User login
Biofeedback in the treatment of heart disease
BIOFEEDBACK: WHAT IS IT?
The term “biofeedback” refers to the instrumentation or training process that allows biologic information to be recorded, displayed, and communicated back to an individual, allowing the individual to make adjustments in physiologic processes that may enhance health or performance. The biofeedback display is analogous to a mirror, in which physiologic processes can be observed and adjusted much as one might adjust a hairstyle or a tie.
In our work with cardiovascular disease patients, biofeedback is a training process that involves a subject or patient, a biofeedback coach or therapist, and state-of-the-art biofeedback equipment. For biofeedback training to be effective, the subject who is trying to learn the skill must be engaged and willing to practice, the coach must be trained in psychophysiology, and the equipment must display accurate readings in real time, allowing the subject to monitor and change physiologic reactions appropriately. The coach teaches the subject about the physiologic parameters, establishes target ranges, and helps the subject learn how to move the physiologic parameters in the right direction.1,2
Training often begins with a session in which a brief mental stress test is followed by a period of relaxation while physiologic parameters are recorded and displayed. This process helps the subject to understand the link between mental processes and physiologic arousal.
Biofeedback training can involve a number of physiologic modalities, including those that reflect autonomic nervous system arousal, such as skin conductance and heart rate variability, and those that are not strictly correlated with autonomic activity, such as surface muscle tension. Each physiologic parameter is recorded by a specific sensor, and all sensors are noninvasive. Sensors feed signals into a computer, where they are processed and amplified, and subjects are able to view the output on a computer screen.
Typically, in our work, there is one screen for the subject, on which a single parameter can be displayed, observed and discussed, and another screen for the coach, on which all parameters are displayed simultaneously. During a single session of biofeedback training, the coach may choose to work on a single parameter or switch between parameters, depending on how much progress is being made with each. In our work with patients, we generally train to simple parameters first, such as respiratory rate, finger temperature, and skin conductance, moving on to surface muscle tension, heart rate, and eventually heart rate variability, which is a more complex concept and more easily understood later in the training process.
It is important that the subject receive positive reinforcement for changing the physiologic parameters, and if the subject struggles too long with one parameter, it is generally useful to go back to a different parameter, where success may be more easily experienced. Ideally, by the end of six to eight training sessions, the subject will be able to make progress on all physiologic parameters, which will track together over time.
BIOFEEDBACK-ASSISTED STRESS MANAGEMENT
Pure biofeedback training consists of operant conditioning. That is, the subject learns to regulate his or her physiology in the right direction because of the feedback, which can be as simple as a pleasant image appearing on a computer screen or as complicated as a car moving faster around a racetrack; pure biofeedback involves changing physiology in response to positive reinforcement of some sort.
In practice, we generally employ biofeedback-assisted stress management (BFSM) rather than pure biofeedback. With BFSM, the subject learns to change physiology in the direction of health and wellness by learning techniques of stress management. The coach teaches the subject various relaxation techniques, such as slow and rhythmic breathing, guided imagery, progressive muscle relaxation, mindfulness, assertiveness, and how to change negative thought patterns. With regular practice, the subject learns to change the physiologic parameters by relaxing the body. For example, instead of instructing the subject to “increase your finger temperature” and assume that the subject will achieve this because doing so will make the light bulb on the screen glow more intensely, the BFSM coach may instead talk with the subject about eliminating stressful thoughts, learning to relax, and the fingers warming in response to the body relaxing.
We distinguish between techniques of stress management, some of which are mentioned above, and psychotherapy, which can certainly be effectively combined with biofeedback, but which we do not provide in our research studies. Coupling stress management techniques with biofeedback helps the subject change physiologic parameters in the direction of wellness and acquire tools that can be used in everyday life when stressful events arise. The objective of BFSM training is not just to change physiology, but also to change the way subjects respond to stressful events in daily life; ie, react to fewer events, react less intensely when they do react, and recover more quickly.
BIOFEEDBACK-ASSISTED STRESS MANAGEMENT IN CARDIOVASCULAR DISEASE
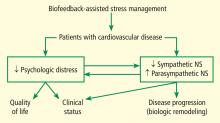
We are currently studying the effects of BFSM in patients with cardiovascular disease, including both heart failure and stable coronary artery disease. Patients with cardiovascular disease often are functionally limited, and they also experience psychologic distress related to physical limitations and other life stressors. Both the physical limitations and the psychologic distress impact quality of life. We hypothesize that BFSM will teach our patients techniques of stress management, both mental and physiologic, that will help relieve their psychologic distress and improve their quality of life. BFSM will also potentially decrease the overactivation of the sympathetic branch of the autonomic nervous system, which is common in cardiovascular disease, and correspondingly upregulate the contribution of the parasympathetic branch of the autonomic nervous system, which should be beneficial.3
A PROMISING TECHNIQUE IN HEART FAILURE
We are currently studying the effects of BFSM in patients with end-stage heart failure who are awaiting heart transplant at Cleveland Clinic.4 As noted in a recent review, biofeedback is a promising technique in heart failure that patients may be able to use to consciously regulate their autonomic nervous systems.5 We hypothesize that BFSM training will interfere with the overactivation of the sympathetic nervous system that is characteristic of heart failure, and that this will reverse the cellular and molecular remodeling that occurs in the failing human heart.
To date, we have enrolled 25 patients; 10 are being studied in our National Institutes of Health–funded Clinic Research Unit and 15 are inpatients. All 25 patients are listed as heart transplant candidates and have given consent for us to study their hearts when they are explanted.
Each patient receives eight sessions with a certified biofeedback therapist. The first and last sessions include mental stress tests, while the remaining six are BFSM training sessions. Patients are assessed at the beginning and end of the study using the 6-minute walk test, the Kansas City Cardiomyopathy Questionnaire, the Medical Outcomes Study 36-Item Short-Form Health Survey (SF-36), and measurement of plasma catecholamines.
The primary end point of the study is the measurement of cellular and molecular markers that have been shown to be altered in the failing human heart, testing the hypothesis that these markers will be reversed in the direction of normal in the BFSM therapy group. These markers are measured in the explanted failing heart when the patient receives a heart transplant.
It is too early to report the results of this study, since only seven patients have undergone transplantation to date. We are encouraged by several early findings, however, and hope these will be validated when the entire group is analyzed.
In early analysis, scores on the Kansas City Cardiomyopathy Questionnaire are improved in the last session compared with the first; patients have shown the ability to learn a slower breathing rate; and they are able to regulate their heart rate variability, as measured by the standard deviation of the N-to-N interval, or SDNN. Most important, measurements in the first seven hearts indicate that there is a degree of biologic remodeling of the failing heart after BFSM that is similar to what we have observed with left ventricular assist devices—hemodynamic pumps that take on the workload of the heart, permitting the heart to rest and recover while the patient is waiting for a transplant.6,7 If BFSM could produce changes in the cellular and molecular properties of the heart that are equal in magnitude to those produced by a mechanical pump, this would be a revolutionary finding in the field of heart-brain medicine.
It should be noted that we are not the first group to study BFSM in patients with heart failure. Moser and colleagues first observed that a single session of skin temperature biofeedback could have significant functional effects in patients with heart failure.8 Bernardi and coworkers showed that merely teaching patients to breathe six times per minute (a large component of BFSM training) improved oxygen saturation and exercise tolerance.9 Swanson and colleagues in 2009 demonstrated that patients with heart failure were able to regulate their heart rate variability, although they observed this only in patients with a left ventricular ejection fraction greater than 30%.10 Our preliminary data demonstrate regulation of heart rate variability in patients with lower ejection fractions, which is promising, but we have also added the biologic component of studying the explanted heart, allowing us to test the hypothesis that BFSM could potentially impact the remodeling process and thus have important therapeutic implications.
TRIAL UNDER WAY IN CORONARY ARTERY DISEASE
In addition to our studies of BFSM in heart failure, we have begun a randomized clinical trial of patients with stable coronary artery disease, type 2 diabetes, or multiple sclerosis. These three patient populations were chosen because evidence from numerous studies suggests that they all involve autonomic nervous system dysregulation as well as an inflammatory process.
It has already been mentioned that BFSM can interfere with overactivation of the sympathetic nervous system and potentially upregulate the contribution of the parasympathetic nervous system, which usually exists in juxtaposition to the sympathetic nervous system. Based on the work of Tracey,11,12 upregulating the parasympathetic nervous system should be antiinflammatory. Thus, we hypothesize that by decreasing both sympathetic nervous system activation and inflammation, BFSM should have an impact on patients with one of these disease states, resulting in improved quality of life and clinical status, reduced anxiety and depression, and changed disease-specific indicators of severity.
We are currently enrolling patients who have coronary artery disease, type 2 diabetes, or multiple sclerosis and randomizing them to groups that will receive either BFSM or usual care. Outcome variables that will be assessed in all patients include heart rate variability; the response of temperature, skin conductance, respiratory rate, and heart rate variability to mental stress; plasma catecholamine levels; plasma C-reactive protein levels; and tumor necrosis factor alpha levels. At the first and last visits, all patients will complete the SF-36, the eight-item Patient Health Questionnaire depression scale (PHQ-8), the Generalized Anxiety Disorder seven-item scale (GAD-7), and a visual analog pain scale. We will also assess disease-specific variables, including heart rate recovery after exercise, plasma lipids, and myeloperoxidase in patients with coronary artery disease; the Multiple Sclerosis Functional Composite (MSFC) test and the Modified Fatigue Impact Scale (MFIS) will be administered to patients with multiple sclerosis; and plasma glucose and hemoglobin A1C will be assessed in patients with type 2 diabetes.
Results of this study will provide data on the potential of BFSM to decrease common markers of autonomic nervous system activation and inflammatory cascades and the effect of those alterations on three specific disease states. To our knowledge, such a randomized study has not been conducted previously; our findings will add significantly to the literature on the mechanism of action of biofeedback-type interventions.
POTENTIAL IMPACT ON DEPRESSION IN CARDIOVASCULAR DISEASE
Depression is increasingly recognized as a component of many cardiovascular diseases; this raises the question of what effect BFSM therapy in cardiovascular disease patients will have on their depression. Of particular importance to this discussion, heart rate variability has been shown to be decreased both in cardiovascular disease and in depression, and BFSM is one treatment that can be used to regulate heart rate variability. Heart rate variability biofeedback has been shown to be useful in treating depression.
Work from Karavidas and colleagues showed that 10 weeks of heart rate variability biofeedback in patients with depression led to significantly improved scores on the Hamilton Depression Scale and the Beck Depression Inventory. Improvement was observed by the fourth week of training, with concurrent increases in the SDNN.13 Siepmann and colleagues also used heart rate variability biofeedback in depressed subjects and demonstrated significant improvement in scores on the Beck Depression Inventory, as well as a concomitant decrease in anxiety.14 In related work, Uhlmann and Fröscher used electroencephalographic biofeedback (also called neurofeedback) in epilepsy patients with depression and measured an increased sense of self control and a decrease in external locus of control; they postulated that biofeedback training provided an important opportunity for success, and thus increased internal control and decreased depression.15
Evidence suggests that BFSM should have an impact on depression in addition to impacting the cardiovascular disease itself, and both should work together to improve quality of life. For this reason we have added a depression inventory to our randomized trial of BFSM in patients who have coronary artery disease, diabetes, or multiple sclerosis.
- McKee MG. Biofeedback: an overview in the context of heart-brain medicine. Cleve Clin J Med 2008; 75(suppl 2):S31–S34.
- Frank DL, Khorshid L, Kiffer JF, Moravec CS, McKee MG. Biofeedback in medicine: who, when, why and how? Ment Health Fam Med 2010; 7:85–91.
- Moravec CS. Biofeedback therapy in cardiovascular disease: rationale and research overview. Cleve Clin J Med 2008; 75(suppl 2):S35–S38.
- McKee MG, Moravec CS. Biofeedback in the treatment of heart failure. Cleve Clin J Med 2010; 77(supp 3): S56–S59.
- Emani S, Binkley PF. Mind-body medicine in chronic heart failure: a translational science challenge. Circ Heart Fail 2010; 3:715–725.
- Ogletree-Hughes ML, Stull LB, Sweet WE, Smedira NG, McCarthy PM, Moravec CS. Mechanical unloading restores beta-adrenergic responsiveness and reverses receptor downregulation in the failing human heart. Circulation 2001; 104:881–886.
- Ogletree ML, Sweet WE, Talerico C, et al. Duration of left ventricular assist device support: effects on abnormal calcium cycling and functional recovery in the failing human heart. J Heart Lung Transplant 2010; 29:554–561.
- Moser DK, Dracup K, Woo MA, Stevenson LW. Voluntary control of vascular tone by using skin-temperature biofeedback-relaxation in patients with advanced heart failure. Altern Ther Health Med 1997; 3:51–59.
- Bernardi L, Porta C, Spicuzza L, et al. Slow breathing increases arterial baroreflex sensitivity in patients with chronic heart failure. Circulation 2002; 105:143–145.
- Swanson KS, Gevirtz RN, Brown M, Spira J, Guarneri E, Stoletniy L. The effect of biofeedback on function in patients with heart failure. Appl Psychophysiol Biofeedback 2009; 34:71–91.
- Tracey KJ. The inflammatory reflex. Nature 2002; 420:853–859.
- Tracey KJ. Reflex control of immunity. Nat Rev Immunol 2009; 9:418–428.
- Karavidas MK, Lehrer PM, Vaschillo E, et al. Preliminary results of an open label study of heart rate variability biofeedback for the treatment of major depression. Appl Psychophysiol Biofeedback 2007; 32:19–30.
- Siepmann M, Aykac V, Unterdörfer J, Petrowski K, Mueck-Weymann M. A pilot study on the effects of heart rate variability biofeedback in patients with depression and in healthy subjects. Appl Psychophysiol Biofeedback 2008; 33:195–201.
- Uhlmann C, Fröscher W. Biofeedback treatment in patients with refractory epilepsy: changes in depression and control orientation. Seizure 2001; 10:34–38.
BIOFEEDBACK: WHAT IS IT?
The term “biofeedback” refers to the instrumentation or training process that allows biologic information to be recorded, displayed, and communicated back to an individual, allowing the individual to make adjustments in physiologic processes that may enhance health or performance. The biofeedback display is analogous to a mirror, in which physiologic processes can be observed and adjusted much as one might adjust a hairstyle or a tie.
In our work with cardiovascular disease patients, biofeedback is a training process that involves a subject or patient, a biofeedback coach or therapist, and state-of-the-art biofeedback equipment. For biofeedback training to be effective, the subject who is trying to learn the skill must be engaged and willing to practice, the coach must be trained in psychophysiology, and the equipment must display accurate readings in real time, allowing the subject to monitor and change physiologic reactions appropriately. The coach teaches the subject about the physiologic parameters, establishes target ranges, and helps the subject learn how to move the physiologic parameters in the right direction.1,2
Training often begins with a session in which a brief mental stress test is followed by a period of relaxation while physiologic parameters are recorded and displayed. This process helps the subject to understand the link between mental processes and physiologic arousal.
Biofeedback training can involve a number of physiologic modalities, including those that reflect autonomic nervous system arousal, such as skin conductance and heart rate variability, and those that are not strictly correlated with autonomic activity, such as surface muscle tension. Each physiologic parameter is recorded by a specific sensor, and all sensors are noninvasive. Sensors feed signals into a computer, where they are processed and amplified, and subjects are able to view the output on a computer screen.
Typically, in our work, there is one screen for the subject, on which a single parameter can be displayed, observed and discussed, and another screen for the coach, on which all parameters are displayed simultaneously. During a single session of biofeedback training, the coach may choose to work on a single parameter or switch between parameters, depending on how much progress is being made with each. In our work with patients, we generally train to simple parameters first, such as respiratory rate, finger temperature, and skin conductance, moving on to surface muscle tension, heart rate, and eventually heart rate variability, which is a more complex concept and more easily understood later in the training process.
It is important that the subject receive positive reinforcement for changing the physiologic parameters, and if the subject struggles too long with one parameter, it is generally useful to go back to a different parameter, where success may be more easily experienced. Ideally, by the end of six to eight training sessions, the subject will be able to make progress on all physiologic parameters, which will track together over time.
BIOFEEDBACK-ASSISTED STRESS MANAGEMENT
Pure biofeedback training consists of operant conditioning. That is, the subject learns to regulate his or her physiology in the right direction because of the feedback, which can be as simple as a pleasant image appearing on a computer screen or as complicated as a car moving faster around a racetrack; pure biofeedback involves changing physiology in response to positive reinforcement of some sort.
In practice, we generally employ biofeedback-assisted stress management (BFSM) rather than pure biofeedback. With BFSM, the subject learns to change physiology in the direction of health and wellness by learning techniques of stress management. The coach teaches the subject various relaxation techniques, such as slow and rhythmic breathing, guided imagery, progressive muscle relaxation, mindfulness, assertiveness, and how to change negative thought patterns. With regular practice, the subject learns to change the physiologic parameters by relaxing the body. For example, instead of instructing the subject to “increase your finger temperature” and assume that the subject will achieve this because doing so will make the light bulb on the screen glow more intensely, the BFSM coach may instead talk with the subject about eliminating stressful thoughts, learning to relax, and the fingers warming in response to the body relaxing.
We distinguish between techniques of stress management, some of which are mentioned above, and psychotherapy, which can certainly be effectively combined with biofeedback, but which we do not provide in our research studies. Coupling stress management techniques with biofeedback helps the subject change physiologic parameters in the direction of wellness and acquire tools that can be used in everyday life when stressful events arise. The objective of BFSM training is not just to change physiology, but also to change the way subjects respond to stressful events in daily life; ie, react to fewer events, react less intensely when they do react, and recover more quickly.
BIOFEEDBACK-ASSISTED STRESS MANAGEMENT IN CARDIOVASCULAR DISEASE
We are currently studying the effects of BFSM in patients with cardiovascular disease, including both heart failure and stable coronary artery disease. Patients with cardiovascular disease often are functionally limited, and they also experience psychologic distress related to physical limitations and other life stressors. Both the physical limitations and the psychologic distress impact quality of life. We hypothesize that BFSM will teach our patients techniques of stress management, both mental and physiologic, that will help relieve their psychologic distress and improve their quality of life. BFSM will also potentially decrease the overactivation of the sympathetic branch of the autonomic nervous system, which is common in cardiovascular disease, and correspondingly upregulate the contribution of the parasympathetic branch of the autonomic nervous system, which should be beneficial.3
A PROMISING TECHNIQUE IN HEART FAILURE
We are currently studying the effects of BFSM in patients with end-stage heart failure who are awaiting heart transplant at Cleveland Clinic.4 As noted in a recent review, biofeedback is a promising technique in heart failure that patients may be able to use to consciously regulate their autonomic nervous systems.5 We hypothesize that BFSM training will interfere with the overactivation of the sympathetic nervous system that is characteristic of heart failure, and that this will reverse the cellular and molecular remodeling that occurs in the failing human heart.
To date, we have enrolled 25 patients; 10 are being studied in our National Institutes of Health–funded Clinic Research Unit and 15 are inpatients. All 25 patients are listed as heart transplant candidates and have given consent for us to study their hearts when they are explanted.
Each patient receives eight sessions with a certified biofeedback therapist. The first and last sessions include mental stress tests, while the remaining six are BFSM training sessions. Patients are assessed at the beginning and end of the study using the 6-minute walk test, the Kansas City Cardiomyopathy Questionnaire, the Medical Outcomes Study 36-Item Short-Form Health Survey (SF-36), and measurement of plasma catecholamines.
The primary end point of the study is the measurement of cellular and molecular markers that have been shown to be altered in the failing human heart, testing the hypothesis that these markers will be reversed in the direction of normal in the BFSM therapy group. These markers are measured in the explanted failing heart when the patient receives a heart transplant.
It is too early to report the results of this study, since only seven patients have undergone transplantation to date. We are encouraged by several early findings, however, and hope these will be validated when the entire group is analyzed.
In early analysis, scores on the Kansas City Cardiomyopathy Questionnaire are improved in the last session compared with the first; patients have shown the ability to learn a slower breathing rate; and they are able to regulate their heart rate variability, as measured by the standard deviation of the N-to-N interval, or SDNN. Most important, measurements in the first seven hearts indicate that there is a degree of biologic remodeling of the failing heart after BFSM that is similar to what we have observed with left ventricular assist devices—hemodynamic pumps that take on the workload of the heart, permitting the heart to rest and recover while the patient is waiting for a transplant.6,7 If BFSM could produce changes in the cellular and molecular properties of the heart that are equal in magnitude to those produced by a mechanical pump, this would be a revolutionary finding in the field of heart-brain medicine.
It should be noted that we are not the first group to study BFSM in patients with heart failure. Moser and colleagues first observed that a single session of skin temperature biofeedback could have significant functional effects in patients with heart failure.8 Bernardi and coworkers showed that merely teaching patients to breathe six times per minute (a large component of BFSM training) improved oxygen saturation and exercise tolerance.9 Swanson and colleagues in 2009 demonstrated that patients with heart failure were able to regulate their heart rate variability, although they observed this only in patients with a left ventricular ejection fraction greater than 30%.10 Our preliminary data demonstrate regulation of heart rate variability in patients with lower ejection fractions, which is promising, but we have also added the biologic component of studying the explanted heart, allowing us to test the hypothesis that BFSM could potentially impact the remodeling process and thus have important therapeutic implications.
TRIAL UNDER WAY IN CORONARY ARTERY DISEASE
In addition to our studies of BFSM in heart failure, we have begun a randomized clinical trial of patients with stable coronary artery disease, type 2 diabetes, or multiple sclerosis. These three patient populations were chosen because evidence from numerous studies suggests that they all involve autonomic nervous system dysregulation as well as an inflammatory process.
It has already been mentioned that BFSM can interfere with overactivation of the sympathetic nervous system and potentially upregulate the contribution of the parasympathetic nervous system, which usually exists in juxtaposition to the sympathetic nervous system. Based on the work of Tracey,11,12 upregulating the parasympathetic nervous system should be antiinflammatory. Thus, we hypothesize that by decreasing both sympathetic nervous system activation and inflammation, BFSM should have an impact on patients with one of these disease states, resulting in improved quality of life and clinical status, reduced anxiety and depression, and changed disease-specific indicators of severity.
We are currently enrolling patients who have coronary artery disease, type 2 diabetes, or multiple sclerosis and randomizing them to groups that will receive either BFSM or usual care. Outcome variables that will be assessed in all patients include heart rate variability; the response of temperature, skin conductance, respiratory rate, and heart rate variability to mental stress; plasma catecholamine levels; plasma C-reactive protein levels; and tumor necrosis factor alpha levels. At the first and last visits, all patients will complete the SF-36, the eight-item Patient Health Questionnaire depression scale (PHQ-8), the Generalized Anxiety Disorder seven-item scale (GAD-7), and a visual analog pain scale. We will also assess disease-specific variables, including heart rate recovery after exercise, plasma lipids, and myeloperoxidase in patients with coronary artery disease; the Multiple Sclerosis Functional Composite (MSFC) test and the Modified Fatigue Impact Scale (MFIS) will be administered to patients with multiple sclerosis; and plasma glucose and hemoglobin A1C will be assessed in patients with type 2 diabetes.
Results of this study will provide data on the potential of BFSM to decrease common markers of autonomic nervous system activation and inflammatory cascades and the effect of those alterations on three specific disease states. To our knowledge, such a randomized study has not been conducted previously; our findings will add significantly to the literature on the mechanism of action of biofeedback-type interventions.
POTENTIAL IMPACT ON DEPRESSION IN CARDIOVASCULAR DISEASE
Depression is increasingly recognized as a component of many cardiovascular diseases; this raises the question of what effect BFSM therapy in cardiovascular disease patients will have on their depression. Of particular importance to this discussion, heart rate variability has been shown to be decreased both in cardiovascular disease and in depression, and BFSM is one treatment that can be used to regulate heart rate variability. Heart rate variability biofeedback has been shown to be useful in treating depression.
Work from Karavidas and colleagues showed that 10 weeks of heart rate variability biofeedback in patients with depression led to significantly improved scores on the Hamilton Depression Scale and the Beck Depression Inventory. Improvement was observed by the fourth week of training, with concurrent increases in the SDNN.13 Siepmann and colleagues also used heart rate variability biofeedback in depressed subjects and demonstrated significant improvement in scores on the Beck Depression Inventory, as well as a concomitant decrease in anxiety.14 In related work, Uhlmann and Fröscher used electroencephalographic biofeedback (also called neurofeedback) in epilepsy patients with depression and measured an increased sense of self control and a decrease in external locus of control; they postulated that biofeedback training provided an important opportunity for success, and thus increased internal control and decreased depression.15
Evidence suggests that BFSM should have an impact on depression in addition to impacting the cardiovascular disease itself, and both should work together to improve quality of life. For this reason we have added a depression inventory to our randomized trial of BFSM in patients who have coronary artery disease, diabetes, or multiple sclerosis.
BIOFEEDBACK: WHAT IS IT?
The term “biofeedback” refers to the instrumentation or training process that allows biologic information to be recorded, displayed, and communicated back to an individual, allowing the individual to make adjustments in physiologic processes that may enhance health or performance. The biofeedback display is analogous to a mirror, in which physiologic processes can be observed and adjusted much as one might adjust a hairstyle or a tie.
In our work with cardiovascular disease patients, biofeedback is a training process that involves a subject or patient, a biofeedback coach or therapist, and state-of-the-art biofeedback equipment. For biofeedback training to be effective, the subject who is trying to learn the skill must be engaged and willing to practice, the coach must be trained in psychophysiology, and the equipment must display accurate readings in real time, allowing the subject to monitor and change physiologic reactions appropriately. The coach teaches the subject about the physiologic parameters, establishes target ranges, and helps the subject learn how to move the physiologic parameters in the right direction.1,2
Training often begins with a session in which a brief mental stress test is followed by a period of relaxation while physiologic parameters are recorded and displayed. This process helps the subject to understand the link between mental processes and physiologic arousal.
Biofeedback training can involve a number of physiologic modalities, including those that reflect autonomic nervous system arousal, such as skin conductance and heart rate variability, and those that are not strictly correlated with autonomic activity, such as surface muscle tension. Each physiologic parameter is recorded by a specific sensor, and all sensors are noninvasive. Sensors feed signals into a computer, where they are processed and amplified, and subjects are able to view the output on a computer screen.
Typically, in our work, there is one screen for the subject, on which a single parameter can be displayed, observed and discussed, and another screen for the coach, on which all parameters are displayed simultaneously. During a single session of biofeedback training, the coach may choose to work on a single parameter or switch between parameters, depending on how much progress is being made with each. In our work with patients, we generally train to simple parameters first, such as respiratory rate, finger temperature, and skin conductance, moving on to surface muscle tension, heart rate, and eventually heart rate variability, which is a more complex concept and more easily understood later in the training process.
It is important that the subject receive positive reinforcement for changing the physiologic parameters, and if the subject struggles too long with one parameter, it is generally useful to go back to a different parameter, where success may be more easily experienced. Ideally, by the end of six to eight training sessions, the subject will be able to make progress on all physiologic parameters, which will track together over time.
BIOFEEDBACK-ASSISTED STRESS MANAGEMENT
Pure biofeedback training consists of operant conditioning. That is, the subject learns to regulate his or her physiology in the right direction because of the feedback, which can be as simple as a pleasant image appearing on a computer screen or as complicated as a car moving faster around a racetrack; pure biofeedback involves changing physiology in response to positive reinforcement of some sort.
In practice, we generally employ biofeedback-assisted stress management (BFSM) rather than pure biofeedback. With BFSM, the subject learns to change physiology in the direction of health and wellness by learning techniques of stress management. The coach teaches the subject various relaxation techniques, such as slow and rhythmic breathing, guided imagery, progressive muscle relaxation, mindfulness, assertiveness, and how to change negative thought patterns. With regular practice, the subject learns to change the physiologic parameters by relaxing the body. For example, instead of instructing the subject to “increase your finger temperature” and assume that the subject will achieve this because doing so will make the light bulb on the screen glow more intensely, the BFSM coach may instead talk with the subject about eliminating stressful thoughts, learning to relax, and the fingers warming in response to the body relaxing.
We distinguish between techniques of stress management, some of which are mentioned above, and psychotherapy, which can certainly be effectively combined with biofeedback, but which we do not provide in our research studies. Coupling stress management techniques with biofeedback helps the subject change physiologic parameters in the direction of wellness and acquire tools that can be used in everyday life when stressful events arise. The objective of BFSM training is not just to change physiology, but also to change the way subjects respond to stressful events in daily life; ie, react to fewer events, react less intensely when they do react, and recover more quickly.
BIOFEEDBACK-ASSISTED STRESS MANAGEMENT IN CARDIOVASCULAR DISEASE
We are currently studying the effects of BFSM in patients with cardiovascular disease, including both heart failure and stable coronary artery disease. Patients with cardiovascular disease often are functionally limited, and they also experience psychologic distress related to physical limitations and other life stressors. Both the physical limitations and the psychologic distress impact quality of life. We hypothesize that BFSM will teach our patients techniques of stress management, both mental and physiologic, that will help relieve their psychologic distress and improve their quality of life. BFSM will also potentially decrease the overactivation of the sympathetic branch of the autonomic nervous system, which is common in cardiovascular disease, and correspondingly upregulate the contribution of the parasympathetic branch of the autonomic nervous system, which should be beneficial.3
A PROMISING TECHNIQUE IN HEART FAILURE
We are currently studying the effects of BFSM in patients with end-stage heart failure who are awaiting heart transplant at Cleveland Clinic.4 As noted in a recent review, biofeedback is a promising technique in heart failure that patients may be able to use to consciously regulate their autonomic nervous systems.5 We hypothesize that BFSM training will interfere with the overactivation of the sympathetic nervous system that is characteristic of heart failure, and that this will reverse the cellular and molecular remodeling that occurs in the failing human heart.
To date, we have enrolled 25 patients; 10 are being studied in our National Institutes of Health–funded Clinic Research Unit and 15 are inpatients. All 25 patients are listed as heart transplant candidates and have given consent for us to study their hearts when they are explanted.
Each patient receives eight sessions with a certified biofeedback therapist. The first and last sessions include mental stress tests, while the remaining six are BFSM training sessions. Patients are assessed at the beginning and end of the study using the 6-minute walk test, the Kansas City Cardiomyopathy Questionnaire, the Medical Outcomes Study 36-Item Short-Form Health Survey (SF-36), and measurement of plasma catecholamines.
The primary end point of the study is the measurement of cellular and molecular markers that have been shown to be altered in the failing human heart, testing the hypothesis that these markers will be reversed in the direction of normal in the BFSM therapy group. These markers are measured in the explanted failing heart when the patient receives a heart transplant.
It is too early to report the results of this study, since only seven patients have undergone transplantation to date. We are encouraged by several early findings, however, and hope these will be validated when the entire group is analyzed.
In early analysis, scores on the Kansas City Cardiomyopathy Questionnaire are improved in the last session compared with the first; patients have shown the ability to learn a slower breathing rate; and they are able to regulate their heart rate variability, as measured by the standard deviation of the N-to-N interval, or SDNN. Most important, measurements in the first seven hearts indicate that there is a degree of biologic remodeling of the failing heart after BFSM that is similar to what we have observed with left ventricular assist devices—hemodynamic pumps that take on the workload of the heart, permitting the heart to rest and recover while the patient is waiting for a transplant.6,7 If BFSM could produce changes in the cellular and molecular properties of the heart that are equal in magnitude to those produced by a mechanical pump, this would be a revolutionary finding in the field of heart-brain medicine.
It should be noted that we are not the first group to study BFSM in patients with heart failure. Moser and colleagues first observed that a single session of skin temperature biofeedback could have significant functional effects in patients with heart failure.8 Bernardi and coworkers showed that merely teaching patients to breathe six times per minute (a large component of BFSM training) improved oxygen saturation and exercise tolerance.9 Swanson and colleagues in 2009 demonstrated that patients with heart failure were able to regulate their heart rate variability, although they observed this only in patients with a left ventricular ejection fraction greater than 30%.10 Our preliminary data demonstrate regulation of heart rate variability in patients with lower ejection fractions, which is promising, but we have also added the biologic component of studying the explanted heart, allowing us to test the hypothesis that BFSM could potentially impact the remodeling process and thus have important therapeutic implications.
TRIAL UNDER WAY IN CORONARY ARTERY DISEASE
In addition to our studies of BFSM in heart failure, we have begun a randomized clinical trial of patients with stable coronary artery disease, type 2 diabetes, or multiple sclerosis. These three patient populations were chosen because evidence from numerous studies suggests that they all involve autonomic nervous system dysregulation as well as an inflammatory process.
It has already been mentioned that BFSM can interfere with overactivation of the sympathetic nervous system and potentially upregulate the contribution of the parasympathetic nervous system, which usually exists in juxtaposition to the sympathetic nervous system. Based on the work of Tracey,11,12 upregulating the parasympathetic nervous system should be antiinflammatory. Thus, we hypothesize that by decreasing both sympathetic nervous system activation and inflammation, BFSM should have an impact on patients with one of these disease states, resulting in improved quality of life and clinical status, reduced anxiety and depression, and changed disease-specific indicators of severity.
We are currently enrolling patients who have coronary artery disease, type 2 diabetes, or multiple sclerosis and randomizing them to groups that will receive either BFSM or usual care. Outcome variables that will be assessed in all patients include heart rate variability; the response of temperature, skin conductance, respiratory rate, and heart rate variability to mental stress; plasma catecholamine levels; plasma C-reactive protein levels; and tumor necrosis factor alpha levels. At the first and last visits, all patients will complete the SF-36, the eight-item Patient Health Questionnaire depression scale (PHQ-8), the Generalized Anxiety Disorder seven-item scale (GAD-7), and a visual analog pain scale. We will also assess disease-specific variables, including heart rate recovery after exercise, plasma lipids, and myeloperoxidase in patients with coronary artery disease; the Multiple Sclerosis Functional Composite (MSFC) test and the Modified Fatigue Impact Scale (MFIS) will be administered to patients with multiple sclerosis; and plasma glucose and hemoglobin A1C will be assessed in patients with type 2 diabetes.
Results of this study will provide data on the potential of BFSM to decrease common markers of autonomic nervous system activation and inflammatory cascades and the effect of those alterations on three specific disease states. To our knowledge, such a randomized study has not been conducted previously; our findings will add significantly to the literature on the mechanism of action of biofeedback-type interventions.
POTENTIAL IMPACT ON DEPRESSION IN CARDIOVASCULAR DISEASE
Depression is increasingly recognized as a component of many cardiovascular diseases; this raises the question of what effect BFSM therapy in cardiovascular disease patients will have on their depression. Of particular importance to this discussion, heart rate variability has been shown to be decreased both in cardiovascular disease and in depression, and BFSM is one treatment that can be used to regulate heart rate variability. Heart rate variability biofeedback has been shown to be useful in treating depression.
Work from Karavidas and colleagues showed that 10 weeks of heart rate variability biofeedback in patients with depression led to significantly improved scores on the Hamilton Depression Scale and the Beck Depression Inventory. Improvement was observed by the fourth week of training, with concurrent increases in the SDNN.13 Siepmann and colleagues also used heart rate variability biofeedback in depressed subjects and demonstrated significant improvement in scores on the Beck Depression Inventory, as well as a concomitant decrease in anxiety.14 In related work, Uhlmann and Fröscher used electroencephalographic biofeedback (also called neurofeedback) in epilepsy patients with depression and measured an increased sense of self control and a decrease in external locus of control; they postulated that biofeedback training provided an important opportunity for success, and thus increased internal control and decreased depression.15
Evidence suggests that BFSM should have an impact on depression in addition to impacting the cardiovascular disease itself, and both should work together to improve quality of life. For this reason we have added a depression inventory to our randomized trial of BFSM in patients who have coronary artery disease, diabetes, or multiple sclerosis.
- McKee MG. Biofeedback: an overview in the context of heart-brain medicine. Cleve Clin J Med 2008; 75(suppl 2):S31–S34.
- Frank DL, Khorshid L, Kiffer JF, Moravec CS, McKee MG. Biofeedback in medicine: who, when, why and how? Ment Health Fam Med 2010; 7:85–91.
- Moravec CS. Biofeedback therapy in cardiovascular disease: rationale and research overview. Cleve Clin J Med 2008; 75(suppl 2):S35–S38.
- McKee MG, Moravec CS. Biofeedback in the treatment of heart failure. Cleve Clin J Med 2010; 77(supp 3): S56–S59.
- Emani S, Binkley PF. Mind-body medicine in chronic heart failure: a translational science challenge. Circ Heart Fail 2010; 3:715–725.
- Ogletree-Hughes ML, Stull LB, Sweet WE, Smedira NG, McCarthy PM, Moravec CS. Mechanical unloading restores beta-adrenergic responsiveness and reverses receptor downregulation in the failing human heart. Circulation 2001; 104:881–886.
- Ogletree ML, Sweet WE, Talerico C, et al. Duration of left ventricular assist device support: effects on abnormal calcium cycling and functional recovery in the failing human heart. J Heart Lung Transplant 2010; 29:554–561.
- Moser DK, Dracup K, Woo MA, Stevenson LW. Voluntary control of vascular tone by using skin-temperature biofeedback-relaxation in patients with advanced heart failure. Altern Ther Health Med 1997; 3:51–59.
- Bernardi L, Porta C, Spicuzza L, et al. Slow breathing increases arterial baroreflex sensitivity in patients with chronic heart failure. Circulation 2002; 105:143–145.
- Swanson KS, Gevirtz RN, Brown M, Spira J, Guarneri E, Stoletniy L. The effect of biofeedback on function in patients with heart failure. Appl Psychophysiol Biofeedback 2009; 34:71–91.
- Tracey KJ. The inflammatory reflex. Nature 2002; 420:853–859.
- Tracey KJ. Reflex control of immunity. Nat Rev Immunol 2009; 9:418–428.
- Karavidas MK, Lehrer PM, Vaschillo E, et al. Preliminary results of an open label study of heart rate variability biofeedback for the treatment of major depression. Appl Psychophysiol Biofeedback 2007; 32:19–30.
- Siepmann M, Aykac V, Unterdörfer J, Petrowski K, Mueck-Weymann M. A pilot study on the effects of heart rate variability biofeedback in patients with depression and in healthy subjects. Appl Psychophysiol Biofeedback 2008; 33:195–201.
- Uhlmann C, Fröscher W. Biofeedback treatment in patients with refractory epilepsy: changes in depression and control orientation. Seizure 2001; 10:34–38.
- McKee MG. Biofeedback: an overview in the context of heart-brain medicine. Cleve Clin J Med 2008; 75(suppl 2):S31–S34.
- Frank DL, Khorshid L, Kiffer JF, Moravec CS, McKee MG. Biofeedback in medicine: who, when, why and how? Ment Health Fam Med 2010; 7:85–91.
- Moravec CS. Biofeedback therapy in cardiovascular disease: rationale and research overview. Cleve Clin J Med 2008; 75(suppl 2):S35–S38.
- McKee MG, Moravec CS. Biofeedback in the treatment of heart failure. Cleve Clin J Med 2010; 77(supp 3): S56–S59.
- Emani S, Binkley PF. Mind-body medicine in chronic heart failure: a translational science challenge. Circ Heart Fail 2010; 3:715–725.
- Ogletree-Hughes ML, Stull LB, Sweet WE, Smedira NG, McCarthy PM, Moravec CS. Mechanical unloading restores beta-adrenergic responsiveness and reverses receptor downregulation in the failing human heart. Circulation 2001; 104:881–886.
- Ogletree ML, Sweet WE, Talerico C, et al. Duration of left ventricular assist device support: effects on abnormal calcium cycling and functional recovery in the failing human heart. J Heart Lung Transplant 2010; 29:554–561.
- Moser DK, Dracup K, Woo MA, Stevenson LW. Voluntary control of vascular tone by using skin-temperature biofeedback-relaxation in patients with advanced heart failure. Altern Ther Health Med 1997; 3:51–59.
- Bernardi L, Porta C, Spicuzza L, et al. Slow breathing increases arterial baroreflex sensitivity in patients with chronic heart failure. Circulation 2002; 105:143–145.
- Swanson KS, Gevirtz RN, Brown M, Spira J, Guarneri E, Stoletniy L. The effect of biofeedback on function in patients with heart failure. Appl Psychophysiol Biofeedback 2009; 34:71–91.
- Tracey KJ. The inflammatory reflex. Nature 2002; 420:853–859.
- Tracey KJ. Reflex control of immunity. Nat Rev Immunol 2009; 9:418–428.
- Karavidas MK, Lehrer PM, Vaschillo E, et al. Preliminary results of an open label study of heart rate variability biofeedback for the treatment of major depression. Appl Psychophysiol Biofeedback 2007; 32:19–30.
- Siepmann M, Aykac V, Unterdörfer J, Petrowski K, Mueck-Weymann M. A pilot study on the effects of heart rate variability biofeedback in patients with depression and in healthy subjects. Appl Psychophysiol Biofeedback 2008; 33:195–201.
- Uhlmann C, Fröscher W. Biofeedback treatment in patients with refractory epilepsy: changes in depression and control orientation. Seizure 2001; 10:34–38.
Biofeedback in coronary artery disease, type 2 diabetes, and multiple sclerosis
Biofeedback in heart failure patients awaiting transplantation
Biofeedback in the treatment of heart failure
BIOFEEDBACK: AN OVERVIEW
Biofeedback is a self-regulation therapy that aims to teach individuals the skills that will allow them to change their physiology in healthy directions.1–3 Biofeedback involves a client, a trained biofeedback coach, and appropriate instrumentation. Sensors are connected to the client, and various physiologic parameters (such as heart rate, blood pressure, and digital peripheral temperature) are displayed on a computer screen. The client is guided through a brief mental stress test and a relaxation exercise to learn to recognize differences between hyperarousal and a more relaxed physiology. Biofeedback training involves a series of sessions in which the goal is to help the client gain control of his or her own physiology by learning relaxation techniques such as deep breathing, progressive muscle relaxation, and guided imagery.1,3,4 Although biofeedback can be used solely as operant conditioning, it is more commonly and more effectively combined with techniques of stress management.
Biofeedback training is commonly (although not exclusively) used to decrease activation of the sympathetic branch of the autonomic nervous system (the “fight or flight” response). The reduction in sympathetic nervous system (SNS) activity is manifest as an increase in digital peripheral temperature and decreases in skin conductance, heart rate, and blood pressure, as well as changes in the frequency distribution of heart rate variability. While the SNS is becoming less activated, the parasympathetic portion of the autonomic nervous system (“rest and digest”) is becoming more involved in regulating body functions. More parasympathetic nervous system (PNS) activation and less SNS activation produces a healthier physiologic state, and thus biofeedback can be used to move the body in the direction of health and wellness.1–4
HEART FAILURE: BIOLOGIC MECHANISMS OF INJURY
Heart failure is the end result of most untreated cardiovascular diseases. Heart failure involves inadequate cardiac pump function, such that appropriate perfusion of end organs does not occur. The process of developing heart failure is a gradual one that begins with compensatory processes. In response to an injury or insult, such as chronic high blood pressure or long-standing coronary artery blockage, the heart compensates by activating various neurohormonal pathways in an attempt to preserve cardiac function and end-organ perfusion.5 When these pathways are activated, they initially help the heart to compensate for the ongoing challenge of increased pressure or decreased tissue oxygenation and allow the cardiovascular system to pump sufficient blood. Over time, however, these compensatory processes become maladaptive. Cellular signaling pathways, which were activated in order to help the heart compensate, actually become as much of a problem as the decreased cardiac function.5
Hyperactivation of the SNS
Chief among these pathways is the SNS, which is the most powerful means by which cardiac function can be augmented.5 In response to decreased cardiac function, cardiac sympathetic nerves are activated, releasing norepinephrine locally, and both norepinephrine and epinephrine increase in the circulating blood. Beta-adrenergic receptors on cardiac myocytes and on vascular smooth muscle cells are stimulated, and the resulting augmentation of cardiac contraction helps the heart to overcome an immediate challenge. If the insult or injury to the heart is acute and time-limited, this system compensates and the situation is resolved. However, chronic activation of the SNS creates more problems than it solves for the failing heart,5,6 including the following:
- Myocardial cells are challenged by the need for increased energy production to support the chronic stimulation
- Oxidative stress ensues
- Receptors are downregulated
- Pathways that result in necrosis and apoptosis are activated
- Myofilament proteins respond to chronically elevated intracellular calcium.
As a result, the heart begins to spiral more quickly into a decompensated state. The toxicity of SNS overactivation is the reason for the success of beta-adrenergic blocking drugs in treating heart failure, but this situation is complicated further by adrenergic receptor polymorphisms and nonhomogeneous responses to beta-blocking agents.6 It is safe to say that the goal of much heart failure therapy is inactivation of the once-compensatory SNS and its resulting biologic effects.
Hypoactivation of the PNS
In addition to hyperactivation of the SNS, heart failure is also accompanied by a decrease in the role of the PNS. Under normal resting conditions, the human heart is governed more by the PNS than the SNS, with the SNS becoming a major source of cardiac control only during periods of decreased cardiac function. In heart failure, however, this relationship is reversed, with the SNS taking over the governing role and PNS input becoming less significant. Studies have suggested that the lack of contribution of the PNS to cardiac regulation in heart failure may be as deleterious as overactivation of the SNS.7 Most recently, stimulation of the vagal nerve has been shown to be beneficial in both animal models8 and humans with heart failure,9 confirming that augmenting PNS activity may be as important as inhibiting SNS activity. Although vagal nerve stimulation may be the first heart failure therapy aimed specifically at the PNS, it is likely that the future will hold more therapies with this goal.
PNS as regulator of inflammation of the failing heart?
It has recently been suggested that beyond its role in regulating cardiac function under baseline conditions, the PNS may participate in regulating the inflammatory state of the failing heart. It has been established since the observations of Packer and colleagues in the early 1990s10 that proinflammatory cytokines such as tumor necrosis factor–alpha, interleukin-6, and interleukin-1 are elevated in the circulation of heart failure patients, that these cytokines are correlated with clinical prognosis, and that they play a role in the activation of deleterious cardiac signaling pathways.11,12 Trials of antiinflammatory therapies in heart failure have been less than successful, but this may be because the complexity of the activation has been underestimated.11 In elegant work reported several years ago, Kevin Tracey’s group showed that stimulation of the vagus nerve could inhibit inflammatory processes associated with sepsis.13,14 Since that time, the reflex activation and inactivation of inflammatory processes by the PNS have become more widely accepted. Although it has not yet been directly demonstrated, it is possible that part of the benefit of vagal nerve stimulation in heart failure will prove to be due to its ability to reduce the chronic inflammatory state of the failing heart.
BIOFEEDBACK IN HEART FAILURE: RATIONALE
The failing heart is characterized by autonomic imbalance (hyperactivation of the SNS and hypoactivation of the PNS) and by a chronic inflammatory state. It has been hypothesized both directly and indirectly that these two major pathophysiologic processes may be intertwined.13–15 Biofeedback-assisted stress management is a therapy that has the potential to interfere with both processes. If the patient with heart failure can be trained to reduce activation of the SNS and to increase control by the PNS, it is likely that the negative consequences of autonomic imbalance will be decreased or possibly even reversed. Whether these effects are limited to quality of life and clinical status, or whether they extend to an effect on myocardial remodeling processes, remains to be established. Since the chronic inflammatory state can also be affected by increasing PNS control of the cardiovascular system, we further hypothesize that biofeedback training may have a direct effect on the inflammatory processes involved in the downward spiral of heart failure.
BIOFEEDBACK IN HEART FAILURE: STUDIES
We are certainly not the first group to hypothesize that self-regulation may have a role in the treatment of cardiovascular diseases in general or heart failure in particular. It has been shown that patients with heart failure manage their disease better and experience less emotional distress when they have a greater sense of control over their condition.16 In addition to giving patients a greater sense of control, some mind-body therapies have been shown to be beneficial in those with heart failure. Pischke et al showed as part of the Multicenter Lifestyle Demonstration Project that patients with left ventricular ejection fractions in the range associated with heart failure (≤ 40%) were able to learn and benefit from stress management techniques equally as well as those with more normal cardiac function.17 Both relaxation training18,19 and meditation20 have been shown to improve quality of life in heart failure patients, but meditation also reduced circulating norepinephrine, a marker of SNS activation.20 Mindfulness training improved clinical symptoms of heart failure and also reduced both anxiety and depression in patients with heart failure.21 Training heart failure patients to breathe more slowly is an intervention that is normally part of biofeedback training, but even when used alone it has resulted in decreased dyspnea,22 increased oxygen saturation,23 and improved exercise tolerance.22,23
To our knowledge, three studies to date have specifically used biofeedback training in patients with documented heart failure. As early as 1997, Moser and colleagues showed that heart failure patients were able to raise their finger temperature in spite of disease-related vascular changes, and that a single session of finger temperature biofeedback resulted in meaningful clinical improvement.24 Luskin et al randomized 33 heart failure patients to either biofeedback-assisted stress management or a control group, and showed improvement with the intervention in perceived stress, emotional distress, exercise tolerance, and depression.25 Most recently, Swanson and colleagues demonstrated improved exercise tolerance after cardiorespiratory biofeedback in patients with higher left ventricular ejection fractions (≥ 31%), although improvement could not be accomplished in those with ejection fractions below 30%.26
ONGOING STUDY IN END-STAGE HEART FAILURE AND FUTURE DIRECTIONS
We are currently involved at Cleveland Clinic in a study of end-stage heart failure patients who are awaiting cardiac transplantation. Each patient is provided with eight sessions of biofeedback training, including respiratory rate, digital peripheral temperature, muscle tension, and heart rate variability. Clinical status, quality of life, and heart failure–specific symptoms are being monitored throughout the training period. Success with biofeedback training is being analyzed, and we are testing the hypothesis that the degree of success in learning self-regulation will predict change in clinical status, quality of life, and the biology of the heart. What is unique to our study is that we will obtain the heart tissue at explant, when the patient receives a cardiac transplant, and we will conduct experiments to determine whether the cellular and molecular phenotype of the heart have been changed by the intervention, particularly components of the SNS, PNS, and inflammatory pathways.
We have been studying human heart failure for many years, and we have previously shown the changes in receptors and signaling pathways that occur in the failing human heart.27–30 We were also among the first to demonstrate that the cellular and molecular changes that occur in the failing human heart are not actually irreversible but can be changed by interventions such as a left ventricular assist device.31–33 Thus we hypothesize that biofeedback training, by interfering with overactivation of the SNS and by allowing the PNS to more adequately contribute to cardiac regulation, will have a meaningful effect on the biology of the failing human heart in addition to improving clinical status and quality of life. We hope to be among the first to demonstrate that effect.
- McKee MG. Biofeedback: an overview in the context of heart-brain medicine. Cleve Clin J Med 2008; 75( suppl 2):S31–S34.
- Moravec CS. Biofeedback therapy in cardiovascular disease: rationale and research overview. Cleve Clin J Med 2008; 75( suppl 2):S35–S38.
- Moss D, McGrady A, Davies TC, Wickramasekera I. Handbook of Mind-Body Medicine for Primary Care. London: Sage Publications; 2003.
- Schwartz MS, Andrasik F. Biofeedback: A Practitioner’s Guide. 3rd ed. New York, NY: Guilford Press; 2003.
- Triposkiadis F, Karayannis G, Giamouzis G, Skoularigis J, Louridas G, Butler J. The sympathetic nervous system in heart failure. J Am Coll Cardiol 2009; 54:1747–1762.
- Floras JS. Sympathetic nervous system activation in human heart failure. J Am Coll Cardiol 2009; 54:375–385.
- Binkley PF, Nunziata E, Haas GJ, Nelson SD, Cody RJ. Parasympathetic withdrawal is an integral component of autonomic imbalance in congestive heart failure: demonstration in human subjects and verification in a paced canine model of ventricular failure. J Am Coll Cardiol 1991; 18:464–472.
- Li M, Zheng C, Sato T, Kawada T, Sugimachi M, Sunugawa K. Vagal nerve stimulation markedly improves long-term survival after chronic heart failure in rats. Circulation 2004; 109:120–124.
- Schwartz PJ, DeFerrari GM, Sanzo A, et al Long term vagal stimulation in patients with advanced heart failure: first experience in man. Eur J Heart Failure 2008; 10:884–891.
- Levine B, Kalman J, Mayer L, Fillit M, Packer M. Elevated circulating levels of tumor necrosis factor in severe chronic heart failure. N Engl J Med 1990; 323:236–241.
- Mann DL. Inflammatory mediators and the failing heart: past, present and the foreseeable future. Circ Res 2002; 91:988–998.
- Parish RC, Evans JD. Inflammation in chronic heart failure. Ann Pharmacother 2008; 42:1002–1016.
- Tracey KJ. The inflammatory reflex. Nature 2002; 420:853–859.
- Tracey KJ. Reflex control of immunity. Nat Rev Immunol 2009; 9:418–428.
- Jankowska EA, Ponikowski P, Piepoli MF, Banasiak W, Anker SD, Poole-Wilson PA. Autonomic imbalance and immune activation in chronic heart failure—pathophysiological links. Cardiovasc Res 2006; 70:434–445.
- Dracup K, Westlake C, Erickson VS, Moser DK, Caldwell ML, Hamilton MA. Perceived control reduces emotional stress in patients with heart failure. J Heart Lung Transplant 2003; 22:90–93.
- Pischke CR, Weidner G, Elliott-Eller M, Ornish D. Lifestyle changes and clinical profile in coronary heart disease patients with an ejection fraction of ≤ 40% or > 40% in the Multicenter Lifestyle Demonstration Project. Eur J Heart Failure 2007; 9:928–934.
- Chang BH, Henricks A, Zhao Y, Rothendler JA, LoCastro JS, Slawsky MT. A relaxation response randomized trial on patients with chronic heart failure. J Cardiopulm Rehab 2005; 25:149–157.
- Yu DSF, Lee DTF, Woo J. Effects of relaxation therapy on psychologic distress and symptom status in older Chinese patients with heart failure. Psychosom Res 2007;427–437.
- Curiati JA, Bocchi E, Freire JO, et al Meditation reduces sympathetic activation and improves the quality of life in elderly patients with optimally treated heart failure: a prospective randomized study. J Altern Complement Med 2005; 11:465–472.
- Sullivan MJ, Wood L, Terry J, et al The support, education and research in chronic heart failure study (SEARCH): a mindfulness-based psychoeducational intervention improves depression and clinical symptoms in patients with chronic heart failure. Am Heart J 2009; 157:84–90.
- Weiner P, Waizman J, Magadle R, Berar-Yanay N, Pelled B. The effect of specific inspiratory muscle training on the sensation of dyspnea and exercise tolerance in patients with congestive heart failure. Clin Cardiol 1999; 22:727–732.
- Bernardi L, Porta C, Spicuzza L, et al Slow breathing increases arterial baroreflex sensitivity in patients with chronic heart failure. Circulation 2002; 105:143–145.
- Moser DK, Dracup K, Woo MA, Stevenson LW. Voluntary control of vascular tone by using skin-temperature biofeedback-relaxation in patients with advanced heart failure. Altern Ther Health Med 1997; 3:51–60.
- Luskin F, Reitz M, Newell K, Quinn TG, Haskell W. A controlled pilot study of stress management training of elderly patients with congestive heart failure. Prev Cardiol 2002; 5:168–172.
- Swanson KS, Gevirtz RN, Brown M, Spira J, Guarneri E, Stoletniy L. The effect of biofeedback on function in patients with heart failure. Appl Psychophysiol Biofeedback 2009; 34:71–91.
- Razeghi P, Mukhopadhyay M, Myers TJ, et al Myocardial tumor necrosis factor alpha expression does not correlate with clinical indices of heart failure in patients on left ventricular assist device support. Ann Thorac Surg 2001; 72:2044–2050.
- Matteo R, Moravec CS. Expression and immunolocalization of annexins IV, V and VI in the failing and non-failing human heart. Cardiovasc Res 2000; 45:961–970.
- Dash R, Frank K, Carr AN, Moravec CS, Kranias EG. Gender influences on sarcoplasmic reticulum calcium handling in the failing human myocardium. J Mol Cell Cardiol 2001; 33:1345–1353.
- DiPaola NR, Sweet WE, Stull LB, Francis GS, Moravec CS. Beta-adrenergic receptors and calcium cycling proteins in normal, hypertrophied and failing human hearts: transition from hypertrophy to failure. J Mol Cell Cardiol 2001; 33:1283–1295.
- Aquila LA, McCarthy PM, Smedira NG, Young JB, Moravec CS. Cytoskeletal structure and recovery in single human cardiac myocytes. J Heart Lung Transplant 2004; 23:954–963.
- Fedak PWM, Moravec CS, McCarthy PM, et al Altered expression of disintegrin metalloproteinases and their inhibitor in human dilated cardiomyopathy. Circulation 2006; 113:238–245.
- Ogletree-Hughes ML, Stull LB, Sweet WE, Smedira NG, McCarthy PM, Moravec CS. Mechanical unloading restores beta adrenergic responsiveness and reverses receptor downregulation in the failing human heart. Circulation 2001; 104:881–886.
BIOFEEDBACK: AN OVERVIEW
Biofeedback is a self-regulation therapy that aims to teach individuals the skills that will allow them to change their physiology in healthy directions.1–3 Biofeedback involves a client, a trained biofeedback coach, and appropriate instrumentation. Sensors are connected to the client, and various physiologic parameters (such as heart rate, blood pressure, and digital peripheral temperature) are displayed on a computer screen. The client is guided through a brief mental stress test and a relaxation exercise to learn to recognize differences between hyperarousal and a more relaxed physiology. Biofeedback training involves a series of sessions in which the goal is to help the client gain control of his or her own physiology by learning relaxation techniques such as deep breathing, progressive muscle relaxation, and guided imagery.1,3,4 Although biofeedback can be used solely as operant conditioning, it is more commonly and more effectively combined with techniques of stress management.
Biofeedback training is commonly (although not exclusively) used to decrease activation of the sympathetic branch of the autonomic nervous system (the “fight or flight” response). The reduction in sympathetic nervous system (SNS) activity is manifest as an increase in digital peripheral temperature and decreases in skin conductance, heart rate, and blood pressure, as well as changes in the frequency distribution of heart rate variability. While the SNS is becoming less activated, the parasympathetic portion of the autonomic nervous system (“rest and digest”) is becoming more involved in regulating body functions. More parasympathetic nervous system (PNS) activation and less SNS activation produces a healthier physiologic state, and thus biofeedback can be used to move the body in the direction of health and wellness.1–4
HEART FAILURE: BIOLOGIC MECHANISMS OF INJURY
Heart failure is the end result of most untreated cardiovascular diseases. Heart failure involves inadequate cardiac pump function, such that appropriate perfusion of end organs does not occur. The process of developing heart failure is a gradual one that begins with compensatory processes. In response to an injury or insult, such as chronic high blood pressure or long-standing coronary artery blockage, the heart compensates by activating various neurohormonal pathways in an attempt to preserve cardiac function and end-organ perfusion.5 When these pathways are activated, they initially help the heart to compensate for the ongoing challenge of increased pressure or decreased tissue oxygenation and allow the cardiovascular system to pump sufficient blood. Over time, however, these compensatory processes become maladaptive. Cellular signaling pathways, which were activated in order to help the heart compensate, actually become as much of a problem as the decreased cardiac function.5
Hyperactivation of the SNS
Chief among these pathways is the SNS, which is the most powerful means by which cardiac function can be augmented.5 In response to decreased cardiac function, cardiac sympathetic nerves are activated, releasing norepinephrine locally, and both norepinephrine and epinephrine increase in the circulating blood. Beta-adrenergic receptors on cardiac myocytes and on vascular smooth muscle cells are stimulated, and the resulting augmentation of cardiac contraction helps the heart to overcome an immediate challenge. If the insult or injury to the heart is acute and time-limited, this system compensates and the situation is resolved. However, chronic activation of the SNS creates more problems than it solves for the failing heart,5,6 including the following:
- Myocardial cells are challenged by the need for increased energy production to support the chronic stimulation
- Oxidative stress ensues
- Receptors are downregulated
- Pathways that result in necrosis and apoptosis are activated
- Myofilament proteins respond to chronically elevated intracellular calcium.
As a result, the heart begins to spiral more quickly into a decompensated state. The toxicity of SNS overactivation is the reason for the success of beta-adrenergic blocking drugs in treating heart failure, but this situation is complicated further by adrenergic receptor polymorphisms and nonhomogeneous responses to beta-blocking agents.6 It is safe to say that the goal of much heart failure therapy is inactivation of the once-compensatory SNS and its resulting biologic effects.
Hypoactivation of the PNS
In addition to hyperactivation of the SNS, heart failure is also accompanied by a decrease in the role of the PNS. Under normal resting conditions, the human heart is governed more by the PNS than the SNS, with the SNS becoming a major source of cardiac control only during periods of decreased cardiac function. In heart failure, however, this relationship is reversed, with the SNS taking over the governing role and PNS input becoming less significant. Studies have suggested that the lack of contribution of the PNS to cardiac regulation in heart failure may be as deleterious as overactivation of the SNS.7 Most recently, stimulation of the vagal nerve has been shown to be beneficial in both animal models8 and humans with heart failure,9 confirming that augmenting PNS activity may be as important as inhibiting SNS activity. Although vagal nerve stimulation may be the first heart failure therapy aimed specifically at the PNS, it is likely that the future will hold more therapies with this goal.
PNS as regulator of inflammation of the failing heart?
It has recently been suggested that beyond its role in regulating cardiac function under baseline conditions, the PNS may participate in regulating the inflammatory state of the failing heart. It has been established since the observations of Packer and colleagues in the early 1990s10 that proinflammatory cytokines such as tumor necrosis factor–alpha, interleukin-6, and interleukin-1 are elevated in the circulation of heart failure patients, that these cytokines are correlated with clinical prognosis, and that they play a role in the activation of deleterious cardiac signaling pathways.11,12 Trials of antiinflammatory therapies in heart failure have been less than successful, but this may be because the complexity of the activation has been underestimated.11 In elegant work reported several years ago, Kevin Tracey’s group showed that stimulation of the vagus nerve could inhibit inflammatory processes associated with sepsis.13,14 Since that time, the reflex activation and inactivation of inflammatory processes by the PNS have become more widely accepted. Although it has not yet been directly demonstrated, it is possible that part of the benefit of vagal nerve stimulation in heart failure will prove to be due to its ability to reduce the chronic inflammatory state of the failing heart.
BIOFEEDBACK IN HEART FAILURE: RATIONALE
The failing heart is characterized by autonomic imbalance (hyperactivation of the SNS and hypoactivation of the PNS) and by a chronic inflammatory state. It has been hypothesized both directly and indirectly that these two major pathophysiologic processes may be intertwined.13–15 Biofeedback-assisted stress management is a therapy that has the potential to interfere with both processes. If the patient with heart failure can be trained to reduce activation of the SNS and to increase control by the PNS, it is likely that the negative consequences of autonomic imbalance will be decreased or possibly even reversed. Whether these effects are limited to quality of life and clinical status, or whether they extend to an effect on myocardial remodeling processes, remains to be established. Since the chronic inflammatory state can also be affected by increasing PNS control of the cardiovascular system, we further hypothesize that biofeedback training may have a direct effect on the inflammatory processes involved in the downward spiral of heart failure.
BIOFEEDBACK IN HEART FAILURE: STUDIES
We are certainly not the first group to hypothesize that self-regulation may have a role in the treatment of cardiovascular diseases in general or heart failure in particular. It has been shown that patients with heart failure manage their disease better and experience less emotional distress when they have a greater sense of control over their condition.16 In addition to giving patients a greater sense of control, some mind-body therapies have been shown to be beneficial in those with heart failure. Pischke et al showed as part of the Multicenter Lifestyle Demonstration Project that patients with left ventricular ejection fractions in the range associated with heart failure (≤ 40%) were able to learn and benefit from stress management techniques equally as well as those with more normal cardiac function.17 Both relaxation training18,19 and meditation20 have been shown to improve quality of life in heart failure patients, but meditation also reduced circulating norepinephrine, a marker of SNS activation.20 Mindfulness training improved clinical symptoms of heart failure and also reduced both anxiety and depression in patients with heart failure.21 Training heart failure patients to breathe more slowly is an intervention that is normally part of biofeedback training, but even when used alone it has resulted in decreased dyspnea,22 increased oxygen saturation,23 and improved exercise tolerance.22,23
To our knowledge, three studies to date have specifically used biofeedback training in patients with documented heart failure. As early as 1997, Moser and colleagues showed that heart failure patients were able to raise their finger temperature in spite of disease-related vascular changes, and that a single session of finger temperature biofeedback resulted in meaningful clinical improvement.24 Luskin et al randomized 33 heart failure patients to either biofeedback-assisted stress management or a control group, and showed improvement with the intervention in perceived stress, emotional distress, exercise tolerance, and depression.25 Most recently, Swanson and colleagues demonstrated improved exercise tolerance after cardiorespiratory biofeedback in patients with higher left ventricular ejection fractions (≥ 31%), although improvement could not be accomplished in those with ejection fractions below 30%.26
ONGOING STUDY IN END-STAGE HEART FAILURE AND FUTURE DIRECTIONS
We are currently involved at Cleveland Clinic in a study of end-stage heart failure patients who are awaiting cardiac transplantation. Each patient is provided with eight sessions of biofeedback training, including respiratory rate, digital peripheral temperature, muscle tension, and heart rate variability. Clinical status, quality of life, and heart failure–specific symptoms are being monitored throughout the training period. Success with biofeedback training is being analyzed, and we are testing the hypothesis that the degree of success in learning self-regulation will predict change in clinical status, quality of life, and the biology of the heart. What is unique to our study is that we will obtain the heart tissue at explant, when the patient receives a cardiac transplant, and we will conduct experiments to determine whether the cellular and molecular phenotype of the heart have been changed by the intervention, particularly components of the SNS, PNS, and inflammatory pathways.
We have been studying human heart failure for many years, and we have previously shown the changes in receptors and signaling pathways that occur in the failing human heart.27–30 We were also among the first to demonstrate that the cellular and molecular changes that occur in the failing human heart are not actually irreversible but can be changed by interventions such as a left ventricular assist device.31–33 Thus we hypothesize that biofeedback training, by interfering with overactivation of the SNS and by allowing the PNS to more adequately contribute to cardiac regulation, will have a meaningful effect on the biology of the failing human heart in addition to improving clinical status and quality of life. We hope to be among the first to demonstrate that effect.
BIOFEEDBACK: AN OVERVIEW
Biofeedback is a self-regulation therapy that aims to teach individuals the skills that will allow them to change their physiology in healthy directions.1–3 Biofeedback involves a client, a trained biofeedback coach, and appropriate instrumentation. Sensors are connected to the client, and various physiologic parameters (such as heart rate, blood pressure, and digital peripheral temperature) are displayed on a computer screen. The client is guided through a brief mental stress test and a relaxation exercise to learn to recognize differences between hyperarousal and a more relaxed physiology. Biofeedback training involves a series of sessions in which the goal is to help the client gain control of his or her own physiology by learning relaxation techniques such as deep breathing, progressive muscle relaxation, and guided imagery.1,3,4 Although biofeedback can be used solely as operant conditioning, it is more commonly and more effectively combined with techniques of stress management.
Biofeedback training is commonly (although not exclusively) used to decrease activation of the sympathetic branch of the autonomic nervous system (the “fight or flight” response). The reduction in sympathetic nervous system (SNS) activity is manifest as an increase in digital peripheral temperature and decreases in skin conductance, heart rate, and blood pressure, as well as changes in the frequency distribution of heart rate variability. While the SNS is becoming less activated, the parasympathetic portion of the autonomic nervous system (“rest and digest”) is becoming more involved in regulating body functions. More parasympathetic nervous system (PNS) activation and less SNS activation produces a healthier physiologic state, and thus biofeedback can be used to move the body in the direction of health and wellness.1–4
HEART FAILURE: BIOLOGIC MECHANISMS OF INJURY
Heart failure is the end result of most untreated cardiovascular diseases. Heart failure involves inadequate cardiac pump function, such that appropriate perfusion of end organs does not occur. The process of developing heart failure is a gradual one that begins with compensatory processes. In response to an injury or insult, such as chronic high blood pressure or long-standing coronary artery blockage, the heart compensates by activating various neurohormonal pathways in an attempt to preserve cardiac function and end-organ perfusion.5 When these pathways are activated, they initially help the heart to compensate for the ongoing challenge of increased pressure or decreased tissue oxygenation and allow the cardiovascular system to pump sufficient blood. Over time, however, these compensatory processes become maladaptive. Cellular signaling pathways, which were activated in order to help the heart compensate, actually become as much of a problem as the decreased cardiac function.5
Hyperactivation of the SNS
Chief among these pathways is the SNS, which is the most powerful means by which cardiac function can be augmented.5 In response to decreased cardiac function, cardiac sympathetic nerves are activated, releasing norepinephrine locally, and both norepinephrine and epinephrine increase in the circulating blood. Beta-adrenergic receptors on cardiac myocytes and on vascular smooth muscle cells are stimulated, and the resulting augmentation of cardiac contraction helps the heart to overcome an immediate challenge. If the insult or injury to the heart is acute and time-limited, this system compensates and the situation is resolved. However, chronic activation of the SNS creates more problems than it solves for the failing heart,5,6 including the following:
- Myocardial cells are challenged by the need for increased energy production to support the chronic stimulation
- Oxidative stress ensues
- Receptors are downregulated
- Pathways that result in necrosis and apoptosis are activated
- Myofilament proteins respond to chronically elevated intracellular calcium.
As a result, the heart begins to spiral more quickly into a decompensated state. The toxicity of SNS overactivation is the reason for the success of beta-adrenergic blocking drugs in treating heart failure, but this situation is complicated further by adrenergic receptor polymorphisms and nonhomogeneous responses to beta-blocking agents.6 It is safe to say that the goal of much heart failure therapy is inactivation of the once-compensatory SNS and its resulting biologic effects.
Hypoactivation of the PNS
In addition to hyperactivation of the SNS, heart failure is also accompanied by a decrease in the role of the PNS. Under normal resting conditions, the human heart is governed more by the PNS than the SNS, with the SNS becoming a major source of cardiac control only during periods of decreased cardiac function. In heart failure, however, this relationship is reversed, with the SNS taking over the governing role and PNS input becoming less significant. Studies have suggested that the lack of contribution of the PNS to cardiac regulation in heart failure may be as deleterious as overactivation of the SNS.7 Most recently, stimulation of the vagal nerve has been shown to be beneficial in both animal models8 and humans with heart failure,9 confirming that augmenting PNS activity may be as important as inhibiting SNS activity. Although vagal nerve stimulation may be the first heart failure therapy aimed specifically at the PNS, it is likely that the future will hold more therapies with this goal.
PNS as regulator of inflammation of the failing heart?
It has recently been suggested that beyond its role in regulating cardiac function under baseline conditions, the PNS may participate in regulating the inflammatory state of the failing heart. It has been established since the observations of Packer and colleagues in the early 1990s10 that proinflammatory cytokines such as tumor necrosis factor–alpha, interleukin-6, and interleukin-1 are elevated in the circulation of heart failure patients, that these cytokines are correlated with clinical prognosis, and that they play a role in the activation of deleterious cardiac signaling pathways.11,12 Trials of antiinflammatory therapies in heart failure have been less than successful, but this may be because the complexity of the activation has been underestimated.11 In elegant work reported several years ago, Kevin Tracey’s group showed that stimulation of the vagus nerve could inhibit inflammatory processes associated with sepsis.13,14 Since that time, the reflex activation and inactivation of inflammatory processes by the PNS have become more widely accepted. Although it has not yet been directly demonstrated, it is possible that part of the benefit of vagal nerve stimulation in heart failure will prove to be due to its ability to reduce the chronic inflammatory state of the failing heart.
BIOFEEDBACK IN HEART FAILURE: RATIONALE
The failing heart is characterized by autonomic imbalance (hyperactivation of the SNS and hypoactivation of the PNS) and by a chronic inflammatory state. It has been hypothesized both directly and indirectly that these two major pathophysiologic processes may be intertwined.13–15 Biofeedback-assisted stress management is a therapy that has the potential to interfere with both processes. If the patient with heart failure can be trained to reduce activation of the SNS and to increase control by the PNS, it is likely that the negative consequences of autonomic imbalance will be decreased or possibly even reversed. Whether these effects are limited to quality of life and clinical status, or whether they extend to an effect on myocardial remodeling processes, remains to be established. Since the chronic inflammatory state can also be affected by increasing PNS control of the cardiovascular system, we further hypothesize that biofeedback training may have a direct effect on the inflammatory processes involved in the downward spiral of heart failure.
BIOFEEDBACK IN HEART FAILURE: STUDIES
We are certainly not the first group to hypothesize that self-regulation may have a role in the treatment of cardiovascular diseases in general or heart failure in particular. It has been shown that patients with heart failure manage their disease better and experience less emotional distress when they have a greater sense of control over their condition.16 In addition to giving patients a greater sense of control, some mind-body therapies have been shown to be beneficial in those with heart failure. Pischke et al showed as part of the Multicenter Lifestyle Demonstration Project that patients with left ventricular ejection fractions in the range associated with heart failure (≤ 40%) were able to learn and benefit from stress management techniques equally as well as those with more normal cardiac function.17 Both relaxation training18,19 and meditation20 have been shown to improve quality of life in heart failure patients, but meditation also reduced circulating norepinephrine, a marker of SNS activation.20 Mindfulness training improved clinical symptoms of heart failure and also reduced both anxiety and depression in patients with heart failure.21 Training heart failure patients to breathe more slowly is an intervention that is normally part of biofeedback training, but even when used alone it has resulted in decreased dyspnea,22 increased oxygen saturation,23 and improved exercise tolerance.22,23
To our knowledge, three studies to date have specifically used biofeedback training in patients with documented heart failure. As early as 1997, Moser and colleagues showed that heart failure patients were able to raise their finger temperature in spite of disease-related vascular changes, and that a single session of finger temperature biofeedback resulted in meaningful clinical improvement.24 Luskin et al randomized 33 heart failure patients to either biofeedback-assisted stress management or a control group, and showed improvement with the intervention in perceived stress, emotional distress, exercise tolerance, and depression.25 Most recently, Swanson and colleagues demonstrated improved exercise tolerance after cardiorespiratory biofeedback in patients with higher left ventricular ejection fractions (≥ 31%), although improvement could not be accomplished in those with ejection fractions below 30%.26
ONGOING STUDY IN END-STAGE HEART FAILURE AND FUTURE DIRECTIONS
We are currently involved at Cleveland Clinic in a study of end-stage heart failure patients who are awaiting cardiac transplantation. Each patient is provided with eight sessions of biofeedback training, including respiratory rate, digital peripheral temperature, muscle tension, and heart rate variability. Clinical status, quality of life, and heart failure–specific symptoms are being monitored throughout the training period. Success with biofeedback training is being analyzed, and we are testing the hypothesis that the degree of success in learning self-regulation will predict change in clinical status, quality of life, and the biology of the heart. What is unique to our study is that we will obtain the heart tissue at explant, when the patient receives a cardiac transplant, and we will conduct experiments to determine whether the cellular and molecular phenotype of the heart have been changed by the intervention, particularly components of the SNS, PNS, and inflammatory pathways.
We have been studying human heart failure for many years, and we have previously shown the changes in receptors and signaling pathways that occur in the failing human heart.27–30 We were also among the first to demonstrate that the cellular and molecular changes that occur in the failing human heart are not actually irreversible but can be changed by interventions such as a left ventricular assist device.31–33 Thus we hypothesize that biofeedback training, by interfering with overactivation of the SNS and by allowing the PNS to more adequately contribute to cardiac regulation, will have a meaningful effect on the biology of the failing human heart in addition to improving clinical status and quality of life. We hope to be among the first to demonstrate that effect.
- McKee MG. Biofeedback: an overview in the context of heart-brain medicine. Cleve Clin J Med 2008; 75( suppl 2):S31–S34.
- Moravec CS. Biofeedback therapy in cardiovascular disease: rationale and research overview. Cleve Clin J Med 2008; 75( suppl 2):S35–S38.
- Moss D, McGrady A, Davies TC, Wickramasekera I. Handbook of Mind-Body Medicine for Primary Care. London: Sage Publications; 2003.
- Schwartz MS, Andrasik F. Biofeedback: A Practitioner’s Guide. 3rd ed. New York, NY: Guilford Press; 2003.
- Triposkiadis F, Karayannis G, Giamouzis G, Skoularigis J, Louridas G, Butler J. The sympathetic nervous system in heart failure. J Am Coll Cardiol 2009; 54:1747–1762.
- Floras JS. Sympathetic nervous system activation in human heart failure. J Am Coll Cardiol 2009; 54:375–385.
- Binkley PF, Nunziata E, Haas GJ, Nelson SD, Cody RJ. Parasympathetic withdrawal is an integral component of autonomic imbalance in congestive heart failure: demonstration in human subjects and verification in a paced canine model of ventricular failure. J Am Coll Cardiol 1991; 18:464–472.
- Li M, Zheng C, Sato T, Kawada T, Sugimachi M, Sunugawa K. Vagal nerve stimulation markedly improves long-term survival after chronic heart failure in rats. Circulation 2004; 109:120–124.
- Schwartz PJ, DeFerrari GM, Sanzo A, et al Long term vagal stimulation in patients with advanced heart failure: first experience in man. Eur J Heart Failure 2008; 10:884–891.
- Levine B, Kalman J, Mayer L, Fillit M, Packer M. Elevated circulating levels of tumor necrosis factor in severe chronic heart failure. N Engl J Med 1990; 323:236–241.
- Mann DL. Inflammatory mediators and the failing heart: past, present and the foreseeable future. Circ Res 2002; 91:988–998.
- Parish RC, Evans JD. Inflammation in chronic heart failure. Ann Pharmacother 2008; 42:1002–1016.
- Tracey KJ. The inflammatory reflex. Nature 2002; 420:853–859.
- Tracey KJ. Reflex control of immunity. Nat Rev Immunol 2009; 9:418–428.
- Jankowska EA, Ponikowski P, Piepoli MF, Banasiak W, Anker SD, Poole-Wilson PA. Autonomic imbalance and immune activation in chronic heart failure—pathophysiological links. Cardiovasc Res 2006; 70:434–445.
- Dracup K, Westlake C, Erickson VS, Moser DK, Caldwell ML, Hamilton MA. Perceived control reduces emotional stress in patients with heart failure. J Heart Lung Transplant 2003; 22:90–93.
- Pischke CR, Weidner G, Elliott-Eller M, Ornish D. Lifestyle changes and clinical profile in coronary heart disease patients with an ejection fraction of ≤ 40% or > 40% in the Multicenter Lifestyle Demonstration Project. Eur J Heart Failure 2007; 9:928–934.
- Chang BH, Henricks A, Zhao Y, Rothendler JA, LoCastro JS, Slawsky MT. A relaxation response randomized trial on patients with chronic heart failure. J Cardiopulm Rehab 2005; 25:149–157.
- Yu DSF, Lee DTF, Woo J. Effects of relaxation therapy on psychologic distress and symptom status in older Chinese patients with heart failure. Psychosom Res 2007;427–437.
- Curiati JA, Bocchi E, Freire JO, et al Meditation reduces sympathetic activation and improves the quality of life in elderly patients with optimally treated heart failure: a prospective randomized study. J Altern Complement Med 2005; 11:465–472.
- Sullivan MJ, Wood L, Terry J, et al The support, education and research in chronic heart failure study (SEARCH): a mindfulness-based psychoeducational intervention improves depression and clinical symptoms in patients with chronic heart failure. Am Heart J 2009; 157:84–90.
- Weiner P, Waizman J, Magadle R, Berar-Yanay N, Pelled B. The effect of specific inspiratory muscle training on the sensation of dyspnea and exercise tolerance in patients with congestive heart failure. Clin Cardiol 1999; 22:727–732.
- Bernardi L, Porta C, Spicuzza L, et al Slow breathing increases arterial baroreflex sensitivity in patients with chronic heart failure. Circulation 2002; 105:143–145.
- Moser DK, Dracup K, Woo MA, Stevenson LW. Voluntary control of vascular tone by using skin-temperature biofeedback-relaxation in patients with advanced heart failure. Altern Ther Health Med 1997; 3:51–60.
- Luskin F, Reitz M, Newell K, Quinn TG, Haskell W. A controlled pilot study of stress management training of elderly patients with congestive heart failure. Prev Cardiol 2002; 5:168–172.
- Swanson KS, Gevirtz RN, Brown M, Spira J, Guarneri E, Stoletniy L. The effect of biofeedback on function in patients with heart failure. Appl Psychophysiol Biofeedback 2009; 34:71–91.
- Razeghi P, Mukhopadhyay M, Myers TJ, et al Myocardial tumor necrosis factor alpha expression does not correlate with clinical indices of heart failure in patients on left ventricular assist device support. Ann Thorac Surg 2001; 72:2044–2050.
- Matteo R, Moravec CS. Expression and immunolocalization of annexins IV, V and VI in the failing and non-failing human heart. Cardiovasc Res 2000; 45:961–970.
- Dash R, Frank K, Carr AN, Moravec CS, Kranias EG. Gender influences on sarcoplasmic reticulum calcium handling in the failing human myocardium. J Mol Cell Cardiol 2001; 33:1345–1353.
- DiPaola NR, Sweet WE, Stull LB, Francis GS, Moravec CS. Beta-adrenergic receptors and calcium cycling proteins in normal, hypertrophied and failing human hearts: transition from hypertrophy to failure. J Mol Cell Cardiol 2001; 33:1283–1295.
- Aquila LA, McCarthy PM, Smedira NG, Young JB, Moravec CS. Cytoskeletal structure and recovery in single human cardiac myocytes. J Heart Lung Transplant 2004; 23:954–963.
- Fedak PWM, Moravec CS, McCarthy PM, et al Altered expression of disintegrin metalloproteinases and their inhibitor in human dilated cardiomyopathy. Circulation 2006; 113:238–245.
- Ogletree-Hughes ML, Stull LB, Sweet WE, Smedira NG, McCarthy PM, Moravec CS. Mechanical unloading restores beta adrenergic responsiveness and reverses receptor downregulation in the failing human heart. Circulation 2001; 104:881–886.
- McKee MG. Biofeedback: an overview in the context of heart-brain medicine. Cleve Clin J Med 2008; 75( suppl 2):S31–S34.
- Moravec CS. Biofeedback therapy in cardiovascular disease: rationale and research overview. Cleve Clin J Med 2008; 75( suppl 2):S35–S38.
- Moss D, McGrady A, Davies TC, Wickramasekera I. Handbook of Mind-Body Medicine for Primary Care. London: Sage Publications; 2003.
- Schwartz MS, Andrasik F. Biofeedback: A Practitioner’s Guide. 3rd ed. New York, NY: Guilford Press; 2003.
- Triposkiadis F, Karayannis G, Giamouzis G, Skoularigis J, Louridas G, Butler J. The sympathetic nervous system in heart failure. J Am Coll Cardiol 2009; 54:1747–1762.
- Floras JS. Sympathetic nervous system activation in human heart failure. J Am Coll Cardiol 2009; 54:375–385.
- Binkley PF, Nunziata E, Haas GJ, Nelson SD, Cody RJ. Parasympathetic withdrawal is an integral component of autonomic imbalance in congestive heart failure: demonstration in human subjects and verification in a paced canine model of ventricular failure. J Am Coll Cardiol 1991; 18:464–472.
- Li M, Zheng C, Sato T, Kawada T, Sugimachi M, Sunugawa K. Vagal nerve stimulation markedly improves long-term survival after chronic heart failure in rats. Circulation 2004; 109:120–124.
- Schwartz PJ, DeFerrari GM, Sanzo A, et al Long term vagal stimulation in patients with advanced heart failure: first experience in man. Eur J Heart Failure 2008; 10:884–891.
- Levine B, Kalman J, Mayer L, Fillit M, Packer M. Elevated circulating levels of tumor necrosis factor in severe chronic heart failure. N Engl J Med 1990; 323:236–241.
- Mann DL. Inflammatory mediators and the failing heart: past, present and the foreseeable future. Circ Res 2002; 91:988–998.
- Parish RC, Evans JD. Inflammation in chronic heart failure. Ann Pharmacother 2008; 42:1002–1016.
- Tracey KJ. The inflammatory reflex. Nature 2002; 420:853–859.
- Tracey KJ. Reflex control of immunity. Nat Rev Immunol 2009; 9:418–428.
- Jankowska EA, Ponikowski P, Piepoli MF, Banasiak W, Anker SD, Poole-Wilson PA. Autonomic imbalance and immune activation in chronic heart failure—pathophysiological links. Cardiovasc Res 2006; 70:434–445.
- Dracup K, Westlake C, Erickson VS, Moser DK, Caldwell ML, Hamilton MA. Perceived control reduces emotional stress in patients with heart failure. J Heart Lung Transplant 2003; 22:90–93.
- Pischke CR, Weidner G, Elliott-Eller M, Ornish D. Lifestyle changes and clinical profile in coronary heart disease patients with an ejection fraction of ≤ 40% or > 40% in the Multicenter Lifestyle Demonstration Project. Eur J Heart Failure 2007; 9:928–934.
- Chang BH, Henricks A, Zhao Y, Rothendler JA, LoCastro JS, Slawsky MT. A relaxation response randomized trial on patients with chronic heart failure. J Cardiopulm Rehab 2005; 25:149–157.
- Yu DSF, Lee DTF, Woo J. Effects of relaxation therapy on psychologic distress and symptom status in older Chinese patients with heart failure. Psychosom Res 2007;427–437.
- Curiati JA, Bocchi E, Freire JO, et al Meditation reduces sympathetic activation and improves the quality of life in elderly patients with optimally treated heart failure: a prospective randomized study. J Altern Complement Med 2005; 11:465–472.
- Sullivan MJ, Wood L, Terry J, et al The support, education and research in chronic heart failure study (SEARCH): a mindfulness-based psychoeducational intervention improves depression and clinical symptoms in patients with chronic heart failure. Am Heart J 2009; 157:84–90.
- Weiner P, Waizman J, Magadle R, Berar-Yanay N, Pelled B. The effect of specific inspiratory muscle training on the sensation of dyspnea and exercise tolerance in patients with congestive heart failure. Clin Cardiol 1999; 22:727–732.
- Bernardi L, Porta C, Spicuzza L, et al Slow breathing increases arterial baroreflex sensitivity in patients with chronic heart failure. Circulation 2002; 105:143–145.
- Moser DK, Dracup K, Woo MA, Stevenson LW. Voluntary control of vascular tone by using skin-temperature biofeedback-relaxation in patients with advanced heart failure. Altern Ther Health Med 1997; 3:51–60.
- Luskin F, Reitz M, Newell K, Quinn TG, Haskell W. A controlled pilot study of stress management training of elderly patients with congestive heart failure. Prev Cardiol 2002; 5:168–172.
- Swanson KS, Gevirtz RN, Brown M, Spira J, Guarneri E, Stoletniy L. The effect of biofeedback on function in patients with heart failure. Appl Psychophysiol Biofeedback 2009; 34:71–91.
- Razeghi P, Mukhopadhyay M, Myers TJ, et al Myocardial tumor necrosis factor alpha expression does not correlate with clinical indices of heart failure in patients on left ventricular assist device support. Ann Thorac Surg 2001; 72:2044–2050.
- Matteo R, Moravec CS. Expression and immunolocalization of annexins IV, V and VI in the failing and non-failing human heart. Cardiovasc Res 2000; 45:961–970.
- Dash R, Frank K, Carr AN, Moravec CS, Kranias EG. Gender influences on sarcoplasmic reticulum calcium handling in the failing human myocardium. J Mol Cell Cardiol 2001; 33:1345–1353.
- DiPaola NR, Sweet WE, Stull LB, Francis GS, Moravec CS. Beta-adrenergic receptors and calcium cycling proteins in normal, hypertrophied and failing human hearts: transition from hypertrophy to failure. J Mol Cell Cardiol 2001; 33:1283–1295.
- Aquila LA, McCarthy PM, Smedira NG, Young JB, Moravec CS. Cytoskeletal structure and recovery in single human cardiac myocytes. J Heart Lung Transplant 2004; 23:954–963.
- Fedak PWM, Moravec CS, McCarthy PM, et al Altered expression of disintegrin metalloproteinases and their inhibitor in human dilated cardiomyopathy. Circulation 2006; 113:238–245.
- Ogletree-Hughes ML, Stull LB, Sweet WE, Smedira NG, McCarthy PM, Moravec CS. Mechanical unloading restores beta adrenergic responsiveness and reverses receptor downregulation in the failing human heart. Circulation 2001; 104:881–886.
Multidisciplinary research in biofeedback
Biofeedback in the treatment of heart failure
Biofeedback-assisted stress management training to reverse myocardial remodeling in patients with end-stage heart failure
Biofeedback therapy in cardiovascular disease: Rationale and research overview
The potential of biofeedback therapies in cardiovascular disease is only recently beginning to be explored in a systematic way. This article reviews the rationale for the use of biofeedback therapy in cardiovascular disease and briefly surveys research on the usefulness of biofeedback for several specific cardiovascular parameters and conditions.
RECOGNIZING THE POTENTIAL OF BIOFEEDBACK IN CARDIOVASCULAR DISEASE
Biofeedback is part of a group of modalities known as “self-regulation therapies,” in which a subject is taught to control the activities of his or her autonomic nervous system. The autonomic nervous system has also been called the “visceral,” “involuntary,” and “automatic” nervous system, which suggests that the physiologic processes governed by this branch of the nervous system are largely beyond conscious control. Until the 1950s, this was largely believed to be true. Physicians and scientists had been convinced that the functions regulated by the sympathetic and parasympathetic branches of the autonomic nervous system, such as digestion, blood pressure, and body temperature, were not amenable to self-regulation.
During the 1950s, however, it became clear that functions of the autonomic nervous system could be controlled by conscious thought and training. Subjects could be taught to correctly perceive and also to control heart rate, blood pressure, skin temperature, and other seemingly involuntary functions. The field of biofeedback and applied psychophysiology became possible with these discoveries and with the advent of technologies capable of measuring physiologic variables with enough sensitivity to detect small changes.
Key role of sympathetic/parasympathetic balance
In cardiovascular medicine, biofeedback has a great deal of therapeutic potential because many diseases of the heart and vasculature involve inappropriate regulation of the autonomic nervous system.
Under normal conditions, the sympathetic branch of the autonomic nervous system serves to augment cardiac function in times of stress, increasing heart rate, contractility, and blood pressure, as well as favoring clotting processes that would be mainly adaptive during the “fight or flight” response. The parasympathetic branch of the autonomic nervous system plays the opposite role during health, exerting a calming influence on cardiovascular function.
Normal cardiovascular function is regulated by a balance between sympathetic and parasympathetic inputs to the heart and blood vessels. Heart rate, for example, is governed by the parasympathetic nervous system under resting conditions, when the intrinsic firing rate of the sinus node is decreased by vagal input. Under stressful conditions, this inhibition is released and sympathetic excitation can increase the heart rate even further. In many pathological cardiac conditions, such as arrhythmias, an imbalance between the two branches of the autonomic nervous system causes at least some of the disease manifestations and often contributes to progression.
Biofeedback as a ‘physiologic beta-blocker’
Another good example is heart failure, where over-activation of the sympathetic nervous system results in many of the phenotypic changes in the myocardium and contributes to the downward spiral from compensatory cardiac hypertrophy to end-stage decompensated failure. The role of sympathetic overactivation in heart failure is clearly evident by the success of beta-adrenergic blocking agents in ameliorating symptoms and delaying disease progression. Given the role of autonomic nervous system dysregulation in cardiovascular diseases, biofeedback therapy has the potential to teach patients a skill that may allow them to decrease activation of their autonomic nervous system, theoretically acting as a “physiologic beta-blocker.”
An adjunct to stress management
The potential of biofeedback to have an impact in the arena of cardiovascular disease has not been well explored. Clinically, biofeedback is often used in the context of stress management programs, but biofeedback is not synonymous with stress management. Stress management programs most commonly involve some type of relaxation training and perhaps cognitive behavioral therapy. Biofeedback can be used to augment relaxation, helping the subject to be more aware of physiologic responses and thus be better able to elicit the relaxation response. Biofeedback can also be used to train subjects to control particular physiologic responses that contribute to symptoms or to disease progression. In cardiovascular disease, although stress management is frequently a component of cardiac rehabilitation programs, the question of whether stress management is more effective with or without biofeedback has not been systematically investigated.
PIONEERING STUDIES OF BIOFEEDBACK IN CARDIOVASCULAR DISEASE
Some of the earliest studies of physiologic regulation using biofeedback were attempted in patients with cardiovascular abnormalities. In 1971, Weiss and Engel reported success in using operant conditioning of heart rate in eight patients with premature ventricular contractions.1 All eight patients were able to achieve some degree of control, and five of the patients were able to decrease the frequency of premature beats, demonstrating increased success over a 21-month follow-up period. Interestingly, use of pharmacologic agents to understand the mechanisms of control suggested that one patient was able to decrease sympathetic control of his heart rate while another increased the parasympathetic influence.
Several years later, Pickering and Gorham reported their work with a single subject, a 31-year-old woman who had a ventricular parasystolic rhythm.2 Using a biofeedback technique, they were able to teach the woman to voluntarily control her heart rate, demonstrating that she could both increase and decrease the rate, avoiding the ranges in which the arrhythmia occurred. In the same year, Benson et al demonstrated that they could teach patients the relaxation response and decrease the incidence of premature ventricular contractions.3 Using Holter monitors for validation, these investigators showed that 4 weeks of relaxation training resulted in 8 of 11 patients being able to control their heart rates sufficiently to have therapeutic impact.
These pioneering studies were very early in the development of the field of biofeedback, but they showed what has been clearly established since—that the input of the autonomic nervous system to the heart can be regulated by biofeedback techniques.
BIOFEEDBACK STUDIES OF SPECIFIC CARDIOVASCULAR PARAMETERS AND DISEASES
A host of parameters for assessment
Many cardiovascular parameters can be used for biofeedback. Commonly these include heart rate, blood pressure, skin temperature, and, more recently, heart rate variability. In each case, the parameter is measured and displayed for the subject, and the subject is taught to make it change in a positive direction through relaxation, thought patterns, imagery, or some combination of techniques. Many times the display of the physiologic parameter and the demonstration that it can be controlled are quite surprising to the subject and lead to an enhanced desire to participate in the therapy.
Heart rate variability: A focus of recent interest
The newest parameter in use, and one that has gained considerable interest in the field of cardiovascular biofeedback, is heart rate variability.4 Heart rate variability refers to the variation within the R-R interval of the electrocardiogram during a fixed cycle. It is associated with adaptiveness of the cardiovascular system, and high variability is believed to be a sign of health. Low variability is associated with a number of disease states. Heart rate variability reflects the balance between sympathetic and parasympathetic input to the heart, and many cardiac disease states have been shown to be associated with low variability. Therapies that increase heart rate variability have been shown to improve prognosis.
On the basis of these observations, heart rate variability biofeedback is used to train patients to increase the variability in their heart rate, using feedback from equipment that records the R-R interval from the electrocardiogram or from blood pulse volume sensors. Patients learn to make the variability greater, primarily by breathing at a resonant frequency, as described by Lehrer et al.5
Several preliminary studies have been conducted with heart rate variability in cardiac patients, but much remains to be understood about its use. In 63 patients with established coronary artery disease, Del Pozo et al showed that six biofeedback sessions coupled with daily practice resulted in significantly increased heart rate variability.6 Similarly, Nolan and colleagues found that five sessions of biofeedback improved symptoms and quality of life in 46 patients with coronary artery disease.7 In 14 patients with heart failure, Luskin et al demonstrated that eight sessions of heart rate variability biofeedback produced reductions in perceived stress and improved function on the 6-minute walk test.8
It remains unclear whether heart rate variability biofeedback has more or less potential than other types of biofeedback in patients with cardiovascular disease, but these preliminary observations suggest that it may be useful in improving symptoms and quality of life.
Biofeedback in hypertension: Despite decades of study, conclusions elusive
Among diseases of the cardiovascular system, biofeedback has been used most frequently in hypertension, where it has been under investigation for more than 30 years, since the early days of biofeedback study.9 The field of biofeedback in hypertension is fraught with difficulties, rendering conclusions about its efficacy difficult.
Biofeedback has been assessed in many different types of hypertension, often within the same study. Essential hypertension and “white coat” hypertension, now known as excessive cardiovascular reactivity, have been most commonly investigated, but with no apparent consensus. The biofeedback techniques used in these studies have ranged from blood pressure biofeedback to electromyography, finger temperature, and skin conductance. More recently, heart rate variability biofeedback has also been used in this population.
In general, biofeedback has been more successful in the treatment of hypertension when respiratory training has been a component of the biofeedback. McGrady has established that certain types of patients with hypertension fare better with biofeedback than others.10 These include patients with higher baseline blood pressure, higher heart rate, cool hands, high electromyographic response, and high plasma renin activity.in short, patients who can be seen to have a high degree of sympathetic arousal.
Blood pressure can be lowered by 6 to 10 mm Hg when biofeedback is effective, which is less of an effect than that observed with most drug therapy for hypertension. Biofeedback does have the advantage, however, of improving overall cardiovascular reactivity and giving the patient a greater sense of control over his or her physical well-being, which may prove valuable in the setting of hypertension. Typically, the most effective interventions for hypertension (and perhaps for cardiovascular disease in general) are individualized for the patient and not protocol-driven. Thus, although biofeedback has potential in hypertension, its efficacy is not proven and systematic trials are lacking.
Biofeedback in heart failure: Targeting sympathetic overactivation
In patients with heart failure, the sympathetic nervous system is overactivated, as noted previously. High levels of plasma norepinephrine correlate with worse prognosis. Decreasing activation of the sympathetic nervous system improves both symptoms and prognosis, as demonstrated in patients taking beta-adrenergic blocking agents or those treated with a left ventricular assist device.
Several studies have suggested that biofeedback may be able to provide a similar reduction in sympathetic nervous system activation in patients with heart failure. Moser and colleagues showed that a single session of skin temperature biofeedback plus relaxation training increased cardiac output in patients with heart failure,11 while studies by Weiner et al,12 Bernardi et al,13 and Mangin et al14 showed that training heart failure patients to breathe more slowly increased their exercise tolerance. Although these studies are preliminary, they support the speculation that if biofeedback can decrease activation of the sympathetic nervous system in patients with heart failure, it may actually cause some degree of remodeling of the failing heart, such as that observed with beta-blockers or left ventricular assist device therapy.
BIOFEEDBACK AND STRESS MANAGEMENT: AN OPPORTUNITY FOR WIDER IMPACT
As mentioned earlier, biofeedback can serve as a component of stress management programs. Biofeedback is often a very effective adjunct to stress management because it teaches the subject to control physiologic reactions that are part of the stress response and gives the subject feedback to suggest that he or she is adequately practicing relaxation. Biofeedback-mediated stress management may actually be the most practical use of biofeedback in the setting of cardiovascular disease because it is easy to practice and can have an effect on large numbers of patients.
Mental stress has been well documented as a significant risk factor for many forms of cardiovascular disease, and stress management programs have been shown to have an impact on disease progression and symptoms. Many studies, including those reported by Sheps et al for the Psychophysiological Investigations of Myocardial Ischemia (PIMI) study,15 have shown that patients who exhibit ischemia in response to a mental stress test have increased mortality from cardiovascular disease. Jiang and colleagues,16 among others, have shown that mental stress predicts cardiac events in patients with lower ejection fractions, and Blumenthal et al17 have repeatedly demonstrated that stress management training reduces the incidence of wall motion abnormalities in patients with cardiovascular disease. Stress management is included in many cardiac rehabilitation programs, and it is likely that routine use of biofeedback as a component of stress management programs would benefit patients with cardiovascular disease, in whom reproducibly decreasing activation of the autonomic nervous system should be helpful.
According to a recent article in the Heart Advisor, 84% of physicians believe that stress is a risk for cardiovascular disease but only 35% say they feel knowledgeable about stress and a mere 5% feel that they succeed in helping stressed patients.18 Anything that could improve these numbers would be beneficial.
CONCLUSIONS
Cardiovascular conditions in which biofeedback has been shown to be helpful include arrhythmias, hypertension, Raynaud phenomenon, ischemia, infarction, and heart failure, but we have barely begun to explore the potential of biofeedback therapy. Given that many cardiovascular diseases involve inappropriate regulation of the autonomic nervous system, instruction in the use of biofeedback to control activation of the sympathetic and parasympathetic nervous systems is likely to be useful in cardiac patients. Systematic trials are needed.
- Weiss T, Engel BT. Operant conditioning of heart rate in patients with premature ventricular contractions. Psychosom Med 1971; 33:301–322.
- Pickering T, Gorham G. Learned heart-rate control by a patient with a ventricular parasystolic rhythm. Lancet 1975; 1:252–253.
- Benson H, Alexander S, Feldman CL. Decreased premature ventricular contractions through use of the relaxation response in patients with stable ischaemic heart disease. Lancet 1975; 2:380–382.
- Kranitz L, Lehrer P. Biofeedback applications in the treatment of cardiovascular diseases. Cardiol Rev 2004; 12:177–181.
- Lehrer PM, Vaschilllo E, Vaschillo B. Resonant frequency biofeedback training to increase cardiac variability: rationale and manual for training. Appl Psychophysiol Biofeedback 2000; 25:177–191.
- Del Pozo JM, Gevirtz RN, Scher B, Guarneri E. Biofeedback treatment increases heart rate variability in patients with known coronary artery disease. Am Heart J 2004; 147:E11.
- Nolan RP, Kamath MV, Floras JS, et al. Heart rate variability biofeedback as a behavioral neurocardiac intervention to enhance vagal heart rate control. Am Heart J 2005; 149:1137.
- Luskin F, Reitz M, Newell K, Quinn TG, Haskell W. A controlled pilot study of stress management training of elderly patients with congestive heart failure. Prev Cardiol 2002; 5:168–172.
- Linden W, Moseley JV. The efficacy of behavioral treatments for hypertension. Appl Psychophysiol Biofeedback 2006; 31:51–63.
- McGrady A. Good news.bad press: applied psychophysiology in cardiovascular disorders. Biofeedback Self Regul 1996; 21:335–346.
- Moser DK, Dracup K, Woo MA, Stevenson LW. Voluntary control of vascular tone by using skin-temperature biofeedback-relaxation in patients with advanced heart failure. Altern Ther Health Med 1997; 3:51–59.
- Weiner P, Waizman J, Magadle R, Berar-Yanay N, Pelled B. The effect of specific inspiratory muscle training on the sensation of dyspnea and exercise tolerance in patients with congestive heart failure. Clin Cardiol 1999; 22:727–732.
- Bernardi L, Porta C, Spicuzza L, et al. Slow breathing increases arterial baroreflex sensitivity in patients with chronic heart failure. Circulation 2002; 105:143–145.
- Mangin L, Monti A, Médigue C, et al. Altered baroreflex gain during voluntary breathing in chronic heart failure. Eur J Heart Fail 2001; 3:189–195.
- Sheps DS, McMahon RP, Becker L, et al. Mental stress-induced ischemia and all-cause mortality in patients with coronary artery disease: results from the Psychophysiological Investigations of Myocardial Ischemia study. Circulation 2002; 105:1780–1784.
- Jiang W, Babyak M, Krantz DS, et al. Mental stress–induced myocardial ischemia and cardiac events. JAMA 1996; 275:1651–1656.
- Blumenthal JA, Sherwood A, Babyak MA, et al. Effects of exercise and stress management training on markers of cardiovascular risk in patients with ischemic heart disease: a randomized controlled trial. JAMA 2005; 293:1626–1634.
- How to stop the toll of stress. Heart Advisor; March 2006:4–5.
The potential of biofeedback therapies in cardiovascular disease is only recently beginning to be explored in a systematic way. This article reviews the rationale for the use of biofeedback therapy in cardiovascular disease and briefly surveys research on the usefulness of biofeedback for several specific cardiovascular parameters and conditions.
RECOGNIZING THE POTENTIAL OF BIOFEEDBACK IN CARDIOVASCULAR DISEASE
Biofeedback is part of a group of modalities known as “self-regulation therapies,” in which a subject is taught to control the activities of his or her autonomic nervous system. The autonomic nervous system has also been called the “visceral,” “involuntary,” and “automatic” nervous system, which suggests that the physiologic processes governed by this branch of the nervous system are largely beyond conscious control. Until the 1950s, this was largely believed to be true. Physicians and scientists had been convinced that the functions regulated by the sympathetic and parasympathetic branches of the autonomic nervous system, such as digestion, blood pressure, and body temperature, were not amenable to self-regulation.
During the 1950s, however, it became clear that functions of the autonomic nervous system could be controlled by conscious thought and training. Subjects could be taught to correctly perceive and also to control heart rate, blood pressure, skin temperature, and other seemingly involuntary functions. The field of biofeedback and applied psychophysiology became possible with these discoveries and with the advent of technologies capable of measuring physiologic variables with enough sensitivity to detect small changes.
Key role of sympathetic/parasympathetic balance
In cardiovascular medicine, biofeedback has a great deal of therapeutic potential because many diseases of the heart and vasculature involve inappropriate regulation of the autonomic nervous system.
Under normal conditions, the sympathetic branch of the autonomic nervous system serves to augment cardiac function in times of stress, increasing heart rate, contractility, and blood pressure, as well as favoring clotting processes that would be mainly adaptive during the “fight or flight” response. The parasympathetic branch of the autonomic nervous system plays the opposite role during health, exerting a calming influence on cardiovascular function.
Normal cardiovascular function is regulated by a balance between sympathetic and parasympathetic inputs to the heart and blood vessels. Heart rate, for example, is governed by the parasympathetic nervous system under resting conditions, when the intrinsic firing rate of the sinus node is decreased by vagal input. Under stressful conditions, this inhibition is released and sympathetic excitation can increase the heart rate even further. In many pathological cardiac conditions, such as arrhythmias, an imbalance between the two branches of the autonomic nervous system causes at least some of the disease manifestations and often contributes to progression.
Biofeedback as a ‘physiologic beta-blocker’
Another good example is heart failure, where over-activation of the sympathetic nervous system results in many of the phenotypic changes in the myocardium and contributes to the downward spiral from compensatory cardiac hypertrophy to end-stage decompensated failure. The role of sympathetic overactivation in heart failure is clearly evident by the success of beta-adrenergic blocking agents in ameliorating symptoms and delaying disease progression. Given the role of autonomic nervous system dysregulation in cardiovascular diseases, biofeedback therapy has the potential to teach patients a skill that may allow them to decrease activation of their autonomic nervous system, theoretically acting as a “physiologic beta-blocker.”
An adjunct to stress management
The potential of biofeedback to have an impact in the arena of cardiovascular disease has not been well explored. Clinically, biofeedback is often used in the context of stress management programs, but biofeedback is not synonymous with stress management. Stress management programs most commonly involve some type of relaxation training and perhaps cognitive behavioral therapy. Biofeedback can be used to augment relaxation, helping the subject to be more aware of physiologic responses and thus be better able to elicit the relaxation response. Biofeedback can also be used to train subjects to control particular physiologic responses that contribute to symptoms or to disease progression. In cardiovascular disease, although stress management is frequently a component of cardiac rehabilitation programs, the question of whether stress management is more effective with or without biofeedback has not been systematically investigated.
PIONEERING STUDIES OF BIOFEEDBACK IN CARDIOVASCULAR DISEASE
Some of the earliest studies of physiologic regulation using biofeedback were attempted in patients with cardiovascular abnormalities. In 1971, Weiss and Engel reported success in using operant conditioning of heart rate in eight patients with premature ventricular contractions.1 All eight patients were able to achieve some degree of control, and five of the patients were able to decrease the frequency of premature beats, demonstrating increased success over a 21-month follow-up period. Interestingly, use of pharmacologic agents to understand the mechanisms of control suggested that one patient was able to decrease sympathetic control of his heart rate while another increased the parasympathetic influence.
Several years later, Pickering and Gorham reported their work with a single subject, a 31-year-old woman who had a ventricular parasystolic rhythm.2 Using a biofeedback technique, they were able to teach the woman to voluntarily control her heart rate, demonstrating that she could both increase and decrease the rate, avoiding the ranges in which the arrhythmia occurred. In the same year, Benson et al demonstrated that they could teach patients the relaxation response and decrease the incidence of premature ventricular contractions.3 Using Holter monitors for validation, these investigators showed that 4 weeks of relaxation training resulted in 8 of 11 patients being able to control their heart rates sufficiently to have therapeutic impact.
These pioneering studies were very early in the development of the field of biofeedback, but they showed what has been clearly established since—that the input of the autonomic nervous system to the heart can be regulated by biofeedback techniques.
BIOFEEDBACK STUDIES OF SPECIFIC CARDIOVASCULAR PARAMETERS AND DISEASES
A host of parameters for assessment
Many cardiovascular parameters can be used for biofeedback. Commonly these include heart rate, blood pressure, skin temperature, and, more recently, heart rate variability. In each case, the parameter is measured and displayed for the subject, and the subject is taught to make it change in a positive direction through relaxation, thought patterns, imagery, or some combination of techniques. Many times the display of the physiologic parameter and the demonstration that it can be controlled are quite surprising to the subject and lead to an enhanced desire to participate in the therapy.
Heart rate variability: A focus of recent interest
The newest parameter in use, and one that has gained considerable interest in the field of cardiovascular biofeedback, is heart rate variability.4 Heart rate variability refers to the variation within the R-R interval of the electrocardiogram during a fixed cycle. It is associated with adaptiveness of the cardiovascular system, and high variability is believed to be a sign of health. Low variability is associated with a number of disease states. Heart rate variability reflects the balance between sympathetic and parasympathetic input to the heart, and many cardiac disease states have been shown to be associated with low variability. Therapies that increase heart rate variability have been shown to improve prognosis.
On the basis of these observations, heart rate variability biofeedback is used to train patients to increase the variability in their heart rate, using feedback from equipment that records the R-R interval from the electrocardiogram or from blood pulse volume sensors. Patients learn to make the variability greater, primarily by breathing at a resonant frequency, as described by Lehrer et al.5
Several preliminary studies have been conducted with heart rate variability in cardiac patients, but much remains to be understood about its use. In 63 patients with established coronary artery disease, Del Pozo et al showed that six biofeedback sessions coupled with daily practice resulted in significantly increased heart rate variability.6 Similarly, Nolan and colleagues found that five sessions of biofeedback improved symptoms and quality of life in 46 patients with coronary artery disease.7 In 14 patients with heart failure, Luskin et al demonstrated that eight sessions of heart rate variability biofeedback produced reductions in perceived stress and improved function on the 6-minute walk test.8
It remains unclear whether heart rate variability biofeedback has more or less potential than other types of biofeedback in patients with cardiovascular disease, but these preliminary observations suggest that it may be useful in improving symptoms and quality of life.
Biofeedback in hypertension: Despite decades of study, conclusions elusive
Among diseases of the cardiovascular system, biofeedback has been used most frequently in hypertension, where it has been under investigation for more than 30 years, since the early days of biofeedback study.9 The field of biofeedback in hypertension is fraught with difficulties, rendering conclusions about its efficacy difficult.
Biofeedback has been assessed in many different types of hypertension, often within the same study. Essential hypertension and “white coat” hypertension, now known as excessive cardiovascular reactivity, have been most commonly investigated, but with no apparent consensus. The biofeedback techniques used in these studies have ranged from blood pressure biofeedback to electromyography, finger temperature, and skin conductance. More recently, heart rate variability biofeedback has also been used in this population.
In general, biofeedback has been more successful in the treatment of hypertension when respiratory training has been a component of the biofeedback. McGrady has established that certain types of patients with hypertension fare better with biofeedback than others.10 These include patients with higher baseline blood pressure, higher heart rate, cool hands, high electromyographic response, and high plasma renin activity.in short, patients who can be seen to have a high degree of sympathetic arousal.
Blood pressure can be lowered by 6 to 10 mm Hg when biofeedback is effective, which is less of an effect than that observed with most drug therapy for hypertension. Biofeedback does have the advantage, however, of improving overall cardiovascular reactivity and giving the patient a greater sense of control over his or her physical well-being, which may prove valuable in the setting of hypertension. Typically, the most effective interventions for hypertension (and perhaps for cardiovascular disease in general) are individualized for the patient and not protocol-driven. Thus, although biofeedback has potential in hypertension, its efficacy is not proven and systematic trials are lacking.
Biofeedback in heart failure: Targeting sympathetic overactivation
In patients with heart failure, the sympathetic nervous system is overactivated, as noted previously. High levels of plasma norepinephrine correlate with worse prognosis. Decreasing activation of the sympathetic nervous system improves both symptoms and prognosis, as demonstrated in patients taking beta-adrenergic blocking agents or those treated with a left ventricular assist device.
Several studies have suggested that biofeedback may be able to provide a similar reduction in sympathetic nervous system activation in patients with heart failure. Moser and colleagues showed that a single session of skin temperature biofeedback plus relaxation training increased cardiac output in patients with heart failure,11 while studies by Weiner et al,12 Bernardi et al,13 and Mangin et al14 showed that training heart failure patients to breathe more slowly increased their exercise tolerance. Although these studies are preliminary, they support the speculation that if biofeedback can decrease activation of the sympathetic nervous system in patients with heart failure, it may actually cause some degree of remodeling of the failing heart, such as that observed with beta-blockers or left ventricular assist device therapy.
BIOFEEDBACK AND STRESS MANAGEMENT: AN OPPORTUNITY FOR WIDER IMPACT
As mentioned earlier, biofeedback can serve as a component of stress management programs. Biofeedback is often a very effective adjunct to stress management because it teaches the subject to control physiologic reactions that are part of the stress response and gives the subject feedback to suggest that he or she is adequately practicing relaxation. Biofeedback-mediated stress management may actually be the most practical use of biofeedback in the setting of cardiovascular disease because it is easy to practice and can have an effect on large numbers of patients.
Mental stress has been well documented as a significant risk factor for many forms of cardiovascular disease, and stress management programs have been shown to have an impact on disease progression and symptoms. Many studies, including those reported by Sheps et al for the Psychophysiological Investigations of Myocardial Ischemia (PIMI) study,15 have shown that patients who exhibit ischemia in response to a mental stress test have increased mortality from cardiovascular disease. Jiang and colleagues,16 among others, have shown that mental stress predicts cardiac events in patients with lower ejection fractions, and Blumenthal et al17 have repeatedly demonstrated that stress management training reduces the incidence of wall motion abnormalities in patients with cardiovascular disease. Stress management is included in many cardiac rehabilitation programs, and it is likely that routine use of biofeedback as a component of stress management programs would benefit patients with cardiovascular disease, in whom reproducibly decreasing activation of the autonomic nervous system should be helpful.
According to a recent article in the Heart Advisor, 84% of physicians believe that stress is a risk for cardiovascular disease but only 35% say they feel knowledgeable about stress and a mere 5% feel that they succeed in helping stressed patients.18 Anything that could improve these numbers would be beneficial.
CONCLUSIONS
Cardiovascular conditions in which biofeedback has been shown to be helpful include arrhythmias, hypertension, Raynaud phenomenon, ischemia, infarction, and heart failure, but we have barely begun to explore the potential of biofeedback therapy. Given that many cardiovascular diseases involve inappropriate regulation of the autonomic nervous system, instruction in the use of biofeedback to control activation of the sympathetic and parasympathetic nervous systems is likely to be useful in cardiac patients. Systematic trials are needed.
The potential of biofeedback therapies in cardiovascular disease is only recently beginning to be explored in a systematic way. This article reviews the rationale for the use of biofeedback therapy in cardiovascular disease and briefly surveys research on the usefulness of biofeedback for several specific cardiovascular parameters and conditions.
RECOGNIZING THE POTENTIAL OF BIOFEEDBACK IN CARDIOVASCULAR DISEASE
Biofeedback is part of a group of modalities known as “self-regulation therapies,” in which a subject is taught to control the activities of his or her autonomic nervous system. The autonomic nervous system has also been called the “visceral,” “involuntary,” and “automatic” nervous system, which suggests that the physiologic processes governed by this branch of the nervous system are largely beyond conscious control. Until the 1950s, this was largely believed to be true. Physicians and scientists had been convinced that the functions regulated by the sympathetic and parasympathetic branches of the autonomic nervous system, such as digestion, blood pressure, and body temperature, were not amenable to self-regulation.
During the 1950s, however, it became clear that functions of the autonomic nervous system could be controlled by conscious thought and training. Subjects could be taught to correctly perceive and also to control heart rate, blood pressure, skin temperature, and other seemingly involuntary functions. The field of biofeedback and applied psychophysiology became possible with these discoveries and with the advent of technologies capable of measuring physiologic variables with enough sensitivity to detect small changes.
Key role of sympathetic/parasympathetic balance
In cardiovascular medicine, biofeedback has a great deal of therapeutic potential because many diseases of the heart and vasculature involve inappropriate regulation of the autonomic nervous system.
Under normal conditions, the sympathetic branch of the autonomic nervous system serves to augment cardiac function in times of stress, increasing heart rate, contractility, and blood pressure, as well as favoring clotting processes that would be mainly adaptive during the “fight or flight” response. The parasympathetic branch of the autonomic nervous system plays the opposite role during health, exerting a calming influence on cardiovascular function.
Normal cardiovascular function is regulated by a balance between sympathetic and parasympathetic inputs to the heart and blood vessels. Heart rate, for example, is governed by the parasympathetic nervous system under resting conditions, when the intrinsic firing rate of the sinus node is decreased by vagal input. Under stressful conditions, this inhibition is released and sympathetic excitation can increase the heart rate even further. In many pathological cardiac conditions, such as arrhythmias, an imbalance between the two branches of the autonomic nervous system causes at least some of the disease manifestations and often contributes to progression.
Biofeedback as a ‘physiologic beta-blocker’
Another good example is heart failure, where over-activation of the sympathetic nervous system results in many of the phenotypic changes in the myocardium and contributes to the downward spiral from compensatory cardiac hypertrophy to end-stage decompensated failure. The role of sympathetic overactivation in heart failure is clearly evident by the success of beta-adrenergic blocking agents in ameliorating symptoms and delaying disease progression. Given the role of autonomic nervous system dysregulation in cardiovascular diseases, biofeedback therapy has the potential to teach patients a skill that may allow them to decrease activation of their autonomic nervous system, theoretically acting as a “physiologic beta-blocker.”
An adjunct to stress management
The potential of biofeedback to have an impact in the arena of cardiovascular disease has not been well explored. Clinically, biofeedback is often used in the context of stress management programs, but biofeedback is not synonymous with stress management. Stress management programs most commonly involve some type of relaxation training and perhaps cognitive behavioral therapy. Biofeedback can be used to augment relaxation, helping the subject to be more aware of physiologic responses and thus be better able to elicit the relaxation response. Biofeedback can also be used to train subjects to control particular physiologic responses that contribute to symptoms or to disease progression. In cardiovascular disease, although stress management is frequently a component of cardiac rehabilitation programs, the question of whether stress management is more effective with or without biofeedback has not been systematically investigated.
PIONEERING STUDIES OF BIOFEEDBACK IN CARDIOVASCULAR DISEASE
Some of the earliest studies of physiologic regulation using biofeedback were attempted in patients with cardiovascular abnormalities. In 1971, Weiss and Engel reported success in using operant conditioning of heart rate in eight patients with premature ventricular contractions.1 All eight patients were able to achieve some degree of control, and five of the patients were able to decrease the frequency of premature beats, demonstrating increased success over a 21-month follow-up period. Interestingly, use of pharmacologic agents to understand the mechanisms of control suggested that one patient was able to decrease sympathetic control of his heart rate while another increased the parasympathetic influence.
Several years later, Pickering and Gorham reported their work with a single subject, a 31-year-old woman who had a ventricular parasystolic rhythm.2 Using a biofeedback technique, they were able to teach the woman to voluntarily control her heart rate, demonstrating that she could both increase and decrease the rate, avoiding the ranges in which the arrhythmia occurred. In the same year, Benson et al demonstrated that they could teach patients the relaxation response and decrease the incidence of premature ventricular contractions.3 Using Holter monitors for validation, these investigators showed that 4 weeks of relaxation training resulted in 8 of 11 patients being able to control their heart rates sufficiently to have therapeutic impact.
These pioneering studies were very early in the development of the field of biofeedback, but they showed what has been clearly established since—that the input of the autonomic nervous system to the heart can be regulated by biofeedback techniques.
BIOFEEDBACK STUDIES OF SPECIFIC CARDIOVASCULAR PARAMETERS AND DISEASES
A host of parameters for assessment
Many cardiovascular parameters can be used for biofeedback. Commonly these include heart rate, blood pressure, skin temperature, and, more recently, heart rate variability. In each case, the parameter is measured and displayed for the subject, and the subject is taught to make it change in a positive direction through relaxation, thought patterns, imagery, or some combination of techniques. Many times the display of the physiologic parameter and the demonstration that it can be controlled are quite surprising to the subject and lead to an enhanced desire to participate in the therapy.
Heart rate variability: A focus of recent interest
The newest parameter in use, and one that has gained considerable interest in the field of cardiovascular biofeedback, is heart rate variability.4 Heart rate variability refers to the variation within the R-R interval of the electrocardiogram during a fixed cycle. It is associated with adaptiveness of the cardiovascular system, and high variability is believed to be a sign of health. Low variability is associated with a number of disease states. Heart rate variability reflects the balance between sympathetic and parasympathetic input to the heart, and many cardiac disease states have been shown to be associated with low variability. Therapies that increase heart rate variability have been shown to improve prognosis.
On the basis of these observations, heart rate variability biofeedback is used to train patients to increase the variability in their heart rate, using feedback from equipment that records the R-R interval from the electrocardiogram or from blood pulse volume sensors. Patients learn to make the variability greater, primarily by breathing at a resonant frequency, as described by Lehrer et al.5
Several preliminary studies have been conducted with heart rate variability in cardiac patients, but much remains to be understood about its use. In 63 patients with established coronary artery disease, Del Pozo et al showed that six biofeedback sessions coupled with daily practice resulted in significantly increased heart rate variability.6 Similarly, Nolan and colleagues found that five sessions of biofeedback improved symptoms and quality of life in 46 patients with coronary artery disease.7 In 14 patients with heart failure, Luskin et al demonstrated that eight sessions of heart rate variability biofeedback produced reductions in perceived stress and improved function on the 6-minute walk test.8
It remains unclear whether heart rate variability biofeedback has more or less potential than other types of biofeedback in patients with cardiovascular disease, but these preliminary observations suggest that it may be useful in improving symptoms and quality of life.
Biofeedback in hypertension: Despite decades of study, conclusions elusive
Among diseases of the cardiovascular system, biofeedback has been used most frequently in hypertension, where it has been under investigation for more than 30 years, since the early days of biofeedback study.9 The field of biofeedback in hypertension is fraught with difficulties, rendering conclusions about its efficacy difficult.
Biofeedback has been assessed in many different types of hypertension, often within the same study. Essential hypertension and “white coat” hypertension, now known as excessive cardiovascular reactivity, have been most commonly investigated, but with no apparent consensus. The biofeedback techniques used in these studies have ranged from blood pressure biofeedback to electromyography, finger temperature, and skin conductance. More recently, heart rate variability biofeedback has also been used in this population.
In general, biofeedback has been more successful in the treatment of hypertension when respiratory training has been a component of the biofeedback. McGrady has established that certain types of patients with hypertension fare better with biofeedback than others.10 These include patients with higher baseline blood pressure, higher heart rate, cool hands, high electromyographic response, and high plasma renin activity.in short, patients who can be seen to have a high degree of sympathetic arousal.
Blood pressure can be lowered by 6 to 10 mm Hg when biofeedback is effective, which is less of an effect than that observed with most drug therapy for hypertension. Biofeedback does have the advantage, however, of improving overall cardiovascular reactivity and giving the patient a greater sense of control over his or her physical well-being, which may prove valuable in the setting of hypertension. Typically, the most effective interventions for hypertension (and perhaps for cardiovascular disease in general) are individualized for the patient and not protocol-driven. Thus, although biofeedback has potential in hypertension, its efficacy is not proven and systematic trials are lacking.
Biofeedback in heart failure: Targeting sympathetic overactivation
In patients with heart failure, the sympathetic nervous system is overactivated, as noted previously. High levels of plasma norepinephrine correlate with worse prognosis. Decreasing activation of the sympathetic nervous system improves both symptoms and prognosis, as demonstrated in patients taking beta-adrenergic blocking agents or those treated with a left ventricular assist device.
Several studies have suggested that biofeedback may be able to provide a similar reduction in sympathetic nervous system activation in patients with heart failure. Moser and colleagues showed that a single session of skin temperature biofeedback plus relaxation training increased cardiac output in patients with heart failure,11 while studies by Weiner et al,12 Bernardi et al,13 and Mangin et al14 showed that training heart failure patients to breathe more slowly increased their exercise tolerance. Although these studies are preliminary, they support the speculation that if biofeedback can decrease activation of the sympathetic nervous system in patients with heart failure, it may actually cause some degree of remodeling of the failing heart, such as that observed with beta-blockers or left ventricular assist device therapy.
BIOFEEDBACK AND STRESS MANAGEMENT: AN OPPORTUNITY FOR WIDER IMPACT
As mentioned earlier, biofeedback can serve as a component of stress management programs. Biofeedback is often a very effective adjunct to stress management because it teaches the subject to control physiologic reactions that are part of the stress response and gives the subject feedback to suggest that he or she is adequately practicing relaxation. Biofeedback-mediated stress management may actually be the most practical use of biofeedback in the setting of cardiovascular disease because it is easy to practice and can have an effect on large numbers of patients.
Mental stress has been well documented as a significant risk factor for many forms of cardiovascular disease, and stress management programs have been shown to have an impact on disease progression and symptoms. Many studies, including those reported by Sheps et al for the Psychophysiological Investigations of Myocardial Ischemia (PIMI) study,15 have shown that patients who exhibit ischemia in response to a mental stress test have increased mortality from cardiovascular disease. Jiang and colleagues,16 among others, have shown that mental stress predicts cardiac events in patients with lower ejection fractions, and Blumenthal et al17 have repeatedly demonstrated that stress management training reduces the incidence of wall motion abnormalities in patients with cardiovascular disease. Stress management is included in many cardiac rehabilitation programs, and it is likely that routine use of biofeedback as a component of stress management programs would benefit patients with cardiovascular disease, in whom reproducibly decreasing activation of the autonomic nervous system should be helpful.
According to a recent article in the Heart Advisor, 84% of physicians believe that stress is a risk for cardiovascular disease but only 35% say they feel knowledgeable about stress and a mere 5% feel that they succeed in helping stressed patients.18 Anything that could improve these numbers would be beneficial.
CONCLUSIONS
Cardiovascular conditions in which biofeedback has been shown to be helpful include arrhythmias, hypertension, Raynaud phenomenon, ischemia, infarction, and heart failure, but we have barely begun to explore the potential of biofeedback therapy. Given that many cardiovascular diseases involve inappropriate regulation of the autonomic nervous system, instruction in the use of biofeedback to control activation of the sympathetic and parasympathetic nervous systems is likely to be useful in cardiac patients. Systematic trials are needed.
- Weiss T, Engel BT. Operant conditioning of heart rate in patients with premature ventricular contractions. Psychosom Med 1971; 33:301–322.
- Pickering T, Gorham G. Learned heart-rate control by a patient with a ventricular parasystolic rhythm. Lancet 1975; 1:252–253.
- Benson H, Alexander S, Feldman CL. Decreased premature ventricular contractions through use of the relaxation response in patients with stable ischaemic heart disease. Lancet 1975; 2:380–382.
- Kranitz L, Lehrer P. Biofeedback applications in the treatment of cardiovascular diseases. Cardiol Rev 2004; 12:177–181.
- Lehrer PM, Vaschilllo E, Vaschillo B. Resonant frequency biofeedback training to increase cardiac variability: rationale and manual for training. Appl Psychophysiol Biofeedback 2000; 25:177–191.
- Del Pozo JM, Gevirtz RN, Scher B, Guarneri E. Biofeedback treatment increases heart rate variability in patients with known coronary artery disease. Am Heart J 2004; 147:E11.
- Nolan RP, Kamath MV, Floras JS, et al. Heart rate variability biofeedback as a behavioral neurocardiac intervention to enhance vagal heart rate control. Am Heart J 2005; 149:1137.
- Luskin F, Reitz M, Newell K, Quinn TG, Haskell W. A controlled pilot study of stress management training of elderly patients with congestive heart failure. Prev Cardiol 2002; 5:168–172.
- Linden W, Moseley JV. The efficacy of behavioral treatments for hypertension. Appl Psychophysiol Biofeedback 2006; 31:51–63.
- McGrady A. Good news.bad press: applied psychophysiology in cardiovascular disorders. Biofeedback Self Regul 1996; 21:335–346.
- Moser DK, Dracup K, Woo MA, Stevenson LW. Voluntary control of vascular tone by using skin-temperature biofeedback-relaxation in patients with advanced heart failure. Altern Ther Health Med 1997; 3:51–59.
- Weiner P, Waizman J, Magadle R, Berar-Yanay N, Pelled B. The effect of specific inspiratory muscle training on the sensation of dyspnea and exercise tolerance in patients with congestive heart failure. Clin Cardiol 1999; 22:727–732.
- Bernardi L, Porta C, Spicuzza L, et al. Slow breathing increases arterial baroreflex sensitivity in patients with chronic heart failure. Circulation 2002; 105:143–145.
- Mangin L, Monti A, Médigue C, et al. Altered baroreflex gain during voluntary breathing in chronic heart failure. Eur J Heart Fail 2001; 3:189–195.
- Sheps DS, McMahon RP, Becker L, et al. Mental stress-induced ischemia and all-cause mortality in patients with coronary artery disease: results from the Psychophysiological Investigations of Myocardial Ischemia study. Circulation 2002; 105:1780–1784.
- Jiang W, Babyak M, Krantz DS, et al. Mental stress–induced myocardial ischemia and cardiac events. JAMA 1996; 275:1651–1656.
- Blumenthal JA, Sherwood A, Babyak MA, et al. Effects of exercise and stress management training on markers of cardiovascular risk in patients with ischemic heart disease: a randomized controlled trial. JAMA 2005; 293:1626–1634.
- How to stop the toll of stress. Heart Advisor; March 2006:4–5.
- Weiss T, Engel BT. Operant conditioning of heart rate in patients with premature ventricular contractions. Psychosom Med 1971; 33:301–322.
- Pickering T, Gorham G. Learned heart-rate control by a patient with a ventricular parasystolic rhythm. Lancet 1975; 1:252–253.
- Benson H, Alexander S, Feldman CL. Decreased premature ventricular contractions through use of the relaxation response in patients with stable ischaemic heart disease. Lancet 1975; 2:380–382.
- Kranitz L, Lehrer P. Biofeedback applications in the treatment of cardiovascular diseases. Cardiol Rev 2004; 12:177–181.
- Lehrer PM, Vaschilllo E, Vaschillo B. Resonant frequency biofeedback training to increase cardiac variability: rationale and manual for training. Appl Psychophysiol Biofeedback 2000; 25:177–191.
- Del Pozo JM, Gevirtz RN, Scher B, Guarneri E. Biofeedback treatment increases heart rate variability in patients with known coronary artery disease. Am Heart J 2004; 147:E11.
- Nolan RP, Kamath MV, Floras JS, et al. Heart rate variability biofeedback as a behavioral neurocardiac intervention to enhance vagal heart rate control. Am Heart J 2005; 149:1137.
- Luskin F, Reitz M, Newell K, Quinn TG, Haskell W. A controlled pilot study of stress management training of elderly patients with congestive heart failure. Prev Cardiol 2002; 5:168–172.
- Linden W, Moseley JV. The efficacy of behavioral treatments for hypertension. Appl Psychophysiol Biofeedback 2006; 31:51–63.
- McGrady A. Good news.bad press: applied psychophysiology in cardiovascular disorders. Biofeedback Self Regul 1996; 21:335–346.
- Moser DK, Dracup K, Woo MA, Stevenson LW. Voluntary control of vascular tone by using skin-temperature biofeedback-relaxation in patients with advanced heart failure. Altern Ther Health Med 1997; 3:51–59.
- Weiner P, Waizman J, Magadle R, Berar-Yanay N, Pelled B. The effect of specific inspiratory muscle training on the sensation of dyspnea and exercise tolerance in patients with congestive heart failure. Clin Cardiol 1999; 22:727–732.
- Bernardi L, Porta C, Spicuzza L, et al. Slow breathing increases arterial baroreflex sensitivity in patients with chronic heart failure. Circulation 2002; 105:143–145.
- Mangin L, Monti A, Médigue C, et al. Altered baroreflex gain during voluntary breathing in chronic heart failure. Eur J Heart Fail 2001; 3:189–195.
- Sheps DS, McMahon RP, Becker L, et al. Mental stress-induced ischemia and all-cause mortality in patients with coronary artery disease: results from the Psychophysiological Investigations of Myocardial Ischemia study. Circulation 2002; 105:1780–1784.
- Jiang W, Babyak M, Krantz DS, et al. Mental stress–induced myocardial ischemia and cardiac events. JAMA 1996; 275:1651–1656.
- Blumenthal JA, Sherwood A, Babyak MA, et al. Effects of exercise and stress management training on markers of cardiovascular risk in patients with ischemic heart disease: a randomized controlled trial. JAMA 2005; 293:1626–1634.
- How to stop the toll of stress. Heart Advisor; March 2006:4–5.