User login
Case Report
A 65-year-old man presented to the dermatology department for treatment of a scaly rash on the face and scalp. A diagnosis of seborrheic dermatitis was made, and he was prescribed ketoconazole cream 2% and shampoo 2%. Two days later, the patient presented to the emergency department for facial swelling and pruritus, which began 1 day after he began using the ketoconazole cream and shampoo. He reported itching and burning on the face that began within several hours of application followed by progressive facial edema. The patient denied shortness of breath or swelling of the tongue. Physical examination revealed mild facial induration with erythematous plaques on the bilateral cheeks, forehead, and eyelids. The patient was instructed to stop using the ketoconazole cream and shampoo. Within several days of discontinuing use of the ketoconazole products, the dermatitis resolved following treatment with oral diphenhydramine and topical desonide.
Review of the patient’s medical record revealed several likely relevant incidences of undiagnosed recurrent dermatitis. Approximately 2 years earlier, the patient had called his primary care provider to report pain, burning, redness, and itching in the right buttock area following use of ketoconazole cream that the physician had prescribed. Allergic contact dermatitis also had been documented in the patient’s dermatology problem list approximately 1.5 years prior to the current presentation, though a likely causative agent was not listed. Approximately 3 months prior to the current presentation, the patient presented with lower leg rash and edema with documentation of possible allergic reaction to ketoconazole cream.
The patient was patch tested several weeks after discontinuation of the ketoconazole products using the 2012 North American Contact Dermatitis Group series (70 allergens), a supplemental series (36 allergens), an antifungal series (10 allergens), and personal products including ketoconazole cream and shampoo (diluted 1:100). Clinically relevant reactions at 72 hours included an extreme reaction (+++) to the patient’s personal ketoconazole cream 2% (E. Fougera & Co)(Figure 1), and strong reactions (++) to purified ketoconazole 5% in petrolatum and ketoconazole cream 2% (E. Fougera & Co) in an antifungal series (Figure 2). A doubtful reaction to methyl methacrylate was not deemed clinically relevant. No reactions were noted to terbinafine cream 1%, clotrimazole cream 1%, nystatin cream, nystatin ointment, econazole nitrate cream 1%, miconazole nitrate cream 2%, tolnaftate cream 1%, or purified clotrimazole 1% in petrolatum.
 |
Figure 1. Reading at 72 hours of patient’s personal products (ketoconazole cream 2% and ketoconazole shampoo 2%). |
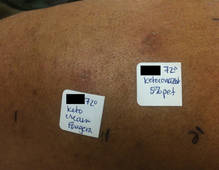 |
Figure 2. Reading at 72 hours of an antifungal series (ketoconazole cream 2% and purified ketocona-zole 5% in petrolatum). |
Comment
Ketoconazole is a widely used antifungal but rarely is reported as a cause of allergic contact dermatitis. Allergies to inactive ingredients, especially vehicles and preservatives, are more common than allergies to ketoconazole itself. In our patient, allergy to inactive ingredients was ruled out by negative reactions to individual constituents and/or negative reactions to other products containing those ingredients. A literature review via Ovid using the search terms ketoconazole, allergic contact dermatitis, and allergy found 4 reports involving 9 documented patients with type IV hypersensitivity to ketoconazole,1-4 and 1 report of 2 patients who developed anaphylaxis from oral ketoconazole.1 Of the 9 dermatitis cases, 3 patients had positive patch tests to only ketoconazole with no reactions to other imidazoles.2,3 Monoallergy to clotrimazole also has been reported.5 A study by Dooms-Goossens et al4 showed that ketoconazole ranked seventh of 11 imidazole derivatives in its frequency to cause allergic contact dermatitis and did not demonstrate statistically significant cross-reactivity with other imidazoles; cross-reactivity usually occurred with miconazole and sulconazole.
Conclusion
This case of contact dermatitis to ketoconazole demonstrates the importance of patch testing with personal products as well as the unpredictability of cross-reactions within the imidazole class of antifungals.
Acknowledgment
This material is the result of work supported with resources and the use of facilities at the Minneapolis Veterans Affairs Health Care System.
1. Garcia-Bravo B, Mazuecos J, Rodriguez-Pichardo A, et al. Hypersensitivity to ketoconazole preparations: study of 4 cases. Contact Dermatitis. 1989;21:346-348.
2. Valsecchi R, Pansera B, di Landro A, et al. Contact dermatitis from ketoconazole. Contact Dermatitis. 1993;29:162.
3. Santucci B, Cannistraci C, Cristaudo A, et al. Contact dermatitis from ketoconazole cream. Contact Dermatitis. 1992;27:274-275.
4. Dooms-Goossens A, Matura M, Drieghe J, et al. Contact allergy to imidazoles used as antimycotic agents. Contact Dermatitis. 1995;33:73-77.
5. Pullen SK, Warshaw EM. Vulvar allergic contact dermatitis from clotrimazole. Dermatitis. 2010;21:59-60.
Case Report
A 65-year-old man presented to the dermatology department for treatment of a scaly rash on the face and scalp. A diagnosis of seborrheic dermatitis was made, and he was prescribed ketoconazole cream 2% and shampoo 2%. Two days later, the patient presented to the emergency department for facial swelling and pruritus, which began 1 day after he began using the ketoconazole cream and shampoo. He reported itching and burning on the face that began within several hours of application followed by progressive facial edema. The patient denied shortness of breath or swelling of the tongue. Physical examination revealed mild facial induration with erythematous plaques on the bilateral cheeks, forehead, and eyelids. The patient was instructed to stop using the ketoconazole cream and shampoo. Within several days of discontinuing use of the ketoconazole products, the dermatitis resolved following treatment with oral diphenhydramine and topical desonide.
Review of the patient’s medical record revealed several likely relevant incidences of undiagnosed recurrent dermatitis. Approximately 2 years earlier, the patient had called his primary care provider to report pain, burning, redness, and itching in the right buttock area following use of ketoconazole cream that the physician had prescribed. Allergic contact dermatitis also had been documented in the patient’s dermatology problem list approximately 1.5 years prior to the current presentation, though a likely causative agent was not listed. Approximately 3 months prior to the current presentation, the patient presented with lower leg rash and edema with documentation of possible allergic reaction to ketoconazole cream.
The patient was patch tested several weeks after discontinuation of the ketoconazole products using the 2012 North American Contact Dermatitis Group series (70 allergens), a supplemental series (36 allergens), an antifungal series (10 allergens), and personal products including ketoconazole cream and shampoo (diluted 1:100). Clinically relevant reactions at 72 hours included an extreme reaction (+++) to the patient’s personal ketoconazole cream 2% (E. Fougera & Co)(Figure 1), and strong reactions (++) to purified ketoconazole 5% in petrolatum and ketoconazole cream 2% (E. Fougera & Co) in an antifungal series (Figure 2). A doubtful reaction to methyl methacrylate was not deemed clinically relevant. No reactions were noted to terbinafine cream 1%, clotrimazole cream 1%, nystatin cream, nystatin ointment, econazole nitrate cream 1%, miconazole nitrate cream 2%, tolnaftate cream 1%, or purified clotrimazole 1% in petrolatum.
 |
Figure 1. Reading at 72 hours of patient’s personal products (ketoconazole cream 2% and ketoconazole shampoo 2%). |
 |
Figure 2. Reading at 72 hours of an antifungal series (ketoconazole cream 2% and purified ketocona-zole 5% in petrolatum). |
Comment
Ketoconazole is a widely used antifungal but rarely is reported as a cause of allergic contact dermatitis. Allergies to inactive ingredients, especially vehicles and preservatives, are more common than allergies to ketoconazole itself. In our patient, allergy to inactive ingredients was ruled out by negative reactions to individual constituents and/or negative reactions to other products containing those ingredients. A literature review via Ovid using the search terms ketoconazole, allergic contact dermatitis, and allergy found 4 reports involving 9 documented patients with type IV hypersensitivity to ketoconazole,1-4 and 1 report of 2 patients who developed anaphylaxis from oral ketoconazole.1 Of the 9 dermatitis cases, 3 patients had positive patch tests to only ketoconazole with no reactions to other imidazoles.2,3 Monoallergy to clotrimazole also has been reported.5 A study by Dooms-Goossens et al4 showed that ketoconazole ranked seventh of 11 imidazole derivatives in its frequency to cause allergic contact dermatitis and did not demonstrate statistically significant cross-reactivity with other imidazoles; cross-reactivity usually occurred with miconazole and sulconazole.
Conclusion
This case of contact dermatitis to ketoconazole demonstrates the importance of patch testing with personal products as well as the unpredictability of cross-reactions within the imidazole class of antifungals.
Acknowledgment
This material is the result of work supported with resources and the use of facilities at the Minneapolis Veterans Affairs Health Care System.
Case Report
A 65-year-old man presented to the dermatology department for treatment of a scaly rash on the face and scalp. A diagnosis of seborrheic dermatitis was made, and he was prescribed ketoconazole cream 2% and shampoo 2%. Two days later, the patient presented to the emergency department for facial swelling and pruritus, which began 1 day after he began using the ketoconazole cream and shampoo. He reported itching and burning on the face that began within several hours of application followed by progressive facial edema. The patient denied shortness of breath or swelling of the tongue. Physical examination revealed mild facial induration with erythematous plaques on the bilateral cheeks, forehead, and eyelids. The patient was instructed to stop using the ketoconazole cream and shampoo. Within several days of discontinuing use of the ketoconazole products, the dermatitis resolved following treatment with oral diphenhydramine and topical desonide.
Review of the patient’s medical record revealed several likely relevant incidences of undiagnosed recurrent dermatitis. Approximately 2 years earlier, the patient had called his primary care provider to report pain, burning, redness, and itching in the right buttock area following use of ketoconazole cream that the physician had prescribed. Allergic contact dermatitis also had been documented in the patient’s dermatology problem list approximately 1.5 years prior to the current presentation, though a likely causative agent was not listed. Approximately 3 months prior to the current presentation, the patient presented with lower leg rash and edema with documentation of possible allergic reaction to ketoconazole cream.
The patient was patch tested several weeks after discontinuation of the ketoconazole products using the 2012 North American Contact Dermatitis Group series (70 allergens), a supplemental series (36 allergens), an antifungal series (10 allergens), and personal products including ketoconazole cream and shampoo (diluted 1:100). Clinically relevant reactions at 72 hours included an extreme reaction (+++) to the patient’s personal ketoconazole cream 2% (E. Fougera & Co)(Figure 1), and strong reactions (++) to purified ketoconazole 5% in petrolatum and ketoconazole cream 2% (E. Fougera & Co) in an antifungal series (Figure 2). A doubtful reaction to methyl methacrylate was not deemed clinically relevant. No reactions were noted to terbinafine cream 1%, clotrimazole cream 1%, nystatin cream, nystatin ointment, econazole nitrate cream 1%, miconazole nitrate cream 2%, tolnaftate cream 1%, or purified clotrimazole 1% in petrolatum.
 |
Figure 1. Reading at 72 hours of patient’s personal products (ketoconazole cream 2% and ketoconazole shampoo 2%). |
 |
Figure 2. Reading at 72 hours of an antifungal series (ketoconazole cream 2% and purified ketocona-zole 5% in petrolatum). |
Comment
Ketoconazole is a widely used antifungal but rarely is reported as a cause of allergic contact dermatitis. Allergies to inactive ingredients, especially vehicles and preservatives, are more common than allergies to ketoconazole itself. In our patient, allergy to inactive ingredients was ruled out by negative reactions to individual constituents and/or negative reactions to other products containing those ingredients. A literature review via Ovid using the search terms ketoconazole, allergic contact dermatitis, and allergy found 4 reports involving 9 documented patients with type IV hypersensitivity to ketoconazole,1-4 and 1 report of 2 patients who developed anaphylaxis from oral ketoconazole.1 Of the 9 dermatitis cases, 3 patients had positive patch tests to only ketoconazole with no reactions to other imidazoles.2,3 Monoallergy to clotrimazole also has been reported.5 A study by Dooms-Goossens et al4 showed that ketoconazole ranked seventh of 11 imidazole derivatives in its frequency to cause allergic contact dermatitis and did not demonstrate statistically significant cross-reactivity with other imidazoles; cross-reactivity usually occurred with miconazole and sulconazole.
Conclusion
This case of contact dermatitis to ketoconazole demonstrates the importance of patch testing with personal products as well as the unpredictability of cross-reactions within the imidazole class of antifungals.
Acknowledgment
This material is the result of work supported with resources and the use of facilities at the Minneapolis Veterans Affairs Health Care System.
1. Garcia-Bravo B, Mazuecos J, Rodriguez-Pichardo A, et al. Hypersensitivity to ketoconazole preparations: study of 4 cases. Contact Dermatitis. 1989;21:346-348.
2. Valsecchi R, Pansera B, di Landro A, et al. Contact dermatitis from ketoconazole. Contact Dermatitis. 1993;29:162.
3. Santucci B, Cannistraci C, Cristaudo A, et al. Contact dermatitis from ketoconazole cream. Contact Dermatitis. 1992;27:274-275.
4. Dooms-Goossens A, Matura M, Drieghe J, et al. Contact allergy to imidazoles used as antimycotic agents. Contact Dermatitis. 1995;33:73-77.
5. Pullen SK, Warshaw EM. Vulvar allergic contact dermatitis from clotrimazole. Dermatitis. 2010;21:59-60.
1. Garcia-Bravo B, Mazuecos J, Rodriguez-Pichardo A, et al. Hypersensitivity to ketoconazole preparations: study of 4 cases. Contact Dermatitis. 1989;21:346-348.
2. Valsecchi R, Pansera B, di Landro A, et al. Contact dermatitis from ketoconazole. Contact Dermatitis. 1993;29:162.
3. Santucci B, Cannistraci C, Cristaudo A, et al. Contact dermatitis from ketoconazole cream. Contact Dermatitis. 1992;27:274-275.
4. Dooms-Goossens A, Matura M, Drieghe J, et al. Contact allergy to imidazoles used as antimycotic agents. Contact Dermatitis. 1995;33:73-77.
5. Pullen SK, Warshaw EM. Vulvar allergic contact dermatitis from clotrimazole. Dermatitis. 2010;21:59-60.
- Contact allergy to topical ketoconazole is rare and its cross-reactivity with other imidazole antifungals is unpredictable.
- Patch testing to personal products often is important for detecting rare allergies.