User login
5 drug interactions you don’t want to miss
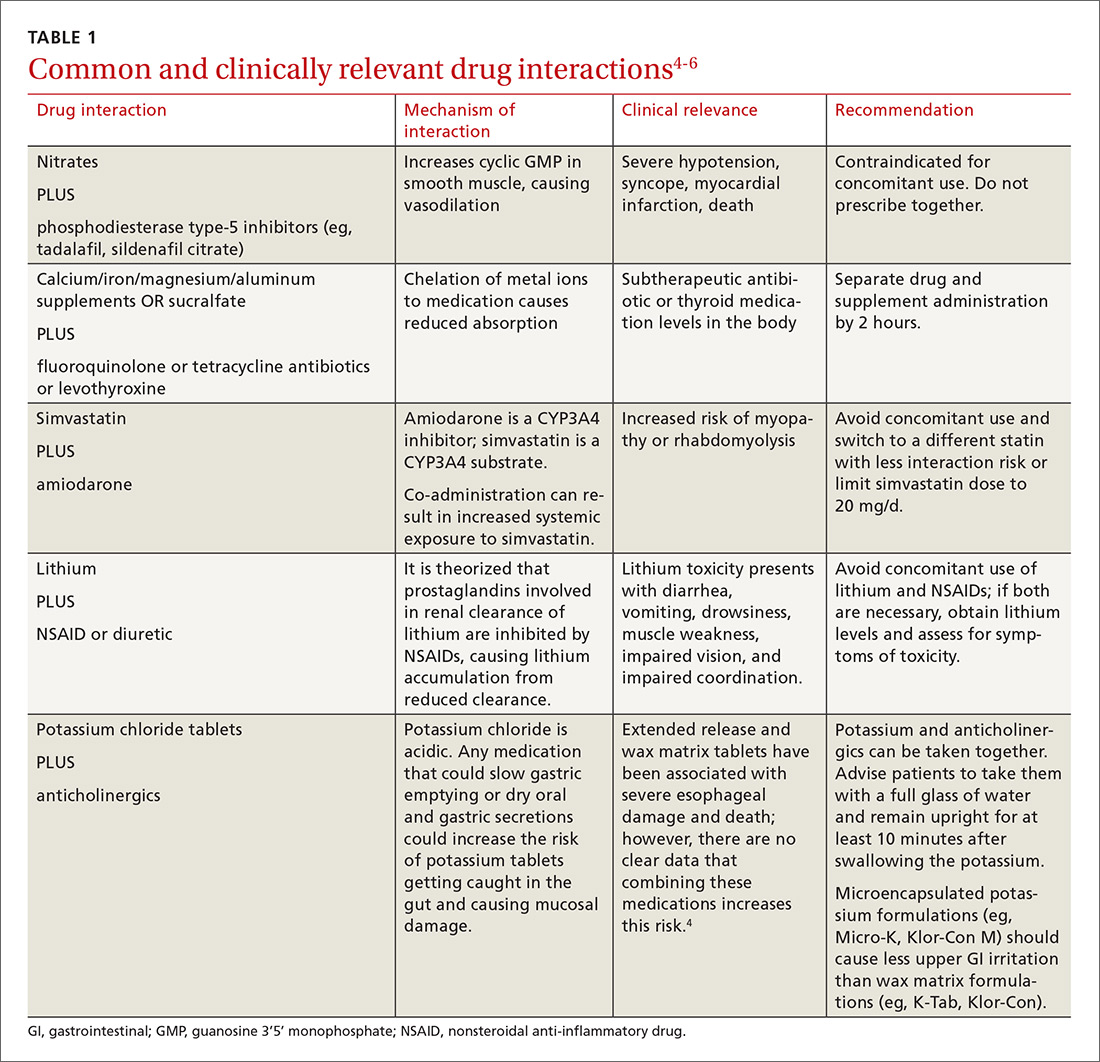
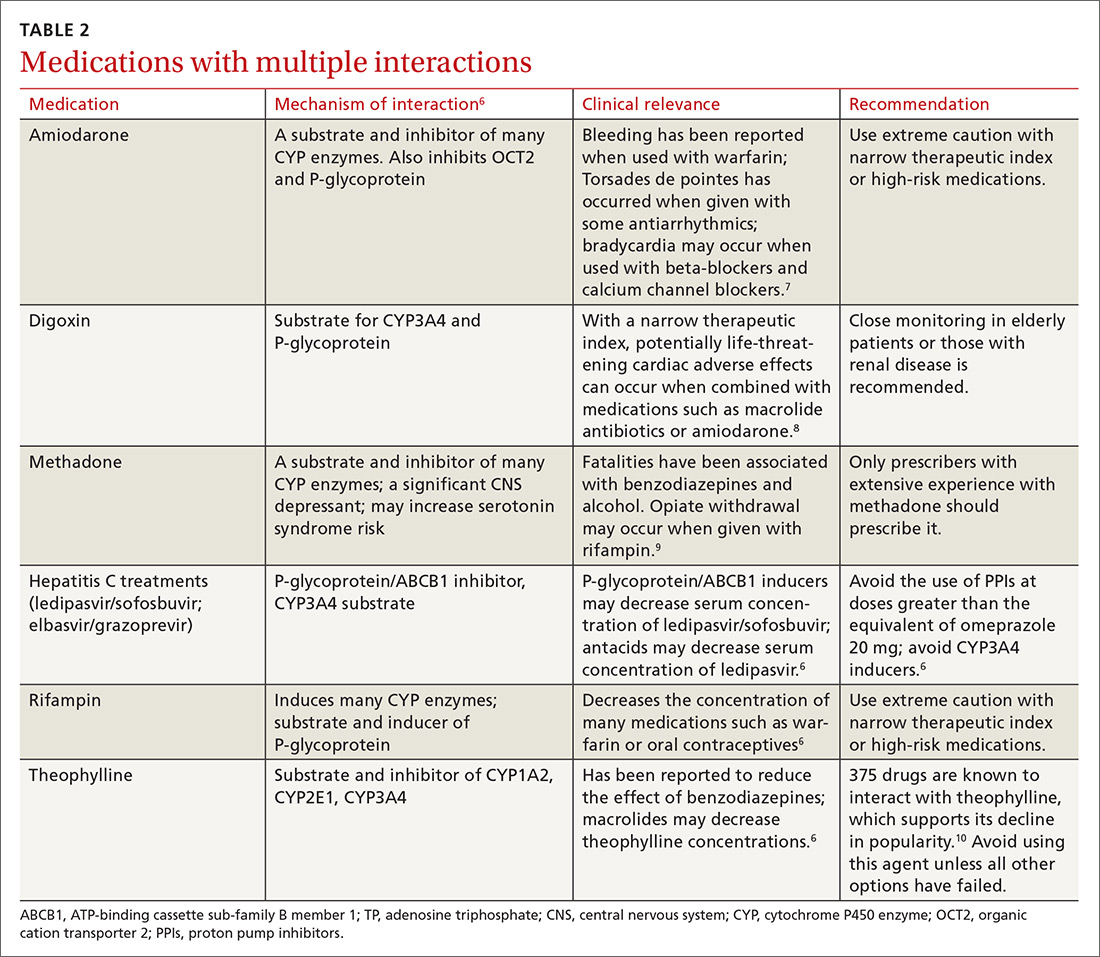
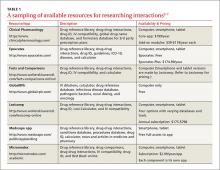
There is a strong relationship between the number of medications taken and the likelihood of a potentially serious drug-drug interaction.1,2 Drug interaction software programs can help alert prescribers to potential problems, but these programs sometimes fail to detect important interactions or generate so many clinically insignificant alerts that they become a nuisance.3 This review provides guidance about 5 clinically relevant drug interactions, including those that are common (TABLE 14-6)—and those that are less common, but no less important (TABLE 26-10).
1. Antiepileptics & contraceptives
Many antiepileptic medications decrease the efficacy of certain contraceptives
Contraception management in women with epilepsy is critical due to potential maternal and fetal complications. Many antiepileptic drugs (AEDs), including carbamazepine, ethosuximide, fosphenytoin, phenobarbital, phenytoin, primidone, topiramate, and valproate, are potentially teratogenic.11 A retrospective, observational study of 115 women of childbearing age who had epilepsy and were seen at a neurology clinic found that 74% were not using documented contraception.11 Of the minority of study participants using contraception, most were using oral contraceptives (OCs) that could potentially interact with AEDs.
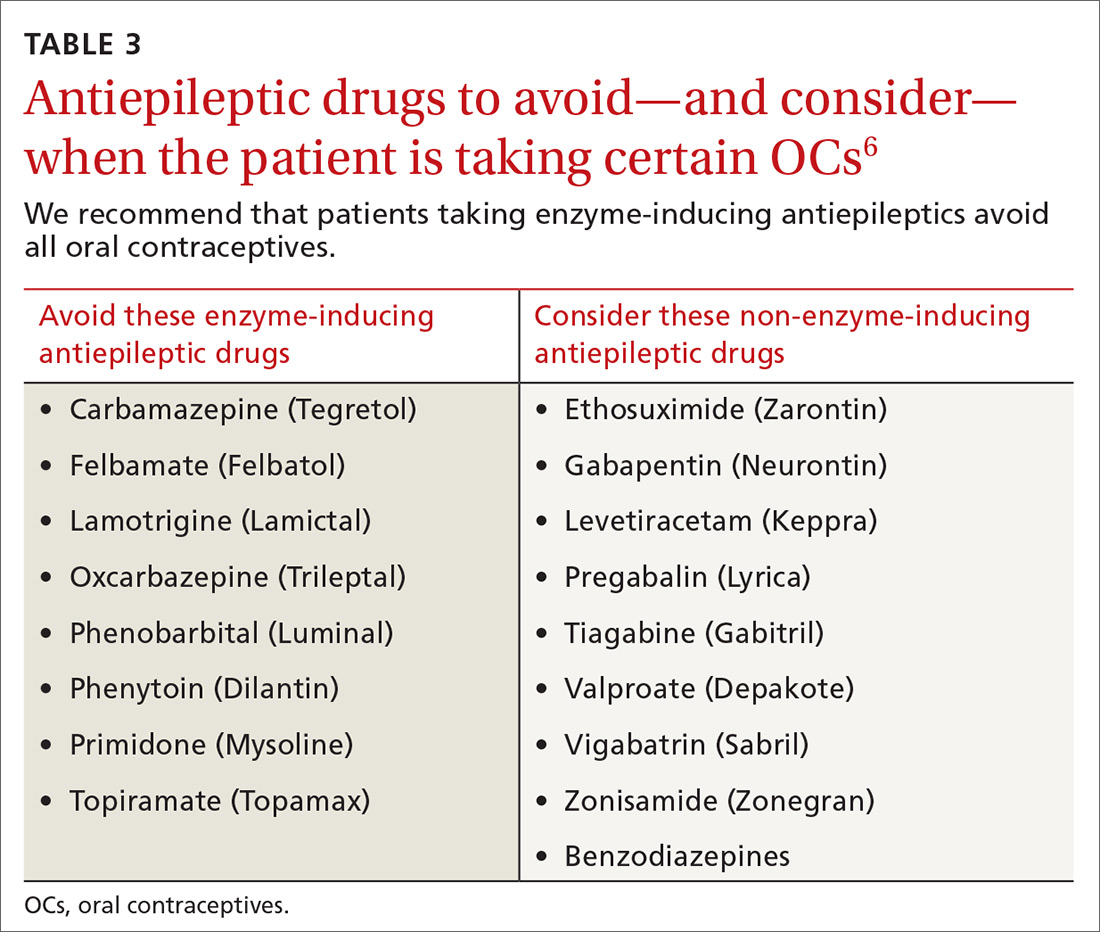
CYP inducers. Estrogen and progesterone are metabolized by the cytochrome P450 3A4 enzyme. Some AEDs induce this enzyme, which can enhance the metabolism of OCs, thus reducing their efficacy.12 It is not known, however, if this interaction results in increased pregnancy rates.13 Most newer AEDs (TABLE 36) do not induce cytochrome P450 3A4 and, thus, do not appear to affect OC efficacy, and may be safer for women with seizure disorders.12 While enzyme-inducing AEDs may decrease the efficacy of progesterone-only OCs and the morning-after pill,12,14,15 progesterone-containing intrauterine devices (IUDs), long-acting progesterone injections, and non-hormonal contraceptive methods appear to be unaffected.14-17
OCs and seizure frequency. There is no strong evidence that OCs affect seizure frequency in epileptic women, although changes in hormone levels during the menstrual cycle do affect seizure susceptibility.12 Combination OCs decrease lamotrigine levels and, therefore, may increase the risk of seizures, but progesterone-only pills do not produce this effect.12,16
Do guidelines exist? There are no specific evidence-based guidelines that pertain to the use of AEDs and contraception together, but some organizations have issued recommendations.
The American College of Obstetricians and Gynecologists recommends using a 30- to 35-mcg estrogen-containing OC rather than a lower dose in women taking an enzyme-inducing AED. The group also recommends using condoms with OCs or using IUDs.18
The American Academy of Neurology suggests that women taking OCs and enzyme-inducing AEDs use an OC containing at least 50 mcg estrogen.19
The National Institute for Health and Care Excellence recommends that women taking enzyme-inducing AEDs avoid progestin-only pills.20
The Faculty of Sexual and Reproductive Healthcare agrees that enzyme-inducing drugs may decrease efficacy and recommend considering IUDs and injectable contraceptive methods.21
2. SSRIs & NSAIDs.
SSRIs increase the GI bleeding risk associated with NSAIDs alone
Nonsteroidal anti-inflammatory drugs (NSAIDs) and selective serotonin reuptake inhibitors (SSRIs) are commonly prescribed worldwide.22,23 A well-established adverse effect of NSAIDs is gastrointestinal (GI) bleeding, and there is increasing evidence that concomitant use of an SSRI can further increase that risk through a variety of mechanisms.23
SSRIs decrease platelet serotonin levels resulting in defective platelet aggregation and impaired hemostasis. Studies have also shown that SSRIs increase gastric acidity, which leads to increased risk of peptic ulcer disease and GI bleeding.23 These mechanisms, combined with the inhibition of gastroprotective prostaglandin cyclooxygenase-1 and platelets by NSAIDs, further potentiate GI bleeding risk.24
Patients at high risk for bleeding with concomitant SSRIs and NSAIDs include older patients, patients with other risk factors for GI bleeding (eg, chronic steroid use), and patients with a history of GI bleeding.23
The evidence. A 2014 meta-analysis found that when SSRIs were used in combination with NSAIDs, the risk of GI bleeding was significantly increased, compared with SSRI monotherapy.23
Case control studies found the risk of upper GI bleeding with SSRIs had a number needed to harm (NNH) of 3177 for a low-risk population and 881 for a high-risk population with an odds ratio (OR) of 1.66 (95% confidence interval [CI], 1.44-1.92; P<.00001).23 When SSRIs were used in combination with NSAIDs, the NNH decreased to 645 for a low-risk population and 179 for a high-risk population (OR=4.25; 95% CI, 2.82-6.42; P<.0001).23
Another meta-analysis found that the OR for bleeding risk increased to 6.33 (95% CI, 3.40-11.8; P<.00001; NNH=106) with concomitant use of NSAIDs and SSRIs, compared with 2.36 (95% CI, 1.44-3.85; P=.0006; NNH=411) for SSRI use alone.25
The studies did not evaluate results based on the indication, dose, or duration of SSRI or NSAID treatment. If both an SSRI and an NSAID must be used, select a cyclooxygenase-2 selective NSAID at the lowest effective dose and consider the addition of a proton pump inhibitor to decrease the risk of a GI bleed.23,26
3. Direct oral anticoagulants and antiepileptics
Don’t use DOACs in patients taking certain antiepileptic medications
Drug interactions with anticoagulants, such as warfarin, are well documented and have been publicized for years, but physicians must also be aware of the potential for interaction between the direct oral anticoagulants (DOACs) and AEDs.
Apixaban, rivaroxaban, and dabigatran appear to interact withthe AEDs carbamazepine, phenytoin, and phenobarbital.27,28 These interactions occur due to AED induction of the CYP3A4 enzyme and effects on the P-glycoprotein (P-gp) efflux pump.27,29 When taken together, the AED induces metabolism and elimination of the DOAC medication to occur more quickly than it would normally, resulting in subtherapeutic concentrations of the DOAC. This could theoretically result in a venous thromboembolic event or stroke.
A caveat. One thing to consider is that studies demonstrating interaction between the DOAC and AED drug classes have been performed in healthy volunteers, making it difficult to extrapolate how this interaction may increase the risk for thrombotic events in other patients.
Some studies demonstrated reductions in drug levels of up to 50% with strong CYP3A4 and P-glycoprotein inducers.30 Common inducers include carbamazepine, rifampin, and St. John’s Wort.6 Patients taking such agents could theoretically have decreased exposure to the DOAC, resulting in an increase in thromboembolic risk.31
4. Statins & certain CYP inhibitors
Combining simvastatin with fibrates warrants extra attention
The efficacy of statin medications in the prevention of atherosclerotic cardiovascular disease (ASCVD) is clear. However, the clinical significance of many identified drug interactions involving statins is difficult to interpret. Interactions that cause increased serum concentrations of statins can increase the risk for liver enzyme elevations and skeletal muscle abnormalities (myalgias to rhabdomyolysis).32 Strong inhibitors of CYP3A4 (amiodarone, cyclosporine, ketoconazole, etc.) significantly increase concentrations of lovastatin, simvastatin, and atorvastatin. Pitavastatin, pravastatin, and rosuvastatin are not susceptible to any CYP-mediated drug interactions;33 therefore, rosuvastatin (a high-intensity statin) is usually recommended over other statins for patients taking strong inhibitors of CYP3A4.
When to limit simvastatin. Doses of simvastatin should not exceed 10 mg/d when combined with diltiazem, dronedarone, or verapamil, and doses should not exceed 20 mg/d when used with amiodarone, amlodipine, or ranolazine.6 These recommendations are in response to results from the SEARCH (Study of the Effectiveness of Additional Reductions in cholesterol and homocysteine) trial, which found a higher incidence of myopathies and rhabdomyolysis in patients taking 80 mg of simvastatin compared with those taking 20-mg doses.34 CYP3A4-inducing medications, especially diltiazem, were thought to also contribute to an increased risk.34
Avoid gemfibrozil with statins. Using fibrates with statins is beneficial for some patients; however, gemfibrozil significantly interacts with statins by inhibiting CYP2C8 and organic anion transporting polypeptide 1B1 (OATP1B1).33 The safer choice is fenofibrate because it does not interfere with statin metabolism and can be safely used in combination with statins.6
A retrospective review of the FDA Adverse Event Reporting System (AERS) database found that 88% of fibrate and statin combinations that resulted in rhabdomyolysis were associated with gemfibrozil/cerivastatin (cerivastatin is no longer available in the United States).35
5. One serotonergic drug & another
Serotonin syndrome is associated with more than just SSRIs
Serotonin syndrome is a constellation of symptoms (hyperthermia, hyperreflexia, muscle clonus, tremor and altered mental status) caused by increases in serotonin levels in the central and peripheral nervous systems that can lead to mild or life-threatening complications such as seizures, muscle breakdown, or hyperthermia. Serotonin syndrome is most likely to occur within 24 hours after a dose increase, after starting a new medication that increases serotonin levels, or after a drug overdose.36
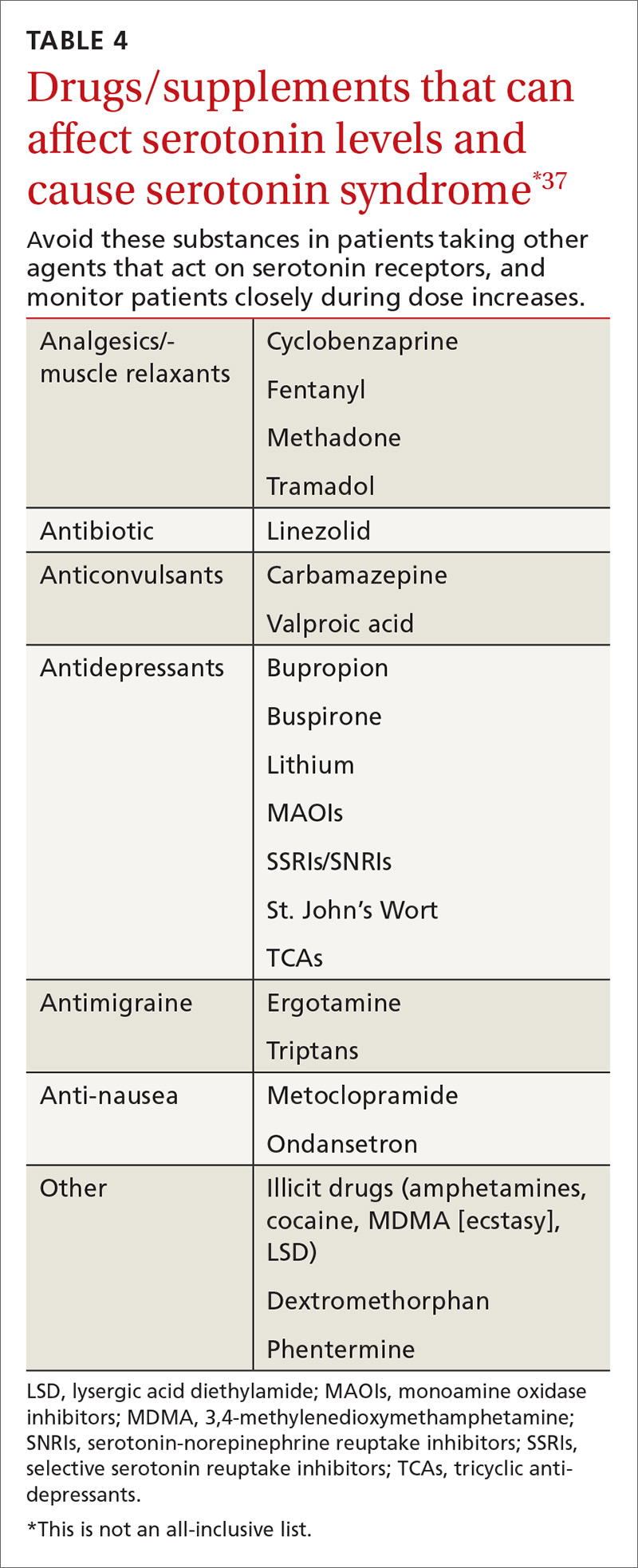
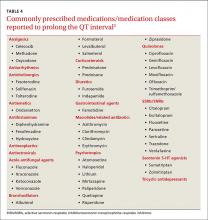
SSRIs are the most commonly reported drug associated with serotonin syndrome; however, other medications (TABLE 437) may be responsible, especially when used in combination with agents that act on serotonin receptors or in patients with impaired metabolism of the drugs being used.37
Other culprits. Serotonergic effects can also be associated with illicit drugs, some nonprescription medications, and supplements. And in March 2016, the FDA issued a warning about the risks of taking opioids with serotonergic medications.38 Although labeling changes have been recommended for all opioids, the cases of serotonin syndrome were reported more often with normal doses of fentanyl and methadone.
There are 2 mechanisms by which drugs may increase a patient’s risk for serotonin syndrome. The first is a pharmacodynamic interaction, which can occur when 2 or more medications act at the same receptor site (serotonin receptors in this example), which may result in an additive or synergistic effect.39
The second mechanism is a pharmacokinetic alteration (an agent alters absorption, distribution, metabolism, or excretion) of CYP enzymes.40 Of the more commonly used antidepressants, citalopram, escitalopram, venlafaxine, and mirtazapine seem to have the least potential for clinically significant pharmacokinetic interactions.41
Guidelines? Currently there are no guidelines for preventing serotonin syndrome. Clinicians should exercise caution in patients at high risk for drug adverse events, such as the elderly, patients taking multiple medications, and patients with comorbidities. Healthy low-risk patients can generally take 2 or 3 serotonergic medications at therapeutic doses without a major risk of harm.
CORRESPONDENCE
Mary Onysko, PharmD, BCPS, 191 East Orchard Road, Suite 200, Littleton, CO 80121; monysko@uwyo.edu.
1. Aparasu R, Baer R, Aparasu A. Clinically important potential drug-drug interactions in outpatient settings. Res Social Adm Pharm. 2007;3:426-437.
2. Johnell K, Klarin I. The relationship between number of drugs and potential drug-drug interactions in the elderly: a study of over 600,000 elderly patients from the Swedish Prescribed Drug Register. Drug Saf. 2007;30:911-918.
3. Pharmacist’s Letter. Online continuing medical education and webinars. Drug interaction overload: Problems and solutions for drug interaction alerts. Volume 2012, Course No. 216. Self-Study Course #120216. Available at: http://pharmacistsletter.therapeuticresearch.com/ce/cecourse.aspx?pc=15-219&quiz=1. Accessed June 9, 2016.
4. PL Detail-Document, Potassium and Anticholinergic Drug Interaction. Pharmacist’s Letter/Prescriber’s Letter. October 2011.
5. Micromedex Solutions. Available at: http://www.micromedexsolutions.com. Accessed May 3, 2016.
6. Lexi-Comp Online. Available at: http://online.lexi.com/lco/action/home. Accessed May 22, 2016.
7. Marcus FI. Drug interactions with amiodarone. Am Heart J. 1983;106(4 Pt 2):924-930.
8. Digoxin: serious drug interactions. Prescrire Int. 2010;19:68-70.
9. McCance-Katz EF, Sullivan LE, Nallani S. Drug interactions of clinical importance among the opioids, methadone and buprenorphine, and other frequently prescribed medications: a review. Am J Addict. 2010;19:4-16.
10. Drugs.com. Theophylline drug interactions. Available at: https://www.drugs.com/drug-interactions/theophylline.html. Accessed June 23, 2016.
11. Bhakta J, Bainbridge J, Borgelt L. Teratogenic medications and concurrent contraceptive use in women of childbearing ability with epilepsy. Epilepsy Behav. 2015;52(Pt A):212-217.
12. Reddy DS. Clinical pharmacokinetic interactions between antiepileptic drugs and hormonal contraceptives. Expert Rev Clin Pharmacol. 2010;3:183-192.
13. Carl JS, Weaver SP, Tweed E. Effect of antiepileptic drugs on oral contraceptives. Am Fam Physician. 2008;78:634-635.
14. O’Brien MD, Guillebaud J. Contraception for women with epilepsy. Epilepsia. 2006;47:1419-1422.
15. Schwenkhagen AM, Stodieck SR. Which contraception for women with epilepsy? Seizure. 2008;17:145-150.
16. Faculty of Sexual and Reproductive Healthcare Clinical Effectiveness Unit. Antiepileptic drugs and contraception. CEU statement. January 2010. Available at: https://www.fsrh.org/standards-and-guidance/documents/ceu-clinical-guidance-drug-interactions-with-hormonal/. Accessed April 25, 2016.
17. Perruca E. Clinically relevant drug interactions with antiepileptic drugs. Br J Clin Pharmacol. 2006;61:246-255.
18. ACOG practice bulletin. Number 73: Use of hormonal contraception in women with coexisting medical conditions. ACOG Committee on Practice Bulletins-Gynecology. Obstet Gynecol. 2006;107:1453-1472.
19. Report of the Quality Standards Subcommittee of the American Academy of Neurology. Practice parameter: management issues for women with epilepsy (summary statement). Neurology. 1998;51:944-948.
20. National Institute for Health and Care Excellence. Do not do recommendation. Available at: https://www.nice.org.uk/donotdo/the-progestogenonly-pill-is-not-recommended-as-reliable-contraception-inwomen-and-girls-taking-enzymeinducing-anti-epileptic-drugs-aeds. Accessed September 21, 2017.
21. Faculty of Sexual and Reproductive Healthcare. Clinical guidance: drug interactions with hormonal contraception. Available at: https://www.fsrh.org/standards-and-guidance/documents/ceu-clinical-guidance-drug-interactions-with-hormonal/. Accessed September 21, 2017.
22. de Jong JCF, van den Berg PB, Tobi H, et al. Combined use of SSRIs and NSAIDs increases the risk of gastrointestinal adverse effects. Br J Clin Pharmacol. 2003;55:591-595.
23. Anglin R, Yuan Y, Moayyedi P, et al. Risk of upper gastrointestinal bleeding with selective serotonin reuptake inhibitors with or without concurrent nonsteroidal anti-inflammatory use: a systematic review and meta-analysis. Am J Gastroenterol. 2014;109:811-819.
24. Mort JR, Aparasu RR, Baer RK, et al. Interaction between selective serotonin reuptake inhibitors and nonsteroidal anti-inflammatory drugs: review of the literature. Pharmacotherapy. 2006;26:1307-1313.
25. Loke YK, Trivedi AN, Singh S. Meta-analysis: gastrointestinal bleeding due to interaction between selective serotonin uptake inhibitors and non-steroidal anti-inflammatory drugs. Aliment Pharmacol Ther. 2008;27:31-40.
26. Venerito M, Wex T, Malfertheiner P. Nonsteroidal anti-inflammatory drug-induced gastroduodenal bleeding: risk factors and prevention strategies. Pharmaceuticals. 2010;3:2225-2237.
27. Boehringer S, Williams CD, Yawn BP, et al. Managing interactions with direct oral anticoagulants (DOACs). Pharmacist’s Letter. May 2016.
28. Johannessen SI, Landmark CJ. Antiepileptic drug interactions – principles and clinical implications. Curr Neuropharmacol. 2010;8:254-267.
29. Mohrien K, Oliphant CS, Self TH. Drug interactions with novel oral anticoagulants. Consultant. 2013;53:918-919. Available at: http://www.consultant360.com/articles/drug-interactions-novel-oral-anticoagulants. Accessed May 3, 2016.
30. Wiggins BS, Northup A, Johnson D, et al. Reduced anticoagulant effect of dabigatran in a patient receiving concomitant phenytoin. Pharmacotherapy. 2016;36:e5-e7.
31. Burnett AE, Mahan CE, Vazquez SR, et al. Guidance for the practical management of the direct oral anticoagulants (DOACs) in VTE treatment. J Thromb Thrombolysis. 2016;41:206-232.
32. Thompson PD, Clarkson P, Karas RH. Statin-associated myopathy. JAMA. 2003;289:1681-1690.
33. Hirota T, Leiri I. Drug-drug interactions that interfere with statin metabolism. Expert Opin Drug Metab Toxicol. 2015;11:1435-1447.
34. Study of the Effectiveness of Additional Reductions in Cholesterol and Homocysteine (SEARCH) Collaborative Group. Intensive lowering of LDL cholesterol wih 80 mg versus 20 mg simvastatin daily in 12,064 survivors of myocardial inffarction: a double-blind randomised trial. Lancet. 2010;376:1658-1669.
35. Jones PH, Davidson MH. Reporting rate of rhabdomyolysis with fenofibrate + statin versus gemfibrozil + any statin. Am J Cardiol. 2005;95:120-122.
36. Birmes P, Coppin D, Schmitt L, et al. Serotonin syndrome: a brief review. CMAJ. 2003;168:1439-1442.
37. Boyer EW, Shannon M. The serotonin syndrome. N Engl J Med. 2005;352:1112-1120.
38. US Food and Drug Administration. FDA Drug Safety Communication: FDA warns about several safety issues with opioid pain medicines; requires label changes. Available at: https://www.fda.gov/Drugs/DrugSafety/ucm489676.htm. Accessed June 15, 2016.
39. Sultana J, Spina E, Trifirò G. Antidepressant use in the elderly: the role of pharmacodynamics and pharmacokinetics in drug safety. Expert Opin Drug Metab Toxicol. 2015;11:883-892.
40. Sproule BA, Naranjo CA, Brenmer KE, et al. Selective serotonin reuptake inhibitors and CNS drug interactions. A critical review of the evidence. Clin Pharmacokinet. 1997;33:454-471.
41. Spina E, Santoro V, D’Arrigo C. Clinically relevant pharmacokinetic drug interactions with second-generation antidepressants: an update. Clin Ther. 2008;30:1206-1227.
There is a strong relationship between the number of medications taken and the likelihood of a potentially serious drug-drug interaction.1,2 Drug interaction software programs can help alert prescribers to potential problems, but these programs sometimes fail to detect important interactions or generate so many clinically insignificant alerts that they become a nuisance.3 This review provides guidance about 5 clinically relevant drug interactions, including those that are common (TABLE 14-6)—and those that are less common, but no less important (TABLE 26-10).
1. Antiepileptics & contraceptives
Many antiepileptic medications decrease the efficacy of certain contraceptives
Contraception management in women with epilepsy is critical due to potential maternal and fetal complications. Many antiepileptic drugs (AEDs), including carbamazepine, ethosuximide, fosphenytoin, phenobarbital, phenytoin, primidone, topiramate, and valproate, are potentially teratogenic.11 A retrospective, observational study of 115 women of childbearing age who had epilepsy and were seen at a neurology clinic found that 74% were not using documented contraception.11 Of the minority of study participants using contraception, most were using oral contraceptives (OCs) that could potentially interact with AEDs.
CYP inducers. Estrogen and progesterone are metabolized by the cytochrome P450 3A4 enzyme. Some AEDs induce this enzyme, which can enhance the metabolism of OCs, thus reducing their efficacy.12 It is not known, however, if this interaction results in increased pregnancy rates.13 Most newer AEDs (TABLE 36) do not induce cytochrome P450 3A4 and, thus, do not appear to affect OC efficacy, and may be safer for women with seizure disorders.12 While enzyme-inducing AEDs may decrease the efficacy of progesterone-only OCs and the morning-after pill,12,14,15 progesterone-containing intrauterine devices (IUDs), long-acting progesterone injections, and non-hormonal contraceptive methods appear to be unaffected.14-17
OCs and seizure frequency. There is no strong evidence that OCs affect seizure frequency in epileptic women, although changes in hormone levels during the menstrual cycle do affect seizure susceptibility.12 Combination OCs decrease lamotrigine levels and, therefore, may increase the risk of seizures, but progesterone-only pills do not produce this effect.12,16
Do guidelines exist? There are no specific evidence-based guidelines that pertain to the use of AEDs and contraception together, but some organizations have issued recommendations.
The American College of Obstetricians and Gynecologists recommends using a 30- to 35-mcg estrogen-containing OC rather than a lower dose in women taking an enzyme-inducing AED. The group also recommends using condoms with OCs or using IUDs.18
The American Academy of Neurology suggests that women taking OCs and enzyme-inducing AEDs use an OC containing at least 50 mcg estrogen.19
The National Institute for Health and Care Excellence recommends that women taking enzyme-inducing AEDs avoid progestin-only pills.20
The Faculty of Sexual and Reproductive Healthcare agrees that enzyme-inducing drugs may decrease efficacy and recommend considering IUDs and injectable contraceptive methods.21
2. SSRIs & NSAIDs.
SSRIs increase the GI bleeding risk associated with NSAIDs alone
Nonsteroidal anti-inflammatory drugs (NSAIDs) and selective serotonin reuptake inhibitors (SSRIs) are commonly prescribed worldwide.22,23 A well-established adverse effect of NSAIDs is gastrointestinal (GI) bleeding, and there is increasing evidence that concomitant use of an SSRI can further increase that risk through a variety of mechanisms.23
SSRIs decrease platelet serotonin levels resulting in defective platelet aggregation and impaired hemostasis. Studies have also shown that SSRIs increase gastric acidity, which leads to increased risk of peptic ulcer disease and GI bleeding.23 These mechanisms, combined with the inhibition of gastroprotective prostaglandin cyclooxygenase-1 and platelets by NSAIDs, further potentiate GI bleeding risk.24
Patients at high risk for bleeding with concomitant SSRIs and NSAIDs include older patients, patients with other risk factors for GI bleeding (eg, chronic steroid use), and patients with a history of GI bleeding.23
The evidence. A 2014 meta-analysis found that when SSRIs were used in combination with NSAIDs, the risk of GI bleeding was significantly increased, compared with SSRI monotherapy.23
Case control studies found the risk of upper GI bleeding with SSRIs had a number needed to harm (NNH) of 3177 for a low-risk population and 881 for a high-risk population with an odds ratio (OR) of 1.66 (95% confidence interval [CI], 1.44-1.92; P<.00001).23 When SSRIs were used in combination with NSAIDs, the NNH decreased to 645 for a low-risk population and 179 for a high-risk population (OR=4.25; 95% CI, 2.82-6.42; P<.0001).23
Another meta-analysis found that the OR for bleeding risk increased to 6.33 (95% CI, 3.40-11.8; P<.00001; NNH=106) with concomitant use of NSAIDs and SSRIs, compared with 2.36 (95% CI, 1.44-3.85; P=.0006; NNH=411) for SSRI use alone.25
The studies did not evaluate results based on the indication, dose, or duration of SSRI or NSAID treatment. If both an SSRI and an NSAID must be used, select a cyclooxygenase-2 selective NSAID at the lowest effective dose and consider the addition of a proton pump inhibitor to decrease the risk of a GI bleed.23,26
3. Direct oral anticoagulants and antiepileptics
Don’t use DOACs in patients taking certain antiepileptic medications
Drug interactions with anticoagulants, such as warfarin, are well documented and have been publicized for years, but physicians must also be aware of the potential for interaction between the direct oral anticoagulants (DOACs) and AEDs.
Apixaban, rivaroxaban, and dabigatran appear to interact withthe AEDs carbamazepine, phenytoin, and phenobarbital.27,28 These interactions occur due to AED induction of the CYP3A4 enzyme and effects on the P-glycoprotein (P-gp) efflux pump.27,29 When taken together, the AED induces metabolism and elimination of the DOAC medication to occur more quickly than it would normally, resulting in subtherapeutic concentrations of the DOAC. This could theoretically result in a venous thromboembolic event or stroke.
A caveat. One thing to consider is that studies demonstrating interaction between the DOAC and AED drug classes have been performed in healthy volunteers, making it difficult to extrapolate how this interaction may increase the risk for thrombotic events in other patients.
Some studies demonstrated reductions in drug levels of up to 50% with strong CYP3A4 and P-glycoprotein inducers.30 Common inducers include carbamazepine, rifampin, and St. John’s Wort.6 Patients taking such agents could theoretically have decreased exposure to the DOAC, resulting in an increase in thromboembolic risk.31
4. Statins & certain CYP inhibitors
Combining simvastatin with fibrates warrants extra attention
The efficacy of statin medications in the prevention of atherosclerotic cardiovascular disease (ASCVD) is clear. However, the clinical significance of many identified drug interactions involving statins is difficult to interpret. Interactions that cause increased serum concentrations of statins can increase the risk for liver enzyme elevations and skeletal muscle abnormalities (myalgias to rhabdomyolysis).32 Strong inhibitors of CYP3A4 (amiodarone, cyclosporine, ketoconazole, etc.) significantly increase concentrations of lovastatin, simvastatin, and atorvastatin. Pitavastatin, pravastatin, and rosuvastatin are not susceptible to any CYP-mediated drug interactions;33 therefore, rosuvastatin (a high-intensity statin) is usually recommended over other statins for patients taking strong inhibitors of CYP3A4.
When to limit simvastatin. Doses of simvastatin should not exceed 10 mg/d when combined with diltiazem, dronedarone, or verapamil, and doses should not exceed 20 mg/d when used with amiodarone, amlodipine, or ranolazine.6 These recommendations are in response to results from the SEARCH (Study of the Effectiveness of Additional Reductions in cholesterol and homocysteine) trial, which found a higher incidence of myopathies and rhabdomyolysis in patients taking 80 mg of simvastatin compared with those taking 20-mg doses.34 CYP3A4-inducing medications, especially diltiazem, were thought to also contribute to an increased risk.34
Avoid gemfibrozil with statins. Using fibrates with statins is beneficial for some patients; however, gemfibrozil significantly interacts with statins by inhibiting CYP2C8 and organic anion transporting polypeptide 1B1 (OATP1B1).33 The safer choice is fenofibrate because it does not interfere with statin metabolism and can be safely used in combination with statins.6
A retrospective review of the FDA Adverse Event Reporting System (AERS) database found that 88% of fibrate and statin combinations that resulted in rhabdomyolysis were associated with gemfibrozil/cerivastatin (cerivastatin is no longer available in the United States).35
5. One serotonergic drug & another
Serotonin syndrome is associated with more than just SSRIs
Serotonin syndrome is a constellation of symptoms (hyperthermia, hyperreflexia, muscle clonus, tremor and altered mental status) caused by increases in serotonin levels in the central and peripheral nervous systems that can lead to mild or life-threatening complications such as seizures, muscle breakdown, or hyperthermia. Serotonin syndrome is most likely to occur within 24 hours after a dose increase, after starting a new medication that increases serotonin levels, or after a drug overdose.36
SSRIs are the most commonly reported drug associated with serotonin syndrome; however, other medications (TABLE 437) may be responsible, especially when used in combination with agents that act on serotonin receptors or in patients with impaired metabolism of the drugs being used.37
Other culprits. Serotonergic effects can also be associated with illicit drugs, some nonprescription medications, and supplements. And in March 2016, the FDA issued a warning about the risks of taking opioids with serotonergic medications.38 Although labeling changes have been recommended for all opioids, the cases of serotonin syndrome were reported more often with normal doses of fentanyl and methadone.
There are 2 mechanisms by which drugs may increase a patient’s risk for serotonin syndrome. The first is a pharmacodynamic interaction, which can occur when 2 or more medications act at the same receptor site (serotonin receptors in this example), which may result in an additive or synergistic effect.39
The second mechanism is a pharmacokinetic alteration (an agent alters absorption, distribution, metabolism, or excretion) of CYP enzymes.40 Of the more commonly used antidepressants, citalopram, escitalopram, venlafaxine, and mirtazapine seem to have the least potential for clinically significant pharmacokinetic interactions.41
Guidelines? Currently there are no guidelines for preventing serotonin syndrome. Clinicians should exercise caution in patients at high risk for drug adverse events, such as the elderly, patients taking multiple medications, and patients with comorbidities. Healthy low-risk patients can generally take 2 or 3 serotonergic medications at therapeutic doses without a major risk of harm.
CORRESPONDENCE
Mary Onysko, PharmD, BCPS, 191 East Orchard Road, Suite 200, Littleton, CO 80121; monysko@uwyo.edu.
There is a strong relationship between the number of medications taken and the likelihood of a potentially serious drug-drug interaction.1,2 Drug interaction software programs can help alert prescribers to potential problems, but these programs sometimes fail to detect important interactions or generate so many clinically insignificant alerts that they become a nuisance.3 This review provides guidance about 5 clinically relevant drug interactions, including those that are common (TABLE 14-6)—and those that are less common, but no less important (TABLE 26-10).
1. Antiepileptics & contraceptives
Many antiepileptic medications decrease the efficacy of certain contraceptives
Contraception management in women with epilepsy is critical due to potential maternal and fetal complications. Many antiepileptic drugs (AEDs), including carbamazepine, ethosuximide, fosphenytoin, phenobarbital, phenytoin, primidone, topiramate, and valproate, are potentially teratogenic.11 A retrospective, observational study of 115 women of childbearing age who had epilepsy and were seen at a neurology clinic found that 74% were not using documented contraception.11 Of the minority of study participants using contraception, most were using oral contraceptives (OCs) that could potentially interact with AEDs.
CYP inducers. Estrogen and progesterone are metabolized by the cytochrome P450 3A4 enzyme. Some AEDs induce this enzyme, which can enhance the metabolism of OCs, thus reducing their efficacy.12 It is not known, however, if this interaction results in increased pregnancy rates.13 Most newer AEDs (TABLE 36) do not induce cytochrome P450 3A4 and, thus, do not appear to affect OC efficacy, and may be safer for women with seizure disorders.12 While enzyme-inducing AEDs may decrease the efficacy of progesterone-only OCs and the morning-after pill,12,14,15 progesterone-containing intrauterine devices (IUDs), long-acting progesterone injections, and non-hormonal contraceptive methods appear to be unaffected.14-17
OCs and seizure frequency. There is no strong evidence that OCs affect seizure frequency in epileptic women, although changes in hormone levels during the menstrual cycle do affect seizure susceptibility.12 Combination OCs decrease lamotrigine levels and, therefore, may increase the risk of seizures, but progesterone-only pills do not produce this effect.12,16
Do guidelines exist? There are no specific evidence-based guidelines that pertain to the use of AEDs and contraception together, but some organizations have issued recommendations.
The American College of Obstetricians and Gynecologists recommends using a 30- to 35-mcg estrogen-containing OC rather than a lower dose in women taking an enzyme-inducing AED. The group also recommends using condoms with OCs or using IUDs.18
The American Academy of Neurology suggests that women taking OCs and enzyme-inducing AEDs use an OC containing at least 50 mcg estrogen.19
The National Institute for Health and Care Excellence recommends that women taking enzyme-inducing AEDs avoid progestin-only pills.20
The Faculty of Sexual and Reproductive Healthcare agrees that enzyme-inducing drugs may decrease efficacy and recommend considering IUDs and injectable contraceptive methods.21
2. SSRIs & NSAIDs.
SSRIs increase the GI bleeding risk associated with NSAIDs alone
Nonsteroidal anti-inflammatory drugs (NSAIDs) and selective serotonin reuptake inhibitors (SSRIs) are commonly prescribed worldwide.22,23 A well-established adverse effect of NSAIDs is gastrointestinal (GI) bleeding, and there is increasing evidence that concomitant use of an SSRI can further increase that risk through a variety of mechanisms.23
SSRIs decrease platelet serotonin levels resulting in defective platelet aggregation and impaired hemostasis. Studies have also shown that SSRIs increase gastric acidity, which leads to increased risk of peptic ulcer disease and GI bleeding.23 These mechanisms, combined with the inhibition of gastroprotective prostaglandin cyclooxygenase-1 and platelets by NSAIDs, further potentiate GI bleeding risk.24
Patients at high risk for bleeding with concomitant SSRIs and NSAIDs include older patients, patients with other risk factors for GI bleeding (eg, chronic steroid use), and patients with a history of GI bleeding.23
The evidence. A 2014 meta-analysis found that when SSRIs were used in combination with NSAIDs, the risk of GI bleeding was significantly increased, compared with SSRI monotherapy.23
Case control studies found the risk of upper GI bleeding with SSRIs had a number needed to harm (NNH) of 3177 for a low-risk population and 881 for a high-risk population with an odds ratio (OR) of 1.66 (95% confidence interval [CI], 1.44-1.92; P<.00001).23 When SSRIs were used in combination with NSAIDs, the NNH decreased to 645 for a low-risk population and 179 for a high-risk population (OR=4.25; 95% CI, 2.82-6.42; P<.0001).23
Another meta-analysis found that the OR for bleeding risk increased to 6.33 (95% CI, 3.40-11.8; P<.00001; NNH=106) with concomitant use of NSAIDs and SSRIs, compared with 2.36 (95% CI, 1.44-3.85; P=.0006; NNH=411) for SSRI use alone.25
The studies did not evaluate results based on the indication, dose, or duration of SSRI or NSAID treatment. If both an SSRI and an NSAID must be used, select a cyclooxygenase-2 selective NSAID at the lowest effective dose and consider the addition of a proton pump inhibitor to decrease the risk of a GI bleed.23,26
3. Direct oral anticoagulants and antiepileptics
Don’t use DOACs in patients taking certain antiepileptic medications
Drug interactions with anticoagulants, such as warfarin, are well documented and have been publicized for years, but physicians must also be aware of the potential for interaction between the direct oral anticoagulants (DOACs) and AEDs.
Apixaban, rivaroxaban, and dabigatran appear to interact withthe AEDs carbamazepine, phenytoin, and phenobarbital.27,28 These interactions occur due to AED induction of the CYP3A4 enzyme and effects on the P-glycoprotein (P-gp) efflux pump.27,29 When taken together, the AED induces metabolism and elimination of the DOAC medication to occur more quickly than it would normally, resulting in subtherapeutic concentrations of the DOAC. This could theoretically result in a venous thromboembolic event or stroke.
A caveat. One thing to consider is that studies demonstrating interaction between the DOAC and AED drug classes have been performed in healthy volunteers, making it difficult to extrapolate how this interaction may increase the risk for thrombotic events in other patients.
Some studies demonstrated reductions in drug levels of up to 50% with strong CYP3A4 and P-glycoprotein inducers.30 Common inducers include carbamazepine, rifampin, and St. John’s Wort.6 Patients taking such agents could theoretically have decreased exposure to the DOAC, resulting in an increase in thromboembolic risk.31
4. Statins & certain CYP inhibitors
Combining simvastatin with fibrates warrants extra attention
The efficacy of statin medications in the prevention of atherosclerotic cardiovascular disease (ASCVD) is clear. However, the clinical significance of many identified drug interactions involving statins is difficult to interpret. Interactions that cause increased serum concentrations of statins can increase the risk for liver enzyme elevations and skeletal muscle abnormalities (myalgias to rhabdomyolysis).32 Strong inhibitors of CYP3A4 (amiodarone, cyclosporine, ketoconazole, etc.) significantly increase concentrations of lovastatin, simvastatin, and atorvastatin. Pitavastatin, pravastatin, and rosuvastatin are not susceptible to any CYP-mediated drug interactions;33 therefore, rosuvastatin (a high-intensity statin) is usually recommended over other statins for patients taking strong inhibitors of CYP3A4.
When to limit simvastatin. Doses of simvastatin should not exceed 10 mg/d when combined with diltiazem, dronedarone, or verapamil, and doses should not exceed 20 mg/d when used with amiodarone, amlodipine, or ranolazine.6 These recommendations are in response to results from the SEARCH (Study of the Effectiveness of Additional Reductions in cholesterol and homocysteine) trial, which found a higher incidence of myopathies and rhabdomyolysis in patients taking 80 mg of simvastatin compared with those taking 20-mg doses.34 CYP3A4-inducing medications, especially diltiazem, were thought to also contribute to an increased risk.34
Avoid gemfibrozil with statins. Using fibrates with statins is beneficial for some patients; however, gemfibrozil significantly interacts with statins by inhibiting CYP2C8 and organic anion transporting polypeptide 1B1 (OATP1B1).33 The safer choice is fenofibrate because it does not interfere with statin metabolism and can be safely used in combination with statins.6
A retrospective review of the FDA Adverse Event Reporting System (AERS) database found that 88% of fibrate and statin combinations that resulted in rhabdomyolysis were associated with gemfibrozil/cerivastatin (cerivastatin is no longer available in the United States).35
5. One serotonergic drug & another
Serotonin syndrome is associated with more than just SSRIs
Serotonin syndrome is a constellation of symptoms (hyperthermia, hyperreflexia, muscle clonus, tremor and altered mental status) caused by increases in serotonin levels in the central and peripheral nervous systems that can lead to mild or life-threatening complications such as seizures, muscle breakdown, or hyperthermia. Serotonin syndrome is most likely to occur within 24 hours after a dose increase, after starting a new medication that increases serotonin levels, or after a drug overdose.36
SSRIs are the most commonly reported drug associated with serotonin syndrome; however, other medications (TABLE 437) may be responsible, especially when used in combination with agents that act on serotonin receptors or in patients with impaired metabolism of the drugs being used.37
Other culprits. Serotonergic effects can also be associated with illicit drugs, some nonprescription medications, and supplements. And in March 2016, the FDA issued a warning about the risks of taking opioids with serotonergic medications.38 Although labeling changes have been recommended for all opioids, the cases of serotonin syndrome were reported more often with normal doses of fentanyl and methadone.
There are 2 mechanisms by which drugs may increase a patient’s risk for serotonin syndrome. The first is a pharmacodynamic interaction, which can occur when 2 or more medications act at the same receptor site (serotonin receptors in this example), which may result in an additive or synergistic effect.39
The second mechanism is a pharmacokinetic alteration (an agent alters absorption, distribution, metabolism, or excretion) of CYP enzymes.40 Of the more commonly used antidepressants, citalopram, escitalopram, venlafaxine, and mirtazapine seem to have the least potential for clinically significant pharmacokinetic interactions.41
Guidelines? Currently there are no guidelines for preventing serotonin syndrome. Clinicians should exercise caution in patients at high risk for drug adverse events, such as the elderly, patients taking multiple medications, and patients with comorbidities. Healthy low-risk patients can generally take 2 or 3 serotonergic medications at therapeutic doses without a major risk of harm.
CORRESPONDENCE
Mary Onysko, PharmD, BCPS, 191 East Orchard Road, Suite 200, Littleton, CO 80121; monysko@uwyo.edu.
1. Aparasu R, Baer R, Aparasu A. Clinically important potential drug-drug interactions in outpatient settings. Res Social Adm Pharm. 2007;3:426-437.
2. Johnell K, Klarin I. The relationship between number of drugs and potential drug-drug interactions in the elderly: a study of over 600,000 elderly patients from the Swedish Prescribed Drug Register. Drug Saf. 2007;30:911-918.
3. Pharmacist’s Letter. Online continuing medical education and webinars. Drug interaction overload: Problems and solutions for drug interaction alerts. Volume 2012, Course No. 216. Self-Study Course #120216. Available at: http://pharmacistsletter.therapeuticresearch.com/ce/cecourse.aspx?pc=15-219&quiz=1. Accessed June 9, 2016.
4. PL Detail-Document, Potassium and Anticholinergic Drug Interaction. Pharmacist’s Letter/Prescriber’s Letter. October 2011.
5. Micromedex Solutions. Available at: http://www.micromedexsolutions.com. Accessed May 3, 2016.
6. Lexi-Comp Online. Available at: http://online.lexi.com/lco/action/home. Accessed May 22, 2016.
7. Marcus FI. Drug interactions with amiodarone. Am Heart J. 1983;106(4 Pt 2):924-930.
8. Digoxin: serious drug interactions. Prescrire Int. 2010;19:68-70.
9. McCance-Katz EF, Sullivan LE, Nallani S. Drug interactions of clinical importance among the opioids, methadone and buprenorphine, and other frequently prescribed medications: a review. Am J Addict. 2010;19:4-16.
10. Drugs.com. Theophylline drug interactions. Available at: https://www.drugs.com/drug-interactions/theophylline.html. Accessed June 23, 2016.
11. Bhakta J, Bainbridge J, Borgelt L. Teratogenic medications and concurrent contraceptive use in women of childbearing ability with epilepsy. Epilepsy Behav. 2015;52(Pt A):212-217.
12. Reddy DS. Clinical pharmacokinetic interactions between antiepileptic drugs and hormonal contraceptives. Expert Rev Clin Pharmacol. 2010;3:183-192.
13. Carl JS, Weaver SP, Tweed E. Effect of antiepileptic drugs on oral contraceptives. Am Fam Physician. 2008;78:634-635.
14. O’Brien MD, Guillebaud J. Contraception for women with epilepsy. Epilepsia. 2006;47:1419-1422.
15. Schwenkhagen AM, Stodieck SR. Which contraception for women with epilepsy? Seizure. 2008;17:145-150.
16. Faculty of Sexual and Reproductive Healthcare Clinical Effectiveness Unit. Antiepileptic drugs and contraception. CEU statement. January 2010. Available at: https://www.fsrh.org/standards-and-guidance/documents/ceu-clinical-guidance-drug-interactions-with-hormonal/. Accessed April 25, 2016.
17. Perruca E. Clinically relevant drug interactions with antiepileptic drugs. Br J Clin Pharmacol. 2006;61:246-255.
18. ACOG practice bulletin. Number 73: Use of hormonal contraception in women with coexisting medical conditions. ACOG Committee on Practice Bulletins-Gynecology. Obstet Gynecol. 2006;107:1453-1472.
19. Report of the Quality Standards Subcommittee of the American Academy of Neurology. Practice parameter: management issues for women with epilepsy (summary statement). Neurology. 1998;51:944-948.
20. National Institute for Health and Care Excellence. Do not do recommendation. Available at: https://www.nice.org.uk/donotdo/the-progestogenonly-pill-is-not-recommended-as-reliable-contraception-inwomen-and-girls-taking-enzymeinducing-anti-epileptic-drugs-aeds. Accessed September 21, 2017.
21. Faculty of Sexual and Reproductive Healthcare. Clinical guidance: drug interactions with hormonal contraception. Available at: https://www.fsrh.org/standards-and-guidance/documents/ceu-clinical-guidance-drug-interactions-with-hormonal/. Accessed September 21, 2017.
22. de Jong JCF, van den Berg PB, Tobi H, et al. Combined use of SSRIs and NSAIDs increases the risk of gastrointestinal adverse effects. Br J Clin Pharmacol. 2003;55:591-595.
23. Anglin R, Yuan Y, Moayyedi P, et al. Risk of upper gastrointestinal bleeding with selective serotonin reuptake inhibitors with or without concurrent nonsteroidal anti-inflammatory use: a systematic review and meta-analysis. Am J Gastroenterol. 2014;109:811-819.
24. Mort JR, Aparasu RR, Baer RK, et al. Interaction between selective serotonin reuptake inhibitors and nonsteroidal anti-inflammatory drugs: review of the literature. Pharmacotherapy. 2006;26:1307-1313.
25. Loke YK, Trivedi AN, Singh S. Meta-analysis: gastrointestinal bleeding due to interaction between selective serotonin uptake inhibitors and non-steroidal anti-inflammatory drugs. Aliment Pharmacol Ther. 2008;27:31-40.
26. Venerito M, Wex T, Malfertheiner P. Nonsteroidal anti-inflammatory drug-induced gastroduodenal bleeding: risk factors and prevention strategies. Pharmaceuticals. 2010;3:2225-2237.
27. Boehringer S, Williams CD, Yawn BP, et al. Managing interactions with direct oral anticoagulants (DOACs). Pharmacist’s Letter. May 2016.
28. Johannessen SI, Landmark CJ. Antiepileptic drug interactions – principles and clinical implications. Curr Neuropharmacol. 2010;8:254-267.
29. Mohrien K, Oliphant CS, Self TH. Drug interactions with novel oral anticoagulants. Consultant. 2013;53:918-919. Available at: http://www.consultant360.com/articles/drug-interactions-novel-oral-anticoagulants. Accessed May 3, 2016.
30. Wiggins BS, Northup A, Johnson D, et al. Reduced anticoagulant effect of dabigatran in a patient receiving concomitant phenytoin. Pharmacotherapy. 2016;36:e5-e7.
31. Burnett AE, Mahan CE, Vazquez SR, et al. Guidance for the practical management of the direct oral anticoagulants (DOACs) in VTE treatment. J Thromb Thrombolysis. 2016;41:206-232.
32. Thompson PD, Clarkson P, Karas RH. Statin-associated myopathy. JAMA. 2003;289:1681-1690.
33. Hirota T, Leiri I. Drug-drug interactions that interfere with statin metabolism. Expert Opin Drug Metab Toxicol. 2015;11:1435-1447.
34. Study of the Effectiveness of Additional Reductions in Cholesterol and Homocysteine (SEARCH) Collaborative Group. Intensive lowering of LDL cholesterol wih 80 mg versus 20 mg simvastatin daily in 12,064 survivors of myocardial inffarction: a double-blind randomised trial. Lancet. 2010;376:1658-1669.
35. Jones PH, Davidson MH. Reporting rate of rhabdomyolysis with fenofibrate + statin versus gemfibrozil + any statin. Am J Cardiol. 2005;95:120-122.
36. Birmes P, Coppin D, Schmitt L, et al. Serotonin syndrome: a brief review. CMAJ. 2003;168:1439-1442.
37. Boyer EW, Shannon M. The serotonin syndrome. N Engl J Med. 2005;352:1112-1120.
38. US Food and Drug Administration. FDA Drug Safety Communication: FDA warns about several safety issues with opioid pain medicines; requires label changes. Available at: https://www.fda.gov/Drugs/DrugSafety/ucm489676.htm. Accessed June 15, 2016.
39. Sultana J, Spina E, Trifirò G. Antidepressant use in the elderly: the role of pharmacodynamics and pharmacokinetics in drug safety. Expert Opin Drug Metab Toxicol. 2015;11:883-892.
40. Sproule BA, Naranjo CA, Brenmer KE, et al. Selective serotonin reuptake inhibitors and CNS drug interactions. A critical review of the evidence. Clin Pharmacokinet. 1997;33:454-471.
41. Spina E, Santoro V, D’Arrigo C. Clinically relevant pharmacokinetic drug interactions with second-generation antidepressants: an update. Clin Ther. 2008;30:1206-1227.
1. Aparasu R, Baer R, Aparasu A. Clinically important potential drug-drug interactions in outpatient settings. Res Social Adm Pharm. 2007;3:426-437.
2. Johnell K, Klarin I. The relationship between number of drugs and potential drug-drug interactions in the elderly: a study of over 600,000 elderly patients from the Swedish Prescribed Drug Register. Drug Saf. 2007;30:911-918.
3. Pharmacist’s Letter. Online continuing medical education and webinars. Drug interaction overload: Problems and solutions for drug interaction alerts. Volume 2012, Course No. 216. Self-Study Course #120216. Available at: http://pharmacistsletter.therapeuticresearch.com/ce/cecourse.aspx?pc=15-219&quiz=1. Accessed June 9, 2016.
4. PL Detail-Document, Potassium and Anticholinergic Drug Interaction. Pharmacist’s Letter/Prescriber’s Letter. October 2011.
5. Micromedex Solutions. Available at: http://www.micromedexsolutions.com. Accessed May 3, 2016.
6. Lexi-Comp Online. Available at: http://online.lexi.com/lco/action/home. Accessed May 22, 2016.
7. Marcus FI. Drug interactions with amiodarone. Am Heart J. 1983;106(4 Pt 2):924-930.
8. Digoxin: serious drug interactions. Prescrire Int. 2010;19:68-70.
9. McCance-Katz EF, Sullivan LE, Nallani S. Drug interactions of clinical importance among the opioids, methadone and buprenorphine, and other frequently prescribed medications: a review. Am J Addict. 2010;19:4-16.
10. Drugs.com. Theophylline drug interactions. Available at: https://www.drugs.com/drug-interactions/theophylline.html. Accessed June 23, 2016.
11. Bhakta J, Bainbridge J, Borgelt L. Teratogenic medications and concurrent contraceptive use in women of childbearing ability with epilepsy. Epilepsy Behav. 2015;52(Pt A):212-217.
12. Reddy DS. Clinical pharmacokinetic interactions between antiepileptic drugs and hormonal contraceptives. Expert Rev Clin Pharmacol. 2010;3:183-192.
13. Carl JS, Weaver SP, Tweed E. Effect of antiepileptic drugs on oral contraceptives. Am Fam Physician. 2008;78:634-635.
14. O’Brien MD, Guillebaud J. Contraception for women with epilepsy. Epilepsia. 2006;47:1419-1422.
15. Schwenkhagen AM, Stodieck SR. Which contraception for women with epilepsy? Seizure. 2008;17:145-150.
16. Faculty of Sexual and Reproductive Healthcare Clinical Effectiveness Unit. Antiepileptic drugs and contraception. CEU statement. January 2010. Available at: https://www.fsrh.org/standards-and-guidance/documents/ceu-clinical-guidance-drug-interactions-with-hormonal/. Accessed April 25, 2016.
17. Perruca E. Clinically relevant drug interactions with antiepileptic drugs. Br J Clin Pharmacol. 2006;61:246-255.
18. ACOG practice bulletin. Number 73: Use of hormonal contraception in women with coexisting medical conditions. ACOG Committee on Practice Bulletins-Gynecology. Obstet Gynecol. 2006;107:1453-1472.
19. Report of the Quality Standards Subcommittee of the American Academy of Neurology. Practice parameter: management issues for women with epilepsy (summary statement). Neurology. 1998;51:944-948.
20. National Institute for Health and Care Excellence. Do not do recommendation. Available at: https://www.nice.org.uk/donotdo/the-progestogenonly-pill-is-not-recommended-as-reliable-contraception-inwomen-and-girls-taking-enzymeinducing-anti-epileptic-drugs-aeds. Accessed September 21, 2017.
21. Faculty of Sexual and Reproductive Healthcare. Clinical guidance: drug interactions with hormonal contraception. Available at: https://www.fsrh.org/standards-and-guidance/documents/ceu-clinical-guidance-drug-interactions-with-hormonal/. Accessed September 21, 2017.
22. de Jong JCF, van den Berg PB, Tobi H, et al. Combined use of SSRIs and NSAIDs increases the risk of gastrointestinal adverse effects. Br J Clin Pharmacol. 2003;55:591-595.
23. Anglin R, Yuan Y, Moayyedi P, et al. Risk of upper gastrointestinal bleeding with selective serotonin reuptake inhibitors with or without concurrent nonsteroidal anti-inflammatory use: a systematic review and meta-analysis. Am J Gastroenterol. 2014;109:811-819.
24. Mort JR, Aparasu RR, Baer RK, et al. Interaction between selective serotonin reuptake inhibitors and nonsteroidal anti-inflammatory drugs: review of the literature. Pharmacotherapy. 2006;26:1307-1313.
25. Loke YK, Trivedi AN, Singh S. Meta-analysis: gastrointestinal bleeding due to interaction between selective serotonin uptake inhibitors and non-steroidal anti-inflammatory drugs. Aliment Pharmacol Ther. 2008;27:31-40.
26. Venerito M, Wex T, Malfertheiner P. Nonsteroidal anti-inflammatory drug-induced gastroduodenal bleeding: risk factors and prevention strategies. Pharmaceuticals. 2010;3:2225-2237.
27. Boehringer S, Williams CD, Yawn BP, et al. Managing interactions with direct oral anticoagulants (DOACs). Pharmacist’s Letter. May 2016.
28. Johannessen SI, Landmark CJ. Antiepileptic drug interactions – principles and clinical implications. Curr Neuropharmacol. 2010;8:254-267.
29. Mohrien K, Oliphant CS, Self TH. Drug interactions with novel oral anticoagulants. Consultant. 2013;53:918-919. Available at: http://www.consultant360.com/articles/drug-interactions-novel-oral-anticoagulants. Accessed May 3, 2016.
30. Wiggins BS, Northup A, Johnson D, et al. Reduced anticoagulant effect of dabigatran in a patient receiving concomitant phenytoin. Pharmacotherapy. 2016;36:e5-e7.
31. Burnett AE, Mahan CE, Vazquez SR, et al. Guidance for the practical management of the direct oral anticoagulants (DOACs) in VTE treatment. J Thromb Thrombolysis. 2016;41:206-232.
32. Thompson PD, Clarkson P, Karas RH. Statin-associated myopathy. JAMA. 2003;289:1681-1690.
33. Hirota T, Leiri I. Drug-drug interactions that interfere with statin metabolism. Expert Opin Drug Metab Toxicol. 2015;11:1435-1447.
34. Study of the Effectiveness of Additional Reductions in Cholesterol and Homocysteine (SEARCH) Collaborative Group. Intensive lowering of LDL cholesterol wih 80 mg versus 20 mg simvastatin daily in 12,064 survivors of myocardial inffarction: a double-blind randomised trial. Lancet. 2010;376:1658-1669.
35. Jones PH, Davidson MH. Reporting rate of rhabdomyolysis with fenofibrate + statin versus gemfibrozil + any statin. Am J Cardiol. 2005;95:120-122.
36. Birmes P, Coppin D, Schmitt L, et al. Serotonin syndrome: a brief review. CMAJ. 2003;168:1439-1442.
37. Boyer EW, Shannon M. The serotonin syndrome. N Engl J Med. 2005;352:1112-1120.
38. US Food and Drug Administration. FDA Drug Safety Communication: FDA warns about several safety issues with opioid pain medicines; requires label changes. Available at: https://www.fda.gov/Drugs/DrugSafety/ucm489676.htm. Accessed June 15, 2016.
39. Sultana J, Spina E, Trifirò G. Antidepressant use in the elderly: the role of pharmacodynamics and pharmacokinetics in drug safety. Expert Opin Drug Metab Toxicol. 2015;11:883-892.
40. Sproule BA, Naranjo CA, Brenmer KE, et al. Selective serotonin reuptake inhibitors and CNS drug interactions. A critical review of the evidence. Clin Pharmacokinet. 1997;33:454-471.
41. Spina E, Santoro V, D’Arrigo C. Clinically relevant pharmacokinetic drug interactions with second-generation antidepressants: an update. Clin Ther. 2008;30:1206-1227.
PRACTICE RECOMMENDATIONS
› Recommend progesterone-containing intrauterine devices or long-acting progesterone injections for women using antiepileptic drugs. B
› Be aware that there is an increased risk of gastrointestinal bleeding when nonsteroidal anti-inflammatory drugs are used with selective serotonin reuptake inhibitors. A
› Do not prescribe novel oral anticoagulants for patients taking carbamazepine, phenytoin, or phenobarbital. B
› Choose fenofibrate over gemfibrozil when combining a fibrate and a statin. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Antibiotic interactions: Answers to 4 common questions
› Avoid preemptive warfarin dose reductions unless you are prescribing trimethoprim/sulfamethoxazole (TMP/SMX) or metronidazole. B
› Recommend a back-up contraceptive method to a woman who is taking a broad-spectrum antibiotic and low-dose OCs—especially if the woman is overweight. C
› Consider using the macrolide, clarithromycin, or the fluoroquinolone, ciprofloxacin, in patients taking medications that prolong QT interval or who are at higher risk for torsades de pointes (TdP). B
› Refrain from cautioning patients taking metronidazole against consuming alcohol. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Despite encouraging data that antibiotic prescribing is on the decline, patients are still prescribed antibiotics frequently, making these agents the 12th most frequently used drug class.1 At the same time, prescribers are caring for patients with increasingly complex drug regimens that provide fertile ground for drug interactions with these antibiotics. And, of course, lifestyle factors such as alcohol consumption are a consideration when any prescription is written.
As pharmacists, we find that certain questions about antibiotic prescribing and interactions come up with frequency. These questions often pertain to the use of warfarin, oral contraceptives, drugs that prolong the QT interval, and alcohol. But conflicting reports about issues such as monitoring international normalized ratio (INR) in patients taking warfarin and antibiotics, and whether (or which) antibiotics decrease the efficacy of oral contraceptives (OCs) can make decision-making challenging.
This review provides evidence-based answers to questions you may have. It also details some reliable sources of information you can consult (TABLE 12-7) when discussing treatment options with other members of the health care team.
1. Which antibiotics are preferable when a patient is taking warfarin, and are preemptive warfarin dose reductions advisable?
The simple answer is that agents with a lower likelihood of affecting the INR, such as penicillin G, clindamycin, and 1st- and 4th-generation cephalosporins, are a good place to start, and whether to preemptively reduce the warfarin dose hinges on the antibiotic being prescribed.
The more detailed answer. The fundamental mechanisms of interaction between warfarin and antibiotics are two-fold:8
- Antimicrobial agents disrupt gastrointestinal flora that synthesize vitamin K.
- Antimicrobials inhibit cytochrome p450 (CYP450) enzymes (primarily CYP2C9 and 3A4), which are responsible for the metabolism of warfarin.
The antibiotics most likely to interfere with warfarin are TMP/SMX, ciprofloxacin, levofloxacin, metronidazole, fluconazole, azithromycin, and clarithromycin (TABLE 2).9,10 Low-risk agents include clindamycin, cephalexin, and penicillin G. When prescribing an antibiotic for a patient taking warfarin, it is important not only to be aware of the agents that should be avoided, but also the agents that do not require more frequent monitoring of INR.
Preemptive warfarin dose reductions? Some physicians make preemptive warfarin dose reductions in an attempt to avoid supratherapeutic INRs in patients being prescribed antibiotics. But the evidence suggests that this step should be considered only in the presence of the antibiotics TMP/SMX and metronidazole.9,11
A 2008 study investigated the anticoagulation effects of a 10% to 20% preemptive warfarin dose reduction vs no dosing change in patients taking TMP/SMX or levofloxacin. The investigators found that the preemptive warfarin dose reduction (intervention) significantly decreased the number of supratherapeutic INR values above 4 when compared to controls (2 of 8 vs 8 of 9).12
In the dose-reduction group, no patients receiving TMP/SMX developed a subtherapeutic INR, whereas 40% (4 of 10 patients) who received levofloxacin developed a subtherapeutic INR.12 The authors of the study concluded that a prophylactic warfarin dose reduction of 10% to 20% is effective in maintaining therapeutic anticoagulation in patients receiving TMP/SMX. They added that while no change in warfarin dosing is necessary with levofloxacin, short-term INR follow-up is a prudent approach to prevent subtherapeutic INRs. Others recommend INR monitoring when antibiotic therapy is started and stopped and whenever the dose is changed.9
A 2010 retrospective, single-center, cohort study looked at patients who were taking metronidazole and warfarin. Researchers compared those who received a preemptive dose reduction of warfarin (mean reduction was 34.6% ± 13.4%) to those who did not and found a statistically significant mean difference in INR of 1.28 (P=.01).13
Almost half (46%) of the patients who did not receive a warfarin dose reduction had an INR >4, whereas none of the patients in the warfarin dose reduction group did (P=.05). Although this secondary outcome was not statistically significant (most likely due to the small sample population [N=20]), the implication is clinically significant. Two patients who reduced their dose had a subtherapeutic INR compared to none of the patients in the control group, which was also not a statistically significant difference.
The authors concluded that a 30% to 35% reduction in mean daily warfarin dose is effective in maintaining therapeutic anticoagulation in patients started on metronidazole.
Significant bleeding events. A retrospective cohort study of slightly more than 22,000 veterans who were prescribed warfarin for ≥30 uninterrupted days and given antibiotics with either a high or low risk for interaction with warfarin were studied for significant bleeding events for one month.10 Ninety-three significant bleeding events occurred in the high-risk group and 36 occurred in the low-risk group over the course of the study. The agent associated with the greatest increased risk of bleeding was TMP/SMX (hazard ratio [HR]=2.09; 95% CI, 1.45-3.02). Of note, metronidazole was not included in this study endpoint.
The study’s secondary endpoint of INR >4 found that 10% of patients taking metronidazole and 8% of patients taking TMP/SMX in addition to warfarin had INRs >4. Almost 10% (9.7%) of patients prescribed fluconazole had a peak INR value >6. Patients taking low-risk antibiotics (clindamycin or cephalexin) had no increased risk of bleeding. Monitoring INR within 3 to 14 days of starting patients on antibiotics was found to decrease the risk of serious bleeding events (HR=0.61; 95% CI, 0.42-0.88). More frequent INR monitoring by itself (without preemptive warfarin dose reductions) is appropriate for other antibiotics, including macrolides, tetracyclines, and some cephalosporins (2nd and 3rd generation).9
THE BOTTOM LINE When prescribing antibiotics for patients taking warfarin, try to choose agents with a lower likelihood of affecting INR such as penicillin G, clindamycin, and 1st- and 4th-generation cephalosporins. With these agents, there is no need for more frequent INR testing or preemptive reductions in warfarin dose. In patients for whom the use of TMP/SMX or metronidazole can’t be avoided, consider reducing the patient’s warfarin dose by 10% to 35% and rechecking the INR 5 days after starting the antibiotic.9,11,12 When prescribing agents such as fluoroquinolones, macrolides, and tetracyclines, do not reduce the patient’s warfarin dose preemptively and recheck INR 5 days after starting therapy.
2. Do antibiotics decrease the efficacy of oral contraceptives?
It’s unlikely, but antibiotics may reduce the efficacy of OCs.
There have been few, but well documented, reports of women using OCs who became pregnant after taking antimicrobials.14 It is recognized that rifampin, an inducer of enzymes that metabolize estrogens, decreases the efficacy of OCs.15 Ketoconazole’s interaction seems less well documented, but combining the agent with low-estrogen (low-dose) OCs warrants caution.16 What is not well understood is whether more common or broad-spectrum antibiotics also increase the risk of OC failure.
Three mechanisms have been proposed:16
- Antimicrobials affect hepatic enzyme induction, which increases metabolism of hormones.
- Broad-spectrum antibiotics reduce gut bacteria, which alters enterohepatic circulation and reduces plasma hormone concentrations.
- Antibiotics increase gastrointestinal motility, which decreases absorption (and reabsorption) of OCs.
A 2007 study found that when physicians and pharmacists were surveyed and asked if broad-spectrum antibiotics have a clinically significant interaction with OCs, 83% of physicians and 89% of pharmacists answered “Yes;”17 however, a large epidemiologic study performed in the United States showed no association between antibiotic use and OC failure.18
After this report, investigators in the Netherlands completed a similar cross-over analysis and found that there was a relationship between the use of antibiotics and breakthrough pregnancy in a population-based prescription database, but that the results didn’t hold for broad-spectrum antibiotics or in a sensitivity analysis.19 Pharmacokinetic studies are also conflicting, as some have shown an effect on serum hormone levels, while others have not.15,20-22
High- vs low-risk agents. Ciprofloxacin did not affect hormone levels in 2 studies.20,21 Rifampin and voriconazole may enhance systemic exposure to OCs.15,22 And erythromycin and azithromycin may interact with OCs, but the clinical significance of this interaction is still unknown.16
Short-courses of TMP/SMX are generally thought to be safe;16 a small study looked at cotrimoxazole 1 g twice daily in 9 women taking long-term OC steroids and found that short courses of the drug were unlikely to cause any adverse effects on contraceptive control.23 Tetracyclines and penicillins were the antibiotics most frequently involved in case reports of pregnancy from the United Kingdom (TABLE 32).16
It is hypothesized that some women may have a higher risk of OC failure than others due to how they metabolize ethinyl estradiol.24 Another hypothesis is that some women have gut flora that is more susceptible to the antibiotic being used. And still another possibility is that lower doses of hormones are being used in OCs than were studied for this interaction.15 Anything that decreases the concentration of these lower-dose OCs is concerning, especially in patients with a higher body mass index (BMI). The few pharmacokinetic studies that have been conducted show that it takes longer for OCs to reach a steady state in obese women and that they have a lower area under the curve (AUC) and maximum estrogen concentration than women with a normal BMI.25
THE BOTTOM LINE Because the degree of variability between patients is unknown and obesity rates are increasing, concern that low-dose OCs may lose efficacy when combined with antibiotics is warranted. While the absolute risk of breakthrough pregnancy seems small, the most conservative approach is to advise patients to use a back-up method of contraception during times of antibiotic use.
3. Which drugs prolong QT intervals?
Macrolides and fluoroquinolones are 2 classes of antibiotics associated with prolonged QT intervals, but other drugs and risk factors are important to consider, as well.
Physicians often receive phone calls from pharmacists warning about drug-drug interactions when they prescribe macrolides or fluoroquinolones for patients already taking medications known to prolong QT intervals or inhibit cytochrome P450 enzymes. Long QT syndrome increases the risk of TdP, a life-threatening arrhythmia. While TdP is rare, its severity warrants a discussion of risk factors and the likelihood of occurrence.
Two QT interval prolonging medications used together in healthy individuals does not warrant a change in therapy. TdP is most likely to occur when 2 or more QT interval prolonging medications are used in a patient who is already at high risk for arrhythmia because of risk factors such as prolonged QT interval at baseline, family history of prolonged QT intervals, female gender, age >60 years, electrolyte abnormalities (hypokalemia, hypomagnesemia, hypocalcemia), underlying comorbid diseases (eg, chronic heart failure, left ventricular hypertrophy, atrial fibrillation), hypertension, bradycardia, and genetic (ion channel) polymorphisms.26,27
Antiarrhythmics and antipsychotics are most commonly associated with drug-induced prolonged QT interval, with most case reports and research being linked to antiarrhythmics (TABLE 42).28 But macrolide and fluoroquinolone antibiotics also have been associated with TdP, although to a lesser extent. In a retrospective analysis of case reports of TdP involving macrolides, erythromycin was present (with or without other medications thought to prolong QT) in 53% of the cases and clarithromycin was involved in 36% of the reports.29
An analysis of 2 studies by the US Food and Drug Administration estimated an occurrence rate of serious cardiac arrhythmias of 46 to 85 per 100,000 users with cardiovascular disease, compared to 5 to 44 per 100,000 users without cardiovascular disease.30 And this may underestimate the actual incidence because spontaneous reporting of adverse effects declines the longer a drug is on the market. Ciprofloxacin is associated with less risk than levofloxacin and gatifloxacin (the latter of which is no longer available in the United States).26
A recent population-based study using data on over 10.6 million people from the Taiwan National Health Insurance Database examined the risk of cardiovascular death among patients using new-generation macrolides, fluoroquinolones, and β-lactam/β-lactamase inhibitors.31 The absolute risk of cardiovascular death per 1000 individuals was 0.06 for clarithromycin, 0.12 for ciprofloxacin, 0.13 for amoxicillin-clavulanate, 0.36 for azithromycin, 0.39 for levofloxacin, and 0.46 for moxifloxacin. The mean interval between first antibiotic use and the adverse cardiac event was <4 days. Not surprisingly, the highest risk was seen in patients with underlying cardiovascular disease.
Another population-based study, this time conducted in Hong Kong, evaluated the cardiovascular safety of clarithromycin compared to that of amoxicillin. Clarithromycin was found to increase the incidence of myocardial infarction, arrhythmia, and cardiac mortality in the short term, with the risk returning to baseline after treatment concluded.32 A binational cohort study of Danish and Swedish adults confirmed that fluoroquinolones (especially ciprofloxacin) do not increase the risk of a serious arrhythmia compared to penicillins.33
THE BOTTOM LINE For patients taking other QT interval prolonging medications or who are at a higher risk for TdP, consider using clarithromycin over erythromycin or azithromycin for a macrolide antibiotic or ciprofloxacin over levofloxacin or moxifloxacin if a fluoroquinolone is warranted. Using 2 drugs that may increase the QT interval is likely safe in the absence of certain risk factors.
4. Should patients avoid alcohol while taking metronidazole?
Probably not.
Warning patients against drinking alcohol while taking metronidazole has been a common practice for years. The mechanism for this theorized interaction was thought to be similar to the interaction between disulfiram and ethanol.34 Disulfiram inhibits hepatic aldehyde dehydrogenase (ALDH) when combined with alcohol, which leads to increased levels of acetaldehyde in the blood and symptoms of flushing, palpitations, nausea, vomiting, headache, and visual disturbances.35 However, multiple studies using rats have found that metronidazole does not inhibit ALDH or increase acetaldehyde concentrations like disulfiram does.34
A 2000 review article discussed 6 cases involving serious metronidazole-ethanol interactions. Ethanol alone was found to explain the reaction in 2 of the cases, and the remaining 4 could be linked to the use of other drugs or disease states.35 A 2002 Finnish study found no statistically significant differences in objective or subjective signs of a disulfiram-like interaction.34 When considering the symptoms associated with the interaction, it is important to remember that many of the symptoms can result from metronidazole therapy alone, regardless of whether other medications or alcohol are used.35
THE BOTTOM LINE Researchers have failed to identify a clinically significant interaction between metronidazole and alcohol. Avoiding alcohol while taking metronidazole does not appear to be necessary.
CORRESPONDENCE
Mary Onysko, PharmD, BCPS, University of Wyoming, School of Pharmacy Health Sciences Center, Room 292, 1000 E. University Avenue, Laramie, WY 82071; monysko@uwyo.edu.
1. Kantor ED, Rehm CD, Haas JS, et al. Trends in prescription drug use among adults in the United States from 1999-2012. JAMA. 2015;314:1818-1831.
2. Lexicomp Online. Clinical Drug Information. Available at: http://www.wolterskluwercdi.com/lexicomp-online/. Accessed December 8, 2015.
3. GlobalRPh: The Clinician’s Ultimate Reference. Available at: http://www.globalrph.com/. Accessed December 8, 2015.
4. Medscape Apps. Available at: http://www.medscape.com/public/applanding. Accessed December 8, 2015.
5. Micromedex Solutions. Academic Institutions. Available at: http://micromedex.com/academic. Accessed December 8, 2015.
6. Patel A. Clinical Pharmacology Mobile-A mobile web app compatible on all smart phones [review] May 31, 2010. Available at: http://www.imedicalapps.com/2010/05/clinical-pharmocology-app-review/. Accessed December 8, 2015.
7. Epocrates. Available at: http://www.epocrates.com/. Accessed December 8, 2015.
8. Baillargeon J, Holmes HM, Lin Y, et al. Concurrent use of warfarin and antibiotics and the risk of bleeding in older adults. Am J Med. 2012;125:183-189.
9. PL Detail-Document #280806. Antimicrobial drug interactions with warfarin. Pharmacist’s Letter/Prescriber’s Letter. August 2012.
10. Lane M, Zeringue A, McDonald J. Serious bleeding events due to warfarin and antibiotic co-prescription in a cohort of veterans. Am J Med. 2014;127:657-663.e2.
11. Hale SF, Lesar TS. Interaction of vitamin K antagonists and trimethoprim-sulfamethoxazole: ignore at your patient’s risk. Drug Metab Drug Interact. 2014;29:53-60.
12. Ahmed A, Stephens JC, Kaus CA, et al. Impact of preemptive warfarin dose reduction on anticoagulation after initiation of trimethoprim-sulfamethoxazole or levofloxacin. J Thromb Thrombolysis. 2008;26:44-48.
13. Holt RK, Anderson EA, Cantrell MA, et al. Preemptive dose reduction of warfarin in patients initiating metronidazole. Drug Metabol Drug Interact. 2010;25:35-39.
14. Hughes BR, Cunliffe WJ. Interactions between the oral contraceptive pill and antibiotics. Br J Dermatol. 1990;122:717-718.
15. Bolt HM. Interactions between clinically used drugs and oral contraceptives. Environ Health Perspect. 1994;102:35-38.
16. Aronson JK. Meyler’s Side Effects of Drugs. 16th ed. The International Encyclopedia of Adverse Drug Reactions and Interactions. Amsterdam, Netherlands: Elsevier; 2016. Available at: http://ac.els-cdn.com/B978044453717101009X/3-s2.0-B978044453717101009X-main.pdf?_tid=b33f6564-9deb-11e5-a8f0-00000aab0f01&acdnat=1449607315_83f5068fc5105226fcc6d7279c083516. Accessed December 8, 2015.
17. Masters KP, Carr BM. Survey of pharmacists and physicians on drug interactions between combined oral contraceptives and broad-spectrum antibiotics. Pharm Pract (Granada). 2009;7:139-144.
18. Toh S, Mitchell AA, Anderka M, et al; National Birth Defects Prevention Study. Antibiotics and oral contraceptive failure—a case-crossover study. Contraception. 2011;83:418-425.
19. Koopmans PC, Bos JH, de Jong van den Berg LT. Are antibiotics related to oral combination contraceptive failures in the Netherlands? A case-crossover study. Pharmacoepidemiol Drug Saf. 2012;21:865-871.
20. Archer JS, Archer DF. Oral contraceptive efficacy and antibiotic interaction: A myth debunked. J Am Acad Dermatol. 2002;46:917–923.
21. Scholten PC, Droppert RM, Zwinkels MGJ, et al. No interaction between ciprofloxacin and an oral contraceptive. Antimicrob Agents Chemother. 1998;42:3266-3268.
22. Andrews E, Damle BD, Fang A, et al. Pharmacokinetics and tolerability of voriconazole and a combination oral contraceptive co-administered in healthy female subjects. Br J Clin Pharmacol. 2008;65:531-539.
23. Grimmer SF, Allen WL, Back DJ, et al. The effect of cotrimoxazole on oral contraceptive steroids in women. Contraception. 1983;28:53-59.
24. Dickinson BD, Altman RD, Nielsen NH, et al; Council on Scientific Affairs, American Medical Association. Drug interactions between oral contraceptives and antibiotics. Obstet Gynecol. 2001;98:853-860.
25. Edelman AB, Cherala G, Stanczyk FZ. Metabolism and pharmacokinetics of contraceptive steroids in obese women: a review. Contraception. 2010;82:314-323.
26. Owens RC Jr, Ambrose PG. Torsades de pointes associated with fluoroquinolones. Pharmacotherapy. 2002;22:663-668.
27. Letsas KP, Efremidis M, Kounas SP, et al. Clinical characteristics of patients with drug-induced QT interval prolongation and torsade de pointes: identification of risk factors. Clin Res Cardiol. 2009;98:208-212.
28. Yap YG, Camm AJ. Drug induced QT prolongation and torsades de pointes. Heart. 2003;89:1363-1372.
29. Shaffer D, Singer S, Korvick J, et al. Concomitant risk factors in reports of torsades de pointes associated with macrolide use: review of the United States Food and Drug Administration adverse event reporting system. Clin Infect Dis. 2002;35:197-200.
30. FDA Briefing Document. Joint Meeting of the Antimicrobial Drugs Advisory Committee and the Drug Safety and Risk Management Advisory Committee. November 5, 2015. Available at: http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/Anti-InfectiveDrugsAdvisoryCommittee/UCM467383.pdf. Accessed June 11, 2016.
31. Chou HW, Wang JL, Chang CH, et al. Risks of cardiac arrhythmia and mortality among patients using new-generation macrolides, fluoroquinolones, and β-lactam/β-lactamase inhibitors: a Taiwanese nationwide study. Clin Infect Dis. 2015;60:566-577.
32. Wong AY, Root A, Douglas IJ, et al. Cardiovascular outcomes associated with use of clarithromycin: population based study. BMJ. 2016;352:h6926.
33. Inghammar M, Svanström H, Melbye M, et al. Oral fluoroquinolone use and serious arrhythmia: bi-national cohort study. BMJ. 2016;352:i843.
34. Visapää JP, Tillonen JS, Kaihovaara PS, et al. Lack of disulfiram-like reaction with metronidazole and ethanol. Ann Pharmacother. 2002;36:971-974. 35. Fjeld H, Raknes G. Is combining metronidazole and alcohol really hazardous? Tidsskr Nor Laegeforen. 2014;134:1661-1663.
› Avoid preemptive warfarin dose reductions unless you are prescribing trimethoprim/sulfamethoxazole (TMP/SMX) or metronidazole. B
› Recommend a back-up contraceptive method to a woman who is taking a broad-spectrum antibiotic and low-dose OCs—especially if the woman is overweight. C
› Consider using the macrolide, clarithromycin, or the fluoroquinolone, ciprofloxacin, in patients taking medications that prolong QT interval or who are at higher risk for torsades de pointes (TdP). B
› Refrain from cautioning patients taking metronidazole against consuming alcohol. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Despite encouraging data that antibiotic prescribing is on the decline, patients are still prescribed antibiotics frequently, making these agents the 12th most frequently used drug class.1 At the same time, prescribers are caring for patients with increasingly complex drug regimens that provide fertile ground for drug interactions with these antibiotics. And, of course, lifestyle factors such as alcohol consumption are a consideration when any prescription is written.
As pharmacists, we find that certain questions about antibiotic prescribing and interactions come up with frequency. These questions often pertain to the use of warfarin, oral contraceptives, drugs that prolong the QT interval, and alcohol. But conflicting reports about issues such as monitoring international normalized ratio (INR) in patients taking warfarin and antibiotics, and whether (or which) antibiotics decrease the efficacy of oral contraceptives (OCs) can make decision-making challenging.
This review provides evidence-based answers to questions you may have. It also details some reliable sources of information you can consult (TABLE 12-7) when discussing treatment options with other members of the health care team.
1. Which antibiotics are preferable when a patient is taking warfarin, and are preemptive warfarin dose reductions advisable?
The simple answer is that agents with a lower likelihood of affecting the INR, such as penicillin G, clindamycin, and 1st- and 4th-generation cephalosporins, are a good place to start, and whether to preemptively reduce the warfarin dose hinges on the antibiotic being prescribed.
The more detailed answer. The fundamental mechanisms of interaction between warfarin and antibiotics are two-fold:8
- Antimicrobial agents disrupt gastrointestinal flora that synthesize vitamin K.
- Antimicrobials inhibit cytochrome p450 (CYP450) enzymes (primarily CYP2C9 and 3A4), which are responsible for the metabolism of warfarin.
The antibiotics most likely to interfere with warfarin are TMP/SMX, ciprofloxacin, levofloxacin, metronidazole, fluconazole, azithromycin, and clarithromycin (TABLE 2).9,10 Low-risk agents include clindamycin, cephalexin, and penicillin G. When prescribing an antibiotic for a patient taking warfarin, it is important not only to be aware of the agents that should be avoided, but also the agents that do not require more frequent monitoring of INR.
Preemptive warfarin dose reductions? Some physicians make preemptive warfarin dose reductions in an attempt to avoid supratherapeutic INRs in patients being prescribed antibiotics. But the evidence suggests that this step should be considered only in the presence of the antibiotics TMP/SMX and metronidazole.9,11
A 2008 study investigated the anticoagulation effects of a 10% to 20% preemptive warfarin dose reduction vs no dosing change in patients taking TMP/SMX or levofloxacin. The investigators found that the preemptive warfarin dose reduction (intervention) significantly decreased the number of supratherapeutic INR values above 4 when compared to controls (2 of 8 vs 8 of 9).12
In the dose-reduction group, no patients receiving TMP/SMX developed a subtherapeutic INR, whereas 40% (4 of 10 patients) who received levofloxacin developed a subtherapeutic INR.12 The authors of the study concluded that a prophylactic warfarin dose reduction of 10% to 20% is effective in maintaining therapeutic anticoagulation in patients receiving TMP/SMX. They added that while no change in warfarin dosing is necessary with levofloxacin, short-term INR follow-up is a prudent approach to prevent subtherapeutic INRs. Others recommend INR monitoring when antibiotic therapy is started and stopped and whenever the dose is changed.9
A 2010 retrospective, single-center, cohort study looked at patients who were taking metronidazole and warfarin. Researchers compared those who received a preemptive dose reduction of warfarin (mean reduction was 34.6% ± 13.4%) to those who did not and found a statistically significant mean difference in INR of 1.28 (P=.01).13
Almost half (46%) of the patients who did not receive a warfarin dose reduction had an INR >4, whereas none of the patients in the warfarin dose reduction group did (P=.05). Although this secondary outcome was not statistically significant (most likely due to the small sample population [N=20]), the implication is clinically significant. Two patients who reduced their dose had a subtherapeutic INR compared to none of the patients in the control group, which was also not a statistically significant difference.
The authors concluded that a 30% to 35% reduction in mean daily warfarin dose is effective in maintaining therapeutic anticoagulation in patients started on metronidazole.
Significant bleeding events. A retrospective cohort study of slightly more than 22,000 veterans who were prescribed warfarin for ≥30 uninterrupted days and given antibiotics with either a high or low risk for interaction with warfarin were studied for significant bleeding events for one month.10 Ninety-three significant bleeding events occurred in the high-risk group and 36 occurred in the low-risk group over the course of the study. The agent associated with the greatest increased risk of bleeding was TMP/SMX (hazard ratio [HR]=2.09; 95% CI, 1.45-3.02). Of note, metronidazole was not included in this study endpoint.
The study’s secondary endpoint of INR >4 found that 10% of patients taking metronidazole and 8% of patients taking TMP/SMX in addition to warfarin had INRs >4. Almost 10% (9.7%) of patients prescribed fluconazole had a peak INR value >6. Patients taking low-risk antibiotics (clindamycin or cephalexin) had no increased risk of bleeding. Monitoring INR within 3 to 14 days of starting patients on antibiotics was found to decrease the risk of serious bleeding events (HR=0.61; 95% CI, 0.42-0.88). More frequent INR monitoring by itself (without preemptive warfarin dose reductions) is appropriate for other antibiotics, including macrolides, tetracyclines, and some cephalosporins (2nd and 3rd generation).9
THE BOTTOM LINE When prescribing antibiotics for patients taking warfarin, try to choose agents with a lower likelihood of affecting INR such as penicillin G, clindamycin, and 1st- and 4th-generation cephalosporins. With these agents, there is no need for more frequent INR testing or preemptive reductions in warfarin dose. In patients for whom the use of TMP/SMX or metronidazole can’t be avoided, consider reducing the patient’s warfarin dose by 10% to 35% and rechecking the INR 5 days after starting the antibiotic.9,11,12 When prescribing agents such as fluoroquinolones, macrolides, and tetracyclines, do not reduce the patient’s warfarin dose preemptively and recheck INR 5 days after starting therapy.
2. Do antibiotics decrease the efficacy of oral contraceptives?
It’s unlikely, but antibiotics may reduce the efficacy of OCs.
There have been few, but well documented, reports of women using OCs who became pregnant after taking antimicrobials.14 It is recognized that rifampin, an inducer of enzymes that metabolize estrogens, decreases the efficacy of OCs.15 Ketoconazole’s interaction seems less well documented, but combining the agent with low-estrogen (low-dose) OCs warrants caution.16 What is not well understood is whether more common or broad-spectrum antibiotics also increase the risk of OC failure.
Three mechanisms have been proposed:16
- Antimicrobials affect hepatic enzyme induction, which increases metabolism of hormones.
- Broad-spectrum antibiotics reduce gut bacteria, which alters enterohepatic circulation and reduces plasma hormone concentrations.
- Antibiotics increase gastrointestinal motility, which decreases absorption (and reabsorption) of OCs.
A 2007 study found that when physicians and pharmacists were surveyed and asked if broad-spectrum antibiotics have a clinically significant interaction with OCs, 83% of physicians and 89% of pharmacists answered “Yes;”17 however, a large epidemiologic study performed in the United States showed no association between antibiotic use and OC failure.18
After this report, investigators in the Netherlands completed a similar cross-over analysis and found that there was a relationship between the use of antibiotics and breakthrough pregnancy in a population-based prescription database, but that the results didn’t hold for broad-spectrum antibiotics or in a sensitivity analysis.19 Pharmacokinetic studies are also conflicting, as some have shown an effect on serum hormone levels, while others have not.15,20-22
High- vs low-risk agents. Ciprofloxacin did not affect hormone levels in 2 studies.20,21 Rifampin and voriconazole may enhance systemic exposure to OCs.15,22 And erythromycin and azithromycin may interact with OCs, but the clinical significance of this interaction is still unknown.16
Short-courses of TMP/SMX are generally thought to be safe;16 a small study looked at cotrimoxazole 1 g twice daily in 9 women taking long-term OC steroids and found that short courses of the drug were unlikely to cause any adverse effects on contraceptive control.23 Tetracyclines and penicillins were the antibiotics most frequently involved in case reports of pregnancy from the United Kingdom (TABLE 32).16
It is hypothesized that some women may have a higher risk of OC failure than others due to how they metabolize ethinyl estradiol.24 Another hypothesis is that some women have gut flora that is more susceptible to the antibiotic being used. And still another possibility is that lower doses of hormones are being used in OCs than were studied for this interaction.15 Anything that decreases the concentration of these lower-dose OCs is concerning, especially in patients with a higher body mass index (BMI). The few pharmacokinetic studies that have been conducted show that it takes longer for OCs to reach a steady state in obese women and that they have a lower area under the curve (AUC) and maximum estrogen concentration than women with a normal BMI.25
THE BOTTOM LINE Because the degree of variability between patients is unknown and obesity rates are increasing, concern that low-dose OCs may lose efficacy when combined with antibiotics is warranted. While the absolute risk of breakthrough pregnancy seems small, the most conservative approach is to advise patients to use a back-up method of contraception during times of antibiotic use.
3. Which drugs prolong QT intervals?
Macrolides and fluoroquinolones are 2 classes of antibiotics associated with prolonged QT intervals, but other drugs and risk factors are important to consider, as well.
Physicians often receive phone calls from pharmacists warning about drug-drug interactions when they prescribe macrolides or fluoroquinolones for patients already taking medications known to prolong QT intervals or inhibit cytochrome P450 enzymes. Long QT syndrome increases the risk of TdP, a life-threatening arrhythmia. While TdP is rare, its severity warrants a discussion of risk factors and the likelihood of occurrence.
Two QT interval prolonging medications used together in healthy individuals does not warrant a change in therapy. TdP is most likely to occur when 2 or more QT interval prolonging medications are used in a patient who is already at high risk for arrhythmia because of risk factors such as prolonged QT interval at baseline, family history of prolonged QT intervals, female gender, age >60 years, electrolyte abnormalities (hypokalemia, hypomagnesemia, hypocalcemia), underlying comorbid diseases (eg, chronic heart failure, left ventricular hypertrophy, atrial fibrillation), hypertension, bradycardia, and genetic (ion channel) polymorphisms.26,27
Antiarrhythmics and antipsychotics are most commonly associated with drug-induced prolonged QT interval, with most case reports and research being linked to antiarrhythmics (TABLE 42).28 But macrolide and fluoroquinolone antibiotics also have been associated with TdP, although to a lesser extent. In a retrospective analysis of case reports of TdP involving macrolides, erythromycin was present (with or without other medications thought to prolong QT) in 53% of the cases and clarithromycin was involved in 36% of the reports.29
An analysis of 2 studies by the US Food and Drug Administration estimated an occurrence rate of serious cardiac arrhythmias of 46 to 85 per 100,000 users with cardiovascular disease, compared to 5 to 44 per 100,000 users without cardiovascular disease.30 And this may underestimate the actual incidence because spontaneous reporting of adverse effects declines the longer a drug is on the market. Ciprofloxacin is associated with less risk than levofloxacin and gatifloxacin (the latter of which is no longer available in the United States).26
A recent population-based study using data on over 10.6 million people from the Taiwan National Health Insurance Database examined the risk of cardiovascular death among patients using new-generation macrolides, fluoroquinolones, and β-lactam/β-lactamase inhibitors.31 The absolute risk of cardiovascular death per 1000 individuals was 0.06 for clarithromycin, 0.12 for ciprofloxacin, 0.13 for amoxicillin-clavulanate, 0.36 for azithromycin, 0.39 for levofloxacin, and 0.46 for moxifloxacin. The mean interval between first antibiotic use and the adverse cardiac event was <4 days. Not surprisingly, the highest risk was seen in patients with underlying cardiovascular disease.
Another population-based study, this time conducted in Hong Kong, evaluated the cardiovascular safety of clarithromycin compared to that of amoxicillin. Clarithromycin was found to increase the incidence of myocardial infarction, arrhythmia, and cardiac mortality in the short term, with the risk returning to baseline after treatment concluded.32 A binational cohort study of Danish and Swedish adults confirmed that fluoroquinolones (especially ciprofloxacin) do not increase the risk of a serious arrhythmia compared to penicillins.33
THE BOTTOM LINE For patients taking other QT interval prolonging medications or who are at a higher risk for TdP, consider using clarithromycin over erythromycin or azithromycin for a macrolide antibiotic or ciprofloxacin over levofloxacin or moxifloxacin if a fluoroquinolone is warranted. Using 2 drugs that may increase the QT interval is likely safe in the absence of certain risk factors.
4. Should patients avoid alcohol while taking metronidazole?
Probably not.
Warning patients against drinking alcohol while taking metronidazole has been a common practice for years. The mechanism for this theorized interaction was thought to be similar to the interaction between disulfiram and ethanol.34 Disulfiram inhibits hepatic aldehyde dehydrogenase (ALDH) when combined with alcohol, which leads to increased levels of acetaldehyde in the blood and symptoms of flushing, palpitations, nausea, vomiting, headache, and visual disturbances.35 However, multiple studies using rats have found that metronidazole does not inhibit ALDH or increase acetaldehyde concentrations like disulfiram does.34
A 2000 review article discussed 6 cases involving serious metronidazole-ethanol interactions. Ethanol alone was found to explain the reaction in 2 of the cases, and the remaining 4 could be linked to the use of other drugs or disease states.35 A 2002 Finnish study found no statistically significant differences in objective or subjective signs of a disulfiram-like interaction.34 When considering the symptoms associated with the interaction, it is important to remember that many of the symptoms can result from metronidazole therapy alone, regardless of whether other medications or alcohol are used.35
THE BOTTOM LINE Researchers have failed to identify a clinically significant interaction between metronidazole and alcohol. Avoiding alcohol while taking metronidazole does not appear to be necessary.
CORRESPONDENCE
Mary Onysko, PharmD, BCPS, University of Wyoming, School of Pharmacy Health Sciences Center, Room 292, 1000 E. University Avenue, Laramie, WY 82071; monysko@uwyo.edu.
› Avoid preemptive warfarin dose reductions unless you are prescribing trimethoprim/sulfamethoxazole (TMP/SMX) or metronidazole. B
› Recommend a back-up contraceptive method to a woman who is taking a broad-spectrum antibiotic and low-dose OCs—especially if the woman is overweight. C
› Consider using the macrolide, clarithromycin, or the fluoroquinolone, ciprofloxacin, in patients taking medications that prolong QT interval or who are at higher risk for torsades de pointes (TdP). B
› Refrain from cautioning patients taking metronidazole against consuming alcohol. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Despite encouraging data that antibiotic prescribing is on the decline, patients are still prescribed antibiotics frequently, making these agents the 12th most frequently used drug class.1 At the same time, prescribers are caring for patients with increasingly complex drug regimens that provide fertile ground for drug interactions with these antibiotics. And, of course, lifestyle factors such as alcohol consumption are a consideration when any prescription is written.
As pharmacists, we find that certain questions about antibiotic prescribing and interactions come up with frequency. These questions often pertain to the use of warfarin, oral contraceptives, drugs that prolong the QT interval, and alcohol. But conflicting reports about issues such as monitoring international normalized ratio (INR) in patients taking warfarin and antibiotics, and whether (or which) antibiotics decrease the efficacy of oral contraceptives (OCs) can make decision-making challenging.
This review provides evidence-based answers to questions you may have. It also details some reliable sources of information you can consult (TABLE 12-7) when discussing treatment options with other members of the health care team.
1. Which antibiotics are preferable when a patient is taking warfarin, and are preemptive warfarin dose reductions advisable?
The simple answer is that agents with a lower likelihood of affecting the INR, such as penicillin G, clindamycin, and 1st- and 4th-generation cephalosporins, are a good place to start, and whether to preemptively reduce the warfarin dose hinges on the antibiotic being prescribed.
The more detailed answer. The fundamental mechanisms of interaction between warfarin and antibiotics are two-fold:8
- Antimicrobial agents disrupt gastrointestinal flora that synthesize vitamin K.
- Antimicrobials inhibit cytochrome p450 (CYP450) enzymes (primarily CYP2C9 and 3A4), which are responsible for the metabolism of warfarin.
The antibiotics most likely to interfere with warfarin are TMP/SMX, ciprofloxacin, levofloxacin, metronidazole, fluconazole, azithromycin, and clarithromycin (TABLE 2).9,10 Low-risk agents include clindamycin, cephalexin, and penicillin G. When prescribing an antibiotic for a patient taking warfarin, it is important not only to be aware of the agents that should be avoided, but also the agents that do not require more frequent monitoring of INR.
Preemptive warfarin dose reductions? Some physicians make preemptive warfarin dose reductions in an attempt to avoid supratherapeutic INRs in patients being prescribed antibiotics. But the evidence suggests that this step should be considered only in the presence of the antibiotics TMP/SMX and metronidazole.9,11
A 2008 study investigated the anticoagulation effects of a 10% to 20% preemptive warfarin dose reduction vs no dosing change in patients taking TMP/SMX or levofloxacin. The investigators found that the preemptive warfarin dose reduction (intervention) significantly decreased the number of supratherapeutic INR values above 4 when compared to controls (2 of 8 vs 8 of 9).12
In the dose-reduction group, no patients receiving TMP/SMX developed a subtherapeutic INR, whereas 40% (4 of 10 patients) who received levofloxacin developed a subtherapeutic INR.12 The authors of the study concluded that a prophylactic warfarin dose reduction of 10% to 20% is effective in maintaining therapeutic anticoagulation in patients receiving TMP/SMX. They added that while no change in warfarin dosing is necessary with levofloxacin, short-term INR follow-up is a prudent approach to prevent subtherapeutic INRs. Others recommend INR monitoring when antibiotic therapy is started and stopped and whenever the dose is changed.9
A 2010 retrospective, single-center, cohort study looked at patients who were taking metronidazole and warfarin. Researchers compared those who received a preemptive dose reduction of warfarin (mean reduction was 34.6% ± 13.4%) to those who did not and found a statistically significant mean difference in INR of 1.28 (P=.01).13
Almost half (46%) of the patients who did not receive a warfarin dose reduction had an INR >4, whereas none of the patients in the warfarin dose reduction group did (P=.05). Although this secondary outcome was not statistically significant (most likely due to the small sample population [N=20]), the implication is clinically significant. Two patients who reduced their dose had a subtherapeutic INR compared to none of the patients in the control group, which was also not a statistically significant difference.
The authors concluded that a 30% to 35% reduction in mean daily warfarin dose is effective in maintaining therapeutic anticoagulation in patients started on metronidazole.
Significant bleeding events. A retrospective cohort study of slightly more than 22,000 veterans who were prescribed warfarin for ≥30 uninterrupted days and given antibiotics with either a high or low risk for interaction with warfarin were studied for significant bleeding events for one month.10 Ninety-three significant bleeding events occurred in the high-risk group and 36 occurred in the low-risk group over the course of the study. The agent associated with the greatest increased risk of bleeding was TMP/SMX (hazard ratio [HR]=2.09; 95% CI, 1.45-3.02). Of note, metronidazole was not included in this study endpoint.
The study’s secondary endpoint of INR >4 found that 10% of patients taking metronidazole and 8% of patients taking TMP/SMX in addition to warfarin had INRs >4. Almost 10% (9.7%) of patients prescribed fluconazole had a peak INR value >6. Patients taking low-risk antibiotics (clindamycin or cephalexin) had no increased risk of bleeding. Monitoring INR within 3 to 14 days of starting patients on antibiotics was found to decrease the risk of serious bleeding events (HR=0.61; 95% CI, 0.42-0.88). More frequent INR monitoring by itself (without preemptive warfarin dose reductions) is appropriate for other antibiotics, including macrolides, tetracyclines, and some cephalosporins (2nd and 3rd generation).9
THE BOTTOM LINE When prescribing antibiotics for patients taking warfarin, try to choose agents with a lower likelihood of affecting INR such as penicillin G, clindamycin, and 1st- and 4th-generation cephalosporins. With these agents, there is no need for more frequent INR testing or preemptive reductions in warfarin dose. In patients for whom the use of TMP/SMX or metronidazole can’t be avoided, consider reducing the patient’s warfarin dose by 10% to 35% and rechecking the INR 5 days after starting the antibiotic.9,11,12 When prescribing agents such as fluoroquinolones, macrolides, and tetracyclines, do not reduce the patient’s warfarin dose preemptively and recheck INR 5 days after starting therapy.
2. Do antibiotics decrease the efficacy of oral contraceptives?
It’s unlikely, but antibiotics may reduce the efficacy of OCs.
There have been few, but well documented, reports of women using OCs who became pregnant after taking antimicrobials.14 It is recognized that rifampin, an inducer of enzymes that metabolize estrogens, decreases the efficacy of OCs.15 Ketoconazole’s interaction seems less well documented, but combining the agent with low-estrogen (low-dose) OCs warrants caution.16 What is not well understood is whether more common or broad-spectrum antibiotics also increase the risk of OC failure.
Three mechanisms have been proposed:16
- Antimicrobials affect hepatic enzyme induction, which increases metabolism of hormones.
- Broad-spectrum antibiotics reduce gut bacteria, which alters enterohepatic circulation and reduces plasma hormone concentrations.
- Antibiotics increase gastrointestinal motility, which decreases absorption (and reabsorption) of OCs.
A 2007 study found that when physicians and pharmacists were surveyed and asked if broad-spectrum antibiotics have a clinically significant interaction with OCs, 83% of physicians and 89% of pharmacists answered “Yes;”17 however, a large epidemiologic study performed in the United States showed no association between antibiotic use and OC failure.18
After this report, investigators in the Netherlands completed a similar cross-over analysis and found that there was a relationship between the use of antibiotics and breakthrough pregnancy in a population-based prescription database, but that the results didn’t hold for broad-spectrum antibiotics or in a sensitivity analysis.19 Pharmacokinetic studies are also conflicting, as some have shown an effect on serum hormone levels, while others have not.15,20-22
High- vs low-risk agents. Ciprofloxacin did not affect hormone levels in 2 studies.20,21 Rifampin and voriconazole may enhance systemic exposure to OCs.15,22 And erythromycin and azithromycin may interact with OCs, but the clinical significance of this interaction is still unknown.16
Short-courses of TMP/SMX are generally thought to be safe;16 a small study looked at cotrimoxazole 1 g twice daily in 9 women taking long-term OC steroids and found that short courses of the drug were unlikely to cause any adverse effects on contraceptive control.23 Tetracyclines and penicillins were the antibiotics most frequently involved in case reports of pregnancy from the United Kingdom (TABLE 32).16
It is hypothesized that some women may have a higher risk of OC failure than others due to how they metabolize ethinyl estradiol.24 Another hypothesis is that some women have gut flora that is more susceptible to the antibiotic being used. And still another possibility is that lower doses of hormones are being used in OCs than were studied for this interaction.15 Anything that decreases the concentration of these lower-dose OCs is concerning, especially in patients with a higher body mass index (BMI). The few pharmacokinetic studies that have been conducted show that it takes longer for OCs to reach a steady state in obese women and that they have a lower area under the curve (AUC) and maximum estrogen concentration than women with a normal BMI.25
THE BOTTOM LINE Because the degree of variability between patients is unknown and obesity rates are increasing, concern that low-dose OCs may lose efficacy when combined with antibiotics is warranted. While the absolute risk of breakthrough pregnancy seems small, the most conservative approach is to advise patients to use a back-up method of contraception during times of antibiotic use.
3. Which drugs prolong QT intervals?
Macrolides and fluoroquinolones are 2 classes of antibiotics associated with prolonged QT intervals, but other drugs and risk factors are important to consider, as well.
Physicians often receive phone calls from pharmacists warning about drug-drug interactions when they prescribe macrolides or fluoroquinolones for patients already taking medications known to prolong QT intervals or inhibit cytochrome P450 enzymes. Long QT syndrome increases the risk of TdP, a life-threatening arrhythmia. While TdP is rare, its severity warrants a discussion of risk factors and the likelihood of occurrence.
Two QT interval prolonging medications used together in healthy individuals does not warrant a change in therapy. TdP is most likely to occur when 2 or more QT interval prolonging medications are used in a patient who is already at high risk for arrhythmia because of risk factors such as prolonged QT interval at baseline, family history of prolonged QT intervals, female gender, age >60 years, electrolyte abnormalities (hypokalemia, hypomagnesemia, hypocalcemia), underlying comorbid diseases (eg, chronic heart failure, left ventricular hypertrophy, atrial fibrillation), hypertension, bradycardia, and genetic (ion channel) polymorphisms.26,27
Antiarrhythmics and antipsychotics are most commonly associated with drug-induced prolonged QT interval, with most case reports and research being linked to antiarrhythmics (TABLE 42).28 But macrolide and fluoroquinolone antibiotics also have been associated with TdP, although to a lesser extent. In a retrospective analysis of case reports of TdP involving macrolides, erythromycin was present (with or without other medications thought to prolong QT) in 53% of the cases and clarithromycin was involved in 36% of the reports.29
An analysis of 2 studies by the US Food and Drug Administration estimated an occurrence rate of serious cardiac arrhythmias of 46 to 85 per 100,000 users with cardiovascular disease, compared to 5 to 44 per 100,000 users without cardiovascular disease.30 And this may underestimate the actual incidence because spontaneous reporting of adverse effects declines the longer a drug is on the market. Ciprofloxacin is associated with less risk than levofloxacin and gatifloxacin (the latter of which is no longer available in the United States).26
A recent population-based study using data on over 10.6 million people from the Taiwan National Health Insurance Database examined the risk of cardiovascular death among patients using new-generation macrolides, fluoroquinolones, and β-lactam/β-lactamase inhibitors.31 The absolute risk of cardiovascular death per 1000 individuals was 0.06 for clarithromycin, 0.12 for ciprofloxacin, 0.13 for amoxicillin-clavulanate, 0.36 for azithromycin, 0.39 for levofloxacin, and 0.46 for moxifloxacin. The mean interval between first antibiotic use and the adverse cardiac event was <4 days. Not surprisingly, the highest risk was seen in patients with underlying cardiovascular disease.
Another population-based study, this time conducted in Hong Kong, evaluated the cardiovascular safety of clarithromycin compared to that of amoxicillin. Clarithromycin was found to increase the incidence of myocardial infarction, arrhythmia, and cardiac mortality in the short term, with the risk returning to baseline after treatment concluded.32 A binational cohort study of Danish and Swedish adults confirmed that fluoroquinolones (especially ciprofloxacin) do not increase the risk of a serious arrhythmia compared to penicillins.33
THE BOTTOM LINE For patients taking other QT interval prolonging medications or who are at a higher risk for TdP, consider using clarithromycin over erythromycin or azithromycin for a macrolide antibiotic or ciprofloxacin over levofloxacin or moxifloxacin if a fluoroquinolone is warranted. Using 2 drugs that may increase the QT interval is likely safe in the absence of certain risk factors.
4. Should patients avoid alcohol while taking metronidazole?
Probably not.
Warning patients against drinking alcohol while taking metronidazole has been a common practice for years. The mechanism for this theorized interaction was thought to be similar to the interaction between disulfiram and ethanol.34 Disulfiram inhibits hepatic aldehyde dehydrogenase (ALDH) when combined with alcohol, which leads to increased levels of acetaldehyde in the blood and symptoms of flushing, palpitations, nausea, vomiting, headache, and visual disturbances.35 However, multiple studies using rats have found that metronidazole does not inhibit ALDH or increase acetaldehyde concentrations like disulfiram does.34
A 2000 review article discussed 6 cases involving serious metronidazole-ethanol interactions. Ethanol alone was found to explain the reaction in 2 of the cases, and the remaining 4 could be linked to the use of other drugs or disease states.35 A 2002 Finnish study found no statistically significant differences in objective or subjective signs of a disulfiram-like interaction.34 When considering the symptoms associated with the interaction, it is important to remember that many of the symptoms can result from metronidazole therapy alone, regardless of whether other medications or alcohol are used.35
THE BOTTOM LINE Researchers have failed to identify a clinically significant interaction between metronidazole and alcohol. Avoiding alcohol while taking metronidazole does not appear to be necessary.
CORRESPONDENCE
Mary Onysko, PharmD, BCPS, University of Wyoming, School of Pharmacy Health Sciences Center, Room 292, 1000 E. University Avenue, Laramie, WY 82071; monysko@uwyo.edu.
1. Kantor ED, Rehm CD, Haas JS, et al. Trends in prescription drug use among adults in the United States from 1999-2012. JAMA. 2015;314:1818-1831.
2. Lexicomp Online. Clinical Drug Information. Available at: http://www.wolterskluwercdi.com/lexicomp-online/. Accessed December 8, 2015.
3. GlobalRPh: The Clinician’s Ultimate Reference. Available at: http://www.globalrph.com/. Accessed December 8, 2015.
4. Medscape Apps. Available at: http://www.medscape.com/public/applanding. Accessed December 8, 2015.
5. Micromedex Solutions. Academic Institutions. Available at: http://micromedex.com/academic. Accessed December 8, 2015.
6. Patel A. Clinical Pharmacology Mobile-A mobile web app compatible on all smart phones [review] May 31, 2010. Available at: http://www.imedicalapps.com/2010/05/clinical-pharmocology-app-review/. Accessed December 8, 2015.
7. Epocrates. Available at: http://www.epocrates.com/. Accessed December 8, 2015.
8. Baillargeon J, Holmes HM, Lin Y, et al. Concurrent use of warfarin and antibiotics and the risk of bleeding in older adults. Am J Med. 2012;125:183-189.
9. PL Detail-Document #280806. Antimicrobial drug interactions with warfarin. Pharmacist’s Letter/Prescriber’s Letter. August 2012.
10. Lane M, Zeringue A, McDonald J. Serious bleeding events due to warfarin and antibiotic co-prescription in a cohort of veterans. Am J Med. 2014;127:657-663.e2.
11. Hale SF, Lesar TS. Interaction of vitamin K antagonists and trimethoprim-sulfamethoxazole: ignore at your patient’s risk. Drug Metab Drug Interact. 2014;29:53-60.
12. Ahmed A, Stephens JC, Kaus CA, et al. Impact of preemptive warfarin dose reduction on anticoagulation after initiation of trimethoprim-sulfamethoxazole or levofloxacin. J Thromb Thrombolysis. 2008;26:44-48.
13. Holt RK, Anderson EA, Cantrell MA, et al. Preemptive dose reduction of warfarin in patients initiating metronidazole. Drug Metabol Drug Interact. 2010;25:35-39.
14. Hughes BR, Cunliffe WJ. Interactions between the oral contraceptive pill and antibiotics. Br J Dermatol. 1990;122:717-718.
15. Bolt HM. Interactions between clinically used drugs and oral contraceptives. Environ Health Perspect. 1994;102:35-38.
16. Aronson JK. Meyler’s Side Effects of Drugs. 16th ed. The International Encyclopedia of Adverse Drug Reactions and Interactions. Amsterdam, Netherlands: Elsevier; 2016. Available at: http://ac.els-cdn.com/B978044453717101009X/3-s2.0-B978044453717101009X-main.pdf?_tid=b33f6564-9deb-11e5-a8f0-00000aab0f01&acdnat=1449607315_83f5068fc5105226fcc6d7279c083516. Accessed December 8, 2015.
17. Masters KP, Carr BM. Survey of pharmacists and physicians on drug interactions between combined oral contraceptives and broad-spectrum antibiotics. Pharm Pract (Granada). 2009;7:139-144.
18. Toh S, Mitchell AA, Anderka M, et al; National Birth Defects Prevention Study. Antibiotics and oral contraceptive failure—a case-crossover study. Contraception. 2011;83:418-425.
19. Koopmans PC, Bos JH, de Jong van den Berg LT. Are antibiotics related to oral combination contraceptive failures in the Netherlands? A case-crossover study. Pharmacoepidemiol Drug Saf. 2012;21:865-871.
20. Archer JS, Archer DF. Oral contraceptive efficacy and antibiotic interaction: A myth debunked. J Am Acad Dermatol. 2002;46:917–923.
21. Scholten PC, Droppert RM, Zwinkels MGJ, et al. No interaction between ciprofloxacin and an oral contraceptive. Antimicrob Agents Chemother. 1998;42:3266-3268.
22. Andrews E, Damle BD, Fang A, et al. Pharmacokinetics and tolerability of voriconazole and a combination oral contraceptive co-administered in healthy female subjects. Br J Clin Pharmacol. 2008;65:531-539.
23. Grimmer SF, Allen WL, Back DJ, et al. The effect of cotrimoxazole on oral contraceptive steroids in women. Contraception. 1983;28:53-59.
24. Dickinson BD, Altman RD, Nielsen NH, et al; Council on Scientific Affairs, American Medical Association. Drug interactions between oral contraceptives and antibiotics. Obstet Gynecol. 2001;98:853-860.
25. Edelman AB, Cherala G, Stanczyk FZ. Metabolism and pharmacokinetics of contraceptive steroids in obese women: a review. Contraception. 2010;82:314-323.
26. Owens RC Jr, Ambrose PG. Torsades de pointes associated with fluoroquinolones. Pharmacotherapy. 2002;22:663-668.
27. Letsas KP, Efremidis M, Kounas SP, et al. Clinical characteristics of patients with drug-induced QT interval prolongation and torsade de pointes: identification of risk factors. Clin Res Cardiol. 2009;98:208-212.
28. Yap YG, Camm AJ. Drug induced QT prolongation and torsades de pointes. Heart. 2003;89:1363-1372.
29. Shaffer D, Singer S, Korvick J, et al. Concomitant risk factors in reports of torsades de pointes associated with macrolide use: review of the United States Food and Drug Administration adverse event reporting system. Clin Infect Dis. 2002;35:197-200.
30. FDA Briefing Document. Joint Meeting of the Antimicrobial Drugs Advisory Committee and the Drug Safety and Risk Management Advisory Committee. November 5, 2015. Available at: http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/Anti-InfectiveDrugsAdvisoryCommittee/UCM467383.pdf. Accessed June 11, 2016.
31. Chou HW, Wang JL, Chang CH, et al. Risks of cardiac arrhythmia and mortality among patients using new-generation macrolides, fluoroquinolones, and β-lactam/β-lactamase inhibitors: a Taiwanese nationwide study. Clin Infect Dis. 2015;60:566-577.
32. Wong AY, Root A, Douglas IJ, et al. Cardiovascular outcomes associated with use of clarithromycin: population based study. BMJ. 2016;352:h6926.
33. Inghammar M, Svanström H, Melbye M, et al. Oral fluoroquinolone use and serious arrhythmia: bi-national cohort study. BMJ. 2016;352:i843.
34. Visapää JP, Tillonen JS, Kaihovaara PS, et al. Lack of disulfiram-like reaction with metronidazole and ethanol. Ann Pharmacother. 2002;36:971-974. 35. Fjeld H, Raknes G. Is combining metronidazole and alcohol really hazardous? Tidsskr Nor Laegeforen. 2014;134:1661-1663.
1. Kantor ED, Rehm CD, Haas JS, et al. Trends in prescription drug use among adults in the United States from 1999-2012. JAMA. 2015;314:1818-1831.
2. Lexicomp Online. Clinical Drug Information. Available at: http://www.wolterskluwercdi.com/lexicomp-online/. Accessed December 8, 2015.
3. GlobalRPh: The Clinician’s Ultimate Reference. Available at: http://www.globalrph.com/. Accessed December 8, 2015.
4. Medscape Apps. Available at: http://www.medscape.com/public/applanding. Accessed December 8, 2015.
5. Micromedex Solutions. Academic Institutions. Available at: http://micromedex.com/academic. Accessed December 8, 2015.
6. Patel A. Clinical Pharmacology Mobile-A mobile web app compatible on all smart phones [review] May 31, 2010. Available at: http://www.imedicalapps.com/2010/05/clinical-pharmocology-app-review/. Accessed December 8, 2015.
7. Epocrates. Available at: http://www.epocrates.com/. Accessed December 8, 2015.
8. Baillargeon J, Holmes HM, Lin Y, et al. Concurrent use of warfarin and antibiotics and the risk of bleeding in older adults. Am J Med. 2012;125:183-189.
9. PL Detail-Document #280806. Antimicrobial drug interactions with warfarin. Pharmacist’s Letter/Prescriber’s Letter. August 2012.
10. Lane M, Zeringue A, McDonald J. Serious bleeding events due to warfarin and antibiotic co-prescription in a cohort of veterans. Am J Med. 2014;127:657-663.e2.
11. Hale SF, Lesar TS. Interaction of vitamin K antagonists and trimethoprim-sulfamethoxazole: ignore at your patient’s risk. Drug Metab Drug Interact. 2014;29:53-60.
12. Ahmed A, Stephens JC, Kaus CA, et al. Impact of preemptive warfarin dose reduction on anticoagulation after initiation of trimethoprim-sulfamethoxazole or levofloxacin. J Thromb Thrombolysis. 2008;26:44-48.
13. Holt RK, Anderson EA, Cantrell MA, et al. Preemptive dose reduction of warfarin in patients initiating metronidazole. Drug Metabol Drug Interact. 2010;25:35-39.
14. Hughes BR, Cunliffe WJ. Interactions between the oral contraceptive pill and antibiotics. Br J Dermatol. 1990;122:717-718.
15. Bolt HM. Interactions between clinically used drugs and oral contraceptives. Environ Health Perspect. 1994;102:35-38.
16. Aronson JK. Meyler’s Side Effects of Drugs. 16th ed. The International Encyclopedia of Adverse Drug Reactions and Interactions. Amsterdam, Netherlands: Elsevier; 2016. Available at: http://ac.els-cdn.com/B978044453717101009X/3-s2.0-B978044453717101009X-main.pdf?_tid=b33f6564-9deb-11e5-a8f0-00000aab0f01&acdnat=1449607315_83f5068fc5105226fcc6d7279c083516. Accessed December 8, 2015.
17. Masters KP, Carr BM. Survey of pharmacists and physicians on drug interactions between combined oral contraceptives and broad-spectrum antibiotics. Pharm Pract (Granada). 2009;7:139-144.
18. Toh S, Mitchell AA, Anderka M, et al; National Birth Defects Prevention Study. Antibiotics and oral contraceptive failure—a case-crossover study. Contraception. 2011;83:418-425.
19. Koopmans PC, Bos JH, de Jong van den Berg LT. Are antibiotics related to oral combination contraceptive failures in the Netherlands? A case-crossover study. Pharmacoepidemiol Drug Saf. 2012;21:865-871.
20. Archer JS, Archer DF. Oral contraceptive efficacy and antibiotic interaction: A myth debunked. J Am Acad Dermatol. 2002;46:917–923.
21. Scholten PC, Droppert RM, Zwinkels MGJ, et al. No interaction between ciprofloxacin and an oral contraceptive. Antimicrob Agents Chemother. 1998;42:3266-3268.
22. Andrews E, Damle BD, Fang A, et al. Pharmacokinetics and tolerability of voriconazole and a combination oral contraceptive co-administered in healthy female subjects. Br J Clin Pharmacol. 2008;65:531-539.
23. Grimmer SF, Allen WL, Back DJ, et al. The effect of cotrimoxazole on oral contraceptive steroids in women. Contraception. 1983;28:53-59.
24. Dickinson BD, Altman RD, Nielsen NH, et al; Council on Scientific Affairs, American Medical Association. Drug interactions between oral contraceptives and antibiotics. Obstet Gynecol. 2001;98:853-860.
25. Edelman AB, Cherala G, Stanczyk FZ. Metabolism and pharmacokinetics of contraceptive steroids in obese women: a review. Contraception. 2010;82:314-323.
26. Owens RC Jr, Ambrose PG. Torsades de pointes associated with fluoroquinolones. Pharmacotherapy. 2002;22:663-668.
27. Letsas KP, Efremidis M, Kounas SP, et al. Clinical characteristics of patients with drug-induced QT interval prolongation and torsade de pointes: identification of risk factors. Clin Res Cardiol. 2009;98:208-212.
28. Yap YG, Camm AJ. Drug induced QT prolongation and torsades de pointes. Heart. 2003;89:1363-1372.
29. Shaffer D, Singer S, Korvick J, et al. Concomitant risk factors in reports of torsades de pointes associated with macrolide use: review of the United States Food and Drug Administration adverse event reporting system. Clin Infect Dis. 2002;35:197-200.
30. FDA Briefing Document. Joint Meeting of the Antimicrobial Drugs Advisory Committee and the Drug Safety and Risk Management Advisory Committee. November 5, 2015. Available at: http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/Anti-InfectiveDrugsAdvisoryCommittee/UCM467383.pdf. Accessed June 11, 2016.
31. Chou HW, Wang JL, Chang CH, et al. Risks of cardiac arrhythmia and mortality among patients using new-generation macrolides, fluoroquinolones, and β-lactam/β-lactamase inhibitors: a Taiwanese nationwide study. Clin Infect Dis. 2015;60:566-577.
32. Wong AY, Root A, Douglas IJ, et al. Cardiovascular outcomes associated with use of clarithromycin: population based study. BMJ. 2016;352:h6926.
33. Inghammar M, Svanström H, Melbye M, et al. Oral fluoroquinolone use and serious arrhythmia: bi-national cohort study. BMJ. 2016;352:i843.
34. Visapää JP, Tillonen JS, Kaihovaara PS, et al. Lack of disulfiram-like reaction with metronidazole and ethanol. Ann Pharmacother. 2002;36:971-974. 35. Fjeld H, Raknes G. Is combining metronidazole and alcohol really hazardous? Tidsskr Nor Laegeforen. 2014;134:1661-1663.
Targeting neuropathic pain: Consider these alternatives
Anticonvulsants, antidepressants, and opioids are the most frequently prescribed medications for neuropathic pain.1 But some patients are unable to tolerate the adverse effects of these drugs, and others achieve only partial pain relief. What can you offer them?
Combinations of prescription medications are generally considered more effective than monotherapy for painful peripheral neuropathy,1 but it is unclear which combinations are best. Alternative therapies—several of which have some evidence of safety and efficacy in treating peripheral neuropathy—are another option. Yet trials with alternative therapies, alone or in combination with prescription drugs, are rarely considered.
In fact, physicians are often unfamiliar with these therapies. Many are concerned about the absence of US Food and Drug Administration approval for alternative therapies and the variability in quality control associated with the lack of oversight, as well. Making recommendations about the duration of therapy also presents a challenge because most studies of supplements are relatively short. What’s more, alternative treatments are rarely covered by third-party payers.
Nonetheless, the therapies detailed in the text and TABLE2-12 that follow are generally well tolerated and appear to be safe. Adding them to your arsenal of therapeutic choices for patients with painful peripheral neuropathy may increase your ability to provide successful treatment.
Acetyl-L-carnitine (ALC)
ALC occurs naturally in the body as L-carnitine and acetyl-carnitine esters, which are converted to carnitines by intracellular enzymes and cell membrane transporters.2 ALC has been studied in patients with neuropathy associated with human immunodeficiency virus (HIV), cancer, and diabetes. Potential mechanisms of action include the correction of a deficiency that may be causing the neuropathy (which sometimes occurs in HIV-positive patients13 or those taking anticonvulsants14), a direct antioxidant effect, or an enhanced response to nerve growth factor.13
ALC can be given intramuscularly (IM) or orally in doses of 2000 to 3000 mg/d. In one randomized placebo-controlled trial (N=333), patients with diabetic neuropathy received 1000 mg IM followed by an oral dose of 2000 mg every day for a year.6 Mean pain scores decreased by 39%, with 67% of those receiving ALC vs 23% of those on placebo showing moderate to marked improvement.
In a pooled analysis (N=1257) of 2 randomized controlled trials (RCTs), patients with diabetes took 1000 mg ALC 3 times daily or placebo for a year.7 Cohort pain scores improved by 40% from baseline in the ALC group compared with a 24% improvement for those in the placebo group.
THE BOTTOM LINE ALC is well tolerated, with minor adverse effects such as headache and nausea reported.6,7 It should not be given to patients taking acenocoumarol or warfarin, however. A major interaction causing an elevated international normalized ratio has been found to occur when either agent is combined with L-carnitine2 and could theoretically occur with ALC, as well. No other drug-drug interactions have been documented.2
Alpha lipoic acid (ALA)
Both a fat- and a water-soluble vitamin that is usually obtained from the diet, ALA regenerates endogenous antioxidants like vitamins C and E and glutathione. It is this regenerative mechanism that it is believed to alleviate diabetic neuropathy.2 ALA 600 mg/d appears to be effective, although studies suggest that intravenous (IV) use is more effective than oral administration.
A meta-analysis of 4 RCTs (N=653), 2 with ALA taken orally and 2 involving IV administration, is a case in point.3 The pooled standardized mean difference estimated from all trials showed a reduction in total symptom scores of −2.26 (95% confidence interval [CI], −3.12 to −1.41; P=.00001), with 0 indicating no symptoms, 3 indicating severe symptoms, and a maximum score of 14.64 if all symptoms were severe and continuous. Subgroup analyses revealed a reduction of −1.78 (95% CI, −2.45 to −1.10; P=.00001) for oral ALA and −2.81 (95% CI, −4.16 to −1.46; P=.0001) for IV administration. Doses >600 mg/d did not improve efficacy, but did increase adverse effects such as nausea, vomiting, and dizziness.
In a multicenter RCT (N=460) of ALA 600 mg/d for 4 years, however, no improvement in the primary endpoint (a composite of neuropathy impairment scores and 7 neurophysiologic tests) was found.15 Although there was a statistically significant improvement in symptoms of neuropathy (−0.68 with ALA compared with +0.61 with placebo), the change was too small to be considered clinically significant.
ALA did slow the progression of neuropathy, however, with 29% of patients in the treatment group experiencing worsening symptoms compared with 38% of those on placebo. There was no difference in tolerability or discontinuation of treatment between the 2 groups.
A recent observational study (N=101) compared the efficacy of pregabalin, carbamazepine, and ALA over a 21-month period.4 Although those taking pregabalin had the best response rate, all 3 treatments led to significant improvement in the burning associated with neuropathic pain.
ALA 100 mg bid has been investigated as part of a 3-drug combination (with pregabalin 75 mg bid and methylcobalamin 750 mcg bid) compared with monotherapy (pregabalin 75 mg bid) in an open randomized study (N=30) for 12 weeks.16 While there was a trend toward improvement in pain relief, sleep interference, and nerve function in the combination therapy group, no statistically significant difference between the 2 groups was found. Nonetheless, more than a third (36%) had a global assessment rating of “excellent” vs one in 5 (20%) of those on pregabalin alone.
THE BOTTOM LINE Overall, ALA is well tolerated; the most common adverse effects are nausea and skin rash. IV administration is more effective than oral administration, but may cause nausea, headache, and an allergic reaction at the injection site.2 ALA does have the potential for an interaction with chemotherapy and thyroid hormone and may decrease the effectiveness of these therapies.2
B vitamins
Deficiencies of vitamin B1 (thiamine), B6 (pyridoxine), B12 (cyanocobalamin), and folate are known causes of neuropathy, and correcting them often improves or eliminates the symptoms.13 Vitamin B12 deficiency is commonly seen in patients taking metformin;14 these patients may benefit from supplementation with B12 1000 mcg/d.
Many of the B vitamins have been studied for treatment of neuropathy, but benfotiamine (a lipid-soluble form of thiamine) is thought to be the best option because it is better absorbed across cell membranes than other B vitamins.9 A Cochrane review found that benfotiamine alone may be effective for both diabetic and alcoholic neuropathy and that short-term use of higher doses of vitamin B complex (25 mg B1 or 320 mg benfotiamine + 50-720 mg B6 + 1000 mcg B12 daily) may reduce neuropathic pain.9
A randomized multicenter trial (N=214) found that adding a supplement containing L-methylfolate 3 mg, pyridoxal 5-phosphate 35 mg, and methylcobalamin 2 mg twice daily to other medications (eg, pregabalin, gabapentin, or duloxetine) improved symptoms of diabetic neuropathy.10 At 24 weeks, those receiving the combination therapy had a 26% decrease in pain symptoms compared with a 15% decrease for those on medication alone, with no significant adverse effects.
THE BOTTOM LINE Overall, vitamin B supplementation is well tolerated and appears to be more effective in relieving neuropathic pain than medication alone.9,14 But larger studies are needed before its efficacy in treating patients who do not have a deficiency can be established.
Capsaicin
Capsaicin, an ingredient found in peppers, works by binding to nociceptors to selectively stimulate afferent C fibers. This causes the release of substance P, a neurotransmitter that mediates pain, leading to its depletion and resulting in desensitization.2 Several meta-analyses and systematic reviews have found that topical capsaicin can be very effective, both as an adjunctive treatment and as monotherapy for neuropathic pain.11,17,18 The concentration used in the studies was 0.075% capsaicin cream, applied 3 to 4 times a day for 6 to 12 weeks, compared with placebo creams. In all categories studied, capsaicin was either statistically significant or trending in its favor, with the exception of adverse effects.
Capsaicin led to an improvement in daily activities and ability to sleep and a reduction in pain as measured with a visual analog scale and physician global evaluation.11,17,18
The most notable adverse effects were a burning sensation on the skin and coughing and sneezing caused by inhalation of dried cream. Although the adverse effects were expected to improve after 2 to 7 days of use, a significant number of participants withdrew from the study.
A 7-study meta-analysis showed the effectiveness of an 8% capsaicin patch for treatment of post-herpetic neuralgia and HIV-associated neuropathy.12 The patch, available only by prescription, was worn every day for 4 weeks (60 minutes daily for post-herpetic neuralgia and 30 minutes a day for HIV-associated neuropathy). The pooled results were statistically significant, but the patch was less effective for patients ages 18 to 40 years and for those of Asian descent. It can be used with other analgesics or as monotherapy, with few adverse reactions.12,19
THE BOTTOM LINE Since capsaicin is a topical medication, there are no relevant drug-drug interactions. Patients should be cautioned to wash their hands after application, however, and to avoid contact with eyes and open wounds.
Gamma linolenic acid (GLA)
Also known as evening primrose oil, GLA is an omega-6 fatty acid that’s an important constituent of neuronal cell membranes—and believed to decrease neuropathic pain by having some anti-inflammatory effects.2 This suggests that therapy with GLA has the potential to improve neuronal phospholipid structure and microcirculation.2
Two placebo-controlled trials (N=22,111) showed improvement in pain scores and multiple neurophysiologic assessments in patients with diabetes treated with GLA (360-480 mg/d).20,21 The treatment was well tolerated, but the beneficial effect was more pronounced in those with less severe diabetes.
THE BOTTOM LINE The dose of GLA studied (8 to 12 capsules daily) could lead to problems with patient adherence. In addition, GLA should be used with caution in patients who are taking antiplatelet medication or have seizure disorders.2
Magnesium (Mg)
Mg is highly involved in multiple enzyme systems throughout the body. Although it is very well absorbed from dietary sources,2 patients with diabetes, liver disease, and hormonal imbalances, as well as the elderly, are often deficient in Mg. It is unclear how this affects peripheral neuropathy.13
Mg may have an antinociceptive effect by decreasing intracellular calcium influx and antagonizing N-methyl-D-aspartate receptors and associated nerve signaling.22 A small RCT (N=80) showed Mg to decrease the severity of neuropathic back pain.22 Patients received Mg sulfate 1 g IV, given over 4 hours, every day for 2 weeks. The infusion was then replaced with Mg oxide 400 mg plus Mg gluconate 100 mg, taken orally twice daily for 4 weeks. An improvement in mean pain score was seen as early as 2 weeks, and scores had decreased by 2.8 points (on a 0-10-point scale) at 6 months.
Another small RCT (N=45) gave patients with neuropathy of postherpetic, traumatic, or surgical (but not diabetic) origin Mg chloride 838 mg orally 3 times a day for 4 weeks.23 The supplement was taken with meals. Mean pain scores in the treatment group decreased by 3 points, but this was not significantly different from the improvement seen in those on placebo.
In a similar study, patients (N=110) with type 1 diabetes and a normal serum Mg but an insufficiency as measured by erythrocyte Mg were given Mg gluconate 300 mg or placebo daily for 5 years.8 The supplement slowed the progression of peripheral neuropathy (only 12% of those receiving Mg gluconate experienced a significant worsening of symptoms over the course of the study, compared with 61% of those in the placebo group), but in most cases, it did not lead to an improvement.
No consistent approach to Mg supplementation has been studied, which makes recommending a particular route, dose, or formulation challenging. There is evidence that oral Mg, particularly in the form of Mg oxide, can cause diarrhea, especially in doses >350 mg/d. Mg gluconate and Mg chloride are better tolerated; Mg carbonate should be avoided due to poor oral absorption.2
BOTTOM LINE Mg supplementation appears to slow the progression of diabetic peripheral neuropathy, but is unsafe for patients with renal dysfunction, cardiac conduction abnormalities, or elevated Mg levels.2 Caution is required, too, when considering Mg supplementation for patients taking anticoagulants, bisphosphonates, digoxin, potassium-sparing diuretics, or tetracycline antibiotics.2
CORRESPONDENCE
Mary Onysko, PharmD, BCPS, University of Wyoming, School of Pharmacy Health Sciences Center, Room 292, 1000 E. University Avenue, Laramie, WY 82071; monysko@uwyo.edu
1. Chaparro LE, Wiffen PJ, Moore RA, et al. Combination pharmacotherapy for the treatment of neuropathic pain in adults. Cochrane Database Syst Rev. 2012:(7):CD008943.
2. Natural Medicines Comprehensive Database. Natural Medicines Comprehensive Database Web site. Available at: http://naturaldatabase.therapeuticresearch.com. Accessed January 4, 2015.
3. Mijnhout GS, Kollen BJ, Alkhalaf A, et al. Alpha lipoic acid for symptomatic peripheral neuropathy in patients with diabetes: a meta-analysis of randomized controlled trials. Int J Endocrinol. 2012;2012:456279.
4. Patel N, Mishra V, Patel P, et al. A study of the use of carbamazepine, pregabalin and alpha lipoic acid in patients of diabetic neuropathy. J Diabetes Metab Disord. 2014;13:62.
5. Bertolotto F, Massone A. Combination of alpha lipoic acid and superoxide dismutase leads to physiological and symptomatic improvements in diabetic neuropathy. Drugs R D. 2012;12:29-34.
6. De Grandis D, Minardi C. Acetyl-L-carnitine (levacecarnine) in the treatment of diabetic neuropathy. A long-term, randomised, double-blind, placebo-controlled study. Drugs R D. 2002;3:223-231.
7. Sima AA, Calvani M, Mehra M, et al; Acetyl-L-Carnitine Study Group. Acetyl-L-carnitine improves pain, nerve regeneration, and vibratory perception in patients with chronic diabetic neuropathy: an analysis of two randomized placebo-controlled trials. Diabetes Care. 2005;28:89-94.
8. De Leeuw, Engelen W, De Block C, et al. Long term magnesium supplementation influences favourably the natural evolution of neuropathy in Mg-depleted type 1 diabetic patients (T1dm). Magnes Res. 2004;17:109-114.
9. Ang CD, Alviar MJM, Dans AL, et al. Vitamin B for treating peripheral neuropathy. Cochrane Database Syst Rev. 2008;(3):CD004573.
10. Fonseca VA, Lavery LA, Thethi TK, et al. Metanx in type 2 diabetes
with peripheral neuropathy: a randomized trial. Am J Med. 2013;126:141-149.
11. Mason L, Moore RA, Derry S, et al. Systematic review of topical capsaicin for the treatment of chronic pain. BMJ. 2004;328:991.
12. Mou J, Paillard F, Turnbull B, et al. Efficacy of Qutenza® (capsaicin) 8% patch for neuropathic pain: a meta-analysis of the Qutenza Clinical Trials Database. Pain. 2013;154:1632-1639.
13. Head KA. Peripheral neuropathy: pathogenic mechanisms and alternative therapies. Altern Med Rev. 2006; 11:294-329.
14. Miranda-Massari JR, Gonzalez MJ, Jimenez FJ, et al. Metabolic correction in the management of diabetic peripheral neuropathy: improving clinical results beyond symptom control. Curr Clin Pharmacol. 2011; 6:260-273.
15. Ziegler D, Low PA, Litchy WJ, et al. Efficacy and safety of antioxidant treatment with a-lipoic acid over 4 years in diabetic polyneuropathy: the NATHAN 1 trial. Diabetes Care. 2011;34:2054-2060.
16. Vasudevan D, Naik MM, Mukaddam QI. Efficacy and safety of methylcobalamin, alpha lipoic acid and pregabalin combination versus pregabalin monotherapy in improving pain and nerve conduction velocity in type 2 diabetes associated impaired peripheral neuropathic condition. [MAINTAIN]: Results of a pilot study. Ann Indian Acad Neurol. 2014;17:19-24.
17. Halat KM, Dennehy CE. Botanicals and dietary supplements in diabetic peripheral neuropathy. J Am Board Fam Pract. 2003;16:47-57.
18. Donofrio P, Walker F, Hunt V, et al. Treatment of painful diabetic neuropathy with topical capsaicin: A multicenter, double-blind, vehicle-controlled study. Arch Int Med. 1991;151:2225-2229.
19. Derry S, Rice ASC, Cole P, et al. Topical capsaicin (high concentration) for chronic neuropathic pain in adults. Cochrane Database Syst Rev. 2013;(2):CD007393.
20. Keen H, Payan J, Allawi J, et al. Treatment of diabetic neuropathy with gamma-linolenic acid. The gamma-Linolenic Acid Multicenter Trial Group. Diabetes Care. 1993;16:8-15.
21. Jamal GA, Carmichael H. The effect of gamma linolenic acid on human diabetic peripheral neuropathy: a double blind placebo controlled trial. Diabetic Med. 1990;7:319-323.
22. Yousef AA, Al-deeb AE. A double-blinded randomised controlled study of the value of sequential intravenous and oral magnesium therapy in patients with chronic low back pain with a neuropathic component. Anaesthesia. 2013;68:260-266.
23. Pickering G, Morel V, Simen E. Oral magnesium treatment in patients with neuropathic pain: a randomized clinical trial. Magnes Res. 2011;24:28-35.
Anticonvulsants, antidepressants, and opioids are the most frequently prescribed medications for neuropathic pain.1 But some patients are unable to tolerate the adverse effects of these drugs, and others achieve only partial pain relief. What can you offer them?
Combinations of prescription medications are generally considered more effective than monotherapy for painful peripheral neuropathy,1 but it is unclear which combinations are best. Alternative therapies—several of which have some evidence of safety and efficacy in treating peripheral neuropathy—are another option. Yet trials with alternative therapies, alone or in combination with prescription drugs, are rarely considered.
In fact, physicians are often unfamiliar with these therapies. Many are concerned about the absence of US Food and Drug Administration approval for alternative therapies and the variability in quality control associated with the lack of oversight, as well. Making recommendations about the duration of therapy also presents a challenge because most studies of supplements are relatively short. What’s more, alternative treatments are rarely covered by third-party payers.
Nonetheless, the therapies detailed in the text and TABLE2-12 that follow are generally well tolerated and appear to be safe. Adding them to your arsenal of therapeutic choices for patients with painful peripheral neuropathy may increase your ability to provide successful treatment.
Acetyl-L-carnitine (ALC)
ALC occurs naturally in the body as L-carnitine and acetyl-carnitine esters, which are converted to carnitines by intracellular enzymes and cell membrane transporters.2 ALC has been studied in patients with neuropathy associated with human immunodeficiency virus (HIV), cancer, and diabetes. Potential mechanisms of action include the correction of a deficiency that may be causing the neuropathy (which sometimes occurs in HIV-positive patients13 or those taking anticonvulsants14), a direct antioxidant effect, or an enhanced response to nerve growth factor.13
ALC can be given intramuscularly (IM) or orally in doses of 2000 to 3000 mg/d. In one randomized placebo-controlled trial (N=333), patients with diabetic neuropathy received 1000 mg IM followed by an oral dose of 2000 mg every day for a year.6 Mean pain scores decreased by 39%, with 67% of those receiving ALC vs 23% of those on placebo showing moderate to marked improvement.
In a pooled analysis (N=1257) of 2 randomized controlled trials (RCTs), patients with diabetes took 1000 mg ALC 3 times daily or placebo for a year.7 Cohort pain scores improved by 40% from baseline in the ALC group compared with a 24% improvement for those in the placebo group.
THE BOTTOM LINE ALC is well tolerated, with minor adverse effects such as headache and nausea reported.6,7 It should not be given to patients taking acenocoumarol or warfarin, however. A major interaction causing an elevated international normalized ratio has been found to occur when either agent is combined with L-carnitine2 and could theoretically occur with ALC, as well. No other drug-drug interactions have been documented.2
Alpha lipoic acid (ALA)
Both a fat- and a water-soluble vitamin that is usually obtained from the diet, ALA regenerates endogenous antioxidants like vitamins C and E and glutathione. It is this regenerative mechanism that it is believed to alleviate diabetic neuropathy.2 ALA 600 mg/d appears to be effective, although studies suggest that intravenous (IV) use is more effective than oral administration.
A meta-analysis of 4 RCTs (N=653), 2 with ALA taken orally and 2 involving IV administration, is a case in point.3 The pooled standardized mean difference estimated from all trials showed a reduction in total symptom scores of −2.26 (95% confidence interval [CI], −3.12 to −1.41; P=.00001), with 0 indicating no symptoms, 3 indicating severe symptoms, and a maximum score of 14.64 if all symptoms were severe and continuous. Subgroup analyses revealed a reduction of −1.78 (95% CI, −2.45 to −1.10; P=.00001) for oral ALA and −2.81 (95% CI, −4.16 to −1.46; P=.0001) for IV administration. Doses >600 mg/d did not improve efficacy, but did increase adverse effects such as nausea, vomiting, and dizziness.
In a multicenter RCT (N=460) of ALA 600 mg/d for 4 years, however, no improvement in the primary endpoint (a composite of neuropathy impairment scores and 7 neurophysiologic tests) was found.15 Although there was a statistically significant improvement in symptoms of neuropathy (−0.68 with ALA compared with +0.61 with placebo), the change was too small to be considered clinically significant.
ALA did slow the progression of neuropathy, however, with 29% of patients in the treatment group experiencing worsening symptoms compared with 38% of those on placebo. There was no difference in tolerability or discontinuation of treatment between the 2 groups.
A recent observational study (N=101) compared the efficacy of pregabalin, carbamazepine, and ALA over a 21-month period.4 Although those taking pregabalin had the best response rate, all 3 treatments led to significant improvement in the burning associated with neuropathic pain.
ALA 100 mg bid has been investigated as part of a 3-drug combination (with pregabalin 75 mg bid and methylcobalamin 750 mcg bid) compared with monotherapy (pregabalin 75 mg bid) in an open randomized study (N=30) for 12 weeks.16 While there was a trend toward improvement in pain relief, sleep interference, and nerve function in the combination therapy group, no statistically significant difference between the 2 groups was found. Nonetheless, more than a third (36%) had a global assessment rating of “excellent” vs one in 5 (20%) of those on pregabalin alone.
THE BOTTOM LINE Overall, ALA is well tolerated; the most common adverse effects are nausea and skin rash. IV administration is more effective than oral administration, but may cause nausea, headache, and an allergic reaction at the injection site.2 ALA does have the potential for an interaction with chemotherapy and thyroid hormone and may decrease the effectiveness of these therapies.2
B vitamins
Deficiencies of vitamin B1 (thiamine), B6 (pyridoxine), B12 (cyanocobalamin), and folate are known causes of neuropathy, and correcting them often improves or eliminates the symptoms.13 Vitamin B12 deficiency is commonly seen in patients taking metformin;14 these patients may benefit from supplementation with B12 1000 mcg/d.
Many of the B vitamins have been studied for treatment of neuropathy, but benfotiamine (a lipid-soluble form of thiamine) is thought to be the best option because it is better absorbed across cell membranes than other B vitamins.9 A Cochrane review found that benfotiamine alone may be effective for both diabetic and alcoholic neuropathy and that short-term use of higher doses of vitamin B complex (25 mg B1 or 320 mg benfotiamine + 50-720 mg B6 + 1000 mcg B12 daily) may reduce neuropathic pain.9
A randomized multicenter trial (N=214) found that adding a supplement containing L-methylfolate 3 mg, pyridoxal 5-phosphate 35 mg, and methylcobalamin 2 mg twice daily to other medications (eg, pregabalin, gabapentin, or duloxetine) improved symptoms of diabetic neuropathy.10 At 24 weeks, those receiving the combination therapy had a 26% decrease in pain symptoms compared with a 15% decrease for those on medication alone, with no significant adverse effects.
THE BOTTOM LINE Overall, vitamin B supplementation is well tolerated and appears to be more effective in relieving neuropathic pain than medication alone.9,14 But larger studies are needed before its efficacy in treating patients who do not have a deficiency can be established.
Capsaicin
Capsaicin, an ingredient found in peppers, works by binding to nociceptors to selectively stimulate afferent C fibers. This causes the release of substance P, a neurotransmitter that mediates pain, leading to its depletion and resulting in desensitization.2 Several meta-analyses and systematic reviews have found that topical capsaicin can be very effective, both as an adjunctive treatment and as monotherapy for neuropathic pain.11,17,18 The concentration used in the studies was 0.075% capsaicin cream, applied 3 to 4 times a day for 6 to 12 weeks, compared with placebo creams. In all categories studied, capsaicin was either statistically significant or trending in its favor, with the exception of adverse effects.
Capsaicin led to an improvement in daily activities and ability to sleep and a reduction in pain as measured with a visual analog scale and physician global evaluation.11,17,18
The most notable adverse effects were a burning sensation on the skin and coughing and sneezing caused by inhalation of dried cream. Although the adverse effects were expected to improve after 2 to 7 days of use, a significant number of participants withdrew from the study.
A 7-study meta-analysis showed the effectiveness of an 8% capsaicin patch for treatment of post-herpetic neuralgia and HIV-associated neuropathy.12 The patch, available only by prescription, was worn every day for 4 weeks (60 minutes daily for post-herpetic neuralgia and 30 minutes a day for HIV-associated neuropathy). The pooled results were statistically significant, but the patch was less effective for patients ages 18 to 40 years and for those of Asian descent. It can be used with other analgesics or as monotherapy, with few adverse reactions.12,19
THE BOTTOM LINE Since capsaicin is a topical medication, there are no relevant drug-drug interactions. Patients should be cautioned to wash their hands after application, however, and to avoid contact with eyes and open wounds.
Gamma linolenic acid (GLA)
Also known as evening primrose oil, GLA is an omega-6 fatty acid that’s an important constituent of neuronal cell membranes—and believed to decrease neuropathic pain by having some anti-inflammatory effects.2 This suggests that therapy with GLA has the potential to improve neuronal phospholipid structure and microcirculation.2
Two placebo-controlled trials (N=22,111) showed improvement in pain scores and multiple neurophysiologic assessments in patients with diabetes treated with GLA (360-480 mg/d).20,21 The treatment was well tolerated, but the beneficial effect was more pronounced in those with less severe diabetes.
THE BOTTOM LINE The dose of GLA studied (8 to 12 capsules daily) could lead to problems with patient adherence. In addition, GLA should be used with caution in patients who are taking antiplatelet medication or have seizure disorders.2
Magnesium (Mg)
Mg is highly involved in multiple enzyme systems throughout the body. Although it is very well absorbed from dietary sources,2 patients with diabetes, liver disease, and hormonal imbalances, as well as the elderly, are often deficient in Mg. It is unclear how this affects peripheral neuropathy.13
Mg may have an antinociceptive effect by decreasing intracellular calcium influx and antagonizing N-methyl-D-aspartate receptors and associated nerve signaling.22 A small RCT (N=80) showed Mg to decrease the severity of neuropathic back pain.22 Patients received Mg sulfate 1 g IV, given over 4 hours, every day for 2 weeks. The infusion was then replaced with Mg oxide 400 mg plus Mg gluconate 100 mg, taken orally twice daily for 4 weeks. An improvement in mean pain score was seen as early as 2 weeks, and scores had decreased by 2.8 points (on a 0-10-point scale) at 6 months.
Another small RCT (N=45) gave patients with neuropathy of postherpetic, traumatic, or surgical (but not diabetic) origin Mg chloride 838 mg orally 3 times a day for 4 weeks.23 The supplement was taken with meals. Mean pain scores in the treatment group decreased by 3 points, but this was not significantly different from the improvement seen in those on placebo.
In a similar study, patients (N=110) with type 1 diabetes and a normal serum Mg but an insufficiency as measured by erythrocyte Mg were given Mg gluconate 300 mg or placebo daily for 5 years.8 The supplement slowed the progression of peripheral neuropathy (only 12% of those receiving Mg gluconate experienced a significant worsening of symptoms over the course of the study, compared with 61% of those in the placebo group), but in most cases, it did not lead to an improvement.
No consistent approach to Mg supplementation has been studied, which makes recommending a particular route, dose, or formulation challenging. There is evidence that oral Mg, particularly in the form of Mg oxide, can cause diarrhea, especially in doses >350 mg/d. Mg gluconate and Mg chloride are better tolerated; Mg carbonate should be avoided due to poor oral absorption.2
BOTTOM LINE Mg supplementation appears to slow the progression of diabetic peripheral neuropathy, but is unsafe for patients with renal dysfunction, cardiac conduction abnormalities, or elevated Mg levels.2 Caution is required, too, when considering Mg supplementation for patients taking anticoagulants, bisphosphonates, digoxin, potassium-sparing diuretics, or tetracycline antibiotics.2
CORRESPONDENCE
Mary Onysko, PharmD, BCPS, University of Wyoming, School of Pharmacy Health Sciences Center, Room 292, 1000 E. University Avenue, Laramie, WY 82071; monysko@uwyo.edu
Anticonvulsants, antidepressants, and opioids are the most frequently prescribed medications for neuropathic pain.1 But some patients are unable to tolerate the adverse effects of these drugs, and others achieve only partial pain relief. What can you offer them?
Combinations of prescription medications are generally considered more effective than monotherapy for painful peripheral neuropathy,1 but it is unclear which combinations are best. Alternative therapies—several of which have some evidence of safety and efficacy in treating peripheral neuropathy—are another option. Yet trials with alternative therapies, alone or in combination with prescription drugs, are rarely considered.
In fact, physicians are often unfamiliar with these therapies. Many are concerned about the absence of US Food and Drug Administration approval for alternative therapies and the variability in quality control associated with the lack of oversight, as well. Making recommendations about the duration of therapy also presents a challenge because most studies of supplements are relatively short. What’s more, alternative treatments are rarely covered by third-party payers.
Nonetheless, the therapies detailed in the text and TABLE2-12 that follow are generally well tolerated and appear to be safe. Adding them to your arsenal of therapeutic choices for patients with painful peripheral neuropathy may increase your ability to provide successful treatment.
Acetyl-L-carnitine (ALC)
ALC occurs naturally in the body as L-carnitine and acetyl-carnitine esters, which are converted to carnitines by intracellular enzymes and cell membrane transporters.2 ALC has been studied in patients with neuropathy associated with human immunodeficiency virus (HIV), cancer, and diabetes. Potential mechanisms of action include the correction of a deficiency that may be causing the neuropathy (which sometimes occurs in HIV-positive patients13 or those taking anticonvulsants14), a direct antioxidant effect, or an enhanced response to nerve growth factor.13
ALC can be given intramuscularly (IM) or orally in doses of 2000 to 3000 mg/d. In one randomized placebo-controlled trial (N=333), patients with diabetic neuropathy received 1000 mg IM followed by an oral dose of 2000 mg every day for a year.6 Mean pain scores decreased by 39%, with 67% of those receiving ALC vs 23% of those on placebo showing moderate to marked improvement.
In a pooled analysis (N=1257) of 2 randomized controlled trials (RCTs), patients with diabetes took 1000 mg ALC 3 times daily or placebo for a year.7 Cohort pain scores improved by 40% from baseline in the ALC group compared with a 24% improvement for those in the placebo group.
THE BOTTOM LINE ALC is well tolerated, with minor adverse effects such as headache and nausea reported.6,7 It should not be given to patients taking acenocoumarol or warfarin, however. A major interaction causing an elevated international normalized ratio has been found to occur when either agent is combined with L-carnitine2 and could theoretically occur with ALC, as well. No other drug-drug interactions have been documented.2
Alpha lipoic acid (ALA)
Both a fat- and a water-soluble vitamin that is usually obtained from the diet, ALA regenerates endogenous antioxidants like vitamins C and E and glutathione. It is this regenerative mechanism that it is believed to alleviate diabetic neuropathy.2 ALA 600 mg/d appears to be effective, although studies suggest that intravenous (IV) use is more effective than oral administration.
A meta-analysis of 4 RCTs (N=653), 2 with ALA taken orally and 2 involving IV administration, is a case in point.3 The pooled standardized mean difference estimated from all trials showed a reduction in total symptom scores of −2.26 (95% confidence interval [CI], −3.12 to −1.41; P=.00001), with 0 indicating no symptoms, 3 indicating severe symptoms, and a maximum score of 14.64 if all symptoms were severe and continuous. Subgroup analyses revealed a reduction of −1.78 (95% CI, −2.45 to −1.10; P=.00001) for oral ALA and −2.81 (95% CI, −4.16 to −1.46; P=.0001) for IV administration. Doses >600 mg/d did not improve efficacy, but did increase adverse effects such as nausea, vomiting, and dizziness.
In a multicenter RCT (N=460) of ALA 600 mg/d for 4 years, however, no improvement in the primary endpoint (a composite of neuropathy impairment scores and 7 neurophysiologic tests) was found.15 Although there was a statistically significant improvement in symptoms of neuropathy (−0.68 with ALA compared with +0.61 with placebo), the change was too small to be considered clinically significant.
ALA did slow the progression of neuropathy, however, with 29% of patients in the treatment group experiencing worsening symptoms compared with 38% of those on placebo. There was no difference in tolerability or discontinuation of treatment between the 2 groups.
A recent observational study (N=101) compared the efficacy of pregabalin, carbamazepine, and ALA over a 21-month period.4 Although those taking pregabalin had the best response rate, all 3 treatments led to significant improvement in the burning associated with neuropathic pain.
ALA 100 mg bid has been investigated as part of a 3-drug combination (with pregabalin 75 mg bid and methylcobalamin 750 mcg bid) compared with monotherapy (pregabalin 75 mg bid) in an open randomized study (N=30) for 12 weeks.16 While there was a trend toward improvement in pain relief, sleep interference, and nerve function in the combination therapy group, no statistically significant difference between the 2 groups was found. Nonetheless, more than a third (36%) had a global assessment rating of “excellent” vs one in 5 (20%) of those on pregabalin alone.
THE BOTTOM LINE Overall, ALA is well tolerated; the most common adverse effects are nausea and skin rash. IV administration is more effective than oral administration, but may cause nausea, headache, and an allergic reaction at the injection site.2 ALA does have the potential for an interaction with chemotherapy and thyroid hormone and may decrease the effectiveness of these therapies.2
B vitamins
Deficiencies of vitamin B1 (thiamine), B6 (pyridoxine), B12 (cyanocobalamin), and folate are known causes of neuropathy, and correcting them often improves or eliminates the symptoms.13 Vitamin B12 deficiency is commonly seen in patients taking metformin;14 these patients may benefit from supplementation with B12 1000 mcg/d.
Many of the B vitamins have been studied for treatment of neuropathy, but benfotiamine (a lipid-soluble form of thiamine) is thought to be the best option because it is better absorbed across cell membranes than other B vitamins.9 A Cochrane review found that benfotiamine alone may be effective for both diabetic and alcoholic neuropathy and that short-term use of higher doses of vitamin B complex (25 mg B1 or 320 mg benfotiamine + 50-720 mg B6 + 1000 mcg B12 daily) may reduce neuropathic pain.9
A randomized multicenter trial (N=214) found that adding a supplement containing L-methylfolate 3 mg, pyridoxal 5-phosphate 35 mg, and methylcobalamin 2 mg twice daily to other medications (eg, pregabalin, gabapentin, or duloxetine) improved symptoms of diabetic neuropathy.10 At 24 weeks, those receiving the combination therapy had a 26% decrease in pain symptoms compared with a 15% decrease for those on medication alone, with no significant adverse effects.
THE BOTTOM LINE Overall, vitamin B supplementation is well tolerated and appears to be more effective in relieving neuropathic pain than medication alone.9,14 But larger studies are needed before its efficacy in treating patients who do not have a deficiency can be established.
Capsaicin
Capsaicin, an ingredient found in peppers, works by binding to nociceptors to selectively stimulate afferent C fibers. This causes the release of substance P, a neurotransmitter that mediates pain, leading to its depletion and resulting in desensitization.2 Several meta-analyses and systematic reviews have found that topical capsaicin can be very effective, both as an adjunctive treatment and as monotherapy for neuropathic pain.11,17,18 The concentration used in the studies was 0.075% capsaicin cream, applied 3 to 4 times a day for 6 to 12 weeks, compared with placebo creams. In all categories studied, capsaicin was either statistically significant or trending in its favor, with the exception of adverse effects.
Capsaicin led to an improvement in daily activities and ability to sleep and a reduction in pain as measured with a visual analog scale and physician global evaluation.11,17,18
The most notable adverse effects were a burning sensation on the skin and coughing and sneezing caused by inhalation of dried cream. Although the adverse effects were expected to improve after 2 to 7 days of use, a significant number of participants withdrew from the study.
A 7-study meta-analysis showed the effectiveness of an 8% capsaicin patch for treatment of post-herpetic neuralgia and HIV-associated neuropathy.12 The patch, available only by prescription, was worn every day for 4 weeks (60 minutes daily for post-herpetic neuralgia and 30 minutes a day for HIV-associated neuropathy). The pooled results were statistically significant, but the patch was less effective for patients ages 18 to 40 years and for those of Asian descent. It can be used with other analgesics or as monotherapy, with few adverse reactions.12,19
THE BOTTOM LINE Since capsaicin is a topical medication, there are no relevant drug-drug interactions. Patients should be cautioned to wash their hands after application, however, and to avoid contact with eyes and open wounds.
Gamma linolenic acid (GLA)
Also known as evening primrose oil, GLA is an omega-6 fatty acid that’s an important constituent of neuronal cell membranes—and believed to decrease neuropathic pain by having some anti-inflammatory effects.2 This suggests that therapy with GLA has the potential to improve neuronal phospholipid structure and microcirculation.2
Two placebo-controlled trials (N=22,111) showed improvement in pain scores and multiple neurophysiologic assessments in patients with diabetes treated with GLA (360-480 mg/d).20,21 The treatment was well tolerated, but the beneficial effect was more pronounced in those with less severe diabetes.
THE BOTTOM LINE The dose of GLA studied (8 to 12 capsules daily) could lead to problems with patient adherence. In addition, GLA should be used with caution in patients who are taking antiplatelet medication or have seizure disorders.2
Magnesium (Mg)
Mg is highly involved in multiple enzyme systems throughout the body. Although it is very well absorbed from dietary sources,2 patients with diabetes, liver disease, and hormonal imbalances, as well as the elderly, are often deficient in Mg. It is unclear how this affects peripheral neuropathy.13
Mg may have an antinociceptive effect by decreasing intracellular calcium influx and antagonizing N-methyl-D-aspartate receptors and associated nerve signaling.22 A small RCT (N=80) showed Mg to decrease the severity of neuropathic back pain.22 Patients received Mg sulfate 1 g IV, given over 4 hours, every day for 2 weeks. The infusion was then replaced with Mg oxide 400 mg plus Mg gluconate 100 mg, taken orally twice daily for 4 weeks. An improvement in mean pain score was seen as early as 2 weeks, and scores had decreased by 2.8 points (on a 0-10-point scale) at 6 months.
Another small RCT (N=45) gave patients with neuropathy of postherpetic, traumatic, or surgical (but not diabetic) origin Mg chloride 838 mg orally 3 times a day for 4 weeks.23 The supplement was taken with meals. Mean pain scores in the treatment group decreased by 3 points, but this was not significantly different from the improvement seen in those on placebo.
In a similar study, patients (N=110) with type 1 diabetes and a normal serum Mg but an insufficiency as measured by erythrocyte Mg were given Mg gluconate 300 mg or placebo daily for 5 years.8 The supplement slowed the progression of peripheral neuropathy (only 12% of those receiving Mg gluconate experienced a significant worsening of symptoms over the course of the study, compared with 61% of those in the placebo group), but in most cases, it did not lead to an improvement.
No consistent approach to Mg supplementation has been studied, which makes recommending a particular route, dose, or formulation challenging. There is evidence that oral Mg, particularly in the form of Mg oxide, can cause diarrhea, especially in doses >350 mg/d. Mg gluconate and Mg chloride are better tolerated; Mg carbonate should be avoided due to poor oral absorption.2
BOTTOM LINE Mg supplementation appears to slow the progression of diabetic peripheral neuropathy, but is unsafe for patients with renal dysfunction, cardiac conduction abnormalities, or elevated Mg levels.2 Caution is required, too, when considering Mg supplementation for patients taking anticoagulants, bisphosphonates, digoxin, potassium-sparing diuretics, or tetracycline antibiotics.2
CORRESPONDENCE
Mary Onysko, PharmD, BCPS, University of Wyoming, School of Pharmacy Health Sciences Center, Room 292, 1000 E. University Avenue, Laramie, WY 82071; monysko@uwyo.edu
1. Chaparro LE, Wiffen PJ, Moore RA, et al. Combination pharmacotherapy for the treatment of neuropathic pain in adults. Cochrane Database Syst Rev. 2012:(7):CD008943.
2. Natural Medicines Comprehensive Database. Natural Medicines Comprehensive Database Web site. Available at: http://naturaldatabase.therapeuticresearch.com. Accessed January 4, 2015.
3. Mijnhout GS, Kollen BJ, Alkhalaf A, et al. Alpha lipoic acid for symptomatic peripheral neuropathy in patients with diabetes: a meta-analysis of randomized controlled trials. Int J Endocrinol. 2012;2012:456279.
4. Patel N, Mishra V, Patel P, et al. A study of the use of carbamazepine, pregabalin and alpha lipoic acid in patients of diabetic neuropathy. J Diabetes Metab Disord. 2014;13:62.
5. Bertolotto F, Massone A. Combination of alpha lipoic acid and superoxide dismutase leads to physiological and symptomatic improvements in diabetic neuropathy. Drugs R D. 2012;12:29-34.
6. De Grandis D, Minardi C. Acetyl-L-carnitine (levacecarnine) in the treatment of diabetic neuropathy. A long-term, randomised, double-blind, placebo-controlled study. Drugs R D. 2002;3:223-231.
7. Sima AA, Calvani M, Mehra M, et al; Acetyl-L-Carnitine Study Group. Acetyl-L-carnitine improves pain, nerve regeneration, and vibratory perception in patients with chronic diabetic neuropathy: an analysis of two randomized placebo-controlled trials. Diabetes Care. 2005;28:89-94.
8. De Leeuw, Engelen W, De Block C, et al. Long term magnesium supplementation influences favourably the natural evolution of neuropathy in Mg-depleted type 1 diabetic patients (T1dm). Magnes Res. 2004;17:109-114.
9. Ang CD, Alviar MJM, Dans AL, et al. Vitamin B for treating peripheral neuropathy. Cochrane Database Syst Rev. 2008;(3):CD004573.
10. Fonseca VA, Lavery LA, Thethi TK, et al. Metanx in type 2 diabetes
with peripheral neuropathy: a randomized trial. Am J Med. 2013;126:141-149.
11. Mason L, Moore RA, Derry S, et al. Systematic review of topical capsaicin for the treatment of chronic pain. BMJ. 2004;328:991.
12. Mou J, Paillard F, Turnbull B, et al. Efficacy of Qutenza® (capsaicin) 8% patch for neuropathic pain: a meta-analysis of the Qutenza Clinical Trials Database. Pain. 2013;154:1632-1639.
13. Head KA. Peripheral neuropathy: pathogenic mechanisms and alternative therapies. Altern Med Rev. 2006; 11:294-329.
14. Miranda-Massari JR, Gonzalez MJ, Jimenez FJ, et al. Metabolic correction in the management of diabetic peripheral neuropathy: improving clinical results beyond symptom control. Curr Clin Pharmacol. 2011; 6:260-273.
15. Ziegler D, Low PA, Litchy WJ, et al. Efficacy and safety of antioxidant treatment with a-lipoic acid over 4 years in diabetic polyneuropathy: the NATHAN 1 trial. Diabetes Care. 2011;34:2054-2060.
16. Vasudevan D, Naik MM, Mukaddam QI. Efficacy and safety of methylcobalamin, alpha lipoic acid and pregabalin combination versus pregabalin monotherapy in improving pain and nerve conduction velocity in type 2 diabetes associated impaired peripheral neuropathic condition. [MAINTAIN]: Results of a pilot study. Ann Indian Acad Neurol. 2014;17:19-24.
17. Halat KM, Dennehy CE. Botanicals and dietary supplements in diabetic peripheral neuropathy. J Am Board Fam Pract. 2003;16:47-57.
18. Donofrio P, Walker F, Hunt V, et al. Treatment of painful diabetic neuropathy with topical capsaicin: A multicenter, double-blind, vehicle-controlled study. Arch Int Med. 1991;151:2225-2229.
19. Derry S, Rice ASC, Cole P, et al. Topical capsaicin (high concentration) for chronic neuropathic pain in adults. Cochrane Database Syst Rev. 2013;(2):CD007393.
20. Keen H, Payan J, Allawi J, et al. Treatment of diabetic neuropathy with gamma-linolenic acid. The gamma-Linolenic Acid Multicenter Trial Group. Diabetes Care. 1993;16:8-15.
21. Jamal GA, Carmichael H. The effect of gamma linolenic acid on human diabetic peripheral neuropathy: a double blind placebo controlled trial. Diabetic Med. 1990;7:319-323.
22. Yousef AA, Al-deeb AE. A double-blinded randomised controlled study of the value of sequential intravenous and oral magnesium therapy in patients with chronic low back pain with a neuropathic component. Anaesthesia. 2013;68:260-266.
23. Pickering G, Morel V, Simen E. Oral magnesium treatment in patients with neuropathic pain: a randomized clinical trial. Magnes Res. 2011;24:28-35.
1. Chaparro LE, Wiffen PJ, Moore RA, et al. Combination pharmacotherapy for the treatment of neuropathic pain in adults. Cochrane Database Syst Rev. 2012:(7):CD008943.
2. Natural Medicines Comprehensive Database. Natural Medicines Comprehensive Database Web site. Available at: http://naturaldatabase.therapeuticresearch.com. Accessed January 4, 2015.
3. Mijnhout GS, Kollen BJ, Alkhalaf A, et al. Alpha lipoic acid for symptomatic peripheral neuropathy in patients with diabetes: a meta-analysis of randomized controlled trials. Int J Endocrinol. 2012;2012:456279.
4. Patel N, Mishra V, Patel P, et al. A study of the use of carbamazepine, pregabalin and alpha lipoic acid in patients of diabetic neuropathy. J Diabetes Metab Disord. 2014;13:62.
5. Bertolotto F, Massone A. Combination of alpha lipoic acid and superoxide dismutase leads to physiological and symptomatic improvements in diabetic neuropathy. Drugs R D. 2012;12:29-34.
6. De Grandis D, Minardi C. Acetyl-L-carnitine (levacecarnine) in the treatment of diabetic neuropathy. A long-term, randomised, double-blind, placebo-controlled study. Drugs R D. 2002;3:223-231.
7. Sima AA, Calvani M, Mehra M, et al; Acetyl-L-Carnitine Study Group. Acetyl-L-carnitine improves pain, nerve regeneration, and vibratory perception in patients with chronic diabetic neuropathy: an analysis of two randomized placebo-controlled trials. Diabetes Care. 2005;28:89-94.
8. De Leeuw, Engelen W, De Block C, et al. Long term magnesium supplementation influences favourably the natural evolution of neuropathy in Mg-depleted type 1 diabetic patients (T1dm). Magnes Res. 2004;17:109-114.
9. Ang CD, Alviar MJM, Dans AL, et al. Vitamin B for treating peripheral neuropathy. Cochrane Database Syst Rev. 2008;(3):CD004573.
10. Fonseca VA, Lavery LA, Thethi TK, et al. Metanx in type 2 diabetes
with peripheral neuropathy: a randomized trial. Am J Med. 2013;126:141-149.
11. Mason L, Moore RA, Derry S, et al. Systematic review of topical capsaicin for the treatment of chronic pain. BMJ. 2004;328:991.
12. Mou J, Paillard F, Turnbull B, et al. Efficacy of Qutenza® (capsaicin) 8% patch for neuropathic pain: a meta-analysis of the Qutenza Clinical Trials Database. Pain. 2013;154:1632-1639.
13. Head KA. Peripheral neuropathy: pathogenic mechanisms and alternative therapies. Altern Med Rev. 2006; 11:294-329.
14. Miranda-Massari JR, Gonzalez MJ, Jimenez FJ, et al. Metabolic correction in the management of diabetic peripheral neuropathy: improving clinical results beyond symptom control. Curr Clin Pharmacol. 2011; 6:260-273.
15. Ziegler D, Low PA, Litchy WJ, et al. Efficacy and safety of antioxidant treatment with a-lipoic acid over 4 years in diabetic polyneuropathy: the NATHAN 1 trial. Diabetes Care. 2011;34:2054-2060.
16. Vasudevan D, Naik MM, Mukaddam QI. Efficacy and safety of methylcobalamin, alpha lipoic acid and pregabalin combination versus pregabalin monotherapy in improving pain and nerve conduction velocity in type 2 diabetes associated impaired peripheral neuropathic condition. [MAINTAIN]: Results of a pilot study. Ann Indian Acad Neurol. 2014;17:19-24.
17. Halat KM, Dennehy CE. Botanicals and dietary supplements in diabetic peripheral neuropathy. J Am Board Fam Pract. 2003;16:47-57.
18. Donofrio P, Walker F, Hunt V, et al. Treatment of painful diabetic neuropathy with topical capsaicin: A multicenter, double-blind, vehicle-controlled study. Arch Int Med. 1991;151:2225-2229.
19. Derry S, Rice ASC, Cole P, et al. Topical capsaicin (high concentration) for chronic neuropathic pain in adults. Cochrane Database Syst Rev. 2013;(2):CD007393.
20. Keen H, Payan J, Allawi J, et al. Treatment of diabetic neuropathy with gamma-linolenic acid. The gamma-Linolenic Acid Multicenter Trial Group. Diabetes Care. 1993;16:8-15.
21. Jamal GA, Carmichael H. The effect of gamma linolenic acid on human diabetic peripheral neuropathy: a double blind placebo controlled trial. Diabetic Med. 1990;7:319-323.
22. Yousef AA, Al-deeb AE. A double-blinded randomised controlled study of the value of sequential intravenous and oral magnesium therapy in patients with chronic low back pain with a neuropathic component. Anaesthesia. 2013;68:260-266.
23. Pickering G, Morel V, Simen E. Oral magnesium treatment in patients with neuropathic pain: a randomized clinical trial. Magnes Res. 2011;24:28-35.
Is a novel anticoagulant right for your patient?
› Consider novel oral anticoagualants (NOACs) for patients who have normal renal function, are comlpiant with medication regimens, and have no history of peptic ulcer or gastrointestinal bleeding. B
› Avoid overlapping warfarin with rivaroxaban or apixaban when transitioning a patient from one anticoagulant to the other, as both agents prolong prothrombin time. B
› When initiating a NOAC, it is not necessary to overlap with a parenteral anticoagulant. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE 1 Sally J is a 72-year-old Caucasian woman who comes to your clinic after being diagnosed with atrial fibrillation (AF). The patient has a 10-year history of type 2 diabetes; she also has a history of hypertension and chronic kidney disease (CKD), with a baseline creatinine clearance of approximately 40 mL/min. Ms. J tells you she knows people who take warfarin and really dislike it. She asks for your opinion of the new anticoagulants she’s seen advertised on TV, and wonders whether one of them would be right for her.
CASE 2 Bobby W, a 35-year-old African American man, was recently diagnosed with deep vein thrombosis (DVT). This was his second clot in 5 years, and occurred after a long flight home from Europe. The patient explains that he leads a very active lifestyle and doesn’t have the time to come in for the monthly international normalized ratio (INR) checks that warfarin requires. What would you recommend for these patients?
Troubled by warfarin’s narrow therapeutic index, numerous medication and dietary interactions, and need for frequent monitoring, patients requiring long-term oral anticoagulation therapy have been seeking alternatives for years. Finally, they have a choice. The US Food and Drug Administration (FDA) approved 3 oral anticoagulants—dabigatran (Pradaxa), rivaroxaban (Xarelto), and apixaban (Eliquis)—in less than 4 years. Known as novel oral anticoagulants (NOACs), they are the first such drugs to enter the market in more than 50 years.1,2
While warfarin inhibits a wide range of clotting factors (including II, VII, IX, and X), NOACs work further down the clotting cascade (TABLE 1).1,3-7 Dabigatran, a direct thrombin inhibitor, only inhibits factor IIa.3,5 Rivaroxaban and apixaban directly inhibit factor Xa and indirectly inhibit factor IIa.3,6,7
There are notable advantages to these newer agents, but some disadvantages that must be considered, as well. Appropriate patient selection, guided by a thorough understanding of the benefits and risks of NOACs, is key.
Stroke prevention in atrial fibrillation
All 3 NOACs are approved for stroke prevention in patients with nonvalvular atrial fibrillation (AF). The approvals are based on a small number of well-designed trials: RE-LY (dabigatran), ROCKET-AF (rivaroxaban), and ARISTOTLE (apixaban).8-10 Compared with warfarin, dabigatran is the only oral anticoagulant with a lower rate of both hemorrhagic and ischemic stroke.8 Both rivaroxaban and apixaban were found to decrease overall stroke risk relative to warfarin, but the difference was driven by a lower risk for hemorrhagic, not ischemic, stroke.9,10
In these trials, overall rates of major bleeding were similar to that of warfarin.8-10 Patients taking warfarin generally experienced higher rates of intracranial hemorrhage but lower rates of gastrointestinal (GI) bleeding than those on NOACs. Relative to warfarin, apixaban was the only NOAC that did not have a higher rate of GI bleeding and the only one with a lower rate of major bleeding.8-10 In addition, apixaban remains the only NOAC found to have a statistically significant decrease in all-cause mortality compared with warfarin.10 Although dabigatran and rivaroxaban were associated with a strong trend towards decreased mortality, both studies were underpowered for this secondary outcome.8,9
Adding NOACs to stroke guidelines. The role of NOACs in the prevention of stroke in patients with nonvalvular AF is beginning to be reflected in newer guidelines. The American College of Chest Physicians (ACCP)’s 2012 guidelines recommend dabigatran over warfarin (grade 2B—weak recommendation; moderate quality evidence) unless the patient is well controlled on warfarin.11 The European Society of Cardiology (ESC)’s 2012 guidelines recommend dabigatran, apixaban, and rivaroxaban as broadly preferable to warfarin, while noting that experience with these agents is limited and appropriate patient selection is important.12
Anticoagulation to treat—and prevent—VTE
The standard of care for acute venous thromboembolism (VTE) is to initiate warfarin along with a parenteral anticoagulant, such as unfractionated heparin, low-molecular-weight heparin (LMWH), or fondaparinux.13 Due to warfarin’s slow onset to peak effect, a parenteral anticoagulant is overlapped for ≥5 days—until warfarin reaches a therapeutic level and can be continued as monotherapy.13 But many patients find subcutaneous delivery of LMWH disagreeable and costly and frequent INR monitoring inconvenient, so the new agents offer notable advantages.
In well-designed studies, dabigatran, rivaroxaban, and apixaban have all been shown to be noninferior to warfarin in the initial treatment of acute DVT and pulmonary embolism (PE).14-17 All 3 agents were also shown to have lower rates of major bleeding than warfarin. Rivaroxaban and apixaban were also superior to warfarin with regard to bleeding events, and dabigatran was noninferior to warfarin for this outcome.14-17
NOACs help prevent recurrence
All 3 NOACs have been studied for long-term prevention of recurrent VTE after 3 to 18 months of anticoagulation, as well. Dabigatran was found in the RE-MEDY trial to be noninferior to warfarin for the risk of recurrent VTE, and to have lower rates of bleeding.18 In separate trials, all 3 agents were superior to placebo in preventing recurrent VTE. Rates of long-term major bleeding were significantly higher than placebo with rivaroxaban and dabigatran, but not with apixaban.15,18,19
Rivaroxaban is the only NOAC to be FDA approved for the treatment of acute DVT and PE, and the ACCP’s 2012 guidelines list it as a viable alternative to parenteral anticoagulation when initiating treatment for acute VTE.6,13 When treating VTE long term, the guidelines continue to recommend warfarin or LMWH rather than dabigatran or rivaroxaban.13 Recommendations may change in coming years, as physicians gain more experience with NOACs and more clinical trials are published.16-19
Starting or converting to NOAC therapy
In patients who have not been on anticoagulant therapy, any NOAC can be initiated immediately, with no need for parenteral, or “bridge” therapy. This is because of the rapid onset of action of the NOACs.12
To transition a patient from warfarin to a NOAC, it is necessary to discontinue warfarin therapy completely and closely monitor INR, then initiate NOAC therapy when INR≤2. No parenteral anticoagulation is necessary (TABLE 2).5-7
If it is necessary to transition a patient from a NOAC to warfarin, the protocol depends on the agent. Because dabigatran has no significant impact on prolongation of prothrombin time (PT), it can be overlapped with warfarin. Rivaroxaban and apixaban have a significant impact on PT prolongation, however, and overlapping either agent with warfarin is not recommended.4 Keep in mind that the recommended dosages for the NOACs are not standardized, and can differ drastically depending on the indication for use as well as on patient-specific factors, including renal function, body weight, and age.
Laboratory monitoring is not required
While warfarin has a great deal of interpatient variability and requires frequent lab monitoring, an oft-cited advantage of the NOACs is that they do not require regular monitoring. However, that also has a downside (TABLE 3).1,3-10,12,14-17,20-22 Monitoring INR in patients on warfarin allows physicians to assess patient compliance. And, if a patient on warfarin requires an invasive procedure, coagulation status and bleeding risk can easily be determined. That is not the case with the NOACs.
While some routine laboratory tests may be elevated in a patient taking a NOAC, the degree of elevation does not correlate well with anticoagulant concentration. And, because each NOAC has a different mechanism of action, different measures will be elevated in a patient taking dabigatran vs apixaban or rivaroxaban.4
Activated partial thromboplastin time (aPTT) is the most readily available lab test to assess the presence or absence of dabigatran.4 A normal aPTT indicates that there is little to no dabigatran present.4 But, while an elevated aPTT suggests the presence of dabigatran, it provides little information about how much.4
PT is a useful test to assess coagulation status in patients on either rivaroxaban or apixaban. A normal PT suggests that minimal amounts (or none) of the NOAC are present in the plasma.4 (A direct thrombin inhibitor assay, calibrated to more accurately assess dabigatran concentration, is being developed for clinical use, but is currently available only for research purposes in the United States; a chromogenic antifactor Xa test specific to apixaban and rivaroxaban is also being developed, but is not yet commercially available.4)
What to do when NOAC reversal is required
Patients often need to stop taking an oral anticoagulation in the days leading up to a planned invasive procedure. In an individual with normal renal function who will undergo a procedure with a standard bleeding risk, a NOAC would generally need to be withheld for one to 2 days prior to surgery, given the relatively short half-life. If a patient has acute renal failure or CKD, however, dabigatran may need to be withheld for a prolonged period (3-6 days) in order to safely proceed to surgery.4,23 NOACs may also need to be withheld for 2 to 6 days prior to any surgery with a high risk for bleeding.4
When speed is of the essence
There is no known antidote to aid in the reversal of dabigatran, rivaroxaban, or apixaban.4 Because of their relatively short half-lives, withholding the medication and providing supportive care is generally sufficient to ensure adequate hemostasis in cases of mild to moderate bleeding.4 If a patient presents with acute ingestion or an overdose, activated charcoal should be administered if the ingestion has occurred within the past 3 hours.4,24 The lack of a clear-cut reversal strategy can be extremely problematic in cases of trauma or life-threatening bleeding, however. (Fresh frozen plasma has not been shown to be effective at reversing NOACs’ effects.4)
In instances of severe bleeding or the need for urgent surgery, a more aggressive approach may be needed. Hemodialysis can be used to assist in the removal of dabigatran, but not rivaroxaban or apixaban.4 However, evidence suggests that the most effective therapy for patients who need rapid reversal of any NOAC is to administer 75 to 80 units/kg of activated prothrombin complex concentrate (aPCC).4,25-27 Recombinant factor VIIa has shown some promise in reversing the anticoagulant effects of these novel agents, but evidence is insufficient to recommend it as first-line therapy at this time.26, 27
Patients are more satisfied
The most obvious advantage of the NOACs as a group compared with warfarin is the lack of need for laboratory monitoring or continuous dose titration. Reliably stable pharmacokinetics make once or twice daily dosing possible. A rapid onset of action negates the need for bridging therapy with parenteral anticoagulants in patients at high risk of thrombosis. This may improve compliance, as many patients are averse to the use of subcutaneous injections or need extensive education before they can safely self-inject. The incidence of heparin-induced thrombocytopenia may also be decreased if unfractionated heparin and LMWH are used less frequently.
NOACs also appear to improve patient satisfaction.20-22 In one study that included patients with AF on dabigatran or warfarin, satisfaction was higher in those taking dabigatran, particularly among those who did not experience significant GI adverse effects.20 Another study showed improved patient satisfaction with rivaroxaban compared with LMWH following lower extremity joint replacement, which led to significantly higher rates of compliance.22
… but problems and pitfalls remain
In addition to the lack of a readily available and clinically validated reversal agent, the absence of a lab test that reliably measures the concentration of NOACs makes it difficult to determine whether patients are following their prescribed regimen.3,4
Medication compliance must be assessed when considering a transition from warfarin to a NOAC. Switching patients with poor INR control on warfarin to a NOAC should be done only after determining that the poor control is not the result of nonadherence. Because of the NOACs’ shorter half-life, patients who don’t take them regularly may be at higher risk for thromboembolic events.1,12
Cost is a serious consideration. While there are some costs associated with the monitoring warfarin requires, the medication itself has been generic for several decades and can be found on many “$4 lists” at pharmacies nationwide. In contrast, all 3 NOACs are available only as branded drugs, and can cost a patient with limited drug coverage anywhere from $250 to $350 per month28—a serious concern, given that the likelihood of noncompliance increases as out-of-pocket costs rise. This was highlighted in a recent study that found patients were twice as likely to discontinue their cholesterol-lowering medication if 100% of the cost was out of pocket, compared with patients who had no prescription copay.29 From the perspective of the US health care system, however, NOACs have been found to be cost effective compared with warfarin, mostly due to the lack of laboratory monitoring.30-32
Adverse effects. The risk of GI bleeds has been shown to be higher in patients taking rivaroxaban and dabigatran vs warfarin.8,9 Dabigatran has also been associated with a significant risk for dyspepsia.5,8,14 In clinical trials, the reported rate for dyspepsia in patients taking dabigatran was 3% to 11%; subsequent investigations have found the incidence to be far higher (33%).8,14,33
Drug interactions. Warfarin has a large number of drug interactions, of course, but because of its long history, these interactions are well established. NOACs also have a number of drug interactions, but the true clinical impact has not yet been established. All 3 agents are substrates for the P-glycoprotein (P-gp) transport system, so any known inhibitors or inducers of the P-gp system should be used cautiously in patients on NOACs.1,3-7,12 Rivaroxaban and apixaban are also substrates for the CYP3A4 hepatic enzyme system, so any drugs known to inhibit or induce this system require caution, as well.1,3-7,12
Who should not take a NOAC?
NOACs should not be prescribed for patients with mechanical heart valves.34 Dabigatran is the only NOAC to have been studied in this patient population, and the phase II trial was stopped prematurely due to increased risk for both bleeding and stroke in patients on dabigatran compared with warfarin.34
Renal impairment must be considered, as well. Do a baseline assessment of renal function in all patients before transitioning them to a NOAC, and periodic reassessment during therapy. While this is important for patients on rivaroxaban and apixaban, it is essential for those on dabigatran, as 80% of the drug is excreted by the kidneys.1,5,12 NOACs have not been adequately assessed in patients with severe renal dysfunction and should be avoided in this patient population. Caution should be exercised in patients with moderate renal dysfunction, as well.5-10,14-19 Apixaban appears to be the safest NOAC for patients with moderate renal dysfunction, as it has the least renal clearance.1,12
Who should take a NOAC?
No well-established criteria for patient selection for NOACs exist, yet appropriate patient selection is crucial. Evidence suggests that NOAC therapy is best suited to those who:
• are relatively young (<65 years)
• have normal renal function
• have poorly controlled INR with warfarin that is unrelated to noncompliance
• are unable to have regular INR monitoring.
Patients best suited for continued use of warfarin would be those whose INR is well controlled, those who have higher goal INR ranges (eg, because of the presence of mechanical heart valves), patients with significant renal dysfunction, and individuals with a history of peptic ulcer disease or GI bleeding. Warfarin may also be the best option for patients with a history of noncompliance and for uninsured or underinsured patients.
CASE 1 Warfarin and any of the NOACs were all feasible options for Ms. J, but apixaban was deemed to be the safest because of her moderate renal dysfunction. However, after she was told that apixaban has little “real world” clinical data, no effective antidote if bleeding were to occur, and a much higher cost than warfarin, she opted for warfarin therapy, despite the laboratory monitoring required.
CASE 2 Mr. W was excited to learn that there were new alternatives to warfarin; he had taken warfarin for 6 months after his last DVT and had a hard time coming in for INR checks. The patient reported that he had no history of bleeding and was compliant with medications. Rivaroxaban was the best option for Mr. W, as it is the only NOAC with FDA approval for the treatment of acute VTE.
CORRESPONDENCE
Jeremy Vandiver, PharmD, BCPS, Swedish Medical Center, Room 3260, 501 East Hampden Avenue, Englewood, CO 80013; jvandiver@uwyo.edu
1. Wittkowsky AK. Novel oral anticoagulants and their role in clinical practice. Pharmacotherapy. 2011;31:1175-1191.
2. Gums JG. Place of dabigatran in contemporary pharmacotherapy. Pharmacotherapy. 2011;31:335-337.
3. Ageno W, Gallus AS, Wittkowsky A, et al. Oral anticoagulant therapy: Antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141(2 suppl):e44S-e88S.
4. Siegal DM, Crowther MA. Acute management of bleeding in patients on novel oral anticoagulants. Eur Heart J. 2013;34:489-496.
5. Pradaxa [package insert]. Ridgefield, CT: Boehringer Ingelheim Pharmaceuticals, Inc; 2010.
6. Xarelto [package insert]. Titusville, NJ: Janssen Pharmaceuticals, Inc; 2011.
7. Eliquis [package insert]. Princeton, NJ: Bristol-Myers Squibb Company; 2012.
8. Connolly SJ, Zekowitz MD, Yusuf S, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139-1151.
9. Patel MR, Mahaffery KW, Garg J, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365:883-891.
10. Granger CB, Alexander JH, McMurray JJV, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365:981-992.
11. You JJ, Singer DE, Howard P, et al. Antithrombotic therapy for atrial fibrillation: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141(2 suppl):e531S-e575S.
12. Camm AJ, Lip GYH, De Caterina R, et al. 2012 focused update of the ESC guidelines for the management of atrial fibrillation. Eur Heart J. 2012;33:2719-2747.
13. Kearon C, Akl EA, Comerota AJ, et al. Antithrombotic therapy for VTE disease: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141(2 suppl):e419S-e494S.
14. Schulman S, Kearon C, Kakkar AK, et al. Dabigatran versus warfarin in the treatment of acute venous thromboembolism. N Engl J Med. 2009;361:2342-2352.
15. Bauersachs R, Berkowitz SD, Brenner B, et al. Rivaroxaban for symptomatic venous thromboembolism. N Engl J Med. 2010;363:2499-2510.
16. Büller HR, Prins MH, Lensin AW, et al. Oral rivaroxaban for the treatment of symptomatic pulmonary embolism. N Engl J Med. 2012;366:1287-1297.
17. Agnelli A, Buller HR, Cohen A, et al. Oral apixaban for the treatment of acute venous thromboembolism. N Engl J Med. 2013;369:799-808.
18. Schulman S, Kearon C, Kakkar AK, et al. Extended use of dabigatran, warfarin, or placebo in venous thromboembolism. N Engl J Med. 2013;368:709-718.
19. Agnelli G, Buller HR, Cohen A, et al. Apixaban for extended treatment of venous thromboembolism. N Engl J Med. 2013;368:699-708.
20. Michel J, Mundell D, Boga T, et al. Dabigatran for anticoagulation in atrial fibrillation–Early clinical experience in a hospital population and comparison to trial data. Heart Lung Circ. 2013;22:50-55.
21. Kendoff D, Perka C, Fritsche HM, et al. Oral thromboprophylaxis following total hip or knee replacement: review and multicentre experience with dabigatran etexilate. Open Orthop J. 2011;5:395-399.
22. Rogers BA, Phillip S, Foote J, et al. Is there adequate provision of venous thromboembolism prophylaxis following hip arthroplasty? An audit and international survey. Ann R Coll Surg Engl. 2010;92:668-672.
23. Healey JS, Eikelboom J, Douketis J, at al. Periprocedural bleeding and thromboembolic events with dabigatran compared with warfarin: results from the randomized evaluation of long-term anticoagulation therapy (RE-LY) randomized trial. Circulation. 2012;126:343-348.
24. van Ryn J, Stangier J, Haertter S, et al. Dabigatran etexilate: a novel, reversible, oral direct thrombin inhibitor: interpretation of coagulation assays and reversal of anticoagulant activity. Thromb Haemost. 2010;103:1116-1127.
25. Eerenberg ES, Kamphuisen PW, Sijpkens MK, et al. Reversal of rivaroxaban and dabigatran by prothrombin complex concentrate: a randomized, placebo-controlled, crossover study in healthy subjects. Circulation. 2011;124:1573-1579.
26. Marlu R, Hodaj E, Paris A, et al. Effect of nonspecific reversal agents on anticoagulant activity of dabigatran and rivaroxaban. A randomised crossover ex vivo study in healthy volunteers. Thromb Haemost. 2012;108:217-224.
27. Escolar G, Fernandez-Gallego V, Arellano-Rodrigo E, et al. Reversal of apixaban induced alterations of hemostasis by different coagulation factor concentrates: studies in vitro with circulating human blood. PLOS ONE. 2013;8:e78696.
28. Drug pricing information. Costco Pharmacy Web site. Available at: http://www2.costco.com/Pharmacy/DrugInformation.aspx?p=1. Accessed December 9, 2013.
29. Schneeweiss S, Patrick AR, Maclure M, et al. Adherence to statin therapy under drug cost sharing in patients with and without myocardial infarction: a population-based natural experiment. Circulation. 2007;115:2128-2135.
30. McKeage K. Dabigatran etexilate: a pharmacoeconomic review of its use in the prevention of stroke and systemic embolism in patients with atrial fibrillation. Pharmacoeconomics. 2012;30:841-55.
31. Lee S, Anglade MW, Pham D, et al. Cost–Effectiveness of rivaroxaban compared to warfarin for stroke prevention in atrial fibrillation. Am J Cardiol. 2012;110:845-851.
32. Kamel H, Easton JD, Johnston SC, et al. Cost-effectiveness of apixaban vs warfarin for secondary stroke prevention in atrial fibrillation. Neurology. 2012;79:1428-1434.
33. Schulman S, Shortt B, Robinson M, et al. Adherence to anticoagulant treatment with dabigatran in a real-world setting. J Thromb Haemost. 2013; 11:1295-1299
34. Eikelboom JW, Connolly SJ, Brueckmann M, et al. Dabigatran versus warfarin in patients with mechanical heart valves. N Engl J Med. 2013;369:1206-1214.
› Consider novel oral anticoagualants (NOACs) for patients who have normal renal function, are comlpiant with medication regimens, and have no history of peptic ulcer or gastrointestinal bleeding. B
› Avoid overlapping warfarin with rivaroxaban or apixaban when transitioning a patient from one anticoagulant to the other, as both agents prolong prothrombin time. B
› When initiating a NOAC, it is not necessary to overlap with a parenteral anticoagulant. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE 1 Sally J is a 72-year-old Caucasian woman who comes to your clinic after being diagnosed with atrial fibrillation (AF). The patient has a 10-year history of type 2 diabetes; she also has a history of hypertension and chronic kidney disease (CKD), with a baseline creatinine clearance of approximately 40 mL/min. Ms. J tells you she knows people who take warfarin and really dislike it. She asks for your opinion of the new anticoagulants she’s seen advertised on TV, and wonders whether one of them would be right for her.
CASE 2 Bobby W, a 35-year-old African American man, was recently diagnosed with deep vein thrombosis (DVT). This was his second clot in 5 years, and occurred after a long flight home from Europe. The patient explains that he leads a very active lifestyle and doesn’t have the time to come in for the monthly international normalized ratio (INR) checks that warfarin requires. What would you recommend for these patients?
Troubled by warfarin’s narrow therapeutic index, numerous medication and dietary interactions, and need for frequent monitoring, patients requiring long-term oral anticoagulation therapy have been seeking alternatives for years. Finally, they have a choice. The US Food and Drug Administration (FDA) approved 3 oral anticoagulants—dabigatran (Pradaxa), rivaroxaban (Xarelto), and apixaban (Eliquis)—in less than 4 years. Known as novel oral anticoagulants (NOACs), they are the first such drugs to enter the market in more than 50 years.1,2
While warfarin inhibits a wide range of clotting factors (including II, VII, IX, and X), NOACs work further down the clotting cascade (TABLE 1).1,3-7 Dabigatran, a direct thrombin inhibitor, only inhibits factor IIa.3,5 Rivaroxaban and apixaban directly inhibit factor Xa and indirectly inhibit factor IIa.3,6,7
There are notable advantages to these newer agents, but some disadvantages that must be considered, as well. Appropriate patient selection, guided by a thorough understanding of the benefits and risks of NOACs, is key.
Stroke prevention in atrial fibrillation
All 3 NOACs are approved for stroke prevention in patients with nonvalvular atrial fibrillation (AF). The approvals are based on a small number of well-designed trials: RE-LY (dabigatran), ROCKET-AF (rivaroxaban), and ARISTOTLE (apixaban).8-10 Compared with warfarin, dabigatran is the only oral anticoagulant with a lower rate of both hemorrhagic and ischemic stroke.8 Both rivaroxaban and apixaban were found to decrease overall stroke risk relative to warfarin, but the difference was driven by a lower risk for hemorrhagic, not ischemic, stroke.9,10
In these trials, overall rates of major bleeding were similar to that of warfarin.8-10 Patients taking warfarin generally experienced higher rates of intracranial hemorrhage but lower rates of gastrointestinal (GI) bleeding than those on NOACs. Relative to warfarin, apixaban was the only NOAC that did not have a higher rate of GI bleeding and the only one with a lower rate of major bleeding.8-10 In addition, apixaban remains the only NOAC found to have a statistically significant decrease in all-cause mortality compared with warfarin.10 Although dabigatran and rivaroxaban were associated with a strong trend towards decreased mortality, both studies were underpowered for this secondary outcome.8,9
Adding NOACs to stroke guidelines. The role of NOACs in the prevention of stroke in patients with nonvalvular AF is beginning to be reflected in newer guidelines. The American College of Chest Physicians (ACCP)’s 2012 guidelines recommend dabigatran over warfarin (grade 2B—weak recommendation; moderate quality evidence) unless the patient is well controlled on warfarin.11 The European Society of Cardiology (ESC)’s 2012 guidelines recommend dabigatran, apixaban, and rivaroxaban as broadly preferable to warfarin, while noting that experience with these agents is limited and appropriate patient selection is important.12
Anticoagulation to treat—and prevent—VTE
The standard of care for acute venous thromboembolism (VTE) is to initiate warfarin along with a parenteral anticoagulant, such as unfractionated heparin, low-molecular-weight heparin (LMWH), or fondaparinux.13 Due to warfarin’s slow onset to peak effect, a parenteral anticoagulant is overlapped for ≥5 days—until warfarin reaches a therapeutic level and can be continued as monotherapy.13 But many patients find subcutaneous delivery of LMWH disagreeable and costly and frequent INR monitoring inconvenient, so the new agents offer notable advantages.
In well-designed studies, dabigatran, rivaroxaban, and apixaban have all been shown to be noninferior to warfarin in the initial treatment of acute DVT and pulmonary embolism (PE).14-17 All 3 agents were also shown to have lower rates of major bleeding than warfarin. Rivaroxaban and apixaban were also superior to warfarin with regard to bleeding events, and dabigatran was noninferior to warfarin for this outcome.14-17
NOACs help prevent recurrence
All 3 NOACs have been studied for long-term prevention of recurrent VTE after 3 to 18 months of anticoagulation, as well. Dabigatran was found in the RE-MEDY trial to be noninferior to warfarin for the risk of recurrent VTE, and to have lower rates of bleeding.18 In separate trials, all 3 agents were superior to placebo in preventing recurrent VTE. Rates of long-term major bleeding were significantly higher than placebo with rivaroxaban and dabigatran, but not with apixaban.15,18,19
Rivaroxaban is the only NOAC to be FDA approved for the treatment of acute DVT and PE, and the ACCP’s 2012 guidelines list it as a viable alternative to parenteral anticoagulation when initiating treatment for acute VTE.6,13 When treating VTE long term, the guidelines continue to recommend warfarin or LMWH rather than dabigatran or rivaroxaban.13 Recommendations may change in coming years, as physicians gain more experience with NOACs and more clinical trials are published.16-19
Starting or converting to NOAC therapy
In patients who have not been on anticoagulant therapy, any NOAC can be initiated immediately, with no need for parenteral, or “bridge” therapy. This is because of the rapid onset of action of the NOACs.12
To transition a patient from warfarin to a NOAC, it is necessary to discontinue warfarin therapy completely and closely monitor INR, then initiate NOAC therapy when INR≤2. No parenteral anticoagulation is necessary (TABLE 2).5-7
If it is necessary to transition a patient from a NOAC to warfarin, the protocol depends on the agent. Because dabigatran has no significant impact on prolongation of prothrombin time (PT), it can be overlapped with warfarin. Rivaroxaban and apixaban have a significant impact on PT prolongation, however, and overlapping either agent with warfarin is not recommended.4 Keep in mind that the recommended dosages for the NOACs are not standardized, and can differ drastically depending on the indication for use as well as on patient-specific factors, including renal function, body weight, and age.
Laboratory monitoring is not required
While warfarin has a great deal of interpatient variability and requires frequent lab monitoring, an oft-cited advantage of the NOACs is that they do not require regular monitoring. However, that also has a downside (TABLE 3).1,3-10,12,14-17,20-22 Monitoring INR in patients on warfarin allows physicians to assess patient compliance. And, if a patient on warfarin requires an invasive procedure, coagulation status and bleeding risk can easily be determined. That is not the case with the NOACs.
While some routine laboratory tests may be elevated in a patient taking a NOAC, the degree of elevation does not correlate well with anticoagulant concentration. And, because each NOAC has a different mechanism of action, different measures will be elevated in a patient taking dabigatran vs apixaban or rivaroxaban.4
Activated partial thromboplastin time (aPTT) is the most readily available lab test to assess the presence or absence of dabigatran.4 A normal aPTT indicates that there is little to no dabigatran present.4 But, while an elevated aPTT suggests the presence of dabigatran, it provides little information about how much.4
PT is a useful test to assess coagulation status in patients on either rivaroxaban or apixaban. A normal PT suggests that minimal amounts (or none) of the NOAC are present in the plasma.4 (A direct thrombin inhibitor assay, calibrated to more accurately assess dabigatran concentration, is being developed for clinical use, but is currently available only for research purposes in the United States; a chromogenic antifactor Xa test specific to apixaban and rivaroxaban is also being developed, but is not yet commercially available.4)
What to do when NOAC reversal is required
Patients often need to stop taking an oral anticoagulation in the days leading up to a planned invasive procedure. In an individual with normal renal function who will undergo a procedure with a standard bleeding risk, a NOAC would generally need to be withheld for one to 2 days prior to surgery, given the relatively short half-life. If a patient has acute renal failure or CKD, however, dabigatran may need to be withheld for a prolonged period (3-6 days) in order to safely proceed to surgery.4,23 NOACs may also need to be withheld for 2 to 6 days prior to any surgery with a high risk for bleeding.4
When speed is of the essence
There is no known antidote to aid in the reversal of dabigatran, rivaroxaban, or apixaban.4 Because of their relatively short half-lives, withholding the medication and providing supportive care is generally sufficient to ensure adequate hemostasis in cases of mild to moderate bleeding.4 If a patient presents with acute ingestion or an overdose, activated charcoal should be administered if the ingestion has occurred within the past 3 hours.4,24 The lack of a clear-cut reversal strategy can be extremely problematic in cases of trauma or life-threatening bleeding, however. (Fresh frozen plasma has not been shown to be effective at reversing NOACs’ effects.4)
In instances of severe bleeding or the need for urgent surgery, a more aggressive approach may be needed. Hemodialysis can be used to assist in the removal of dabigatran, but not rivaroxaban or apixaban.4 However, evidence suggests that the most effective therapy for patients who need rapid reversal of any NOAC is to administer 75 to 80 units/kg of activated prothrombin complex concentrate (aPCC).4,25-27 Recombinant factor VIIa has shown some promise in reversing the anticoagulant effects of these novel agents, but evidence is insufficient to recommend it as first-line therapy at this time.26, 27
Patients are more satisfied
The most obvious advantage of the NOACs as a group compared with warfarin is the lack of need for laboratory monitoring or continuous dose titration. Reliably stable pharmacokinetics make once or twice daily dosing possible. A rapid onset of action negates the need for bridging therapy with parenteral anticoagulants in patients at high risk of thrombosis. This may improve compliance, as many patients are averse to the use of subcutaneous injections or need extensive education before they can safely self-inject. The incidence of heparin-induced thrombocytopenia may also be decreased if unfractionated heparin and LMWH are used less frequently.
NOACs also appear to improve patient satisfaction.20-22 In one study that included patients with AF on dabigatran or warfarin, satisfaction was higher in those taking dabigatran, particularly among those who did not experience significant GI adverse effects.20 Another study showed improved patient satisfaction with rivaroxaban compared with LMWH following lower extremity joint replacement, which led to significantly higher rates of compliance.22
… but problems and pitfalls remain
In addition to the lack of a readily available and clinically validated reversal agent, the absence of a lab test that reliably measures the concentration of NOACs makes it difficult to determine whether patients are following their prescribed regimen.3,4
Medication compliance must be assessed when considering a transition from warfarin to a NOAC. Switching patients with poor INR control on warfarin to a NOAC should be done only after determining that the poor control is not the result of nonadherence. Because of the NOACs’ shorter half-life, patients who don’t take them regularly may be at higher risk for thromboembolic events.1,12
Cost is a serious consideration. While there are some costs associated with the monitoring warfarin requires, the medication itself has been generic for several decades and can be found on many “$4 lists” at pharmacies nationwide. In contrast, all 3 NOACs are available only as branded drugs, and can cost a patient with limited drug coverage anywhere from $250 to $350 per month28—a serious concern, given that the likelihood of noncompliance increases as out-of-pocket costs rise. This was highlighted in a recent study that found patients were twice as likely to discontinue their cholesterol-lowering medication if 100% of the cost was out of pocket, compared with patients who had no prescription copay.29 From the perspective of the US health care system, however, NOACs have been found to be cost effective compared with warfarin, mostly due to the lack of laboratory monitoring.30-32
Adverse effects. The risk of GI bleeds has been shown to be higher in patients taking rivaroxaban and dabigatran vs warfarin.8,9 Dabigatran has also been associated with a significant risk for dyspepsia.5,8,14 In clinical trials, the reported rate for dyspepsia in patients taking dabigatran was 3% to 11%; subsequent investigations have found the incidence to be far higher (33%).8,14,33
Drug interactions. Warfarin has a large number of drug interactions, of course, but because of its long history, these interactions are well established. NOACs also have a number of drug interactions, but the true clinical impact has not yet been established. All 3 agents are substrates for the P-glycoprotein (P-gp) transport system, so any known inhibitors or inducers of the P-gp system should be used cautiously in patients on NOACs.1,3-7,12 Rivaroxaban and apixaban are also substrates for the CYP3A4 hepatic enzyme system, so any drugs known to inhibit or induce this system require caution, as well.1,3-7,12
Who should not take a NOAC?
NOACs should not be prescribed for patients with mechanical heart valves.34 Dabigatran is the only NOAC to have been studied in this patient population, and the phase II trial was stopped prematurely due to increased risk for both bleeding and stroke in patients on dabigatran compared with warfarin.34
Renal impairment must be considered, as well. Do a baseline assessment of renal function in all patients before transitioning them to a NOAC, and periodic reassessment during therapy. While this is important for patients on rivaroxaban and apixaban, it is essential for those on dabigatran, as 80% of the drug is excreted by the kidneys.1,5,12 NOACs have not been adequately assessed in patients with severe renal dysfunction and should be avoided in this patient population. Caution should be exercised in patients with moderate renal dysfunction, as well.5-10,14-19 Apixaban appears to be the safest NOAC for patients with moderate renal dysfunction, as it has the least renal clearance.1,12
Who should take a NOAC?
No well-established criteria for patient selection for NOACs exist, yet appropriate patient selection is crucial. Evidence suggests that NOAC therapy is best suited to those who:
• are relatively young (<65 years)
• have normal renal function
• have poorly controlled INR with warfarin that is unrelated to noncompliance
• are unable to have regular INR monitoring.
Patients best suited for continued use of warfarin would be those whose INR is well controlled, those who have higher goal INR ranges (eg, because of the presence of mechanical heart valves), patients with significant renal dysfunction, and individuals with a history of peptic ulcer disease or GI bleeding. Warfarin may also be the best option for patients with a history of noncompliance and for uninsured or underinsured patients.
CASE 1 Warfarin and any of the NOACs were all feasible options for Ms. J, but apixaban was deemed to be the safest because of her moderate renal dysfunction. However, after she was told that apixaban has little “real world” clinical data, no effective antidote if bleeding were to occur, and a much higher cost than warfarin, she opted for warfarin therapy, despite the laboratory monitoring required.
CASE 2 Mr. W was excited to learn that there were new alternatives to warfarin; he had taken warfarin for 6 months after his last DVT and had a hard time coming in for INR checks. The patient reported that he had no history of bleeding and was compliant with medications. Rivaroxaban was the best option for Mr. W, as it is the only NOAC with FDA approval for the treatment of acute VTE.
CORRESPONDENCE
Jeremy Vandiver, PharmD, BCPS, Swedish Medical Center, Room 3260, 501 East Hampden Avenue, Englewood, CO 80013; jvandiver@uwyo.edu
› Consider novel oral anticoagualants (NOACs) for patients who have normal renal function, are comlpiant with medication regimens, and have no history of peptic ulcer or gastrointestinal bleeding. B
› Avoid overlapping warfarin with rivaroxaban or apixaban when transitioning a patient from one anticoagulant to the other, as both agents prolong prothrombin time. B
› When initiating a NOAC, it is not necessary to overlap with a parenteral anticoagulant. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE 1 Sally J is a 72-year-old Caucasian woman who comes to your clinic after being diagnosed with atrial fibrillation (AF). The patient has a 10-year history of type 2 diabetes; she also has a history of hypertension and chronic kidney disease (CKD), with a baseline creatinine clearance of approximately 40 mL/min. Ms. J tells you she knows people who take warfarin and really dislike it. She asks for your opinion of the new anticoagulants she’s seen advertised on TV, and wonders whether one of them would be right for her.
CASE 2 Bobby W, a 35-year-old African American man, was recently diagnosed with deep vein thrombosis (DVT). This was his second clot in 5 years, and occurred after a long flight home from Europe. The patient explains that he leads a very active lifestyle and doesn’t have the time to come in for the monthly international normalized ratio (INR) checks that warfarin requires. What would you recommend for these patients?
Troubled by warfarin’s narrow therapeutic index, numerous medication and dietary interactions, and need for frequent monitoring, patients requiring long-term oral anticoagulation therapy have been seeking alternatives for years. Finally, they have a choice. The US Food and Drug Administration (FDA) approved 3 oral anticoagulants—dabigatran (Pradaxa), rivaroxaban (Xarelto), and apixaban (Eliquis)—in less than 4 years. Known as novel oral anticoagulants (NOACs), they are the first such drugs to enter the market in more than 50 years.1,2
While warfarin inhibits a wide range of clotting factors (including II, VII, IX, and X), NOACs work further down the clotting cascade (TABLE 1).1,3-7 Dabigatran, a direct thrombin inhibitor, only inhibits factor IIa.3,5 Rivaroxaban and apixaban directly inhibit factor Xa and indirectly inhibit factor IIa.3,6,7
There are notable advantages to these newer agents, but some disadvantages that must be considered, as well. Appropriate patient selection, guided by a thorough understanding of the benefits and risks of NOACs, is key.
Stroke prevention in atrial fibrillation
All 3 NOACs are approved for stroke prevention in patients with nonvalvular atrial fibrillation (AF). The approvals are based on a small number of well-designed trials: RE-LY (dabigatran), ROCKET-AF (rivaroxaban), and ARISTOTLE (apixaban).8-10 Compared with warfarin, dabigatran is the only oral anticoagulant with a lower rate of both hemorrhagic and ischemic stroke.8 Both rivaroxaban and apixaban were found to decrease overall stroke risk relative to warfarin, but the difference was driven by a lower risk for hemorrhagic, not ischemic, stroke.9,10
In these trials, overall rates of major bleeding were similar to that of warfarin.8-10 Patients taking warfarin generally experienced higher rates of intracranial hemorrhage but lower rates of gastrointestinal (GI) bleeding than those on NOACs. Relative to warfarin, apixaban was the only NOAC that did not have a higher rate of GI bleeding and the only one with a lower rate of major bleeding.8-10 In addition, apixaban remains the only NOAC found to have a statistically significant decrease in all-cause mortality compared with warfarin.10 Although dabigatran and rivaroxaban were associated with a strong trend towards decreased mortality, both studies were underpowered for this secondary outcome.8,9
Adding NOACs to stroke guidelines. The role of NOACs in the prevention of stroke in patients with nonvalvular AF is beginning to be reflected in newer guidelines. The American College of Chest Physicians (ACCP)’s 2012 guidelines recommend dabigatran over warfarin (grade 2B—weak recommendation; moderate quality evidence) unless the patient is well controlled on warfarin.11 The European Society of Cardiology (ESC)’s 2012 guidelines recommend dabigatran, apixaban, and rivaroxaban as broadly preferable to warfarin, while noting that experience with these agents is limited and appropriate patient selection is important.12
Anticoagulation to treat—and prevent—VTE
The standard of care for acute venous thromboembolism (VTE) is to initiate warfarin along with a parenteral anticoagulant, such as unfractionated heparin, low-molecular-weight heparin (LMWH), or fondaparinux.13 Due to warfarin’s slow onset to peak effect, a parenteral anticoagulant is overlapped for ≥5 days—until warfarin reaches a therapeutic level and can be continued as monotherapy.13 But many patients find subcutaneous delivery of LMWH disagreeable and costly and frequent INR monitoring inconvenient, so the new agents offer notable advantages.
In well-designed studies, dabigatran, rivaroxaban, and apixaban have all been shown to be noninferior to warfarin in the initial treatment of acute DVT and pulmonary embolism (PE).14-17 All 3 agents were also shown to have lower rates of major bleeding than warfarin. Rivaroxaban and apixaban were also superior to warfarin with regard to bleeding events, and dabigatran was noninferior to warfarin for this outcome.14-17
NOACs help prevent recurrence
All 3 NOACs have been studied for long-term prevention of recurrent VTE after 3 to 18 months of anticoagulation, as well. Dabigatran was found in the RE-MEDY trial to be noninferior to warfarin for the risk of recurrent VTE, and to have lower rates of bleeding.18 In separate trials, all 3 agents were superior to placebo in preventing recurrent VTE. Rates of long-term major bleeding were significantly higher than placebo with rivaroxaban and dabigatran, but not with apixaban.15,18,19
Rivaroxaban is the only NOAC to be FDA approved for the treatment of acute DVT and PE, and the ACCP’s 2012 guidelines list it as a viable alternative to parenteral anticoagulation when initiating treatment for acute VTE.6,13 When treating VTE long term, the guidelines continue to recommend warfarin or LMWH rather than dabigatran or rivaroxaban.13 Recommendations may change in coming years, as physicians gain more experience with NOACs and more clinical trials are published.16-19
Starting or converting to NOAC therapy
In patients who have not been on anticoagulant therapy, any NOAC can be initiated immediately, with no need for parenteral, or “bridge” therapy. This is because of the rapid onset of action of the NOACs.12
To transition a patient from warfarin to a NOAC, it is necessary to discontinue warfarin therapy completely and closely monitor INR, then initiate NOAC therapy when INR≤2. No parenteral anticoagulation is necessary (TABLE 2).5-7
If it is necessary to transition a patient from a NOAC to warfarin, the protocol depends on the agent. Because dabigatran has no significant impact on prolongation of prothrombin time (PT), it can be overlapped with warfarin. Rivaroxaban and apixaban have a significant impact on PT prolongation, however, and overlapping either agent with warfarin is not recommended.4 Keep in mind that the recommended dosages for the NOACs are not standardized, and can differ drastically depending on the indication for use as well as on patient-specific factors, including renal function, body weight, and age.
Laboratory monitoring is not required
While warfarin has a great deal of interpatient variability and requires frequent lab monitoring, an oft-cited advantage of the NOACs is that they do not require regular monitoring. However, that also has a downside (TABLE 3).1,3-10,12,14-17,20-22 Monitoring INR in patients on warfarin allows physicians to assess patient compliance. And, if a patient on warfarin requires an invasive procedure, coagulation status and bleeding risk can easily be determined. That is not the case with the NOACs.
While some routine laboratory tests may be elevated in a patient taking a NOAC, the degree of elevation does not correlate well with anticoagulant concentration. And, because each NOAC has a different mechanism of action, different measures will be elevated in a patient taking dabigatran vs apixaban or rivaroxaban.4
Activated partial thromboplastin time (aPTT) is the most readily available lab test to assess the presence or absence of dabigatran.4 A normal aPTT indicates that there is little to no dabigatran present.4 But, while an elevated aPTT suggests the presence of dabigatran, it provides little information about how much.4
PT is a useful test to assess coagulation status in patients on either rivaroxaban or apixaban. A normal PT suggests that minimal amounts (or none) of the NOAC are present in the plasma.4 (A direct thrombin inhibitor assay, calibrated to more accurately assess dabigatran concentration, is being developed for clinical use, but is currently available only for research purposes in the United States; a chromogenic antifactor Xa test specific to apixaban and rivaroxaban is also being developed, but is not yet commercially available.4)
What to do when NOAC reversal is required
Patients often need to stop taking an oral anticoagulation in the days leading up to a planned invasive procedure. In an individual with normal renal function who will undergo a procedure with a standard bleeding risk, a NOAC would generally need to be withheld for one to 2 days prior to surgery, given the relatively short half-life. If a patient has acute renal failure or CKD, however, dabigatran may need to be withheld for a prolonged period (3-6 days) in order to safely proceed to surgery.4,23 NOACs may also need to be withheld for 2 to 6 days prior to any surgery with a high risk for bleeding.4
When speed is of the essence
There is no known antidote to aid in the reversal of dabigatran, rivaroxaban, or apixaban.4 Because of their relatively short half-lives, withholding the medication and providing supportive care is generally sufficient to ensure adequate hemostasis in cases of mild to moderate bleeding.4 If a patient presents with acute ingestion or an overdose, activated charcoal should be administered if the ingestion has occurred within the past 3 hours.4,24 The lack of a clear-cut reversal strategy can be extremely problematic in cases of trauma or life-threatening bleeding, however. (Fresh frozen plasma has not been shown to be effective at reversing NOACs’ effects.4)
In instances of severe bleeding or the need for urgent surgery, a more aggressive approach may be needed. Hemodialysis can be used to assist in the removal of dabigatran, but not rivaroxaban or apixaban.4 However, evidence suggests that the most effective therapy for patients who need rapid reversal of any NOAC is to administer 75 to 80 units/kg of activated prothrombin complex concentrate (aPCC).4,25-27 Recombinant factor VIIa has shown some promise in reversing the anticoagulant effects of these novel agents, but evidence is insufficient to recommend it as first-line therapy at this time.26, 27
Patients are more satisfied
The most obvious advantage of the NOACs as a group compared with warfarin is the lack of need for laboratory monitoring or continuous dose titration. Reliably stable pharmacokinetics make once or twice daily dosing possible. A rapid onset of action negates the need for bridging therapy with parenteral anticoagulants in patients at high risk of thrombosis. This may improve compliance, as many patients are averse to the use of subcutaneous injections or need extensive education before they can safely self-inject. The incidence of heparin-induced thrombocytopenia may also be decreased if unfractionated heparin and LMWH are used less frequently.
NOACs also appear to improve patient satisfaction.20-22 In one study that included patients with AF on dabigatran or warfarin, satisfaction was higher in those taking dabigatran, particularly among those who did not experience significant GI adverse effects.20 Another study showed improved patient satisfaction with rivaroxaban compared with LMWH following lower extremity joint replacement, which led to significantly higher rates of compliance.22
… but problems and pitfalls remain
In addition to the lack of a readily available and clinically validated reversal agent, the absence of a lab test that reliably measures the concentration of NOACs makes it difficult to determine whether patients are following their prescribed regimen.3,4
Medication compliance must be assessed when considering a transition from warfarin to a NOAC. Switching patients with poor INR control on warfarin to a NOAC should be done only after determining that the poor control is not the result of nonadherence. Because of the NOACs’ shorter half-life, patients who don’t take them regularly may be at higher risk for thromboembolic events.1,12
Cost is a serious consideration. While there are some costs associated with the monitoring warfarin requires, the medication itself has been generic for several decades and can be found on many “$4 lists” at pharmacies nationwide. In contrast, all 3 NOACs are available only as branded drugs, and can cost a patient with limited drug coverage anywhere from $250 to $350 per month28—a serious concern, given that the likelihood of noncompliance increases as out-of-pocket costs rise. This was highlighted in a recent study that found patients were twice as likely to discontinue their cholesterol-lowering medication if 100% of the cost was out of pocket, compared with patients who had no prescription copay.29 From the perspective of the US health care system, however, NOACs have been found to be cost effective compared with warfarin, mostly due to the lack of laboratory monitoring.30-32
Adverse effects. The risk of GI bleeds has been shown to be higher in patients taking rivaroxaban and dabigatran vs warfarin.8,9 Dabigatran has also been associated with a significant risk for dyspepsia.5,8,14 In clinical trials, the reported rate for dyspepsia in patients taking dabigatran was 3% to 11%; subsequent investigations have found the incidence to be far higher (33%).8,14,33
Drug interactions. Warfarin has a large number of drug interactions, of course, but because of its long history, these interactions are well established. NOACs also have a number of drug interactions, but the true clinical impact has not yet been established. All 3 agents are substrates for the P-glycoprotein (P-gp) transport system, so any known inhibitors or inducers of the P-gp system should be used cautiously in patients on NOACs.1,3-7,12 Rivaroxaban and apixaban are also substrates for the CYP3A4 hepatic enzyme system, so any drugs known to inhibit or induce this system require caution, as well.1,3-7,12
Who should not take a NOAC?
NOACs should not be prescribed for patients with mechanical heart valves.34 Dabigatran is the only NOAC to have been studied in this patient population, and the phase II trial was stopped prematurely due to increased risk for both bleeding and stroke in patients on dabigatran compared with warfarin.34
Renal impairment must be considered, as well. Do a baseline assessment of renal function in all patients before transitioning them to a NOAC, and periodic reassessment during therapy. While this is important for patients on rivaroxaban and apixaban, it is essential for those on dabigatran, as 80% of the drug is excreted by the kidneys.1,5,12 NOACs have not been adequately assessed in patients with severe renal dysfunction and should be avoided in this patient population. Caution should be exercised in patients with moderate renal dysfunction, as well.5-10,14-19 Apixaban appears to be the safest NOAC for patients with moderate renal dysfunction, as it has the least renal clearance.1,12
Who should take a NOAC?
No well-established criteria for patient selection for NOACs exist, yet appropriate patient selection is crucial. Evidence suggests that NOAC therapy is best suited to those who:
• are relatively young (<65 years)
• have normal renal function
• have poorly controlled INR with warfarin that is unrelated to noncompliance
• are unable to have regular INR monitoring.
Patients best suited for continued use of warfarin would be those whose INR is well controlled, those who have higher goal INR ranges (eg, because of the presence of mechanical heart valves), patients with significant renal dysfunction, and individuals with a history of peptic ulcer disease or GI bleeding. Warfarin may also be the best option for patients with a history of noncompliance and for uninsured or underinsured patients.
CASE 1 Warfarin and any of the NOACs were all feasible options for Ms. J, but apixaban was deemed to be the safest because of her moderate renal dysfunction. However, after she was told that apixaban has little “real world” clinical data, no effective antidote if bleeding were to occur, and a much higher cost than warfarin, she opted for warfarin therapy, despite the laboratory monitoring required.
CASE 2 Mr. W was excited to learn that there were new alternatives to warfarin; he had taken warfarin for 6 months after his last DVT and had a hard time coming in for INR checks. The patient reported that he had no history of bleeding and was compliant with medications. Rivaroxaban was the best option for Mr. W, as it is the only NOAC with FDA approval for the treatment of acute VTE.
CORRESPONDENCE
Jeremy Vandiver, PharmD, BCPS, Swedish Medical Center, Room 3260, 501 East Hampden Avenue, Englewood, CO 80013; jvandiver@uwyo.edu
1. Wittkowsky AK. Novel oral anticoagulants and their role in clinical practice. Pharmacotherapy. 2011;31:1175-1191.
2. Gums JG. Place of dabigatran in contemporary pharmacotherapy. Pharmacotherapy. 2011;31:335-337.
3. Ageno W, Gallus AS, Wittkowsky A, et al. Oral anticoagulant therapy: Antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141(2 suppl):e44S-e88S.
4. Siegal DM, Crowther MA. Acute management of bleeding in patients on novel oral anticoagulants. Eur Heart J. 2013;34:489-496.
5. Pradaxa [package insert]. Ridgefield, CT: Boehringer Ingelheim Pharmaceuticals, Inc; 2010.
6. Xarelto [package insert]. Titusville, NJ: Janssen Pharmaceuticals, Inc; 2011.
7. Eliquis [package insert]. Princeton, NJ: Bristol-Myers Squibb Company; 2012.
8. Connolly SJ, Zekowitz MD, Yusuf S, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139-1151.
9. Patel MR, Mahaffery KW, Garg J, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365:883-891.
10. Granger CB, Alexander JH, McMurray JJV, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365:981-992.
11. You JJ, Singer DE, Howard P, et al. Antithrombotic therapy for atrial fibrillation: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141(2 suppl):e531S-e575S.
12. Camm AJ, Lip GYH, De Caterina R, et al. 2012 focused update of the ESC guidelines for the management of atrial fibrillation. Eur Heart J. 2012;33:2719-2747.
13. Kearon C, Akl EA, Comerota AJ, et al. Antithrombotic therapy for VTE disease: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141(2 suppl):e419S-e494S.
14. Schulman S, Kearon C, Kakkar AK, et al. Dabigatran versus warfarin in the treatment of acute venous thromboembolism. N Engl J Med. 2009;361:2342-2352.
15. Bauersachs R, Berkowitz SD, Brenner B, et al. Rivaroxaban for symptomatic venous thromboembolism. N Engl J Med. 2010;363:2499-2510.
16. Büller HR, Prins MH, Lensin AW, et al. Oral rivaroxaban for the treatment of symptomatic pulmonary embolism. N Engl J Med. 2012;366:1287-1297.
17. Agnelli A, Buller HR, Cohen A, et al. Oral apixaban for the treatment of acute venous thromboembolism. N Engl J Med. 2013;369:799-808.
18. Schulman S, Kearon C, Kakkar AK, et al. Extended use of dabigatran, warfarin, or placebo in venous thromboembolism. N Engl J Med. 2013;368:709-718.
19. Agnelli G, Buller HR, Cohen A, et al. Apixaban for extended treatment of venous thromboembolism. N Engl J Med. 2013;368:699-708.
20. Michel J, Mundell D, Boga T, et al. Dabigatran for anticoagulation in atrial fibrillation–Early clinical experience in a hospital population and comparison to trial data. Heart Lung Circ. 2013;22:50-55.
21. Kendoff D, Perka C, Fritsche HM, et al. Oral thromboprophylaxis following total hip or knee replacement: review and multicentre experience with dabigatran etexilate. Open Orthop J. 2011;5:395-399.
22. Rogers BA, Phillip S, Foote J, et al. Is there adequate provision of venous thromboembolism prophylaxis following hip arthroplasty? An audit and international survey. Ann R Coll Surg Engl. 2010;92:668-672.
23. Healey JS, Eikelboom J, Douketis J, at al. Periprocedural bleeding and thromboembolic events with dabigatran compared with warfarin: results from the randomized evaluation of long-term anticoagulation therapy (RE-LY) randomized trial. Circulation. 2012;126:343-348.
24. van Ryn J, Stangier J, Haertter S, et al. Dabigatran etexilate: a novel, reversible, oral direct thrombin inhibitor: interpretation of coagulation assays and reversal of anticoagulant activity. Thromb Haemost. 2010;103:1116-1127.
25. Eerenberg ES, Kamphuisen PW, Sijpkens MK, et al. Reversal of rivaroxaban and dabigatran by prothrombin complex concentrate: a randomized, placebo-controlled, crossover study in healthy subjects. Circulation. 2011;124:1573-1579.
26. Marlu R, Hodaj E, Paris A, et al. Effect of nonspecific reversal agents on anticoagulant activity of dabigatran and rivaroxaban. A randomised crossover ex vivo study in healthy volunteers. Thromb Haemost. 2012;108:217-224.
27. Escolar G, Fernandez-Gallego V, Arellano-Rodrigo E, et al. Reversal of apixaban induced alterations of hemostasis by different coagulation factor concentrates: studies in vitro with circulating human blood. PLOS ONE. 2013;8:e78696.
28. Drug pricing information. Costco Pharmacy Web site. Available at: http://www2.costco.com/Pharmacy/DrugInformation.aspx?p=1. Accessed December 9, 2013.
29. Schneeweiss S, Patrick AR, Maclure M, et al. Adherence to statin therapy under drug cost sharing in patients with and without myocardial infarction: a population-based natural experiment. Circulation. 2007;115:2128-2135.
30. McKeage K. Dabigatran etexilate: a pharmacoeconomic review of its use in the prevention of stroke and systemic embolism in patients with atrial fibrillation. Pharmacoeconomics. 2012;30:841-55.
31. Lee S, Anglade MW, Pham D, et al. Cost–Effectiveness of rivaroxaban compared to warfarin for stroke prevention in atrial fibrillation. Am J Cardiol. 2012;110:845-851.
32. Kamel H, Easton JD, Johnston SC, et al. Cost-effectiveness of apixaban vs warfarin for secondary stroke prevention in atrial fibrillation. Neurology. 2012;79:1428-1434.
33. Schulman S, Shortt B, Robinson M, et al. Adherence to anticoagulant treatment with dabigatran in a real-world setting. J Thromb Haemost. 2013; 11:1295-1299
34. Eikelboom JW, Connolly SJ, Brueckmann M, et al. Dabigatran versus warfarin in patients with mechanical heart valves. N Engl J Med. 2013;369:1206-1214.
1. Wittkowsky AK. Novel oral anticoagulants and their role in clinical practice. Pharmacotherapy. 2011;31:1175-1191.
2. Gums JG. Place of dabigatran in contemporary pharmacotherapy. Pharmacotherapy. 2011;31:335-337.
3. Ageno W, Gallus AS, Wittkowsky A, et al. Oral anticoagulant therapy: Antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141(2 suppl):e44S-e88S.
4. Siegal DM, Crowther MA. Acute management of bleeding in patients on novel oral anticoagulants. Eur Heart J. 2013;34:489-496.
5. Pradaxa [package insert]. Ridgefield, CT: Boehringer Ingelheim Pharmaceuticals, Inc; 2010.
6. Xarelto [package insert]. Titusville, NJ: Janssen Pharmaceuticals, Inc; 2011.
7. Eliquis [package insert]. Princeton, NJ: Bristol-Myers Squibb Company; 2012.
8. Connolly SJ, Zekowitz MD, Yusuf S, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139-1151.
9. Patel MR, Mahaffery KW, Garg J, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365:883-891.
10. Granger CB, Alexander JH, McMurray JJV, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365:981-992.
11. You JJ, Singer DE, Howard P, et al. Antithrombotic therapy for atrial fibrillation: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141(2 suppl):e531S-e575S.
12. Camm AJ, Lip GYH, De Caterina R, et al. 2012 focused update of the ESC guidelines for the management of atrial fibrillation. Eur Heart J. 2012;33:2719-2747.
13. Kearon C, Akl EA, Comerota AJ, et al. Antithrombotic therapy for VTE disease: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141(2 suppl):e419S-e494S.
14. Schulman S, Kearon C, Kakkar AK, et al. Dabigatran versus warfarin in the treatment of acute venous thromboembolism. N Engl J Med. 2009;361:2342-2352.
15. Bauersachs R, Berkowitz SD, Brenner B, et al. Rivaroxaban for symptomatic venous thromboembolism. N Engl J Med. 2010;363:2499-2510.
16. Büller HR, Prins MH, Lensin AW, et al. Oral rivaroxaban for the treatment of symptomatic pulmonary embolism. N Engl J Med. 2012;366:1287-1297.
17. Agnelli A, Buller HR, Cohen A, et al. Oral apixaban for the treatment of acute venous thromboembolism. N Engl J Med. 2013;369:799-808.
18. Schulman S, Kearon C, Kakkar AK, et al. Extended use of dabigatran, warfarin, or placebo in venous thromboembolism. N Engl J Med. 2013;368:709-718.
19. Agnelli G, Buller HR, Cohen A, et al. Apixaban for extended treatment of venous thromboembolism. N Engl J Med. 2013;368:699-708.
20. Michel J, Mundell D, Boga T, et al. Dabigatran for anticoagulation in atrial fibrillation–Early clinical experience in a hospital population and comparison to trial data. Heart Lung Circ. 2013;22:50-55.
21. Kendoff D, Perka C, Fritsche HM, et al. Oral thromboprophylaxis following total hip or knee replacement: review and multicentre experience with dabigatran etexilate. Open Orthop J. 2011;5:395-399.
22. Rogers BA, Phillip S, Foote J, et al. Is there adequate provision of venous thromboembolism prophylaxis following hip arthroplasty? An audit and international survey. Ann R Coll Surg Engl. 2010;92:668-672.
23. Healey JS, Eikelboom J, Douketis J, at al. Periprocedural bleeding and thromboembolic events with dabigatran compared with warfarin: results from the randomized evaluation of long-term anticoagulation therapy (RE-LY) randomized trial. Circulation. 2012;126:343-348.
24. van Ryn J, Stangier J, Haertter S, et al. Dabigatran etexilate: a novel, reversible, oral direct thrombin inhibitor: interpretation of coagulation assays and reversal of anticoagulant activity. Thromb Haemost. 2010;103:1116-1127.
25. Eerenberg ES, Kamphuisen PW, Sijpkens MK, et al. Reversal of rivaroxaban and dabigatran by prothrombin complex concentrate: a randomized, placebo-controlled, crossover study in healthy subjects. Circulation. 2011;124:1573-1579.
26. Marlu R, Hodaj E, Paris A, et al. Effect of nonspecific reversal agents on anticoagulant activity of dabigatran and rivaroxaban. A randomised crossover ex vivo study in healthy volunteers. Thromb Haemost. 2012;108:217-224.
27. Escolar G, Fernandez-Gallego V, Arellano-Rodrigo E, et al. Reversal of apixaban induced alterations of hemostasis by different coagulation factor concentrates: studies in vitro with circulating human blood. PLOS ONE. 2013;8:e78696.
28. Drug pricing information. Costco Pharmacy Web site. Available at: http://www2.costco.com/Pharmacy/DrugInformation.aspx?p=1. Accessed December 9, 2013.
29. Schneeweiss S, Patrick AR, Maclure M, et al. Adherence to statin therapy under drug cost sharing in patients with and without myocardial infarction: a population-based natural experiment. Circulation. 2007;115:2128-2135.
30. McKeage K. Dabigatran etexilate: a pharmacoeconomic review of its use in the prevention of stroke and systemic embolism in patients with atrial fibrillation. Pharmacoeconomics. 2012;30:841-55.
31. Lee S, Anglade MW, Pham D, et al. Cost–Effectiveness of rivaroxaban compared to warfarin for stroke prevention in atrial fibrillation. Am J Cardiol. 2012;110:845-851.
32. Kamel H, Easton JD, Johnston SC, et al. Cost-effectiveness of apixaban vs warfarin for secondary stroke prevention in atrial fibrillation. Neurology. 2012;79:1428-1434.
33. Schulman S, Shortt B, Robinson M, et al. Adherence to anticoagulant treatment with dabigatran in a real-world setting. J Thromb Haemost. 2013; 11:1295-1299
34. Eikelboom JW, Connolly SJ, Brueckmann M, et al. Dabigatran versus warfarin in patients with mechanical heart valves. N Engl J Med. 2013;369:1206-1214.