User login
To the Editor:
Hailey-Hailey disease (HHD)(also known as familial benign chronic pemphigus) is an inherited autosomal-dominant condition in the family of chronic bullous diseases. It is characterized by flaccid blisters, erosions, and macerated vegetative plaques with a predilection for intertriginous sites. Lesions often are weeping, painful, pruritic, and malodorous, leading to decreased quality of life for patients. Complications of this chronic disease include an increased risk for secondary infection and malignant transformation to squamous cell carcinoma.1
Treatment of HHD remains difficult. Topical steroids, oral steroids, and ablative techniques such as dermabrasion and ablative lasers are the most widely reported therapies. OnabotulinumtoxinA has been described as a successful treatment for patients with HHD, including for disease recalcitrant to other therapies.2 We describe 2 patients with HHD who responded to treatment with intralesional onabotulinumtoxinA injections with and without adjuvant oral glycopyrrolate.
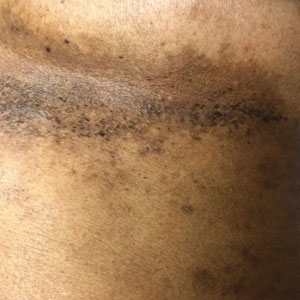
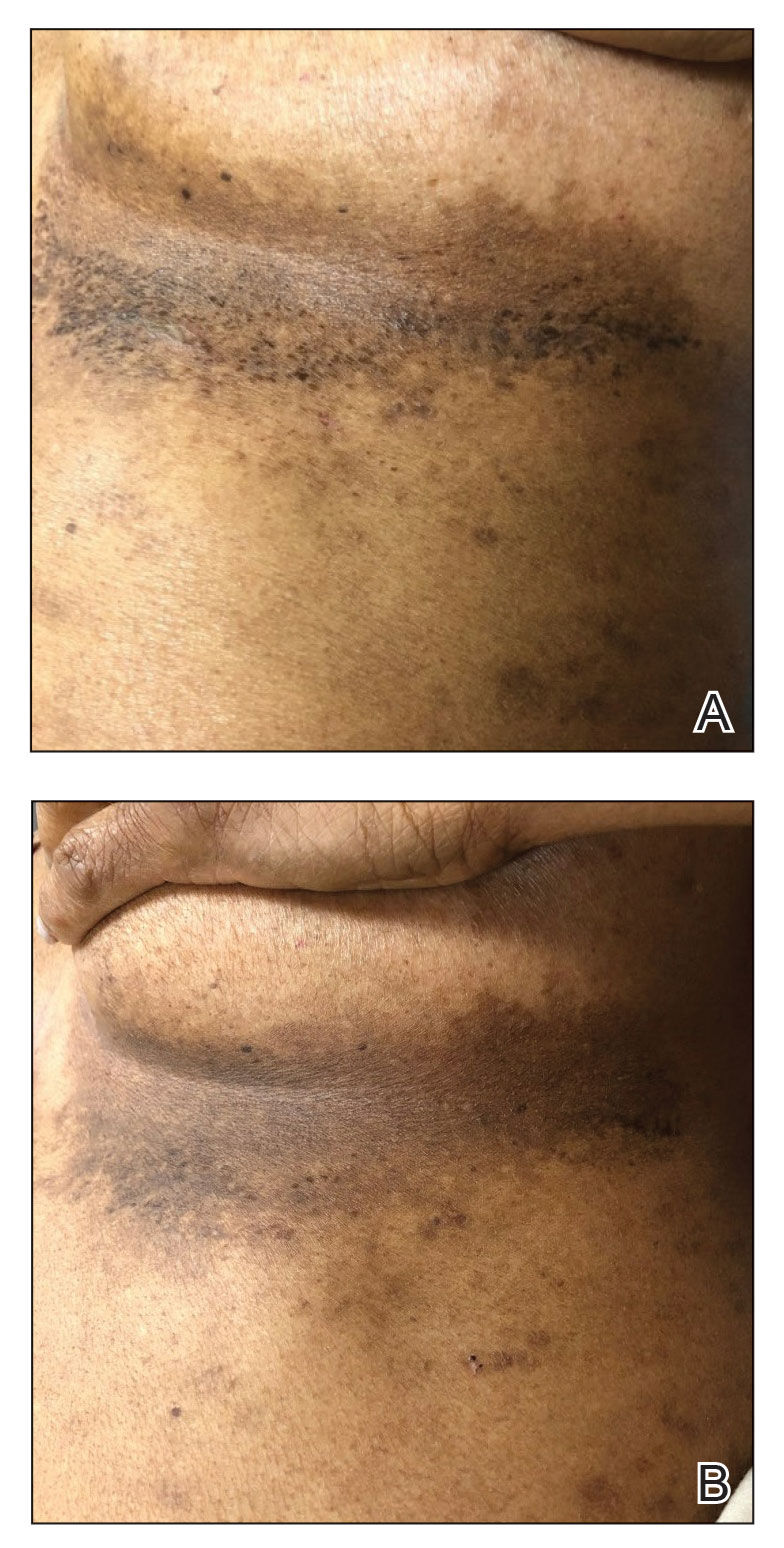
A 54-year-old woman presented with painful flaccid blisters under the breasts (Figure 1A) and in the axillae and groin of 3 weeks’ duration. Biopsy results from this initial visit were consistent with a diagnosis of HHD. The patient reported that the onset of blisters coincided with episodes of severe hyperhidrosis. Therapy with topical and oral steroids, antifungals, antibiotics, and topical aluminum chloride failed to achieve adequate disease control. After a discussion of the risks and benefits, the patient agreed to treatment with injections of onabotulinumtoxinA. At months 0, 3, and 6, the patient received 50 U of onabotulinumtoxinA under the breasts and in the axillae and the groin, for a total of 250 U each session. Each injection consisted of 2.5 U of onabotulinumtoxinA spaced 1-cm apart. Clinical improvement was noted within 2 weeks of initiating neuromodulator therapy. Follow-up at 9 months demonstrated improvement (Figure 1B); however, complete clearance was not achieved, and the patient required ongoing treatment with onabotulinumtoxinA every 3 months.
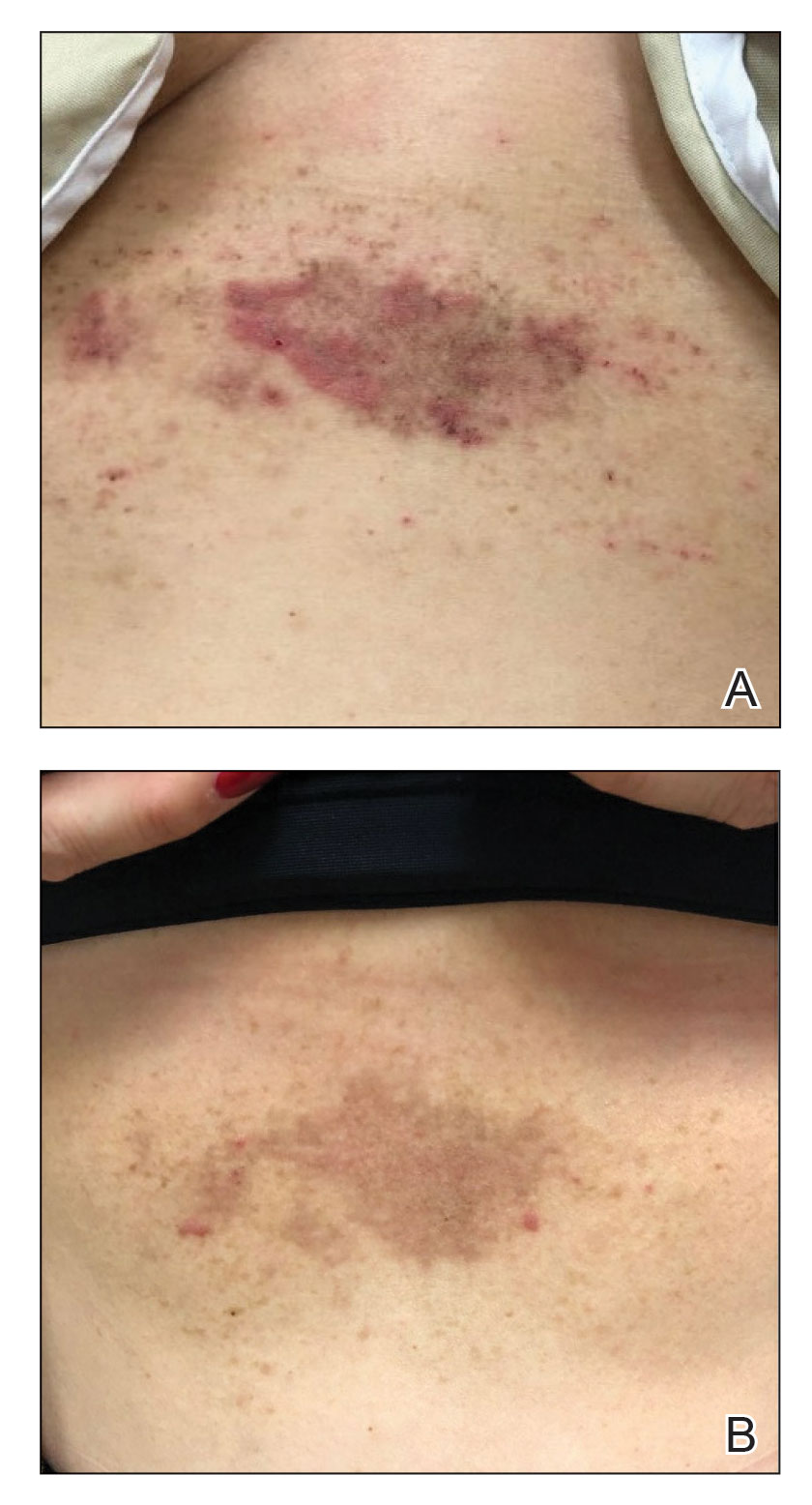
A 43-year-old woman presented with erythematous eroded plaques of the antecubital fossae, axillae, and chest (Figure 2A) of 10 years’ duration. A biopsy from an outside provider demonstrated findings consistent with a diagnosis of HHD. Prior therapies included topical and oral steroids. After a discussion of the risks and benefits, the patient was treated with onabotulinumtoxinA injections in combination with oral glycopyrrolate 5 mg daily. She received 30 U of onabotulinumtoxinA to each axilla, 10 U to each antecubital fossa, and 20 U to the central chest. At 1 month follow-up, the patient reported great improvement in lesion burden and active disease (Figure 2B). Nine months after treatment, her HHD was in complete remission with glycopyrrolate alone and she did not require further therapy with onabotulinumtoxinA.
Hailey-Hailey disease has been attributed to mutations of the ATPase secretory pathway Ca2+ transporting 1 gene, ATP2C1, that lead to aberrations in calcium signaling and subsequent impaired adhesion between keratinocytes.2 These compromised cell-cell connections are worsened by the presence of humidity, causing further acantholysis. Chemical denervation of the sweat glands with botulinum toxin has been postulated to improve HHD by reducing moisture in vulnerable areas. Our 2 cases add to the existing literature documenting tangible clinical results that correlate with this hypothesis.3-5
Our second case is unique in that the patient achieved rapid improvement using a combination of onabotulinumtoxinA and glycopyrrolate therapy. Both onabotulinumtoxinA and glycopyrrolate inhibit acetylcholine signaling that is required for sweat production; however, each drug exerts its effect on different zones of the cholinergic pathway, which may partially account for the synergistic effect of onabotulinumtoxinA and glycopyrrolate to improve HHD, as sweating is dually inhibited by the 2 drugs. Additionally, the combined local and systemic administration of these anticholinergic medications may further potentiate the sweat blockade, particularly in areas most prone to disease.
Botulinum toxin for the treatment of HHD is an effective monotherapy. The addition of an oral anticholinergic to local neuromodulator injections may speed symptom resolution and sustain disease remission. Further studies to evaluate this combination are warranted.
- Palmer DD, Perry HO. Benign familial chronic pemphigus. Arch Dermatol. 1962;86:493-502. doi:10.1001/archderm.1962.01590100107020
- Farahnik B, Blattner CM, Mortazie MB, et al. Interventional treatments for Hailey-Hailey disease. J Am Acad Dermatol. 2017;76:551-558.e553. doi:10.1016/j.jaad.2016.08.039
- Bessa GR, Glaziovine TC, Manzoni AP, et al. Hailey-Hailey disease treatment with botulinum toxin type A. An Bras Dermatol. 2010;85:717-722. doi:10.1590/s0365-05962010000500021
- Lapiere JC, Hirsh A, Gordon KB, et al. Botulinum toxin type A for the treatment of axillary Hailey-Hailey disease. Dermatol Surg. 2000;26:371-374. doi:10.1046/j.1524-4725.2000.99278.x
- Koeyers WJ, Van Der Geer S, Krekels G. Botulinum toxin type A as an adjuvant treatment modality for extensive Hailey-Hailey disease. J Dermatolog Treat. 2008;19:251-254. doi:10.1080/09546630801955135
To the Editor:
Hailey-Hailey disease (HHD)(also known as familial benign chronic pemphigus) is an inherited autosomal-dominant condition in the family of chronic bullous diseases. It is characterized by flaccid blisters, erosions, and macerated vegetative plaques with a predilection for intertriginous sites. Lesions often are weeping, painful, pruritic, and malodorous, leading to decreased quality of life for patients. Complications of this chronic disease include an increased risk for secondary infection and malignant transformation to squamous cell carcinoma.1
Treatment of HHD remains difficult. Topical steroids, oral steroids, and ablative techniques such as dermabrasion and ablative lasers are the most widely reported therapies. OnabotulinumtoxinA has been described as a successful treatment for patients with HHD, including for disease recalcitrant to other therapies.2 We describe 2 patients with HHD who responded to treatment with intralesional onabotulinumtoxinA injections with and without adjuvant oral glycopyrrolate.
A 54-year-old woman presented with painful flaccid blisters under the breasts (Figure 1A) and in the axillae and groin of 3 weeks’ duration. Biopsy results from this initial visit were consistent with a diagnosis of HHD. The patient reported that the onset of blisters coincided with episodes of severe hyperhidrosis. Therapy with topical and oral steroids, antifungals, antibiotics, and topical aluminum chloride failed to achieve adequate disease control. After a discussion of the risks and benefits, the patient agreed to treatment with injections of onabotulinumtoxinA. At months 0, 3, and 6, the patient received 50 U of onabotulinumtoxinA under the breasts and in the axillae and the groin, for a total of 250 U each session. Each injection consisted of 2.5 U of onabotulinumtoxinA spaced 1-cm apart. Clinical improvement was noted within 2 weeks of initiating neuromodulator therapy. Follow-up at 9 months demonstrated improvement (Figure 1B); however, complete clearance was not achieved, and the patient required ongoing treatment with onabotulinumtoxinA every 3 months.

A 43-year-old woman presented with erythematous eroded plaques of the antecubital fossae, axillae, and chest (Figure 2A) of 10 years’ duration. A biopsy from an outside provider demonstrated findings consistent with a diagnosis of HHD. Prior therapies included topical and oral steroids. After a discussion of the risks and benefits, the patient was treated with onabotulinumtoxinA injections in combination with oral glycopyrrolate 5 mg daily. She received 30 U of onabotulinumtoxinA to each axilla, 10 U to each antecubital fossa, and 20 U to the central chest. At 1 month follow-up, the patient reported great improvement in lesion burden and active disease (Figure 2B). Nine months after treatment, her HHD was in complete remission with glycopyrrolate alone and she did not require further therapy with onabotulinumtoxinA.

Hailey-Hailey disease has been attributed to mutations of the ATPase secretory pathway Ca2+ transporting 1 gene, ATP2C1, that lead to aberrations in calcium signaling and subsequent impaired adhesion between keratinocytes.2 These compromised cell-cell connections are worsened by the presence of humidity, causing further acantholysis. Chemical denervation of the sweat glands with botulinum toxin has been postulated to improve HHD by reducing moisture in vulnerable areas. Our 2 cases add to the existing literature documenting tangible clinical results that correlate with this hypothesis.3-5
Our second case is unique in that the patient achieved rapid improvement using a combination of onabotulinumtoxinA and glycopyrrolate therapy. Both onabotulinumtoxinA and glycopyrrolate inhibit acetylcholine signaling that is required for sweat production; however, each drug exerts its effect on different zones of the cholinergic pathway, which may partially account for the synergistic effect of onabotulinumtoxinA and glycopyrrolate to improve HHD, as sweating is dually inhibited by the 2 drugs. Additionally, the combined local and systemic administration of these anticholinergic medications may further potentiate the sweat blockade, particularly in areas most prone to disease.
Botulinum toxin for the treatment of HHD is an effective monotherapy. The addition of an oral anticholinergic to local neuromodulator injections may speed symptom resolution and sustain disease remission. Further studies to evaluate this combination are warranted.
To the Editor:
Hailey-Hailey disease (HHD)(also known as familial benign chronic pemphigus) is an inherited autosomal-dominant condition in the family of chronic bullous diseases. It is characterized by flaccid blisters, erosions, and macerated vegetative plaques with a predilection for intertriginous sites. Lesions often are weeping, painful, pruritic, and malodorous, leading to decreased quality of life for patients. Complications of this chronic disease include an increased risk for secondary infection and malignant transformation to squamous cell carcinoma.1
Treatment of HHD remains difficult. Topical steroids, oral steroids, and ablative techniques such as dermabrasion and ablative lasers are the most widely reported therapies. OnabotulinumtoxinA has been described as a successful treatment for patients with HHD, including for disease recalcitrant to other therapies.2 We describe 2 patients with HHD who responded to treatment with intralesional onabotulinumtoxinA injections with and without adjuvant oral glycopyrrolate.
A 54-year-old woman presented with painful flaccid blisters under the breasts (Figure 1A) and in the axillae and groin of 3 weeks’ duration. Biopsy results from this initial visit were consistent with a diagnosis of HHD. The patient reported that the onset of blisters coincided with episodes of severe hyperhidrosis. Therapy with topical and oral steroids, antifungals, antibiotics, and topical aluminum chloride failed to achieve adequate disease control. After a discussion of the risks and benefits, the patient agreed to treatment with injections of onabotulinumtoxinA. At months 0, 3, and 6, the patient received 50 U of onabotulinumtoxinA under the breasts and in the axillae and the groin, for a total of 250 U each session. Each injection consisted of 2.5 U of onabotulinumtoxinA spaced 1-cm apart. Clinical improvement was noted within 2 weeks of initiating neuromodulator therapy. Follow-up at 9 months demonstrated improvement (Figure 1B); however, complete clearance was not achieved, and the patient required ongoing treatment with onabotulinumtoxinA every 3 months.

A 43-year-old woman presented with erythematous eroded plaques of the antecubital fossae, axillae, and chest (Figure 2A) of 10 years’ duration. A biopsy from an outside provider demonstrated findings consistent with a diagnosis of HHD. Prior therapies included topical and oral steroids. After a discussion of the risks and benefits, the patient was treated with onabotulinumtoxinA injections in combination with oral glycopyrrolate 5 mg daily. She received 30 U of onabotulinumtoxinA to each axilla, 10 U to each antecubital fossa, and 20 U to the central chest. At 1 month follow-up, the patient reported great improvement in lesion burden and active disease (Figure 2B). Nine months after treatment, her HHD was in complete remission with glycopyrrolate alone and she did not require further therapy with onabotulinumtoxinA.

Hailey-Hailey disease has been attributed to mutations of the ATPase secretory pathway Ca2+ transporting 1 gene, ATP2C1, that lead to aberrations in calcium signaling and subsequent impaired adhesion between keratinocytes.2 These compromised cell-cell connections are worsened by the presence of humidity, causing further acantholysis. Chemical denervation of the sweat glands with botulinum toxin has been postulated to improve HHD by reducing moisture in vulnerable areas. Our 2 cases add to the existing literature documenting tangible clinical results that correlate with this hypothesis.3-5
Our second case is unique in that the patient achieved rapid improvement using a combination of onabotulinumtoxinA and glycopyrrolate therapy. Both onabotulinumtoxinA and glycopyrrolate inhibit acetylcholine signaling that is required for sweat production; however, each drug exerts its effect on different zones of the cholinergic pathway, which may partially account for the synergistic effect of onabotulinumtoxinA and glycopyrrolate to improve HHD, as sweating is dually inhibited by the 2 drugs. Additionally, the combined local and systemic administration of these anticholinergic medications may further potentiate the sweat blockade, particularly in areas most prone to disease.
Botulinum toxin for the treatment of HHD is an effective monotherapy. The addition of an oral anticholinergic to local neuromodulator injections may speed symptom resolution and sustain disease remission. Further studies to evaluate this combination are warranted.
- Palmer DD, Perry HO. Benign familial chronic pemphigus. Arch Dermatol. 1962;86:493-502. doi:10.1001/archderm.1962.01590100107020
- Farahnik B, Blattner CM, Mortazie MB, et al. Interventional treatments for Hailey-Hailey disease. J Am Acad Dermatol. 2017;76:551-558.e553. doi:10.1016/j.jaad.2016.08.039
- Bessa GR, Glaziovine TC, Manzoni AP, et al. Hailey-Hailey disease treatment with botulinum toxin type A. An Bras Dermatol. 2010;85:717-722. doi:10.1590/s0365-05962010000500021
- Lapiere JC, Hirsh A, Gordon KB, et al. Botulinum toxin type A for the treatment of axillary Hailey-Hailey disease. Dermatol Surg. 2000;26:371-374. doi:10.1046/j.1524-4725.2000.99278.x
- Koeyers WJ, Van Der Geer S, Krekels G. Botulinum toxin type A as an adjuvant treatment modality for extensive Hailey-Hailey disease. J Dermatolog Treat. 2008;19:251-254. doi:10.1080/09546630801955135
- Palmer DD, Perry HO. Benign familial chronic pemphigus. Arch Dermatol. 1962;86:493-502. doi:10.1001/archderm.1962.01590100107020
- Farahnik B, Blattner CM, Mortazie MB, et al. Interventional treatments for Hailey-Hailey disease. J Am Acad Dermatol. 2017;76:551-558.e553. doi:10.1016/j.jaad.2016.08.039
- Bessa GR, Glaziovine TC, Manzoni AP, et al. Hailey-Hailey disease treatment with botulinum toxin type A. An Bras Dermatol. 2010;85:717-722. doi:10.1590/s0365-05962010000500021
- Lapiere JC, Hirsh A, Gordon KB, et al. Botulinum toxin type A for the treatment of axillary Hailey-Hailey disease. Dermatol Surg. 2000;26:371-374. doi:10.1046/j.1524-4725.2000.99278.x
- Koeyers WJ, Van Der Geer S, Krekels G. Botulinum toxin type A as an adjuvant treatment modality for extensive Hailey-Hailey disease. J Dermatolog Treat. 2008;19:251-254. doi:10.1080/09546630801955135
Practice Points
- Hailey-Hailey disease is associated with decreased quality of life for patients, and current treatment options are limited.
- A combination of local neuromodulator injections and systemic oral anticholinergic therapy may provide sustained disease remission compared to neuromodulator therapy alone.