User login
Healing after rotator cuff repair (RCR) can be challenging, especially in cases of large and massive tears, revision repairs, and tendons with poor tissue quality.1-3 Poor tissue quality is associated with increased risk for recurrent tears, independent of age and tear size.3 Various techniques have been used to improve tendon fixation strength in these difficult situations, including augmented suture configurations (eg, massive cuff stitches, rip-stop stitches) and tissue grafts (eg, acellular dermal matrix).4-9 Clinical studies have found improved healing rates for larger tears and revision repairs using acellular dermal matrix grafts.6,10 Synthetic patches are another option for RCR augmentation, but limited clinical data and biomechanical evidence support use of synthetic grafts as an augment for RCRs.11-13
Polyhydroxyalkanoates (PHAs) are a class of biodegradable polymers that have been used as orthopedic devices, tissue scaffolds, patches, and other applications with increasing frequency over the past decade.14 In the laboratory, these implanted materials have been shown to support cell migration and growth.15 The PHA family of polymers typically degrades by hydrolytic and bacterial depolymerase mechanisms over 52-plus weeks in vivo.14PHA grafts have been studied in the setting of RCR. An expanded polytetrafluoroethylene scaffold was shown to improve repair mechanics when used as a bursal side graft in an in vitro ovine model.11 The graft increased tendon footprint contact pressure and failure loads by almost 180 N. In clinical studies, poly-L-lactic acid augmentations have been used to reinforce massive RCRs. Lenart and colleagues16 found that 38% of 16 patients with such tears had an intact rotator cuff at 1.2-year follow-up, and improvement in clinical scores. Proctor13 reported on use of a poly-L-lactic acid retrograde patch for reinforcement of massive tears with both single- and double-row repairs in 18 patients. The cohort had more favorable rates of intact cuffs at 12 months (83%) and 42 months (78%), and ASES (American Shoulder and Elbow Surgeons) scores improved from 25 before surgery to 82 at latest follow-up after surgery.
RCR augmentation traditionally has been performed with an open or mini-open technique.6 Recently, several authors have reported on arthroscopic techniques for augmentation with either acellular dermal matrix or synthetic grafts.13,17,18 Most techniques have involved “bridging” with a graft or patch used to stress-shield a single-row repair.8,9,13 This bridging typically involves placing several sutures medial to where the anchor repair stitches pass through the tendon. An alternative is to pass the repair stitches through both the tendon and the graft.17-19 The overall volume of tissue incorporated into the repair stitches (rotator cuff plus graft) is increased with the augmented technique relative to the bridging technique. Both can be technically challenging, but the augmented technique may be easier to perform arthroscopically.9,19 Regardless, these techniques are complicated and require a higher level of arthroscopic skills compared with those required in arthroscopic RCR without a graft. Simplifying arthroscopic graft augmentation likely will increase its utility because, even for skilled surgeons, adding a graft can increase operative time by 20 to 30 minutes. Simplification will also extend use of the technique to surgeons with less experience and proficiency with arthroscopic repair.
We developed a simple method for augmenting single-row RCR with a strip of bioresorbable soft-tissue scaffold. We also conducted a study to evaluate the initial biomechanical properties of single-row RCR in cadaveric shoulder specimens augmented with PHA mesh (BioFiber; Tornier) graft as compared with single-row RCR without augmentation. Both cyclic gap formation and ultimate failure loads and displacement were quantified. We hypothesized that the augmented RCRs would have decreased gap formation and increased ultimate failure loads compared with nonaugmented RCRs. This study was exempt from having to obtain Institutional Review B
Methods
Eight pairs of fresh-frozen cadaver humeri (6 male, 2 female; mean [SD] age, 61 [9] years) were dissected of all soft tissue (except rotator cuff) by Dr. Tashjian, a board-certified, fellowship-trained orthopedic surgeon. There were no qualitative differences in tendon condition between tendons within a pair. The supraspinatus muscle and tendon were separated from the other rotator cuff muscles. The infraspinatus, subscapularis, and teres minor were removed from the humerus. Last, the supraspinatus was resected at its insertion. Humeral pairs were then randomized into augmented and nonaugmented RCRs within each pair.
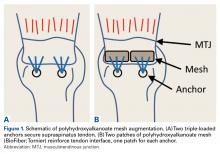
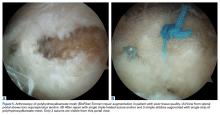
In the nonaugmented group, the supraspinatus was reattached to its insertion in a single-row RCR with 2 triple-loaded suture anchors (5.5-mm Insite FT Ti, No. 2 Force Fiber suture; Tornier) and 6 simple stitches (Figure 1A). Anchors were placed midway between the articular margin and the lateral edge of the greater tuberosity at about 45° to the bone surface.
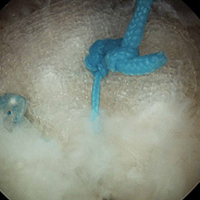
In the contralateral shoulders, augmented RCRs were performed. Specimens were prepared exactly as they were for the nonaugmented RCRs, including anchor placement and suture passage. Before knot tying, RCRs were augmented with 2 strips of 13-mm × 23-mm PHA mesh (BioFiber) (Figure 1B). One strip was used to augment the 3 sutures of each anchor, overlying the residual tendon, to reinforce the tendon–knot interface. After each suture was passed through the supraspinatus tendon from the intra-articular surface, the stitch was passed through the strip of PHA mesh. Stitches were separated by 5 mm in each mesh strip. All 6 sutures were then tied with a Revo knot between the free end of each suture leg and the leg that passed through the tendon and mesh.
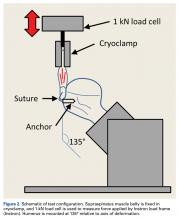
Each humerus was transected at the midshaft and potted and mounted in an Instron 1331 load frame with Model 8800 controller (Instron). A cryoclamp was used to grasp the supraspinatus muscle belly above the musculotendinous junction (Figure 2).
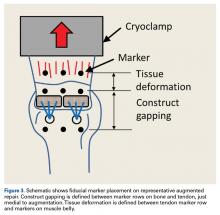
Three rows of 2-mm fiducial markers were affixed to the bone, tendon, and muscle belly with cyanoacrylate for tracking with a digital video system (DMAS Version 6.5; Spicatek) (Figure 3).21
A 0.1-MPa pre-stress (applied force/tendon cross-sectional area) was applied to each construct to determine the starting position for the deformation profile. Each repair underwent 1000 cycles of uniaxial load-controlled displacement between 0.1 and 1.0 MPa of effective stress at 1 Hz. Effective stress was determined as the ratio of applied force to cross-sectional area of the tendon at harvest to normalize the applied loads between tendons of varying size. During cyclic testing, gapping of more than 5 mm was defined as construct failure.22 After cyclic loading, each construct was loaded to failure at 1.0 mm/s. Ultimate failure load was defined as the highest load achieved at the maximum displacement before rapid decline in load supported by the construct.
Statistical Analysis
Paired t tests were used to compare the matched pairs of constructs. For all tests, significance was set at P ≤ .05. Post hoc power was calculated for significant results using G*Power Version 3.1.6.23 All data are presented as means (SDs).
Results
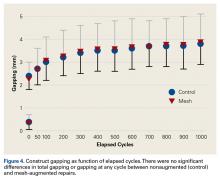
After 1000 cycles of displacement, mean (SD) gapping was 3.8 (0.9) mm for the nonaugmented repairs and 3.9 (1.1) mm for the PHA mesh–augmented repairs (P = .879) (Figure 4).
For the nonaugmented repairs, mean (SD) failure displacement was 6.3 (1.7) mm, and mean (SD) ultimate failure load was 472.1 (120.3) N. For the PHA-augmented repairs, failure displacement was 5.5 (1.9) mm, and ultimate failure load was 571.2 (173.0) N. There was no difference in failure displacement (P = .393), but there was a difference in ultimate failure load (P = .042; power = 0.57). During failure testing, mean (SD) tissue deformation was higher (P = .012; power = 0.83) for the PHA-augmented repairs, 1.2 (0.7) mm, than for the nonaugmented repairs, 0.8 (0.5) mm. Failures, which were consistent within pairs, were caused by tissue failure, with sutures pulling through the tissue (4 pairs) or single anchor pullout before ultimate tissue failure (4 pairs). Of the 4 failures with anchor pullout, 3 had anterior anchor pullout, and 1 had posterior anchor pullout. In all specimens with anchor pullout, the second anchor remained stable, and ultimate failure occurred with tissue tearing at the suture interface. There were no significant differences in any metrics between specimens that failed with intact anchors and specimens with single anchor pullout (P ≥ .122). Therefore, both groups were pooled for the failure analysis.
Discussion
RCR augmentation with a synthetic graft is a viable option for improving fixation strength of supraspinatus repairs, as shown in otherwise healthy tendon in the present study. Our hypothesis that there would be decreased gap formation with graft augmentation was not supported, whereas the hypothesis of increased failure loads with graft augmentation was supported. These findings may also be applicable in cases of large tears, revisions, and tendons with poor tissue quality. Simplification of graft application techniques will allow quick and easy arthroscopic augmentation.
Studies of RCRs for large or massive tears have reported retear rates of 25% to 79%.24-26 Latissimus dorsi tendon transfers also show promise in posterosuperior RCRs, with failure rates near 10%.27,28 Although use of PHA patches in RCR augmentation is relatively new, short-term and midterm failure rates are in the range of 20% to 60% in the few small cohorts currently being studied.13,16 It is possible that these rates may improve as indications, surgical experience, and techniques for use of PHA patches are further refined. Regardless, with PHA currently being used in practice, it is important to quantify the biomechanics of the augmentation as a baseline for its performance in reinforcing the tendon–suture interface.
We determined that the initial fixation strength of single-row repairs was higher with the addition of PHA synthetic grafts using a very simple technique. Single-row triple-loaded anchor repairs already provide high initial mechanical strength, and our results are similar to those of another study of this technique.29 Despite the already high mechanical strength of a triple-loaded anchor repair, PHA mesh increased ultimate strength by about 100 N (~25%). Of note, tissue elongation during failure was higher (P = .012; power = 0.83) in the PHA-augmented group (1.2 mm) than in the nonaugmented group (0.8 mm). This was not surprising—failure loads were almost 100 N higher in the PHA-augmented group than in the nonaugmented group. Consequently, much higher forces were placed on the muscle belly, likely resulting in additional elongation of the intact tissue medial to the repair construct.
The ultimate failure loads in our study compare favorably with the biomechanical strength of augmented repairs reported by others.8,9,18 Barber and colleagues18 evaluated an augmented single-row repair with 2 double-loaded suture anchors and an acellular dermal matrix graft. The ultimate failure load of the augmented repairs was 325 N. In contrast, Omae and colleagues8 tested a bridging single-row repair using 2 double-loaded suture anchors and an acellular dermal matrix graft. Ultimate failure load of the augmented repairs was 560 N, similar to our finding. Last, Shea and colleagues9 evaluated a bridging single-row repair using 2 double-loaded suture anchors and an acellular dermal matrix graft, with ultimate failure load of 429 N. The techniques in all 3 studies can be performed arthroscopically but are challenging and require multiple extra sutures and anchors that need management and tying. Our technique provides similar initial fixation strength, has no requirement for extra sutures or anchors, and is very simple to perform.
The supraspinatus tendon is estimated to fail between 800 N and 1000 N.30,31 Biomechanical shoulder simulators use supraspinatus forces in the range of 20 N to 200 N for scapular plane abduction.32-36 Therefore, the single-row repair failures in our study fell between functional and full-thickness failure loads. Studies on the mechanics of degenerated human supraspinatus tendon are limited, but there is evidence the mechanical properties of these tissues are inferior to those of healthy tendon.37 A 100-N increase in failure loads with PHA augmentation may prove highly significant in reinforcing the suture–tendon interface in degenerated tendons.
Adding the mesh did not have any effect on gapping at the repair site after cyclic loading. This finding suggests that construct gapping under cyclic loading is not a function of a reinforced knot–tendon interface but is instead caused by microtearing and cinching of the suture constructs in relation to the underlying bone. Tissue elongation likely was not a strong contributor to overall cyclic gapping, as elongation did not differ between the nonaugmented and augmented repairs (0.5 mm vs 0.7 mm; P = .276) and was small relative to the nearly 4 mm of construct gapping. Gapping may be affected by healing and integration of the mesh into the repaired tendon over time, but this effect could not be captured in the present study. Patients are initially immobilized and passive shoulder motion gradually introduced, in stark contrast to the immediate loading protocol in the present study. Regardless, the 25% increase in overall strength may be clinically important, especially in cases of difficult repair or poor tissue quality.
Our technique simplifies arthroscopic augmentation—stitches are passed through the rotator cuff in simple fashion. Before being tied, the limbs that were passed through the rotator cuff are removed through a cannula and then passed through the synthetic graft.
Study Limitations
This study had several limitations. First, it was a cadaveric biomechanical study that evaluated only time-zero biomechanical properties. Loads were normalized to tendon size, specimens were randomized between sides, and paired specimens were used to minimize the effects of tendon and bone quality on outcome metrics. In addition, donor tendons were representative of otherwise healthy tissue. Chronic tears and associated resorption/atrophy could have affected the magnitude of forces and gapping detected in this study. Theoretically, over time the tendon tissue will adhere to and grow into the mesh, which could minimize potential differences. Studies are needed to determine the effects of healing on long-term repair strength in affected patients. Last, all constructs were performed in open fashion to improve repeatability of construct placement and provide accessibility for Instron testing. Our technique did not directly replicate the arthroscopic approach, but, unlike other augmentation techniques, it is so simple that transition to all-arthroscopic augmentation is realistic.
Patch augmentation increases the cost of materials and operative time and should be considered a limitation of its utility. We do not recommend augmentation in all RCRs, as it likely is cost-ineffective. Instead, we recommend augmentation in cases of poor tissue quality, which could lead to healing failure, revision surgery, and higher overall patient costs beyond the cost of adding augmentation. Similarly, we recommend augmentation for revision cases in which tendon healing has failed and tissue quality is poor. The goal is to prevent another failure.
Conclusion
PHA graft augmentation of single-row triple-loaded anchor repairs of the supraspinatus tendon improves the overall ultimate load to failure by 25%. There was no difference in gap formation after cyclic loading between augmented and nonaugmented repairs. This technique for arthroscopic augmentation can be used to improve initial biomechanical repair strength in tears at risk for failure.
Am J Orthop. 2016;45(7):E527-E533. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Galatz LM, Ball CM, Teefey SA, Middleton WD, Yamaguchi K. The outcome and repair integrity of completely arthroscopically repaired large and massive rotator cuff tears. J Bone Joint Surg Am. 2004;86(2):219-224.
2. Keener JD, Wei AS, Kim HM, et al. Revision arthroscopic rotator cuff repair: repair integrity and clinical outcome. J Bone Joint Surg Am. 2010;92(3):590-598.
3. Nho SJ, Brown BS, Lyman S, Adler RS, Altchek DW, MacGillivray JD. Prospective analysis of arthroscopic rotator cuff repair: prognostic factors affecting clinical and ultrasound outcome. J Shoulder Elbow Surg. 2009;18(1):13-20.
4. Barber FA, Herbert MA, Schroeder FA, Aziz-Jacobo J, Mays MM, Rapley JH. Biomechanical advantages of triple-loaded suture anchors compared with double-row rotator cuff repairs. Arthroscopy. 2010;26(3):316-323.
5. Burkhart SS, Denard PJ, Konicek J, Hanypsiak BT. Biomechanical validation of load-sharing rip-stop fixation for the repair of tissue-deficient rotator cuff tears. Am J Sports Med. 2014;42(2):457-462.
6. Gupta AK, Hug K, Boggess B, Gavigan M, Toth AP. Massive or 2-tendon rotator cuff tears in active patients with minimal glenohumeral arthritis: clinical and radiographic outcomes of reconstruction using dermal tissue matrix xenograft. Am J Sports Med. 2013;41(4):872-879.
7. Ma CB, MacGillivray JD, Clabeaux J, Lee S, Otis JC. Biomechanical evaluation of arthroscopic rotator cuff stitches. J Bone Joint Surg Am. 2004;86(6):1211-1216.
8. Omae H, Steinmann SP, Zhao C, et al. Biomechanical effect of rotator cuff augmentation with an acellular dermal matrix graft: a cadaver study. Clin Biomech. 2012;27(8):789-792.
9. Shea KP, Obopilwe E, Sperling JW, Iannotti JP. A biomechanical analysis of gap formation and failure mechanics of a xenograft-reinforced rotator cuff repair in a cadaveric model. J Shoulder Elbow Surg. 2012;21(8):1072-1079.
10. Agrawal V. Healing rates for challenging rotator cuff tears utilizing an acellular human dermal reinforcement graft. Int J Shoulder Surg. 2012;6(2):36-44.
11. Beimers L, Lam PH, Murrell GA. The biomechanical effects of polytetrafluoroethylene suture augmentations in lateral-row rotator cuff repairs in an ovine model. J Shoulder Elbow Surg. 2014;23(10):1545-1552.
12. McCarron JA, Milks RA, Chen X, Iannotti JP, Derwin KA. Improved time-zero biomechanical properties using poly-L-lactic acid graft augmentation in a cadaveric rotator cuff repair model. J Shoulder Elbow Surg. 2010;19(5):688-696.
13. Proctor CS. Long-term successful arthroscopic repair of large and massive rotator cuff tears with a functional and degradable reinforcement device. J Shoulder Elbow Surg. 2014;23(10):1508-1513.
14. Misra SK, Valappil SP, Roy I, Boccaccini AR. Polyhydroxyalkanoate (PHA)/inorganic phase composites for tissue engineering applications. Biomacromolecules. 2006;7(8):2249-2258.
15. Ellis G, Cano P, Jadraque M, et al. Laser microperforated biodegradable microbial polyhydroxyalkanoate substrates for tissue repair strategies: an infrared microspectroscopy study. Anal Bioanal Chem. 2011;399(7):2379-2388.
16. Lenart BA, Martens KA, Kearns KA, Gillespie RJ, Zoga AC, Williams GR. Treatment of massive and recurrent rotator cuff tears augmented with a poly-l-lactide graft, a preliminary study. J Shoulder Elbow Surg. 2015;24(6):915-921.
17. Barber FA, Burns JP, Deutsch A, Labbé MR, Litchfield RB. A prospective, randomized evaluation of acellular human dermal matrix augmentation for arthroscopic rotator cuff repair. Arthroscopy. 2012;28(1):8-15.
18. Barber FA, Herbert MA, Boothby MH. Ultimate tensile failure loads of a human dermal allograft rotator cuff augmentation. Arthroscopy. 2008;24(1):20-24.
19. Gilot GJ, Attia AK, Alvarez AM. Arthroscopic repair of rotator cuff tears using extracellular matrix graft. Arthrosc Tech. 2014;3(4):e487-e489.
20. Barber FA, Coons DA, Ruiz-Suarez M. Cyclic load testing of biodegradable suture anchors containing 2 high-strength sutures. Arthroscopy. 2007;23(4):355-360.
21. Kullar RS, Reagan JM, Kolz CW, Burks RT, Henninger HB. Suture placement near the musculotendinous junction in the supraspinatus: implications for rotator cuff repair. Am J Sports Med. 2015;43(1):57-62.
22. Burkhart SS, Diaz Pagàn JL, Wirth MA, Athanasiou KA. Cyclic loading of anchor-based rotator cuff repairs: confirmation of the tension overload phenomenon and comparison of suture anchor fixation with transosseous fixation. Arthroscopy. 1997;13(6):720-724.
23. Faul F, Erdfelder E, Lang AG, Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39(2):175-191.
24. Greenspoon JA, Petri M, Warth RJ, Millett PJ. Massive rotator cuff tears: pathomechanics, current treatment options, and clinical outcomes. J Shoulder Elbow Surg. 2015;24(9):1493-1505.
25. Hein J, Reilly JM, Chae J, Maerz T, Anderson K. Retear rates after arthroscopic single-row, double-row, and suture bridge rotator cuff repair at a minimum of 1 year of imaging follow-up: a systematic review. Arthroscopy. 2015;31(11):2274-2281.
26. Henry P, Wasserstein D, Park S, et al. Arthroscopic repair for chronic massive rotator cuff tears: a systematic review. Arthroscopy. 2015;31(12):2472-2480.
27. El-Azab HM, Rott O, Irlenbusch U. Long-term follow-up after latissimus dorsi transfer for irreparable posterosuperior rotator cuff tears. J Bone Joint Surg Am. 2015;97(6):462-469.
28. Gerber C, Rahm SA, Catanzaro S, Farshad M, Moor BK. Latissimus dorsi tendon transfer for treatment of irreparable posterosuperior rotator cuff tears: long-term results at a minimum follow-up of ten years. J Bone Joint Surg Am. 2013;95(21):1920-1926.
29. Coons DA, Barber FA, Herbert MA. Triple-loaded single-anchor stitch configurations: an analysis of cyclically loaded suture–tendon interface security. Arthroscopy. 2006;22(11):1154-1158.
30. Itoi E, Berglund LJ, Grabowski JJ, et al. Tensile properties of the supraspinatus tendon. J Orthop Res. 1995;13(4):578-584.
31. Matsuhashi T, Hooke AW, Zhao KD, et al. Tensile properties of a morphologically split supraspinatus tendon. Clin Anat. 2014;27(5):702-706.
32. Apreleva M, Parsons IM 4th, Warner JJ, Fu FH, Woo SL. Experimental investigation of reaction forces at the glenohumeral joint during active abduction. J Shoulder Elbow Surg. 2000;9(5):409-417.
33. Giles JW, Ferreira LM, Athwal GS, Johnson JA. Development and performance evaluation of a multi-PID muscle loading driven in vitro active-motion shoulder simulator and application to assessing reverse total shoulder arthroplasty. J Biomech Eng. 2014;136(12):121007.
34. Hansen ML, Otis JC, Johnson JS, Cordasco FA, Craig EV, Warren RF. Biomechanics of massive rotator cuff tears: implications for treatment. J Bone Joint Surg Am. 2008;90(2):316-325.
35. Henninger HB, Barg A, Anderson AE, Bachus KN, Tashjian RZ, Burks RT. Effect of deltoid tension and humeral version in reverse total shoulder arthroplasty: a biomechanical study. J Shoulder Elbow Surg. 2012;21(4):483-490.
36. Mihata T, Gates J, McGarry MH, Lee J, Kinoshita M, Lee TQ. Effect of rotator cuff muscle imbalance on forceful internal impingement and peel-back of the superior labrum: a cadaveric study. Am J Sports Med. 2009;37(11):2222-2227.
37. Sano H, Ishii H, Yeadon A, Backman DS, Brunet JA, Uhthoff HK. Degeneration at the insertion weakens the tensile strength of the supraspinatus tendon: a comparative mechanical and histologic study of the bone–tendon complex. J Orthop Res. 1997;15(5):719-726.
Healing after rotator cuff repair (RCR) can be challenging, especially in cases of large and massive tears, revision repairs, and tendons with poor tissue quality.1-3 Poor tissue quality is associated with increased risk for recurrent tears, independent of age and tear size.3 Various techniques have been used to improve tendon fixation strength in these difficult situations, including augmented suture configurations (eg, massive cuff stitches, rip-stop stitches) and tissue grafts (eg, acellular dermal matrix).4-9 Clinical studies have found improved healing rates for larger tears and revision repairs using acellular dermal matrix grafts.6,10 Synthetic patches are another option for RCR augmentation, but limited clinical data and biomechanical evidence support use of synthetic grafts as an augment for RCRs.11-13
Polyhydroxyalkanoates (PHAs) are a class of biodegradable polymers that have been used as orthopedic devices, tissue scaffolds, patches, and other applications with increasing frequency over the past decade.14 In the laboratory, these implanted materials have been shown to support cell migration and growth.15 The PHA family of polymers typically degrades by hydrolytic and bacterial depolymerase mechanisms over 52-plus weeks in vivo.14PHA grafts have been studied in the setting of RCR. An expanded polytetrafluoroethylene scaffold was shown to improve repair mechanics when used as a bursal side graft in an in vitro ovine model.11 The graft increased tendon footprint contact pressure and failure loads by almost 180 N. In clinical studies, poly-L-lactic acid augmentations have been used to reinforce massive RCRs. Lenart and colleagues16 found that 38% of 16 patients with such tears had an intact rotator cuff at 1.2-year follow-up, and improvement in clinical scores. Proctor13 reported on use of a poly-L-lactic acid retrograde patch for reinforcement of massive tears with both single- and double-row repairs in 18 patients. The cohort had more favorable rates of intact cuffs at 12 months (83%) and 42 months (78%), and ASES (American Shoulder and Elbow Surgeons) scores improved from 25 before surgery to 82 at latest follow-up after surgery.
RCR augmentation traditionally has been performed with an open or mini-open technique.6 Recently, several authors have reported on arthroscopic techniques for augmentation with either acellular dermal matrix or synthetic grafts.13,17,18 Most techniques have involved “bridging” with a graft or patch used to stress-shield a single-row repair.8,9,13 This bridging typically involves placing several sutures medial to where the anchor repair stitches pass through the tendon. An alternative is to pass the repair stitches through both the tendon and the graft.17-19 The overall volume of tissue incorporated into the repair stitches (rotator cuff plus graft) is increased with the augmented technique relative to the bridging technique. Both can be technically challenging, but the augmented technique may be easier to perform arthroscopically.9,19 Regardless, these techniques are complicated and require a higher level of arthroscopic skills compared with those required in arthroscopic RCR without a graft. Simplifying arthroscopic graft augmentation likely will increase its utility because, even for skilled surgeons, adding a graft can increase operative time by 20 to 30 minutes. Simplification will also extend use of the technique to surgeons with less experience and proficiency with arthroscopic repair.
We developed a simple method for augmenting single-row RCR with a strip of bioresorbable soft-tissue scaffold. We also conducted a study to evaluate the initial biomechanical properties of single-row RCR in cadaveric shoulder specimens augmented with PHA mesh (BioFiber; Tornier) graft as compared with single-row RCR without augmentation. Both cyclic gap formation and ultimate failure loads and displacement were quantified. We hypothesized that the augmented RCRs would have decreased gap formation and increased ultimate failure loads compared with nonaugmented RCRs. This study was exempt from having to obtain Institutional Review B
Methods
Eight pairs of fresh-frozen cadaver humeri (6 male, 2 female; mean [SD] age, 61 [9] years) were dissected of all soft tissue (except rotator cuff) by Dr. Tashjian, a board-certified, fellowship-trained orthopedic surgeon. There were no qualitative differences in tendon condition between tendons within a pair. The supraspinatus muscle and tendon were separated from the other rotator cuff muscles. The infraspinatus, subscapularis, and teres minor were removed from the humerus. Last, the supraspinatus was resected at its insertion. Humeral pairs were then randomized into augmented and nonaugmented RCRs within each pair.
In the nonaugmented group, the supraspinatus was reattached to its insertion in a single-row RCR with 2 triple-loaded suture anchors (5.5-mm Insite FT Ti, No. 2 Force Fiber suture; Tornier) and 6 simple stitches (Figure 1A). Anchors were placed midway between the articular margin and the lateral edge of the greater tuberosity at about 45° to the bone surface.
In the contralateral shoulders, augmented RCRs were performed. Specimens were prepared exactly as they were for the nonaugmented RCRs, including anchor placement and suture passage. Before knot tying, RCRs were augmented with 2 strips of 13-mm × 23-mm PHA mesh (BioFiber) (Figure 1B). One strip was used to augment the 3 sutures of each anchor, overlying the residual tendon, to reinforce the tendon–knot interface. After each suture was passed through the supraspinatus tendon from the intra-articular surface, the stitch was passed through the strip of PHA mesh. Stitches were separated by 5 mm in each mesh strip. All 6 sutures were then tied with a Revo knot between the free end of each suture leg and the leg that passed through the tendon and mesh.
Each humerus was transected at the midshaft and potted and mounted in an Instron 1331 load frame with Model 8800 controller (Instron). A cryoclamp was used to grasp the supraspinatus muscle belly above the musculotendinous junction (Figure 2).
Three rows of 2-mm fiducial markers were affixed to the bone, tendon, and muscle belly with cyanoacrylate for tracking with a digital video system (DMAS Version 6.5; Spicatek) (Figure 3).21
A 0.1-MPa pre-stress (applied force/tendon cross-sectional area) was applied to each construct to determine the starting position for the deformation profile. Each repair underwent 1000 cycles of uniaxial load-controlled displacement between 0.1 and 1.0 MPa of effective stress at 1 Hz. Effective stress was determined as the ratio of applied force to cross-sectional area of the tendon at harvest to normalize the applied loads between tendons of varying size. During cyclic testing, gapping of more than 5 mm was defined as construct failure.22 After cyclic loading, each construct was loaded to failure at 1.0 mm/s. Ultimate failure load was defined as the highest load achieved at the maximum displacement before rapid decline in load supported by the construct.
Statistical Analysis
Paired t tests were used to compare the matched pairs of constructs. For all tests, significance was set at P ≤ .05. Post hoc power was calculated for significant results using G*Power Version 3.1.6.23 All data are presented as means (SDs).
Results
After 1000 cycles of displacement, mean (SD) gapping was 3.8 (0.9) mm for the nonaugmented repairs and 3.9 (1.1) mm for the PHA mesh–augmented repairs (P = .879) (Figure 4).
For the nonaugmented repairs, mean (SD) failure displacement was 6.3 (1.7) mm, and mean (SD) ultimate failure load was 472.1 (120.3) N. For the PHA-augmented repairs, failure displacement was 5.5 (1.9) mm, and ultimate failure load was 571.2 (173.0) N. There was no difference in failure displacement (P = .393), but there was a difference in ultimate failure load (P = .042; power = 0.57). During failure testing, mean (SD) tissue deformation was higher (P = .012; power = 0.83) for the PHA-augmented repairs, 1.2 (0.7) mm, than for the nonaugmented repairs, 0.8 (0.5) mm. Failures, which were consistent within pairs, were caused by tissue failure, with sutures pulling through the tissue (4 pairs) or single anchor pullout before ultimate tissue failure (4 pairs). Of the 4 failures with anchor pullout, 3 had anterior anchor pullout, and 1 had posterior anchor pullout. In all specimens with anchor pullout, the second anchor remained stable, and ultimate failure occurred with tissue tearing at the suture interface. There were no significant differences in any metrics between specimens that failed with intact anchors and specimens with single anchor pullout (P ≥ .122). Therefore, both groups were pooled for the failure analysis.
Discussion
RCR augmentation with a synthetic graft is a viable option for improving fixation strength of supraspinatus repairs, as shown in otherwise healthy tendon in the present study. Our hypothesis that there would be decreased gap formation with graft augmentation was not supported, whereas the hypothesis of increased failure loads with graft augmentation was supported. These findings may also be applicable in cases of large tears, revisions, and tendons with poor tissue quality. Simplification of graft application techniques will allow quick and easy arthroscopic augmentation.
Studies of RCRs for large or massive tears have reported retear rates of 25% to 79%.24-26 Latissimus dorsi tendon transfers also show promise in posterosuperior RCRs, with failure rates near 10%.27,28 Although use of PHA patches in RCR augmentation is relatively new, short-term and midterm failure rates are in the range of 20% to 60% in the few small cohorts currently being studied.13,16 It is possible that these rates may improve as indications, surgical experience, and techniques for use of PHA patches are further refined. Regardless, with PHA currently being used in practice, it is important to quantify the biomechanics of the augmentation as a baseline for its performance in reinforcing the tendon–suture interface.
We determined that the initial fixation strength of single-row repairs was higher with the addition of PHA synthetic grafts using a very simple technique. Single-row triple-loaded anchor repairs already provide high initial mechanical strength, and our results are similar to those of another study of this technique.29 Despite the already high mechanical strength of a triple-loaded anchor repair, PHA mesh increased ultimate strength by about 100 N (~25%). Of note, tissue elongation during failure was higher (P = .012; power = 0.83) in the PHA-augmented group (1.2 mm) than in the nonaugmented group (0.8 mm). This was not surprising—failure loads were almost 100 N higher in the PHA-augmented group than in the nonaugmented group. Consequently, much higher forces were placed on the muscle belly, likely resulting in additional elongation of the intact tissue medial to the repair construct.
The ultimate failure loads in our study compare favorably with the biomechanical strength of augmented repairs reported by others.8,9,18 Barber and colleagues18 evaluated an augmented single-row repair with 2 double-loaded suture anchors and an acellular dermal matrix graft. The ultimate failure load of the augmented repairs was 325 N. In contrast, Omae and colleagues8 tested a bridging single-row repair using 2 double-loaded suture anchors and an acellular dermal matrix graft. Ultimate failure load of the augmented repairs was 560 N, similar to our finding. Last, Shea and colleagues9 evaluated a bridging single-row repair using 2 double-loaded suture anchors and an acellular dermal matrix graft, with ultimate failure load of 429 N. The techniques in all 3 studies can be performed arthroscopically but are challenging and require multiple extra sutures and anchors that need management and tying. Our technique provides similar initial fixation strength, has no requirement for extra sutures or anchors, and is very simple to perform.
The supraspinatus tendon is estimated to fail between 800 N and 1000 N.30,31 Biomechanical shoulder simulators use supraspinatus forces in the range of 20 N to 200 N for scapular plane abduction.32-36 Therefore, the single-row repair failures in our study fell between functional and full-thickness failure loads. Studies on the mechanics of degenerated human supraspinatus tendon are limited, but there is evidence the mechanical properties of these tissues are inferior to those of healthy tendon.37 A 100-N increase in failure loads with PHA augmentation may prove highly significant in reinforcing the suture–tendon interface in degenerated tendons.
Adding the mesh did not have any effect on gapping at the repair site after cyclic loading. This finding suggests that construct gapping under cyclic loading is not a function of a reinforced knot–tendon interface but is instead caused by microtearing and cinching of the suture constructs in relation to the underlying bone. Tissue elongation likely was not a strong contributor to overall cyclic gapping, as elongation did not differ between the nonaugmented and augmented repairs (0.5 mm vs 0.7 mm; P = .276) and was small relative to the nearly 4 mm of construct gapping. Gapping may be affected by healing and integration of the mesh into the repaired tendon over time, but this effect could not be captured in the present study. Patients are initially immobilized and passive shoulder motion gradually introduced, in stark contrast to the immediate loading protocol in the present study. Regardless, the 25% increase in overall strength may be clinically important, especially in cases of difficult repair or poor tissue quality.
Our technique simplifies arthroscopic augmentation—stitches are passed through the rotator cuff in simple fashion. Before being tied, the limbs that were passed through the rotator cuff are removed through a cannula and then passed through the synthetic graft.
Study Limitations
This study had several limitations. First, it was a cadaveric biomechanical study that evaluated only time-zero biomechanical properties. Loads were normalized to tendon size, specimens were randomized between sides, and paired specimens were used to minimize the effects of tendon and bone quality on outcome metrics. In addition, donor tendons were representative of otherwise healthy tissue. Chronic tears and associated resorption/atrophy could have affected the magnitude of forces and gapping detected in this study. Theoretically, over time the tendon tissue will adhere to and grow into the mesh, which could minimize potential differences. Studies are needed to determine the effects of healing on long-term repair strength in affected patients. Last, all constructs were performed in open fashion to improve repeatability of construct placement and provide accessibility for Instron testing. Our technique did not directly replicate the arthroscopic approach, but, unlike other augmentation techniques, it is so simple that transition to all-arthroscopic augmentation is realistic.
Patch augmentation increases the cost of materials and operative time and should be considered a limitation of its utility. We do not recommend augmentation in all RCRs, as it likely is cost-ineffective. Instead, we recommend augmentation in cases of poor tissue quality, which could lead to healing failure, revision surgery, and higher overall patient costs beyond the cost of adding augmentation. Similarly, we recommend augmentation for revision cases in which tendon healing has failed and tissue quality is poor. The goal is to prevent another failure.
Conclusion
PHA graft augmentation of single-row triple-loaded anchor repairs of the supraspinatus tendon improves the overall ultimate load to failure by 25%. There was no difference in gap formation after cyclic loading between augmented and nonaugmented repairs. This technique for arthroscopic augmentation can be used to improve initial biomechanical repair strength in tears at risk for failure.
Am J Orthop. 2016;45(7):E527-E533. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
Healing after rotator cuff repair (RCR) can be challenging, especially in cases of large and massive tears, revision repairs, and tendons with poor tissue quality.1-3 Poor tissue quality is associated with increased risk for recurrent tears, independent of age and tear size.3 Various techniques have been used to improve tendon fixation strength in these difficult situations, including augmented suture configurations (eg, massive cuff stitches, rip-stop stitches) and tissue grafts (eg, acellular dermal matrix).4-9 Clinical studies have found improved healing rates for larger tears and revision repairs using acellular dermal matrix grafts.6,10 Synthetic patches are another option for RCR augmentation, but limited clinical data and biomechanical evidence support use of synthetic grafts as an augment for RCRs.11-13
Polyhydroxyalkanoates (PHAs) are a class of biodegradable polymers that have been used as orthopedic devices, tissue scaffolds, patches, and other applications with increasing frequency over the past decade.14 In the laboratory, these implanted materials have been shown to support cell migration and growth.15 The PHA family of polymers typically degrades by hydrolytic and bacterial depolymerase mechanisms over 52-plus weeks in vivo.14PHA grafts have been studied in the setting of RCR. An expanded polytetrafluoroethylene scaffold was shown to improve repair mechanics when used as a bursal side graft in an in vitro ovine model.11 The graft increased tendon footprint contact pressure and failure loads by almost 180 N. In clinical studies, poly-L-lactic acid augmentations have been used to reinforce massive RCRs. Lenart and colleagues16 found that 38% of 16 patients with such tears had an intact rotator cuff at 1.2-year follow-up, and improvement in clinical scores. Proctor13 reported on use of a poly-L-lactic acid retrograde patch for reinforcement of massive tears with both single- and double-row repairs in 18 patients. The cohort had more favorable rates of intact cuffs at 12 months (83%) and 42 months (78%), and ASES (American Shoulder and Elbow Surgeons) scores improved from 25 before surgery to 82 at latest follow-up after surgery.
RCR augmentation traditionally has been performed with an open or mini-open technique.6 Recently, several authors have reported on arthroscopic techniques for augmentation with either acellular dermal matrix or synthetic grafts.13,17,18 Most techniques have involved “bridging” with a graft or patch used to stress-shield a single-row repair.8,9,13 This bridging typically involves placing several sutures medial to where the anchor repair stitches pass through the tendon. An alternative is to pass the repair stitches through both the tendon and the graft.17-19 The overall volume of tissue incorporated into the repair stitches (rotator cuff plus graft) is increased with the augmented technique relative to the bridging technique. Both can be technically challenging, but the augmented technique may be easier to perform arthroscopically.9,19 Regardless, these techniques are complicated and require a higher level of arthroscopic skills compared with those required in arthroscopic RCR without a graft. Simplifying arthroscopic graft augmentation likely will increase its utility because, even for skilled surgeons, adding a graft can increase operative time by 20 to 30 minutes. Simplification will also extend use of the technique to surgeons with less experience and proficiency with arthroscopic repair.
We developed a simple method for augmenting single-row RCR with a strip of bioresorbable soft-tissue scaffold. We also conducted a study to evaluate the initial biomechanical properties of single-row RCR in cadaveric shoulder specimens augmented with PHA mesh (BioFiber; Tornier) graft as compared with single-row RCR without augmentation. Both cyclic gap formation and ultimate failure loads and displacement were quantified. We hypothesized that the augmented RCRs would have decreased gap formation and increased ultimate failure loads compared with nonaugmented RCRs. This study was exempt from having to obtain Institutional Review B
Methods
Eight pairs of fresh-frozen cadaver humeri (6 male, 2 female; mean [SD] age, 61 [9] years) were dissected of all soft tissue (except rotator cuff) by Dr. Tashjian, a board-certified, fellowship-trained orthopedic surgeon. There were no qualitative differences in tendon condition between tendons within a pair. The supraspinatus muscle and tendon were separated from the other rotator cuff muscles. The infraspinatus, subscapularis, and teres minor were removed from the humerus. Last, the supraspinatus was resected at its insertion. Humeral pairs were then randomized into augmented and nonaugmented RCRs within each pair.
In the nonaugmented group, the supraspinatus was reattached to its insertion in a single-row RCR with 2 triple-loaded suture anchors (5.5-mm Insite FT Ti, No. 2 Force Fiber suture; Tornier) and 6 simple stitches (Figure 1A). Anchors were placed midway between the articular margin and the lateral edge of the greater tuberosity at about 45° to the bone surface.
In the contralateral shoulders, augmented RCRs were performed. Specimens were prepared exactly as they were for the nonaugmented RCRs, including anchor placement and suture passage. Before knot tying, RCRs were augmented with 2 strips of 13-mm × 23-mm PHA mesh (BioFiber) (Figure 1B). One strip was used to augment the 3 sutures of each anchor, overlying the residual tendon, to reinforce the tendon–knot interface. After each suture was passed through the supraspinatus tendon from the intra-articular surface, the stitch was passed through the strip of PHA mesh. Stitches were separated by 5 mm in each mesh strip. All 6 sutures were then tied with a Revo knot between the free end of each suture leg and the leg that passed through the tendon and mesh.
Each humerus was transected at the midshaft and potted and mounted in an Instron 1331 load frame with Model 8800 controller (Instron). A cryoclamp was used to grasp the supraspinatus muscle belly above the musculotendinous junction (Figure 2).
Three rows of 2-mm fiducial markers were affixed to the bone, tendon, and muscle belly with cyanoacrylate for tracking with a digital video system (DMAS Version 6.5; Spicatek) (Figure 3).21
A 0.1-MPa pre-stress (applied force/tendon cross-sectional area) was applied to each construct to determine the starting position for the deformation profile. Each repair underwent 1000 cycles of uniaxial load-controlled displacement between 0.1 and 1.0 MPa of effective stress at 1 Hz. Effective stress was determined as the ratio of applied force to cross-sectional area of the tendon at harvest to normalize the applied loads between tendons of varying size. During cyclic testing, gapping of more than 5 mm was defined as construct failure.22 After cyclic loading, each construct was loaded to failure at 1.0 mm/s. Ultimate failure load was defined as the highest load achieved at the maximum displacement before rapid decline in load supported by the construct.
Statistical Analysis
Paired t tests were used to compare the matched pairs of constructs. For all tests, significance was set at P ≤ .05. Post hoc power was calculated for significant results using G*Power Version 3.1.6.23 All data are presented as means (SDs).
Results
After 1000 cycles of displacement, mean (SD) gapping was 3.8 (0.9) mm for the nonaugmented repairs and 3.9 (1.1) mm for the PHA mesh–augmented repairs (P = .879) (Figure 4).
For the nonaugmented repairs, mean (SD) failure displacement was 6.3 (1.7) mm, and mean (SD) ultimate failure load was 472.1 (120.3) N. For the PHA-augmented repairs, failure displacement was 5.5 (1.9) mm, and ultimate failure load was 571.2 (173.0) N. There was no difference in failure displacement (P = .393), but there was a difference in ultimate failure load (P = .042; power = 0.57). During failure testing, mean (SD) tissue deformation was higher (P = .012; power = 0.83) for the PHA-augmented repairs, 1.2 (0.7) mm, than for the nonaugmented repairs, 0.8 (0.5) mm. Failures, which were consistent within pairs, were caused by tissue failure, with sutures pulling through the tissue (4 pairs) or single anchor pullout before ultimate tissue failure (4 pairs). Of the 4 failures with anchor pullout, 3 had anterior anchor pullout, and 1 had posterior anchor pullout. In all specimens with anchor pullout, the second anchor remained stable, and ultimate failure occurred with tissue tearing at the suture interface. There were no significant differences in any metrics between specimens that failed with intact anchors and specimens with single anchor pullout (P ≥ .122). Therefore, both groups were pooled for the failure analysis.
Discussion
RCR augmentation with a synthetic graft is a viable option for improving fixation strength of supraspinatus repairs, as shown in otherwise healthy tendon in the present study. Our hypothesis that there would be decreased gap formation with graft augmentation was not supported, whereas the hypothesis of increased failure loads with graft augmentation was supported. These findings may also be applicable in cases of large tears, revisions, and tendons with poor tissue quality. Simplification of graft application techniques will allow quick and easy arthroscopic augmentation.
Studies of RCRs for large or massive tears have reported retear rates of 25% to 79%.24-26 Latissimus dorsi tendon transfers also show promise in posterosuperior RCRs, with failure rates near 10%.27,28 Although use of PHA patches in RCR augmentation is relatively new, short-term and midterm failure rates are in the range of 20% to 60% in the few small cohorts currently being studied.13,16 It is possible that these rates may improve as indications, surgical experience, and techniques for use of PHA patches are further refined. Regardless, with PHA currently being used in practice, it is important to quantify the biomechanics of the augmentation as a baseline for its performance in reinforcing the tendon–suture interface.
We determined that the initial fixation strength of single-row repairs was higher with the addition of PHA synthetic grafts using a very simple technique. Single-row triple-loaded anchor repairs already provide high initial mechanical strength, and our results are similar to those of another study of this technique.29 Despite the already high mechanical strength of a triple-loaded anchor repair, PHA mesh increased ultimate strength by about 100 N (~25%). Of note, tissue elongation during failure was higher (P = .012; power = 0.83) in the PHA-augmented group (1.2 mm) than in the nonaugmented group (0.8 mm). This was not surprising—failure loads were almost 100 N higher in the PHA-augmented group than in the nonaugmented group. Consequently, much higher forces were placed on the muscle belly, likely resulting in additional elongation of the intact tissue medial to the repair construct.
The ultimate failure loads in our study compare favorably with the biomechanical strength of augmented repairs reported by others.8,9,18 Barber and colleagues18 evaluated an augmented single-row repair with 2 double-loaded suture anchors and an acellular dermal matrix graft. The ultimate failure load of the augmented repairs was 325 N. In contrast, Omae and colleagues8 tested a bridging single-row repair using 2 double-loaded suture anchors and an acellular dermal matrix graft. Ultimate failure load of the augmented repairs was 560 N, similar to our finding. Last, Shea and colleagues9 evaluated a bridging single-row repair using 2 double-loaded suture anchors and an acellular dermal matrix graft, with ultimate failure load of 429 N. The techniques in all 3 studies can be performed arthroscopically but are challenging and require multiple extra sutures and anchors that need management and tying. Our technique provides similar initial fixation strength, has no requirement for extra sutures or anchors, and is very simple to perform.
The supraspinatus tendon is estimated to fail between 800 N and 1000 N.30,31 Biomechanical shoulder simulators use supraspinatus forces in the range of 20 N to 200 N for scapular plane abduction.32-36 Therefore, the single-row repair failures in our study fell between functional and full-thickness failure loads. Studies on the mechanics of degenerated human supraspinatus tendon are limited, but there is evidence the mechanical properties of these tissues are inferior to those of healthy tendon.37 A 100-N increase in failure loads with PHA augmentation may prove highly significant in reinforcing the suture–tendon interface in degenerated tendons.
Adding the mesh did not have any effect on gapping at the repair site after cyclic loading. This finding suggests that construct gapping under cyclic loading is not a function of a reinforced knot–tendon interface but is instead caused by microtearing and cinching of the suture constructs in relation to the underlying bone. Tissue elongation likely was not a strong contributor to overall cyclic gapping, as elongation did not differ between the nonaugmented and augmented repairs (0.5 mm vs 0.7 mm; P = .276) and was small relative to the nearly 4 mm of construct gapping. Gapping may be affected by healing and integration of the mesh into the repaired tendon over time, but this effect could not be captured in the present study. Patients are initially immobilized and passive shoulder motion gradually introduced, in stark contrast to the immediate loading protocol in the present study. Regardless, the 25% increase in overall strength may be clinically important, especially in cases of difficult repair or poor tissue quality.
Our technique simplifies arthroscopic augmentation—stitches are passed through the rotator cuff in simple fashion. Before being tied, the limbs that were passed through the rotator cuff are removed through a cannula and then passed through the synthetic graft.
Study Limitations
This study had several limitations. First, it was a cadaveric biomechanical study that evaluated only time-zero biomechanical properties. Loads were normalized to tendon size, specimens were randomized between sides, and paired specimens were used to minimize the effects of tendon and bone quality on outcome metrics. In addition, donor tendons were representative of otherwise healthy tissue. Chronic tears and associated resorption/atrophy could have affected the magnitude of forces and gapping detected in this study. Theoretically, over time the tendon tissue will adhere to and grow into the mesh, which could minimize potential differences. Studies are needed to determine the effects of healing on long-term repair strength in affected patients. Last, all constructs were performed in open fashion to improve repeatability of construct placement and provide accessibility for Instron testing. Our technique did not directly replicate the arthroscopic approach, but, unlike other augmentation techniques, it is so simple that transition to all-arthroscopic augmentation is realistic.
Patch augmentation increases the cost of materials and operative time and should be considered a limitation of its utility. We do not recommend augmentation in all RCRs, as it likely is cost-ineffective. Instead, we recommend augmentation in cases of poor tissue quality, which could lead to healing failure, revision surgery, and higher overall patient costs beyond the cost of adding augmentation. Similarly, we recommend augmentation for revision cases in which tendon healing has failed and tissue quality is poor. The goal is to prevent another failure.
Conclusion
PHA graft augmentation of single-row triple-loaded anchor repairs of the supraspinatus tendon improves the overall ultimate load to failure by 25%. There was no difference in gap formation after cyclic loading between augmented and nonaugmented repairs. This technique for arthroscopic augmentation can be used to improve initial biomechanical repair strength in tears at risk for failure.
Am J Orthop. 2016;45(7):E527-E533. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Galatz LM, Ball CM, Teefey SA, Middleton WD, Yamaguchi K. The outcome and repair integrity of completely arthroscopically repaired large and massive rotator cuff tears. J Bone Joint Surg Am. 2004;86(2):219-224.
2. Keener JD, Wei AS, Kim HM, et al. Revision arthroscopic rotator cuff repair: repair integrity and clinical outcome. J Bone Joint Surg Am. 2010;92(3):590-598.
3. Nho SJ, Brown BS, Lyman S, Adler RS, Altchek DW, MacGillivray JD. Prospective analysis of arthroscopic rotator cuff repair: prognostic factors affecting clinical and ultrasound outcome. J Shoulder Elbow Surg. 2009;18(1):13-20.
4. Barber FA, Herbert MA, Schroeder FA, Aziz-Jacobo J, Mays MM, Rapley JH. Biomechanical advantages of triple-loaded suture anchors compared with double-row rotator cuff repairs. Arthroscopy. 2010;26(3):316-323.
5. Burkhart SS, Denard PJ, Konicek J, Hanypsiak BT. Biomechanical validation of load-sharing rip-stop fixation for the repair of tissue-deficient rotator cuff tears. Am J Sports Med. 2014;42(2):457-462.
6. Gupta AK, Hug K, Boggess B, Gavigan M, Toth AP. Massive or 2-tendon rotator cuff tears in active patients with minimal glenohumeral arthritis: clinical and radiographic outcomes of reconstruction using dermal tissue matrix xenograft. Am J Sports Med. 2013;41(4):872-879.
7. Ma CB, MacGillivray JD, Clabeaux J, Lee S, Otis JC. Biomechanical evaluation of arthroscopic rotator cuff stitches. J Bone Joint Surg Am. 2004;86(6):1211-1216.
8. Omae H, Steinmann SP, Zhao C, et al. Biomechanical effect of rotator cuff augmentation with an acellular dermal matrix graft: a cadaver study. Clin Biomech. 2012;27(8):789-792.
9. Shea KP, Obopilwe E, Sperling JW, Iannotti JP. A biomechanical analysis of gap formation and failure mechanics of a xenograft-reinforced rotator cuff repair in a cadaveric model. J Shoulder Elbow Surg. 2012;21(8):1072-1079.
10. Agrawal V. Healing rates for challenging rotator cuff tears utilizing an acellular human dermal reinforcement graft. Int J Shoulder Surg. 2012;6(2):36-44.
11. Beimers L, Lam PH, Murrell GA. The biomechanical effects of polytetrafluoroethylene suture augmentations in lateral-row rotator cuff repairs in an ovine model. J Shoulder Elbow Surg. 2014;23(10):1545-1552.
12. McCarron JA, Milks RA, Chen X, Iannotti JP, Derwin KA. Improved time-zero biomechanical properties using poly-L-lactic acid graft augmentation in a cadaveric rotator cuff repair model. J Shoulder Elbow Surg. 2010;19(5):688-696.
13. Proctor CS. Long-term successful arthroscopic repair of large and massive rotator cuff tears with a functional and degradable reinforcement device. J Shoulder Elbow Surg. 2014;23(10):1508-1513.
14. Misra SK, Valappil SP, Roy I, Boccaccini AR. Polyhydroxyalkanoate (PHA)/inorganic phase composites for tissue engineering applications. Biomacromolecules. 2006;7(8):2249-2258.
15. Ellis G, Cano P, Jadraque M, et al. Laser microperforated biodegradable microbial polyhydroxyalkanoate substrates for tissue repair strategies: an infrared microspectroscopy study. Anal Bioanal Chem. 2011;399(7):2379-2388.
16. Lenart BA, Martens KA, Kearns KA, Gillespie RJ, Zoga AC, Williams GR. Treatment of massive and recurrent rotator cuff tears augmented with a poly-l-lactide graft, a preliminary study. J Shoulder Elbow Surg. 2015;24(6):915-921.
17. Barber FA, Burns JP, Deutsch A, Labbé MR, Litchfield RB. A prospective, randomized evaluation of acellular human dermal matrix augmentation for arthroscopic rotator cuff repair. Arthroscopy. 2012;28(1):8-15.
18. Barber FA, Herbert MA, Boothby MH. Ultimate tensile failure loads of a human dermal allograft rotator cuff augmentation. Arthroscopy. 2008;24(1):20-24.
19. Gilot GJ, Attia AK, Alvarez AM. Arthroscopic repair of rotator cuff tears using extracellular matrix graft. Arthrosc Tech. 2014;3(4):e487-e489.
20. Barber FA, Coons DA, Ruiz-Suarez M. Cyclic load testing of biodegradable suture anchors containing 2 high-strength sutures. Arthroscopy. 2007;23(4):355-360.
21. Kullar RS, Reagan JM, Kolz CW, Burks RT, Henninger HB. Suture placement near the musculotendinous junction in the supraspinatus: implications for rotator cuff repair. Am J Sports Med. 2015;43(1):57-62.
22. Burkhart SS, Diaz Pagàn JL, Wirth MA, Athanasiou KA. Cyclic loading of anchor-based rotator cuff repairs: confirmation of the tension overload phenomenon and comparison of suture anchor fixation with transosseous fixation. Arthroscopy. 1997;13(6):720-724.
23. Faul F, Erdfelder E, Lang AG, Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39(2):175-191.
24. Greenspoon JA, Petri M, Warth RJ, Millett PJ. Massive rotator cuff tears: pathomechanics, current treatment options, and clinical outcomes. J Shoulder Elbow Surg. 2015;24(9):1493-1505.
25. Hein J, Reilly JM, Chae J, Maerz T, Anderson K. Retear rates after arthroscopic single-row, double-row, and suture bridge rotator cuff repair at a minimum of 1 year of imaging follow-up: a systematic review. Arthroscopy. 2015;31(11):2274-2281.
26. Henry P, Wasserstein D, Park S, et al. Arthroscopic repair for chronic massive rotator cuff tears: a systematic review. Arthroscopy. 2015;31(12):2472-2480.
27. El-Azab HM, Rott O, Irlenbusch U. Long-term follow-up after latissimus dorsi transfer for irreparable posterosuperior rotator cuff tears. J Bone Joint Surg Am. 2015;97(6):462-469.
28. Gerber C, Rahm SA, Catanzaro S, Farshad M, Moor BK. Latissimus dorsi tendon transfer for treatment of irreparable posterosuperior rotator cuff tears: long-term results at a minimum follow-up of ten years. J Bone Joint Surg Am. 2013;95(21):1920-1926.
29. Coons DA, Barber FA, Herbert MA. Triple-loaded single-anchor stitch configurations: an analysis of cyclically loaded suture–tendon interface security. Arthroscopy. 2006;22(11):1154-1158.
30. Itoi E, Berglund LJ, Grabowski JJ, et al. Tensile properties of the supraspinatus tendon. J Orthop Res. 1995;13(4):578-584.
31. Matsuhashi T, Hooke AW, Zhao KD, et al. Tensile properties of a morphologically split supraspinatus tendon. Clin Anat. 2014;27(5):702-706.
32. Apreleva M, Parsons IM 4th, Warner JJ, Fu FH, Woo SL. Experimental investigation of reaction forces at the glenohumeral joint during active abduction. J Shoulder Elbow Surg. 2000;9(5):409-417.
33. Giles JW, Ferreira LM, Athwal GS, Johnson JA. Development and performance evaluation of a multi-PID muscle loading driven in vitro active-motion shoulder simulator and application to assessing reverse total shoulder arthroplasty. J Biomech Eng. 2014;136(12):121007.
34. Hansen ML, Otis JC, Johnson JS, Cordasco FA, Craig EV, Warren RF. Biomechanics of massive rotator cuff tears: implications for treatment. J Bone Joint Surg Am. 2008;90(2):316-325.
35. Henninger HB, Barg A, Anderson AE, Bachus KN, Tashjian RZ, Burks RT. Effect of deltoid tension and humeral version in reverse total shoulder arthroplasty: a biomechanical study. J Shoulder Elbow Surg. 2012;21(4):483-490.
36. Mihata T, Gates J, McGarry MH, Lee J, Kinoshita M, Lee TQ. Effect of rotator cuff muscle imbalance on forceful internal impingement and peel-back of the superior labrum: a cadaveric study. Am J Sports Med. 2009;37(11):2222-2227.
37. Sano H, Ishii H, Yeadon A, Backman DS, Brunet JA, Uhthoff HK. Degeneration at the insertion weakens the tensile strength of the supraspinatus tendon: a comparative mechanical and histologic study of the bone–tendon complex. J Orthop Res. 1997;15(5):719-726.
1. Galatz LM, Ball CM, Teefey SA, Middleton WD, Yamaguchi K. The outcome and repair integrity of completely arthroscopically repaired large and massive rotator cuff tears. J Bone Joint Surg Am. 2004;86(2):219-224.
2. Keener JD, Wei AS, Kim HM, et al. Revision arthroscopic rotator cuff repair: repair integrity and clinical outcome. J Bone Joint Surg Am. 2010;92(3):590-598.
3. Nho SJ, Brown BS, Lyman S, Adler RS, Altchek DW, MacGillivray JD. Prospective analysis of arthroscopic rotator cuff repair: prognostic factors affecting clinical and ultrasound outcome. J Shoulder Elbow Surg. 2009;18(1):13-20.
4. Barber FA, Herbert MA, Schroeder FA, Aziz-Jacobo J, Mays MM, Rapley JH. Biomechanical advantages of triple-loaded suture anchors compared with double-row rotator cuff repairs. Arthroscopy. 2010;26(3):316-323.
5. Burkhart SS, Denard PJ, Konicek J, Hanypsiak BT. Biomechanical validation of load-sharing rip-stop fixation for the repair of tissue-deficient rotator cuff tears. Am J Sports Med. 2014;42(2):457-462.
6. Gupta AK, Hug K, Boggess B, Gavigan M, Toth AP. Massive or 2-tendon rotator cuff tears in active patients with minimal glenohumeral arthritis: clinical and radiographic outcomes of reconstruction using dermal tissue matrix xenograft. Am J Sports Med. 2013;41(4):872-879.
7. Ma CB, MacGillivray JD, Clabeaux J, Lee S, Otis JC. Biomechanical evaluation of arthroscopic rotator cuff stitches. J Bone Joint Surg Am. 2004;86(6):1211-1216.
8. Omae H, Steinmann SP, Zhao C, et al. Biomechanical effect of rotator cuff augmentation with an acellular dermal matrix graft: a cadaver study. Clin Biomech. 2012;27(8):789-792.
9. Shea KP, Obopilwe E, Sperling JW, Iannotti JP. A biomechanical analysis of gap formation and failure mechanics of a xenograft-reinforced rotator cuff repair in a cadaveric model. J Shoulder Elbow Surg. 2012;21(8):1072-1079.
10. Agrawal V. Healing rates for challenging rotator cuff tears utilizing an acellular human dermal reinforcement graft. Int J Shoulder Surg. 2012;6(2):36-44.
11. Beimers L, Lam PH, Murrell GA. The biomechanical effects of polytetrafluoroethylene suture augmentations in lateral-row rotator cuff repairs in an ovine model. J Shoulder Elbow Surg. 2014;23(10):1545-1552.
12. McCarron JA, Milks RA, Chen X, Iannotti JP, Derwin KA. Improved time-zero biomechanical properties using poly-L-lactic acid graft augmentation in a cadaveric rotator cuff repair model. J Shoulder Elbow Surg. 2010;19(5):688-696.
13. Proctor CS. Long-term successful arthroscopic repair of large and massive rotator cuff tears with a functional and degradable reinforcement device. J Shoulder Elbow Surg. 2014;23(10):1508-1513.
14. Misra SK, Valappil SP, Roy I, Boccaccini AR. Polyhydroxyalkanoate (PHA)/inorganic phase composites for tissue engineering applications. Biomacromolecules. 2006;7(8):2249-2258.
15. Ellis G, Cano P, Jadraque M, et al. Laser microperforated biodegradable microbial polyhydroxyalkanoate substrates for tissue repair strategies: an infrared microspectroscopy study. Anal Bioanal Chem. 2011;399(7):2379-2388.
16. Lenart BA, Martens KA, Kearns KA, Gillespie RJ, Zoga AC, Williams GR. Treatment of massive and recurrent rotator cuff tears augmented with a poly-l-lactide graft, a preliminary study. J Shoulder Elbow Surg. 2015;24(6):915-921.
17. Barber FA, Burns JP, Deutsch A, Labbé MR, Litchfield RB. A prospective, randomized evaluation of acellular human dermal matrix augmentation for arthroscopic rotator cuff repair. Arthroscopy. 2012;28(1):8-15.
18. Barber FA, Herbert MA, Boothby MH. Ultimate tensile failure loads of a human dermal allograft rotator cuff augmentation. Arthroscopy. 2008;24(1):20-24.
19. Gilot GJ, Attia AK, Alvarez AM. Arthroscopic repair of rotator cuff tears using extracellular matrix graft. Arthrosc Tech. 2014;3(4):e487-e489.
20. Barber FA, Coons DA, Ruiz-Suarez M. Cyclic load testing of biodegradable suture anchors containing 2 high-strength sutures. Arthroscopy. 2007;23(4):355-360.
21. Kullar RS, Reagan JM, Kolz CW, Burks RT, Henninger HB. Suture placement near the musculotendinous junction in the supraspinatus: implications for rotator cuff repair. Am J Sports Med. 2015;43(1):57-62.
22. Burkhart SS, Diaz Pagàn JL, Wirth MA, Athanasiou KA. Cyclic loading of anchor-based rotator cuff repairs: confirmation of the tension overload phenomenon and comparison of suture anchor fixation with transosseous fixation. Arthroscopy. 1997;13(6):720-724.
23. Faul F, Erdfelder E, Lang AG, Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39(2):175-191.
24. Greenspoon JA, Petri M, Warth RJ, Millett PJ. Massive rotator cuff tears: pathomechanics, current treatment options, and clinical outcomes. J Shoulder Elbow Surg. 2015;24(9):1493-1505.
25. Hein J, Reilly JM, Chae J, Maerz T, Anderson K. Retear rates after arthroscopic single-row, double-row, and suture bridge rotator cuff repair at a minimum of 1 year of imaging follow-up: a systematic review. Arthroscopy. 2015;31(11):2274-2281.
26. Henry P, Wasserstein D, Park S, et al. Arthroscopic repair for chronic massive rotator cuff tears: a systematic review. Arthroscopy. 2015;31(12):2472-2480.
27. El-Azab HM, Rott O, Irlenbusch U. Long-term follow-up after latissimus dorsi transfer for irreparable posterosuperior rotator cuff tears. J Bone Joint Surg Am. 2015;97(6):462-469.
28. Gerber C, Rahm SA, Catanzaro S, Farshad M, Moor BK. Latissimus dorsi tendon transfer for treatment of irreparable posterosuperior rotator cuff tears: long-term results at a minimum follow-up of ten years. J Bone Joint Surg Am. 2013;95(21):1920-1926.
29. Coons DA, Barber FA, Herbert MA. Triple-loaded single-anchor stitch configurations: an analysis of cyclically loaded suture–tendon interface security. Arthroscopy. 2006;22(11):1154-1158.
30. Itoi E, Berglund LJ, Grabowski JJ, et al. Tensile properties of the supraspinatus tendon. J Orthop Res. 1995;13(4):578-584.
31. Matsuhashi T, Hooke AW, Zhao KD, et al. Tensile properties of a morphologically split supraspinatus tendon. Clin Anat. 2014;27(5):702-706.
32. Apreleva M, Parsons IM 4th, Warner JJ, Fu FH, Woo SL. Experimental investigation of reaction forces at the glenohumeral joint during active abduction. J Shoulder Elbow Surg. 2000;9(5):409-417.
33. Giles JW, Ferreira LM, Athwal GS, Johnson JA. Development and performance evaluation of a multi-PID muscle loading driven in vitro active-motion shoulder simulator and application to assessing reverse total shoulder arthroplasty. J Biomech Eng. 2014;136(12):121007.
34. Hansen ML, Otis JC, Johnson JS, Cordasco FA, Craig EV, Warren RF. Biomechanics of massive rotator cuff tears: implications for treatment. J Bone Joint Surg Am. 2008;90(2):316-325.
35. Henninger HB, Barg A, Anderson AE, Bachus KN, Tashjian RZ, Burks RT. Effect of deltoid tension and humeral version in reverse total shoulder arthroplasty: a biomechanical study. J Shoulder Elbow Surg. 2012;21(4):483-490.
36. Mihata T, Gates J, McGarry MH, Lee J, Kinoshita M, Lee TQ. Effect of rotator cuff muscle imbalance on forceful internal impingement and peel-back of the superior labrum: a cadaveric study. Am J Sports Med. 2009;37(11):2222-2227.
37. Sano H, Ishii H, Yeadon A, Backman DS, Brunet JA, Uhthoff HK. Degeneration at the insertion weakens the tensile strength of the supraspinatus tendon: a comparative mechanical and histologic study of the bone–tendon complex. J Orthop Res. 1997;15(5):719-726.