User login
A 46‐year‐old African American man presented to the emergency department with severe chest pain that awakened him from sleep. The pain was substernal, radiated to the neck and back, and was continuous, lasting approximately 1 hour. It was associated with nausea, diaphoresis, and dizziness. The patient denied dyspnea, orthopnea, paroxysmal nocturnal dyspnea, dysphagia, odynophagia, vomiting, fever, chills, or headache. He denied recent recreational drug use. He works as a landscaper.
Substernal chest pain radiating to the neck and back implicates structures in the middle mediastinum, chiefly the heart, aorta, esophagus, pulmonary arteries, and mediastinal pleura. The presence of nausea and diaphoresis suggests a vagal response to pain.
The sudden onset of symptoms and the lack of fevers and chills make infectious causes in the mediastinum such as mediastinitis and pneumonia less likely. Acute pulmonary embolism often presents with pleuritic pain and dyspnea, features not present in this patient.
The absence of odynophagia and dysphagia makes esophageal rupture or perforation of esophageal diverticula unlikely. Likewise, the absence of dyspnea, orthopnea, and paroxysmal nocturnal dyspnea makes it unlikely to be acute left ventricular failure from a sudden rise in left atrial pressure. Such a scenario may occur in the setting of a myocardial infarction or rupture of a papillary muscle, chordae tendineae, or sinus of Valsalva.
Dissection of the aorta with or without involvement of the aortic root merits strong consideration. Dissection involving the carotid or vertebral arteries could explain the patient's dizziness. Physical stresses in a landscaper may contribute to elevation in blood pressure and set the stage for an aortic dissection, especially with other risk factors.
The patient has a history of a positive PPD and was treated with isoniazid for 6 months. His mother had a history of hypertension and died from a myocardial infarction at age 64. His father's medical history is unknown. He has a history of alcohol abuse but has been abstinent for more than a year. He smoked marijuana and tobacco occasionally, with a 15 pack‐year cigarette history. He also stated that the last time he used cocaine was 1 year prior to admission.
That his mother succumbed to a myocardial infarction as well as having been hypertensive could be important family history risk factors given the patient's symptoms. Furthermore, the concurrent use of alcohol and tobacco by the patient increases his risk of developing severe hypertension. The use of cocaine is associated with sudden elevations in systemic blood pressure, which predispose to intimal damage in the aorta, especially if other risk factors are present. Marijuana smoking has been implicated in pulmonary aspergillosis, but this sudden presentation in the absence of pulmonary symptoms makes it most unlikely. Optimal therapy of a positive PPD should not predispose the patient to acute exudative pericarditis. Thus far the features suggest an acute vascular episode without significant compromise of cardiac output.
The patient was alert and in mild distress from chest discomfort. He was afebrile, with a blood pressure of 136/64 in the right arm and 139/63 in the left arm. Heart rate was 60 beats per minute, and the respiratory rate was 12 beats per minute, with an oxygen saturation of 99% while breathing room air. Examination of the head and neck revealed no signs of trauma. Jugular venous waveforms were normal, and carotid artery pulsations had normal strength and upstroke without audible bruits. The lungs were clear to auscultation. Heart sounds were notable for a 3/6 diastolic murmur heard best at the right sternal border. Rate and rhythm were regular, and the apical impulse was sustained but not displaced. The peripheral pulses were present and equal in quality throughout. The findings of the abdominal exam were normal, and the digital rectal examination was negative for occult blood. The findings of the neurologic, musculoskeletal, and dermatologic exams also were normal.
A slightly elevated pulse pressure without a significant difference in upper extremity blood pressure could be a result of aortic regurgitation, sinus of Valsalva rupture, or a high‐cardiac‐output state, as seen in thyrotoxicosis, anemia, or arteriovenous fistula.
The presence of a 3/6 diastolic murmur at the sternal border, however, favors conditions that cause aortic valve regurgitation and, less commonly, pulmonary valve regurgitation, or turbulent flow across the tricuspid valve. The murmur of pulmonary and aortic valve regurgitation can be difficult to distinguish by auscultation; however, the absence of other findings such as a right ventricular heave, elevated jugular venous pressure, or primary lung disease do not support a pulmonary valve etiology. Turbulent flow across the tricuspid valve can be seen in high‐output states, large atrial septal defects, and tricuspid stenosis. This type of murmur is heard best at the lower sternal border and tends to increase with inspiration. An early diastolic murmur would suggest aortic regurgitation, either from aortic valve or aortic root pathology. Aortic regurgitation would contribute to a sustained apical impulse.
Clear lungs and the absence of tachycardia suggest that left ventricle function is not severely compromised. These findings argue against acute rupture of the sinus of Valsalva, a condition that normally causes biventricular failure. The presence of equal peripheral pulses does not exclude the diagnosis of aortic dissection, as the dissection may have occurred proximal to the origins of the right innominate and the left subclavian arteries.
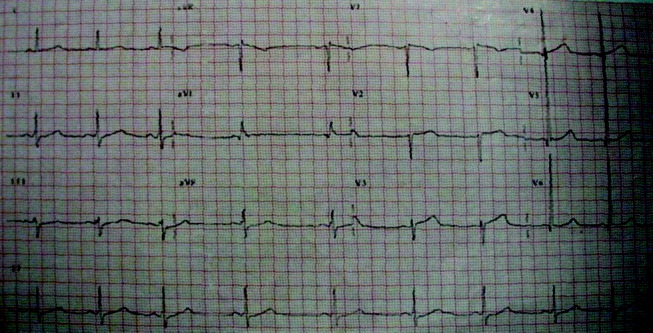
Initial laboratory studies revealed a hematocrit of 34.9, a leukocyte count of 6800/mm3, and a platelet count of 195 000/mm3. The levels of serum electrolytes, serum creatinine, blood urea nitrogen, and initial cardiac enzymes were normal. The urine drug screen was negative. The electrocardiogram showed evidence of left ventricular hypertrophy (Figure 1), and portable chest radiography revealed an enlarged cardiac silhouette and a widened mediastinum (Figure 2).
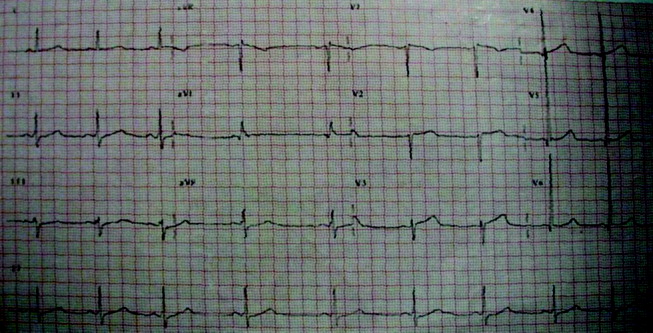
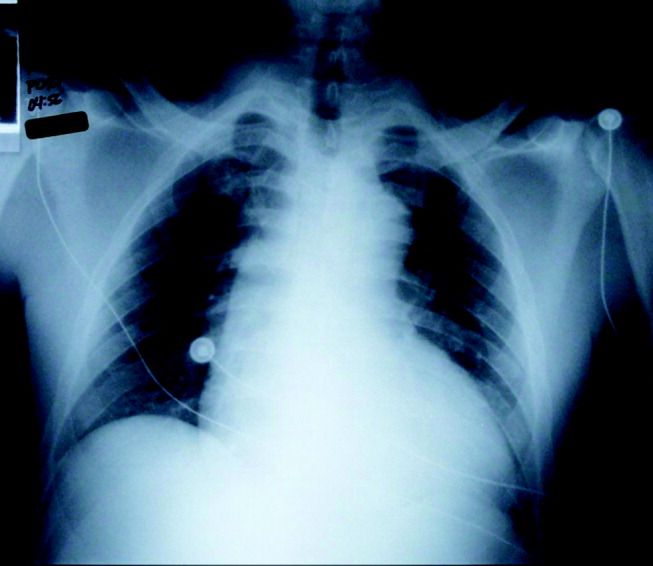
The presence of a widened mediastinum is consistent with aortic dissection but may also suggest a mass, aortic aneurysm, infiltrative disease, or a collection of fluid (eg, blood). A normal level of cardiac enzymes and the absence of ischemic findings on electrocardiography make myocardial infarction less likely. Given his hemodynamic stability, he is unlikely to have suffered cardiac rupture; however, he may still have a dissection of the aorta or a rupture of the sinus of Valsalva. The slight decline in hematocrit is compatible with either a mediastinal or pericardial collection of blood.
The patient was emergently sent for computerized axial tomography of the chest, which revealed a dilated aortic root at 6 cm but no evidence of aortic dissection (Figure 3). The lung fields were normal.
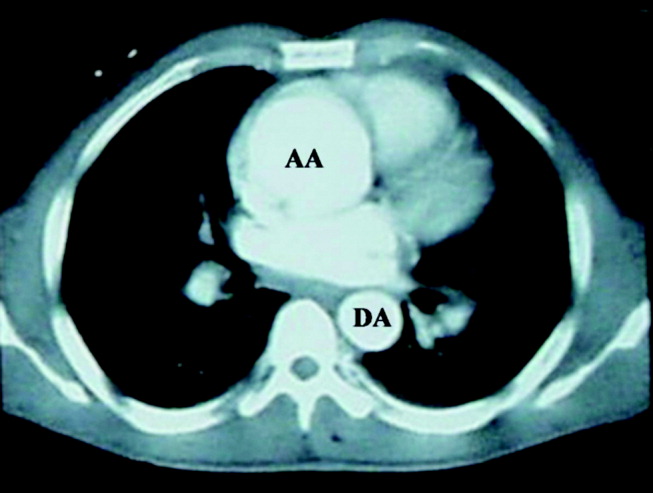
Despite a negative test, the presence of severe acute chest pain in the presence of a widened mediastinum is still concerning for aortic dissection. If the dissection involved the aortic valve annulus, the resulting acute regurgitation can not be severe, given the absence of left ventricular heart failure. Likewise, the presence of normal lung fields suggests the patient has no acute elevation of left ventricular end diastolic pressures such as is seen in ventricle septal defect, papillary muscle dysfunction, or acute mitral valvular lesion.
Cardiology consultants were emergently consulted and performed a transesophageal echocardiogram that confirmed a dilated aortic root (6.3 cm) without evidence of dissection. The patient was noted to have moderate to severe aortic regurgitation and a dilated left ventricle with moderate hypertrophy. A trace effusion was noted, and the left ventricular ejection fraction was estimated to be 50%.
The presence of moderate to severe aortic regurgitation with a dilated aortic root suggests 3 possibilities: undiagnosed dissection of the aortic root with preexisting aortic insufficiency (eg, bicuspid aortic valve, rheumatic valve disease, previous endocarditis); infectious (eg, syphilitic) or noninfectious (eg, ankylosing spondylitis, Takayasu's arteritis) meso‐aortitis causing aortic dilatation and subsequent regurgitation; or, finally, connective tissue diseases (eg, Marfan's syndrome, Ehlers‐Danlos), which can cause premature degeneration of the aortic media. The acuity of the patient's symptoms and the lack of systemic findings make a connective tissue or inflammatory disease unlikely. The clinical index of suspicion for aortic dissection needs to remain very high, as failure to make an expedient diagnosis may lead to complications and a deleterious outcome. Aortography may help to define this lesion.
The cardiothoracic surgical team was consulted and recommended aortic root and valve replacement. The patient was observed overnight and scheduled for preoperative cardiac catheterization the following morning. Aortogram revealed moderate to severe aortic insufficiency and a small dissection flap on the lesser curvature of the aorta, above the left coronary ostia (Figure 4). Coronary angiography revealed a 50% stenosis of the ostia of the first and second diagonal arteries, with no other flow‐limiting lesions.
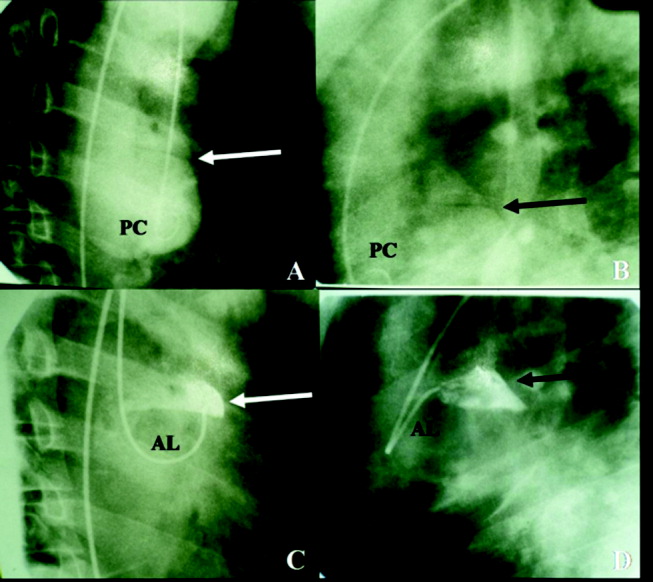
The study has clearly demonstrated that the patient suffered an acute aortic dissection without involvement of the aortic annulus. Given the absence of left ventricular failure, it appears the aortic regurgitation was chronic and secondary to a previously existing aortic aneurysm. Asymptomatic congenital defects of the meso‐aorta such as cystic medical necrosis can predispose to aortic dissection at a relatively young age. However, this patient's condition may have been aggravated by drug abuse, paroxysmal elevation of blood pressure during landscaping, or other risk factors. Surgical correction of the dissection and aortic regurgitation is necessary.
The patient underwent aortic valve replacement with a 25‐mm St. Jude mechanical valve and an ascending aortic transection repair with a 32‐mm Dacron tube graft. On postoperative day 9, the patient was discharged home in stable condition.
COMMENTARY
Ensuring both accuracy and efficiency when making a diagnosis can be difficult, particularly when patients present in an atypical fashion or when diagnostic testing yields inconclusive results. Thus, a physician must sift through each clinical clue, remembering that although certain findings are pathognomonic for a disease process, a constellation of signs and symptoms when present can be equally diagnostic.
The initial likelihood of disease (ie, pretest probability) is generated by the history and physical and laboratory examinations. If the pretest probability is high and the subsequent diagnostic test is positive, the modified likelihood of disease (post‐test probability) is nearly 100%. If, however, the pretest probability is high and the diagnostic test is negative, the likelihood of disease is less clear. In these situations, the physician can either review the initial findings that generated the pretest probability or perform an additional diagnostic test of higher sensitivity.
In this exercise, a 46‐year‐old man presented to the emergency department with severe chest pain and findings characteristic of aortic dissection. The physicians appropriately sent him for chest computerized tomography (CT) because of a high pretest likelihood of aortic dissection. Because this test did not confirm the presence of aortic dissection, the patient underwent transesophageal echocardiography (TEE), a test of equal or greater sensitivity.1, 2 This test was also negative; however, a small dissection flap was subsequently found during cardiac catheterization. In this case, a test of lower sensitivity and specificity confirmed the diagnosis of dissection,3 demonstrating the possibility that either CT or TEE can be misinterpreted. Indeed, a final review of the TEE, showed the dissection flap, albeit small, had been missed. In this case, a diagnostic error was made that delayed the diagnosis when sufficient information was available earlier. Diagnostic errors are prevalent in medical practice and are commonly the result of numerous factors, though cognitive problems appear to be the largest contributor to this process.4 In particular, faulty synthesis of information, rather than inadequate medical knowledge, is the most common cause of cognitive medical errors. The error made in this case likely falls under the subcategory of faulty test detection or perception and subsequent premature closure (failure to consider other possibilities once an initial diagnosis of uncomplicated aortic aneurysm had been reached).4
Though medical errors cannot be completely eliminated, cases such as this should be reviewed to understand the cognitive processes that may lead to an erroneous diagnosis. In addition to the false‐negative TEE finding, this patient also had a coexisting condition that may have preoccupied the medical team. The thoracic aortic aneurysm seen by all imaging modalities was large and required intervention regardless of the presence of a dissection. However, this chronic condition became the focus of treatment, and the acute event that precipitated admission was missed. Perhaps if the primary team maintained a very high index of suspicion for dissection and conveyed this to the cardiology consultants, a meticulous review of the TEE would have then followed that may have uncovered the subtle findings of the dissection flap.
Fortunately, definitive treatment with surgical aortic root and valve replacement was performed in a timely manner, as the consequences of a delayed diagnosis in this situation could have been catastrophic. The mortality rate of a type A dissection is extremely high initially (1%‐2% per hour),5, 6 and thus surgical intervention is typically performed immediately after the diagnosis and the extent of this disease is established, rather than the following morning.7
This case highlights not only the problems resulting in diagnostic errors but also exemplifies the thought process required to make a challenging diagnosis. Our case discussant was able to avoid cognitive pitfalls by presenting a broad differential diagnosis and reevaluating the diagnosis with each additional piece of information provided.8 An experienced clinician should realize that patients with an extremely high pretest probability of disease and a negative diagnostic test should be further investigated, regardless of the test sensitivity. Furthermore, time‐honored methods such as history taking, physical examination. and thoughtful analyses should remain critical tools in the process of reaching an accurate diagnosis despite technological advances in diagnostic testing.
- ,,, et al.The diagnosis of thoracic aortic dissection by noninvasive imaging procedures.N Engl J Med.1993;328(1):1–9.
- ,,, et al.Accuracy of biplane and multiplane transesophageal echocardiography in diagnosis of typical acute aortic dissection and intramural hematoma.J Am Coll Cardiol.1996;28:627–636.
- ,.Aortic dissection: new frontiers in diagnosis and management: Part I: from etiology to diagnostic strategies.Circulation.2003;108:628–635.
- ,,.Diagnostic error in internal medicine.Arch Intern Med.2005;165:1493–1499.
- ,,.Dissecting aneurysm of the aorta: a review of 505 cases.Medicine (Baltimore).1958;37(3):217–279.
- ,,, et al.The International Registry of Acute Aortic Dissection (IRAD): new insights into an old disease.JAMA.2000;283:897–903.
- ,.Surgery of the thoracic aorta.N Engl J Med.1997;336:1876–1888.
- .Clinical reasoning in medicine. In:Higgs J,Jones MA, eds.Clinical Reasoning in the Health Professions.Woburn, Mass:Butterworth‐Heinemann;1995:49–59.
A 46‐year‐old African American man presented to the emergency department with severe chest pain that awakened him from sleep. The pain was substernal, radiated to the neck and back, and was continuous, lasting approximately 1 hour. It was associated with nausea, diaphoresis, and dizziness. The patient denied dyspnea, orthopnea, paroxysmal nocturnal dyspnea, dysphagia, odynophagia, vomiting, fever, chills, or headache. He denied recent recreational drug use. He works as a landscaper.
Substernal chest pain radiating to the neck and back implicates structures in the middle mediastinum, chiefly the heart, aorta, esophagus, pulmonary arteries, and mediastinal pleura. The presence of nausea and diaphoresis suggests a vagal response to pain.
The sudden onset of symptoms and the lack of fevers and chills make infectious causes in the mediastinum such as mediastinitis and pneumonia less likely. Acute pulmonary embolism often presents with pleuritic pain and dyspnea, features not present in this patient.
The absence of odynophagia and dysphagia makes esophageal rupture or perforation of esophageal diverticula unlikely. Likewise, the absence of dyspnea, orthopnea, and paroxysmal nocturnal dyspnea makes it unlikely to be acute left ventricular failure from a sudden rise in left atrial pressure. Such a scenario may occur in the setting of a myocardial infarction or rupture of a papillary muscle, chordae tendineae, or sinus of Valsalva.
Dissection of the aorta with or without involvement of the aortic root merits strong consideration. Dissection involving the carotid or vertebral arteries could explain the patient's dizziness. Physical stresses in a landscaper may contribute to elevation in blood pressure and set the stage for an aortic dissection, especially with other risk factors.
The patient has a history of a positive PPD and was treated with isoniazid for 6 months. His mother had a history of hypertension and died from a myocardial infarction at age 64. His father's medical history is unknown. He has a history of alcohol abuse but has been abstinent for more than a year. He smoked marijuana and tobacco occasionally, with a 15 pack‐year cigarette history. He also stated that the last time he used cocaine was 1 year prior to admission.
That his mother succumbed to a myocardial infarction as well as having been hypertensive could be important family history risk factors given the patient's symptoms. Furthermore, the concurrent use of alcohol and tobacco by the patient increases his risk of developing severe hypertension. The use of cocaine is associated with sudden elevations in systemic blood pressure, which predispose to intimal damage in the aorta, especially if other risk factors are present. Marijuana smoking has been implicated in pulmonary aspergillosis, but this sudden presentation in the absence of pulmonary symptoms makes it most unlikely. Optimal therapy of a positive PPD should not predispose the patient to acute exudative pericarditis. Thus far the features suggest an acute vascular episode without significant compromise of cardiac output.
The patient was alert and in mild distress from chest discomfort. He was afebrile, with a blood pressure of 136/64 in the right arm and 139/63 in the left arm. Heart rate was 60 beats per minute, and the respiratory rate was 12 beats per minute, with an oxygen saturation of 99% while breathing room air. Examination of the head and neck revealed no signs of trauma. Jugular venous waveforms were normal, and carotid artery pulsations had normal strength and upstroke without audible bruits. The lungs were clear to auscultation. Heart sounds were notable for a 3/6 diastolic murmur heard best at the right sternal border. Rate and rhythm were regular, and the apical impulse was sustained but not displaced. The peripheral pulses were present and equal in quality throughout. The findings of the abdominal exam were normal, and the digital rectal examination was negative for occult blood. The findings of the neurologic, musculoskeletal, and dermatologic exams also were normal.
A slightly elevated pulse pressure without a significant difference in upper extremity blood pressure could be a result of aortic regurgitation, sinus of Valsalva rupture, or a high‐cardiac‐output state, as seen in thyrotoxicosis, anemia, or arteriovenous fistula.
The presence of a 3/6 diastolic murmur at the sternal border, however, favors conditions that cause aortic valve regurgitation and, less commonly, pulmonary valve regurgitation, or turbulent flow across the tricuspid valve. The murmur of pulmonary and aortic valve regurgitation can be difficult to distinguish by auscultation; however, the absence of other findings such as a right ventricular heave, elevated jugular venous pressure, or primary lung disease do not support a pulmonary valve etiology. Turbulent flow across the tricuspid valve can be seen in high‐output states, large atrial septal defects, and tricuspid stenosis. This type of murmur is heard best at the lower sternal border and tends to increase with inspiration. An early diastolic murmur would suggest aortic regurgitation, either from aortic valve or aortic root pathology. Aortic regurgitation would contribute to a sustained apical impulse.
Clear lungs and the absence of tachycardia suggest that left ventricle function is not severely compromised. These findings argue against acute rupture of the sinus of Valsalva, a condition that normally causes biventricular failure. The presence of equal peripheral pulses does not exclude the diagnosis of aortic dissection, as the dissection may have occurred proximal to the origins of the right innominate and the left subclavian arteries.
Initial laboratory studies revealed a hematocrit of 34.9, a leukocyte count of 6800/mm3, and a platelet count of 195 000/mm3. The levels of serum electrolytes, serum creatinine, blood urea nitrogen, and initial cardiac enzymes were normal. The urine drug screen was negative. The electrocardiogram showed evidence of left ventricular hypertrophy (Figure 1), and portable chest radiography revealed an enlarged cardiac silhouette and a widened mediastinum (Figure 2).


The presence of a widened mediastinum is consistent with aortic dissection but may also suggest a mass, aortic aneurysm, infiltrative disease, or a collection of fluid (eg, blood). A normal level of cardiac enzymes and the absence of ischemic findings on electrocardiography make myocardial infarction less likely. Given his hemodynamic stability, he is unlikely to have suffered cardiac rupture; however, he may still have a dissection of the aorta or a rupture of the sinus of Valsalva. The slight decline in hematocrit is compatible with either a mediastinal or pericardial collection of blood.
The patient was emergently sent for computerized axial tomography of the chest, which revealed a dilated aortic root at 6 cm but no evidence of aortic dissection (Figure 3). The lung fields were normal.

Despite a negative test, the presence of severe acute chest pain in the presence of a widened mediastinum is still concerning for aortic dissection. If the dissection involved the aortic valve annulus, the resulting acute regurgitation can not be severe, given the absence of left ventricular heart failure. Likewise, the presence of normal lung fields suggests the patient has no acute elevation of left ventricular end diastolic pressures such as is seen in ventricle septal defect, papillary muscle dysfunction, or acute mitral valvular lesion.
Cardiology consultants were emergently consulted and performed a transesophageal echocardiogram that confirmed a dilated aortic root (6.3 cm) without evidence of dissection. The patient was noted to have moderate to severe aortic regurgitation and a dilated left ventricle with moderate hypertrophy. A trace effusion was noted, and the left ventricular ejection fraction was estimated to be 50%.
The presence of moderate to severe aortic regurgitation with a dilated aortic root suggests 3 possibilities: undiagnosed dissection of the aortic root with preexisting aortic insufficiency (eg, bicuspid aortic valve, rheumatic valve disease, previous endocarditis); infectious (eg, syphilitic) or noninfectious (eg, ankylosing spondylitis, Takayasu's arteritis) meso‐aortitis causing aortic dilatation and subsequent regurgitation; or, finally, connective tissue diseases (eg, Marfan's syndrome, Ehlers‐Danlos), which can cause premature degeneration of the aortic media. The acuity of the patient's symptoms and the lack of systemic findings make a connective tissue or inflammatory disease unlikely. The clinical index of suspicion for aortic dissection needs to remain very high, as failure to make an expedient diagnosis may lead to complications and a deleterious outcome. Aortography may help to define this lesion.
The cardiothoracic surgical team was consulted and recommended aortic root and valve replacement. The patient was observed overnight and scheduled for preoperative cardiac catheterization the following morning. Aortogram revealed moderate to severe aortic insufficiency and a small dissection flap on the lesser curvature of the aorta, above the left coronary ostia (Figure 4). Coronary angiography revealed a 50% stenosis of the ostia of the first and second diagonal arteries, with no other flow‐limiting lesions.

The study has clearly demonstrated that the patient suffered an acute aortic dissection without involvement of the aortic annulus. Given the absence of left ventricular failure, it appears the aortic regurgitation was chronic and secondary to a previously existing aortic aneurysm. Asymptomatic congenital defects of the meso‐aorta such as cystic medical necrosis can predispose to aortic dissection at a relatively young age. However, this patient's condition may have been aggravated by drug abuse, paroxysmal elevation of blood pressure during landscaping, or other risk factors. Surgical correction of the dissection and aortic regurgitation is necessary.
The patient underwent aortic valve replacement with a 25‐mm St. Jude mechanical valve and an ascending aortic transection repair with a 32‐mm Dacron tube graft. On postoperative day 9, the patient was discharged home in stable condition.
COMMENTARY
Ensuring both accuracy and efficiency when making a diagnosis can be difficult, particularly when patients present in an atypical fashion or when diagnostic testing yields inconclusive results. Thus, a physician must sift through each clinical clue, remembering that although certain findings are pathognomonic for a disease process, a constellation of signs and symptoms when present can be equally diagnostic.
The initial likelihood of disease (ie, pretest probability) is generated by the history and physical and laboratory examinations. If the pretest probability is high and the subsequent diagnostic test is positive, the modified likelihood of disease (post‐test probability) is nearly 100%. If, however, the pretest probability is high and the diagnostic test is negative, the likelihood of disease is less clear. In these situations, the physician can either review the initial findings that generated the pretest probability or perform an additional diagnostic test of higher sensitivity.
In this exercise, a 46‐year‐old man presented to the emergency department with severe chest pain and findings characteristic of aortic dissection. The physicians appropriately sent him for chest computerized tomography (CT) because of a high pretest likelihood of aortic dissection. Because this test did not confirm the presence of aortic dissection, the patient underwent transesophageal echocardiography (TEE), a test of equal or greater sensitivity.1, 2 This test was also negative; however, a small dissection flap was subsequently found during cardiac catheterization. In this case, a test of lower sensitivity and specificity confirmed the diagnosis of dissection,3 demonstrating the possibility that either CT or TEE can be misinterpreted. Indeed, a final review of the TEE, showed the dissection flap, albeit small, had been missed. In this case, a diagnostic error was made that delayed the diagnosis when sufficient information was available earlier. Diagnostic errors are prevalent in medical practice and are commonly the result of numerous factors, though cognitive problems appear to be the largest contributor to this process.4 In particular, faulty synthesis of information, rather than inadequate medical knowledge, is the most common cause of cognitive medical errors. The error made in this case likely falls under the subcategory of faulty test detection or perception and subsequent premature closure (failure to consider other possibilities once an initial diagnosis of uncomplicated aortic aneurysm had been reached).4
Though medical errors cannot be completely eliminated, cases such as this should be reviewed to understand the cognitive processes that may lead to an erroneous diagnosis. In addition to the false‐negative TEE finding, this patient also had a coexisting condition that may have preoccupied the medical team. The thoracic aortic aneurysm seen by all imaging modalities was large and required intervention regardless of the presence of a dissection. However, this chronic condition became the focus of treatment, and the acute event that precipitated admission was missed. Perhaps if the primary team maintained a very high index of suspicion for dissection and conveyed this to the cardiology consultants, a meticulous review of the TEE would have then followed that may have uncovered the subtle findings of the dissection flap.
Fortunately, definitive treatment with surgical aortic root and valve replacement was performed in a timely manner, as the consequences of a delayed diagnosis in this situation could have been catastrophic. The mortality rate of a type A dissection is extremely high initially (1%‐2% per hour),5, 6 and thus surgical intervention is typically performed immediately after the diagnosis and the extent of this disease is established, rather than the following morning.7
This case highlights not only the problems resulting in diagnostic errors but also exemplifies the thought process required to make a challenging diagnosis. Our case discussant was able to avoid cognitive pitfalls by presenting a broad differential diagnosis and reevaluating the diagnosis with each additional piece of information provided.8 An experienced clinician should realize that patients with an extremely high pretest probability of disease and a negative diagnostic test should be further investigated, regardless of the test sensitivity. Furthermore, time‐honored methods such as history taking, physical examination. and thoughtful analyses should remain critical tools in the process of reaching an accurate diagnosis despite technological advances in diagnostic testing.
A 46‐year‐old African American man presented to the emergency department with severe chest pain that awakened him from sleep. The pain was substernal, radiated to the neck and back, and was continuous, lasting approximately 1 hour. It was associated with nausea, diaphoresis, and dizziness. The patient denied dyspnea, orthopnea, paroxysmal nocturnal dyspnea, dysphagia, odynophagia, vomiting, fever, chills, or headache. He denied recent recreational drug use. He works as a landscaper.
Substernal chest pain radiating to the neck and back implicates structures in the middle mediastinum, chiefly the heart, aorta, esophagus, pulmonary arteries, and mediastinal pleura. The presence of nausea and diaphoresis suggests a vagal response to pain.
The sudden onset of symptoms and the lack of fevers and chills make infectious causes in the mediastinum such as mediastinitis and pneumonia less likely. Acute pulmonary embolism often presents with pleuritic pain and dyspnea, features not present in this patient.
The absence of odynophagia and dysphagia makes esophageal rupture or perforation of esophageal diverticula unlikely. Likewise, the absence of dyspnea, orthopnea, and paroxysmal nocturnal dyspnea makes it unlikely to be acute left ventricular failure from a sudden rise in left atrial pressure. Such a scenario may occur in the setting of a myocardial infarction or rupture of a papillary muscle, chordae tendineae, or sinus of Valsalva.
Dissection of the aorta with or without involvement of the aortic root merits strong consideration. Dissection involving the carotid or vertebral arteries could explain the patient's dizziness. Physical stresses in a landscaper may contribute to elevation in blood pressure and set the stage for an aortic dissection, especially with other risk factors.
The patient has a history of a positive PPD and was treated with isoniazid for 6 months. His mother had a history of hypertension and died from a myocardial infarction at age 64. His father's medical history is unknown. He has a history of alcohol abuse but has been abstinent for more than a year. He smoked marijuana and tobacco occasionally, with a 15 pack‐year cigarette history. He also stated that the last time he used cocaine was 1 year prior to admission.
That his mother succumbed to a myocardial infarction as well as having been hypertensive could be important family history risk factors given the patient's symptoms. Furthermore, the concurrent use of alcohol and tobacco by the patient increases his risk of developing severe hypertension. The use of cocaine is associated with sudden elevations in systemic blood pressure, which predispose to intimal damage in the aorta, especially if other risk factors are present. Marijuana smoking has been implicated in pulmonary aspergillosis, but this sudden presentation in the absence of pulmonary symptoms makes it most unlikely. Optimal therapy of a positive PPD should not predispose the patient to acute exudative pericarditis. Thus far the features suggest an acute vascular episode without significant compromise of cardiac output.
The patient was alert and in mild distress from chest discomfort. He was afebrile, with a blood pressure of 136/64 in the right arm and 139/63 in the left arm. Heart rate was 60 beats per minute, and the respiratory rate was 12 beats per minute, with an oxygen saturation of 99% while breathing room air. Examination of the head and neck revealed no signs of trauma. Jugular venous waveforms were normal, and carotid artery pulsations had normal strength and upstroke without audible bruits. The lungs were clear to auscultation. Heart sounds were notable for a 3/6 diastolic murmur heard best at the right sternal border. Rate and rhythm were regular, and the apical impulse was sustained but not displaced. The peripheral pulses were present and equal in quality throughout. The findings of the abdominal exam were normal, and the digital rectal examination was negative for occult blood. The findings of the neurologic, musculoskeletal, and dermatologic exams also were normal.
A slightly elevated pulse pressure without a significant difference in upper extremity blood pressure could be a result of aortic regurgitation, sinus of Valsalva rupture, or a high‐cardiac‐output state, as seen in thyrotoxicosis, anemia, or arteriovenous fistula.
The presence of a 3/6 diastolic murmur at the sternal border, however, favors conditions that cause aortic valve regurgitation and, less commonly, pulmonary valve regurgitation, or turbulent flow across the tricuspid valve. The murmur of pulmonary and aortic valve regurgitation can be difficult to distinguish by auscultation; however, the absence of other findings such as a right ventricular heave, elevated jugular venous pressure, or primary lung disease do not support a pulmonary valve etiology. Turbulent flow across the tricuspid valve can be seen in high‐output states, large atrial septal defects, and tricuspid stenosis. This type of murmur is heard best at the lower sternal border and tends to increase with inspiration. An early diastolic murmur would suggest aortic regurgitation, either from aortic valve or aortic root pathology. Aortic regurgitation would contribute to a sustained apical impulse.
Clear lungs and the absence of tachycardia suggest that left ventricle function is not severely compromised. These findings argue against acute rupture of the sinus of Valsalva, a condition that normally causes biventricular failure. The presence of equal peripheral pulses does not exclude the diagnosis of aortic dissection, as the dissection may have occurred proximal to the origins of the right innominate and the left subclavian arteries.
Initial laboratory studies revealed a hematocrit of 34.9, a leukocyte count of 6800/mm3, and a platelet count of 195 000/mm3. The levels of serum electrolytes, serum creatinine, blood urea nitrogen, and initial cardiac enzymes were normal. The urine drug screen was negative. The electrocardiogram showed evidence of left ventricular hypertrophy (Figure 1), and portable chest radiography revealed an enlarged cardiac silhouette and a widened mediastinum (Figure 2).


The presence of a widened mediastinum is consistent with aortic dissection but may also suggest a mass, aortic aneurysm, infiltrative disease, or a collection of fluid (eg, blood). A normal level of cardiac enzymes and the absence of ischemic findings on electrocardiography make myocardial infarction less likely. Given his hemodynamic stability, he is unlikely to have suffered cardiac rupture; however, he may still have a dissection of the aorta or a rupture of the sinus of Valsalva. The slight decline in hematocrit is compatible with either a mediastinal or pericardial collection of blood.
The patient was emergently sent for computerized axial tomography of the chest, which revealed a dilated aortic root at 6 cm but no evidence of aortic dissection (Figure 3). The lung fields were normal.

Despite a negative test, the presence of severe acute chest pain in the presence of a widened mediastinum is still concerning for aortic dissection. If the dissection involved the aortic valve annulus, the resulting acute regurgitation can not be severe, given the absence of left ventricular heart failure. Likewise, the presence of normal lung fields suggests the patient has no acute elevation of left ventricular end diastolic pressures such as is seen in ventricle septal defect, papillary muscle dysfunction, or acute mitral valvular lesion.
Cardiology consultants were emergently consulted and performed a transesophageal echocardiogram that confirmed a dilated aortic root (6.3 cm) without evidence of dissection. The patient was noted to have moderate to severe aortic regurgitation and a dilated left ventricle with moderate hypertrophy. A trace effusion was noted, and the left ventricular ejection fraction was estimated to be 50%.
The presence of moderate to severe aortic regurgitation with a dilated aortic root suggests 3 possibilities: undiagnosed dissection of the aortic root with preexisting aortic insufficiency (eg, bicuspid aortic valve, rheumatic valve disease, previous endocarditis); infectious (eg, syphilitic) or noninfectious (eg, ankylosing spondylitis, Takayasu's arteritis) meso‐aortitis causing aortic dilatation and subsequent regurgitation; or, finally, connective tissue diseases (eg, Marfan's syndrome, Ehlers‐Danlos), which can cause premature degeneration of the aortic media. The acuity of the patient's symptoms and the lack of systemic findings make a connective tissue or inflammatory disease unlikely. The clinical index of suspicion for aortic dissection needs to remain very high, as failure to make an expedient diagnosis may lead to complications and a deleterious outcome. Aortography may help to define this lesion.
The cardiothoracic surgical team was consulted and recommended aortic root and valve replacement. The patient was observed overnight and scheduled for preoperative cardiac catheterization the following morning. Aortogram revealed moderate to severe aortic insufficiency and a small dissection flap on the lesser curvature of the aorta, above the left coronary ostia (Figure 4). Coronary angiography revealed a 50% stenosis of the ostia of the first and second diagonal arteries, with no other flow‐limiting lesions.

The study has clearly demonstrated that the patient suffered an acute aortic dissection without involvement of the aortic annulus. Given the absence of left ventricular failure, it appears the aortic regurgitation was chronic and secondary to a previously existing aortic aneurysm. Asymptomatic congenital defects of the meso‐aorta such as cystic medical necrosis can predispose to aortic dissection at a relatively young age. However, this patient's condition may have been aggravated by drug abuse, paroxysmal elevation of blood pressure during landscaping, or other risk factors. Surgical correction of the dissection and aortic regurgitation is necessary.
The patient underwent aortic valve replacement with a 25‐mm St. Jude mechanical valve and an ascending aortic transection repair with a 32‐mm Dacron tube graft. On postoperative day 9, the patient was discharged home in stable condition.
COMMENTARY
Ensuring both accuracy and efficiency when making a diagnosis can be difficult, particularly when patients present in an atypical fashion or when diagnostic testing yields inconclusive results. Thus, a physician must sift through each clinical clue, remembering that although certain findings are pathognomonic for a disease process, a constellation of signs and symptoms when present can be equally diagnostic.
The initial likelihood of disease (ie, pretest probability) is generated by the history and physical and laboratory examinations. If the pretest probability is high and the subsequent diagnostic test is positive, the modified likelihood of disease (post‐test probability) is nearly 100%. If, however, the pretest probability is high and the diagnostic test is negative, the likelihood of disease is less clear. In these situations, the physician can either review the initial findings that generated the pretest probability or perform an additional diagnostic test of higher sensitivity.
In this exercise, a 46‐year‐old man presented to the emergency department with severe chest pain and findings characteristic of aortic dissection. The physicians appropriately sent him for chest computerized tomography (CT) because of a high pretest likelihood of aortic dissection. Because this test did not confirm the presence of aortic dissection, the patient underwent transesophageal echocardiography (TEE), a test of equal or greater sensitivity.1, 2 This test was also negative; however, a small dissection flap was subsequently found during cardiac catheterization. In this case, a test of lower sensitivity and specificity confirmed the diagnosis of dissection,3 demonstrating the possibility that either CT or TEE can be misinterpreted. Indeed, a final review of the TEE, showed the dissection flap, albeit small, had been missed. In this case, a diagnostic error was made that delayed the diagnosis when sufficient information was available earlier. Diagnostic errors are prevalent in medical practice and are commonly the result of numerous factors, though cognitive problems appear to be the largest contributor to this process.4 In particular, faulty synthesis of information, rather than inadequate medical knowledge, is the most common cause of cognitive medical errors. The error made in this case likely falls under the subcategory of faulty test detection or perception and subsequent premature closure (failure to consider other possibilities once an initial diagnosis of uncomplicated aortic aneurysm had been reached).4
Though medical errors cannot be completely eliminated, cases such as this should be reviewed to understand the cognitive processes that may lead to an erroneous diagnosis. In addition to the false‐negative TEE finding, this patient also had a coexisting condition that may have preoccupied the medical team. The thoracic aortic aneurysm seen by all imaging modalities was large and required intervention regardless of the presence of a dissection. However, this chronic condition became the focus of treatment, and the acute event that precipitated admission was missed. Perhaps if the primary team maintained a very high index of suspicion for dissection and conveyed this to the cardiology consultants, a meticulous review of the TEE would have then followed that may have uncovered the subtle findings of the dissection flap.
Fortunately, definitive treatment with surgical aortic root and valve replacement was performed in a timely manner, as the consequences of a delayed diagnosis in this situation could have been catastrophic. The mortality rate of a type A dissection is extremely high initially (1%‐2% per hour),5, 6 and thus surgical intervention is typically performed immediately after the diagnosis and the extent of this disease is established, rather than the following morning.7
This case highlights not only the problems resulting in diagnostic errors but also exemplifies the thought process required to make a challenging diagnosis. Our case discussant was able to avoid cognitive pitfalls by presenting a broad differential diagnosis and reevaluating the diagnosis with each additional piece of information provided.8 An experienced clinician should realize that patients with an extremely high pretest probability of disease and a negative diagnostic test should be further investigated, regardless of the test sensitivity. Furthermore, time‐honored methods such as history taking, physical examination. and thoughtful analyses should remain critical tools in the process of reaching an accurate diagnosis despite technological advances in diagnostic testing.
- ,,, et al.The diagnosis of thoracic aortic dissection by noninvasive imaging procedures.N Engl J Med.1993;328(1):1–9.
- ,,, et al.Accuracy of biplane and multiplane transesophageal echocardiography in diagnosis of typical acute aortic dissection and intramural hematoma.J Am Coll Cardiol.1996;28:627–636.
- ,.Aortic dissection: new frontiers in diagnosis and management: Part I: from etiology to diagnostic strategies.Circulation.2003;108:628–635.
- ,,.Diagnostic error in internal medicine.Arch Intern Med.2005;165:1493–1499.
- ,,.Dissecting aneurysm of the aorta: a review of 505 cases.Medicine (Baltimore).1958;37(3):217–279.
- ,,, et al.The International Registry of Acute Aortic Dissection (IRAD): new insights into an old disease.JAMA.2000;283:897–903.
- ,.Surgery of the thoracic aorta.N Engl J Med.1997;336:1876–1888.
- .Clinical reasoning in medicine. In:Higgs J,Jones MA, eds.Clinical Reasoning in the Health Professions.Woburn, Mass:Butterworth‐Heinemann;1995:49–59.
- ,,, et al.The diagnosis of thoracic aortic dissection by noninvasive imaging procedures.N Engl J Med.1993;328(1):1–9.
- ,,, et al.Accuracy of biplane and multiplane transesophageal echocardiography in diagnosis of typical acute aortic dissection and intramural hematoma.J Am Coll Cardiol.1996;28:627–636.
- ,.Aortic dissection: new frontiers in diagnosis and management: Part I: from etiology to diagnostic strategies.Circulation.2003;108:628–635.
- ,,.Diagnostic error in internal medicine.Arch Intern Med.2005;165:1493–1499.
- ,,.Dissecting aneurysm of the aorta: a review of 505 cases.Medicine (Baltimore).1958;37(3):217–279.
- ,,, et al.The International Registry of Acute Aortic Dissection (IRAD): new insights into an old disease.JAMA.2000;283:897–903.
- ,.Surgery of the thoracic aorta.N Engl J Med.1997;336:1876–1888.
- .Clinical reasoning in medicine. In:Higgs J,Jones MA, eds.Clinical Reasoning in the Health Professions.Woburn, Mass:Butterworth‐Heinemann;1995:49–59.