User login
5 drug interactions you don’t want to miss
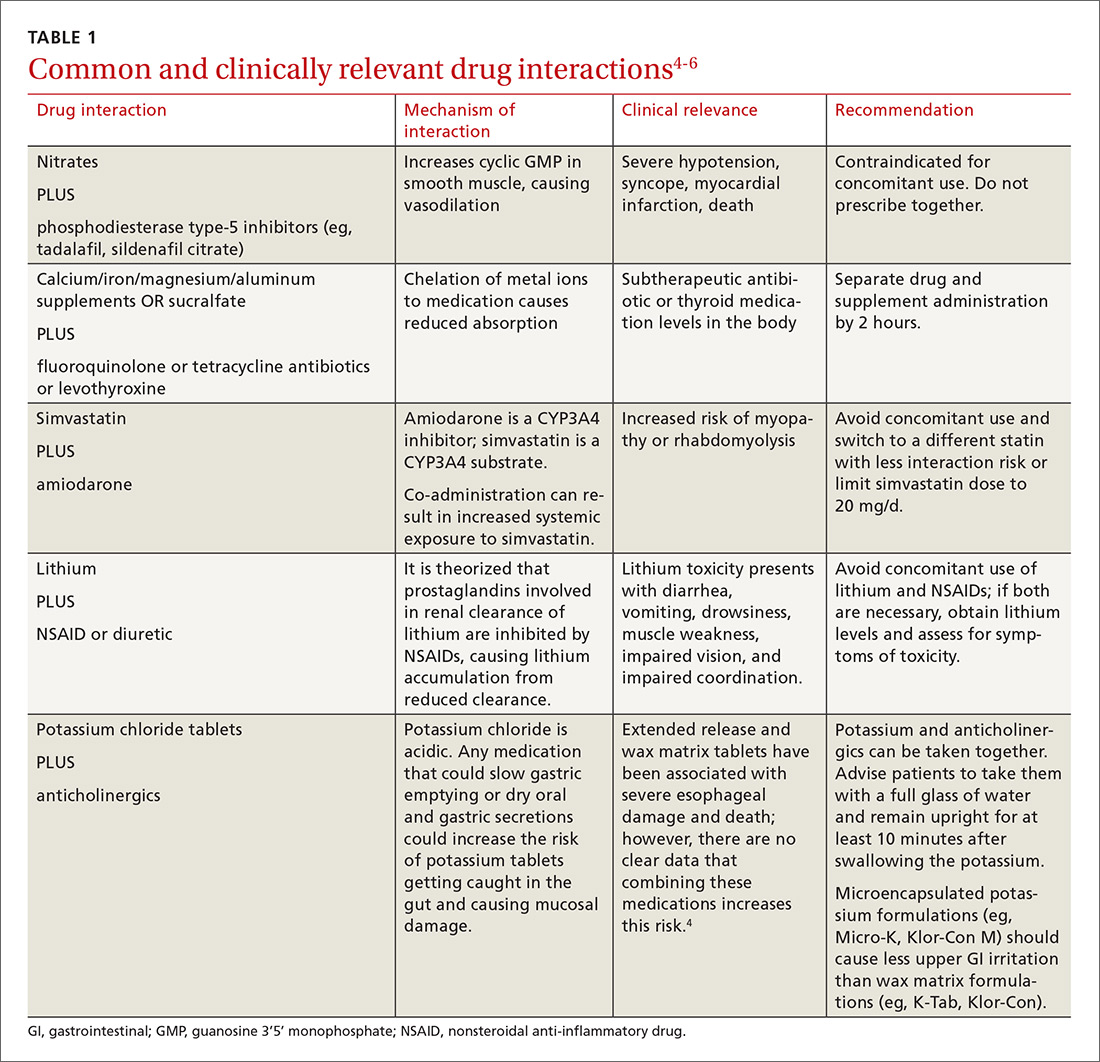
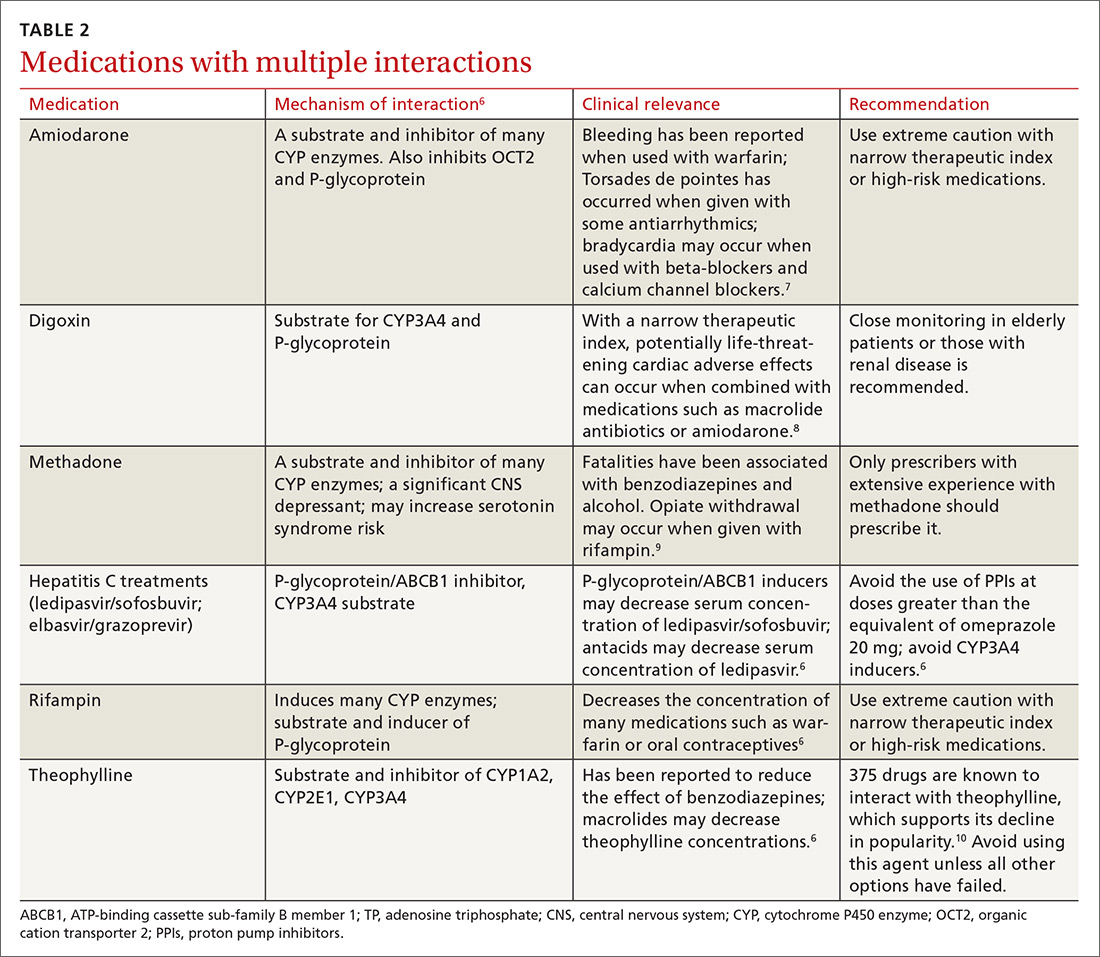
There is a strong relationship between the number of medications taken and the likelihood of a potentially serious drug-drug interaction.1,2 Drug interaction software programs can help alert prescribers to potential problems, but these programs sometimes fail to detect important interactions or generate so many clinically insignificant alerts that they become a nuisance.3 This review provides guidance about 5 clinically relevant drug interactions, including those that are common (TABLE 14-6)—and those that are less common, but no less important (TABLE 26-10).
1. Antiepileptics & contraceptives
Many antiepileptic medications decrease the efficacy of certain contraceptives
Contraception management in women with epilepsy is critical due to potential maternal and fetal complications. Many antiepileptic drugs (AEDs), including carbamazepine, ethosuximide, fosphenytoin, phenobarbital, phenytoin, primidone, topiramate, and valproate, are potentially teratogenic.11 A retrospective, observational study of 115 women of childbearing age who had epilepsy and were seen at a neurology clinic found that 74% were not using documented contraception.11 Of the minority of study participants using contraception, most were using oral contraceptives (OCs) that could potentially interact with AEDs.
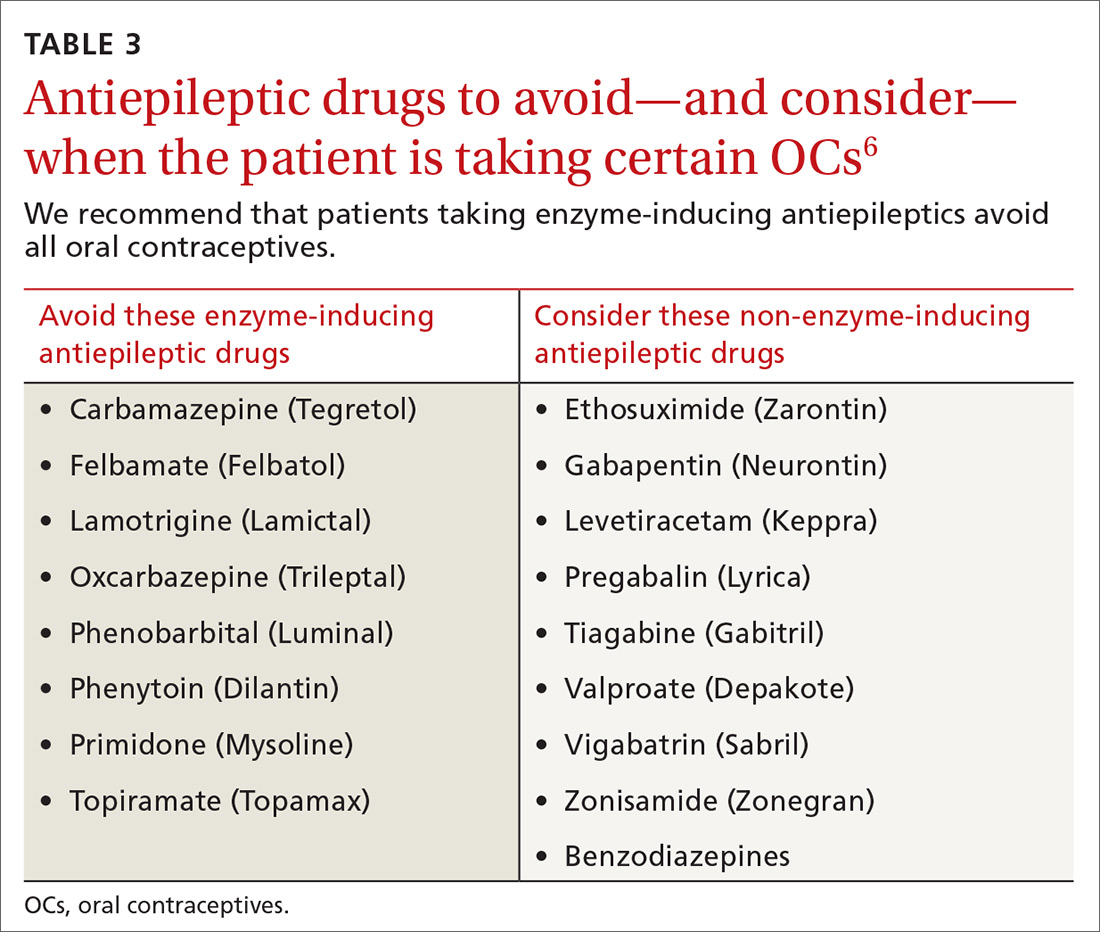
CYP inducers. Estrogen and progesterone are metabolized by the cytochrome P450 3A4 enzyme. Some AEDs induce this enzyme, which can enhance the metabolism of OCs, thus reducing their efficacy.12 It is not known, however, if this interaction results in increased pregnancy rates.13 Most newer AEDs (TABLE 36) do not induce cytochrome P450 3A4 and, thus, do not appear to affect OC efficacy, and may be safer for women with seizure disorders.12 While enzyme-inducing AEDs may decrease the efficacy of progesterone-only OCs and the morning-after pill,12,14,15 progesterone-containing intrauterine devices (IUDs), long-acting progesterone injections, and non-hormonal contraceptive methods appear to be unaffected.14-17
OCs and seizure frequency. There is no strong evidence that OCs affect seizure frequency in epileptic women, although changes in hormone levels during the menstrual cycle do affect seizure susceptibility.12 Combination OCs decrease lamotrigine levels and, therefore, may increase the risk of seizures, but progesterone-only pills do not produce this effect.12,16
Do guidelines exist? There are no specific evidence-based guidelines that pertain to the use of AEDs and contraception together, but some organizations have issued recommendations.
The American College of Obstetricians and Gynecologists recommends using a 30- to 35-mcg estrogen-containing OC rather than a lower dose in women taking an enzyme-inducing AED. The group also recommends using condoms with OCs or using IUDs.18
The American Academy of Neurology suggests that women taking OCs and enzyme-inducing AEDs use an OC containing at least 50 mcg estrogen.19
The National Institute for Health and Care Excellence recommends that women taking enzyme-inducing AEDs avoid progestin-only pills.20
The Faculty of Sexual and Reproductive Healthcare agrees that enzyme-inducing drugs may decrease efficacy and recommend considering IUDs and injectable contraceptive methods.21
2. SSRIs & NSAIDs.
SSRIs increase the GI bleeding risk associated with NSAIDs alone
Nonsteroidal anti-inflammatory drugs (NSAIDs) and selective serotonin reuptake inhibitors (SSRIs) are commonly prescribed worldwide.22,23 A well-established adverse effect of NSAIDs is gastrointestinal (GI) bleeding, and there is increasing evidence that concomitant use of an SSRI can further increase that risk through a variety of mechanisms.23
SSRIs decrease platelet serotonin levels resulting in defective platelet aggregation and impaired hemostasis. Studies have also shown that SSRIs increase gastric acidity, which leads to increased risk of peptic ulcer disease and GI bleeding.23 These mechanisms, combined with the inhibition of gastroprotective prostaglandin cyclooxygenase-1 and platelets by NSAIDs, further potentiate GI bleeding risk.24
Patients at high risk for bleeding with concomitant SSRIs and NSAIDs include older patients, patients with other risk factors for GI bleeding (eg, chronic steroid use), and patients with a history of GI bleeding.23
The evidence. A 2014 meta-analysis found that when SSRIs were used in combination with NSAIDs, the risk of GI bleeding was significantly increased, compared with SSRI monotherapy.23
Case control studies found the risk of upper GI bleeding with SSRIs had a number needed to harm (NNH) of 3177 for a low-risk population and 881 for a high-risk population with an odds ratio (OR) of 1.66 (95% confidence interval [CI], 1.44-1.92; P<.00001).23 When SSRIs were used in combination with NSAIDs, the NNH decreased to 645 for a low-risk population and 179 for a high-risk population (OR=4.25; 95% CI, 2.82-6.42; P<.0001).23
Another meta-analysis found that the OR for bleeding risk increased to 6.33 (95% CI, 3.40-11.8; P<.00001; NNH=106) with concomitant use of NSAIDs and SSRIs, compared with 2.36 (95% CI, 1.44-3.85; P=.0006; NNH=411) for SSRI use alone.25
The studies did not evaluate results based on the indication, dose, or duration of SSRI or NSAID treatment. If both an SSRI and an NSAID must be used, select a cyclooxygenase-2 selective NSAID at the lowest effective dose and consider the addition of a proton pump inhibitor to decrease the risk of a GI bleed.23,26
3. Direct oral anticoagulants and antiepileptics
Don’t use DOACs in patients taking certain antiepileptic medications
Drug interactions with anticoagulants, such as warfarin, are well documented and have been publicized for years, but physicians must also be aware of the potential for interaction between the direct oral anticoagulants (DOACs) and AEDs.
Apixaban, rivaroxaban, and dabigatran appear to interact withthe AEDs carbamazepine, phenytoin, and phenobarbital.27,28 These interactions occur due to AED induction of the CYP3A4 enzyme and effects on the P-glycoprotein (P-gp) efflux pump.27,29 When taken together, the AED induces metabolism and elimination of the DOAC medication to occur more quickly than it would normally, resulting in subtherapeutic concentrations of the DOAC. This could theoretically result in a venous thromboembolic event or stroke.
A caveat. One thing to consider is that studies demonstrating interaction between the DOAC and AED drug classes have been performed in healthy volunteers, making it difficult to extrapolate how this interaction may increase the risk for thrombotic events in other patients.
Some studies demonstrated reductions in drug levels of up to 50% with strong CYP3A4 and P-glycoprotein inducers.30 Common inducers include carbamazepine, rifampin, and St. John’s Wort.6 Patients taking such agents could theoretically have decreased exposure to the DOAC, resulting in an increase in thromboembolic risk.31
4. Statins & certain CYP inhibitors
Combining simvastatin with fibrates warrants extra attention
The efficacy of statin medications in the prevention of atherosclerotic cardiovascular disease (ASCVD) is clear. However, the clinical significance of many identified drug interactions involving statins is difficult to interpret. Interactions that cause increased serum concentrations of statins can increase the risk for liver enzyme elevations and skeletal muscle abnormalities (myalgias to rhabdomyolysis).32 Strong inhibitors of CYP3A4 (amiodarone, cyclosporine, ketoconazole, etc.) significantly increase concentrations of lovastatin, simvastatin, and atorvastatin. Pitavastatin, pravastatin, and rosuvastatin are not susceptible to any CYP-mediated drug interactions;33 therefore, rosuvastatin (a high-intensity statin) is usually recommended over other statins for patients taking strong inhibitors of CYP3A4.
When to limit simvastatin. Doses of simvastatin should not exceed 10 mg/d when combined with diltiazem, dronedarone, or verapamil, and doses should not exceed 20 mg/d when used with amiodarone, amlodipine, or ranolazine.6 These recommendations are in response to results from the SEARCH (Study of the Effectiveness of Additional Reductions in cholesterol and homocysteine) trial, which found a higher incidence of myopathies and rhabdomyolysis in patients taking 80 mg of simvastatin compared with those taking 20-mg doses.34 CYP3A4-inducing medications, especially diltiazem, were thought to also contribute to an increased risk.34
Avoid gemfibrozil with statins. Using fibrates with statins is beneficial for some patients; however, gemfibrozil significantly interacts with statins by inhibiting CYP2C8 and organic anion transporting polypeptide 1B1 (OATP1B1).33 The safer choice is fenofibrate because it does not interfere with statin metabolism and can be safely used in combination with statins.6
A retrospective review of the FDA Adverse Event Reporting System (AERS) database found that 88% of fibrate and statin combinations that resulted in rhabdomyolysis were associated with gemfibrozil/cerivastatin (cerivastatin is no longer available in the United States).35
5. One serotonergic drug & another
Serotonin syndrome is associated with more than just SSRIs
Serotonin syndrome is a constellation of symptoms (hyperthermia, hyperreflexia, muscle clonus, tremor and altered mental status) caused by increases in serotonin levels in the central and peripheral nervous systems that can lead to mild or life-threatening complications such as seizures, muscle breakdown, or hyperthermia. Serotonin syndrome is most likely to occur within 24 hours after a dose increase, after starting a new medication that increases serotonin levels, or after a drug overdose.36
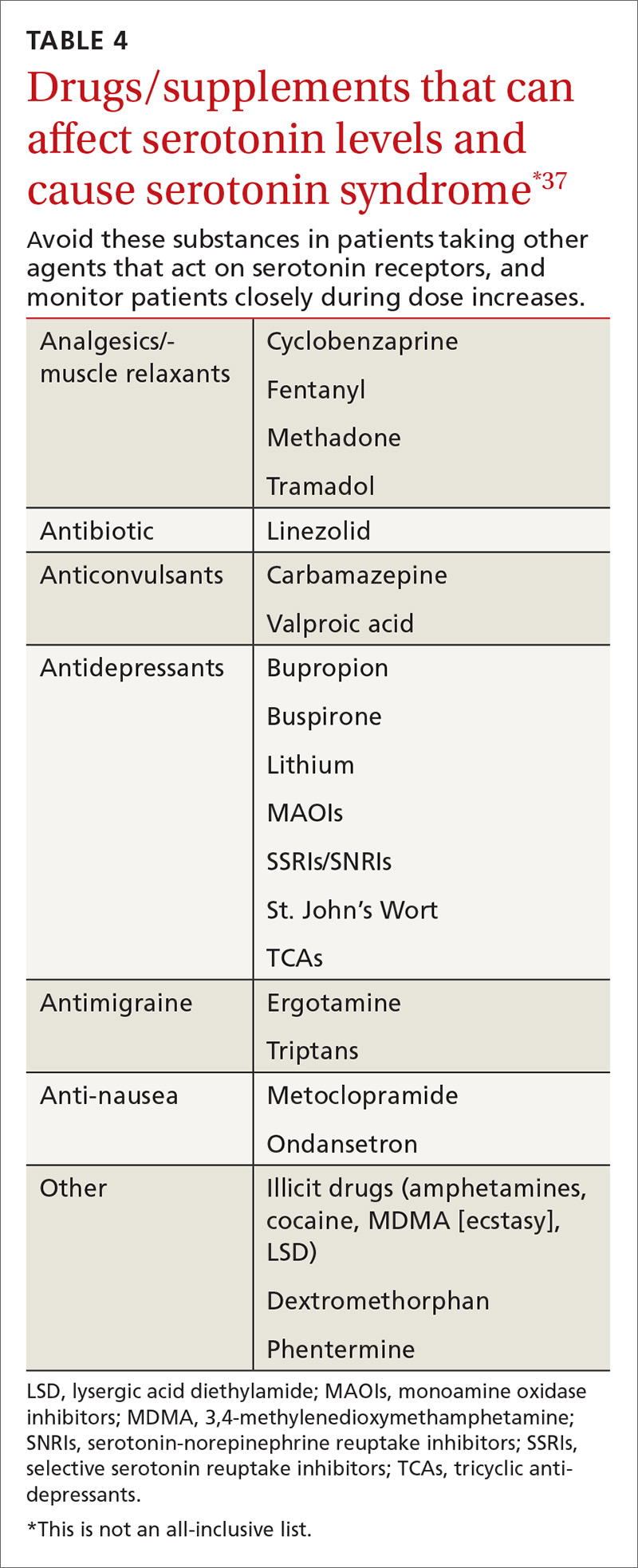
SSRIs are the most commonly reported drug associated with serotonin syndrome; however, other medications (TABLE 437) may be responsible, especially when used in combination with agents that act on serotonin receptors or in patients with impaired metabolism of the drugs being used.37
Other culprits. Serotonergic effects can also be associated with illicit drugs, some nonprescription medications, and supplements. And in March 2016, the FDA issued a warning about the risks of taking opioids with serotonergic medications.38 Although labeling changes have been recommended for all opioids, the cases of serotonin syndrome were reported more often with normal doses of fentanyl and methadone.
There are 2 mechanisms by which drugs may increase a patient’s risk for serotonin syndrome. The first is a pharmacodynamic interaction, which can occur when 2 or more medications act at the same receptor site (serotonin receptors in this example), which may result in an additive or synergistic effect.39
The second mechanism is a pharmacokinetic alteration (an agent alters absorption, distribution, metabolism, or excretion) of CYP enzymes.40 Of the more commonly used antidepressants, citalopram, escitalopram, venlafaxine, and mirtazapine seem to have the least potential for clinically significant pharmacokinetic interactions.41
Guidelines? Currently there are no guidelines for preventing serotonin syndrome. Clinicians should exercise caution in patients at high risk for drug adverse events, such as the elderly, patients taking multiple medications, and patients with comorbidities. Healthy low-risk patients can generally take 2 or 3 serotonergic medications at therapeutic doses without a major risk of harm.
CORRESPONDENCE
Mary Onysko, PharmD, BCPS, 191 East Orchard Road, Suite 200, Littleton, CO 80121; monysko@uwyo.edu.
1. Aparasu R, Baer R, Aparasu A. Clinically important potential drug-drug interactions in outpatient settings. Res Social Adm Pharm. 2007;3:426-437.
2. Johnell K, Klarin I. The relationship between number of drugs and potential drug-drug interactions in the elderly: a study of over 600,000 elderly patients from the Swedish Prescribed Drug Register. Drug Saf. 2007;30:911-918.
3. Pharmacist’s Letter. Online continuing medical education and webinars. Drug interaction overload: Problems and solutions for drug interaction alerts. Volume 2012, Course No. 216. Self-Study Course #120216. Available at: http://pharmacistsletter.therapeuticresearch.com/ce/cecourse.aspx?pc=15-219&quiz=1. Accessed June 9, 2016.
4. PL Detail-Document, Potassium and Anticholinergic Drug Interaction. Pharmacist’s Letter/Prescriber’s Letter. October 2011.
5. Micromedex Solutions. Available at: http://www.micromedexsolutions.com. Accessed May 3, 2016.
6. Lexi-Comp Online. Available at: http://online.lexi.com/lco/action/home. Accessed May 22, 2016.
7. Marcus FI. Drug interactions with amiodarone. Am Heart J. 1983;106(4 Pt 2):924-930.
8. Digoxin: serious drug interactions. Prescrire Int. 2010;19:68-70.
9. McCance-Katz EF, Sullivan LE, Nallani S. Drug interactions of clinical importance among the opioids, methadone and buprenorphine, and other frequently prescribed medications: a review. Am J Addict. 2010;19:4-16.
10. Drugs.com. Theophylline drug interactions. Available at: https://www.drugs.com/drug-interactions/theophylline.html. Accessed June 23, 2016.
11. Bhakta J, Bainbridge J, Borgelt L. Teratogenic medications and concurrent contraceptive use in women of childbearing ability with epilepsy. Epilepsy Behav. 2015;52(Pt A):212-217.
12. Reddy DS. Clinical pharmacokinetic interactions between antiepileptic drugs and hormonal contraceptives. Expert Rev Clin Pharmacol. 2010;3:183-192.
13. Carl JS, Weaver SP, Tweed E. Effect of antiepileptic drugs on oral contraceptives. Am Fam Physician. 2008;78:634-635.
14. O’Brien MD, Guillebaud J. Contraception for women with epilepsy. Epilepsia. 2006;47:1419-1422.
15. Schwenkhagen AM, Stodieck SR. Which contraception for women with epilepsy? Seizure. 2008;17:145-150.
16. Faculty of Sexual and Reproductive Healthcare Clinical Effectiveness Unit. Antiepileptic drugs and contraception. CEU statement. January 2010. Available at: https://www.fsrh.org/standards-and-guidance/documents/ceu-clinical-guidance-drug-interactions-with-hormonal/. Accessed April 25, 2016.
17. Perruca E. Clinically relevant drug interactions with antiepileptic drugs. Br J Clin Pharmacol. 2006;61:246-255.
18. ACOG practice bulletin. Number 73: Use of hormonal contraception in women with coexisting medical conditions. ACOG Committee on Practice Bulletins-Gynecology. Obstet Gynecol. 2006;107:1453-1472.
19. Report of the Quality Standards Subcommittee of the American Academy of Neurology. Practice parameter: management issues for women with epilepsy (summary statement). Neurology. 1998;51:944-948.
20. National Institute for Health and Care Excellence. Do not do recommendation. Available at: https://www.nice.org.uk/donotdo/the-progestogenonly-pill-is-not-recommended-as-reliable-contraception-inwomen-and-girls-taking-enzymeinducing-anti-epileptic-drugs-aeds. Accessed September 21, 2017.
21. Faculty of Sexual and Reproductive Healthcare. Clinical guidance: drug interactions with hormonal contraception. Available at: https://www.fsrh.org/standards-and-guidance/documents/ceu-clinical-guidance-drug-interactions-with-hormonal/. Accessed September 21, 2017.
22. de Jong JCF, van den Berg PB, Tobi H, et al. Combined use of SSRIs and NSAIDs increases the risk of gastrointestinal adverse effects. Br J Clin Pharmacol. 2003;55:591-595.
23. Anglin R, Yuan Y, Moayyedi P, et al. Risk of upper gastrointestinal bleeding with selective serotonin reuptake inhibitors with or without concurrent nonsteroidal anti-inflammatory use: a systematic review and meta-analysis. Am J Gastroenterol. 2014;109:811-819.
24. Mort JR, Aparasu RR, Baer RK, et al. Interaction between selective serotonin reuptake inhibitors and nonsteroidal anti-inflammatory drugs: review of the literature. Pharmacotherapy. 2006;26:1307-1313.
25. Loke YK, Trivedi AN, Singh S. Meta-analysis: gastrointestinal bleeding due to interaction between selective serotonin uptake inhibitors and non-steroidal anti-inflammatory drugs. Aliment Pharmacol Ther. 2008;27:31-40.
26. Venerito M, Wex T, Malfertheiner P. Nonsteroidal anti-inflammatory drug-induced gastroduodenal bleeding: risk factors and prevention strategies. Pharmaceuticals. 2010;3:2225-2237.
27. Boehringer S, Williams CD, Yawn BP, et al. Managing interactions with direct oral anticoagulants (DOACs). Pharmacist’s Letter. May 2016.
28. Johannessen SI, Landmark CJ. Antiepileptic drug interactions – principles and clinical implications. Curr Neuropharmacol. 2010;8:254-267.
29. Mohrien K, Oliphant CS, Self TH. Drug interactions with novel oral anticoagulants. Consultant. 2013;53:918-919. Available at: http://www.consultant360.com/articles/drug-interactions-novel-oral-anticoagulants. Accessed May 3, 2016.
30. Wiggins BS, Northup A, Johnson D, et al. Reduced anticoagulant effect of dabigatran in a patient receiving concomitant phenytoin. Pharmacotherapy. 2016;36:e5-e7.
31. Burnett AE, Mahan CE, Vazquez SR, et al. Guidance for the practical management of the direct oral anticoagulants (DOACs) in VTE treatment. J Thromb Thrombolysis. 2016;41:206-232.
32. Thompson PD, Clarkson P, Karas RH. Statin-associated myopathy. JAMA. 2003;289:1681-1690.
33. Hirota T, Leiri I. Drug-drug interactions that interfere with statin metabolism. Expert Opin Drug Metab Toxicol. 2015;11:1435-1447.
34. Study of the Effectiveness of Additional Reductions in Cholesterol and Homocysteine (SEARCH) Collaborative Group. Intensive lowering of LDL cholesterol wih 80 mg versus 20 mg simvastatin daily in 12,064 survivors of myocardial inffarction: a double-blind randomised trial. Lancet. 2010;376:1658-1669.
35. Jones PH, Davidson MH. Reporting rate of rhabdomyolysis with fenofibrate + statin versus gemfibrozil + any statin. Am J Cardiol. 2005;95:120-122.
36. Birmes P, Coppin D, Schmitt L, et al. Serotonin syndrome: a brief review. CMAJ. 2003;168:1439-1442.
37. Boyer EW, Shannon M. The serotonin syndrome. N Engl J Med. 2005;352:1112-1120.
38. US Food and Drug Administration. FDA Drug Safety Communication: FDA warns about several safety issues with opioid pain medicines; requires label changes. Available at: https://www.fda.gov/Drugs/DrugSafety/ucm489676.htm. Accessed June 15, 2016.
39. Sultana J, Spina E, Trifirò G. Antidepressant use in the elderly: the role of pharmacodynamics and pharmacokinetics in drug safety. Expert Opin Drug Metab Toxicol. 2015;11:883-892.
40. Sproule BA, Naranjo CA, Brenmer KE, et al. Selective serotonin reuptake inhibitors and CNS drug interactions. A critical review of the evidence. Clin Pharmacokinet. 1997;33:454-471.
41. Spina E, Santoro V, D’Arrigo C. Clinically relevant pharmacokinetic drug interactions with second-generation antidepressants: an update. Clin Ther. 2008;30:1206-1227.
There is a strong relationship between the number of medications taken and the likelihood of a potentially serious drug-drug interaction.1,2 Drug interaction software programs can help alert prescribers to potential problems, but these programs sometimes fail to detect important interactions or generate so many clinically insignificant alerts that they become a nuisance.3 This review provides guidance about 5 clinically relevant drug interactions, including those that are common (TABLE 14-6)—and those that are less common, but no less important (TABLE 26-10).
1. Antiepileptics & contraceptives
Many antiepileptic medications decrease the efficacy of certain contraceptives
Contraception management in women with epilepsy is critical due to potential maternal and fetal complications. Many antiepileptic drugs (AEDs), including carbamazepine, ethosuximide, fosphenytoin, phenobarbital, phenytoin, primidone, topiramate, and valproate, are potentially teratogenic.11 A retrospective, observational study of 115 women of childbearing age who had epilepsy and were seen at a neurology clinic found that 74% were not using documented contraception.11 Of the minority of study participants using contraception, most were using oral contraceptives (OCs) that could potentially interact with AEDs.
CYP inducers. Estrogen and progesterone are metabolized by the cytochrome P450 3A4 enzyme. Some AEDs induce this enzyme, which can enhance the metabolism of OCs, thus reducing their efficacy.12 It is not known, however, if this interaction results in increased pregnancy rates.13 Most newer AEDs (TABLE 36) do not induce cytochrome P450 3A4 and, thus, do not appear to affect OC efficacy, and may be safer for women with seizure disorders.12 While enzyme-inducing AEDs may decrease the efficacy of progesterone-only OCs and the morning-after pill,12,14,15 progesterone-containing intrauterine devices (IUDs), long-acting progesterone injections, and non-hormonal contraceptive methods appear to be unaffected.14-17
OCs and seizure frequency. There is no strong evidence that OCs affect seizure frequency in epileptic women, although changes in hormone levels during the menstrual cycle do affect seizure susceptibility.12 Combination OCs decrease lamotrigine levels and, therefore, may increase the risk of seizures, but progesterone-only pills do not produce this effect.12,16
Do guidelines exist? There are no specific evidence-based guidelines that pertain to the use of AEDs and contraception together, but some organizations have issued recommendations.
The American College of Obstetricians and Gynecologists recommends using a 30- to 35-mcg estrogen-containing OC rather than a lower dose in women taking an enzyme-inducing AED. The group also recommends using condoms with OCs or using IUDs.18
The American Academy of Neurology suggests that women taking OCs and enzyme-inducing AEDs use an OC containing at least 50 mcg estrogen.19
The National Institute for Health and Care Excellence recommends that women taking enzyme-inducing AEDs avoid progestin-only pills.20
The Faculty of Sexual and Reproductive Healthcare agrees that enzyme-inducing drugs may decrease efficacy and recommend considering IUDs and injectable contraceptive methods.21
2. SSRIs & NSAIDs.
SSRIs increase the GI bleeding risk associated with NSAIDs alone
Nonsteroidal anti-inflammatory drugs (NSAIDs) and selective serotonin reuptake inhibitors (SSRIs) are commonly prescribed worldwide.22,23 A well-established adverse effect of NSAIDs is gastrointestinal (GI) bleeding, and there is increasing evidence that concomitant use of an SSRI can further increase that risk through a variety of mechanisms.23
SSRIs decrease platelet serotonin levels resulting in defective platelet aggregation and impaired hemostasis. Studies have also shown that SSRIs increase gastric acidity, which leads to increased risk of peptic ulcer disease and GI bleeding.23 These mechanisms, combined with the inhibition of gastroprotective prostaglandin cyclooxygenase-1 and platelets by NSAIDs, further potentiate GI bleeding risk.24
Patients at high risk for bleeding with concomitant SSRIs and NSAIDs include older patients, patients with other risk factors for GI bleeding (eg, chronic steroid use), and patients with a history of GI bleeding.23
The evidence. A 2014 meta-analysis found that when SSRIs were used in combination with NSAIDs, the risk of GI bleeding was significantly increased, compared with SSRI monotherapy.23
Case control studies found the risk of upper GI bleeding with SSRIs had a number needed to harm (NNH) of 3177 for a low-risk population and 881 for a high-risk population with an odds ratio (OR) of 1.66 (95% confidence interval [CI], 1.44-1.92; P<.00001).23 When SSRIs were used in combination with NSAIDs, the NNH decreased to 645 for a low-risk population and 179 for a high-risk population (OR=4.25; 95% CI, 2.82-6.42; P<.0001).23
Another meta-analysis found that the OR for bleeding risk increased to 6.33 (95% CI, 3.40-11.8; P<.00001; NNH=106) with concomitant use of NSAIDs and SSRIs, compared with 2.36 (95% CI, 1.44-3.85; P=.0006; NNH=411) for SSRI use alone.25
The studies did not evaluate results based on the indication, dose, or duration of SSRI or NSAID treatment. If both an SSRI and an NSAID must be used, select a cyclooxygenase-2 selective NSAID at the lowest effective dose and consider the addition of a proton pump inhibitor to decrease the risk of a GI bleed.23,26
3. Direct oral anticoagulants and antiepileptics
Don’t use DOACs in patients taking certain antiepileptic medications
Drug interactions with anticoagulants, such as warfarin, are well documented and have been publicized for years, but physicians must also be aware of the potential for interaction between the direct oral anticoagulants (DOACs) and AEDs.
Apixaban, rivaroxaban, and dabigatran appear to interact withthe AEDs carbamazepine, phenytoin, and phenobarbital.27,28 These interactions occur due to AED induction of the CYP3A4 enzyme and effects on the P-glycoprotein (P-gp) efflux pump.27,29 When taken together, the AED induces metabolism and elimination of the DOAC medication to occur more quickly than it would normally, resulting in subtherapeutic concentrations of the DOAC. This could theoretically result in a venous thromboembolic event or stroke.
A caveat. One thing to consider is that studies demonstrating interaction between the DOAC and AED drug classes have been performed in healthy volunteers, making it difficult to extrapolate how this interaction may increase the risk for thrombotic events in other patients.
Some studies demonstrated reductions in drug levels of up to 50% with strong CYP3A4 and P-glycoprotein inducers.30 Common inducers include carbamazepine, rifampin, and St. John’s Wort.6 Patients taking such agents could theoretically have decreased exposure to the DOAC, resulting in an increase in thromboembolic risk.31
4. Statins & certain CYP inhibitors
Combining simvastatin with fibrates warrants extra attention
The efficacy of statin medications in the prevention of atherosclerotic cardiovascular disease (ASCVD) is clear. However, the clinical significance of many identified drug interactions involving statins is difficult to interpret. Interactions that cause increased serum concentrations of statins can increase the risk for liver enzyme elevations and skeletal muscle abnormalities (myalgias to rhabdomyolysis).32 Strong inhibitors of CYP3A4 (amiodarone, cyclosporine, ketoconazole, etc.) significantly increase concentrations of lovastatin, simvastatin, and atorvastatin. Pitavastatin, pravastatin, and rosuvastatin are not susceptible to any CYP-mediated drug interactions;33 therefore, rosuvastatin (a high-intensity statin) is usually recommended over other statins for patients taking strong inhibitors of CYP3A4.
When to limit simvastatin. Doses of simvastatin should not exceed 10 mg/d when combined with diltiazem, dronedarone, or verapamil, and doses should not exceed 20 mg/d when used with amiodarone, amlodipine, or ranolazine.6 These recommendations are in response to results from the SEARCH (Study of the Effectiveness of Additional Reductions in cholesterol and homocysteine) trial, which found a higher incidence of myopathies and rhabdomyolysis in patients taking 80 mg of simvastatin compared with those taking 20-mg doses.34 CYP3A4-inducing medications, especially diltiazem, were thought to also contribute to an increased risk.34
Avoid gemfibrozil with statins. Using fibrates with statins is beneficial for some patients; however, gemfibrozil significantly interacts with statins by inhibiting CYP2C8 and organic anion transporting polypeptide 1B1 (OATP1B1).33 The safer choice is fenofibrate because it does not interfere with statin metabolism and can be safely used in combination with statins.6
A retrospective review of the FDA Adverse Event Reporting System (AERS) database found that 88% of fibrate and statin combinations that resulted in rhabdomyolysis were associated with gemfibrozil/cerivastatin (cerivastatin is no longer available in the United States).35
5. One serotonergic drug & another
Serotonin syndrome is associated with more than just SSRIs
Serotonin syndrome is a constellation of symptoms (hyperthermia, hyperreflexia, muscle clonus, tremor and altered mental status) caused by increases in serotonin levels in the central and peripheral nervous systems that can lead to mild or life-threatening complications such as seizures, muscle breakdown, or hyperthermia. Serotonin syndrome is most likely to occur within 24 hours after a dose increase, after starting a new medication that increases serotonin levels, or after a drug overdose.36
SSRIs are the most commonly reported drug associated with serotonin syndrome; however, other medications (TABLE 437) may be responsible, especially when used in combination with agents that act on serotonin receptors or in patients with impaired metabolism of the drugs being used.37
Other culprits. Serotonergic effects can also be associated with illicit drugs, some nonprescription medications, and supplements. And in March 2016, the FDA issued a warning about the risks of taking opioids with serotonergic medications.38 Although labeling changes have been recommended for all opioids, the cases of serotonin syndrome were reported more often with normal doses of fentanyl and methadone.
There are 2 mechanisms by which drugs may increase a patient’s risk for serotonin syndrome. The first is a pharmacodynamic interaction, which can occur when 2 or more medications act at the same receptor site (serotonin receptors in this example), which may result in an additive or synergistic effect.39
The second mechanism is a pharmacokinetic alteration (an agent alters absorption, distribution, metabolism, or excretion) of CYP enzymes.40 Of the more commonly used antidepressants, citalopram, escitalopram, venlafaxine, and mirtazapine seem to have the least potential for clinically significant pharmacokinetic interactions.41
Guidelines? Currently there are no guidelines for preventing serotonin syndrome. Clinicians should exercise caution in patients at high risk for drug adverse events, such as the elderly, patients taking multiple medications, and patients with comorbidities. Healthy low-risk patients can generally take 2 or 3 serotonergic medications at therapeutic doses without a major risk of harm.
CORRESPONDENCE
Mary Onysko, PharmD, BCPS, 191 East Orchard Road, Suite 200, Littleton, CO 80121; monysko@uwyo.edu.
There is a strong relationship between the number of medications taken and the likelihood of a potentially serious drug-drug interaction.1,2 Drug interaction software programs can help alert prescribers to potential problems, but these programs sometimes fail to detect important interactions or generate so many clinically insignificant alerts that they become a nuisance.3 This review provides guidance about 5 clinically relevant drug interactions, including those that are common (TABLE 14-6)—and those that are less common, but no less important (TABLE 26-10).
1. Antiepileptics & contraceptives
Many antiepileptic medications decrease the efficacy of certain contraceptives
Contraception management in women with epilepsy is critical due to potential maternal and fetal complications. Many antiepileptic drugs (AEDs), including carbamazepine, ethosuximide, fosphenytoin, phenobarbital, phenytoin, primidone, topiramate, and valproate, are potentially teratogenic.11 A retrospective, observational study of 115 women of childbearing age who had epilepsy and were seen at a neurology clinic found that 74% were not using documented contraception.11 Of the minority of study participants using contraception, most were using oral contraceptives (OCs) that could potentially interact with AEDs.
CYP inducers. Estrogen and progesterone are metabolized by the cytochrome P450 3A4 enzyme. Some AEDs induce this enzyme, which can enhance the metabolism of OCs, thus reducing their efficacy.12 It is not known, however, if this interaction results in increased pregnancy rates.13 Most newer AEDs (TABLE 36) do not induce cytochrome P450 3A4 and, thus, do not appear to affect OC efficacy, and may be safer for women with seizure disorders.12 While enzyme-inducing AEDs may decrease the efficacy of progesterone-only OCs and the morning-after pill,12,14,15 progesterone-containing intrauterine devices (IUDs), long-acting progesterone injections, and non-hormonal contraceptive methods appear to be unaffected.14-17
OCs and seizure frequency. There is no strong evidence that OCs affect seizure frequency in epileptic women, although changes in hormone levels during the menstrual cycle do affect seizure susceptibility.12 Combination OCs decrease lamotrigine levels and, therefore, may increase the risk of seizures, but progesterone-only pills do not produce this effect.12,16
Do guidelines exist? There are no specific evidence-based guidelines that pertain to the use of AEDs and contraception together, but some organizations have issued recommendations.
The American College of Obstetricians and Gynecologists recommends using a 30- to 35-mcg estrogen-containing OC rather than a lower dose in women taking an enzyme-inducing AED. The group also recommends using condoms with OCs or using IUDs.18
The American Academy of Neurology suggests that women taking OCs and enzyme-inducing AEDs use an OC containing at least 50 mcg estrogen.19
The National Institute for Health and Care Excellence recommends that women taking enzyme-inducing AEDs avoid progestin-only pills.20
The Faculty of Sexual and Reproductive Healthcare agrees that enzyme-inducing drugs may decrease efficacy and recommend considering IUDs and injectable contraceptive methods.21
2. SSRIs & NSAIDs.
SSRIs increase the GI bleeding risk associated with NSAIDs alone
Nonsteroidal anti-inflammatory drugs (NSAIDs) and selective serotonin reuptake inhibitors (SSRIs) are commonly prescribed worldwide.22,23 A well-established adverse effect of NSAIDs is gastrointestinal (GI) bleeding, and there is increasing evidence that concomitant use of an SSRI can further increase that risk through a variety of mechanisms.23
SSRIs decrease platelet serotonin levels resulting in defective platelet aggregation and impaired hemostasis. Studies have also shown that SSRIs increase gastric acidity, which leads to increased risk of peptic ulcer disease and GI bleeding.23 These mechanisms, combined with the inhibition of gastroprotective prostaglandin cyclooxygenase-1 and platelets by NSAIDs, further potentiate GI bleeding risk.24
Patients at high risk for bleeding with concomitant SSRIs and NSAIDs include older patients, patients with other risk factors for GI bleeding (eg, chronic steroid use), and patients with a history of GI bleeding.23
The evidence. A 2014 meta-analysis found that when SSRIs were used in combination with NSAIDs, the risk of GI bleeding was significantly increased, compared with SSRI monotherapy.23
Case control studies found the risk of upper GI bleeding with SSRIs had a number needed to harm (NNH) of 3177 for a low-risk population and 881 for a high-risk population with an odds ratio (OR) of 1.66 (95% confidence interval [CI], 1.44-1.92; P<.00001).23 When SSRIs were used in combination with NSAIDs, the NNH decreased to 645 for a low-risk population and 179 for a high-risk population (OR=4.25; 95% CI, 2.82-6.42; P<.0001).23
Another meta-analysis found that the OR for bleeding risk increased to 6.33 (95% CI, 3.40-11.8; P<.00001; NNH=106) with concomitant use of NSAIDs and SSRIs, compared with 2.36 (95% CI, 1.44-3.85; P=.0006; NNH=411) for SSRI use alone.25
The studies did not evaluate results based on the indication, dose, or duration of SSRI or NSAID treatment. If both an SSRI and an NSAID must be used, select a cyclooxygenase-2 selective NSAID at the lowest effective dose and consider the addition of a proton pump inhibitor to decrease the risk of a GI bleed.23,26
3. Direct oral anticoagulants and antiepileptics
Don’t use DOACs in patients taking certain antiepileptic medications
Drug interactions with anticoagulants, such as warfarin, are well documented and have been publicized for years, but physicians must also be aware of the potential for interaction between the direct oral anticoagulants (DOACs) and AEDs.
Apixaban, rivaroxaban, and dabigatran appear to interact withthe AEDs carbamazepine, phenytoin, and phenobarbital.27,28 These interactions occur due to AED induction of the CYP3A4 enzyme and effects on the P-glycoprotein (P-gp) efflux pump.27,29 When taken together, the AED induces metabolism and elimination of the DOAC medication to occur more quickly than it would normally, resulting in subtherapeutic concentrations of the DOAC. This could theoretically result in a venous thromboembolic event or stroke.
A caveat. One thing to consider is that studies demonstrating interaction between the DOAC and AED drug classes have been performed in healthy volunteers, making it difficult to extrapolate how this interaction may increase the risk for thrombotic events in other patients.
Some studies demonstrated reductions in drug levels of up to 50% with strong CYP3A4 and P-glycoprotein inducers.30 Common inducers include carbamazepine, rifampin, and St. John’s Wort.6 Patients taking such agents could theoretically have decreased exposure to the DOAC, resulting in an increase in thromboembolic risk.31
4. Statins & certain CYP inhibitors
Combining simvastatin with fibrates warrants extra attention
The efficacy of statin medications in the prevention of atherosclerotic cardiovascular disease (ASCVD) is clear. However, the clinical significance of many identified drug interactions involving statins is difficult to interpret. Interactions that cause increased serum concentrations of statins can increase the risk for liver enzyme elevations and skeletal muscle abnormalities (myalgias to rhabdomyolysis).32 Strong inhibitors of CYP3A4 (amiodarone, cyclosporine, ketoconazole, etc.) significantly increase concentrations of lovastatin, simvastatin, and atorvastatin. Pitavastatin, pravastatin, and rosuvastatin are not susceptible to any CYP-mediated drug interactions;33 therefore, rosuvastatin (a high-intensity statin) is usually recommended over other statins for patients taking strong inhibitors of CYP3A4.
When to limit simvastatin. Doses of simvastatin should not exceed 10 mg/d when combined with diltiazem, dronedarone, or verapamil, and doses should not exceed 20 mg/d when used with amiodarone, amlodipine, or ranolazine.6 These recommendations are in response to results from the SEARCH (Study of the Effectiveness of Additional Reductions in cholesterol and homocysteine) trial, which found a higher incidence of myopathies and rhabdomyolysis in patients taking 80 mg of simvastatin compared with those taking 20-mg doses.34 CYP3A4-inducing medications, especially diltiazem, were thought to also contribute to an increased risk.34
Avoid gemfibrozil with statins. Using fibrates with statins is beneficial for some patients; however, gemfibrozil significantly interacts with statins by inhibiting CYP2C8 and organic anion transporting polypeptide 1B1 (OATP1B1).33 The safer choice is fenofibrate because it does not interfere with statin metabolism and can be safely used in combination with statins.6
A retrospective review of the FDA Adverse Event Reporting System (AERS) database found that 88% of fibrate and statin combinations that resulted in rhabdomyolysis were associated with gemfibrozil/cerivastatin (cerivastatin is no longer available in the United States).35
5. One serotonergic drug & another
Serotonin syndrome is associated with more than just SSRIs
Serotonin syndrome is a constellation of symptoms (hyperthermia, hyperreflexia, muscle clonus, tremor and altered mental status) caused by increases in serotonin levels in the central and peripheral nervous systems that can lead to mild or life-threatening complications such as seizures, muscle breakdown, or hyperthermia. Serotonin syndrome is most likely to occur within 24 hours after a dose increase, after starting a new medication that increases serotonin levels, or after a drug overdose.36
SSRIs are the most commonly reported drug associated with serotonin syndrome; however, other medications (TABLE 437) may be responsible, especially when used in combination with agents that act on serotonin receptors or in patients with impaired metabolism of the drugs being used.37
Other culprits. Serotonergic effects can also be associated with illicit drugs, some nonprescription medications, and supplements. And in March 2016, the FDA issued a warning about the risks of taking opioids with serotonergic medications.38 Although labeling changes have been recommended for all opioids, the cases of serotonin syndrome were reported more often with normal doses of fentanyl and methadone.
There are 2 mechanisms by which drugs may increase a patient’s risk for serotonin syndrome. The first is a pharmacodynamic interaction, which can occur when 2 or more medications act at the same receptor site (serotonin receptors in this example), which may result in an additive or synergistic effect.39
The second mechanism is a pharmacokinetic alteration (an agent alters absorption, distribution, metabolism, or excretion) of CYP enzymes.40 Of the more commonly used antidepressants, citalopram, escitalopram, venlafaxine, and mirtazapine seem to have the least potential for clinically significant pharmacokinetic interactions.41
Guidelines? Currently there are no guidelines for preventing serotonin syndrome. Clinicians should exercise caution in patients at high risk for drug adverse events, such as the elderly, patients taking multiple medications, and patients with comorbidities. Healthy low-risk patients can generally take 2 or 3 serotonergic medications at therapeutic doses without a major risk of harm.
CORRESPONDENCE
Mary Onysko, PharmD, BCPS, 191 East Orchard Road, Suite 200, Littleton, CO 80121; monysko@uwyo.edu.
1. Aparasu R, Baer R, Aparasu A. Clinically important potential drug-drug interactions in outpatient settings. Res Social Adm Pharm. 2007;3:426-437.
2. Johnell K, Klarin I. The relationship between number of drugs and potential drug-drug interactions in the elderly: a study of over 600,000 elderly patients from the Swedish Prescribed Drug Register. Drug Saf. 2007;30:911-918.
3. Pharmacist’s Letter. Online continuing medical education and webinars. Drug interaction overload: Problems and solutions for drug interaction alerts. Volume 2012, Course No. 216. Self-Study Course #120216. Available at: http://pharmacistsletter.therapeuticresearch.com/ce/cecourse.aspx?pc=15-219&quiz=1. Accessed June 9, 2016.
4. PL Detail-Document, Potassium and Anticholinergic Drug Interaction. Pharmacist’s Letter/Prescriber’s Letter. October 2011.
5. Micromedex Solutions. Available at: http://www.micromedexsolutions.com. Accessed May 3, 2016.
6. Lexi-Comp Online. Available at: http://online.lexi.com/lco/action/home. Accessed May 22, 2016.
7. Marcus FI. Drug interactions with amiodarone. Am Heart J. 1983;106(4 Pt 2):924-930.
8. Digoxin: serious drug interactions. Prescrire Int. 2010;19:68-70.
9. McCance-Katz EF, Sullivan LE, Nallani S. Drug interactions of clinical importance among the opioids, methadone and buprenorphine, and other frequently prescribed medications: a review. Am J Addict. 2010;19:4-16.
10. Drugs.com. Theophylline drug interactions. Available at: https://www.drugs.com/drug-interactions/theophylline.html. Accessed June 23, 2016.
11. Bhakta J, Bainbridge J, Borgelt L. Teratogenic medications and concurrent contraceptive use in women of childbearing ability with epilepsy. Epilepsy Behav. 2015;52(Pt A):212-217.
12. Reddy DS. Clinical pharmacokinetic interactions between antiepileptic drugs and hormonal contraceptives. Expert Rev Clin Pharmacol. 2010;3:183-192.
13. Carl JS, Weaver SP, Tweed E. Effect of antiepileptic drugs on oral contraceptives. Am Fam Physician. 2008;78:634-635.
14. O’Brien MD, Guillebaud J. Contraception for women with epilepsy. Epilepsia. 2006;47:1419-1422.
15. Schwenkhagen AM, Stodieck SR. Which contraception for women with epilepsy? Seizure. 2008;17:145-150.
16. Faculty of Sexual and Reproductive Healthcare Clinical Effectiveness Unit. Antiepileptic drugs and contraception. CEU statement. January 2010. Available at: https://www.fsrh.org/standards-and-guidance/documents/ceu-clinical-guidance-drug-interactions-with-hormonal/. Accessed April 25, 2016.
17. Perruca E. Clinically relevant drug interactions with antiepileptic drugs. Br J Clin Pharmacol. 2006;61:246-255.
18. ACOG practice bulletin. Number 73: Use of hormonal contraception in women with coexisting medical conditions. ACOG Committee on Practice Bulletins-Gynecology. Obstet Gynecol. 2006;107:1453-1472.
19. Report of the Quality Standards Subcommittee of the American Academy of Neurology. Practice parameter: management issues for women with epilepsy (summary statement). Neurology. 1998;51:944-948.
20. National Institute for Health and Care Excellence. Do not do recommendation. Available at: https://www.nice.org.uk/donotdo/the-progestogenonly-pill-is-not-recommended-as-reliable-contraception-inwomen-and-girls-taking-enzymeinducing-anti-epileptic-drugs-aeds. Accessed September 21, 2017.
21. Faculty of Sexual and Reproductive Healthcare. Clinical guidance: drug interactions with hormonal contraception. Available at: https://www.fsrh.org/standards-and-guidance/documents/ceu-clinical-guidance-drug-interactions-with-hormonal/. Accessed September 21, 2017.
22. de Jong JCF, van den Berg PB, Tobi H, et al. Combined use of SSRIs and NSAIDs increases the risk of gastrointestinal adverse effects. Br J Clin Pharmacol. 2003;55:591-595.
23. Anglin R, Yuan Y, Moayyedi P, et al. Risk of upper gastrointestinal bleeding with selective serotonin reuptake inhibitors with or without concurrent nonsteroidal anti-inflammatory use: a systematic review and meta-analysis. Am J Gastroenterol. 2014;109:811-819.
24. Mort JR, Aparasu RR, Baer RK, et al. Interaction between selective serotonin reuptake inhibitors and nonsteroidal anti-inflammatory drugs: review of the literature. Pharmacotherapy. 2006;26:1307-1313.
25. Loke YK, Trivedi AN, Singh S. Meta-analysis: gastrointestinal bleeding due to interaction between selective serotonin uptake inhibitors and non-steroidal anti-inflammatory drugs. Aliment Pharmacol Ther. 2008;27:31-40.
26. Venerito M, Wex T, Malfertheiner P. Nonsteroidal anti-inflammatory drug-induced gastroduodenal bleeding: risk factors and prevention strategies. Pharmaceuticals. 2010;3:2225-2237.
27. Boehringer S, Williams CD, Yawn BP, et al. Managing interactions with direct oral anticoagulants (DOACs). Pharmacist’s Letter. May 2016.
28. Johannessen SI, Landmark CJ. Antiepileptic drug interactions – principles and clinical implications. Curr Neuropharmacol. 2010;8:254-267.
29. Mohrien K, Oliphant CS, Self TH. Drug interactions with novel oral anticoagulants. Consultant. 2013;53:918-919. Available at: http://www.consultant360.com/articles/drug-interactions-novel-oral-anticoagulants. Accessed May 3, 2016.
30. Wiggins BS, Northup A, Johnson D, et al. Reduced anticoagulant effect of dabigatran in a patient receiving concomitant phenytoin. Pharmacotherapy. 2016;36:e5-e7.
31. Burnett AE, Mahan CE, Vazquez SR, et al. Guidance for the practical management of the direct oral anticoagulants (DOACs) in VTE treatment. J Thromb Thrombolysis. 2016;41:206-232.
32. Thompson PD, Clarkson P, Karas RH. Statin-associated myopathy. JAMA. 2003;289:1681-1690.
33. Hirota T, Leiri I. Drug-drug interactions that interfere with statin metabolism. Expert Opin Drug Metab Toxicol. 2015;11:1435-1447.
34. Study of the Effectiveness of Additional Reductions in Cholesterol and Homocysteine (SEARCH) Collaborative Group. Intensive lowering of LDL cholesterol wih 80 mg versus 20 mg simvastatin daily in 12,064 survivors of myocardial inffarction: a double-blind randomised trial. Lancet. 2010;376:1658-1669.
35. Jones PH, Davidson MH. Reporting rate of rhabdomyolysis with fenofibrate + statin versus gemfibrozil + any statin. Am J Cardiol. 2005;95:120-122.
36. Birmes P, Coppin D, Schmitt L, et al. Serotonin syndrome: a brief review. CMAJ. 2003;168:1439-1442.
37. Boyer EW, Shannon M. The serotonin syndrome. N Engl J Med. 2005;352:1112-1120.
38. US Food and Drug Administration. FDA Drug Safety Communication: FDA warns about several safety issues with opioid pain medicines; requires label changes. Available at: https://www.fda.gov/Drugs/DrugSafety/ucm489676.htm. Accessed June 15, 2016.
39. Sultana J, Spina E, Trifirò G. Antidepressant use in the elderly: the role of pharmacodynamics and pharmacokinetics in drug safety. Expert Opin Drug Metab Toxicol. 2015;11:883-892.
40. Sproule BA, Naranjo CA, Brenmer KE, et al. Selective serotonin reuptake inhibitors and CNS drug interactions. A critical review of the evidence. Clin Pharmacokinet. 1997;33:454-471.
41. Spina E, Santoro V, D’Arrigo C. Clinically relevant pharmacokinetic drug interactions with second-generation antidepressants: an update. Clin Ther. 2008;30:1206-1227.
1. Aparasu R, Baer R, Aparasu A. Clinically important potential drug-drug interactions in outpatient settings. Res Social Adm Pharm. 2007;3:426-437.
2. Johnell K, Klarin I. The relationship between number of drugs and potential drug-drug interactions in the elderly: a study of over 600,000 elderly patients from the Swedish Prescribed Drug Register. Drug Saf. 2007;30:911-918.
3. Pharmacist’s Letter. Online continuing medical education and webinars. Drug interaction overload: Problems and solutions for drug interaction alerts. Volume 2012, Course No. 216. Self-Study Course #120216. Available at: http://pharmacistsletter.therapeuticresearch.com/ce/cecourse.aspx?pc=15-219&quiz=1. Accessed June 9, 2016.
4. PL Detail-Document, Potassium and Anticholinergic Drug Interaction. Pharmacist’s Letter/Prescriber’s Letter. October 2011.
5. Micromedex Solutions. Available at: http://www.micromedexsolutions.com. Accessed May 3, 2016.
6. Lexi-Comp Online. Available at: http://online.lexi.com/lco/action/home. Accessed May 22, 2016.
7. Marcus FI. Drug interactions with amiodarone. Am Heart J. 1983;106(4 Pt 2):924-930.
8. Digoxin: serious drug interactions. Prescrire Int. 2010;19:68-70.
9. McCance-Katz EF, Sullivan LE, Nallani S. Drug interactions of clinical importance among the opioids, methadone and buprenorphine, and other frequently prescribed medications: a review. Am J Addict. 2010;19:4-16.
10. Drugs.com. Theophylline drug interactions. Available at: https://www.drugs.com/drug-interactions/theophylline.html. Accessed June 23, 2016.
11. Bhakta J, Bainbridge J, Borgelt L. Teratogenic medications and concurrent contraceptive use in women of childbearing ability with epilepsy. Epilepsy Behav. 2015;52(Pt A):212-217.
12. Reddy DS. Clinical pharmacokinetic interactions between antiepileptic drugs and hormonal contraceptives. Expert Rev Clin Pharmacol. 2010;3:183-192.
13. Carl JS, Weaver SP, Tweed E. Effect of antiepileptic drugs on oral contraceptives. Am Fam Physician. 2008;78:634-635.
14. O’Brien MD, Guillebaud J. Contraception for women with epilepsy. Epilepsia. 2006;47:1419-1422.
15. Schwenkhagen AM, Stodieck SR. Which contraception for women with epilepsy? Seizure. 2008;17:145-150.
16. Faculty of Sexual and Reproductive Healthcare Clinical Effectiveness Unit. Antiepileptic drugs and contraception. CEU statement. January 2010. Available at: https://www.fsrh.org/standards-and-guidance/documents/ceu-clinical-guidance-drug-interactions-with-hormonal/. Accessed April 25, 2016.
17. Perruca E. Clinically relevant drug interactions with antiepileptic drugs. Br J Clin Pharmacol. 2006;61:246-255.
18. ACOG practice bulletin. Number 73: Use of hormonal contraception in women with coexisting medical conditions. ACOG Committee on Practice Bulletins-Gynecology. Obstet Gynecol. 2006;107:1453-1472.
19. Report of the Quality Standards Subcommittee of the American Academy of Neurology. Practice parameter: management issues for women with epilepsy (summary statement). Neurology. 1998;51:944-948.
20. National Institute for Health and Care Excellence. Do not do recommendation. Available at: https://www.nice.org.uk/donotdo/the-progestogenonly-pill-is-not-recommended-as-reliable-contraception-inwomen-and-girls-taking-enzymeinducing-anti-epileptic-drugs-aeds. Accessed September 21, 2017.
21. Faculty of Sexual and Reproductive Healthcare. Clinical guidance: drug interactions with hormonal contraception. Available at: https://www.fsrh.org/standards-and-guidance/documents/ceu-clinical-guidance-drug-interactions-with-hormonal/. Accessed September 21, 2017.
22. de Jong JCF, van den Berg PB, Tobi H, et al. Combined use of SSRIs and NSAIDs increases the risk of gastrointestinal adverse effects. Br J Clin Pharmacol. 2003;55:591-595.
23. Anglin R, Yuan Y, Moayyedi P, et al. Risk of upper gastrointestinal bleeding with selective serotonin reuptake inhibitors with or without concurrent nonsteroidal anti-inflammatory use: a systematic review and meta-analysis. Am J Gastroenterol. 2014;109:811-819.
24. Mort JR, Aparasu RR, Baer RK, et al. Interaction between selective serotonin reuptake inhibitors and nonsteroidal anti-inflammatory drugs: review of the literature. Pharmacotherapy. 2006;26:1307-1313.
25. Loke YK, Trivedi AN, Singh S. Meta-analysis: gastrointestinal bleeding due to interaction between selective serotonin uptake inhibitors and non-steroidal anti-inflammatory drugs. Aliment Pharmacol Ther. 2008;27:31-40.
26. Venerito M, Wex T, Malfertheiner P. Nonsteroidal anti-inflammatory drug-induced gastroduodenal bleeding: risk factors and prevention strategies. Pharmaceuticals. 2010;3:2225-2237.
27. Boehringer S, Williams CD, Yawn BP, et al. Managing interactions with direct oral anticoagulants (DOACs). Pharmacist’s Letter. May 2016.
28. Johannessen SI, Landmark CJ. Antiepileptic drug interactions – principles and clinical implications. Curr Neuropharmacol. 2010;8:254-267.
29. Mohrien K, Oliphant CS, Self TH. Drug interactions with novel oral anticoagulants. Consultant. 2013;53:918-919. Available at: http://www.consultant360.com/articles/drug-interactions-novel-oral-anticoagulants. Accessed May 3, 2016.
30. Wiggins BS, Northup A, Johnson D, et al. Reduced anticoagulant effect of dabigatran in a patient receiving concomitant phenytoin. Pharmacotherapy. 2016;36:e5-e7.
31. Burnett AE, Mahan CE, Vazquez SR, et al. Guidance for the practical management of the direct oral anticoagulants (DOACs) in VTE treatment. J Thromb Thrombolysis. 2016;41:206-232.
32. Thompson PD, Clarkson P, Karas RH. Statin-associated myopathy. JAMA. 2003;289:1681-1690.
33. Hirota T, Leiri I. Drug-drug interactions that interfere with statin metabolism. Expert Opin Drug Metab Toxicol. 2015;11:1435-1447.
34. Study of the Effectiveness of Additional Reductions in Cholesterol and Homocysteine (SEARCH) Collaborative Group. Intensive lowering of LDL cholesterol wih 80 mg versus 20 mg simvastatin daily in 12,064 survivors of myocardial inffarction: a double-blind randomised trial. Lancet. 2010;376:1658-1669.
35. Jones PH, Davidson MH. Reporting rate of rhabdomyolysis with fenofibrate + statin versus gemfibrozil + any statin. Am J Cardiol. 2005;95:120-122.
36. Birmes P, Coppin D, Schmitt L, et al. Serotonin syndrome: a brief review. CMAJ. 2003;168:1439-1442.
37. Boyer EW, Shannon M. The serotonin syndrome. N Engl J Med. 2005;352:1112-1120.
38. US Food and Drug Administration. FDA Drug Safety Communication: FDA warns about several safety issues with opioid pain medicines; requires label changes. Available at: https://www.fda.gov/Drugs/DrugSafety/ucm489676.htm. Accessed June 15, 2016.
39. Sultana J, Spina E, Trifirò G. Antidepressant use in the elderly: the role of pharmacodynamics and pharmacokinetics in drug safety. Expert Opin Drug Metab Toxicol. 2015;11:883-892.
40. Sproule BA, Naranjo CA, Brenmer KE, et al. Selective serotonin reuptake inhibitors and CNS drug interactions. A critical review of the evidence. Clin Pharmacokinet. 1997;33:454-471.
41. Spina E, Santoro V, D’Arrigo C. Clinically relevant pharmacokinetic drug interactions with second-generation antidepressants: an update. Clin Ther. 2008;30:1206-1227.
PRACTICE RECOMMENDATIONS
› Recommend progesterone-containing intrauterine devices or long-acting progesterone injections for women using antiepileptic drugs. B
› Be aware that there is an increased risk of gastrointestinal bleeding when nonsteroidal anti-inflammatory drugs are used with selective serotonin reuptake inhibitors. A
› Do not prescribe novel oral anticoagulants for patients taking carbamazepine, phenytoin, or phenobarbital. B
› Choose fenofibrate over gemfibrozil when combining a fibrate and a statin. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series