User login
› Avoid preemptive warfarin dose reductions unless you are prescribing trimethoprim/sulfamethoxazole (TMP/SMX) or metronidazole. B
› Recommend a back-up contraceptive method to a woman who is taking a broad-spectrum antibiotic and low-dose OCs—especially if the woman is overweight. C
› Consider using the macrolide, clarithromycin, or the fluoroquinolone, ciprofloxacin, in patients taking medications that prolong QT interval or who are at higher risk for torsades de pointes (TdP). B
› Refrain from cautioning patients taking metronidazole against consuming alcohol. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Despite encouraging data that antibiotic prescribing is on the decline, patients are still prescribed antibiotics frequently, making these agents the 12th most frequently used drug class.1 At the same time, prescribers are caring for patients with increasingly complex drug regimens that provide fertile ground for drug interactions with these antibiotics. And, of course, lifestyle factors such as alcohol consumption are a consideration when any prescription is written.
As pharmacists, we find that certain questions about antibiotic prescribing and interactions come up with frequency. These questions often pertain to the use of warfarin, oral contraceptives, drugs that prolong the QT interval, and alcohol. But conflicting reports about issues such as monitoring international normalized ratio (INR) in patients taking warfarin and antibiotics, and whether (or which) antibiotics decrease the efficacy of oral contraceptives (OCs) can make decision-making challenging.
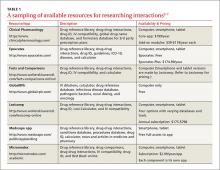
This review provides evidence-based answers to questions you may have. It also details some reliable sources of information you can consult (TABLE 12-7) when discussing treatment options with other members of the health care team.
1. Which antibiotics are preferable when a patient is taking warfarin, and are preemptive warfarin dose reductions advisable?
The simple answer is that agents with a lower likelihood of affecting the INR, such as penicillin G, clindamycin, and 1st- and 4th-generation cephalosporins, are a good place to start, and whether to preemptively reduce the warfarin dose hinges on the antibiotic being prescribed.
The more detailed answer. The fundamental mechanisms of interaction between warfarin and antibiotics are two-fold:8
- Antimicrobial agents disrupt gastrointestinal flora that synthesize vitamin K.
- Antimicrobials inhibit cytochrome p450 (CYP450) enzymes (primarily CYP2C9 and 3A4), which are responsible for the metabolism of warfarin.
The antibiotics most likely to interfere with warfarin are TMP/SMX, ciprofloxacin, levofloxacin, metronidazole, fluconazole, azithromycin, and clarithromycin (TABLE 2).9,10 Low-risk agents include clindamycin, cephalexin, and penicillin G. When prescribing an antibiotic for a patient taking warfarin, it is important not only to be aware of the agents that should be avoided, but also the agents that do not require more frequent monitoring of INR.
Preemptive warfarin dose reductions? Some physicians make preemptive warfarin dose reductions in an attempt to avoid supratherapeutic INRs in patients being prescribed antibiotics. But the evidence suggests that this step should be considered only in the presence of the antibiotics TMP/SMX and metronidazole.9,11
A 2008 study investigated the anticoagulation effects of a 10% to 20% preemptive warfarin dose reduction vs no dosing change in patients taking TMP/SMX or levofloxacin. The investigators found that the preemptive warfarin dose reduction (intervention) significantly decreased the number of supratherapeutic INR values above 4 when compared to controls (2 of 8 vs 8 of 9).12
In the dose-reduction group, no patients receiving TMP/SMX developed a subtherapeutic INR, whereas 40% (4 of 10 patients) who received levofloxacin developed a subtherapeutic INR.12 The authors of the study concluded that a prophylactic warfarin dose reduction of 10% to 20% is effective in maintaining therapeutic anticoagulation in patients receiving TMP/SMX. They added that while no change in warfarin dosing is necessary with levofloxacin, short-term INR follow-up is a prudent approach to prevent subtherapeutic INRs. Others recommend INR monitoring when antibiotic therapy is started and stopped and whenever the dose is changed.9
A 2010 retrospective, single-center, cohort study looked at patients who were taking metronidazole and warfarin. Researchers compared those who received a preemptive dose reduction of warfarin (mean reduction was 34.6% ± 13.4%) to those who did not and found a statistically significant mean difference in INR of 1.28 (P=.01).13
Almost half (46%) of the patients who did not receive a warfarin dose reduction had an INR >4, whereas none of the patients in the warfarin dose reduction group did (P=.05). Although this secondary outcome was not statistically significant (most likely due to the small sample population [N=20]), the implication is clinically significant. Two patients who reduced their dose had a subtherapeutic INR compared to none of the patients in the control group, which was also not a statistically significant difference.
The authors concluded that a 30% to 35% reduction in mean daily warfarin dose is effective in maintaining therapeutic anticoagulation in patients started on metronidazole.
Significant bleeding events. A retrospective cohort study of slightly more than 22,000 veterans who were prescribed warfarin for ≥30 uninterrupted days and given antibiotics with either a high or low risk for interaction with warfarin were studied for significant bleeding events for one month.10 Ninety-three significant bleeding events occurred in the high-risk group and 36 occurred in the low-risk group over the course of the study. The agent associated with the greatest increased risk of bleeding was TMP/SMX (hazard ratio [HR]=2.09; 95% CI, 1.45-3.02). Of note, metronidazole was not included in this study endpoint.
The study’s secondary endpoint of INR >4 found that 10% of patients taking metronidazole and 8% of patients taking TMP/SMX in addition to warfarin had INRs >4. Almost 10% (9.7%) of patients prescribed fluconazole had a peak INR value >6. Patients taking low-risk antibiotics (clindamycin or cephalexin) had no increased risk of bleeding. Monitoring INR within 3 to 14 days of starting patients on antibiotics was found to decrease the risk of serious bleeding events (HR=0.61; 95% CI, 0.42-0.88). More frequent INR monitoring by itself (without preemptive warfarin dose reductions) is appropriate for other antibiotics, including macrolides, tetracyclines, and some cephalosporins (2nd and 3rd generation).9
THE BOTTOM LINE When prescribing antibiotics for patients taking warfarin, try to choose agents with a lower likelihood of affecting INR such as penicillin G, clindamycin, and 1st- and 4th-generation cephalosporins. With these agents, there is no need for more frequent INR testing or preemptive reductions in warfarin dose. In patients for whom the use of TMP/SMX or metronidazole can’t be avoided, consider reducing the patient’s warfarin dose by 10% to 35% and rechecking the INR 5 days after starting the antibiotic.9,11,12 When prescribing agents such as fluoroquinolones, macrolides, and tetracyclines, do not reduce the patient’s warfarin dose preemptively and recheck INR 5 days after starting therapy.
2. Do antibiotics decrease the efficacy of oral contraceptives?
It’s unlikely, but antibiotics may reduce the efficacy of OCs.
There have been few, but well documented, reports of women using OCs who became pregnant after taking antimicrobials.14 It is recognized that rifampin, an inducer of enzymes that metabolize estrogens, decreases the efficacy of OCs.15 Ketoconazole’s interaction seems less well documented, but combining the agent with low-estrogen (low-dose) OCs warrants caution.16 What is not well understood is whether more common or broad-spectrum antibiotics also increase the risk of OC failure.
Three mechanisms have been proposed:16
- Antimicrobials affect hepatic enzyme induction, which increases metabolism of hormones.
- Broad-spectrum antibiotics reduce gut bacteria, which alters enterohepatic circulation and reduces plasma hormone concentrations.
- Antibiotics increase gastrointestinal motility, which decreases absorption (and reabsorption) of OCs.
A 2007 study found that when physicians and pharmacists were surveyed and asked if broad-spectrum antibiotics have a clinically significant interaction with OCs, 83% of physicians and 89% of pharmacists answered “Yes;”17 however, a large epidemiologic study performed in the United States showed no association between antibiotic use and OC failure.18
After this report, investigators in the Netherlands completed a similar cross-over analysis and found that there was a relationship between the use of antibiotics and breakthrough pregnancy in a population-based prescription database, but that the results didn’t hold for broad-spectrum antibiotics or in a sensitivity analysis.19 Pharmacokinetic studies are also conflicting, as some have shown an effect on serum hormone levels, while others have not.15,20-22
High- vs low-risk agents. Ciprofloxacin did not affect hormone levels in 2 studies.20,21 Rifampin and voriconazole may enhance systemic exposure to OCs.15,22 And erythromycin and azithromycin may interact with OCs, but the clinical significance of this interaction is still unknown.16
Short-courses of TMP/SMX are generally thought to be safe;16 a small study looked at cotrimoxazole 1 g twice daily in 9 women taking long-term OC steroids and found that short courses of the drug were unlikely to cause any adverse effects on contraceptive control.23 Tetracyclines and penicillins were the antibiotics most frequently involved in case reports of pregnancy from the United Kingdom (TABLE 32).16
It is hypothesized that some women may have a higher risk of OC failure than others due to how they metabolize ethinyl estradiol.24 Another hypothesis is that some women have gut flora that is more susceptible to the antibiotic being used. And still another possibility is that lower doses of hormones are being used in OCs than were studied for this interaction.15 Anything that decreases the concentration of these lower-dose OCs is concerning, especially in patients with a higher body mass index (BMI). The few pharmacokinetic studies that have been conducted show that it takes longer for OCs to reach a steady state in obese women and that they have a lower area under the curve (AUC) and maximum estrogen concentration than women with a normal BMI.25
THE BOTTOM LINE Because the degree of variability between patients is unknown and obesity rates are increasing, concern that low-dose OCs may lose efficacy when combined with antibiotics is warranted. While the absolute risk of breakthrough pregnancy seems small, the most conservative approach is to advise patients to use a back-up method of contraception during times of antibiotic use.
3. Which drugs prolong QT intervals?
Macrolides and fluoroquinolones are 2 classes of antibiotics associated with prolonged QT intervals, but other drugs and risk factors are important to consider, as well.
Physicians often receive phone calls from pharmacists warning about drug-drug interactions when they prescribe macrolides or fluoroquinolones for patients already taking medications known to prolong QT intervals or inhibit cytochrome P450 enzymes. Long QT syndrome increases the risk of TdP, a life-threatening arrhythmia. While TdP is rare, its severity warrants a discussion of risk factors and the likelihood of occurrence.
Two QT interval prolonging medications used together in healthy individuals does not warrant a change in therapy. TdP is most likely to occur when 2 or more QT interval prolonging medications are used in a patient who is already at high risk for arrhythmia because of risk factors such as prolonged QT interval at baseline, family history of prolonged QT intervals, female gender, age >60 years, electrolyte abnormalities (hypokalemia, hypomagnesemia, hypocalcemia), underlying comorbid diseases (eg, chronic heart failure, left ventricular hypertrophy, atrial fibrillation), hypertension, bradycardia, and genetic (ion channel) polymorphisms.26,27
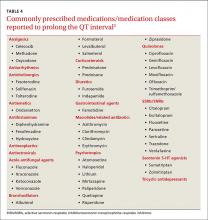
Antiarrhythmics and antipsychotics are most commonly associated with drug-induced prolonged QT interval, with most case reports and research being linked to antiarrhythmics (TABLE 42).28 But macrolide and fluoroquinolone antibiotics also have been associated with TdP, although to a lesser extent. In a retrospective analysis of case reports of TdP involving macrolides, erythromycin was present (with or without other medications thought to prolong QT) in 53% of the cases and clarithromycin was involved in 36% of the reports.29
An analysis of 2 studies by the US Food and Drug Administration estimated an occurrence rate of serious cardiac arrhythmias of 46 to 85 per 100,000 users with cardiovascular disease, compared to 5 to 44 per 100,000 users without cardiovascular disease.30 And this may underestimate the actual incidence because spontaneous reporting of adverse effects declines the longer a drug is on the market. Ciprofloxacin is associated with less risk than levofloxacin and gatifloxacin (the latter of which is no longer available in the United States).26
A recent population-based study using data on over 10.6 million people from the Taiwan National Health Insurance Database examined the risk of cardiovascular death among patients using new-generation macrolides, fluoroquinolones, and β-lactam/β-lactamase inhibitors.31 The absolute risk of cardiovascular death per 1000 individuals was 0.06 for clarithromycin, 0.12 for ciprofloxacin, 0.13 for amoxicillin-clavulanate, 0.36 for azithromycin, 0.39 for levofloxacin, and 0.46 for moxifloxacin. The mean interval between first antibiotic use and the adverse cardiac event was <4 days. Not surprisingly, the highest risk was seen in patients with underlying cardiovascular disease.
Another population-based study, this time conducted in Hong Kong, evaluated the cardiovascular safety of clarithromycin compared to that of amoxicillin. Clarithromycin was found to increase the incidence of myocardial infarction, arrhythmia, and cardiac mortality in the short term, with the risk returning to baseline after treatment concluded.32 A binational cohort study of Danish and Swedish adults confirmed that fluoroquinolones (especially ciprofloxacin) do not increase the risk of a serious arrhythmia compared to penicillins.33
THE BOTTOM LINE For patients taking other QT interval prolonging medications or who are at a higher risk for TdP, consider using clarithromycin over erythromycin or azithromycin for a macrolide antibiotic or ciprofloxacin over levofloxacin or moxifloxacin if a fluoroquinolone is warranted. Using 2 drugs that may increase the QT interval is likely safe in the absence of certain risk factors.
4. Should patients avoid alcohol while taking metronidazole?
Probably not.
Warning patients against drinking alcohol while taking metronidazole has been a common practice for years. The mechanism for this theorized interaction was thought to be similar to the interaction between disulfiram and ethanol.34 Disulfiram inhibits hepatic aldehyde dehydrogenase (ALDH) when combined with alcohol, which leads to increased levels of acetaldehyde in the blood and symptoms of flushing, palpitations, nausea, vomiting, headache, and visual disturbances.35 However, multiple studies using rats have found that metronidazole does not inhibit ALDH or increase acetaldehyde concentrations like disulfiram does.34
A 2000 review article discussed 6 cases involving serious metronidazole-ethanol interactions. Ethanol alone was found to explain the reaction in 2 of the cases, and the remaining 4 could be linked to the use of other drugs or disease states.35 A 2002 Finnish study found no statistically significant differences in objective or subjective signs of a disulfiram-like interaction.34 When considering the symptoms associated with the interaction, it is important to remember that many of the symptoms can result from metronidazole therapy alone, regardless of whether other medications or alcohol are used.35
THE BOTTOM LINE Researchers have failed to identify a clinically significant interaction between metronidazole and alcohol. Avoiding alcohol while taking metronidazole does not appear to be necessary.
CORRESPONDENCE
Mary Onysko, PharmD, BCPS, University of Wyoming, School of Pharmacy Health Sciences Center, Room 292, 1000 E. University Avenue, Laramie, WY 82071; monysko@uwyo.edu.
1. Kantor ED, Rehm CD, Haas JS, et al. Trends in prescription drug use among adults in the United States from 1999-2012. JAMA. 2015;314:1818-1831.
2. Lexicomp Online. Clinical Drug Information. Available at: http://www.wolterskluwercdi.com/lexicomp-online/. Accessed December 8, 2015.
3. GlobalRPh: The Clinician’s Ultimate Reference. Available at: http://www.globalrph.com/. Accessed December 8, 2015.
4. Medscape Apps. Available at: http://www.medscape.com/public/applanding. Accessed December 8, 2015.
5. Micromedex Solutions. Academic Institutions. Available at: http://micromedex.com/academic. Accessed December 8, 2015.
6. Patel A. Clinical Pharmacology Mobile-A mobile web app compatible on all smart phones [review] May 31, 2010. Available at: http://www.imedicalapps.com/2010/05/clinical-pharmocology-app-review/. Accessed December 8, 2015.
7. Epocrates. Available at: http://www.epocrates.com/. Accessed December 8, 2015.
8. Baillargeon J, Holmes HM, Lin Y, et al. Concurrent use of warfarin and antibiotics and the risk of bleeding in older adults. Am J Med. 2012;125:183-189.
9. PL Detail-Document #280806. Antimicrobial drug interactions with warfarin. Pharmacist’s Letter/Prescriber’s Letter. August 2012.
10. Lane M, Zeringue A, McDonald J. Serious bleeding events due to warfarin and antibiotic co-prescription in a cohort of veterans. Am J Med. 2014;127:657-663.e2.
11. Hale SF, Lesar TS. Interaction of vitamin K antagonists and trimethoprim-sulfamethoxazole: ignore at your patient’s risk. Drug Metab Drug Interact. 2014;29:53-60.
12. Ahmed A, Stephens JC, Kaus CA, et al. Impact of preemptive warfarin dose reduction on anticoagulation after initiation of trimethoprim-sulfamethoxazole or levofloxacin. J Thromb Thrombolysis. 2008;26:44-48.
13. Holt RK, Anderson EA, Cantrell MA, et al. Preemptive dose reduction of warfarin in patients initiating metronidazole. Drug Metabol Drug Interact. 2010;25:35-39.
14. Hughes BR, Cunliffe WJ. Interactions between the oral contraceptive pill and antibiotics. Br J Dermatol. 1990;122:717-718.
15. Bolt HM. Interactions between clinically used drugs and oral contraceptives. Environ Health Perspect. 1994;102:35-38.
16. Aronson JK. Meyler’s Side Effects of Drugs. 16th ed. The International Encyclopedia of Adverse Drug Reactions and Interactions. Amsterdam, Netherlands: Elsevier; 2016. Available at: http://ac.els-cdn.com/B978044453717101009X/3-s2.0-B978044453717101009X-main.pdf?_tid=b33f6564-9deb-11e5-a8f0-00000aab0f01&acdnat=1449607315_83f5068fc5105226fcc6d7279c083516. Accessed December 8, 2015.
17. Masters KP, Carr BM. Survey of pharmacists and physicians on drug interactions between combined oral contraceptives and broad-spectrum antibiotics. Pharm Pract (Granada). 2009;7:139-144.
18. Toh S, Mitchell AA, Anderka M, et al; National Birth Defects Prevention Study. Antibiotics and oral contraceptive failure—a case-crossover study. Contraception. 2011;83:418-425.
19. Koopmans PC, Bos JH, de Jong van den Berg LT. Are antibiotics related to oral combination contraceptive failures in the Netherlands? A case-crossover study. Pharmacoepidemiol Drug Saf. 2012;21:865-871.
20. Archer JS, Archer DF. Oral contraceptive efficacy and antibiotic interaction: A myth debunked. J Am Acad Dermatol. 2002;46:917–923.
21. Scholten PC, Droppert RM, Zwinkels MGJ, et al. No interaction between ciprofloxacin and an oral contraceptive. Antimicrob Agents Chemother. 1998;42:3266-3268.
22. Andrews E, Damle BD, Fang A, et al. Pharmacokinetics and tolerability of voriconazole and a combination oral contraceptive co-administered in healthy female subjects. Br J Clin Pharmacol. 2008;65:531-539.
23. Grimmer SF, Allen WL, Back DJ, et al. The effect of cotrimoxazole on oral contraceptive steroids in women. Contraception. 1983;28:53-59.
24. Dickinson BD, Altman RD, Nielsen NH, et al; Council on Scientific Affairs, American Medical Association. Drug interactions between oral contraceptives and antibiotics. Obstet Gynecol. 2001;98:853-860.
25. Edelman AB, Cherala G, Stanczyk FZ. Metabolism and pharmacokinetics of contraceptive steroids in obese women: a review. Contraception. 2010;82:314-323.
26. Owens RC Jr, Ambrose PG. Torsades de pointes associated with fluoroquinolones. Pharmacotherapy. 2002;22:663-668.
27. Letsas KP, Efremidis M, Kounas SP, et al. Clinical characteristics of patients with drug-induced QT interval prolongation and torsade de pointes: identification of risk factors. Clin Res Cardiol. 2009;98:208-212.
28. Yap YG, Camm AJ. Drug induced QT prolongation and torsades de pointes. Heart. 2003;89:1363-1372.
29. Shaffer D, Singer S, Korvick J, et al. Concomitant risk factors in reports of torsades de pointes associated with macrolide use: review of the United States Food and Drug Administration adverse event reporting system. Clin Infect Dis. 2002;35:197-200.
30. FDA Briefing Document. Joint Meeting of the Antimicrobial Drugs Advisory Committee and the Drug Safety and Risk Management Advisory Committee. November 5, 2015. Available at: http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/Anti-InfectiveDrugsAdvisoryCommittee/UCM467383.pdf. Accessed June 11, 2016.
31. Chou HW, Wang JL, Chang CH, et al. Risks of cardiac arrhythmia and mortality among patients using new-generation macrolides, fluoroquinolones, and β-lactam/β-lactamase inhibitors: a Taiwanese nationwide study. Clin Infect Dis. 2015;60:566-577.
32. Wong AY, Root A, Douglas IJ, et al. Cardiovascular outcomes associated with use of clarithromycin: population based study. BMJ. 2016;352:h6926.
33. Inghammar M, Svanström H, Melbye M, et al. Oral fluoroquinolone use and serious arrhythmia: bi-national cohort study. BMJ. 2016;352:i843.
34. Visapää JP, Tillonen JS, Kaihovaara PS, et al. Lack of disulfiram-like reaction with metronidazole and ethanol. Ann Pharmacother. 2002;36:971-974. 35. Fjeld H, Raknes G. Is combining metronidazole and alcohol really hazardous? Tidsskr Nor Laegeforen. 2014;134:1661-1663.
› Avoid preemptive warfarin dose reductions unless you are prescribing trimethoprim/sulfamethoxazole (TMP/SMX) or metronidazole. B
› Recommend a back-up contraceptive method to a woman who is taking a broad-spectrum antibiotic and low-dose OCs—especially if the woman is overweight. C
› Consider using the macrolide, clarithromycin, or the fluoroquinolone, ciprofloxacin, in patients taking medications that prolong QT interval or who are at higher risk for torsades de pointes (TdP). B
› Refrain from cautioning patients taking metronidazole against consuming alcohol. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Despite encouraging data that antibiotic prescribing is on the decline, patients are still prescribed antibiotics frequently, making these agents the 12th most frequently used drug class.1 At the same time, prescribers are caring for patients with increasingly complex drug regimens that provide fertile ground for drug interactions with these antibiotics. And, of course, lifestyle factors such as alcohol consumption are a consideration when any prescription is written.
As pharmacists, we find that certain questions about antibiotic prescribing and interactions come up with frequency. These questions often pertain to the use of warfarin, oral contraceptives, drugs that prolong the QT interval, and alcohol. But conflicting reports about issues such as monitoring international normalized ratio (INR) in patients taking warfarin and antibiotics, and whether (or which) antibiotics decrease the efficacy of oral contraceptives (OCs) can make decision-making challenging.
This review provides evidence-based answers to questions you may have. It also details some reliable sources of information you can consult (TABLE 12-7) when discussing treatment options with other members of the health care team.
1. Which antibiotics are preferable when a patient is taking warfarin, and are preemptive warfarin dose reductions advisable?
The simple answer is that agents with a lower likelihood of affecting the INR, such as penicillin G, clindamycin, and 1st- and 4th-generation cephalosporins, are a good place to start, and whether to preemptively reduce the warfarin dose hinges on the antibiotic being prescribed.
The more detailed answer. The fundamental mechanisms of interaction between warfarin and antibiotics are two-fold:8
- Antimicrobial agents disrupt gastrointestinal flora that synthesize vitamin K.
- Antimicrobials inhibit cytochrome p450 (CYP450) enzymes (primarily CYP2C9 and 3A4), which are responsible for the metabolism of warfarin.
The antibiotics most likely to interfere with warfarin are TMP/SMX, ciprofloxacin, levofloxacin, metronidazole, fluconazole, azithromycin, and clarithromycin (TABLE 2).9,10 Low-risk agents include clindamycin, cephalexin, and penicillin G. When prescribing an antibiotic for a patient taking warfarin, it is important not only to be aware of the agents that should be avoided, but also the agents that do not require more frequent monitoring of INR.
Preemptive warfarin dose reductions? Some physicians make preemptive warfarin dose reductions in an attempt to avoid supratherapeutic INRs in patients being prescribed antibiotics. But the evidence suggests that this step should be considered only in the presence of the antibiotics TMP/SMX and metronidazole.9,11
A 2008 study investigated the anticoagulation effects of a 10% to 20% preemptive warfarin dose reduction vs no dosing change in patients taking TMP/SMX or levofloxacin. The investigators found that the preemptive warfarin dose reduction (intervention) significantly decreased the number of supratherapeutic INR values above 4 when compared to controls (2 of 8 vs 8 of 9).12
In the dose-reduction group, no patients receiving TMP/SMX developed a subtherapeutic INR, whereas 40% (4 of 10 patients) who received levofloxacin developed a subtherapeutic INR.12 The authors of the study concluded that a prophylactic warfarin dose reduction of 10% to 20% is effective in maintaining therapeutic anticoagulation in patients receiving TMP/SMX. They added that while no change in warfarin dosing is necessary with levofloxacin, short-term INR follow-up is a prudent approach to prevent subtherapeutic INRs. Others recommend INR monitoring when antibiotic therapy is started and stopped and whenever the dose is changed.9
A 2010 retrospective, single-center, cohort study looked at patients who were taking metronidazole and warfarin. Researchers compared those who received a preemptive dose reduction of warfarin (mean reduction was 34.6% ± 13.4%) to those who did not and found a statistically significant mean difference in INR of 1.28 (P=.01).13
Almost half (46%) of the patients who did not receive a warfarin dose reduction had an INR >4, whereas none of the patients in the warfarin dose reduction group did (P=.05). Although this secondary outcome was not statistically significant (most likely due to the small sample population [N=20]), the implication is clinically significant. Two patients who reduced their dose had a subtherapeutic INR compared to none of the patients in the control group, which was also not a statistically significant difference.
The authors concluded that a 30% to 35% reduction in mean daily warfarin dose is effective in maintaining therapeutic anticoagulation in patients started on metronidazole.
Significant bleeding events. A retrospective cohort study of slightly more than 22,000 veterans who were prescribed warfarin for ≥30 uninterrupted days and given antibiotics with either a high or low risk for interaction with warfarin were studied for significant bleeding events for one month.10 Ninety-three significant bleeding events occurred in the high-risk group and 36 occurred in the low-risk group over the course of the study. The agent associated with the greatest increased risk of bleeding was TMP/SMX (hazard ratio [HR]=2.09; 95% CI, 1.45-3.02). Of note, metronidazole was not included in this study endpoint.
The study’s secondary endpoint of INR >4 found that 10% of patients taking metronidazole and 8% of patients taking TMP/SMX in addition to warfarin had INRs >4. Almost 10% (9.7%) of patients prescribed fluconazole had a peak INR value >6. Patients taking low-risk antibiotics (clindamycin or cephalexin) had no increased risk of bleeding. Monitoring INR within 3 to 14 days of starting patients on antibiotics was found to decrease the risk of serious bleeding events (HR=0.61; 95% CI, 0.42-0.88). More frequent INR monitoring by itself (without preemptive warfarin dose reductions) is appropriate for other antibiotics, including macrolides, tetracyclines, and some cephalosporins (2nd and 3rd generation).9
THE BOTTOM LINE When prescribing antibiotics for patients taking warfarin, try to choose agents with a lower likelihood of affecting INR such as penicillin G, clindamycin, and 1st- and 4th-generation cephalosporins. With these agents, there is no need for more frequent INR testing or preemptive reductions in warfarin dose. In patients for whom the use of TMP/SMX or metronidazole can’t be avoided, consider reducing the patient’s warfarin dose by 10% to 35% and rechecking the INR 5 days after starting the antibiotic.9,11,12 When prescribing agents such as fluoroquinolones, macrolides, and tetracyclines, do not reduce the patient’s warfarin dose preemptively and recheck INR 5 days after starting therapy.
2. Do antibiotics decrease the efficacy of oral contraceptives?
It’s unlikely, but antibiotics may reduce the efficacy of OCs.
There have been few, but well documented, reports of women using OCs who became pregnant after taking antimicrobials.14 It is recognized that rifampin, an inducer of enzymes that metabolize estrogens, decreases the efficacy of OCs.15 Ketoconazole’s interaction seems less well documented, but combining the agent with low-estrogen (low-dose) OCs warrants caution.16 What is not well understood is whether more common or broad-spectrum antibiotics also increase the risk of OC failure.
Three mechanisms have been proposed:16
- Antimicrobials affect hepatic enzyme induction, which increases metabolism of hormones.
- Broad-spectrum antibiotics reduce gut bacteria, which alters enterohepatic circulation and reduces plasma hormone concentrations.
- Antibiotics increase gastrointestinal motility, which decreases absorption (and reabsorption) of OCs.
A 2007 study found that when physicians and pharmacists were surveyed and asked if broad-spectrum antibiotics have a clinically significant interaction with OCs, 83% of physicians and 89% of pharmacists answered “Yes;”17 however, a large epidemiologic study performed in the United States showed no association between antibiotic use and OC failure.18
After this report, investigators in the Netherlands completed a similar cross-over analysis and found that there was a relationship between the use of antibiotics and breakthrough pregnancy in a population-based prescription database, but that the results didn’t hold for broad-spectrum antibiotics or in a sensitivity analysis.19 Pharmacokinetic studies are also conflicting, as some have shown an effect on serum hormone levels, while others have not.15,20-22
High- vs low-risk agents. Ciprofloxacin did not affect hormone levels in 2 studies.20,21 Rifampin and voriconazole may enhance systemic exposure to OCs.15,22 And erythromycin and azithromycin may interact with OCs, but the clinical significance of this interaction is still unknown.16
Short-courses of TMP/SMX are generally thought to be safe;16 a small study looked at cotrimoxazole 1 g twice daily in 9 women taking long-term OC steroids and found that short courses of the drug were unlikely to cause any adverse effects on contraceptive control.23 Tetracyclines and penicillins were the antibiotics most frequently involved in case reports of pregnancy from the United Kingdom (TABLE 32).16
It is hypothesized that some women may have a higher risk of OC failure than others due to how they metabolize ethinyl estradiol.24 Another hypothesis is that some women have gut flora that is more susceptible to the antibiotic being used. And still another possibility is that lower doses of hormones are being used in OCs than were studied for this interaction.15 Anything that decreases the concentration of these lower-dose OCs is concerning, especially in patients with a higher body mass index (BMI). The few pharmacokinetic studies that have been conducted show that it takes longer for OCs to reach a steady state in obese women and that they have a lower area under the curve (AUC) and maximum estrogen concentration than women with a normal BMI.25
THE BOTTOM LINE Because the degree of variability between patients is unknown and obesity rates are increasing, concern that low-dose OCs may lose efficacy when combined with antibiotics is warranted. While the absolute risk of breakthrough pregnancy seems small, the most conservative approach is to advise patients to use a back-up method of contraception during times of antibiotic use.
3. Which drugs prolong QT intervals?
Macrolides and fluoroquinolones are 2 classes of antibiotics associated with prolonged QT intervals, but other drugs and risk factors are important to consider, as well.
Physicians often receive phone calls from pharmacists warning about drug-drug interactions when they prescribe macrolides or fluoroquinolones for patients already taking medications known to prolong QT intervals or inhibit cytochrome P450 enzymes. Long QT syndrome increases the risk of TdP, a life-threatening arrhythmia. While TdP is rare, its severity warrants a discussion of risk factors and the likelihood of occurrence.
Two QT interval prolonging medications used together in healthy individuals does not warrant a change in therapy. TdP is most likely to occur when 2 or more QT interval prolonging medications are used in a patient who is already at high risk for arrhythmia because of risk factors such as prolonged QT interval at baseline, family history of prolonged QT intervals, female gender, age >60 years, electrolyte abnormalities (hypokalemia, hypomagnesemia, hypocalcemia), underlying comorbid diseases (eg, chronic heart failure, left ventricular hypertrophy, atrial fibrillation), hypertension, bradycardia, and genetic (ion channel) polymorphisms.26,27
Antiarrhythmics and antipsychotics are most commonly associated with drug-induced prolonged QT interval, with most case reports and research being linked to antiarrhythmics (TABLE 42).28 But macrolide and fluoroquinolone antibiotics also have been associated with TdP, although to a lesser extent. In a retrospective analysis of case reports of TdP involving macrolides, erythromycin was present (with or without other medications thought to prolong QT) in 53% of the cases and clarithromycin was involved in 36% of the reports.29
An analysis of 2 studies by the US Food and Drug Administration estimated an occurrence rate of serious cardiac arrhythmias of 46 to 85 per 100,000 users with cardiovascular disease, compared to 5 to 44 per 100,000 users without cardiovascular disease.30 And this may underestimate the actual incidence because spontaneous reporting of adverse effects declines the longer a drug is on the market. Ciprofloxacin is associated with less risk than levofloxacin and gatifloxacin (the latter of which is no longer available in the United States).26
A recent population-based study using data on over 10.6 million people from the Taiwan National Health Insurance Database examined the risk of cardiovascular death among patients using new-generation macrolides, fluoroquinolones, and β-lactam/β-lactamase inhibitors.31 The absolute risk of cardiovascular death per 1000 individuals was 0.06 for clarithromycin, 0.12 for ciprofloxacin, 0.13 for amoxicillin-clavulanate, 0.36 for azithromycin, 0.39 for levofloxacin, and 0.46 for moxifloxacin. The mean interval between first antibiotic use and the adverse cardiac event was <4 days. Not surprisingly, the highest risk was seen in patients with underlying cardiovascular disease.
Another population-based study, this time conducted in Hong Kong, evaluated the cardiovascular safety of clarithromycin compared to that of amoxicillin. Clarithromycin was found to increase the incidence of myocardial infarction, arrhythmia, and cardiac mortality in the short term, with the risk returning to baseline after treatment concluded.32 A binational cohort study of Danish and Swedish adults confirmed that fluoroquinolones (especially ciprofloxacin) do not increase the risk of a serious arrhythmia compared to penicillins.33
THE BOTTOM LINE For patients taking other QT interval prolonging medications or who are at a higher risk for TdP, consider using clarithromycin over erythromycin or azithromycin for a macrolide antibiotic or ciprofloxacin over levofloxacin or moxifloxacin if a fluoroquinolone is warranted. Using 2 drugs that may increase the QT interval is likely safe in the absence of certain risk factors.
4. Should patients avoid alcohol while taking metronidazole?
Probably not.
Warning patients against drinking alcohol while taking metronidazole has been a common practice for years. The mechanism for this theorized interaction was thought to be similar to the interaction between disulfiram and ethanol.34 Disulfiram inhibits hepatic aldehyde dehydrogenase (ALDH) when combined with alcohol, which leads to increased levels of acetaldehyde in the blood and symptoms of flushing, palpitations, nausea, vomiting, headache, and visual disturbances.35 However, multiple studies using rats have found that metronidazole does not inhibit ALDH or increase acetaldehyde concentrations like disulfiram does.34
A 2000 review article discussed 6 cases involving serious metronidazole-ethanol interactions. Ethanol alone was found to explain the reaction in 2 of the cases, and the remaining 4 could be linked to the use of other drugs or disease states.35 A 2002 Finnish study found no statistically significant differences in objective or subjective signs of a disulfiram-like interaction.34 When considering the symptoms associated with the interaction, it is important to remember that many of the symptoms can result from metronidazole therapy alone, regardless of whether other medications or alcohol are used.35
THE BOTTOM LINE Researchers have failed to identify a clinically significant interaction between metronidazole and alcohol. Avoiding alcohol while taking metronidazole does not appear to be necessary.
CORRESPONDENCE
Mary Onysko, PharmD, BCPS, University of Wyoming, School of Pharmacy Health Sciences Center, Room 292, 1000 E. University Avenue, Laramie, WY 82071; monysko@uwyo.edu.
› Avoid preemptive warfarin dose reductions unless you are prescribing trimethoprim/sulfamethoxazole (TMP/SMX) or metronidazole. B
› Recommend a back-up contraceptive method to a woman who is taking a broad-spectrum antibiotic and low-dose OCs—especially if the woman is overweight. C
› Consider using the macrolide, clarithromycin, or the fluoroquinolone, ciprofloxacin, in patients taking medications that prolong QT interval or who are at higher risk for torsades de pointes (TdP). B
› Refrain from cautioning patients taking metronidazole against consuming alcohol. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Despite encouraging data that antibiotic prescribing is on the decline, patients are still prescribed antibiotics frequently, making these agents the 12th most frequently used drug class.1 At the same time, prescribers are caring for patients with increasingly complex drug regimens that provide fertile ground for drug interactions with these antibiotics. And, of course, lifestyle factors such as alcohol consumption are a consideration when any prescription is written.
As pharmacists, we find that certain questions about antibiotic prescribing and interactions come up with frequency. These questions often pertain to the use of warfarin, oral contraceptives, drugs that prolong the QT interval, and alcohol. But conflicting reports about issues such as monitoring international normalized ratio (INR) in patients taking warfarin and antibiotics, and whether (or which) antibiotics decrease the efficacy of oral contraceptives (OCs) can make decision-making challenging.
This review provides evidence-based answers to questions you may have. It also details some reliable sources of information you can consult (TABLE 12-7) when discussing treatment options with other members of the health care team.
1. Which antibiotics are preferable when a patient is taking warfarin, and are preemptive warfarin dose reductions advisable?
The simple answer is that agents with a lower likelihood of affecting the INR, such as penicillin G, clindamycin, and 1st- and 4th-generation cephalosporins, are a good place to start, and whether to preemptively reduce the warfarin dose hinges on the antibiotic being prescribed.
The more detailed answer. The fundamental mechanisms of interaction between warfarin and antibiotics are two-fold:8
- Antimicrobial agents disrupt gastrointestinal flora that synthesize vitamin K.
- Antimicrobials inhibit cytochrome p450 (CYP450) enzymes (primarily CYP2C9 and 3A4), which are responsible for the metabolism of warfarin.
The antibiotics most likely to interfere with warfarin are TMP/SMX, ciprofloxacin, levofloxacin, metronidazole, fluconazole, azithromycin, and clarithromycin (TABLE 2).9,10 Low-risk agents include clindamycin, cephalexin, and penicillin G. When prescribing an antibiotic for a patient taking warfarin, it is important not only to be aware of the agents that should be avoided, but also the agents that do not require more frequent monitoring of INR.
Preemptive warfarin dose reductions? Some physicians make preemptive warfarin dose reductions in an attempt to avoid supratherapeutic INRs in patients being prescribed antibiotics. But the evidence suggests that this step should be considered only in the presence of the antibiotics TMP/SMX and metronidazole.9,11
A 2008 study investigated the anticoagulation effects of a 10% to 20% preemptive warfarin dose reduction vs no dosing change in patients taking TMP/SMX or levofloxacin. The investigators found that the preemptive warfarin dose reduction (intervention) significantly decreased the number of supratherapeutic INR values above 4 when compared to controls (2 of 8 vs 8 of 9).12
In the dose-reduction group, no patients receiving TMP/SMX developed a subtherapeutic INR, whereas 40% (4 of 10 patients) who received levofloxacin developed a subtherapeutic INR.12 The authors of the study concluded that a prophylactic warfarin dose reduction of 10% to 20% is effective in maintaining therapeutic anticoagulation in patients receiving TMP/SMX. They added that while no change in warfarin dosing is necessary with levofloxacin, short-term INR follow-up is a prudent approach to prevent subtherapeutic INRs. Others recommend INR monitoring when antibiotic therapy is started and stopped and whenever the dose is changed.9
A 2010 retrospective, single-center, cohort study looked at patients who were taking metronidazole and warfarin. Researchers compared those who received a preemptive dose reduction of warfarin (mean reduction was 34.6% ± 13.4%) to those who did not and found a statistically significant mean difference in INR of 1.28 (P=.01).13
Almost half (46%) of the patients who did not receive a warfarin dose reduction had an INR >4, whereas none of the patients in the warfarin dose reduction group did (P=.05). Although this secondary outcome was not statistically significant (most likely due to the small sample population [N=20]), the implication is clinically significant. Two patients who reduced their dose had a subtherapeutic INR compared to none of the patients in the control group, which was also not a statistically significant difference.
The authors concluded that a 30% to 35% reduction in mean daily warfarin dose is effective in maintaining therapeutic anticoagulation in patients started on metronidazole.
Significant bleeding events. A retrospective cohort study of slightly more than 22,000 veterans who were prescribed warfarin for ≥30 uninterrupted days and given antibiotics with either a high or low risk for interaction with warfarin were studied for significant bleeding events for one month.10 Ninety-three significant bleeding events occurred in the high-risk group and 36 occurred in the low-risk group over the course of the study. The agent associated with the greatest increased risk of bleeding was TMP/SMX (hazard ratio [HR]=2.09; 95% CI, 1.45-3.02). Of note, metronidazole was not included in this study endpoint.
The study’s secondary endpoint of INR >4 found that 10% of patients taking metronidazole and 8% of patients taking TMP/SMX in addition to warfarin had INRs >4. Almost 10% (9.7%) of patients prescribed fluconazole had a peak INR value >6. Patients taking low-risk antibiotics (clindamycin or cephalexin) had no increased risk of bleeding. Monitoring INR within 3 to 14 days of starting patients on antibiotics was found to decrease the risk of serious bleeding events (HR=0.61; 95% CI, 0.42-0.88). More frequent INR monitoring by itself (without preemptive warfarin dose reductions) is appropriate for other antibiotics, including macrolides, tetracyclines, and some cephalosporins (2nd and 3rd generation).9
THE BOTTOM LINE When prescribing antibiotics for patients taking warfarin, try to choose agents with a lower likelihood of affecting INR such as penicillin G, clindamycin, and 1st- and 4th-generation cephalosporins. With these agents, there is no need for more frequent INR testing or preemptive reductions in warfarin dose. In patients for whom the use of TMP/SMX or metronidazole can’t be avoided, consider reducing the patient’s warfarin dose by 10% to 35% and rechecking the INR 5 days after starting the antibiotic.9,11,12 When prescribing agents such as fluoroquinolones, macrolides, and tetracyclines, do not reduce the patient’s warfarin dose preemptively and recheck INR 5 days after starting therapy.
2. Do antibiotics decrease the efficacy of oral contraceptives?
It’s unlikely, but antibiotics may reduce the efficacy of OCs.
There have been few, but well documented, reports of women using OCs who became pregnant after taking antimicrobials.14 It is recognized that rifampin, an inducer of enzymes that metabolize estrogens, decreases the efficacy of OCs.15 Ketoconazole’s interaction seems less well documented, but combining the agent with low-estrogen (low-dose) OCs warrants caution.16 What is not well understood is whether more common or broad-spectrum antibiotics also increase the risk of OC failure.
Three mechanisms have been proposed:16
- Antimicrobials affect hepatic enzyme induction, which increases metabolism of hormones.
- Broad-spectrum antibiotics reduce gut bacteria, which alters enterohepatic circulation and reduces plasma hormone concentrations.
- Antibiotics increase gastrointestinal motility, which decreases absorption (and reabsorption) of OCs.
A 2007 study found that when physicians and pharmacists were surveyed and asked if broad-spectrum antibiotics have a clinically significant interaction with OCs, 83% of physicians and 89% of pharmacists answered “Yes;”17 however, a large epidemiologic study performed in the United States showed no association between antibiotic use and OC failure.18
After this report, investigators in the Netherlands completed a similar cross-over analysis and found that there was a relationship between the use of antibiotics and breakthrough pregnancy in a population-based prescription database, but that the results didn’t hold for broad-spectrum antibiotics or in a sensitivity analysis.19 Pharmacokinetic studies are also conflicting, as some have shown an effect on serum hormone levels, while others have not.15,20-22
High- vs low-risk agents. Ciprofloxacin did not affect hormone levels in 2 studies.20,21 Rifampin and voriconazole may enhance systemic exposure to OCs.15,22 And erythromycin and azithromycin may interact with OCs, but the clinical significance of this interaction is still unknown.16
Short-courses of TMP/SMX are generally thought to be safe;16 a small study looked at cotrimoxazole 1 g twice daily in 9 women taking long-term OC steroids and found that short courses of the drug were unlikely to cause any adverse effects on contraceptive control.23 Tetracyclines and penicillins were the antibiotics most frequently involved in case reports of pregnancy from the United Kingdom (TABLE 32).16
It is hypothesized that some women may have a higher risk of OC failure than others due to how they metabolize ethinyl estradiol.24 Another hypothesis is that some women have gut flora that is more susceptible to the antibiotic being used. And still another possibility is that lower doses of hormones are being used in OCs than were studied for this interaction.15 Anything that decreases the concentration of these lower-dose OCs is concerning, especially in patients with a higher body mass index (BMI). The few pharmacokinetic studies that have been conducted show that it takes longer for OCs to reach a steady state in obese women and that they have a lower area under the curve (AUC) and maximum estrogen concentration than women with a normal BMI.25
THE BOTTOM LINE Because the degree of variability between patients is unknown and obesity rates are increasing, concern that low-dose OCs may lose efficacy when combined with antibiotics is warranted. While the absolute risk of breakthrough pregnancy seems small, the most conservative approach is to advise patients to use a back-up method of contraception during times of antibiotic use.
3. Which drugs prolong QT intervals?
Macrolides and fluoroquinolones are 2 classes of antibiotics associated with prolonged QT intervals, but other drugs and risk factors are important to consider, as well.
Physicians often receive phone calls from pharmacists warning about drug-drug interactions when they prescribe macrolides or fluoroquinolones for patients already taking medications known to prolong QT intervals or inhibit cytochrome P450 enzymes. Long QT syndrome increases the risk of TdP, a life-threatening arrhythmia. While TdP is rare, its severity warrants a discussion of risk factors and the likelihood of occurrence.
Two QT interval prolonging medications used together in healthy individuals does not warrant a change in therapy. TdP is most likely to occur when 2 or more QT interval prolonging medications are used in a patient who is already at high risk for arrhythmia because of risk factors such as prolonged QT interval at baseline, family history of prolonged QT intervals, female gender, age >60 years, electrolyte abnormalities (hypokalemia, hypomagnesemia, hypocalcemia), underlying comorbid diseases (eg, chronic heart failure, left ventricular hypertrophy, atrial fibrillation), hypertension, bradycardia, and genetic (ion channel) polymorphisms.26,27
Antiarrhythmics and antipsychotics are most commonly associated with drug-induced prolonged QT interval, with most case reports and research being linked to antiarrhythmics (TABLE 42).28 But macrolide and fluoroquinolone antibiotics also have been associated with TdP, although to a lesser extent. In a retrospective analysis of case reports of TdP involving macrolides, erythromycin was present (with or without other medications thought to prolong QT) in 53% of the cases and clarithromycin was involved in 36% of the reports.29
An analysis of 2 studies by the US Food and Drug Administration estimated an occurrence rate of serious cardiac arrhythmias of 46 to 85 per 100,000 users with cardiovascular disease, compared to 5 to 44 per 100,000 users without cardiovascular disease.30 And this may underestimate the actual incidence because spontaneous reporting of adverse effects declines the longer a drug is on the market. Ciprofloxacin is associated with less risk than levofloxacin and gatifloxacin (the latter of which is no longer available in the United States).26
A recent population-based study using data on over 10.6 million people from the Taiwan National Health Insurance Database examined the risk of cardiovascular death among patients using new-generation macrolides, fluoroquinolones, and β-lactam/β-lactamase inhibitors.31 The absolute risk of cardiovascular death per 1000 individuals was 0.06 for clarithromycin, 0.12 for ciprofloxacin, 0.13 for amoxicillin-clavulanate, 0.36 for azithromycin, 0.39 for levofloxacin, and 0.46 for moxifloxacin. The mean interval between first antibiotic use and the adverse cardiac event was <4 days. Not surprisingly, the highest risk was seen in patients with underlying cardiovascular disease.
Another population-based study, this time conducted in Hong Kong, evaluated the cardiovascular safety of clarithromycin compared to that of amoxicillin. Clarithromycin was found to increase the incidence of myocardial infarction, arrhythmia, and cardiac mortality in the short term, with the risk returning to baseline after treatment concluded.32 A binational cohort study of Danish and Swedish adults confirmed that fluoroquinolones (especially ciprofloxacin) do not increase the risk of a serious arrhythmia compared to penicillins.33
THE BOTTOM LINE For patients taking other QT interval prolonging medications or who are at a higher risk for TdP, consider using clarithromycin over erythromycin or azithromycin for a macrolide antibiotic or ciprofloxacin over levofloxacin or moxifloxacin if a fluoroquinolone is warranted. Using 2 drugs that may increase the QT interval is likely safe in the absence of certain risk factors.
4. Should patients avoid alcohol while taking metronidazole?
Probably not.
Warning patients against drinking alcohol while taking metronidazole has been a common practice for years. The mechanism for this theorized interaction was thought to be similar to the interaction between disulfiram and ethanol.34 Disulfiram inhibits hepatic aldehyde dehydrogenase (ALDH) when combined with alcohol, which leads to increased levels of acetaldehyde in the blood and symptoms of flushing, palpitations, nausea, vomiting, headache, and visual disturbances.35 However, multiple studies using rats have found that metronidazole does not inhibit ALDH or increase acetaldehyde concentrations like disulfiram does.34
A 2000 review article discussed 6 cases involving serious metronidazole-ethanol interactions. Ethanol alone was found to explain the reaction in 2 of the cases, and the remaining 4 could be linked to the use of other drugs or disease states.35 A 2002 Finnish study found no statistically significant differences in objective or subjective signs of a disulfiram-like interaction.34 When considering the symptoms associated with the interaction, it is important to remember that many of the symptoms can result from metronidazole therapy alone, regardless of whether other medications or alcohol are used.35
THE BOTTOM LINE Researchers have failed to identify a clinically significant interaction between metronidazole and alcohol. Avoiding alcohol while taking metronidazole does not appear to be necessary.
CORRESPONDENCE
Mary Onysko, PharmD, BCPS, University of Wyoming, School of Pharmacy Health Sciences Center, Room 292, 1000 E. University Avenue, Laramie, WY 82071; monysko@uwyo.edu.
1. Kantor ED, Rehm CD, Haas JS, et al. Trends in prescription drug use among adults in the United States from 1999-2012. JAMA. 2015;314:1818-1831.
2. Lexicomp Online. Clinical Drug Information. Available at: http://www.wolterskluwercdi.com/lexicomp-online/. Accessed December 8, 2015.
3. GlobalRPh: The Clinician’s Ultimate Reference. Available at: http://www.globalrph.com/. Accessed December 8, 2015.
4. Medscape Apps. Available at: http://www.medscape.com/public/applanding. Accessed December 8, 2015.
5. Micromedex Solutions. Academic Institutions. Available at: http://micromedex.com/academic. Accessed December 8, 2015.
6. Patel A. Clinical Pharmacology Mobile-A mobile web app compatible on all smart phones [review] May 31, 2010. Available at: http://www.imedicalapps.com/2010/05/clinical-pharmocology-app-review/. Accessed December 8, 2015.
7. Epocrates. Available at: http://www.epocrates.com/. Accessed December 8, 2015.
8. Baillargeon J, Holmes HM, Lin Y, et al. Concurrent use of warfarin and antibiotics and the risk of bleeding in older adults. Am J Med. 2012;125:183-189.
9. PL Detail-Document #280806. Antimicrobial drug interactions with warfarin. Pharmacist’s Letter/Prescriber’s Letter. August 2012.
10. Lane M, Zeringue A, McDonald J. Serious bleeding events due to warfarin and antibiotic co-prescription in a cohort of veterans. Am J Med. 2014;127:657-663.e2.
11. Hale SF, Lesar TS. Interaction of vitamin K antagonists and trimethoprim-sulfamethoxazole: ignore at your patient’s risk. Drug Metab Drug Interact. 2014;29:53-60.
12. Ahmed A, Stephens JC, Kaus CA, et al. Impact of preemptive warfarin dose reduction on anticoagulation after initiation of trimethoprim-sulfamethoxazole or levofloxacin. J Thromb Thrombolysis. 2008;26:44-48.
13. Holt RK, Anderson EA, Cantrell MA, et al. Preemptive dose reduction of warfarin in patients initiating metronidazole. Drug Metabol Drug Interact. 2010;25:35-39.
14. Hughes BR, Cunliffe WJ. Interactions between the oral contraceptive pill and antibiotics. Br J Dermatol. 1990;122:717-718.
15. Bolt HM. Interactions between clinically used drugs and oral contraceptives. Environ Health Perspect. 1994;102:35-38.
16. Aronson JK. Meyler’s Side Effects of Drugs. 16th ed. The International Encyclopedia of Adverse Drug Reactions and Interactions. Amsterdam, Netherlands: Elsevier; 2016. Available at: http://ac.els-cdn.com/B978044453717101009X/3-s2.0-B978044453717101009X-main.pdf?_tid=b33f6564-9deb-11e5-a8f0-00000aab0f01&acdnat=1449607315_83f5068fc5105226fcc6d7279c083516. Accessed December 8, 2015.
17. Masters KP, Carr BM. Survey of pharmacists and physicians on drug interactions between combined oral contraceptives and broad-spectrum antibiotics. Pharm Pract (Granada). 2009;7:139-144.
18. Toh S, Mitchell AA, Anderka M, et al; National Birth Defects Prevention Study. Antibiotics and oral contraceptive failure—a case-crossover study. Contraception. 2011;83:418-425.
19. Koopmans PC, Bos JH, de Jong van den Berg LT. Are antibiotics related to oral combination contraceptive failures in the Netherlands? A case-crossover study. Pharmacoepidemiol Drug Saf. 2012;21:865-871.
20. Archer JS, Archer DF. Oral contraceptive efficacy and antibiotic interaction: A myth debunked. J Am Acad Dermatol. 2002;46:917–923.
21. Scholten PC, Droppert RM, Zwinkels MGJ, et al. No interaction between ciprofloxacin and an oral contraceptive. Antimicrob Agents Chemother. 1998;42:3266-3268.
22. Andrews E, Damle BD, Fang A, et al. Pharmacokinetics and tolerability of voriconazole and a combination oral contraceptive co-administered in healthy female subjects. Br J Clin Pharmacol. 2008;65:531-539.
23. Grimmer SF, Allen WL, Back DJ, et al. The effect of cotrimoxazole on oral contraceptive steroids in women. Contraception. 1983;28:53-59.
24. Dickinson BD, Altman RD, Nielsen NH, et al; Council on Scientific Affairs, American Medical Association. Drug interactions between oral contraceptives and antibiotics. Obstet Gynecol. 2001;98:853-860.
25. Edelman AB, Cherala G, Stanczyk FZ. Metabolism and pharmacokinetics of contraceptive steroids in obese women: a review. Contraception. 2010;82:314-323.
26. Owens RC Jr, Ambrose PG. Torsades de pointes associated with fluoroquinolones. Pharmacotherapy. 2002;22:663-668.
27. Letsas KP, Efremidis M, Kounas SP, et al. Clinical characteristics of patients with drug-induced QT interval prolongation and torsade de pointes: identification of risk factors. Clin Res Cardiol. 2009;98:208-212.
28. Yap YG, Camm AJ. Drug induced QT prolongation and torsades de pointes. Heart. 2003;89:1363-1372.
29. Shaffer D, Singer S, Korvick J, et al. Concomitant risk factors in reports of torsades de pointes associated with macrolide use: review of the United States Food and Drug Administration adverse event reporting system. Clin Infect Dis. 2002;35:197-200.
30. FDA Briefing Document. Joint Meeting of the Antimicrobial Drugs Advisory Committee and the Drug Safety and Risk Management Advisory Committee. November 5, 2015. Available at: http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/Anti-InfectiveDrugsAdvisoryCommittee/UCM467383.pdf. Accessed June 11, 2016.
31. Chou HW, Wang JL, Chang CH, et al. Risks of cardiac arrhythmia and mortality among patients using new-generation macrolides, fluoroquinolones, and β-lactam/β-lactamase inhibitors: a Taiwanese nationwide study. Clin Infect Dis. 2015;60:566-577.
32. Wong AY, Root A, Douglas IJ, et al. Cardiovascular outcomes associated with use of clarithromycin: population based study. BMJ. 2016;352:h6926.
33. Inghammar M, Svanström H, Melbye M, et al. Oral fluoroquinolone use and serious arrhythmia: bi-national cohort study. BMJ. 2016;352:i843.
34. Visapää JP, Tillonen JS, Kaihovaara PS, et al. Lack of disulfiram-like reaction with metronidazole and ethanol. Ann Pharmacother. 2002;36:971-974. 35. Fjeld H, Raknes G. Is combining metronidazole and alcohol really hazardous? Tidsskr Nor Laegeforen. 2014;134:1661-1663.
1. Kantor ED, Rehm CD, Haas JS, et al. Trends in prescription drug use among adults in the United States from 1999-2012. JAMA. 2015;314:1818-1831.
2. Lexicomp Online. Clinical Drug Information. Available at: http://www.wolterskluwercdi.com/lexicomp-online/. Accessed December 8, 2015.
3. GlobalRPh: The Clinician’s Ultimate Reference. Available at: http://www.globalrph.com/. Accessed December 8, 2015.
4. Medscape Apps. Available at: http://www.medscape.com/public/applanding. Accessed December 8, 2015.
5. Micromedex Solutions. Academic Institutions. Available at: http://micromedex.com/academic. Accessed December 8, 2015.
6. Patel A. Clinical Pharmacology Mobile-A mobile web app compatible on all smart phones [review] May 31, 2010. Available at: http://www.imedicalapps.com/2010/05/clinical-pharmocology-app-review/. Accessed December 8, 2015.
7. Epocrates. Available at: http://www.epocrates.com/. Accessed December 8, 2015.
8. Baillargeon J, Holmes HM, Lin Y, et al. Concurrent use of warfarin and antibiotics and the risk of bleeding in older adults. Am J Med. 2012;125:183-189.
9. PL Detail-Document #280806. Antimicrobial drug interactions with warfarin. Pharmacist’s Letter/Prescriber’s Letter. August 2012.
10. Lane M, Zeringue A, McDonald J. Serious bleeding events due to warfarin and antibiotic co-prescription in a cohort of veterans. Am J Med. 2014;127:657-663.e2.
11. Hale SF, Lesar TS. Interaction of vitamin K antagonists and trimethoprim-sulfamethoxazole: ignore at your patient’s risk. Drug Metab Drug Interact. 2014;29:53-60.
12. Ahmed A, Stephens JC, Kaus CA, et al. Impact of preemptive warfarin dose reduction on anticoagulation after initiation of trimethoprim-sulfamethoxazole or levofloxacin. J Thromb Thrombolysis. 2008;26:44-48.
13. Holt RK, Anderson EA, Cantrell MA, et al. Preemptive dose reduction of warfarin in patients initiating metronidazole. Drug Metabol Drug Interact. 2010;25:35-39.
14. Hughes BR, Cunliffe WJ. Interactions between the oral contraceptive pill and antibiotics. Br J Dermatol. 1990;122:717-718.
15. Bolt HM. Interactions between clinically used drugs and oral contraceptives. Environ Health Perspect. 1994;102:35-38.
16. Aronson JK. Meyler’s Side Effects of Drugs. 16th ed. The International Encyclopedia of Adverse Drug Reactions and Interactions. Amsterdam, Netherlands: Elsevier; 2016. Available at: http://ac.els-cdn.com/B978044453717101009X/3-s2.0-B978044453717101009X-main.pdf?_tid=b33f6564-9deb-11e5-a8f0-00000aab0f01&acdnat=1449607315_83f5068fc5105226fcc6d7279c083516. Accessed December 8, 2015.
17. Masters KP, Carr BM. Survey of pharmacists and physicians on drug interactions between combined oral contraceptives and broad-spectrum antibiotics. Pharm Pract (Granada). 2009;7:139-144.
18. Toh S, Mitchell AA, Anderka M, et al; National Birth Defects Prevention Study. Antibiotics and oral contraceptive failure—a case-crossover study. Contraception. 2011;83:418-425.
19. Koopmans PC, Bos JH, de Jong van den Berg LT. Are antibiotics related to oral combination contraceptive failures in the Netherlands? A case-crossover study. Pharmacoepidemiol Drug Saf. 2012;21:865-871.
20. Archer JS, Archer DF. Oral contraceptive efficacy and antibiotic interaction: A myth debunked. J Am Acad Dermatol. 2002;46:917–923.
21. Scholten PC, Droppert RM, Zwinkels MGJ, et al. No interaction between ciprofloxacin and an oral contraceptive. Antimicrob Agents Chemother. 1998;42:3266-3268.
22. Andrews E, Damle BD, Fang A, et al. Pharmacokinetics and tolerability of voriconazole and a combination oral contraceptive co-administered in healthy female subjects. Br J Clin Pharmacol. 2008;65:531-539.
23. Grimmer SF, Allen WL, Back DJ, et al. The effect of cotrimoxazole on oral contraceptive steroids in women. Contraception. 1983;28:53-59.
24. Dickinson BD, Altman RD, Nielsen NH, et al; Council on Scientific Affairs, American Medical Association. Drug interactions between oral contraceptives and antibiotics. Obstet Gynecol. 2001;98:853-860.
25. Edelman AB, Cherala G, Stanczyk FZ. Metabolism and pharmacokinetics of contraceptive steroids in obese women: a review. Contraception. 2010;82:314-323.
26. Owens RC Jr, Ambrose PG. Torsades de pointes associated with fluoroquinolones. Pharmacotherapy. 2002;22:663-668.
27. Letsas KP, Efremidis M, Kounas SP, et al. Clinical characteristics of patients with drug-induced QT interval prolongation and torsade de pointes: identification of risk factors. Clin Res Cardiol. 2009;98:208-212.
28. Yap YG, Camm AJ. Drug induced QT prolongation and torsades de pointes. Heart. 2003;89:1363-1372.
29. Shaffer D, Singer S, Korvick J, et al. Concomitant risk factors in reports of torsades de pointes associated with macrolide use: review of the United States Food and Drug Administration adverse event reporting system. Clin Infect Dis. 2002;35:197-200.
30. FDA Briefing Document. Joint Meeting of the Antimicrobial Drugs Advisory Committee and the Drug Safety and Risk Management Advisory Committee. November 5, 2015. Available at: http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/Anti-InfectiveDrugsAdvisoryCommittee/UCM467383.pdf. Accessed June 11, 2016.
31. Chou HW, Wang JL, Chang CH, et al. Risks of cardiac arrhythmia and mortality among patients using new-generation macrolides, fluoroquinolones, and β-lactam/β-lactamase inhibitors: a Taiwanese nationwide study. Clin Infect Dis. 2015;60:566-577.
32. Wong AY, Root A, Douglas IJ, et al. Cardiovascular outcomes associated with use of clarithromycin: population based study. BMJ. 2016;352:h6926.
33. Inghammar M, Svanström H, Melbye M, et al. Oral fluoroquinolone use and serious arrhythmia: bi-national cohort study. BMJ. 2016;352:i843.
34. Visapää JP, Tillonen JS, Kaihovaara PS, et al. Lack of disulfiram-like reaction with metronidazole and ethanol. Ann Pharmacother. 2002;36:971-974. 35. Fjeld H, Raknes G. Is combining metronidazole and alcohol really hazardous? Tidsskr Nor Laegeforen. 2014;134:1661-1663.