD, and obsessive-compulsive disorder (OCD).67 Although this medication has reported treatment benefits for patients with phobia, panic disorder,15 chronic pain,68 Tourette syndrome,69 premature ejaculation, anorexia nervosa,70 cataplexy,49 and enuresis,71 it is FDA-approved only for the treatment of OCD.72 Clomipramine may also be beneficial for pain and headache, possibly because of its anti-inflammatory action.73 The anticholinergic effects of clomipramine may add to its efficacy in depression.
The pathophysiology of MDD is connected to hyperactivity of the HPA axis and elevated cortisol levels. Higher clomipramine plasma levels show a linear correlation with lower cortisol secretion and levels, possibly aiding in the treatment of MDD and anxiety.74 The higher the blood level, the more pronounced clomipramine’s therapeutic effect across multiple domains.75
IV infusion of clomipramine produces the highest concentration in the shortest time, but overall, research does not necessarily support increased efficacy of IV over oral administration. There is evidence suggesting that subgroups of patients with severe, treatment-refractory OCD may benefit from IV agents and research suggests a faster onset of action.76 Faster onset of symptom relief is the basis for IV clomipramine use. In patients with OCD, it can take several months for oral medications to produce therapeutic benefits; not all patients can tolerate this. In such scenarios, IV administration may be considered, though it is not appropriate for routine use until more research is available. Patients with treatment-resistant OCD who have exhausted other means of symptom relief may also be candidates for IV treatment.
The adverse effects of IV clomipramine are no different from oral use, though they may be more pronounced. A pretreatment cardiac exam is desirable because clomipramine, like other TCAs, may be cardiotoxic. The anticholinergic adverse effects of TCAs are well known to clinicians77 and partially explained in the scopolamine section of this article. It is not advisable to combine clomipramine with other TCAs or serotonin reuptake inhibitors. Clomipramine also should not be combined with monoamine oxidase inhibitors, though such a combination was reported in medical literature.78 Combination with antiarrhythmics such as lidocaine or opioids such as fentanyl or and tramadol is highly discouraged (fentanyl and tramadol also have serotonergic effects).79
Continue to: Clomipramine for IV use is not commercially available...
Clomipramine for IV use is not commercially available and must be sterilely compounded. The usual course of treatment is a series of 3 infusions: 150 mg on Day 1, 200 mg on Day 2 or Day 3, and 250 mg on Day 3, Day 4, or Day 5, depending on tolerability. A protocol with a 50 mg/d starting dose and titration up to a maximum dose of 225 mg/d over 5 to 7 days has been suggested for inpatient settings.67 Titration to 250 mg is more common.80
A longer series may be performed, but this increases the likelihood of adverse effects. Booster and maintenance treatments are also completed when required. Cardiac monitoring is mandatory.
Vortioxetine and citalopram
IV treatment of depression with
Injections and blocks
Three interventional approaches to treatment that possess psychotherapeutic potential include stellate ganglion blocks (SGBs), glabellar BT injections, and trigger point injections (TPIs). None of these are FDA-approved for psychiatric treatment.
Stellate ganglion blocks
The sympathetic nervous system is involved in autonomic hyperarousal and is at the core of posttraumatic symptomatology.83 Insomnia, anxiety, irritability, hypervigilance, and other excitatory CNS events are connected to the sympathetic nervous system and amygdala activation is commonly observed in those exposed to extreme stress or traumatic events.84
Continue to: SGBs were first performed 100 years ago...
SGBs were first performed 100 years ago and reported to have beneficial psychiatric effects at the end of the 1940s. In 1998 in Finland, improvement of posttraumatic stress disorder (PTSD) symptoms was observed accidentally via thoracic level spine blocks.85 In 2006, cervical level sympathetic blocks were shown to be effective for PTSD symptom control.86 By the end of 2010, Veterans Administration hospitals adopted SGBs to treat veterans with PTSD.87,88 The first multisite, randomized clinical trial of
Since the stellate ganglion is connected to the amygdala, SGB has also been assessed for treating anxiety and depression.89,90 Outside of PTSD, SGBs are used to treat complex regional pain syndrome,91 phantom limb pain, trigeminal neuralgia,92 intractable angina,93 and postherpetic neuralgia in the head, neck, upper chest, or arms.94 The procedure consists of an injection of a local anesthetic through a 25-gauge needle into the stellate sympathetic ganglion at the C6 or C7 vertebral levels. An injection into C6 is considered safer because of specific cervical spine anatomy. Ideally, fluoroscopic guidance or ultrasound is used to guide needle insertion.95
A severe drop in blood pressure may be associated with SGBs and is mitigated by IV hydration. Other adverse effects include red eyes, drooping of the eyelids, nasal congestion, hoarseness, difficulty swallowing, a sensation of a “lump” in the throat, and a sensation of warmth or tingling in the arm or hand. Bilateral SGB is contraindicated due to the danger of respiratory arrest.96
Glabellar BT injections
OnabotulinumtoxinA (BT) injection was first approved for therapeutic use in 1989 for eye muscle disorders such as strabismus97 and blepharospasm.98 It was later approved for several other indications, including cosmetic use, hyperhidrosis, migraine prevention, neurogenic bladder disorder, overactive bladder, urinary incontinence, and spasticity.99-104 BT is used off-label for achalasia and sialorrhea.105,106 Its mechanism of action is primarily attributed to muscle paralysis by blocking presynaptic acetylcholine release into neuromuscular junctions.107
Facial BT injections are usually administered for cosmetic purposes, but smoothing forehead wrinkles and frown lines (the glabellar region of the face) both have antidepressant effects.108 BT injections into the glabellar region also demonstrate antidepressant effects, particularly in patients with comorbid migraines and MDD.109 Early case observations supported the independent benefit of the toxin on MDD when the toxin was injected into the glabellar region.110,111 The most frequent protocol involves injections in the procerus and corrugator muscles.
Continue to: The facial feedback/emotional proprioception hypothesis...
The facial feedback/emotional proprioception hypothesis has dominated thinking about the mood-improving effects of BT. The theory is that blocking muscular expression of sadness (especially in the face) interrupts the experience of sadness; therefore, depression subsides.112,113 However, BT injections in the muscles involved in the smile and an expression of positive emotions (lateral part of the musculus orbicularis oculi) have been associated with increased MDD scores.114 Thus, the mechanism clearly involves more than the cosmetic effect, since facial muscle injections in rats also have antidepressant effects.115
The use of progressive muscle relaxation is well-established in psychiatric treatment. The investigated conditions of increased muscle tone, especially torticollis and blepharospasm, are associated with MDD, and it may be speculated that proprioceptive feedback from the affected muscles may be causally involved in this association.116-118 Activity of the corrugator muscle has been positively associated with increased amygdala activity.119 This suggests a potential similar mechanism to that hypothesized for SGB.
Alternatively, BT is commonly used to treat chronic conditions that may contribute to depression; its success in relieving the underlying problem may indirectly relieve MDD. Thus, in a postmarketing safety evaluation of BT, MDD was demonstrated 40% to 88% less often by patients treated with BT for 6 of the 8 conditions and injection sites, such as in spasms and spasticity of arms and legs, torticollis and neck pain, and axilla and palm injections for hyperhidrosis. In a parotid and submandibular glands BT injection subcohort, no patients experienced depressive symptoms.120
Medicinal BT is generally considered safe. The most common adverse effects are hypersensitivity, injection site reactions, and other adverse effects specific to the injection site.121 Additionally, the cosmetic effects are transient, given the nature of the medication.
Trigger point injections
TPIs in the neck and shoulders are frequently used to treat tension headaches and various referred pain locations in the face and arms. Tension and depression frequently overlap in clinical practice.122 Relieving muscle tension (with resulting trigger points) improves muscle function and mood.
Continue to: The higher the number of active...
The higher the number of active trigger points (TPs), the greater the physical burden of headache and the higher the anxiety level. Gender differences in TP prevalence and TPI efficacy have been described in the literature. For example, the number of active TPs seems directly associated with anxiety levels in women but not in men.123 Although TPs appear to be more closely associated with anxiety than depression,124 depression associated with muscle tension does improve with TPIs. European studies have demonstrated a decrease in scores on the Hamilton Depression Rating Scale and Hamilton Anxiety Rating Scale following TPI treatment.125 The effect may be indirect, as when a patient’s pain is relieved, sleep and other psychiatric symptoms improve.126
A randomized controlled trial by Castro Sánchez et al127 demonstrated that dry needling therapy in patients with fibromyalgia syndrome (FMS) showed improvements in pain pressure thresholds, body pain, vitality, and social function, as well as total FMS symptoms, quality of sleep, anxiety, hospital anxiety and depression, general pain intensity, and fatigue.
Myofascial pain syndrome, catastrophizing, and muscle tension are common in patients with depression, anxiety, and somatization. Local TPI therapy aimed at inactivating pain generators is supported by moderate quality evidence. All manner of therapies have been described, including injection of saline, corticosteroids, local anesthetic agents, and dry needling. BT injections in chronic TPs are also practiced, though no specific injection therapy has been reliably shown to be more advantageous than another. The benefits of TPIs may be derived from the needle itself rather than from any specific substance injected. Stimulation of a local twitch response with direct needling of the TP appears of importance. There is no established consensus regarding the number of injection points, frequency of administration, and volume or type of injectate.128
Adverse effects of TPIs relate to the nature of the invasive therapy, with the risk of tissue damage and bleeding. Pneumothorax risk is present with needle insertion at the neck and thorax.129 Patients with diabetes may experience variations in blood sugar control if steroids are used.
Bottom Line
Interventional treatment modalities that may have a role in psychiatric treatment include IV administration of ketamine, aducanumab, lecanemab, brexanolone, magnesium, scopolamine, and clomipramine. Other interventional approaches include stellate ganglion blocks, glabellar botulinum toxin injections, and trigger point injections.
Related Resources
- Dokucu ME, Janicak PG. Nontraditional therapies for treatment-resistant depression. Current Psychiatry. 2021; 20(9):38-43,49. doi:10.12788/cp.0166
- Kim J, Khoury R, Grossberg GT. Botulinum toxin: emerging psychiatric indications. Current Psychiatry. 2018;17(12):8-18.
Drug Brand Names
Aducanumab • Aduhelm
Aripiprazole • Abilify
Aripiprazole lauroxil • Aristada
Brexanolone • Zulresso
Buprenorphine • Sublocade
Citalopram • Celexa
Clomipramine • Anafranil
Diazepam • Valium
Droperidol • Inapsine
Esketamine • Spravato
Fentanyl • Actiq
Fluphenazine decanoate • Modecate
Fluphenazine hydrochloride • Prolixin
Haloperidol decanoate • Haldol decanoate
Haloperidol lactate • Haldol
Ketamine • Ketalar
Lecanemab • Leqembi
Lidocaine • Xylocaine
Lorazepam • Ativan
Loxapine inhaled • Adasuve
Naltrexone • Vivitrol
Magnesium sulfate • Sulfamag
Midazolam • Versed
Olanzapine • Zyprexa
OnabotulinumtoxinA injection • Botox
Paliperidone • Invega Hafyera, Invega Sustenna, Invega Trinza
Rapamycin • Rapamune, Sirolimus
Risperidone • Perseris
Risperidone microspheres • Risperdal Consta, Rykindo
Scopolamine • Hyoscine
Tramadol • Conzip
Vortioxetine • Trintellix
Ziprasidone • Geodon
1. Vincent KM, Ryan M, Palmer E, et al. Interventional psychiatry. Postgrad Med. 2020;132(7):573-574.
2. Allen MH, Feifel D, Lesem MD, et al. Efficacy and safety of loxapine for inhalation in the treatment of agitation in patients with schizophrenia: a randomized, double-blind, placebo-controlled trial. J Clin Psychiatry. 2011;72(10):1313-1321.
3. Kwentus J, Riesenberg RA, Marandi M, et al. Rapid acute treatment of agitation in patients with bipolar I disorder: a multicenter, randomized, placebo-controlled clinical trial with inhaled loxapine. Bipolar Disord. 2012;14(1):31-40.
4. Lee JD, Nunes EV Jr, Novo P, et al. Comparative effectiveness of extended-release naltrexone versus buprenorphine-naloxone for opioid relapse prevention (X:BOT): a multicentre, open-label, randomised controlled trial. Lancet. 2018;391(10118):309-318.
5. Haight BR, Learned SM, Laffont CM, et al. Efficacy and safety of a monthly buprenorphine depot injection for opioid use disorder: a multicentre, randomised, double‐blind, placebo‐controlled, phase 3 trial. Lancet. 2019;393(10173):778-790.
6. Andorn A, Graham J, Csernansky J, et al. Monthly extended-release risperidone (RBP-7000) in the treatment of schizophrenia: results from the phase 3 program. J Clin Psychopharmacol. 2019;39(5):428-433.
7. Dundee TW. Twenty-five years of ketamine. A report of an international meeting. Anaesthesia. 1990;45(2):159. doi:10.1111/j.1365-2044.1990.tb14287.x
8. White PF, Way WL, Trevor AJ. Ketamine--its pharmacology and therapeutic uses. Anesthesiology. 1982;56(2):119-136. doi:10.1097/00000542-198202000-00007
9. Zanos P, Gould TD. Mechanisms of ketamine action as an antidepressant. Mol Psychiatry. 2018;23(4):801-811.
10. Molero P, Ramos-Quiroga JA, Martin-Santos R, et al. Antidepressant efficacy and tolerability of ketamine and esketamine: a critical review. CNS Drugs. 2018;32(5):411-420. doi:10.1007/s40263-018-0519-3
11. Williams NR, Heifets BD, Blasey C, et al. Attenuation of antidepressant effects of ketamine by opioid receptor antagonism. Am J Psychiatry. 2018;175(12):1205-1215.
12. Witkin JM, Martin AE, Golani LK, et al. Rapid-acting antidepressants. Adv Pharmacol. 2019;86:47-96.
13. Strayer RJ, Nelson LS. Adverse events associated with ketamine for procedural sedation in adults. Am J Emerg Med. 2008;26(9):985-1028. doi:10.1016/j.ajem.2007.12.005
14. Frye MA, Blier P, Tye SJ. Concomitant benzodiazepine use attenuates ketamine response: implications for large scale study design and clinical development. J Clin Psychopharmacol. 2015;35(3):334-336.
15. Fava M, Freeman MP, Flynn M, et al. Double-blind, placebo-controlled, dose-ranging trial of intravenous ketamine as adjunctive therapy in treatment-resistant depression (TRD). Mol Psychiatry. 2020;25(7):1592-1603.
16. Bahji A, Vazquez GH, Zarate CA Jr. Comparative efficacy of racemic ketamine and esketamine for depression: a systematic review and meta-analysis. J Affect Disord. 2021;278:542-555. Erratum in: J Affect Disord. 2021;281:1001.
17. Brendle M, Robison R, Malone DC. Cost-effectiveness of esketamine nasal spray compared to intravenous ketamine for patients with treatment-resistant depression in the US utilizing clinical trial efficacy and real-world effectiveness estimates. J Affect Disord. 2022;319:388-396.
18. Dhillon S. Aducanumab: first approval. Drugs. 2021;81(12):1437-1443. Erratum in: Drugs. 2021;81(14):1701.
19. van Dyck CH, Swanson CJ, Aisen P, et al. Lecanemab in early Alzheimer’s disease. N Engl J Med. 2023;388(1):9-21. doi:10.1056/NEJMoa2212948
20. Sevigny J, Chiao P, Bussière T, et al. The antibody aducanumab reduces Aβ plaques in Alzheimer’s disease. Nature. 2016;537(7618):50-56. Update in: Nature. 2017;546(7659):564.
21. Fillit H, Green A. Aducanumab and the FDA – where are we now? Nat Rev Neurol. 2021;17(3):129-130.
22. Reardon S. FDA approves Alzheimer’s drug lecanemab amid safety concerns. Nature. 2023;613(7943):227-228. doi:10.1038/d41586-023-00030-3
23. McDade E, Cummings JL, Dhadda S, et al. Lecanemab in patients with early Alzheimer’s disease: detailed results on biomarker, cognitive, and clinical effects from the randomized and open-label extension of the phase 2 proof-of-concept study. Alzheimers Res Ther. 2022;14(1):191. doi:10.1186/s13195-022-01124-2
24. Mintun MA, Lo AC, Evans CD, et al. Donanemab in early Alzheimer’s disease. N Engl J Med. 2021;384(18):1691-1704.
25. Luisi S, Petraglia F, Benedetto C, et al. Serum allopregnanolone levels in pregnant women: changes during pregnancy, at delivery, and in hypertensive patients. J Clin Endocrinol Metab. 2000;85(7):2429-2433.
26. Meltzer-Brody S, Colquhoun H, Riesenberg R, et al. Brexanolone injection in post-partum depression: two multicentre, double-blind, randomised, placebo-controlled, phase 3 trials. Lancet. 2018;392(10152):1058-1070.
27. Powell JG, Garland S, Preston K, et al. Brexanolone (Zulresso): finally, an FDA-approved treatment for postpartum depression. Ann Pharmacother. 2020;54(2):157-163.
28. Patterson R, Krohn H, Richardson E, et al. A brexanolone treatment program at an academic medical center: patient selection, 90-day posttreatment outcomes, and lessons learned. J Acad Consult Liaison Psychiatry. 2022;63(1):14-22.
29. World Health Organization. WHO model list of essential medicines - 22nd list (2021). World Health Organization. September 30, 2021. Accessed April 7, 2023. https://www.who.int/publications/i/item/WHO-MHP-HPS-EML-2021.02
30. Eby GA, Eby KL, Mruk H. Magnesium and major depression. In: Vink R, Nechifor M, eds. Magnesium in the Central Nervous System. University of Adelaide Press; 2011.
31. Plant TM, Zeleznik AJ. Knobil and Neill’s Physiology of Reproduction. 4th ed. Elsevier Inc.; 2015:2503-2550.
32. Sidebotham D, Le Grice IJ. Physiology and pathophysiology. In: Sidebotham D, McKee A, Gillham M, Levy J. Cardiothoracic Critical Care. Elsevier, Inc.; 2007:3-27.
33. Duley L, Gülmezoglu AM, Henderson-Smart DJ, et al. Magnesium sulphate and other anticonvulsants for women with pre-eclampsia. Cochrane Database Syst Rev. 2010;2010(11):CD000025.
34. Emergency supply of medicines. In: British National Formulary. British Medical Association, Royal Pharmaceutical Society; 2015:6. Accessed April 7, 2023. https://www.academia.edu/35076015/british_national_formulary_2015_pdf
35. Kwofie K, Wolfson AB. Intravenous magnesium sulfate for acute asthma exacerbation in children and adults. Am Fam Physician. 2021;103(4):245-246.
36. Patniyot IR, Gelfand AA. Acute treatment therapies for pediatric migraine: a qualitative systematic review. Headache. 2016;56(1):49-70.
37. Wang X, Du X, Yang H, et al. Use of intravenous magnesium sulfate among patients with acute myocardial infarction in China from 2001 to 2015: China PEACE-Retrospective AMI Study. BMJ Open. 2020;10(3):e033269.
38. Karhu E, Atlas SE, Jinrun G, et al. Intravenous infusion of magnesium sulfate is not associated with cardiovascular, liver, kidney, and metabolic toxicity in adults. J Clin Transl Res. 2018;4(1):47-55.
39. Noah L, Pickering G, Mazur A, et al. Impact of magnesium supplementation, in combination with vitamin B6, on stress and magnesium status: secondary data from a randomized controlled trial. Magnes Res. 2020;33(3):45-57.
40. Erstad BL, Cotugno CL. Management of alcohol withdrawal. Am J Health Syst Pharm. 1995;52(7):697-709.
41. Abumaria N, Luo L, Ahn M, et al. Magnesium supplement enhances spatial-context pattern separation and prevents fear overgeneralization. Behav Pharmacol. 2013;24(4):255-263.
42. Kirov GK, Tsachev KN. Magnesium, schizophrenia and manic-depressive disease. Neuropsychobiology. 1990;23(2):79-81.
43. Botturi A, Ciappolino V, Delvecchio G, et al. The role and the effect of magnesium in mental disorders: a systematic review. Nutrients. 2020;12(6):1661.
44. Kirkland AE, Sarlo GL, Holton KF. The role of magnesium in neurological disorders. Nutrients. 2018;10(6):730.
45. Magnesium sulfate intravenous side effects by likelihood and severity. WebMD. Accessed April 9, 2023. https://www.webmd.com/drugs/2/drug-149570/magnesium-sulfate-intravenous/details/list-sideeffects
46. Scopolamine base transdermal system – uses, side effects, and more. WebMD. Accessed April 9, 2023. https://www.webmd.com/drugs/2/drug-14032/scopolamine-transdermal/details
47. Bolden C, Cusack B, Richelson E. Antagonism by antimuscarinic and neuroleptic compounds at the five cloned human muscarinic cholinergic receptors expressed in Chinese hamster ovary cells. J Pharmacol Exp Ther. 1992;260(2):576-580.
48. Janowsky DS, el-Yousef MK, Davis JM, et al. A cholinergic-adrenergic hypothesis of mania and depression. Lancet. 1972;2(7778):632-635.
49. Janowsky DS, Risch SC, Gillin JC. Adrenergic-cholinergic balance and the treatment of affective disorders. Prog Neuropsychopharmacol Biol Psychiatry. 1983;7(2-3):297-307.
50. Gershon S, Shaw FH. Psychiatric sequelae of chronic exposure to organophosphorous insecticides. Lancet. 1972;1(7191):1371-1374.
51. Davis KL, Berger PA, Hollister LE, et al. Physostigmine in mania. Arch Gen Psychiatry. 1978;35(1):119-122.
52. Wang JC, Hinrichs AL, Stock H, et al. Evidence of common and specific genetic effects: association of the muscarinic acetylcholine receptor M2 (CHRM2) gene with alcohol dependence and major depressive syndrome. Hum Mol Genet. 2004;13(17):1903-1911.
53. Brown RG. Effects of antidepressants and anticholinergics in a mouse “behavioral despair” test. Eur J Pharmacol. 1979;58(3):331-334.
54. Porsolt RD, Le Pichon M, Jalfre M. Depression: a new animal model sensitive to antidepressant treatments. Nature. 1977;266(5604):730-732.
55. Ji CX, Zhang JJ. Effect of scopolamine on depression in mice. Abstract in English. Yao Xue Xue Bao. 2011;46(4):400-405.
56. Furey ML, Drevets WC. Antidepressant efficacy of the antimuscarinic drug scopolamine: a randomized, placebo-controlled clinical trial. Arch Gen Psychiatry. 2006;63(10):1121-1129.
57. Drevets WC, Furey ML. Replication of scopolamine’s antidepressant efficacy in major depressive disorder: a randomized, placebo-controlled clinical trial. Biol Psychiatry. 2010;67(5):432-438.
58. Furey ML, Khanna A, Hoffman EM, et al. Scopolamine produces larger antidepressant and antianxiety effects in women than in men. Neuropsychopharmacology. 2010;35(12):2479-2488.
59. Gibbs RB, Gabor R, Cox T, et al. Effects of raloxifene and estradiol on hippocampal acetylcholine release and spatial learning in the rat. Psychoneuroendocrinology. 2004;29(6):741-748.
60. Pongrac JL, Gibbs RB, Defranco DB. Estrogen-mediated regulation of cholinergic expression in basal forebrain neurons requires extracellular-signal-regulated kinase activity. Neuroscience. 2004;124(4):809-816.
61. Daniel JM, Dohanich GP. Acetylcholine mediates the estrogen-induced increase in NMDA receptor binding in CA1 of the hippocampus and the associated improvement in working memory. J Neurosci. 2001;21(17):6949-6956.
62. Gerhard DM, Wohleb ES, Duman RS. Emerging treatment mechanisms for depression: focus on glutamate and synaptic plasticity. Drug Discov Today. 2016;21(3):454-464.
63. Voderholzer U. Sleep deprivation and antidepressant treatment. Dialogues Clin Neurosci. 2003;5(4):366-369.
64. Hasselmann H. Scopolamine and depression: a role for muscarinic antagonism? CNS Neurol Disord Drug Targets. 2014;13(4):673-683.
65. Transderm scopolamine [prescribing information]. Warren, NJ: GSK Consumer Healthcare; 2019.
66. Jaffe RJ, Novakovic V, Peselow ED. Scopolamine as an antidepressant: a systematic review. Clin Neuropharmacol. 2013;36(1):24-26.
67. Karameh WK, Khani M. Intravenous clomipramine for treatment-resistant obsessive-compulsive disorder. Int J Neuropsychopharmacol. 2015;19(2):pyv084.
68. Andrews ET, Beattie RM, Tighe MP. Functional abdominal pain: what clinicians need to know. Arch Dis Child. 2020;105(10):938-944. doi:10.1136/archdischild-2020-318825
69. Aliane V, Pérez S, Bohren Y, et al. Key role of striatal cholinergic interneurons in processes leading to arrest of motor stereotypies. Brain. 2011;134(Pt 1):110-118. doi:10.1093/brain/awq285
70. Tzavara ET, Bymaster FP, Davis RJ, et al. M4 muscarinic receptors regulate the dynamics of cholinergic and dopaminergic neurotransmission: relevance to the pathophysiology and treatment of related CNS pathologies. FASEB J. 2004;18(12):1410-1412. doi:10.1096/fj.04-1575fje
71. Korczyn AD, Kish I. The mechanism of imipramine in enuresis nocturna. Clin Exp Pharmacol Physiol. 1979;6(1):31-35. doi:10.1111/j.1440-1681.1979.tb00004.x
72. Trimble MR. Worldwide use of clomipramine. J Clin Psychiatry. 1990;51(Suppl):51-54; discussion 55-58.
73. Gong W, Zhang S, Zong Y, et al. Involvement of the microglial NLRP3 inflammasome in the anti-inflammatory effect of the antidepressant clomipramine. J Affect Disord. 2019;254:15-25.
74. Piwowarska J, Wrzosek M, Radziwon’-Zaleska M. Serum cortisol concentration in patients with major depression after treatment with clomipramine. Pharmacol Rep. 2009;61(4):604-611.
75. Danish University Antidepressant Group (DUAG). Clomipramine dose-effect study in patients with depression: clinical end points and pharmacokinetics. Clin Pharmacol Ther. 1999;66(2):152-165.
76. Moukaddam NJ, Hirschfeld RMA. Intravenous antidepressants: a review. Depress Anxiety. 2004;19(1):1-9.
77. Gerretsen P, Pollock BG. Rediscovering adverse anticholinergic effects. J Clin Psychiatry. 2011;72(6):869-870. doi:10.4088/JCP.11ac07093
78. Thomas SJ, Shin M, McInnis MG, et al. Combination therapy with monoamine oxidase inhibitors and other antidepressants or stimulants: strategies for the management of treatment-resistant depression. Pharmacotherapy. 2015;35(4):433-449. doi:10.1002/phar.1576
79. Robles LA. Serotonin syndrome induced by fentanyl in a child: case report. Clin Neuropharmacol. 2015;38(5):206-208. doi:10.1097/WNF.0000000000000100
80. Fallon BA, Liebowitz MR, Campeas R, et al. Intravenous clomipramine for obsessive-compulsive disorder refractory to oral clomipramine: a placebo-controlled study. Arch Gen Psychiatry. 1998;55(10):918-924.
81. Vieta E, Florea I, Schmidt SN, et al. Intravenous vortioxetine to accelerate onset of effect in major depressive disorder: a 2-week, randomized, double-blind, placebo-controlled study. Int Clin Psychopharmacol. 2019;34(4):153-160.
82. Kasper S, Müller-Spahn F. Intravenous antidepressant treatment: focus on citalopram. Eur Arch Psychiatry Clin Neurosci. 2002;252(3):105-109.
83. Togay B, El-Mallakh RS. Posttraumatic stress disorder: from pathophysiology to pharmacology. Current Psychiatry. 2020;19(5):33-39.
84. Adhikari A, Lerner TN, Finkelstein J, et al. Basomedial amygdala mediates top-down control of anxiety and fear. Nature. 2015;527(7577):179-185. doi:10.1038/nature15698
85. Lipov E. In search of an effective treatment for combat-related post-traumatic stress disorder (PTSD): can the stellate ganglion block be the answer? Pain Pract. 2010;10(4):265-266.
86. Lipov E, Ritchie EC. A review of the use of stellate ganglion block in the treatment of PTSD. Curr Psychiatry Rep. 2015;17(8):599.
87. Olmsted KLR, Bartoszek M, McLean B, et al. Effect of stellate ganglion block treatment on posttraumatic stress disorder symptoms: a randomized clinical trial. JAMA Psychiatry. 2020;77(2):130-138.
88. Lipov E, Candido K. The successful use of left-sided stellate ganglion block in patients that fail to respond to right-sided stellate ganglion block for the treatment of post-traumatic stress disorder symptoms: a retrospective analysis of 205 patients. Mil Med. 2021;186(11-12):319-320.
89. Li Y, Loshak H. Stellate ganglion block for the treatment of post-traumatic stress disorder, depression, and anxiety. Canadian J Health Technol. 2021;1(3):1-30.
90. Kerzner J, Liu H, Demchenko I, et al. Stellate ganglion block for psychiatric disorders: a systematic review of the clinical research landscape. Chronic Stress (Thousand Oaks). 2021;5:24705470211055176.
91. Wie C, Gupta R, Maloney J, et al. Interventional modalities to treat complex regional pain syndrome. Curr Pain Headache Rep. 2021;25(2):10. doi:10.1007/s11916-020-00904-5
92. Chaturvedi A, Dash HH. Sympathetic blockade for the relief of chronic pain. J Indian Med Assoc. 2001;99(12):698-703.
93. Chester M, Hammond C. Leach A. Long-term benefits of stellate ganglion block in severe chronic refractory angina. Pain. 2000;87(1):103-105. doi:10.1016/S0304-3959(00)00270-0
94. Jeon Y. Therapeutic potential of stellate ganglion block in orofacial pain: a mini review. J Dent Anesth Pain Med. 2016;16(3):159-163. doi:10.17245/jdapm.2016.16.3.159
95. Shan HH, Chen HF, Ni Y, et al. Effects of stellate ganglion block through different approaches under guidance of ultrasound. Front Surg. 2022;8:797793. doi:10.3389/fsurg.2021.797793
96. Goel V, Patwardhan AM, Ibrahim M, et al. Complications associated with stellate ganglion nerve block: a systematic review. Reg Anesth Pain Med. 2019;rapm-2018-100127. doi:10.1136/rapm-2018-100127
97. Rowe FJ, Noonan CP. Botulinum toxin for the treatment of strabismus. Cochrane Database Syst Rev. 2017;3(3):CD006499.
98. Roggenkämper P, Jost WH, Bihari K, et al. Efficacy and safety of a new botulinum toxin type A free of complexing proteins in the treatment of blepharospasm. J Neural Transm (Vienna). 2006;113(3):303-312.
99. Heckmann M, Ceballos-Baumann AO, Plewig G; Hyperhidrosis Study Group. Botulinum toxin A for axillary hyperhidrosis (excessive sweating). N Engl J Med. 2001;344(7):488-493.
100. Carruthers JA, Lowe NJ, Menter MA, et al. A multicenter, double-blind, randomized, placebo-controlled study of the efficacy and safety of botulinum toxin type A in the treatment of glabellar lines. J Am Acad Dermatol. 2002;46(6):840-849.
101. Schurch B, de Sèze M, Denys P, et al. Botulinum toxin type A is a safe and effective treatment for neurogenic urinary incontinence: results of a single treatment, randomized, placebo controlled 6-month study. J Urol. 2005;174:196–200.
102. Aurora SK, Winner P, Freeman MC, et al. OnabotulinumtoxinA for treatment of chronic migraine: Pooled analyses of the 56-week PREEMPT clinical program. Headache. 2011;51(9):1358-1373.
103. Dashtipour K, Chen JJ, Walker HW, et al. Systematic literature review of abobotulinumtoxinA in clinical trials for adult upper limb spasticity. Am J Phys Med Rehabil. 2015;94(3):229-238.
104. Nitti VW, Dmochowski R, Herschorn S, et al. OnabotulinumtoxinA for the treatment of patients with overactive bladder and urinary incontinence: results of a phase 3, randomized, placebo-controlled trial. J Urol. 2017;197(2S):S216-S223.
105. Jongerius PH, van den Hoogen FJA, van Limbeek J, et al. Effect of botulinum toxin in the treatment of drooling: a controlled clinical trial. Pediatrics. 2004;114(3):620-627.
106. Zaninotto, G. Annese V, Costantini M, et al. Randomized controlled trial of botulinum toxin versus laparoscopic heller myotomy for esophageal achalasia. Ann Surg. 2004;239(3):364-370.
107. Dressler D, Adib Saberi F. Botulinum toxin: mechanisms of action. Eur Neurol. 2005;53:3-9.
108. Lewis MB, Bowler PJ. Botulinum toxin cosmetic therapy correlates with a more positive mood. J Cosmet Dermatol. 2009;8(1):24-26.
109. Affatato O, Moulin TC, Pisanu C, et al. High efficacy of onabotulinumtoxinA treatment in patients with comorbid migraine and depression: a meta-analysis. J Transl Med. 2021;19(1):133.
110. Finzi E, Wasserman E. Treatment of depression with botulinum toxin A: a case series. Dermatol Surg. 2006;32(5):645-649; discussion 649-650.
111. Schulze J, Neumann I, Magid M, et al. Botulinum toxin for the management of depression: an updated review of the evidence and meta-analysis. J Psychiatr Res. 2021;135:332-340.
112. Finzi E, Rosenthal NE. Emotional proprioception: treatment of depression with afferent facial feedback. J Psychiatr Res. 2016;80:93-96.
113. Söderkvist S, Ohlén K, Dimberg U. How the experience of emotion is modulated by facial feedback. J Nonverbal Behav. 2018;42(1):129-151.
114. Lewis, MB. The interactions between botulinum-toxin-based facial treatments and embodied emotions. Sci Rep. 2018;8(1):14720.
115. Li Y, Liu J, Liu X, et al. Antidepressant-like action of single facial injection of botulinum neurotoxin A is associated with augmented 5-HT levels and BDNF/ERK/CREB pathways in mouse brain. Neurosci Bull. 2019;35(4):661-672. Erratum in: Neurosci Bull. 2019;35(4):779-780.
116. Gündel H, Wolf A, Xidara V, et al. High psychiatric comorbidity in spasmodic torticollis: a controlled study. J Nerv Ment Dis. 2003;191(7):465-473.
117. Hall TA, McGwin G Jr, Searcey K, et al. Health-related quality of life and psychosocial characteristics of patients with benign essential blepharospasm. Arch Ophthalmol. 2006;124(1):116-119.
118. Ceylan D, Erer S, Zarifog˘lu M, et al. Evaluation of anxiety and depression scales and quality of life in cervical dystonia patients on botulinum toxin therapy and their relatives. Neurol Sci. 2019;40(4):725-731.
119. Heller AS, Lapate RC, Mayer KE, et al. The face of negative affect: trial-by-trial corrugator responses to negative pictures are positively associated with amygdala and negatively associated with ventromedial prefrontal cortex activity. J Cogn Neurosci. 2014;26(9):2102-2110.
120. Makunts T, Wollmer MA, Abagyan R. Postmarketing safety surveillance data reveals antidepressant effects of botulinum toxin across various indications and injection sites. Sci Rep. 2020;10(1):12851.
121. Ahsanuddin S, Roy S, Nasser W, et al. Adverse events associated with botox as reported in a Food and Drug Administration database. Aesthetic Plast Surg. 2021;45(3):1201-1209. doi:10.1007/s00266-020-02027-z
122. Kashif M, Tahir S, Ashfaq F, et al. Association of myofascial trigger points in neck and shoulder region with depression, anxiety, and stress among university students. J Pak Med Assoc. 2021;71(9):2139-2142.
123. Cigarán-Méndez M, Jiménez-Antona C, Parás-Bravo P, et al. Active trigger points are associated with anxiety and widespread pressure pain sensitivity in women, but not men, with tension type headache. Pain Pract. 2019;19(5):522-529.
124. Palacios-Ceña M, Castaldo M, Wang K, et al. Relationship of active trigger points with related disability and anxiety in people with tension-type headache. Medicine (Baltimore). 2017;96(13):e6548.
125. Karadas Ö, Inan LE, Ulas Ü, et al. Efficacy of local lidocaine application on anxiety and depression and its curative effect on patients with chronic tension-type headache. Eur Neurol. 2013;70(1-2):95-101.
126. Gerwin RD. Classification, epidemiology and natural history of myofascial pain syndrome. Curr Pain Headache Rep. 2001;5(5):412-420.
127. Castro Sánchez AM, García López H, Fernández Sánchez M, et al. Improvement in clinical outcomes after dry needling versus myofascial release on pain pressure thresholds, quality of life, fatigue, pain intensity, quality of sleep, anxiety, and depression in patients with fibromyalgia syndrome. Disabil Rehabil. 2019;41(19):2235-2246.
128. Healy GM, Finn DP, O’Gorman DA, et al. Pretreatment anxiety and pain acceptance are associated with response to trigger point injection therapy for chronic myofascial pain. Pain Med. 2015;16(10):1955-1966.
129. Morjaria JB, Lakshminarayana UB, Liu-Shiu-Cheong P, et al. Pneumothorax: a tale of pain or spontaneity. Ther Adv Chronic Dis. 2014;5(6):269-273.
Advances in the understanding of neurobiological and neuropsychiatric pathophysiology have opened new avenues of treatment for psychiatric patients. Historically, with a few exceptions, most psychiatric medications have been administered orally. However, many of the newer treatments require some form of specialized administration because they cannot be taken orally due to their chemical property (such as aducanumab); because there is the need to produce stable blood levels of the medication (such as brexanolone); because oral administration greatly diminished efficacy (such as oral vs IV magnesium or scopolamine), or because the treatment is focused on specific brain structures. This need for specialized administration has created a subspecialty called interventional psychiatry.
Part 1 of this 2-part article provides an overview of 1 type of interventional psychiatry: parenterally administered medications, including those administered via IV. We also describe 3 other interventional approaches to treatment: stellate ganglion blocks, glabellar botulinum toxin (BT) injections, and trigger point injections. In Part 2 we will review interventional approaches that involve neuromodulation.
Parenteral medications in psychiatry
In general, IV and IM medications can be more potent that oral medications due to their overall faster onset of action and higher blood concentrations. These more invasive forms of administration can have significant limitations, such as a risk of infection at the injection site, the need to be administered in a medical setting, additional time, and patient discomfort.

Table 1 lists short-acting injectable medications used in psychiatry, and Table 2 lists long-acting injectable medications. Parenteral administration of antipsychotics is performed to alleviate acute agitation or for chronic symptom control. These medications generally are not considered interventional treatments, but could be classified as such due to their invasive nature.1 Furthermore, inhalable loxapine—which is indicated for managing acute agitation—requires a Risk Evaluation and Mitigation Strategy program consisting of 2 hours observation and monitoring of respiratory status.2,3 Other indications for parenteral treatments include IM naltrexone extended release4 and subcutaneous injections of buprenorphine extended release5 and risperidone.6
IV administration
Most IV treatments described in this article are not FDA-approved for psychiatric treatment. Despite this, many interventional psychiatric treatments are part of clinical practice. IV infusion of ketamine is the most widely known and most researched of these. Table 3 lists other IV treatments that could be used as psychiatric treatment.
Ketamine
Since the early 1960s, ketamine has been used as a surgical anesthetic for animals. In the United States, it was approved for human surgical anesthesia in 1970. It was widely used during the Vietnam War due to its lack of inhibition of respiratory drive; medical staff first noticed an improvement in depressive symptoms and the resolution of suicidal ideation in patients who received ketamine. This led to further research on ketamine, particularly to determine its application in treatment-resistant depression (TRD) and other conditions.7 IV ketamine administration is most widely researched, but IM injections, intranasal sprays, and lozenges are also available. The dissociative properties of ketamine have led to its recreational use.8
Hypotheses for the mechanism of action of ketamine as an antidepressant include direct synaptic or extrasynaptic (GluN2B-selective), N-methyl-
Continue to: Ketamine is a schedule...
Ketamine is a schedule III medication with addictive properties. Delirium, panic attacks, hallucinations, nightmares, dysphoria, and paranoia may occur during and after use.13 Premedication with benzodiazepines, most notably lorazepam, is occasionally used to minimize ketamine’s adverse effects, but this generally is not recommended because doing so reduces ketamine’s antidepressant effects.14 Driving and operating heavy machinery is contraindicated after IV infusion. The usual protocol involves an IV infusion of ketamine 0.4 mg/kg to 1 mg/kg dosing over 1 hour. Doses between 0.4 mg/kg and 0.6 mg/kg are most common. Ketamine has a therapeutic window; doses >0.5 mg/kg are progressively less effective.15 Unlike the recommendation after esketamine administration, after receiving ketamine, patients remain in the care of their treatment team for <2 hours.
Esketamine, the S enantiomer of ketamine, was FDA-approved for TRD as an intranasal formulation. Esketamine is more commonly used than IV ketamine because it is FDA-approved and typically covered by insurance, but it may not be as effective.16 An economic analysis by Brendle et al17 suggested insurance companies would lower costs if they covered ketamine infusions vs intranasal esketamine.
Aducanumab and lecanemab
The most recent FDA-approved interventional agents are aducanumab and lecanemab, which are indicated for treating Alzheimer disease.18,19 Both are human monoclonal antibodies that bind selectively and with high affinity to amyloid beta plaque aggregates and promote their removal by Fc receptor–mediated phagocytosis.20
FDA approval of aducanumab and lecanemab was controversial. Initially, aducanumab’s safety monitoring board performed a futility analysis that suggested aducanumab was unlikely to separate from placebo, and the research was stopped.21 The manufacturer petitioned the FDA to consider the medication for accelerated approval on the basis of biomarker data showing that amyloid beta plaque aggregates become smaller. Current FDA approval is temporary to allow patients access to this potentially beneficial agent, but the manufacturer must supply clinical evidence that the reduction of amyloid beta plaques is associated with desirable changes in the course of Alzheimer disease, or risk losing the approval.
Lecanemab is also a human monoclonal antibody intended to remove amyloid beta plaques that was FDA-approved under the accelerated approval pathway.22 Unlike aducanumab, lecanemab demonstrated a statistically significant (although clinically imperceptible) reduction in the rate of cognitive decline; it did not show cognitive improvement.23 Lecanemab also significantly reduced amyloid beta plaques.23
Continue to: Aducanumab and lecanemab are generally...
Aducanumab and lecanemab are generally not covered by insurance and typically cost >$26,000 annually. Both are administered by IV infusion once a month. More monoclonal antibody medications for treating early Alzheimer disease are in the late stages of development, most notably donanebab.24 Observations during clinical trials found that in the later stages of Alzheimer disease, forceful removal of plaques by the autoimmune process damages neurons, while in less dense deposits of early dementia such removal is not harmful to the cells and prevents amyloid buildup.
Brexanolone is an aqueous formulation of allopregnanolone, a major metabolite of progesterone and a positive allosteric modulator of GABA-A receptors.25 Its levels are maximal at the end of the third trimester of pregnancy and fall rapidly following delivery. Research showed a 3-day infusion was rapidly and significantly effective for treating postpartum depression26 and brexanolone received FDA approval for this indication in March 2019.27 However, various administrative, economic, insurance, and other hurdles make it difficult for patients to access this treatment. Despite its rapid onset of action (usually 48 hours), brexanolone takes an average of 15 days to go through the prior authorization process.28 In addition to the need for prior authorization, the main impediment to the use of brexanolone is the 3-day infusion schedule, which greatly magnifies the cost but is partially circumvented by the availability of dedicated outpatient centers.
Magnesium
Magnesium is on the World Health Organization’s Model List of Essential Medicines.29 There has been extensive research on the use of magnesium sulfate for psychiatric indications, especially for depression.30 Magnesium functions similarly to calcium channel blockers by competitively blocking intracellular calcium channels, decreasing calcium availability, and inhibiting smooth muscle contractility.31 It also competes with calcium at the motor end plate, reducing excitation by inhibiting the release of acetylcholine.32 This property is used for high-dose IV magnesium treatment of impending preterm labor in obstetrics. Magnesium sulfate is the drug of choice in treating eclamptic seizures and preventing seizures in severe preeclampsia or gestational hypertension with severe features.33 It is also used to treat torsade de pointes, severe asthma exacerbations, constipation, and barium poisoning.34 Beneficial use in asthma treatment35 and the treatment of migraine36 have also been reported.
IV magnesium in myocardial infarction may be harmful,37 though outside of acute cardiac events, magnesium is found to be safe.38
Oral magnesium sulfate is a common over-the-counter anxiety remedy. As a general cell stabilizer (mediated by the reduction of intracellular calcium), magnesium is potentially beneficial outside of its muscle-relaxing properties, although muscle relaxing can benefit anxious patients. It is used to treat anxiety,39 alcohol withdrawal,40 and fear.41 Low magnesium blood levels are found in patients with depression, schizophrenia,42 and attention-deficit/hyperactivity disorder.43 However, it is important to note that the therapeutic effect of magnesium when treating anxiety and headache is independent of preinfusion magnesium blood levels.43
Continue to: The efficacy of oral magnesium...
The efficacy of oral magnesium is not robust. However, IV administration has a pronounced beneficial effect as an abortive and preventative treatment in many patients with anxiety.44
IV administration of magnesium can produce adverse effects, including flushing, sweating, hypotension, depressed reflexes, flaccid paralysis, hypothermia, circulatory collapse, and cardiac and CNS depression. These complications are very rare and dose-dependent.45 Magnesium is excreted by the kidneys, and dosing must be decreased in patients with kidney failure. The most common adverse effect is local burning along the vein upon infusion; small doses of IV lidocaine can remedy this. Hot flashes are also common.45
Various dosing strategies are available. In patients with anxiety, application dosing is based on the recommended preeclampsia IV dose of 4 g diluted in 250 mL of 5% dextrose. Much higher doses may be used in obstetrics. Unlike in obstetrics, for psychiatric indications, magnesium is administered over 60 to 90 minutes. Heart monitoring is recommended.
Scopolamine
Scopolamine is primarily used to relieve nausea, vomiting, and dizziness associated with motion sickness and recovery from anesthesia. It is also used in ophthalmology and in patients with excessive sweating. In off-label and experimental applications, scopolamine has been used in patients with TRD.46
Scopolamine is an anticholinergic medication. It is a nonselective antagonist at muscarinic receptors.47 Tricyclic antidepressants (TCAs) possess strong anticholinergic function. Newer generations of antidepressants were designed specifically not to have this function because it was believed to be an unwanted and potentially dangerous adverse effect. However, data suggest that anticholinergic action is important in decreasing depressive symptoms. Several hypotheses of anticholinergic effects on depression have been published since the 1970s. They include the cholinergic-adrenergic hypothesis,48 acetylcholine predominance relative to adrenergic action hypothesis,49 and insecticide poisoning observations.50 Centrally acting physostigmine (but not peripherally acting neostigmine) was reported to control mania.48,51 A genetic connection between the M2acetylcholine receptor in patients with major depressive disorder (MDD) and alcohol use disorder is also suggestive.52
Continue to: Multiple animal studies show...
Multiple animal studies show direct improvement in mobility and a decrease in despair upon introducing anticholinergic substances.53-55 The cholinergic theory of depression has been studied in several controlled clinical human studies.56,57 Use of a short-acting anticholinergic glycopyrrolate during electroconvulsive therapy (ECT) may contribute to the procedure’s efficacy.
Human research shows scopolamine has a higher efficacy as an antidepressant and anti-anxiety medication in women than in men,58 possibly because estrogen increases the activity of choline acetyltransferase and release of acetylcholine.59,60 M2receptors mediate estrogen influence on the NMDAR, which may explain the anticholinergic effects of depression treatments in women.61
Another proposed mechanism of action of scopolamine is a potent inhibition of the NMDAR.62 Rapid treatments of depression may be based on this mechanism. Examples of such treatments include IV ketamine and sleep deprivation.63 IV scopolamine shows potency in treating MDD and bipolar depression. This treatment should be reserved for patients who do not respond to or are not candidates for other usual treatment modalities of MDD and for the most severe cases. Scopolamine is 30 times more potent than amitriptyline in anticholinergic effect and reportedly produces sustained improvement in MDD.64
Scopolamine has no black-box warnings. It has not been studied in pregnant women and is not recommended for use during pregnancy. Due to possible negative cardiovascular effects, a normal electrocardiogram is required before the start of treatment. Exercise caution in patients with glaucoma, benign prostatic enlargement, gastroparesis, unstable cardiovascular status, or severe renal impairment.
Treatment with scopolamine is not indicated for patients with myasthenia gravis, psychosis, or seizures. Patients must be off potassium for 3 days before beginning scopolamine treatment. Patients should consult with their cardiologist before having a scopolamine infusion. Adverse reactions may include psychosis, tachycardia, seizures, paralytic ileus, and glaucoma exacerbation. The most common adverse effects of scopolamine infusion treatment include drowsiness, dry mouth, blurred vision, lightheadedness, and dizziness. Due to possible drowsiness, operating motor vehicles or heavy machinery must be avoided on the day of treatment.65 Overall, the adverse effects of scopolamine are preventable and manageable, and its antidepressant efficacy is noteworthy.66
Continue to: Treatment typically consists of 3 consecutive infusions...
Treatment typically consists of 3 consecutive infusions of 4 mcg/kg separated by 3 to 5 days.56 It is possible to have a longer treatment course if the patient experiences only partial improvement. Repeated courses or maintenance treatment (similar to ECT maintenance) are utilized in some patients if indicated. Cardiac monitoring is mandatory.
Clomipramine
Clomipramine, a TCA, acts as a preferential inhibitor of 5-hydroxytryptamine uptake and has proven effective in managing depression, TRD, and obsessive-compulsive disorder (OCD).67 Although this medication has reported treatment benefits for patients with phobia, panic disorder,15 chronic pain,68 Tourette syndrome,69 premature ejaculation, anorexia nervosa,70 cataplexy,49 and enuresis,71 it is FDA-approved only for the treatment of OCD.72 Clomipramine may also be beneficial for pain and headache, possibly because of its anti-inflammatory action.73 The anticholinergic effects of clomipramine may add to its efficacy in depression.
The pathophysiology of MDD is connected to hyperactivity of the HPA axis and elevated cortisol levels. Higher clomipramine plasma levels show a linear correlation with lower cortisol secretion and levels, possibly aiding in the treatment of MDD and anxiety.74 The higher the blood level, the more pronounced clomipramine’s therapeutic effect across multiple domains.75
IV infusion of clomipramine produces the highest concentration in the shortest time, but overall, research does not necessarily support increased efficacy of IV over oral administration. There is evidence suggesting that subgroups of patients with severe, treatment-refractory OCD may benefit from IV agents and research suggests a faster onset of action.76 Faster onset of symptom relief is the basis for IV clomipramine use. In patients with OCD, it can take several months for oral medications to produce therapeutic benefits; not all patients can tolerate this. In such scenarios, IV administration may be considered, though it is not appropriate for routine use until more research is available. Patients with treatment-resistant OCD who have exhausted other means of symptom relief may also be candidates for IV treatment.
The adverse effects of IV clomipramine are no different from oral use, though they may be more pronounced. A pretreatment cardiac exam is desirable because clomipramine, like other TCAs, may be cardiotoxic. The anticholinergic adverse effects of TCAs are well known to clinicians77 and partially explained in the scopolamine section of this article. It is not advisable to combine clomipramine with other TCAs or serotonin reuptake inhibitors. Clomipramine also should not be combined with monoamine oxidase inhibitors, though such a combination was reported in medical literature.78 Combination with antiarrhythmics such as lidocaine or opioids such as fentanyl or and tramadol is highly discouraged (fentanyl and tramadol also have serotonergic effects).79
Continue to: Clomipramine for IV use is not commercially available...
Clomipramine for IV use is not commercially available and must be sterilely compounded. The usual course of treatment is a series of 3 infusions: 150 mg on Day 1, 200 mg on Day 2 or Day 3, and 250 mg on Day 3, Day 4, or Day 5, depending on tolerability. A protocol with a 50 mg/d starting dose and titration up to a maximum dose of 225 mg/d over 5 to 7 days has been suggested for inpatient settings.67 Titration to 250 mg is more common.80
A longer series may be performed, but this increases the likelihood of adverse effects. Booster and maintenance treatments are also completed when required. Cardiac monitoring is mandatory.
Vortioxetine and citalopram
IV treatment of depression with
Injections and blocks
Three interventional approaches to treatment that possess psychotherapeutic potential include stellate ganglion blocks (SGBs), glabellar BT injections, and trigger point injections (TPIs). None of these are FDA-approved for psychiatric treatment.
Stellate ganglion blocks
The sympathetic nervous system is involved in autonomic hyperarousal and is at the core of posttraumatic symptomatology.83 Insomnia, anxiety, irritability, hypervigilance, and other excitatory CNS events are connected to the sympathetic nervous system and amygdala activation is commonly observed in those exposed to extreme stress or traumatic events.84
Continue to: SGBs were first performed 100 years ago...
SGBs were first performed 100 years ago and reported to have beneficial psychiatric effects at the end of the 1940s. In 1998 in Finland, improvement of posttraumatic stress disorder (PTSD) symptoms was observed accidentally via thoracic level spine blocks.85 In 2006, cervical level sympathetic blocks were shown to be effective for PTSD symptom control.86 By the end of 2010, Veterans Administration hospitals adopted SGBs to treat veterans with PTSD.87,88 The first multisite, randomized clinical trial of
Since the stellate ganglion is connected to the amygdala, SGB has also been assessed for treating anxiety and depression.89,90 Outside of PTSD, SGBs are used to treat complex regional pain syndrome,91 phantom limb pain, trigeminal neuralgia,92 intractable angina,93 and postherpetic neuralgia in the head, neck, upper chest, or arms.94 The procedure consists of an injection of a local anesthetic through a 25-gauge needle into the stellate sympathetic ganglion at the C6 or C7 vertebral levels. An injection into C6 is considered safer because of specific cervical spine anatomy. Ideally, fluoroscopic guidance or ultrasound is used to guide needle insertion.95
A severe drop in blood pressure may be associated with SGBs and is mitigated by IV hydration. Other adverse effects include red eyes, drooping of the eyelids, nasal congestion, hoarseness, difficulty swallowing, a sensation of a “lump” in the throat, and a sensation of warmth or tingling in the arm or hand. Bilateral SGB is contraindicated due to the danger of respiratory arrest.96
Glabellar BT injections
OnabotulinumtoxinA (BT) injection was first approved for therapeutic use in 1989 for eye muscle disorders such as strabismus97 and blepharospasm.98 It was later approved for several other indications, including cosmetic use, hyperhidrosis, migraine prevention, neurogenic bladder disorder, overactive bladder, urinary incontinence, and spasticity.99-104 BT is used off-label for achalasia and sialorrhea.105,106 Its mechanism of action is primarily attributed to muscle paralysis by blocking presynaptic acetylcholine release into neuromuscular junctions.107
Facial BT injections are usually administered for cosmetic purposes, but smoothing forehead wrinkles and frown lines (the glabellar region of the face) both have antidepressant effects.108 BT injections into the glabellar region also demonstrate antidepressant effects, particularly in patients with comorbid migraines and MDD.109 Early case observations supported the independent benefit of the toxin on MDD when the toxin was injected into the glabellar region.110,111 The most frequent protocol involves injections in the procerus and corrugator muscles.
Continue to: The facial feedback/emotional proprioception hypothesis...
The facial feedback/emotional proprioception hypothesis has dominated thinking about the mood-improving effects of BT. The theory is that blocking muscular expression of sadness (especially in the face) interrupts the experience of sadness; therefore, depression subsides.112,113 However, BT injections in the muscles involved in the smile and an expression of positive emotions (lateral part of the musculus orbicularis oculi) have been associated with increased MDD scores.114 Thus, the mechanism clearly involves more than the cosmetic effect, since facial muscle injections in rats also have antidepressant effects.115
The use of progressive muscle relaxation is well-established in psychiatric treatment. The investigated conditions of increased muscle tone, especially torticollis and blepharospasm, are associated with MDD, and it may be speculated that proprioceptive feedback from the affected muscles may be causally involved in this association.116-118 Activity of the corrugator muscle has been positively associated with increased amygdala activity.119 This suggests a potential similar mechanism to that hypothesized for SGB.
Alternatively, BT is commonly used to treat chronic conditions that may contribute to depression; its success in relieving the underlying problem may indirectly relieve MDD. Thus, in a postmarketing safety evaluation of BT, MDD was demonstrated 40% to 88% less often by patients treated with BT for 6 of the 8 conditions and injection sites, such as in spasms and spasticity of arms and legs, torticollis and neck pain, and axilla and palm injections for hyperhidrosis. In a parotid and submandibular glands BT injection subcohort, no patients experienced depressive symptoms.120
Medicinal BT is generally considered safe. The most common adverse effects are hypersensitivity, injection site reactions, and other adverse effects specific to the injection site.121 Additionally, the cosmetic effects are transient, given the nature of the medication.
Trigger point injections
TPIs in the neck and shoulders are frequently used to treat tension headaches and various referred pain locations in the face and arms. Tension and depression frequently overlap in clinical practice.122 Relieving muscle tension (with resulting trigger points) improves muscle function and mood.
Continue to: The higher the number of active...
The higher the number of active trigger points (TPs), the greater the physical burden of headache and the higher the anxiety level. Gender differences in TP prevalence and TPI efficacy have been described in the literature. For example, the number of active TPs seems directly associated with anxiety levels in women but not in men.123 Although TPs appear to be more closely associated with anxiety than depression,124 depression associated with muscle tension does improve with TPIs. European studies have demonstrated a decrease in scores on the Hamilton Depression Rating Scale and Hamilton Anxiety Rating Scale following TPI treatment.125 The effect may be indirect, as when a patient’s pain is relieved, sleep and other psychiatric symptoms improve.126
A randomized controlled trial by Castro Sánchez et al127 demonstrated that dry needling therapy in patients with fibromyalgia syndrome (FMS) showed improvements in pain pressure thresholds, body pain, vitality, and social function, as well as total FMS symptoms, quality of sleep, anxiety, hospital anxiety and depression, general pain intensity, and fatigue.
Myofascial pain syndrome, catastrophizing, and muscle tension are common in patients with depression, anxiety, and somatization. Local TPI therapy aimed at inactivating pain generators is supported by moderate quality evidence. All manner of therapies have been described, including injection of saline, corticosteroids, local anesthetic agents, and dry needling. BT injections in chronic TPs are also practiced, though no specific injection therapy has been reliably shown to be more advantageous than another. The benefits of TPIs may be derived from the needle itself rather than from any specific substance injected. Stimulation of a local twitch response with direct needling of the TP appears of importance. There is no established consensus regarding the number of injection points, frequency of administration, and volume or type of injectate.128
Adverse effects of TPIs relate to the nature of the invasive therapy, with the risk of tissue damage and bleeding. Pneumothorax risk is present with needle insertion at the neck and thorax.129 Patients with diabetes may experience variations in blood sugar control if steroids are used.
Bottom Line
Interventional treatment modalities that may have a role in psychiatric treatment include IV administration of ketamine, aducanumab, lecanemab, brexanolone, magnesium, scopolamine, and clomipramine. Other interventional approaches include stellate ganglion blocks, glabellar botulinum toxin injections, and trigger point injections.
Related Resources
- Dokucu ME, Janicak PG. Nontraditional therapies for treatment-resistant depression. Current Psychiatry. 2021; 20(9):38-43,49. doi:10.12788/cp.0166
- Kim J, Khoury R, Grossberg GT. Botulinum toxin: emerging psychiatric indications. Current Psychiatry. 2018;17(12):8-18.
Drug Brand Names
Aducanumab • Aduhelm
Aripiprazole • Abilify
Aripiprazole lauroxil • Aristada
Brexanolone • Zulresso
Buprenorphine • Sublocade
Citalopram • Celexa
Clomipramine • Anafranil
Diazepam • Valium
Droperidol • Inapsine
Esketamine • Spravato
Fentanyl • Actiq
Fluphenazine decanoate • Modecate
Fluphenazine hydrochloride • Prolixin
Haloperidol decanoate • Haldol decanoate
Haloperidol lactate • Haldol
Ketamine • Ketalar
Lecanemab • Leqembi
Lidocaine • Xylocaine
Lorazepam • Ativan
Loxapine inhaled • Adasuve
Naltrexone • Vivitrol
Magnesium sulfate • Sulfamag
Midazolam • Versed
Olanzapine • Zyprexa
OnabotulinumtoxinA injection • Botox
Paliperidone • Invega Hafyera, Invega Sustenna, Invega Trinza
Rapamycin • Rapamune, Sirolimus
Risperidone • Perseris
Risperidone microspheres • Risperdal Consta, Rykindo
Scopolamine • Hyoscine
Tramadol • Conzip
Vortioxetine • Trintellix
Ziprasidone • Geodon
Advances in the understanding of neurobiological and neuropsychiatric pathophysiology have opened new avenues of treatment for psychiatric patients. Historically, with a few exceptions, most psychiatric medications have been administered orally. However, many of the newer treatments require some form of specialized administration because they cannot be taken orally due to their chemical property (such as aducanumab); because there is the need to produce stable blood levels of the medication (such as brexanolone); because oral administration greatly diminished efficacy (such as oral vs IV magnesium or scopolamine), or because the treatment is focused on specific brain structures. This need for specialized administration has created a subspecialty called interventional psychiatry.
Part 1 of this 2-part article provides an overview of 1 type of interventional psychiatry: parenterally administered medications, including those administered via IV. We also describe 3 other interventional approaches to treatment: stellate ganglion blocks, glabellar botulinum toxin (BT) injections, and trigger point injections. In Part 2 we will review interventional approaches that involve neuromodulation.
Parenteral medications in psychiatry
In general, IV and IM medications can be more potent that oral medications due to their overall faster onset of action and higher blood concentrations. These more invasive forms of administration can have significant limitations, such as a risk of infection at the injection site, the need to be administered in a medical setting, additional time, and patient discomfort.

Table 1 lists short-acting injectable medications used in psychiatry, and Table 2 lists long-acting injectable medications. Parenteral administration of antipsychotics is performed to alleviate acute agitation or for chronic symptom control. These medications generally are not considered interventional treatments, but could be classified as such due to their invasive nature.1 Furthermore, inhalable loxapine—which is indicated for managing acute agitation—requires a Risk Evaluation and Mitigation Strategy program consisting of 2 hours observation and monitoring of respiratory status.2,3 Other indications for parenteral treatments include IM naltrexone extended release4 and subcutaneous injections of buprenorphine extended release5 and risperidone.6
IV administration
Most IV treatments described in this article are not FDA-approved for psychiatric treatment. Despite this, many interventional psychiatric treatments are part of clinical practice. IV infusion of ketamine is the most widely known and most researched of these. Table 3 lists other IV treatments that could be used as psychiatric treatment.
Ketamine
Since the early 1960s, ketamine has been used as a surgical anesthetic for animals. In the United States, it was approved for human surgical anesthesia in 1970. It was widely used during the Vietnam War due to its lack of inhibition of respiratory drive; medical staff first noticed an improvement in depressive symptoms and the resolution of suicidal ideation in patients who received ketamine. This led to further research on ketamine, particularly to determine its application in treatment-resistant depression (TRD) and other conditions.7 IV ketamine administration is most widely researched, but IM injections, intranasal sprays, and lozenges are also available. The dissociative properties of ketamine have led to its recreational use.8
Hypotheses for the mechanism of action of ketamine as an antidepressant include direct synaptic or extrasynaptic (GluN2B-selective), N-methyl-
Continue to: Ketamine is a schedule...
Ketamine is a schedule III medication with addictive properties. Delirium, panic attacks, hallucinations, nightmares, dysphoria, and paranoia may occur during and after use.13 Premedication with benzodiazepines, most notably lorazepam, is occasionally used to minimize ketamine’s adverse effects, but this generally is not recommended because doing so reduces ketamine’s antidepressant effects.14 Driving and operating heavy machinery is contraindicated after IV infusion. The usual protocol involves an IV infusion of ketamine 0.4 mg/kg to 1 mg/kg dosing over 1 hour. Doses between 0.4 mg/kg and 0.6 mg/kg are most common. Ketamine has a therapeutic window; doses >0.5 mg/kg are progressively less effective.15 Unlike the recommendation after esketamine administration, after receiving ketamine, patients remain in the care of their treatment team for <2 hours.
Esketamine, the S enantiomer of ketamine, was FDA-approved for TRD as an intranasal formulation. Esketamine is more commonly used than IV ketamine because it is FDA-approved and typically covered by insurance, but it may not be as effective.16 An economic analysis by Brendle et al17 suggested insurance companies would lower costs if they covered ketamine infusions vs intranasal esketamine.
Aducanumab and lecanemab
The most recent FDA-approved interventional agents are aducanumab and lecanemab, which are indicated for treating Alzheimer disease.18,19 Both are human monoclonal antibodies that bind selectively and with high affinity to amyloid beta plaque aggregates and promote their removal by Fc receptor–mediated phagocytosis.20
FDA approval of aducanumab and lecanemab was controversial. Initially, aducanumab’s safety monitoring board performed a futility analysis that suggested aducanumab was unlikely to separate from placebo, and the research was stopped.21 The manufacturer petitioned the FDA to consider the medication for accelerated approval on the basis of biomarker data showing that amyloid beta plaque aggregates become smaller. Current FDA approval is temporary to allow patients access to this potentially beneficial agent, but the manufacturer must supply clinical evidence that the reduction of amyloid beta plaques is associated with desirable changes in the course of Alzheimer disease, or risk losing the approval.
Lecanemab is also a human monoclonal antibody intended to remove amyloid beta plaques that was FDA-approved under the accelerated approval pathway.22 Unlike aducanumab, lecanemab demonstrated a statistically significant (although clinically imperceptible) reduction in the rate of cognitive decline; it did not show cognitive improvement.23 Lecanemab also significantly reduced amyloid beta plaques.23
Continue to: Aducanumab and lecanemab are generally...
Aducanumab and lecanemab are generally not covered by insurance and typically cost >$26,000 annually. Both are administered by IV infusion once a month. More monoclonal antibody medications for treating early Alzheimer disease are in the late stages of development, most notably donanebab.24 Observations during clinical trials found that in the later stages of Alzheimer disease, forceful removal of plaques by the autoimmune process damages neurons, while in less dense deposits of early dementia such removal is not harmful to the cells and prevents amyloid buildup.
Brexanolone is an aqueous formulation of allopregnanolone, a major metabolite of progesterone and a positive allosteric modulator of GABA-A receptors.25 Its levels are maximal at the end of the third trimester of pregnancy and fall rapidly following delivery. Research showed a 3-day infusion was rapidly and significantly effective for treating postpartum depression26 and brexanolone received FDA approval for this indication in March 2019.27 However, various administrative, economic, insurance, and other hurdles make it difficult for patients to access this treatment. Despite its rapid onset of action (usually 48 hours), brexanolone takes an average of 15 days to go through the prior authorization process.28 In addition to the need for prior authorization, the main impediment to the use of brexanolone is the 3-day infusion schedule, which greatly magnifies the cost but is partially circumvented by the availability of dedicated outpatient centers.
Magnesium
Magnesium is on the World Health Organization’s Model List of Essential Medicines.29 There has been extensive research on the use of magnesium sulfate for psychiatric indications, especially for depression.30 Magnesium functions similarly to calcium channel blockers by competitively blocking intracellular calcium channels, decreasing calcium availability, and inhibiting smooth muscle contractility.31 It also competes with calcium at the motor end plate, reducing excitation by inhibiting the release of acetylcholine.32 This property is used for high-dose IV magnesium treatment of impending preterm labor in obstetrics. Magnesium sulfate is the drug of choice in treating eclamptic seizures and preventing seizures in severe preeclampsia or gestational hypertension with severe features.33 It is also used to treat torsade de pointes, severe asthma exacerbations, constipation, and barium poisoning.34 Beneficial use in asthma treatment35 and the treatment of migraine36 have also been reported.
IV magnesium in myocardial infarction may be harmful,37 though outside of acute cardiac events, magnesium is found to be safe.38
Oral magnesium sulfate is a common over-the-counter anxiety remedy. As a general cell stabilizer (mediated by the reduction of intracellular calcium), magnesium is potentially beneficial outside of its muscle-relaxing properties, although muscle relaxing can benefit anxious patients. It is used to treat anxiety,39 alcohol withdrawal,40 and fear.41 Low magnesium blood levels are found in patients with depression, schizophrenia,42 and attention-deficit/hyperactivity disorder.43 However, it is important to note that the therapeutic effect of magnesium when treating anxiety and headache is independent of preinfusion magnesium blood levels.43
Continue to: The efficacy of oral magnesium...
The efficacy of oral magnesium is not robust. However, IV administration has a pronounced beneficial effect as an abortive and preventative treatment in many patients with anxiety.44
IV administration of magnesium can produce adverse effects, including flushing, sweating, hypotension, depressed reflexes, flaccid paralysis, hypothermia, circulatory collapse, and cardiac and CNS depression. These complications are very rare and dose-dependent.45 Magnesium is excreted by the kidneys, and dosing must be decreased in patients with kidney failure. The most common adverse effect is local burning along the vein upon infusion; small doses of IV lidocaine can remedy this. Hot flashes are also common.45
Various dosing strategies are available. In patients with anxiety, application dosing is based on the recommended preeclampsia IV dose of 4 g diluted in 250 mL of 5% dextrose. Much higher doses may be used in obstetrics. Unlike in obstetrics, for psychiatric indications, magnesium is administered over 60 to 90 minutes. Heart monitoring is recommended.
Scopolamine
Scopolamine is primarily used to relieve nausea, vomiting, and dizziness associated with motion sickness and recovery from anesthesia. It is also used in ophthalmology and in patients with excessive sweating. In off-label and experimental applications, scopolamine has been used in patients with TRD.46
Scopolamine is an anticholinergic medication. It is a nonselective antagonist at muscarinic receptors.47 Tricyclic antidepressants (TCAs) possess strong anticholinergic function. Newer generations of antidepressants were designed specifically not to have this function because it was believed to be an unwanted and potentially dangerous adverse effect. However, data suggest that anticholinergic action is important in decreasing depressive symptoms. Several hypotheses of anticholinergic effects on depression have been published since the 1970s. They include the cholinergic-adrenergic hypothesis,48 acetylcholine predominance relative to adrenergic action hypothesis,49 and insecticide poisoning observations.50 Centrally acting physostigmine (but not peripherally acting neostigmine) was reported to control mania.48,51 A genetic connection between the M2acetylcholine receptor in patients with major depressive disorder (MDD) and alcohol use disorder is also suggestive.52
Continue to: Multiple animal studies show...
Multiple animal studies show direct improvement in mobility and a decrease in despair upon introducing anticholinergic substances.53-55 The cholinergic theory of depression has been studied in several controlled clinical human studies.56,57 Use of a short-acting anticholinergic glycopyrrolate during electroconvulsive therapy (ECT) may contribute to the procedure’s efficacy.
Human research shows scopolamine has a higher efficacy as an antidepressant and anti-anxiety medication in women than in men,58 possibly because estrogen increases the activity of choline acetyltransferase and release of acetylcholine.59,60 M2receptors mediate estrogen influence on the NMDAR, which may explain the anticholinergic effects of depression treatments in women.61
Another proposed mechanism of action of scopolamine is a potent inhibition of the NMDAR.62 Rapid treatments of depression may be based on this mechanism. Examples of such treatments include IV ketamine and sleep deprivation.63 IV scopolamine shows potency in treating MDD and bipolar depression. This treatment should be reserved for patients who do not respond to or are not candidates for other usual treatment modalities of MDD and for the most severe cases. Scopolamine is 30 times more potent than amitriptyline in anticholinergic effect and reportedly produces sustained improvement in MDD.64
Scopolamine has no black-box warnings. It has not been studied in pregnant women and is not recommended for use during pregnancy. Due to possible negative cardiovascular effects, a normal electrocardiogram is required before the start of treatment. Exercise caution in patients with glaucoma, benign prostatic enlargement, gastroparesis, unstable cardiovascular status, or severe renal impairment.
Treatment with scopolamine is not indicated for patients with myasthenia gravis, psychosis, or seizures. Patients must be off potassium for 3 days before beginning scopolamine treatment. Patients should consult with their cardiologist before having a scopolamine infusion. Adverse reactions may include psychosis, tachycardia, seizures, paralytic ileus, and glaucoma exacerbation. The most common adverse effects of scopolamine infusion treatment include drowsiness, dry mouth, blurred vision, lightheadedness, and dizziness. Due to possible drowsiness, operating motor vehicles or heavy machinery must be avoided on the day of treatment.65 Overall, the adverse effects of scopolamine are preventable and manageable, and its antidepressant efficacy is noteworthy.66
Continue to: Treatment typically consists of 3 consecutive infusions...
Treatment typically consists of 3 consecutive infusions of 4 mcg/kg separated by 3 to 5 days.56 It is possible to have a longer treatment course if the patient experiences only partial improvement. Repeated courses or maintenance treatment (similar to ECT maintenance) are utilized in some patients if indicated. Cardiac monitoring is mandatory.
Clomipramine
Clomipramine, a TCA, acts as a preferential inhibitor of 5-hydroxytryptamine uptake and has proven effective in managing depression, TRD, and obsessive-compulsive disorder (OCD).67 Although this medication has reported treatment benefits for patients with phobia, panic disorder,15 chronic pain,68 Tourette syndrome,69 premature ejaculation, anorexia nervosa,70 cataplexy,49 and enuresis,71 it is FDA-approved only for the treatment of OCD.72 Clomipramine may also be beneficial for pain and headache, possibly because of its anti-inflammatory action.73 The anticholinergic effects of clomipramine may add to its efficacy in depression.
The pathophysiology of MDD is connected to hyperactivity of the HPA axis and elevated cortisol levels. Higher clomipramine plasma levels show a linear correlation with lower cortisol secretion and levels, possibly aiding in the treatment of MDD and anxiety.74 The higher the blood level, the more pronounced clomipramine’s therapeutic effect across multiple domains.75
IV infusion of clomipramine produces the highest concentration in the shortest time, but overall, research does not necessarily support increased efficacy of IV over oral administration. There is evidence suggesting that subgroups of patients with severe, treatment-refractory OCD may benefit from IV agents and research suggests a faster onset of action.76 Faster onset of symptom relief is the basis for IV clomipramine use. In patients with OCD, it can take several months for oral medications to produce therapeutic benefits; not all patients can tolerate this. In such scenarios, IV administration may be considered, though it is not appropriate for routine use until more research is available. Patients with treatment-resistant OCD who have exhausted other means of symptom relief may also be candidates for IV treatment.
The adverse effects of IV clomipramine are no different from oral use, though they may be more pronounced. A pretreatment cardiac exam is desirable because clomipramine, like other TCAs, may be cardiotoxic. The anticholinergic adverse effects of TCAs are well known to clinicians77 and partially explained in the scopolamine section of this article. It is not advisable to combine clomipramine with other TCAs or serotonin reuptake inhibitors. Clomipramine also should not be combined with monoamine oxidase inhibitors, though such a combination was reported in medical literature.78 Combination with antiarrhythmics such as lidocaine or opioids such as fentanyl or and tramadol is highly discouraged (fentanyl and tramadol also have serotonergic effects).79
Continue to: Clomipramine for IV use is not commercially available...
Clomipramine for IV use is not commercially available and must be sterilely compounded. The usual course of treatment is a series of 3 infusions: 150 mg on Day 1, 200 mg on Day 2 or Day 3, and 250 mg on Day 3, Day 4, or Day 5, depending on tolerability. A protocol with a 50 mg/d starting dose and titration up to a maximum dose of 225 mg/d over 5 to 7 days has been suggested for inpatient settings.67 Titration to 250 mg is more common.80
A longer series may be performed, but this increases the likelihood of adverse effects. Booster and maintenance treatments are also completed when required. Cardiac monitoring is mandatory.
Vortioxetine and citalopram
IV treatment of depression with
Injections and blocks
Three interventional approaches to treatment that possess psychotherapeutic potential include stellate ganglion blocks (SGBs), glabellar BT injections, and trigger point injections (TPIs). None of these are FDA-approved for psychiatric treatment.
Stellate ganglion blocks
The sympathetic nervous system is involved in autonomic hyperarousal and is at the core of posttraumatic symptomatology.83 Insomnia, anxiety, irritability, hypervigilance, and other excitatory CNS events are connected to the sympathetic nervous system and amygdala activation is commonly observed in those exposed to extreme stress or traumatic events.84
Continue to: SGBs were first performed 100 years ago...
SGBs were first performed 100 years ago and reported to have beneficial psychiatric effects at the end of the 1940s. In 1998 in Finland, improvement of posttraumatic stress disorder (PTSD) symptoms was observed accidentally via thoracic level spine blocks.85 In 2006, cervical level sympathetic blocks were shown to be effective for PTSD symptom control.86 By the end of 2010, Veterans Administration hospitals adopted SGBs to treat veterans with PTSD.87,88 The first multisite, randomized clinical trial of
Since the stellate ganglion is connected to the amygdala, SGB has also been assessed for treating anxiety and depression.89,90 Outside of PTSD, SGBs are used to treat complex regional pain syndrome,91 phantom limb pain, trigeminal neuralgia,92 intractable angina,93 and postherpetic neuralgia in the head, neck, upper chest, or arms.94 The procedure consists of an injection of a local anesthetic through a 25-gauge needle into the stellate sympathetic ganglion at the C6 or C7 vertebral levels. An injection into C6 is considered safer because of specific cervical spine anatomy. Ideally, fluoroscopic guidance or ultrasound is used to guide needle insertion.95
A severe drop in blood pressure may be associated with SGBs and is mitigated by IV hydration. Other adverse effects include red eyes, drooping of the eyelids, nasal congestion, hoarseness, difficulty swallowing, a sensation of a “lump” in the throat, and a sensation of warmth or tingling in the arm or hand. Bilateral SGB is contraindicated due to the danger of respiratory arrest.96
Glabellar BT injections
OnabotulinumtoxinA (BT) injection was first approved for therapeutic use in 1989 for eye muscle disorders such as strabismus97 and blepharospasm.98 It was later approved for several other indications, including cosmetic use, hyperhidrosis, migraine prevention, neurogenic bladder disorder, overactive bladder, urinary incontinence, and spasticity.99-104 BT is used off-label for achalasia and sialorrhea.105,106 Its mechanism of action is primarily attributed to muscle paralysis by blocking presynaptic acetylcholine release into neuromuscular junctions.107
Facial BT injections are usually administered for cosmetic purposes, but smoothing forehead wrinkles and frown lines (the glabellar region of the face) both have antidepressant effects.108 BT injections into the glabellar region also demonstrate antidepressant effects, particularly in patients with comorbid migraines and MDD.109 Early case observations supported the independent benefit of the toxin on MDD when the toxin was injected into the glabellar region.110,111 The most frequent protocol involves injections in the procerus and corrugator muscles.
Continue to: The facial feedback/emotional proprioception hypothesis...
The facial feedback/emotional proprioception hypothesis has dominated thinking about the mood-improving effects of BT. The theory is that blocking muscular expression of sadness (especially in the face) interrupts the experience of sadness; therefore, depression subsides.112,113 However, BT injections in the muscles involved in the smile and an expression of positive emotions (lateral part of the musculus orbicularis oculi) have been associated with increased MDD scores.114 Thus, the mechanism clearly involves more than the cosmetic effect, since facial muscle injections in rats also have antidepressant effects.115
The use of progressive muscle relaxation is well-established in psychiatric treatment. The investigated conditions of increased muscle tone, especially torticollis and blepharospasm, are associated with MDD, and it may be speculated that proprioceptive feedback from the affected muscles may be causally involved in this association.116-118 Activity of the corrugator muscle has been positively associated with increased amygdala activity.119 This suggests a potential similar mechanism to that hypothesized for SGB.
Alternatively, BT is commonly used to treat chronic conditions that may contribute to depression; its success in relieving the underlying problem may indirectly relieve MDD. Thus, in a postmarketing safety evaluation of BT, MDD was demonstrated 40% to 88% less often by patients treated with BT for 6 of the 8 conditions and injection sites, such as in spasms and spasticity of arms and legs, torticollis and neck pain, and axilla and palm injections for hyperhidrosis. In a parotid and submandibular glands BT injection subcohort, no patients experienced depressive symptoms.120
Medicinal BT is generally considered safe. The most common adverse effects are hypersensitivity, injection site reactions, and other adverse effects specific to the injection site.121 Additionally, the cosmetic effects are transient, given the nature of the medication.
Trigger point injections
TPIs in the neck and shoulders are frequently used to treat tension headaches and various referred pain locations in the face and arms. Tension and depression frequently overlap in clinical practice.122 Relieving muscle tension (with resulting trigger points) improves muscle function and mood.
Continue to: The higher the number of active...
The higher the number of active trigger points (TPs), the greater the physical burden of headache and the higher the anxiety level. Gender differences in TP prevalence and TPI efficacy have been described in the literature. For example, the number of active TPs seems directly associated with anxiety levels in women but not in men.123 Although TPs appear to be more closely associated with anxiety than depression,124 depression associated with muscle tension does improve with TPIs. European studies have demonstrated a decrease in scores on the Hamilton Depression Rating Scale and Hamilton Anxiety Rating Scale following TPI treatment.125 The effect may be indirect, as when a patient’s pain is relieved, sleep and other psychiatric symptoms improve.126
A randomized controlled trial by Castro Sánchez et al127 demonstrated that dry needling therapy in patients with fibromyalgia syndrome (FMS) showed improvements in pain pressure thresholds, body pain, vitality, and social function, as well as total FMS symptoms, quality of sleep, anxiety, hospital anxiety and depression, general pain intensity, and fatigue.
Myofascial pain syndrome, catastrophizing, and muscle tension are common in patients with depression, anxiety, and somatization. Local TPI therapy aimed at inactivating pain generators is supported by moderate quality evidence. All manner of therapies have been described, including injection of saline, corticosteroids, local anesthetic agents, and dry needling. BT injections in chronic TPs are also practiced, though no specific injection therapy has been reliably shown to be more advantageous than another. The benefits of TPIs may be derived from the needle itself rather than from any specific substance injected. Stimulation of a local twitch response with direct needling of the TP appears of importance. There is no established consensus regarding the number of injection points, frequency of administration, and volume or type of injectate.128
Adverse effects of TPIs relate to the nature of the invasive therapy, with the risk of tissue damage and bleeding. Pneumothorax risk is present with needle insertion at the neck and thorax.129 Patients with diabetes may experience variations in blood sugar control if steroids are used.
Bottom Line
Interventional treatment modalities that may have a role in psychiatric treatment include IV administration of ketamine, aducanumab, lecanemab, brexanolone, magnesium, scopolamine, and clomipramine. Other interventional approaches include stellate ganglion blocks, glabellar botulinum toxin injections, and trigger point injections.
Related Resources
- Dokucu ME, Janicak PG. Nontraditional therapies for treatment-resistant depression. Current Psychiatry. 2021; 20(9):38-43,49. doi:10.12788/cp.0166
- Kim J, Khoury R, Grossberg GT. Botulinum toxin: emerging psychiatric indications. Current Psychiatry. 2018;17(12):8-18.
Drug Brand Names
Aducanumab • Aduhelm
Aripiprazole • Abilify
Aripiprazole lauroxil • Aristada
Brexanolone • Zulresso
Buprenorphine • Sublocade
Citalopram • Celexa
Clomipramine • Anafranil
Diazepam • Valium
Droperidol • Inapsine
Esketamine • Spravato
Fentanyl • Actiq
Fluphenazine decanoate • Modecate
Fluphenazine hydrochloride • Prolixin
Haloperidol decanoate • Haldol decanoate
Haloperidol lactate • Haldol
Ketamine • Ketalar
Lecanemab • Leqembi
Lidocaine • Xylocaine
Lorazepam • Ativan
Loxapine inhaled • Adasuve
Naltrexone • Vivitrol
Magnesium sulfate • Sulfamag
Midazolam • Versed
Olanzapine • Zyprexa
OnabotulinumtoxinA injection • Botox
Paliperidone • Invega Hafyera, Invega Sustenna, Invega Trinza
Rapamycin • Rapamune, Sirolimus
Risperidone • Perseris
Risperidone microspheres • Risperdal Consta, Rykindo
Scopolamine • Hyoscine
Tramadol • Conzip
Vortioxetine • Trintellix
Ziprasidone • Geodon
1. Vincent KM, Ryan M, Palmer E, et al. Interventional psychiatry. Postgrad Med. 2020;132(7):573-574.
2. Allen MH, Feifel D, Lesem MD, et al. Efficacy and safety of loxapine for inhalation in the treatment of agitation in patients with schizophrenia: a randomized, double-blind, placebo-controlled trial. J Clin Psychiatry. 2011;72(10):1313-1321.
3. Kwentus J, Riesenberg RA, Marandi M, et al. Rapid acute treatment of agitation in patients with bipolar I disorder: a multicenter, randomized, placebo-controlled clinical trial with inhaled loxapine. Bipolar Disord. 2012;14(1):31-40.
4. Lee JD, Nunes EV Jr, Novo P, et al. Comparative effectiveness of extended-release naltrexone versus buprenorphine-naloxone for opioid relapse prevention (X:BOT): a multicentre, open-label, randomised controlled trial. Lancet. 2018;391(10118):309-318.
5. Haight BR, Learned SM, Laffont CM, et al. Efficacy and safety of a monthly buprenorphine depot injection for opioid use disorder: a multicentre, randomised, double‐blind, placebo‐controlled, phase 3 trial. Lancet. 2019;393(10173):778-790.
6. Andorn A, Graham J, Csernansky J, et al. Monthly extended-release risperidone (RBP-7000) in the treatment of schizophrenia: results from the phase 3 program. J Clin Psychopharmacol. 2019;39(5):428-433.
7. Dundee TW. Twenty-five years of ketamine. A report of an international meeting. Anaesthesia. 1990;45(2):159. doi:10.1111/j.1365-2044.1990.tb14287.x
8. White PF, Way WL, Trevor AJ. Ketamine--its pharmacology and therapeutic uses. Anesthesiology. 1982;56(2):119-136. doi:10.1097/00000542-198202000-00007
9. Zanos P, Gould TD. Mechanisms of ketamine action as an antidepressant. Mol Psychiatry. 2018;23(4):801-811.
10. Molero P, Ramos-Quiroga JA, Martin-Santos R, et al. Antidepressant efficacy and tolerability of ketamine and esketamine: a critical review. CNS Drugs. 2018;32(5):411-420. doi:10.1007/s40263-018-0519-3
11. Williams NR, Heifets BD, Blasey C, et al. Attenuation of antidepressant effects of ketamine by opioid receptor antagonism. Am J Psychiatry. 2018;175(12):1205-1215.
12. Witkin JM, Martin AE, Golani LK, et al. Rapid-acting antidepressants. Adv Pharmacol. 2019;86:47-96.
13. Strayer RJ, Nelson LS. Adverse events associated with ketamine for procedural sedation in adults. Am J Emerg Med. 2008;26(9):985-1028. doi:10.1016/j.ajem.2007.12.005
14. Frye MA, Blier P, Tye SJ. Concomitant benzodiazepine use attenuates ketamine response: implications for large scale study design and clinical development. J Clin Psychopharmacol. 2015;35(3):334-336.
15. Fava M, Freeman MP, Flynn M, et al. Double-blind, placebo-controlled, dose-ranging trial of intravenous ketamine as adjunctive therapy in treatment-resistant depression (TRD). Mol Psychiatry. 2020;25(7):1592-1603.
16. Bahji A, Vazquez GH, Zarate CA Jr. Comparative efficacy of racemic ketamine and esketamine for depression: a systematic review and meta-analysis. J Affect Disord. 2021;278:542-555. Erratum in: J Affect Disord. 2021;281:1001.
17. Brendle M, Robison R, Malone DC. Cost-effectiveness of esketamine nasal spray compared to intravenous ketamine for patients with treatment-resistant depression in the US utilizing clinical trial efficacy and real-world effectiveness estimates. J Affect Disord. 2022;319:388-396.
18. Dhillon S. Aducanumab: first approval. Drugs. 2021;81(12):1437-1443. Erratum in: Drugs. 2021;81(14):1701.
19. van Dyck CH, Swanson CJ, Aisen P, et al. Lecanemab in early Alzheimer’s disease. N Engl J Med. 2023;388(1):9-21. doi:10.1056/NEJMoa2212948
20. Sevigny J, Chiao P, Bussière T, et al. The antibody aducanumab reduces Aβ plaques in Alzheimer’s disease. Nature. 2016;537(7618):50-56. Update in: Nature. 2017;546(7659):564.
21. Fillit H, Green A. Aducanumab and the FDA – where are we now? Nat Rev Neurol. 2021;17(3):129-130.
22. Reardon S. FDA approves Alzheimer’s drug lecanemab amid safety concerns. Nature. 2023;613(7943):227-228. doi:10.1038/d41586-023-00030-3
23. McDade E, Cummings JL, Dhadda S, et al. Lecanemab in patients with early Alzheimer’s disease: detailed results on biomarker, cognitive, and clinical effects from the randomized and open-label extension of the phase 2 proof-of-concept study. Alzheimers Res Ther. 2022;14(1):191. doi:10.1186/s13195-022-01124-2
24. Mintun MA, Lo AC, Evans CD, et al. Donanemab in early Alzheimer’s disease. N Engl J Med. 2021;384(18):1691-1704.
25. Luisi S, Petraglia F, Benedetto C, et al. Serum allopregnanolone levels in pregnant women: changes during pregnancy, at delivery, and in hypertensive patients. J Clin Endocrinol Metab. 2000;85(7):2429-2433.
26. Meltzer-Brody S, Colquhoun H, Riesenberg R, et al. Brexanolone injection in post-partum depression: two multicentre, double-blind, randomised, placebo-controlled, phase 3 trials. Lancet. 2018;392(10152):1058-1070.
27. Powell JG, Garland S, Preston K, et al. Brexanolone (Zulresso): finally, an FDA-approved treatment for postpartum depression. Ann Pharmacother. 2020;54(2):157-163.
28. Patterson R, Krohn H, Richardson E, et al. A brexanolone treatment program at an academic medical center: patient selection, 90-day posttreatment outcomes, and lessons learned. J Acad Consult Liaison Psychiatry. 2022;63(1):14-22.
29. World Health Organization. WHO model list of essential medicines - 22nd list (2021). World Health Organization. September 30, 2021. Accessed April 7, 2023. https://www.who.int/publications/i/item/WHO-MHP-HPS-EML-2021.02
30. Eby GA, Eby KL, Mruk H. Magnesium and major depression. In: Vink R, Nechifor M, eds. Magnesium in the Central Nervous System. University of Adelaide Press; 2011.
31. Plant TM, Zeleznik AJ. Knobil and Neill’s Physiology of Reproduction. 4th ed. Elsevier Inc.; 2015:2503-2550.
32. Sidebotham D, Le Grice IJ. Physiology and pathophysiology. In: Sidebotham D, McKee A, Gillham M, Levy J. Cardiothoracic Critical Care. Elsevier, Inc.; 2007:3-27.
33. Duley L, Gülmezoglu AM, Henderson-Smart DJ, et al. Magnesium sulphate and other anticonvulsants for women with pre-eclampsia. Cochrane Database Syst Rev. 2010;2010(11):CD000025.
34. Emergency supply of medicines. In: British National Formulary. British Medical Association, Royal Pharmaceutical Society; 2015:6. Accessed April 7, 2023. https://www.academia.edu/35076015/british_national_formulary_2015_pdf
35. Kwofie K, Wolfson AB. Intravenous magnesium sulfate for acute asthma exacerbation in children and adults. Am Fam Physician. 2021;103(4):245-246.
36. Patniyot IR, Gelfand AA. Acute treatment therapies for pediatric migraine: a qualitative systematic review. Headache. 2016;56(1):49-70.
37. Wang X, Du X, Yang H, et al. Use of intravenous magnesium sulfate among patients with acute myocardial infarction in China from 2001 to 2015: China PEACE-Retrospective AMI Study. BMJ Open. 2020;10(3):e033269.
38. Karhu E, Atlas SE, Jinrun G, et al. Intravenous infusion of magnesium sulfate is not associated with cardiovascular, liver, kidney, and metabolic toxicity in adults. J Clin Transl Res. 2018;4(1):47-55.
39. Noah L, Pickering G, Mazur A, et al. Impact of magnesium supplementation, in combination with vitamin B6, on stress and magnesium status: secondary data from a randomized controlled trial. Magnes Res. 2020;33(3):45-57.
40. Erstad BL, Cotugno CL. Management of alcohol withdrawal. Am J Health Syst Pharm. 1995;52(7):697-709.
41. Abumaria N, Luo L, Ahn M, et al. Magnesium supplement enhances spatial-context pattern separation and prevents fear overgeneralization. Behav Pharmacol. 2013;24(4):255-263.
42. Kirov GK, Tsachev KN. Magnesium, schizophrenia and manic-depressive disease. Neuropsychobiology. 1990;23(2):79-81.
43. Botturi A, Ciappolino V, Delvecchio G, et al. The role and the effect of magnesium in mental disorders: a systematic review. Nutrients. 2020;12(6):1661.
44. Kirkland AE, Sarlo GL, Holton KF. The role of magnesium in neurological disorders. Nutrients. 2018;10(6):730.
45. Magnesium sulfate intravenous side effects by likelihood and severity. WebMD. Accessed April 9, 2023. https://www.webmd.com/drugs/2/drug-149570/magnesium-sulfate-intravenous/details/list-sideeffects
46. Scopolamine base transdermal system – uses, side effects, and more. WebMD. Accessed April 9, 2023. https://www.webmd.com/drugs/2/drug-14032/scopolamine-transdermal/details
47. Bolden C, Cusack B, Richelson E. Antagonism by antimuscarinic and neuroleptic compounds at the five cloned human muscarinic cholinergic receptors expressed in Chinese hamster ovary cells. J Pharmacol Exp Ther. 1992;260(2):576-580.
48. Janowsky DS, el-Yousef MK, Davis JM, et al. A cholinergic-adrenergic hypothesis of mania and depression. Lancet. 1972;2(7778):632-635.
49. Janowsky DS, Risch SC, Gillin JC. Adrenergic-cholinergic balance and the treatment of affective disorders. Prog Neuropsychopharmacol Biol Psychiatry. 1983;7(2-3):297-307.
50. Gershon S, Shaw FH. Psychiatric sequelae of chronic exposure to organophosphorous insecticides. Lancet. 1972;1(7191):1371-1374.
51. Davis KL, Berger PA, Hollister LE, et al. Physostigmine in mania. Arch Gen Psychiatry. 1978;35(1):119-122.
52. Wang JC, Hinrichs AL, Stock H, et al. Evidence of common and specific genetic effects: association of the muscarinic acetylcholine receptor M2 (CHRM2) gene with alcohol dependence and major depressive syndrome. Hum Mol Genet. 2004;13(17):1903-1911.
53. Brown RG. Effects of antidepressants and anticholinergics in a mouse “behavioral despair” test. Eur J Pharmacol. 1979;58(3):331-334.
54. Porsolt RD, Le Pichon M, Jalfre M. Depression: a new animal model sensitive to antidepressant treatments. Nature. 1977;266(5604):730-732.
55. Ji CX, Zhang JJ. Effect of scopolamine on depression in mice. Abstract in English. Yao Xue Xue Bao. 2011;46(4):400-405.
56. Furey ML, Drevets WC. Antidepressant efficacy of the antimuscarinic drug scopolamine: a randomized, placebo-controlled clinical trial. Arch Gen Psychiatry. 2006;63(10):1121-1129.
57. Drevets WC, Furey ML. Replication of scopolamine’s antidepressant efficacy in major depressive disorder: a randomized, placebo-controlled clinical trial. Biol Psychiatry. 2010;67(5):432-438.
58. Furey ML, Khanna A, Hoffman EM, et al. Scopolamine produces larger antidepressant and antianxiety effects in women than in men. Neuropsychopharmacology. 2010;35(12):2479-2488.
59. Gibbs RB, Gabor R, Cox T, et al. Effects of raloxifene and estradiol on hippocampal acetylcholine release and spatial learning in the rat. Psychoneuroendocrinology. 2004;29(6):741-748.
60. Pongrac JL, Gibbs RB, Defranco DB. Estrogen-mediated regulation of cholinergic expression in basal forebrain neurons requires extracellular-signal-regulated kinase activity. Neuroscience. 2004;124(4):809-816.
61. Daniel JM, Dohanich GP. Acetylcholine mediates the estrogen-induced increase in NMDA receptor binding in CA1 of the hippocampus and the associated improvement in working memory. J Neurosci. 2001;21(17):6949-6956.
62. Gerhard DM, Wohleb ES, Duman RS. Emerging treatment mechanisms for depression: focus on glutamate and synaptic plasticity. Drug Discov Today. 2016;21(3):454-464.
63. Voderholzer U. Sleep deprivation and antidepressant treatment. Dialogues Clin Neurosci. 2003;5(4):366-369.
64. Hasselmann H. Scopolamine and depression: a role for muscarinic antagonism? CNS Neurol Disord Drug Targets. 2014;13(4):673-683.
65. Transderm scopolamine [prescribing information]. Warren, NJ: GSK Consumer Healthcare; 2019.
66. Jaffe RJ, Novakovic V, Peselow ED. Scopolamine as an antidepressant: a systematic review. Clin Neuropharmacol. 2013;36(1):24-26.
67. Karameh WK, Khani M. Intravenous clomipramine for treatment-resistant obsessive-compulsive disorder. Int J Neuropsychopharmacol. 2015;19(2):pyv084.
68. Andrews ET, Beattie RM, Tighe MP. Functional abdominal pain: what clinicians need to know. Arch Dis Child. 2020;105(10):938-944. doi:10.1136/archdischild-2020-318825
69. Aliane V, Pérez S, Bohren Y, et al. Key role of striatal cholinergic interneurons in processes leading to arrest of motor stereotypies. Brain. 2011;134(Pt 1):110-118. doi:10.1093/brain/awq285
70. Tzavara ET, Bymaster FP, Davis RJ, et al. M4 muscarinic receptors regulate the dynamics of cholinergic and dopaminergic neurotransmission: relevance to the pathophysiology and treatment of related CNS pathologies. FASEB J. 2004;18(12):1410-1412. doi:10.1096/fj.04-1575fje
71. Korczyn AD, Kish I. The mechanism of imipramine in enuresis nocturna. Clin Exp Pharmacol Physiol. 1979;6(1):31-35. doi:10.1111/j.1440-1681.1979.tb00004.x
72. Trimble MR. Worldwide use of clomipramine. J Clin Psychiatry. 1990;51(Suppl):51-54; discussion 55-58.
73. Gong W, Zhang S, Zong Y, et al. Involvement of the microglial NLRP3 inflammasome in the anti-inflammatory effect of the antidepressant clomipramine. J Affect Disord. 2019;254:15-25.
74. Piwowarska J, Wrzosek M, Radziwon’-Zaleska M. Serum cortisol concentration in patients with major depression after treatment with clomipramine. Pharmacol Rep. 2009;61(4):604-611.
75. Danish University Antidepressant Group (DUAG). Clomipramine dose-effect study in patients with depression: clinical end points and pharmacokinetics. Clin Pharmacol Ther. 1999;66(2):152-165.
76. Moukaddam NJ, Hirschfeld RMA. Intravenous antidepressants: a review. Depress Anxiety. 2004;19(1):1-9.
77. Gerretsen P, Pollock BG. Rediscovering adverse anticholinergic effects. J Clin Psychiatry. 2011;72(6):869-870. doi:10.4088/JCP.11ac07093
78. Thomas SJ, Shin M, McInnis MG, et al. Combination therapy with monoamine oxidase inhibitors and other antidepressants or stimulants: strategies for the management of treatment-resistant depression. Pharmacotherapy. 2015;35(4):433-449. doi:10.1002/phar.1576
79. Robles LA. Serotonin syndrome induced by fentanyl in a child: case report. Clin Neuropharmacol. 2015;38(5):206-208. doi:10.1097/WNF.0000000000000100
80. Fallon BA, Liebowitz MR, Campeas R, et al. Intravenous clomipramine for obsessive-compulsive disorder refractory to oral clomipramine: a placebo-controlled study. Arch Gen Psychiatry. 1998;55(10):918-924.
81. Vieta E, Florea I, Schmidt SN, et al. Intravenous vortioxetine to accelerate onset of effect in major depressive disorder: a 2-week, randomized, double-blind, placebo-controlled study. Int Clin Psychopharmacol. 2019;34(4):153-160.
82. Kasper S, Müller-Spahn F. Intravenous antidepressant treatment: focus on citalopram. Eur Arch Psychiatry Clin Neurosci. 2002;252(3):105-109.
83. Togay B, El-Mallakh RS. Posttraumatic stress disorder: from pathophysiology to pharmacology. Current Psychiatry. 2020;19(5):33-39.
84. Adhikari A, Lerner TN, Finkelstein J, et al. Basomedial amygdala mediates top-down control of anxiety and fear. Nature. 2015;527(7577):179-185. doi:10.1038/nature15698
85. Lipov E. In search of an effective treatment for combat-related post-traumatic stress disorder (PTSD): can the stellate ganglion block be the answer? Pain Pract. 2010;10(4):265-266.
86. Lipov E, Ritchie EC. A review of the use of stellate ganglion block in the treatment of PTSD. Curr Psychiatry Rep. 2015;17(8):599.
87. Olmsted KLR, Bartoszek M, McLean B, et al. Effect of stellate ganglion block treatment on posttraumatic stress disorder symptoms: a randomized clinical trial. JAMA Psychiatry. 2020;77(2):130-138.
88. Lipov E, Candido K. The successful use of left-sided stellate ganglion block in patients that fail to respond to right-sided stellate ganglion block for the treatment of post-traumatic stress disorder symptoms: a retrospective analysis of 205 patients. Mil Med. 2021;186(11-12):319-320.
89. Li Y, Loshak H. Stellate ganglion block for the treatment of post-traumatic stress disorder, depression, and anxiety. Canadian J Health Technol. 2021;1(3):1-30.
90. Kerzner J, Liu H, Demchenko I, et al. Stellate ganglion block for psychiatric disorders: a systematic review of the clinical research landscape. Chronic Stress (Thousand Oaks). 2021;5:24705470211055176.
91. Wie C, Gupta R, Maloney J, et al. Interventional modalities to treat complex regional pain syndrome. Curr Pain Headache Rep. 2021;25(2):10. doi:10.1007/s11916-020-00904-5
92. Chaturvedi A, Dash HH. Sympathetic blockade for the relief of chronic pain. J Indian Med Assoc. 2001;99(12):698-703.
93. Chester M, Hammond C. Leach A. Long-term benefits of stellate ganglion block in severe chronic refractory angina. Pain. 2000;87(1):103-105. doi:10.1016/S0304-3959(00)00270-0
94. Jeon Y. Therapeutic potential of stellate ganglion block in orofacial pain: a mini review. J Dent Anesth Pain Med. 2016;16(3):159-163. doi:10.17245/jdapm.2016.16.3.159
95. Shan HH, Chen HF, Ni Y, et al. Effects of stellate ganglion block through different approaches under guidance of ultrasound. Front Surg. 2022;8:797793. doi:10.3389/fsurg.2021.797793
96. Goel V, Patwardhan AM, Ibrahim M, et al. Complications associated with stellate ganglion nerve block: a systematic review. Reg Anesth Pain Med. 2019;rapm-2018-100127. doi:10.1136/rapm-2018-100127
97. Rowe FJ, Noonan CP. Botulinum toxin for the treatment of strabismus. Cochrane Database Syst Rev. 2017;3(3):CD006499.
98. Roggenkämper P, Jost WH, Bihari K, et al. Efficacy and safety of a new botulinum toxin type A free of complexing proteins in the treatment of blepharospasm. J Neural Transm (Vienna). 2006;113(3):303-312.
99. Heckmann M, Ceballos-Baumann AO, Plewig G; Hyperhidrosis Study Group. Botulinum toxin A for axillary hyperhidrosis (excessive sweating). N Engl J Med. 2001;344(7):488-493.
100. Carruthers JA, Lowe NJ, Menter MA, et al. A multicenter, double-blind, randomized, placebo-controlled study of the efficacy and safety of botulinum toxin type A in the treatment of glabellar lines. J Am Acad Dermatol. 2002;46(6):840-849.
101. Schurch B, de Sèze M, Denys P, et al. Botulinum toxin type A is a safe and effective treatment for neurogenic urinary incontinence: results of a single treatment, randomized, placebo controlled 6-month study. J Urol. 2005;174:196–200.
102. Aurora SK, Winner P, Freeman MC, et al. OnabotulinumtoxinA for treatment of chronic migraine: Pooled analyses of the 56-week PREEMPT clinical program. Headache. 2011;51(9):1358-1373.
103. Dashtipour K, Chen JJ, Walker HW, et al. Systematic literature review of abobotulinumtoxinA in clinical trials for adult upper limb spasticity. Am J Phys Med Rehabil. 2015;94(3):229-238.
104. Nitti VW, Dmochowski R, Herschorn S, et al. OnabotulinumtoxinA for the treatment of patients with overactive bladder and urinary incontinence: results of a phase 3, randomized, placebo-controlled trial. J Urol. 2017;197(2S):S216-S223.
105. Jongerius PH, van den Hoogen FJA, van Limbeek J, et al. Effect of botulinum toxin in the treatment of drooling: a controlled clinical trial. Pediatrics. 2004;114(3):620-627.
106. Zaninotto, G. Annese V, Costantini M, et al. Randomized controlled trial of botulinum toxin versus laparoscopic heller myotomy for esophageal achalasia. Ann Surg. 2004;239(3):364-370.
107. Dressler D, Adib Saberi F. Botulinum toxin: mechanisms of action. Eur Neurol. 2005;53:3-9.
108. Lewis MB, Bowler PJ. Botulinum toxin cosmetic therapy correlates with a more positive mood. J Cosmet Dermatol. 2009;8(1):24-26.
109. Affatato O, Moulin TC, Pisanu C, et al. High efficacy of onabotulinumtoxinA treatment in patients with comorbid migraine and depression: a meta-analysis. J Transl Med. 2021;19(1):133.
110. Finzi E, Wasserman E. Treatment of depression with botulinum toxin A: a case series. Dermatol Surg. 2006;32(5):645-649; discussion 649-650.
111. Schulze J, Neumann I, Magid M, et al. Botulinum toxin for the management of depression: an updated review of the evidence and meta-analysis. J Psychiatr Res. 2021;135:332-340.
112. Finzi E, Rosenthal NE. Emotional proprioception: treatment of depression with afferent facial feedback. J Psychiatr Res. 2016;80:93-96.
113. Söderkvist S, Ohlén K, Dimberg U. How the experience of emotion is modulated by facial feedback. J Nonverbal Behav. 2018;42(1):129-151.
114. Lewis, MB. The interactions between botulinum-toxin-based facial treatments and embodied emotions. Sci Rep. 2018;8(1):14720.
115. Li Y, Liu J, Liu X, et al. Antidepressant-like action of single facial injection of botulinum neurotoxin A is associated with augmented 5-HT levels and BDNF/ERK/CREB pathways in mouse brain. Neurosci Bull. 2019;35(4):661-672. Erratum in: Neurosci Bull. 2019;35(4):779-780.
116. Gündel H, Wolf A, Xidara V, et al. High psychiatric comorbidity in spasmodic torticollis: a controlled study. J Nerv Ment Dis. 2003;191(7):465-473.
117. Hall TA, McGwin G Jr, Searcey K, et al. Health-related quality of life and psychosocial characteristics of patients with benign essential blepharospasm. Arch Ophthalmol. 2006;124(1):116-119.
118. Ceylan D, Erer S, Zarifog˘lu M, et al. Evaluation of anxiety and depression scales and quality of life in cervical dystonia patients on botulinum toxin therapy and their relatives. Neurol Sci. 2019;40(4):725-731.
119. Heller AS, Lapate RC, Mayer KE, et al. The face of negative affect: trial-by-trial corrugator responses to negative pictures are positively associated with amygdala and negatively associated with ventromedial prefrontal cortex activity. J Cogn Neurosci. 2014;26(9):2102-2110.
120. Makunts T, Wollmer MA, Abagyan R. Postmarketing safety surveillance data reveals antidepressant effects of botulinum toxin across various indications and injection sites. Sci Rep. 2020;10(1):12851.
121. Ahsanuddin S, Roy S, Nasser W, et al. Adverse events associated with botox as reported in a Food and Drug Administration database. Aesthetic Plast Surg. 2021;45(3):1201-1209. doi:10.1007/s00266-020-02027-z
122. Kashif M, Tahir S, Ashfaq F, et al. Association of myofascial trigger points in neck and shoulder region with depression, anxiety, and stress among university students. J Pak Med Assoc. 2021;71(9):2139-2142.
123. Cigarán-Méndez M, Jiménez-Antona C, Parás-Bravo P, et al. Active trigger points are associated with anxiety and widespread pressure pain sensitivity in women, but not men, with tension type headache. Pain Pract. 2019;19(5):522-529.
124. Palacios-Ceña M, Castaldo M, Wang K, et al. Relationship of active trigger points with related disability and anxiety in people with tension-type headache. Medicine (Baltimore). 2017;96(13):e6548.
125. Karadas Ö, Inan LE, Ulas Ü, et al. Efficacy of local lidocaine application on anxiety and depression and its curative effect on patients with chronic tension-type headache. Eur Neurol. 2013;70(1-2):95-101.
126. Gerwin RD. Classification, epidemiology and natural history of myofascial pain syndrome. Curr Pain Headache Rep. 2001;5(5):412-420.
127. Castro Sánchez AM, García López H, Fernández Sánchez M, et al. Improvement in clinical outcomes after dry needling versus myofascial release on pain pressure thresholds, quality of life, fatigue, pain intensity, quality of sleep, anxiety, and depression in patients with fibromyalgia syndrome. Disabil Rehabil. 2019;41(19):2235-2246.
128. Healy GM, Finn DP, O’Gorman DA, et al. Pretreatment anxiety and pain acceptance are associated with response to trigger point injection therapy for chronic myofascial pain. Pain Med. 2015;16(10):1955-1966.
129. Morjaria JB, Lakshminarayana UB, Liu-Shiu-Cheong P, et al. Pneumothorax: a tale of pain or spontaneity. Ther Adv Chronic Dis. 2014;5(6):269-273.
1. Vincent KM, Ryan M, Palmer E, et al. Interventional psychiatry. Postgrad Med. 2020;132(7):573-574.
2. Allen MH, Feifel D, Lesem MD, et al. Efficacy and safety of loxapine for inhalation in the treatment of agitation in patients with schizophrenia: a randomized, double-blind, placebo-controlled trial. J Clin Psychiatry. 2011;72(10):1313-1321.
3. Kwentus J, Riesenberg RA, Marandi M, et al. Rapid acute treatment of agitation in patients with bipolar I disorder: a multicenter, randomized, placebo-controlled clinical trial with inhaled loxapine. Bipolar Disord. 2012;14(1):31-40.
4. Lee JD, Nunes EV Jr, Novo P, et al. Comparative effectiveness of extended-release naltrexone versus buprenorphine-naloxone for opioid relapse prevention (X:BOT): a multicentre, open-label, randomised controlled trial. Lancet. 2018;391(10118):309-318.
5. Haight BR, Learned SM, Laffont CM, et al. Efficacy and safety of a monthly buprenorphine depot injection for opioid use disorder: a multicentre, randomised, double‐blind, placebo‐controlled, phase 3 trial. Lancet. 2019;393(10173):778-790.
6. Andorn A, Graham J, Csernansky J, et al. Monthly extended-release risperidone (RBP-7000) in the treatment of schizophrenia: results from the phase 3 program. J Clin Psychopharmacol. 2019;39(5):428-433.
7. Dundee TW. Twenty-five years of ketamine. A report of an international meeting. Anaesthesia. 1990;45(2):159. doi:10.1111/j.1365-2044.1990.tb14287.x
8. White PF, Way WL, Trevor AJ. Ketamine--its pharmacology and therapeutic uses. Anesthesiology. 1982;56(2):119-136. doi:10.1097/00000542-198202000-00007
9. Zanos P, Gould TD. Mechanisms of ketamine action as an antidepressant. Mol Psychiatry. 2018;23(4):801-811.
10. Molero P, Ramos-Quiroga JA, Martin-Santos R, et al. Antidepressant efficacy and tolerability of ketamine and esketamine: a critical review. CNS Drugs. 2018;32(5):411-420. doi:10.1007/s40263-018-0519-3
11. Williams NR, Heifets BD, Blasey C, et al. Attenuation of antidepressant effects of ketamine by opioid receptor antagonism. Am J Psychiatry. 2018;175(12):1205-1215.
12. Witkin JM, Martin AE, Golani LK, et al. Rapid-acting antidepressants. Adv Pharmacol. 2019;86:47-96.
13. Strayer RJ, Nelson LS. Adverse events associated with ketamine for procedural sedation in adults. Am J Emerg Med. 2008;26(9):985-1028. doi:10.1016/j.ajem.2007.12.005
14. Frye MA, Blier P, Tye SJ. Concomitant benzodiazepine use attenuates ketamine response: implications for large scale study design and clinical development. J Clin Psychopharmacol. 2015;35(3):334-336.
15. Fava M, Freeman MP, Flynn M, et al. Double-blind, placebo-controlled, dose-ranging trial of intravenous ketamine as adjunctive therapy in treatment-resistant depression (TRD). Mol Psychiatry. 2020;25(7):1592-1603.
16. Bahji A, Vazquez GH, Zarate CA Jr. Comparative efficacy of racemic ketamine and esketamine for depression: a systematic review and meta-analysis. J Affect Disord. 2021;278:542-555. Erratum in: J Affect Disord. 2021;281:1001.
17. Brendle M, Robison R, Malone DC. Cost-effectiveness of esketamine nasal spray compared to intravenous ketamine for patients with treatment-resistant depression in the US utilizing clinical trial efficacy and real-world effectiveness estimates. J Affect Disord. 2022;319:388-396.
18. Dhillon S. Aducanumab: first approval. Drugs. 2021;81(12):1437-1443. Erratum in: Drugs. 2021;81(14):1701.
19. van Dyck CH, Swanson CJ, Aisen P, et al. Lecanemab in early Alzheimer’s disease. N Engl J Med. 2023;388(1):9-21. doi:10.1056/NEJMoa2212948
20. Sevigny J, Chiao P, Bussière T, et al. The antibody aducanumab reduces Aβ plaques in Alzheimer’s disease. Nature. 2016;537(7618):50-56. Update in: Nature. 2017;546(7659):564.
21. Fillit H, Green A. Aducanumab and the FDA – where are we now? Nat Rev Neurol. 2021;17(3):129-130.
22. Reardon S. FDA approves Alzheimer’s drug lecanemab amid safety concerns. Nature. 2023;613(7943):227-228. doi:10.1038/d41586-023-00030-3
23. McDade E, Cummings JL, Dhadda S, et al. Lecanemab in patients with early Alzheimer’s disease: detailed results on biomarker, cognitive, and clinical effects from the randomized and open-label extension of the phase 2 proof-of-concept study. Alzheimers Res Ther. 2022;14(1):191. doi:10.1186/s13195-022-01124-2
24. Mintun MA, Lo AC, Evans CD, et al. Donanemab in early Alzheimer’s disease. N Engl J Med. 2021;384(18):1691-1704.
25. Luisi S, Petraglia F, Benedetto C, et al. Serum allopregnanolone levels in pregnant women: changes during pregnancy, at delivery, and in hypertensive patients. J Clin Endocrinol Metab. 2000;85(7):2429-2433.
26. Meltzer-Brody S, Colquhoun H, Riesenberg R, et al. Brexanolone injection in post-partum depression: two multicentre, double-blind, randomised, placebo-controlled, phase 3 trials. Lancet. 2018;392(10152):1058-1070.
27. Powell JG, Garland S, Preston K, et al. Brexanolone (Zulresso): finally, an FDA-approved treatment for postpartum depression. Ann Pharmacother. 2020;54(2):157-163.
28. Patterson R, Krohn H, Richardson E, et al. A brexanolone treatment program at an academic medical center: patient selection, 90-day posttreatment outcomes, and lessons learned. J Acad Consult Liaison Psychiatry. 2022;63(1):14-22.
29. World Health Organization. WHO model list of essential medicines - 22nd list (2021). World Health Organization. September 30, 2021. Accessed April 7, 2023. https://www.who.int/publications/i/item/WHO-MHP-HPS-EML-2021.02
30. Eby GA, Eby KL, Mruk H. Magnesium and major depression. In: Vink R, Nechifor M, eds. Magnesium in the Central Nervous System. University of Adelaide Press; 2011.
31. Plant TM, Zeleznik AJ. Knobil and Neill’s Physiology of Reproduction. 4th ed. Elsevier Inc.; 2015:2503-2550.
32. Sidebotham D, Le Grice IJ. Physiology and pathophysiology. In: Sidebotham D, McKee A, Gillham M, Levy J. Cardiothoracic Critical Care. Elsevier, Inc.; 2007:3-27.
33. Duley L, Gülmezoglu AM, Henderson-Smart DJ, et al. Magnesium sulphate and other anticonvulsants for women with pre-eclampsia. Cochrane Database Syst Rev. 2010;2010(11):CD000025.
34. Emergency supply of medicines. In: British National Formulary. British Medical Association, Royal Pharmaceutical Society; 2015:6. Accessed April 7, 2023. https://www.academia.edu/35076015/british_national_formulary_2015_pdf
35. Kwofie K, Wolfson AB. Intravenous magnesium sulfate for acute asthma exacerbation in children and adults. Am Fam Physician. 2021;103(4):245-246.
36. Patniyot IR, Gelfand AA. Acute treatment therapies for pediatric migraine: a qualitative systematic review. Headache. 2016;56(1):49-70.
37. Wang X, Du X, Yang H, et al. Use of intravenous magnesium sulfate among patients with acute myocardial infarction in China from 2001 to 2015: China PEACE-Retrospective AMI Study. BMJ Open. 2020;10(3):e033269.
38. Karhu E, Atlas SE, Jinrun G, et al. Intravenous infusion of magnesium sulfate is not associated with cardiovascular, liver, kidney, and metabolic toxicity in adults. J Clin Transl Res. 2018;4(1):47-55.
39. Noah L, Pickering G, Mazur A, et al. Impact of magnesium supplementation, in combination with vitamin B6, on stress and magnesium status: secondary data from a randomized controlled trial. Magnes Res. 2020;33(3):45-57.
40. Erstad BL, Cotugno CL. Management of alcohol withdrawal. Am J Health Syst Pharm. 1995;52(7):697-709.
41. Abumaria N, Luo L, Ahn M, et al. Magnesium supplement enhances spatial-context pattern separation and prevents fear overgeneralization. Behav Pharmacol. 2013;24(4):255-263.
42. Kirov GK, Tsachev KN. Magnesium, schizophrenia and manic-depressive disease. Neuropsychobiology. 1990;23(2):79-81.
43. Botturi A, Ciappolino V, Delvecchio G, et al. The role and the effect of magnesium in mental disorders: a systematic review. Nutrients. 2020;12(6):1661.
44. Kirkland AE, Sarlo GL, Holton KF. The role of magnesium in neurological disorders. Nutrients. 2018;10(6):730.
45. Magnesium sulfate intravenous side effects by likelihood and severity. WebMD. Accessed April 9, 2023. https://www.webmd.com/drugs/2/drug-149570/magnesium-sulfate-intravenous/details/list-sideeffects
46. Scopolamine base transdermal system – uses, side effects, and more. WebMD. Accessed April 9, 2023. https://www.webmd.com/drugs/2/drug-14032/scopolamine-transdermal/details
47. Bolden C, Cusack B, Richelson E. Antagonism by antimuscarinic and neuroleptic compounds at the five cloned human muscarinic cholinergic receptors expressed in Chinese hamster ovary cells. J Pharmacol Exp Ther. 1992;260(2):576-580.
48. Janowsky DS, el-Yousef MK, Davis JM, et al. A cholinergic-adrenergic hypothesis of mania and depression. Lancet. 1972;2(7778):632-635.
49. Janowsky DS, Risch SC, Gillin JC. Adrenergic-cholinergic balance and the treatment of affective disorders. Prog Neuropsychopharmacol Biol Psychiatry. 1983;7(2-3):297-307.
50. Gershon S, Shaw FH. Psychiatric sequelae of chronic exposure to organophosphorous insecticides. Lancet. 1972;1(7191):1371-1374.
51. Davis KL, Berger PA, Hollister LE, et al. Physostigmine in mania. Arch Gen Psychiatry. 1978;35(1):119-122.
52. Wang JC, Hinrichs AL, Stock H, et al. Evidence of common and specific genetic effects: association of the muscarinic acetylcholine receptor M2 (CHRM2) gene with alcohol dependence and major depressive syndrome. Hum Mol Genet. 2004;13(17):1903-1911.
53. Brown RG. Effects of antidepressants and anticholinergics in a mouse “behavioral despair” test. Eur J Pharmacol. 1979;58(3):331-334.
54. Porsolt RD, Le Pichon M, Jalfre M. Depression: a new animal model sensitive to antidepressant treatments. Nature. 1977;266(5604):730-732.
55. Ji CX, Zhang JJ. Effect of scopolamine on depression in mice. Abstract in English. Yao Xue Xue Bao. 2011;46(4):400-405.
56. Furey ML, Drevets WC. Antidepressant efficacy of the antimuscarinic drug scopolamine: a randomized, placebo-controlled clinical trial. Arch Gen Psychiatry. 2006;63(10):1121-1129.
57. Drevets WC, Furey ML. Replication of scopolamine’s antidepressant efficacy in major depressive disorder: a randomized, placebo-controlled clinical trial. Biol Psychiatry. 2010;67(5):432-438.
58. Furey ML, Khanna A, Hoffman EM, et al. Scopolamine produces larger antidepressant and antianxiety effects in women than in men. Neuropsychopharmacology. 2010;35(12):2479-2488.
59. Gibbs RB, Gabor R, Cox T, et al. Effects of raloxifene and estradiol on hippocampal acetylcholine release and spatial learning in the rat. Psychoneuroendocrinology. 2004;29(6):741-748.
60. Pongrac JL, Gibbs RB, Defranco DB. Estrogen-mediated regulation of cholinergic expression in basal forebrain neurons requires extracellular-signal-regulated kinase activity. Neuroscience. 2004;124(4):809-816.
61. Daniel JM, Dohanich GP. Acetylcholine mediates the estrogen-induced increase in NMDA receptor binding in CA1 of the hippocampus and the associated improvement in working memory. J Neurosci. 2001;21(17):6949-6956.
62. Gerhard DM, Wohleb ES, Duman RS. Emerging treatment mechanisms for depression: focus on glutamate and synaptic plasticity. Drug Discov Today. 2016;21(3):454-464.
63. Voderholzer U. Sleep deprivation and antidepressant treatment. Dialogues Clin Neurosci. 2003;5(4):366-369.
64. Hasselmann H. Scopolamine and depression: a role for muscarinic antagonism? CNS Neurol Disord Drug Targets. 2014;13(4):673-683.
65. Transderm scopolamine [prescribing information]. Warren, NJ: GSK Consumer Healthcare; 2019.
66. Jaffe RJ, Novakovic V, Peselow ED. Scopolamine as an antidepressant: a systematic review. Clin Neuropharmacol. 2013;36(1):24-26.
67. Karameh WK, Khani M. Intravenous clomipramine for treatment-resistant obsessive-compulsive disorder. Int J Neuropsychopharmacol. 2015;19(2):pyv084.
68. Andrews ET, Beattie RM, Tighe MP. Functional abdominal pain: what clinicians need to know. Arch Dis Child. 2020;105(10):938-944. doi:10.1136/archdischild-2020-318825
69. Aliane V, Pérez S, Bohren Y, et al. Key role of striatal cholinergic interneurons in processes leading to arrest of motor stereotypies. Brain. 2011;134(Pt 1):110-118. doi:10.1093/brain/awq285
70. Tzavara ET, Bymaster FP, Davis RJ, et al. M4 muscarinic receptors regulate the dynamics of cholinergic and dopaminergic neurotransmission: relevance to the pathophysiology and treatment of related CNS pathologies. FASEB J. 2004;18(12):1410-1412. doi:10.1096/fj.04-1575fje
71. Korczyn AD, Kish I. The mechanism of imipramine in enuresis nocturna. Clin Exp Pharmacol Physiol. 1979;6(1):31-35. doi:10.1111/j.1440-1681.1979.tb00004.x
72. Trimble MR. Worldwide use of clomipramine. J Clin Psychiatry. 1990;51(Suppl):51-54; discussion 55-58.
73. Gong W, Zhang S, Zong Y, et al. Involvement of the microglial NLRP3 inflammasome in the anti-inflammatory effect of the antidepressant clomipramine. J Affect Disord. 2019;254:15-25.
74. Piwowarska J, Wrzosek M, Radziwon’-Zaleska M. Serum cortisol concentration in patients with major depression after treatment with clomipramine. Pharmacol Rep. 2009;61(4):604-611.
75. Danish University Antidepressant Group (DUAG). Clomipramine dose-effect study in patients with depression: clinical end points and pharmacokinetics. Clin Pharmacol Ther. 1999;66(2):152-165.
76. Moukaddam NJ, Hirschfeld RMA. Intravenous antidepressants: a review. Depress Anxiety. 2004;19(1):1-9.
77. Gerretsen P, Pollock BG. Rediscovering adverse anticholinergic effects. J Clin Psychiatry. 2011;72(6):869-870. doi:10.4088/JCP.11ac07093
78. Thomas SJ, Shin M, McInnis MG, et al. Combination therapy with monoamine oxidase inhibitors and other antidepressants or stimulants: strategies for the management of treatment-resistant depression. Pharmacotherapy. 2015;35(4):433-449. doi:10.1002/phar.1576
79. Robles LA. Serotonin syndrome induced by fentanyl in a child: case report. Clin Neuropharmacol. 2015;38(5):206-208. doi:10.1097/WNF.0000000000000100
80. Fallon BA, Liebowitz MR, Campeas R, et al. Intravenous clomipramine for obsessive-compulsive disorder refractory to oral clomipramine: a placebo-controlled study. Arch Gen Psychiatry. 1998;55(10):918-924.
81. Vieta E, Florea I, Schmidt SN, et al. Intravenous vortioxetine to accelerate onset of effect in major depressive disorder: a 2-week, randomized, double-blind, placebo-controlled study. Int Clin Psychopharmacol. 2019;34(4):153-160.
82. Kasper S, Müller-Spahn F. Intravenous antidepressant treatment: focus on citalopram. Eur Arch Psychiatry Clin Neurosci. 2002;252(3):105-109.
83. Togay B, El-Mallakh RS. Posttraumatic stress disorder: from pathophysiology to pharmacology. Current Psychiatry. 2020;19(5):33-39.
84. Adhikari A, Lerner TN, Finkelstein J, et al. Basomedial amygdala mediates top-down control of anxiety and fear. Nature. 2015;527(7577):179-185. doi:10.1038/nature15698
85. Lipov E. In search of an effective treatment for combat-related post-traumatic stress disorder (PTSD): can the stellate ganglion block be the answer? Pain Pract. 2010;10(4):265-266.
86. Lipov E, Ritchie EC. A review of the use of stellate ganglion block in the treatment of PTSD. Curr Psychiatry Rep. 2015;17(8):599.
87. Olmsted KLR, Bartoszek M, McLean B, et al. Effect of stellate ganglion block treatment on posttraumatic stress disorder symptoms: a randomized clinical trial. JAMA Psychiatry. 2020;77(2):130-138.
88. Lipov E, Candido K. The successful use of left-sided stellate ganglion block in patients that fail to respond to right-sided stellate ganglion block for the treatment of post-traumatic stress disorder symptoms: a retrospective analysis of 205 patients. Mil Med. 2021;186(11-12):319-320.
89. Li Y, Loshak H. Stellate ganglion block for the treatment of post-traumatic stress disorder, depression, and anxiety. Canadian J Health Technol. 2021;1(3):1-30.
90. Kerzner J, Liu H, Demchenko I, et al. Stellate ganglion block for psychiatric disorders: a systematic review of the clinical research landscape. Chronic Stress (Thousand Oaks). 2021;5:24705470211055176.
91. Wie C, Gupta R, Maloney J, et al. Interventional modalities to treat complex regional pain syndrome. Curr Pain Headache Rep. 2021;25(2):10. doi:10.1007/s11916-020-00904-5
92. Chaturvedi A, Dash HH. Sympathetic blockade for the relief of chronic pain. J Indian Med Assoc. 2001;99(12):698-703.
93. Chester M, Hammond C. Leach A. Long-term benefits of stellate ganglion block in severe chronic refractory angina. Pain. 2000;87(1):103-105. doi:10.1016/S0304-3959(00)00270-0
94. Jeon Y. Therapeutic potential of stellate ganglion block in orofacial pain: a mini review. J Dent Anesth Pain Med. 2016;16(3):159-163. doi:10.17245/jdapm.2016.16.3.159
95. Shan HH, Chen HF, Ni Y, et al. Effects of stellate ganglion block through different approaches under guidance of ultrasound. Front Surg. 2022;8:797793. doi:10.3389/fsurg.2021.797793
96. Goel V, Patwardhan AM, Ibrahim M, et al. Complications associated with stellate ganglion nerve block: a systematic review. Reg Anesth Pain Med. 2019;rapm-2018-100127. doi:10.1136/rapm-2018-100127
97. Rowe FJ, Noonan CP. Botulinum toxin for the treatment of strabismus. Cochrane Database Syst Rev. 2017;3(3):CD006499.
98. Roggenkämper P, Jost WH, Bihari K, et al. Efficacy and safety of a new botulinum toxin type A free of complexing proteins in the treatment of blepharospasm. J Neural Transm (Vienna). 2006;113(3):303-312.
99. Heckmann M, Ceballos-Baumann AO, Plewig G; Hyperhidrosis Study Group. Botulinum toxin A for axillary hyperhidrosis (excessive sweating). N Engl J Med. 2001;344(7):488-493.
100. Carruthers JA, Lowe NJ, Menter MA, et al. A multicenter, double-blind, randomized, placebo-controlled study of the efficacy and safety of botulinum toxin type A in the treatment of glabellar lines. J Am Acad Dermatol. 2002;46(6):840-849.
101. Schurch B, de Sèze M, Denys P, et al. Botulinum toxin type A is a safe and effective treatment for neurogenic urinary incontinence: results of a single treatment, randomized, placebo controlled 6-month study. J Urol. 2005;174:196–200.
102. Aurora SK, Winner P, Freeman MC, et al. OnabotulinumtoxinA for treatment of chronic migraine: Pooled analyses of the 56-week PREEMPT clinical program. Headache. 2011;51(9):1358-1373.
103. Dashtipour K, Chen JJ, Walker HW, et al. Systematic literature review of abobotulinumtoxinA in clinical trials for adult upper limb spasticity. Am J Phys Med Rehabil. 2015;94(3):229-238.
104. Nitti VW, Dmochowski R, Herschorn S, et al. OnabotulinumtoxinA for the treatment of patients with overactive bladder and urinary incontinence: results of a phase 3, randomized, placebo-controlled trial. J Urol. 2017;197(2S):S216-S223.
105. Jongerius PH, van den Hoogen FJA, van Limbeek J, et al. Effect of botulinum toxin in the treatment of drooling: a controlled clinical trial. Pediatrics. 2004;114(3):620-627.
106. Zaninotto, G. Annese V, Costantini M, et al. Randomized controlled trial of botulinum toxin versus laparoscopic heller myotomy for esophageal achalasia. Ann Surg. 2004;239(3):364-370.
107. Dressler D, Adib Saberi F. Botulinum toxin: mechanisms of action. Eur Neurol. 2005;53:3-9.
108. Lewis MB, Bowler PJ. Botulinum toxin cosmetic therapy correlates with a more positive mood. J Cosmet Dermatol. 2009;8(1):24-26.
109. Affatato O, Moulin TC, Pisanu C, et al. High efficacy of onabotulinumtoxinA treatment in patients with comorbid migraine and depression: a meta-analysis. J Transl Med. 2021;19(1):133.
110. Finzi E, Wasserman E. Treatment of depression with botulinum toxin A: a case series. Dermatol Surg. 2006;32(5):645-649; discussion 649-650.
111. Schulze J, Neumann I, Magid M, et al. Botulinum toxin for the management of depression: an updated review of the evidence and meta-analysis. J Psychiatr Res. 2021;135:332-340.
112. Finzi E, Rosenthal NE. Emotional proprioception: treatment of depression with afferent facial feedback. J Psychiatr Res. 2016;80:93-96.
113. Söderkvist S, Ohlén K, Dimberg U. How the experience of emotion is modulated by facial feedback. J Nonverbal Behav. 2018;42(1):129-151.
114. Lewis, MB. The interactions between botulinum-toxin-based facial treatments and embodied emotions. Sci Rep. 2018;8(1):14720.
115. Li Y, Liu J, Liu X, et al. Antidepressant-like action of single facial injection of botulinum neurotoxin A is associated with augmented 5-HT levels and BDNF/ERK/CREB pathways in mouse brain. Neurosci Bull. 2019;35(4):661-672. Erratum in: Neurosci Bull. 2019;35(4):779-780.
116. Gündel H, Wolf A, Xidara V, et al. High psychiatric comorbidity in spasmodic torticollis: a controlled study. J Nerv Ment Dis. 2003;191(7):465-473.
117. Hall TA, McGwin G Jr, Searcey K, et al. Health-related quality of life and psychosocial characteristics of patients with benign essential blepharospasm. Arch Ophthalmol. 2006;124(1):116-119.
118. Ceylan D, Erer S, Zarifog˘lu M, et al. Evaluation of anxiety and depression scales and quality of life in cervical dystonia patients on botulinum toxin therapy and their relatives. Neurol Sci. 2019;40(4):725-731.
119. Heller AS, Lapate RC, Mayer KE, et al. The face of negative affect: trial-by-trial corrugator responses to negative pictures are positively associated with amygdala and negatively associated with ventromedial prefrontal cortex activity. J Cogn Neurosci. 2014;26(9):2102-2110.
120. Makunts T, Wollmer MA, Abagyan R. Postmarketing safety surveillance data reveals antidepressant effects of botulinum toxin across various indications and injection sites. Sci Rep. 2020;10(1):12851.
121. Ahsanuddin S, Roy S, Nasser W, et al. Adverse events associated with botox as reported in a Food and Drug Administration database. Aesthetic Plast Surg. 2021;45(3):1201-1209. doi:10.1007/s00266-020-02027-z
122. Kashif M, Tahir S, Ashfaq F, et al. Association of myofascial trigger points in neck and shoulder region with depression, anxiety, and stress among university students. J Pak Med Assoc. 2021;71(9):2139-2142.
123. Cigarán-Méndez M, Jiménez-Antona C, Parás-Bravo P, et al. Active trigger points are associated with anxiety and widespread pressure pain sensitivity in women, but not men, with tension type headache. Pain Pract. 2019;19(5):522-529.
124. Palacios-Ceña M, Castaldo M, Wang K, et al. Relationship of active trigger points with related disability and anxiety in people with tension-type headache. Medicine (Baltimore). 2017;96(13):e6548.
125. Karadas Ö, Inan LE, Ulas Ü, et al. Efficacy of local lidocaine application on anxiety and depression and its curative effect on patients with chronic tension-type headache. Eur Neurol. 2013;70(1-2):95-101.
126. Gerwin RD. Classification, epidemiology and natural history of myofascial pain syndrome. Curr Pain Headache Rep. 2001;5(5):412-420.
127. Castro Sánchez AM, García López H, Fernández Sánchez M, et al. Improvement in clinical outcomes after dry needling versus myofascial release on pain pressure thresholds, quality of life, fatigue, pain intensity, quality of sleep, anxiety, and depression in patients with fibromyalgia syndrome. Disabil Rehabil. 2019;41(19):2235-2246.
128. Healy GM, Finn DP, O’Gorman DA, et al. Pretreatment anxiety and pain acceptance are associated with response to trigger point injection therapy for chronic myofascial pain. Pain Med. 2015;16(10):1955-1966.
129. Morjaria JB, Lakshminarayana UB, Liu-Shiu-Cheong P, et al. Pneumothorax: a tale of pain or spontaneity. Ther Adv Chronic Dis. 2014;5(6):269-273.
Bipolar disorder: The foundational role of mood stabilizers
Bipolar disorder (BD) is a recurrent, life-long psychiatric illness affecting nearly 2% of the world population1,2 that is characterized by episodes of mania and depression interspersed among periods of relative mood stability.3 The illness causes an enormous health burden, which makes understanding its pathophysiology and treatment patterns a substantial priority.4 In the 1950s, lithium was found to be effective for treating acute manic episodes and preventing relapse in BD.5 Since then, valproate and carbamazepine also have been FDA-approved for treating mania.6,7 Antipsychotics have also shown evidence of efficacy in BD treatment,8,9 particularly for use in acute settings for more rapid effect or for a limited duration,10 which has led some to refer to them as “mood stabilizers.”11
In this article, we describe changes in trends of prescribing medications to treat BD, the role of ion dysregulation in the disorder, and how a better understanding of this dysregulation might impact the choice of treatment.
Changes in pharmacotherapy for bipolar disorder
From 1997 through 2016, the use of lithium for BD decreased from >30% of patients to 17.6% (with a nadir of 13.9% from 2009 to 2012).12 Over the same period, the use of nonlithium mood stabilizers decreased from 30.4% to approximately 4.8%, while second-generation antipsychotic (SGAs) use increased from 12.4% to 50.4%.12 Distressingly, antidepressant use increased from approximately 47% to 56.8%, and antidepressant use without concomitant mood stabilizers increased from 38% to 40.8%, although the rate of antidepressants without either a mood stabilizer or an antipsychotic remained relatively stable (14.9% to 16.8%).12 In randomized trials, when added to mood stabilizers, antidepressants have consistently failed to separate from placebo,13-15 but they can destabilize the illness, resulting in increases in mania, depression, and subsyndromal mixed symptoms.16-18
It is easy to understand clinicians’ attempts to address their patients’ distress due to depressive symptoms that do not resolve with mood stabilizers.19,20 Similarly, the increased use of antipsychotics is driven by evidence that antipsychotics are effective for treating bipolar depression and preventing the recurrence of manic and (for some antipsychotics) depressive episodes.21,22 However, long-term antipsychotic use causes brain volume change in patients with schizophrenia23 or major depressive disorder24 and in nonhuman primates25,26; metabolic abnormalities27-31; and cardiovascular adverse effects.32 Antipsychotics are believed to be associated with withdrawal psychosis.33,34 In the head-to-head Clinical Health Outcomes Initiative in Comparative Effectiveness for Bipolar Disorder (Bipolar CHOICE) study, quetiapine was as effective as lithium but associated with more adverse effects.35 Importantly, the estimated disability-adjusted life years of patients with BD increased by 54.4% from 6.02 million in 1990 to 9.29 million in 2017, which is greater than the increase in the incidence of BD (47.74%) over the same time.36 This means that despite the dramatic increase in treatment options for people with BD, functional outcomes have declined.
One major difference between antipsychotics and mood stabilizers is that antipsychotics do not alter the underlying abnormal pathology of BD.37 An ideal pharmacologic intervention is one that corrects a known pathophysiologic anomaly of the condition being treated. There are no demonstrated abnormalities in the dopamine or serotonin systems in individuals with BD, but long-term use of antipsychotics may create dopaminergic alterations.33 One of the most reproducible biomarkers associated with manic and bipolar depressed mood states is increased intracellular sodium38,39 and reduced ability to correct a sodium challenge.40-42 By normalizing intracellular sodium levels, lithium and the mood-stabilizing anticonvulsants uniquely and specifically counter known physiologic abnormalities in patients with BD.37,43
The role of ion dysregulation
The pathophysiology of BD remains elusive. A multitude of lines of evidence link BD to abnormal neuroimaging findings,22,44,45 oxidative stress,46 inflammation,47 and mitochondrial disease,48 but there is still no unifying understanding of these findings. Ion dysregulation appears to be central to understanding and treating BD.38,39
Despite extensive genetic studies, no genes have been identified that mediate >5% of the risk for BD. Nonetheless, 74% of all genes identified as mediating risk for BD code for proteins essential for the regulation of ion transport and membrane potential.49 The 2 genes that contribute the greatest risk are CACNA1C and ANK3, which code for a calcium channel and a cytoskeletal protein, respectively.50ANK3 codes for ankyrin G, which plays a role in proper coupling of the voltage-gated sodium channels to the cytoskeleton.51 An additional risk gene, TRANK1, contains multiple ankyrin-like repeat domains, which suggests some shared functions with ANK3.52 More importantly, the most reproducible pathophysiologic findings in BD are dysregulation of sodium, potassium, hydrogen, and calcium transport, with consequent alteration of depolarization potential, neuronal excitability, and calcium-mediated processes.38,39,53-56 For example, increased sodium and calcium within cells have been observed in both mania and bipolar depression, and these levels normalize during euthymia. All medications that are effective for treating BD may reduce intracellular sodium or calcium; traditional mood stabilizers do so directly by inhibiting voltage-sensitive sodium channels in an activity-dependent manner or displacing intracellular sodium,43,57 whereas antipsychotics do so indirectly by increasing sodium pump activity through inhibition of second messengers of the dopamine D2 family of receptors.37
Continue to: The extent of ion dysregulation...
The extent of ion dysregulation is directly associated with the expressed mood state of the illness. A small reduction in the activity of the sodium pump results in a small increase in intracellular sodium (approximately 10 mM).39,58 This led to the hypothesis that increased intracellular sodium causes the transmembrane potential to increase closer to membrane depolarization threshold, which increases excitability of affected neurons.38,39,58 Neurons are likely to fire and propagate signals more easily, which may manifest as symptoms of mania, such as increased energy, activity, lability, excitability, irritability, tangentiality, and looseness of associations. As the process of increased intracellular sodium progresses, a minority of neurons are expected to have their transmembrane potentials depolarize sufficiently for the resting membrane potential to go beyond threshold potential.59 Such neurons are in a state of constant depolarization (also known as depolarization block), which disrupts neuronal circuits. The difficulty in progression of these signals results in the classic bipolar depression symptoms of low energy, reduced activity, and slowing of all brain activity that is seen as psychomotor slowing.38
Implications for treatment
Medications for treating bipolar illness include lithium, anticonvulsants, benzodiazepines, first-generation antipsychotics, and SGAs.37,43
Mood stabilizers (lithium and certain anticonvulsants) correct the previously mentioned sodium abnormality by reducing sodium entry into the cell in an activity-dependent manner.43 As the only agents that directly address a known pathophysiologic abnormality, they are foundational in the treatment of BD.60 Lithium effectively treats acute mania and prevents relapse.61 It preferentially targets the active neurons, entering through both voltage-responsive and neurotransmitter-coupled channels.43,62 This results in an increase of intracellular lithium concentrations to as much as 8 times that of the extracellular concentration.63 These ions displace intracellular sodium ions in a 1:1 ratio, which results in a reduced intracellular sodium concentration that reduces the excitability of neurons.43,57,62
Substantial evidence supports the use of valproic acid for initial and maintenance treatment of BD.64 It inhibits the voltage-sensitive sodium channel when the channel is open, which results in an activity-dependent action that selectively impacts rapidly firing neurons.43 The voltage-gated sodium channels exist nearly exclusively on the axon, beyond the hillock65; as such, valproic acid will only inhibit neurons that fire, whereas lithium accumulates throughout the neuron and will affect depolarization in the neuronal soma as well as the firing in the axon.43 Additionally, valproic acid has been observed to enhance gamma-aminobutyric acid (GABA) levels and transmission.43,66,67 A meta-analysis that included 6 randomized controlled trials illustrated that, acutely, valproate was not different from lithium’s overall efficacy (RR 1.02; 95% CI, 0.87 to 1.20), but was associated with reduced dropout rates compared with placebo or lithium (RR 0.82; 95% CI, 0.71 to 0.95 and RR 0.87; 95% CI, 0.77 to 0.98, respectively).64
Lamotrigine is an anticonvulsant used for initial and maintenance treatment of BD, with greater efficacy for depressive episodes68; it also has notable effect for treating bipolar depression, although it is not FDA-approved for this indication.69 Lamotrigine inhibits sodium influx by binding to open voltage-gated sodium channels70 but also appears to reduce N-methyl-D-aspartate–mediated sodium entry,71 thereby acting both prehillock and posthillock.
Continue to: Carbamazepine is an anticonvulsant...
Carbamazepine is an anticonvulsant FDA-approved for treating BD.7 Like valproate, it acts by inhibiting voltage-gated sodium channels in an activity-dependent manner,72 which means it preferentially inhibits the most active neurons and those with higher intracellular sodium.43
Benzodiazepines, which have shown to be effective for treating acute mania,73 potentiate synaptic GABA receptors accruing an elevation in intracellular chloride influx.74 Despite acute efficacy, benzodiazepine use is limited because these agents are associated with worsening long-term, substance use–related outcomes.75,76
Antipsychotics are effective for treating mood disorders,60,76 and their use has been rising dramatically.12 The antimanic effect of all antipsychotics is believed to be mediated through dopamine D2 blockade, since use of a dose sufficient to block D2 receptors is required, and haloperidol, which acts exclusively on the D2 receptor, is equal to SGAs in its antimanic effect.77 Blockade of the D2 receptor will increase the activity of the sodium pump (sodium and potassium-activated adenosine triphosphatase) thus reducing intracellular sodium and calcium concentrations.37 When antipsychotics are used as antidepressants, they are generally used at doses lower than those used to treat mania.78
Antipsychotics are effective for treating BD, and may work more quickly than other agents for treating acute mania.79 However, maintenance or prevention trials tend to favor mood stabilizers.35,60,80 Several add-on studies have found the combination of a mood stabilizer plus an antipsychotic is superior to a mood stabilizer alone or an antipsychotic alone.81
An argument for mood stabilizers
Evidence suggests mood stabilizers and other approaches, such as antipsychotics, are almost equivalent for treating acute mania, with a small clinical advantage of mood stabilizers for preventing relapse. In general, current treatment guidelines do not distinguish mood stabilizers from antipsychotics as the first-line treatment.82 Over the past 20 years, antipsychotic use has increased while mood stabilizer use has decreased, so that presently a patient with BD is more likely to be prescribed an antipsychotic than a mood stabilizer.12 Over the same time, dysfunction among patients with BD has increased.33 Antipsychotics are appealing because they appear to be equally effective and generally well tolerated. But these agents cause problems that are difficult to see in routine visits, such as metabolic27-31 and cardiovascular adverse effects29 as well as reductions in brain volume.23-26 Mechanistic research suggests that mood stabilizers directly correct known pathophysiologic anomalies with additional protective effects, whereas antipsychotics appear to create new abnormalities and contribute to medical problems. Clinicians need to look beyond the similarities in acute efficacy and make a more broadly supported, evidence-based choice for managing BD, which clearly places mood stabilizers as the first-line agent and antipsychotics as reasonable alternatives. At a minimum, mood stabilizers should be viewed as the foundation to which antipsychotics can be added.
Bottom Line
Traditional mood stabilizers—lithium and some anticonvulsants—are the only agents that directly address physiologic abnormalities associated with both mania and bipolar depression, including mood state–associated elevations of intracellular sodium. Because of their specificity, these agents maximize mood stabilization and minimize adverse effects.
Related Resources
- Karas A, Stummer L, Freedberg A. Psychiatric and nonpsychiatric indications for mood stabilizers and select antiepileptics. Current Psychiatry. 2022;21(4):34-38. doi:10.12788/cp.0230
- Koch J. Mood stabilizers: balancing tolerability, serum levels, and dosage. Current Psychiatry. 2021;20(7):37-40. doi:10.12788/cp.0147
Drug Brand Names
Carbamazepine • Tegretol
Haloperidol • Haldol
Lamotrigine • Lamictal
Lithium • Eskalith, Lithobid
Quetiapine • Seroquel
Valproate • Depakote, Depakene
1. Whiteford HA, Degenhardt L, Rehm J, et al. Global burden of disease attributable to mental and substance use disorders: findings from the Global Burden of Disease Study 2010. Lancet. 2013;382(9904):1575-1586. doi:10.1016/S0140-6736(13)61611-6
2. Merikangas KR, Jin R, He JP, et al. Prevalence and correlates of bipolar spectrum disorder in the world mental health survey initiative. Arch Gen Psychiatry. 2011;68(3):241-251. doi:10.1001/archgenpsychiatry.2011.12
3. Müller JK, Leweke FM. Bipolar disorder: clinical overview. Article in English, German. Med Monatsschr Pharm. 2016;39(9):363-369.
4. Smith DJ, Whitham EA, Ghaemi SN. Bipolar disorder. Handb Clin Neurol. 2012;106:251-263. doi:10.1016/B978-0-444-52002-9.00015-2
5. Goodwin FK, Ghaemi SN. The impact of the discovery of lithium on psychiatric thought and practice in the USA and Europe. Aust N Z J Psychiatry. 1999;33 Suppl:S54-S64. doi:10.1111/j.1440-1614.1999.00669.x
6. Pope HG, McElroy SL, Keck PE, et al. Valproate in the treatment of acute mania. A placebo-controlled study. Arch Gen Psychiatry. 1991;48(1):62-68. doi:10.1001/archpsyc.1991.01810250064008
7. Weisler RH, Keck PE Jr, Swann AC, et al. Extended-release carbamazepine capsules as monotherapy for acute mania in bipolar disorder: a multicenter, randomized, double-blind, placebo-controlled trial. J Clin Psychiatry. 2005;66(3):323-330. doi:10.4088/jcp.v66n0308
8. Tarr GP, Glue P, Herbison P. Comparative efficacy and acceptability of mood stabilizer and second generation antipsychotic monotherapy for acute mania--a systematic review and meta-analysis. J Affect Disord. 2011;134(1-3):14-19. doi:10.1016/j.jad.2010.11.009
9. Pahwa M, Sleem A, Elsayed OH, et al. New antipsychotic medications in the last decade. Curr Psychiatry Rep. 2021;23(12):87.
10. Correll CU, Sheridan EM, DelBello MP. Antipsychotic and mood stabilizer efficacy and tolerability in pediatric and adult patients with bipolar I mania: a comparative analysis of acute, randomized, placebo-controlled trials. Bipolar Disord. 2010;12(2):116-141. doi:10.1111/j.1399-5618.2010.00798.x
11. Rybakowski JK. Two generations of mood stabilizers. Int J Neuropsychopharmacol. 2007;10:709-711. doi:10.1017/s146114570700795x
12. Rhee TG, Olfson M, Nierenberg AA, et al. 20-year trends in the pharmacologic treatment of bipolar disorder by psychiatrists in outpatient care settings. Am J Psychiatry. 2020;177(8):706-715. doi:10.1176/appi.ajp.2020.19091000
13. El-Mallakh RS. Adjunctive antidepressant treatment for bipolar depression. N Engl J Med. 2007;357(6):615; author reply 615-616.
14. Sachs GS, Nierenberg AA, Calabrese JR, et al. Effectiveness of adjunctive antidepressant treatment for bipolar depression. N Engl J Med. 2007;356(17):1711-1722. doi:10.1056/NEJMoa064135
15. Ghaemi SN, Whitham EA, Vohringer PA, et al. Citalopram for acute and preventive efficacy in bipolar depression (CAPE-BD): a randomized, double-blind, placebo-controlled trial. J Clin Psychiatry. 2021;82(1):19m13136. doi:10.4088/JCP.19m13136
16. El-Mallakh RS, Ghaemi SN, Sagduyu K, et al. Antidepressant-associated chronic irritable dysphoria (ACID) in STEP-BD patients. J Affect Disord. 2008;111(2-3):372-377. doi:10.1016/j.jad.2008.03.025
17. Ghaemi SN, Ostacher MM, El-Mallakh RS, et al. Antidepressant discontinuation in bipolar depression: a systematic treatment enhancement program for bipolar disorder (STEP-BD) randomized clinical trial of long-term effectiveness and safety. J Clin Psychiatry. 2010;71(4):372-380.
18. Strejilevich SA, Martino DJ, Marengo E, et al. Long-term worsening of bipolar disorder related with frequency of antidepressant exposure. Ann Clin Psychiatry. 2011;23(3):186-192.
19. Pacchiarotti I, Bond DJ, Baldessarini RJ, et al. The International Society of Bipolar Disorders (ISBD) task force report on antidepressant use in bipolar disorders. Am J Psychiatry. 2013;170(11):1249-1262. doi:10.1176/appi.ajp.2013.13020185
20. McIntyre RS, Calabrese JR. Bipolar depression: the clinical characteristics and unmet needs of a complex disorder. Curr Med Res Opin. 2019;35(11):1993-2005.
21. Fornaro M, Stubbs B, De Berardis D, et al. Atypical antipsychotics in the treatment of acute bipolar depression with mixed features: a systematic review and exploratory meta-analysis of placebo-controlled clinical trials. Int J Mol Sci. 2016;17(2):241. doi:10.3390/ijms17020241
22. Lindström L, Lindström E, Nilsson M, et al. Maintenance therapy with second generation antipsychotics for bipolar disorder – a systematic review and meta-analysis. J Affect Disord. 2017;213:138-150. doi:10.1016/j.jad.2017.02.012
23. Ho BC, Andreasen NC, Ziebell S, et al. Long-term antipsychotic treatment and brain volumes: a longitudinal study of first-episode schizophrenia. Arch Gen Psychiatry. 2011;68(2):128-137. doi:010.1001/archgenpsychiatry.2010.199
24. Voineskos AN, Mulsant BH, Dickie EW, et al. Effects of antipsychotic medication on brain structure in patients with major depressive disorder and psychotic features: neuroimaging findings in the context of a randomized placebo-controlled clinical trial. JAMA Psychiatry. 2020;77(7):674-683. doi:10.1001/jamapsychiatry.2020.0036
25. Konopaske GT, Bolo NR, Basu AC, et al. Time-dependent effects of haloperidol on glutamine and GABA homeostasis and astrocyte activity in the rat brain. Psychopharmacology (Berl). 2013;230(1):57-67. doi:10.1007/s00213-013-3136-3
26. Dorph-Petersen KA, Pierri JN, Perel JM, et al. The influence of chronic exposure to antipsychotic medications on brain size before and after tissue fixation: a comparison of haloperidol and olanzapine in macaque monkeys. Neuropsychopharmacology. 2005;30(9):1649-1661. doi:10.1038/sj.npp.1300710
27. McIntyre RS, Mancini DA, Basile VS, et al. Antipsychotic-induced weight gain: bipolar disorder and leptin. J Clin Psychopharmacol. 2003;23(4):323-327. doi:10.1097/01.jcp.0000085403.08426.f4
28. McIntyre RS, Konarski JZ, Wilkins K, et al. Obesity in bipolar disorder and major depressive disorder: results from a national community health survey on mental health and well-being. Can J Psychiatry. 2006;51(5):274-280. doi:10.1177/070674370605100502
29. McIntyre RS, Cha DS, Kim RD, et al. A review of FDA-approved treatment options in bipolar depression. CNS Spectr. 2013;18(Suppl 1):4-20. doi:10.1017/S1092852913000746
30. Barton BB, Segger F, Fischer K, et al. Update on weight-gain caused by antipsychotics: a systematic review and meta-analysis. Expert Opin Drug Saf. 2020;19(3):295-314. doi:10.1080/14740338.2020.1713091
31. Doane MJ, Bessonova L, Friedler HS, et al. Weight gain and comorbidities associated with oral second-generation antipsychotics: analysis of real-world data for patients with schizophrenia or bipolar I disorder. BMC Psychiatry. 2022;22(1):114. doi:10.1186/s12888-022-03758-w
32. Buckley NA, Sanders P. Cardiovascular adverse effects of antipsychotic drugs. Drug Saf. 2000;23(3):215-228. doi:10.2165/00002018-200023030-00004
33. Ali Z, Roque A, El-Mallakh RS. A unifying theory for the pathoetiologic mechanism of tardive dyskinesia. Med Hypotheses. 2020;140:109682. doi:10.1016/j.mehy.2020.109682
34. Sleem A, El-Mallakh RS. Adaptive changes to antipsychotics: their consequences and how to avoid them. Curr Psychiatry. 2022;21(7):46-50,52. doi: 10.12788/cp.0262
35. Nierenberg AA, McElroy SL, Friedman ES, et al. Bipolar CHOICE (Clinical Health Outcomes Initiative in Comparative Effectiveness): a pragmatic 6-month trial of lithium versus quetiapine for bipolar disorder. J Clin Psychiatry. 2016;77(1):90-99. doi:10.4088/JCP.14m09349
36. He H, Hu C, Ren Z, et al. Trends in the incidence and DALYs of bipolar disorder at global, regional, and national levels: results from the global burden of disease study 2017. J Psychiatr Res. 2020;125:96-105. doi:10.1016/j.jpsychires.2020.03.015
37. Roberts RJ, Repass R, El-Mallakh RS. Effect of dopamine on intracellular sodium: a common pathway for pharmacological mechanism of action in bipolar illness. World J Biol Psychiatry. 2010;11(2 Pt 2):181-187. doi:10.1080/15622970902718774
38. El-Mallakh RS, Wyatt RJ. The Na, K-ATPase hypothesis for bipolar illness. Biol Psychiatry. 1995;37(4):235-244. doi:10.1016/0006-3223(94)00201-D
39. El-Mallakh RS, Yff T, Gao Y. Ion dysregulation in the pathogenesis of bipolar disorder. Ann Depress Anxiety. 2016;3(1):1076.
40. Li R, El-Mallakh RS. Differential response of bipolar and normal control lymphoblastoid cell sodium pump to ethacrynic acid. J Affect Disord. 2004;80(1):11-17. doi:10.1016/S0165-0327(03)00044-2
41. Woodruff DB, El-Mallakh RS, Thiruvengadam AP. Validation of a diagnostic screening blood test for bipolar disorder. Ann Clin Psychiatry. 2012;24(2):135-139.
42. Gao Y, Lohano K, Delamere NA, et al. Ethanol normalizes glutamate-induced elevation of intracellular sodium in olfactory neuroepithelial progenitors from subjects with bipolar illness but not nonbipolar controls: biologic evidence for the self-medication hypothesis. Bipolar Disord. 2019;21(2):179-181. doi:10.1111/bdi.12737
43. El-Mallakh RS, Huff MO. Mood stabilizers and ion regulation. Harv Rev Psychiatry. 2001;9(1):23-32. doi:10.1080/10673220127873
44. Phillips ML, Swartz HA. A critical appraisal of neuroimaging studies of bipolar disorder: toward a new conceptualization of underlying neural circuitry and a road map for future research. Am J Psychiatry. 2014;171(8):829-843. doi:10.1176/appi.ajp.2014.13081008
45. Hibar DP, Westlye LT, Doan NT, et al. Cortical abnormalities in bipolar disorder: an MRI analysis of 6503 individuals from the ENIGMA Bipolar Disorder Working Group. Mol Psychiatry. 2018;23(4):932-942. doi:10.1038/mp.2017.73
46. Brown NC, Andreazza AC, Young LT. An updated meta-analysis of oxidative stress markers in bipolar disorder. Psychiatry Res. 2014;218(1-2):61-68. doi:10.1016/j.psychres.2014.04.005
47. Benedetti F, Aggio V, Pratesi ML, et al. Neuroinflammation in bipolar depression. Front Psychiatry. 2020;11:71. doi:10.3389/fpsyt.2020.00071
48. Andreazza AC, Duong A, Young LT. Bipolar disorder as a mitochondrial disease. Biol Psychiatry. 2018;83(9):720-721. doi:10.1016/j.biopsych.2017.09.018
49. Askland KD. Toward a biaxial model of “bipolar” affective disorders: further exploration of genetic, molecular and cellular substrates. J Affect Disord. 2006;94(1-3):35-66. doi:10.1016/j.jad.2006.01.033
50. Ferreira MA, O’Donovan MC, Meng YA, et al; Wellcome Trust Case Control Consortium. Collaborative genome-wide association analysis supports a role for ANK3 and CACNA1C in bipolar disorder. Nat Genet 2008;40(9):1056-1058. doi:10.1038/ng.209
51. Salvi AM, Bays JL, Mackin SR, et al. Ankyrin G organizes membrane components to promote coupling of cell mechanics and glucose uptake. Nat Cell Biol. 2021;23(5):457-466. doi:10.1038/s41556-021-00677-y
52. Gargus JJ. Ion channel functional candidate genes in multigenic neuropsychiatric disease. Biol Psychiatry. 2006;60(2):177-185. doi:10.1016/j.biopsych.2005.12.008
53. Dubovsky SL, Murphy J, Thomas M, et al. Abnormal intracellular calcium ion concentration in platelets and lymphocytes of bipolar patients. Am J Psychiatry 1992;149(1):118-120. doi:10.1176/ajp.149.1.118
54. Blaustein MP. Physiological effects of endogenous ouabain: control of intracellular Ca2+ stores and cell responsiveness. Am J Physiol. 1993;264(6 Pt 1):C1367–C1387. doi:10.1152/ajpcell.1993.264.6.C1367
55. El-Mallakh RS, Li R, Worth CA, et al. Leukocyte transmembrane potential in bipolar illness. J Affect Disord. 1996;41(1):33-37. doi:10.1016/0165-0327(96)00063-8
56. El-Mallakh RS, Gao Y, You P. Role of endogenous ouabain in the etiology of bipolar disorder. Int J Bipolar Disord. 2021;9(1):6. doi:10.1186/s40345-020-00213-1
57. Huang X, Lei Z, El‐Mallakh RS. Lithium normalizes elevated intracellular sodium. Bipolar Disord. 2007;9(3):298-300. doi:10.1111/j.1399-5618.2007.00429.x
58. Shaw DM. Mineral metabolism, mania, and melancholia. Br Med J. 1966;2(5508):262-267. doi:10.1136/bmj.2.5508.262
59. Qian K, Yu N, Tucker KR, et al. Mathematical analysis of depolarization block mediated by slow inactivation of fast sodium channels in midbrain dopamine neurons. J Neurophysiol. 2014;112(11):2779-2790. doi:10.1152/jn.00578.2014
60. Sleem A, El-Mallakh RS. Advances in the psychopharmacotherapy of bipolar disorder type I. Exp Opin Pharmacother. 2021;22(10):1267-1290. doi:10.1080/14656566.2021.1893306
61. Malhi GS., Tanious M, Das P, et al. Potential mechanisms of action of lithium in bipolar disorder. CNS Drugs. 2013;27(2):135-153. doi:10.1007/s40263-013-0039-0
62. Armett CJ, Ritchie JM. On the permeability of mammalian non-myelinated fibers to sodium and to lithium ions. J Physiol. 1963;165(1):130-140. doi:10.1113/jphysiol.1963.sp007047
63. Kabakov AY, Karkanias NB, Lenox RH, et al. Synapse-specific accumulation of lithium in intracellular microdomains: a model for uncoupling coincidence detection in the brain. Synapse. 1998;28(4):271-279. doi:10.1002/(SICI)1098-2396(199804)28:4<271::AID-SYN2>3.0.CO;2-6
64. Cipriani A, Reid K, Young AH, et al. Valproic acid, valproate and divalproex in the maintenance treatment of bipolar disorder. Cochrane Database Syst Rev. 2013;2013(10):CD003196. doi:10.1002/14651858.CD003196.pub2
65. Lai HC, Jan LY. The distribution and targeting of neuronal voltage-gated ion channels. Nat Rev Neurosci. 2006;7(7):548-562. doi:10.1038/nrn1938
66. Löscher W, Schmidt D. Increase of human plasma GABA by sodium valproate. Epilepsia. 1980;21(6):611-615. doi:10.1111/j.1528-1157.1980.tb04314.x
67. Owens MJ, Nemeroff CB. Pharmacology of valproate. Psychopharmacol Bull. 2003;37(Suppl 2):17-24.
68. Calabrese JR, Vieta E, Shelton MD. Latest maintenance data on lamotrigine in bipolar disorder. Eur Neuropsychopharmacol. 2003;13(Suppl 2):S57-S66. doi:10.1016/s0924-977x(03)00079-8
69. Geddes JR, Calabrese JR, Goodwin GM. Lamotrigine for treatment of bipolar depression: independent meta-analysis and meta-regression of individual patient data from five randomised trials. Br J Psychiatry. 2009;194(1):4-9. doi:10.1192/bjp.bp.107.048504
70. Nakatani Y, Masuko H, Amano T. Effect of lamotrigine on Na(v)1.4 voltage-gated sodium channels. J Pharmacol Sci. 2013;123(2):203-206. doi:10.1254/jphs.13116sc
71. Ramadan E, Basselin M, Rao JS, et al. Lamotrigine blocks NMDA receptor-initiated arachidonic acid signalling in rat brain: implications for its efficacy in bipolar disorder. Int J Neuropsychopharmacol. 2012;15(7):931-943. doi:10.1017/S1461145711001003
72. Jo S, Bean BP. Sidedness of carbamazepine accessibility to voltage-gated sodium channels. Mol Pharmacol. 2014;85(2):381-387. doi:10.1124/mol.113.090472
73. Curtin F, Schulz P. Clonazepam and lorazepam in acute mania: a Bayesian meta-analysis. J Affect Disord 2004;78(3):201-208. doi:10.1016/S0165-0327(02)00317-8
74. Edwards R, Stephenson U, Flewett T. Clonazepam in acute mania: a double blind trial. Aust N Z J Psychiatry 1991;25(2):238-242. doi:10.3109/00048679109077740
75. Lin SC, Chen CC, Chen YH, et al. Benzodiazepine prescription among patients with severe mental illness and co-occurring alcohol abuse/dependence in Taiwan. Hum Psychopharmacol. 2011;26(3):201-207. doi:10.1002/hup.1193
76. Prisciandaro JJ, Brown DG, Brady KT, et al. Comorbid anxiety disorders and baseline medication regimens predict clinical outcomes in individuals with co-occurring bipolar disorder and alcohol dependence: results of a randomized controlled trial. Psychiatry Res. 2011;188(3):361-365. doi:10.1016/j.psychres.2011.04.030
77. Ashok AH, Marques TR, Jauhar S, et al. The dopamine hypothesis of bipolar affective disorder: the state of the art and implications for treatment. Mol Psychiatry. 2017;22(5):666-679. doi:10.1038/mp.2017.16
78. Roberts RJ, Lohano KK, El-Mallakh RS. Antipsychotics as antidepressants. Asia Pac Psychiatry. 2016;8(3):179-188. doi:10.1111/appy.12186
79. Cipriani A, Barbui C, Salanti G, et al. Comparative efficacy and acceptability of antimanic drugs in acute mania: a multiple-treatments meta-analysis. Lancet. 2011;378(9799):1306-1315. doi:10.1016/S0140-6736(11)60873-8
80. Hayes JF, Marston L, Walters K, et al. Lithium vs. valproate vs. olanzapine vs. quetiapine as maintenance monotherapy for bipolar disorder: a population-based UK cohort study using electronic health records. World Psychiatry. 2016;15(1):53-58. doi:10.1002/wps.20298
81. Geddes JR, Gardiner A, Rendell J, et al. Comparative evaluation of quetiapine plus lamotrigine combination versus quetiapine monotherapy (and folic acid versus placebo) in bipolar depression (CEQUEL): a 2 × 2 factorial randomised trial. Lancet Psychiatry. 2016;3(1):31239. doi:10.1016/S2215-0366(15)00450-2
82. Goodwin GM, Haddad PM, Ferrier IN, et al. Evidence-based guidelines for treating bipolar disorder: revised third edition recommendations from the British Association for Psychopharmacology. J Psychopharmacol. 2016;30(6):495-553. doi:10.1177/0269881116636545
Bipolar disorder (BD) is a recurrent, life-long psychiatric illness affecting nearly 2% of the world population1,2 that is characterized by episodes of mania and depression interspersed among periods of relative mood stability.3 The illness causes an enormous health burden, which makes understanding its pathophysiology and treatment patterns a substantial priority.4 In the 1950s, lithium was found to be effective for treating acute manic episodes and preventing relapse in BD.5 Since then, valproate and carbamazepine also have been FDA-approved for treating mania.6,7 Antipsychotics have also shown evidence of efficacy in BD treatment,8,9 particularly for use in acute settings for more rapid effect or for a limited duration,10 which has led some to refer to them as “mood stabilizers.”11
In this article, we describe changes in trends of prescribing medications to treat BD, the role of ion dysregulation in the disorder, and how a better understanding of this dysregulation might impact the choice of treatment.
Changes in pharmacotherapy for bipolar disorder
From 1997 through 2016, the use of lithium for BD decreased from >30% of patients to 17.6% (with a nadir of 13.9% from 2009 to 2012).12 Over the same period, the use of nonlithium mood stabilizers decreased from 30.4% to approximately 4.8%, while second-generation antipsychotic (SGAs) use increased from 12.4% to 50.4%.12 Distressingly, antidepressant use increased from approximately 47% to 56.8%, and antidepressant use without concomitant mood stabilizers increased from 38% to 40.8%, although the rate of antidepressants without either a mood stabilizer or an antipsychotic remained relatively stable (14.9% to 16.8%).12 In randomized trials, when added to mood stabilizers, antidepressants have consistently failed to separate from placebo,13-15 but they can destabilize the illness, resulting in increases in mania, depression, and subsyndromal mixed symptoms.16-18
It is easy to understand clinicians’ attempts to address their patients’ distress due to depressive symptoms that do not resolve with mood stabilizers.19,20 Similarly, the increased use of antipsychotics is driven by evidence that antipsychotics are effective for treating bipolar depression and preventing the recurrence of manic and (for some antipsychotics) depressive episodes.21,22 However, long-term antipsychotic use causes brain volume change in patients with schizophrenia23 or major depressive disorder24 and in nonhuman primates25,26; metabolic abnormalities27-31; and cardiovascular adverse effects.32 Antipsychotics are believed to be associated with withdrawal psychosis.33,34 In the head-to-head Clinical Health Outcomes Initiative in Comparative Effectiveness for Bipolar Disorder (Bipolar CHOICE) study, quetiapine was as effective as lithium but associated with more adverse effects.35 Importantly, the estimated disability-adjusted life years of patients with BD increased by 54.4% from 6.02 million in 1990 to 9.29 million in 2017, which is greater than the increase in the incidence of BD (47.74%) over the same time.36 This means that despite the dramatic increase in treatment options for people with BD, functional outcomes have declined.
One major difference between antipsychotics and mood stabilizers is that antipsychotics do not alter the underlying abnormal pathology of BD.37 An ideal pharmacologic intervention is one that corrects a known pathophysiologic anomaly of the condition being treated. There are no demonstrated abnormalities in the dopamine or serotonin systems in individuals with BD, but long-term use of antipsychotics may create dopaminergic alterations.33 One of the most reproducible biomarkers associated with manic and bipolar depressed mood states is increased intracellular sodium38,39 and reduced ability to correct a sodium challenge.40-42 By normalizing intracellular sodium levels, lithium and the mood-stabilizing anticonvulsants uniquely and specifically counter known physiologic abnormalities in patients with BD.37,43
The role of ion dysregulation
The pathophysiology of BD remains elusive. A multitude of lines of evidence link BD to abnormal neuroimaging findings,22,44,45 oxidative stress,46 inflammation,47 and mitochondrial disease,48 but there is still no unifying understanding of these findings. Ion dysregulation appears to be central to understanding and treating BD.38,39
Despite extensive genetic studies, no genes have been identified that mediate >5% of the risk for BD. Nonetheless, 74% of all genes identified as mediating risk for BD code for proteins essential for the regulation of ion transport and membrane potential.49 The 2 genes that contribute the greatest risk are CACNA1C and ANK3, which code for a calcium channel and a cytoskeletal protein, respectively.50ANK3 codes for ankyrin G, which plays a role in proper coupling of the voltage-gated sodium channels to the cytoskeleton.51 An additional risk gene, TRANK1, contains multiple ankyrin-like repeat domains, which suggests some shared functions with ANK3.52 More importantly, the most reproducible pathophysiologic findings in BD are dysregulation of sodium, potassium, hydrogen, and calcium transport, with consequent alteration of depolarization potential, neuronal excitability, and calcium-mediated processes.38,39,53-56 For example, increased sodium and calcium within cells have been observed in both mania and bipolar depression, and these levels normalize during euthymia. All medications that are effective for treating BD may reduce intracellular sodium or calcium; traditional mood stabilizers do so directly by inhibiting voltage-sensitive sodium channels in an activity-dependent manner or displacing intracellular sodium,43,57 whereas antipsychotics do so indirectly by increasing sodium pump activity through inhibition of second messengers of the dopamine D2 family of receptors.37
Continue to: The extent of ion dysregulation...
The extent of ion dysregulation is directly associated with the expressed mood state of the illness. A small reduction in the activity of the sodium pump results in a small increase in intracellular sodium (approximately 10 mM).39,58 This led to the hypothesis that increased intracellular sodium causes the transmembrane potential to increase closer to membrane depolarization threshold, which increases excitability of affected neurons.38,39,58 Neurons are likely to fire and propagate signals more easily, which may manifest as symptoms of mania, such as increased energy, activity, lability, excitability, irritability, tangentiality, and looseness of associations. As the process of increased intracellular sodium progresses, a minority of neurons are expected to have their transmembrane potentials depolarize sufficiently for the resting membrane potential to go beyond threshold potential.59 Such neurons are in a state of constant depolarization (also known as depolarization block), which disrupts neuronal circuits. The difficulty in progression of these signals results in the classic bipolar depression symptoms of low energy, reduced activity, and slowing of all brain activity that is seen as psychomotor slowing.38
Implications for treatment
Medications for treating bipolar illness include lithium, anticonvulsants, benzodiazepines, first-generation antipsychotics, and SGAs.37,43
Mood stabilizers (lithium and certain anticonvulsants) correct the previously mentioned sodium abnormality by reducing sodium entry into the cell in an activity-dependent manner.43 As the only agents that directly address a known pathophysiologic abnormality, they are foundational in the treatment of BD.60 Lithium effectively treats acute mania and prevents relapse.61 It preferentially targets the active neurons, entering through both voltage-responsive and neurotransmitter-coupled channels.43,62 This results in an increase of intracellular lithium concentrations to as much as 8 times that of the extracellular concentration.63 These ions displace intracellular sodium ions in a 1:1 ratio, which results in a reduced intracellular sodium concentration that reduces the excitability of neurons.43,57,62
Substantial evidence supports the use of valproic acid for initial and maintenance treatment of BD.64 It inhibits the voltage-sensitive sodium channel when the channel is open, which results in an activity-dependent action that selectively impacts rapidly firing neurons.43 The voltage-gated sodium channels exist nearly exclusively on the axon, beyond the hillock65; as such, valproic acid will only inhibit neurons that fire, whereas lithium accumulates throughout the neuron and will affect depolarization in the neuronal soma as well as the firing in the axon.43 Additionally, valproic acid has been observed to enhance gamma-aminobutyric acid (GABA) levels and transmission.43,66,67 A meta-analysis that included 6 randomized controlled trials illustrated that, acutely, valproate was not different from lithium’s overall efficacy (RR 1.02; 95% CI, 0.87 to 1.20), but was associated with reduced dropout rates compared with placebo or lithium (RR 0.82; 95% CI, 0.71 to 0.95 and RR 0.87; 95% CI, 0.77 to 0.98, respectively).64
Lamotrigine is an anticonvulsant used for initial and maintenance treatment of BD, with greater efficacy for depressive episodes68; it also has notable effect for treating bipolar depression, although it is not FDA-approved for this indication.69 Lamotrigine inhibits sodium influx by binding to open voltage-gated sodium channels70 but also appears to reduce N-methyl-D-aspartate–mediated sodium entry,71 thereby acting both prehillock and posthillock.
Continue to: Carbamazepine is an anticonvulsant...
Carbamazepine is an anticonvulsant FDA-approved for treating BD.7 Like valproate, it acts by inhibiting voltage-gated sodium channels in an activity-dependent manner,72 which means it preferentially inhibits the most active neurons and those with higher intracellular sodium.43
Benzodiazepines, which have shown to be effective for treating acute mania,73 potentiate synaptic GABA receptors accruing an elevation in intracellular chloride influx.74 Despite acute efficacy, benzodiazepine use is limited because these agents are associated with worsening long-term, substance use–related outcomes.75,76
Antipsychotics are effective for treating mood disorders,60,76 and their use has been rising dramatically.12 The antimanic effect of all antipsychotics is believed to be mediated through dopamine D2 blockade, since use of a dose sufficient to block D2 receptors is required, and haloperidol, which acts exclusively on the D2 receptor, is equal to SGAs in its antimanic effect.77 Blockade of the D2 receptor will increase the activity of the sodium pump (sodium and potassium-activated adenosine triphosphatase) thus reducing intracellular sodium and calcium concentrations.37 When antipsychotics are used as antidepressants, they are generally used at doses lower than those used to treat mania.78
Antipsychotics are effective for treating BD, and may work more quickly than other agents for treating acute mania.79 However, maintenance or prevention trials tend to favor mood stabilizers.35,60,80 Several add-on studies have found the combination of a mood stabilizer plus an antipsychotic is superior to a mood stabilizer alone or an antipsychotic alone.81
An argument for mood stabilizers
Evidence suggests mood stabilizers and other approaches, such as antipsychotics, are almost equivalent for treating acute mania, with a small clinical advantage of mood stabilizers for preventing relapse. In general, current treatment guidelines do not distinguish mood stabilizers from antipsychotics as the first-line treatment.82 Over the past 20 years, antipsychotic use has increased while mood stabilizer use has decreased, so that presently a patient with BD is more likely to be prescribed an antipsychotic than a mood stabilizer.12 Over the same time, dysfunction among patients with BD has increased.33 Antipsychotics are appealing because they appear to be equally effective and generally well tolerated. But these agents cause problems that are difficult to see in routine visits, such as metabolic27-31 and cardiovascular adverse effects29 as well as reductions in brain volume.23-26 Mechanistic research suggests that mood stabilizers directly correct known pathophysiologic anomalies with additional protective effects, whereas antipsychotics appear to create new abnormalities and contribute to medical problems. Clinicians need to look beyond the similarities in acute efficacy and make a more broadly supported, evidence-based choice for managing BD, which clearly places mood stabilizers as the first-line agent and antipsychotics as reasonable alternatives. At a minimum, mood stabilizers should be viewed as the foundation to which antipsychotics can be added.
Bottom Line
Traditional mood stabilizers—lithium and some anticonvulsants—are the only agents that directly address physiologic abnormalities associated with both mania and bipolar depression, including mood state–associated elevations of intracellular sodium. Because of their specificity, these agents maximize mood stabilization and minimize adverse effects.
Related Resources
- Karas A, Stummer L, Freedberg A. Psychiatric and nonpsychiatric indications for mood stabilizers and select antiepileptics. Current Psychiatry. 2022;21(4):34-38. doi:10.12788/cp.0230
- Koch J. Mood stabilizers: balancing tolerability, serum levels, and dosage. Current Psychiatry. 2021;20(7):37-40. doi:10.12788/cp.0147
Drug Brand Names
Carbamazepine • Tegretol
Haloperidol • Haldol
Lamotrigine • Lamictal
Lithium • Eskalith, Lithobid
Quetiapine • Seroquel
Valproate • Depakote, Depakene
Bipolar disorder (BD) is a recurrent, life-long psychiatric illness affecting nearly 2% of the world population1,2 that is characterized by episodes of mania and depression interspersed among periods of relative mood stability.3 The illness causes an enormous health burden, which makes understanding its pathophysiology and treatment patterns a substantial priority.4 In the 1950s, lithium was found to be effective for treating acute manic episodes and preventing relapse in BD.5 Since then, valproate and carbamazepine also have been FDA-approved for treating mania.6,7 Antipsychotics have also shown evidence of efficacy in BD treatment,8,9 particularly for use in acute settings for more rapid effect or for a limited duration,10 which has led some to refer to them as “mood stabilizers.”11
In this article, we describe changes in trends of prescribing medications to treat BD, the role of ion dysregulation in the disorder, and how a better understanding of this dysregulation might impact the choice of treatment.
Changes in pharmacotherapy for bipolar disorder
From 1997 through 2016, the use of lithium for BD decreased from >30% of patients to 17.6% (with a nadir of 13.9% from 2009 to 2012).12 Over the same period, the use of nonlithium mood stabilizers decreased from 30.4% to approximately 4.8%, while second-generation antipsychotic (SGAs) use increased from 12.4% to 50.4%.12 Distressingly, antidepressant use increased from approximately 47% to 56.8%, and antidepressant use without concomitant mood stabilizers increased from 38% to 40.8%, although the rate of antidepressants without either a mood stabilizer or an antipsychotic remained relatively stable (14.9% to 16.8%).12 In randomized trials, when added to mood stabilizers, antidepressants have consistently failed to separate from placebo,13-15 but they can destabilize the illness, resulting in increases in mania, depression, and subsyndromal mixed symptoms.16-18
It is easy to understand clinicians’ attempts to address their patients’ distress due to depressive symptoms that do not resolve with mood stabilizers.19,20 Similarly, the increased use of antipsychotics is driven by evidence that antipsychotics are effective for treating bipolar depression and preventing the recurrence of manic and (for some antipsychotics) depressive episodes.21,22 However, long-term antipsychotic use causes brain volume change in patients with schizophrenia23 or major depressive disorder24 and in nonhuman primates25,26; metabolic abnormalities27-31; and cardiovascular adverse effects.32 Antipsychotics are believed to be associated with withdrawal psychosis.33,34 In the head-to-head Clinical Health Outcomes Initiative in Comparative Effectiveness for Bipolar Disorder (Bipolar CHOICE) study, quetiapine was as effective as lithium but associated with more adverse effects.35 Importantly, the estimated disability-adjusted life years of patients with BD increased by 54.4% from 6.02 million in 1990 to 9.29 million in 2017, which is greater than the increase in the incidence of BD (47.74%) over the same time.36 This means that despite the dramatic increase in treatment options for people with BD, functional outcomes have declined.
One major difference between antipsychotics and mood stabilizers is that antipsychotics do not alter the underlying abnormal pathology of BD.37 An ideal pharmacologic intervention is one that corrects a known pathophysiologic anomaly of the condition being treated. There are no demonstrated abnormalities in the dopamine or serotonin systems in individuals with BD, but long-term use of antipsychotics may create dopaminergic alterations.33 One of the most reproducible biomarkers associated with manic and bipolar depressed mood states is increased intracellular sodium38,39 and reduced ability to correct a sodium challenge.40-42 By normalizing intracellular sodium levels, lithium and the mood-stabilizing anticonvulsants uniquely and specifically counter known physiologic abnormalities in patients with BD.37,43
The role of ion dysregulation
The pathophysiology of BD remains elusive. A multitude of lines of evidence link BD to abnormal neuroimaging findings,22,44,45 oxidative stress,46 inflammation,47 and mitochondrial disease,48 but there is still no unifying understanding of these findings. Ion dysregulation appears to be central to understanding and treating BD.38,39
Despite extensive genetic studies, no genes have been identified that mediate >5% of the risk for BD. Nonetheless, 74% of all genes identified as mediating risk for BD code for proteins essential for the regulation of ion transport and membrane potential.49 The 2 genes that contribute the greatest risk are CACNA1C and ANK3, which code for a calcium channel and a cytoskeletal protein, respectively.50ANK3 codes for ankyrin G, which plays a role in proper coupling of the voltage-gated sodium channels to the cytoskeleton.51 An additional risk gene, TRANK1, contains multiple ankyrin-like repeat domains, which suggests some shared functions with ANK3.52 More importantly, the most reproducible pathophysiologic findings in BD are dysregulation of sodium, potassium, hydrogen, and calcium transport, with consequent alteration of depolarization potential, neuronal excitability, and calcium-mediated processes.38,39,53-56 For example, increased sodium and calcium within cells have been observed in both mania and bipolar depression, and these levels normalize during euthymia. All medications that are effective for treating BD may reduce intracellular sodium or calcium; traditional mood stabilizers do so directly by inhibiting voltage-sensitive sodium channels in an activity-dependent manner or displacing intracellular sodium,43,57 whereas antipsychotics do so indirectly by increasing sodium pump activity through inhibition of second messengers of the dopamine D2 family of receptors.37
Continue to: The extent of ion dysregulation...
The extent of ion dysregulation is directly associated with the expressed mood state of the illness. A small reduction in the activity of the sodium pump results in a small increase in intracellular sodium (approximately 10 mM).39,58 This led to the hypothesis that increased intracellular sodium causes the transmembrane potential to increase closer to membrane depolarization threshold, which increases excitability of affected neurons.38,39,58 Neurons are likely to fire and propagate signals more easily, which may manifest as symptoms of mania, such as increased energy, activity, lability, excitability, irritability, tangentiality, and looseness of associations. As the process of increased intracellular sodium progresses, a minority of neurons are expected to have their transmembrane potentials depolarize sufficiently for the resting membrane potential to go beyond threshold potential.59 Such neurons are in a state of constant depolarization (also known as depolarization block), which disrupts neuronal circuits. The difficulty in progression of these signals results in the classic bipolar depression symptoms of low energy, reduced activity, and slowing of all brain activity that is seen as psychomotor slowing.38
Implications for treatment
Medications for treating bipolar illness include lithium, anticonvulsants, benzodiazepines, first-generation antipsychotics, and SGAs.37,43
Mood stabilizers (lithium and certain anticonvulsants) correct the previously mentioned sodium abnormality by reducing sodium entry into the cell in an activity-dependent manner.43 As the only agents that directly address a known pathophysiologic abnormality, they are foundational in the treatment of BD.60 Lithium effectively treats acute mania and prevents relapse.61 It preferentially targets the active neurons, entering through both voltage-responsive and neurotransmitter-coupled channels.43,62 This results in an increase of intracellular lithium concentrations to as much as 8 times that of the extracellular concentration.63 These ions displace intracellular sodium ions in a 1:1 ratio, which results in a reduced intracellular sodium concentration that reduces the excitability of neurons.43,57,62
Substantial evidence supports the use of valproic acid for initial and maintenance treatment of BD.64 It inhibits the voltage-sensitive sodium channel when the channel is open, which results in an activity-dependent action that selectively impacts rapidly firing neurons.43 The voltage-gated sodium channels exist nearly exclusively on the axon, beyond the hillock65; as such, valproic acid will only inhibit neurons that fire, whereas lithium accumulates throughout the neuron and will affect depolarization in the neuronal soma as well as the firing in the axon.43 Additionally, valproic acid has been observed to enhance gamma-aminobutyric acid (GABA) levels and transmission.43,66,67 A meta-analysis that included 6 randomized controlled trials illustrated that, acutely, valproate was not different from lithium’s overall efficacy (RR 1.02; 95% CI, 0.87 to 1.20), but was associated with reduced dropout rates compared with placebo or lithium (RR 0.82; 95% CI, 0.71 to 0.95 and RR 0.87; 95% CI, 0.77 to 0.98, respectively).64
Lamotrigine is an anticonvulsant used for initial and maintenance treatment of BD, with greater efficacy for depressive episodes68; it also has notable effect for treating bipolar depression, although it is not FDA-approved for this indication.69 Lamotrigine inhibits sodium influx by binding to open voltage-gated sodium channels70 but also appears to reduce N-methyl-D-aspartate–mediated sodium entry,71 thereby acting both prehillock and posthillock.
Continue to: Carbamazepine is an anticonvulsant...
Carbamazepine is an anticonvulsant FDA-approved for treating BD.7 Like valproate, it acts by inhibiting voltage-gated sodium channels in an activity-dependent manner,72 which means it preferentially inhibits the most active neurons and those with higher intracellular sodium.43
Benzodiazepines, which have shown to be effective for treating acute mania,73 potentiate synaptic GABA receptors accruing an elevation in intracellular chloride influx.74 Despite acute efficacy, benzodiazepine use is limited because these agents are associated with worsening long-term, substance use–related outcomes.75,76
Antipsychotics are effective for treating mood disorders,60,76 and their use has been rising dramatically.12 The antimanic effect of all antipsychotics is believed to be mediated through dopamine D2 blockade, since use of a dose sufficient to block D2 receptors is required, and haloperidol, which acts exclusively on the D2 receptor, is equal to SGAs in its antimanic effect.77 Blockade of the D2 receptor will increase the activity of the sodium pump (sodium and potassium-activated adenosine triphosphatase) thus reducing intracellular sodium and calcium concentrations.37 When antipsychotics are used as antidepressants, they are generally used at doses lower than those used to treat mania.78
Antipsychotics are effective for treating BD, and may work more quickly than other agents for treating acute mania.79 However, maintenance or prevention trials tend to favor mood stabilizers.35,60,80 Several add-on studies have found the combination of a mood stabilizer plus an antipsychotic is superior to a mood stabilizer alone or an antipsychotic alone.81
An argument for mood stabilizers
Evidence suggests mood stabilizers and other approaches, such as antipsychotics, are almost equivalent for treating acute mania, with a small clinical advantage of mood stabilizers for preventing relapse. In general, current treatment guidelines do not distinguish mood stabilizers from antipsychotics as the first-line treatment.82 Over the past 20 years, antipsychotic use has increased while mood stabilizer use has decreased, so that presently a patient with BD is more likely to be prescribed an antipsychotic than a mood stabilizer.12 Over the same time, dysfunction among patients with BD has increased.33 Antipsychotics are appealing because they appear to be equally effective and generally well tolerated. But these agents cause problems that are difficult to see in routine visits, such as metabolic27-31 and cardiovascular adverse effects29 as well as reductions in brain volume.23-26 Mechanistic research suggests that mood stabilizers directly correct known pathophysiologic anomalies with additional protective effects, whereas antipsychotics appear to create new abnormalities and contribute to medical problems. Clinicians need to look beyond the similarities in acute efficacy and make a more broadly supported, evidence-based choice for managing BD, which clearly places mood stabilizers as the first-line agent and antipsychotics as reasonable alternatives. At a minimum, mood stabilizers should be viewed as the foundation to which antipsychotics can be added.
Bottom Line
Traditional mood stabilizers—lithium and some anticonvulsants—are the only agents that directly address physiologic abnormalities associated with both mania and bipolar depression, including mood state–associated elevations of intracellular sodium. Because of their specificity, these agents maximize mood stabilization and minimize adverse effects.
Related Resources
- Karas A, Stummer L, Freedberg A. Psychiatric and nonpsychiatric indications for mood stabilizers and select antiepileptics. Current Psychiatry. 2022;21(4):34-38. doi:10.12788/cp.0230
- Koch J. Mood stabilizers: balancing tolerability, serum levels, and dosage. Current Psychiatry. 2021;20(7):37-40. doi:10.12788/cp.0147
Drug Brand Names
Carbamazepine • Tegretol
Haloperidol • Haldol
Lamotrigine • Lamictal
Lithium • Eskalith, Lithobid
Quetiapine • Seroquel
Valproate • Depakote, Depakene
1. Whiteford HA, Degenhardt L, Rehm J, et al. Global burden of disease attributable to mental and substance use disorders: findings from the Global Burden of Disease Study 2010. Lancet. 2013;382(9904):1575-1586. doi:10.1016/S0140-6736(13)61611-6
2. Merikangas KR, Jin R, He JP, et al. Prevalence and correlates of bipolar spectrum disorder in the world mental health survey initiative. Arch Gen Psychiatry. 2011;68(3):241-251. doi:10.1001/archgenpsychiatry.2011.12
3. Müller JK, Leweke FM. Bipolar disorder: clinical overview. Article in English, German. Med Monatsschr Pharm. 2016;39(9):363-369.
4. Smith DJ, Whitham EA, Ghaemi SN. Bipolar disorder. Handb Clin Neurol. 2012;106:251-263. doi:10.1016/B978-0-444-52002-9.00015-2
5. Goodwin FK, Ghaemi SN. The impact of the discovery of lithium on psychiatric thought and practice in the USA and Europe. Aust N Z J Psychiatry. 1999;33 Suppl:S54-S64. doi:10.1111/j.1440-1614.1999.00669.x
6. Pope HG, McElroy SL, Keck PE, et al. Valproate in the treatment of acute mania. A placebo-controlled study. Arch Gen Psychiatry. 1991;48(1):62-68. doi:10.1001/archpsyc.1991.01810250064008
7. Weisler RH, Keck PE Jr, Swann AC, et al. Extended-release carbamazepine capsules as monotherapy for acute mania in bipolar disorder: a multicenter, randomized, double-blind, placebo-controlled trial. J Clin Psychiatry. 2005;66(3):323-330. doi:10.4088/jcp.v66n0308
8. Tarr GP, Glue P, Herbison P. Comparative efficacy and acceptability of mood stabilizer and second generation antipsychotic monotherapy for acute mania--a systematic review and meta-analysis. J Affect Disord. 2011;134(1-3):14-19. doi:10.1016/j.jad.2010.11.009
9. Pahwa M, Sleem A, Elsayed OH, et al. New antipsychotic medications in the last decade. Curr Psychiatry Rep. 2021;23(12):87.
10. Correll CU, Sheridan EM, DelBello MP. Antipsychotic and mood stabilizer efficacy and tolerability in pediatric and adult patients with bipolar I mania: a comparative analysis of acute, randomized, placebo-controlled trials. Bipolar Disord. 2010;12(2):116-141. doi:10.1111/j.1399-5618.2010.00798.x
11. Rybakowski JK. Two generations of mood stabilizers. Int J Neuropsychopharmacol. 2007;10:709-711. doi:10.1017/s146114570700795x
12. Rhee TG, Olfson M, Nierenberg AA, et al. 20-year trends in the pharmacologic treatment of bipolar disorder by psychiatrists in outpatient care settings. Am J Psychiatry. 2020;177(8):706-715. doi:10.1176/appi.ajp.2020.19091000
13. El-Mallakh RS. Adjunctive antidepressant treatment for bipolar depression. N Engl J Med. 2007;357(6):615; author reply 615-616.
14. Sachs GS, Nierenberg AA, Calabrese JR, et al. Effectiveness of adjunctive antidepressant treatment for bipolar depression. N Engl J Med. 2007;356(17):1711-1722. doi:10.1056/NEJMoa064135
15. Ghaemi SN, Whitham EA, Vohringer PA, et al. Citalopram for acute and preventive efficacy in bipolar depression (CAPE-BD): a randomized, double-blind, placebo-controlled trial. J Clin Psychiatry. 2021;82(1):19m13136. doi:10.4088/JCP.19m13136
16. El-Mallakh RS, Ghaemi SN, Sagduyu K, et al. Antidepressant-associated chronic irritable dysphoria (ACID) in STEP-BD patients. J Affect Disord. 2008;111(2-3):372-377. doi:10.1016/j.jad.2008.03.025
17. Ghaemi SN, Ostacher MM, El-Mallakh RS, et al. Antidepressant discontinuation in bipolar depression: a systematic treatment enhancement program for bipolar disorder (STEP-BD) randomized clinical trial of long-term effectiveness and safety. J Clin Psychiatry. 2010;71(4):372-380.
18. Strejilevich SA, Martino DJ, Marengo E, et al. Long-term worsening of bipolar disorder related with frequency of antidepressant exposure. Ann Clin Psychiatry. 2011;23(3):186-192.
19. Pacchiarotti I, Bond DJ, Baldessarini RJ, et al. The International Society of Bipolar Disorders (ISBD) task force report on antidepressant use in bipolar disorders. Am J Psychiatry. 2013;170(11):1249-1262. doi:10.1176/appi.ajp.2013.13020185
20. McIntyre RS, Calabrese JR. Bipolar depression: the clinical characteristics and unmet needs of a complex disorder. Curr Med Res Opin. 2019;35(11):1993-2005.
21. Fornaro M, Stubbs B, De Berardis D, et al. Atypical antipsychotics in the treatment of acute bipolar depression with mixed features: a systematic review and exploratory meta-analysis of placebo-controlled clinical trials. Int J Mol Sci. 2016;17(2):241. doi:10.3390/ijms17020241
22. Lindström L, Lindström E, Nilsson M, et al. Maintenance therapy with second generation antipsychotics for bipolar disorder – a systematic review and meta-analysis. J Affect Disord. 2017;213:138-150. doi:10.1016/j.jad.2017.02.012
23. Ho BC, Andreasen NC, Ziebell S, et al. Long-term antipsychotic treatment and brain volumes: a longitudinal study of first-episode schizophrenia. Arch Gen Psychiatry. 2011;68(2):128-137. doi:010.1001/archgenpsychiatry.2010.199
24. Voineskos AN, Mulsant BH, Dickie EW, et al. Effects of antipsychotic medication on brain structure in patients with major depressive disorder and psychotic features: neuroimaging findings in the context of a randomized placebo-controlled clinical trial. JAMA Psychiatry. 2020;77(7):674-683. doi:10.1001/jamapsychiatry.2020.0036
25. Konopaske GT, Bolo NR, Basu AC, et al. Time-dependent effects of haloperidol on glutamine and GABA homeostasis and astrocyte activity in the rat brain. Psychopharmacology (Berl). 2013;230(1):57-67. doi:10.1007/s00213-013-3136-3
26. Dorph-Petersen KA, Pierri JN, Perel JM, et al. The influence of chronic exposure to antipsychotic medications on brain size before and after tissue fixation: a comparison of haloperidol and olanzapine in macaque monkeys. Neuropsychopharmacology. 2005;30(9):1649-1661. doi:10.1038/sj.npp.1300710
27. McIntyre RS, Mancini DA, Basile VS, et al. Antipsychotic-induced weight gain: bipolar disorder and leptin. J Clin Psychopharmacol. 2003;23(4):323-327. doi:10.1097/01.jcp.0000085403.08426.f4
28. McIntyre RS, Konarski JZ, Wilkins K, et al. Obesity in bipolar disorder and major depressive disorder: results from a national community health survey on mental health and well-being. Can J Psychiatry. 2006;51(5):274-280. doi:10.1177/070674370605100502
29. McIntyre RS, Cha DS, Kim RD, et al. A review of FDA-approved treatment options in bipolar depression. CNS Spectr. 2013;18(Suppl 1):4-20. doi:10.1017/S1092852913000746
30. Barton BB, Segger F, Fischer K, et al. Update on weight-gain caused by antipsychotics: a systematic review and meta-analysis. Expert Opin Drug Saf. 2020;19(3):295-314. doi:10.1080/14740338.2020.1713091
31. Doane MJ, Bessonova L, Friedler HS, et al. Weight gain and comorbidities associated with oral second-generation antipsychotics: analysis of real-world data for patients with schizophrenia or bipolar I disorder. BMC Psychiatry. 2022;22(1):114. doi:10.1186/s12888-022-03758-w
32. Buckley NA, Sanders P. Cardiovascular adverse effects of antipsychotic drugs. Drug Saf. 2000;23(3):215-228. doi:10.2165/00002018-200023030-00004
33. Ali Z, Roque A, El-Mallakh RS. A unifying theory for the pathoetiologic mechanism of tardive dyskinesia. Med Hypotheses. 2020;140:109682. doi:10.1016/j.mehy.2020.109682
34. Sleem A, El-Mallakh RS. Adaptive changes to antipsychotics: their consequences and how to avoid them. Curr Psychiatry. 2022;21(7):46-50,52. doi: 10.12788/cp.0262
35. Nierenberg AA, McElroy SL, Friedman ES, et al. Bipolar CHOICE (Clinical Health Outcomes Initiative in Comparative Effectiveness): a pragmatic 6-month trial of lithium versus quetiapine for bipolar disorder. J Clin Psychiatry. 2016;77(1):90-99. doi:10.4088/JCP.14m09349
36. He H, Hu C, Ren Z, et al. Trends in the incidence and DALYs of bipolar disorder at global, regional, and national levels: results from the global burden of disease study 2017. J Psychiatr Res. 2020;125:96-105. doi:10.1016/j.jpsychires.2020.03.015
37. Roberts RJ, Repass R, El-Mallakh RS. Effect of dopamine on intracellular sodium: a common pathway for pharmacological mechanism of action in bipolar illness. World J Biol Psychiatry. 2010;11(2 Pt 2):181-187. doi:10.1080/15622970902718774
38. El-Mallakh RS, Wyatt RJ. The Na, K-ATPase hypothesis for bipolar illness. Biol Psychiatry. 1995;37(4):235-244. doi:10.1016/0006-3223(94)00201-D
39. El-Mallakh RS, Yff T, Gao Y. Ion dysregulation in the pathogenesis of bipolar disorder. Ann Depress Anxiety. 2016;3(1):1076.
40. Li R, El-Mallakh RS. Differential response of bipolar and normal control lymphoblastoid cell sodium pump to ethacrynic acid. J Affect Disord. 2004;80(1):11-17. doi:10.1016/S0165-0327(03)00044-2
41. Woodruff DB, El-Mallakh RS, Thiruvengadam AP. Validation of a diagnostic screening blood test for bipolar disorder. Ann Clin Psychiatry. 2012;24(2):135-139.
42. Gao Y, Lohano K, Delamere NA, et al. Ethanol normalizes glutamate-induced elevation of intracellular sodium in olfactory neuroepithelial progenitors from subjects with bipolar illness but not nonbipolar controls: biologic evidence for the self-medication hypothesis. Bipolar Disord. 2019;21(2):179-181. doi:10.1111/bdi.12737
43. El-Mallakh RS, Huff MO. Mood stabilizers and ion regulation. Harv Rev Psychiatry. 2001;9(1):23-32. doi:10.1080/10673220127873
44. Phillips ML, Swartz HA. A critical appraisal of neuroimaging studies of bipolar disorder: toward a new conceptualization of underlying neural circuitry and a road map for future research. Am J Psychiatry. 2014;171(8):829-843. doi:10.1176/appi.ajp.2014.13081008
45. Hibar DP, Westlye LT, Doan NT, et al. Cortical abnormalities in bipolar disorder: an MRI analysis of 6503 individuals from the ENIGMA Bipolar Disorder Working Group. Mol Psychiatry. 2018;23(4):932-942. doi:10.1038/mp.2017.73
46. Brown NC, Andreazza AC, Young LT. An updated meta-analysis of oxidative stress markers in bipolar disorder. Psychiatry Res. 2014;218(1-2):61-68. doi:10.1016/j.psychres.2014.04.005
47. Benedetti F, Aggio V, Pratesi ML, et al. Neuroinflammation in bipolar depression. Front Psychiatry. 2020;11:71. doi:10.3389/fpsyt.2020.00071
48. Andreazza AC, Duong A, Young LT. Bipolar disorder as a mitochondrial disease. Biol Psychiatry. 2018;83(9):720-721. doi:10.1016/j.biopsych.2017.09.018
49. Askland KD. Toward a biaxial model of “bipolar” affective disorders: further exploration of genetic, molecular and cellular substrates. J Affect Disord. 2006;94(1-3):35-66. doi:10.1016/j.jad.2006.01.033
50. Ferreira MA, O’Donovan MC, Meng YA, et al; Wellcome Trust Case Control Consortium. Collaborative genome-wide association analysis supports a role for ANK3 and CACNA1C in bipolar disorder. Nat Genet 2008;40(9):1056-1058. doi:10.1038/ng.209
51. Salvi AM, Bays JL, Mackin SR, et al. Ankyrin G organizes membrane components to promote coupling of cell mechanics and glucose uptake. Nat Cell Biol. 2021;23(5):457-466. doi:10.1038/s41556-021-00677-y
52. Gargus JJ. Ion channel functional candidate genes in multigenic neuropsychiatric disease. Biol Psychiatry. 2006;60(2):177-185. doi:10.1016/j.biopsych.2005.12.008
53. Dubovsky SL, Murphy J, Thomas M, et al. Abnormal intracellular calcium ion concentration in platelets and lymphocytes of bipolar patients. Am J Psychiatry 1992;149(1):118-120. doi:10.1176/ajp.149.1.118
54. Blaustein MP. Physiological effects of endogenous ouabain: control of intracellular Ca2+ stores and cell responsiveness. Am J Physiol. 1993;264(6 Pt 1):C1367–C1387. doi:10.1152/ajpcell.1993.264.6.C1367
55. El-Mallakh RS, Li R, Worth CA, et al. Leukocyte transmembrane potential in bipolar illness. J Affect Disord. 1996;41(1):33-37. doi:10.1016/0165-0327(96)00063-8
56. El-Mallakh RS, Gao Y, You P. Role of endogenous ouabain in the etiology of bipolar disorder. Int J Bipolar Disord. 2021;9(1):6. doi:10.1186/s40345-020-00213-1
57. Huang X, Lei Z, El‐Mallakh RS. Lithium normalizes elevated intracellular sodium. Bipolar Disord. 2007;9(3):298-300. doi:10.1111/j.1399-5618.2007.00429.x
58. Shaw DM. Mineral metabolism, mania, and melancholia. Br Med J. 1966;2(5508):262-267. doi:10.1136/bmj.2.5508.262
59. Qian K, Yu N, Tucker KR, et al. Mathematical analysis of depolarization block mediated by slow inactivation of fast sodium channels in midbrain dopamine neurons. J Neurophysiol. 2014;112(11):2779-2790. doi:10.1152/jn.00578.2014
60. Sleem A, El-Mallakh RS. Advances in the psychopharmacotherapy of bipolar disorder type I. Exp Opin Pharmacother. 2021;22(10):1267-1290. doi:10.1080/14656566.2021.1893306
61. Malhi GS., Tanious M, Das P, et al. Potential mechanisms of action of lithium in bipolar disorder. CNS Drugs. 2013;27(2):135-153. doi:10.1007/s40263-013-0039-0
62. Armett CJ, Ritchie JM. On the permeability of mammalian non-myelinated fibers to sodium and to lithium ions. J Physiol. 1963;165(1):130-140. doi:10.1113/jphysiol.1963.sp007047
63. Kabakov AY, Karkanias NB, Lenox RH, et al. Synapse-specific accumulation of lithium in intracellular microdomains: a model for uncoupling coincidence detection in the brain. Synapse. 1998;28(4):271-279. doi:10.1002/(SICI)1098-2396(199804)28:4<271::AID-SYN2>3.0.CO;2-6
64. Cipriani A, Reid K, Young AH, et al. Valproic acid, valproate and divalproex in the maintenance treatment of bipolar disorder. Cochrane Database Syst Rev. 2013;2013(10):CD003196. doi:10.1002/14651858.CD003196.pub2
65. Lai HC, Jan LY. The distribution and targeting of neuronal voltage-gated ion channels. Nat Rev Neurosci. 2006;7(7):548-562. doi:10.1038/nrn1938
66. Löscher W, Schmidt D. Increase of human plasma GABA by sodium valproate. Epilepsia. 1980;21(6):611-615. doi:10.1111/j.1528-1157.1980.tb04314.x
67. Owens MJ, Nemeroff CB. Pharmacology of valproate. Psychopharmacol Bull. 2003;37(Suppl 2):17-24.
68. Calabrese JR, Vieta E, Shelton MD. Latest maintenance data on lamotrigine in bipolar disorder. Eur Neuropsychopharmacol. 2003;13(Suppl 2):S57-S66. doi:10.1016/s0924-977x(03)00079-8
69. Geddes JR, Calabrese JR, Goodwin GM. Lamotrigine for treatment of bipolar depression: independent meta-analysis and meta-regression of individual patient data from five randomised trials. Br J Psychiatry. 2009;194(1):4-9. doi:10.1192/bjp.bp.107.048504
70. Nakatani Y, Masuko H, Amano T. Effect of lamotrigine on Na(v)1.4 voltage-gated sodium channels. J Pharmacol Sci. 2013;123(2):203-206. doi:10.1254/jphs.13116sc
71. Ramadan E, Basselin M, Rao JS, et al. Lamotrigine blocks NMDA receptor-initiated arachidonic acid signalling in rat brain: implications for its efficacy in bipolar disorder. Int J Neuropsychopharmacol. 2012;15(7):931-943. doi:10.1017/S1461145711001003
72. Jo S, Bean BP. Sidedness of carbamazepine accessibility to voltage-gated sodium channels. Mol Pharmacol. 2014;85(2):381-387. doi:10.1124/mol.113.090472
73. Curtin F, Schulz P. Clonazepam and lorazepam in acute mania: a Bayesian meta-analysis. J Affect Disord 2004;78(3):201-208. doi:10.1016/S0165-0327(02)00317-8
74. Edwards R, Stephenson U, Flewett T. Clonazepam in acute mania: a double blind trial. Aust N Z J Psychiatry 1991;25(2):238-242. doi:10.3109/00048679109077740
75. Lin SC, Chen CC, Chen YH, et al. Benzodiazepine prescription among patients with severe mental illness and co-occurring alcohol abuse/dependence in Taiwan. Hum Psychopharmacol. 2011;26(3):201-207. doi:10.1002/hup.1193
76. Prisciandaro JJ, Brown DG, Brady KT, et al. Comorbid anxiety disorders and baseline medication regimens predict clinical outcomes in individuals with co-occurring bipolar disorder and alcohol dependence: results of a randomized controlled trial. Psychiatry Res. 2011;188(3):361-365. doi:10.1016/j.psychres.2011.04.030
77. Ashok AH, Marques TR, Jauhar S, et al. The dopamine hypothesis of bipolar affective disorder: the state of the art and implications for treatment. Mol Psychiatry. 2017;22(5):666-679. doi:10.1038/mp.2017.16
78. Roberts RJ, Lohano KK, El-Mallakh RS. Antipsychotics as antidepressants. Asia Pac Psychiatry. 2016;8(3):179-188. doi:10.1111/appy.12186
79. Cipriani A, Barbui C, Salanti G, et al. Comparative efficacy and acceptability of antimanic drugs in acute mania: a multiple-treatments meta-analysis. Lancet. 2011;378(9799):1306-1315. doi:10.1016/S0140-6736(11)60873-8
80. Hayes JF, Marston L, Walters K, et al. Lithium vs. valproate vs. olanzapine vs. quetiapine as maintenance monotherapy for bipolar disorder: a population-based UK cohort study using electronic health records. World Psychiatry. 2016;15(1):53-58. doi:10.1002/wps.20298
81. Geddes JR, Gardiner A, Rendell J, et al. Comparative evaluation of quetiapine plus lamotrigine combination versus quetiapine monotherapy (and folic acid versus placebo) in bipolar depression (CEQUEL): a 2 × 2 factorial randomised trial. Lancet Psychiatry. 2016;3(1):31239. doi:10.1016/S2215-0366(15)00450-2
82. Goodwin GM, Haddad PM, Ferrier IN, et al. Evidence-based guidelines for treating bipolar disorder: revised third edition recommendations from the British Association for Psychopharmacology. J Psychopharmacol. 2016;30(6):495-553. doi:10.1177/0269881116636545
1. Whiteford HA, Degenhardt L, Rehm J, et al. Global burden of disease attributable to mental and substance use disorders: findings from the Global Burden of Disease Study 2010. Lancet. 2013;382(9904):1575-1586. doi:10.1016/S0140-6736(13)61611-6
2. Merikangas KR, Jin R, He JP, et al. Prevalence and correlates of bipolar spectrum disorder in the world mental health survey initiative. Arch Gen Psychiatry. 2011;68(3):241-251. doi:10.1001/archgenpsychiatry.2011.12
3. Müller JK, Leweke FM. Bipolar disorder: clinical overview. Article in English, German. Med Monatsschr Pharm. 2016;39(9):363-369.
4. Smith DJ, Whitham EA, Ghaemi SN. Bipolar disorder. Handb Clin Neurol. 2012;106:251-263. doi:10.1016/B978-0-444-52002-9.00015-2
5. Goodwin FK, Ghaemi SN. The impact of the discovery of lithium on psychiatric thought and practice in the USA and Europe. Aust N Z J Psychiatry. 1999;33 Suppl:S54-S64. doi:10.1111/j.1440-1614.1999.00669.x
6. Pope HG, McElroy SL, Keck PE, et al. Valproate in the treatment of acute mania. A placebo-controlled study. Arch Gen Psychiatry. 1991;48(1):62-68. doi:10.1001/archpsyc.1991.01810250064008
7. Weisler RH, Keck PE Jr, Swann AC, et al. Extended-release carbamazepine capsules as monotherapy for acute mania in bipolar disorder: a multicenter, randomized, double-blind, placebo-controlled trial. J Clin Psychiatry. 2005;66(3):323-330. doi:10.4088/jcp.v66n0308
8. Tarr GP, Glue P, Herbison P. Comparative efficacy and acceptability of mood stabilizer and second generation antipsychotic monotherapy for acute mania--a systematic review and meta-analysis. J Affect Disord. 2011;134(1-3):14-19. doi:10.1016/j.jad.2010.11.009
9. Pahwa M, Sleem A, Elsayed OH, et al. New antipsychotic medications in the last decade. Curr Psychiatry Rep. 2021;23(12):87.
10. Correll CU, Sheridan EM, DelBello MP. Antipsychotic and mood stabilizer efficacy and tolerability in pediatric and adult patients with bipolar I mania: a comparative analysis of acute, randomized, placebo-controlled trials. Bipolar Disord. 2010;12(2):116-141. doi:10.1111/j.1399-5618.2010.00798.x
11. Rybakowski JK. Two generations of mood stabilizers. Int J Neuropsychopharmacol. 2007;10:709-711. doi:10.1017/s146114570700795x
12. Rhee TG, Olfson M, Nierenberg AA, et al. 20-year trends in the pharmacologic treatment of bipolar disorder by psychiatrists in outpatient care settings. Am J Psychiatry. 2020;177(8):706-715. doi:10.1176/appi.ajp.2020.19091000
13. El-Mallakh RS. Adjunctive antidepressant treatment for bipolar depression. N Engl J Med. 2007;357(6):615; author reply 615-616.
14. Sachs GS, Nierenberg AA, Calabrese JR, et al. Effectiveness of adjunctive antidepressant treatment for bipolar depression. N Engl J Med. 2007;356(17):1711-1722. doi:10.1056/NEJMoa064135
15. Ghaemi SN, Whitham EA, Vohringer PA, et al. Citalopram for acute and preventive efficacy in bipolar depression (CAPE-BD): a randomized, double-blind, placebo-controlled trial. J Clin Psychiatry. 2021;82(1):19m13136. doi:10.4088/JCP.19m13136
16. El-Mallakh RS, Ghaemi SN, Sagduyu K, et al. Antidepressant-associated chronic irritable dysphoria (ACID) in STEP-BD patients. J Affect Disord. 2008;111(2-3):372-377. doi:10.1016/j.jad.2008.03.025
17. Ghaemi SN, Ostacher MM, El-Mallakh RS, et al. Antidepressant discontinuation in bipolar depression: a systematic treatment enhancement program for bipolar disorder (STEP-BD) randomized clinical trial of long-term effectiveness and safety. J Clin Psychiatry. 2010;71(4):372-380.
18. Strejilevich SA, Martino DJ, Marengo E, et al. Long-term worsening of bipolar disorder related with frequency of antidepressant exposure. Ann Clin Psychiatry. 2011;23(3):186-192.
19. Pacchiarotti I, Bond DJ, Baldessarini RJ, et al. The International Society of Bipolar Disorders (ISBD) task force report on antidepressant use in bipolar disorders. Am J Psychiatry. 2013;170(11):1249-1262. doi:10.1176/appi.ajp.2013.13020185
20. McIntyre RS, Calabrese JR. Bipolar depression: the clinical characteristics and unmet needs of a complex disorder. Curr Med Res Opin. 2019;35(11):1993-2005.
21. Fornaro M, Stubbs B, De Berardis D, et al. Atypical antipsychotics in the treatment of acute bipolar depression with mixed features: a systematic review and exploratory meta-analysis of placebo-controlled clinical trials. Int J Mol Sci. 2016;17(2):241. doi:10.3390/ijms17020241
22. Lindström L, Lindström E, Nilsson M, et al. Maintenance therapy with second generation antipsychotics for bipolar disorder – a systematic review and meta-analysis. J Affect Disord. 2017;213:138-150. doi:10.1016/j.jad.2017.02.012
23. Ho BC, Andreasen NC, Ziebell S, et al. Long-term antipsychotic treatment and brain volumes: a longitudinal study of first-episode schizophrenia. Arch Gen Psychiatry. 2011;68(2):128-137. doi:010.1001/archgenpsychiatry.2010.199
24. Voineskos AN, Mulsant BH, Dickie EW, et al. Effects of antipsychotic medication on brain structure in patients with major depressive disorder and psychotic features: neuroimaging findings in the context of a randomized placebo-controlled clinical trial. JAMA Psychiatry. 2020;77(7):674-683. doi:10.1001/jamapsychiatry.2020.0036
25. Konopaske GT, Bolo NR, Basu AC, et al. Time-dependent effects of haloperidol on glutamine and GABA homeostasis and astrocyte activity in the rat brain. Psychopharmacology (Berl). 2013;230(1):57-67. doi:10.1007/s00213-013-3136-3
26. Dorph-Petersen KA, Pierri JN, Perel JM, et al. The influence of chronic exposure to antipsychotic medications on brain size before and after tissue fixation: a comparison of haloperidol and olanzapine in macaque monkeys. Neuropsychopharmacology. 2005;30(9):1649-1661. doi:10.1038/sj.npp.1300710
27. McIntyre RS, Mancini DA, Basile VS, et al. Antipsychotic-induced weight gain: bipolar disorder and leptin. J Clin Psychopharmacol. 2003;23(4):323-327. doi:10.1097/01.jcp.0000085403.08426.f4
28. McIntyre RS, Konarski JZ, Wilkins K, et al. Obesity in bipolar disorder and major depressive disorder: results from a national community health survey on mental health and well-being. Can J Psychiatry. 2006;51(5):274-280. doi:10.1177/070674370605100502
29. McIntyre RS, Cha DS, Kim RD, et al. A review of FDA-approved treatment options in bipolar depression. CNS Spectr. 2013;18(Suppl 1):4-20. doi:10.1017/S1092852913000746
30. Barton BB, Segger F, Fischer K, et al. Update on weight-gain caused by antipsychotics: a systematic review and meta-analysis. Expert Opin Drug Saf. 2020;19(3):295-314. doi:10.1080/14740338.2020.1713091
31. Doane MJ, Bessonova L, Friedler HS, et al. Weight gain and comorbidities associated with oral second-generation antipsychotics: analysis of real-world data for patients with schizophrenia or bipolar I disorder. BMC Psychiatry. 2022;22(1):114. doi:10.1186/s12888-022-03758-w
32. Buckley NA, Sanders P. Cardiovascular adverse effects of antipsychotic drugs. Drug Saf. 2000;23(3):215-228. doi:10.2165/00002018-200023030-00004
33. Ali Z, Roque A, El-Mallakh RS. A unifying theory for the pathoetiologic mechanism of tardive dyskinesia. Med Hypotheses. 2020;140:109682. doi:10.1016/j.mehy.2020.109682
34. Sleem A, El-Mallakh RS. Adaptive changes to antipsychotics: their consequences and how to avoid them. Curr Psychiatry. 2022;21(7):46-50,52. doi: 10.12788/cp.0262
35. Nierenberg AA, McElroy SL, Friedman ES, et al. Bipolar CHOICE (Clinical Health Outcomes Initiative in Comparative Effectiveness): a pragmatic 6-month trial of lithium versus quetiapine for bipolar disorder. J Clin Psychiatry. 2016;77(1):90-99. doi:10.4088/JCP.14m09349
36. He H, Hu C, Ren Z, et al. Trends in the incidence and DALYs of bipolar disorder at global, regional, and national levels: results from the global burden of disease study 2017. J Psychiatr Res. 2020;125:96-105. doi:10.1016/j.jpsychires.2020.03.015
37. Roberts RJ, Repass R, El-Mallakh RS. Effect of dopamine on intracellular sodium: a common pathway for pharmacological mechanism of action in bipolar illness. World J Biol Psychiatry. 2010;11(2 Pt 2):181-187. doi:10.1080/15622970902718774
38. El-Mallakh RS, Wyatt RJ. The Na, K-ATPase hypothesis for bipolar illness. Biol Psychiatry. 1995;37(4):235-244. doi:10.1016/0006-3223(94)00201-D
39. El-Mallakh RS, Yff T, Gao Y. Ion dysregulation in the pathogenesis of bipolar disorder. Ann Depress Anxiety. 2016;3(1):1076.
40. Li R, El-Mallakh RS. Differential response of bipolar and normal control lymphoblastoid cell sodium pump to ethacrynic acid. J Affect Disord. 2004;80(1):11-17. doi:10.1016/S0165-0327(03)00044-2
41. Woodruff DB, El-Mallakh RS, Thiruvengadam AP. Validation of a diagnostic screening blood test for bipolar disorder. Ann Clin Psychiatry. 2012;24(2):135-139.
42. Gao Y, Lohano K, Delamere NA, et al. Ethanol normalizes glutamate-induced elevation of intracellular sodium in olfactory neuroepithelial progenitors from subjects with bipolar illness but not nonbipolar controls: biologic evidence for the self-medication hypothesis. Bipolar Disord. 2019;21(2):179-181. doi:10.1111/bdi.12737
43. El-Mallakh RS, Huff MO. Mood stabilizers and ion regulation. Harv Rev Psychiatry. 2001;9(1):23-32. doi:10.1080/10673220127873
44. Phillips ML, Swartz HA. A critical appraisal of neuroimaging studies of bipolar disorder: toward a new conceptualization of underlying neural circuitry and a road map for future research. Am J Psychiatry. 2014;171(8):829-843. doi:10.1176/appi.ajp.2014.13081008
45. Hibar DP, Westlye LT, Doan NT, et al. Cortical abnormalities in bipolar disorder: an MRI analysis of 6503 individuals from the ENIGMA Bipolar Disorder Working Group. Mol Psychiatry. 2018;23(4):932-942. doi:10.1038/mp.2017.73
46. Brown NC, Andreazza AC, Young LT. An updated meta-analysis of oxidative stress markers in bipolar disorder. Psychiatry Res. 2014;218(1-2):61-68. doi:10.1016/j.psychres.2014.04.005
47. Benedetti F, Aggio V, Pratesi ML, et al. Neuroinflammation in bipolar depression. Front Psychiatry. 2020;11:71. doi:10.3389/fpsyt.2020.00071
48. Andreazza AC, Duong A, Young LT. Bipolar disorder as a mitochondrial disease. Biol Psychiatry. 2018;83(9):720-721. doi:10.1016/j.biopsych.2017.09.018
49. Askland KD. Toward a biaxial model of “bipolar” affective disorders: further exploration of genetic, molecular and cellular substrates. J Affect Disord. 2006;94(1-3):35-66. doi:10.1016/j.jad.2006.01.033
50. Ferreira MA, O’Donovan MC, Meng YA, et al; Wellcome Trust Case Control Consortium. Collaborative genome-wide association analysis supports a role for ANK3 and CACNA1C in bipolar disorder. Nat Genet 2008;40(9):1056-1058. doi:10.1038/ng.209
51. Salvi AM, Bays JL, Mackin SR, et al. Ankyrin G organizes membrane components to promote coupling of cell mechanics and glucose uptake. Nat Cell Biol. 2021;23(5):457-466. doi:10.1038/s41556-021-00677-y
52. Gargus JJ. Ion channel functional candidate genes in multigenic neuropsychiatric disease. Biol Psychiatry. 2006;60(2):177-185. doi:10.1016/j.biopsych.2005.12.008
53. Dubovsky SL, Murphy J, Thomas M, et al. Abnormal intracellular calcium ion concentration in platelets and lymphocytes of bipolar patients. Am J Psychiatry 1992;149(1):118-120. doi:10.1176/ajp.149.1.118
54. Blaustein MP. Physiological effects of endogenous ouabain: control of intracellular Ca2+ stores and cell responsiveness. Am J Physiol. 1993;264(6 Pt 1):C1367–C1387. doi:10.1152/ajpcell.1993.264.6.C1367
55. El-Mallakh RS, Li R, Worth CA, et al. Leukocyte transmembrane potential in bipolar illness. J Affect Disord. 1996;41(1):33-37. doi:10.1016/0165-0327(96)00063-8
56. El-Mallakh RS, Gao Y, You P. Role of endogenous ouabain in the etiology of bipolar disorder. Int J Bipolar Disord. 2021;9(1):6. doi:10.1186/s40345-020-00213-1
57. Huang X, Lei Z, El‐Mallakh RS. Lithium normalizes elevated intracellular sodium. Bipolar Disord. 2007;9(3):298-300. doi:10.1111/j.1399-5618.2007.00429.x
58. Shaw DM. Mineral metabolism, mania, and melancholia. Br Med J. 1966;2(5508):262-267. doi:10.1136/bmj.2.5508.262
59. Qian K, Yu N, Tucker KR, et al. Mathematical analysis of depolarization block mediated by slow inactivation of fast sodium channels in midbrain dopamine neurons. J Neurophysiol. 2014;112(11):2779-2790. doi:10.1152/jn.00578.2014
60. Sleem A, El-Mallakh RS. Advances in the psychopharmacotherapy of bipolar disorder type I. Exp Opin Pharmacother. 2021;22(10):1267-1290. doi:10.1080/14656566.2021.1893306
61. Malhi GS., Tanious M, Das P, et al. Potential mechanisms of action of lithium in bipolar disorder. CNS Drugs. 2013;27(2):135-153. doi:10.1007/s40263-013-0039-0
62. Armett CJ, Ritchie JM. On the permeability of mammalian non-myelinated fibers to sodium and to lithium ions. J Physiol. 1963;165(1):130-140. doi:10.1113/jphysiol.1963.sp007047
63. Kabakov AY, Karkanias NB, Lenox RH, et al. Synapse-specific accumulation of lithium in intracellular microdomains: a model for uncoupling coincidence detection in the brain. Synapse. 1998;28(4):271-279. doi:10.1002/(SICI)1098-2396(199804)28:4<271::AID-SYN2>3.0.CO;2-6
64. Cipriani A, Reid K, Young AH, et al. Valproic acid, valproate and divalproex in the maintenance treatment of bipolar disorder. Cochrane Database Syst Rev. 2013;2013(10):CD003196. doi:10.1002/14651858.CD003196.pub2
65. Lai HC, Jan LY. The distribution and targeting of neuronal voltage-gated ion channels. Nat Rev Neurosci. 2006;7(7):548-562. doi:10.1038/nrn1938
66. Löscher W, Schmidt D. Increase of human plasma GABA by sodium valproate. Epilepsia. 1980;21(6):611-615. doi:10.1111/j.1528-1157.1980.tb04314.x
67. Owens MJ, Nemeroff CB. Pharmacology of valproate. Psychopharmacol Bull. 2003;37(Suppl 2):17-24.
68. Calabrese JR, Vieta E, Shelton MD. Latest maintenance data on lamotrigine in bipolar disorder. Eur Neuropsychopharmacol. 2003;13(Suppl 2):S57-S66. doi:10.1016/s0924-977x(03)00079-8
69. Geddes JR, Calabrese JR, Goodwin GM. Lamotrigine for treatment of bipolar depression: independent meta-analysis and meta-regression of individual patient data from five randomised trials. Br J Psychiatry. 2009;194(1):4-9. doi:10.1192/bjp.bp.107.048504
70. Nakatani Y, Masuko H, Amano T. Effect of lamotrigine on Na(v)1.4 voltage-gated sodium channels. J Pharmacol Sci. 2013;123(2):203-206. doi:10.1254/jphs.13116sc
71. Ramadan E, Basselin M, Rao JS, et al. Lamotrigine blocks NMDA receptor-initiated arachidonic acid signalling in rat brain: implications for its efficacy in bipolar disorder. Int J Neuropsychopharmacol. 2012;15(7):931-943. doi:10.1017/S1461145711001003
72. Jo S, Bean BP. Sidedness of carbamazepine accessibility to voltage-gated sodium channels. Mol Pharmacol. 2014;85(2):381-387. doi:10.1124/mol.113.090472
73. Curtin F, Schulz P. Clonazepam and lorazepam in acute mania: a Bayesian meta-analysis. J Affect Disord 2004;78(3):201-208. doi:10.1016/S0165-0327(02)00317-8
74. Edwards R, Stephenson U, Flewett T. Clonazepam in acute mania: a double blind trial. Aust N Z J Psychiatry 1991;25(2):238-242. doi:10.3109/00048679109077740
75. Lin SC, Chen CC, Chen YH, et al. Benzodiazepine prescription among patients with severe mental illness and co-occurring alcohol abuse/dependence in Taiwan. Hum Psychopharmacol. 2011;26(3):201-207. doi:10.1002/hup.1193
76. Prisciandaro JJ, Brown DG, Brady KT, et al. Comorbid anxiety disorders and baseline medication regimens predict clinical outcomes in individuals with co-occurring bipolar disorder and alcohol dependence: results of a randomized controlled trial. Psychiatry Res. 2011;188(3):361-365. doi:10.1016/j.psychres.2011.04.030
77. Ashok AH, Marques TR, Jauhar S, et al. The dopamine hypothesis of bipolar affective disorder: the state of the art and implications for treatment. Mol Psychiatry. 2017;22(5):666-679. doi:10.1038/mp.2017.16
78. Roberts RJ, Lohano KK, El-Mallakh RS. Antipsychotics as antidepressants. Asia Pac Psychiatry. 2016;8(3):179-188. doi:10.1111/appy.12186
79. Cipriani A, Barbui C, Salanti G, et al. Comparative efficacy and acceptability of antimanic drugs in acute mania: a multiple-treatments meta-analysis. Lancet. 2011;378(9799):1306-1315. doi:10.1016/S0140-6736(11)60873-8
80. Hayes JF, Marston L, Walters K, et al. Lithium vs. valproate vs. olanzapine vs. quetiapine as maintenance monotherapy for bipolar disorder: a population-based UK cohort study using electronic health records. World Psychiatry. 2016;15(1):53-58. doi:10.1002/wps.20298
81. Geddes JR, Gardiner A, Rendell J, et al. Comparative evaluation of quetiapine plus lamotrigine combination versus quetiapine monotherapy (and folic acid versus placebo) in bipolar depression (CEQUEL): a 2 × 2 factorial randomised trial. Lancet Psychiatry. 2016;3(1):31239. doi:10.1016/S2215-0366(15)00450-2
82. Goodwin GM, Haddad PM, Ferrier IN, et al. Evidence-based guidelines for treating bipolar disorder: revised third edition recommendations from the British Association for Psychopharmacology. J Psychopharmacol. 2016;30(6):495-553. doi:10.1177/0269881116636545
Adaptive changes to antipsychotics: How to avoid the consequences
While our understanding of the mechanisms of psychosis continues to evolve beyond the dopamine hypothesis, the key role of dopamine in psychosis and its treatment has not faded.1 Over time, the dopamine hypothesis of schizophrenia has evolved from focusing on dopamine hyperactivity to specifying the regional abnormalities in the brain with subcortical hyperdopaminergia and prefrontal hypodopaminergia.2 Despite this divergence in dopaminergic function, antipsychotic medications that block dopamine D2 receptors (D2R) remain central to treating psychotic symptoms and preventing relapse.3,4 Notably, antipsychotics block both presynaptic and postsynaptic receptors affecting the regulation of dopamine synthesis and release in the brain.5,6
Chronic dopamine D2R blockade with antipsychotics induces adaptive changes that can contribute to both acute and chronic adverse effects. In this article, we discuss these changes, and steps clinicians can take to minimize their occurrence.
Dopamine D2R: A primer
There are 5 types of dopamine receptors, numbered D1 through D5, but there are only 2 families of dopamine receptors: the D1 family (D1 and D5), and the D2 family (D2, D3, and D4). All dopamine receptors are G protein–coupled, but the D2 family of receptors generally increases protein kinase A (PKA) as the second messenger, whereas the D1 family increases cyclic adenosine monophosphate (cAMP) as the second messenger.5 There are 2 distinct variants of the D2R of 2 different lengths made from the same gene (DRD2) via posttranslational modification. The long isoform of D2R (D2L) has an additional 29 amino acids compared to the short isoform (D2S).7 Additional evidence points to a third splice variant called D2Longer that arises from aberrant RNA splicing and contains 2 more amino acids than D2L; its relevance is not known.8
The D2L isoform is the primary postsynaptic receptor, expressed more in the striatum and nucleus accumbens (NAc) targeted by dopaminergic afferents. The D2S isoform, however, is predominantly presynaptic, more densely expressed on cell bodies and projection axons of the dopaminergic neurons of the midbrain and hypothalamus.9 Each isoform contributes differentially to the therapeutic and adverse effects of antipsychotics, and evidence from animal studies suggests that D2L is the main variant responsible for drug-induced parkinsonism.10 The D2S acts as the principal autoreceptor for the dopaminergic system.5,11,12
Autoreceptors regulate dopamine transmission. Dopamine itself and D2R agonists are reported to have higher affinity and potency with D2S. Activation of these autoreceptors is a negative feedback mechanism that decreases dopamine release. Similarly, when they are blocked (such as with use of an antipsychotic), there is an increase in dopamine release. Additionally, these autoreceptors modulate several key processes:
- neuronal firing rate by activating potassium conductance
- dopamine synthesis by downregulating the expression of tyrosine hydroxylase (TH) enzyme (the rate-limiting step)
- exocytotic release of dopamine and other neurotransmitters
- dopamine reuptake via increasing the activity of the dopamine transporter (DAT).12
Consequences of antipsychotic D2R blockade
Most antipsychotics begin to produce a therapeutic antipsychotic effect at 65% to 75% occupancy of the D2Rs.3 This level also produces an optimal balance between clinical efficacy and a lower incidence of adverse effects.3 A higher D2R occupancy by both first-generation (FGA) and second-generation (SGA) pure antagonist antipsychotics can lead to parkinsonism.
Parkinsonism is associated with the subsequent appearance of one of the most distressing consequences of long-term antipsychotic treatment, tardive dyskinesia (TD).13 TD is an iatrogenic, usually late-onset syndrome consisting of persistent, involuntary, and repetitive movements. It classically involves the highly innervated striated muscles of the tongue, mouth, face, and fingers, though it can also involve the trunk and extremities.14 It occurs secondary to chronic exposure to dopamine receptor–blocking agents, including dopaminergic antiemetics.15 The prevalence of TD is higher in patients treated long-term with FGAs (30.0% to 32.4%) than in those treated with SGAs (13.1% to 20.7%) due to serotonin 5HT2A blockade that results in increased dopamine release in the basal ganglia.16
Continue to: Dopamine supersenstivity psychosis...
Dopamine supersensitivity psychosis (DSP) is a term that describes the clinical iatrogenic phenomenon that might be observed with long-term antipsychotic treatment. DSP is suggested to be strongly associated with treatment failure/resistance in schizophrenia.17,18 Manifestations of DSP include development of antipsychotic drug tolerance that undermines treatment efficacy, rebound psychosis during or after treatment discontinuation, and the presence of TD. Like TD, it may be reversed temporarily by increasing the dose of the antipsychotic.18
DSP and (more extensively) TD are commonly hypothesized to result from the postsynaptic dopamine receptor supersensitivity that develops because of chronic D2Rs blockade by antipsychotics. Neostriatal dopamine receptor supersensitivity is believed to lead to TD, while mesolimbic supersensitivity leads to DSP.19 Supersensitivity has traditionally been believed to be due to upregulation of postsynaptic D2R number and sensitivity.20,21 However, both TD and DSP are more likely a consequence of a host of compensatory neurobiological adaptations across the synapse that include:
- postsynaptic increase in the number of D2Rs that amplifies the dopamine signal
- an increased number of synapses, dendritic spines, and perforated synapses (seen in animal models), all of which lead to a potentiated dopamine signal
- presynaptic changes with higher levels of dopamine released into the synapse via an increase in quantal size as postsynaptic D2Rs blockade results in more dopamine becoming available in the synapse for recycling via the dopamine transporter
- increased dopamine turnover due to presynaptic D2S autoreceptor blockade.22
So if giving a D2R blocking agent for a long time increases the dopamine signal, at least in some patients, what can the clinician do to treat the psychosis, and not cause changes in the brain that could lead to TD or DSP?
Partial agonist antipsychotics and biased agonism of D2Rs
One approach to try to avoid the compensatory changes to dopamine blockade might be to use a D2R partial agonist.18,23 For example, aripiprazole is a partial agonist at the D2R commonly used to manage schizophrenia and bipolar disorder. It possesses greater affinity at the D2R compared with the serotonin 2A (5-hydroxytryptamine, 5HT2A) serotonin receptor. Unlike full antagonists, aripiprazole requires exceptionally high D2 receptor occupancy (approximately 90%) to be at a clinically effective antipsychotic dose.24,25 This is a general requirement for all D2R partial agonists.26
A partial agonist generally has to possess greater affinity to the receptor than the neurotransmitter with which it is competing. Aripiprazole has more than twice the affinity to D2R than dopamine. Other partial agonists have similarly high, or higher, D2R affinity. Effective antipsychotic partial agonists stimulate the D2Rs at approximately 30% ± 10% the maximal signal achieved with dopamine. This is essentially equivalent to having approximately 70% receptor occupancy with a full antagonist, except it is built into how the molecule works. Having this low-grade partial activation of D2Rs creates multiple receptor-mediated actions:
- reduction of cAMP accumulation
- antagonism to guanosine 5’-0-(3-thio) triphosphate (GTPgamma S) binding with relatively less recruitment of beta-arrestin 2 (these diverging effects on G protein are the definition of biased agonism)
- antagonism of G protein activation of K+ channels (GIRK) activity
- agonism for the inhibition of TH.
Continue to: Additionally, aripiprazole was found...
Additionally, aripiprazole was found to be associated with a lesser increase in dopamine turnover than full antagonist antipsychotics (Figure27) and decreased DAT binding density in NAc and the ventral tegmental area (VTA). The distinctive pharmacologic profile and biased agonism of this drug could be attributed to its ability to activate presynaptic D2 autoreceptors, which, as previously mentioned, regulate dopamine release via negative feedback mechanism.5,25 Cariprazine, another D2R partial agonist, has similar doubling of dopamine turnover.28
Activation of presynaptic D2S receptors ultimately leads to decreased dopamine synthesis and release, which combats or prevents the brain adaptations regarding dopamine supersensitivity and D2Rs upregulation. While TD can still occur occasionally with aripiprazole or other partial agonists,29,30 animal studies show that administration of methamphetamine significantly lowers locomotor response and the density of striatal D2Rs in a group treated with aripiprazole compared to a group treated with haloperidol.31 Aripiprazole also improved the supersensitivity parameters induced by chronic treatment with haloperidol, which suggests that it is associated with reduced dopamine supersensitivity.31 Similarly, in human studies, partial agonists appear to have a lower rate of parkinsonism and TD.32,33 One study reported that aripiprazole was associated with a significant improvement of TD in more than 50% of patients after 24 weeks of treatment.34
Lumateperone’s unique pharmacologic profile
Lumateperone is a newer antipsychotic that was FDA-approved in December 2019 for the treatment of adults with schizophrenia35 and more recently for the treatment of bipolar depression.36 It possesses a unique combination of pharmacologic properties; it is a postsynaptic D2R antagonist and a presynaptic D2R partial agonist.27
Interestingly, lumateperone has regional selectivity. It increases dopamine release in the medial prefrontal cortex (where D2R is rare) but not in the nigrostriatal pathways.27,37 It does not increase TH phosphorylation (which would increase dopamine concentration) or dopamine turnover in the striatum (Figure27). In a preclinical functional activity assay of lumateperone, the lack of change of dopamine turnover with lumateperone resembles placebo and is even less than that observed with aripiprazole (Figure27). This effect is consistent with partial agonism at the presynaptic D2S, where the stimulation of that receptor prevents the concomitant increase in dopamine synthesis and release that occurs when that receptor is blocked.
It is believed that the lack of increase in dopamine turnover is one of the reasons that lumateperone postsynaptic D2R occupancy is exceptionally low at clinically effective doses. In a positron emission tomography study analyzing posttreatment scans after approximately 2 weeks of a 60 mg/d dose, the mean peak striatal D2R occupancy was approximately 40%,38 which is remarkably lower than the 65% to 75% blockade needed for purely antagonist D2R antipsychotics.3 This low receptor occupancy appears to mediate the low incidence of parkinsonism and prolactin release seen with lumateperone.
Continue to: Take-home points
Take-home points
Adaptive upregulation of dopamine neurotransmission underlies acute adverse effects such as parkinsonism and is also key for delayed consequences such as TD, and possibly the development of treatment resistance. Adaptive upregulation results from an increase in postsynaptic dopamine receptors, numbers of synapses, and dopamine release. The latter has been demonstrated to be greatest with full antagonists, less with partial agonists, and not present with lumateperone, which is a postsynaptic antagonist but a presynaptic partial agonist (Figure27). Reducing adaptive upregulation can reduce both acute and long-term consequences of dopamine blockade. Early use of agents that minimize these adaptive changes, such as a postsynaptic partial agonist (aripiprazole, brexpiprazole, or cariprazine) or a presynaptic partial agonist (lumateperone), appears to be a reasonable clinical option.
Bottom Line
Chronic dopamine D2 receptor blockade with antipsychotics induces adaptive changes that can contribute to both acute and chronic adverse effects. The most severe of these are tardive dyskinesia (TD) and dopamine supersensitivity psychosis (DSP). The use of agents that mitigate these changes, such as the partial D2 agonists aripiprazole, brexpiprazole, and cariprazine and the postsynaptic antagonist/presynaptic partial agonist lumateperone, can potentially reduce these adaptive changes and reduce the likelihood of TD and DSP.
Related Resources
- Citrome L. Aripiprazole, brexpiprazole, and cariprazine: not all the same. Current Psychiatry. 2018;17(4):24-33,43.
- Meyer JM. Lumateperone for schizophrenia. Current Psychiatry. 2020;19(2):33-39.
Drug Brand Names
Aripiprazole • Abilify
Brexpiprazole • Rexulti
Cariprazine • Vraylar
Haloperidol • Haldol
Lumateperone • Caplyta
Methamphetamine • Desoxyn
Risperidone • Risperdal
1. Stahl SM. Beyond the dopamine hypothesis of schizophrenia to three neural networks of psychosis: dopamine, serotonin, and glutamate. CNS Spectr. 2018;23(3):187-191.
2. Howes OD, Kapur S. The dopamine hypothesis of schizophrenia: version III--the final common pathway. Schizophr Bull. 2009;35(3):549-562.
3. Ginovart N, Kapur S. Role of dopamine D2 receptors for antipsychotic activity. Handb Exp Pharmacol. 2012;(212):27-52.
4. Madras BK. History of the discovery of the antipsychotic dopamine D2 receptor: a basis for the dopamine hypothesis of schizophrenia. J Hist Neurosci. 2013;22(1):62-78.
5. Beaulieu JM, Gainetdinov RR. The physiology, signaling, and pharmacology of dopamine receptors. Pharmacol Rev. 201;63(1):182-217.
6. Martel JC, Gatti McArthur S. Dopamine receptor subtypes, physiology and pharmacology: new ligands and concepts in schizophrenia. Front Pharmacol. 2020;11:1003.
7. Monsma FJ Jr, McVittie LD, Gerfen CR, et al. Multiple D2 dopamine receptors produced by alternative RNA splicing. Nature. 1989;342(6252):926-929.
8. Seeman P, Nam D, Ulpian C, et al. New dopamine receptor, D2(Longer), with unique TG splice site, in human brain. Brain Res Mol Brain Res. 2000;76(1):132-141.
9. Khan ZU, Mrzljak L, Gutierrez A, et al. Prominence of the dopamine D2 short isoform in dopaminergic pathways. Proc Natl Acad Sci U S A. 1998;95(13):7731-7736.
10. Xu R, Hranilovic D, Fetsko LA, et al. Dopamine D2S and D2L receptors may differentially contribute to the actions of antipsychotic and psychotic agents in mice. Mol Psychiatry. 2002;7(10):1075-1082.
11. Anzalone A, Lizardi-Ortiz JE, Ramos M, et al. Dual control of dopamine synthesis and release by presynaptic and postsynaptic dopamine D2 receptors. J Neurosci. 2012;32(26):9023-9034.
12. Ford CP. The role of D2-autoreceptors in regulating dopamine neuron activity and transmission. Neuroscience. 2014;282:13-22.
13. Stroup TS, Gray N. Management of common adverse effects of antipsychotic medications. World Psychiatry. 2018;17(3):341-356.
14. El-Mallakh RS, Pant B, Caudill R, et al. Does peripheral neuropathy allow for the clinical expression of tardive dyskinesia by unmasking central nervous system changes? Med Hypotheses. 2001;57:210-215.
15. Citrome L, Saklad SR. Revisiting tardive dyskinesia: focusing on the basics of identification and treatment. J Clin Psychiatry. 2020;81(2):TV18059AH3C.
16. Carbon M, Kane JM, Leucht S, et al. Tardive dyskinesia risk with first- and second-generation antipsychotics in comparative randomized controlled trials: a meta-analysis. World Psychiatry. 2018;17(3):330-340.
17. Samaha AN, Seeman P, Stewart J, et al. “Breakthrough” dopamine supersensitivity during ongoing antipsychotic treatment leads to treatment failure over time. J Neurosci. 2007;27(11):2979-2986.
18. Yin J, Barr AM, Ramos-Miguel A, et al. Antipsychotic induced dopamine supersensitivity psychosis: a comprehensive review. Curr Neuropharmacol. 2017;15(1):174-183.
19. Chouinard G, Jones BD, Annable L. Neuroleptic-induced supersensitivity psychosis. Am J Psychiatry. 1978;135(11):1409-1410.
20. Burt DR, Creese I, Snyder SH. Antischizophrenic drugs: chronic treatment elevates dopamine receptor binding in brain. Science. 1977;196(4287):326-328.
21. Silvestri S, Seeman MV, Negrete JC, et al. Increased dopamine D2 receptor binding after long-term treatment with antipsychotics in humans: a clinical PET study. Psychopharmacology (Berl). 2000;152(2):174-180.
22. Ali Z, Roque A, El-Mallakh RS. A unifying theory for the pathoetiologic mechanism of tardive dyskinesia. Med Hypotheses. 2020;140:109682.
23. Lieberman JA. Dopamine partial agonists: a new class of antipsychotic. CNS Drugs. 2004;18(4):251-267.
24. Mailman RB, Murthy V. Third generation antipsychotic drugs: partial agonism or receptor functional selectivity? Curr Pharm Des. 2010;16(5):488-501.
25. Tuplin EW, Holahan MR. Aripiprazole, a drug that displays partial agonism and functional selectivity. Curr Neuropharmacol. 2017;15(8):1192-1207.
26. Hart XM, Schmitz CN, Gründer G. Molecular imaging of dopamine partial agonists in humans: implications for clinical practice. Front Psychiatry. 2022;13:832209.
27. Snyder GL, Vanover KE, Zhu H, et al. Functional profile of a novel modulator of serotonin, dopamine, and glutamate neurotransmission. Psychopharmacology (Berl). 2015;232(3):605-621.
28. Kiss B, Horváth A, Némethy Z, et al. Cariprazine (RGH-188), a dopamine D(3) receptor-preferring, D(3)/D(2) dopamine receptor antagonist-partial agonist antipsychotic candidate: in vitro and neurochemical profile. J Pharmacol Exp Ther. 2010;333(1):328-340.
29. Abbasian C, Power P. A case of aripiprazole and tardive dyskinesia. J Psychopharmacol. 2009;23(2):214-215.
30. Peña MS, Yaltho TC, Jankovic J. Tardive dyskinesia and other movement disorders secondary to aripiprazole. Mov Disord. 2011;26(1):147-152.
31. Tadokoro S, Okamura N, Sekine Y, et al. Chronic treatment with aripiprazole prevents development of dopamine supersensitivity and potentially supersensitivity psychosis. Schizophr Bull. 2012;38(5):1012-1020.
32. Kang NR, Kim MD. Tardive dyskinesia: treatment with aripiprazole. Clin Psychopharmacol Neurosci. 2011;9(1):1-8.
33. Frankel JS, Schwartz TL. Brexpiprazole and cariprazine: distinguishing two new atypical antipsychotics from the original dopamine stabilizer aripiprazole. Ther Adv Psychopharmacol. 2017;7(1):29-41.
34. Chan CH, Chan HY, Chen YC. Switching antipsychotic treatment to aripiprazole in psychotic patients with neuroleptic-induced tardive dyskinesia: a 24-week follow-up study. Int Clin Psychopharmacol. 2018;33(3):155-162.
35. Blair HA. Lumateperone: first approval. Drugs. 2020;80(4):417-423.
36. Calabrese JR, Durgam S, Satlin A, et al. Efficacy and safety of Lumateperone for major depressive episodes associated with bipolar I or bipolar II disorder: a phase 3 randomized placebo-controlled trial. Am J Psychiatry. 2021;178(12):1098-1106.
37. Nakai S, Hirose T, Uwahodo Y, et al. Diminished catalepsy and dopamine metabolism distinguish aripiprazole from haloperidol or risperidone. Eur J Pharmacol. 2003;472(12):89-97.
38. Vanover KE, Davis RE, Zhou Y, et al. Dopamine D2 receptor occupancy of lumateperone (ITI-007): a positron emission tomography study in patients with schizophrenia. Neuropsychopharmacology. 2019;44(3):598-605.
While our understanding of the mechanisms of psychosis continues to evolve beyond the dopamine hypothesis, the key role of dopamine in psychosis and its treatment has not faded.1 Over time, the dopamine hypothesis of schizophrenia has evolved from focusing on dopamine hyperactivity to specifying the regional abnormalities in the brain with subcortical hyperdopaminergia and prefrontal hypodopaminergia.2 Despite this divergence in dopaminergic function, antipsychotic medications that block dopamine D2 receptors (D2R) remain central to treating psychotic symptoms and preventing relapse.3,4 Notably, antipsychotics block both presynaptic and postsynaptic receptors affecting the regulation of dopamine synthesis and release in the brain.5,6
Chronic dopamine D2R blockade with antipsychotics induces adaptive changes that can contribute to both acute and chronic adverse effects. In this article, we discuss these changes, and steps clinicians can take to minimize their occurrence.
Dopamine D2R: A primer
There are 5 types of dopamine receptors, numbered D1 through D5, but there are only 2 families of dopamine receptors: the D1 family (D1 and D5), and the D2 family (D2, D3, and D4). All dopamine receptors are G protein–coupled, but the D2 family of receptors generally increases protein kinase A (PKA) as the second messenger, whereas the D1 family increases cyclic adenosine monophosphate (cAMP) as the second messenger.5 There are 2 distinct variants of the D2R of 2 different lengths made from the same gene (DRD2) via posttranslational modification. The long isoform of D2R (D2L) has an additional 29 amino acids compared to the short isoform (D2S).7 Additional evidence points to a third splice variant called D2Longer that arises from aberrant RNA splicing and contains 2 more amino acids than D2L; its relevance is not known.8
The D2L isoform is the primary postsynaptic receptor, expressed more in the striatum and nucleus accumbens (NAc) targeted by dopaminergic afferents. The D2S isoform, however, is predominantly presynaptic, more densely expressed on cell bodies and projection axons of the dopaminergic neurons of the midbrain and hypothalamus.9 Each isoform contributes differentially to the therapeutic and adverse effects of antipsychotics, and evidence from animal studies suggests that D2L is the main variant responsible for drug-induced parkinsonism.10 The D2S acts as the principal autoreceptor for the dopaminergic system.5,11,12
Autoreceptors regulate dopamine transmission. Dopamine itself and D2R agonists are reported to have higher affinity and potency with D2S. Activation of these autoreceptors is a negative feedback mechanism that decreases dopamine release. Similarly, when they are blocked (such as with use of an antipsychotic), there is an increase in dopamine release. Additionally, these autoreceptors modulate several key processes:
- neuronal firing rate by activating potassium conductance
- dopamine synthesis by downregulating the expression of tyrosine hydroxylase (TH) enzyme (the rate-limiting step)
- exocytotic release of dopamine and other neurotransmitters
- dopamine reuptake via increasing the activity of the dopamine transporter (DAT).12
Consequences of antipsychotic D2R blockade
Most antipsychotics begin to produce a therapeutic antipsychotic effect at 65% to 75% occupancy of the D2Rs.3 This level also produces an optimal balance between clinical efficacy and a lower incidence of adverse effects.3 A higher D2R occupancy by both first-generation (FGA) and second-generation (SGA) pure antagonist antipsychotics can lead to parkinsonism.
Parkinsonism is associated with the subsequent appearance of one of the most distressing consequences of long-term antipsychotic treatment, tardive dyskinesia (TD).13 TD is an iatrogenic, usually late-onset syndrome consisting of persistent, involuntary, and repetitive movements. It classically involves the highly innervated striated muscles of the tongue, mouth, face, and fingers, though it can also involve the trunk and extremities.14 It occurs secondary to chronic exposure to dopamine receptor–blocking agents, including dopaminergic antiemetics.15 The prevalence of TD is higher in patients treated long-term with FGAs (30.0% to 32.4%) than in those treated with SGAs (13.1% to 20.7%) due to serotonin 5HT2A blockade that results in increased dopamine release in the basal ganglia.16
Continue to: Dopamine supersenstivity psychosis...
Dopamine supersensitivity psychosis (DSP) is a term that describes the clinical iatrogenic phenomenon that might be observed with long-term antipsychotic treatment. DSP is suggested to be strongly associated with treatment failure/resistance in schizophrenia.17,18 Manifestations of DSP include development of antipsychotic drug tolerance that undermines treatment efficacy, rebound psychosis during or after treatment discontinuation, and the presence of TD. Like TD, it may be reversed temporarily by increasing the dose of the antipsychotic.18
DSP and (more extensively) TD are commonly hypothesized to result from the postsynaptic dopamine receptor supersensitivity that develops because of chronic D2Rs blockade by antipsychotics. Neostriatal dopamine receptor supersensitivity is believed to lead to TD, while mesolimbic supersensitivity leads to DSP.19 Supersensitivity has traditionally been believed to be due to upregulation of postsynaptic D2R number and sensitivity.20,21 However, both TD and DSP are more likely a consequence of a host of compensatory neurobiological adaptations across the synapse that include:
- postsynaptic increase in the number of D2Rs that amplifies the dopamine signal
- an increased number of synapses, dendritic spines, and perforated synapses (seen in animal models), all of which lead to a potentiated dopamine signal
- presynaptic changes with higher levels of dopamine released into the synapse via an increase in quantal size as postsynaptic D2Rs blockade results in more dopamine becoming available in the synapse for recycling via the dopamine transporter
- increased dopamine turnover due to presynaptic D2S autoreceptor blockade.22
So if giving a D2R blocking agent for a long time increases the dopamine signal, at least in some patients, what can the clinician do to treat the psychosis, and not cause changes in the brain that could lead to TD or DSP?
Partial agonist antipsychotics and biased agonism of D2Rs
One approach to try to avoid the compensatory changes to dopamine blockade might be to use a D2R partial agonist.18,23 For example, aripiprazole is a partial agonist at the D2R commonly used to manage schizophrenia and bipolar disorder. It possesses greater affinity at the D2R compared with the serotonin 2A (5-hydroxytryptamine, 5HT2A) serotonin receptor. Unlike full antagonists, aripiprazole requires exceptionally high D2 receptor occupancy (approximately 90%) to be at a clinically effective antipsychotic dose.24,25 This is a general requirement for all D2R partial agonists.26
A partial agonist generally has to possess greater affinity to the receptor than the neurotransmitter with which it is competing. Aripiprazole has more than twice the affinity to D2R than dopamine. Other partial agonists have similarly high, or higher, D2R affinity. Effective antipsychotic partial agonists stimulate the D2Rs at approximately 30% ± 10% the maximal signal achieved with dopamine. This is essentially equivalent to having approximately 70% receptor occupancy with a full antagonist, except it is built into how the molecule works. Having this low-grade partial activation of D2Rs creates multiple receptor-mediated actions:
- reduction of cAMP accumulation
- antagonism to guanosine 5’-0-(3-thio) triphosphate (GTPgamma S) binding with relatively less recruitment of beta-arrestin 2 (these diverging effects on G protein are the definition of biased agonism)
- antagonism of G protein activation of K+ channels (GIRK) activity
- agonism for the inhibition of TH.
Continue to: Additionally, aripiprazole was found...
Additionally, aripiprazole was found to be associated with a lesser increase in dopamine turnover than full antagonist antipsychotics (Figure27) and decreased DAT binding density in NAc and the ventral tegmental area (VTA). The distinctive pharmacologic profile and biased agonism of this drug could be attributed to its ability to activate presynaptic D2 autoreceptors, which, as previously mentioned, regulate dopamine release via negative feedback mechanism.5,25 Cariprazine, another D2R partial agonist, has similar doubling of dopamine turnover.28
Activation of presynaptic D2S receptors ultimately leads to decreased dopamine synthesis and release, which combats or prevents the brain adaptations regarding dopamine supersensitivity and D2Rs upregulation. While TD can still occur occasionally with aripiprazole or other partial agonists,29,30 animal studies show that administration of methamphetamine significantly lowers locomotor response and the density of striatal D2Rs in a group treated with aripiprazole compared to a group treated with haloperidol.31 Aripiprazole also improved the supersensitivity parameters induced by chronic treatment with haloperidol, which suggests that it is associated with reduced dopamine supersensitivity.31 Similarly, in human studies, partial agonists appear to have a lower rate of parkinsonism and TD.32,33 One study reported that aripiprazole was associated with a significant improvement of TD in more than 50% of patients after 24 weeks of treatment.34
Lumateperone’s unique pharmacologic profile
Lumateperone is a newer antipsychotic that was FDA-approved in December 2019 for the treatment of adults with schizophrenia35 and more recently for the treatment of bipolar depression.36 It possesses a unique combination of pharmacologic properties; it is a postsynaptic D2R antagonist and a presynaptic D2R partial agonist.27
Interestingly, lumateperone has regional selectivity. It increases dopamine release in the medial prefrontal cortex (where D2R is rare) but not in the nigrostriatal pathways.27,37 It does not increase TH phosphorylation (which would increase dopamine concentration) or dopamine turnover in the striatum (Figure27). In a preclinical functional activity assay of lumateperone, the lack of change of dopamine turnover with lumateperone resembles placebo and is even less than that observed with aripiprazole (Figure27). This effect is consistent with partial agonism at the presynaptic D2S, where the stimulation of that receptor prevents the concomitant increase in dopamine synthesis and release that occurs when that receptor is blocked.
It is believed that the lack of increase in dopamine turnover is one of the reasons that lumateperone postsynaptic D2R occupancy is exceptionally low at clinically effective doses. In a positron emission tomography study analyzing posttreatment scans after approximately 2 weeks of a 60 mg/d dose, the mean peak striatal D2R occupancy was approximately 40%,38 which is remarkably lower than the 65% to 75% blockade needed for purely antagonist D2R antipsychotics.3 This low receptor occupancy appears to mediate the low incidence of parkinsonism and prolactin release seen with lumateperone.
Continue to: Take-home points
Take-home points
Adaptive upregulation of dopamine neurotransmission underlies acute adverse effects such as parkinsonism and is also key for delayed consequences such as TD, and possibly the development of treatment resistance. Adaptive upregulation results from an increase in postsynaptic dopamine receptors, numbers of synapses, and dopamine release. The latter has been demonstrated to be greatest with full antagonists, less with partial agonists, and not present with lumateperone, which is a postsynaptic antagonist but a presynaptic partial agonist (Figure27). Reducing adaptive upregulation can reduce both acute and long-term consequences of dopamine blockade. Early use of agents that minimize these adaptive changes, such as a postsynaptic partial agonist (aripiprazole, brexpiprazole, or cariprazine) or a presynaptic partial agonist (lumateperone), appears to be a reasonable clinical option.
Bottom Line
Chronic dopamine D2 receptor blockade with antipsychotics induces adaptive changes that can contribute to both acute and chronic adverse effects. The most severe of these are tardive dyskinesia (TD) and dopamine supersensitivity psychosis (DSP). The use of agents that mitigate these changes, such as the partial D2 agonists aripiprazole, brexpiprazole, and cariprazine and the postsynaptic antagonist/presynaptic partial agonist lumateperone, can potentially reduce these adaptive changes and reduce the likelihood of TD and DSP.
Related Resources
- Citrome L. Aripiprazole, brexpiprazole, and cariprazine: not all the same. Current Psychiatry. 2018;17(4):24-33,43.
- Meyer JM. Lumateperone for schizophrenia. Current Psychiatry. 2020;19(2):33-39.
Drug Brand Names
Aripiprazole • Abilify
Brexpiprazole • Rexulti
Cariprazine • Vraylar
Haloperidol • Haldol
Lumateperone • Caplyta
Methamphetamine • Desoxyn
Risperidone • Risperdal
While our understanding of the mechanisms of psychosis continues to evolve beyond the dopamine hypothesis, the key role of dopamine in psychosis and its treatment has not faded.1 Over time, the dopamine hypothesis of schizophrenia has evolved from focusing on dopamine hyperactivity to specifying the regional abnormalities in the brain with subcortical hyperdopaminergia and prefrontal hypodopaminergia.2 Despite this divergence in dopaminergic function, antipsychotic medications that block dopamine D2 receptors (D2R) remain central to treating psychotic symptoms and preventing relapse.3,4 Notably, antipsychotics block both presynaptic and postsynaptic receptors affecting the regulation of dopamine synthesis and release in the brain.5,6
Chronic dopamine D2R blockade with antipsychotics induces adaptive changes that can contribute to both acute and chronic adverse effects. In this article, we discuss these changes, and steps clinicians can take to minimize their occurrence.
Dopamine D2R: A primer
There are 5 types of dopamine receptors, numbered D1 through D5, but there are only 2 families of dopamine receptors: the D1 family (D1 and D5), and the D2 family (D2, D3, and D4). All dopamine receptors are G protein–coupled, but the D2 family of receptors generally increases protein kinase A (PKA) as the second messenger, whereas the D1 family increases cyclic adenosine monophosphate (cAMP) as the second messenger.5 There are 2 distinct variants of the D2R of 2 different lengths made from the same gene (DRD2) via posttranslational modification. The long isoform of D2R (D2L) has an additional 29 amino acids compared to the short isoform (D2S).7 Additional evidence points to a third splice variant called D2Longer that arises from aberrant RNA splicing and contains 2 more amino acids than D2L; its relevance is not known.8
The D2L isoform is the primary postsynaptic receptor, expressed more in the striatum and nucleus accumbens (NAc) targeted by dopaminergic afferents. The D2S isoform, however, is predominantly presynaptic, more densely expressed on cell bodies and projection axons of the dopaminergic neurons of the midbrain and hypothalamus.9 Each isoform contributes differentially to the therapeutic and adverse effects of antipsychotics, and evidence from animal studies suggests that D2L is the main variant responsible for drug-induced parkinsonism.10 The D2S acts as the principal autoreceptor for the dopaminergic system.5,11,12
Autoreceptors regulate dopamine transmission. Dopamine itself and D2R agonists are reported to have higher affinity and potency with D2S. Activation of these autoreceptors is a negative feedback mechanism that decreases dopamine release. Similarly, when they are blocked (such as with use of an antipsychotic), there is an increase in dopamine release. Additionally, these autoreceptors modulate several key processes:
- neuronal firing rate by activating potassium conductance
- dopamine synthesis by downregulating the expression of tyrosine hydroxylase (TH) enzyme (the rate-limiting step)
- exocytotic release of dopamine and other neurotransmitters
- dopamine reuptake via increasing the activity of the dopamine transporter (DAT).12
Consequences of antipsychotic D2R blockade
Most antipsychotics begin to produce a therapeutic antipsychotic effect at 65% to 75% occupancy of the D2Rs.3 This level also produces an optimal balance between clinical efficacy and a lower incidence of adverse effects.3 A higher D2R occupancy by both first-generation (FGA) and second-generation (SGA) pure antagonist antipsychotics can lead to parkinsonism.
Parkinsonism is associated with the subsequent appearance of one of the most distressing consequences of long-term antipsychotic treatment, tardive dyskinesia (TD).13 TD is an iatrogenic, usually late-onset syndrome consisting of persistent, involuntary, and repetitive movements. It classically involves the highly innervated striated muscles of the tongue, mouth, face, and fingers, though it can also involve the trunk and extremities.14 It occurs secondary to chronic exposure to dopamine receptor–blocking agents, including dopaminergic antiemetics.15 The prevalence of TD is higher in patients treated long-term with FGAs (30.0% to 32.4%) than in those treated with SGAs (13.1% to 20.7%) due to serotonin 5HT2A blockade that results in increased dopamine release in the basal ganglia.16
Continue to: Dopamine supersenstivity psychosis...
Dopamine supersensitivity psychosis (DSP) is a term that describes the clinical iatrogenic phenomenon that might be observed with long-term antipsychotic treatment. DSP is suggested to be strongly associated with treatment failure/resistance in schizophrenia.17,18 Manifestations of DSP include development of antipsychotic drug tolerance that undermines treatment efficacy, rebound psychosis during or after treatment discontinuation, and the presence of TD. Like TD, it may be reversed temporarily by increasing the dose of the antipsychotic.18
DSP and (more extensively) TD are commonly hypothesized to result from the postsynaptic dopamine receptor supersensitivity that develops because of chronic D2Rs blockade by antipsychotics. Neostriatal dopamine receptor supersensitivity is believed to lead to TD, while mesolimbic supersensitivity leads to DSP.19 Supersensitivity has traditionally been believed to be due to upregulation of postsynaptic D2R number and sensitivity.20,21 However, both TD and DSP are more likely a consequence of a host of compensatory neurobiological adaptations across the synapse that include:
- postsynaptic increase in the number of D2Rs that amplifies the dopamine signal
- an increased number of synapses, dendritic spines, and perforated synapses (seen in animal models), all of which lead to a potentiated dopamine signal
- presynaptic changes with higher levels of dopamine released into the synapse via an increase in quantal size as postsynaptic D2Rs blockade results in more dopamine becoming available in the synapse for recycling via the dopamine transporter
- increased dopamine turnover due to presynaptic D2S autoreceptor blockade.22
So if giving a D2R blocking agent for a long time increases the dopamine signal, at least in some patients, what can the clinician do to treat the psychosis, and not cause changes in the brain that could lead to TD or DSP?
Partial agonist antipsychotics and biased agonism of D2Rs
One approach to try to avoid the compensatory changes to dopamine blockade might be to use a D2R partial agonist.18,23 For example, aripiprazole is a partial agonist at the D2R commonly used to manage schizophrenia and bipolar disorder. It possesses greater affinity at the D2R compared with the serotonin 2A (5-hydroxytryptamine, 5HT2A) serotonin receptor. Unlike full antagonists, aripiprazole requires exceptionally high D2 receptor occupancy (approximately 90%) to be at a clinically effective antipsychotic dose.24,25 This is a general requirement for all D2R partial agonists.26
A partial agonist generally has to possess greater affinity to the receptor than the neurotransmitter with which it is competing. Aripiprazole has more than twice the affinity to D2R than dopamine. Other partial agonists have similarly high, or higher, D2R affinity. Effective antipsychotic partial agonists stimulate the D2Rs at approximately 30% ± 10% the maximal signal achieved with dopamine. This is essentially equivalent to having approximately 70% receptor occupancy with a full antagonist, except it is built into how the molecule works. Having this low-grade partial activation of D2Rs creates multiple receptor-mediated actions:
- reduction of cAMP accumulation
- antagonism to guanosine 5’-0-(3-thio) triphosphate (GTPgamma S) binding with relatively less recruitment of beta-arrestin 2 (these diverging effects on G protein are the definition of biased agonism)
- antagonism of G protein activation of K+ channels (GIRK) activity
- agonism for the inhibition of TH.
Continue to: Additionally, aripiprazole was found...
Additionally, aripiprazole was found to be associated with a lesser increase in dopamine turnover than full antagonist antipsychotics (Figure27) and decreased DAT binding density in NAc and the ventral tegmental area (VTA). The distinctive pharmacologic profile and biased agonism of this drug could be attributed to its ability to activate presynaptic D2 autoreceptors, which, as previously mentioned, regulate dopamine release via negative feedback mechanism.5,25 Cariprazine, another D2R partial agonist, has similar doubling of dopamine turnover.28
Activation of presynaptic D2S receptors ultimately leads to decreased dopamine synthesis and release, which combats or prevents the brain adaptations regarding dopamine supersensitivity and D2Rs upregulation. While TD can still occur occasionally with aripiprazole or other partial agonists,29,30 animal studies show that administration of methamphetamine significantly lowers locomotor response and the density of striatal D2Rs in a group treated with aripiprazole compared to a group treated with haloperidol.31 Aripiprazole also improved the supersensitivity parameters induced by chronic treatment with haloperidol, which suggests that it is associated with reduced dopamine supersensitivity.31 Similarly, in human studies, partial agonists appear to have a lower rate of parkinsonism and TD.32,33 One study reported that aripiprazole was associated with a significant improvement of TD in more than 50% of patients after 24 weeks of treatment.34
Lumateperone’s unique pharmacologic profile
Lumateperone is a newer antipsychotic that was FDA-approved in December 2019 for the treatment of adults with schizophrenia35 and more recently for the treatment of bipolar depression.36 It possesses a unique combination of pharmacologic properties; it is a postsynaptic D2R antagonist and a presynaptic D2R partial agonist.27
Interestingly, lumateperone has regional selectivity. It increases dopamine release in the medial prefrontal cortex (where D2R is rare) but not in the nigrostriatal pathways.27,37 It does not increase TH phosphorylation (which would increase dopamine concentration) or dopamine turnover in the striatum (Figure27). In a preclinical functional activity assay of lumateperone, the lack of change of dopamine turnover with lumateperone resembles placebo and is even less than that observed with aripiprazole (Figure27). This effect is consistent with partial agonism at the presynaptic D2S, where the stimulation of that receptor prevents the concomitant increase in dopamine synthesis and release that occurs when that receptor is blocked.
It is believed that the lack of increase in dopamine turnover is one of the reasons that lumateperone postsynaptic D2R occupancy is exceptionally low at clinically effective doses. In a positron emission tomography study analyzing posttreatment scans after approximately 2 weeks of a 60 mg/d dose, the mean peak striatal D2R occupancy was approximately 40%,38 which is remarkably lower than the 65% to 75% blockade needed for purely antagonist D2R antipsychotics.3 This low receptor occupancy appears to mediate the low incidence of parkinsonism and prolactin release seen with lumateperone.
Continue to: Take-home points
Take-home points
Adaptive upregulation of dopamine neurotransmission underlies acute adverse effects such as parkinsonism and is also key for delayed consequences such as TD, and possibly the development of treatment resistance. Adaptive upregulation results from an increase in postsynaptic dopamine receptors, numbers of synapses, and dopamine release. The latter has been demonstrated to be greatest with full antagonists, less with partial agonists, and not present with lumateperone, which is a postsynaptic antagonist but a presynaptic partial agonist (Figure27). Reducing adaptive upregulation can reduce both acute and long-term consequences of dopamine blockade. Early use of agents that minimize these adaptive changes, such as a postsynaptic partial agonist (aripiprazole, brexpiprazole, or cariprazine) or a presynaptic partial agonist (lumateperone), appears to be a reasonable clinical option.
Bottom Line
Chronic dopamine D2 receptor blockade with antipsychotics induces adaptive changes that can contribute to both acute and chronic adverse effects. The most severe of these are tardive dyskinesia (TD) and dopamine supersensitivity psychosis (DSP). The use of agents that mitigate these changes, such as the partial D2 agonists aripiprazole, brexpiprazole, and cariprazine and the postsynaptic antagonist/presynaptic partial agonist lumateperone, can potentially reduce these adaptive changes and reduce the likelihood of TD and DSP.
Related Resources
- Citrome L. Aripiprazole, brexpiprazole, and cariprazine: not all the same. Current Psychiatry. 2018;17(4):24-33,43.
- Meyer JM. Lumateperone for schizophrenia. Current Psychiatry. 2020;19(2):33-39.
Drug Brand Names
Aripiprazole • Abilify
Brexpiprazole • Rexulti
Cariprazine • Vraylar
Haloperidol • Haldol
Lumateperone • Caplyta
Methamphetamine • Desoxyn
Risperidone • Risperdal
1. Stahl SM. Beyond the dopamine hypothesis of schizophrenia to three neural networks of psychosis: dopamine, serotonin, and glutamate. CNS Spectr. 2018;23(3):187-191.
2. Howes OD, Kapur S. The dopamine hypothesis of schizophrenia: version III--the final common pathway. Schizophr Bull. 2009;35(3):549-562.
3. Ginovart N, Kapur S. Role of dopamine D2 receptors for antipsychotic activity. Handb Exp Pharmacol. 2012;(212):27-52.
4. Madras BK. History of the discovery of the antipsychotic dopamine D2 receptor: a basis for the dopamine hypothesis of schizophrenia. J Hist Neurosci. 2013;22(1):62-78.
5. Beaulieu JM, Gainetdinov RR. The physiology, signaling, and pharmacology of dopamine receptors. Pharmacol Rev. 201;63(1):182-217.
6. Martel JC, Gatti McArthur S. Dopamine receptor subtypes, physiology and pharmacology: new ligands and concepts in schizophrenia. Front Pharmacol. 2020;11:1003.
7. Monsma FJ Jr, McVittie LD, Gerfen CR, et al. Multiple D2 dopamine receptors produced by alternative RNA splicing. Nature. 1989;342(6252):926-929.
8. Seeman P, Nam D, Ulpian C, et al. New dopamine receptor, D2(Longer), with unique TG splice site, in human brain. Brain Res Mol Brain Res. 2000;76(1):132-141.
9. Khan ZU, Mrzljak L, Gutierrez A, et al. Prominence of the dopamine D2 short isoform in dopaminergic pathways. Proc Natl Acad Sci U S A. 1998;95(13):7731-7736.
10. Xu R, Hranilovic D, Fetsko LA, et al. Dopamine D2S and D2L receptors may differentially contribute to the actions of antipsychotic and psychotic agents in mice. Mol Psychiatry. 2002;7(10):1075-1082.
11. Anzalone A, Lizardi-Ortiz JE, Ramos M, et al. Dual control of dopamine synthesis and release by presynaptic and postsynaptic dopamine D2 receptors. J Neurosci. 2012;32(26):9023-9034.
12. Ford CP. The role of D2-autoreceptors in regulating dopamine neuron activity and transmission. Neuroscience. 2014;282:13-22.
13. Stroup TS, Gray N. Management of common adverse effects of antipsychotic medications. World Psychiatry. 2018;17(3):341-356.
14. El-Mallakh RS, Pant B, Caudill R, et al. Does peripheral neuropathy allow for the clinical expression of tardive dyskinesia by unmasking central nervous system changes? Med Hypotheses. 2001;57:210-215.
15. Citrome L, Saklad SR. Revisiting tardive dyskinesia: focusing on the basics of identification and treatment. J Clin Psychiatry. 2020;81(2):TV18059AH3C.
16. Carbon M, Kane JM, Leucht S, et al. Tardive dyskinesia risk with first- and second-generation antipsychotics in comparative randomized controlled trials: a meta-analysis. World Psychiatry. 2018;17(3):330-340.
17. Samaha AN, Seeman P, Stewart J, et al. “Breakthrough” dopamine supersensitivity during ongoing antipsychotic treatment leads to treatment failure over time. J Neurosci. 2007;27(11):2979-2986.
18. Yin J, Barr AM, Ramos-Miguel A, et al. Antipsychotic induced dopamine supersensitivity psychosis: a comprehensive review. Curr Neuropharmacol. 2017;15(1):174-183.
19. Chouinard G, Jones BD, Annable L. Neuroleptic-induced supersensitivity psychosis. Am J Psychiatry. 1978;135(11):1409-1410.
20. Burt DR, Creese I, Snyder SH. Antischizophrenic drugs: chronic treatment elevates dopamine receptor binding in brain. Science. 1977;196(4287):326-328.
21. Silvestri S, Seeman MV, Negrete JC, et al. Increased dopamine D2 receptor binding after long-term treatment with antipsychotics in humans: a clinical PET study. Psychopharmacology (Berl). 2000;152(2):174-180.
22. Ali Z, Roque A, El-Mallakh RS. A unifying theory for the pathoetiologic mechanism of tardive dyskinesia. Med Hypotheses. 2020;140:109682.
23. Lieberman JA. Dopamine partial agonists: a new class of antipsychotic. CNS Drugs. 2004;18(4):251-267.
24. Mailman RB, Murthy V. Third generation antipsychotic drugs: partial agonism or receptor functional selectivity? Curr Pharm Des. 2010;16(5):488-501.
25. Tuplin EW, Holahan MR. Aripiprazole, a drug that displays partial agonism and functional selectivity. Curr Neuropharmacol. 2017;15(8):1192-1207.
26. Hart XM, Schmitz CN, Gründer G. Molecular imaging of dopamine partial agonists in humans: implications for clinical practice. Front Psychiatry. 2022;13:832209.
27. Snyder GL, Vanover KE, Zhu H, et al. Functional profile of a novel modulator of serotonin, dopamine, and glutamate neurotransmission. Psychopharmacology (Berl). 2015;232(3):605-621.
28. Kiss B, Horváth A, Némethy Z, et al. Cariprazine (RGH-188), a dopamine D(3) receptor-preferring, D(3)/D(2) dopamine receptor antagonist-partial agonist antipsychotic candidate: in vitro and neurochemical profile. J Pharmacol Exp Ther. 2010;333(1):328-340.
29. Abbasian C, Power P. A case of aripiprazole and tardive dyskinesia. J Psychopharmacol. 2009;23(2):214-215.
30. Peña MS, Yaltho TC, Jankovic J. Tardive dyskinesia and other movement disorders secondary to aripiprazole. Mov Disord. 2011;26(1):147-152.
31. Tadokoro S, Okamura N, Sekine Y, et al. Chronic treatment with aripiprazole prevents development of dopamine supersensitivity and potentially supersensitivity psychosis. Schizophr Bull. 2012;38(5):1012-1020.
32. Kang NR, Kim MD. Tardive dyskinesia: treatment with aripiprazole. Clin Psychopharmacol Neurosci. 2011;9(1):1-8.
33. Frankel JS, Schwartz TL. Brexpiprazole and cariprazine: distinguishing two new atypical antipsychotics from the original dopamine stabilizer aripiprazole. Ther Adv Psychopharmacol. 2017;7(1):29-41.
34. Chan CH, Chan HY, Chen YC. Switching antipsychotic treatment to aripiprazole in psychotic patients with neuroleptic-induced tardive dyskinesia: a 24-week follow-up study. Int Clin Psychopharmacol. 2018;33(3):155-162.
35. Blair HA. Lumateperone: first approval. Drugs. 2020;80(4):417-423.
36. Calabrese JR, Durgam S, Satlin A, et al. Efficacy and safety of Lumateperone for major depressive episodes associated with bipolar I or bipolar II disorder: a phase 3 randomized placebo-controlled trial. Am J Psychiatry. 2021;178(12):1098-1106.
37. Nakai S, Hirose T, Uwahodo Y, et al. Diminished catalepsy and dopamine metabolism distinguish aripiprazole from haloperidol or risperidone. Eur J Pharmacol. 2003;472(12):89-97.
38. Vanover KE, Davis RE, Zhou Y, et al. Dopamine D2 receptor occupancy of lumateperone (ITI-007): a positron emission tomography study in patients with schizophrenia. Neuropsychopharmacology. 2019;44(3):598-605.
1. Stahl SM. Beyond the dopamine hypothesis of schizophrenia to three neural networks of psychosis: dopamine, serotonin, and glutamate. CNS Spectr. 2018;23(3):187-191.
2. Howes OD, Kapur S. The dopamine hypothesis of schizophrenia: version III--the final common pathway. Schizophr Bull. 2009;35(3):549-562.
3. Ginovart N, Kapur S. Role of dopamine D2 receptors for antipsychotic activity. Handb Exp Pharmacol. 2012;(212):27-52.
4. Madras BK. History of the discovery of the antipsychotic dopamine D2 receptor: a basis for the dopamine hypothesis of schizophrenia. J Hist Neurosci. 2013;22(1):62-78.
5. Beaulieu JM, Gainetdinov RR. The physiology, signaling, and pharmacology of dopamine receptors. Pharmacol Rev. 201;63(1):182-217.
6. Martel JC, Gatti McArthur S. Dopamine receptor subtypes, physiology and pharmacology: new ligands and concepts in schizophrenia. Front Pharmacol. 2020;11:1003.
7. Monsma FJ Jr, McVittie LD, Gerfen CR, et al. Multiple D2 dopamine receptors produced by alternative RNA splicing. Nature. 1989;342(6252):926-929.
8. Seeman P, Nam D, Ulpian C, et al. New dopamine receptor, D2(Longer), with unique TG splice site, in human brain. Brain Res Mol Brain Res. 2000;76(1):132-141.
9. Khan ZU, Mrzljak L, Gutierrez A, et al. Prominence of the dopamine D2 short isoform in dopaminergic pathways. Proc Natl Acad Sci U S A. 1998;95(13):7731-7736.
10. Xu R, Hranilovic D, Fetsko LA, et al. Dopamine D2S and D2L receptors may differentially contribute to the actions of antipsychotic and psychotic agents in mice. Mol Psychiatry. 2002;7(10):1075-1082.
11. Anzalone A, Lizardi-Ortiz JE, Ramos M, et al. Dual control of dopamine synthesis and release by presynaptic and postsynaptic dopamine D2 receptors. J Neurosci. 2012;32(26):9023-9034.
12. Ford CP. The role of D2-autoreceptors in regulating dopamine neuron activity and transmission. Neuroscience. 2014;282:13-22.
13. Stroup TS, Gray N. Management of common adverse effects of antipsychotic medications. World Psychiatry. 2018;17(3):341-356.
14. El-Mallakh RS, Pant B, Caudill R, et al. Does peripheral neuropathy allow for the clinical expression of tardive dyskinesia by unmasking central nervous system changes? Med Hypotheses. 2001;57:210-215.
15. Citrome L, Saklad SR. Revisiting tardive dyskinesia: focusing on the basics of identification and treatment. J Clin Psychiatry. 2020;81(2):TV18059AH3C.
16. Carbon M, Kane JM, Leucht S, et al. Tardive dyskinesia risk with first- and second-generation antipsychotics in comparative randomized controlled trials: a meta-analysis. World Psychiatry. 2018;17(3):330-340.
17. Samaha AN, Seeman P, Stewart J, et al. “Breakthrough” dopamine supersensitivity during ongoing antipsychotic treatment leads to treatment failure over time. J Neurosci. 2007;27(11):2979-2986.
18. Yin J, Barr AM, Ramos-Miguel A, et al. Antipsychotic induced dopamine supersensitivity psychosis: a comprehensive review. Curr Neuropharmacol. 2017;15(1):174-183.
19. Chouinard G, Jones BD, Annable L. Neuroleptic-induced supersensitivity psychosis. Am J Psychiatry. 1978;135(11):1409-1410.
20. Burt DR, Creese I, Snyder SH. Antischizophrenic drugs: chronic treatment elevates dopamine receptor binding in brain. Science. 1977;196(4287):326-328.
21. Silvestri S, Seeman MV, Negrete JC, et al. Increased dopamine D2 receptor binding after long-term treatment with antipsychotics in humans: a clinical PET study. Psychopharmacology (Berl). 2000;152(2):174-180.
22. Ali Z, Roque A, El-Mallakh RS. A unifying theory for the pathoetiologic mechanism of tardive dyskinesia. Med Hypotheses. 2020;140:109682.
23. Lieberman JA. Dopamine partial agonists: a new class of antipsychotic. CNS Drugs. 2004;18(4):251-267.
24. Mailman RB, Murthy V. Third generation antipsychotic drugs: partial agonism or receptor functional selectivity? Curr Pharm Des. 2010;16(5):488-501.
25. Tuplin EW, Holahan MR. Aripiprazole, a drug that displays partial agonism and functional selectivity. Curr Neuropharmacol. 2017;15(8):1192-1207.
26. Hart XM, Schmitz CN, Gründer G. Molecular imaging of dopamine partial agonists in humans: implications for clinical practice. Front Psychiatry. 2022;13:832209.
27. Snyder GL, Vanover KE, Zhu H, et al. Functional profile of a novel modulator of serotonin, dopamine, and glutamate neurotransmission. Psychopharmacology (Berl). 2015;232(3):605-621.
28. Kiss B, Horváth A, Némethy Z, et al. Cariprazine (RGH-188), a dopamine D(3) receptor-preferring, D(3)/D(2) dopamine receptor antagonist-partial agonist antipsychotic candidate: in vitro and neurochemical profile. J Pharmacol Exp Ther. 2010;333(1):328-340.
29. Abbasian C, Power P. A case of aripiprazole and tardive dyskinesia. J Psychopharmacol. 2009;23(2):214-215.
30. Peña MS, Yaltho TC, Jankovic J. Tardive dyskinesia and other movement disorders secondary to aripiprazole. Mov Disord. 2011;26(1):147-152.
31. Tadokoro S, Okamura N, Sekine Y, et al. Chronic treatment with aripiprazole prevents development of dopamine supersensitivity and potentially supersensitivity psychosis. Schizophr Bull. 2012;38(5):1012-1020.
32. Kang NR, Kim MD. Tardive dyskinesia: treatment with aripiprazole. Clin Psychopharmacol Neurosci. 2011;9(1):1-8.
33. Frankel JS, Schwartz TL. Brexpiprazole and cariprazine: distinguishing two new atypical antipsychotics from the original dopamine stabilizer aripiprazole. Ther Adv Psychopharmacol. 2017;7(1):29-41.
34. Chan CH, Chan HY, Chen YC. Switching antipsychotic treatment to aripiprazole in psychotic patients with neuroleptic-induced tardive dyskinesia: a 24-week follow-up study. Int Clin Psychopharmacol. 2018;33(3):155-162.
35. Blair HA. Lumateperone: first approval. Drugs. 2020;80(4):417-423.
36. Calabrese JR, Durgam S, Satlin A, et al. Efficacy and safety of Lumateperone for major depressive episodes associated with bipolar I or bipolar II disorder: a phase 3 randomized placebo-controlled trial. Am J Psychiatry. 2021;178(12):1098-1106.
37. Nakai S, Hirose T, Uwahodo Y, et al. Diminished catalepsy and dopamine metabolism distinguish aripiprazole from haloperidol or risperidone. Eur J Pharmacol. 2003;472(12):89-97.
38. Vanover KE, Davis RE, Zhou Y, et al. Dopamine D2 receptor occupancy of lumateperone (ITI-007): a positron emission tomography study in patients with schizophrenia. Neuropsychopharmacology. 2019;44(3):598-605.
Serotonin-mediated anxiety: How to recognize and treat it
Sara R. Abell, MD, and Rif S. El-Mallakh, MD
Individuals with anxiety will experience frequent or chronic excessive worry, nervousness, a sense of unease, a feeling of being unfocused, and distress, which result in functional impairment.1 Frequently, anxiety is accompanied by restlessness or muscle tension. Generalized anxiety disorder is one of the most common psychiatric diagnoses in the United States and has a prevalence of 2% to 6% globally.2 Although research has been conducted regarding anxiety’s pathogenesis, to date a firm consensus on its etiology has not been reached.3 It is likely multifactorial, with environmental and biologic components.
One area of focus has been neurotransmitters and the possible role they play in the pathogenesis of anxiety. Specifically, the monoamine neurotransmitters have been implicated in the clinical manifestations of anxiety. Among the amines, normal roles include stimulating the autonomic nervous system and regulating numerous cognitive phenomena, such as volition and emotion. Many psychiatric medications modify aminergic transmission, and many current anxiety medications target amine neurotransmitters. Medications that target histamine, serotonin, norepinephrine, and dopamine all play a role in treating anxiety.
In this article, we focus on serotonin (5-hydroxytryptamine, 5-HT) as a mediator of anxiety and on excessive synaptic 5-HT as the cause of anxiety. We discuss how 5-HT–mediated anxiety can be identified and offer some solutions for its treatment.
The amine neurotransmitters
There are 6 amine neurotransmitters in the CNS. These are derived from tyrosine (dopamine [DA], norepinephrine [NE], and epinephrine), histidine (histamine), and tryptophan (serotonin [5-HT] and melatonin). In addition to their physiologic actions, amines have been implicated in both acute and chronic anxiety. Excessive DA stimulation has been linked with fear4,5; NE elevations are central to hypervigilance and hyperarousal of posttraumatic stress disorder6; and histamine may mediate emotional memories involved in fear and anxiety.7 Understanding the normal function of 5-HT will aid in understanding its potential problematic role (Box,8-18page 38).
How serotonin-mediated anxiety presents
“Anxiety” is a collection of signs and symptoms that likely represent multiple processes and have the common characteristic of being subjectively unpleasant, with a subjective wish for the feeling to end. The expression of anxiety disorders is quite diverse and ranges from brief episodes such as panic attacks (which may be mediated, in part, by epinephrine/NE19) to lifelong stereotypic obsessions and compulsions (which may be mediated, in part, by DA and modified by 5-HT20,21). Biochemical separation of the anxiety disorders is key to achieving tailored treatment.6 Towards this end, it is important to investigate the phenomenon of serotonin-mediated anxiety.
Because clinicians are familiar with reductions of anxiety as selective serotonin reuptake inhibitors (SSRIs) increase 5-HT levels in the synapse, it is difficult to conceptualize serotonin-mediated anxiety. However, many of the effects at postsynaptic 5-HT receptors may be biphasic.15-18 Serotonin-mediated anxiety appears to occur when levels of 5-HT (or stimulation of 5-HT receptors) are particularly high. This is most frequently seen in patients who genetically have high synaptic 5-HT (by virtue of the short form of the 5-HT transporter),22 whose synaptic 5-HT is further increased by treatment with an SSRI,23 and who are experiencing a stressor that yet further increases their synaptic 5-HT.24 However, it may occur in some individuals with only 2 of these 3 conditions.Clinically, individuals with serotonin-mediated anxiety will usually appear calm. The anxiety they are experiencing is not exhibited in any way in the motor system (ie, they do not appear restless, do not pace, muscle tone is not increased, etc.). However, they will generally complain of an internal agitation, a sense of a negative internal energy. Frequently, they will use descriptions such as “I feel I could jump out of my skin.” As previously mentioned, this is usually in the setting of some environmental stress, in addition to either a pharmacologic (SSRI) or genetic (short form of the 5-HT transporter) reason for increasing synaptic 5-HT, or both.
Almost always, interventions that block multiple postsynaptic 5-HT receptors or discontinuation of the SSRI (if applicable) will alleviate the anxiety, quickly or more slowly, respectively. Sublingual asenapine, which at low doses can block 5-HT2C (Ki = 0.03 nM), 5-HT2A (Ki = 0.07 nM), 5-HT7 (Ki = 0.11 nM), 5-HT2B (Ki = 0.18 nM), and 5-HT6 (Ki = 0.25 nM),25,26 and which will produce peak plasma levels within 10 minutes,27 usually is quite effective.
Box
Serotonin (5-HT) arises from neurons in the raphe nuclei of the rostral pons and projects superiorly to the cerebral cortex and inferiorly to the spinal cord.8 It works in an inhibitory or excitatory manner depending on which receptors are activated. In the periphery, 5-HT influences intestinal peristalsis, sensory modulation, gland function, thermoregulation, blood pressure, platelet aggregation, and sexual behavior,9 all actions that produce potential adverse effects of serotonin reuptake– inhibiting antidepressants. In the CNS, 5-HT plays a role in attention bias; decision-making; sleep and wakefulness; and mood regulation. In short, serotonin can be viewed as mediating emotional motivation.10
Serotonin alters neuroplasticity. During development, 5-HT stimulates creation of new synapses and increases the density of synaptic webs. It has a direct stimulatory effect on the length of dendrites, their branching, and their myelination.11 In the CNS, it plays a role in dendritic arborization. Animal studies with rats have shown that lesioning highly concentrated 5-HT areas at early ages resulted in an adult brain with a lower number of neurons and a less complex web of dendrites.12,13 In situations of emotional stress, it is theorized that low levels of 5-HT lead to a reduced ability to deal with emotional stressors due to lower levels of complexity in synaptic connections.
Serotonin has also been implicated in mediating some aspects of dopamine-related actions, such as locomotion, reward, and threat avoidance. This is believed to contribute to the beneficial effect of 5-HT2A blockade by secondgeneration antipsychotics (SGAs).14 Blockade of other 5-HT receptors, such as 5-HT1A, 5-HT2C, 5-HT6, and 5-HT7, may also contribute to the antipsychotic action of SGAs.14
Serotonin receptors are found throughout the body, and 14 subtypes have been identified.9 Excitatory and inhibitory action of 5-HT depends on the receptor, and the actions of 5-HT can differ with the same receptor at different concentrations. This is because serotonin’s effects are biphasic and concentration-dependent, meaning that levels of 5-HT in the synapse will dictate the downstream effect of receptor agonism or antagonism. Animal models have shown that low-dose agonism of 5-HT receptors causes vasoconstriction of the coronary arteries, and high doses cause relaxation. This response has also been demonstrated in the vasculature of the kidneys and the smooth muscle of the trachea. Additionally, 5-HT works in conjunction with histamine to produce a biphasic response in the colonic arteries and veins in situations of endothelial damage.15
Most relevant to this discussion are 5-HT’s actions in mood regulation and behavior. Low 5-HT states result in less behavioral inhibition, leading to higher impulse control failures and aggression. Experiments in mice with deficient serotonergic brain regions show hypoactivity, extended daytime sleep, anxiety, and depressive behaviors.13 Serotonin’s behavioral effects are also biphasic. For example, lowdose antagonism with trazodone of 5-HT receptors demonstrated a pro-aggressive behavioral effect, while high-dose antagonism is anti-aggressive.15 Similar biphasic effects may result in either induction or reduction of anxiety with agents that block or excite certain 5-HT receptors.16-18
Continue to: A key difference: No motor system involvement...
A key difference: No motor system involvement
What distinguishes 5-HT from the other amine transmitters as a mediator of anxiety is the lack of involvement of the motor system. Multiple studies in rats illustrate that exogenously augmenting 5-HT has no effect on levels of locomotor activity. Dopamine depletion is well-characterized in the motor dysfunction of Parkinson’s disease, and DA excess can cause repetitive, stereotyped movements, such as seen in tardive dyskinesia or Huntington’s disease.8 In humans, serotonin-mediated anxiety is usually without a motoric component; patients appear calm but complain of extreme anxiety or agitation. Agitation has been reported after initiation of an SSRI,29 and is more likely to occur in patients with the short form of the 5-HT transporter.30 Motoric activation has been reported in some of these studies, but does not seem to cluster with the complaint of agitation.29 The reduced number of available transporters means a chronic steady-state elevation of serotonin, because less serotonin is being removed from the synapse after it is released. This is one of the reasons patients with the short form of the 5-HT transporter may be more susceptible to serotonin-mediated anxiety.
What you need to keep in mind
Pharmacologic treatment of anxiety begins with an SSRI, a serotonin-norepinephrine reuptake inhibitor (SNRI), or buspirone. Second-line treatments include hydroxyzine, gabapentin, pregabalin, and quetiapine.3,31 However, clinicians need to be aware that a fraction of their patients will report anxiety that will not have any external manifestations, but will be experienced as an unpleasant internal energy. These patients may report an increase in their anxiety levels when started on an SSRI or SNRI.29,30 This anxiety is most likely mediated by increases of synaptic 5-HT. This occurs because many serotonergic receptors may have a biphasic response, so that too much stimulation is experienced as excessive internal energy.16-18 In such patients, blockade of key 5-HT receptors may reduce that internal agitation. The advantage of recognizing serotonin-mediated anxiety is that one can specifically tailor treatment to address the patient’s specific physiology.
It is important to note that the anxiolytic effect of asenapine is specific to patients with serotonin-mediated anxiety. Unlike quetiapine, which is effective as augmentation therapy in generalized anxiety disorder,31 asenapine does not appear to reduce anxiety in patients with schizophrenia32 or borderline personality disorder33 when administered for other reasons. However, it may reduce anxiety in patients with the short form of the 5-HT transporter.30,34
Bottom Line
Serotonin-mediated anxiety occurs when levels of synaptic serotonin (5-HT) are high. Patients with serotonin-mediated anxiety appear calm but will report experiencing an unpleasant internal energy. Interventions that block multiple postsynaptic 5-HT receptors or discontinuation of a selective serotonin reuptake inhibitor (if applicable) will alleviate the anxiety.
Related Resource
• Bhatt NV. Anxiety disorders. https://emedicine.medscape. com/article/286227-overview
Drug Brand Names
Asenapine • Saphris, Secuado
Gabapentin • Neurontin
Hydroxyzine • Vistaril
Pregabalin • Lyrica
Quetiapine • Seroquel
Trazodone • Oleptro
1. Shelton CI. Diagnosis and management of anxiety disorders. J Am Osteopath Assoc. 2004;104(3 Suppl 3):S2-S5.
2. Ruscio AM, Hallion LS, Lim CCW, et al. Cross-sectional comparison of the epidemiology of DSM-5 generalized anxiety disorder across the globe. JAMA Psychiatry. 2017;74(5):465-475.
3. Locke AB, Kirst N, Shultz CG. Diagnosis and management of generalized anxiety disorder and panic disorder in adults. Am Fam Physician. 2015;91(9):617-624.
4. Hariri AR, Mattay VS, Tessitore A, et al. Dextroamphetamine modulates the response of the human amygdala. Neuropsychopharmacology. 2002;27(6):1036-1040.
5. Colombo AC, de Oliveira AR, Reimer AE, et al. Dopaminergic mechanisms underlying catalepsy, fear and anxiety: do they interact? Behav Brain Res. 2013;257:201-207.
6. Togay B, El-Mallakh RS. Posttraumatic stress disorder: from pathophysiology to pharmacology. Curr Psychiatry. 2020;19(5):33-39.
7. Provensi G, Passani MB, Costa A, et al. Neuronal histamine and the memory of emotionally salient events. Br J Pharmacol. 2020;177(3):557-569.
8. Purves D, Augustine GJ, Fitzpatrick D, et al (eds). Neuroscience. 2nd ed. Sinauer Associates; 2001.
9. Pytliak M, Vargová V, Mechírová V, et al. Serotonin receptors – from molecular biology to clinical applications. Physiol Res. 2011;60(1):15-25.
10. Meneses A, Liy-Salmeron G. Serotonin and emotion, learning and memory. Rev Neurosci. 2012;23(5-6):543-553.
11. Whitaker-Azmitia PM. Serotonin and brain development: role in human developmental diseases. Brain Res Bull. 2001;56(5):479-485.
12. Towle AC, Breese GR, Mueller RA, et al. Early postnatal administration of 5,7-DHT: effects on serotonergic neurons and terminals. Brain Res. 1984;310(1):67-75.
13. Rok-Bujko P, Krzs´cik P, Szyndler J, et al. The influence of neonatal serotonin depletion on emotional and exploratory behaviours in rats. Behav Brain Res. 2012;226(1):87-95.
14. Meltzer HY. The role of serotonin in antipsychotic drug action. Neuropsychopharmacology. 1999;21(2 Suppl):106S-115S.
15. Calabrese EJ. 5-Hydroxytryptamine (serotonin): biphasic dose responses. Crit Rev Toxicol. 2001;31(4-5):553-561.
16. Zuardi AW. 5-HT-related drugs and human experimental anxiety. Neurosci Biobehav Rev. 1990;14(4):507-510.
17. Sánchez C, Meier E. Behavioral profiles of SSRIs in animal models of depression, anxiety and aggression. Are they all alike? Psychopharmacology (Berl). 1997;129(3):197-205.
18. Koek W, Mitchell NC, Daws LC. Biphasic effects of selective serotonin reuptake inhibitors on anxiety: rapid reversal of escitalopram’s anxiogenic effects in the novelty-induced hypophagia test in mice? Behav Pharmacol. 2018;29(4):365-369.
19. van Zijderveld GA, Veltman DJ, van Dyck R, et al. Epinephrine-induced panic attacks and hyperventilation. J Psychiatr Res. 1999;33(1):73-78.
20. Ho EV, Thompson SL, Katzka WR, et al. Clinically effective OCD treatment prevents 5-HT1B receptor-induced repetitive behavior and striatal activation. Psychopharmacology (Berl). 2016;233(1):57-70.
21. Stein DJ, Costa DLC, Lochner C, et al. Obsessive-compulsive disorder. Nat Rev Dis Primers. 2019;5(1):52.
22. Luddington NS, Mandadapu A, Husk M, et al. Clinical implications of genetic variation in the serotonin transporter promoter region: a review. Prim Care Companion J Clin Psychiatry. 2009;11(3):93-102.
23. Stahl SM. Mechanism of action of serotonin selective reuptake inhibitors. Serotonin receptors and pathways mediate therapeutic effects and side effects. J Affect Disord. 1998;51(3):215-235.
24. Chaouloff F, Berton O, Mormède P. Serotonin and stress. Neuropsychopharmacology. 1999;21(2 Suppl):28S-32S.
25. Siafis S, Tzachanis D, Samara M, et al. Antipsychotic drugs: From receptor-binding profiles to metabolic side effects. Curr Neuropharmacol. 2018;16(8):1210-1223.
26. Carrithers B, El-Mallakh RS. Transdermal asenapine in schizophrenia: a systematic review. Patient Prefer Adherence. 2020;14:1541-1551.
27. Citrome L. Asenapine review, part I: chemistry, receptor affinity profile, pharmacokinetics and metabolism. Expert Opin Drug Metab Toxicol. 2014;10(6):893-903.
28. Pratts M, Citrome L, Grant W, et al. A single-dose, randomized, double-blind, placebo-controlled trial of sublingual asenapine for acute agitation. Acta Psychiatr Scand. 2014;130(1):61-68.
29. Biswas AB, Bhaumik S, Branford D. Treatment-emergent behavioural side effects with selective serotonin re-uptake inhibitors in adults with learning disabilities. Hum Psychopharmacol. 2001;16(2):133-137.
30. Perlis RH, Mischoulon D, Smoller JW, et al. Serotonin transporter polymorphisms and adverse effects with fluoxetine treatment. Biol Psychiatry. 2003;54(9):879-883.
31. Ipser JC, Carey P, Dhansay Y, et al. Pharmacotherapy augmentation strategies in treatment-resistant anxiety disorders. Cochrane Database Syst Rev. 2006;(4):CD005473.
32. Kane JM, Mackle M, Snow-Adami L, et al. A randomized placebo-controlled trial of asenapine for the prevention of relapse of schizophrenia after long-term treatment. J Clin Psychiatry. 2011;72(3):349-355.
33. Bozzatello P, Rocca P, Uscinska M, et al. Efficacy and tolerability of asenapine compared with olanzapine in borderline personality disorder: an open-label randomized controlled trial. CNS Drugs. 2017;31(9):809-819.
34. El-Mallakh RS, Nuss S, Gao D, et al. Asenapine in the treatment of bipolar depression. Psychopharmacol Bull. 2020;50(1):8-18.
Sara R. Abell, MD, and Rif S. El-Mallakh, MD
Individuals with anxiety will experience frequent or chronic excessive worry, nervousness, a sense of unease, a feeling of being unfocused, and distress, which result in functional impairment.1 Frequently, anxiety is accompanied by restlessness or muscle tension. Generalized anxiety disorder is one of the most common psychiatric diagnoses in the United States and has a prevalence of 2% to 6% globally.2 Although research has been conducted regarding anxiety’s pathogenesis, to date a firm consensus on its etiology has not been reached.3 It is likely multifactorial, with environmental and biologic components.
One area of focus has been neurotransmitters and the possible role they play in the pathogenesis of anxiety. Specifically, the monoamine neurotransmitters have been implicated in the clinical manifestations of anxiety. Among the amines, normal roles include stimulating the autonomic nervous system and regulating numerous cognitive phenomena, such as volition and emotion. Many psychiatric medications modify aminergic transmission, and many current anxiety medications target amine neurotransmitters. Medications that target histamine, serotonin, norepinephrine, and dopamine all play a role in treating anxiety.
In this article, we focus on serotonin (5-hydroxytryptamine, 5-HT) as a mediator of anxiety and on excessive synaptic 5-HT as the cause of anxiety. We discuss how 5-HT–mediated anxiety can be identified and offer some solutions for its treatment.
The amine neurotransmitters
There are 6 amine neurotransmitters in the CNS. These are derived from tyrosine (dopamine [DA], norepinephrine [NE], and epinephrine), histidine (histamine), and tryptophan (serotonin [5-HT] and melatonin). In addition to their physiologic actions, amines have been implicated in both acute and chronic anxiety. Excessive DA stimulation has been linked with fear4,5; NE elevations are central to hypervigilance and hyperarousal of posttraumatic stress disorder6; and histamine may mediate emotional memories involved in fear and anxiety.7 Understanding the normal function of 5-HT will aid in understanding its potential problematic role (Box,8-18page 38).
How serotonin-mediated anxiety presents
“Anxiety” is a collection of signs and symptoms that likely represent multiple processes and have the common characteristic of being subjectively unpleasant, with a subjective wish for the feeling to end. The expression of anxiety disorders is quite diverse and ranges from brief episodes such as panic attacks (which may be mediated, in part, by epinephrine/NE19) to lifelong stereotypic obsessions and compulsions (which may be mediated, in part, by DA and modified by 5-HT20,21). Biochemical separation of the anxiety disorders is key to achieving tailored treatment.6 Towards this end, it is important to investigate the phenomenon of serotonin-mediated anxiety.
Because clinicians are familiar with reductions of anxiety as selective serotonin reuptake inhibitors (SSRIs) increase 5-HT levels in the synapse, it is difficult to conceptualize serotonin-mediated anxiety. However, many of the effects at postsynaptic 5-HT receptors may be biphasic.15-18 Serotonin-mediated anxiety appears to occur when levels of 5-HT (or stimulation of 5-HT receptors) are particularly high. This is most frequently seen in patients who genetically have high synaptic 5-HT (by virtue of the short form of the 5-HT transporter),22 whose synaptic 5-HT is further increased by treatment with an SSRI,23 and who are experiencing a stressor that yet further increases their synaptic 5-HT.24 However, it may occur in some individuals with only 2 of these 3 conditions.Clinically, individuals with serotonin-mediated anxiety will usually appear calm. The anxiety they are experiencing is not exhibited in any way in the motor system (ie, they do not appear restless, do not pace, muscle tone is not increased, etc.). However, they will generally complain of an internal agitation, a sense of a negative internal energy. Frequently, they will use descriptions such as “I feel I could jump out of my skin.” As previously mentioned, this is usually in the setting of some environmental stress, in addition to either a pharmacologic (SSRI) or genetic (short form of the 5-HT transporter) reason for increasing synaptic 5-HT, or both.
Almost always, interventions that block multiple postsynaptic 5-HT receptors or discontinuation of the SSRI (if applicable) will alleviate the anxiety, quickly or more slowly, respectively. Sublingual asenapine, which at low doses can block 5-HT2C (Ki = 0.03 nM), 5-HT2A (Ki = 0.07 nM), 5-HT7 (Ki = 0.11 nM), 5-HT2B (Ki = 0.18 nM), and 5-HT6 (Ki = 0.25 nM),25,26 and which will produce peak plasma levels within 10 minutes,27 usually is quite effective.
Box
Serotonin (5-HT) arises from neurons in the raphe nuclei of the rostral pons and projects superiorly to the cerebral cortex and inferiorly to the spinal cord.8 It works in an inhibitory or excitatory manner depending on which receptors are activated. In the periphery, 5-HT influences intestinal peristalsis, sensory modulation, gland function, thermoregulation, blood pressure, platelet aggregation, and sexual behavior,9 all actions that produce potential adverse effects of serotonin reuptake– inhibiting antidepressants. In the CNS, 5-HT plays a role in attention bias; decision-making; sleep and wakefulness; and mood regulation. In short, serotonin can be viewed as mediating emotional motivation.10
Serotonin alters neuroplasticity. During development, 5-HT stimulates creation of new synapses and increases the density of synaptic webs. It has a direct stimulatory effect on the length of dendrites, their branching, and their myelination.11 In the CNS, it plays a role in dendritic arborization. Animal studies with rats have shown that lesioning highly concentrated 5-HT areas at early ages resulted in an adult brain with a lower number of neurons and a less complex web of dendrites.12,13 In situations of emotional stress, it is theorized that low levels of 5-HT lead to a reduced ability to deal with emotional stressors due to lower levels of complexity in synaptic connections.
Serotonin has also been implicated in mediating some aspects of dopamine-related actions, such as locomotion, reward, and threat avoidance. This is believed to contribute to the beneficial effect of 5-HT2A blockade by secondgeneration antipsychotics (SGAs).14 Blockade of other 5-HT receptors, such as 5-HT1A, 5-HT2C, 5-HT6, and 5-HT7, may also contribute to the antipsychotic action of SGAs.14
Serotonin receptors are found throughout the body, and 14 subtypes have been identified.9 Excitatory and inhibitory action of 5-HT depends on the receptor, and the actions of 5-HT can differ with the same receptor at different concentrations. This is because serotonin’s effects are biphasic and concentration-dependent, meaning that levels of 5-HT in the synapse will dictate the downstream effect of receptor agonism or antagonism. Animal models have shown that low-dose agonism of 5-HT receptors causes vasoconstriction of the coronary arteries, and high doses cause relaxation. This response has also been demonstrated in the vasculature of the kidneys and the smooth muscle of the trachea. Additionally, 5-HT works in conjunction with histamine to produce a biphasic response in the colonic arteries and veins in situations of endothelial damage.15
Most relevant to this discussion are 5-HT’s actions in mood regulation and behavior. Low 5-HT states result in less behavioral inhibition, leading to higher impulse control failures and aggression. Experiments in mice with deficient serotonergic brain regions show hypoactivity, extended daytime sleep, anxiety, and depressive behaviors.13 Serotonin’s behavioral effects are also biphasic. For example, lowdose antagonism with trazodone of 5-HT receptors demonstrated a pro-aggressive behavioral effect, while high-dose antagonism is anti-aggressive.15 Similar biphasic effects may result in either induction or reduction of anxiety with agents that block or excite certain 5-HT receptors.16-18
Continue to: A key difference: No motor system involvement...
A key difference: No motor system involvement
What distinguishes 5-HT from the other amine transmitters as a mediator of anxiety is the lack of involvement of the motor system. Multiple studies in rats illustrate that exogenously augmenting 5-HT has no effect on levels of locomotor activity. Dopamine depletion is well-characterized in the motor dysfunction of Parkinson’s disease, and DA excess can cause repetitive, stereotyped movements, such as seen in tardive dyskinesia or Huntington’s disease.8 In humans, serotonin-mediated anxiety is usually without a motoric component; patients appear calm but complain of extreme anxiety or agitation. Agitation has been reported after initiation of an SSRI,29 and is more likely to occur in patients with the short form of the 5-HT transporter.30 Motoric activation has been reported in some of these studies, but does not seem to cluster with the complaint of agitation.29 The reduced number of available transporters means a chronic steady-state elevation of serotonin, because less serotonin is being removed from the synapse after it is released. This is one of the reasons patients with the short form of the 5-HT transporter may be more susceptible to serotonin-mediated anxiety.
What you need to keep in mind
Pharmacologic treatment of anxiety begins with an SSRI, a serotonin-norepinephrine reuptake inhibitor (SNRI), or buspirone. Second-line treatments include hydroxyzine, gabapentin, pregabalin, and quetiapine.3,31 However, clinicians need to be aware that a fraction of their patients will report anxiety that will not have any external manifestations, but will be experienced as an unpleasant internal energy. These patients may report an increase in their anxiety levels when started on an SSRI or SNRI.29,30 This anxiety is most likely mediated by increases of synaptic 5-HT. This occurs because many serotonergic receptors may have a biphasic response, so that too much stimulation is experienced as excessive internal energy.16-18 In such patients, blockade of key 5-HT receptors may reduce that internal agitation. The advantage of recognizing serotonin-mediated anxiety is that one can specifically tailor treatment to address the patient’s specific physiology.
It is important to note that the anxiolytic effect of asenapine is specific to patients with serotonin-mediated anxiety. Unlike quetiapine, which is effective as augmentation therapy in generalized anxiety disorder,31 asenapine does not appear to reduce anxiety in patients with schizophrenia32 or borderline personality disorder33 when administered for other reasons. However, it may reduce anxiety in patients with the short form of the 5-HT transporter.30,34
Bottom Line
Serotonin-mediated anxiety occurs when levels of synaptic serotonin (5-HT) are high. Patients with serotonin-mediated anxiety appear calm but will report experiencing an unpleasant internal energy. Interventions that block multiple postsynaptic 5-HT receptors or discontinuation of a selective serotonin reuptake inhibitor (if applicable) will alleviate the anxiety.
Related Resource
• Bhatt NV. Anxiety disorders. https://emedicine.medscape. com/article/286227-overview
Drug Brand Names
Asenapine • Saphris, Secuado
Gabapentin • Neurontin
Hydroxyzine • Vistaril
Pregabalin • Lyrica
Quetiapine • Seroquel
Trazodone • Oleptro
Sara R. Abell, MD, and Rif S. El-Mallakh, MD
Individuals with anxiety will experience frequent or chronic excessive worry, nervousness, a sense of unease, a feeling of being unfocused, and distress, which result in functional impairment.1 Frequently, anxiety is accompanied by restlessness or muscle tension. Generalized anxiety disorder is one of the most common psychiatric diagnoses in the United States and has a prevalence of 2% to 6% globally.2 Although research has been conducted regarding anxiety’s pathogenesis, to date a firm consensus on its etiology has not been reached.3 It is likely multifactorial, with environmental and biologic components.
One area of focus has been neurotransmitters and the possible role they play in the pathogenesis of anxiety. Specifically, the monoamine neurotransmitters have been implicated in the clinical manifestations of anxiety. Among the amines, normal roles include stimulating the autonomic nervous system and regulating numerous cognitive phenomena, such as volition and emotion. Many psychiatric medications modify aminergic transmission, and many current anxiety medications target amine neurotransmitters. Medications that target histamine, serotonin, norepinephrine, and dopamine all play a role in treating anxiety.
In this article, we focus on serotonin (5-hydroxytryptamine, 5-HT) as a mediator of anxiety and on excessive synaptic 5-HT as the cause of anxiety. We discuss how 5-HT–mediated anxiety can be identified and offer some solutions for its treatment.
The amine neurotransmitters
There are 6 amine neurotransmitters in the CNS. These are derived from tyrosine (dopamine [DA], norepinephrine [NE], and epinephrine), histidine (histamine), and tryptophan (serotonin [5-HT] and melatonin). In addition to their physiologic actions, amines have been implicated in both acute and chronic anxiety. Excessive DA stimulation has been linked with fear4,5; NE elevations are central to hypervigilance and hyperarousal of posttraumatic stress disorder6; and histamine may mediate emotional memories involved in fear and anxiety.7 Understanding the normal function of 5-HT will aid in understanding its potential problematic role (Box,8-18page 38).
How serotonin-mediated anxiety presents
“Anxiety” is a collection of signs and symptoms that likely represent multiple processes and have the common characteristic of being subjectively unpleasant, with a subjective wish for the feeling to end. The expression of anxiety disorders is quite diverse and ranges from brief episodes such as panic attacks (which may be mediated, in part, by epinephrine/NE19) to lifelong stereotypic obsessions and compulsions (which may be mediated, in part, by DA and modified by 5-HT20,21). Biochemical separation of the anxiety disorders is key to achieving tailored treatment.6 Towards this end, it is important to investigate the phenomenon of serotonin-mediated anxiety.
Because clinicians are familiar with reductions of anxiety as selective serotonin reuptake inhibitors (SSRIs) increase 5-HT levels in the synapse, it is difficult to conceptualize serotonin-mediated anxiety. However, many of the effects at postsynaptic 5-HT receptors may be biphasic.15-18 Serotonin-mediated anxiety appears to occur when levels of 5-HT (or stimulation of 5-HT receptors) are particularly high. This is most frequently seen in patients who genetically have high synaptic 5-HT (by virtue of the short form of the 5-HT transporter),22 whose synaptic 5-HT is further increased by treatment with an SSRI,23 and who are experiencing a stressor that yet further increases their synaptic 5-HT.24 However, it may occur in some individuals with only 2 of these 3 conditions.Clinically, individuals with serotonin-mediated anxiety will usually appear calm. The anxiety they are experiencing is not exhibited in any way in the motor system (ie, they do not appear restless, do not pace, muscle tone is not increased, etc.). However, they will generally complain of an internal agitation, a sense of a negative internal energy. Frequently, they will use descriptions such as “I feel I could jump out of my skin.” As previously mentioned, this is usually in the setting of some environmental stress, in addition to either a pharmacologic (SSRI) or genetic (short form of the 5-HT transporter) reason for increasing synaptic 5-HT, or both.
Almost always, interventions that block multiple postsynaptic 5-HT receptors or discontinuation of the SSRI (if applicable) will alleviate the anxiety, quickly or more slowly, respectively. Sublingual asenapine, which at low doses can block 5-HT2C (Ki = 0.03 nM), 5-HT2A (Ki = 0.07 nM), 5-HT7 (Ki = 0.11 nM), 5-HT2B (Ki = 0.18 nM), and 5-HT6 (Ki = 0.25 nM),25,26 and which will produce peak plasma levels within 10 minutes,27 usually is quite effective.
Box
Serotonin (5-HT) arises from neurons in the raphe nuclei of the rostral pons and projects superiorly to the cerebral cortex and inferiorly to the spinal cord.8 It works in an inhibitory or excitatory manner depending on which receptors are activated. In the periphery, 5-HT influences intestinal peristalsis, sensory modulation, gland function, thermoregulation, blood pressure, platelet aggregation, and sexual behavior,9 all actions that produce potential adverse effects of serotonin reuptake– inhibiting antidepressants. In the CNS, 5-HT plays a role in attention bias; decision-making; sleep and wakefulness; and mood regulation. In short, serotonin can be viewed as mediating emotional motivation.10
Serotonin alters neuroplasticity. During development, 5-HT stimulates creation of new synapses and increases the density of synaptic webs. It has a direct stimulatory effect on the length of dendrites, their branching, and their myelination.11 In the CNS, it plays a role in dendritic arborization. Animal studies with rats have shown that lesioning highly concentrated 5-HT areas at early ages resulted in an adult brain with a lower number of neurons and a less complex web of dendrites.12,13 In situations of emotional stress, it is theorized that low levels of 5-HT lead to a reduced ability to deal with emotional stressors due to lower levels of complexity in synaptic connections.
Serotonin has also been implicated in mediating some aspects of dopamine-related actions, such as locomotion, reward, and threat avoidance. This is believed to contribute to the beneficial effect of 5-HT2A blockade by secondgeneration antipsychotics (SGAs).14 Blockade of other 5-HT receptors, such as 5-HT1A, 5-HT2C, 5-HT6, and 5-HT7, may also contribute to the antipsychotic action of SGAs.14
Serotonin receptors are found throughout the body, and 14 subtypes have been identified.9 Excitatory and inhibitory action of 5-HT depends on the receptor, and the actions of 5-HT can differ with the same receptor at different concentrations. This is because serotonin’s effects are biphasic and concentration-dependent, meaning that levels of 5-HT in the synapse will dictate the downstream effect of receptor agonism or antagonism. Animal models have shown that low-dose agonism of 5-HT receptors causes vasoconstriction of the coronary arteries, and high doses cause relaxation. This response has also been demonstrated in the vasculature of the kidneys and the smooth muscle of the trachea. Additionally, 5-HT works in conjunction with histamine to produce a biphasic response in the colonic arteries and veins in situations of endothelial damage.15
Most relevant to this discussion are 5-HT’s actions in mood regulation and behavior. Low 5-HT states result in less behavioral inhibition, leading to higher impulse control failures and aggression. Experiments in mice with deficient serotonergic brain regions show hypoactivity, extended daytime sleep, anxiety, and depressive behaviors.13 Serotonin’s behavioral effects are also biphasic. For example, lowdose antagonism with trazodone of 5-HT receptors demonstrated a pro-aggressive behavioral effect, while high-dose antagonism is anti-aggressive.15 Similar biphasic effects may result in either induction or reduction of anxiety with agents that block or excite certain 5-HT receptors.16-18
Continue to: A key difference: No motor system involvement...
A key difference: No motor system involvement
What distinguishes 5-HT from the other amine transmitters as a mediator of anxiety is the lack of involvement of the motor system. Multiple studies in rats illustrate that exogenously augmenting 5-HT has no effect on levels of locomotor activity. Dopamine depletion is well-characterized in the motor dysfunction of Parkinson’s disease, and DA excess can cause repetitive, stereotyped movements, such as seen in tardive dyskinesia or Huntington’s disease.8 In humans, serotonin-mediated anxiety is usually without a motoric component; patients appear calm but complain of extreme anxiety or agitation. Agitation has been reported after initiation of an SSRI,29 and is more likely to occur in patients with the short form of the 5-HT transporter.30 Motoric activation has been reported in some of these studies, but does not seem to cluster with the complaint of agitation.29 The reduced number of available transporters means a chronic steady-state elevation of serotonin, because less serotonin is being removed from the synapse after it is released. This is one of the reasons patients with the short form of the 5-HT transporter may be more susceptible to serotonin-mediated anxiety.
What you need to keep in mind
Pharmacologic treatment of anxiety begins with an SSRI, a serotonin-norepinephrine reuptake inhibitor (SNRI), or buspirone. Second-line treatments include hydroxyzine, gabapentin, pregabalin, and quetiapine.3,31 However, clinicians need to be aware that a fraction of their patients will report anxiety that will not have any external manifestations, but will be experienced as an unpleasant internal energy. These patients may report an increase in their anxiety levels when started on an SSRI or SNRI.29,30 This anxiety is most likely mediated by increases of synaptic 5-HT. This occurs because many serotonergic receptors may have a biphasic response, so that too much stimulation is experienced as excessive internal energy.16-18 In such patients, blockade of key 5-HT receptors may reduce that internal agitation. The advantage of recognizing serotonin-mediated anxiety is that one can specifically tailor treatment to address the patient’s specific physiology.
It is important to note that the anxiolytic effect of asenapine is specific to patients with serotonin-mediated anxiety. Unlike quetiapine, which is effective as augmentation therapy in generalized anxiety disorder,31 asenapine does not appear to reduce anxiety in patients with schizophrenia32 or borderline personality disorder33 when administered for other reasons. However, it may reduce anxiety in patients with the short form of the 5-HT transporter.30,34
Bottom Line
Serotonin-mediated anxiety occurs when levels of synaptic serotonin (5-HT) are high. Patients with serotonin-mediated anxiety appear calm but will report experiencing an unpleasant internal energy. Interventions that block multiple postsynaptic 5-HT receptors or discontinuation of a selective serotonin reuptake inhibitor (if applicable) will alleviate the anxiety.
Related Resource
• Bhatt NV. Anxiety disorders. https://emedicine.medscape. com/article/286227-overview
Drug Brand Names
Asenapine • Saphris, Secuado
Gabapentin • Neurontin
Hydroxyzine • Vistaril
Pregabalin • Lyrica
Quetiapine • Seroquel
Trazodone • Oleptro
1. Shelton CI. Diagnosis and management of anxiety disorders. J Am Osteopath Assoc. 2004;104(3 Suppl 3):S2-S5.
2. Ruscio AM, Hallion LS, Lim CCW, et al. Cross-sectional comparison of the epidemiology of DSM-5 generalized anxiety disorder across the globe. JAMA Psychiatry. 2017;74(5):465-475.
3. Locke AB, Kirst N, Shultz CG. Diagnosis and management of generalized anxiety disorder and panic disorder in adults. Am Fam Physician. 2015;91(9):617-624.
4. Hariri AR, Mattay VS, Tessitore A, et al. Dextroamphetamine modulates the response of the human amygdala. Neuropsychopharmacology. 2002;27(6):1036-1040.
5. Colombo AC, de Oliveira AR, Reimer AE, et al. Dopaminergic mechanisms underlying catalepsy, fear and anxiety: do they interact? Behav Brain Res. 2013;257:201-207.
6. Togay B, El-Mallakh RS. Posttraumatic stress disorder: from pathophysiology to pharmacology. Curr Psychiatry. 2020;19(5):33-39.
7. Provensi G, Passani MB, Costa A, et al. Neuronal histamine and the memory of emotionally salient events. Br J Pharmacol. 2020;177(3):557-569.
8. Purves D, Augustine GJ, Fitzpatrick D, et al (eds). Neuroscience. 2nd ed. Sinauer Associates; 2001.
9. Pytliak M, Vargová V, Mechírová V, et al. Serotonin receptors – from molecular biology to clinical applications. Physiol Res. 2011;60(1):15-25.
10. Meneses A, Liy-Salmeron G. Serotonin and emotion, learning and memory. Rev Neurosci. 2012;23(5-6):543-553.
11. Whitaker-Azmitia PM. Serotonin and brain development: role in human developmental diseases. Brain Res Bull. 2001;56(5):479-485.
12. Towle AC, Breese GR, Mueller RA, et al. Early postnatal administration of 5,7-DHT: effects on serotonergic neurons and terminals. Brain Res. 1984;310(1):67-75.
13. Rok-Bujko P, Krzs´cik P, Szyndler J, et al. The influence of neonatal serotonin depletion on emotional and exploratory behaviours in rats. Behav Brain Res. 2012;226(1):87-95.
14. Meltzer HY. The role of serotonin in antipsychotic drug action. Neuropsychopharmacology. 1999;21(2 Suppl):106S-115S.
15. Calabrese EJ. 5-Hydroxytryptamine (serotonin): biphasic dose responses. Crit Rev Toxicol. 2001;31(4-5):553-561.
16. Zuardi AW. 5-HT-related drugs and human experimental anxiety. Neurosci Biobehav Rev. 1990;14(4):507-510.
17. Sánchez C, Meier E. Behavioral profiles of SSRIs in animal models of depression, anxiety and aggression. Are they all alike? Psychopharmacology (Berl). 1997;129(3):197-205.
18. Koek W, Mitchell NC, Daws LC. Biphasic effects of selective serotonin reuptake inhibitors on anxiety: rapid reversal of escitalopram’s anxiogenic effects in the novelty-induced hypophagia test in mice? Behav Pharmacol. 2018;29(4):365-369.
19. van Zijderveld GA, Veltman DJ, van Dyck R, et al. Epinephrine-induced panic attacks and hyperventilation. J Psychiatr Res. 1999;33(1):73-78.
20. Ho EV, Thompson SL, Katzka WR, et al. Clinically effective OCD treatment prevents 5-HT1B receptor-induced repetitive behavior and striatal activation. Psychopharmacology (Berl). 2016;233(1):57-70.
21. Stein DJ, Costa DLC, Lochner C, et al. Obsessive-compulsive disorder. Nat Rev Dis Primers. 2019;5(1):52.
22. Luddington NS, Mandadapu A, Husk M, et al. Clinical implications of genetic variation in the serotonin transporter promoter region: a review. Prim Care Companion J Clin Psychiatry. 2009;11(3):93-102.
23. Stahl SM. Mechanism of action of serotonin selective reuptake inhibitors. Serotonin receptors and pathways mediate therapeutic effects and side effects. J Affect Disord. 1998;51(3):215-235.
24. Chaouloff F, Berton O, Mormède P. Serotonin and stress. Neuropsychopharmacology. 1999;21(2 Suppl):28S-32S.
25. Siafis S, Tzachanis D, Samara M, et al. Antipsychotic drugs: From receptor-binding profiles to metabolic side effects. Curr Neuropharmacol. 2018;16(8):1210-1223.
26. Carrithers B, El-Mallakh RS. Transdermal asenapine in schizophrenia: a systematic review. Patient Prefer Adherence. 2020;14:1541-1551.
27. Citrome L. Asenapine review, part I: chemistry, receptor affinity profile, pharmacokinetics and metabolism. Expert Opin Drug Metab Toxicol. 2014;10(6):893-903.
28. Pratts M, Citrome L, Grant W, et al. A single-dose, randomized, double-blind, placebo-controlled trial of sublingual asenapine for acute agitation. Acta Psychiatr Scand. 2014;130(1):61-68.
29. Biswas AB, Bhaumik S, Branford D. Treatment-emergent behavioural side effects with selective serotonin re-uptake inhibitors in adults with learning disabilities. Hum Psychopharmacol. 2001;16(2):133-137.
30. Perlis RH, Mischoulon D, Smoller JW, et al. Serotonin transporter polymorphisms and adverse effects with fluoxetine treatment. Biol Psychiatry. 2003;54(9):879-883.
31. Ipser JC, Carey P, Dhansay Y, et al. Pharmacotherapy augmentation strategies in treatment-resistant anxiety disorders. Cochrane Database Syst Rev. 2006;(4):CD005473.
32. Kane JM, Mackle M, Snow-Adami L, et al. A randomized placebo-controlled trial of asenapine for the prevention of relapse of schizophrenia after long-term treatment. J Clin Psychiatry. 2011;72(3):349-355.
33. Bozzatello P, Rocca P, Uscinska M, et al. Efficacy and tolerability of asenapine compared with olanzapine in borderline personality disorder: an open-label randomized controlled trial. CNS Drugs. 2017;31(9):809-819.
34. El-Mallakh RS, Nuss S, Gao D, et al. Asenapine in the treatment of bipolar depression. Psychopharmacol Bull. 2020;50(1):8-18.
1. Shelton CI. Diagnosis and management of anxiety disorders. J Am Osteopath Assoc. 2004;104(3 Suppl 3):S2-S5.
2. Ruscio AM, Hallion LS, Lim CCW, et al. Cross-sectional comparison of the epidemiology of DSM-5 generalized anxiety disorder across the globe. JAMA Psychiatry. 2017;74(5):465-475.
3. Locke AB, Kirst N, Shultz CG. Diagnosis and management of generalized anxiety disorder and panic disorder in adults. Am Fam Physician. 2015;91(9):617-624.
4. Hariri AR, Mattay VS, Tessitore A, et al. Dextroamphetamine modulates the response of the human amygdala. Neuropsychopharmacology. 2002;27(6):1036-1040.
5. Colombo AC, de Oliveira AR, Reimer AE, et al. Dopaminergic mechanisms underlying catalepsy, fear and anxiety: do they interact? Behav Brain Res. 2013;257:201-207.
6. Togay B, El-Mallakh RS. Posttraumatic stress disorder: from pathophysiology to pharmacology. Curr Psychiatry. 2020;19(5):33-39.
7. Provensi G, Passani MB, Costa A, et al. Neuronal histamine and the memory of emotionally salient events. Br J Pharmacol. 2020;177(3):557-569.
8. Purves D, Augustine GJ, Fitzpatrick D, et al (eds). Neuroscience. 2nd ed. Sinauer Associates; 2001.
9. Pytliak M, Vargová V, Mechírová V, et al. Serotonin receptors – from molecular biology to clinical applications. Physiol Res. 2011;60(1):15-25.
10. Meneses A, Liy-Salmeron G. Serotonin and emotion, learning and memory. Rev Neurosci. 2012;23(5-6):543-553.
11. Whitaker-Azmitia PM. Serotonin and brain development: role in human developmental diseases. Brain Res Bull. 2001;56(5):479-485.
12. Towle AC, Breese GR, Mueller RA, et al. Early postnatal administration of 5,7-DHT: effects on serotonergic neurons and terminals. Brain Res. 1984;310(1):67-75.
13. Rok-Bujko P, Krzs´cik P, Szyndler J, et al. The influence of neonatal serotonin depletion on emotional and exploratory behaviours in rats. Behav Brain Res. 2012;226(1):87-95.
14. Meltzer HY. The role of serotonin in antipsychotic drug action. Neuropsychopharmacology. 1999;21(2 Suppl):106S-115S.
15. Calabrese EJ. 5-Hydroxytryptamine (serotonin): biphasic dose responses. Crit Rev Toxicol. 2001;31(4-5):553-561.
16. Zuardi AW. 5-HT-related drugs and human experimental anxiety. Neurosci Biobehav Rev. 1990;14(4):507-510.
17. Sánchez C, Meier E. Behavioral profiles of SSRIs in animal models of depression, anxiety and aggression. Are they all alike? Psychopharmacology (Berl). 1997;129(3):197-205.
18. Koek W, Mitchell NC, Daws LC. Biphasic effects of selective serotonin reuptake inhibitors on anxiety: rapid reversal of escitalopram’s anxiogenic effects in the novelty-induced hypophagia test in mice? Behav Pharmacol. 2018;29(4):365-369.
19. van Zijderveld GA, Veltman DJ, van Dyck R, et al. Epinephrine-induced panic attacks and hyperventilation. J Psychiatr Res. 1999;33(1):73-78.
20. Ho EV, Thompson SL, Katzka WR, et al. Clinically effective OCD treatment prevents 5-HT1B receptor-induced repetitive behavior and striatal activation. Psychopharmacology (Berl). 2016;233(1):57-70.
21. Stein DJ, Costa DLC, Lochner C, et al. Obsessive-compulsive disorder. Nat Rev Dis Primers. 2019;5(1):52.
22. Luddington NS, Mandadapu A, Husk M, et al. Clinical implications of genetic variation in the serotonin transporter promoter region: a review. Prim Care Companion J Clin Psychiatry. 2009;11(3):93-102.
23. Stahl SM. Mechanism of action of serotonin selective reuptake inhibitors. Serotonin receptors and pathways mediate therapeutic effects and side effects. J Affect Disord. 1998;51(3):215-235.
24. Chaouloff F, Berton O, Mormède P. Serotonin and stress. Neuropsychopharmacology. 1999;21(2 Suppl):28S-32S.
25. Siafis S, Tzachanis D, Samara M, et al. Antipsychotic drugs: From receptor-binding profiles to metabolic side effects. Curr Neuropharmacol. 2018;16(8):1210-1223.
26. Carrithers B, El-Mallakh RS. Transdermal asenapine in schizophrenia: a systematic review. Patient Prefer Adherence. 2020;14:1541-1551.
27. Citrome L. Asenapine review, part I: chemistry, receptor affinity profile, pharmacokinetics and metabolism. Expert Opin Drug Metab Toxicol. 2014;10(6):893-903.
28. Pratts M, Citrome L, Grant W, et al. A single-dose, randomized, double-blind, placebo-controlled trial of sublingual asenapine for acute agitation. Acta Psychiatr Scand. 2014;130(1):61-68.
29. Biswas AB, Bhaumik S, Branford D. Treatment-emergent behavioural side effects with selective serotonin re-uptake inhibitors in adults with learning disabilities. Hum Psychopharmacol. 2001;16(2):133-137.
30. Perlis RH, Mischoulon D, Smoller JW, et al. Serotonin transporter polymorphisms and adverse effects with fluoxetine treatment. Biol Psychiatry. 2003;54(9):879-883.
31. Ipser JC, Carey P, Dhansay Y, et al. Pharmacotherapy augmentation strategies in treatment-resistant anxiety disorders. Cochrane Database Syst Rev. 2006;(4):CD005473.
32. Kane JM, Mackle M, Snow-Adami L, et al. A randomized placebo-controlled trial of asenapine for the prevention of relapse of schizophrenia after long-term treatment. J Clin Psychiatry. 2011;72(3):349-355.
33. Bozzatello P, Rocca P, Uscinska M, et al. Efficacy and tolerability of asenapine compared with olanzapine in borderline personality disorder: an open-label randomized controlled trial. CNS Drugs. 2017;31(9):809-819.
34. El-Mallakh RS, Nuss S, Gao D, et al. Asenapine in the treatment of bipolar depression. Psychopharmacol Bull. 2020;50(1):8-18.
Lithium and kidney disease: Understand the risks
Lithium is one of the most widely used mood stabilizers and is considered a first-line treatment for bipolar disorder because of its proven antimanic and prophylactic effects.1 This medication also can reduce the risk of suicide in patients with bipolar disorder.2 However, it has a narrow therapeutic index. While lithium has many reversible adverse effects—such as tremors, gastrointestinal disturbance, and thyroid dysfunction—its perceived irreversible nephrotoxic effects makes some clinicians hesitant to prescribe it.3,4 In this article, we describe the relationship between lithium and nephrotoxicity, explain the apparent contradiction in published research regarding this topic, and offer suggestions for how to determine whether you should continue treatment with lithium for a patient who develops renal changes.
A lithium dilemma
Many psychiatrists have faced the dilemma of whether to discontinue lithium upon the appearance of glomerular renal changes and risk exposing patients to relapse or suicide, or to continue prescribing lithium and risk development of end stage renal disease (ESRD). Discontinuing lithium is not associated with the reversal of renal changes and kidney recovery,5 and exposes patients to psychiatric risks, such as mood recurrence and increased risk of suicide.6 Switching from lithium to another mood stabilizer is associated with a host of adverse effects, including diabetes mellitus and weight gain, and mood stabilizer use is not associated with reduced renal risk in patients with bipolar disorder.5 For example, Markowitz et al6 evaluated 24 patients with renal insufficiency after an average of 13.6 years of chronic lithium treatment. Despite stopping lithium, 8 patients out of the 19 available for follow-up (42%) developed ESRD.6 This study also found that serum creatinine levels >2.5 mg/dL are a predictor of progression to ESRD.6
Discontinuing lithium is associated with high rates of mood recurrence (60% to 70%), especially for patients who had been stable on lithium for years.7,8 If lithium is tapered slowly, the risk of mood recurrence may drop to approximately 42% over the subsequent 18 months, but this is nearly 3-fold greater than the risk of mood recurrence in patients with good response to valproate who are switched to another mood stabilizer (16.7%, c2 = 4.3, P = .048),9 which suggests that stopping lithium is particularly problematic. Considering the lifetime consequences of bipolar illness, for most patients who have been receiving lithium for a long time, the recommendation is to continue lithium.10,11
The reasons for conflicting evidence
Many studies indicate that there is either no statistically significant association or a very low association between lithium and developing ESRD,12-16 while others suggest that long-term lithium treatment increases the risk of chronic nephropathy to a clinically relevant degree (note that these arguments are not mutually exclusive).6,17-22 Much of this confusion has to do with not making a distinction between renal tubular dysfunction, which occurs early and in approximately one-half of patients treated with lithium,23 and glomerular dysfunction, which occurs late and is associated with reductions in glomerular filtration and ESRD.24 Adding to the confusion is that even without lithium, the rate of renal disease in patients with mood disorders is 2- to 3-fold higher than that of the general population.25 Lithium treatment is associated with a rate that is higher still,25-27 but this effect is erroneously exaggerated in studies that examined patients treated with lithium without comparison to a mood-disorder control group.
Renal tubular dysfunction presents as diabetes insipidus with polyuria and polydipsia, which is related to a reduced ability to concentrate the urine.28 Reduced glomerular filtration rate (GFR) as a consequence of lithium treatment occurs in 15% of patients23 and represents approximately 0.22% of patients on dialysis.18 Lithium-related reduction in GFR is a slowly progressive process that typically requires >20 years of lithium use to result in ESRD.18 While some decline in GFR may be seen within 1 year after starting lithium, the average age of patients who develop ESRD is 65 years.6 Interestingly, short-term animal studies have suggested that lithium may have antiproteinuric, protective, and pro-reparative effects in acute kidney injury.29
Anatomical anomalies in lithium-related glomerular dysfunction
In a study conducted before improved imaging technology was developed, Markowitz et al6 used renal biopsy to evaluate lithium-related nephropathy in 24 patients.6 Findings revealed chronic tubulointerstitial nephritis in all patients, along with a wide range of abnormalities, including tubular atrophy and interstitial fibrosis interspersed with microcyst formation arising from distal tubules or collecting ducts.6 Focal segmental glomerulosclerosis (FSGS) was found in 50% of patients. This might have been a result of nephron loss and compensatory hypertrophy of surviving nephrons, which suggests that FSGS is possibly a post-adaptive effect (rather than a direct damaging effect) of lithium on the glomerulus. The most noticeable finding was the appearance of microcysts in 62.5% of patients.6 It is important to note that these biopsy techniques sampled a relatively small fraction of the kidney volume, and that microcysts might have been more prevalent.
Recently, noninvasive imaging techniques have been used to detect microcysts in patients developing lithium-related nephropathy. While ultrasound and computed tomography (CT) can detect renal microcysts, magnetic resonance imaging (MRI), specifically the half-Fourier acquisition single-shot turbo spin-echo T2-weighted and gadolinium-enhanced (FISP three-dimensional MR angiographic) sequence, is the best noninvasive technology to demonstrate the presence of renal microcysts of a diameter of 1 to 2 mm.30 Ultrasound is sometimes difficult to utilize because while classic cysts appear as anechoic, lithium-induced microcysts may have the appearance of small echogenic foci.31,32 When evaluated by CT, renal microcysts may appear as hypodense lesions.
Continue to: Recent small studies...
Recent small studies have shown that MRI can detect renal microcysts in approximately 100% of patients who are receiving chronic lithium treatment and have renal insufficiency. One MRI study found renal microcysts in all 16 patients.33 In another MRI study of 4 patients, all were positive for renal microcysts.34 The relationship between MRI findings and renal function impairment in patients receiving long-term lithium therapy is still not clear; however, 1 study that examined 35 patients who received lithium reported that the number of cysts is generally related to the duration of lithium therapy.35 Thus, microcysts seem to present long before the elevation in creatinine, and nearly always present in patients with some glomerular dysfunction.
Cystic renal lesions have a wide variety of differential diagnoses, including simple renal cysts; glomerulocystic kidney disease; medullary cystic kidney disease and acquired cystic kidney disease; and multicystic dysplastic kidney and autosomal dominant polycystic kidney disease.36 In patients who have a long history of lithium use, lithium-related nephrotoxicity should be added to the differential diagnosis. The ubiquitous presence of renal microcysts and their relationship to duration of lithium exposure and renal function suggest that they may be intimately related to lithium-related ESRD.37
This association appears to be sufficiently reliable and clinicians can use T2-weighted MRI to determine if renal dysfunction is related to lithium. Lithium-related renal microcysts are visualized as multiple bilateral hyperintense foci with a diameter of 1 to 3 mm that involve both the cortex and medulla, tend to be symmetrically distributed throughout the kidney, and are associated with normal-sized kidneys.33,36 Large cysts are unlikely to be related to lithium; only microcysts are associated with lithium treatment. For examples of how these cysts appear on MRI, see Figure 1, Figure 2, and Figure 3. The exact mechanism of lithium-related nephrotoxicity is unclear, but may be related to the mTOR (mammalian target of rapamycin) pathway or GSK-3beta (glycogen synthase kinase-3beta) (Box6,37-44).
Box 1
The exact mechanism of lithium-related nephrotoxicity is unclear. The mTOR (mammalian target of rapamycin) pathway is an intracellular signaling pathway important in controlling cell proliferation and cell growth via the mTOR complex 1 (mTORC1). Researchers have hypothesized that the mTOR pathway may be responsible for lithium-induced microcysts.38 One study found that mTOR signaling is activated in the renal collecting ducts of mice that received long-term lithium.38 After the same mice received rapamycin (sirolimus), an allosteric inhibitor of mTOR, lithium-induced proliferation of medullary collecting duct cells (microcysts) was reversed.38
Additionally, GSK-3beta (glycogen synthase kinase-3beta), which is expressed in the adult kidney and is a target for lithium, appears to have a role in this pathology. GSK-3beta is involved in multiple biologic processes, including immunomodulation, embryologic development, and tissue injury and repair. It has the ability to promote apoptosis and inhibit proliferation.39 At therapeutic levels, lithium can inhibit GSK-3beta activity by phosphorylation of the serine 9 residue pGSK-3beta-s9.40 This action is believed to play a role in lithium’s neuroprotective properties, specifically through inhibiting the proapoptotic effects of GSK-3beta.41,42 Ironically, this antiapoptotic mechanism of lithium may be associated with its renal adverse effects.
Researchers have proposed that lithium enters distal nephron segments, inhibiting GSK-3beta and disrupting the balance between proliferative and apoptotic signals. The appearance of microcysts may be related to lithium’s antiapoptotic effect. In patients who received chronic treatment with lithium, their kidneys displayed multiple cortical microcysts immunopositive for GSK-3beta.43 Lithium may prevent the clearance of older renal tubular cells that would typically have been removed by normal apoptotic processes.37 As more of these tubular cells accumulate, they invaginate and form a cyst.37 As cysts accumulate during 20 years of treatment, the volume that the cysts occupy within the normal-sized and unyielding renal capsule displaces and injures otherwise healthy renal tissue, in a process similar to injury due to hydrocephalus in the brain.37
Interestingly, if the antiapoptotic mechanism of lithium-induced microcysts is true, it is possible that mood stabilizers that also have antiapoptotic properties (such as valproic acid) would also increase the risk of renal microcysts.44 This may underlie the observation that nearly one-half of patients continue to experience progression of renal disease after discontinuing lithium.6
Take-home points
In patients receiving chronic lithium treatment, it can take 20 years to produce a significant reduction in GFR. Switching patients who respond to lithium to other mood-stabilizing agents is associated with a significantly increased risk for mood recurrence and adverse consequences from the alternate medication. Because ESRD may occur more frequently in patients with mood disorders than in the general population, renal disease may be misattributed to lithium use. In approximately one-half of patients, renal disease will continue to progress after discontinuing lithium, which essentially eliminates the benefit of switching medications. This means that the decision to switch a patient who has responded well to lithium treatment for a decade or more to an alternate agent to avoid progression to ESRD may be associated with a very high potential cost but limited benefit.
One solution might be to more accurately identify patients with lithium-related glomerular disease, so that the potential benefit of switching may outweigh potential harm. The presence of renal microcysts on MRI of the kidney may be used to provide some of that reassurance. On renal biopsy, >60% of patients will have documented microcysts, and on MRI, it may approach 100%. The presence of microcysts provides potential evidence that reduced glomerular function is related to lithium. However, the absence of renal microcysts may not be as instructive—a negative MRI of the kidneys may not be sufficient evidence to rule out lithium as the culprit.
Continue to: Bottom Line
Bottom Line
Lithium is an effective treatment for bipolar disorder, but its perceived irreversible nephrotoxic effects make some clinicians hesitant to prescribe it. Discontinuing lithium or switching to another medication also carries risks. For most patients who have been receiving lithium for a long time, the recommendation is to obtain a renal MRI and to cautiously continue lithium if the patient does not have microcysts.
Related Resources
- Hayes JF, Osborn DPJ, Francis E, et al. Prediction of individuals at high risk of chronic kidney disease during treatment with lithium for bipolar disorder. BMC Med. 2021;19(1):99. doi: 10.1186/s12916-021-01964-z
- Pelekanos M, Foo K. A resident’s guide to lithium. Current Psychiatry. 2021;20(4):e3-e7. doi:10.12788/cp.0113
Drug Brand Names
Lithium • Eskalith, Lithobid
Sirolimus • Rapamune
Valproate • Depacon
1. Severus E, Bauer M, Geddes J. Efficacy and effectiveness of lithium in the long-term treatment of bipolar disorders: an update 2018. Pharamacopsychiatry. 2018;51(5):173-176.
2. Smith KA, Cipriani A. Lithium and suicide in mood disorders: updated meta-review of the scientific literature. Bipolar Disord. 2017;19(7):575-586.
3. El-Mallakh RS. Lithium: actions and mechanisms. Progress in Psychiatry Series, 50. American Psychiatric Press; 1996.
4. Gitlin M. Why is not lithium prescribed more often? Here are the reasons. J Psychiatry Neurol Sci. 2016, 29:293-297.
5. Kessing LV, Feldt-Rasmussen B, Andersen PK, et al. Continuation of lithium after a diagnosis of chronic kidney disease. Acta Psychiatr Scand. 2017;136(6):615-622.
6. Markowitz GS, Radhakrishnan J, Kambham N, et al. Lithium nephrotoxicity: a progressive combined glomerular and tubulointerstitial nephropathy. J Am Soc Nephrol. 2000;11(8):1439-1448.
7. Faedda GL, Tondo L, Baldessarini RJ, et al. Outcome after rapid vs gradual discontinuation of lithium treatment in bipolar disorders. Arch Gen Psychiatry. 1993;50(6):448-455.
8. Yazici O, Kora K, Polat A, et al. Controlled lithium discontinuation in bipolar patients with good response to long-term lithium prophylaxis. J Affect Disord. 2004;80(2-3):269-271.
9. Rosso G, Solia F, Albert U, et al. Affective recurrences in bipolar disorder after switching from lithium to valproate or vice versa: a series of 57 cases. J Clin Psychopharmacol. 2017;37(2):278-281.
10. Werneke U, Ott M, Renberg ES, et al. A decision analysis of long-term lithium treatment and the risk of renal failure. Acta Psychiatr Scand. 2012;126(3):186-197.
11. Sani G, Perugi G, Tondo L. Treatment of bipolar disorder in a lifetime perspective: is lithium still the best choice? Clin Drug Investig. 2017;37(8):713-727.
12. Vestergaard P, Amdisen A. Lithium treatment and kidney function: a follow-up study of 237 patients in long-term treatment. Acta Psychiatr Scand. 1981;63(4):333-345.
13. Walker RG, Bennett WM, Davies BM, et al. Structural and functional effects of long-term lithium therapy. Kidney Int Suppl. 1982;11:S13-S19.
14. Coskunol H, Vahip S, Mees ED, et al. Renal side-effects of long-term lithium treatment. J Affect Disord. 1997;43(1):5-10.
15. Paul R, Minay J, Cardwell C, et al. Meta-analysis of the effects of lithium usage on serum creatinine levels. J Psychopharmacol. 2010;24(10):1425-1431.
16. McKnight RF, Adida M, Budge K, et al. Lithium toxicity profile: a systematic review and meta-analysis. Lancet. 2012;379(9817):721-728.
17. Turan T, Esel E, Tokgöz B, et al. Effects of short- and long-term lithium treatment on kidney functioning in patients with bipolar mood disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2002;26(3):561-565.
18. Presne C, Fakhouri F, Noël LH, et al. Lithium-induced nephropathy: rate of progression and prognostic factors. Kidney Int. 2003;64(2):585-592.
19. McCann SM, Daly J, Kelly CB. The impact of long-term lithium treatment on renal function in an outpatient population. Ulster Med J. 2008;77(2):102-105.
20. Kripalani M, Shawcross J, Reilly J, et al. Lithium and chronic kidney disease. BMJ. 2009;339:b2452. doi: 10.1136/bmj.b2452
21. Bendz H, Schön S, Attman PO, et al. Renal failure occurs in chronic lithium treatment but is uncommon. Kidney Int. 2010;77(3):219-224. doi: 10.1038/ki.2009.433
22. Aiff H, Attman PO, Aurell M, et al. The impact of modern treatment principles may have eliminated lithium-induced renal failure. J Psychopharmacol. 2014; 28(2):151-154.
23. Boton R, Gaviria M, Batlle DC. Prevalence, pathogenesis, and treatment of renal dysfunction associated with chronic lithium therapy. Am J Kidney Dis. 1987;10(5):329-345.
24. Bocchetta A, Ardau R, Fanni T, et al. Renal function during long-term lithium treatment: a cross-sectional and longitudinal study. BMC Med. 2015, 21;13:12. doi: 10.1186/s12916-014-0249-4
25. Tredget J, Kirov A, Kirov G. Effects of chronic lithium treatment on renal function. J Affect Disord. 2010;126(3):436-440.
26. Adam WR, Schweitzer I, Walker BG. Trade-off between the benefits of lithium treatment and the risk of chronic kidney disease. Nephrology. 2012,17(8):776-779.
27. Azab AN, Shnaider A, Osher Y, et al. Lithium nephrotoxicity. Int J Bipolar Disord. 2015;3(1):1-9.
28. Trepiccione F, Christensen BM. Lithium-induced nephrogenic diabetes insipidus: new clinical and experimental findings. J Nephrol. 2010;23 Suppl 16:S43-S48.
29. Gong R, Wang P, Dworkin L. What we need to know about the effect of lithium on the kidney. Am J Physiol Renal Physiol. 2016;311(6):F1168-F1171. doi: 10.1152/ajprenal.00145.2016
30. Golshayan D, Nseir G, Venetz JP, et al. MR imaging as a specific diagnostic tool for bilateral microcysts in chronic lithium nephropathy. Kidney Int. 2012;81(6):601. doi: 10.1038/ki.2011.449
31. Di Salvo DN, Park J, Laing FC. Lithium nephropathy: Unique sonographic findings. J Ultrasound Med. 2012;31(4):637-644.
32. Jon´czyk-Potoczna K, Abramowicz M, Chłopocka-Woz´niak M, et al. Renal sonography in bipolar patients on long-term lithium treatment. J Clin Ultrasound. 2016;44(6):354-359.
33. Farres MT, Ronco P, Saadoun D, et al. Chronic lithium nephropathy: MR imaging for diagnosis. Radiol. 2003;229(2):570-574.
34. Roque A, Herédia V, Ramalho M, et al. MR findings of lithium-related kidney disease: preliminary observations in four patients. Abdom Imaging. 2012;37(1):140-146.
35. Farshchian N, Farnia V, Aghaiani M, et al. MRI findings and renal function in patients on long-term lithium therapy. Eur Psychiatry. 2013; 28(Sl):1. doi: 10.1016/S0924-9338(13)77306-1
36. Wood CG 3rd, Stromberg LJ 3rd, Harmath CB, et al. CT and MR imaging for evaluation of cystic renal lesions and diseases. Radiographics. 2015;35(1):125-141.
37. Khan M, El-Mallakh RS. Renal microcysts and lithium. Int J Psychiatry Med. 2015;50(3):290-298.
38. Gao Y, Romero-Aleshire MJ, Cai Q, et al. Rapamycin inhibition of mTORC1 reverses lithium-induced proliferation of renal collecting duct cells. Am J Physiol Renal Physiol. 2013;305(8):1201-1208.
39. Pap M, Cooper GM. Role of glycogen synthase kinase-3 in the phosphatidylinositol 3-Kinase/Akt cell survival pathway. J Biol Chem. 1998:273(32):19929-19932.
40. Stambolic V, Ruel L, Woodgett JR. Lithium inhibits glycogen synthase kinase-3 activity and mimics wingless signalling in intact cells. Curr Biol. 1996;6(12):1664-1668.
41. Rao R. Glycogen synthase kinase-3 regulation of urinary concentrating ability. Curr Opin Nephrol Hypertens. 2012;21(5):541-546.
42. Diniz BS, Machado Vieira R, Forlenza OV. Lithium and neuroprotection: translational evidence and implications for the treatment of neuropsychiatric disorders. Neuropsychiatr Dis Treat. 2013;9:493-500. doi: 10.2147/NDT.S33086
43. Kjaersgaard G, Madsen K, Marcussen N, et al. Tissue injury after lithium treatment in human and rat postnatal kidney involves glycogen synthase kinase-3β-positive epithelium. Am J Physiol Renal Physiol. 2012;302(4):455-465.
44. Zhang C, Zhu J, Zhang J, et al. Neuroprotective and anti-apoptotic effects of valproic acid on adult rat cerebral cortex through ERK and Akt signaling pathway at acute phase of traumatic brain injury. Brain Res. 2014;1555:1-9. doi: 10.1016/j.brainres.2014.01.051
Lithium is one of the most widely used mood stabilizers and is considered a first-line treatment for bipolar disorder because of its proven antimanic and prophylactic effects.1 This medication also can reduce the risk of suicide in patients with bipolar disorder.2 However, it has a narrow therapeutic index. While lithium has many reversible adverse effects—such as tremors, gastrointestinal disturbance, and thyroid dysfunction—its perceived irreversible nephrotoxic effects makes some clinicians hesitant to prescribe it.3,4 In this article, we describe the relationship between lithium and nephrotoxicity, explain the apparent contradiction in published research regarding this topic, and offer suggestions for how to determine whether you should continue treatment with lithium for a patient who develops renal changes.
A lithium dilemma
Many psychiatrists have faced the dilemma of whether to discontinue lithium upon the appearance of glomerular renal changes and risk exposing patients to relapse or suicide, or to continue prescribing lithium and risk development of end stage renal disease (ESRD). Discontinuing lithium is not associated with the reversal of renal changes and kidney recovery,5 and exposes patients to psychiatric risks, such as mood recurrence and increased risk of suicide.6 Switching from lithium to another mood stabilizer is associated with a host of adverse effects, including diabetes mellitus and weight gain, and mood stabilizer use is not associated with reduced renal risk in patients with bipolar disorder.5 For example, Markowitz et al6 evaluated 24 patients with renal insufficiency after an average of 13.6 years of chronic lithium treatment. Despite stopping lithium, 8 patients out of the 19 available for follow-up (42%) developed ESRD.6 This study also found that serum creatinine levels >2.5 mg/dL are a predictor of progression to ESRD.6
Discontinuing lithium is associated with high rates of mood recurrence (60% to 70%), especially for patients who had been stable on lithium for years.7,8 If lithium is tapered slowly, the risk of mood recurrence may drop to approximately 42% over the subsequent 18 months, but this is nearly 3-fold greater than the risk of mood recurrence in patients with good response to valproate who are switched to another mood stabilizer (16.7%, c2 = 4.3, P = .048),9 which suggests that stopping lithium is particularly problematic. Considering the lifetime consequences of bipolar illness, for most patients who have been receiving lithium for a long time, the recommendation is to continue lithium.10,11
The reasons for conflicting evidence
Many studies indicate that there is either no statistically significant association or a very low association between lithium and developing ESRD,12-16 while others suggest that long-term lithium treatment increases the risk of chronic nephropathy to a clinically relevant degree (note that these arguments are not mutually exclusive).6,17-22 Much of this confusion has to do with not making a distinction between renal tubular dysfunction, which occurs early and in approximately one-half of patients treated with lithium,23 and glomerular dysfunction, which occurs late and is associated with reductions in glomerular filtration and ESRD.24 Adding to the confusion is that even without lithium, the rate of renal disease in patients with mood disorders is 2- to 3-fold higher than that of the general population.25 Lithium treatment is associated with a rate that is higher still,25-27 but this effect is erroneously exaggerated in studies that examined patients treated with lithium without comparison to a mood-disorder control group.
Renal tubular dysfunction presents as diabetes insipidus with polyuria and polydipsia, which is related to a reduced ability to concentrate the urine.28 Reduced glomerular filtration rate (GFR) as a consequence of lithium treatment occurs in 15% of patients23 and represents approximately 0.22% of patients on dialysis.18 Lithium-related reduction in GFR is a slowly progressive process that typically requires >20 years of lithium use to result in ESRD.18 While some decline in GFR may be seen within 1 year after starting lithium, the average age of patients who develop ESRD is 65 years.6 Interestingly, short-term animal studies have suggested that lithium may have antiproteinuric, protective, and pro-reparative effects in acute kidney injury.29
Anatomical anomalies in lithium-related glomerular dysfunction
In a study conducted before improved imaging technology was developed, Markowitz et al6 used renal biopsy to evaluate lithium-related nephropathy in 24 patients.6 Findings revealed chronic tubulointerstitial nephritis in all patients, along with a wide range of abnormalities, including tubular atrophy and interstitial fibrosis interspersed with microcyst formation arising from distal tubules or collecting ducts.6 Focal segmental glomerulosclerosis (FSGS) was found in 50% of patients. This might have been a result of nephron loss and compensatory hypertrophy of surviving nephrons, which suggests that FSGS is possibly a post-adaptive effect (rather than a direct damaging effect) of lithium on the glomerulus. The most noticeable finding was the appearance of microcysts in 62.5% of patients.6 It is important to note that these biopsy techniques sampled a relatively small fraction of the kidney volume, and that microcysts might have been more prevalent.
Recently, noninvasive imaging techniques have been used to detect microcysts in patients developing lithium-related nephropathy. While ultrasound and computed tomography (CT) can detect renal microcysts, magnetic resonance imaging (MRI), specifically the half-Fourier acquisition single-shot turbo spin-echo T2-weighted and gadolinium-enhanced (FISP three-dimensional MR angiographic) sequence, is the best noninvasive technology to demonstrate the presence of renal microcysts of a diameter of 1 to 2 mm.30 Ultrasound is sometimes difficult to utilize because while classic cysts appear as anechoic, lithium-induced microcysts may have the appearance of small echogenic foci.31,32 When evaluated by CT, renal microcysts may appear as hypodense lesions.
Continue to: Recent small studies...
Recent small studies have shown that MRI can detect renal microcysts in approximately 100% of patients who are receiving chronic lithium treatment and have renal insufficiency. One MRI study found renal microcysts in all 16 patients.33 In another MRI study of 4 patients, all were positive for renal microcysts.34 The relationship between MRI findings and renal function impairment in patients receiving long-term lithium therapy is still not clear; however, 1 study that examined 35 patients who received lithium reported that the number of cysts is generally related to the duration of lithium therapy.35 Thus, microcysts seem to present long before the elevation in creatinine, and nearly always present in patients with some glomerular dysfunction.
Cystic renal lesions have a wide variety of differential diagnoses, including simple renal cysts; glomerulocystic kidney disease; medullary cystic kidney disease and acquired cystic kidney disease; and multicystic dysplastic kidney and autosomal dominant polycystic kidney disease.36 In patients who have a long history of lithium use, lithium-related nephrotoxicity should be added to the differential diagnosis. The ubiquitous presence of renal microcysts and their relationship to duration of lithium exposure and renal function suggest that they may be intimately related to lithium-related ESRD.37
This association appears to be sufficiently reliable and clinicians can use T2-weighted MRI to determine if renal dysfunction is related to lithium. Lithium-related renal microcysts are visualized as multiple bilateral hyperintense foci with a diameter of 1 to 3 mm that involve both the cortex and medulla, tend to be symmetrically distributed throughout the kidney, and are associated with normal-sized kidneys.33,36 Large cysts are unlikely to be related to lithium; only microcysts are associated with lithium treatment. For examples of how these cysts appear on MRI, see Figure 1, Figure 2, and Figure 3. The exact mechanism of lithium-related nephrotoxicity is unclear, but may be related to the mTOR (mammalian target of rapamycin) pathway or GSK-3beta (glycogen synthase kinase-3beta) (Box6,37-44).
Box 1
The exact mechanism of lithium-related nephrotoxicity is unclear. The mTOR (mammalian target of rapamycin) pathway is an intracellular signaling pathway important in controlling cell proliferation and cell growth via the mTOR complex 1 (mTORC1). Researchers have hypothesized that the mTOR pathway may be responsible for lithium-induced microcysts.38 One study found that mTOR signaling is activated in the renal collecting ducts of mice that received long-term lithium.38 After the same mice received rapamycin (sirolimus), an allosteric inhibitor of mTOR, lithium-induced proliferation of medullary collecting duct cells (microcysts) was reversed.38
Additionally, GSK-3beta (glycogen synthase kinase-3beta), which is expressed in the adult kidney and is a target for lithium, appears to have a role in this pathology. GSK-3beta is involved in multiple biologic processes, including immunomodulation, embryologic development, and tissue injury and repair. It has the ability to promote apoptosis and inhibit proliferation.39 At therapeutic levels, lithium can inhibit GSK-3beta activity by phosphorylation of the serine 9 residue pGSK-3beta-s9.40 This action is believed to play a role in lithium’s neuroprotective properties, specifically through inhibiting the proapoptotic effects of GSK-3beta.41,42 Ironically, this antiapoptotic mechanism of lithium may be associated with its renal adverse effects.
Researchers have proposed that lithium enters distal nephron segments, inhibiting GSK-3beta and disrupting the balance between proliferative and apoptotic signals. The appearance of microcysts may be related to lithium’s antiapoptotic effect. In patients who received chronic treatment with lithium, their kidneys displayed multiple cortical microcysts immunopositive for GSK-3beta.43 Lithium may prevent the clearance of older renal tubular cells that would typically have been removed by normal apoptotic processes.37 As more of these tubular cells accumulate, they invaginate and form a cyst.37 As cysts accumulate during 20 years of treatment, the volume that the cysts occupy within the normal-sized and unyielding renal capsule displaces and injures otherwise healthy renal tissue, in a process similar to injury due to hydrocephalus in the brain.37
Interestingly, if the antiapoptotic mechanism of lithium-induced microcysts is true, it is possible that mood stabilizers that also have antiapoptotic properties (such as valproic acid) would also increase the risk of renal microcysts.44 This may underlie the observation that nearly one-half of patients continue to experience progression of renal disease after discontinuing lithium.6
Take-home points
In patients receiving chronic lithium treatment, it can take 20 years to produce a significant reduction in GFR. Switching patients who respond to lithium to other mood-stabilizing agents is associated with a significantly increased risk for mood recurrence and adverse consequences from the alternate medication. Because ESRD may occur more frequently in patients with mood disorders than in the general population, renal disease may be misattributed to lithium use. In approximately one-half of patients, renal disease will continue to progress after discontinuing lithium, which essentially eliminates the benefit of switching medications. This means that the decision to switch a patient who has responded well to lithium treatment for a decade or more to an alternate agent to avoid progression to ESRD may be associated with a very high potential cost but limited benefit.
One solution might be to more accurately identify patients with lithium-related glomerular disease, so that the potential benefit of switching may outweigh potential harm. The presence of renal microcysts on MRI of the kidney may be used to provide some of that reassurance. On renal biopsy, >60% of patients will have documented microcysts, and on MRI, it may approach 100%. The presence of microcysts provides potential evidence that reduced glomerular function is related to lithium. However, the absence of renal microcysts may not be as instructive—a negative MRI of the kidneys may not be sufficient evidence to rule out lithium as the culprit.
Continue to: Bottom Line
Bottom Line
Lithium is an effective treatment for bipolar disorder, but its perceived irreversible nephrotoxic effects make some clinicians hesitant to prescribe it. Discontinuing lithium or switching to another medication also carries risks. For most patients who have been receiving lithium for a long time, the recommendation is to obtain a renal MRI and to cautiously continue lithium if the patient does not have microcysts.
Related Resources
- Hayes JF, Osborn DPJ, Francis E, et al. Prediction of individuals at high risk of chronic kidney disease during treatment with lithium for bipolar disorder. BMC Med. 2021;19(1):99. doi: 10.1186/s12916-021-01964-z
- Pelekanos M, Foo K. A resident’s guide to lithium. Current Psychiatry. 2021;20(4):e3-e7. doi:10.12788/cp.0113
Drug Brand Names
Lithium • Eskalith, Lithobid
Sirolimus • Rapamune
Valproate • Depacon
Lithium is one of the most widely used mood stabilizers and is considered a first-line treatment for bipolar disorder because of its proven antimanic and prophylactic effects.1 This medication also can reduce the risk of suicide in patients with bipolar disorder.2 However, it has a narrow therapeutic index. While lithium has many reversible adverse effects—such as tremors, gastrointestinal disturbance, and thyroid dysfunction—its perceived irreversible nephrotoxic effects makes some clinicians hesitant to prescribe it.3,4 In this article, we describe the relationship between lithium and nephrotoxicity, explain the apparent contradiction in published research regarding this topic, and offer suggestions for how to determine whether you should continue treatment with lithium for a patient who develops renal changes.
A lithium dilemma
Many psychiatrists have faced the dilemma of whether to discontinue lithium upon the appearance of glomerular renal changes and risk exposing patients to relapse or suicide, or to continue prescribing lithium and risk development of end stage renal disease (ESRD). Discontinuing lithium is not associated with the reversal of renal changes and kidney recovery,5 and exposes patients to psychiatric risks, such as mood recurrence and increased risk of suicide.6 Switching from lithium to another mood stabilizer is associated with a host of adverse effects, including diabetes mellitus and weight gain, and mood stabilizer use is not associated with reduced renal risk in patients with bipolar disorder.5 For example, Markowitz et al6 evaluated 24 patients with renal insufficiency after an average of 13.6 years of chronic lithium treatment. Despite stopping lithium, 8 patients out of the 19 available for follow-up (42%) developed ESRD.6 This study also found that serum creatinine levels >2.5 mg/dL are a predictor of progression to ESRD.6
Discontinuing lithium is associated with high rates of mood recurrence (60% to 70%), especially for patients who had been stable on lithium for years.7,8 If lithium is tapered slowly, the risk of mood recurrence may drop to approximately 42% over the subsequent 18 months, but this is nearly 3-fold greater than the risk of mood recurrence in patients with good response to valproate who are switched to another mood stabilizer (16.7%, c2 = 4.3, P = .048),9 which suggests that stopping lithium is particularly problematic. Considering the lifetime consequences of bipolar illness, for most patients who have been receiving lithium for a long time, the recommendation is to continue lithium.10,11
The reasons for conflicting evidence
Many studies indicate that there is either no statistically significant association or a very low association between lithium and developing ESRD,12-16 while others suggest that long-term lithium treatment increases the risk of chronic nephropathy to a clinically relevant degree (note that these arguments are not mutually exclusive).6,17-22 Much of this confusion has to do with not making a distinction between renal tubular dysfunction, which occurs early and in approximately one-half of patients treated with lithium,23 and glomerular dysfunction, which occurs late and is associated with reductions in glomerular filtration and ESRD.24 Adding to the confusion is that even without lithium, the rate of renal disease in patients with mood disorders is 2- to 3-fold higher than that of the general population.25 Lithium treatment is associated with a rate that is higher still,25-27 but this effect is erroneously exaggerated in studies that examined patients treated with lithium without comparison to a mood-disorder control group.
Renal tubular dysfunction presents as diabetes insipidus with polyuria and polydipsia, which is related to a reduced ability to concentrate the urine.28 Reduced glomerular filtration rate (GFR) as a consequence of lithium treatment occurs in 15% of patients23 and represents approximately 0.22% of patients on dialysis.18 Lithium-related reduction in GFR is a slowly progressive process that typically requires >20 years of lithium use to result in ESRD.18 While some decline in GFR may be seen within 1 year after starting lithium, the average age of patients who develop ESRD is 65 years.6 Interestingly, short-term animal studies have suggested that lithium may have antiproteinuric, protective, and pro-reparative effects in acute kidney injury.29
Anatomical anomalies in lithium-related glomerular dysfunction
In a study conducted before improved imaging technology was developed, Markowitz et al6 used renal biopsy to evaluate lithium-related nephropathy in 24 patients.6 Findings revealed chronic tubulointerstitial nephritis in all patients, along with a wide range of abnormalities, including tubular atrophy and interstitial fibrosis interspersed with microcyst formation arising from distal tubules or collecting ducts.6 Focal segmental glomerulosclerosis (FSGS) was found in 50% of patients. This might have been a result of nephron loss and compensatory hypertrophy of surviving nephrons, which suggests that FSGS is possibly a post-adaptive effect (rather than a direct damaging effect) of lithium on the glomerulus. The most noticeable finding was the appearance of microcysts in 62.5% of patients.6 It is important to note that these biopsy techniques sampled a relatively small fraction of the kidney volume, and that microcysts might have been more prevalent.
Recently, noninvasive imaging techniques have been used to detect microcysts in patients developing lithium-related nephropathy. While ultrasound and computed tomography (CT) can detect renal microcysts, magnetic resonance imaging (MRI), specifically the half-Fourier acquisition single-shot turbo spin-echo T2-weighted and gadolinium-enhanced (FISP three-dimensional MR angiographic) sequence, is the best noninvasive technology to demonstrate the presence of renal microcysts of a diameter of 1 to 2 mm.30 Ultrasound is sometimes difficult to utilize because while classic cysts appear as anechoic, lithium-induced microcysts may have the appearance of small echogenic foci.31,32 When evaluated by CT, renal microcysts may appear as hypodense lesions.
Continue to: Recent small studies...
Recent small studies have shown that MRI can detect renal microcysts in approximately 100% of patients who are receiving chronic lithium treatment and have renal insufficiency. One MRI study found renal microcysts in all 16 patients.33 In another MRI study of 4 patients, all were positive for renal microcysts.34 The relationship between MRI findings and renal function impairment in patients receiving long-term lithium therapy is still not clear; however, 1 study that examined 35 patients who received lithium reported that the number of cysts is generally related to the duration of lithium therapy.35 Thus, microcysts seem to present long before the elevation in creatinine, and nearly always present in patients with some glomerular dysfunction.
Cystic renal lesions have a wide variety of differential diagnoses, including simple renal cysts; glomerulocystic kidney disease; medullary cystic kidney disease and acquired cystic kidney disease; and multicystic dysplastic kidney and autosomal dominant polycystic kidney disease.36 In patients who have a long history of lithium use, lithium-related nephrotoxicity should be added to the differential diagnosis. The ubiquitous presence of renal microcysts and their relationship to duration of lithium exposure and renal function suggest that they may be intimately related to lithium-related ESRD.37
This association appears to be sufficiently reliable and clinicians can use T2-weighted MRI to determine if renal dysfunction is related to lithium. Lithium-related renal microcysts are visualized as multiple bilateral hyperintense foci with a diameter of 1 to 3 mm that involve both the cortex and medulla, tend to be symmetrically distributed throughout the kidney, and are associated with normal-sized kidneys.33,36 Large cysts are unlikely to be related to lithium; only microcysts are associated with lithium treatment. For examples of how these cysts appear on MRI, see Figure 1, Figure 2, and Figure 3. The exact mechanism of lithium-related nephrotoxicity is unclear, but may be related to the mTOR (mammalian target of rapamycin) pathway or GSK-3beta (glycogen synthase kinase-3beta) (Box6,37-44).
Box 1
The exact mechanism of lithium-related nephrotoxicity is unclear. The mTOR (mammalian target of rapamycin) pathway is an intracellular signaling pathway important in controlling cell proliferation and cell growth via the mTOR complex 1 (mTORC1). Researchers have hypothesized that the mTOR pathway may be responsible for lithium-induced microcysts.38 One study found that mTOR signaling is activated in the renal collecting ducts of mice that received long-term lithium.38 After the same mice received rapamycin (sirolimus), an allosteric inhibitor of mTOR, lithium-induced proliferation of medullary collecting duct cells (microcysts) was reversed.38
Additionally, GSK-3beta (glycogen synthase kinase-3beta), which is expressed in the adult kidney and is a target for lithium, appears to have a role in this pathology. GSK-3beta is involved in multiple biologic processes, including immunomodulation, embryologic development, and tissue injury and repair. It has the ability to promote apoptosis and inhibit proliferation.39 At therapeutic levels, lithium can inhibit GSK-3beta activity by phosphorylation of the serine 9 residue pGSK-3beta-s9.40 This action is believed to play a role in lithium’s neuroprotective properties, specifically through inhibiting the proapoptotic effects of GSK-3beta.41,42 Ironically, this antiapoptotic mechanism of lithium may be associated with its renal adverse effects.
Researchers have proposed that lithium enters distal nephron segments, inhibiting GSK-3beta and disrupting the balance between proliferative and apoptotic signals. The appearance of microcysts may be related to lithium’s antiapoptotic effect. In patients who received chronic treatment with lithium, their kidneys displayed multiple cortical microcysts immunopositive for GSK-3beta.43 Lithium may prevent the clearance of older renal tubular cells that would typically have been removed by normal apoptotic processes.37 As more of these tubular cells accumulate, they invaginate and form a cyst.37 As cysts accumulate during 20 years of treatment, the volume that the cysts occupy within the normal-sized and unyielding renal capsule displaces and injures otherwise healthy renal tissue, in a process similar to injury due to hydrocephalus in the brain.37
Interestingly, if the antiapoptotic mechanism of lithium-induced microcysts is true, it is possible that mood stabilizers that also have antiapoptotic properties (such as valproic acid) would also increase the risk of renal microcysts.44 This may underlie the observation that nearly one-half of patients continue to experience progression of renal disease after discontinuing lithium.6
Take-home points
In patients receiving chronic lithium treatment, it can take 20 years to produce a significant reduction in GFR. Switching patients who respond to lithium to other mood-stabilizing agents is associated with a significantly increased risk for mood recurrence and adverse consequences from the alternate medication. Because ESRD may occur more frequently in patients with mood disorders than in the general population, renal disease may be misattributed to lithium use. In approximately one-half of patients, renal disease will continue to progress after discontinuing lithium, which essentially eliminates the benefit of switching medications. This means that the decision to switch a patient who has responded well to lithium treatment for a decade or more to an alternate agent to avoid progression to ESRD may be associated with a very high potential cost but limited benefit.
One solution might be to more accurately identify patients with lithium-related glomerular disease, so that the potential benefit of switching may outweigh potential harm. The presence of renal microcysts on MRI of the kidney may be used to provide some of that reassurance. On renal biopsy, >60% of patients will have documented microcysts, and on MRI, it may approach 100%. The presence of microcysts provides potential evidence that reduced glomerular function is related to lithium. However, the absence of renal microcysts may not be as instructive—a negative MRI of the kidneys may not be sufficient evidence to rule out lithium as the culprit.
Continue to: Bottom Line
Bottom Line
Lithium is an effective treatment for bipolar disorder, but its perceived irreversible nephrotoxic effects make some clinicians hesitant to prescribe it. Discontinuing lithium or switching to another medication also carries risks. For most patients who have been receiving lithium for a long time, the recommendation is to obtain a renal MRI and to cautiously continue lithium if the patient does not have microcysts.
Related Resources
- Hayes JF, Osborn DPJ, Francis E, et al. Prediction of individuals at high risk of chronic kidney disease during treatment with lithium for bipolar disorder. BMC Med. 2021;19(1):99. doi: 10.1186/s12916-021-01964-z
- Pelekanos M, Foo K. A resident’s guide to lithium. Current Psychiatry. 2021;20(4):e3-e7. doi:10.12788/cp.0113
Drug Brand Names
Lithium • Eskalith, Lithobid
Sirolimus • Rapamune
Valproate • Depacon
1. Severus E, Bauer M, Geddes J. Efficacy and effectiveness of lithium in the long-term treatment of bipolar disorders: an update 2018. Pharamacopsychiatry. 2018;51(5):173-176.
2. Smith KA, Cipriani A. Lithium and suicide in mood disorders: updated meta-review of the scientific literature. Bipolar Disord. 2017;19(7):575-586.
3. El-Mallakh RS. Lithium: actions and mechanisms. Progress in Psychiatry Series, 50. American Psychiatric Press; 1996.
4. Gitlin M. Why is not lithium prescribed more often? Here are the reasons. J Psychiatry Neurol Sci. 2016, 29:293-297.
5. Kessing LV, Feldt-Rasmussen B, Andersen PK, et al. Continuation of lithium after a diagnosis of chronic kidney disease. Acta Psychiatr Scand. 2017;136(6):615-622.
6. Markowitz GS, Radhakrishnan J, Kambham N, et al. Lithium nephrotoxicity: a progressive combined glomerular and tubulointerstitial nephropathy. J Am Soc Nephrol. 2000;11(8):1439-1448.
7. Faedda GL, Tondo L, Baldessarini RJ, et al. Outcome after rapid vs gradual discontinuation of lithium treatment in bipolar disorders. Arch Gen Psychiatry. 1993;50(6):448-455.
8. Yazici O, Kora K, Polat A, et al. Controlled lithium discontinuation in bipolar patients with good response to long-term lithium prophylaxis. J Affect Disord. 2004;80(2-3):269-271.
9. Rosso G, Solia F, Albert U, et al. Affective recurrences in bipolar disorder after switching from lithium to valproate or vice versa: a series of 57 cases. J Clin Psychopharmacol. 2017;37(2):278-281.
10. Werneke U, Ott M, Renberg ES, et al. A decision analysis of long-term lithium treatment and the risk of renal failure. Acta Psychiatr Scand. 2012;126(3):186-197.
11. Sani G, Perugi G, Tondo L. Treatment of bipolar disorder in a lifetime perspective: is lithium still the best choice? Clin Drug Investig. 2017;37(8):713-727.
12. Vestergaard P, Amdisen A. Lithium treatment and kidney function: a follow-up study of 237 patients in long-term treatment. Acta Psychiatr Scand. 1981;63(4):333-345.
13. Walker RG, Bennett WM, Davies BM, et al. Structural and functional effects of long-term lithium therapy. Kidney Int Suppl. 1982;11:S13-S19.
14. Coskunol H, Vahip S, Mees ED, et al. Renal side-effects of long-term lithium treatment. J Affect Disord. 1997;43(1):5-10.
15. Paul R, Minay J, Cardwell C, et al. Meta-analysis of the effects of lithium usage on serum creatinine levels. J Psychopharmacol. 2010;24(10):1425-1431.
16. McKnight RF, Adida M, Budge K, et al. Lithium toxicity profile: a systematic review and meta-analysis. Lancet. 2012;379(9817):721-728.
17. Turan T, Esel E, Tokgöz B, et al. Effects of short- and long-term lithium treatment on kidney functioning in patients with bipolar mood disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2002;26(3):561-565.
18. Presne C, Fakhouri F, Noël LH, et al. Lithium-induced nephropathy: rate of progression and prognostic factors. Kidney Int. 2003;64(2):585-592.
19. McCann SM, Daly J, Kelly CB. The impact of long-term lithium treatment on renal function in an outpatient population. Ulster Med J. 2008;77(2):102-105.
20. Kripalani M, Shawcross J, Reilly J, et al. Lithium and chronic kidney disease. BMJ. 2009;339:b2452. doi: 10.1136/bmj.b2452
21. Bendz H, Schön S, Attman PO, et al. Renal failure occurs in chronic lithium treatment but is uncommon. Kidney Int. 2010;77(3):219-224. doi: 10.1038/ki.2009.433
22. Aiff H, Attman PO, Aurell M, et al. The impact of modern treatment principles may have eliminated lithium-induced renal failure. J Psychopharmacol. 2014; 28(2):151-154.
23. Boton R, Gaviria M, Batlle DC. Prevalence, pathogenesis, and treatment of renal dysfunction associated with chronic lithium therapy. Am J Kidney Dis. 1987;10(5):329-345.
24. Bocchetta A, Ardau R, Fanni T, et al. Renal function during long-term lithium treatment: a cross-sectional and longitudinal study. BMC Med. 2015, 21;13:12. doi: 10.1186/s12916-014-0249-4
25. Tredget J, Kirov A, Kirov G. Effects of chronic lithium treatment on renal function. J Affect Disord. 2010;126(3):436-440.
26. Adam WR, Schweitzer I, Walker BG. Trade-off between the benefits of lithium treatment and the risk of chronic kidney disease. Nephrology. 2012,17(8):776-779.
27. Azab AN, Shnaider A, Osher Y, et al. Lithium nephrotoxicity. Int J Bipolar Disord. 2015;3(1):1-9.
28. Trepiccione F, Christensen BM. Lithium-induced nephrogenic diabetes insipidus: new clinical and experimental findings. J Nephrol. 2010;23 Suppl 16:S43-S48.
29. Gong R, Wang P, Dworkin L. What we need to know about the effect of lithium on the kidney. Am J Physiol Renal Physiol. 2016;311(6):F1168-F1171. doi: 10.1152/ajprenal.00145.2016
30. Golshayan D, Nseir G, Venetz JP, et al. MR imaging as a specific diagnostic tool for bilateral microcysts in chronic lithium nephropathy. Kidney Int. 2012;81(6):601. doi: 10.1038/ki.2011.449
31. Di Salvo DN, Park J, Laing FC. Lithium nephropathy: Unique sonographic findings. J Ultrasound Med. 2012;31(4):637-644.
32. Jon´czyk-Potoczna K, Abramowicz M, Chłopocka-Woz´niak M, et al. Renal sonography in bipolar patients on long-term lithium treatment. J Clin Ultrasound. 2016;44(6):354-359.
33. Farres MT, Ronco P, Saadoun D, et al. Chronic lithium nephropathy: MR imaging for diagnosis. Radiol. 2003;229(2):570-574.
34. Roque A, Herédia V, Ramalho M, et al. MR findings of lithium-related kidney disease: preliminary observations in four patients. Abdom Imaging. 2012;37(1):140-146.
35. Farshchian N, Farnia V, Aghaiani M, et al. MRI findings and renal function in patients on long-term lithium therapy. Eur Psychiatry. 2013; 28(Sl):1. doi: 10.1016/S0924-9338(13)77306-1
36. Wood CG 3rd, Stromberg LJ 3rd, Harmath CB, et al. CT and MR imaging for evaluation of cystic renal lesions and diseases. Radiographics. 2015;35(1):125-141.
37. Khan M, El-Mallakh RS. Renal microcysts and lithium. Int J Psychiatry Med. 2015;50(3):290-298.
38. Gao Y, Romero-Aleshire MJ, Cai Q, et al. Rapamycin inhibition of mTORC1 reverses lithium-induced proliferation of renal collecting duct cells. Am J Physiol Renal Physiol. 2013;305(8):1201-1208.
39. Pap M, Cooper GM. Role of glycogen synthase kinase-3 in the phosphatidylinositol 3-Kinase/Akt cell survival pathway. J Biol Chem. 1998:273(32):19929-19932.
40. Stambolic V, Ruel L, Woodgett JR. Lithium inhibits glycogen synthase kinase-3 activity and mimics wingless signalling in intact cells. Curr Biol. 1996;6(12):1664-1668.
41. Rao R. Glycogen synthase kinase-3 regulation of urinary concentrating ability. Curr Opin Nephrol Hypertens. 2012;21(5):541-546.
42. Diniz BS, Machado Vieira R, Forlenza OV. Lithium and neuroprotection: translational evidence and implications for the treatment of neuropsychiatric disorders. Neuropsychiatr Dis Treat. 2013;9:493-500. doi: 10.2147/NDT.S33086
43. Kjaersgaard G, Madsen K, Marcussen N, et al. Tissue injury after lithium treatment in human and rat postnatal kidney involves glycogen synthase kinase-3β-positive epithelium. Am J Physiol Renal Physiol. 2012;302(4):455-465.
44. Zhang C, Zhu J, Zhang J, et al. Neuroprotective and anti-apoptotic effects of valproic acid on adult rat cerebral cortex through ERK and Akt signaling pathway at acute phase of traumatic brain injury. Brain Res. 2014;1555:1-9. doi: 10.1016/j.brainres.2014.01.051
1. Severus E, Bauer M, Geddes J. Efficacy and effectiveness of lithium in the long-term treatment of bipolar disorders: an update 2018. Pharamacopsychiatry. 2018;51(5):173-176.
2. Smith KA, Cipriani A. Lithium and suicide in mood disorders: updated meta-review of the scientific literature. Bipolar Disord. 2017;19(7):575-586.
3. El-Mallakh RS. Lithium: actions and mechanisms. Progress in Psychiatry Series, 50. American Psychiatric Press; 1996.
4. Gitlin M. Why is not lithium prescribed more often? Here are the reasons. J Psychiatry Neurol Sci. 2016, 29:293-297.
5. Kessing LV, Feldt-Rasmussen B, Andersen PK, et al. Continuation of lithium after a diagnosis of chronic kidney disease. Acta Psychiatr Scand. 2017;136(6):615-622.
6. Markowitz GS, Radhakrishnan J, Kambham N, et al. Lithium nephrotoxicity: a progressive combined glomerular and tubulointerstitial nephropathy. J Am Soc Nephrol. 2000;11(8):1439-1448.
7. Faedda GL, Tondo L, Baldessarini RJ, et al. Outcome after rapid vs gradual discontinuation of lithium treatment in bipolar disorders. Arch Gen Psychiatry. 1993;50(6):448-455.
8. Yazici O, Kora K, Polat A, et al. Controlled lithium discontinuation in bipolar patients with good response to long-term lithium prophylaxis. J Affect Disord. 2004;80(2-3):269-271.
9. Rosso G, Solia F, Albert U, et al. Affective recurrences in bipolar disorder after switching from lithium to valproate or vice versa: a series of 57 cases. J Clin Psychopharmacol. 2017;37(2):278-281.
10. Werneke U, Ott M, Renberg ES, et al. A decision analysis of long-term lithium treatment and the risk of renal failure. Acta Psychiatr Scand. 2012;126(3):186-197.
11. Sani G, Perugi G, Tondo L. Treatment of bipolar disorder in a lifetime perspective: is lithium still the best choice? Clin Drug Investig. 2017;37(8):713-727.
12. Vestergaard P, Amdisen A. Lithium treatment and kidney function: a follow-up study of 237 patients in long-term treatment. Acta Psychiatr Scand. 1981;63(4):333-345.
13. Walker RG, Bennett WM, Davies BM, et al. Structural and functional effects of long-term lithium therapy. Kidney Int Suppl. 1982;11:S13-S19.
14. Coskunol H, Vahip S, Mees ED, et al. Renal side-effects of long-term lithium treatment. J Affect Disord. 1997;43(1):5-10.
15. Paul R, Minay J, Cardwell C, et al. Meta-analysis of the effects of lithium usage on serum creatinine levels. J Psychopharmacol. 2010;24(10):1425-1431.
16. McKnight RF, Adida M, Budge K, et al. Lithium toxicity profile: a systematic review and meta-analysis. Lancet. 2012;379(9817):721-728.
17. Turan T, Esel E, Tokgöz B, et al. Effects of short- and long-term lithium treatment on kidney functioning in patients with bipolar mood disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2002;26(3):561-565.
18. Presne C, Fakhouri F, Noël LH, et al. Lithium-induced nephropathy: rate of progression and prognostic factors. Kidney Int. 2003;64(2):585-592.
19. McCann SM, Daly J, Kelly CB. The impact of long-term lithium treatment on renal function in an outpatient population. Ulster Med J. 2008;77(2):102-105.
20. Kripalani M, Shawcross J, Reilly J, et al. Lithium and chronic kidney disease. BMJ. 2009;339:b2452. doi: 10.1136/bmj.b2452
21. Bendz H, Schön S, Attman PO, et al. Renal failure occurs in chronic lithium treatment but is uncommon. Kidney Int. 2010;77(3):219-224. doi: 10.1038/ki.2009.433
22. Aiff H, Attman PO, Aurell M, et al. The impact of modern treatment principles may have eliminated lithium-induced renal failure. J Psychopharmacol. 2014; 28(2):151-154.
23. Boton R, Gaviria M, Batlle DC. Prevalence, pathogenesis, and treatment of renal dysfunction associated with chronic lithium therapy. Am J Kidney Dis. 1987;10(5):329-345.
24. Bocchetta A, Ardau R, Fanni T, et al. Renal function during long-term lithium treatment: a cross-sectional and longitudinal study. BMC Med. 2015, 21;13:12. doi: 10.1186/s12916-014-0249-4
25. Tredget J, Kirov A, Kirov G. Effects of chronic lithium treatment on renal function. J Affect Disord. 2010;126(3):436-440.
26. Adam WR, Schweitzer I, Walker BG. Trade-off between the benefits of lithium treatment and the risk of chronic kidney disease. Nephrology. 2012,17(8):776-779.
27. Azab AN, Shnaider A, Osher Y, et al. Lithium nephrotoxicity. Int J Bipolar Disord. 2015;3(1):1-9.
28. Trepiccione F, Christensen BM. Lithium-induced nephrogenic diabetes insipidus: new clinical and experimental findings. J Nephrol. 2010;23 Suppl 16:S43-S48.
29. Gong R, Wang P, Dworkin L. What we need to know about the effect of lithium on the kidney. Am J Physiol Renal Physiol. 2016;311(6):F1168-F1171. doi: 10.1152/ajprenal.00145.2016
30. Golshayan D, Nseir G, Venetz JP, et al. MR imaging as a specific diagnostic tool for bilateral microcysts in chronic lithium nephropathy. Kidney Int. 2012;81(6):601. doi: 10.1038/ki.2011.449
31. Di Salvo DN, Park J, Laing FC. Lithium nephropathy: Unique sonographic findings. J Ultrasound Med. 2012;31(4):637-644.
32. Jon´czyk-Potoczna K, Abramowicz M, Chłopocka-Woz´niak M, et al. Renal sonography in bipolar patients on long-term lithium treatment. J Clin Ultrasound. 2016;44(6):354-359.
33. Farres MT, Ronco P, Saadoun D, et al. Chronic lithium nephropathy: MR imaging for diagnosis. Radiol. 2003;229(2):570-574.
34. Roque A, Herédia V, Ramalho M, et al. MR findings of lithium-related kidney disease: preliminary observations in four patients. Abdom Imaging. 2012;37(1):140-146.
35. Farshchian N, Farnia V, Aghaiani M, et al. MRI findings and renal function in patients on long-term lithium therapy. Eur Psychiatry. 2013; 28(Sl):1. doi: 10.1016/S0924-9338(13)77306-1
36. Wood CG 3rd, Stromberg LJ 3rd, Harmath CB, et al. CT and MR imaging for evaluation of cystic renal lesions and diseases. Radiographics. 2015;35(1):125-141.
37. Khan M, El-Mallakh RS. Renal microcysts and lithium. Int J Psychiatry Med. 2015;50(3):290-298.
38. Gao Y, Romero-Aleshire MJ, Cai Q, et al. Rapamycin inhibition of mTORC1 reverses lithium-induced proliferation of renal collecting duct cells. Am J Physiol Renal Physiol. 2013;305(8):1201-1208.
39. Pap M, Cooper GM. Role of glycogen synthase kinase-3 in the phosphatidylinositol 3-Kinase/Akt cell survival pathway. J Biol Chem. 1998:273(32):19929-19932.
40. Stambolic V, Ruel L, Woodgett JR. Lithium inhibits glycogen synthase kinase-3 activity and mimics wingless signalling in intact cells. Curr Biol. 1996;6(12):1664-1668.
41. Rao R. Glycogen synthase kinase-3 regulation of urinary concentrating ability. Curr Opin Nephrol Hypertens. 2012;21(5):541-546.
42. Diniz BS, Machado Vieira R, Forlenza OV. Lithium and neuroprotection: translational evidence and implications for the treatment of neuropsychiatric disorders. Neuropsychiatr Dis Treat. 2013;9:493-500. doi: 10.2147/NDT.S33086
43. Kjaersgaard G, Madsen K, Marcussen N, et al. Tissue injury after lithium treatment in human and rat postnatal kidney involves glycogen synthase kinase-3β-positive epithelium. Am J Physiol Renal Physiol. 2012;302(4):455-465.
44. Zhang C, Zhu J, Zhang J, et al. Neuroprotective and anti-apoptotic effects of valproic acid on adult rat cerebral cortex through ERK and Akt signaling pathway at acute phase of traumatic brain injury. Brain Res. 2014;1555:1-9. doi: 10.1016/j.brainres.2014.01.051
Suvorexant: An option for preventing delirium?
Delirium is characterized by a disturbance of consciousness or cognition that typically has a rapid onset and fluctuating course.1 Up to 42% of hospitalized geriatric patients experience delirium.1 Approximately 10% to 31% of these patients have the condition upon admission, and the remainder develop it during their hospitalization.1 Unfortunately, options for preventing or treating delirium are limited. Benzodiazepines and antipsychotic medications have been used to treat problematic behaviors associated with delirium, but they do not effectively reduce the occurrence, duration, or severity of this condition.2,3
Recent evidence suggests that suvorexant, which is FDA-approved for insomnia, may be useful for preventing delirium. Suvorexant—a dual orexin receptor (OX1R, OX2R) antagonist—promotes sleep onset and maintenance, and is associated with normal measures of sleep activity such as rapid eye movement (REM) sleep, non-REM sleep, and sleep stage–specific electroencephalographic profiles.4 Here we review 3 studies that evaluated suvorexant for preventing delirium.
Hatta et al.5 In this randomized, placebo-controlled, blinded, multicenter study, 72 patients (age 65 to 89) newly admitted to an ICU were randomized to suvorexant, 15 mg/d, (n = 36) or placebo (n = 36) for 3 days.5 None of the patients taking suvorexant developed delirium, whereas 17% (6 patients) in the placebo group did (P = .025).5
Azuma et al.6 In this 7-day, blinded, randomized study of 70 adult patients (age ≥20) admitted to an ICU, 34 participants received suvorexant (15 mg nightly for age <65, 20 mg nightly for age ≥65) and the rest received treatment as usual (TAU). Suvorexant was associated with a lower incidence of delirium symptoms (n = 6, 17.6%) compared with TAU (n = 17, 47.2%) (P = .011).6 The onset of delirium was earlier in the TAU group (P < .05).6
Hatta et al.7 In this large prospective, observational study of adults (age >65), 526 patients with significant risk factors for delirium were prescribed suvorexant and/or ramelteon. Approximately 16% of the patients who received either or both of these medications met DSM-5 criteria for delirium, compared with 24% who did not receive these medications (P = .005).7
Acknowledgment
The authors thank Jakob Evans, BS, for compiling much of the research for this article.
1. Siddiqi N, House AO, Holmes JD. Occurrence and outcome of delirium in medical in-patients: a systematic literature review. Age Ageing. 2006;35(4):350-364.
2. Lonergan E, Luxenberg J, Areosa Sastre A. Benzodiazepines for delirium. Cochrane Database Syst Rev. 2009;2009(4):CD006379.
3. Burry L, Mehta S, Perreault MM, et al. Antipsychotics for treatment of delirium in hospitalised non-ICU patients. Cochrane Database Syst Rev. 2018;6(6):CD005594.
4. Coleman PJ, Gotter AL, Herring WJ, et al. The discovery of suvorexant, the first orexin receptor drug for insomnia. Annu Rev Pharmacol Toxicol. 2017;57:509-533.
5. Hatta K, Kishi Y, Wada K, et al. Preventive effects of suvorexant on delirium: a randomized placebo-controlled trial. J Clin Psychiatry. 2017;78(8):e970-e979.
6. Azuma K, Takaesu Y, Soeda H, et al. Ability of suvorexant to prevent delirium in patients in the intensive care unit: a randomized controlled trial. Acute Med Surg. 2018;5(4):362-368.
7. Hatta K, Kishi Y, Wada K, et al. Real-world effectiveness of ramelteon and suvorexant for delirium prevention in 948 patients with delirium risk factors. J Clin Psychiatry. 2019;81(1):19m12865. doi: 10.4088/JCP.19m12865
Delirium is characterized by a disturbance of consciousness or cognition that typically has a rapid onset and fluctuating course.1 Up to 42% of hospitalized geriatric patients experience delirium.1 Approximately 10% to 31% of these patients have the condition upon admission, and the remainder develop it during their hospitalization.1 Unfortunately, options for preventing or treating delirium are limited. Benzodiazepines and antipsychotic medications have been used to treat problematic behaviors associated with delirium, but they do not effectively reduce the occurrence, duration, or severity of this condition.2,3
Recent evidence suggests that suvorexant, which is FDA-approved for insomnia, may be useful for preventing delirium. Suvorexant—a dual orexin receptor (OX1R, OX2R) antagonist—promotes sleep onset and maintenance, and is associated with normal measures of sleep activity such as rapid eye movement (REM) sleep, non-REM sleep, and sleep stage–specific electroencephalographic profiles.4 Here we review 3 studies that evaluated suvorexant for preventing delirium.
Hatta et al.5 In this randomized, placebo-controlled, blinded, multicenter study, 72 patients (age 65 to 89) newly admitted to an ICU were randomized to suvorexant, 15 mg/d, (n = 36) or placebo (n = 36) for 3 days.5 None of the patients taking suvorexant developed delirium, whereas 17% (6 patients) in the placebo group did (P = .025).5
Azuma et al.6 In this 7-day, blinded, randomized study of 70 adult patients (age ≥20) admitted to an ICU, 34 participants received suvorexant (15 mg nightly for age <65, 20 mg nightly for age ≥65) and the rest received treatment as usual (TAU). Suvorexant was associated with a lower incidence of delirium symptoms (n = 6, 17.6%) compared with TAU (n = 17, 47.2%) (P = .011).6 The onset of delirium was earlier in the TAU group (P < .05).6
Hatta et al.7 In this large prospective, observational study of adults (age >65), 526 patients with significant risk factors for delirium were prescribed suvorexant and/or ramelteon. Approximately 16% of the patients who received either or both of these medications met DSM-5 criteria for delirium, compared with 24% who did not receive these medications (P = .005).7
Acknowledgment
The authors thank Jakob Evans, BS, for compiling much of the research for this article.
Delirium is characterized by a disturbance of consciousness or cognition that typically has a rapid onset and fluctuating course.1 Up to 42% of hospitalized geriatric patients experience delirium.1 Approximately 10% to 31% of these patients have the condition upon admission, and the remainder develop it during their hospitalization.1 Unfortunately, options for preventing or treating delirium are limited. Benzodiazepines and antipsychotic medications have been used to treat problematic behaviors associated with delirium, but they do not effectively reduce the occurrence, duration, or severity of this condition.2,3
Recent evidence suggests that suvorexant, which is FDA-approved for insomnia, may be useful for preventing delirium. Suvorexant—a dual orexin receptor (OX1R, OX2R) antagonist—promotes sleep onset and maintenance, and is associated with normal measures of sleep activity such as rapid eye movement (REM) sleep, non-REM sleep, and sleep stage–specific electroencephalographic profiles.4 Here we review 3 studies that evaluated suvorexant for preventing delirium.
Hatta et al.5 In this randomized, placebo-controlled, blinded, multicenter study, 72 patients (age 65 to 89) newly admitted to an ICU were randomized to suvorexant, 15 mg/d, (n = 36) or placebo (n = 36) for 3 days.5 None of the patients taking suvorexant developed delirium, whereas 17% (6 patients) in the placebo group did (P = .025).5
Azuma et al.6 In this 7-day, blinded, randomized study of 70 adult patients (age ≥20) admitted to an ICU, 34 participants received suvorexant (15 mg nightly for age <65, 20 mg nightly for age ≥65) and the rest received treatment as usual (TAU). Suvorexant was associated with a lower incidence of delirium symptoms (n = 6, 17.6%) compared with TAU (n = 17, 47.2%) (P = .011).6 The onset of delirium was earlier in the TAU group (P < .05).6
Hatta et al.7 In this large prospective, observational study of adults (age >65), 526 patients with significant risk factors for delirium were prescribed suvorexant and/or ramelteon. Approximately 16% of the patients who received either or both of these medications met DSM-5 criteria for delirium, compared with 24% who did not receive these medications (P = .005).7
Acknowledgment
The authors thank Jakob Evans, BS, for compiling much of the research for this article.
1. Siddiqi N, House AO, Holmes JD. Occurrence and outcome of delirium in medical in-patients: a systematic literature review. Age Ageing. 2006;35(4):350-364.
2. Lonergan E, Luxenberg J, Areosa Sastre A. Benzodiazepines for delirium. Cochrane Database Syst Rev. 2009;2009(4):CD006379.
3. Burry L, Mehta S, Perreault MM, et al. Antipsychotics for treatment of delirium in hospitalised non-ICU patients. Cochrane Database Syst Rev. 2018;6(6):CD005594.
4. Coleman PJ, Gotter AL, Herring WJ, et al. The discovery of suvorexant, the first orexin receptor drug for insomnia. Annu Rev Pharmacol Toxicol. 2017;57:509-533.
5. Hatta K, Kishi Y, Wada K, et al. Preventive effects of suvorexant on delirium: a randomized placebo-controlled trial. J Clin Psychiatry. 2017;78(8):e970-e979.
6. Azuma K, Takaesu Y, Soeda H, et al. Ability of suvorexant to prevent delirium in patients in the intensive care unit: a randomized controlled trial. Acute Med Surg. 2018;5(4):362-368.
7. Hatta K, Kishi Y, Wada K, et al. Real-world effectiveness of ramelteon and suvorexant for delirium prevention in 948 patients with delirium risk factors. J Clin Psychiatry. 2019;81(1):19m12865. doi: 10.4088/JCP.19m12865
1. Siddiqi N, House AO, Holmes JD. Occurrence and outcome of delirium in medical in-patients: a systematic literature review. Age Ageing. 2006;35(4):350-364.
2. Lonergan E, Luxenberg J, Areosa Sastre A. Benzodiazepines for delirium. Cochrane Database Syst Rev. 2009;2009(4):CD006379.
3. Burry L, Mehta S, Perreault MM, et al. Antipsychotics for treatment of delirium in hospitalised non-ICU patients. Cochrane Database Syst Rev. 2018;6(6):CD005594.
4. Coleman PJ, Gotter AL, Herring WJ, et al. The discovery of suvorexant, the first orexin receptor drug for insomnia. Annu Rev Pharmacol Toxicol. 2017;57:509-533.
5. Hatta K, Kishi Y, Wada K, et al. Preventive effects of suvorexant on delirium: a randomized placebo-controlled trial. J Clin Psychiatry. 2017;78(8):e970-e979.
6. Azuma K, Takaesu Y, Soeda H, et al. Ability of suvorexant to prevent delirium in patients in the intensive care unit: a randomized controlled trial. Acute Med Surg. 2018;5(4):362-368.
7. Hatta K, Kishi Y, Wada K, et al. Real-world effectiveness of ramelteon and suvorexant for delirium prevention in 948 patients with delirium risk factors. J Clin Psychiatry. 2019;81(1):19m12865. doi: 10.4088/JCP.19m12865
Posttraumatic stress disorder: From pathophysiology to pharmacology
Posttraumatic stress disorder (PTSD) occurs acutely and chronically in the aftermath of severe and potentially life-threatening trauma.1 The prevalence of PTSD varies significantly across countries and by type of trauma (Box1-7).
Box
In the general population, the prevalence of posttraumatic stress disorder (PTSD) varies from as low as 0.3% in China to as high as 6.1% in New Zealand1 and 6.8% in the United States.2 These rates are actually much lower than expected when one considers that severe trauma is experienced by 60.7% of men and 51.2% of women.3,4 Although the majority of individuals exposed to trauma experience emotional distress immediately following a traumatic event, most of them do not develop PTSD.5
It appears that the context of trauma is important: 12% to 15% of veterans experience PTSD, compared with 19% to 75% of crime victims and 80% of rape victims.1 The lifetime risk for PTSD is twice as high in women as it is in men,6 and genetic vulnerability may play a role. For example, twin studies showed that approximately 30% of the risk for PTSD may be mediated by genetic predisposition.7
Individuals who develop PTSD experience a wide range of symptoms.8 These can be categorized as PTSD-specific symptoms, or nonspecific symptoms. PTSD-specific symptoms include nightmares, flashbacks, dissociative reactions, hyperreactivity or hyperarousal, distress with reminders of trauma, and avoidance of trauma-related physical reminders and thoughts/feelings (Table8). Nonspecific symptoms include depressive and anxiety symptoms and significant problems in social, relationship, or work situations.8
While successful treatment necessitates taking all of these symptoms into account, understanding the pathophysiology of PTSD can inform a more focused and rational treatment approach. In this article, we describe some key pathophysiologic PTSD studies, and focus on PTSD-specific psychopathology to inform treatment.
Brain systems implicated in PTSD
Neuropeptide Y (NPY) is an anxiolytic endogenous peptide that has connections to the hypothalamic-pituitary-adrenal (HPA) axis. Its levels can be modulated by stress.9 Preclinical and clinical studies strongly support a potential role of NPY dysfunction in the pathophysiology of PTSD. Lower concentrations of NPY increase susceptibility to PTSD in combat veterans10 and in animal models.11 Three single-nucleotide polymorphisms (SNPs) appear to mediate this effect.12 These findings strongly support pharmaceutical targeting this system as a useful therapeutic approach.13,14 Indeed, intranasal NPY administered as a single dose reduces anxiety in animal models15 and in humans,16 but this work has not yet translated into clinical tools.
Corticotropin-releasing hormone receptor (CRHR1) gene. Corticotropin-releasing hormone has been implicated in PTSD.17 Corticotropin-releasing hormone receptors (CRHR) are important mediators in response to stress.18,19 They bind corticotropin-releasing hormone and contribute to the integration of autonomic, behavioral, and immune responses to stress.20 Single-nucleotide polymorphisms in the regulatory portion of the CRHR1 gene are associated with an increased risk for depression in adults who have a history of child abuse.21
The CRHR1 receptor antagonist GSK561679 is an investigational agent for the treatment of mood and anxiety disorders.22 In exploratory studies,23,24 GSK561679 was found to inhibit fear-potentiated startle in patients with PTSD, but not overall PTSD symptoms, although a subset of women with a specific genetic variant of the CRHR1 gene (rs110402) experienced significant benefit.25,26 This suggests that we must learn more about this system before we proceed.27
Brain-derived neurotrophic factor (BDNF). The synthesis of BDNF is influenced by neuronal activity in the brain and plays a role in synaptic transmission and plasticity.28 Brain-derived neurotrophic factor is encoded by the BDNF gene, which has been implicated in stress vulnerability.29 A common SNP in the pro-region of the human BDNF gene results in a valine-to-methionine substitution at the 66th amino acid (Val66Met). The functional Val66Met polymorphism may have a role in the risk of developing PTSD. However, not all studies support this finding. One study found that an SNP with a resulting Val66Met polymorphism is associated with adult PTSD symptoms after childhood abuse, while a meta-analysis of 7 studies did not confirm this.30,31 We need to learn more about BDNF before we proceed.32
Continue to: Serotonin transporter (5-HTT) gene
Serotonin transporter (5-HTT) gene. Serotonin transporter is a monoamine transporter protein that terminates the neurotransmitter signal by transporting serotonin from the synaptic cleft back into the presynaptic neuron. It is encoded by the SLC6A4 gene, which resides on the long arm of chromosome 17(17q11.1-q12). It is a large gene with 31 kilo bases and 14 separate exons (transcribed regions).33,34
This gene has several variants. The best-studied is a variation in the promoter region. A 44-bp insertion or deletion yields the “long” and “short” alleles, respectively. The proteins produced by the 2 alleles are identical, but the amount of expressed protein is different. The short allele (“S”) is associated with a nearly 50% reduction in 5-HTT expression in both homozygotes and heterozygotes.35 A greater incidence of serotonin transporter promoter region (5-HTTLPR) S has been found in individuals with PTSD compared with those without PTSD,36-38 and 5-HTTLPR S increases the risk of PTSD in individuals with low social support39 or after very few traumatic events.40 The short allele variant is also associated with depression in individuals who face adversity.35,41
The overrepresentation of the short form of 5-HTTLPR in individuals who develop PTSD may represent a potential problem with current treatment paradigms, in which an antidepressant is the first-line treatment, because this allele is associated with reduced response to antidepressants.42,43 More distressing is the possible association of this allele with increased suicide risk, particularly violent suicide44 or repeated suicide attempts.45
Furthermore, a functional MRI study of patients who were anxious revealed that in individuals with the short allele, administration of
Catechol-o-methyltransferase (COMT) is one of the enzymes that degrades catecholamines such as dopamine, epinephrine, and norepinephrine (NE).47 In humans, COMT protein is encoded by the COMT gene. This gene is associated with allelic variants; the best-studied of these is Val158Met. COMT Val158Met polymorphism (rs4860) has been linked to deficits in stress response and emotional resilience.48,49 Val158Met is associated with a 40% reduction in enzyme activity and slower catalysis of catecholamines, resulting in increases in catecholamines levels in the brain, which may increase the risk of developing PTSD.50 Individuals homozygous for this SNP (Met/Met) are highly susceptible to develop PTSD independently of the severity of the trauma they experienced.51 The Val158Met polymorphism may be associated with other abnormalities, such as cognitive problems with specific frontal cortical activity, and also with improved antidepressant response (valine homozygotes less responsive than methionine homozygotes).52 This gene is available on gene testing profiles.
Continue to: The role of norepinephrine in PTSD
The role of norepinephrine in PTSD
Perhaps the greatest advance in the understanding of the pathophysiology of PTSD relates to changes in brain NE. The HPA axis is responsible for coordinating the hormonal response to stress. Dysregulation of this axis and increased activity of the central and peripheral noradrenergic systems are usually observed in patients with PTSD.53 Several monoamine neurotransmitters are important in the regulation and function of the HPA axis. Norepinephrine plays a major role in stress.
The clinical PTSD-specific criteria are all descriptions of excessive noradrenergic tone.54 For example, hypervigilance and hyperstartle are clearly anticipated as evidence of NE stimulation. Flashbacks, particularly those that might be precipitated by environmental cues, also can be a manifestation of the vigilance induced by NE. Sleep disturbances (insomnia and nightmares) are present; insomnia is reported more often than nightmares.55 Increased catecholamine levels, particularly NE, are a feature of sleep disturbances associated with middle insomnia. Dreams can be remembered only if you wake up during dreaming. Catecholamines do not change the content of dreams, just recall.56
In a study of central noradrenergic tone in patients with PTSD, 6 hourly CSF samples were collected from 11 male combat veterans with PTSD and 8 healthy controls.57 Participants with PTSD had significantly higher CSF NE concentrations (0.55 ± 0.17 pmol/ml vs 0.39 ± 0.16 pmol/mL in the PTSD and control groups, respectively; F = 4.49, P < .05).57 Overall PTSD symptoms correlated significantly with CSF NE levels (r = 0.82, P <.005), and PTSD-specific symptoms such as avoidance (r = 0.79, P = .004). Intrusive thoughts (r = 0.57, P = .07) and hyperarousal (r = 0.54, P = .09) were also related.57 This relationship is unique; patients with PTSD with predominant depressive symptoms do not have elevated plasma NE levels.58
In the human brain, there are 3 main groups of NE receptors: alpha-1 receptors, alpha-2 receptors, and beta receptors.59 Alpha-1 receptors (alpha-1A, alpha-1B, and alpha-1D) are postsynaptic and mediate increase in inositol trisphosphate (IP3) and intracellular calcium (Ca2+). Alpha-2 receptors (alpha-2A, alpha-2B, alpha-2C) in the CNS are presynaptic autoreceptors and serve to reduce NE release. Beta receptors (beta-1, beta-2, beta-3) inhibit cyclic adenosine monophosphate (cAMP) production.59 The effects of inhibition of alpha or beta receptors are different. Inhibition of beta receptors is associated with depressive symptoms and depressive syndrome, inhibition of peripheral beta receptors is associated with reductions in anxiety (generally reduction of pulse, sweating, tremor),60 and inhibition of central alpha-1 receptors is associated with reduced PTSD symptoms.61
Choice of agents for PTSD-specific symptoms
As outlined in the Table,8 PTSD is characterized by 3 types of symptoms that are specific for PTSD. Trauma-focused psychotherapy62,63 and selective serotonin reuptake inhibitors (SSRIs)64 are considered first-line therapy for PTSD. Only
Continue to: Serotonin transporter promoter...
Serotonin transporter promoter region gene short-type variants, which possibly increase an individual’s predisposition to developing PTSD, may explain the abundance of depressive symptoms in this condition and the subdued response to antidepressants. Specifically, an anticipated preponderance of these alleles may be associated with poorer outcomes. Non-SSRI treatments, such as low-dose
On the other hand, animal models support antagonism of the postsynaptic alpha-1 adrenergic receptor of the CNS as a target for PTSD treatment.71 Although
Quetiapine might be another non-SSRI option for treating patients with PTSD. It is an antagonist with high affinity tothehistamine-1 receptor at low doses. Norquetiapine is an alpha-2 antagonist that increases brain NE levels. Both quetiapine and norquetiapine are alpha-1 antagonists. There is no beta blockade and no SSRI effect, but some 5HT2A blockade, which may be anxiolytic. Compared with placebo, an average quetiapine dose of 258 mg/d resulted in significantly greater reductions in Clinician-Administered PTSD Scale total score, re-experiencing score, and hyperarousal score.73
Unfortunately, none of the non-SSRI options have been adequately evaluated. For now, clinicians need to continue to use SSRIs, and researchers need to continue to explore mechanism-guided alternatives.
Bottom Line
Understanding the mechanisms of the pathophysiology of posttraumatic stress disorder (PTSD) may allow clinicians to “jump ahead” of clinical studies and FDA indications. Clinicians may reasonably use alpha-1 antagonists (eg, prazosin, quetiapine) for general clinical improvement of patients with PTSD, particularly for PTSD-specific symptoms. Using antihistamines to reduce anxiety (especially in patients who have the COMT Val158Met polymorphism) may also be reasonable.
Related Resources
- North CS, Hong BA, Downs DL. PTSD: a systematic approach to diagnosis and treatment. Current Psychiatry. 2018;17(4):35-43.
- Zhang Y, Ren R, Sanford LD, et al. The effects of prazosin on sleep disturbances in post-traumatic stress disorder: a systematic review and meta-analysis. Sleep Med. 2019; 67:225-231.
Drug Brand Names
Aripiprazole • Abilify
Citalopram • Celexa
Paroxetine • Paxil
Prazosin • Minipress
Quetiapine • Seroquel
Sertraline • Zoloft
1. Javidi H, Yadollahie M. Post-traumatic stress disorder. Int J Occup Environ Med. 2012;3(1):2-9.
2. Kessler RC, Chiu WT, Demler O, et al. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62(6):617-627.
3. Kessler RC, Sonnega A, Bromet E, et al. Posttraumatic stress disorder in the National Comorbidity Survey. Arch Gen Psychiatry. 1995;52(12):1048-1060.
4. Breslau N, Kessler RC, Chilcoat HD, et al. Trauma and posttraumatic stress disorder in the community: the 1996 Detroit Area Survey of Trauma. Arch Gen Psychiatry. 1998;55(7):626-632.
5. Cerda M, Sagdeo A, Johnson J, et al. Genetic and environmental influences on psychiatric comorbidity: a systematic review. J Affect Disord. 2010;126(1-2):14-38.
6. Yehuda R, Hoge CW, McFarlane AC, et al. Post-traumatic stress disorder. Nat Rev Dis Primers. 2015;1:15057. doi: 10.1038/nrdp.2015.57.
7. True WR, Rice J, Eisen SA, et al. A twin study of genetic and environmental contributions to liability for posttraumatic stress symptoms. Arch Gen Psychiatry. 1993;50(4):257-264.
8. Diagnostic and statistical manual of mental disorders. 5th ed. Washington, DC: American Psychiatric Association; 2013:271-280.
9. Reichmann F, Holzer P. Neuropeptide Y: a stressful review. Neuropeptides. 2016;55:99-109.
10. Yehuda R, Brand S, Yang RK. Plasma neuropeptide Y concentrations in combat exposed veterans: relationship to trauma exposure, recovery from PTSD, and coping. Biol Psychiatry. 2006;59(7):660-663.
11. Cohen H, Liu T, Kozlovsky N, et al. The neuropeptide Y (NPY)-ergic system is associated with behavioral resilience to stress exposure in an animal model of post-traumatic stress disorder. Neuropsychopharmacology. 2012;37(2):350-363.
12. Donner J, Sipilä T, Ripatti S, et al. Support for involvement of glutamate decarboxylase 1 and neuropeptide Y in anxiety susceptibility. Am J Med Genet B Neuropsychiatr Genet. 2012;159B(3):316-327.
13. Schmeltzer SN, Herman JP, Sah R. Neuropeptide Y (NPY) and posttraumatic stress disorder (PTSD): a translational update. Exp Neurol. 2016;284(pt B):196-210.
14. Kautz M, Charney DS, Murrough JW. Neuropeptide Y, resilience, and PTSD therapeutics. Neurosci Lett. 2017;649:164-169.
15. Serova LI, Laukova M, Alaluf LG, et al. Intranasal neuropeptide Y reverses anxiety and depressive-like behavior impaired by single prolonged stress PTSD model. Eur Neuropsychopharmacol. 2014;24(1):142-147.
16. Sayed S, Van Dam NT, Horn SR, et al. A randomized dose-ranging study of neuropeptide Y in patients with posttraumatic stress disorder. Int J Neuropsychopharmacol. 2018;21(1):3-11.
17. Toth M, Flandreau EI, Deslauriers J, et al. Overexpression of forebrain CRH during early life increases trauma susceptibility in adulthood. Neuropsychopharmacology. 2016;41(6):1681-1690.
18. White S, Acierno R, Ruggiero KJ, et al. Association of CRHR1 variants and posttraumatic stress symptoms in hurricane exposed adults. J Anxiety Disord. 2013;27(7):678-683.
19. Wolf EJ, Mitchell KS, Logue MW, et al. Corticotropin releasing hormone receptor 2 (CRHR-2) gene is associated with decreased risk and severity of posttraumatic stress disorder in women. Depress Anxiety. 2013;30(12):1161-1169.
20. Whitnall MH. Regulation of the hypothalamic corticotropin-releasing hormone neurosecretory system. Prog Neurobiol. 1993;40(5):573-629.
21. Bradley RG, Binder EB, Epstein MP, et al. Influence of child abuse on adult depression: moderation by the corticotropin-releasing hormone receptor gene. Arch Gen Psychiatry. 2008;65(2):190-200.
22. Tellew JE, Lanier M, Moorjani M, et al. Discovery of NBI-77860/GSK561679, a potent corticotropin-releasing factor (CRF1) receptor antagonist with improved pharmacokinetic properties. Bioorg Med Chem Lett. 2010;20(24):7259-7264.
23. Dunlop BW, Rothbaum BO, Binder EB, et al. Evaluation of a corticotropin releasing hormone type 1 receptor antagonist in women with posttraumatic stress disorder: study protocol for a randomized controlled trial. Trials. 2014;15:240. doi: 10.1186/1745-6215-15-240.
24. Jovanovic T, Duncan EJ, Kaye J, et al. Psychophysiological treatment outcomes: Corticotropin-releasing factor type 1 receptor antagonist increases inhibition of fear-potentiated startle in PTSD patients. Psychophysiology. 2019:e13356. doi: 10.1111/psyp.13356.
25. Dunlop BW, Binder EB, Iosifescu D, et al. Corticotropin-releasing factor receptor 1 antagonism is ineffective for women with posttraumatic stress disorder. Biol Psychiatry. 2017;82(12):866-874.
26. Pape JC, Carrillo-Roa T, Rothbaum BO, et al. DNA methylation levels are associated with CRF1 receptor antagonist treatment outcome in women with post-traumatic stress disorder. Clin Epigenetics. 2018;10(1):136. doi: 10.1186/s13148-018-0569-x.
27. Murrough JW, Charney DS. Corticotropin-releasing factor type 1 receptor antagonists for stress-related disorders: time to call it quits? Biol Psychiatry. 2017;82(12):858-860.
28. Leal G, Bramham CR, Duarte CB. BDNF and hippocampal synaptic plasticity. Vitam Horm. 2017;104:153-195.
29. Groves JO. Is it time to reassess the BDNF hypothesis of depression? Mol Psychiatry. 2007;12:1079-1088.
30. Frustaci A, Pozzi G, Gianfagna F, et al. Meta-analysis of the brain-derived neurotrophic factor gene (BDNF) Val66Met polymorphism in anxiety disorders and anxiety-related personality traits. Neuropsychobiology. 2008;58(3-4):163-170.
31. Gatt JM, Nemeroff CB, Dobson-Stone C, et al. Interactions between BDNF Val66Met polymorphism and early life stress predict brain and arousal pathways to syndromal depression and anxiety. Mol Psychiatry. 2009;14(7):681-695.
32. Ragen BJ, Seidel J, Chollak C, et al. Investigational drugs under development for the treatment of PTSD. Expert Opin Investig Drugs. 2015;24(5):659-672.
33. Caspi A, Sugden K, Moffitt TE, et al. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science. 2003;301(5631):386-389.
34. Murphy DL, Fox MA, Timpano KR, et al. How the serotonin story is being rewritten by new gene-based discoveries principally related to SLC6A4, the serotonin transporter gene, which functions to influence all cellular serotonin systems. Neuropharmacology. 2008;55(6):932-960.
35. Luddington NS, Mandadapu A, Husk M, et al. Clinical implications of genetic variation in the serotonin transporter promoter region: a review. Prim Care Compan J Clin Psychiatry. 2009;11:(3):93-102.
36. Lee HJ, Lee MS, Kang RH, et al. Influence of the serotonin transporter promoter gene polymorphism on susceptibility to posttraumatic stress disorder. Depress Anxiety. 2005;21(3):135-139.
37. Liu Y, Garrett ME, Dennis MF, et al. An examination of the association between 5-HTTLPR, combat exposure, and PTSD diagnosis among U.S. veterans. PLoS One. 2015;10(3):e0119998. doi: 10.1371/journal.pone.0119998.
38. Mehta D, Voisey J, Bruenig D, et al. Transcriptome analysis reveals novel genes and immune networks dysregulated in veterans with PTSD. Brain Behav Immun. 2018;74:133-142. doi: 10.1016/j.bbi.2018.08.014.
39. Kilpatrick DG, Koenen KC, Ruggiero KJ, et al. The serotonin transporter genotype and social support and moderation of posttraumatic stress disorder and depression in hurricane-exposed adults. Am J Psychiatry. 2007;164(11):1693-1699.
40. Kolassa IT, Ertl V, Eckart C, et al. Association study of trauma load and SLC6A4 promoter polymorphism in posttraumatic stress disorder: evidence from survivors of the Rwandan genocide. J Clin Psychiatry. 2010;71(5):543-547.
41. Bryant RA, Felmingham KL, Falconer EM, et al. Preliminary evidence of the short allele of the serotonin transporter gene predicting poor response to cognitive behavior therapy in posttraumatic stress disorder. Biol Psychiatry. 2010;67(12):1217-1219.
42. Mrazek DA, Rush AJ, Biernacka JM, et al. SLC6A4 variation and citalopram response. Am J Med Genet B Neuropsychiatr Genet. 2009;150B(3):341-351.
43. Shiroma PR, Drews MS, Geske JR, et al. SLC6A4 polymorphisms and age of onset in late-life depression on treatment outcomes with citalopram: a Sequenced Treatment Alternatives to Relieve Depression (STAR*D) report. Am J Geriatr Psychiatry. 2014;22(11):1140-1148.
44. Fanelli G, Serretti A. The influence of the serotonin transporter gene 5-HTTLPR polymorphism on suicidal behaviors: a meta-analysis. Prog Neuropsychopharmacol Biol Psychiatry. 2019;88:375-387.
45. Courtet P, Picot MC, Bellivier F, et al. Serotonin transporter gene may be involved in short-term risk of subsequent suicide attempts. Biol Psychiatry. 2003;55(1):46-51.
46. Outhred T, Das P, Dobson-Stone C, et al. The impact of 5-HTTLPR on acute serotonin transporter blockade by escitalopram on emotion processing: Preliminary findings from a randomised, crossover fMRI study. Aust NZ J Psychiatry. 2014;48(12):1115-1125.
47. Lachman HM, Papolos DF, Saito T, et al. Human catechol-O-methyltransferase pharmacogenetics: description of a functional polymorphism and its potential application to neuropsychiatric disorders. Pharmacogenetics. 1996;6(3):243-250.
48. Valente NL, Vallada H, Cordeiro Q, et al. Catechol-O-methyltransferase (COMT) val158met polymorphism as a risk factor for PTSD after urban violence. J Mol Neurosci. 2011;43(3):516-523.
49. van Rooij SJ, Stevens JS, Ely TD, et al. Childhood trauma and COMT genotype interact to increase hippocampal activation in resilient individuals. Front Psychiatry. 2016;7:156. doi: 10.3389/fpsyt.2016.00156.
50. Wu G, Feder A, Cohen H, et al. Understanding resilience. Front Behav Neuroscience. 2013;7:10. doi: 10.3389/fnbeh.2013.00010.
51. Kolassa I, Kolassa S, Ertl V, et al. The risk of posttraumatic stress disorder after trauma depends on traumatic load and the catechol-O-methyltransferase Val(158)Met polymorphism. Biol Psychiatry. 2010;67(4):304-308.
52. Bruder GE, Keilp JG, Xu H, et al. Catechol-O-methyltransferase (COMT) genotypes and working memory: associations with differing cognitive operations. Biol Psychiatry. 2005;58(11):901-907.
53. Strawn JR, Geracioti TD Jr. Noradrenergic dysfunction and the psychopharmacology of posttraumatic stress disorder. Depress Anxiety. 2008;25(3):260-271.
54. Hendrickson RC, Raskind MA. Noradrenergic dysregulation in the pathophysiology of PTSD. Exp Neurol. 2016;284(pt B):181-195.
55. Neylan TC, Marmar CR, Metzler TJ, et al. Sleep disturbances in the Vietnam generation: findings from a nationally representative sample of male Vietnam veterans. Am J Psychiatry. 1998;155(7):929-933.
56. Roehrs TA, Roth T. Hyperarousal in insomnia and hypnotic dose escalation. Sleep Med. 2016;23:16-20.
57. Geracioti TD Jr, Baker DG, Ekhator NN, et al. CSF Norepinephrine concentrations in posttraumatic stress disorder. Am J Psychiatry. 2001;158(8):1227-1230.
58. Yehuda R, Siever LJ, Teicher MH, et al. Plasma norepinephrine and 3-methoxy-4-hydroxyphenylglycol concentrations and severity of depression in combat posttraumatic stress disorder and major depressive disorder. Biol Psychiatry. 1998;44(1):56-63.
59. Molinoff PB. Alpha- and beta-adrenergic receptor subtypes properties, distribution and regulation. Drugs. 1984;28(suppl 2):1-15.
60. El-Mallakh RS. The use of beta-blockers in psychiatry. Res Staff Phys. 1989;35:49-52,59,62.
61. Raskind MA, Peterson K, Williams T, et al. A trial of prazosin for combat trauma PTSD with nightmares in active-duty soldiers returned from Iraq and Afghanistan. Am J Psychiatry. 2013;170(9):1003-1010.
62. Bisson JI, Roberts NP, Andrew M, et al. Psychological therapies for chronic post-traumatic stress disorder (PTSD) in adults. Cochrane Database Syst Rev. 2013;(12):CD003388.
63. Steenkamp MM, Litz BT, Hoge CW, et al. Psychotherapy for military-related PTSD: a review of randomized clinical trials. JAMA. 2015;314(5):489-500.
64. Hoskins M, Pearce J, Bethell A, et al. Pharmacotherapy for post-traumatic stress disorder: systematic review and meta-analysis. Br J Psychiatry. 2015;206(2):93-100.
65. Belkin MR, Schwartz TL. Alpha-2 receptor agonists for the treatment of posttraumatic stress disorder. Drugs Context. 2015;4:212286. doi: 10.7573/dic.212286.
66. Brady K, Pearlstein T, Asnis GM, et al. Efficacy and safety of sertraline treatment of posttraumatic stress disorder: a randomized controlled trial. JAMA. 2000;283(14):1837-1844.
67. Davidson JRT, Landerman LR, Farfel GM, et al. Characterizing the effects of sertraline in post-traumatic stress disorder. Psychol Med. 2002;32(4):661-670.
68. Hertzberg MA, Feldman ME, Beckham JC, et al. Lack of efficacy for fluoxetine in PTSD: a placebo controlled trial in combat veterans. Ann Clin Psychiatry. 2000;12(2):101-105.
69. Friedman MJ, Marmar CR, Baker DG, et al. Randomized, double-blind comparison of sertraline and placebo for posttraumatic stress disorder in a Department of Veterans Affairs setting. J Clin Psychiatry. 2007;68(5):711-720.
70. Mello MF, Costa MCP, Schoedl AF, et al. Aripiprazole in the treatment of posttraumatic stress disorder: an open-label trial. Rev Bras Psiquiatr. 2008;30(4):358-361.
71. Birnbaum S, Gobeske KT, Auerbach J, et al. A role for norepinephrine in stress-induced cognitive deficits: alpha-1-adrenoceptor mediation in the prefrontal cortex. Biol Psychiatry. 1999;46(9):1266-1274.
72. Ahmadpanah M, Sabzeiee P, Hosseini SM, et al. Comparing the effect of prazosin and hydroxyzine on sleep quality in patients suffering from posttraumatic stress disorder. Neuropsychobiology. 2014;69(4):235-242.
73. Villarreal G, Hamner MB, Cañive JM, et al. Efficacy of quetiapine monotherapy in posttraumatic stress disorder: a randomized, placebo-controlled trial. Am J Psychiatry. 2016;173(12):1205-1212.
Posttraumatic stress disorder (PTSD) occurs acutely and chronically in the aftermath of severe and potentially life-threatening trauma.1 The prevalence of PTSD varies significantly across countries and by type of trauma (Box1-7).
Box
In the general population, the prevalence of posttraumatic stress disorder (PTSD) varies from as low as 0.3% in China to as high as 6.1% in New Zealand1 and 6.8% in the United States.2 These rates are actually much lower than expected when one considers that severe trauma is experienced by 60.7% of men and 51.2% of women.3,4 Although the majority of individuals exposed to trauma experience emotional distress immediately following a traumatic event, most of them do not develop PTSD.5
It appears that the context of trauma is important: 12% to 15% of veterans experience PTSD, compared with 19% to 75% of crime victims and 80% of rape victims.1 The lifetime risk for PTSD is twice as high in women as it is in men,6 and genetic vulnerability may play a role. For example, twin studies showed that approximately 30% of the risk for PTSD may be mediated by genetic predisposition.7
Individuals who develop PTSD experience a wide range of symptoms.8 These can be categorized as PTSD-specific symptoms, or nonspecific symptoms. PTSD-specific symptoms include nightmares, flashbacks, dissociative reactions, hyperreactivity or hyperarousal, distress with reminders of trauma, and avoidance of trauma-related physical reminders and thoughts/feelings (Table8). Nonspecific symptoms include depressive and anxiety symptoms and significant problems in social, relationship, or work situations.8
While successful treatment necessitates taking all of these symptoms into account, understanding the pathophysiology of PTSD can inform a more focused and rational treatment approach. In this article, we describe some key pathophysiologic PTSD studies, and focus on PTSD-specific psychopathology to inform treatment.
Brain systems implicated in PTSD
Neuropeptide Y (NPY) is an anxiolytic endogenous peptide that has connections to the hypothalamic-pituitary-adrenal (HPA) axis. Its levels can be modulated by stress.9 Preclinical and clinical studies strongly support a potential role of NPY dysfunction in the pathophysiology of PTSD. Lower concentrations of NPY increase susceptibility to PTSD in combat veterans10 and in animal models.11 Three single-nucleotide polymorphisms (SNPs) appear to mediate this effect.12 These findings strongly support pharmaceutical targeting this system as a useful therapeutic approach.13,14 Indeed, intranasal NPY administered as a single dose reduces anxiety in animal models15 and in humans,16 but this work has not yet translated into clinical tools.
Corticotropin-releasing hormone receptor (CRHR1) gene. Corticotropin-releasing hormone has been implicated in PTSD.17 Corticotropin-releasing hormone receptors (CRHR) are important mediators in response to stress.18,19 They bind corticotropin-releasing hormone and contribute to the integration of autonomic, behavioral, and immune responses to stress.20 Single-nucleotide polymorphisms in the regulatory portion of the CRHR1 gene are associated with an increased risk for depression in adults who have a history of child abuse.21
The CRHR1 receptor antagonist GSK561679 is an investigational agent for the treatment of mood and anxiety disorders.22 In exploratory studies,23,24 GSK561679 was found to inhibit fear-potentiated startle in patients with PTSD, but not overall PTSD symptoms, although a subset of women with a specific genetic variant of the CRHR1 gene (rs110402) experienced significant benefit.25,26 This suggests that we must learn more about this system before we proceed.27
Brain-derived neurotrophic factor (BDNF). The synthesis of BDNF is influenced by neuronal activity in the brain and plays a role in synaptic transmission and plasticity.28 Brain-derived neurotrophic factor is encoded by the BDNF gene, which has been implicated in stress vulnerability.29 A common SNP in the pro-region of the human BDNF gene results in a valine-to-methionine substitution at the 66th amino acid (Val66Met). The functional Val66Met polymorphism may have a role in the risk of developing PTSD. However, not all studies support this finding. One study found that an SNP with a resulting Val66Met polymorphism is associated with adult PTSD symptoms after childhood abuse, while a meta-analysis of 7 studies did not confirm this.30,31 We need to learn more about BDNF before we proceed.32
Continue to: Serotonin transporter (5-HTT) gene
Serotonin transporter (5-HTT) gene. Serotonin transporter is a monoamine transporter protein that terminates the neurotransmitter signal by transporting serotonin from the synaptic cleft back into the presynaptic neuron. It is encoded by the SLC6A4 gene, which resides on the long arm of chromosome 17(17q11.1-q12). It is a large gene with 31 kilo bases and 14 separate exons (transcribed regions).33,34
This gene has several variants. The best-studied is a variation in the promoter region. A 44-bp insertion or deletion yields the “long” and “short” alleles, respectively. The proteins produced by the 2 alleles are identical, but the amount of expressed protein is different. The short allele (“S”) is associated with a nearly 50% reduction in 5-HTT expression in both homozygotes and heterozygotes.35 A greater incidence of serotonin transporter promoter region (5-HTTLPR) S has been found in individuals with PTSD compared with those without PTSD,36-38 and 5-HTTLPR S increases the risk of PTSD in individuals with low social support39 or after very few traumatic events.40 The short allele variant is also associated with depression in individuals who face adversity.35,41
The overrepresentation of the short form of 5-HTTLPR in individuals who develop PTSD may represent a potential problem with current treatment paradigms, in which an antidepressant is the first-line treatment, because this allele is associated with reduced response to antidepressants.42,43 More distressing is the possible association of this allele with increased suicide risk, particularly violent suicide44 or repeated suicide attempts.45
Furthermore, a functional MRI study of patients who were anxious revealed that in individuals with the short allele, administration of
Catechol-o-methyltransferase (COMT) is one of the enzymes that degrades catecholamines such as dopamine, epinephrine, and norepinephrine (NE).47 In humans, COMT protein is encoded by the COMT gene. This gene is associated with allelic variants; the best-studied of these is Val158Met. COMT Val158Met polymorphism (rs4860) has been linked to deficits in stress response and emotional resilience.48,49 Val158Met is associated with a 40% reduction in enzyme activity and slower catalysis of catecholamines, resulting in increases in catecholamines levels in the brain, which may increase the risk of developing PTSD.50 Individuals homozygous for this SNP (Met/Met) are highly susceptible to develop PTSD independently of the severity of the trauma they experienced.51 The Val158Met polymorphism may be associated with other abnormalities, such as cognitive problems with specific frontal cortical activity, and also with improved antidepressant response (valine homozygotes less responsive than methionine homozygotes).52 This gene is available on gene testing profiles.
Continue to: The role of norepinephrine in PTSD
The role of norepinephrine in PTSD
Perhaps the greatest advance in the understanding of the pathophysiology of PTSD relates to changes in brain NE. The HPA axis is responsible for coordinating the hormonal response to stress. Dysregulation of this axis and increased activity of the central and peripheral noradrenergic systems are usually observed in patients with PTSD.53 Several monoamine neurotransmitters are important in the regulation and function of the HPA axis. Norepinephrine plays a major role in stress.
The clinical PTSD-specific criteria are all descriptions of excessive noradrenergic tone.54 For example, hypervigilance and hyperstartle are clearly anticipated as evidence of NE stimulation. Flashbacks, particularly those that might be precipitated by environmental cues, also can be a manifestation of the vigilance induced by NE. Sleep disturbances (insomnia and nightmares) are present; insomnia is reported more often than nightmares.55 Increased catecholamine levels, particularly NE, are a feature of sleep disturbances associated with middle insomnia. Dreams can be remembered only if you wake up during dreaming. Catecholamines do not change the content of dreams, just recall.56
In a study of central noradrenergic tone in patients with PTSD, 6 hourly CSF samples were collected from 11 male combat veterans with PTSD and 8 healthy controls.57 Participants with PTSD had significantly higher CSF NE concentrations (0.55 ± 0.17 pmol/ml vs 0.39 ± 0.16 pmol/mL in the PTSD and control groups, respectively; F = 4.49, P < .05).57 Overall PTSD symptoms correlated significantly with CSF NE levels (r = 0.82, P <.005), and PTSD-specific symptoms such as avoidance (r = 0.79, P = .004). Intrusive thoughts (r = 0.57, P = .07) and hyperarousal (r = 0.54, P = .09) were also related.57 This relationship is unique; patients with PTSD with predominant depressive symptoms do not have elevated plasma NE levels.58
In the human brain, there are 3 main groups of NE receptors: alpha-1 receptors, alpha-2 receptors, and beta receptors.59 Alpha-1 receptors (alpha-1A, alpha-1B, and alpha-1D) are postsynaptic and mediate increase in inositol trisphosphate (IP3) and intracellular calcium (Ca2+). Alpha-2 receptors (alpha-2A, alpha-2B, alpha-2C) in the CNS are presynaptic autoreceptors and serve to reduce NE release. Beta receptors (beta-1, beta-2, beta-3) inhibit cyclic adenosine monophosphate (cAMP) production.59 The effects of inhibition of alpha or beta receptors are different. Inhibition of beta receptors is associated with depressive symptoms and depressive syndrome, inhibition of peripheral beta receptors is associated with reductions in anxiety (generally reduction of pulse, sweating, tremor),60 and inhibition of central alpha-1 receptors is associated with reduced PTSD symptoms.61
Choice of agents for PTSD-specific symptoms
As outlined in the Table,8 PTSD is characterized by 3 types of symptoms that are specific for PTSD. Trauma-focused psychotherapy62,63 and selective serotonin reuptake inhibitors (SSRIs)64 are considered first-line therapy for PTSD. Only
Continue to: Serotonin transporter promoter...
Serotonin transporter promoter region gene short-type variants, which possibly increase an individual’s predisposition to developing PTSD, may explain the abundance of depressive symptoms in this condition and the subdued response to antidepressants. Specifically, an anticipated preponderance of these alleles may be associated with poorer outcomes. Non-SSRI treatments, such as low-dose
On the other hand, animal models support antagonism of the postsynaptic alpha-1 adrenergic receptor of the CNS as a target for PTSD treatment.71 Although
Quetiapine might be another non-SSRI option for treating patients with PTSD. It is an antagonist with high affinity tothehistamine-1 receptor at low doses. Norquetiapine is an alpha-2 antagonist that increases brain NE levels. Both quetiapine and norquetiapine are alpha-1 antagonists. There is no beta blockade and no SSRI effect, but some 5HT2A blockade, which may be anxiolytic. Compared with placebo, an average quetiapine dose of 258 mg/d resulted in significantly greater reductions in Clinician-Administered PTSD Scale total score, re-experiencing score, and hyperarousal score.73
Unfortunately, none of the non-SSRI options have been adequately evaluated. For now, clinicians need to continue to use SSRIs, and researchers need to continue to explore mechanism-guided alternatives.
Bottom Line
Understanding the mechanisms of the pathophysiology of posttraumatic stress disorder (PTSD) may allow clinicians to “jump ahead” of clinical studies and FDA indications. Clinicians may reasonably use alpha-1 antagonists (eg, prazosin, quetiapine) for general clinical improvement of patients with PTSD, particularly for PTSD-specific symptoms. Using antihistamines to reduce anxiety (especially in patients who have the COMT Val158Met polymorphism) may also be reasonable.
Related Resources
- North CS, Hong BA, Downs DL. PTSD: a systematic approach to diagnosis and treatment. Current Psychiatry. 2018;17(4):35-43.
- Zhang Y, Ren R, Sanford LD, et al. The effects of prazosin on sleep disturbances in post-traumatic stress disorder: a systematic review and meta-analysis. Sleep Med. 2019; 67:225-231.
Drug Brand Names
Aripiprazole • Abilify
Citalopram • Celexa
Paroxetine • Paxil
Prazosin • Minipress
Quetiapine • Seroquel
Sertraline • Zoloft
Posttraumatic stress disorder (PTSD) occurs acutely and chronically in the aftermath of severe and potentially life-threatening trauma.1 The prevalence of PTSD varies significantly across countries and by type of trauma (Box1-7).
Box
In the general population, the prevalence of posttraumatic stress disorder (PTSD) varies from as low as 0.3% in China to as high as 6.1% in New Zealand1 and 6.8% in the United States.2 These rates are actually much lower than expected when one considers that severe trauma is experienced by 60.7% of men and 51.2% of women.3,4 Although the majority of individuals exposed to trauma experience emotional distress immediately following a traumatic event, most of them do not develop PTSD.5
It appears that the context of trauma is important: 12% to 15% of veterans experience PTSD, compared with 19% to 75% of crime victims and 80% of rape victims.1 The lifetime risk for PTSD is twice as high in women as it is in men,6 and genetic vulnerability may play a role. For example, twin studies showed that approximately 30% of the risk for PTSD may be mediated by genetic predisposition.7
Individuals who develop PTSD experience a wide range of symptoms.8 These can be categorized as PTSD-specific symptoms, or nonspecific symptoms. PTSD-specific symptoms include nightmares, flashbacks, dissociative reactions, hyperreactivity or hyperarousal, distress with reminders of trauma, and avoidance of trauma-related physical reminders and thoughts/feelings (Table8). Nonspecific symptoms include depressive and anxiety symptoms and significant problems in social, relationship, or work situations.8
While successful treatment necessitates taking all of these symptoms into account, understanding the pathophysiology of PTSD can inform a more focused and rational treatment approach. In this article, we describe some key pathophysiologic PTSD studies, and focus on PTSD-specific psychopathology to inform treatment.
Brain systems implicated in PTSD
Neuropeptide Y (NPY) is an anxiolytic endogenous peptide that has connections to the hypothalamic-pituitary-adrenal (HPA) axis. Its levels can be modulated by stress.9 Preclinical and clinical studies strongly support a potential role of NPY dysfunction in the pathophysiology of PTSD. Lower concentrations of NPY increase susceptibility to PTSD in combat veterans10 and in animal models.11 Three single-nucleotide polymorphisms (SNPs) appear to mediate this effect.12 These findings strongly support pharmaceutical targeting this system as a useful therapeutic approach.13,14 Indeed, intranasal NPY administered as a single dose reduces anxiety in animal models15 and in humans,16 but this work has not yet translated into clinical tools.
Corticotropin-releasing hormone receptor (CRHR1) gene. Corticotropin-releasing hormone has been implicated in PTSD.17 Corticotropin-releasing hormone receptors (CRHR) are important mediators in response to stress.18,19 They bind corticotropin-releasing hormone and contribute to the integration of autonomic, behavioral, and immune responses to stress.20 Single-nucleotide polymorphisms in the regulatory portion of the CRHR1 gene are associated with an increased risk for depression in adults who have a history of child abuse.21
The CRHR1 receptor antagonist GSK561679 is an investigational agent for the treatment of mood and anxiety disorders.22 In exploratory studies,23,24 GSK561679 was found to inhibit fear-potentiated startle in patients with PTSD, but not overall PTSD symptoms, although a subset of women with a specific genetic variant of the CRHR1 gene (rs110402) experienced significant benefit.25,26 This suggests that we must learn more about this system before we proceed.27
Brain-derived neurotrophic factor (BDNF). The synthesis of BDNF is influenced by neuronal activity in the brain and plays a role in synaptic transmission and plasticity.28 Brain-derived neurotrophic factor is encoded by the BDNF gene, which has been implicated in stress vulnerability.29 A common SNP in the pro-region of the human BDNF gene results in a valine-to-methionine substitution at the 66th amino acid (Val66Met). The functional Val66Met polymorphism may have a role in the risk of developing PTSD. However, not all studies support this finding. One study found that an SNP with a resulting Val66Met polymorphism is associated with adult PTSD symptoms after childhood abuse, while a meta-analysis of 7 studies did not confirm this.30,31 We need to learn more about BDNF before we proceed.32
Continue to: Serotonin transporter (5-HTT) gene
Serotonin transporter (5-HTT) gene. Serotonin transporter is a monoamine transporter protein that terminates the neurotransmitter signal by transporting serotonin from the synaptic cleft back into the presynaptic neuron. It is encoded by the SLC6A4 gene, which resides on the long arm of chromosome 17(17q11.1-q12). It is a large gene with 31 kilo bases and 14 separate exons (transcribed regions).33,34
This gene has several variants. The best-studied is a variation in the promoter region. A 44-bp insertion or deletion yields the “long” and “short” alleles, respectively. The proteins produced by the 2 alleles are identical, but the amount of expressed protein is different. The short allele (“S”) is associated with a nearly 50% reduction in 5-HTT expression in both homozygotes and heterozygotes.35 A greater incidence of serotonin transporter promoter region (5-HTTLPR) S has been found in individuals with PTSD compared with those without PTSD,36-38 and 5-HTTLPR S increases the risk of PTSD in individuals with low social support39 or after very few traumatic events.40 The short allele variant is also associated with depression in individuals who face adversity.35,41
The overrepresentation of the short form of 5-HTTLPR in individuals who develop PTSD may represent a potential problem with current treatment paradigms, in which an antidepressant is the first-line treatment, because this allele is associated with reduced response to antidepressants.42,43 More distressing is the possible association of this allele with increased suicide risk, particularly violent suicide44 or repeated suicide attempts.45
Furthermore, a functional MRI study of patients who were anxious revealed that in individuals with the short allele, administration of
Catechol-o-methyltransferase (COMT) is one of the enzymes that degrades catecholamines such as dopamine, epinephrine, and norepinephrine (NE).47 In humans, COMT protein is encoded by the COMT gene. This gene is associated with allelic variants; the best-studied of these is Val158Met. COMT Val158Met polymorphism (rs4860) has been linked to deficits in stress response and emotional resilience.48,49 Val158Met is associated with a 40% reduction in enzyme activity and slower catalysis of catecholamines, resulting in increases in catecholamines levels in the brain, which may increase the risk of developing PTSD.50 Individuals homozygous for this SNP (Met/Met) are highly susceptible to develop PTSD independently of the severity of the trauma they experienced.51 The Val158Met polymorphism may be associated with other abnormalities, such as cognitive problems with specific frontal cortical activity, and also with improved antidepressant response (valine homozygotes less responsive than methionine homozygotes).52 This gene is available on gene testing profiles.
Continue to: The role of norepinephrine in PTSD
The role of norepinephrine in PTSD
Perhaps the greatest advance in the understanding of the pathophysiology of PTSD relates to changes in brain NE. The HPA axis is responsible for coordinating the hormonal response to stress. Dysregulation of this axis and increased activity of the central and peripheral noradrenergic systems are usually observed in patients with PTSD.53 Several monoamine neurotransmitters are important in the regulation and function of the HPA axis. Norepinephrine plays a major role in stress.
The clinical PTSD-specific criteria are all descriptions of excessive noradrenergic tone.54 For example, hypervigilance and hyperstartle are clearly anticipated as evidence of NE stimulation. Flashbacks, particularly those that might be precipitated by environmental cues, also can be a manifestation of the vigilance induced by NE. Sleep disturbances (insomnia and nightmares) are present; insomnia is reported more often than nightmares.55 Increased catecholamine levels, particularly NE, are a feature of sleep disturbances associated with middle insomnia. Dreams can be remembered only if you wake up during dreaming. Catecholamines do not change the content of dreams, just recall.56
In a study of central noradrenergic tone in patients with PTSD, 6 hourly CSF samples were collected from 11 male combat veterans with PTSD and 8 healthy controls.57 Participants with PTSD had significantly higher CSF NE concentrations (0.55 ± 0.17 pmol/ml vs 0.39 ± 0.16 pmol/mL in the PTSD and control groups, respectively; F = 4.49, P < .05).57 Overall PTSD symptoms correlated significantly with CSF NE levels (r = 0.82, P <.005), and PTSD-specific symptoms such as avoidance (r = 0.79, P = .004). Intrusive thoughts (r = 0.57, P = .07) and hyperarousal (r = 0.54, P = .09) were also related.57 This relationship is unique; patients with PTSD with predominant depressive symptoms do not have elevated plasma NE levels.58
In the human brain, there are 3 main groups of NE receptors: alpha-1 receptors, alpha-2 receptors, and beta receptors.59 Alpha-1 receptors (alpha-1A, alpha-1B, and alpha-1D) are postsynaptic and mediate increase in inositol trisphosphate (IP3) and intracellular calcium (Ca2+). Alpha-2 receptors (alpha-2A, alpha-2B, alpha-2C) in the CNS are presynaptic autoreceptors and serve to reduce NE release. Beta receptors (beta-1, beta-2, beta-3) inhibit cyclic adenosine monophosphate (cAMP) production.59 The effects of inhibition of alpha or beta receptors are different. Inhibition of beta receptors is associated with depressive symptoms and depressive syndrome, inhibition of peripheral beta receptors is associated with reductions in anxiety (generally reduction of pulse, sweating, tremor),60 and inhibition of central alpha-1 receptors is associated with reduced PTSD symptoms.61
Choice of agents for PTSD-specific symptoms
As outlined in the Table,8 PTSD is characterized by 3 types of symptoms that are specific for PTSD. Trauma-focused psychotherapy62,63 and selective serotonin reuptake inhibitors (SSRIs)64 are considered first-line therapy for PTSD. Only
Continue to: Serotonin transporter promoter...
Serotonin transporter promoter region gene short-type variants, which possibly increase an individual’s predisposition to developing PTSD, may explain the abundance of depressive symptoms in this condition and the subdued response to antidepressants. Specifically, an anticipated preponderance of these alleles may be associated with poorer outcomes. Non-SSRI treatments, such as low-dose
On the other hand, animal models support antagonism of the postsynaptic alpha-1 adrenergic receptor of the CNS as a target for PTSD treatment.71 Although
Quetiapine might be another non-SSRI option for treating patients with PTSD. It is an antagonist with high affinity tothehistamine-1 receptor at low doses. Norquetiapine is an alpha-2 antagonist that increases brain NE levels. Both quetiapine and norquetiapine are alpha-1 antagonists. There is no beta blockade and no SSRI effect, but some 5HT2A blockade, which may be anxiolytic. Compared with placebo, an average quetiapine dose of 258 mg/d resulted in significantly greater reductions in Clinician-Administered PTSD Scale total score, re-experiencing score, and hyperarousal score.73
Unfortunately, none of the non-SSRI options have been adequately evaluated. For now, clinicians need to continue to use SSRIs, and researchers need to continue to explore mechanism-guided alternatives.
Bottom Line
Understanding the mechanisms of the pathophysiology of posttraumatic stress disorder (PTSD) may allow clinicians to “jump ahead” of clinical studies and FDA indications. Clinicians may reasonably use alpha-1 antagonists (eg, prazosin, quetiapine) for general clinical improvement of patients with PTSD, particularly for PTSD-specific symptoms. Using antihistamines to reduce anxiety (especially in patients who have the COMT Val158Met polymorphism) may also be reasonable.
Related Resources
- North CS, Hong BA, Downs DL. PTSD: a systematic approach to diagnosis and treatment. Current Psychiatry. 2018;17(4):35-43.
- Zhang Y, Ren R, Sanford LD, et al. The effects of prazosin on sleep disturbances in post-traumatic stress disorder: a systematic review and meta-analysis. Sleep Med. 2019; 67:225-231.
Drug Brand Names
Aripiprazole • Abilify
Citalopram • Celexa
Paroxetine • Paxil
Prazosin • Minipress
Quetiapine • Seroquel
Sertraline • Zoloft
1. Javidi H, Yadollahie M. Post-traumatic stress disorder. Int J Occup Environ Med. 2012;3(1):2-9.
2. Kessler RC, Chiu WT, Demler O, et al. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62(6):617-627.
3. Kessler RC, Sonnega A, Bromet E, et al. Posttraumatic stress disorder in the National Comorbidity Survey. Arch Gen Psychiatry. 1995;52(12):1048-1060.
4. Breslau N, Kessler RC, Chilcoat HD, et al. Trauma and posttraumatic stress disorder in the community: the 1996 Detroit Area Survey of Trauma. Arch Gen Psychiatry. 1998;55(7):626-632.
5. Cerda M, Sagdeo A, Johnson J, et al. Genetic and environmental influences on psychiatric comorbidity: a systematic review. J Affect Disord. 2010;126(1-2):14-38.
6. Yehuda R, Hoge CW, McFarlane AC, et al. Post-traumatic stress disorder. Nat Rev Dis Primers. 2015;1:15057. doi: 10.1038/nrdp.2015.57.
7. True WR, Rice J, Eisen SA, et al. A twin study of genetic and environmental contributions to liability for posttraumatic stress symptoms. Arch Gen Psychiatry. 1993;50(4):257-264.
8. Diagnostic and statistical manual of mental disorders. 5th ed. Washington, DC: American Psychiatric Association; 2013:271-280.
9. Reichmann F, Holzer P. Neuropeptide Y: a stressful review. Neuropeptides. 2016;55:99-109.
10. Yehuda R, Brand S, Yang RK. Plasma neuropeptide Y concentrations in combat exposed veterans: relationship to trauma exposure, recovery from PTSD, and coping. Biol Psychiatry. 2006;59(7):660-663.
11. Cohen H, Liu T, Kozlovsky N, et al. The neuropeptide Y (NPY)-ergic system is associated with behavioral resilience to stress exposure in an animal model of post-traumatic stress disorder. Neuropsychopharmacology. 2012;37(2):350-363.
12. Donner J, Sipilä T, Ripatti S, et al. Support for involvement of glutamate decarboxylase 1 and neuropeptide Y in anxiety susceptibility. Am J Med Genet B Neuropsychiatr Genet. 2012;159B(3):316-327.
13. Schmeltzer SN, Herman JP, Sah R. Neuropeptide Y (NPY) and posttraumatic stress disorder (PTSD): a translational update. Exp Neurol. 2016;284(pt B):196-210.
14. Kautz M, Charney DS, Murrough JW. Neuropeptide Y, resilience, and PTSD therapeutics. Neurosci Lett. 2017;649:164-169.
15. Serova LI, Laukova M, Alaluf LG, et al. Intranasal neuropeptide Y reverses anxiety and depressive-like behavior impaired by single prolonged stress PTSD model. Eur Neuropsychopharmacol. 2014;24(1):142-147.
16. Sayed S, Van Dam NT, Horn SR, et al. A randomized dose-ranging study of neuropeptide Y in patients with posttraumatic stress disorder. Int J Neuropsychopharmacol. 2018;21(1):3-11.
17. Toth M, Flandreau EI, Deslauriers J, et al. Overexpression of forebrain CRH during early life increases trauma susceptibility in adulthood. Neuropsychopharmacology. 2016;41(6):1681-1690.
18. White S, Acierno R, Ruggiero KJ, et al. Association of CRHR1 variants and posttraumatic stress symptoms in hurricane exposed adults. J Anxiety Disord. 2013;27(7):678-683.
19. Wolf EJ, Mitchell KS, Logue MW, et al. Corticotropin releasing hormone receptor 2 (CRHR-2) gene is associated with decreased risk and severity of posttraumatic stress disorder in women. Depress Anxiety. 2013;30(12):1161-1169.
20. Whitnall MH. Regulation of the hypothalamic corticotropin-releasing hormone neurosecretory system. Prog Neurobiol. 1993;40(5):573-629.
21. Bradley RG, Binder EB, Epstein MP, et al. Influence of child abuse on adult depression: moderation by the corticotropin-releasing hormone receptor gene. Arch Gen Psychiatry. 2008;65(2):190-200.
22. Tellew JE, Lanier M, Moorjani M, et al. Discovery of NBI-77860/GSK561679, a potent corticotropin-releasing factor (CRF1) receptor antagonist with improved pharmacokinetic properties. Bioorg Med Chem Lett. 2010;20(24):7259-7264.
23. Dunlop BW, Rothbaum BO, Binder EB, et al. Evaluation of a corticotropin releasing hormone type 1 receptor antagonist in women with posttraumatic stress disorder: study protocol for a randomized controlled trial. Trials. 2014;15:240. doi: 10.1186/1745-6215-15-240.
24. Jovanovic T, Duncan EJ, Kaye J, et al. Psychophysiological treatment outcomes: Corticotropin-releasing factor type 1 receptor antagonist increases inhibition of fear-potentiated startle in PTSD patients. Psychophysiology. 2019:e13356. doi: 10.1111/psyp.13356.
25. Dunlop BW, Binder EB, Iosifescu D, et al. Corticotropin-releasing factor receptor 1 antagonism is ineffective for women with posttraumatic stress disorder. Biol Psychiatry. 2017;82(12):866-874.
26. Pape JC, Carrillo-Roa T, Rothbaum BO, et al. DNA methylation levels are associated with CRF1 receptor antagonist treatment outcome in women with post-traumatic stress disorder. Clin Epigenetics. 2018;10(1):136. doi: 10.1186/s13148-018-0569-x.
27. Murrough JW, Charney DS. Corticotropin-releasing factor type 1 receptor antagonists for stress-related disorders: time to call it quits? Biol Psychiatry. 2017;82(12):858-860.
28. Leal G, Bramham CR, Duarte CB. BDNF and hippocampal synaptic plasticity. Vitam Horm. 2017;104:153-195.
29. Groves JO. Is it time to reassess the BDNF hypothesis of depression? Mol Psychiatry. 2007;12:1079-1088.
30. Frustaci A, Pozzi G, Gianfagna F, et al. Meta-analysis of the brain-derived neurotrophic factor gene (BDNF) Val66Met polymorphism in anxiety disorders and anxiety-related personality traits. Neuropsychobiology. 2008;58(3-4):163-170.
31. Gatt JM, Nemeroff CB, Dobson-Stone C, et al. Interactions between BDNF Val66Met polymorphism and early life stress predict brain and arousal pathways to syndromal depression and anxiety. Mol Psychiatry. 2009;14(7):681-695.
32. Ragen BJ, Seidel J, Chollak C, et al. Investigational drugs under development for the treatment of PTSD. Expert Opin Investig Drugs. 2015;24(5):659-672.
33. Caspi A, Sugden K, Moffitt TE, et al. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science. 2003;301(5631):386-389.
34. Murphy DL, Fox MA, Timpano KR, et al. How the serotonin story is being rewritten by new gene-based discoveries principally related to SLC6A4, the serotonin transporter gene, which functions to influence all cellular serotonin systems. Neuropharmacology. 2008;55(6):932-960.
35. Luddington NS, Mandadapu A, Husk M, et al. Clinical implications of genetic variation in the serotonin transporter promoter region: a review. Prim Care Compan J Clin Psychiatry. 2009;11:(3):93-102.
36. Lee HJ, Lee MS, Kang RH, et al. Influence of the serotonin transporter promoter gene polymorphism on susceptibility to posttraumatic stress disorder. Depress Anxiety. 2005;21(3):135-139.
37. Liu Y, Garrett ME, Dennis MF, et al. An examination of the association between 5-HTTLPR, combat exposure, and PTSD diagnosis among U.S. veterans. PLoS One. 2015;10(3):e0119998. doi: 10.1371/journal.pone.0119998.
38. Mehta D, Voisey J, Bruenig D, et al. Transcriptome analysis reveals novel genes and immune networks dysregulated in veterans with PTSD. Brain Behav Immun. 2018;74:133-142. doi: 10.1016/j.bbi.2018.08.014.
39. Kilpatrick DG, Koenen KC, Ruggiero KJ, et al. The serotonin transporter genotype and social support and moderation of posttraumatic stress disorder and depression in hurricane-exposed adults. Am J Psychiatry. 2007;164(11):1693-1699.
40. Kolassa IT, Ertl V, Eckart C, et al. Association study of trauma load and SLC6A4 promoter polymorphism in posttraumatic stress disorder: evidence from survivors of the Rwandan genocide. J Clin Psychiatry. 2010;71(5):543-547.
41. Bryant RA, Felmingham KL, Falconer EM, et al. Preliminary evidence of the short allele of the serotonin transporter gene predicting poor response to cognitive behavior therapy in posttraumatic stress disorder. Biol Psychiatry. 2010;67(12):1217-1219.
42. Mrazek DA, Rush AJ, Biernacka JM, et al. SLC6A4 variation and citalopram response. Am J Med Genet B Neuropsychiatr Genet. 2009;150B(3):341-351.
43. Shiroma PR, Drews MS, Geske JR, et al. SLC6A4 polymorphisms and age of onset in late-life depression on treatment outcomes with citalopram: a Sequenced Treatment Alternatives to Relieve Depression (STAR*D) report. Am J Geriatr Psychiatry. 2014;22(11):1140-1148.
44. Fanelli G, Serretti A. The influence of the serotonin transporter gene 5-HTTLPR polymorphism on suicidal behaviors: a meta-analysis. Prog Neuropsychopharmacol Biol Psychiatry. 2019;88:375-387.
45. Courtet P, Picot MC, Bellivier F, et al. Serotonin transporter gene may be involved in short-term risk of subsequent suicide attempts. Biol Psychiatry. 2003;55(1):46-51.
46. Outhred T, Das P, Dobson-Stone C, et al. The impact of 5-HTTLPR on acute serotonin transporter blockade by escitalopram on emotion processing: Preliminary findings from a randomised, crossover fMRI study. Aust NZ J Psychiatry. 2014;48(12):1115-1125.
47. Lachman HM, Papolos DF, Saito T, et al. Human catechol-O-methyltransferase pharmacogenetics: description of a functional polymorphism and its potential application to neuropsychiatric disorders. Pharmacogenetics. 1996;6(3):243-250.
48. Valente NL, Vallada H, Cordeiro Q, et al. Catechol-O-methyltransferase (COMT) val158met polymorphism as a risk factor for PTSD after urban violence. J Mol Neurosci. 2011;43(3):516-523.
49. van Rooij SJ, Stevens JS, Ely TD, et al. Childhood trauma and COMT genotype interact to increase hippocampal activation in resilient individuals. Front Psychiatry. 2016;7:156. doi: 10.3389/fpsyt.2016.00156.
50. Wu G, Feder A, Cohen H, et al. Understanding resilience. Front Behav Neuroscience. 2013;7:10. doi: 10.3389/fnbeh.2013.00010.
51. Kolassa I, Kolassa S, Ertl V, et al. The risk of posttraumatic stress disorder after trauma depends on traumatic load and the catechol-O-methyltransferase Val(158)Met polymorphism. Biol Psychiatry. 2010;67(4):304-308.
52. Bruder GE, Keilp JG, Xu H, et al. Catechol-O-methyltransferase (COMT) genotypes and working memory: associations with differing cognitive operations. Biol Psychiatry. 2005;58(11):901-907.
53. Strawn JR, Geracioti TD Jr. Noradrenergic dysfunction and the psychopharmacology of posttraumatic stress disorder. Depress Anxiety. 2008;25(3):260-271.
54. Hendrickson RC, Raskind MA. Noradrenergic dysregulation in the pathophysiology of PTSD. Exp Neurol. 2016;284(pt B):181-195.
55. Neylan TC, Marmar CR, Metzler TJ, et al. Sleep disturbances in the Vietnam generation: findings from a nationally representative sample of male Vietnam veterans. Am J Psychiatry. 1998;155(7):929-933.
56. Roehrs TA, Roth T. Hyperarousal in insomnia and hypnotic dose escalation. Sleep Med. 2016;23:16-20.
57. Geracioti TD Jr, Baker DG, Ekhator NN, et al. CSF Norepinephrine concentrations in posttraumatic stress disorder. Am J Psychiatry. 2001;158(8):1227-1230.
58. Yehuda R, Siever LJ, Teicher MH, et al. Plasma norepinephrine and 3-methoxy-4-hydroxyphenylglycol concentrations and severity of depression in combat posttraumatic stress disorder and major depressive disorder. Biol Psychiatry. 1998;44(1):56-63.
59. Molinoff PB. Alpha- and beta-adrenergic receptor subtypes properties, distribution and regulation. Drugs. 1984;28(suppl 2):1-15.
60. El-Mallakh RS. The use of beta-blockers in psychiatry. Res Staff Phys. 1989;35:49-52,59,62.
61. Raskind MA, Peterson K, Williams T, et al. A trial of prazosin for combat trauma PTSD with nightmares in active-duty soldiers returned from Iraq and Afghanistan. Am J Psychiatry. 2013;170(9):1003-1010.
62. Bisson JI, Roberts NP, Andrew M, et al. Psychological therapies for chronic post-traumatic stress disorder (PTSD) in adults. Cochrane Database Syst Rev. 2013;(12):CD003388.
63. Steenkamp MM, Litz BT, Hoge CW, et al. Psychotherapy for military-related PTSD: a review of randomized clinical trials. JAMA. 2015;314(5):489-500.
64. Hoskins M, Pearce J, Bethell A, et al. Pharmacotherapy for post-traumatic stress disorder: systematic review and meta-analysis. Br J Psychiatry. 2015;206(2):93-100.
65. Belkin MR, Schwartz TL. Alpha-2 receptor agonists for the treatment of posttraumatic stress disorder. Drugs Context. 2015;4:212286. doi: 10.7573/dic.212286.
66. Brady K, Pearlstein T, Asnis GM, et al. Efficacy and safety of sertraline treatment of posttraumatic stress disorder: a randomized controlled trial. JAMA. 2000;283(14):1837-1844.
67. Davidson JRT, Landerman LR, Farfel GM, et al. Characterizing the effects of sertraline in post-traumatic stress disorder. Psychol Med. 2002;32(4):661-670.
68. Hertzberg MA, Feldman ME, Beckham JC, et al. Lack of efficacy for fluoxetine in PTSD: a placebo controlled trial in combat veterans. Ann Clin Psychiatry. 2000;12(2):101-105.
69. Friedman MJ, Marmar CR, Baker DG, et al. Randomized, double-blind comparison of sertraline and placebo for posttraumatic stress disorder in a Department of Veterans Affairs setting. J Clin Psychiatry. 2007;68(5):711-720.
70. Mello MF, Costa MCP, Schoedl AF, et al. Aripiprazole in the treatment of posttraumatic stress disorder: an open-label trial. Rev Bras Psiquiatr. 2008;30(4):358-361.
71. Birnbaum S, Gobeske KT, Auerbach J, et al. A role for norepinephrine in stress-induced cognitive deficits: alpha-1-adrenoceptor mediation in the prefrontal cortex. Biol Psychiatry. 1999;46(9):1266-1274.
72. Ahmadpanah M, Sabzeiee P, Hosseini SM, et al. Comparing the effect of prazosin and hydroxyzine on sleep quality in patients suffering from posttraumatic stress disorder. Neuropsychobiology. 2014;69(4):235-242.
73. Villarreal G, Hamner MB, Cañive JM, et al. Efficacy of quetiapine monotherapy in posttraumatic stress disorder: a randomized, placebo-controlled trial. Am J Psychiatry. 2016;173(12):1205-1212.
1. Javidi H, Yadollahie M. Post-traumatic stress disorder. Int J Occup Environ Med. 2012;3(1):2-9.
2. Kessler RC, Chiu WT, Demler O, et al. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62(6):617-627.
3. Kessler RC, Sonnega A, Bromet E, et al. Posttraumatic stress disorder in the National Comorbidity Survey. Arch Gen Psychiatry. 1995;52(12):1048-1060.
4. Breslau N, Kessler RC, Chilcoat HD, et al. Trauma and posttraumatic stress disorder in the community: the 1996 Detroit Area Survey of Trauma. Arch Gen Psychiatry. 1998;55(7):626-632.
5. Cerda M, Sagdeo A, Johnson J, et al. Genetic and environmental influences on psychiatric comorbidity: a systematic review. J Affect Disord. 2010;126(1-2):14-38.
6. Yehuda R, Hoge CW, McFarlane AC, et al. Post-traumatic stress disorder. Nat Rev Dis Primers. 2015;1:15057. doi: 10.1038/nrdp.2015.57.
7. True WR, Rice J, Eisen SA, et al. A twin study of genetic and environmental contributions to liability for posttraumatic stress symptoms. Arch Gen Psychiatry. 1993;50(4):257-264.
8. Diagnostic and statistical manual of mental disorders. 5th ed. Washington, DC: American Psychiatric Association; 2013:271-280.
9. Reichmann F, Holzer P. Neuropeptide Y: a stressful review. Neuropeptides. 2016;55:99-109.
10. Yehuda R, Brand S, Yang RK. Plasma neuropeptide Y concentrations in combat exposed veterans: relationship to trauma exposure, recovery from PTSD, and coping. Biol Psychiatry. 2006;59(7):660-663.
11. Cohen H, Liu T, Kozlovsky N, et al. The neuropeptide Y (NPY)-ergic system is associated with behavioral resilience to stress exposure in an animal model of post-traumatic stress disorder. Neuropsychopharmacology. 2012;37(2):350-363.
12. Donner J, Sipilä T, Ripatti S, et al. Support for involvement of glutamate decarboxylase 1 and neuropeptide Y in anxiety susceptibility. Am J Med Genet B Neuropsychiatr Genet. 2012;159B(3):316-327.
13. Schmeltzer SN, Herman JP, Sah R. Neuropeptide Y (NPY) and posttraumatic stress disorder (PTSD): a translational update. Exp Neurol. 2016;284(pt B):196-210.
14. Kautz M, Charney DS, Murrough JW. Neuropeptide Y, resilience, and PTSD therapeutics. Neurosci Lett. 2017;649:164-169.
15. Serova LI, Laukova M, Alaluf LG, et al. Intranasal neuropeptide Y reverses anxiety and depressive-like behavior impaired by single prolonged stress PTSD model. Eur Neuropsychopharmacol. 2014;24(1):142-147.
16. Sayed S, Van Dam NT, Horn SR, et al. A randomized dose-ranging study of neuropeptide Y in patients with posttraumatic stress disorder. Int J Neuropsychopharmacol. 2018;21(1):3-11.
17. Toth M, Flandreau EI, Deslauriers J, et al. Overexpression of forebrain CRH during early life increases trauma susceptibility in adulthood. Neuropsychopharmacology. 2016;41(6):1681-1690.
18. White S, Acierno R, Ruggiero KJ, et al. Association of CRHR1 variants and posttraumatic stress symptoms in hurricane exposed adults. J Anxiety Disord. 2013;27(7):678-683.
19. Wolf EJ, Mitchell KS, Logue MW, et al. Corticotropin releasing hormone receptor 2 (CRHR-2) gene is associated with decreased risk and severity of posttraumatic stress disorder in women. Depress Anxiety. 2013;30(12):1161-1169.
20. Whitnall MH. Regulation of the hypothalamic corticotropin-releasing hormone neurosecretory system. Prog Neurobiol. 1993;40(5):573-629.
21. Bradley RG, Binder EB, Epstein MP, et al. Influence of child abuse on adult depression: moderation by the corticotropin-releasing hormone receptor gene. Arch Gen Psychiatry. 2008;65(2):190-200.
22. Tellew JE, Lanier M, Moorjani M, et al. Discovery of NBI-77860/GSK561679, a potent corticotropin-releasing factor (CRF1) receptor antagonist with improved pharmacokinetic properties. Bioorg Med Chem Lett. 2010;20(24):7259-7264.
23. Dunlop BW, Rothbaum BO, Binder EB, et al. Evaluation of a corticotropin releasing hormone type 1 receptor antagonist in women with posttraumatic stress disorder: study protocol for a randomized controlled trial. Trials. 2014;15:240. doi: 10.1186/1745-6215-15-240.
24. Jovanovic T, Duncan EJ, Kaye J, et al. Psychophysiological treatment outcomes: Corticotropin-releasing factor type 1 receptor antagonist increases inhibition of fear-potentiated startle in PTSD patients. Psychophysiology. 2019:e13356. doi: 10.1111/psyp.13356.
25. Dunlop BW, Binder EB, Iosifescu D, et al. Corticotropin-releasing factor receptor 1 antagonism is ineffective for women with posttraumatic stress disorder. Biol Psychiatry. 2017;82(12):866-874.
26. Pape JC, Carrillo-Roa T, Rothbaum BO, et al. DNA methylation levels are associated with CRF1 receptor antagonist treatment outcome in women with post-traumatic stress disorder. Clin Epigenetics. 2018;10(1):136. doi: 10.1186/s13148-018-0569-x.
27. Murrough JW, Charney DS. Corticotropin-releasing factor type 1 receptor antagonists for stress-related disorders: time to call it quits? Biol Psychiatry. 2017;82(12):858-860.
28. Leal G, Bramham CR, Duarte CB. BDNF and hippocampal synaptic plasticity. Vitam Horm. 2017;104:153-195.
29. Groves JO. Is it time to reassess the BDNF hypothesis of depression? Mol Psychiatry. 2007;12:1079-1088.
30. Frustaci A, Pozzi G, Gianfagna F, et al. Meta-analysis of the brain-derived neurotrophic factor gene (BDNF) Val66Met polymorphism in anxiety disorders and anxiety-related personality traits. Neuropsychobiology. 2008;58(3-4):163-170.
31. Gatt JM, Nemeroff CB, Dobson-Stone C, et al. Interactions between BDNF Val66Met polymorphism and early life stress predict brain and arousal pathways to syndromal depression and anxiety. Mol Psychiatry. 2009;14(7):681-695.
32. Ragen BJ, Seidel J, Chollak C, et al. Investigational drugs under development for the treatment of PTSD. Expert Opin Investig Drugs. 2015;24(5):659-672.
33. Caspi A, Sugden K, Moffitt TE, et al. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science. 2003;301(5631):386-389.
34. Murphy DL, Fox MA, Timpano KR, et al. How the serotonin story is being rewritten by new gene-based discoveries principally related to SLC6A4, the serotonin transporter gene, which functions to influence all cellular serotonin systems. Neuropharmacology. 2008;55(6):932-960.
35. Luddington NS, Mandadapu A, Husk M, et al. Clinical implications of genetic variation in the serotonin transporter promoter region: a review. Prim Care Compan J Clin Psychiatry. 2009;11:(3):93-102.
36. Lee HJ, Lee MS, Kang RH, et al. Influence of the serotonin transporter promoter gene polymorphism on susceptibility to posttraumatic stress disorder. Depress Anxiety. 2005;21(3):135-139.
37. Liu Y, Garrett ME, Dennis MF, et al. An examination of the association between 5-HTTLPR, combat exposure, and PTSD diagnosis among U.S. veterans. PLoS One. 2015;10(3):e0119998. doi: 10.1371/journal.pone.0119998.
38. Mehta D, Voisey J, Bruenig D, et al. Transcriptome analysis reveals novel genes and immune networks dysregulated in veterans with PTSD. Brain Behav Immun. 2018;74:133-142. doi: 10.1016/j.bbi.2018.08.014.
39. Kilpatrick DG, Koenen KC, Ruggiero KJ, et al. The serotonin transporter genotype and social support and moderation of posttraumatic stress disorder and depression in hurricane-exposed adults. Am J Psychiatry. 2007;164(11):1693-1699.
40. Kolassa IT, Ertl V, Eckart C, et al. Association study of trauma load and SLC6A4 promoter polymorphism in posttraumatic stress disorder: evidence from survivors of the Rwandan genocide. J Clin Psychiatry. 2010;71(5):543-547.
41. Bryant RA, Felmingham KL, Falconer EM, et al. Preliminary evidence of the short allele of the serotonin transporter gene predicting poor response to cognitive behavior therapy in posttraumatic stress disorder. Biol Psychiatry. 2010;67(12):1217-1219.
42. Mrazek DA, Rush AJ, Biernacka JM, et al. SLC6A4 variation and citalopram response. Am J Med Genet B Neuropsychiatr Genet. 2009;150B(3):341-351.
43. Shiroma PR, Drews MS, Geske JR, et al. SLC6A4 polymorphisms and age of onset in late-life depression on treatment outcomes with citalopram: a Sequenced Treatment Alternatives to Relieve Depression (STAR*D) report. Am J Geriatr Psychiatry. 2014;22(11):1140-1148.
44. Fanelli G, Serretti A. The influence of the serotonin transporter gene 5-HTTLPR polymorphism on suicidal behaviors: a meta-analysis. Prog Neuropsychopharmacol Biol Psychiatry. 2019;88:375-387.
45. Courtet P, Picot MC, Bellivier F, et al. Serotonin transporter gene may be involved in short-term risk of subsequent suicide attempts. Biol Psychiatry. 2003;55(1):46-51.
46. Outhred T, Das P, Dobson-Stone C, et al. The impact of 5-HTTLPR on acute serotonin transporter blockade by escitalopram on emotion processing: Preliminary findings from a randomised, crossover fMRI study. Aust NZ J Psychiatry. 2014;48(12):1115-1125.
47. Lachman HM, Papolos DF, Saito T, et al. Human catechol-O-methyltransferase pharmacogenetics: description of a functional polymorphism and its potential application to neuropsychiatric disorders. Pharmacogenetics. 1996;6(3):243-250.
48. Valente NL, Vallada H, Cordeiro Q, et al. Catechol-O-methyltransferase (COMT) val158met polymorphism as a risk factor for PTSD after urban violence. J Mol Neurosci. 2011;43(3):516-523.
49. van Rooij SJ, Stevens JS, Ely TD, et al. Childhood trauma and COMT genotype interact to increase hippocampal activation in resilient individuals. Front Psychiatry. 2016;7:156. doi: 10.3389/fpsyt.2016.00156.
50. Wu G, Feder A, Cohen H, et al. Understanding resilience. Front Behav Neuroscience. 2013;7:10. doi: 10.3389/fnbeh.2013.00010.
51. Kolassa I, Kolassa S, Ertl V, et al. The risk of posttraumatic stress disorder after trauma depends on traumatic load and the catechol-O-methyltransferase Val(158)Met polymorphism. Biol Psychiatry. 2010;67(4):304-308.
52. Bruder GE, Keilp JG, Xu H, et al. Catechol-O-methyltransferase (COMT) genotypes and working memory: associations with differing cognitive operations. Biol Psychiatry. 2005;58(11):901-907.
53. Strawn JR, Geracioti TD Jr. Noradrenergic dysfunction and the psychopharmacology of posttraumatic stress disorder. Depress Anxiety. 2008;25(3):260-271.
54. Hendrickson RC, Raskind MA. Noradrenergic dysregulation in the pathophysiology of PTSD. Exp Neurol. 2016;284(pt B):181-195.
55. Neylan TC, Marmar CR, Metzler TJ, et al. Sleep disturbances in the Vietnam generation: findings from a nationally representative sample of male Vietnam veterans. Am J Psychiatry. 1998;155(7):929-933.
56. Roehrs TA, Roth T. Hyperarousal in insomnia and hypnotic dose escalation. Sleep Med. 2016;23:16-20.
57. Geracioti TD Jr, Baker DG, Ekhator NN, et al. CSF Norepinephrine concentrations in posttraumatic stress disorder. Am J Psychiatry. 2001;158(8):1227-1230.
58. Yehuda R, Siever LJ, Teicher MH, et al. Plasma norepinephrine and 3-methoxy-4-hydroxyphenylglycol concentrations and severity of depression in combat posttraumatic stress disorder and major depressive disorder. Biol Psychiatry. 1998;44(1):56-63.
59. Molinoff PB. Alpha- and beta-adrenergic receptor subtypes properties, distribution and regulation. Drugs. 1984;28(suppl 2):1-15.
60. El-Mallakh RS. The use of beta-blockers in psychiatry. Res Staff Phys. 1989;35:49-52,59,62.
61. Raskind MA, Peterson K, Williams T, et al. A trial of prazosin for combat trauma PTSD with nightmares in active-duty soldiers returned from Iraq and Afghanistan. Am J Psychiatry. 2013;170(9):1003-1010.
62. Bisson JI, Roberts NP, Andrew M, et al. Psychological therapies for chronic post-traumatic stress disorder (PTSD) in adults. Cochrane Database Syst Rev. 2013;(12):CD003388.
63. Steenkamp MM, Litz BT, Hoge CW, et al. Psychotherapy for military-related PTSD: a review of randomized clinical trials. JAMA. 2015;314(5):489-500.
64. Hoskins M, Pearce J, Bethell A, et al. Pharmacotherapy for post-traumatic stress disorder: systematic review and meta-analysis. Br J Psychiatry. 2015;206(2):93-100.
65. Belkin MR, Schwartz TL. Alpha-2 receptor agonists for the treatment of posttraumatic stress disorder. Drugs Context. 2015;4:212286. doi: 10.7573/dic.212286.
66. Brady K, Pearlstein T, Asnis GM, et al. Efficacy and safety of sertraline treatment of posttraumatic stress disorder: a randomized controlled trial. JAMA. 2000;283(14):1837-1844.
67. Davidson JRT, Landerman LR, Farfel GM, et al. Characterizing the effects of sertraline in post-traumatic stress disorder. Psychol Med. 2002;32(4):661-670.
68. Hertzberg MA, Feldman ME, Beckham JC, et al. Lack of efficacy for fluoxetine in PTSD: a placebo controlled trial in combat veterans. Ann Clin Psychiatry. 2000;12(2):101-105.
69. Friedman MJ, Marmar CR, Baker DG, et al. Randomized, double-blind comparison of sertraline and placebo for posttraumatic stress disorder in a Department of Veterans Affairs setting. J Clin Psychiatry. 2007;68(5):711-720.
70. Mello MF, Costa MCP, Schoedl AF, et al. Aripiprazole in the treatment of posttraumatic stress disorder: an open-label trial. Rev Bras Psiquiatr. 2008;30(4):358-361.
71. Birnbaum S, Gobeske KT, Auerbach J, et al. A role for norepinephrine in stress-induced cognitive deficits: alpha-1-adrenoceptor mediation in the prefrontal cortex. Biol Psychiatry. 1999;46(9):1266-1274.
72. Ahmadpanah M, Sabzeiee P, Hosseini SM, et al. Comparing the effect of prazosin and hydroxyzine on sleep quality in patients suffering from posttraumatic stress disorder. Neuropsychobiology. 2014;69(4):235-242.
73. Villarreal G, Hamner MB, Cañive JM, et al. Efficacy of quetiapine monotherapy in posttraumatic stress disorder: a randomized, placebo-controlled trial. Am J Psychiatry. 2016;173(12):1205-1212.
Receptor occupancy and drug response: Understanding the relationship
Most clinicians do not think about receptor occupancy when they prescribe a medication. Most simply assume that if they use a low dose of a medication, they will get a small effect, and if they use a higher dose, they will get a larger effect. However, this is frequently not accurate. Clinicians need to understand the relationship between receptor occupancy and drug response.
In general, when an antagonist of a neurotransmitter receptor is used, it must occupy a minimum of 65% to 70% of the target receptor to be effective. This is clearly the case when the target is a postsynaptic receptor, such as the dopamine D2 receptor.1-3 Similarly, despite significant variability in antidepressant response,4 blockade of 65% to 80% of presynaptic transport proteins—such as the serotonin reuptake pumps when considering serotoninergic antidepressants,5,6 or the norepinephrine reuptake pumps when considering noradrenergic agents such as nortriptyline7—is necessary for these medications to be effective.
It is reasonable to think of the drug response of such agents as following a “threshold” model (Figure 1). This model makes 2 predictions. The first prediction is that a low dose of the drug might result in <50% receptor occupancy, but is not associated with a smaller response; it is simply ineffective. The second prediction is that a very high dose of the drug (eg, one that may exceed 90% receptor occupancy) does not result in any additional benefit, but may cause additional adverse consequences.8
Alternatively, agonist medications, such as benzodiazepines or opiates, have their efficacy in a continuous dose-dependent fashion (Figure 2). Titrating these medications for clinical response is necessary, and minimal effective doses are highly individual. Agonist medications will not be addressed further in this article.
In this article, the term “response” is used to denote the average (population) symptom change in a study population. This term is not used as clinicians often use it to mean that their specific patient’s illness has improved, or that the patient has gone into remission. Furthermore, the information described in this article does not optimize clinical outcome, but instead is intended to help clinicians optimize the use of their pharmacologic tools.
Minimal effective dose
Medications that have a threshold for activity will display that clinically in a minimal effective dose (Table 13,9 and Table 25). The minimal effective dose of medications that act by blocking a neurotransmitter receptor is usually the dose that achieves 65% to 80% receptor occupancy in typical individuals (Table 25). The minimal effective doses for antipsychotics are listed in Table 1.3,9 These doses are known to occupy approximately 65% to 70% of postsynaptic D2 receptors in living humans as confirmed by positron emission tomography (PET) scans.10 Similar minimal effective doses can be determined for serotonin-reuptake inhibiting (SRI) antidepressants (Table 25). In placebo-controlled trials, doses that were smaller than the minimal effective dose did not provide any benefit.
There are important caveats to this. First is the use of partial agonists. Depending on the level of intrinsic activity of a partial agonist and clinical goal, the clinician may aim for a different level of receptor occupancy. For example, aripiprazole will act as a dopamine agonist at lower concentrations, but blocks the receptor at higher concentrations.11 Unlike antagonist antipsychotics, which require only 65% to 70% D2 receptor occupancy to be effective, aripiprazole receptor binding at effective antipsychotic doses is 90% to 95%.12-14 Since aripiprazole has an intrinsic activity of approximately 30% (ie, when it binds, it stimulates the D2 receptor to about 30% of the effect of dopamine binding to the receptor15), binding to 90% of the receptors, and displacing endogenous dopamine, allows aripiprazole to replace the background or tonic tone of dopamine, which has been measured at 19% in people with schizophrenia and 9% in controls.16 Clinically, this still appears as the minimal effective dose achieving maximal response17-19 without significant parkinsonism despite >90% receptor occupancy.12
Continue to: The second caveat is...
The second caveat is the action of low D2 receptor affinity antipsychotics, such as clozapine and quetiapine. These agents generally achieve adequate D2 receptor occupancy for only a brief period of time.20 It has been suggested that continuous receptor occupancy at ≥65% may not be necessary to obtain antipsychotic control.21,22 There may also be specific limbic and cortical (vs striatal) D2 receptor selectivity by clozapine23 compared with other second-generation antipsychotics such as risperidone and olanzapine,24,25 although this point remains debatable.26 Furthermore, the antipsychotic efficacy of low D2 receptor affinity drugs is unreliable, even in controlled, blinded studies (eg, a failed large quetiapine study27). Thus far, the actual antipsychotic mechanism of these agents is yet to be fully understood.
Minimal effective dose achieves maximal response
An interesting aspect of the threshold phenomenon of drug response is that once the minimal effective dose is reached, maximal response is achieved. In other words, there is no additional efficacy with additional dose increases. This is readily demonstrated in some studies in which patients were randomly assigned to different fixed doses or dose ranges. In these studies, there was generally no difference in response rates of different doses, so that once 65% to 80% receptor occupancy is achieved, minimal and maximal clinical response is simultaneously reached.18,28,29
For example, in the original risperidone studies, 6 mg/d was essentially equivalent to 16 mg/d.28 Similarly, lurasidone, 40 mg/d, achieves approximately 65% D2 occupancy.30 When the daily dose is increased to 120 mg, there is no additional benefit in controlling psychosis in schizophrenia.29 This pattern is also seen in partial agonists, where there are no differences between lower and higher doses in terms of response.18
Upon reading this, many clinicians may think “I don’t care what the studies say, I have seen additional benefits with additional doses.” There are several explanations for this. One is that individual patients have genetic variants that may prevent them from responding in a typical fashion. Hints of this are seen in an apparent disconnect between dosage and drug levels, so that it is not surprising that drug levels are a much better predictor of receptor occupancy than dosage.31 Nonetheless, as previously pointed out, for a population, dosage does predict receptor occupancy and outcome. However, for individuals, genetic variations make dosages less reliable. For example, ultrarapid metabolizers of cytochrome P450 (CYP) 2D6 may discontinue risperidone due to nonresponse, or require a higher dose or longer time period to respond.32,33 Similarly, patients who smoke may require an increase in doses of CYP1A2 substrates such as clozapine and olanzapine.34
Alternatively, the clinician may note improvement in mood, sleep, appetite, or other symptoms at lower doses, and then note additional improvements in psychosis or mania at higher doses.3 This occurs due to the varying affinity of different receptors. For example, in bipolar depression trials that used quetiapine in a fixed-dose design, patients who received 300 or 600 mg/d responded in the same fashion, with no additional benefit in improving depression with the higher dose.35 Similarly, in a flexible dose range study that evaluated lurasidone in bipolar depression, an average dose of 34 mg/d (range 20 to 60 mg/d) and an average dose of 83 mg/d (range 80 to 120 mg/d) both resulted in the same response (a 15.4-point reduction in depression ratings and an effect size of 0.51).36 For both quetiapine and lurasidone, higher doses are generally required to control psychosis.29,37 Note that for lurasidone, agitation, but not psychosis, improves with higher doses, which suggests that recruitment of additional receptors results in improvement in a different set of symptoms.9
Continue to: Clinical implications
Clinical implications
The implications for clinicians are relatively clear. Knowing the minimal effective doses for depression, psychosis, or mania informs the target dose. If improvement is seen at lower doses, the clinician needs to assess the profile of symptoms that improved, potential drug–drug interactions, or potential irregularities in the patient’s metabolic pathways. Clinicians need to increase doses above the minimally effective dose carefully, and expend additional effort in analyzing changes in their patient’s symptoms and adverse effects; this analysis should be performed with skepticism and willingness to reduce a dosage if no additional benefit is seen. Attention to these receptor-symptom interactions will improve response and reduce adverse consequences in the majority of patients.
Related Resource
- Lako IM, van den Heuvel ER, Knegtering H, et al. Estimating dopamine D2 receptor occupancy for doses of 8 antipsychotics: a meta-analysis. J Clin Psychopharmacol. 2013;33(5):675-681.
Drug Brand Names
Aripiprazole • Abilify
Asenapine • Saphris
Citalopram • Celexa
Clozapine • Clozaril
Fluoxetine • Prozac
Haloperidol • Haldol
Iloperidone • Fanapt
Lurasidone • Latuda
Nortriptyline • Pamelor
Olanzapine • Zyprexa
Paroxetine • Paxil
Quetiapine • Seroquel
Risperidone • Risperdal
Sertraline • Zoloft
Venlafaxine • Effexor
Ziprasidone • Geodon
1. Farde L, Nordström AL, Wiesel FA, et al. Positron emission tomographic analysis of central D1 and D2 dopamine receptor occupancy in patients treated with classical neuroleptics and clozapine. Relation to extrapyramidal side effects. Arch Gen Psychiatry. 1992;49(7):538-544.
2. Kapur S, Zipursky R, Jones C, et al. Relationship between dopamine D(2) occupancy, clinical response, and side effects: a double-blind PET study of first-episode schizophrenia. Am J Psychiatry. 2000;157(4):514-520.
3. Roberts RJ, Lohano KK, El-Mallakh RS. Antipsychotics as antidepressants. Asia Pacific Psychiatry. 2016;8(3):179-188.
4. Quitkin FM, Rabkin JG, Gerald J, et al. Validity of clinical trials of antidepressants. Am J Psychiatry. 2000;157(3):327-337.
5. Meyer JH, Wilson AA, Sagrati S, et al. Serotonin transporter occupancy of five selective serotonin reuptake inhibitors at different doses: an [11C]DASB positron emission tomography study. Am J Psychiatry. 2004;161(5):826-835.
6. Lundberg J, Tiger M, Landén M, et al. Serotonin transporter occupancy with TCAs and SSRIs: a PET study in patients with major depressive disorder. Int J Neuropsychopharmacol. 2012;15(8):1167-1172.
7. Takano H, Arakawa R, Nogami T, et al. Norepinephrine transporter occupancy by nortriptyline in patients with depression: a positron emission tomography study with (S,S)-[¹8F]FMeNER-D2. Int J Neuropsychopharmacol. 2014;17(4):553-560.
8. Johnson M, Kozielska M, Pilla Reddy V, et al. Dopamine D2 receptor occupancy as a predictor of catalepsy in rats: a pharmacokinetic-pharmacodynamic modeling approach. Pharm Res. 2014;31(10):2605-2617.
9. Allen MH, Citrome L, Pikalov A, et al. Efficacy of lurasidone in the treatment of agitation: a post hoc analysis of five short-term studies in acutely ill patients with schizophrenia. Gen Hosp Psychiatry. 2017;47:75-82.
10. Sekine M, Maeda J, Shimada H, et al. Central nervous system drug evaluation using positron emission tomography. Clin Psychopharmacol Neurosci. 2011;9(1):9-16.
11. Ma GF, Raivio N, Sabrià J, et al. Agonist and antagonist effects of aripiprazole on D2-like receptors controlling rat brain dopamine synthesis depend on the dopaminergic tone. Int J Neuropsychopharmacol. 2014;18(4):pii: pyu046. doi: 10.1093/ijnp/pyu046.
12. Yokoi F, Gründer G, Biziere K, et al. Dopamine D2 and D3 receptor occupancy in normal humans treated with the antipsychotic drug aripiprazole (OPC 14597): a study using positron emission tomography and [11C]raclopride. Neuropsychopharmacology. 2002;27(2):248-259.
13. Gründer G, Carlsson A, Wong DF. Mechanism of new antipsychotic medications: occupancy is not just antagonism. Arch Gen Psychiatry. 2003;60(10):974-977.
14. Mamo D, Graff A, Mizrahi R, et al. Differential effects of aripiprazole on D(2), 5-HT(2), and 5-HT(1A)receptor occupancy in patients with schizophrenia: a triple tracer PET study. Am J Psychiatry. 2007;164(9):1411-1417.
15. Weiden PJ, Preskorn SH, Fahnestock PA, et al. Translating the psychopharmacology of antipsychotics to individualized treatment for severe mental illness: a roadmap. J Clin Psychiatry. 2007;68(suppl 7):1-48.
16. Abi-Dargham A, Rodenhiser J, Printz D, et al. Increased baseline occupancy of D2 receptors by dopamine in schizophrenia. Proc Natl Acad Sci U S A. 2000;97(14):8104-8109.
17. Kane JM, Carson WH, Saha AR, et al. Efficacy and safety of aripiprazole and haloperidol versus placebo in patients with schizophrenia and schizoaffective disorder. J Clin Psychiatry. 2002;63(9):763-771.
18. Potkin SG, Saha AR, Kujawa MJ, et al. Aripiprazole, an antipsychotic with a novel mechanism of action, and risperidone vs placebo in patients with schizophrenia and schizoaffective disorder. Arch Gen Psychiatry. 2003;60(7):681-690.
19. Cutler AJ, Marcus RN, Hardy SA, et al. The efficacy and safety of lower doses of aripiprazole for the treatment of patients with acute exacerbation of schizophrenia. CNS Spectr. 2006;11(9):691-702; quiz 719.
20. Gründer G, Landvogt C, Vernaleken I, et al. The striatal and extrastriatal D2/D3 receptor-binding profile of clozapine in patients with schizophrenia. Neuropsychopharmacology. 2006;31(5):1027-1035.
21. Mizuno Y, Bies RR, Remington G, et al. Dopamine D2 receptor occupancy with risperidone or olanzapine during maintenance treatment of schizophrenia: a cross-sectional study. Prog Neuropsychopharmacol Biol Psychiatry. 2012;37(1):182-187.
22. Moriguchi S, Bies RR, Remington G, et al. Estimated dopamine D2 receptor occupancy and remission in schizophrenia: analysis of the CATIE data. J Clin Psychopharmacol. 2013;33(5):682-685.
23. Pilowsky LS, Mulligan RS, Acton PD, et al. Limbic selectivity of clozapine. Lancet. 1997;350(9076):490-491.
24. Ito H, Arakawa R, Takahashi H, et al. No regional difference in dopamine D2 receptor occupancy by the second-generation antipsychotic drug risperidone in humans: a positron emission tomography study. Int J Neuropsychopharmacol. 2009;12(5):667-675.
25. Arakawa R, Ito H, Okumura M, et al. Extrastriatal dopamine D(2) receptor occupancy in olanzapine-treated patients with schizophrenia. Eur Arch Psychiatry Clin Neurosci. 2010;260(4):345-350.
26. Xiberas X, Martinot JL, Mallet L, et al. Extrastriatal and striatal D(2) dopamine receptor blockade with haloperidol or new antipsychotic drugs in patients with schizophrenia. Br J Psychiatry. 2001;179:503-508.
27. Cutler AJ, Tran-Johnson T, Kalali A, et al. A failed 6-week, randomized, double-blind, placebo-controlled study of once-daily extended release quetiapine fumarate in patients with acute schizophrenia: lessons learned. Psychopharmacol Bull. 2010;43(4):37-69.
28. Marder SR, Meibach RC. Risperidone in the treatment of schizophrenia. Am J Psychiatry. 1994;151(6):825-835.
29. Meltzer HY, Cucchiaro J, Silva R, et al. Lurasidone in the treatment of schizophrenia: a randomized, double-blind, placebo- and olanzapine-controlled study. Am J Psychiatry. 2011;168(9):957-967.
30. Wong DF, Kuwabara H, Brašic JR, et al. Determination of dopamine D2 receptor occupancy by lurasidone using positron emission tomography in healthy male subjects. Psychopharmacology (Berl). 2013;229(2):245-252.
31. Potkin SG, Keator DB, Kesler-West ML, et al. D2 receptor occupancy following lurasidone treatment in patients with schizophrenia or schizoaffective disorder. CNS Spectr. 2014;19(2):176-181.
32. de Leon J, Susce MT, Pan RM, et al. The CYP2D6 poor metabolizer phenotype may be associated with risperidone adverse drug reactions and discontinuation. J Clin Psychiatry. 2005;66(1):15-27.
33. de Leon J, Susce MT, Pan RM, et al. A study of genetic (CYP2D6 and ABCB1) and environmental (drug inhibitors and inducers) variables that may influence plasma risperidone levels. Pharmacopsychiatry. 2007;40(3):93-102.
34. Narahari A, El-Mallakh RS, Kolikonda MK, et al. How coffee and cigarettes can affect the response to psychopharmacotherapy. Current Psychiatry. 2015;14(10):79-80.
35. Calabrese JR, Keck PE Jr, Macfadden W, et al. A randomized, double-blind, placebo-controlled trial of quetiapine in the treatment of bipolar I or II depression. Am J Psychiatry. 2005;162(7):1351-1360.
36. Loebel A, Cucchiaro J, Silva R, et al. Lurasidone monotherapy in the treatment of bipolar I depression: a randomized, double-blind, placebo-controlled study. Am J Psychiatry. 2014;171(2):160-168.
37. Lindenmayer JP, Brown D, Liu S, et al. The efficacy and tolerability of once-daily extended release quetiapine fumarate in hospitalized patients with acute schizophrenia: a 6-week randomized, double-blind, placebo-controlled study. Psychopharmacol Bull. 2008;41(3):11-35.
Most clinicians do not think about receptor occupancy when they prescribe a medication. Most simply assume that if they use a low dose of a medication, they will get a small effect, and if they use a higher dose, they will get a larger effect. However, this is frequently not accurate. Clinicians need to understand the relationship between receptor occupancy and drug response.
In general, when an antagonist of a neurotransmitter receptor is used, it must occupy a minimum of 65% to 70% of the target receptor to be effective. This is clearly the case when the target is a postsynaptic receptor, such as the dopamine D2 receptor.1-3 Similarly, despite significant variability in antidepressant response,4 blockade of 65% to 80% of presynaptic transport proteins—such as the serotonin reuptake pumps when considering serotoninergic antidepressants,5,6 or the norepinephrine reuptake pumps when considering noradrenergic agents such as nortriptyline7—is necessary for these medications to be effective.
It is reasonable to think of the drug response of such agents as following a “threshold” model (Figure 1). This model makes 2 predictions. The first prediction is that a low dose of the drug might result in <50% receptor occupancy, but is not associated with a smaller response; it is simply ineffective. The second prediction is that a very high dose of the drug (eg, one that may exceed 90% receptor occupancy) does not result in any additional benefit, but may cause additional adverse consequences.8
Alternatively, agonist medications, such as benzodiazepines or opiates, have their efficacy in a continuous dose-dependent fashion (Figure 2). Titrating these medications for clinical response is necessary, and minimal effective doses are highly individual. Agonist medications will not be addressed further in this article.
In this article, the term “response” is used to denote the average (population) symptom change in a study population. This term is not used as clinicians often use it to mean that their specific patient’s illness has improved, or that the patient has gone into remission. Furthermore, the information described in this article does not optimize clinical outcome, but instead is intended to help clinicians optimize the use of their pharmacologic tools.
Minimal effective dose
Medications that have a threshold for activity will display that clinically in a minimal effective dose (Table 13,9 and Table 25). The minimal effective dose of medications that act by blocking a neurotransmitter receptor is usually the dose that achieves 65% to 80% receptor occupancy in typical individuals (Table 25). The minimal effective doses for antipsychotics are listed in Table 1.3,9 These doses are known to occupy approximately 65% to 70% of postsynaptic D2 receptors in living humans as confirmed by positron emission tomography (PET) scans.10 Similar minimal effective doses can be determined for serotonin-reuptake inhibiting (SRI) antidepressants (Table 25). In placebo-controlled trials, doses that were smaller than the minimal effective dose did not provide any benefit.
There are important caveats to this. First is the use of partial agonists. Depending on the level of intrinsic activity of a partial agonist and clinical goal, the clinician may aim for a different level of receptor occupancy. For example, aripiprazole will act as a dopamine agonist at lower concentrations, but blocks the receptor at higher concentrations.11 Unlike antagonist antipsychotics, which require only 65% to 70% D2 receptor occupancy to be effective, aripiprazole receptor binding at effective antipsychotic doses is 90% to 95%.12-14 Since aripiprazole has an intrinsic activity of approximately 30% (ie, when it binds, it stimulates the D2 receptor to about 30% of the effect of dopamine binding to the receptor15), binding to 90% of the receptors, and displacing endogenous dopamine, allows aripiprazole to replace the background or tonic tone of dopamine, which has been measured at 19% in people with schizophrenia and 9% in controls.16 Clinically, this still appears as the minimal effective dose achieving maximal response17-19 without significant parkinsonism despite >90% receptor occupancy.12
Continue to: The second caveat is...
The second caveat is the action of low D2 receptor affinity antipsychotics, such as clozapine and quetiapine. These agents generally achieve adequate D2 receptor occupancy for only a brief period of time.20 It has been suggested that continuous receptor occupancy at ≥65% may not be necessary to obtain antipsychotic control.21,22 There may also be specific limbic and cortical (vs striatal) D2 receptor selectivity by clozapine23 compared with other second-generation antipsychotics such as risperidone and olanzapine,24,25 although this point remains debatable.26 Furthermore, the antipsychotic efficacy of low D2 receptor affinity drugs is unreliable, even in controlled, blinded studies (eg, a failed large quetiapine study27). Thus far, the actual antipsychotic mechanism of these agents is yet to be fully understood.
Minimal effective dose achieves maximal response
An interesting aspect of the threshold phenomenon of drug response is that once the minimal effective dose is reached, maximal response is achieved. In other words, there is no additional efficacy with additional dose increases. This is readily demonstrated in some studies in which patients were randomly assigned to different fixed doses or dose ranges. In these studies, there was generally no difference in response rates of different doses, so that once 65% to 80% receptor occupancy is achieved, minimal and maximal clinical response is simultaneously reached.18,28,29
For example, in the original risperidone studies, 6 mg/d was essentially equivalent to 16 mg/d.28 Similarly, lurasidone, 40 mg/d, achieves approximately 65% D2 occupancy.30 When the daily dose is increased to 120 mg, there is no additional benefit in controlling psychosis in schizophrenia.29 This pattern is also seen in partial agonists, where there are no differences between lower and higher doses in terms of response.18
Upon reading this, many clinicians may think “I don’t care what the studies say, I have seen additional benefits with additional doses.” There are several explanations for this. One is that individual patients have genetic variants that may prevent them from responding in a typical fashion. Hints of this are seen in an apparent disconnect between dosage and drug levels, so that it is not surprising that drug levels are a much better predictor of receptor occupancy than dosage.31 Nonetheless, as previously pointed out, for a population, dosage does predict receptor occupancy and outcome. However, for individuals, genetic variations make dosages less reliable. For example, ultrarapid metabolizers of cytochrome P450 (CYP) 2D6 may discontinue risperidone due to nonresponse, or require a higher dose or longer time period to respond.32,33 Similarly, patients who smoke may require an increase in doses of CYP1A2 substrates such as clozapine and olanzapine.34
Alternatively, the clinician may note improvement in mood, sleep, appetite, or other symptoms at lower doses, and then note additional improvements in psychosis or mania at higher doses.3 This occurs due to the varying affinity of different receptors. For example, in bipolar depression trials that used quetiapine in a fixed-dose design, patients who received 300 or 600 mg/d responded in the same fashion, with no additional benefit in improving depression with the higher dose.35 Similarly, in a flexible dose range study that evaluated lurasidone in bipolar depression, an average dose of 34 mg/d (range 20 to 60 mg/d) and an average dose of 83 mg/d (range 80 to 120 mg/d) both resulted in the same response (a 15.4-point reduction in depression ratings and an effect size of 0.51).36 For both quetiapine and lurasidone, higher doses are generally required to control psychosis.29,37 Note that for lurasidone, agitation, but not psychosis, improves with higher doses, which suggests that recruitment of additional receptors results in improvement in a different set of symptoms.9
Continue to: Clinical implications
Clinical implications
The implications for clinicians are relatively clear. Knowing the minimal effective doses for depression, psychosis, or mania informs the target dose. If improvement is seen at lower doses, the clinician needs to assess the profile of symptoms that improved, potential drug–drug interactions, or potential irregularities in the patient’s metabolic pathways. Clinicians need to increase doses above the minimally effective dose carefully, and expend additional effort in analyzing changes in their patient’s symptoms and adverse effects; this analysis should be performed with skepticism and willingness to reduce a dosage if no additional benefit is seen. Attention to these receptor-symptom interactions will improve response and reduce adverse consequences in the majority of patients.
Related Resource
- Lako IM, van den Heuvel ER, Knegtering H, et al. Estimating dopamine D2 receptor occupancy for doses of 8 antipsychotics: a meta-analysis. J Clin Psychopharmacol. 2013;33(5):675-681.
Drug Brand Names
Aripiprazole • Abilify
Asenapine • Saphris
Citalopram • Celexa
Clozapine • Clozaril
Fluoxetine • Prozac
Haloperidol • Haldol
Iloperidone • Fanapt
Lurasidone • Latuda
Nortriptyline • Pamelor
Olanzapine • Zyprexa
Paroxetine • Paxil
Quetiapine • Seroquel
Risperidone • Risperdal
Sertraline • Zoloft
Venlafaxine • Effexor
Ziprasidone • Geodon
Most clinicians do not think about receptor occupancy when they prescribe a medication. Most simply assume that if they use a low dose of a medication, they will get a small effect, and if they use a higher dose, they will get a larger effect. However, this is frequently not accurate. Clinicians need to understand the relationship between receptor occupancy and drug response.
In general, when an antagonist of a neurotransmitter receptor is used, it must occupy a minimum of 65% to 70% of the target receptor to be effective. This is clearly the case when the target is a postsynaptic receptor, such as the dopamine D2 receptor.1-3 Similarly, despite significant variability in antidepressant response,4 blockade of 65% to 80% of presynaptic transport proteins—such as the serotonin reuptake pumps when considering serotoninergic antidepressants,5,6 or the norepinephrine reuptake pumps when considering noradrenergic agents such as nortriptyline7—is necessary for these medications to be effective.
It is reasonable to think of the drug response of such agents as following a “threshold” model (Figure 1). This model makes 2 predictions. The first prediction is that a low dose of the drug might result in <50% receptor occupancy, but is not associated with a smaller response; it is simply ineffective. The second prediction is that a very high dose of the drug (eg, one that may exceed 90% receptor occupancy) does not result in any additional benefit, but may cause additional adverse consequences.8
Alternatively, agonist medications, such as benzodiazepines or opiates, have their efficacy in a continuous dose-dependent fashion (Figure 2). Titrating these medications for clinical response is necessary, and minimal effective doses are highly individual. Agonist medications will not be addressed further in this article.
In this article, the term “response” is used to denote the average (population) symptom change in a study population. This term is not used as clinicians often use it to mean that their specific patient’s illness has improved, or that the patient has gone into remission. Furthermore, the information described in this article does not optimize clinical outcome, but instead is intended to help clinicians optimize the use of their pharmacologic tools.
Minimal effective dose
Medications that have a threshold for activity will display that clinically in a minimal effective dose (Table 13,9 and Table 25). The minimal effective dose of medications that act by blocking a neurotransmitter receptor is usually the dose that achieves 65% to 80% receptor occupancy in typical individuals (Table 25). The minimal effective doses for antipsychotics are listed in Table 1.3,9 These doses are known to occupy approximately 65% to 70% of postsynaptic D2 receptors in living humans as confirmed by positron emission tomography (PET) scans.10 Similar minimal effective doses can be determined for serotonin-reuptake inhibiting (SRI) antidepressants (Table 25). In placebo-controlled trials, doses that were smaller than the minimal effective dose did not provide any benefit.
There are important caveats to this. First is the use of partial agonists. Depending on the level of intrinsic activity of a partial agonist and clinical goal, the clinician may aim for a different level of receptor occupancy. For example, aripiprazole will act as a dopamine agonist at lower concentrations, but blocks the receptor at higher concentrations.11 Unlike antagonist antipsychotics, which require only 65% to 70% D2 receptor occupancy to be effective, aripiprazole receptor binding at effective antipsychotic doses is 90% to 95%.12-14 Since aripiprazole has an intrinsic activity of approximately 30% (ie, when it binds, it stimulates the D2 receptor to about 30% of the effect of dopamine binding to the receptor15), binding to 90% of the receptors, and displacing endogenous dopamine, allows aripiprazole to replace the background or tonic tone of dopamine, which has been measured at 19% in people with schizophrenia and 9% in controls.16 Clinically, this still appears as the minimal effective dose achieving maximal response17-19 without significant parkinsonism despite >90% receptor occupancy.12
Continue to: The second caveat is...
The second caveat is the action of low D2 receptor affinity antipsychotics, such as clozapine and quetiapine. These agents generally achieve adequate D2 receptor occupancy for only a brief period of time.20 It has been suggested that continuous receptor occupancy at ≥65% may not be necessary to obtain antipsychotic control.21,22 There may also be specific limbic and cortical (vs striatal) D2 receptor selectivity by clozapine23 compared with other second-generation antipsychotics such as risperidone and olanzapine,24,25 although this point remains debatable.26 Furthermore, the antipsychotic efficacy of low D2 receptor affinity drugs is unreliable, even in controlled, blinded studies (eg, a failed large quetiapine study27). Thus far, the actual antipsychotic mechanism of these agents is yet to be fully understood.
Minimal effective dose achieves maximal response
An interesting aspect of the threshold phenomenon of drug response is that once the minimal effective dose is reached, maximal response is achieved. In other words, there is no additional efficacy with additional dose increases. This is readily demonstrated in some studies in which patients were randomly assigned to different fixed doses or dose ranges. In these studies, there was generally no difference in response rates of different doses, so that once 65% to 80% receptor occupancy is achieved, minimal and maximal clinical response is simultaneously reached.18,28,29
For example, in the original risperidone studies, 6 mg/d was essentially equivalent to 16 mg/d.28 Similarly, lurasidone, 40 mg/d, achieves approximately 65% D2 occupancy.30 When the daily dose is increased to 120 mg, there is no additional benefit in controlling psychosis in schizophrenia.29 This pattern is also seen in partial agonists, where there are no differences between lower and higher doses in terms of response.18
Upon reading this, many clinicians may think “I don’t care what the studies say, I have seen additional benefits with additional doses.” There are several explanations for this. One is that individual patients have genetic variants that may prevent them from responding in a typical fashion. Hints of this are seen in an apparent disconnect between dosage and drug levels, so that it is not surprising that drug levels are a much better predictor of receptor occupancy than dosage.31 Nonetheless, as previously pointed out, for a population, dosage does predict receptor occupancy and outcome. However, for individuals, genetic variations make dosages less reliable. For example, ultrarapid metabolizers of cytochrome P450 (CYP) 2D6 may discontinue risperidone due to nonresponse, or require a higher dose or longer time period to respond.32,33 Similarly, patients who smoke may require an increase in doses of CYP1A2 substrates such as clozapine and olanzapine.34
Alternatively, the clinician may note improvement in mood, sleep, appetite, or other symptoms at lower doses, and then note additional improvements in psychosis or mania at higher doses.3 This occurs due to the varying affinity of different receptors. For example, in bipolar depression trials that used quetiapine in a fixed-dose design, patients who received 300 or 600 mg/d responded in the same fashion, with no additional benefit in improving depression with the higher dose.35 Similarly, in a flexible dose range study that evaluated lurasidone in bipolar depression, an average dose of 34 mg/d (range 20 to 60 mg/d) and an average dose of 83 mg/d (range 80 to 120 mg/d) both resulted in the same response (a 15.4-point reduction in depression ratings and an effect size of 0.51).36 For both quetiapine and lurasidone, higher doses are generally required to control psychosis.29,37 Note that for lurasidone, agitation, but not psychosis, improves with higher doses, which suggests that recruitment of additional receptors results in improvement in a different set of symptoms.9
Continue to: Clinical implications
Clinical implications
The implications for clinicians are relatively clear. Knowing the minimal effective doses for depression, psychosis, or mania informs the target dose. If improvement is seen at lower doses, the clinician needs to assess the profile of symptoms that improved, potential drug–drug interactions, or potential irregularities in the patient’s metabolic pathways. Clinicians need to increase doses above the minimally effective dose carefully, and expend additional effort in analyzing changes in their patient’s symptoms and adverse effects; this analysis should be performed with skepticism and willingness to reduce a dosage if no additional benefit is seen. Attention to these receptor-symptom interactions will improve response and reduce adverse consequences in the majority of patients.
Related Resource
- Lako IM, van den Heuvel ER, Knegtering H, et al. Estimating dopamine D2 receptor occupancy for doses of 8 antipsychotics: a meta-analysis. J Clin Psychopharmacol. 2013;33(5):675-681.
Drug Brand Names
Aripiprazole • Abilify
Asenapine • Saphris
Citalopram • Celexa
Clozapine • Clozaril
Fluoxetine • Prozac
Haloperidol • Haldol
Iloperidone • Fanapt
Lurasidone • Latuda
Nortriptyline • Pamelor
Olanzapine • Zyprexa
Paroxetine • Paxil
Quetiapine • Seroquel
Risperidone • Risperdal
Sertraline • Zoloft
Venlafaxine • Effexor
Ziprasidone • Geodon
1. Farde L, Nordström AL, Wiesel FA, et al. Positron emission tomographic analysis of central D1 and D2 dopamine receptor occupancy in patients treated with classical neuroleptics and clozapine. Relation to extrapyramidal side effects. Arch Gen Psychiatry. 1992;49(7):538-544.
2. Kapur S, Zipursky R, Jones C, et al. Relationship between dopamine D(2) occupancy, clinical response, and side effects: a double-blind PET study of first-episode schizophrenia. Am J Psychiatry. 2000;157(4):514-520.
3. Roberts RJ, Lohano KK, El-Mallakh RS. Antipsychotics as antidepressants. Asia Pacific Psychiatry. 2016;8(3):179-188.
4. Quitkin FM, Rabkin JG, Gerald J, et al. Validity of clinical trials of antidepressants. Am J Psychiatry. 2000;157(3):327-337.
5. Meyer JH, Wilson AA, Sagrati S, et al. Serotonin transporter occupancy of five selective serotonin reuptake inhibitors at different doses: an [11C]DASB positron emission tomography study. Am J Psychiatry. 2004;161(5):826-835.
6. Lundberg J, Tiger M, Landén M, et al. Serotonin transporter occupancy with TCAs and SSRIs: a PET study in patients with major depressive disorder. Int J Neuropsychopharmacol. 2012;15(8):1167-1172.
7. Takano H, Arakawa R, Nogami T, et al. Norepinephrine transporter occupancy by nortriptyline in patients with depression: a positron emission tomography study with (S,S)-[¹8F]FMeNER-D2. Int J Neuropsychopharmacol. 2014;17(4):553-560.
8. Johnson M, Kozielska M, Pilla Reddy V, et al. Dopamine D2 receptor occupancy as a predictor of catalepsy in rats: a pharmacokinetic-pharmacodynamic modeling approach. Pharm Res. 2014;31(10):2605-2617.
9. Allen MH, Citrome L, Pikalov A, et al. Efficacy of lurasidone in the treatment of agitation: a post hoc analysis of five short-term studies in acutely ill patients with schizophrenia. Gen Hosp Psychiatry. 2017;47:75-82.
10. Sekine M, Maeda J, Shimada H, et al. Central nervous system drug evaluation using positron emission tomography. Clin Psychopharmacol Neurosci. 2011;9(1):9-16.
11. Ma GF, Raivio N, Sabrià J, et al. Agonist and antagonist effects of aripiprazole on D2-like receptors controlling rat brain dopamine synthesis depend on the dopaminergic tone. Int J Neuropsychopharmacol. 2014;18(4):pii: pyu046. doi: 10.1093/ijnp/pyu046.
12. Yokoi F, Gründer G, Biziere K, et al. Dopamine D2 and D3 receptor occupancy in normal humans treated with the antipsychotic drug aripiprazole (OPC 14597): a study using positron emission tomography and [11C]raclopride. Neuropsychopharmacology. 2002;27(2):248-259.
13. Gründer G, Carlsson A, Wong DF. Mechanism of new antipsychotic medications: occupancy is not just antagonism. Arch Gen Psychiatry. 2003;60(10):974-977.
14. Mamo D, Graff A, Mizrahi R, et al. Differential effects of aripiprazole on D(2), 5-HT(2), and 5-HT(1A)receptor occupancy in patients with schizophrenia: a triple tracer PET study. Am J Psychiatry. 2007;164(9):1411-1417.
15. Weiden PJ, Preskorn SH, Fahnestock PA, et al. Translating the psychopharmacology of antipsychotics to individualized treatment for severe mental illness: a roadmap. J Clin Psychiatry. 2007;68(suppl 7):1-48.
16. Abi-Dargham A, Rodenhiser J, Printz D, et al. Increased baseline occupancy of D2 receptors by dopamine in schizophrenia. Proc Natl Acad Sci U S A. 2000;97(14):8104-8109.
17. Kane JM, Carson WH, Saha AR, et al. Efficacy and safety of aripiprazole and haloperidol versus placebo in patients with schizophrenia and schizoaffective disorder. J Clin Psychiatry. 2002;63(9):763-771.
18. Potkin SG, Saha AR, Kujawa MJ, et al. Aripiprazole, an antipsychotic with a novel mechanism of action, and risperidone vs placebo in patients with schizophrenia and schizoaffective disorder. Arch Gen Psychiatry. 2003;60(7):681-690.
19. Cutler AJ, Marcus RN, Hardy SA, et al. The efficacy and safety of lower doses of aripiprazole for the treatment of patients with acute exacerbation of schizophrenia. CNS Spectr. 2006;11(9):691-702; quiz 719.
20. Gründer G, Landvogt C, Vernaleken I, et al. The striatal and extrastriatal D2/D3 receptor-binding profile of clozapine in patients with schizophrenia. Neuropsychopharmacology. 2006;31(5):1027-1035.
21. Mizuno Y, Bies RR, Remington G, et al. Dopamine D2 receptor occupancy with risperidone or olanzapine during maintenance treatment of schizophrenia: a cross-sectional study. Prog Neuropsychopharmacol Biol Psychiatry. 2012;37(1):182-187.
22. Moriguchi S, Bies RR, Remington G, et al. Estimated dopamine D2 receptor occupancy and remission in schizophrenia: analysis of the CATIE data. J Clin Psychopharmacol. 2013;33(5):682-685.
23. Pilowsky LS, Mulligan RS, Acton PD, et al. Limbic selectivity of clozapine. Lancet. 1997;350(9076):490-491.
24. Ito H, Arakawa R, Takahashi H, et al. No regional difference in dopamine D2 receptor occupancy by the second-generation antipsychotic drug risperidone in humans: a positron emission tomography study. Int J Neuropsychopharmacol. 2009;12(5):667-675.
25. Arakawa R, Ito H, Okumura M, et al. Extrastriatal dopamine D(2) receptor occupancy in olanzapine-treated patients with schizophrenia. Eur Arch Psychiatry Clin Neurosci. 2010;260(4):345-350.
26. Xiberas X, Martinot JL, Mallet L, et al. Extrastriatal and striatal D(2) dopamine receptor blockade with haloperidol or new antipsychotic drugs in patients with schizophrenia. Br J Psychiatry. 2001;179:503-508.
27. Cutler AJ, Tran-Johnson T, Kalali A, et al. A failed 6-week, randomized, double-blind, placebo-controlled study of once-daily extended release quetiapine fumarate in patients with acute schizophrenia: lessons learned. Psychopharmacol Bull. 2010;43(4):37-69.
28. Marder SR, Meibach RC. Risperidone in the treatment of schizophrenia. Am J Psychiatry. 1994;151(6):825-835.
29. Meltzer HY, Cucchiaro J, Silva R, et al. Lurasidone in the treatment of schizophrenia: a randomized, double-blind, placebo- and olanzapine-controlled study. Am J Psychiatry. 2011;168(9):957-967.
30. Wong DF, Kuwabara H, Brašic JR, et al. Determination of dopamine D2 receptor occupancy by lurasidone using positron emission tomography in healthy male subjects. Psychopharmacology (Berl). 2013;229(2):245-252.
31. Potkin SG, Keator DB, Kesler-West ML, et al. D2 receptor occupancy following lurasidone treatment in patients with schizophrenia or schizoaffective disorder. CNS Spectr. 2014;19(2):176-181.
32. de Leon J, Susce MT, Pan RM, et al. The CYP2D6 poor metabolizer phenotype may be associated with risperidone adverse drug reactions and discontinuation. J Clin Psychiatry. 2005;66(1):15-27.
33. de Leon J, Susce MT, Pan RM, et al. A study of genetic (CYP2D6 and ABCB1) and environmental (drug inhibitors and inducers) variables that may influence plasma risperidone levels. Pharmacopsychiatry. 2007;40(3):93-102.
34. Narahari A, El-Mallakh RS, Kolikonda MK, et al. How coffee and cigarettes can affect the response to psychopharmacotherapy. Current Psychiatry. 2015;14(10):79-80.
35. Calabrese JR, Keck PE Jr, Macfadden W, et al. A randomized, double-blind, placebo-controlled trial of quetiapine in the treatment of bipolar I or II depression. Am J Psychiatry. 2005;162(7):1351-1360.
36. Loebel A, Cucchiaro J, Silva R, et al. Lurasidone monotherapy in the treatment of bipolar I depression: a randomized, double-blind, placebo-controlled study. Am J Psychiatry. 2014;171(2):160-168.
37. Lindenmayer JP, Brown D, Liu S, et al. The efficacy and tolerability of once-daily extended release quetiapine fumarate in hospitalized patients with acute schizophrenia: a 6-week randomized, double-blind, placebo-controlled study. Psychopharmacol Bull. 2008;41(3):11-35.
1. Farde L, Nordström AL, Wiesel FA, et al. Positron emission tomographic analysis of central D1 and D2 dopamine receptor occupancy in patients treated with classical neuroleptics and clozapine. Relation to extrapyramidal side effects. Arch Gen Psychiatry. 1992;49(7):538-544.
2. Kapur S, Zipursky R, Jones C, et al. Relationship between dopamine D(2) occupancy, clinical response, and side effects: a double-blind PET study of first-episode schizophrenia. Am J Psychiatry. 2000;157(4):514-520.
3. Roberts RJ, Lohano KK, El-Mallakh RS. Antipsychotics as antidepressants. Asia Pacific Psychiatry. 2016;8(3):179-188.
4. Quitkin FM, Rabkin JG, Gerald J, et al. Validity of clinical trials of antidepressants. Am J Psychiatry. 2000;157(3):327-337.
5. Meyer JH, Wilson AA, Sagrati S, et al. Serotonin transporter occupancy of five selective serotonin reuptake inhibitors at different doses: an [11C]DASB positron emission tomography study. Am J Psychiatry. 2004;161(5):826-835.
6. Lundberg J, Tiger M, Landén M, et al. Serotonin transporter occupancy with TCAs and SSRIs: a PET study in patients with major depressive disorder. Int J Neuropsychopharmacol. 2012;15(8):1167-1172.
7. Takano H, Arakawa R, Nogami T, et al. Norepinephrine transporter occupancy by nortriptyline in patients with depression: a positron emission tomography study with (S,S)-[¹8F]FMeNER-D2. Int J Neuropsychopharmacol. 2014;17(4):553-560.
8. Johnson M, Kozielska M, Pilla Reddy V, et al. Dopamine D2 receptor occupancy as a predictor of catalepsy in rats: a pharmacokinetic-pharmacodynamic modeling approach. Pharm Res. 2014;31(10):2605-2617.
9. Allen MH, Citrome L, Pikalov A, et al. Efficacy of lurasidone in the treatment of agitation: a post hoc analysis of five short-term studies in acutely ill patients with schizophrenia. Gen Hosp Psychiatry. 2017;47:75-82.
10. Sekine M, Maeda J, Shimada H, et al. Central nervous system drug evaluation using positron emission tomography. Clin Psychopharmacol Neurosci. 2011;9(1):9-16.
11. Ma GF, Raivio N, Sabrià J, et al. Agonist and antagonist effects of aripiprazole on D2-like receptors controlling rat brain dopamine synthesis depend on the dopaminergic tone. Int J Neuropsychopharmacol. 2014;18(4):pii: pyu046. doi: 10.1093/ijnp/pyu046.
12. Yokoi F, Gründer G, Biziere K, et al. Dopamine D2 and D3 receptor occupancy in normal humans treated with the antipsychotic drug aripiprazole (OPC 14597): a study using positron emission tomography and [11C]raclopride. Neuropsychopharmacology. 2002;27(2):248-259.
13. Gründer G, Carlsson A, Wong DF. Mechanism of new antipsychotic medications: occupancy is not just antagonism. Arch Gen Psychiatry. 2003;60(10):974-977.
14. Mamo D, Graff A, Mizrahi R, et al. Differential effects of aripiprazole on D(2), 5-HT(2), and 5-HT(1A)receptor occupancy in patients with schizophrenia: a triple tracer PET study. Am J Psychiatry. 2007;164(9):1411-1417.
15. Weiden PJ, Preskorn SH, Fahnestock PA, et al. Translating the psychopharmacology of antipsychotics to individualized treatment for severe mental illness: a roadmap. J Clin Psychiatry. 2007;68(suppl 7):1-48.
16. Abi-Dargham A, Rodenhiser J, Printz D, et al. Increased baseline occupancy of D2 receptors by dopamine in schizophrenia. Proc Natl Acad Sci U S A. 2000;97(14):8104-8109.
17. Kane JM, Carson WH, Saha AR, et al. Efficacy and safety of aripiprazole and haloperidol versus placebo in patients with schizophrenia and schizoaffective disorder. J Clin Psychiatry. 2002;63(9):763-771.
18. Potkin SG, Saha AR, Kujawa MJ, et al. Aripiprazole, an antipsychotic with a novel mechanism of action, and risperidone vs placebo in patients with schizophrenia and schizoaffective disorder. Arch Gen Psychiatry. 2003;60(7):681-690.
19. Cutler AJ, Marcus RN, Hardy SA, et al. The efficacy and safety of lower doses of aripiprazole for the treatment of patients with acute exacerbation of schizophrenia. CNS Spectr. 2006;11(9):691-702; quiz 719.
20. Gründer G, Landvogt C, Vernaleken I, et al. The striatal and extrastriatal D2/D3 receptor-binding profile of clozapine in patients with schizophrenia. Neuropsychopharmacology. 2006;31(5):1027-1035.
21. Mizuno Y, Bies RR, Remington G, et al. Dopamine D2 receptor occupancy with risperidone or olanzapine during maintenance treatment of schizophrenia: a cross-sectional study. Prog Neuropsychopharmacol Biol Psychiatry. 2012;37(1):182-187.
22. Moriguchi S, Bies RR, Remington G, et al. Estimated dopamine D2 receptor occupancy and remission in schizophrenia: analysis of the CATIE data. J Clin Psychopharmacol. 2013;33(5):682-685.
23. Pilowsky LS, Mulligan RS, Acton PD, et al. Limbic selectivity of clozapine. Lancet. 1997;350(9076):490-491.
24. Ito H, Arakawa R, Takahashi H, et al. No regional difference in dopamine D2 receptor occupancy by the second-generation antipsychotic drug risperidone in humans: a positron emission tomography study. Int J Neuropsychopharmacol. 2009;12(5):667-675.
25. Arakawa R, Ito H, Okumura M, et al. Extrastriatal dopamine D(2) receptor occupancy in olanzapine-treated patients with schizophrenia. Eur Arch Psychiatry Clin Neurosci. 2010;260(4):345-350.
26. Xiberas X, Martinot JL, Mallet L, et al. Extrastriatal and striatal D(2) dopamine receptor blockade with haloperidol or new antipsychotic drugs in patients with schizophrenia. Br J Psychiatry. 2001;179:503-508.
27. Cutler AJ, Tran-Johnson T, Kalali A, et al. A failed 6-week, randomized, double-blind, placebo-controlled study of once-daily extended release quetiapine fumarate in patients with acute schizophrenia: lessons learned. Psychopharmacol Bull. 2010;43(4):37-69.
28. Marder SR, Meibach RC. Risperidone in the treatment of schizophrenia. Am J Psychiatry. 1994;151(6):825-835.
29. Meltzer HY, Cucchiaro J, Silva R, et al. Lurasidone in the treatment of schizophrenia: a randomized, double-blind, placebo- and olanzapine-controlled study. Am J Psychiatry. 2011;168(9):957-967.
30. Wong DF, Kuwabara H, Brašic JR, et al. Determination of dopamine D2 receptor occupancy by lurasidone using positron emission tomography in healthy male subjects. Psychopharmacology (Berl). 2013;229(2):245-252.
31. Potkin SG, Keator DB, Kesler-West ML, et al. D2 receptor occupancy following lurasidone treatment in patients with schizophrenia or schizoaffective disorder. CNS Spectr. 2014;19(2):176-181.
32. de Leon J, Susce MT, Pan RM, et al. The CYP2D6 poor metabolizer phenotype may be associated with risperidone adverse drug reactions and discontinuation. J Clin Psychiatry. 2005;66(1):15-27.
33. de Leon J, Susce MT, Pan RM, et al. A study of genetic (CYP2D6 and ABCB1) and environmental (drug inhibitors and inducers) variables that may influence plasma risperidone levels. Pharmacopsychiatry. 2007;40(3):93-102.
34. Narahari A, El-Mallakh RS, Kolikonda MK, et al. How coffee and cigarettes can affect the response to psychopharmacotherapy. Current Psychiatry. 2015;14(10):79-80.
35. Calabrese JR, Keck PE Jr, Macfadden W, et al. A randomized, double-blind, placebo-controlled trial of quetiapine in the treatment of bipolar I or II depression. Am J Psychiatry. 2005;162(7):1351-1360.
36. Loebel A, Cucchiaro J, Silva R, et al. Lurasidone monotherapy in the treatment of bipolar I depression: a randomized, double-blind, placebo-controlled study. Am J Psychiatry. 2014;171(2):160-168.
37. Lindenmayer JP, Brown D, Liu S, et al. The efficacy and tolerability of once-daily extended release quetiapine fumarate in hospitalized patients with acute schizophrenia: a 6-week randomized, double-blind, placebo-controlled study. Psychopharmacol Bull. 2008;41(3):11-35.