User login
When treating chronic psychotic disorders, U.S. psychiatrists generally prefer second-generation antipsychotics (SGAs) to first-generation antipsychotics (FGAs) because of widely held views1,2 that SGAs:
- are more effective for negative and cognitive symptoms
- produce fewer troublesome side effects
- help patients realize a better quality of life.
These beliefs have been challenged by two large-scale, government-supported studies: the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) in the United States3-6 and more recently the Cost Utility of the Latest Antipsychotic Drugs in Schizophrenia Study (CUtLASS) from the United Kingdom.7,8
CATIE and CUtLASS data suggest that the SGA advantage has been exaggerated, if in fact such an advantage exists. Other Current Psychiatry articles for the clinical practitioner have discussed the CATIE findings.9-11 This article addresses the CUtLASS results in the context of the trial’s methodology, using information from the primary publications7,8 and technical report.12
Cutlass study
Design. CUtLASS included 2 “bands” (Table 1):
- Band 1 compared the clinical usefulness and cost effectiveness of FGAs and SGAs in treating schizophrenia7
- Band 2 compared the effectiveness of clozapine versus other SGAs in treating refractory schizophrenia.8
CUtLASS Band 1 was not as extensive in scope as CATIE, and its design had some important differences (Table 2). Patients were referred for participation because their psychiatrists were considering a change in antipsychotic medication to address adverse effects or inadequate response. Fewer patients were recruited than expected—40% of the planned sample during 30 months of recruitment—but researchers considered the size sufficient to compare the effectiveness of FGAs and SGAs.
Patients were randomly assigned to treatment with an antipsychotic class, either:
- an FGA (1 of 11 options—including 5 depot formulations—chosen by the treating clinician)
- or an SGA (risperidone, olanzapine, quetiapine, or amisulpride, also chosen by the clinician).
Physicians and patients were not blinded to the medications used. They could choose medications within patients’ assigned classes and switch as needed in ways that mimicked clinical practice. Trained assessors, who were blinded to the medications being used, evaluated the patients after 12, 26, and 52 weeks.
Quality of life was the primary outcome measure.13 Secondary measures included symptoms, side effects, patient satisfaction, and cost of care.
Band 1 results. Patients assigned to the SGA or FGA classes showed no significant differences in quality of life measures or schizophrenia symptoms. If anything, the findings slightly favored the FGAs.
Patient satisfaction and overall cost of care were similar, and rates of extrapyramidal symptoms (EPS), tardive dyskinesia, and akathisia did not differ significantly.
Clozapine comparison. In CUtLASS band 2, a different sample of 136 schizophrenia patients who had responded poorly to ≥2 antipsychotics was randomly assigned to clozapine or one of the above four SGAs. During the 1-year comparison trial, clozapine:
- was found to be significantly more effective (P=0.01) in managing patients’ symptoms, as measured by total Positive and Negative Syndrome Scale (PANSS) score
- showed a trend (P=0.08) towards providing these treatment-resistant patients with a better quality of life.8
Table 1
Summary of CUtLASS trial design and results
Band 1
|
Band 2
|
| CUtLASS: Cost Utility of the Latest Antipsychotic Drugs in Schizophrenia Study |
| FGA: First-generation antipsychotic |
| PANSS: Positive and Negative Syndrome Scale |
| SGA: Second-generation antipsychotic |
Table 2
Comparing designs of the CUtLASS and CATIE schizophrenia trials
| CUtLASS | CATIE | |
|---|---|---|
| Trial duration | 12 months | 18 months |
| Clinical sites | 14 (United Kingdom) | 57 (United States) |
| Number of Subjects | 227 | 1,460 |
| Gender and age | 68% male; mean age 41 | 74% male; mean age 41 |
| Mental illness duration (mean) | 14 years | 16 years |
| Diagnosis | 75% schizophrenia | 100% schizophrenia |
| First-episode patients included? | Yes (13% of sample) | No |
| % of patients receiving antipsychotics at enrollment | 99% | 74% |
| Baseline PANSS score (mean) | 82% FGAs; 40% depot | 15% FGAs; <5% depot |
| Baseline PANSS score | 72.2 | 75.7 |
| Baseline EPS scores | Low | Low |
| Antipsychotic options in randomization | 2 classes (SGA or FGA) (50% of subjects assigned to an FGA) | 4 SGAs, 1 FGA (20% of subjects assigned to an FGA) |
| % of subjects given sulpiride | 49% | 0% |
| Administration methodology | Medication blinded to raters but not to patients and physicians | Medication blinded to patients and physicians |
| Primary outcome | Quality of life | Discontinuation of medication |
| Long-acting antipsychotic option? | Yes | No |
| Antipsychotic switching | All patients switched agents; 49% changed antipsychotic class | 15% stayed on some agent |
| CATIE: Clinical Antipsychotic Trials of Intervention Effectiveness | ||
| CUtLASS: Cost Utility of the Latest Antipsychotic Drugs in Schizophrenia Study | ||
| EPS: Extrapyramidal symptom | ||
| FGA: First-generation antipsychotic | ||
| PANSS: Positive and Negative Syndrome Scale | ||
| SGA: Second-generation antipsychotic | ||
Comparing catie, cutlass data
The CUtLASS findings are not identical to those of CATIE phase 114 but are remarkably similar: no differences in effectiveness were seen between FGAs and SGA when treating patients with chronic schizophrenia.15,16
CUtLASS investigators concluded that “in people with schizophrenia whose medication is changed for clinical reasons, there is no disadvantage across 1 year in terms of quality of life, symptoms, or associated costs of care in using FGAs rather than nonclozapine SGAs.”7
By confirming CATIE’s results, is CUtLASS the final word on antipsychotic treatment of chronic schizophrenia? Or is it just another piece of the puzzle? CATIE and CUtLASS add much to our knowledge, but methodologic “flies in the ointment” plague all clinical trials. We must consider potential biases and confounding factors to properly interpret and apply their findings.
Although the CUtLASS trial was well-constructed and executed, its conclusions—like those of CATIE—merit careful scrutiny. Its patient recruitment methods and study design involved choices and compromises that are appropriate to evaluate17,18 as we weigh CUtLASS’ contribution to the SGA/FGA debate (Table 3).
Table 3
‘Flies in the ointment’ of the CUtLASS trial design
| Who was studied |
|
| What was compared |
|
| Other Issues |
|
| CUtLASS: Cost Utility of the Latest Antipsychotic Drugs in Schizophrenia Study | |
| EPS: Extrapyramidal symptom | |
| FGA: First-generation antipsychotic | |
| SGA: Second-generation antipsychotic | |
Who was studied?
Selection questions. CUtLASS researchers had problems recruiting patients for their study, in part because clinicians were reluctant to expose their patients to a 50% probability of being assigned to an FGA. Only 40% of the targeted sample was recruited, and participating clinicians referred only 20% to 37% of their eligible patients to the study.12 Thus, one could ask:
- Were enrolled subjects truly representative of the population from which they were drawn?
- Or did selection bias result in a disproportionate inclusion of individuals with certain characteristics?
Is it possible, for example, that clinicians preferentially referred medication-noncompliant patients to CUtLASS because they believed the benefits of depot FGAs—such as more assured adherence—would compensate for the potential benefits of SGAs—better efficacy/tolerability?19
Treatment resistance. Although patients were randomly assigned to FGAs or SGAs, a significantly greater proportion of those whose antipsychotics were being changed because of treatment resistance were assigned to receive SGAs. Treatment resistance was one reason that 88% of subjects in the SGA arm were referred to the trial, compared with 70% of subjects in the FGA arm (P<0.01).12 The extent to which this differential assignment may have biased results against SGAs is unclear.
EPS risk. CUtLASS-1 patients had been ill a mean of 14 years and had low baseline EPS rates despite receiving long-term antipsychotics (primarily FGAs). Even so, FGAs and SGAs showed similar rates of akathisia and other EPS. Thus—as with the CATIE results—the extent to which CUtLASS-1 findings may apply beyond chronic schizophrenia patients at relatively low risk for EPS is unclear.11,17
Impact of switching. Although patients were referred to CUtLASS because of adverse effects or inadequate response to one or more antipsychotics, they were only moderately ill (mean PANSS total score 72)20 and probably were deriving some benefit from their baseline antipsychotics. Before randomization, 82% of patients were receiving an FGA and 19% an SGA. Consequently, a far larger percentage of patients in the SGA group had to switch to a different medication class as the trial began.
As observed in CATIE, switching antipsychotics often has short-term negative consequences for patients,21 although switching classes (as in CUtLASS) may have had a different impact than switching individual antipsychotics (as in CATIE). If unequal antipsychotic switching rates in the two arms differentially affected patients’ quality of life, we would expect to see this effect emerge at the 12-week assessment, which is precisely where the greatest difference in Quality of Life Scale (QLS)13 scores appeared.
The mean QLS score for patients in the SGA arm was 2.6 points lower than in the FGA group at 12 weeks. This difference disappeared and, in fact, reversed at 26 weeks, but this 12-week effect had a strong impact on results of the 52-week intent-to-treat analysis. CUtLASS—like CATIE—might exemplify the risks of switching patients from treatment with partially effective antipsychotics.22
What was compared?
Classes vs individual drugs. The decision in CUtLASS-1 to compare antipsychotic classes rather than individual agents makes it difficult to interpret its findings. Antipsychotics are not homogeneous; clear differences exist within both the SGA and FGA classes in terms of individual agents’ efficacy and tolerability, and each SGA has a reasonably well-established and different side-effect profile.23
Sulpiride was the most commonly used FGA in CUtLASS-1 (by 49% of FGA patients). Sulpiride has some unusual attributes—such as lower EPS liability—and is not available in the United States. Thus, including this agent might have affected how applicable CUtLASS findings are to clinical practice in the United States.
Oral vs depot delivery. Individuals assigned to an FGA could receive either oral or long-acting depot medication, whereas those assigned to an SGA could receive only oral medication. At baseline, 84 of 227 CUtLASS-1 participants were receiving a depot antipsychotic, which was discontinued during randomization in 72 patients. During the 1-year study, the number of patients receiving a depot antipsychotic tripled from 12 to 35, suggesting the usefulness of long-acting agents in this population.19
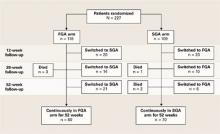
Cross-class switching. Although participating physicians and their patients were urged to stay within assigned antipsychotic classes at least for the first 12 weeks and ideally for 1 year, a high rate of cross-class switching occurred (Figure). At the 52-week assessment, 51 of 118 patients (43%) in the intent-to-treat FGA group were receiving SGAs instead.
The CUtLASS authors’ assert that the trial refutes the hypothesis that using SGAs is superior to using FGAs in improving quality of life. This conclusion is difficult to justify when so many patients assigned to the FGA class actually were receiving SGAs. The conclusion is further weakened if differential switching rates put SGAs at a disadvantage in the first 12 weeks of the trial.
A more accurate conclusion of the intent-to-treat comparison appears in the technical report: “There was no statistically significant difference in terms of quality of life or symptoms over 1 year in commencing [italics added] conventional antipsychotic drugs rather than new atypical drugs.”12
Figure CUtLASS-1: Did switching rate affect trial outcome?
The high rate of cross-class medication switching in CUtLASS-1 may have weakened the study’s conclusion that virtually no difference in effectiveness exists between first- and second-generation antipsychotics. At the 52-week assessment, 51 of 118 patients (43%) in the intent-to-treat FGA group were receiving SGAs instead. Not shown in the figure is that 4 of the total 55 patients who switched from FGAs to SGAs had switched back to FGAs by the 52-week assessment.
CUtLASS: Cost Utility of the Latest Antipsychotic Drugs in Schizophrenia Study
FGA: First-generation antipsychotic
SGA: Second-generation antipsychotic
Source: Adapted from reference 7, Figure 1
Clinical implications
Notwithstanding these cautionary notes, CUtLASS-1 findings add to the questions raised by CATIE about the relative effectiveness of SGAs and FGAs. At a minimum, the data indicate that the SGA advantage has been overstated or oversimplified and that FGAs may be suitable options for meeting the needs of some patients with psychosis (particularly those at low risk for EPS).
Depot antipsychotics. CUtLASS also suggests a wider role for long-acting antipsychotics in chronic psychotic disorders, beyond treating patients with severe nonadherence.19,23 The number of patients receiving long-acting agents tripled over the 1-year study.12
Clozapine. Both CATIE and CUtLASS-2 confirmed clozapine’s superior efficacy for patients with treatment-resistant psychotic illness (Table 4). CUtLASS-2 also reaffirmed the challenges of clozapine’s metabolic and other side effects, such as sedation, hypotension, and hypersalivation.
All-cause discontinuation was significantly higher (P<0.05) in patients taking clozapine (73%) than in those taking other SGAs (52%). Even so, clozapine-group patients achieved significantly greater symptom reduction and tended toward a higher quality of life than other SGA-group patients.
Table 4
Clinical ‘pearls’ from the CUtLASS trial data
|
| CUtLASS: Cost Utility of the Latest Antipsychotic Drugs in Schizophrenia Study |
| EPS: Extrapyramidal symptom |
| FGA: First-generation antipsychotic |
| SGA: Second-generation antipsychotic |
Overview. In conclusion, one can reasonably conclude from analyzing the CATIE and CUtLASS data that:
- FGA-SGA differences are not as great as previously thought.
- Substantial differences exist among agents within both antipsychotic classes, particularly in side effect profiles.
- Neither study disproves the following presumed benefit of SGAs: that compared with FGAs, SGAs provide an equivalent antipsychotic effect and pose a lower risk of problems related to unmitigated dopamine blockade—such as EPS, dysphoria, bradyphrenia, neuroleptic-induced deficit syndrome, and tardive dyskinesia.11
- To use antipsychotics effectively and optimize individual treatment, consider the CATIE and CUtLASS trials in the contexts of their designs and the results of other studies of patients with chronic schizophrenia.
Related resources
- Heres S, Davis J, Maino K, et al. Why olanzapine beats risperidone, risperidone beats quetiapine, and quetiapine beats olanzapine: An exploratory analysis of head-head comparison studies of second-generation antipsychotics. Am J Psychiatry 2006;163:185-94.
Drug brand names
- Clozapine • Clozaril
- Quetiapine • Seroquel
- Olanzapine • Zyprexa
- Risperidone • Risperdal
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Kane JM, Leucht S, Carpenter D, et al. Expert consensus guideline series: optimizing pharmacologic treatment of psychotic disorders. J Clin Psychiatry 2003;64(suppl 12):1-100.
2. Tandon R, Fleischhacker WW. Comparative efficacy of antipsychotics in the treatment of schizophrenia: a critical assessment. Schizophr Res 2005;79:145-55.
3. Lieberman JA, Stroup TS, McEvoy JP, et al. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med 2005;353:1209-23.
4. McEvoy JP, Lieberman JA, Stroup TS, et al. Effectiveness of clozapine versus olanzapine, quetiapine, and risperidone in patients with chronic schizophrenia who did not respond to prior antipsychotic treatment. Am J Psychiatry 2006;163:600-10.
5. Stroup TS, Lieberman JA, McEvoy JP, et al. Effectiveness of olanzapine, quetiapine, risperidone, and ziprasidone in patients with chronic schizophrenia following discontinuation of a previous atypical antipsychotic. Am J Psychiatry 2006;163:611-22.
6. Rosenheck RA, Leslie DL, Sindelar J, et al. Cost-effectiveness of second-generation antipsychotics and perphenazine in a randomized trial of treatment for chronic schizophrenia. Am J Psychiatry 2006;163:2080-9.
7. Jones PB, Barnes T, Davies L, et al. Randomized controlled trial of the effect on quality of life of second- vs first-generation antipsychotic drugs in schizophrenia: Cost Utility of the Latest Antipsychotic Drugs in Schizophrenia Study (CUtLASS). Arch Gen Psychiatry 2006;63:1079-87.
8. Lewis SW, Barnes TRE, Davies L, et al. Randomised controlled trial of effect of prescription of clozapine versus other second-generation antipsychotic drugs in resistant schizophrenia. Schizophr Bull 2006;32:715-23.
9. Nasrallah HA. CATIE’s surprises: in antipsychotics’ square-off, were there winners or losers? Current Psychiatry 2006;5(2):49-65.
10. Buckley PF. Which antipsychotic do I choose next? CATIE phase 2 offers insights on efficacy and tolerability. Current Psychiatry 2006;5(9):27-43.
11. Tandon R, Constantine R. Avoiding EPS is key to realizing ‘atypical’ benefits. Current Psychiatry 2006;5(11):35-45.
12. Lewis SW, Davies L, Jones PB, et al. Randomised controlled trials of conventional antipsychotic versus new atypical drugs, and new atypical drugs versus clozapine, in people with schizophrenia responding poorly to, or intolerant of, current drug treatment. Health Technology Assessment 2006;10(#17):1-182.Available at http://www.hta.ac.uk/project/1078.asp. Accessed January 3, 2007.
13. Heinrichs DW, Hanlon TE, Carpenter WT. The Quality of Life Scale: an instrument for assessing the schizophrenic deficit syndrome. Schizophr Bull 1984;10:388-98.
14. Tandon R, Davis JM, Carpenter WT. CATIE, CUtLASS, and the FGA-SGA debate (letter). Arch Gen Psychiatry 2007 (in press).
15. Lieberman J. Comparative effectiveness of antipsychotic drugs: a commentary on the Cost Utility of the Latest Antipsychotic Drugs in Schizophrenia Study and Clinical Antipsychotic Trials of Intervention Effectiveness. Arch Gen Psychiatry 2006;63:1069-72.
16. Rosenheck RA. Outcomes, costs, and policy caution: a commentary on the Cost Utility of the Latest Antipsychotic Drugs in Schizophrenia Study. Arch Gen Psychiatry 2006;63:1074-6.
17. Tandon R. Comparative effectiveness of antipsychotics in the treatment of schizophrenia: what CATIE tells us—Parts 1 and 2. International Drug Therapy Newsletter 2006;41(7,9):51-8,67-74.
18. Meltzer HY, Bobo WV. Interpreting the efficacy findings in the CATIE study: what clinicians should know. CNS Spectrums 2006;11(suppl 7):14-24.
19. Kane JM. Review of treatments that can ameliorate nonadherence in patients with schizophrenia. J Clin Psychiatry 2006;67(suppl 5):9-14.
20. Leucht S, Kane JM, Kissling W, et al. What does the PANSS mean? Schizophr Res 2006;79:231-8.
21. Essock SM, Covell NH, Davis SM, et al. Effectiveness of switching antipsychotic medications. Am J Psychiatry 2006;163:2090-5.
22. Davis JM, Marder S, Tamminga CA. Switch or stay? Am J Psychiatry 2006;163:2032-3.
23. Tandon R, Targum SD, Nasrallah HA, et al. Strategies for maximizing clinical effectiveness in the treatment of schizophrenia. Journal of Psychiatric Practice 2006;12:348-63.
When treating chronic psychotic disorders, U.S. psychiatrists generally prefer second-generation antipsychotics (SGAs) to first-generation antipsychotics (FGAs) because of widely held views1,2 that SGAs:
- are more effective for negative and cognitive symptoms
- produce fewer troublesome side effects
- help patients realize a better quality of life.
These beliefs have been challenged by two large-scale, government-supported studies: the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) in the United States3-6 and more recently the Cost Utility of the Latest Antipsychotic Drugs in Schizophrenia Study (CUtLASS) from the United Kingdom.7,8
CATIE and CUtLASS data suggest that the SGA advantage has been exaggerated, if in fact such an advantage exists. Other Current Psychiatry articles for the clinical practitioner have discussed the CATIE findings.9-11 This article addresses the CUtLASS results in the context of the trial’s methodology, using information from the primary publications7,8 and technical report.12
Cutlass study
Design. CUtLASS included 2 “bands” (Table 1):
- Band 1 compared the clinical usefulness and cost effectiveness of FGAs and SGAs in treating schizophrenia7
- Band 2 compared the effectiveness of clozapine versus other SGAs in treating refractory schizophrenia.8
CUtLASS Band 1 was not as extensive in scope as CATIE, and its design had some important differences (Table 2). Patients were referred for participation because their psychiatrists were considering a change in antipsychotic medication to address adverse effects or inadequate response. Fewer patients were recruited than expected—40% of the planned sample during 30 months of recruitment—but researchers considered the size sufficient to compare the effectiveness of FGAs and SGAs.
Patients were randomly assigned to treatment with an antipsychotic class, either:
- an FGA (1 of 11 options—including 5 depot formulations—chosen by the treating clinician)
- or an SGA (risperidone, olanzapine, quetiapine, or amisulpride, also chosen by the clinician).
Physicians and patients were not blinded to the medications used. They could choose medications within patients’ assigned classes and switch as needed in ways that mimicked clinical practice. Trained assessors, who were blinded to the medications being used, evaluated the patients after 12, 26, and 52 weeks.
Quality of life was the primary outcome measure.13 Secondary measures included symptoms, side effects, patient satisfaction, and cost of care.
Band 1 results. Patients assigned to the SGA or FGA classes showed no significant differences in quality of life measures or schizophrenia symptoms. If anything, the findings slightly favored the FGAs.
Patient satisfaction and overall cost of care were similar, and rates of extrapyramidal symptoms (EPS), tardive dyskinesia, and akathisia did not differ significantly.
Clozapine comparison. In CUtLASS band 2, a different sample of 136 schizophrenia patients who had responded poorly to ≥2 antipsychotics was randomly assigned to clozapine or one of the above four SGAs. During the 1-year comparison trial, clozapine:
- was found to be significantly more effective (P=0.01) in managing patients’ symptoms, as measured by total Positive and Negative Syndrome Scale (PANSS) score
- showed a trend (P=0.08) towards providing these treatment-resistant patients with a better quality of life.8
Table 1
Summary of CUtLASS trial design and results
Band 1
|
Band 2
|
| CUtLASS: Cost Utility of the Latest Antipsychotic Drugs in Schizophrenia Study |
| FGA: First-generation antipsychotic |
| PANSS: Positive and Negative Syndrome Scale |
| SGA: Second-generation antipsychotic |
Table 2
Comparing designs of the CUtLASS and CATIE schizophrenia trials
| CUtLASS | CATIE | |
|---|---|---|
| Trial duration | 12 months | 18 months |
| Clinical sites | 14 (United Kingdom) | 57 (United States) |
| Number of Subjects | 227 | 1,460 |
| Gender and age | 68% male; mean age 41 | 74% male; mean age 41 |
| Mental illness duration (mean) | 14 years | 16 years |
| Diagnosis | 75% schizophrenia | 100% schizophrenia |
| First-episode patients included? | Yes (13% of sample) | No |
| % of patients receiving antipsychotics at enrollment | 99% | 74% |
| Baseline PANSS score (mean) | 82% FGAs; 40% depot | 15% FGAs; <5% depot |
| Baseline PANSS score | 72.2 | 75.7 |
| Baseline EPS scores | Low | Low |
| Antipsychotic options in randomization | 2 classes (SGA or FGA) (50% of subjects assigned to an FGA) | 4 SGAs, 1 FGA (20% of subjects assigned to an FGA) |
| % of subjects given sulpiride | 49% | 0% |
| Administration methodology | Medication blinded to raters but not to patients and physicians | Medication blinded to patients and physicians |
| Primary outcome | Quality of life | Discontinuation of medication |
| Long-acting antipsychotic option? | Yes | No |
| Antipsychotic switching | All patients switched agents; 49% changed antipsychotic class | 15% stayed on some agent |
| CATIE: Clinical Antipsychotic Trials of Intervention Effectiveness | ||
| CUtLASS: Cost Utility of the Latest Antipsychotic Drugs in Schizophrenia Study | ||
| EPS: Extrapyramidal symptom | ||
| FGA: First-generation antipsychotic | ||
| PANSS: Positive and Negative Syndrome Scale | ||
| SGA: Second-generation antipsychotic | ||
Comparing catie, cutlass data
The CUtLASS findings are not identical to those of CATIE phase 114 but are remarkably similar: no differences in effectiveness were seen between FGAs and SGA when treating patients with chronic schizophrenia.15,16
CUtLASS investigators concluded that “in people with schizophrenia whose medication is changed for clinical reasons, there is no disadvantage across 1 year in terms of quality of life, symptoms, or associated costs of care in using FGAs rather than nonclozapine SGAs.”7
By confirming CATIE’s results, is CUtLASS the final word on antipsychotic treatment of chronic schizophrenia? Or is it just another piece of the puzzle? CATIE and CUtLASS add much to our knowledge, but methodologic “flies in the ointment” plague all clinical trials. We must consider potential biases and confounding factors to properly interpret and apply their findings.
Although the CUtLASS trial was well-constructed and executed, its conclusions—like those of CATIE—merit careful scrutiny. Its patient recruitment methods and study design involved choices and compromises that are appropriate to evaluate17,18 as we weigh CUtLASS’ contribution to the SGA/FGA debate (Table 3).
Table 3
‘Flies in the ointment’ of the CUtLASS trial design
| Who was studied |
|
| What was compared |
|
| Other Issues |
|
| CUtLASS: Cost Utility of the Latest Antipsychotic Drugs in Schizophrenia Study | |
| EPS: Extrapyramidal symptom | |
| FGA: First-generation antipsychotic | |
| SGA: Second-generation antipsychotic | |
Who was studied?
Selection questions. CUtLASS researchers had problems recruiting patients for their study, in part because clinicians were reluctant to expose their patients to a 50% probability of being assigned to an FGA. Only 40% of the targeted sample was recruited, and participating clinicians referred only 20% to 37% of their eligible patients to the study.12 Thus, one could ask:
- Were enrolled subjects truly representative of the population from which they were drawn?
- Or did selection bias result in a disproportionate inclusion of individuals with certain characteristics?
Is it possible, for example, that clinicians preferentially referred medication-noncompliant patients to CUtLASS because they believed the benefits of depot FGAs—such as more assured adherence—would compensate for the potential benefits of SGAs—better efficacy/tolerability?19
Treatment resistance. Although patients were randomly assigned to FGAs or SGAs, a significantly greater proportion of those whose antipsychotics were being changed because of treatment resistance were assigned to receive SGAs. Treatment resistance was one reason that 88% of subjects in the SGA arm were referred to the trial, compared with 70% of subjects in the FGA arm (P<0.01).12 The extent to which this differential assignment may have biased results against SGAs is unclear.
EPS risk. CUtLASS-1 patients had been ill a mean of 14 years and had low baseline EPS rates despite receiving long-term antipsychotics (primarily FGAs). Even so, FGAs and SGAs showed similar rates of akathisia and other EPS. Thus—as with the CATIE results—the extent to which CUtLASS-1 findings may apply beyond chronic schizophrenia patients at relatively low risk for EPS is unclear.11,17
Impact of switching. Although patients were referred to CUtLASS because of adverse effects or inadequate response to one or more antipsychotics, they were only moderately ill (mean PANSS total score 72)20 and probably were deriving some benefit from their baseline antipsychotics. Before randomization, 82% of patients were receiving an FGA and 19% an SGA. Consequently, a far larger percentage of patients in the SGA group had to switch to a different medication class as the trial began.
As observed in CATIE, switching antipsychotics often has short-term negative consequences for patients,21 although switching classes (as in CUtLASS) may have had a different impact than switching individual antipsychotics (as in CATIE). If unequal antipsychotic switching rates in the two arms differentially affected patients’ quality of life, we would expect to see this effect emerge at the 12-week assessment, which is precisely where the greatest difference in Quality of Life Scale (QLS)13 scores appeared.
The mean QLS score for patients in the SGA arm was 2.6 points lower than in the FGA group at 12 weeks. This difference disappeared and, in fact, reversed at 26 weeks, but this 12-week effect had a strong impact on results of the 52-week intent-to-treat analysis. CUtLASS—like CATIE—might exemplify the risks of switching patients from treatment with partially effective antipsychotics.22
What was compared?
Classes vs individual drugs. The decision in CUtLASS-1 to compare antipsychotic classes rather than individual agents makes it difficult to interpret its findings. Antipsychotics are not homogeneous; clear differences exist within both the SGA and FGA classes in terms of individual agents’ efficacy and tolerability, and each SGA has a reasonably well-established and different side-effect profile.23
Sulpiride was the most commonly used FGA in CUtLASS-1 (by 49% of FGA patients). Sulpiride has some unusual attributes—such as lower EPS liability—and is not available in the United States. Thus, including this agent might have affected how applicable CUtLASS findings are to clinical practice in the United States.
Oral vs depot delivery. Individuals assigned to an FGA could receive either oral or long-acting depot medication, whereas those assigned to an SGA could receive only oral medication. At baseline, 84 of 227 CUtLASS-1 participants were receiving a depot antipsychotic, which was discontinued during randomization in 72 patients. During the 1-year study, the number of patients receiving a depot antipsychotic tripled from 12 to 35, suggesting the usefulness of long-acting agents in this population.19
Cross-class switching. Although participating physicians and their patients were urged to stay within assigned antipsychotic classes at least for the first 12 weeks and ideally for 1 year, a high rate of cross-class switching occurred (Figure). At the 52-week assessment, 51 of 118 patients (43%) in the intent-to-treat FGA group were receiving SGAs instead.
The CUtLASS authors’ assert that the trial refutes the hypothesis that using SGAs is superior to using FGAs in improving quality of life. This conclusion is difficult to justify when so many patients assigned to the FGA class actually were receiving SGAs. The conclusion is further weakened if differential switching rates put SGAs at a disadvantage in the first 12 weeks of the trial.
A more accurate conclusion of the intent-to-treat comparison appears in the technical report: “There was no statistically significant difference in terms of quality of life or symptoms over 1 year in commencing [italics added] conventional antipsychotic drugs rather than new atypical drugs.”12
Figure CUtLASS-1: Did switching rate affect trial outcome?
The high rate of cross-class medication switching in CUtLASS-1 may have weakened the study’s conclusion that virtually no difference in effectiveness exists between first- and second-generation antipsychotics. At the 52-week assessment, 51 of 118 patients (43%) in the intent-to-treat FGA group were receiving SGAs instead. Not shown in the figure is that 4 of the total 55 patients who switched from FGAs to SGAs had switched back to FGAs by the 52-week assessment.
CUtLASS: Cost Utility of the Latest Antipsychotic Drugs in Schizophrenia Study
FGA: First-generation antipsychotic
SGA: Second-generation antipsychotic
Source: Adapted from reference 7, Figure 1
Clinical implications
Notwithstanding these cautionary notes, CUtLASS-1 findings add to the questions raised by CATIE about the relative effectiveness of SGAs and FGAs. At a minimum, the data indicate that the SGA advantage has been overstated or oversimplified and that FGAs may be suitable options for meeting the needs of some patients with psychosis (particularly those at low risk for EPS).
Depot antipsychotics. CUtLASS also suggests a wider role for long-acting antipsychotics in chronic psychotic disorders, beyond treating patients with severe nonadherence.19,23 The number of patients receiving long-acting agents tripled over the 1-year study.12
Clozapine. Both CATIE and CUtLASS-2 confirmed clozapine’s superior efficacy for patients with treatment-resistant psychotic illness (Table 4). CUtLASS-2 also reaffirmed the challenges of clozapine’s metabolic and other side effects, such as sedation, hypotension, and hypersalivation.
All-cause discontinuation was significantly higher (P<0.05) in patients taking clozapine (73%) than in those taking other SGAs (52%). Even so, clozapine-group patients achieved significantly greater symptom reduction and tended toward a higher quality of life than other SGA-group patients.
Table 4
Clinical ‘pearls’ from the CUtLASS trial data
|
| CUtLASS: Cost Utility of the Latest Antipsychotic Drugs in Schizophrenia Study |
| EPS: Extrapyramidal symptom |
| FGA: First-generation antipsychotic |
| SGA: Second-generation antipsychotic |
Overview. In conclusion, one can reasonably conclude from analyzing the CATIE and CUtLASS data that:
- FGA-SGA differences are not as great as previously thought.
- Substantial differences exist among agents within both antipsychotic classes, particularly in side effect profiles.
- Neither study disproves the following presumed benefit of SGAs: that compared with FGAs, SGAs provide an equivalent antipsychotic effect and pose a lower risk of problems related to unmitigated dopamine blockade—such as EPS, dysphoria, bradyphrenia, neuroleptic-induced deficit syndrome, and tardive dyskinesia.11
- To use antipsychotics effectively and optimize individual treatment, consider the CATIE and CUtLASS trials in the contexts of their designs and the results of other studies of patients with chronic schizophrenia.
Related resources
- Heres S, Davis J, Maino K, et al. Why olanzapine beats risperidone, risperidone beats quetiapine, and quetiapine beats olanzapine: An exploratory analysis of head-head comparison studies of second-generation antipsychotics. Am J Psychiatry 2006;163:185-94.
Drug brand names
- Clozapine • Clozaril
- Quetiapine • Seroquel
- Olanzapine • Zyprexa
- Risperidone • Risperdal
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
When treating chronic psychotic disorders, U.S. psychiatrists generally prefer second-generation antipsychotics (SGAs) to first-generation antipsychotics (FGAs) because of widely held views1,2 that SGAs:
- are more effective for negative and cognitive symptoms
- produce fewer troublesome side effects
- help patients realize a better quality of life.
These beliefs have been challenged by two large-scale, government-supported studies: the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) in the United States3-6 and more recently the Cost Utility of the Latest Antipsychotic Drugs in Schizophrenia Study (CUtLASS) from the United Kingdom.7,8
CATIE and CUtLASS data suggest that the SGA advantage has been exaggerated, if in fact such an advantage exists. Other Current Psychiatry articles for the clinical practitioner have discussed the CATIE findings.9-11 This article addresses the CUtLASS results in the context of the trial’s methodology, using information from the primary publications7,8 and technical report.12
Cutlass study
Design. CUtLASS included 2 “bands” (Table 1):
- Band 1 compared the clinical usefulness and cost effectiveness of FGAs and SGAs in treating schizophrenia7
- Band 2 compared the effectiveness of clozapine versus other SGAs in treating refractory schizophrenia.8
CUtLASS Band 1 was not as extensive in scope as CATIE, and its design had some important differences (Table 2). Patients were referred for participation because their psychiatrists were considering a change in antipsychotic medication to address adverse effects or inadequate response. Fewer patients were recruited than expected—40% of the planned sample during 30 months of recruitment—but researchers considered the size sufficient to compare the effectiveness of FGAs and SGAs.
Patients were randomly assigned to treatment with an antipsychotic class, either:
- an FGA (1 of 11 options—including 5 depot formulations—chosen by the treating clinician)
- or an SGA (risperidone, olanzapine, quetiapine, or amisulpride, also chosen by the clinician).
Physicians and patients were not blinded to the medications used. They could choose medications within patients’ assigned classes and switch as needed in ways that mimicked clinical practice. Trained assessors, who were blinded to the medications being used, evaluated the patients after 12, 26, and 52 weeks.
Quality of life was the primary outcome measure.13 Secondary measures included symptoms, side effects, patient satisfaction, and cost of care.
Band 1 results. Patients assigned to the SGA or FGA classes showed no significant differences in quality of life measures or schizophrenia symptoms. If anything, the findings slightly favored the FGAs.
Patient satisfaction and overall cost of care were similar, and rates of extrapyramidal symptoms (EPS), tardive dyskinesia, and akathisia did not differ significantly.
Clozapine comparison. In CUtLASS band 2, a different sample of 136 schizophrenia patients who had responded poorly to ≥2 antipsychotics was randomly assigned to clozapine or one of the above four SGAs. During the 1-year comparison trial, clozapine:
- was found to be significantly more effective (P=0.01) in managing patients’ symptoms, as measured by total Positive and Negative Syndrome Scale (PANSS) score
- showed a trend (P=0.08) towards providing these treatment-resistant patients with a better quality of life.8
Table 1
Summary of CUtLASS trial design and results
Band 1
|
Band 2
|
| CUtLASS: Cost Utility of the Latest Antipsychotic Drugs in Schizophrenia Study |
| FGA: First-generation antipsychotic |
| PANSS: Positive and Negative Syndrome Scale |
| SGA: Second-generation antipsychotic |
Table 2
Comparing designs of the CUtLASS and CATIE schizophrenia trials
| CUtLASS | CATIE | |
|---|---|---|
| Trial duration | 12 months | 18 months |
| Clinical sites | 14 (United Kingdom) | 57 (United States) |
| Number of Subjects | 227 | 1,460 |
| Gender and age | 68% male; mean age 41 | 74% male; mean age 41 |
| Mental illness duration (mean) | 14 years | 16 years |
| Diagnosis | 75% schizophrenia | 100% schizophrenia |
| First-episode patients included? | Yes (13% of sample) | No |
| % of patients receiving antipsychotics at enrollment | 99% | 74% |
| Baseline PANSS score (mean) | 82% FGAs; 40% depot | 15% FGAs; <5% depot |
| Baseline PANSS score | 72.2 | 75.7 |
| Baseline EPS scores | Low | Low |
| Antipsychotic options in randomization | 2 classes (SGA or FGA) (50% of subjects assigned to an FGA) | 4 SGAs, 1 FGA (20% of subjects assigned to an FGA) |
| % of subjects given sulpiride | 49% | 0% |
| Administration methodology | Medication blinded to raters but not to patients and physicians | Medication blinded to patients and physicians |
| Primary outcome | Quality of life | Discontinuation of medication |
| Long-acting antipsychotic option? | Yes | No |
| Antipsychotic switching | All patients switched agents; 49% changed antipsychotic class | 15% stayed on some agent |
| CATIE: Clinical Antipsychotic Trials of Intervention Effectiveness | ||
| CUtLASS: Cost Utility of the Latest Antipsychotic Drugs in Schizophrenia Study | ||
| EPS: Extrapyramidal symptom | ||
| FGA: First-generation antipsychotic | ||
| PANSS: Positive and Negative Syndrome Scale | ||
| SGA: Second-generation antipsychotic | ||
Comparing catie, cutlass data
The CUtLASS findings are not identical to those of CATIE phase 114 but are remarkably similar: no differences in effectiveness were seen between FGAs and SGA when treating patients with chronic schizophrenia.15,16
CUtLASS investigators concluded that “in people with schizophrenia whose medication is changed for clinical reasons, there is no disadvantage across 1 year in terms of quality of life, symptoms, or associated costs of care in using FGAs rather than nonclozapine SGAs.”7
By confirming CATIE’s results, is CUtLASS the final word on antipsychotic treatment of chronic schizophrenia? Or is it just another piece of the puzzle? CATIE and CUtLASS add much to our knowledge, but methodologic “flies in the ointment” plague all clinical trials. We must consider potential biases and confounding factors to properly interpret and apply their findings.
Although the CUtLASS trial was well-constructed and executed, its conclusions—like those of CATIE—merit careful scrutiny. Its patient recruitment methods and study design involved choices and compromises that are appropriate to evaluate17,18 as we weigh CUtLASS’ contribution to the SGA/FGA debate (Table 3).
Table 3
‘Flies in the ointment’ of the CUtLASS trial design
| Who was studied |
|
| What was compared |
|
| Other Issues |
|
| CUtLASS: Cost Utility of the Latest Antipsychotic Drugs in Schizophrenia Study | |
| EPS: Extrapyramidal symptom | |
| FGA: First-generation antipsychotic | |
| SGA: Second-generation antipsychotic | |
Who was studied?
Selection questions. CUtLASS researchers had problems recruiting patients for their study, in part because clinicians were reluctant to expose their patients to a 50% probability of being assigned to an FGA. Only 40% of the targeted sample was recruited, and participating clinicians referred only 20% to 37% of their eligible patients to the study.12 Thus, one could ask:
- Were enrolled subjects truly representative of the population from which they were drawn?
- Or did selection bias result in a disproportionate inclusion of individuals with certain characteristics?
Is it possible, for example, that clinicians preferentially referred medication-noncompliant patients to CUtLASS because they believed the benefits of depot FGAs—such as more assured adherence—would compensate for the potential benefits of SGAs—better efficacy/tolerability?19
Treatment resistance. Although patients were randomly assigned to FGAs or SGAs, a significantly greater proportion of those whose antipsychotics were being changed because of treatment resistance were assigned to receive SGAs. Treatment resistance was one reason that 88% of subjects in the SGA arm were referred to the trial, compared with 70% of subjects in the FGA arm (P<0.01).12 The extent to which this differential assignment may have biased results against SGAs is unclear.
EPS risk. CUtLASS-1 patients had been ill a mean of 14 years and had low baseline EPS rates despite receiving long-term antipsychotics (primarily FGAs). Even so, FGAs and SGAs showed similar rates of akathisia and other EPS. Thus—as with the CATIE results—the extent to which CUtLASS-1 findings may apply beyond chronic schizophrenia patients at relatively low risk for EPS is unclear.11,17
Impact of switching. Although patients were referred to CUtLASS because of adverse effects or inadequate response to one or more antipsychotics, they were only moderately ill (mean PANSS total score 72)20 and probably were deriving some benefit from their baseline antipsychotics. Before randomization, 82% of patients were receiving an FGA and 19% an SGA. Consequently, a far larger percentage of patients in the SGA group had to switch to a different medication class as the trial began.
As observed in CATIE, switching antipsychotics often has short-term negative consequences for patients,21 although switching classes (as in CUtLASS) may have had a different impact than switching individual antipsychotics (as in CATIE). If unequal antipsychotic switching rates in the two arms differentially affected patients’ quality of life, we would expect to see this effect emerge at the 12-week assessment, which is precisely where the greatest difference in Quality of Life Scale (QLS)13 scores appeared.
The mean QLS score for patients in the SGA arm was 2.6 points lower than in the FGA group at 12 weeks. This difference disappeared and, in fact, reversed at 26 weeks, but this 12-week effect had a strong impact on results of the 52-week intent-to-treat analysis. CUtLASS—like CATIE—might exemplify the risks of switching patients from treatment with partially effective antipsychotics.22
What was compared?
Classes vs individual drugs. The decision in CUtLASS-1 to compare antipsychotic classes rather than individual agents makes it difficult to interpret its findings. Antipsychotics are not homogeneous; clear differences exist within both the SGA and FGA classes in terms of individual agents’ efficacy and tolerability, and each SGA has a reasonably well-established and different side-effect profile.23
Sulpiride was the most commonly used FGA in CUtLASS-1 (by 49% of FGA patients). Sulpiride has some unusual attributes—such as lower EPS liability—and is not available in the United States. Thus, including this agent might have affected how applicable CUtLASS findings are to clinical practice in the United States.
Oral vs depot delivery. Individuals assigned to an FGA could receive either oral or long-acting depot medication, whereas those assigned to an SGA could receive only oral medication. At baseline, 84 of 227 CUtLASS-1 participants were receiving a depot antipsychotic, which was discontinued during randomization in 72 patients. During the 1-year study, the number of patients receiving a depot antipsychotic tripled from 12 to 35, suggesting the usefulness of long-acting agents in this population.19
Cross-class switching. Although participating physicians and their patients were urged to stay within assigned antipsychotic classes at least for the first 12 weeks and ideally for 1 year, a high rate of cross-class switching occurred (Figure). At the 52-week assessment, 51 of 118 patients (43%) in the intent-to-treat FGA group were receiving SGAs instead.
The CUtLASS authors’ assert that the trial refutes the hypothesis that using SGAs is superior to using FGAs in improving quality of life. This conclusion is difficult to justify when so many patients assigned to the FGA class actually were receiving SGAs. The conclusion is further weakened if differential switching rates put SGAs at a disadvantage in the first 12 weeks of the trial.
A more accurate conclusion of the intent-to-treat comparison appears in the technical report: “There was no statistically significant difference in terms of quality of life or symptoms over 1 year in commencing [italics added] conventional antipsychotic drugs rather than new atypical drugs.”12
Figure CUtLASS-1: Did switching rate affect trial outcome?
The high rate of cross-class medication switching in CUtLASS-1 may have weakened the study’s conclusion that virtually no difference in effectiveness exists between first- and second-generation antipsychotics. At the 52-week assessment, 51 of 118 patients (43%) in the intent-to-treat FGA group were receiving SGAs instead. Not shown in the figure is that 4 of the total 55 patients who switched from FGAs to SGAs had switched back to FGAs by the 52-week assessment.
CUtLASS: Cost Utility of the Latest Antipsychotic Drugs in Schizophrenia Study
FGA: First-generation antipsychotic
SGA: Second-generation antipsychotic
Source: Adapted from reference 7, Figure 1
Clinical implications
Notwithstanding these cautionary notes, CUtLASS-1 findings add to the questions raised by CATIE about the relative effectiveness of SGAs and FGAs. At a minimum, the data indicate that the SGA advantage has been overstated or oversimplified and that FGAs may be suitable options for meeting the needs of some patients with psychosis (particularly those at low risk for EPS).
Depot antipsychotics. CUtLASS also suggests a wider role for long-acting antipsychotics in chronic psychotic disorders, beyond treating patients with severe nonadherence.19,23 The number of patients receiving long-acting agents tripled over the 1-year study.12
Clozapine. Both CATIE and CUtLASS-2 confirmed clozapine’s superior efficacy for patients with treatment-resistant psychotic illness (Table 4). CUtLASS-2 also reaffirmed the challenges of clozapine’s metabolic and other side effects, such as sedation, hypotension, and hypersalivation.
All-cause discontinuation was significantly higher (P<0.05) in patients taking clozapine (73%) than in those taking other SGAs (52%). Even so, clozapine-group patients achieved significantly greater symptom reduction and tended toward a higher quality of life than other SGA-group patients.
Table 4
Clinical ‘pearls’ from the CUtLASS trial data
|
| CUtLASS: Cost Utility of the Latest Antipsychotic Drugs in Schizophrenia Study |
| EPS: Extrapyramidal symptom |
| FGA: First-generation antipsychotic |
| SGA: Second-generation antipsychotic |
Overview. In conclusion, one can reasonably conclude from analyzing the CATIE and CUtLASS data that:
- FGA-SGA differences are not as great as previously thought.
- Substantial differences exist among agents within both antipsychotic classes, particularly in side effect profiles.
- Neither study disproves the following presumed benefit of SGAs: that compared with FGAs, SGAs provide an equivalent antipsychotic effect and pose a lower risk of problems related to unmitigated dopamine blockade—such as EPS, dysphoria, bradyphrenia, neuroleptic-induced deficit syndrome, and tardive dyskinesia.11
- To use antipsychotics effectively and optimize individual treatment, consider the CATIE and CUtLASS trials in the contexts of their designs and the results of other studies of patients with chronic schizophrenia.
Related resources
- Heres S, Davis J, Maino K, et al. Why olanzapine beats risperidone, risperidone beats quetiapine, and quetiapine beats olanzapine: An exploratory analysis of head-head comparison studies of second-generation antipsychotics. Am J Psychiatry 2006;163:185-94.
Drug brand names
- Clozapine • Clozaril
- Quetiapine • Seroquel
- Olanzapine • Zyprexa
- Risperidone • Risperdal
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Kane JM, Leucht S, Carpenter D, et al. Expert consensus guideline series: optimizing pharmacologic treatment of psychotic disorders. J Clin Psychiatry 2003;64(suppl 12):1-100.
2. Tandon R, Fleischhacker WW. Comparative efficacy of antipsychotics in the treatment of schizophrenia: a critical assessment. Schizophr Res 2005;79:145-55.
3. Lieberman JA, Stroup TS, McEvoy JP, et al. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med 2005;353:1209-23.
4. McEvoy JP, Lieberman JA, Stroup TS, et al. Effectiveness of clozapine versus olanzapine, quetiapine, and risperidone in patients with chronic schizophrenia who did not respond to prior antipsychotic treatment. Am J Psychiatry 2006;163:600-10.
5. Stroup TS, Lieberman JA, McEvoy JP, et al. Effectiveness of olanzapine, quetiapine, risperidone, and ziprasidone in patients with chronic schizophrenia following discontinuation of a previous atypical antipsychotic. Am J Psychiatry 2006;163:611-22.
6. Rosenheck RA, Leslie DL, Sindelar J, et al. Cost-effectiveness of second-generation antipsychotics and perphenazine in a randomized trial of treatment for chronic schizophrenia. Am J Psychiatry 2006;163:2080-9.
7. Jones PB, Barnes T, Davies L, et al. Randomized controlled trial of the effect on quality of life of second- vs first-generation antipsychotic drugs in schizophrenia: Cost Utility of the Latest Antipsychotic Drugs in Schizophrenia Study (CUtLASS). Arch Gen Psychiatry 2006;63:1079-87.
8. Lewis SW, Barnes TRE, Davies L, et al. Randomised controlled trial of effect of prescription of clozapine versus other second-generation antipsychotic drugs in resistant schizophrenia. Schizophr Bull 2006;32:715-23.
9. Nasrallah HA. CATIE’s surprises: in antipsychotics’ square-off, were there winners or losers? Current Psychiatry 2006;5(2):49-65.
10. Buckley PF. Which antipsychotic do I choose next? CATIE phase 2 offers insights on efficacy and tolerability. Current Psychiatry 2006;5(9):27-43.
11. Tandon R, Constantine R. Avoiding EPS is key to realizing ‘atypical’ benefits. Current Psychiatry 2006;5(11):35-45.
12. Lewis SW, Davies L, Jones PB, et al. Randomised controlled trials of conventional antipsychotic versus new atypical drugs, and new atypical drugs versus clozapine, in people with schizophrenia responding poorly to, or intolerant of, current drug treatment. Health Technology Assessment 2006;10(#17):1-182.Available at http://www.hta.ac.uk/project/1078.asp. Accessed January 3, 2007.
13. Heinrichs DW, Hanlon TE, Carpenter WT. The Quality of Life Scale: an instrument for assessing the schizophrenic deficit syndrome. Schizophr Bull 1984;10:388-98.
14. Tandon R, Davis JM, Carpenter WT. CATIE, CUtLASS, and the FGA-SGA debate (letter). Arch Gen Psychiatry 2007 (in press).
15. Lieberman J. Comparative effectiveness of antipsychotic drugs: a commentary on the Cost Utility of the Latest Antipsychotic Drugs in Schizophrenia Study and Clinical Antipsychotic Trials of Intervention Effectiveness. Arch Gen Psychiatry 2006;63:1069-72.
16. Rosenheck RA. Outcomes, costs, and policy caution: a commentary on the Cost Utility of the Latest Antipsychotic Drugs in Schizophrenia Study. Arch Gen Psychiatry 2006;63:1074-6.
17. Tandon R. Comparative effectiveness of antipsychotics in the treatment of schizophrenia: what CATIE tells us—Parts 1 and 2. International Drug Therapy Newsletter 2006;41(7,9):51-8,67-74.
18. Meltzer HY, Bobo WV. Interpreting the efficacy findings in the CATIE study: what clinicians should know. CNS Spectrums 2006;11(suppl 7):14-24.
19. Kane JM. Review of treatments that can ameliorate nonadherence in patients with schizophrenia. J Clin Psychiatry 2006;67(suppl 5):9-14.
20. Leucht S, Kane JM, Kissling W, et al. What does the PANSS mean? Schizophr Res 2006;79:231-8.
21. Essock SM, Covell NH, Davis SM, et al. Effectiveness of switching antipsychotic medications. Am J Psychiatry 2006;163:2090-5.
22. Davis JM, Marder S, Tamminga CA. Switch or stay? Am J Psychiatry 2006;163:2032-3.
23. Tandon R, Targum SD, Nasrallah HA, et al. Strategies for maximizing clinical effectiveness in the treatment of schizophrenia. Journal of Psychiatric Practice 2006;12:348-63.
1. Kane JM, Leucht S, Carpenter D, et al. Expert consensus guideline series: optimizing pharmacologic treatment of psychotic disorders. J Clin Psychiatry 2003;64(suppl 12):1-100.
2. Tandon R, Fleischhacker WW. Comparative efficacy of antipsychotics in the treatment of schizophrenia: a critical assessment. Schizophr Res 2005;79:145-55.
3. Lieberman JA, Stroup TS, McEvoy JP, et al. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med 2005;353:1209-23.
4. McEvoy JP, Lieberman JA, Stroup TS, et al. Effectiveness of clozapine versus olanzapine, quetiapine, and risperidone in patients with chronic schizophrenia who did not respond to prior antipsychotic treatment. Am J Psychiatry 2006;163:600-10.
5. Stroup TS, Lieberman JA, McEvoy JP, et al. Effectiveness of olanzapine, quetiapine, risperidone, and ziprasidone in patients with chronic schizophrenia following discontinuation of a previous atypical antipsychotic. Am J Psychiatry 2006;163:611-22.
6. Rosenheck RA, Leslie DL, Sindelar J, et al. Cost-effectiveness of second-generation antipsychotics and perphenazine in a randomized trial of treatment for chronic schizophrenia. Am J Psychiatry 2006;163:2080-9.
7. Jones PB, Barnes T, Davies L, et al. Randomized controlled trial of the effect on quality of life of second- vs first-generation antipsychotic drugs in schizophrenia: Cost Utility of the Latest Antipsychotic Drugs in Schizophrenia Study (CUtLASS). Arch Gen Psychiatry 2006;63:1079-87.
8. Lewis SW, Barnes TRE, Davies L, et al. Randomised controlled trial of effect of prescription of clozapine versus other second-generation antipsychotic drugs in resistant schizophrenia. Schizophr Bull 2006;32:715-23.
9. Nasrallah HA. CATIE’s surprises: in antipsychotics’ square-off, were there winners or losers? Current Psychiatry 2006;5(2):49-65.
10. Buckley PF. Which antipsychotic do I choose next? CATIE phase 2 offers insights on efficacy and tolerability. Current Psychiatry 2006;5(9):27-43.
11. Tandon R, Constantine R. Avoiding EPS is key to realizing ‘atypical’ benefits. Current Psychiatry 2006;5(11):35-45.
12. Lewis SW, Davies L, Jones PB, et al. Randomised controlled trials of conventional antipsychotic versus new atypical drugs, and new atypical drugs versus clozapine, in people with schizophrenia responding poorly to, or intolerant of, current drug treatment. Health Technology Assessment 2006;10(#17):1-182.Available at http://www.hta.ac.uk/project/1078.asp. Accessed January 3, 2007.
13. Heinrichs DW, Hanlon TE, Carpenter WT. The Quality of Life Scale: an instrument for assessing the schizophrenic deficit syndrome. Schizophr Bull 1984;10:388-98.
14. Tandon R, Davis JM, Carpenter WT. CATIE, CUtLASS, and the FGA-SGA debate (letter). Arch Gen Psychiatry 2007 (in press).
15. Lieberman J. Comparative effectiveness of antipsychotic drugs: a commentary on the Cost Utility of the Latest Antipsychotic Drugs in Schizophrenia Study and Clinical Antipsychotic Trials of Intervention Effectiveness. Arch Gen Psychiatry 2006;63:1069-72.
16. Rosenheck RA. Outcomes, costs, and policy caution: a commentary on the Cost Utility of the Latest Antipsychotic Drugs in Schizophrenia Study. Arch Gen Psychiatry 2006;63:1074-6.
17. Tandon R. Comparative effectiveness of antipsychotics in the treatment of schizophrenia: what CATIE tells us—Parts 1 and 2. International Drug Therapy Newsletter 2006;41(7,9):51-8,67-74.
18. Meltzer HY, Bobo WV. Interpreting the efficacy findings in the CATIE study: what clinicians should know. CNS Spectrums 2006;11(suppl 7):14-24.
19. Kane JM. Review of treatments that can ameliorate nonadherence in patients with schizophrenia. J Clin Psychiatry 2006;67(suppl 5):9-14.
20. Leucht S, Kane JM, Kissling W, et al. What does the PANSS mean? Schizophr Res 2006;79:231-8.
21. Essock SM, Covell NH, Davis SM, et al. Effectiveness of switching antipsychotic medications. Am J Psychiatry 2006;163:2090-5.
22. Davis JM, Marder S, Tamminga CA. Switch or stay? Am J Psychiatry 2006;163:2032-3.
23. Tandon R, Targum SD, Nasrallah HA, et al. Strategies for maximizing clinical effectiveness in the treatment of schizophrenia. Journal of Psychiatric Practice 2006;12:348-63.