User login
Beta-cell therapies for type 1 diabetes: Transplants and bionics
With intensive insulin regimens and home blood glucose monitoring, patients with type 1 diabetes are controlling their blood glucose better than in the past. Nevertheless, glucose regulation is still imperfect and tedious, and striving for tight glycemic control poses the risk of hypoglycemia.
Prominent among the challenges are the sheer numbers involved. Some 1.25 million Americans have type 1 diabetes, and another 30 million have type 2, but only about 7,000 to 8,000 pancreases are available for transplant each year.1 While awaiting a breakthrough—perhaps involving stem cells, perhaps involving organs obtained from animals—an insulin pump may offer better diabetes control for many. Another possibility is a closed-loop system with a continuous glucose monitor that drives a dual-infusion pump, delivering insulin when glucose levels rise too high, and glucagon when they dip too low.
DIABETES WAS KNOWN IN ANCIENT TIMES
About 3,000 years ago, Egyptians described the syndrome of thirst, emaciation, and sweet urine that attracted ants. The term diabetes (Greek for siphon) was first recorded in 1425; mellitus (Latin for sweet with honey) was not added until 1675.
In 1857, Bernard hypothesized that diabetes was caused by overproduction of glucose in the liver. This idea was replaced in 1889, when Mering and Minkowski proposed the dysfunctional pancreas theory that eventually led to the discovery of the beta cell.2
In 1921, Banting and Best isolated insulin, and for the past 100 years subcutaneous insulin replacement has been the mainstay of treatment. But starting about 50 years ago, researchers have been looking for safe and long-lasting ways to replace beta cells and eliminate the need for exogenous insulin replacement.
TRANSPLANTING THE WHOLE PANCREAS
The first whole-pancreas transplant was performed in 1966 by Kelly et al,3 followed by 13 more by 1973.4 These first transplant grafts were short-lived, with only 1 graft surviving longer than 1 year. Since then, more than 12,000 pancreases have been transplanted worldwide, as refinements in surgical techniques and immunosuppressive therapies have improved patient and graft survival rates.4
Today, most pancreas transplants are in patients who have both type 1 diabetes and end-stage renal disease due to diabetic nephropathy, and most receive both a kidney and a pancreas at the same time. Far fewer patients receive a pancreas after previously receiving a kidney, or receive a pancreas alone.
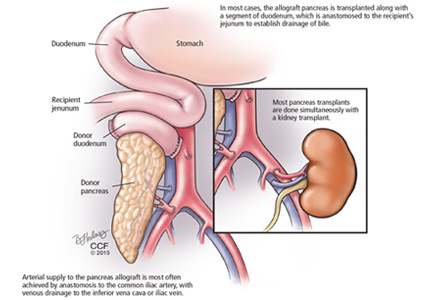
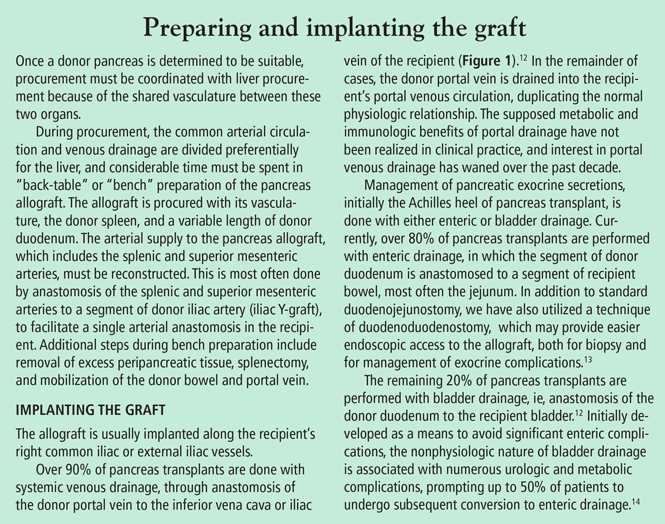
The bile duct of the transplanted pancreas is usually routed into the patient’s small intestine, as nature intended, and less often into the bladder. Although bladder drainage is associated with urinary complications, it has the advantage of allowing measurement of pancreatic amylase levels in the urine to monitor for graft rejection. With simultaneous pancreas and kidney transplant, the serum creatinine concentration can also be monitored for rejection of the kidney graft.
Current immunosuppressive regimens vary but generally consist of anti-T-cell antibodies at the time of surgery, followed by lifelong treatment with the combination of a calcineurin inhibitor (cyclosporine or tacrolimus) and an antimetabolite (mycophenolate mofetil or azathioprine).
Outcomes are good. The rates of patient and graft survival are highest with simultaneous pancreas-kidney transplant, and somewhat lower with pancreas-after-kidney and pancreas-alone transplant.
Benefits of pancreas transplant
Most recipients can stop taking insulin immediately after the procedure, and their hemoglobin A1c levels normalize and stay low for the life of the graft. Lipid levels also decrease, although this has not been directly correlated with lower risk of vascular disease.4
Transplant also reduces or eliminates some complications of diabetes, including retinopathy, nephropathy, cardiomyopathy, and gastropathy.
For example, in patients undergoing simultaneous pancreas-kidney transplant, diabetic nephropathy does not recur in the new kidney. Fioretto et al5 reported that nephropathy lesions reversed during the 10 years after pancreas transplant.
Kennedy et al6,7 found that preexisting diabetic neuropathy improved slightly (although neurologic status did not completely return to normal) over a period of up to 42 months in a group of patients who received a pancreas transplant, whereas it tended to worsen in a control group. Both groups were assessed at baseline and at 12 and 24 months, with a subgroup followed through 42 months, and they underwent testing of motor, sensory, and autonomic function.6,7
Disadvantages of pancreas transplant
Disadvantages of whole-pancreas transplant include hypoglycemia (usually mild), adverse effects of immunosuppression, potential for surgical complications including an increased rate of death in the first 90 days after the procedure, and cost.
In an analysis comparing the 5-year estimated costs of dialysis, kidney transplant alone from cadavers or live donors, or simultaneous pancreas-kidney transplant for diabetic patients with end-stage renal disease, the least expensive option was kidney transplant from a live donor.8 The most expensive option was simultaneous pancreas-kidney transplant, but quality of life was better with this option. The analysis did not consider the potential cost of long-term treatments for complications related to diabetes that could be saved with a pancreas transplant.
Data conflict regarding the risk of death with different types of pancreas transplants. A retrospective cohort study of data from 124 US transplant centers reported in 2003 found higher mortality rates in pancreas-alone transplant recipients than in patients on a transplant waiting list receiving conventional therapy.9 In contrast, a 2004 study reported that after the first 90 days, when the risk of death was clearly higher, mortality rates were lower after simultaneous pancreas-kidney transplant and pancreas-after-kidney transplant.10 After pancreas-alone transplant, however, mortality rates were higher than with exogenous insulin therapy.
Although outcomes have improved, fewer patients with type 1 diabetes are undergoing pancreas transplant in recent years.
Interestingly, more simultaneous pancreas-kidney transplants are being successfully performed in patients with type 2 diabetes, who now account for 8% of all simultaneous pancreas-kidney transplant recipients.11 Outcomes of pancreas transplant appear to be similar regardless of diabetes type.
Bottom line
Pancreas transplant is a viable option for certain cases of complicated diabetes.
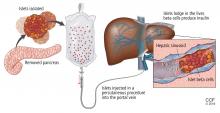
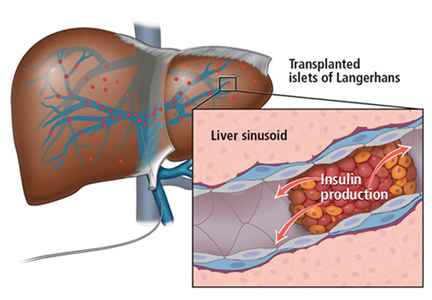
TRANSPLANTING ISLET CELLS
Despite its successes, pancreas transplant is major surgery and requires lifetime immunosuppression. Research is ongoing into a less-invasive procedure that, it is hoped, would require less immunosuppression: transplanting islets by themselves.
Islet autotransplant after pancreatectomy
For some patients with chronic pancreatitis, the only option to relieve chronic pain, narcotic dependence, and poor quality of life is to remove the pancreas. In the past, this desperate measure would instantly and inevitably cause diabetes, but not anymore.
Alpha cells and glucagon are a different story; a complication of islet transplant is hypoglycemia. In 2016, Lin et al12 reported spontaneous hypoglycemia in 6 of 12 patients who maintained insulin independence after autotransplant of islets. Although the transplanted islets had functional alpha cells that could in theory produce glucagon, as well as beta cells that produce insulin and C-peptide, apparently the alpha cells were not secreting glucagon in response to the hypoglycemia.
Location may matter. Gupta et al,13 in a 1997 study in dogs, found that more hypoglycemia occurs if islets are autotransplanted into the liver than if they are transplanted into the peritoneal cavity. A possible explanation may have to do with the glycemic environment of the liver.
Islet allotransplant
Islets can also be taken from cadaver donors and transplanted into patients with type 1 diabetes, who do not have enough working beta cells.
Success of allotransplant increased after the publication of observational data from the program in Edmonton in Canada, in which 7 consecutive patients with type 1 diabetes achieved initial insulin independence after islet allotransplant using steroid-free immunosuppression.14 Six recipients required islets from 2 donors, and 1 required islets from 4 donors, so they all received large volumes of at least 11,000 islet equivalents (IEQ) per kilogram of body weight.
In a subsequent report from the same team,15 16 (44%) of 36 patients remained insulin-free at 1 year, and C-peptide secretion was detectable in 70% at 2 years. But despite the elevated C-peptide levels, only 5 patients remained insulin-independent by 2 years. Lower hemoglobin A1c levels and decreases in hypoglycemic events from baseline also were noted.
The Clinical Islet Transplantation Consortium (CITC)16 and Collaborative Islet Transplant Registry (CITR)17 were established in 2004 to combine data and resources from centers around the world, including several that specialize in islet isolation and purification. Currently, more than 80 studies are being conducted.
The CITC and CITR now have data on more than 1,000 allogeneic islet transplant recipients (islet transplant alone, after kidney transplant, or simultaneous with it). The primary outcomes are hemoglobin A1c levels below 7% fasting C-peptide levels 0.3 ng/mL or higher, and fasting blood glucose of 60 to 140 mg/dL with no severe hypoglycemic events. The best results for islet-alone transplant have been in recipients over age 35 who received at least 325,000 IEQs with use of tumor necrosis factor antagonists for induction and calcineurin inhibitors or mammalian target of rapamycin (mTOR) inhibitors for maintenance.17
The best success for islet-after-kidney transplant was achieved with the same protocol but with insulin given to the donor during hospitalization before pancreas procurement. For participants with favorable factors, a hemoglobin A1c at or below 6.5% was achieved in about 80% at 1 year after last infusion, with more than 80% maintaining their fasting blood glucose level goals. About 70% of these patients were insulin-independent at 1 year. Hypoglycemia unawareness resolved in these patients even 5 years after infusion. Although there were no deaths or disabilities related to these transplants, bleeding occurred in 1 of 15 procedures. There was also a notable decline in estimated glomerular filtration rates with calcineurin inhibitor-based immunosuppression.17
Making islets go farther
One of the greatest challenges to islet transplant is the need for multiple donors to provide enough islet cells to overcome the loss of cells during transplant. Pancreases are already in short supply, and if each recipient needs more than 1, this makes the shortage worse. Some centers have achieved transplant with fewer donors,18,19 possibly by selecting pancreases from young donors who had a high body mass index and more islet cells, and harvesting and using them with a shorter cold ischemic time.
The number of viable, functioning islet cells drastically decreases after transplant, especially when transplanted into the portal system. This phenomenon is linked to an instant, blood-mediated inflammatory reaction involving antibody binding, complement and coagulation cascade activation, and platelet aggregation. The reaction, part of the innate immune system, damages the islet cells and leads to insulin dumping and early graft loss in studies in vitro and in vivo. Another factor affecting the survival of the graft cells is the low oxygen tension in the portal system.
For this reason, sites such as the pancreas, gastric submucosa, genitourinary tract, muscle, omentum, bone marrow, kidney capsule, peritoneum, anterior eye chamber, testis, and thymus are being explored.20
To create a more supportive environment for the transplanted cells, biotechnicians are trying to encapsulate islets in a semipermeable membrane that would protect them from the immune system while still allowing oxygen, nutrients, waste products, and, critically, insulin to diffuse in and out. Currently, no site or encapsulated product has been more successful than the current practice of implanting naked islets in the portal system.20
Bottom line
Without advances in transplant sites or increasing the yield of islet cells to allow single-donor transplants, islet cell allotransplant will not be feasible for most patients with type 1 diabetes.
Xenotransplant: Can pig cells make up the shortage?
Use of animal kidneys (xenotransplant) is a potential solution to the shortage of human organs for transplant.
In theory, pigs could be a source. Porcine insulin is similar to human insulin (differing by only 1 amino acid), and it should be possible to breed “knockout” pigs that lack the antigens responsible for acute humoral rejection.21
On the other hand, transplant of porcine islets poses several immunologic, physiologic, ethical, legal, and infectious concerns. For example, porcine tissue could carry pig viruses, such as porcine endogenous retroviruses.21 And even if the pigs are genetically modified, patients will still require immunosuppressive therapy.
A review of 17 studies of pig islet xenotransplant into nonhuman primates found that in 5 of the studies (4 using diabetic primates) the grafts survived at least 3 months.22 Of these, 1 study used encapsulation, and the rest used intensive and toxic immunosuppression.
More research is needed to make xenotransplant a clinical option.
Transplanting stem cells or beta cells grown from stem cells
Stem cells provide an exciting potential alternative to the limited donor pool. During the past decade, several studies have shown success using human pluripotent stem cells (embryonic stem cells and human-induced pluripotent stem cells), mesenchymal stem cells isolated from adult tissues, and directly programmed somatic cells. Researchers have created stable cultures of pluripotent stem cells from embryonic stem cells, which could possibly be produced on a large scale and banked.23
Human pluripotent stem cells derived from pancreatic progenitors have been shown to mature into more functional, islet-like structures in vivo. They transform into subtypes of islet cells including alpha, beta, and delta cells, ghrelin-producing cells, and pancreatic polypeptide hormone-producing cells. This process takes 2 to 6 weeks. In mice, these cells have been shown to maintain glucose homeostasis.24 Phase 1 and 2 trials in humans are now being conducted.
Pagliuca et al25 generated functional human pancreatic beta cells in vitro from embryonic stem cells. Rezania et al24 reversed diabetes with insulin-producing cells derived in vitro from human pluripotent stem cells. The techniques used in these studies contributed to the success of a study by Vegas et al,26 who achieved successful long-term glycemic control in mice using polymer-encapsulated human stem cell-derived beta cells.
Reversal of autoimmunity is an important step that needs to be overcome in stem cell transplant for type 1 diabetes. Nikolic et al27 have achieved mixed allogeneic chimerism across major histocompatibility complex barriers with nonmyeloablative conditioning in advanced-diabetic nonobese diabetic mice. However, conditioning alone (ie, without bone marrow transplant) does not permit acceptance of allogeneic islets and does not reverse autoimmunity or allow islet regeneration.28 Adding allogeneic bone marrow transplant to conditioned nonobese diabetic mice leads to tolerance to the donor and reverses autoimmunity.
THE ‘BIONIC’ PANCREAS
While we wait for advances in islet cell transplant, improved insulin pumps hold promise.
One such experimental device, the iLet (Beta Bionics, Boston, MA), designed by Damiano et al, consists of 2 infusion pumps (1 for insulin, 1 for glucagon) linked to a continuous glucose monitor via a smartphone app.
The monitor measures the glucose level every 5 minutes and transmits the information wirelessly to the phone app, which calculates the amount of insulin and glucagon required to stabilize the blood glucose: more insulin if too high, more glucagon if too low. The phone transmits this information to the pumps.
Dubbed the “bionic” pancreas, this closed-loop system frees patients from the tasks of measuring their glucose multiple times a day, calculating the appropriate dose, and giving multiple insulin injections.
The 2016 summer camp study29 followed 19 preteens wearing the bionic pancreas for 5 days. During this time, the patients had lower mean glucose levels and less hypoglycemia than during control periods. No episodes of severe hypoglycemia were recorded.
El-Khatib et al30 randomly assigned 43 patients to treatment with either the bihormonal bionic pancreas or usual care (a conventional insulin pump or a sensor-augmented insulin pump) for 11 days, followed by 11 days of the opposite treatment. All participants continued their normal activities. The bionic pancreas system was superior to the insulin pump in terms of the mean glucose concentration and mean time in the hypoglycemic range (P < .0001 for both results).
Bottom line
As the search continues for better solutions, advances in technology such as the bionic pancreas could provide a safer (ie, less hypoglycemic) and more successful alternative for insulin replacement in the near future.
- American Diabetes Association. Statistics about diabetes: overall numbers, diabetes and prediabetes. www.diabetes.org/diabetes-basics/statistics/. Accessed November 6, 2018.
- Ahmed AM. History of diabetes mellitus. Saudi Med J 2002; 23(4):373–378. pmid:11953758
- Kelly WD, Lillehei RC, Merkel FK, Idezuki Y, Goetz FC. Allotransplantation of the pancreas and duodenum along with the kidney in diabetic nephropathy. Surgery 1967; 61:827–837. pmid: 5338113
- Sutherland DE, Gruessner RW, Dunn DL, et al. Lessons learned from more than 1,000 pancreas transplants at a single institution. Ann Surg 2001; 233(4):463–501. pmid:11303130
- Fioretto P, Steffes MW, Sutherland DE, Goetz FC, Mauer M. Reversal of lesions of diabetic nephropathy after pancreas transplantation. N Engl J Med 1998; 339(2):69–75. doi:10.1056/NEJM199807093390202
- Kennedy WR, Navarro X, Goetz FC, Sutherland DE, Najarian JS. Effects of pancreatic transplantation on diabetic neuropathy. N Engl J Med 1990; 322(15):1031–1037. doi:10.1056/NEJM199004123221503
- Kennedy WR, Navarro X, Sutherland DER. Neuropathy profile of diabetic patients in a pancreas transplantation program. Neurology 1995; 45(4):773–780. pmid:7723969
- Douzdjian V, Ferrara D, Silvestri G. Treatment strategies for insulin-dependent diabetics with ESRD: a cost-effectiveness decision analysis model. Am J Kidney Dis 1998; 31(5):794–802. pmid:9590189
- Venstrom JM, McBride MA, Rother KI, Hirshberg B, Orchard TJ, Harlan DM. Survival after pancreas transplantation in patients with diabetes and preserved kidney function. JAMA 2003; 290(21):2817–2823. doi:10.1001/jama.290.21.2817
- Gruessner RW, Sutherland DE, Gruessner AC. Mortality assessment for pancreas transplants. Am J Transplant 2004; 4(12):2018–2026. doi:10.1111/j.1600-6143.2004.00667.x
- Redfield RR, Scalea JR, Odorico JS. Simultaneous pancreas and kidney transplantation: current trends and future directions. Curr Opin Organ Transplant 2015; 20(1):94-102. doi:10.1097/MOT.0000000000000146
- Lin YK, Faiman C, Johnston PC, et al. Spontaneous hypoglycemia after islet autotransplantation for chronic pancreatitis. J Clin Endocrinol Metab 2016; 101(10):3669–3675. doi:10.1210/jc.2016-2111
- Gupta V, Wahoff DC, Rooney DP, et al. The defective glucagon response from transplanted intrahepatic pancreatic islets during hypoglycemia is transplantation site-determined. Diabetes 1997; 46(1):28–33. pmid:8971077
- Shapiro AM, Lakey JR, Ryan EA, et al. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med 2000; 343(4):230–238. doi:10.1056/NEJM200007273430401
- Shapiro AM, Ricordi C, Hering BJ, et al. International trial of the Edmonton protocol for islet transplantation. N Engl J Med 2006; 355(13):1318–1330. doi:10.1056/NEJMoa061267
- Clinical Islet Transplantation (CIT) Consortium. www.citisletstudy.org. Accessed November 6, 2018.
- Collaborative Islet Transplantation Registry (CITR). CITR 10th Annual Report. https://citregistry.org/system/files/10th_AR.pdf. Accessed November 6, 2018.
- Hering BJ, Kandaswamy R, Harmon JV, et al. Transplantation of cultured islets from two-layer preserved pancreases in type 1 diabetes with anti-CD3 antibody. Am J Transplant 2004; 4(3):390–401. pmid:14961992
- Posselt AM, Bellin MD, Tavakol M, et al. Islet transplantation in type 1 diabetics using an immunosuppressive protocol based on the anti-LFA-1 antibody efalizumab. Am J Transplant 2010; 10(8):1870–1880. doi:10.1111/j.1600-6143.2010.03073.x
- Cantarelli E, Piemonti L. Alternative transplantation sites for pancreatic islet grafts. Curr Diab Rep 2011; 11(5):364–374. doi:10.1007/s11892-011-0216-9
- Cooper DK, Gollackner B, Knosalla C, Teranishi K. Xenotransplantation—how far have we come? Transpl Immunol 2002; 9(2–4):251–256. pmid:12180839
- Marigliano M, Bertera S, Grupillo M, Trucco M, Bottino R. Pig-to-nonhuman primates pancreatic islet xenotransplantation: an overview. Curr Diab Rep 2011; 11(5):402–412. doi:10.1007/s11892-011-0213-z
- Bartlett ST, Markmann JF, Johnson P, et al. Report from IPITA-TTS opinion leaders meeting on the future of beta-cell replacement. Transplantation 2016; 100(suppl 2):S1–S44. doi:10.1097/TP.0000000000001055
- Rezania A, Bruin JE, Arora P, et al. Reversal of diabetes with insulin-producing cells derived in vitro from human pluripotent stem cells. Nat Biotechnol 2014; 32(11):1121–1133. doi:10.1038/nbt.3033
- Pagliuca FW, Millman JR, Gurtler M, et al. Generation of functional human pancreatic beta cells in vitro. Cell 2014; 159(2):428–439. doi:10.1016/j.cell.2014.09.040
- Vegas AJ, Veiseh O, Gurtler M, et al. Long-term glycemic control using polymer-encapsulated human stem cell-derived beta cells in immune-competent mice. Nat Med 2016; 22(3):306–311. doi:10.1038/nm.4030
- Nikolic B, Takeuchi Y, Leykin I, Fudaba Y, Smith RN, Sykes M. Mixed hematopoietic chimerism allows cure of autoimmune tolerance and reversal of autoimmunity. Diabetes 2004; 53(2):376–383. pmid:14747288
- Li HW, Sykes M. Emerging concepts in haematopoietic cell transplantation. Nat Rev Immunol 2012; 12(6):403–416. doi:10.1038/nri3226
- Russell SJ, Hillard MA, Balliro C, et al. Day and night glycaemic control with a bionic pancreas versus conventional insulin pump therapy in preadolescent children with type 1 diabetes: a randomised crossover trial. Lancet Diabetes Endocrinol 2016; 4(3):233–243. doi:10.1016/S2213-8587(15)00489-1
- El-Khatib FH, Balliro C, Hillard MA, et al. Home use of a bihormonal bionic pancreas versus insulin pump therapy in adults with type 1 diabetes: a multicenter randomized crossover trial. Lancet 2017; 389(10067):369–380. doi:10.1016/S0140-6736(16)32567-3
With intensive insulin regimens and home blood glucose monitoring, patients with type 1 diabetes are controlling their blood glucose better than in the past. Nevertheless, glucose regulation is still imperfect and tedious, and striving for tight glycemic control poses the risk of hypoglycemia.
Prominent among the challenges are the sheer numbers involved. Some 1.25 million Americans have type 1 diabetes, and another 30 million have type 2, but only about 7,000 to 8,000 pancreases are available for transplant each year.1 While awaiting a breakthrough—perhaps involving stem cells, perhaps involving organs obtained from animals—an insulin pump may offer better diabetes control for many. Another possibility is a closed-loop system with a continuous glucose monitor that drives a dual-infusion pump, delivering insulin when glucose levels rise too high, and glucagon when they dip too low.
DIABETES WAS KNOWN IN ANCIENT TIMES
About 3,000 years ago, Egyptians described the syndrome of thirst, emaciation, and sweet urine that attracted ants. The term diabetes (Greek for siphon) was first recorded in 1425; mellitus (Latin for sweet with honey) was not added until 1675.
In 1857, Bernard hypothesized that diabetes was caused by overproduction of glucose in the liver. This idea was replaced in 1889, when Mering and Minkowski proposed the dysfunctional pancreas theory that eventually led to the discovery of the beta cell.2
In 1921, Banting and Best isolated insulin, and for the past 100 years subcutaneous insulin replacement has been the mainstay of treatment. But starting about 50 years ago, researchers have been looking for safe and long-lasting ways to replace beta cells and eliminate the need for exogenous insulin replacement.
TRANSPLANTING THE WHOLE PANCREAS
The first whole-pancreas transplant was performed in 1966 by Kelly et al,3 followed by 13 more by 1973.4 These first transplant grafts were short-lived, with only 1 graft surviving longer than 1 year. Since then, more than 12,000 pancreases have been transplanted worldwide, as refinements in surgical techniques and immunosuppressive therapies have improved patient and graft survival rates.4
Today, most pancreas transplants are in patients who have both type 1 diabetes and end-stage renal disease due to diabetic nephropathy, and most receive both a kidney and a pancreas at the same time. Far fewer patients receive a pancreas after previously receiving a kidney, or receive a pancreas alone.
The bile duct of the transplanted pancreas is usually routed into the patient’s small intestine, as nature intended, and less often into the bladder. Although bladder drainage is associated with urinary complications, it has the advantage of allowing measurement of pancreatic amylase levels in the urine to monitor for graft rejection. With simultaneous pancreas and kidney transplant, the serum creatinine concentration can also be monitored for rejection of the kidney graft.
Current immunosuppressive regimens vary but generally consist of anti-T-cell antibodies at the time of surgery, followed by lifelong treatment with the combination of a calcineurin inhibitor (cyclosporine or tacrolimus) and an antimetabolite (mycophenolate mofetil or azathioprine).
Outcomes are good. The rates of patient and graft survival are highest with simultaneous pancreas-kidney transplant, and somewhat lower with pancreas-after-kidney and pancreas-alone transplant.
Benefits of pancreas transplant
Most recipients can stop taking insulin immediately after the procedure, and their hemoglobin A1c levels normalize and stay low for the life of the graft. Lipid levels also decrease, although this has not been directly correlated with lower risk of vascular disease.4
Transplant also reduces or eliminates some complications of diabetes, including retinopathy, nephropathy, cardiomyopathy, and gastropathy.
For example, in patients undergoing simultaneous pancreas-kidney transplant, diabetic nephropathy does not recur in the new kidney. Fioretto et al5 reported that nephropathy lesions reversed during the 10 years after pancreas transplant.
Kennedy et al6,7 found that preexisting diabetic neuropathy improved slightly (although neurologic status did not completely return to normal) over a period of up to 42 months in a group of patients who received a pancreas transplant, whereas it tended to worsen in a control group. Both groups were assessed at baseline and at 12 and 24 months, with a subgroup followed through 42 months, and they underwent testing of motor, sensory, and autonomic function.6,7
Disadvantages of pancreas transplant
Disadvantages of whole-pancreas transplant include hypoglycemia (usually mild), adverse effects of immunosuppression, potential for surgical complications including an increased rate of death in the first 90 days after the procedure, and cost.
In an analysis comparing the 5-year estimated costs of dialysis, kidney transplant alone from cadavers or live donors, or simultaneous pancreas-kidney transplant for diabetic patients with end-stage renal disease, the least expensive option was kidney transplant from a live donor.8 The most expensive option was simultaneous pancreas-kidney transplant, but quality of life was better with this option. The analysis did not consider the potential cost of long-term treatments for complications related to diabetes that could be saved with a pancreas transplant.
Data conflict regarding the risk of death with different types of pancreas transplants. A retrospective cohort study of data from 124 US transplant centers reported in 2003 found higher mortality rates in pancreas-alone transplant recipients than in patients on a transplant waiting list receiving conventional therapy.9 In contrast, a 2004 study reported that after the first 90 days, when the risk of death was clearly higher, mortality rates were lower after simultaneous pancreas-kidney transplant and pancreas-after-kidney transplant.10 After pancreas-alone transplant, however, mortality rates were higher than with exogenous insulin therapy.
Although outcomes have improved, fewer patients with type 1 diabetes are undergoing pancreas transplant in recent years.
Interestingly, more simultaneous pancreas-kidney transplants are being successfully performed in patients with type 2 diabetes, who now account for 8% of all simultaneous pancreas-kidney transplant recipients.11 Outcomes of pancreas transplant appear to be similar regardless of diabetes type.
Bottom line
Pancreas transplant is a viable option for certain cases of complicated diabetes.
TRANSPLANTING ISLET CELLS
Despite its successes, pancreas transplant is major surgery and requires lifetime immunosuppression. Research is ongoing into a less-invasive procedure that, it is hoped, would require less immunosuppression: transplanting islets by themselves.
Islet autotransplant after pancreatectomy
For some patients with chronic pancreatitis, the only option to relieve chronic pain, narcotic dependence, and poor quality of life is to remove the pancreas. In the past, this desperate measure would instantly and inevitably cause diabetes, but not anymore.
Alpha cells and glucagon are a different story; a complication of islet transplant is hypoglycemia. In 2016, Lin et al12 reported spontaneous hypoglycemia in 6 of 12 patients who maintained insulin independence after autotransplant of islets. Although the transplanted islets had functional alpha cells that could in theory produce glucagon, as well as beta cells that produce insulin and C-peptide, apparently the alpha cells were not secreting glucagon in response to the hypoglycemia.
Location may matter. Gupta et al,13 in a 1997 study in dogs, found that more hypoglycemia occurs if islets are autotransplanted into the liver than if they are transplanted into the peritoneal cavity. A possible explanation may have to do with the glycemic environment of the liver.
Islet allotransplant
Islets can also be taken from cadaver donors and transplanted into patients with type 1 diabetes, who do not have enough working beta cells.
Success of allotransplant increased after the publication of observational data from the program in Edmonton in Canada, in which 7 consecutive patients with type 1 diabetes achieved initial insulin independence after islet allotransplant using steroid-free immunosuppression.14 Six recipients required islets from 2 donors, and 1 required islets from 4 donors, so they all received large volumes of at least 11,000 islet equivalents (IEQ) per kilogram of body weight.
In a subsequent report from the same team,15 16 (44%) of 36 patients remained insulin-free at 1 year, and C-peptide secretion was detectable in 70% at 2 years. But despite the elevated C-peptide levels, only 5 patients remained insulin-independent by 2 years. Lower hemoglobin A1c levels and decreases in hypoglycemic events from baseline also were noted.
The Clinical Islet Transplantation Consortium (CITC)16 and Collaborative Islet Transplant Registry (CITR)17 were established in 2004 to combine data and resources from centers around the world, including several that specialize in islet isolation and purification. Currently, more than 80 studies are being conducted.
The CITC and CITR now have data on more than 1,000 allogeneic islet transplant recipients (islet transplant alone, after kidney transplant, or simultaneous with it). The primary outcomes are hemoglobin A1c levels below 7% fasting C-peptide levels 0.3 ng/mL or higher, and fasting blood glucose of 60 to 140 mg/dL with no severe hypoglycemic events. The best results for islet-alone transplant have been in recipients over age 35 who received at least 325,000 IEQs with use of tumor necrosis factor antagonists for induction and calcineurin inhibitors or mammalian target of rapamycin (mTOR) inhibitors for maintenance.17
The best success for islet-after-kidney transplant was achieved with the same protocol but with insulin given to the donor during hospitalization before pancreas procurement. For participants with favorable factors, a hemoglobin A1c at or below 6.5% was achieved in about 80% at 1 year after last infusion, with more than 80% maintaining their fasting blood glucose level goals. About 70% of these patients were insulin-independent at 1 year. Hypoglycemia unawareness resolved in these patients even 5 years after infusion. Although there were no deaths or disabilities related to these transplants, bleeding occurred in 1 of 15 procedures. There was also a notable decline in estimated glomerular filtration rates with calcineurin inhibitor-based immunosuppression.17
Making islets go farther
One of the greatest challenges to islet transplant is the need for multiple donors to provide enough islet cells to overcome the loss of cells during transplant. Pancreases are already in short supply, and if each recipient needs more than 1, this makes the shortage worse. Some centers have achieved transplant with fewer donors,18,19 possibly by selecting pancreases from young donors who had a high body mass index and more islet cells, and harvesting and using them with a shorter cold ischemic time.
The number of viable, functioning islet cells drastically decreases after transplant, especially when transplanted into the portal system. This phenomenon is linked to an instant, blood-mediated inflammatory reaction involving antibody binding, complement and coagulation cascade activation, and platelet aggregation. The reaction, part of the innate immune system, damages the islet cells and leads to insulin dumping and early graft loss in studies in vitro and in vivo. Another factor affecting the survival of the graft cells is the low oxygen tension in the portal system.
For this reason, sites such as the pancreas, gastric submucosa, genitourinary tract, muscle, omentum, bone marrow, kidney capsule, peritoneum, anterior eye chamber, testis, and thymus are being explored.20
To create a more supportive environment for the transplanted cells, biotechnicians are trying to encapsulate islets in a semipermeable membrane that would protect them from the immune system while still allowing oxygen, nutrients, waste products, and, critically, insulin to diffuse in and out. Currently, no site or encapsulated product has been more successful than the current practice of implanting naked islets in the portal system.20
Bottom line
Without advances in transplant sites or increasing the yield of islet cells to allow single-donor transplants, islet cell allotransplant will not be feasible for most patients with type 1 diabetes.
Xenotransplant: Can pig cells make up the shortage?
Use of animal kidneys (xenotransplant) is a potential solution to the shortage of human organs for transplant.
In theory, pigs could be a source. Porcine insulin is similar to human insulin (differing by only 1 amino acid), and it should be possible to breed “knockout” pigs that lack the antigens responsible for acute humoral rejection.21
On the other hand, transplant of porcine islets poses several immunologic, physiologic, ethical, legal, and infectious concerns. For example, porcine tissue could carry pig viruses, such as porcine endogenous retroviruses.21 And even if the pigs are genetically modified, patients will still require immunosuppressive therapy.
A review of 17 studies of pig islet xenotransplant into nonhuman primates found that in 5 of the studies (4 using diabetic primates) the grafts survived at least 3 months.22 Of these, 1 study used encapsulation, and the rest used intensive and toxic immunosuppression.
More research is needed to make xenotransplant a clinical option.
Transplanting stem cells or beta cells grown from stem cells
Stem cells provide an exciting potential alternative to the limited donor pool. During the past decade, several studies have shown success using human pluripotent stem cells (embryonic stem cells and human-induced pluripotent stem cells), mesenchymal stem cells isolated from adult tissues, and directly programmed somatic cells. Researchers have created stable cultures of pluripotent stem cells from embryonic stem cells, which could possibly be produced on a large scale and banked.23
Human pluripotent stem cells derived from pancreatic progenitors have been shown to mature into more functional, islet-like structures in vivo. They transform into subtypes of islet cells including alpha, beta, and delta cells, ghrelin-producing cells, and pancreatic polypeptide hormone-producing cells. This process takes 2 to 6 weeks. In mice, these cells have been shown to maintain glucose homeostasis.24 Phase 1 and 2 trials in humans are now being conducted.
Pagliuca et al25 generated functional human pancreatic beta cells in vitro from embryonic stem cells. Rezania et al24 reversed diabetes with insulin-producing cells derived in vitro from human pluripotent stem cells. The techniques used in these studies contributed to the success of a study by Vegas et al,26 who achieved successful long-term glycemic control in mice using polymer-encapsulated human stem cell-derived beta cells.
Reversal of autoimmunity is an important step that needs to be overcome in stem cell transplant for type 1 diabetes. Nikolic et al27 have achieved mixed allogeneic chimerism across major histocompatibility complex barriers with nonmyeloablative conditioning in advanced-diabetic nonobese diabetic mice. However, conditioning alone (ie, without bone marrow transplant) does not permit acceptance of allogeneic islets and does not reverse autoimmunity or allow islet regeneration.28 Adding allogeneic bone marrow transplant to conditioned nonobese diabetic mice leads to tolerance to the donor and reverses autoimmunity.
THE ‘BIONIC’ PANCREAS
While we wait for advances in islet cell transplant, improved insulin pumps hold promise.
One such experimental device, the iLet (Beta Bionics, Boston, MA), designed by Damiano et al, consists of 2 infusion pumps (1 for insulin, 1 for glucagon) linked to a continuous glucose monitor via a smartphone app.
The monitor measures the glucose level every 5 minutes and transmits the information wirelessly to the phone app, which calculates the amount of insulin and glucagon required to stabilize the blood glucose: more insulin if too high, more glucagon if too low. The phone transmits this information to the pumps.
Dubbed the “bionic” pancreas, this closed-loop system frees patients from the tasks of measuring their glucose multiple times a day, calculating the appropriate dose, and giving multiple insulin injections.
The 2016 summer camp study29 followed 19 preteens wearing the bionic pancreas for 5 days. During this time, the patients had lower mean glucose levels and less hypoglycemia than during control periods. No episodes of severe hypoglycemia were recorded.
El-Khatib et al30 randomly assigned 43 patients to treatment with either the bihormonal bionic pancreas or usual care (a conventional insulin pump or a sensor-augmented insulin pump) for 11 days, followed by 11 days of the opposite treatment. All participants continued their normal activities. The bionic pancreas system was superior to the insulin pump in terms of the mean glucose concentration and mean time in the hypoglycemic range (P < .0001 for both results).
Bottom line
As the search continues for better solutions, advances in technology such as the bionic pancreas could provide a safer (ie, less hypoglycemic) and more successful alternative for insulin replacement in the near future.
With intensive insulin regimens and home blood glucose monitoring, patients with type 1 diabetes are controlling their blood glucose better than in the past. Nevertheless, glucose regulation is still imperfect and tedious, and striving for tight glycemic control poses the risk of hypoglycemia.
Prominent among the challenges are the sheer numbers involved. Some 1.25 million Americans have type 1 diabetes, and another 30 million have type 2, but only about 7,000 to 8,000 pancreases are available for transplant each year.1 While awaiting a breakthrough—perhaps involving stem cells, perhaps involving organs obtained from animals—an insulin pump may offer better diabetes control for many. Another possibility is a closed-loop system with a continuous glucose monitor that drives a dual-infusion pump, delivering insulin when glucose levels rise too high, and glucagon when they dip too low.
DIABETES WAS KNOWN IN ANCIENT TIMES
About 3,000 years ago, Egyptians described the syndrome of thirst, emaciation, and sweet urine that attracted ants. The term diabetes (Greek for siphon) was first recorded in 1425; mellitus (Latin for sweet with honey) was not added until 1675.
In 1857, Bernard hypothesized that diabetes was caused by overproduction of glucose in the liver. This idea was replaced in 1889, when Mering and Minkowski proposed the dysfunctional pancreas theory that eventually led to the discovery of the beta cell.2
In 1921, Banting and Best isolated insulin, and for the past 100 years subcutaneous insulin replacement has been the mainstay of treatment. But starting about 50 years ago, researchers have been looking for safe and long-lasting ways to replace beta cells and eliminate the need for exogenous insulin replacement.
TRANSPLANTING THE WHOLE PANCREAS
The first whole-pancreas transplant was performed in 1966 by Kelly et al,3 followed by 13 more by 1973.4 These first transplant grafts were short-lived, with only 1 graft surviving longer than 1 year. Since then, more than 12,000 pancreases have been transplanted worldwide, as refinements in surgical techniques and immunosuppressive therapies have improved patient and graft survival rates.4
Today, most pancreas transplants are in patients who have both type 1 diabetes and end-stage renal disease due to diabetic nephropathy, and most receive both a kidney and a pancreas at the same time. Far fewer patients receive a pancreas after previously receiving a kidney, or receive a pancreas alone.
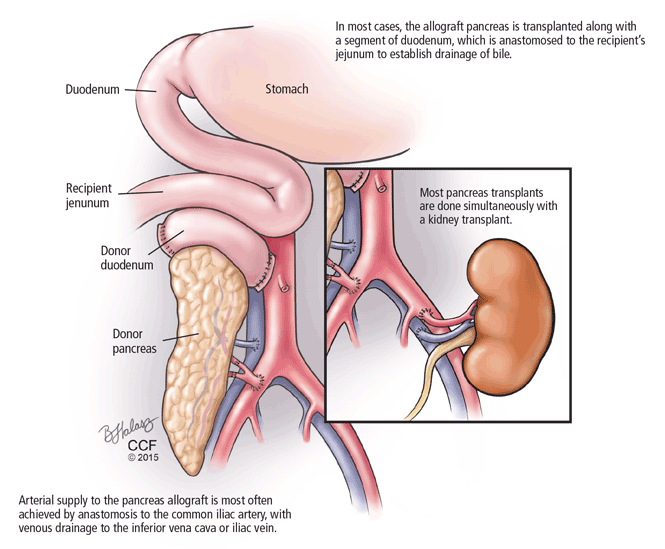
The bile duct of the transplanted pancreas is usually routed into the patient’s small intestine, as nature intended, and less often into the bladder. Although bladder drainage is associated with urinary complications, it has the advantage of allowing measurement of pancreatic amylase levels in the urine to monitor for graft rejection. With simultaneous pancreas and kidney transplant, the serum creatinine concentration can also be monitored for rejection of the kidney graft.
Current immunosuppressive regimens vary but generally consist of anti-T-cell antibodies at the time of surgery, followed by lifelong treatment with the combination of a calcineurin inhibitor (cyclosporine or tacrolimus) and an antimetabolite (mycophenolate mofetil or azathioprine).
Outcomes are good. The rates of patient and graft survival are highest with simultaneous pancreas-kidney transplant, and somewhat lower with pancreas-after-kidney and pancreas-alone transplant.
Benefits of pancreas transplant
Most recipients can stop taking insulin immediately after the procedure, and their hemoglobin A1c levels normalize and stay low for the life of the graft. Lipid levels also decrease, although this has not been directly correlated with lower risk of vascular disease.4
Transplant also reduces or eliminates some complications of diabetes, including retinopathy, nephropathy, cardiomyopathy, and gastropathy.
For example, in patients undergoing simultaneous pancreas-kidney transplant, diabetic nephropathy does not recur in the new kidney. Fioretto et al5 reported that nephropathy lesions reversed during the 10 years after pancreas transplant.
Kennedy et al6,7 found that preexisting diabetic neuropathy improved slightly (although neurologic status did not completely return to normal) over a period of up to 42 months in a group of patients who received a pancreas transplant, whereas it tended to worsen in a control group. Both groups were assessed at baseline and at 12 and 24 months, with a subgroup followed through 42 months, and they underwent testing of motor, sensory, and autonomic function.6,7
Disadvantages of pancreas transplant
Disadvantages of whole-pancreas transplant include hypoglycemia (usually mild), adverse effects of immunosuppression, potential for surgical complications including an increased rate of death in the first 90 days after the procedure, and cost.
In an analysis comparing the 5-year estimated costs of dialysis, kidney transplant alone from cadavers or live donors, or simultaneous pancreas-kidney transplant for diabetic patients with end-stage renal disease, the least expensive option was kidney transplant from a live donor.8 The most expensive option was simultaneous pancreas-kidney transplant, but quality of life was better with this option. The analysis did not consider the potential cost of long-term treatments for complications related to diabetes that could be saved with a pancreas transplant.
Data conflict regarding the risk of death with different types of pancreas transplants. A retrospective cohort study of data from 124 US transplant centers reported in 2003 found higher mortality rates in pancreas-alone transplant recipients than in patients on a transplant waiting list receiving conventional therapy.9 In contrast, a 2004 study reported that after the first 90 days, when the risk of death was clearly higher, mortality rates were lower after simultaneous pancreas-kidney transplant and pancreas-after-kidney transplant.10 After pancreas-alone transplant, however, mortality rates were higher than with exogenous insulin therapy.
Although outcomes have improved, fewer patients with type 1 diabetes are undergoing pancreas transplant in recent years.
Interestingly, more simultaneous pancreas-kidney transplants are being successfully performed in patients with type 2 diabetes, who now account for 8% of all simultaneous pancreas-kidney transplant recipients.11 Outcomes of pancreas transplant appear to be similar regardless of diabetes type.
Bottom line
Pancreas transplant is a viable option for certain cases of complicated diabetes.
TRANSPLANTING ISLET CELLS
Despite its successes, pancreas transplant is major surgery and requires lifetime immunosuppression. Research is ongoing into a less-invasive procedure that, it is hoped, would require less immunosuppression: transplanting islets by themselves.
Islet autotransplant after pancreatectomy
For some patients with chronic pancreatitis, the only option to relieve chronic pain, narcotic dependence, and poor quality of life is to remove the pancreas. In the past, this desperate measure would instantly and inevitably cause diabetes, but not anymore.
Alpha cells and glucagon are a different story; a complication of islet transplant is hypoglycemia. In 2016, Lin et al12 reported spontaneous hypoglycemia in 6 of 12 patients who maintained insulin independence after autotransplant of islets. Although the transplanted islets had functional alpha cells that could in theory produce glucagon, as well as beta cells that produce insulin and C-peptide, apparently the alpha cells were not secreting glucagon in response to the hypoglycemia.
Location may matter. Gupta et al,13 in a 1997 study in dogs, found that more hypoglycemia occurs if islets are autotransplanted into the liver than if they are transplanted into the peritoneal cavity. A possible explanation may have to do with the glycemic environment of the liver.
Islet allotransplant
Islets can also be taken from cadaver donors and transplanted into patients with type 1 diabetes, who do not have enough working beta cells.
Success of allotransplant increased after the publication of observational data from the program in Edmonton in Canada, in which 7 consecutive patients with type 1 diabetes achieved initial insulin independence after islet allotransplant using steroid-free immunosuppression.14 Six recipients required islets from 2 donors, and 1 required islets from 4 donors, so they all received large volumes of at least 11,000 islet equivalents (IEQ) per kilogram of body weight.
In a subsequent report from the same team,15 16 (44%) of 36 patients remained insulin-free at 1 year, and C-peptide secretion was detectable in 70% at 2 years. But despite the elevated C-peptide levels, only 5 patients remained insulin-independent by 2 years. Lower hemoglobin A1c levels and decreases in hypoglycemic events from baseline also were noted.
The Clinical Islet Transplantation Consortium (CITC)16 and Collaborative Islet Transplant Registry (CITR)17 were established in 2004 to combine data and resources from centers around the world, including several that specialize in islet isolation and purification. Currently, more than 80 studies are being conducted.
The CITC and CITR now have data on more than 1,000 allogeneic islet transplant recipients (islet transplant alone, after kidney transplant, or simultaneous with it). The primary outcomes are hemoglobin A1c levels below 7% fasting C-peptide levels 0.3 ng/mL or higher, and fasting blood glucose of 60 to 140 mg/dL with no severe hypoglycemic events. The best results for islet-alone transplant have been in recipients over age 35 who received at least 325,000 IEQs with use of tumor necrosis factor antagonists for induction and calcineurin inhibitors or mammalian target of rapamycin (mTOR) inhibitors for maintenance.17
The best success for islet-after-kidney transplant was achieved with the same protocol but with insulin given to the donor during hospitalization before pancreas procurement. For participants with favorable factors, a hemoglobin A1c at or below 6.5% was achieved in about 80% at 1 year after last infusion, with more than 80% maintaining their fasting blood glucose level goals. About 70% of these patients were insulin-independent at 1 year. Hypoglycemia unawareness resolved in these patients even 5 years after infusion. Although there were no deaths or disabilities related to these transplants, bleeding occurred in 1 of 15 procedures. There was also a notable decline in estimated glomerular filtration rates with calcineurin inhibitor-based immunosuppression.17
Making islets go farther
One of the greatest challenges to islet transplant is the need for multiple donors to provide enough islet cells to overcome the loss of cells during transplant. Pancreases are already in short supply, and if each recipient needs more than 1, this makes the shortage worse. Some centers have achieved transplant with fewer donors,18,19 possibly by selecting pancreases from young donors who had a high body mass index and more islet cells, and harvesting and using them with a shorter cold ischemic time.
The number of viable, functioning islet cells drastically decreases after transplant, especially when transplanted into the portal system. This phenomenon is linked to an instant, blood-mediated inflammatory reaction involving antibody binding, complement and coagulation cascade activation, and platelet aggregation. The reaction, part of the innate immune system, damages the islet cells and leads to insulin dumping and early graft loss in studies in vitro and in vivo. Another factor affecting the survival of the graft cells is the low oxygen tension in the portal system.
For this reason, sites such as the pancreas, gastric submucosa, genitourinary tract, muscle, omentum, bone marrow, kidney capsule, peritoneum, anterior eye chamber, testis, and thymus are being explored.20
To create a more supportive environment for the transplanted cells, biotechnicians are trying to encapsulate islets in a semipermeable membrane that would protect them from the immune system while still allowing oxygen, nutrients, waste products, and, critically, insulin to diffuse in and out. Currently, no site or encapsulated product has been more successful than the current practice of implanting naked islets in the portal system.20
Bottom line
Without advances in transplant sites or increasing the yield of islet cells to allow single-donor transplants, islet cell allotransplant will not be feasible for most patients with type 1 diabetes.
Xenotransplant: Can pig cells make up the shortage?
Use of animal kidneys (xenotransplant) is a potential solution to the shortage of human organs for transplant.
In theory, pigs could be a source. Porcine insulin is similar to human insulin (differing by only 1 amino acid), and it should be possible to breed “knockout” pigs that lack the antigens responsible for acute humoral rejection.21
On the other hand, transplant of porcine islets poses several immunologic, physiologic, ethical, legal, and infectious concerns. For example, porcine tissue could carry pig viruses, such as porcine endogenous retroviruses.21 And even if the pigs are genetically modified, patients will still require immunosuppressive therapy.
A review of 17 studies of pig islet xenotransplant into nonhuman primates found that in 5 of the studies (4 using diabetic primates) the grafts survived at least 3 months.22 Of these, 1 study used encapsulation, and the rest used intensive and toxic immunosuppression.
More research is needed to make xenotransplant a clinical option.
Transplanting stem cells or beta cells grown from stem cells
Stem cells provide an exciting potential alternative to the limited donor pool. During the past decade, several studies have shown success using human pluripotent stem cells (embryonic stem cells and human-induced pluripotent stem cells), mesenchymal stem cells isolated from adult tissues, and directly programmed somatic cells. Researchers have created stable cultures of pluripotent stem cells from embryonic stem cells, which could possibly be produced on a large scale and banked.23
Human pluripotent stem cells derived from pancreatic progenitors have been shown to mature into more functional, islet-like structures in vivo. They transform into subtypes of islet cells including alpha, beta, and delta cells, ghrelin-producing cells, and pancreatic polypeptide hormone-producing cells. This process takes 2 to 6 weeks. In mice, these cells have been shown to maintain glucose homeostasis.24 Phase 1 and 2 trials in humans are now being conducted.
Pagliuca et al25 generated functional human pancreatic beta cells in vitro from embryonic stem cells. Rezania et al24 reversed diabetes with insulin-producing cells derived in vitro from human pluripotent stem cells. The techniques used in these studies contributed to the success of a study by Vegas et al,26 who achieved successful long-term glycemic control in mice using polymer-encapsulated human stem cell-derived beta cells.
Reversal of autoimmunity is an important step that needs to be overcome in stem cell transplant for type 1 diabetes. Nikolic et al27 have achieved mixed allogeneic chimerism across major histocompatibility complex barriers with nonmyeloablative conditioning in advanced-diabetic nonobese diabetic mice. However, conditioning alone (ie, without bone marrow transplant) does not permit acceptance of allogeneic islets and does not reverse autoimmunity or allow islet regeneration.28 Adding allogeneic bone marrow transplant to conditioned nonobese diabetic mice leads to tolerance to the donor and reverses autoimmunity.
THE ‘BIONIC’ PANCREAS
While we wait for advances in islet cell transplant, improved insulin pumps hold promise.
One such experimental device, the iLet (Beta Bionics, Boston, MA), designed by Damiano et al, consists of 2 infusion pumps (1 for insulin, 1 for glucagon) linked to a continuous glucose monitor via a smartphone app.
The monitor measures the glucose level every 5 minutes and transmits the information wirelessly to the phone app, which calculates the amount of insulin and glucagon required to stabilize the blood glucose: more insulin if too high, more glucagon if too low. The phone transmits this information to the pumps.
Dubbed the “bionic” pancreas, this closed-loop system frees patients from the tasks of measuring their glucose multiple times a day, calculating the appropriate dose, and giving multiple insulin injections.
The 2016 summer camp study29 followed 19 preteens wearing the bionic pancreas for 5 days. During this time, the patients had lower mean glucose levels and less hypoglycemia than during control periods. No episodes of severe hypoglycemia were recorded.
El-Khatib et al30 randomly assigned 43 patients to treatment with either the bihormonal bionic pancreas or usual care (a conventional insulin pump or a sensor-augmented insulin pump) for 11 days, followed by 11 days of the opposite treatment. All participants continued their normal activities. The bionic pancreas system was superior to the insulin pump in terms of the mean glucose concentration and mean time in the hypoglycemic range (P < .0001 for both results).
Bottom line
As the search continues for better solutions, advances in technology such as the bionic pancreas could provide a safer (ie, less hypoglycemic) and more successful alternative for insulin replacement in the near future.
- American Diabetes Association. Statistics about diabetes: overall numbers, diabetes and prediabetes. www.diabetes.org/diabetes-basics/statistics/. Accessed November 6, 2018.
- Ahmed AM. History of diabetes mellitus. Saudi Med J 2002; 23(4):373–378. pmid:11953758
- Kelly WD, Lillehei RC, Merkel FK, Idezuki Y, Goetz FC. Allotransplantation of the pancreas and duodenum along with the kidney in diabetic nephropathy. Surgery 1967; 61:827–837. pmid: 5338113
- Sutherland DE, Gruessner RW, Dunn DL, et al. Lessons learned from more than 1,000 pancreas transplants at a single institution. Ann Surg 2001; 233(4):463–501. pmid:11303130
- Fioretto P, Steffes MW, Sutherland DE, Goetz FC, Mauer M. Reversal of lesions of diabetic nephropathy after pancreas transplantation. N Engl J Med 1998; 339(2):69–75. doi:10.1056/NEJM199807093390202
- Kennedy WR, Navarro X, Goetz FC, Sutherland DE, Najarian JS. Effects of pancreatic transplantation on diabetic neuropathy. N Engl J Med 1990; 322(15):1031–1037. doi:10.1056/NEJM199004123221503
- Kennedy WR, Navarro X, Sutherland DER. Neuropathy profile of diabetic patients in a pancreas transplantation program. Neurology 1995; 45(4):773–780. pmid:7723969
- Douzdjian V, Ferrara D, Silvestri G. Treatment strategies for insulin-dependent diabetics with ESRD: a cost-effectiveness decision analysis model. Am J Kidney Dis 1998; 31(5):794–802. pmid:9590189
- Venstrom JM, McBride MA, Rother KI, Hirshberg B, Orchard TJ, Harlan DM. Survival after pancreas transplantation in patients with diabetes and preserved kidney function. JAMA 2003; 290(21):2817–2823. doi:10.1001/jama.290.21.2817
- Gruessner RW, Sutherland DE, Gruessner AC. Mortality assessment for pancreas transplants. Am J Transplant 2004; 4(12):2018–2026. doi:10.1111/j.1600-6143.2004.00667.x
- Redfield RR, Scalea JR, Odorico JS. Simultaneous pancreas and kidney transplantation: current trends and future directions. Curr Opin Organ Transplant 2015; 20(1):94-102. doi:10.1097/MOT.0000000000000146
- Lin YK, Faiman C, Johnston PC, et al. Spontaneous hypoglycemia after islet autotransplantation for chronic pancreatitis. J Clin Endocrinol Metab 2016; 101(10):3669–3675. doi:10.1210/jc.2016-2111
- Gupta V, Wahoff DC, Rooney DP, et al. The defective glucagon response from transplanted intrahepatic pancreatic islets during hypoglycemia is transplantation site-determined. Diabetes 1997; 46(1):28–33. pmid:8971077
- Shapiro AM, Lakey JR, Ryan EA, et al. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med 2000; 343(4):230–238. doi:10.1056/NEJM200007273430401
- Shapiro AM, Ricordi C, Hering BJ, et al. International trial of the Edmonton protocol for islet transplantation. N Engl J Med 2006; 355(13):1318–1330. doi:10.1056/NEJMoa061267
- Clinical Islet Transplantation (CIT) Consortium. www.citisletstudy.org. Accessed November 6, 2018.
- Collaborative Islet Transplantation Registry (CITR). CITR 10th Annual Report. https://citregistry.org/system/files/10th_AR.pdf. Accessed November 6, 2018.
- Hering BJ, Kandaswamy R, Harmon JV, et al. Transplantation of cultured islets from two-layer preserved pancreases in type 1 diabetes with anti-CD3 antibody. Am J Transplant 2004; 4(3):390–401. pmid:14961992
- Posselt AM, Bellin MD, Tavakol M, et al. Islet transplantation in type 1 diabetics using an immunosuppressive protocol based on the anti-LFA-1 antibody efalizumab. Am J Transplant 2010; 10(8):1870–1880. doi:10.1111/j.1600-6143.2010.03073.x
- Cantarelli E, Piemonti L. Alternative transplantation sites for pancreatic islet grafts. Curr Diab Rep 2011; 11(5):364–374. doi:10.1007/s11892-011-0216-9
- Cooper DK, Gollackner B, Knosalla C, Teranishi K. Xenotransplantation—how far have we come? Transpl Immunol 2002; 9(2–4):251–256. pmid:12180839
- Marigliano M, Bertera S, Grupillo M, Trucco M, Bottino R. Pig-to-nonhuman primates pancreatic islet xenotransplantation: an overview. Curr Diab Rep 2011; 11(5):402–412. doi:10.1007/s11892-011-0213-z
- Bartlett ST, Markmann JF, Johnson P, et al. Report from IPITA-TTS opinion leaders meeting on the future of beta-cell replacement. Transplantation 2016; 100(suppl 2):S1–S44. doi:10.1097/TP.0000000000001055
- Rezania A, Bruin JE, Arora P, et al. Reversal of diabetes with insulin-producing cells derived in vitro from human pluripotent stem cells. Nat Biotechnol 2014; 32(11):1121–1133. doi:10.1038/nbt.3033
- Pagliuca FW, Millman JR, Gurtler M, et al. Generation of functional human pancreatic beta cells in vitro. Cell 2014; 159(2):428–439. doi:10.1016/j.cell.2014.09.040
- Vegas AJ, Veiseh O, Gurtler M, et al. Long-term glycemic control using polymer-encapsulated human stem cell-derived beta cells in immune-competent mice. Nat Med 2016; 22(3):306–311. doi:10.1038/nm.4030
- Nikolic B, Takeuchi Y, Leykin I, Fudaba Y, Smith RN, Sykes M. Mixed hematopoietic chimerism allows cure of autoimmune tolerance and reversal of autoimmunity. Diabetes 2004; 53(2):376–383. pmid:14747288
- Li HW, Sykes M. Emerging concepts in haematopoietic cell transplantation. Nat Rev Immunol 2012; 12(6):403–416. doi:10.1038/nri3226
- Russell SJ, Hillard MA, Balliro C, et al. Day and night glycaemic control with a bionic pancreas versus conventional insulin pump therapy in preadolescent children with type 1 diabetes: a randomised crossover trial. Lancet Diabetes Endocrinol 2016; 4(3):233–243. doi:10.1016/S2213-8587(15)00489-1
- El-Khatib FH, Balliro C, Hillard MA, et al. Home use of a bihormonal bionic pancreas versus insulin pump therapy in adults with type 1 diabetes: a multicenter randomized crossover trial. Lancet 2017; 389(10067):369–380. doi:10.1016/S0140-6736(16)32567-3
- American Diabetes Association. Statistics about diabetes: overall numbers, diabetes and prediabetes. www.diabetes.org/diabetes-basics/statistics/. Accessed November 6, 2018.
- Ahmed AM. History of diabetes mellitus. Saudi Med J 2002; 23(4):373–378. pmid:11953758
- Kelly WD, Lillehei RC, Merkel FK, Idezuki Y, Goetz FC. Allotransplantation of the pancreas and duodenum along with the kidney in diabetic nephropathy. Surgery 1967; 61:827–837. pmid: 5338113
- Sutherland DE, Gruessner RW, Dunn DL, et al. Lessons learned from more than 1,000 pancreas transplants at a single institution. Ann Surg 2001; 233(4):463–501. pmid:11303130
- Fioretto P, Steffes MW, Sutherland DE, Goetz FC, Mauer M. Reversal of lesions of diabetic nephropathy after pancreas transplantation. N Engl J Med 1998; 339(2):69–75. doi:10.1056/NEJM199807093390202
- Kennedy WR, Navarro X, Goetz FC, Sutherland DE, Najarian JS. Effects of pancreatic transplantation on diabetic neuropathy. N Engl J Med 1990; 322(15):1031–1037. doi:10.1056/NEJM199004123221503
- Kennedy WR, Navarro X, Sutherland DER. Neuropathy profile of diabetic patients in a pancreas transplantation program. Neurology 1995; 45(4):773–780. pmid:7723969
- Douzdjian V, Ferrara D, Silvestri G. Treatment strategies for insulin-dependent diabetics with ESRD: a cost-effectiveness decision analysis model. Am J Kidney Dis 1998; 31(5):794–802. pmid:9590189
- Venstrom JM, McBride MA, Rother KI, Hirshberg B, Orchard TJ, Harlan DM. Survival after pancreas transplantation in patients with diabetes and preserved kidney function. JAMA 2003; 290(21):2817–2823. doi:10.1001/jama.290.21.2817
- Gruessner RW, Sutherland DE, Gruessner AC. Mortality assessment for pancreas transplants. Am J Transplant 2004; 4(12):2018–2026. doi:10.1111/j.1600-6143.2004.00667.x
- Redfield RR, Scalea JR, Odorico JS. Simultaneous pancreas and kidney transplantation: current trends and future directions. Curr Opin Organ Transplant 2015; 20(1):94-102. doi:10.1097/MOT.0000000000000146
- Lin YK, Faiman C, Johnston PC, et al. Spontaneous hypoglycemia after islet autotransplantation for chronic pancreatitis. J Clin Endocrinol Metab 2016; 101(10):3669–3675. doi:10.1210/jc.2016-2111
- Gupta V, Wahoff DC, Rooney DP, et al. The defective glucagon response from transplanted intrahepatic pancreatic islets during hypoglycemia is transplantation site-determined. Diabetes 1997; 46(1):28–33. pmid:8971077
- Shapiro AM, Lakey JR, Ryan EA, et al. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med 2000; 343(4):230–238. doi:10.1056/NEJM200007273430401
- Shapiro AM, Ricordi C, Hering BJ, et al. International trial of the Edmonton protocol for islet transplantation. N Engl J Med 2006; 355(13):1318–1330. doi:10.1056/NEJMoa061267
- Clinical Islet Transplantation (CIT) Consortium. www.citisletstudy.org. Accessed November 6, 2018.
- Collaborative Islet Transplantation Registry (CITR). CITR 10th Annual Report. https://citregistry.org/system/files/10th_AR.pdf. Accessed November 6, 2018.
- Hering BJ, Kandaswamy R, Harmon JV, et al. Transplantation of cultured islets from two-layer preserved pancreases in type 1 diabetes with anti-CD3 antibody. Am J Transplant 2004; 4(3):390–401. pmid:14961992
- Posselt AM, Bellin MD, Tavakol M, et al. Islet transplantation in type 1 diabetics using an immunosuppressive protocol based on the anti-LFA-1 antibody efalizumab. Am J Transplant 2010; 10(8):1870–1880. doi:10.1111/j.1600-6143.2010.03073.x
- Cantarelli E, Piemonti L. Alternative transplantation sites for pancreatic islet grafts. Curr Diab Rep 2011; 11(5):364–374. doi:10.1007/s11892-011-0216-9
- Cooper DK, Gollackner B, Knosalla C, Teranishi K. Xenotransplantation—how far have we come? Transpl Immunol 2002; 9(2–4):251–256. pmid:12180839
- Marigliano M, Bertera S, Grupillo M, Trucco M, Bottino R. Pig-to-nonhuman primates pancreatic islet xenotransplantation: an overview. Curr Diab Rep 2011; 11(5):402–412. doi:10.1007/s11892-011-0213-z
- Bartlett ST, Markmann JF, Johnson P, et al. Report from IPITA-TTS opinion leaders meeting on the future of beta-cell replacement. Transplantation 2016; 100(suppl 2):S1–S44. doi:10.1097/TP.0000000000001055
- Rezania A, Bruin JE, Arora P, et al. Reversal of diabetes with insulin-producing cells derived in vitro from human pluripotent stem cells. Nat Biotechnol 2014; 32(11):1121–1133. doi:10.1038/nbt.3033
- Pagliuca FW, Millman JR, Gurtler M, et al. Generation of functional human pancreatic beta cells in vitro. Cell 2014; 159(2):428–439. doi:10.1016/j.cell.2014.09.040
- Vegas AJ, Veiseh O, Gurtler M, et al. Long-term glycemic control using polymer-encapsulated human stem cell-derived beta cells in immune-competent mice. Nat Med 2016; 22(3):306–311. doi:10.1038/nm.4030
- Nikolic B, Takeuchi Y, Leykin I, Fudaba Y, Smith RN, Sykes M. Mixed hematopoietic chimerism allows cure of autoimmune tolerance and reversal of autoimmunity. Diabetes 2004; 53(2):376–383. pmid:14747288
- Li HW, Sykes M. Emerging concepts in haematopoietic cell transplantation. Nat Rev Immunol 2012; 12(6):403–416. doi:10.1038/nri3226
- Russell SJ, Hillard MA, Balliro C, et al. Day and night glycaemic control with a bionic pancreas versus conventional insulin pump therapy in preadolescent children with type 1 diabetes: a randomised crossover trial. Lancet Diabetes Endocrinol 2016; 4(3):233–243. doi:10.1016/S2213-8587(15)00489-1
- El-Khatib FH, Balliro C, Hillard MA, et al. Home use of a bihormonal bionic pancreas versus insulin pump therapy in adults with type 1 diabetes: a multicenter randomized crossover trial. Lancet 2017; 389(10067):369–380. doi:10.1016/S0140-6736(16)32567-3
KEY POINTS
- Most pancreas transplant recipients become insulin-independent immediately.
- A key drawback to islet transplant is the need for multiple donors to provide enough islet cells to achieve insulin independence.
- As with other organs for transplant, the need for donor pancreases far outnumbers the supply. Stem cells or beta cells grown from stem cells may avoid this problem. Another potential solution is to use organs from animals, possibly pigs, but much more work is needed to make these procedures viable.
- While we await a breakthrough in beta-cell therapy, a bionic pancreas may be the answer for a number of patients.
Pancreas transplant for diabetes mellitus
Pancreas transplant is the only long-term diabetes treatment that consistently results in normal hemoglobin A1c levels without the risk of severe hypoglycemia. Additionally, pancreas transplant may prevent, halt, or even reverse the complications of diabetes.
Here, we explore the indications, options, and outcomes of pancreas transplant as a treatment for diabetes mellitus.
DIABETES IS COMMON, AND OFTEN NOT WELL CONTROLLED
Diabetes mellitus affects more than 25 million people in the United States (8.3% of the population) and is the leading cause of kidney failure, nontraumatic lower-limb amputation, and adult-onset blindness. In 2007, nearly $116 billion was spent on diabetes treatment, not counting another $58 billion in indirect costs such as disability, work loss, and premature death.1
Despite the tremendous expenditure in human, material, and financial resources, only about 50% of patients achieve their diabetes treatment goals. In 2013, a large US population-based study2 reported that 52.2% of patients were achieving the American Diabetes Association treatment goal of hemoglobin A1c lower than 7%. A similar study in South Korea3 found that 45.6% were at this goal.
Most of the patients in these studies had type 2 diabetes, and the data suggested that attaining glycemic goals is more difficult in insulin-treated patients. Studies of patients with type 1 diabetes found hemoglobin A1c levels lower than 7% in only 8.1% of hospitalized patients with type 1 diabetes, and in only 13% in an outpatient diabetes clinic.4,5
YET RATES OF PANCREAS TRANSPLANT ARE DECLINING
Pancreas transplant was first performed more than 40 years ago at the University of Minnesota.6 Since then, dramatic changes in immunosuppression, organ preservation, surgical technique, and donor and recipient selection have brought about significant progress.
Currently, more than 13,000 patients are alive with a functioning pancreas allograft. After reaching a peak in 2004, the annual number of pancreas transplants performed in the United States has declined steadily, whereas the procedure continues to increase in popularity outside North America.7 The primary reason for the decline is recognition of donor factors that lead to success—surgeons are refusing to transplant organs they might have accepted previously, because experience suggests they would yield poor results. In the United States, 1,043 pancreas transplants were performed in 2012, and more than 3,100 patients were on the waiting list.8
Islet cell transplant—a different procedure involving harvesting, encapsulating, and implanting insulin-producing beta cells—has not gained widespread application due to very low long-term success rates.
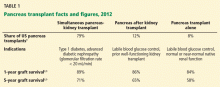
THREE CATEGORIES OF PANCREAS TRANSPLANT
Pancreas transplant can be categorized according to whether the patient is also receiving or has already received a kidney graft (Table 1).
Simultaneous kidney and pancreas transplant is performed in patients who have type 1 diabetes with advanced chronic kidney disease due to diabetic nephropathy. This remains the most commonly performed type, accounting for 79% of all pancreas transplants in 2012.8
Pancreas-after-kidney transplant is most often done after a living-donor kidney transplant. This procedure accounted for most of the increase in pancreas transplants during the first decade of the 2000s. However, the number of these procedures has steadily decreased since 2004, and in 2012 accounted for only 12% of pancreas transplants.8
Pancreas transplant alone is performed in nonuremic diabetic patients who have labile blood sugar control. Performed in patients with preserved renal function but severe complications of “brittle” diabetes, such as hypoglycemic unawareness, this type accounts for 8% of pancreas transplants.9
Indications for pancreas transplant
A small number of these procedures are done for indications unrelated to diabetes mellitus. In most of these cases, the pancreas is transplanted as part of a multivisceral transplant to facilitate the technical (surgical) aspect of the procedure—the pancreas, liver, stomach, gallbladder, and part of the intestines are transplanted en bloc to maintain the native vasculature. Very infrequently, pancreas transplant is done to replace exocrine pancreatic function.
A small, select group of patients with type 2 diabetes and low body mass index (BMI) may be eligible for pancreas transplant, and they accounted for 8.2% of active candidates in 2012.8 However, most pancreas transplants are performed in patients with type 1 diabetes.
WHAT MAKES A GOOD ALLOGRAFT?
Pancreas allografts are procured as whole organs from brain-dead organ donors. Relatively few pancreas allografts (3.1% in 2012) are from cardiac-death donors, because of concern about warm ischemic injury during the period of circulatory arrest.8
Proper donor selection is critical to the success of pancreas transplant, as donor factors including medical history, age, BMI, and cause of death can significantly affect the outcome. In general, transplant of a pancreas allograft from a young donor (age < 30) with excellent organ function, low BMI, and traumatic cause of death provides the best chance of success.
The Pancreas Donor Risk Index (PDRI)10 was developed after analysis of objective donor criteria, transplant type, and ischemic time in grafts transplanted between 2000 and 2006. One-year graft survival was directly related to the PDRI and ranged between 77% and 87% in recipients of “standard” pancreas allografts (PDRI score of 1.0). Use of grafts from the highest (worst) three quintiles of PDRI (PDRI score > 1.16) was associated with 1-year graft survival rates of 67% to 82%, significantly inferior to that seen with “higher- quality” grafts, again emphasizing the need for rigorous donor selection.10
In addition to these objective measures, visual assessment of pancreas quality at the time of procurement remains an equally important predictor of success. Determination of subjective features, such as fatty infiltration and glandular fibrosis, requires surgical experience developed over several years. In a 2010 analysis, dissatisfaction with the quality of the donor graft on inspection accounted for more than 80% of refusals of potential pancreas donors.11 These studies illustrate an ill-defined aspect of pancreas transplant, ie, even when the pancreas donor is perceived to be suitable, the outcome may be markedly different.
SURGICAL COMPLICATIONS
Surgical complications have long been considered a limiting factor in the growth of pancreas transplant. Technical failure or loss of the graft within 90 days is most commonly due to graft thrombosis, leakage of the enteric anastomosis, or severe peripancreatic infection. The rate of technical failure has declined across all recipient categories and is currently about 9%.8
DO RECIPIENT FACTORS AFFECT OUTCOMES?
As mentioned above, the PDRI identifies donor factors that influence the 1-year graft survival rate. Recipient factors are also thought to play a role, although the influence of these factors has not been consistently demonstrated.
Humar et al15 found that recipient obesity (defined in this study as BMI > 25 kg/m2) and donor age over 40 were risk factors for early laparotomy after pancreas transplant.15 Moreover, patients undergoing early laparotomy had poorer graft survival outcomes.
This finding was reinforced by an analysis of 5,725 primary simultaneous pancreas-kidney recipients between 2000 and 2007. Obesity (BMI 30 ≥ kg/m2) was associated with increased rates of patient death, pancreas graft loss, and kidney graft loss at 3 years.16
More recently, Finger et al17 did not find a statistically significant association between recipient BMI and technical failure, but they did notice a trend toward increased graft loss with a BMI greater than 25 kg/m2. Similarly, others have not found a clear adverse association between recipient BMI and pancreas graft survival.
Intuitively, obesity and other recipient factors such as age, vascular disease, duration of diabetes, and dialysis should influence pancreas graft survival but have not been shown in analyses to carry an adverse effect.18 The inability to consistently find adverse effects of recipient characteristics is most likely due to the relative similarity between the vast majority of pancreas transplant recipients and the relatively small numbers of adverse events. In 98 consecutive pancreas transplants at our center between 2009 and 2014, the technical loss rate was 1.8% (unpublished data).
Acute rejection most commonly occurs during the first year and is usually reversible. More than 1 year after transplant, graft loss is due to chronic rejection, and death is usually from underlying cardiovascular disease.
The immunosuppressive regimens used in pancreas transplant are similar to those in kidney transplant. Since the pancreas is considered to be more immunogenic than other organs, most centers employ a strategy of induction immunosuppression with T-cell–depleting or interleukin 2-receptor antibodies. Maintenance immunosuppression consists of a calcineurin inhibitor (tacrolimus or cyclosporine), an antimetabolite (mycophenolate), and a corticosteroid.8
Immunosuppressive complications occur at a rate similar to that seen in other solid-organ transplants and include an increased risk of opportunistic infection and malignancy. The risk of these complications must be balanced against the patient’s risk of health decline with dialysis and insulin-based therapies.
OVERALL OUTCOMES ARE GOOD
The success rate of pancreas transplant is currently at its highest since the inception of the procedure. The unadjusted patient survival rate for all groups is over 96% at 1 year, and over 80% at 5 years.8 One-year patient survival after pancreas transplant alone, at better than 96%, is the highest of all organ transplant procedures.9
Several recently published single-center reviews of pancreas transplant since 2000 report patient survival rates of 96% to 100% at 1 year and 88% to 100% at 5 years.19–22 This variability is likely closely linked to donor and recipient selection, as centers performing smaller numbers of transplants tend to be more selective and, in turn, report higher patient survival rates.19,21
Long-term patient survival outcomes can be gathered from larger, registry-based reviews, accepting limitations in assessing causes of patient death. Siskind et al23 analyzed the outcomes of 20,854 US pancreas transplants done between 1996 and 2012 and found the 10-year patient survival rate ranged from 43% to 77% and was highly dependent on patient age at the time of the procedure.23 Patient survival after transplant must be balanced against the generally poor long-term survival prospects of diabetic patients on dialysis.
By type of transplant, pancreas graft survival rates at 1 year are 89% for simultaneous pancreas-kidney transplant, 86% for pancreas-after-kidney transplant, and 84% for pancreas-alone transplant. Graft survival rates at 5 years are 71% for simultaneous pancreas-kidney transplant, 65% for pancreas-after-kidney transplant, and 58% for pancreas-alone transplant.8,9
Simultaneous pancreas-kidney transplant has been shown to improve the survival rate compared with cadaveric kidney transplant alone in patients with type 1 diabetes and chronic kidney disease.24,25 The survival benefit of isolated pancreas transplant (after kidney transplant and alone) is not evident at 4-year follow-up compared with patients on the waiting list. However, the benefit for the individual patient must be considered by weighing the incapacities experienced with insulin-based treatments against the risks of surgery and immunosuppression.26,27 For patients who have experienced frequent and significant hypoglycemic episodes, particularly those requiring third-party assistance, pancreas transplant can be a lifesaving procedure.
Effects on secondary diabetic complications
Notwithstanding the effect on the patient’s life span, data from several studies of long-term pancreas transplant recipients suggest that secondary diabetic complications can be halted or even improved. Most of these studies examined the effect of restoring euglycemia in nephropathy and the subsequent influence on renal function.
Effect on renal function. Kleinclauss et al28 examined renal allograft function in type 1 diabetic recipients of living-donor kidney transplants. Comparing kidney allograft survival and function in patients who received a subsequent pancreas-after-kidney transplant vs those who did not, graft survival was superior after 5 years, and the estimated glomerular filtration rate was 10 mL/min higher in pancreas-after-kidney recipients.28 This improvement in renal function was not seen immediately after the pancreas transplant but became evident more than 4 years after establishment of normoglycemia. Somewhat similarly, reversal of diabetic changes in native kidney biopsies has been seen 10 years after pancreas transplant.29
Effect on neuropathy. In other studies, reversal of autonomic neuropathy and hypoglycemic unawareness and improvements in peripheral sensory-motor neuropathy have also been observed.30–32
Effect on retinopathy. Improvements in early-stage nonproliferative diabetic retinopathy and laser-treated proliferative lesions have been seen, even within short periods of follow-up.33 Other groups have shown a significantly higher proportion of improvement or stability of advanced diabetic retinopathy at 3 years after simultaneous pancreas-kidney transplant, compared with kidney transplant alone in patients with type 1 diabetes.34
Effect on heart disease. Salutary effects on cardiovascular risk factors and amelioration of cardiac morphology and functional cardiac indices have been seen within the first posttransplant year.35 Moreover, with longer follow-up (nearly 4 years), simultaneous pancreas-kidney recipients with functioning pancreas grafts were found to have less progression of coronary atherosclerosis than simultaneous pancreas-kidney recipients with early pancreas graft loss.36 These data provide a potential pathophysiologic mechanism for the long-term survival advantage seen in uremic type 1 diabetic patients undergoing simultaneous pancreas-kidney transplant.
In the aggregate, these findings suggest that, in the absence of surgical and immunosuppression-related complications, a functioning pancreas allograft can alter the progress of diabetic complications. As an extension of these results, pancreas transplant done earlier in the course of diabetes may have an even greater impact.
- Centers for Disease Control and Prevention (CDC). National diabetes fact sheet: national estimates and general information on diabetes and prediabetes in the United States, 2011. www.cdc.gov/diabetes/pubs/pdf/ndfs_2011.pdf. Accessed August 12, 2015.
- Ali MK, Bullard KM, Saaddine JB, Cowie CC, Imperatore G, Gregg EW. Achievement of goals in US diabetes care, 1999–2010. N Engl J Med 2013; 368:1613–1624.
- Jeon JY, Kim DJ, Ko SH, et al; Taskforce Team of Diabetes Fact Sheet of the Korean Diabetes Association. Current status of glycemic control of patients with diabetes in Korea: the fifth Korea national health and nutrition examination survey. Diabetes Metab J 2014; 38:197–203.
- Govan L, Wu O, Briggs A, et al; Scottish Diabetes Research Network Epidemiology Group. Achieved levels of HbA1c and likelihood of hospital admission in people with type 1 diabetes in the Scottish population: a study from the Scottish Diabetes Research Network Epidemiology Group. Diabetes Care 2011; 34:1992–1997.
- Bryant W, Greenfield JR, Chisholm DJ, Campbell LV. Diabetes guidelines: easier to preach than to practise? Med J Aust 2006; 185:305–309.
- Kelly WD, Lillehei RC, Merkel FK, Idezuki Y, Goetz FC. Allotransplantation of the pancreas and duodenum along with the kidney in diabetic nephropathy. Surgery 1967; 61:827–837.
- Gruessner AC, Gruessner RW. Pancreas transplant outcomes for United States and non United States cases as reported to the United Network for Organ Sharing and the International Pancreas Transplant Registry as of December 2011. Clin Transpl 2012: 23–40.
- Israni AK, Skeans MA, Gustafson SK, et al. OPTN/SRTR 2012 Annual Data Report: pancreas. Am J Transplant 2014; 14(suppl 1):45–68
- Gruessner RW, Gruessner AC. Pancreas transplant alone: a procedure coming of age. Diabetes Care 2013; 36:2440–2447.
- Axelrod DA, Sung RS, Meyer KH, Wolfe RA, Kaufman DB. Systematic evaluation of pancreas allograft quality, outcomes and geographic variation in utilization. Am J Transplant 2010; 10:837–845.
- Wiseman AC, Wainright JL, Sleeman E, et al. An analysis of the lack of donor pancreas utilization from younger adult organ donors. Transplantation 2010; 90:475–480.
- Gruessner RW, Gruessner AC. The current state of pancreas transplantation. Nat Rev Endocrinol 2013; 9:555–562.
- Gunasekaran G, Wee A, Rabets J, Winans C, Krishnamurthi V. Duodenoduodenostomy in pancreas transplantation. Clin Transplant 2012; 26:550–557.
- Sollinger HW, Odorico JS, Becker YT, D’Alessandro AM, Pirsch JD. One thousand simultaneous pancreas-kidney transplants at a single center with 22-year follow-up. Ann Surg 2009; 250:618–630.
- Humar A, Kandaswamy R, Granger D, Gruessner RW, Gruessner AC, Sutherland DE. Decreased surgical risks of pancreas transplantation in the modern era. Ann Surg 2000; 231:269–275.
- Sampaio MS, Reddy PN, Kuo HT, et al. Obesity was associated with inferior outcomes in simultaneous pancreas kidney transplant. Transplantation 2010; 89:1117–1125.
- Finger EB, Radosevich DM, Dunn TB, et al. A composite risk model for predicting technical failure in pancreas transplantation. Am J Transplant 2013; 13:1840–1849.
- Fridell JA, Mangus RS, Taber TE, et al. Growth of a nation part II: impact of recipient obesity on whole-organ pancreas transplantation. Clin Transplant 2011; 25:E366–E374.
- Tai DS, Hong J, Busuttil RW, Lipshutz GS. Low rates of short- and long-term graft loss after kidney-pancreas transplant from a single center. JAMA Surg 2013; 148:368–373.
- Bazerbachi F, Selzner M, Marquez MA, et al. Pancreas-after-kidney versus synchronous pancreas-kidney transplantation: comparison of intermediate-term results. Transplantation 2013; 95:489–494.
- Laftavi MR, Pankewycz O, Gruessner A, et al. Long-term outcomes of pancreas after kidney transplantation in small centers: is it justified? Transplant Proc 2014; 46:1920–1923.
- Stratta RJ, Farney AC, Orlando G, Farooq U, Al-Shraideh Y, Rogers J. Similar results with solitary pancreas transplantation compared with simultaneous pancreas-kidney transplantation in the new millennium. Transplant Proc 2014; 46:1924–1927.
- Siskind E, Maloney C, Akerman M, et al. An analysis of pancreas transplantation outcomes based on age groupings—an update of the UNOS database. Clin Transplant 2014; 28:990–994.
- Ojo AO, Meier-Kriesche HU, Hanson JA, et al. The impact of simultaneous pancreas-kidney transplantation on long-term patient survival. Transplantation 2001; 71:82–90.
- Reddy KS, Stablein D, Taranto S, et al. Long-term survival following simultaneous kidney-pancreas transplantation versus kidney transplantation alone in patients with type 1 diabetes mellitus and renal failure. Am J Kidney Dis 2003; 41:464–470.
- Venstrom JM, McBride MA, Rother KI, Hirshberg B, Orchard TJ, Harlan DM. Survival after pancreas transplantation in patients with diabetes and preserved kidney function. JAMA 2003; 290:2817–2823.
- Gruessner RW, Sutherland DE, Gruessner AC. Mortality assessment for pancreas transplants. Am J Transplant 2004; 4:2018–2026.
- Kleinclauss F, Fauda M, Sutherland DE, et al. Pancreas after living donor kidney transplants in diabetic patients: impact on long-term kidney graft function. Clin Transplant 2009; 23:437–446.
- Fioretto P, Steffes MW, Sutherland DE, Goetz FC, Mauer M. Reversal of lesions of diabetic nephropathy after pancreas transplantation. N Engl J Med 1998; 339:69–75.
- Landgraf R. Impact of pancreas transplantation on diabetic secondary complications and quality of life. Diabetologia 1996; 39:1415–1424.
- Robertson RP. Update on transplanting beta cells for reversing type 1 diabetes. Endocrinol Metab Clin North Am 2010; 39:655–667.
- Robertson RP, Holohan TV, Genuth S. Therapeutic controversy: pancreas transplantation for type I diabetes. J Clin Endocrinol Metab 1998; 83:1868–1674.
- Giannarelli R, Coppelli A, Sartini MS, et al. Pancreas transplant alone has beneficial effects on retinopathy in type 1 diabetic patients. Diabetologia 2006; 49:2977–2982.
- Koznarová R, Saudek F, Sosna T, et al. Beneficial effect of pancreas and kidney transplantation on advanced diabetic retinopathy. Cell Transplant 2000; 9:903–908.
- Coppelli A, Giannarelli R, Mariotti R, et al. Pancreas transplant alone determines early improvement of cardiovascular risk factors and cardiac function in type 1 diabetic patients. Transplantation 2003; 76:974–976.
- Jukema JW, Smets YF, van der Pijl JW, et al. Impact of simultaneous pancreas and kidney transplantation on progression of coronary atherosclerosis in patients with end-stage renal failure due to type 1 diabetes. Diabetes Care 2002; 25:906–911.
Pancreas transplant is the only long-term diabetes treatment that consistently results in normal hemoglobin A1c levels without the risk of severe hypoglycemia. Additionally, pancreas transplant may prevent, halt, or even reverse the complications of diabetes.
Here, we explore the indications, options, and outcomes of pancreas transplant as a treatment for diabetes mellitus.
DIABETES IS COMMON, AND OFTEN NOT WELL CONTROLLED
Diabetes mellitus affects more than 25 million people in the United States (8.3% of the population) and is the leading cause of kidney failure, nontraumatic lower-limb amputation, and adult-onset blindness. In 2007, nearly $116 billion was spent on diabetes treatment, not counting another $58 billion in indirect costs such as disability, work loss, and premature death.1
Despite the tremendous expenditure in human, material, and financial resources, only about 50% of patients achieve their diabetes treatment goals. In 2013, a large US population-based study2 reported that 52.2% of patients were achieving the American Diabetes Association treatment goal of hemoglobin A1c lower than 7%. A similar study in South Korea3 found that 45.6% were at this goal.
Most of the patients in these studies had type 2 diabetes, and the data suggested that attaining glycemic goals is more difficult in insulin-treated patients. Studies of patients with type 1 diabetes found hemoglobin A1c levels lower than 7% in only 8.1% of hospitalized patients with type 1 diabetes, and in only 13% in an outpatient diabetes clinic.4,5
YET RATES OF PANCREAS TRANSPLANT ARE DECLINING
Pancreas transplant was first performed more than 40 years ago at the University of Minnesota.6 Since then, dramatic changes in immunosuppression, organ preservation, surgical technique, and donor and recipient selection have brought about significant progress.
Currently, more than 13,000 patients are alive with a functioning pancreas allograft. After reaching a peak in 2004, the annual number of pancreas transplants performed in the United States has declined steadily, whereas the procedure continues to increase in popularity outside North America.7 The primary reason for the decline is recognition of donor factors that lead to success—surgeons are refusing to transplant organs they might have accepted previously, because experience suggests they would yield poor results. In the United States, 1,043 pancreas transplants were performed in 2012, and more than 3,100 patients were on the waiting list.8
Islet cell transplant—a different procedure involving harvesting, encapsulating, and implanting insulin-producing beta cells—has not gained widespread application due to very low long-term success rates.
THREE CATEGORIES OF PANCREAS TRANSPLANT
Pancreas transplant can be categorized according to whether the patient is also receiving or has already received a kidney graft (Table 1).
Simultaneous kidney and pancreas transplant is performed in patients who have type 1 diabetes with advanced chronic kidney disease due to diabetic nephropathy. This remains the most commonly performed type, accounting for 79% of all pancreas transplants in 2012.8
Pancreas-after-kidney transplant is most often done after a living-donor kidney transplant. This procedure accounted for most of the increase in pancreas transplants during the first decade of the 2000s. However, the number of these procedures has steadily decreased since 2004, and in 2012 accounted for only 12% of pancreas transplants.8
Pancreas transplant alone is performed in nonuremic diabetic patients who have labile blood sugar control. Performed in patients with preserved renal function but severe complications of “brittle” diabetes, such as hypoglycemic unawareness, this type accounts for 8% of pancreas transplants.9
Indications for pancreas transplant
A small number of these procedures are done for indications unrelated to diabetes mellitus. In most of these cases, the pancreas is transplanted as part of a multivisceral transplant to facilitate the technical (surgical) aspect of the procedure—the pancreas, liver, stomach, gallbladder, and part of the intestines are transplanted en bloc to maintain the native vasculature. Very infrequently, pancreas transplant is done to replace exocrine pancreatic function.
A small, select group of patients with type 2 diabetes and low body mass index (BMI) may be eligible for pancreas transplant, and they accounted for 8.2% of active candidates in 2012.8 However, most pancreas transplants are performed in patients with type 1 diabetes.
WHAT MAKES A GOOD ALLOGRAFT?
Pancreas allografts are procured as whole organs from brain-dead organ donors. Relatively few pancreas allografts (3.1% in 2012) are from cardiac-death donors, because of concern about warm ischemic injury during the period of circulatory arrest.8


Proper donor selection is critical to the success of pancreas transplant, as donor factors including medical history, age, BMI, and cause of death can significantly affect the outcome. In general, transplant of a pancreas allograft from a young donor (age < 30) with excellent organ function, low BMI, and traumatic cause of death provides the best chance of success.
The Pancreas Donor Risk Index (PDRI)10 was developed after analysis of objective donor criteria, transplant type, and ischemic time in grafts transplanted between 2000 and 2006. One-year graft survival was directly related to the PDRI and ranged between 77% and 87% in recipients of “standard” pancreas allografts (PDRI score of 1.0). Use of grafts from the highest (worst) three quintiles of PDRI (PDRI score > 1.16) was associated with 1-year graft survival rates of 67% to 82%, significantly inferior to that seen with “higher- quality” grafts, again emphasizing the need for rigorous donor selection.10
In addition to these objective measures, visual assessment of pancreas quality at the time of procurement remains an equally important predictor of success. Determination of subjective features, such as fatty infiltration and glandular fibrosis, requires surgical experience developed over several years. In a 2010 analysis, dissatisfaction with the quality of the donor graft on inspection accounted for more than 80% of refusals of potential pancreas donors.11 These studies illustrate an ill-defined aspect of pancreas transplant, ie, even when the pancreas donor is perceived to be suitable, the outcome may be markedly different.
SURGICAL COMPLICATIONS
Surgical complications have long been considered a limiting factor in the growth of pancreas transplant. Technical failure or loss of the graft within 90 days is most commonly due to graft thrombosis, leakage of the enteric anastomosis, or severe peripancreatic infection. The rate of technical failure has declined across all recipient categories and is currently about 9%.8
DO RECIPIENT FACTORS AFFECT OUTCOMES?
As mentioned above, the PDRI identifies donor factors that influence the 1-year graft survival rate. Recipient factors are also thought to play a role, although the influence of these factors has not been consistently demonstrated.
Humar et al15 found that recipient obesity (defined in this study as BMI > 25 kg/m2) and donor age over 40 were risk factors for early laparotomy after pancreas transplant.15 Moreover, patients undergoing early laparotomy had poorer graft survival outcomes.
This finding was reinforced by an analysis of 5,725 primary simultaneous pancreas-kidney recipients between 2000 and 2007. Obesity (BMI 30 ≥ kg/m2) was associated with increased rates of patient death, pancreas graft loss, and kidney graft loss at 3 years.16
More recently, Finger et al17 did not find a statistically significant association between recipient BMI and technical failure, but they did notice a trend toward increased graft loss with a BMI greater than 25 kg/m2. Similarly, others have not found a clear adverse association between recipient BMI and pancreas graft survival.
Intuitively, obesity and other recipient factors such as age, vascular disease, duration of diabetes, and dialysis should influence pancreas graft survival but have not been shown in analyses to carry an adverse effect.18 The inability to consistently find adverse effects of recipient characteristics is most likely due to the relative similarity between the vast majority of pancreas transplant recipients and the relatively small numbers of adverse events. In 98 consecutive pancreas transplants at our center between 2009 and 2014, the technical loss rate was 1.8% (unpublished data).
Acute rejection most commonly occurs during the first year and is usually reversible. More than 1 year after transplant, graft loss is due to chronic rejection, and death is usually from underlying cardiovascular disease.
The immunosuppressive regimens used in pancreas transplant are similar to those in kidney transplant. Since the pancreas is considered to be more immunogenic than other organs, most centers employ a strategy of induction immunosuppression with T-cell–depleting or interleukin 2-receptor antibodies. Maintenance immunosuppression consists of a calcineurin inhibitor (tacrolimus or cyclosporine), an antimetabolite (mycophenolate), and a corticosteroid.8
Immunosuppressive complications occur at a rate similar to that seen in other solid-organ transplants and include an increased risk of opportunistic infection and malignancy. The risk of these complications must be balanced against the patient’s risk of health decline with dialysis and insulin-based therapies.
OVERALL OUTCOMES ARE GOOD
The success rate of pancreas transplant is currently at its highest since the inception of the procedure. The unadjusted patient survival rate for all groups is over 96% at 1 year, and over 80% at 5 years.8 One-year patient survival after pancreas transplant alone, at better than 96%, is the highest of all organ transplant procedures.9
Several recently published single-center reviews of pancreas transplant since 2000 report patient survival rates of 96% to 100% at 1 year and 88% to 100% at 5 years.19–22 This variability is likely closely linked to donor and recipient selection, as centers performing smaller numbers of transplants tend to be more selective and, in turn, report higher patient survival rates.19,21
Long-term patient survival outcomes can be gathered from larger, registry-based reviews, accepting limitations in assessing causes of patient death. Siskind et al23 analyzed the outcomes of 20,854 US pancreas transplants done between 1996 and 2012 and found the 10-year patient survival rate ranged from 43% to 77% and was highly dependent on patient age at the time of the procedure.23 Patient survival after transplant must be balanced against the generally poor long-term survival prospects of diabetic patients on dialysis.
By type of transplant, pancreas graft survival rates at 1 year are 89% for simultaneous pancreas-kidney transplant, 86% for pancreas-after-kidney transplant, and 84% for pancreas-alone transplant. Graft survival rates at 5 years are 71% for simultaneous pancreas-kidney transplant, 65% for pancreas-after-kidney transplant, and 58% for pancreas-alone transplant.8,9
Simultaneous pancreas-kidney transplant has been shown to improve the survival rate compared with cadaveric kidney transplant alone in patients with type 1 diabetes and chronic kidney disease.24,25 The survival benefit of isolated pancreas transplant (after kidney transplant and alone) is not evident at 4-year follow-up compared with patients on the waiting list. However, the benefit for the individual patient must be considered by weighing the incapacities experienced with insulin-based treatments against the risks of surgery and immunosuppression.26,27 For patients who have experienced frequent and significant hypoglycemic episodes, particularly those requiring third-party assistance, pancreas transplant can be a lifesaving procedure.
Effects on secondary diabetic complications
Notwithstanding the effect on the patient’s life span, data from several studies of long-term pancreas transplant recipients suggest that secondary diabetic complications can be halted or even improved. Most of these studies examined the effect of restoring euglycemia in nephropathy and the subsequent influence on renal function.
Effect on renal function. Kleinclauss et al28 examined renal allograft function in type 1 diabetic recipients of living-donor kidney transplants. Comparing kidney allograft survival and function in patients who received a subsequent pancreas-after-kidney transplant vs those who did not, graft survival was superior after 5 years, and the estimated glomerular filtration rate was 10 mL/min higher in pancreas-after-kidney recipients.28 This improvement in renal function was not seen immediately after the pancreas transplant but became evident more than 4 years after establishment of normoglycemia. Somewhat similarly, reversal of diabetic changes in native kidney biopsies has been seen 10 years after pancreas transplant.29
Effect on neuropathy. In other studies, reversal of autonomic neuropathy and hypoglycemic unawareness and improvements in peripheral sensory-motor neuropathy have also been observed.30–32
Effect on retinopathy. Improvements in early-stage nonproliferative diabetic retinopathy and laser-treated proliferative lesions have been seen, even within short periods of follow-up.33 Other groups have shown a significantly higher proportion of improvement or stability of advanced diabetic retinopathy at 3 years after simultaneous pancreas-kidney transplant, compared with kidney transplant alone in patients with type 1 diabetes.34
Effect on heart disease. Salutary effects on cardiovascular risk factors and amelioration of cardiac morphology and functional cardiac indices have been seen within the first posttransplant year.35 Moreover, with longer follow-up (nearly 4 years), simultaneous pancreas-kidney recipients with functioning pancreas grafts were found to have less progression of coronary atherosclerosis than simultaneous pancreas-kidney recipients with early pancreas graft loss.36 These data provide a potential pathophysiologic mechanism for the long-term survival advantage seen in uremic type 1 diabetic patients undergoing simultaneous pancreas-kidney transplant.
In the aggregate, these findings suggest that, in the absence of surgical and immunosuppression-related complications, a functioning pancreas allograft can alter the progress of diabetic complications. As an extension of these results, pancreas transplant done earlier in the course of diabetes may have an even greater impact.
Pancreas transplant is the only long-term diabetes treatment that consistently results in normal hemoglobin A1c levels without the risk of severe hypoglycemia. Additionally, pancreas transplant may prevent, halt, or even reverse the complications of diabetes.
Here, we explore the indications, options, and outcomes of pancreas transplant as a treatment for diabetes mellitus.
DIABETES IS COMMON, AND OFTEN NOT WELL CONTROLLED
Diabetes mellitus affects more than 25 million people in the United States (8.3% of the population) and is the leading cause of kidney failure, nontraumatic lower-limb amputation, and adult-onset blindness. In 2007, nearly $116 billion was spent on diabetes treatment, not counting another $58 billion in indirect costs such as disability, work loss, and premature death.1
Despite the tremendous expenditure in human, material, and financial resources, only about 50% of patients achieve their diabetes treatment goals. In 2013, a large US population-based study2 reported that 52.2% of patients were achieving the American Diabetes Association treatment goal of hemoglobin A1c lower than 7%. A similar study in South Korea3 found that 45.6% were at this goal.
Most of the patients in these studies had type 2 diabetes, and the data suggested that attaining glycemic goals is more difficult in insulin-treated patients. Studies of patients with type 1 diabetes found hemoglobin A1c levels lower than 7% in only 8.1% of hospitalized patients with type 1 diabetes, and in only 13% in an outpatient diabetes clinic.4,5
YET RATES OF PANCREAS TRANSPLANT ARE DECLINING
Pancreas transplant was first performed more than 40 years ago at the University of Minnesota.6 Since then, dramatic changes in immunosuppression, organ preservation, surgical technique, and donor and recipient selection have brought about significant progress.
Currently, more than 13,000 patients are alive with a functioning pancreas allograft. After reaching a peak in 2004, the annual number of pancreas transplants performed in the United States has declined steadily, whereas the procedure continues to increase in popularity outside North America.7 The primary reason for the decline is recognition of donor factors that lead to success—surgeons are refusing to transplant organs they might have accepted previously, because experience suggests they would yield poor results. In the United States, 1,043 pancreas transplants were performed in 2012, and more than 3,100 patients were on the waiting list.8
Islet cell transplant—a different procedure involving harvesting, encapsulating, and implanting insulin-producing beta cells—has not gained widespread application due to very low long-term success rates.
THREE CATEGORIES OF PANCREAS TRANSPLANT
Pancreas transplant can be categorized according to whether the patient is also receiving or has already received a kidney graft (Table 1).
Simultaneous kidney and pancreas transplant is performed in patients who have type 1 diabetes with advanced chronic kidney disease due to diabetic nephropathy. This remains the most commonly performed type, accounting for 79% of all pancreas transplants in 2012.8
Pancreas-after-kidney transplant is most often done after a living-donor kidney transplant. This procedure accounted for most of the increase in pancreas transplants during the first decade of the 2000s. However, the number of these procedures has steadily decreased since 2004, and in 2012 accounted for only 12% of pancreas transplants.8
Pancreas transplant alone is performed in nonuremic diabetic patients who have labile blood sugar control. Performed in patients with preserved renal function but severe complications of “brittle” diabetes, such as hypoglycemic unawareness, this type accounts for 8% of pancreas transplants.9
Indications for pancreas transplant
A small number of these procedures are done for indications unrelated to diabetes mellitus. In most of these cases, the pancreas is transplanted as part of a multivisceral transplant to facilitate the technical (surgical) aspect of the procedure—the pancreas, liver, stomach, gallbladder, and part of the intestines are transplanted en bloc to maintain the native vasculature. Very infrequently, pancreas transplant is done to replace exocrine pancreatic function.
A small, select group of patients with type 2 diabetes and low body mass index (BMI) may be eligible for pancreas transplant, and they accounted for 8.2% of active candidates in 2012.8 However, most pancreas transplants are performed in patients with type 1 diabetes.
WHAT MAKES A GOOD ALLOGRAFT?
Pancreas allografts are procured as whole organs from brain-dead organ donors. Relatively few pancreas allografts (3.1% in 2012) are from cardiac-death donors, because of concern about warm ischemic injury during the period of circulatory arrest.8


Proper donor selection is critical to the success of pancreas transplant, as donor factors including medical history, age, BMI, and cause of death can significantly affect the outcome. In general, transplant of a pancreas allograft from a young donor (age < 30) with excellent organ function, low BMI, and traumatic cause of death provides the best chance of success.
The Pancreas Donor Risk Index (PDRI)10 was developed after analysis of objective donor criteria, transplant type, and ischemic time in grafts transplanted between 2000 and 2006. One-year graft survival was directly related to the PDRI and ranged between 77% and 87% in recipients of “standard” pancreas allografts (PDRI score of 1.0). Use of grafts from the highest (worst) three quintiles of PDRI (PDRI score > 1.16) was associated with 1-year graft survival rates of 67% to 82%, significantly inferior to that seen with “higher- quality” grafts, again emphasizing the need for rigorous donor selection.10
In addition to these objective measures, visual assessment of pancreas quality at the time of procurement remains an equally important predictor of success. Determination of subjective features, such as fatty infiltration and glandular fibrosis, requires surgical experience developed over several years. In a 2010 analysis, dissatisfaction with the quality of the donor graft on inspection accounted for more than 80% of refusals of potential pancreas donors.11 These studies illustrate an ill-defined aspect of pancreas transplant, ie, even when the pancreas donor is perceived to be suitable, the outcome may be markedly different.
SURGICAL COMPLICATIONS
Surgical complications have long been considered a limiting factor in the growth of pancreas transplant. Technical failure or loss of the graft within 90 days is most commonly due to graft thrombosis, leakage of the enteric anastomosis, or severe peripancreatic infection. The rate of technical failure has declined across all recipient categories and is currently about 9%.8
DO RECIPIENT FACTORS AFFECT OUTCOMES?
As mentioned above, the PDRI identifies donor factors that influence the 1-year graft survival rate. Recipient factors are also thought to play a role, although the influence of these factors has not been consistently demonstrated.
Humar et al15 found that recipient obesity (defined in this study as BMI > 25 kg/m2) and donor age over 40 were risk factors for early laparotomy after pancreas transplant.15 Moreover, patients undergoing early laparotomy had poorer graft survival outcomes.
This finding was reinforced by an analysis of 5,725 primary simultaneous pancreas-kidney recipients between 2000 and 2007. Obesity (BMI 30 ≥ kg/m2) was associated with increased rates of patient death, pancreas graft loss, and kidney graft loss at 3 years.16
More recently, Finger et al17 did not find a statistically significant association between recipient BMI and technical failure, but they did notice a trend toward increased graft loss with a BMI greater than 25 kg/m2. Similarly, others have not found a clear adverse association between recipient BMI and pancreas graft survival.
Intuitively, obesity and other recipient factors such as age, vascular disease, duration of diabetes, and dialysis should influence pancreas graft survival but have not been shown in analyses to carry an adverse effect.18 The inability to consistently find adverse effects of recipient characteristics is most likely due to the relative similarity between the vast majority of pancreas transplant recipients and the relatively small numbers of adverse events. In 98 consecutive pancreas transplants at our center between 2009 and 2014, the technical loss rate was 1.8% (unpublished data).
Acute rejection most commonly occurs during the first year and is usually reversible. More than 1 year after transplant, graft loss is due to chronic rejection, and death is usually from underlying cardiovascular disease.
The immunosuppressive regimens used in pancreas transplant are similar to those in kidney transplant. Since the pancreas is considered to be more immunogenic than other organs, most centers employ a strategy of induction immunosuppression with T-cell–depleting or interleukin 2-receptor antibodies. Maintenance immunosuppression consists of a calcineurin inhibitor (tacrolimus or cyclosporine), an antimetabolite (mycophenolate), and a corticosteroid.8
Immunosuppressive complications occur at a rate similar to that seen in other solid-organ transplants and include an increased risk of opportunistic infection and malignancy. The risk of these complications must be balanced against the patient’s risk of health decline with dialysis and insulin-based therapies.
OVERALL OUTCOMES ARE GOOD
The success rate of pancreas transplant is currently at its highest since the inception of the procedure. The unadjusted patient survival rate for all groups is over 96% at 1 year, and over 80% at 5 years.8 One-year patient survival after pancreas transplant alone, at better than 96%, is the highest of all organ transplant procedures.9
Several recently published single-center reviews of pancreas transplant since 2000 report patient survival rates of 96% to 100% at 1 year and 88% to 100% at 5 years.19–22 This variability is likely closely linked to donor and recipient selection, as centers performing smaller numbers of transplants tend to be more selective and, in turn, report higher patient survival rates.19,21
Long-term patient survival outcomes can be gathered from larger, registry-based reviews, accepting limitations in assessing causes of patient death. Siskind et al23 analyzed the outcomes of 20,854 US pancreas transplants done between 1996 and 2012 and found the 10-year patient survival rate ranged from 43% to 77% and was highly dependent on patient age at the time of the procedure.23 Patient survival after transplant must be balanced against the generally poor long-term survival prospects of diabetic patients on dialysis.
By type of transplant, pancreas graft survival rates at 1 year are 89% for simultaneous pancreas-kidney transplant, 86% for pancreas-after-kidney transplant, and 84% for pancreas-alone transplant. Graft survival rates at 5 years are 71% for simultaneous pancreas-kidney transplant, 65% for pancreas-after-kidney transplant, and 58% for pancreas-alone transplant.8,9
Simultaneous pancreas-kidney transplant has been shown to improve the survival rate compared with cadaveric kidney transplant alone in patients with type 1 diabetes and chronic kidney disease.24,25 The survival benefit of isolated pancreas transplant (after kidney transplant and alone) is not evident at 4-year follow-up compared with patients on the waiting list. However, the benefit for the individual patient must be considered by weighing the incapacities experienced with insulin-based treatments against the risks of surgery and immunosuppression.26,27 For patients who have experienced frequent and significant hypoglycemic episodes, particularly those requiring third-party assistance, pancreas transplant can be a lifesaving procedure.
Effects on secondary diabetic complications
Notwithstanding the effect on the patient’s life span, data from several studies of long-term pancreas transplant recipients suggest that secondary diabetic complications can be halted or even improved. Most of these studies examined the effect of restoring euglycemia in nephropathy and the subsequent influence on renal function.
Effect on renal function. Kleinclauss et al28 examined renal allograft function in type 1 diabetic recipients of living-donor kidney transplants. Comparing kidney allograft survival and function in patients who received a subsequent pancreas-after-kidney transplant vs those who did not, graft survival was superior after 5 years, and the estimated glomerular filtration rate was 10 mL/min higher in pancreas-after-kidney recipients.28 This improvement in renal function was not seen immediately after the pancreas transplant but became evident more than 4 years after establishment of normoglycemia. Somewhat similarly, reversal of diabetic changes in native kidney biopsies has been seen 10 years after pancreas transplant.29
Effect on neuropathy. In other studies, reversal of autonomic neuropathy and hypoglycemic unawareness and improvements in peripheral sensory-motor neuropathy have also been observed.30–32
Effect on retinopathy. Improvements in early-stage nonproliferative diabetic retinopathy and laser-treated proliferative lesions have been seen, even within short periods of follow-up.33 Other groups have shown a significantly higher proportion of improvement or stability of advanced diabetic retinopathy at 3 years after simultaneous pancreas-kidney transplant, compared with kidney transplant alone in patients with type 1 diabetes.34
Effect on heart disease. Salutary effects on cardiovascular risk factors and amelioration of cardiac morphology and functional cardiac indices have been seen within the first posttransplant year.35 Moreover, with longer follow-up (nearly 4 years), simultaneous pancreas-kidney recipients with functioning pancreas grafts were found to have less progression of coronary atherosclerosis than simultaneous pancreas-kidney recipients with early pancreas graft loss.36 These data provide a potential pathophysiologic mechanism for the long-term survival advantage seen in uremic type 1 diabetic patients undergoing simultaneous pancreas-kidney transplant.
In the aggregate, these findings suggest that, in the absence of surgical and immunosuppression-related complications, a functioning pancreas allograft can alter the progress of diabetic complications. As an extension of these results, pancreas transplant done earlier in the course of diabetes may have an even greater impact.
- Centers for Disease Control and Prevention (CDC). National diabetes fact sheet: national estimates and general information on diabetes and prediabetes in the United States, 2011. www.cdc.gov/diabetes/pubs/pdf/ndfs_2011.pdf. Accessed August 12, 2015.
- Ali MK, Bullard KM, Saaddine JB, Cowie CC, Imperatore G, Gregg EW. Achievement of goals in US diabetes care, 1999–2010. N Engl J Med 2013; 368:1613–1624.
- Jeon JY, Kim DJ, Ko SH, et al; Taskforce Team of Diabetes Fact Sheet of the Korean Diabetes Association. Current status of glycemic control of patients with diabetes in Korea: the fifth Korea national health and nutrition examination survey. Diabetes Metab J 2014; 38:197–203.
- Govan L, Wu O, Briggs A, et al; Scottish Diabetes Research Network Epidemiology Group. Achieved levels of HbA1c and likelihood of hospital admission in people with type 1 diabetes in the Scottish population: a study from the Scottish Diabetes Research Network Epidemiology Group. Diabetes Care 2011; 34:1992–1997.
- Bryant W, Greenfield JR, Chisholm DJ, Campbell LV. Diabetes guidelines: easier to preach than to practise? Med J Aust 2006; 185:305–309.
- Kelly WD, Lillehei RC, Merkel FK, Idezuki Y, Goetz FC. Allotransplantation of the pancreas and duodenum along with the kidney in diabetic nephropathy. Surgery 1967; 61:827–837.
- Gruessner AC, Gruessner RW. Pancreas transplant outcomes for United States and non United States cases as reported to the United Network for Organ Sharing and the International Pancreas Transplant Registry as of December 2011. Clin Transpl 2012: 23–40.
- Israni AK, Skeans MA, Gustafson SK, et al. OPTN/SRTR 2012 Annual Data Report: pancreas. Am J Transplant 2014; 14(suppl 1):45–68
- Gruessner RW, Gruessner AC. Pancreas transplant alone: a procedure coming of age. Diabetes Care 2013; 36:2440–2447.
- Axelrod DA, Sung RS, Meyer KH, Wolfe RA, Kaufman DB. Systematic evaluation of pancreas allograft quality, outcomes and geographic variation in utilization. Am J Transplant 2010; 10:837–845.
- Wiseman AC, Wainright JL, Sleeman E, et al. An analysis of the lack of donor pancreas utilization from younger adult organ donors. Transplantation 2010; 90:475–480.
- Gruessner RW, Gruessner AC. The current state of pancreas transplantation. Nat Rev Endocrinol 2013; 9:555–562.
- Gunasekaran G, Wee A, Rabets J, Winans C, Krishnamurthi V. Duodenoduodenostomy in pancreas transplantation. Clin Transplant 2012; 26:550–557.
- Sollinger HW, Odorico JS, Becker YT, D’Alessandro AM, Pirsch JD. One thousand simultaneous pancreas-kidney transplants at a single center with 22-year follow-up. Ann Surg 2009; 250:618–630.
- Humar A, Kandaswamy R, Granger D, Gruessner RW, Gruessner AC, Sutherland DE. Decreased surgical risks of pancreas transplantation in the modern era. Ann Surg 2000; 231:269–275.
- Sampaio MS, Reddy PN, Kuo HT, et al. Obesity was associated with inferior outcomes in simultaneous pancreas kidney transplant. Transplantation 2010; 89:1117–1125.
- Finger EB, Radosevich DM, Dunn TB, et al. A composite risk model for predicting technical failure in pancreas transplantation. Am J Transplant 2013; 13:1840–1849.
- Fridell JA, Mangus RS, Taber TE, et al. Growth of a nation part II: impact of recipient obesity on whole-organ pancreas transplantation. Clin Transplant 2011; 25:E366–E374.
- Tai DS, Hong J, Busuttil RW, Lipshutz GS. Low rates of short- and long-term graft loss after kidney-pancreas transplant from a single center. JAMA Surg 2013; 148:368–373.
- Bazerbachi F, Selzner M, Marquez MA, et al. Pancreas-after-kidney versus synchronous pancreas-kidney transplantation: comparison of intermediate-term results. Transplantation 2013; 95:489–494.
- Laftavi MR, Pankewycz O, Gruessner A, et al. Long-term outcomes of pancreas after kidney transplantation in small centers: is it justified? Transplant Proc 2014; 46:1920–1923.
- Stratta RJ, Farney AC, Orlando G, Farooq U, Al-Shraideh Y, Rogers J. Similar results with solitary pancreas transplantation compared with simultaneous pancreas-kidney transplantation in the new millennium. Transplant Proc 2014; 46:1924–1927.
- Siskind E, Maloney C, Akerman M, et al. An analysis of pancreas transplantation outcomes based on age groupings—an update of the UNOS database. Clin Transplant 2014; 28:990–994.
- Ojo AO, Meier-Kriesche HU, Hanson JA, et al. The impact of simultaneous pancreas-kidney transplantation on long-term patient survival. Transplantation 2001; 71:82–90.
- Reddy KS, Stablein D, Taranto S, et al. Long-term survival following simultaneous kidney-pancreas transplantation versus kidney transplantation alone in patients with type 1 diabetes mellitus and renal failure. Am J Kidney Dis 2003; 41:464–470.
- Venstrom JM, McBride MA, Rother KI, Hirshberg B, Orchard TJ, Harlan DM. Survival after pancreas transplantation in patients with diabetes and preserved kidney function. JAMA 2003; 290:2817–2823.
- Gruessner RW, Sutherland DE, Gruessner AC. Mortality assessment for pancreas transplants. Am J Transplant 2004; 4:2018–2026.
- Kleinclauss F, Fauda M, Sutherland DE, et al. Pancreas after living donor kidney transplants in diabetic patients: impact on long-term kidney graft function. Clin Transplant 2009; 23:437–446.
- Fioretto P, Steffes MW, Sutherland DE, Goetz FC, Mauer M. Reversal of lesions of diabetic nephropathy after pancreas transplantation. N Engl J Med 1998; 339:69–75.
- Landgraf R. Impact of pancreas transplantation on diabetic secondary complications and quality of life. Diabetologia 1996; 39:1415–1424.
- Robertson RP. Update on transplanting beta cells for reversing type 1 diabetes. Endocrinol Metab Clin North Am 2010; 39:655–667.
- Robertson RP, Holohan TV, Genuth S. Therapeutic controversy: pancreas transplantation for type I diabetes. J Clin Endocrinol Metab 1998; 83:1868–1674.
- Giannarelli R, Coppelli A, Sartini MS, et al. Pancreas transplant alone has beneficial effects on retinopathy in type 1 diabetic patients. Diabetologia 2006; 49:2977–2982.
- Koznarová R, Saudek F, Sosna T, et al. Beneficial effect of pancreas and kidney transplantation on advanced diabetic retinopathy. Cell Transplant 2000; 9:903–908.
- Coppelli A, Giannarelli R, Mariotti R, et al. Pancreas transplant alone determines early improvement of cardiovascular risk factors and cardiac function in type 1 diabetic patients. Transplantation 2003; 76:974–976.
- Jukema JW, Smets YF, van der Pijl JW, et al. Impact of simultaneous pancreas and kidney transplantation on progression of coronary atherosclerosis in patients with end-stage renal failure due to type 1 diabetes. Diabetes Care 2002; 25:906–911.
- Centers for Disease Control and Prevention (CDC). National diabetes fact sheet: national estimates and general information on diabetes and prediabetes in the United States, 2011. www.cdc.gov/diabetes/pubs/pdf/ndfs_2011.pdf. Accessed August 12, 2015.
- Ali MK, Bullard KM, Saaddine JB, Cowie CC, Imperatore G, Gregg EW. Achievement of goals in US diabetes care, 1999–2010. N Engl J Med 2013; 368:1613–1624.
- Jeon JY, Kim DJ, Ko SH, et al; Taskforce Team of Diabetes Fact Sheet of the Korean Diabetes Association. Current status of glycemic control of patients with diabetes in Korea: the fifth Korea national health and nutrition examination survey. Diabetes Metab J 2014; 38:197–203.
- Govan L, Wu O, Briggs A, et al; Scottish Diabetes Research Network Epidemiology Group. Achieved levels of HbA1c and likelihood of hospital admission in people with type 1 diabetes in the Scottish population: a study from the Scottish Diabetes Research Network Epidemiology Group. Diabetes Care 2011; 34:1992–1997.
- Bryant W, Greenfield JR, Chisholm DJ, Campbell LV. Diabetes guidelines: easier to preach than to practise? Med J Aust 2006; 185:305–309.
- Kelly WD, Lillehei RC, Merkel FK, Idezuki Y, Goetz FC. Allotransplantation of the pancreas and duodenum along with the kidney in diabetic nephropathy. Surgery 1967; 61:827–837.
- Gruessner AC, Gruessner RW. Pancreas transplant outcomes for United States and non United States cases as reported to the United Network for Organ Sharing and the International Pancreas Transplant Registry as of December 2011. Clin Transpl 2012: 23–40.
- Israni AK, Skeans MA, Gustafson SK, et al. OPTN/SRTR 2012 Annual Data Report: pancreas. Am J Transplant 2014; 14(suppl 1):45–68
- Gruessner RW, Gruessner AC. Pancreas transplant alone: a procedure coming of age. Diabetes Care 2013; 36:2440–2447.
- Axelrod DA, Sung RS, Meyer KH, Wolfe RA, Kaufman DB. Systematic evaluation of pancreas allograft quality, outcomes and geographic variation in utilization. Am J Transplant 2010; 10:837–845.
- Wiseman AC, Wainright JL, Sleeman E, et al. An analysis of the lack of donor pancreas utilization from younger adult organ donors. Transplantation 2010; 90:475–480.
- Gruessner RW, Gruessner AC. The current state of pancreas transplantation. Nat Rev Endocrinol 2013; 9:555–562.
- Gunasekaran G, Wee A, Rabets J, Winans C, Krishnamurthi V. Duodenoduodenostomy in pancreas transplantation. Clin Transplant 2012; 26:550–557.
- Sollinger HW, Odorico JS, Becker YT, D’Alessandro AM, Pirsch JD. One thousand simultaneous pancreas-kidney transplants at a single center with 22-year follow-up. Ann Surg 2009; 250:618–630.
- Humar A, Kandaswamy R, Granger D, Gruessner RW, Gruessner AC, Sutherland DE. Decreased surgical risks of pancreas transplantation in the modern era. Ann Surg 2000; 231:269–275.
- Sampaio MS, Reddy PN, Kuo HT, et al. Obesity was associated with inferior outcomes in simultaneous pancreas kidney transplant. Transplantation 2010; 89:1117–1125.
- Finger EB, Radosevich DM, Dunn TB, et al. A composite risk model for predicting technical failure in pancreas transplantation. Am J Transplant 2013; 13:1840–1849.
- Fridell JA, Mangus RS, Taber TE, et al. Growth of a nation part II: impact of recipient obesity on whole-organ pancreas transplantation. Clin Transplant 2011; 25:E366–E374.
- Tai DS, Hong J, Busuttil RW, Lipshutz GS. Low rates of short- and long-term graft loss after kidney-pancreas transplant from a single center. JAMA Surg 2013; 148:368–373.
- Bazerbachi F, Selzner M, Marquez MA, et al. Pancreas-after-kidney versus synchronous pancreas-kidney transplantation: comparison of intermediate-term results. Transplantation 2013; 95:489–494.
- Laftavi MR, Pankewycz O, Gruessner A, et al. Long-term outcomes of pancreas after kidney transplantation in small centers: is it justified? Transplant Proc 2014; 46:1920–1923.
- Stratta RJ, Farney AC, Orlando G, Farooq U, Al-Shraideh Y, Rogers J. Similar results with solitary pancreas transplantation compared with simultaneous pancreas-kidney transplantation in the new millennium. Transplant Proc 2014; 46:1924–1927.
- Siskind E, Maloney C, Akerman M, et al. An analysis of pancreas transplantation outcomes based on age groupings—an update of the UNOS database. Clin Transplant 2014; 28:990–994.
- Ojo AO, Meier-Kriesche HU, Hanson JA, et al. The impact of simultaneous pancreas-kidney transplantation on long-term patient survival. Transplantation 2001; 71:82–90.
- Reddy KS, Stablein D, Taranto S, et al. Long-term survival following simultaneous kidney-pancreas transplantation versus kidney transplantation alone in patients with type 1 diabetes mellitus and renal failure. Am J Kidney Dis 2003; 41:464–470.
- Venstrom JM, McBride MA, Rother KI, Hirshberg B, Orchard TJ, Harlan DM. Survival after pancreas transplantation in patients with diabetes and preserved kidney function. JAMA 2003; 290:2817–2823.
- Gruessner RW, Sutherland DE, Gruessner AC. Mortality assessment for pancreas transplants. Am J Transplant 2004; 4:2018–2026.
- Kleinclauss F, Fauda M, Sutherland DE, et al. Pancreas after living donor kidney transplants in diabetic patients: impact on long-term kidney graft function. Clin Transplant 2009; 23:437–446.
- Fioretto P, Steffes MW, Sutherland DE, Goetz FC, Mauer M. Reversal of lesions of diabetic nephropathy after pancreas transplantation. N Engl J Med 1998; 339:69–75.
- Landgraf R. Impact of pancreas transplantation on diabetic secondary complications and quality of life. Diabetologia 1996; 39:1415–1424.
- Robertson RP. Update on transplanting beta cells for reversing type 1 diabetes. Endocrinol Metab Clin North Am 2010; 39:655–667.
- Robertson RP, Holohan TV, Genuth S. Therapeutic controversy: pancreas transplantation for type I diabetes. J Clin Endocrinol Metab 1998; 83:1868–1674.
- Giannarelli R, Coppelli A, Sartini MS, et al. Pancreas transplant alone has beneficial effects on retinopathy in type 1 diabetic patients. Diabetologia 2006; 49:2977–2982.
- Koznarová R, Saudek F, Sosna T, et al. Beneficial effect of pancreas and kidney transplantation on advanced diabetic retinopathy. Cell Transplant 2000; 9:903–908.
- Coppelli A, Giannarelli R, Mariotti R, et al. Pancreas transplant alone determines early improvement of cardiovascular risk factors and cardiac function in type 1 diabetic patients. Transplantation 2003; 76:974–976.
- Jukema JW, Smets YF, van der Pijl JW, et al. Impact of simultaneous pancreas and kidney transplantation on progression of coronary atherosclerosis in patients with end-stage renal failure due to type 1 diabetes. Diabetes Care 2002; 25:906–911.
KEY POINTS
- Current options are simultaneous pancreas-kidney transplant, pancreas-after-kidney transplant, and pancreas-alone transplant.
- Simultaneous pancreas-kidney transplant provides a significant survival benefit over insulin- and dialysis-based therapies.
- Isolated pancreas transplant for diabetic patients without uremia can prevent hypoglycemic unawareness.