User login
• When evaluating patients with suspected cutaneous lupus erythematosus, use multiple criteria—including histologic and immunofluorescent biopsy findings and American College of Rheumatology criteria—to rule out systemic disease. C
• Cancer screening with a careful history and physical examination is recommended for all adult patients whom you suspect of having dermatomyositis. C
• Suspect mixed connective tissue disease in patients with skin findings characteristic of varying auto-immune disorders appearing sequentially over several months or years. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Many systemic conditions are accompanied by skin manifestations. This is especially true for connective tissue disorders, for which dermatologic findings are often the key to diagnosis.
In this review, we describe the dermatologic findings of some well-known connective tissue disorders. The text and photographs in the pages that follow will help you hone your diagnostic skills, leading to earlier treatment and, possibly, better outcomes.
Lupus erythematosus: Cutaneous and systemic disease often overlap
Lupus erythematosus (LE), a chronic, inflammatory autoimmune condition that primarily affects women in their 20s and 30s, may initially present as a systemic disease or in a purely cutaneous form. However, most patients with systemic LE have some skin manifestations, and those with cutaneous LE often have—or subsequently develop—systemic involvement.1 Thus, recognizing the cutaneous manifestations of LE will not only aid in diagnosis, but will help you identify patients at risk for systemic disease.
Cutaneous LE has 4 subtypes
There are 4 subcategories of cutaneous LE—acute, subacute, chronic, and intermittent.2 Each is differentiated by the appearance of the lesions (TABLE 1), histology, and serological markers.1 Photosensitivity is common to all the subcategories to varying degrees.
Acute cutaneous lupus erythematosus (ACLE) is typically characterized by the classic malar “butterfly” rash, an erythematous eruption of macules or edematous papules over the bridge of the nose and cheek.3 Although this presentation is most common, there are variations—1 in which the lesions cover other exposed areas (commonly including the “V” of the chest, the extensor surface of the arms, and the hands), and a rare form in which toxic epidermal necrolysis-like blistering occurs.1,4 These skin changes—which generally last anywhere from a few hours to several weeks—typically resolve without scarring, although pigment changes can occur.5
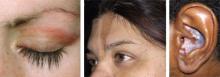
Left: Dermatomyositis is the underlying cause of the heliotrope discoloration on this patient’s upper eyelid.
Center: Linear morphea is associated with the lesion on this patient’s face—called en coup de sabre because it resembles the mark caused by the stroke of a sword in a duel.
Right: Discoid lupus erythematosus causes hypopigmentation and scarring.Patients with ACLE have a predisposition to systemic LE; unlike those with other forms of cutaneous LE, 40% to 90% will have double-stranded DNA (dsDNA) autoantibodies.3,6
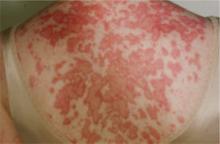
Subacute cutaneous lupus erythematosus (SCLE), which usually affects middle-aged Caucasian women, is characterized by erythematous papulosquamous (psoriasis-like) eruptions or annular lesions with raised red borders and central clearing—or both. These lesions, which are nonscarring, lack in-duration, and rarely affect the scalp or face, appear suddenly, usually after exposure to sunlight (FIGURE 1)5,7-9 or certain drugs. Hydrochlorothiazide, terbinafine, calcium channel blockers, and angiotensin-converting enzyme inhibitors are common offenders.1,6
FIGURE 1
Annular lesions in subacute cutaneous lupus erythematosus
Ring-like lesions with raised red borders and central clearing on the back of a patient with subacute cutaneous lupus erythematosus.
SCLE is often associated with extracutaneous symptoms such as arthritis and myalgias,1,8 but patients are at relatively low risk for severe systemic manifestations.5,8,10 Serology is often notable, with anti-Ro (SS-A) antibodies present in 70% to 90% of patients and anti-La (SS-B) autoantibodies found in 30% to 50%.1,11
Chronic cutaneous lupus erythematosus (CCLE) also occurs predominantly in females, at a ratio as high as 5 to 1.12 There are 3 variations of CCLE: discoid lupus erythematosus (DLE), LE profundus, and chilblain LE (TABLE 1). DLE, characterized by alopecia, skin atrophy, and dyspigmentation, is the most common and affects patients of all ages and ethnic groups.9,13,14 (See image above.)
DLE lesions typically begin as erythematous papules and plaques with scale. As the disorder progresses, the lesions spread, causing follicular plugging, peripheral hyperpigmentation and central hypopigmentation, telangiectasia, and atrophy.9,15 In some cases, patients develop thickened, scarred skin and permanent scarring alopecia.15 Prompt recognition of DLE is particularly important, as early referral and treatment may reduce the likelihood of permanent scarring alopecia and pigment changes.11
Intermittent cutaneous lupus erythematosus (ICLE), a relatively new subtype of cutaneous LE, is represented by a rare condition—lupus erythematosus tumidus (LET)—reported in <100 cases worldwide. LET is characterized by succulent, erythematous, and edematous plaques on sun-exposed parts of the body.1,16
TABLE 1
Cutaneous lupus erythematosus: Recognizing the subtypes1
| Acute cutaneous lupus erythematosus (ACLE) |
|---|
| Localized: erythematous macules or papules over the bridge of the nose and cheek, with sparing of the nasolabial folds (“butterfly rash”) |
| Generalized: similar erythematous lesions over other photodistributed parts of the body, including the neck, chest, arms, and hands |
| Toxic epidermal necrolysis-like (TEN-like): blistering and epidermal cleavage in photodistributed parts of the body |
| Subacute cutaneous lupus erythematosus (SCLE) |
| Erythematous papulosquamous (psoriasis-like) eruptions or annular (ring-like) lesions with raised red borders and central clearing, occurring symmetrically and suddenly after sunlight exposure on photo-distributed body parts; the scalp and face are rarely affected |
| Chronic cutaneous lupus erythematosus (CCLE) |
| Discoid lupus erythematosus: erythematous papules and plaques with associated scale, spreading centrifugally with follicular plugging, pigment change, telangiectasia, and atrophy; scarring alopecia can occur |
| Lupus erythematosus profundus: tender, erythematous nodules and plaques, usually involving the proximal extremities, trunk, breasts, buttocks, and face |
| Chilblain lupus erythematosus: tender, erythematous nodules and plaques, occurring in acral areas often in response to cold |
| Intermittent cutaneous lupus erythematosus (ICLE) |
| Lupus erythematosus tumidus: succulent, erythematous, and edematous plaques found on photodistributed parts of the body |
Cutaneous LE diagnosis and treatment: Start with ACR criteria
When evaluating patients with suspected cutaneous LE, it is important not only to identify the subtype, but also to rule out systemic disease using criteria established by the American College of Rheumatology (ACR).17 Notably, 4 of the 11 diagnostic criteria for systemic disease involve visual clues, including malar rash, discoid rash, photosensitivity, and oral ulcerations. Laboratory evidence of systemic disease may include a positive antinuclear antibody, anti-dsDNA, or anti-Sm autoantibody test results, as well as hematologic abnormalities described in the ACR guidelines.
Ultimately, a diagnosis of cutaneous LE should be based on the patient’s history and physical exam, autoantibody profile, and histologic and immunofluorescent biopsy findings. A rheumatologic evaluation may help to determine which patients have systemic disease, as the ACR criteria may overdiagnose systemic LE in those with predominantly skin changes.6
Treatment of cutaneous LE is based on the subtype and extent of disease, with potent topical corticosteroids, in combination with antimalarial agents, being the primary therapies. ACLE skin lesions generally respond best to systemic corticosteroids and immunosuppressive agents (such as azathioprine or cyclophosphamide) that are used to control underlying systemic disease. SCLE can be managed with topical corticosteroids; however, patients typically also require systemic treatment, often with hydroxychloroquine, for optimal control. DLE, the most common form of CCLE, is managed in a similar fashion, with a greater role for intralesional corticosteroid injections. Lesions associated with ICLE, which often resolve spontaneously, may be treated with topical corticosteroids and antimalarials.16 It is also important to advise all patients with cutaneous LE to use a broad-spectrum sunscreen, as ultraviolet (UV) exposure can induce or exacerbate the lesions.9
Dermatomyositis: Rare but serious
Dermatomyositis is an idiopathic inflammatory myopathy characterized by chronic muscle inflammation, symmetric proximal muscle weakness, and distinct cutaneous findings. In addition to possible cardiac and pulmonary complications, dysphagia, and joint contractures, dermatomyositis is associated with cancer, with up to 25% of affected adults having an underlying occult malignancy.18
Dermatomyositis is a rare condition, with a prevalence of only 1 to 10 cases per million adults and 3.2 cases per million children.19 It has a bimodal age distribution, with most juvenile cases affecting children between the ages of 5 and 14 years and most adult cases developing in the fifth and sixth decades of life. Women are affected twice as often as men.18
Suspect dermatomyositis when you see any of the following signs:
- A heliotrope rash: violaceous macules and patches, with or without edema, symmetrically on the periorbital skin, present early in the disease course in 30% to 60% of patients.19 (See image)
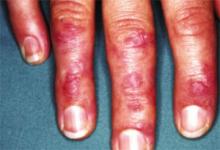
- Gottron’s papules: violaceous papules on the dorsal interphalangeal and metacarpophalangeal joints of the hands, elbows, and knees, occurring in as many as 70% of patients (FIGURE 2).19,20
- Gottron’s sign: nonscaling, violaceous erythematous macules and plaques occurring symmetrically in the same distribution as Gottron’s papules, but with sparing of the interphalangeal spaces.20
- Periungual erythema and telangiectasias: redness and dilation of the blood vessels in the skin surrounding the nail plate.
- The shawl and V-signs: erythematous macular eruptions occurring in a “shawl” pattern on the shoulders, arms, and upper back and in a V-shaped pattern on the anterior neck and chest.
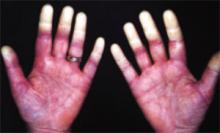
- Mechanic’s hand: extensive scaling, fissuring, and roughening of the palmar aspect of the hand.20,21
- Poikiloderma vasculare atrophicans: circumscribed violaceous erythema with thinning of the skin, prominent telangiectasias, and a mottled pattern of hypo- and hyperpigmentation, typically occurring on the anterior neck, chest, posterior shoulders, back, and buttocks, years after onset of the disease.21-24
- Cutaneous calcification (calcinosis cutis): usually on the buttocks, elbows, knees, and traumatized areas, affecting 30% to 70% of children with dermatomyositis, but only 10% of adults with the disorder.20,25-27
FIGURE 2
Gottron’s papules in dermatomyositis
Violaceous papules on the dorsal interphalangeal and metacarpophalangeal joints of the hands are a common manifestation of dermatomyositis.
Bohan and Peter criteria help with dermatomyositis diagnosis
A diagnosis of dermatomyositis can be established using the following criteria developed by Bohan and Peter in 1975: (1) symmetric muscle weakness; (2) elevation of muscle enzymes, most notably creatinine phosphokinase; (3) evidence of inflammation on muscle biopsy; (4) electromyographic features of myositis; and (5) characteristic dermatologic signs, including a heliotrope rash and Gottron’s papules.28,29 A definitive diagnosis can be made when the patient meets 3 of the first 4 criteria, as well as the fifth.
Corticosteroids are the mainstay of treatment for dermatomyositis, although the dose and duration are subject to debate. Cutaneous manifestations of dermatomyositis are commonly treated with topical corticosteroids and oral hydroxychloroquine, as well as emollients and antipruritic agents.
Cancer screening. All adults with signs and symptoms of dermatomyositis should undergo cancer screening. The most common malignancies are ovarian cancer, gastric cancer, and lymphoma.21
Photoprotection. As with cutaneous LE, UV exposure can exacerbate dermatomyositis, and patients should use a broad-spectrum sunscreen.
Scleroderma: Localized and systemic disease
The major cutaneous manifestation of scleroderma is that of thickened, leathery, bound-down skin, seen in both localized and systemic disease. In both cases, the lesions typically evolve through 3 characteristic stages: the initial inflammatory and edematous stage; a fibrotic stage, during which the skin lesions appear hard, tight, and hidebound; and the final, atrophic stage. However, not all lesions progress to this final stage.8
Localized scleroderma (also known as morphea) affects roughly 1 in 100,000 people. It is more prevalent in females than males, with a ratio as high as 3 to 1.30-33 Cutaneous findings vary, and numerous clinical presentations are possible. The most widely used classification system, the Mayo Clinic Classification, recognizes 5 subtypes: plaque morphea (the most common), generalized morphea, linear morphea, deep morphea, and bullous morphea (TABLE 2).34 The mean age of onset depends on the subtype, and ranges from 12 years for linear morphea to 45 years for deep morphea.35
While systemic involvement is not common with localized scleroderma, extracutaneous manifestations have been reported in up to 25% of cases.31,36,37 In a 2005 multicenter study of 750 pediatric patients with localized scleroderma, the most frequently reported extracutaneous symptom was arthritis, found in 12% of patients.36 Less frequent findings included neurologic symptoms such as seizures and headaches, vascular changes such as deep vein thrombosis, and gastrointestinal, cardiac, and renal conditions. Although the precise etiology of localized scleroderma is unknown, it is thought to be associated with trauma, prior infection by Borrelia burgdorferi, chronic venous insufficiency, and irradiation for breast cancer.30,38,39
TABLE 2
Localized scleroderma: What to look for8,34
|
Systemic scleroderma is a heterogenous disorder
Commonly called systemic sclerosis (SSc), systemic scleroderma is characterized by proliferative vascular lesions; fibrosis of internal organs, including the lungs, heart, kidneys, and gastrointestinal tract; and distinct cutaneous manifestations.3 SSc affects about 240 people per million adults, mostly between the ages of 30 and 50 years, with women affected 3 times as often as men.40-42
The cause of SSc is unknown, although a genetic predisposition is likely. Environmental factors likely play a role in triggering the disease, with possible causative factors including cytomegalovirus and other viral infections and exposure to certain chemicals.43
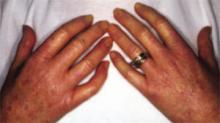
As in localized scleroderma, cutaneous manifestations are a prominent part of SSc and typically develop early on. Raynaud’s phenomenon (RP) (FIGURE 3), which occurs in 90% to 98% of patients with SSc,44 can develop in association with other cutaneous findings or precede additional skin changes by months or years.
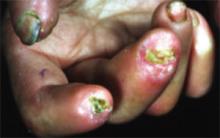
Other early skin changes include nonpitting edema of the fingers and toes, which creates a sausage-like appearance. This is typically followed by hardening and thickening of the skin in these areas, which can result in highly disabling sclerodactyly (FIGURE 4).45 Patients can also develop painful ulcerations on their fingertips and knuckles (rat bite necroses) due to local ischemia and vascular insufficiency. This may be complicated by secondary bacterial infection, gangrene, and acroosteolysis, leading to articular deformities and dissolution of terminal phalanges (FIGURE 5).45-48
On the face, SSc is characterized early on by periorbital edema and later by the development of a beaked nose, a reduction in the size of the mouth (microstomia) with radial furrowing, thinning of the lips, and telangiectasias.45,49 As the sclerosis worsens, patients are frequently left with expressionless, mask-like faces.48
Other common cutaneous manifestations of SSc include a “salt and pepper” appearance, with alternating areas of hypo- and hyperpigmented skin. Additionally, patients may suffer from a loss of hair follicles, severely dry skin, and pruritus.45,48
There are 2 major subsets of SSc—limited cutaneous SSc and diffuse cutaneous SSc. While they can be differentiated based on the history of symptoms, the appearance and extent of cutaneous involvement, and certain serological makers, the most important difference is the speed at which the disease progresses and its severity: Limited cutaneous SSc typically progresses slowly, while diffuse cutaneous SSc is characterized by a relatively rapid onset of disease, with skin and internal organ involvement likely to be severe.49
Scleroderma diagnosis and treatment: Vascular changes are an important clue
Skin changes seen in localized scleroderma and SSc can be clinically and histologically similar, making it difficult to arrive at a definitive diagnosis.
One clinical clue is that in localized scleroderma, vascular changes, such as RP and periungual nailfold telangiectasia, are typically absent. In addition, cutaneous changes in the hands and fingers, such as sclerodactyly, are more characteristic of systemic disease.10
SSc can be diagnosed using criteria proposed by the ACR, which have been shown to be highly sensitive (97%) and specific (98%).50 The major criterion is proximal scleroderma—symmetric thickening, tightening, and induration of the skin of the fingers and areas proximal to the metacarpophalangeal or metatarsophalangeal joints, which may include the trunk, neck, and face. Minor criteria include (1) sclerodactyly, (2) digital pitting scars of fingertips or loss of substance of the distal finger pad, and (3) bilateral basilar pulmonary fibrosis. Diagnosis is based on the presence of either the major criterion or 2 of the 3 minor criteria.50
Numerous therapies are available for localized scleroderma, including topical, intralesional, and systemic corticosteroids, topical tacrolimus, hydroxychloroquine, topical and systemic calcipotriol, penicillamine, sulfasalazine, interferon-[H9253], methotrexate, phototherapy with UV light, and imiquimod.
SSc skin manifestations, which can be severe and disabling, can be treated with a wide range of therapies. Topical or systemic corticosteroids; topical calcineurin inhibitors; systemic immunosuppressive agents such as methotrexate, cyclophosphamide, cyclosporine, and D-penicillamine; and phototherapy have all had varying success at reducing hardening of the skin.51-54 Other skin manifestations, including RP, dryness and itching, pigment changes, digital ulcerations, calcifications, and telangiectasias, should be managed as needed, with various supplemental treatment options available.45 These may include emollients, antihistamines, and topical corticosteroids for dryness and itching; laser therapy for telangiectasias; and corticosteroid injection, laser therapy, or surgery for calcifications. However, there is limited evidence of efficacy for most of these options.45
FIGURE 3
An attack of Raynaud’s phenomenon
Raynaud’s phenomenon, characterized by blanching of the distal fingertips, is shown here in a patient with systemic sclerosis.
FIGURE 4
Sclerodactyly in a patient with systemic sclerosis
Hardening and thickening of the skin can result in highly disabling sclerodactyly in patients with systemic sclerosis.
FIGURE 5
Dissolution of terminal phalanges
Bony resorption and ulceration have led to the loss of distal phalanges in this patient with systemic sclerosis.
Mixed connective tissue disease has features of several disorders
Mixed connective tissue disease (MCTD) is an apparently distinct rheumatologic condition characterized by a combination of clinical features of systemic LE, dermatomyositis, scleroderma, polymyositis, and rheumatoid arthritis. The presence of high titers of a unique autoantibody, anti-U1-RNP,54-57 aids in diagnosis.
Although precise prevalence data are lacking, MCTD is thought to occur in about 1 in 10,000 people.58 The disease is much more common in women, with a female-to-male ratio as high as 7 to 1, and generally occurs in the second or third decade of life.58-60
The clinical manifestations of MCTD typically evolve, with overlapping features of various autoimmune disorders appearing sequentially over several months to years.57 Early in the course of MCTD, patients commonly experience fatigue, polyarthritis, hand edema, and RP. In time, virtually every organ system may be involved. Pulmonary hypertension is a major cause of death.59,61
Unlike many other connective tissue disorders, MCTD lacks any distinct cutaneous findings. In addition to those mentioned earlier, prominent skin findings include swelling of the fingers, sclerodactyly, and the acute malar eruptions and discoid plaques typically associated with LE.56,57,62 Cutaneous manifestations associated with dermatomyositis and scleroderma may also be seen, particularly juxta-articular calcinosis. The mucous membranes may be involved, as well, resulting in nasal perforation, buccal and urogenital ulcerations, and sicca complex.58,63,64
MCTD diagnosis and treatment: Look for these serology and clinical findings
Diagnosing MCTD can be clinically challenging, as signs and symptoms of the disease commonly evolve over time. The Alarcon-Segovia criteria—largely regarded as the best diagnostic tool for MCTD65,66—include 1 serologic finding (elevated anti-U1-RNP [titer ≥1:1600]) and 5 clinical findings (RP, edema of the hands, synovitis, myositis, and acrosclerosis). Diagnosis requires the presence of the serologic criterion and ≥3 of the 5 clinical criteria.
Treatment of the cutaneous manifestations should be based on the effectiveness of therapies for similar skin findings seen in other disorders. In treating MCTD (or any other connective tissue disorder), a team that includes nurses, physical and occupational therapists, primary care physicians, and specialists in dermatology and rheumatology is essential for an optimal outcome.
CORRESPONDENCE Christian R. Halvorson, MD, Mercy Medical Center, Department of Medicine, 301 St. Paul Place, Baltimore, MD 21202; crhalvor@gmail.com
1. Renner R, Sticherling M. The different faces of cutaneous lupus erythematosus. G Ital Dermatol Venereol. 2009;144:135-147.
2. Kuhn A, Ruzicka T. Classification of cutaneous lupus erythematosus. In: Kuhn A, Lehmann P, Ruzicka T, eds. Cutaneous Lupus Erythematosus. New York: Springer; 2004:53–57.
3. Costner MI, Grau RH. Update on connective tissue diseases in dermatology. Semin Cutan Med Surg. 2006;25:207-220.
4. Ting W, Stone MS, Racila D, et al. Toxic epidermal necrolysis-like acute cutaneous lupus erythematosus and the spectrum of the acute syndrome of apoptotic pan-epidermolysis (ASAP): a case report, concept review and proposal for new classification of lupus erythematosus vesiculobullous skin lesions. Lupus. 2004;13:941-950.
5. Jorizzo JL, Carroll CL, Sangueza OP. Lupus erythematosus. In: Bolognia JL, Jorizzo JL, Rapini RP, eds. Dermatology. 2nd ed. Spain: Elsevier Limited; 2008.
6. Kuhn A, Sticherling M, Bonsmann G. Clinical manifestations of cutaneous lupus erythematosus. J Dtsch Dermatol Ges. 2007;5:1124-1137.
7. Sontheimer RD, Thomas JR, Gilliam JN. Subacute cutaneous lupus erythematosus: a cutaneous marker for a distinct lupus erythematosus subset. Arch Dermatol. 1979;115:1409-1415.
8. Sontheimer RD. Skin manifestations of systemic autoimmune connective tissue disease: diagnostics and therapeutics. Best Pract Res Clin Rheumatol. 2004;18:429-462.
9. Panjwani S. Early diagnosis and treatment of discoid lupus erythematosus. J Am Board Fam Med. 2009;22:206-213.
10. Sontheimer RD, Provost TT. Lupus erythematosus. In: Sontheimer RD, Provost TT, eds. Cutaneous Manifestations of Rheumatic Diseases. Baltimore: Williams & Wilkins; 1996.
11. Mond CB, Peterson MG, Rothfield NF. Correlation of antiro antibody with photosensitivity rash in systemic lupus erythematosus patients. Arthritis Rheum. 1989;32:202-204.
12. Jacyk WK, Damisah M. Discoid lupus erythematosus in the Nigerians. Br J Dermatol. 1979;100:131-135.
13. Callen JP. Cutaneous lupus erythematosus: A personal approach to management. Australas J Dermatol. 2006;47:13-27.
14. Gilliam JN, Sontheimer RD. Distinctive cutaneous subsets in the spectrum of lupus erythematosus. J Am Acad Dermatol. 1981;4:471-475.
15. Farley-Loftus R, Mahlberg M, Merola J, et al. Generalized discoid lupus erythematosus. Dermatol Online J. 2009;15:18.-
16. Kuhn A, Richter-Hintz D, Oslislo C, et al. Lupus erythematosus tumidus—a neglected subset of cutaneous lupus erythematosus: report of 40 cases. Arch Dermatol. 2000;136:1033-1041.
17. American College of Rheumatology. The 1982 revised criteria for classification of systemic lupus erythematosus. Available at: http://www.rheumatology.org/practice/clinical/classification/SLE/sle.asp. Accessed September 21, 2010.
18. Na SJ, Kim SM, Sunwoo IN, et al. Clinical characteristics and outcomes of juvenile and adult dermatomyositis. J Korean Med Sci. 2009;24:715-721.
19. Kovacs SO, Kovacs SC. Dermatomyositis. J Am Acad Dermatol. 1998;39:899-922.
20. Koler RA, Montemarano A. Dermatomyositis. Am Fam Physician. 2001;64:1565-1572.
21. Sunkureddi P, Nguyen-Oghalai TU, Jarvis JL, et al. Signs of dermatomyositis. Hosp Physician. 2005;41:41-44.
22. Iorizzo LJ, 3rd, Jorizzo JL. The treatment and prognosis of dermatomyositis: an updated review. J Am Acad Dermatol. 2008;59:99-112.
23. Jorizzo JL, Carroll CL, Sangueza OP. Dermatomyositis. In: Bolognia JL, Jorizzo JL, Rapini RP, eds. Dermatology. 2nd ed. Spain: Elsevier Limited; 2008:575–583.
24. Sontheimer RD. Dermatomyositis. In: Freedberg IM, Eisen AZ, Wolff K, et al, eds. Fitzpatrick’s Dermatology in General Medicine. 5th ed. New York: McGraw-Hill; 1999:2009–2020.
25. Halbert AR. Juvenile dermatomyositis. Australas J Dermatol. 1996;37:106-108.
26. Orlow SJ, Watsky KL, Bolognia JL. Skin and bones II. J Am Acad Dermatol. 1991;25:447-462.
27. Bowyer SL, Blane CE, Sullivan DB, et al. Childhood dermatomyositis: factors predicting functional outcome and development of dystrophic calcification. J Pediatr. 1983;103:882-888.
28. Bohan A, Peter JB. Polymyositis and dermatomyositis (second of two parts). N Engl J Med. 1975;292:403-407.
29. Bohan A, Peter JB. Polymyositis and dermatomyositis (first of two parts). N Engl J Med. 1975;292:344-347.
30. Vancheeswaran R, Black CM, David J, et al. Childhood-onset scleroderma: is it different from adult-onset disease? Arthritis Rheum. 1996;39:1041-1049.
31. Zulian F, Athreya BH, Laxer R, et al. Juvenile localized scleroderma: Clinical and epidemiological features in 750 children. An international study. Rheumatology (Oxford). 2006;45:614-620.
32. Marzano AV, Menni S, Parodi A, et al. Localized scleroderma in adults and children. clinical and laboratory investigations on 239 cases. Eur J Dermatol. 2003;13:171-176.
33. Uziel Y, Krafchik BR, Silverman ED, et al. Localized scleroderma in childhood: a report of 30 cases. Semin Arthritis Rheum. 1994;23:328-340.
34. Peterson LS, Nelson AM, Su WP. Classification of morphea (localized scleroderma). Mayo Clin Proc. 1995;70:1068-1076.
35. Peterson LS, Nelson AM, Su WP, et al. The epidemiology of morphea (localized scleroderma) in Olmsted County 1960-1993. J Rheumatol. 1997;24:73-80.
36. Zulian F, Vallongo C, Woo P, et al. Localized scleroderma in childhood is not just a skin disease. Arthritis Rheum. 2005;52:2873-2881.
37. Dehen L, Roujeau JC, Cosnes A, et al. Internal involvement in localized scleroderma. Medicine (Baltimore). 1994;73:241-245.
38. Ludwig RJ, Werner RJ, Winker W, et al. Chronic venous insufficiency - a potential trigger for localized scleroderma. J Eur Acad Dermatol Venereol. 2006;20:96-99.
39. Aberer E, Neumann R, Stanek G. Is localised scleroderma a borrelia infection? Lancet. 1985;2:278.-
40. Krishnan E, Furst DE. Systemic sclerosis mortality in the United States: 1979-1998. Eur J Epidemiol. 2005;20:855-861.
41. Silman AJ. Scleroderma—demographics and survival. J Rheumatol Suppl. 1997;48:58-61.
42. Medsger TA,, Jr, Masi AT. Epidemiology of systemic sclerosis (scleroderma). Ann Intern Med. 1971;74:714-721.
43. Charles C, Clements P, Furst DE. Systemic sclerosis: Hypothesis-driven treatment strategies. Lancet. 2006;367:1683-1691.
44. Maricq HR, Harper FE, Khan MM, et al. Microvascular abnormalities as possible predictors of disease subsets in Raynaud phenomenon and early connective tissue disease. Clin Exp Rheumatol. 1983;1:195-205.
45. Krieg T, Takehara K. Skin disease: A cardinal feature of systemic sclerosis. Rheumatology (Oxford). 2009;48 (suppl 3):S14-S18.
46. Ingraham KM, Steen VD. Morbidity of digital tip ulcerations in scleroderma. Arthritis Rheum. 2006;54(9 suppl.):F78.-
47. Ferri C, Valentini G, Cozzi F, et al. Systemic sclerosis: Demographic, clinical, and serologic features and survival in 1,012 italian patients. Medicine (Baltimore). 2002;81:139-153.
48. Haustein UF. Systemic sclerosis - scleroderma. Dermatol Online J. 2002;8.-
49. Fitzpatrick TB, Johnson RA, Klaus W, et al. In Colour Atlas and Synopsis of Clinical Dermatology. 4th ed. New York: McGraw-Hill; 2001:368–369.
50. Preliminary criteria for the classification of systemic sclerosis (scleroderma). subcommittee for scleroderma criteria of the American Rheumatism Association diagnostic and therapeutic criteria committee. Arthritis Rheum. 1980;23:581-590.
51. Valentini G, Paone C, La Montagna G, et al. Low-dose intravenous cyclophosphamide in systemic sclerosis: an open prospective efficacy study in patients with early diffuse disease. Scand J Rheumatol. 2006;35:35-38.
52. Steen VD, Medsger TA, Jr. Improvement in skin thickening in systemic sclerosis associated with improved survival. Arthritis Rheum. 2001;44:2828-2835.
53. Basso M, Filaci G, Cutolo M, et al. Long-term treatment of patients affected by systemic sclerosis with cyclosporin A. Ann Ital Med Int. 2001;16:233-239.
54. Morton SJ, Powell RJ. Cyclosporin and tacrolimus: their use in a routine clinical setting for scleroderma. Rheumatology (Oxford). 2000;39:865-869.
55. Kasukawa R. Mixed connective tissue disease. Intern Med. 1999;38:386-393.
56. Bennett RM, O’Connell DJ. Mixed connective tisssue disease: A clinicopathologic study of 20 cases. Semin Arthritis Rheum. 1980;10:25-51.
57. Sharp GC, Irvin WS, Tan EM, et al. Mixed connective tissue disease—an apparently distinct rheumatic disease syndrome associated with a specific antibody to an extractable nuclear antigen (ENA). Am J Med. 1972;52:148-159.
58. Venables PJ. Mixed connective tissue disease. Lupus. 2006;15:132-137.
59. Farhey Y, Hess EV. Mixed connective tissue disease. Arthritis Care Res. 1997;10:333-342.
60. Alarcon-Segovia D. Mixed connective tissue disease - a decade of growing pains. J Rheumatol. 1981;8:535-540.
61. Ueda N, Mimura K, Maeda H, et al. Mixed connective tissue disease with fatal pulmonary hypertension and a review of literature. Virchows Arch A Pathol Anat Histopathol. 1984;404:335-340.
62. Bennett RM. Mixed connective tissue disease and other overlap syndromes. In: Kelley WN, Harris EDJ, Ruddy SH, et al, eds. Textbook of Rheumatology. 4th ed. Philadelphia: WB Saunders; 1993:1061.
63. Nakashima M, Suzuki K, Okada M, et al. Panniculitis in a patient with mixed connective tissue disease. Mod Rheumatol. 2004;14:250-253.
64. Magro CM, Crowson AN, Regauer S. Mixed connective tissue disease. A clinical, histologic, and immunofluorescence study of eight cases. Am J Dermatopathol. 1997;19:206-213.
65. Amigues JM, Cantagrel A, Abbal M, et al. Comparative study of 4 diagnosis criteria sets for mixed connective tissue disease in patients with anti-RNP antibodies. autoimmunity group of the hospitals of toulouse. J Rheumatol. 1996;23:2055-2062.
66. Alarcon-Segovia D, Cardiel MH. Comparison between 3 diagnostic criteria for mixed connective tissue disease. study of 593 patients. J Rheumatol. 1989;16:328-334.
• When evaluating patients with suspected cutaneous lupus erythematosus, use multiple criteria—including histologic and immunofluorescent biopsy findings and American College of Rheumatology criteria—to rule out systemic disease. C
• Cancer screening with a careful history and physical examination is recommended for all adult patients whom you suspect of having dermatomyositis. C
• Suspect mixed connective tissue disease in patients with skin findings characteristic of varying auto-immune disorders appearing sequentially over several months or years. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Many systemic conditions are accompanied by skin manifestations. This is especially true for connective tissue disorders, for which dermatologic findings are often the key to diagnosis.
In this review, we describe the dermatologic findings of some well-known connective tissue disorders. The text and photographs in the pages that follow will help you hone your diagnostic skills, leading to earlier treatment and, possibly, better outcomes.
Lupus erythematosus: Cutaneous and systemic disease often overlap
Lupus erythematosus (LE), a chronic, inflammatory autoimmune condition that primarily affects women in their 20s and 30s, may initially present as a systemic disease or in a purely cutaneous form. However, most patients with systemic LE have some skin manifestations, and those with cutaneous LE often have—or subsequently develop—systemic involvement.1 Thus, recognizing the cutaneous manifestations of LE will not only aid in diagnosis, but will help you identify patients at risk for systemic disease.
Cutaneous LE has 4 subtypes
There are 4 subcategories of cutaneous LE—acute, subacute, chronic, and intermittent.2 Each is differentiated by the appearance of the lesions (TABLE 1), histology, and serological markers.1 Photosensitivity is common to all the subcategories to varying degrees.
Acute cutaneous lupus erythematosus (ACLE) is typically characterized by the classic malar “butterfly” rash, an erythematous eruption of macules or edematous papules over the bridge of the nose and cheek.3 Although this presentation is most common, there are variations—1 in which the lesions cover other exposed areas (commonly including the “V” of the chest, the extensor surface of the arms, and the hands), and a rare form in which toxic epidermal necrolysis-like blistering occurs.1,4 These skin changes—which generally last anywhere from a few hours to several weeks—typically resolve without scarring, although pigment changes can occur.5
Left: Dermatomyositis is the underlying cause of the heliotrope discoloration on this patient’s upper eyelid.
Center: Linear morphea is associated with the lesion on this patient’s face—called en coup de sabre because it resembles the mark caused by the stroke of a sword in a duel.
Right: Discoid lupus erythematosus causes hypopigmentation and scarring.Patients with ACLE have a predisposition to systemic LE; unlike those with other forms of cutaneous LE, 40% to 90% will have double-stranded DNA (dsDNA) autoantibodies.3,6
Subacute cutaneous lupus erythematosus (SCLE), which usually affects middle-aged Caucasian women, is characterized by erythematous papulosquamous (psoriasis-like) eruptions or annular lesions with raised red borders and central clearing—or both. These lesions, which are nonscarring, lack in-duration, and rarely affect the scalp or face, appear suddenly, usually after exposure to sunlight (FIGURE 1)5,7-9 or certain drugs. Hydrochlorothiazide, terbinafine, calcium channel blockers, and angiotensin-converting enzyme inhibitors are common offenders.1,6
FIGURE 1
Annular lesions in subacute cutaneous lupus erythematosus
Ring-like lesions with raised red borders and central clearing on the back of a patient with subacute cutaneous lupus erythematosus.
SCLE is often associated with extracutaneous symptoms such as arthritis and myalgias,1,8 but patients are at relatively low risk for severe systemic manifestations.5,8,10 Serology is often notable, with anti-Ro (SS-A) antibodies present in 70% to 90% of patients and anti-La (SS-B) autoantibodies found in 30% to 50%.1,11
Chronic cutaneous lupus erythematosus (CCLE) also occurs predominantly in females, at a ratio as high as 5 to 1.12 There are 3 variations of CCLE: discoid lupus erythematosus (DLE), LE profundus, and chilblain LE (TABLE 1). DLE, characterized by alopecia, skin atrophy, and dyspigmentation, is the most common and affects patients of all ages and ethnic groups.9,13,14 (See image above.)
DLE lesions typically begin as erythematous papules and plaques with scale. As the disorder progresses, the lesions spread, causing follicular plugging, peripheral hyperpigmentation and central hypopigmentation, telangiectasia, and atrophy.9,15 In some cases, patients develop thickened, scarred skin and permanent scarring alopecia.15 Prompt recognition of DLE is particularly important, as early referral and treatment may reduce the likelihood of permanent scarring alopecia and pigment changes.11
Intermittent cutaneous lupus erythematosus (ICLE), a relatively new subtype of cutaneous LE, is represented by a rare condition—lupus erythematosus tumidus (LET)—reported in <100 cases worldwide. LET is characterized by succulent, erythematous, and edematous plaques on sun-exposed parts of the body.1,16
TABLE 1
Cutaneous lupus erythematosus: Recognizing the subtypes1
| Acute cutaneous lupus erythematosus (ACLE) |
|---|
| Localized: erythematous macules or papules over the bridge of the nose and cheek, with sparing of the nasolabial folds (“butterfly rash”) |
| Generalized: similar erythematous lesions over other photodistributed parts of the body, including the neck, chest, arms, and hands |
| Toxic epidermal necrolysis-like (TEN-like): blistering and epidermal cleavage in photodistributed parts of the body |
| Subacute cutaneous lupus erythematosus (SCLE) |
| Erythematous papulosquamous (psoriasis-like) eruptions or annular (ring-like) lesions with raised red borders and central clearing, occurring symmetrically and suddenly after sunlight exposure on photo-distributed body parts; the scalp and face are rarely affected |
| Chronic cutaneous lupus erythematosus (CCLE) |
| Discoid lupus erythematosus: erythematous papules and plaques with associated scale, spreading centrifugally with follicular plugging, pigment change, telangiectasia, and atrophy; scarring alopecia can occur |
| Lupus erythematosus profundus: tender, erythematous nodules and plaques, usually involving the proximal extremities, trunk, breasts, buttocks, and face |
| Chilblain lupus erythematosus: tender, erythematous nodules and plaques, occurring in acral areas often in response to cold |
| Intermittent cutaneous lupus erythematosus (ICLE) |
| Lupus erythematosus tumidus: succulent, erythematous, and edematous plaques found on photodistributed parts of the body |
Cutaneous LE diagnosis and treatment: Start with ACR criteria
When evaluating patients with suspected cutaneous LE, it is important not only to identify the subtype, but also to rule out systemic disease using criteria established by the American College of Rheumatology (ACR).17 Notably, 4 of the 11 diagnostic criteria for systemic disease involve visual clues, including malar rash, discoid rash, photosensitivity, and oral ulcerations. Laboratory evidence of systemic disease may include a positive antinuclear antibody, anti-dsDNA, or anti-Sm autoantibody test results, as well as hematologic abnormalities described in the ACR guidelines.
Ultimately, a diagnosis of cutaneous LE should be based on the patient’s history and physical exam, autoantibody profile, and histologic and immunofluorescent biopsy findings. A rheumatologic evaluation may help to determine which patients have systemic disease, as the ACR criteria may overdiagnose systemic LE in those with predominantly skin changes.6
Treatment of cutaneous LE is based on the subtype and extent of disease, with potent topical corticosteroids, in combination with antimalarial agents, being the primary therapies. ACLE skin lesions generally respond best to systemic corticosteroids and immunosuppressive agents (such as azathioprine or cyclophosphamide) that are used to control underlying systemic disease. SCLE can be managed with topical corticosteroids; however, patients typically also require systemic treatment, often with hydroxychloroquine, for optimal control. DLE, the most common form of CCLE, is managed in a similar fashion, with a greater role for intralesional corticosteroid injections. Lesions associated with ICLE, which often resolve spontaneously, may be treated with topical corticosteroids and antimalarials.16 It is also important to advise all patients with cutaneous LE to use a broad-spectrum sunscreen, as ultraviolet (UV) exposure can induce or exacerbate the lesions.9
Dermatomyositis: Rare but serious
Dermatomyositis is an idiopathic inflammatory myopathy characterized by chronic muscle inflammation, symmetric proximal muscle weakness, and distinct cutaneous findings. In addition to possible cardiac and pulmonary complications, dysphagia, and joint contractures, dermatomyositis is associated with cancer, with up to 25% of affected adults having an underlying occult malignancy.18
Dermatomyositis is a rare condition, with a prevalence of only 1 to 10 cases per million adults and 3.2 cases per million children.19 It has a bimodal age distribution, with most juvenile cases affecting children between the ages of 5 and 14 years and most adult cases developing in the fifth and sixth decades of life. Women are affected twice as often as men.18
Suspect dermatomyositis when you see any of the following signs:
- A heliotrope rash: violaceous macules and patches, with or without edema, symmetrically on the periorbital skin, present early in the disease course in 30% to 60% of patients.19 (See image)
- Gottron’s papules: violaceous papules on the dorsal interphalangeal and metacarpophalangeal joints of the hands, elbows, and knees, occurring in as many as 70% of patients (FIGURE 2).19,20
- Gottron’s sign: nonscaling, violaceous erythematous macules and plaques occurring symmetrically in the same distribution as Gottron’s papules, but with sparing of the interphalangeal spaces.20
- Periungual erythema and telangiectasias: redness and dilation of the blood vessels in the skin surrounding the nail plate.
- The shawl and V-signs: erythematous macular eruptions occurring in a “shawl” pattern on the shoulders, arms, and upper back and in a V-shaped pattern on the anterior neck and chest.
- Mechanic’s hand: extensive scaling, fissuring, and roughening of the palmar aspect of the hand.20,21
- Poikiloderma vasculare atrophicans: circumscribed violaceous erythema with thinning of the skin, prominent telangiectasias, and a mottled pattern of hypo- and hyperpigmentation, typically occurring on the anterior neck, chest, posterior shoulders, back, and buttocks, years after onset of the disease.21-24
- Cutaneous calcification (calcinosis cutis): usually on the buttocks, elbows, knees, and traumatized areas, affecting 30% to 70% of children with dermatomyositis, but only 10% of adults with the disorder.20,25-27
FIGURE 2
Gottron’s papules in dermatomyositis
Violaceous papules on the dorsal interphalangeal and metacarpophalangeal joints of the hands are a common manifestation of dermatomyositis.
Bohan and Peter criteria help with dermatomyositis diagnosis
A diagnosis of dermatomyositis can be established using the following criteria developed by Bohan and Peter in 1975: (1) symmetric muscle weakness; (2) elevation of muscle enzymes, most notably creatinine phosphokinase; (3) evidence of inflammation on muscle biopsy; (4) electromyographic features of myositis; and (5) characteristic dermatologic signs, including a heliotrope rash and Gottron’s papules.28,29 A definitive diagnosis can be made when the patient meets 3 of the first 4 criteria, as well as the fifth.
Corticosteroids are the mainstay of treatment for dermatomyositis, although the dose and duration are subject to debate. Cutaneous manifestations of dermatomyositis are commonly treated with topical corticosteroids and oral hydroxychloroquine, as well as emollients and antipruritic agents.
Cancer screening. All adults with signs and symptoms of dermatomyositis should undergo cancer screening. The most common malignancies are ovarian cancer, gastric cancer, and lymphoma.21
Photoprotection. As with cutaneous LE, UV exposure can exacerbate dermatomyositis, and patients should use a broad-spectrum sunscreen.
Scleroderma: Localized and systemic disease
The major cutaneous manifestation of scleroderma is that of thickened, leathery, bound-down skin, seen in both localized and systemic disease. In both cases, the lesions typically evolve through 3 characteristic stages: the initial inflammatory and edematous stage; a fibrotic stage, during which the skin lesions appear hard, tight, and hidebound; and the final, atrophic stage. However, not all lesions progress to this final stage.8
Localized scleroderma (also known as morphea) affects roughly 1 in 100,000 people. It is more prevalent in females than males, with a ratio as high as 3 to 1.30-33 Cutaneous findings vary, and numerous clinical presentations are possible. The most widely used classification system, the Mayo Clinic Classification, recognizes 5 subtypes: plaque morphea (the most common), generalized morphea, linear morphea, deep morphea, and bullous morphea (TABLE 2).34 The mean age of onset depends on the subtype, and ranges from 12 years for linear morphea to 45 years for deep morphea.35
While systemic involvement is not common with localized scleroderma, extracutaneous manifestations have been reported in up to 25% of cases.31,36,37 In a 2005 multicenter study of 750 pediatric patients with localized scleroderma, the most frequently reported extracutaneous symptom was arthritis, found in 12% of patients.36 Less frequent findings included neurologic symptoms such as seizures and headaches, vascular changes such as deep vein thrombosis, and gastrointestinal, cardiac, and renal conditions. Although the precise etiology of localized scleroderma is unknown, it is thought to be associated with trauma, prior infection by Borrelia burgdorferi, chronic venous insufficiency, and irradiation for breast cancer.30,38,39
TABLE 2
Localized scleroderma: What to look for8,34
|
Systemic scleroderma is a heterogenous disorder
Commonly called systemic sclerosis (SSc), systemic scleroderma is characterized by proliferative vascular lesions; fibrosis of internal organs, including the lungs, heart, kidneys, and gastrointestinal tract; and distinct cutaneous manifestations.3 SSc affects about 240 people per million adults, mostly between the ages of 30 and 50 years, with women affected 3 times as often as men.40-42
The cause of SSc is unknown, although a genetic predisposition is likely. Environmental factors likely play a role in triggering the disease, with possible causative factors including cytomegalovirus and other viral infections and exposure to certain chemicals.43
As in localized scleroderma, cutaneous manifestations are a prominent part of SSc and typically develop early on. Raynaud’s phenomenon (RP) (FIGURE 3), which occurs in 90% to 98% of patients with SSc,44 can develop in association with other cutaneous findings or precede additional skin changes by months or years.
Other early skin changes include nonpitting edema of the fingers and toes, which creates a sausage-like appearance. This is typically followed by hardening and thickening of the skin in these areas, which can result in highly disabling sclerodactyly (FIGURE 4).45 Patients can also develop painful ulcerations on their fingertips and knuckles (rat bite necroses) due to local ischemia and vascular insufficiency. This may be complicated by secondary bacterial infection, gangrene, and acroosteolysis, leading to articular deformities and dissolution of terminal phalanges (FIGURE 5).45-48
On the face, SSc is characterized early on by periorbital edema and later by the development of a beaked nose, a reduction in the size of the mouth (microstomia) with radial furrowing, thinning of the lips, and telangiectasias.45,49 As the sclerosis worsens, patients are frequently left with expressionless, mask-like faces.48
Other common cutaneous manifestations of SSc include a “salt and pepper” appearance, with alternating areas of hypo- and hyperpigmented skin. Additionally, patients may suffer from a loss of hair follicles, severely dry skin, and pruritus.45,48
There are 2 major subsets of SSc—limited cutaneous SSc and diffuse cutaneous SSc. While they can be differentiated based on the history of symptoms, the appearance and extent of cutaneous involvement, and certain serological makers, the most important difference is the speed at which the disease progresses and its severity: Limited cutaneous SSc typically progresses slowly, while diffuse cutaneous SSc is characterized by a relatively rapid onset of disease, with skin and internal organ involvement likely to be severe.49
Scleroderma diagnosis and treatment: Vascular changes are an important clue
Skin changes seen in localized scleroderma and SSc can be clinically and histologically similar, making it difficult to arrive at a definitive diagnosis.
One clinical clue is that in localized scleroderma, vascular changes, such as RP and periungual nailfold telangiectasia, are typically absent. In addition, cutaneous changes in the hands and fingers, such as sclerodactyly, are more characteristic of systemic disease.10
SSc can be diagnosed using criteria proposed by the ACR, which have been shown to be highly sensitive (97%) and specific (98%).50 The major criterion is proximal scleroderma—symmetric thickening, tightening, and induration of the skin of the fingers and areas proximal to the metacarpophalangeal or metatarsophalangeal joints, which may include the trunk, neck, and face. Minor criteria include (1) sclerodactyly, (2) digital pitting scars of fingertips or loss of substance of the distal finger pad, and (3) bilateral basilar pulmonary fibrosis. Diagnosis is based on the presence of either the major criterion or 2 of the 3 minor criteria.50
Numerous therapies are available for localized scleroderma, including topical, intralesional, and systemic corticosteroids, topical tacrolimus, hydroxychloroquine, topical and systemic calcipotriol, penicillamine, sulfasalazine, interferon-[H9253], methotrexate, phototherapy with UV light, and imiquimod.
SSc skin manifestations, which can be severe and disabling, can be treated with a wide range of therapies. Topical or systemic corticosteroids; topical calcineurin inhibitors; systemic immunosuppressive agents such as methotrexate, cyclophosphamide, cyclosporine, and D-penicillamine; and phototherapy have all had varying success at reducing hardening of the skin.51-54 Other skin manifestations, including RP, dryness and itching, pigment changes, digital ulcerations, calcifications, and telangiectasias, should be managed as needed, with various supplemental treatment options available.45 These may include emollients, antihistamines, and topical corticosteroids for dryness and itching; laser therapy for telangiectasias; and corticosteroid injection, laser therapy, or surgery for calcifications. However, there is limited evidence of efficacy for most of these options.45
FIGURE 3
An attack of Raynaud’s phenomenon
Raynaud’s phenomenon, characterized by blanching of the distal fingertips, is shown here in a patient with systemic sclerosis.
FIGURE 4
Sclerodactyly in a patient with systemic sclerosis
Hardening and thickening of the skin can result in highly disabling sclerodactyly in patients with systemic sclerosis.
FIGURE 5
Dissolution of terminal phalanges
Bony resorption and ulceration have led to the loss of distal phalanges in this patient with systemic sclerosis.
Mixed connective tissue disease has features of several disorders
Mixed connective tissue disease (MCTD) is an apparently distinct rheumatologic condition characterized by a combination of clinical features of systemic LE, dermatomyositis, scleroderma, polymyositis, and rheumatoid arthritis. The presence of high titers of a unique autoantibody, anti-U1-RNP,54-57 aids in diagnosis.
Although precise prevalence data are lacking, MCTD is thought to occur in about 1 in 10,000 people.58 The disease is much more common in women, with a female-to-male ratio as high as 7 to 1, and generally occurs in the second or third decade of life.58-60
The clinical manifestations of MCTD typically evolve, with overlapping features of various autoimmune disorders appearing sequentially over several months to years.57 Early in the course of MCTD, patients commonly experience fatigue, polyarthritis, hand edema, and RP. In time, virtually every organ system may be involved. Pulmonary hypertension is a major cause of death.59,61
Unlike many other connective tissue disorders, MCTD lacks any distinct cutaneous findings. In addition to those mentioned earlier, prominent skin findings include swelling of the fingers, sclerodactyly, and the acute malar eruptions and discoid plaques typically associated with LE.56,57,62 Cutaneous manifestations associated with dermatomyositis and scleroderma may also be seen, particularly juxta-articular calcinosis. The mucous membranes may be involved, as well, resulting in nasal perforation, buccal and urogenital ulcerations, and sicca complex.58,63,64
MCTD diagnosis and treatment: Look for these serology and clinical findings
Diagnosing MCTD can be clinically challenging, as signs and symptoms of the disease commonly evolve over time. The Alarcon-Segovia criteria—largely regarded as the best diagnostic tool for MCTD65,66—include 1 serologic finding (elevated anti-U1-RNP [titer ≥1:1600]) and 5 clinical findings (RP, edema of the hands, synovitis, myositis, and acrosclerosis). Diagnosis requires the presence of the serologic criterion and ≥3 of the 5 clinical criteria.
Treatment of the cutaneous manifestations should be based on the effectiveness of therapies for similar skin findings seen in other disorders. In treating MCTD (or any other connective tissue disorder), a team that includes nurses, physical and occupational therapists, primary care physicians, and specialists in dermatology and rheumatology is essential for an optimal outcome.
CORRESPONDENCE Christian R. Halvorson, MD, Mercy Medical Center, Department of Medicine, 301 St. Paul Place, Baltimore, MD 21202; crhalvor@gmail.com
• When evaluating patients with suspected cutaneous lupus erythematosus, use multiple criteria—including histologic and immunofluorescent biopsy findings and American College of Rheumatology criteria—to rule out systemic disease. C
• Cancer screening with a careful history and physical examination is recommended for all adult patients whom you suspect of having dermatomyositis. C
• Suspect mixed connective tissue disease in patients with skin findings characteristic of varying auto-immune disorders appearing sequentially over several months or years. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Many systemic conditions are accompanied by skin manifestations. This is especially true for connective tissue disorders, for which dermatologic findings are often the key to diagnosis.
In this review, we describe the dermatologic findings of some well-known connective tissue disorders. The text and photographs in the pages that follow will help you hone your diagnostic skills, leading to earlier treatment and, possibly, better outcomes.
Lupus erythematosus: Cutaneous and systemic disease often overlap
Lupus erythematosus (LE), a chronic, inflammatory autoimmune condition that primarily affects women in their 20s and 30s, may initially present as a systemic disease or in a purely cutaneous form. However, most patients with systemic LE have some skin manifestations, and those with cutaneous LE often have—or subsequently develop—systemic involvement.1 Thus, recognizing the cutaneous manifestations of LE will not only aid in diagnosis, but will help you identify patients at risk for systemic disease.
Cutaneous LE has 4 subtypes
There are 4 subcategories of cutaneous LE—acute, subacute, chronic, and intermittent.2 Each is differentiated by the appearance of the lesions (TABLE 1), histology, and serological markers.1 Photosensitivity is common to all the subcategories to varying degrees.
Acute cutaneous lupus erythematosus (ACLE) is typically characterized by the classic malar “butterfly” rash, an erythematous eruption of macules or edematous papules over the bridge of the nose and cheek.3 Although this presentation is most common, there are variations—1 in which the lesions cover other exposed areas (commonly including the “V” of the chest, the extensor surface of the arms, and the hands), and a rare form in which toxic epidermal necrolysis-like blistering occurs.1,4 These skin changes—which generally last anywhere from a few hours to several weeks—typically resolve without scarring, although pigment changes can occur.5
Left: Dermatomyositis is the underlying cause of the heliotrope discoloration on this patient’s upper eyelid.
Center: Linear morphea is associated with the lesion on this patient’s face—called en coup de sabre because it resembles the mark caused by the stroke of a sword in a duel.
Right: Discoid lupus erythematosus causes hypopigmentation and scarring.Patients with ACLE have a predisposition to systemic LE; unlike those with other forms of cutaneous LE, 40% to 90% will have double-stranded DNA (dsDNA) autoantibodies.3,6
Subacute cutaneous lupus erythematosus (SCLE), which usually affects middle-aged Caucasian women, is characterized by erythematous papulosquamous (psoriasis-like) eruptions or annular lesions with raised red borders and central clearing—or both. These lesions, which are nonscarring, lack in-duration, and rarely affect the scalp or face, appear suddenly, usually after exposure to sunlight (FIGURE 1)5,7-9 or certain drugs. Hydrochlorothiazide, terbinafine, calcium channel blockers, and angiotensin-converting enzyme inhibitors are common offenders.1,6
FIGURE 1
Annular lesions in subacute cutaneous lupus erythematosus
Ring-like lesions with raised red borders and central clearing on the back of a patient with subacute cutaneous lupus erythematosus.
SCLE is often associated with extracutaneous symptoms such as arthritis and myalgias,1,8 but patients are at relatively low risk for severe systemic manifestations.5,8,10 Serology is often notable, with anti-Ro (SS-A) antibodies present in 70% to 90% of patients and anti-La (SS-B) autoantibodies found in 30% to 50%.1,11
Chronic cutaneous lupus erythematosus (CCLE) also occurs predominantly in females, at a ratio as high as 5 to 1.12 There are 3 variations of CCLE: discoid lupus erythematosus (DLE), LE profundus, and chilblain LE (TABLE 1). DLE, characterized by alopecia, skin atrophy, and dyspigmentation, is the most common and affects patients of all ages and ethnic groups.9,13,14 (See image above.)
DLE lesions typically begin as erythematous papules and plaques with scale. As the disorder progresses, the lesions spread, causing follicular plugging, peripheral hyperpigmentation and central hypopigmentation, telangiectasia, and atrophy.9,15 In some cases, patients develop thickened, scarred skin and permanent scarring alopecia.15 Prompt recognition of DLE is particularly important, as early referral and treatment may reduce the likelihood of permanent scarring alopecia and pigment changes.11
Intermittent cutaneous lupus erythematosus (ICLE), a relatively new subtype of cutaneous LE, is represented by a rare condition—lupus erythematosus tumidus (LET)—reported in <100 cases worldwide. LET is characterized by succulent, erythematous, and edematous plaques on sun-exposed parts of the body.1,16
TABLE 1
Cutaneous lupus erythematosus: Recognizing the subtypes1
| Acute cutaneous lupus erythematosus (ACLE) |
|---|
| Localized: erythematous macules or papules over the bridge of the nose and cheek, with sparing of the nasolabial folds (“butterfly rash”) |
| Generalized: similar erythematous lesions over other photodistributed parts of the body, including the neck, chest, arms, and hands |
| Toxic epidermal necrolysis-like (TEN-like): blistering and epidermal cleavage in photodistributed parts of the body |
| Subacute cutaneous lupus erythematosus (SCLE) |
| Erythematous papulosquamous (psoriasis-like) eruptions or annular (ring-like) lesions with raised red borders and central clearing, occurring symmetrically and suddenly after sunlight exposure on photo-distributed body parts; the scalp and face are rarely affected |
| Chronic cutaneous lupus erythematosus (CCLE) |
| Discoid lupus erythematosus: erythematous papules and plaques with associated scale, spreading centrifugally with follicular plugging, pigment change, telangiectasia, and atrophy; scarring alopecia can occur |
| Lupus erythematosus profundus: tender, erythematous nodules and plaques, usually involving the proximal extremities, trunk, breasts, buttocks, and face |
| Chilblain lupus erythematosus: tender, erythematous nodules and plaques, occurring in acral areas often in response to cold |
| Intermittent cutaneous lupus erythematosus (ICLE) |
| Lupus erythematosus tumidus: succulent, erythematous, and edematous plaques found on photodistributed parts of the body |
Cutaneous LE diagnosis and treatment: Start with ACR criteria
When evaluating patients with suspected cutaneous LE, it is important not only to identify the subtype, but also to rule out systemic disease using criteria established by the American College of Rheumatology (ACR).17 Notably, 4 of the 11 diagnostic criteria for systemic disease involve visual clues, including malar rash, discoid rash, photosensitivity, and oral ulcerations. Laboratory evidence of systemic disease may include a positive antinuclear antibody, anti-dsDNA, or anti-Sm autoantibody test results, as well as hematologic abnormalities described in the ACR guidelines.
Ultimately, a diagnosis of cutaneous LE should be based on the patient’s history and physical exam, autoantibody profile, and histologic and immunofluorescent biopsy findings. A rheumatologic evaluation may help to determine which patients have systemic disease, as the ACR criteria may overdiagnose systemic LE in those with predominantly skin changes.6
Treatment of cutaneous LE is based on the subtype and extent of disease, with potent topical corticosteroids, in combination with antimalarial agents, being the primary therapies. ACLE skin lesions generally respond best to systemic corticosteroids and immunosuppressive agents (such as azathioprine or cyclophosphamide) that are used to control underlying systemic disease. SCLE can be managed with topical corticosteroids; however, patients typically also require systemic treatment, often with hydroxychloroquine, for optimal control. DLE, the most common form of CCLE, is managed in a similar fashion, with a greater role for intralesional corticosteroid injections. Lesions associated with ICLE, which often resolve spontaneously, may be treated with topical corticosteroids and antimalarials.16 It is also important to advise all patients with cutaneous LE to use a broad-spectrum sunscreen, as ultraviolet (UV) exposure can induce or exacerbate the lesions.9
Dermatomyositis: Rare but serious
Dermatomyositis is an idiopathic inflammatory myopathy characterized by chronic muscle inflammation, symmetric proximal muscle weakness, and distinct cutaneous findings. In addition to possible cardiac and pulmonary complications, dysphagia, and joint contractures, dermatomyositis is associated with cancer, with up to 25% of affected adults having an underlying occult malignancy.18
Dermatomyositis is a rare condition, with a prevalence of only 1 to 10 cases per million adults and 3.2 cases per million children.19 It has a bimodal age distribution, with most juvenile cases affecting children between the ages of 5 and 14 years and most adult cases developing in the fifth and sixth decades of life. Women are affected twice as often as men.18
Suspect dermatomyositis when you see any of the following signs:
- A heliotrope rash: violaceous macules and patches, with or without edema, symmetrically on the periorbital skin, present early in the disease course in 30% to 60% of patients.19 (See image)
- Gottron’s papules: violaceous papules on the dorsal interphalangeal and metacarpophalangeal joints of the hands, elbows, and knees, occurring in as many as 70% of patients (FIGURE 2).19,20
- Gottron’s sign: nonscaling, violaceous erythematous macules and plaques occurring symmetrically in the same distribution as Gottron’s papules, but with sparing of the interphalangeal spaces.20
- Periungual erythema and telangiectasias: redness and dilation of the blood vessels in the skin surrounding the nail plate.
- The shawl and V-signs: erythematous macular eruptions occurring in a “shawl” pattern on the shoulders, arms, and upper back and in a V-shaped pattern on the anterior neck and chest.
- Mechanic’s hand: extensive scaling, fissuring, and roughening of the palmar aspect of the hand.20,21
- Poikiloderma vasculare atrophicans: circumscribed violaceous erythema with thinning of the skin, prominent telangiectasias, and a mottled pattern of hypo- and hyperpigmentation, typically occurring on the anterior neck, chest, posterior shoulders, back, and buttocks, years after onset of the disease.21-24
- Cutaneous calcification (calcinosis cutis): usually on the buttocks, elbows, knees, and traumatized areas, affecting 30% to 70% of children with dermatomyositis, but only 10% of adults with the disorder.20,25-27
FIGURE 2
Gottron’s papules in dermatomyositis
Violaceous papules on the dorsal interphalangeal and metacarpophalangeal joints of the hands are a common manifestation of dermatomyositis.
Bohan and Peter criteria help with dermatomyositis diagnosis
A diagnosis of dermatomyositis can be established using the following criteria developed by Bohan and Peter in 1975: (1) symmetric muscle weakness; (2) elevation of muscle enzymes, most notably creatinine phosphokinase; (3) evidence of inflammation on muscle biopsy; (4) electromyographic features of myositis; and (5) characteristic dermatologic signs, including a heliotrope rash and Gottron’s papules.28,29 A definitive diagnosis can be made when the patient meets 3 of the first 4 criteria, as well as the fifth.
Corticosteroids are the mainstay of treatment for dermatomyositis, although the dose and duration are subject to debate. Cutaneous manifestations of dermatomyositis are commonly treated with topical corticosteroids and oral hydroxychloroquine, as well as emollients and antipruritic agents.
Cancer screening. All adults with signs and symptoms of dermatomyositis should undergo cancer screening. The most common malignancies are ovarian cancer, gastric cancer, and lymphoma.21
Photoprotection. As with cutaneous LE, UV exposure can exacerbate dermatomyositis, and patients should use a broad-spectrum sunscreen.
Scleroderma: Localized and systemic disease
The major cutaneous manifestation of scleroderma is that of thickened, leathery, bound-down skin, seen in both localized and systemic disease. In both cases, the lesions typically evolve through 3 characteristic stages: the initial inflammatory and edematous stage; a fibrotic stage, during which the skin lesions appear hard, tight, and hidebound; and the final, atrophic stage. However, not all lesions progress to this final stage.8
Localized scleroderma (also known as morphea) affects roughly 1 in 100,000 people. It is more prevalent in females than males, with a ratio as high as 3 to 1.30-33 Cutaneous findings vary, and numerous clinical presentations are possible. The most widely used classification system, the Mayo Clinic Classification, recognizes 5 subtypes: plaque morphea (the most common), generalized morphea, linear morphea, deep morphea, and bullous morphea (TABLE 2).34 The mean age of onset depends on the subtype, and ranges from 12 years for linear morphea to 45 years for deep morphea.35
While systemic involvement is not common with localized scleroderma, extracutaneous manifestations have been reported in up to 25% of cases.31,36,37 In a 2005 multicenter study of 750 pediatric patients with localized scleroderma, the most frequently reported extracutaneous symptom was arthritis, found in 12% of patients.36 Less frequent findings included neurologic symptoms such as seizures and headaches, vascular changes such as deep vein thrombosis, and gastrointestinal, cardiac, and renal conditions. Although the precise etiology of localized scleroderma is unknown, it is thought to be associated with trauma, prior infection by Borrelia burgdorferi, chronic venous insufficiency, and irradiation for breast cancer.30,38,39
TABLE 2
Localized scleroderma: What to look for8,34
|
Systemic scleroderma is a heterogenous disorder
Commonly called systemic sclerosis (SSc), systemic scleroderma is characterized by proliferative vascular lesions; fibrosis of internal organs, including the lungs, heart, kidneys, and gastrointestinal tract; and distinct cutaneous manifestations.3 SSc affects about 240 people per million adults, mostly between the ages of 30 and 50 years, with women affected 3 times as often as men.40-42
The cause of SSc is unknown, although a genetic predisposition is likely. Environmental factors likely play a role in triggering the disease, with possible causative factors including cytomegalovirus and other viral infections and exposure to certain chemicals.43
As in localized scleroderma, cutaneous manifestations are a prominent part of SSc and typically develop early on. Raynaud’s phenomenon (RP) (FIGURE 3), which occurs in 90% to 98% of patients with SSc,44 can develop in association with other cutaneous findings or precede additional skin changes by months or years.
Other early skin changes include nonpitting edema of the fingers and toes, which creates a sausage-like appearance. This is typically followed by hardening and thickening of the skin in these areas, which can result in highly disabling sclerodactyly (FIGURE 4).45 Patients can also develop painful ulcerations on their fingertips and knuckles (rat bite necroses) due to local ischemia and vascular insufficiency. This may be complicated by secondary bacterial infection, gangrene, and acroosteolysis, leading to articular deformities and dissolution of terminal phalanges (FIGURE 5).45-48
On the face, SSc is characterized early on by periorbital edema and later by the development of a beaked nose, a reduction in the size of the mouth (microstomia) with radial furrowing, thinning of the lips, and telangiectasias.45,49 As the sclerosis worsens, patients are frequently left with expressionless, mask-like faces.48
Other common cutaneous manifestations of SSc include a “salt and pepper” appearance, with alternating areas of hypo- and hyperpigmented skin. Additionally, patients may suffer from a loss of hair follicles, severely dry skin, and pruritus.45,48
There are 2 major subsets of SSc—limited cutaneous SSc and diffuse cutaneous SSc. While they can be differentiated based on the history of symptoms, the appearance and extent of cutaneous involvement, and certain serological makers, the most important difference is the speed at which the disease progresses and its severity: Limited cutaneous SSc typically progresses slowly, while diffuse cutaneous SSc is characterized by a relatively rapid onset of disease, with skin and internal organ involvement likely to be severe.49
Scleroderma diagnosis and treatment: Vascular changes are an important clue
Skin changes seen in localized scleroderma and SSc can be clinically and histologically similar, making it difficult to arrive at a definitive diagnosis.
One clinical clue is that in localized scleroderma, vascular changes, such as RP and periungual nailfold telangiectasia, are typically absent. In addition, cutaneous changes in the hands and fingers, such as sclerodactyly, are more characteristic of systemic disease.10
SSc can be diagnosed using criteria proposed by the ACR, which have been shown to be highly sensitive (97%) and specific (98%).50 The major criterion is proximal scleroderma—symmetric thickening, tightening, and induration of the skin of the fingers and areas proximal to the metacarpophalangeal or metatarsophalangeal joints, which may include the trunk, neck, and face. Minor criteria include (1) sclerodactyly, (2) digital pitting scars of fingertips or loss of substance of the distal finger pad, and (3) bilateral basilar pulmonary fibrosis. Diagnosis is based on the presence of either the major criterion or 2 of the 3 minor criteria.50
Numerous therapies are available for localized scleroderma, including topical, intralesional, and systemic corticosteroids, topical tacrolimus, hydroxychloroquine, topical and systemic calcipotriol, penicillamine, sulfasalazine, interferon-[H9253], methotrexate, phototherapy with UV light, and imiquimod.
SSc skin manifestations, which can be severe and disabling, can be treated with a wide range of therapies. Topical or systemic corticosteroids; topical calcineurin inhibitors; systemic immunosuppressive agents such as methotrexate, cyclophosphamide, cyclosporine, and D-penicillamine; and phototherapy have all had varying success at reducing hardening of the skin.51-54 Other skin manifestations, including RP, dryness and itching, pigment changes, digital ulcerations, calcifications, and telangiectasias, should be managed as needed, with various supplemental treatment options available.45 These may include emollients, antihistamines, and topical corticosteroids for dryness and itching; laser therapy for telangiectasias; and corticosteroid injection, laser therapy, or surgery for calcifications. However, there is limited evidence of efficacy for most of these options.45
FIGURE 3
An attack of Raynaud’s phenomenon
Raynaud’s phenomenon, characterized by blanching of the distal fingertips, is shown here in a patient with systemic sclerosis.
FIGURE 4
Sclerodactyly in a patient with systemic sclerosis
Hardening and thickening of the skin can result in highly disabling sclerodactyly in patients with systemic sclerosis.
FIGURE 5
Dissolution of terminal phalanges
Bony resorption and ulceration have led to the loss of distal phalanges in this patient with systemic sclerosis.
Mixed connective tissue disease has features of several disorders
Mixed connective tissue disease (MCTD) is an apparently distinct rheumatologic condition characterized by a combination of clinical features of systemic LE, dermatomyositis, scleroderma, polymyositis, and rheumatoid arthritis. The presence of high titers of a unique autoantibody, anti-U1-RNP,54-57 aids in diagnosis.
Although precise prevalence data are lacking, MCTD is thought to occur in about 1 in 10,000 people.58 The disease is much more common in women, with a female-to-male ratio as high as 7 to 1, and generally occurs in the second or third decade of life.58-60
The clinical manifestations of MCTD typically evolve, with overlapping features of various autoimmune disorders appearing sequentially over several months to years.57 Early in the course of MCTD, patients commonly experience fatigue, polyarthritis, hand edema, and RP. In time, virtually every organ system may be involved. Pulmonary hypertension is a major cause of death.59,61
Unlike many other connective tissue disorders, MCTD lacks any distinct cutaneous findings. In addition to those mentioned earlier, prominent skin findings include swelling of the fingers, sclerodactyly, and the acute malar eruptions and discoid plaques typically associated with LE.56,57,62 Cutaneous manifestations associated with dermatomyositis and scleroderma may also be seen, particularly juxta-articular calcinosis. The mucous membranes may be involved, as well, resulting in nasal perforation, buccal and urogenital ulcerations, and sicca complex.58,63,64
MCTD diagnosis and treatment: Look for these serology and clinical findings
Diagnosing MCTD can be clinically challenging, as signs and symptoms of the disease commonly evolve over time. The Alarcon-Segovia criteria—largely regarded as the best diagnostic tool for MCTD65,66—include 1 serologic finding (elevated anti-U1-RNP [titer ≥1:1600]) and 5 clinical findings (RP, edema of the hands, synovitis, myositis, and acrosclerosis). Diagnosis requires the presence of the serologic criterion and ≥3 of the 5 clinical criteria.
Treatment of the cutaneous manifestations should be based on the effectiveness of therapies for similar skin findings seen in other disorders. In treating MCTD (or any other connective tissue disorder), a team that includes nurses, physical and occupational therapists, primary care physicians, and specialists in dermatology and rheumatology is essential for an optimal outcome.
CORRESPONDENCE Christian R. Halvorson, MD, Mercy Medical Center, Department of Medicine, 301 St. Paul Place, Baltimore, MD 21202; crhalvor@gmail.com
1. Renner R, Sticherling M. The different faces of cutaneous lupus erythematosus. G Ital Dermatol Venereol. 2009;144:135-147.
2. Kuhn A, Ruzicka T. Classification of cutaneous lupus erythematosus. In: Kuhn A, Lehmann P, Ruzicka T, eds. Cutaneous Lupus Erythematosus. New York: Springer; 2004:53–57.
3. Costner MI, Grau RH. Update on connective tissue diseases in dermatology. Semin Cutan Med Surg. 2006;25:207-220.
4. Ting W, Stone MS, Racila D, et al. Toxic epidermal necrolysis-like acute cutaneous lupus erythematosus and the spectrum of the acute syndrome of apoptotic pan-epidermolysis (ASAP): a case report, concept review and proposal for new classification of lupus erythematosus vesiculobullous skin lesions. Lupus. 2004;13:941-950.
5. Jorizzo JL, Carroll CL, Sangueza OP. Lupus erythematosus. In: Bolognia JL, Jorizzo JL, Rapini RP, eds. Dermatology. 2nd ed. Spain: Elsevier Limited; 2008.
6. Kuhn A, Sticherling M, Bonsmann G. Clinical manifestations of cutaneous lupus erythematosus. J Dtsch Dermatol Ges. 2007;5:1124-1137.
7. Sontheimer RD, Thomas JR, Gilliam JN. Subacute cutaneous lupus erythematosus: a cutaneous marker for a distinct lupus erythematosus subset. Arch Dermatol. 1979;115:1409-1415.
8. Sontheimer RD. Skin manifestations of systemic autoimmune connective tissue disease: diagnostics and therapeutics. Best Pract Res Clin Rheumatol. 2004;18:429-462.
9. Panjwani S. Early diagnosis and treatment of discoid lupus erythematosus. J Am Board Fam Med. 2009;22:206-213.
10. Sontheimer RD, Provost TT. Lupus erythematosus. In: Sontheimer RD, Provost TT, eds. Cutaneous Manifestations of Rheumatic Diseases. Baltimore: Williams & Wilkins; 1996.
11. Mond CB, Peterson MG, Rothfield NF. Correlation of antiro antibody with photosensitivity rash in systemic lupus erythematosus patients. Arthritis Rheum. 1989;32:202-204.
12. Jacyk WK, Damisah M. Discoid lupus erythematosus in the Nigerians. Br J Dermatol. 1979;100:131-135.
13. Callen JP. Cutaneous lupus erythematosus: A personal approach to management. Australas J Dermatol. 2006;47:13-27.
14. Gilliam JN, Sontheimer RD. Distinctive cutaneous subsets in the spectrum of lupus erythematosus. J Am Acad Dermatol. 1981;4:471-475.
15. Farley-Loftus R, Mahlberg M, Merola J, et al. Generalized discoid lupus erythematosus. Dermatol Online J. 2009;15:18.-
16. Kuhn A, Richter-Hintz D, Oslislo C, et al. Lupus erythematosus tumidus—a neglected subset of cutaneous lupus erythematosus: report of 40 cases. Arch Dermatol. 2000;136:1033-1041.
17. American College of Rheumatology. The 1982 revised criteria for classification of systemic lupus erythematosus. Available at: http://www.rheumatology.org/practice/clinical/classification/SLE/sle.asp. Accessed September 21, 2010.
18. Na SJ, Kim SM, Sunwoo IN, et al. Clinical characteristics and outcomes of juvenile and adult dermatomyositis. J Korean Med Sci. 2009;24:715-721.
19. Kovacs SO, Kovacs SC. Dermatomyositis. J Am Acad Dermatol. 1998;39:899-922.
20. Koler RA, Montemarano A. Dermatomyositis. Am Fam Physician. 2001;64:1565-1572.
21. Sunkureddi P, Nguyen-Oghalai TU, Jarvis JL, et al. Signs of dermatomyositis. Hosp Physician. 2005;41:41-44.
22. Iorizzo LJ, 3rd, Jorizzo JL. The treatment and prognosis of dermatomyositis: an updated review. J Am Acad Dermatol. 2008;59:99-112.
23. Jorizzo JL, Carroll CL, Sangueza OP. Dermatomyositis. In: Bolognia JL, Jorizzo JL, Rapini RP, eds. Dermatology. 2nd ed. Spain: Elsevier Limited; 2008:575–583.
24. Sontheimer RD. Dermatomyositis. In: Freedberg IM, Eisen AZ, Wolff K, et al, eds. Fitzpatrick’s Dermatology in General Medicine. 5th ed. New York: McGraw-Hill; 1999:2009–2020.
25. Halbert AR. Juvenile dermatomyositis. Australas J Dermatol. 1996;37:106-108.
26. Orlow SJ, Watsky KL, Bolognia JL. Skin and bones II. J Am Acad Dermatol. 1991;25:447-462.
27. Bowyer SL, Blane CE, Sullivan DB, et al. Childhood dermatomyositis: factors predicting functional outcome and development of dystrophic calcification. J Pediatr. 1983;103:882-888.
28. Bohan A, Peter JB. Polymyositis and dermatomyositis (second of two parts). N Engl J Med. 1975;292:403-407.
29. Bohan A, Peter JB. Polymyositis and dermatomyositis (first of two parts). N Engl J Med. 1975;292:344-347.
30. Vancheeswaran R, Black CM, David J, et al. Childhood-onset scleroderma: is it different from adult-onset disease? Arthritis Rheum. 1996;39:1041-1049.
31. Zulian F, Athreya BH, Laxer R, et al. Juvenile localized scleroderma: Clinical and epidemiological features in 750 children. An international study. Rheumatology (Oxford). 2006;45:614-620.
32. Marzano AV, Menni S, Parodi A, et al. Localized scleroderma in adults and children. clinical and laboratory investigations on 239 cases. Eur J Dermatol. 2003;13:171-176.
33. Uziel Y, Krafchik BR, Silverman ED, et al. Localized scleroderma in childhood: a report of 30 cases. Semin Arthritis Rheum. 1994;23:328-340.
34. Peterson LS, Nelson AM, Su WP. Classification of morphea (localized scleroderma). Mayo Clin Proc. 1995;70:1068-1076.
35. Peterson LS, Nelson AM, Su WP, et al. The epidemiology of morphea (localized scleroderma) in Olmsted County 1960-1993. J Rheumatol. 1997;24:73-80.
36. Zulian F, Vallongo C, Woo P, et al. Localized scleroderma in childhood is not just a skin disease. Arthritis Rheum. 2005;52:2873-2881.
37. Dehen L, Roujeau JC, Cosnes A, et al. Internal involvement in localized scleroderma. Medicine (Baltimore). 1994;73:241-245.
38. Ludwig RJ, Werner RJ, Winker W, et al. Chronic venous insufficiency - a potential trigger for localized scleroderma. J Eur Acad Dermatol Venereol. 2006;20:96-99.
39. Aberer E, Neumann R, Stanek G. Is localised scleroderma a borrelia infection? Lancet. 1985;2:278.-
40. Krishnan E, Furst DE. Systemic sclerosis mortality in the United States: 1979-1998. Eur J Epidemiol. 2005;20:855-861.
41. Silman AJ. Scleroderma—demographics and survival. J Rheumatol Suppl. 1997;48:58-61.
42. Medsger TA,, Jr, Masi AT. Epidemiology of systemic sclerosis (scleroderma). Ann Intern Med. 1971;74:714-721.
43. Charles C, Clements P, Furst DE. Systemic sclerosis: Hypothesis-driven treatment strategies. Lancet. 2006;367:1683-1691.
44. Maricq HR, Harper FE, Khan MM, et al. Microvascular abnormalities as possible predictors of disease subsets in Raynaud phenomenon and early connective tissue disease. Clin Exp Rheumatol. 1983;1:195-205.
45. Krieg T, Takehara K. Skin disease: A cardinal feature of systemic sclerosis. Rheumatology (Oxford). 2009;48 (suppl 3):S14-S18.
46. Ingraham KM, Steen VD. Morbidity of digital tip ulcerations in scleroderma. Arthritis Rheum. 2006;54(9 suppl.):F78.-
47. Ferri C, Valentini G, Cozzi F, et al. Systemic sclerosis: Demographic, clinical, and serologic features and survival in 1,012 italian patients. Medicine (Baltimore). 2002;81:139-153.
48. Haustein UF. Systemic sclerosis - scleroderma. Dermatol Online J. 2002;8.-
49. Fitzpatrick TB, Johnson RA, Klaus W, et al. In Colour Atlas and Synopsis of Clinical Dermatology. 4th ed. New York: McGraw-Hill; 2001:368–369.
50. Preliminary criteria for the classification of systemic sclerosis (scleroderma). subcommittee for scleroderma criteria of the American Rheumatism Association diagnostic and therapeutic criteria committee. Arthritis Rheum. 1980;23:581-590.
51. Valentini G, Paone C, La Montagna G, et al. Low-dose intravenous cyclophosphamide in systemic sclerosis: an open prospective efficacy study in patients with early diffuse disease. Scand J Rheumatol. 2006;35:35-38.
52. Steen VD, Medsger TA, Jr. Improvement in skin thickening in systemic sclerosis associated with improved survival. Arthritis Rheum. 2001;44:2828-2835.
53. Basso M, Filaci G, Cutolo M, et al. Long-term treatment of patients affected by systemic sclerosis with cyclosporin A. Ann Ital Med Int. 2001;16:233-239.
54. Morton SJ, Powell RJ. Cyclosporin and tacrolimus: their use in a routine clinical setting for scleroderma. Rheumatology (Oxford). 2000;39:865-869.
55. Kasukawa R. Mixed connective tissue disease. Intern Med. 1999;38:386-393.
56. Bennett RM, O’Connell DJ. Mixed connective tisssue disease: A clinicopathologic study of 20 cases. Semin Arthritis Rheum. 1980;10:25-51.
57. Sharp GC, Irvin WS, Tan EM, et al. Mixed connective tissue disease—an apparently distinct rheumatic disease syndrome associated with a specific antibody to an extractable nuclear antigen (ENA). Am J Med. 1972;52:148-159.
58. Venables PJ. Mixed connective tissue disease. Lupus. 2006;15:132-137.
59. Farhey Y, Hess EV. Mixed connective tissue disease. Arthritis Care Res. 1997;10:333-342.
60. Alarcon-Segovia D. Mixed connective tissue disease - a decade of growing pains. J Rheumatol. 1981;8:535-540.
61. Ueda N, Mimura K, Maeda H, et al. Mixed connective tissue disease with fatal pulmonary hypertension and a review of literature. Virchows Arch A Pathol Anat Histopathol. 1984;404:335-340.
62. Bennett RM. Mixed connective tissue disease and other overlap syndromes. In: Kelley WN, Harris EDJ, Ruddy SH, et al, eds. Textbook of Rheumatology. 4th ed. Philadelphia: WB Saunders; 1993:1061.
63. Nakashima M, Suzuki K, Okada M, et al. Panniculitis in a patient with mixed connective tissue disease. Mod Rheumatol. 2004;14:250-253.
64. Magro CM, Crowson AN, Regauer S. Mixed connective tissue disease. A clinical, histologic, and immunofluorescence study of eight cases. Am J Dermatopathol. 1997;19:206-213.
65. Amigues JM, Cantagrel A, Abbal M, et al. Comparative study of 4 diagnosis criteria sets for mixed connective tissue disease in patients with anti-RNP antibodies. autoimmunity group of the hospitals of toulouse. J Rheumatol. 1996;23:2055-2062.
66. Alarcon-Segovia D, Cardiel MH. Comparison between 3 diagnostic criteria for mixed connective tissue disease. study of 593 patients. J Rheumatol. 1989;16:328-334.
1. Renner R, Sticherling M. The different faces of cutaneous lupus erythematosus. G Ital Dermatol Venereol. 2009;144:135-147.
2. Kuhn A, Ruzicka T. Classification of cutaneous lupus erythematosus. In: Kuhn A, Lehmann P, Ruzicka T, eds. Cutaneous Lupus Erythematosus. New York: Springer; 2004:53–57.
3. Costner MI, Grau RH. Update on connective tissue diseases in dermatology. Semin Cutan Med Surg. 2006;25:207-220.
4. Ting W, Stone MS, Racila D, et al. Toxic epidermal necrolysis-like acute cutaneous lupus erythematosus and the spectrum of the acute syndrome of apoptotic pan-epidermolysis (ASAP): a case report, concept review and proposal for new classification of lupus erythematosus vesiculobullous skin lesions. Lupus. 2004;13:941-950.
5. Jorizzo JL, Carroll CL, Sangueza OP. Lupus erythematosus. In: Bolognia JL, Jorizzo JL, Rapini RP, eds. Dermatology. 2nd ed. Spain: Elsevier Limited; 2008.
6. Kuhn A, Sticherling M, Bonsmann G. Clinical manifestations of cutaneous lupus erythematosus. J Dtsch Dermatol Ges. 2007;5:1124-1137.
7. Sontheimer RD, Thomas JR, Gilliam JN. Subacute cutaneous lupus erythematosus: a cutaneous marker for a distinct lupus erythematosus subset. Arch Dermatol. 1979;115:1409-1415.
8. Sontheimer RD. Skin manifestations of systemic autoimmune connective tissue disease: diagnostics and therapeutics. Best Pract Res Clin Rheumatol. 2004;18:429-462.
9. Panjwani S. Early diagnosis and treatment of discoid lupus erythematosus. J Am Board Fam Med. 2009;22:206-213.
10. Sontheimer RD, Provost TT. Lupus erythematosus. In: Sontheimer RD, Provost TT, eds. Cutaneous Manifestations of Rheumatic Diseases. Baltimore: Williams & Wilkins; 1996.
11. Mond CB, Peterson MG, Rothfield NF. Correlation of antiro antibody with photosensitivity rash in systemic lupus erythematosus patients. Arthritis Rheum. 1989;32:202-204.
12. Jacyk WK, Damisah M. Discoid lupus erythematosus in the Nigerians. Br J Dermatol. 1979;100:131-135.
13. Callen JP. Cutaneous lupus erythematosus: A personal approach to management. Australas J Dermatol. 2006;47:13-27.
14. Gilliam JN, Sontheimer RD. Distinctive cutaneous subsets in the spectrum of lupus erythematosus. J Am Acad Dermatol. 1981;4:471-475.
15. Farley-Loftus R, Mahlberg M, Merola J, et al. Generalized discoid lupus erythematosus. Dermatol Online J. 2009;15:18.-
16. Kuhn A, Richter-Hintz D, Oslislo C, et al. Lupus erythematosus tumidus—a neglected subset of cutaneous lupus erythematosus: report of 40 cases. Arch Dermatol. 2000;136:1033-1041.
17. American College of Rheumatology. The 1982 revised criteria for classification of systemic lupus erythematosus. Available at: http://www.rheumatology.org/practice/clinical/classification/SLE/sle.asp. Accessed September 21, 2010.
18. Na SJ, Kim SM, Sunwoo IN, et al. Clinical characteristics and outcomes of juvenile and adult dermatomyositis. J Korean Med Sci. 2009;24:715-721.
19. Kovacs SO, Kovacs SC. Dermatomyositis. J Am Acad Dermatol. 1998;39:899-922.
20. Koler RA, Montemarano A. Dermatomyositis. Am Fam Physician. 2001;64:1565-1572.
21. Sunkureddi P, Nguyen-Oghalai TU, Jarvis JL, et al. Signs of dermatomyositis. Hosp Physician. 2005;41:41-44.
22. Iorizzo LJ, 3rd, Jorizzo JL. The treatment and prognosis of dermatomyositis: an updated review. J Am Acad Dermatol. 2008;59:99-112.
23. Jorizzo JL, Carroll CL, Sangueza OP. Dermatomyositis. In: Bolognia JL, Jorizzo JL, Rapini RP, eds. Dermatology. 2nd ed. Spain: Elsevier Limited; 2008:575–583.
24. Sontheimer RD. Dermatomyositis. In: Freedberg IM, Eisen AZ, Wolff K, et al, eds. Fitzpatrick’s Dermatology in General Medicine. 5th ed. New York: McGraw-Hill; 1999:2009–2020.
25. Halbert AR. Juvenile dermatomyositis. Australas J Dermatol. 1996;37:106-108.
26. Orlow SJ, Watsky KL, Bolognia JL. Skin and bones II. J Am Acad Dermatol. 1991;25:447-462.
27. Bowyer SL, Blane CE, Sullivan DB, et al. Childhood dermatomyositis: factors predicting functional outcome and development of dystrophic calcification. J Pediatr. 1983;103:882-888.
28. Bohan A, Peter JB. Polymyositis and dermatomyositis (second of two parts). N Engl J Med. 1975;292:403-407.
29. Bohan A, Peter JB. Polymyositis and dermatomyositis (first of two parts). N Engl J Med. 1975;292:344-347.
30. Vancheeswaran R, Black CM, David J, et al. Childhood-onset scleroderma: is it different from adult-onset disease? Arthritis Rheum. 1996;39:1041-1049.
31. Zulian F, Athreya BH, Laxer R, et al. Juvenile localized scleroderma: Clinical and epidemiological features in 750 children. An international study. Rheumatology (Oxford). 2006;45:614-620.
32. Marzano AV, Menni S, Parodi A, et al. Localized scleroderma in adults and children. clinical and laboratory investigations on 239 cases. Eur J Dermatol. 2003;13:171-176.
33. Uziel Y, Krafchik BR, Silverman ED, et al. Localized scleroderma in childhood: a report of 30 cases. Semin Arthritis Rheum. 1994;23:328-340.
34. Peterson LS, Nelson AM, Su WP. Classification of morphea (localized scleroderma). Mayo Clin Proc. 1995;70:1068-1076.
35. Peterson LS, Nelson AM, Su WP, et al. The epidemiology of morphea (localized scleroderma) in Olmsted County 1960-1993. J Rheumatol. 1997;24:73-80.
36. Zulian F, Vallongo C, Woo P, et al. Localized scleroderma in childhood is not just a skin disease. Arthritis Rheum. 2005;52:2873-2881.
37. Dehen L, Roujeau JC, Cosnes A, et al. Internal involvement in localized scleroderma. Medicine (Baltimore). 1994;73:241-245.
38. Ludwig RJ, Werner RJ, Winker W, et al. Chronic venous insufficiency - a potential trigger for localized scleroderma. J Eur Acad Dermatol Venereol. 2006;20:96-99.
39. Aberer E, Neumann R, Stanek G. Is localised scleroderma a borrelia infection? Lancet. 1985;2:278.-
40. Krishnan E, Furst DE. Systemic sclerosis mortality in the United States: 1979-1998. Eur J Epidemiol. 2005;20:855-861.
41. Silman AJ. Scleroderma—demographics and survival. J Rheumatol Suppl. 1997;48:58-61.
42. Medsger TA,, Jr, Masi AT. Epidemiology of systemic sclerosis (scleroderma). Ann Intern Med. 1971;74:714-721.
43. Charles C, Clements P, Furst DE. Systemic sclerosis: Hypothesis-driven treatment strategies. Lancet. 2006;367:1683-1691.
44. Maricq HR, Harper FE, Khan MM, et al. Microvascular abnormalities as possible predictors of disease subsets in Raynaud phenomenon and early connective tissue disease. Clin Exp Rheumatol. 1983;1:195-205.
45. Krieg T, Takehara K. Skin disease: A cardinal feature of systemic sclerosis. Rheumatology (Oxford). 2009;48 (suppl 3):S14-S18.
46. Ingraham KM, Steen VD. Morbidity of digital tip ulcerations in scleroderma. Arthritis Rheum. 2006;54(9 suppl.):F78.-
47. Ferri C, Valentini G, Cozzi F, et al. Systemic sclerosis: Demographic, clinical, and serologic features and survival in 1,012 italian patients. Medicine (Baltimore). 2002;81:139-153.
48. Haustein UF. Systemic sclerosis - scleroderma. Dermatol Online J. 2002;8.-
49. Fitzpatrick TB, Johnson RA, Klaus W, et al. In Colour Atlas and Synopsis of Clinical Dermatology. 4th ed. New York: McGraw-Hill; 2001:368–369.
50. Preliminary criteria for the classification of systemic sclerosis (scleroderma). subcommittee for scleroderma criteria of the American Rheumatism Association diagnostic and therapeutic criteria committee. Arthritis Rheum. 1980;23:581-590.
51. Valentini G, Paone C, La Montagna G, et al. Low-dose intravenous cyclophosphamide in systemic sclerosis: an open prospective efficacy study in patients with early diffuse disease. Scand J Rheumatol. 2006;35:35-38.
52. Steen VD, Medsger TA, Jr. Improvement in skin thickening in systemic sclerosis associated with improved survival. Arthritis Rheum. 2001;44:2828-2835.
53. Basso M, Filaci G, Cutolo M, et al. Long-term treatment of patients affected by systemic sclerosis with cyclosporin A. Ann Ital Med Int. 2001;16:233-239.
54. Morton SJ, Powell RJ. Cyclosporin and tacrolimus: their use in a routine clinical setting for scleroderma. Rheumatology (Oxford). 2000;39:865-869.
55. Kasukawa R. Mixed connective tissue disease. Intern Med. 1999;38:386-393.
56. Bennett RM, O’Connell DJ. Mixed connective tisssue disease: A clinicopathologic study of 20 cases. Semin Arthritis Rheum. 1980;10:25-51.
57. Sharp GC, Irvin WS, Tan EM, et al. Mixed connective tissue disease—an apparently distinct rheumatic disease syndrome associated with a specific antibody to an extractable nuclear antigen (ENA). Am J Med. 1972;52:148-159.
58. Venables PJ. Mixed connective tissue disease. Lupus. 2006;15:132-137.
59. Farhey Y, Hess EV. Mixed connective tissue disease. Arthritis Care Res. 1997;10:333-342.
60. Alarcon-Segovia D. Mixed connective tissue disease - a decade of growing pains. J Rheumatol. 1981;8:535-540.
61. Ueda N, Mimura K, Maeda H, et al. Mixed connective tissue disease with fatal pulmonary hypertension and a review of literature. Virchows Arch A Pathol Anat Histopathol. 1984;404:335-340.
62. Bennett RM. Mixed connective tissue disease and other overlap syndromes. In: Kelley WN, Harris EDJ, Ruddy SH, et al, eds. Textbook of Rheumatology. 4th ed. Philadelphia: WB Saunders; 1993:1061.
63. Nakashima M, Suzuki K, Okada M, et al. Panniculitis in a patient with mixed connective tissue disease. Mod Rheumatol. 2004;14:250-253.
64. Magro CM, Crowson AN, Regauer S. Mixed connective tissue disease. A clinical, histologic, and immunofluorescence study of eight cases. Am J Dermatopathol. 1997;19:206-213.
65. Amigues JM, Cantagrel A, Abbal M, et al. Comparative study of 4 diagnosis criteria sets for mixed connective tissue disease in patients with anti-RNP antibodies. autoimmunity group of the hospitals of toulouse. J Rheumatol. 1996;23:2055-2062.
66. Alarcon-Segovia D, Cardiel MH. Comparison between 3 diagnostic criteria for mixed connective tissue disease. study of 593 patients. J Rheumatol. 1989;16:328-334.