User login
CHICAGO – Canagliflozin outperformed sitagliptin for the treatment of patients with type 2 diabetes in the first randomized head-to-head comparison of drugs representing the two different classes of oral antidiabetic medications.
Canagliflozin (Invokana) received approval from the Food and Drug Administration in March as the first in a new class of medications for type 2 diabetes known as sodium glucose co-transporter 2 (SGLT2) inhibitors. SGLT2 is responsible for most glucose reabsorption in the kidney, so its inhibition boosts urinary glucose excretion. Other SGLT2 inhibitors are coming through the developmental pipeline.
In a phase III, double-blind, 52-week clinical trial, canagliflozin improved glycemic control, lowered body weight, and reduced blood pressure to a significantly greater extent than did sitagliptin (Januvia), a dipeptidyl peptidase-4 (DPP-4) inhibitor, Dr. Fernando Lavalle Gonzalez reported at the annual scientific sessions of the American Diabetes Association.
The multicenter four-armed study included 1,020 type 2 patients with diabetes who had inadequate glycemic control on metformin monotherapy. They were randomized to canagliflozin at 100 or 300 mg/day, sitagliptin at 100 mg/day, or 26 weeks of placebo followed by 26 weeks on sitagliptin at 100 mg/day. All participants continued on metformin.
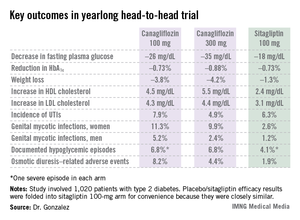
Reductions in fasting plasma glucose at 52 weeks averaged 26.2 mg/dL in the lower-dose canagliflozin group and 35.2 mg/dL in the higher-dose group, both significantly greater than the 17.7-mg/dL decrease in patients on sitagliptin.
In addition to the better outcomes seen with canagliflozin in terms of the major efficacy endpoints involving glycemic control, weight loss, and blood pressure, the SGLT2 inhibitor resulted in a bigger boost in HDL cholesterol (see chart). On the downside, both canagliflozin and sitagliptin resulted in modest increases in low-density lipoprotein (LDL), noted Dr. Gonzalez, an endocrinologist at the Autonomous University of Nuevo Leon in Monterrey, Mexico.
Canagliflozin was associated with significantly higher rates of genital mycotic infections in both men and women, as well as more adverse events related to osmotic diuresis. The infections were symptomatic, easily diagnosed, and readily treated with topical or oral antifungals. The adverse events related to increased urination were typically mild. These side effects led to very few study discontinuations, according to Dr. Gonzalez.
He said mechanistic studies have identified two factors as being responsible for the clinically meaningful reductions in blood pressure seen with canagliflozin in this study, which amounted to a mean 3.5 mm Hg decrease in systolic blood pressure at 100 mg/day and a 4.7 mm Hg reduction at 300 mg/day. It appears that about half of the blood pressure reduction is due to the negative salt and water balance, on the order of 750-1,000 cc, occurring in the first 3-4 days of treatment, and the other half is related to the weight loss accompanying canagliflozin therapy.
Session chair Dr. Ralph A. DeFronzo, who is involved in SGLT2 inhibitor research, said a widespread misconception exists that the medications are associated with an increased risk of urinary tract infections.
"It’s very commonly stated that this class of drugs is associated with an increase in UTIs [urinary tract infections]. In fact, if you look at the data rather than what’s said, this really in my opinion doesn’t hold up. You can see it in this study, where there’s no significant difference between the groups," said Dr. DeFronzo, professor of medicine and director of the diabetes division at the University of Texas Health Science Center, San Antonio.
He also put into perspective the increase in LDL seen with canagliflozin.
"Looking across the studies, with canagliflozin at the 100-mg dose the rise in LDL is about 4 mg/dL, and it’s about 8 mg/dL at the 300-mg dose. So if you’re truly treating your patients to goal and they’re at an LDL of 70 mg/dL, in the worst case scenario you’d be going from 70 to 78 mg/dL. I think many of us here in the audience probably wouldn’t even increase the dose of a statin, but if you did up it by one dose, you’d get the LDL back down to 70," he said.
Dr. DeFronzo reported serving on advisory panels for Janssen, which markets canagliflozin, as well as for Amylin Pharmaceuticals, Boehringer Ingelheim, Bristol-Myers Squibb, Novo Nordisk, and Takeda. Dr. Gonzalez is on speakers bureaus and advisory panels for Janssen and numerous other companies.
Invokana, Food and Drug Administration, FDA approval, SGLT2,
CHICAGO – Canagliflozin outperformed sitagliptin for the treatment of patients with type 2 diabetes in the first randomized head-to-head comparison of drugs representing the two different classes of oral antidiabetic medications.
Canagliflozin (Invokana) received approval from the Food and Drug Administration in March as the first in a new class of medications for type 2 diabetes known as sodium glucose co-transporter 2 (SGLT2) inhibitors. SGLT2 is responsible for most glucose reabsorption in the kidney, so its inhibition boosts urinary glucose excretion. Other SGLT2 inhibitors are coming through the developmental pipeline.

In a phase III, double-blind, 52-week clinical trial, canagliflozin improved glycemic control, lowered body weight, and reduced blood pressure to a significantly greater extent than did sitagliptin (Januvia), a dipeptidyl peptidase-4 (DPP-4) inhibitor, Dr. Fernando Lavalle Gonzalez reported at the annual scientific sessions of the American Diabetes Association.
The multicenter four-armed study included 1,020 type 2 patients with diabetes who had inadequate glycemic control on metformin monotherapy. They were randomized to canagliflozin at 100 or 300 mg/day, sitagliptin at 100 mg/day, or 26 weeks of placebo followed by 26 weeks on sitagliptin at 100 mg/day. All participants continued on metformin.
Reductions in fasting plasma glucose at 52 weeks averaged 26.2 mg/dL in the lower-dose canagliflozin group and 35.2 mg/dL in the higher-dose group, both significantly greater than the 17.7-mg/dL decrease in patients on sitagliptin.
In addition to the better outcomes seen with canagliflozin in terms of the major efficacy endpoints involving glycemic control, weight loss, and blood pressure, the SGLT2 inhibitor resulted in a bigger boost in HDL cholesterol (see chart). On the downside, both canagliflozin and sitagliptin resulted in modest increases in low-density lipoprotein (LDL), noted Dr. Gonzalez, an endocrinologist at the Autonomous University of Nuevo Leon in Monterrey, Mexico.
Canagliflozin was associated with significantly higher rates of genital mycotic infections in both men and women, as well as more adverse events related to osmotic diuresis. The infections were symptomatic, easily diagnosed, and readily treated with topical or oral antifungals. The adverse events related to increased urination were typically mild. These side effects led to very few study discontinuations, according to Dr. Gonzalez.
He said mechanistic studies have identified two factors as being responsible for the clinically meaningful reductions in blood pressure seen with canagliflozin in this study, which amounted to a mean 3.5 mm Hg decrease in systolic blood pressure at 100 mg/day and a 4.7 mm Hg reduction at 300 mg/day. It appears that about half of the blood pressure reduction is due to the negative salt and water balance, on the order of 750-1,000 cc, occurring in the first 3-4 days of treatment, and the other half is related to the weight loss accompanying canagliflozin therapy.
Session chair Dr. Ralph A. DeFronzo, who is involved in SGLT2 inhibitor research, said a widespread misconception exists that the medications are associated with an increased risk of urinary tract infections.
"It’s very commonly stated that this class of drugs is associated with an increase in UTIs [urinary tract infections]. In fact, if you look at the data rather than what’s said, this really in my opinion doesn’t hold up. You can see it in this study, where there’s no significant difference between the groups," said Dr. DeFronzo, professor of medicine and director of the diabetes division at the University of Texas Health Science Center, San Antonio.
He also put into perspective the increase in LDL seen with canagliflozin.
"Looking across the studies, with canagliflozin at the 100-mg dose the rise in LDL is about 4 mg/dL, and it’s about 8 mg/dL at the 300-mg dose. So if you’re truly treating your patients to goal and they’re at an LDL of 70 mg/dL, in the worst case scenario you’d be going from 70 to 78 mg/dL. I think many of us here in the audience probably wouldn’t even increase the dose of a statin, but if you did up it by one dose, you’d get the LDL back down to 70," he said.
Dr. DeFronzo reported serving on advisory panels for Janssen, which markets canagliflozin, as well as for Amylin Pharmaceuticals, Boehringer Ingelheim, Bristol-Myers Squibb, Novo Nordisk, and Takeda. Dr. Gonzalez is on speakers bureaus and advisory panels for Janssen and numerous other companies.
CHICAGO – Canagliflozin outperformed sitagliptin for the treatment of patients with type 2 diabetes in the first randomized head-to-head comparison of drugs representing the two different classes of oral antidiabetic medications.
Canagliflozin (Invokana) received approval from the Food and Drug Administration in March as the first in a new class of medications for type 2 diabetes known as sodium glucose co-transporter 2 (SGLT2) inhibitors. SGLT2 is responsible for most glucose reabsorption in the kidney, so its inhibition boosts urinary glucose excretion. Other SGLT2 inhibitors are coming through the developmental pipeline.

In a phase III, double-blind, 52-week clinical trial, canagliflozin improved glycemic control, lowered body weight, and reduced blood pressure to a significantly greater extent than did sitagliptin (Januvia), a dipeptidyl peptidase-4 (DPP-4) inhibitor, Dr. Fernando Lavalle Gonzalez reported at the annual scientific sessions of the American Diabetes Association.
The multicenter four-armed study included 1,020 type 2 patients with diabetes who had inadequate glycemic control on metformin monotherapy. They were randomized to canagliflozin at 100 or 300 mg/day, sitagliptin at 100 mg/day, or 26 weeks of placebo followed by 26 weeks on sitagliptin at 100 mg/day. All participants continued on metformin.
Reductions in fasting plasma glucose at 52 weeks averaged 26.2 mg/dL in the lower-dose canagliflozin group and 35.2 mg/dL in the higher-dose group, both significantly greater than the 17.7-mg/dL decrease in patients on sitagliptin.
In addition to the better outcomes seen with canagliflozin in terms of the major efficacy endpoints involving glycemic control, weight loss, and blood pressure, the SGLT2 inhibitor resulted in a bigger boost in HDL cholesterol (see chart). On the downside, both canagliflozin and sitagliptin resulted in modest increases in low-density lipoprotein (LDL), noted Dr. Gonzalez, an endocrinologist at the Autonomous University of Nuevo Leon in Monterrey, Mexico.
Canagliflozin was associated with significantly higher rates of genital mycotic infections in both men and women, as well as more adverse events related to osmotic diuresis. The infections were symptomatic, easily diagnosed, and readily treated with topical or oral antifungals. The adverse events related to increased urination were typically mild. These side effects led to very few study discontinuations, according to Dr. Gonzalez.
He said mechanistic studies have identified two factors as being responsible for the clinically meaningful reductions in blood pressure seen with canagliflozin in this study, which amounted to a mean 3.5 mm Hg decrease in systolic blood pressure at 100 mg/day and a 4.7 mm Hg reduction at 300 mg/day. It appears that about half of the blood pressure reduction is due to the negative salt and water balance, on the order of 750-1,000 cc, occurring in the first 3-4 days of treatment, and the other half is related to the weight loss accompanying canagliflozin therapy.
Session chair Dr. Ralph A. DeFronzo, who is involved in SGLT2 inhibitor research, said a widespread misconception exists that the medications are associated with an increased risk of urinary tract infections.
"It’s very commonly stated that this class of drugs is associated with an increase in UTIs [urinary tract infections]. In fact, if you look at the data rather than what’s said, this really in my opinion doesn’t hold up. You can see it in this study, where there’s no significant difference between the groups," said Dr. DeFronzo, professor of medicine and director of the diabetes division at the University of Texas Health Science Center, San Antonio.
He also put into perspective the increase in LDL seen with canagliflozin.
"Looking across the studies, with canagliflozin at the 100-mg dose the rise in LDL is about 4 mg/dL, and it’s about 8 mg/dL at the 300-mg dose. So if you’re truly treating your patients to goal and they’re at an LDL of 70 mg/dL, in the worst case scenario you’d be going from 70 to 78 mg/dL. I think many of us here in the audience probably wouldn’t even increase the dose of a statin, but if you did up it by one dose, you’d get the LDL back down to 70," he said.
Dr. DeFronzo reported serving on advisory panels for Janssen, which markets canagliflozin, as well as for Amylin Pharmaceuticals, Boehringer Ingelheim, Bristol-Myers Squibb, Novo Nordisk, and Takeda. Dr. Gonzalez is on speakers bureaus and advisory panels for Janssen and numerous other companies.
Invokana, Food and Drug Administration, FDA approval, SGLT2,
Invokana, Food and Drug Administration, FDA approval, SGLT2,
AT THE ADA ANNUAL SCIENTIFIC SESSIONS
Major finding: Patients with type 2 diabetes averaged a 26.2-mg/dL reduction from baseline in fasting plasma glucose after 52 weeks on canagliflozin at 100 mg/day and a 35.2-mg/dL decrease on the sodium glucose transporter 2 inhibitor at 300 mg/day, both superior to the 17.7-mg/dL reduction seen with sitagliptin.
Data source: A 52-week, double-blind, multicenter, phase III randomized trial involving 1,020 patients with type 2 diabetes who had inadequate glycemic control with metformin monotherapy.
Disclosures: Dr. DeFronzo reported serving on advisory panels for Janssen, which markets canagliflozin, as well as for Amylin Pharmaceuticals, Boehringer Ingelheim, Bristol-Myers Squibb, Novo Nordisk, and Takeda. Dr. Gonzalez is on speakers bureaus and advisory panels for Janssen and numerous other companies.