User login
Multispecialty Opioid Risk Reduction Program Targeting Chronic Pain and Addiction Management in Veterans
Chronic pain significantly affects 100 million Americans.1,2 Pain accounts for $560 to $635 billion in annual financial costs to society, including health care costs and loss of productivity (ie, days missed from work, hours of work lost, and lower wages).2,3 Although pain prevalence exceeds other chronic diseases, such as diabetes mellitus, cancer, and heart disease, it lacks a sufficient body of evidence-based research and guidelines on the underlying mechanisms, valid methods of assessment, and comparative effectiveness of treatments to effectively implement into clinical practice.2,4 Prevention and treatment of pain are often delayed, inaccessible, or inadequate.2 Primary care providers (PCPs) are most often sought for pain management and treat about 52% of chronic pain patients.2,3,5 Veterans are especially vulnerable to chronic pain and are at risk for inadequate treatment.2
Background
There is an epidemic of drug abuse and mortality from opioid prescription medication.6 In the US, rates of overdose deaths from prescription opioids were 6.1 per 100,000 for men and 4.2 per 100,000 for women in 2017. Opioids were involved in 47,600 overdose deaths in 2017, accounting for 67.8% of all drug overdose deaths.7
A large number of patients on long-term opioids have preexisting substance use disorders and/or psychiatric disease, further complicating chronic pain management.8-10 Prescription opioid use has been the precursor for about 80% of people who are now heroin addicts.11 Iatrogenic addiction from prescription medications isn’t easily captured by standard addiction criteria. Consequently, in patients who are on opioid therapy for prolonged periods, separating complex opioid dependence from addiction is difficult.12 Improved addiction screening and risk mitigation strategies are needed along with aggressive treatment monitoring to curb the opioid epidemic.
Opioid Management in Primary Care
The majority of opioid medications are prescribed by PCPs, which is magnified in the US Department of Veterans Affairs (VA) health care system due to the high prevalence of service-related injuries.3,13 The VA is at the forefront of addressing the complexities of opioid addiction through several initiatives.14 The ability to offer the frequent visits needed to safely manage patients prescribed opioids and the integration of mental health and addiction treatment are often lacking in non-VA primary care clinics. Therefore, a key to solving the opioid crisis is developing these capabilities so they can be delivered within the primary care setting. There is substantial evidence in support of nonopioid alternatives to chronic pain management, including other pharmacologic approaches, exercise, physical therapy, acupuncture, weight loss, smoking cessation, chiropractic care, cognitive behavioral therapy (CBT), and other integrative health modalities.
A 2009 VA directive mandated the development of a comprehensive, integrated, systemwide approach to pain management.15 The VA Stepped-Care Biopsychosocial Model for Pain Management is dependent on timely access to secondary consultation from pain medicine, behavioral health, physical medicine, and other specialty consultation.15
History of VHA SCAN-ECHO Model
The Specialty Care Access Network–Extension for Community Health Outcomes (SCAN-ECHO) is a Veterans Health Administration (VHA) adaptation of a program that originated at the University of Mexico.16,17 The SCAN-ECHO model uses a multisite videoconferencing network to provide specialty care consultations to PCPs and patient aligned care teams (PACTs). During the 60- to 90-minute weekly sessions, case presentations are analyzed in real time so that over time, the PCPs gain knowledge, competency, and confidence in learning how to handle complex chronic conditions.
Since its implementation, the SCAN-ECHO program has been adopted across the VHA in a variety of specialties. One program, the SCAN-ECHO for Pain Management (SCAN-ECHO-PM) was implemented in 7 VHA networks in 31 states, spanning 47 medical centers and 148 community-based outpatient clinics (CBOCs).18 The SCAN-ECHO-PM program successfully conducted 257 multidisciplinary pain consultations between 2011 and 2013, resulting in increased initiation of nonopioid medications.18
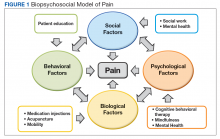
The aim of this article is to describe the implementation of a multicomponent primary care-based pain clinic with a fully integrated mental health service and addiction service at the North Florida/South Georgia Veterans Health System (NF/SGVHS). A practiced-based intervention of the biopsychosocial model with robust patient engagement has guided the development of the NF/SGVHS pain clinic (Figure 1).4,19
Pain CLinic
NF/SGVHS comprises the Malcom Randall and Lake City VA medical centers (VAMCs) hospitals, 3 satellite outpatient clinics, and 8 CBOCs. Spanning 33 counties in North Florida and 19 counties in South Georgia, the NF/SGVHS serves more than 140,000 patients. In 2010, the Malcom Randall VAMC established a multidisciplinary primary care pain clinic to manage veterans at high-risk for noncancer chronic pain and addiction. The noncancer pain policy was revised after garnering support from stakeholders who treat chronic pain, including the chiefs of psychiatry, rehabilitation medicine, neurosurgery, psychology, interventional pain, pharmacy, nursing, addiction medicine, and primary care. The clinic is staffed by primary care physicians trained in internal medicine and family medicine and is structured with 1-hour first visits, and 30-minute follow-up visits to allow enough time for comprehensive evaluation while meeting the needs for close follow-up support.
All physicians in the clinic have buprenorphine prescribing credentials to aid in the management of opioid addiction, as some patients feel more comfortable receiving addiction treatment in a primary care setting. The multimodal care model consists of several services that include addiction psychiatrists, interventional pain specialists, pain psychologists, and pain pharmacologists who coordinate the care to the veterans. The addiction psychiatrists offer a full range of services with inpatient residential and outpatient programs. Through recurring meetings with primary care pain clinic staff, the addiction psychiatrists are available to discuss use of buprenorphine and arrange follow-up for patients with complex pain addiction. There is ongoing collaboration to develop the best care plan that meets the patient’s needs for chronic pain, addiction, and/or mental health issues. The interventional pain service has 3 fellowship-trained pain care providers who deliver comprehensive evaluation, pharmacologic recommendations, and a full range of interventional and complementary therapies with an emphasis on objective functional improvement. Pain care providers offer alternatives to patients who are being weaned from opioids and support the multidisciplinary patient engagement model.
The pain psychology program, established in 2011, delivers CBT to 5 onsite locations and 5 telehealth locations. The service includes an advanced CBT program and a couples CBT program. The pharmacy pain fellowship program provides staff for an outpatient e-consult pain management service and an inpatient pharmacy consult service. Harnessing pain specialty pharmacists, the pharmacy service addresses pharmacokinetic issues, urine drug screen (UDS) results, opioid tapering and discharge planning for pain, addiction and mental health needs. The NF/SGVHS Primary Care Pain Clinic was established to support PCPs who did not feel comfortable managing chronic pain patients. These patients were typically on high-dose opioid therapy (> 100-mg morphine equivalent daily doses [MEDDs]); patients with a history of opioid addiction; patients with an addiction to opioids combined with benzodiazepines; and patients with comorbid medical issues (eg, sleep apnea), which complicated their management. The process of addressing opioid safety in these complex pain patients can be labor intensive and generally cannot be accomplished in a brief visit in a primary care setting where many other medical problems often need to be addressed.
Most patients on high-dose opioids are fearful of any changes in their medications. The difficult conversation regarding opioid safety is a lengthy one and frequently will occur over multiple visits. In addition, safely tapering opioids requires frequent follow-up to provide psychological support and to address withdrawal and mental health issues that may arise. As opioids are tapered, the clinic reinforces improved pain care through a multimodal biopsychosocial model. All veterans receiving pain care outside the VA are monitored annually to assure they are receiving evidence-based pain care as defined by the biopsychosocial model.
Education
Since 2011, the NF/SGVHS SCAN-ECHO pain and addiction educational forum has created > 50 hours of approved annual continuing medical education (CME) on pain management and addiction for PCPs. Initially, the 1-hour weekly educational audioconferences presented a pain management case along with related topics and involved specialists from interventional pain, physical therapy, psychiatry, nursing, neurology, and psychology departments. In 2013, in conjunction with the VA SCAN-ECHO program of Hunter Holmes McGuire VAMC in Richmond, Virginia, and Walter Reed National Military Medical Center in Bethesda, Maryland, the audioconference was expanded to 2 days each week with additional topics on addiction management. Residency and fellowship rotations were developed that specifically targeted fellows from psychiatry, pharmacology, and interventional pain departments.
Currently, an 8-session pain school is delivered onsite and at 7 telehealth locations. The school is a collaborative effort involving interventional pain, psychology, pharmacy, nutrition, and the primary care pain clinic staff. As the cornerstone of the program, the pain school stresses the biopsychosocial patient engagement model.
Program Evaluation
The VA is equipped with multiple telehealth service networks that allow for the delivery of programs, such as the pain school, a pain psychology program, and a yoga program, onsite or offsite. The VA Computerized Patient Record System (CPRS) manages electronic health records, allowing for rapid chart review and e-consults. The NF/SGVHS Pain Management Program provides about 1500 e-consults yearly. The CPRS includes templates with pain metrics to help PCPs deliver pain care more efficiently and evaluate performance measures. This system also allows for the capture of data to track improvements in the care of the veterans served.
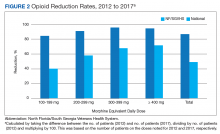
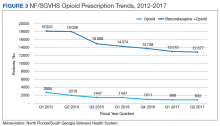
From 2012 to 2017, more than 5000 NF/SGVHS patients were weaned from opioids. Overall, there was an 87% reduction in patients receiving opioids ( ≥ 100-mg MEDDs) within the NF/SGVHS, which is significantly more than the 49% seen nationally across the VHA (Figure 2). Percent reduction was calculated by taking the difference in number of patients receiving opioids in 2012 and 2017, dividing by the number of patients receiving opioids in 2012 and multiplying by 100. The largest proportion of opioid dose reductions for NF/SGVHS and VHA patients, respectively, were seen in 300-mg to 399-mg MEDDs (95% vs 67%, respectively); followed by ≥ 400-mg MEDDs (94% vs 71%, respectively); 200-mg to 299-mg MEDDs (91% vs 58%, respectively); and 100-mg to 199-mg MEDDs (84% vs 40%, respectively). When examining NF/SGVHS trends over time, there has been a consistent decline in patients prescribed opioids (18 223 in 2012 compared with 12 877 in 2017) with similar trends in benzodiazepine-opioid combination therapy (2694 in 2012 compared with 833 in 2017) (Figure 3).
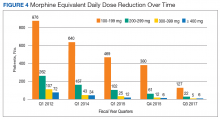
Similar declines are seen when patients are stratified by the MEDD (Figure 4). From 2012 to 2017, 92% of the patients were successfully tapered off doses ≥ 400-mg MEDD (2012, n = 72; 2017, n = 6), and tapered off 300-mg to 399-mg MEDD (2012, n = 107; 2017, n = 5); 95% were tapered off 200-mg to 299-mg MEDD (2012, n = 262; 2017, n = 22); and 86% were tapered off 100-mg to 199-mg MEDD (2012, n = 876; 2017; n = 127).
Conclusion
Successful integration of primary care with mental health and addiction services is paramount to aggressively taper patients with chronic pain from opioids. There is evidence that drug dependence and chronic pain should be treated like other chronic illness.20 Both chronic pain and addiction can be treated with a multidimensional self-management approach. In view of the high incidence of mental health and addiction associated with opioid use, it makes sense that an integrated, 1-stop pain and addiction clinic that understands and addresses both issues is more likely to improve patient outcomes.
Acknowledgments
This material is the result of work supported by the resources and facilities at the North Florida/South Georgia Veterans Health System, Geriatric Research Education Clinical Center in Gainesville, Florida.
1. Dueñas M, Ojeda B, Salazar A, Mico JA, Failde I. A review of chronic pain impact on patients, their social environment and the health care system. J Pain Res. 2016;9:457-467.
2. Institute of Medicine (US) Committee on Advancing Pain Research, Care, and Education. Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research. Washington, DC: Institute of Medicine; 2011.
3. Breuer B, Cruciani R, Portenoy RK. Pain management by primary care physicians, pain physicians, chiropractors, and acupuncturists: a national survey. South Med J. 2010;103(8):738-747.
4. Gatchel RJ, McGeary DD, McGeary CA, Lippe B. Interdisciplinary chronic pain management: past, present, and future. Am Psychol. 2014;69(2):119-130.
5. Meghani SH, Polomano RC, Tait RC, Vallerand AH, Anderson KO, Gallagher RM. Advancing a national agenda to eliminate disparities in pain care: directions for health policy, education, practice, and research. Pain Med. 2012;13(1):5-28.
6. McHugh RK, Nielsen S, Weiss RD. Prescription drug abuse: from epidemiology to public policy. J Subst Abuse Treat. 2015;48(1):1-7.
7. Scholl L, Seth P, Kariisa M, Wilson N, Baldwin G. Drug and opioid-involved overdose deaths-United States, 2013-2017. MMWR Morb Mortal Wkly Rep. 2018;67(5152):1419-1427.
8. Edlund MJ, Martin BC, Devries A, Fan MY, Braden JB, Sullivan MD. Trends in use of opioids for chronic noncancer pain among individuals with mental health and substance use disorders: the TROUP study. Clin J Pain. 2010;26(1):1-8.
9. Højsted J, Sjøgren P. Addiction to opioids in chronic pain patients: a literature review. Eur J Pain. 2007;11(5):490-518.
10. Seal KH, Shi Y, Cohen G, et al. Association of mental health disorders with prescription opioids and high-risk opioid use in US veterans of Iraq and Afghanistan. JAMA. 2012;307(9):940-947.
11. Kolodny A, Courtwright DT, Hwang CS, et al. The prescription opioid and heroin crisis: a public health approach to an epidemic of addiction. Annu Rev Public Health. 2015;36:559-574.
12. Ballantyne JC, Sullivan MD, Kolodny A. Opioid dependence vs addiction: a distinction without a difference? Arch Intern Med. 2012;172(17):1342-1343.
13. Levy B, Paulozzi L, Mack KA, Jones CM. Trends in opioid analgesic-prescribing rates by specialty, U.S., 2007-2012. Am J Prev Med. 2015;49(3):409-413.
14. Gellad WF, Good CB, Shulkin DJ. Addressing the opioid epidemic in the United States: lessons from the Department of Veterans Affairs. JAMA Intern Med. 2017;177(5):611-612.
15. US Department of Veterans Affairs. Veteran Health Administration Directive 2009-053, Pain Management. https://www.va.gov/painmanagement/docs/vha09paindirective.pdf. Published October 28, 2009. Accessed August 19, 2019.
16. Arora S, Geppert CM, Kalishman S, et al. Academic health center management of chronic diseases through knowledge networks: Project ECHO. Acad Med. 2007;82(2):154-160.
17. Kirsh S, Su GL, Sales A, Jain R. Access to outpatient specialty care: solutions from an integrated health care system. Am J Med Qual. 2015;30(1):88-90.
18. Frank JW, Carey EP, Fagan KM, et al. Evaluation of a telementoring intervention for pain management in the Veterans Health Administration. Pain Med. 2015;16(6):1090-1100.
19. Fillingim RB. Individual differences in pain: understanding the mosaic that makes pain personal. Pain. 2017;158 (suppl 1):S11-S18.
20. McLellan AT, Lewis DC, O’Brien CP, Kleber HD. Drug dependence, a chronic medical illness: implications for treatment, insurance, and outcomes evaluation. JAMA. 2000;284(13):1689-1695.
Chronic pain significantly affects 100 million Americans.1,2 Pain accounts for $560 to $635 billion in annual financial costs to society, including health care costs and loss of productivity (ie, days missed from work, hours of work lost, and lower wages).2,3 Although pain prevalence exceeds other chronic diseases, such as diabetes mellitus, cancer, and heart disease, it lacks a sufficient body of evidence-based research and guidelines on the underlying mechanisms, valid methods of assessment, and comparative effectiveness of treatments to effectively implement into clinical practice.2,4 Prevention and treatment of pain are often delayed, inaccessible, or inadequate.2 Primary care providers (PCPs) are most often sought for pain management and treat about 52% of chronic pain patients.2,3,5 Veterans are especially vulnerable to chronic pain and are at risk for inadequate treatment.2
Background
There is an epidemic of drug abuse and mortality from opioid prescription medication.6 In the US, rates of overdose deaths from prescription opioids were 6.1 per 100,000 for men and 4.2 per 100,000 for women in 2017. Opioids were involved in 47,600 overdose deaths in 2017, accounting for 67.8% of all drug overdose deaths.7
A large number of patients on long-term opioids have preexisting substance use disorders and/or psychiatric disease, further complicating chronic pain management.8-10 Prescription opioid use has been the precursor for about 80% of people who are now heroin addicts.11 Iatrogenic addiction from prescription medications isn’t easily captured by standard addiction criteria. Consequently, in patients who are on opioid therapy for prolonged periods, separating complex opioid dependence from addiction is difficult.12 Improved addiction screening and risk mitigation strategies are needed along with aggressive treatment monitoring to curb the opioid epidemic.
Opioid Management in Primary Care
The majority of opioid medications are prescribed by PCPs, which is magnified in the US Department of Veterans Affairs (VA) health care system due to the high prevalence of service-related injuries.3,13 The VA is at the forefront of addressing the complexities of opioid addiction through several initiatives.14 The ability to offer the frequent visits needed to safely manage patients prescribed opioids and the integration of mental health and addiction treatment are often lacking in non-VA primary care clinics. Therefore, a key to solving the opioid crisis is developing these capabilities so they can be delivered within the primary care setting. There is substantial evidence in support of nonopioid alternatives to chronic pain management, including other pharmacologic approaches, exercise, physical therapy, acupuncture, weight loss, smoking cessation, chiropractic care, cognitive behavioral therapy (CBT), and other integrative health modalities.
A 2009 VA directive mandated the development of a comprehensive, integrated, systemwide approach to pain management.15 The VA Stepped-Care Biopsychosocial Model for Pain Management is dependent on timely access to secondary consultation from pain medicine, behavioral health, physical medicine, and other specialty consultation.15
History of VHA SCAN-ECHO Model
The Specialty Care Access Network–Extension for Community Health Outcomes (SCAN-ECHO) is a Veterans Health Administration (VHA) adaptation of a program that originated at the University of Mexico.16,17 The SCAN-ECHO model uses a multisite videoconferencing network to provide specialty care consultations to PCPs and patient aligned care teams (PACTs). During the 60- to 90-minute weekly sessions, case presentations are analyzed in real time so that over time, the PCPs gain knowledge, competency, and confidence in learning how to handle complex chronic conditions.
Since its implementation, the SCAN-ECHO program has been adopted across the VHA in a variety of specialties. One program, the SCAN-ECHO for Pain Management (SCAN-ECHO-PM) was implemented in 7 VHA networks in 31 states, spanning 47 medical centers and 148 community-based outpatient clinics (CBOCs).18 The SCAN-ECHO-PM program successfully conducted 257 multidisciplinary pain consultations between 2011 and 2013, resulting in increased initiation of nonopioid medications.18
The aim of this article is to describe the implementation of a multicomponent primary care-based pain clinic with a fully integrated mental health service and addiction service at the North Florida/South Georgia Veterans Health System (NF/SGVHS). A practiced-based intervention of the biopsychosocial model with robust patient engagement has guided the development of the NF/SGVHS pain clinic (Figure 1).4,19
Pain CLinic
NF/SGVHS comprises the Malcom Randall and Lake City VA medical centers (VAMCs) hospitals, 3 satellite outpatient clinics, and 8 CBOCs. Spanning 33 counties in North Florida and 19 counties in South Georgia, the NF/SGVHS serves more than 140,000 patients. In 2010, the Malcom Randall VAMC established a multidisciplinary primary care pain clinic to manage veterans at high-risk for noncancer chronic pain and addiction. The noncancer pain policy was revised after garnering support from stakeholders who treat chronic pain, including the chiefs of psychiatry, rehabilitation medicine, neurosurgery, psychology, interventional pain, pharmacy, nursing, addiction medicine, and primary care. The clinic is staffed by primary care physicians trained in internal medicine and family medicine and is structured with 1-hour first visits, and 30-minute follow-up visits to allow enough time for comprehensive evaluation while meeting the needs for close follow-up support.
All physicians in the clinic have buprenorphine prescribing credentials to aid in the management of opioid addiction, as some patients feel more comfortable receiving addiction treatment in a primary care setting. The multimodal care model consists of several services that include addiction psychiatrists, interventional pain specialists, pain psychologists, and pain pharmacologists who coordinate the care to the veterans. The addiction psychiatrists offer a full range of services with inpatient residential and outpatient programs. Through recurring meetings with primary care pain clinic staff, the addiction psychiatrists are available to discuss use of buprenorphine and arrange follow-up for patients with complex pain addiction. There is ongoing collaboration to develop the best care plan that meets the patient’s needs for chronic pain, addiction, and/or mental health issues. The interventional pain service has 3 fellowship-trained pain care providers who deliver comprehensive evaluation, pharmacologic recommendations, and a full range of interventional and complementary therapies with an emphasis on objective functional improvement. Pain care providers offer alternatives to patients who are being weaned from opioids and support the multidisciplinary patient engagement model.
The pain psychology program, established in 2011, delivers CBT to 5 onsite locations and 5 telehealth locations. The service includes an advanced CBT program and a couples CBT program. The pharmacy pain fellowship program provides staff for an outpatient e-consult pain management service and an inpatient pharmacy consult service. Harnessing pain specialty pharmacists, the pharmacy service addresses pharmacokinetic issues, urine drug screen (UDS) results, opioid tapering and discharge planning for pain, addiction and mental health needs. The NF/SGVHS Primary Care Pain Clinic was established to support PCPs who did not feel comfortable managing chronic pain patients. These patients were typically on high-dose opioid therapy (> 100-mg morphine equivalent daily doses [MEDDs]); patients with a history of opioid addiction; patients with an addiction to opioids combined with benzodiazepines; and patients with comorbid medical issues (eg, sleep apnea), which complicated their management. The process of addressing opioid safety in these complex pain patients can be labor intensive and generally cannot be accomplished in a brief visit in a primary care setting where many other medical problems often need to be addressed.
Most patients on high-dose opioids are fearful of any changes in their medications. The difficult conversation regarding opioid safety is a lengthy one and frequently will occur over multiple visits. In addition, safely tapering opioids requires frequent follow-up to provide psychological support and to address withdrawal and mental health issues that may arise. As opioids are tapered, the clinic reinforces improved pain care through a multimodal biopsychosocial model. All veterans receiving pain care outside the VA are monitored annually to assure they are receiving evidence-based pain care as defined by the biopsychosocial model.
Education
Since 2011, the NF/SGVHS SCAN-ECHO pain and addiction educational forum has created > 50 hours of approved annual continuing medical education (CME) on pain management and addiction for PCPs. Initially, the 1-hour weekly educational audioconferences presented a pain management case along with related topics and involved specialists from interventional pain, physical therapy, psychiatry, nursing, neurology, and psychology departments. In 2013, in conjunction with the VA SCAN-ECHO program of Hunter Holmes McGuire VAMC in Richmond, Virginia, and Walter Reed National Military Medical Center in Bethesda, Maryland, the audioconference was expanded to 2 days each week with additional topics on addiction management. Residency and fellowship rotations were developed that specifically targeted fellows from psychiatry, pharmacology, and interventional pain departments.
Currently, an 8-session pain school is delivered onsite and at 7 telehealth locations. The school is a collaborative effort involving interventional pain, psychology, pharmacy, nutrition, and the primary care pain clinic staff. As the cornerstone of the program, the pain school stresses the biopsychosocial patient engagement model.
Program Evaluation
The VA is equipped with multiple telehealth service networks that allow for the delivery of programs, such as the pain school, a pain psychology program, and a yoga program, onsite or offsite. The VA Computerized Patient Record System (CPRS) manages electronic health records, allowing for rapid chart review and e-consults. The NF/SGVHS Pain Management Program provides about 1500 e-consults yearly. The CPRS includes templates with pain metrics to help PCPs deliver pain care more efficiently and evaluate performance measures. This system also allows for the capture of data to track improvements in the care of the veterans served.
From 2012 to 2017, more than 5000 NF/SGVHS patients were weaned from opioids. Overall, there was an 87% reduction in patients receiving opioids ( ≥ 100-mg MEDDs) within the NF/SGVHS, which is significantly more than the 49% seen nationally across the VHA (Figure 2). Percent reduction was calculated by taking the difference in number of patients receiving opioids in 2012 and 2017, dividing by the number of patients receiving opioids in 2012 and multiplying by 100. The largest proportion of opioid dose reductions for NF/SGVHS and VHA patients, respectively, were seen in 300-mg to 399-mg MEDDs (95% vs 67%, respectively); followed by ≥ 400-mg MEDDs (94% vs 71%, respectively); 200-mg to 299-mg MEDDs (91% vs 58%, respectively); and 100-mg to 199-mg MEDDs (84% vs 40%, respectively). When examining NF/SGVHS trends over time, there has been a consistent decline in patients prescribed opioids (18 223 in 2012 compared with 12 877 in 2017) with similar trends in benzodiazepine-opioid combination therapy (2694 in 2012 compared with 833 in 2017) (Figure 3).
Similar declines are seen when patients are stratified by the MEDD (Figure 4). From 2012 to 2017, 92% of the patients were successfully tapered off doses ≥ 400-mg MEDD (2012, n = 72; 2017, n = 6), and tapered off 300-mg to 399-mg MEDD (2012, n = 107; 2017, n = 5); 95% were tapered off 200-mg to 299-mg MEDD (2012, n = 262; 2017, n = 22); and 86% were tapered off 100-mg to 199-mg MEDD (2012, n = 876; 2017; n = 127).
Conclusion
Successful integration of primary care with mental health and addiction services is paramount to aggressively taper patients with chronic pain from opioids. There is evidence that drug dependence and chronic pain should be treated like other chronic illness.20 Both chronic pain and addiction can be treated with a multidimensional self-management approach. In view of the high incidence of mental health and addiction associated with opioid use, it makes sense that an integrated, 1-stop pain and addiction clinic that understands and addresses both issues is more likely to improve patient outcomes.
Acknowledgments
This material is the result of work supported by the resources and facilities at the North Florida/South Georgia Veterans Health System, Geriatric Research Education Clinical Center in Gainesville, Florida.
Chronic pain significantly affects 100 million Americans.1,2 Pain accounts for $560 to $635 billion in annual financial costs to society, including health care costs and loss of productivity (ie, days missed from work, hours of work lost, and lower wages).2,3 Although pain prevalence exceeds other chronic diseases, such as diabetes mellitus, cancer, and heart disease, it lacks a sufficient body of evidence-based research and guidelines on the underlying mechanisms, valid methods of assessment, and comparative effectiveness of treatments to effectively implement into clinical practice.2,4 Prevention and treatment of pain are often delayed, inaccessible, or inadequate.2 Primary care providers (PCPs) are most often sought for pain management and treat about 52% of chronic pain patients.2,3,5 Veterans are especially vulnerable to chronic pain and are at risk for inadequate treatment.2
Background
There is an epidemic of drug abuse and mortality from opioid prescription medication.6 In the US, rates of overdose deaths from prescription opioids were 6.1 per 100,000 for men and 4.2 per 100,000 for women in 2017. Opioids were involved in 47,600 overdose deaths in 2017, accounting for 67.8% of all drug overdose deaths.7
A large number of patients on long-term opioids have preexisting substance use disorders and/or psychiatric disease, further complicating chronic pain management.8-10 Prescription opioid use has been the precursor for about 80% of people who are now heroin addicts.11 Iatrogenic addiction from prescription medications isn’t easily captured by standard addiction criteria. Consequently, in patients who are on opioid therapy for prolonged periods, separating complex opioid dependence from addiction is difficult.12 Improved addiction screening and risk mitigation strategies are needed along with aggressive treatment monitoring to curb the opioid epidemic.
Opioid Management in Primary Care
The majority of opioid medications are prescribed by PCPs, which is magnified in the US Department of Veterans Affairs (VA) health care system due to the high prevalence of service-related injuries.3,13 The VA is at the forefront of addressing the complexities of opioid addiction through several initiatives.14 The ability to offer the frequent visits needed to safely manage patients prescribed opioids and the integration of mental health and addiction treatment are often lacking in non-VA primary care clinics. Therefore, a key to solving the opioid crisis is developing these capabilities so they can be delivered within the primary care setting. There is substantial evidence in support of nonopioid alternatives to chronic pain management, including other pharmacologic approaches, exercise, physical therapy, acupuncture, weight loss, smoking cessation, chiropractic care, cognitive behavioral therapy (CBT), and other integrative health modalities.
A 2009 VA directive mandated the development of a comprehensive, integrated, systemwide approach to pain management.15 The VA Stepped-Care Biopsychosocial Model for Pain Management is dependent on timely access to secondary consultation from pain medicine, behavioral health, physical medicine, and other specialty consultation.15
History of VHA SCAN-ECHO Model
The Specialty Care Access Network–Extension for Community Health Outcomes (SCAN-ECHO) is a Veterans Health Administration (VHA) adaptation of a program that originated at the University of Mexico.16,17 The SCAN-ECHO model uses a multisite videoconferencing network to provide specialty care consultations to PCPs and patient aligned care teams (PACTs). During the 60- to 90-minute weekly sessions, case presentations are analyzed in real time so that over time, the PCPs gain knowledge, competency, and confidence in learning how to handle complex chronic conditions.
Since its implementation, the SCAN-ECHO program has been adopted across the VHA in a variety of specialties. One program, the SCAN-ECHO for Pain Management (SCAN-ECHO-PM) was implemented in 7 VHA networks in 31 states, spanning 47 medical centers and 148 community-based outpatient clinics (CBOCs).18 The SCAN-ECHO-PM program successfully conducted 257 multidisciplinary pain consultations between 2011 and 2013, resulting in increased initiation of nonopioid medications.18
The aim of this article is to describe the implementation of a multicomponent primary care-based pain clinic with a fully integrated mental health service and addiction service at the North Florida/South Georgia Veterans Health System (NF/SGVHS). A practiced-based intervention of the biopsychosocial model with robust patient engagement has guided the development of the NF/SGVHS pain clinic (Figure 1).4,19
Pain CLinic
NF/SGVHS comprises the Malcom Randall and Lake City VA medical centers (VAMCs) hospitals, 3 satellite outpatient clinics, and 8 CBOCs. Spanning 33 counties in North Florida and 19 counties in South Georgia, the NF/SGVHS serves more than 140,000 patients. In 2010, the Malcom Randall VAMC established a multidisciplinary primary care pain clinic to manage veterans at high-risk for noncancer chronic pain and addiction. The noncancer pain policy was revised after garnering support from stakeholders who treat chronic pain, including the chiefs of psychiatry, rehabilitation medicine, neurosurgery, psychology, interventional pain, pharmacy, nursing, addiction medicine, and primary care. The clinic is staffed by primary care physicians trained in internal medicine and family medicine and is structured with 1-hour first visits, and 30-minute follow-up visits to allow enough time for comprehensive evaluation while meeting the needs for close follow-up support.
All physicians in the clinic have buprenorphine prescribing credentials to aid in the management of opioid addiction, as some patients feel more comfortable receiving addiction treatment in a primary care setting. The multimodal care model consists of several services that include addiction psychiatrists, interventional pain specialists, pain psychologists, and pain pharmacologists who coordinate the care to the veterans. The addiction psychiatrists offer a full range of services with inpatient residential and outpatient programs. Through recurring meetings with primary care pain clinic staff, the addiction psychiatrists are available to discuss use of buprenorphine and arrange follow-up for patients with complex pain addiction. There is ongoing collaboration to develop the best care plan that meets the patient’s needs for chronic pain, addiction, and/or mental health issues. The interventional pain service has 3 fellowship-trained pain care providers who deliver comprehensive evaluation, pharmacologic recommendations, and a full range of interventional and complementary therapies with an emphasis on objective functional improvement. Pain care providers offer alternatives to patients who are being weaned from opioids and support the multidisciplinary patient engagement model.
The pain psychology program, established in 2011, delivers CBT to 5 onsite locations and 5 telehealth locations. The service includes an advanced CBT program and a couples CBT program. The pharmacy pain fellowship program provides staff for an outpatient e-consult pain management service and an inpatient pharmacy consult service. Harnessing pain specialty pharmacists, the pharmacy service addresses pharmacokinetic issues, urine drug screen (UDS) results, opioid tapering and discharge planning for pain, addiction and mental health needs. The NF/SGVHS Primary Care Pain Clinic was established to support PCPs who did not feel comfortable managing chronic pain patients. These patients were typically on high-dose opioid therapy (> 100-mg morphine equivalent daily doses [MEDDs]); patients with a history of opioid addiction; patients with an addiction to opioids combined with benzodiazepines; and patients with comorbid medical issues (eg, sleep apnea), which complicated their management. The process of addressing opioid safety in these complex pain patients can be labor intensive and generally cannot be accomplished in a brief visit in a primary care setting where many other medical problems often need to be addressed.
Most patients on high-dose opioids are fearful of any changes in their medications. The difficult conversation regarding opioid safety is a lengthy one and frequently will occur over multiple visits. In addition, safely tapering opioids requires frequent follow-up to provide psychological support and to address withdrawal and mental health issues that may arise. As opioids are tapered, the clinic reinforces improved pain care through a multimodal biopsychosocial model. All veterans receiving pain care outside the VA are monitored annually to assure they are receiving evidence-based pain care as defined by the biopsychosocial model.
Education
Since 2011, the NF/SGVHS SCAN-ECHO pain and addiction educational forum has created > 50 hours of approved annual continuing medical education (CME) on pain management and addiction for PCPs. Initially, the 1-hour weekly educational audioconferences presented a pain management case along with related topics and involved specialists from interventional pain, physical therapy, psychiatry, nursing, neurology, and psychology departments. In 2013, in conjunction with the VA SCAN-ECHO program of Hunter Holmes McGuire VAMC in Richmond, Virginia, and Walter Reed National Military Medical Center in Bethesda, Maryland, the audioconference was expanded to 2 days each week with additional topics on addiction management. Residency and fellowship rotations were developed that specifically targeted fellows from psychiatry, pharmacology, and interventional pain departments.
Currently, an 8-session pain school is delivered onsite and at 7 telehealth locations. The school is a collaborative effort involving interventional pain, psychology, pharmacy, nutrition, and the primary care pain clinic staff. As the cornerstone of the program, the pain school stresses the biopsychosocial patient engagement model.
Program Evaluation
The VA is equipped with multiple telehealth service networks that allow for the delivery of programs, such as the pain school, a pain psychology program, and a yoga program, onsite or offsite. The VA Computerized Patient Record System (CPRS) manages electronic health records, allowing for rapid chart review and e-consults. The NF/SGVHS Pain Management Program provides about 1500 e-consults yearly. The CPRS includes templates with pain metrics to help PCPs deliver pain care more efficiently and evaluate performance measures. This system also allows for the capture of data to track improvements in the care of the veterans served.
From 2012 to 2017, more than 5000 NF/SGVHS patients were weaned from opioids. Overall, there was an 87% reduction in patients receiving opioids ( ≥ 100-mg MEDDs) within the NF/SGVHS, which is significantly more than the 49% seen nationally across the VHA (Figure 2). Percent reduction was calculated by taking the difference in number of patients receiving opioids in 2012 and 2017, dividing by the number of patients receiving opioids in 2012 and multiplying by 100. The largest proportion of opioid dose reductions for NF/SGVHS and VHA patients, respectively, were seen in 300-mg to 399-mg MEDDs (95% vs 67%, respectively); followed by ≥ 400-mg MEDDs (94% vs 71%, respectively); 200-mg to 299-mg MEDDs (91% vs 58%, respectively); and 100-mg to 199-mg MEDDs (84% vs 40%, respectively). When examining NF/SGVHS trends over time, there has been a consistent decline in patients prescribed opioids (18 223 in 2012 compared with 12 877 in 2017) with similar trends in benzodiazepine-opioid combination therapy (2694 in 2012 compared with 833 in 2017) (Figure 3).
Similar declines are seen when patients are stratified by the MEDD (Figure 4). From 2012 to 2017, 92% of the patients were successfully tapered off doses ≥ 400-mg MEDD (2012, n = 72; 2017, n = 6), and tapered off 300-mg to 399-mg MEDD (2012, n = 107; 2017, n = 5); 95% were tapered off 200-mg to 299-mg MEDD (2012, n = 262; 2017, n = 22); and 86% were tapered off 100-mg to 199-mg MEDD (2012, n = 876; 2017; n = 127).
Conclusion
Successful integration of primary care with mental health and addiction services is paramount to aggressively taper patients with chronic pain from opioids. There is evidence that drug dependence and chronic pain should be treated like other chronic illness.20 Both chronic pain and addiction can be treated with a multidimensional self-management approach. In view of the high incidence of mental health and addiction associated with opioid use, it makes sense that an integrated, 1-stop pain and addiction clinic that understands and addresses both issues is more likely to improve patient outcomes.
Acknowledgments
This material is the result of work supported by the resources and facilities at the North Florida/South Georgia Veterans Health System, Geriatric Research Education Clinical Center in Gainesville, Florida.
1. Dueñas M, Ojeda B, Salazar A, Mico JA, Failde I. A review of chronic pain impact on patients, their social environment and the health care system. J Pain Res. 2016;9:457-467.
2. Institute of Medicine (US) Committee on Advancing Pain Research, Care, and Education. Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research. Washington, DC: Institute of Medicine; 2011.
3. Breuer B, Cruciani R, Portenoy RK. Pain management by primary care physicians, pain physicians, chiropractors, and acupuncturists: a national survey. South Med J. 2010;103(8):738-747.
4. Gatchel RJ, McGeary DD, McGeary CA, Lippe B. Interdisciplinary chronic pain management: past, present, and future. Am Psychol. 2014;69(2):119-130.
5. Meghani SH, Polomano RC, Tait RC, Vallerand AH, Anderson KO, Gallagher RM. Advancing a national agenda to eliminate disparities in pain care: directions for health policy, education, practice, and research. Pain Med. 2012;13(1):5-28.
6. McHugh RK, Nielsen S, Weiss RD. Prescription drug abuse: from epidemiology to public policy. J Subst Abuse Treat. 2015;48(1):1-7.
7. Scholl L, Seth P, Kariisa M, Wilson N, Baldwin G. Drug and opioid-involved overdose deaths-United States, 2013-2017. MMWR Morb Mortal Wkly Rep. 2018;67(5152):1419-1427.
8. Edlund MJ, Martin BC, Devries A, Fan MY, Braden JB, Sullivan MD. Trends in use of opioids for chronic noncancer pain among individuals with mental health and substance use disorders: the TROUP study. Clin J Pain. 2010;26(1):1-8.
9. Højsted J, Sjøgren P. Addiction to opioids in chronic pain patients: a literature review. Eur J Pain. 2007;11(5):490-518.
10. Seal KH, Shi Y, Cohen G, et al. Association of mental health disorders with prescription opioids and high-risk opioid use in US veterans of Iraq and Afghanistan. JAMA. 2012;307(9):940-947.
11. Kolodny A, Courtwright DT, Hwang CS, et al. The prescription opioid and heroin crisis: a public health approach to an epidemic of addiction. Annu Rev Public Health. 2015;36:559-574.
12. Ballantyne JC, Sullivan MD, Kolodny A. Opioid dependence vs addiction: a distinction without a difference? Arch Intern Med. 2012;172(17):1342-1343.
13. Levy B, Paulozzi L, Mack KA, Jones CM. Trends in opioid analgesic-prescribing rates by specialty, U.S., 2007-2012. Am J Prev Med. 2015;49(3):409-413.
14. Gellad WF, Good CB, Shulkin DJ. Addressing the opioid epidemic in the United States: lessons from the Department of Veterans Affairs. JAMA Intern Med. 2017;177(5):611-612.
15. US Department of Veterans Affairs. Veteran Health Administration Directive 2009-053, Pain Management. https://www.va.gov/painmanagement/docs/vha09paindirective.pdf. Published October 28, 2009. Accessed August 19, 2019.
16. Arora S, Geppert CM, Kalishman S, et al. Academic health center management of chronic diseases through knowledge networks: Project ECHO. Acad Med. 2007;82(2):154-160.
17. Kirsh S, Su GL, Sales A, Jain R. Access to outpatient specialty care: solutions from an integrated health care system. Am J Med Qual. 2015;30(1):88-90.
18. Frank JW, Carey EP, Fagan KM, et al. Evaluation of a telementoring intervention for pain management in the Veterans Health Administration. Pain Med. 2015;16(6):1090-1100.
19. Fillingim RB. Individual differences in pain: understanding the mosaic that makes pain personal. Pain. 2017;158 (suppl 1):S11-S18.
20. McLellan AT, Lewis DC, O’Brien CP, Kleber HD. Drug dependence, a chronic medical illness: implications for treatment, insurance, and outcomes evaluation. JAMA. 2000;284(13):1689-1695.
1. Dueñas M, Ojeda B, Salazar A, Mico JA, Failde I. A review of chronic pain impact on patients, their social environment and the health care system. J Pain Res. 2016;9:457-467.
2. Institute of Medicine (US) Committee on Advancing Pain Research, Care, and Education. Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research. Washington, DC: Institute of Medicine; 2011.
3. Breuer B, Cruciani R, Portenoy RK. Pain management by primary care physicians, pain physicians, chiropractors, and acupuncturists: a national survey. South Med J. 2010;103(8):738-747.
4. Gatchel RJ, McGeary DD, McGeary CA, Lippe B. Interdisciplinary chronic pain management: past, present, and future. Am Psychol. 2014;69(2):119-130.
5. Meghani SH, Polomano RC, Tait RC, Vallerand AH, Anderson KO, Gallagher RM. Advancing a national agenda to eliminate disparities in pain care: directions for health policy, education, practice, and research. Pain Med. 2012;13(1):5-28.
6. McHugh RK, Nielsen S, Weiss RD. Prescription drug abuse: from epidemiology to public policy. J Subst Abuse Treat. 2015;48(1):1-7.
7. Scholl L, Seth P, Kariisa M, Wilson N, Baldwin G. Drug and opioid-involved overdose deaths-United States, 2013-2017. MMWR Morb Mortal Wkly Rep. 2018;67(5152):1419-1427.
8. Edlund MJ, Martin BC, Devries A, Fan MY, Braden JB, Sullivan MD. Trends in use of opioids for chronic noncancer pain among individuals with mental health and substance use disorders: the TROUP study. Clin J Pain. 2010;26(1):1-8.
9. Højsted J, Sjøgren P. Addiction to opioids in chronic pain patients: a literature review. Eur J Pain. 2007;11(5):490-518.
10. Seal KH, Shi Y, Cohen G, et al. Association of mental health disorders with prescription opioids and high-risk opioid use in US veterans of Iraq and Afghanistan. JAMA. 2012;307(9):940-947.
11. Kolodny A, Courtwright DT, Hwang CS, et al. The prescription opioid and heroin crisis: a public health approach to an epidemic of addiction. Annu Rev Public Health. 2015;36:559-574.
12. Ballantyne JC, Sullivan MD, Kolodny A. Opioid dependence vs addiction: a distinction without a difference? Arch Intern Med. 2012;172(17):1342-1343.
13. Levy B, Paulozzi L, Mack KA, Jones CM. Trends in opioid analgesic-prescribing rates by specialty, U.S., 2007-2012. Am J Prev Med. 2015;49(3):409-413.
14. Gellad WF, Good CB, Shulkin DJ. Addressing the opioid epidemic in the United States: lessons from the Department of Veterans Affairs. JAMA Intern Med. 2017;177(5):611-612.
15. US Department of Veterans Affairs. Veteran Health Administration Directive 2009-053, Pain Management. https://www.va.gov/painmanagement/docs/vha09paindirective.pdf. Published October 28, 2009. Accessed August 19, 2019.
16. Arora S, Geppert CM, Kalishman S, et al. Academic health center management of chronic diseases through knowledge networks: Project ECHO. Acad Med. 2007;82(2):154-160.
17. Kirsh S, Su GL, Sales A, Jain R. Access to outpatient specialty care: solutions from an integrated health care system. Am J Med Qual. 2015;30(1):88-90.
18. Frank JW, Carey EP, Fagan KM, et al. Evaluation of a telementoring intervention for pain management in the Veterans Health Administration. Pain Med. 2015;16(6):1090-1100.
19. Fillingim RB. Individual differences in pain: understanding the mosaic that makes pain personal. Pain. 2017;158 (suppl 1):S11-S18.
20. McLellan AT, Lewis DC, O’Brien CP, Kleber HD. Drug dependence, a chronic medical illness: implications for treatment, insurance, and outcomes evaluation. JAMA. 2000;284(13):1689-1695.
Worsening agitation and hallucinations: Could it be PTSD?
CASE Confusion, hallucinations
Mr. G, age 57, is brought to the emergency department (ED) from a hospice care facility for worsening agitation and psychosis over 2 days. His wife, who accompanies him, describes a 2-month onset of “confusion” with occasional visual hallucinations. She says that at baseline Mr. G was alert and oriented and able to engage appropriately in conversations. The hospice facility administered emergency medications, including unknown dosages of haloperidol and chlorpromazine, the morning before transfer to the ED.
Mr. G has a history of posttraumatic stress disorder (PTSD), anxiety, and depression that has been managed for 6 years with several trials of antidepressant monotherapy, including fluoxetine, citalopram, mirtazapine, bupropion, and augmentation using aripiprazole, risperidone, topiramate, and zolpidem. At the time of this hospital presentation, his symptoms are controlled on clonazepam, 2 mg/d, and trazodone, 50 mg/d. For his pain attributed to non-small cell lung cancer (NSCLC), he receives methadone, 25 mg, 6 times a day, and hydromorphone, 8 mg, every 4 hours as needed, for breakthrough pain. Mr. G underwent a right upper lobectomy 5 years ago and neurosurgery with a right suboccipital craniectomy for right-sided cerebellar metastatic tumor measuring 2 × 1 × 0.6 cm, along with chemotherapy and radiation for metastasis in the brain 1 year ago. His last chemotherapy session was 3 months ago.
In the ED, Mr. G is sedated and oriented only to person and his wife. He is observed mumbling incoherently. Abnormal vital signs and laboratory findings are elevated pulse, 97 beats per minute; mild anemia, 13.5 g/dL hemoglobin and 40.8% hematocrit; an elevated glucose of 136 mg/dL; and small amounts of blood, trace ketones, and hyaline casts in urinalysis. Vital signs, laboratory resu
In addition to psychotropic and pain medication, Mr. G is taking cyclobenzaprine, 5 mg, every 6 hours as needed, for muscle spasms; docusate, 200 mg/d; enoxaparin, 100 mg/1mL, every 12 hours; folic acid, 1 mg/d; gabapentin, 600 mg, 3 times daily; lidocaine ointment, twice daily as needed, for pain; omeprazole, 80 mg/d; ondansetron, 4 mg, every 8 hours as needed, for nausea; and tamsulosin, 0.4 mg/d.
What is your differential diagnosis for Mr. G?
a) brain metastases
b) infection
c) PTSD
d) polypharmacy
e) benzodiazepine withdrawal
The authors’ observations
Altered mental status (AMS), or acute confusional state, describes an individual who fails to interact with environmental stimuli in an appropriate, anticipated manner. The disturbance usually is acute and transient.1 Often providers struggle to obtain relevant facts about a patient’s history of illness and must use laboratory and diagnostic data to determine the underlying cause of the patient’s disorientation.
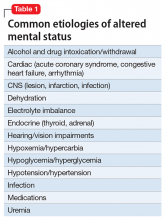
Mental status includes 2 components: arousal and awareness. Arousal refers to a person’s wakeful state and how an individual responds to his (her) surroundings. Impairment in arousal can result in variable states including lethargy, drowsiness, and even coma. Awareness, on the other hand, is an individual’s perception of his environment, including orientation to surroundings, executive functioning, and memory. Although arousal level is controlled by the reticular activating system of the brainstem, awareness of consciousness is mediated at the cortical level. Mr. G experienced increased arousal and AMS with a clear change in behavior from his baseline. With increasing frequency of hallucinations and agitated behaviors, several tests must be ordered to determine the etiology of his altered mentation (Table 1).
Which test would you order next?
a) urine drug screen (UDS)
b) chest CT with pulmonary embolism protocol
c) CT of the head
d) blood cultures
e) chest radiography
EVALUATION Awake, still confused
The ED physician orders a UDS, non-contrasted CT of the head, and chest radiography for preliminary workup investigating the cause of Mr. G’s AMS. UDS is negative for illicit substances. The non-contrasted CT of the head shows a stable, right cerebellar hemisphere lesion from a prior lung metastasis. Mr. G’s chest radiography reading describes an ill-defined opacity at the left lung base.
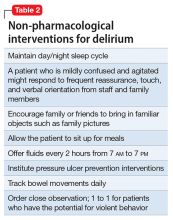
Mr. G is admitted to the medical service and is started on dexamethasone, 8 mg/d, for his NSCLC with brain metastasis. Clonazepam is continued to prevent benzodiazepine withdrawal. The psychiatry and palliative care teams are consulted to determine if Mr. G’s PTSD symptoms and/or opioids are contributing to his AMS and psychosis. After evaluation, the psychiatry team recommends decreasing clonazepam to 0.5 mg, twice daily, starting olanzapine, 5 mg, every 12 hours, for agitation and psychosis involving auditory and visual hallucinations as well as paranoid themes related to food contamination, and using non-pharmacologic interventions for delirium treatment (Table 2). In a prospective, randomized controlled trial of olanzapine vs haloperidol, clinical improvement in delirious states was seen in individuals who received either antipsychotic medication; however, haloperidol was associated with extrapyramidal side effects. Therefore, olanzapine is a safe alternative to haloperidol in delirious patients.2
The psychiatry consult service suspects delirium due to polypharmacy or Mr. G’s metastatic brain lesion. However, other collaborating treatment teams feel that Mr. G’s presentation was precipitated by an exacerbation of PTSD symptoms because of the observed psychotic themes, in addition to metabolic encephalopathy. Acute stress disorder can present with emotional numbing, depersonalization, reduced awareness of surroundings, or dissociative amnesia. However, Mr. G has not experienced PTSD symptoms involving mental status changes with fluctuating orientation in the past nor has he displayed persistent dissociation during outpatient psychiatric care. Therefore, it is unlikely that PTSD is the primary cause of his hospital admission.
The palliative care team recommends switching Mr. G’s pain medications to methadone, 20 mg, every 6 hours, to reduce possibility that opioids are contributing to his delirious state. Mr. G’s medical providers report that the chest radiography is suspicious for pneumonia and start him on levofloxacin, 500 mg/d.
The authors’ observations
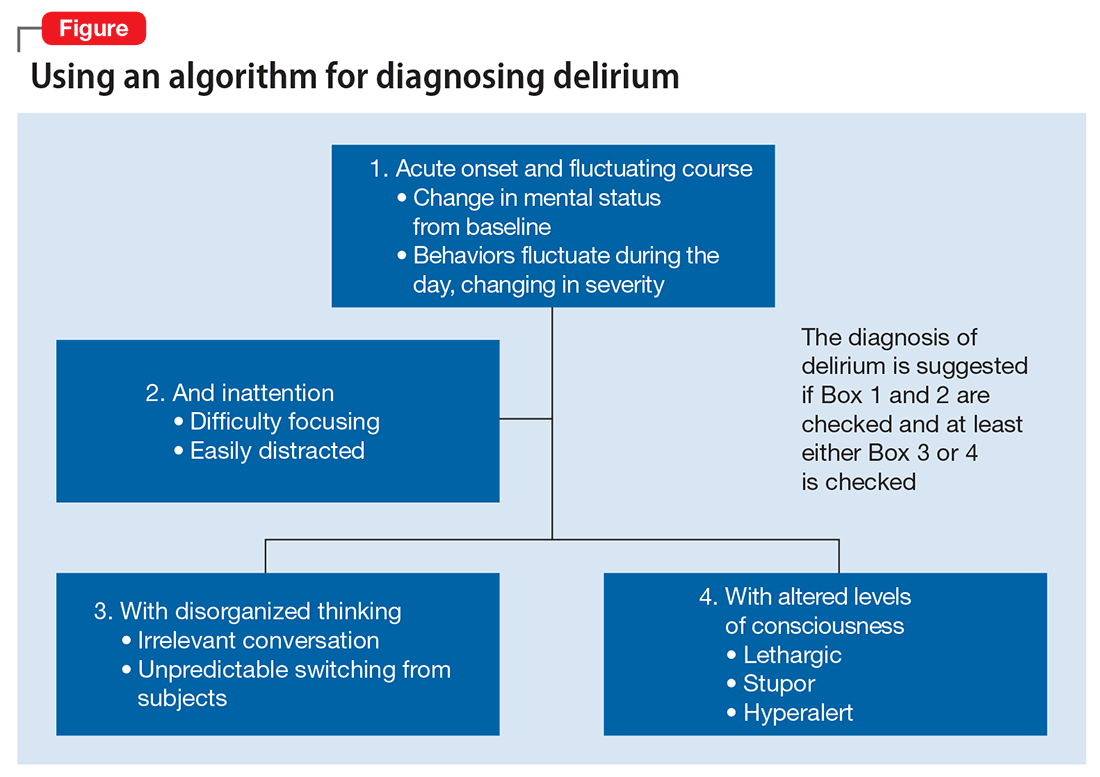
DSM-5 criteria for delirium has 4 components:
- disturbance in attention and awareness
- change in cognition
- the disturbance develops over a short period of time
- there is evidence that the disturbance is a direct consequence of a medical condition, medication, or substance, or more than 1 cause.3
Mr. G presented with multi-factorial delirium, and as a result, all underlying contributions, including infection, polypharmacy, brain metastasis, and steroids needed to be considered. Treating delirium requires investigating the underlying cause and keeping the patient safe in the process (Figure). Mr. G was agitated at presentation; therefore, low-dosage olanzapine was initiated to address the imbalance between the cholinergic and dopaminergic systems in the CNS, which are thought to be the mechanism behind delirious presentations.
In Mr. G’s case, methadone was lowered, with continual monitoring and evaluation for his comfort. Infections, specifically urinary tract infections and pneumonia, can cause delirium states and must be treated with appropriate antibiotics. Metastatic tumors have been known to precipitate changes in mental status and can be ruled out via imaging. In Mr. G’s case, his metastatic lesion remained stable from prior radiographic studies.
TREATMENT Delirium resolves
Mr. G slowly responds to multi-modal treatment including decreased opioids and benzodiazepines and the use of low-dosage antipsychotics. He begins to return to baseline with antibiotic administration. By hospital day 5, Mr. G is alert and oriented. He notes resolution of his auditory and visual hallucinations and denies any persistent paranoia or delusions. The medical team observes Mr. G is having difficulty swallowing with meals, and orders a speech therapy evaluation. After assessment, the team suspects that aspiration pneumonia could have precipitated Mr. G’s initial decline and recommends a mechanic diet with thin liquids to reduce the risk of future aspiration.
Mr. G is discharged home in his wife’s care with home hospice to continue end-of-life care. His medication regimen includes olanzapine, 10 mg/d, to continue until his next outpatient appointment, trazodone, 50 mg/d, for depression and PTSD symptoms, and clonazepam is decreased to 0.5 mg, at bedtime, for anxiety.
The authors’ observations
Mr. G’s case highlights the importance of fully evaluating all common underlying causes of delirium. The etiology of delirium is more likely to be missed in medically complex patients or in patients with a history of psychiatric illness. Palliative care patients have several risk factors for delirium, such as benzodiazepine or opioid treatment, dementia, and organic diseases such as brain metastasis.6 A recent study assessed the frequency of delirium in cancer patients admitted to an inpatient palliative unit and found that 71% of individuals had a diagnosis of delirium at admission and 26% developed delirium afterward.7 Despite the increased likelihood of developing delirium, more than one-half of palliative patients have delirium that is missed by their primary providers.8 Similarly, patients with documented psychiatric illness were approximately 2.5 times more likely to have overlooked delirium compared with patients without psychiatric illness.9
Risk and prevention
Patients with risk factors for delirium—which includes sedative and narcotic usage, advanced cancer, older age, prolonged hospital stays, surgical procedures, and/or cognitive impairment—should receive interventions to prevent delirium. However, if symptoms of AMS are present, providers should perform a complete workup for underlying causes of delirium. Remembering that individuals with delirium have an impaired ability to voice symptoms, such as dyspnea, dysuria, and headache, clinicians should have a high index of suspicion for delirium in patients at heightened risk.10
Perhaps most important, teams treating patients at high risk for delirium should employ preventive measures to reduce the development of delirium. Although more studies are needed to clarify the role of drug therapies for preventing delirium, there is strong evidence for several non-pharmacotherapeutic interventions including:
- frequent orientation activities
- early mobilization
- maintaining healthy sleep–wake cycles
- minimizing the use of psychoactive drugs and frequently reviewing the medication regimen
- allowing use of eyeglasses and hearing aids
- treating volume depletion.10
These preventive measures are important when treating delirium, such as minimizing Mr. G’s use of benzodiazepine and opioids—medications known to contribute to iatrogenic delirium.
A delirium diagnosis portends grave adverse outcomes. Research has shown significant associations with morbidity and mortality, financial and emotional burden, and prolonged hospitalizations. Often, symptoms of delirium persist for months and patients do not recover completely. However, studies have found that when underlying causes are treated effectively, delirium is more likely to be reversible.11
The prompt diagnosis of delirium with good interdisciplinary communication can reduce the risk of these adverse outcomes.12 Consultation-liaison psychiatrists are well positioned to facilitate the diagnoses of delirium and play a role in educating other health care providers of the importance of prevention, early symptom recognition, full workup, and effective treatment of its underlying causes.
1. Posner JB, Saper CB, Schiff ND, et al. Plum and Posner’s diagnosis of stupor and coma. New York, NY: Oxford University Press; 2007.
2. Skrobik YK, Bergeron N, Dumont M, et al. Olanzapine vs haldoperidol: treating delirium in a critical care setting. Intensive Care Med. 2004;30(3):444-449.
3. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
4. Lonergan E, Luxenberg J, Areosa Sastre A, et al. Benzodiazepines for delirium. Cochrane Database Syst Rev. 2009;(1):CD006379. doi: 10.1002/14651858.CD006379.pub2.
5. Vella-Brincat J, Macleod AD. Adverse effects of opioids on the central nervous system of palliative care patients. J Pain Palliat Care Pharmacother. 2007;21(1):15-25.
6. Grassi L, Caraceni A, Mitchell AJ, et al. Management of delirium in palliative care: a review. Curr Psychiatry Rep. 2015;17(3):550.
7. de la Cruz M, Ransing V, Yennu S, et al. The frequency, characteristics, and outcomes among cancer patients with delirium admitted to an acute palliative care unit. Oncologist. 2015;20(12):1425-1431.
8. de la Cruz, M, Fan J, Yennu S, et al. The frequency of missed delirium in patients referred to palliative care in a comprehensive cancer center. Support Care Cancer. 2015;23(8):2427-2433.
9. Swigart SE, Kishi Y, Thurber S, et al. Misdiagnosed delirium in patient referrals to a university-based hospital psychiatry department. Psychosomatics. 2008;49(2):104-108.
10. Inouye SK, Bogardus ST Jr, Charpentier PA, et al. A multicomponent intervention to prevent delirium in hospitalized older patients. N Engl J Med. 1999;340(9):669-676.
11. Dasgupta M, Hillier LM. Factors associated with prolonged delirium: a systematic review. Int Psychogeriatr. 2010;22(3):373-394.
12. Detweiler MB, Kenneth A, Bader G, et al. Can improved intra- and inter-team communication reduce missed delirium? Psychiatr Q. 2014;85(2):211-224.
CASE Confusion, hallucinations
Mr. G, age 57, is brought to the emergency department (ED) from a hospice care facility for worsening agitation and psychosis over 2 days. His wife, who accompanies him, describes a 2-month onset of “confusion” with occasional visual hallucinations. She says that at baseline Mr. G was alert and oriented and able to engage appropriately in conversations. The hospice facility administered emergency medications, including unknown dosages of haloperidol and chlorpromazine, the morning before transfer to the ED.
Mr. G has a history of posttraumatic stress disorder (PTSD), anxiety, and depression that has been managed for 6 years with several trials of antidepressant monotherapy, including fluoxetine, citalopram, mirtazapine, bupropion, and augmentation using aripiprazole, risperidone, topiramate, and zolpidem. At the time of this hospital presentation, his symptoms are controlled on clonazepam, 2 mg/d, and trazodone, 50 mg/d. For his pain attributed to non-small cell lung cancer (NSCLC), he receives methadone, 25 mg, 6 times a day, and hydromorphone, 8 mg, every 4 hours as needed, for breakthrough pain. Mr. G underwent a right upper lobectomy 5 years ago and neurosurgery with a right suboccipital craniectomy for right-sided cerebellar metastatic tumor measuring 2 × 1 × 0.6 cm, along with chemotherapy and radiation for metastasis in the brain 1 year ago. His last chemotherapy session was 3 months ago.
In the ED, Mr. G is sedated and oriented only to person and his wife. He is observed mumbling incoherently. Abnormal vital signs and laboratory findings are elevated pulse, 97 beats per minute; mild anemia, 13.5 g/dL hemoglobin and 40.8% hematocrit; an elevated glucose of 136 mg/dL; and small amounts of blood, trace ketones, and hyaline casts in urinalysis. Vital signs, laboratory resu
In addition to psychotropic and pain medication, Mr. G is taking cyclobenzaprine, 5 mg, every 6 hours as needed, for muscle spasms; docusate, 200 mg/d; enoxaparin, 100 mg/1mL, every 12 hours; folic acid, 1 mg/d; gabapentin, 600 mg, 3 times daily; lidocaine ointment, twice daily as needed, for pain; omeprazole, 80 mg/d; ondansetron, 4 mg, every 8 hours as needed, for nausea; and tamsulosin, 0.4 mg/d.
What is your differential diagnosis for Mr. G?
a) brain metastases
b) infection
c) PTSD
d) polypharmacy
e) benzodiazepine withdrawal
The authors’ observations
Altered mental status (AMS), or acute confusional state, describes an individual who fails to interact with environmental stimuli in an appropriate, anticipated manner. The disturbance usually is acute and transient.1 Often providers struggle to obtain relevant facts about a patient’s history of illness and must use laboratory and diagnostic data to determine the underlying cause of the patient’s disorientation.
Mental status includes 2 components: arousal and awareness. Arousal refers to a person’s wakeful state and how an individual responds to his (her) surroundings. Impairment in arousal can result in variable states including lethargy, drowsiness, and even coma. Awareness, on the other hand, is an individual’s perception of his environment, including orientation to surroundings, executive functioning, and memory. Although arousal level is controlled by the reticular activating system of the brainstem, awareness of consciousness is mediated at the cortical level. Mr. G experienced increased arousal and AMS with a clear change in behavior from his baseline. With increasing frequency of hallucinations and agitated behaviors, several tests must be ordered to determine the etiology of his altered mentation (Table 1).
Which test would you order next?
a) urine drug screen (UDS)
b) chest CT with pulmonary embolism protocol
c) CT of the head
d) blood cultures
e) chest radiography
EVALUATION Awake, still confused
The ED physician orders a UDS, non-contrasted CT of the head, and chest radiography for preliminary workup investigating the cause of Mr. G’s AMS. UDS is negative for illicit substances. The non-contrasted CT of the head shows a stable, right cerebellar hemisphere lesion from a prior lung metastasis. Mr. G’s chest radiography reading describes an ill-defined opacity at the left lung base.
Mr. G is admitted to the medical service and is started on dexamethasone, 8 mg/d, for his NSCLC with brain metastasis. Clonazepam is continued to prevent benzodiazepine withdrawal. The psychiatry and palliative care teams are consulted to determine if Mr. G’s PTSD symptoms and/or opioids are contributing to his AMS and psychosis. After evaluation, the psychiatry team recommends decreasing clonazepam to 0.5 mg, twice daily, starting olanzapine, 5 mg, every 12 hours, for agitation and psychosis involving auditory and visual hallucinations as well as paranoid themes related to food contamination, and using non-pharmacologic interventions for delirium treatment (Table 2). In a prospective, randomized controlled trial of olanzapine vs haloperidol, clinical improvement in delirious states was seen in individuals who received either antipsychotic medication; however, haloperidol was associated with extrapyramidal side effects. Therefore, olanzapine is a safe alternative to haloperidol in delirious patients.2
The psychiatry consult service suspects delirium due to polypharmacy or Mr. G’s metastatic brain lesion. However, other collaborating treatment teams feel that Mr. G’s presentation was precipitated by an exacerbation of PTSD symptoms because of the observed psychotic themes, in addition to metabolic encephalopathy. Acute stress disorder can present with emotional numbing, depersonalization, reduced awareness of surroundings, or dissociative amnesia. However, Mr. G has not experienced PTSD symptoms involving mental status changes with fluctuating orientation in the past nor has he displayed persistent dissociation during outpatient psychiatric care. Therefore, it is unlikely that PTSD is the primary cause of his hospital admission.
The palliative care team recommends switching Mr. G’s pain medications to methadone, 20 mg, every 6 hours, to reduce possibility that opioids are contributing to his delirious state. Mr. G’s medical providers report that the chest radiography is suspicious for pneumonia and start him on levofloxacin, 500 mg/d.
The authors’ observations
DSM-5 criteria for delirium has 4 components:
- disturbance in attention and awareness
- change in cognition
- the disturbance develops over a short period of time
- there is evidence that the disturbance is a direct consequence of a medical condition, medication, or substance, or more than 1 cause.3
Mr. G presented with multi-factorial delirium, and as a result, all underlying contributions, including infection, polypharmacy, brain metastasis, and steroids needed to be considered. Treating delirium requires investigating the underlying cause and keeping the patient safe in the process (Figure). Mr. G was agitated at presentation; therefore, low-dosage olanzapine was initiated to address the imbalance between the cholinergic and dopaminergic systems in the CNS, which are thought to be the mechanism behind delirious presentations.
In Mr. G’s case, methadone was lowered, with continual monitoring and evaluation for his comfort. Infections, specifically urinary tract infections and pneumonia, can cause delirium states and must be treated with appropriate antibiotics. Metastatic tumors have been known to precipitate changes in mental status and can be ruled out via imaging. In Mr. G’s case, his metastatic lesion remained stable from prior radiographic studies.
TREATMENT Delirium resolves
Mr. G slowly responds to multi-modal treatment including decreased opioids and benzodiazepines and the use of low-dosage antipsychotics. He begins to return to baseline with antibiotic administration. By hospital day 5, Mr. G is alert and oriented. He notes resolution of his auditory and visual hallucinations and denies any persistent paranoia or delusions. The medical team observes Mr. G is having difficulty swallowing with meals, and orders a speech therapy evaluation. After assessment, the team suspects that aspiration pneumonia could have precipitated Mr. G’s initial decline and recommends a mechanic diet with thin liquids to reduce the risk of future aspiration.
Mr. G is discharged home in his wife’s care with home hospice to continue end-of-life care. His medication regimen includes olanzapine, 10 mg/d, to continue until his next outpatient appointment, trazodone, 50 mg/d, for depression and PTSD symptoms, and clonazepam is decreased to 0.5 mg, at bedtime, for anxiety.
The authors’ observations
Mr. G’s case highlights the importance of fully evaluating all common underlying causes of delirium. The etiology of delirium is more likely to be missed in medically complex patients or in patients with a history of psychiatric illness. Palliative care patients have several risk factors for delirium, such as benzodiazepine or opioid treatment, dementia, and organic diseases such as brain metastasis.6 A recent study assessed the frequency of delirium in cancer patients admitted to an inpatient palliative unit and found that 71% of individuals had a diagnosis of delirium at admission and 26% developed delirium afterward.7 Despite the increased likelihood of developing delirium, more than one-half of palliative patients have delirium that is missed by their primary providers.8 Similarly, patients with documented psychiatric illness were approximately 2.5 times more likely to have overlooked delirium compared with patients without psychiatric illness.9
Risk and prevention
Patients with risk factors for delirium—which includes sedative and narcotic usage, advanced cancer, older age, prolonged hospital stays, surgical procedures, and/or cognitive impairment—should receive interventions to prevent delirium. However, if symptoms of AMS are present, providers should perform a complete workup for underlying causes of delirium. Remembering that individuals with delirium have an impaired ability to voice symptoms, such as dyspnea, dysuria, and headache, clinicians should have a high index of suspicion for delirium in patients at heightened risk.10
Perhaps most important, teams treating patients at high risk for delirium should employ preventive measures to reduce the development of delirium. Although more studies are needed to clarify the role of drug therapies for preventing delirium, there is strong evidence for several non-pharmacotherapeutic interventions including:
- frequent orientation activities
- early mobilization
- maintaining healthy sleep–wake cycles
- minimizing the use of psychoactive drugs and frequently reviewing the medication regimen
- allowing use of eyeglasses and hearing aids
- treating volume depletion.10
These preventive measures are important when treating delirium, such as minimizing Mr. G’s use of benzodiazepine and opioids—medications known to contribute to iatrogenic delirium.
A delirium diagnosis portends grave adverse outcomes. Research has shown significant associations with morbidity and mortality, financial and emotional burden, and prolonged hospitalizations. Often, symptoms of delirium persist for months and patients do not recover completely. However, studies have found that when underlying causes are treated effectively, delirium is more likely to be reversible.11
The prompt diagnosis of delirium with good interdisciplinary communication can reduce the risk of these adverse outcomes.12 Consultation-liaison psychiatrists are well positioned to facilitate the diagnoses of delirium and play a role in educating other health care providers of the importance of prevention, early symptom recognition, full workup, and effective treatment of its underlying causes.
CASE Confusion, hallucinations
Mr. G, age 57, is brought to the emergency department (ED) from a hospice care facility for worsening agitation and psychosis over 2 days. His wife, who accompanies him, describes a 2-month onset of “confusion” with occasional visual hallucinations. She says that at baseline Mr. G was alert and oriented and able to engage appropriately in conversations. The hospice facility administered emergency medications, including unknown dosages of haloperidol and chlorpromazine, the morning before transfer to the ED.
Mr. G has a history of posttraumatic stress disorder (PTSD), anxiety, and depression that has been managed for 6 years with several trials of antidepressant monotherapy, including fluoxetine, citalopram, mirtazapine, bupropion, and augmentation using aripiprazole, risperidone, topiramate, and zolpidem. At the time of this hospital presentation, his symptoms are controlled on clonazepam, 2 mg/d, and trazodone, 50 mg/d. For his pain attributed to non-small cell lung cancer (NSCLC), he receives methadone, 25 mg, 6 times a day, and hydromorphone, 8 mg, every 4 hours as needed, for breakthrough pain. Mr. G underwent a right upper lobectomy 5 years ago and neurosurgery with a right suboccipital craniectomy for right-sided cerebellar metastatic tumor measuring 2 × 1 × 0.6 cm, along with chemotherapy and radiation for metastasis in the brain 1 year ago. His last chemotherapy session was 3 months ago.
In the ED, Mr. G is sedated and oriented only to person and his wife. He is observed mumbling incoherently. Abnormal vital signs and laboratory findings are elevated pulse, 97 beats per minute; mild anemia, 13.5 g/dL hemoglobin and 40.8% hematocrit; an elevated glucose of 136 mg/dL; and small amounts of blood, trace ketones, and hyaline casts in urinalysis. Vital signs, laboratory resu
In addition to psychotropic and pain medication, Mr. G is taking cyclobenzaprine, 5 mg, every 6 hours as needed, for muscle spasms; docusate, 200 mg/d; enoxaparin, 100 mg/1mL, every 12 hours; folic acid, 1 mg/d; gabapentin, 600 mg, 3 times daily; lidocaine ointment, twice daily as needed, for pain; omeprazole, 80 mg/d; ondansetron, 4 mg, every 8 hours as needed, for nausea; and tamsulosin, 0.4 mg/d.
What is your differential diagnosis for Mr. G?
a) brain metastases
b) infection
c) PTSD
d) polypharmacy
e) benzodiazepine withdrawal
The authors’ observations
Altered mental status (AMS), or acute confusional state, describes an individual who fails to interact with environmental stimuli in an appropriate, anticipated manner. The disturbance usually is acute and transient.1 Often providers struggle to obtain relevant facts about a patient’s history of illness and must use laboratory and diagnostic data to determine the underlying cause of the patient’s disorientation.
Mental status includes 2 components: arousal and awareness. Arousal refers to a person’s wakeful state and how an individual responds to his (her) surroundings. Impairment in arousal can result in variable states including lethargy, drowsiness, and even coma. Awareness, on the other hand, is an individual’s perception of his environment, including orientation to surroundings, executive functioning, and memory. Although arousal level is controlled by the reticular activating system of the brainstem, awareness of consciousness is mediated at the cortical level. Mr. G experienced increased arousal and AMS with a clear change in behavior from his baseline. With increasing frequency of hallucinations and agitated behaviors, several tests must be ordered to determine the etiology of his altered mentation (Table 1).
Which test would you order next?
a) urine drug screen (UDS)
b) chest CT with pulmonary embolism protocol
c) CT of the head
d) blood cultures
e) chest radiography
EVALUATION Awake, still confused
The ED physician orders a UDS, non-contrasted CT of the head, and chest radiography for preliminary workup investigating the cause of Mr. G’s AMS. UDS is negative for illicit substances. The non-contrasted CT of the head shows a stable, right cerebellar hemisphere lesion from a prior lung metastasis. Mr. G’s chest radiography reading describes an ill-defined opacity at the left lung base.
Mr. G is admitted to the medical service and is started on dexamethasone, 8 mg/d, for his NSCLC with brain metastasis. Clonazepam is continued to prevent benzodiazepine withdrawal. The psychiatry and palliative care teams are consulted to determine if Mr. G’s PTSD symptoms and/or opioids are contributing to his AMS and psychosis. After evaluation, the psychiatry team recommends decreasing clonazepam to 0.5 mg, twice daily, starting olanzapine, 5 mg, every 12 hours, for agitation and psychosis involving auditory and visual hallucinations as well as paranoid themes related to food contamination, and using non-pharmacologic interventions for delirium treatment (Table 2). In a prospective, randomized controlled trial of olanzapine vs haloperidol, clinical improvement in delirious states was seen in individuals who received either antipsychotic medication; however, haloperidol was associated with extrapyramidal side effects. Therefore, olanzapine is a safe alternative to haloperidol in delirious patients.2
The psychiatry consult service suspects delirium due to polypharmacy or Mr. G’s metastatic brain lesion. However, other collaborating treatment teams feel that Mr. G’s presentation was precipitated by an exacerbation of PTSD symptoms because of the observed psychotic themes, in addition to metabolic encephalopathy. Acute stress disorder can present with emotional numbing, depersonalization, reduced awareness of surroundings, or dissociative amnesia. However, Mr. G has not experienced PTSD symptoms involving mental status changes with fluctuating orientation in the past nor has he displayed persistent dissociation during outpatient psychiatric care. Therefore, it is unlikely that PTSD is the primary cause of his hospital admission.
The palliative care team recommends switching Mr. G’s pain medications to methadone, 20 mg, every 6 hours, to reduce possibility that opioids are contributing to his delirious state. Mr. G’s medical providers report that the chest radiography is suspicious for pneumonia and start him on levofloxacin, 500 mg/d.
The authors’ observations
DSM-5 criteria for delirium has 4 components:
- disturbance in attention and awareness
- change in cognition
- the disturbance develops over a short period of time
- there is evidence that the disturbance is a direct consequence of a medical condition, medication, or substance, or more than 1 cause.3
Mr. G presented with multi-factorial delirium, and as a result, all underlying contributions, including infection, polypharmacy, brain metastasis, and steroids needed to be considered. Treating delirium requires investigating the underlying cause and keeping the patient safe in the process (Figure). Mr. G was agitated at presentation; therefore, low-dosage olanzapine was initiated to address the imbalance between the cholinergic and dopaminergic systems in the CNS, which are thought to be the mechanism behind delirious presentations.
In Mr. G’s case, methadone was lowered, with continual monitoring and evaluation for his comfort. Infections, specifically urinary tract infections and pneumonia, can cause delirium states and must be treated with appropriate antibiotics. Metastatic tumors have been known to precipitate changes in mental status and can be ruled out via imaging. In Mr. G’s case, his metastatic lesion remained stable from prior radiographic studies.
TREATMENT Delirium resolves
Mr. G slowly responds to multi-modal treatment including decreased opioids and benzodiazepines and the use of low-dosage antipsychotics. He begins to return to baseline with antibiotic administration. By hospital day 5, Mr. G is alert and oriented. He notes resolution of his auditory and visual hallucinations and denies any persistent paranoia or delusions. The medical team observes Mr. G is having difficulty swallowing with meals, and orders a speech therapy evaluation. After assessment, the team suspects that aspiration pneumonia could have precipitated Mr. G’s initial decline and recommends a mechanic diet with thin liquids to reduce the risk of future aspiration.
Mr. G is discharged home in his wife’s care with home hospice to continue end-of-life care. His medication regimen includes olanzapine, 10 mg/d, to continue until his next outpatient appointment, trazodone, 50 mg/d, for depression and PTSD symptoms, and clonazepam is decreased to 0.5 mg, at bedtime, for anxiety.
The authors’ observations
Mr. G’s case highlights the importance of fully evaluating all common underlying causes of delirium. The etiology of delirium is more likely to be missed in medically complex patients or in patients with a history of psychiatric illness. Palliative care patients have several risk factors for delirium, such as benzodiazepine or opioid treatment, dementia, and organic diseases such as brain metastasis.6 A recent study assessed the frequency of delirium in cancer patients admitted to an inpatient palliative unit and found that 71% of individuals had a diagnosis of delirium at admission and 26% developed delirium afterward.7 Despite the increased likelihood of developing delirium, more than one-half of palliative patients have delirium that is missed by their primary providers.8 Similarly, patients with documented psychiatric illness were approximately 2.5 times more likely to have overlooked delirium compared with patients without psychiatric illness.9
Risk and prevention
Patients with risk factors for delirium—which includes sedative and narcotic usage, advanced cancer, older age, prolonged hospital stays, surgical procedures, and/or cognitive impairment—should receive interventions to prevent delirium. However, if symptoms of AMS are present, providers should perform a complete workup for underlying causes of delirium. Remembering that individuals with delirium have an impaired ability to voice symptoms, such as dyspnea, dysuria, and headache, clinicians should have a high index of suspicion for delirium in patients at heightened risk.10
Perhaps most important, teams treating patients at high risk for delirium should employ preventive measures to reduce the development of delirium. Although more studies are needed to clarify the role of drug therapies for preventing delirium, there is strong evidence for several non-pharmacotherapeutic interventions including:
- frequent orientation activities
- early mobilization
- maintaining healthy sleep–wake cycles
- minimizing the use of psychoactive drugs and frequently reviewing the medication regimen
- allowing use of eyeglasses and hearing aids
- treating volume depletion.10
These preventive measures are important when treating delirium, such as minimizing Mr. G’s use of benzodiazepine and opioids—medications known to contribute to iatrogenic delirium.
A delirium diagnosis portends grave adverse outcomes. Research has shown significant associations with morbidity and mortality, financial and emotional burden, and prolonged hospitalizations. Often, symptoms of delirium persist for months and patients do not recover completely. However, studies have found that when underlying causes are treated effectively, delirium is more likely to be reversible.11
The prompt diagnosis of delirium with good interdisciplinary communication can reduce the risk of these adverse outcomes.12 Consultation-liaison psychiatrists are well positioned to facilitate the diagnoses of delirium and play a role in educating other health care providers of the importance of prevention, early symptom recognition, full workup, and effective treatment of its underlying causes.
1. Posner JB, Saper CB, Schiff ND, et al. Plum and Posner’s diagnosis of stupor and coma. New York, NY: Oxford University Press; 2007.
2. Skrobik YK, Bergeron N, Dumont M, et al. Olanzapine vs haldoperidol: treating delirium in a critical care setting. Intensive Care Med. 2004;30(3):444-449.
3. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
4. Lonergan E, Luxenberg J, Areosa Sastre A, et al. Benzodiazepines for delirium. Cochrane Database Syst Rev. 2009;(1):CD006379. doi: 10.1002/14651858.CD006379.pub2.
5. Vella-Brincat J, Macleod AD. Adverse effects of opioids on the central nervous system of palliative care patients. J Pain Palliat Care Pharmacother. 2007;21(1):15-25.
6. Grassi L, Caraceni A, Mitchell AJ, et al. Management of delirium in palliative care: a review. Curr Psychiatry Rep. 2015;17(3):550.
7. de la Cruz M, Ransing V, Yennu S, et al. The frequency, characteristics, and outcomes among cancer patients with delirium admitted to an acute palliative care unit. Oncologist. 2015;20(12):1425-1431.
8. de la Cruz, M, Fan J, Yennu S, et al. The frequency of missed delirium in patients referred to palliative care in a comprehensive cancer center. Support Care Cancer. 2015;23(8):2427-2433.
9. Swigart SE, Kishi Y, Thurber S, et al. Misdiagnosed delirium in patient referrals to a university-based hospital psychiatry department. Psychosomatics. 2008;49(2):104-108.
10. Inouye SK, Bogardus ST Jr, Charpentier PA, et al. A multicomponent intervention to prevent delirium in hospitalized older patients. N Engl J Med. 1999;340(9):669-676.
11. Dasgupta M, Hillier LM. Factors associated with prolonged delirium: a systematic review. Int Psychogeriatr. 2010;22(3):373-394.
12. Detweiler MB, Kenneth A, Bader G, et al. Can improved intra- and inter-team communication reduce missed delirium? Psychiatr Q. 2014;85(2):211-224.
1. Posner JB, Saper CB, Schiff ND, et al. Plum and Posner’s diagnosis of stupor and coma. New York, NY: Oxford University Press; 2007.
2. Skrobik YK, Bergeron N, Dumont M, et al. Olanzapine vs haldoperidol: treating delirium in a critical care setting. Intensive Care Med. 2004;30(3):444-449.
3. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
4. Lonergan E, Luxenberg J, Areosa Sastre A, et al. Benzodiazepines for delirium. Cochrane Database Syst Rev. 2009;(1):CD006379. doi: 10.1002/14651858.CD006379.pub2.
5. Vella-Brincat J, Macleod AD. Adverse effects of opioids on the central nervous system of palliative care patients. J Pain Palliat Care Pharmacother. 2007;21(1):15-25.
6. Grassi L, Caraceni A, Mitchell AJ, et al. Management of delirium in palliative care: a review. Curr Psychiatry Rep. 2015;17(3):550.
7. de la Cruz M, Ransing V, Yennu S, et al. The frequency, characteristics, and outcomes among cancer patients with delirium admitted to an acute palliative care unit. Oncologist. 2015;20(12):1425-1431.
8. de la Cruz, M, Fan J, Yennu S, et al. The frequency of missed delirium in patients referred to palliative care in a comprehensive cancer center. Support Care Cancer. 2015;23(8):2427-2433.
9. Swigart SE, Kishi Y, Thurber S, et al. Misdiagnosed delirium in patient referrals to a university-based hospital psychiatry department. Psychosomatics. 2008;49(2):104-108.
10. Inouye SK, Bogardus ST Jr, Charpentier PA, et al. A multicomponent intervention to prevent delirium in hospitalized older patients. N Engl J Med. 1999;340(9):669-676.
11. Dasgupta M, Hillier LM. Factors associated with prolonged delirium: a systematic review. Int Psychogeriatr. 2010;22(3):373-394.
12. Detweiler MB, Kenneth A, Bader G, et al. Can improved intra- and inter-team communication reduce missed delirium? Psychiatr Q. 2014;85(2):211-224.
What psychiatrists must know to make the mandated transition to ICD-10
Just as psychiatrists are adapting to DSM-5, they have to cope with implementation of the 10th edition of the International Statistical Classification of Diseases and Related Health Problems (ICD-10). This challenge raises questions: What is the importance of understanding ICD-10? How will it affect the practice of psychiatry?
Furthermore, how does ICD-10 relate to DSM-5 and Current Procedural Terminology (CPT)? How does it differ from ICD-9? What are the ICD-10-Clinical Modification (CM) and ICD-10-Procedures (PCS)?Learning the essence of the changes, and understanding what impact they have on your clinical work, are necessary to ensure that your practice keeps pace with professional and legal standards of care. The effort involved is not onerous, however, and can improve the quality and efficiency of your care and how you document it.
In this article, we provide you with an overview of ICD-10; highlight major changes of the new classification; explain its relevance to clinical practice; and offer guidelines for implementing it effectively. We also emphasize that a good understanding of DSM-5 facilitates appreciation of ICD-10 and makes its implementation fairly easy and straightforward.
To begin, we provide a glossary of ICD-related terms and a review of additional definitions, distinctions, and dates (Box).1-6
Major changes from ICD-9
No question: ICD-10 is going to significantly influence your practice and your reimbursement. Furthermore, a number of revisions in ICD-10 have the potential to meaningfully improve clinical documentation and communication and to enhance your ability to precisely describe the complexity of your patients—with implications for billing.
ICD-10 differs from ICD-9 in organization, structure, code composition, and level of detail. In addition, ICD-10 makes some changes in terminology and definitions, with the goal of improving precision.
ICD-10 also is much larger than ICD-9.The total number of medical diagnostic codes has increased more than 5-fold—from approximately 13,000 to 69,000. This expansion allows for greater specificity in diagnosis and enables differentiation of an initial clinical encounter from a subsequent encounter.
To accommodate the expansion in the number of codes, the 5-digit numeric codes used in ICD-9 have been replaced in ICD-10 by 7-digit alphanumeric codes:
- the first digit always is a letter
- the second and third digits are numbers followed by a decimal point
- the fourth though seventh digits can be letters or numbers
- the first 3 digits denote the diagnostic category
- the fourth through sixth digits provide diagnostic detail
- the seventh digit provides information about the nature of the encounter (eg, initial, subsequent, or sequel, denoted respectively by “A,” “D,” and “S” in the seventh digit).
The number of 3-digit categories for psychiatric disorders has increased from 30 in ICD-9 (290-319) to 100 in ICD-10 (F00-F99). Only the first 5 digits are used for the section on mental disorders in ICD-10, with the first digit always “F” and the second digit a number denoting the broad type of disorders. The second and third digits in conjunction define the major category of the disorder; the fourth and fifth digits provide additional descriptive detail about the disorder (Table).
ICD-9 ‘V’ codes are out
What were called “V” codes in ICD-9—factors that influence health status and contact with health services—have been replaced by “Z” codes in ICD-10. These “Z” codes provide greater detail and precision than “V” codes provided.
Examples of “Z” codes relevant to psychiatry are:
Z00 General psychiatric examination (eg, of a person who does not have a complaint or diagnosis)
Z03 Examination for suspected mental and behavioral disorder
Z04 Examination for medicolegal or other purposes; Z04.8 is relevant laboratory testing, such as drug testing of urine or blood
Z50 Care involving rehabilitation (substance use disorder, etc.)
Z60 Problem related to social environment
Z61 Problem related to negative life events in childhood
Z63 Problem related to primary support group, including family circumstances
Z64-Z65 Problem related to other psychosocial circumstances
Z70-Z71 Condition requiring counseling, not elsewhere classified
Z73 Problem related to difficulty with life management (burnout, stress, role conflict, etc.)
Z75 Problem related to medical facilities and other aspects of health care (eg, awaiting admission)
Z81 Family history of mental or behavioral disorders
Z85-Z91 Personal history of various disorders (must be absent or in full remission at the moment); Z86.51, for example, refers to a history of combat and operational stress reaction.
Greater precision is now possible when coding for treatment-related adverse effects. A particular adverse effect now is coded under the relevant system, along with its attribution to the specific substance. Obesity attributable to antipsychotic treatment,7,8 for example, is coded as E66.1.
Integrating DSM-5 and ICD-10
Because DSM-5 lists corresponding ICD-10-CM codes for all disorders, you will find it much easier than other physicians to implement ICD-10. DSM-5 includes ICD-9-CM and ICD-10-CM codes for each DSM-5 disorder (for example, the ICD-9-CM code for schizophrenia is 295.x; the ICD-10-CM code is F20.9).9
Furthermore, a number of changes from ICD-9-CM to ICD-10-CM enable documentation of greater diagnostic specificity; for example, DSM-5 schizoaffective disorder, bipolar type, and schizoaffective disorder, depressive type, are distinctly coded as F25.0 and F25.1, respectively, in ICD-10-CM, whereas both were coded as 295.7 in ICD-9-CM.10
You will continue to use DSM-5 criteria to guide your diagnostic process, translating the DSM-5 diagnosis (diagnoses) into corresponding ICD-10-CM codes. Experience with DSM-5 substantially simplifies the transition to ICD-10.
Key differences between DSM-5 and ICD-10
There are notable differences in organization and content between DSM-5 and ICD-10.
The 20 chapters in DSM-5 begin with neurodevelopmental disorders; neurocognitive disorders are toward the end (ie, childhood to late life). In contrast, neurocognitive disorders (ie, “dementia”) appear at the beginning of ICD-10; neurodevelopmental disorders are at the end.
Elimination of schizophrenia subtypes in DSM-5 necessitates coding of all schizophrenia as F20.9 in ICD-10-CM because F20.0-F20.8 are specific subtypes. DSM-5 schizophreniform disorder is coded F20.81.
Substance abuse and substance dependence continue to be separate in ICD-10-CM, but they are combined in a single category of substance use disorders in DSM-5. The correct ICD-10-CM code (ie, abuse vs dependence) is determined by the severity of the substance use disorder: “Mild” coding as abuse (F1x.1) and “moderate” and “severe” coding as dependence (F2x.2), with x denoting the substance abused.
There can be multiple applicable diagnoses associated with a clinical encounter, as there was with ICD-9-CM. Give precedence to the diagnosis that best represents the nature of the presenting problem; list other diagnoses in the order of their relevance. DSM-5 and ICD-10-CM are similar in this regard.
ICD-10-CM uses only subtypes, in contrast to the use of subtypes and specifiers in DSM-5 to describe variability in disorders across patients. It is possible, however, to code certain DSM-5 specifiers in ICD-10-CM. (This is discussed in the “Recording Procedures” section of the DSM-5 text and summarized at the beginning of the manual, and appears in the “Appendix.”) To code the catatonia specifier in the context of schizoaffective disorder, depressive type, for example, use ICD-10-CM code F25.1 for the disorder and add code F06.1 for the catatonia specifier.11
How will ICD-10 affect your practice?
As of October 1, 2015, all health care facilities were to have become ICD-10 compliant. Furthermore, any Health Insurance Portability and Accountability Act-covered entity must use ICD-10-CM codes if it expects to be reimbursed for health care services.
Mental health practitioners might think that the transition from ICD-9-CM to ICD-10-CM involves only billers and coders, not them. They are wrong. All clinicians are responsible for documenting their diagnostic and treatment services properly. Medical records must contain adequate information to support any diagnostic (ICD-10-CM) and treatment (CPT) codes that are applied to a given clinical encounter.
The greater detail and specificity that are provided by ICD-10-CM allow more accurate recording of clinical complexity, which, in turn, influences reimbursement. However, good documentation is necessary for proper coding. Because clinicians are ultimately responsible for proper diagnostic coding, good understanding of ICD-10-CM is essential to be able to code properly.
Similar to the expansion of ICD-10-CM (from volumes 1 and 2 of ICD-9-CM), ICD-10-PCS has undergone similar expansion (from volume 3 of ICD-9-CM), with a corresponding increase in specificity. For example, there are now 5 distinct codes for electroconvulsive therapy (GZB0ZZZ-GZB4ZZZ) that distinguish unilateral from bilateral electrode placement and single from multiple stimulations.
DSM-5 will continue to be the frameworkfor psychiatric assessment and diagnosis. ICD-10-CM will be the coding system to accurately denote DSM-5 diagnoses. The Centers for Medicare and Medicaid Services (CMS) and the National Center for Health Statistics recognize DSM-5 as the means to identify proper ICD-10-CM codes for mental disorders. CMS also has announced that, although ICD-10-CM codes are necessary for reimbursement, use of an incorrect code will not be the basis for denying a Medicare claim for 1 year.
Making ICD-10 part of practice
Here are several keys to implementing ICD-10 with minimum pain and maximum benefit.
Multiple diagnosis codes should be listed in the order of their relevance to the clinical encounter.
Visit type. The seventh character of the ICD-10-CM code denotes the type of visit (initial, subsequent, or sequela) and must be provided:
- An initial encounter is one in which the patient first receives active treatment.
- A subsequent encounter refers to a follow-up visit in which the patient receives routine care during the healing or recovery phase.
- A sequel encounter is one in which a patient receives treatment for complications or conditions that arise as a direct result of the initial condition.
The transition to ICD-10 should be facilitated by adoption of DSM-5. Continue using DSM-5 to determine the correct diagnosis or diagnoses of the mental disorder, then apply the corresponding ICD-10-CM code(s). The better you understand and apply DSM-5, the more precise you can be in utilizing the greater specificity and accuracy afforded by ICD-10-CM coding.
Document well. Good understanding of the structure and organization of ICD-10-CM facilitates efficient, comprehensive documentation. This, in turn, will foster better clinical communication and appropriate reimbursement.
Know your payers—in particular, their policies regarding differential reimbursement for clinical complexity (based on ICD-10-CM/PCS). Medical practices that are part of an accountable care organization, and those that have risk-adjusted contracts must pay special attention to documenting clinical complexity when coding.
Know your electronic health care record, understand what tools it offers to efficiently translate DSM-5 diagnoses into appropriate ICD-10-CM codes, and use those tools efficiently.
Review your medical record documentation for the top 20 conditions in your practice, in the context of their definition in ICD-10-CM.
If you have coders who do ICD-10-CM coding for you, review a few patient charts with them to compare your sense of the patient’s clinical complexity and their coding based on your documentation.
Changes in DSM-5 have encouraged clinicians to improve their assessment of patients and provide measurement-based care. The significant changes in ICD-10-CM should provide the impetus for you to hone your ability to provide documentation. Sufficient flexibility exists within guidelines to permit individualization of the style of documentation.
Because all DSM-5 diagnoses map to appropriate ICD-10-CM codes, effective use of DSM-5 should make the transition to ICD-10 easy.
Bottom Line
Compared with ICD-9, definitions of mental health diagnoses have been improved in ICD-10, and more elaborate code descriptions in ICD-10-CM provide for greater precision when you report a diagnosis. The result? More accurate and efficient documentation of the care you provide and better reimbursement. Understanding what impact the changes in ICD-10 will have on your clinical work will ensure that your practice keeps pace with professional and legal standards of care.
Related Resources
• Blue Cross Blue Shield of Michigan ICD-10 update: mental and behavioral health ICD-10-CM codes. http://www.bcbsm.com/content/dam/public/Providers/Documents/help/faqs/icd10-update-mentalhealth.pdf.
• American Psychiatric Association ICD-10 tutorial. http://www.psychiatry.org/psychiatrists/practice/dsm/icd-10.
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Diagnostic and statistical manual of mental disorders, 5th edition. Washington DC: American Psychiatric Association; 2013.
2. World Health Organization. The ICD-10 classification of mental and behavioral disorders: clinical descriptions and diagnostic guidelines. Geneva, Switzerland: World Health Organization; 1992.
3. American Medical Association. ICD-10-CM 2016: the complete official code set. Chicago, IL: American Medical Association; 2015.
4. American Medical Association. CPT-2016, professional edition. Chicago, IL: American Medical Association; 2015.
5. American Medical Association. ICD-10-CM expert for physicians 2016: the complete official code set. Chicago, IL: American Medical Association; 2015.
6. American Medical Association. ICD-10-PCS mapping to ICD-9-CM volume 3. Chicago, IL: American Medical Association; 2015.
7. Tandon R, Halbreich U. The second-generation ‘atypical’ antipsychotics: similar efficacy but different neuroendocrine side-effects. Psychoneuroendocrinology. 2003;28(suppl 1):1-7.
8. Tandon R. Antipsychotics in the treatment of schizophrenia: an overview. J Clin Psychiatry. 2011;72(suppl 1):4-8.
9. Tandon R, Gaebel W, Barch DM, et al. Definition and description of schizophrenia in the DSM-5. Schizophr Res. 2013;150(1):3-10.
10. Malaspina D, Owens MJ, Heckers S, et al. Schizoaffective disorder in the DSM-5. Schizophr Res. 2013;150(1):21-25.
11. Tandon R, Heckers S, Bustillo J, et al. Catatonia in DSM-5. Schizophr Res. 2013;150(1):26-30.
Just as psychiatrists are adapting to DSM-5, they have to cope with implementation of the 10th edition of the International Statistical Classification of Diseases and Related Health Problems (ICD-10). This challenge raises questions: What is the importance of understanding ICD-10? How will it affect the practice of psychiatry?
Furthermore, how does ICD-10 relate to DSM-5 and Current Procedural Terminology (CPT)? How does it differ from ICD-9? What are the ICD-10-Clinical Modification (CM) and ICD-10-Procedures (PCS)?Learning the essence of the changes, and understanding what impact they have on your clinical work, are necessary to ensure that your practice keeps pace with professional and legal standards of care. The effort involved is not onerous, however, and can improve the quality and efficiency of your care and how you document it.
In this article, we provide you with an overview of ICD-10; highlight major changes of the new classification; explain its relevance to clinical practice; and offer guidelines for implementing it effectively. We also emphasize that a good understanding of DSM-5 facilitates appreciation of ICD-10 and makes its implementation fairly easy and straightforward.
To begin, we provide a glossary of ICD-related terms and a review of additional definitions, distinctions, and dates (Box).1-6
Major changes from ICD-9
No question: ICD-10 is going to significantly influence your practice and your reimbursement. Furthermore, a number of revisions in ICD-10 have the potential to meaningfully improve clinical documentation and communication and to enhance your ability to precisely describe the complexity of your patients—with implications for billing.
ICD-10 differs from ICD-9 in organization, structure, code composition, and level of detail. In addition, ICD-10 makes some changes in terminology and definitions, with the goal of improving precision.
ICD-10 also is much larger than ICD-9.The total number of medical diagnostic codes has increased more than 5-fold—from approximately 13,000 to 69,000. This expansion allows for greater specificity in diagnosis and enables differentiation of an initial clinical encounter from a subsequent encounter.
To accommodate the expansion in the number of codes, the 5-digit numeric codes used in ICD-9 have been replaced in ICD-10 by 7-digit alphanumeric codes:
- the first digit always is a letter
- the second and third digits are numbers followed by a decimal point
- the fourth though seventh digits can be letters or numbers
- the first 3 digits denote the diagnostic category
- the fourth through sixth digits provide diagnostic detail
- the seventh digit provides information about the nature of the encounter (eg, initial, subsequent, or sequel, denoted respectively by “A,” “D,” and “S” in the seventh digit).
The number of 3-digit categories for psychiatric disorders has increased from 30 in ICD-9 (290-319) to 100 in ICD-10 (F00-F99). Only the first 5 digits are used for the section on mental disorders in ICD-10, with the first digit always “F” and the second digit a number denoting the broad type of disorders. The second and third digits in conjunction define the major category of the disorder; the fourth and fifth digits provide additional descriptive detail about the disorder (Table).
ICD-9 ‘V’ codes are out
What were called “V” codes in ICD-9—factors that influence health status and contact with health services—have been replaced by “Z” codes in ICD-10. These “Z” codes provide greater detail and precision than “V” codes provided.
Examples of “Z” codes relevant to psychiatry are:
Z00 General psychiatric examination (eg, of a person who does not have a complaint or diagnosis)
Z03 Examination for suspected mental and behavioral disorder
Z04 Examination for medicolegal or other purposes; Z04.8 is relevant laboratory testing, such as drug testing of urine or blood
Z50 Care involving rehabilitation (substance use disorder, etc.)
Z60 Problem related to social environment
Z61 Problem related to negative life events in childhood
Z63 Problem related to primary support group, including family circumstances
Z64-Z65 Problem related to other psychosocial circumstances
Z70-Z71 Condition requiring counseling, not elsewhere classified
Z73 Problem related to difficulty with life management (burnout, stress, role conflict, etc.)
Z75 Problem related to medical facilities and other aspects of health care (eg, awaiting admission)
Z81 Family history of mental or behavioral disorders
Z85-Z91 Personal history of various disorders (must be absent or in full remission at the moment); Z86.51, for example, refers to a history of combat and operational stress reaction.
Greater precision is now possible when coding for treatment-related adverse effects. A particular adverse effect now is coded under the relevant system, along with its attribution to the specific substance. Obesity attributable to antipsychotic treatment,7,8 for example, is coded as E66.1.
Integrating DSM-5 and ICD-10
Because DSM-5 lists corresponding ICD-10-CM codes for all disorders, you will find it much easier than other physicians to implement ICD-10. DSM-5 includes ICD-9-CM and ICD-10-CM codes for each DSM-5 disorder (for example, the ICD-9-CM code for schizophrenia is 295.x; the ICD-10-CM code is F20.9).9
Furthermore, a number of changes from ICD-9-CM to ICD-10-CM enable documentation of greater diagnostic specificity; for example, DSM-5 schizoaffective disorder, bipolar type, and schizoaffective disorder, depressive type, are distinctly coded as F25.0 and F25.1, respectively, in ICD-10-CM, whereas both were coded as 295.7 in ICD-9-CM.10
You will continue to use DSM-5 criteria to guide your diagnostic process, translating the DSM-5 diagnosis (diagnoses) into corresponding ICD-10-CM codes. Experience with DSM-5 substantially simplifies the transition to ICD-10.
Key differences between DSM-5 and ICD-10
There are notable differences in organization and content between DSM-5 and ICD-10.
The 20 chapters in DSM-5 begin with neurodevelopmental disorders; neurocognitive disorders are toward the end (ie, childhood to late life). In contrast, neurocognitive disorders (ie, “dementia”) appear at the beginning of ICD-10; neurodevelopmental disorders are at the end.
Elimination of schizophrenia subtypes in DSM-5 necessitates coding of all schizophrenia as F20.9 in ICD-10-CM because F20.0-F20.8 are specific subtypes. DSM-5 schizophreniform disorder is coded F20.81.
Substance abuse and substance dependence continue to be separate in ICD-10-CM, but they are combined in a single category of substance use disorders in DSM-5. The correct ICD-10-CM code (ie, abuse vs dependence) is determined by the severity of the substance use disorder: “Mild” coding as abuse (F1x.1) and “moderate” and “severe” coding as dependence (F2x.2), with x denoting the substance abused.
There can be multiple applicable diagnoses associated with a clinical encounter, as there was with ICD-9-CM. Give precedence to the diagnosis that best represents the nature of the presenting problem; list other diagnoses in the order of their relevance. DSM-5 and ICD-10-CM are similar in this regard.
ICD-10-CM uses only subtypes, in contrast to the use of subtypes and specifiers in DSM-5 to describe variability in disorders across patients. It is possible, however, to code certain DSM-5 specifiers in ICD-10-CM. (This is discussed in the “Recording Procedures” section of the DSM-5 text and summarized at the beginning of the manual, and appears in the “Appendix.”) To code the catatonia specifier in the context of schizoaffective disorder, depressive type, for example, use ICD-10-CM code F25.1 for the disorder and add code F06.1 for the catatonia specifier.11
How will ICD-10 affect your practice?
As of October 1, 2015, all health care facilities were to have become ICD-10 compliant. Furthermore, any Health Insurance Portability and Accountability Act-covered entity must use ICD-10-CM codes if it expects to be reimbursed for health care services.
Mental health practitioners might think that the transition from ICD-9-CM to ICD-10-CM involves only billers and coders, not them. They are wrong. All clinicians are responsible for documenting their diagnostic and treatment services properly. Medical records must contain adequate information to support any diagnostic (ICD-10-CM) and treatment (CPT) codes that are applied to a given clinical encounter.
The greater detail and specificity that are provided by ICD-10-CM allow more accurate recording of clinical complexity, which, in turn, influences reimbursement. However, good documentation is necessary for proper coding. Because clinicians are ultimately responsible for proper diagnostic coding, good understanding of ICD-10-CM is essential to be able to code properly.
Similar to the expansion of ICD-10-CM (from volumes 1 and 2 of ICD-9-CM), ICD-10-PCS has undergone similar expansion (from volume 3 of ICD-9-CM), with a corresponding increase in specificity. For example, there are now 5 distinct codes for electroconvulsive therapy (GZB0ZZZ-GZB4ZZZ) that distinguish unilateral from bilateral electrode placement and single from multiple stimulations.
DSM-5 will continue to be the frameworkfor psychiatric assessment and diagnosis. ICD-10-CM will be the coding system to accurately denote DSM-5 diagnoses. The Centers for Medicare and Medicaid Services (CMS) and the National Center for Health Statistics recognize DSM-5 as the means to identify proper ICD-10-CM codes for mental disorders. CMS also has announced that, although ICD-10-CM codes are necessary for reimbursement, use of an incorrect code will not be the basis for denying a Medicare claim for 1 year.
Making ICD-10 part of practice
Here are several keys to implementing ICD-10 with minimum pain and maximum benefit.
Multiple diagnosis codes should be listed in the order of their relevance to the clinical encounter.
Visit type. The seventh character of the ICD-10-CM code denotes the type of visit (initial, subsequent, or sequela) and must be provided:
- An initial encounter is one in which the patient first receives active treatment.
- A subsequent encounter refers to a follow-up visit in which the patient receives routine care during the healing or recovery phase.
- A sequel encounter is one in which a patient receives treatment for complications or conditions that arise as a direct result of the initial condition.
The transition to ICD-10 should be facilitated by adoption of DSM-5. Continue using DSM-5 to determine the correct diagnosis or diagnoses of the mental disorder, then apply the corresponding ICD-10-CM code(s). The better you understand and apply DSM-5, the more precise you can be in utilizing the greater specificity and accuracy afforded by ICD-10-CM coding.
Document well. Good understanding of the structure and organization of ICD-10-CM facilitates efficient, comprehensive documentation. This, in turn, will foster better clinical communication and appropriate reimbursement.
Know your payers—in particular, their policies regarding differential reimbursement for clinical complexity (based on ICD-10-CM/PCS). Medical practices that are part of an accountable care organization, and those that have risk-adjusted contracts must pay special attention to documenting clinical complexity when coding.
Know your electronic health care record, understand what tools it offers to efficiently translate DSM-5 diagnoses into appropriate ICD-10-CM codes, and use those tools efficiently.
Review your medical record documentation for the top 20 conditions in your practice, in the context of their definition in ICD-10-CM.
If you have coders who do ICD-10-CM coding for you, review a few patient charts with them to compare your sense of the patient’s clinical complexity and their coding based on your documentation.
Changes in DSM-5 have encouraged clinicians to improve their assessment of patients and provide measurement-based care. The significant changes in ICD-10-CM should provide the impetus for you to hone your ability to provide documentation. Sufficient flexibility exists within guidelines to permit individualization of the style of documentation.
Because all DSM-5 diagnoses map to appropriate ICD-10-CM codes, effective use of DSM-5 should make the transition to ICD-10 easy.
Bottom Line
Compared with ICD-9, definitions of mental health diagnoses have been improved in ICD-10, and more elaborate code descriptions in ICD-10-CM provide for greater precision when you report a diagnosis. The result? More accurate and efficient documentation of the care you provide and better reimbursement. Understanding what impact the changes in ICD-10 will have on your clinical work will ensure that your practice keeps pace with professional and legal standards of care.
Related Resources
• Blue Cross Blue Shield of Michigan ICD-10 update: mental and behavioral health ICD-10-CM codes. http://www.bcbsm.com/content/dam/public/Providers/Documents/help/faqs/icd10-update-mentalhealth.pdf.
• American Psychiatric Association ICD-10 tutorial. http://www.psychiatry.org/psychiatrists/practice/dsm/icd-10.
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
Just as psychiatrists are adapting to DSM-5, they have to cope with implementation of the 10th edition of the International Statistical Classification of Diseases and Related Health Problems (ICD-10). This challenge raises questions: What is the importance of understanding ICD-10? How will it affect the practice of psychiatry?
Furthermore, how does ICD-10 relate to DSM-5 and Current Procedural Terminology (CPT)? How does it differ from ICD-9? What are the ICD-10-Clinical Modification (CM) and ICD-10-Procedures (PCS)?Learning the essence of the changes, and understanding what impact they have on your clinical work, are necessary to ensure that your practice keeps pace with professional and legal standards of care. The effort involved is not onerous, however, and can improve the quality and efficiency of your care and how you document it.
In this article, we provide you with an overview of ICD-10; highlight major changes of the new classification; explain its relevance to clinical practice; and offer guidelines for implementing it effectively. We also emphasize that a good understanding of DSM-5 facilitates appreciation of ICD-10 and makes its implementation fairly easy and straightforward.
To begin, we provide a glossary of ICD-related terms and a review of additional definitions, distinctions, and dates (Box).1-6
Major changes from ICD-9
No question: ICD-10 is going to significantly influence your practice and your reimbursement. Furthermore, a number of revisions in ICD-10 have the potential to meaningfully improve clinical documentation and communication and to enhance your ability to precisely describe the complexity of your patients—with implications for billing.
ICD-10 differs from ICD-9 in organization, structure, code composition, and level of detail. In addition, ICD-10 makes some changes in terminology and definitions, with the goal of improving precision.
ICD-10 also is much larger than ICD-9.The total number of medical diagnostic codes has increased more than 5-fold—from approximately 13,000 to 69,000. This expansion allows for greater specificity in diagnosis and enables differentiation of an initial clinical encounter from a subsequent encounter.
To accommodate the expansion in the number of codes, the 5-digit numeric codes used in ICD-9 have been replaced in ICD-10 by 7-digit alphanumeric codes:
- the first digit always is a letter
- the second and third digits are numbers followed by a decimal point
- the fourth though seventh digits can be letters or numbers
- the first 3 digits denote the diagnostic category
- the fourth through sixth digits provide diagnostic detail
- the seventh digit provides information about the nature of the encounter (eg, initial, subsequent, or sequel, denoted respectively by “A,” “D,” and “S” in the seventh digit).
The number of 3-digit categories for psychiatric disorders has increased from 30 in ICD-9 (290-319) to 100 in ICD-10 (F00-F99). Only the first 5 digits are used for the section on mental disorders in ICD-10, with the first digit always “F” and the second digit a number denoting the broad type of disorders. The second and third digits in conjunction define the major category of the disorder; the fourth and fifth digits provide additional descriptive detail about the disorder (Table).
ICD-9 ‘V’ codes are out
What were called “V” codes in ICD-9—factors that influence health status and contact with health services—have been replaced by “Z” codes in ICD-10. These “Z” codes provide greater detail and precision than “V” codes provided.
Examples of “Z” codes relevant to psychiatry are:
Z00 General psychiatric examination (eg, of a person who does not have a complaint or diagnosis)
Z03 Examination for suspected mental and behavioral disorder
Z04 Examination for medicolegal or other purposes; Z04.8 is relevant laboratory testing, such as drug testing of urine or blood
Z50 Care involving rehabilitation (substance use disorder, etc.)
Z60 Problem related to social environment
Z61 Problem related to negative life events in childhood
Z63 Problem related to primary support group, including family circumstances
Z64-Z65 Problem related to other psychosocial circumstances
Z70-Z71 Condition requiring counseling, not elsewhere classified
Z73 Problem related to difficulty with life management (burnout, stress, role conflict, etc.)
Z75 Problem related to medical facilities and other aspects of health care (eg, awaiting admission)
Z81 Family history of mental or behavioral disorders
Z85-Z91 Personal history of various disorders (must be absent or in full remission at the moment); Z86.51, for example, refers to a history of combat and operational stress reaction.
Greater precision is now possible when coding for treatment-related adverse effects. A particular adverse effect now is coded under the relevant system, along with its attribution to the specific substance. Obesity attributable to antipsychotic treatment,7,8 for example, is coded as E66.1.
Integrating DSM-5 and ICD-10
Because DSM-5 lists corresponding ICD-10-CM codes for all disorders, you will find it much easier than other physicians to implement ICD-10. DSM-5 includes ICD-9-CM and ICD-10-CM codes for each DSM-5 disorder (for example, the ICD-9-CM code for schizophrenia is 295.x; the ICD-10-CM code is F20.9).9
Furthermore, a number of changes from ICD-9-CM to ICD-10-CM enable documentation of greater diagnostic specificity; for example, DSM-5 schizoaffective disorder, bipolar type, and schizoaffective disorder, depressive type, are distinctly coded as F25.0 and F25.1, respectively, in ICD-10-CM, whereas both were coded as 295.7 in ICD-9-CM.10
You will continue to use DSM-5 criteria to guide your diagnostic process, translating the DSM-5 diagnosis (diagnoses) into corresponding ICD-10-CM codes. Experience with DSM-5 substantially simplifies the transition to ICD-10.
Key differences between DSM-5 and ICD-10
There are notable differences in organization and content between DSM-5 and ICD-10.
The 20 chapters in DSM-5 begin with neurodevelopmental disorders; neurocognitive disorders are toward the end (ie, childhood to late life). In contrast, neurocognitive disorders (ie, “dementia”) appear at the beginning of ICD-10; neurodevelopmental disorders are at the end.
Elimination of schizophrenia subtypes in DSM-5 necessitates coding of all schizophrenia as F20.9 in ICD-10-CM because F20.0-F20.8 are specific subtypes. DSM-5 schizophreniform disorder is coded F20.81.
Substance abuse and substance dependence continue to be separate in ICD-10-CM, but they are combined in a single category of substance use disorders in DSM-5. The correct ICD-10-CM code (ie, abuse vs dependence) is determined by the severity of the substance use disorder: “Mild” coding as abuse (F1x.1) and “moderate” and “severe” coding as dependence (F2x.2), with x denoting the substance abused.
There can be multiple applicable diagnoses associated with a clinical encounter, as there was with ICD-9-CM. Give precedence to the diagnosis that best represents the nature of the presenting problem; list other diagnoses in the order of their relevance. DSM-5 and ICD-10-CM are similar in this regard.
ICD-10-CM uses only subtypes, in contrast to the use of subtypes and specifiers in DSM-5 to describe variability in disorders across patients. It is possible, however, to code certain DSM-5 specifiers in ICD-10-CM. (This is discussed in the “Recording Procedures” section of the DSM-5 text and summarized at the beginning of the manual, and appears in the “Appendix.”) To code the catatonia specifier in the context of schizoaffective disorder, depressive type, for example, use ICD-10-CM code F25.1 for the disorder and add code F06.1 for the catatonia specifier.11
How will ICD-10 affect your practice?
As of October 1, 2015, all health care facilities were to have become ICD-10 compliant. Furthermore, any Health Insurance Portability and Accountability Act-covered entity must use ICD-10-CM codes if it expects to be reimbursed for health care services.
Mental health practitioners might think that the transition from ICD-9-CM to ICD-10-CM involves only billers and coders, not them. They are wrong. All clinicians are responsible for documenting their diagnostic and treatment services properly. Medical records must contain adequate information to support any diagnostic (ICD-10-CM) and treatment (CPT) codes that are applied to a given clinical encounter.
The greater detail and specificity that are provided by ICD-10-CM allow more accurate recording of clinical complexity, which, in turn, influences reimbursement. However, good documentation is necessary for proper coding. Because clinicians are ultimately responsible for proper diagnostic coding, good understanding of ICD-10-CM is essential to be able to code properly.
Similar to the expansion of ICD-10-CM (from volumes 1 and 2 of ICD-9-CM), ICD-10-PCS has undergone similar expansion (from volume 3 of ICD-9-CM), with a corresponding increase in specificity. For example, there are now 5 distinct codes for electroconvulsive therapy (GZB0ZZZ-GZB4ZZZ) that distinguish unilateral from bilateral electrode placement and single from multiple stimulations.
DSM-5 will continue to be the frameworkfor psychiatric assessment and diagnosis. ICD-10-CM will be the coding system to accurately denote DSM-5 diagnoses. The Centers for Medicare and Medicaid Services (CMS) and the National Center for Health Statistics recognize DSM-5 as the means to identify proper ICD-10-CM codes for mental disorders. CMS also has announced that, although ICD-10-CM codes are necessary for reimbursement, use of an incorrect code will not be the basis for denying a Medicare claim for 1 year.
Making ICD-10 part of practice
Here are several keys to implementing ICD-10 with minimum pain and maximum benefit.
Multiple diagnosis codes should be listed in the order of their relevance to the clinical encounter.
Visit type. The seventh character of the ICD-10-CM code denotes the type of visit (initial, subsequent, or sequela) and must be provided:
- An initial encounter is one in which the patient first receives active treatment.
- A subsequent encounter refers to a follow-up visit in which the patient receives routine care during the healing or recovery phase.
- A sequel encounter is one in which a patient receives treatment for complications or conditions that arise as a direct result of the initial condition.
The transition to ICD-10 should be facilitated by adoption of DSM-5. Continue using DSM-5 to determine the correct diagnosis or diagnoses of the mental disorder, then apply the corresponding ICD-10-CM code(s). The better you understand and apply DSM-5, the more precise you can be in utilizing the greater specificity and accuracy afforded by ICD-10-CM coding.
Document well. Good understanding of the structure and organization of ICD-10-CM facilitates efficient, comprehensive documentation. This, in turn, will foster better clinical communication and appropriate reimbursement.
Know your payers—in particular, their policies regarding differential reimbursement for clinical complexity (based on ICD-10-CM/PCS). Medical practices that are part of an accountable care organization, and those that have risk-adjusted contracts must pay special attention to documenting clinical complexity when coding.
Know your electronic health care record, understand what tools it offers to efficiently translate DSM-5 diagnoses into appropriate ICD-10-CM codes, and use those tools efficiently.
Review your medical record documentation for the top 20 conditions in your practice, in the context of their definition in ICD-10-CM.
If you have coders who do ICD-10-CM coding for you, review a few patient charts with them to compare your sense of the patient’s clinical complexity and their coding based on your documentation.
Changes in DSM-5 have encouraged clinicians to improve their assessment of patients and provide measurement-based care. The significant changes in ICD-10-CM should provide the impetus for you to hone your ability to provide documentation. Sufficient flexibility exists within guidelines to permit individualization of the style of documentation.
Because all DSM-5 diagnoses map to appropriate ICD-10-CM codes, effective use of DSM-5 should make the transition to ICD-10 easy.
Bottom Line
Compared with ICD-9, definitions of mental health diagnoses have been improved in ICD-10, and more elaborate code descriptions in ICD-10-CM provide for greater precision when you report a diagnosis. The result? More accurate and efficient documentation of the care you provide and better reimbursement. Understanding what impact the changes in ICD-10 will have on your clinical work will ensure that your practice keeps pace with professional and legal standards of care.
Related Resources
• Blue Cross Blue Shield of Michigan ICD-10 update: mental and behavioral health ICD-10-CM codes. http://www.bcbsm.com/content/dam/public/Providers/Documents/help/faqs/icd10-update-mentalhealth.pdf.
• American Psychiatric Association ICD-10 tutorial. http://www.psychiatry.org/psychiatrists/practice/dsm/icd-10.
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Diagnostic and statistical manual of mental disorders, 5th edition. Washington DC: American Psychiatric Association; 2013.
2. World Health Organization. The ICD-10 classification of mental and behavioral disorders: clinical descriptions and diagnostic guidelines. Geneva, Switzerland: World Health Organization; 1992.
3. American Medical Association. ICD-10-CM 2016: the complete official code set. Chicago, IL: American Medical Association; 2015.
4. American Medical Association. CPT-2016, professional edition. Chicago, IL: American Medical Association; 2015.
5. American Medical Association. ICD-10-CM expert for physicians 2016: the complete official code set. Chicago, IL: American Medical Association; 2015.
6. American Medical Association. ICD-10-PCS mapping to ICD-9-CM volume 3. Chicago, IL: American Medical Association; 2015.
7. Tandon R, Halbreich U. The second-generation ‘atypical’ antipsychotics: similar efficacy but different neuroendocrine side-effects. Psychoneuroendocrinology. 2003;28(suppl 1):1-7.
8. Tandon R. Antipsychotics in the treatment of schizophrenia: an overview. J Clin Psychiatry. 2011;72(suppl 1):4-8.
9. Tandon R, Gaebel W, Barch DM, et al. Definition and description of schizophrenia in the DSM-5. Schizophr Res. 2013;150(1):3-10.
10. Malaspina D, Owens MJ, Heckers S, et al. Schizoaffective disorder in the DSM-5. Schizophr Res. 2013;150(1):21-25.
11. Tandon R, Heckers S, Bustillo J, et al. Catatonia in DSM-5. Schizophr Res. 2013;150(1):26-30.
1. Diagnostic and statistical manual of mental disorders, 5th edition. Washington DC: American Psychiatric Association; 2013.
2. World Health Organization. The ICD-10 classification of mental and behavioral disorders: clinical descriptions and diagnostic guidelines. Geneva, Switzerland: World Health Organization; 1992.
3. American Medical Association. ICD-10-CM 2016: the complete official code set. Chicago, IL: American Medical Association; 2015.
4. American Medical Association. CPT-2016, professional edition. Chicago, IL: American Medical Association; 2015.
5. American Medical Association. ICD-10-CM expert for physicians 2016: the complete official code set. Chicago, IL: American Medical Association; 2015.
6. American Medical Association. ICD-10-PCS mapping to ICD-9-CM volume 3. Chicago, IL: American Medical Association; 2015.
7. Tandon R, Halbreich U. The second-generation ‘atypical’ antipsychotics: similar efficacy but different neuroendocrine side-effects. Psychoneuroendocrinology. 2003;28(suppl 1):1-7.
8. Tandon R. Antipsychotics in the treatment of schizophrenia: an overview. J Clin Psychiatry. 2011;72(suppl 1):4-8.
9. Tandon R, Gaebel W, Barch DM, et al. Definition and description of schizophrenia in the DSM-5. Schizophr Res. 2013;150(1):3-10.
10. Malaspina D, Owens MJ, Heckers S, et al. Schizoaffective disorder in the DSM-5. Schizophr Res. 2013;150(1):21-25.
11. Tandon R, Heckers S, Bustillo J, et al. Catatonia in DSM-5. Schizophr Res. 2013;150(1):26-30.
Getting ready for DSM-5: Psychotic disorders
Discuss this article at www.facebook.com/CurrentPsychiatry
In DSM-IV,1 the section on schizophrenia and other psychotic disorders includes schizophrenia (with 5 subtypes), schizophreniform disorder, schizoaffective disorder, delusional disorder, shared psychotic disorder, brief psychotic disorder, substance-induced psychotic disorder, psychotic disorder due to a general medical condition, and psychotic disorder not otherwise specified. As we consider proposed changes to DSM-5 (Table 1),2 it is useful to consider limitations in our current construct of schizophrenia.
Table 1
Psychotic disorders in DSM-5: Summary of proposed changes
| Replace existing subtypes with dimensions |
| Include diagnosis of attenuated psychosis syndrome |
| Modify criteria for schizoaffective disorder |
| ‘Delink’ catatonia from schizophrenia |
| Source: Reference 2 |
First, many etiological factors and pathophysiological processes appear relevant to what we consider schizophrenia and it is almost certain that our construct of schizophrenia encompasses not one but numerous diseases with a shared phenotype.3-5
Second, the boundary between schizophrenia and schizoaffective disorder is imprecisely defined, and a proportion of patients with schizophrenia with some mood symptoms may inappropriately receive a schizoaffective disorder diagnosis. This is compounded by the poor reliability and low diagnostic stability of a schizoaffective disorder diagnosis.6-8
Third, the current classic schizophrenia subtypes provide an inadequate description of the enormous heterogeneity of this condition. Additionally, subtype stability is low, and only the paranoid and undifferentiated subtypes are used frequently in clinical practice.
Fourth, the prominence given to Schneiderian first-rank symptoms (“bizarre” delusions or “special” hallucinations) appears misplaced.
Fifth, the current construct of schizophrenia inadequately describes the major psychopathological dimensions of the condition and stages of its evolution.8,9
Finally, the current clinical construct of schizophrenia does not match neurobiological markers and genetic findings or specific pharmacological treatment provided.5,10 Proposed DSM-5 revisions2 to the definition of schizophrenia to address these limitations are summarized below.
Schizophrenia syndrome
Proposed changes to the diagnostic criteria for schizophrenia are modest and continuity with DSM-IV is broadly maintained. Two modest changes to criterion A (active phase symptoms) are proposed:
- Eliminate special treatment of bizarre delusions and other Schneiderian first-rank symptoms. In DSM-IV, only 1 criterion A is required if it is a bizarre delusion or hallucination. Because Schneiderian first-rank symptoms do not have diagnostic specificity and diagnosing “bizarreness” of delusions and hallucinations has low reliability, it is proposed that these positive symptoms be treated like any other with regard to their diagnostic implications.
- Require that at least 1 of the 2 symptoms required to meet criterion A be delusions, hallucinations, or disorganized thinking. These are core positive symptoms diagnosed with high reliability and might reasonably be considered necessary for a reliable schizophrenia diagnosis.
Subtypes
The DSM-5 proposal for describing schizophrenia advocates eliminating DSM-IV schizophrenia subtypes. These subtypes have limited diagnostic stability, low reliability, and poor validity. Furthermore, except for the paranoid and undifferentiated subtypes, other subtypes rarely are used in most mental health care systems.
Schizoaffective disorder
Characterizing patients with both psychotic and mood symptoms either concurrently or at different points during their illness always has been controversial. In DSM-I and DSM-II, a diagnosis of schizophrenia, schizoaffective subtype, generally was recommended for such patients. DSM-III reversed this recommendation and specified that schizophrenia was to be diagnosed only in the absence of prominent mood symptoms. Furthermore, in DSM-III, diagnosing schizoaffective disorder was strongly discouraged, and it was the only condition in DSM-III without operational criteria. Schizoaffective disorder saw a revival in DSM-III-R that has continued through DSM-IV. In fact, in many mental health care systems, almost one-third of patients with psychotic symptoms receive a schizoaffective disorder diagnosis. One of the insidious changes to the definition of schizoaffective disorder from DSM-III to DSM-IV is that it moved from being a lifetime diagnosis to a cross-sectional diagnosis—ie, in DSM-IV, only mood/psychotic symptoms in the current episode are considered, and the longitudinal course of these symptoms in the patient’s life are ignored. The current DSM-5 proposal attempts to improve reliability of this diagnosis by providing more specific criteria and is reconceptualizing schizoaffective disorder as a longitudinal diagnosis. To this end, the most significant proposed change is to criterion C of schizoaffective disorder, which attempts to demarcate schizoaffective disorder from schizophrenia with prominent mood symptoms. Criterion C will be revised to state “symptoms that meet criteria for a mood episode are present for a majority (>50%) of the total duration of the active and residual periods of the illness.”2
Psychopathological dimensions
Schizophrenic illness is characterized by several psychopathological domains, with a distinctive course, patterns of treatment-response, and prognostic implications. The relative severity of symptom dimensions—positive, negative, mood, disorganization, motor, and cognitive—vary among patients and also within patients at different stages of their illness. Measuring the relative severity of these symptom dimensions throughout the illness course can provide clinicians with useful information about the nature of a patient’s schizophrenic illness and the specific impact of treatment on different aspects of his or her illness (Table 2). In addition to being clinically useful, dimensional measurement also should improve schizophrenia research because having dimensional information will permit studies on etiology and pathogenesis that cut across current diagnostic categories. Although field trials are evaluating 9 dimensions—delusions, hallucinations, disorganization, depression, mania, cognitive impairment, restricted emotional expression, avolition, and psychomotor—it is likely that fewer dimensions will be recommended for DSM-5, based on reliability results of these trials, clinical utility, and logistic feasibility in routine clinical settings.
Table 2
Goals of a dimensional approach to schizophrenia
| Better understanding of schizophrenia |
| Distinct dimensions of illness |
| Distinct stages of illness |
| Elucidation of neurobiology |
| More precise delineation of etiology |
| More refined treatment development |
| Direction at specific dimension-endophenotype |
| Stage-specific treatment |
| Novel treatment targets |
Attenuated psychosis syndrome
Some clinicians and researchers believe that many patients with schizophrenia experience unsatisfactory outcomes because we identify the illness and initiate treatment after substantial brain tissue damage has occurred. Introducing attenuated psychosis syndrome will support clinicians’ efforts to recognize mild psychotic symptoms early in their evolution and to monitor—and if necessary, intervene—during these crucial early stages. Risks include possible stigma and inappropriate use of medications and other treatments. This controversial proposal is being field tested. It is unclear if this category will be included in DSM-5 and if it does, whether it will be in the main text or the appendix.
Catatonia
Catatonia will be used as a specifier for various psychotic disorders, major mood disorders, and associated with a general medical condition. Additionally, the same criteria will be used to diagnose catatonia across DSM-5. Catatonia Not Elsewhere Classified might be added as a residual category for other conditions in which a clear catatonic syndrome is present and the parent disorder has not yet been identified.2
Other psychotic disorders
Relatively minor changes are proposed in criteria for other disorders in this section. There are likely to be changes in the text, however, that incorporate new information about these conditions generated since publication of DSM-IV-TR in 2000. Some proposed changes include:
- deleting shared delusional disorder (folie à deux) as a separate diagnosis and instead characterizing it as a specifier for delusional disorder
- clarifying the distinction between substance-induced psychotic disorder and other psychotic disorders accompanied by comorbid substance use.
Current status of DSM-5
Field trials are being completed and their results remain to be analyzed. Major changes being evaluated in the field trials include:
- the impact of the change in concept and criteria for schizoaffective disorder
- the addition of a series of psychopathology dimensions
- the impact of adding attenuated psychosis syndrome as a new class.
Changes proposed by the Psychosis Disorders Work Group are intended to increase clinical utility (fewer diagnoses, better demarcation between disorders, greater treatment relevance [dimensions]) and modestly improve validity (more consistent with current information about the nature of various psychotic disorders), while retaining reliability in diagnosing various psychotic disorders (and improving it for schizoaffective disorder). Proposed changes are modest by and large but hope to set a better stage for a future etiopathophysiological classification.
The Psychosis Disorders Work Group’s recommendations are posted on the DSM-5 Web site2 at www.dsm5.org and are being reviewed by 2 expert committees established by the American Psychiatric Association Board of Trustees: a Scientific Review Committee and a Clinical and Public Health Implications Committee. Based on the results of the field trials, ongoing reviews, and other emerging data and discussions, additional changes to the current DSM-5 proposals may occur. DSM-5 is likely to be finalized in early 2013 and the published manual will be released in May 2013.
Related Resources
- American Psychiatric Association. DSM-5 Development. www.dsm5.org.
- Woods SW, McGlashan TH. The risk-benefit ratio of the proposed DSM-5 attenuated psychosis syndrome. Am J Psychiatry. 2011;168(12):1338.
Disclosure
Dr. Tandon is a member of the DSM-5 Psychotic Disorders Work Group. He is solely responsible for the content of this article.
1. Diagnostic and statistical manual of mental disorders, 4th ed. Washington, DC: American Psychiatric Association; 1994.
2. American Psychiatric Association. DSM-5 development. http://www.dsm5.org. Accessed March 19, 2011.
3. Tandon R, Keshavan MS, Nasrallah HA. Schizophrenia “just the facts”: what we know in 2008. Part 1: overview. Schizophr Res. 2008;100(1-3):4-19.
4. Tandon R, Keshavan MS, Nasrallah HA. Schizophrenia “just the facts” what we know in 2008. 2: epidemiology and etiology. Schizophr Res. 2008;102(1-3):1-18.
5. Keshavan MS, Tandon R, Boutros N, et al. Schizophrenia, “just the facts” what we know in 2008. Part 3: neurobiology. Schizophr Res. 2008;106(2-3):89-107.
6. Tandon R, Maj M. Nosological status and definition of schizophrenia. Some considerations for DSM-V and ICD-11. Asian Journal of Psychiatry. 2008;1(2):22-27.
7. Fiedorowicz JG, Epping EA, Flaum M. Toward defining schizophrenia as a more useful clinical construct. Curr Psychiatry Rep. 2008;10(4):344-351.
8. Tandon R, Nasrallah HA, Keshavan MS. Schizophrenia “just the facts” 4. Clinical features and conceptualization. Schizophr Res. 2009;110(1-3):1-23.
9. McGorry PD. Risk syndromes clinical staging, and DSM V: new diagnostic infrastructure for early intervention in psychiatry. Schizophr Res. 2010;120(1-3):49-53.
10. Tandon R, Nasrallah HA, Keshavan MS. Schizophrenia “just the facts” 5. Treatment and prevention. Past, present, and future. Schizophr Res. 2010;122(1-3):1-23.
Discuss this article at www.facebook.com/CurrentPsychiatry
In DSM-IV,1 the section on schizophrenia and other psychotic disorders includes schizophrenia (with 5 subtypes), schizophreniform disorder, schizoaffective disorder, delusional disorder, shared psychotic disorder, brief psychotic disorder, substance-induced psychotic disorder, psychotic disorder due to a general medical condition, and psychotic disorder not otherwise specified. As we consider proposed changes to DSM-5 (Table 1),2 it is useful to consider limitations in our current construct of schizophrenia.
Table 1
Psychotic disorders in DSM-5: Summary of proposed changes
| Replace existing subtypes with dimensions |
| Include diagnosis of attenuated psychosis syndrome |
| Modify criteria for schizoaffective disorder |
| ‘Delink’ catatonia from schizophrenia |
| Source: Reference 2 |
First, many etiological factors and pathophysiological processes appear relevant to what we consider schizophrenia and it is almost certain that our construct of schizophrenia encompasses not one but numerous diseases with a shared phenotype.3-5
Second, the boundary between schizophrenia and schizoaffective disorder is imprecisely defined, and a proportion of patients with schizophrenia with some mood symptoms may inappropriately receive a schizoaffective disorder diagnosis. This is compounded by the poor reliability and low diagnostic stability of a schizoaffective disorder diagnosis.6-8
Third, the current classic schizophrenia subtypes provide an inadequate description of the enormous heterogeneity of this condition. Additionally, subtype stability is low, and only the paranoid and undifferentiated subtypes are used frequently in clinical practice.
Fourth, the prominence given to Schneiderian first-rank symptoms (“bizarre” delusions or “special” hallucinations) appears misplaced.
Fifth, the current construct of schizophrenia inadequately describes the major psychopathological dimensions of the condition and stages of its evolution.8,9
Finally, the current clinical construct of schizophrenia does not match neurobiological markers and genetic findings or specific pharmacological treatment provided.5,10 Proposed DSM-5 revisions2 to the definition of schizophrenia to address these limitations are summarized below.
Schizophrenia syndrome
Proposed changes to the diagnostic criteria for schizophrenia are modest and continuity with DSM-IV is broadly maintained. Two modest changes to criterion A (active phase symptoms) are proposed:
- Eliminate special treatment of bizarre delusions and other Schneiderian first-rank symptoms. In DSM-IV, only 1 criterion A is required if it is a bizarre delusion or hallucination. Because Schneiderian first-rank symptoms do not have diagnostic specificity and diagnosing “bizarreness” of delusions and hallucinations has low reliability, it is proposed that these positive symptoms be treated like any other with regard to their diagnostic implications.
- Require that at least 1 of the 2 symptoms required to meet criterion A be delusions, hallucinations, or disorganized thinking. These are core positive symptoms diagnosed with high reliability and might reasonably be considered necessary for a reliable schizophrenia diagnosis.
Subtypes
The DSM-5 proposal for describing schizophrenia advocates eliminating DSM-IV schizophrenia subtypes. These subtypes have limited diagnostic stability, low reliability, and poor validity. Furthermore, except for the paranoid and undifferentiated subtypes, other subtypes rarely are used in most mental health care systems.
Schizoaffective disorder
Characterizing patients with both psychotic and mood symptoms either concurrently or at different points during their illness always has been controversial. In DSM-I and DSM-II, a diagnosis of schizophrenia, schizoaffective subtype, generally was recommended for such patients. DSM-III reversed this recommendation and specified that schizophrenia was to be diagnosed only in the absence of prominent mood symptoms. Furthermore, in DSM-III, diagnosing schizoaffective disorder was strongly discouraged, and it was the only condition in DSM-III without operational criteria. Schizoaffective disorder saw a revival in DSM-III-R that has continued through DSM-IV. In fact, in many mental health care systems, almost one-third of patients with psychotic symptoms receive a schizoaffective disorder diagnosis. One of the insidious changes to the definition of schizoaffective disorder from DSM-III to DSM-IV is that it moved from being a lifetime diagnosis to a cross-sectional diagnosis—ie, in DSM-IV, only mood/psychotic symptoms in the current episode are considered, and the longitudinal course of these symptoms in the patient’s life are ignored. The current DSM-5 proposal attempts to improve reliability of this diagnosis by providing more specific criteria and is reconceptualizing schizoaffective disorder as a longitudinal diagnosis. To this end, the most significant proposed change is to criterion C of schizoaffective disorder, which attempts to demarcate schizoaffective disorder from schizophrenia with prominent mood symptoms. Criterion C will be revised to state “symptoms that meet criteria for a mood episode are present for a majority (>50%) of the total duration of the active and residual periods of the illness.”2
Psychopathological dimensions
Schizophrenic illness is characterized by several psychopathological domains, with a distinctive course, patterns of treatment-response, and prognostic implications. The relative severity of symptom dimensions—positive, negative, mood, disorganization, motor, and cognitive—vary among patients and also within patients at different stages of their illness. Measuring the relative severity of these symptom dimensions throughout the illness course can provide clinicians with useful information about the nature of a patient’s schizophrenic illness and the specific impact of treatment on different aspects of his or her illness (Table 2). In addition to being clinically useful, dimensional measurement also should improve schizophrenia research because having dimensional information will permit studies on etiology and pathogenesis that cut across current diagnostic categories. Although field trials are evaluating 9 dimensions—delusions, hallucinations, disorganization, depression, mania, cognitive impairment, restricted emotional expression, avolition, and psychomotor—it is likely that fewer dimensions will be recommended for DSM-5, based on reliability results of these trials, clinical utility, and logistic feasibility in routine clinical settings.
Table 2
Goals of a dimensional approach to schizophrenia
| Better understanding of schizophrenia |
| Distinct dimensions of illness |
| Distinct stages of illness |
| Elucidation of neurobiology |
| More precise delineation of etiology |
| More refined treatment development |
| Direction at specific dimension-endophenotype |
| Stage-specific treatment |
| Novel treatment targets |
Attenuated psychosis syndrome
Some clinicians and researchers believe that many patients with schizophrenia experience unsatisfactory outcomes because we identify the illness and initiate treatment after substantial brain tissue damage has occurred. Introducing attenuated psychosis syndrome will support clinicians’ efforts to recognize mild psychotic symptoms early in their evolution and to monitor—and if necessary, intervene—during these crucial early stages. Risks include possible stigma and inappropriate use of medications and other treatments. This controversial proposal is being field tested. It is unclear if this category will be included in DSM-5 and if it does, whether it will be in the main text or the appendix.
Catatonia
Catatonia will be used as a specifier for various psychotic disorders, major mood disorders, and associated with a general medical condition. Additionally, the same criteria will be used to diagnose catatonia across DSM-5. Catatonia Not Elsewhere Classified might be added as a residual category for other conditions in which a clear catatonic syndrome is present and the parent disorder has not yet been identified.2
Other psychotic disorders
Relatively minor changes are proposed in criteria for other disorders in this section. There are likely to be changes in the text, however, that incorporate new information about these conditions generated since publication of DSM-IV-TR in 2000. Some proposed changes include:
- deleting shared delusional disorder (folie à deux) as a separate diagnosis and instead characterizing it as a specifier for delusional disorder
- clarifying the distinction between substance-induced psychotic disorder and other psychotic disorders accompanied by comorbid substance use.
Current status of DSM-5
Field trials are being completed and their results remain to be analyzed. Major changes being evaluated in the field trials include:
- the impact of the change in concept and criteria for schizoaffective disorder
- the addition of a series of psychopathology dimensions
- the impact of adding attenuated psychosis syndrome as a new class.
Changes proposed by the Psychosis Disorders Work Group are intended to increase clinical utility (fewer diagnoses, better demarcation between disorders, greater treatment relevance [dimensions]) and modestly improve validity (more consistent with current information about the nature of various psychotic disorders), while retaining reliability in diagnosing various psychotic disorders (and improving it for schizoaffective disorder). Proposed changes are modest by and large but hope to set a better stage for a future etiopathophysiological classification.
The Psychosis Disorders Work Group’s recommendations are posted on the DSM-5 Web site2 at www.dsm5.org and are being reviewed by 2 expert committees established by the American Psychiatric Association Board of Trustees: a Scientific Review Committee and a Clinical and Public Health Implications Committee. Based on the results of the field trials, ongoing reviews, and other emerging data and discussions, additional changes to the current DSM-5 proposals may occur. DSM-5 is likely to be finalized in early 2013 and the published manual will be released in May 2013.
Related Resources
- American Psychiatric Association. DSM-5 Development. www.dsm5.org.
- Woods SW, McGlashan TH. The risk-benefit ratio of the proposed DSM-5 attenuated psychosis syndrome. Am J Psychiatry. 2011;168(12):1338.
Disclosure
Dr. Tandon is a member of the DSM-5 Psychotic Disorders Work Group. He is solely responsible for the content of this article.
Discuss this article at www.facebook.com/CurrentPsychiatry
In DSM-IV,1 the section on schizophrenia and other psychotic disorders includes schizophrenia (with 5 subtypes), schizophreniform disorder, schizoaffective disorder, delusional disorder, shared psychotic disorder, brief psychotic disorder, substance-induced psychotic disorder, psychotic disorder due to a general medical condition, and psychotic disorder not otherwise specified. As we consider proposed changes to DSM-5 (Table 1),2 it is useful to consider limitations in our current construct of schizophrenia.
Table 1
Psychotic disorders in DSM-5: Summary of proposed changes
| Replace existing subtypes with dimensions |
| Include diagnosis of attenuated psychosis syndrome |
| Modify criteria for schizoaffective disorder |
| ‘Delink’ catatonia from schizophrenia |
| Source: Reference 2 |
First, many etiological factors and pathophysiological processes appear relevant to what we consider schizophrenia and it is almost certain that our construct of schizophrenia encompasses not one but numerous diseases with a shared phenotype.3-5
Second, the boundary between schizophrenia and schizoaffective disorder is imprecisely defined, and a proportion of patients with schizophrenia with some mood symptoms may inappropriately receive a schizoaffective disorder diagnosis. This is compounded by the poor reliability and low diagnostic stability of a schizoaffective disorder diagnosis.6-8
Third, the current classic schizophrenia subtypes provide an inadequate description of the enormous heterogeneity of this condition. Additionally, subtype stability is low, and only the paranoid and undifferentiated subtypes are used frequently in clinical practice.
Fourth, the prominence given to Schneiderian first-rank symptoms (“bizarre” delusions or “special” hallucinations) appears misplaced.
Fifth, the current construct of schizophrenia inadequately describes the major psychopathological dimensions of the condition and stages of its evolution.8,9
Finally, the current clinical construct of schizophrenia does not match neurobiological markers and genetic findings or specific pharmacological treatment provided.5,10 Proposed DSM-5 revisions2 to the definition of schizophrenia to address these limitations are summarized below.
Schizophrenia syndrome
Proposed changes to the diagnostic criteria for schizophrenia are modest and continuity with DSM-IV is broadly maintained. Two modest changes to criterion A (active phase symptoms) are proposed:
- Eliminate special treatment of bizarre delusions and other Schneiderian first-rank symptoms. In DSM-IV, only 1 criterion A is required if it is a bizarre delusion or hallucination. Because Schneiderian first-rank symptoms do not have diagnostic specificity and diagnosing “bizarreness” of delusions and hallucinations has low reliability, it is proposed that these positive symptoms be treated like any other with regard to their diagnostic implications.
- Require that at least 1 of the 2 symptoms required to meet criterion A be delusions, hallucinations, or disorganized thinking. These are core positive symptoms diagnosed with high reliability and might reasonably be considered necessary for a reliable schizophrenia diagnosis.
Subtypes
The DSM-5 proposal for describing schizophrenia advocates eliminating DSM-IV schizophrenia subtypes. These subtypes have limited diagnostic stability, low reliability, and poor validity. Furthermore, except for the paranoid and undifferentiated subtypes, other subtypes rarely are used in most mental health care systems.
Schizoaffective disorder
Characterizing patients with both psychotic and mood symptoms either concurrently or at different points during their illness always has been controversial. In DSM-I and DSM-II, a diagnosis of schizophrenia, schizoaffective subtype, generally was recommended for such patients. DSM-III reversed this recommendation and specified that schizophrenia was to be diagnosed only in the absence of prominent mood symptoms. Furthermore, in DSM-III, diagnosing schizoaffective disorder was strongly discouraged, and it was the only condition in DSM-III without operational criteria. Schizoaffective disorder saw a revival in DSM-III-R that has continued through DSM-IV. In fact, in many mental health care systems, almost one-third of patients with psychotic symptoms receive a schizoaffective disorder diagnosis. One of the insidious changes to the definition of schizoaffective disorder from DSM-III to DSM-IV is that it moved from being a lifetime diagnosis to a cross-sectional diagnosis—ie, in DSM-IV, only mood/psychotic symptoms in the current episode are considered, and the longitudinal course of these symptoms in the patient’s life are ignored. The current DSM-5 proposal attempts to improve reliability of this diagnosis by providing more specific criteria and is reconceptualizing schizoaffective disorder as a longitudinal diagnosis. To this end, the most significant proposed change is to criterion C of schizoaffective disorder, which attempts to demarcate schizoaffective disorder from schizophrenia with prominent mood symptoms. Criterion C will be revised to state “symptoms that meet criteria for a mood episode are present for a majority (>50%) of the total duration of the active and residual periods of the illness.”2
Psychopathological dimensions
Schizophrenic illness is characterized by several psychopathological domains, with a distinctive course, patterns of treatment-response, and prognostic implications. The relative severity of symptom dimensions—positive, negative, mood, disorganization, motor, and cognitive—vary among patients and also within patients at different stages of their illness. Measuring the relative severity of these symptom dimensions throughout the illness course can provide clinicians with useful information about the nature of a patient’s schizophrenic illness and the specific impact of treatment on different aspects of his or her illness (Table 2). In addition to being clinically useful, dimensional measurement also should improve schizophrenia research because having dimensional information will permit studies on etiology and pathogenesis that cut across current diagnostic categories. Although field trials are evaluating 9 dimensions—delusions, hallucinations, disorganization, depression, mania, cognitive impairment, restricted emotional expression, avolition, and psychomotor—it is likely that fewer dimensions will be recommended for DSM-5, based on reliability results of these trials, clinical utility, and logistic feasibility in routine clinical settings.
Table 2
Goals of a dimensional approach to schizophrenia
| Better understanding of schizophrenia |
| Distinct dimensions of illness |
| Distinct stages of illness |
| Elucidation of neurobiology |
| More precise delineation of etiology |
| More refined treatment development |
| Direction at specific dimension-endophenotype |
| Stage-specific treatment |
| Novel treatment targets |
Attenuated psychosis syndrome
Some clinicians and researchers believe that many patients with schizophrenia experience unsatisfactory outcomes because we identify the illness and initiate treatment after substantial brain tissue damage has occurred. Introducing attenuated psychosis syndrome will support clinicians’ efforts to recognize mild psychotic symptoms early in their evolution and to monitor—and if necessary, intervene—during these crucial early stages. Risks include possible stigma and inappropriate use of medications and other treatments. This controversial proposal is being field tested. It is unclear if this category will be included in DSM-5 and if it does, whether it will be in the main text or the appendix.
Catatonia
Catatonia will be used as a specifier for various psychotic disorders, major mood disorders, and associated with a general medical condition. Additionally, the same criteria will be used to diagnose catatonia across DSM-5. Catatonia Not Elsewhere Classified might be added as a residual category for other conditions in which a clear catatonic syndrome is present and the parent disorder has not yet been identified.2
Other psychotic disorders
Relatively minor changes are proposed in criteria for other disorders in this section. There are likely to be changes in the text, however, that incorporate new information about these conditions generated since publication of DSM-IV-TR in 2000. Some proposed changes include:
- deleting shared delusional disorder (folie à deux) as a separate diagnosis and instead characterizing it as a specifier for delusional disorder
- clarifying the distinction between substance-induced psychotic disorder and other psychotic disorders accompanied by comorbid substance use.
Current status of DSM-5
Field trials are being completed and their results remain to be analyzed. Major changes being evaluated in the field trials include:
- the impact of the change in concept and criteria for schizoaffective disorder
- the addition of a series of psychopathology dimensions
- the impact of adding attenuated psychosis syndrome as a new class.
Changes proposed by the Psychosis Disorders Work Group are intended to increase clinical utility (fewer diagnoses, better demarcation between disorders, greater treatment relevance [dimensions]) and modestly improve validity (more consistent with current information about the nature of various psychotic disorders), while retaining reliability in diagnosing various psychotic disorders (and improving it for schizoaffective disorder). Proposed changes are modest by and large but hope to set a better stage for a future etiopathophysiological classification.
The Psychosis Disorders Work Group’s recommendations are posted on the DSM-5 Web site2 at www.dsm5.org and are being reviewed by 2 expert committees established by the American Psychiatric Association Board of Trustees: a Scientific Review Committee and a Clinical and Public Health Implications Committee. Based on the results of the field trials, ongoing reviews, and other emerging data and discussions, additional changes to the current DSM-5 proposals may occur. DSM-5 is likely to be finalized in early 2013 and the published manual will be released in May 2013.
Related Resources
- American Psychiatric Association. DSM-5 Development. www.dsm5.org.
- Woods SW, McGlashan TH. The risk-benefit ratio of the proposed DSM-5 attenuated psychosis syndrome. Am J Psychiatry. 2011;168(12):1338.
Disclosure
Dr. Tandon is a member of the DSM-5 Psychotic Disorders Work Group. He is solely responsible for the content of this article.
1. Diagnostic and statistical manual of mental disorders, 4th ed. Washington, DC: American Psychiatric Association; 1994.
2. American Psychiatric Association. DSM-5 development. http://www.dsm5.org. Accessed March 19, 2011.
3. Tandon R, Keshavan MS, Nasrallah HA. Schizophrenia “just the facts”: what we know in 2008. Part 1: overview. Schizophr Res. 2008;100(1-3):4-19.
4. Tandon R, Keshavan MS, Nasrallah HA. Schizophrenia “just the facts” what we know in 2008. 2: epidemiology and etiology. Schizophr Res. 2008;102(1-3):1-18.
5. Keshavan MS, Tandon R, Boutros N, et al. Schizophrenia, “just the facts” what we know in 2008. Part 3: neurobiology. Schizophr Res. 2008;106(2-3):89-107.
6. Tandon R, Maj M. Nosological status and definition of schizophrenia. Some considerations for DSM-V and ICD-11. Asian Journal of Psychiatry. 2008;1(2):22-27.
7. Fiedorowicz JG, Epping EA, Flaum M. Toward defining schizophrenia as a more useful clinical construct. Curr Psychiatry Rep. 2008;10(4):344-351.
8. Tandon R, Nasrallah HA, Keshavan MS. Schizophrenia “just the facts” 4. Clinical features and conceptualization. Schizophr Res. 2009;110(1-3):1-23.
9. McGorry PD. Risk syndromes clinical staging, and DSM V: new diagnostic infrastructure for early intervention in psychiatry. Schizophr Res. 2010;120(1-3):49-53.
10. Tandon R, Nasrallah HA, Keshavan MS. Schizophrenia “just the facts” 5. Treatment and prevention. Past, present, and future. Schizophr Res. 2010;122(1-3):1-23.
1. Diagnostic and statistical manual of mental disorders, 4th ed. Washington, DC: American Psychiatric Association; 1994.
2. American Psychiatric Association. DSM-5 development. http://www.dsm5.org. Accessed March 19, 2011.
3. Tandon R, Keshavan MS, Nasrallah HA. Schizophrenia “just the facts”: what we know in 2008. Part 1: overview. Schizophr Res. 2008;100(1-3):4-19.
4. Tandon R, Keshavan MS, Nasrallah HA. Schizophrenia “just the facts” what we know in 2008. 2: epidemiology and etiology. Schizophr Res. 2008;102(1-3):1-18.
5. Keshavan MS, Tandon R, Boutros N, et al. Schizophrenia, “just the facts” what we know in 2008. Part 3: neurobiology. Schizophr Res. 2008;106(2-3):89-107.
6. Tandon R, Maj M. Nosological status and definition of schizophrenia. Some considerations for DSM-V and ICD-11. Asian Journal of Psychiatry. 2008;1(2):22-27.
7. Fiedorowicz JG, Epping EA, Flaum M. Toward defining schizophrenia as a more useful clinical construct. Curr Psychiatry Rep. 2008;10(4):344-351.
8. Tandon R, Nasrallah HA, Keshavan MS. Schizophrenia “just the facts” 4. Clinical features and conceptualization. Schizophr Res. 2009;110(1-3):1-23.
9. McGorry PD. Risk syndromes clinical staging, and DSM V: new diagnostic infrastructure for early intervention in psychiatry. Schizophr Res. 2010;120(1-3):49-53.
10. Tandon R, Nasrallah HA, Keshavan MS. Schizophrenia “just the facts” 5. Treatment and prevention. Past, present, and future. Schizophr Res. 2010;122(1-3):1-23.
Getting ready for DSM-5: Part 1
Discuss this article at www.facebook.com/CurrentPsychiatry
Work on the fifth edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-5)—scheduled to be published in May 2013—has been ongoing for more than a decade. Momentous advances in genetics and brain imaging since publication of DSM-IV in 1994 have generated optimism that an improved understanding of the neurobiologic underpinnings of psychiatric disorders might lead to a paradigm shift from the current descriptive classification system to a more scientific etiopathophysiological system similar to that used by other medical specialities.1
Some fear that any changes to our current classification system may be premature and could make an already complex system even more unwieldy.2 Scores of articles about the content and process of DSM-5 and several critiques and commentaries on the topic have been published. The American Psychiatric Association (APA) has made the DSM-5 process transparent by posting frequent updates to the DSM-5 Development Web site (www.dsm5.org), seeking feedback from the psychiatric community and the public, and presenting progress reports by members of the DSM-5 Task Force at scientific meetings.
There have been few discussions on the implications of DSM-5 from the practicing clinician’s vantage point, which I seek to present in this series of articles, the remainder of which will be published here, at CurrentPsychiatry.com. In this article, I:
- provide a brief history of psychiatric classification, focusing on the origins and evolution of the DSM system
- summarize the limitations of DSM-IV
- note the challenges and tensions in the construction of DSM-5
- review the DSM-5 process
- outline its current status
- discuss the organization and content of future articles in this series.
Although I am a member of the DSM-5 Psychotic Disorders Work Group, I am solely responsible for the content and any opinions that I offer in this article and series. All details of DSM-5 that I discuss are publicly available at www.dsm5.org. I’ve been a clinician and clinical researcher for >25 years, and my opinions are colored by the need for clarity, rigor, clinical relevance, and a disdain for overly speculative thinking.
Evolution of DSM
A nosological system (system of classification of disease) enables clinicians to provide specific treatments for medical causes of human disease and/or disability with precise and predictable effects and guide patients and families about the likely course and outcome. Such classification systems also are used by:
- researchers, to learn more about the nature of the conditions being classified and develop better treatments for them
- health care systems, to provide optimal health care and track its appropriate provision
- insurance companies, to provide appropriate reimbursement for health care
- health product developers, including pharmaceutical companies, to develop health care products and promote their appropriate utilization
- government agencies, to determine health priorities and apportionment of health care resources
- public health agencies, to track the distribution of health and disease in communities around the world.
An ideal classification system would meet all constituents’ needs while perfectly mapping natural disease entities with distinct etiology and pathophysiology (validity), consistently allow all users to reach the same diagnosis (reliability), and provide clinicians with clear guidance about treatment and likely course for each of the entities (utility), with the list of entities being mutually exclusive and collectively exhaustive (coverage).
The current nosological system for psychiatric disorders originated in the late 19th and early 20th centuries and culminated in the first edition of the Diagnostic and Statistical Manual of Mental Disorders3 released in 1952 and a section related to mental disorders (section V) in the sixth revision of the International Classification of Disease (ICD).4 Whereas DSM focuses exclusively on mental disorders, the ICD is a general medical classification system that began covering mental disorders with its sixth revision in 1949. In subsequent revisions (ICD-7 through -10 and DSM-II through -IV), substantial changes in diagnostic criteria have been made, although the systems’ basic structure has been retained. Table 1 describes major changes from DSM-I through DSM-IV-TR.3,5-9 DSM and ICD both are being revised; DSM-5 is scheduled to be released in 2013 and ICD-11 is to be finalized by 2016.
Table 1
Conceptual development of DSM-I to DSM-IV-TR
| Version | Comments |
|---|---|
| DSM-I (1952)3 | Presumed etiology. 106 diagnoses |
| DSM-II (1968)5 | Glossary definitions. 185 diagnoses |
| DSM-III (1980)6 | Paradigm shift. Explicit criteria. Emphasis on reliability. 265 diagnoses |
| DSM-III-R (1987)7 | Modest changes. Blunted hierarchies. Clarifications. 292 diagnoses |
| DSM-IV (1994)8 | Modest changes. More blunted hierarchies. 361 diagnoses |
| DSM-IV-TR (2000)9 | Only text revision. 361 diagnostic conditions |
What do clinicians need?
Similar to ICD-10, DSM-IV is marked by considerable complexity, variable validity, limited clinical and research utility, and problems of burgeoning comorbidity.10 Efforts to revise DSM seek to address these limitations. From a clinician’s perspective, the most challenging aspects of DSM-IV derive from its complexity—which makes clinical application difficult—and its limited clinical utility, which is exemplified by artificial comorbidity,11 frequent use of “not otherwise specified” (NOS), and relative treatment nonspecificity with reference to diagnosis.
As clinicians, we want a nosological system that is easy to use, can guide treatment decisions, provides useful information about likely disease course and outcomes, and allows us to easily communicate about disease nature with patients, families, payers, and health care administrators. Additionally, although good validity and reliability are desirable for clinicians, adequate coverage of psychiatric disease—the listed conditions should be collectively exhaustive and mutually exclusive—is particularly valued. Finally, we want a diagnostic system that allows us to explain the reasoning behind psychiatric diagnoses and related treatment in lay terms to patients and their families.
DSM-5 development to date
DSM-5 development has been a collaborative effort led by the APA and involves the National Institute of Mental Health, the National Institute on Drug Abuse, the National Institute on Alcohol Abuse and Alcoholism, and the World Health Organization (WHO). Between 1999 and 2002, 3 work conferences resulted in a series of white papers that identified gaps and research needs.1 Between 2003 and 2008, the American Psychiatric Institute for Research and Education, the National Institute of Health, and the WHO organized 13 international conferences to review a wide range of nosologic issues; the proceedings have been compiled into 13 monographs (11 published and 2 in press) and >125 scientific articles that serve as key reference sources for the DSM-5 process. For a continually updated list of these publications, see www.dsm5.org/Research.
In 2006, DSM-5 Task Force Chair David J. Kupfer, MD and Vice Chair Darrel A. Regier, MD, MPH were appointed and began selecting members of the DSM-5 Task Force, a process that was completed in 2007. Members of the 13 diagnostic area Work Groups (Table 2) were selected and the Work Groups were constituted in 2008. All 168 Task Force and Work Group members were vetted to ensure that they met standards of minimum conflict of interest and broad representation. Membership includes diverse professional representation from academia and mental health; 75% of members are from the United States. Six cross-cutting study groups have deliberated on a range of common issues, including:
- spectrum disorders
- lifespan and development
- gender and cross-cultural
- psychiatric/general medicine interface
- impairment and disability assessment
- diagnostic measurement and assessment.
Additionally, >300 external advisors with special expertise have participated in the process. Since 2008, each of the Work Groups has conducted extensive literature reviews of all assigned disorders, evaluated what works and what doesn’t work in DSM-IV-TR, assessed new research developments and clinical issues that have arisen since publication of DSM-IV-TR in 1994, and developed research plans to investigate critical issues utilizing systematic reviews and secondary data analyses. Based on these analyses, each Work Group proposed draft diagnostic criteria for its disorders, using a strict protocol for criteria revisions such as addition or deletion of disorders and changes to existing diagnostic criteria. These draft diagnostic criteria were first presented on www.dsm5.org in late 2009 through early 2010. Based on input from other Work Groups, the Task Force, several external groups, and the public, the Work Groups revised these criteria and prioritized necessary field trials to evaluate key recommendations. Phase I of the field trials began in 2010.12 Results of these field trials are being compiled and analyzed.
The DSM-5 Work Groups have met via teleconference 1 to 2 times a month and in-person twice a year, with significant communication between meetings. Work Group chairs are members of the Task Force, which has equally frequent meetings. Reports of DSM-5 deliberations have been presented at hundreds of professional meetings and described in >200 scientific publications. Comprehensive information and ongoing updates on DSM-5 and a list of publications and meetings are provided at www.dsm5.org.
Public input has been sought and the Work Groups have received and processed >10,000 comments. In 2010, the APA Board of Trustees appointed a Scientific Review Committee to evaluate the scientific merit and clinical impact of the Work Group recommendations and comment on the strength of the evidence advanced in support of each proposed revision. In 2011, the Board of Trustees appointed a Clinical and Public Health Committee to evaluate the clinical utility and public health significance of the proposed revisions. The APA and WHO have shared information and assessments in an effort to harmonize diagnostic criteria between DSM-5 and ICD-11.
Initial hopes that DSM-5 could represent a paradigm shift toward an etiopathophysiological classification of psychiatric disorders have been tempered by recognition of the limitations of our current neurobiologic understanding of psychiatric disorders. Therefore, the focus for DSM-5 has shifted from validity enhancements to improved clinical utility while building a framework that better lends itself to a future etiopathophysiological nosology.13-18 Whereas dimensional assessments are likely to be added across various diagnostic categories, a primarily categorical nosology will be retained and the proposed criterion changes are relatively modest. The results of our enhanced knowledge about the neurobiologic underpinnings of psychiatric disorders will not be reflected in diagnostic criteria, but in the significant revisions to the DSM text.
Our DSM-5 series
Subsequent articles in this series—which will be published here, at CurrentPsychiatry.com—will discuss specific proposed DSM-5 changes in 13 groups of disorders (Table 2) and their clinical implications. These articles also will address the relationship of DSM to ICD, issues with dimensional classification, and the importance of and challenges in precise diagnostic measurement.
Table 2
DSM-5 Work Groups
| Attention-deficit/hyperactivity disorder and disruptive behaviors |
| Anxiety, obsessive-compulsive, posttraumatic, and dissociative disorders |
| Disorders in childhood and adolescence |
| Eating disorders |
| Mood disorders |
| Neurocognitive disorders |
| Neurodevelopmental disorders |
| Personality and personality disorders |
| Psychotic disorders |
| Sexual and gender identity disorders |
| Sleep-wake disorders |
| Somatic distress disorders |
| Substance-related disorders |
Related Resources
- American Psychiatric Association. DSM-5 Development. www.dsm5.org.
- Black DW, Zimmerman M. Redefining personality disorders: Proposed revisions for DSM-5. Current Psychiatry. 2011;10(9):26-38.
Disclosure
Dr. Tandon is a member of the DSM-5 Psychotic Disorders Work Group. He reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Kupfer DJ, First MB, Regier DA. eds. A research agenda for DSM-V. Washington, DC: American Psychiatric Association; 2002.
2. Frances A. Whither DSM-V? Br J Psychiatry. 2009;195(5):391-392.
3. Diagnostic and statistical manual of mental disorders, 1st ed. Washington DC: American Psychiatric Association; 1952.
4. World Health Organization. Manual of the international statistical classification of diseases injuries and causes of death, 6th revision (ICD-6). Geneva, Switzerland: World Health Organization; 1949.
5. Diagnostic and statistical manual of mental disorders, 2nd ed. Washington DC: American Psychiatric Association; 1968.
6. Diagnostic and statistical manual of mental disorders, 3rd ed. Washington DC: American Psychiatric Association; 1980.
7. Diagnostic and statistical manual of mental disorders, 3rd ed, rev. Washington, DC: American Psychiatric Association; 1987.
8. Diagnostic and statistical manual of mental disorders, 4th ed. Washington DC: American Psychiatric Association; 1994.
9. Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington DC: American Psychiatric Association; 2000.
10. Kendell RE, Jablensky A. Distinguishing between the validity and utility of psychiatric diagnoses. Am J Psychiatry. 2003;160(1):4-12.
11. Maj M. “Psychiatric comorbidity”: an artifact of current diagnostic systems? Br J Psychiatry. 2005;186:182-184.
12. Kraemer HC, Kupfer DJ, Narrow WE, et al. Moving toward DSM-5: the field trials. Am J Psychiatry. 2010;167(10):1158-1160.
13. Hyman SE. The diagnosis of mental disorders: the problem of reification. Annu Rev Clin Psychol. 2010;6:155-179.
14. Tandon R, Nasrallah HA, Keshavan MS. Schizophrenia “just the facts” 4. Clinical features and conceptualization. Schizophr Res. 2009;110(1-3):1-23.
15. Insel T, Cuthbert B, Garvey M, et al. Research domain criteria (RDoC): toward a new classification framework for research on mental disorders. Am J Psychiatry. 2010;167(7):748-751.
16. Kendler KS, First MB. Alternative futures for the DSM revision process: iteration versus paradigm shift. Br J Psychiatry. 2010;197(4):263-265.
17. Kupfer DJ, Regier DA. Neuroscience clinical evidence, and the future of psychiatric classification in DSM-5. Am J Psychiatry. 2011;168(7):672-674.
18. Regier DA, Kuhl EA, Narrow WE, et al. Research planning for the future of psychiatric diagnosis [published online ahead of print June 13, 2011]. Eur Psychiatry. doi:10.1016/j.eurpsy.2009.11.013.
Discuss this article at www.facebook.com/CurrentPsychiatry
Work on the fifth edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-5)—scheduled to be published in May 2013—has been ongoing for more than a decade. Momentous advances in genetics and brain imaging since publication of DSM-IV in 1994 have generated optimism that an improved understanding of the neurobiologic underpinnings of psychiatric disorders might lead to a paradigm shift from the current descriptive classification system to a more scientific etiopathophysiological system similar to that used by other medical specialities.1
Some fear that any changes to our current classification system may be premature and could make an already complex system even more unwieldy.2 Scores of articles about the content and process of DSM-5 and several critiques and commentaries on the topic have been published. The American Psychiatric Association (APA) has made the DSM-5 process transparent by posting frequent updates to the DSM-5 Development Web site (www.dsm5.org), seeking feedback from the psychiatric community and the public, and presenting progress reports by members of the DSM-5 Task Force at scientific meetings.
There have been few discussions on the implications of DSM-5 from the practicing clinician’s vantage point, which I seek to present in this series of articles, the remainder of which will be published here, at CurrentPsychiatry.com. In this article, I:
- provide a brief history of psychiatric classification, focusing on the origins and evolution of the DSM system
- summarize the limitations of DSM-IV
- note the challenges and tensions in the construction of DSM-5
- review the DSM-5 process
- outline its current status
- discuss the organization and content of future articles in this series.
Although I am a member of the DSM-5 Psychotic Disorders Work Group, I am solely responsible for the content and any opinions that I offer in this article and series. All details of DSM-5 that I discuss are publicly available at www.dsm5.org. I’ve been a clinician and clinical researcher for >25 years, and my opinions are colored by the need for clarity, rigor, clinical relevance, and a disdain for overly speculative thinking.
Evolution of DSM
A nosological system (system of classification of disease) enables clinicians to provide specific treatments for medical causes of human disease and/or disability with precise and predictable effects and guide patients and families about the likely course and outcome. Such classification systems also are used by:
- researchers, to learn more about the nature of the conditions being classified and develop better treatments for them
- health care systems, to provide optimal health care and track its appropriate provision
- insurance companies, to provide appropriate reimbursement for health care
- health product developers, including pharmaceutical companies, to develop health care products and promote their appropriate utilization
- government agencies, to determine health priorities and apportionment of health care resources
- public health agencies, to track the distribution of health and disease in communities around the world.
An ideal classification system would meet all constituents’ needs while perfectly mapping natural disease entities with distinct etiology and pathophysiology (validity), consistently allow all users to reach the same diagnosis (reliability), and provide clinicians with clear guidance about treatment and likely course for each of the entities (utility), with the list of entities being mutually exclusive and collectively exhaustive (coverage).
The current nosological system for psychiatric disorders originated in the late 19th and early 20th centuries and culminated in the first edition of the Diagnostic and Statistical Manual of Mental Disorders3 released in 1952 and a section related to mental disorders (section V) in the sixth revision of the International Classification of Disease (ICD).4 Whereas DSM focuses exclusively on mental disorders, the ICD is a general medical classification system that began covering mental disorders with its sixth revision in 1949. In subsequent revisions (ICD-7 through -10 and DSM-II through -IV), substantial changes in diagnostic criteria have been made, although the systems’ basic structure has been retained. Table 1 describes major changes from DSM-I through DSM-IV-TR.3,5-9 DSM and ICD both are being revised; DSM-5 is scheduled to be released in 2013 and ICD-11 is to be finalized by 2016.
Table 1
Conceptual development of DSM-I to DSM-IV-TR
| Version | Comments |
|---|---|
| DSM-I (1952)3 | Presumed etiology. 106 diagnoses |
| DSM-II (1968)5 | Glossary definitions. 185 diagnoses |
| DSM-III (1980)6 | Paradigm shift. Explicit criteria. Emphasis on reliability. 265 diagnoses |
| DSM-III-R (1987)7 | Modest changes. Blunted hierarchies. Clarifications. 292 diagnoses |
| DSM-IV (1994)8 | Modest changes. More blunted hierarchies. 361 diagnoses |
| DSM-IV-TR (2000)9 | Only text revision. 361 diagnostic conditions |
What do clinicians need?
Similar to ICD-10, DSM-IV is marked by considerable complexity, variable validity, limited clinical and research utility, and problems of burgeoning comorbidity.10 Efforts to revise DSM seek to address these limitations. From a clinician’s perspective, the most challenging aspects of DSM-IV derive from its complexity—which makes clinical application difficult—and its limited clinical utility, which is exemplified by artificial comorbidity,11 frequent use of “not otherwise specified” (NOS), and relative treatment nonspecificity with reference to diagnosis.
As clinicians, we want a nosological system that is easy to use, can guide treatment decisions, provides useful information about likely disease course and outcomes, and allows us to easily communicate about disease nature with patients, families, payers, and health care administrators. Additionally, although good validity and reliability are desirable for clinicians, adequate coverage of psychiatric disease—the listed conditions should be collectively exhaustive and mutually exclusive—is particularly valued. Finally, we want a diagnostic system that allows us to explain the reasoning behind psychiatric diagnoses and related treatment in lay terms to patients and their families.
DSM-5 development to date
DSM-5 development has been a collaborative effort led by the APA and involves the National Institute of Mental Health, the National Institute on Drug Abuse, the National Institute on Alcohol Abuse and Alcoholism, and the World Health Organization (WHO). Between 1999 and 2002, 3 work conferences resulted in a series of white papers that identified gaps and research needs.1 Between 2003 and 2008, the American Psychiatric Institute for Research and Education, the National Institute of Health, and the WHO organized 13 international conferences to review a wide range of nosologic issues; the proceedings have been compiled into 13 monographs (11 published and 2 in press) and >125 scientific articles that serve as key reference sources for the DSM-5 process. For a continually updated list of these publications, see www.dsm5.org/Research.
In 2006, DSM-5 Task Force Chair David J. Kupfer, MD and Vice Chair Darrel A. Regier, MD, MPH were appointed and began selecting members of the DSM-5 Task Force, a process that was completed in 2007. Members of the 13 diagnostic area Work Groups (Table 2) were selected and the Work Groups were constituted in 2008. All 168 Task Force and Work Group members were vetted to ensure that they met standards of minimum conflict of interest and broad representation. Membership includes diverse professional representation from academia and mental health; 75% of members are from the United States. Six cross-cutting study groups have deliberated on a range of common issues, including:
- spectrum disorders
- lifespan and development
- gender and cross-cultural
- psychiatric/general medicine interface
- impairment and disability assessment
- diagnostic measurement and assessment.
Additionally, >300 external advisors with special expertise have participated in the process. Since 2008, each of the Work Groups has conducted extensive literature reviews of all assigned disorders, evaluated what works and what doesn’t work in DSM-IV-TR, assessed new research developments and clinical issues that have arisen since publication of DSM-IV-TR in 1994, and developed research plans to investigate critical issues utilizing systematic reviews and secondary data analyses. Based on these analyses, each Work Group proposed draft diagnostic criteria for its disorders, using a strict protocol for criteria revisions such as addition or deletion of disorders and changes to existing diagnostic criteria. These draft diagnostic criteria were first presented on www.dsm5.org in late 2009 through early 2010. Based on input from other Work Groups, the Task Force, several external groups, and the public, the Work Groups revised these criteria and prioritized necessary field trials to evaluate key recommendations. Phase I of the field trials began in 2010.12 Results of these field trials are being compiled and analyzed.
The DSM-5 Work Groups have met via teleconference 1 to 2 times a month and in-person twice a year, with significant communication between meetings. Work Group chairs are members of the Task Force, which has equally frequent meetings. Reports of DSM-5 deliberations have been presented at hundreds of professional meetings and described in >200 scientific publications. Comprehensive information and ongoing updates on DSM-5 and a list of publications and meetings are provided at www.dsm5.org.
Public input has been sought and the Work Groups have received and processed >10,000 comments. In 2010, the APA Board of Trustees appointed a Scientific Review Committee to evaluate the scientific merit and clinical impact of the Work Group recommendations and comment on the strength of the evidence advanced in support of each proposed revision. In 2011, the Board of Trustees appointed a Clinical and Public Health Committee to evaluate the clinical utility and public health significance of the proposed revisions. The APA and WHO have shared information and assessments in an effort to harmonize diagnostic criteria between DSM-5 and ICD-11.
Initial hopes that DSM-5 could represent a paradigm shift toward an etiopathophysiological classification of psychiatric disorders have been tempered by recognition of the limitations of our current neurobiologic understanding of psychiatric disorders. Therefore, the focus for DSM-5 has shifted from validity enhancements to improved clinical utility while building a framework that better lends itself to a future etiopathophysiological nosology.13-18 Whereas dimensional assessments are likely to be added across various diagnostic categories, a primarily categorical nosology will be retained and the proposed criterion changes are relatively modest. The results of our enhanced knowledge about the neurobiologic underpinnings of psychiatric disorders will not be reflected in diagnostic criteria, but in the significant revisions to the DSM text.
Our DSM-5 series
Subsequent articles in this series—which will be published here, at CurrentPsychiatry.com—will discuss specific proposed DSM-5 changes in 13 groups of disorders (Table 2) and their clinical implications. These articles also will address the relationship of DSM to ICD, issues with dimensional classification, and the importance of and challenges in precise diagnostic measurement.
Table 2
DSM-5 Work Groups
| Attention-deficit/hyperactivity disorder and disruptive behaviors |
| Anxiety, obsessive-compulsive, posttraumatic, and dissociative disorders |
| Disorders in childhood and adolescence |
| Eating disorders |
| Mood disorders |
| Neurocognitive disorders |
| Neurodevelopmental disorders |
| Personality and personality disorders |
| Psychotic disorders |
| Sexual and gender identity disorders |
| Sleep-wake disorders |
| Somatic distress disorders |
| Substance-related disorders |
Related Resources
- American Psychiatric Association. DSM-5 Development. www.dsm5.org.
- Black DW, Zimmerman M. Redefining personality disorders: Proposed revisions for DSM-5. Current Psychiatry. 2011;10(9):26-38.
Disclosure
Dr. Tandon is a member of the DSM-5 Psychotic Disorders Work Group. He reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Discuss this article at www.facebook.com/CurrentPsychiatry
Work on the fifth edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-5)—scheduled to be published in May 2013—has been ongoing for more than a decade. Momentous advances in genetics and brain imaging since publication of DSM-IV in 1994 have generated optimism that an improved understanding of the neurobiologic underpinnings of psychiatric disorders might lead to a paradigm shift from the current descriptive classification system to a more scientific etiopathophysiological system similar to that used by other medical specialities.1
Some fear that any changes to our current classification system may be premature and could make an already complex system even more unwieldy.2 Scores of articles about the content and process of DSM-5 and several critiques and commentaries on the topic have been published. The American Psychiatric Association (APA) has made the DSM-5 process transparent by posting frequent updates to the DSM-5 Development Web site (www.dsm5.org), seeking feedback from the psychiatric community and the public, and presenting progress reports by members of the DSM-5 Task Force at scientific meetings.
There have been few discussions on the implications of DSM-5 from the practicing clinician’s vantage point, which I seek to present in this series of articles, the remainder of which will be published here, at CurrentPsychiatry.com. In this article, I:
- provide a brief history of psychiatric classification, focusing on the origins and evolution of the DSM system
- summarize the limitations of DSM-IV
- note the challenges and tensions in the construction of DSM-5
- review the DSM-5 process
- outline its current status
- discuss the organization and content of future articles in this series.
Although I am a member of the DSM-5 Psychotic Disorders Work Group, I am solely responsible for the content and any opinions that I offer in this article and series. All details of DSM-5 that I discuss are publicly available at www.dsm5.org. I’ve been a clinician and clinical researcher for >25 years, and my opinions are colored by the need for clarity, rigor, clinical relevance, and a disdain for overly speculative thinking.
Evolution of DSM
A nosological system (system of classification of disease) enables clinicians to provide specific treatments for medical causes of human disease and/or disability with precise and predictable effects and guide patients and families about the likely course and outcome. Such classification systems also are used by:
- researchers, to learn more about the nature of the conditions being classified and develop better treatments for them
- health care systems, to provide optimal health care and track its appropriate provision
- insurance companies, to provide appropriate reimbursement for health care
- health product developers, including pharmaceutical companies, to develop health care products and promote their appropriate utilization
- government agencies, to determine health priorities and apportionment of health care resources
- public health agencies, to track the distribution of health and disease in communities around the world.
An ideal classification system would meet all constituents’ needs while perfectly mapping natural disease entities with distinct etiology and pathophysiology (validity), consistently allow all users to reach the same diagnosis (reliability), and provide clinicians with clear guidance about treatment and likely course for each of the entities (utility), with the list of entities being mutually exclusive and collectively exhaustive (coverage).
The current nosological system for psychiatric disorders originated in the late 19th and early 20th centuries and culminated in the first edition of the Diagnostic and Statistical Manual of Mental Disorders3 released in 1952 and a section related to mental disorders (section V) in the sixth revision of the International Classification of Disease (ICD).4 Whereas DSM focuses exclusively on mental disorders, the ICD is a general medical classification system that began covering mental disorders with its sixth revision in 1949. In subsequent revisions (ICD-7 through -10 and DSM-II through -IV), substantial changes in diagnostic criteria have been made, although the systems’ basic structure has been retained. Table 1 describes major changes from DSM-I through DSM-IV-TR.3,5-9 DSM and ICD both are being revised; DSM-5 is scheduled to be released in 2013 and ICD-11 is to be finalized by 2016.
Table 1
Conceptual development of DSM-I to DSM-IV-TR
| Version | Comments |
|---|---|
| DSM-I (1952)3 | Presumed etiology. 106 diagnoses |
| DSM-II (1968)5 | Glossary definitions. 185 diagnoses |
| DSM-III (1980)6 | Paradigm shift. Explicit criteria. Emphasis on reliability. 265 diagnoses |
| DSM-III-R (1987)7 | Modest changes. Blunted hierarchies. Clarifications. 292 diagnoses |
| DSM-IV (1994)8 | Modest changes. More blunted hierarchies. 361 diagnoses |
| DSM-IV-TR (2000)9 | Only text revision. 361 diagnostic conditions |
What do clinicians need?
Similar to ICD-10, DSM-IV is marked by considerable complexity, variable validity, limited clinical and research utility, and problems of burgeoning comorbidity.10 Efforts to revise DSM seek to address these limitations. From a clinician’s perspective, the most challenging aspects of DSM-IV derive from its complexity—which makes clinical application difficult—and its limited clinical utility, which is exemplified by artificial comorbidity,11 frequent use of “not otherwise specified” (NOS), and relative treatment nonspecificity with reference to diagnosis.
As clinicians, we want a nosological system that is easy to use, can guide treatment decisions, provides useful information about likely disease course and outcomes, and allows us to easily communicate about disease nature with patients, families, payers, and health care administrators. Additionally, although good validity and reliability are desirable for clinicians, adequate coverage of psychiatric disease—the listed conditions should be collectively exhaustive and mutually exclusive—is particularly valued. Finally, we want a diagnostic system that allows us to explain the reasoning behind psychiatric diagnoses and related treatment in lay terms to patients and their families.
DSM-5 development to date
DSM-5 development has been a collaborative effort led by the APA and involves the National Institute of Mental Health, the National Institute on Drug Abuse, the National Institute on Alcohol Abuse and Alcoholism, and the World Health Organization (WHO). Between 1999 and 2002, 3 work conferences resulted in a series of white papers that identified gaps and research needs.1 Between 2003 and 2008, the American Psychiatric Institute for Research and Education, the National Institute of Health, and the WHO organized 13 international conferences to review a wide range of nosologic issues; the proceedings have been compiled into 13 monographs (11 published and 2 in press) and >125 scientific articles that serve as key reference sources for the DSM-5 process. For a continually updated list of these publications, see www.dsm5.org/Research.
In 2006, DSM-5 Task Force Chair David J. Kupfer, MD and Vice Chair Darrel A. Regier, MD, MPH were appointed and began selecting members of the DSM-5 Task Force, a process that was completed in 2007. Members of the 13 diagnostic area Work Groups (Table 2) were selected and the Work Groups were constituted in 2008. All 168 Task Force and Work Group members were vetted to ensure that they met standards of minimum conflict of interest and broad representation. Membership includes diverse professional representation from academia and mental health; 75% of members are from the United States. Six cross-cutting study groups have deliberated on a range of common issues, including:
- spectrum disorders
- lifespan and development
- gender and cross-cultural
- psychiatric/general medicine interface
- impairment and disability assessment
- diagnostic measurement and assessment.
Additionally, >300 external advisors with special expertise have participated in the process. Since 2008, each of the Work Groups has conducted extensive literature reviews of all assigned disorders, evaluated what works and what doesn’t work in DSM-IV-TR, assessed new research developments and clinical issues that have arisen since publication of DSM-IV-TR in 1994, and developed research plans to investigate critical issues utilizing systematic reviews and secondary data analyses. Based on these analyses, each Work Group proposed draft diagnostic criteria for its disorders, using a strict protocol for criteria revisions such as addition or deletion of disorders and changes to existing diagnostic criteria. These draft diagnostic criteria were first presented on www.dsm5.org in late 2009 through early 2010. Based on input from other Work Groups, the Task Force, several external groups, and the public, the Work Groups revised these criteria and prioritized necessary field trials to evaluate key recommendations. Phase I of the field trials began in 2010.12 Results of these field trials are being compiled and analyzed.
The DSM-5 Work Groups have met via teleconference 1 to 2 times a month and in-person twice a year, with significant communication between meetings. Work Group chairs are members of the Task Force, which has equally frequent meetings. Reports of DSM-5 deliberations have been presented at hundreds of professional meetings and described in >200 scientific publications. Comprehensive information and ongoing updates on DSM-5 and a list of publications and meetings are provided at www.dsm5.org.
Public input has been sought and the Work Groups have received and processed >10,000 comments. In 2010, the APA Board of Trustees appointed a Scientific Review Committee to evaluate the scientific merit and clinical impact of the Work Group recommendations and comment on the strength of the evidence advanced in support of each proposed revision. In 2011, the Board of Trustees appointed a Clinical and Public Health Committee to evaluate the clinical utility and public health significance of the proposed revisions. The APA and WHO have shared information and assessments in an effort to harmonize diagnostic criteria between DSM-5 and ICD-11.
Initial hopes that DSM-5 could represent a paradigm shift toward an etiopathophysiological classification of psychiatric disorders have been tempered by recognition of the limitations of our current neurobiologic understanding of psychiatric disorders. Therefore, the focus for DSM-5 has shifted from validity enhancements to improved clinical utility while building a framework that better lends itself to a future etiopathophysiological nosology.13-18 Whereas dimensional assessments are likely to be added across various diagnostic categories, a primarily categorical nosology will be retained and the proposed criterion changes are relatively modest. The results of our enhanced knowledge about the neurobiologic underpinnings of psychiatric disorders will not be reflected in diagnostic criteria, but in the significant revisions to the DSM text.
Our DSM-5 series
Subsequent articles in this series—which will be published here, at CurrentPsychiatry.com—will discuss specific proposed DSM-5 changes in 13 groups of disorders (Table 2) and their clinical implications. These articles also will address the relationship of DSM to ICD, issues with dimensional classification, and the importance of and challenges in precise diagnostic measurement.
Table 2
DSM-5 Work Groups
| Attention-deficit/hyperactivity disorder and disruptive behaviors |
| Anxiety, obsessive-compulsive, posttraumatic, and dissociative disorders |
| Disorders in childhood and adolescence |
| Eating disorders |
| Mood disorders |
| Neurocognitive disorders |
| Neurodevelopmental disorders |
| Personality and personality disorders |
| Psychotic disorders |
| Sexual and gender identity disorders |
| Sleep-wake disorders |
| Somatic distress disorders |
| Substance-related disorders |
Related Resources
- American Psychiatric Association. DSM-5 Development. www.dsm5.org.
- Black DW, Zimmerman M. Redefining personality disorders: Proposed revisions for DSM-5. Current Psychiatry. 2011;10(9):26-38.
Disclosure
Dr. Tandon is a member of the DSM-5 Psychotic Disorders Work Group. He reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Kupfer DJ, First MB, Regier DA. eds. A research agenda for DSM-V. Washington, DC: American Psychiatric Association; 2002.
2. Frances A. Whither DSM-V? Br J Psychiatry. 2009;195(5):391-392.
3. Diagnostic and statistical manual of mental disorders, 1st ed. Washington DC: American Psychiatric Association; 1952.
4. World Health Organization. Manual of the international statistical classification of diseases injuries and causes of death, 6th revision (ICD-6). Geneva, Switzerland: World Health Organization; 1949.
5. Diagnostic and statistical manual of mental disorders, 2nd ed. Washington DC: American Psychiatric Association; 1968.
6. Diagnostic and statistical manual of mental disorders, 3rd ed. Washington DC: American Psychiatric Association; 1980.
7. Diagnostic and statistical manual of mental disorders, 3rd ed, rev. Washington, DC: American Psychiatric Association; 1987.
8. Diagnostic and statistical manual of mental disorders, 4th ed. Washington DC: American Psychiatric Association; 1994.
9. Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington DC: American Psychiatric Association; 2000.
10. Kendell RE, Jablensky A. Distinguishing between the validity and utility of psychiatric diagnoses. Am J Psychiatry. 2003;160(1):4-12.
11. Maj M. “Psychiatric comorbidity”: an artifact of current diagnostic systems? Br J Psychiatry. 2005;186:182-184.
12. Kraemer HC, Kupfer DJ, Narrow WE, et al. Moving toward DSM-5: the field trials. Am J Psychiatry. 2010;167(10):1158-1160.
13. Hyman SE. The diagnosis of mental disorders: the problem of reification. Annu Rev Clin Psychol. 2010;6:155-179.
14. Tandon R, Nasrallah HA, Keshavan MS. Schizophrenia “just the facts” 4. Clinical features and conceptualization. Schizophr Res. 2009;110(1-3):1-23.
15. Insel T, Cuthbert B, Garvey M, et al. Research domain criteria (RDoC): toward a new classification framework for research on mental disorders. Am J Psychiatry. 2010;167(7):748-751.
16. Kendler KS, First MB. Alternative futures for the DSM revision process: iteration versus paradigm shift. Br J Psychiatry. 2010;197(4):263-265.
17. Kupfer DJ, Regier DA. Neuroscience clinical evidence, and the future of psychiatric classification in DSM-5. Am J Psychiatry. 2011;168(7):672-674.
18. Regier DA, Kuhl EA, Narrow WE, et al. Research planning for the future of psychiatric diagnosis [published online ahead of print June 13, 2011]. Eur Psychiatry. doi:10.1016/j.eurpsy.2009.11.013.
1. Kupfer DJ, First MB, Regier DA. eds. A research agenda for DSM-V. Washington, DC: American Psychiatric Association; 2002.
2. Frances A. Whither DSM-V? Br J Psychiatry. 2009;195(5):391-392.
3. Diagnostic and statistical manual of mental disorders, 1st ed. Washington DC: American Psychiatric Association; 1952.
4. World Health Organization. Manual of the international statistical classification of diseases injuries and causes of death, 6th revision (ICD-6). Geneva, Switzerland: World Health Organization; 1949.
5. Diagnostic and statistical manual of mental disorders, 2nd ed. Washington DC: American Psychiatric Association; 1968.
6. Diagnostic and statistical manual of mental disorders, 3rd ed. Washington DC: American Psychiatric Association; 1980.
7. Diagnostic and statistical manual of mental disorders, 3rd ed, rev. Washington, DC: American Psychiatric Association; 1987.
8. Diagnostic and statistical manual of mental disorders, 4th ed. Washington DC: American Psychiatric Association; 1994.
9. Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington DC: American Psychiatric Association; 2000.
10. Kendell RE, Jablensky A. Distinguishing between the validity and utility of psychiatric diagnoses. Am J Psychiatry. 2003;160(1):4-12.
11. Maj M. “Psychiatric comorbidity”: an artifact of current diagnostic systems? Br J Psychiatry. 2005;186:182-184.
12. Kraemer HC, Kupfer DJ, Narrow WE, et al. Moving toward DSM-5: the field trials. Am J Psychiatry. 2010;167(10):1158-1160.
13. Hyman SE. The diagnosis of mental disorders: the problem of reification. Annu Rev Clin Psychol. 2010;6:155-179.
14. Tandon R, Nasrallah HA, Keshavan MS. Schizophrenia “just the facts” 4. Clinical features and conceptualization. Schizophr Res. 2009;110(1-3):1-23.
15. Insel T, Cuthbert B, Garvey M, et al. Research domain criteria (RDoC): toward a new classification framework for research on mental disorders. Am J Psychiatry. 2010;167(7):748-751.
16. Kendler KS, First MB. Alternative futures for the DSM revision process: iteration versus paradigm shift. Br J Psychiatry. 2010;197(4):263-265.
17. Kupfer DJ, Regier DA. Neuroscience clinical evidence, and the future of psychiatric classification in DSM-5. Am J Psychiatry. 2011;168(7):672-674.
18. Regier DA, Kuhl EA, Narrow WE, et al. Research planning for the future of psychiatric diagnosis [published online ahead of print June 13, 2011]. Eur Psychiatry. doi:10.1016/j.eurpsy.2009.11.013.
Antipsychotics equivalent? CUtLASS renews the debate
When treating chronic psychotic disorders, U.S. psychiatrists generally prefer second-generation antipsychotics (SGAs) to first-generation antipsychotics (FGAs) because of widely held views1,2 that SGAs:
- are more effective for negative and cognitive symptoms
- produce fewer troublesome side effects
- help patients realize a better quality of life.
These beliefs have been challenged by two large-scale, government-supported studies: the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) in the United States3-6 and more recently the Cost Utility of the Latest Antipsychotic Drugs in Schizophrenia Study (CUtLASS) from the United Kingdom.7,8
CATIE and CUtLASS data suggest that the SGA advantage has been exaggerated, if in fact such an advantage exists. Other Current Psychiatry articles for the clinical practitioner have discussed the CATIE findings.9-11 This article addresses the CUtLASS results in the context of the trial’s methodology, using information from the primary publications7,8 and technical report.12
Cutlass study
Design. CUtLASS included 2 “bands” (Table 1):
- Band 1 compared the clinical usefulness and cost effectiveness of FGAs and SGAs in treating schizophrenia7
- Band 2 compared the effectiveness of clozapine versus other SGAs in treating refractory schizophrenia.8
CUtLASS Band 1 was not as extensive in scope as CATIE, and its design had some important differences (Table 2). Patients were referred for participation because their psychiatrists were considering a change in antipsychotic medication to address adverse effects or inadequate response. Fewer patients were recruited than expected—40% of the planned sample during 30 months of recruitment—but researchers considered the size sufficient to compare the effectiveness of FGAs and SGAs.
Patients were randomly assigned to treatment with an antipsychotic class, either:
- an FGA (1 of 11 options—including 5 depot formulations—chosen by the treating clinician)
- or an SGA (risperidone, olanzapine, quetiapine, or amisulpride, also chosen by the clinician).
Physicians and patients were not blinded to the medications used. They could choose medications within patients’ assigned classes and switch as needed in ways that mimicked clinical practice. Trained assessors, who were blinded to the medications being used, evaluated the patients after 12, 26, and 52 weeks.
Quality of life was the primary outcome measure.13 Secondary measures included symptoms, side effects, patient satisfaction, and cost of care.
Band 1 results. Patients assigned to the SGA or FGA classes showed no significant differences in quality of life measures or schizophrenia symptoms. If anything, the findings slightly favored the FGAs.
Patient satisfaction and overall cost of care were similar, and rates of extrapyramidal symptoms (EPS), tardive dyskinesia, and akathisia did not differ significantly.
Clozapine comparison. In CUtLASS band 2, a different sample of 136 schizophrenia patients who had responded poorly to ≥2 antipsychotics was randomly assigned to clozapine or one of the above four SGAs. During the 1-year comparison trial, clozapine:
- was found to be significantly more effective (P=0.01) in managing patients’ symptoms, as measured by total Positive and Negative Syndrome Scale (PANSS) score
- showed a trend (P=0.08) towards providing these treatment-resistant patients with a better quality of life.8
Table 1
Summary of CUtLASS trial design and results
Band 1
|
Band 2
|
| CUtLASS: Cost Utility of the Latest Antipsychotic Drugs in Schizophrenia Study |
| FGA: First-generation antipsychotic |
| PANSS: Positive and Negative Syndrome Scale |
| SGA: Second-generation antipsychotic |
Table 2
Comparing designs of the CUtLASS and CATIE schizophrenia trials
| CUtLASS | CATIE | |
|---|---|---|
| Trial duration | 12 months | 18 months |
| Clinical sites | 14 (United Kingdom) | 57 (United States) |
| Number of Subjects | 227 | 1,460 |
| Gender and age | 68% male; mean age 41 | 74% male; mean age 41 |
| Mental illness duration (mean) | 14 years | 16 years |
| Diagnosis | 75% schizophrenia | 100% schizophrenia |
| First-episode patients included? | Yes (13% of sample) | No |
| % of patients receiving antipsychotics at enrollment | 99% | 74% |
| Baseline PANSS score (mean) | 82% FGAs; 40% depot | 15% FGAs; <5% depot |
| Baseline PANSS score | 72.2 | 75.7 |
| Baseline EPS scores | Low | Low |
| Antipsychotic options in randomization | 2 classes (SGA or FGA) (50% of subjects assigned to an FGA) | 4 SGAs, 1 FGA (20% of subjects assigned to an FGA) |
| % of subjects given sulpiride | 49% | 0% |
| Administration methodology | Medication blinded to raters but not to patients and physicians | Medication blinded to patients and physicians |
| Primary outcome | Quality of life | Discontinuation of medication |
| Long-acting antipsychotic option? | Yes | No |
| Antipsychotic switching | All patients switched agents; 49% changed antipsychotic class | 15% stayed on some agent |
| CATIE: Clinical Antipsychotic Trials of Intervention Effectiveness | ||
| CUtLASS: Cost Utility of the Latest Antipsychotic Drugs in Schizophrenia Study | ||
| EPS: Extrapyramidal symptom | ||
| FGA: First-generation antipsychotic | ||
| PANSS: Positive and Negative Syndrome Scale | ||
| SGA: Second-generation antipsychotic | ||
Comparing catie, cutlass data
The CUtLASS findings are not identical to those of CATIE phase 114 but are remarkably similar: no differences in effectiveness were seen between FGAs and SGA when treating patients with chronic schizophrenia.15,16
CUtLASS investigators concluded that “in people with schizophrenia whose medication is changed for clinical reasons, there is no disadvantage across 1 year in terms of quality of life, symptoms, or associated costs of care in using FGAs rather than nonclozapine SGAs.”7
By confirming CATIE’s results, is CUtLASS the final word on antipsychotic treatment of chronic schizophrenia? Or is it just another piece of the puzzle? CATIE and CUtLASS add much to our knowledge, but methodologic “flies in the ointment” plague all clinical trials. We must consider potential biases and confounding factors to properly interpret and apply their findings.
Although the CUtLASS trial was well-constructed and executed, its conclusions—like those of CATIE—merit careful scrutiny. Its patient recruitment methods and study design involved choices and compromises that are appropriate to evaluate17,18 as we weigh CUtLASS’ contribution to the SGA/FGA debate (Table 3).
Table 3
‘Flies in the ointment’ of the CUtLASS trial design
| Who was studied |
|
| What was compared |
|
| Other Issues |
|
| CUtLASS: Cost Utility of the Latest Antipsychotic Drugs in Schizophrenia Study | |
| EPS: Extrapyramidal symptom | |
| FGA: First-generation antipsychotic | |
| SGA: Second-generation antipsychotic | |
Who was studied?
Selection questions. CUtLASS researchers had problems recruiting patients for their study, in part because clinicians were reluctant to expose their patients to a 50% probability of being assigned to an FGA. Only 40% of the targeted sample was recruited, and participating clinicians referred only 20% to 37% of their eligible patients to the study.12 Thus, one could ask:
- Were enrolled subjects truly representative of the population from which they were drawn?
- Or did selection bias result in a disproportionate inclusion of individuals with certain characteristics?
Is it possible, for example, that clinicians preferentially referred medication-noncompliant patients to CUtLASS because they believed the benefits of depot FGAs—such as more assured adherence—would compensate for the potential benefits of SGAs—better efficacy/tolerability?19
Treatment resistance. Although patients were randomly assigned to FGAs or SGAs, a significantly greater proportion of those whose antipsychotics were being changed because of treatment resistance were assigned to receive SGAs. Treatment resistance was one reason that 88% of subjects in the SGA arm were referred to the trial, compared with 70% of subjects in the FGA arm (P<0.01).12 The extent to which this differential assignment may have biased results against SGAs is unclear.
EPS risk. CUtLASS-1 patients had been ill a mean of 14 years and had low baseline EPS rates despite receiving long-term antipsychotics (primarily FGAs). Even so, FGAs and SGAs showed similar rates of akathisia and other EPS. Thus—as with the CATIE results—the extent to which CUtLASS-1 findings may apply beyond chronic schizophrenia patients at relatively low risk for EPS is unclear.11,17
Impact of switching. Although patients were referred to CUtLASS because of adverse effects or inadequate response to one or more antipsychotics, they were only moderately ill (mean PANSS total score 72)20 and probably were deriving some benefit from their baseline antipsychotics. Before randomization, 82% of patients were receiving an FGA and 19% an SGA. Consequently, a far larger percentage of patients in the SGA group had to switch to a different medication class as the trial began.
As observed in CATIE, switching antipsychotics often has short-term negative consequences for patients,21 although switching classes (as in CUtLASS) may have had a different impact than switching individual antipsychotics (as in CATIE). If unequal antipsychotic switching rates in the two arms differentially affected patients’ quality of life, we would expect to see this effect emerge at the 12-week assessment, which is precisely where the greatest difference in Quality of Life Scale (QLS)13 scores appeared.
The mean QLS score for patients in the SGA arm was 2.6 points lower than in the FGA group at 12 weeks. This difference disappeared and, in fact, reversed at 26 weeks, but this 12-week effect had a strong impact on results of the 52-week intent-to-treat analysis. CUtLASS—like CATIE—might exemplify the risks of switching patients from treatment with partially effective antipsychotics.22
What was compared?
Classes vs individual drugs. The decision in CUtLASS-1 to compare antipsychotic classes rather than individual agents makes it difficult to interpret its findings. Antipsychotics are not homogeneous; clear differences exist within both the SGA and FGA classes in terms of individual agents’ efficacy and tolerability, and each SGA has a reasonably well-established and different side-effect profile.23
Sulpiride was the most commonly used FGA in CUtLASS-1 (by 49% of FGA patients). Sulpiride has some unusual attributes—such as lower EPS liability—and is not available in the United States. Thus, including this agent might have affected how applicable CUtLASS findings are to clinical practice in the United States.
Oral vs depot delivery. Individuals assigned to an FGA could receive either oral or long-acting depot medication, whereas those assigned to an SGA could receive only oral medication. At baseline, 84 of 227 CUtLASS-1 participants were receiving a depot antipsychotic, which was discontinued during randomization in 72 patients. During the 1-year study, the number of patients receiving a depot antipsychotic tripled from 12 to 35, suggesting the usefulness of long-acting agents in this population.19
Cross-class switching. Although participating physicians and their patients were urged to stay within assigned antipsychotic classes at least for the first 12 weeks and ideally for 1 year, a high rate of cross-class switching occurred (Figure). At the 52-week assessment, 51 of 118 patients (43%) in the intent-to-treat FGA group were receiving SGAs instead.
The CUtLASS authors’ assert that the trial refutes the hypothesis that using SGAs is superior to using FGAs in improving quality of life. This conclusion is difficult to justify when so many patients assigned to the FGA class actually were receiving SGAs. The conclusion is further weakened if differential switching rates put SGAs at a disadvantage in the first 12 weeks of the trial.
A more accurate conclusion of the intent-to-treat comparison appears in the technical report: “There was no statistically significant difference in terms of quality of life or symptoms over 1 year in commencing [italics added] conventional antipsychotic drugs rather than new atypical drugs.”12
Figure CUtLASS-1: Did switching rate affect trial outcome?
The high rate of cross-class medication switching in CUtLASS-1 may have weakened the study’s conclusion that virtually no difference in effectiveness exists between first- and second-generation antipsychotics. At the 52-week assessment, 51 of 118 patients (43%) in the intent-to-treat FGA group were receiving SGAs instead. Not shown in the figure is that 4 of the total 55 patients who switched from FGAs to SGAs had switched back to FGAs by the 52-week assessment.
CUtLASS: Cost Utility of the Latest Antipsychotic Drugs in Schizophrenia Study
FGA: First-generation antipsychotic
SGA: Second-generation antipsychotic
Source: Adapted from reference 7, Figure 1
Clinical implications
Notwithstanding these cautionary notes, CUtLASS-1 findings add to the questions raised by CATIE about the relative effectiveness of SGAs and FGAs. At a minimum, the data indicate that the SGA advantage has been overstated or oversimplified and that FGAs may be suitable options for meeting the needs of some patients with psychosis (particularly those at low risk for EPS).
Depot antipsychotics. CUtLASS also suggests a wider role for long-acting antipsychotics in chronic psychotic disorders, beyond treating patients with severe nonadherence.19,23 The number of patients receiving long-acting agents tripled over the 1-year study.12
Clozapine. Both CATIE and CUtLASS-2 confirmed clozapine’s superior efficacy for patients with treatment-resistant psychotic illness (Table 4). CUtLASS-2 also reaffirmed the challenges of clozapine’s metabolic and other side effects, such as sedation, hypotension, and hypersalivation.
All-cause discontinuation was significantly higher (P<0.05) in patients taking clozapine (73%) than in those taking other SGAs (52%). Even so, clozapine-group patients achieved significantly greater symptom reduction and tended toward a higher quality of life than other SGA-group patients.
Table 4
Clinical ‘pearls’ from the CUtLASS trial data
|
| CUtLASS: Cost Utility of the Latest Antipsychotic Drugs in Schizophrenia Study |
| EPS: Extrapyramidal symptom |
| FGA: First-generation antipsychotic |
| SGA: Second-generation antipsychotic |
Overview. In conclusion, one can reasonably conclude from analyzing the CATIE and CUtLASS data that:
- FGA-SGA differences are not as great as previously thought.
- Substantial differences exist among agents within both antipsychotic classes, particularly in side effect profiles.
- Neither study disproves the following presumed benefit of SGAs: that compared with FGAs, SGAs provide an equivalent antipsychotic effect and pose a lower risk of problems related to unmitigated dopamine blockade—such as EPS, dysphoria, bradyphrenia, neuroleptic-induced deficit syndrome, and tardive dyskinesia.11
- To use antipsychotics effectively and optimize individual treatment, consider the CATIE and CUtLASS trials in the contexts of their designs and the results of other studies of patients with chronic schizophrenia.
Related resources
- Heres S, Davis J, Maino K, et al. Why olanzapine beats risperidone, risperidone beats quetiapine, and quetiapine beats olanzapine: An exploratory analysis of head-head comparison studies of second-generation antipsychotics. Am J Psychiatry 2006;163:185-94.
Drug brand names
- Clozapine • Clozaril
- Quetiapine • Seroquel
- Olanzapine • Zyprexa
- Risperidone • Risperdal
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Kane JM, Leucht S, Carpenter D, et al. Expert consensus guideline series: optimizing pharmacologic treatment of psychotic disorders. J Clin Psychiatry 2003;64(suppl 12):1-100.
2. Tandon R, Fleischhacker WW. Comparative efficacy of antipsychotics in the treatment of schizophrenia: a critical assessment. Schizophr Res 2005;79:145-55.
3. Lieberman JA, Stroup TS, McEvoy JP, et al. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med 2005;353:1209-23.
4. McEvoy JP, Lieberman JA, Stroup TS, et al. Effectiveness of clozapine versus olanzapine, quetiapine, and risperidone in patients with chronic schizophrenia who did not respond to prior antipsychotic treatment. Am J Psychiatry 2006;163:600-10.
5. Stroup TS, Lieberman JA, McEvoy JP, et al. Effectiveness of olanzapine, quetiapine, risperidone, and ziprasidone in patients with chronic schizophrenia following discontinuation of a previous atypical antipsychotic. Am J Psychiatry 2006;163:611-22.
6. Rosenheck RA, Leslie DL, Sindelar J, et al. Cost-effectiveness of second-generation antipsychotics and perphenazine in a randomized trial of treatment for chronic schizophrenia. Am J Psychiatry 2006;163:2080-9.
7. Jones PB, Barnes T, Davies L, et al. Randomized controlled trial of the effect on quality of life of second- vs first-generation antipsychotic drugs in schizophrenia: Cost Utility of the Latest Antipsychotic Drugs in Schizophrenia Study (CUtLASS). Arch Gen Psychiatry 2006;63:1079-87.
8. Lewis SW, Barnes TRE, Davies L, et al. Randomised controlled trial of effect of prescription of clozapine versus other second-generation antipsychotic drugs in resistant schizophrenia. Schizophr Bull 2006;32:715-23.
9. Nasrallah HA. CATIE’s surprises: in antipsychotics’ square-off, were there winners or losers? Current Psychiatry 2006;5(2):49-65.
10. Buckley PF. Which antipsychotic do I choose next? CATIE phase 2 offers insights on efficacy and tolerability. Current Psychiatry 2006;5(9):27-43.
11. Tandon R, Constantine R. Avoiding EPS is key to realizing ‘atypical’ benefits. Current Psychiatry 2006;5(11):35-45.
12. Lewis SW, Davies L, Jones PB, et al. Randomised controlled trials of conventional antipsychotic versus new atypical drugs, and new atypical drugs versus clozapine, in people with schizophrenia responding poorly to, or intolerant of, current drug treatment. Health Technology Assessment 2006;10(#17):1-182.Available at http://www.hta.ac.uk/project/1078.asp. Accessed January 3, 2007.
13. Heinrichs DW, Hanlon TE, Carpenter WT. The Quality of Life Scale: an instrument for assessing the schizophrenic deficit syndrome. Schizophr Bull 1984;10:388-98.
14. Tandon R, Davis JM, Carpenter WT. CATIE, CUtLASS, and the FGA-SGA debate (letter). Arch Gen Psychiatry 2007 (in press).
15. Lieberman J. Comparative effectiveness of antipsychotic drugs: a commentary on the Cost Utility of the Latest Antipsychotic Drugs in Schizophrenia Study and Clinical Antipsychotic Trials of Intervention Effectiveness. Arch Gen Psychiatry 2006;63:1069-72.
16. Rosenheck RA. Outcomes, costs, and policy caution: a commentary on the Cost Utility of the Latest Antipsychotic Drugs in Schizophrenia Study. Arch Gen Psychiatry 2006;63:1074-6.
17. Tandon R. Comparative effectiveness of antipsychotics in the treatment of schizophrenia: what CATIE tells us—Parts 1 and 2. International Drug Therapy Newsletter 2006;41(7,9):51-8,67-74.
18. Meltzer HY, Bobo WV. Interpreting the efficacy findings in the CATIE study: what clinicians should know. CNS Spectrums 2006;11(suppl 7):14-24.
19. Kane JM. Review of treatments that can ameliorate nonadherence in patients with schizophrenia. J Clin Psychiatry 2006;67(suppl 5):9-14.
20. Leucht S, Kane JM, Kissling W, et al. What does the PANSS mean? Schizophr Res 2006;79:231-8.
21. Essock SM, Covell NH, Davis SM, et al. Effectiveness of switching antipsychotic medications. Am J Psychiatry 2006;163:2090-5.
22. Davis JM, Marder S, Tamminga CA. Switch or stay? Am J Psychiatry 2006;163:2032-3.
23. Tandon R, Targum SD, Nasrallah HA, et al. Strategies for maximizing clinical effectiveness in the treatment of schizophrenia. Journal of Psychiatric Practice 2006;12:348-63.
When treating chronic psychotic disorders, U.S. psychiatrists generally prefer second-generation antipsychotics (SGAs) to first-generation antipsychotics (FGAs) because of widely held views1,2 that SGAs:
- are more effective for negative and cognitive symptoms
- produce fewer troublesome side effects
- help patients realize a better quality of life.
These beliefs have been challenged by two large-scale, government-supported studies: the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) in the United States3-6 and more recently the Cost Utility of the Latest Antipsychotic Drugs in Schizophrenia Study (CUtLASS) from the United Kingdom.7,8
CATIE and CUtLASS data suggest that the SGA advantage has been exaggerated, if in fact such an advantage exists. Other Current Psychiatry articles for the clinical practitioner have discussed the CATIE findings.9-11 This article addresses the CUtLASS results in the context of the trial’s methodology, using information from the primary publications7,8 and technical report.12
Cutlass study
Design. CUtLASS included 2 “bands” (Table 1):
- Band 1 compared the clinical usefulness and cost effectiveness of FGAs and SGAs in treating schizophrenia7
- Band 2 compared the effectiveness of clozapine versus other SGAs in treating refractory schizophrenia.8
CUtLASS Band 1 was not as extensive in scope as CATIE, and its design had some important differences (Table 2). Patients were referred for participation because their psychiatrists were considering a change in antipsychotic medication to address adverse effects or inadequate response. Fewer patients were recruited than expected—40% of the planned sample during 30 months of recruitment—but researchers considered the size sufficient to compare the effectiveness of FGAs and SGAs.
Patients were randomly assigned to treatment with an antipsychotic class, either:
- an FGA (1 of 11 options—including 5 depot formulations—chosen by the treating clinician)
- or an SGA (risperidone, olanzapine, quetiapine, or amisulpride, also chosen by the clinician).
Physicians and patients were not blinded to the medications used. They could choose medications within patients’ assigned classes and switch as needed in ways that mimicked clinical practice. Trained assessors, who were blinded to the medications being used, evaluated the patients after 12, 26, and 52 weeks.
Quality of life was the primary outcome measure.13 Secondary measures included symptoms, side effects, patient satisfaction, and cost of care.
Band 1 results. Patients assigned to the SGA or FGA classes showed no significant differences in quality of life measures or schizophrenia symptoms. If anything, the findings slightly favored the FGAs.
Patient satisfaction and overall cost of care were similar, and rates of extrapyramidal symptoms (EPS), tardive dyskinesia, and akathisia did not differ significantly.
Clozapine comparison. In CUtLASS band 2, a different sample of 136 schizophrenia patients who had responded poorly to ≥2 antipsychotics was randomly assigned to clozapine or one of the above four SGAs. During the 1-year comparison trial, clozapine:
- was found to be significantly more effective (P=0.01) in managing patients’ symptoms, as measured by total Positive and Negative Syndrome Scale (PANSS) score
- showed a trend (P=0.08) towards providing these treatment-resistant patients with a better quality of life.8
Table 1
Summary of CUtLASS trial design and results
Band 1
|
Band 2
|
| CUtLASS: Cost Utility of the Latest Antipsychotic Drugs in Schizophrenia Study |
| FGA: First-generation antipsychotic |
| PANSS: Positive and Negative Syndrome Scale |
| SGA: Second-generation antipsychotic |
Table 2
Comparing designs of the CUtLASS and CATIE schizophrenia trials
| CUtLASS | CATIE | |
|---|---|---|
| Trial duration | 12 months | 18 months |
| Clinical sites | 14 (United Kingdom) | 57 (United States) |
| Number of Subjects | 227 | 1,460 |
| Gender and age | 68% male; mean age 41 | 74% male; mean age 41 |
| Mental illness duration (mean) | 14 years | 16 years |
| Diagnosis | 75% schizophrenia | 100% schizophrenia |
| First-episode patients included? | Yes (13% of sample) | No |
| % of patients receiving antipsychotics at enrollment | 99% | 74% |
| Baseline PANSS score (mean) | 82% FGAs; 40% depot | 15% FGAs; <5% depot |
| Baseline PANSS score | 72.2 | 75.7 |
| Baseline EPS scores | Low | Low |
| Antipsychotic options in randomization | 2 classes (SGA or FGA) (50% of subjects assigned to an FGA) | 4 SGAs, 1 FGA (20% of subjects assigned to an FGA) |
| % of subjects given sulpiride | 49% | 0% |
| Administration methodology | Medication blinded to raters but not to patients and physicians | Medication blinded to patients and physicians |
| Primary outcome | Quality of life | Discontinuation of medication |
| Long-acting antipsychotic option? | Yes | No |
| Antipsychotic switching | All patients switched agents; 49% changed antipsychotic class | 15% stayed on some agent |
| CATIE: Clinical Antipsychotic Trials of Intervention Effectiveness | ||
| CUtLASS: Cost Utility of the Latest Antipsychotic Drugs in Schizophrenia Study | ||
| EPS: Extrapyramidal symptom | ||
| FGA: First-generation antipsychotic | ||
| PANSS: Positive and Negative Syndrome Scale | ||
| SGA: Second-generation antipsychotic | ||
Comparing catie, cutlass data
The CUtLASS findings are not identical to those of CATIE phase 114 but are remarkably similar: no differences in effectiveness were seen between FGAs and SGA when treating patients with chronic schizophrenia.15,16
CUtLASS investigators concluded that “in people with schizophrenia whose medication is changed for clinical reasons, there is no disadvantage across 1 year in terms of quality of life, symptoms, or associated costs of care in using FGAs rather than nonclozapine SGAs.”7
By confirming CATIE’s results, is CUtLASS the final word on antipsychotic treatment of chronic schizophrenia? Or is it just another piece of the puzzle? CATIE and CUtLASS add much to our knowledge, but methodologic “flies in the ointment” plague all clinical trials. We must consider potential biases and confounding factors to properly interpret and apply their findings.
Although the CUtLASS trial was well-constructed and executed, its conclusions—like those of CATIE—merit careful scrutiny. Its patient recruitment methods and study design involved choices and compromises that are appropriate to evaluate17,18 as we weigh CUtLASS’ contribution to the SGA/FGA debate (Table 3).
Table 3
‘Flies in the ointment’ of the CUtLASS trial design
| Who was studied |
|
| What was compared |
|
| Other Issues |
|
| CUtLASS: Cost Utility of the Latest Antipsychotic Drugs in Schizophrenia Study | |
| EPS: Extrapyramidal symptom | |
| FGA: First-generation antipsychotic | |
| SGA: Second-generation antipsychotic | |
Who was studied?
Selection questions. CUtLASS researchers had problems recruiting patients for their study, in part because clinicians were reluctant to expose their patients to a 50% probability of being assigned to an FGA. Only 40% of the targeted sample was recruited, and participating clinicians referred only 20% to 37% of their eligible patients to the study.12 Thus, one could ask:
- Were enrolled subjects truly representative of the population from which they were drawn?
- Or did selection bias result in a disproportionate inclusion of individuals with certain characteristics?
Is it possible, for example, that clinicians preferentially referred medication-noncompliant patients to CUtLASS because they believed the benefits of depot FGAs—such as more assured adherence—would compensate for the potential benefits of SGAs—better efficacy/tolerability?19
Treatment resistance. Although patients were randomly assigned to FGAs or SGAs, a significantly greater proportion of those whose antipsychotics were being changed because of treatment resistance were assigned to receive SGAs. Treatment resistance was one reason that 88% of subjects in the SGA arm were referred to the trial, compared with 70% of subjects in the FGA arm (P<0.01).12 The extent to which this differential assignment may have biased results against SGAs is unclear.
EPS risk. CUtLASS-1 patients had been ill a mean of 14 years and had low baseline EPS rates despite receiving long-term antipsychotics (primarily FGAs). Even so, FGAs and SGAs showed similar rates of akathisia and other EPS. Thus—as with the CATIE results—the extent to which CUtLASS-1 findings may apply beyond chronic schizophrenia patients at relatively low risk for EPS is unclear.11,17
Impact of switching. Although patients were referred to CUtLASS because of adverse effects or inadequate response to one or more antipsychotics, they were only moderately ill (mean PANSS total score 72)20 and probably were deriving some benefit from their baseline antipsychotics. Before randomization, 82% of patients were receiving an FGA and 19% an SGA. Consequently, a far larger percentage of patients in the SGA group had to switch to a different medication class as the trial began.
As observed in CATIE, switching antipsychotics often has short-term negative consequences for patients,21 although switching classes (as in CUtLASS) may have had a different impact than switching individual antipsychotics (as in CATIE). If unequal antipsychotic switching rates in the two arms differentially affected patients’ quality of life, we would expect to see this effect emerge at the 12-week assessment, which is precisely where the greatest difference in Quality of Life Scale (QLS)13 scores appeared.
The mean QLS score for patients in the SGA arm was 2.6 points lower than in the FGA group at 12 weeks. This difference disappeared and, in fact, reversed at 26 weeks, but this 12-week effect had a strong impact on results of the 52-week intent-to-treat analysis. CUtLASS—like CATIE—might exemplify the risks of switching patients from treatment with partially effective antipsychotics.22
What was compared?
Classes vs individual drugs. The decision in CUtLASS-1 to compare antipsychotic classes rather than individual agents makes it difficult to interpret its findings. Antipsychotics are not homogeneous; clear differences exist within both the SGA and FGA classes in terms of individual agents’ efficacy and tolerability, and each SGA has a reasonably well-established and different side-effect profile.23
Sulpiride was the most commonly used FGA in CUtLASS-1 (by 49% of FGA patients). Sulpiride has some unusual attributes—such as lower EPS liability—and is not available in the United States. Thus, including this agent might have affected how applicable CUtLASS findings are to clinical practice in the United States.
Oral vs depot delivery. Individuals assigned to an FGA could receive either oral or long-acting depot medication, whereas those assigned to an SGA could receive only oral medication. At baseline, 84 of 227 CUtLASS-1 participants were receiving a depot antipsychotic, which was discontinued during randomization in 72 patients. During the 1-year study, the number of patients receiving a depot antipsychotic tripled from 12 to 35, suggesting the usefulness of long-acting agents in this population.19
Cross-class switching. Although participating physicians and their patients were urged to stay within assigned antipsychotic classes at least for the first 12 weeks and ideally for 1 year, a high rate of cross-class switching occurred (Figure). At the 52-week assessment, 51 of 118 patients (43%) in the intent-to-treat FGA group were receiving SGAs instead.
The CUtLASS authors’ assert that the trial refutes the hypothesis that using SGAs is superior to using FGAs in improving quality of life. This conclusion is difficult to justify when so many patients assigned to the FGA class actually were receiving SGAs. The conclusion is further weakened if differential switching rates put SGAs at a disadvantage in the first 12 weeks of the trial.
A more accurate conclusion of the intent-to-treat comparison appears in the technical report: “There was no statistically significant difference in terms of quality of life or symptoms over 1 year in commencing [italics added] conventional antipsychotic drugs rather than new atypical drugs.”12
Figure CUtLASS-1: Did switching rate affect trial outcome?
The high rate of cross-class medication switching in CUtLASS-1 may have weakened the study’s conclusion that virtually no difference in effectiveness exists between first- and second-generation antipsychotics. At the 52-week assessment, 51 of 118 patients (43%) in the intent-to-treat FGA group were receiving SGAs instead. Not shown in the figure is that 4 of the total 55 patients who switched from FGAs to SGAs had switched back to FGAs by the 52-week assessment.
CUtLASS: Cost Utility of the Latest Antipsychotic Drugs in Schizophrenia Study
FGA: First-generation antipsychotic
SGA: Second-generation antipsychotic
Source: Adapted from reference 7, Figure 1
Clinical implications
Notwithstanding these cautionary notes, CUtLASS-1 findings add to the questions raised by CATIE about the relative effectiveness of SGAs and FGAs. At a minimum, the data indicate that the SGA advantage has been overstated or oversimplified and that FGAs may be suitable options for meeting the needs of some patients with psychosis (particularly those at low risk for EPS).
Depot antipsychotics. CUtLASS also suggests a wider role for long-acting antipsychotics in chronic psychotic disorders, beyond treating patients with severe nonadherence.19,23 The number of patients receiving long-acting agents tripled over the 1-year study.12
Clozapine. Both CATIE and CUtLASS-2 confirmed clozapine’s superior efficacy for patients with treatment-resistant psychotic illness (Table 4). CUtLASS-2 also reaffirmed the challenges of clozapine’s metabolic and other side effects, such as sedation, hypotension, and hypersalivation.
All-cause discontinuation was significantly higher (P<0.05) in patients taking clozapine (73%) than in those taking other SGAs (52%). Even so, clozapine-group patients achieved significantly greater symptom reduction and tended toward a higher quality of life than other SGA-group patients.
Table 4
Clinical ‘pearls’ from the CUtLASS trial data
|
| CUtLASS: Cost Utility of the Latest Antipsychotic Drugs in Schizophrenia Study |
| EPS: Extrapyramidal symptom |
| FGA: First-generation antipsychotic |
| SGA: Second-generation antipsychotic |
Overview. In conclusion, one can reasonably conclude from analyzing the CATIE and CUtLASS data that:
- FGA-SGA differences are not as great as previously thought.
- Substantial differences exist among agents within both antipsychotic classes, particularly in side effect profiles.
- Neither study disproves the following presumed benefit of SGAs: that compared with FGAs, SGAs provide an equivalent antipsychotic effect and pose a lower risk of problems related to unmitigated dopamine blockade—such as EPS, dysphoria, bradyphrenia, neuroleptic-induced deficit syndrome, and tardive dyskinesia.11
- To use antipsychotics effectively and optimize individual treatment, consider the CATIE and CUtLASS trials in the contexts of their designs and the results of other studies of patients with chronic schizophrenia.
Related resources
- Heres S, Davis J, Maino K, et al. Why olanzapine beats risperidone, risperidone beats quetiapine, and quetiapine beats olanzapine: An exploratory analysis of head-head comparison studies of second-generation antipsychotics. Am J Psychiatry 2006;163:185-94.
Drug brand names
- Clozapine • Clozaril
- Quetiapine • Seroquel
- Olanzapine • Zyprexa
- Risperidone • Risperdal
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
When treating chronic psychotic disorders, U.S. psychiatrists generally prefer second-generation antipsychotics (SGAs) to first-generation antipsychotics (FGAs) because of widely held views1,2 that SGAs:
- are more effective for negative and cognitive symptoms
- produce fewer troublesome side effects
- help patients realize a better quality of life.
These beliefs have been challenged by two large-scale, government-supported studies: the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) in the United States3-6 and more recently the Cost Utility of the Latest Antipsychotic Drugs in Schizophrenia Study (CUtLASS) from the United Kingdom.7,8
CATIE and CUtLASS data suggest that the SGA advantage has been exaggerated, if in fact such an advantage exists. Other Current Psychiatry articles for the clinical practitioner have discussed the CATIE findings.9-11 This article addresses the CUtLASS results in the context of the trial’s methodology, using information from the primary publications7,8 and technical report.12
Cutlass study
Design. CUtLASS included 2 “bands” (Table 1):
- Band 1 compared the clinical usefulness and cost effectiveness of FGAs and SGAs in treating schizophrenia7
- Band 2 compared the effectiveness of clozapine versus other SGAs in treating refractory schizophrenia.8
CUtLASS Band 1 was not as extensive in scope as CATIE, and its design had some important differences (Table 2). Patients were referred for participation because their psychiatrists were considering a change in antipsychotic medication to address adverse effects or inadequate response. Fewer patients were recruited than expected—40% of the planned sample during 30 months of recruitment—but researchers considered the size sufficient to compare the effectiveness of FGAs and SGAs.
Patients were randomly assigned to treatment with an antipsychotic class, either:
- an FGA (1 of 11 options—including 5 depot formulations—chosen by the treating clinician)
- or an SGA (risperidone, olanzapine, quetiapine, or amisulpride, also chosen by the clinician).
Physicians and patients were not blinded to the medications used. They could choose medications within patients’ assigned classes and switch as needed in ways that mimicked clinical practice. Trained assessors, who were blinded to the medications being used, evaluated the patients after 12, 26, and 52 weeks.
Quality of life was the primary outcome measure.13 Secondary measures included symptoms, side effects, patient satisfaction, and cost of care.
Band 1 results. Patients assigned to the SGA or FGA classes showed no significant differences in quality of life measures or schizophrenia symptoms. If anything, the findings slightly favored the FGAs.
Patient satisfaction and overall cost of care were similar, and rates of extrapyramidal symptoms (EPS), tardive dyskinesia, and akathisia did not differ significantly.
Clozapine comparison. In CUtLASS band 2, a different sample of 136 schizophrenia patients who had responded poorly to ≥2 antipsychotics was randomly assigned to clozapine or one of the above four SGAs. During the 1-year comparison trial, clozapine:
- was found to be significantly more effective (P=0.01) in managing patients’ symptoms, as measured by total Positive and Negative Syndrome Scale (PANSS) score
- showed a trend (P=0.08) towards providing these treatment-resistant patients with a better quality of life.8
Table 1
Summary of CUtLASS trial design and results
Band 1
|
Band 2
|
| CUtLASS: Cost Utility of the Latest Antipsychotic Drugs in Schizophrenia Study |
| FGA: First-generation antipsychotic |
| PANSS: Positive and Negative Syndrome Scale |
| SGA: Second-generation antipsychotic |
Table 2
Comparing designs of the CUtLASS and CATIE schizophrenia trials
| CUtLASS | CATIE | |
|---|---|---|
| Trial duration | 12 months | 18 months |
| Clinical sites | 14 (United Kingdom) | 57 (United States) |
| Number of Subjects | 227 | 1,460 |
| Gender and age | 68% male; mean age 41 | 74% male; mean age 41 |
| Mental illness duration (mean) | 14 years | 16 years |
| Diagnosis | 75% schizophrenia | 100% schizophrenia |
| First-episode patients included? | Yes (13% of sample) | No |
| % of patients receiving antipsychotics at enrollment | 99% | 74% |
| Baseline PANSS score (mean) | 82% FGAs; 40% depot | 15% FGAs; <5% depot |
| Baseline PANSS score | 72.2 | 75.7 |
| Baseline EPS scores | Low | Low |
| Antipsychotic options in randomization | 2 classes (SGA or FGA) (50% of subjects assigned to an FGA) | 4 SGAs, 1 FGA (20% of subjects assigned to an FGA) |
| % of subjects given sulpiride | 49% | 0% |
| Administration methodology | Medication blinded to raters but not to patients and physicians | Medication blinded to patients and physicians |
| Primary outcome | Quality of life | Discontinuation of medication |
| Long-acting antipsychotic option? | Yes | No |
| Antipsychotic switching | All patients switched agents; 49% changed antipsychotic class | 15% stayed on some agent |
| CATIE: Clinical Antipsychotic Trials of Intervention Effectiveness | ||
| CUtLASS: Cost Utility of the Latest Antipsychotic Drugs in Schizophrenia Study | ||
| EPS: Extrapyramidal symptom | ||
| FGA: First-generation antipsychotic | ||
| PANSS: Positive and Negative Syndrome Scale | ||
| SGA: Second-generation antipsychotic | ||
Comparing catie, cutlass data
The CUtLASS findings are not identical to those of CATIE phase 114 but are remarkably similar: no differences in effectiveness were seen between FGAs and SGA when treating patients with chronic schizophrenia.15,16
CUtLASS investigators concluded that “in people with schizophrenia whose medication is changed for clinical reasons, there is no disadvantage across 1 year in terms of quality of life, symptoms, or associated costs of care in using FGAs rather than nonclozapine SGAs.”7
By confirming CATIE’s results, is CUtLASS the final word on antipsychotic treatment of chronic schizophrenia? Or is it just another piece of the puzzle? CATIE and CUtLASS add much to our knowledge, but methodologic “flies in the ointment” plague all clinical trials. We must consider potential biases and confounding factors to properly interpret and apply their findings.
Although the CUtLASS trial was well-constructed and executed, its conclusions—like those of CATIE—merit careful scrutiny. Its patient recruitment methods and study design involved choices and compromises that are appropriate to evaluate17,18 as we weigh CUtLASS’ contribution to the SGA/FGA debate (Table 3).
Table 3
‘Flies in the ointment’ of the CUtLASS trial design
| Who was studied |
|
| What was compared |
|
| Other Issues |
|
| CUtLASS: Cost Utility of the Latest Antipsychotic Drugs in Schizophrenia Study | |
| EPS: Extrapyramidal symptom | |
| FGA: First-generation antipsychotic | |
| SGA: Second-generation antipsychotic | |
Who was studied?
Selection questions. CUtLASS researchers had problems recruiting patients for their study, in part because clinicians were reluctant to expose their patients to a 50% probability of being assigned to an FGA. Only 40% of the targeted sample was recruited, and participating clinicians referred only 20% to 37% of their eligible patients to the study.12 Thus, one could ask:
- Were enrolled subjects truly representative of the population from which they were drawn?
- Or did selection bias result in a disproportionate inclusion of individuals with certain characteristics?
Is it possible, for example, that clinicians preferentially referred medication-noncompliant patients to CUtLASS because they believed the benefits of depot FGAs—such as more assured adherence—would compensate for the potential benefits of SGAs—better efficacy/tolerability?19
Treatment resistance. Although patients were randomly assigned to FGAs or SGAs, a significantly greater proportion of those whose antipsychotics were being changed because of treatment resistance were assigned to receive SGAs. Treatment resistance was one reason that 88% of subjects in the SGA arm were referred to the trial, compared with 70% of subjects in the FGA arm (P<0.01).12 The extent to which this differential assignment may have biased results against SGAs is unclear.
EPS risk. CUtLASS-1 patients had been ill a mean of 14 years and had low baseline EPS rates despite receiving long-term antipsychotics (primarily FGAs). Even so, FGAs and SGAs showed similar rates of akathisia and other EPS. Thus—as with the CATIE results—the extent to which CUtLASS-1 findings may apply beyond chronic schizophrenia patients at relatively low risk for EPS is unclear.11,17
Impact of switching. Although patients were referred to CUtLASS because of adverse effects or inadequate response to one or more antipsychotics, they were only moderately ill (mean PANSS total score 72)20 and probably were deriving some benefit from their baseline antipsychotics. Before randomization, 82% of patients were receiving an FGA and 19% an SGA. Consequently, a far larger percentage of patients in the SGA group had to switch to a different medication class as the trial began.
As observed in CATIE, switching antipsychotics often has short-term negative consequences for patients,21 although switching classes (as in CUtLASS) may have had a different impact than switching individual antipsychotics (as in CATIE). If unequal antipsychotic switching rates in the two arms differentially affected patients’ quality of life, we would expect to see this effect emerge at the 12-week assessment, which is precisely where the greatest difference in Quality of Life Scale (QLS)13 scores appeared.
The mean QLS score for patients in the SGA arm was 2.6 points lower than in the FGA group at 12 weeks. This difference disappeared and, in fact, reversed at 26 weeks, but this 12-week effect had a strong impact on results of the 52-week intent-to-treat analysis. CUtLASS—like CATIE—might exemplify the risks of switching patients from treatment with partially effective antipsychotics.22
What was compared?
Classes vs individual drugs. The decision in CUtLASS-1 to compare antipsychotic classes rather than individual agents makes it difficult to interpret its findings. Antipsychotics are not homogeneous; clear differences exist within both the SGA and FGA classes in terms of individual agents’ efficacy and tolerability, and each SGA has a reasonably well-established and different side-effect profile.23
Sulpiride was the most commonly used FGA in CUtLASS-1 (by 49% of FGA patients). Sulpiride has some unusual attributes—such as lower EPS liability—and is not available in the United States. Thus, including this agent might have affected how applicable CUtLASS findings are to clinical practice in the United States.
Oral vs depot delivery. Individuals assigned to an FGA could receive either oral or long-acting depot medication, whereas those assigned to an SGA could receive only oral medication. At baseline, 84 of 227 CUtLASS-1 participants were receiving a depot antipsychotic, which was discontinued during randomization in 72 patients. During the 1-year study, the number of patients receiving a depot antipsychotic tripled from 12 to 35, suggesting the usefulness of long-acting agents in this population.19
Cross-class switching. Although participating physicians and their patients were urged to stay within assigned antipsychotic classes at least for the first 12 weeks and ideally for 1 year, a high rate of cross-class switching occurred (Figure). At the 52-week assessment, 51 of 118 patients (43%) in the intent-to-treat FGA group were receiving SGAs instead.
The CUtLASS authors’ assert that the trial refutes the hypothesis that using SGAs is superior to using FGAs in improving quality of life. This conclusion is difficult to justify when so many patients assigned to the FGA class actually were receiving SGAs. The conclusion is further weakened if differential switching rates put SGAs at a disadvantage in the first 12 weeks of the trial.
A more accurate conclusion of the intent-to-treat comparison appears in the technical report: “There was no statistically significant difference in terms of quality of life or symptoms over 1 year in commencing [italics added] conventional antipsychotic drugs rather than new atypical drugs.”12
Figure CUtLASS-1: Did switching rate affect trial outcome?
The high rate of cross-class medication switching in CUtLASS-1 may have weakened the study’s conclusion that virtually no difference in effectiveness exists between first- and second-generation antipsychotics. At the 52-week assessment, 51 of 118 patients (43%) in the intent-to-treat FGA group were receiving SGAs instead. Not shown in the figure is that 4 of the total 55 patients who switched from FGAs to SGAs had switched back to FGAs by the 52-week assessment.
CUtLASS: Cost Utility of the Latest Antipsychotic Drugs in Schizophrenia Study
FGA: First-generation antipsychotic
SGA: Second-generation antipsychotic
Source: Adapted from reference 7, Figure 1
Clinical implications
Notwithstanding these cautionary notes, CUtLASS-1 findings add to the questions raised by CATIE about the relative effectiveness of SGAs and FGAs. At a minimum, the data indicate that the SGA advantage has been overstated or oversimplified and that FGAs may be suitable options for meeting the needs of some patients with psychosis (particularly those at low risk for EPS).
Depot antipsychotics. CUtLASS also suggests a wider role for long-acting antipsychotics in chronic psychotic disorders, beyond treating patients with severe nonadherence.19,23 The number of patients receiving long-acting agents tripled over the 1-year study.12
Clozapine. Both CATIE and CUtLASS-2 confirmed clozapine’s superior efficacy for patients with treatment-resistant psychotic illness (Table 4). CUtLASS-2 also reaffirmed the challenges of clozapine’s metabolic and other side effects, such as sedation, hypotension, and hypersalivation.
All-cause discontinuation was significantly higher (P<0.05) in patients taking clozapine (73%) than in those taking other SGAs (52%). Even so, clozapine-group patients achieved significantly greater symptom reduction and tended toward a higher quality of life than other SGA-group patients.
Table 4
Clinical ‘pearls’ from the CUtLASS trial data
|
| CUtLASS: Cost Utility of the Latest Antipsychotic Drugs in Schizophrenia Study |
| EPS: Extrapyramidal symptom |
| FGA: First-generation antipsychotic |
| SGA: Second-generation antipsychotic |
Overview. In conclusion, one can reasonably conclude from analyzing the CATIE and CUtLASS data that:
- FGA-SGA differences are not as great as previously thought.
- Substantial differences exist among agents within both antipsychotic classes, particularly in side effect profiles.
- Neither study disproves the following presumed benefit of SGAs: that compared with FGAs, SGAs provide an equivalent antipsychotic effect and pose a lower risk of problems related to unmitigated dopamine blockade—such as EPS, dysphoria, bradyphrenia, neuroleptic-induced deficit syndrome, and tardive dyskinesia.11
- To use antipsychotics effectively and optimize individual treatment, consider the CATIE and CUtLASS trials in the contexts of their designs and the results of other studies of patients with chronic schizophrenia.
Related resources
- Heres S, Davis J, Maino K, et al. Why olanzapine beats risperidone, risperidone beats quetiapine, and quetiapine beats olanzapine: An exploratory analysis of head-head comparison studies of second-generation antipsychotics. Am J Psychiatry 2006;163:185-94.
Drug brand names
- Clozapine • Clozaril
- Quetiapine • Seroquel
- Olanzapine • Zyprexa
- Risperidone • Risperdal
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Kane JM, Leucht S, Carpenter D, et al. Expert consensus guideline series: optimizing pharmacologic treatment of psychotic disorders. J Clin Psychiatry 2003;64(suppl 12):1-100.
2. Tandon R, Fleischhacker WW. Comparative efficacy of antipsychotics in the treatment of schizophrenia: a critical assessment. Schizophr Res 2005;79:145-55.
3. Lieberman JA, Stroup TS, McEvoy JP, et al. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med 2005;353:1209-23.
4. McEvoy JP, Lieberman JA, Stroup TS, et al. Effectiveness of clozapine versus olanzapine, quetiapine, and risperidone in patients with chronic schizophrenia who did not respond to prior antipsychotic treatment. Am J Psychiatry 2006;163:600-10.
5. Stroup TS, Lieberman JA, McEvoy JP, et al. Effectiveness of olanzapine, quetiapine, risperidone, and ziprasidone in patients with chronic schizophrenia following discontinuation of a previous atypical antipsychotic. Am J Psychiatry 2006;163:611-22.
6. Rosenheck RA, Leslie DL, Sindelar J, et al. Cost-effectiveness of second-generation antipsychotics and perphenazine in a randomized trial of treatment for chronic schizophrenia. Am J Psychiatry 2006;163:2080-9.
7. Jones PB, Barnes T, Davies L, et al. Randomized controlled trial of the effect on quality of life of second- vs first-generation antipsychotic drugs in schizophrenia: Cost Utility of the Latest Antipsychotic Drugs in Schizophrenia Study (CUtLASS). Arch Gen Psychiatry 2006;63:1079-87.
8. Lewis SW, Barnes TRE, Davies L, et al. Randomised controlled trial of effect of prescription of clozapine versus other second-generation antipsychotic drugs in resistant schizophrenia. Schizophr Bull 2006;32:715-23.
9. Nasrallah HA. CATIE’s surprises: in antipsychotics’ square-off, were there winners or losers? Current Psychiatry 2006;5(2):49-65.
10. Buckley PF. Which antipsychotic do I choose next? CATIE phase 2 offers insights on efficacy and tolerability. Current Psychiatry 2006;5(9):27-43.
11. Tandon R, Constantine R. Avoiding EPS is key to realizing ‘atypical’ benefits. Current Psychiatry 2006;5(11):35-45.
12. Lewis SW, Davies L, Jones PB, et al. Randomised controlled trials of conventional antipsychotic versus new atypical drugs, and new atypical drugs versus clozapine, in people with schizophrenia responding poorly to, or intolerant of, current drug treatment. Health Technology Assessment 2006;10(#17):1-182.Available at http://www.hta.ac.uk/project/1078.asp. Accessed January 3, 2007.
13. Heinrichs DW, Hanlon TE, Carpenter WT. The Quality of Life Scale: an instrument for assessing the schizophrenic deficit syndrome. Schizophr Bull 1984;10:388-98.
14. Tandon R, Davis JM, Carpenter WT. CATIE, CUtLASS, and the FGA-SGA debate (letter). Arch Gen Psychiatry 2007 (in press).
15. Lieberman J. Comparative effectiveness of antipsychotic drugs: a commentary on the Cost Utility of the Latest Antipsychotic Drugs in Schizophrenia Study and Clinical Antipsychotic Trials of Intervention Effectiveness. Arch Gen Psychiatry 2006;63:1069-72.
16. Rosenheck RA. Outcomes, costs, and policy caution: a commentary on the Cost Utility of the Latest Antipsychotic Drugs in Schizophrenia Study. Arch Gen Psychiatry 2006;63:1074-6.
17. Tandon R. Comparative effectiveness of antipsychotics in the treatment of schizophrenia: what CATIE tells us—Parts 1 and 2. International Drug Therapy Newsletter 2006;41(7,9):51-8,67-74.
18. Meltzer HY, Bobo WV. Interpreting the efficacy findings in the CATIE study: what clinicians should know. CNS Spectrums 2006;11(suppl 7):14-24.
19. Kane JM. Review of treatments that can ameliorate nonadherence in patients with schizophrenia. J Clin Psychiatry 2006;67(suppl 5):9-14.
20. Leucht S, Kane JM, Kissling W, et al. What does the PANSS mean? Schizophr Res 2006;79:231-8.
21. Essock SM, Covell NH, Davis SM, et al. Effectiveness of switching antipsychotic medications. Am J Psychiatry 2006;163:2090-5.
22. Davis JM, Marder S, Tamminga CA. Switch or stay? Am J Psychiatry 2006;163:2032-3.
23. Tandon R, Targum SD, Nasrallah HA, et al. Strategies for maximizing clinical effectiveness in the treatment of schizophrenia. Journal of Psychiatric Practice 2006;12:348-63.
1. Kane JM, Leucht S, Carpenter D, et al. Expert consensus guideline series: optimizing pharmacologic treatment of psychotic disorders. J Clin Psychiatry 2003;64(suppl 12):1-100.
2. Tandon R, Fleischhacker WW. Comparative efficacy of antipsychotics in the treatment of schizophrenia: a critical assessment. Schizophr Res 2005;79:145-55.
3. Lieberman JA, Stroup TS, McEvoy JP, et al. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med 2005;353:1209-23.
4. McEvoy JP, Lieberman JA, Stroup TS, et al. Effectiveness of clozapine versus olanzapine, quetiapine, and risperidone in patients with chronic schizophrenia who did not respond to prior antipsychotic treatment. Am J Psychiatry 2006;163:600-10.
5. Stroup TS, Lieberman JA, McEvoy JP, et al. Effectiveness of olanzapine, quetiapine, risperidone, and ziprasidone in patients with chronic schizophrenia following discontinuation of a previous atypical antipsychotic. Am J Psychiatry 2006;163:611-22.
6. Rosenheck RA, Leslie DL, Sindelar J, et al. Cost-effectiveness of second-generation antipsychotics and perphenazine in a randomized trial of treatment for chronic schizophrenia. Am J Psychiatry 2006;163:2080-9.
7. Jones PB, Barnes T, Davies L, et al. Randomized controlled trial of the effect on quality of life of second- vs first-generation antipsychotic drugs in schizophrenia: Cost Utility of the Latest Antipsychotic Drugs in Schizophrenia Study (CUtLASS). Arch Gen Psychiatry 2006;63:1079-87.
8. Lewis SW, Barnes TRE, Davies L, et al. Randomised controlled trial of effect of prescription of clozapine versus other second-generation antipsychotic drugs in resistant schizophrenia. Schizophr Bull 2006;32:715-23.
9. Nasrallah HA. CATIE’s surprises: in antipsychotics’ square-off, were there winners or losers? Current Psychiatry 2006;5(2):49-65.
10. Buckley PF. Which antipsychotic do I choose next? CATIE phase 2 offers insights on efficacy and tolerability. Current Psychiatry 2006;5(9):27-43.
11. Tandon R, Constantine R. Avoiding EPS is key to realizing ‘atypical’ benefits. Current Psychiatry 2006;5(11):35-45.
12. Lewis SW, Davies L, Jones PB, et al. Randomised controlled trials of conventional antipsychotic versus new atypical drugs, and new atypical drugs versus clozapine, in people with schizophrenia responding poorly to, or intolerant of, current drug treatment. Health Technology Assessment 2006;10(#17):1-182.Available at http://www.hta.ac.uk/project/1078.asp. Accessed January 3, 2007.
13. Heinrichs DW, Hanlon TE, Carpenter WT. The Quality of Life Scale: an instrument for assessing the schizophrenic deficit syndrome. Schizophr Bull 1984;10:388-98.
14. Tandon R, Davis JM, Carpenter WT. CATIE, CUtLASS, and the FGA-SGA debate (letter). Arch Gen Psychiatry 2007 (in press).
15. Lieberman J. Comparative effectiveness of antipsychotic drugs: a commentary on the Cost Utility of the Latest Antipsychotic Drugs in Schizophrenia Study and Clinical Antipsychotic Trials of Intervention Effectiveness. Arch Gen Psychiatry 2006;63:1069-72.
16. Rosenheck RA. Outcomes, costs, and policy caution: a commentary on the Cost Utility of the Latest Antipsychotic Drugs in Schizophrenia Study. Arch Gen Psychiatry 2006;63:1074-6.
17. Tandon R. Comparative effectiveness of antipsychotics in the treatment of schizophrenia: what CATIE tells us—Parts 1 and 2. International Drug Therapy Newsletter 2006;41(7,9):51-8,67-74.
18. Meltzer HY, Bobo WV. Interpreting the efficacy findings in the CATIE study: what clinicians should know. CNS Spectrums 2006;11(suppl 7):14-24.
19. Kane JM. Review of treatments that can ameliorate nonadherence in patients with schizophrenia. J Clin Psychiatry 2006;67(suppl 5):9-14.
20. Leucht S, Kane JM, Kissling W, et al. What does the PANSS mean? Schizophr Res 2006;79:231-8.
21. Essock SM, Covell NH, Davis SM, et al. Effectiveness of switching antipsychotic medications. Am J Psychiatry 2006;163:2090-5.
22. Davis JM, Marder S, Tamminga CA. Switch or stay? Am J Psychiatry 2006;163:2032-3.
23. Tandon R, Targum SD, Nasrallah HA, et al. Strategies for maximizing clinical effectiveness in the treatment of schizophrenia. Journal of Psychiatric Practice 2006;12:348-63.
Avoiding EPS is key to realizing ‘atypical’ benefits
Many findings of the Clinical Antipsychotic Trials of Intervention Effectiveness in schizophrenia (CATIE) were unexpected,1,2 but one was arguably the most surprising. It was that schizophrenia patients showed similar rates of extrapyramidal symptoms (EPS), whether treated with a first-generation antipsychotic (FGA) or any of four second-generation antipsychotics (SGAs).
This finding in CATIE phase 1 runs contrary to the understanding that SGAs, compared with FGAs, provide a broader spectrum of efficacy with significantly fewer motor side effects. A substantial body of evidence and virtually all schizophrenia treatment guidelines3-5 support this prevailing view.
Did earlier schizophrenia treatment studies misinform us, or was CATIE’s comparison of FGAs and SGAs “flawed”?6,7 This article attempts to reconcile the divergent findings about antipsychotics and EPS and reveals a clinical pearl that suggests how to provide optimum antipsychotic therapy to schizophrenia patients.
What did catie find?
CATIE was a three-phase, 18-month, randomized controlled clinical trial designed to evaluate the effectiveness of five SGAs (risperidone, olanzapine, quetiapine, ziprasidone, and clozapine) and two FGAs (perphenazine and fluphenazine) in treating schizophrenia. Findings from phases 1 and 2 have been published or presented (Table 1),2,8-9 and results from phase 3 are awaited.
Table 1
5 key findings from CATIE phases 1 and 2
|
CATIE phase 1 found no difference in efficacy, safety/tolerability, or effectiveness among perphenazine, risperidone, ziprasidone, and quetiapine. Soon-to-be-published data also will show no significant difference in cognitive effects among patients receiving perphenazine or any of four SGAs (risperidone, olanzapine, quetiapine, or ziprasidone).8 Because no FGA was used in CATIE phase 2,9-11 its results added little to phase 1 observations about how “typical” and “atypical” antipsychotics compare.
‘Atypicals’ and EPS. By definition, a reduced tendency to cause EPS (such as parkinsonism, dystonia, akathisia, and akinesia) distinguishes SGAs from FGAs. In fact, SGAs were called “atypical” because they disproved the belief that EPS are an unavoidable consequence of drugs that produce an antipsychotic effect.12,13 The CATIE trial’s inability to detect a difference in EPS rates between typical and atypical antipsychotics (Table 2)2 is therefore the study’s most surprising finding.
Table 2
CATIE: Similar EPS rates with perphenazine and SGAs*
| EPS measurement | Perphenazine-treated patients | SGA-treated patients |
|---|---|---|
| Increased mean Simpson-Angus Scale score | 6% | 4% to 8% |
| Increased AIMS global severity score | 17% | 13% to 16% |
| Increased Barnes Akathisia Rating Scale score | 7% | 5% to 9% |
| Anticholinergic added | 10% | 3% to 9% |
| * Differences were not statistically significant | ||
| EPS: extrapyramidal side effects | ||
| SGA: second-generation antipsychotic | ||
| AIMS: Abnormal Involuntary Movement Scale | ||
| Source: Reference 2 | ||
Making sense of catie
Most studies suggest consistent differences between FGAs and SGAs in risk of EPS and tardive dyskinesia.14-16 One explanation for CATIE’s discrepant findings may be that the use of high-dose, high-potency haloperidol as the typical comparator in pre-CATIE studies magnified differences between FGAs and SGAs.17,18
Conversely, CATIE researchers minimized this difference by studying a population of schizophrenia patients at an unusually low risk for EPS. The study design:
- assigned 231 patients with a history of tardive dyskinesia to an SGA, without the opportunity to be randomly assigned to an FGA
- excluded patients with first-episode schizophrenia
- enrolled patients who had been treated with antipsychotics for an average of 14 years without a history of significant adverse effects from study treatments.19
Just as prior studies might have exaggerated the EPS advantage for SGAs, CATIE might have minimized the FGA-SGA difference by studying a low-risk cohort in a way that reduced the trial’s ability to detect such differences.
Interpretation. How can we reconcile the absence of a difference between FGAs and SGAs in EPS liability in CATIE with the preponderance of data suggesting otherwise? It appears that SGAs may be less likely to cause EPS than FGAs, but this difference is not evident in all populations. Furthermore, SGAs and FGAs differ in their ability to provide an adequate antipsychotic effect without EPS.
Among FGAs, low-potency agents are less likely to cause EPS or require concomitant anticholinergics than high-potency agents. Among SGAs, the gradient of EPS liability appears to be risperidone > olanzapine, aripiprazole, ziprasidone > quetiapine > clozapine (Figure). Clinically, these pharmacologic differences interact with physiologic differences in EPS vulnerability—some patients are more liable to develop EPS than others. Individuals who are more susceptible to developing EPS are more likely to benefit from antipsychotics with lower EPS liability.
CATIE found no difference among the various FGAs and SGAs with regard to overall efficacy, effects on cognition, and occurrence of tardive dyskinesia in treating chronic schizophrenia. Perhaps it was CATIE’s failure to find a difference in EPS that explains its inability to demonstrate FGA-SGA differences in cognition and other effectiveness domains.
Figure Dose-response curves: Antipsychotic vs extrapyramidal effects
All FGAs and SGAs produce an equivalent antipsychotic effect (red), but they vary in the degree of separation between dosages at which their antipsychotic and extrapyramidal effects occur.
Source: Adapted from reference 13
What catie tells us
The exaggerated view of SGAs as uniformly more efficacious, safer, and better tolerated than FGAs needs to be revised. At the same time, however, the results of CATIE should not be over-interpreted. They tell us that if the four phase 1 SGAs and the FGA perphenazine are used at certain dosages in a particular manner in a specific schizophrenia population—chronic, moderately ill, without tardive dyskinesia—then no differences might be expected among these antipsychotics. But CATIE’s findings might not generalize beyond individuals with schizophrenia at low risk for EPS.
CATIE underlines the importance of achieving an adequate antipsychotic effect without EPS and without using anticholinergics. Clinical consequences of EPS extend beyond motor manifestations and include:
- worse cognition (bradyphrenia)
- worse negative symptoms (neuroleptic-induced deficit syndrome)
- worse depression and suicidality (neuroleptic dysphoria)
- higher risk of tardive dyskinesia.20
SGAs’ presumed ability to provide broader efficacy—cognition, negative symptoms, dysphoria—and lower risk of tardive dyskinesia appears to be driven by their lower EPS liability in association with an equivalent antipsychotic effect. Evidence for an SGA advantage independent of this effect is weak.21,22
Thus, CATIE’s inability to find an FGA-SGA difference in EPS might explain its failure to observe an FGA-SGA difference in cognition and other effectiveness domains.
The clinical pearl
Avoiding EPS and anticholinergics appears to be the key to improving cognition, dysphoria, and negative symptoms with FGAs and SGAs. SGAs’ ability to achieve an equivalent antipsychotic effect without EPS also seems related to their lower risk of tardive dyskinesia.
SGAs’ main advantage may be their greater ease of achieving an adequate antipsychotic effect without EPS or the need to add an anticholinergic to treat or prevent EPS. This comes from the broader separation between dosages at which SGAs produce their antipsychotic versus EPS effects, compared with FGAs (Figure).13
In clinical practice, then, we must achieve an adequate antipsychotic effect for our patients without EPS—whether we are using FGAs or SGAs—to obtain “atypical” benefits. The purported benefits of an “atypical” antipsychotic are not unique to a particular class of agents but relate to achieving a good antipsychotic effect without EPS—and the SGAs are better able to accomplish this than the FGAs.
Careful EPS monitoring is crucial to achieving optimal antipsychotic therapy. Reduced emphasis on EPS in the past decade (in awareness of EPS and training to detect symptoms) and overlap between behavioral aspects of EPS and psychopathology need to be addressed.
CATIE confirms clinical observations that:
Different agents are associated with different adverse effects, which can make achieving maximum efficacy and safety/tolerability challenging.
But differences among antipsychotics and heterogeneity in individual response and vulnerabilities may allow us to optimize treatment.
Different agents at different dosages may provide the best outcomes for individual patients, and the optimal agent and/or dosage can vary in the same patient at different stages of the illness. The CATIE trial contributes to evidence that guides our efforts to provide optimal antipsychotic treatment of schizophrenia (Table 3). Its “surprising” findings are most useful when considered in the context of the database to which it adds.25
Table 3
Treating chronic schizophrenia: 4 clinical tips from CATIE
| Minimizing extrapyramidal symptoms (EPS) is essential, whether using FGAs or SGAs |
| Avoiding EPS and not using adjunctive anticholinergics is the key to SGAs’ purported benefits, such better cognition, less dysphoria, lower negative symptom burden, and lower risk of tardive dyskinesia |
| Antipsychotic dosing is key to accomplishing an adequate antipsychotic effect without EPS |
| Match the antipsychotic choice and dosage to the individual patient’s vulnerability, then make adjustments based on response |
Related resources
- Tandon R. Comparative effectiveness of antipsychotics in the treatment of schizophrenia: What does CATIE tell us? Parts 1 and 2. Int Drug Ther Newsl 2006;41:51-8;67-74.
- Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) schizophrenia study. www.catie.unc.edu/schizophrenia.
Drug brand names
- Clozapine • Clozaril
- Fluphenazine • Permitil
- Olanzapine • Zyprexa
- Perphenazine • Trilafon
- Risperidone • Risperdal
- Quetiapine • Seroquel
- Ziprasidone • Geodon
Disclosures
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Nasrallah HA. CATIE’s surprises. Current Psychiatry 2006;5(2):48-65.
2. Lieberman JA, Stroup ST, McEvoy JP, et al. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med 2005;353:1209-23.
3. Kane JM, Leucht S, Carpenter D, et al. The expert consensus guideline series: optimizing pharmacologic treatment of psychotic disorders. J Clin Psychiatry 2003;64(suppl 12):1-100.
4. Miller AL, Hall CS, Buchanan RW, et al. The Texas Medication Algorithm Project antipsychotic algorithm for schizophrenia: 2003 update. J Clin Psychiatry 2004;65:500-8.
5. American Psychiatric Association. Practice guideline for the treatment of patients with schizophrenia, 2nd ed. Am J Psychiatry 2004;161(suppl 2):1-56.
6. Kane JM. Commentary on the CATIE trial. J Clin Psychiatry 2006;67:831-2.
7. Glick ID. Understanding the results of CATIE in the context of the field. CNS Spectrums 2006;1(suppl 7):40-7.
8. Keefe RSE. Neurocognitive effects of antipsychotic medications in patients with chronic schizophrenia in the CATIE trial. Paper presented at: 61st Society of Biological Psychiatry annual meeting; May 18-20, 2006; Toronto, Canada.
9. McEvoy JP, Lieberman JA, Stroup TS, et al. Effectiveness of clozapine versus olanzapine, quetiapine, and risperidone in patients with chronic schizophrenia who did not respond to prior antipsychotic treatment. Am J Psychiatry 2006;163:600-10.
10. Stroup TS, Lieberman JA, McEvoy JP, et al. Effectiveness of olanzapine, quetiapine, risperidone, and ziprasidone in patients with chronic schizophrenia following discontinuation of a previous atypical antipsychotic. Am J Psychiatry 2006;163:611-22.
11. Buckley PF. Which antipsychotic do I choose next? CATIE phase 2 offers insights on efficacy and tolerability. Current Psychiatry 2006;5(9):27-43.
12. Casey DE. Motor and mental aspects of EPS. Int Clin Psychopharmacol 1995;10:105-14.
13. Jibson MD, Tandon R. New atypical antipsychotic medications. J Psychiatr Res 1998;32:215-28.
14. Pierre JM. Extrapyramidal symptoms with atypical antipsychotics. Drug Safety 2005;28:191-208.
15. Kelly DL, Conley RR, Carpenter WT. First-episode schizophrenia: A focus on pharmacological treatment and safety considerations. Drugs 2005;65:1113-38.
16. Correll CU, Leucht S, Kane JM. Lower risk for tardive dyskinesia associated with second-generation antipsychotics: a systematic review of 1-year studies. Am J Psychiatry 2004;161:414-25.
17. Geddes J, Freemantle N, Harrison P, et al. Atypical antipsychotics in the treatment of schizophrenia: a systematic overview and meta-regression analysis. BMJ 2000;231:1371-6.
18. Hugenholtz GW, Heerdink ER, Stolker JJ, et al. Haloperidol dose when used as active comparator in randomized controlled trials with atypical antipsychotics in schizophrenia: comparison with officially recommended doses. J Clin Psychiatry 2006;67:897-903.
19. Casey D. Implications of the CATIE trial on treatment: extrapyramidal symptoms. CNS Spectrums 2006;11(suppl 7):25-31.
20. Tandon R. Jibson MD: Extrapyramidal side effects of antipsychotic treatment: Scope of problem and impact on outcome. Ann Clin Psychiatry 2002;14:123-9.
21. Thornton AE, Snellenberg JXV, Sepehry AA, Honer WG. The impact of atypical antipsychotic medications on long-term memory dysfunction in schizophrenia spectrum disorder: a quantitative review. J Psychopharmacol 2006;20:335-46.
22. Carpenter WT, Gold JM. Another view of therapy for cognition in schizophrenia. Biol Psychiatry 2002;51:972-8.
23. Davis JM, Chen N. Dose response and dose equivalence of antipsychotics. J Clin Psychopharmacol 2004;24:192-208.
24. Tandon R, Nasrallah HA. Subjecting meta-analyses to closer scrutiny: Little support for differential efficacy among second-generation antipsychotics at equivalent doses. Arch Gen Psychiatry 2006;62:935-7.
25. Tandon R. Comparing antipsychotic efficacy. Am J Psychiatry 2006;163:1645.-
Many findings of the Clinical Antipsychotic Trials of Intervention Effectiveness in schizophrenia (CATIE) were unexpected,1,2 but one was arguably the most surprising. It was that schizophrenia patients showed similar rates of extrapyramidal symptoms (EPS), whether treated with a first-generation antipsychotic (FGA) or any of four second-generation antipsychotics (SGAs).
This finding in CATIE phase 1 runs contrary to the understanding that SGAs, compared with FGAs, provide a broader spectrum of efficacy with significantly fewer motor side effects. A substantial body of evidence and virtually all schizophrenia treatment guidelines3-5 support this prevailing view.
Did earlier schizophrenia treatment studies misinform us, or was CATIE’s comparison of FGAs and SGAs “flawed”?6,7 This article attempts to reconcile the divergent findings about antipsychotics and EPS and reveals a clinical pearl that suggests how to provide optimum antipsychotic therapy to schizophrenia patients.
What did catie find?
CATIE was a three-phase, 18-month, randomized controlled clinical trial designed to evaluate the effectiveness of five SGAs (risperidone, olanzapine, quetiapine, ziprasidone, and clozapine) and two FGAs (perphenazine and fluphenazine) in treating schizophrenia. Findings from phases 1 and 2 have been published or presented (Table 1),2,8-9 and results from phase 3 are awaited.
Table 1
5 key findings from CATIE phases 1 and 2
|
CATIE phase 1 found no difference in efficacy, safety/tolerability, or effectiveness among perphenazine, risperidone, ziprasidone, and quetiapine. Soon-to-be-published data also will show no significant difference in cognitive effects among patients receiving perphenazine or any of four SGAs (risperidone, olanzapine, quetiapine, or ziprasidone).8 Because no FGA was used in CATIE phase 2,9-11 its results added little to phase 1 observations about how “typical” and “atypical” antipsychotics compare.
‘Atypicals’ and EPS. By definition, a reduced tendency to cause EPS (such as parkinsonism, dystonia, akathisia, and akinesia) distinguishes SGAs from FGAs. In fact, SGAs were called “atypical” because they disproved the belief that EPS are an unavoidable consequence of drugs that produce an antipsychotic effect.12,13 The CATIE trial’s inability to detect a difference in EPS rates between typical and atypical antipsychotics (Table 2)2 is therefore the study’s most surprising finding.
Table 2
CATIE: Similar EPS rates with perphenazine and SGAs*
| EPS measurement | Perphenazine-treated patients | SGA-treated patients |
|---|---|---|
| Increased mean Simpson-Angus Scale score | 6% | 4% to 8% |
| Increased AIMS global severity score | 17% | 13% to 16% |
| Increased Barnes Akathisia Rating Scale score | 7% | 5% to 9% |
| Anticholinergic added | 10% | 3% to 9% |
| * Differences were not statistically significant | ||
| EPS: extrapyramidal side effects | ||
| SGA: second-generation antipsychotic | ||
| AIMS: Abnormal Involuntary Movement Scale | ||
| Source: Reference 2 | ||
Making sense of catie
Most studies suggest consistent differences between FGAs and SGAs in risk of EPS and tardive dyskinesia.14-16 One explanation for CATIE’s discrepant findings may be that the use of high-dose, high-potency haloperidol as the typical comparator in pre-CATIE studies magnified differences between FGAs and SGAs.17,18
Conversely, CATIE researchers minimized this difference by studying a population of schizophrenia patients at an unusually low risk for EPS. The study design:
- assigned 231 patients with a history of tardive dyskinesia to an SGA, without the opportunity to be randomly assigned to an FGA
- excluded patients with first-episode schizophrenia
- enrolled patients who had been treated with antipsychotics for an average of 14 years without a history of significant adverse effects from study treatments.19
Just as prior studies might have exaggerated the EPS advantage for SGAs, CATIE might have minimized the FGA-SGA difference by studying a low-risk cohort in a way that reduced the trial’s ability to detect such differences.
Interpretation. How can we reconcile the absence of a difference between FGAs and SGAs in EPS liability in CATIE with the preponderance of data suggesting otherwise? It appears that SGAs may be less likely to cause EPS than FGAs, but this difference is not evident in all populations. Furthermore, SGAs and FGAs differ in their ability to provide an adequate antipsychotic effect without EPS.
Among FGAs, low-potency agents are less likely to cause EPS or require concomitant anticholinergics than high-potency agents. Among SGAs, the gradient of EPS liability appears to be risperidone > olanzapine, aripiprazole, ziprasidone > quetiapine > clozapine (Figure). Clinically, these pharmacologic differences interact with physiologic differences in EPS vulnerability—some patients are more liable to develop EPS than others. Individuals who are more susceptible to developing EPS are more likely to benefit from antipsychotics with lower EPS liability.
CATIE found no difference among the various FGAs and SGAs with regard to overall efficacy, effects on cognition, and occurrence of tardive dyskinesia in treating chronic schizophrenia. Perhaps it was CATIE’s failure to find a difference in EPS that explains its inability to demonstrate FGA-SGA differences in cognition and other effectiveness domains.
Figure Dose-response curves: Antipsychotic vs extrapyramidal effects
All FGAs and SGAs produce an equivalent antipsychotic effect (red), but they vary in the degree of separation between dosages at which their antipsychotic and extrapyramidal effects occur.
Source: Adapted from reference 13
What catie tells us
The exaggerated view of SGAs as uniformly more efficacious, safer, and better tolerated than FGAs needs to be revised. At the same time, however, the results of CATIE should not be over-interpreted. They tell us that if the four phase 1 SGAs and the FGA perphenazine are used at certain dosages in a particular manner in a specific schizophrenia population—chronic, moderately ill, without tardive dyskinesia—then no differences might be expected among these antipsychotics. But CATIE’s findings might not generalize beyond individuals with schizophrenia at low risk for EPS.
CATIE underlines the importance of achieving an adequate antipsychotic effect without EPS and without using anticholinergics. Clinical consequences of EPS extend beyond motor manifestations and include:
- worse cognition (bradyphrenia)
- worse negative symptoms (neuroleptic-induced deficit syndrome)
- worse depression and suicidality (neuroleptic dysphoria)
- higher risk of tardive dyskinesia.20
SGAs’ presumed ability to provide broader efficacy—cognition, negative symptoms, dysphoria—and lower risk of tardive dyskinesia appears to be driven by their lower EPS liability in association with an equivalent antipsychotic effect. Evidence for an SGA advantage independent of this effect is weak.21,22
Thus, CATIE’s inability to find an FGA-SGA difference in EPS might explain its failure to observe an FGA-SGA difference in cognition and other effectiveness domains.
The clinical pearl
Avoiding EPS and anticholinergics appears to be the key to improving cognition, dysphoria, and negative symptoms with FGAs and SGAs. SGAs’ ability to achieve an equivalent antipsychotic effect without EPS also seems related to their lower risk of tardive dyskinesia.
SGAs’ main advantage may be their greater ease of achieving an adequate antipsychotic effect without EPS or the need to add an anticholinergic to treat or prevent EPS. This comes from the broader separation between dosages at which SGAs produce their antipsychotic versus EPS effects, compared with FGAs (Figure).13
In clinical practice, then, we must achieve an adequate antipsychotic effect for our patients without EPS—whether we are using FGAs or SGAs—to obtain “atypical” benefits. The purported benefits of an “atypical” antipsychotic are not unique to a particular class of agents but relate to achieving a good antipsychotic effect without EPS—and the SGAs are better able to accomplish this than the FGAs.
Careful EPS monitoring is crucial to achieving optimal antipsychotic therapy. Reduced emphasis on EPS in the past decade (in awareness of EPS and training to detect symptoms) and overlap between behavioral aspects of EPS and psychopathology need to be addressed.
CATIE confirms clinical observations that:
Different agents are associated with different adverse effects, which can make achieving maximum efficacy and safety/tolerability challenging.
But differences among antipsychotics and heterogeneity in individual response and vulnerabilities may allow us to optimize treatment.
Different agents at different dosages may provide the best outcomes for individual patients, and the optimal agent and/or dosage can vary in the same patient at different stages of the illness. The CATIE trial contributes to evidence that guides our efforts to provide optimal antipsychotic treatment of schizophrenia (Table 3). Its “surprising” findings are most useful when considered in the context of the database to which it adds.25
Table 3
Treating chronic schizophrenia: 4 clinical tips from CATIE
| Minimizing extrapyramidal symptoms (EPS) is essential, whether using FGAs or SGAs |
| Avoiding EPS and not using adjunctive anticholinergics is the key to SGAs’ purported benefits, such better cognition, less dysphoria, lower negative symptom burden, and lower risk of tardive dyskinesia |
| Antipsychotic dosing is key to accomplishing an adequate antipsychotic effect without EPS |
| Match the antipsychotic choice and dosage to the individual patient’s vulnerability, then make adjustments based on response |
Related resources
- Tandon R. Comparative effectiveness of antipsychotics in the treatment of schizophrenia: What does CATIE tell us? Parts 1 and 2. Int Drug Ther Newsl 2006;41:51-8;67-74.
- Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) schizophrenia study. www.catie.unc.edu/schizophrenia.
Drug brand names
- Clozapine • Clozaril
- Fluphenazine • Permitil
- Olanzapine • Zyprexa
- Perphenazine • Trilafon
- Risperidone • Risperdal
- Quetiapine • Seroquel
- Ziprasidone • Geodon
Disclosures
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Many findings of the Clinical Antipsychotic Trials of Intervention Effectiveness in schizophrenia (CATIE) were unexpected,1,2 but one was arguably the most surprising. It was that schizophrenia patients showed similar rates of extrapyramidal symptoms (EPS), whether treated with a first-generation antipsychotic (FGA) or any of four second-generation antipsychotics (SGAs).
This finding in CATIE phase 1 runs contrary to the understanding that SGAs, compared with FGAs, provide a broader spectrum of efficacy with significantly fewer motor side effects. A substantial body of evidence and virtually all schizophrenia treatment guidelines3-5 support this prevailing view.
Did earlier schizophrenia treatment studies misinform us, or was CATIE’s comparison of FGAs and SGAs “flawed”?6,7 This article attempts to reconcile the divergent findings about antipsychotics and EPS and reveals a clinical pearl that suggests how to provide optimum antipsychotic therapy to schizophrenia patients.
What did catie find?
CATIE was a three-phase, 18-month, randomized controlled clinical trial designed to evaluate the effectiveness of five SGAs (risperidone, olanzapine, quetiapine, ziprasidone, and clozapine) and two FGAs (perphenazine and fluphenazine) in treating schizophrenia. Findings from phases 1 and 2 have been published or presented (Table 1),2,8-9 and results from phase 3 are awaited.
Table 1
5 key findings from CATIE phases 1 and 2
|
CATIE phase 1 found no difference in efficacy, safety/tolerability, or effectiveness among perphenazine, risperidone, ziprasidone, and quetiapine. Soon-to-be-published data also will show no significant difference in cognitive effects among patients receiving perphenazine or any of four SGAs (risperidone, olanzapine, quetiapine, or ziprasidone).8 Because no FGA was used in CATIE phase 2,9-11 its results added little to phase 1 observations about how “typical” and “atypical” antipsychotics compare.
‘Atypicals’ and EPS. By definition, a reduced tendency to cause EPS (such as parkinsonism, dystonia, akathisia, and akinesia) distinguishes SGAs from FGAs. In fact, SGAs were called “atypical” because they disproved the belief that EPS are an unavoidable consequence of drugs that produce an antipsychotic effect.12,13 The CATIE trial’s inability to detect a difference in EPS rates between typical and atypical antipsychotics (Table 2)2 is therefore the study’s most surprising finding.
Table 2
CATIE: Similar EPS rates with perphenazine and SGAs*
| EPS measurement | Perphenazine-treated patients | SGA-treated patients |
|---|---|---|
| Increased mean Simpson-Angus Scale score | 6% | 4% to 8% |
| Increased AIMS global severity score | 17% | 13% to 16% |
| Increased Barnes Akathisia Rating Scale score | 7% | 5% to 9% |
| Anticholinergic added | 10% | 3% to 9% |
| * Differences were not statistically significant | ||
| EPS: extrapyramidal side effects | ||
| SGA: second-generation antipsychotic | ||
| AIMS: Abnormal Involuntary Movement Scale | ||
| Source: Reference 2 | ||
Making sense of catie
Most studies suggest consistent differences between FGAs and SGAs in risk of EPS and tardive dyskinesia.14-16 One explanation for CATIE’s discrepant findings may be that the use of high-dose, high-potency haloperidol as the typical comparator in pre-CATIE studies magnified differences between FGAs and SGAs.17,18
Conversely, CATIE researchers minimized this difference by studying a population of schizophrenia patients at an unusually low risk for EPS. The study design:
- assigned 231 patients with a history of tardive dyskinesia to an SGA, without the opportunity to be randomly assigned to an FGA
- excluded patients with first-episode schizophrenia
- enrolled patients who had been treated with antipsychotics for an average of 14 years without a history of significant adverse effects from study treatments.19
Just as prior studies might have exaggerated the EPS advantage for SGAs, CATIE might have minimized the FGA-SGA difference by studying a low-risk cohort in a way that reduced the trial’s ability to detect such differences.
Interpretation. How can we reconcile the absence of a difference between FGAs and SGAs in EPS liability in CATIE with the preponderance of data suggesting otherwise? It appears that SGAs may be less likely to cause EPS than FGAs, but this difference is not evident in all populations. Furthermore, SGAs and FGAs differ in their ability to provide an adequate antipsychotic effect without EPS.
Among FGAs, low-potency agents are less likely to cause EPS or require concomitant anticholinergics than high-potency agents. Among SGAs, the gradient of EPS liability appears to be risperidone > olanzapine, aripiprazole, ziprasidone > quetiapine > clozapine (Figure). Clinically, these pharmacologic differences interact with physiologic differences in EPS vulnerability—some patients are more liable to develop EPS than others. Individuals who are more susceptible to developing EPS are more likely to benefit from antipsychotics with lower EPS liability.
CATIE found no difference among the various FGAs and SGAs with regard to overall efficacy, effects on cognition, and occurrence of tardive dyskinesia in treating chronic schizophrenia. Perhaps it was CATIE’s failure to find a difference in EPS that explains its inability to demonstrate FGA-SGA differences in cognition and other effectiveness domains.
Figure Dose-response curves: Antipsychotic vs extrapyramidal effects
All FGAs and SGAs produce an equivalent antipsychotic effect (red), but they vary in the degree of separation between dosages at which their antipsychotic and extrapyramidal effects occur.
Source: Adapted from reference 13
What catie tells us
The exaggerated view of SGAs as uniformly more efficacious, safer, and better tolerated than FGAs needs to be revised. At the same time, however, the results of CATIE should not be over-interpreted. They tell us that if the four phase 1 SGAs and the FGA perphenazine are used at certain dosages in a particular manner in a specific schizophrenia population—chronic, moderately ill, without tardive dyskinesia—then no differences might be expected among these antipsychotics. But CATIE’s findings might not generalize beyond individuals with schizophrenia at low risk for EPS.
CATIE underlines the importance of achieving an adequate antipsychotic effect without EPS and without using anticholinergics. Clinical consequences of EPS extend beyond motor manifestations and include:
- worse cognition (bradyphrenia)
- worse negative symptoms (neuroleptic-induced deficit syndrome)
- worse depression and suicidality (neuroleptic dysphoria)
- higher risk of tardive dyskinesia.20
SGAs’ presumed ability to provide broader efficacy—cognition, negative symptoms, dysphoria—and lower risk of tardive dyskinesia appears to be driven by their lower EPS liability in association with an equivalent antipsychotic effect. Evidence for an SGA advantage independent of this effect is weak.21,22
Thus, CATIE’s inability to find an FGA-SGA difference in EPS might explain its failure to observe an FGA-SGA difference in cognition and other effectiveness domains.
The clinical pearl
Avoiding EPS and anticholinergics appears to be the key to improving cognition, dysphoria, and negative symptoms with FGAs and SGAs. SGAs’ ability to achieve an equivalent antipsychotic effect without EPS also seems related to their lower risk of tardive dyskinesia.
SGAs’ main advantage may be their greater ease of achieving an adequate antipsychotic effect without EPS or the need to add an anticholinergic to treat or prevent EPS. This comes from the broader separation between dosages at which SGAs produce their antipsychotic versus EPS effects, compared with FGAs (Figure).13
In clinical practice, then, we must achieve an adequate antipsychotic effect for our patients without EPS—whether we are using FGAs or SGAs—to obtain “atypical” benefits. The purported benefits of an “atypical” antipsychotic are not unique to a particular class of agents but relate to achieving a good antipsychotic effect without EPS—and the SGAs are better able to accomplish this than the FGAs.
Careful EPS monitoring is crucial to achieving optimal antipsychotic therapy. Reduced emphasis on EPS in the past decade (in awareness of EPS and training to detect symptoms) and overlap between behavioral aspects of EPS and psychopathology need to be addressed.
CATIE confirms clinical observations that:
Different agents are associated with different adverse effects, which can make achieving maximum efficacy and safety/tolerability challenging.
But differences among antipsychotics and heterogeneity in individual response and vulnerabilities may allow us to optimize treatment.
Different agents at different dosages may provide the best outcomes for individual patients, and the optimal agent and/or dosage can vary in the same patient at different stages of the illness. The CATIE trial contributes to evidence that guides our efforts to provide optimal antipsychotic treatment of schizophrenia (Table 3). Its “surprising” findings are most useful when considered in the context of the database to which it adds.25
Table 3
Treating chronic schizophrenia: 4 clinical tips from CATIE
| Minimizing extrapyramidal symptoms (EPS) is essential, whether using FGAs or SGAs |
| Avoiding EPS and not using adjunctive anticholinergics is the key to SGAs’ purported benefits, such better cognition, less dysphoria, lower negative symptom burden, and lower risk of tardive dyskinesia |
| Antipsychotic dosing is key to accomplishing an adequate antipsychotic effect without EPS |
| Match the antipsychotic choice and dosage to the individual patient’s vulnerability, then make adjustments based on response |
Related resources
- Tandon R. Comparative effectiveness of antipsychotics in the treatment of schizophrenia: What does CATIE tell us? Parts 1 and 2. Int Drug Ther Newsl 2006;41:51-8;67-74.
- Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) schizophrenia study. www.catie.unc.edu/schizophrenia.
Drug brand names
- Clozapine • Clozaril
- Fluphenazine • Permitil
- Olanzapine • Zyprexa
- Perphenazine • Trilafon
- Risperidone • Risperdal
- Quetiapine • Seroquel
- Ziprasidone • Geodon
Disclosures
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Nasrallah HA. CATIE’s surprises. Current Psychiatry 2006;5(2):48-65.
2. Lieberman JA, Stroup ST, McEvoy JP, et al. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med 2005;353:1209-23.
3. Kane JM, Leucht S, Carpenter D, et al. The expert consensus guideline series: optimizing pharmacologic treatment of psychotic disorders. J Clin Psychiatry 2003;64(suppl 12):1-100.
4. Miller AL, Hall CS, Buchanan RW, et al. The Texas Medication Algorithm Project antipsychotic algorithm for schizophrenia: 2003 update. J Clin Psychiatry 2004;65:500-8.
5. American Psychiatric Association. Practice guideline for the treatment of patients with schizophrenia, 2nd ed. Am J Psychiatry 2004;161(suppl 2):1-56.
6. Kane JM. Commentary on the CATIE trial. J Clin Psychiatry 2006;67:831-2.
7. Glick ID. Understanding the results of CATIE in the context of the field. CNS Spectrums 2006;1(suppl 7):40-7.
8. Keefe RSE. Neurocognitive effects of antipsychotic medications in patients with chronic schizophrenia in the CATIE trial. Paper presented at: 61st Society of Biological Psychiatry annual meeting; May 18-20, 2006; Toronto, Canada.
9. McEvoy JP, Lieberman JA, Stroup TS, et al. Effectiveness of clozapine versus olanzapine, quetiapine, and risperidone in patients with chronic schizophrenia who did not respond to prior antipsychotic treatment. Am J Psychiatry 2006;163:600-10.
10. Stroup TS, Lieberman JA, McEvoy JP, et al. Effectiveness of olanzapine, quetiapine, risperidone, and ziprasidone in patients with chronic schizophrenia following discontinuation of a previous atypical antipsychotic. Am J Psychiatry 2006;163:611-22.
11. Buckley PF. Which antipsychotic do I choose next? CATIE phase 2 offers insights on efficacy and tolerability. Current Psychiatry 2006;5(9):27-43.
12. Casey DE. Motor and mental aspects of EPS. Int Clin Psychopharmacol 1995;10:105-14.
13. Jibson MD, Tandon R. New atypical antipsychotic medications. J Psychiatr Res 1998;32:215-28.
14. Pierre JM. Extrapyramidal symptoms with atypical antipsychotics. Drug Safety 2005;28:191-208.
15. Kelly DL, Conley RR, Carpenter WT. First-episode schizophrenia: A focus on pharmacological treatment and safety considerations. Drugs 2005;65:1113-38.
16. Correll CU, Leucht S, Kane JM. Lower risk for tardive dyskinesia associated with second-generation antipsychotics: a systematic review of 1-year studies. Am J Psychiatry 2004;161:414-25.
17. Geddes J, Freemantle N, Harrison P, et al. Atypical antipsychotics in the treatment of schizophrenia: a systematic overview and meta-regression analysis. BMJ 2000;231:1371-6.
18. Hugenholtz GW, Heerdink ER, Stolker JJ, et al. Haloperidol dose when used as active comparator in randomized controlled trials with atypical antipsychotics in schizophrenia: comparison with officially recommended doses. J Clin Psychiatry 2006;67:897-903.
19. Casey D. Implications of the CATIE trial on treatment: extrapyramidal symptoms. CNS Spectrums 2006;11(suppl 7):25-31.
20. Tandon R. Jibson MD: Extrapyramidal side effects of antipsychotic treatment: Scope of problem and impact on outcome. Ann Clin Psychiatry 2002;14:123-9.
21. Thornton AE, Snellenberg JXV, Sepehry AA, Honer WG. The impact of atypical antipsychotic medications on long-term memory dysfunction in schizophrenia spectrum disorder: a quantitative review. J Psychopharmacol 2006;20:335-46.
22. Carpenter WT, Gold JM. Another view of therapy for cognition in schizophrenia. Biol Psychiatry 2002;51:972-8.
23. Davis JM, Chen N. Dose response and dose equivalence of antipsychotics. J Clin Psychopharmacol 2004;24:192-208.
24. Tandon R, Nasrallah HA. Subjecting meta-analyses to closer scrutiny: Little support for differential efficacy among second-generation antipsychotics at equivalent doses. Arch Gen Psychiatry 2006;62:935-7.
25. Tandon R. Comparing antipsychotic efficacy. Am J Psychiatry 2006;163:1645.-
1. Nasrallah HA. CATIE’s surprises. Current Psychiatry 2006;5(2):48-65.
2. Lieberman JA, Stroup ST, McEvoy JP, et al. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med 2005;353:1209-23.
3. Kane JM, Leucht S, Carpenter D, et al. The expert consensus guideline series: optimizing pharmacologic treatment of psychotic disorders. J Clin Psychiatry 2003;64(suppl 12):1-100.
4. Miller AL, Hall CS, Buchanan RW, et al. The Texas Medication Algorithm Project antipsychotic algorithm for schizophrenia: 2003 update. J Clin Psychiatry 2004;65:500-8.
5. American Psychiatric Association. Practice guideline for the treatment of patients with schizophrenia, 2nd ed. Am J Psychiatry 2004;161(suppl 2):1-56.
6. Kane JM. Commentary on the CATIE trial. J Clin Psychiatry 2006;67:831-2.
7. Glick ID. Understanding the results of CATIE in the context of the field. CNS Spectrums 2006;1(suppl 7):40-7.
8. Keefe RSE. Neurocognitive effects of antipsychotic medications in patients with chronic schizophrenia in the CATIE trial. Paper presented at: 61st Society of Biological Psychiatry annual meeting; May 18-20, 2006; Toronto, Canada.
9. McEvoy JP, Lieberman JA, Stroup TS, et al. Effectiveness of clozapine versus olanzapine, quetiapine, and risperidone in patients with chronic schizophrenia who did not respond to prior antipsychotic treatment. Am J Psychiatry 2006;163:600-10.
10. Stroup TS, Lieberman JA, McEvoy JP, et al. Effectiveness of olanzapine, quetiapine, risperidone, and ziprasidone in patients with chronic schizophrenia following discontinuation of a previous atypical antipsychotic. Am J Psychiatry 2006;163:611-22.
11. Buckley PF. Which antipsychotic do I choose next? CATIE phase 2 offers insights on efficacy and tolerability. Current Psychiatry 2006;5(9):27-43.
12. Casey DE. Motor and mental aspects of EPS. Int Clin Psychopharmacol 1995;10:105-14.
13. Jibson MD, Tandon R. New atypical antipsychotic medications. J Psychiatr Res 1998;32:215-28.
14. Pierre JM. Extrapyramidal symptoms with atypical antipsychotics. Drug Safety 2005;28:191-208.
15. Kelly DL, Conley RR, Carpenter WT. First-episode schizophrenia: A focus on pharmacological treatment and safety considerations. Drugs 2005;65:1113-38.
16. Correll CU, Leucht S, Kane JM. Lower risk for tardive dyskinesia associated with second-generation antipsychotics: a systematic review of 1-year studies. Am J Psychiatry 2004;161:414-25.
17. Geddes J, Freemantle N, Harrison P, et al. Atypical antipsychotics in the treatment of schizophrenia: a systematic overview and meta-regression analysis. BMJ 2000;231:1371-6.
18. Hugenholtz GW, Heerdink ER, Stolker JJ, et al. Haloperidol dose when used as active comparator in randomized controlled trials with atypical antipsychotics in schizophrenia: comparison with officially recommended doses. J Clin Psychiatry 2006;67:897-903.
19. Casey D. Implications of the CATIE trial on treatment: extrapyramidal symptoms. CNS Spectrums 2006;11(suppl 7):25-31.
20. Tandon R. Jibson MD: Extrapyramidal side effects of antipsychotic treatment: Scope of problem and impact on outcome. Ann Clin Psychiatry 2002;14:123-9.
21. Thornton AE, Snellenberg JXV, Sepehry AA, Honer WG. The impact of atypical antipsychotic medications on long-term memory dysfunction in schizophrenia spectrum disorder: a quantitative review. J Psychopharmacol 2006;20:335-46.
22. Carpenter WT, Gold JM. Another view of therapy for cognition in schizophrenia. Biol Psychiatry 2002;51:972-8.
23. Davis JM, Chen N. Dose response and dose equivalence of antipsychotics. J Clin Psychopharmacol 2004;24:192-208.
24. Tandon R, Nasrallah HA. Subjecting meta-analyses to closer scrutiny: Little support for differential efficacy among second-generation antipsychotics at equivalent doses. Arch Gen Psychiatry 2006;62:935-7.
25. Tandon R. Comparing antipsychotic efficacy. Am J Psychiatry 2006;163:1645.-
Negative symptoms of schizophrenia: How to treat them most effectively
Negative symptoms are the major contributor to low function levels and debilitation in most patients with schizophrenia. Poorly motivated patients cannot function adequately at school or work. Relationships with family and friends decay in the face of unresponsive affect and inattention to social cues. Personal interests yield to the dampening influences of anhedonia, apathy, and inattention.
Yet because active psychosis is the most common cause of hospital admission, a primary goal of treatment—and sometimes the only objective of pharmacologic treatment—is to eliminate or reduce positive symptoms. And although controlling positive symptoms is remarkably effective in reducing hospitalizations, patients’ functional capacity improves only minimally as psychosis abates. Even with optimal antipsychotic treatment, negative symptoms tend to persist.
For psychiatrists, the three major challenges of schizophrenia’s negative symptoms are their modest therapeutic response, pervasiveness, and diminution of patients’ quality of life. To help you manage negative symptoms, we suggest the following approach to their assessment and treatment.
Importance of negative symptoms
Schizophrenia is a heterogeneous disorder characterized by positive, negative, cognitive, and mood symptoms. The relative severity of these four pathologic domains varies from case to case and within the same individual over time. Though related, these domains have distinct underlying mechanisms and are differentially related to functional capacity and quality of life. They also show different patterns of response to treatment. Whereas positive symptoms refer to new psychological experiences outside the range of normal (e.g., delusions, hallucinations, suspiciousness, disorganized thinking), negative symptoms represent loss of normal function.
Negative symptoms include blunting of affect, poverty of speech and thought, apathy, anhedonia, reduced social drive, loss of motivation, lack of social interest, and inattention to social or cognitive input. These symptoms have devastating consequences on patients’ lives, and only modest progress has been made in treating them effectively.
From negative to positive. Early investigators1,2 considered negative symptoms to represent the fundamental defect of schizophrenia. Over the years, however, the importance of negative symptoms was progressively downplayed. Positive symptoms were increasingly emphasized because:
- positive symptoms have a more dramatic and easily recognized presentation
- negative symptoms are more difficult to reliably define and document
- antipsychotics, which revolutionized schizophrenia treatment, produce their most dramatic improvement in positive symptoms.
Renewed interest. The almost universal presence and relative persistence of negative symptoms, and the fact that they represent the most debilitating and refractory aspect of schizophrenic psychopathology, make them difficult to ignore. Consequently, interest in negative symptoms resurged in the 1980s-90s, with intense efforts to better understand them and treat them more effectively.3-5
Table
SCHIZOPHRENIA’S NEGATIVE SYMPTOMS: PRIMARY AND SECONDARY COMPONENTS
| Primary Associated with positive symptoms Deficit or primary enduring symptoms (premorbid and deteriorative) |
| Secondary Associated with extrapyramidal symptoms, depression, or environmental deprivation |
| Source: Adapted from DeQuardo JR, Tandon R. J Psychiatr Res 1998;32 (3-4):229-42. |
Negative symptoms are now better (but still incompletely) understood, and their treatment has improved but is still inadequate. Because intense effort yielded only modest success, researchers and clinicians have again begun to pay less attention to negative symptoms and shifted their focus to cognition in schizophrenia. Negative symptoms remain relevant, however, because they constitute the main barrier to a better quality of life for patients with schizophrenia.
Assessment for negative symptoms
The four major clinical subgroups of negative symptoms are affective, communicative, conational, and relational.
Affective. Blunted affect—including deficits in facial expression, eye contact, gestures, and voice pattern—is perhaps the most conspicuous negative symptom. In mild form, gestures may seem artificial or mechanical, and the voice is stilted or lacks normal inflection. Patients with severe blunted affect may appear devoid of facial expression or communicative gestures. They may sit impassively with little spontaneous movement, speak in a monotone, and gaze blankly in no particular direction.
Even when conversation becomes emotional, the patient’s affect does not adjust appropriately to reflect his or her feelings. Nor does the patient display even a basic level of understanding or responsiveness that typically characterize casual human interactions. The ability to experience pleasure (anhedonia) and sense of caring (apathy) are also reduced.
Communicative. The patient’s speech may be reduced in quantity (poverty of speech) and information (poverty of content of speech). In mild forms of impoverished speech (alogia), the patient makes brief, unelaborated statements; in the more severe form, the patient can be virtually mute. Whatever speech is present tends to be vague and overly generalized. Periods of silence may occur, either before the patient answers a question (increased latency) or in the midst of a response (blocking).
Conational. The patient may show a lack of drive or goal-directed behavior (avolition). Personal grooming may be poor. Physical activity may be limited. Patients typically have great difficulty following a work schedule or hospital ward routine. They fail to initiate activities, participate grudgingly, and require frequent direction and encouragement.
Continue to: Relational
Relational. Interest in social activities and relationships is reduced (asociality). Even enjoyable and recreational activities are neglected. Interpersonal relations may be of little interest. Friendships become rare and shallow, with little sharing of intimacy. Contacts with family are neglected. Sexual interest declines. As symptoms progress, patients become increasingly isolated.
Primary and secondary symptoms
Negative symptoms are an intrinsic component of schizophrenic psychopathology, and they can also be caused by secondary factors (Table).6,7 Distinguishing between primary and secondary causes of negative symptoms can help you select appropriate treatment in specific clinical situations.
Primary symptoms. From a longitudinal perspective, the three major components of primary negative symptoms are:
- premorbid negative symptoms (present prior to psychosis onset and associated with poor premorbid functioning)
- psychotic-phase, nonenduring negative symptoms that fluctuate with positive symptoms around periods of psychotic exacerbation
- deteriorative negative symptoms that intensify following each psychotic exacerbation and reflect a decline from premorbid levels of functioning.
Though little can be done to treat the premorbid component, psychotic-phase negative symptoms improve along with positive symptoms (although more slowly).8,9 Therefore, the best strategy for managing negative symptoms is to treat positive symptoms more effectively. Although there is no specific treatment for deteriorative negative symptoms, the severity of this component appears to be related to the “toxicity of psychosis” and can be reduced by early, effective antipsychotic treatment.10,11
Secondary negative symptoms occur in association with (and presumably are caused by) factors such as depression, extrapyramidal symptoms (EPS), and environmental deprivation. Secondary negative symptoms usually respond to treatment of the underlying cause.
Assessment
Symptom severity. Assessing the severity of a patient’s negative symptoms on an ongoing basis is a most important first step towards optimal treatment:
- Our objective is to improve patients’ function and quality of life, and negative symptoms compromise both of these more than any other factor.
- Ongoing assessment can track whether prescribed treatments are improving or worsening a patient’s symptoms.
Tools to assess the severity of negative symptoms include the Brief Psychiatric Rating Scale (BPRS) and Positive and Negative Symptom Scale (PANSS).12 The Scale for the Assessment of Negative Symptoms (SANS)13 measures them exclusively, and others such as the Schedule for the Deficit Syndrome (SDS)14 attempt to classify them into subgroups.
Discussing these instruments is beyond the scope of this article, but they differ greatly in their approach to assessing negative symptoms. Instead of using cumbersome assessment instruments, however, we recommend that you focus on two to four of a patient’s “target” symptoms or behaviors and note their severity on an ongoing basis.
Contributing factors. Determining the overall contribution of different factors to a patient’s negative symptoms allows us to target treatments. Sorting out these relative factors can be difficult, however. For example:
- In a patient on antipsychotic treatment who is experiencing psychotic symptoms (eg, persecutory delusions), depressive symptoms, and prominent negative symptoms, the clinician can only guess whether the negative symptoms are primary or secondary.
- In a patient who is socially withdrawn and delusional, withdrawal may be secondary to delusions or may represent a primary negative symptom.
- In a patient on typical antipsychotics, a flat affect may be caused by antipsychotic-induced EPS or it may be a primary negative symptom.
- A disorganized patient with schizophrenia and depression is often unable to convey his or her feelings coherently, so that negative symptoms secondary to affective disturbance may often be mistaken as primary.
Even in research settings, the distinction between primary and secondary symptoms is quite unreliable; nevertheless, it is of great clinical importance. Two strategies may be helpful:
- Consider whether symptoms are specific to the presumed etiology, such as guilt and sadness in depression or cogwheeling and tremor in EPS.
- Treat empirically, and monitor whether negative symptoms improve. If they improve with antidepressant treatment, for example, then depression was the presumable cause. If they improve with anticholinergics, they were presumably secondary to EPS.
Treatment
Negative symptoms are generally viewed as treatment-resistant, but evidence suggests that they do respond to pharmacologic and social interventions (Box). Most responsive to treatment are negative symptoms that occur in association with positive symptoms (psychotic-phase) and secondary negative symptoms caused by neuroleptic medication, depression, or lack of stimulation.
The most effective treatment for secondary symptoms is to target the underlying cause. Neuroleptic-induced akinesia may respond to anticholinergic agents, reduction in antipsychotic dose, or a change in antipsychotic. Using one of the newer-generation antipsychotics (clozapine, risperidone, olanzapine, quetiapine, or ziprasidone) may prevent EPS.
Apsychosocial approach to schizophrenia builds on relationships between the patient and others and may involve social skills training, vocational rehabilitation, and psychotherapy. Activity-oriented therapies appear to be significantly more effective than verbal therapies.
Goals of psychosocial therapy:
- set realistic expectations for the patient
- stay active in treatment in the face of a protracted illness
- create a benign and supportive environment for the patient and caregivers.
Social skills training, designed to help the patient correctly perceive and respond to social situations, is the most widely studied and applied psychosocial intervention. The training is similar to that used in educational settings but focuses on remedying social rather than academic deficits. In schizophrenia, skills training programs address living skills, communication, conflict resolution, vocational skills, etc.
In early studies of social skills training, patients and their families described enhanced social adjustment, and hospitalization rates improved. More recent studies have confirmed improved social adjustment and relapse rates but suggest that overall symptom improvement is modest.
Continue to: Comorbid depression
Comorbid depression may require adding an antidepressant, or it may respond directly to an antipsychotic. Lack of stimulation is best handled by placing the patient in a more appropriately stimulating (but not overstimulating) and supportive environment. Nonenduring primary or psychotic-phase negative symptoms respond to effective antipsychotic treatment of the positive symptoms.
Atypical antipsychotics. Conventional antipsychotics (e.g., haloperidol, chlorpromazine) clearly offer some benefit in treating negative symptoms, but they have a much greater effect on positive symptoms.15 Using higher-than-appropriate doses diminishes their effect on negative symptoms and may result in severe EPS.
Two-thirds of the approximately 35 studies comparing conventional and atypical antipsychotics in treating negative symptoms have found atypicals to be significantly more effective (regardless of which atypical was used). In general, atypical antipsychotics improve negative symptoms by about 25%, compared with 10 to 15% improvement with conventional agents.16,17
Much of the greater benefit with atypicals appears to be related to their at least equivalent ability to improve positive symptoms without causing EPS. Consequently, the key to improved patient outcomes is appropriate dosing of atypical antipsychotics that reduces positive symptoms optimally without EPS and without the need for an anticholinergic (Figure).
Whether the greater improvement with atypical agents implies an improvement in primary versus secondary negative symptoms is academic.18 From the patient’s perspective, the greater reduction in negative symptoms is meaningful, regardless of why it occurs.
Other medications. Secondary negative symptoms are most effectively treated with medications directed at the primary etiology. For EPS, change the antipsychotic, reduce the dosage, or add an anticholinergic. For depression, try an antidepressant (preferably a selective serotonin reuptake inhibitor). If a likely contributing factor can be identified, then initiate specific treatment.
Figure
ANTIPSYCHOTICS IMPROVE NEGATIVE SYMPTOMS THROUGH THEIR EFFECT ON PSYCHOSIS
Source: Adapted from Tandon et al. J Psychiatric Res. 1993;27:341-347.
Antipsychotics improve negative symptoms through their effect on positive (psychotic) symptoms, but they do not affect secondary components—such as environmental deprivation and depression—or the primary components of deterioration and premorbid symptoms. Typical and atypical antipsychotics have similar effects on positive symptoms, but atypical antipsychotics carry a lower risk of extrapyramidal side effects.
Empiric therapy—trying one agent and then another in an effort to reduce negative symptoms—is appropriate if done systematically and sequentially. Medications that are found not to be helpful should be discontinued. Electroconvulsive therapy is not effective in treating negative symptoms.
Related resources
- Greden JF, Tandon R (eds). Negative schizophrenic symptoms: pathophysiology and clinical implications. Washington, DC: American Psychiatric Press, 1991.
- Keefe RSE, McEvoy JP (eds). Negative symptom and cognitive deficit treatment response in schizophrenia. Washington, DC: American Psychiatric Press, 2001.
Drug brand names
- Chlorpromazine • Thorazine
- Clozapine • Clozaril
- Haloperidol • Haldol
- Olanzapine • Zyprexa
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Ziprasidone • Geodon
1. Kraepelin E. Dementia praecox and paraphrenia. Translated by Barclay RM, Robertson GM. Edinburgh: E&S Livingstone; 1919.
2. Bleuler E. Dementia praecox or the group of schizophrenias. Translated by Zinkin H. New York: International Universities Press; 1911.
3. Crow TJ. Molecular pathology of schizophrenia: More than one disease process. Br Med J. 1980;280:66-68.
4. Andreasen NC. Negative symptoms in schizophrenia: definition and reliability. Arch Gen Psychiatry. 1982;39:784-788.
5. Carpenter WT Jr, Heinrichs DW, Alphs LD. Treatment of negative symptoms. Schizophrenia Bull. 1985;11:440-452.
6. Carpenter WT, Jr, Heinrichs DW, Wagman AMI. Deficit and nondeficit forms of schizophrenia: the concept. Am J Psychiatry. 1988;145:578-583.
7. DeQuardo JR, Tandon R. Do atypical antipsychotic medications favorably alter the long-term course of schizophrenia? J Psychiatric Res. 1998;32:229-242.
8. Tandon R, Greden JF. Cholinergic hyperactivity and negative schizophrenic symptoms. Arch Gen Psychiatry. 1989;46:745-753.
9. Tandon R, et al. Covariance of positive and negative symptoms during neuroleptic treatment in schizophrenia: a replication. Biol Psychiatry. 1993;34(7):495-497.
10. Tandon R, Milner K, Jibson MD. Antipsychotics from theory to practice: integrating clinical and basic data. J Clin Psychiatry. 1999;60(suppl 8):21-28.
11. Jibson MD, Tandon R. Treatment of schizophrenia. Psych Clin North Am Annual of Drug Therapy. 2000;7:83-113.
12. Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS). Schizophrenia Bull. 1987;13:261-276.
13. Andreasen NC. Scale for the Assessment of Negative Symptoms (SANS). Iowa City: University of Iowa; 1983.
14. Kirkpatrick B, Buchanan RW, McKenney PD, Alphs LD, Carpenter WT, Jr. The Schedule for the Deficit Syndrome: an instrument for research in schizophrenia. Psychiatry Res. 1989;30(2):119-124.
15. Meltzer HY, Sommers AA, Luchins DJ. The effect of neuroleptics and other psychotropic drugs on negative symptoms in schizophrenia. J Clin Psychopharmacol. 1986;6:329-338.
16. Kane J, Honigfeld G, Singer J, Meltzer H. Clozapine for the treatment-resistant schizophrenic: a double-blind comparison with chlorpromazine. Arch Gen Psychiatry. 1988;45(9):789-796.
17. Tandon R, Goldman R, DeQuardo JR, et al. Positive and negative symptoms covary during clozapine treatment in schizophrenia. J Psychiatric Res. 1993;27:341-347.
18. Breier A, Buchanan RW, Kirkpatrick B, et al. Effect of clozapine on positive and negative symptoms in outpatients with schizophrenia. Am J Psychiatry. 1994;151(1):20-26.
Negative symptoms are the major contributor to low function levels and debilitation in most patients with schizophrenia. Poorly motivated patients cannot function adequately at school or work. Relationships with family and friends decay in the face of unresponsive affect and inattention to social cues. Personal interests yield to the dampening influences of anhedonia, apathy, and inattention.
Yet because active psychosis is the most common cause of hospital admission, a primary goal of treatment—and sometimes the only objective of pharmacologic treatment—is to eliminate or reduce positive symptoms. And although controlling positive symptoms is remarkably effective in reducing hospitalizations, patients’ functional capacity improves only minimally as psychosis abates. Even with optimal antipsychotic treatment, negative symptoms tend to persist.
For psychiatrists, the three major challenges of schizophrenia’s negative symptoms are their modest therapeutic response, pervasiveness, and diminution of patients’ quality of life. To help you manage negative symptoms, we suggest the following approach to their assessment and treatment.
Importance of negative symptoms
Schizophrenia is a heterogeneous disorder characterized by positive, negative, cognitive, and mood symptoms. The relative severity of these four pathologic domains varies from case to case and within the same individual over time. Though related, these domains have distinct underlying mechanisms and are differentially related to functional capacity and quality of life. They also show different patterns of response to treatment. Whereas positive symptoms refer to new psychological experiences outside the range of normal (e.g., delusions, hallucinations, suspiciousness, disorganized thinking), negative symptoms represent loss of normal function.
Negative symptoms include blunting of affect, poverty of speech and thought, apathy, anhedonia, reduced social drive, loss of motivation, lack of social interest, and inattention to social or cognitive input. These symptoms have devastating consequences on patients’ lives, and only modest progress has been made in treating them effectively.
From negative to positive. Early investigators1,2 considered negative symptoms to represent the fundamental defect of schizophrenia. Over the years, however, the importance of negative symptoms was progressively downplayed. Positive symptoms were increasingly emphasized because:
- positive symptoms have a more dramatic and easily recognized presentation
- negative symptoms are more difficult to reliably define and document
- antipsychotics, which revolutionized schizophrenia treatment, produce their most dramatic improvement in positive symptoms.
Renewed interest. The almost universal presence and relative persistence of negative symptoms, and the fact that they represent the most debilitating and refractory aspect of schizophrenic psychopathology, make them difficult to ignore. Consequently, interest in negative symptoms resurged in the 1980s-90s, with intense efforts to better understand them and treat them more effectively.3-5
Table
SCHIZOPHRENIA’S NEGATIVE SYMPTOMS: PRIMARY AND SECONDARY COMPONENTS
| Primary Associated with positive symptoms Deficit or primary enduring symptoms (premorbid and deteriorative) |
| Secondary Associated with extrapyramidal symptoms, depression, or environmental deprivation |
| Source: Adapted from DeQuardo JR, Tandon R. J Psychiatr Res 1998;32 (3-4):229-42. |
Negative symptoms are now better (but still incompletely) understood, and their treatment has improved but is still inadequate. Because intense effort yielded only modest success, researchers and clinicians have again begun to pay less attention to negative symptoms and shifted their focus to cognition in schizophrenia. Negative symptoms remain relevant, however, because they constitute the main barrier to a better quality of life for patients with schizophrenia.
Assessment for negative symptoms
The four major clinical subgroups of negative symptoms are affective, communicative, conational, and relational.
Affective. Blunted affect—including deficits in facial expression, eye contact, gestures, and voice pattern—is perhaps the most conspicuous negative symptom. In mild form, gestures may seem artificial or mechanical, and the voice is stilted or lacks normal inflection. Patients with severe blunted affect may appear devoid of facial expression or communicative gestures. They may sit impassively with little spontaneous movement, speak in a monotone, and gaze blankly in no particular direction.
Even when conversation becomes emotional, the patient’s affect does not adjust appropriately to reflect his or her feelings. Nor does the patient display even a basic level of understanding or responsiveness that typically characterize casual human interactions. The ability to experience pleasure (anhedonia) and sense of caring (apathy) are also reduced.
Communicative. The patient’s speech may be reduced in quantity (poverty of speech) and information (poverty of content of speech). In mild forms of impoverished speech (alogia), the patient makes brief, unelaborated statements; in the more severe form, the patient can be virtually mute. Whatever speech is present tends to be vague and overly generalized. Periods of silence may occur, either before the patient answers a question (increased latency) or in the midst of a response (blocking).
Conational. The patient may show a lack of drive or goal-directed behavior (avolition). Personal grooming may be poor. Physical activity may be limited. Patients typically have great difficulty following a work schedule or hospital ward routine. They fail to initiate activities, participate grudgingly, and require frequent direction and encouragement.
Continue to: Relational
Relational. Interest in social activities and relationships is reduced (asociality). Even enjoyable and recreational activities are neglected. Interpersonal relations may be of little interest. Friendships become rare and shallow, with little sharing of intimacy. Contacts with family are neglected. Sexual interest declines. As symptoms progress, patients become increasingly isolated.
Primary and secondary symptoms
Negative symptoms are an intrinsic component of schizophrenic psychopathology, and they can also be caused by secondary factors (Table).6,7 Distinguishing between primary and secondary causes of negative symptoms can help you select appropriate treatment in specific clinical situations.
Primary symptoms. From a longitudinal perspective, the three major components of primary negative symptoms are:
- premorbid negative symptoms (present prior to psychosis onset and associated with poor premorbid functioning)
- psychotic-phase, nonenduring negative symptoms that fluctuate with positive symptoms around periods of psychotic exacerbation
- deteriorative negative symptoms that intensify following each psychotic exacerbation and reflect a decline from premorbid levels of functioning.
Though little can be done to treat the premorbid component, psychotic-phase negative symptoms improve along with positive symptoms (although more slowly).8,9 Therefore, the best strategy for managing negative symptoms is to treat positive symptoms more effectively. Although there is no specific treatment for deteriorative negative symptoms, the severity of this component appears to be related to the “toxicity of psychosis” and can be reduced by early, effective antipsychotic treatment.10,11
Secondary negative symptoms occur in association with (and presumably are caused by) factors such as depression, extrapyramidal symptoms (EPS), and environmental deprivation. Secondary negative symptoms usually respond to treatment of the underlying cause.
Assessment
Symptom severity. Assessing the severity of a patient’s negative symptoms on an ongoing basis is a most important first step towards optimal treatment:
- Our objective is to improve patients’ function and quality of life, and negative symptoms compromise both of these more than any other factor.
- Ongoing assessment can track whether prescribed treatments are improving or worsening a patient’s symptoms.
Tools to assess the severity of negative symptoms include the Brief Psychiatric Rating Scale (BPRS) and Positive and Negative Symptom Scale (PANSS).12 The Scale for the Assessment of Negative Symptoms (SANS)13 measures them exclusively, and others such as the Schedule for the Deficit Syndrome (SDS)14 attempt to classify them into subgroups.
Discussing these instruments is beyond the scope of this article, but they differ greatly in their approach to assessing negative symptoms. Instead of using cumbersome assessment instruments, however, we recommend that you focus on two to four of a patient’s “target” symptoms or behaviors and note their severity on an ongoing basis.
Contributing factors. Determining the overall contribution of different factors to a patient’s negative symptoms allows us to target treatments. Sorting out these relative factors can be difficult, however. For example:
- In a patient on antipsychotic treatment who is experiencing psychotic symptoms (eg, persecutory delusions), depressive symptoms, and prominent negative symptoms, the clinician can only guess whether the negative symptoms are primary or secondary.
- In a patient who is socially withdrawn and delusional, withdrawal may be secondary to delusions or may represent a primary negative symptom.
- In a patient on typical antipsychotics, a flat affect may be caused by antipsychotic-induced EPS or it may be a primary negative symptom.
- A disorganized patient with schizophrenia and depression is often unable to convey his or her feelings coherently, so that negative symptoms secondary to affective disturbance may often be mistaken as primary.
Even in research settings, the distinction between primary and secondary symptoms is quite unreliable; nevertheless, it is of great clinical importance. Two strategies may be helpful:
- Consider whether symptoms are specific to the presumed etiology, such as guilt and sadness in depression or cogwheeling and tremor in EPS.
- Treat empirically, and monitor whether negative symptoms improve. If they improve with antidepressant treatment, for example, then depression was the presumable cause. If they improve with anticholinergics, they were presumably secondary to EPS.
Treatment
Negative symptoms are generally viewed as treatment-resistant, but evidence suggests that they do respond to pharmacologic and social interventions (Box). Most responsive to treatment are negative symptoms that occur in association with positive symptoms (psychotic-phase) and secondary negative symptoms caused by neuroleptic medication, depression, or lack of stimulation.
The most effective treatment for secondary symptoms is to target the underlying cause. Neuroleptic-induced akinesia may respond to anticholinergic agents, reduction in antipsychotic dose, or a change in antipsychotic. Using one of the newer-generation antipsychotics (clozapine, risperidone, olanzapine, quetiapine, or ziprasidone) may prevent EPS.
Apsychosocial approach to schizophrenia builds on relationships between the patient and others and may involve social skills training, vocational rehabilitation, and psychotherapy. Activity-oriented therapies appear to be significantly more effective than verbal therapies.
Goals of psychosocial therapy:
- set realistic expectations for the patient
- stay active in treatment in the face of a protracted illness
- create a benign and supportive environment for the patient and caregivers.
Social skills training, designed to help the patient correctly perceive and respond to social situations, is the most widely studied and applied psychosocial intervention. The training is similar to that used in educational settings but focuses on remedying social rather than academic deficits. In schizophrenia, skills training programs address living skills, communication, conflict resolution, vocational skills, etc.
In early studies of social skills training, patients and their families described enhanced social adjustment, and hospitalization rates improved. More recent studies have confirmed improved social adjustment and relapse rates but suggest that overall symptom improvement is modest.
Continue to: Comorbid depression
Comorbid depression may require adding an antidepressant, or it may respond directly to an antipsychotic. Lack of stimulation is best handled by placing the patient in a more appropriately stimulating (but not overstimulating) and supportive environment. Nonenduring primary or psychotic-phase negative symptoms respond to effective antipsychotic treatment of the positive symptoms.
Atypical antipsychotics. Conventional antipsychotics (e.g., haloperidol, chlorpromazine) clearly offer some benefit in treating negative symptoms, but they have a much greater effect on positive symptoms.15 Using higher-than-appropriate doses diminishes their effect on negative symptoms and may result in severe EPS.
Two-thirds of the approximately 35 studies comparing conventional and atypical antipsychotics in treating negative symptoms have found atypicals to be significantly more effective (regardless of which atypical was used). In general, atypical antipsychotics improve negative symptoms by about 25%, compared with 10 to 15% improvement with conventional agents.16,17
Much of the greater benefit with atypicals appears to be related to their at least equivalent ability to improve positive symptoms without causing EPS. Consequently, the key to improved patient outcomes is appropriate dosing of atypical antipsychotics that reduces positive symptoms optimally without EPS and without the need for an anticholinergic (Figure).
Whether the greater improvement with atypical agents implies an improvement in primary versus secondary negative symptoms is academic.18 From the patient’s perspective, the greater reduction in negative symptoms is meaningful, regardless of why it occurs.
Other medications. Secondary negative symptoms are most effectively treated with medications directed at the primary etiology. For EPS, change the antipsychotic, reduce the dosage, or add an anticholinergic. For depression, try an antidepressant (preferably a selective serotonin reuptake inhibitor). If a likely contributing factor can be identified, then initiate specific treatment.
Figure
ANTIPSYCHOTICS IMPROVE NEGATIVE SYMPTOMS THROUGH THEIR EFFECT ON PSYCHOSIS
Source: Adapted from Tandon et al. J Psychiatric Res. 1993;27:341-347.
Antipsychotics improve negative symptoms through their effect on positive (psychotic) symptoms, but they do not affect secondary components—such as environmental deprivation and depression—or the primary components of deterioration and premorbid symptoms. Typical and atypical antipsychotics have similar effects on positive symptoms, but atypical antipsychotics carry a lower risk of extrapyramidal side effects.
Empiric therapy—trying one agent and then another in an effort to reduce negative symptoms—is appropriate if done systematically and sequentially. Medications that are found not to be helpful should be discontinued. Electroconvulsive therapy is not effective in treating negative symptoms.
Related resources
- Greden JF, Tandon R (eds). Negative schizophrenic symptoms: pathophysiology and clinical implications. Washington, DC: American Psychiatric Press, 1991.
- Keefe RSE, McEvoy JP (eds). Negative symptom and cognitive deficit treatment response in schizophrenia. Washington, DC: American Psychiatric Press, 2001.
Drug brand names
- Chlorpromazine • Thorazine
- Clozapine • Clozaril
- Haloperidol • Haldol
- Olanzapine • Zyprexa
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Ziprasidone • Geodon
Negative symptoms are the major contributor to low function levels and debilitation in most patients with schizophrenia. Poorly motivated patients cannot function adequately at school or work. Relationships with family and friends decay in the face of unresponsive affect and inattention to social cues. Personal interests yield to the dampening influences of anhedonia, apathy, and inattention.
Yet because active psychosis is the most common cause of hospital admission, a primary goal of treatment—and sometimes the only objective of pharmacologic treatment—is to eliminate or reduce positive symptoms. And although controlling positive symptoms is remarkably effective in reducing hospitalizations, patients’ functional capacity improves only minimally as psychosis abates. Even with optimal antipsychotic treatment, negative symptoms tend to persist.
For psychiatrists, the three major challenges of schizophrenia’s negative symptoms are their modest therapeutic response, pervasiveness, and diminution of patients’ quality of life. To help you manage negative symptoms, we suggest the following approach to their assessment and treatment.
Importance of negative symptoms
Schizophrenia is a heterogeneous disorder characterized by positive, negative, cognitive, and mood symptoms. The relative severity of these four pathologic domains varies from case to case and within the same individual over time. Though related, these domains have distinct underlying mechanisms and are differentially related to functional capacity and quality of life. They also show different patterns of response to treatment. Whereas positive symptoms refer to new psychological experiences outside the range of normal (e.g., delusions, hallucinations, suspiciousness, disorganized thinking), negative symptoms represent loss of normal function.
Negative symptoms include blunting of affect, poverty of speech and thought, apathy, anhedonia, reduced social drive, loss of motivation, lack of social interest, and inattention to social or cognitive input. These symptoms have devastating consequences on patients’ lives, and only modest progress has been made in treating them effectively.
From negative to positive. Early investigators1,2 considered negative symptoms to represent the fundamental defect of schizophrenia. Over the years, however, the importance of negative symptoms was progressively downplayed. Positive symptoms were increasingly emphasized because:
- positive symptoms have a more dramatic and easily recognized presentation
- negative symptoms are more difficult to reliably define and document
- antipsychotics, which revolutionized schizophrenia treatment, produce their most dramatic improvement in positive symptoms.
Renewed interest. The almost universal presence and relative persistence of negative symptoms, and the fact that they represent the most debilitating and refractory aspect of schizophrenic psychopathology, make them difficult to ignore. Consequently, interest in negative symptoms resurged in the 1980s-90s, with intense efforts to better understand them and treat them more effectively.3-5
Table
SCHIZOPHRENIA’S NEGATIVE SYMPTOMS: PRIMARY AND SECONDARY COMPONENTS
| Primary Associated with positive symptoms Deficit or primary enduring symptoms (premorbid and deteriorative) |
| Secondary Associated with extrapyramidal symptoms, depression, or environmental deprivation |
| Source: Adapted from DeQuardo JR, Tandon R. J Psychiatr Res 1998;32 (3-4):229-42. |
Negative symptoms are now better (but still incompletely) understood, and their treatment has improved but is still inadequate. Because intense effort yielded only modest success, researchers and clinicians have again begun to pay less attention to negative symptoms and shifted their focus to cognition in schizophrenia. Negative symptoms remain relevant, however, because they constitute the main barrier to a better quality of life for patients with schizophrenia.
Assessment for negative symptoms
The four major clinical subgroups of negative symptoms are affective, communicative, conational, and relational.
Affective. Blunted affect—including deficits in facial expression, eye contact, gestures, and voice pattern—is perhaps the most conspicuous negative symptom. In mild form, gestures may seem artificial or mechanical, and the voice is stilted or lacks normal inflection. Patients with severe blunted affect may appear devoid of facial expression or communicative gestures. They may sit impassively with little spontaneous movement, speak in a monotone, and gaze blankly in no particular direction.
Even when conversation becomes emotional, the patient’s affect does not adjust appropriately to reflect his or her feelings. Nor does the patient display even a basic level of understanding or responsiveness that typically characterize casual human interactions. The ability to experience pleasure (anhedonia) and sense of caring (apathy) are also reduced.
Communicative. The patient’s speech may be reduced in quantity (poverty of speech) and information (poverty of content of speech). In mild forms of impoverished speech (alogia), the patient makes brief, unelaborated statements; in the more severe form, the patient can be virtually mute. Whatever speech is present tends to be vague and overly generalized. Periods of silence may occur, either before the patient answers a question (increased latency) or in the midst of a response (blocking).
Conational. The patient may show a lack of drive or goal-directed behavior (avolition). Personal grooming may be poor. Physical activity may be limited. Patients typically have great difficulty following a work schedule or hospital ward routine. They fail to initiate activities, participate grudgingly, and require frequent direction and encouragement.
Continue to: Relational
Relational. Interest in social activities and relationships is reduced (asociality). Even enjoyable and recreational activities are neglected. Interpersonal relations may be of little interest. Friendships become rare and shallow, with little sharing of intimacy. Contacts with family are neglected. Sexual interest declines. As symptoms progress, patients become increasingly isolated.
Primary and secondary symptoms
Negative symptoms are an intrinsic component of schizophrenic psychopathology, and they can also be caused by secondary factors (Table).6,7 Distinguishing between primary and secondary causes of negative symptoms can help you select appropriate treatment in specific clinical situations.
Primary symptoms. From a longitudinal perspective, the three major components of primary negative symptoms are:
- premorbid negative symptoms (present prior to psychosis onset and associated with poor premorbid functioning)
- psychotic-phase, nonenduring negative symptoms that fluctuate with positive symptoms around periods of psychotic exacerbation
- deteriorative negative symptoms that intensify following each psychotic exacerbation and reflect a decline from premorbid levels of functioning.
Though little can be done to treat the premorbid component, psychotic-phase negative symptoms improve along with positive symptoms (although more slowly).8,9 Therefore, the best strategy for managing negative symptoms is to treat positive symptoms more effectively. Although there is no specific treatment for deteriorative negative symptoms, the severity of this component appears to be related to the “toxicity of psychosis” and can be reduced by early, effective antipsychotic treatment.10,11
Secondary negative symptoms occur in association with (and presumably are caused by) factors such as depression, extrapyramidal symptoms (EPS), and environmental deprivation. Secondary negative symptoms usually respond to treatment of the underlying cause.
Assessment
Symptom severity. Assessing the severity of a patient’s negative symptoms on an ongoing basis is a most important first step towards optimal treatment:
- Our objective is to improve patients’ function and quality of life, and negative symptoms compromise both of these more than any other factor.
- Ongoing assessment can track whether prescribed treatments are improving or worsening a patient’s symptoms.
Tools to assess the severity of negative symptoms include the Brief Psychiatric Rating Scale (BPRS) and Positive and Negative Symptom Scale (PANSS).12 The Scale for the Assessment of Negative Symptoms (SANS)13 measures them exclusively, and others such as the Schedule for the Deficit Syndrome (SDS)14 attempt to classify them into subgroups.
Discussing these instruments is beyond the scope of this article, but they differ greatly in their approach to assessing negative symptoms. Instead of using cumbersome assessment instruments, however, we recommend that you focus on two to four of a patient’s “target” symptoms or behaviors and note their severity on an ongoing basis.
Contributing factors. Determining the overall contribution of different factors to a patient’s negative symptoms allows us to target treatments. Sorting out these relative factors can be difficult, however. For example:
- In a patient on antipsychotic treatment who is experiencing psychotic symptoms (eg, persecutory delusions), depressive symptoms, and prominent negative symptoms, the clinician can only guess whether the negative symptoms are primary or secondary.
- In a patient who is socially withdrawn and delusional, withdrawal may be secondary to delusions or may represent a primary negative symptom.
- In a patient on typical antipsychotics, a flat affect may be caused by antipsychotic-induced EPS or it may be a primary negative symptom.
- A disorganized patient with schizophrenia and depression is often unable to convey his or her feelings coherently, so that negative symptoms secondary to affective disturbance may often be mistaken as primary.
Even in research settings, the distinction between primary and secondary symptoms is quite unreliable; nevertheless, it is of great clinical importance. Two strategies may be helpful:
- Consider whether symptoms are specific to the presumed etiology, such as guilt and sadness in depression or cogwheeling and tremor in EPS.
- Treat empirically, and monitor whether negative symptoms improve. If they improve with antidepressant treatment, for example, then depression was the presumable cause. If they improve with anticholinergics, they were presumably secondary to EPS.
Treatment
Negative symptoms are generally viewed as treatment-resistant, but evidence suggests that they do respond to pharmacologic and social interventions (Box). Most responsive to treatment are negative symptoms that occur in association with positive symptoms (psychotic-phase) and secondary negative symptoms caused by neuroleptic medication, depression, or lack of stimulation.
The most effective treatment for secondary symptoms is to target the underlying cause. Neuroleptic-induced akinesia may respond to anticholinergic agents, reduction in antipsychotic dose, or a change in antipsychotic. Using one of the newer-generation antipsychotics (clozapine, risperidone, olanzapine, quetiapine, or ziprasidone) may prevent EPS.
Apsychosocial approach to schizophrenia builds on relationships between the patient and others and may involve social skills training, vocational rehabilitation, and psychotherapy. Activity-oriented therapies appear to be significantly more effective than verbal therapies.
Goals of psychosocial therapy:
- set realistic expectations for the patient
- stay active in treatment in the face of a protracted illness
- create a benign and supportive environment for the patient and caregivers.
Social skills training, designed to help the patient correctly perceive and respond to social situations, is the most widely studied and applied psychosocial intervention. The training is similar to that used in educational settings but focuses on remedying social rather than academic deficits. In schizophrenia, skills training programs address living skills, communication, conflict resolution, vocational skills, etc.
In early studies of social skills training, patients and their families described enhanced social adjustment, and hospitalization rates improved. More recent studies have confirmed improved social adjustment and relapse rates but suggest that overall symptom improvement is modest.
Continue to: Comorbid depression
Comorbid depression may require adding an antidepressant, or it may respond directly to an antipsychotic. Lack of stimulation is best handled by placing the patient in a more appropriately stimulating (but not overstimulating) and supportive environment. Nonenduring primary or psychotic-phase negative symptoms respond to effective antipsychotic treatment of the positive symptoms.
Atypical antipsychotics. Conventional antipsychotics (e.g., haloperidol, chlorpromazine) clearly offer some benefit in treating negative symptoms, but they have a much greater effect on positive symptoms.15 Using higher-than-appropriate doses diminishes their effect on negative symptoms and may result in severe EPS.
Two-thirds of the approximately 35 studies comparing conventional and atypical antipsychotics in treating negative symptoms have found atypicals to be significantly more effective (regardless of which atypical was used). In general, atypical antipsychotics improve negative symptoms by about 25%, compared with 10 to 15% improvement with conventional agents.16,17
Much of the greater benefit with atypicals appears to be related to their at least equivalent ability to improve positive symptoms without causing EPS. Consequently, the key to improved patient outcomes is appropriate dosing of atypical antipsychotics that reduces positive symptoms optimally without EPS and without the need for an anticholinergic (Figure).
Whether the greater improvement with atypical agents implies an improvement in primary versus secondary negative symptoms is academic.18 From the patient’s perspective, the greater reduction in negative symptoms is meaningful, regardless of why it occurs.
Other medications. Secondary negative symptoms are most effectively treated with medications directed at the primary etiology. For EPS, change the antipsychotic, reduce the dosage, or add an anticholinergic. For depression, try an antidepressant (preferably a selective serotonin reuptake inhibitor). If a likely contributing factor can be identified, then initiate specific treatment.
Figure
ANTIPSYCHOTICS IMPROVE NEGATIVE SYMPTOMS THROUGH THEIR EFFECT ON PSYCHOSIS
Source: Adapted from Tandon et al. J Psychiatric Res. 1993;27:341-347.
Antipsychotics improve negative symptoms through their effect on positive (psychotic) symptoms, but they do not affect secondary components—such as environmental deprivation and depression—or the primary components of deterioration and premorbid symptoms. Typical and atypical antipsychotics have similar effects on positive symptoms, but atypical antipsychotics carry a lower risk of extrapyramidal side effects.
Empiric therapy—trying one agent and then another in an effort to reduce negative symptoms—is appropriate if done systematically and sequentially. Medications that are found not to be helpful should be discontinued. Electroconvulsive therapy is not effective in treating negative symptoms.
Related resources
- Greden JF, Tandon R (eds). Negative schizophrenic symptoms: pathophysiology and clinical implications. Washington, DC: American Psychiatric Press, 1991.
- Keefe RSE, McEvoy JP (eds). Negative symptom and cognitive deficit treatment response in schizophrenia. Washington, DC: American Psychiatric Press, 2001.
Drug brand names
- Chlorpromazine • Thorazine
- Clozapine • Clozaril
- Haloperidol • Haldol
- Olanzapine • Zyprexa
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Ziprasidone • Geodon
1. Kraepelin E. Dementia praecox and paraphrenia. Translated by Barclay RM, Robertson GM. Edinburgh: E&S Livingstone; 1919.
2. Bleuler E. Dementia praecox or the group of schizophrenias. Translated by Zinkin H. New York: International Universities Press; 1911.
3. Crow TJ. Molecular pathology of schizophrenia: More than one disease process. Br Med J. 1980;280:66-68.
4. Andreasen NC. Negative symptoms in schizophrenia: definition and reliability. Arch Gen Psychiatry. 1982;39:784-788.
5. Carpenter WT Jr, Heinrichs DW, Alphs LD. Treatment of negative symptoms. Schizophrenia Bull. 1985;11:440-452.
6. Carpenter WT, Jr, Heinrichs DW, Wagman AMI. Deficit and nondeficit forms of schizophrenia: the concept. Am J Psychiatry. 1988;145:578-583.
7. DeQuardo JR, Tandon R. Do atypical antipsychotic medications favorably alter the long-term course of schizophrenia? J Psychiatric Res. 1998;32:229-242.
8. Tandon R, Greden JF. Cholinergic hyperactivity and negative schizophrenic symptoms. Arch Gen Psychiatry. 1989;46:745-753.
9. Tandon R, et al. Covariance of positive and negative symptoms during neuroleptic treatment in schizophrenia: a replication. Biol Psychiatry. 1993;34(7):495-497.
10. Tandon R, Milner K, Jibson MD. Antipsychotics from theory to practice: integrating clinical and basic data. J Clin Psychiatry. 1999;60(suppl 8):21-28.
11. Jibson MD, Tandon R. Treatment of schizophrenia. Psych Clin North Am Annual of Drug Therapy. 2000;7:83-113.
12. Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS). Schizophrenia Bull. 1987;13:261-276.
13. Andreasen NC. Scale for the Assessment of Negative Symptoms (SANS). Iowa City: University of Iowa; 1983.
14. Kirkpatrick B, Buchanan RW, McKenney PD, Alphs LD, Carpenter WT, Jr. The Schedule for the Deficit Syndrome: an instrument for research in schizophrenia. Psychiatry Res. 1989;30(2):119-124.
15. Meltzer HY, Sommers AA, Luchins DJ. The effect of neuroleptics and other psychotropic drugs on negative symptoms in schizophrenia. J Clin Psychopharmacol. 1986;6:329-338.
16. Kane J, Honigfeld G, Singer J, Meltzer H. Clozapine for the treatment-resistant schizophrenic: a double-blind comparison with chlorpromazine. Arch Gen Psychiatry. 1988;45(9):789-796.
17. Tandon R, Goldman R, DeQuardo JR, et al. Positive and negative symptoms covary during clozapine treatment in schizophrenia. J Psychiatric Res. 1993;27:341-347.
18. Breier A, Buchanan RW, Kirkpatrick B, et al. Effect of clozapine on positive and negative symptoms in outpatients with schizophrenia. Am J Psychiatry. 1994;151(1):20-26.
1. Kraepelin E. Dementia praecox and paraphrenia. Translated by Barclay RM, Robertson GM. Edinburgh: E&S Livingstone; 1919.
2. Bleuler E. Dementia praecox or the group of schizophrenias. Translated by Zinkin H. New York: International Universities Press; 1911.
3. Crow TJ. Molecular pathology of schizophrenia: More than one disease process. Br Med J. 1980;280:66-68.
4. Andreasen NC. Negative symptoms in schizophrenia: definition and reliability. Arch Gen Psychiatry. 1982;39:784-788.
5. Carpenter WT Jr, Heinrichs DW, Alphs LD. Treatment of negative symptoms. Schizophrenia Bull. 1985;11:440-452.
6. Carpenter WT, Jr, Heinrichs DW, Wagman AMI. Deficit and nondeficit forms of schizophrenia: the concept. Am J Psychiatry. 1988;145:578-583.
7. DeQuardo JR, Tandon R. Do atypical antipsychotic medications favorably alter the long-term course of schizophrenia? J Psychiatric Res. 1998;32:229-242.
8. Tandon R, Greden JF. Cholinergic hyperactivity and negative schizophrenic symptoms. Arch Gen Psychiatry. 1989;46:745-753.
9. Tandon R, et al. Covariance of positive and negative symptoms during neuroleptic treatment in schizophrenia: a replication. Biol Psychiatry. 1993;34(7):495-497.
10. Tandon R, Milner K, Jibson MD. Antipsychotics from theory to practice: integrating clinical and basic data. J Clin Psychiatry. 1999;60(suppl 8):21-28.
11. Jibson MD, Tandon R. Treatment of schizophrenia. Psych Clin North Am Annual of Drug Therapy. 2000;7:83-113.
12. Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS). Schizophrenia Bull. 1987;13:261-276.
13. Andreasen NC. Scale for the Assessment of Negative Symptoms (SANS). Iowa City: University of Iowa; 1983.
14. Kirkpatrick B, Buchanan RW, McKenney PD, Alphs LD, Carpenter WT, Jr. The Schedule for the Deficit Syndrome: an instrument for research in schizophrenia. Psychiatry Res. 1989;30(2):119-124.
15. Meltzer HY, Sommers AA, Luchins DJ. The effect of neuroleptics and other psychotropic drugs on negative symptoms in schizophrenia. J Clin Psychopharmacol. 1986;6:329-338.
16. Kane J, Honigfeld G, Singer J, Meltzer H. Clozapine for the treatment-resistant schizophrenic: a double-blind comparison with chlorpromazine. Arch Gen Psychiatry. 1988;45(9):789-796.
17. Tandon R, Goldman R, DeQuardo JR, et al. Positive and negative symptoms covary during clozapine treatment in schizophrenia. J Psychiatric Res. 1993;27:341-347.
18. Breier A, Buchanan RW, Kirkpatrick B, et al. Effect of clozapine on positive and negative symptoms in outpatients with schizophrenia. Am J Psychiatry. 1994;151(1):20-26.