User login
Biofeedback in coronary artery disease, type 2 diabetes, and multiple sclerosis
Prevalence of anxiety and type D personality in an outpatient ICD clinic
Anxiety and type D personality in ICD patients: Impact of shocks
Gender differences prominent in linking anxiety to long-term mortality among the elderly
Depression and heart disease: What do we know, and where are we headed?
Depression is a risk factor for heart disease, and in patients with heart disease, it is a risk factor for complications and death. Unfortunately, in the trials performed to date, treating depression in cardiac patients did not lead to lower rates of recurrent cardiovascular events or death. Nevertheless, we recommend that clinicians systematically screen for it in their heart patients, in view of the benefits of antidepressant therapy.
In this article we review key epidemiologic and psychosocial studies, the mechanistic links between depression and heart disease, and recent intervention trials. We also offer practical management advice and address the continued need for guidelines and risk stratification in the treatment of depressed cardiac patients.
After we submitted our review article, the American Heart Association (AHA)1 released a consensus document recommending that health care providers screen for and treat depression in patients with coronary heart disease. We will discuss the same screening tests that have been recommended by the AHA.
DEPRESSION AND HEART DISEASE: COMMON AND LINKED
Depression and heart disease are very common and often coexist: the prevalence of depression in various heart conditions ranges from 15% to 20%.1–3 According to data from the World Health Organization, by the year 2020 depression will be the second-leading cause of disability in developed countries (after heart disease).4
The World Health Survey5 showed that depression worsens health more than angina, arthritis, asthma, or diabetes. Furthermore, patients with severe mental illness have a higher risk of dying from heart disease and stroke.6
SOME HEART DISEASE RISK FACTORS ARE PSYCHOSOCIAL
In the 1980s, the “type A” personality (ambitious, aggressive, hostile, and competitive, with a chronic sense of urgency) was linked to heart disease.7 Later studies differed as to whether the entire set of features is valid as a collective risk factor for progressive heart disease,8 but hostility remains a validated risk factor and a focus of behavior modification.9,10
Other psychosocial risk factors have been implicated,11,12 one of which is social isolation.9,13 Another is the “type D” personality, which includes a tendency to experience negative emotions across time and situations coupled with social inhibition and which is believed to be more valid than the type A personality as a risk factor for cardiac disease.14,15
The INTERHEART study16 gathered data about attributable risk in the development of myocardial infarction (MI) in 52 countries in a case-control fashion. Psychosocial factors including stress, low generalized locus of control (ie, the perceived inability to control one’s life), and depression accounted for 32.5% of the attributable risk for an MI.17 This would mean that they account for slightly less attributable risk than that of lifetime smoking but more than that of hypertension and obesity.
Job stress increases the risk of initial coronary heart disease18 and also the risk of recurrent cardiac events after a first MI.19 Even though numerous psychosocial risk factors have been associated with coronary heart disease, including anxiety,20,21 depression is perhaps the best studied.
PROSPECTIVE STUDIES OF DEPRESSION AND HEART DISEASE
To examine the impact of depression in coronary heart disease, prospective studies have been done in healthy people and in patients with established cardiovascular disease who develop depression.22
In healthy people, depression increases the risk of coronary disease
The 1996 Epidemiologic Catchment Area study23 found that people with major depression had a risk of MI four times higher than the norm, and people with 2 weeks of sadness or dysphoria had a risk two times higher.
A subsequent meta-analysis of 11 studies,24 which included 36,000 patients, found that the overall relative risk of developing heart disease in depressed but healthy people was 1.64.
A meta-analysis by Van der Kooy et al25 of 28 epidemiologic studies with nearly 80,000 patients showed depression to be an independent risk factor for cardiovascular disease.
Wulsin and Singal26 performed a systematic review to see if depression increases the risk of coronary disease. In 10 studies with a follow-up of more than 4 years, the relative risk in people with depression was 1.64, which was less than that in active smokers (2.5) but more than that in passive smokers (1.25).
Depression can also exacerbate the classic risk factors for coronary disease, such as smoking, diabetes, obesity, and physical inactivity. 27
A 2007 study from Sweden28 prospectively followed patients who were hospitalized for depression. The odds ratio of developing an acute MI was 2.9, and the risk persisted for decades after the initial hospitalization.
A prospective United Kingdom cohort study of people initially free of heart disease revealed major depression to be associated with a higher rate of death from ischemic heart disease.29 Specifically, patients who had depression currently or in the past 12 months had a 2.7 times higher risk of dying than those who had never had depression or who had had it more than 12 months previously.
In existing heart disease, depression predicts recurrent events, death
Carney et el30 found that patients with major depressive disorder had a higher incidence of new cardiac events in the 12 months after undergoing cardiac catheterization than those without major depressive disorder.
Frasure-Smith et al,31 in a landmark study, showed that patients who were depressed at 1 week after an MI were three to four times more likely to die in the next 6 months than nondepressed post-MI patients. Even after 18 months, depression remained an independent risk factor for cardiac-related death.32
In longer studies (with up to 19.4 years of follow-up), depression was associated with higher rates of death from cardiac and all causes in patients with coronary artery disease.33 Lespérance et al34 found that in MI patients, the higher the Beck Depression Inventory score at the time of hospital admission, the higher the 5-year death rate.
Using meta-analysis, Barth et al35 found the risk of dying in the first 2 years after initial assessment to be twice as high in depressed cardiac patients as in nondepressed cardiac patients (odds ratio 2.24).
Van Melle et al36 reviewed 22 studies and found that in the 2 years after an MI, depressed patients had a 2 to 2.5 times higher risk of dying of a cardiac or any other cause than did nondepressed patients.
Depression also predicts higher morbidity and mortality rates in patients undergoing coronary artery bypass grafting,37,38 patients with congestive heart failure,39 and heart transplant recipients.40
MEDICAL ILLNESS CAN PREDISPOSE TO DEPRESSION, AND VICE VERSA
Medical illnesses can predispose a patient to develop depression. Specifically, compared with healthy people, cardiac patients appear to be at greater risk of developing depression for many years after the initial medical diagnosis is made.41
Katon et al42 reviewed 31 studies involving 16,922 patients, that assessed the impact of depression and anxiety in chronic medical illnesses such as heart disease, diabetes, pulmonary disease, and arthritis. After the severity of the medical disorder was controlled for, patients with depression and anxiety reported a higher number of medical symptoms.
DEPRESSION WORSENS QUALITY OF LIFE AND ADHERENCE TO TREATMENT
Depressed patients perceive their health status and quality of life negatively. In the Heart and Soul study,43 depressive symptoms and low exercise capacity—but not low ejection fraction or ischemia—were significantly associated with perceived deterioration of health in patients with coronary artery disease.
After an MI, patients who take their cardiac drugs properly have a better chance of survival.44,45 Clinical depression can worsen compliance with cardiac medication regimens,46 and reducing depression increases medication adherence overall.47 Not surprisingly, depressed patients also adhere less well to other recommendations,48 including modifying the diet, exercising, stopping smoking, and attending cardiac rehabilitation programs. 49
PLAUSIBLE MECHANISMS LINK DEPRESSION AND HEART DISEASE
Traditional cardiac risk factors such as smoking, high cholesterol, hypertension, diabetes, and obesity tend to cluster in depressed patients. 50 Other mechanisms linking depression and heart disease are reviewed below.51,52
Autonomic imbalance
Excessive sympathetic stimulation or diminished vagal stimulation or both are associated with higher rates of morbidity and death.53
Lack of variability in the heart rate reflects a sympathetic-vagal imbalance and is a risk factor for ventricular arrhythmias and sudden cardiac death in patients with cardiovascular disease.54 Carney et al55 reported that patients with coronary artery disease and depression had significantly less heart rate variability than nondepressed cardiac patients. Similarly, after an MI, depressed patients had significantly less heart rate variability than nondepressed patients,56 implying that low heart rate variability may mediate the adverse effect of depression on survival after an MI.57
In the Heart and Soul study, Gehi et al58 found no distinct relationship between heart rate variability and depression. However, in the same study, de Jong et al59 did find specific somatic symptoms of depression to be associated with lower heart rate variability, although cognitive symptoms were not.
Platelet activation, endothelial dysfunction
Depressed patients have been found to have exaggerated platelet reactivity.60 Plasma levels of platelet factor IV and beta-thromboglobulin, markers of platelet activation, are higher in depressed patients with ischemic heart disease than in nondepressed patients with ischemic heart disease and in control patients.61 This activation of platelets can lead to vascular damage and thrombosis.
In a subset study of the Sertraline Anti-Depressant Heart Attack Randomized Trial (SADHART), depressed MI patients were treated with sertraline (Zoloft), a selective serotonin reuptake inhibitor (SSRI), and had substantially less platelet and endothelial biomarker release.62
Depressed cardiac patients also have impaired flow-mediated dilation of the brachial artery, a sign of endothelial dysfunction.63 Although a recent study did not find coronary endothelial dysfunction in depressed patients who did not have cardiac disease, these patients had more clustering of other cardiac risk factors.64
Hypothalamic-pituitary-adrenocortical and sympathetic adrenal medullary activation
High cortisol levels can accelerate the development of hypertension and atherosclerosis and result in endothelial vascular injury. Sympathoadrenal activation in turn can lead to higher levels of catecholamines, predisposing to vasoconstriction, a rapid heart rate, and platelet activation. Depressed patients have more activation of the hypothalamic-pituitary-adrenocortical and sympathetic adrenal medullary systems,51,65 yet another plausible mechanism for worse clinical outcomes in depressed cardiac patients.
Sudden emotional stress can cause transient left ventricular dysfunction, even in people without coronary disease, an effect that may be mediated by elevated plasma catecholamine levels.66
Inflammatory cytokines
Inflammatory cytokines play a key role in the development of atherosclerosis.67 C-reactive protein, an acute-phase reactant produced in hepatocytes, can be induced by cytokines such as interleukin 6. Damage to endothelial tissues leads to the release of inflammatory cytokines, including interleukin 1, interleukin 6, and tumor tumor necrosis factor alpha.
Depressed patients have higher levels of these inflammatory markers.68,69 A prospective study reported direct correlations between depression scores and C-reactive protein levels in post-MI patients.70 The Heart and Soul study, however, did not confirm that coronary patients have more inflammation if they have depression,71 indicating that the relationship is complex and is perhaps more evident in specific types of depression.72
Anticholinergic inflammatory pathway
Tracey73 proposed a theory that vagal tone inhibits the release of inflammatory cytokines. This has important implications for treatment, as exercise, biofeedback, and meditation can stimulate the vagus nerve and therefore have beneficial anti-inflammatory effects.74
Polymorphism in the serotonin transport promoter region gene
Research is focusing on the serotonin transport promoter region gene (5-HTTLPR).75 The gene exists in two forms, a long one and a less-effective short one that appears to predispose to depression.76
Nakatani et al77 showed that MI patients were more likely to become depressed and to have subsequent cardiac events if one or both of their alleles of this gene were short. Otte et al,78 using Heart and Soul study data, found that patients with a short allele had a higher likelihood of depression, higher perceived levels of stress, and higher urinary norepinephrine secretion. However, the long allele genotype may be associated with a higher risk of developing an MI.79
Our knowledge of the genetic interplay of depression and cardiovascular disease is still in its infancy, and further studies are needed to clarify these findings.
IN TRIALS, LESS DEPRESSION BUT NO EFFECT ON DEATHS, RECURRENT MI
Major behavioral and drug trials conducted in the last 15 years have focused on how to best treat depression in cardiac patients.80–85
The Montreal Heart Attack Readjustment Trial (MHART)81 used telephone calls and home nursing visits to explore and monitor psychological distress for up to 1 year after an MI. The overall trial did not show these interventions to have any impact on survival compared with usual care. In fact, in women receiving the telephone intervention, there was a trend toward higher rates of cardiac and all-cause death, which was quite unexpected. Uncovering stresses and problems without resolving them, rather than encouraging patients to place these on the “back burner,” may partially explain these results.
SADHART82 studied the safety of sertraline in depressed post-MI patients. No major differences in cardiac function were noted between the sertraline and placebo groups, showing that sertraline was safe for these patients. The sertraline group had fewer cardiovascular events, but the difference was not statistically significant.
The Enhancing Recovery in Coronary Heart Disease (ENRICHD) study83 was primarily designed to see whether a psychosocial intervention would decrease deaths in depressed cardiac patients. Much to the chagrin of behavioral medicine, the group undergoing cognitive behavioral therapy did not have a higher rate of event-free survival, although the intervention had a favorable impact on depression and social support.
The Myocardial Infarction Depression Intervention Trial (MIND-IT)84 looked at whether the antidepressant mirtazapine (Remeron) would improve long-term depression and cardiovascular outcomes in depressed post-MI patients. In 18 months of follow-up, neither objective was obtained.
The Cardiac Randomized Evaluation of Antidepressant and Psychotherapy Efficacy (CREATE) trial85 tested the efficacy of the SSRI citalopram (Celexa) and interpersonal therapy in a short-term intervention. Here, the antidepressant was superior to placebo in the primary outcome of treating depression, but interpersonal therapy had no advantage over “clinical management,” ie, a shorter, 20-minute supportive intervention.
Common threads in these studies.
- In ENRICHD and MIND-IT, patients whose depression did not respond to treatment were at higher risk of cardiac events during follow-up.86–88
- In SADHART and CREATE, which used drug treatment, the antidepressant response was more robust in patients with a history of depression before their heart attacks, suggesting that a patient with recurrent depression at the time of a cardiac event should receive medication for it.85,89
CLINICAL RECOMMENDATIONS
Use a depression screening tool
Ziegelstein et al90 recently studied the ability of clinical personnel to detect depression in hospitalized MI patients. If a screening tool was not used, the results were abysmal, indicating the need to use formal screening for symptoms of depression in acute MI patients.
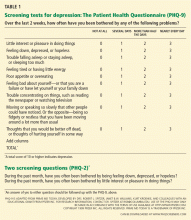
Many self-rating scales are available, among which are the Beck Depression Inventory (BDI) and the Hospital Anxiety and Depression Scale (HADS). Others are:
The PHQ-2 consists of the two first questions of the PHQ-9, which deal with mood and lack of pleasure. A cut-off score of 3 or higher has a sensitivity of 83% and a specificity of 92%,96 fulfilling the need for a quick and reliable depression screening tool. The clinician can also ask for a yes-or-no answer to the two questions of the PHQ-2 (Table 1). A yes to either of the two questions is up to 90% sensitive and 75% specific.92,97
When to suspect depression in cardiac patients
Which type of psychotherapy is best?
The negative results of psychosocial interventions (phone calls and home visits from a nurse) in MHART and of cognitive behavioral therapy in ENRICHD raise questions about which type of psychotherapy is best for depression in heart disease. CREATE found that 50-minute weekly sessions of interpersonal psychotherapy were no more beneficial than clinical management, ie, 20-minute weekly sessions that focused on compliance with treatment and education about depression and overall management. Perhaps a type of therapy akin to “clinical management” in this study or the brief behavior-based and targeted therapy used in the Improving Mood Promoting Access to Collaborative Care Treatment (IMPACT) trials of depression in primary care99 could be designed specifically to treat depression in cardiac disease. However, it is also quite possible that treatments that focus on uncovering stresses or problems may not be timely for these patients.
Which therapy is best for women is another area of consideration. In MHART, even after 5 years of follow-up,100 women who received the psychosocial support intervention did marginally worse. In the ENRICHD study, women did not experience a benefit from cognitive behavioral therapy. Further studies must address sex differences in response to different therapies.
SSRIs seem to be better than other antidepressants for cardiac patients
Before SSRIs were available, tricyclic antidepressants were the mainstays. Subsequent analysis showed the tricyclics to have an unfavorable risk-benefit profile in cardiac patients,101 and since other types of antidepressants are available, tricyclics should be avoided altogether in cardiac patients.102
Whether the SSRIs actually decrease one’s risk of death in heart disease is still an issue of debate, but there are encouraging signs. In SADHART, the rate of death and recurrent nonfatal MI was 20% lower in the patients randomized to receive sertraline, although the difference was not statistically significant.82 In ENRICHD, patients who did not respond to cognitive behavioral treatment or had severe depression could receive sertraline or other antidepressant drugs on a nonrandomized basis, and those who did had a 42% lower incidence of death or recurrent MI.103
The SADHART and CREATE trials provide convincing evidence of the cardiac safety and antidepressant efficacy of two SSRIs (sertraline and citalopram) in depressed cardiac patients. Mirtazapine, studied in MIND-IT, was not effective in treating depression in cardiac patients, although it had a better adverse effect and safety profile than tricyclic antidepressants. 104
Clinical observations indicate that SSRIs are associated with less risk of MI than non-SSRI drugs.105,106 During hospitalization for acute coronary syndromes, patients on SSRIs had lower rates of recurrent ischemia and heart failure but higher bleeding rates than patients not taking SSRIs.107 In a retrospective study of patients undergoing coronary artery bypass grafting, those on an SSRI before surgery had higher rates of death and rehospitalization.108 Being on antidepressant medication could be interpreted as a surrogate marker of having more severe depression before surgery; this issue clearly requires further study.
Given current observations and recent data from interventional trials coupled with the safe drug-interaction profile of sertraline and citalopram, these two SSRIs are recommended for treating depression in cardiac patients. If the patient is also receiving an anticoagulant, one should monitor for bleeding, as all SSRIs are associated with a prolonged bleeding time. Monitoring for rare cases of hyponatremia and bradycardia should also be part of early follow-up.
Do cardiac drugs have psychiatric effects?
Some concerns have arisen about cardiovascular drugs causing or aggravating psychiatric conditions.
Statins were once suspected of causing clinical depression or even suicide. However, subsequent studies have not substantiated this.109,110 In fact, long-term statin use has been associated with improved psychological wellbeing. 111 Whether the favorable psychological profile is due to an improved lifestyle, a direct noncholesterol effect, or an immunomodulatory effect has yet to be determined.
Beta-blockers have been suspected of increasing depression and fatigue. Robust metaanalyses have shown no increased risk of depressive symptoms but a small increased risk of fatigue and sexual dysfunction.112 Observational trials in the first year post-MI have shown no differences between beta-blocker users and nonusers in depressive symptoms or depressive disorders.113
Statins and beta-blockers offer both immense cardiac benefit and low risk, and both may be prescribed with confidence in depressed cardiac patients.
Refer patients for cardiac rehabilitation
The American Association of Cardiovascular and Pulmonary Rehabilitation strongly recommends screening cardiac patients for depression and referring them to cardiac rehabilitation programs.114 Typical programs run 12 weeks, affording an opportunity to further listen to and assess the patient and to promote general wellness via nutrition, stress management, and exercise.
These interventions by themselves can favorably affect depression. Blumenthal and colleagues,115 in the Standard Medical Intervention and Long-Term Exercise (SMILE) study, found that exercise was as effective as drug treatment in reducing depression. In addition, stress management as a psychosocial treatment in cardiac rehabilitation can reduce death rates in cardiac patients.116
Unfortunately, many patients who are eligible for cardiac rehabilitation programs do not avail themselves of them.117
Our algorithm
FUTURE DIRECTIONS FOR RESEARCH
Can we predict the course of depression?
We need to identify better which patients will have a spontaneous remission of their depressive symptoms after a cardiac event, which patients will linger with depression, and which patients will best respond to treatment. Risk stratification, using the psychiatric history, symptoms and severity of depression, and genetic predisposition118 might allow improved targeted therapies.
Does depression cause cardiac disease?
The link between depression and heart disease can be seen as merely an association. In the interventional trials performed to date, we have not yet seen a reduction in cardiac deaths when depression was treated, challenging any assumption of a causal relationship between depression and heart disease. The debate about association vs cause is germane to behavioral medicine,119 and the better we understand the mechanistic pathways, the better we can advise patients and treat depression comorbid with heart disease.
Behavioral medicine is currently measuring the aspects of depression associated with cardiac disease, including the spectrum of somatic (body) and affective (mood) symptoms120 and specific areas such as sympathetic arousal and early morning insomnia.121 If we can determine the depression subtype that carries a worse cardiac prognosis, we may untangle the biobehavioral links that bidirectionally bridge clinical depression and cardiac disease.
Another area of interest, emotional vitality (a positive state associated with interest, enthusiasm, excitement, and energy for living) has been shown to protect against coronary heart disease122 and holds much promise.
In the plenary lecture of the Academy of Psychosomatic Medicine in 2006, Frasure-Smith spoke of the “pleiotropism” of our antidepressant interventions on the various risk factors in depressed cardiac patients.123 We need behavioral medicine studies that elucidate these mechanisms, guiding more precise treatments as well as novel therapies. Omega-3 fatty acids, which benefit heart disease and clinical depression,124 will be used in a randomized controlled trial by Lespérance and colleagues.125 We await the results of this exciting research.
Will treating depression help in other types of heart disease?
The SADHART-CHF trial is examining whether 12 weeks of sertraline therapy is better than placebo in preventing death and improving cardiac outcomes in patients with chronic heart failure and comorbid major depressive disorder. It was to be completed in the fall of 2008. The results and experience of this study will help in designing future interventional trials to reduce the risk of depression in cardiovascular diseases.
We also await the results of a National Heart, Lung, and Blood Institute (NHLBI) trial, “Bypassing the Blues,” which is studying the treatment of depression after cardiac bypass surgery. This study should provide further insights into management of the depressed cardiac patient. Further prognostic studies in cardiac patients are also needed using the PHQ-9 and its shorter version, PHQ-2.
Current and future guidelines
For years our European colleagues have been ahead of us in recognizing depression screening and stress management as key to cardiac disease-prevention strategies.126 The NHLBI nicely outlined recommendations on the assessment and treatment of depression in cardiovascular patients.127 The just-published AHA Science Advisory should further encourage clinicians to screen and treat depression in the patient population.1 As our knowledge grows, we look forward to future evidence-based guidelines for depressed cardiac patients.
- Lichtman JH, Bigger JT, Blumenthal JA, et al. Depression and coronary heart disease. Recommendations for screening, referral, and treatment. Circulation 2008; 118:1768–1775.
- Jiang W, Glassman A, Krishnan R, O’Connor CM, Califf RM. Depression and ischemic heart disease: what have we learned so far and what must we do in the future? Am Heart J 2005; 150:54–78.
- Freedland KE, Rich MW, Skala JA, Carney RM, Davila-Roman VG, Jaffe AS. Prevalence of depression in hospitalized patients with congestive heart failure. Psychosom Med 2003; 65:119–128.
- Murray CJ, Lopez AD, Murray CJ, Lopez AD. Global mortality, disability, and the contribution of risk factors: Global Burden of Disease Study. Lancet 1997; 349:1436–1442.
- Moussavi S, Chatterji S, Verdes E, Tandon A, Patel V, Ustun B. Depression, chronic diseases, and decrements in health: results from the World Health Surveys. Lancet 2007; 370:851–858.
- Osborn DP, Levy G, Nazareth I, Petersen I, Islam A, King MB. Relative risk of cardiovascular and cancer mortality in people with severe mental illness from the United Kingdom’s general practice research database. Arch Gen Psychiatry 2007; 64:242–249.
- Friedman M, Thoresen CE, Gill JJ, et al. Alteration of type A behavior and its effect on cardiac recurrences in post myocardial infarction patients: summary results of the recurrent coronary prevention project. Am Heart J 1986; 112:653–665.
- Ragland DR, Brand RJ. Type A behavior and mortality from coronary heart disease. N Engl J Med 1988; 318:65–69.
- Rozanski A, Blumenthal JA, Kaplan J. Impact of psychological factors on the pathogenesis of cardiovascular disease and implications for therapy. Circulation 1999; 99:2192–2217.
- Iribarren C, Sidney S, Bild DE, et al. Association of hostility with coronary artery calcification in young adults: the CARDIA study. Coronary Artery Risk Development in Young Adults. JAMA 2000; 283:2546–2551.
- Williams RB, Barefoot JC, Schneiderman N. Psychosocial risk factors for cardiovascular disease: more than one culprit at work. JAMA 2003; 290:2190–2192.
- Rozanski A, Blumenthal JA, Davidson KW, Saab PG, Kubzansky L. The epidemiology, pathophysiology, and management of psychosocial risk factors in cardiac practice: the emerging field of behavioral cardiology. J Am Coll Cardiol 2005; 45:637–651.
- Hemingway H, Marmot M. Evidence based cardiology: psychosocial factors in the aetiology and prognosis of coronary heart disease: systematic review of prospective cohort studies. BMJ 1999; 318:1460–1467.
- Denollet J. DS14: Standard assessment of negative affectivity, social inhibition, and type D personality. Psychosom Med 2005; 67:89–97.
- Steptoe A, Molloy GJ. Personality and heart disease. Heart 2007; 93:783–784.
- Yusuf S, Hawken S, Ôunpuu S, et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet 2004; 364:937–952.
- Rosengren A, Hawken S, Ounpuu S, et al. Association of psychosocial risk factors with risk of acute myocardial infarction in 11119 cases and 13648 controls from 52 countries (the INTERHEART study): case-control study. Lancet 2004; 364:953–962.
- Kuper H, Marmot M. Job strain, job demands, decision latitude, and risk of coronary heart disease within the Whitehall II study. J Epidemiol Community Health 2003; 57:147–153.
- Aboa-Eboule C, Brisson C, Maunsell E, et al. Job strain and risk of acute recurrent coronary heart disease events. JAMA 2007; 298:1652–1660.
- Frasure-Smith N, Lespérance F. Depression and anxiety as predictors of 2-year cardiac events in patients with stable coronary artery disease. Arch Gen Psychiatry 2008; 65:62–71.
- Shen B-J, Avivi YE, Todaro JF, et al. Anxiety characteristics independently and prospectively predict myocardial infarction in men. J Am Coll Cardiol 2008; 51:113–119.
- Carney RM, Freedland KE. Depression, mortality, and medical morbidity in patients with coronary heart disease. Biol Psychiatry 2003; 54:241–247.
- Pratt LA, Ford DE, Crum RM, Armenian HK, Gallo JJ, Eaton WW. Depression, psychotropic medication, and risk of myocardial infarction: prospective data from the Baltimore ECA follow-up. Circulation 1996; 94:3123–3129.
- Rugulies R. Depression as a predictor for coronary heart disease. A review and meta-analysis. Am J Prev Med 2002; 23:51–61.
- Van der Kooy K, van Hout H, Marwijk H, Marten H, Stehouwer C, Beekman A. Depression and the risk for cardiovascular diseases: systematic review and meta analysis. Int J Geriatr Psychiatry 2007; 22:613–626.
- Wulsin LR, Singal BM. Do depressive symptoms increase the risk for the onset of coronary disease? A systematic quantitative review. Psychosom Med 2003; 65:201–210.
- Wulsin LR. Is depression a major risk factor for coronary disease? A systematic review of the epidemiologic evidence. Harv Rev Psychiatry 2004; 12:79–93.
- Janszky I, Ahlbom A, Hallqvist J, Ahnve S. Hospitalization for depression is associated with an increased risk for myocardial infarction not explained by lifestyle, lipids, coagulation, and inflammation: The SHEEP Study. Biol Psychiatry 2007; 62:25–32.
- Surtees PG, Wainwright NWJ, Luben RN, Wareham NJ, Bingham SA, Khaw K-T. Depression and ischemic heart disease mortality: evidence from the EPIC-Norfolk United Kingdom Prospective Cohort Study. Am J Psychiatry 2008; 165:515–523.
- Carney RM, Rich MW, Freedland KE, et al. Major depressive disorder predicts cardiac events in patients with coronary artery disease. Psychosom Med 1988; 50:627–633.
- Frasure-Smith N, Lespérance F, Talajic M. Depression following myocardial infarction. Impact on 6-month survival. JAMA 1993; 270:1819–1825.
- Frasure-Smith N, Lespérance F, Talajic M. Depression and 18-month prognosis after myocardial infarction. Circulation 1995; 91:999–1005.
- Barefoot JC, Helms MJ, Mark DB, et al. Depression and long-term mortality risk in patients with coronary artery disease. Am J Cardiol 1996; 78:613–617.
- Lespérance F, Frasure-Smith N, Talajic M, Bourassa MG. Five-year risk of cardiac mortality in relation to initial severity and one-year changes in depression symptoms after myocardial infarction. Circulation 2002; 105:1049–1053.
- Barth J, Schumacher M, Herrmann-Lingen C. Depression as a risk factor for mortality in patients with coronary heart disease: a meta-analysis. Psychosom Med 2004; 66:802–813.
- van Melle JP, de Jonge P, Spijkerman TA, et al. Prognostic association of depression following myocardial infarction with mortality and cardiovascular events: a meta-analysis. Psychosom Med 2004; 66:814–822.
- Blumenthal JA, Lett HS, Babyak MA, et al. Depression as a risk factor for mortality after coronary artery bypass surgery. Lancet 2003; 362:604–609.
- Sullivan MD, LaCroix AZ, Spertus JA, Hecht J, Russo J. Depression predicts revascularization procedures for 5 years after coronary angiography. Psychosom Med 2003; 65:229–236.
- Jiang W, Alexander J, Christopher E, et al. Relationship of depression to increased risk of mortality and rehospitalization in patients with congestive heart failure. Arch Intern Med 2001; 161:1849–1856.
- Zipfel S, Schneider A, Wild B, et al. Effect of depressive symptoms on survival after heart transplantation. Psychosom Med 2002; 64:740–747.
- Polsky D, Doshi JA, Marcus S, et al. Long-term risk for depressive symptoms after a medical diagnosis. Arch Intern Med 2005; 165:1260–1266.
- Katon W, Lin EHB, Kroenke K. The association of depression and anxiety with medical symptom burden in patients with chronic medical illness. Gen Hosp Psychiatry 2007; 29:147–155.
- Ruo B, Rumsfeld JS, Hlatky MA, Liu H, Browner WS, Whooley MA. Depressive symptoms and health-related quality of life: the Heart and Soul Study. JAMA 2003; 290:215–221.
- Rasmussen JN, Chong A, Alter DA. Relationship between adherence to evidence-based pharmacotherapy and long-term mortality after acute myocardial infarction. JAMA 2007; 297:177–186.
- Gehi AK, Ali S, Na B, Whooley MA. Self-reported medication adherence and cardiovascular events in patients with stable coronary heart disease: the Heart and Soul Study. Arch Intern Med 2007; 167:1798–1803.
- Gehi A, Haas D, Pipkin S, Whooley MA. Depression and medication adherence in outpatients with coronary heart disease: findings from the Heart and Soul Study. Arch Intern Med 2005; 165:2508–2513.
- Rieckmann N, Gerin W, Kronish IM, et al. Course of depressive symptoms and medication adherence after acute coronary syndromes: an electronic medication monitoring study. J Am Coll Cardiol 2006; 48:2218–2222.
- Ziegelstein RC, Fauerbach JA, Stevens SS, Romanelli J, Richter DP, Bush DE. Patients with depression are less likely to follow recommendations to reduce cardiac risk during recovery from a myocardial infarction. Arch Intern Med 2000; 160:1818–1823.
- Kronish IM, Rieckmann N, Halm EA, et al. Persistent depression affects adherence to secondary prevention behaviors after acute coronary syndromes. J Gen Intern Med 2006; 21:1178–1183.
- Joynt KE, Whellan DJ, O’Connor CM. Depression and cardiovascular disease: mechanisms of interaction. Biol Psychiatry 2003; 54:248–261.
- Musselman DL, Evans DL, Nemeroff CB. The relationship of depression to cardiovascular disease: epidemiology, biology, and treatment. Arch Gen Psychiatry 1998; 55:580–592.
- Lespérance F, Frasure-Smith N. Depression and heart disease. Cleve Clin J Med 2007; 74(suppl 1):S63–S66.
- Curtis BM. Autonomic tone as a cardiovascular risk factor: the dangers of chronic fight or flight. Mayo Clin Proc 2002; 77:45–54.
- Topol EJ. Textbook of Cardiovascular Medicine, 2nd ed. Philadelphia: Lippincott Williams & Williams 2002.
- Carney RM, Saunders RD, Freedland KE, Stein P, Rich MW, Jaffe AS. Association of depression with reduced heart rate variability in coronary artery disease. Am J Cardiol 1995; 76:562–564.
- Carney RM, Blumenthal JA, Stein PK, et al. Depression, heart rate variability, and acute myocardial infarction. Circulation 2001; 104:2024–2028.
- Carney RM, Blumenthal JA, Freedland KE, et al. Low heart rate variability and the effect of depression on post-myocardial infarction mortality. Arch Intern Med 2005; 165:1486–1491.
- Gehi A, Mangano D, Pipkin S, Browner WS, Whooley MA. Depression and heart rate variability in patients with stable coronary heart disease: findings from the Heart and Soul Study. Arch Gen Psychiatry 2005; 62:661–666.
- de Jonge P, Mangano D, Whooley MA. Differential association of cognitive and somatic depressive symptoms with heart rate variability in patients with stable coronary heart disease: findings from the Heart and Soul Study. Psychosom Med 2007; 69:735–739.
- Musselman DL, Tomer A, Manatunga AK, et al. Exaggerated platelet reactivity in major depression. Am J Psychiatry 1996; 153:1313–1317.
- Laghrissi-Thode F, Wagner WR, Pollock BG, Johnson PC, Finkel MS. Elevated platelet factor 4 and beta-thromboglobulin plasma levels in depressed patients with ischemic heart disease. Biol Psychiatry 1997; 42:290–295.
- Serebruany VL, Glassman AH, Malinin AI, et al. Platelet/endothelial biomarkers in depressed patients treated with the selective serotonin reuptake inhibitor sertraline after acute coronary events: the Sertraline AntiDepressant Heart Attack Randomized Trial (SADHART) Platelet Substudy. Circulation 2003; 108:939–944.
- Sherwood A, Hinderliter AL, Watkins LL, Waugh RA, Blumenthal JA. Impaired endothelial function in coronary heart disease patients with depressive symptomatology. J Am Coll Cardiol 2005; 46:656–659.
- Yang EH, Lerman S, Lennon RJ, Simari RD, Lerman LO, Lerman A. Relation of depression to coronary endothelial function. Am J Cardiol 2007; 99:1134–1136.
- Otte C, Neylan TC, Pipkin SS, Browner WS, Whooley MA. Depressive symptoms and 24-hour urinary norepinephrine excretion levels in patients with coronary disease: findings from the Heart and Soul Study. Am J Psychiatry 2005; 162:2139–2145.
- Wittstein IS, Thiemann DR, Lima JAC, et al. Neurohumoral features of myocardial stunning due to sudden emotional stress. N Engl J Med 2005; 352:539–548.
- Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med 2005; 352:1685–1695.
- Miller GE, Stetler CA, Carney RM, Freedland KE, Banks WA. Clinical depression and inflammatory risk markers for coronary heart disease. Am J Cardiol 2002; 90:1279–1283.
- Empana JP, Sykes DH, Luc G, et al. Contributions of depressive mood and circulating inflammatory markers to coronary heart disease in healthy European men: the Prospective Epidemiological Study of Myocardial Infarction (PRIME). Circulation 2005; 111:2299–2305.
- Frasure-Smith N, Lespérance F, Irwin MR, Sauvé C, Lespérance J, Théroux P. Depression, C-reactive protein and two-year major adverse cardiac events in men after acute coronary syndromes. Biol Psychiatry 2007; 62:302–308.
- Whooley MA, Caska CM, Hendrickson BE, Rourke MA, Ho J, Ali S. Depression and inflammation in patients with coronary heart disease: findings from the Heart and Soul Study. Biol Psychiatry 2007; 62:314–320.
- Glassman AH, Miller GE. Where there is depression, there is inflammation…sometimes! Biol Psychiatry 2007; 62:280–281.
- Tracey KJ. Physiology and immunology of the cholinergic antiinflammatory pathway. J Clin Invest 2007; 117:289–296.
- Tracey KJ. The inflammatory reflex. Nature 2002; 420:853–859.
- McCaffery JM, Frasure-Smith N, Dubé M-P, et al. Common genetic vulnerability to depressive symptoms and coronary artery disease: a review and development of candidate genes related to inflammation and serotonin. Psychosom Med 2006; 68:187–200.
- Caspi A, Sugden K, Moffitt TE, et al. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science 2003; 301:386–389.
- Nakatani D, Sato H, Sakata Y, et al. Influence of serotonin transporter gene polymorphism on depressive symptoms and new cardiac events after acute myocardial infarction. Am Heart J 2005; 150:652–658.
- Otte C, McCaffery J, Ali S, Whooley MA. Association of a serotonin transporter polymorphism (5-HTTLPR) with depression, perceived stress, and norepinephrine in patients with coronary disease: the Heart and Soul Study. Am J Psychiatry 2007; 164:1379–1384.
- Fumeron F, Betoulle D, Nicaud V, et al. Serotonin transporter gene polymorphism and myocardial infarction: Etude Cas-Témoins de l’Infarctus du Myocarde (ECTIM). Circulation 2002; 105:2943–2945.
- Rivelli S, Jiang W. Depression and ischemic heart disease: what have we learned from clinical trials? Curr Opin Cardiol 2007; 22:286–291.
- Frasure-Smith N, Lespérance F, Prince RH, et al. Randomised trial of home-based psychosocial nursing intervention for patients recovering from myocardial infarction. Lancet 1997; 350:473–479.
- Glassman AH, O’Connor CM, Califf RM, et al. Sertraline treatment of major depression in patients with acute MI or unstable angina. JAMA 2002; 288:701–709.
- Writing Committee for the EI. Effects of treating depression and low perceived social support on clinical events after myocardial infarction: the Enhancing Recovery in Coronary Heart Disease Patients (ENRICHD) randomized trial. JAMA 2003; 289:3106–3116.
- van Melle JP, de Jonge P, Honig A, et al. Effects of antidepressant treatment following myocardial infarction. Br J Psychiatry 2007; 190:460–466.
- Lespérance F, Frasure-Smith N, Koszycki D, et al. Effects of citalopram and interpersonal psychotherapy on depression in patients with coronary artery disease: the Canadian Cardiac Randomized Evaluation of Antidepressant and Psychotherapy Efficacy (CREATE) trial. JAMA 2007; 297:367–379.
- Carney RM, Blumenthal JA, Freedland KE, et al. Depression and late mortality after myocardial infarction in the Enhancing Recovery in Coronary Heart Disease (ENRICHD) Study. Psychosom Med 2004; 66:466–474.
- de Jonge P, Honig A, van Melle JP, et al. Nonresponse to treatment for depression following myocardial infarction: association with subsequent cardiac events. Am J Psychiatry 2007; 164:1371–1378.
- Carney RM, Freedland KE. Depression and coronary heart disease: more pieces of the puzzle. Am J Psychiatry 2007; 164:1307–1309.
- Glassman AH, Bigger JT, Gaffney M, Shapiro PA, Swenson JR. Onset of major depression associated with acute coronary syndromes: relationship of onset, major depressive disorder history, and episode severity to sertraline benefit. Arch Gen Psychiatry 2006; 63:283–288.
- Ziegelstein RC, Kim SY, Kao D, et al. Can doctors and nurses recognize depression in patients hospitalized with an acute myocardial infarction in the absence of formal screening? Psychosom Med 2005; 67:393–397.
- Kroenke K, Spitzer RL, Williams JBW. The PHQ-9. Validity of a brief depression severity measure. J Gen Intern Med 2001; 16:606–613.
- Löwe B, Unutzer J, Callahan CM, Perkins AJ, Kroenke K. Monitoring depression treatment outcomes with the Patient Health Questionnaire-9. Med Care 2004; 42:1194–1201.
- Parashar S, Rumsfeld JS, Spertus JA, et al. Time course of depression and outcome of myocardial infarction. Arch Intern Med 2006; 166:2035–2043.
- Amin AA, Jones AMH, Nugent K, Rumsfeld JS, Spertus JA. The prevalence of unrecognized depression in patients with acute coronary syndrome. Am Heart J 2006; 152:928–934.
- McManus D, Pipkin SS, Whooley MA. Screening for depression in patients with coronary heart disease (data from the Heart and Soul Study). Am J Cardiol 2005; 96:1076–1081.
- Kroenke K, Spitzer RL, Williams JB. The Patient Health Questionnaire-2: validity of a two-item depression screener. Med Care 2003; 41:1284–1292.
- Spitzer RL, Williams JB, Kroenke K, et al. Utility of a new procedure for diagnosing mental disorders in primary care. The PRIME-MD 1000 study. JAMA 1994; 272:1749–1756.
- Lespérance F, Frasure-Smith N. Depression in patients with cardiac disease. J Psychosom Res 2000; 48:379–391.
- Hunkeler EM, Katon W, Tang L, et al. Long term outcomes from the IMPACT randomised trial for depressed elderly patients in primary care. BMJ 2006; 332:259–263.
- Frasure-Smith N, Lespérance F, Gravel G, Masson A, Juneau M, Bourassa MG. Long-term survival differences among low-anxious, high-anxious and repressive copers enrolled in the Montreal Heart Attack Readjustment Trial. Psychosom Med 2002; 64:571–579.
- Glassman AH, Roose SP, Bigger JT. The safety of tricyclic antidepressants in cardiac patients: risk-benefit reconsidered. JAMA 1993; 269:2673–2675.
- Cohen HW, Gibson G, Alderman MH. Excess risk of myocardial infarction in patients treated with antidepressant medications: association with use of tricyclic agents. Am J Med 2000; 108:2–8.
- Taylor CB, Youngblood ME, Catellier D, et al. Effects of antidepressant medication on morbidity and mortality in depressed patients after myocardial infarction. Arch Gen Psychiatry 2005; 62:792–798.
- Montgomery SA. Safety of mirtazapine: a review. Int Clin Psychopharmacol 1995; 10(suppl 4):37–45.
- Sauer WH, Berlin JA, Kimmel SE. Selective serotonin reuptake inhibitors and myocardial infarction. Circulation 2001; 104:1894–1898.
- Sauer WH, Berlin JA, Kimmel SE. Effect of antidepressants and their relative affinity for the serotonin transporter on the risk of myocardial infarction. Circulation 2003; 108:32–36.
- Ziegelstein RC, Meuchel J, Kim TJ, et al. Selective serotonin reuptake inhibitor use by patients with acute coronary syndromes. Am J Med 2007; 120:525–530.
- Xiong GL, Jiang W, Clare R, et al. Prognosis of patients taking selective serotonin reuptake inhibitors before coronary artery bypass grafting. Am J Cardiol 2006; 98:42–47.
- Yang C-C, Jick SS, Jick H. Lipid-lowering drugs and the risk of depression and suicidal behavior. Arch Intern Med 2003; 163:1926–1932.
- Callreus T, Agerskov Andersen U, Hallas J, Andersen M. Cardiovascular drugs and the risk of suicide: a nested case-control study. Eur J Clin Pharmacol 2007; 63:591–596.
- Young-Xu Y, Chan KA, Liao JK, Ravid S, Blatt CM. Long-term statin use and psychological well-being. J Am Coll Cardiol 2003; 42:690–697.
- Ko DT, Hebert PR, Coffey CS, Sedrakyan A, Curtis JP, Krumholz HM. Beta-blocker therapy and symptoms of depression, fatigue, and sexual dysfunction. JAMA 2002; 288:351–357.
- van Melle JP, Verbeek D, van den Berg MP, Ormel J, van der Line MR, de Jonge P. Beta-blockers and depression after myocardial infarction. J Am Coll Cardiol 2006; 48:2209–2214.
- Thomas RJ, King M, Lui K, et al. AACVPR/ACC/AHA 2007 performance measures on cardiac rehabilitation for referral to and delivery of cardiac rehabilitation/secondary prevention services. J Am Coll Cardiol 2007; 50:1400–1433.
- Blumenthal JA, Babyak MA, Doraiswamy PM, et al. Exercise and pharmacotherapy in the treatment of major depressive disorder. Psychosom Med 2007; 69:587–596.
- Linden W, Phillips MJ, Leclerc J. Psychological treatment of cardiac patients: a meta-analysis. Eur Heart J 2007; 28:2972–2984.
- Centers for Disease Control and Prevention. Receipt of outpatient cardiac rehabilitation among heart attack survivors—United States, 2005. JAMA 2008; 299:1534–1536.
- Williams RB. Treating depression after myocardial infarction: can selecting patients on the basis of genetic susceptibility improve psychiatric and medical outcomes? Am Heart J 2005; 150:617–619.
- Schneiderman N, Williams RB. The great debate editorial, revisited. Psychosom Med 2006; 68:636–638.
- de Jonge P, Ormel J, van den Brink RHS, et al. Symptom dimensions of depression following myocardial infarction and their relationship with somatic health status and cardiovascular prognosis. Am J Psychiatry 2006; 163:138–144.
- Fraguas R, Iosifescu DV, Alpert J, et al. Major depressive disorder and comorbid cardiac disease: is there a depressive subtype with greater cardiovascular morbidity? Results from the STAR*D Study. Psychosomatics 2007; 48:418–425.
- Kubzansky LD, Thurston RC. Emotional vitality and incident coronary heart disease: benefits of healthy psychological functioning. Arch Gen Psychiatry 2007; 64:1393–1401.
- Frasure-Smith N. Reflections on depression as a cardiac risk factor Academy of Psychosomatic Medicine, 53rd Annual Meeting, Tucson, Arizona, 2006.
- Frasure-Smith N, Lespérance F. Major depression is associated with lower omega-3 fatty acid levels in patients with recent acute coronary syndromes. Biol Psychiatry 2004; 55:891–896.
- Lespérance F. Annual Research Award Lecture Academy of Psychosomatic Medicine, Amelia Island, Florida, 2007.
- Graham I, Atar D, Borch-Johnsen K, et al. European guidelines on cardiovascular disease prevention in clinical practice: executive summary. Eur Heart J 2007; 28:2375–2414.
- Davidson KW, Kupfer DJ, Bigger JT, et al. Assessment and treatment of depression in patients with cardiovascular disease: National Heart, Lung, and Blood Institute Working Group Report. Psychosom Med 2006; 68:645–650.
Depression is a risk factor for heart disease, and in patients with heart disease, it is a risk factor for complications and death. Unfortunately, in the trials performed to date, treating depression in cardiac patients did not lead to lower rates of recurrent cardiovascular events or death. Nevertheless, we recommend that clinicians systematically screen for it in their heart patients, in view of the benefits of antidepressant therapy.
In this article we review key epidemiologic and psychosocial studies, the mechanistic links between depression and heart disease, and recent intervention trials. We also offer practical management advice and address the continued need for guidelines and risk stratification in the treatment of depressed cardiac patients.
After we submitted our review article, the American Heart Association (AHA)1 released a consensus document recommending that health care providers screen for and treat depression in patients with coronary heart disease. We will discuss the same screening tests that have been recommended by the AHA.
DEPRESSION AND HEART DISEASE: COMMON AND LINKED
Depression and heart disease are very common and often coexist: the prevalence of depression in various heart conditions ranges from 15% to 20%.1–3 According to data from the World Health Organization, by the year 2020 depression will be the second-leading cause of disability in developed countries (after heart disease).4
The World Health Survey5 showed that depression worsens health more than angina, arthritis, asthma, or diabetes. Furthermore, patients with severe mental illness have a higher risk of dying from heart disease and stroke.6
SOME HEART DISEASE RISK FACTORS ARE PSYCHOSOCIAL
In the 1980s, the “type A” personality (ambitious, aggressive, hostile, and competitive, with a chronic sense of urgency) was linked to heart disease.7 Later studies differed as to whether the entire set of features is valid as a collective risk factor for progressive heart disease,8 but hostility remains a validated risk factor and a focus of behavior modification.9,10
Other psychosocial risk factors have been implicated,11,12 one of which is social isolation.9,13 Another is the “type D” personality, which includes a tendency to experience negative emotions across time and situations coupled with social inhibition and which is believed to be more valid than the type A personality as a risk factor for cardiac disease.14,15
The INTERHEART study16 gathered data about attributable risk in the development of myocardial infarction (MI) in 52 countries in a case-control fashion. Psychosocial factors including stress, low generalized locus of control (ie, the perceived inability to control one’s life), and depression accounted for 32.5% of the attributable risk for an MI.17 This would mean that they account for slightly less attributable risk than that of lifetime smoking but more than that of hypertension and obesity.
Job stress increases the risk of initial coronary heart disease18 and also the risk of recurrent cardiac events after a first MI.19 Even though numerous psychosocial risk factors have been associated with coronary heart disease, including anxiety,20,21 depression is perhaps the best studied.
PROSPECTIVE STUDIES OF DEPRESSION AND HEART DISEASE
To examine the impact of depression in coronary heart disease, prospective studies have been done in healthy people and in patients with established cardiovascular disease who develop depression.22
In healthy people, depression increases the risk of coronary disease
The 1996 Epidemiologic Catchment Area study23 found that people with major depression had a risk of MI four times higher than the norm, and people with 2 weeks of sadness or dysphoria had a risk two times higher.
A subsequent meta-analysis of 11 studies,24 which included 36,000 patients, found that the overall relative risk of developing heart disease in depressed but healthy people was 1.64.
A meta-analysis by Van der Kooy et al25 of 28 epidemiologic studies with nearly 80,000 patients showed depression to be an independent risk factor for cardiovascular disease.
Wulsin and Singal26 performed a systematic review to see if depression increases the risk of coronary disease. In 10 studies with a follow-up of more than 4 years, the relative risk in people with depression was 1.64, which was less than that in active smokers (2.5) but more than that in passive smokers (1.25).
Depression can also exacerbate the classic risk factors for coronary disease, such as smoking, diabetes, obesity, and physical inactivity. 27
A 2007 study from Sweden28 prospectively followed patients who were hospitalized for depression. The odds ratio of developing an acute MI was 2.9, and the risk persisted for decades after the initial hospitalization.
A prospective United Kingdom cohort study of people initially free of heart disease revealed major depression to be associated with a higher rate of death from ischemic heart disease.29 Specifically, patients who had depression currently or in the past 12 months had a 2.7 times higher risk of dying than those who had never had depression or who had had it more than 12 months previously.
In existing heart disease, depression predicts recurrent events, death
Carney et el30 found that patients with major depressive disorder had a higher incidence of new cardiac events in the 12 months after undergoing cardiac catheterization than those without major depressive disorder.
Frasure-Smith et al,31 in a landmark study, showed that patients who were depressed at 1 week after an MI were three to four times more likely to die in the next 6 months than nondepressed post-MI patients. Even after 18 months, depression remained an independent risk factor for cardiac-related death.32
In longer studies (with up to 19.4 years of follow-up), depression was associated with higher rates of death from cardiac and all causes in patients with coronary artery disease.33 Lespérance et al34 found that in MI patients, the higher the Beck Depression Inventory score at the time of hospital admission, the higher the 5-year death rate.
Using meta-analysis, Barth et al35 found the risk of dying in the first 2 years after initial assessment to be twice as high in depressed cardiac patients as in nondepressed cardiac patients (odds ratio 2.24).
Van Melle et al36 reviewed 22 studies and found that in the 2 years after an MI, depressed patients had a 2 to 2.5 times higher risk of dying of a cardiac or any other cause than did nondepressed patients.
Depression also predicts higher morbidity and mortality rates in patients undergoing coronary artery bypass grafting,37,38 patients with congestive heart failure,39 and heart transplant recipients.40
MEDICAL ILLNESS CAN PREDISPOSE TO DEPRESSION, AND VICE VERSA
Medical illnesses can predispose a patient to develop depression. Specifically, compared with healthy people, cardiac patients appear to be at greater risk of developing depression for many years after the initial medical diagnosis is made.41
Katon et al42 reviewed 31 studies involving 16,922 patients, that assessed the impact of depression and anxiety in chronic medical illnesses such as heart disease, diabetes, pulmonary disease, and arthritis. After the severity of the medical disorder was controlled for, patients with depression and anxiety reported a higher number of medical symptoms.
DEPRESSION WORSENS QUALITY OF LIFE AND ADHERENCE TO TREATMENT
Depressed patients perceive their health status and quality of life negatively. In the Heart and Soul study,43 depressive symptoms and low exercise capacity—but not low ejection fraction or ischemia—were significantly associated with perceived deterioration of health in patients with coronary artery disease.
After an MI, patients who take their cardiac drugs properly have a better chance of survival.44,45 Clinical depression can worsen compliance with cardiac medication regimens,46 and reducing depression increases medication adherence overall.47 Not surprisingly, depressed patients also adhere less well to other recommendations,48 including modifying the diet, exercising, stopping smoking, and attending cardiac rehabilitation programs. 49
PLAUSIBLE MECHANISMS LINK DEPRESSION AND HEART DISEASE
Traditional cardiac risk factors such as smoking, high cholesterol, hypertension, diabetes, and obesity tend to cluster in depressed patients. 50 Other mechanisms linking depression and heart disease are reviewed below.51,52
Autonomic imbalance
Excessive sympathetic stimulation or diminished vagal stimulation or both are associated with higher rates of morbidity and death.53
Lack of variability in the heart rate reflects a sympathetic-vagal imbalance and is a risk factor for ventricular arrhythmias and sudden cardiac death in patients with cardiovascular disease.54 Carney et al55 reported that patients with coronary artery disease and depression had significantly less heart rate variability than nondepressed cardiac patients. Similarly, after an MI, depressed patients had significantly less heart rate variability than nondepressed patients,56 implying that low heart rate variability may mediate the adverse effect of depression on survival after an MI.57
In the Heart and Soul study, Gehi et al58 found no distinct relationship between heart rate variability and depression. However, in the same study, de Jong et al59 did find specific somatic symptoms of depression to be associated with lower heart rate variability, although cognitive symptoms were not.
Platelet activation, endothelial dysfunction
Depressed patients have been found to have exaggerated platelet reactivity.60 Plasma levels of platelet factor IV and beta-thromboglobulin, markers of platelet activation, are higher in depressed patients with ischemic heart disease than in nondepressed patients with ischemic heart disease and in control patients.61 This activation of platelets can lead to vascular damage and thrombosis.
In a subset study of the Sertraline Anti-Depressant Heart Attack Randomized Trial (SADHART), depressed MI patients were treated with sertraline (Zoloft), a selective serotonin reuptake inhibitor (SSRI), and had substantially less platelet and endothelial biomarker release.62
Depressed cardiac patients also have impaired flow-mediated dilation of the brachial artery, a sign of endothelial dysfunction.63 Although a recent study did not find coronary endothelial dysfunction in depressed patients who did not have cardiac disease, these patients had more clustering of other cardiac risk factors.64
Hypothalamic-pituitary-adrenocortical and sympathetic adrenal medullary activation
High cortisol levels can accelerate the development of hypertension and atherosclerosis and result in endothelial vascular injury. Sympathoadrenal activation in turn can lead to higher levels of catecholamines, predisposing to vasoconstriction, a rapid heart rate, and platelet activation. Depressed patients have more activation of the hypothalamic-pituitary-adrenocortical and sympathetic adrenal medullary systems,51,65 yet another plausible mechanism for worse clinical outcomes in depressed cardiac patients.
Sudden emotional stress can cause transient left ventricular dysfunction, even in people without coronary disease, an effect that may be mediated by elevated plasma catecholamine levels.66
Inflammatory cytokines
Inflammatory cytokines play a key role in the development of atherosclerosis.67 C-reactive protein, an acute-phase reactant produced in hepatocytes, can be induced by cytokines such as interleukin 6. Damage to endothelial tissues leads to the release of inflammatory cytokines, including interleukin 1, interleukin 6, and tumor tumor necrosis factor alpha.
Depressed patients have higher levels of these inflammatory markers.68,69 A prospective study reported direct correlations between depression scores and C-reactive protein levels in post-MI patients.70 The Heart and Soul study, however, did not confirm that coronary patients have more inflammation if they have depression,71 indicating that the relationship is complex and is perhaps more evident in specific types of depression.72
Anticholinergic inflammatory pathway
Tracey73 proposed a theory that vagal tone inhibits the release of inflammatory cytokines. This has important implications for treatment, as exercise, biofeedback, and meditation can stimulate the vagus nerve and therefore have beneficial anti-inflammatory effects.74
Polymorphism in the serotonin transport promoter region gene
Research is focusing on the serotonin transport promoter region gene (5-HTTLPR).75 The gene exists in two forms, a long one and a less-effective short one that appears to predispose to depression.76
Nakatani et al77 showed that MI patients were more likely to become depressed and to have subsequent cardiac events if one or both of their alleles of this gene were short. Otte et al,78 using Heart and Soul study data, found that patients with a short allele had a higher likelihood of depression, higher perceived levels of stress, and higher urinary norepinephrine secretion. However, the long allele genotype may be associated with a higher risk of developing an MI.79
Our knowledge of the genetic interplay of depression and cardiovascular disease is still in its infancy, and further studies are needed to clarify these findings.
IN TRIALS, LESS DEPRESSION BUT NO EFFECT ON DEATHS, RECURRENT MI
Major behavioral and drug trials conducted in the last 15 years have focused on how to best treat depression in cardiac patients.80–85
The Montreal Heart Attack Readjustment Trial (MHART)81 used telephone calls and home nursing visits to explore and monitor psychological distress for up to 1 year after an MI. The overall trial did not show these interventions to have any impact on survival compared with usual care. In fact, in women receiving the telephone intervention, there was a trend toward higher rates of cardiac and all-cause death, which was quite unexpected. Uncovering stresses and problems without resolving them, rather than encouraging patients to place these on the “back burner,” may partially explain these results.
SADHART82 studied the safety of sertraline in depressed post-MI patients. No major differences in cardiac function were noted between the sertraline and placebo groups, showing that sertraline was safe for these patients. The sertraline group had fewer cardiovascular events, but the difference was not statistically significant.
The Enhancing Recovery in Coronary Heart Disease (ENRICHD) study83 was primarily designed to see whether a psychosocial intervention would decrease deaths in depressed cardiac patients. Much to the chagrin of behavioral medicine, the group undergoing cognitive behavioral therapy did not have a higher rate of event-free survival, although the intervention had a favorable impact on depression and social support.
The Myocardial Infarction Depression Intervention Trial (MIND-IT)84 looked at whether the antidepressant mirtazapine (Remeron) would improve long-term depression and cardiovascular outcomes in depressed post-MI patients. In 18 months of follow-up, neither objective was obtained.
The Cardiac Randomized Evaluation of Antidepressant and Psychotherapy Efficacy (CREATE) trial85 tested the efficacy of the SSRI citalopram (Celexa) and interpersonal therapy in a short-term intervention. Here, the antidepressant was superior to placebo in the primary outcome of treating depression, but interpersonal therapy had no advantage over “clinical management,” ie, a shorter, 20-minute supportive intervention.
Common threads in these studies.
- In ENRICHD and MIND-IT, patients whose depression did not respond to treatment were at higher risk of cardiac events during follow-up.86–88
- In SADHART and CREATE, which used drug treatment, the antidepressant response was more robust in patients with a history of depression before their heart attacks, suggesting that a patient with recurrent depression at the time of a cardiac event should receive medication for it.85,89
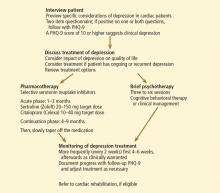
CLINICAL RECOMMENDATIONS
Use a depression screening tool
Ziegelstein et al90 recently studied the ability of clinical personnel to detect depression in hospitalized MI patients. If a screening tool was not used, the results were abysmal, indicating the need to use formal screening for symptoms of depression in acute MI patients.
Many self-rating scales are available, among which are the Beck Depression Inventory (BDI) and the Hospital Anxiety and Depression Scale (HADS). Others are:
The PHQ-2 consists of the two first questions of the PHQ-9, which deal with mood and lack of pleasure. A cut-off score of 3 or higher has a sensitivity of 83% and a specificity of 92%,96 fulfilling the need for a quick and reliable depression screening tool. The clinician can also ask for a yes-or-no answer to the two questions of the PHQ-2 (Table 1). A yes to either of the two questions is up to 90% sensitive and 75% specific.92,97
When to suspect depression in cardiac patients
Which type of psychotherapy is best?
The negative results of psychosocial interventions (phone calls and home visits from a nurse) in MHART and of cognitive behavioral therapy in ENRICHD raise questions about which type of psychotherapy is best for depression in heart disease. CREATE found that 50-minute weekly sessions of interpersonal psychotherapy were no more beneficial than clinical management, ie, 20-minute weekly sessions that focused on compliance with treatment and education about depression and overall management. Perhaps a type of therapy akin to “clinical management” in this study or the brief behavior-based and targeted therapy used in the Improving Mood Promoting Access to Collaborative Care Treatment (IMPACT) trials of depression in primary care99 could be designed specifically to treat depression in cardiac disease. However, it is also quite possible that treatments that focus on uncovering stresses or problems may not be timely for these patients.
Which therapy is best for women is another area of consideration. In MHART, even after 5 years of follow-up,100 women who received the psychosocial support intervention did marginally worse. In the ENRICHD study, women did not experience a benefit from cognitive behavioral therapy. Further studies must address sex differences in response to different therapies.
SSRIs seem to be better than other antidepressants for cardiac patients
Before SSRIs were available, tricyclic antidepressants were the mainstays. Subsequent analysis showed the tricyclics to have an unfavorable risk-benefit profile in cardiac patients,101 and since other types of antidepressants are available, tricyclics should be avoided altogether in cardiac patients.102
Whether the SSRIs actually decrease one’s risk of death in heart disease is still an issue of debate, but there are encouraging signs. In SADHART, the rate of death and recurrent nonfatal MI was 20% lower in the patients randomized to receive sertraline, although the difference was not statistically significant.82 In ENRICHD, patients who did not respond to cognitive behavioral treatment or had severe depression could receive sertraline or other antidepressant drugs on a nonrandomized basis, and those who did had a 42% lower incidence of death or recurrent MI.103
The SADHART and CREATE trials provide convincing evidence of the cardiac safety and antidepressant efficacy of two SSRIs (sertraline and citalopram) in depressed cardiac patients. Mirtazapine, studied in MIND-IT, was not effective in treating depression in cardiac patients, although it had a better adverse effect and safety profile than tricyclic antidepressants. 104
Clinical observations indicate that SSRIs are associated with less risk of MI than non-SSRI drugs.105,106 During hospitalization for acute coronary syndromes, patients on SSRIs had lower rates of recurrent ischemia and heart failure but higher bleeding rates than patients not taking SSRIs.107 In a retrospective study of patients undergoing coronary artery bypass grafting, those on an SSRI before surgery had higher rates of death and rehospitalization.108 Being on antidepressant medication could be interpreted as a surrogate marker of having more severe depression before surgery; this issue clearly requires further study.
Given current observations and recent data from interventional trials coupled with the safe drug-interaction profile of sertraline and citalopram, these two SSRIs are recommended for treating depression in cardiac patients. If the patient is also receiving an anticoagulant, one should monitor for bleeding, as all SSRIs are associated with a prolonged bleeding time. Monitoring for rare cases of hyponatremia and bradycardia should also be part of early follow-up.
Do cardiac drugs have psychiatric effects?
Some concerns have arisen about cardiovascular drugs causing or aggravating psychiatric conditions.
Statins were once suspected of causing clinical depression or even suicide. However, subsequent studies have not substantiated this.109,110 In fact, long-term statin use has been associated with improved psychological wellbeing. 111 Whether the favorable psychological profile is due to an improved lifestyle, a direct noncholesterol effect, or an immunomodulatory effect has yet to be determined.
Beta-blockers have been suspected of increasing depression and fatigue. Robust metaanalyses have shown no increased risk of depressive symptoms but a small increased risk of fatigue and sexual dysfunction.112 Observational trials in the first year post-MI have shown no differences between beta-blocker users and nonusers in depressive symptoms or depressive disorders.113
Statins and beta-blockers offer both immense cardiac benefit and low risk, and both may be prescribed with confidence in depressed cardiac patients.
Refer patients for cardiac rehabilitation
The American Association of Cardiovascular and Pulmonary Rehabilitation strongly recommends screening cardiac patients for depression and referring them to cardiac rehabilitation programs.114 Typical programs run 12 weeks, affording an opportunity to further listen to and assess the patient and to promote general wellness via nutrition, stress management, and exercise.
These interventions by themselves can favorably affect depression. Blumenthal and colleagues,115 in the Standard Medical Intervention and Long-Term Exercise (SMILE) study, found that exercise was as effective as drug treatment in reducing depression. In addition, stress management as a psychosocial treatment in cardiac rehabilitation can reduce death rates in cardiac patients.116
Unfortunately, many patients who are eligible for cardiac rehabilitation programs do not avail themselves of them.117
Our algorithm
FUTURE DIRECTIONS FOR RESEARCH
Can we predict the course of depression?
We need to identify better which patients will have a spontaneous remission of their depressive symptoms after a cardiac event, which patients will linger with depression, and which patients will best respond to treatment. Risk stratification, using the psychiatric history, symptoms and severity of depression, and genetic predisposition118 might allow improved targeted therapies.
Does depression cause cardiac disease?
The link between depression and heart disease can be seen as merely an association. In the interventional trials performed to date, we have not yet seen a reduction in cardiac deaths when depression was treated, challenging any assumption of a causal relationship between depression and heart disease. The debate about association vs cause is germane to behavioral medicine,119 and the better we understand the mechanistic pathways, the better we can advise patients and treat depression comorbid with heart disease.
Behavioral medicine is currently measuring the aspects of depression associated with cardiac disease, including the spectrum of somatic (body) and affective (mood) symptoms120 and specific areas such as sympathetic arousal and early morning insomnia.121 If we can determine the depression subtype that carries a worse cardiac prognosis, we may untangle the biobehavioral links that bidirectionally bridge clinical depression and cardiac disease.
Another area of interest, emotional vitality (a positive state associated with interest, enthusiasm, excitement, and energy for living) has been shown to protect against coronary heart disease122 and holds much promise.
In the plenary lecture of the Academy of Psychosomatic Medicine in 2006, Frasure-Smith spoke of the “pleiotropism” of our antidepressant interventions on the various risk factors in depressed cardiac patients.123 We need behavioral medicine studies that elucidate these mechanisms, guiding more precise treatments as well as novel therapies. Omega-3 fatty acids, which benefit heart disease and clinical depression,124 will be used in a randomized controlled trial by Lespérance and colleagues.125 We await the results of this exciting research.
Will treating depression help in other types of heart disease?
The SADHART-CHF trial is examining whether 12 weeks of sertraline therapy is better than placebo in preventing death and improving cardiac outcomes in patients with chronic heart failure and comorbid major depressive disorder. It was to be completed in the fall of 2008. The results and experience of this study will help in designing future interventional trials to reduce the risk of depression in cardiovascular diseases.
We also await the results of a National Heart, Lung, and Blood Institute (NHLBI) trial, “Bypassing the Blues,” which is studying the treatment of depression after cardiac bypass surgery. This study should provide further insights into management of the depressed cardiac patient. Further prognostic studies in cardiac patients are also needed using the PHQ-9 and its shorter version, PHQ-2.
Current and future guidelines
For years our European colleagues have been ahead of us in recognizing depression screening and stress management as key to cardiac disease-prevention strategies.126 The NHLBI nicely outlined recommendations on the assessment and treatment of depression in cardiovascular patients.127 The just-published AHA Science Advisory should further encourage clinicians to screen and treat depression in the patient population.1 As our knowledge grows, we look forward to future evidence-based guidelines for depressed cardiac patients.
Depression is a risk factor for heart disease, and in patients with heart disease, it is a risk factor for complications and death. Unfortunately, in the trials performed to date, treating depression in cardiac patients did not lead to lower rates of recurrent cardiovascular events or death. Nevertheless, we recommend that clinicians systematically screen for it in their heart patients, in view of the benefits of antidepressant therapy.
In this article we review key epidemiologic and psychosocial studies, the mechanistic links between depression and heart disease, and recent intervention trials. We also offer practical management advice and address the continued need for guidelines and risk stratification in the treatment of depressed cardiac patients.
After we submitted our review article, the American Heart Association (AHA)1 released a consensus document recommending that health care providers screen for and treat depression in patients with coronary heart disease. We will discuss the same screening tests that have been recommended by the AHA.
DEPRESSION AND HEART DISEASE: COMMON AND LINKED
Depression and heart disease are very common and often coexist: the prevalence of depression in various heart conditions ranges from 15% to 20%.1–3 According to data from the World Health Organization, by the year 2020 depression will be the second-leading cause of disability in developed countries (after heart disease).4
The World Health Survey5 showed that depression worsens health more than angina, arthritis, asthma, or diabetes. Furthermore, patients with severe mental illness have a higher risk of dying from heart disease and stroke.6
SOME HEART DISEASE RISK FACTORS ARE PSYCHOSOCIAL
In the 1980s, the “type A” personality (ambitious, aggressive, hostile, and competitive, with a chronic sense of urgency) was linked to heart disease.7 Later studies differed as to whether the entire set of features is valid as a collective risk factor for progressive heart disease,8 but hostility remains a validated risk factor and a focus of behavior modification.9,10
Other psychosocial risk factors have been implicated,11,12 one of which is social isolation.9,13 Another is the “type D” personality, which includes a tendency to experience negative emotions across time and situations coupled with social inhibition and which is believed to be more valid than the type A personality as a risk factor for cardiac disease.14,15
The INTERHEART study16 gathered data about attributable risk in the development of myocardial infarction (MI) in 52 countries in a case-control fashion. Psychosocial factors including stress, low generalized locus of control (ie, the perceived inability to control one’s life), and depression accounted for 32.5% of the attributable risk for an MI.17 This would mean that they account for slightly less attributable risk than that of lifetime smoking but more than that of hypertension and obesity.
Job stress increases the risk of initial coronary heart disease18 and also the risk of recurrent cardiac events after a first MI.19 Even though numerous psychosocial risk factors have been associated with coronary heart disease, including anxiety,20,21 depression is perhaps the best studied.
PROSPECTIVE STUDIES OF DEPRESSION AND HEART DISEASE
To examine the impact of depression in coronary heart disease, prospective studies have been done in healthy people and in patients with established cardiovascular disease who develop depression.22
In healthy people, depression increases the risk of coronary disease
The 1996 Epidemiologic Catchment Area study23 found that people with major depression had a risk of MI four times higher than the norm, and people with 2 weeks of sadness or dysphoria had a risk two times higher.
A subsequent meta-analysis of 11 studies,24 which included 36,000 patients, found that the overall relative risk of developing heart disease in depressed but healthy people was 1.64.
A meta-analysis by Van der Kooy et al25 of 28 epidemiologic studies with nearly 80,000 patients showed depression to be an independent risk factor for cardiovascular disease.
Wulsin and Singal26 performed a systematic review to see if depression increases the risk of coronary disease. In 10 studies with a follow-up of more than 4 years, the relative risk in people with depression was 1.64, which was less than that in active smokers (2.5) but more than that in passive smokers (1.25).
Depression can also exacerbate the classic risk factors for coronary disease, such as smoking, diabetes, obesity, and physical inactivity. 27
A 2007 study from Sweden28 prospectively followed patients who were hospitalized for depression. The odds ratio of developing an acute MI was 2.9, and the risk persisted for decades after the initial hospitalization.
A prospective United Kingdom cohort study of people initially free of heart disease revealed major depression to be associated with a higher rate of death from ischemic heart disease.29 Specifically, patients who had depression currently or in the past 12 months had a 2.7 times higher risk of dying than those who had never had depression or who had had it more than 12 months previously.
In existing heart disease, depression predicts recurrent events, death
Carney et el30 found that patients with major depressive disorder had a higher incidence of new cardiac events in the 12 months after undergoing cardiac catheterization than those without major depressive disorder.
Frasure-Smith et al,31 in a landmark study, showed that patients who were depressed at 1 week after an MI were three to four times more likely to die in the next 6 months than nondepressed post-MI patients. Even after 18 months, depression remained an independent risk factor for cardiac-related death.32
In longer studies (with up to 19.4 years of follow-up), depression was associated with higher rates of death from cardiac and all causes in patients with coronary artery disease.33 Lespérance et al34 found that in MI patients, the higher the Beck Depression Inventory score at the time of hospital admission, the higher the 5-year death rate.
Using meta-analysis, Barth et al35 found the risk of dying in the first 2 years after initial assessment to be twice as high in depressed cardiac patients as in nondepressed cardiac patients (odds ratio 2.24).
Van Melle et al36 reviewed 22 studies and found that in the 2 years after an MI, depressed patients had a 2 to 2.5 times higher risk of dying of a cardiac or any other cause than did nondepressed patients.
Depression also predicts higher morbidity and mortality rates in patients undergoing coronary artery bypass grafting,37,38 patients with congestive heart failure,39 and heart transplant recipients.40
MEDICAL ILLNESS CAN PREDISPOSE TO DEPRESSION, AND VICE VERSA
Medical illnesses can predispose a patient to develop depression. Specifically, compared with healthy people, cardiac patients appear to be at greater risk of developing depression for many years after the initial medical diagnosis is made.41
Katon et al42 reviewed 31 studies involving 16,922 patients, that assessed the impact of depression and anxiety in chronic medical illnesses such as heart disease, diabetes, pulmonary disease, and arthritis. After the severity of the medical disorder was controlled for, patients with depression and anxiety reported a higher number of medical symptoms.
DEPRESSION WORSENS QUALITY OF LIFE AND ADHERENCE TO TREATMENT
Depressed patients perceive their health status and quality of life negatively. In the Heart and Soul study,43 depressive symptoms and low exercise capacity—but not low ejection fraction or ischemia—were significantly associated with perceived deterioration of health in patients with coronary artery disease.
After an MI, patients who take their cardiac drugs properly have a better chance of survival.44,45 Clinical depression can worsen compliance with cardiac medication regimens,46 and reducing depression increases medication adherence overall.47 Not surprisingly, depressed patients also adhere less well to other recommendations,48 including modifying the diet, exercising, stopping smoking, and attending cardiac rehabilitation programs. 49
PLAUSIBLE MECHANISMS LINK DEPRESSION AND HEART DISEASE
Traditional cardiac risk factors such as smoking, high cholesterol, hypertension, diabetes, and obesity tend to cluster in depressed patients. 50 Other mechanisms linking depression and heart disease are reviewed below.51,52
Autonomic imbalance
Excessive sympathetic stimulation or diminished vagal stimulation or both are associated with higher rates of morbidity and death.53
Lack of variability in the heart rate reflects a sympathetic-vagal imbalance and is a risk factor for ventricular arrhythmias and sudden cardiac death in patients with cardiovascular disease.54 Carney et al55 reported that patients with coronary artery disease and depression had significantly less heart rate variability than nondepressed cardiac patients. Similarly, after an MI, depressed patients had significantly less heart rate variability than nondepressed patients,56 implying that low heart rate variability may mediate the adverse effect of depression on survival after an MI.57
In the Heart and Soul study, Gehi et al58 found no distinct relationship between heart rate variability and depression. However, in the same study, de Jong et al59 did find specific somatic symptoms of depression to be associated with lower heart rate variability, although cognitive symptoms were not.
Platelet activation, endothelial dysfunction
Depressed patients have been found to have exaggerated platelet reactivity.60 Plasma levels of platelet factor IV and beta-thromboglobulin, markers of platelet activation, are higher in depressed patients with ischemic heart disease than in nondepressed patients with ischemic heart disease and in control patients.61 This activation of platelets can lead to vascular damage and thrombosis.
In a subset study of the Sertraline Anti-Depressant Heart Attack Randomized Trial (SADHART), depressed MI patients were treated with sertraline (Zoloft), a selective serotonin reuptake inhibitor (SSRI), and had substantially less platelet and endothelial biomarker release.62
Depressed cardiac patients also have impaired flow-mediated dilation of the brachial artery, a sign of endothelial dysfunction.63 Although a recent study did not find coronary endothelial dysfunction in depressed patients who did not have cardiac disease, these patients had more clustering of other cardiac risk factors.64
Hypothalamic-pituitary-adrenocortical and sympathetic adrenal medullary activation
High cortisol levels can accelerate the development of hypertension and atherosclerosis and result in endothelial vascular injury. Sympathoadrenal activation in turn can lead to higher levels of catecholamines, predisposing to vasoconstriction, a rapid heart rate, and platelet activation. Depressed patients have more activation of the hypothalamic-pituitary-adrenocortical and sympathetic adrenal medullary systems,51,65 yet another plausible mechanism for worse clinical outcomes in depressed cardiac patients.
Sudden emotional stress can cause transient left ventricular dysfunction, even in people without coronary disease, an effect that may be mediated by elevated plasma catecholamine levels.66
Inflammatory cytokines
Inflammatory cytokines play a key role in the development of atherosclerosis.67 C-reactive protein, an acute-phase reactant produced in hepatocytes, can be induced by cytokines such as interleukin 6. Damage to endothelial tissues leads to the release of inflammatory cytokines, including interleukin 1, interleukin 6, and tumor tumor necrosis factor alpha.
Depressed patients have higher levels of these inflammatory markers.68,69 A prospective study reported direct correlations between depression scores and C-reactive protein levels in post-MI patients.70 The Heart and Soul study, however, did not confirm that coronary patients have more inflammation if they have depression,71 indicating that the relationship is complex and is perhaps more evident in specific types of depression.72
Anticholinergic inflammatory pathway
Tracey73 proposed a theory that vagal tone inhibits the release of inflammatory cytokines. This has important implications for treatment, as exercise, biofeedback, and meditation can stimulate the vagus nerve and therefore have beneficial anti-inflammatory effects.74
Polymorphism in the serotonin transport promoter region gene
Research is focusing on the serotonin transport promoter region gene (5-HTTLPR).75 The gene exists in two forms, a long one and a less-effective short one that appears to predispose to depression.76
Nakatani et al77 showed that MI patients were more likely to become depressed and to have subsequent cardiac events if one or both of their alleles of this gene were short. Otte et al,78 using Heart and Soul study data, found that patients with a short allele had a higher likelihood of depression, higher perceived levels of stress, and higher urinary norepinephrine secretion. However, the long allele genotype may be associated with a higher risk of developing an MI.79
Our knowledge of the genetic interplay of depression and cardiovascular disease is still in its infancy, and further studies are needed to clarify these findings.
IN TRIALS, LESS DEPRESSION BUT NO EFFECT ON DEATHS, RECURRENT MI
Major behavioral and drug trials conducted in the last 15 years have focused on how to best treat depression in cardiac patients.80–85
The Montreal Heart Attack Readjustment Trial (MHART)81 used telephone calls and home nursing visits to explore and monitor psychological distress for up to 1 year after an MI. The overall trial did not show these interventions to have any impact on survival compared with usual care. In fact, in women receiving the telephone intervention, there was a trend toward higher rates of cardiac and all-cause death, which was quite unexpected. Uncovering stresses and problems without resolving them, rather than encouraging patients to place these on the “back burner,” may partially explain these results.
SADHART82 studied the safety of sertraline in depressed post-MI patients. No major differences in cardiac function were noted between the sertraline and placebo groups, showing that sertraline was safe for these patients. The sertraline group had fewer cardiovascular events, but the difference was not statistically significant.
The Enhancing Recovery in Coronary Heart Disease (ENRICHD) study83 was primarily designed to see whether a psychosocial intervention would decrease deaths in depressed cardiac patients. Much to the chagrin of behavioral medicine, the group undergoing cognitive behavioral therapy did not have a higher rate of event-free survival, although the intervention had a favorable impact on depression and social support.
The Myocardial Infarction Depression Intervention Trial (MIND-IT)84 looked at whether the antidepressant mirtazapine (Remeron) would improve long-term depression and cardiovascular outcomes in depressed post-MI patients. In 18 months of follow-up, neither objective was obtained.
The Cardiac Randomized Evaluation of Antidepressant and Psychotherapy Efficacy (CREATE) trial85 tested the efficacy of the SSRI citalopram (Celexa) and interpersonal therapy in a short-term intervention. Here, the antidepressant was superior to placebo in the primary outcome of treating depression, but interpersonal therapy had no advantage over “clinical management,” ie, a shorter, 20-minute supportive intervention.
Common threads in these studies.
- In ENRICHD and MIND-IT, patients whose depression did not respond to treatment were at higher risk of cardiac events during follow-up.86–88
- In SADHART and CREATE, which used drug treatment, the antidepressant response was more robust in patients with a history of depression before their heart attacks, suggesting that a patient with recurrent depression at the time of a cardiac event should receive medication for it.85,89
CLINICAL RECOMMENDATIONS
Use a depression screening tool
Ziegelstein et al90 recently studied the ability of clinical personnel to detect depression in hospitalized MI patients. If a screening tool was not used, the results were abysmal, indicating the need to use formal screening for symptoms of depression in acute MI patients.
Many self-rating scales are available, among which are the Beck Depression Inventory (BDI) and the Hospital Anxiety and Depression Scale (HADS). Others are:
The PHQ-2 consists of the two first questions of the PHQ-9, which deal with mood and lack of pleasure. A cut-off score of 3 or higher has a sensitivity of 83% and a specificity of 92%,96 fulfilling the need for a quick and reliable depression screening tool. The clinician can also ask for a yes-or-no answer to the two questions of the PHQ-2 (Table 1). A yes to either of the two questions is up to 90% sensitive and 75% specific.92,97
When to suspect depression in cardiac patients
Which type of psychotherapy is best?
The negative results of psychosocial interventions (phone calls and home visits from a nurse) in MHART and of cognitive behavioral therapy in ENRICHD raise questions about which type of psychotherapy is best for depression in heart disease. CREATE found that 50-minute weekly sessions of interpersonal psychotherapy were no more beneficial than clinical management, ie, 20-minute weekly sessions that focused on compliance with treatment and education about depression and overall management. Perhaps a type of therapy akin to “clinical management” in this study or the brief behavior-based and targeted therapy used in the Improving Mood Promoting Access to Collaborative Care Treatment (IMPACT) trials of depression in primary care99 could be designed specifically to treat depression in cardiac disease. However, it is also quite possible that treatments that focus on uncovering stresses or problems may not be timely for these patients.
Which therapy is best for women is another area of consideration. In MHART, even after 5 years of follow-up,100 women who received the psychosocial support intervention did marginally worse. In the ENRICHD study, women did not experience a benefit from cognitive behavioral therapy. Further studies must address sex differences in response to different therapies.
SSRIs seem to be better than other antidepressants for cardiac patients
Before SSRIs were available, tricyclic antidepressants were the mainstays. Subsequent analysis showed the tricyclics to have an unfavorable risk-benefit profile in cardiac patients,101 and since other types of antidepressants are available, tricyclics should be avoided altogether in cardiac patients.102
Whether the SSRIs actually decrease one’s risk of death in heart disease is still an issue of debate, but there are encouraging signs. In SADHART, the rate of death and recurrent nonfatal MI was 20% lower in the patients randomized to receive sertraline, although the difference was not statistically significant.82 In ENRICHD, patients who did not respond to cognitive behavioral treatment or had severe depression could receive sertraline or other antidepressant drugs on a nonrandomized basis, and those who did had a 42% lower incidence of death or recurrent MI.103
The SADHART and CREATE trials provide convincing evidence of the cardiac safety and antidepressant efficacy of two SSRIs (sertraline and citalopram) in depressed cardiac patients. Mirtazapine, studied in MIND-IT, was not effective in treating depression in cardiac patients, although it had a better adverse effect and safety profile than tricyclic antidepressants. 104
Clinical observations indicate that SSRIs are associated with less risk of MI than non-SSRI drugs.105,106 During hospitalization for acute coronary syndromes, patients on SSRIs had lower rates of recurrent ischemia and heart failure but higher bleeding rates than patients not taking SSRIs.107 In a retrospective study of patients undergoing coronary artery bypass grafting, those on an SSRI before surgery had higher rates of death and rehospitalization.108 Being on antidepressant medication could be interpreted as a surrogate marker of having more severe depression before surgery; this issue clearly requires further study.
Given current observations and recent data from interventional trials coupled with the safe drug-interaction profile of sertraline and citalopram, these two SSRIs are recommended for treating depression in cardiac patients. If the patient is also receiving an anticoagulant, one should monitor for bleeding, as all SSRIs are associated with a prolonged bleeding time. Monitoring for rare cases of hyponatremia and bradycardia should also be part of early follow-up.
Do cardiac drugs have psychiatric effects?
Some concerns have arisen about cardiovascular drugs causing or aggravating psychiatric conditions.
Statins were once suspected of causing clinical depression or even suicide. However, subsequent studies have not substantiated this.109,110 In fact, long-term statin use has been associated with improved psychological wellbeing. 111 Whether the favorable psychological profile is due to an improved lifestyle, a direct noncholesterol effect, or an immunomodulatory effect has yet to be determined.
Beta-blockers have been suspected of increasing depression and fatigue. Robust metaanalyses have shown no increased risk of depressive symptoms but a small increased risk of fatigue and sexual dysfunction.112 Observational trials in the first year post-MI have shown no differences between beta-blocker users and nonusers in depressive symptoms or depressive disorders.113
Statins and beta-blockers offer both immense cardiac benefit and low risk, and both may be prescribed with confidence in depressed cardiac patients.
Refer patients for cardiac rehabilitation
The American Association of Cardiovascular and Pulmonary Rehabilitation strongly recommends screening cardiac patients for depression and referring them to cardiac rehabilitation programs.114 Typical programs run 12 weeks, affording an opportunity to further listen to and assess the patient and to promote general wellness via nutrition, stress management, and exercise.
These interventions by themselves can favorably affect depression. Blumenthal and colleagues,115 in the Standard Medical Intervention and Long-Term Exercise (SMILE) study, found that exercise was as effective as drug treatment in reducing depression. In addition, stress management as a psychosocial treatment in cardiac rehabilitation can reduce death rates in cardiac patients.116
Unfortunately, many patients who are eligible for cardiac rehabilitation programs do not avail themselves of them.117
Our algorithm
FUTURE DIRECTIONS FOR RESEARCH
Can we predict the course of depression?
We need to identify better which patients will have a spontaneous remission of their depressive symptoms after a cardiac event, which patients will linger with depression, and which patients will best respond to treatment. Risk stratification, using the psychiatric history, symptoms and severity of depression, and genetic predisposition118 might allow improved targeted therapies.
Does depression cause cardiac disease?
The link between depression and heart disease can be seen as merely an association. In the interventional trials performed to date, we have not yet seen a reduction in cardiac deaths when depression was treated, challenging any assumption of a causal relationship between depression and heart disease. The debate about association vs cause is germane to behavioral medicine,119 and the better we understand the mechanistic pathways, the better we can advise patients and treat depression comorbid with heart disease.
Behavioral medicine is currently measuring the aspects of depression associated with cardiac disease, including the spectrum of somatic (body) and affective (mood) symptoms120 and specific areas such as sympathetic arousal and early morning insomnia.121 If we can determine the depression subtype that carries a worse cardiac prognosis, we may untangle the biobehavioral links that bidirectionally bridge clinical depression and cardiac disease.
Another area of interest, emotional vitality (a positive state associated with interest, enthusiasm, excitement, and energy for living) has been shown to protect against coronary heart disease122 and holds much promise.
In the plenary lecture of the Academy of Psychosomatic Medicine in 2006, Frasure-Smith spoke of the “pleiotropism” of our antidepressant interventions on the various risk factors in depressed cardiac patients.123 We need behavioral medicine studies that elucidate these mechanisms, guiding more precise treatments as well as novel therapies. Omega-3 fatty acids, which benefit heart disease and clinical depression,124 will be used in a randomized controlled trial by Lespérance and colleagues.125 We await the results of this exciting research.
Will treating depression help in other types of heart disease?
The SADHART-CHF trial is examining whether 12 weeks of sertraline therapy is better than placebo in preventing death and improving cardiac outcomes in patients with chronic heart failure and comorbid major depressive disorder. It was to be completed in the fall of 2008. The results and experience of this study will help in designing future interventional trials to reduce the risk of depression in cardiovascular diseases.
We also await the results of a National Heart, Lung, and Blood Institute (NHLBI) trial, “Bypassing the Blues,” which is studying the treatment of depression after cardiac bypass surgery. This study should provide further insights into management of the depressed cardiac patient. Further prognostic studies in cardiac patients are also needed using the PHQ-9 and its shorter version, PHQ-2.
Current and future guidelines
For years our European colleagues have been ahead of us in recognizing depression screening and stress management as key to cardiac disease-prevention strategies.126 The NHLBI nicely outlined recommendations on the assessment and treatment of depression in cardiovascular patients.127 The just-published AHA Science Advisory should further encourage clinicians to screen and treat depression in the patient population.1 As our knowledge grows, we look forward to future evidence-based guidelines for depressed cardiac patients.
- Lichtman JH, Bigger JT, Blumenthal JA, et al. Depression and coronary heart disease. Recommendations for screening, referral, and treatment. Circulation 2008; 118:1768–1775.
- Jiang W, Glassman A, Krishnan R, O’Connor CM, Califf RM. Depression and ischemic heart disease: what have we learned so far and what must we do in the future? Am Heart J 2005; 150:54–78.
- Freedland KE, Rich MW, Skala JA, Carney RM, Davila-Roman VG, Jaffe AS. Prevalence of depression in hospitalized patients with congestive heart failure. Psychosom Med 2003; 65:119–128.
- Murray CJ, Lopez AD, Murray CJ, Lopez AD. Global mortality, disability, and the contribution of risk factors: Global Burden of Disease Study. Lancet 1997; 349:1436–1442.
- Moussavi S, Chatterji S, Verdes E, Tandon A, Patel V, Ustun B. Depression, chronic diseases, and decrements in health: results from the World Health Surveys. Lancet 2007; 370:851–858.
- Osborn DP, Levy G, Nazareth I, Petersen I, Islam A, King MB. Relative risk of cardiovascular and cancer mortality in people with severe mental illness from the United Kingdom’s general practice research database. Arch Gen Psychiatry 2007; 64:242–249.
- Friedman M, Thoresen CE, Gill JJ, et al. Alteration of type A behavior and its effect on cardiac recurrences in post myocardial infarction patients: summary results of the recurrent coronary prevention project. Am Heart J 1986; 112:653–665.
- Ragland DR, Brand RJ. Type A behavior and mortality from coronary heart disease. N Engl J Med 1988; 318:65–69.
- Rozanski A, Blumenthal JA, Kaplan J. Impact of psychological factors on the pathogenesis of cardiovascular disease and implications for therapy. Circulation 1999; 99:2192–2217.
- Iribarren C, Sidney S, Bild DE, et al. Association of hostility with coronary artery calcification in young adults: the CARDIA study. Coronary Artery Risk Development in Young Adults. JAMA 2000; 283:2546–2551.
- Williams RB, Barefoot JC, Schneiderman N. Psychosocial risk factors for cardiovascular disease: more than one culprit at work. JAMA 2003; 290:2190–2192.
- Rozanski A, Blumenthal JA, Davidson KW, Saab PG, Kubzansky L. The epidemiology, pathophysiology, and management of psychosocial risk factors in cardiac practice: the emerging field of behavioral cardiology. J Am Coll Cardiol 2005; 45:637–651.
- Hemingway H, Marmot M. Evidence based cardiology: psychosocial factors in the aetiology and prognosis of coronary heart disease: systematic review of prospective cohort studies. BMJ 1999; 318:1460–1467.
- Denollet J. DS14: Standard assessment of negative affectivity, social inhibition, and type D personality. Psychosom Med 2005; 67:89–97.
- Steptoe A, Molloy GJ. Personality and heart disease. Heart 2007; 93:783–784.
- Yusuf S, Hawken S, Ôunpuu S, et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet 2004; 364:937–952.
- Rosengren A, Hawken S, Ounpuu S, et al. Association of psychosocial risk factors with risk of acute myocardial infarction in 11119 cases and 13648 controls from 52 countries (the INTERHEART study): case-control study. Lancet 2004; 364:953–962.
- Kuper H, Marmot M. Job strain, job demands, decision latitude, and risk of coronary heart disease within the Whitehall II study. J Epidemiol Community Health 2003; 57:147–153.
- Aboa-Eboule C, Brisson C, Maunsell E, et al. Job strain and risk of acute recurrent coronary heart disease events. JAMA 2007; 298:1652–1660.
- Frasure-Smith N, Lespérance F. Depression and anxiety as predictors of 2-year cardiac events in patients with stable coronary artery disease. Arch Gen Psychiatry 2008; 65:62–71.
- Shen B-J, Avivi YE, Todaro JF, et al. Anxiety characteristics independently and prospectively predict myocardial infarction in men. J Am Coll Cardiol 2008; 51:113–119.
- Carney RM, Freedland KE. Depression, mortality, and medical morbidity in patients with coronary heart disease. Biol Psychiatry 2003; 54:241–247.
- Pratt LA, Ford DE, Crum RM, Armenian HK, Gallo JJ, Eaton WW. Depression, psychotropic medication, and risk of myocardial infarction: prospective data from the Baltimore ECA follow-up. Circulation 1996; 94:3123–3129.
- Rugulies R. Depression as a predictor for coronary heart disease. A review and meta-analysis. Am J Prev Med 2002; 23:51–61.
- Van der Kooy K, van Hout H, Marwijk H, Marten H, Stehouwer C, Beekman A. Depression and the risk for cardiovascular diseases: systematic review and meta analysis. Int J Geriatr Psychiatry 2007; 22:613–626.
- Wulsin LR, Singal BM. Do depressive symptoms increase the risk for the onset of coronary disease? A systematic quantitative review. Psychosom Med 2003; 65:201–210.
- Wulsin LR. Is depression a major risk factor for coronary disease? A systematic review of the epidemiologic evidence. Harv Rev Psychiatry 2004; 12:79–93.
- Janszky I, Ahlbom A, Hallqvist J, Ahnve S. Hospitalization for depression is associated with an increased risk for myocardial infarction not explained by lifestyle, lipids, coagulation, and inflammation: The SHEEP Study. Biol Psychiatry 2007; 62:25–32.
- Surtees PG, Wainwright NWJ, Luben RN, Wareham NJ, Bingham SA, Khaw K-T. Depression and ischemic heart disease mortality: evidence from the EPIC-Norfolk United Kingdom Prospective Cohort Study. Am J Psychiatry 2008; 165:515–523.
- Carney RM, Rich MW, Freedland KE, et al. Major depressive disorder predicts cardiac events in patients with coronary artery disease. Psychosom Med 1988; 50:627–633.
- Frasure-Smith N, Lespérance F, Talajic M. Depression following myocardial infarction. Impact on 6-month survival. JAMA 1993; 270:1819–1825.
- Frasure-Smith N, Lespérance F, Talajic M. Depression and 18-month prognosis after myocardial infarction. Circulation 1995; 91:999–1005.
- Barefoot JC, Helms MJ, Mark DB, et al. Depression and long-term mortality risk in patients with coronary artery disease. Am J Cardiol 1996; 78:613–617.
- Lespérance F, Frasure-Smith N, Talajic M, Bourassa MG. Five-year risk of cardiac mortality in relation to initial severity and one-year changes in depression symptoms after myocardial infarction. Circulation 2002; 105:1049–1053.
- Barth J, Schumacher M, Herrmann-Lingen C. Depression as a risk factor for mortality in patients with coronary heart disease: a meta-analysis. Psychosom Med 2004; 66:802–813.
- van Melle JP, de Jonge P, Spijkerman TA, et al. Prognostic association of depression following myocardial infarction with mortality and cardiovascular events: a meta-analysis. Psychosom Med 2004; 66:814–822.
- Blumenthal JA, Lett HS, Babyak MA, et al. Depression as a risk factor for mortality after coronary artery bypass surgery. Lancet 2003; 362:604–609.
- Sullivan MD, LaCroix AZ, Spertus JA, Hecht J, Russo J. Depression predicts revascularization procedures for 5 years after coronary angiography. Psychosom Med 2003; 65:229–236.
- Jiang W, Alexander J, Christopher E, et al. Relationship of depression to increased risk of mortality and rehospitalization in patients with congestive heart failure. Arch Intern Med 2001; 161:1849–1856.
- Zipfel S, Schneider A, Wild B, et al. Effect of depressive symptoms on survival after heart transplantation. Psychosom Med 2002; 64:740–747.
- Polsky D, Doshi JA, Marcus S, et al. Long-term risk for depressive symptoms after a medical diagnosis. Arch Intern Med 2005; 165:1260–1266.
- Katon W, Lin EHB, Kroenke K. The association of depression and anxiety with medical symptom burden in patients with chronic medical illness. Gen Hosp Psychiatry 2007; 29:147–155.
- Ruo B, Rumsfeld JS, Hlatky MA, Liu H, Browner WS, Whooley MA. Depressive symptoms and health-related quality of life: the Heart and Soul Study. JAMA 2003; 290:215–221.
- Rasmussen JN, Chong A, Alter DA. Relationship between adherence to evidence-based pharmacotherapy and long-term mortality after acute myocardial infarction. JAMA 2007; 297:177–186.
- Gehi AK, Ali S, Na B, Whooley MA. Self-reported medication adherence and cardiovascular events in patients with stable coronary heart disease: the Heart and Soul Study. Arch Intern Med 2007; 167:1798–1803.
- Gehi A, Haas D, Pipkin S, Whooley MA. Depression and medication adherence in outpatients with coronary heart disease: findings from the Heart and Soul Study. Arch Intern Med 2005; 165:2508–2513.
- Rieckmann N, Gerin W, Kronish IM, et al. Course of depressive symptoms and medication adherence after acute coronary syndromes: an electronic medication monitoring study. J Am Coll Cardiol 2006; 48:2218–2222.
- Ziegelstein RC, Fauerbach JA, Stevens SS, Romanelli J, Richter DP, Bush DE. Patients with depression are less likely to follow recommendations to reduce cardiac risk during recovery from a myocardial infarction. Arch Intern Med 2000; 160:1818–1823.
- Kronish IM, Rieckmann N, Halm EA, et al. Persistent depression affects adherence to secondary prevention behaviors after acute coronary syndromes. J Gen Intern Med 2006; 21:1178–1183.
- Joynt KE, Whellan DJ, O’Connor CM. Depression and cardiovascular disease: mechanisms of interaction. Biol Psychiatry 2003; 54:248–261.
- Musselman DL, Evans DL, Nemeroff CB. The relationship of depression to cardiovascular disease: epidemiology, biology, and treatment. Arch Gen Psychiatry 1998; 55:580–592.
- Lespérance F, Frasure-Smith N. Depression and heart disease. Cleve Clin J Med 2007; 74(suppl 1):S63–S66.
- Curtis BM. Autonomic tone as a cardiovascular risk factor: the dangers of chronic fight or flight. Mayo Clin Proc 2002; 77:45–54.
- Topol EJ. Textbook of Cardiovascular Medicine, 2nd ed. Philadelphia: Lippincott Williams & Williams 2002.
- Carney RM, Saunders RD, Freedland KE, Stein P, Rich MW, Jaffe AS. Association of depression with reduced heart rate variability in coronary artery disease. Am J Cardiol 1995; 76:562–564.
- Carney RM, Blumenthal JA, Stein PK, et al. Depression, heart rate variability, and acute myocardial infarction. Circulation 2001; 104:2024–2028.
- Carney RM, Blumenthal JA, Freedland KE, et al. Low heart rate variability and the effect of depression on post-myocardial infarction mortality. Arch Intern Med 2005; 165:1486–1491.
- Gehi A, Mangano D, Pipkin S, Browner WS, Whooley MA. Depression and heart rate variability in patients with stable coronary heart disease: findings from the Heart and Soul Study. Arch Gen Psychiatry 2005; 62:661–666.
- de Jonge P, Mangano D, Whooley MA. Differential association of cognitive and somatic depressive symptoms with heart rate variability in patients with stable coronary heart disease: findings from the Heart and Soul Study. Psychosom Med 2007; 69:735–739.
- Musselman DL, Tomer A, Manatunga AK, et al. Exaggerated platelet reactivity in major depression. Am J Psychiatry 1996; 153:1313–1317.
- Laghrissi-Thode F, Wagner WR, Pollock BG, Johnson PC, Finkel MS. Elevated platelet factor 4 and beta-thromboglobulin plasma levels in depressed patients with ischemic heart disease. Biol Psychiatry 1997; 42:290–295.
- Serebruany VL, Glassman AH, Malinin AI, et al. Platelet/endothelial biomarkers in depressed patients treated with the selective serotonin reuptake inhibitor sertraline after acute coronary events: the Sertraline AntiDepressant Heart Attack Randomized Trial (SADHART) Platelet Substudy. Circulation 2003; 108:939–944.
- Sherwood A, Hinderliter AL, Watkins LL, Waugh RA, Blumenthal JA. Impaired endothelial function in coronary heart disease patients with depressive symptomatology. J Am Coll Cardiol 2005; 46:656–659.
- Yang EH, Lerman S, Lennon RJ, Simari RD, Lerman LO, Lerman A. Relation of depression to coronary endothelial function. Am J Cardiol 2007; 99:1134–1136.
- Otte C, Neylan TC, Pipkin SS, Browner WS, Whooley MA. Depressive symptoms and 24-hour urinary norepinephrine excretion levels in patients with coronary disease: findings from the Heart and Soul Study. Am J Psychiatry 2005; 162:2139–2145.
- Wittstein IS, Thiemann DR, Lima JAC, et al. Neurohumoral features of myocardial stunning due to sudden emotional stress. N Engl J Med 2005; 352:539–548.
- Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med 2005; 352:1685–1695.
- Miller GE, Stetler CA, Carney RM, Freedland KE, Banks WA. Clinical depression and inflammatory risk markers for coronary heart disease. Am J Cardiol 2002; 90:1279–1283.
- Empana JP, Sykes DH, Luc G, et al. Contributions of depressive mood and circulating inflammatory markers to coronary heart disease in healthy European men: the Prospective Epidemiological Study of Myocardial Infarction (PRIME). Circulation 2005; 111:2299–2305.
- Frasure-Smith N, Lespérance F, Irwin MR, Sauvé C, Lespérance J, Théroux P. Depression, C-reactive protein and two-year major adverse cardiac events in men after acute coronary syndromes. Biol Psychiatry 2007; 62:302–308.
- Whooley MA, Caska CM, Hendrickson BE, Rourke MA, Ho J, Ali S. Depression and inflammation in patients with coronary heart disease: findings from the Heart and Soul Study. Biol Psychiatry 2007; 62:314–320.
- Glassman AH, Miller GE. Where there is depression, there is inflammation…sometimes! Biol Psychiatry 2007; 62:280–281.
- Tracey KJ. Physiology and immunology of the cholinergic antiinflammatory pathway. J Clin Invest 2007; 117:289–296.
- Tracey KJ. The inflammatory reflex. Nature 2002; 420:853–859.
- McCaffery JM, Frasure-Smith N, Dubé M-P, et al. Common genetic vulnerability to depressive symptoms and coronary artery disease: a review and development of candidate genes related to inflammation and serotonin. Psychosom Med 2006; 68:187–200.
- Caspi A, Sugden K, Moffitt TE, et al. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science 2003; 301:386–389.
- Nakatani D, Sato H, Sakata Y, et al. Influence of serotonin transporter gene polymorphism on depressive symptoms and new cardiac events after acute myocardial infarction. Am Heart J 2005; 150:652–658.
- Otte C, McCaffery J, Ali S, Whooley MA. Association of a serotonin transporter polymorphism (5-HTTLPR) with depression, perceived stress, and norepinephrine in patients with coronary disease: the Heart and Soul Study. Am J Psychiatry 2007; 164:1379–1384.
- Fumeron F, Betoulle D, Nicaud V, et al. Serotonin transporter gene polymorphism and myocardial infarction: Etude Cas-Témoins de l’Infarctus du Myocarde (ECTIM). Circulation 2002; 105:2943–2945.
- Rivelli S, Jiang W. Depression and ischemic heart disease: what have we learned from clinical trials? Curr Opin Cardiol 2007; 22:286–291.
- Frasure-Smith N, Lespérance F, Prince RH, et al. Randomised trial of home-based psychosocial nursing intervention for patients recovering from myocardial infarction. Lancet 1997; 350:473–479.
- Glassman AH, O’Connor CM, Califf RM, et al. Sertraline treatment of major depression in patients with acute MI or unstable angina. JAMA 2002; 288:701–709.
- Writing Committee for the EI. Effects of treating depression and low perceived social support on clinical events after myocardial infarction: the Enhancing Recovery in Coronary Heart Disease Patients (ENRICHD) randomized trial. JAMA 2003; 289:3106–3116.
- van Melle JP, de Jonge P, Honig A, et al. Effects of antidepressant treatment following myocardial infarction. Br J Psychiatry 2007; 190:460–466.
- Lespérance F, Frasure-Smith N, Koszycki D, et al. Effects of citalopram and interpersonal psychotherapy on depression in patients with coronary artery disease: the Canadian Cardiac Randomized Evaluation of Antidepressant and Psychotherapy Efficacy (CREATE) trial. JAMA 2007; 297:367–379.
- Carney RM, Blumenthal JA, Freedland KE, et al. Depression and late mortality after myocardial infarction in the Enhancing Recovery in Coronary Heart Disease (ENRICHD) Study. Psychosom Med 2004; 66:466–474.
- de Jonge P, Honig A, van Melle JP, et al. Nonresponse to treatment for depression following myocardial infarction: association with subsequent cardiac events. Am J Psychiatry 2007; 164:1371–1378.
- Carney RM, Freedland KE. Depression and coronary heart disease: more pieces of the puzzle. Am J Psychiatry 2007; 164:1307–1309.
- Glassman AH, Bigger JT, Gaffney M, Shapiro PA, Swenson JR. Onset of major depression associated with acute coronary syndromes: relationship of onset, major depressive disorder history, and episode severity to sertraline benefit. Arch Gen Psychiatry 2006; 63:283–288.
- Ziegelstein RC, Kim SY, Kao D, et al. Can doctors and nurses recognize depression in patients hospitalized with an acute myocardial infarction in the absence of formal screening? Psychosom Med 2005; 67:393–397.
- Kroenke K, Spitzer RL, Williams JBW. The PHQ-9. Validity of a brief depression severity measure. J Gen Intern Med 2001; 16:606–613.
- Löwe B, Unutzer J, Callahan CM, Perkins AJ, Kroenke K. Monitoring depression treatment outcomes with the Patient Health Questionnaire-9. Med Care 2004; 42:1194–1201.
- Parashar S, Rumsfeld JS, Spertus JA, et al. Time course of depression and outcome of myocardial infarction. Arch Intern Med 2006; 166:2035–2043.
- Amin AA, Jones AMH, Nugent K, Rumsfeld JS, Spertus JA. The prevalence of unrecognized depression in patients with acute coronary syndrome. Am Heart J 2006; 152:928–934.
- McManus D, Pipkin SS, Whooley MA. Screening for depression in patients with coronary heart disease (data from the Heart and Soul Study). Am J Cardiol 2005; 96:1076–1081.
- Kroenke K, Spitzer RL, Williams JB. The Patient Health Questionnaire-2: validity of a two-item depression screener. Med Care 2003; 41:1284–1292.
- Spitzer RL, Williams JB, Kroenke K, et al. Utility of a new procedure for diagnosing mental disorders in primary care. The PRIME-MD 1000 study. JAMA 1994; 272:1749–1756.
- Lespérance F, Frasure-Smith N. Depression in patients with cardiac disease. J Psychosom Res 2000; 48:379–391.
- Hunkeler EM, Katon W, Tang L, et al. Long term outcomes from the IMPACT randomised trial for depressed elderly patients in primary care. BMJ 2006; 332:259–263.
- Frasure-Smith N, Lespérance F, Gravel G, Masson A, Juneau M, Bourassa MG. Long-term survival differences among low-anxious, high-anxious and repressive copers enrolled in the Montreal Heart Attack Readjustment Trial. Psychosom Med 2002; 64:571–579.
- Glassman AH, Roose SP, Bigger JT. The safety of tricyclic antidepressants in cardiac patients: risk-benefit reconsidered. JAMA 1993; 269:2673–2675.
- Cohen HW, Gibson G, Alderman MH. Excess risk of myocardial infarction in patients treated with antidepressant medications: association with use of tricyclic agents. Am J Med 2000; 108:2–8.
- Taylor CB, Youngblood ME, Catellier D, et al. Effects of antidepressant medication on morbidity and mortality in depressed patients after myocardial infarction. Arch Gen Psychiatry 2005; 62:792–798.
- Montgomery SA. Safety of mirtazapine: a review. Int Clin Psychopharmacol 1995; 10(suppl 4):37–45.
- Sauer WH, Berlin JA, Kimmel SE. Selective serotonin reuptake inhibitors and myocardial infarction. Circulation 2001; 104:1894–1898.
- Sauer WH, Berlin JA, Kimmel SE. Effect of antidepressants and their relative affinity for the serotonin transporter on the risk of myocardial infarction. Circulation 2003; 108:32–36.
- Ziegelstein RC, Meuchel J, Kim TJ, et al. Selective serotonin reuptake inhibitor use by patients with acute coronary syndromes. Am J Med 2007; 120:525–530.
- Xiong GL, Jiang W, Clare R, et al. Prognosis of patients taking selective serotonin reuptake inhibitors before coronary artery bypass grafting. Am J Cardiol 2006; 98:42–47.
- Yang C-C, Jick SS, Jick H. Lipid-lowering drugs and the risk of depression and suicidal behavior. Arch Intern Med 2003; 163:1926–1932.
- Callreus T, Agerskov Andersen U, Hallas J, Andersen M. Cardiovascular drugs and the risk of suicide: a nested case-control study. Eur J Clin Pharmacol 2007; 63:591–596.
- Young-Xu Y, Chan KA, Liao JK, Ravid S, Blatt CM. Long-term statin use and psychological well-being. J Am Coll Cardiol 2003; 42:690–697.
- Ko DT, Hebert PR, Coffey CS, Sedrakyan A, Curtis JP, Krumholz HM. Beta-blocker therapy and symptoms of depression, fatigue, and sexual dysfunction. JAMA 2002; 288:351–357.
- van Melle JP, Verbeek D, van den Berg MP, Ormel J, van der Line MR, de Jonge P. Beta-blockers and depression after myocardial infarction. J Am Coll Cardiol 2006; 48:2209–2214.
- Thomas RJ, King M, Lui K, et al. AACVPR/ACC/AHA 2007 performance measures on cardiac rehabilitation for referral to and delivery of cardiac rehabilitation/secondary prevention services. J Am Coll Cardiol 2007; 50:1400–1433.
- Blumenthal JA, Babyak MA, Doraiswamy PM, et al. Exercise and pharmacotherapy in the treatment of major depressive disorder. Psychosom Med 2007; 69:587–596.
- Linden W, Phillips MJ, Leclerc J. Psychological treatment of cardiac patients: a meta-analysis. Eur Heart J 2007; 28:2972–2984.
- Centers for Disease Control and Prevention. Receipt of outpatient cardiac rehabilitation among heart attack survivors—United States, 2005. JAMA 2008; 299:1534–1536.
- Williams RB. Treating depression after myocardial infarction: can selecting patients on the basis of genetic susceptibility improve psychiatric and medical outcomes? Am Heart J 2005; 150:617–619.
- Schneiderman N, Williams RB. The great debate editorial, revisited. Psychosom Med 2006; 68:636–638.
- de Jonge P, Ormel J, van den Brink RHS, et al. Symptom dimensions of depression following myocardial infarction and their relationship with somatic health status and cardiovascular prognosis. Am J Psychiatry 2006; 163:138–144.
- Fraguas R, Iosifescu DV, Alpert J, et al. Major depressive disorder and comorbid cardiac disease: is there a depressive subtype with greater cardiovascular morbidity? Results from the STAR*D Study. Psychosomatics 2007; 48:418–425.
- Kubzansky LD, Thurston RC. Emotional vitality and incident coronary heart disease: benefits of healthy psychological functioning. Arch Gen Psychiatry 2007; 64:1393–1401.
- Frasure-Smith N. Reflections on depression as a cardiac risk factor Academy of Psychosomatic Medicine, 53rd Annual Meeting, Tucson, Arizona, 2006.
- Frasure-Smith N, Lespérance F. Major depression is associated with lower omega-3 fatty acid levels in patients with recent acute coronary syndromes. Biol Psychiatry 2004; 55:891–896.
- Lespérance F. Annual Research Award Lecture Academy of Psychosomatic Medicine, Amelia Island, Florida, 2007.
- Graham I, Atar D, Borch-Johnsen K, et al. European guidelines on cardiovascular disease prevention in clinical practice: executive summary. Eur Heart J 2007; 28:2375–2414.
- Davidson KW, Kupfer DJ, Bigger JT, et al. Assessment and treatment of depression in patients with cardiovascular disease: National Heart, Lung, and Blood Institute Working Group Report. Psychosom Med 2006; 68:645–650.
- Lichtman JH, Bigger JT, Blumenthal JA, et al. Depression and coronary heart disease. Recommendations for screening, referral, and treatment. Circulation 2008; 118:1768–1775.
- Jiang W, Glassman A, Krishnan R, O’Connor CM, Califf RM. Depression and ischemic heart disease: what have we learned so far and what must we do in the future? Am Heart J 2005; 150:54–78.
- Freedland KE, Rich MW, Skala JA, Carney RM, Davila-Roman VG, Jaffe AS. Prevalence of depression in hospitalized patients with congestive heart failure. Psychosom Med 2003; 65:119–128.
- Murray CJ, Lopez AD, Murray CJ, Lopez AD. Global mortality, disability, and the contribution of risk factors: Global Burden of Disease Study. Lancet 1997; 349:1436–1442.
- Moussavi S, Chatterji S, Verdes E, Tandon A, Patel V, Ustun B. Depression, chronic diseases, and decrements in health: results from the World Health Surveys. Lancet 2007; 370:851–858.
- Osborn DP, Levy G, Nazareth I, Petersen I, Islam A, King MB. Relative risk of cardiovascular and cancer mortality in people with severe mental illness from the United Kingdom’s general practice research database. Arch Gen Psychiatry 2007; 64:242–249.
- Friedman M, Thoresen CE, Gill JJ, et al. Alteration of type A behavior and its effect on cardiac recurrences in post myocardial infarction patients: summary results of the recurrent coronary prevention project. Am Heart J 1986; 112:653–665.
- Ragland DR, Brand RJ. Type A behavior and mortality from coronary heart disease. N Engl J Med 1988; 318:65–69.
- Rozanski A, Blumenthal JA, Kaplan J. Impact of psychological factors on the pathogenesis of cardiovascular disease and implications for therapy. Circulation 1999; 99:2192–2217.
- Iribarren C, Sidney S, Bild DE, et al. Association of hostility with coronary artery calcification in young adults: the CARDIA study. Coronary Artery Risk Development in Young Adults. JAMA 2000; 283:2546–2551.
- Williams RB, Barefoot JC, Schneiderman N. Psychosocial risk factors for cardiovascular disease: more than one culprit at work. JAMA 2003; 290:2190–2192.
- Rozanski A, Blumenthal JA, Davidson KW, Saab PG, Kubzansky L. The epidemiology, pathophysiology, and management of psychosocial risk factors in cardiac practice: the emerging field of behavioral cardiology. J Am Coll Cardiol 2005; 45:637–651.
- Hemingway H, Marmot M. Evidence based cardiology: psychosocial factors in the aetiology and prognosis of coronary heart disease: systematic review of prospective cohort studies. BMJ 1999; 318:1460–1467.
- Denollet J. DS14: Standard assessment of negative affectivity, social inhibition, and type D personality. Psychosom Med 2005; 67:89–97.
- Steptoe A, Molloy GJ. Personality and heart disease. Heart 2007; 93:783–784.
- Yusuf S, Hawken S, Ôunpuu S, et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet 2004; 364:937–952.
- Rosengren A, Hawken S, Ounpuu S, et al. Association of psychosocial risk factors with risk of acute myocardial infarction in 11119 cases and 13648 controls from 52 countries (the INTERHEART study): case-control study. Lancet 2004; 364:953–962.
- Kuper H, Marmot M. Job strain, job demands, decision latitude, and risk of coronary heart disease within the Whitehall II study. J Epidemiol Community Health 2003; 57:147–153.
- Aboa-Eboule C, Brisson C, Maunsell E, et al. Job strain and risk of acute recurrent coronary heart disease events. JAMA 2007; 298:1652–1660.
- Frasure-Smith N, Lespérance F. Depression and anxiety as predictors of 2-year cardiac events in patients with stable coronary artery disease. Arch Gen Psychiatry 2008; 65:62–71.
- Shen B-J, Avivi YE, Todaro JF, et al. Anxiety characteristics independently and prospectively predict myocardial infarction in men. J Am Coll Cardiol 2008; 51:113–119.
- Carney RM, Freedland KE. Depression, mortality, and medical morbidity in patients with coronary heart disease. Biol Psychiatry 2003; 54:241–247.
- Pratt LA, Ford DE, Crum RM, Armenian HK, Gallo JJ, Eaton WW. Depression, psychotropic medication, and risk of myocardial infarction: prospective data from the Baltimore ECA follow-up. Circulation 1996; 94:3123–3129.
- Rugulies R. Depression as a predictor for coronary heart disease. A review and meta-analysis. Am J Prev Med 2002; 23:51–61.
- Van der Kooy K, van Hout H, Marwijk H, Marten H, Stehouwer C, Beekman A. Depression and the risk for cardiovascular diseases: systematic review and meta analysis. Int J Geriatr Psychiatry 2007; 22:613–626.
- Wulsin LR, Singal BM. Do depressive symptoms increase the risk for the onset of coronary disease? A systematic quantitative review. Psychosom Med 2003; 65:201–210.
- Wulsin LR. Is depression a major risk factor for coronary disease? A systematic review of the epidemiologic evidence. Harv Rev Psychiatry 2004; 12:79–93.
- Janszky I, Ahlbom A, Hallqvist J, Ahnve S. Hospitalization for depression is associated with an increased risk for myocardial infarction not explained by lifestyle, lipids, coagulation, and inflammation: The SHEEP Study. Biol Psychiatry 2007; 62:25–32.
- Surtees PG, Wainwright NWJ, Luben RN, Wareham NJ, Bingham SA, Khaw K-T. Depression and ischemic heart disease mortality: evidence from the EPIC-Norfolk United Kingdom Prospective Cohort Study. Am J Psychiatry 2008; 165:515–523.
- Carney RM, Rich MW, Freedland KE, et al. Major depressive disorder predicts cardiac events in patients with coronary artery disease. Psychosom Med 1988; 50:627–633.
- Frasure-Smith N, Lespérance F, Talajic M. Depression following myocardial infarction. Impact on 6-month survival. JAMA 1993; 270:1819–1825.
- Frasure-Smith N, Lespérance F, Talajic M. Depression and 18-month prognosis after myocardial infarction. Circulation 1995; 91:999–1005.
- Barefoot JC, Helms MJ, Mark DB, et al. Depression and long-term mortality risk in patients with coronary artery disease. Am J Cardiol 1996; 78:613–617.
- Lespérance F, Frasure-Smith N, Talajic M, Bourassa MG. Five-year risk of cardiac mortality in relation to initial severity and one-year changes in depression symptoms after myocardial infarction. Circulation 2002; 105:1049–1053.
- Barth J, Schumacher M, Herrmann-Lingen C. Depression as a risk factor for mortality in patients with coronary heart disease: a meta-analysis. Psychosom Med 2004; 66:802–813.
- van Melle JP, de Jonge P, Spijkerman TA, et al. Prognostic association of depression following myocardial infarction with mortality and cardiovascular events: a meta-analysis. Psychosom Med 2004; 66:814–822.
- Blumenthal JA, Lett HS, Babyak MA, et al. Depression as a risk factor for mortality after coronary artery bypass surgery. Lancet 2003; 362:604–609.
- Sullivan MD, LaCroix AZ, Spertus JA, Hecht J, Russo J. Depression predicts revascularization procedures for 5 years after coronary angiography. Psychosom Med 2003; 65:229–236.
- Jiang W, Alexander J, Christopher E, et al. Relationship of depression to increased risk of mortality and rehospitalization in patients with congestive heart failure. Arch Intern Med 2001; 161:1849–1856.
- Zipfel S, Schneider A, Wild B, et al. Effect of depressive symptoms on survival after heart transplantation. Psychosom Med 2002; 64:740–747.
- Polsky D, Doshi JA, Marcus S, et al. Long-term risk for depressive symptoms after a medical diagnosis. Arch Intern Med 2005; 165:1260–1266.
- Katon W, Lin EHB, Kroenke K. The association of depression and anxiety with medical symptom burden in patients with chronic medical illness. Gen Hosp Psychiatry 2007; 29:147–155.
- Ruo B, Rumsfeld JS, Hlatky MA, Liu H, Browner WS, Whooley MA. Depressive symptoms and health-related quality of life: the Heart and Soul Study. JAMA 2003; 290:215–221.
- Rasmussen JN, Chong A, Alter DA. Relationship between adherence to evidence-based pharmacotherapy and long-term mortality after acute myocardial infarction. JAMA 2007; 297:177–186.
- Gehi AK, Ali S, Na B, Whooley MA. Self-reported medication adherence and cardiovascular events in patients with stable coronary heart disease: the Heart and Soul Study. Arch Intern Med 2007; 167:1798–1803.
- Gehi A, Haas D, Pipkin S, Whooley MA. Depression and medication adherence in outpatients with coronary heart disease: findings from the Heart and Soul Study. Arch Intern Med 2005; 165:2508–2513.
- Rieckmann N, Gerin W, Kronish IM, et al. Course of depressive symptoms and medication adherence after acute coronary syndromes: an electronic medication monitoring study. J Am Coll Cardiol 2006; 48:2218–2222.
- Ziegelstein RC, Fauerbach JA, Stevens SS, Romanelli J, Richter DP, Bush DE. Patients with depression are less likely to follow recommendations to reduce cardiac risk during recovery from a myocardial infarction. Arch Intern Med 2000; 160:1818–1823.
- Kronish IM, Rieckmann N, Halm EA, et al. Persistent depression affects adherence to secondary prevention behaviors after acute coronary syndromes. J Gen Intern Med 2006; 21:1178–1183.
- Joynt KE, Whellan DJ, O’Connor CM. Depression and cardiovascular disease: mechanisms of interaction. Biol Psychiatry 2003; 54:248–261.
- Musselman DL, Evans DL, Nemeroff CB. The relationship of depression to cardiovascular disease: epidemiology, biology, and treatment. Arch Gen Psychiatry 1998; 55:580–592.
- Lespérance F, Frasure-Smith N. Depression and heart disease. Cleve Clin J Med 2007; 74(suppl 1):S63–S66.
- Curtis BM. Autonomic tone as a cardiovascular risk factor: the dangers of chronic fight or flight. Mayo Clin Proc 2002; 77:45–54.
- Topol EJ. Textbook of Cardiovascular Medicine, 2nd ed. Philadelphia: Lippincott Williams & Williams 2002.
- Carney RM, Saunders RD, Freedland KE, Stein P, Rich MW, Jaffe AS. Association of depression with reduced heart rate variability in coronary artery disease. Am J Cardiol 1995; 76:562–564.
- Carney RM, Blumenthal JA, Stein PK, et al. Depression, heart rate variability, and acute myocardial infarction. Circulation 2001; 104:2024–2028.
- Carney RM, Blumenthal JA, Freedland KE, et al. Low heart rate variability and the effect of depression on post-myocardial infarction mortality. Arch Intern Med 2005; 165:1486–1491.
- Gehi A, Mangano D, Pipkin S, Browner WS, Whooley MA. Depression and heart rate variability in patients with stable coronary heart disease: findings from the Heart and Soul Study. Arch Gen Psychiatry 2005; 62:661–666.
- de Jonge P, Mangano D, Whooley MA. Differential association of cognitive and somatic depressive symptoms with heart rate variability in patients with stable coronary heart disease: findings from the Heart and Soul Study. Psychosom Med 2007; 69:735–739.
- Musselman DL, Tomer A, Manatunga AK, et al. Exaggerated platelet reactivity in major depression. Am J Psychiatry 1996; 153:1313–1317.
- Laghrissi-Thode F, Wagner WR, Pollock BG, Johnson PC, Finkel MS. Elevated platelet factor 4 and beta-thromboglobulin plasma levels in depressed patients with ischemic heart disease. Biol Psychiatry 1997; 42:290–295.
- Serebruany VL, Glassman AH, Malinin AI, et al. Platelet/endothelial biomarkers in depressed patients treated with the selective serotonin reuptake inhibitor sertraline after acute coronary events: the Sertraline AntiDepressant Heart Attack Randomized Trial (SADHART) Platelet Substudy. Circulation 2003; 108:939–944.
- Sherwood A, Hinderliter AL, Watkins LL, Waugh RA, Blumenthal JA. Impaired endothelial function in coronary heart disease patients with depressive symptomatology. J Am Coll Cardiol 2005; 46:656–659.
- Yang EH, Lerman S, Lennon RJ, Simari RD, Lerman LO, Lerman A. Relation of depression to coronary endothelial function. Am J Cardiol 2007; 99:1134–1136.
- Otte C, Neylan TC, Pipkin SS, Browner WS, Whooley MA. Depressive symptoms and 24-hour urinary norepinephrine excretion levels in patients with coronary disease: findings from the Heart and Soul Study. Am J Psychiatry 2005; 162:2139–2145.
- Wittstein IS, Thiemann DR, Lima JAC, et al. Neurohumoral features of myocardial stunning due to sudden emotional stress. N Engl J Med 2005; 352:539–548.
- Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med 2005; 352:1685–1695.
- Miller GE, Stetler CA, Carney RM, Freedland KE, Banks WA. Clinical depression and inflammatory risk markers for coronary heart disease. Am J Cardiol 2002; 90:1279–1283.
- Empana JP, Sykes DH, Luc G, et al. Contributions of depressive mood and circulating inflammatory markers to coronary heart disease in healthy European men: the Prospective Epidemiological Study of Myocardial Infarction (PRIME). Circulation 2005; 111:2299–2305.
- Frasure-Smith N, Lespérance F, Irwin MR, Sauvé C, Lespérance J, Théroux P. Depression, C-reactive protein and two-year major adverse cardiac events in men after acute coronary syndromes. Biol Psychiatry 2007; 62:302–308.
- Whooley MA, Caska CM, Hendrickson BE, Rourke MA, Ho J, Ali S. Depression and inflammation in patients with coronary heart disease: findings from the Heart and Soul Study. Biol Psychiatry 2007; 62:314–320.
- Glassman AH, Miller GE. Where there is depression, there is inflammation…sometimes! Biol Psychiatry 2007; 62:280–281.
- Tracey KJ. Physiology and immunology of the cholinergic antiinflammatory pathway. J Clin Invest 2007; 117:289–296.
- Tracey KJ. The inflammatory reflex. Nature 2002; 420:853–859.
- McCaffery JM, Frasure-Smith N, Dubé M-P, et al. Common genetic vulnerability to depressive symptoms and coronary artery disease: a review and development of candidate genes related to inflammation and serotonin. Psychosom Med 2006; 68:187–200.
- Caspi A, Sugden K, Moffitt TE, et al. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science 2003; 301:386–389.
- Nakatani D, Sato H, Sakata Y, et al. Influence of serotonin transporter gene polymorphism on depressive symptoms and new cardiac events after acute myocardial infarction. Am Heart J 2005; 150:652–658.
- Otte C, McCaffery J, Ali S, Whooley MA. Association of a serotonin transporter polymorphism (5-HTTLPR) with depression, perceived stress, and norepinephrine in patients with coronary disease: the Heart and Soul Study. Am J Psychiatry 2007; 164:1379–1384.
- Fumeron F, Betoulle D, Nicaud V, et al. Serotonin transporter gene polymorphism and myocardial infarction: Etude Cas-Témoins de l’Infarctus du Myocarde (ECTIM). Circulation 2002; 105:2943–2945.
- Rivelli S, Jiang W. Depression and ischemic heart disease: what have we learned from clinical trials? Curr Opin Cardiol 2007; 22:286–291.
- Frasure-Smith N, Lespérance F, Prince RH, et al. Randomised trial of home-based psychosocial nursing intervention for patients recovering from myocardial infarction. Lancet 1997; 350:473–479.
- Glassman AH, O’Connor CM, Califf RM, et al. Sertraline treatment of major depression in patients with acute MI or unstable angina. JAMA 2002; 288:701–709.
- Writing Committee for the EI. Effects of treating depression and low perceived social support on clinical events after myocardial infarction: the Enhancing Recovery in Coronary Heart Disease Patients (ENRICHD) randomized trial. JAMA 2003; 289:3106–3116.
- van Melle JP, de Jonge P, Honig A, et al. Effects of antidepressant treatment following myocardial infarction. Br J Psychiatry 2007; 190:460–466.
- Lespérance F, Frasure-Smith N, Koszycki D, et al. Effects of citalopram and interpersonal psychotherapy on depression in patients with coronary artery disease: the Canadian Cardiac Randomized Evaluation of Antidepressant and Psychotherapy Efficacy (CREATE) trial. JAMA 2007; 297:367–379.
- Carney RM, Blumenthal JA, Freedland KE, et al. Depression and late mortality after myocardial infarction in the Enhancing Recovery in Coronary Heart Disease (ENRICHD) Study. Psychosom Med 2004; 66:466–474.
- de Jonge P, Honig A, van Melle JP, et al. Nonresponse to treatment for depression following myocardial infarction: association with subsequent cardiac events. Am J Psychiatry 2007; 164:1371–1378.
- Carney RM, Freedland KE. Depression and coronary heart disease: more pieces of the puzzle. Am J Psychiatry 2007; 164:1307–1309.
- Glassman AH, Bigger JT, Gaffney M, Shapiro PA, Swenson JR. Onset of major depression associated with acute coronary syndromes: relationship of onset, major depressive disorder history, and episode severity to sertraline benefit. Arch Gen Psychiatry 2006; 63:283–288.
- Ziegelstein RC, Kim SY, Kao D, et al. Can doctors and nurses recognize depression in patients hospitalized with an acute myocardial infarction in the absence of formal screening? Psychosom Med 2005; 67:393–397.
- Kroenke K, Spitzer RL, Williams JBW. The PHQ-9. Validity of a brief depression severity measure. J Gen Intern Med 2001; 16:606–613.
- Löwe B, Unutzer J, Callahan CM, Perkins AJ, Kroenke K. Monitoring depression treatment outcomes with the Patient Health Questionnaire-9. Med Care 2004; 42:1194–1201.
- Parashar S, Rumsfeld JS, Spertus JA, et al. Time course of depression and outcome of myocardial infarction. Arch Intern Med 2006; 166:2035–2043.
- Amin AA, Jones AMH, Nugent K, Rumsfeld JS, Spertus JA. The prevalence of unrecognized depression in patients with acute coronary syndrome. Am Heart J 2006; 152:928–934.
- McManus D, Pipkin SS, Whooley MA. Screening for depression in patients with coronary heart disease (data from the Heart and Soul Study). Am J Cardiol 2005; 96:1076–1081.
- Kroenke K, Spitzer RL, Williams JB. The Patient Health Questionnaire-2: validity of a two-item depression screener. Med Care 2003; 41:1284–1292.
- Spitzer RL, Williams JB, Kroenke K, et al. Utility of a new procedure for diagnosing mental disorders in primary care. The PRIME-MD 1000 study. JAMA 1994; 272:1749–1756.
- Lespérance F, Frasure-Smith N. Depression in patients with cardiac disease. J Psychosom Res 2000; 48:379–391.
- Hunkeler EM, Katon W, Tang L, et al. Long term outcomes from the IMPACT randomised trial for depressed elderly patients in primary care. BMJ 2006; 332:259–263.
- Frasure-Smith N, Lespérance F, Gravel G, Masson A, Juneau M, Bourassa MG. Long-term survival differences among low-anxious, high-anxious and repressive copers enrolled in the Montreal Heart Attack Readjustment Trial. Psychosom Med 2002; 64:571–579.
- Glassman AH, Roose SP, Bigger JT. The safety of tricyclic antidepressants in cardiac patients: risk-benefit reconsidered. JAMA 1993; 269:2673–2675.
- Cohen HW, Gibson G, Alderman MH. Excess risk of myocardial infarction in patients treated with antidepressant medications: association with use of tricyclic agents. Am J Med 2000; 108:2–8.
- Taylor CB, Youngblood ME, Catellier D, et al. Effects of antidepressant medication on morbidity and mortality in depressed patients after myocardial infarction. Arch Gen Psychiatry 2005; 62:792–798.
- Montgomery SA. Safety of mirtazapine: a review. Int Clin Psychopharmacol 1995; 10(suppl 4):37–45.
- Sauer WH, Berlin JA, Kimmel SE. Selective serotonin reuptake inhibitors and myocardial infarction. Circulation 2001; 104:1894–1898.
- Sauer WH, Berlin JA, Kimmel SE. Effect of antidepressants and their relative affinity for the serotonin transporter on the risk of myocardial infarction. Circulation 2003; 108:32–36.
- Ziegelstein RC, Meuchel J, Kim TJ, et al. Selective serotonin reuptake inhibitor use by patients with acute coronary syndromes. Am J Med 2007; 120:525–530.
- Xiong GL, Jiang W, Clare R, et al. Prognosis of patients taking selective serotonin reuptake inhibitors before coronary artery bypass grafting. Am J Cardiol 2006; 98:42–47.
- Yang C-C, Jick SS, Jick H. Lipid-lowering drugs and the risk of depression and suicidal behavior. Arch Intern Med 2003; 163:1926–1932.
- Callreus T, Agerskov Andersen U, Hallas J, Andersen M. Cardiovascular drugs and the risk of suicide: a nested case-control study. Eur J Clin Pharmacol 2007; 63:591–596.
- Young-Xu Y, Chan KA, Liao JK, Ravid S, Blatt CM. Long-term statin use and psychological well-being. J Am Coll Cardiol 2003; 42:690–697.
- Ko DT, Hebert PR, Coffey CS, Sedrakyan A, Curtis JP, Krumholz HM. Beta-blocker therapy and symptoms of depression, fatigue, and sexual dysfunction. JAMA 2002; 288:351–357.
- van Melle JP, Verbeek D, van den Berg MP, Ormel J, van der Line MR, de Jonge P. Beta-blockers and depression after myocardial infarction. J Am Coll Cardiol 2006; 48:2209–2214.
- Thomas RJ, King M, Lui K, et al. AACVPR/ACC/AHA 2007 performance measures on cardiac rehabilitation for referral to and delivery of cardiac rehabilitation/secondary prevention services. J Am Coll Cardiol 2007; 50:1400–1433.
- Blumenthal JA, Babyak MA, Doraiswamy PM, et al. Exercise and pharmacotherapy in the treatment of major depressive disorder. Psychosom Med 2007; 69:587–596.
- Linden W, Phillips MJ, Leclerc J. Psychological treatment of cardiac patients: a meta-analysis. Eur Heart J 2007; 28:2972–2984.
- Centers for Disease Control and Prevention. Receipt of outpatient cardiac rehabilitation among heart attack survivors—United States, 2005. JAMA 2008; 299:1534–1536.
- Williams RB. Treating depression after myocardial infarction: can selecting patients on the basis of genetic susceptibility improve psychiatric and medical outcomes? Am Heart J 2005; 150:617–619.
- Schneiderman N, Williams RB. The great debate editorial, revisited. Psychosom Med 2006; 68:636–638.
- de Jonge P, Ormel J, van den Brink RHS, et al. Symptom dimensions of depression following myocardial infarction and their relationship with somatic health status and cardiovascular prognosis. Am J Psychiatry 2006; 163:138–144.
- Fraguas R, Iosifescu DV, Alpert J, et al. Major depressive disorder and comorbid cardiac disease: is there a depressive subtype with greater cardiovascular morbidity? Results from the STAR*D Study. Psychosomatics 2007; 48:418–425.
- Kubzansky LD, Thurston RC. Emotional vitality and incident coronary heart disease: benefits of healthy psychological functioning. Arch Gen Psychiatry 2007; 64:1393–1401.
- Frasure-Smith N. Reflections on depression as a cardiac risk factor Academy of Psychosomatic Medicine, 53rd Annual Meeting, Tucson, Arizona, 2006.
- Frasure-Smith N, Lespérance F. Major depression is associated with lower omega-3 fatty acid levels in patients with recent acute coronary syndromes. Biol Psychiatry 2004; 55:891–896.
- Lespérance F. Annual Research Award Lecture Academy of Psychosomatic Medicine, Amelia Island, Florida, 2007.
- Graham I, Atar D, Borch-Johnsen K, et al. European guidelines on cardiovascular disease prevention in clinical practice: executive summary. Eur Heart J 2007; 28:2375–2414.
- Davidson KW, Kupfer DJ, Bigger JT, et al. Assessment and treatment of depression in patients with cardiovascular disease: National Heart, Lung, and Blood Institute Working Group Report. Psychosom Med 2006; 68:645–650.
KEY POINTS
- Depression is a risk factor for new cardiac disease and has a detrimental impact in established cardiac disease.
- Numerous mechanistic pathways have been implicated.
- In clinical trials, drug therapy and psychotherapy have not clearly decreased the rate of cardiac death in depressed cardiac patients, but they did improve depression, adherence to drug therapy, and quality of life.
- Clinicians should routinely screen for depression in cardiac patients and should not hesitate to treat it.
- Eligible patients should routinely be referred to cardiac rehabilitation programs.
Case study in heart-brain interplay: A 53-year-old woman recovering from mitral valve repair
CARDIAC CASE PRESENTATION
A 53-year-old woman, a malpractice lawyer, with a history of mitral valve prolapse was diagnosed with severe mitral regurgitation and referred for mitral valve repair.
History and examination
The patient had no other cardiac history. She reported jogging 2 to 3 miles daily and playing tennis regularly, but over the past few months she had become more fatigued during her jogs, to the point that she occasionally had to reduce her pace and even shorten the duration of her runs.
On her initial visit, she expressed surprise regarding the severity of her mitral valve disease, as she had always been healthy. She seemed somewhat nervous but appropriately concerned about the impending surgery, and questioned whether she would be able to return to her previous level of activity. She also mentioned that she hoped the timing of the surgery would permit her to attend her son’s college graduation in 9 weeks.
Her medical history was notable for mitral valve prolapse. She had a history of panic attacks, for which she occasionally took alprazolam. There was no family history of cardiac disease. She did not use tobacco and occasionally consumed alcohol. A review of systems was negative.
Her physical examination was unremarkable except for a grade 4/6 holosystolic murmur at the apex that radiated to the axilla, which was consistent with the mitral regurgitation.
A transthoracic echocardiogram demonstrated mitral regurgitation that extended through the left atrium back into the pulmonary veins. The left ventricular ejection fraction was 50%, which is considered low-normal. The degree of mitral regurgitation was 4+. No other significant valvular disease was observed.
An electrocardiogram revealed a normal sinus rhythm. Per our routine, the patient underwent cardiac catheterization, which showed normal coronary arteries.
An uncomplicated repair, but slow recovery
The mitral valve repair was performed without complications. The course in the intensive care unit was uncomplicated, and the patient was quickly extubated and transferred to a regular nursing floor.
On the nursing floor, controlling the patient’s pain was difficult. She refused to use her incentive spirometer and initially refused to ambulate or even move from her bed to a chair. She was quite tearful.
A postoperative transthoracic echocardiogram revealed a satisfactorily repaired mitral valve with no mitral regurgitation. Her ejection fraction decreased to 40%, which is not unusual after mitral valve surgery.
Her hospital course was notable for an episode of shortness of breath and tachycardia. Sinus tachycardia was evident on review of the telemetry strips. A repeat echocardiogram showed no changes compared with the prior postoperative echocardiogram. Spiral computed tomography was negative for pulmonary embolism.
Pain control remained difficult. The patient expressed concern about the postoperative decrease in her ejection fraction; she was reassured that a decrease in ejection fraction was not unusual, but she remained tearful. The family expressed concern because the patient “wasn’t acting like herself,” and her ambulation and use of her incentive spirometer continued to be minimal, which had the potential to hamper her recovery and rehabilitation. For these reasons, a psychiatric consult was requested and the patient was seen prior to discharge from the hospital on postoperative day 6.
Wound check at 1 week postdischarge
A routine wound check was performed 1 week after discharge, at which time the patient was still reporting pain that was more severe than would be expected at her postoperative stage. She reported concern about drainage from the incision. She said that she was unable to do much walking or stair climbing, and she reported sleeping in the guest bedroom on the first floor of her house because she was unable to negotiate the stairs to her bedroom.
A check of the wound showed minimal serous drainage at the inferior aspect and was consistent with normal wound healing. The slow progress of her recovery was a concern, as was the possible contribution of her anxiety to this slow progress, so we kept our psychiatric colleagues informed about the patient’s recovery.
Follow-up at postoperative week 4
At the follow-up visit at postoperative week 4, the patient reported still being in pain, although the pain had improved, and complained of constant fatigue and shortness of breath that prevented her from returning to work. She had been discharged on lisinopril and admitted to occasional medication noncompliance. She said that if she did not improve dramatically and quickly, she would not be able to attend her son’s graduation.
We considered the possibility of new ischemia, a large pleural effusion, postpericardiotomy syndrome, constrictive pericarditis, or a mitral valve leak as potential causes of her symptoms. A chest radiograph was obtained, which demonstrated a small left pleural effusion, and an echocardiogram showed that her ejection fraction remained at 40% and the mitral valve repair remained intact. The patient had a psychiatric visit scheduled later on the day of this follow-up visit and was referred to the cardiac rehabilitation program, to start on week 6 of her postoperative care.
PSYCHIATRIC CASE PRESENTATION
At the time of the first psychiatric consult, postoperative day 6, the patient’s chart was reviewed, detailing her presentation and hospital course as described above. The chart confirmed one episode of “panic” following surgery while the patient was on telemetry, showing only sinus tachycardia. This episode was successfully treated with 1 mg of lorazepam. She expressed a fear of “losing it,” which is how she characterized her panicky state during the hospital stay, punctuated by the feeling that she was not in control. The nursing staff reported that she was distressed and irritable. Her husband also confirmed that the patient “was not herself.”
Her baseline functioning was high; she is a partner in a law firm and is customarily “in control.” Before the interview began, the patient had several questions ready, including how quickly she would heal, how soon she could return to work and resume her normal activities, the reason for her low ejection fraction despite having mitral valve surgery, and whether or not she would be able to attend her son’s graduation. Even though she knew the psychiatry consult had been ordered, she was not very receptive to it at first and was more focused on her physical symptoms.
Psychiatric history
Her psychiatric history was significant for fear of heights and panic attacks, but she had been able to conquer each. She overcame performance anxiety in high school and was able to be a successful malpractice attorney, deliberating cases in court. She had never seen a psychiatrist or mental health professional, and had never been on psychotropic medications, although for the past couple of years she had been using 0.5 mg of alprazolam to treat flight anxiety. She admitted to postpartum depression that lasted about 2 months; no treatment was sought at the time, and the depression resolved.
Family and personal history
Her mother was a teacher and a “professional worrier,” and her father is a retired lawyer. She reported resolving to “suck it up” during times of adversity during childhood, but her childhood was otherwise unremarkable. She is an only child and finished at the top of her class at law school.
Review of symptoms
An assessment of depressive symptoms using the mnemonic SIGECAPS (disturbance of sleep; disturbance of interest; presence of guilt; disturbance of energy, concentration, or appetite; increased or decreased psychomotor activity; ideas of suicide) elicited low energy levels, decreased concentration, and a “slowed down” feeling. The WART (withdrawal, anhedonia, rumination, tearfulness) scale, used to assess depressive symptoms in the medically ill, showed the patient to be withdrawn and tearful at times.
Mental status examination
The patient was polite, professionally courteous, and sitting up in bed. Her vital signs were stable (heart rate, 70 beats per minute; blood pressure, 122/72 mm Hg) and her mood was “fine,” although she had many concerns about her physical health. Her affect was serious, constricted, and controlling. Her thought process was linear and organized, and her thought content revealed no psychosis, suicidal ideations, or overt hopelessness. She admitted that she was slightly anxious and overwhelmed, and that this anxiety precipitated her “panic” on telemetry and tearfulness, but she believed (and asked for assurance) that this level of anxiety was normal following surgery.
Diagnosis and recommendations
By the end of the consultation, we were able to make a series of recommendations. We arrived at a diagnosis of adjustment disorder with anxious features, and we agreed to treat her with alprazolam at a dose of 0.5 mg twice daily as needed. We provided education about mood and anxiety disorders in cardiac patients. We explained that her postpartum depression was a risk factor for future depression. We discussed coping strategies and relaxation techniques, and scheduled a follow-up appointment with her primary care physician for further monitoring of her mood and anxiety.
One week postdischarge
The cardiology team communicated with us after her wound check at postdischarge week 1. At this time, she was still having pain and was concerned about excessive wound drainage even though it was found to be minimal. The cardiology team was concerned because her progress was slow and she appeared anxious and tense. A follow-up psychiatry consultation was arranged for the patient’s next postoperative visit.
Follow-up at postoperative week 4
At her scheduled psychiatric visit at postoperative week 4, the patient was a little surprised to see the fellow, as she expected to see the staff psychiatrist. She appeared tense and frustrated, was fixated on her echocardiogram and her physical symptoms, and reported that she was not yet back to work. She was preoccupied with her son’s graduation that was coming up and wondered if she would be able to attend and celebrate it.
We administered the Patient Health Questionnaire depression scale, and the patient’s score of 11 indicated moderate depression. Treatment options, including psychotherapy and pharmacotherapy with a selective serotonin reuptake inhibitor (SSRI), were reviewed with the patient. A call to the cardiology team revealed that her ejection fraction was fairly typical for a patient who has had a mitral valve repair but that the continued fatigue was not normal, leading us to suspect that depression may be the actual cause of her fatigue. She remarked, “Let’s see how the cardiac rehabilitation program goes and then we’ll talk about medications for depression.”
Cardiac rehabilitation at postoperative week 6
The patient was entered into the cardiac rehabilitation program, and she was administered a Short Form–36 (SF-36) health survey, which showed a low mental summary score and a low physical component summary score (low scores connote worse health and/or more disability). She was referred to the psychiatrist at the cardiovascular behavioral health clinic for further assessment of her mood as she commenced the cardiac rehabilitation program.
DISCUSSION OF THE CASE
To explore management options in this case and discuss the insights it provides into heart-brain interactions, the case presentation was followed by an interactive discussion (moderated by Dr. James B. Young, Department of Cardiovascular Medicine and Chairman, Division of Medicine at Cleveland Clinic) between the physicians who presented the case and the Heart-Brain Summit audience.
Dr. James Young: Let’s begin by considering whether there were some red flags that may have been apparent up front to predict that this patient might have been challenging in the postoperative period. I think one red flag was the diagnosis of mitral valve prolapse itself, which has been known to occur in type A personalities, who tend to exhibit catecholamine excess and sympathetic nervous system arousal that activates the autonomic nervous system.
Also, I’d be interested to know a few more findings from the patient’s physical examination. Was she thin? Did she have a narrow anteroposterior diameter? Did she have pectus excavatum? Did she have arachnodactyly tendencies? These are important characteristics that might have flagged the anxiety up front, as psychosomatic manifestations of patients with mitral valve prolapse were identified—and hotly debated—20 to 30 years ago. Although the link between mitral valve prolapse and personality type has fallen out of favor in cardiology circles, it clearly seems to describe this patient. The history of anxiety, panic, and possibly agoraphobia has been well described in patients with mitral valve prolapse and excematous degeneration.
I’d like to pose the following questions to the audience. What do you do with this patient now? Do you push medication therapy? Do you push psychotherapy? What is the next step?
Comment from audience: You haven’t excluded the post-pump syndrome. This patient is very bright and it wouldn’t take much of an insult to impair her sufficiently so that she would interpret the world in a different way. From my point of view, she needs sophisticated neuropsychological testing soon.
Dr. Young: That’s a good point. We know that cardiopulmonary bypass is associated with difficulties and problems that have been underreported in the past.
Comment from audience: The last thing that this patient wants to admit or even allude to is a psychological problem. She is the last one who’s going to even hint at it, which makes it very easy to miss. Look at how she reacted when she heard that there was a psychiatrist in the room. These patients are not necessarily well disposed to completing screening tests because they recognize that somebody is trying to identify a psychological problem. I don’t know that I have the answer, but I think that we should avoid browbeating ourselves for the problem.
Dr. Young: I want to mention the cultural anthropology of physicians and how it affects our approach to treatment. I like being a cardiologist because I write prescriptions for drugs that have proven to be useful, such as beta-blockers and ACE inhibitors, among others. From this experience came my earlier question, “Should we give this patient a drug?” The cardiologist’s focus—perhaps excessive focus—on pharmacologic solutions may not be the best way to approach this patient. You allude to some important issues about screening a patient for diseases that can be more easily treated.
Comment from audience: I have seen such situations as a result of drug interactions; many of our patients are on multiple drugs when they leave the hospital. The other issue to consider is sleep deprivation, with or without sleep apnea.
Dr. Young: Many complications, particularly in patients with heart failure, are related to disordered sleep, which certainly causes some heart-brain dysfunction. What about the drugs?
Dr. Thomas Callahan: We considered the effects of her medications, which included an ACE inhibitor and her analgesics. We also considered the lingering effects of anesthesia or other medications that she might have been receiving.
Dr. Young: Remember, she was reporting considerable pain. I suspect that she was on a cocktail of pain medications that might have been contributing to her difficulties.
Comment from audience: Morphine’s effects tend to be stronger in women than in men. The other issue is the 10% drop in ejection fraction after the surgery. This patient may be thinking, “Why did I go through all of this if my ejection fraction is going to be worse?”
Dr. Callahan: A drop in the ejection fraction, especially after mitral valve repair, is common. We often address it with patients preoperatively, but perhaps not with everyone, and perhaps not clearly enough.
Dr. Young: Also, this is an example of a patient who had heart failure going into the operation, but “heart failure” would be the worst term to use with this particular patient. An ejection fraction of 50% is not normal for a patient with 4+ mitral regurgitation and, as Dr. Callahan suggested, when you take away the mitral regurgitation, you dump a little more load on the left ventricle, and the ejection fraction will go down. We see this all the time, although I admit that cardiologists or cardiac surgeons don’t necessarily do the best job of discussing these subtleties with patients. Something we can take away from this case is a sense of the importance of improving our communications with patients about what they might expect postoperatively, although it still needs to be tailored to the individual patient. If this patient had understood the pathophysiology behind the drop in ejection fraction, it may have helped her. Other patients, on the other hand, may not require detailed conversations about this phenomenon.
Comment from audience: It was mentioned several times that the husband said the patient was not herself. Did you interact with the husband and the son to get a sense of the long-term dynamics of this family? It seems that there may have been some issues with the family dynamics.
Dr. Ubaid Khokhar: That’s a good question, although no underlying dynamics seemed apparent. The husband and son’s primary concern was that the patient’s previous characteristics of perfectionism and always being “in control” were so much in contrast with the tearful episodes she was having now. “She is not the same,” is how they kept phrasing it. However, there were no other significant changes—no rumination about suicide, no overt unwillingness to go along with treatment, or anything like that.
Comment from audience: I believe strongly that this patient was depressed, although she did not admit it. She had four of the five symptoms. She did not admit to a depressed mood but was tearful, which you reported at every postoperative visit. This is a sign of depression. We know very well that anxiety and depression often are present in tandem, especially in patients with high baseline anxiety. When they have more stress in their lives, they tend to get depressed.
I agree with the preceding comments that drug interactions are a potential worry; however, a few of the SSRIs have favorable drug-drug interaction profiles. I would urge this patient to try SSRI therapy. If she rejected this by responding, “I’m not depressed,” you could point out that SSRIs work very well for anxiety. Alprazolam is not a good medication for anxiety because it has a very short half-life, which can leave patients with an increase in anxious feelings after the medication is cleared from their system but before their next dose.
In addition to SSRI therapy as a first-line approach, I would try stress management, biofeedback, or even psychosupportive therapy that relies on patient education to help this patient understand her condition and take back control.
CASE OUTCOME
Our initial approach with this patient was the path of least resistance. Very good points have been made by the discussants and members of the audience. This patient was attached to alprazolam because it was the only psychotropic medication that she had ever taken. For this reason, she was discharged on alprazolam even though it wasn’t the ideal medication. As pointed out by the audience, the patient was quite resistant to the concept of having depression superimposed on a history of anxiety. In the cardiac rehabilitation setting she was again reassured by the exercise physiologists that her heart was doing well. A cardiologist personally reviewed the echocardiographic reports and films with the patient, pointed out the absence of unusual abnormalities with her heart, and suggested that something else was causing her symptoms. This direct explanation and reassurance from the cardiologist facilitated the patient’s ability to entertain depression as a comorbid condition.
At the visit with the psychiatrist in the cardiac rehabilitation program, the patient finally accepted that her lack of confidence could also be a symptom of depression. We repeated the Patient Health Questionnaire, which still showed moderate depression, and we started her on an SSRI, citalopram. About 3 weeks later, she began to regain her confidence, and she was able to attend and host her son’s graduation. By 8 weeks after the start of antidepressant therapy, a repeat Patient Health Questionnaire showed no evidence of depression.
Her progress, both physically and emotionally, was quite pronounced during the 12-week cardiac rehabilitation program. Her physical stamina improved, her fatigue abated, and her sense of confidence was restored. She successfully returned to work and her family concurred that she had returned to her “old self.” She benefited from the stress management and lifestyle seminars that were offered in the cardiac rehabilitation program, and her exit SF-36 scores were much improved. The patient pleasantly surprised us all by taking the initiative of forming a monthly women’s support group for coping with heart surgery.
She completed a 9-month course of the SSRI, with the depression in full remission, and has continued to follow up with her cardiologist and her exercise regimen.
CARDIAC CASE PRESENTATION
A 53-year-old woman, a malpractice lawyer, with a history of mitral valve prolapse was diagnosed with severe mitral regurgitation and referred for mitral valve repair.
History and examination
The patient had no other cardiac history. She reported jogging 2 to 3 miles daily and playing tennis regularly, but over the past few months she had become more fatigued during her jogs, to the point that she occasionally had to reduce her pace and even shorten the duration of her runs.
On her initial visit, she expressed surprise regarding the severity of her mitral valve disease, as she had always been healthy. She seemed somewhat nervous but appropriately concerned about the impending surgery, and questioned whether she would be able to return to her previous level of activity. She also mentioned that she hoped the timing of the surgery would permit her to attend her son’s college graduation in 9 weeks.
Her medical history was notable for mitral valve prolapse. She had a history of panic attacks, for which she occasionally took alprazolam. There was no family history of cardiac disease. She did not use tobacco and occasionally consumed alcohol. A review of systems was negative.
Her physical examination was unremarkable except for a grade 4/6 holosystolic murmur at the apex that radiated to the axilla, which was consistent with the mitral regurgitation.
A transthoracic echocardiogram demonstrated mitral regurgitation that extended through the left atrium back into the pulmonary veins. The left ventricular ejection fraction was 50%, which is considered low-normal. The degree of mitral regurgitation was 4+. No other significant valvular disease was observed.
An electrocardiogram revealed a normal sinus rhythm. Per our routine, the patient underwent cardiac catheterization, which showed normal coronary arteries.
An uncomplicated repair, but slow recovery
The mitral valve repair was performed without complications. The course in the intensive care unit was uncomplicated, and the patient was quickly extubated and transferred to a regular nursing floor.
On the nursing floor, controlling the patient’s pain was difficult. She refused to use her incentive spirometer and initially refused to ambulate or even move from her bed to a chair. She was quite tearful.
A postoperative transthoracic echocardiogram revealed a satisfactorily repaired mitral valve with no mitral regurgitation. Her ejection fraction decreased to 40%, which is not unusual after mitral valve surgery.
Her hospital course was notable for an episode of shortness of breath and tachycardia. Sinus tachycardia was evident on review of the telemetry strips. A repeat echocardiogram showed no changes compared with the prior postoperative echocardiogram. Spiral computed tomography was negative for pulmonary embolism.
Pain control remained difficult. The patient expressed concern about the postoperative decrease in her ejection fraction; she was reassured that a decrease in ejection fraction was not unusual, but she remained tearful. The family expressed concern because the patient “wasn’t acting like herself,” and her ambulation and use of her incentive spirometer continued to be minimal, which had the potential to hamper her recovery and rehabilitation. For these reasons, a psychiatric consult was requested and the patient was seen prior to discharge from the hospital on postoperative day 6.
Wound check at 1 week postdischarge
A routine wound check was performed 1 week after discharge, at which time the patient was still reporting pain that was more severe than would be expected at her postoperative stage. She reported concern about drainage from the incision. She said that she was unable to do much walking or stair climbing, and she reported sleeping in the guest bedroom on the first floor of her house because she was unable to negotiate the stairs to her bedroom.
A check of the wound showed minimal serous drainage at the inferior aspect and was consistent with normal wound healing. The slow progress of her recovery was a concern, as was the possible contribution of her anxiety to this slow progress, so we kept our psychiatric colleagues informed about the patient’s recovery.
Follow-up at postoperative week 4
At the follow-up visit at postoperative week 4, the patient reported still being in pain, although the pain had improved, and complained of constant fatigue and shortness of breath that prevented her from returning to work. She had been discharged on lisinopril and admitted to occasional medication noncompliance. She said that if she did not improve dramatically and quickly, she would not be able to attend her son’s graduation.
We considered the possibility of new ischemia, a large pleural effusion, postpericardiotomy syndrome, constrictive pericarditis, or a mitral valve leak as potential causes of her symptoms. A chest radiograph was obtained, which demonstrated a small left pleural effusion, and an echocardiogram showed that her ejection fraction remained at 40% and the mitral valve repair remained intact. The patient had a psychiatric visit scheduled later on the day of this follow-up visit and was referred to the cardiac rehabilitation program, to start on week 6 of her postoperative care.
PSYCHIATRIC CASE PRESENTATION
At the time of the first psychiatric consult, postoperative day 6, the patient’s chart was reviewed, detailing her presentation and hospital course as described above. The chart confirmed one episode of “panic” following surgery while the patient was on telemetry, showing only sinus tachycardia. This episode was successfully treated with 1 mg of lorazepam. She expressed a fear of “losing it,” which is how she characterized her panicky state during the hospital stay, punctuated by the feeling that she was not in control. The nursing staff reported that she was distressed and irritable. Her husband also confirmed that the patient “was not herself.”
Her baseline functioning was high; she is a partner in a law firm and is customarily “in control.” Before the interview began, the patient had several questions ready, including how quickly she would heal, how soon she could return to work and resume her normal activities, the reason for her low ejection fraction despite having mitral valve surgery, and whether or not she would be able to attend her son’s graduation. Even though she knew the psychiatry consult had been ordered, she was not very receptive to it at first and was more focused on her physical symptoms.
Psychiatric history
Her psychiatric history was significant for fear of heights and panic attacks, but she had been able to conquer each. She overcame performance anxiety in high school and was able to be a successful malpractice attorney, deliberating cases in court. She had never seen a psychiatrist or mental health professional, and had never been on psychotropic medications, although for the past couple of years she had been using 0.5 mg of alprazolam to treat flight anxiety. She admitted to postpartum depression that lasted about 2 months; no treatment was sought at the time, and the depression resolved.
Family and personal history
Her mother was a teacher and a “professional worrier,” and her father is a retired lawyer. She reported resolving to “suck it up” during times of adversity during childhood, but her childhood was otherwise unremarkable. She is an only child and finished at the top of her class at law school.
Review of symptoms
An assessment of depressive symptoms using the mnemonic SIGECAPS (disturbance of sleep; disturbance of interest; presence of guilt; disturbance of energy, concentration, or appetite; increased or decreased psychomotor activity; ideas of suicide) elicited low energy levels, decreased concentration, and a “slowed down” feeling. The WART (withdrawal, anhedonia, rumination, tearfulness) scale, used to assess depressive symptoms in the medically ill, showed the patient to be withdrawn and tearful at times.
Mental status examination
The patient was polite, professionally courteous, and sitting up in bed. Her vital signs were stable (heart rate, 70 beats per minute; blood pressure, 122/72 mm Hg) and her mood was “fine,” although she had many concerns about her physical health. Her affect was serious, constricted, and controlling. Her thought process was linear and organized, and her thought content revealed no psychosis, suicidal ideations, or overt hopelessness. She admitted that she was slightly anxious and overwhelmed, and that this anxiety precipitated her “panic” on telemetry and tearfulness, but she believed (and asked for assurance) that this level of anxiety was normal following surgery.
Diagnosis and recommendations
By the end of the consultation, we were able to make a series of recommendations. We arrived at a diagnosis of adjustment disorder with anxious features, and we agreed to treat her with alprazolam at a dose of 0.5 mg twice daily as needed. We provided education about mood and anxiety disorders in cardiac patients. We explained that her postpartum depression was a risk factor for future depression. We discussed coping strategies and relaxation techniques, and scheduled a follow-up appointment with her primary care physician for further monitoring of her mood and anxiety.
One week postdischarge
The cardiology team communicated with us after her wound check at postdischarge week 1. At this time, she was still having pain and was concerned about excessive wound drainage even though it was found to be minimal. The cardiology team was concerned because her progress was slow and she appeared anxious and tense. A follow-up psychiatry consultation was arranged for the patient’s next postoperative visit.
Follow-up at postoperative week 4
At her scheduled psychiatric visit at postoperative week 4, the patient was a little surprised to see the fellow, as she expected to see the staff psychiatrist. She appeared tense and frustrated, was fixated on her echocardiogram and her physical symptoms, and reported that she was not yet back to work. She was preoccupied with her son’s graduation that was coming up and wondered if she would be able to attend and celebrate it.
We administered the Patient Health Questionnaire depression scale, and the patient’s score of 11 indicated moderate depression. Treatment options, including psychotherapy and pharmacotherapy with a selective serotonin reuptake inhibitor (SSRI), were reviewed with the patient. A call to the cardiology team revealed that her ejection fraction was fairly typical for a patient who has had a mitral valve repair but that the continued fatigue was not normal, leading us to suspect that depression may be the actual cause of her fatigue. She remarked, “Let’s see how the cardiac rehabilitation program goes and then we’ll talk about medications for depression.”
Cardiac rehabilitation at postoperative week 6
The patient was entered into the cardiac rehabilitation program, and she was administered a Short Form–36 (SF-36) health survey, which showed a low mental summary score and a low physical component summary score (low scores connote worse health and/or more disability). She was referred to the psychiatrist at the cardiovascular behavioral health clinic for further assessment of her mood as she commenced the cardiac rehabilitation program.
DISCUSSION OF THE CASE
To explore management options in this case and discuss the insights it provides into heart-brain interactions, the case presentation was followed by an interactive discussion (moderated by Dr. James B. Young, Department of Cardiovascular Medicine and Chairman, Division of Medicine at Cleveland Clinic) between the physicians who presented the case and the Heart-Brain Summit audience.
Dr. James Young: Let’s begin by considering whether there were some red flags that may have been apparent up front to predict that this patient might have been challenging in the postoperative period. I think one red flag was the diagnosis of mitral valve prolapse itself, which has been known to occur in type A personalities, who tend to exhibit catecholamine excess and sympathetic nervous system arousal that activates the autonomic nervous system.
Also, I’d be interested to know a few more findings from the patient’s physical examination. Was she thin? Did she have a narrow anteroposterior diameter? Did she have pectus excavatum? Did she have arachnodactyly tendencies? These are important characteristics that might have flagged the anxiety up front, as psychosomatic manifestations of patients with mitral valve prolapse were identified—and hotly debated—20 to 30 years ago. Although the link between mitral valve prolapse and personality type has fallen out of favor in cardiology circles, it clearly seems to describe this patient. The history of anxiety, panic, and possibly agoraphobia has been well described in patients with mitral valve prolapse and excematous degeneration.
I’d like to pose the following questions to the audience. What do you do with this patient now? Do you push medication therapy? Do you push psychotherapy? What is the next step?
Comment from audience: You haven’t excluded the post-pump syndrome. This patient is very bright and it wouldn’t take much of an insult to impair her sufficiently so that she would interpret the world in a different way. From my point of view, she needs sophisticated neuropsychological testing soon.
Dr. Young: That’s a good point. We know that cardiopulmonary bypass is associated with difficulties and problems that have been underreported in the past.
Comment from audience: The last thing that this patient wants to admit or even allude to is a psychological problem. She is the last one who’s going to even hint at it, which makes it very easy to miss. Look at how she reacted when she heard that there was a psychiatrist in the room. These patients are not necessarily well disposed to completing screening tests because they recognize that somebody is trying to identify a psychological problem. I don’t know that I have the answer, but I think that we should avoid browbeating ourselves for the problem.
Dr. Young: I want to mention the cultural anthropology of physicians and how it affects our approach to treatment. I like being a cardiologist because I write prescriptions for drugs that have proven to be useful, such as beta-blockers and ACE inhibitors, among others. From this experience came my earlier question, “Should we give this patient a drug?” The cardiologist’s focus—perhaps excessive focus—on pharmacologic solutions may not be the best way to approach this patient. You allude to some important issues about screening a patient for diseases that can be more easily treated.
Comment from audience: I have seen such situations as a result of drug interactions; many of our patients are on multiple drugs when they leave the hospital. The other issue to consider is sleep deprivation, with or without sleep apnea.
Dr. Young: Many complications, particularly in patients with heart failure, are related to disordered sleep, which certainly causes some heart-brain dysfunction. What about the drugs?
Dr. Thomas Callahan: We considered the effects of her medications, which included an ACE inhibitor and her analgesics. We also considered the lingering effects of anesthesia or other medications that she might have been receiving.
Dr. Young: Remember, she was reporting considerable pain. I suspect that she was on a cocktail of pain medications that might have been contributing to her difficulties.
Comment from audience: Morphine’s effects tend to be stronger in women than in men. The other issue is the 10% drop in ejection fraction after the surgery. This patient may be thinking, “Why did I go through all of this if my ejection fraction is going to be worse?”
Dr. Callahan: A drop in the ejection fraction, especially after mitral valve repair, is common. We often address it with patients preoperatively, but perhaps not with everyone, and perhaps not clearly enough.
Dr. Young: Also, this is an example of a patient who had heart failure going into the operation, but “heart failure” would be the worst term to use with this particular patient. An ejection fraction of 50% is not normal for a patient with 4+ mitral regurgitation and, as Dr. Callahan suggested, when you take away the mitral regurgitation, you dump a little more load on the left ventricle, and the ejection fraction will go down. We see this all the time, although I admit that cardiologists or cardiac surgeons don’t necessarily do the best job of discussing these subtleties with patients. Something we can take away from this case is a sense of the importance of improving our communications with patients about what they might expect postoperatively, although it still needs to be tailored to the individual patient. If this patient had understood the pathophysiology behind the drop in ejection fraction, it may have helped her. Other patients, on the other hand, may not require detailed conversations about this phenomenon.
Comment from audience: It was mentioned several times that the husband said the patient was not herself. Did you interact with the husband and the son to get a sense of the long-term dynamics of this family? It seems that there may have been some issues with the family dynamics.
Dr. Ubaid Khokhar: That’s a good question, although no underlying dynamics seemed apparent. The husband and son’s primary concern was that the patient’s previous characteristics of perfectionism and always being “in control” were so much in contrast with the tearful episodes she was having now. “She is not the same,” is how they kept phrasing it. However, there were no other significant changes—no rumination about suicide, no overt unwillingness to go along with treatment, or anything like that.
Comment from audience: I believe strongly that this patient was depressed, although she did not admit it. She had four of the five symptoms. She did not admit to a depressed mood but was tearful, which you reported at every postoperative visit. This is a sign of depression. We know very well that anxiety and depression often are present in tandem, especially in patients with high baseline anxiety. When they have more stress in their lives, they tend to get depressed.
I agree with the preceding comments that drug interactions are a potential worry; however, a few of the SSRIs have favorable drug-drug interaction profiles. I would urge this patient to try SSRI therapy. If she rejected this by responding, “I’m not depressed,” you could point out that SSRIs work very well for anxiety. Alprazolam is not a good medication for anxiety because it has a very short half-life, which can leave patients with an increase in anxious feelings after the medication is cleared from their system but before their next dose.
In addition to SSRI therapy as a first-line approach, I would try stress management, biofeedback, or even psychosupportive therapy that relies on patient education to help this patient understand her condition and take back control.
CASE OUTCOME
Our initial approach with this patient was the path of least resistance. Very good points have been made by the discussants and members of the audience. This patient was attached to alprazolam because it was the only psychotropic medication that she had ever taken. For this reason, she was discharged on alprazolam even though it wasn’t the ideal medication. As pointed out by the audience, the patient was quite resistant to the concept of having depression superimposed on a history of anxiety. In the cardiac rehabilitation setting she was again reassured by the exercise physiologists that her heart was doing well. A cardiologist personally reviewed the echocardiographic reports and films with the patient, pointed out the absence of unusual abnormalities with her heart, and suggested that something else was causing her symptoms. This direct explanation and reassurance from the cardiologist facilitated the patient’s ability to entertain depression as a comorbid condition.
At the visit with the psychiatrist in the cardiac rehabilitation program, the patient finally accepted that her lack of confidence could also be a symptom of depression. We repeated the Patient Health Questionnaire, which still showed moderate depression, and we started her on an SSRI, citalopram. About 3 weeks later, she began to regain her confidence, and she was able to attend and host her son’s graduation. By 8 weeks after the start of antidepressant therapy, a repeat Patient Health Questionnaire showed no evidence of depression.
Her progress, both physically and emotionally, was quite pronounced during the 12-week cardiac rehabilitation program. Her physical stamina improved, her fatigue abated, and her sense of confidence was restored. She successfully returned to work and her family concurred that she had returned to her “old self.” She benefited from the stress management and lifestyle seminars that were offered in the cardiac rehabilitation program, and her exit SF-36 scores were much improved. The patient pleasantly surprised us all by taking the initiative of forming a monthly women’s support group for coping with heart surgery.
She completed a 9-month course of the SSRI, with the depression in full remission, and has continued to follow up with her cardiologist and her exercise regimen.
CARDIAC CASE PRESENTATION
A 53-year-old woman, a malpractice lawyer, with a history of mitral valve prolapse was diagnosed with severe mitral regurgitation and referred for mitral valve repair.
History and examination
The patient had no other cardiac history. She reported jogging 2 to 3 miles daily and playing tennis regularly, but over the past few months she had become more fatigued during her jogs, to the point that she occasionally had to reduce her pace and even shorten the duration of her runs.
On her initial visit, she expressed surprise regarding the severity of her mitral valve disease, as she had always been healthy. She seemed somewhat nervous but appropriately concerned about the impending surgery, and questioned whether she would be able to return to her previous level of activity. She also mentioned that she hoped the timing of the surgery would permit her to attend her son’s college graduation in 9 weeks.
Her medical history was notable for mitral valve prolapse. She had a history of panic attacks, for which she occasionally took alprazolam. There was no family history of cardiac disease. She did not use tobacco and occasionally consumed alcohol. A review of systems was negative.
Her physical examination was unremarkable except for a grade 4/6 holosystolic murmur at the apex that radiated to the axilla, which was consistent with the mitral regurgitation.
A transthoracic echocardiogram demonstrated mitral regurgitation that extended through the left atrium back into the pulmonary veins. The left ventricular ejection fraction was 50%, which is considered low-normal. The degree of mitral regurgitation was 4+. No other significant valvular disease was observed.
An electrocardiogram revealed a normal sinus rhythm. Per our routine, the patient underwent cardiac catheterization, which showed normal coronary arteries.
An uncomplicated repair, but slow recovery
The mitral valve repair was performed without complications. The course in the intensive care unit was uncomplicated, and the patient was quickly extubated and transferred to a regular nursing floor.
On the nursing floor, controlling the patient’s pain was difficult. She refused to use her incentive spirometer and initially refused to ambulate or even move from her bed to a chair. She was quite tearful.
A postoperative transthoracic echocardiogram revealed a satisfactorily repaired mitral valve with no mitral regurgitation. Her ejection fraction decreased to 40%, which is not unusual after mitral valve surgery.
Her hospital course was notable for an episode of shortness of breath and tachycardia. Sinus tachycardia was evident on review of the telemetry strips. A repeat echocardiogram showed no changes compared with the prior postoperative echocardiogram. Spiral computed tomography was negative for pulmonary embolism.
Pain control remained difficult. The patient expressed concern about the postoperative decrease in her ejection fraction; she was reassured that a decrease in ejection fraction was not unusual, but she remained tearful. The family expressed concern because the patient “wasn’t acting like herself,” and her ambulation and use of her incentive spirometer continued to be minimal, which had the potential to hamper her recovery and rehabilitation. For these reasons, a psychiatric consult was requested and the patient was seen prior to discharge from the hospital on postoperative day 6.
Wound check at 1 week postdischarge
A routine wound check was performed 1 week after discharge, at which time the patient was still reporting pain that was more severe than would be expected at her postoperative stage. She reported concern about drainage from the incision. She said that she was unable to do much walking or stair climbing, and she reported sleeping in the guest bedroom on the first floor of her house because she was unable to negotiate the stairs to her bedroom.
A check of the wound showed minimal serous drainage at the inferior aspect and was consistent with normal wound healing. The slow progress of her recovery was a concern, as was the possible contribution of her anxiety to this slow progress, so we kept our psychiatric colleagues informed about the patient’s recovery.
Follow-up at postoperative week 4
At the follow-up visit at postoperative week 4, the patient reported still being in pain, although the pain had improved, and complained of constant fatigue and shortness of breath that prevented her from returning to work. She had been discharged on lisinopril and admitted to occasional medication noncompliance. She said that if she did not improve dramatically and quickly, she would not be able to attend her son’s graduation.
We considered the possibility of new ischemia, a large pleural effusion, postpericardiotomy syndrome, constrictive pericarditis, or a mitral valve leak as potential causes of her symptoms. A chest radiograph was obtained, which demonstrated a small left pleural effusion, and an echocardiogram showed that her ejection fraction remained at 40% and the mitral valve repair remained intact. The patient had a psychiatric visit scheduled later on the day of this follow-up visit and was referred to the cardiac rehabilitation program, to start on week 6 of her postoperative care.
PSYCHIATRIC CASE PRESENTATION
At the time of the first psychiatric consult, postoperative day 6, the patient’s chart was reviewed, detailing her presentation and hospital course as described above. The chart confirmed one episode of “panic” following surgery while the patient was on telemetry, showing only sinus tachycardia. This episode was successfully treated with 1 mg of lorazepam. She expressed a fear of “losing it,” which is how she characterized her panicky state during the hospital stay, punctuated by the feeling that she was not in control. The nursing staff reported that she was distressed and irritable. Her husband also confirmed that the patient “was not herself.”
Her baseline functioning was high; she is a partner in a law firm and is customarily “in control.” Before the interview began, the patient had several questions ready, including how quickly she would heal, how soon she could return to work and resume her normal activities, the reason for her low ejection fraction despite having mitral valve surgery, and whether or not she would be able to attend her son’s graduation. Even though she knew the psychiatry consult had been ordered, she was not very receptive to it at first and was more focused on her physical symptoms.
Psychiatric history
Her psychiatric history was significant for fear of heights and panic attacks, but she had been able to conquer each. She overcame performance anxiety in high school and was able to be a successful malpractice attorney, deliberating cases in court. She had never seen a psychiatrist or mental health professional, and had never been on psychotropic medications, although for the past couple of years she had been using 0.5 mg of alprazolam to treat flight anxiety. She admitted to postpartum depression that lasted about 2 months; no treatment was sought at the time, and the depression resolved.
Family and personal history
Her mother was a teacher and a “professional worrier,” and her father is a retired lawyer. She reported resolving to “suck it up” during times of adversity during childhood, but her childhood was otherwise unremarkable. She is an only child and finished at the top of her class at law school.
Review of symptoms
An assessment of depressive symptoms using the mnemonic SIGECAPS (disturbance of sleep; disturbance of interest; presence of guilt; disturbance of energy, concentration, or appetite; increased or decreased psychomotor activity; ideas of suicide) elicited low energy levels, decreased concentration, and a “slowed down” feeling. The WART (withdrawal, anhedonia, rumination, tearfulness) scale, used to assess depressive symptoms in the medically ill, showed the patient to be withdrawn and tearful at times.
Mental status examination
The patient was polite, professionally courteous, and sitting up in bed. Her vital signs were stable (heart rate, 70 beats per minute; blood pressure, 122/72 mm Hg) and her mood was “fine,” although she had many concerns about her physical health. Her affect was serious, constricted, and controlling. Her thought process was linear and organized, and her thought content revealed no psychosis, suicidal ideations, or overt hopelessness. She admitted that she was slightly anxious and overwhelmed, and that this anxiety precipitated her “panic” on telemetry and tearfulness, but she believed (and asked for assurance) that this level of anxiety was normal following surgery.
Diagnosis and recommendations
By the end of the consultation, we were able to make a series of recommendations. We arrived at a diagnosis of adjustment disorder with anxious features, and we agreed to treat her with alprazolam at a dose of 0.5 mg twice daily as needed. We provided education about mood and anxiety disorders in cardiac patients. We explained that her postpartum depression was a risk factor for future depression. We discussed coping strategies and relaxation techniques, and scheduled a follow-up appointment with her primary care physician for further monitoring of her mood and anxiety.
One week postdischarge
The cardiology team communicated with us after her wound check at postdischarge week 1. At this time, she was still having pain and was concerned about excessive wound drainage even though it was found to be minimal. The cardiology team was concerned because her progress was slow and she appeared anxious and tense. A follow-up psychiatry consultation was arranged for the patient’s next postoperative visit.
Follow-up at postoperative week 4
At her scheduled psychiatric visit at postoperative week 4, the patient was a little surprised to see the fellow, as she expected to see the staff psychiatrist. She appeared tense and frustrated, was fixated on her echocardiogram and her physical symptoms, and reported that she was not yet back to work. She was preoccupied with her son’s graduation that was coming up and wondered if she would be able to attend and celebrate it.
We administered the Patient Health Questionnaire depression scale, and the patient’s score of 11 indicated moderate depression. Treatment options, including psychotherapy and pharmacotherapy with a selective serotonin reuptake inhibitor (SSRI), were reviewed with the patient. A call to the cardiology team revealed that her ejection fraction was fairly typical for a patient who has had a mitral valve repair but that the continued fatigue was not normal, leading us to suspect that depression may be the actual cause of her fatigue. She remarked, “Let’s see how the cardiac rehabilitation program goes and then we’ll talk about medications for depression.”
Cardiac rehabilitation at postoperative week 6
The patient was entered into the cardiac rehabilitation program, and she was administered a Short Form–36 (SF-36) health survey, which showed a low mental summary score and a low physical component summary score (low scores connote worse health and/or more disability). She was referred to the psychiatrist at the cardiovascular behavioral health clinic for further assessment of her mood as she commenced the cardiac rehabilitation program.
DISCUSSION OF THE CASE
To explore management options in this case and discuss the insights it provides into heart-brain interactions, the case presentation was followed by an interactive discussion (moderated by Dr. James B. Young, Department of Cardiovascular Medicine and Chairman, Division of Medicine at Cleveland Clinic) between the physicians who presented the case and the Heart-Brain Summit audience.
Dr. James Young: Let’s begin by considering whether there were some red flags that may have been apparent up front to predict that this patient might have been challenging in the postoperative period. I think one red flag was the diagnosis of mitral valve prolapse itself, which has been known to occur in type A personalities, who tend to exhibit catecholamine excess and sympathetic nervous system arousal that activates the autonomic nervous system.
Also, I’d be interested to know a few more findings from the patient’s physical examination. Was she thin? Did she have a narrow anteroposterior diameter? Did she have pectus excavatum? Did she have arachnodactyly tendencies? These are important characteristics that might have flagged the anxiety up front, as psychosomatic manifestations of patients with mitral valve prolapse were identified—and hotly debated—20 to 30 years ago. Although the link between mitral valve prolapse and personality type has fallen out of favor in cardiology circles, it clearly seems to describe this patient. The history of anxiety, panic, and possibly agoraphobia has been well described in patients with mitral valve prolapse and excematous degeneration.
I’d like to pose the following questions to the audience. What do you do with this patient now? Do you push medication therapy? Do you push psychotherapy? What is the next step?
Comment from audience: You haven’t excluded the post-pump syndrome. This patient is very bright and it wouldn’t take much of an insult to impair her sufficiently so that she would interpret the world in a different way. From my point of view, she needs sophisticated neuropsychological testing soon.
Dr. Young: That’s a good point. We know that cardiopulmonary bypass is associated with difficulties and problems that have been underreported in the past.
Comment from audience: The last thing that this patient wants to admit or even allude to is a psychological problem. She is the last one who’s going to even hint at it, which makes it very easy to miss. Look at how she reacted when she heard that there was a psychiatrist in the room. These patients are not necessarily well disposed to completing screening tests because they recognize that somebody is trying to identify a psychological problem. I don’t know that I have the answer, but I think that we should avoid browbeating ourselves for the problem.
Dr. Young: I want to mention the cultural anthropology of physicians and how it affects our approach to treatment. I like being a cardiologist because I write prescriptions for drugs that have proven to be useful, such as beta-blockers and ACE inhibitors, among others. From this experience came my earlier question, “Should we give this patient a drug?” The cardiologist’s focus—perhaps excessive focus—on pharmacologic solutions may not be the best way to approach this patient. You allude to some important issues about screening a patient for diseases that can be more easily treated.
Comment from audience: I have seen such situations as a result of drug interactions; many of our patients are on multiple drugs when they leave the hospital. The other issue to consider is sleep deprivation, with or without sleep apnea.
Dr. Young: Many complications, particularly in patients with heart failure, are related to disordered sleep, which certainly causes some heart-brain dysfunction. What about the drugs?
Dr. Thomas Callahan: We considered the effects of her medications, which included an ACE inhibitor and her analgesics. We also considered the lingering effects of anesthesia or other medications that she might have been receiving.
Dr. Young: Remember, she was reporting considerable pain. I suspect that she was on a cocktail of pain medications that might have been contributing to her difficulties.
Comment from audience: Morphine’s effects tend to be stronger in women than in men. The other issue is the 10% drop in ejection fraction after the surgery. This patient may be thinking, “Why did I go through all of this if my ejection fraction is going to be worse?”
Dr. Callahan: A drop in the ejection fraction, especially after mitral valve repair, is common. We often address it with patients preoperatively, but perhaps not with everyone, and perhaps not clearly enough.
Dr. Young: Also, this is an example of a patient who had heart failure going into the operation, but “heart failure” would be the worst term to use with this particular patient. An ejection fraction of 50% is not normal for a patient with 4+ mitral regurgitation and, as Dr. Callahan suggested, when you take away the mitral regurgitation, you dump a little more load on the left ventricle, and the ejection fraction will go down. We see this all the time, although I admit that cardiologists or cardiac surgeons don’t necessarily do the best job of discussing these subtleties with patients. Something we can take away from this case is a sense of the importance of improving our communications with patients about what they might expect postoperatively, although it still needs to be tailored to the individual patient. If this patient had understood the pathophysiology behind the drop in ejection fraction, it may have helped her. Other patients, on the other hand, may not require detailed conversations about this phenomenon.
Comment from audience: It was mentioned several times that the husband said the patient was not herself. Did you interact with the husband and the son to get a sense of the long-term dynamics of this family? It seems that there may have been some issues with the family dynamics.
Dr. Ubaid Khokhar: That’s a good question, although no underlying dynamics seemed apparent. The husband and son’s primary concern was that the patient’s previous characteristics of perfectionism and always being “in control” were so much in contrast with the tearful episodes she was having now. “She is not the same,” is how they kept phrasing it. However, there were no other significant changes—no rumination about suicide, no overt unwillingness to go along with treatment, or anything like that.
Comment from audience: I believe strongly that this patient was depressed, although she did not admit it. She had four of the five symptoms. She did not admit to a depressed mood but was tearful, which you reported at every postoperative visit. This is a sign of depression. We know very well that anxiety and depression often are present in tandem, especially in patients with high baseline anxiety. When they have more stress in their lives, they tend to get depressed.
I agree with the preceding comments that drug interactions are a potential worry; however, a few of the SSRIs have favorable drug-drug interaction profiles. I would urge this patient to try SSRI therapy. If she rejected this by responding, “I’m not depressed,” you could point out that SSRIs work very well for anxiety. Alprazolam is not a good medication for anxiety because it has a very short half-life, which can leave patients with an increase in anxious feelings after the medication is cleared from their system but before their next dose.
In addition to SSRI therapy as a first-line approach, I would try stress management, biofeedback, or even psychosupportive therapy that relies on patient education to help this patient understand her condition and take back control.
CASE OUTCOME
Our initial approach with this patient was the path of least resistance. Very good points have been made by the discussants and members of the audience. This patient was attached to alprazolam because it was the only psychotropic medication that she had ever taken. For this reason, she was discharged on alprazolam even though it wasn’t the ideal medication. As pointed out by the audience, the patient was quite resistant to the concept of having depression superimposed on a history of anxiety. In the cardiac rehabilitation setting she was again reassured by the exercise physiologists that her heart was doing well. A cardiologist personally reviewed the echocardiographic reports and films with the patient, pointed out the absence of unusual abnormalities with her heart, and suggested that something else was causing her symptoms. This direct explanation and reassurance from the cardiologist facilitated the patient’s ability to entertain depression as a comorbid condition.
At the visit with the psychiatrist in the cardiac rehabilitation program, the patient finally accepted that her lack of confidence could also be a symptom of depression. We repeated the Patient Health Questionnaire, which still showed moderate depression, and we started her on an SSRI, citalopram. About 3 weeks later, she began to regain her confidence, and she was able to attend and host her son’s graduation. By 8 weeks after the start of antidepressant therapy, a repeat Patient Health Questionnaire showed no evidence of depression.
Her progress, both physically and emotionally, was quite pronounced during the 12-week cardiac rehabilitation program. Her physical stamina improved, her fatigue abated, and her sense of confidence was restored. She successfully returned to work and her family concurred that she had returned to her “old self.” She benefited from the stress management and lifestyle seminars that were offered in the cardiac rehabilitation program, and her exit SF-36 scores were much improved. The patient pleasantly surprised us all by taking the initiative of forming a monthly women’s support group for coping with heart surgery.
She completed a 9-month course of the SSRI, with the depression in full remission, and has continued to follow up with her cardiologist and her exercise regimen.