User login
BIOFEEDBACK: WHAT IS IT?
The term “biofeedback” refers to the instrumentation or training process that allows biologic information to be recorded, displayed, and communicated back to an individual, allowing the individual to make adjustments in physiologic processes that may enhance health or performance. The biofeedback display is analogous to a mirror, in which physiologic processes can be observed and adjusted much as one might adjust a hairstyle or a tie.
In our work with cardiovascular disease patients, biofeedback is a training process that involves a subject or patient, a biofeedback coach or therapist, and state-of-the-art biofeedback equipment. For biofeedback training to be effective, the subject who is trying to learn the skill must be engaged and willing to practice, the coach must be trained in psychophysiology, and the equipment must display accurate readings in real time, allowing the subject to monitor and change physiologic reactions appropriately. The coach teaches the subject about the physiologic parameters, establishes target ranges, and helps the subject learn how to move the physiologic parameters in the right direction.1,2
Training often begins with a session in which a brief mental stress test is followed by a period of relaxation while physiologic parameters are recorded and displayed. This process helps the subject to understand the link between mental processes and physiologic arousal.
Biofeedback training can involve a number of physiologic modalities, including those that reflect autonomic nervous system arousal, such as skin conductance and heart rate variability, and those that are not strictly correlated with autonomic activity, such as surface muscle tension. Each physiologic parameter is recorded by a specific sensor, and all sensors are noninvasive. Sensors feed signals into a computer, where they are processed and amplified, and subjects are able to view the output on a computer screen.
Typically, in our work, there is one screen for the subject, on which a single parameter can be displayed, observed and discussed, and another screen for the coach, on which all parameters are displayed simultaneously. During a single session of biofeedback training, the coach may choose to work on a single parameter or switch between parameters, depending on how much progress is being made with each. In our work with patients, we generally train to simple parameters first, such as respiratory rate, finger temperature, and skin conductance, moving on to surface muscle tension, heart rate, and eventually heart rate variability, which is a more complex concept and more easily understood later in the training process.
It is important that the subject receive positive reinforcement for changing the physiologic parameters, and if the subject struggles too long with one parameter, it is generally useful to go back to a different parameter, where success may be more easily experienced. Ideally, by the end of six to eight training sessions, the subject will be able to make progress on all physiologic parameters, which will track together over time.
BIOFEEDBACK-ASSISTED STRESS MANAGEMENT
Pure biofeedback training consists of operant conditioning. That is, the subject learns to regulate his or her physiology in the right direction because of the feedback, which can be as simple as a pleasant image appearing on a computer screen or as complicated as a car moving faster around a racetrack; pure biofeedback involves changing physiology in response to positive reinforcement of some sort.
In practice, we generally employ biofeedback-assisted stress management (BFSM) rather than pure biofeedback. With BFSM, the subject learns to change physiology in the direction of health and wellness by learning techniques of stress management. The coach teaches the subject various relaxation techniques, such as slow and rhythmic breathing, guided imagery, progressive muscle relaxation, mindfulness, assertiveness, and how to change negative thought patterns. With regular practice, the subject learns to change the physiologic parameters by relaxing the body. For example, instead of instructing the subject to “increase your finger temperature” and assume that the subject will achieve this because doing so will make the light bulb on the screen glow more intensely, the BFSM coach may instead talk with the subject about eliminating stressful thoughts, learning to relax, and the fingers warming in response to the body relaxing.
We distinguish between techniques of stress management, some of which are mentioned above, and psychotherapy, which can certainly be effectively combined with biofeedback, but which we do not provide in our research studies. Coupling stress management techniques with biofeedback helps the subject change physiologic parameters in the direction of wellness and acquire tools that can be used in everyday life when stressful events arise. The objective of BFSM training is not just to change physiology, but also to change the way subjects respond to stressful events in daily life; ie, react to fewer events, react less intensely when they do react, and recover more quickly.
BIOFEEDBACK-ASSISTED STRESS MANAGEMENT IN CARDIOVASCULAR DISEASE
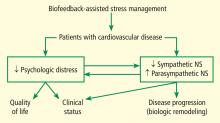
We are currently studying the effects of BFSM in patients with cardiovascular disease, including both heart failure and stable coronary artery disease. Patients with cardiovascular disease often are functionally limited, and they also experience psychologic distress related to physical limitations and other life stressors. Both the physical limitations and the psychologic distress impact quality of life. We hypothesize that BFSM will teach our patients techniques of stress management, both mental and physiologic, that will help relieve their psychologic distress and improve their quality of life. BFSM will also potentially decrease the overactivation of the sympathetic branch of the autonomic nervous system, which is common in cardiovascular disease, and correspondingly upregulate the contribution of the parasympathetic branch of the autonomic nervous system, which should be beneficial.3
A PROMISING TECHNIQUE IN HEART FAILURE
We are currently studying the effects of BFSM in patients with end-stage heart failure who are awaiting heart transplant at Cleveland Clinic.4 As noted in a recent review, biofeedback is a promising technique in heart failure that patients may be able to use to consciously regulate their autonomic nervous systems.5 We hypothesize that BFSM training will interfere with the overactivation of the sympathetic nervous system that is characteristic of heart failure, and that this will reverse the cellular and molecular remodeling that occurs in the failing human heart.
To date, we have enrolled 25 patients; 10 are being studied in our National Institutes of Health–funded Clinic Research Unit and 15 are inpatients. All 25 patients are listed as heart transplant candidates and have given consent for us to study their hearts when they are explanted.
Each patient receives eight sessions with a certified biofeedback therapist. The first and last sessions include mental stress tests, while the remaining six are BFSM training sessions. Patients are assessed at the beginning and end of the study using the 6-minute walk test, the Kansas City Cardiomyopathy Questionnaire, the Medical Outcomes Study 36-Item Short-Form Health Survey (SF-36), and measurement of plasma catecholamines.
The primary end point of the study is the measurement of cellular and molecular markers that have been shown to be altered in the failing human heart, testing the hypothesis that these markers will be reversed in the direction of normal in the BFSM therapy group. These markers are measured in the explanted failing heart when the patient receives a heart transplant.
It is too early to report the results of this study, since only seven patients have undergone transplantation to date. We are encouraged by several early findings, however, and hope these will be validated when the entire group is analyzed.
In early analysis, scores on the Kansas City Cardiomyopathy Questionnaire are improved in the last session compared with the first; patients have shown the ability to learn a slower breathing rate; and they are able to regulate their heart rate variability, as measured by the standard deviation of the N-to-N interval, or SDNN. Most important, measurements in the first seven hearts indicate that there is a degree of biologic remodeling of the failing heart after BFSM that is similar to what we have observed with left ventricular assist devices—hemodynamic pumps that take on the workload of the heart, permitting the heart to rest and recover while the patient is waiting for a transplant.6,7 If BFSM could produce changes in the cellular and molecular properties of the heart that are equal in magnitude to those produced by a mechanical pump, this would be a revolutionary finding in the field of heart-brain medicine.
It should be noted that we are not the first group to study BFSM in patients with heart failure. Moser and colleagues first observed that a single session of skin temperature biofeedback could have significant functional effects in patients with heart failure.8 Bernardi and coworkers showed that merely teaching patients to breathe six times per minute (a large component of BFSM training) improved oxygen saturation and exercise tolerance.9 Swanson and colleagues in 2009 demonstrated that patients with heart failure were able to regulate their heart rate variability, although they observed this only in patients with a left ventricular ejection fraction greater than 30%.10 Our preliminary data demonstrate regulation of heart rate variability in patients with lower ejection fractions, which is promising, but we have also added the biologic component of studying the explanted heart, allowing us to test the hypothesis that BFSM could potentially impact the remodeling process and thus have important therapeutic implications.
TRIAL UNDER WAY IN CORONARY ARTERY DISEASE
In addition to our studies of BFSM in heart failure, we have begun a randomized clinical trial of patients with stable coronary artery disease, type 2 diabetes, or multiple sclerosis. These three patient populations were chosen because evidence from numerous studies suggests that they all involve autonomic nervous system dysregulation as well as an inflammatory process.
It has already been mentioned that BFSM can interfere with overactivation of the sympathetic nervous system and potentially upregulate the contribution of the parasympathetic nervous system, which usually exists in juxtaposition to the sympathetic nervous system. Based on the work of Tracey,11,12 upregulating the parasympathetic nervous system should be antiinflammatory. Thus, we hypothesize that by decreasing both sympathetic nervous system activation and inflammation, BFSM should have an impact on patients with one of these disease states, resulting in improved quality of life and clinical status, reduced anxiety and depression, and changed disease-specific indicators of severity.
We are currently enrolling patients who have coronary artery disease, type 2 diabetes, or multiple sclerosis and randomizing them to groups that will receive either BFSM or usual care. Outcome variables that will be assessed in all patients include heart rate variability; the response of temperature, skin conductance, respiratory rate, and heart rate variability to mental stress; plasma catecholamine levels; plasma C-reactive protein levels; and tumor necrosis factor alpha levels. At the first and last visits, all patients will complete the SF-36, the eight-item Patient Health Questionnaire depression scale (PHQ-8), the Generalized Anxiety Disorder seven-item scale (GAD-7), and a visual analog pain scale. We will also assess disease-specific variables, including heart rate recovery after exercise, plasma lipids, and myeloperoxidase in patients with coronary artery disease; the Multiple Sclerosis Functional Composite (MSFC) test and the Modified Fatigue Impact Scale (MFIS) will be administered to patients with multiple sclerosis; and plasma glucose and hemoglobin A1C will be assessed in patients with type 2 diabetes.
Results of this study will provide data on the potential of BFSM to decrease common markers of autonomic nervous system activation and inflammatory cascades and the effect of those alterations on three specific disease states. To our knowledge, such a randomized study has not been conducted previously; our findings will add significantly to the literature on the mechanism of action of biofeedback-type interventions.
POTENTIAL IMPACT ON DEPRESSION IN CARDIOVASCULAR DISEASE
Depression is increasingly recognized as a component of many cardiovascular diseases; this raises the question of what effect BFSM therapy in cardiovascular disease patients will have on their depression. Of particular importance to this discussion, heart rate variability has been shown to be decreased both in cardiovascular disease and in depression, and BFSM is one treatment that can be used to regulate heart rate variability. Heart rate variability biofeedback has been shown to be useful in treating depression.
Work from Karavidas and colleagues showed that 10 weeks of heart rate variability biofeedback in patients with depression led to significantly improved scores on the Hamilton Depression Scale and the Beck Depression Inventory. Improvement was observed by the fourth week of training, with concurrent increases in the SDNN.13 Siepmann and colleagues also used heart rate variability biofeedback in depressed subjects and demonstrated significant improvement in scores on the Beck Depression Inventory, as well as a concomitant decrease in anxiety.14 In related work, Uhlmann and Fröscher used electroencephalographic biofeedback (also called neurofeedback) in epilepsy patients with depression and measured an increased sense of self control and a decrease in external locus of control; they postulated that biofeedback training provided an important opportunity for success, and thus increased internal control and decreased depression.15
Evidence suggests that BFSM should have an impact on depression in addition to impacting the cardiovascular disease itself, and both should work together to improve quality of life. For this reason we have added a depression inventory to our randomized trial of BFSM in patients who have coronary artery disease, diabetes, or multiple sclerosis.
- McKee MG. Biofeedback: an overview in the context of heart-brain medicine. Cleve Clin J Med 2008; 75(suppl 2):S31–S34.
- Frank DL, Khorshid L, Kiffer JF, Moravec CS, McKee MG. Biofeedback in medicine: who, when, why and how? Ment Health Fam Med 2010; 7:85–91.
- Moravec CS. Biofeedback therapy in cardiovascular disease: rationale and research overview. Cleve Clin J Med 2008; 75(suppl 2):S35–S38.
- McKee MG, Moravec CS. Biofeedback in the treatment of heart failure. Cleve Clin J Med 2010; 77(supp 3): S56–S59.
- Emani S, Binkley PF. Mind-body medicine in chronic heart failure: a translational science challenge. Circ Heart Fail 2010; 3:715–725.
- Ogletree-Hughes ML, Stull LB, Sweet WE, Smedira NG, McCarthy PM, Moravec CS. Mechanical unloading restores beta-adrenergic responsiveness and reverses receptor downregulation in the failing human heart. Circulation 2001; 104:881–886.
- Ogletree ML, Sweet WE, Talerico C, et al. Duration of left ventricular assist device support: effects on abnormal calcium cycling and functional recovery in the failing human heart. J Heart Lung Transplant 2010; 29:554–561.
- Moser DK, Dracup K, Woo MA, Stevenson LW. Voluntary control of vascular tone by using skin-temperature biofeedback-relaxation in patients with advanced heart failure. Altern Ther Health Med 1997; 3:51–59.
- Bernardi L, Porta C, Spicuzza L, et al. Slow breathing increases arterial baroreflex sensitivity in patients with chronic heart failure. Circulation 2002; 105:143–145.
- Swanson KS, Gevirtz RN, Brown M, Spira J, Guarneri E, Stoletniy L. The effect of biofeedback on function in patients with heart failure. Appl Psychophysiol Biofeedback 2009; 34:71–91.
- Tracey KJ. The inflammatory reflex. Nature 2002; 420:853–859.
- Tracey KJ. Reflex control of immunity. Nat Rev Immunol 2009; 9:418–428.
- Karavidas MK, Lehrer PM, Vaschillo E, et al. Preliminary results of an open label study of heart rate variability biofeedback for the treatment of major depression. Appl Psychophysiol Biofeedback 2007; 32:19–30.
- Siepmann M, Aykac V, Unterdörfer J, Petrowski K, Mueck-Weymann M. A pilot study on the effects of heart rate variability biofeedback in patients with depression and in healthy subjects. Appl Psychophysiol Biofeedback 2008; 33:195–201.
- Uhlmann C, Fröscher W. Biofeedback treatment in patients with refractory epilepsy: changes in depression and control orientation. Seizure 2001; 10:34–38.
BIOFEEDBACK: WHAT IS IT?
The term “biofeedback” refers to the instrumentation or training process that allows biologic information to be recorded, displayed, and communicated back to an individual, allowing the individual to make adjustments in physiologic processes that may enhance health or performance. The biofeedback display is analogous to a mirror, in which physiologic processes can be observed and adjusted much as one might adjust a hairstyle or a tie.
In our work with cardiovascular disease patients, biofeedback is a training process that involves a subject or patient, a biofeedback coach or therapist, and state-of-the-art biofeedback equipment. For biofeedback training to be effective, the subject who is trying to learn the skill must be engaged and willing to practice, the coach must be trained in psychophysiology, and the equipment must display accurate readings in real time, allowing the subject to monitor and change physiologic reactions appropriately. The coach teaches the subject about the physiologic parameters, establishes target ranges, and helps the subject learn how to move the physiologic parameters in the right direction.1,2
Training often begins with a session in which a brief mental stress test is followed by a period of relaxation while physiologic parameters are recorded and displayed. This process helps the subject to understand the link between mental processes and physiologic arousal.
Biofeedback training can involve a number of physiologic modalities, including those that reflect autonomic nervous system arousal, such as skin conductance and heart rate variability, and those that are not strictly correlated with autonomic activity, such as surface muscle tension. Each physiologic parameter is recorded by a specific sensor, and all sensors are noninvasive. Sensors feed signals into a computer, where they are processed and amplified, and subjects are able to view the output on a computer screen.
Typically, in our work, there is one screen for the subject, on which a single parameter can be displayed, observed and discussed, and another screen for the coach, on which all parameters are displayed simultaneously. During a single session of biofeedback training, the coach may choose to work on a single parameter or switch between parameters, depending on how much progress is being made with each. In our work with patients, we generally train to simple parameters first, such as respiratory rate, finger temperature, and skin conductance, moving on to surface muscle tension, heart rate, and eventually heart rate variability, which is a more complex concept and more easily understood later in the training process.
It is important that the subject receive positive reinforcement for changing the physiologic parameters, and if the subject struggles too long with one parameter, it is generally useful to go back to a different parameter, where success may be more easily experienced. Ideally, by the end of six to eight training sessions, the subject will be able to make progress on all physiologic parameters, which will track together over time.
BIOFEEDBACK-ASSISTED STRESS MANAGEMENT
Pure biofeedback training consists of operant conditioning. That is, the subject learns to regulate his or her physiology in the right direction because of the feedback, which can be as simple as a pleasant image appearing on a computer screen or as complicated as a car moving faster around a racetrack; pure biofeedback involves changing physiology in response to positive reinforcement of some sort.
In practice, we generally employ biofeedback-assisted stress management (BFSM) rather than pure biofeedback. With BFSM, the subject learns to change physiology in the direction of health and wellness by learning techniques of stress management. The coach teaches the subject various relaxation techniques, such as slow and rhythmic breathing, guided imagery, progressive muscle relaxation, mindfulness, assertiveness, and how to change negative thought patterns. With regular practice, the subject learns to change the physiologic parameters by relaxing the body. For example, instead of instructing the subject to “increase your finger temperature” and assume that the subject will achieve this because doing so will make the light bulb on the screen glow more intensely, the BFSM coach may instead talk with the subject about eliminating stressful thoughts, learning to relax, and the fingers warming in response to the body relaxing.
We distinguish between techniques of stress management, some of which are mentioned above, and psychotherapy, which can certainly be effectively combined with biofeedback, but which we do not provide in our research studies. Coupling stress management techniques with biofeedback helps the subject change physiologic parameters in the direction of wellness and acquire tools that can be used in everyday life when stressful events arise. The objective of BFSM training is not just to change physiology, but also to change the way subjects respond to stressful events in daily life; ie, react to fewer events, react less intensely when they do react, and recover more quickly.
BIOFEEDBACK-ASSISTED STRESS MANAGEMENT IN CARDIOVASCULAR DISEASE
We are currently studying the effects of BFSM in patients with cardiovascular disease, including both heart failure and stable coronary artery disease. Patients with cardiovascular disease often are functionally limited, and they also experience psychologic distress related to physical limitations and other life stressors. Both the physical limitations and the psychologic distress impact quality of life. We hypothesize that BFSM will teach our patients techniques of stress management, both mental and physiologic, that will help relieve their psychologic distress and improve their quality of life. BFSM will also potentially decrease the overactivation of the sympathetic branch of the autonomic nervous system, which is common in cardiovascular disease, and correspondingly upregulate the contribution of the parasympathetic branch of the autonomic nervous system, which should be beneficial.3
A PROMISING TECHNIQUE IN HEART FAILURE
We are currently studying the effects of BFSM in patients with end-stage heart failure who are awaiting heart transplant at Cleveland Clinic.4 As noted in a recent review, biofeedback is a promising technique in heart failure that patients may be able to use to consciously regulate their autonomic nervous systems.5 We hypothesize that BFSM training will interfere with the overactivation of the sympathetic nervous system that is characteristic of heart failure, and that this will reverse the cellular and molecular remodeling that occurs in the failing human heart.
To date, we have enrolled 25 patients; 10 are being studied in our National Institutes of Health–funded Clinic Research Unit and 15 are inpatients. All 25 patients are listed as heart transplant candidates and have given consent for us to study their hearts when they are explanted.
Each patient receives eight sessions with a certified biofeedback therapist. The first and last sessions include mental stress tests, while the remaining six are BFSM training sessions. Patients are assessed at the beginning and end of the study using the 6-minute walk test, the Kansas City Cardiomyopathy Questionnaire, the Medical Outcomes Study 36-Item Short-Form Health Survey (SF-36), and measurement of plasma catecholamines.
The primary end point of the study is the measurement of cellular and molecular markers that have been shown to be altered in the failing human heart, testing the hypothesis that these markers will be reversed in the direction of normal in the BFSM therapy group. These markers are measured in the explanted failing heart when the patient receives a heart transplant.
It is too early to report the results of this study, since only seven patients have undergone transplantation to date. We are encouraged by several early findings, however, and hope these will be validated when the entire group is analyzed.
In early analysis, scores on the Kansas City Cardiomyopathy Questionnaire are improved in the last session compared with the first; patients have shown the ability to learn a slower breathing rate; and they are able to regulate their heart rate variability, as measured by the standard deviation of the N-to-N interval, or SDNN. Most important, measurements in the first seven hearts indicate that there is a degree of biologic remodeling of the failing heart after BFSM that is similar to what we have observed with left ventricular assist devices—hemodynamic pumps that take on the workload of the heart, permitting the heart to rest and recover while the patient is waiting for a transplant.6,7 If BFSM could produce changes in the cellular and molecular properties of the heart that are equal in magnitude to those produced by a mechanical pump, this would be a revolutionary finding in the field of heart-brain medicine.
It should be noted that we are not the first group to study BFSM in patients with heart failure. Moser and colleagues first observed that a single session of skin temperature biofeedback could have significant functional effects in patients with heart failure.8 Bernardi and coworkers showed that merely teaching patients to breathe six times per minute (a large component of BFSM training) improved oxygen saturation and exercise tolerance.9 Swanson and colleagues in 2009 demonstrated that patients with heart failure were able to regulate their heart rate variability, although they observed this only in patients with a left ventricular ejection fraction greater than 30%.10 Our preliminary data demonstrate regulation of heart rate variability in patients with lower ejection fractions, which is promising, but we have also added the biologic component of studying the explanted heart, allowing us to test the hypothesis that BFSM could potentially impact the remodeling process and thus have important therapeutic implications.
TRIAL UNDER WAY IN CORONARY ARTERY DISEASE
In addition to our studies of BFSM in heart failure, we have begun a randomized clinical trial of patients with stable coronary artery disease, type 2 diabetes, or multiple sclerosis. These three patient populations were chosen because evidence from numerous studies suggests that they all involve autonomic nervous system dysregulation as well as an inflammatory process.
It has already been mentioned that BFSM can interfere with overactivation of the sympathetic nervous system and potentially upregulate the contribution of the parasympathetic nervous system, which usually exists in juxtaposition to the sympathetic nervous system. Based on the work of Tracey,11,12 upregulating the parasympathetic nervous system should be antiinflammatory. Thus, we hypothesize that by decreasing both sympathetic nervous system activation and inflammation, BFSM should have an impact on patients with one of these disease states, resulting in improved quality of life and clinical status, reduced anxiety and depression, and changed disease-specific indicators of severity.
We are currently enrolling patients who have coronary artery disease, type 2 diabetes, or multiple sclerosis and randomizing them to groups that will receive either BFSM or usual care. Outcome variables that will be assessed in all patients include heart rate variability; the response of temperature, skin conductance, respiratory rate, and heart rate variability to mental stress; plasma catecholamine levels; plasma C-reactive protein levels; and tumor necrosis factor alpha levels. At the first and last visits, all patients will complete the SF-36, the eight-item Patient Health Questionnaire depression scale (PHQ-8), the Generalized Anxiety Disorder seven-item scale (GAD-7), and a visual analog pain scale. We will also assess disease-specific variables, including heart rate recovery after exercise, plasma lipids, and myeloperoxidase in patients with coronary artery disease; the Multiple Sclerosis Functional Composite (MSFC) test and the Modified Fatigue Impact Scale (MFIS) will be administered to patients with multiple sclerosis; and plasma glucose and hemoglobin A1C will be assessed in patients with type 2 diabetes.
Results of this study will provide data on the potential of BFSM to decrease common markers of autonomic nervous system activation and inflammatory cascades and the effect of those alterations on three specific disease states. To our knowledge, such a randomized study has not been conducted previously; our findings will add significantly to the literature on the mechanism of action of biofeedback-type interventions.
POTENTIAL IMPACT ON DEPRESSION IN CARDIOVASCULAR DISEASE
Depression is increasingly recognized as a component of many cardiovascular diseases; this raises the question of what effect BFSM therapy in cardiovascular disease patients will have on their depression. Of particular importance to this discussion, heart rate variability has been shown to be decreased both in cardiovascular disease and in depression, and BFSM is one treatment that can be used to regulate heart rate variability. Heart rate variability biofeedback has been shown to be useful in treating depression.
Work from Karavidas and colleagues showed that 10 weeks of heart rate variability biofeedback in patients with depression led to significantly improved scores on the Hamilton Depression Scale and the Beck Depression Inventory. Improvement was observed by the fourth week of training, with concurrent increases in the SDNN.13 Siepmann and colleagues also used heart rate variability biofeedback in depressed subjects and demonstrated significant improvement in scores on the Beck Depression Inventory, as well as a concomitant decrease in anxiety.14 In related work, Uhlmann and Fröscher used electroencephalographic biofeedback (also called neurofeedback) in epilepsy patients with depression and measured an increased sense of self control and a decrease in external locus of control; they postulated that biofeedback training provided an important opportunity for success, and thus increased internal control and decreased depression.15
Evidence suggests that BFSM should have an impact on depression in addition to impacting the cardiovascular disease itself, and both should work together to improve quality of life. For this reason we have added a depression inventory to our randomized trial of BFSM in patients who have coronary artery disease, diabetes, or multiple sclerosis.
BIOFEEDBACK: WHAT IS IT?
The term “biofeedback” refers to the instrumentation or training process that allows biologic information to be recorded, displayed, and communicated back to an individual, allowing the individual to make adjustments in physiologic processes that may enhance health or performance. The biofeedback display is analogous to a mirror, in which physiologic processes can be observed and adjusted much as one might adjust a hairstyle or a tie.
In our work with cardiovascular disease patients, biofeedback is a training process that involves a subject or patient, a biofeedback coach or therapist, and state-of-the-art biofeedback equipment. For biofeedback training to be effective, the subject who is trying to learn the skill must be engaged and willing to practice, the coach must be trained in psychophysiology, and the equipment must display accurate readings in real time, allowing the subject to monitor and change physiologic reactions appropriately. The coach teaches the subject about the physiologic parameters, establishes target ranges, and helps the subject learn how to move the physiologic parameters in the right direction.1,2
Training often begins with a session in which a brief mental stress test is followed by a period of relaxation while physiologic parameters are recorded and displayed. This process helps the subject to understand the link between mental processes and physiologic arousal.
Biofeedback training can involve a number of physiologic modalities, including those that reflect autonomic nervous system arousal, such as skin conductance and heart rate variability, and those that are not strictly correlated with autonomic activity, such as surface muscle tension. Each physiologic parameter is recorded by a specific sensor, and all sensors are noninvasive. Sensors feed signals into a computer, where they are processed and amplified, and subjects are able to view the output on a computer screen.
Typically, in our work, there is one screen for the subject, on which a single parameter can be displayed, observed and discussed, and another screen for the coach, on which all parameters are displayed simultaneously. During a single session of biofeedback training, the coach may choose to work on a single parameter or switch between parameters, depending on how much progress is being made with each. In our work with patients, we generally train to simple parameters first, such as respiratory rate, finger temperature, and skin conductance, moving on to surface muscle tension, heart rate, and eventually heart rate variability, which is a more complex concept and more easily understood later in the training process.
It is important that the subject receive positive reinforcement for changing the physiologic parameters, and if the subject struggles too long with one parameter, it is generally useful to go back to a different parameter, where success may be more easily experienced. Ideally, by the end of six to eight training sessions, the subject will be able to make progress on all physiologic parameters, which will track together over time.
BIOFEEDBACK-ASSISTED STRESS MANAGEMENT
Pure biofeedback training consists of operant conditioning. That is, the subject learns to regulate his or her physiology in the right direction because of the feedback, which can be as simple as a pleasant image appearing on a computer screen or as complicated as a car moving faster around a racetrack; pure biofeedback involves changing physiology in response to positive reinforcement of some sort.
In practice, we generally employ biofeedback-assisted stress management (BFSM) rather than pure biofeedback. With BFSM, the subject learns to change physiology in the direction of health and wellness by learning techniques of stress management. The coach teaches the subject various relaxation techniques, such as slow and rhythmic breathing, guided imagery, progressive muscle relaxation, mindfulness, assertiveness, and how to change negative thought patterns. With regular practice, the subject learns to change the physiologic parameters by relaxing the body. For example, instead of instructing the subject to “increase your finger temperature” and assume that the subject will achieve this because doing so will make the light bulb on the screen glow more intensely, the BFSM coach may instead talk with the subject about eliminating stressful thoughts, learning to relax, and the fingers warming in response to the body relaxing.
We distinguish between techniques of stress management, some of which are mentioned above, and psychotherapy, which can certainly be effectively combined with biofeedback, but which we do not provide in our research studies. Coupling stress management techniques with biofeedback helps the subject change physiologic parameters in the direction of wellness and acquire tools that can be used in everyday life when stressful events arise. The objective of BFSM training is not just to change physiology, but also to change the way subjects respond to stressful events in daily life; ie, react to fewer events, react less intensely when they do react, and recover more quickly.
BIOFEEDBACK-ASSISTED STRESS MANAGEMENT IN CARDIOVASCULAR DISEASE
We are currently studying the effects of BFSM in patients with cardiovascular disease, including both heart failure and stable coronary artery disease. Patients with cardiovascular disease often are functionally limited, and they also experience psychologic distress related to physical limitations and other life stressors. Both the physical limitations and the psychologic distress impact quality of life. We hypothesize that BFSM will teach our patients techniques of stress management, both mental and physiologic, that will help relieve their psychologic distress and improve their quality of life. BFSM will also potentially decrease the overactivation of the sympathetic branch of the autonomic nervous system, which is common in cardiovascular disease, and correspondingly upregulate the contribution of the parasympathetic branch of the autonomic nervous system, which should be beneficial.3
A PROMISING TECHNIQUE IN HEART FAILURE
We are currently studying the effects of BFSM in patients with end-stage heart failure who are awaiting heart transplant at Cleveland Clinic.4 As noted in a recent review, biofeedback is a promising technique in heart failure that patients may be able to use to consciously regulate their autonomic nervous systems.5 We hypothesize that BFSM training will interfere with the overactivation of the sympathetic nervous system that is characteristic of heart failure, and that this will reverse the cellular and molecular remodeling that occurs in the failing human heart.
To date, we have enrolled 25 patients; 10 are being studied in our National Institutes of Health–funded Clinic Research Unit and 15 are inpatients. All 25 patients are listed as heart transplant candidates and have given consent for us to study their hearts when they are explanted.
Each patient receives eight sessions with a certified biofeedback therapist. The first and last sessions include mental stress tests, while the remaining six are BFSM training sessions. Patients are assessed at the beginning and end of the study using the 6-minute walk test, the Kansas City Cardiomyopathy Questionnaire, the Medical Outcomes Study 36-Item Short-Form Health Survey (SF-36), and measurement of plasma catecholamines.
The primary end point of the study is the measurement of cellular and molecular markers that have been shown to be altered in the failing human heart, testing the hypothesis that these markers will be reversed in the direction of normal in the BFSM therapy group. These markers are measured in the explanted failing heart when the patient receives a heart transplant.
It is too early to report the results of this study, since only seven patients have undergone transplantation to date. We are encouraged by several early findings, however, and hope these will be validated when the entire group is analyzed.
In early analysis, scores on the Kansas City Cardiomyopathy Questionnaire are improved in the last session compared with the first; patients have shown the ability to learn a slower breathing rate; and they are able to regulate their heart rate variability, as measured by the standard deviation of the N-to-N interval, or SDNN. Most important, measurements in the first seven hearts indicate that there is a degree of biologic remodeling of the failing heart after BFSM that is similar to what we have observed with left ventricular assist devices—hemodynamic pumps that take on the workload of the heart, permitting the heart to rest and recover while the patient is waiting for a transplant.6,7 If BFSM could produce changes in the cellular and molecular properties of the heart that are equal in magnitude to those produced by a mechanical pump, this would be a revolutionary finding in the field of heart-brain medicine.
It should be noted that we are not the first group to study BFSM in patients with heart failure. Moser and colleagues first observed that a single session of skin temperature biofeedback could have significant functional effects in patients with heart failure.8 Bernardi and coworkers showed that merely teaching patients to breathe six times per minute (a large component of BFSM training) improved oxygen saturation and exercise tolerance.9 Swanson and colleagues in 2009 demonstrated that patients with heart failure were able to regulate their heart rate variability, although they observed this only in patients with a left ventricular ejection fraction greater than 30%.10 Our preliminary data demonstrate regulation of heart rate variability in patients with lower ejection fractions, which is promising, but we have also added the biologic component of studying the explanted heart, allowing us to test the hypothesis that BFSM could potentially impact the remodeling process and thus have important therapeutic implications.
TRIAL UNDER WAY IN CORONARY ARTERY DISEASE
In addition to our studies of BFSM in heart failure, we have begun a randomized clinical trial of patients with stable coronary artery disease, type 2 diabetes, or multiple sclerosis. These three patient populations were chosen because evidence from numerous studies suggests that they all involve autonomic nervous system dysregulation as well as an inflammatory process.
It has already been mentioned that BFSM can interfere with overactivation of the sympathetic nervous system and potentially upregulate the contribution of the parasympathetic nervous system, which usually exists in juxtaposition to the sympathetic nervous system. Based on the work of Tracey,11,12 upregulating the parasympathetic nervous system should be antiinflammatory. Thus, we hypothesize that by decreasing both sympathetic nervous system activation and inflammation, BFSM should have an impact on patients with one of these disease states, resulting in improved quality of life and clinical status, reduced anxiety and depression, and changed disease-specific indicators of severity.
We are currently enrolling patients who have coronary artery disease, type 2 diabetes, or multiple sclerosis and randomizing them to groups that will receive either BFSM or usual care. Outcome variables that will be assessed in all patients include heart rate variability; the response of temperature, skin conductance, respiratory rate, and heart rate variability to mental stress; plasma catecholamine levels; plasma C-reactive protein levels; and tumor necrosis factor alpha levels. At the first and last visits, all patients will complete the SF-36, the eight-item Patient Health Questionnaire depression scale (PHQ-8), the Generalized Anxiety Disorder seven-item scale (GAD-7), and a visual analog pain scale. We will also assess disease-specific variables, including heart rate recovery after exercise, plasma lipids, and myeloperoxidase in patients with coronary artery disease; the Multiple Sclerosis Functional Composite (MSFC) test and the Modified Fatigue Impact Scale (MFIS) will be administered to patients with multiple sclerosis; and plasma glucose and hemoglobin A1C will be assessed in patients with type 2 diabetes.
Results of this study will provide data on the potential of BFSM to decrease common markers of autonomic nervous system activation and inflammatory cascades and the effect of those alterations on three specific disease states. To our knowledge, such a randomized study has not been conducted previously; our findings will add significantly to the literature on the mechanism of action of biofeedback-type interventions.
POTENTIAL IMPACT ON DEPRESSION IN CARDIOVASCULAR DISEASE
Depression is increasingly recognized as a component of many cardiovascular diseases; this raises the question of what effect BFSM therapy in cardiovascular disease patients will have on their depression. Of particular importance to this discussion, heart rate variability has been shown to be decreased both in cardiovascular disease and in depression, and BFSM is one treatment that can be used to regulate heart rate variability. Heart rate variability biofeedback has been shown to be useful in treating depression.
Work from Karavidas and colleagues showed that 10 weeks of heart rate variability biofeedback in patients with depression led to significantly improved scores on the Hamilton Depression Scale and the Beck Depression Inventory. Improvement was observed by the fourth week of training, with concurrent increases in the SDNN.13 Siepmann and colleagues also used heart rate variability biofeedback in depressed subjects and demonstrated significant improvement in scores on the Beck Depression Inventory, as well as a concomitant decrease in anxiety.14 In related work, Uhlmann and Fröscher used electroencephalographic biofeedback (also called neurofeedback) in epilepsy patients with depression and measured an increased sense of self control and a decrease in external locus of control; they postulated that biofeedback training provided an important opportunity for success, and thus increased internal control and decreased depression.15
Evidence suggests that BFSM should have an impact on depression in addition to impacting the cardiovascular disease itself, and both should work together to improve quality of life. For this reason we have added a depression inventory to our randomized trial of BFSM in patients who have coronary artery disease, diabetes, or multiple sclerosis.
- McKee MG. Biofeedback: an overview in the context of heart-brain medicine. Cleve Clin J Med 2008; 75(suppl 2):S31–S34.
- Frank DL, Khorshid L, Kiffer JF, Moravec CS, McKee MG. Biofeedback in medicine: who, when, why and how? Ment Health Fam Med 2010; 7:85–91.
- Moravec CS. Biofeedback therapy in cardiovascular disease: rationale and research overview. Cleve Clin J Med 2008; 75(suppl 2):S35–S38.
- McKee MG, Moravec CS. Biofeedback in the treatment of heart failure. Cleve Clin J Med 2010; 77(supp 3): S56–S59.
- Emani S, Binkley PF. Mind-body medicine in chronic heart failure: a translational science challenge. Circ Heart Fail 2010; 3:715–725.
- Ogletree-Hughes ML, Stull LB, Sweet WE, Smedira NG, McCarthy PM, Moravec CS. Mechanical unloading restores beta-adrenergic responsiveness and reverses receptor downregulation in the failing human heart. Circulation 2001; 104:881–886.
- Ogletree ML, Sweet WE, Talerico C, et al. Duration of left ventricular assist device support: effects on abnormal calcium cycling and functional recovery in the failing human heart. J Heart Lung Transplant 2010; 29:554–561.
- Moser DK, Dracup K, Woo MA, Stevenson LW. Voluntary control of vascular tone by using skin-temperature biofeedback-relaxation in patients with advanced heart failure. Altern Ther Health Med 1997; 3:51–59.
- Bernardi L, Porta C, Spicuzza L, et al. Slow breathing increases arterial baroreflex sensitivity in patients with chronic heart failure. Circulation 2002; 105:143–145.
- Swanson KS, Gevirtz RN, Brown M, Spira J, Guarneri E, Stoletniy L. The effect of biofeedback on function in patients with heart failure. Appl Psychophysiol Biofeedback 2009; 34:71–91.
- Tracey KJ. The inflammatory reflex. Nature 2002; 420:853–859.
- Tracey KJ. Reflex control of immunity. Nat Rev Immunol 2009; 9:418–428.
- Karavidas MK, Lehrer PM, Vaschillo E, et al. Preliminary results of an open label study of heart rate variability biofeedback for the treatment of major depression. Appl Psychophysiol Biofeedback 2007; 32:19–30.
- Siepmann M, Aykac V, Unterdörfer J, Petrowski K, Mueck-Weymann M. A pilot study on the effects of heart rate variability biofeedback in patients with depression and in healthy subjects. Appl Psychophysiol Biofeedback 2008; 33:195–201.
- Uhlmann C, Fröscher W. Biofeedback treatment in patients with refractory epilepsy: changes in depression and control orientation. Seizure 2001; 10:34–38.
- McKee MG. Biofeedback: an overview in the context of heart-brain medicine. Cleve Clin J Med 2008; 75(suppl 2):S31–S34.
- Frank DL, Khorshid L, Kiffer JF, Moravec CS, McKee MG. Biofeedback in medicine: who, when, why and how? Ment Health Fam Med 2010; 7:85–91.
- Moravec CS. Biofeedback therapy in cardiovascular disease: rationale and research overview. Cleve Clin J Med 2008; 75(suppl 2):S35–S38.
- McKee MG, Moravec CS. Biofeedback in the treatment of heart failure. Cleve Clin J Med 2010; 77(supp 3): S56–S59.
- Emani S, Binkley PF. Mind-body medicine in chronic heart failure: a translational science challenge. Circ Heart Fail 2010; 3:715–725.
- Ogletree-Hughes ML, Stull LB, Sweet WE, Smedira NG, McCarthy PM, Moravec CS. Mechanical unloading restores beta-adrenergic responsiveness and reverses receptor downregulation in the failing human heart. Circulation 2001; 104:881–886.
- Ogletree ML, Sweet WE, Talerico C, et al. Duration of left ventricular assist device support: effects on abnormal calcium cycling and functional recovery in the failing human heart. J Heart Lung Transplant 2010; 29:554–561.
- Moser DK, Dracup K, Woo MA, Stevenson LW. Voluntary control of vascular tone by using skin-temperature biofeedback-relaxation in patients with advanced heart failure. Altern Ther Health Med 1997; 3:51–59.
- Bernardi L, Porta C, Spicuzza L, et al. Slow breathing increases arterial baroreflex sensitivity in patients with chronic heart failure. Circulation 2002; 105:143–145.
- Swanson KS, Gevirtz RN, Brown M, Spira J, Guarneri E, Stoletniy L. The effect of biofeedback on function in patients with heart failure. Appl Psychophysiol Biofeedback 2009; 34:71–91.
- Tracey KJ. The inflammatory reflex. Nature 2002; 420:853–859.
- Tracey KJ. Reflex control of immunity. Nat Rev Immunol 2009; 9:418–428.
- Karavidas MK, Lehrer PM, Vaschillo E, et al. Preliminary results of an open label study of heart rate variability biofeedback for the treatment of major depression. Appl Psychophysiol Biofeedback 2007; 32:19–30.
- Siepmann M, Aykac V, Unterdörfer J, Petrowski K, Mueck-Weymann M. A pilot study on the effects of heart rate variability biofeedback in patients with depression and in healthy subjects. Appl Psychophysiol Biofeedback 2008; 33:195–201.
- Uhlmann C, Fröscher W. Biofeedback treatment in patients with refractory epilepsy: changes in depression and control orientation. Seizure 2001; 10:34–38.