User login
Until recently it had been argued that hospitalization was not the time in the life of a patient to insist on tight glycemic control. Hyperglycemia was understood to be a consequence of medical stress.1 It was well known that infection, sepsis, or other medical stress might exacerbate hyperglycemia or promote a diabetic crisis.25 Admittedly, the severity of hyperglycemia among patients who have diabetes was thought to predict the risk for hospitalization with infection as well as the outcome of the infectious condition.68 However, until recently strict glycemic control in the hospital was not strongly advocated because hypoglycemia might occur and might be directly and uniquely traceable to actions taken in the hospital.9, 10 Furthermore, the complications of diabetes were thought to be divided into acute metabolic emergencies and chronic tissue complications such as polyneuropathy, retinopathy, nephropathy, and macrovascular disease that would have evolved over a period longer than the duration of hospitalization, and the possibility that short‐term hyperglycemia might affect outcomes was considered unproven.
The purpose of this article is to define the specific populations, disorders, and hospital settings for which there now is strong evidence that short‐term glycemic control will affect the outcome of a course of hospital treatment.
PHYSIOLOGIC LINK BETWEEN HYPERGLYCEMIA AND ADVERSE OUTCOMES
Five years ago a caregiver would not have been likely to think of glycemic control as a contributing factor when considering specific complex problems such as pump failure or arrhythmia after cardiac surgery, long‐term mortality after myocardial infarction, acute renal failure, the need for transfusion during treatment of a complicated surgical illness, or prolonged dependence on a ventilator in the surgical ICU. Although it now known that these and other complications are linked to hospital hyperglycemia, the mechanisms of harm are several steps removed from the hyperglycemia itself. The causes of these adverse outcomes are multifactorial. The causal dependence of the injury on hyperglycemia is not easy to see. In fact, it required randomized prospectively designed trials to convincingly demonstrate the contributory role of hyperglycemia to these and other adverse outcomes.
Now that this link has been convincingly demonstrated, there is intense interest in discovering the probable mechanisms by which control of hyperglycemia and specifically the use of insulin might improve outcomes.1114 Mortality, predominantly sepsis related, was the primary outcome for which the Leuven, Belgium, report of 2001 on the surgical ICU showed improvement.15 Simplistically, in seeking a mechanism of benefit with respect to sepsis, it might be argued that if gross hyperglycemia were prevented in patients with surgical wounds, improvement of host defenses against infective organisms might be expected.1622 However, additional mechanisms of protection probably should be invoked, including those by which glycemic control and specifically insulin therapy affect endothelial function and the coagulation pathway, thus improving the ability of a patient to withstand and recover from sepsis. Insulin promotes beneficial nitric oxide synthase activation (e‐NOS) in capillary endothelium.2326 In patients with prolonged critical illness, intensive insulin therapy lowers ICAM‐1 levels, reflecting reduced endothelial activation. Whereas e‐NOS exerts a beneficial endothelial effect, hepatic iNOS activation is harmful. One proposed mechanism of benefit from adequate insulin therapy is suppression of excessive hepatic iNOS‐induced release of circulating NO, which might contribute to endothelial dysfunction, organ failure, and death.27
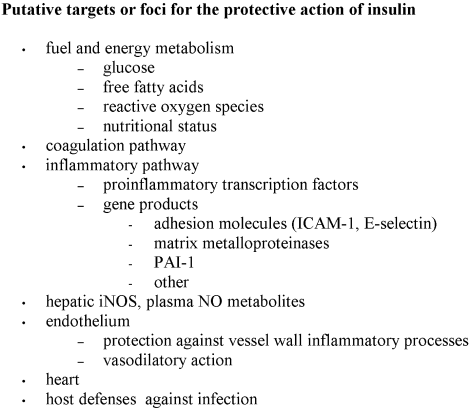
Additional proposed mechanisms of hyperglycemia‐induced harm to hospitalized patients, some potentially specifically reduced by insulin therapy, resemble those discussed in relation to macrovascular disease and include activation of inflammatory cytokines, matrix metalloproteinases, and adhesion molecules, and the adverse arrhythmogenic effects of elevated circulating nonesterified fatty acids (Figure 1).2834
CRITICAL WINDOW OF TIME
The results of several retrospectively or prospectively conducted single‐institution observational studies suggest there is a critical window of time within which clinicians must get it right, when attempting glycemic control or else jeopardize therapeutic goals, such as duration of remission in treatment of acute lymphocytic leukemia35 or avoidance of acute rejection in renal allograft surgery.36 Additionally, delayed risk of infection appears to be linked to previous glycemic control during specific early time frames surrounding surgery, renal transplantation, admission for trauma, and induction chemotherapy for leukemia (Table 1).3541 For patients who have diabetes, the potential to reduce the consequences of infections through intensified glycemic control probably begins prior to admission and in the hospital has still not been fully realized.4244
| Patients | Ascertainment of hyperglycemia | Delayed events among patients with early hyperglycemia |
|---|---|---|
| 100 postoperative uninfected diabetic patients undergoing elective surgery, monitored prospectively37 | First postoperative day | Postoperative nosocomial infection rate within 14 days was 2.7 times higher for patients having at least one BG > 220 mg/dL (33.3% vs. 11.5%). |
| 990 historical controls and 595 patients in the interventional group of postoperative cardiac diabetic patients40 | First 2 postoperative days | Incidence of deep sternal wound infection was reduced from 2.4% to 1.5% (P < 0.02) after introduction of protocol to maintain mean BG < 200 mg/dL. |
| 423 renal allograft recipients receiving their first cadaveric transplant36 | First 100 hours; first day. | A mean of 10.8 2.3 days after transplantation, 70% of patients developed postoperative infection, and after a mean of 7.7 2.6 days, 40% developed acute rejection. Every patient with mean BG over 200 mg/dL during the first 100 hours developed postoperative infection. On the first postoperative day the mean BG had been 248.4 mg/dL among those developing infection and 167.4 mg/dL among those without infection (P < .001), and the mean BG had been 270 mmol/L among those developing rejection and 194 mmol/L among those without rejection. |
| 516 trauma patients admitted to the ICU38 | Either of first 2 hospital days | Hyperglycemia 200 mg/dL was associated with a higher infection rate (32% vs. 22%, P = .04) and with greater mortality (34% vs. 13%, P < .0001) |
| 275 patients having lower‐extremity peripheral vascular surgery39 | First 48 hours | Postoperative infections within 30 days were 5.1 times more frequent in the top quartile for BG versus the lowest quartile (confidence interval 1.6‐17.1, P = .007). |
| 278 adult patients receiving induction chemotherapy for acute lymphocytic leukemia35 | First 30 days | Hyperglycemia defined as 2 BG 200 mg/dL was associated with a greater likelihood of sepsis (16.5% vs. 8.0%, P = .03) or any complicated infection (38.8% vs. 25.1%, P = .016), shorter duration of complete remission (24 vs. 52 months), and with shorter median survival (29 vs. 88 months, P = .001). |
KEY STUDIES SHOWING CLINICAL BENEFIT OF TIGHT GLYCEMIC CONTROL
A summary of several key studies that demonstrated the clinical benefit of tight glycemic control is shown in Table 2. These studies successfully separated the intensively and conventionally managed study groups according blood glucose. Although trials using glucose‐insulin‐potassium infusions (GIK) such that blood glucose was lowered have shown benefit for patients who have had myocardial infarctions4547 or cardiac surgery,48 not all GIK studies have yielded positive results. The negative results of the CREATE‐ECLA study suggest that GIK therapy per se is not beneficial unless it reduces blood glucose.49 In the setting of acute myocardial infarction, the DIGAMI 2 trial and the HI‐5 trials failed to achieve the intended separation between treatment groups.50, 51 It has been speculated that if insulin is delivered so as to not achieve normoglycemia, then hyperinsulinemia in the presence of hyperglycemia actually may be proinflammatory.52
| Population and/or setting and patients | Study design and/or intervention | Principal findings |
|---|---|---|
| Patients with diabetes mellitus and with hyperglycemia > 11 mmol/L or similar hyperglycemia without known diabetes who had had acute myocardial within the preceding 24 hours.4547 There were 620 patients, of whom 15 of the controls and 10 patients in the intensive group had no prior diagnosis of diabetes. | Prospective, randomized, controlled clinical trial. Controls received standard treatment. Treatment group received infusion of glucose‐insulin for at least 24 hours, followed by multiple injections of subcutaneous insulin for at least 3 months. | Overall mortality after 1 year was l9% in the insulin group compared to 26% among controls (P < .05). The most frequent cause of death of all patients was congestive heart failure. The benefit continued for at least 3.5 years, with an absolute reduction in mortality of 11%. |
| Diabetic cardiac surgery population.40, 5557 To date, 5510 cardiac surgery patients who were either admitted or discharged with the diagnosis of diabetes have been studied. | Prospective nonrandomized study of the effects of hyperglycemia and its pharmacologic reduction with intensive intravenous insulin regimens on outcomes. | The 3‐day average of perioperative BG (3‐BG) correlated with mortality (P < .0001, odds ratio 2.0 per 50 mg/dL increase in 3‐BG). Continuous intravenous insulin infusion independently reduced risk of death 60% (RR = 0.4, P < .001). Length of stay in the CABG population increased by 1 day for every increase of 50 mg/dL in 3‐BG. |
| Critically ill surgical patients receiving mechanical ventilation. Overall, 13% of 1548 participants had previously diagnosed diabetes. | Prospective, randomized, controlled clinical trial. Intravenous insulin infusion targeting BG 80‐110 mg/dL was initiated in the intensive group for BG > 110 mg/dL. Intravenous insulin infusion targeting BG 180‐200 was initiated in the conventional group for BG > 215 mg/dL. A whole‐blood glucose method using a gas analyzer was employed to determine arterial blood glucose. | In the group assigned to intensive insulin therapy mortality was reduced during intensive care from 8.0% with conventional treatment to 4.6% (P < .04), or a risk reduction of 42%. In the group remaining in the ICU for more than 5 days, mortality was reduced from 20.2% with conventional treatment to 10.6% with intensive insulin therapy; P = .005. The intensive group experienced reductions in overall in‐hospital mortality of 34%, in bloodstream infections of 46%, and in acute renal failure requiring dialysis or hemofiltration of 41%. Also reduced were the need for red‐cell transfusions, critical‐illness neuropathy, duration of mechanical ventilation, and length of stay in the ICU. |
| Critically ill medical patients. The intention to treat group included 1200 participants, of whom 16.9% had a history of diabetes.54 | Randomized controlled clinical trial, as above. | In the intensive group, in‐hospital mortality was not significantly reduced, and among the 433 who stayed in the ICU for less than 3 days, mortality was actually higher. However, in the intention‐to‐treat group there was reduction in newly acquired kidney injury, prolonged mechanical ventilation, and length of stay in the ICU and in the hospital. Among the 767 patients who stayed in the ICU for 3 or more days, intensive insulin therapy reduced in‐hospital mortality from 52.5% to 43.0%. |
| Patients within 24 hours of having an acute stroke and who had poststroke hyperglycemia. The results of the first 452 patients recruited to the GIST‐UK study showed that 15.3% had previously recognized diabetes.62 | Randomized controlled clinical trial. The intensive group received glucose‐potassium‐insulin (GKI) infusion, and the conventional group received saline infusion. | Although mean glucose declined in both groups, the GKI infusion safely achieved separation of groups by blood glucose. Outcomes are pending. |
The findings of the negative intensive insulin studies do not offset the evidence favoring glycemic control, derived from studies that actually achieved the lowering of blood glucose in the intensive groups, such as the successful prospectively designed trials in myocardial infarction or surgical or medical intensive care units15, 45, 47, 53, 54 and the long‐running, large, prospectively monitored Portland series utilizing insulin infusion for cardiac surgery,40, 5557 which have demonstrated the beneficial effects of euglycemia on mortality and morbidity, or the findings of Krinsley, reported elsewhere in this issue.58, 59 The well‐recognized correlation between outcomes of acute stroke and poststroke hyperglycemia has led to the design of a multicenter trial of glucose‐insulin‐potassium infusion for stroke, in which separation of groups by blood glucose has been achieved.6062 The success of GIK therapy in controlling hyperglycemia depends in part on the particular formulation of the infusion as it matches patient needs, and it is probable that insulin infusion following stroke is capable of safely achieving even tighter glycemic control than GIK.63 Intravenous insulin infusion therapy is more difficult to conduct than GIK therapy. However, because of concern about the proinflammatory and prothrombotic effects of hyperglycemia and recognition of the occasional failure of GIK infusions to control hyperglycemia and of the anti‐inflammatory, antithrombotic, and vasodilatory actions of insulin, there have been calls for additional trials of insulin infusions (as opposed to glucose‐insulin infusions) for both acute myocardial infarction and stroke.52, 64
HYPOGLYCEMIA
Serious or fatal sequelae of hypoglycemia are the principal safety risks in intensive insulin management.9, 65 Case ascertainment cannot be assured by glucose averaging methods but instead requires a method of searching for isolated episodes of hypoglycemia.10 One of the most dreaded consequences of nonfatal hypoglycemia is permanent impairment of intellectual function. Because many euglycemic medically ill patients experience alteration of sensorium while acutely ill, there is a risk that the consequences of an actual episode of severe hypoglycemia will be ascribed to other comorbidities, overlooking or misattributing the altered cognitive function that may persist at discharge to causes other than the obvious iatrogenic one. Because there is an increased risk of hypoglycemia during intensive insulin therapy, controversy has arisen over glycemic targets, especially among critically ill nonsurgical patients.54, 6669
On the other hand, the consequences of hypoglycemia during intensive intravenous insulin therapy in a surgical ICU were said to be negligible.15 In hospitalized elderly patients, hypoglycemia in association with increased mortality risk may not be an independent predictor.70 In the critical care unit, predictors of hypoglycemia are identifiable after the introduction of strict glycemic control that include not only insulin therapy but also CVVH treatment with bicarbonate substitution fluid, discontinuation of nutrition without insulin adjustment, prior diagnosis of diabetes mellitus, sepsis, and the need for inotropic or vasopressor drugs.71 A prudent policy for the future would be not only to treat hypoglycemia promptly when it does occur, with attention to subsequent monitoring to avoid relapse or recurrence, but also to actively introduce strategies for predicting the risk of hypoglycemia and preventing it, especially when high risk is identified.10 The fear of hypoglycemia should not paralyze efforts to achieve better glycemic control in hospitalized patients.
UNANSWERED QUESTIONS AND VISION FOR THE FUTURE
On general wards, 38% of admitted patients may have hyperglycemia.72 As shown in the Leuven, Belgium, study, to achieve the target BG of less than 110 mg/dL in the intensive group in the surgical critical care unit, it was necessary to administer intravenous insulin infusion to essentially all patients. In a study that utilized continuous glucose monitoring, normoglycemia in patients in the intensive care unit was achieved as little as 22% of the time.73
An actively debated subject is how best to assess hospital performance in glycemic control (glucometrics). Hospitals need to show satisfactory control of variability between patients and within the treatment course of individual patients. That is, it is not sufficient to be satisfied with reasonable results of average blood glucose, using blood glucose as the unit of observation (where n is the number of blood glucose determinations). Alternatives are to use patient day or individual patient as a unit of observation (where n is the number of patient days and the number of patients, respectively). Time‐weighting methods are cumbersome but increase the validity of the averaging method used. When the patient is the unit of observation, possible measures include a per‐patient blood glucose average, percentage of blood glucose measurements with certain ranges, time spent within certain blood glucose ranges, or area under the curve of blood glucose versus time. Caveats about glucometrics pertain to both intravenous insulin infusion and also subcutaneous insulin management.74
Although awaiting additional evidence, diabetes experts have widely accepted the proposition that hospitals should focus on prevention of hyperglycemia as an important patient safety factor.75 Although the target range for glycemic control remains controversial, many students of the subject have endorsed the recommendations mentioned in the lead article in this issue, with the understanding that these criteria were developed from the results of the Leuven, Belgium, study, in which a whole blood glucose analyzer was used for measurement, and that the method of measurement of blood glucose must be considered in interpreting applicability of the target range at individual hospitals, many of which use plasma‐correlated methods yielding higher results. However, in new settings and for medical conditions that have not yet been rigorously evaluated by clinical trials, it is an unanswered question whether intensification of glycemic control can be achieved safely outside the critical care unit and, if so, what type of insulin therapy should be used and for what conditions the benefits would outweigh the risks and justify the costs.
Most inpatient management probably will continue to be conducted using subcutaneous injection therapy,7682 designed to match carbohydrate exposure through the appropriate use of analogs or conventional insulin products. One argument for the use of insulin analogs in the hospital, using basal‐prandial‐correction therapy, is the probability of reducing hypoglycemia and getting closer to target range control among patients who are eating but who are at risk for abrupt suspension of meals. Aggressive subcutaneous management strategies are likely to be most effective when standardized protocols, order sets, and informative computerized order entry systems gain widespread hospital acceptance.
If the importance of gaining glycemic control is highly time dependent and if hyperglycemia is uncontrolled, then a strong argument can be made for routine use of intravenous insulin infusion. For appropriately selected patients, intravenous insulin infusion is cost effective,83, 84 and its use can be extended to appropriate patients outside the critical care unit.8587 Many hospitals have protocols for intravenous insulin but use them only sporadically. For patients who already are in the intensive care setting, it is imperative to develop policies that require introduction of intravenous insulin infusion at a given glycemic threshold. On general wards that lack sufficient staffing to conduct intravenous insulin therapy, it is appropriate either to transfer candidate patients to a ward that has adequate staffing when medical condition requires improved control or to develop policies and procedures that will extend the use of intravenous insulin infusion to general wards. In the future, new technologies can be envisioned that will unburden nursing staff, making intravenous insulin infusion realizable as the treatment of choice for hemodynamically stable patients in most hospital settings. These technologies will include continuous monitoring of blood glucose, dose‐defining algorithms, and the eventual development of a fully automated closed‐loop system of monitoring and delivery that might automatically match insulin delivery to carbohydrate exposure and patient insulin sensitivity.8893
- ,,.Stress‐induced hyperglycemia.Crit Care Clin.2001;17(1):107–124.
- ,,,,.Hyperosmolarity and acidosis in diabetes mellitus: a three‐year experience in Rhode Island.J Gen Intern Med.1991:495–502.
- ,,.Prognostic factors in the diabetic hyperosmolar state.J Am Geriatr Soc.1987;35:737–741.
- ,,.Predisposing factors for the diabetic hyperosmolar state.Arch Intern Med.1987;147:499–501.
- .The diabetic hyperosmolar state.Clin Geriatr Med.1990;6:797–806.
- ,,,,,.Bacteremia in adult diabetic patients.Diabetes Care.1991;14(2):89–94.
- ,,,,,.Influence of diabetes mellitus and glycaemic control on the characteristics and outcome of common infections.Diabet Med.1996:457–463.
- ,,.Diabetes and the risk of infection‐related mortality in the U.S.Diabetes Care.2001;24:1044–1049.
- ,,.Hypoglycemia in hospitalized patients.N Engl J Med.1986;315:1245–1250.
- ,,, et al.Hospital hypoglycemia: not only treatment but also prevention.Endocr Pract.2004;10(suppl 2):71–80.
- .Effect of insulin therapy on nonglycemic variables during acute illness.Endocr Pract.2004;10(suppl 2):63–70.
- .Molecular mechanisms by which metabolic control may improve outcomes.Endocr Pract.2004;10(suppl 2):57–62.
- ,,,,.Insulin infusion in acute illness.J Clin Invest.2005;115:2069–2072.
- ,,.Intensive insulin therapy in the intensive care unit: update on clinical impact and mechanisms of action.Endocr Pract.2006;12(suppl 3):14–21.
- ,,, et al.Intensive insulin therapy in critically ill patients.N Engl J Med.2001;345:1359–1367.
- ,,.Impaired granulocyte adherence. A reversible defect in host defense in patients with poorly controlled diabetes.Diabetes.1978;27:677–681.
- ,,.Impaired leukocyte function in patients with poorly controlled diabetes.Diabetes.1974;23(1):9–15.
- ,,, et al.The effect of diabetes mellitus on chemotactic and bactericidal activity of human polymorphonuclear leukocytes.Diabetes Res Clin Pract.1987;4:27–35.
- ,,,,.Polymorphonuclear leukocytes in non‐insulin‐dependent diabetes mellitus: abnormalities in metabolism and function.Ann Intern Med.1995;123:919–924.
- ,,,,.Agonist‐dependent failure of neutrophil function in diabetes correlates with extent of hyperglycemia.J Leukoc Biol.2001;70:395–404.
- ,,,,,.Insulin increases neutrophil count and phagocytic capacity after cardiac surgery.Anesth Analg.2002;94:1113–1119.
- ,,,.In vivo evidences that insulin regulates human polymorphonuclear neutrophil functions.J Leukoc Biol.2004;76:1104–1110.
- ,.Insulin‐stimulated production of nitric oxide is inhibited by wortmannin. Direct measurement in vascular endothelial cells.J Clin Invest.1996;98:894–898.
- ,,,,.Insulin‐mediated skeletal muscle vasodilation is nitric oxide dependent. A novel action of insulin to increase nitric oxide release.J Clin Invest.1994;94:1172–1179.
- ,.Effect of insulin on human aortic endothelial nitric oxide synthase.Metabolism.2000;49(2):147–50.
- ,.Nitric oxide, platelet function, myocardial infarction and reperfusion therapies.Heart Fail Rev.2003;8(1):47–54.
- ,,, et al.Intensive insulin therapy protects the endothelium of critically ill patients.J Clin Invest.2005;115:2277–2286.
- ,,, et al.Elevated circulating free fatty acid levels impair endothelium‐dependent vasodilation.J Clin Invest.1997;100:1230–1239.
- ,,, et al.Insulin inhibits intranuclear nuclear factor kappaB and stimulates IkappaB in mononuclear cells in obese subjects: evidence for an anti‐inflammatory effect?J Clin Endocrinol Metab.2001;86:3257–3265.
- ,,.The anti‐inflammatory and potential anti‐atherogenic effect of insulin: a new paradigm.Diabetologia.2002;45:924–930.
- ,,,.The potential influence of inflammation and insulin resistance on the pathogenesis and treatment of atherosclerosis‐related complications in type 2 diabetes.J Clin Endocrinol Metab.2003;88:2422–2429.
- ,,.The potential therapeutic role of insulin in acute myocardial infarction in patients admitted to intensive care and in those with unspecified hyperglycemia.Diabetes Care.2003;26:516–519.
- ,,.Insulin treatment improves the systemic inflammatory reaction to severe trauma.Ann Surg.2004;239:553–560.
- ,,, et al.Anti‐inflammatory and profibrinolytic effect of insulin in acute ST‐segment‐elevation myocardial infarction.Circulation.2004;109:849–854.
- .Relation between the duration of remission and hyperglycemia in induction chemotherapy for acute lymphocytic leukemia.Cancer.2004;100:1179–1185.
- ,,,,.Early peri‐operative glycaemic control and allograft rejection in patients with diabetes mellitus: a pilot study.Transplantation.2001;72:1321–1324.
- ,,, et al.Early postoperative glucose control predicts nosocomial infection rate in diabetic patients.J Parenter Enteral Nutr.1998;22(2):77–81.
- ,,,,.Relationship of early hyperglycemia to mortality in trauma patients.J Trauma.2004;56:1058–1062.
- ,,,,.Early post‐operative glucose levels are an independent risk factor for infection after peripheral vascular surgery. A retrospective study.Eur J Vasc Endovasc Surg.2004;5:520–525.
- ,,,.Continuous intravenous insulin infusion reduces the incidence of deep sternal wound infection in diabetic patients after cardiac surgical procedures.Ann Thorac Surg.1999;67:352–362.
- ,.Effects on outcome of in‐hospital transition from intravenous insulin infusion to subcutaneous therapy.Am J Cardiol.2006;98:557–564.
- ,,,.The impact of diabetes in patients with necrotizing soft tissue infections.Surg Infect.2005;6:427–438.
- ,,,.Infections in patients with diabetes mellitus.N Engl J Med.1999;341:1906–1912.
- ,,.Quantifying the risk of infectious diseases for people with diabetes.Diabetes Care.2003;26:510–513.
- ,,, et al.Randomized trial of insulin‐glucose infusion followed by subcutaneous insulin treatment in diabetic patients with acute myocardial infarction (DIGAMI study): effects on mortality at 1 year.J Am Coll Cardiol.1995;26:57–65.
- ,,, et al.Effects of insulin treatment on cause‐specific one‐year mortality and morbidity in diabetic patients with acute myocardial infarction.Eur Heart J.1996;17:1337–1344.
- .Prospective randomised study of intensive insulin treatment on long term survival after acute myocardial infarction in patients with diabetes mellitus.DIGAMI (Diabetes Mellitus, Insulin Glucose Infusion in Acute Myocardial Infarction) Study Group.BMJ.1997;314:1512–1515.
- ,,,,,.Tight glycemic control in diabetic coronary artery bypass graft patients improves perioperative outcomes and decreases recurrent ischemic events.Circulation.2004;109:1497–1502.
- .Effect of Glucose‐Insulin‐Potassium Infusion on Mortality in Patients With Acute ST‐Segment Elevation Myocardial Infarction: The CREATE‐ECLA Randomized Controlled Trial.JAMA.2005;293:437–446.
- ,,, et al.Intense metabolic control by means of insulin in patients with diabetes mellitus and acute myocardial infarction (DIGAMI 2): effects on mortality and morbidity.Eur Heart J.2005;26:650–661.
- ,,.The Hyperglycemia: Intensive Insulin Infusion In Infarction (HI‐5) Study: A randomized controlled trial of insulin infusion therapy for myocardial infarction.Diabetes Care.2006;29:765–770.
- .Inpatient diabetes: review of data from the cardiac care unit.Endocr Pract.2006;12(suppl 3):27–34.
- ,.Reduction of nosocomial infections in the surgical intensive‐care unit by strict glycemic control.Endocr Pract.2004;10(suppl 2):46–52.
- ,,, et al.Intensive insulin therapy in the medical ICU.N Engl J Med.2006;354:449–461.
- ,,.Glucose control lowers the risk of wound infection in diabetics after open heart operations.Ann Thorac Surg.1997;63:356–361.
- ,,, et al.Continuous insulin infusion reduces mortality in patients with diabetes undergoing coronary artery bypass grafting.J Thorac Cardiovasc Surg.2003;125:1007–1021.
- ,.Clinical effects of hyperglycemia in the cardiac surgery population: the Portland diabetic project.Endocr Pract.2006;12(suppl 3):22–26.
- .Effect of an intensive glucose management protocol on the mortality of critically ill adult patients.Mayo Clin Proc.2004;79:992–1000.
- .Decreased mortality of critically ill patients with the use of an intensive glycemic management protocol.Mayo Clin Proc.2003;78:1471–1478.
- ,,, et al.Admission glucose level and clinical outcomes in the NINDS rt‐PA Stroke. Trial.Neurology.2002;59:669–74.
- ,,,,,.Glucose potassium insulin infusions in the treatment of acute stroke patients with mild to moderate hyperglycemia: the Glucose Insulin in Stroke. Trial (GIST).Stroke.1999;30:793–799.
- ,,,.Poststroke hyperglycemia: natural history and immediate management.Stroke.2004;35(1):122–126.
- ,,,.IV insulin during acute cerebral infarction in diabetic patients.Neurology.2004;62:1441–1442.
- ,,,.Hyperglycemia, insulin, and acute ischemic stroke: a mechanistic justification for a trial of insulin infusion therapy.Stroke.2006;37(1):267–273.
- ,,.Hypoglycemia and cardiac arrest in a critically ill patient on strict glycemic control.Anesth Analg.2006;102:549–551.
- ,,.Strict glucose control in the critically ill.Br Med J.2006;332:865–866.
- .Intensive Insulin in Intensive Care.N Engl J Med.2006;354:516–518.
- ,.Counterpoint: Inpatient glucose management: a premature call to arms?Diabetes Care.2005;28:976–979.
- ,,,.Glucose control and mortality in critically ill patients.JAMA.2003;290:2041–2047.
- ,,, et al.Hypoglycemia as a predictor of mortality in hospitalized elderly patients.Arch Intern Med.2003;163:1825–1829.
- ,,, et al.Predisposing factors for hypoglycemia in the intensive care unit.Crit Care Med.2006;34:96–101.
- ,,,,,.Hyperglycemia: an independent marker of in‐hospital mortality in patients with undiagnosed diabetes.J Clin Endocrinol Metab.2002;87:978–982.
- ,,,.Intensive insulin therapy in the intensive care unit: assessment by continuous glucose monitoring.Diabetes Care.2006;29:1750–1756.
- ,,,.No patient left behind: evaluation and design of intravenous insulin infusion algorithms.Endocr Pract.2006;12(suppl 3):72–78.
- ,,, et al.Management of diabetes and hyperglycemia in hospitals.Diabetes Care.2004;27:553–591.
- ,,,.Eliminating Inpatient Sliding‐Scale Insulin: A reeducation project with medical house staff.Diabetes Care.2005;28:1008–1011.
- ,,.70/30 Insulin algorithm versus sliding scale insulin.Ann Pharmacother.2005;39:1606–1609.
- ,.Hospital management of hyperglycemia.Clin Diabetes.2004;22(2):81–88.
- ,.Subcutaneous insulin therapy in the hospital setting: issues, concerns, and implementation.Endocr Pract.2004;10(suppl 2):81–88.
- ,,,,.Hyperglycemia in the hospital.Diabetes Spectr.2005;18(1):20–27.
- .Insulin Analogues.N Engl J Med.2005;352:174–183.
- .Insulin management of diabetic patients on general medical and surgical floors.Endocr Pract.2006;12(suppl 3):86–90.
- ,,, et al.Improved perioperative glycemic control by continuous insulin infusion under supervision of an endocrinologist does not increase costs in patients with diabetes.Endocr Pract.2004;10(2):112–118.
- ,,.Effect of hyperglycemia and continuous intravenous insulin infusions on outcomes of cardiac surgical procedures: the Portland Diabetic Project.Endocr Pract.2004;10(suppl 2):21–33.
- ,,,.New insulin infusion protocol improves blood glucose control in hospitalized patients without increasing hypoglycemia.J Qual Patient Saf.2005;31:141–147.
- ,,,,.Optimizing hospital use of intravenous insulin therapy: improved management of hyperglycemia and error reduction with a new nomogram.Endocr Pract.2005;11:240–253.
- ,,,,.Implementation of a new intravenous insulin method on intermediate‐care units in hospitalized patients.Diabetes Educ.2005;31:818–823.
- ,,.Clinical results of an updated insulin infusion protocol in critically ill patients.Diabetes Spectr.2005;18(3):188–191.
- ,,.Glucommander: A computer‐directed intravenous insulin system shown to be safe, simple, and effective in 120,618 h of operation.Diabetes Care.2005;28:2418–2423.
- ,,, et al.Improving hyperglycemia management in the intensive care unit: preliminary report of a nurse‐driven quality improvement project using a redesigned insulin infusion algorithm.Diabetes Educ.2006;32:394–403.
- ,,, et al.Performance of a dose‐defining insulin infusion protocol among trauma service ICU admissions.Diabetes Technol Ther.2006;8:476–488.
- ,,, et al.A simple insulin‐nutrition protocol for tight glycemic control in critical illness: development and protocol comparison.Diabetes Technol Ther.2006;8:191–206.
- ,,, et al.Evaluation of the impact of chiropodist care in the secondary prevention of foot ulcerations in diabetic subjects.Diabetes Care.2003;26:1691–1695.
Until recently it had been argued that hospitalization was not the time in the life of a patient to insist on tight glycemic control. Hyperglycemia was understood to be a consequence of medical stress.1 It was well known that infection, sepsis, or other medical stress might exacerbate hyperglycemia or promote a diabetic crisis.25 Admittedly, the severity of hyperglycemia among patients who have diabetes was thought to predict the risk for hospitalization with infection as well as the outcome of the infectious condition.68 However, until recently strict glycemic control in the hospital was not strongly advocated because hypoglycemia might occur and might be directly and uniquely traceable to actions taken in the hospital.9, 10 Furthermore, the complications of diabetes were thought to be divided into acute metabolic emergencies and chronic tissue complications such as polyneuropathy, retinopathy, nephropathy, and macrovascular disease that would have evolved over a period longer than the duration of hospitalization, and the possibility that short‐term hyperglycemia might affect outcomes was considered unproven.
The purpose of this article is to define the specific populations, disorders, and hospital settings for which there now is strong evidence that short‐term glycemic control will affect the outcome of a course of hospital treatment.
PHYSIOLOGIC LINK BETWEEN HYPERGLYCEMIA AND ADVERSE OUTCOMES
Five years ago a caregiver would not have been likely to think of glycemic control as a contributing factor when considering specific complex problems such as pump failure or arrhythmia after cardiac surgery, long‐term mortality after myocardial infarction, acute renal failure, the need for transfusion during treatment of a complicated surgical illness, or prolonged dependence on a ventilator in the surgical ICU. Although it now known that these and other complications are linked to hospital hyperglycemia, the mechanisms of harm are several steps removed from the hyperglycemia itself. The causes of these adverse outcomes are multifactorial. The causal dependence of the injury on hyperglycemia is not easy to see. In fact, it required randomized prospectively designed trials to convincingly demonstrate the contributory role of hyperglycemia to these and other adverse outcomes.
Now that this link has been convincingly demonstrated, there is intense interest in discovering the probable mechanisms by which control of hyperglycemia and specifically the use of insulin might improve outcomes.1114 Mortality, predominantly sepsis related, was the primary outcome for which the Leuven, Belgium, report of 2001 on the surgical ICU showed improvement.15 Simplistically, in seeking a mechanism of benefit with respect to sepsis, it might be argued that if gross hyperglycemia were prevented in patients with surgical wounds, improvement of host defenses against infective organisms might be expected.1622 However, additional mechanisms of protection probably should be invoked, including those by which glycemic control and specifically insulin therapy affect endothelial function and the coagulation pathway, thus improving the ability of a patient to withstand and recover from sepsis. Insulin promotes beneficial nitric oxide synthase activation (e‐NOS) in capillary endothelium.2326 In patients with prolonged critical illness, intensive insulin therapy lowers ICAM‐1 levels, reflecting reduced endothelial activation. Whereas e‐NOS exerts a beneficial endothelial effect, hepatic iNOS activation is harmful. One proposed mechanism of benefit from adequate insulin therapy is suppression of excessive hepatic iNOS‐induced release of circulating NO, which might contribute to endothelial dysfunction, organ failure, and death.27
Additional proposed mechanisms of hyperglycemia‐induced harm to hospitalized patients, some potentially specifically reduced by insulin therapy, resemble those discussed in relation to macrovascular disease and include activation of inflammatory cytokines, matrix metalloproteinases, and adhesion molecules, and the adverse arrhythmogenic effects of elevated circulating nonesterified fatty acids (Figure 1).2834

CRITICAL WINDOW OF TIME
The results of several retrospectively or prospectively conducted single‐institution observational studies suggest there is a critical window of time within which clinicians must get it right, when attempting glycemic control or else jeopardize therapeutic goals, such as duration of remission in treatment of acute lymphocytic leukemia35 or avoidance of acute rejection in renal allograft surgery.36 Additionally, delayed risk of infection appears to be linked to previous glycemic control during specific early time frames surrounding surgery, renal transplantation, admission for trauma, and induction chemotherapy for leukemia (Table 1).3541 For patients who have diabetes, the potential to reduce the consequences of infections through intensified glycemic control probably begins prior to admission and in the hospital has still not been fully realized.4244
| Patients | Ascertainment of hyperglycemia | Delayed events among patients with early hyperglycemia |
|---|---|---|
| 100 postoperative uninfected diabetic patients undergoing elective surgery, monitored prospectively37 | First postoperative day | Postoperative nosocomial infection rate within 14 days was 2.7 times higher for patients having at least one BG > 220 mg/dL (33.3% vs. 11.5%). |
| 990 historical controls and 595 patients in the interventional group of postoperative cardiac diabetic patients40 | First 2 postoperative days | Incidence of deep sternal wound infection was reduced from 2.4% to 1.5% (P < 0.02) after introduction of protocol to maintain mean BG < 200 mg/dL. |
| 423 renal allograft recipients receiving their first cadaveric transplant36 | First 100 hours; first day. | A mean of 10.8 2.3 days after transplantation, 70% of patients developed postoperative infection, and after a mean of 7.7 2.6 days, 40% developed acute rejection. Every patient with mean BG over 200 mg/dL during the first 100 hours developed postoperative infection. On the first postoperative day the mean BG had been 248.4 mg/dL among those developing infection and 167.4 mg/dL among those without infection (P < .001), and the mean BG had been 270 mmol/L among those developing rejection and 194 mmol/L among those without rejection. |
| 516 trauma patients admitted to the ICU38 | Either of first 2 hospital days | Hyperglycemia 200 mg/dL was associated with a higher infection rate (32% vs. 22%, P = .04) and with greater mortality (34% vs. 13%, P < .0001) |
| 275 patients having lower‐extremity peripheral vascular surgery39 | First 48 hours | Postoperative infections within 30 days were 5.1 times more frequent in the top quartile for BG versus the lowest quartile (confidence interval 1.6‐17.1, P = .007). |
| 278 adult patients receiving induction chemotherapy for acute lymphocytic leukemia35 | First 30 days | Hyperglycemia defined as 2 BG 200 mg/dL was associated with a greater likelihood of sepsis (16.5% vs. 8.0%, P = .03) or any complicated infection (38.8% vs. 25.1%, P = .016), shorter duration of complete remission (24 vs. 52 months), and with shorter median survival (29 vs. 88 months, P = .001). |
KEY STUDIES SHOWING CLINICAL BENEFIT OF TIGHT GLYCEMIC CONTROL
A summary of several key studies that demonstrated the clinical benefit of tight glycemic control is shown in Table 2. These studies successfully separated the intensively and conventionally managed study groups according blood glucose. Although trials using glucose‐insulin‐potassium infusions (GIK) such that blood glucose was lowered have shown benefit for patients who have had myocardial infarctions4547 or cardiac surgery,48 not all GIK studies have yielded positive results. The negative results of the CREATE‐ECLA study suggest that GIK therapy per se is not beneficial unless it reduces blood glucose.49 In the setting of acute myocardial infarction, the DIGAMI 2 trial and the HI‐5 trials failed to achieve the intended separation between treatment groups.50, 51 It has been speculated that if insulin is delivered so as to not achieve normoglycemia, then hyperinsulinemia in the presence of hyperglycemia actually may be proinflammatory.52
| Population and/or setting and patients | Study design and/or intervention | Principal findings |
|---|---|---|
| Patients with diabetes mellitus and with hyperglycemia > 11 mmol/L or similar hyperglycemia without known diabetes who had had acute myocardial within the preceding 24 hours.4547 There were 620 patients, of whom 15 of the controls and 10 patients in the intensive group had no prior diagnosis of diabetes. | Prospective, randomized, controlled clinical trial. Controls received standard treatment. Treatment group received infusion of glucose‐insulin for at least 24 hours, followed by multiple injections of subcutaneous insulin for at least 3 months. | Overall mortality after 1 year was l9% in the insulin group compared to 26% among controls (P < .05). The most frequent cause of death of all patients was congestive heart failure. The benefit continued for at least 3.5 years, with an absolute reduction in mortality of 11%. |
| Diabetic cardiac surgery population.40, 5557 To date, 5510 cardiac surgery patients who were either admitted or discharged with the diagnosis of diabetes have been studied. | Prospective nonrandomized study of the effects of hyperglycemia and its pharmacologic reduction with intensive intravenous insulin regimens on outcomes. | The 3‐day average of perioperative BG (3‐BG) correlated with mortality (P < .0001, odds ratio 2.0 per 50 mg/dL increase in 3‐BG). Continuous intravenous insulin infusion independently reduced risk of death 60% (RR = 0.4, P < .001). Length of stay in the CABG population increased by 1 day for every increase of 50 mg/dL in 3‐BG. |
| Critically ill surgical patients receiving mechanical ventilation. Overall, 13% of 1548 participants had previously diagnosed diabetes. | Prospective, randomized, controlled clinical trial. Intravenous insulin infusion targeting BG 80‐110 mg/dL was initiated in the intensive group for BG > 110 mg/dL. Intravenous insulin infusion targeting BG 180‐200 was initiated in the conventional group for BG > 215 mg/dL. A whole‐blood glucose method using a gas analyzer was employed to determine arterial blood glucose. | In the group assigned to intensive insulin therapy mortality was reduced during intensive care from 8.0% with conventional treatment to 4.6% (P < .04), or a risk reduction of 42%. In the group remaining in the ICU for more than 5 days, mortality was reduced from 20.2% with conventional treatment to 10.6% with intensive insulin therapy; P = .005. The intensive group experienced reductions in overall in‐hospital mortality of 34%, in bloodstream infections of 46%, and in acute renal failure requiring dialysis or hemofiltration of 41%. Also reduced were the need for red‐cell transfusions, critical‐illness neuropathy, duration of mechanical ventilation, and length of stay in the ICU. |
| Critically ill medical patients. The intention to treat group included 1200 participants, of whom 16.9% had a history of diabetes.54 | Randomized controlled clinical trial, as above. | In the intensive group, in‐hospital mortality was not significantly reduced, and among the 433 who stayed in the ICU for less than 3 days, mortality was actually higher. However, in the intention‐to‐treat group there was reduction in newly acquired kidney injury, prolonged mechanical ventilation, and length of stay in the ICU and in the hospital. Among the 767 patients who stayed in the ICU for 3 or more days, intensive insulin therapy reduced in‐hospital mortality from 52.5% to 43.0%. |
| Patients within 24 hours of having an acute stroke and who had poststroke hyperglycemia. The results of the first 452 patients recruited to the GIST‐UK study showed that 15.3% had previously recognized diabetes.62 | Randomized controlled clinical trial. The intensive group received glucose‐potassium‐insulin (GKI) infusion, and the conventional group received saline infusion. | Although mean glucose declined in both groups, the GKI infusion safely achieved separation of groups by blood glucose. Outcomes are pending. |
The findings of the negative intensive insulin studies do not offset the evidence favoring glycemic control, derived from studies that actually achieved the lowering of blood glucose in the intensive groups, such as the successful prospectively designed trials in myocardial infarction or surgical or medical intensive care units15, 45, 47, 53, 54 and the long‐running, large, prospectively monitored Portland series utilizing insulin infusion for cardiac surgery,40, 5557 which have demonstrated the beneficial effects of euglycemia on mortality and morbidity, or the findings of Krinsley, reported elsewhere in this issue.58, 59 The well‐recognized correlation between outcomes of acute stroke and poststroke hyperglycemia has led to the design of a multicenter trial of glucose‐insulin‐potassium infusion for stroke, in which separation of groups by blood glucose has been achieved.6062 The success of GIK therapy in controlling hyperglycemia depends in part on the particular formulation of the infusion as it matches patient needs, and it is probable that insulin infusion following stroke is capable of safely achieving even tighter glycemic control than GIK.63 Intravenous insulin infusion therapy is more difficult to conduct than GIK therapy. However, because of concern about the proinflammatory and prothrombotic effects of hyperglycemia and recognition of the occasional failure of GIK infusions to control hyperglycemia and of the anti‐inflammatory, antithrombotic, and vasodilatory actions of insulin, there have been calls for additional trials of insulin infusions (as opposed to glucose‐insulin infusions) for both acute myocardial infarction and stroke.52, 64
HYPOGLYCEMIA
Serious or fatal sequelae of hypoglycemia are the principal safety risks in intensive insulin management.9, 65 Case ascertainment cannot be assured by glucose averaging methods but instead requires a method of searching for isolated episodes of hypoglycemia.10 One of the most dreaded consequences of nonfatal hypoglycemia is permanent impairment of intellectual function. Because many euglycemic medically ill patients experience alteration of sensorium while acutely ill, there is a risk that the consequences of an actual episode of severe hypoglycemia will be ascribed to other comorbidities, overlooking or misattributing the altered cognitive function that may persist at discharge to causes other than the obvious iatrogenic one. Because there is an increased risk of hypoglycemia during intensive insulin therapy, controversy has arisen over glycemic targets, especially among critically ill nonsurgical patients.54, 6669
On the other hand, the consequences of hypoglycemia during intensive intravenous insulin therapy in a surgical ICU were said to be negligible.15 In hospitalized elderly patients, hypoglycemia in association with increased mortality risk may not be an independent predictor.70 In the critical care unit, predictors of hypoglycemia are identifiable after the introduction of strict glycemic control that include not only insulin therapy but also CVVH treatment with bicarbonate substitution fluid, discontinuation of nutrition without insulin adjustment, prior diagnosis of diabetes mellitus, sepsis, and the need for inotropic or vasopressor drugs.71 A prudent policy for the future would be not only to treat hypoglycemia promptly when it does occur, with attention to subsequent monitoring to avoid relapse or recurrence, but also to actively introduce strategies for predicting the risk of hypoglycemia and preventing it, especially when high risk is identified.10 The fear of hypoglycemia should not paralyze efforts to achieve better glycemic control in hospitalized patients.
UNANSWERED QUESTIONS AND VISION FOR THE FUTURE
On general wards, 38% of admitted patients may have hyperglycemia.72 As shown in the Leuven, Belgium, study, to achieve the target BG of less than 110 mg/dL in the intensive group in the surgical critical care unit, it was necessary to administer intravenous insulin infusion to essentially all patients. In a study that utilized continuous glucose monitoring, normoglycemia in patients in the intensive care unit was achieved as little as 22% of the time.73
An actively debated subject is how best to assess hospital performance in glycemic control (glucometrics). Hospitals need to show satisfactory control of variability between patients and within the treatment course of individual patients. That is, it is not sufficient to be satisfied with reasonable results of average blood glucose, using blood glucose as the unit of observation (where n is the number of blood glucose determinations). Alternatives are to use patient day or individual patient as a unit of observation (where n is the number of patient days and the number of patients, respectively). Time‐weighting methods are cumbersome but increase the validity of the averaging method used. When the patient is the unit of observation, possible measures include a per‐patient blood glucose average, percentage of blood glucose measurements with certain ranges, time spent within certain blood glucose ranges, or area under the curve of blood glucose versus time. Caveats about glucometrics pertain to both intravenous insulin infusion and also subcutaneous insulin management.74
Although awaiting additional evidence, diabetes experts have widely accepted the proposition that hospitals should focus on prevention of hyperglycemia as an important patient safety factor.75 Although the target range for glycemic control remains controversial, many students of the subject have endorsed the recommendations mentioned in the lead article in this issue, with the understanding that these criteria were developed from the results of the Leuven, Belgium, study, in which a whole blood glucose analyzer was used for measurement, and that the method of measurement of blood glucose must be considered in interpreting applicability of the target range at individual hospitals, many of which use plasma‐correlated methods yielding higher results. However, in new settings and for medical conditions that have not yet been rigorously evaluated by clinical trials, it is an unanswered question whether intensification of glycemic control can be achieved safely outside the critical care unit and, if so, what type of insulin therapy should be used and for what conditions the benefits would outweigh the risks and justify the costs.
Most inpatient management probably will continue to be conducted using subcutaneous injection therapy,7682 designed to match carbohydrate exposure through the appropriate use of analogs or conventional insulin products. One argument for the use of insulin analogs in the hospital, using basal‐prandial‐correction therapy, is the probability of reducing hypoglycemia and getting closer to target range control among patients who are eating but who are at risk for abrupt suspension of meals. Aggressive subcutaneous management strategies are likely to be most effective when standardized protocols, order sets, and informative computerized order entry systems gain widespread hospital acceptance.
If the importance of gaining glycemic control is highly time dependent and if hyperglycemia is uncontrolled, then a strong argument can be made for routine use of intravenous insulin infusion. For appropriately selected patients, intravenous insulin infusion is cost effective,83, 84 and its use can be extended to appropriate patients outside the critical care unit.8587 Many hospitals have protocols for intravenous insulin but use them only sporadically. For patients who already are in the intensive care setting, it is imperative to develop policies that require introduction of intravenous insulin infusion at a given glycemic threshold. On general wards that lack sufficient staffing to conduct intravenous insulin therapy, it is appropriate either to transfer candidate patients to a ward that has adequate staffing when medical condition requires improved control or to develop policies and procedures that will extend the use of intravenous insulin infusion to general wards. In the future, new technologies can be envisioned that will unburden nursing staff, making intravenous insulin infusion realizable as the treatment of choice for hemodynamically stable patients in most hospital settings. These technologies will include continuous monitoring of blood glucose, dose‐defining algorithms, and the eventual development of a fully automated closed‐loop system of monitoring and delivery that might automatically match insulin delivery to carbohydrate exposure and patient insulin sensitivity.8893
Until recently it had been argued that hospitalization was not the time in the life of a patient to insist on tight glycemic control. Hyperglycemia was understood to be a consequence of medical stress.1 It was well known that infection, sepsis, or other medical stress might exacerbate hyperglycemia or promote a diabetic crisis.25 Admittedly, the severity of hyperglycemia among patients who have diabetes was thought to predict the risk for hospitalization with infection as well as the outcome of the infectious condition.68 However, until recently strict glycemic control in the hospital was not strongly advocated because hypoglycemia might occur and might be directly and uniquely traceable to actions taken in the hospital.9, 10 Furthermore, the complications of diabetes were thought to be divided into acute metabolic emergencies and chronic tissue complications such as polyneuropathy, retinopathy, nephropathy, and macrovascular disease that would have evolved over a period longer than the duration of hospitalization, and the possibility that short‐term hyperglycemia might affect outcomes was considered unproven.
The purpose of this article is to define the specific populations, disorders, and hospital settings for which there now is strong evidence that short‐term glycemic control will affect the outcome of a course of hospital treatment.
PHYSIOLOGIC LINK BETWEEN HYPERGLYCEMIA AND ADVERSE OUTCOMES
Five years ago a caregiver would not have been likely to think of glycemic control as a contributing factor when considering specific complex problems such as pump failure or arrhythmia after cardiac surgery, long‐term mortality after myocardial infarction, acute renal failure, the need for transfusion during treatment of a complicated surgical illness, or prolonged dependence on a ventilator in the surgical ICU. Although it now known that these and other complications are linked to hospital hyperglycemia, the mechanisms of harm are several steps removed from the hyperglycemia itself. The causes of these adverse outcomes are multifactorial. The causal dependence of the injury on hyperglycemia is not easy to see. In fact, it required randomized prospectively designed trials to convincingly demonstrate the contributory role of hyperglycemia to these and other adverse outcomes.
Now that this link has been convincingly demonstrated, there is intense interest in discovering the probable mechanisms by which control of hyperglycemia and specifically the use of insulin might improve outcomes.1114 Mortality, predominantly sepsis related, was the primary outcome for which the Leuven, Belgium, report of 2001 on the surgical ICU showed improvement.15 Simplistically, in seeking a mechanism of benefit with respect to sepsis, it might be argued that if gross hyperglycemia were prevented in patients with surgical wounds, improvement of host defenses against infective organisms might be expected.1622 However, additional mechanisms of protection probably should be invoked, including those by which glycemic control and specifically insulin therapy affect endothelial function and the coagulation pathway, thus improving the ability of a patient to withstand and recover from sepsis. Insulin promotes beneficial nitric oxide synthase activation (e‐NOS) in capillary endothelium.2326 In patients with prolonged critical illness, intensive insulin therapy lowers ICAM‐1 levels, reflecting reduced endothelial activation. Whereas e‐NOS exerts a beneficial endothelial effect, hepatic iNOS activation is harmful. One proposed mechanism of benefit from adequate insulin therapy is suppression of excessive hepatic iNOS‐induced release of circulating NO, which might contribute to endothelial dysfunction, organ failure, and death.27
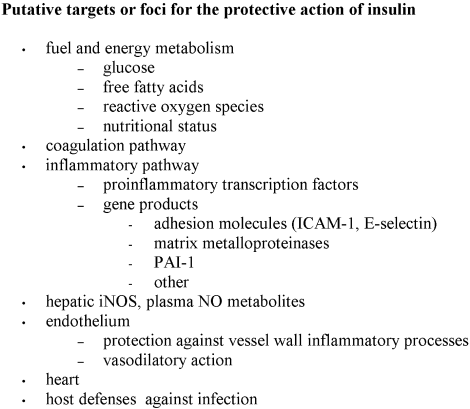
Additional proposed mechanisms of hyperglycemia‐induced harm to hospitalized patients, some potentially specifically reduced by insulin therapy, resemble those discussed in relation to macrovascular disease and include activation of inflammatory cytokines, matrix metalloproteinases, and adhesion molecules, and the adverse arrhythmogenic effects of elevated circulating nonesterified fatty acids (Figure 1).2834

CRITICAL WINDOW OF TIME
The results of several retrospectively or prospectively conducted single‐institution observational studies suggest there is a critical window of time within which clinicians must get it right, when attempting glycemic control or else jeopardize therapeutic goals, such as duration of remission in treatment of acute lymphocytic leukemia35 or avoidance of acute rejection in renal allograft surgery.36 Additionally, delayed risk of infection appears to be linked to previous glycemic control during specific early time frames surrounding surgery, renal transplantation, admission for trauma, and induction chemotherapy for leukemia (Table 1).3541 For patients who have diabetes, the potential to reduce the consequences of infections through intensified glycemic control probably begins prior to admission and in the hospital has still not been fully realized.4244
| Patients | Ascertainment of hyperglycemia | Delayed events among patients with early hyperglycemia |
|---|---|---|
| 100 postoperative uninfected diabetic patients undergoing elective surgery, monitored prospectively37 | First postoperative day | Postoperative nosocomial infection rate within 14 days was 2.7 times higher for patients having at least one BG > 220 mg/dL (33.3% vs. 11.5%). |
| 990 historical controls and 595 patients in the interventional group of postoperative cardiac diabetic patients40 | First 2 postoperative days | Incidence of deep sternal wound infection was reduced from 2.4% to 1.5% (P < 0.02) after introduction of protocol to maintain mean BG < 200 mg/dL. |
| 423 renal allograft recipients receiving their first cadaveric transplant36 | First 100 hours; first day. | A mean of 10.8 2.3 days after transplantation, 70% of patients developed postoperative infection, and after a mean of 7.7 2.6 days, 40% developed acute rejection. Every patient with mean BG over 200 mg/dL during the first 100 hours developed postoperative infection. On the first postoperative day the mean BG had been 248.4 mg/dL among those developing infection and 167.4 mg/dL among those without infection (P < .001), and the mean BG had been 270 mmol/L among those developing rejection and 194 mmol/L among those without rejection. |
| 516 trauma patients admitted to the ICU38 | Either of first 2 hospital days | Hyperglycemia 200 mg/dL was associated with a higher infection rate (32% vs. 22%, P = .04) and with greater mortality (34% vs. 13%, P < .0001) |
| 275 patients having lower‐extremity peripheral vascular surgery39 | First 48 hours | Postoperative infections within 30 days were 5.1 times more frequent in the top quartile for BG versus the lowest quartile (confidence interval 1.6‐17.1, P = .007). |
| 278 adult patients receiving induction chemotherapy for acute lymphocytic leukemia35 | First 30 days | Hyperglycemia defined as 2 BG 200 mg/dL was associated with a greater likelihood of sepsis (16.5% vs. 8.0%, P = .03) or any complicated infection (38.8% vs. 25.1%, P = .016), shorter duration of complete remission (24 vs. 52 months), and with shorter median survival (29 vs. 88 months, P = .001). |
KEY STUDIES SHOWING CLINICAL BENEFIT OF TIGHT GLYCEMIC CONTROL
A summary of several key studies that demonstrated the clinical benefit of tight glycemic control is shown in Table 2. These studies successfully separated the intensively and conventionally managed study groups according blood glucose. Although trials using glucose‐insulin‐potassium infusions (GIK) such that blood glucose was lowered have shown benefit for patients who have had myocardial infarctions4547 or cardiac surgery,48 not all GIK studies have yielded positive results. The negative results of the CREATE‐ECLA study suggest that GIK therapy per se is not beneficial unless it reduces blood glucose.49 In the setting of acute myocardial infarction, the DIGAMI 2 trial and the HI‐5 trials failed to achieve the intended separation between treatment groups.50, 51 It has been speculated that if insulin is delivered so as to not achieve normoglycemia, then hyperinsulinemia in the presence of hyperglycemia actually may be proinflammatory.52
| Population and/or setting and patients | Study design and/or intervention | Principal findings |
|---|---|---|
| Patients with diabetes mellitus and with hyperglycemia > 11 mmol/L or similar hyperglycemia without known diabetes who had had acute myocardial within the preceding 24 hours.4547 There were 620 patients, of whom 15 of the controls and 10 patients in the intensive group had no prior diagnosis of diabetes. | Prospective, randomized, controlled clinical trial. Controls received standard treatment. Treatment group received infusion of glucose‐insulin for at least 24 hours, followed by multiple injections of subcutaneous insulin for at least 3 months. | Overall mortality after 1 year was l9% in the insulin group compared to 26% among controls (P < .05). The most frequent cause of death of all patients was congestive heart failure. The benefit continued for at least 3.5 years, with an absolute reduction in mortality of 11%. |
| Diabetic cardiac surgery population.40, 5557 To date, 5510 cardiac surgery patients who were either admitted or discharged with the diagnosis of diabetes have been studied. | Prospective nonrandomized study of the effects of hyperglycemia and its pharmacologic reduction with intensive intravenous insulin regimens on outcomes. | The 3‐day average of perioperative BG (3‐BG) correlated with mortality (P < .0001, odds ratio 2.0 per 50 mg/dL increase in 3‐BG). Continuous intravenous insulin infusion independently reduced risk of death 60% (RR = 0.4, P < .001). Length of stay in the CABG population increased by 1 day for every increase of 50 mg/dL in 3‐BG. |
| Critically ill surgical patients receiving mechanical ventilation. Overall, 13% of 1548 participants had previously diagnosed diabetes. | Prospective, randomized, controlled clinical trial. Intravenous insulin infusion targeting BG 80‐110 mg/dL was initiated in the intensive group for BG > 110 mg/dL. Intravenous insulin infusion targeting BG 180‐200 was initiated in the conventional group for BG > 215 mg/dL. A whole‐blood glucose method using a gas analyzer was employed to determine arterial blood glucose. | In the group assigned to intensive insulin therapy mortality was reduced during intensive care from 8.0% with conventional treatment to 4.6% (P < .04), or a risk reduction of 42%. In the group remaining in the ICU for more than 5 days, mortality was reduced from 20.2% with conventional treatment to 10.6% with intensive insulin therapy; P = .005. The intensive group experienced reductions in overall in‐hospital mortality of 34%, in bloodstream infections of 46%, and in acute renal failure requiring dialysis or hemofiltration of 41%. Also reduced were the need for red‐cell transfusions, critical‐illness neuropathy, duration of mechanical ventilation, and length of stay in the ICU. |
| Critically ill medical patients. The intention to treat group included 1200 participants, of whom 16.9% had a history of diabetes.54 | Randomized controlled clinical trial, as above. | In the intensive group, in‐hospital mortality was not significantly reduced, and among the 433 who stayed in the ICU for less than 3 days, mortality was actually higher. However, in the intention‐to‐treat group there was reduction in newly acquired kidney injury, prolonged mechanical ventilation, and length of stay in the ICU and in the hospital. Among the 767 patients who stayed in the ICU for 3 or more days, intensive insulin therapy reduced in‐hospital mortality from 52.5% to 43.0%. |
| Patients within 24 hours of having an acute stroke and who had poststroke hyperglycemia. The results of the first 452 patients recruited to the GIST‐UK study showed that 15.3% had previously recognized diabetes.62 | Randomized controlled clinical trial. The intensive group received glucose‐potassium‐insulin (GKI) infusion, and the conventional group received saline infusion. | Although mean glucose declined in both groups, the GKI infusion safely achieved separation of groups by blood glucose. Outcomes are pending. |
The findings of the negative intensive insulin studies do not offset the evidence favoring glycemic control, derived from studies that actually achieved the lowering of blood glucose in the intensive groups, such as the successful prospectively designed trials in myocardial infarction or surgical or medical intensive care units15, 45, 47, 53, 54 and the long‐running, large, prospectively monitored Portland series utilizing insulin infusion for cardiac surgery,40, 5557 which have demonstrated the beneficial effects of euglycemia on mortality and morbidity, or the findings of Krinsley, reported elsewhere in this issue.58, 59 The well‐recognized correlation between outcomes of acute stroke and poststroke hyperglycemia has led to the design of a multicenter trial of glucose‐insulin‐potassium infusion for stroke, in which separation of groups by blood glucose has been achieved.6062 The success of GIK therapy in controlling hyperglycemia depends in part on the particular formulation of the infusion as it matches patient needs, and it is probable that insulin infusion following stroke is capable of safely achieving even tighter glycemic control than GIK.63 Intravenous insulin infusion therapy is more difficult to conduct than GIK therapy. However, because of concern about the proinflammatory and prothrombotic effects of hyperglycemia and recognition of the occasional failure of GIK infusions to control hyperglycemia and of the anti‐inflammatory, antithrombotic, and vasodilatory actions of insulin, there have been calls for additional trials of insulin infusions (as opposed to glucose‐insulin infusions) for both acute myocardial infarction and stroke.52, 64
HYPOGLYCEMIA
Serious or fatal sequelae of hypoglycemia are the principal safety risks in intensive insulin management.9, 65 Case ascertainment cannot be assured by glucose averaging methods but instead requires a method of searching for isolated episodes of hypoglycemia.10 One of the most dreaded consequences of nonfatal hypoglycemia is permanent impairment of intellectual function. Because many euglycemic medically ill patients experience alteration of sensorium while acutely ill, there is a risk that the consequences of an actual episode of severe hypoglycemia will be ascribed to other comorbidities, overlooking or misattributing the altered cognitive function that may persist at discharge to causes other than the obvious iatrogenic one. Because there is an increased risk of hypoglycemia during intensive insulin therapy, controversy has arisen over glycemic targets, especially among critically ill nonsurgical patients.54, 6669
On the other hand, the consequences of hypoglycemia during intensive intravenous insulin therapy in a surgical ICU were said to be negligible.15 In hospitalized elderly patients, hypoglycemia in association with increased mortality risk may not be an independent predictor.70 In the critical care unit, predictors of hypoglycemia are identifiable after the introduction of strict glycemic control that include not only insulin therapy but also CVVH treatment with bicarbonate substitution fluid, discontinuation of nutrition without insulin adjustment, prior diagnosis of diabetes mellitus, sepsis, and the need for inotropic or vasopressor drugs.71 A prudent policy for the future would be not only to treat hypoglycemia promptly when it does occur, with attention to subsequent monitoring to avoid relapse or recurrence, but also to actively introduce strategies for predicting the risk of hypoglycemia and preventing it, especially when high risk is identified.10 The fear of hypoglycemia should not paralyze efforts to achieve better glycemic control in hospitalized patients.
UNANSWERED QUESTIONS AND VISION FOR THE FUTURE
On general wards, 38% of admitted patients may have hyperglycemia.72 As shown in the Leuven, Belgium, study, to achieve the target BG of less than 110 mg/dL in the intensive group in the surgical critical care unit, it was necessary to administer intravenous insulin infusion to essentially all patients. In a study that utilized continuous glucose monitoring, normoglycemia in patients in the intensive care unit was achieved as little as 22% of the time.73
An actively debated subject is how best to assess hospital performance in glycemic control (glucometrics). Hospitals need to show satisfactory control of variability between patients and within the treatment course of individual patients. That is, it is not sufficient to be satisfied with reasonable results of average blood glucose, using blood glucose as the unit of observation (where n is the number of blood glucose determinations). Alternatives are to use patient day or individual patient as a unit of observation (where n is the number of patient days and the number of patients, respectively). Time‐weighting methods are cumbersome but increase the validity of the averaging method used. When the patient is the unit of observation, possible measures include a per‐patient blood glucose average, percentage of blood glucose measurements with certain ranges, time spent within certain blood glucose ranges, or area under the curve of blood glucose versus time. Caveats about glucometrics pertain to both intravenous insulin infusion and also subcutaneous insulin management.74
Although awaiting additional evidence, diabetes experts have widely accepted the proposition that hospitals should focus on prevention of hyperglycemia as an important patient safety factor.75 Although the target range for glycemic control remains controversial, many students of the subject have endorsed the recommendations mentioned in the lead article in this issue, with the understanding that these criteria were developed from the results of the Leuven, Belgium, study, in which a whole blood glucose analyzer was used for measurement, and that the method of measurement of blood glucose must be considered in interpreting applicability of the target range at individual hospitals, many of which use plasma‐correlated methods yielding higher results. However, in new settings and for medical conditions that have not yet been rigorously evaluated by clinical trials, it is an unanswered question whether intensification of glycemic control can be achieved safely outside the critical care unit and, if so, what type of insulin therapy should be used and for what conditions the benefits would outweigh the risks and justify the costs.
Most inpatient management probably will continue to be conducted using subcutaneous injection therapy,7682 designed to match carbohydrate exposure through the appropriate use of analogs or conventional insulin products. One argument for the use of insulin analogs in the hospital, using basal‐prandial‐correction therapy, is the probability of reducing hypoglycemia and getting closer to target range control among patients who are eating but who are at risk for abrupt suspension of meals. Aggressive subcutaneous management strategies are likely to be most effective when standardized protocols, order sets, and informative computerized order entry systems gain widespread hospital acceptance.
If the importance of gaining glycemic control is highly time dependent and if hyperglycemia is uncontrolled, then a strong argument can be made for routine use of intravenous insulin infusion. For appropriately selected patients, intravenous insulin infusion is cost effective,83, 84 and its use can be extended to appropriate patients outside the critical care unit.8587 Many hospitals have protocols for intravenous insulin but use them only sporadically. For patients who already are in the intensive care setting, it is imperative to develop policies that require introduction of intravenous insulin infusion at a given glycemic threshold. On general wards that lack sufficient staffing to conduct intravenous insulin therapy, it is appropriate either to transfer candidate patients to a ward that has adequate staffing when medical condition requires improved control or to develop policies and procedures that will extend the use of intravenous insulin infusion to general wards. In the future, new technologies can be envisioned that will unburden nursing staff, making intravenous insulin infusion realizable as the treatment of choice for hemodynamically stable patients in most hospital settings. These technologies will include continuous monitoring of blood glucose, dose‐defining algorithms, and the eventual development of a fully automated closed‐loop system of monitoring and delivery that might automatically match insulin delivery to carbohydrate exposure and patient insulin sensitivity.8893
- ,,.Stress‐induced hyperglycemia.Crit Care Clin.2001;17(1):107–124.
- ,,,,.Hyperosmolarity and acidosis in diabetes mellitus: a three‐year experience in Rhode Island.J Gen Intern Med.1991:495–502.
- ,,.Prognostic factors in the diabetic hyperosmolar state.J Am Geriatr Soc.1987;35:737–741.
- ,,.Predisposing factors for the diabetic hyperosmolar state.Arch Intern Med.1987;147:499–501.
- .The diabetic hyperosmolar state.Clin Geriatr Med.1990;6:797–806.
- ,,,,,.Bacteremia in adult diabetic patients.Diabetes Care.1991;14(2):89–94.
- ,,,,,.Influence of diabetes mellitus and glycaemic control on the characteristics and outcome of common infections.Diabet Med.1996:457–463.
- ,,.Diabetes and the risk of infection‐related mortality in the U.S.Diabetes Care.2001;24:1044–1049.
- ,,.Hypoglycemia in hospitalized patients.N Engl J Med.1986;315:1245–1250.
- ,,, et al.Hospital hypoglycemia: not only treatment but also prevention.Endocr Pract.2004;10(suppl 2):71–80.
- .Effect of insulin therapy on nonglycemic variables during acute illness.Endocr Pract.2004;10(suppl 2):63–70.
- .Molecular mechanisms by which metabolic control may improve outcomes.Endocr Pract.2004;10(suppl 2):57–62.
- ,,,,.Insulin infusion in acute illness.J Clin Invest.2005;115:2069–2072.
- ,,.Intensive insulin therapy in the intensive care unit: update on clinical impact and mechanisms of action.Endocr Pract.2006;12(suppl 3):14–21.
- ,,, et al.Intensive insulin therapy in critically ill patients.N Engl J Med.2001;345:1359–1367.
- ,,.Impaired granulocyte adherence. A reversible defect in host defense in patients with poorly controlled diabetes.Diabetes.1978;27:677–681.
- ,,.Impaired leukocyte function in patients with poorly controlled diabetes.Diabetes.1974;23(1):9–15.
- ,,, et al.The effect of diabetes mellitus on chemotactic and bactericidal activity of human polymorphonuclear leukocytes.Diabetes Res Clin Pract.1987;4:27–35.
- ,,,,.Polymorphonuclear leukocytes in non‐insulin‐dependent diabetes mellitus: abnormalities in metabolism and function.Ann Intern Med.1995;123:919–924.
- ,,,,.Agonist‐dependent failure of neutrophil function in diabetes correlates with extent of hyperglycemia.J Leukoc Biol.2001;70:395–404.
- ,,,,,.Insulin increases neutrophil count and phagocytic capacity after cardiac surgery.Anesth Analg.2002;94:1113–1119.
- ,,,.In vivo evidences that insulin regulates human polymorphonuclear neutrophil functions.J Leukoc Biol.2004;76:1104–1110.
- ,.Insulin‐stimulated production of nitric oxide is inhibited by wortmannin. Direct measurement in vascular endothelial cells.J Clin Invest.1996;98:894–898.
- ,,,,.Insulin‐mediated skeletal muscle vasodilation is nitric oxide dependent. A novel action of insulin to increase nitric oxide release.J Clin Invest.1994;94:1172–1179.
- ,.Effect of insulin on human aortic endothelial nitric oxide synthase.Metabolism.2000;49(2):147–50.
- ,.Nitric oxide, platelet function, myocardial infarction and reperfusion therapies.Heart Fail Rev.2003;8(1):47–54.
- ,,, et al.Intensive insulin therapy protects the endothelium of critically ill patients.J Clin Invest.2005;115:2277–2286.
- ,,, et al.Elevated circulating free fatty acid levels impair endothelium‐dependent vasodilation.J Clin Invest.1997;100:1230–1239.
- ,,, et al.Insulin inhibits intranuclear nuclear factor kappaB and stimulates IkappaB in mononuclear cells in obese subjects: evidence for an anti‐inflammatory effect?J Clin Endocrinol Metab.2001;86:3257–3265.
- ,,.The anti‐inflammatory and potential anti‐atherogenic effect of insulin: a new paradigm.Diabetologia.2002;45:924–930.
- ,,,.The potential influence of inflammation and insulin resistance on the pathogenesis and treatment of atherosclerosis‐related complications in type 2 diabetes.J Clin Endocrinol Metab.2003;88:2422–2429.
- ,,.The potential therapeutic role of insulin in acute myocardial infarction in patients admitted to intensive care and in those with unspecified hyperglycemia.Diabetes Care.2003;26:516–519.
- ,,.Insulin treatment improves the systemic inflammatory reaction to severe trauma.Ann Surg.2004;239:553–560.
- ,,, et al.Anti‐inflammatory and profibrinolytic effect of insulin in acute ST‐segment‐elevation myocardial infarction.Circulation.2004;109:849–854.
- .Relation between the duration of remission and hyperglycemia in induction chemotherapy for acute lymphocytic leukemia.Cancer.2004;100:1179–1185.
- ,,,,.Early peri‐operative glycaemic control and allograft rejection in patients with diabetes mellitus: a pilot study.Transplantation.2001;72:1321–1324.
- ,,, et al.Early postoperative glucose control predicts nosocomial infection rate in diabetic patients.J Parenter Enteral Nutr.1998;22(2):77–81.
- ,,,,.Relationship of early hyperglycemia to mortality in trauma patients.J Trauma.2004;56:1058–1062.
- ,,,,.Early post‐operative glucose levels are an independent risk factor for infection after peripheral vascular surgery. A retrospective study.Eur J Vasc Endovasc Surg.2004;5:520–525.
- ,,,.Continuous intravenous insulin infusion reduces the incidence of deep sternal wound infection in diabetic patients after cardiac surgical procedures.Ann Thorac Surg.1999;67:352–362.
- ,.Effects on outcome of in‐hospital transition from intravenous insulin infusion to subcutaneous therapy.Am J Cardiol.2006;98:557–564.
- ,,,.The impact of diabetes in patients with necrotizing soft tissue infections.Surg Infect.2005;6:427–438.
- ,,,.Infections in patients with diabetes mellitus.N Engl J Med.1999;341:1906–1912.
- ,,.Quantifying the risk of infectious diseases for people with diabetes.Diabetes Care.2003;26:510–513.
- ,,, et al.Randomized trial of insulin‐glucose infusion followed by subcutaneous insulin treatment in diabetic patients with acute myocardial infarction (DIGAMI study): effects on mortality at 1 year.J Am Coll Cardiol.1995;26:57–65.
- ,,, et al.Effects of insulin treatment on cause‐specific one‐year mortality and morbidity in diabetic patients with acute myocardial infarction.Eur Heart J.1996;17:1337–1344.
- .Prospective randomised study of intensive insulin treatment on long term survival after acute myocardial infarction in patients with diabetes mellitus.DIGAMI (Diabetes Mellitus, Insulin Glucose Infusion in Acute Myocardial Infarction) Study Group.BMJ.1997;314:1512–1515.
- ,,,,,.Tight glycemic control in diabetic coronary artery bypass graft patients improves perioperative outcomes and decreases recurrent ischemic events.Circulation.2004;109:1497–1502.
- .Effect of Glucose‐Insulin‐Potassium Infusion on Mortality in Patients With Acute ST‐Segment Elevation Myocardial Infarction: The CREATE‐ECLA Randomized Controlled Trial.JAMA.2005;293:437–446.
- ,,, et al.Intense metabolic control by means of insulin in patients with diabetes mellitus and acute myocardial infarction (DIGAMI 2): effects on mortality and morbidity.Eur Heart J.2005;26:650–661.
- ,,.The Hyperglycemia: Intensive Insulin Infusion In Infarction (HI‐5) Study: A randomized controlled trial of insulin infusion therapy for myocardial infarction.Diabetes Care.2006;29:765–770.
- .Inpatient diabetes: review of data from the cardiac care unit.Endocr Pract.2006;12(suppl 3):27–34.
- ,.Reduction of nosocomial infections in the surgical intensive‐care unit by strict glycemic control.Endocr Pract.2004;10(suppl 2):46–52.
- ,,, et al.Intensive insulin therapy in the medical ICU.N Engl J Med.2006;354:449–461.
- ,,.Glucose control lowers the risk of wound infection in diabetics after open heart operations.Ann Thorac Surg.1997;63:356–361.
- ,,, et al.Continuous insulin infusion reduces mortality in patients with diabetes undergoing coronary artery bypass grafting.J Thorac Cardiovasc Surg.2003;125:1007–1021.
- ,.Clinical effects of hyperglycemia in the cardiac surgery population: the Portland diabetic project.Endocr Pract.2006;12(suppl 3):22–26.
- .Effect of an intensive glucose management protocol on the mortality of critically ill adult patients.Mayo Clin Proc.2004;79:992–1000.
- .Decreased mortality of critically ill patients with the use of an intensive glycemic management protocol.Mayo Clin Proc.2003;78:1471–1478.
- ,,, et al.Admission glucose level and clinical outcomes in the NINDS rt‐PA Stroke. Trial.Neurology.2002;59:669–74.
- ,,,,,.Glucose potassium insulin infusions in the treatment of acute stroke patients with mild to moderate hyperglycemia: the Glucose Insulin in Stroke. Trial (GIST).Stroke.1999;30:793–799.
- ,,,.Poststroke hyperglycemia: natural history and immediate management.Stroke.2004;35(1):122–126.
- ,,,.IV insulin during acute cerebral infarction in diabetic patients.Neurology.2004;62:1441–1442.
- ,,,.Hyperglycemia, insulin, and acute ischemic stroke: a mechanistic justification for a trial of insulin infusion therapy.Stroke.2006;37(1):267–273.
- ,,.Hypoglycemia and cardiac arrest in a critically ill patient on strict glycemic control.Anesth Analg.2006;102:549–551.
- ,,.Strict glucose control in the critically ill.Br Med J.2006;332:865–866.
- .Intensive Insulin in Intensive Care.N Engl J Med.2006;354:516–518.
- ,.Counterpoint: Inpatient glucose management: a premature call to arms?Diabetes Care.2005;28:976–979.
- ,,,.Glucose control and mortality in critically ill patients.JAMA.2003;290:2041–2047.
- ,,, et al.Hypoglycemia as a predictor of mortality in hospitalized elderly patients.Arch Intern Med.2003;163:1825–1829.
- ,,, et al.Predisposing factors for hypoglycemia in the intensive care unit.Crit Care Med.2006;34:96–101.
- ,,,,,.Hyperglycemia: an independent marker of in‐hospital mortality in patients with undiagnosed diabetes.J Clin Endocrinol Metab.2002;87:978–982.
- ,,,.Intensive insulin therapy in the intensive care unit: assessment by continuous glucose monitoring.Diabetes Care.2006;29:1750–1756.
- ,,,.No patient left behind: evaluation and design of intravenous insulin infusion algorithms.Endocr Pract.2006;12(suppl 3):72–78.
- ,,, et al.Management of diabetes and hyperglycemia in hospitals.Diabetes Care.2004;27:553–591.
- ,,,.Eliminating Inpatient Sliding‐Scale Insulin: A reeducation project with medical house staff.Diabetes Care.2005;28:1008–1011.
- ,,.70/30 Insulin algorithm versus sliding scale insulin.Ann Pharmacother.2005;39:1606–1609.
- ,.Hospital management of hyperglycemia.Clin Diabetes.2004;22(2):81–88.
- ,.Subcutaneous insulin therapy in the hospital setting: issues, concerns, and implementation.Endocr Pract.2004;10(suppl 2):81–88.
- ,,,,.Hyperglycemia in the hospital.Diabetes Spectr.2005;18(1):20–27.
- .Insulin Analogues.N Engl J Med.2005;352:174–183.
- .Insulin management of diabetic patients on general medical and surgical floors.Endocr Pract.2006;12(suppl 3):86–90.
- ,,, et al.Improved perioperative glycemic control by continuous insulin infusion under supervision of an endocrinologist does not increase costs in patients with diabetes.Endocr Pract.2004;10(2):112–118.
- ,,.Effect of hyperglycemia and continuous intravenous insulin infusions on outcomes of cardiac surgical procedures: the Portland Diabetic Project.Endocr Pract.2004;10(suppl 2):21–33.
- ,,,.New insulin infusion protocol improves blood glucose control in hospitalized patients without increasing hypoglycemia.J Qual Patient Saf.2005;31:141–147.
- ,,,,.Optimizing hospital use of intravenous insulin therapy: improved management of hyperglycemia and error reduction with a new nomogram.Endocr Pract.2005;11:240–253.
- ,,,,.Implementation of a new intravenous insulin method on intermediate‐care units in hospitalized patients.Diabetes Educ.2005;31:818–823.
- ,,.Clinical results of an updated insulin infusion protocol in critically ill patients.Diabetes Spectr.2005;18(3):188–191.
- ,,.Glucommander: A computer‐directed intravenous insulin system shown to be safe, simple, and effective in 120,618 h of operation.Diabetes Care.2005;28:2418–2423.
- ,,, et al.Improving hyperglycemia management in the intensive care unit: preliminary report of a nurse‐driven quality improvement project using a redesigned insulin infusion algorithm.Diabetes Educ.2006;32:394–403.
- ,,, et al.Performance of a dose‐defining insulin infusion protocol among trauma service ICU admissions.Diabetes Technol Ther.2006;8:476–488.
- ,,, et al.A simple insulin‐nutrition protocol for tight glycemic control in critical illness: development and protocol comparison.Diabetes Technol Ther.2006;8:191–206.
- ,,, et al.Evaluation of the impact of chiropodist care in the secondary prevention of foot ulcerations in diabetic subjects.Diabetes Care.2003;26:1691–1695.
- ,,.Stress‐induced hyperglycemia.Crit Care Clin.2001;17(1):107–124.
- ,,,,.Hyperosmolarity and acidosis in diabetes mellitus: a three‐year experience in Rhode Island.J Gen Intern Med.1991:495–502.
- ,,.Prognostic factors in the diabetic hyperosmolar state.J Am Geriatr Soc.1987;35:737–741.
- ,,.Predisposing factors for the diabetic hyperosmolar state.Arch Intern Med.1987;147:499–501.
- .The diabetic hyperosmolar state.Clin Geriatr Med.1990;6:797–806.
- ,,,,,.Bacteremia in adult diabetic patients.Diabetes Care.1991;14(2):89–94.
- ,,,,,.Influence of diabetes mellitus and glycaemic control on the characteristics and outcome of common infections.Diabet Med.1996:457–463.
- ,,.Diabetes and the risk of infection‐related mortality in the U.S.Diabetes Care.2001;24:1044–1049.
- ,,.Hypoglycemia in hospitalized patients.N Engl J Med.1986;315:1245–1250.
- ,,, et al.Hospital hypoglycemia: not only treatment but also prevention.Endocr Pract.2004;10(suppl 2):71–80.
- .Effect of insulin therapy on nonglycemic variables during acute illness.Endocr Pract.2004;10(suppl 2):63–70.
- .Molecular mechanisms by which metabolic control may improve outcomes.Endocr Pract.2004;10(suppl 2):57–62.
- ,,,,.Insulin infusion in acute illness.J Clin Invest.2005;115:2069–2072.
- ,,.Intensive insulin therapy in the intensive care unit: update on clinical impact and mechanisms of action.Endocr Pract.2006;12(suppl 3):14–21.
- ,,, et al.Intensive insulin therapy in critically ill patients.N Engl J Med.2001;345:1359–1367.
- ,,.Impaired granulocyte adherence. A reversible defect in host defense in patients with poorly controlled diabetes.Diabetes.1978;27:677–681.
- ,,.Impaired leukocyte function in patients with poorly controlled diabetes.Diabetes.1974;23(1):9–15.
- ,,, et al.The effect of diabetes mellitus on chemotactic and bactericidal activity of human polymorphonuclear leukocytes.Diabetes Res Clin Pract.1987;4:27–35.
- ,,,,.Polymorphonuclear leukocytes in non‐insulin‐dependent diabetes mellitus: abnormalities in metabolism and function.Ann Intern Med.1995;123:919–924.
- ,,,,.Agonist‐dependent failure of neutrophil function in diabetes correlates with extent of hyperglycemia.J Leukoc Biol.2001;70:395–404.
- ,,,,,.Insulin increases neutrophil count and phagocytic capacity after cardiac surgery.Anesth Analg.2002;94:1113–1119.
- ,,,.In vivo evidences that insulin regulates human polymorphonuclear neutrophil functions.J Leukoc Biol.2004;76:1104–1110.
- ,.Insulin‐stimulated production of nitric oxide is inhibited by wortmannin. Direct measurement in vascular endothelial cells.J Clin Invest.1996;98:894–898.
- ,,,,.Insulin‐mediated skeletal muscle vasodilation is nitric oxide dependent. A novel action of insulin to increase nitric oxide release.J Clin Invest.1994;94:1172–1179.
- ,.Effect of insulin on human aortic endothelial nitric oxide synthase.Metabolism.2000;49(2):147–50.
- ,.Nitric oxide, platelet function, myocardial infarction and reperfusion therapies.Heart Fail Rev.2003;8(1):47–54.
- ,,, et al.Intensive insulin therapy protects the endothelium of critically ill patients.J Clin Invest.2005;115:2277–2286.
- ,,, et al.Elevated circulating free fatty acid levels impair endothelium‐dependent vasodilation.J Clin Invest.1997;100:1230–1239.
- ,,, et al.Insulin inhibits intranuclear nuclear factor kappaB and stimulates IkappaB in mononuclear cells in obese subjects: evidence for an anti‐inflammatory effect?J Clin Endocrinol Metab.2001;86:3257–3265.
- ,,.The anti‐inflammatory and potential anti‐atherogenic effect of insulin: a new paradigm.Diabetologia.2002;45:924–930.
- ,,,.The potential influence of inflammation and insulin resistance on the pathogenesis and treatment of atherosclerosis‐related complications in type 2 diabetes.J Clin Endocrinol Metab.2003;88:2422–2429.
- ,,.The potential therapeutic role of insulin in acute myocardial infarction in patients admitted to intensive care and in those with unspecified hyperglycemia.Diabetes Care.2003;26:516–519.
- ,,.Insulin treatment improves the systemic inflammatory reaction to severe trauma.Ann Surg.2004;239:553–560.
- ,,, et al.Anti‐inflammatory and profibrinolytic effect of insulin in acute ST‐segment‐elevation myocardial infarction.Circulation.2004;109:849–854.
- .Relation between the duration of remission and hyperglycemia in induction chemotherapy for acute lymphocytic leukemia.Cancer.2004;100:1179–1185.
- ,,,,.Early peri‐operative glycaemic control and allograft rejection in patients with diabetes mellitus: a pilot study.Transplantation.2001;72:1321–1324.
- ,,, et al.Early postoperative glucose control predicts nosocomial infection rate in diabetic patients.J Parenter Enteral Nutr.1998;22(2):77–81.
- ,,,,.Relationship of early hyperglycemia to mortality in trauma patients.J Trauma.2004;56:1058–1062.
- ,,,,.Early post‐operative glucose levels are an independent risk factor for infection after peripheral vascular surgery. A retrospective study.Eur J Vasc Endovasc Surg.2004;5:520–525.
- ,,,.Continuous intravenous insulin infusion reduces the incidence of deep sternal wound infection in diabetic patients after cardiac surgical procedures.Ann Thorac Surg.1999;67:352–362.
- ,.Effects on outcome of in‐hospital transition from intravenous insulin infusion to subcutaneous therapy.Am J Cardiol.2006;98:557–564.
- ,,,.The impact of diabetes in patients with necrotizing soft tissue infections.Surg Infect.2005;6:427–438.
- ,,,.Infections in patients with diabetes mellitus.N Engl J Med.1999;341:1906–1912.
- ,,.Quantifying the risk of infectious diseases for people with diabetes.Diabetes Care.2003;26:510–513.
- ,,, et al.Randomized trial of insulin‐glucose infusion followed by subcutaneous insulin treatment in diabetic patients with acute myocardial infarction (DIGAMI study): effects on mortality at 1 year.J Am Coll Cardiol.1995;26:57–65.
- ,,, et al.Effects of insulin treatment on cause‐specific one‐year mortality and morbidity in diabetic patients with acute myocardial infarction.Eur Heart J.1996;17:1337–1344.
- .Prospective randomised study of intensive insulin treatment on long term survival after acute myocardial infarction in patients with diabetes mellitus.DIGAMI (Diabetes Mellitus, Insulin Glucose Infusion in Acute Myocardial Infarction) Study Group.BMJ.1997;314:1512–1515.
- ,,,,,.Tight glycemic control in diabetic coronary artery bypass graft patients improves perioperative outcomes and decreases recurrent ischemic events.Circulation.2004;109:1497–1502.
- .Effect of Glucose‐Insulin‐Potassium Infusion on Mortality in Patients With Acute ST‐Segment Elevation Myocardial Infarction: The CREATE‐ECLA Randomized Controlled Trial.JAMA.2005;293:437–446.
- ,,, et al.Intense metabolic control by means of insulin in patients with diabetes mellitus and acute myocardial infarction (DIGAMI 2): effects on mortality and morbidity.Eur Heart J.2005;26:650–661.
- ,,.The Hyperglycemia: Intensive Insulin Infusion In Infarction (HI‐5) Study: A randomized controlled trial of insulin infusion therapy for myocardial infarction.Diabetes Care.2006;29:765–770.
- .Inpatient diabetes: review of data from the cardiac care unit.Endocr Pract.2006;12(suppl 3):27–34.
- ,.Reduction of nosocomial infections in the surgical intensive‐care unit by strict glycemic control.Endocr Pract.2004;10(suppl 2):46–52.
- ,,, et al.Intensive insulin therapy in the medical ICU.N Engl J Med.2006;354:449–461.
- ,,.Glucose control lowers the risk of wound infection in diabetics after open heart operations.Ann Thorac Surg.1997;63:356–361.
- ,,, et al.Continuous insulin infusion reduces mortality in patients with diabetes undergoing coronary artery bypass grafting.J Thorac Cardiovasc Surg.2003;125:1007–1021.
- ,.Clinical effects of hyperglycemia in the cardiac surgery population: the Portland diabetic project.Endocr Pract.2006;12(suppl 3):22–26.
- .Effect of an intensive glucose management protocol on the mortality of critically ill adult patients.Mayo Clin Proc.2004;79:992–1000.
- .Decreased mortality of critically ill patients with the use of an intensive glycemic management protocol.Mayo Clin Proc.2003;78:1471–1478.
- ,,, et al.Admission glucose level and clinical outcomes in the NINDS rt‐PA Stroke. Trial.Neurology.2002;59:669–74.
- ,,,,,.Glucose potassium insulin infusions in the treatment of acute stroke patients with mild to moderate hyperglycemia: the Glucose Insulin in Stroke. Trial (GIST).Stroke.1999;30:793–799.
- ,,,.Poststroke hyperglycemia: natural history and immediate management.Stroke.2004;35(1):122–126.
- ,,,.IV insulin during acute cerebral infarction in diabetic patients.Neurology.2004;62:1441–1442.
- ,,,.Hyperglycemia, insulin, and acute ischemic stroke: a mechanistic justification for a trial of insulin infusion therapy.Stroke.2006;37(1):267–273.
- ,,.Hypoglycemia and cardiac arrest in a critically ill patient on strict glycemic control.Anesth Analg.2006;102:549–551.
- ,,.Strict glucose control in the critically ill.Br Med J.2006;332:865–866.
- .Intensive Insulin in Intensive Care.N Engl J Med.2006;354:516–518.
- ,.Counterpoint: Inpatient glucose management: a premature call to arms?Diabetes Care.2005;28:976–979.
- ,,,.Glucose control and mortality in critically ill patients.JAMA.2003;290:2041–2047.
- ,,, et al.Hypoglycemia as a predictor of mortality in hospitalized elderly patients.Arch Intern Med.2003;163:1825–1829.
- ,,, et al.Predisposing factors for hypoglycemia in the intensive care unit.Crit Care Med.2006;34:96–101.
- ,,,,,.Hyperglycemia: an independent marker of in‐hospital mortality in patients with undiagnosed diabetes.J Clin Endocrinol Metab.2002;87:978–982.
- ,,,.Intensive insulin therapy in the intensive care unit: assessment by continuous glucose monitoring.Diabetes Care.2006;29:1750–1756.
- ,,,.No patient left behind: evaluation and design of intravenous insulin infusion algorithms.Endocr Pract.2006;12(suppl 3):72–78.
- ,,, et al.Management of diabetes and hyperglycemia in hospitals.Diabetes Care.2004;27:553–591.
- ,,,.Eliminating Inpatient Sliding‐Scale Insulin: A reeducation project with medical house staff.Diabetes Care.2005;28:1008–1011.
- ,,.70/30 Insulin algorithm versus sliding scale insulin.Ann Pharmacother.2005;39:1606–1609.
- ,.Hospital management of hyperglycemia.Clin Diabetes.2004;22(2):81–88.
- ,.Subcutaneous insulin therapy in the hospital setting: issues, concerns, and implementation.Endocr Pract.2004;10(suppl 2):81–88.
- ,,,,.Hyperglycemia in the hospital.Diabetes Spectr.2005;18(1):20–27.
- .Insulin Analogues.N Engl J Med.2005;352:174–183.
- .Insulin management of diabetic patients on general medical and surgical floors.Endocr Pract.2006;12(suppl 3):86–90.
- ,,, et al.Improved perioperative glycemic control by continuous insulin infusion under supervision of an endocrinologist does not increase costs in patients with diabetes.Endocr Pract.2004;10(2):112–118.
- ,,.Effect of hyperglycemia and continuous intravenous insulin infusions on outcomes of cardiac surgical procedures: the Portland Diabetic Project.Endocr Pract.2004;10(suppl 2):21–33.
- ,,,.New insulin infusion protocol improves blood glucose control in hospitalized patients without increasing hypoglycemia.J Qual Patient Saf.2005;31:141–147.
- ,,,,.Optimizing hospital use of intravenous insulin therapy: improved management of hyperglycemia and error reduction with a new nomogram.Endocr Pract.2005;11:240–253.
- ,,,,.Implementation of a new intravenous insulin method on intermediate‐care units in hospitalized patients.Diabetes Educ.2005;31:818–823.
- ,,.Clinical results of an updated insulin infusion protocol in critically ill patients.Diabetes Spectr.2005;18(3):188–191.
- ,,.Glucommander: A computer‐directed intravenous insulin system shown to be safe, simple, and effective in 120,618 h of operation.Diabetes Care.2005;28:2418–2423.
- ,,, et al.Improving hyperglycemia management in the intensive care unit: preliminary report of a nurse‐driven quality improvement project using a redesigned insulin infusion algorithm.Diabetes Educ.2006;32:394–403.
- ,,, et al.Performance of a dose‐defining insulin infusion protocol among trauma service ICU admissions.Diabetes Technol Ther.2006;8:476–488.
- ,,, et al.A simple insulin‐nutrition protocol for tight glycemic control in critical illness: development and protocol comparison.Diabetes Technol Ther.2006;8:191–206.
- ,,, et al.Evaluation of the impact of chiropodist care in the secondary prevention of foot ulcerations in diabetic subjects.Diabetes Care.2003;26:1691–1695.