User login
A 26‐year‐old woman presented with a 1‐week history of epigastric and left upper quadrant pain associated with nausea and vomiting. She also described 3 weeks of constant substernal chest pain, dyspnea, and decreased exercise tolerance.
Her medical history was significant for a pituitary macroadenoma diagnosed 6 years previously that had been treated with cabergoline. She had a miscarriage 7 years ago but gave birth to a healthy child 5 months prior to admission. She had smoked 2 cigarettes per day for the last 7 years. She denied alcohol or illicit drug use. Her mother had sickle cell trait.
On admission, her heart rate was 112 beats/minute, blood pressure was 110/80 mm Hg, and respiratory rate was 26 per minute. Jugular venous distension was not appreciated. She had decreased breath sounds over the right lung base. The apical impulse was palpated in the left sixth intercostal space 1 cm lateral to the midclavicular line, and a 2/6 holosystolic murmur was auscultated at the left lower sternal border. No other murmurs or S3 or S4 gallop could be appreciated. There were no vascular or immunological phenomena suggestive of infective endocarditis. She had abdominal tenderness in the epigastrium and bilateral upper quadrants. There was no lower extremity edema, and the extremities were well perfused.
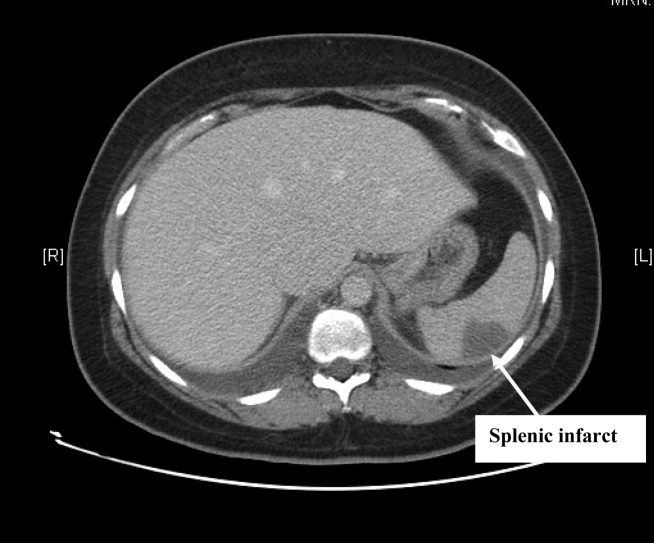
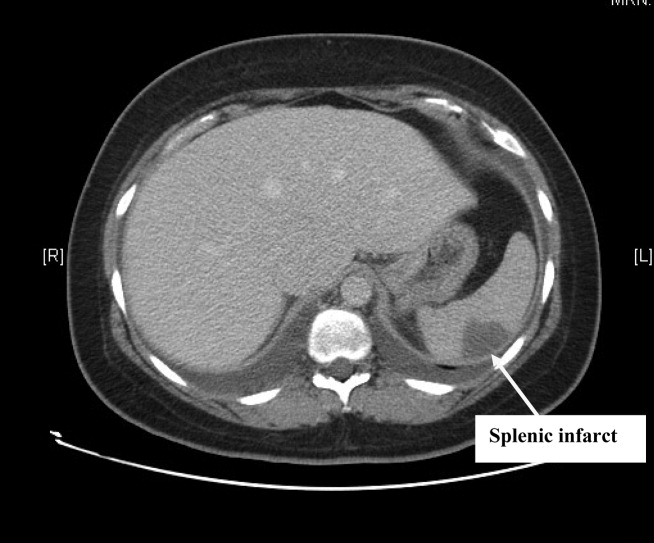
Complete blood count, electrolytes, and liver, renal, and coagulation profiles were normal. Her chest x‐ray revealed cardiomegaly and bilateral pleural effusions. EKG showed sinus tachycardia and nonspecific T‐wave changes. To further evaluate her abdominal pain, a CT scan of the abdomen and pelvis (Fig. 1) was ordered. This revealed a 3 by 1.8 cm splenic infarct. Because of her respiratory symptoms and tachycardia, a pulmonary embolism was suspected but was ruled out with a CT angiogram of the chest.
She was diagnosed with new‐onset heart failure and a splenic infarct. However, it was unclear if the 2 problems were linked. Possible etiologies of the splenic infarct included thrombus from hypercoagulable state (given her prior miscarriage, postpartum state), infarct from hemoglobinopathy (given her family history), septic emboli from infective endocarditis, and peripartum cardiomyopathy associated with embolism to the spleen.
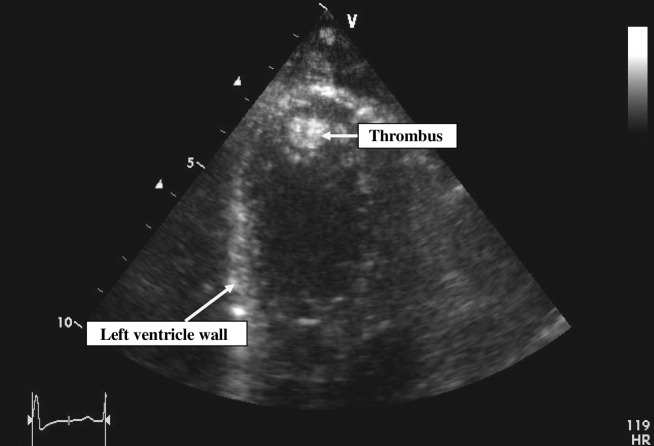
Pain control, empiric antibiotics, and intravenous diuretics were started. Twelve hours later, the patient's dyspnea and chest pain resolved. Her blood culture results were negative, and hemoglobin electrophoresis was normal. Results of a hypercoagulable workup for an arterial thrombus that included lupus anticoagulant, anticardiolipin antibodies, and antibodies to 2‐glycoprotein‐I were negative. The echocardiogram (Fig. 2) showed a dilated left ventricle with an ejection fraction (EF) of 10%15%, normal valvular morphology without vegetations, moderate mitral and tricuspid regurgitation, and a 1‐cm left ventricular thrombus and 3 small adjacent thrombi.
Based on the echocardiographic data, recent pregnancy, and absence of other risk factors for heart failure, a diagnosis was made of peripartum cardiomyopathy with left ventricular thrombi and subsequent embolization to the spleen.
Standard heart failure therapy including diuretics, beta‐blockers, and angiotensin‐converting enzyme inhibitors and anticoagulation with warfarin were started. Within 24 hours, the patient was asymptomatic except for minimal abdominal pain. The patient was discharged home in a stable condition the following day. At her outpatient follow‐up 3 months later, she was well compensated and asymptomatic.
DISCUSSION
Using the search terms peripartum cardiomyopathy, cardiomyopathy, thromboembolism, and postpartum period, we performed a MEDLINE search of the English literature from 1950 to 2007. We did not find any reported cases of splenic infarction complicating peripartum cardiomyopathy.
Peripartum cardiomyopathy (PPCM) is a form of dilated cardiomyopathy that occurs as a complication of pregnancy. It can present with heart failure in the last month of pregnancy or within 5 months after delivery.1, 2 The incidence of PPCM is unknown but has been estimated at 1 in 30004000 live births.3
Our patient met the criteria for PPCM as set forth by the National Heart, Lung, and Blood Institute (NHLBI), in conjunction with the Office of Rare Disease of the National Institutes of Health in April 1997.3 To establish a diagnosis of PPCM, 4 criteria have to be met:
-
Development of heart failure in the last month of pregnancy or within 5 months after delivery;
-
Absence of an identifiable cause of heart failure;
-
Absence of recognizable heart disease prior to the last month of pregnancy; and
-
Left ventricular systolic dysfunction demonstrated by echocardiographic variables such as depressed shortening fraction or left ventricular ejection fraction < 45%.
Thromboembolism has been reported with an incidence of 4% to 30% in peripartum cardiomyopathy.4 In our literature review, we found several case reports of thromboembolic phenomena complicating peripartum cardiomyopathy. These included lower extremity arterial thromboembolism with compromised circulation,5 cerebral embolism,6 and acute myocardial infarction secondary to coronary artery embolism.7 This is the first reported case of splenic artery embolization leading to splenic infarct as a complication of peripartum cardiomyopathy.
Because of the high risk of thromboembolism, the NHLBI recommends that anticoagulation be added to the standard heart failure treatment of PPCM patients with an ejection fraction of less than 35%,3 although there are no prospective randomized clinical trial data to support this recommendation. For anticoagulation, heparin is generally used in the antepartum period and warfarin in the postpartum period. It has been recommended that anticoagulation be continued for as long as the cardiomegaly persists.1
In addition to anticoagulation for PPCM patients with an EF < 35%, standard heart failure therapy includes salt restriction, diuretics, and beta‐blockers. Angiotensin‐converting enzyme inhibitors can be teratogenic during pregnancy but can be used after delivery. Hydralazine can be used safely during pregnancy as an alternative to angiotensin‐converting enzyme inhibitors. Patients failing maximal medical management may be candidates for cardiac transplantation.
Recommendations regarding subsequent pregnancies seem related to the return of ventricular size and function. Patients whose heart size does not return to normal should be strongly advised to avoid future pregnancies.2, 3 Patients who recover ventricular function may have deterioration of left ventricular function with future pregnancies.8 They should be counseled about the risk and closely monitored for development of heart failure if they become pregnant again.
- ,.Peripartum cardiomyopathy.Circulation.1971;44:964–968.
- ,,, et al.Natural course of peripartum cardiomyopathy.Circulation.1971;44:1053–1061.
- ,,, et al.Peripartum Cardiomyopathy. National Heart, Lung, and Blood Institute and Office of Rare Diseases (National Institutes of Health) Workshop Recommendations and Review.JAMA.2000;283:1183–1188.
- .Peripartum cardiomyopathy.N Engl J Med.1985;312:1432–1437.
- ,,, et al.Peripartum cardiomyopathy presenting as lower extremity arterial thromboembolism.J Reprod Med.2000;45:351–353.
- ,,, et al.Cerebral embolism as the initial manifestation of peripartum cardiomyopathy.Neurology.1982;32:668–671.
- ,,, et al.Peripartum cardiomyopathy presenting as an acute myocardial infarction.Mayo Clin Proc.2002;77:500–501.
- ,,, et al.Recurrent peripartum cardiomyopathy.Eur J Obstet Gynecol Reprod Biol.1998;76:29–30.
A 26‐year‐old woman presented with a 1‐week history of epigastric and left upper quadrant pain associated with nausea and vomiting. She also described 3 weeks of constant substernal chest pain, dyspnea, and decreased exercise tolerance.
Her medical history was significant for a pituitary macroadenoma diagnosed 6 years previously that had been treated with cabergoline. She had a miscarriage 7 years ago but gave birth to a healthy child 5 months prior to admission. She had smoked 2 cigarettes per day for the last 7 years. She denied alcohol or illicit drug use. Her mother had sickle cell trait.
On admission, her heart rate was 112 beats/minute, blood pressure was 110/80 mm Hg, and respiratory rate was 26 per minute. Jugular venous distension was not appreciated. She had decreased breath sounds over the right lung base. The apical impulse was palpated in the left sixth intercostal space 1 cm lateral to the midclavicular line, and a 2/6 holosystolic murmur was auscultated at the left lower sternal border. No other murmurs or S3 or S4 gallop could be appreciated. There were no vascular or immunological phenomena suggestive of infective endocarditis. She had abdominal tenderness in the epigastrium and bilateral upper quadrants. There was no lower extremity edema, and the extremities were well perfused.
Complete blood count, electrolytes, and liver, renal, and coagulation profiles were normal. Her chest x‐ray revealed cardiomegaly and bilateral pleural effusions. EKG showed sinus tachycardia and nonspecific T‐wave changes. To further evaluate her abdominal pain, a CT scan of the abdomen and pelvis (Fig. 1) was ordered. This revealed a 3 by 1.8 cm splenic infarct. Because of her respiratory symptoms and tachycardia, a pulmonary embolism was suspected but was ruled out with a CT angiogram of the chest.

She was diagnosed with new‐onset heart failure and a splenic infarct. However, it was unclear if the 2 problems were linked. Possible etiologies of the splenic infarct included thrombus from hypercoagulable state (given her prior miscarriage, postpartum state), infarct from hemoglobinopathy (given her family history), septic emboli from infective endocarditis, and peripartum cardiomyopathy associated with embolism to the spleen.
Pain control, empiric antibiotics, and intravenous diuretics were started. Twelve hours later, the patient's dyspnea and chest pain resolved. Her blood culture results were negative, and hemoglobin electrophoresis was normal. Results of a hypercoagulable workup for an arterial thrombus that included lupus anticoagulant, anticardiolipin antibodies, and antibodies to 2‐glycoprotein‐I were negative. The echocardiogram (Fig. 2) showed a dilated left ventricle with an ejection fraction (EF) of 10%15%, normal valvular morphology without vegetations, moderate mitral and tricuspid regurgitation, and a 1‐cm left ventricular thrombus and 3 small adjacent thrombi.

Based on the echocardiographic data, recent pregnancy, and absence of other risk factors for heart failure, a diagnosis was made of peripartum cardiomyopathy with left ventricular thrombi and subsequent embolization to the spleen.
Standard heart failure therapy including diuretics, beta‐blockers, and angiotensin‐converting enzyme inhibitors and anticoagulation with warfarin were started. Within 24 hours, the patient was asymptomatic except for minimal abdominal pain. The patient was discharged home in a stable condition the following day. At her outpatient follow‐up 3 months later, she was well compensated and asymptomatic.
DISCUSSION
Using the search terms peripartum cardiomyopathy, cardiomyopathy, thromboembolism, and postpartum period, we performed a MEDLINE search of the English literature from 1950 to 2007. We did not find any reported cases of splenic infarction complicating peripartum cardiomyopathy.
Peripartum cardiomyopathy (PPCM) is a form of dilated cardiomyopathy that occurs as a complication of pregnancy. It can present with heart failure in the last month of pregnancy or within 5 months after delivery.1, 2 The incidence of PPCM is unknown but has been estimated at 1 in 30004000 live births.3
Our patient met the criteria for PPCM as set forth by the National Heart, Lung, and Blood Institute (NHLBI), in conjunction with the Office of Rare Disease of the National Institutes of Health in April 1997.3 To establish a diagnosis of PPCM, 4 criteria have to be met:
-
Development of heart failure in the last month of pregnancy or within 5 months after delivery;
-
Absence of an identifiable cause of heart failure;
-
Absence of recognizable heart disease prior to the last month of pregnancy; and
-
Left ventricular systolic dysfunction demonstrated by echocardiographic variables such as depressed shortening fraction or left ventricular ejection fraction < 45%.
Thromboembolism has been reported with an incidence of 4% to 30% in peripartum cardiomyopathy.4 In our literature review, we found several case reports of thromboembolic phenomena complicating peripartum cardiomyopathy. These included lower extremity arterial thromboembolism with compromised circulation,5 cerebral embolism,6 and acute myocardial infarction secondary to coronary artery embolism.7 This is the first reported case of splenic artery embolization leading to splenic infarct as a complication of peripartum cardiomyopathy.
Because of the high risk of thromboembolism, the NHLBI recommends that anticoagulation be added to the standard heart failure treatment of PPCM patients with an ejection fraction of less than 35%,3 although there are no prospective randomized clinical trial data to support this recommendation. For anticoagulation, heparin is generally used in the antepartum period and warfarin in the postpartum period. It has been recommended that anticoagulation be continued for as long as the cardiomegaly persists.1
In addition to anticoagulation for PPCM patients with an EF < 35%, standard heart failure therapy includes salt restriction, diuretics, and beta‐blockers. Angiotensin‐converting enzyme inhibitors can be teratogenic during pregnancy but can be used after delivery. Hydralazine can be used safely during pregnancy as an alternative to angiotensin‐converting enzyme inhibitors. Patients failing maximal medical management may be candidates for cardiac transplantation.
Recommendations regarding subsequent pregnancies seem related to the return of ventricular size and function. Patients whose heart size does not return to normal should be strongly advised to avoid future pregnancies.2, 3 Patients who recover ventricular function may have deterioration of left ventricular function with future pregnancies.8 They should be counseled about the risk and closely monitored for development of heart failure if they become pregnant again.
A 26‐year‐old woman presented with a 1‐week history of epigastric and left upper quadrant pain associated with nausea and vomiting. She also described 3 weeks of constant substernal chest pain, dyspnea, and decreased exercise tolerance.
Her medical history was significant for a pituitary macroadenoma diagnosed 6 years previously that had been treated with cabergoline. She had a miscarriage 7 years ago but gave birth to a healthy child 5 months prior to admission. She had smoked 2 cigarettes per day for the last 7 years. She denied alcohol or illicit drug use. Her mother had sickle cell trait.
On admission, her heart rate was 112 beats/minute, blood pressure was 110/80 mm Hg, and respiratory rate was 26 per minute. Jugular venous distension was not appreciated. She had decreased breath sounds over the right lung base. The apical impulse was palpated in the left sixth intercostal space 1 cm lateral to the midclavicular line, and a 2/6 holosystolic murmur was auscultated at the left lower sternal border. No other murmurs or S3 or S4 gallop could be appreciated. There were no vascular or immunological phenomena suggestive of infective endocarditis. She had abdominal tenderness in the epigastrium and bilateral upper quadrants. There was no lower extremity edema, and the extremities were well perfused.
Complete blood count, electrolytes, and liver, renal, and coagulation profiles were normal. Her chest x‐ray revealed cardiomegaly and bilateral pleural effusions. EKG showed sinus tachycardia and nonspecific T‐wave changes. To further evaluate her abdominal pain, a CT scan of the abdomen and pelvis (Fig. 1) was ordered. This revealed a 3 by 1.8 cm splenic infarct. Because of her respiratory symptoms and tachycardia, a pulmonary embolism was suspected but was ruled out with a CT angiogram of the chest.

She was diagnosed with new‐onset heart failure and a splenic infarct. However, it was unclear if the 2 problems were linked. Possible etiologies of the splenic infarct included thrombus from hypercoagulable state (given her prior miscarriage, postpartum state), infarct from hemoglobinopathy (given her family history), septic emboli from infective endocarditis, and peripartum cardiomyopathy associated with embolism to the spleen.
Pain control, empiric antibiotics, and intravenous diuretics were started. Twelve hours later, the patient's dyspnea and chest pain resolved. Her blood culture results were negative, and hemoglobin electrophoresis was normal. Results of a hypercoagulable workup for an arterial thrombus that included lupus anticoagulant, anticardiolipin antibodies, and antibodies to 2‐glycoprotein‐I were negative. The echocardiogram (Fig. 2) showed a dilated left ventricle with an ejection fraction (EF) of 10%15%, normal valvular morphology without vegetations, moderate mitral and tricuspid regurgitation, and a 1‐cm left ventricular thrombus and 3 small adjacent thrombi.

Based on the echocardiographic data, recent pregnancy, and absence of other risk factors for heart failure, a diagnosis was made of peripartum cardiomyopathy with left ventricular thrombi and subsequent embolization to the spleen.
Standard heart failure therapy including diuretics, beta‐blockers, and angiotensin‐converting enzyme inhibitors and anticoagulation with warfarin were started. Within 24 hours, the patient was asymptomatic except for minimal abdominal pain. The patient was discharged home in a stable condition the following day. At her outpatient follow‐up 3 months later, she was well compensated and asymptomatic.
DISCUSSION
Using the search terms peripartum cardiomyopathy, cardiomyopathy, thromboembolism, and postpartum period, we performed a MEDLINE search of the English literature from 1950 to 2007. We did not find any reported cases of splenic infarction complicating peripartum cardiomyopathy.
Peripartum cardiomyopathy (PPCM) is a form of dilated cardiomyopathy that occurs as a complication of pregnancy. It can present with heart failure in the last month of pregnancy or within 5 months after delivery.1, 2 The incidence of PPCM is unknown but has been estimated at 1 in 30004000 live births.3
Our patient met the criteria for PPCM as set forth by the National Heart, Lung, and Blood Institute (NHLBI), in conjunction with the Office of Rare Disease of the National Institutes of Health in April 1997.3 To establish a diagnosis of PPCM, 4 criteria have to be met:
-
Development of heart failure in the last month of pregnancy or within 5 months after delivery;
-
Absence of an identifiable cause of heart failure;
-
Absence of recognizable heart disease prior to the last month of pregnancy; and
-
Left ventricular systolic dysfunction demonstrated by echocardiographic variables such as depressed shortening fraction or left ventricular ejection fraction < 45%.
Thromboembolism has been reported with an incidence of 4% to 30% in peripartum cardiomyopathy.4 In our literature review, we found several case reports of thromboembolic phenomena complicating peripartum cardiomyopathy. These included lower extremity arterial thromboembolism with compromised circulation,5 cerebral embolism,6 and acute myocardial infarction secondary to coronary artery embolism.7 This is the first reported case of splenic artery embolization leading to splenic infarct as a complication of peripartum cardiomyopathy.
Because of the high risk of thromboembolism, the NHLBI recommends that anticoagulation be added to the standard heart failure treatment of PPCM patients with an ejection fraction of less than 35%,3 although there are no prospective randomized clinical trial data to support this recommendation. For anticoagulation, heparin is generally used in the antepartum period and warfarin in the postpartum period. It has been recommended that anticoagulation be continued for as long as the cardiomegaly persists.1
In addition to anticoagulation for PPCM patients with an EF < 35%, standard heart failure therapy includes salt restriction, diuretics, and beta‐blockers. Angiotensin‐converting enzyme inhibitors can be teratogenic during pregnancy but can be used after delivery. Hydralazine can be used safely during pregnancy as an alternative to angiotensin‐converting enzyme inhibitors. Patients failing maximal medical management may be candidates for cardiac transplantation.
Recommendations regarding subsequent pregnancies seem related to the return of ventricular size and function. Patients whose heart size does not return to normal should be strongly advised to avoid future pregnancies.2, 3 Patients who recover ventricular function may have deterioration of left ventricular function with future pregnancies.8 They should be counseled about the risk and closely monitored for development of heart failure if they become pregnant again.
- ,.Peripartum cardiomyopathy.Circulation.1971;44:964–968.
- ,,, et al.Natural course of peripartum cardiomyopathy.Circulation.1971;44:1053–1061.
- ,,, et al.Peripartum Cardiomyopathy. National Heart, Lung, and Blood Institute and Office of Rare Diseases (National Institutes of Health) Workshop Recommendations and Review.JAMA.2000;283:1183–1188.
- .Peripartum cardiomyopathy.N Engl J Med.1985;312:1432–1437.
- ,,, et al.Peripartum cardiomyopathy presenting as lower extremity arterial thromboembolism.J Reprod Med.2000;45:351–353.
- ,,, et al.Cerebral embolism as the initial manifestation of peripartum cardiomyopathy.Neurology.1982;32:668–671.
- ,,, et al.Peripartum cardiomyopathy presenting as an acute myocardial infarction.Mayo Clin Proc.2002;77:500–501.
- ,,, et al.Recurrent peripartum cardiomyopathy.Eur J Obstet Gynecol Reprod Biol.1998;76:29–30.
- ,.Peripartum cardiomyopathy.Circulation.1971;44:964–968.
- ,,, et al.Natural course of peripartum cardiomyopathy.Circulation.1971;44:1053–1061.
- ,,, et al.Peripartum Cardiomyopathy. National Heart, Lung, and Blood Institute and Office of Rare Diseases (National Institutes of Health) Workshop Recommendations and Review.JAMA.2000;283:1183–1188.
- .Peripartum cardiomyopathy.N Engl J Med.1985;312:1432–1437.
- ,,, et al.Peripartum cardiomyopathy presenting as lower extremity arterial thromboembolism.J Reprod Med.2000;45:351–353.
- ,,, et al.Cerebral embolism as the initial manifestation of peripartum cardiomyopathy.Neurology.1982;32:668–671.
- ,,, et al.Peripartum cardiomyopathy presenting as an acute myocardial infarction.Mayo Clin Proc.2002;77:500–501.
- ,,, et al.Recurrent peripartum cardiomyopathy.Eur J Obstet Gynecol Reprod Biol.1998;76:29–30.