User login
A street medicine view of tobacco use in patients with schizophrenia
Editor’s note: Readers’ Forum is a department for correspondence from readers that is not in response to articles published in
Throughout my psychiatric clerkship, I (JWF) participated in street medicine, the practice of providing care to patients (typically those who are homeless) at the location they currently reside, such as in a homeless encampment or community shelter. Our clinical team drove to locations that provided housing for patients diagnosed with schizophrenia, where we assisted with medications and blood draws. I remember pulling up the first day and seeing someone outside smoking a cigarette. I soon learned that many people living in such situations were smokers, and that among the substances they used, tobacco was the most common.
One patient said the cigarettes helped him manage the “voices in his head” as well as some of the adverse effects from medication, such as parkinsonism and akathisia. I asked my attending physician about this and she explained that for some patients, using tobacco was a way to mitigate the positive symptoms of schizophrenia and make the adverse effects of their therapy, particularly extrapyramidal symptoms (EPS), more bearable. By the end of my 2-week rotation, I was sure of a trend: our patients with schizophrenia smoked incessantly. Near the end of my rotation, I asked a patient, “Why do you smoke”? The patient looked at me, puzzled, and replied: “I just do.” This exchange only piqued my curiosity, and I could not help but wonder: what is the relationship between tobacco use and schizophrenia? How is tobacco use related to the pathophysiology of schizophrenia? Does tobacco use among patients with schizophrenia ameliorate aspects of their psychosis? Street medicine offered me a window into a biomedically intriguing question, and I wanted to learn more.
What smoking does for patients with schizophrenia
The high prevalence of smoking among patients with schizophrenia (50% to 88%) greatly exceeds the rates of smoking among patients with other psychiatric illnesses.1,2 The role of smoking in relation to schizophrenia and other psychoses is multidimensional, and evidence implicates smoking as a risk factor for schizophrenia.3,4
Two mechanisms may help explain tobacco use in patients with schizophrenia: reducing the adverse effects of antipsychotic medications and promoting neural transmission of dopamine. Second-generation antipsychotics (SGAs) are a first-line treatment, but they can produce EPS, metabolic dysregulation, and blood disorders such as hyponatremia and (rarely) agranulocytosis (1% with clozapine).5 Compared to those who are nonsmokers, patients with schizophrenia who smoke are more likely to experience more severe symptoms (eg, hallucinations and delusions) and less severe EPS.5,6 Research suggests that exposure to polycyclic aromatic hydrocarbons released during smoking induces cytochrome P450 1A2, an enzyme that metabolizes antipsychotic medications such as haloperidol, clozapine, and olanzapine. Increased metabolism results in lower serum concentrations of antipsychotics, lower efficacy, and more severe positive symptoms.5,6
Additionally, tobacco is an activator of nicotinic acetylcholine receptors (nAChR).6 When these receptors become activated, dopamine is released. Dopamine serves as a mediator of reward for nicotine use. In the context of schizophrenia, tobacco use opposes the mechanism of action of SGAs, which is to block neural transmission of dopamine.6 The etiology of EPS is related to the blockade of postsynaptic dopamine release in the striatum.6 By activating nAChR, smoking induces a downstream release of dopamine that can alleviate iatrogenic EPS by restoring neural transmission of dopamine.6 Nicotine may also modulate alpha-7 nicotinic receptor dysfunction, and improve the ability to filter out irrelevant environmental stimuli (impaired sensory gating), which can be overwhelming for patients with schizophrenia. It also can improve cognitive dysfunction and attention by inducing the release of dopamine in mesocortical pathways.7 The implications of this neural pathway are significant because smoking is significantly greater in tobacco users who are diagnosed with schizophrenia compared to tobacco users who lack a psychiatric diagnosis.6,7 Smoking may enhance dopaminergic neural transmission to a far greater extent in tobacco users with schizophrenia compared to tobacco users who do not develop schizophrenia, which suggests intrinsic differences at the neuronal level. Neural differences between tobacco users with or without schizophrenia may synergize with smoking in clinically and biologically meaningful ways. These pathways require further research to support or disprove these hypotheses.
Aside from the dopaminergic system, mechanisms influencing tobacco use among patients with schizophrenia may also be related to nicotine’s mild antidepressant effects. Evidence suggests a clinically meaningful association between nicotine dependence and mood disorders, and this association may be due to the antidepressant effects of nicotine.8-13 Patients with schizophrenia may experience respite from depressive symptoms through their tobacco use, eventually leading to nicotine dependence.
Continue to: Treatment of schizophrenia...
Treatment of schizophrenia involves multimodal management of a patient’s life, including reducing maladaptive habits that are harmful to health. Chronic smoking in patients with schizophrenia is associated not only with atherosclerosis and cardiovascular disease, but also with poor neurologic functioning, such as significant impairment in attention, working memory, learning, executive function, reasoning, problem-solving and speed of processing.14 One study found that in patients with schizophrenia, smoking increased the 20-year cardiovascular mortality risk by 86%.15
Despite challenges to abstinence, smoking cessation should be discussed with these patients, especially given the high prevalence of smoking among this vulnerable population. Bupropion and varenicline have been studied in the context of smoking cessation among patients with schizophrenia. Data on varenicline are mixed. Smokers with schizophrenia who received bupropion showed higher rates of abstinence from smoking compared to those who received placebo.16
As part of the biopsychosocial model of clinical care, sociodemographic factors must be considered in assessing the relationship between tobacco use and schizophrenia, because a large proportion of patients diagnosed with schizophrenia are members of underrepresented minority groups.17 A PubMed database search using keywords “African American” or “Black,” “tobacco,” and “schizophrenia” located only 12 studies, most of which lacked relevance to this question. Han et al18 is 1 of the few studies to investigate sociodemographic factors as they relate to tobacco use among adults with psychoses. Social determinants of health and other confounding variables also need defining to truly distinguish causation from correlation, especially regarding tobacco use and its association with other health risk behaviors.19
Without the street medicine component of the medical school training I received, the pattern of smoking among patients with schizophrenia may have remained invisible or insignificant to me, as tobacco use is not permitted in the inpatient and outpatient academic settings. This experience not only raised insightful questions, but also emphasized the clinical value of seeing patients within their living environment.
1. Patkar AA, Gopalakrishnan R, Lundy A, et al. Relationship between tobacco smoking and positive and negative symptoms in schizophrenia. J Nerv Ment Dis. 2002;190(9):604-610. doi:10.1097/00005053-200209000-00005
2. Ding JB, Hu K. Cigarette smoking and schizophrenia: etiology, clinical, pharmacological, and treatment implications. Schizophr Res Treatment. 2021;2021:7698030. doi:10.1155/2021/7698030
3. Kendler KS, Lönn SL, Sundquist J, et al. Smoking and schizophrenia in population cohorts of Swedish women and men: a prospective co-relative control study. Am J Psychiatry. 2015;172(11):1092-1100. doi:10.1176/appi.ajp.2015.15010126
4. Patel KR, Cherian J, Gohil K, et al. Schizophrenia: overview and treatment options. P T. 2014;39(9):638-645.
5. King M, Jones R, Petersen I, et al. Cigarette smoking as a risk factor for schizophrenia or all non-affective psychoses. Psychol Med. 2021;51(8):1373-1381. doi:10.1017/S0033291720000136
6. Sagud M, Mihaljevic Peles A, Pivac N, et al. Smoking in schizophrenia: recent findings about an old problem. Curr Opin Psychiatry. 2019;32(5):402-408. doi:10.1097/YCO.0000000000000529
7. Quigley H, MacCabe JH. The relationship between nicotine and psychosis. Ther Adv Psychopharmacol. 2019;9:2045125319859969. doi:10.1177/2045125319859969
8. Balfour DJ, Ridley DL. The effects of nicotine on neural pathways implicated in depression: a factor in nicotine addiction? Pharmacol Biochem Behav. 2000;66(1):79-85. doi:10.1016/s0091-3057(00)00205-7
9. Wang P, Abdin E, Asharani PV, et al. Nicotine dependence in patients with major depressive disorder and psychotic disorders and its relationship with quality of life. Int J Environ Res Public Health. 2021;18(24):13035. doi:10.3390/ijerph182413035
10. Popik P, Krawczyk M, Kos T, et al. Nicotine produces antidepressant-like actions: behavioral and neurochemical evidence. Eur J Pharmacol. 2005;515(1-3):128-133. doi:10.1016/j.ejphar.2005.04.009
11. Quattrocki E, Baird A, Yurgelun-Todd D. Biological aspects of the link between smoking and depression. Harv Rev Psychiatry. 2000;8(3):99-110.
12. Pal A, Balhara YP. A review of impact of tobacco use on patients with co-occurring psychiatric disorders. Tob Use Insights. 2016;9:7-12. doi:10.4137/TUI.S32201
13. Prochaska JJ, Das S, Young-Wolff KC. Smoking, mental illness, and public health. Annu Rev Public Health. 2017;38:165-185. doi:10.1146/annurev-publhealth-031816-044618
14. Coustals N, Martelli C, Brunet-Lecomte M, et al. Chronic smoking and cognition in patients with schizophrenia: a meta-analysis. Schizophr Res. 2020;222:113-121. doi:10.1016/j.schres.2020.03.071
15. Stolz PA, Wehring HJ, Liu F, et al. Effects of cigarette smoking and clozapine treatment on 20-year all-cause & cardiovascular mortality in schizophrenia. Psychiatr Q. 2019;90(2):351-359. doi:10.1007/s11126-018-9621-4
16. Tsoi DT, Porwal M, Webster AC. Interventions for smoking cessation and reduction in individuals with schizophrenia. Cochrane Database Syst Rev. 2013;2013(2):CD007253. doi:10.1002/14651858.CD007253.pub3
17. Heun-Johnson H, Menchine M, Axeen S, et al. Association between race/ethnicity and disparities in health care use before first-episode psychosis among privately insured young patients. JAMA Psychiatry. 2021;78(3):311-319. doi:10.1001/jamapsychiatry.2020.3995
18. Han B, Aung TW, Volkow ND, et al. Tobacco use, nicotine dependence, and cessation methods in us adults with psychosis. JAMA Netw Open. 2023;6(3):e234995. doi:10.1001/jamanetworkopen.2023.4995
19. Peltzer K, Pengpid S. Tobacco use and associated mental symptoms and health risk behaviours amongst individuals 15 years or older in South Africa. S Afr J Psychiatr. 2020;26:1499. doi:10.4102/sajpsychiatry.v26.i0.1499
Editor’s note: Readers’ Forum is a department for correspondence from readers that is not in response to articles published in
Throughout my psychiatric clerkship, I (JWF) participated in street medicine, the practice of providing care to patients (typically those who are homeless) at the location they currently reside, such as in a homeless encampment or community shelter. Our clinical team drove to locations that provided housing for patients diagnosed with schizophrenia, where we assisted with medications and blood draws. I remember pulling up the first day and seeing someone outside smoking a cigarette. I soon learned that many people living in such situations were smokers, and that among the substances they used, tobacco was the most common.
One patient said the cigarettes helped him manage the “voices in his head” as well as some of the adverse effects from medication, such as parkinsonism and akathisia. I asked my attending physician about this and she explained that for some patients, using tobacco was a way to mitigate the positive symptoms of schizophrenia and make the adverse effects of their therapy, particularly extrapyramidal symptoms (EPS), more bearable. By the end of my 2-week rotation, I was sure of a trend: our patients with schizophrenia smoked incessantly. Near the end of my rotation, I asked a patient, “Why do you smoke”? The patient looked at me, puzzled, and replied: “I just do.” This exchange only piqued my curiosity, and I could not help but wonder: what is the relationship between tobacco use and schizophrenia? How is tobacco use related to the pathophysiology of schizophrenia? Does tobacco use among patients with schizophrenia ameliorate aspects of their psychosis? Street medicine offered me a window into a biomedically intriguing question, and I wanted to learn more.
What smoking does for patients with schizophrenia
The high prevalence of smoking among patients with schizophrenia (50% to 88%) greatly exceeds the rates of smoking among patients with other psychiatric illnesses.1,2 The role of smoking in relation to schizophrenia and other psychoses is multidimensional, and evidence implicates smoking as a risk factor for schizophrenia.3,4
Two mechanisms may help explain tobacco use in patients with schizophrenia: reducing the adverse effects of antipsychotic medications and promoting neural transmission of dopamine. Second-generation antipsychotics (SGAs) are a first-line treatment, but they can produce EPS, metabolic dysregulation, and blood disorders such as hyponatremia and (rarely) agranulocytosis (1% with clozapine).5 Compared to those who are nonsmokers, patients with schizophrenia who smoke are more likely to experience more severe symptoms (eg, hallucinations and delusions) and less severe EPS.5,6 Research suggests that exposure to polycyclic aromatic hydrocarbons released during smoking induces cytochrome P450 1A2, an enzyme that metabolizes antipsychotic medications such as haloperidol, clozapine, and olanzapine. Increased metabolism results in lower serum concentrations of antipsychotics, lower efficacy, and more severe positive symptoms.5,6
Additionally, tobacco is an activator of nicotinic acetylcholine receptors (nAChR).6 When these receptors become activated, dopamine is released. Dopamine serves as a mediator of reward for nicotine use. In the context of schizophrenia, tobacco use opposes the mechanism of action of SGAs, which is to block neural transmission of dopamine.6 The etiology of EPS is related to the blockade of postsynaptic dopamine release in the striatum.6 By activating nAChR, smoking induces a downstream release of dopamine that can alleviate iatrogenic EPS by restoring neural transmission of dopamine.6 Nicotine may also modulate alpha-7 nicotinic receptor dysfunction, and improve the ability to filter out irrelevant environmental stimuli (impaired sensory gating), which can be overwhelming for patients with schizophrenia. It also can improve cognitive dysfunction and attention by inducing the release of dopamine in mesocortical pathways.7 The implications of this neural pathway are significant because smoking is significantly greater in tobacco users who are diagnosed with schizophrenia compared to tobacco users who lack a psychiatric diagnosis.6,7 Smoking may enhance dopaminergic neural transmission to a far greater extent in tobacco users with schizophrenia compared to tobacco users who do not develop schizophrenia, which suggests intrinsic differences at the neuronal level. Neural differences between tobacco users with or without schizophrenia may synergize with smoking in clinically and biologically meaningful ways. These pathways require further research to support or disprove these hypotheses.
Aside from the dopaminergic system, mechanisms influencing tobacco use among patients with schizophrenia may also be related to nicotine’s mild antidepressant effects. Evidence suggests a clinically meaningful association between nicotine dependence and mood disorders, and this association may be due to the antidepressant effects of nicotine.8-13 Patients with schizophrenia may experience respite from depressive symptoms through their tobacco use, eventually leading to nicotine dependence.
Continue to: Treatment of schizophrenia...
Treatment of schizophrenia involves multimodal management of a patient’s life, including reducing maladaptive habits that are harmful to health. Chronic smoking in patients with schizophrenia is associated not only with atherosclerosis and cardiovascular disease, but also with poor neurologic functioning, such as significant impairment in attention, working memory, learning, executive function, reasoning, problem-solving and speed of processing.14 One study found that in patients with schizophrenia, smoking increased the 20-year cardiovascular mortality risk by 86%.15
Despite challenges to abstinence, smoking cessation should be discussed with these patients, especially given the high prevalence of smoking among this vulnerable population. Bupropion and varenicline have been studied in the context of smoking cessation among patients with schizophrenia. Data on varenicline are mixed. Smokers with schizophrenia who received bupropion showed higher rates of abstinence from smoking compared to those who received placebo.16
As part of the biopsychosocial model of clinical care, sociodemographic factors must be considered in assessing the relationship between tobacco use and schizophrenia, because a large proportion of patients diagnosed with schizophrenia are members of underrepresented minority groups.17 A PubMed database search using keywords “African American” or “Black,” “tobacco,” and “schizophrenia” located only 12 studies, most of which lacked relevance to this question. Han et al18 is 1 of the few studies to investigate sociodemographic factors as they relate to tobacco use among adults with psychoses. Social determinants of health and other confounding variables also need defining to truly distinguish causation from correlation, especially regarding tobacco use and its association with other health risk behaviors.19
Without the street medicine component of the medical school training I received, the pattern of smoking among patients with schizophrenia may have remained invisible or insignificant to me, as tobacco use is not permitted in the inpatient and outpatient academic settings. This experience not only raised insightful questions, but also emphasized the clinical value of seeing patients within their living environment.
Editor’s note: Readers’ Forum is a department for correspondence from readers that is not in response to articles published in
Throughout my psychiatric clerkship, I (JWF) participated in street medicine, the practice of providing care to patients (typically those who are homeless) at the location they currently reside, such as in a homeless encampment or community shelter. Our clinical team drove to locations that provided housing for patients diagnosed with schizophrenia, where we assisted with medications and blood draws. I remember pulling up the first day and seeing someone outside smoking a cigarette. I soon learned that many people living in such situations were smokers, and that among the substances they used, tobacco was the most common.
One patient said the cigarettes helped him manage the “voices in his head” as well as some of the adverse effects from medication, such as parkinsonism and akathisia. I asked my attending physician about this and she explained that for some patients, using tobacco was a way to mitigate the positive symptoms of schizophrenia and make the adverse effects of their therapy, particularly extrapyramidal symptoms (EPS), more bearable. By the end of my 2-week rotation, I was sure of a trend: our patients with schizophrenia smoked incessantly. Near the end of my rotation, I asked a patient, “Why do you smoke”? The patient looked at me, puzzled, and replied: “I just do.” This exchange only piqued my curiosity, and I could not help but wonder: what is the relationship between tobacco use and schizophrenia? How is tobacco use related to the pathophysiology of schizophrenia? Does tobacco use among patients with schizophrenia ameliorate aspects of their psychosis? Street medicine offered me a window into a biomedically intriguing question, and I wanted to learn more.
What smoking does for patients with schizophrenia
The high prevalence of smoking among patients with schizophrenia (50% to 88%) greatly exceeds the rates of smoking among patients with other psychiatric illnesses.1,2 The role of smoking in relation to schizophrenia and other psychoses is multidimensional, and evidence implicates smoking as a risk factor for schizophrenia.3,4
Two mechanisms may help explain tobacco use in patients with schizophrenia: reducing the adverse effects of antipsychotic medications and promoting neural transmission of dopamine. Second-generation antipsychotics (SGAs) are a first-line treatment, but they can produce EPS, metabolic dysregulation, and blood disorders such as hyponatremia and (rarely) agranulocytosis (1% with clozapine).5 Compared to those who are nonsmokers, patients with schizophrenia who smoke are more likely to experience more severe symptoms (eg, hallucinations and delusions) and less severe EPS.5,6 Research suggests that exposure to polycyclic aromatic hydrocarbons released during smoking induces cytochrome P450 1A2, an enzyme that metabolizes antipsychotic medications such as haloperidol, clozapine, and olanzapine. Increased metabolism results in lower serum concentrations of antipsychotics, lower efficacy, and more severe positive symptoms.5,6
Additionally, tobacco is an activator of nicotinic acetylcholine receptors (nAChR).6 When these receptors become activated, dopamine is released. Dopamine serves as a mediator of reward for nicotine use. In the context of schizophrenia, tobacco use opposes the mechanism of action of SGAs, which is to block neural transmission of dopamine.6 The etiology of EPS is related to the blockade of postsynaptic dopamine release in the striatum.6 By activating nAChR, smoking induces a downstream release of dopamine that can alleviate iatrogenic EPS by restoring neural transmission of dopamine.6 Nicotine may also modulate alpha-7 nicotinic receptor dysfunction, and improve the ability to filter out irrelevant environmental stimuli (impaired sensory gating), which can be overwhelming for patients with schizophrenia. It also can improve cognitive dysfunction and attention by inducing the release of dopamine in mesocortical pathways.7 The implications of this neural pathway are significant because smoking is significantly greater in tobacco users who are diagnosed with schizophrenia compared to tobacco users who lack a psychiatric diagnosis.6,7 Smoking may enhance dopaminergic neural transmission to a far greater extent in tobacco users with schizophrenia compared to tobacco users who do not develop schizophrenia, which suggests intrinsic differences at the neuronal level. Neural differences between tobacco users with or without schizophrenia may synergize with smoking in clinically and biologically meaningful ways. These pathways require further research to support or disprove these hypotheses.
Aside from the dopaminergic system, mechanisms influencing tobacco use among patients with schizophrenia may also be related to nicotine’s mild antidepressant effects. Evidence suggests a clinically meaningful association between nicotine dependence and mood disorders, and this association may be due to the antidepressant effects of nicotine.8-13 Patients with schizophrenia may experience respite from depressive symptoms through their tobacco use, eventually leading to nicotine dependence.
Continue to: Treatment of schizophrenia...
Treatment of schizophrenia involves multimodal management of a patient’s life, including reducing maladaptive habits that are harmful to health. Chronic smoking in patients with schizophrenia is associated not only with atherosclerosis and cardiovascular disease, but also with poor neurologic functioning, such as significant impairment in attention, working memory, learning, executive function, reasoning, problem-solving and speed of processing.14 One study found that in patients with schizophrenia, smoking increased the 20-year cardiovascular mortality risk by 86%.15
Despite challenges to abstinence, smoking cessation should be discussed with these patients, especially given the high prevalence of smoking among this vulnerable population. Bupropion and varenicline have been studied in the context of smoking cessation among patients with schizophrenia. Data on varenicline are mixed. Smokers with schizophrenia who received bupropion showed higher rates of abstinence from smoking compared to those who received placebo.16
As part of the biopsychosocial model of clinical care, sociodemographic factors must be considered in assessing the relationship between tobacco use and schizophrenia, because a large proportion of patients diagnosed with schizophrenia are members of underrepresented minority groups.17 A PubMed database search using keywords “African American” or “Black,” “tobacco,” and “schizophrenia” located only 12 studies, most of which lacked relevance to this question. Han et al18 is 1 of the few studies to investigate sociodemographic factors as they relate to tobacco use among adults with psychoses. Social determinants of health and other confounding variables also need defining to truly distinguish causation from correlation, especially regarding tobacco use and its association with other health risk behaviors.19
Without the street medicine component of the medical school training I received, the pattern of smoking among patients with schizophrenia may have remained invisible or insignificant to me, as tobacco use is not permitted in the inpatient and outpatient academic settings. This experience not only raised insightful questions, but also emphasized the clinical value of seeing patients within their living environment.
1. Patkar AA, Gopalakrishnan R, Lundy A, et al. Relationship between tobacco smoking and positive and negative symptoms in schizophrenia. J Nerv Ment Dis. 2002;190(9):604-610. doi:10.1097/00005053-200209000-00005
2. Ding JB, Hu K. Cigarette smoking and schizophrenia: etiology, clinical, pharmacological, and treatment implications. Schizophr Res Treatment. 2021;2021:7698030. doi:10.1155/2021/7698030
3. Kendler KS, Lönn SL, Sundquist J, et al. Smoking and schizophrenia in population cohorts of Swedish women and men: a prospective co-relative control study. Am J Psychiatry. 2015;172(11):1092-1100. doi:10.1176/appi.ajp.2015.15010126
4. Patel KR, Cherian J, Gohil K, et al. Schizophrenia: overview and treatment options. P T. 2014;39(9):638-645.
5. King M, Jones R, Petersen I, et al. Cigarette smoking as a risk factor for schizophrenia or all non-affective psychoses. Psychol Med. 2021;51(8):1373-1381. doi:10.1017/S0033291720000136
6. Sagud M, Mihaljevic Peles A, Pivac N, et al. Smoking in schizophrenia: recent findings about an old problem. Curr Opin Psychiatry. 2019;32(5):402-408. doi:10.1097/YCO.0000000000000529
7. Quigley H, MacCabe JH. The relationship between nicotine and psychosis. Ther Adv Psychopharmacol. 2019;9:2045125319859969. doi:10.1177/2045125319859969
8. Balfour DJ, Ridley DL. The effects of nicotine on neural pathways implicated in depression: a factor in nicotine addiction? Pharmacol Biochem Behav. 2000;66(1):79-85. doi:10.1016/s0091-3057(00)00205-7
9. Wang P, Abdin E, Asharani PV, et al. Nicotine dependence in patients with major depressive disorder and psychotic disorders and its relationship with quality of life. Int J Environ Res Public Health. 2021;18(24):13035. doi:10.3390/ijerph182413035
10. Popik P, Krawczyk M, Kos T, et al. Nicotine produces antidepressant-like actions: behavioral and neurochemical evidence. Eur J Pharmacol. 2005;515(1-3):128-133. doi:10.1016/j.ejphar.2005.04.009
11. Quattrocki E, Baird A, Yurgelun-Todd D. Biological aspects of the link between smoking and depression. Harv Rev Psychiatry. 2000;8(3):99-110.
12. Pal A, Balhara YP. A review of impact of tobacco use on patients with co-occurring psychiatric disorders. Tob Use Insights. 2016;9:7-12. doi:10.4137/TUI.S32201
13. Prochaska JJ, Das S, Young-Wolff KC. Smoking, mental illness, and public health. Annu Rev Public Health. 2017;38:165-185. doi:10.1146/annurev-publhealth-031816-044618
14. Coustals N, Martelli C, Brunet-Lecomte M, et al. Chronic smoking and cognition in patients with schizophrenia: a meta-analysis. Schizophr Res. 2020;222:113-121. doi:10.1016/j.schres.2020.03.071
15. Stolz PA, Wehring HJ, Liu F, et al. Effects of cigarette smoking and clozapine treatment on 20-year all-cause & cardiovascular mortality in schizophrenia. Psychiatr Q. 2019;90(2):351-359. doi:10.1007/s11126-018-9621-4
16. Tsoi DT, Porwal M, Webster AC. Interventions for smoking cessation and reduction in individuals with schizophrenia. Cochrane Database Syst Rev. 2013;2013(2):CD007253. doi:10.1002/14651858.CD007253.pub3
17. Heun-Johnson H, Menchine M, Axeen S, et al. Association between race/ethnicity and disparities in health care use before first-episode psychosis among privately insured young patients. JAMA Psychiatry. 2021;78(3):311-319. doi:10.1001/jamapsychiatry.2020.3995
18. Han B, Aung TW, Volkow ND, et al. Tobacco use, nicotine dependence, and cessation methods in us adults with psychosis. JAMA Netw Open. 2023;6(3):e234995. doi:10.1001/jamanetworkopen.2023.4995
19. Peltzer K, Pengpid S. Tobacco use and associated mental symptoms and health risk behaviours amongst individuals 15 years or older in South Africa. S Afr J Psychiatr. 2020;26:1499. doi:10.4102/sajpsychiatry.v26.i0.1499
1. Patkar AA, Gopalakrishnan R, Lundy A, et al. Relationship between tobacco smoking and positive and negative symptoms in schizophrenia. J Nerv Ment Dis. 2002;190(9):604-610. doi:10.1097/00005053-200209000-00005
2. Ding JB, Hu K. Cigarette smoking and schizophrenia: etiology, clinical, pharmacological, and treatment implications. Schizophr Res Treatment. 2021;2021:7698030. doi:10.1155/2021/7698030
3. Kendler KS, Lönn SL, Sundquist J, et al. Smoking and schizophrenia in population cohorts of Swedish women and men: a prospective co-relative control study. Am J Psychiatry. 2015;172(11):1092-1100. doi:10.1176/appi.ajp.2015.15010126
4. Patel KR, Cherian J, Gohil K, et al. Schizophrenia: overview and treatment options. P T. 2014;39(9):638-645.
5. King M, Jones R, Petersen I, et al. Cigarette smoking as a risk factor for schizophrenia or all non-affective psychoses. Psychol Med. 2021;51(8):1373-1381. doi:10.1017/S0033291720000136
6. Sagud M, Mihaljevic Peles A, Pivac N, et al. Smoking in schizophrenia: recent findings about an old problem. Curr Opin Psychiatry. 2019;32(5):402-408. doi:10.1097/YCO.0000000000000529
7. Quigley H, MacCabe JH. The relationship between nicotine and psychosis. Ther Adv Psychopharmacol. 2019;9:2045125319859969. doi:10.1177/2045125319859969
8. Balfour DJ, Ridley DL. The effects of nicotine on neural pathways implicated in depression: a factor in nicotine addiction? Pharmacol Biochem Behav. 2000;66(1):79-85. doi:10.1016/s0091-3057(00)00205-7
9. Wang P, Abdin E, Asharani PV, et al. Nicotine dependence in patients with major depressive disorder and psychotic disorders and its relationship with quality of life. Int J Environ Res Public Health. 2021;18(24):13035. doi:10.3390/ijerph182413035
10. Popik P, Krawczyk M, Kos T, et al. Nicotine produces antidepressant-like actions: behavioral and neurochemical evidence. Eur J Pharmacol. 2005;515(1-3):128-133. doi:10.1016/j.ejphar.2005.04.009
11. Quattrocki E, Baird A, Yurgelun-Todd D. Biological aspects of the link between smoking and depression. Harv Rev Psychiatry. 2000;8(3):99-110.
12. Pal A, Balhara YP. A review of impact of tobacco use on patients with co-occurring psychiatric disorders. Tob Use Insights. 2016;9:7-12. doi:10.4137/TUI.S32201
13. Prochaska JJ, Das S, Young-Wolff KC. Smoking, mental illness, and public health. Annu Rev Public Health. 2017;38:165-185. doi:10.1146/annurev-publhealth-031816-044618
14. Coustals N, Martelli C, Brunet-Lecomte M, et al. Chronic smoking and cognition in patients with schizophrenia: a meta-analysis. Schizophr Res. 2020;222:113-121. doi:10.1016/j.schres.2020.03.071
15. Stolz PA, Wehring HJ, Liu F, et al. Effects of cigarette smoking and clozapine treatment on 20-year all-cause & cardiovascular mortality in schizophrenia. Psychiatr Q. 2019;90(2):351-359. doi:10.1007/s11126-018-9621-4
16. Tsoi DT, Porwal M, Webster AC. Interventions for smoking cessation and reduction in individuals with schizophrenia. Cochrane Database Syst Rev. 2013;2013(2):CD007253. doi:10.1002/14651858.CD007253.pub3
17. Heun-Johnson H, Menchine M, Axeen S, et al. Association between race/ethnicity and disparities in health care use before first-episode psychosis among privately insured young patients. JAMA Psychiatry. 2021;78(3):311-319. doi:10.1001/jamapsychiatry.2020.3995
18. Han B, Aung TW, Volkow ND, et al. Tobacco use, nicotine dependence, and cessation methods in us adults with psychosis. JAMA Netw Open. 2023;6(3):e234995. doi:10.1001/jamanetworkopen.2023.4995
19. Peltzer K, Pengpid S. Tobacco use and associated mental symptoms and health risk behaviours amongst individuals 15 years or older in South Africa. S Afr J Psychiatr. 2020;26:1499. doi:10.4102/sajpsychiatry.v26.i0.1499
A Severe Case of Paliperidone Palmitate-Induced Parkinsonism Leading to Prolonged Hospitalization: Opportunities for Improvement
Many patients with psychiatric illness have difficulty with medication adherence. Patients with impaired reality testing especially are at risk.
Keck and McElroy evaluated 141 patients who were initially hospitalized for bipolar disorder prospectively over 1 year to assess adherence with medication. During the follow-up period, 71 patients (51%) were partially or totally nonadherent with medication as prescribed. The most commonly cited reason for nonadherence was denial of need.1
Clinicians and patients face additional challenges due to the deleterious effects of relapse in the setting of both schizophrenia and bipolar disorder. Almost all oral antipsychotic or mood stabilizer medications require a minimum dosing schedule to effectively treat these disorders, and some of these oral medications require regular laboratory monitoring. Moreover, some of the agents can have serious adverse effects (AEs), such as seizure or withdrawal, if stopped abruptly. Social support from family or friends may improve adherence, but many psychiatric outpatients have a smaller social support network than do patients without psychiatric illnesses.2
Long-acting injectable (LAI) antipsychotics have been available for the past 40 years. These medications have provided clinicians with an additional option for patients with schizophrenia or bipolar disorder who are nonadherent to their medication treatment plans or who desire an administration choice that is more convenient than daily oral pills.3-7 Some clinical practice guidelines recommend considering LAIs as a maintenance treatment for schizophrenia.5 Like the rest of the pharmacopoeia, these formulations have AEs, such as extrapyramidal symptoms (EPS), weight gain, and metabolic syndrome.1 The longer half-life of these drugs may make such effects difficult to reverse.
This article presents a case of the use of depot formulation paliperidone palmitate in an elderly patient with bipolar disorder who was previously on high-dose oral second generation antipsychotics. He developed severe parkinsonism during a protracted hospitalization that ended in death.
Case Presentation
Mr. W was a 68-year-old homeless white male with a history of coronary artery disease status-post coronary artery bypass surgery, obstructive sleep apnea, and bipolar 1 disorder who presented to a large rural VAMC emergency department (ED) as a transfer from an outside hospital (OSH). He originally presented at the OSH for vomiting and diarrhea, but while there, he was placed under involuntary psychiatric commitment. The involuntary commitment form noted him to be tangential and disorganized; he was found walking about the ED without clothes. During the initial psychiatry interview, the clinician noted a disorganized thought process. When asked about circumstances leading to admission, he stated he was “a scuba diver, pilot, actor, submarine commander.” He also reported he had given “seminars to 6,000 people,” he held a psychology degree, and he came from a family that owned part of the island of Kodiak, Alaska. Mr. W stated he had no mental health history and believed psychiatry was witchcraft. He reported having no hallucinations and stated he heard the voice of god. He also reported to have met god multiple times and to have been married to a supermodel.
Mr. W’s chart demonstrated a history of mental illness over 30 years and that he previously was prescribed psychiatric medications. He had multiple inpatient psychiatric admissions with grandiose ideations, disorganized behaviors, and hypersexuality. He had been prescribed quetiapine, divalproex, lithium, carbamazepine, and lorazepam. He was formally diagnosed in the past with bipolar 1 disorder. There also was a family history of psychiatric illness. His mother had received electroconvulsive therapy, and both parents had alcohol substance use disorder.
Mr. W had been homeless for 20 years and had several psychiatric admissions during this period. Mr. W also had chronic difficulty with obtaining food and taking medications as prescribed. Sometimes he would only be able to eat 1 to 2 meals per day. He often changed location and had lived in at least 7 different states. Currently, he was estranged from his family. About 19 years ago, his sister reported that the veteran had told her that he was Jesus Christ, per clinical records. His estranged sister’s statement was corroborated by past psychology consult records citing episodes of the patient hearing god 30 and 26 years before the current admission. His second ex-wife cited inappropriate sexual behavior in front of their children. He had difficulty in school, failed at least 2 grades, and joined the U.S. Navy in tenth grade. A Neurobehavioral Cognitive Status Examination given 19 years ago showed mild impairment on attention and severe impairment in memory.
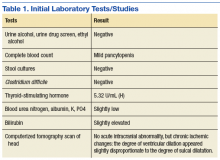
The physical examination on presentation to the OSH was unremarkable. Mr. W did not cooperate with formal neurocognitive testing, and he consistently made errors during orientation testing. Complete blood count from a OSH ED laboratory test was remarkable for a mild pancytopenia with a leukocyte count of 3,100 cells/mcL, hemoglobin 13.1 g/dL, and hematocrit 38.4%. Red cell distribution width was within normal limits at 13.5%. Stool cultures showed normal fecal flora and no salmonella, shigella, or campylobacter. Thyroid-stimulating hormone (TSH) was slightly elevated at 5.32 U/mL. An electrocardiogram showed a QTc interval of 412 ms. A computerized tomography scan of his head showed no acute intracranial abnormality along with chronic ischemic changes in the brain (Table 1). Presumed cause of his nausea and diarrhea was viral gastroenteritis likely acquired at a homeless shelter.
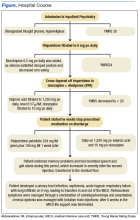
Once stabilized, Mr. W was admitted to the VA hospital inpatient psychiatry unit under involuntary commitment for acute mania. Risperidone 0.25 mg orally twice a day was started for mood stabilization and psychosis along with trazodone 50 mg orally as needed for insomnia. Despite upward titration and change in frequency of the risperidone dose, Mr. W’s manic episode persisted. He remained on the psychiatric floor for 2 months (Figure). His TSH and free T4 were monitored during his stay, and levothyroxine was started. Risperidone was titrated to 8 mg/d. Mr. W’s Young Mania Rating Scale (YMRS) score decreased from 30 to 24. Mr. W had a mild improvement in irritability and speech rate but little change in elevated mood and delusional content.
He continued to endorse “speaking to god 16 times” even at the highest risperidone dose. The treatment team prescribed dissolvable risperidone tablets secondary to diversion concerns. In addition, the team added benztropine 0.5 mg once a day after observing a stooped posture and decreased arm swing. Mr. W noted risperidone made him “lethargic” and that his “body did not need” it. After 1 month of treatment with risperidone, the treatment team decided to cross taper the veteran from risperidone to a combination of olanzapine and divalproex secondary to inadequate treatment response.
The inpatient team started Mr. W on oral disintegrating tablets of olanzapine 5 mg once a day, and oral divalproex 1,000 mg once a day. An intramuscular backup of olanzapine was made available if oral medication was refused. Divalproex was titrated to 1,250 mg once a day to target a serum level of 61.7 µg/mL, and olanzapine was titrated to 10 mg once a day. After 9 days, the veteran showed moderate improvement in mania symptoms with a YMRS score < 20, indicating the absence of mania. However, the veteran made it very clear that he would stop taking the prescribed medication on discharge. The team elected to initiate a LAI.
The veteran received his first injection of the LAI psychiatric medication paliperidone palmitate 234 mg and a second 156-mg injection of the same medication 1 week later as per loading protocol. He was concurrently on daily oral divalproex 1,250 mg and olanzapine 10 mg. Mr. W continued to note he felt sedated during this period; his sedation worsened after the second injection. He also began to forget the location of his room and developed mumbled speech. His gait deteriorated to where he required a walker 6 days after injection and a wheelchair 3 days later. He became incontinent of urine and feces. Mr. W exhibited masked facies with severe drooling. This eventually progressed to difficulty swallowing. At the advice of speech pathology, he was downgraded to a pureed and nectar-thick liquid diet. He required assistance with meals.
Because of his sedation and parkinsonism symptoms, he was tapered off both olanzapine and divalproex. His last dose of olanzapine was on the date of his first injection and last dose of divalproex was 15 days after the second injection. The benztropine, which was originally given to counteract the effects of risperidone monotherapy, was discontinued over concern of anticholinergic load and sedation. The neurology consultant recommended carbidopa 25 mg and levodopa 100 mg 3 times per day for treatment of parkinsonism symptoms. Mr. W was only able to take 1 dose because of trouble swallowing. Twenty days after his second injection, a rapid response team (local clinical team 1 step below a code team) was called as Mr. W was unusually lethargic and unable to eat. He was then transferred to the medical floor.
While on the medical floor, dobhoff tube access was established for nutrition and to allow administration of carbidopa and levodopa. Mr. W could still speak at this time and was distraught. He stated, “I don’t know why god would do this to me.” Further workup was performed to look for other etiologies of the patient’s change in status. Creatinine kinase testing, lumbar puncture with cerebral spinal fluid (CSF) bacterial culture, CSF cryptococcal testing, and syphilis antigens were all negative. Magnetic resonance imaging of the brain demonstrated diffuse cerebral atrophy with widened cistern and sulci resulting in ex vacuo dilatation.
Neurology thought that the ventriculomegaly did not have features of normal pressure hydrocephalus and was secondary to chronic ischemic demyelination caused by chronic malnutrition. During follow-up visits, the veteran was less and less verbal. It progressed to where he answered questions only in grunts. Eight days after transfer to the medical floor, Mr. W was noted to have his neck locked in a laterally rotated position with clonus of the sternocleidomastoid. Due to concern about possibility of neck dystonia and the poor adherence of the patient with carbidopa and levodopa given orally, the psychiatric team made the recommendation to start benztropine 1 mg given twice a day, delivered via the dobhoff tube to treat both the parkinsonism and dystonia. The following day Mr. W failed a repeat swallow study and was no longer allowed to receive anything orally.
Mild icterus and jaundice were noted on physical examination along with transaminitis and elevated bilirubin. He developed a fever. Thirteen days after transfer to the medical floor, blood cultures revealed Klebsiella septicemia. Benztropine was discontinued at this time because of concern the medication was causing or exacerbating the fever. While being treated for Klebsiella sepsis, the psychiatry team addressed his continued hypophonia, inability to ambulate, masked facies, and neck dystonia with diphenhydramine 50 mg given intramuscular (IM) twice per day.
Mr. W developed several more iatrogenic complications near this time, including urinary tract infection septicemia and acute hypoxic respiratory failure with lung infiltrate on X-ray, requiring ventilator support. His clinical status led to a number of transfers in and out of the medical intensive care unit (MICU). During this time, his parkinsonism symptoms were managed through a combination of carbidopa and levodopa and amantadine. Cervical dystonia was managed with botulism toxin injections. Mr. W spent 6 weeks in the MICU until the decision was made to terminate life support, and he was taken off the ventilator. He died shortly thereafter. Autopsy findings suggested that Mr. W had severe Alzheimer disease.
Discussion
Following the IM injection of paliperidone palmitate, Mr. W had a complicated hospital stay resulting in his demise from sepsis and multiorgan failure. Severe immobilization, rigidity, and dystonia prevented Mr. W from conducting activities of daily living, which resulted in invasive interventions, such as continued foley catheterization. His sepsis was likely secondary to aspiration, catheterization, and eventual ventilation—all iatrogenic complications. Previous estimates in the U.S. have suggested a total of 225,000 deaths per year from iatrogenic causes.8
There are several areas of concern. Clearly, Mr. W had severe illness that greatly affected his life. He was estranged from family and had endured a 2-decade period of homelessness. He deserved effective treatment for his psychiatric illness to relieve his suffering. His long period of mental illness without effective treatment very likely biased the initial treatment team toward an aggressive approach.
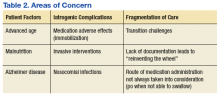
Fragmented Care
The prolonged hospital stay and multiple complications directly led to fragmentation in Mr. W’s care. He was hospitalized for months on 3 different main services: psychiatry, medicine, and the MICU. Even when he remained on the same service, the primary members of his treatment team changed every few weeks. Many different specialties were consulted and reconsulted. Members of the specialty consult teams changed throughout the hospitalization as well. Given the nature of the local clinical administration, Mr. W likely received the most consistent team members from the attendings on the psychiatry consult-liaison service (who do not rotate) and from a local subspecialty delirium consult team (all members stay consistent except pharmacy residents).
Documentation of clinical reasoning behind treatment decisions was not ideal and occasionally lacking. This led to a tendency to “reinvent the wheel” with Mr. W’s treatment approach every few weeks. It was not until Mr. W had spent a significant amount of time on the medical service that an interdisciplinary treatment team meeting involving medicine, psychiatry, nursing, delirium, and neurology experts occurred. Although the interdisciplinary meeting helped by reviewing the hospital course, agreeing on a likely cause of the symptoms, and creating a treatment plan going forward, Mr. W was not able to recover.
Even when team members were stable, communication in a timely fashion did not always occur. At several points, expert recommendations were delayed by a day or more. Difficulties in treatment implementation were not communicated back to the specialty teams. The most significant example was a delay in recognition when Mr. W could no longer take oral pills secondary to the parkinsonism. Many days passed before an alternative liquid or dissolved medication was recommended on 2 separate occasions.
Subspecialty Consult
Addressing these documentation, communication, and transition challenges is neither easy nor unique to this large rural VA medical center. The authors have attempted to address this in the local system with the creation of a delirium team subspecialty consult service. Team members do not rotate and are able to follow patients throughout their hospital course. At the time of Mr. W’s hospitalization, the team included representatives from nursing, psychiatry, and occasionally pharmacy. Since then, it has expanded to include geriatrics and medicine. In addition to delirium being a marker for complex patients at risk for hospital complications, medical reasons for an extended length of stay could serve as a trigger for a referral to such a team of experts. In Mr. W’s case, that could have led to interdisciplinary consultation up to 2 months before it occurred. This may have led to a much better outcome.
Secondary parkinsonism is most notable with the typical antipychotics. The prevalence can vary between 50% and 75% and may be higher within the elderly population. However, all antipsychotics have a chance of demonstrating EPS. Risperidone has a low incidence at low doses; studies have shown dose-related parkinsonism at doses of 2 to 6 mg/d. Significant risk of parkinsonism is further exacerbated when drug-drug interactions are considered.9 Concurrently receiving 2 antipsychotics, olanzapine and paliperidone, initially caused the EPS. The veteran’s cerebral atrophy from significant malnutrition related to chronic homelessness, and the presence of Alzheimer disease only identified postmortem exacerbated this AE. Further complicating the management of the EPS, paliperidone palmitate has a long half-life of 25 to 49 days.9 Simply discontinuing the medication did not remove it from Mr. W’s system. Paliperidone would have continued to be present for months.
Conclusion
In this case, aggressive changes in the antipsychotic medications in a short period led to Mr. W effectively having 3 different agents in his system at the same time. This significantly elevated his risk of AEs, including parkinsonism. The clinician must be vigilant to further recognize the initial symptoms of parkinsonism on clinical presentation. Administration of clinical scales, such as the Simpson-Angus Extrapyramidal Side Effect, can help in these situations.10 Malnutrition and increased age can predispose patients to neurolepticAEs, so treatment teams should exercise caution when administering antipsychotics in such a population. Pharmacokinetic changes in all major organ systems from aging result in higher and more variable drug concentrations. This leads to an increased sensitivity to drugs and AEs.9
Given the increasing geriatric patient population in the U.S., treating mania in the elderly will become more common. Providers should carefully consider the risks vs benefit ratio for each individual because a serious adverse reaction may result in detrimental consequences. Even with severe symptoms leading to a bias toward an aggressive approach, it may be better to “start low and go slow.” Early inclusion of interdisciplinary expertise should be sought in complex cases.
1. Keck PE Jr, McElroy SL, Strakowski SM, Bourne ML, West SA. Compliance with maintenance treatment in bipolar disorder. Psychopharmacol Bull. 1997;33(1):87-91.
2. Henderson S, Duncan-Jones P, McAuley H, Ritchie K. The patient’s primary group. Br J Psychiatry. 1978;132:74-86.
3. Buoli M, Ciappolino V, Altamura AC. Paliperidone palmitate depot in the long-term treatment of psychotic bipolar disorder: a case series. Clin Neuropharmacol. 2015;38(5):209-211.
4. Chou YH, Chu PC, Wu SW, et al. A systematic review and experts’ consensus for long-acting injectable antipsychotics in bipolar disorder. Clin Psychopharmacol Neurosci. 2015;13(2):121-128.
5. Kishi T, Oya K, Iwata N. Long-acting injectable antipsychotics for prevention of relapse in bipolar disorder: a systematic review and meta-analysis of randomized controlled trials. Int J Neuropsychopharmacol. 2016;19(9):1-10.
6. Llorca PM, Abbar M, Courtet P, Guillaume S, Lancrenon S, Samalin L. Guidelines for the use and management of long-acting injectable antipsychotics in serious mental illness. BMC Psychiatry. 2013;13:340.
7. Spanarello S, La Ferla T. The pharmacokinetics of long-acting antipsychotic medications. Curr Clin Pharmacol. 2014;9(3):310-317.
8. Starfield B. Is US health really the best in the world? JAMA. 2000;284(4):483-485.
9. Labbate LA, Fava M, Rosenbaum JF, Arana GW. Handbook of Psychiatric Drug Therapy. 6th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2010.
10. Simpson GM, Angus JW. A rating scale for extrapyramidal side effects. Acta Psychiatr Scand Suppl. 1970;212:11-19.
Many patients with psychiatric illness have difficulty with medication adherence. Patients with impaired reality testing especially are at risk.
Keck and McElroy evaluated 141 patients who were initially hospitalized for bipolar disorder prospectively over 1 year to assess adherence with medication. During the follow-up period, 71 patients (51%) were partially or totally nonadherent with medication as prescribed. The most commonly cited reason for nonadherence was denial of need.1
Clinicians and patients face additional challenges due to the deleterious effects of relapse in the setting of both schizophrenia and bipolar disorder. Almost all oral antipsychotic or mood stabilizer medications require a minimum dosing schedule to effectively treat these disorders, and some of these oral medications require regular laboratory monitoring. Moreover, some of the agents can have serious adverse effects (AEs), such as seizure or withdrawal, if stopped abruptly. Social support from family or friends may improve adherence, but many psychiatric outpatients have a smaller social support network than do patients without psychiatric illnesses.2
Long-acting injectable (LAI) antipsychotics have been available for the past 40 years. These medications have provided clinicians with an additional option for patients with schizophrenia or bipolar disorder who are nonadherent to their medication treatment plans or who desire an administration choice that is more convenient than daily oral pills.3-7 Some clinical practice guidelines recommend considering LAIs as a maintenance treatment for schizophrenia.5 Like the rest of the pharmacopoeia, these formulations have AEs, such as extrapyramidal symptoms (EPS), weight gain, and metabolic syndrome.1 The longer half-life of these drugs may make such effects difficult to reverse.
This article presents a case of the use of depot formulation paliperidone palmitate in an elderly patient with bipolar disorder who was previously on high-dose oral second generation antipsychotics. He developed severe parkinsonism during a protracted hospitalization that ended in death.
Case Presentation
Mr. W was a 68-year-old homeless white male with a history of coronary artery disease status-post coronary artery bypass surgery, obstructive sleep apnea, and bipolar 1 disorder who presented to a large rural VAMC emergency department (ED) as a transfer from an outside hospital (OSH). He originally presented at the OSH for vomiting and diarrhea, but while there, he was placed under involuntary psychiatric commitment. The involuntary commitment form noted him to be tangential and disorganized; he was found walking about the ED without clothes. During the initial psychiatry interview, the clinician noted a disorganized thought process. When asked about circumstances leading to admission, he stated he was “a scuba diver, pilot, actor, submarine commander.” He also reported he had given “seminars to 6,000 people,” he held a psychology degree, and he came from a family that owned part of the island of Kodiak, Alaska. Mr. W stated he had no mental health history and believed psychiatry was witchcraft. He reported having no hallucinations and stated he heard the voice of god. He also reported to have met god multiple times and to have been married to a supermodel.
Mr. W’s chart demonstrated a history of mental illness over 30 years and that he previously was prescribed psychiatric medications. He had multiple inpatient psychiatric admissions with grandiose ideations, disorganized behaviors, and hypersexuality. He had been prescribed quetiapine, divalproex, lithium, carbamazepine, and lorazepam. He was formally diagnosed in the past with bipolar 1 disorder. There also was a family history of psychiatric illness. His mother had received electroconvulsive therapy, and both parents had alcohol substance use disorder.
Mr. W had been homeless for 20 years and had several psychiatric admissions during this period. Mr. W also had chronic difficulty with obtaining food and taking medications as prescribed. Sometimes he would only be able to eat 1 to 2 meals per day. He often changed location and had lived in at least 7 different states. Currently, he was estranged from his family. About 19 years ago, his sister reported that the veteran had told her that he was Jesus Christ, per clinical records. His estranged sister’s statement was corroborated by past psychology consult records citing episodes of the patient hearing god 30 and 26 years before the current admission. His second ex-wife cited inappropriate sexual behavior in front of their children. He had difficulty in school, failed at least 2 grades, and joined the U.S. Navy in tenth grade. A Neurobehavioral Cognitive Status Examination given 19 years ago showed mild impairment on attention and severe impairment in memory.
The physical examination on presentation to the OSH was unremarkable. Mr. W did not cooperate with formal neurocognitive testing, and he consistently made errors during orientation testing. Complete blood count from a OSH ED laboratory test was remarkable for a mild pancytopenia with a leukocyte count of 3,100 cells/mcL, hemoglobin 13.1 g/dL, and hematocrit 38.4%. Red cell distribution width was within normal limits at 13.5%. Stool cultures showed normal fecal flora and no salmonella, shigella, or campylobacter. Thyroid-stimulating hormone (TSH) was slightly elevated at 5.32 U/mL. An electrocardiogram showed a QTc interval of 412 ms. A computerized tomography scan of his head showed no acute intracranial abnormality along with chronic ischemic changes in the brain (Table 1). Presumed cause of his nausea and diarrhea was viral gastroenteritis likely acquired at a homeless shelter.
Once stabilized, Mr. W was admitted to the VA hospital inpatient psychiatry unit under involuntary commitment for acute mania. Risperidone 0.25 mg orally twice a day was started for mood stabilization and psychosis along with trazodone 50 mg orally as needed for insomnia. Despite upward titration and change in frequency of the risperidone dose, Mr. W’s manic episode persisted. He remained on the psychiatric floor for 2 months (Figure). His TSH and free T4 were monitored during his stay, and levothyroxine was started. Risperidone was titrated to 8 mg/d. Mr. W’s Young Mania Rating Scale (YMRS) score decreased from 30 to 24. Mr. W had a mild improvement in irritability and speech rate but little change in elevated mood and delusional content.
He continued to endorse “speaking to god 16 times” even at the highest risperidone dose. The treatment team prescribed dissolvable risperidone tablets secondary to diversion concerns. In addition, the team added benztropine 0.5 mg once a day after observing a stooped posture and decreased arm swing. Mr. W noted risperidone made him “lethargic” and that his “body did not need” it. After 1 month of treatment with risperidone, the treatment team decided to cross taper the veteran from risperidone to a combination of olanzapine and divalproex secondary to inadequate treatment response.
The inpatient team started Mr. W on oral disintegrating tablets of olanzapine 5 mg once a day, and oral divalproex 1,000 mg once a day. An intramuscular backup of olanzapine was made available if oral medication was refused. Divalproex was titrated to 1,250 mg once a day to target a serum level of 61.7 µg/mL, and olanzapine was titrated to 10 mg once a day. After 9 days, the veteran showed moderate improvement in mania symptoms with a YMRS score < 20, indicating the absence of mania. However, the veteran made it very clear that he would stop taking the prescribed medication on discharge. The team elected to initiate a LAI.
The veteran received his first injection of the LAI psychiatric medication paliperidone palmitate 234 mg and a second 156-mg injection of the same medication 1 week later as per loading protocol. He was concurrently on daily oral divalproex 1,250 mg and olanzapine 10 mg. Mr. W continued to note he felt sedated during this period; his sedation worsened after the second injection. He also began to forget the location of his room and developed mumbled speech. His gait deteriorated to where he required a walker 6 days after injection and a wheelchair 3 days later. He became incontinent of urine and feces. Mr. W exhibited masked facies with severe drooling. This eventually progressed to difficulty swallowing. At the advice of speech pathology, he was downgraded to a pureed and nectar-thick liquid diet. He required assistance with meals.
Because of his sedation and parkinsonism symptoms, he was tapered off both olanzapine and divalproex. His last dose of olanzapine was on the date of his first injection and last dose of divalproex was 15 days after the second injection. The benztropine, which was originally given to counteract the effects of risperidone monotherapy, was discontinued over concern of anticholinergic load and sedation. The neurology consultant recommended carbidopa 25 mg and levodopa 100 mg 3 times per day for treatment of parkinsonism symptoms. Mr. W was only able to take 1 dose because of trouble swallowing. Twenty days after his second injection, a rapid response team (local clinical team 1 step below a code team) was called as Mr. W was unusually lethargic and unable to eat. He was then transferred to the medical floor.
While on the medical floor, dobhoff tube access was established for nutrition and to allow administration of carbidopa and levodopa. Mr. W could still speak at this time and was distraught. He stated, “I don’t know why god would do this to me.” Further workup was performed to look for other etiologies of the patient’s change in status. Creatinine kinase testing, lumbar puncture with cerebral spinal fluid (CSF) bacterial culture, CSF cryptococcal testing, and syphilis antigens were all negative. Magnetic resonance imaging of the brain demonstrated diffuse cerebral atrophy with widened cistern and sulci resulting in ex vacuo dilatation.
Neurology thought that the ventriculomegaly did not have features of normal pressure hydrocephalus and was secondary to chronic ischemic demyelination caused by chronic malnutrition. During follow-up visits, the veteran was less and less verbal. It progressed to where he answered questions only in grunts. Eight days after transfer to the medical floor, Mr. W was noted to have his neck locked in a laterally rotated position with clonus of the sternocleidomastoid. Due to concern about possibility of neck dystonia and the poor adherence of the patient with carbidopa and levodopa given orally, the psychiatric team made the recommendation to start benztropine 1 mg given twice a day, delivered via the dobhoff tube to treat both the parkinsonism and dystonia. The following day Mr. W failed a repeat swallow study and was no longer allowed to receive anything orally.
Mild icterus and jaundice were noted on physical examination along with transaminitis and elevated bilirubin. He developed a fever. Thirteen days after transfer to the medical floor, blood cultures revealed Klebsiella septicemia. Benztropine was discontinued at this time because of concern the medication was causing or exacerbating the fever. While being treated for Klebsiella sepsis, the psychiatry team addressed his continued hypophonia, inability to ambulate, masked facies, and neck dystonia with diphenhydramine 50 mg given intramuscular (IM) twice per day.
Mr. W developed several more iatrogenic complications near this time, including urinary tract infection septicemia and acute hypoxic respiratory failure with lung infiltrate on X-ray, requiring ventilator support. His clinical status led to a number of transfers in and out of the medical intensive care unit (MICU). During this time, his parkinsonism symptoms were managed through a combination of carbidopa and levodopa and amantadine. Cervical dystonia was managed with botulism toxin injections. Mr. W spent 6 weeks in the MICU until the decision was made to terminate life support, and he was taken off the ventilator. He died shortly thereafter. Autopsy findings suggested that Mr. W had severe Alzheimer disease.
Discussion
Following the IM injection of paliperidone palmitate, Mr. W had a complicated hospital stay resulting in his demise from sepsis and multiorgan failure. Severe immobilization, rigidity, and dystonia prevented Mr. W from conducting activities of daily living, which resulted in invasive interventions, such as continued foley catheterization. His sepsis was likely secondary to aspiration, catheterization, and eventual ventilation—all iatrogenic complications. Previous estimates in the U.S. have suggested a total of 225,000 deaths per year from iatrogenic causes.8
There are several areas of concern. Clearly, Mr. W had severe illness that greatly affected his life. He was estranged from family and had endured a 2-decade period of homelessness. He deserved effective treatment for his psychiatric illness to relieve his suffering. His long period of mental illness without effective treatment very likely biased the initial treatment team toward an aggressive approach.
Fragmented Care
The prolonged hospital stay and multiple complications directly led to fragmentation in Mr. W’s care. He was hospitalized for months on 3 different main services: psychiatry, medicine, and the MICU. Even when he remained on the same service, the primary members of his treatment team changed every few weeks. Many different specialties were consulted and reconsulted. Members of the specialty consult teams changed throughout the hospitalization as well. Given the nature of the local clinical administration, Mr. W likely received the most consistent team members from the attendings on the psychiatry consult-liaison service (who do not rotate) and from a local subspecialty delirium consult team (all members stay consistent except pharmacy residents).
Documentation of clinical reasoning behind treatment decisions was not ideal and occasionally lacking. This led to a tendency to “reinvent the wheel” with Mr. W’s treatment approach every few weeks. It was not until Mr. W had spent a significant amount of time on the medical service that an interdisciplinary treatment team meeting involving medicine, psychiatry, nursing, delirium, and neurology experts occurred. Although the interdisciplinary meeting helped by reviewing the hospital course, agreeing on a likely cause of the symptoms, and creating a treatment plan going forward, Mr. W was not able to recover.
Even when team members were stable, communication in a timely fashion did not always occur. At several points, expert recommendations were delayed by a day or more. Difficulties in treatment implementation were not communicated back to the specialty teams. The most significant example was a delay in recognition when Mr. W could no longer take oral pills secondary to the parkinsonism. Many days passed before an alternative liquid or dissolved medication was recommended on 2 separate occasions.
Subspecialty Consult
Addressing these documentation, communication, and transition challenges is neither easy nor unique to this large rural VA medical center. The authors have attempted to address this in the local system with the creation of a delirium team subspecialty consult service. Team members do not rotate and are able to follow patients throughout their hospital course. At the time of Mr. W’s hospitalization, the team included representatives from nursing, psychiatry, and occasionally pharmacy. Since then, it has expanded to include geriatrics and medicine. In addition to delirium being a marker for complex patients at risk for hospital complications, medical reasons for an extended length of stay could serve as a trigger for a referral to such a team of experts. In Mr. W’s case, that could have led to interdisciplinary consultation up to 2 months before it occurred. This may have led to a much better outcome.
Secondary parkinsonism is most notable with the typical antipychotics. The prevalence can vary between 50% and 75% and may be higher within the elderly population. However, all antipsychotics have a chance of demonstrating EPS. Risperidone has a low incidence at low doses; studies have shown dose-related parkinsonism at doses of 2 to 6 mg/d. Significant risk of parkinsonism is further exacerbated when drug-drug interactions are considered.9 Concurrently receiving 2 antipsychotics, olanzapine and paliperidone, initially caused the EPS. The veteran’s cerebral atrophy from significant malnutrition related to chronic homelessness, and the presence of Alzheimer disease only identified postmortem exacerbated this AE. Further complicating the management of the EPS, paliperidone palmitate has a long half-life of 25 to 49 days.9 Simply discontinuing the medication did not remove it from Mr. W’s system. Paliperidone would have continued to be present for months.
Conclusion
In this case, aggressive changes in the antipsychotic medications in a short period led to Mr. W effectively having 3 different agents in his system at the same time. This significantly elevated his risk of AEs, including parkinsonism. The clinician must be vigilant to further recognize the initial symptoms of parkinsonism on clinical presentation. Administration of clinical scales, such as the Simpson-Angus Extrapyramidal Side Effect, can help in these situations.10 Malnutrition and increased age can predispose patients to neurolepticAEs, so treatment teams should exercise caution when administering antipsychotics in such a population. Pharmacokinetic changes in all major organ systems from aging result in higher and more variable drug concentrations. This leads to an increased sensitivity to drugs and AEs.9
Given the increasing geriatric patient population in the U.S., treating mania in the elderly will become more common. Providers should carefully consider the risks vs benefit ratio for each individual because a serious adverse reaction may result in detrimental consequences. Even with severe symptoms leading to a bias toward an aggressive approach, it may be better to “start low and go slow.” Early inclusion of interdisciplinary expertise should be sought in complex cases.
Many patients with psychiatric illness have difficulty with medication adherence. Patients with impaired reality testing especially are at risk.
Keck and McElroy evaluated 141 patients who were initially hospitalized for bipolar disorder prospectively over 1 year to assess adherence with medication. During the follow-up period, 71 patients (51%) were partially or totally nonadherent with medication as prescribed. The most commonly cited reason for nonadherence was denial of need.1
Clinicians and patients face additional challenges due to the deleterious effects of relapse in the setting of both schizophrenia and bipolar disorder. Almost all oral antipsychotic or mood stabilizer medications require a minimum dosing schedule to effectively treat these disorders, and some of these oral medications require regular laboratory monitoring. Moreover, some of the agents can have serious adverse effects (AEs), such as seizure or withdrawal, if stopped abruptly. Social support from family or friends may improve adherence, but many psychiatric outpatients have a smaller social support network than do patients without psychiatric illnesses.2
Long-acting injectable (LAI) antipsychotics have been available for the past 40 years. These medications have provided clinicians with an additional option for patients with schizophrenia or bipolar disorder who are nonadherent to their medication treatment plans or who desire an administration choice that is more convenient than daily oral pills.3-7 Some clinical practice guidelines recommend considering LAIs as a maintenance treatment for schizophrenia.5 Like the rest of the pharmacopoeia, these formulations have AEs, such as extrapyramidal symptoms (EPS), weight gain, and metabolic syndrome.1 The longer half-life of these drugs may make such effects difficult to reverse.
This article presents a case of the use of depot formulation paliperidone palmitate in an elderly patient with bipolar disorder who was previously on high-dose oral second generation antipsychotics. He developed severe parkinsonism during a protracted hospitalization that ended in death.
Case Presentation
Mr. W was a 68-year-old homeless white male with a history of coronary artery disease status-post coronary artery bypass surgery, obstructive sleep apnea, and bipolar 1 disorder who presented to a large rural VAMC emergency department (ED) as a transfer from an outside hospital (OSH). He originally presented at the OSH for vomiting and diarrhea, but while there, he was placed under involuntary psychiatric commitment. The involuntary commitment form noted him to be tangential and disorganized; he was found walking about the ED without clothes. During the initial psychiatry interview, the clinician noted a disorganized thought process. When asked about circumstances leading to admission, he stated he was “a scuba diver, pilot, actor, submarine commander.” He also reported he had given “seminars to 6,000 people,” he held a psychology degree, and he came from a family that owned part of the island of Kodiak, Alaska. Mr. W stated he had no mental health history and believed psychiatry was witchcraft. He reported having no hallucinations and stated he heard the voice of god. He also reported to have met god multiple times and to have been married to a supermodel.
Mr. W’s chart demonstrated a history of mental illness over 30 years and that he previously was prescribed psychiatric medications. He had multiple inpatient psychiatric admissions with grandiose ideations, disorganized behaviors, and hypersexuality. He had been prescribed quetiapine, divalproex, lithium, carbamazepine, and lorazepam. He was formally diagnosed in the past with bipolar 1 disorder. There also was a family history of psychiatric illness. His mother had received electroconvulsive therapy, and both parents had alcohol substance use disorder.
Mr. W had been homeless for 20 years and had several psychiatric admissions during this period. Mr. W also had chronic difficulty with obtaining food and taking medications as prescribed. Sometimes he would only be able to eat 1 to 2 meals per day. He often changed location and had lived in at least 7 different states. Currently, he was estranged from his family. About 19 years ago, his sister reported that the veteran had told her that he was Jesus Christ, per clinical records. His estranged sister’s statement was corroborated by past psychology consult records citing episodes of the patient hearing god 30 and 26 years before the current admission. His second ex-wife cited inappropriate sexual behavior in front of their children. He had difficulty in school, failed at least 2 grades, and joined the U.S. Navy in tenth grade. A Neurobehavioral Cognitive Status Examination given 19 years ago showed mild impairment on attention and severe impairment in memory.
The physical examination on presentation to the OSH was unremarkable. Mr. W did not cooperate with formal neurocognitive testing, and he consistently made errors during orientation testing. Complete blood count from a OSH ED laboratory test was remarkable for a mild pancytopenia with a leukocyte count of 3,100 cells/mcL, hemoglobin 13.1 g/dL, and hematocrit 38.4%. Red cell distribution width was within normal limits at 13.5%. Stool cultures showed normal fecal flora and no salmonella, shigella, or campylobacter. Thyroid-stimulating hormone (TSH) was slightly elevated at 5.32 U/mL. An electrocardiogram showed a QTc interval of 412 ms. A computerized tomography scan of his head showed no acute intracranial abnormality along with chronic ischemic changes in the brain (Table 1). Presumed cause of his nausea and diarrhea was viral gastroenteritis likely acquired at a homeless shelter.
Once stabilized, Mr. W was admitted to the VA hospital inpatient psychiatry unit under involuntary commitment for acute mania. Risperidone 0.25 mg orally twice a day was started for mood stabilization and psychosis along with trazodone 50 mg orally as needed for insomnia. Despite upward titration and change in frequency of the risperidone dose, Mr. W’s manic episode persisted. He remained on the psychiatric floor for 2 months (Figure). His TSH and free T4 were monitored during his stay, and levothyroxine was started. Risperidone was titrated to 8 mg/d. Mr. W’s Young Mania Rating Scale (YMRS) score decreased from 30 to 24. Mr. W had a mild improvement in irritability and speech rate but little change in elevated mood and delusional content.
He continued to endorse “speaking to god 16 times” even at the highest risperidone dose. The treatment team prescribed dissolvable risperidone tablets secondary to diversion concerns. In addition, the team added benztropine 0.5 mg once a day after observing a stooped posture and decreased arm swing. Mr. W noted risperidone made him “lethargic” and that his “body did not need” it. After 1 month of treatment with risperidone, the treatment team decided to cross taper the veteran from risperidone to a combination of olanzapine and divalproex secondary to inadequate treatment response.
The inpatient team started Mr. W on oral disintegrating tablets of olanzapine 5 mg once a day, and oral divalproex 1,000 mg once a day. An intramuscular backup of olanzapine was made available if oral medication was refused. Divalproex was titrated to 1,250 mg once a day to target a serum level of 61.7 µg/mL, and olanzapine was titrated to 10 mg once a day. After 9 days, the veteran showed moderate improvement in mania symptoms with a YMRS score < 20, indicating the absence of mania. However, the veteran made it very clear that he would stop taking the prescribed medication on discharge. The team elected to initiate a LAI.
The veteran received his first injection of the LAI psychiatric medication paliperidone palmitate 234 mg and a second 156-mg injection of the same medication 1 week later as per loading protocol. He was concurrently on daily oral divalproex 1,250 mg and olanzapine 10 mg. Mr. W continued to note he felt sedated during this period; his sedation worsened after the second injection. He also began to forget the location of his room and developed mumbled speech. His gait deteriorated to where he required a walker 6 days after injection and a wheelchair 3 days later. He became incontinent of urine and feces. Mr. W exhibited masked facies with severe drooling. This eventually progressed to difficulty swallowing. At the advice of speech pathology, he was downgraded to a pureed and nectar-thick liquid diet. He required assistance with meals.
Because of his sedation and parkinsonism symptoms, he was tapered off both olanzapine and divalproex. His last dose of olanzapine was on the date of his first injection and last dose of divalproex was 15 days after the second injection. The benztropine, which was originally given to counteract the effects of risperidone monotherapy, was discontinued over concern of anticholinergic load and sedation. The neurology consultant recommended carbidopa 25 mg and levodopa 100 mg 3 times per day for treatment of parkinsonism symptoms. Mr. W was only able to take 1 dose because of trouble swallowing. Twenty days after his second injection, a rapid response team (local clinical team 1 step below a code team) was called as Mr. W was unusually lethargic and unable to eat. He was then transferred to the medical floor.
While on the medical floor, dobhoff tube access was established for nutrition and to allow administration of carbidopa and levodopa. Mr. W could still speak at this time and was distraught. He stated, “I don’t know why god would do this to me.” Further workup was performed to look for other etiologies of the patient’s change in status. Creatinine kinase testing, lumbar puncture with cerebral spinal fluid (CSF) bacterial culture, CSF cryptococcal testing, and syphilis antigens were all negative. Magnetic resonance imaging of the brain demonstrated diffuse cerebral atrophy with widened cistern and sulci resulting in ex vacuo dilatation.
Neurology thought that the ventriculomegaly did not have features of normal pressure hydrocephalus and was secondary to chronic ischemic demyelination caused by chronic malnutrition. During follow-up visits, the veteran was less and less verbal. It progressed to where he answered questions only in grunts. Eight days after transfer to the medical floor, Mr. W was noted to have his neck locked in a laterally rotated position with clonus of the sternocleidomastoid. Due to concern about possibility of neck dystonia and the poor adherence of the patient with carbidopa and levodopa given orally, the psychiatric team made the recommendation to start benztropine 1 mg given twice a day, delivered via the dobhoff tube to treat both the parkinsonism and dystonia. The following day Mr. W failed a repeat swallow study and was no longer allowed to receive anything orally.
Mild icterus and jaundice were noted on physical examination along with transaminitis and elevated bilirubin. He developed a fever. Thirteen days after transfer to the medical floor, blood cultures revealed Klebsiella septicemia. Benztropine was discontinued at this time because of concern the medication was causing or exacerbating the fever. While being treated for Klebsiella sepsis, the psychiatry team addressed his continued hypophonia, inability to ambulate, masked facies, and neck dystonia with diphenhydramine 50 mg given intramuscular (IM) twice per day.
Mr. W developed several more iatrogenic complications near this time, including urinary tract infection septicemia and acute hypoxic respiratory failure with lung infiltrate on X-ray, requiring ventilator support. His clinical status led to a number of transfers in and out of the medical intensive care unit (MICU). During this time, his parkinsonism symptoms were managed through a combination of carbidopa and levodopa and amantadine. Cervical dystonia was managed with botulism toxin injections. Mr. W spent 6 weeks in the MICU until the decision was made to terminate life support, and he was taken off the ventilator. He died shortly thereafter. Autopsy findings suggested that Mr. W had severe Alzheimer disease.
Discussion
Following the IM injection of paliperidone palmitate, Mr. W had a complicated hospital stay resulting in his demise from sepsis and multiorgan failure. Severe immobilization, rigidity, and dystonia prevented Mr. W from conducting activities of daily living, which resulted in invasive interventions, such as continued foley catheterization. His sepsis was likely secondary to aspiration, catheterization, and eventual ventilation—all iatrogenic complications. Previous estimates in the U.S. have suggested a total of 225,000 deaths per year from iatrogenic causes.8
There are several areas of concern. Clearly, Mr. W had severe illness that greatly affected his life. He was estranged from family and had endured a 2-decade period of homelessness. He deserved effective treatment for his psychiatric illness to relieve his suffering. His long period of mental illness without effective treatment very likely biased the initial treatment team toward an aggressive approach.
Fragmented Care
The prolonged hospital stay and multiple complications directly led to fragmentation in Mr. W’s care. He was hospitalized for months on 3 different main services: psychiatry, medicine, and the MICU. Even when he remained on the same service, the primary members of his treatment team changed every few weeks. Many different specialties were consulted and reconsulted. Members of the specialty consult teams changed throughout the hospitalization as well. Given the nature of the local clinical administration, Mr. W likely received the most consistent team members from the attendings on the psychiatry consult-liaison service (who do not rotate) and from a local subspecialty delirium consult team (all members stay consistent except pharmacy residents).
Documentation of clinical reasoning behind treatment decisions was not ideal and occasionally lacking. This led to a tendency to “reinvent the wheel” with Mr. W’s treatment approach every few weeks. It was not until Mr. W had spent a significant amount of time on the medical service that an interdisciplinary treatment team meeting involving medicine, psychiatry, nursing, delirium, and neurology experts occurred. Although the interdisciplinary meeting helped by reviewing the hospital course, agreeing on a likely cause of the symptoms, and creating a treatment plan going forward, Mr. W was not able to recover.
Even when team members were stable, communication in a timely fashion did not always occur. At several points, expert recommendations were delayed by a day or more. Difficulties in treatment implementation were not communicated back to the specialty teams. The most significant example was a delay in recognition when Mr. W could no longer take oral pills secondary to the parkinsonism. Many days passed before an alternative liquid or dissolved medication was recommended on 2 separate occasions.
Subspecialty Consult
Addressing these documentation, communication, and transition challenges is neither easy nor unique to this large rural VA medical center. The authors have attempted to address this in the local system with the creation of a delirium team subspecialty consult service. Team members do not rotate and are able to follow patients throughout their hospital course. At the time of Mr. W’s hospitalization, the team included representatives from nursing, psychiatry, and occasionally pharmacy. Since then, it has expanded to include geriatrics and medicine. In addition to delirium being a marker for complex patients at risk for hospital complications, medical reasons for an extended length of stay could serve as a trigger for a referral to such a team of experts. In Mr. W’s case, that could have led to interdisciplinary consultation up to 2 months before it occurred. This may have led to a much better outcome.
Secondary parkinsonism is most notable with the typical antipychotics. The prevalence can vary between 50% and 75% and may be higher within the elderly population. However, all antipsychotics have a chance of demonstrating EPS. Risperidone has a low incidence at low doses; studies have shown dose-related parkinsonism at doses of 2 to 6 mg/d. Significant risk of parkinsonism is further exacerbated when drug-drug interactions are considered.9 Concurrently receiving 2 antipsychotics, olanzapine and paliperidone, initially caused the EPS. The veteran’s cerebral atrophy from significant malnutrition related to chronic homelessness, and the presence of Alzheimer disease only identified postmortem exacerbated this AE. Further complicating the management of the EPS, paliperidone palmitate has a long half-life of 25 to 49 days.9 Simply discontinuing the medication did not remove it from Mr. W’s system. Paliperidone would have continued to be present for months.
Conclusion
In this case, aggressive changes in the antipsychotic medications in a short period led to Mr. W effectively having 3 different agents in his system at the same time. This significantly elevated his risk of AEs, including parkinsonism. The clinician must be vigilant to further recognize the initial symptoms of parkinsonism on clinical presentation. Administration of clinical scales, such as the Simpson-Angus Extrapyramidal Side Effect, can help in these situations.10 Malnutrition and increased age can predispose patients to neurolepticAEs, so treatment teams should exercise caution when administering antipsychotics in such a population. Pharmacokinetic changes in all major organ systems from aging result in higher and more variable drug concentrations. This leads to an increased sensitivity to drugs and AEs.9
Given the increasing geriatric patient population in the U.S., treating mania in the elderly will become more common. Providers should carefully consider the risks vs benefit ratio for each individual because a serious adverse reaction may result in detrimental consequences. Even with severe symptoms leading to a bias toward an aggressive approach, it may be better to “start low and go slow.” Early inclusion of interdisciplinary expertise should be sought in complex cases.
1. Keck PE Jr, McElroy SL, Strakowski SM, Bourne ML, West SA. Compliance with maintenance treatment in bipolar disorder. Psychopharmacol Bull. 1997;33(1):87-91.
2. Henderson S, Duncan-Jones P, McAuley H, Ritchie K. The patient’s primary group. Br J Psychiatry. 1978;132:74-86.
3. Buoli M, Ciappolino V, Altamura AC. Paliperidone palmitate depot in the long-term treatment of psychotic bipolar disorder: a case series. Clin Neuropharmacol. 2015;38(5):209-211.
4. Chou YH, Chu PC, Wu SW, et al. A systematic review and experts’ consensus for long-acting injectable antipsychotics in bipolar disorder. Clin Psychopharmacol Neurosci. 2015;13(2):121-128.
5. Kishi T, Oya K, Iwata N. Long-acting injectable antipsychotics for prevention of relapse in bipolar disorder: a systematic review and meta-analysis of randomized controlled trials. Int J Neuropsychopharmacol. 2016;19(9):1-10.
6. Llorca PM, Abbar M, Courtet P, Guillaume S, Lancrenon S, Samalin L. Guidelines for the use and management of long-acting injectable antipsychotics in serious mental illness. BMC Psychiatry. 2013;13:340.
7. Spanarello S, La Ferla T. The pharmacokinetics of long-acting antipsychotic medications. Curr Clin Pharmacol. 2014;9(3):310-317.
8. Starfield B. Is US health really the best in the world? JAMA. 2000;284(4):483-485.
9. Labbate LA, Fava M, Rosenbaum JF, Arana GW. Handbook of Psychiatric Drug Therapy. 6th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2010.
10. Simpson GM, Angus JW. A rating scale for extrapyramidal side effects. Acta Psychiatr Scand Suppl. 1970;212:11-19.
1. Keck PE Jr, McElroy SL, Strakowski SM, Bourne ML, West SA. Compliance with maintenance treatment in bipolar disorder. Psychopharmacol Bull. 1997;33(1):87-91.
2. Henderson S, Duncan-Jones P, McAuley H, Ritchie K. The patient’s primary group. Br J Psychiatry. 1978;132:74-86.
3. Buoli M, Ciappolino V, Altamura AC. Paliperidone palmitate depot in the long-term treatment of psychotic bipolar disorder: a case series. Clin Neuropharmacol. 2015;38(5):209-211.
4. Chou YH, Chu PC, Wu SW, et al. A systematic review and experts’ consensus for long-acting injectable antipsychotics in bipolar disorder. Clin Psychopharmacol Neurosci. 2015;13(2):121-128.
5. Kishi T, Oya K, Iwata N. Long-acting injectable antipsychotics for prevention of relapse in bipolar disorder: a systematic review and meta-analysis of randomized controlled trials. Int J Neuropsychopharmacol. 2016;19(9):1-10.
6. Llorca PM, Abbar M, Courtet P, Guillaume S, Lancrenon S, Samalin L. Guidelines for the use and management of long-acting injectable antipsychotics in serious mental illness. BMC Psychiatry. 2013;13:340.
7. Spanarello S, La Ferla T. The pharmacokinetics of long-acting antipsychotic medications. Curr Clin Pharmacol. 2014;9(3):310-317.
8. Starfield B. Is US health really the best in the world? JAMA. 2000;284(4):483-485.
9. Labbate LA, Fava M, Rosenbaum JF, Arana GW. Handbook of Psychiatric Drug Therapy. 6th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2010.
10. Simpson GM, Angus JW. A rating scale for extrapyramidal side effects. Acta Psychiatr Scand Suppl. 1970;212:11-19.
Worsening agitation and hallucinations: Could it be PTSD?
CASE Confusion, hallucinations
Mr. G, age 57, is brought to the emergency department (ED) from a hospice care facility for worsening agitation and psychosis over 2 days. His wife, who accompanies him, describes a 2-month onset of “confusion” with occasional visual hallucinations. She says that at baseline Mr. G was alert and oriented and able to engage appropriately in conversations. The hospice facility administered emergency medications, including unknown dosages of haloperidol and chlorpromazine, the morning before transfer to the ED.
Mr. G has a history of posttraumatic stress disorder (PTSD), anxiety, and depression that has been managed for 6 years with several trials of antidepressant monotherapy, including fluoxetine, citalopram, mirtazapine, bupropion, and augmentation using aripiprazole, risperidone, topiramate, and zolpidem. At the time of this hospital presentation, his symptoms are controlled on clonazepam, 2 mg/d, and trazodone, 50 mg/d. For his pain attributed to non-small cell lung cancer (NSCLC), he receives methadone, 25 mg, 6 times a day, and hydromorphone, 8 mg, every 4 hours as needed, for breakthrough pain. Mr. G underwent a right upper lobectomy 5 years ago and neurosurgery with a right suboccipital craniectomy for right-sided cerebellar metastatic tumor measuring 2 × 1 × 0.6 cm, along with chemotherapy and radiation for metastasis in the brain 1 year ago. His last chemotherapy session was 3 months ago.
In the ED, Mr. G is sedated and oriented only to person and his wife. He is observed mumbling incoherently. Abnormal vital signs and laboratory findings are elevated pulse, 97 beats per minute; mild anemia, 13.5 g/dL hemoglobin and 40.8% hematocrit; an elevated glucose of 136 mg/dL; and small amounts of blood, trace ketones, and hyaline casts in urinalysis. Vital signs, laboratory resu
In addition to psychotropic and pain medication, Mr. G is taking cyclobenzaprine, 5 mg, every 6 hours as needed, for muscle spasms; docusate, 200 mg/d; enoxaparin, 100 mg/1mL, every 12 hours; folic acid, 1 mg/d; gabapentin, 600 mg, 3 times daily; lidocaine ointment, twice daily as needed, for pain; omeprazole, 80 mg/d; ondansetron, 4 mg, every 8 hours as needed, for nausea; and tamsulosin, 0.4 mg/d.
What is your differential diagnosis for Mr. G?
a) brain metastases
b) infection
c) PTSD
d) polypharmacy
e) benzodiazepine withdrawal
The authors’ observations
Altered mental status (AMS), or acute confusional state, describes an individual who fails to interact with environmental stimuli in an appropriate, anticipated manner. The disturbance usually is acute and transient.1 Often providers struggle to obtain relevant facts about a patient’s history of illness and must use laboratory and diagnostic data to determine the underlying cause of the patient’s disorientation.
Mental status includes 2 components: arousal and awareness. Arousal refers to a person’s wakeful state and how an individual responds to his (her) surroundings. Impairment in arousal can result in variable states including lethargy, drowsiness, and even coma. Awareness, on the other hand, is an individual’s perception of his environment, including orientation to surroundings, executive functioning, and memory. Although arousal level is controlled by the reticular activating system of the brainstem, awareness of consciousness is mediated at the cortical level. Mr. G experienced increased arousal and AMS with a clear change in behavior from his baseline. With increasing frequency of hallucinations and agitated behaviors, several tests must be ordered to determine the etiology of his altered mentation (Table 1).
Which test would you order next?
a) urine drug screen (UDS)
b) chest CT with pulmonary embolism protocol
c) CT of the head
d) blood cultures
e) chest radiography
EVALUATION Awake, still confused
The ED physician orders a UDS, non-contrasted CT of the head, and chest radiography for preliminary workup investigating the cause of Mr. G’s AMS. UDS is negative for illicit substances. The non-contrasted CT of the head shows a stable, right cerebellar hemisphere lesion from a prior lung metastasis. Mr. G’s chest radiography reading describes an ill-defined opacity at the left lung base.
Mr. G is admitted to the medical service and is started on dexamethasone, 8 mg/d, for his NSCLC with brain metastasis. Clonazepam is continued to prevent benzodiazepine withdrawal. The psychiatry and palliative care teams are consulted to determine if Mr. G’s PTSD symptoms and/or opioids are contributing to his AMS and psychosis. After evaluation, the psychiatry team recommends decreasing clonazepam to 0.5 mg, twice daily, starting olanzapine, 5 mg, every 12 hours, for agitation and psychosis involving auditory and visual hallucinations as well as paranoid themes related to food contamination, and using non-pharmacologic interventions for delirium treatment (Table 2). In a prospective, randomized controlled trial of olanzapine vs haloperidol, clinical improvement in delirious states was seen in individuals who received either antipsychotic medication; however, haloperidol was associated with extrapyramidal side effects. Therefore, olanzapine is a safe alternative to haloperidol in delirious patients.2
The psychiatry consult service suspects delirium due to polypharmacy or Mr. G’s metastatic brain lesion. However, other collaborating treatment teams feel that Mr. G’s presentation was precipitated by an exacerbation of PTSD symptoms because of the observed psychotic themes, in addition to metabolic encephalopathy. Acute stress disorder can present with emotional numbing, depersonalization, reduced awareness of surroundings, or dissociative amnesia. However, Mr. G has not experienced PTSD symptoms involving mental status changes with fluctuating orientation in the past nor has he displayed persistent dissociation during outpatient psychiatric care. Therefore, it is unlikely that PTSD is the primary cause of his hospital admission.
The palliative care team recommends switching Mr. G’s pain medications to methadone, 20 mg, every 6 hours, to reduce possibility that opioids are contributing to his delirious state. Mr. G’s medical providers report that the chest radiography is suspicious for pneumonia and start him on levofloxacin, 500 mg/d.
The authors’ observations
DSM-5 criteria for delirium has 4 components:
- disturbance in attention and awareness
- change in cognition
- the disturbance develops over a short period of time
- there is evidence that the disturbance is a direct consequence of a medical condition, medication, or substance, or more than 1 cause.3
Mr. G presented with multi-factorial delirium, and as a result, all underlying contributions, including infection, polypharmacy, brain metastasis, and steroids needed to be considered. Treating delirium requires investigating the underlying cause and keeping the patient safe in the process (Figure). Mr. G was agitated at presentation; therefore, low-dosage olanzapine was initiated to address the imbalance between the cholinergic and dopaminergic systems in the CNS, which are thought to be the mechanism behind delirious presentations.
In Mr. G’s case, methadone was lowered, with continual monitoring and evaluation for his comfort. Infections, specifically urinary tract infections and pneumonia, can cause delirium states and must be treated with appropriate antibiotics. Metastatic tumors have been known to precipitate changes in mental status and can be ruled out via imaging. In Mr. G’s case, his metastatic lesion remained stable from prior radiographic studies.
TREATMENT Delirium resolves
Mr. G slowly responds to multi-modal treatment including decreased opioids and benzodiazepines and the use of low-dosage antipsychotics. He begins to return to baseline with antibiotic administration. By hospital day 5, Mr. G is alert and oriented. He notes resolution of his auditory and visual hallucinations and denies any persistent paranoia or delusions. The medical team observes Mr. G is having difficulty swallowing with meals, and orders a speech therapy evaluation. After assessment, the team suspects that aspiration pneumonia could have precipitated Mr. G’s initial decline and recommends a mechanic diet with thin liquids to reduce the risk of future aspiration.
Mr. G is discharged home in his wife’s care with home hospice to continue end-of-life care. His medication regimen includes olanzapine, 10 mg/d, to continue until his next outpatient appointment, trazodone, 50 mg/d, for depression and PTSD symptoms, and clonazepam is decreased to 0.5 mg, at bedtime, for anxiety.
The authors’ observations
Mr. G’s case highlights the importance of fully evaluating all common underlying causes of delirium. The etiology of delirium is more likely to be missed in medically complex patients or in patients with a history of psychiatric illness. Palliative care patients have several risk factors for delirium, such as benzodiazepine or opioid treatment, dementia, and organic diseases such as brain metastasis.6 A recent study assessed the frequency of delirium in cancer patients admitted to an inpatient palliative unit and found that 71% of individuals had a diagnosis of delirium at admission and 26% developed delirium afterward.7 Despite the increased likelihood of developing delirium, more than one-half of palliative patients have delirium that is missed by their primary providers.8 Similarly, patients with documented psychiatric illness were approximately 2.5 times more likely to have overlooked delirium compared with patients without psychiatric illness.9
Risk and prevention
Patients with risk factors for delirium—which includes sedative and narcotic usage, advanced cancer, older age, prolonged hospital stays, surgical procedures, and/or cognitive impairment—should receive interventions to prevent delirium. However, if symptoms of AMS are present, providers should perform a complete workup for underlying causes of delirium. Remembering that individuals with delirium have an impaired ability to voice symptoms, such as dyspnea, dysuria, and headache, clinicians should have a high index of suspicion for delirium in patients at heightened risk.10
Perhaps most important, teams treating patients at high risk for delirium should employ preventive measures to reduce the development of delirium. Although more studies are needed to clarify the role of drug therapies for preventing delirium, there is strong evidence for several non-pharmacotherapeutic interventions including:
- frequent orientation activities
- early mobilization
- maintaining healthy sleep–wake cycles
- minimizing the use of psychoactive drugs and frequently reviewing the medication regimen
- allowing use of eyeglasses and hearing aids
- treating volume depletion.10
These preventive measures are important when treating delirium, such as minimizing Mr. G’s use of benzodiazepine and opioids—medications known to contribute to iatrogenic delirium.
A delirium diagnosis portends grave adverse outcomes. Research has shown significant associations with morbidity and mortality, financial and emotional burden, and prolonged hospitalizations. Often, symptoms of delirium persist for months and patients do not recover completely. However, studies have found that when underlying causes are treated effectively, delirium is more likely to be reversible.11
The prompt diagnosis of delirium with good interdisciplinary communication can reduce the risk of these adverse outcomes.12 Consultation-liaison psychiatrists are well positioned to facilitate the diagnoses of delirium and play a role in educating other health care providers of the importance of prevention, early symptom recognition, full workup, and effective treatment of its underlying causes.
1. Posner JB, Saper CB, Schiff ND, et al. Plum and Posner’s diagnosis of stupor and coma. New York, NY: Oxford University Press; 2007.
2. Skrobik YK, Bergeron N, Dumont M, et al. Olanzapine vs haldoperidol: treating delirium in a critical care setting. Intensive Care Med. 2004;30(3):444-449.
3. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
4. Lonergan E, Luxenberg J, Areosa Sastre A, et al. Benzodiazepines for delirium. Cochrane Database Syst Rev. 2009;(1):CD006379. doi: 10.1002/14651858.CD006379.pub2.
5. Vella-Brincat J, Macleod AD. Adverse effects of opioids on the central nervous system of palliative care patients. J Pain Palliat Care Pharmacother. 2007;21(1):15-25.
6. Grassi L, Caraceni A, Mitchell AJ, et al. Management of delirium in palliative care: a review. Curr Psychiatry Rep. 2015;17(3):550.
7. de la Cruz M, Ransing V, Yennu S, et al. The frequency, characteristics, and outcomes among cancer patients with delirium admitted to an acute palliative care unit. Oncologist. 2015;20(12):1425-1431.
8. de la Cruz, M, Fan J, Yennu S, et al. The frequency of missed delirium in patients referred to palliative care in a comprehensive cancer center. Support Care Cancer. 2015;23(8):2427-2433.
9. Swigart SE, Kishi Y, Thurber S, et al. Misdiagnosed delirium in patient referrals to a university-based hospital psychiatry department. Psychosomatics. 2008;49(2):104-108.
10. Inouye SK, Bogardus ST Jr, Charpentier PA, et al. A multicomponent intervention to prevent delirium in hospitalized older patients. N Engl J Med. 1999;340(9):669-676.
11. Dasgupta M, Hillier LM. Factors associated with prolonged delirium: a systematic review. Int Psychogeriatr. 2010;22(3):373-394.
12. Detweiler MB, Kenneth A, Bader G, et al. Can improved intra- and inter-team communication reduce missed delirium? Psychiatr Q. 2014;85(2):211-224.
CASE Confusion, hallucinations
Mr. G, age 57, is brought to the emergency department (ED) from a hospice care facility for worsening agitation and psychosis over 2 days. His wife, who accompanies him, describes a 2-month onset of “confusion” with occasional visual hallucinations. She says that at baseline Mr. G was alert and oriented and able to engage appropriately in conversations. The hospice facility administered emergency medications, including unknown dosages of haloperidol and chlorpromazine, the morning before transfer to the ED.
Mr. G has a history of posttraumatic stress disorder (PTSD), anxiety, and depression that has been managed for 6 years with several trials of antidepressant monotherapy, including fluoxetine, citalopram, mirtazapine, bupropion, and augmentation using aripiprazole, risperidone, topiramate, and zolpidem. At the time of this hospital presentation, his symptoms are controlled on clonazepam, 2 mg/d, and trazodone, 50 mg/d. For his pain attributed to non-small cell lung cancer (NSCLC), he receives methadone, 25 mg, 6 times a day, and hydromorphone, 8 mg, every 4 hours as needed, for breakthrough pain. Mr. G underwent a right upper lobectomy 5 years ago and neurosurgery with a right suboccipital craniectomy for right-sided cerebellar metastatic tumor measuring 2 × 1 × 0.6 cm, along with chemotherapy and radiation for metastasis in the brain 1 year ago. His last chemotherapy session was 3 months ago.
In the ED, Mr. G is sedated and oriented only to person and his wife. He is observed mumbling incoherently. Abnormal vital signs and laboratory findings are elevated pulse, 97 beats per minute; mild anemia, 13.5 g/dL hemoglobin and 40.8% hematocrit; an elevated glucose of 136 mg/dL; and small amounts of blood, trace ketones, and hyaline casts in urinalysis. Vital signs, laboratory resu
In addition to psychotropic and pain medication, Mr. G is taking cyclobenzaprine, 5 mg, every 6 hours as needed, for muscle spasms; docusate, 200 mg/d; enoxaparin, 100 mg/1mL, every 12 hours; folic acid, 1 mg/d; gabapentin, 600 mg, 3 times daily; lidocaine ointment, twice daily as needed, for pain; omeprazole, 80 mg/d; ondansetron, 4 mg, every 8 hours as needed, for nausea; and tamsulosin, 0.4 mg/d.
What is your differential diagnosis for Mr. G?
a) brain metastases
b) infection
c) PTSD
d) polypharmacy
e) benzodiazepine withdrawal
The authors’ observations
Altered mental status (AMS), or acute confusional state, describes an individual who fails to interact with environmental stimuli in an appropriate, anticipated manner. The disturbance usually is acute and transient.1 Often providers struggle to obtain relevant facts about a patient’s history of illness and must use laboratory and diagnostic data to determine the underlying cause of the patient’s disorientation.
Mental status includes 2 components: arousal and awareness. Arousal refers to a person’s wakeful state and how an individual responds to his (her) surroundings. Impairment in arousal can result in variable states including lethargy, drowsiness, and even coma. Awareness, on the other hand, is an individual’s perception of his environment, including orientation to surroundings, executive functioning, and memory. Although arousal level is controlled by the reticular activating system of the brainstem, awareness of consciousness is mediated at the cortical level. Mr. G experienced increased arousal and AMS with a clear change in behavior from his baseline. With increasing frequency of hallucinations and agitated behaviors, several tests must be ordered to determine the etiology of his altered mentation (Table 1).
Which test would you order next?
a) urine drug screen (UDS)
b) chest CT with pulmonary embolism protocol
c) CT of the head
d) blood cultures
e) chest radiography
EVALUATION Awake, still confused
The ED physician orders a UDS, non-contrasted CT of the head, and chest radiography for preliminary workup investigating the cause of Mr. G’s AMS. UDS is negative for illicit substances. The non-contrasted CT of the head shows a stable, right cerebellar hemisphere lesion from a prior lung metastasis. Mr. G’s chest radiography reading describes an ill-defined opacity at the left lung base.
Mr. G is admitted to the medical service and is started on dexamethasone, 8 mg/d, for his NSCLC with brain metastasis. Clonazepam is continued to prevent benzodiazepine withdrawal. The psychiatry and palliative care teams are consulted to determine if Mr. G’s PTSD symptoms and/or opioids are contributing to his AMS and psychosis. After evaluation, the psychiatry team recommends decreasing clonazepam to 0.5 mg, twice daily, starting olanzapine, 5 mg, every 12 hours, for agitation and psychosis involving auditory and visual hallucinations as well as paranoid themes related to food contamination, and using non-pharmacologic interventions for delirium treatment (Table 2). In a prospective, randomized controlled trial of olanzapine vs haloperidol, clinical improvement in delirious states was seen in individuals who received either antipsychotic medication; however, haloperidol was associated with extrapyramidal side effects. Therefore, olanzapine is a safe alternative to haloperidol in delirious patients.2
The psychiatry consult service suspects delirium due to polypharmacy or Mr. G’s metastatic brain lesion. However, other collaborating treatment teams feel that Mr. G’s presentation was precipitated by an exacerbation of PTSD symptoms because of the observed psychotic themes, in addition to metabolic encephalopathy. Acute stress disorder can present with emotional numbing, depersonalization, reduced awareness of surroundings, or dissociative amnesia. However, Mr. G has not experienced PTSD symptoms involving mental status changes with fluctuating orientation in the past nor has he displayed persistent dissociation during outpatient psychiatric care. Therefore, it is unlikely that PTSD is the primary cause of his hospital admission.
The palliative care team recommends switching Mr. G’s pain medications to methadone, 20 mg, every 6 hours, to reduce possibility that opioids are contributing to his delirious state. Mr. G’s medical providers report that the chest radiography is suspicious for pneumonia and start him on levofloxacin, 500 mg/d.
The authors’ observations
DSM-5 criteria for delirium has 4 components:
- disturbance in attention and awareness
- change in cognition
- the disturbance develops over a short period of time
- there is evidence that the disturbance is a direct consequence of a medical condition, medication, or substance, or more than 1 cause.3
Mr. G presented with multi-factorial delirium, and as a result, all underlying contributions, including infection, polypharmacy, brain metastasis, and steroids needed to be considered. Treating delirium requires investigating the underlying cause and keeping the patient safe in the process (Figure). Mr. G was agitated at presentation; therefore, low-dosage olanzapine was initiated to address the imbalance between the cholinergic and dopaminergic systems in the CNS, which are thought to be the mechanism behind delirious presentations.
In Mr. G’s case, methadone was lowered, with continual monitoring and evaluation for his comfort. Infections, specifically urinary tract infections and pneumonia, can cause delirium states and must be treated with appropriate antibiotics. Metastatic tumors have been known to precipitate changes in mental status and can be ruled out via imaging. In Mr. G’s case, his metastatic lesion remained stable from prior radiographic studies.
TREATMENT Delirium resolves
Mr. G slowly responds to multi-modal treatment including decreased opioids and benzodiazepines and the use of low-dosage antipsychotics. He begins to return to baseline with antibiotic administration. By hospital day 5, Mr. G is alert and oriented. He notes resolution of his auditory and visual hallucinations and denies any persistent paranoia or delusions. The medical team observes Mr. G is having difficulty swallowing with meals, and orders a speech therapy evaluation. After assessment, the team suspects that aspiration pneumonia could have precipitated Mr. G’s initial decline and recommends a mechanic diet with thin liquids to reduce the risk of future aspiration.
Mr. G is discharged home in his wife’s care with home hospice to continue end-of-life care. His medication regimen includes olanzapine, 10 mg/d, to continue until his next outpatient appointment, trazodone, 50 mg/d, for depression and PTSD symptoms, and clonazepam is decreased to 0.5 mg, at bedtime, for anxiety.
The authors’ observations
Mr. G’s case highlights the importance of fully evaluating all common underlying causes of delirium. The etiology of delirium is more likely to be missed in medically complex patients or in patients with a history of psychiatric illness. Palliative care patients have several risk factors for delirium, such as benzodiazepine or opioid treatment, dementia, and organic diseases such as brain metastasis.6 A recent study assessed the frequency of delirium in cancer patients admitted to an inpatient palliative unit and found that 71% of individuals had a diagnosis of delirium at admission and 26% developed delirium afterward.7 Despite the increased likelihood of developing delirium, more than one-half of palliative patients have delirium that is missed by their primary providers.8 Similarly, patients with documented psychiatric illness were approximately 2.5 times more likely to have overlooked delirium compared with patients without psychiatric illness.9
Risk and prevention
Patients with risk factors for delirium—which includes sedative and narcotic usage, advanced cancer, older age, prolonged hospital stays, surgical procedures, and/or cognitive impairment—should receive interventions to prevent delirium. However, if symptoms of AMS are present, providers should perform a complete workup for underlying causes of delirium. Remembering that individuals with delirium have an impaired ability to voice symptoms, such as dyspnea, dysuria, and headache, clinicians should have a high index of suspicion for delirium in patients at heightened risk.10
Perhaps most important, teams treating patients at high risk for delirium should employ preventive measures to reduce the development of delirium. Although more studies are needed to clarify the role of drug therapies for preventing delirium, there is strong evidence for several non-pharmacotherapeutic interventions including:
- frequent orientation activities
- early mobilization
- maintaining healthy sleep–wake cycles
- minimizing the use of psychoactive drugs and frequently reviewing the medication regimen
- allowing use of eyeglasses and hearing aids
- treating volume depletion.10
These preventive measures are important when treating delirium, such as minimizing Mr. G’s use of benzodiazepine and opioids—medications known to contribute to iatrogenic delirium.
A delirium diagnosis portends grave adverse outcomes. Research has shown significant associations with morbidity and mortality, financial and emotional burden, and prolonged hospitalizations. Often, symptoms of delirium persist for months and patients do not recover completely. However, studies have found that when underlying causes are treated effectively, delirium is more likely to be reversible.11
The prompt diagnosis of delirium with good interdisciplinary communication can reduce the risk of these adverse outcomes.12 Consultation-liaison psychiatrists are well positioned to facilitate the diagnoses of delirium and play a role in educating other health care providers of the importance of prevention, early symptom recognition, full workup, and effective treatment of its underlying causes.
CASE Confusion, hallucinations
Mr. G, age 57, is brought to the emergency department (ED) from a hospice care facility for worsening agitation and psychosis over 2 days. His wife, who accompanies him, describes a 2-month onset of “confusion” with occasional visual hallucinations. She says that at baseline Mr. G was alert and oriented and able to engage appropriately in conversations. The hospice facility administered emergency medications, including unknown dosages of haloperidol and chlorpromazine, the morning before transfer to the ED.
Mr. G has a history of posttraumatic stress disorder (PTSD), anxiety, and depression that has been managed for 6 years with several trials of antidepressant monotherapy, including fluoxetine, citalopram, mirtazapine, bupropion, and augmentation using aripiprazole, risperidone, topiramate, and zolpidem. At the time of this hospital presentation, his symptoms are controlled on clonazepam, 2 mg/d, and trazodone, 50 mg/d. For his pain attributed to non-small cell lung cancer (NSCLC), he receives methadone, 25 mg, 6 times a day, and hydromorphone, 8 mg, every 4 hours as needed, for breakthrough pain. Mr. G underwent a right upper lobectomy 5 years ago and neurosurgery with a right suboccipital craniectomy for right-sided cerebellar metastatic tumor measuring 2 × 1 × 0.6 cm, along with chemotherapy and radiation for metastasis in the brain 1 year ago. His last chemotherapy session was 3 months ago.
In the ED, Mr. G is sedated and oriented only to person and his wife. He is observed mumbling incoherently. Abnormal vital signs and laboratory findings are elevated pulse, 97 beats per minute; mild anemia, 13.5 g/dL hemoglobin and 40.8% hematocrit; an elevated glucose of 136 mg/dL; and small amounts of blood, trace ketones, and hyaline casts in urinalysis. Vital signs, laboratory resu
In addition to psychotropic and pain medication, Mr. G is taking cyclobenzaprine, 5 mg, every 6 hours as needed, for muscle spasms; docusate, 200 mg/d; enoxaparin, 100 mg/1mL, every 12 hours; folic acid, 1 mg/d; gabapentin, 600 mg, 3 times daily; lidocaine ointment, twice daily as needed, for pain; omeprazole, 80 mg/d; ondansetron, 4 mg, every 8 hours as needed, for nausea; and tamsulosin, 0.4 mg/d.
What is your differential diagnosis for Mr. G?
a) brain metastases
b) infection
c) PTSD
d) polypharmacy
e) benzodiazepine withdrawal
The authors’ observations
Altered mental status (AMS), or acute confusional state, describes an individual who fails to interact with environmental stimuli in an appropriate, anticipated manner. The disturbance usually is acute and transient.1 Often providers struggle to obtain relevant facts about a patient’s history of illness and must use laboratory and diagnostic data to determine the underlying cause of the patient’s disorientation.
Mental status includes 2 components: arousal and awareness. Arousal refers to a person’s wakeful state and how an individual responds to his (her) surroundings. Impairment in arousal can result in variable states including lethargy, drowsiness, and even coma. Awareness, on the other hand, is an individual’s perception of his environment, including orientation to surroundings, executive functioning, and memory. Although arousal level is controlled by the reticular activating system of the brainstem, awareness of consciousness is mediated at the cortical level. Mr. G experienced increased arousal and AMS with a clear change in behavior from his baseline. With increasing frequency of hallucinations and agitated behaviors, several tests must be ordered to determine the etiology of his altered mentation (Table 1).
Which test would you order next?
a) urine drug screen (UDS)
b) chest CT with pulmonary embolism protocol
c) CT of the head
d) blood cultures
e) chest radiography
EVALUATION Awake, still confused
The ED physician orders a UDS, non-contrasted CT of the head, and chest radiography for preliminary workup investigating the cause of Mr. G’s AMS. UDS is negative for illicit substances. The non-contrasted CT of the head shows a stable, right cerebellar hemisphere lesion from a prior lung metastasis. Mr. G’s chest radiography reading describes an ill-defined opacity at the left lung base.
Mr. G is admitted to the medical service and is started on dexamethasone, 8 mg/d, for his NSCLC with brain metastasis. Clonazepam is continued to prevent benzodiazepine withdrawal. The psychiatry and palliative care teams are consulted to determine if Mr. G’s PTSD symptoms and/or opioids are contributing to his AMS and psychosis. After evaluation, the psychiatry team recommends decreasing clonazepam to 0.5 mg, twice daily, starting olanzapine, 5 mg, every 12 hours, for agitation and psychosis involving auditory and visual hallucinations as well as paranoid themes related to food contamination, and using non-pharmacologic interventions for delirium treatment (Table 2). In a prospective, randomized controlled trial of olanzapine vs haloperidol, clinical improvement in delirious states was seen in individuals who received either antipsychotic medication; however, haloperidol was associated with extrapyramidal side effects. Therefore, olanzapine is a safe alternative to haloperidol in delirious patients.2
The psychiatry consult service suspects delirium due to polypharmacy or Mr. G’s metastatic brain lesion. However, other collaborating treatment teams feel that Mr. G’s presentation was precipitated by an exacerbation of PTSD symptoms because of the observed psychotic themes, in addition to metabolic encephalopathy. Acute stress disorder can present with emotional numbing, depersonalization, reduced awareness of surroundings, or dissociative amnesia. However, Mr. G has not experienced PTSD symptoms involving mental status changes with fluctuating orientation in the past nor has he displayed persistent dissociation during outpatient psychiatric care. Therefore, it is unlikely that PTSD is the primary cause of his hospital admission.
The palliative care team recommends switching Mr. G’s pain medications to methadone, 20 mg, every 6 hours, to reduce possibility that opioids are contributing to his delirious state. Mr. G’s medical providers report that the chest radiography is suspicious for pneumonia and start him on levofloxacin, 500 mg/d.
The authors’ observations
DSM-5 criteria for delirium has 4 components:
- disturbance in attention and awareness
- change in cognition
- the disturbance develops over a short period of time
- there is evidence that the disturbance is a direct consequence of a medical condition, medication, or substance, or more than 1 cause.3
Mr. G presented with multi-factorial delirium, and as a result, all underlying contributions, including infection, polypharmacy, brain metastasis, and steroids needed to be considered. Treating delirium requires investigating the underlying cause and keeping the patient safe in the process (Figure). Mr. G was agitated at presentation; therefore, low-dosage olanzapine was initiated to address the imbalance between the cholinergic and dopaminergic systems in the CNS, which are thought to be the mechanism behind delirious presentations.
In Mr. G’s case, methadone was lowered, with continual monitoring and evaluation for his comfort. Infections, specifically urinary tract infections and pneumonia, can cause delirium states and must be treated with appropriate antibiotics. Metastatic tumors have been known to precipitate changes in mental status and can be ruled out via imaging. In Mr. G’s case, his metastatic lesion remained stable from prior radiographic studies.
TREATMENT Delirium resolves
Mr. G slowly responds to multi-modal treatment including decreased opioids and benzodiazepines and the use of low-dosage antipsychotics. He begins to return to baseline with antibiotic administration. By hospital day 5, Mr. G is alert and oriented. He notes resolution of his auditory and visual hallucinations and denies any persistent paranoia or delusions. The medical team observes Mr. G is having difficulty swallowing with meals, and orders a speech therapy evaluation. After assessment, the team suspects that aspiration pneumonia could have precipitated Mr. G’s initial decline and recommends a mechanic diet with thin liquids to reduce the risk of future aspiration.
Mr. G is discharged home in his wife’s care with home hospice to continue end-of-life care. His medication regimen includes olanzapine, 10 mg/d, to continue until his next outpatient appointment, trazodone, 50 mg/d, for depression and PTSD symptoms, and clonazepam is decreased to 0.5 mg, at bedtime, for anxiety.
The authors’ observations
Mr. G’s case highlights the importance of fully evaluating all common underlying causes of delirium. The etiology of delirium is more likely to be missed in medically complex patients or in patients with a history of psychiatric illness. Palliative care patients have several risk factors for delirium, such as benzodiazepine or opioid treatment, dementia, and organic diseases such as brain metastasis.6 A recent study assessed the frequency of delirium in cancer patients admitted to an inpatient palliative unit and found that 71% of individuals had a diagnosis of delirium at admission and 26% developed delirium afterward.7 Despite the increased likelihood of developing delirium, more than one-half of palliative patients have delirium that is missed by their primary providers.8 Similarly, patients with documented psychiatric illness were approximately 2.5 times more likely to have overlooked delirium compared with patients without psychiatric illness.9
Risk and prevention
Patients with risk factors for delirium—which includes sedative and narcotic usage, advanced cancer, older age, prolonged hospital stays, surgical procedures, and/or cognitive impairment—should receive interventions to prevent delirium. However, if symptoms of AMS are present, providers should perform a complete workup for underlying causes of delirium. Remembering that individuals with delirium have an impaired ability to voice symptoms, such as dyspnea, dysuria, and headache, clinicians should have a high index of suspicion for delirium in patients at heightened risk.10
Perhaps most important, teams treating patients at high risk for delirium should employ preventive measures to reduce the development of delirium. Although more studies are needed to clarify the role of drug therapies for preventing delirium, there is strong evidence for several non-pharmacotherapeutic interventions including:
- frequent orientation activities
- early mobilization
- maintaining healthy sleep–wake cycles
- minimizing the use of psychoactive drugs and frequently reviewing the medication regimen
- allowing use of eyeglasses and hearing aids
- treating volume depletion.10
These preventive measures are important when treating delirium, such as minimizing Mr. G’s use of benzodiazepine and opioids—medications known to contribute to iatrogenic delirium.
A delirium diagnosis portends grave adverse outcomes. Research has shown significant associations with morbidity and mortality, financial and emotional burden, and prolonged hospitalizations. Often, symptoms of delirium persist for months and patients do not recover completely. However, studies have found that when underlying causes are treated effectively, delirium is more likely to be reversible.11
The prompt diagnosis of delirium with good interdisciplinary communication can reduce the risk of these adverse outcomes.12 Consultation-liaison psychiatrists are well positioned to facilitate the diagnoses of delirium and play a role in educating other health care providers of the importance of prevention, early symptom recognition, full workup, and effective treatment of its underlying causes.
1. Posner JB, Saper CB, Schiff ND, et al. Plum and Posner’s diagnosis of stupor and coma. New York, NY: Oxford University Press; 2007.
2. Skrobik YK, Bergeron N, Dumont M, et al. Olanzapine vs haldoperidol: treating delirium in a critical care setting. Intensive Care Med. 2004;30(3):444-449.
3. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
4. Lonergan E, Luxenberg J, Areosa Sastre A, et al. Benzodiazepines for delirium. Cochrane Database Syst Rev. 2009;(1):CD006379. doi: 10.1002/14651858.CD006379.pub2.
5. Vella-Brincat J, Macleod AD. Adverse effects of opioids on the central nervous system of palliative care patients. J Pain Palliat Care Pharmacother. 2007;21(1):15-25.
6. Grassi L, Caraceni A, Mitchell AJ, et al. Management of delirium in palliative care: a review. Curr Psychiatry Rep. 2015;17(3):550.
7. de la Cruz M, Ransing V, Yennu S, et al. The frequency, characteristics, and outcomes among cancer patients with delirium admitted to an acute palliative care unit. Oncologist. 2015;20(12):1425-1431.
8. de la Cruz, M, Fan J, Yennu S, et al. The frequency of missed delirium in patients referred to palliative care in a comprehensive cancer center. Support Care Cancer. 2015;23(8):2427-2433.
9. Swigart SE, Kishi Y, Thurber S, et al. Misdiagnosed delirium in patient referrals to a university-based hospital psychiatry department. Psychosomatics. 2008;49(2):104-108.
10. Inouye SK, Bogardus ST Jr, Charpentier PA, et al. A multicomponent intervention to prevent delirium in hospitalized older patients. N Engl J Med. 1999;340(9):669-676.
11. Dasgupta M, Hillier LM. Factors associated with prolonged delirium: a systematic review. Int Psychogeriatr. 2010;22(3):373-394.
12. Detweiler MB, Kenneth A, Bader G, et al. Can improved intra- and inter-team communication reduce missed delirium? Psychiatr Q. 2014;85(2):211-224.
1. Posner JB, Saper CB, Schiff ND, et al. Plum and Posner’s diagnosis of stupor and coma. New York, NY: Oxford University Press; 2007.
2. Skrobik YK, Bergeron N, Dumont M, et al. Olanzapine vs haldoperidol: treating delirium in a critical care setting. Intensive Care Med. 2004;30(3):444-449.
3. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
4. Lonergan E, Luxenberg J, Areosa Sastre A, et al. Benzodiazepines for delirium. Cochrane Database Syst Rev. 2009;(1):CD006379. doi: 10.1002/14651858.CD006379.pub2.
5. Vella-Brincat J, Macleod AD. Adverse effects of opioids on the central nervous system of palliative care patients. J Pain Palliat Care Pharmacother. 2007;21(1):15-25.
6. Grassi L, Caraceni A, Mitchell AJ, et al. Management of delirium in palliative care: a review. Curr Psychiatry Rep. 2015;17(3):550.
7. de la Cruz M, Ransing V, Yennu S, et al. The frequency, characteristics, and outcomes among cancer patients with delirium admitted to an acute palliative care unit. Oncologist. 2015;20(12):1425-1431.
8. de la Cruz, M, Fan J, Yennu S, et al. The frequency of missed delirium in patients referred to palliative care in a comprehensive cancer center. Support Care Cancer. 2015;23(8):2427-2433.
9. Swigart SE, Kishi Y, Thurber S, et al. Misdiagnosed delirium in patient referrals to a university-based hospital psychiatry department. Psychosomatics. 2008;49(2):104-108.
10. Inouye SK, Bogardus ST Jr, Charpentier PA, et al. A multicomponent intervention to prevent delirium in hospitalized older patients. N Engl J Med. 1999;340(9):669-676.
11. Dasgupta M, Hillier LM. Factors associated with prolonged delirium: a systematic review. Int Psychogeriatr. 2010;22(3):373-394.
12. Detweiler MB, Kenneth A, Bader G, et al. Can improved intra- and inter-team communication reduce missed delirium? Psychiatr Q. 2014;85(2):211-224.
No evidence of pregnancy, but she is suicidal and depressed after ‘my baby died’
CASE Depressed after she says her baby died
Ms. R, age 50, is an African-American woman who presents to a psychiatric hospital under an involuntary commitment executed by local law enforcement. Her sister called the authorities because Ms. R reportedly told her that she is “very depressed” and wants to “end [her] life” by taking an overdose of medications after the death of her newborn 1 week earlier.
Ms. R states that she delivered a child at “full term” in the emergency department of an outside community hospital, and that her current psychiatric symptoms began after the child died from “SIDS” [sudden infant death syndrome] shortly after birth.
Ms. R describes depressive symptoms including depressed mood, anhedonia, decreased energy, feelings of guilt, decreased concentration, poor sleep, and suicidal ideation. She denies substance use or a medical condition that could have induced these symptoms, and denies symptoms of mania, anxiety, or psychosis at admission or during the previous year.
Ms. R reports a history of manic episodes that includes periods of elevated mood or irritability, impulsivity, increased energy, excessive spending despite negative consequences, lack of need for sleep, rapid thoughts, and rapid speech that impaired her social and occupational functioning. Her most recent manic episode was approximately 3 years before this admission. She reports a previous suicide attempt and a history of physical abuse from a former intimate partner.
Neither the findings of a physical examination nor the results of a screening test for serum β-human chorionic gonadotropin (βHCG) are consistent with pregnancy. Ms. R’s medical record reveals that she was hospitalized for a “cardiac workup” a week earlier and requested investigation of possible pregnancy, which was negative. Records also reveal that she had a hysterectomy 10 years earlier.
Although Ms. R’s sister and boyfriend support her claim of pregnancy, the patient’s young adult son refutes it and states that she “does stuff like this for attention.” Her son also reports receiving a forged sonogram picture that his mother found online 1 month earlier. Ms. R presents an obituary from a local newspaper for the child but, on further investigation, the photograph of the infant was discovered to be of another child, also obtained online. Ms. R’s family denies knowledge of potential external reward Ms. R could gain by claiming to be pregnant.
Which of the following diagnoses can be considered after Ms. R’s initial presentation?
a) somatic symptom disorder
b) major depressive disorder
c) bipolar I disorder
d) delusional disorder
The authors’ observations
Ms. R reported the recent death of a newborn that was incompatible with her medical history. Her family members revealed that Ms. R made an active effort to deceive them about the reported pregnancy. She also exhibited symptoms of a major depressive episode in the context of previous manic episodes and expressed suicidal ideation.
The first step in the diagnostic pathway was to rule out possible medical explanations, including pregnancy, which could account for the patient’s symptoms. Although the serum βHCG level usually returns to non-pregnant levels 2 to 4 weeks after delivery, it can take even longer in some women.1 The absence of βHCG along with the recorded history of hysterectomy indicated that Ms. R was not pregnant at the time of testing or within the preceding few weeks. Once medical anomalies and substance use were ruled out, further classification of the psychiatric condition was undertaken.
One aspect of establishing a diagnosis for Ms. R is determining the presence of psychosis (eg, delusional thinking) (Table 1). Ms. R deliberately fabricated evidence of her pregnancy and manipulated family members, which indicated a low likelihood of delusions and supported a diagnostic alternative to psychosis.
Ms. R has a well-described history of manic episodes with current symptoms of a major depressive episode. The treatment team makes a diagnosis of bipolar I disorder, most recent episode depressed. The depressive symptoms Ms. R described were consistent with bipolar depression but did not explain her report of a pregnancy that is inconsistent with reality.
As is the case with Ms. R, diagnostic clarity often requires observation and evaluation over time. Building a strong therapeutic relationship with Ms. R in the context of an appropriate treatment plan allows the treatment team to explore the origin, motivations, and evolution of her thought content while managing her illness.
Confronting a patient about her false claims is likely to result in which of the following?
a) spontaneous resolution of symptoms
b) improved therapeutic alliance
c) degradation of the patient’s coping mechanism
d) violent outbursts by the patient
EVALUATION Confrontation
At admission, Ms. R remains resolute that she was pregnant and is suffering immense psychological distress secondary to the death of her child. Early in the treatment course, she is confronted with evidence indicating that her pregnancy was impossible. Shortly after this interaction, nursing staff alerts the treating physician that Ms. R experienced a “seizure-like spell” characterized by gross non-stereotyped jerking of the upper extremities, intact orientation, retention of bowel and bladder function, and coherent speech consistent with a diagnosis of pseudoseizure.2
Ms. R is transferred to a tertiary care facility for neurologic evaluation and observation. Ms. R repeatedly presents a photograph that she claims to be of her deceased child and implores the allied treatment team to advocate for discharge. Evaluation of Ms. R’s neurologic symptoms revealed no medical explanation for the “seizure-like spell” and she is transferred to the inpatient psychiatric hospital.
Upon return to the inpatient psychiatric unit, Ms. R receives intensive psychological exploration of her symptoms, thought content, and the foundation of her pregnancy claim. Within days, she acknowledges that the pregnancy was “not real” and that she was conscious of this fact in the months prior to hospitalization. She cites turmoil in her romantic relationship as the primary stimulus for her actions.
The authors’ observations
Ms. R’s reported pregnancy was not a delusion, but rather a deceitful exposition constructed with appropriate reality testing and a conscious awareness of the manipulation. This eliminated delusions as the explanation of her pregnancy claim. Although Ms. R initially rejected evidence refuting her belief of pregnancy, she recognized and accepted reality with appropriate intervention.
Factitious disorder vs malingering
Factitious disorder and malingering can present with intentional induction or report of symptoms or signs of a physical abnormality:
Factitious disorder imposed on the self is a willful misrepresentation or fabrication of signs or symptoms of an illness by a person in the absence of obvious personal gain that cannot be explained by a separate physical or mental illness (Table 2).3,4
Malingering is the intentional production or exaggeration of physical or psychological signs or symptoms with obvious secondary gain.
Malingering can be excluded in Ms. R’s case: She did not gain external reward by falsely reporting pregnancy. Although DSM-IV-TR (Table 2) assumes that the motivation for the patient with factitious disorder is to assume the sick role, DSM-5 merely states that the she (he) should present themselves as ill, impaired, or injured.3,4
Ms. R’s treatment team diagnosed factitious disorder imposed on self after careful exclusion of other causes for her symptoms. Bipolar I disorder, most recent episode depressed, also was diagnosed after considering Ms. R’s previous history of manic episodes and depressive symptoms at presentation.
Factitious disorder and other psychiatric conditions often are comorbid. Bipolar disorder, as in Ms. R’s case, as well as major depressive disorder commonly are comorbid with factitious disorder. It is also important to note that factitious disorder often occurs in the context of a personality disorder.5
Which of the following medications are FDA-approved for treating factitious disorder?
a) olanzapine-fluoxetine combination
b) lurasidone
c) valproic acid
d) all of the above
e) no medications are approved for treating factitious disorder
TREATMENT Support, drug therapy
Treatment of Ms. R’s factitious disorder consists of psychological interventions via psychotherapy and strengthening of social support. She participates in daily individual therapy sessions as well as several group therapy activities. Ms. R engages with her social worker to facilitate a successful transition to an appropriate support network and access community resources to aid her wellness.
The treatment team feels that her diagnosis of bipolar I disorder, most recent episode depressed, warrants pharmacologic intervention. Ms. R agrees to begin a mood stabilizer, valproic acid, instead of medications FDA-approved to treat bipolar depression, such as lurasidone or quetiapine, because she reports good efficacy and tolerability when she took it during a major depressive episode approximately 4 years earlier.
Valproic acid is started at 250 mg/d and increased to 1,000 mg/d. Ms. R tolerates the medication without observed or reported adverse effects.
The authors’ observations
Managing factitious disorder can be challenging; patients can evoke strong feelings of countertransference during treatment.3,6,7 Providers might feel that the patient does not need to be treated, or that the patient is “not really sick.” This may induce anger and animosity toward the patient (therapeutic nihilism).8 These negative emotions are likely to disrupt the patient–provider relationship and exacerbate the patient’s symptoms.
It is generally accepted that the patient should be made aware of the treatment plan, in an indirect and tactful way, so that the patient does not feel “outed.” Unmasking the patient—the process of instilling insight—is a delicate step and can be a stressful time for the patient.9 A confrontational approach often places the patient’s sick role in doubt and does not address the pathological aspect of the disorder.
It is rare for a patient to admit to fabricating symptoms; confronted, the patient is likely to double their efforts to maintain the rouse of a fictional disease.10,11 It is important for the treatment team to be aware that patients frequently leave the treatment facility against medical advice, seek a different provider, or even pursue legal action for defamation against the treating physician.
Treating comorbid medical and psychiatric conditions is important for successful management of a patient with factitious disorder. Initiating valproic acid to address Ms. R’s bipolar depression contributed to her overall psychiatric stability. Initial treatment with a medication that is FDA-approved for treating bipolar depression, such as lurasidone, quetiapine, or olanzapine-fluoxetine combination, should be considered as an alternative. We chose valproic acid for Ms. R because of its previous efficacy, good tolerability, and the patient’s high level of comfort with the medication.
Which of the following are risk factors for factitious disorder?
a) lengthy medical treatments or hospitalizations as a child
b) female sex
c) experience as a health care worker
d) all of the above
OUTCOME Stabilization
Successful treatment during Ms. R’s inpatient psychiatric admission results in improved insight, remission of suicidal ideation, and stabilization of mood lability. She is discharged to the care of her family with a plan to follow up with a psychotherapist and psychiatrist. Continued administration of valproic acid continues to be effective after discharge.
Ms. R engages in frequent follow-up with outpatient psychiatric services. She remains engaged in psychotherapy and psychiatric care 1 year after discharge. Ms. R has made no report of pregnancy or required hospitalization during this time. She expresses trust in the mental health care system and acknowledges the role treatment played in her improvement.
1. Reyes FI, Winter JS, Faiman C. Postpartum disappearance of chorionic gonadotropin from the maternal and neonatal circulations. Am J Obstet Gynecol. 1985;153(5):486-489.
2. Avbersek A, Sisodiya S. Does the primary literature provide support for clinical signs used to distinguish psychogenic nonepileptic seizures from epileptic seizures? J Neurol Neurosurg Psychiatry. 2010;81(7):719-725.
3. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
4. Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington, DC: American Psychiatric Association; 2000.
5. Kapfhammer HP, Rothenhausler HM, Dietrich E, et al. Artifactual disorders—between deception and self-mutilation. Experiences in consultation psychiatry at a university clinic [in German]. Nervenarzt. 1998;69(5):401-409.
6. Feldman MD, Feldman JM. Tangled in the web: countertransference in the therapy of factitious disorders. Int J Psychiatry Med. 1995;25(4):389-399.
7. Wedel KR. A therapeutic confrontation approach to treating patients with factitious illness. Soc Work. 1971;16(2):69-73.
8. Feldman MD, Hamilton JC, Deemer HN. Factitious disorder. In: Phillips KA, ed. Somatoform and factitious disorder. Washington, DC: American Psychiatric Press; 2001:129-159.
9. Scher LM, Knudsen P, Leamon M. Somatic symptom and related disorders. In: Hales RE, Yudofsky SC, Weiss Roberts L, eds. The American Publishing Psychiatric Publishing textbook of psychiatry. Arlington, VA: American Psychiatric Publishing; 2014:531-556.
10. Lipsitt DR. Introduction. In: Feldman MD, Eisendrath SJ, eds. The spectrum of factitious disorders. Washington, DC: American Psychiatric Press; 1996:xix-xxviii.
11. van der Feltz-Cornelis CM. Confronting patients about a factitious disorder [in Dutch]. Ned Tidjschr Geneeskd. 2000;144(12):545-548.
CASE Depressed after she says her baby died
Ms. R, age 50, is an African-American woman who presents to a psychiatric hospital under an involuntary commitment executed by local law enforcement. Her sister called the authorities because Ms. R reportedly told her that she is “very depressed” and wants to “end [her] life” by taking an overdose of medications after the death of her newborn 1 week earlier.
Ms. R states that she delivered a child at “full term” in the emergency department of an outside community hospital, and that her current psychiatric symptoms began after the child died from “SIDS” [sudden infant death syndrome] shortly after birth.
Ms. R describes depressive symptoms including depressed mood, anhedonia, decreased energy, feelings of guilt, decreased concentration, poor sleep, and suicidal ideation. She denies substance use or a medical condition that could have induced these symptoms, and denies symptoms of mania, anxiety, or psychosis at admission or during the previous year.
Ms. R reports a history of manic episodes that includes periods of elevated mood or irritability, impulsivity, increased energy, excessive spending despite negative consequences, lack of need for sleep, rapid thoughts, and rapid speech that impaired her social and occupational functioning. Her most recent manic episode was approximately 3 years before this admission. She reports a previous suicide attempt and a history of physical abuse from a former intimate partner.
Neither the findings of a physical examination nor the results of a screening test for serum β-human chorionic gonadotropin (βHCG) are consistent with pregnancy. Ms. R’s medical record reveals that she was hospitalized for a “cardiac workup” a week earlier and requested investigation of possible pregnancy, which was negative. Records also reveal that she had a hysterectomy 10 years earlier.
Although Ms. R’s sister and boyfriend support her claim of pregnancy, the patient’s young adult son refutes it and states that she “does stuff like this for attention.” Her son also reports receiving a forged sonogram picture that his mother found online 1 month earlier. Ms. R presents an obituary from a local newspaper for the child but, on further investigation, the photograph of the infant was discovered to be of another child, also obtained online. Ms. R’s family denies knowledge of potential external reward Ms. R could gain by claiming to be pregnant.
Which of the following diagnoses can be considered after Ms. R’s initial presentation?
a) somatic symptom disorder
b) major depressive disorder
c) bipolar I disorder
d) delusional disorder
The authors’ observations
Ms. R reported the recent death of a newborn that was incompatible with her medical history. Her family members revealed that Ms. R made an active effort to deceive them about the reported pregnancy. She also exhibited symptoms of a major depressive episode in the context of previous manic episodes and expressed suicidal ideation.
The first step in the diagnostic pathway was to rule out possible medical explanations, including pregnancy, which could account for the patient’s symptoms. Although the serum βHCG level usually returns to non-pregnant levels 2 to 4 weeks after delivery, it can take even longer in some women.1 The absence of βHCG along with the recorded history of hysterectomy indicated that Ms. R was not pregnant at the time of testing or within the preceding few weeks. Once medical anomalies and substance use were ruled out, further classification of the psychiatric condition was undertaken.
One aspect of establishing a diagnosis for Ms. R is determining the presence of psychosis (eg, delusional thinking) (Table 1). Ms. R deliberately fabricated evidence of her pregnancy and manipulated family members, which indicated a low likelihood of delusions and supported a diagnostic alternative to psychosis.
Ms. R has a well-described history of manic episodes with current symptoms of a major depressive episode. The treatment team makes a diagnosis of bipolar I disorder, most recent episode depressed. The depressive symptoms Ms. R described were consistent with bipolar depression but did not explain her report of a pregnancy that is inconsistent with reality.
As is the case with Ms. R, diagnostic clarity often requires observation and evaluation over time. Building a strong therapeutic relationship with Ms. R in the context of an appropriate treatment plan allows the treatment team to explore the origin, motivations, and evolution of her thought content while managing her illness.
Confronting a patient about her false claims is likely to result in which of the following?
a) spontaneous resolution of symptoms
b) improved therapeutic alliance
c) degradation of the patient’s coping mechanism
d) violent outbursts by the patient
EVALUATION Confrontation
At admission, Ms. R remains resolute that she was pregnant and is suffering immense psychological distress secondary to the death of her child. Early in the treatment course, she is confronted with evidence indicating that her pregnancy was impossible. Shortly after this interaction, nursing staff alerts the treating physician that Ms. R experienced a “seizure-like spell” characterized by gross non-stereotyped jerking of the upper extremities, intact orientation, retention of bowel and bladder function, and coherent speech consistent with a diagnosis of pseudoseizure.2
Ms. R is transferred to a tertiary care facility for neurologic evaluation and observation. Ms. R repeatedly presents a photograph that she claims to be of her deceased child and implores the allied treatment team to advocate for discharge. Evaluation of Ms. R’s neurologic symptoms revealed no medical explanation for the “seizure-like spell” and she is transferred to the inpatient psychiatric hospital.
Upon return to the inpatient psychiatric unit, Ms. R receives intensive psychological exploration of her symptoms, thought content, and the foundation of her pregnancy claim. Within days, she acknowledges that the pregnancy was “not real” and that she was conscious of this fact in the months prior to hospitalization. She cites turmoil in her romantic relationship as the primary stimulus for her actions.
The authors’ observations
Ms. R’s reported pregnancy was not a delusion, but rather a deceitful exposition constructed with appropriate reality testing and a conscious awareness of the manipulation. This eliminated delusions as the explanation of her pregnancy claim. Although Ms. R initially rejected evidence refuting her belief of pregnancy, she recognized and accepted reality with appropriate intervention.
Factitious disorder vs malingering
Factitious disorder and malingering can present with intentional induction or report of symptoms or signs of a physical abnormality:
Factitious disorder imposed on the self is a willful misrepresentation or fabrication of signs or symptoms of an illness by a person in the absence of obvious personal gain that cannot be explained by a separate physical or mental illness (Table 2).3,4
Malingering is the intentional production or exaggeration of physical or psychological signs or symptoms with obvious secondary gain.
Malingering can be excluded in Ms. R’s case: She did not gain external reward by falsely reporting pregnancy. Although DSM-IV-TR (Table 2) assumes that the motivation for the patient with factitious disorder is to assume the sick role, DSM-5 merely states that the she (he) should present themselves as ill, impaired, or injured.3,4
Ms. R’s treatment team diagnosed factitious disorder imposed on self after careful exclusion of other causes for her symptoms. Bipolar I disorder, most recent episode depressed, also was diagnosed after considering Ms. R’s previous history of manic episodes and depressive symptoms at presentation.
Factitious disorder and other psychiatric conditions often are comorbid. Bipolar disorder, as in Ms. R’s case, as well as major depressive disorder commonly are comorbid with factitious disorder. It is also important to note that factitious disorder often occurs in the context of a personality disorder.5
Which of the following medications are FDA-approved for treating factitious disorder?
a) olanzapine-fluoxetine combination
b) lurasidone
c) valproic acid
d) all of the above
e) no medications are approved for treating factitious disorder
TREATMENT Support, drug therapy
Treatment of Ms. R’s factitious disorder consists of psychological interventions via psychotherapy and strengthening of social support. She participates in daily individual therapy sessions as well as several group therapy activities. Ms. R engages with her social worker to facilitate a successful transition to an appropriate support network and access community resources to aid her wellness.
The treatment team feels that her diagnosis of bipolar I disorder, most recent episode depressed, warrants pharmacologic intervention. Ms. R agrees to begin a mood stabilizer, valproic acid, instead of medications FDA-approved to treat bipolar depression, such as lurasidone or quetiapine, because she reports good efficacy and tolerability when she took it during a major depressive episode approximately 4 years earlier.
Valproic acid is started at 250 mg/d and increased to 1,000 mg/d. Ms. R tolerates the medication without observed or reported adverse effects.
The authors’ observations
Managing factitious disorder can be challenging; patients can evoke strong feelings of countertransference during treatment.3,6,7 Providers might feel that the patient does not need to be treated, or that the patient is “not really sick.” This may induce anger and animosity toward the patient (therapeutic nihilism).8 These negative emotions are likely to disrupt the patient–provider relationship and exacerbate the patient’s symptoms.
It is generally accepted that the patient should be made aware of the treatment plan, in an indirect and tactful way, so that the patient does not feel “outed.” Unmasking the patient—the process of instilling insight—is a delicate step and can be a stressful time for the patient.9 A confrontational approach often places the patient’s sick role in doubt and does not address the pathological aspect of the disorder.
It is rare for a patient to admit to fabricating symptoms; confronted, the patient is likely to double their efforts to maintain the rouse of a fictional disease.10,11 It is important for the treatment team to be aware that patients frequently leave the treatment facility against medical advice, seek a different provider, or even pursue legal action for defamation against the treating physician.
Treating comorbid medical and psychiatric conditions is important for successful management of a patient with factitious disorder. Initiating valproic acid to address Ms. R’s bipolar depression contributed to her overall psychiatric stability. Initial treatment with a medication that is FDA-approved for treating bipolar depression, such as lurasidone, quetiapine, or olanzapine-fluoxetine combination, should be considered as an alternative. We chose valproic acid for Ms. R because of its previous efficacy, good tolerability, and the patient’s high level of comfort with the medication.
Which of the following are risk factors for factitious disorder?
a) lengthy medical treatments or hospitalizations as a child
b) female sex
c) experience as a health care worker
d) all of the above
OUTCOME Stabilization
Successful treatment during Ms. R’s inpatient psychiatric admission results in improved insight, remission of suicidal ideation, and stabilization of mood lability. She is discharged to the care of her family with a plan to follow up with a psychotherapist and psychiatrist. Continued administration of valproic acid continues to be effective after discharge.
Ms. R engages in frequent follow-up with outpatient psychiatric services. She remains engaged in psychotherapy and psychiatric care 1 year after discharge. Ms. R has made no report of pregnancy or required hospitalization during this time. She expresses trust in the mental health care system and acknowledges the role treatment played in her improvement.
CASE Depressed after she says her baby died
Ms. R, age 50, is an African-American woman who presents to a psychiatric hospital under an involuntary commitment executed by local law enforcement. Her sister called the authorities because Ms. R reportedly told her that she is “very depressed” and wants to “end [her] life” by taking an overdose of medications after the death of her newborn 1 week earlier.
Ms. R states that she delivered a child at “full term” in the emergency department of an outside community hospital, and that her current psychiatric symptoms began after the child died from “SIDS” [sudden infant death syndrome] shortly after birth.
Ms. R describes depressive symptoms including depressed mood, anhedonia, decreased energy, feelings of guilt, decreased concentration, poor sleep, and suicidal ideation. She denies substance use or a medical condition that could have induced these symptoms, and denies symptoms of mania, anxiety, or psychosis at admission or during the previous year.
Ms. R reports a history of manic episodes that includes periods of elevated mood or irritability, impulsivity, increased energy, excessive spending despite negative consequences, lack of need for sleep, rapid thoughts, and rapid speech that impaired her social and occupational functioning. Her most recent manic episode was approximately 3 years before this admission. She reports a previous suicide attempt and a history of physical abuse from a former intimate partner.
Neither the findings of a physical examination nor the results of a screening test for serum β-human chorionic gonadotropin (βHCG) are consistent with pregnancy. Ms. R’s medical record reveals that she was hospitalized for a “cardiac workup” a week earlier and requested investigation of possible pregnancy, which was negative. Records also reveal that she had a hysterectomy 10 years earlier.
Although Ms. R’s sister and boyfriend support her claim of pregnancy, the patient’s young adult son refutes it and states that she “does stuff like this for attention.” Her son also reports receiving a forged sonogram picture that his mother found online 1 month earlier. Ms. R presents an obituary from a local newspaper for the child but, on further investigation, the photograph of the infant was discovered to be of another child, also obtained online. Ms. R’s family denies knowledge of potential external reward Ms. R could gain by claiming to be pregnant.
Which of the following diagnoses can be considered after Ms. R’s initial presentation?
a) somatic symptom disorder
b) major depressive disorder
c) bipolar I disorder
d) delusional disorder
The authors’ observations
Ms. R reported the recent death of a newborn that was incompatible with her medical history. Her family members revealed that Ms. R made an active effort to deceive them about the reported pregnancy. She also exhibited symptoms of a major depressive episode in the context of previous manic episodes and expressed suicidal ideation.
The first step in the diagnostic pathway was to rule out possible medical explanations, including pregnancy, which could account for the patient’s symptoms. Although the serum βHCG level usually returns to non-pregnant levels 2 to 4 weeks after delivery, it can take even longer in some women.1 The absence of βHCG along with the recorded history of hysterectomy indicated that Ms. R was not pregnant at the time of testing or within the preceding few weeks. Once medical anomalies and substance use were ruled out, further classification of the psychiatric condition was undertaken.
One aspect of establishing a diagnosis for Ms. R is determining the presence of psychosis (eg, delusional thinking) (Table 1). Ms. R deliberately fabricated evidence of her pregnancy and manipulated family members, which indicated a low likelihood of delusions and supported a diagnostic alternative to psychosis.
Ms. R has a well-described history of manic episodes with current symptoms of a major depressive episode. The treatment team makes a diagnosis of bipolar I disorder, most recent episode depressed. The depressive symptoms Ms. R described were consistent with bipolar depression but did not explain her report of a pregnancy that is inconsistent with reality.
As is the case with Ms. R, diagnostic clarity often requires observation and evaluation over time. Building a strong therapeutic relationship with Ms. R in the context of an appropriate treatment plan allows the treatment team to explore the origin, motivations, and evolution of her thought content while managing her illness.
Confronting a patient about her false claims is likely to result in which of the following?
a) spontaneous resolution of symptoms
b) improved therapeutic alliance
c) degradation of the patient’s coping mechanism
d) violent outbursts by the patient
EVALUATION Confrontation
At admission, Ms. R remains resolute that she was pregnant and is suffering immense psychological distress secondary to the death of her child. Early in the treatment course, she is confronted with evidence indicating that her pregnancy was impossible. Shortly after this interaction, nursing staff alerts the treating physician that Ms. R experienced a “seizure-like spell” characterized by gross non-stereotyped jerking of the upper extremities, intact orientation, retention of bowel and bladder function, and coherent speech consistent with a diagnosis of pseudoseizure.2
Ms. R is transferred to a tertiary care facility for neurologic evaluation and observation. Ms. R repeatedly presents a photograph that she claims to be of her deceased child and implores the allied treatment team to advocate for discharge. Evaluation of Ms. R’s neurologic symptoms revealed no medical explanation for the “seizure-like spell” and she is transferred to the inpatient psychiatric hospital.
Upon return to the inpatient psychiatric unit, Ms. R receives intensive psychological exploration of her symptoms, thought content, and the foundation of her pregnancy claim. Within days, she acknowledges that the pregnancy was “not real” and that she was conscious of this fact in the months prior to hospitalization. She cites turmoil in her romantic relationship as the primary stimulus for her actions.
The authors’ observations
Ms. R’s reported pregnancy was not a delusion, but rather a deceitful exposition constructed with appropriate reality testing and a conscious awareness of the manipulation. This eliminated delusions as the explanation of her pregnancy claim. Although Ms. R initially rejected evidence refuting her belief of pregnancy, she recognized and accepted reality with appropriate intervention.
Factitious disorder vs malingering
Factitious disorder and malingering can present with intentional induction or report of symptoms or signs of a physical abnormality:
Factitious disorder imposed on the self is a willful misrepresentation or fabrication of signs or symptoms of an illness by a person in the absence of obvious personal gain that cannot be explained by a separate physical or mental illness (Table 2).3,4
Malingering is the intentional production or exaggeration of physical or psychological signs or symptoms with obvious secondary gain.
Malingering can be excluded in Ms. R’s case: She did not gain external reward by falsely reporting pregnancy. Although DSM-IV-TR (Table 2) assumes that the motivation for the patient with factitious disorder is to assume the sick role, DSM-5 merely states that the she (he) should present themselves as ill, impaired, or injured.3,4
Ms. R’s treatment team diagnosed factitious disorder imposed on self after careful exclusion of other causes for her symptoms. Bipolar I disorder, most recent episode depressed, also was diagnosed after considering Ms. R’s previous history of manic episodes and depressive symptoms at presentation.
Factitious disorder and other psychiatric conditions often are comorbid. Bipolar disorder, as in Ms. R’s case, as well as major depressive disorder commonly are comorbid with factitious disorder. It is also important to note that factitious disorder often occurs in the context of a personality disorder.5
Which of the following medications are FDA-approved for treating factitious disorder?
a) olanzapine-fluoxetine combination
b) lurasidone
c) valproic acid
d) all of the above
e) no medications are approved for treating factitious disorder
TREATMENT Support, drug therapy
Treatment of Ms. R’s factitious disorder consists of psychological interventions via psychotherapy and strengthening of social support. She participates in daily individual therapy sessions as well as several group therapy activities. Ms. R engages with her social worker to facilitate a successful transition to an appropriate support network and access community resources to aid her wellness.
The treatment team feels that her diagnosis of bipolar I disorder, most recent episode depressed, warrants pharmacologic intervention. Ms. R agrees to begin a mood stabilizer, valproic acid, instead of medications FDA-approved to treat bipolar depression, such as lurasidone or quetiapine, because she reports good efficacy and tolerability when she took it during a major depressive episode approximately 4 years earlier.
Valproic acid is started at 250 mg/d and increased to 1,000 mg/d. Ms. R tolerates the medication without observed or reported adverse effects.
The authors’ observations
Managing factitious disorder can be challenging; patients can evoke strong feelings of countertransference during treatment.3,6,7 Providers might feel that the patient does not need to be treated, or that the patient is “not really sick.” This may induce anger and animosity toward the patient (therapeutic nihilism).8 These negative emotions are likely to disrupt the patient–provider relationship and exacerbate the patient’s symptoms.
It is generally accepted that the patient should be made aware of the treatment plan, in an indirect and tactful way, so that the patient does not feel “outed.” Unmasking the patient—the process of instilling insight—is a delicate step and can be a stressful time for the patient.9 A confrontational approach often places the patient’s sick role in doubt and does not address the pathological aspect of the disorder.
It is rare for a patient to admit to fabricating symptoms; confronted, the patient is likely to double their efforts to maintain the rouse of a fictional disease.10,11 It is important for the treatment team to be aware that patients frequently leave the treatment facility against medical advice, seek a different provider, or even pursue legal action for defamation against the treating physician.
Treating comorbid medical and psychiatric conditions is important for successful management of a patient with factitious disorder. Initiating valproic acid to address Ms. R’s bipolar depression contributed to her overall psychiatric stability. Initial treatment with a medication that is FDA-approved for treating bipolar depression, such as lurasidone, quetiapine, or olanzapine-fluoxetine combination, should be considered as an alternative. We chose valproic acid for Ms. R because of its previous efficacy, good tolerability, and the patient’s high level of comfort with the medication.
Which of the following are risk factors for factitious disorder?
a) lengthy medical treatments or hospitalizations as a child
b) female sex
c) experience as a health care worker
d) all of the above
OUTCOME Stabilization
Successful treatment during Ms. R’s inpatient psychiatric admission results in improved insight, remission of suicidal ideation, and stabilization of mood lability. She is discharged to the care of her family with a plan to follow up with a psychotherapist and psychiatrist. Continued administration of valproic acid continues to be effective after discharge.
Ms. R engages in frequent follow-up with outpatient psychiatric services. She remains engaged in psychotherapy and psychiatric care 1 year after discharge. Ms. R has made no report of pregnancy or required hospitalization during this time. She expresses trust in the mental health care system and acknowledges the role treatment played in her improvement.
1. Reyes FI, Winter JS, Faiman C. Postpartum disappearance of chorionic gonadotropin from the maternal and neonatal circulations. Am J Obstet Gynecol. 1985;153(5):486-489.
2. Avbersek A, Sisodiya S. Does the primary literature provide support for clinical signs used to distinguish psychogenic nonepileptic seizures from epileptic seizures? J Neurol Neurosurg Psychiatry. 2010;81(7):719-725.
3. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
4. Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington, DC: American Psychiatric Association; 2000.
5. Kapfhammer HP, Rothenhausler HM, Dietrich E, et al. Artifactual disorders—between deception and self-mutilation. Experiences in consultation psychiatry at a university clinic [in German]. Nervenarzt. 1998;69(5):401-409.
6. Feldman MD, Feldman JM. Tangled in the web: countertransference in the therapy of factitious disorders. Int J Psychiatry Med. 1995;25(4):389-399.
7. Wedel KR. A therapeutic confrontation approach to treating patients with factitious illness. Soc Work. 1971;16(2):69-73.
8. Feldman MD, Hamilton JC, Deemer HN. Factitious disorder. In: Phillips KA, ed. Somatoform and factitious disorder. Washington, DC: American Psychiatric Press; 2001:129-159.
9. Scher LM, Knudsen P, Leamon M. Somatic symptom and related disorders. In: Hales RE, Yudofsky SC, Weiss Roberts L, eds. The American Publishing Psychiatric Publishing textbook of psychiatry. Arlington, VA: American Psychiatric Publishing; 2014:531-556.
10. Lipsitt DR. Introduction. In: Feldman MD, Eisendrath SJ, eds. The spectrum of factitious disorders. Washington, DC: American Psychiatric Press; 1996:xix-xxviii.
11. van der Feltz-Cornelis CM. Confronting patients about a factitious disorder [in Dutch]. Ned Tidjschr Geneeskd. 2000;144(12):545-548.
1. Reyes FI, Winter JS, Faiman C. Postpartum disappearance of chorionic gonadotropin from the maternal and neonatal circulations. Am J Obstet Gynecol. 1985;153(5):486-489.
2. Avbersek A, Sisodiya S. Does the primary literature provide support for clinical signs used to distinguish psychogenic nonepileptic seizures from epileptic seizures? J Neurol Neurosurg Psychiatry. 2010;81(7):719-725.
3. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
4. Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington, DC: American Psychiatric Association; 2000.
5. Kapfhammer HP, Rothenhausler HM, Dietrich E, et al. Artifactual disorders—between deception and self-mutilation. Experiences in consultation psychiatry at a university clinic [in German]. Nervenarzt. 1998;69(5):401-409.
6. Feldman MD, Feldman JM. Tangled in the web: countertransference in the therapy of factitious disorders. Int J Psychiatry Med. 1995;25(4):389-399.
7. Wedel KR. A therapeutic confrontation approach to treating patients with factitious illness. Soc Work. 1971;16(2):69-73.
8. Feldman MD, Hamilton JC, Deemer HN. Factitious disorder. In: Phillips KA, ed. Somatoform and factitious disorder. Washington, DC: American Psychiatric Press; 2001:129-159.
9. Scher LM, Knudsen P, Leamon M. Somatic symptom and related disorders. In: Hales RE, Yudofsky SC, Weiss Roberts L, eds. The American Publishing Psychiatric Publishing textbook of psychiatry. Arlington, VA: American Psychiatric Publishing; 2014:531-556.
10. Lipsitt DR. Introduction. In: Feldman MD, Eisendrath SJ, eds. The spectrum of factitious disorders. Washington, DC: American Psychiatric Press; 1996:xix-xxviii.
11. van der Feltz-Cornelis CM. Confronting patients about a factitious disorder [in Dutch]. Ned Tidjschr Geneeskd. 2000;144(12):545-548.