User login
Biofeedback in the treatment of heart disease
BIOFEEDBACK: WHAT IS IT?
The term “biofeedback” refers to the instrumentation or training process that allows biologic information to be recorded, displayed, and communicated back to an individual, allowing the individual to make adjustments in physiologic processes that may enhance health or performance. The biofeedback display is analogous to a mirror, in which physiologic processes can be observed and adjusted much as one might adjust a hairstyle or a tie.
In our work with cardiovascular disease patients, biofeedback is a training process that involves a subject or patient, a biofeedback coach or therapist, and state-of-the-art biofeedback equipment. For biofeedback training to be effective, the subject who is trying to learn the skill must be engaged and willing to practice, the coach must be trained in psychophysiology, and the equipment must display accurate readings in real time, allowing the subject to monitor and change physiologic reactions appropriately. The coach teaches the subject about the physiologic parameters, establishes target ranges, and helps the subject learn how to move the physiologic parameters in the right direction.1,2
Training often begins with a session in which a brief mental stress test is followed by a period of relaxation while physiologic parameters are recorded and displayed. This process helps the subject to understand the link between mental processes and physiologic arousal.
Biofeedback training can involve a number of physiologic modalities, including those that reflect autonomic nervous system arousal, such as skin conductance and heart rate variability, and those that are not strictly correlated with autonomic activity, such as surface muscle tension. Each physiologic parameter is recorded by a specific sensor, and all sensors are noninvasive. Sensors feed signals into a computer, where they are processed and amplified, and subjects are able to view the output on a computer screen.
Typically, in our work, there is one screen for the subject, on which a single parameter can be displayed, observed and discussed, and another screen for the coach, on which all parameters are displayed simultaneously. During a single session of biofeedback training, the coach may choose to work on a single parameter or switch between parameters, depending on how much progress is being made with each. In our work with patients, we generally train to simple parameters first, such as respiratory rate, finger temperature, and skin conductance, moving on to surface muscle tension, heart rate, and eventually heart rate variability, which is a more complex concept and more easily understood later in the training process.
It is important that the subject receive positive reinforcement for changing the physiologic parameters, and if the subject struggles too long with one parameter, it is generally useful to go back to a different parameter, where success may be more easily experienced. Ideally, by the end of six to eight training sessions, the subject will be able to make progress on all physiologic parameters, which will track together over time.
BIOFEEDBACK-ASSISTED STRESS MANAGEMENT
Pure biofeedback training consists of operant conditioning. That is, the subject learns to regulate his or her physiology in the right direction because of the feedback, which can be as simple as a pleasant image appearing on a computer screen or as complicated as a car moving faster around a racetrack; pure biofeedback involves changing physiology in response to positive reinforcement of some sort.
In practice, we generally employ biofeedback-assisted stress management (BFSM) rather than pure biofeedback. With BFSM, the subject learns to change physiology in the direction of health and wellness by learning techniques of stress management. The coach teaches the subject various relaxation techniques, such as slow and rhythmic breathing, guided imagery, progressive muscle relaxation, mindfulness, assertiveness, and how to change negative thought patterns. With regular practice, the subject learns to change the physiologic parameters by relaxing the body. For example, instead of instructing the subject to “increase your finger temperature” and assume that the subject will achieve this because doing so will make the light bulb on the screen glow more intensely, the BFSM coach may instead talk with the subject about eliminating stressful thoughts, learning to relax, and the fingers warming in response to the body relaxing.
We distinguish between techniques of stress management, some of which are mentioned above, and psychotherapy, which can certainly be effectively combined with biofeedback, but which we do not provide in our research studies. Coupling stress management techniques with biofeedback helps the subject change physiologic parameters in the direction of wellness and acquire tools that can be used in everyday life when stressful events arise. The objective of BFSM training is not just to change physiology, but also to change the way subjects respond to stressful events in daily life; ie, react to fewer events, react less intensely when they do react, and recover more quickly.
BIOFEEDBACK-ASSISTED STRESS MANAGEMENT IN CARDIOVASCULAR DISEASE
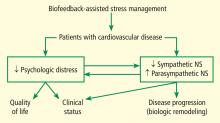
We are currently studying the effects of BFSM in patients with cardiovascular disease, including both heart failure and stable coronary artery disease. Patients with cardiovascular disease often are functionally limited, and they also experience psychologic distress related to physical limitations and other life stressors. Both the physical limitations and the psychologic distress impact quality of life. We hypothesize that BFSM will teach our patients techniques of stress management, both mental and physiologic, that will help relieve their psychologic distress and improve their quality of life. BFSM will also potentially decrease the overactivation of the sympathetic branch of the autonomic nervous system, which is common in cardiovascular disease, and correspondingly upregulate the contribution of the parasympathetic branch of the autonomic nervous system, which should be beneficial.3
A PROMISING TECHNIQUE IN HEART FAILURE
We are currently studying the effects of BFSM in patients with end-stage heart failure who are awaiting heart transplant at Cleveland Clinic.4 As noted in a recent review, biofeedback is a promising technique in heart failure that patients may be able to use to consciously regulate their autonomic nervous systems.5 We hypothesize that BFSM training will interfere with the overactivation of the sympathetic nervous system that is characteristic of heart failure, and that this will reverse the cellular and molecular remodeling that occurs in the failing human heart.
To date, we have enrolled 25 patients; 10 are being studied in our National Institutes of Health–funded Clinic Research Unit and 15 are inpatients. All 25 patients are listed as heart transplant candidates and have given consent for us to study their hearts when they are explanted.
Each patient receives eight sessions with a certified biofeedback therapist. The first and last sessions include mental stress tests, while the remaining six are BFSM training sessions. Patients are assessed at the beginning and end of the study using the 6-minute walk test, the Kansas City Cardiomyopathy Questionnaire, the Medical Outcomes Study 36-Item Short-Form Health Survey (SF-36), and measurement of plasma catecholamines.
The primary end point of the study is the measurement of cellular and molecular markers that have been shown to be altered in the failing human heart, testing the hypothesis that these markers will be reversed in the direction of normal in the BFSM therapy group. These markers are measured in the explanted failing heart when the patient receives a heart transplant.
It is too early to report the results of this study, since only seven patients have undergone transplantation to date. We are encouraged by several early findings, however, and hope these will be validated when the entire group is analyzed.
In early analysis, scores on the Kansas City Cardiomyopathy Questionnaire are improved in the last session compared with the first; patients have shown the ability to learn a slower breathing rate; and they are able to regulate their heart rate variability, as measured by the standard deviation of the N-to-N interval, or SDNN. Most important, measurements in the first seven hearts indicate that there is a degree of biologic remodeling of the failing heart after BFSM that is similar to what we have observed with left ventricular assist devices—hemodynamic pumps that take on the workload of the heart, permitting the heart to rest and recover while the patient is waiting for a transplant.6,7 If BFSM could produce changes in the cellular and molecular properties of the heart that are equal in magnitude to those produced by a mechanical pump, this would be a revolutionary finding in the field of heart-brain medicine.
It should be noted that we are not the first group to study BFSM in patients with heart failure. Moser and colleagues first observed that a single session of skin temperature biofeedback could have significant functional effects in patients with heart failure.8 Bernardi and coworkers showed that merely teaching patients to breathe six times per minute (a large component of BFSM training) improved oxygen saturation and exercise tolerance.9 Swanson and colleagues in 2009 demonstrated that patients with heart failure were able to regulate their heart rate variability, although they observed this only in patients with a left ventricular ejection fraction greater than 30%.10 Our preliminary data demonstrate regulation of heart rate variability in patients with lower ejection fractions, which is promising, but we have also added the biologic component of studying the explanted heart, allowing us to test the hypothesis that BFSM could potentially impact the remodeling process and thus have important therapeutic implications.
TRIAL UNDER WAY IN CORONARY ARTERY DISEASE
In addition to our studies of BFSM in heart failure, we have begun a randomized clinical trial of patients with stable coronary artery disease, type 2 diabetes, or multiple sclerosis. These three patient populations were chosen because evidence from numerous studies suggests that they all involve autonomic nervous system dysregulation as well as an inflammatory process.
It has already been mentioned that BFSM can interfere with overactivation of the sympathetic nervous system and potentially upregulate the contribution of the parasympathetic nervous system, which usually exists in juxtaposition to the sympathetic nervous system. Based on the work of Tracey,11,12 upregulating the parasympathetic nervous system should be antiinflammatory. Thus, we hypothesize that by decreasing both sympathetic nervous system activation and inflammation, BFSM should have an impact on patients with one of these disease states, resulting in improved quality of life and clinical status, reduced anxiety and depression, and changed disease-specific indicators of severity.
We are currently enrolling patients who have coronary artery disease, type 2 diabetes, or multiple sclerosis and randomizing them to groups that will receive either BFSM or usual care. Outcome variables that will be assessed in all patients include heart rate variability; the response of temperature, skin conductance, respiratory rate, and heart rate variability to mental stress; plasma catecholamine levels; plasma C-reactive protein levels; and tumor necrosis factor alpha levels. At the first and last visits, all patients will complete the SF-36, the eight-item Patient Health Questionnaire depression scale (PHQ-8), the Generalized Anxiety Disorder seven-item scale (GAD-7), and a visual analog pain scale. We will also assess disease-specific variables, including heart rate recovery after exercise, plasma lipids, and myeloperoxidase in patients with coronary artery disease; the Multiple Sclerosis Functional Composite (MSFC) test and the Modified Fatigue Impact Scale (MFIS) will be administered to patients with multiple sclerosis; and plasma glucose and hemoglobin A1C will be assessed in patients with type 2 diabetes.
Results of this study will provide data on the potential of BFSM to decrease common markers of autonomic nervous system activation and inflammatory cascades and the effect of those alterations on three specific disease states. To our knowledge, such a randomized study has not been conducted previously; our findings will add significantly to the literature on the mechanism of action of biofeedback-type interventions.
POTENTIAL IMPACT ON DEPRESSION IN CARDIOVASCULAR DISEASE
Depression is increasingly recognized as a component of many cardiovascular diseases; this raises the question of what effect BFSM therapy in cardiovascular disease patients will have on their depression. Of particular importance to this discussion, heart rate variability has been shown to be decreased both in cardiovascular disease and in depression, and BFSM is one treatment that can be used to regulate heart rate variability. Heart rate variability biofeedback has been shown to be useful in treating depression.
Work from Karavidas and colleagues showed that 10 weeks of heart rate variability biofeedback in patients with depression led to significantly improved scores on the Hamilton Depression Scale and the Beck Depression Inventory. Improvement was observed by the fourth week of training, with concurrent increases in the SDNN.13 Siepmann and colleagues also used heart rate variability biofeedback in depressed subjects and demonstrated significant improvement in scores on the Beck Depression Inventory, as well as a concomitant decrease in anxiety.14 In related work, Uhlmann and Fröscher used electroencephalographic biofeedback (also called neurofeedback) in epilepsy patients with depression and measured an increased sense of self control and a decrease in external locus of control; they postulated that biofeedback training provided an important opportunity for success, and thus increased internal control and decreased depression.15
Evidence suggests that BFSM should have an impact on depression in addition to impacting the cardiovascular disease itself, and both should work together to improve quality of life. For this reason we have added a depression inventory to our randomized trial of BFSM in patients who have coronary artery disease, diabetes, or multiple sclerosis.
- McKee MG. Biofeedback: an overview in the context of heart-brain medicine. Cleve Clin J Med 2008; 75(suppl 2):S31–S34.
- Frank DL, Khorshid L, Kiffer JF, Moravec CS, McKee MG. Biofeedback in medicine: who, when, why and how? Ment Health Fam Med 2010; 7:85–91.
- Moravec CS. Biofeedback therapy in cardiovascular disease: rationale and research overview. Cleve Clin J Med 2008; 75(suppl 2):S35–S38.
- McKee MG, Moravec CS. Biofeedback in the treatment of heart failure. Cleve Clin J Med 2010; 77(supp 3): S56–S59.
- Emani S, Binkley PF. Mind-body medicine in chronic heart failure: a translational science challenge. Circ Heart Fail 2010; 3:715–725.
- Ogletree-Hughes ML, Stull LB, Sweet WE, Smedira NG, McCarthy PM, Moravec CS. Mechanical unloading restores beta-adrenergic responsiveness and reverses receptor downregulation in the failing human heart. Circulation 2001; 104:881–886.
- Ogletree ML, Sweet WE, Talerico C, et al. Duration of left ventricular assist device support: effects on abnormal calcium cycling and functional recovery in the failing human heart. J Heart Lung Transplant 2010; 29:554–561.
- Moser DK, Dracup K, Woo MA, Stevenson LW. Voluntary control of vascular tone by using skin-temperature biofeedback-relaxation in patients with advanced heart failure. Altern Ther Health Med 1997; 3:51–59.
- Bernardi L, Porta C, Spicuzza L, et al. Slow breathing increases arterial baroreflex sensitivity in patients with chronic heart failure. Circulation 2002; 105:143–145.
- Swanson KS, Gevirtz RN, Brown M, Spira J, Guarneri E, Stoletniy L. The effect of biofeedback on function in patients with heart failure. Appl Psychophysiol Biofeedback 2009; 34:71–91.
- Tracey KJ. The inflammatory reflex. Nature 2002; 420:853–859.
- Tracey KJ. Reflex control of immunity. Nat Rev Immunol 2009; 9:418–428.
- Karavidas MK, Lehrer PM, Vaschillo E, et al. Preliminary results of an open label study of heart rate variability biofeedback for the treatment of major depression. Appl Psychophysiol Biofeedback 2007; 32:19–30.
- Siepmann M, Aykac V, Unterdörfer J, Petrowski K, Mueck-Weymann M. A pilot study on the effects of heart rate variability biofeedback in patients with depression and in healthy subjects. Appl Psychophysiol Biofeedback 2008; 33:195–201.
- Uhlmann C, Fröscher W. Biofeedback treatment in patients with refractory epilepsy: changes in depression and control orientation. Seizure 2001; 10:34–38.
BIOFEEDBACK: WHAT IS IT?
The term “biofeedback” refers to the instrumentation or training process that allows biologic information to be recorded, displayed, and communicated back to an individual, allowing the individual to make adjustments in physiologic processes that may enhance health or performance. The biofeedback display is analogous to a mirror, in which physiologic processes can be observed and adjusted much as one might adjust a hairstyle or a tie.
In our work with cardiovascular disease patients, biofeedback is a training process that involves a subject or patient, a biofeedback coach or therapist, and state-of-the-art biofeedback equipment. For biofeedback training to be effective, the subject who is trying to learn the skill must be engaged and willing to practice, the coach must be trained in psychophysiology, and the equipment must display accurate readings in real time, allowing the subject to monitor and change physiologic reactions appropriately. The coach teaches the subject about the physiologic parameters, establishes target ranges, and helps the subject learn how to move the physiologic parameters in the right direction.1,2
Training often begins with a session in which a brief mental stress test is followed by a period of relaxation while physiologic parameters are recorded and displayed. This process helps the subject to understand the link between mental processes and physiologic arousal.
Biofeedback training can involve a number of physiologic modalities, including those that reflect autonomic nervous system arousal, such as skin conductance and heart rate variability, and those that are not strictly correlated with autonomic activity, such as surface muscle tension. Each physiologic parameter is recorded by a specific sensor, and all sensors are noninvasive. Sensors feed signals into a computer, where they are processed and amplified, and subjects are able to view the output on a computer screen.
Typically, in our work, there is one screen for the subject, on which a single parameter can be displayed, observed and discussed, and another screen for the coach, on which all parameters are displayed simultaneously. During a single session of biofeedback training, the coach may choose to work on a single parameter or switch between parameters, depending on how much progress is being made with each. In our work with patients, we generally train to simple parameters first, such as respiratory rate, finger temperature, and skin conductance, moving on to surface muscle tension, heart rate, and eventually heart rate variability, which is a more complex concept and more easily understood later in the training process.
It is important that the subject receive positive reinforcement for changing the physiologic parameters, and if the subject struggles too long with one parameter, it is generally useful to go back to a different parameter, where success may be more easily experienced. Ideally, by the end of six to eight training sessions, the subject will be able to make progress on all physiologic parameters, which will track together over time.
BIOFEEDBACK-ASSISTED STRESS MANAGEMENT
Pure biofeedback training consists of operant conditioning. That is, the subject learns to regulate his or her physiology in the right direction because of the feedback, which can be as simple as a pleasant image appearing on a computer screen or as complicated as a car moving faster around a racetrack; pure biofeedback involves changing physiology in response to positive reinforcement of some sort.
In practice, we generally employ biofeedback-assisted stress management (BFSM) rather than pure biofeedback. With BFSM, the subject learns to change physiology in the direction of health and wellness by learning techniques of stress management. The coach teaches the subject various relaxation techniques, such as slow and rhythmic breathing, guided imagery, progressive muscle relaxation, mindfulness, assertiveness, and how to change negative thought patterns. With regular practice, the subject learns to change the physiologic parameters by relaxing the body. For example, instead of instructing the subject to “increase your finger temperature” and assume that the subject will achieve this because doing so will make the light bulb on the screen glow more intensely, the BFSM coach may instead talk with the subject about eliminating stressful thoughts, learning to relax, and the fingers warming in response to the body relaxing.
We distinguish between techniques of stress management, some of which are mentioned above, and psychotherapy, which can certainly be effectively combined with biofeedback, but which we do not provide in our research studies. Coupling stress management techniques with biofeedback helps the subject change physiologic parameters in the direction of wellness and acquire tools that can be used in everyday life when stressful events arise. The objective of BFSM training is not just to change physiology, but also to change the way subjects respond to stressful events in daily life; ie, react to fewer events, react less intensely when they do react, and recover more quickly.
BIOFEEDBACK-ASSISTED STRESS MANAGEMENT IN CARDIOVASCULAR DISEASE
We are currently studying the effects of BFSM in patients with cardiovascular disease, including both heart failure and stable coronary artery disease. Patients with cardiovascular disease often are functionally limited, and they also experience psychologic distress related to physical limitations and other life stressors. Both the physical limitations and the psychologic distress impact quality of life. We hypothesize that BFSM will teach our patients techniques of stress management, both mental and physiologic, that will help relieve their psychologic distress and improve their quality of life. BFSM will also potentially decrease the overactivation of the sympathetic branch of the autonomic nervous system, which is common in cardiovascular disease, and correspondingly upregulate the contribution of the parasympathetic branch of the autonomic nervous system, which should be beneficial.3
A PROMISING TECHNIQUE IN HEART FAILURE
We are currently studying the effects of BFSM in patients with end-stage heart failure who are awaiting heart transplant at Cleveland Clinic.4 As noted in a recent review, biofeedback is a promising technique in heart failure that patients may be able to use to consciously regulate their autonomic nervous systems.5 We hypothesize that BFSM training will interfere with the overactivation of the sympathetic nervous system that is characteristic of heart failure, and that this will reverse the cellular and molecular remodeling that occurs in the failing human heart.
To date, we have enrolled 25 patients; 10 are being studied in our National Institutes of Health–funded Clinic Research Unit and 15 are inpatients. All 25 patients are listed as heart transplant candidates and have given consent for us to study their hearts when they are explanted.
Each patient receives eight sessions with a certified biofeedback therapist. The first and last sessions include mental stress tests, while the remaining six are BFSM training sessions. Patients are assessed at the beginning and end of the study using the 6-minute walk test, the Kansas City Cardiomyopathy Questionnaire, the Medical Outcomes Study 36-Item Short-Form Health Survey (SF-36), and measurement of plasma catecholamines.
The primary end point of the study is the measurement of cellular and molecular markers that have been shown to be altered in the failing human heart, testing the hypothesis that these markers will be reversed in the direction of normal in the BFSM therapy group. These markers are measured in the explanted failing heart when the patient receives a heart transplant.
It is too early to report the results of this study, since only seven patients have undergone transplantation to date. We are encouraged by several early findings, however, and hope these will be validated when the entire group is analyzed.
In early analysis, scores on the Kansas City Cardiomyopathy Questionnaire are improved in the last session compared with the first; patients have shown the ability to learn a slower breathing rate; and they are able to regulate their heart rate variability, as measured by the standard deviation of the N-to-N interval, or SDNN. Most important, measurements in the first seven hearts indicate that there is a degree of biologic remodeling of the failing heart after BFSM that is similar to what we have observed with left ventricular assist devices—hemodynamic pumps that take on the workload of the heart, permitting the heart to rest and recover while the patient is waiting for a transplant.6,7 If BFSM could produce changes in the cellular and molecular properties of the heart that are equal in magnitude to those produced by a mechanical pump, this would be a revolutionary finding in the field of heart-brain medicine.
It should be noted that we are not the first group to study BFSM in patients with heart failure. Moser and colleagues first observed that a single session of skin temperature biofeedback could have significant functional effects in patients with heart failure.8 Bernardi and coworkers showed that merely teaching patients to breathe six times per minute (a large component of BFSM training) improved oxygen saturation and exercise tolerance.9 Swanson and colleagues in 2009 demonstrated that patients with heart failure were able to regulate their heart rate variability, although they observed this only in patients with a left ventricular ejection fraction greater than 30%.10 Our preliminary data demonstrate regulation of heart rate variability in patients with lower ejection fractions, which is promising, but we have also added the biologic component of studying the explanted heart, allowing us to test the hypothesis that BFSM could potentially impact the remodeling process and thus have important therapeutic implications.
TRIAL UNDER WAY IN CORONARY ARTERY DISEASE
In addition to our studies of BFSM in heart failure, we have begun a randomized clinical trial of patients with stable coronary artery disease, type 2 diabetes, or multiple sclerosis. These three patient populations were chosen because evidence from numerous studies suggests that they all involve autonomic nervous system dysregulation as well as an inflammatory process.
It has already been mentioned that BFSM can interfere with overactivation of the sympathetic nervous system and potentially upregulate the contribution of the parasympathetic nervous system, which usually exists in juxtaposition to the sympathetic nervous system. Based on the work of Tracey,11,12 upregulating the parasympathetic nervous system should be antiinflammatory. Thus, we hypothesize that by decreasing both sympathetic nervous system activation and inflammation, BFSM should have an impact on patients with one of these disease states, resulting in improved quality of life and clinical status, reduced anxiety and depression, and changed disease-specific indicators of severity.
We are currently enrolling patients who have coronary artery disease, type 2 diabetes, or multiple sclerosis and randomizing them to groups that will receive either BFSM or usual care. Outcome variables that will be assessed in all patients include heart rate variability; the response of temperature, skin conductance, respiratory rate, and heart rate variability to mental stress; plasma catecholamine levels; plasma C-reactive protein levels; and tumor necrosis factor alpha levels. At the first and last visits, all patients will complete the SF-36, the eight-item Patient Health Questionnaire depression scale (PHQ-8), the Generalized Anxiety Disorder seven-item scale (GAD-7), and a visual analog pain scale. We will also assess disease-specific variables, including heart rate recovery after exercise, plasma lipids, and myeloperoxidase in patients with coronary artery disease; the Multiple Sclerosis Functional Composite (MSFC) test and the Modified Fatigue Impact Scale (MFIS) will be administered to patients with multiple sclerosis; and plasma glucose and hemoglobin A1C will be assessed in patients with type 2 diabetes.
Results of this study will provide data on the potential of BFSM to decrease common markers of autonomic nervous system activation and inflammatory cascades and the effect of those alterations on three specific disease states. To our knowledge, such a randomized study has not been conducted previously; our findings will add significantly to the literature on the mechanism of action of biofeedback-type interventions.
POTENTIAL IMPACT ON DEPRESSION IN CARDIOVASCULAR DISEASE
Depression is increasingly recognized as a component of many cardiovascular diseases; this raises the question of what effect BFSM therapy in cardiovascular disease patients will have on their depression. Of particular importance to this discussion, heart rate variability has been shown to be decreased both in cardiovascular disease and in depression, and BFSM is one treatment that can be used to regulate heart rate variability. Heart rate variability biofeedback has been shown to be useful in treating depression.
Work from Karavidas and colleagues showed that 10 weeks of heart rate variability biofeedback in patients with depression led to significantly improved scores on the Hamilton Depression Scale and the Beck Depression Inventory. Improvement was observed by the fourth week of training, with concurrent increases in the SDNN.13 Siepmann and colleagues also used heart rate variability biofeedback in depressed subjects and demonstrated significant improvement in scores on the Beck Depression Inventory, as well as a concomitant decrease in anxiety.14 In related work, Uhlmann and Fröscher used electroencephalographic biofeedback (also called neurofeedback) in epilepsy patients with depression and measured an increased sense of self control and a decrease in external locus of control; they postulated that biofeedback training provided an important opportunity for success, and thus increased internal control and decreased depression.15
Evidence suggests that BFSM should have an impact on depression in addition to impacting the cardiovascular disease itself, and both should work together to improve quality of life. For this reason we have added a depression inventory to our randomized trial of BFSM in patients who have coronary artery disease, diabetes, or multiple sclerosis.
BIOFEEDBACK: WHAT IS IT?
The term “biofeedback” refers to the instrumentation or training process that allows biologic information to be recorded, displayed, and communicated back to an individual, allowing the individual to make adjustments in physiologic processes that may enhance health or performance. The biofeedback display is analogous to a mirror, in which physiologic processes can be observed and adjusted much as one might adjust a hairstyle or a tie.
In our work with cardiovascular disease patients, biofeedback is a training process that involves a subject or patient, a biofeedback coach or therapist, and state-of-the-art biofeedback equipment. For biofeedback training to be effective, the subject who is trying to learn the skill must be engaged and willing to practice, the coach must be trained in psychophysiology, and the equipment must display accurate readings in real time, allowing the subject to monitor and change physiologic reactions appropriately. The coach teaches the subject about the physiologic parameters, establishes target ranges, and helps the subject learn how to move the physiologic parameters in the right direction.1,2
Training often begins with a session in which a brief mental stress test is followed by a period of relaxation while physiologic parameters are recorded and displayed. This process helps the subject to understand the link between mental processes and physiologic arousal.
Biofeedback training can involve a number of physiologic modalities, including those that reflect autonomic nervous system arousal, such as skin conductance and heart rate variability, and those that are not strictly correlated with autonomic activity, such as surface muscle tension. Each physiologic parameter is recorded by a specific sensor, and all sensors are noninvasive. Sensors feed signals into a computer, where they are processed and amplified, and subjects are able to view the output on a computer screen.
Typically, in our work, there is one screen for the subject, on which a single parameter can be displayed, observed and discussed, and another screen for the coach, on which all parameters are displayed simultaneously. During a single session of biofeedback training, the coach may choose to work on a single parameter or switch between parameters, depending on how much progress is being made with each. In our work with patients, we generally train to simple parameters first, such as respiratory rate, finger temperature, and skin conductance, moving on to surface muscle tension, heart rate, and eventually heart rate variability, which is a more complex concept and more easily understood later in the training process.
It is important that the subject receive positive reinforcement for changing the physiologic parameters, and if the subject struggles too long with one parameter, it is generally useful to go back to a different parameter, where success may be more easily experienced. Ideally, by the end of six to eight training sessions, the subject will be able to make progress on all physiologic parameters, which will track together over time.
BIOFEEDBACK-ASSISTED STRESS MANAGEMENT
Pure biofeedback training consists of operant conditioning. That is, the subject learns to regulate his or her physiology in the right direction because of the feedback, which can be as simple as a pleasant image appearing on a computer screen or as complicated as a car moving faster around a racetrack; pure biofeedback involves changing physiology in response to positive reinforcement of some sort.
In practice, we generally employ biofeedback-assisted stress management (BFSM) rather than pure biofeedback. With BFSM, the subject learns to change physiology in the direction of health and wellness by learning techniques of stress management. The coach teaches the subject various relaxation techniques, such as slow and rhythmic breathing, guided imagery, progressive muscle relaxation, mindfulness, assertiveness, and how to change negative thought patterns. With regular practice, the subject learns to change the physiologic parameters by relaxing the body. For example, instead of instructing the subject to “increase your finger temperature” and assume that the subject will achieve this because doing so will make the light bulb on the screen glow more intensely, the BFSM coach may instead talk with the subject about eliminating stressful thoughts, learning to relax, and the fingers warming in response to the body relaxing.
We distinguish between techniques of stress management, some of which are mentioned above, and psychotherapy, which can certainly be effectively combined with biofeedback, but which we do not provide in our research studies. Coupling stress management techniques with biofeedback helps the subject change physiologic parameters in the direction of wellness and acquire tools that can be used in everyday life when stressful events arise. The objective of BFSM training is not just to change physiology, but also to change the way subjects respond to stressful events in daily life; ie, react to fewer events, react less intensely when they do react, and recover more quickly.
BIOFEEDBACK-ASSISTED STRESS MANAGEMENT IN CARDIOVASCULAR DISEASE
We are currently studying the effects of BFSM in patients with cardiovascular disease, including both heart failure and stable coronary artery disease. Patients with cardiovascular disease often are functionally limited, and they also experience psychologic distress related to physical limitations and other life stressors. Both the physical limitations and the psychologic distress impact quality of life. We hypothesize that BFSM will teach our patients techniques of stress management, both mental and physiologic, that will help relieve their psychologic distress and improve their quality of life. BFSM will also potentially decrease the overactivation of the sympathetic branch of the autonomic nervous system, which is common in cardiovascular disease, and correspondingly upregulate the contribution of the parasympathetic branch of the autonomic nervous system, which should be beneficial.3
A PROMISING TECHNIQUE IN HEART FAILURE
We are currently studying the effects of BFSM in patients with end-stage heart failure who are awaiting heart transplant at Cleveland Clinic.4 As noted in a recent review, biofeedback is a promising technique in heart failure that patients may be able to use to consciously regulate their autonomic nervous systems.5 We hypothesize that BFSM training will interfere with the overactivation of the sympathetic nervous system that is characteristic of heart failure, and that this will reverse the cellular and molecular remodeling that occurs in the failing human heart.
To date, we have enrolled 25 patients; 10 are being studied in our National Institutes of Health–funded Clinic Research Unit and 15 are inpatients. All 25 patients are listed as heart transplant candidates and have given consent for us to study their hearts when they are explanted.
Each patient receives eight sessions with a certified biofeedback therapist. The first and last sessions include mental stress tests, while the remaining six are BFSM training sessions. Patients are assessed at the beginning and end of the study using the 6-minute walk test, the Kansas City Cardiomyopathy Questionnaire, the Medical Outcomes Study 36-Item Short-Form Health Survey (SF-36), and measurement of plasma catecholamines.
The primary end point of the study is the measurement of cellular and molecular markers that have been shown to be altered in the failing human heart, testing the hypothesis that these markers will be reversed in the direction of normal in the BFSM therapy group. These markers are measured in the explanted failing heart when the patient receives a heart transplant.
It is too early to report the results of this study, since only seven patients have undergone transplantation to date. We are encouraged by several early findings, however, and hope these will be validated when the entire group is analyzed.
In early analysis, scores on the Kansas City Cardiomyopathy Questionnaire are improved in the last session compared with the first; patients have shown the ability to learn a slower breathing rate; and they are able to regulate their heart rate variability, as measured by the standard deviation of the N-to-N interval, or SDNN. Most important, measurements in the first seven hearts indicate that there is a degree of biologic remodeling of the failing heart after BFSM that is similar to what we have observed with left ventricular assist devices—hemodynamic pumps that take on the workload of the heart, permitting the heart to rest and recover while the patient is waiting for a transplant.6,7 If BFSM could produce changes in the cellular and molecular properties of the heart that are equal in magnitude to those produced by a mechanical pump, this would be a revolutionary finding in the field of heart-brain medicine.
It should be noted that we are not the first group to study BFSM in patients with heart failure. Moser and colleagues first observed that a single session of skin temperature biofeedback could have significant functional effects in patients with heart failure.8 Bernardi and coworkers showed that merely teaching patients to breathe six times per minute (a large component of BFSM training) improved oxygen saturation and exercise tolerance.9 Swanson and colleagues in 2009 demonstrated that patients with heart failure were able to regulate their heart rate variability, although they observed this only in patients with a left ventricular ejection fraction greater than 30%.10 Our preliminary data demonstrate regulation of heart rate variability in patients with lower ejection fractions, which is promising, but we have also added the biologic component of studying the explanted heart, allowing us to test the hypothesis that BFSM could potentially impact the remodeling process and thus have important therapeutic implications.
TRIAL UNDER WAY IN CORONARY ARTERY DISEASE
In addition to our studies of BFSM in heart failure, we have begun a randomized clinical trial of patients with stable coronary artery disease, type 2 diabetes, or multiple sclerosis. These three patient populations were chosen because evidence from numerous studies suggests that they all involve autonomic nervous system dysregulation as well as an inflammatory process.
It has already been mentioned that BFSM can interfere with overactivation of the sympathetic nervous system and potentially upregulate the contribution of the parasympathetic nervous system, which usually exists in juxtaposition to the sympathetic nervous system. Based on the work of Tracey,11,12 upregulating the parasympathetic nervous system should be antiinflammatory. Thus, we hypothesize that by decreasing both sympathetic nervous system activation and inflammation, BFSM should have an impact on patients with one of these disease states, resulting in improved quality of life and clinical status, reduced anxiety and depression, and changed disease-specific indicators of severity.
We are currently enrolling patients who have coronary artery disease, type 2 diabetes, or multiple sclerosis and randomizing them to groups that will receive either BFSM or usual care. Outcome variables that will be assessed in all patients include heart rate variability; the response of temperature, skin conductance, respiratory rate, and heart rate variability to mental stress; plasma catecholamine levels; plasma C-reactive protein levels; and tumor necrosis factor alpha levels. At the first and last visits, all patients will complete the SF-36, the eight-item Patient Health Questionnaire depression scale (PHQ-8), the Generalized Anxiety Disorder seven-item scale (GAD-7), and a visual analog pain scale. We will also assess disease-specific variables, including heart rate recovery after exercise, plasma lipids, and myeloperoxidase in patients with coronary artery disease; the Multiple Sclerosis Functional Composite (MSFC) test and the Modified Fatigue Impact Scale (MFIS) will be administered to patients with multiple sclerosis; and plasma glucose and hemoglobin A1C will be assessed in patients with type 2 diabetes.
Results of this study will provide data on the potential of BFSM to decrease common markers of autonomic nervous system activation and inflammatory cascades and the effect of those alterations on three specific disease states. To our knowledge, such a randomized study has not been conducted previously; our findings will add significantly to the literature on the mechanism of action of biofeedback-type interventions.
POTENTIAL IMPACT ON DEPRESSION IN CARDIOVASCULAR DISEASE
Depression is increasingly recognized as a component of many cardiovascular diseases; this raises the question of what effect BFSM therapy in cardiovascular disease patients will have on their depression. Of particular importance to this discussion, heart rate variability has been shown to be decreased both in cardiovascular disease and in depression, and BFSM is one treatment that can be used to regulate heart rate variability. Heart rate variability biofeedback has been shown to be useful in treating depression.
Work from Karavidas and colleagues showed that 10 weeks of heart rate variability biofeedback in patients with depression led to significantly improved scores on the Hamilton Depression Scale and the Beck Depression Inventory. Improvement was observed by the fourth week of training, with concurrent increases in the SDNN.13 Siepmann and colleagues also used heart rate variability biofeedback in depressed subjects and demonstrated significant improvement in scores on the Beck Depression Inventory, as well as a concomitant decrease in anxiety.14 In related work, Uhlmann and Fröscher used electroencephalographic biofeedback (also called neurofeedback) in epilepsy patients with depression and measured an increased sense of self control and a decrease in external locus of control; they postulated that biofeedback training provided an important opportunity for success, and thus increased internal control and decreased depression.15
Evidence suggests that BFSM should have an impact on depression in addition to impacting the cardiovascular disease itself, and both should work together to improve quality of life. For this reason we have added a depression inventory to our randomized trial of BFSM in patients who have coronary artery disease, diabetes, or multiple sclerosis.
- McKee MG. Biofeedback: an overview in the context of heart-brain medicine. Cleve Clin J Med 2008; 75(suppl 2):S31–S34.
- Frank DL, Khorshid L, Kiffer JF, Moravec CS, McKee MG. Biofeedback in medicine: who, when, why and how? Ment Health Fam Med 2010; 7:85–91.
- Moravec CS. Biofeedback therapy in cardiovascular disease: rationale and research overview. Cleve Clin J Med 2008; 75(suppl 2):S35–S38.
- McKee MG, Moravec CS. Biofeedback in the treatment of heart failure. Cleve Clin J Med 2010; 77(supp 3): S56–S59.
- Emani S, Binkley PF. Mind-body medicine in chronic heart failure: a translational science challenge. Circ Heart Fail 2010; 3:715–725.
- Ogletree-Hughes ML, Stull LB, Sweet WE, Smedira NG, McCarthy PM, Moravec CS. Mechanical unloading restores beta-adrenergic responsiveness and reverses receptor downregulation in the failing human heart. Circulation 2001; 104:881–886.
- Ogletree ML, Sweet WE, Talerico C, et al. Duration of left ventricular assist device support: effects on abnormal calcium cycling and functional recovery in the failing human heart. J Heart Lung Transplant 2010; 29:554–561.
- Moser DK, Dracup K, Woo MA, Stevenson LW. Voluntary control of vascular tone by using skin-temperature biofeedback-relaxation in patients with advanced heart failure. Altern Ther Health Med 1997; 3:51–59.
- Bernardi L, Porta C, Spicuzza L, et al. Slow breathing increases arterial baroreflex sensitivity in patients with chronic heart failure. Circulation 2002; 105:143–145.
- Swanson KS, Gevirtz RN, Brown M, Spira J, Guarneri E, Stoletniy L. The effect of biofeedback on function in patients with heart failure. Appl Psychophysiol Biofeedback 2009; 34:71–91.
- Tracey KJ. The inflammatory reflex. Nature 2002; 420:853–859.
- Tracey KJ. Reflex control of immunity. Nat Rev Immunol 2009; 9:418–428.
- Karavidas MK, Lehrer PM, Vaschillo E, et al. Preliminary results of an open label study of heart rate variability biofeedback for the treatment of major depression. Appl Psychophysiol Biofeedback 2007; 32:19–30.
- Siepmann M, Aykac V, Unterdörfer J, Petrowski K, Mueck-Weymann M. A pilot study on the effects of heart rate variability biofeedback in patients with depression and in healthy subjects. Appl Psychophysiol Biofeedback 2008; 33:195–201.
- Uhlmann C, Fröscher W. Biofeedback treatment in patients with refractory epilepsy: changes in depression and control orientation. Seizure 2001; 10:34–38.
- McKee MG. Biofeedback: an overview in the context of heart-brain medicine. Cleve Clin J Med 2008; 75(suppl 2):S31–S34.
- Frank DL, Khorshid L, Kiffer JF, Moravec CS, McKee MG. Biofeedback in medicine: who, when, why and how? Ment Health Fam Med 2010; 7:85–91.
- Moravec CS. Biofeedback therapy in cardiovascular disease: rationale and research overview. Cleve Clin J Med 2008; 75(suppl 2):S35–S38.
- McKee MG, Moravec CS. Biofeedback in the treatment of heart failure. Cleve Clin J Med 2010; 77(supp 3): S56–S59.
- Emani S, Binkley PF. Mind-body medicine in chronic heart failure: a translational science challenge. Circ Heart Fail 2010; 3:715–725.
- Ogletree-Hughes ML, Stull LB, Sweet WE, Smedira NG, McCarthy PM, Moravec CS. Mechanical unloading restores beta-adrenergic responsiveness and reverses receptor downregulation in the failing human heart. Circulation 2001; 104:881–886.
- Ogletree ML, Sweet WE, Talerico C, et al. Duration of left ventricular assist device support: effects on abnormal calcium cycling and functional recovery in the failing human heart. J Heart Lung Transplant 2010; 29:554–561.
- Moser DK, Dracup K, Woo MA, Stevenson LW. Voluntary control of vascular tone by using skin-temperature biofeedback-relaxation in patients with advanced heart failure. Altern Ther Health Med 1997; 3:51–59.
- Bernardi L, Porta C, Spicuzza L, et al. Slow breathing increases arterial baroreflex sensitivity in patients with chronic heart failure. Circulation 2002; 105:143–145.
- Swanson KS, Gevirtz RN, Brown M, Spira J, Guarneri E, Stoletniy L. The effect of biofeedback on function in patients with heart failure. Appl Psychophysiol Biofeedback 2009; 34:71–91.
- Tracey KJ. The inflammatory reflex. Nature 2002; 420:853–859.
- Tracey KJ. Reflex control of immunity. Nat Rev Immunol 2009; 9:418–428.
- Karavidas MK, Lehrer PM, Vaschillo E, et al. Preliminary results of an open label study of heart rate variability biofeedback for the treatment of major depression. Appl Psychophysiol Biofeedback 2007; 32:19–30.
- Siepmann M, Aykac V, Unterdörfer J, Petrowski K, Mueck-Weymann M. A pilot study on the effects of heart rate variability biofeedback in patients with depression and in healthy subjects. Appl Psychophysiol Biofeedback 2008; 33:195–201.
- Uhlmann C, Fröscher W. Biofeedback treatment in patients with refractory epilepsy: changes in depression and control orientation. Seizure 2001; 10:34–38.
Stress in medicine: Strategies for caregivers, patients, clinicians—The burdens of caregiver stress
The number of people in the United States who spend a significant part of each week working as unpaid caregivers is considerable, and the toll exacted for such work is high. Understanding the profi le of the caregiver, the nature of the duties performed, the stress imposed by such duties, and the consequences of the stress can assist the clinician in recognizing the caregiver in need of intervention.
A PROFILE OF THE CAREGIVER
A recent survey estimated that more than 65 million Americans provide unpaid assistance annually to older adults with disabilities.1 The value of that labor has been estimated at $306 billion annually, or nearly double the combined cost of home health care and nursing home care.2,3
The typical caregiver is a woman, about 48 years old, with some college education, who spends 20 hours or more each week providing unpaid care to someone aged 50 years or older.1 The recipients of care often have long-term physical disabilities; mental confusion or emotional problems frequently complicate care.
PSYCHOLOGIC AND PHYSICAL COSTS
Caregiving may take a toll on the caregiver in a variety of ways: behavioral, in the form of alcohol or substance use4; psychologic, in the form of depression or other mental health problems5; and physical, in the form of chronic health conditions and impaired immune response.6 About three-fifths of caregivers report fair or poor health, compared with one-third of noncaregivers, and caregivers have approximately twice as many chronic conditions, such as heart disease, cancer, arthritis, and diabetes, compared with noncaregivers.2,7 Caregiving also exacts a financial toll, as employees who are caregivers cost their employers $13.4 billion more per year in health care expenditures.8 In addition, absenteeism, workday interruptions, and shifts from full-time to part-time work by caregivers cost businesses between $17.1 and $33.6 billion per year.9
The cost of caregiving is higher for women, who exhibit higher levels of anxiety and depression and lower levels of subjective well-being, life satisfaction, and physical health.10,11 The stress of caregiving has also been identified as a risk factor for morbidity among older (66 to 96 years old) caregivers, who have a 63% greater mortality than noncaregivers of the same age.12
PSYCHOSOCIAL STRESS, UNHEALTHY BEHAVIORS, AND ILLNESS ARE LINKED
Psychosocial stress is a predictor of disease and can lead to unhealthy behaviors such as smoking, substance abuse, overeating, poor nutrition, and a sedentary lifestyle; these, in turn, can lead to physical and psychiatric illness. Behaviors adopted initially as coping skills may persist to become chronic, thereby promoting either continued wellness (in the case of healthy coping behaviors) or worsening levels of illness (in the case of unhealthy coping behaviors).
McEwen and Gianaros13 have suggested that these stress mechanisms arise from patterns of communication between the brain and the autonomic, cardiovascular, and immune systems, which mutually influenceone another. These so-called bidirectional stress processes affect cognition, experience, and behavior.
An integrated model of stress that maps the bidirectional causal pathways among psychosocial stressors, resulting unhealthy behaviors, and illness is needed. Although the steps from unhealthy behaviors to illness are fairly well understood, the links from psychosocial stress, such as those exhibited by caregivers, to unhealthy behaviors are not as clear. Several mediators are under study:
- Personality mediators can be either ameliorative (resilience, self-confi dence, self-control, optimism, high self-esteem, a sense of mastery, and finding meaning in life) or exacerbating (neuroticism and inhibition, which together form the so-called type D personality).
- Environmental mediators include social support, financial support, a history of a significant life change, and trauma early in life, which may increase one’s subsequent vulnerability to unhealthy behaviors.
- Biologic mediators may include prolonged sympathetic activation and enhanced platelet activation, caused by increased levels of depression and anxiety in chronically stressed caregivers.14
IMPLICATIONS FOR INTERVENTION
A significant percentage of caregivers do not need a clinician’s intervention to help them cope with stress or unhealthy coping skills. Among caregivers aged 50 years or older, 47% indicated in a recent study that the burden of caregiving is low (ie, 1 or 2 on a 5-point scale).1 Those who respond to stressors as challenges rather than threats tend to be resilient people who exert control over their lives, often through meditation or similar techniques, and have a strong social support network. Many report that caregiving provides them with an opportunity to act in accordance with their values and feel helpful rather than helpless.
Cognitive-behavioral interventions to alleviate stress-related symptoms appear to be more effective if offered as individual rather than group therapy. Teaching caregivers effective coping strategies, rather than merely providing social support, has been shown to improve caregiver psychologic health.15 Chief among the goals of intervention should be to alter brain function and instill optimism, a sense of control and self-esteem.13
- The National Alliance for Caregiving, in collaboration with the American Association of Retired Persons. Caregiving in the U.S. 2009. National Alliance for Caregiving Web site. http://www.caregiving.org/data/Caregiving_in_the_US_2009_full_report.pdf. Published November 2009. Accessed March 21, 2011.
- Family Caregiver Alliance. Caregiver health. A population at risk. National Alliance for Caregiving Web site. http://www.caregiver.org/caregiver/jsp/content_node.jsp?nodeid=1822. Published 2006. Accessed March 21, 2011.
- Family Caregiver Alliance. Prevalence, hours, and economic value of family caregiving, updated state-by-state analysis of 2004 national estimates. National Alliance for Caregiving Web site. http://www.caregiver.org/caregiver/jsp/content/pdfs/State_Caregiving_Data_Amo_20061107.pdf. Published 2006. Accessed March 21, 2011.
- Evercare. Study of caregivers in decline: a close-up look at the health risks of caring for a loved one. National Alliance for Caregiving Web site. http://www.caregiving.org/data/Caregivers%20in%20Decline%20Study-FINAL-lowres.pdf. Published 2006. Accessed March 21, 2011.
- Pinquart M, Sörensen S. Differences between caregivers and noncaregivers in psychological health and physical health: a metaanalysis. Psychol Aging 2003; 18:250–267.
- Vitaliano PP, Zhang J, Scanlan JM. Is caregiving hazardous to one’s physical health? A meta-analysis. Psychol Bull 2003; 129:946–972.
- Ho A, Collins S, Davis K, Doty M. A look at working-age caregivers’ roles, health concerns, and need for support (issue brief). New York, NY: The Commonwealth Fund; 2005.
- MetLife study of working caregivers and employer health care costs. MetLife Web site. http://www.metlife.com/assets/cao/mmi/publications/studies/2010/mmi-working-caregivers-employers-health-carecosts.pdf. Published July 2006. Accessed March 21, 2011.
- MetLife caregiving cost study: productivity losses to U.S. business. National Alliance for Caregiving Web site. http://www.caregiving. org/data/Caregiver%20Cost%20Study.pdf. Published July 2006. Accessed March 21, 2011.
- Pinquart M, Sörensen S. Gender differences in caregiver stressors, social resources, and health: an updated meta-analysis. J Gerontol B Psychol Sci Soc Sci 2006; 61:P33–P45.
- Johnson RW, Wiener JM. A profi le of frail older Americans and their caregivers. Urban Institute Web site. http://www.urban.org/UploadedPDF/311284_older_americans.pdf. Published February 2006. Accessed March 21, 2011.
- Schulz R, Beach SR. Caregiving as a risk factor for mortality: the caregiver health effects study. JAMA 1999; 282:2215–2219.
- McEwen BS, Gianaros PJ. Central role of the brain in stress and adaptation: links to socioeconomic status, health, and disease. Ann NY Acad Sci 2010; 1186:190–222.
- Aschbacher K, Mills PJ, von Känel R, et al. Effects of depressive and anxious symptoms on norepinephrine and platelet P-selectin responses to acute psychological stress among elderly caregivers. Brain Behav Immun 2008; 22:493–502.
- Selwood A, Johnston K, Katona C, Lyketsos C, Livingston G. Systematic review of the effect of psychological interventions on family caregivers of people with dementia. J Affect Disord 2007; 101:75–89.
The number of people in the United States who spend a significant part of each week working as unpaid caregivers is considerable, and the toll exacted for such work is high. Understanding the profi le of the caregiver, the nature of the duties performed, the stress imposed by such duties, and the consequences of the stress can assist the clinician in recognizing the caregiver in need of intervention.
A PROFILE OF THE CAREGIVER
A recent survey estimated that more than 65 million Americans provide unpaid assistance annually to older adults with disabilities.1 The value of that labor has been estimated at $306 billion annually, or nearly double the combined cost of home health care and nursing home care.2,3
The typical caregiver is a woman, about 48 years old, with some college education, who spends 20 hours or more each week providing unpaid care to someone aged 50 years or older.1 The recipients of care often have long-term physical disabilities; mental confusion or emotional problems frequently complicate care.
PSYCHOLOGIC AND PHYSICAL COSTS
Caregiving may take a toll on the caregiver in a variety of ways: behavioral, in the form of alcohol or substance use4; psychologic, in the form of depression or other mental health problems5; and physical, in the form of chronic health conditions and impaired immune response.6 About three-fifths of caregivers report fair or poor health, compared with one-third of noncaregivers, and caregivers have approximately twice as many chronic conditions, such as heart disease, cancer, arthritis, and diabetes, compared with noncaregivers.2,7 Caregiving also exacts a financial toll, as employees who are caregivers cost their employers $13.4 billion more per year in health care expenditures.8 In addition, absenteeism, workday interruptions, and shifts from full-time to part-time work by caregivers cost businesses between $17.1 and $33.6 billion per year.9
The cost of caregiving is higher for women, who exhibit higher levels of anxiety and depression and lower levels of subjective well-being, life satisfaction, and physical health.10,11 The stress of caregiving has also been identified as a risk factor for morbidity among older (66 to 96 years old) caregivers, who have a 63% greater mortality than noncaregivers of the same age.12
PSYCHOSOCIAL STRESS, UNHEALTHY BEHAVIORS, AND ILLNESS ARE LINKED
Psychosocial stress is a predictor of disease and can lead to unhealthy behaviors such as smoking, substance abuse, overeating, poor nutrition, and a sedentary lifestyle; these, in turn, can lead to physical and psychiatric illness. Behaviors adopted initially as coping skills may persist to become chronic, thereby promoting either continued wellness (in the case of healthy coping behaviors) or worsening levels of illness (in the case of unhealthy coping behaviors).
McEwen and Gianaros13 have suggested that these stress mechanisms arise from patterns of communication between the brain and the autonomic, cardiovascular, and immune systems, which mutually influenceone another. These so-called bidirectional stress processes affect cognition, experience, and behavior.
An integrated model of stress that maps the bidirectional causal pathways among psychosocial stressors, resulting unhealthy behaviors, and illness is needed. Although the steps from unhealthy behaviors to illness are fairly well understood, the links from psychosocial stress, such as those exhibited by caregivers, to unhealthy behaviors are not as clear. Several mediators are under study:
- Personality mediators can be either ameliorative (resilience, self-confi dence, self-control, optimism, high self-esteem, a sense of mastery, and finding meaning in life) or exacerbating (neuroticism and inhibition, which together form the so-called type D personality).
- Environmental mediators include social support, financial support, a history of a significant life change, and trauma early in life, which may increase one’s subsequent vulnerability to unhealthy behaviors.
- Biologic mediators may include prolonged sympathetic activation and enhanced platelet activation, caused by increased levels of depression and anxiety in chronically stressed caregivers.14
IMPLICATIONS FOR INTERVENTION
A significant percentage of caregivers do not need a clinician’s intervention to help them cope with stress or unhealthy coping skills. Among caregivers aged 50 years or older, 47% indicated in a recent study that the burden of caregiving is low (ie, 1 or 2 on a 5-point scale).1 Those who respond to stressors as challenges rather than threats tend to be resilient people who exert control over their lives, often through meditation or similar techniques, and have a strong social support network. Many report that caregiving provides them with an opportunity to act in accordance with their values and feel helpful rather than helpless.
Cognitive-behavioral interventions to alleviate stress-related symptoms appear to be more effective if offered as individual rather than group therapy. Teaching caregivers effective coping strategies, rather than merely providing social support, has been shown to improve caregiver psychologic health.15 Chief among the goals of intervention should be to alter brain function and instill optimism, a sense of control and self-esteem.13
The number of people in the United States who spend a significant part of each week working as unpaid caregivers is considerable, and the toll exacted for such work is high. Understanding the profi le of the caregiver, the nature of the duties performed, the stress imposed by such duties, and the consequences of the stress can assist the clinician in recognizing the caregiver in need of intervention.
A PROFILE OF THE CAREGIVER
A recent survey estimated that more than 65 million Americans provide unpaid assistance annually to older adults with disabilities.1 The value of that labor has been estimated at $306 billion annually, or nearly double the combined cost of home health care and nursing home care.2,3
The typical caregiver is a woman, about 48 years old, with some college education, who spends 20 hours or more each week providing unpaid care to someone aged 50 years or older.1 The recipients of care often have long-term physical disabilities; mental confusion or emotional problems frequently complicate care.
PSYCHOLOGIC AND PHYSICAL COSTS
Caregiving may take a toll on the caregiver in a variety of ways: behavioral, in the form of alcohol or substance use4; psychologic, in the form of depression or other mental health problems5; and physical, in the form of chronic health conditions and impaired immune response.6 About three-fifths of caregivers report fair or poor health, compared with one-third of noncaregivers, and caregivers have approximately twice as many chronic conditions, such as heart disease, cancer, arthritis, and diabetes, compared with noncaregivers.2,7 Caregiving also exacts a financial toll, as employees who are caregivers cost their employers $13.4 billion more per year in health care expenditures.8 In addition, absenteeism, workday interruptions, and shifts from full-time to part-time work by caregivers cost businesses between $17.1 and $33.6 billion per year.9
The cost of caregiving is higher for women, who exhibit higher levels of anxiety and depression and lower levels of subjective well-being, life satisfaction, and physical health.10,11 The stress of caregiving has also been identified as a risk factor for morbidity among older (66 to 96 years old) caregivers, who have a 63% greater mortality than noncaregivers of the same age.12
PSYCHOSOCIAL STRESS, UNHEALTHY BEHAVIORS, AND ILLNESS ARE LINKED
Psychosocial stress is a predictor of disease and can lead to unhealthy behaviors such as smoking, substance abuse, overeating, poor nutrition, and a sedentary lifestyle; these, in turn, can lead to physical and psychiatric illness. Behaviors adopted initially as coping skills may persist to become chronic, thereby promoting either continued wellness (in the case of healthy coping behaviors) or worsening levels of illness (in the case of unhealthy coping behaviors).
McEwen and Gianaros13 have suggested that these stress mechanisms arise from patterns of communication between the brain and the autonomic, cardiovascular, and immune systems, which mutually influenceone another. These so-called bidirectional stress processes affect cognition, experience, and behavior.
An integrated model of stress that maps the bidirectional causal pathways among psychosocial stressors, resulting unhealthy behaviors, and illness is needed. Although the steps from unhealthy behaviors to illness are fairly well understood, the links from psychosocial stress, such as those exhibited by caregivers, to unhealthy behaviors are not as clear. Several mediators are under study:
- Personality mediators can be either ameliorative (resilience, self-confi dence, self-control, optimism, high self-esteem, a sense of mastery, and finding meaning in life) or exacerbating (neuroticism and inhibition, which together form the so-called type D personality).
- Environmental mediators include social support, financial support, a history of a significant life change, and trauma early in life, which may increase one’s subsequent vulnerability to unhealthy behaviors.
- Biologic mediators may include prolonged sympathetic activation and enhanced platelet activation, caused by increased levels of depression and anxiety in chronically stressed caregivers.14
IMPLICATIONS FOR INTERVENTION
A significant percentage of caregivers do not need a clinician’s intervention to help them cope with stress or unhealthy coping skills. Among caregivers aged 50 years or older, 47% indicated in a recent study that the burden of caregiving is low (ie, 1 or 2 on a 5-point scale).1 Those who respond to stressors as challenges rather than threats tend to be resilient people who exert control over their lives, often through meditation or similar techniques, and have a strong social support network. Many report that caregiving provides them with an opportunity to act in accordance with their values and feel helpful rather than helpless.
Cognitive-behavioral interventions to alleviate stress-related symptoms appear to be more effective if offered as individual rather than group therapy. Teaching caregivers effective coping strategies, rather than merely providing social support, has been shown to improve caregiver psychologic health.15 Chief among the goals of intervention should be to alter brain function and instill optimism, a sense of control and self-esteem.13
- The National Alliance for Caregiving, in collaboration with the American Association of Retired Persons. Caregiving in the U.S. 2009. National Alliance for Caregiving Web site. http://www.caregiving.org/data/Caregiving_in_the_US_2009_full_report.pdf. Published November 2009. Accessed March 21, 2011.
- Family Caregiver Alliance. Caregiver health. A population at risk. National Alliance for Caregiving Web site. http://www.caregiver.org/caregiver/jsp/content_node.jsp?nodeid=1822. Published 2006. Accessed March 21, 2011.
- Family Caregiver Alliance. Prevalence, hours, and economic value of family caregiving, updated state-by-state analysis of 2004 national estimates. National Alliance for Caregiving Web site. http://www.caregiver.org/caregiver/jsp/content/pdfs/State_Caregiving_Data_Amo_20061107.pdf. Published 2006. Accessed March 21, 2011.
- Evercare. Study of caregivers in decline: a close-up look at the health risks of caring for a loved one. National Alliance for Caregiving Web site. http://www.caregiving.org/data/Caregivers%20in%20Decline%20Study-FINAL-lowres.pdf. Published 2006. Accessed March 21, 2011.
- Pinquart M, Sörensen S. Differences between caregivers and noncaregivers in psychological health and physical health: a metaanalysis. Psychol Aging 2003; 18:250–267.
- Vitaliano PP, Zhang J, Scanlan JM. Is caregiving hazardous to one’s physical health? A meta-analysis. Psychol Bull 2003; 129:946–972.
- Ho A, Collins S, Davis K, Doty M. A look at working-age caregivers’ roles, health concerns, and need for support (issue brief). New York, NY: The Commonwealth Fund; 2005.
- MetLife study of working caregivers and employer health care costs. MetLife Web site. http://www.metlife.com/assets/cao/mmi/publications/studies/2010/mmi-working-caregivers-employers-health-carecosts.pdf. Published July 2006. Accessed March 21, 2011.
- MetLife caregiving cost study: productivity losses to U.S. business. National Alliance for Caregiving Web site. http://www.caregiving. org/data/Caregiver%20Cost%20Study.pdf. Published July 2006. Accessed March 21, 2011.
- Pinquart M, Sörensen S. Gender differences in caregiver stressors, social resources, and health: an updated meta-analysis. J Gerontol B Psychol Sci Soc Sci 2006; 61:P33–P45.
- Johnson RW, Wiener JM. A profi le of frail older Americans and their caregivers. Urban Institute Web site. http://www.urban.org/UploadedPDF/311284_older_americans.pdf. Published February 2006. Accessed March 21, 2011.
- Schulz R, Beach SR. Caregiving as a risk factor for mortality: the caregiver health effects study. JAMA 1999; 282:2215–2219.
- McEwen BS, Gianaros PJ. Central role of the brain in stress and adaptation: links to socioeconomic status, health, and disease. Ann NY Acad Sci 2010; 1186:190–222.
- Aschbacher K, Mills PJ, von Känel R, et al. Effects of depressive and anxious symptoms on norepinephrine and platelet P-selectin responses to acute psychological stress among elderly caregivers. Brain Behav Immun 2008; 22:493–502.
- Selwood A, Johnston K, Katona C, Lyketsos C, Livingston G. Systematic review of the effect of psychological interventions on family caregivers of people with dementia. J Affect Disord 2007; 101:75–89.
- The National Alliance for Caregiving, in collaboration with the American Association of Retired Persons. Caregiving in the U.S. 2009. National Alliance for Caregiving Web site. http://www.caregiving.org/data/Caregiving_in_the_US_2009_full_report.pdf. Published November 2009. Accessed March 21, 2011.
- Family Caregiver Alliance. Caregiver health. A population at risk. National Alliance for Caregiving Web site. http://www.caregiver.org/caregiver/jsp/content_node.jsp?nodeid=1822. Published 2006. Accessed March 21, 2011.
- Family Caregiver Alliance. Prevalence, hours, and economic value of family caregiving, updated state-by-state analysis of 2004 national estimates. National Alliance for Caregiving Web site. http://www.caregiver.org/caregiver/jsp/content/pdfs/State_Caregiving_Data_Amo_20061107.pdf. Published 2006. Accessed March 21, 2011.
- Evercare. Study of caregivers in decline: a close-up look at the health risks of caring for a loved one. National Alliance for Caregiving Web site. http://www.caregiving.org/data/Caregivers%20in%20Decline%20Study-FINAL-lowres.pdf. Published 2006. Accessed March 21, 2011.
- Pinquart M, Sörensen S. Differences between caregivers and noncaregivers in psychological health and physical health: a metaanalysis. Psychol Aging 2003; 18:250–267.
- Vitaliano PP, Zhang J, Scanlan JM. Is caregiving hazardous to one’s physical health? A meta-analysis. Psychol Bull 2003; 129:946–972.
- Ho A, Collins S, Davis K, Doty M. A look at working-age caregivers’ roles, health concerns, and need for support (issue brief). New York, NY: The Commonwealth Fund; 2005.
- MetLife study of working caregivers and employer health care costs. MetLife Web site. http://www.metlife.com/assets/cao/mmi/publications/studies/2010/mmi-working-caregivers-employers-health-carecosts.pdf. Published July 2006. Accessed March 21, 2011.
- MetLife caregiving cost study: productivity losses to U.S. business. National Alliance for Caregiving Web site. http://www.caregiving. org/data/Caregiver%20Cost%20Study.pdf. Published July 2006. Accessed March 21, 2011.
- Pinquart M, Sörensen S. Gender differences in caregiver stressors, social resources, and health: an updated meta-analysis. J Gerontol B Psychol Sci Soc Sci 2006; 61:P33–P45.
- Johnson RW, Wiener JM. A profi le of frail older Americans and their caregivers. Urban Institute Web site. http://www.urban.org/UploadedPDF/311284_older_americans.pdf. Published February 2006. Accessed March 21, 2011.
- Schulz R, Beach SR. Caregiving as a risk factor for mortality: the caregiver health effects study. JAMA 1999; 282:2215–2219.
- McEwen BS, Gianaros PJ. Central role of the brain in stress and adaptation: links to socioeconomic status, health, and disease. Ann NY Acad Sci 2010; 1186:190–222.
- Aschbacher K, Mills PJ, von Känel R, et al. Effects of depressive and anxious symptoms on norepinephrine and platelet P-selectin responses to acute psychological stress among elderly caregivers. Brain Behav Immun 2008; 22:493–502.
- Selwood A, Johnston K, Katona C, Lyketsos C, Livingston G. Systematic review of the effect of psychological interventions on family caregivers of people with dementia. J Affect Disord 2007; 101:75–89.
Stress in medicine: Strategies for caregivers, patients, clinicians—Panel discussion
Question from audience: Why does the Cleveland Clinic start its healing services program preoperatively rather than postoperatively?
Dr. Gillinov: We have a fairly well defined preoperative set of medical tests, and during this process nurses present patients with materials that explain the experience, and nurses and doctors make themselves available in special classes to answer patients’ questions. In doing so, we have increasingly identified patients preoperatively who have stress or problems.
Last week I saw a woman who had a leaking mitral valve, but her symptoms were out of proportion to her disease. She had loss of energy and appetite, and she wasn’t eating much. She was depressed and our team picked that up. She actually never had to undergo surgery. We referred her to a psychologist and, according to her son, she started to feel better. By starting preoperatively, we’re sometimes able to pick out things that we should treat instead of heart disease.
We also provide guided imagery and massage preoperatively.
Dr. Duffy: Healing services is on standing preoperative orders at the hospital. The team goes in proactively and asks, “In addition to your open heart surgery on Wednesday, is there anything we can do to support your emotional and spiritual journey here today?”
Terminology also matters. The term “healing services” is a safe umbrella under which we include biofeedback as one of the services, but it encompasses pastoral care, hospice care, and palliative care. The way it’s integrated into a care model is important. If it’s reserved for end of life, it might be viewed as defective or as a death sentence, so we want the healing services team to be proactive.
Question from audience: How does the primary care physician fit into all of this? I believe that if the physicians in the hospital want to gain patient confidence, they’ll show that they’re communicating well with the primary care physician.
Dr. Gevirtz: The primary care physicians are incredibly open to this idea. They have 12 minutes to deal with people with fibromyalgia, irritable bowel syndrome, chronic pain, noncardiac chest pain, etc. What are they going to do in 12 minutes? They’re grateful if they have a handoff, especially if it’s in the Clinic itself.
Question from audience: Are there any thoughts on making biofeedback part of general training rather than using it just for patients who’ve already experienced trauma?
Dr. Gevirtz: We did a study in which we showed that a biofeedback technician in the primary care setting saved the health maintenance system quite a lot of money, but the administration couldn’t decide whose territory to take to give us an office, so it ended the program.
Dr. Russoniello: How we enable greater access to our intervention is an important question. I see people quit the program if they can’t get access to biofeedback. In an effort to enhance compliance, we’ve incorporated biofeedback into video games, working with a couple of private companies to develop them.The idea is that persons playing the video game can accrue points to enhance their overall score if they perform paced breathing or some other form of biofeedback. Early indications from focus groups are that people will like this.
We have already shown in randomized controlled clinical studies of depression and anxiety that certain video games can improve mood and decrease stress.There is a big movement to get products in people’s hands to help them manage their health.
Question from audience: How much overlap is there between biofeedback methodologies—enhancing heart rate variability, vagal withdrawal, neurofeedback, and electroencephalographic feedback—in the systems you’re targeting and what are the unique contributions of each?
Dr. Gevirtz: We follow a stepped-care model. We start with the simplest and move on to the more complicated technologies. Two published studies with long-term followup showed the effectiveness of a learned breathing technique in alleviating noncardiac chest pain. Simple biofeedback wasn’t even needed. Three years later, the patients were better than they were at the end of the actual training. If you can do it simply, then you do it, and if it doesn’t work, then move on to more and more complicated techniques, with neurofeedback being the last resort.
Question from audience: Has anybody measured the physical impact of stimulating multiple systems on the study subject? In other words, can it be damaging to overstimulate these systems at the same time?
Dr. Gevirtz: We’ve been trying to do that. Recurrent abdominal pain or functional abdominal pain is the most common complaint to pediatric gastroenterologists. We have 1,800 patients a year who make it to the children’s hospital level with this complaint. These are kids who are suffering with very great pain and we we’re pretty sure it’s an autonomically mediated kind of phenomenon. We’re able to measure vagal activity in these kids in ambulatory settings at school and have found very little vagal activity before treatment. After training, they were able to restore vagal activity, and it correlated at the level of 0.63 with a reduction of symptoms. I think it’s important to try to tie the physiology to symptoms. It’s not always easy to do but we’re trying.
Question from audience: I’d like to pick up on two topics that Dr. Duffy raised: the business of medicine and the proposal for informed hope rather than an informed consent before surgery. Something that I see with patients and families at times is this magical expectation promoted by the business side that medicine can do these amazing and wonderful things and doesn’t have any sort of weaknesses. I wonder what role unrealistic expectations promoted by the media, advertising, and others may play in the stress of patients, caregivers, and physicians who need to try to meet the expectations of infallible medicine?
Dr. Duffy: We’ve spun so far the other way with our advanced technology that we’ve lost the human side, especially the concept of a relationship and giving people hope even though they have a terminal condition. It’s a balance between the art and the business of medicine. It’s about setting realistic expectations and realistic hope.
Question from audience: Why does the Cleveland Clinic start its healing services program preoperatively rather than postoperatively?
Dr. Gillinov: We have a fairly well defined preoperative set of medical tests, and during this process nurses present patients with materials that explain the experience, and nurses and doctors make themselves available in special classes to answer patients’ questions. In doing so, we have increasingly identified patients preoperatively who have stress or problems.
Last week I saw a woman who had a leaking mitral valve, but her symptoms were out of proportion to her disease. She had loss of energy and appetite, and she wasn’t eating much. She was depressed and our team picked that up. She actually never had to undergo surgery. We referred her to a psychologist and, according to her son, she started to feel better. By starting preoperatively, we’re sometimes able to pick out things that we should treat instead of heart disease.
We also provide guided imagery and massage preoperatively.
Dr. Duffy: Healing services is on standing preoperative orders at the hospital. The team goes in proactively and asks, “In addition to your open heart surgery on Wednesday, is there anything we can do to support your emotional and spiritual journey here today?”
Terminology also matters. The term “healing services” is a safe umbrella under which we include biofeedback as one of the services, but it encompasses pastoral care, hospice care, and palliative care. The way it’s integrated into a care model is important. If it’s reserved for end of life, it might be viewed as defective or as a death sentence, so we want the healing services team to be proactive.
Question from audience: How does the primary care physician fit into all of this? I believe that if the physicians in the hospital want to gain patient confidence, they’ll show that they’re communicating well with the primary care physician.
Dr. Gevirtz: The primary care physicians are incredibly open to this idea. They have 12 minutes to deal with people with fibromyalgia, irritable bowel syndrome, chronic pain, noncardiac chest pain, etc. What are they going to do in 12 minutes? They’re grateful if they have a handoff, especially if it’s in the Clinic itself.
Question from audience: Are there any thoughts on making biofeedback part of general training rather than using it just for patients who’ve already experienced trauma?
Dr. Gevirtz: We did a study in which we showed that a biofeedback technician in the primary care setting saved the health maintenance system quite a lot of money, but the administration couldn’t decide whose territory to take to give us an office, so it ended the program.
Dr. Russoniello: How we enable greater access to our intervention is an important question. I see people quit the program if they can’t get access to biofeedback. In an effort to enhance compliance, we’ve incorporated biofeedback into video games, working with a couple of private companies to develop them.The idea is that persons playing the video game can accrue points to enhance their overall score if they perform paced breathing or some other form of biofeedback. Early indications from focus groups are that people will like this.
We have already shown in randomized controlled clinical studies of depression and anxiety that certain video games can improve mood and decrease stress.There is a big movement to get products in people’s hands to help them manage their health.
Question from audience: How much overlap is there between biofeedback methodologies—enhancing heart rate variability, vagal withdrawal, neurofeedback, and electroencephalographic feedback—in the systems you’re targeting and what are the unique contributions of each?
Dr. Gevirtz: We follow a stepped-care model. We start with the simplest and move on to the more complicated technologies. Two published studies with long-term followup showed the effectiveness of a learned breathing technique in alleviating noncardiac chest pain. Simple biofeedback wasn’t even needed. Three years later, the patients were better than they were at the end of the actual training. If you can do it simply, then you do it, and if it doesn’t work, then move on to more and more complicated techniques, with neurofeedback being the last resort.
Question from audience: Has anybody measured the physical impact of stimulating multiple systems on the study subject? In other words, can it be damaging to overstimulate these systems at the same time?
Dr. Gevirtz: We’ve been trying to do that. Recurrent abdominal pain or functional abdominal pain is the most common complaint to pediatric gastroenterologists. We have 1,800 patients a year who make it to the children’s hospital level with this complaint. These are kids who are suffering with very great pain and we we’re pretty sure it’s an autonomically mediated kind of phenomenon. We’re able to measure vagal activity in these kids in ambulatory settings at school and have found very little vagal activity before treatment. After training, they were able to restore vagal activity, and it correlated at the level of 0.63 with a reduction of symptoms. I think it’s important to try to tie the physiology to symptoms. It’s not always easy to do but we’re trying.
Question from audience: I’d like to pick up on two topics that Dr. Duffy raised: the business of medicine and the proposal for informed hope rather than an informed consent before surgery. Something that I see with patients and families at times is this magical expectation promoted by the business side that medicine can do these amazing and wonderful things and doesn’t have any sort of weaknesses. I wonder what role unrealistic expectations promoted by the media, advertising, and others may play in the stress of patients, caregivers, and physicians who need to try to meet the expectations of infallible medicine?
Dr. Duffy: We’ve spun so far the other way with our advanced technology that we’ve lost the human side, especially the concept of a relationship and giving people hope even though they have a terminal condition. It’s a balance between the art and the business of medicine. It’s about setting realistic expectations and realistic hope.
Question from audience: Why does the Cleveland Clinic start its healing services program preoperatively rather than postoperatively?
Dr. Gillinov: We have a fairly well defined preoperative set of medical tests, and during this process nurses present patients with materials that explain the experience, and nurses and doctors make themselves available in special classes to answer patients’ questions. In doing so, we have increasingly identified patients preoperatively who have stress or problems.
Last week I saw a woman who had a leaking mitral valve, but her symptoms were out of proportion to her disease. She had loss of energy and appetite, and she wasn’t eating much. She was depressed and our team picked that up. She actually never had to undergo surgery. We referred her to a psychologist and, according to her son, she started to feel better. By starting preoperatively, we’re sometimes able to pick out things that we should treat instead of heart disease.
We also provide guided imagery and massage preoperatively.
Dr. Duffy: Healing services is on standing preoperative orders at the hospital. The team goes in proactively and asks, “In addition to your open heart surgery on Wednesday, is there anything we can do to support your emotional and spiritual journey here today?”
Terminology also matters. The term “healing services” is a safe umbrella under which we include biofeedback as one of the services, but it encompasses pastoral care, hospice care, and palliative care. The way it’s integrated into a care model is important. If it’s reserved for end of life, it might be viewed as defective or as a death sentence, so we want the healing services team to be proactive.
Question from audience: How does the primary care physician fit into all of this? I believe that if the physicians in the hospital want to gain patient confidence, they’ll show that they’re communicating well with the primary care physician.
Dr. Gevirtz: The primary care physicians are incredibly open to this idea. They have 12 minutes to deal with people with fibromyalgia, irritable bowel syndrome, chronic pain, noncardiac chest pain, etc. What are they going to do in 12 minutes? They’re grateful if they have a handoff, especially if it’s in the Clinic itself.
Question from audience: Are there any thoughts on making biofeedback part of general training rather than using it just for patients who’ve already experienced trauma?
Dr. Gevirtz: We did a study in which we showed that a biofeedback technician in the primary care setting saved the health maintenance system quite a lot of money, but the administration couldn’t decide whose territory to take to give us an office, so it ended the program.
Dr. Russoniello: How we enable greater access to our intervention is an important question. I see people quit the program if they can’t get access to biofeedback. In an effort to enhance compliance, we’ve incorporated biofeedback into video games, working with a couple of private companies to develop them.The idea is that persons playing the video game can accrue points to enhance their overall score if they perform paced breathing or some other form of biofeedback. Early indications from focus groups are that people will like this.
We have already shown in randomized controlled clinical studies of depression and anxiety that certain video games can improve mood and decrease stress.There is a big movement to get products in people’s hands to help them manage their health.
Question from audience: How much overlap is there between biofeedback methodologies—enhancing heart rate variability, vagal withdrawal, neurofeedback, and electroencephalographic feedback—in the systems you’re targeting and what are the unique contributions of each?
Dr. Gevirtz: We follow a stepped-care model. We start with the simplest and move on to the more complicated technologies. Two published studies with long-term followup showed the effectiveness of a learned breathing technique in alleviating noncardiac chest pain. Simple biofeedback wasn’t even needed. Three years later, the patients were better than they were at the end of the actual training. If you can do it simply, then you do it, and if it doesn’t work, then move on to more and more complicated techniques, with neurofeedback being the last resort.
Question from audience: Has anybody measured the physical impact of stimulating multiple systems on the study subject? In other words, can it be damaging to overstimulate these systems at the same time?
Dr. Gevirtz: We’ve been trying to do that. Recurrent abdominal pain or functional abdominal pain is the most common complaint to pediatric gastroenterologists. We have 1,800 patients a year who make it to the children’s hospital level with this complaint. These are kids who are suffering with very great pain and we we’re pretty sure it’s an autonomically mediated kind of phenomenon. We’re able to measure vagal activity in these kids in ambulatory settings at school and have found very little vagal activity before treatment. After training, they were able to restore vagal activity, and it correlated at the level of 0.63 with a reduction of symptoms. I think it’s important to try to tie the physiology to symptoms. It’s not always easy to do but we’re trying.
Question from audience: I’d like to pick up on two topics that Dr. Duffy raised: the business of medicine and the proposal for informed hope rather than an informed consent before surgery. Something that I see with patients and families at times is this magical expectation promoted by the business side that medicine can do these amazing and wonderful things and doesn’t have any sort of weaknesses. I wonder what role unrealistic expectations promoted by the media, advertising, and others may play in the stress of patients, caregivers, and physicians who need to try to meet the expectations of infallible medicine?
Dr. Duffy: We’ve spun so far the other way with our advanced technology that we’ve lost the human side, especially the concept of a relationship and giving people hope even though they have a terminal condition. It’s a balance between the art and the business of medicine. It’s about setting realistic expectations and realistic hope.
Biofeedback in coronary artery disease, type 2 diabetes, and multiple sclerosis
Biofeedback in heart failure patients awaiting transplantation
Biofeedback in the treatment of heart failure
BIOFEEDBACK: AN OVERVIEW
Biofeedback is a self-regulation therapy that aims to teach individuals the skills that will allow them to change their physiology in healthy directions.1–3 Biofeedback involves a client, a trained biofeedback coach, and appropriate instrumentation. Sensors are connected to the client, and various physiologic parameters (such as heart rate, blood pressure, and digital peripheral temperature) are displayed on a computer screen. The client is guided through a brief mental stress test and a relaxation exercise to learn to recognize differences between hyperarousal and a more relaxed physiology. Biofeedback training involves a series of sessions in which the goal is to help the client gain control of his or her own physiology by learning relaxation techniques such as deep breathing, progressive muscle relaxation, and guided imagery.1,3,4 Although biofeedback can be used solely as operant conditioning, it is more commonly and more effectively combined with techniques of stress management.
Biofeedback training is commonly (although not exclusively) used to decrease activation of the sympathetic branch of the autonomic nervous system (the “fight or flight” response). The reduction in sympathetic nervous system (SNS) activity is manifest as an increase in digital peripheral temperature and decreases in skin conductance, heart rate, and blood pressure, as well as changes in the frequency distribution of heart rate variability. While the SNS is becoming less activated, the parasympathetic portion of the autonomic nervous system (“rest and digest”) is becoming more involved in regulating body functions. More parasympathetic nervous system (PNS) activation and less SNS activation produces a healthier physiologic state, and thus biofeedback can be used to move the body in the direction of health and wellness.1–4
HEART FAILURE: BIOLOGIC MECHANISMS OF INJURY
Heart failure is the end result of most untreated cardiovascular diseases. Heart failure involves inadequate cardiac pump function, such that appropriate perfusion of end organs does not occur. The process of developing heart failure is a gradual one that begins with compensatory processes. In response to an injury or insult, such as chronic high blood pressure or long-standing coronary artery blockage, the heart compensates by activating various neurohormonal pathways in an attempt to preserve cardiac function and end-organ perfusion.5 When these pathways are activated, they initially help the heart to compensate for the ongoing challenge of increased pressure or decreased tissue oxygenation and allow the cardiovascular system to pump sufficient blood. Over time, however, these compensatory processes become maladaptive. Cellular signaling pathways, which were activated in order to help the heart compensate, actually become as much of a problem as the decreased cardiac function.5
Hyperactivation of the SNS
Chief among these pathways is the SNS, which is the most powerful means by which cardiac function can be augmented.5 In response to decreased cardiac function, cardiac sympathetic nerves are activated, releasing norepinephrine locally, and both norepinephrine and epinephrine increase in the circulating blood. Beta-adrenergic receptors on cardiac myocytes and on vascular smooth muscle cells are stimulated, and the resulting augmentation of cardiac contraction helps the heart to overcome an immediate challenge. If the insult or injury to the heart is acute and time-limited, this system compensates and the situation is resolved. However, chronic activation of the SNS creates more problems than it solves for the failing heart,5,6 including the following:
- Myocardial cells are challenged by the need for increased energy production to support the chronic stimulation
- Oxidative stress ensues
- Receptors are downregulated
- Pathways that result in necrosis and apoptosis are activated
- Myofilament proteins respond to chronically elevated intracellular calcium.
As a result, the heart begins to spiral more quickly into a decompensated state. The toxicity of SNS overactivation is the reason for the success of beta-adrenergic blocking drugs in treating heart failure, but this situation is complicated further by adrenergic receptor polymorphisms and nonhomogeneous responses to beta-blocking agents.6 It is safe to say that the goal of much heart failure therapy is inactivation of the once-compensatory SNS and its resulting biologic effects.
Hypoactivation of the PNS
In addition to hyperactivation of the SNS, heart failure is also accompanied by a decrease in the role of the PNS. Under normal resting conditions, the human heart is governed more by the PNS than the SNS, with the SNS becoming a major source of cardiac control only during periods of decreased cardiac function. In heart failure, however, this relationship is reversed, with the SNS taking over the governing role and PNS input becoming less significant. Studies have suggested that the lack of contribution of the PNS to cardiac regulation in heart failure may be as deleterious as overactivation of the SNS.7 Most recently, stimulation of the vagal nerve has been shown to be beneficial in both animal models8 and humans with heart failure,9 confirming that augmenting PNS activity may be as important as inhibiting SNS activity. Although vagal nerve stimulation may be the first heart failure therapy aimed specifically at the PNS, it is likely that the future will hold more therapies with this goal.
PNS as regulator of inflammation of the failing heart?
It has recently been suggested that beyond its role in regulating cardiac function under baseline conditions, the PNS may participate in regulating the inflammatory state of the failing heart. It has been established since the observations of Packer and colleagues in the early 1990s10 that proinflammatory cytokines such as tumor necrosis factor–alpha, interleukin-6, and interleukin-1 are elevated in the circulation of heart failure patients, that these cytokines are correlated with clinical prognosis, and that they play a role in the activation of deleterious cardiac signaling pathways.11,12 Trials of antiinflammatory therapies in heart failure have been less than successful, but this may be because the complexity of the activation has been underestimated.11 In elegant work reported several years ago, Kevin Tracey’s group showed that stimulation of the vagus nerve could inhibit inflammatory processes associated with sepsis.13,14 Since that time, the reflex activation and inactivation of inflammatory processes by the PNS have become more widely accepted. Although it has not yet been directly demonstrated, it is possible that part of the benefit of vagal nerve stimulation in heart failure will prove to be due to its ability to reduce the chronic inflammatory state of the failing heart.
BIOFEEDBACK IN HEART FAILURE: RATIONALE
The failing heart is characterized by autonomic imbalance (hyperactivation of the SNS and hypoactivation of the PNS) and by a chronic inflammatory state. It has been hypothesized both directly and indirectly that these two major pathophysiologic processes may be intertwined.13–15 Biofeedback-assisted stress management is a therapy that has the potential to interfere with both processes. If the patient with heart failure can be trained to reduce activation of the SNS and to increase control by the PNS, it is likely that the negative consequences of autonomic imbalance will be decreased or possibly even reversed. Whether these effects are limited to quality of life and clinical status, or whether they extend to an effect on myocardial remodeling processes, remains to be established. Since the chronic inflammatory state can also be affected by increasing PNS control of the cardiovascular system, we further hypothesize that biofeedback training may have a direct effect on the inflammatory processes involved in the downward spiral of heart failure.
BIOFEEDBACK IN HEART FAILURE: STUDIES
We are certainly not the first group to hypothesize that self-regulation may have a role in the treatment of cardiovascular diseases in general or heart failure in particular. It has been shown that patients with heart failure manage their disease better and experience less emotional distress when they have a greater sense of control over their condition.16 In addition to giving patients a greater sense of control, some mind-body therapies have been shown to be beneficial in those with heart failure. Pischke et al showed as part of the Multicenter Lifestyle Demonstration Project that patients with left ventricular ejection fractions in the range associated with heart failure (≤ 40%) were able to learn and benefit from stress management techniques equally as well as those with more normal cardiac function.17 Both relaxation training18,19 and meditation20 have been shown to improve quality of life in heart failure patients, but meditation also reduced circulating norepinephrine, a marker of SNS activation.20 Mindfulness training improved clinical symptoms of heart failure and also reduced both anxiety and depression in patients with heart failure.21 Training heart failure patients to breathe more slowly is an intervention that is normally part of biofeedback training, but even when used alone it has resulted in decreased dyspnea,22 increased oxygen saturation,23 and improved exercise tolerance.22,23
To our knowledge, three studies to date have specifically used biofeedback training in patients with documented heart failure. As early as 1997, Moser and colleagues showed that heart failure patients were able to raise their finger temperature in spite of disease-related vascular changes, and that a single session of finger temperature biofeedback resulted in meaningful clinical improvement.24 Luskin et al randomized 33 heart failure patients to either biofeedback-assisted stress management or a control group, and showed improvement with the intervention in perceived stress, emotional distress, exercise tolerance, and depression.25 Most recently, Swanson and colleagues demonstrated improved exercise tolerance after cardiorespiratory biofeedback in patients with higher left ventricular ejection fractions (≥ 31%), although improvement could not be accomplished in those with ejection fractions below 30%.26
ONGOING STUDY IN END-STAGE HEART FAILURE AND FUTURE DIRECTIONS
We are currently involved at Cleveland Clinic in a study of end-stage heart failure patients who are awaiting cardiac transplantation. Each patient is provided with eight sessions of biofeedback training, including respiratory rate, digital peripheral temperature, muscle tension, and heart rate variability. Clinical status, quality of life, and heart failure–specific symptoms are being monitored throughout the training period. Success with biofeedback training is being analyzed, and we are testing the hypothesis that the degree of success in learning self-regulation will predict change in clinical status, quality of life, and the biology of the heart. What is unique to our study is that we will obtain the heart tissue at explant, when the patient receives a cardiac transplant, and we will conduct experiments to determine whether the cellular and molecular phenotype of the heart have been changed by the intervention, particularly components of the SNS, PNS, and inflammatory pathways.
We have been studying human heart failure for many years, and we have previously shown the changes in receptors and signaling pathways that occur in the failing human heart.27–30 We were also among the first to demonstrate that the cellular and molecular changes that occur in the failing human heart are not actually irreversible but can be changed by interventions such as a left ventricular assist device.31–33 Thus we hypothesize that biofeedback training, by interfering with overactivation of the SNS and by allowing the PNS to more adequately contribute to cardiac regulation, will have a meaningful effect on the biology of the failing human heart in addition to improving clinical status and quality of life. We hope to be among the first to demonstrate that effect.
- McKee MG. Biofeedback: an overview in the context of heart-brain medicine. Cleve Clin J Med 2008; 75( suppl 2):S31–S34.
- Moravec CS. Biofeedback therapy in cardiovascular disease: rationale and research overview. Cleve Clin J Med 2008; 75( suppl 2):S35–S38.
- Moss D, McGrady A, Davies TC, Wickramasekera I. Handbook of Mind-Body Medicine for Primary Care. London: Sage Publications; 2003.
- Schwartz MS, Andrasik F. Biofeedback: A Practitioner’s Guide. 3rd ed. New York, NY: Guilford Press; 2003.
- Triposkiadis F, Karayannis G, Giamouzis G, Skoularigis J, Louridas G, Butler J. The sympathetic nervous system in heart failure. J Am Coll Cardiol 2009; 54:1747–1762.
- Floras JS. Sympathetic nervous system activation in human heart failure. J Am Coll Cardiol 2009; 54:375–385.
- Binkley PF, Nunziata E, Haas GJ, Nelson SD, Cody RJ. Parasympathetic withdrawal is an integral component of autonomic imbalance in congestive heart failure: demonstration in human subjects and verification in a paced canine model of ventricular failure. J Am Coll Cardiol 1991; 18:464–472.
- Li M, Zheng C, Sato T, Kawada T, Sugimachi M, Sunugawa K. Vagal nerve stimulation markedly improves long-term survival after chronic heart failure in rats. Circulation 2004; 109:120–124.
- Schwartz PJ, DeFerrari GM, Sanzo A, et al Long term vagal stimulation in patients with advanced heart failure: first experience in man. Eur J Heart Failure 2008; 10:884–891.
- Levine B, Kalman J, Mayer L, Fillit M, Packer M. Elevated circulating levels of tumor necrosis factor in severe chronic heart failure. N Engl J Med 1990; 323:236–241.
- Mann DL. Inflammatory mediators and the failing heart: past, present and the foreseeable future. Circ Res 2002; 91:988–998.
- Parish RC, Evans JD. Inflammation in chronic heart failure. Ann Pharmacother 2008; 42:1002–1016.
- Tracey KJ. The inflammatory reflex. Nature 2002; 420:853–859.
- Tracey KJ. Reflex control of immunity. Nat Rev Immunol 2009; 9:418–428.
- Jankowska EA, Ponikowski P, Piepoli MF, Banasiak W, Anker SD, Poole-Wilson PA. Autonomic imbalance and immune activation in chronic heart failure—pathophysiological links. Cardiovasc Res 2006; 70:434–445.
- Dracup K, Westlake C, Erickson VS, Moser DK, Caldwell ML, Hamilton MA. Perceived control reduces emotional stress in patients with heart failure. J Heart Lung Transplant 2003; 22:90–93.
- Pischke CR, Weidner G, Elliott-Eller M, Ornish D. Lifestyle changes and clinical profile in coronary heart disease patients with an ejection fraction of ≤ 40% or > 40% in the Multicenter Lifestyle Demonstration Project. Eur J Heart Failure 2007; 9:928–934.
- Chang BH, Henricks A, Zhao Y, Rothendler JA, LoCastro JS, Slawsky MT. A relaxation response randomized trial on patients with chronic heart failure. J Cardiopulm Rehab 2005; 25:149–157.
- Yu DSF, Lee DTF, Woo J. Effects of relaxation therapy on psychologic distress and symptom status in older Chinese patients with heart failure. Psychosom Res 2007;427–437.
- Curiati JA, Bocchi E, Freire JO, et al Meditation reduces sympathetic activation and improves the quality of life in elderly patients with optimally treated heart failure: a prospective randomized study. J Altern Complement Med 2005; 11:465–472.
- Sullivan MJ, Wood L, Terry J, et al The support, education and research in chronic heart failure study (SEARCH): a mindfulness-based psychoeducational intervention improves depression and clinical symptoms in patients with chronic heart failure. Am Heart J 2009; 157:84–90.
- Weiner P, Waizman J, Magadle R, Berar-Yanay N, Pelled B. The effect of specific inspiratory muscle training on the sensation of dyspnea and exercise tolerance in patients with congestive heart failure. Clin Cardiol 1999; 22:727–732.
- Bernardi L, Porta C, Spicuzza L, et al Slow breathing increases arterial baroreflex sensitivity in patients with chronic heart failure. Circulation 2002; 105:143–145.
- Moser DK, Dracup K, Woo MA, Stevenson LW. Voluntary control of vascular tone by using skin-temperature biofeedback-relaxation in patients with advanced heart failure. Altern Ther Health Med 1997; 3:51–60.
- Luskin F, Reitz M, Newell K, Quinn TG, Haskell W. A controlled pilot study of stress management training of elderly patients with congestive heart failure. Prev Cardiol 2002; 5:168–172.
- Swanson KS, Gevirtz RN, Brown M, Spira J, Guarneri E, Stoletniy L. The effect of biofeedback on function in patients with heart failure. Appl Psychophysiol Biofeedback 2009; 34:71–91.
- Razeghi P, Mukhopadhyay M, Myers TJ, et al Myocardial tumor necrosis factor alpha expression does not correlate with clinical indices of heart failure in patients on left ventricular assist device support. Ann Thorac Surg 2001; 72:2044–2050.
- Matteo R, Moravec CS. Expression and immunolocalization of annexins IV, V and VI in the failing and non-failing human heart. Cardiovasc Res 2000; 45:961–970.
- Dash R, Frank K, Carr AN, Moravec CS, Kranias EG. Gender influences on sarcoplasmic reticulum calcium handling in the failing human myocardium. J Mol Cell Cardiol 2001; 33:1345–1353.
- DiPaola NR, Sweet WE, Stull LB, Francis GS, Moravec CS. Beta-adrenergic receptors and calcium cycling proteins in normal, hypertrophied and failing human hearts: transition from hypertrophy to failure. J Mol Cell Cardiol 2001; 33:1283–1295.
- Aquila LA, McCarthy PM, Smedira NG, Young JB, Moravec CS. Cytoskeletal structure and recovery in single human cardiac myocytes. J Heart Lung Transplant 2004; 23:954–963.
- Fedak PWM, Moravec CS, McCarthy PM, et al Altered expression of disintegrin metalloproteinases and their inhibitor in human dilated cardiomyopathy. Circulation 2006; 113:238–245.
- Ogletree-Hughes ML, Stull LB, Sweet WE, Smedira NG, McCarthy PM, Moravec CS. Mechanical unloading restores beta adrenergic responsiveness and reverses receptor downregulation in the failing human heart. Circulation 2001; 104:881–886.
BIOFEEDBACK: AN OVERVIEW
Biofeedback is a self-regulation therapy that aims to teach individuals the skills that will allow them to change their physiology in healthy directions.1–3 Biofeedback involves a client, a trained biofeedback coach, and appropriate instrumentation. Sensors are connected to the client, and various physiologic parameters (such as heart rate, blood pressure, and digital peripheral temperature) are displayed on a computer screen. The client is guided through a brief mental stress test and a relaxation exercise to learn to recognize differences between hyperarousal and a more relaxed physiology. Biofeedback training involves a series of sessions in which the goal is to help the client gain control of his or her own physiology by learning relaxation techniques such as deep breathing, progressive muscle relaxation, and guided imagery.1,3,4 Although biofeedback can be used solely as operant conditioning, it is more commonly and more effectively combined with techniques of stress management.
Biofeedback training is commonly (although not exclusively) used to decrease activation of the sympathetic branch of the autonomic nervous system (the “fight or flight” response). The reduction in sympathetic nervous system (SNS) activity is manifest as an increase in digital peripheral temperature and decreases in skin conductance, heart rate, and blood pressure, as well as changes in the frequency distribution of heart rate variability. While the SNS is becoming less activated, the parasympathetic portion of the autonomic nervous system (“rest and digest”) is becoming more involved in regulating body functions. More parasympathetic nervous system (PNS) activation and less SNS activation produces a healthier physiologic state, and thus biofeedback can be used to move the body in the direction of health and wellness.1–4
HEART FAILURE: BIOLOGIC MECHANISMS OF INJURY
Heart failure is the end result of most untreated cardiovascular diseases. Heart failure involves inadequate cardiac pump function, such that appropriate perfusion of end organs does not occur. The process of developing heart failure is a gradual one that begins with compensatory processes. In response to an injury or insult, such as chronic high blood pressure or long-standing coronary artery blockage, the heart compensates by activating various neurohormonal pathways in an attempt to preserve cardiac function and end-organ perfusion.5 When these pathways are activated, they initially help the heart to compensate for the ongoing challenge of increased pressure or decreased tissue oxygenation and allow the cardiovascular system to pump sufficient blood. Over time, however, these compensatory processes become maladaptive. Cellular signaling pathways, which were activated in order to help the heart compensate, actually become as much of a problem as the decreased cardiac function.5
Hyperactivation of the SNS
Chief among these pathways is the SNS, which is the most powerful means by which cardiac function can be augmented.5 In response to decreased cardiac function, cardiac sympathetic nerves are activated, releasing norepinephrine locally, and both norepinephrine and epinephrine increase in the circulating blood. Beta-adrenergic receptors on cardiac myocytes and on vascular smooth muscle cells are stimulated, and the resulting augmentation of cardiac contraction helps the heart to overcome an immediate challenge. If the insult or injury to the heart is acute and time-limited, this system compensates and the situation is resolved. However, chronic activation of the SNS creates more problems than it solves for the failing heart,5,6 including the following:
- Myocardial cells are challenged by the need for increased energy production to support the chronic stimulation
- Oxidative stress ensues
- Receptors are downregulated
- Pathways that result in necrosis and apoptosis are activated
- Myofilament proteins respond to chronically elevated intracellular calcium.
As a result, the heart begins to spiral more quickly into a decompensated state. The toxicity of SNS overactivation is the reason for the success of beta-adrenergic blocking drugs in treating heart failure, but this situation is complicated further by adrenergic receptor polymorphisms and nonhomogeneous responses to beta-blocking agents.6 It is safe to say that the goal of much heart failure therapy is inactivation of the once-compensatory SNS and its resulting biologic effects.
Hypoactivation of the PNS
In addition to hyperactivation of the SNS, heart failure is also accompanied by a decrease in the role of the PNS. Under normal resting conditions, the human heart is governed more by the PNS than the SNS, with the SNS becoming a major source of cardiac control only during periods of decreased cardiac function. In heart failure, however, this relationship is reversed, with the SNS taking over the governing role and PNS input becoming less significant. Studies have suggested that the lack of contribution of the PNS to cardiac regulation in heart failure may be as deleterious as overactivation of the SNS.7 Most recently, stimulation of the vagal nerve has been shown to be beneficial in both animal models8 and humans with heart failure,9 confirming that augmenting PNS activity may be as important as inhibiting SNS activity. Although vagal nerve stimulation may be the first heart failure therapy aimed specifically at the PNS, it is likely that the future will hold more therapies with this goal.
PNS as regulator of inflammation of the failing heart?
It has recently been suggested that beyond its role in regulating cardiac function under baseline conditions, the PNS may participate in regulating the inflammatory state of the failing heart. It has been established since the observations of Packer and colleagues in the early 1990s10 that proinflammatory cytokines such as tumor necrosis factor–alpha, interleukin-6, and interleukin-1 are elevated in the circulation of heart failure patients, that these cytokines are correlated with clinical prognosis, and that they play a role in the activation of deleterious cardiac signaling pathways.11,12 Trials of antiinflammatory therapies in heart failure have been less than successful, but this may be because the complexity of the activation has been underestimated.11 In elegant work reported several years ago, Kevin Tracey’s group showed that stimulation of the vagus nerve could inhibit inflammatory processes associated with sepsis.13,14 Since that time, the reflex activation and inactivation of inflammatory processes by the PNS have become more widely accepted. Although it has not yet been directly demonstrated, it is possible that part of the benefit of vagal nerve stimulation in heart failure will prove to be due to its ability to reduce the chronic inflammatory state of the failing heart.
BIOFEEDBACK IN HEART FAILURE: RATIONALE
The failing heart is characterized by autonomic imbalance (hyperactivation of the SNS and hypoactivation of the PNS) and by a chronic inflammatory state. It has been hypothesized both directly and indirectly that these two major pathophysiologic processes may be intertwined.13–15 Biofeedback-assisted stress management is a therapy that has the potential to interfere with both processes. If the patient with heart failure can be trained to reduce activation of the SNS and to increase control by the PNS, it is likely that the negative consequences of autonomic imbalance will be decreased or possibly even reversed. Whether these effects are limited to quality of life and clinical status, or whether they extend to an effect on myocardial remodeling processes, remains to be established. Since the chronic inflammatory state can also be affected by increasing PNS control of the cardiovascular system, we further hypothesize that biofeedback training may have a direct effect on the inflammatory processes involved in the downward spiral of heart failure.
BIOFEEDBACK IN HEART FAILURE: STUDIES
We are certainly not the first group to hypothesize that self-regulation may have a role in the treatment of cardiovascular diseases in general or heart failure in particular. It has been shown that patients with heart failure manage their disease better and experience less emotional distress when they have a greater sense of control over their condition.16 In addition to giving patients a greater sense of control, some mind-body therapies have been shown to be beneficial in those with heart failure. Pischke et al showed as part of the Multicenter Lifestyle Demonstration Project that patients with left ventricular ejection fractions in the range associated with heart failure (≤ 40%) were able to learn and benefit from stress management techniques equally as well as those with more normal cardiac function.17 Both relaxation training18,19 and meditation20 have been shown to improve quality of life in heart failure patients, but meditation also reduced circulating norepinephrine, a marker of SNS activation.20 Mindfulness training improved clinical symptoms of heart failure and also reduced both anxiety and depression in patients with heart failure.21 Training heart failure patients to breathe more slowly is an intervention that is normally part of biofeedback training, but even when used alone it has resulted in decreased dyspnea,22 increased oxygen saturation,23 and improved exercise tolerance.22,23
To our knowledge, three studies to date have specifically used biofeedback training in patients with documented heart failure. As early as 1997, Moser and colleagues showed that heart failure patients were able to raise their finger temperature in spite of disease-related vascular changes, and that a single session of finger temperature biofeedback resulted in meaningful clinical improvement.24 Luskin et al randomized 33 heart failure patients to either biofeedback-assisted stress management or a control group, and showed improvement with the intervention in perceived stress, emotional distress, exercise tolerance, and depression.25 Most recently, Swanson and colleagues demonstrated improved exercise tolerance after cardiorespiratory biofeedback in patients with higher left ventricular ejection fractions (≥ 31%), although improvement could not be accomplished in those with ejection fractions below 30%.26
ONGOING STUDY IN END-STAGE HEART FAILURE AND FUTURE DIRECTIONS
We are currently involved at Cleveland Clinic in a study of end-stage heart failure patients who are awaiting cardiac transplantation. Each patient is provided with eight sessions of biofeedback training, including respiratory rate, digital peripheral temperature, muscle tension, and heart rate variability. Clinical status, quality of life, and heart failure–specific symptoms are being monitored throughout the training period. Success with biofeedback training is being analyzed, and we are testing the hypothesis that the degree of success in learning self-regulation will predict change in clinical status, quality of life, and the biology of the heart. What is unique to our study is that we will obtain the heart tissue at explant, when the patient receives a cardiac transplant, and we will conduct experiments to determine whether the cellular and molecular phenotype of the heart have been changed by the intervention, particularly components of the SNS, PNS, and inflammatory pathways.
We have been studying human heart failure for many years, and we have previously shown the changes in receptors and signaling pathways that occur in the failing human heart.27–30 We were also among the first to demonstrate that the cellular and molecular changes that occur in the failing human heart are not actually irreversible but can be changed by interventions such as a left ventricular assist device.31–33 Thus we hypothesize that biofeedback training, by interfering with overactivation of the SNS and by allowing the PNS to more adequately contribute to cardiac regulation, will have a meaningful effect on the biology of the failing human heart in addition to improving clinical status and quality of life. We hope to be among the first to demonstrate that effect.
BIOFEEDBACK: AN OVERVIEW
Biofeedback is a self-regulation therapy that aims to teach individuals the skills that will allow them to change their physiology in healthy directions.1–3 Biofeedback involves a client, a trained biofeedback coach, and appropriate instrumentation. Sensors are connected to the client, and various physiologic parameters (such as heart rate, blood pressure, and digital peripheral temperature) are displayed on a computer screen. The client is guided through a brief mental stress test and a relaxation exercise to learn to recognize differences between hyperarousal and a more relaxed physiology. Biofeedback training involves a series of sessions in which the goal is to help the client gain control of his or her own physiology by learning relaxation techniques such as deep breathing, progressive muscle relaxation, and guided imagery.1,3,4 Although biofeedback can be used solely as operant conditioning, it is more commonly and more effectively combined with techniques of stress management.
Biofeedback training is commonly (although not exclusively) used to decrease activation of the sympathetic branch of the autonomic nervous system (the “fight or flight” response). The reduction in sympathetic nervous system (SNS) activity is manifest as an increase in digital peripheral temperature and decreases in skin conductance, heart rate, and blood pressure, as well as changes in the frequency distribution of heart rate variability. While the SNS is becoming less activated, the parasympathetic portion of the autonomic nervous system (“rest and digest”) is becoming more involved in regulating body functions. More parasympathetic nervous system (PNS) activation and less SNS activation produces a healthier physiologic state, and thus biofeedback can be used to move the body in the direction of health and wellness.1–4
HEART FAILURE: BIOLOGIC MECHANISMS OF INJURY
Heart failure is the end result of most untreated cardiovascular diseases. Heart failure involves inadequate cardiac pump function, such that appropriate perfusion of end organs does not occur. The process of developing heart failure is a gradual one that begins with compensatory processes. In response to an injury or insult, such as chronic high blood pressure or long-standing coronary artery blockage, the heart compensates by activating various neurohormonal pathways in an attempt to preserve cardiac function and end-organ perfusion.5 When these pathways are activated, they initially help the heart to compensate for the ongoing challenge of increased pressure or decreased tissue oxygenation and allow the cardiovascular system to pump sufficient blood. Over time, however, these compensatory processes become maladaptive. Cellular signaling pathways, which were activated in order to help the heart compensate, actually become as much of a problem as the decreased cardiac function.5
Hyperactivation of the SNS
Chief among these pathways is the SNS, which is the most powerful means by which cardiac function can be augmented.5 In response to decreased cardiac function, cardiac sympathetic nerves are activated, releasing norepinephrine locally, and both norepinephrine and epinephrine increase in the circulating blood. Beta-adrenergic receptors on cardiac myocytes and on vascular smooth muscle cells are stimulated, and the resulting augmentation of cardiac contraction helps the heart to overcome an immediate challenge. If the insult or injury to the heart is acute and time-limited, this system compensates and the situation is resolved. However, chronic activation of the SNS creates more problems than it solves for the failing heart,5,6 including the following:
- Myocardial cells are challenged by the need for increased energy production to support the chronic stimulation
- Oxidative stress ensues
- Receptors are downregulated
- Pathways that result in necrosis and apoptosis are activated
- Myofilament proteins respond to chronically elevated intracellular calcium.
As a result, the heart begins to spiral more quickly into a decompensated state. The toxicity of SNS overactivation is the reason for the success of beta-adrenergic blocking drugs in treating heart failure, but this situation is complicated further by adrenergic receptor polymorphisms and nonhomogeneous responses to beta-blocking agents.6 It is safe to say that the goal of much heart failure therapy is inactivation of the once-compensatory SNS and its resulting biologic effects.
Hypoactivation of the PNS
In addition to hyperactivation of the SNS, heart failure is also accompanied by a decrease in the role of the PNS. Under normal resting conditions, the human heart is governed more by the PNS than the SNS, with the SNS becoming a major source of cardiac control only during periods of decreased cardiac function. In heart failure, however, this relationship is reversed, with the SNS taking over the governing role and PNS input becoming less significant. Studies have suggested that the lack of contribution of the PNS to cardiac regulation in heart failure may be as deleterious as overactivation of the SNS.7 Most recently, stimulation of the vagal nerve has been shown to be beneficial in both animal models8 and humans with heart failure,9 confirming that augmenting PNS activity may be as important as inhibiting SNS activity. Although vagal nerve stimulation may be the first heart failure therapy aimed specifically at the PNS, it is likely that the future will hold more therapies with this goal.
PNS as regulator of inflammation of the failing heart?
It has recently been suggested that beyond its role in regulating cardiac function under baseline conditions, the PNS may participate in regulating the inflammatory state of the failing heart. It has been established since the observations of Packer and colleagues in the early 1990s10 that proinflammatory cytokines such as tumor necrosis factor–alpha, interleukin-6, and interleukin-1 are elevated in the circulation of heart failure patients, that these cytokines are correlated with clinical prognosis, and that they play a role in the activation of deleterious cardiac signaling pathways.11,12 Trials of antiinflammatory therapies in heart failure have been less than successful, but this may be because the complexity of the activation has been underestimated.11 In elegant work reported several years ago, Kevin Tracey’s group showed that stimulation of the vagus nerve could inhibit inflammatory processes associated with sepsis.13,14 Since that time, the reflex activation and inactivation of inflammatory processes by the PNS have become more widely accepted. Although it has not yet been directly demonstrated, it is possible that part of the benefit of vagal nerve stimulation in heart failure will prove to be due to its ability to reduce the chronic inflammatory state of the failing heart.
BIOFEEDBACK IN HEART FAILURE: RATIONALE
The failing heart is characterized by autonomic imbalance (hyperactivation of the SNS and hypoactivation of the PNS) and by a chronic inflammatory state. It has been hypothesized both directly and indirectly that these two major pathophysiologic processes may be intertwined.13–15 Biofeedback-assisted stress management is a therapy that has the potential to interfere with both processes. If the patient with heart failure can be trained to reduce activation of the SNS and to increase control by the PNS, it is likely that the negative consequences of autonomic imbalance will be decreased or possibly even reversed. Whether these effects are limited to quality of life and clinical status, or whether they extend to an effect on myocardial remodeling processes, remains to be established. Since the chronic inflammatory state can also be affected by increasing PNS control of the cardiovascular system, we further hypothesize that biofeedback training may have a direct effect on the inflammatory processes involved in the downward spiral of heart failure.
BIOFEEDBACK IN HEART FAILURE: STUDIES
We are certainly not the first group to hypothesize that self-regulation may have a role in the treatment of cardiovascular diseases in general or heart failure in particular. It has been shown that patients with heart failure manage their disease better and experience less emotional distress when they have a greater sense of control over their condition.16 In addition to giving patients a greater sense of control, some mind-body therapies have been shown to be beneficial in those with heart failure. Pischke et al showed as part of the Multicenter Lifestyle Demonstration Project that patients with left ventricular ejection fractions in the range associated with heart failure (≤ 40%) were able to learn and benefit from stress management techniques equally as well as those with more normal cardiac function.17 Both relaxation training18,19 and meditation20 have been shown to improve quality of life in heart failure patients, but meditation also reduced circulating norepinephrine, a marker of SNS activation.20 Mindfulness training improved clinical symptoms of heart failure and also reduced both anxiety and depression in patients with heart failure.21 Training heart failure patients to breathe more slowly is an intervention that is normally part of biofeedback training, but even when used alone it has resulted in decreased dyspnea,22 increased oxygen saturation,23 and improved exercise tolerance.22,23
To our knowledge, three studies to date have specifically used biofeedback training in patients with documented heart failure. As early as 1997, Moser and colleagues showed that heart failure patients were able to raise their finger temperature in spite of disease-related vascular changes, and that a single session of finger temperature biofeedback resulted in meaningful clinical improvement.24 Luskin et al randomized 33 heart failure patients to either biofeedback-assisted stress management or a control group, and showed improvement with the intervention in perceived stress, emotional distress, exercise tolerance, and depression.25 Most recently, Swanson and colleagues demonstrated improved exercise tolerance after cardiorespiratory biofeedback in patients with higher left ventricular ejection fractions (≥ 31%), although improvement could not be accomplished in those with ejection fractions below 30%.26
ONGOING STUDY IN END-STAGE HEART FAILURE AND FUTURE DIRECTIONS
We are currently involved at Cleveland Clinic in a study of end-stage heart failure patients who are awaiting cardiac transplantation. Each patient is provided with eight sessions of biofeedback training, including respiratory rate, digital peripheral temperature, muscle tension, and heart rate variability. Clinical status, quality of life, and heart failure–specific symptoms are being monitored throughout the training period. Success with biofeedback training is being analyzed, and we are testing the hypothesis that the degree of success in learning self-regulation will predict change in clinical status, quality of life, and the biology of the heart. What is unique to our study is that we will obtain the heart tissue at explant, when the patient receives a cardiac transplant, and we will conduct experiments to determine whether the cellular and molecular phenotype of the heart have been changed by the intervention, particularly components of the SNS, PNS, and inflammatory pathways.
We have been studying human heart failure for many years, and we have previously shown the changes in receptors and signaling pathways that occur in the failing human heart.27–30 We were also among the first to demonstrate that the cellular and molecular changes that occur in the failing human heart are not actually irreversible but can be changed by interventions such as a left ventricular assist device.31–33 Thus we hypothesize that biofeedback training, by interfering with overactivation of the SNS and by allowing the PNS to more adequately contribute to cardiac regulation, will have a meaningful effect on the biology of the failing human heart in addition to improving clinical status and quality of life. We hope to be among the first to demonstrate that effect.
- McKee MG. Biofeedback: an overview in the context of heart-brain medicine. Cleve Clin J Med 2008; 75( suppl 2):S31–S34.
- Moravec CS. Biofeedback therapy in cardiovascular disease: rationale and research overview. Cleve Clin J Med 2008; 75( suppl 2):S35–S38.
- Moss D, McGrady A, Davies TC, Wickramasekera I. Handbook of Mind-Body Medicine for Primary Care. London: Sage Publications; 2003.
- Schwartz MS, Andrasik F. Biofeedback: A Practitioner’s Guide. 3rd ed. New York, NY: Guilford Press; 2003.
- Triposkiadis F, Karayannis G, Giamouzis G, Skoularigis J, Louridas G, Butler J. The sympathetic nervous system in heart failure. J Am Coll Cardiol 2009; 54:1747–1762.
- Floras JS. Sympathetic nervous system activation in human heart failure. J Am Coll Cardiol 2009; 54:375–385.
- Binkley PF, Nunziata E, Haas GJ, Nelson SD, Cody RJ. Parasympathetic withdrawal is an integral component of autonomic imbalance in congestive heart failure: demonstration in human subjects and verification in a paced canine model of ventricular failure. J Am Coll Cardiol 1991; 18:464–472.
- Li M, Zheng C, Sato T, Kawada T, Sugimachi M, Sunugawa K. Vagal nerve stimulation markedly improves long-term survival after chronic heart failure in rats. Circulation 2004; 109:120–124.
- Schwartz PJ, DeFerrari GM, Sanzo A, et al Long term vagal stimulation in patients with advanced heart failure: first experience in man. Eur J Heart Failure 2008; 10:884–891.
- Levine B, Kalman J, Mayer L, Fillit M, Packer M. Elevated circulating levels of tumor necrosis factor in severe chronic heart failure. N Engl J Med 1990; 323:236–241.
- Mann DL. Inflammatory mediators and the failing heart: past, present and the foreseeable future. Circ Res 2002; 91:988–998.
- Parish RC, Evans JD. Inflammation in chronic heart failure. Ann Pharmacother 2008; 42:1002–1016.
- Tracey KJ. The inflammatory reflex. Nature 2002; 420:853–859.
- Tracey KJ. Reflex control of immunity. Nat Rev Immunol 2009; 9:418–428.
- Jankowska EA, Ponikowski P, Piepoli MF, Banasiak W, Anker SD, Poole-Wilson PA. Autonomic imbalance and immune activation in chronic heart failure—pathophysiological links. Cardiovasc Res 2006; 70:434–445.
- Dracup K, Westlake C, Erickson VS, Moser DK, Caldwell ML, Hamilton MA. Perceived control reduces emotional stress in patients with heart failure. J Heart Lung Transplant 2003; 22:90–93.
- Pischke CR, Weidner G, Elliott-Eller M, Ornish D. Lifestyle changes and clinical profile in coronary heart disease patients with an ejection fraction of ≤ 40% or > 40% in the Multicenter Lifestyle Demonstration Project. Eur J Heart Failure 2007; 9:928–934.
- Chang BH, Henricks A, Zhao Y, Rothendler JA, LoCastro JS, Slawsky MT. A relaxation response randomized trial on patients with chronic heart failure. J Cardiopulm Rehab 2005; 25:149–157.
- Yu DSF, Lee DTF, Woo J. Effects of relaxation therapy on psychologic distress and symptom status in older Chinese patients with heart failure. Psychosom Res 2007;427–437.
- Curiati JA, Bocchi E, Freire JO, et al Meditation reduces sympathetic activation and improves the quality of life in elderly patients with optimally treated heart failure: a prospective randomized study. J Altern Complement Med 2005; 11:465–472.
- Sullivan MJ, Wood L, Terry J, et al The support, education and research in chronic heart failure study (SEARCH): a mindfulness-based psychoeducational intervention improves depression and clinical symptoms in patients with chronic heart failure. Am Heart J 2009; 157:84–90.
- Weiner P, Waizman J, Magadle R, Berar-Yanay N, Pelled B. The effect of specific inspiratory muscle training on the sensation of dyspnea and exercise tolerance in patients with congestive heart failure. Clin Cardiol 1999; 22:727–732.
- Bernardi L, Porta C, Spicuzza L, et al Slow breathing increases arterial baroreflex sensitivity in patients with chronic heart failure. Circulation 2002; 105:143–145.
- Moser DK, Dracup K, Woo MA, Stevenson LW. Voluntary control of vascular tone by using skin-temperature biofeedback-relaxation in patients with advanced heart failure. Altern Ther Health Med 1997; 3:51–60.
- Luskin F, Reitz M, Newell K, Quinn TG, Haskell W. A controlled pilot study of stress management training of elderly patients with congestive heart failure. Prev Cardiol 2002; 5:168–172.
- Swanson KS, Gevirtz RN, Brown M, Spira J, Guarneri E, Stoletniy L. The effect of biofeedback on function in patients with heart failure. Appl Psychophysiol Biofeedback 2009; 34:71–91.
- Razeghi P, Mukhopadhyay M, Myers TJ, et al Myocardial tumor necrosis factor alpha expression does not correlate with clinical indices of heart failure in patients on left ventricular assist device support. Ann Thorac Surg 2001; 72:2044–2050.
- Matteo R, Moravec CS. Expression and immunolocalization of annexins IV, V and VI in the failing and non-failing human heart. Cardiovasc Res 2000; 45:961–970.
- Dash R, Frank K, Carr AN, Moravec CS, Kranias EG. Gender influences on sarcoplasmic reticulum calcium handling in the failing human myocardium. J Mol Cell Cardiol 2001; 33:1345–1353.
- DiPaola NR, Sweet WE, Stull LB, Francis GS, Moravec CS. Beta-adrenergic receptors and calcium cycling proteins in normal, hypertrophied and failing human hearts: transition from hypertrophy to failure. J Mol Cell Cardiol 2001; 33:1283–1295.
- Aquila LA, McCarthy PM, Smedira NG, Young JB, Moravec CS. Cytoskeletal structure and recovery in single human cardiac myocytes. J Heart Lung Transplant 2004; 23:954–963.
- Fedak PWM, Moravec CS, McCarthy PM, et al Altered expression of disintegrin metalloproteinases and their inhibitor in human dilated cardiomyopathy. Circulation 2006; 113:238–245.
- Ogletree-Hughes ML, Stull LB, Sweet WE, Smedira NG, McCarthy PM, Moravec CS. Mechanical unloading restores beta adrenergic responsiveness and reverses receptor downregulation in the failing human heart. Circulation 2001; 104:881–886.
- McKee MG. Biofeedback: an overview in the context of heart-brain medicine. Cleve Clin J Med 2008; 75( suppl 2):S31–S34.
- Moravec CS. Biofeedback therapy in cardiovascular disease: rationale and research overview. Cleve Clin J Med 2008; 75( suppl 2):S35–S38.
- Moss D, McGrady A, Davies TC, Wickramasekera I. Handbook of Mind-Body Medicine for Primary Care. London: Sage Publications; 2003.
- Schwartz MS, Andrasik F. Biofeedback: A Practitioner’s Guide. 3rd ed. New York, NY: Guilford Press; 2003.
- Triposkiadis F, Karayannis G, Giamouzis G, Skoularigis J, Louridas G, Butler J. The sympathetic nervous system in heart failure. J Am Coll Cardiol 2009; 54:1747–1762.
- Floras JS. Sympathetic nervous system activation in human heart failure. J Am Coll Cardiol 2009; 54:375–385.
- Binkley PF, Nunziata E, Haas GJ, Nelson SD, Cody RJ. Parasympathetic withdrawal is an integral component of autonomic imbalance in congestive heart failure: demonstration in human subjects and verification in a paced canine model of ventricular failure. J Am Coll Cardiol 1991; 18:464–472.
- Li M, Zheng C, Sato T, Kawada T, Sugimachi M, Sunugawa K. Vagal nerve stimulation markedly improves long-term survival after chronic heart failure in rats. Circulation 2004; 109:120–124.
- Schwartz PJ, DeFerrari GM, Sanzo A, et al Long term vagal stimulation in patients with advanced heart failure: first experience in man. Eur J Heart Failure 2008; 10:884–891.
- Levine B, Kalman J, Mayer L, Fillit M, Packer M. Elevated circulating levels of tumor necrosis factor in severe chronic heart failure. N Engl J Med 1990; 323:236–241.
- Mann DL. Inflammatory mediators and the failing heart: past, present and the foreseeable future. Circ Res 2002; 91:988–998.
- Parish RC, Evans JD. Inflammation in chronic heart failure. Ann Pharmacother 2008; 42:1002–1016.
- Tracey KJ. The inflammatory reflex. Nature 2002; 420:853–859.
- Tracey KJ. Reflex control of immunity. Nat Rev Immunol 2009; 9:418–428.
- Jankowska EA, Ponikowski P, Piepoli MF, Banasiak W, Anker SD, Poole-Wilson PA. Autonomic imbalance and immune activation in chronic heart failure—pathophysiological links. Cardiovasc Res 2006; 70:434–445.
- Dracup K, Westlake C, Erickson VS, Moser DK, Caldwell ML, Hamilton MA. Perceived control reduces emotional stress in patients with heart failure. J Heart Lung Transplant 2003; 22:90–93.
- Pischke CR, Weidner G, Elliott-Eller M, Ornish D. Lifestyle changes and clinical profile in coronary heart disease patients with an ejection fraction of ≤ 40% or > 40% in the Multicenter Lifestyle Demonstration Project. Eur J Heart Failure 2007; 9:928–934.
- Chang BH, Henricks A, Zhao Y, Rothendler JA, LoCastro JS, Slawsky MT. A relaxation response randomized trial on patients with chronic heart failure. J Cardiopulm Rehab 2005; 25:149–157.
- Yu DSF, Lee DTF, Woo J. Effects of relaxation therapy on psychologic distress and symptom status in older Chinese patients with heart failure. Psychosom Res 2007;427–437.
- Curiati JA, Bocchi E, Freire JO, et al Meditation reduces sympathetic activation and improves the quality of life in elderly patients with optimally treated heart failure: a prospective randomized study. J Altern Complement Med 2005; 11:465–472.
- Sullivan MJ, Wood L, Terry J, et al The support, education and research in chronic heart failure study (SEARCH): a mindfulness-based psychoeducational intervention improves depression and clinical symptoms in patients with chronic heart failure. Am Heart J 2009; 157:84–90.
- Weiner P, Waizman J, Magadle R, Berar-Yanay N, Pelled B. The effect of specific inspiratory muscle training on the sensation of dyspnea and exercise tolerance in patients with congestive heart failure. Clin Cardiol 1999; 22:727–732.
- Bernardi L, Porta C, Spicuzza L, et al Slow breathing increases arterial baroreflex sensitivity in patients with chronic heart failure. Circulation 2002; 105:143–145.
- Moser DK, Dracup K, Woo MA, Stevenson LW. Voluntary control of vascular tone by using skin-temperature biofeedback-relaxation in patients with advanced heart failure. Altern Ther Health Med 1997; 3:51–60.
- Luskin F, Reitz M, Newell K, Quinn TG, Haskell W. A controlled pilot study of stress management training of elderly patients with congestive heart failure. Prev Cardiol 2002; 5:168–172.
- Swanson KS, Gevirtz RN, Brown M, Spira J, Guarneri E, Stoletniy L. The effect of biofeedback on function in patients with heart failure. Appl Psychophysiol Biofeedback 2009; 34:71–91.
- Razeghi P, Mukhopadhyay M, Myers TJ, et al Myocardial tumor necrosis factor alpha expression does not correlate with clinical indices of heart failure in patients on left ventricular assist device support. Ann Thorac Surg 2001; 72:2044–2050.
- Matteo R, Moravec CS. Expression and immunolocalization of annexins IV, V and VI in the failing and non-failing human heart. Cardiovasc Res 2000; 45:961–970.
- Dash R, Frank K, Carr AN, Moravec CS, Kranias EG. Gender influences on sarcoplasmic reticulum calcium handling in the failing human myocardium. J Mol Cell Cardiol 2001; 33:1345–1353.
- DiPaola NR, Sweet WE, Stull LB, Francis GS, Moravec CS. Beta-adrenergic receptors and calcium cycling proteins in normal, hypertrophied and failing human hearts: transition from hypertrophy to failure. J Mol Cell Cardiol 2001; 33:1283–1295.
- Aquila LA, McCarthy PM, Smedira NG, Young JB, Moravec CS. Cytoskeletal structure and recovery in single human cardiac myocytes. J Heart Lung Transplant 2004; 23:954–963.
- Fedak PWM, Moravec CS, McCarthy PM, et al Altered expression of disintegrin metalloproteinases and their inhibitor in human dilated cardiomyopathy. Circulation 2006; 113:238–245.
- Ogletree-Hughes ML, Stull LB, Sweet WE, Smedira NG, McCarthy PM, Moravec CS. Mechanical unloading restores beta adrenergic responsiveness and reverses receptor downregulation in the failing human heart. Circulation 2001; 104:881–886.
Biofeedback in the treatment of heart failure
Biofeedback-assisted stress management training to reverse myocardial remodeling in patients with end-stage heart failure
Biofeedback: An overview in the context of heart-brain medicine
Clinical biofeedback therapy is one of the many new approaches in health care aimed at helping individuals take responsibility for their well-being, including responsibility for the cognitive, emotional, and behavioral changes needed to effect healthy physiologic change. This article provides a brief survey of biofeedback therapy by defining what biofeedback involves, reviewing the various modalities that it can serve to monitor, discussing major models of biofeedback therapy, and outlining criteria for evaluating the efficacy of biofeedback interventions.
BIOFEEDBACK: BOTH PROCESS AND INSTRUMENTATION
Biofeedback refers to both a process and the instrumentation used in that process.
The process is one of learning to use physiologic information that is monitored and “fed back” through biofeedback instruments. The term dates from 1969, when it was coined to describe laboratory procedures that had been developed in the 1940s in which research subjects learned to modify heart rate, blood flow, and other physiologic functions that were not normally thought of as being subject to conscious control. Feedback itself has been present through much of human history, particularly through the use of mirrored surfaces to practice the expression of emotion.1
Biofeedback instruments monitor one or more physiologic processes, measure what is monitored and transform that measurement into auditory and/or visual signals, and present what is monitored and measured in a simple, direct, and immediate way. Biofeedback equipment typically is noninvasive. The instruments provide continuous monitoring and transformation of physiologic data into understandable feedback for the patient being monitored. Current computerized instruments can provide simultaneous displays and recording of multiple channels of physiologic information. The goal is to enable the individual being monitored to change some physiologic process, guided by the information provided by the biofeedback equipment. How many training sessions are necessary varies with the individual and the disorder, ranging from a few to 50 or more. Our experience is that the great majority of patients obtain benefit in 8 to 12 sessions.
MULTIPLE MODALITIES FOR MONITORING
Multiple modalities can be monitored via biofeedback. Surface electromyography is perhaps the most commonly used instrumentation. Other commonly used measures in a psychophysiologic/biofeedback assessment are respiration rate and depth, skin surface temperature (particularly at the fingertips), cardiovascular reactivity (particularly heart rate and blood pressure), and electrodermal response.2
Feedback of real-time physiologic data is limited only by one’s creativity and technological capabilities. Most of the early noncomputerized equipment provided feedback through the onset and offset of sounds, the changing of tones and volume, the turning on and off of lights, and digital numeric displays indicating both the direction of change and absolute values (such as digital peripheral temperature). Current computerized equipment uses such feedback features as computer games, which the patient “wins” by reaching a goal (such as a systolic blood pressure level below 130 mm Hg), mandalas that can be filled in with colors of the patient’s choosing as he or she progresses in the desired direction, and complex computer-generated figures and graphs.
Electroencephalographic biofeedback (neurofeedback) has become a separate area of study and application, with particular use in the treatment of attention deficit disorder. A baseline electroencephalogram is used in neurofeedback assessment to identify abnormal patterns, and follow-up training is provided to teach the patient to change these patterns in a healthy direction.3
More recently, heart rate variability has come into use as a measure of adaptability or autonomic balance. Soviet scientists were the first to study heart rate variability biofeedback, working with cosmonauts in measuring autonomic function. They found that the low-frequency (0.1-Hz) bands produced the highest frequency-specific oscillations in heart rate variability, and training typically proceeds in increasing amplitude of the low-frequency band (also called the baroreceptor band). Because diminished heart rate variability is a predictor of increased risk for cardiac mortality, teaching patients to increase heart rate variability made sense. The training involves instruction in breathing at an identified resonant frequency that is related to optimal low-frequency band power.4
LEARNING AND MODELS OF BIOFEEDBACK
Accurate feedback facilitates the learning of any skill, whether it be sinking a golf putt, solving an algebra problem, or controlling physiologic behavior. A man playing darts blindfolded is unlikely to achieve as good a score as he would with the blindfold off, because feedback makes a difference.5
Four conditions are important for effective learning;5 the learner must:
- Have the capacity to respond
- Be motivated to learn
- Be positively reinforced for learning
- Be given accurate information about the results of the learning effort.
Direct feedback learning model
The direct feedback learning model assumes that adding feedback to the other important conditions of learning will result in a patient gaining control of the relevant physiology being targeted. This model has been used in treating many disorders, including Raynaud phenomenon and urinary and fecal incontinence.
Biofeedback training in this model may involve a coach/instructor/therapist only to the extent of explaining the equipment and its use. In other words, the coach “teaches the patient how to use the mirror.” More commonly.particularly for training in lowered arousal for patients in whom stress reactivity is a significant factor in the development and maintenance of excessive (sympathetic nervous system) arousal that leads to symptoms.a skilled therapist is present. The therapist not only teaches the patient how to use information from biofeedback instruments but also guides the patient in identifying and changing cognitive, emotional, and behavioral patterns that contribute to excessive reactivity. The relationship of physiologic reactivity to the subject matter under discussion also helps diagnostically in identifying stressful areas of life, particularly in psychophysiologic responders who are repressive and denying and who are not good at identifying the stressors in their lives. The equipment becomes a mirror that lets the patient see a problem that he or she had not identified as such.5
Therapeutic/stress-management/biofeedback model
When treating patients with disordered physiology (including autonomic imbalance) in the therapeutic/stress-management/biofeedback model, it is essential to understand each patient as an individual. In this model, stress management and psychotherapeutic interventions address particular vulnerabilities that lead to excessive arousal. This approach starts with a psychophysiologic assessment in which resting levels of relevant physiologic dimensions are measured; this is followed by imposition of stressors to measure reactivity and then by a recovery period in which rate and extent of recovery are measured. An interview and psychological test help determine which cognitive, emotional and behavioral patterns contribute to vulnerability. Patients typically respond well to this approach. It is common for patients to use such descriptions as, “I break out in a cold sweat when I’m stressed,” or “I feel heartsick when I’m stressed,” which suggests that the notion of mind-body interaction resonates with patients.6
CRITERIA FOR EVALUATING EFFICACY OF BIOFEEDBACK INTERVENTIONS
Several years ago a task force of the Association for Applied Psychophysiology and Biofeedback and the Society for Neuronal Regulation published criteria for evaluating the clinical efficacy of biofeedback/psychophysiologic interventions.7 These criteria are detailed below.3,7
Level 1: Not empirically supported
This designation applies to interventions supported only by anecdotal reports and/or case studies in non–peer-reviewed venues (ie, not empirically supported).
Level 2: Possibly efficacious
This applies to interventions supported by at least one study of sufficient statistical power with well-identified outcome measures but which lacked randomized assignment to a control condition internal to the study.
Level 3: Probably efficacious
This applies to interventions supported by multiple observational studies, clinical studies, wait-list–controlled studies, and within-subject and intrasubject replication studies that demonstrate efficacy.
Level 4: Efficacious
a. In a comparison with a no-treatment control group, alternative treatment group, or sham (placebo) control using randomized assignment, the intervention is shown to be statistically significantly superior to the control condition, or the intervention is equivalent to a treatment of established efficacy in a study with sufficient power to detect moderate differences, and
b. The studies have been conducted with a population treated for a specific problem, for whom inclusion criteria are delineated in a reliable, operationally defined manner, and
c. The study used valid and clearly specified outcome measures related to the problem being treated, and
d. The data were subjected to appropriate data analysis, and
e. The diagnostic and treatment variables and procedures were clearly defined in a manner that permits replication of the study by independent researchers, and
f. The superiority or equivalence of the intervention has been shown in at least two independent research settings.
Level 5: Efficacious and specific
This designation applies when the intervention has been shown to be superior to credible sham therapy, pill therapy, or alternative bona fide treatment in at least two independent research settings.
Efficacy ratings for specific disorders
Despite high standards, biofeedback thrives
The above criteria represent high standards. Since biofeedback training is often more like physical therapy or learning a language, double-blind protocols usually are not feasible, nor is sham training. Moreover, the effectiveness of training is perhaps even more difficult to assess in daily practice, with the inevitable multiplicity of confounding variables. Nevertheless, biofeedback training for many disorders is standing the test of both time and outcomes research, and it is increasingly embraced by the public and recognized by health care insurers and professionals alike.
- Gaarder KR, Montgomery PS. Clinical biofeedback: a procedural manual. Baltimore, MD: Williams and Wilkins; 1977.
- Schwartz MS, Andrasik F. Biofeedback: a practitioner’s guide. 3rd ed. New York, NY: Guilford Press; 2003.
- Yucha C, Gilbert C. Evidence-based practice in biofeedback and neurofeedback. Wheat Ridge, CO: Association for Applied Psychophysiology and Biofeedback; 2004.
- Lehrer PM, Vaschillo E, Lu SE, et al. Heart rate variability biofeedback: effects of age on heart rate variability, baroreflex gain, and asthma. Chest 2006; 129:278–284.
- McKee MG. Contributions of psychophysiologic monitoring to diagnosis and treatment of chronic head pain: a case study. Headache Q 1991; II(4):327–330.
- McKee MG. Using biofeedback and self-control techniques to prevent heart attacks. Psychiatr Ann 1978; 8:10.
- Moss D, Gunkelman J. Task Force Report on methodology and empirically supported treatments: introduction. Appl Psychophysiol Biofeedback 2002; 27:271–272.
- Weatherall M. Biofeedback or pelvic floor muscle exercises for female genuine stress incontinence: a meta-analysis of trials identified in a systematic review. BJU Int 1999; 83:1015–1016.
- Wenck LS, Leu PW, D’Amato RC. Evaluating the efficacy of a biofeedback intervention to reduce children’s anxiety. J Clin Psychol 1996; 52:469–473.
- Kaiser DA, Othmer S. Effect of neurofeedback on variables of attention in a large multi-center trial. J Neurother 2000; 4:5–15.
- Silberstein SD, for the US Headache Consortium. Practice parameter: evidence-based guidelines for migraine headache (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 2000; 55:754–762.
- Yucha CB, Clark L, Smith M, Uris P, Lafleur B, Duval S. The effect of biofeedback in hypertension. Appl Nurs Res 2001; 14:29–35.
- Crider AB, Glaros AG. A meta-analysis of EMG biofeedback treatment of temporomandibular disorders. J Orofac Pain 1999; 13:29–37.
- Van Kampen M, De Weerdt W, Van Poppel H, De Ridder D, Feys H, Baert L. Effect of pelvic-floor re-education on duration and degree of incontinence after radical prostatectomy: a randomised controlled trial. Lancet 2000; 355:98–102.
- Saxby E, Peniston EG. Alpha-theta brainwave neurofeedback training: an effective treatment for male and female alcoholics with depressive symptoms. J Clin Psychol 1995; 51:685–693.
- Lavigne JV, Ross CK, Berry SL, Hayford JR, Pachman LM. Evaluation of a psychological treatment package for treating pain in juvenile rheumatoid arthritis. Arthritis Care Res 1992; 5:101–110.
- Humphreys PA, Gevirtz RN. Treatment of recurrent abdominal pain: components analysis of four treatment protocols. J Pediatr Gastroenterol Nutr 2000; 31:47–51.
- Kotchoubey B, Strehl U, Uhlmann C, et al. Modification of slow cortical potentials in patients with refractory epilepsy: a controlled outcome study. Epilepsia 2001; 42:406–416.
- Chiarioni G, Bassotti G, Stanganini S, Vantini I, Whitehead WE. Sensory retraining is key to biofeedback therapy for formed stool fecal incontinence. Am J Gastroenterol 2002; 97:109–117.
- Labbé EE. Treatment of childhood migraine with autogenic training and skin temperature biofeedback: a component analysis. Headache 1995; 35:10–13.
- Morin CM, Hauri PJ, Espie CA, Spielman AJ, Buysse DJ, Bootzin RR. Nonpharmacologic treatment of chronic insomnia. An American Academy of Sleep Medicine review. Sleep 1999; 22:1134–1156.
- Thornton K. Improvement/rehabilitation of memory functioning with neurotherapy/QEEG biofeedback. J Head Trauma Rehabil 2000; 15:1285–1296.
- Bergeron S, Binik YM, Khalifé S, et al. A randomized comparison of group cognitive–behavioral therapy, surface electromyographic biofeedback, and vestibulectomy in the treatment of dyspareunia resulting from vulvar vestibulitis. Pain 2001; 91:297–306.
Clinical biofeedback therapy is one of the many new approaches in health care aimed at helping individuals take responsibility for their well-being, including responsibility for the cognitive, emotional, and behavioral changes needed to effect healthy physiologic change. This article provides a brief survey of biofeedback therapy by defining what biofeedback involves, reviewing the various modalities that it can serve to monitor, discussing major models of biofeedback therapy, and outlining criteria for evaluating the efficacy of biofeedback interventions.
BIOFEEDBACK: BOTH PROCESS AND INSTRUMENTATION
Biofeedback refers to both a process and the instrumentation used in that process.
The process is one of learning to use physiologic information that is monitored and “fed back” through biofeedback instruments. The term dates from 1969, when it was coined to describe laboratory procedures that had been developed in the 1940s in which research subjects learned to modify heart rate, blood flow, and other physiologic functions that were not normally thought of as being subject to conscious control. Feedback itself has been present through much of human history, particularly through the use of mirrored surfaces to practice the expression of emotion.1
Biofeedback instruments monitor one or more physiologic processes, measure what is monitored and transform that measurement into auditory and/or visual signals, and present what is monitored and measured in a simple, direct, and immediate way. Biofeedback equipment typically is noninvasive. The instruments provide continuous monitoring and transformation of physiologic data into understandable feedback for the patient being monitored. Current computerized instruments can provide simultaneous displays and recording of multiple channels of physiologic information. The goal is to enable the individual being monitored to change some physiologic process, guided by the information provided by the biofeedback equipment. How many training sessions are necessary varies with the individual and the disorder, ranging from a few to 50 or more. Our experience is that the great majority of patients obtain benefit in 8 to 12 sessions.
MULTIPLE MODALITIES FOR MONITORING
Multiple modalities can be monitored via biofeedback. Surface electromyography is perhaps the most commonly used instrumentation. Other commonly used measures in a psychophysiologic/biofeedback assessment are respiration rate and depth, skin surface temperature (particularly at the fingertips), cardiovascular reactivity (particularly heart rate and blood pressure), and electrodermal response.2
Feedback of real-time physiologic data is limited only by one’s creativity and technological capabilities. Most of the early noncomputerized equipment provided feedback through the onset and offset of sounds, the changing of tones and volume, the turning on and off of lights, and digital numeric displays indicating both the direction of change and absolute values (such as digital peripheral temperature). Current computerized equipment uses such feedback features as computer games, which the patient “wins” by reaching a goal (such as a systolic blood pressure level below 130 mm Hg), mandalas that can be filled in with colors of the patient’s choosing as he or she progresses in the desired direction, and complex computer-generated figures and graphs.
Electroencephalographic biofeedback (neurofeedback) has become a separate area of study and application, with particular use in the treatment of attention deficit disorder. A baseline electroencephalogram is used in neurofeedback assessment to identify abnormal patterns, and follow-up training is provided to teach the patient to change these patterns in a healthy direction.3
More recently, heart rate variability has come into use as a measure of adaptability or autonomic balance. Soviet scientists were the first to study heart rate variability biofeedback, working with cosmonauts in measuring autonomic function. They found that the low-frequency (0.1-Hz) bands produced the highest frequency-specific oscillations in heart rate variability, and training typically proceeds in increasing amplitude of the low-frequency band (also called the baroreceptor band). Because diminished heart rate variability is a predictor of increased risk for cardiac mortality, teaching patients to increase heart rate variability made sense. The training involves instruction in breathing at an identified resonant frequency that is related to optimal low-frequency band power.4
LEARNING AND MODELS OF BIOFEEDBACK
Accurate feedback facilitates the learning of any skill, whether it be sinking a golf putt, solving an algebra problem, or controlling physiologic behavior. A man playing darts blindfolded is unlikely to achieve as good a score as he would with the blindfold off, because feedback makes a difference.5
Four conditions are important for effective learning;5 the learner must:
- Have the capacity to respond
- Be motivated to learn
- Be positively reinforced for learning
- Be given accurate information about the results of the learning effort.
Direct feedback learning model
The direct feedback learning model assumes that adding feedback to the other important conditions of learning will result in a patient gaining control of the relevant physiology being targeted. This model has been used in treating many disorders, including Raynaud phenomenon and urinary and fecal incontinence.
Biofeedback training in this model may involve a coach/instructor/therapist only to the extent of explaining the equipment and its use. In other words, the coach “teaches the patient how to use the mirror.” More commonly.particularly for training in lowered arousal for patients in whom stress reactivity is a significant factor in the development and maintenance of excessive (sympathetic nervous system) arousal that leads to symptoms.a skilled therapist is present. The therapist not only teaches the patient how to use information from biofeedback instruments but also guides the patient in identifying and changing cognitive, emotional, and behavioral patterns that contribute to excessive reactivity. The relationship of physiologic reactivity to the subject matter under discussion also helps diagnostically in identifying stressful areas of life, particularly in psychophysiologic responders who are repressive and denying and who are not good at identifying the stressors in their lives. The equipment becomes a mirror that lets the patient see a problem that he or she had not identified as such.5
Therapeutic/stress-management/biofeedback model
When treating patients with disordered physiology (including autonomic imbalance) in the therapeutic/stress-management/biofeedback model, it is essential to understand each patient as an individual. In this model, stress management and psychotherapeutic interventions address particular vulnerabilities that lead to excessive arousal. This approach starts with a psychophysiologic assessment in which resting levels of relevant physiologic dimensions are measured; this is followed by imposition of stressors to measure reactivity and then by a recovery period in which rate and extent of recovery are measured. An interview and psychological test help determine which cognitive, emotional and behavioral patterns contribute to vulnerability. Patients typically respond well to this approach. It is common for patients to use such descriptions as, “I break out in a cold sweat when I’m stressed,” or “I feel heartsick when I’m stressed,” which suggests that the notion of mind-body interaction resonates with patients.6
CRITERIA FOR EVALUATING EFFICACY OF BIOFEEDBACK INTERVENTIONS
Several years ago a task force of the Association for Applied Psychophysiology and Biofeedback and the Society for Neuronal Regulation published criteria for evaluating the clinical efficacy of biofeedback/psychophysiologic interventions.7 These criteria are detailed below.3,7
Level 1: Not empirically supported
This designation applies to interventions supported only by anecdotal reports and/or case studies in non–peer-reviewed venues (ie, not empirically supported).
Level 2: Possibly efficacious
This applies to interventions supported by at least one study of sufficient statistical power with well-identified outcome measures but which lacked randomized assignment to a control condition internal to the study.
Level 3: Probably efficacious
This applies to interventions supported by multiple observational studies, clinical studies, wait-list–controlled studies, and within-subject and intrasubject replication studies that demonstrate efficacy.
Level 4: Efficacious
a. In a comparison with a no-treatment control group, alternative treatment group, or sham (placebo) control using randomized assignment, the intervention is shown to be statistically significantly superior to the control condition, or the intervention is equivalent to a treatment of established efficacy in a study with sufficient power to detect moderate differences, and
b. The studies have been conducted with a population treated for a specific problem, for whom inclusion criteria are delineated in a reliable, operationally defined manner, and
c. The study used valid and clearly specified outcome measures related to the problem being treated, and
d. The data were subjected to appropriate data analysis, and
e. The diagnostic and treatment variables and procedures were clearly defined in a manner that permits replication of the study by independent researchers, and
f. The superiority or equivalence of the intervention has been shown in at least two independent research settings.
Level 5: Efficacious and specific
This designation applies when the intervention has been shown to be superior to credible sham therapy, pill therapy, or alternative bona fide treatment in at least two independent research settings.
Efficacy ratings for specific disorders
Despite high standards, biofeedback thrives
The above criteria represent high standards. Since biofeedback training is often more like physical therapy or learning a language, double-blind protocols usually are not feasible, nor is sham training. Moreover, the effectiveness of training is perhaps even more difficult to assess in daily practice, with the inevitable multiplicity of confounding variables. Nevertheless, biofeedback training for many disorders is standing the test of both time and outcomes research, and it is increasingly embraced by the public and recognized by health care insurers and professionals alike.
Clinical biofeedback therapy is one of the many new approaches in health care aimed at helping individuals take responsibility for their well-being, including responsibility for the cognitive, emotional, and behavioral changes needed to effect healthy physiologic change. This article provides a brief survey of biofeedback therapy by defining what biofeedback involves, reviewing the various modalities that it can serve to monitor, discussing major models of biofeedback therapy, and outlining criteria for evaluating the efficacy of biofeedback interventions.
BIOFEEDBACK: BOTH PROCESS AND INSTRUMENTATION
Biofeedback refers to both a process and the instrumentation used in that process.
The process is one of learning to use physiologic information that is monitored and “fed back” through biofeedback instruments. The term dates from 1969, when it was coined to describe laboratory procedures that had been developed in the 1940s in which research subjects learned to modify heart rate, blood flow, and other physiologic functions that were not normally thought of as being subject to conscious control. Feedback itself has been present through much of human history, particularly through the use of mirrored surfaces to practice the expression of emotion.1
Biofeedback instruments monitor one or more physiologic processes, measure what is monitored and transform that measurement into auditory and/or visual signals, and present what is monitored and measured in a simple, direct, and immediate way. Biofeedback equipment typically is noninvasive. The instruments provide continuous monitoring and transformation of physiologic data into understandable feedback for the patient being monitored. Current computerized instruments can provide simultaneous displays and recording of multiple channels of physiologic information. The goal is to enable the individual being monitored to change some physiologic process, guided by the information provided by the biofeedback equipment. How many training sessions are necessary varies with the individual and the disorder, ranging from a few to 50 or more. Our experience is that the great majority of patients obtain benefit in 8 to 12 sessions.
MULTIPLE MODALITIES FOR MONITORING
Multiple modalities can be monitored via biofeedback. Surface electromyography is perhaps the most commonly used instrumentation. Other commonly used measures in a psychophysiologic/biofeedback assessment are respiration rate and depth, skin surface temperature (particularly at the fingertips), cardiovascular reactivity (particularly heart rate and blood pressure), and electrodermal response.2
Feedback of real-time physiologic data is limited only by one’s creativity and technological capabilities. Most of the early noncomputerized equipment provided feedback through the onset and offset of sounds, the changing of tones and volume, the turning on and off of lights, and digital numeric displays indicating both the direction of change and absolute values (such as digital peripheral temperature). Current computerized equipment uses such feedback features as computer games, which the patient “wins” by reaching a goal (such as a systolic blood pressure level below 130 mm Hg), mandalas that can be filled in with colors of the patient’s choosing as he or she progresses in the desired direction, and complex computer-generated figures and graphs.
Electroencephalographic biofeedback (neurofeedback) has become a separate area of study and application, with particular use in the treatment of attention deficit disorder. A baseline electroencephalogram is used in neurofeedback assessment to identify abnormal patterns, and follow-up training is provided to teach the patient to change these patterns in a healthy direction.3
More recently, heart rate variability has come into use as a measure of adaptability or autonomic balance. Soviet scientists were the first to study heart rate variability biofeedback, working with cosmonauts in measuring autonomic function. They found that the low-frequency (0.1-Hz) bands produced the highest frequency-specific oscillations in heart rate variability, and training typically proceeds in increasing amplitude of the low-frequency band (also called the baroreceptor band). Because diminished heart rate variability is a predictor of increased risk for cardiac mortality, teaching patients to increase heart rate variability made sense. The training involves instruction in breathing at an identified resonant frequency that is related to optimal low-frequency band power.4
LEARNING AND MODELS OF BIOFEEDBACK
Accurate feedback facilitates the learning of any skill, whether it be sinking a golf putt, solving an algebra problem, or controlling physiologic behavior. A man playing darts blindfolded is unlikely to achieve as good a score as he would with the blindfold off, because feedback makes a difference.5
Four conditions are important for effective learning;5 the learner must:
- Have the capacity to respond
- Be motivated to learn
- Be positively reinforced for learning
- Be given accurate information about the results of the learning effort.
Direct feedback learning model
The direct feedback learning model assumes that adding feedback to the other important conditions of learning will result in a patient gaining control of the relevant physiology being targeted. This model has been used in treating many disorders, including Raynaud phenomenon and urinary and fecal incontinence.
Biofeedback training in this model may involve a coach/instructor/therapist only to the extent of explaining the equipment and its use. In other words, the coach “teaches the patient how to use the mirror.” More commonly.particularly for training in lowered arousal for patients in whom stress reactivity is a significant factor in the development and maintenance of excessive (sympathetic nervous system) arousal that leads to symptoms.a skilled therapist is present. The therapist not only teaches the patient how to use information from biofeedback instruments but also guides the patient in identifying and changing cognitive, emotional, and behavioral patterns that contribute to excessive reactivity. The relationship of physiologic reactivity to the subject matter under discussion also helps diagnostically in identifying stressful areas of life, particularly in psychophysiologic responders who are repressive and denying and who are not good at identifying the stressors in their lives. The equipment becomes a mirror that lets the patient see a problem that he or she had not identified as such.5
Therapeutic/stress-management/biofeedback model
When treating patients with disordered physiology (including autonomic imbalance) in the therapeutic/stress-management/biofeedback model, it is essential to understand each patient as an individual. In this model, stress management and psychotherapeutic interventions address particular vulnerabilities that lead to excessive arousal. This approach starts with a psychophysiologic assessment in which resting levels of relevant physiologic dimensions are measured; this is followed by imposition of stressors to measure reactivity and then by a recovery period in which rate and extent of recovery are measured. An interview and psychological test help determine which cognitive, emotional and behavioral patterns contribute to vulnerability. Patients typically respond well to this approach. It is common for patients to use such descriptions as, “I break out in a cold sweat when I’m stressed,” or “I feel heartsick when I’m stressed,” which suggests that the notion of mind-body interaction resonates with patients.6
CRITERIA FOR EVALUATING EFFICACY OF BIOFEEDBACK INTERVENTIONS
Several years ago a task force of the Association for Applied Psychophysiology and Biofeedback and the Society for Neuronal Regulation published criteria for evaluating the clinical efficacy of biofeedback/psychophysiologic interventions.7 These criteria are detailed below.3,7
Level 1: Not empirically supported
This designation applies to interventions supported only by anecdotal reports and/or case studies in non–peer-reviewed venues (ie, not empirically supported).
Level 2: Possibly efficacious
This applies to interventions supported by at least one study of sufficient statistical power with well-identified outcome measures but which lacked randomized assignment to a control condition internal to the study.
Level 3: Probably efficacious
This applies to interventions supported by multiple observational studies, clinical studies, wait-list–controlled studies, and within-subject and intrasubject replication studies that demonstrate efficacy.
Level 4: Efficacious
a. In a comparison with a no-treatment control group, alternative treatment group, or sham (placebo) control using randomized assignment, the intervention is shown to be statistically significantly superior to the control condition, or the intervention is equivalent to a treatment of established efficacy in a study with sufficient power to detect moderate differences, and
b. The studies have been conducted with a population treated for a specific problem, for whom inclusion criteria are delineated in a reliable, operationally defined manner, and
c. The study used valid and clearly specified outcome measures related to the problem being treated, and
d. The data were subjected to appropriate data analysis, and
e. The diagnostic and treatment variables and procedures were clearly defined in a manner that permits replication of the study by independent researchers, and
f. The superiority or equivalence of the intervention has been shown in at least two independent research settings.
Level 5: Efficacious and specific
This designation applies when the intervention has been shown to be superior to credible sham therapy, pill therapy, or alternative bona fide treatment in at least two independent research settings.
Efficacy ratings for specific disorders
Despite high standards, biofeedback thrives
The above criteria represent high standards. Since biofeedback training is often more like physical therapy or learning a language, double-blind protocols usually are not feasible, nor is sham training. Moreover, the effectiveness of training is perhaps even more difficult to assess in daily practice, with the inevitable multiplicity of confounding variables. Nevertheless, biofeedback training for many disorders is standing the test of both time and outcomes research, and it is increasingly embraced by the public and recognized by health care insurers and professionals alike.
- Gaarder KR, Montgomery PS. Clinical biofeedback: a procedural manual. Baltimore, MD: Williams and Wilkins; 1977.
- Schwartz MS, Andrasik F. Biofeedback: a practitioner’s guide. 3rd ed. New York, NY: Guilford Press; 2003.
- Yucha C, Gilbert C. Evidence-based practice in biofeedback and neurofeedback. Wheat Ridge, CO: Association for Applied Psychophysiology and Biofeedback; 2004.
- Lehrer PM, Vaschillo E, Lu SE, et al. Heart rate variability biofeedback: effects of age on heart rate variability, baroreflex gain, and asthma. Chest 2006; 129:278–284.
- McKee MG. Contributions of psychophysiologic monitoring to diagnosis and treatment of chronic head pain: a case study. Headache Q 1991; II(4):327–330.
- McKee MG. Using biofeedback and self-control techniques to prevent heart attacks. Psychiatr Ann 1978; 8:10.
- Moss D, Gunkelman J. Task Force Report on methodology and empirically supported treatments: introduction. Appl Psychophysiol Biofeedback 2002; 27:271–272.
- Weatherall M. Biofeedback or pelvic floor muscle exercises for female genuine stress incontinence: a meta-analysis of trials identified in a systematic review. BJU Int 1999; 83:1015–1016.
- Wenck LS, Leu PW, D’Amato RC. Evaluating the efficacy of a biofeedback intervention to reduce children’s anxiety. J Clin Psychol 1996; 52:469–473.
- Kaiser DA, Othmer S. Effect of neurofeedback on variables of attention in a large multi-center trial. J Neurother 2000; 4:5–15.
- Silberstein SD, for the US Headache Consortium. Practice parameter: evidence-based guidelines for migraine headache (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 2000; 55:754–762.
- Yucha CB, Clark L, Smith M, Uris P, Lafleur B, Duval S. The effect of biofeedback in hypertension. Appl Nurs Res 2001; 14:29–35.
- Crider AB, Glaros AG. A meta-analysis of EMG biofeedback treatment of temporomandibular disorders. J Orofac Pain 1999; 13:29–37.
- Van Kampen M, De Weerdt W, Van Poppel H, De Ridder D, Feys H, Baert L. Effect of pelvic-floor re-education on duration and degree of incontinence after radical prostatectomy: a randomised controlled trial. Lancet 2000; 355:98–102.
- Saxby E, Peniston EG. Alpha-theta brainwave neurofeedback training: an effective treatment for male and female alcoholics with depressive symptoms. J Clin Psychol 1995; 51:685–693.
- Lavigne JV, Ross CK, Berry SL, Hayford JR, Pachman LM. Evaluation of a psychological treatment package for treating pain in juvenile rheumatoid arthritis. Arthritis Care Res 1992; 5:101–110.
- Humphreys PA, Gevirtz RN. Treatment of recurrent abdominal pain: components analysis of four treatment protocols. J Pediatr Gastroenterol Nutr 2000; 31:47–51.
- Kotchoubey B, Strehl U, Uhlmann C, et al. Modification of slow cortical potentials in patients with refractory epilepsy: a controlled outcome study. Epilepsia 2001; 42:406–416.
- Chiarioni G, Bassotti G, Stanganini S, Vantini I, Whitehead WE. Sensory retraining is key to biofeedback therapy for formed stool fecal incontinence. Am J Gastroenterol 2002; 97:109–117.
- Labbé EE. Treatment of childhood migraine with autogenic training and skin temperature biofeedback: a component analysis. Headache 1995; 35:10–13.
- Morin CM, Hauri PJ, Espie CA, Spielman AJ, Buysse DJ, Bootzin RR. Nonpharmacologic treatment of chronic insomnia. An American Academy of Sleep Medicine review. Sleep 1999; 22:1134–1156.
- Thornton K. Improvement/rehabilitation of memory functioning with neurotherapy/QEEG biofeedback. J Head Trauma Rehabil 2000; 15:1285–1296.
- Bergeron S, Binik YM, Khalifé S, et al. A randomized comparison of group cognitive–behavioral therapy, surface electromyographic biofeedback, and vestibulectomy in the treatment of dyspareunia resulting from vulvar vestibulitis. Pain 2001; 91:297–306.
- Gaarder KR, Montgomery PS. Clinical biofeedback: a procedural manual. Baltimore, MD: Williams and Wilkins; 1977.
- Schwartz MS, Andrasik F. Biofeedback: a practitioner’s guide. 3rd ed. New York, NY: Guilford Press; 2003.
- Yucha C, Gilbert C. Evidence-based practice in biofeedback and neurofeedback. Wheat Ridge, CO: Association for Applied Psychophysiology and Biofeedback; 2004.
- Lehrer PM, Vaschillo E, Lu SE, et al. Heart rate variability biofeedback: effects of age on heart rate variability, baroreflex gain, and asthma. Chest 2006; 129:278–284.
- McKee MG. Contributions of psychophysiologic monitoring to diagnosis and treatment of chronic head pain: a case study. Headache Q 1991; II(4):327–330.
- McKee MG. Using biofeedback and self-control techniques to prevent heart attacks. Psychiatr Ann 1978; 8:10.
- Moss D, Gunkelman J. Task Force Report on methodology and empirically supported treatments: introduction. Appl Psychophysiol Biofeedback 2002; 27:271–272.
- Weatherall M. Biofeedback or pelvic floor muscle exercises for female genuine stress incontinence: a meta-analysis of trials identified in a systematic review. BJU Int 1999; 83:1015–1016.
- Wenck LS, Leu PW, D’Amato RC. Evaluating the efficacy of a biofeedback intervention to reduce children’s anxiety. J Clin Psychol 1996; 52:469–473.
- Kaiser DA, Othmer S. Effect of neurofeedback on variables of attention in a large multi-center trial. J Neurother 2000; 4:5–15.
- Silberstein SD, for the US Headache Consortium. Practice parameter: evidence-based guidelines for migraine headache (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 2000; 55:754–762.
- Yucha CB, Clark L, Smith M, Uris P, Lafleur B, Duval S. The effect of biofeedback in hypertension. Appl Nurs Res 2001; 14:29–35.
- Crider AB, Glaros AG. A meta-analysis of EMG biofeedback treatment of temporomandibular disorders. J Orofac Pain 1999; 13:29–37.
- Van Kampen M, De Weerdt W, Van Poppel H, De Ridder D, Feys H, Baert L. Effect of pelvic-floor re-education on duration and degree of incontinence after radical prostatectomy: a randomised controlled trial. Lancet 2000; 355:98–102.
- Saxby E, Peniston EG. Alpha-theta brainwave neurofeedback training: an effective treatment for male and female alcoholics with depressive symptoms. J Clin Psychol 1995; 51:685–693.
- Lavigne JV, Ross CK, Berry SL, Hayford JR, Pachman LM. Evaluation of a psychological treatment package for treating pain in juvenile rheumatoid arthritis. Arthritis Care Res 1992; 5:101–110.
- Humphreys PA, Gevirtz RN. Treatment of recurrent abdominal pain: components analysis of four treatment protocols. J Pediatr Gastroenterol Nutr 2000; 31:47–51.
- Kotchoubey B, Strehl U, Uhlmann C, et al. Modification of slow cortical potentials in patients with refractory epilepsy: a controlled outcome study. Epilepsia 2001; 42:406–416.
- Chiarioni G, Bassotti G, Stanganini S, Vantini I, Whitehead WE. Sensory retraining is key to biofeedback therapy for formed stool fecal incontinence. Am J Gastroenterol 2002; 97:109–117.
- Labbé EE. Treatment of childhood migraine with autogenic training and skin temperature biofeedback: a component analysis. Headache 1995; 35:10–13.
- Morin CM, Hauri PJ, Espie CA, Spielman AJ, Buysse DJ, Bootzin RR. Nonpharmacologic treatment of chronic insomnia. An American Academy of Sleep Medicine review. Sleep 1999; 22:1134–1156.
- Thornton K. Improvement/rehabilitation of memory functioning with neurotherapy/QEEG biofeedback. J Head Trauma Rehabil 2000; 15:1285–1296.
- Bergeron S, Binik YM, Khalifé S, et al. A randomized comparison of group cognitive–behavioral therapy, surface electromyographic biofeedback, and vestibulectomy in the treatment of dyspareunia resulting from vulvar vestibulitis. Pain 2001; 91:297–306.