User login
Bipolar disorder: The foundational role of mood stabilizers
Bipolar disorder (BD) is a recurrent, life-long psychiatric illness affecting nearly 2% of the world population1,2 that is characterized by episodes of mania and depression interspersed among periods of relative mood stability.3 The illness causes an enormous health burden, which makes understanding its pathophysiology and treatment patterns a substantial priority.4 In the 1950s, lithium was found to be effective for treating acute manic episodes and preventing relapse in BD.5 Since then, valproate and carbamazepine also have been FDA-approved for treating mania.6,7 Antipsychotics have also shown evidence of efficacy in BD treatment,8,9 particularly for use in acute settings for more rapid effect or for a limited duration,10 which has led some to refer to them as “mood stabilizers.”11
In this article, we describe changes in trends of prescribing medications to treat BD, the role of ion dysregulation in the disorder, and how a better understanding of this dysregulation might impact the choice of treatment.
Changes in pharmacotherapy for bipolar disorder
From 1997 through 2016, the use of lithium for BD decreased from >30% of patients to 17.6% (with a nadir of 13.9% from 2009 to 2012).12 Over the same period, the use of nonlithium mood stabilizers decreased from 30.4% to approximately 4.8%, while second-generation antipsychotic (SGAs) use increased from 12.4% to 50.4%.12 Distressingly, antidepressant use increased from approximately 47% to 56.8%, and antidepressant use without concomitant mood stabilizers increased from 38% to 40.8%, although the rate of antidepressants without either a mood stabilizer or an antipsychotic remained relatively stable (14.9% to 16.8%).12 In randomized trials, when added to mood stabilizers, antidepressants have consistently failed to separate from placebo,13-15 but they can destabilize the illness, resulting in increases in mania, depression, and subsyndromal mixed symptoms.16-18
It is easy to understand clinicians’ attempts to address their patients’ distress due to depressive symptoms that do not resolve with mood stabilizers.19,20 Similarly, the increased use of antipsychotics is driven by evidence that antipsychotics are effective for treating bipolar depression and preventing the recurrence of manic and (for some antipsychotics) depressive episodes.21,22 However, long-term antipsychotic use causes brain volume change in patients with schizophrenia23 or major depressive disorder24 and in nonhuman primates25,26; metabolic abnormalities27-31; and cardiovascular adverse effects.32 Antipsychotics are believed to be associated with withdrawal psychosis.33,34 In the head-to-head Clinical Health Outcomes Initiative in Comparative Effectiveness for Bipolar Disorder (Bipolar CHOICE) study, quetiapine was as effective as lithium but associated with more adverse effects.35 Importantly, the estimated disability-adjusted life years of patients with BD increased by 54.4% from 6.02 million in 1990 to 9.29 million in 2017, which is greater than the increase in the incidence of BD (47.74%) over the same time.36 This means that despite the dramatic increase in treatment options for people with BD, functional outcomes have declined.
One major difference between antipsychotics and mood stabilizers is that antipsychotics do not alter the underlying abnormal pathology of BD.37 An ideal pharmacologic intervention is one that corrects a known pathophysiologic anomaly of the condition being treated. There are no demonstrated abnormalities in the dopamine or serotonin systems in individuals with BD, but long-term use of antipsychotics may create dopaminergic alterations.33 One of the most reproducible biomarkers associated with manic and bipolar depressed mood states is increased intracellular sodium38,39 and reduced ability to correct a sodium challenge.40-42 By normalizing intracellular sodium levels, lithium and the mood-stabilizing anticonvulsants uniquely and specifically counter known physiologic abnormalities in patients with BD.37,43
The role of ion dysregulation
The pathophysiology of BD remains elusive. A multitude of lines of evidence link BD to abnormal neuroimaging findings,22,44,45 oxidative stress,46 inflammation,47 and mitochondrial disease,48 but there is still no unifying understanding of these findings. Ion dysregulation appears to be central to understanding and treating BD.38,39
Despite extensive genetic studies, no genes have been identified that mediate >5% of the risk for BD. Nonetheless, 74% of all genes identified as mediating risk for BD code for proteins essential for the regulation of ion transport and membrane potential.49 The 2 genes that contribute the greatest risk are CACNA1C and ANK3, which code for a calcium channel and a cytoskeletal protein, respectively.50ANK3 codes for ankyrin G, which plays a role in proper coupling of the voltage-gated sodium channels to the cytoskeleton.51 An additional risk gene, TRANK1, contains multiple ankyrin-like repeat domains, which suggests some shared functions with ANK3.52 More importantly, the most reproducible pathophysiologic findings in BD are dysregulation of sodium, potassium, hydrogen, and calcium transport, with consequent alteration of depolarization potential, neuronal excitability, and calcium-mediated processes.38,39,53-56 For example, increased sodium and calcium within cells have been observed in both mania and bipolar depression, and these levels normalize during euthymia. All medications that are effective for treating BD may reduce intracellular sodium or calcium; traditional mood stabilizers do so directly by inhibiting voltage-sensitive sodium channels in an activity-dependent manner or displacing intracellular sodium,43,57 whereas antipsychotics do so indirectly by increasing sodium pump activity through inhibition of second messengers of the dopamine D2 family of receptors.37
Continue to: The extent of ion dysregulation...
The extent of ion dysregulation is directly associated with the expressed mood state of the illness. A small reduction in the activity of the sodium pump results in a small increase in intracellular sodium (approximately 10 mM).39,58 This led to the hypothesis that increased intracellular sodium causes the transmembrane potential to increase closer to membrane depolarization threshold, which increases excitability of affected neurons.38,39,58 Neurons are likely to fire and propagate signals more easily, which may manifest as symptoms of mania, such as increased energy, activity, lability, excitability, irritability, tangentiality, and looseness of associations. As the process of increased intracellular sodium progresses, a minority of neurons are expected to have their transmembrane potentials depolarize sufficiently for the resting membrane potential to go beyond threshold potential.59 Such neurons are in a state of constant depolarization (also known as depolarization block), which disrupts neuronal circuits. The difficulty in progression of these signals results in the classic bipolar depression symptoms of low energy, reduced activity, and slowing of all brain activity that is seen as psychomotor slowing.38
Implications for treatment
Medications for treating bipolar illness include lithium, anticonvulsants, benzodiazepines, first-generation antipsychotics, and SGAs.37,43
Mood stabilizers (lithium and certain anticonvulsants) correct the previously mentioned sodium abnormality by reducing sodium entry into the cell in an activity-dependent manner.43 As the only agents that directly address a known pathophysiologic abnormality, they are foundational in the treatment of BD.60 Lithium effectively treats acute mania and prevents relapse.61 It preferentially targets the active neurons, entering through both voltage-responsive and neurotransmitter-coupled channels.43,62 This results in an increase of intracellular lithium concentrations to as much as 8 times that of the extracellular concentration.63 These ions displace intracellular sodium ions in a 1:1 ratio, which results in a reduced intracellular sodium concentration that reduces the excitability of neurons.43,57,62
Substantial evidence supports the use of valproic acid for initial and maintenance treatment of BD.64 It inhibits the voltage-sensitive sodium channel when the channel is open, which results in an activity-dependent action that selectively impacts rapidly firing neurons.43 The voltage-gated sodium channels exist nearly exclusively on the axon, beyond the hillock65; as such, valproic acid will only inhibit neurons that fire, whereas lithium accumulates throughout the neuron and will affect depolarization in the neuronal soma as well as the firing in the axon.43 Additionally, valproic acid has been observed to enhance gamma-aminobutyric acid (GABA) levels and transmission.43,66,67 A meta-analysis that included 6 randomized controlled trials illustrated that, acutely, valproate was not different from lithium’s overall efficacy (RR 1.02; 95% CI, 0.87 to 1.20), but was associated with reduced dropout rates compared with placebo or lithium (RR 0.82; 95% CI, 0.71 to 0.95 and RR 0.87; 95% CI, 0.77 to 0.98, respectively).64
Lamotrigine is an anticonvulsant used for initial and maintenance treatment of BD, with greater efficacy for depressive episodes68; it also has notable effect for treating bipolar depression, although it is not FDA-approved for this indication.69 Lamotrigine inhibits sodium influx by binding to open voltage-gated sodium channels70 but also appears to reduce N-methyl-D-aspartate–mediated sodium entry,71 thereby acting both prehillock and posthillock.
Continue to: Carbamazepine is an anticonvulsant...
Carbamazepine is an anticonvulsant FDA-approved for treating BD.7 Like valproate, it acts by inhibiting voltage-gated sodium channels in an activity-dependent manner,72 which means it preferentially inhibits the most active neurons and those with higher intracellular sodium.43
Benzodiazepines, which have shown to be effective for treating acute mania,73 potentiate synaptic GABA receptors accruing an elevation in intracellular chloride influx.74 Despite acute efficacy, benzodiazepine use is limited because these agents are associated with worsening long-term, substance use–related outcomes.75,76
Antipsychotics are effective for treating mood disorders,60,76 and their use has been rising dramatically.12 The antimanic effect of all antipsychotics is believed to be mediated through dopamine D2 blockade, since use of a dose sufficient to block D2 receptors is required, and haloperidol, which acts exclusively on the D2 receptor, is equal to SGAs in its antimanic effect.77 Blockade of the D2 receptor will increase the activity of the sodium pump (sodium and potassium-activated adenosine triphosphatase) thus reducing intracellular sodium and calcium concentrations.37 When antipsychotics are used as antidepressants, they are generally used at doses lower than those used to treat mania.78
Antipsychotics are effective for treating BD, and may work more quickly than other agents for treating acute mania.79 However, maintenance or prevention trials tend to favor mood stabilizers.35,60,80 Several add-on studies have found the combination of a mood stabilizer plus an antipsychotic is superior to a mood stabilizer alone or an antipsychotic alone.81
An argument for mood stabilizers
Evidence suggests mood stabilizers and other approaches, such as antipsychotics, are almost equivalent for treating acute mania, with a small clinical advantage of mood stabilizers for preventing relapse. In general, current treatment guidelines do not distinguish mood stabilizers from antipsychotics as the first-line treatment.82 Over the past 20 years, antipsychotic use has increased while mood stabilizer use has decreased, so that presently a patient with BD is more likely to be prescribed an antipsychotic than a mood stabilizer.12 Over the same time, dysfunction among patients with BD has increased.33 Antipsychotics are appealing because they appear to be equally effective and generally well tolerated. But these agents cause problems that are difficult to see in routine visits, such as metabolic27-31 and cardiovascular adverse effects29 as well as reductions in brain volume.23-26 Mechanistic research suggests that mood stabilizers directly correct known pathophysiologic anomalies with additional protective effects, whereas antipsychotics appear to create new abnormalities and contribute to medical problems. Clinicians need to look beyond the similarities in acute efficacy and make a more broadly supported, evidence-based choice for managing BD, which clearly places mood stabilizers as the first-line agent and antipsychotics as reasonable alternatives. At a minimum, mood stabilizers should be viewed as the foundation to which antipsychotics can be added.
Bottom Line
Traditional mood stabilizers—lithium and some anticonvulsants—are the only agents that directly address physiologic abnormalities associated with both mania and bipolar depression, including mood state–associated elevations of intracellular sodium. Because of their specificity, these agents maximize mood stabilization and minimize adverse effects.
Related Resources
- Karas A, Stummer L, Freedberg A. Psychiatric and nonpsychiatric indications for mood stabilizers and select antiepileptics. Current Psychiatry. 2022;21(4):34-38. doi:10.12788/cp.0230
- Koch J. Mood stabilizers: balancing tolerability, serum levels, and dosage. Current Psychiatry. 2021;20(7):37-40. doi:10.12788/cp.0147
Drug Brand Names
Carbamazepine • Tegretol
Haloperidol • Haldol
Lamotrigine • Lamictal
Lithium • Eskalith, Lithobid
Quetiapine • Seroquel
Valproate • Depakote, Depakene
1. Whiteford HA, Degenhardt L, Rehm J, et al. Global burden of disease attributable to mental and substance use disorders: findings from the Global Burden of Disease Study 2010. Lancet. 2013;382(9904):1575-1586. doi:10.1016/S0140-6736(13)61611-6
2. Merikangas KR, Jin R, He JP, et al. Prevalence and correlates of bipolar spectrum disorder in the world mental health survey initiative. Arch Gen Psychiatry. 2011;68(3):241-251. doi:10.1001/archgenpsychiatry.2011.12
3. Müller JK, Leweke FM. Bipolar disorder: clinical overview. Article in English, German. Med Monatsschr Pharm. 2016;39(9):363-369.
4. Smith DJ, Whitham EA, Ghaemi SN. Bipolar disorder. Handb Clin Neurol. 2012;106:251-263. doi:10.1016/B978-0-444-52002-9.00015-2
5. Goodwin FK, Ghaemi SN. The impact of the discovery of lithium on psychiatric thought and practice in the USA and Europe. Aust N Z J Psychiatry. 1999;33 Suppl:S54-S64. doi:10.1111/j.1440-1614.1999.00669.x
6. Pope HG, McElroy SL, Keck PE, et al. Valproate in the treatment of acute mania. A placebo-controlled study. Arch Gen Psychiatry. 1991;48(1):62-68. doi:10.1001/archpsyc.1991.01810250064008
7. Weisler RH, Keck PE Jr, Swann AC, et al. Extended-release carbamazepine capsules as monotherapy for acute mania in bipolar disorder: a multicenter, randomized, double-blind, placebo-controlled trial. J Clin Psychiatry. 2005;66(3):323-330. doi:10.4088/jcp.v66n0308
8. Tarr GP, Glue P, Herbison P. Comparative efficacy and acceptability of mood stabilizer and second generation antipsychotic monotherapy for acute mania--a systematic review and meta-analysis. J Affect Disord. 2011;134(1-3):14-19. doi:10.1016/j.jad.2010.11.009
9. Pahwa M, Sleem A, Elsayed OH, et al. New antipsychotic medications in the last decade. Curr Psychiatry Rep. 2021;23(12):87.
10. Correll CU, Sheridan EM, DelBello MP. Antipsychotic and mood stabilizer efficacy and tolerability in pediatric and adult patients with bipolar I mania: a comparative analysis of acute, randomized, placebo-controlled trials. Bipolar Disord. 2010;12(2):116-141. doi:10.1111/j.1399-5618.2010.00798.x
11. Rybakowski JK. Two generations of mood stabilizers. Int J Neuropsychopharmacol. 2007;10:709-711. doi:10.1017/s146114570700795x
12. Rhee TG, Olfson M, Nierenberg AA, et al. 20-year trends in the pharmacologic treatment of bipolar disorder by psychiatrists in outpatient care settings. Am J Psychiatry. 2020;177(8):706-715. doi:10.1176/appi.ajp.2020.19091000
13. El-Mallakh RS. Adjunctive antidepressant treatment for bipolar depression. N Engl J Med. 2007;357(6):615; author reply 615-616.
14. Sachs GS, Nierenberg AA, Calabrese JR, et al. Effectiveness of adjunctive antidepressant treatment for bipolar depression. N Engl J Med. 2007;356(17):1711-1722. doi:10.1056/NEJMoa064135
15. Ghaemi SN, Whitham EA, Vohringer PA, et al. Citalopram for acute and preventive efficacy in bipolar depression (CAPE-BD): a randomized, double-blind, placebo-controlled trial. J Clin Psychiatry. 2021;82(1):19m13136. doi:10.4088/JCP.19m13136
16. El-Mallakh RS, Ghaemi SN, Sagduyu K, et al. Antidepressant-associated chronic irritable dysphoria (ACID) in STEP-BD patients. J Affect Disord. 2008;111(2-3):372-377. doi:10.1016/j.jad.2008.03.025
17. Ghaemi SN, Ostacher MM, El-Mallakh RS, et al. Antidepressant discontinuation in bipolar depression: a systematic treatment enhancement program for bipolar disorder (STEP-BD) randomized clinical trial of long-term effectiveness and safety. J Clin Psychiatry. 2010;71(4):372-380.
18. Strejilevich SA, Martino DJ, Marengo E, et al. Long-term worsening of bipolar disorder related with frequency of antidepressant exposure. Ann Clin Psychiatry. 2011;23(3):186-192.
19. Pacchiarotti I, Bond DJ, Baldessarini RJ, et al. The International Society of Bipolar Disorders (ISBD) task force report on antidepressant use in bipolar disorders. Am J Psychiatry. 2013;170(11):1249-1262. doi:10.1176/appi.ajp.2013.13020185
20. McIntyre RS, Calabrese JR. Bipolar depression: the clinical characteristics and unmet needs of a complex disorder. Curr Med Res Opin. 2019;35(11):1993-2005.
21. Fornaro M, Stubbs B, De Berardis D, et al. Atypical antipsychotics in the treatment of acute bipolar depression with mixed features: a systematic review and exploratory meta-analysis of placebo-controlled clinical trials. Int J Mol Sci. 2016;17(2):241. doi:10.3390/ijms17020241
22. Lindström L, Lindström E, Nilsson M, et al. Maintenance therapy with second generation antipsychotics for bipolar disorder – a systematic review and meta-analysis. J Affect Disord. 2017;213:138-150. doi:10.1016/j.jad.2017.02.012
23. Ho BC, Andreasen NC, Ziebell S, et al. Long-term antipsychotic treatment and brain volumes: a longitudinal study of first-episode schizophrenia. Arch Gen Psychiatry. 2011;68(2):128-137. doi:010.1001/archgenpsychiatry.2010.199
24. Voineskos AN, Mulsant BH, Dickie EW, et al. Effects of antipsychotic medication on brain structure in patients with major depressive disorder and psychotic features: neuroimaging findings in the context of a randomized placebo-controlled clinical trial. JAMA Psychiatry. 2020;77(7):674-683. doi:10.1001/jamapsychiatry.2020.0036
25. Konopaske GT, Bolo NR, Basu AC, et al. Time-dependent effects of haloperidol on glutamine and GABA homeostasis and astrocyte activity in the rat brain. Psychopharmacology (Berl). 2013;230(1):57-67. doi:10.1007/s00213-013-3136-3
26. Dorph-Petersen KA, Pierri JN, Perel JM, et al. The influence of chronic exposure to antipsychotic medications on brain size before and after tissue fixation: a comparison of haloperidol and olanzapine in macaque monkeys. Neuropsychopharmacology. 2005;30(9):1649-1661. doi:10.1038/sj.npp.1300710
27. McIntyre RS, Mancini DA, Basile VS, et al. Antipsychotic-induced weight gain: bipolar disorder and leptin. J Clin Psychopharmacol. 2003;23(4):323-327. doi:10.1097/01.jcp.0000085403.08426.f4
28. McIntyre RS, Konarski JZ, Wilkins K, et al. Obesity in bipolar disorder and major depressive disorder: results from a national community health survey on mental health and well-being. Can J Psychiatry. 2006;51(5):274-280. doi:10.1177/070674370605100502
29. McIntyre RS, Cha DS, Kim RD, et al. A review of FDA-approved treatment options in bipolar depression. CNS Spectr. 2013;18(Suppl 1):4-20. doi:10.1017/S1092852913000746
30. Barton BB, Segger F, Fischer K, et al. Update on weight-gain caused by antipsychotics: a systematic review and meta-analysis. Expert Opin Drug Saf. 2020;19(3):295-314. doi:10.1080/14740338.2020.1713091
31. Doane MJ, Bessonova L, Friedler HS, et al. Weight gain and comorbidities associated with oral second-generation antipsychotics: analysis of real-world data for patients with schizophrenia or bipolar I disorder. BMC Psychiatry. 2022;22(1):114. doi:10.1186/s12888-022-03758-w
32. Buckley NA, Sanders P. Cardiovascular adverse effects of antipsychotic drugs. Drug Saf. 2000;23(3):215-228. doi:10.2165/00002018-200023030-00004
33. Ali Z, Roque A, El-Mallakh RS. A unifying theory for the pathoetiologic mechanism of tardive dyskinesia. Med Hypotheses. 2020;140:109682. doi:10.1016/j.mehy.2020.109682
34. Sleem A, El-Mallakh RS. Adaptive changes to antipsychotics: their consequences and how to avoid them. Curr Psychiatry. 2022;21(7):46-50,52. doi: 10.12788/cp.0262
35. Nierenberg AA, McElroy SL, Friedman ES, et al. Bipolar CHOICE (Clinical Health Outcomes Initiative in Comparative Effectiveness): a pragmatic 6-month trial of lithium versus quetiapine for bipolar disorder. J Clin Psychiatry. 2016;77(1):90-99. doi:10.4088/JCP.14m09349
36. He H, Hu C, Ren Z, et al. Trends in the incidence and DALYs of bipolar disorder at global, regional, and national levels: results from the global burden of disease study 2017. J Psychiatr Res. 2020;125:96-105. doi:10.1016/j.jpsychires.2020.03.015
37. Roberts RJ, Repass R, El-Mallakh RS. Effect of dopamine on intracellular sodium: a common pathway for pharmacological mechanism of action in bipolar illness. World J Biol Psychiatry. 2010;11(2 Pt 2):181-187. doi:10.1080/15622970902718774
38. El-Mallakh RS, Wyatt RJ. The Na, K-ATPase hypothesis for bipolar illness. Biol Psychiatry. 1995;37(4):235-244. doi:10.1016/0006-3223(94)00201-D
39. El-Mallakh RS, Yff T, Gao Y. Ion dysregulation in the pathogenesis of bipolar disorder. Ann Depress Anxiety. 2016;3(1):1076.
40. Li R, El-Mallakh RS. Differential response of bipolar and normal control lymphoblastoid cell sodium pump to ethacrynic acid. J Affect Disord. 2004;80(1):11-17. doi:10.1016/S0165-0327(03)00044-2
41. Woodruff DB, El-Mallakh RS, Thiruvengadam AP. Validation of a diagnostic screening blood test for bipolar disorder. Ann Clin Psychiatry. 2012;24(2):135-139.
42. Gao Y, Lohano K, Delamere NA, et al. Ethanol normalizes glutamate-induced elevation of intracellular sodium in olfactory neuroepithelial progenitors from subjects with bipolar illness but not nonbipolar controls: biologic evidence for the self-medication hypothesis. Bipolar Disord. 2019;21(2):179-181. doi:10.1111/bdi.12737
43. El-Mallakh RS, Huff MO. Mood stabilizers and ion regulation. Harv Rev Psychiatry. 2001;9(1):23-32. doi:10.1080/10673220127873
44. Phillips ML, Swartz HA. A critical appraisal of neuroimaging studies of bipolar disorder: toward a new conceptualization of underlying neural circuitry and a road map for future research. Am J Psychiatry. 2014;171(8):829-843. doi:10.1176/appi.ajp.2014.13081008
45. Hibar DP, Westlye LT, Doan NT, et al. Cortical abnormalities in bipolar disorder: an MRI analysis of 6503 individuals from the ENIGMA Bipolar Disorder Working Group. Mol Psychiatry. 2018;23(4):932-942. doi:10.1038/mp.2017.73
46. Brown NC, Andreazza AC, Young LT. An updated meta-analysis of oxidative stress markers in bipolar disorder. Psychiatry Res. 2014;218(1-2):61-68. doi:10.1016/j.psychres.2014.04.005
47. Benedetti F, Aggio V, Pratesi ML, et al. Neuroinflammation in bipolar depression. Front Psychiatry. 2020;11:71. doi:10.3389/fpsyt.2020.00071
48. Andreazza AC, Duong A, Young LT. Bipolar disorder as a mitochondrial disease. Biol Psychiatry. 2018;83(9):720-721. doi:10.1016/j.biopsych.2017.09.018
49. Askland KD. Toward a biaxial model of “bipolar” affective disorders: further exploration of genetic, molecular and cellular substrates. J Affect Disord. 2006;94(1-3):35-66. doi:10.1016/j.jad.2006.01.033
50. Ferreira MA, O’Donovan MC, Meng YA, et al; Wellcome Trust Case Control Consortium. Collaborative genome-wide association analysis supports a role for ANK3 and CACNA1C in bipolar disorder. Nat Genet 2008;40(9):1056-1058. doi:10.1038/ng.209
51. Salvi AM, Bays JL, Mackin SR, et al. Ankyrin G organizes membrane components to promote coupling of cell mechanics and glucose uptake. Nat Cell Biol. 2021;23(5):457-466. doi:10.1038/s41556-021-00677-y
52. Gargus JJ. Ion channel functional candidate genes in multigenic neuropsychiatric disease. Biol Psychiatry. 2006;60(2):177-185. doi:10.1016/j.biopsych.2005.12.008
53. Dubovsky SL, Murphy J, Thomas M, et al. Abnormal intracellular calcium ion concentration in platelets and lymphocytes of bipolar patients. Am J Psychiatry 1992;149(1):118-120. doi:10.1176/ajp.149.1.118
54. Blaustein MP. Physiological effects of endogenous ouabain: control of intracellular Ca2+ stores and cell responsiveness. Am J Physiol. 1993;264(6 Pt 1):C1367–C1387. doi:10.1152/ajpcell.1993.264.6.C1367
55. El-Mallakh RS, Li R, Worth CA, et al. Leukocyte transmembrane potential in bipolar illness. J Affect Disord. 1996;41(1):33-37. doi:10.1016/0165-0327(96)00063-8
56. El-Mallakh RS, Gao Y, You P. Role of endogenous ouabain in the etiology of bipolar disorder. Int J Bipolar Disord. 2021;9(1):6. doi:10.1186/s40345-020-00213-1
57. Huang X, Lei Z, El‐Mallakh RS. Lithium normalizes elevated intracellular sodium. Bipolar Disord. 2007;9(3):298-300. doi:10.1111/j.1399-5618.2007.00429.x
58. Shaw DM. Mineral metabolism, mania, and melancholia. Br Med J. 1966;2(5508):262-267. doi:10.1136/bmj.2.5508.262
59. Qian K, Yu N, Tucker KR, et al. Mathematical analysis of depolarization block mediated by slow inactivation of fast sodium channels in midbrain dopamine neurons. J Neurophysiol. 2014;112(11):2779-2790. doi:10.1152/jn.00578.2014
60. Sleem A, El-Mallakh RS. Advances in the psychopharmacotherapy of bipolar disorder type I. Exp Opin Pharmacother. 2021;22(10):1267-1290. doi:10.1080/14656566.2021.1893306
61. Malhi GS., Tanious M, Das P, et al. Potential mechanisms of action of lithium in bipolar disorder. CNS Drugs. 2013;27(2):135-153. doi:10.1007/s40263-013-0039-0
62. Armett CJ, Ritchie JM. On the permeability of mammalian non-myelinated fibers to sodium and to lithium ions. J Physiol. 1963;165(1):130-140. doi:10.1113/jphysiol.1963.sp007047
63. Kabakov AY, Karkanias NB, Lenox RH, et al. Synapse-specific accumulation of lithium in intracellular microdomains: a model for uncoupling coincidence detection in the brain. Synapse. 1998;28(4):271-279. doi:10.1002/(SICI)1098-2396(199804)28:4<271::AID-SYN2>3.0.CO;2-6
64. Cipriani A, Reid K, Young AH, et al. Valproic acid, valproate and divalproex in the maintenance treatment of bipolar disorder. Cochrane Database Syst Rev. 2013;2013(10):CD003196. doi:10.1002/14651858.CD003196.pub2
65. Lai HC, Jan LY. The distribution and targeting of neuronal voltage-gated ion channels. Nat Rev Neurosci. 2006;7(7):548-562. doi:10.1038/nrn1938
66. Löscher W, Schmidt D. Increase of human plasma GABA by sodium valproate. Epilepsia. 1980;21(6):611-615. doi:10.1111/j.1528-1157.1980.tb04314.x
67. Owens MJ, Nemeroff CB. Pharmacology of valproate. Psychopharmacol Bull. 2003;37(Suppl 2):17-24.
68. Calabrese JR, Vieta E, Shelton MD. Latest maintenance data on lamotrigine in bipolar disorder. Eur Neuropsychopharmacol. 2003;13(Suppl 2):S57-S66. doi:10.1016/s0924-977x(03)00079-8
69. Geddes JR, Calabrese JR, Goodwin GM. Lamotrigine for treatment of bipolar depression: independent meta-analysis and meta-regression of individual patient data from five randomised trials. Br J Psychiatry. 2009;194(1):4-9. doi:10.1192/bjp.bp.107.048504
70. Nakatani Y, Masuko H, Amano T. Effect of lamotrigine on Na(v)1.4 voltage-gated sodium channels. J Pharmacol Sci. 2013;123(2):203-206. doi:10.1254/jphs.13116sc
71. Ramadan E, Basselin M, Rao JS, et al. Lamotrigine blocks NMDA receptor-initiated arachidonic acid signalling in rat brain: implications for its efficacy in bipolar disorder. Int J Neuropsychopharmacol. 2012;15(7):931-943. doi:10.1017/S1461145711001003
72. Jo S, Bean BP. Sidedness of carbamazepine accessibility to voltage-gated sodium channels. Mol Pharmacol. 2014;85(2):381-387. doi:10.1124/mol.113.090472
73. Curtin F, Schulz P. Clonazepam and lorazepam in acute mania: a Bayesian meta-analysis. J Affect Disord 2004;78(3):201-208. doi:10.1016/S0165-0327(02)00317-8
74. Edwards R, Stephenson U, Flewett T. Clonazepam in acute mania: a double blind trial. Aust N Z J Psychiatry 1991;25(2):238-242. doi:10.3109/00048679109077740
75. Lin SC, Chen CC, Chen YH, et al. Benzodiazepine prescription among patients with severe mental illness and co-occurring alcohol abuse/dependence in Taiwan. Hum Psychopharmacol. 2011;26(3):201-207. doi:10.1002/hup.1193
76. Prisciandaro JJ, Brown DG, Brady KT, et al. Comorbid anxiety disorders and baseline medication regimens predict clinical outcomes in individuals with co-occurring bipolar disorder and alcohol dependence: results of a randomized controlled trial. Psychiatry Res. 2011;188(3):361-365. doi:10.1016/j.psychres.2011.04.030
77. Ashok AH, Marques TR, Jauhar S, et al. The dopamine hypothesis of bipolar affective disorder: the state of the art and implications for treatment. Mol Psychiatry. 2017;22(5):666-679. doi:10.1038/mp.2017.16
78. Roberts RJ, Lohano KK, El-Mallakh RS. Antipsychotics as antidepressants. Asia Pac Psychiatry. 2016;8(3):179-188. doi:10.1111/appy.12186
79. Cipriani A, Barbui C, Salanti G, et al. Comparative efficacy and acceptability of antimanic drugs in acute mania: a multiple-treatments meta-analysis. Lancet. 2011;378(9799):1306-1315. doi:10.1016/S0140-6736(11)60873-8
80. Hayes JF, Marston L, Walters K, et al. Lithium vs. valproate vs. olanzapine vs. quetiapine as maintenance monotherapy for bipolar disorder: a population-based UK cohort study using electronic health records. World Psychiatry. 2016;15(1):53-58. doi:10.1002/wps.20298
81. Geddes JR, Gardiner A, Rendell J, et al. Comparative evaluation of quetiapine plus lamotrigine combination versus quetiapine monotherapy (and folic acid versus placebo) in bipolar depression (CEQUEL): a 2 × 2 factorial randomised trial. Lancet Psychiatry. 2016;3(1):31239. doi:10.1016/S2215-0366(15)00450-2
82. Goodwin GM, Haddad PM, Ferrier IN, et al. Evidence-based guidelines for treating bipolar disorder: revised third edition recommendations from the British Association for Psychopharmacology. J Psychopharmacol. 2016;30(6):495-553. doi:10.1177/0269881116636545
Bipolar disorder (BD) is a recurrent, life-long psychiatric illness affecting nearly 2% of the world population1,2 that is characterized by episodes of mania and depression interspersed among periods of relative mood stability.3 The illness causes an enormous health burden, which makes understanding its pathophysiology and treatment patterns a substantial priority.4 In the 1950s, lithium was found to be effective for treating acute manic episodes and preventing relapse in BD.5 Since then, valproate and carbamazepine also have been FDA-approved for treating mania.6,7 Antipsychotics have also shown evidence of efficacy in BD treatment,8,9 particularly for use in acute settings for more rapid effect or for a limited duration,10 which has led some to refer to them as “mood stabilizers.”11
In this article, we describe changes in trends of prescribing medications to treat BD, the role of ion dysregulation in the disorder, and how a better understanding of this dysregulation might impact the choice of treatment.
Changes in pharmacotherapy for bipolar disorder
From 1997 through 2016, the use of lithium for BD decreased from >30% of patients to 17.6% (with a nadir of 13.9% from 2009 to 2012).12 Over the same period, the use of nonlithium mood stabilizers decreased from 30.4% to approximately 4.8%, while second-generation antipsychotic (SGAs) use increased from 12.4% to 50.4%.12 Distressingly, antidepressant use increased from approximately 47% to 56.8%, and antidepressant use without concomitant mood stabilizers increased from 38% to 40.8%, although the rate of antidepressants without either a mood stabilizer or an antipsychotic remained relatively stable (14.9% to 16.8%).12 In randomized trials, when added to mood stabilizers, antidepressants have consistently failed to separate from placebo,13-15 but they can destabilize the illness, resulting in increases in mania, depression, and subsyndromal mixed symptoms.16-18
It is easy to understand clinicians’ attempts to address their patients’ distress due to depressive symptoms that do not resolve with mood stabilizers.19,20 Similarly, the increased use of antipsychotics is driven by evidence that antipsychotics are effective for treating bipolar depression and preventing the recurrence of manic and (for some antipsychotics) depressive episodes.21,22 However, long-term antipsychotic use causes brain volume change in patients with schizophrenia23 or major depressive disorder24 and in nonhuman primates25,26; metabolic abnormalities27-31; and cardiovascular adverse effects.32 Antipsychotics are believed to be associated with withdrawal psychosis.33,34 In the head-to-head Clinical Health Outcomes Initiative in Comparative Effectiveness for Bipolar Disorder (Bipolar CHOICE) study, quetiapine was as effective as lithium but associated with more adverse effects.35 Importantly, the estimated disability-adjusted life years of patients with BD increased by 54.4% from 6.02 million in 1990 to 9.29 million in 2017, which is greater than the increase in the incidence of BD (47.74%) over the same time.36 This means that despite the dramatic increase in treatment options for people with BD, functional outcomes have declined.
One major difference between antipsychotics and mood stabilizers is that antipsychotics do not alter the underlying abnormal pathology of BD.37 An ideal pharmacologic intervention is one that corrects a known pathophysiologic anomaly of the condition being treated. There are no demonstrated abnormalities in the dopamine or serotonin systems in individuals with BD, but long-term use of antipsychotics may create dopaminergic alterations.33 One of the most reproducible biomarkers associated with manic and bipolar depressed mood states is increased intracellular sodium38,39 and reduced ability to correct a sodium challenge.40-42 By normalizing intracellular sodium levels, lithium and the mood-stabilizing anticonvulsants uniquely and specifically counter known physiologic abnormalities in patients with BD.37,43
The role of ion dysregulation
The pathophysiology of BD remains elusive. A multitude of lines of evidence link BD to abnormal neuroimaging findings,22,44,45 oxidative stress,46 inflammation,47 and mitochondrial disease,48 but there is still no unifying understanding of these findings. Ion dysregulation appears to be central to understanding and treating BD.38,39
Despite extensive genetic studies, no genes have been identified that mediate >5% of the risk for BD. Nonetheless, 74% of all genes identified as mediating risk for BD code for proteins essential for the regulation of ion transport and membrane potential.49 The 2 genes that contribute the greatest risk are CACNA1C and ANK3, which code for a calcium channel and a cytoskeletal protein, respectively.50ANK3 codes for ankyrin G, which plays a role in proper coupling of the voltage-gated sodium channels to the cytoskeleton.51 An additional risk gene, TRANK1, contains multiple ankyrin-like repeat domains, which suggests some shared functions with ANK3.52 More importantly, the most reproducible pathophysiologic findings in BD are dysregulation of sodium, potassium, hydrogen, and calcium transport, with consequent alteration of depolarization potential, neuronal excitability, and calcium-mediated processes.38,39,53-56 For example, increased sodium and calcium within cells have been observed in both mania and bipolar depression, and these levels normalize during euthymia. All medications that are effective for treating BD may reduce intracellular sodium or calcium; traditional mood stabilizers do so directly by inhibiting voltage-sensitive sodium channels in an activity-dependent manner or displacing intracellular sodium,43,57 whereas antipsychotics do so indirectly by increasing sodium pump activity through inhibition of second messengers of the dopamine D2 family of receptors.37
Continue to: The extent of ion dysregulation...
The extent of ion dysregulation is directly associated with the expressed mood state of the illness. A small reduction in the activity of the sodium pump results in a small increase in intracellular sodium (approximately 10 mM).39,58 This led to the hypothesis that increased intracellular sodium causes the transmembrane potential to increase closer to membrane depolarization threshold, which increases excitability of affected neurons.38,39,58 Neurons are likely to fire and propagate signals more easily, which may manifest as symptoms of mania, such as increased energy, activity, lability, excitability, irritability, tangentiality, and looseness of associations. As the process of increased intracellular sodium progresses, a minority of neurons are expected to have their transmembrane potentials depolarize sufficiently for the resting membrane potential to go beyond threshold potential.59 Such neurons are in a state of constant depolarization (also known as depolarization block), which disrupts neuronal circuits. The difficulty in progression of these signals results in the classic bipolar depression symptoms of low energy, reduced activity, and slowing of all brain activity that is seen as psychomotor slowing.38
Implications for treatment
Medications for treating bipolar illness include lithium, anticonvulsants, benzodiazepines, first-generation antipsychotics, and SGAs.37,43
Mood stabilizers (lithium and certain anticonvulsants) correct the previously mentioned sodium abnormality by reducing sodium entry into the cell in an activity-dependent manner.43 As the only agents that directly address a known pathophysiologic abnormality, they are foundational in the treatment of BD.60 Lithium effectively treats acute mania and prevents relapse.61 It preferentially targets the active neurons, entering through both voltage-responsive and neurotransmitter-coupled channels.43,62 This results in an increase of intracellular lithium concentrations to as much as 8 times that of the extracellular concentration.63 These ions displace intracellular sodium ions in a 1:1 ratio, which results in a reduced intracellular sodium concentration that reduces the excitability of neurons.43,57,62
Substantial evidence supports the use of valproic acid for initial and maintenance treatment of BD.64 It inhibits the voltage-sensitive sodium channel when the channel is open, which results in an activity-dependent action that selectively impacts rapidly firing neurons.43 The voltage-gated sodium channels exist nearly exclusively on the axon, beyond the hillock65; as such, valproic acid will only inhibit neurons that fire, whereas lithium accumulates throughout the neuron and will affect depolarization in the neuronal soma as well as the firing in the axon.43 Additionally, valproic acid has been observed to enhance gamma-aminobutyric acid (GABA) levels and transmission.43,66,67 A meta-analysis that included 6 randomized controlled trials illustrated that, acutely, valproate was not different from lithium’s overall efficacy (RR 1.02; 95% CI, 0.87 to 1.20), but was associated with reduced dropout rates compared with placebo or lithium (RR 0.82; 95% CI, 0.71 to 0.95 and RR 0.87; 95% CI, 0.77 to 0.98, respectively).64
Lamotrigine is an anticonvulsant used for initial and maintenance treatment of BD, with greater efficacy for depressive episodes68; it also has notable effect for treating bipolar depression, although it is not FDA-approved for this indication.69 Lamotrigine inhibits sodium influx by binding to open voltage-gated sodium channels70 but also appears to reduce N-methyl-D-aspartate–mediated sodium entry,71 thereby acting both prehillock and posthillock.
Continue to: Carbamazepine is an anticonvulsant...
Carbamazepine is an anticonvulsant FDA-approved for treating BD.7 Like valproate, it acts by inhibiting voltage-gated sodium channels in an activity-dependent manner,72 which means it preferentially inhibits the most active neurons and those with higher intracellular sodium.43
Benzodiazepines, which have shown to be effective for treating acute mania,73 potentiate synaptic GABA receptors accruing an elevation in intracellular chloride influx.74 Despite acute efficacy, benzodiazepine use is limited because these agents are associated with worsening long-term, substance use–related outcomes.75,76
Antipsychotics are effective for treating mood disorders,60,76 and their use has been rising dramatically.12 The antimanic effect of all antipsychotics is believed to be mediated through dopamine D2 blockade, since use of a dose sufficient to block D2 receptors is required, and haloperidol, which acts exclusively on the D2 receptor, is equal to SGAs in its antimanic effect.77 Blockade of the D2 receptor will increase the activity of the sodium pump (sodium and potassium-activated adenosine triphosphatase) thus reducing intracellular sodium and calcium concentrations.37 When antipsychotics are used as antidepressants, they are generally used at doses lower than those used to treat mania.78
Antipsychotics are effective for treating BD, and may work more quickly than other agents for treating acute mania.79 However, maintenance or prevention trials tend to favor mood stabilizers.35,60,80 Several add-on studies have found the combination of a mood stabilizer plus an antipsychotic is superior to a mood stabilizer alone or an antipsychotic alone.81
An argument for mood stabilizers
Evidence suggests mood stabilizers and other approaches, such as antipsychotics, are almost equivalent for treating acute mania, with a small clinical advantage of mood stabilizers for preventing relapse. In general, current treatment guidelines do not distinguish mood stabilizers from antipsychotics as the first-line treatment.82 Over the past 20 years, antipsychotic use has increased while mood stabilizer use has decreased, so that presently a patient with BD is more likely to be prescribed an antipsychotic than a mood stabilizer.12 Over the same time, dysfunction among patients with BD has increased.33 Antipsychotics are appealing because they appear to be equally effective and generally well tolerated. But these agents cause problems that are difficult to see in routine visits, such as metabolic27-31 and cardiovascular adverse effects29 as well as reductions in brain volume.23-26 Mechanistic research suggests that mood stabilizers directly correct known pathophysiologic anomalies with additional protective effects, whereas antipsychotics appear to create new abnormalities and contribute to medical problems. Clinicians need to look beyond the similarities in acute efficacy and make a more broadly supported, evidence-based choice for managing BD, which clearly places mood stabilizers as the first-line agent and antipsychotics as reasonable alternatives. At a minimum, mood stabilizers should be viewed as the foundation to which antipsychotics can be added.
Bottom Line
Traditional mood stabilizers—lithium and some anticonvulsants—are the only agents that directly address physiologic abnormalities associated with both mania and bipolar depression, including mood state–associated elevations of intracellular sodium. Because of their specificity, these agents maximize mood stabilization and minimize adverse effects.
Related Resources
- Karas A, Stummer L, Freedberg A. Psychiatric and nonpsychiatric indications for mood stabilizers and select antiepileptics. Current Psychiatry. 2022;21(4):34-38. doi:10.12788/cp.0230
- Koch J. Mood stabilizers: balancing tolerability, serum levels, and dosage. Current Psychiatry. 2021;20(7):37-40. doi:10.12788/cp.0147
Drug Brand Names
Carbamazepine • Tegretol
Haloperidol • Haldol
Lamotrigine • Lamictal
Lithium • Eskalith, Lithobid
Quetiapine • Seroquel
Valproate • Depakote, Depakene
Bipolar disorder (BD) is a recurrent, life-long psychiatric illness affecting nearly 2% of the world population1,2 that is characterized by episodes of mania and depression interspersed among periods of relative mood stability.3 The illness causes an enormous health burden, which makes understanding its pathophysiology and treatment patterns a substantial priority.4 In the 1950s, lithium was found to be effective for treating acute manic episodes and preventing relapse in BD.5 Since then, valproate and carbamazepine also have been FDA-approved for treating mania.6,7 Antipsychotics have also shown evidence of efficacy in BD treatment,8,9 particularly for use in acute settings for more rapid effect or for a limited duration,10 which has led some to refer to them as “mood stabilizers.”11
In this article, we describe changes in trends of prescribing medications to treat BD, the role of ion dysregulation in the disorder, and how a better understanding of this dysregulation might impact the choice of treatment.
Changes in pharmacotherapy for bipolar disorder
From 1997 through 2016, the use of lithium for BD decreased from >30% of patients to 17.6% (with a nadir of 13.9% from 2009 to 2012).12 Over the same period, the use of nonlithium mood stabilizers decreased from 30.4% to approximately 4.8%, while second-generation antipsychotic (SGAs) use increased from 12.4% to 50.4%.12 Distressingly, antidepressant use increased from approximately 47% to 56.8%, and antidepressant use without concomitant mood stabilizers increased from 38% to 40.8%, although the rate of antidepressants without either a mood stabilizer or an antipsychotic remained relatively stable (14.9% to 16.8%).12 In randomized trials, when added to mood stabilizers, antidepressants have consistently failed to separate from placebo,13-15 but they can destabilize the illness, resulting in increases in mania, depression, and subsyndromal mixed symptoms.16-18
It is easy to understand clinicians’ attempts to address their patients’ distress due to depressive symptoms that do not resolve with mood stabilizers.19,20 Similarly, the increased use of antipsychotics is driven by evidence that antipsychotics are effective for treating bipolar depression and preventing the recurrence of manic and (for some antipsychotics) depressive episodes.21,22 However, long-term antipsychotic use causes brain volume change in patients with schizophrenia23 or major depressive disorder24 and in nonhuman primates25,26; metabolic abnormalities27-31; and cardiovascular adverse effects.32 Antipsychotics are believed to be associated with withdrawal psychosis.33,34 In the head-to-head Clinical Health Outcomes Initiative in Comparative Effectiveness for Bipolar Disorder (Bipolar CHOICE) study, quetiapine was as effective as lithium but associated with more adverse effects.35 Importantly, the estimated disability-adjusted life years of patients with BD increased by 54.4% from 6.02 million in 1990 to 9.29 million in 2017, which is greater than the increase in the incidence of BD (47.74%) over the same time.36 This means that despite the dramatic increase in treatment options for people with BD, functional outcomes have declined.
One major difference between antipsychotics and mood stabilizers is that antipsychotics do not alter the underlying abnormal pathology of BD.37 An ideal pharmacologic intervention is one that corrects a known pathophysiologic anomaly of the condition being treated. There are no demonstrated abnormalities in the dopamine or serotonin systems in individuals with BD, but long-term use of antipsychotics may create dopaminergic alterations.33 One of the most reproducible biomarkers associated with manic and bipolar depressed mood states is increased intracellular sodium38,39 and reduced ability to correct a sodium challenge.40-42 By normalizing intracellular sodium levels, lithium and the mood-stabilizing anticonvulsants uniquely and specifically counter known physiologic abnormalities in patients with BD.37,43
The role of ion dysregulation
The pathophysiology of BD remains elusive. A multitude of lines of evidence link BD to abnormal neuroimaging findings,22,44,45 oxidative stress,46 inflammation,47 and mitochondrial disease,48 but there is still no unifying understanding of these findings. Ion dysregulation appears to be central to understanding and treating BD.38,39
Despite extensive genetic studies, no genes have been identified that mediate >5% of the risk for BD. Nonetheless, 74% of all genes identified as mediating risk for BD code for proteins essential for the regulation of ion transport and membrane potential.49 The 2 genes that contribute the greatest risk are CACNA1C and ANK3, which code for a calcium channel and a cytoskeletal protein, respectively.50ANK3 codes for ankyrin G, which plays a role in proper coupling of the voltage-gated sodium channels to the cytoskeleton.51 An additional risk gene, TRANK1, contains multiple ankyrin-like repeat domains, which suggests some shared functions with ANK3.52 More importantly, the most reproducible pathophysiologic findings in BD are dysregulation of sodium, potassium, hydrogen, and calcium transport, with consequent alteration of depolarization potential, neuronal excitability, and calcium-mediated processes.38,39,53-56 For example, increased sodium and calcium within cells have been observed in both mania and bipolar depression, and these levels normalize during euthymia. All medications that are effective for treating BD may reduce intracellular sodium or calcium; traditional mood stabilizers do so directly by inhibiting voltage-sensitive sodium channels in an activity-dependent manner or displacing intracellular sodium,43,57 whereas antipsychotics do so indirectly by increasing sodium pump activity through inhibition of second messengers of the dopamine D2 family of receptors.37
Continue to: The extent of ion dysregulation...
The extent of ion dysregulation is directly associated with the expressed mood state of the illness. A small reduction in the activity of the sodium pump results in a small increase in intracellular sodium (approximately 10 mM).39,58 This led to the hypothesis that increased intracellular sodium causes the transmembrane potential to increase closer to membrane depolarization threshold, which increases excitability of affected neurons.38,39,58 Neurons are likely to fire and propagate signals more easily, which may manifest as symptoms of mania, such as increased energy, activity, lability, excitability, irritability, tangentiality, and looseness of associations. As the process of increased intracellular sodium progresses, a minority of neurons are expected to have their transmembrane potentials depolarize sufficiently for the resting membrane potential to go beyond threshold potential.59 Such neurons are in a state of constant depolarization (also known as depolarization block), which disrupts neuronal circuits. The difficulty in progression of these signals results in the classic bipolar depression symptoms of low energy, reduced activity, and slowing of all brain activity that is seen as psychomotor slowing.38
Implications for treatment
Medications for treating bipolar illness include lithium, anticonvulsants, benzodiazepines, first-generation antipsychotics, and SGAs.37,43
Mood stabilizers (lithium and certain anticonvulsants) correct the previously mentioned sodium abnormality by reducing sodium entry into the cell in an activity-dependent manner.43 As the only agents that directly address a known pathophysiologic abnormality, they are foundational in the treatment of BD.60 Lithium effectively treats acute mania and prevents relapse.61 It preferentially targets the active neurons, entering through both voltage-responsive and neurotransmitter-coupled channels.43,62 This results in an increase of intracellular lithium concentrations to as much as 8 times that of the extracellular concentration.63 These ions displace intracellular sodium ions in a 1:1 ratio, which results in a reduced intracellular sodium concentration that reduces the excitability of neurons.43,57,62
Substantial evidence supports the use of valproic acid for initial and maintenance treatment of BD.64 It inhibits the voltage-sensitive sodium channel when the channel is open, which results in an activity-dependent action that selectively impacts rapidly firing neurons.43 The voltage-gated sodium channels exist nearly exclusively on the axon, beyond the hillock65; as such, valproic acid will only inhibit neurons that fire, whereas lithium accumulates throughout the neuron and will affect depolarization in the neuronal soma as well as the firing in the axon.43 Additionally, valproic acid has been observed to enhance gamma-aminobutyric acid (GABA) levels and transmission.43,66,67 A meta-analysis that included 6 randomized controlled trials illustrated that, acutely, valproate was not different from lithium’s overall efficacy (RR 1.02; 95% CI, 0.87 to 1.20), but was associated with reduced dropout rates compared with placebo or lithium (RR 0.82; 95% CI, 0.71 to 0.95 and RR 0.87; 95% CI, 0.77 to 0.98, respectively).64
Lamotrigine is an anticonvulsant used for initial and maintenance treatment of BD, with greater efficacy for depressive episodes68; it also has notable effect for treating bipolar depression, although it is not FDA-approved for this indication.69 Lamotrigine inhibits sodium influx by binding to open voltage-gated sodium channels70 but also appears to reduce N-methyl-D-aspartate–mediated sodium entry,71 thereby acting both prehillock and posthillock.
Continue to: Carbamazepine is an anticonvulsant...
Carbamazepine is an anticonvulsant FDA-approved for treating BD.7 Like valproate, it acts by inhibiting voltage-gated sodium channels in an activity-dependent manner,72 which means it preferentially inhibits the most active neurons and those with higher intracellular sodium.43
Benzodiazepines, which have shown to be effective for treating acute mania,73 potentiate synaptic GABA receptors accruing an elevation in intracellular chloride influx.74 Despite acute efficacy, benzodiazepine use is limited because these agents are associated with worsening long-term, substance use–related outcomes.75,76
Antipsychotics are effective for treating mood disorders,60,76 and their use has been rising dramatically.12 The antimanic effect of all antipsychotics is believed to be mediated through dopamine D2 blockade, since use of a dose sufficient to block D2 receptors is required, and haloperidol, which acts exclusively on the D2 receptor, is equal to SGAs in its antimanic effect.77 Blockade of the D2 receptor will increase the activity of the sodium pump (sodium and potassium-activated adenosine triphosphatase) thus reducing intracellular sodium and calcium concentrations.37 When antipsychotics are used as antidepressants, they are generally used at doses lower than those used to treat mania.78
Antipsychotics are effective for treating BD, and may work more quickly than other agents for treating acute mania.79 However, maintenance or prevention trials tend to favor mood stabilizers.35,60,80 Several add-on studies have found the combination of a mood stabilizer plus an antipsychotic is superior to a mood stabilizer alone or an antipsychotic alone.81
An argument for mood stabilizers
Evidence suggests mood stabilizers and other approaches, such as antipsychotics, are almost equivalent for treating acute mania, with a small clinical advantage of mood stabilizers for preventing relapse. In general, current treatment guidelines do not distinguish mood stabilizers from antipsychotics as the first-line treatment.82 Over the past 20 years, antipsychotic use has increased while mood stabilizer use has decreased, so that presently a patient with BD is more likely to be prescribed an antipsychotic than a mood stabilizer.12 Over the same time, dysfunction among patients with BD has increased.33 Antipsychotics are appealing because they appear to be equally effective and generally well tolerated. But these agents cause problems that are difficult to see in routine visits, such as metabolic27-31 and cardiovascular adverse effects29 as well as reductions in brain volume.23-26 Mechanistic research suggests that mood stabilizers directly correct known pathophysiologic anomalies with additional protective effects, whereas antipsychotics appear to create new abnormalities and contribute to medical problems. Clinicians need to look beyond the similarities in acute efficacy and make a more broadly supported, evidence-based choice for managing BD, which clearly places mood stabilizers as the first-line agent and antipsychotics as reasonable alternatives. At a minimum, mood stabilizers should be viewed as the foundation to which antipsychotics can be added.
Bottom Line
Traditional mood stabilizers—lithium and some anticonvulsants—are the only agents that directly address physiologic abnormalities associated with both mania and bipolar depression, including mood state–associated elevations of intracellular sodium. Because of their specificity, these agents maximize mood stabilization and minimize adverse effects.
Related Resources
- Karas A, Stummer L, Freedberg A. Psychiatric and nonpsychiatric indications for mood stabilizers and select antiepileptics. Current Psychiatry. 2022;21(4):34-38. doi:10.12788/cp.0230
- Koch J. Mood stabilizers: balancing tolerability, serum levels, and dosage. Current Psychiatry. 2021;20(7):37-40. doi:10.12788/cp.0147
Drug Brand Names
Carbamazepine • Tegretol
Haloperidol • Haldol
Lamotrigine • Lamictal
Lithium • Eskalith, Lithobid
Quetiapine • Seroquel
Valproate • Depakote, Depakene
1. Whiteford HA, Degenhardt L, Rehm J, et al. Global burden of disease attributable to mental and substance use disorders: findings from the Global Burden of Disease Study 2010. Lancet. 2013;382(9904):1575-1586. doi:10.1016/S0140-6736(13)61611-6
2. Merikangas KR, Jin R, He JP, et al. Prevalence and correlates of bipolar spectrum disorder in the world mental health survey initiative. Arch Gen Psychiatry. 2011;68(3):241-251. doi:10.1001/archgenpsychiatry.2011.12
3. Müller JK, Leweke FM. Bipolar disorder: clinical overview. Article in English, German. Med Monatsschr Pharm. 2016;39(9):363-369.
4. Smith DJ, Whitham EA, Ghaemi SN. Bipolar disorder. Handb Clin Neurol. 2012;106:251-263. doi:10.1016/B978-0-444-52002-9.00015-2
5. Goodwin FK, Ghaemi SN. The impact of the discovery of lithium on psychiatric thought and practice in the USA and Europe. Aust N Z J Psychiatry. 1999;33 Suppl:S54-S64. doi:10.1111/j.1440-1614.1999.00669.x
6. Pope HG, McElroy SL, Keck PE, et al. Valproate in the treatment of acute mania. A placebo-controlled study. Arch Gen Psychiatry. 1991;48(1):62-68. doi:10.1001/archpsyc.1991.01810250064008
7. Weisler RH, Keck PE Jr, Swann AC, et al. Extended-release carbamazepine capsules as monotherapy for acute mania in bipolar disorder: a multicenter, randomized, double-blind, placebo-controlled trial. J Clin Psychiatry. 2005;66(3):323-330. doi:10.4088/jcp.v66n0308
8. Tarr GP, Glue P, Herbison P. Comparative efficacy and acceptability of mood stabilizer and second generation antipsychotic monotherapy for acute mania--a systematic review and meta-analysis. J Affect Disord. 2011;134(1-3):14-19. doi:10.1016/j.jad.2010.11.009
9. Pahwa M, Sleem A, Elsayed OH, et al. New antipsychotic medications in the last decade. Curr Psychiatry Rep. 2021;23(12):87.
10. Correll CU, Sheridan EM, DelBello MP. Antipsychotic and mood stabilizer efficacy and tolerability in pediatric and adult patients with bipolar I mania: a comparative analysis of acute, randomized, placebo-controlled trials. Bipolar Disord. 2010;12(2):116-141. doi:10.1111/j.1399-5618.2010.00798.x
11. Rybakowski JK. Two generations of mood stabilizers. Int J Neuropsychopharmacol. 2007;10:709-711. doi:10.1017/s146114570700795x
12. Rhee TG, Olfson M, Nierenberg AA, et al. 20-year trends in the pharmacologic treatment of bipolar disorder by psychiatrists in outpatient care settings. Am J Psychiatry. 2020;177(8):706-715. doi:10.1176/appi.ajp.2020.19091000
13. El-Mallakh RS. Adjunctive antidepressant treatment for bipolar depression. N Engl J Med. 2007;357(6):615; author reply 615-616.
14. Sachs GS, Nierenberg AA, Calabrese JR, et al. Effectiveness of adjunctive antidepressant treatment for bipolar depression. N Engl J Med. 2007;356(17):1711-1722. doi:10.1056/NEJMoa064135
15. Ghaemi SN, Whitham EA, Vohringer PA, et al. Citalopram for acute and preventive efficacy in bipolar depression (CAPE-BD): a randomized, double-blind, placebo-controlled trial. J Clin Psychiatry. 2021;82(1):19m13136. doi:10.4088/JCP.19m13136
16. El-Mallakh RS, Ghaemi SN, Sagduyu K, et al. Antidepressant-associated chronic irritable dysphoria (ACID) in STEP-BD patients. J Affect Disord. 2008;111(2-3):372-377. doi:10.1016/j.jad.2008.03.025
17. Ghaemi SN, Ostacher MM, El-Mallakh RS, et al. Antidepressant discontinuation in bipolar depression: a systematic treatment enhancement program for bipolar disorder (STEP-BD) randomized clinical trial of long-term effectiveness and safety. J Clin Psychiatry. 2010;71(4):372-380.
18. Strejilevich SA, Martino DJ, Marengo E, et al. Long-term worsening of bipolar disorder related with frequency of antidepressant exposure. Ann Clin Psychiatry. 2011;23(3):186-192.
19. Pacchiarotti I, Bond DJ, Baldessarini RJ, et al. The International Society of Bipolar Disorders (ISBD) task force report on antidepressant use in bipolar disorders. Am J Psychiatry. 2013;170(11):1249-1262. doi:10.1176/appi.ajp.2013.13020185
20. McIntyre RS, Calabrese JR. Bipolar depression: the clinical characteristics and unmet needs of a complex disorder. Curr Med Res Opin. 2019;35(11):1993-2005.
21. Fornaro M, Stubbs B, De Berardis D, et al. Atypical antipsychotics in the treatment of acute bipolar depression with mixed features: a systematic review and exploratory meta-analysis of placebo-controlled clinical trials. Int J Mol Sci. 2016;17(2):241. doi:10.3390/ijms17020241
22. Lindström L, Lindström E, Nilsson M, et al. Maintenance therapy with second generation antipsychotics for bipolar disorder – a systematic review and meta-analysis. J Affect Disord. 2017;213:138-150. doi:10.1016/j.jad.2017.02.012
23. Ho BC, Andreasen NC, Ziebell S, et al. Long-term antipsychotic treatment and brain volumes: a longitudinal study of first-episode schizophrenia. Arch Gen Psychiatry. 2011;68(2):128-137. doi:010.1001/archgenpsychiatry.2010.199
24. Voineskos AN, Mulsant BH, Dickie EW, et al. Effects of antipsychotic medication on brain structure in patients with major depressive disorder and psychotic features: neuroimaging findings in the context of a randomized placebo-controlled clinical trial. JAMA Psychiatry. 2020;77(7):674-683. doi:10.1001/jamapsychiatry.2020.0036
25. Konopaske GT, Bolo NR, Basu AC, et al. Time-dependent effects of haloperidol on glutamine and GABA homeostasis and astrocyte activity in the rat brain. Psychopharmacology (Berl). 2013;230(1):57-67. doi:10.1007/s00213-013-3136-3
26. Dorph-Petersen KA, Pierri JN, Perel JM, et al. The influence of chronic exposure to antipsychotic medications on brain size before and after tissue fixation: a comparison of haloperidol and olanzapine in macaque monkeys. Neuropsychopharmacology. 2005;30(9):1649-1661. doi:10.1038/sj.npp.1300710
27. McIntyre RS, Mancini DA, Basile VS, et al. Antipsychotic-induced weight gain: bipolar disorder and leptin. J Clin Psychopharmacol. 2003;23(4):323-327. doi:10.1097/01.jcp.0000085403.08426.f4
28. McIntyre RS, Konarski JZ, Wilkins K, et al. Obesity in bipolar disorder and major depressive disorder: results from a national community health survey on mental health and well-being. Can J Psychiatry. 2006;51(5):274-280. doi:10.1177/070674370605100502
29. McIntyre RS, Cha DS, Kim RD, et al. A review of FDA-approved treatment options in bipolar depression. CNS Spectr. 2013;18(Suppl 1):4-20. doi:10.1017/S1092852913000746
30. Barton BB, Segger F, Fischer K, et al. Update on weight-gain caused by antipsychotics: a systematic review and meta-analysis. Expert Opin Drug Saf. 2020;19(3):295-314. doi:10.1080/14740338.2020.1713091
31. Doane MJ, Bessonova L, Friedler HS, et al. Weight gain and comorbidities associated with oral second-generation antipsychotics: analysis of real-world data for patients with schizophrenia or bipolar I disorder. BMC Psychiatry. 2022;22(1):114. doi:10.1186/s12888-022-03758-w
32. Buckley NA, Sanders P. Cardiovascular adverse effects of antipsychotic drugs. Drug Saf. 2000;23(3):215-228. doi:10.2165/00002018-200023030-00004
33. Ali Z, Roque A, El-Mallakh RS. A unifying theory for the pathoetiologic mechanism of tardive dyskinesia. Med Hypotheses. 2020;140:109682. doi:10.1016/j.mehy.2020.109682
34. Sleem A, El-Mallakh RS. Adaptive changes to antipsychotics: their consequences and how to avoid them. Curr Psychiatry. 2022;21(7):46-50,52. doi: 10.12788/cp.0262
35. Nierenberg AA, McElroy SL, Friedman ES, et al. Bipolar CHOICE (Clinical Health Outcomes Initiative in Comparative Effectiveness): a pragmatic 6-month trial of lithium versus quetiapine for bipolar disorder. J Clin Psychiatry. 2016;77(1):90-99. doi:10.4088/JCP.14m09349
36. He H, Hu C, Ren Z, et al. Trends in the incidence and DALYs of bipolar disorder at global, regional, and national levels: results from the global burden of disease study 2017. J Psychiatr Res. 2020;125:96-105. doi:10.1016/j.jpsychires.2020.03.015
37. Roberts RJ, Repass R, El-Mallakh RS. Effect of dopamine on intracellular sodium: a common pathway for pharmacological mechanism of action in bipolar illness. World J Biol Psychiatry. 2010;11(2 Pt 2):181-187. doi:10.1080/15622970902718774
38. El-Mallakh RS, Wyatt RJ. The Na, K-ATPase hypothesis for bipolar illness. Biol Psychiatry. 1995;37(4):235-244. doi:10.1016/0006-3223(94)00201-D
39. El-Mallakh RS, Yff T, Gao Y. Ion dysregulation in the pathogenesis of bipolar disorder. Ann Depress Anxiety. 2016;3(1):1076.
40. Li R, El-Mallakh RS. Differential response of bipolar and normal control lymphoblastoid cell sodium pump to ethacrynic acid. J Affect Disord. 2004;80(1):11-17. doi:10.1016/S0165-0327(03)00044-2
41. Woodruff DB, El-Mallakh RS, Thiruvengadam AP. Validation of a diagnostic screening blood test for bipolar disorder. Ann Clin Psychiatry. 2012;24(2):135-139.
42. Gao Y, Lohano K, Delamere NA, et al. Ethanol normalizes glutamate-induced elevation of intracellular sodium in olfactory neuroepithelial progenitors from subjects with bipolar illness but not nonbipolar controls: biologic evidence for the self-medication hypothesis. Bipolar Disord. 2019;21(2):179-181. doi:10.1111/bdi.12737
43. El-Mallakh RS, Huff MO. Mood stabilizers and ion regulation. Harv Rev Psychiatry. 2001;9(1):23-32. doi:10.1080/10673220127873
44. Phillips ML, Swartz HA. A critical appraisal of neuroimaging studies of bipolar disorder: toward a new conceptualization of underlying neural circuitry and a road map for future research. Am J Psychiatry. 2014;171(8):829-843. doi:10.1176/appi.ajp.2014.13081008
45. Hibar DP, Westlye LT, Doan NT, et al. Cortical abnormalities in bipolar disorder: an MRI analysis of 6503 individuals from the ENIGMA Bipolar Disorder Working Group. Mol Psychiatry. 2018;23(4):932-942. doi:10.1038/mp.2017.73
46. Brown NC, Andreazza AC, Young LT. An updated meta-analysis of oxidative stress markers in bipolar disorder. Psychiatry Res. 2014;218(1-2):61-68. doi:10.1016/j.psychres.2014.04.005
47. Benedetti F, Aggio V, Pratesi ML, et al. Neuroinflammation in bipolar depression. Front Psychiatry. 2020;11:71. doi:10.3389/fpsyt.2020.00071
48. Andreazza AC, Duong A, Young LT. Bipolar disorder as a mitochondrial disease. Biol Psychiatry. 2018;83(9):720-721. doi:10.1016/j.biopsych.2017.09.018
49. Askland KD. Toward a biaxial model of “bipolar” affective disorders: further exploration of genetic, molecular and cellular substrates. J Affect Disord. 2006;94(1-3):35-66. doi:10.1016/j.jad.2006.01.033
50. Ferreira MA, O’Donovan MC, Meng YA, et al; Wellcome Trust Case Control Consortium. Collaborative genome-wide association analysis supports a role for ANK3 and CACNA1C in bipolar disorder. Nat Genet 2008;40(9):1056-1058. doi:10.1038/ng.209
51. Salvi AM, Bays JL, Mackin SR, et al. Ankyrin G organizes membrane components to promote coupling of cell mechanics and glucose uptake. Nat Cell Biol. 2021;23(5):457-466. doi:10.1038/s41556-021-00677-y
52. Gargus JJ. Ion channel functional candidate genes in multigenic neuropsychiatric disease. Biol Psychiatry. 2006;60(2):177-185. doi:10.1016/j.biopsych.2005.12.008
53. Dubovsky SL, Murphy J, Thomas M, et al. Abnormal intracellular calcium ion concentration in platelets and lymphocytes of bipolar patients. Am J Psychiatry 1992;149(1):118-120. doi:10.1176/ajp.149.1.118
54. Blaustein MP. Physiological effects of endogenous ouabain: control of intracellular Ca2+ stores and cell responsiveness. Am J Physiol. 1993;264(6 Pt 1):C1367–C1387. doi:10.1152/ajpcell.1993.264.6.C1367
55. El-Mallakh RS, Li R, Worth CA, et al. Leukocyte transmembrane potential in bipolar illness. J Affect Disord. 1996;41(1):33-37. doi:10.1016/0165-0327(96)00063-8
56. El-Mallakh RS, Gao Y, You P. Role of endogenous ouabain in the etiology of bipolar disorder. Int J Bipolar Disord. 2021;9(1):6. doi:10.1186/s40345-020-00213-1
57. Huang X, Lei Z, El‐Mallakh RS. Lithium normalizes elevated intracellular sodium. Bipolar Disord. 2007;9(3):298-300. doi:10.1111/j.1399-5618.2007.00429.x
58. Shaw DM. Mineral metabolism, mania, and melancholia. Br Med J. 1966;2(5508):262-267. doi:10.1136/bmj.2.5508.262
59. Qian K, Yu N, Tucker KR, et al. Mathematical analysis of depolarization block mediated by slow inactivation of fast sodium channels in midbrain dopamine neurons. J Neurophysiol. 2014;112(11):2779-2790. doi:10.1152/jn.00578.2014
60. Sleem A, El-Mallakh RS. Advances in the psychopharmacotherapy of bipolar disorder type I. Exp Opin Pharmacother. 2021;22(10):1267-1290. doi:10.1080/14656566.2021.1893306
61. Malhi GS., Tanious M, Das P, et al. Potential mechanisms of action of lithium in bipolar disorder. CNS Drugs. 2013;27(2):135-153. doi:10.1007/s40263-013-0039-0
62. Armett CJ, Ritchie JM. On the permeability of mammalian non-myelinated fibers to sodium and to lithium ions. J Physiol. 1963;165(1):130-140. doi:10.1113/jphysiol.1963.sp007047
63. Kabakov AY, Karkanias NB, Lenox RH, et al. Synapse-specific accumulation of lithium in intracellular microdomains: a model for uncoupling coincidence detection in the brain. Synapse. 1998;28(4):271-279. doi:10.1002/(SICI)1098-2396(199804)28:4<271::AID-SYN2>3.0.CO;2-6
64. Cipriani A, Reid K, Young AH, et al. Valproic acid, valproate and divalproex in the maintenance treatment of bipolar disorder. Cochrane Database Syst Rev. 2013;2013(10):CD003196. doi:10.1002/14651858.CD003196.pub2
65. Lai HC, Jan LY. The distribution and targeting of neuronal voltage-gated ion channels. Nat Rev Neurosci. 2006;7(7):548-562. doi:10.1038/nrn1938
66. Löscher W, Schmidt D. Increase of human plasma GABA by sodium valproate. Epilepsia. 1980;21(6):611-615. doi:10.1111/j.1528-1157.1980.tb04314.x
67. Owens MJ, Nemeroff CB. Pharmacology of valproate. Psychopharmacol Bull. 2003;37(Suppl 2):17-24.
68. Calabrese JR, Vieta E, Shelton MD. Latest maintenance data on lamotrigine in bipolar disorder. Eur Neuropsychopharmacol. 2003;13(Suppl 2):S57-S66. doi:10.1016/s0924-977x(03)00079-8
69. Geddes JR, Calabrese JR, Goodwin GM. Lamotrigine for treatment of bipolar depression: independent meta-analysis and meta-regression of individual patient data from five randomised trials. Br J Psychiatry. 2009;194(1):4-9. doi:10.1192/bjp.bp.107.048504
70. Nakatani Y, Masuko H, Amano T. Effect of lamotrigine on Na(v)1.4 voltage-gated sodium channels. J Pharmacol Sci. 2013;123(2):203-206. doi:10.1254/jphs.13116sc
71. Ramadan E, Basselin M, Rao JS, et al. Lamotrigine blocks NMDA receptor-initiated arachidonic acid signalling in rat brain: implications for its efficacy in bipolar disorder. Int J Neuropsychopharmacol. 2012;15(7):931-943. doi:10.1017/S1461145711001003
72. Jo S, Bean BP. Sidedness of carbamazepine accessibility to voltage-gated sodium channels. Mol Pharmacol. 2014;85(2):381-387. doi:10.1124/mol.113.090472
73. Curtin F, Schulz P. Clonazepam and lorazepam in acute mania: a Bayesian meta-analysis. J Affect Disord 2004;78(3):201-208. doi:10.1016/S0165-0327(02)00317-8
74. Edwards R, Stephenson U, Flewett T. Clonazepam in acute mania: a double blind trial. Aust N Z J Psychiatry 1991;25(2):238-242. doi:10.3109/00048679109077740
75. Lin SC, Chen CC, Chen YH, et al. Benzodiazepine prescription among patients with severe mental illness and co-occurring alcohol abuse/dependence in Taiwan. Hum Psychopharmacol. 2011;26(3):201-207. doi:10.1002/hup.1193
76. Prisciandaro JJ, Brown DG, Brady KT, et al. Comorbid anxiety disorders and baseline medication regimens predict clinical outcomes in individuals with co-occurring bipolar disorder and alcohol dependence: results of a randomized controlled trial. Psychiatry Res. 2011;188(3):361-365. doi:10.1016/j.psychres.2011.04.030
77. Ashok AH, Marques TR, Jauhar S, et al. The dopamine hypothesis of bipolar affective disorder: the state of the art and implications for treatment. Mol Psychiatry. 2017;22(5):666-679. doi:10.1038/mp.2017.16
78. Roberts RJ, Lohano KK, El-Mallakh RS. Antipsychotics as antidepressants. Asia Pac Psychiatry. 2016;8(3):179-188. doi:10.1111/appy.12186
79. Cipriani A, Barbui C, Salanti G, et al. Comparative efficacy and acceptability of antimanic drugs in acute mania: a multiple-treatments meta-analysis. Lancet. 2011;378(9799):1306-1315. doi:10.1016/S0140-6736(11)60873-8
80. Hayes JF, Marston L, Walters K, et al. Lithium vs. valproate vs. olanzapine vs. quetiapine as maintenance monotherapy for bipolar disorder: a population-based UK cohort study using electronic health records. World Psychiatry. 2016;15(1):53-58. doi:10.1002/wps.20298
81. Geddes JR, Gardiner A, Rendell J, et al. Comparative evaluation of quetiapine plus lamotrigine combination versus quetiapine monotherapy (and folic acid versus placebo) in bipolar depression (CEQUEL): a 2 × 2 factorial randomised trial. Lancet Psychiatry. 2016;3(1):31239. doi:10.1016/S2215-0366(15)00450-2
82. Goodwin GM, Haddad PM, Ferrier IN, et al. Evidence-based guidelines for treating bipolar disorder: revised third edition recommendations from the British Association for Psychopharmacology. J Psychopharmacol. 2016;30(6):495-553. doi:10.1177/0269881116636545
1. Whiteford HA, Degenhardt L, Rehm J, et al. Global burden of disease attributable to mental and substance use disorders: findings from the Global Burden of Disease Study 2010. Lancet. 2013;382(9904):1575-1586. doi:10.1016/S0140-6736(13)61611-6
2. Merikangas KR, Jin R, He JP, et al. Prevalence and correlates of bipolar spectrum disorder in the world mental health survey initiative. Arch Gen Psychiatry. 2011;68(3):241-251. doi:10.1001/archgenpsychiatry.2011.12
3. Müller JK, Leweke FM. Bipolar disorder: clinical overview. Article in English, German. Med Monatsschr Pharm. 2016;39(9):363-369.
4. Smith DJ, Whitham EA, Ghaemi SN. Bipolar disorder. Handb Clin Neurol. 2012;106:251-263. doi:10.1016/B978-0-444-52002-9.00015-2
5. Goodwin FK, Ghaemi SN. The impact of the discovery of lithium on psychiatric thought and practice in the USA and Europe. Aust N Z J Psychiatry. 1999;33 Suppl:S54-S64. doi:10.1111/j.1440-1614.1999.00669.x
6. Pope HG, McElroy SL, Keck PE, et al. Valproate in the treatment of acute mania. A placebo-controlled study. Arch Gen Psychiatry. 1991;48(1):62-68. doi:10.1001/archpsyc.1991.01810250064008
7. Weisler RH, Keck PE Jr, Swann AC, et al. Extended-release carbamazepine capsules as monotherapy for acute mania in bipolar disorder: a multicenter, randomized, double-blind, placebo-controlled trial. J Clin Psychiatry. 2005;66(3):323-330. doi:10.4088/jcp.v66n0308
8. Tarr GP, Glue P, Herbison P. Comparative efficacy and acceptability of mood stabilizer and second generation antipsychotic monotherapy for acute mania--a systematic review and meta-analysis. J Affect Disord. 2011;134(1-3):14-19. doi:10.1016/j.jad.2010.11.009
9. Pahwa M, Sleem A, Elsayed OH, et al. New antipsychotic medications in the last decade. Curr Psychiatry Rep. 2021;23(12):87.
10. Correll CU, Sheridan EM, DelBello MP. Antipsychotic and mood stabilizer efficacy and tolerability in pediatric and adult patients with bipolar I mania: a comparative analysis of acute, randomized, placebo-controlled trials. Bipolar Disord. 2010;12(2):116-141. doi:10.1111/j.1399-5618.2010.00798.x
11. Rybakowski JK. Two generations of mood stabilizers. Int J Neuropsychopharmacol. 2007;10:709-711. doi:10.1017/s146114570700795x
12. Rhee TG, Olfson M, Nierenberg AA, et al. 20-year trends in the pharmacologic treatment of bipolar disorder by psychiatrists in outpatient care settings. Am J Psychiatry. 2020;177(8):706-715. doi:10.1176/appi.ajp.2020.19091000
13. El-Mallakh RS. Adjunctive antidepressant treatment for bipolar depression. N Engl J Med. 2007;357(6):615; author reply 615-616.
14. Sachs GS, Nierenberg AA, Calabrese JR, et al. Effectiveness of adjunctive antidepressant treatment for bipolar depression. N Engl J Med. 2007;356(17):1711-1722. doi:10.1056/NEJMoa064135
15. Ghaemi SN, Whitham EA, Vohringer PA, et al. Citalopram for acute and preventive efficacy in bipolar depression (CAPE-BD): a randomized, double-blind, placebo-controlled trial. J Clin Psychiatry. 2021;82(1):19m13136. doi:10.4088/JCP.19m13136
16. El-Mallakh RS, Ghaemi SN, Sagduyu K, et al. Antidepressant-associated chronic irritable dysphoria (ACID) in STEP-BD patients. J Affect Disord. 2008;111(2-3):372-377. doi:10.1016/j.jad.2008.03.025
17. Ghaemi SN, Ostacher MM, El-Mallakh RS, et al. Antidepressant discontinuation in bipolar depression: a systematic treatment enhancement program for bipolar disorder (STEP-BD) randomized clinical trial of long-term effectiveness and safety. J Clin Psychiatry. 2010;71(4):372-380.
18. Strejilevich SA, Martino DJ, Marengo E, et al. Long-term worsening of bipolar disorder related with frequency of antidepressant exposure. Ann Clin Psychiatry. 2011;23(3):186-192.
19. Pacchiarotti I, Bond DJ, Baldessarini RJ, et al. The International Society of Bipolar Disorders (ISBD) task force report on antidepressant use in bipolar disorders. Am J Psychiatry. 2013;170(11):1249-1262. doi:10.1176/appi.ajp.2013.13020185
20. McIntyre RS, Calabrese JR. Bipolar depression: the clinical characteristics and unmet needs of a complex disorder. Curr Med Res Opin. 2019;35(11):1993-2005.
21. Fornaro M, Stubbs B, De Berardis D, et al. Atypical antipsychotics in the treatment of acute bipolar depression with mixed features: a systematic review and exploratory meta-analysis of placebo-controlled clinical trials. Int J Mol Sci. 2016;17(2):241. doi:10.3390/ijms17020241
22. Lindström L, Lindström E, Nilsson M, et al. Maintenance therapy with second generation antipsychotics for bipolar disorder – a systematic review and meta-analysis. J Affect Disord. 2017;213:138-150. doi:10.1016/j.jad.2017.02.012
23. Ho BC, Andreasen NC, Ziebell S, et al. Long-term antipsychotic treatment and brain volumes: a longitudinal study of first-episode schizophrenia. Arch Gen Psychiatry. 2011;68(2):128-137. doi:010.1001/archgenpsychiatry.2010.199
24. Voineskos AN, Mulsant BH, Dickie EW, et al. Effects of antipsychotic medication on brain structure in patients with major depressive disorder and psychotic features: neuroimaging findings in the context of a randomized placebo-controlled clinical trial. JAMA Psychiatry. 2020;77(7):674-683. doi:10.1001/jamapsychiatry.2020.0036
25. Konopaske GT, Bolo NR, Basu AC, et al. Time-dependent effects of haloperidol on glutamine and GABA homeostasis and astrocyte activity in the rat brain. Psychopharmacology (Berl). 2013;230(1):57-67. doi:10.1007/s00213-013-3136-3
26. Dorph-Petersen KA, Pierri JN, Perel JM, et al. The influence of chronic exposure to antipsychotic medications on brain size before and after tissue fixation: a comparison of haloperidol and olanzapine in macaque monkeys. Neuropsychopharmacology. 2005;30(9):1649-1661. doi:10.1038/sj.npp.1300710
27. McIntyre RS, Mancini DA, Basile VS, et al. Antipsychotic-induced weight gain: bipolar disorder and leptin. J Clin Psychopharmacol. 2003;23(4):323-327. doi:10.1097/01.jcp.0000085403.08426.f4
28. McIntyre RS, Konarski JZ, Wilkins K, et al. Obesity in bipolar disorder and major depressive disorder: results from a national community health survey on mental health and well-being. Can J Psychiatry. 2006;51(5):274-280. doi:10.1177/070674370605100502
29. McIntyre RS, Cha DS, Kim RD, et al. A review of FDA-approved treatment options in bipolar depression. CNS Spectr. 2013;18(Suppl 1):4-20. doi:10.1017/S1092852913000746
30. Barton BB, Segger F, Fischer K, et al. Update on weight-gain caused by antipsychotics: a systematic review and meta-analysis. Expert Opin Drug Saf. 2020;19(3):295-314. doi:10.1080/14740338.2020.1713091
31. Doane MJ, Bessonova L, Friedler HS, et al. Weight gain and comorbidities associated with oral second-generation antipsychotics: analysis of real-world data for patients with schizophrenia or bipolar I disorder. BMC Psychiatry. 2022;22(1):114. doi:10.1186/s12888-022-03758-w
32. Buckley NA, Sanders P. Cardiovascular adverse effects of antipsychotic drugs. Drug Saf. 2000;23(3):215-228. doi:10.2165/00002018-200023030-00004
33. Ali Z, Roque A, El-Mallakh RS. A unifying theory for the pathoetiologic mechanism of tardive dyskinesia. Med Hypotheses. 2020;140:109682. doi:10.1016/j.mehy.2020.109682
34. Sleem A, El-Mallakh RS. Adaptive changes to antipsychotics: their consequences and how to avoid them. Curr Psychiatry. 2022;21(7):46-50,52. doi: 10.12788/cp.0262
35. Nierenberg AA, McElroy SL, Friedman ES, et al. Bipolar CHOICE (Clinical Health Outcomes Initiative in Comparative Effectiveness): a pragmatic 6-month trial of lithium versus quetiapine for bipolar disorder. J Clin Psychiatry. 2016;77(1):90-99. doi:10.4088/JCP.14m09349
36. He H, Hu C, Ren Z, et al. Trends in the incidence and DALYs of bipolar disorder at global, regional, and national levels: results from the global burden of disease study 2017. J Psychiatr Res. 2020;125:96-105. doi:10.1016/j.jpsychires.2020.03.015
37. Roberts RJ, Repass R, El-Mallakh RS. Effect of dopamine on intracellular sodium: a common pathway for pharmacological mechanism of action in bipolar illness. World J Biol Psychiatry. 2010;11(2 Pt 2):181-187. doi:10.1080/15622970902718774
38. El-Mallakh RS, Wyatt RJ. The Na, K-ATPase hypothesis for bipolar illness. Biol Psychiatry. 1995;37(4):235-244. doi:10.1016/0006-3223(94)00201-D
39. El-Mallakh RS, Yff T, Gao Y. Ion dysregulation in the pathogenesis of bipolar disorder. Ann Depress Anxiety. 2016;3(1):1076.
40. Li R, El-Mallakh RS. Differential response of bipolar and normal control lymphoblastoid cell sodium pump to ethacrynic acid. J Affect Disord. 2004;80(1):11-17. doi:10.1016/S0165-0327(03)00044-2
41. Woodruff DB, El-Mallakh RS, Thiruvengadam AP. Validation of a diagnostic screening blood test for bipolar disorder. Ann Clin Psychiatry. 2012;24(2):135-139.
42. Gao Y, Lohano K, Delamere NA, et al. Ethanol normalizes glutamate-induced elevation of intracellular sodium in olfactory neuroepithelial progenitors from subjects with bipolar illness but not nonbipolar controls: biologic evidence for the self-medication hypothesis. Bipolar Disord. 2019;21(2):179-181. doi:10.1111/bdi.12737
43. El-Mallakh RS, Huff MO. Mood stabilizers and ion regulation. Harv Rev Psychiatry. 2001;9(1):23-32. doi:10.1080/10673220127873
44. Phillips ML, Swartz HA. A critical appraisal of neuroimaging studies of bipolar disorder: toward a new conceptualization of underlying neural circuitry and a road map for future research. Am J Psychiatry. 2014;171(8):829-843. doi:10.1176/appi.ajp.2014.13081008
45. Hibar DP, Westlye LT, Doan NT, et al. Cortical abnormalities in bipolar disorder: an MRI analysis of 6503 individuals from the ENIGMA Bipolar Disorder Working Group. Mol Psychiatry. 2018;23(4):932-942. doi:10.1038/mp.2017.73
46. Brown NC, Andreazza AC, Young LT. An updated meta-analysis of oxidative stress markers in bipolar disorder. Psychiatry Res. 2014;218(1-2):61-68. doi:10.1016/j.psychres.2014.04.005
47. Benedetti F, Aggio V, Pratesi ML, et al. Neuroinflammation in bipolar depression. Front Psychiatry. 2020;11:71. doi:10.3389/fpsyt.2020.00071
48. Andreazza AC, Duong A, Young LT. Bipolar disorder as a mitochondrial disease. Biol Psychiatry. 2018;83(9):720-721. doi:10.1016/j.biopsych.2017.09.018
49. Askland KD. Toward a biaxial model of “bipolar” affective disorders: further exploration of genetic, molecular and cellular substrates. J Affect Disord. 2006;94(1-3):35-66. doi:10.1016/j.jad.2006.01.033
50. Ferreira MA, O’Donovan MC, Meng YA, et al; Wellcome Trust Case Control Consortium. Collaborative genome-wide association analysis supports a role for ANK3 and CACNA1C in bipolar disorder. Nat Genet 2008;40(9):1056-1058. doi:10.1038/ng.209
51. Salvi AM, Bays JL, Mackin SR, et al. Ankyrin G organizes membrane components to promote coupling of cell mechanics and glucose uptake. Nat Cell Biol. 2021;23(5):457-466. doi:10.1038/s41556-021-00677-y
52. Gargus JJ. Ion channel functional candidate genes in multigenic neuropsychiatric disease. Biol Psychiatry. 2006;60(2):177-185. doi:10.1016/j.biopsych.2005.12.008
53. Dubovsky SL, Murphy J, Thomas M, et al. Abnormal intracellular calcium ion concentration in platelets and lymphocytes of bipolar patients. Am J Psychiatry 1992;149(1):118-120. doi:10.1176/ajp.149.1.118
54. Blaustein MP. Physiological effects of endogenous ouabain: control of intracellular Ca2+ stores and cell responsiveness. Am J Physiol. 1993;264(6 Pt 1):C1367–C1387. doi:10.1152/ajpcell.1993.264.6.C1367
55. El-Mallakh RS, Li R, Worth CA, et al. Leukocyte transmembrane potential in bipolar illness. J Affect Disord. 1996;41(1):33-37. doi:10.1016/0165-0327(96)00063-8
56. El-Mallakh RS, Gao Y, You P. Role of endogenous ouabain in the etiology of bipolar disorder. Int J Bipolar Disord. 2021;9(1):6. doi:10.1186/s40345-020-00213-1
57. Huang X, Lei Z, El‐Mallakh RS. Lithium normalizes elevated intracellular sodium. Bipolar Disord. 2007;9(3):298-300. doi:10.1111/j.1399-5618.2007.00429.x
58. Shaw DM. Mineral metabolism, mania, and melancholia. Br Med J. 1966;2(5508):262-267. doi:10.1136/bmj.2.5508.262
59. Qian K, Yu N, Tucker KR, et al. Mathematical analysis of depolarization block mediated by slow inactivation of fast sodium channels in midbrain dopamine neurons. J Neurophysiol. 2014;112(11):2779-2790. doi:10.1152/jn.00578.2014
60. Sleem A, El-Mallakh RS. Advances in the psychopharmacotherapy of bipolar disorder type I. Exp Opin Pharmacother. 2021;22(10):1267-1290. doi:10.1080/14656566.2021.1893306
61. Malhi GS., Tanious M, Das P, et al. Potential mechanisms of action of lithium in bipolar disorder. CNS Drugs. 2013;27(2):135-153. doi:10.1007/s40263-013-0039-0
62. Armett CJ, Ritchie JM. On the permeability of mammalian non-myelinated fibers to sodium and to lithium ions. J Physiol. 1963;165(1):130-140. doi:10.1113/jphysiol.1963.sp007047
63. Kabakov AY, Karkanias NB, Lenox RH, et al. Synapse-specific accumulation of lithium in intracellular microdomains: a model for uncoupling coincidence detection in the brain. Synapse. 1998;28(4):271-279. doi:10.1002/(SICI)1098-2396(199804)28:4<271::AID-SYN2>3.0.CO;2-6
64. Cipriani A, Reid K, Young AH, et al. Valproic acid, valproate and divalproex in the maintenance treatment of bipolar disorder. Cochrane Database Syst Rev. 2013;2013(10):CD003196. doi:10.1002/14651858.CD003196.pub2
65. Lai HC, Jan LY. The distribution and targeting of neuronal voltage-gated ion channels. Nat Rev Neurosci. 2006;7(7):548-562. doi:10.1038/nrn1938
66. Löscher W, Schmidt D. Increase of human plasma GABA by sodium valproate. Epilepsia. 1980;21(6):611-615. doi:10.1111/j.1528-1157.1980.tb04314.x
67. Owens MJ, Nemeroff CB. Pharmacology of valproate. Psychopharmacol Bull. 2003;37(Suppl 2):17-24.
68. Calabrese JR, Vieta E, Shelton MD. Latest maintenance data on lamotrigine in bipolar disorder. Eur Neuropsychopharmacol. 2003;13(Suppl 2):S57-S66. doi:10.1016/s0924-977x(03)00079-8
69. Geddes JR, Calabrese JR, Goodwin GM. Lamotrigine for treatment of bipolar depression: independent meta-analysis and meta-regression of individual patient data from five randomised trials. Br J Psychiatry. 2009;194(1):4-9. doi:10.1192/bjp.bp.107.048504
70. Nakatani Y, Masuko H, Amano T. Effect of lamotrigine on Na(v)1.4 voltage-gated sodium channels. J Pharmacol Sci. 2013;123(2):203-206. doi:10.1254/jphs.13116sc
71. Ramadan E, Basselin M, Rao JS, et al. Lamotrigine blocks NMDA receptor-initiated arachidonic acid signalling in rat brain: implications for its efficacy in bipolar disorder. Int J Neuropsychopharmacol. 2012;15(7):931-943. doi:10.1017/S1461145711001003
72. Jo S, Bean BP. Sidedness of carbamazepine accessibility to voltage-gated sodium channels. Mol Pharmacol. 2014;85(2):381-387. doi:10.1124/mol.113.090472
73. Curtin F, Schulz P. Clonazepam and lorazepam in acute mania: a Bayesian meta-analysis. J Affect Disord 2004;78(3):201-208. doi:10.1016/S0165-0327(02)00317-8
74. Edwards R, Stephenson U, Flewett T. Clonazepam in acute mania: a double blind trial. Aust N Z J Psychiatry 1991;25(2):238-242. doi:10.3109/00048679109077740
75. Lin SC, Chen CC, Chen YH, et al. Benzodiazepine prescription among patients with severe mental illness and co-occurring alcohol abuse/dependence in Taiwan. Hum Psychopharmacol. 2011;26(3):201-207. doi:10.1002/hup.1193
76. Prisciandaro JJ, Brown DG, Brady KT, et al. Comorbid anxiety disorders and baseline medication regimens predict clinical outcomes in individuals with co-occurring bipolar disorder and alcohol dependence: results of a randomized controlled trial. Psychiatry Res. 2011;188(3):361-365. doi:10.1016/j.psychres.2011.04.030
77. Ashok AH, Marques TR, Jauhar S, et al. The dopamine hypothesis of bipolar affective disorder: the state of the art and implications for treatment. Mol Psychiatry. 2017;22(5):666-679. doi:10.1038/mp.2017.16
78. Roberts RJ, Lohano KK, El-Mallakh RS. Antipsychotics as antidepressants. Asia Pac Psychiatry. 2016;8(3):179-188. doi:10.1111/appy.12186
79. Cipriani A, Barbui C, Salanti G, et al. Comparative efficacy and acceptability of antimanic drugs in acute mania: a multiple-treatments meta-analysis. Lancet. 2011;378(9799):1306-1315. doi:10.1016/S0140-6736(11)60873-8
80. Hayes JF, Marston L, Walters K, et al. Lithium vs. valproate vs. olanzapine vs. quetiapine as maintenance monotherapy for bipolar disorder: a population-based UK cohort study using electronic health records. World Psychiatry. 2016;15(1):53-58. doi:10.1002/wps.20298
81. Geddes JR, Gardiner A, Rendell J, et al. Comparative evaluation of quetiapine plus lamotrigine combination versus quetiapine monotherapy (and folic acid versus placebo) in bipolar depression (CEQUEL): a 2 × 2 factorial randomised trial. Lancet Psychiatry. 2016;3(1):31239. doi:10.1016/S2215-0366(15)00450-2
82. Goodwin GM, Haddad PM, Ferrier IN, et al. Evidence-based guidelines for treating bipolar disorder: revised third edition recommendations from the British Association for Psychopharmacology. J Psychopharmacol. 2016;30(6):495-553. doi:10.1177/0269881116636545
Faulty fences: Blood-brain barrier dysfunction in schizophrenia
The blood-brain barrier (BBB) is an essential barrier of closely spaced cells that regulates entry into the CNS. What passes should be highly regulated to protect the brain from potentially harmful peripheral cells or molecules from the rest of the body. However, research has revealed that the BBB is pathologically permeable in several disease states, including schizophrenia, epilepsy, traumatic brain injury, autism, and DiGeorge syndrome (22q11.2 deletion syndrome, which often presents with symptoms of schizophrenia).1,2 In this article, we discuss potential markers of BBB dysfunction, the consequences of a porous BBB, the effect of BBB permeability on microglial activation, and possible treatment implications.
Detecting a BBB leak
The BBB is composed of microvascular endothelial cell units. Adherens junctions, astrocyte endfeet, and pericytes are all part of these units, but tight junctions have the most significant role in BBB barrier function. Tight junction protein composition varies depending on the location of the endothelium. In the BBB, they are primarily composed of claudin-5, occludin, zonulin, and junction adhesion molecules (JAMs) (Figure). Claudins and occludins are especially important components of the tight junction because they span plasma membranes.3
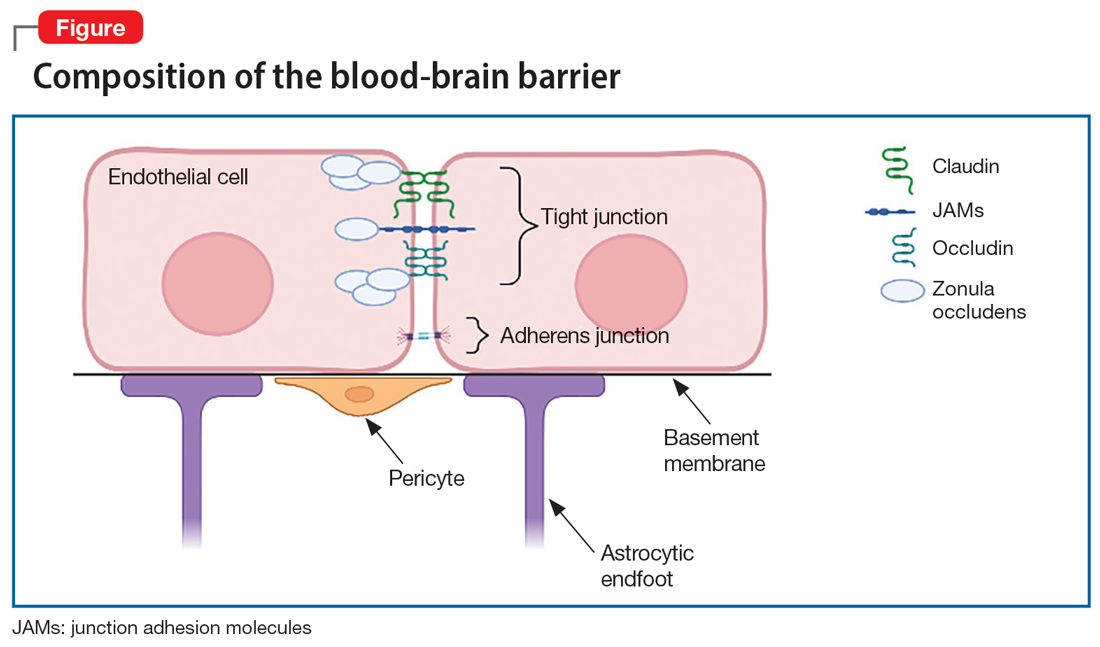
Researchers began to suspect tight junction permeability in schizophrenia while searching for schizophrenia biomarkers. For example, S100B is a marker of astrocytic reactivity to damage. It is increased in schizophrenia, major depressive disorder, and bipolar disorder.4 Studies found elevated S100B specifically in drug-free patients with schizophrenia,5 which prompted research suggesting it could predict the severity of negative symptoms.6 The accuracy of S100B as a biomarker was later complicated by the finding that adipose tissue also secretes S100B. This is problematic due to the high rates of comorbid obesity in psychiatric populations.2
Perhaps a better biomarker is the ratio of albumin in the CSF vs that in peripheral serum. The CSF-to-blood albumin ratio (Q-Alb) is widely considered an acceptable marker of BBB dysfunction because albumin must cross the BBB to alter the ratio. Studies have found a high Q-Alb in neurodegenerative disorders such as multiple sclerosis as well as in schizophrenia, which suggests that some level of BBB dysfunction is occurring. Although the Q-Alb may change slightly when confounded by antipsychotic use or with CSF flow changes,2,4 both S100B and Q-Alb elevation are sufficient for further investigation into tight junction alteration in schizophrenia.
Claudin-5 is a promising factor in detecting BBB permeability. Claudin-5 is deleted in DiGeorge syndrome, which is highly comorbid with schizophrenia and psychosis.1 Mouse knockdown studies show that full suppression of claudin-5 results in psychotic symptoms before fatal seizures,2,7 but a partial absence may enable psychotic symptoms. The same study showed that normally continuous claudin-5 was patchy along blood vessels in the affected sample.7 Follow-up experiments suggest that loss of claudin-5 in schizophrenia is especially prominent in the hippocampus, and there is mixed evidence of a decrease in the prefrontal cortex.8
Outside of claudin-5 alone, JAM-A plays a more regulatory role. It is upstream from an enhancer protein gene that serves as a transcription factor for the claudin-5 promoter, so when JAM-A is deleted, there is less claudin-5.9 However, while this decrease in claudin-5 may be pathological, there could still be various upstream changes that lead to schizophrenia.
What are the consequences of a porous BBB?
Although it is well established that the BBB passes small molecules and solutes, there is significant evidence of inflammatory trafficking in disease states. The BBB moves proinflammatory cytokines, alters transporters, and may even let white blood cells (WBCs) pass through. Immune cell infiltration has different requirements depending on the cell type. T cells rely on integrins, vascular cell adhesion molecule 1 (vCAM1), and intercellular adhesion molecule 1 (iCAM1) for binding, rolling, adhering, crossing, and migration to sites of inflammation.10,11 Both iCAM1 and vCAM1 are elevated in schizophrenia compared to other psychiatric disorders (such as unipolar depression) and correlate with other biomarkers. For example, vCAM1, responsible for recruitment and crossing, is correlated with a high Q-Alb.12 Primarily produced by astrocytes and endothelial cells, iCAM1 plays the largest role in crossing the BBB and migration. Postmortem tissue demonstrates that cytokines upregulate iCAM1 mRNA at the BBB in schizophrenia.13 Increased cytokines are well documented in the inflammatory model of schizophrenia. Interestingly, decreasing claudin-5 also upregulates iCAM1 production.14 Therefore, low baseline claudin-5 may contribute to additional inflammation and symptoms.
Continue to: BBB permeability also results...
BBB permeability also results in a certain pattern of leukocyte and cytokine activity. Interleukin-1 (IL-1), IL-6, and tumor necrosis factor–alpha can all cross the BBB during neuroimmune inflammation,10 but there are abnormal heightened and sustained responses of these molecules in schizophrenia. IL-6 is a proinflammatory cytokine in both acute and chronic inflammation that is expressed by astrocytes, endothelial cells, and microglia.15 IL-6 and its soluble receptor are both elevated in schizophrenia and are associated with white matter degeneration16,17 and an increase in vCAM1.15 This implies that while neuroinflammation in schizophrenia is occurring, additional leukocytes are being recruited and secreting their own cytokines in a chronic destructive positive feedback loop. Meanwhile, atypical IL-10 levels can no longer maintain balanced levels of inflammatory molecules,16 which leads to reduced control of inflammation.
Genetics and immunohistochemistry suggest that the BBB allows the passage of excess B cells and T cells in schizophrenia. Cytokines from WBCs or the BBB during inflammation recruit these additional infiltrating lymphocytes. In gene-wide association studies, there are several genes in schizophrenia important for B cells and T cells in addition to inflammation that interact in a proinflammatory network.16 These cells are also diffusely found in the white matter18 and hippocampal tissue19 of patients with schizophrenia. Taken together, an increase in adhesion molecules, WBCs, and cytokine crosstalk supports a leaky BBB as an important component of the inflammatory model of schizophrenia.
The role of microglia in BBB dysfunction
The effect of BBB permeability on microglial activation is an important caveat in the current research. Although several reports have linked neuroinflammation to confirmed microglial activation in schizophrenia, there is not enough evidence to claim that the BBB alone is the missing link between these theories. Some research suggests that chronic release of cytokines such as IL-6 from macrophages and T cells could increase migration across the BBB for microglial activation.16,20 However, positron emission tomography has shown mixed results at best. Translocator protein (TSPO) is expressed by microglia that are actively secreting cytokines.21 Researchers tracking TSPO changes in relation to BBB alteration have not seen elevated binding in schizophrenia, change due to stage of disease course, or differentiation from low-grade inflammation.21-24 Moreover, TSPO may be confounded by antipsychotic use25 and microglial expression did not correlate with any changes in adhesion molecules.13 TSPO is not an ideal indicator of microglial activation due to BBB breakdown, but that does not bar the possibility of at least a partial contribution to the development of schizophrenia.
Corsi-Zuelli et al26 created a model that attempts to merge BBB permeability and microglial activation through a different medium—T regulatory cells (TRegs). They write that if TRegs mediate interactions between astrocytes and microglia, their hypofunction would impose a prolonged T cell response. The increased access to a high level of IL-6 and its soluble receptors may keep the TRegs hypofunctional in schizophrenia and promote T cell conversion to inflammatory cell types. Experimentally, TReg induction reversed some psychotic symptoms, and greater TReg expression was associated with fewer negative symptoms.26 In an already insufficient BBB, more access to cytokines and leukocytes would sustain inflammation and microglial secretions.
In addition to the issues described regarding the BBB, the blood-CSF barrier at the choroid plexus may also be insufficient in schizophrenia (Box27-31).
Box
The choroid plexus’ primary role is to make CSF, but it also secretes cytokines and to some extent serves as a barrier. Unlike the blood-brain barrier (BBB), the blood-CSF barrier is composed of endothelial cells with fenestrations as well as tight junctions, which make the blood-CSF barrier overall more permeable.27,28 The most unusual finding regarding the choroid plexus in schizophrenia is size. The choroid plexus is physically larger in patients with schizophrenia, and to a lesser extent, in their first-degree relatives.29 A larger choroid plexus is correlated with more severe cognitive symptoms, increased risk for psychosis via biological stress, and significantly higher interleukin-6 (IL-6).27,29 The increased thickness could be an attempt to compensate for hyperactivity and toxic processes in a permeable environment. More circulating cytokines such as IL-6 and tumor necrosis factor–alpha from microglia can trigger an increase in intercellular adhesion molecule 1, resulting in leukocyte attachment and entry.30 Less claudin-5 at the choroid plexus in schizophrenia implicates similar permissive effects as seen at the BBB.31 Although the contribution of blood-CSF barrier dysfunction to schizophrenia requires further study, reduced barrier function outside the BBB is a viable line of inquiry.
Continue to: Caveats about this research
Caveats about this research
There are 3 important points to note about the current research concerning abnormal BBB permeability:
1. BBB dysfunction may exist only in a subset of people diagnosed with schizophrenia. In most human studies, only some patients with schizophrenia demonstrated alterations that suggested pathological BBB permeability. In addition, even when there is BBB dysfunction, it could be a secondary phenomenon, rather than a primary etiologic process.
2. Patient demographics across studies have not always been adequately described. Potential confounds such as obesity, smoking, or antipsychotic use were not consistently recorded or examined as a possible factor.
3. Currently available biomarkers are not perfect. Cytokine elevation, S100B, and Q-Alb are indirect measures of BBB disruption and are found in other disorders. Therefore, they only support the theory of BBB dysfunction in schizophrenia, rather than prove it. They are also not reliable markers for schizophrenia alone. Researchers have pointed out that these markers and proteins work in concert, which necessitates a network analysis approach.16 More research regarding the details of permeability is required to establish more reliable biomarkers and tailored treatment.
Treatment implications
One of the first treatment directions that comes to mind is managing the gaps in the BBB via tight junctions. Presently, there are no FDA-approved medications for altering tight junction proteins, but researchers are exploring potential agents that can induce claudin-5 and reduce inflammation.14 While we wait for such a medication, patients may benefit from existing anti-inflammatory treatments to control immune infiltration and its products. Various anti-inflammatory agents—including cyclooxygenase inhibitors,
Bottom Line
Recent research has revealed that the blood-brain barrier (BBB) is pathologically permeable in several disease states, including schizophrenia. Better characterization of the leaky BBB in schizophrenia has enormous potential in helping us understand how current theories fit together and could serve as a missing puzzle piece in treating schizophrenia.
Related Resources
- Levine A, Strawn JR. The brain’s Twitter system: neuronal extracellular vesicles. Current Psychiatry. 2022;21(6):9-11, 17-19,27. doi:10.12788/cp.0257
Drug Brand Names
Minocycline • Dynacin, Minocin
1. Li Y, Xia Y, Zhu H, et al. Investigation of neurodevelopmental deficits of 22 q11.2 deletion syndrome with a patient-iPSC-derived blood-brain barrier model. Cells. 2021;10(10):2576. doi:10.3390/cells10102576
2. Kealy J, Greene C, Campbell M. Blood-brain barrier regulation in psychiatric disorders. Neurosci Lett. 2020;726:133664. doi:10.1016/j.neulet.2018.06.033
3. Ballabh P, Braun A, Nedergaard M. The blood-brain barrier: an overview: structure, regulation, and clinical implications. Neurobiol Dis. 2004;16(1):1-13. doi:10.1016/j.nbd.2003.12.016
4. Futtrup J, Margolinsky R, Benros ME, et al. Blood-brain barrier pathology in patients with severe mental disorders: a systematic review and meta-analysis of biomarkers in case-control studies. Brain Behav Immun Health. 2020;6:100102. doi:10.1016/j.bbih.2020.100102
5. Chen S, Tian L, Chen N, et al. Cognitive dysfunction correlates with elevated serum S100B concentration in drug-free acutely relapsed patients with schizophrenia. Psychiatry Res. 2017;247:6-11. doi:10.1016/j.psychres.2016.09.029
6. Wu YF, Sytwu HK, Lung FW. Human aquaporin 4 gene polymorphisms and haplotypes are associated with serum S100B level and negative symptoms of schizophrenia in a southern Chinese Han population. Front Psychiatry. 2018;9:657. doi:10.3389/fpsyt.2018.00657
7. Greene C, Kealy J, Humphries MM, et al. Dose-dependent expression of claudin-5 is a modifying factor in schizophrenia. Mol Psychiatry. 2018;23(11):2156-2166. doi:10.1038/MP.2017.156
8. Greene C, Hanley N, Campbell M. Blood-brain barrier associated tight junction disruption is a hallmark feature of major psychiatric disorders. Transl Psychiatry. 2020;10(1):373. doi:10.1038/s41398-020-01054-3
9. Kakogiannos N, Ferrari L, Giampietro C, et al. JAM-A acts via C/EBP-α to promote claudin-5 expression and enhance endothelial barrier function. Circ Res. 2020:1056-1073. doi:10.1161/CIRCRESAHA.120.316742
10. Erickson MA, Dohi K, Banks WA. Neuroinflammation: a common pathway in CNS diseases as mediated at the blood-brain barrier. Neuroimmunomodulation. 2012;19(2):121-130. doi:10.1159/000330247
11. Ao LY, Yan YY, Zhou L, et al. Immune cells after ischemic stroke onset: roles, migration, and target intervention. J Mol Neurosci. 2018;66(3):342-355. doi:10.1007/s12031-018-1173-4
12. Meixensberger S, Kuzior H, Fiebich BL, et al. Upregulation of sICAM-1 and sVCAM-1 levels in the cerebrospinal fluid of patients with schizophrenia spectrum disorders. Diagnostics (Basel). 2021;11(7):1134. doi:10.3390diagnostics11071134
13. Cai HQ, Catts VS, Webster MJ, et al. Increased macrophages and changed brain endothelial cell gene expression in the frontal cortex of people with schizophrenia displaying inflammation. Mol Psychiatry. 2020;25(4):761-775. doi:10.1038/s41380-018-0235-x
14. Greene C, Hanley N, Reschke CR, et al. Microvascular stabilization via blood-brain barrier regulation prevents seizure activity. Nat Commun. 2022;13(1):2003. doi:10.1038/s41467-022-29657-y
15. García-Juárez M, Camacho-Morales A. Defining the role of anti- and pro-inflammatory outcomes of interleukin-6 in mental health. Neuroscience. 2022;492:32-46. doi:10.1016/j.neuroscience.2022.03.020
16. Pong S, Karmacharya R, Sofman M, et al. The role of brain microvascular endothelial cell and blood-brain barrier dysfunction in schizophrenia. Complex Psychiatry. 2020;6(1-2):30-46. doi:10.1159/000511552
17. Patel A, Zhu Y, Kuzhikandathil EV, et al. Soluble interleukin-6 receptor induces motor stereotypies and co-localizes with gp130 in regions linked to cortico-striato-thalamo-cortical circuits. PLoS One. 2012;7(7): e41623. doi:10.1371/journal.pone.0041623
18. Schlaaff K, Dobrowolny H, Frodl T, et al. Increased densities of T and B lymphocytes indicate neuroinflammation in subgroups of schizophrenia and mood disorder patients. Brain Behav Immun. 2020;88:497-506. doi:10.1016/j.bbi.2020.04.021
19. Busse S, Busse M, Schiltz K, et al. Different distribution patterns of lymphocytes and microglia in the hippocampus of patients with residual versus paranoid schizophrenia: further evidence for disease course-related immune alterations? Brain Behav Immun. 2012;26(8):1273-1279. doi:10.1016/j.bbi.2012.08.005
20. Na KS, Jung HY, Kim YK. The role of pro-inflammatory cytokines in the neuroinflammation and neurogenesis of schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2014;48:277-286. doi:10.1016/j.pnpbp.2012.10.022
21. Conen S, Gregory CJ, Hinz R, et al. Neuroinflammation as measured by positron emission tomography in patients with recent onset and established schizophrenia: implications for immune pathogenesis. Mol Psychiatry. 2021;26(9):5398-5406. doi:10.1038/S41380-020-0829-Y
22. Najjar S, Pahlajani S, De Sanctis V, et al. Neurovascular unit dysfunction and blood-brain barrier hyperpermeability contribute to schizophrenia neurobiology: a theoretical integration of clinical and experimental evidence. Front Psychiatry. 2017;8:83. doi:10.3389/fpsyt.2017.00083
23. Pinjari OF, Dasgupta SK, Okusaga OO. Plasma soluble P-selectin, interleukin-6 and S100B protein in patients with schizophrenia: a pilot study. Psychiatr Q. 2022;93(1):335-345. doi:10.1007/s11126-021-09954-3
24. Di Biase MA, Zalesky A, O’keefe G, et al. PET imaging of putative microglial activation in individuals at ultra-high risk for psychosis, recently diagnosed and chronically ill with schizophrenia. Transl Psychiatry. 2017;7(8):e1225. doi:10.1038/tp.2017.193
25. Holmes SE, Hinz R, Drake RJ, et al. In vivo imaging of brain microglial activity in antipsychotic-free and medicated schizophrenia: a [11C](R)-PK11195 positron emission tomography study. Mol Psychiatry. 2016;21(12):1672-1679. doi:10.1038/mp.2016.180
26. Corsi-Zuelli F, Deakin B, de Lima MHF, et al. T regulatory cells as a potential therapeutic target in psychosis? Current challenges and future perspectives. Brain Behav Immun Health. 2021;17:100330. doi:10.1016/j.bbih.2021.100330
27. Bannai D, Lutz O, Lizano P. Neuroimaging considerations when investigating choroid plexus morphology in idiopathic psychosis. Schizophr Res. 2020;224:19-21. doi:10.1016/j.schres.2020.07.013
28. Hladky SB, Barrand MA. Fluid and ion transfer across the blood-brain and blood-cerebrospinal fluid barriers; a comparative account of mechanisms and roles. Fluids Barriers CNS. 2016;13(1):19. doi:10.1186/s12987-016-0040-3
29. Lizano P, Lutz O, Ling G, et al. Association of choroid plexus enlargement with cognitive, inflammatory, and structural phenotypes across the psychosis spectrum. Am J Psychiatry. 2019;176(7):564-572. doi:10.1176/appi.ajp.2019.18070825
30. Castellani G, Contarini G, Mereu M, et al. Dopamine-mediated immunomodulation affects choroid plexus function. Brain Behav Immun. 2019;81:138-150. doi:10.1016/j.bbi.2019.06.006
31. Bitanihirwe BKY, Lizano P, Woo TW. Deconstructing the functional neuroanatomy of the choroid plexus: an ontogenetic perspective for studying neurodevelopmental and neuropsychiatric disorders. Mol Psychiatry. 2022;1-10. doi:10.1038/s41380-022-01623-6
The blood-brain barrier (BBB) is an essential barrier of closely spaced cells that regulates entry into the CNS. What passes should be highly regulated to protect the brain from potentially harmful peripheral cells or molecules from the rest of the body. However, research has revealed that the BBB is pathologically permeable in several disease states, including schizophrenia, epilepsy, traumatic brain injury, autism, and DiGeorge syndrome (22q11.2 deletion syndrome, which often presents with symptoms of schizophrenia).1,2 In this article, we discuss potential markers of BBB dysfunction, the consequences of a porous BBB, the effect of BBB permeability on microglial activation, and possible treatment implications.
Detecting a BBB leak
The BBB is composed of microvascular endothelial cell units. Adherens junctions, astrocyte endfeet, and pericytes are all part of these units, but tight junctions have the most significant role in BBB barrier function. Tight junction protein composition varies depending on the location of the endothelium. In the BBB, they are primarily composed of claudin-5, occludin, zonulin, and junction adhesion molecules (JAMs) (Figure). Claudins and occludins are especially important components of the tight junction because they span plasma membranes.3

Researchers began to suspect tight junction permeability in schizophrenia while searching for schizophrenia biomarkers. For example, S100B is a marker of astrocytic reactivity to damage. It is increased in schizophrenia, major depressive disorder, and bipolar disorder.4 Studies found elevated S100B specifically in drug-free patients with schizophrenia,5 which prompted research suggesting it could predict the severity of negative symptoms.6 The accuracy of S100B as a biomarker was later complicated by the finding that adipose tissue also secretes S100B. This is problematic due to the high rates of comorbid obesity in psychiatric populations.2
Perhaps a better biomarker is the ratio of albumin in the CSF vs that in peripheral serum. The CSF-to-blood albumin ratio (Q-Alb) is widely considered an acceptable marker of BBB dysfunction because albumin must cross the BBB to alter the ratio. Studies have found a high Q-Alb in neurodegenerative disorders such as multiple sclerosis as well as in schizophrenia, which suggests that some level of BBB dysfunction is occurring. Although the Q-Alb may change slightly when confounded by antipsychotic use or with CSF flow changes,2,4 both S100B and Q-Alb elevation are sufficient for further investigation into tight junction alteration in schizophrenia.
Claudin-5 is a promising factor in detecting BBB permeability. Claudin-5 is deleted in DiGeorge syndrome, which is highly comorbid with schizophrenia and psychosis.1 Mouse knockdown studies show that full suppression of claudin-5 results in psychotic symptoms before fatal seizures,2,7 but a partial absence may enable psychotic symptoms. The same study showed that normally continuous claudin-5 was patchy along blood vessels in the affected sample.7 Follow-up experiments suggest that loss of claudin-5 in schizophrenia is especially prominent in the hippocampus, and there is mixed evidence of a decrease in the prefrontal cortex.8
Outside of claudin-5 alone, JAM-A plays a more regulatory role. It is upstream from an enhancer protein gene that serves as a transcription factor for the claudin-5 promoter, so when JAM-A is deleted, there is less claudin-5.9 However, while this decrease in claudin-5 may be pathological, there could still be various upstream changes that lead to schizophrenia.
What are the consequences of a porous BBB?
Although it is well established that the BBB passes small molecules and solutes, there is significant evidence of inflammatory trafficking in disease states. The BBB moves proinflammatory cytokines, alters transporters, and may even let white blood cells (WBCs) pass through. Immune cell infiltration has different requirements depending on the cell type. T cells rely on integrins, vascular cell adhesion molecule 1 (vCAM1), and intercellular adhesion molecule 1 (iCAM1) for binding, rolling, adhering, crossing, and migration to sites of inflammation.10,11 Both iCAM1 and vCAM1 are elevated in schizophrenia compared to other psychiatric disorders (such as unipolar depression) and correlate with other biomarkers. For example, vCAM1, responsible for recruitment and crossing, is correlated with a high Q-Alb.12 Primarily produced by astrocytes and endothelial cells, iCAM1 plays the largest role in crossing the BBB and migration. Postmortem tissue demonstrates that cytokines upregulate iCAM1 mRNA at the BBB in schizophrenia.13 Increased cytokines are well documented in the inflammatory model of schizophrenia. Interestingly, decreasing claudin-5 also upregulates iCAM1 production.14 Therefore, low baseline claudin-5 may contribute to additional inflammation and symptoms.
Continue to: BBB permeability also results...
BBB permeability also results in a certain pattern of leukocyte and cytokine activity. Interleukin-1 (IL-1), IL-6, and tumor necrosis factor–alpha can all cross the BBB during neuroimmune inflammation,10 but there are abnormal heightened and sustained responses of these molecules in schizophrenia. IL-6 is a proinflammatory cytokine in both acute and chronic inflammation that is expressed by astrocytes, endothelial cells, and microglia.15 IL-6 and its soluble receptor are both elevated in schizophrenia and are associated with white matter degeneration16,17 and an increase in vCAM1.15 This implies that while neuroinflammation in schizophrenia is occurring, additional leukocytes are being recruited and secreting their own cytokines in a chronic destructive positive feedback loop. Meanwhile, atypical IL-10 levels can no longer maintain balanced levels of inflammatory molecules,16 which leads to reduced control of inflammation.
Genetics and immunohistochemistry suggest that the BBB allows the passage of excess B cells and T cells in schizophrenia. Cytokines from WBCs or the BBB during inflammation recruit these additional infiltrating lymphocytes. In gene-wide association studies, there are several genes in schizophrenia important for B cells and T cells in addition to inflammation that interact in a proinflammatory network.16 These cells are also diffusely found in the white matter18 and hippocampal tissue19 of patients with schizophrenia. Taken together, an increase in adhesion molecules, WBCs, and cytokine crosstalk supports a leaky BBB as an important component of the inflammatory model of schizophrenia.
The role of microglia in BBB dysfunction
The effect of BBB permeability on microglial activation is an important caveat in the current research. Although several reports have linked neuroinflammation to confirmed microglial activation in schizophrenia, there is not enough evidence to claim that the BBB alone is the missing link between these theories. Some research suggests that chronic release of cytokines such as IL-6 from macrophages and T cells could increase migration across the BBB for microglial activation.16,20 However, positron emission tomography has shown mixed results at best. Translocator protein (TSPO) is expressed by microglia that are actively secreting cytokines.21 Researchers tracking TSPO changes in relation to BBB alteration have not seen elevated binding in schizophrenia, change due to stage of disease course, or differentiation from low-grade inflammation.21-24 Moreover, TSPO may be confounded by antipsychotic use25 and microglial expression did not correlate with any changes in adhesion molecules.13 TSPO is not an ideal indicator of microglial activation due to BBB breakdown, but that does not bar the possibility of at least a partial contribution to the development of schizophrenia.
Corsi-Zuelli et al26 created a model that attempts to merge BBB permeability and microglial activation through a different medium—T regulatory cells (TRegs). They write that if TRegs mediate interactions between astrocytes and microglia, their hypofunction would impose a prolonged T cell response. The increased access to a high level of IL-6 and its soluble receptors may keep the TRegs hypofunctional in schizophrenia and promote T cell conversion to inflammatory cell types. Experimentally, TReg induction reversed some psychotic symptoms, and greater TReg expression was associated with fewer negative symptoms.26 In an already insufficient BBB, more access to cytokines and leukocytes would sustain inflammation and microglial secretions.
In addition to the issues described regarding the BBB, the blood-CSF barrier at the choroid plexus may also be insufficient in schizophrenia (Box27-31).
Box
The choroid plexus’ primary role is to make CSF, but it also secretes cytokines and to some extent serves as a barrier. Unlike the blood-brain barrier (BBB), the blood-CSF barrier is composed of endothelial cells with fenestrations as well as tight junctions, which make the blood-CSF barrier overall more permeable.27,28 The most unusual finding regarding the choroid plexus in schizophrenia is size. The choroid plexus is physically larger in patients with schizophrenia, and to a lesser extent, in their first-degree relatives.29 A larger choroid plexus is correlated with more severe cognitive symptoms, increased risk for psychosis via biological stress, and significantly higher interleukin-6 (IL-6).27,29 The increased thickness could be an attempt to compensate for hyperactivity and toxic processes in a permeable environment. More circulating cytokines such as IL-6 and tumor necrosis factor–alpha from microglia can trigger an increase in intercellular adhesion molecule 1, resulting in leukocyte attachment and entry.30 Less claudin-5 at the choroid plexus in schizophrenia implicates similar permissive effects as seen at the BBB.31 Although the contribution of blood-CSF barrier dysfunction to schizophrenia requires further study, reduced barrier function outside the BBB is a viable line of inquiry.
Continue to: Caveats about this research
Caveats about this research
There are 3 important points to note about the current research concerning abnormal BBB permeability:
1. BBB dysfunction may exist only in a subset of people diagnosed with schizophrenia. In most human studies, only some patients with schizophrenia demonstrated alterations that suggested pathological BBB permeability. In addition, even when there is BBB dysfunction, it could be a secondary phenomenon, rather than a primary etiologic process.
2. Patient demographics across studies have not always been adequately described. Potential confounds such as obesity, smoking, or antipsychotic use were not consistently recorded or examined as a possible factor.
3. Currently available biomarkers are not perfect. Cytokine elevation, S100B, and Q-Alb are indirect measures of BBB disruption and are found in other disorders. Therefore, they only support the theory of BBB dysfunction in schizophrenia, rather than prove it. They are also not reliable markers for schizophrenia alone. Researchers have pointed out that these markers and proteins work in concert, which necessitates a network analysis approach.16 More research regarding the details of permeability is required to establish more reliable biomarkers and tailored treatment.
Treatment implications
One of the first treatment directions that comes to mind is managing the gaps in the BBB via tight junctions. Presently, there are no FDA-approved medications for altering tight junction proteins, but researchers are exploring potential agents that can induce claudin-5 and reduce inflammation.14 While we wait for such a medication, patients may benefit from existing anti-inflammatory treatments to control immune infiltration and its products. Various anti-inflammatory agents—including cyclooxygenase inhibitors,
Bottom Line
Recent research has revealed that the blood-brain barrier (BBB) is pathologically permeable in several disease states, including schizophrenia. Better characterization of the leaky BBB in schizophrenia has enormous potential in helping us understand how current theories fit together and could serve as a missing puzzle piece in treating schizophrenia.
Related Resources
- Levine A, Strawn JR. The brain’s Twitter system: neuronal extracellular vesicles. Current Psychiatry. 2022;21(6):9-11, 17-19,27. doi:10.12788/cp.0257
Drug Brand Names
Minocycline • Dynacin, Minocin
The blood-brain barrier (BBB) is an essential barrier of closely spaced cells that regulates entry into the CNS. What passes should be highly regulated to protect the brain from potentially harmful peripheral cells or molecules from the rest of the body. However, research has revealed that the BBB is pathologically permeable in several disease states, including schizophrenia, epilepsy, traumatic brain injury, autism, and DiGeorge syndrome (22q11.2 deletion syndrome, which often presents with symptoms of schizophrenia).1,2 In this article, we discuss potential markers of BBB dysfunction, the consequences of a porous BBB, the effect of BBB permeability on microglial activation, and possible treatment implications.
Detecting a BBB leak
The BBB is composed of microvascular endothelial cell units. Adherens junctions, astrocyte endfeet, and pericytes are all part of these units, but tight junctions have the most significant role in BBB barrier function. Tight junction protein composition varies depending on the location of the endothelium. In the BBB, they are primarily composed of claudin-5, occludin, zonulin, and junction adhesion molecules (JAMs) (Figure). Claudins and occludins are especially important components of the tight junction because they span plasma membranes.3

Researchers began to suspect tight junction permeability in schizophrenia while searching for schizophrenia biomarkers. For example, S100B is a marker of astrocytic reactivity to damage. It is increased in schizophrenia, major depressive disorder, and bipolar disorder.4 Studies found elevated S100B specifically in drug-free patients with schizophrenia,5 which prompted research suggesting it could predict the severity of negative symptoms.6 The accuracy of S100B as a biomarker was later complicated by the finding that adipose tissue also secretes S100B. This is problematic due to the high rates of comorbid obesity in psychiatric populations.2
Perhaps a better biomarker is the ratio of albumin in the CSF vs that in peripheral serum. The CSF-to-blood albumin ratio (Q-Alb) is widely considered an acceptable marker of BBB dysfunction because albumin must cross the BBB to alter the ratio. Studies have found a high Q-Alb in neurodegenerative disorders such as multiple sclerosis as well as in schizophrenia, which suggests that some level of BBB dysfunction is occurring. Although the Q-Alb may change slightly when confounded by antipsychotic use or with CSF flow changes,2,4 both S100B and Q-Alb elevation are sufficient for further investigation into tight junction alteration in schizophrenia.
Claudin-5 is a promising factor in detecting BBB permeability. Claudin-5 is deleted in DiGeorge syndrome, which is highly comorbid with schizophrenia and psychosis.1 Mouse knockdown studies show that full suppression of claudin-5 results in psychotic symptoms before fatal seizures,2,7 but a partial absence may enable psychotic symptoms. The same study showed that normally continuous claudin-5 was patchy along blood vessels in the affected sample.7 Follow-up experiments suggest that loss of claudin-5 in schizophrenia is especially prominent in the hippocampus, and there is mixed evidence of a decrease in the prefrontal cortex.8
Outside of claudin-5 alone, JAM-A plays a more regulatory role. It is upstream from an enhancer protein gene that serves as a transcription factor for the claudin-5 promoter, so when JAM-A is deleted, there is less claudin-5.9 However, while this decrease in claudin-5 may be pathological, there could still be various upstream changes that lead to schizophrenia.
What are the consequences of a porous BBB?
Although it is well established that the BBB passes small molecules and solutes, there is significant evidence of inflammatory trafficking in disease states. The BBB moves proinflammatory cytokines, alters transporters, and may even let white blood cells (WBCs) pass through. Immune cell infiltration has different requirements depending on the cell type. T cells rely on integrins, vascular cell adhesion molecule 1 (vCAM1), and intercellular adhesion molecule 1 (iCAM1) for binding, rolling, adhering, crossing, and migration to sites of inflammation.10,11 Both iCAM1 and vCAM1 are elevated in schizophrenia compared to other psychiatric disorders (such as unipolar depression) and correlate with other biomarkers. For example, vCAM1, responsible for recruitment and crossing, is correlated with a high Q-Alb.12 Primarily produced by astrocytes and endothelial cells, iCAM1 plays the largest role in crossing the BBB and migration. Postmortem tissue demonstrates that cytokines upregulate iCAM1 mRNA at the BBB in schizophrenia.13 Increased cytokines are well documented in the inflammatory model of schizophrenia. Interestingly, decreasing claudin-5 also upregulates iCAM1 production.14 Therefore, low baseline claudin-5 may contribute to additional inflammation and symptoms.
Continue to: BBB permeability also results...
BBB permeability also results in a certain pattern of leukocyte and cytokine activity. Interleukin-1 (IL-1), IL-6, and tumor necrosis factor–alpha can all cross the BBB during neuroimmune inflammation,10 but there are abnormal heightened and sustained responses of these molecules in schizophrenia. IL-6 is a proinflammatory cytokine in both acute and chronic inflammation that is expressed by astrocytes, endothelial cells, and microglia.15 IL-6 and its soluble receptor are both elevated in schizophrenia and are associated with white matter degeneration16,17 and an increase in vCAM1.15 This implies that while neuroinflammation in schizophrenia is occurring, additional leukocytes are being recruited and secreting their own cytokines in a chronic destructive positive feedback loop. Meanwhile, atypical IL-10 levels can no longer maintain balanced levels of inflammatory molecules,16 which leads to reduced control of inflammation.
Genetics and immunohistochemistry suggest that the BBB allows the passage of excess B cells and T cells in schizophrenia. Cytokines from WBCs or the BBB during inflammation recruit these additional infiltrating lymphocytes. In gene-wide association studies, there are several genes in schizophrenia important for B cells and T cells in addition to inflammation that interact in a proinflammatory network.16 These cells are also diffusely found in the white matter18 and hippocampal tissue19 of patients with schizophrenia. Taken together, an increase in adhesion molecules, WBCs, and cytokine crosstalk supports a leaky BBB as an important component of the inflammatory model of schizophrenia.
The role of microglia in BBB dysfunction
The effect of BBB permeability on microglial activation is an important caveat in the current research. Although several reports have linked neuroinflammation to confirmed microglial activation in schizophrenia, there is not enough evidence to claim that the BBB alone is the missing link between these theories. Some research suggests that chronic release of cytokines such as IL-6 from macrophages and T cells could increase migration across the BBB for microglial activation.16,20 However, positron emission tomography has shown mixed results at best. Translocator protein (TSPO) is expressed by microglia that are actively secreting cytokines.21 Researchers tracking TSPO changes in relation to BBB alteration have not seen elevated binding in schizophrenia, change due to stage of disease course, or differentiation from low-grade inflammation.21-24 Moreover, TSPO may be confounded by antipsychotic use25 and microglial expression did not correlate with any changes in adhesion molecules.13 TSPO is not an ideal indicator of microglial activation due to BBB breakdown, but that does not bar the possibility of at least a partial contribution to the development of schizophrenia.
Corsi-Zuelli et al26 created a model that attempts to merge BBB permeability and microglial activation through a different medium—T regulatory cells (TRegs). They write that if TRegs mediate interactions between astrocytes and microglia, their hypofunction would impose a prolonged T cell response. The increased access to a high level of IL-6 and its soluble receptors may keep the TRegs hypofunctional in schizophrenia and promote T cell conversion to inflammatory cell types. Experimentally, TReg induction reversed some psychotic symptoms, and greater TReg expression was associated with fewer negative symptoms.26 In an already insufficient BBB, more access to cytokines and leukocytes would sustain inflammation and microglial secretions.
In addition to the issues described regarding the BBB, the blood-CSF barrier at the choroid plexus may also be insufficient in schizophrenia (Box27-31).
Box
The choroid plexus’ primary role is to make CSF, but it also secretes cytokines and to some extent serves as a barrier. Unlike the blood-brain barrier (BBB), the blood-CSF barrier is composed of endothelial cells with fenestrations as well as tight junctions, which make the blood-CSF barrier overall more permeable.27,28 The most unusual finding regarding the choroid plexus in schizophrenia is size. The choroid plexus is physically larger in patients with schizophrenia, and to a lesser extent, in their first-degree relatives.29 A larger choroid plexus is correlated with more severe cognitive symptoms, increased risk for psychosis via biological stress, and significantly higher interleukin-6 (IL-6).27,29 The increased thickness could be an attempt to compensate for hyperactivity and toxic processes in a permeable environment. More circulating cytokines such as IL-6 and tumor necrosis factor–alpha from microglia can trigger an increase in intercellular adhesion molecule 1, resulting in leukocyte attachment and entry.30 Less claudin-5 at the choroid plexus in schizophrenia implicates similar permissive effects as seen at the BBB.31 Although the contribution of blood-CSF barrier dysfunction to schizophrenia requires further study, reduced barrier function outside the BBB is a viable line of inquiry.
Continue to: Caveats about this research
Caveats about this research
There are 3 important points to note about the current research concerning abnormal BBB permeability:
1. BBB dysfunction may exist only in a subset of people diagnosed with schizophrenia. In most human studies, only some patients with schizophrenia demonstrated alterations that suggested pathological BBB permeability. In addition, even when there is BBB dysfunction, it could be a secondary phenomenon, rather than a primary etiologic process.
2. Patient demographics across studies have not always been adequately described. Potential confounds such as obesity, smoking, or antipsychotic use were not consistently recorded or examined as a possible factor.
3. Currently available biomarkers are not perfect. Cytokine elevation, S100B, and Q-Alb are indirect measures of BBB disruption and are found in other disorders. Therefore, they only support the theory of BBB dysfunction in schizophrenia, rather than prove it. They are also not reliable markers for schizophrenia alone. Researchers have pointed out that these markers and proteins work in concert, which necessitates a network analysis approach.16 More research regarding the details of permeability is required to establish more reliable biomarkers and tailored treatment.
Treatment implications
One of the first treatment directions that comes to mind is managing the gaps in the BBB via tight junctions. Presently, there are no FDA-approved medications for altering tight junction proteins, but researchers are exploring potential agents that can induce claudin-5 and reduce inflammation.14 While we wait for such a medication, patients may benefit from existing anti-inflammatory treatments to control immune infiltration and its products. Various anti-inflammatory agents—including cyclooxygenase inhibitors,
Bottom Line
Recent research has revealed that the blood-brain barrier (BBB) is pathologically permeable in several disease states, including schizophrenia. Better characterization of the leaky BBB in schizophrenia has enormous potential in helping us understand how current theories fit together and could serve as a missing puzzle piece in treating schizophrenia.
Related Resources
- Levine A, Strawn JR. The brain’s Twitter system: neuronal extracellular vesicles. Current Psychiatry. 2022;21(6):9-11, 17-19,27. doi:10.12788/cp.0257
Drug Brand Names
Minocycline • Dynacin, Minocin
1. Li Y, Xia Y, Zhu H, et al. Investigation of neurodevelopmental deficits of 22 q11.2 deletion syndrome with a patient-iPSC-derived blood-brain barrier model. Cells. 2021;10(10):2576. doi:10.3390/cells10102576
2. Kealy J, Greene C, Campbell M. Blood-brain barrier regulation in psychiatric disorders. Neurosci Lett. 2020;726:133664. doi:10.1016/j.neulet.2018.06.033
3. Ballabh P, Braun A, Nedergaard M. The blood-brain barrier: an overview: structure, regulation, and clinical implications. Neurobiol Dis. 2004;16(1):1-13. doi:10.1016/j.nbd.2003.12.016
4. Futtrup J, Margolinsky R, Benros ME, et al. Blood-brain barrier pathology in patients with severe mental disorders: a systematic review and meta-analysis of biomarkers in case-control studies. Brain Behav Immun Health. 2020;6:100102. doi:10.1016/j.bbih.2020.100102
5. Chen S, Tian L, Chen N, et al. Cognitive dysfunction correlates with elevated serum S100B concentration in drug-free acutely relapsed patients with schizophrenia. Psychiatry Res. 2017;247:6-11. doi:10.1016/j.psychres.2016.09.029
6. Wu YF, Sytwu HK, Lung FW. Human aquaporin 4 gene polymorphisms and haplotypes are associated with serum S100B level and negative symptoms of schizophrenia in a southern Chinese Han population. Front Psychiatry. 2018;9:657. doi:10.3389/fpsyt.2018.00657
7. Greene C, Kealy J, Humphries MM, et al. Dose-dependent expression of claudin-5 is a modifying factor in schizophrenia. Mol Psychiatry. 2018;23(11):2156-2166. doi:10.1038/MP.2017.156
8. Greene C, Hanley N, Campbell M. Blood-brain barrier associated tight junction disruption is a hallmark feature of major psychiatric disorders. Transl Psychiatry. 2020;10(1):373. doi:10.1038/s41398-020-01054-3
9. Kakogiannos N, Ferrari L, Giampietro C, et al. JAM-A acts via C/EBP-α to promote claudin-5 expression and enhance endothelial barrier function. Circ Res. 2020:1056-1073. doi:10.1161/CIRCRESAHA.120.316742
10. Erickson MA, Dohi K, Banks WA. Neuroinflammation: a common pathway in CNS diseases as mediated at the blood-brain barrier. Neuroimmunomodulation. 2012;19(2):121-130. doi:10.1159/000330247
11. Ao LY, Yan YY, Zhou L, et al. Immune cells after ischemic stroke onset: roles, migration, and target intervention. J Mol Neurosci. 2018;66(3):342-355. doi:10.1007/s12031-018-1173-4
12. Meixensberger S, Kuzior H, Fiebich BL, et al. Upregulation of sICAM-1 and sVCAM-1 levels in the cerebrospinal fluid of patients with schizophrenia spectrum disorders. Diagnostics (Basel). 2021;11(7):1134. doi:10.3390diagnostics11071134
13. Cai HQ, Catts VS, Webster MJ, et al. Increased macrophages and changed brain endothelial cell gene expression in the frontal cortex of people with schizophrenia displaying inflammation. Mol Psychiatry. 2020;25(4):761-775. doi:10.1038/s41380-018-0235-x
14. Greene C, Hanley N, Reschke CR, et al. Microvascular stabilization via blood-brain barrier regulation prevents seizure activity. Nat Commun. 2022;13(1):2003. doi:10.1038/s41467-022-29657-y
15. García-Juárez M, Camacho-Morales A. Defining the role of anti- and pro-inflammatory outcomes of interleukin-6 in mental health. Neuroscience. 2022;492:32-46. doi:10.1016/j.neuroscience.2022.03.020
16. Pong S, Karmacharya R, Sofman M, et al. The role of brain microvascular endothelial cell and blood-brain barrier dysfunction in schizophrenia. Complex Psychiatry. 2020;6(1-2):30-46. doi:10.1159/000511552
17. Patel A, Zhu Y, Kuzhikandathil EV, et al. Soluble interleukin-6 receptor induces motor stereotypies and co-localizes with gp130 in regions linked to cortico-striato-thalamo-cortical circuits. PLoS One. 2012;7(7): e41623. doi:10.1371/journal.pone.0041623
18. Schlaaff K, Dobrowolny H, Frodl T, et al. Increased densities of T and B lymphocytes indicate neuroinflammation in subgroups of schizophrenia and mood disorder patients. Brain Behav Immun. 2020;88:497-506. doi:10.1016/j.bbi.2020.04.021
19. Busse S, Busse M, Schiltz K, et al. Different distribution patterns of lymphocytes and microglia in the hippocampus of patients with residual versus paranoid schizophrenia: further evidence for disease course-related immune alterations? Brain Behav Immun. 2012;26(8):1273-1279. doi:10.1016/j.bbi.2012.08.005
20. Na KS, Jung HY, Kim YK. The role of pro-inflammatory cytokines in the neuroinflammation and neurogenesis of schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2014;48:277-286. doi:10.1016/j.pnpbp.2012.10.022
21. Conen S, Gregory CJ, Hinz R, et al. Neuroinflammation as measured by positron emission tomography in patients with recent onset and established schizophrenia: implications for immune pathogenesis. Mol Psychiatry. 2021;26(9):5398-5406. doi:10.1038/S41380-020-0829-Y
22. Najjar S, Pahlajani S, De Sanctis V, et al. Neurovascular unit dysfunction and blood-brain barrier hyperpermeability contribute to schizophrenia neurobiology: a theoretical integration of clinical and experimental evidence. Front Psychiatry. 2017;8:83. doi:10.3389/fpsyt.2017.00083
23. Pinjari OF, Dasgupta SK, Okusaga OO. Plasma soluble P-selectin, interleukin-6 and S100B protein in patients with schizophrenia: a pilot study. Psychiatr Q. 2022;93(1):335-345. doi:10.1007/s11126-021-09954-3
24. Di Biase MA, Zalesky A, O’keefe G, et al. PET imaging of putative microglial activation in individuals at ultra-high risk for psychosis, recently diagnosed and chronically ill with schizophrenia. Transl Psychiatry. 2017;7(8):e1225. doi:10.1038/tp.2017.193
25. Holmes SE, Hinz R, Drake RJ, et al. In vivo imaging of brain microglial activity in antipsychotic-free and medicated schizophrenia: a [11C](R)-PK11195 positron emission tomography study. Mol Psychiatry. 2016;21(12):1672-1679. doi:10.1038/mp.2016.180
26. Corsi-Zuelli F, Deakin B, de Lima MHF, et al. T regulatory cells as a potential therapeutic target in psychosis? Current challenges and future perspectives. Brain Behav Immun Health. 2021;17:100330. doi:10.1016/j.bbih.2021.100330
27. Bannai D, Lutz O, Lizano P. Neuroimaging considerations when investigating choroid plexus morphology in idiopathic psychosis. Schizophr Res. 2020;224:19-21. doi:10.1016/j.schres.2020.07.013
28. Hladky SB, Barrand MA. Fluid and ion transfer across the blood-brain and blood-cerebrospinal fluid barriers; a comparative account of mechanisms and roles. Fluids Barriers CNS. 2016;13(1):19. doi:10.1186/s12987-016-0040-3
29. Lizano P, Lutz O, Ling G, et al. Association of choroid plexus enlargement with cognitive, inflammatory, and structural phenotypes across the psychosis spectrum. Am J Psychiatry. 2019;176(7):564-572. doi:10.1176/appi.ajp.2019.18070825
30. Castellani G, Contarini G, Mereu M, et al. Dopamine-mediated immunomodulation affects choroid plexus function. Brain Behav Immun. 2019;81:138-150. doi:10.1016/j.bbi.2019.06.006
31. Bitanihirwe BKY, Lizano P, Woo TW. Deconstructing the functional neuroanatomy of the choroid plexus: an ontogenetic perspective for studying neurodevelopmental and neuropsychiatric disorders. Mol Psychiatry. 2022;1-10. doi:10.1038/s41380-022-01623-6
1. Li Y, Xia Y, Zhu H, et al. Investigation of neurodevelopmental deficits of 22 q11.2 deletion syndrome with a patient-iPSC-derived blood-brain barrier model. Cells. 2021;10(10):2576. doi:10.3390/cells10102576
2. Kealy J, Greene C, Campbell M. Blood-brain barrier regulation in psychiatric disorders. Neurosci Lett. 2020;726:133664. doi:10.1016/j.neulet.2018.06.033
3. Ballabh P, Braun A, Nedergaard M. The blood-brain barrier: an overview: structure, regulation, and clinical implications. Neurobiol Dis. 2004;16(1):1-13. doi:10.1016/j.nbd.2003.12.016
4. Futtrup J, Margolinsky R, Benros ME, et al. Blood-brain barrier pathology in patients with severe mental disorders: a systematic review and meta-analysis of biomarkers in case-control studies. Brain Behav Immun Health. 2020;6:100102. doi:10.1016/j.bbih.2020.100102
5. Chen S, Tian L, Chen N, et al. Cognitive dysfunction correlates with elevated serum S100B concentration in drug-free acutely relapsed patients with schizophrenia. Psychiatry Res. 2017;247:6-11. doi:10.1016/j.psychres.2016.09.029
6. Wu YF, Sytwu HK, Lung FW. Human aquaporin 4 gene polymorphisms and haplotypes are associated with serum S100B level and negative symptoms of schizophrenia in a southern Chinese Han population. Front Psychiatry. 2018;9:657. doi:10.3389/fpsyt.2018.00657
7. Greene C, Kealy J, Humphries MM, et al. Dose-dependent expression of claudin-5 is a modifying factor in schizophrenia. Mol Psychiatry. 2018;23(11):2156-2166. doi:10.1038/MP.2017.156
8. Greene C, Hanley N, Campbell M. Blood-brain barrier associated tight junction disruption is a hallmark feature of major psychiatric disorders. Transl Psychiatry. 2020;10(1):373. doi:10.1038/s41398-020-01054-3
9. Kakogiannos N, Ferrari L, Giampietro C, et al. JAM-A acts via C/EBP-α to promote claudin-5 expression and enhance endothelial barrier function. Circ Res. 2020:1056-1073. doi:10.1161/CIRCRESAHA.120.316742
10. Erickson MA, Dohi K, Banks WA. Neuroinflammation: a common pathway in CNS diseases as mediated at the blood-brain barrier. Neuroimmunomodulation. 2012;19(2):121-130. doi:10.1159/000330247
11. Ao LY, Yan YY, Zhou L, et al. Immune cells after ischemic stroke onset: roles, migration, and target intervention. J Mol Neurosci. 2018;66(3):342-355. doi:10.1007/s12031-018-1173-4
12. Meixensberger S, Kuzior H, Fiebich BL, et al. Upregulation of sICAM-1 and sVCAM-1 levels in the cerebrospinal fluid of patients with schizophrenia spectrum disorders. Diagnostics (Basel). 2021;11(7):1134. doi:10.3390diagnostics11071134
13. Cai HQ, Catts VS, Webster MJ, et al. Increased macrophages and changed brain endothelial cell gene expression in the frontal cortex of people with schizophrenia displaying inflammation. Mol Psychiatry. 2020;25(4):761-775. doi:10.1038/s41380-018-0235-x
14. Greene C, Hanley N, Reschke CR, et al. Microvascular stabilization via blood-brain barrier regulation prevents seizure activity. Nat Commun. 2022;13(1):2003. doi:10.1038/s41467-022-29657-y
15. García-Juárez M, Camacho-Morales A. Defining the role of anti- and pro-inflammatory outcomes of interleukin-6 in mental health. Neuroscience. 2022;492:32-46. doi:10.1016/j.neuroscience.2022.03.020
16. Pong S, Karmacharya R, Sofman M, et al. The role of brain microvascular endothelial cell and blood-brain barrier dysfunction in schizophrenia. Complex Psychiatry. 2020;6(1-2):30-46. doi:10.1159/000511552
17. Patel A, Zhu Y, Kuzhikandathil EV, et al. Soluble interleukin-6 receptor induces motor stereotypies and co-localizes with gp130 in regions linked to cortico-striato-thalamo-cortical circuits. PLoS One. 2012;7(7): e41623. doi:10.1371/journal.pone.0041623
18. Schlaaff K, Dobrowolny H, Frodl T, et al. Increased densities of T and B lymphocytes indicate neuroinflammation in subgroups of schizophrenia and mood disorder patients. Brain Behav Immun. 2020;88:497-506. doi:10.1016/j.bbi.2020.04.021
19. Busse S, Busse M, Schiltz K, et al. Different distribution patterns of lymphocytes and microglia in the hippocampus of patients with residual versus paranoid schizophrenia: further evidence for disease course-related immune alterations? Brain Behav Immun. 2012;26(8):1273-1279. doi:10.1016/j.bbi.2012.08.005
20. Na KS, Jung HY, Kim YK. The role of pro-inflammatory cytokines in the neuroinflammation and neurogenesis of schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2014;48:277-286. doi:10.1016/j.pnpbp.2012.10.022
21. Conen S, Gregory CJ, Hinz R, et al. Neuroinflammation as measured by positron emission tomography in patients with recent onset and established schizophrenia: implications for immune pathogenesis. Mol Psychiatry. 2021;26(9):5398-5406. doi:10.1038/S41380-020-0829-Y
22. Najjar S, Pahlajani S, De Sanctis V, et al. Neurovascular unit dysfunction and blood-brain barrier hyperpermeability contribute to schizophrenia neurobiology: a theoretical integration of clinical and experimental evidence. Front Psychiatry. 2017;8:83. doi:10.3389/fpsyt.2017.00083
23. Pinjari OF, Dasgupta SK, Okusaga OO. Plasma soluble P-selectin, interleukin-6 and S100B protein in patients with schizophrenia: a pilot study. Psychiatr Q. 2022;93(1):335-345. doi:10.1007/s11126-021-09954-3
24. Di Biase MA, Zalesky A, O’keefe G, et al. PET imaging of putative microglial activation in individuals at ultra-high risk for psychosis, recently diagnosed and chronically ill with schizophrenia. Transl Psychiatry. 2017;7(8):e1225. doi:10.1038/tp.2017.193
25. Holmes SE, Hinz R, Drake RJ, et al. In vivo imaging of brain microglial activity in antipsychotic-free and medicated schizophrenia: a [11C](R)-PK11195 positron emission tomography study. Mol Psychiatry. 2016;21(12):1672-1679. doi:10.1038/mp.2016.180
26. Corsi-Zuelli F, Deakin B, de Lima MHF, et al. T regulatory cells as a potential therapeutic target in psychosis? Current challenges and future perspectives. Brain Behav Immun Health. 2021;17:100330. doi:10.1016/j.bbih.2021.100330
27. Bannai D, Lutz O, Lizano P. Neuroimaging considerations when investigating choroid plexus morphology in idiopathic psychosis. Schizophr Res. 2020;224:19-21. doi:10.1016/j.schres.2020.07.013
28. Hladky SB, Barrand MA. Fluid and ion transfer across the blood-brain and blood-cerebrospinal fluid barriers; a comparative account of mechanisms and roles. Fluids Barriers CNS. 2016;13(1):19. doi:10.1186/s12987-016-0040-3
29. Lizano P, Lutz O, Ling G, et al. Association of choroid plexus enlargement with cognitive, inflammatory, and structural phenotypes across the psychosis spectrum. Am J Psychiatry. 2019;176(7):564-572. doi:10.1176/appi.ajp.2019.18070825
30. Castellani G, Contarini G, Mereu M, et al. Dopamine-mediated immunomodulation affects choroid plexus function. Brain Behav Immun. 2019;81:138-150. doi:10.1016/j.bbi.2019.06.006
31. Bitanihirwe BKY, Lizano P, Woo TW. Deconstructing the functional neuroanatomy of the choroid plexus: an ontogenetic perspective for studying neurodevelopmental and neuropsychiatric disorders. Mol Psychiatry. 2022;1-10. doi:10.1038/s41380-022-01623-6
Adaptive changes to antipsychotics: How to avoid the consequences
While our understanding of the mechanisms of psychosis continues to evolve beyond the dopamine hypothesis, the key role of dopamine in psychosis and its treatment has not faded.1 Over time, the dopamine hypothesis of schizophrenia has evolved from focusing on dopamine hyperactivity to specifying the regional abnormalities in the brain with subcortical hyperdopaminergia and prefrontal hypodopaminergia.2 Despite this divergence in dopaminergic function, antipsychotic medications that block dopamine D2 receptors (D2R) remain central to treating psychotic symptoms and preventing relapse.3,4 Notably, antipsychotics block both presynaptic and postsynaptic receptors affecting the regulation of dopamine synthesis and release in the brain.5,6
Chronic dopamine D2R blockade with antipsychotics induces adaptive changes that can contribute to both acute and chronic adverse effects. In this article, we discuss these changes, and steps clinicians can take to minimize their occurrence.
Dopamine D2R: A primer
There are 5 types of dopamine receptors, numbered D1 through D5, but there are only 2 families of dopamine receptors: the D1 family (D1 and D5), and the D2 family (D2, D3, and D4). All dopamine receptors are G protein–coupled, but the D2 family of receptors generally increases protein kinase A (PKA) as the second messenger, whereas the D1 family increases cyclic adenosine monophosphate (cAMP) as the second messenger.5 There are 2 distinct variants of the D2R of 2 different lengths made from the same gene (DRD2) via posttranslational modification. The long isoform of D2R (D2L) has an additional 29 amino acids compared to the short isoform (D2S).7 Additional evidence points to a third splice variant called D2Longer that arises from aberrant RNA splicing and contains 2 more amino acids than D2L; its relevance is not known.8
The D2L isoform is the primary postsynaptic receptor, expressed more in the striatum and nucleus accumbens (NAc) targeted by dopaminergic afferents. The D2S isoform, however, is predominantly presynaptic, more densely expressed on cell bodies and projection axons of the dopaminergic neurons of the midbrain and hypothalamus.9 Each isoform contributes differentially to the therapeutic and adverse effects of antipsychotics, and evidence from animal studies suggests that D2L is the main variant responsible for drug-induced parkinsonism.10 The D2S acts as the principal autoreceptor for the dopaminergic system.5,11,12
Autoreceptors regulate dopamine transmission. Dopamine itself and D2R agonists are reported to have higher affinity and potency with D2S. Activation of these autoreceptors is a negative feedback mechanism that decreases dopamine release. Similarly, when they are blocked (such as with use of an antipsychotic), there is an increase in dopamine release. Additionally, these autoreceptors modulate several key processes:
- neuronal firing rate by activating potassium conductance
- dopamine synthesis by downregulating the expression of tyrosine hydroxylase (TH) enzyme (the rate-limiting step)
- exocytotic release of dopamine and other neurotransmitters
- dopamine reuptake via increasing the activity of the dopamine transporter (DAT).12
Consequences of antipsychotic D2R blockade
Most antipsychotics begin to produce a therapeutic antipsychotic effect at 65% to 75% occupancy of the D2Rs.3 This level also produces an optimal balance between clinical efficacy and a lower incidence of adverse effects.3 A higher D2R occupancy by both first-generation (FGA) and second-generation (SGA) pure antagonist antipsychotics can lead to parkinsonism.
Parkinsonism is associated with the subsequent appearance of one of the most distressing consequences of long-term antipsychotic treatment, tardive dyskinesia (TD).13 TD is an iatrogenic, usually late-onset syndrome consisting of persistent, involuntary, and repetitive movements. It classically involves the highly innervated striated muscles of the tongue, mouth, face, and fingers, though it can also involve the trunk and extremities.14 It occurs secondary to chronic exposure to dopamine receptor–blocking agents, including dopaminergic antiemetics.15 The prevalence of TD is higher in patients treated long-term with FGAs (30.0% to 32.4%) than in those treated with SGAs (13.1% to 20.7%) due to serotonin 5HT2A blockade that results in increased dopamine release in the basal ganglia.16
Continue to: Dopamine supersenstivity psychosis...
Dopamine supersensitivity psychosis (DSP) is a term that describes the clinical iatrogenic phenomenon that might be observed with long-term antipsychotic treatment. DSP is suggested to be strongly associated with treatment failure/resistance in schizophrenia.17,18 Manifestations of DSP include development of antipsychotic drug tolerance that undermines treatment efficacy, rebound psychosis during or after treatment discontinuation, and the presence of TD. Like TD, it may be reversed temporarily by increasing the dose of the antipsychotic.18
DSP and (more extensively) TD are commonly hypothesized to result from the postsynaptic dopamine receptor supersensitivity that develops because of chronic D2Rs blockade by antipsychotics. Neostriatal dopamine receptor supersensitivity is believed to lead to TD, while mesolimbic supersensitivity leads to DSP.19 Supersensitivity has traditionally been believed to be due to upregulation of postsynaptic D2R number and sensitivity.20,21 However, both TD and DSP are more likely a consequence of a host of compensatory neurobiological adaptations across the synapse that include:
- postsynaptic increase in the number of D2Rs that amplifies the dopamine signal
- an increased number of synapses, dendritic spines, and perforated synapses (seen in animal models), all of which lead to a potentiated dopamine signal
- presynaptic changes with higher levels of dopamine released into the synapse via an increase in quantal size as postsynaptic D2Rs blockade results in more dopamine becoming available in the synapse for recycling via the dopamine transporter
- increased dopamine turnover due to presynaptic D2S autoreceptor blockade.22
So if giving a D2R blocking agent for a long time increases the dopamine signal, at least in some patients, what can the clinician do to treat the psychosis, and not cause changes in the brain that could lead to TD or DSP?
Partial agonist antipsychotics and biased agonism of D2Rs
One approach to try to avoid the compensatory changes to dopamine blockade might be to use a D2R partial agonist.18,23 For example, aripiprazole is a partial agonist at the D2R commonly used to manage schizophrenia and bipolar disorder. It possesses greater affinity at the D2R compared with the serotonin 2A (5-hydroxytryptamine, 5HT2A) serotonin receptor. Unlike full antagonists, aripiprazole requires exceptionally high D2 receptor occupancy (approximately 90%) to be at a clinically effective antipsychotic dose.24,25 This is a general requirement for all D2R partial agonists.26
A partial agonist generally has to possess greater affinity to the receptor than the neurotransmitter with which it is competing. Aripiprazole has more than twice the affinity to D2R than dopamine. Other partial agonists have similarly high, or higher, D2R affinity. Effective antipsychotic partial agonists stimulate the D2Rs at approximately 30% ± 10% the maximal signal achieved with dopamine. This is essentially equivalent to having approximately 70% receptor occupancy with a full antagonist, except it is built into how the molecule works. Having this low-grade partial activation of D2Rs creates multiple receptor-mediated actions:
- reduction of cAMP accumulation
- antagonism to guanosine 5’-0-(3-thio) triphosphate (GTPgamma S) binding with relatively less recruitment of beta-arrestin 2 (these diverging effects on G protein are the definition of biased agonism)
- antagonism of G protein activation of K+ channels (GIRK) activity
- agonism for the inhibition of TH.
Continue to: Additionally, aripiprazole was found...
Additionally, aripiprazole was found to be associated with a lesser increase in dopamine turnover than full antagonist antipsychotics (Figure27) and decreased DAT binding density in NAc and the ventral tegmental area (VTA). The distinctive pharmacologic profile and biased agonism of this drug could be attributed to its ability to activate presynaptic D2 autoreceptors, which, as previously mentioned, regulate dopamine release via negative feedback mechanism.5,25 Cariprazine, another D2R partial agonist, has similar doubling of dopamine turnover.28
Activation of presynaptic D2S receptors ultimately leads to decreased dopamine synthesis and release, which combats or prevents the brain adaptations regarding dopamine supersensitivity and D2Rs upregulation. While TD can still occur occasionally with aripiprazole or other partial agonists,29,30 animal studies show that administration of methamphetamine significantly lowers locomotor response and the density of striatal D2Rs in a group treated with aripiprazole compared to a group treated with haloperidol.31 Aripiprazole also improved the supersensitivity parameters induced by chronic treatment with haloperidol, which suggests that it is associated with reduced dopamine supersensitivity.31 Similarly, in human studies, partial agonists appear to have a lower rate of parkinsonism and TD.32,33 One study reported that aripiprazole was associated with a significant improvement of TD in more than 50% of patients after 24 weeks of treatment.34
Lumateperone’s unique pharmacologic profile
Lumateperone is a newer antipsychotic that was FDA-approved in December 2019 for the treatment of adults with schizophrenia35 and more recently for the treatment of bipolar depression.36 It possesses a unique combination of pharmacologic properties; it is a postsynaptic D2R antagonist and a presynaptic D2R partial agonist.27
Interestingly, lumateperone has regional selectivity. It increases dopamine release in the medial prefrontal cortex (where D2R is rare) but not in the nigrostriatal pathways.27,37 It does not increase TH phosphorylation (which would increase dopamine concentration) or dopamine turnover in the striatum (Figure27). In a preclinical functional activity assay of lumateperone, the lack of change of dopamine turnover with lumateperone resembles placebo and is even less than that observed with aripiprazole (Figure27). This effect is consistent with partial agonism at the presynaptic D2S, where the stimulation of that receptor prevents the concomitant increase in dopamine synthesis and release that occurs when that receptor is blocked.
It is believed that the lack of increase in dopamine turnover is one of the reasons that lumateperone postsynaptic D2R occupancy is exceptionally low at clinically effective doses. In a positron emission tomography study analyzing posttreatment scans after approximately 2 weeks of a 60 mg/d dose, the mean peak striatal D2R occupancy was approximately 40%,38 which is remarkably lower than the 65% to 75% blockade needed for purely antagonist D2R antipsychotics.3 This low receptor occupancy appears to mediate the low incidence of parkinsonism and prolactin release seen with lumateperone.
Continue to: Take-home points
Take-home points
Adaptive upregulation of dopamine neurotransmission underlies acute adverse effects such as parkinsonism and is also key for delayed consequences such as TD, and possibly the development of treatment resistance. Adaptive upregulation results from an increase in postsynaptic dopamine receptors, numbers of synapses, and dopamine release. The latter has been demonstrated to be greatest with full antagonists, less with partial agonists, and not present with lumateperone, which is a postsynaptic antagonist but a presynaptic partial agonist (Figure27). Reducing adaptive upregulation can reduce both acute and long-term consequences of dopamine blockade. Early use of agents that minimize these adaptive changes, such as a postsynaptic partial agonist (aripiprazole, brexpiprazole, or cariprazine) or a presynaptic partial agonist (lumateperone), appears to be a reasonable clinical option.
Bottom Line
Chronic dopamine D2 receptor blockade with antipsychotics induces adaptive changes that can contribute to both acute and chronic adverse effects. The most severe of these are tardive dyskinesia (TD) and dopamine supersensitivity psychosis (DSP). The use of agents that mitigate these changes, such as the partial D2 agonists aripiprazole, brexpiprazole, and cariprazine and the postsynaptic antagonist/presynaptic partial agonist lumateperone, can potentially reduce these adaptive changes and reduce the likelihood of TD and DSP.
Related Resources
- Citrome L. Aripiprazole, brexpiprazole, and cariprazine: not all the same. Current Psychiatry. 2018;17(4):24-33,43.
- Meyer JM. Lumateperone for schizophrenia. Current Psychiatry. 2020;19(2):33-39.
Drug Brand Names
Aripiprazole • Abilify
Brexpiprazole • Rexulti
Cariprazine • Vraylar
Haloperidol • Haldol
Lumateperone • Caplyta
Methamphetamine • Desoxyn
Risperidone • Risperdal
1. Stahl SM. Beyond the dopamine hypothesis of schizophrenia to three neural networks of psychosis: dopamine, serotonin, and glutamate. CNS Spectr. 2018;23(3):187-191.
2. Howes OD, Kapur S. The dopamine hypothesis of schizophrenia: version III--the final common pathway. Schizophr Bull. 2009;35(3):549-562.
3. Ginovart N, Kapur S. Role of dopamine D2 receptors for antipsychotic activity. Handb Exp Pharmacol. 2012;(212):27-52.
4. Madras BK. History of the discovery of the antipsychotic dopamine D2 receptor: a basis for the dopamine hypothesis of schizophrenia. J Hist Neurosci. 2013;22(1):62-78.
5. Beaulieu JM, Gainetdinov RR. The physiology, signaling, and pharmacology of dopamine receptors. Pharmacol Rev. 201;63(1):182-217.
6. Martel JC, Gatti McArthur S. Dopamine receptor subtypes, physiology and pharmacology: new ligands and concepts in schizophrenia. Front Pharmacol. 2020;11:1003.
7. Monsma FJ Jr, McVittie LD, Gerfen CR, et al. Multiple D2 dopamine receptors produced by alternative RNA splicing. Nature. 1989;342(6252):926-929.
8. Seeman P, Nam D, Ulpian C, et al. New dopamine receptor, D2(Longer), with unique TG splice site, in human brain. Brain Res Mol Brain Res. 2000;76(1):132-141.
9. Khan ZU, Mrzljak L, Gutierrez A, et al. Prominence of the dopamine D2 short isoform in dopaminergic pathways. Proc Natl Acad Sci U S A. 1998;95(13):7731-7736.
10. Xu R, Hranilovic D, Fetsko LA, et al. Dopamine D2S and D2L receptors may differentially contribute to the actions of antipsychotic and psychotic agents in mice. Mol Psychiatry. 2002;7(10):1075-1082.
11. Anzalone A, Lizardi-Ortiz JE, Ramos M, et al. Dual control of dopamine synthesis and release by presynaptic and postsynaptic dopamine D2 receptors. J Neurosci. 2012;32(26):9023-9034.
12. Ford CP. The role of D2-autoreceptors in regulating dopamine neuron activity and transmission. Neuroscience. 2014;282:13-22.
13. Stroup TS, Gray N. Management of common adverse effects of antipsychotic medications. World Psychiatry. 2018;17(3):341-356.
14. El-Mallakh RS, Pant B, Caudill R, et al. Does peripheral neuropathy allow for the clinical expression of tardive dyskinesia by unmasking central nervous system changes? Med Hypotheses. 2001;57:210-215.
15. Citrome L, Saklad SR. Revisiting tardive dyskinesia: focusing on the basics of identification and treatment. J Clin Psychiatry. 2020;81(2):TV18059AH3C.
16. Carbon M, Kane JM, Leucht S, et al. Tardive dyskinesia risk with first- and second-generation antipsychotics in comparative randomized controlled trials: a meta-analysis. World Psychiatry. 2018;17(3):330-340.
17. Samaha AN, Seeman P, Stewart J, et al. “Breakthrough” dopamine supersensitivity during ongoing antipsychotic treatment leads to treatment failure over time. J Neurosci. 2007;27(11):2979-2986.
18. Yin J, Barr AM, Ramos-Miguel A, et al. Antipsychotic induced dopamine supersensitivity psychosis: a comprehensive review. Curr Neuropharmacol. 2017;15(1):174-183.
19. Chouinard G, Jones BD, Annable L. Neuroleptic-induced supersensitivity psychosis. Am J Psychiatry. 1978;135(11):1409-1410.
20. Burt DR, Creese I, Snyder SH. Antischizophrenic drugs: chronic treatment elevates dopamine receptor binding in brain. Science. 1977;196(4287):326-328.
21. Silvestri S, Seeman MV, Negrete JC, et al. Increased dopamine D2 receptor binding after long-term treatment with antipsychotics in humans: a clinical PET study. Psychopharmacology (Berl). 2000;152(2):174-180.
22. Ali Z, Roque A, El-Mallakh RS. A unifying theory for the pathoetiologic mechanism of tardive dyskinesia. Med Hypotheses. 2020;140:109682.
23. Lieberman JA. Dopamine partial agonists: a new class of antipsychotic. CNS Drugs. 2004;18(4):251-267.
24. Mailman RB, Murthy V. Third generation antipsychotic drugs: partial agonism or receptor functional selectivity? Curr Pharm Des. 2010;16(5):488-501.
25. Tuplin EW, Holahan MR. Aripiprazole, a drug that displays partial agonism and functional selectivity. Curr Neuropharmacol. 2017;15(8):1192-1207.
26. Hart XM, Schmitz CN, Gründer G. Molecular imaging of dopamine partial agonists in humans: implications for clinical practice. Front Psychiatry. 2022;13:832209.
27. Snyder GL, Vanover KE, Zhu H, et al. Functional profile of a novel modulator of serotonin, dopamine, and glutamate neurotransmission. Psychopharmacology (Berl). 2015;232(3):605-621.
28. Kiss B, Horváth A, Némethy Z, et al. Cariprazine (RGH-188), a dopamine D(3) receptor-preferring, D(3)/D(2) dopamine receptor antagonist-partial agonist antipsychotic candidate: in vitro and neurochemical profile. J Pharmacol Exp Ther. 2010;333(1):328-340.
29. Abbasian C, Power P. A case of aripiprazole and tardive dyskinesia. J Psychopharmacol. 2009;23(2):214-215.
30. Peña MS, Yaltho TC, Jankovic J. Tardive dyskinesia and other movement disorders secondary to aripiprazole. Mov Disord. 2011;26(1):147-152.
31. Tadokoro S, Okamura N, Sekine Y, et al. Chronic treatment with aripiprazole prevents development of dopamine supersensitivity and potentially supersensitivity psychosis. Schizophr Bull. 2012;38(5):1012-1020.
32. Kang NR, Kim MD. Tardive dyskinesia: treatment with aripiprazole. Clin Psychopharmacol Neurosci. 2011;9(1):1-8.
33. Frankel JS, Schwartz TL. Brexpiprazole and cariprazine: distinguishing two new atypical antipsychotics from the original dopamine stabilizer aripiprazole. Ther Adv Psychopharmacol. 2017;7(1):29-41.
34. Chan CH, Chan HY, Chen YC. Switching antipsychotic treatment to aripiprazole in psychotic patients with neuroleptic-induced tardive dyskinesia: a 24-week follow-up study. Int Clin Psychopharmacol. 2018;33(3):155-162.
35. Blair HA. Lumateperone: first approval. Drugs. 2020;80(4):417-423.
36. Calabrese JR, Durgam S, Satlin A, et al. Efficacy and safety of Lumateperone for major depressive episodes associated with bipolar I or bipolar II disorder: a phase 3 randomized placebo-controlled trial. Am J Psychiatry. 2021;178(12):1098-1106.
37. Nakai S, Hirose T, Uwahodo Y, et al. Diminished catalepsy and dopamine metabolism distinguish aripiprazole from haloperidol or risperidone. Eur J Pharmacol. 2003;472(12):89-97.
38. Vanover KE, Davis RE, Zhou Y, et al. Dopamine D2 receptor occupancy of lumateperone (ITI-007): a positron emission tomography study in patients with schizophrenia. Neuropsychopharmacology. 2019;44(3):598-605.
While our understanding of the mechanisms of psychosis continues to evolve beyond the dopamine hypothesis, the key role of dopamine in psychosis and its treatment has not faded.1 Over time, the dopamine hypothesis of schizophrenia has evolved from focusing on dopamine hyperactivity to specifying the regional abnormalities in the brain with subcortical hyperdopaminergia and prefrontal hypodopaminergia.2 Despite this divergence in dopaminergic function, antipsychotic medications that block dopamine D2 receptors (D2R) remain central to treating psychotic symptoms and preventing relapse.3,4 Notably, antipsychotics block both presynaptic and postsynaptic receptors affecting the regulation of dopamine synthesis and release in the brain.5,6
Chronic dopamine D2R blockade with antipsychotics induces adaptive changes that can contribute to both acute and chronic adverse effects. In this article, we discuss these changes, and steps clinicians can take to minimize their occurrence.
Dopamine D2R: A primer
There are 5 types of dopamine receptors, numbered D1 through D5, but there are only 2 families of dopamine receptors: the D1 family (D1 and D5), and the D2 family (D2, D3, and D4). All dopamine receptors are G protein–coupled, but the D2 family of receptors generally increases protein kinase A (PKA) as the second messenger, whereas the D1 family increases cyclic adenosine monophosphate (cAMP) as the second messenger.5 There are 2 distinct variants of the D2R of 2 different lengths made from the same gene (DRD2) via posttranslational modification. The long isoform of D2R (D2L) has an additional 29 amino acids compared to the short isoform (D2S).7 Additional evidence points to a third splice variant called D2Longer that arises from aberrant RNA splicing and contains 2 more amino acids than D2L; its relevance is not known.8
The D2L isoform is the primary postsynaptic receptor, expressed more in the striatum and nucleus accumbens (NAc) targeted by dopaminergic afferents. The D2S isoform, however, is predominantly presynaptic, more densely expressed on cell bodies and projection axons of the dopaminergic neurons of the midbrain and hypothalamus.9 Each isoform contributes differentially to the therapeutic and adverse effects of antipsychotics, and evidence from animal studies suggests that D2L is the main variant responsible for drug-induced parkinsonism.10 The D2S acts as the principal autoreceptor for the dopaminergic system.5,11,12
Autoreceptors regulate dopamine transmission. Dopamine itself and D2R agonists are reported to have higher affinity and potency with D2S. Activation of these autoreceptors is a negative feedback mechanism that decreases dopamine release. Similarly, when they are blocked (such as with use of an antipsychotic), there is an increase in dopamine release. Additionally, these autoreceptors modulate several key processes:
- neuronal firing rate by activating potassium conductance
- dopamine synthesis by downregulating the expression of tyrosine hydroxylase (TH) enzyme (the rate-limiting step)
- exocytotic release of dopamine and other neurotransmitters
- dopamine reuptake via increasing the activity of the dopamine transporter (DAT).12
Consequences of antipsychotic D2R blockade
Most antipsychotics begin to produce a therapeutic antipsychotic effect at 65% to 75% occupancy of the D2Rs.3 This level also produces an optimal balance between clinical efficacy and a lower incidence of adverse effects.3 A higher D2R occupancy by both first-generation (FGA) and second-generation (SGA) pure antagonist antipsychotics can lead to parkinsonism.
Parkinsonism is associated with the subsequent appearance of one of the most distressing consequences of long-term antipsychotic treatment, tardive dyskinesia (TD).13 TD is an iatrogenic, usually late-onset syndrome consisting of persistent, involuntary, and repetitive movements. It classically involves the highly innervated striated muscles of the tongue, mouth, face, and fingers, though it can also involve the trunk and extremities.14 It occurs secondary to chronic exposure to dopamine receptor–blocking agents, including dopaminergic antiemetics.15 The prevalence of TD is higher in patients treated long-term with FGAs (30.0% to 32.4%) than in those treated with SGAs (13.1% to 20.7%) due to serotonin 5HT2A blockade that results in increased dopamine release in the basal ganglia.16
Continue to: Dopamine supersenstivity psychosis...
Dopamine supersensitivity psychosis (DSP) is a term that describes the clinical iatrogenic phenomenon that might be observed with long-term antipsychotic treatment. DSP is suggested to be strongly associated with treatment failure/resistance in schizophrenia.17,18 Manifestations of DSP include development of antipsychotic drug tolerance that undermines treatment efficacy, rebound psychosis during or after treatment discontinuation, and the presence of TD. Like TD, it may be reversed temporarily by increasing the dose of the antipsychotic.18
DSP and (more extensively) TD are commonly hypothesized to result from the postsynaptic dopamine receptor supersensitivity that develops because of chronic D2Rs blockade by antipsychotics. Neostriatal dopamine receptor supersensitivity is believed to lead to TD, while mesolimbic supersensitivity leads to DSP.19 Supersensitivity has traditionally been believed to be due to upregulation of postsynaptic D2R number and sensitivity.20,21 However, both TD and DSP are more likely a consequence of a host of compensatory neurobiological adaptations across the synapse that include:
- postsynaptic increase in the number of D2Rs that amplifies the dopamine signal
- an increased number of synapses, dendritic spines, and perforated synapses (seen in animal models), all of which lead to a potentiated dopamine signal
- presynaptic changes with higher levels of dopamine released into the synapse via an increase in quantal size as postsynaptic D2Rs blockade results in more dopamine becoming available in the synapse for recycling via the dopamine transporter
- increased dopamine turnover due to presynaptic D2S autoreceptor blockade.22
So if giving a D2R blocking agent for a long time increases the dopamine signal, at least in some patients, what can the clinician do to treat the psychosis, and not cause changes in the brain that could lead to TD or DSP?
Partial agonist antipsychotics and biased agonism of D2Rs
One approach to try to avoid the compensatory changes to dopamine blockade might be to use a D2R partial agonist.18,23 For example, aripiprazole is a partial agonist at the D2R commonly used to manage schizophrenia and bipolar disorder. It possesses greater affinity at the D2R compared with the serotonin 2A (5-hydroxytryptamine, 5HT2A) serotonin receptor. Unlike full antagonists, aripiprazole requires exceptionally high D2 receptor occupancy (approximately 90%) to be at a clinically effective antipsychotic dose.24,25 This is a general requirement for all D2R partial agonists.26
A partial agonist generally has to possess greater affinity to the receptor than the neurotransmitter with which it is competing. Aripiprazole has more than twice the affinity to D2R than dopamine. Other partial agonists have similarly high, or higher, D2R affinity. Effective antipsychotic partial agonists stimulate the D2Rs at approximately 30% ± 10% the maximal signal achieved with dopamine. This is essentially equivalent to having approximately 70% receptor occupancy with a full antagonist, except it is built into how the molecule works. Having this low-grade partial activation of D2Rs creates multiple receptor-mediated actions:
- reduction of cAMP accumulation
- antagonism to guanosine 5’-0-(3-thio) triphosphate (GTPgamma S) binding with relatively less recruitment of beta-arrestin 2 (these diverging effects on G protein are the definition of biased agonism)
- antagonism of G protein activation of K+ channels (GIRK) activity
- agonism for the inhibition of TH.
Continue to: Additionally, aripiprazole was found...
Additionally, aripiprazole was found to be associated with a lesser increase in dopamine turnover than full antagonist antipsychotics (Figure27) and decreased DAT binding density in NAc and the ventral tegmental area (VTA). The distinctive pharmacologic profile and biased agonism of this drug could be attributed to its ability to activate presynaptic D2 autoreceptors, which, as previously mentioned, regulate dopamine release via negative feedback mechanism.5,25 Cariprazine, another D2R partial agonist, has similar doubling of dopamine turnover.28
Activation of presynaptic D2S receptors ultimately leads to decreased dopamine synthesis and release, which combats or prevents the brain adaptations regarding dopamine supersensitivity and D2Rs upregulation. While TD can still occur occasionally with aripiprazole or other partial agonists,29,30 animal studies show that administration of methamphetamine significantly lowers locomotor response and the density of striatal D2Rs in a group treated with aripiprazole compared to a group treated with haloperidol.31 Aripiprazole also improved the supersensitivity parameters induced by chronic treatment with haloperidol, which suggests that it is associated with reduced dopamine supersensitivity.31 Similarly, in human studies, partial agonists appear to have a lower rate of parkinsonism and TD.32,33 One study reported that aripiprazole was associated with a significant improvement of TD in more than 50% of patients after 24 weeks of treatment.34
Lumateperone’s unique pharmacologic profile
Lumateperone is a newer antipsychotic that was FDA-approved in December 2019 for the treatment of adults with schizophrenia35 and more recently for the treatment of bipolar depression.36 It possesses a unique combination of pharmacologic properties; it is a postsynaptic D2R antagonist and a presynaptic D2R partial agonist.27
Interestingly, lumateperone has regional selectivity. It increases dopamine release in the medial prefrontal cortex (where D2R is rare) but not in the nigrostriatal pathways.27,37 It does not increase TH phosphorylation (which would increase dopamine concentration) or dopamine turnover in the striatum (Figure27). In a preclinical functional activity assay of lumateperone, the lack of change of dopamine turnover with lumateperone resembles placebo and is even less than that observed with aripiprazole (Figure27). This effect is consistent with partial agonism at the presynaptic D2S, where the stimulation of that receptor prevents the concomitant increase in dopamine synthesis and release that occurs when that receptor is blocked.
It is believed that the lack of increase in dopamine turnover is one of the reasons that lumateperone postsynaptic D2R occupancy is exceptionally low at clinically effective doses. In a positron emission tomography study analyzing posttreatment scans after approximately 2 weeks of a 60 mg/d dose, the mean peak striatal D2R occupancy was approximately 40%,38 which is remarkably lower than the 65% to 75% blockade needed for purely antagonist D2R antipsychotics.3 This low receptor occupancy appears to mediate the low incidence of parkinsonism and prolactin release seen with lumateperone.
Continue to: Take-home points
Take-home points
Adaptive upregulation of dopamine neurotransmission underlies acute adverse effects such as parkinsonism and is also key for delayed consequences such as TD, and possibly the development of treatment resistance. Adaptive upregulation results from an increase in postsynaptic dopamine receptors, numbers of synapses, and dopamine release. The latter has been demonstrated to be greatest with full antagonists, less with partial agonists, and not present with lumateperone, which is a postsynaptic antagonist but a presynaptic partial agonist (Figure27). Reducing adaptive upregulation can reduce both acute and long-term consequences of dopamine blockade. Early use of agents that minimize these adaptive changes, such as a postsynaptic partial agonist (aripiprazole, brexpiprazole, or cariprazine) or a presynaptic partial agonist (lumateperone), appears to be a reasonable clinical option.
Bottom Line
Chronic dopamine D2 receptor blockade with antipsychotics induces adaptive changes that can contribute to both acute and chronic adverse effects. The most severe of these are tardive dyskinesia (TD) and dopamine supersensitivity psychosis (DSP). The use of agents that mitigate these changes, such as the partial D2 agonists aripiprazole, brexpiprazole, and cariprazine and the postsynaptic antagonist/presynaptic partial agonist lumateperone, can potentially reduce these adaptive changes and reduce the likelihood of TD and DSP.
Related Resources
- Citrome L. Aripiprazole, brexpiprazole, and cariprazine: not all the same. Current Psychiatry. 2018;17(4):24-33,43.
- Meyer JM. Lumateperone for schizophrenia. Current Psychiatry. 2020;19(2):33-39.
Drug Brand Names
Aripiprazole • Abilify
Brexpiprazole • Rexulti
Cariprazine • Vraylar
Haloperidol • Haldol
Lumateperone • Caplyta
Methamphetamine • Desoxyn
Risperidone • Risperdal
While our understanding of the mechanisms of psychosis continues to evolve beyond the dopamine hypothesis, the key role of dopamine in psychosis and its treatment has not faded.1 Over time, the dopamine hypothesis of schizophrenia has evolved from focusing on dopamine hyperactivity to specifying the regional abnormalities in the brain with subcortical hyperdopaminergia and prefrontal hypodopaminergia.2 Despite this divergence in dopaminergic function, antipsychotic medications that block dopamine D2 receptors (D2R) remain central to treating psychotic symptoms and preventing relapse.3,4 Notably, antipsychotics block both presynaptic and postsynaptic receptors affecting the regulation of dopamine synthesis and release in the brain.5,6
Chronic dopamine D2R blockade with antipsychotics induces adaptive changes that can contribute to both acute and chronic adverse effects. In this article, we discuss these changes, and steps clinicians can take to minimize their occurrence.
Dopamine D2R: A primer
There are 5 types of dopamine receptors, numbered D1 through D5, but there are only 2 families of dopamine receptors: the D1 family (D1 and D5), and the D2 family (D2, D3, and D4). All dopamine receptors are G protein–coupled, but the D2 family of receptors generally increases protein kinase A (PKA) as the second messenger, whereas the D1 family increases cyclic adenosine monophosphate (cAMP) as the second messenger.5 There are 2 distinct variants of the D2R of 2 different lengths made from the same gene (DRD2) via posttranslational modification. The long isoform of D2R (D2L) has an additional 29 amino acids compared to the short isoform (D2S).7 Additional evidence points to a third splice variant called D2Longer that arises from aberrant RNA splicing and contains 2 more amino acids than D2L; its relevance is not known.8
The D2L isoform is the primary postsynaptic receptor, expressed more in the striatum and nucleus accumbens (NAc) targeted by dopaminergic afferents. The D2S isoform, however, is predominantly presynaptic, more densely expressed on cell bodies and projection axons of the dopaminergic neurons of the midbrain and hypothalamus.9 Each isoform contributes differentially to the therapeutic and adverse effects of antipsychotics, and evidence from animal studies suggests that D2L is the main variant responsible for drug-induced parkinsonism.10 The D2S acts as the principal autoreceptor for the dopaminergic system.5,11,12
Autoreceptors regulate dopamine transmission. Dopamine itself and D2R agonists are reported to have higher affinity and potency with D2S. Activation of these autoreceptors is a negative feedback mechanism that decreases dopamine release. Similarly, when they are blocked (such as with use of an antipsychotic), there is an increase in dopamine release. Additionally, these autoreceptors modulate several key processes:
- neuronal firing rate by activating potassium conductance
- dopamine synthesis by downregulating the expression of tyrosine hydroxylase (TH) enzyme (the rate-limiting step)
- exocytotic release of dopamine and other neurotransmitters
- dopamine reuptake via increasing the activity of the dopamine transporter (DAT).12
Consequences of antipsychotic D2R blockade
Most antipsychotics begin to produce a therapeutic antipsychotic effect at 65% to 75% occupancy of the D2Rs.3 This level also produces an optimal balance between clinical efficacy and a lower incidence of adverse effects.3 A higher D2R occupancy by both first-generation (FGA) and second-generation (SGA) pure antagonist antipsychotics can lead to parkinsonism.
Parkinsonism is associated with the subsequent appearance of one of the most distressing consequences of long-term antipsychotic treatment, tardive dyskinesia (TD).13 TD is an iatrogenic, usually late-onset syndrome consisting of persistent, involuntary, and repetitive movements. It classically involves the highly innervated striated muscles of the tongue, mouth, face, and fingers, though it can also involve the trunk and extremities.14 It occurs secondary to chronic exposure to dopamine receptor–blocking agents, including dopaminergic antiemetics.15 The prevalence of TD is higher in patients treated long-term with FGAs (30.0% to 32.4%) than in those treated with SGAs (13.1% to 20.7%) due to serotonin 5HT2A blockade that results in increased dopamine release in the basal ganglia.16
Continue to: Dopamine supersenstivity psychosis...
Dopamine supersensitivity psychosis (DSP) is a term that describes the clinical iatrogenic phenomenon that might be observed with long-term antipsychotic treatment. DSP is suggested to be strongly associated with treatment failure/resistance in schizophrenia.17,18 Manifestations of DSP include development of antipsychotic drug tolerance that undermines treatment efficacy, rebound psychosis during or after treatment discontinuation, and the presence of TD. Like TD, it may be reversed temporarily by increasing the dose of the antipsychotic.18
DSP and (more extensively) TD are commonly hypothesized to result from the postsynaptic dopamine receptor supersensitivity that develops because of chronic D2Rs blockade by antipsychotics. Neostriatal dopamine receptor supersensitivity is believed to lead to TD, while mesolimbic supersensitivity leads to DSP.19 Supersensitivity has traditionally been believed to be due to upregulation of postsynaptic D2R number and sensitivity.20,21 However, both TD and DSP are more likely a consequence of a host of compensatory neurobiological adaptations across the synapse that include:
- postsynaptic increase in the number of D2Rs that amplifies the dopamine signal
- an increased number of synapses, dendritic spines, and perforated synapses (seen in animal models), all of which lead to a potentiated dopamine signal
- presynaptic changes with higher levels of dopamine released into the synapse via an increase in quantal size as postsynaptic D2Rs blockade results in more dopamine becoming available in the synapse for recycling via the dopamine transporter
- increased dopamine turnover due to presynaptic D2S autoreceptor blockade.22
So if giving a D2R blocking agent for a long time increases the dopamine signal, at least in some patients, what can the clinician do to treat the psychosis, and not cause changes in the brain that could lead to TD or DSP?
Partial agonist antipsychotics and biased agonism of D2Rs
One approach to try to avoid the compensatory changes to dopamine blockade might be to use a D2R partial agonist.18,23 For example, aripiprazole is a partial agonist at the D2R commonly used to manage schizophrenia and bipolar disorder. It possesses greater affinity at the D2R compared with the serotonin 2A (5-hydroxytryptamine, 5HT2A) serotonin receptor. Unlike full antagonists, aripiprazole requires exceptionally high D2 receptor occupancy (approximately 90%) to be at a clinically effective antipsychotic dose.24,25 This is a general requirement for all D2R partial agonists.26
A partial agonist generally has to possess greater affinity to the receptor than the neurotransmitter with which it is competing. Aripiprazole has more than twice the affinity to D2R than dopamine. Other partial agonists have similarly high, or higher, D2R affinity. Effective antipsychotic partial agonists stimulate the D2Rs at approximately 30% ± 10% the maximal signal achieved with dopamine. This is essentially equivalent to having approximately 70% receptor occupancy with a full antagonist, except it is built into how the molecule works. Having this low-grade partial activation of D2Rs creates multiple receptor-mediated actions:
- reduction of cAMP accumulation
- antagonism to guanosine 5’-0-(3-thio) triphosphate (GTPgamma S) binding with relatively less recruitment of beta-arrestin 2 (these diverging effects on G protein are the definition of biased agonism)
- antagonism of G protein activation of K+ channels (GIRK) activity
- agonism for the inhibition of TH.
Continue to: Additionally, aripiprazole was found...
Additionally, aripiprazole was found to be associated with a lesser increase in dopamine turnover than full antagonist antipsychotics (Figure27) and decreased DAT binding density in NAc and the ventral tegmental area (VTA). The distinctive pharmacologic profile and biased agonism of this drug could be attributed to its ability to activate presynaptic D2 autoreceptors, which, as previously mentioned, regulate dopamine release via negative feedback mechanism.5,25 Cariprazine, another D2R partial agonist, has similar doubling of dopamine turnover.28
Activation of presynaptic D2S receptors ultimately leads to decreased dopamine synthesis and release, which combats or prevents the brain adaptations regarding dopamine supersensitivity and D2Rs upregulation. While TD can still occur occasionally with aripiprazole or other partial agonists,29,30 animal studies show that administration of methamphetamine significantly lowers locomotor response and the density of striatal D2Rs in a group treated with aripiprazole compared to a group treated with haloperidol.31 Aripiprazole also improved the supersensitivity parameters induced by chronic treatment with haloperidol, which suggests that it is associated with reduced dopamine supersensitivity.31 Similarly, in human studies, partial agonists appear to have a lower rate of parkinsonism and TD.32,33 One study reported that aripiprazole was associated with a significant improvement of TD in more than 50% of patients after 24 weeks of treatment.34
Lumateperone’s unique pharmacologic profile
Lumateperone is a newer antipsychotic that was FDA-approved in December 2019 for the treatment of adults with schizophrenia35 and more recently for the treatment of bipolar depression.36 It possesses a unique combination of pharmacologic properties; it is a postsynaptic D2R antagonist and a presynaptic D2R partial agonist.27
Interestingly, lumateperone has regional selectivity. It increases dopamine release in the medial prefrontal cortex (where D2R is rare) but not in the nigrostriatal pathways.27,37 It does not increase TH phosphorylation (which would increase dopamine concentration) or dopamine turnover in the striatum (Figure27). In a preclinical functional activity assay of lumateperone, the lack of change of dopamine turnover with lumateperone resembles placebo and is even less than that observed with aripiprazole (Figure27). This effect is consistent with partial agonism at the presynaptic D2S, where the stimulation of that receptor prevents the concomitant increase in dopamine synthesis and release that occurs when that receptor is blocked.
It is believed that the lack of increase in dopamine turnover is one of the reasons that lumateperone postsynaptic D2R occupancy is exceptionally low at clinically effective doses. In a positron emission tomography study analyzing posttreatment scans after approximately 2 weeks of a 60 mg/d dose, the mean peak striatal D2R occupancy was approximately 40%,38 which is remarkably lower than the 65% to 75% blockade needed for purely antagonist D2R antipsychotics.3 This low receptor occupancy appears to mediate the low incidence of parkinsonism and prolactin release seen with lumateperone.
Continue to: Take-home points
Take-home points
Adaptive upregulation of dopamine neurotransmission underlies acute adverse effects such as parkinsonism and is also key for delayed consequences such as TD, and possibly the development of treatment resistance. Adaptive upregulation results from an increase in postsynaptic dopamine receptors, numbers of synapses, and dopamine release. The latter has been demonstrated to be greatest with full antagonists, less with partial agonists, and not present with lumateperone, which is a postsynaptic antagonist but a presynaptic partial agonist (Figure27). Reducing adaptive upregulation can reduce both acute and long-term consequences of dopamine blockade. Early use of agents that minimize these adaptive changes, such as a postsynaptic partial agonist (aripiprazole, brexpiprazole, or cariprazine) or a presynaptic partial agonist (lumateperone), appears to be a reasonable clinical option.
Bottom Line
Chronic dopamine D2 receptor blockade with antipsychotics induces adaptive changes that can contribute to both acute and chronic adverse effects. The most severe of these are tardive dyskinesia (TD) and dopamine supersensitivity psychosis (DSP). The use of agents that mitigate these changes, such as the partial D2 agonists aripiprazole, brexpiprazole, and cariprazine and the postsynaptic antagonist/presynaptic partial agonist lumateperone, can potentially reduce these adaptive changes and reduce the likelihood of TD and DSP.
Related Resources
- Citrome L. Aripiprazole, brexpiprazole, and cariprazine: not all the same. Current Psychiatry. 2018;17(4):24-33,43.
- Meyer JM. Lumateperone for schizophrenia. Current Psychiatry. 2020;19(2):33-39.
Drug Brand Names
Aripiprazole • Abilify
Brexpiprazole • Rexulti
Cariprazine • Vraylar
Haloperidol • Haldol
Lumateperone • Caplyta
Methamphetamine • Desoxyn
Risperidone • Risperdal
1. Stahl SM. Beyond the dopamine hypothesis of schizophrenia to three neural networks of psychosis: dopamine, serotonin, and glutamate. CNS Spectr. 2018;23(3):187-191.
2. Howes OD, Kapur S. The dopamine hypothesis of schizophrenia: version III--the final common pathway. Schizophr Bull. 2009;35(3):549-562.
3. Ginovart N, Kapur S. Role of dopamine D2 receptors for antipsychotic activity. Handb Exp Pharmacol. 2012;(212):27-52.
4. Madras BK. History of the discovery of the antipsychotic dopamine D2 receptor: a basis for the dopamine hypothesis of schizophrenia. J Hist Neurosci. 2013;22(1):62-78.
5. Beaulieu JM, Gainetdinov RR. The physiology, signaling, and pharmacology of dopamine receptors. Pharmacol Rev. 201;63(1):182-217.
6. Martel JC, Gatti McArthur S. Dopamine receptor subtypes, physiology and pharmacology: new ligands and concepts in schizophrenia. Front Pharmacol. 2020;11:1003.
7. Monsma FJ Jr, McVittie LD, Gerfen CR, et al. Multiple D2 dopamine receptors produced by alternative RNA splicing. Nature. 1989;342(6252):926-929.
8. Seeman P, Nam D, Ulpian C, et al. New dopamine receptor, D2(Longer), with unique TG splice site, in human brain. Brain Res Mol Brain Res. 2000;76(1):132-141.
9. Khan ZU, Mrzljak L, Gutierrez A, et al. Prominence of the dopamine D2 short isoform in dopaminergic pathways. Proc Natl Acad Sci U S A. 1998;95(13):7731-7736.
10. Xu R, Hranilovic D, Fetsko LA, et al. Dopamine D2S and D2L receptors may differentially contribute to the actions of antipsychotic and psychotic agents in mice. Mol Psychiatry. 2002;7(10):1075-1082.
11. Anzalone A, Lizardi-Ortiz JE, Ramos M, et al. Dual control of dopamine synthesis and release by presynaptic and postsynaptic dopamine D2 receptors. J Neurosci. 2012;32(26):9023-9034.
12. Ford CP. The role of D2-autoreceptors in regulating dopamine neuron activity and transmission. Neuroscience. 2014;282:13-22.
13. Stroup TS, Gray N. Management of common adverse effects of antipsychotic medications. World Psychiatry. 2018;17(3):341-356.
14. El-Mallakh RS, Pant B, Caudill R, et al. Does peripheral neuropathy allow for the clinical expression of tardive dyskinesia by unmasking central nervous system changes? Med Hypotheses. 2001;57:210-215.
15. Citrome L, Saklad SR. Revisiting tardive dyskinesia: focusing on the basics of identification and treatment. J Clin Psychiatry. 2020;81(2):TV18059AH3C.
16. Carbon M, Kane JM, Leucht S, et al. Tardive dyskinesia risk with first- and second-generation antipsychotics in comparative randomized controlled trials: a meta-analysis. World Psychiatry. 2018;17(3):330-340.
17. Samaha AN, Seeman P, Stewart J, et al. “Breakthrough” dopamine supersensitivity during ongoing antipsychotic treatment leads to treatment failure over time. J Neurosci. 2007;27(11):2979-2986.
18. Yin J, Barr AM, Ramos-Miguel A, et al. Antipsychotic induced dopamine supersensitivity psychosis: a comprehensive review. Curr Neuropharmacol. 2017;15(1):174-183.
19. Chouinard G, Jones BD, Annable L. Neuroleptic-induced supersensitivity psychosis. Am J Psychiatry. 1978;135(11):1409-1410.
20. Burt DR, Creese I, Snyder SH. Antischizophrenic drugs: chronic treatment elevates dopamine receptor binding in brain. Science. 1977;196(4287):326-328.
21. Silvestri S, Seeman MV, Negrete JC, et al. Increased dopamine D2 receptor binding after long-term treatment with antipsychotics in humans: a clinical PET study. Psychopharmacology (Berl). 2000;152(2):174-180.
22. Ali Z, Roque A, El-Mallakh RS. A unifying theory for the pathoetiologic mechanism of tardive dyskinesia. Med Hypotheses. 2020;140:109682.
23. Lieberman JA. Dopamine partial agonists: a new class of antipsychotic. CNS Drugs. 2004;18(4):251-267.
24. Mailman RB, Murthy V. Third generation antipsychotic drugs: partial agonism or receptor functional selectivity? Curr Pharm Des. 2010;16(5):488-501.
25. Tuplin EW, Holahan MR. Aripiprazole, a drug that displays partial agonism and functional selectivity. Curr Neuropharmacol. 2017;15(8):1192-1207.
26. Hart XM, Schmitz CN, Gründer G. Molecular imaging of dopamine partial agonists in humans: implications for clinical practice. Front Psychiatry. 2022;13:832209.
27. Snyder GL, Vanover KE, Zhu H, et al. Functional profile of a novel modulator of serotonin, dopamine, and glutamate neurotransmission. Psychopharmacology (Berl). 2015;232(3):605-621.
28. Kiss B, Horváth A, Némethy Z, et al. Cariprazine (RGH-188), a dopamine D(3) receptor-preferring, D(3)/D(2) dopamine receptor antagonist-partial agonist antipsychotic candidate: in vitro and neurochemical profile. J Pharmacol Exp Ther. 2010;333(1):328-340.
29. Abbasian C, Power P. A case of aripiprazole and tardive dyskinesia. J Psychopharmacol. 2009;23(2):214-215.
30. Peña MS, Yaltho TC, Jankovic J. Tardive dyskinesia and other movement disorders secondary to aripiprazole. Mov Disord. 2011;26(1):147-152.
31. Tadokoro S, Okamura N, Sekine Y, et al. Chronic treatment with aripiprazole prevents development of dopamine supersensitivity and potentially supersensitivity psychosis. Schizophr Bull. 2012;38(5):1012-1020.
32. Kang NR, Kim MD. Tardive dyskinesia: treatment with aripiprazole. Clin Psychopharmacol Neurosci. 2011;9(1):1-8.
33. Frankel JS, Schwartz TL. Brexpiprazole and cariprazine: distinguishing two new atypical antipsychotics from the original dopamine stabilizer aripiprazole. Ther Adv Psychopharmacol. 2017;7(1):29-41.
34. Chan CH, Chan HY, Chen YC. Switching antipsychotic treatment to aripiprazole in psychotic patients with neuroleptic-induced tardive dyskinesia: a 24-week follow-up study. Int Clin Psychopharmacol. 2018;33(3):155-162.
35. Blair HA. Lumateperone: first approval. Drugs. 2020;80(4):417-423.
36. Calabrese JR, Durgam S, Satlin A, et al. Efficacy and safety of Lumateperone for major depressive episodes associated with bipolar I or bipolar II disorder: a phase 3 randomized placebo-controlled trial. Am J Psychiatry. 2021;178(12):1098-1106.
37. Nakai S, Hirose T, Uwahodo Y, et al. Diminished catalepsy and dopamine metabolism distinguish aripiprazole from haloperidol or risperidone. Eur J Pharmacol. 2003;472(12):89-97.
38. Vanover KE, Davis RE, Zhou Y, et al. Dopamine D2 receptor occupancy of lumateperone (ITI-007): a positron emission tomography study in patients with schizophrenia. Neuropsychopharmacology. 2019;44(3):598-605.
1. Stahl SM. Beyond the dopamine hypothesis of schizophrenia to three neural networks of psychosis: dopamine, serotonin, and glutamate. CNS Spectr. 2018;23(3):187-191.
2. Howes OD, Kapur S. The dopamine hypothesis of schizophrenia: version III--the final common pathway. Schizophr Bull. 2009;35(3):549-562.
3. Ginovart N, Kapur S. Role of dopamine D2 receptors for antipsychotic activity. Handb Exp Pharmacol. 2012;(212):27-52.
4. Madras BK. History of the discovery of the antipsychotic dopamine D2 receptor: a basis for the dopamine hypothesis of schizophrenia. J Hist Neurosci. 2013;22(1):62-78.
5. Beaulieu JM, Gainetdinov RR. The physiology, signaling, and pharmacology of dopamine receptors. Pharmacol Rev. 201;63(1):182-217.
6. Martel JC, Gatti McArthur S. Dopamine receptor subtypes, physiology and pharmacology: new ligands and concepts in schizophrenia. Front Pharmacol. 2020;11:1003.
7. Monsma FJ Jr, McVittie LD, Gerfen CR, et al. Multiple D2 dopamine receptors produced by alternative RNA splicing. Nature. 1989;342(6252):926-929.
8. Seeman P, Nam D, Ulpian C, et al. New dopamine receptor, D2(Longer), with unique TG splice site, in human brain. Brain Res Mol Brain Res. 2000;76(1):132-141.
9. Khan ZU, Mrzljak L, Gutierrez A, et al. Prominence of the dopamine D2 short isoform in dopaminergic pathways. Proc Natl Acad Sci U S A. 1998;95(13):7731-7736.
10. Xu R, Hranilovic D, Fetsko LA, et al. Dopamine D2S and D2L receptors may differentially contribute to the actions of antipsychotic and psychotic agents in mice. Mol Psychiatry. 2002;7(10):1075-1082.
11. Anzalone A, Lizardi-Ortiz JE, Ramos M, et al. Dual control of dopamine synthesis and release by presynaptic and postsynaptic dopamine D2 receptors. J Neurosci. 2012;32(26):9023-9034.
12. Ford CP. The role of D2-autoreceptors in regulating dopamine neuron activity and transmission. Neuroscience. 2014;282:13-22.
13. Stroup TS, Gray N. Management of common adverse effects of antipsychotic medications. World Psychiatry. 2018;17(3):341-356.
14. El-Mallakh RS, Pant B, Caudill R, et al. Does peripheral neuropathy allow for the clinical expression of tardive dyskinesia by unmasking central nervous system changes? Med Hypotheses. 2001;57:210-215.
15. Citrome L, Saklad SR. Revisiting tardive dyskinesia: focusing on the basics of identification and treatment. J Clin Psychiatry. 2020;81(2):TV18059AH3C.
16. Carbon M, Kane JM, Leucht S, et al. Tardive dyskinesia risk with first- and second-generation antipsychotics in comparative randomized controlled trials: a meta-analysis. World Psychiatry. 2018;17(3):330-340.
17. Samaha AN, Seeman P, Stewart J, et al. “Breakthrough” dopamine supersensitivity during ongoing antipsychotic treatment leads to treatment failure over time. J Neurosci. 2007;27(11):2979-2986.
18. Yin J, Barr AM, Ramos-Miguel A, et al. Antipsychotic induced dopamine supersensitivity psychosis: a comprehensive review. Curr Neuropharmacol. 2017;15(1):174-183.
19. Chouinard G, Jones BD, Annable L. Neuroleptic-induced supersensitivity psychosis. Am J Psychiatry. 1978;135(11):1409-1410.
20. Burt DR, Creese I, Snyder SH. Antischizophrenic drugs: chronic treatment elevates dopamine receptor binding in brain. Science. 1977;196(4287):326-328.
21. Silvestri S, Seeman MV, Negrete JC, et al. Increased dopamine D2 receptor binding after long-term treatment with antipsychotics in humans: a clinical PET study. Psychopharmacology (Berl). 2000;152(2):174-180.
22. Ali Z, Roque A, El-Mallakh RS. A unifying theory for the pathoetiologic mechanism of tardive dyskinesia. Med Hypotheses. 2020;140:109682.
23. Lieberman JA. Dopamine partial agonists: a new class of antipsychotic. CNS Drugs. 2004;18(4):251-267.
24. Mailman RB, Murthy V. Third generation antipsychotic drugs: partial agonism or receptor functional selectivity? Curr Pharm Des. 2010;16(5):488-501.
25. Tuplin EW, Holahan MR. Aripiprazole, a drug that displays partial agonism and functional selectivity. Curr Neuropharmacol. 2017;15(8):1192-1207.
26. Hart XM, Schmitz CN, Gründer G. Molecular imaging of dopamine partial agonists in humans: implications for clinical practice. Front Psychiatry. 2022;13:832209.
27. Snyder GL, Vanover KE, Zhu H, et al. Functional profile of a novel modulator of serotonin, dopamine, and glutamate neurotransmission. Psychopharmacology (Berl). 2015;232(3):605-621.
28. Kiss B, Horváth A, Némethy Z, et al. Cariprazine (RGH-188), a dopamine D(3) receptor-preferring, D(3)/D(2) dopamine receptor antagonist-partial agonist antipsychotic candidate: in vitro and neurochemical profile. J Pharmacol Exp Ther. 2010;333(1):328-340.
29. Abbasian C, Power P. A case of aripiprazole and tardive dyskinesia. J Psychopharmacol. 2009;23(2):214-215.
30. Peña MS, Yaltho TC, Jankovic J. Tardive dyskinesia and other movement disorders secondary to aripiprazole. Mov Disord. 2011;26(1):147-152.
31. Tadokoro S, Okamura N, Sekine Y, et al. Chronic treatment with aripiprazole prevents development of dopamine supersensitivity and potentially supersensitivity psychosis. Schizophr Bull. 2012;38(5):1012-1020.
32. Kang NR, Kim MD. Tardive dyskinesia: treatment with aripiprazole. Clin Psychopharmacol Neurosci. 2011;9(1):1-8.
33. Frankel JS, Schwartz TL. Brexpiprazole and cariprazine: distinguishing two new atypical antipsychotics from the original dopamine stabilizer aripiprazole. Ther Adv Psychopharmacol. 2017;7(1):29-41.
34. Chan CH, Chan HY, Chen YC. Switching antipsychotic treatment to aripiprazole in psychotic patients with neuroleptic-induced tardive dyskinesia: a 24-week follow-up study. Int Clin Psychopharmacol. 2018;33(3):155-162.
35. Blair HA. Lumateperone: first approval. Drugs. 2020;80(4):417-423.
36. Calabrese JR, Durgam S, Satlin A, et al. Efficacy and safety of Lumateperone for major depressive episodes associated with bipolar I or bipolar II disorder: a phase 3 randomized placebo-controlled trial. Am J Psychiatry. 2021;178(12):1098-1106.
37. Nakai S, Hirose T, Uwahodo Y, et al. Diminished catalepsy and dopamine metabolism distinguish aripiprazole from haloperidol or risperidone. Eur J Pharmacol. 2003;472(12):89-97.
38. Vanover KE, Davis RE, Zhou Y, et al. Dopamine D2 receptor occupancy of lumateperone (ITI-007): a positron emission tomography study in patients with schizophrenia. Neuropsychopharmacology. 2019;44(3):598-605.
The brain’s Twitter system: Neuronal extracellular vesicles
Twitter, a microblogging and social networking service, has become a “go-to’” for conversations, updates, breaking news, and sharing the more mundane aspects of our lives. Tweets, which were lengthened from 140 to 280 characters in 2017, rapidly communicate and disseminate information to a wide audience. Generally, tweets are visible to everyone, though users can mute and block other users from viewing their tweets. Spikes in tweets and tweeting frequency reflect hyper-current events: the last minutes of the Super Bowl, certification of an election, or a new movie release. In fact, social scientists have analyzed tweet frequencies to examine the impact of local and national events. However, few are aware that like celebrities, politicians, influencers, and ordinary citizens, the human brain also tweets.
In this article, we describe the components of the brain’s “Twitter” system, how it works, and how it might someday be used to improve the diagnosis and treatment of psychiatric disorders.
Brain tweets
The brain’s Twitter system involves extracellular vesicles (EVs), tiny (<1 µm) membrane-bound vesicles that are released from neurons, glia, and other neuronal cells (Table). These EVs cross the blood-brain barrier and facilitate cell-to-cell communication within and among tissues (Figure 1).
First described in the 1980s,1 EVs are secreted by a diverse array of cells: mast cells reticulocytes, epithelial cells, immune cells, neurons, glia, and oligodendrocytes. Like tweets, EVs rapidly disseminate packets of information throughout the brain and body and direct the molecular activity of recipient cells in both health and disease. These “brain tweets” contain short, circumscribed messages, and the characters are the EV cargos: RNAs, proteins, lipids, and metabolites. Like a Twitter feed, EVs cast a wide communication net across the body, much of which finds its way to the blood. As neuroscientists, we can follow these tweets by isolating tissue-derived EVs in plasma and examining their surface molecules and cargo. By following this Twitter feed, we can tap into important molecular communications and identify “trending” (evolving) pathological processes, and perhaps use the brain Twitter feed to improve diagnosis and treatments. We can pinpoint, in the blood, signals from CNS processes, down to the level of identifying EV cargos from specific brain cell types.
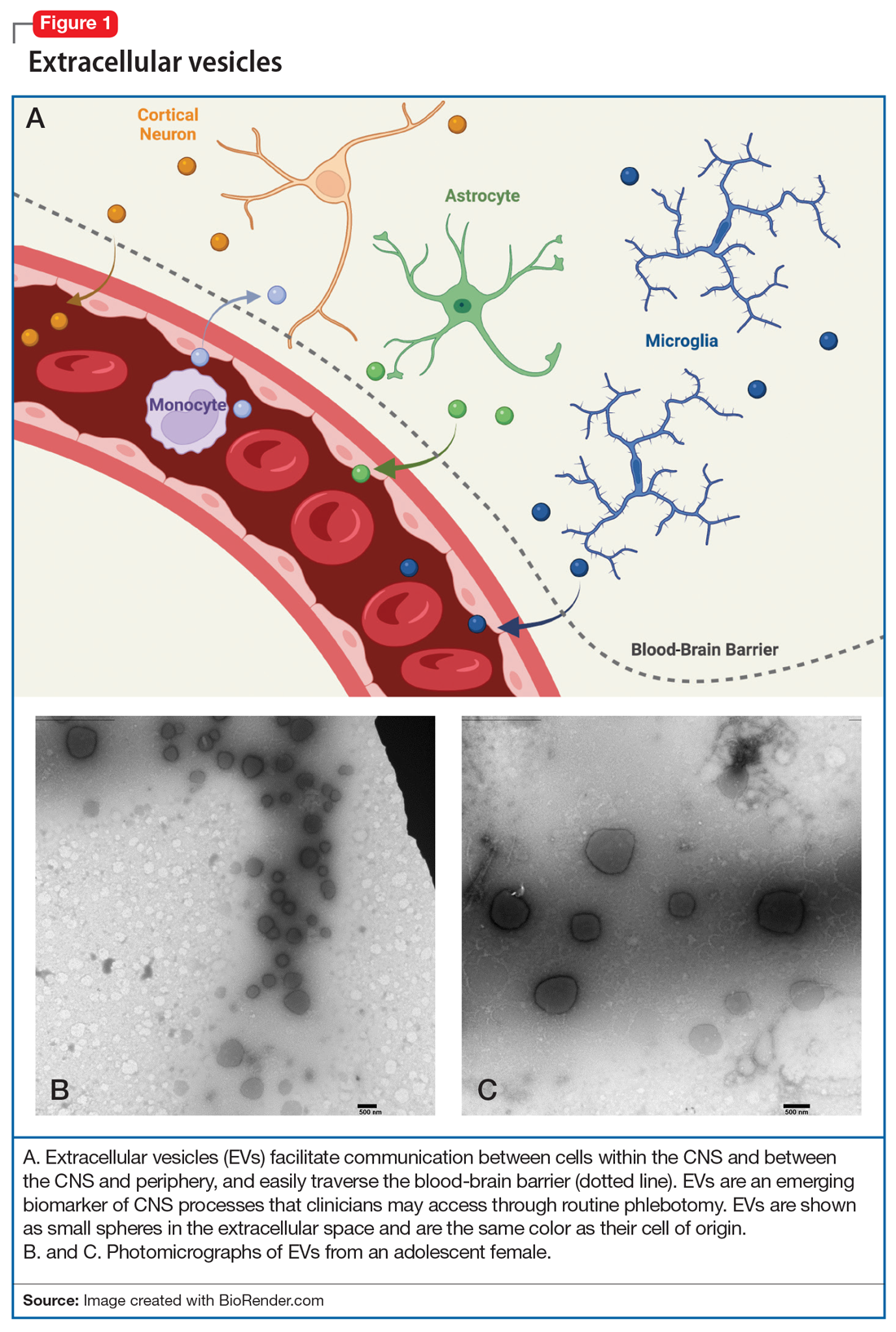
Within the CNS, EVs are secreted by neurons, where they may modulate synaptic plasticity and transfer molecular cargo among neurons. EVs also facilitate communication between neurons and glia, maintain homeostasis, trigger neuroprotective processes, and even regulate synaptic transmission.2
What’s in a brain tweet?
To discuss what’s in a brain tweet, we must first understand how a brain tweet is composed. EVs are pinched off from membranes of intercellular structures (eg, golgi or endoplasmic reticulum) or pinched off directly from cell membranes, where upon release they become EVs. There is a complex cellular machinery that transports what ultimately becomes an EV to the cell membrane.3 EVs contain unique mixtures of lipids, proteins, and nucleic acids (eg, microRNA [miRNA], mRNA, and noncoding RNA).4 To date, nearly 10,000 proteins, 11,000 lipids, 3,500 mRNAs, and 3,000 miRNAs have been identified as cargos in extracellular vesicles (Figure 1). Similar to how the release of EVs is dependent on complex intracellular machinery, the packing of these contents into what will become the EV involves a parallel set of complex machinery that is largely directed by endosomal sorting complexes required for transport (ESCRT) proteins.5 Of interest, when viruses attack cells, they hijack this EV packaging system to package and release new viruses. EVs vary in size, shape, and density; this variation is related to the cell origin, among other things. EVs also differ in their membrane lipid composition and in terms of transmembrane proteins as well as the proteins that facilitate EV binding to target cells (Figure 2).6 Ultimately, these exosomes are taken up by the recipient cells.
EV-facilitated neuron-to-neuron tweets have been implicated in neuronal growth and differentiation.7 EV-driven communication between cells also can decrease dendrite growth and can trigger microglia to prune synapses.8 EVs from glial cells may promote neuronal integrity, directly boost presynaptic glutamate release,9 or even, through miRNAs, change the expression of glutamate receptors.10 EVs from astrocytes transport proteins that enable neuronal repair, while EVs from microglia regulate neuronal homeostasis. EV cargos—lipids, proteins, and miRNAs—from neurons modify signal transduction and protein expression in recipient cells. Taken together, data suggest that EVs facilitate anterograde and retrograde transfer of signals across synapses,7,11 a putative mechanism for driving synaptic plasticity,12 which is a process implicated in the therapeutic efficacy of psychotropic medications and psychotherapies.
Continue to: #Targets and #neuron
#Targets and #neuron
Adding a hashtag to a tweet links it to other tweets, just as membrane features of EVs direct how EVs link to target cells. When these EVs bind to target cells, they fuse and release their cargo into the target cell (Figure 2). These directed cargo—whether mRNA, proteins, or other molecules—can direct the recipient cell to modify its firing rate (in the case of neurons), alter transmitter release, and increase or decrease expression of various genes. The targeting process is complex, and our understanding of this process is evolving. Briefly, integrin, lipid composition, glycans (eg, polysaccharides), and tetraspanin components of EVs influence their affinity for specific target cells.13 Recently, we have been able to read these hashtags and isolate cell-specific, neuron-derived EVs. Immunoadsorption techniques that leverage antibodies against L1 cell adhesion molecule protein (L1CAM(+)), primarily expressed in neurons, can identify neuronally-derived EVs (Figure 3). The specific EVs contain cargos of neuronal origin and provide a “window” into molecular processes in the brain by way of the blood (or other peripheral fluids). In following the neuronal tweets, we can follow molecular measures of important brain molecules in biofluids outside the CNS, including saliva and potentially urine (Figure 1B and 1C). In following these specific neuronal Twitter feeds, we can gain critical insights into specific brain processes.
EVs in psychiatric disorders
EVs are implicated in neuroinflammation,14 neurogenesis, synaptic plasticity, and epigenetic regulation—all processes that are involved in the pathophysiology of psychiatric disorders. Postmortem research suggests that EVs in the brain carry proinflammatory molecules from microglia, as well as secretions of regulatory miRNA that are responsible for synaptic plasticity and dendritic growth in depression, bipolar disorder, schizophrenia, and addiction. In addition, second-generation antipsychotics change the composition of EV cargos in the brain, altering their RNA, protein, and lipid content, often reflecting profound changes in gene expression in various cells in the CNS. In our lab, we have identified several molecules in plasma EVs, both lipids and miRNA, that can potentially predict the response to treatment of pediatric anxiety with selective serotonin reuptake inhibitors as well as opiate addiction.15
Further, given our increasing understanding of the way in which EV cargo reflects neuronal physiology as well as the potential pathophysiologic states of cells (including neurons), studying EVs’ molecular content can identify molecular messages—in blood—that are derived from the neurons in the brain. Having the tools to examine molecular brain regulators or other markers of disease progression (eg, beta amyloid) or brain health (eg, brain-derived neurotrophic factor) may advance our understanding and treatment of psychiatric disorders and create opportunities for precision medicine driven by biological rather than ethnologic and phenomenological markers. Whereas in the not-too-distant past molecular processes in the brain were only accessible through invasive measures—such as brain biopsy or through a lumbar puncture—studying CNS-derived EVs in blood offers us an opportunity to gain access to brain molecular signatures with relative ease. Often, these molecular signatures predate clinical changes by years or months, allowing us the prospects of potentially identifying and treating CNS disorders early on, possibly even before the onset of symptoms.
Therapeutic use of the Twitter feed
EV may be used to alter brain receptor structures in a targeted way to facilitate treatment of various psychiatric disorders. One example is a proof-of-concept study in mice in which administration of artificially manufactured EVs led to a decrease of opioid receptor mu.16 This was done by constructing EVs that carry neuron-specific rabies viral glycoprotein (RVG) peptide on the membrane surface to deliver mu opioid receptor small interfering RNA into the brain. This resulted in downregulation of mu opioid receptor and a decrease in morphine relapse.16
Additional ways in which EVs can be used therapeutically is via targeted drug delivery CNS methods. EVs may represent the next generation of treatment by allowing not only medication transport into the CNS,17 but also by facilitating directed CNS transport. What if we could use a molecular hashtag to send a dopaminergic agent to the substantia nigra of a patient with Parkinson disease but avoid sending that same treatment to the limbic cortex, where it might produce perceptual disturbances or hallucinations? In the future, EVs may help clinicians access the CNS, which is traditionally restricted by the blood brain barrier, and make it easier to achieve CNS concentrations of medications13 while decreasing medication exposure in other parts of the body. The therapeutic potential of EVs for medication delivery and regenerative medicine is awe-inspiring. Several studies have modified EVs to improve their therapeutic properties and to target delivery to specific cells13 by leveraging EV surface markers.18
Future directions for EVs
A better understanding of neuron-derived EVs may eventually help us abandon nosology-based diagnostic criteria and adopt molecular-based diagnostic approaches in psychiatry. It may allow us to consider a molecular synaptic etiology of psychiatric disorders, and diagnose patients based on synaptic pathology utilizing “neuron-derived EV liquid biopsies.” Such a shift would align psychiatry with other medical fields in which diagnosis and treatment are often based on biopsies and blood tests. Because proteins in EVs often exist in their native states, intact with their posttranslational modifications, they provide a window into testing their actual in vivo functioning. EVs have an immense potential to revolutionize psychiatric diagnosis, facilitate precision treatment, predict response, and discover much-needed novel therapeutics.
Bottom Line
Much like a tweet, extracellular vesicles (EVs) encode short messages that are transmit ted efficiently throughout the CNS and body. They may represent a reservoir for CNS-specific biomarkers that can be is olated from plasma to guide psychiatric diagnosis and treatment. EVs represent a new frontier in the molecular study of psychiatric illness.
Related Resources
Vesiclepedia. www.microvesicles.org/
1. Harding C, Heuser J, Stahl P. Receptor-mediated endocytosis of transferrin and recycling of the transferrin receptor in rat reticulocytes. J Cell Biol. 1983;97(2):329-339. doi:10.1083/jcb.97.2.329
2. Huo L, Du X, Li X, et al. The emerging role of neural cell-derived exosomes in intercellular communication in health and neurodegenerative diseases. Front Neurosci. 2021;15:738442. doi:10.3389/fnins.2021
3. Raposo G, Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol. 2013;200(4):373-83. doi: 10.1083/jcb.201211138
4. Keerthikumar S, Chisanga D, Ariyaratne D, et al. ExoCarta: a web-based compendium of exosomal cargo. J Mol Biol. 2016;428(4):688-692. doi:10.1016/j.jmb.2015.09.019
5. Babst M. A protein’s final ESCRT. Traffic. 2005;6(1):2-9. doi:10.1111/j.1600-0854.2004.00246.x
6. Anakor E, Le Gall L, Dumonceaux J, et al. Exosomes in ageing and motor neurone disease: biogenesis, uptake mechanisms, modifications in disease and uses in the development of biomarkers and therapeutics. Cells. 2021;10(11)29-30. doi:10.3390/cells10112930
7. Chivet M, Javalet C, Hemming F, et al. Exosomes as a novel way of interneuronal communication. Biochem Soc Trans. 2013;41(1):241-244. doi:10.1042/BST20120266
8. Liu HY, Huang CM, Hung YF, et al. The microRNAs Let7c and miR21 are recognized by neuronal Toll-like receptor 7 to restrict dendritic growth of neurons. Exp Neurol. 2015;269:202-212. doi:10.1016/j.expneurol.2015.04.011
9. Antonucci F, Turola E, Riganti L, et al. Microvesicles released from microglia stimulate synaptic activity via enhanced sphingolipid metabolism. EMBO J. 2012;31(5):1231-1240. doi:10.1038/emboj.2011.489
10. Goncalves MB, Malmqvist T, Clarke E, et al. Neuronal RARβ signaling modulates PTEN activity directly in neurons and via exosome transfer in astrocytes to prevent glial scar formation and induce spinal cord regeneration. J Neurosci. 2015;35(47):15731-15745. doi:10.1523/JNEUROSCI.1339-15.2015
11. Korkut C, Li Y, Koles K, et al. Regulation of postsynaptic retrograde signaling by presynaptic exosome release. Neuron. 2013;77(6):1039-1046. doi:10.1016/j.neuron.2013.01.013
12. Chivet M, Javalet C, Laulagnier K, et al. Exosomes secreted by cortical neurons upon glutamatergic synapse activation specifically interact with neurons. J Extracell Vesicles. 2014;3(1):24722. doi:10.3402/jev.v3
13. Dickens AM, Tovar-Y-Romo LB, Yoo SW, et al. Astrocyte-shed extracellular vesicles regulate the peripheral leukocyte response to inflammatory brain lesions. Sci Signal. 2017;10(473). doi:10.1126/scisignal.aai7696
14. Strawn J, Levine A. Treatment response biomarkers in anxiety disorders: from neuroimaging to neuronally-derived extracellular vesicles and beyond. Biomark Neuropsychiatry. 2020;3:100024.
15. Liu Y, Li D, Liu Z, et al. Targeted exosome-mediated delivery of opioid receptor Mu siRNA for the treatment of morphine relapse. Sci Rep. 2015;5:17543. doi:10.1038/srep17543
16. Shahjin F, Chand S, Yelamanchili S V. Extracellular vesicles as drug delivery vehicles to the central nervous system. J Neuroimmune Pharmacol. 2020;15(3):443-458. doi:10.1007/s11481-019-09875-w
17. Murphy DE, de Jong OG, Brouwer M, et al. Extracellular vesicle-based therapeutics: natural versus engineered targeting and trafficking. Exp Mol Med. 2019;51(3):1-12. doi:10.1038/s12276-019-0223-5
18. Meng W, He C, Hao Y, et al. Prospects and challenges of extracellular vesicle-based drug delivery system: considering cell source. Drug Deliv. 2020;27(1):585-598. doi:10.1080/10717544.2020.1748758
Twitter, a microblogging and social networking service, has become a “go-to’” for conversations, updates, breaking news, and sharing the more mundane aspects of our lives. Tweets, which were lengthened from 140 to 280 characters in 2017, rapidly communicate and disseminate information to a wide audience. Generally, tweets are visible to everyone, though users can mute and block other users from viewing their tweets. Spikes in tweets and tweeting frequency reflect hyper-current events: the last minutes of the Super Bowl, certification of an election, or a new movie release. In fact, social scientists have analyzed tweet frequencies to examine the impact of local and national events. However, few are aware that like celebrities, politicians, influencers, and ordinary citizens, the human brain also tweets.
In this article, we describe the components of the brain’s “Twitter” system, how it works, and how it might someday be used to improve the diagnosis and treatment of psychiatric disorders.
Brain tweets
The brain’s Twitter system involves extracellular vesicles (EVs), tiny (<1 µm) membrane-bound vesicles that are released from neurons, glia, and other neuronal cells (Table). These EVs cross the blood-brain barrier and facilitate cell-to-cell communication within and among tissues (Figure 1).
First described in the 1980s,1 EVs are secreted by a diverse array of cells: mast cells reticulocytes, epithelial cells, immune cells, neurons, glia, and oligodendrocytes. Like tweets, EVs rapidly disseminate packets of information throughout the brain and body and direct the molecular activity of recipient cells in both health and disease. These “brain tweets” contain short, circumscribed messages, and the characters are the EV cargos: RNAs, proteins, lipids, and metabolites. Like a Twitter feed, EVs cast a wide communication net across the body, much of which finds its way to the blood. As neuroscientists, we can follow these tweets by isolating tissue-derived EVs in plasma and examining their surface molecules and cargo. By following this Twitter feed, we can tap into important molecular communications and identify “trending” (evolving) pathological processes, and perhaps use the brain Twitter feed to improve diagnosis and treatments. We can pinpoint, in the blood, signals from CNS processes, down to the level of identifying EV cargos from specific brain cell types.

Within the CNS, EVs are secreted by neurons, where they may modulate synaptic plasticity and transfer molecular cargo among neurons. EVs also facilitate communication between neurons and glia, maintain homeostasis, trigger neuroprotective processes, and even regulate synaptic transmission.2
What’s in a brain tweet?
To discuss what’s in a brain tweet, we must first understand how a brain tweet is composed. EVs are pinched off from membranes of intercellular structures (eg, golgi or endoplasmic reticulum) or pinched off directly from cell membranes, where upon release they become EVs. There is a complex cellular machinery that transports what ultimately becomes an EV to the cell membrane.3 EVs contain unique mixtures of lipids, proteins, and nucleic acids (eg, microRNA [miRNA], mRNA, and noncoding RNA).4 To date, nearly 10,000 proteins, 11,000 lipids, 3,500 mRNAs, and 3,000 miRNAs have been identified as cargos in extracellular vesicles (Figure 1). Similar to how the release of EVs is dependent on complex intracellular machinery, the packing of these contents into what will become the EV involves a parallel set of complex machinery that is largely directed by endosomal sorting complexes required for transport (ESCRT) proteins.5 Of interest, when viruses attack cells, they hijack this EV packaging system to package and release new viruses. EVs vary in size, shape, and density; this variation is related to the cell origin, among other things. EVs also differ in their membrane lipid composition and in terms of transmembrane proteins as well as the proteins that facilitate EV binding to target cells (Figure 2).6 Ultimately, these exosomes are taken up by the recipient cells.

EV-facilitated neuron-to-neuron tweets have been implicated in neuronal growth and differentiation.7 EV-driven communication between cells also can decrease dendrite growth and can trigger microglia to prune synapses.8 EVs from glial cells may promote neuronal integrity, directly boost presynaptic glutamate release,9 or even, through miRNAs, change the expression of glutamate receptors.10 EVs from astrocytes transport proteins that enable neuronal repair, while EVs from microglia regulate neuronal homeostasis. EV cargos—lipids, proteins, and miRNAs—from neurons modify signal transduction and protein expression in recipient cells. Taken together, data suggest that EVs facilitate anterograde and retrograde transfer of signals across synapses,7,11 a putative mechanism for driving synaptic plasticity,12 which is a process implicated in the therapeutic efficacy of psychotropic medications and psychotherapies.
Continue to: #Targets and #neuron
#Targets and #neuron
Adding a hashtag to a tweet links it to other tweets, just as membrane features of EVs direct how EVs link to target cells. When these EVs bind to target cells, they fuse and release their cargo into the target cell (Figure 2). These directed cargo—whether mRNA, proteins, or other molecules—can direct the recipient cell to modify its firing rate (in the case of neurons), alter transmitter release, and increase or decrease expression of various genes. The targeting process is complex, and our understanding of this process is evolving. Briefly, integrin, lipid composition, glycans (eg, polysaccharides), and tetraspanin components of EVs influence their affinity for specific target cells.13 Recently, we have been able to read these hashtags and isolate cell-specific, neuron-derived EVs. Immunoadsorption techniques that leverage antibodies against L1 cell adhesion molecule protein (L1CAM(+)), primarily expressed in neurons, can identify neuronally-derived EVs (Figure 3). The specific EVs contain cargos of neuronal origin and provide a “window” into molecular processes in the brain by way of the blood (or other peripheral fluids). In following the neuronal tweets, we can follow molecular measures of important brain molecules in biofluids outside the CNS, including saliva and potentially urine (Figure 1B and 1C). In following these specific neuronal Twitter feeds, we can gain critical insights into specific brain processes.

EVs in psychiatric disorders
EVs are implicated in neuroinflammation,14 neurogenesis, synaptic plasticity, and epigenetic regulation—all processes that are involved in the pathophysiology of psychiatric disorders. Postmortem research suggests that EVs in the brain carry proinflammatory molecules from microglia, as well as secretions of regulatory miRNA that are responsible for synaptic plasticity and dendritic growth in depression, bipolar disorder, schizophrenia, and addiction. In addition, second-generation antipsychotics change the composition of EV cargos in the brain, altering their RNA, protein, and lipid content, often reflecting profound changes in gene expression in various cells in the CNS. In our lab, we have identified several molecules in plasma EVs, both lipids and miRNA, that can potentially predict the response to treatment of pediatric anxiety with selective serotonin reuptake inhibitors as well as opiate addiction.15
Further, given our increasing understanding of the way in which EV cargo reflects neuronal physiology as well as the potential pathophysiologic states of cells (including neurons), studying EVs’ molecular content can identify molecular messages—in blood—that are derived from the neurons in the brain. Having the tools to examine molecular brain regulators or other markers of disease progression (eg, beta amyloid) or brain health (eg, brain-derived neurotrophic factor) may advance our understanding and treatment of psychiatric disorders and create opportunities for precision medicine driven by biological rather than ethnologic and phenomenological markers. Whereas in the not-too-distant past molecular processes in the brain were only accessible through invasive measures—such as brain biopsy or through a lumbar puncture—studying CNS-derived EVs in blood offers us an opportunity to gain access to brain molecular signatures with relative ease. Often, these molecular signatures predate clinical changes by years or months, allowing us the prospects of potentially identifying and treating CNS disorders early on, possibly even before the onset of symptoms.
Therapeutic use of the Twitter feed
EV may be used to alter brain receptor structures in a targeted way to facilitate treatment of various psychiatric disorders. One example is a proof-of-concept study in mice in which administration of artificially manufactured EVs led to a decrease of opioid receptor mu.16 This was done by constructing EVs that carry neuron-specific rabies viral glycoprotein (RVG) peptide on the membrane surface to deliver mu opioid receptor small interfering RNA into the brain. This resulted in downregulation of mu opioid receptor and a decrease in morphine relapse.16
Additional ways in which EVs can be used therapeutically is via targeted drug delivery CNS methods. EVs may represent the next generation of treatment by allowing not only medication transport into the CNS,17 but also by facilitating directed CNS transport. What if we could use a molecular hashtag to send a dopaminergic agent to the substantia nigra of a patient with Parkinson disease but avoid sending that same treatment to the limbic cortex, where it might produce perceptual disturbances or hallucinations? In the future, EVs may help clinicians access the CNS, which is traditionally restricted by the blood brain barrier, and make it easier to achieve CNS concentrations of medications13 while decreasing medication exposure in other parts of the body. The therapeutic potential of EVs for medication delivery and regenerative medicine is awe-inspiring. Several studies have modified EVs to improve their therapeutic properties and to target delivery to specific cells13 by leveraging EV surface markers.18
Future directions for EVs
A better understanding of neuron-derived EVs may eventually help us abandon nosology-based diagnostic criteria and adopt molecular-based diagnostic approaches in psychiatry. It may allow us to consider a molecular synaptic etiology of psychiatric disorders, and diagnose patients based on synaptic pathology utilizing “neuron-derived EV liquid biopsies.” Such a shift would align psychiatry with other medical fields in which diagnosis and treatment are often based on biopsies and blood tests. Because proteins in EVs often exist in their native states, intact with their posttranslational modifications, they provide a window into testing their actual in vivo functioning. EVs have an immense potential to revolutionize psychiatric diagnosis, facilitate precision treatment, predict response, and discover much-needed novel therapeutics.
Bottom Line
Much like a tweet, extracellular vesicles (EVs) encode short messages that are transmit ted efficiently throughout the CNS and body. They may represent a reservoir for CNS-specific biomarkers that can be is olated from plasma to guide psychiatric diagnosis and treatment. EVs represent a new frontier in the molecular study of psychiatric illness.
Related Resources
Vesiclepedia. www.microvesicles.org/
Twitter, a microblogging and social networking service, has become a “go-to’” for conversations, updates, breaking news, and sharing the more mundane aspects of our lives. Tweets, which were lengthened from 140 to 280 characters in 2017, rapidly communicate and disseminate information to a wide audience. Generally, tweets are visible to everyone, though users can mute and block other users from viewing their tweets. Spikes in tweets and tweeting frequency reflect hyper-current events: the last minutes of the Super Bowl, certification of an election, or a new movie release. In fact, social scientists have analyzed tweet frequencies to examine the impact of local and national events. However, few are aware that like celebrities, politicians, influencers, and ordinary citizens, the human brain also tweets.
In this article, we describe the components of the brain’s “Twitter” system, how it works, and how it might someday be used to improve the diagnosis and treatment of psychiatric disorders.
Brain tweets
The brain’s Twitter system involves extracellular vesicles (EVs), tiny (<1 µm) membrane-bound vesicles that are released from neurons, glia, and other neuronal cells (Table). These EVs cross the blood-brain barrier and facilitate cell-to-cell communication within and among tissues (Figure 1).
First described in the 1980s,1 EVs are secreted by a diverse array of cells: mast cells reticulocytes, epithelial cells, immune cells, neurons, glia, and oligodendrocytes. Like tweets, EVs rapidly disseminate packets of information throughout the brain and body and direct the molecular activity of recipient cells in both health and disease. These “brain tweets” contain short, circumscribed messages, and the characters are the EV cargos: RNAs, proteins, lipids, and metabolites. Like a Twitter feed, EVs cast a wide communication net across the body, much of which finds its way to the blood. As neuroscientists, we can follow these tweets by isolating tissue-derived EVs in plasma and examining their surface molecules and cargo. By following this Twitter feed, we can tap into important molecular communications and identify “trending” (evolving) pathological processes, and perhaps use the brain Twitter feed to improve diagnosis and treatments. We can pinpoint, in the blood, signals from CNS processes, down to the level of identifying EV cargos from specific brain cell types.

Within the CNS, EVs are secreted by neurons, where they may modulate synaptic plasticity and transfer molecular cargo among neurons. EVs also facilitate communication between neurons and glia, maintain homeostasis, trigger neuroprotective processes, and even regulate synaptic transmission.2
What’s in a brain tweet?
To discuss what’s in a brain tweet, we must first understand how a brain tweet is composed. EVs are pinched off from membranes of intercellular structures (eg, golgi or endoplasmic reticulum) or pinched off directly from cell membranes, where upon release they become EVs. There is a complex cellular machinery that transports what ultimately becomes an EV to the cell membrane.3 EVs contain unique mixtures of lipids, proteins, and nucleic acids (eg, microRNA [miRNA], mRNA, and noncoding RNA).4 To date, nearly 10,000 proteins, 11,000 lipids, 3,500 mRNAs, and 3,000 miRNAs have been identified as cargos in extracellular vesicles (Figure 1). Similar to how the release of EVs is dependent on complex intracellular machinery, the packing of these contents into what will become the EV involves a parallel set of complex machinery that is largely directed by endosomal sorting complexes required for transport (ESCRT) proteins.5 Of interest, when viruses attack cells, they hijack this EV packaging system to package and release new viruses. EVs vary in size, shape, and density; this variation is related to the cell origin, among other things. EVs also differ in their membrane lipid composition and in terms of transmembrane proteins as well as the proteins that facilitate EV binding to target cells (Figure 2).6 Ultimately, these exosomes are taken up by the recipient cells.

EV-facilitated neuron-to-neuron tweets have been implicated in neuronal growth and differentiation.7 EV-driven communication between cells also can decrease dendrite growth and can trigger microglia to prune synapses.8 EVs from glial cells may promote neuronal integrity, directly boost presynaptic glutamate release,9 or even, through miRNAs, change the expression of glutamate receptors.10 EVs from astrocytes transport proteins that enable neuronal repair, while EVs from microglia regulate neuronal homeostasis. EV cargos—lipids, proteins, and miRNAs—from neurons modify signal transduction and protein expression in recipient cells. Taken together, data suggest that EVs facilitate anterograde and retrograde transfer of signals across synapses,7,11 a putative mechanism for driving synaptic plasticity,12 which is a process implicated in the therapeutic efficacy of psychotropic medications and psychotherapies.
Continue to: #Targets and #neuron
#Targets and #neuron
Adding a hashtag to a tweet links it to other tweets, just as membrane features of EVs direct how EVs link to target cells. When these EVs bind to target cells, they fuse and release their cargo into the target cell (Figure 2). These directed cargo—whether mRNA, proteins, or other molecules—can direct the recipient cell to modify its firing rate (in the case of neurons), alter transmitter release, and increase or decrease expression of various genes. The targeting process is complex, and our understanding of this process is evolving. Briefly, integrin, lipid composition, glycans (eg, polysaccharides), and tetraspanin components of EVs influence their affinity for specific target cells.13 Recently, we have been able to read these hashtags and isolate cell-specific, neuron-derived EVs. Immunoadsorption techniques that leverage antibodies against L1 cell adhesion molecule protein (L1CAM(+)), primarily expressed in neurons, can identify neuronally-derived EVs (Figure 3). The specific EVs contain cargos of neuronal origin and provide a “window” into molecular processes in the brain by way of the blood (or other peripheral fluids). In following the neuronal tweets, we can follow molecular measures of important brain molecules in biofluids outside the CNS, including saliva and potentially urine (Figure 1B and 1C). In following these specific neuronal Twitter feeds, we can gain critical insights into specific brain processes.

EVs in psychiatric disorders
EVs are implicated in neuroinflammation,14 neurogenesis, synaptic plasticity, and epigenetic regulation—all processes that are involved in the pathophysiology of psychiatric disorders. Postmortem research suggests that EVs in the brain carry proinflammatory molecules from microglia, as well as secretions of regulatory miRNA that are responsible for synaptic plasticity and dendritic growth in depression, bipolar disorder, schizophrenia, and addiction. In addition, second-generation antipsychotics change the composition of EV cargos in the brain, altering their RNA, protein, and lipid content, often reflecting profound changes in gene expression in various cells in the CNS. In our lab, we have identified several molecules in plasma EVs, both lipids and miRNA, that can potentially predict the response to treatment of pediatric anxiety with selective serotonin reuptake inhibitors as well as opiate addiction.15
Further, given our increasing understanding of the way in which EV cargo reflects neuronal physiology as well as the potential pathophysiologic states of cells (including neurons), studying EVs’ molecular content can identify molecular messages—in blood—that are derived from the neurons in the brain. Having the tools to examine molecular brain regulators or other markers of disease progression (eg, beta amyloid) or brain health (eg, brain-derived neurotrophic factor) may advance our understanding and treatment of psychiatric disorders and create opportunities for precision medicine driven by biological rather than ethnologic and phenomenological markers. Whereas in the not-too-distant past molecular processes in the brain were only accessible through invasive measures—such as brain biopsy or through a lumbar puncture—studying CNS-derived EVs in blood offers us an opportunity to gain access to brain molecular signatures with relative ease. Often, these molecular signatures predate clinical changes by years or months, allowing us the prospects of potentially identifying and treating CNS disorders early on, possibly even before the onset of symptoms.
Therapeutic use of the Twitter feed
EV may be used to alter brain receptor structures in a targeted way to facilitate treatment of various psychiatric disorders. One example is a proof-of-concept study in mice in which administration of artificially manufactured EVs led to a decrease of opioid receptor mu.16 This was done by constructing EVs that carry neuron-specific rabies viral glycoprotein (RVG) peptide on the membrane surface to deliver mu opioid receptor small interfering RNA into the brain. This resulted in downregulation of mu opioid receptor and a decrease in morphine relapse.16
Additional ways in which EVs can be used therapeutically is via targeted drug delivery CNS methods. EVs may represent the next generation of treatment by allowing not only medication transport into the CNS,17 but also by facilitating directed CNS transport. What if we could use a molecular hashtag to send a dopaminergic agent to the substantia nigra of a patient with Parkinson disease but avoid sending that same treatment to the limbic cortex, where it might produce perceptual disturbances or hallucinations? In the future, EVs may help clinicians access the CNS, which is traditionally restricted by the blood brain barrier, and make it easier to achieve CNS concentrations of medications13 while decreasing medication exposure in other parts of the body. The therapeutic potential of EVs for medication delivery and regenerative medicine is awe-inspiring. Several studies have modified EVs to improve their therapeutic properties and to target delivery to specific cells13 by leveraging EV surface markers.18
Future directions for EVs
A better understanding of neuron-derived EVs may eventually help us abandon nosology-based diagnostic criteria and adopt molecular-based diagnostic approaches in psychiatry. It may allow us to consider a molecular synaptic etiology of psychiatric disorders, and diagnose patients based on synaptic pathology utilizing “neuron-derived EV liquid biopsies.” Such a shift would align psychiatry with other medical fields in which diagnosis and treatment are often based on biopsies and blood tests. Because proteins in EVs often exist in their native states, intact with their posttranslational modifications, they provide a window into testing their actual in vivo functioning. EVs have an immense potential to revolutionize psychiatric diagnosis, facilitate precision treatment, predict response, and discover much-needed novel therapeutics.
Bottom Line
Much like a tweet, extracellular vesicles (EVs) encode short messages that are transmit ted efficiently throughout the CNS and body. They may represent a reservoir for CNS-specific biomarkers that can be is olated from plasma to guide psychiatric diagnosis and treatment. EVs represent a new frontier in the molecular study of psychiatric illness.
Related Resources
Vesiclepedia. www.microvesicles.org/
1. Harding C, Heuser J, Stahl P. Receptor-mediated endocytosis of transferrin and recycling of the transferrin receptor in rat reticulocytes. J Cell Biol. 1983;97(2):329-339. doi:10.1083/jcb.97.2.329
2. Huo L, Du X, Li X, et al. The emerging role of neural cell-derived exosomes in intercellular communication in health and neurodegenerative diseases. Front Neurosci. 2021;15:738442. doi:10.3389/fnins.2021
3. Raposo G, Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol. 2013;200(4):373-83. doi: 10.1083/jcb.201211138
4. Keerthikumar S, Chisanga D, Ariyaratne D, et al. ExoCarta: a web-based compendium of exosomal cargo. J Mol Biol. 2016;428(4):688-692. doi:10.1016/j.jmb.2015.09.019
5. Babst M. A protein’s final ESCRT. Traffic. 2005;6(1):2-9. doi:10.1111/j.1600-0854.2004.00246.x
6. Anakor E, Le Gall L, Dumonceaux J, et al. Exosomes in ageing and motor neurone disease: biogenesis, uptake mechanisms, modifications in disease and uses in the development of biomarkers and therapeutics. Cells. 2021;10(11)29-30. doi:10.3390/cells10112930
7. Chivet M, Javalet C, Hemming F, et al. Exosomes as a novel way of interneuronal communication. Biochem Soc Trans. 2013;41(1):241-244. doi:10.1042/BST20120266
8. Liu HY, Huang CM, Hung YF, et al. The microRNAs Let7c and miR21 are recognized by neuronal Toll-like receptor 7 to restrict dendritic growth of neurons. Exp Neurol. 2015;269:202-212. doi:10.1016/j.expneurol.2015.04.011
9. Antonucci F, Turola E, Riganti L, et al. Microvesicles released from microglia stimulate synaptic activity via enhanced sphingolipid metabolism. EMBO J. 2012;31(5):1231-1240. doi:10.1038/emboj.2011.489
10. Goncalves MB, Malmqvist T, Clarke E, et al. Neuronal RARβ signaling modulates PTEN activity directly in neurons and via exosome transfer in astrocytes to prevent glial scar formation and induce spinal cord regeneration. J Neurosci. 2015;35(47):15731-15745. doi:10.1523/JNEUROSCI.1339-15.2015
11. Korkut C, Li Y, Koles K, et al. Regulation of postsynaptic retrograde signaling by presynaptic exosome release. Neuron. 2013;77(6):1039-1046. doi:10.1016/j.neuron.2013.01.013
12. Chivet M, Javalet C, Laulagnier K, et al. Exosomes secreted by cortical neurons upon glutamatergic synapse activation specifically interact with neurons. J Extracell Vesicles. 2014;3(1):24722. doi:10.3402/jev.v3
13. Dickens AM, Tovar-Y-Romo LB, Yoo SW, et al. Astrocyte-shed extracellular vesicles regulate the peripheral leukocyte response to inflammatory brain lesions. Sci Signal. 2017;10(473). doi:10.1126/scisignal.aai7696
14. Strawn J, Levine A. Treatment response biomarkers in anxiety disorders: from neuroimaging to neuronally-derived extracellular vesicles and beyond. Biomark Neuropsychiatry. 2020;3:100024.
15. Liu Y, Li D, Liu Z, et al. Targeted exosome-mediated delivery of opioid receptor Mu siRNA for the treatment of morphine relapse. Sci Rep. 2015;5:17543. doi:10.1038/srep17543
16. Shahjin F, Chand S, Yelamanchili S V. Extracellular vesicles as drug delivery vehicles to the central nervous system. J Neuroimmune Pharmacol. 2020;15(3):443-458. doi:10.1007/s11481-019-09875-w
17. Murphy DE, de Jong OG, Brouwer M, et al. Extracellular vesicle-based therapeutics: natural versus engineered targeting and trafficking. Exp Mol Med. 2019;51(3):1-12. doi:10.1038/s12276-019-0223-5
18. Meng W, He C, Hao Y, et al. Prospects and challenges of extracellular vesicle-based drug delivery system: considering cell source. Drug Deliv. 2020;27(1):585-598. doi:10.1080/10717544.2020.1748758
1. Harding C, Heuser J, Stahl P. Receptor-mediated endocytosis of transferrin and recycling of the transferrin receptor in rat reticulocytes. J Cell Biol. 1983;97(2):329-339. doi:10.1083/jcb.97.2.329
2. Huo L, Du X, Li X, et al. The emerging role of neural cell-derived exosomes in intercellular communication in health and neurodegenerative diseases. Front Neurosci. 2021;15:738442. doi:10.3389/fnins.2021
3. Raposo G, Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol. 2013;200(4):373-83. doi: 10.1083/jcb.201211138
4. Keerthikumar S, Chisanga D, Ariyaratne D, et al. ExoCarta: a web-based compendium of exosomal cargo. J Mol Biol. 2016;428(4):688-692. doi:10.1016/j.jmb.2015.09.019
5. Babst M. A protein’s final ESCRT. Traffic. 2005;6(1):2-9. doi:10.1111/j.1600-0854.2004.00246.x
6. Anakor E, Le Gall L, Dumonceaux J, et al. Exosomes in ageing and motor neurone disease: biogenesis, uptake mechanisms, modifications in disease and uses in the development of biomarkers and therapeutics. Cells. 2021;10(11)29-30. doi:10.3390/cells10112930
7. Chivet M, Javalet C, Hemming F, et al. Exosomes as a novel way of interneuronal communication. Biochem Soc Trans. 2013;41(1):241-244. doi:10.1042/BST20120266
8. Liu HY, Huang CM, Hung YF, et al. The microRNAs Let7c and miR21 are recognized by neuronal Toll-like receptor 7 to restrict dendritic growth of neurons. Exp Neurol. 2015;269:202-212. doi:10.1016/j.expneurol.2015.04.011
9. Antonucci F, Turola E, Riganti L, et al. Microvesicles released from microglia stimulate synaptic activity via enhanced sphingolipid metabolism. EMBO J. 2012;31(5):1231-1240. doi:10.1038/emboj.2011.489
10. Goncalves MB, Malmqvist T, Clarke E, et al. Neuronal RARβ signaling modulates PTEN activity directly in neurons and via exosome transfer in astrocytes to prevent glial scar formation and induce spinal cord regeneration. J Neurosci. 2015;35(47):15731-15745. doi:10.1523/JNEUROSCI.1339-15.2015
11. Korkut C, Li Y, Koles K, et al. Regulation of postsynaptic retrograde signaling by presynaptic exosome release. Neuron. 2013;77(6):1039-1046. doi:10.1016/j.neuron.2013.01.013
12. Chivet M, Javalet C, Laulagnier K, et al. Exosomes secreted by cortical neurons upon glutamatergic synapse activation specifically interact with neurons. J Extracell Vesicles. 2014;3(1):24722. doi:10.3402/jev.v3
13. Dickens AM, Tovar-Y-Romo LB, Yoo SW, et al. Astrocyte-shed extracellular vesicles regulate the peripheral leukocyte response to inflammatory brain lesions. Sci Signal. 2017;10(473). doi:10.1126/scisignal.aai7696
14. Strawn J, Levine A. Treatment response biomarkers in anxiety disorders: from neuroimaging to neuronally-derived extracellular vesicles and beyond. Biomark Neuropsychiatry. 2020;3:100024.
15. Liu Y, Li D, Liu Z, et al. Targeted exosome-mediated delivery of opioid receptor Mu siRNA for the treatment of morphine relapse. Sci Rep. 2015;5:17543. doi:10.1038/srep17543
16. Shahjin F, Chand S, Yelamanchili S V. Extracellular vesicles as drug delivery vehicles to the central nervous system. J Neuroimmune Pharmacol. 2020;15(3):443-458. doi:10.1007/s11481-019-09875-w
17. Murphy DE, de Jong OG, Brouwer M, et al. Extracellular vesicle-based therapeutics: natural versus engineered targeting and trafficking. Exp Mol Med. 2019;51(3):1-12. doi:10.1038/s12276-019-0223-5
18. Meng W, He C, Hao Y, et al. Prospects and challenges of extracellular vesicle-based drug delivery system: considering cell source. Drug Deliv. 2020;27(1):585-598. doi:10.1080/10717544.2020.1748758
Serotonin-mediated anxiety: How to recognize and treat it
Sara R. Abell, MD, and Rif S. El-Mallakh, MD
Individuals with anxiety will experience frequent or chronic excessive worry, nervousness, a sense of unease, a feeling of being unfocused, and distress, which result in functional impairment.1 Frequently, anxiety is accompanied by restlessness or muscle tension. Generalized anxiety disorder is one of the most common psychiatric diagnoses in the United States and has a prevalence of 2% to 6% globally.2 Although research has been conducted regarding anxiety’s pathogenesis, to date a firm consensus on its etiology has not been reached.3 It is likely multifactorial, with environmental and biologic components.
One area of focus has been neurotransmitters and the possible role they play in the pathogenesis of anxiety. Specifically, the monoamine neurotransmitters have been implicated in the clinical manifestations of anxiety. Among the amines, normal roles include stimulating the autonomic nervous system and regulating numerous cognitive phenomena, such as volition and emotion. Many psychiatric medications modify aminergic transmission, and many current anxiety medications target amine neurotransmitters. Medications that target histamine, serotonin, norepinephrine, and dopamine all play a role in treating anxiety.
In this article, we focus on serotonin (5-hydroxytryptamine, 5-HT) as a mediator of anxiety and on excessive synaptic 5-HT as the cause of anxiety. We discuss how 5-HT–mediated anxiety can be identified and offer some solutions for its treatment.
The amine neurotransmitters
There are 6 amine neurotransmitters in the CNS. These are derived from tyrosine (dopamine [DA], norepinephrine [NE], and epinephrine), histidine (histamine), and tryptophan (serotonin [5-HT] and melatonin). In addition to their physiologic actions, amines have been implicated in both acute and chronic anxiety. Excessive DA stimulation has been linked with fear4,5; NE elevations are central to hypervigilance and hyperarousal of posttraumatic stress disorder6; and histamine may mediate emotional memories involved in fear and anxiety.7 Understanding the normal function of 5-HT will aid in understanding its potential problematic role (Box,8-18page 38).
How serotonin-mediated anxiety presents
“Anxiety” is a collection of signs and symptoms that likely represent multiple processes and have the common characteristic of being subjectively unpleasant, with a subjective wish for the feeling to end. The expression of anxiety disorders is quite diverse and ranges from brief episodes such as panic attacks (which may be mediated, in part, by epinephrine/NE19) to lifelong stereotypic obsessions and compulsions (which may be mediated, in part, by DA and modified by 5-HT20,21). Biochemical separation of the anxiety disorders is key to achieving tailored treatment.6 Towards this end, it is important to investigate the phenomenon of serotonin-mediated anxiety.
Because clinicians are familiar with reductions of anxiety as selective serotonin reuptake inhibitors (SSRIs) increase 5-HT levels in the synapse, it is difficult to conceptualize serotonin-mediated anxiety. However, many of the effects at postsynaptic 5-HT receptors may be biphasic.15-18 Serotonin-mediated anxiety appears to occur when levels of 5-HT (or stimulation of 5-HT receptors) are particularly high. This is most frequently seen in patients who genetically have high synaptic 5-HT (by virtue of the short form of the 5-HT transporter),22 whose synaptic 5-HT is further increased by treatment with an SSRI,23 and who are experiencing a stressor that yet further increases their synaptic 5-HT.24 However, it may occur in some individuals with only 2 of these 3 conditions.Clinically, individuals with serotonin-mediated anxiety will usually appear calm. The anxiety they are experiencing is not exhibited in any way in the motor system (ie, they do not appear restless, do not pace, muscle tone is not increased, etc.). However, they will generally complain of an internal agitation, a sense of a negative internal energy. Frequently, they will use descriptions such as “I feel I could jump out of my skin.” As previously mentioned, this is usually in the setting of some environmental stress, in addition to either a pharmacologic (SSRI) or genetic (short form of the 5-HT transporter) reason for increasing synaptic 5-HT, or both.
Almost always, interventions that block multiple postsynaptic 5-HT receptors or discontinuation of the SSRI (if applicable) will alleviate the anxiety, quickly or more slowly, respectively. Sublingual asenapine, which at low doses can block 5-HT2C (Ki = 0.03 nM), 5-HT2A (Ki = 0.07 nM), 5-HT7 (Ki = 0.11 nM), 5-HT2B (Ki = 0.18 nM), and 5-HT6 (Ki = 0.25 nM),25,26 and which will produce peak plasma levels within 10 minutes,27 usually is quite effective.
Box
Serotonin (5-HT) arises from neurons in the raphe nuclei of the rostral pons and projects superiorly to the cerebral cortex and inferiorly to the spinal cord.8 It works in an inhibitory or excitatory manner depending on which receptors are activated. In the periphery, 5-HT influences intestinal peristalsis, sensory modulation, gland function, thermoregulation, blood pressure, platelet aggregation, and sexual behavior,9 all actions that produce potential adverse effects of serotonin reuptake– inhibiting antidepressants. In the CNS, 5-HT plays a role in attention bias; decision-making; sleep and wakefulness; and mood regulation. In short, serotonin can be viewed as mediating emotional motivation.10
Serotonin alters neuroplasticity. During development, 5-HT stimulates creation of new synapses and increases the density of synaptic webs. It has a direct stimulatory effect on the length of dendrites, their branching, and their myelination.11 In the CNS, it plays a role in dendritic arborization. Animal studies with rats have shown that lesioning highly concentrated 5-HT areas at early ages resulted in an adult brain with a lower number of neurons and a less complex web of dendrites.12,13 In situations of emotional stress, it is theorized that low levels of 5-HT lead to a reduced ability to deal with emotional stressors due to lower levels of complexity in synaptic connections.
Serotonin has also been implicated in mediating some aspects of dopamine-related actions, such as locomotion, reward, and threat avoidance. This is believed to contribute to the beneficial effect of 5-HT2A blockade by secondgeneration antipsychotics (SGAs).14 Blockade of other 5-HT receptors, such as 5-HT1A, 5-HT2C, 5-HT6, and 5-HT7, may also contribute to the antipsychotic action of SGAs.14
Serotonin receptors are found throughout the body, and 14 subtypes have been identified.9 Excitatory and inhibitory action of 5-HT depends on the receptor, and the actions of 5-HT can differ with the same receptor at different concentrations. This is because serotonin’s effects are biphasic and concentration-dependent, meaning that levels of 5-HT in the synapse will dictate the downstream effect of receptor agonism or antagonism. Animal models have shown that low-dose agonism of 5-HT receptors causes vasoconstriction of the coronary arteries, and high doses cause relaxation. This response has also been demonstrated in the vasculature of the kidneys and the smooth muscle of the trachea. Additionally, 5-HT works in conjunction with histamine to produce a biphasic response in the colonic arteries and veins in situations of endothelial damage.15
Most relevant to this discussion are 5-HT’s actions in mood regulation and behavior. Low 5-HT states result in less behavioral inhibition, leading to higher impulse control failures and aggression. Experiments in mice with deficient serotonergic brain regions show hypoactivity, extended daytime sleep, anxiety, and depressive behaviors.13 Serotonin’s behavioral effects are also biphasic. For example, lowdose antagonism with trazodone of 5-HT receptors demonstrated a pro-aggressive behavioral effect, while high-dose antagonism is anti-aggressive.15 Similar biphasic effects may result in either induction or reduction of anxiety with agents that block or excite certain 5-HT receptors.16-18
Continue to: A key difference: No motor system involvement...
A key difference: No motor system involvement
What distinguishes 5-HT from the other amine transmitters as a mediator of anxiety is the lack of involvement of the motor system. Multiple studies in rats illustrate that exogenously augmenting 5-HT has no effect on levels of locomotor activity. Dopamine depletion is well-characterized in the motor dysfunction of Parkinson’s disease, and DA excess can cause repetitive, stereotyped movements, such as seen in tardive dyskinesia or Huntington’s disease.8 In humans, serotonin-mediated anxiety is usually without a motoric component; patients appear calm but complain of extreme anxiety or agitation. Agitation has been reported after initiation of an SSRI,29 and is more likely to occur in patients with the short form of the 5-HT transporter.30 Motoric activation has been reported in some of these studies, but does not seem to cluster with the complaint of agitation.29 The reduced number of available transporters means a chronic steady-state elevation of serotonin, because less serotonin is being removed from the synapse after it is released. This is one of the reasons patients with the short form of the 5-HT transporter may be more susceptible to serotonin-mediated anxiety.
What you need to keep in mind
Pharmacologic treatment of anxiety begins with an SSRI, a serotonin-norepinephrine reuptake inhibitor (SNRI), or buspirone. Second-line treatments include hydroxyzine, gabapentin, pregabalin, and quetiapine.3,31 However, clinicians need to be aware that a fraction of their patients will report anxiety that will not have any external manifestations, but will be experienced as an unpleasant internal energy. These patients may report an increase in their anxiety levels when started on an SSRI or SNRI.29,30 This anxiety is most likely mediated by increases of synaptic 5-HT. This occurs because many serotonergic receptors may have a biphasic response, so that too much stimulation is experienced as excessive internal energy.16-18 In such patients, blockade of key 5-HT receptors may reduce that internal agitation. The advantage of recognizing serotonin-mediated anxiety is that one can specifically tailor treatment to address the patient’s specific physiology.
It is important to note that the anxiolytic effect of asenapine is specific to patients with serotonin-mediated anxiety. Unlike quetiapine, which is effective as augmentation therapy in generalized anxiety disorder,31 asenapine does not appear to reduce anxiety in patients with schizophrenia32 or borderline personality disorder33 when administered for other reasons. However, it may reduce anxiety in patients with the short form of the 5-HT transporter.30,34
Bottom Line
Serotonin-mediated anxiety occurs when levels of synaptic serotonin (5-HT) are high. Patients with serotonin-mediated anxiety appear calm but will report experiencing an unpleasant internal energy. Interventions that block multiple postsynaptic 5-HT receptors or discontinuation of a selective serotonin reuptake inhibitor (if applicable) will alleviate the anxiety.
Related Resource
• Bhatt NV. Anxiety disorders. https://emedicine.medscape. com/article/286227-overview
Drug Brand Names
Asenapine • Saphris, Secuado
Gabapentin • Neurontin
Hydroxyzine • Vistaril
Pregabalin • Lyrica
Quetiapine • Seroquel
Trazodone • Oleptro
1. Shelton CI. Diagnosis and management of anxiety disorders. J Am Osteopath Assoc. 2004;104(3 Suppl 3):S2-S5.
2. Ruscio AM, Hallion LS, Lim CCW, et al. Cross-sectional comparison of the epidemiology of DSM-5 generalized anxiety disorder across the globe. JAMA Psychiatry. 2017;74(5):465-475.
3. Locke AB, Kirst N, Shultz CG. Diagnosis and management of generalized anxiety disorder and panic disorder in adults. Am Fam Physician. 2015;91(9):617-624.
4. Hariri AR, Mattay VS, Tessitore A, et al. Dextroamphetamine modulates the response of the human amygdala. Neuropsychopharmacology. 2002;27(6):1036-1040.
5. Colombo AC, de Oliveira AR, Reimer AE, et al. Dopaminergic mechanisms underlying catalepsy, fear and anxiety: do they interact? Behav Brain Res. 2013;257:201-207.
6. Togay B, El-Mallakh RS. Posttraumatic stress disorder: from pathophysiology to pharmacology. Curr Psychiatry. 2020;19(5):33-39.
7. Provensi G, Passani MB, Costa A, et al. Neuronal histamine and the memory of emotionally salient events. Br J Pharmacol. 2020;177(3):557-569.
8. Purves D, Augustine GJ, Fitzpatrick D, et al (eds). Neuroscience. 2nd ed. Sinauer Associates; 2001.
9. Pytliak M, Vargová V, Mechírová V, et al. Serotonin receptors – from molecular biology to clinical applications. Physiol Res. 2011;60(1):15-25.
10. Meneses A, Liy-Salmeron G. Serotonin and emotion, learning and memory. Rev Neurosci. 2012;23(5-6):543-553.
11. Whitaker-Azmitia PM. Serotonin and brain development: role in human developmental diseases. Brain Res Bull. 2001;56(5):479-485.
12. Towle AC, Breese GR, Mueller RA, et al. Early postnatal administration of 5,7-DHT: effects on serotonergic neurons and terminals. Brain Res. 1984;310(1):67-75.
13. Rok-Bujko P, Krzs´cik P, Szyndler J, et al. The influence of neonatal serotonin depletion on emotional and exploratory behaviours in rats. Behav Brain Res. 2012;226(1):87-95.
14. Meltzer HY. The role of serotonin in antipsychotic drug action. Neuropsychopharmacology. 1999;21(2 Suppl):106S-115S.
15. Calabrese EJ. 5-Hydroxytryptamine (serotonin): biphasic dose responses. Crit Rev Toxicol. 2001;31(4-5):553-561.
16. Zuardi AW. 5-HT-related drugs and human experimental anxiety. Neurosci Biobehav Rev. 1990;14(4):507-510.
17. Sánchez C, Meier E. Behavioral profiles of SSRIs in animal models of depression, anxiety and aggression. Are they all alike? Psychopharmacology (Berl). 1997;129(3):197-205.
18. Koek W, Mitchell NC, Daws LC. Biphasic effects of selective serotonin reuptake inhibitors on anxiety: rapid reversal of escitalopram’s anxiogenic effects in the novelty-induced hypophagia test in mice? Behav Pharmacol. 2018;29(4):365-369.
19. van Zijderveld GA, Veltman DJ, van Dyck R, et al. Epinephrine-induced panic attacks and hyperventilation. J Psychiatr Res. 1999;33(1):73-78.
20. Ho EV, Thompson SL, Katzka WR, et al. Clinically effective OCD treatment prevents 5-HT1B receptor-induced repetitive behavior and striatal activation. Psychopharmacology (Berl). 2016;233(1):57-70.
21. Stein DJ, Costa DLC, Lochner C, et al. Obsessive-compulsive disorder. Nat Rev Dis Primers. 2019;5(1):52.
22. Luddington NS, Mandadapu A, Husk M, et al. Clinical implications of genetic variation in the serotonin transporter promoter region: a review. Prim Care Companion J Clin Psychiatry. 2009;11(3):93-102.
23. Stahl SM. Mechanism of action of serotonin selective reuptake inhibitors. Serotonin receptors and pathways mediate therapeutic effects and side effects. J Affect Disord. 1998;51(3):215-235.
24. Chaouloff F, Berton O, Mormède P. Serotonin and stress. Neuropsychopharmacology. 1999;21(2 Suppl):28S-32S.
25. Siafis S, Tzachanis D, Samara M, et al. Antipsychotic drugs: From receptor-binding profiles to metabolic side effects. Curr Neuropharmacol. 2018;16(8):1210-1223.
26. Carrithers B, El-Mallakh RS. Transdermal asenapine in schizophrenia: a systematic review. Patient Prefer Adherence. 2020;14:1541-1551.
27. Citrome L. Asenapine review, part I: chemistry, receptor affinity profile, pharmacokinetics and metabolism. Expert Opin Drug Metab Toxicol. 2014;10(6):893-903.
28. Pratts M, Citrome L, Grant W, et al. A single-dose, randomized, double-blind, placebo-controlled trial of sublingual asenapine for acute agitation. Acta Psychiatr Scand. 2014;130(1):61-68.
29. Biswas AB, Bhaumik S, Branford D. Treatment-emergent behavioural side effects with selective serotonin re-uptake inhibitors in adults with learning disabilities. Hum Psychopharmacol. 2001;16(2):133-137.
30. Perlis RH, Mischoulon D, Smoller JW, et al. Serotonin transporter polymorphisms and adverse effects with fluoxetine treatment. Biol Psychiatry. 2003;54(9):879-883.
31. Ipser JC, Carey P, Dhansay Y, et al. Pharmacotherapy augmentation strategies in treatment-resistant anxiety disorders. Cochrane Database Syst Rev. 2006;(4):CD005473.
32. Kane JM, Mackle M, Snow-Adami L, et al. A randomized placebo-controlled trial of asenapine for the prevention of relapse of schizophrenia after long-term treatment. J Clin Psychiatry. 2011;72(3):349-355.
33. Bozzatello P, Rocca P, Uscinska M, et al. Efficacy and tolerability of asenapine compared with olanzapine in borderline personality disorder: an open-label randomized controlled trial. CNS Drugs. 2017;31(9):809-819.
34. El-Mallakh RS, Nuss S, Gao D, et al. Asenapine in the treatment of bipolar depression. Psychopharmacol Bull. 2020;50(1):8-18.
Sara R. Abell, MD, and Rif S. El-Mallakh, MD
Individuals with anxiety will experience frequent or chronic excessive worry, nervousness, a sense of unease, a feeling of being unfocused, and distress, which result in functional impairment.1 Frequently, anxiety is accompanied by restlessness or muscle tension. Generalized anxiety disorder is one of the most common psychiatric diagnoses in the United States and has a prevalence of 2% to 6% globally.2 Although research has been conducted regarding anxiety’s pathogenesis, to date a firm consensus on its etiology has not been reached.3 It is likely multifactorial, with environmental and biologic components.
One area of focus has been neurotransmitters and the possible role they play in the pathogenesis of anxiety. Specifically, the monoamine neurotransmitters have been implicated in the clinical manifestations of anxiety. Among the amines, normal roles include stimulating the autonomic nervous system and regulating numerous cognitive phenomena, such as volition and emotion. Many psychiatric medications modify aminergic transmission, and many current anxiety medications target amine neurotransmitters. Medications that target histamine, serotonin, norepinephrine, and dopamine all play a role in treating anxiety.
In this article, we focus on serotonin (5-hydroxytryptamine, 5-HT) as a mediator of anxiety and on excessive synaptic 5-HT as the cause of anxiety. We discuss how 5-HT–mediated anxiety can be identified and offer some solutions for its treatment.
The amine neurotransmitters
There are 6 amine neurotransmitters in the CNS. These are derived from tyrosine (dopamine [DA], norepinephrine [NE], and epinephrine), histidine (histamine), and tryptophan (serotonin [5-HT] and melatonin). In addition to their physiologic actions, amines have been implicated in both acute and chronic anxiety. Excessive DA stimulation has been linked with fear4,5; NE elevations are central to hypervigilance and hyperarousal of posttraumatic stress disorder6; and histamine may mediate emotional memories involved in fear and anxiety.7 Understanding the normal function of 5-HT will aid in understanding its potential problematic role (Box,8-18page 38).
How serotonin-mediated anxiety presents
“Anxiety” is a collection of signs and symptoms that likely represent multiple processes and have the common characteristic of being subjectively unpleasant, with a subjective wish for the feeling to end. The expression of anxiety disorders is quite diverse and ranges from brief episodes such as panic attacks (which may be mediated, in part, by epinephrine/NE19) to lifelong stereotypic obsessions and compulsions (which may be mediated, in part, by DA and modified by 5-HT20,21). Biochemical separation of the anxiety disorders is key to achieving tailored treatment.6 Towards this end, it is important to investigate the phenomenon of serotonin-mediated anxiety.
Because clinicians are familiar with reductions of anxiety as selective serotonin reuptake inhibitors (SSRIs) increase 5-HT levels in the synapse, it is difficult to conceptualize serotonin-mediated anxiety. However, many of the effects at postsynaptic 5-HT receptors may be biphasic.15-18 Serotonin-mediated anxiety appears to occur when levels of 5-HT (or stimulation of 5-HT receptors) are particularly high. This is most frequently seen in patients who genetically have high synaptic 5-HT (by virtue of the short form of the 5-HT transporter),22 whose synaptic 5-HT is further increased by treatment with an SSRI,23 and who are experiencing a stressor that yet further increases their synaptic 5-HT.24 However, it may occur in some individuals with only 2 of these 3 conditions.Clinically, individuals with serotonin-mediated anxiety will usually appear calm. The anxiety they are experiencing is not exhibited in any way in the motor system (ie, they do not appear restless, do not pace, muscle tone is not increased, etc.). However, they will generally complain of an internal agitation, a sense of a negative internal energy. Frequently, they will use descriptions such as “I feel I could jump out of my skin.” As previously mentioned, this is usually in the setting of some environmental stress, in addition to either a pharmacologic (SSRI) or genetic (short form of the 5-HT transporter) reason for increasing synaptic 5-HT, or both.
Almost always, interventions that block multiple postsynaptic 5-HT receptors or discontinuation of the SSRI (if applicable) will alleviate the anxiety, quickly or more slowly, respectively. Sublingual asenapine, which at low doses can block 5-HT2C (Ki = 0.03 nM), 5-HT2A (Ki = 0.07 nM), 5-HT7 (Ki = 0.11 nM), 5-HT2B (Ki = 0.18 nM), and 5-HT6 (Ki = 0.25 nM),25,26 and which will produce peak plasma levels within 10 minutes,27 usually is quite effective.
Box
Serotonin (5-HT) arises from neurons in the raphe nuclei of the rostral pons and projects superiorly to the cerebral cortex and inferiorly to the spinal cord.8 It works in an inhibitory or excitatory manner depending on which receptors are activated. In the periphery, 5-HT influences intestinal peristalsis, sensory modulation, gland function, thermoregulation, blood pressure, platelet aggregation, and sexual behavior,9 all actions that produce potential adverse effects of serotonin reuptake– inhibiting antidepressants. In the CNS, 5-HT plays a role in attention bias; decision-making; sleep and wakefulness; and mood regulation. In short, serotonin can be viewed as mediating emotional motivation.10
Serotonin alters neuroplasticity. During development, 5-HT stimulates creation of new synapses and increases the density of synaptic webs. It has a direct stimulatory effect on the length of dendrites, their branching, and their myelination.11 In the CNS, it plays a role in dendritic arborization. Animal studies with rats have shown that lesioning highly concentrated 5-HT areas at early ages resulted in an adult brain with a lower number of neurons and a less complex web of dendrites.12,13 In situations of emotional stress, it is theorized that low levels of 5-HT lead to a reduced ability to deal with emotional stressors due to lower levels of complexity in synaptic connections.
Serotonin has also been implicated in mediating some aspects of dopamine-related actions, such as locomotion, reward, and threat avoidance. This is believed to contribute to the beneficial effect of 5-HT2A blockade by secondgeneration antipsychotics (SGAs).14 Blockade of other 5-HT receptors, such as 5-HT1A, 5-HT2C, 5-HT6, and 5-HT7, may also contribute to the antipsychotic action of SGAs.14
Serotonin receptors are found throughout the body, and 14 subtypes have been identified.9 Excitatory and inhibitory action of 5-HT depends on the receptor, and the actions of 5-HT can differ with the same receptor at different concentrations. This is because serotonin’s effects are biphasic and concentration-dependent, meaning that levels of 5-HT in the synapse will dictate the downstream effect of receptor agonism or antagonism. Animal models have shown that low-dose agonism of 5-HT receptors causes vasoconstriction of the coronary arteries, and high doses cause relaxation. This response has also been demonstrated in the vasculature of the kidneys and the smooth muscle of the trachea. Additionally, 5-HT works in conjunction with histamine to produce a biphasic response in the colonic arteries and veins in situations of endothelial damage.15
Most relevant to this discussion are 5-HT’s actions in mood regulation and behavior. Low 5-HT states result in less behavioral inhibition, leading to higher impulse control failures and aggression. Experiments in mice with deficient serotonergic brain regions show hypoactivity, extended daytime sleep, anxiety, and depressive behaviors.13 Serotonin’s behavioral effects are also biphasic. For example, lowdose antagonism with trazodone of 5-HT receptors demonstrated a pro-aggressive behavioral effect, while high-dose antagonism is anti-aggressive.15 Similar biphasic effects may result in either induction or reduction of anxiety with agents that block or excite certain 5-HT receptors.16-18
Continue to: A key difference: No motor system involvement...
A key difference: No motor system involvement
What distinguishes 5-HT from the other amine transmitters as a mediator of anxiety is the lack of involvement of the motor system. Multiple studies in rats illustrate that exogenously augmenting 5-HT has no effect on levels of locomotor activity. Dopamine depletion is well-characterized in the motor dysfunction of Parkinson’s disease, and DA excess can cause repetitive, stereotyped movements, such as seen in tardive dyskinesia or Huntington’s disease.8 In humans, serotonin-mediated anxiety is usually without a motoric component; patients appear calm but complain of extreme anxiety or agitation. Agitation has been reported after initiation of an SSRI,29 and is more likely to occur in patients with the short form of the 5-HT transporter.30 Motoric activation has been reported in some of these studies, but does not seem to cluster with the complaint of agitation.29 The reduced number of available transporters means a chronic steady-state elevation of serotonin, because less serotonin is being removed from the synapse after it is released. This is one of the reasons patients with the short form of the 5-HT transporter may be more susceptible to serotonin-mediated anxiety.
What you need to keep in mind
Pharmacologic treatment of anxiety begins with an SSRI, a serotonin-norepinephrine reuptake inhibitor (SNRI), or buspirone. Second-line treatments include hydroxyzine, gabapentin, pregabalin, and quetiapine.3,31 However, clinicians need to be aware that a fraction of their patients will report anxiety that will not have any external manifestations, but will be experienced as an unpleasant internal energy. These patients may report an increase in their anxiety levels when started on an SSRI or SNRI.29,30 This anxiety is most likely mediated by increases of synaptic 5-HT. This occurs because many serotonergic receptors may have a biphasic response, so that too much stimulation is experienced as excessive internal energy.16-18 In such patients, blockade of key 5-HT receptors may reduce that internal agitation. The advantage of recognizing serotonin-mediated anxiety is that one can specifically tailor treatment to address the patient’s specific physiology.
It is important to note that the anxiolytic effect of asenapine is specific to patients with serotonin-mediated anxiety. Unlike quetiapine, which is effective as augmentation therapy in generalized anxiety disorder,31 asenapine does not appear to reduce anxiety in patients with schizophrenia32 or borderline personality disorder33 when administered for other reasons. However, it may reduce anxiety in patients with the short form of the 5-HT transporter.30,34
Bottom Line
Serotonin-mediated anxiety occurs when levels of synaptic serotonin (5-HT) are high. Patients with serotonin-mediated anxiety appear calm but will report experiencing an unpleasant internal energy. Interventions that block multiple postsynaptic 5-HT receptors or discontinuation of a selective serotonin reuptake inhibitor (if applicable) will alleviate the anxiety.
Related Resource
• Bhatt NV. Anxiety disorders. https://emedicine.medscape. com/article/286227-overview
Drug Brand Names
Asenapine • Saphris, Secuado
Gabapentin • Neurontin
Hydroxyzine • Vistaril
Pregabalin • Lyrica
Quetiapine • Seroquel
Trazodone • Oleptro
Sara R. Abell, MD, and Rif S. El-Mallakh, MD
Individuals with anxiety will experience frequent or chronic excessive worry, nervousness, a sense of unease, a feeling of being unfocused, and distress, which result in functional impairment.1 Frequently, anxiety is accompanied by restlessness or muscle tension. Generalized anxiety disorder is one of the most common psychiatric diagnoses in the United States and has a prevalence of 2% to 6% globally.2 Although research has been conducted regarding anxiety’s pathogenesis, to date a firm consensus on its etiology has not been reached.3 It is likely multifactorial, with environmental and biologic components.
One area of focus has been neurotransmitters and the possible role they play in the pathogenesis of anxiety. Specifically, the monoamine neurotransmitters have been implicated in the clinical manifestations of anxiety. Among the amines, normal roles include stimulating the autonomic nervous system and regulating numerous cognitive phenomena, such as volition and emotion. Many psychiatric medications modify aminergic transmission, and many current anxiety medications target amine neurotransmitters. Medications that target histamine, serotonin, norepinephrine, and dopamine all play a role in treating anxiety.
In this article, we focus on serotonin (5-hydroxytryptamine, 5-HT) as a mediator of anxiety and on excessive synaptic 5-HT as the cause of anxiety. We discuss how 5-HT–mediated anxiety can be identified and offer some solutions for its treatment.
The amine neurotransmitters
There are 6 amine neurotransmitters in the CNS. These are derived from tyrosine (dopamine [DA], norepinephrine [NE], and epinephrine), histidine (histamine), and tryptophan (serotonin [5-HT] and melatonin). In addition to their physiologic actions, amines have been implicated in both acute and chronic anxiety. Excessive DA stimulation has been linked with fear4,5; NE elevations are central to hypervigilance and hyperarousal of posttraumatic stress disorder6; and histamine may mediate emotional memories involved in fear and anxiety.7 Understanding the normal function of 5-HT will aid in understanding its potential problematic role (Box,8-18page 38).
How serotonin-mediated anxiety presents
“Anxiety” is a collection of signs and symptoms that likely represent multiple processes and have the common characteristic of being subjectively unpleasant, with a subjective wish for the feeling to end. The expression of anxiety disorders is quite diverse and ranges from brief episodes such as panic attacks (which may be mediated, in part, by epinephrine/NE19) to lifelong stereotypic obsessions and compulsions (which may be mediated, in part, by DA and modified by 5-HT20,21). Biochemical separation of the anxiety disorders is key to achieving tailored treatment.6 Towards this end, it is important to investigate the phenomenon of serotonin-mediated anxiety.
Because clinicians are familiar with reductions of anxiety as selective serotonin reuptake inhibitors (SSRIs) increase 5-HT levels in the synapse, it is difficult to conceptualize serotonin-mediated anxiety. However, many of the effects at postsynaptic 5-HT receptors may be biphasic.15-18 Serotonin-mediated anxiety appears to occur when levels of 5-HT (or stimulation of 5-HT receptors) are particularly high. This is most frequently seen in patients who genetically have high synaptic 5-HT (by virtue of the short form of the 5-HT transporter),22 whose synaptic 5-HT is further increased by treatment with an SSRI,23 and who are experiencing a stressor that yet further increases their synaptic 5-HT.24 However, it may occur in some individuals with only 2 of these 3 conditions.Clinically, individuals with serotonin-mediated anxiety will usually appear calm. The anxiety they are experiencing is not exhibited in any way in the motor system (ie, they do not appear restless, do not pace, muscle tone is not increased, etc.). However, they will generally complain of an internal agitation, a sense of a negative internal energy. Frequently, they will use descriptions such as “I feel I could jump out of my skin.” As previously mentioned, this is usually in the setting of some environmental stress, in addition to either a pharmacologic (SSRI) or genetic (short form of the 5-HT transporter) reason for increasing synaptic 5-HT, or both.
Almost always, interventions that block multiple postsynaptic 5-HT receptors or discontinuation of the SSRI (if applicable) will alleviate the anxiety, quickly or more slowly, respectively. Sublingual asenapine, which at low doses can block 5-HT2C (Ki = 0.03 nM), 5-HT2A (Ki = 0.07 nM), 5-HT7 (Ki = 0.11 nM), 5-HT2B (Ki = 0.18 nM), and 5-HT6 (Ki = 0.25 nM),25,26 and which will produce peak plasma levels within 10 minutes,27 usually is quite effective.
Box
Serotonin (5-HT) arises from neurons in the raphe nuclei of the rostral pons and projects superiorly to the cerebral cortex and inferiorly to the spinal cord.8 It works in an inhibitory or excitatory manner depending on which receptors are activated. In the periphery, 5-HT influences intestinal peristalsis, sensory modulation, gland function, thermoregulation, blood pressure, platelet aggregation, and sexual behavior,9 all actions that produce potential adverse effects of serotonin reuptake– inhibiting antidepressants. In the CNS, 5-HT plays a role in attention bias; decision-making; sleep and wakefulness; and mood regulation. In short, serotonin can be viewed as mediating emotional motivation.10
Serotonin alters neuroplasticity. During development, 5-HT stimulates creation of new synapses and increases the density of synaptic webs. It has a direct stimulatory effect on the length of dendrites, their branching, and their myelination.11 In the CNS, it plays a role in dendritic arborization. Animal studies with rats have shown that lesioning highly concentrated 5-HT areas at early ages resulted in an adult brain with a lower number of neurons and a less complex web of dendrites.12,13 In situations of emotional stress, it is theorized that low levels of 5-HT lead to a reduced ability to deal with emotional stressors due to lower levels of complexity in synaptic connections.
Serotonin has also been implicated in mediating some aspects of dopamine-related actions, such as locomotion, reward, and threat avoidance. This is believed to contribute to the beneficial effect of 5-HT2A blockade by secondgeneration antipsychotics (SGAs).14 Blockade of other 5-HT receptors, such as 5-HT1A, 5-HT2C, 5-HT6, and 5-HT7, may also contribute to the antipsychotic action of SGAs.14
Serotonin receptors are found throughout the body, and 14 subtypes have been identified.9 Excitatory and inhibitory action of 5-HT depends on the receptor, and the actions of 5-HT can differ with the same receptor at different concentrations. This is because serotonin’s effects are biphasic and concentration-dependent, meaning that levels of 5-HT in the synapse will dictate the downstream effect of receptor agonism or antagonism. Animal models have shown that low-dose agonism of 5-HT receptors causes vasoconstriction of the coronary arteries, and high doses cause relaxation. This response has also been demonstrated in the vasculature of the kidneys and the smooth muscle of the trachea. Additionally, 5-HT works in conjunction with histamine to produce a biphasic response in the colonic arteries and veins in situations of endothelial damage.15
Most relevant to this discussion are 5-HT’s actions in mood regulation and behavior. Low 5-HT states result in less behavioral inhibition, leading to higher impulse control failures and aggression. Experiments in mice with deficient serotonergic brain regions show hypoactivity, extended daytime sleep, anxiety, and depressive behaviors.13 Serotonin’s behavioral effects are also biphasic. For example, lowdose antagonism with trazodone of 5-HT receptors demonstrated a pro-aggressive behavioral effect, while high-dose antagonism is anti-aggressive.15 Similar biphasic effects may result in either induction or reduction of anxiety with agents that block or excite certain 5-HT receptors.16-18
Continue to: A key difference: No motor system involvement...
A key difference: No motor system involvement
What distinguishes 5-HT from the other amine transmitters as a mediator of anxiety is the lack of involvement of the motor system. Multiple studies in rats illustrate that exogenously augmenting 5-HT has no effect on levels of locomotor activity. Dopamine depletion is well-characterized in the motor dysfunction of Parkinson’s disease, and DA excess can cause repetitive, stereotyped movements, such as seen in tardive dyskinesia or Huntington’s disease.8 In humans, serotonin-mediated anxiety is usually without a motoric component; patients appear calm but complain of extreme anxiety or agitation. Agitation has been reported after initiation of an SSRI,29 and is more likely to occur in patients with the short form of the 5-HT transporter.30 Motoric activation has been reported in some of these studies, but does not seem to cluster with the complaint of agitation.29 The reduced number of available transporters means a chronic steady-state elevation of serotonin, because less serotonin is being removed from the synapse after it is released. This is one of the reasons patients with the short form of the 5-HT transporter may be more susceptible to serotonin-mediated anxiety.
What you need to keep in mind
Pharmacologic treatment of anxiety begins with an SSRI, a serotonin-norepinephrine reuptake inhibitor (SNRI), or buspirone. Second-line treatments include hydroxyzine, gabapentin, pregabalin, and quetiapine.3,31 However, clinicians need to be aware that a fraction of their patients will report anxiety that will not have any external manifestations, but will be experienced as an unpleasant internal energy. These patients may report an increase in their anxiety levels when started on an SSRI or SNRI.29,30 This anxiety is most likely mediated by increases of synaptic 5-HT. This occurs because many serotonergic receptors may have a biphasic response, so that too much stimulation is experienced as excessive internal energy.16-18 In such patients, blockade of key 5-HT receptors may reduce that internal agitation. The advantage of recognizing serotonin-mediated anxiety is that one can specifically tailor treatment to address the patient’s specific physiology.
It is important to note that the anxiolytic effect of asenapine is specific to patients with serotonin-mediated anxiety. Unlike quetiapine, which is effective as augmentation therapy in generalized anxiety disorder,31 asenapine does not appear to reduce anxiety in patients with schizophrenia32 or borderline personality disorder33 when administered for other reasons. However, it may reduce anxiety in patients with the short form of the 5-HT transporter.30,34
Bottom Line
Serotonin-mediated anxiety occurs when levels of synaptic serotonin (5-HT) are high. Patients with serotonin-mediated anxiety appear calm but will report experiencing an unpleasant internal energy. Interventions that block multiple postsynaptic 5-HT receptors or discontinuation of a selective serotonin reuptake inhibitor (if applicable) will alleviate the anxiety.
Related Resource
• Bhatt NV. Anxiety disorders. https://emedicine.medscape. com/article/286227-overview
Drug Brand Names
Asenapine • Saphris, Secuado
Gabapentin • Neurontin
Hydroxyzine • Vistaril
Pregabalin • Lyrica
Quetiapine • Seroquel
Trazodone • Oleptro
1. Shelton CI. Diagnosis and management of anxiety disorders. J Am Osteopath Assoc. 2004;104(3 Suppl 3):S2-S5.
2. Ruscio AM, Hallion LS, Lim CCW, et al. Cross-sectional comparison of the epidemiology of DSM-5 generalized anxiety disorder across the globe. JAMA Psychiatry. 2017;74(5):465-475.
3. Locke AB, Kirst N, Shultz CG. Diagnosis and management of generalized anxiety disorder and panic disorder in adults. Am Fam Physician. 2015;91(9):617-624.
4. Hariri AR, Mattay VS, Tessitore A, et al. Dextroamphetamine modulates the response of the human amygdala. Neuropsychopharmacology. 2002;27(6):1036-1040.
5. Colombo AC, de Oliveira AR, Reimer AE, et al. Dopaminergic mechanisms underlying catalepsy, fear and anxiety: do they interact? Behav Brain Res. 2013;257:201-207.
6. Togay B, El-Mallakh RS. Posttraumatic stress disorder: from pathophysiology to pharmacology. Curr Psychiatry. 2020;19(5):33-39.
7. Provensi G, Passani MB, Costa A, et al. Neuronal histamine and the memory of emotionally salient events. Br J Pharmacol. 2020;177(3):557-569.
8. Purves D, Augustine GJ, Fitzpatrick D, et al (eds). Neuroscience. 2nd ed. Sinauer Associates; 2001.
9. Pytliak M, Vargová V, Mechírová V, et al. Serotonin receptors – from molecular biology to clinical applications. Physiol Res. 2011;60(1):15-25.
10. Meneses A, Liy-Salmeron G. Serotonin and emotion, learning and memory. Rev Neurosci. 2012;23(5-6):543-553.
11. Whitaker-Azmitia PM. Serotonin and brain development: role in human developmental diseases. Brain Res Bull. 2001;56(5):479-485.
12. Towle AC, Breese GR, Mueller RA, et al. Early postnatal administration of 5,7-DHT: effects on serotonergic neurons and terminals. Brain Res. 1984;310(1):67-75.
13. Rok-Bujko P, Krzs´cik P, Szyndler J, et al. The influence of neonatal serotonin depletion on emotional and exploratory behaviours in rats. Behav Brain Res. 2012;226(1):87-95.
14. Meltzer HY. The role of serotonin in antipsychotic drug action. Neuropsychopharmacology. 1999;21(2 Suppl):106S-115S.
15. Calabrese EJ. 5-Hydroxytryptamine (serotonin): biphasic dose responses. Crit Rev Toxicol. 2001;31(4-5):553-561.
16. Zuardi AW. 5-HT-related drugs and human experimental anxiety. Neurosci Biobehav Rev. 1990;14(4):507-510.
17. Sánchez C, Meier E. Behavioral profiles of SSRIs in animal models of depression, anxiety and aggression. Are they all alike? Psychopharmacology (Berl). 1997;129(3):197-205.
18. Koek W, Mitchell NC, Daws LC. Biphasic effects of selective serotonin reuptake inhibitors on anxiety: rapid reversal of escitalopram’s anxiogenic effects in the novelty-induced hypophagia test in mice? Behav Pharmacol. 2018;29(4):365-369.
19. van Zijderveld GA, Veltman DJ, van Dyck R, et al. Epinephrine-induced panic attacks and hyperventilation. J Psychiatr Res. 1999;33(1):73-78.
20. Ho EV, Thompson SL, Katzka WR, et al. Clinically effective OCD treatment prevents 5-HT1B receptor-induced repetitive behavior and striatal activation. Psychopharmacology (Berl). 2016;233(1):57-70.
21. Stein DJ, Costa DLC, Lochner C, et al. Obsessive-compulsive disorder. Nat Rev Dis Primers. 2019;5(1):52.
22. Luddington NS, Mandadapu A, Husk M, et al. Clinical implications of genetic variation in the serotonin transporter promoter region: a review. Prim Care Companion J Clin Psychiatry. 2009;11(3):93-102.
23. Stahl SM. Mechanism of action of serotonin selective reuptake inhibitors. Serotonin receptors and pathways mediate therapeutic effects and side effects. J Affect Disord. 1998;51(3):215-235.
24. Chaouloff F, Berton O, Mormède P. Serotonin and stress. Neuropsychopharmacology. 1999;21(2 Suppl):28S-32S.
25. Siafis S, Tzachanis D, Samara M, et al. Antipsychotic drugs: From receptor-binding profiles to metabolic side effects. Curr Neuropharmacol. 2018;16(8):1210-1223.
26. Carrithers B, El-Mallakh RS. Transdermal asenapine in schizophrenia: a systematic review. Patient Prefer Adherence. 2020;14:1541-1551.
27. Citrome L. Asenapine review, part I: chemistry, receptor affinity profile, pharmacokinetics and metabolism. Expert Opin Drug Metab Toxicol. 2014;10(6):893-903.
28. Pratts M, Citrome L, Grant W, et al. A single-dose, randomized, double-blind, placebo-controlled trial of sublingual asenapine for acute agitation. Acta Psychiatr Scand. 2014;130(1):61-68.
29. Biswas AB, Bhaumik S, Branford D. Treatment-emergent behavioural side effects with selective serotonin re-uptake inhibitors in adults with learning disabilities. Hum Psychopharmacol. 2001;16(2):133-137.
30. Perlis RH, Mischoulon D, Smoller JW, et al. Serotonin transporter polymorphisms and adverse effects with fluoxetine treatment. Biol Psychiatry. 2003;54(9):879-883.
31. Ipser JC, Carey P, Dhansay Y, et al. Pharmacotherapy augmentation strategies in treatment-resistant anxiety disorders. Cochrane Database Syst Rev. 2006;(4):CD005473.
32. Kane JM, Mackle M, Snow-Adami L, et al. A randomized placebo-controlled trial of asenapine for the prevention of relapse of schizophrenia after long-term treatment. J Clin Psychiatry. 2011;72(3):349-355.
33. Bozzatello P, Rocca P, Uscinska M, et al. Efficacy and tolerability of asenapine compared with olanzapine in borderline personality disorder: an open-label randomized controlled trial. CNS Drugs. 2017;31(9):809-819.
34. El-Mallakh RS, Nuss S, Gao D, et al. Asenapine in the treatment of bipolar depression. Psychopharmacol Bull. 2020;50(1):8-18.
1. Shelton CI. Diagnosis and management of anxiety disorders. J Am Osteopath Assoc. 2004;104(3 Suppl 3):S2-S5.
2. Ruscio AM, Hallion LS, Lim CCW, et al. Cross-sectional comparison of the epidemiology of DSM-5 generalized anxiety disorder across the globe. JAMA Psychiatry. 2017;74(5):465-475.
3. Locke AB, Kirst N, Shultz CG. Diagnosis and management of generalized anxiety disorder and panic disorder in adults. Am Fam Physician. 2015;91(9):617-624.
4. Hariri AR, Mattay VS, Tessitore A, et al. Dextroamphetamine modulates the response of the human amygdala. Neuropsychopharmacology. 2002;27(6):1036-1040.
5. Colombo AC, de Oliveira AR, Reimer AE, et al. Dopaminergic mechanisms underlying catalepsy, fear and anxiety: do they interact? Behav Brain Res. 2013;257:201-207.
6. Togay B, El-Mallakh RS. Posttraumatic stress disorder: from pathophysiology to pharmacology. Curr Psychiatry. 2020;19(5):33-39.
7. Provensi G, Passani MB, Costa A, et al. Neuronal histamine and the memory of emotionally salient events. Br J Pharmacol. 2020;177(3):557-569.
8. Purves D, Augustine GJ, Fitzpatrick D, et al (eds). Neuroscience. 2nd ed. Sinauer Associates; 2001.
9. Pytliak M, Vargová V, Mechírová V, et al. Serotonin receptors – from molecular biology to clinical applications. Physiol Res. 2011;60(1):15-25.
10. Meneses A, Liy-Salmeron G. Serotonin and emotion, learning and memory. Rev Neurosci. 2012;23(5-6):543-553.
11. Whitaker-Azmitia PM. Serotonin and brain development: role in human developmental diseases. Brain Res Bull. 2001;56(5):479-485.
12. Towle AC, Breese GR, Mueller RA, et al. Early postnatal administration of 5,7-DHT: effects on serotonergic neurons and terminals. Brain Res. 1984;310(1):67-75.
13. Rok-Bujko P, Krzs´cik P, Szyndler J, et al. The influence of neonatal serotonin depletion on emotional and exploratory behaviours in rats. Behav Brain Res. 2012;226(1):87-95.
14. Meltzer HY. The role of serotonin in antipsychotic drug action. Neuropsychopharmacology. 1999;21(2 Suppl):106S-115S.
15. Calabrese EJ. 5-Hydroxytryptamine (serotonin): biphasic dose responses. Crit Rev Toxicol. 2001;31(4-5):553-561.
16. Zuardi AW. 5-HT-related drugs and human experimental anxiety. Neurosci Biobehav Rev. 1990;14(4):507-510.
17. Sánchez C, Meier E. Behavioral profiles of SSRIs in animal models of depression, anxiety and aggression. Are they all alike? Psychopharmacology (Berl). 1997;129(3):197-205.
18. Koek W, Mitchell NC, Daws LC. Biphasic effects of selective serotonin reuptake inhibitors on anxiety: rapid reversal of escitalopram’s anxiogenic effects in the novelty-induced hypophagia test in mice? Behav Pharmacol. 2018;29(4):365-369.
19. van Zijderveld GA, Veltman DJ, van Dyck R, et al. Epinephrine-induced panic attacks and hyperventilation. J Psychiatr Res. 1999;33(1):73-78.
20. Ho EV, Thompson SL, Katzka WR, et al. Clinically effective OCD treatment prevents 5-HT1B receptor-induced repetitive behavior and striatal activation. Psychopharmacology (Berl). 2016;233(1):57-70.
21. Stein DJ, Costa DLC, Lochner C, et al. Obsessive-compulsive disorder. Nat Rev Dis Primers. 2019;5(1):52.
22. Luddington NS, Mandadapu A, Husk M, et al. Clinical implications of genetic variation in the serotonin transporter promoter region: a review. Prim Care Companion J Clin Psychiatry. 2009;11(3):93-102.
23. Stahl SM. Mechanism of action of serotonin selective reuptake inhibitors. Serotonin receptors and pathways mediate therapeutic effects and side effects. J Affect Disord. 1998;51(3):215-235.
24. Chaouloff F, Berton O, Mormède P. Serotonin and stress. Neuropsychopharmacology. 1999;21(2 Suppl):28S-32S.
25. Siafis S, Tzachanis D, Samara M, et al. Antipsychotic drugs: From receptor-binding profiles to metabolic side effects. Curr Neuropharmacol. 2018;16(8):1210-1223.
26. Carrithers B, El-Mallakh RS. Transdermal asenapine in schizophrenia: a systematic review. Patient Prefer Adherence. 2020;14:1541-1551.
27. Citrome L. Asenapine review, part I: chemistry, receptor affinity profile, pharmacokinetics and metabolism. Expert Opin Drug Metab Toxicol. 2014;10(6):893-903.
28. Pratts M, Citrome L, Grant W, et al. A single-dose, randomized, double-blind, placebo-controlled trial of sublingual asenapine for acute agitation. Acta Psychiatr Scand. 2014;130(1):61-68.
29. Biswas AB, Bhaumik S, Branford D. Treatment-emergent behavioural side effects with selective serotonin re-uptake inhibitors in adults with learning disabilities. Hum Psychopharmacol. 2001;16(2):133-137.
30. Perlis RH, Mischoulon D, Smoller JW, et al. Serotonin transporter polymorphisms and adverse effects with fluoxetine treatment. Biol Psychiatry. 2003;54(9):879-883.
31. Ipser JC, Carey P, Dhansay Y, et al. Pharmacotherapy augmentation strategies in treatment-resistant anxiety disorders. Cochrane Database Syst Rev. 2006;(4):CD005473.
32. Kane JM, Mackle M, Snow-Adami L, et al. A randomized placebo-controlled trial of asenapine for the prevention of relapse of schizophrenia after long-term treatment. J Clin Psychiatry. 2011;72(3):349-355.
33. Bozzatello P, Rocca P, Uscinska M, et al. Efficacy and tolerability of asenapine compared with olanzapine in borderline personality disorder: an open-label randomized controlled trial. CNS Drugs. 2017;31(9):809-819.
34. El-Mallakh RS, Nuss S, Gao D, et al. Asenapine in the treatment of bipolar depression. Psychopharmacol Bull. 2020;50(1):8-18.