User login
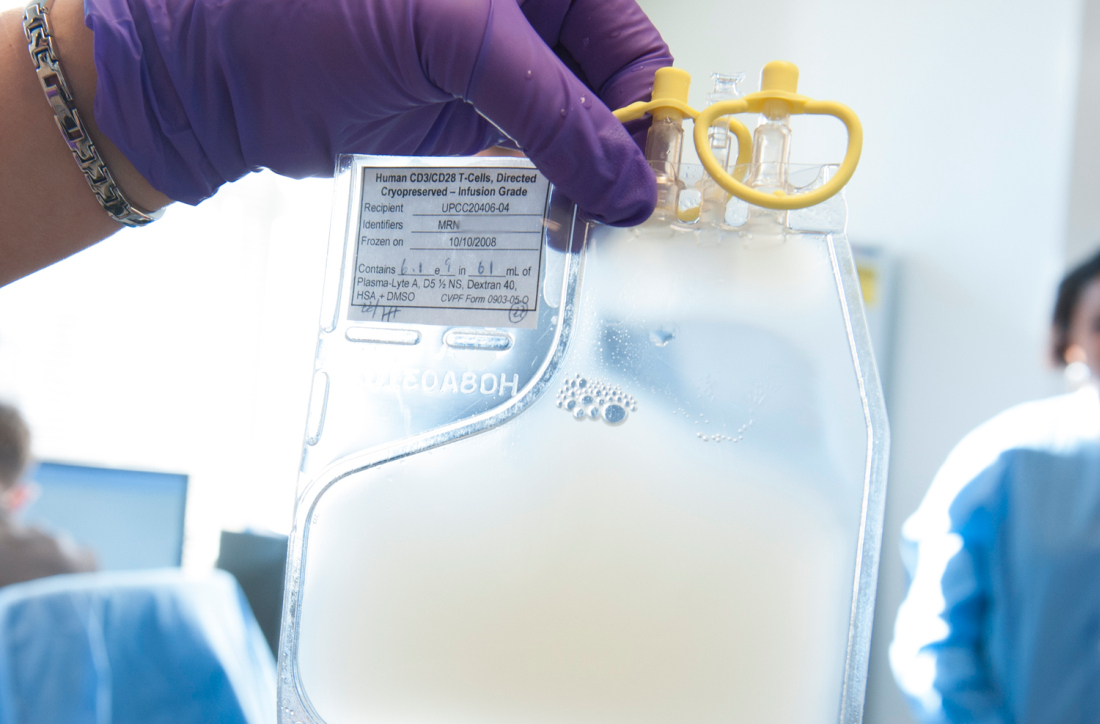
Hospitals could get a payment bump for administering chimeric antigen receptor (CAR) T-cell therapies under a proposed rule issued by the Centers for Medicare & Medicaid Services.
The proposal calls for raising the new technology add-on payment (NTAP) associated with the therapies from 50% of the technology to 65%, an increase from $186,500 to $242,450.
Beginning with discharges on Oct. 1, 2019, if discharge costs involving a new medical service or technology exceed the full Medicare Severity Diagnosis-Related Group (DRG) payment, Medicare would make an add-on payment of either 65% of the cost of the new medical service or technology, or 65% of the amount by which the costs of the case exceed the standard DRG payment, whichever is less.
Roy Silverstein, MD, president of the American Society of Hematology, said the group was pleased that the CMS is examining its existing payment policies to identify more realistic ways to account for the costs of administering CAR T-cell therapies.
“While ASH had originally suggested a higher [new technology] payment, any increase is an improvement,” Dr. Silverstein said in a statement. “While the proposal from CMS is promising, it is not a one-stop solution for making CAR T more accessible to patients. Just as these therapies are innovative, it is going to take some innovation on the part of CMS to develop a plan that equitably compensates providers and institutions so that offering the therapy is sustainable.”
The agency’s proposal follows an August 2018 final rule by the CMS that set a new payment scheme for inpatient administration of two CAR T-cell therapies. The rule categorized CAR T-cell therapies under the umbrella of the renamed Medicare Severity–Diagnosis Related Groups (MS-DRG) 016 – Autologous Bone Marrow Transplant with CC/MCC or T-cell Immunotherapy – and assigned ICD-10 PCS procedure codes XW033C3 and XW043C3 to the use of axicabtagene ciloleucel (Yescarta) and tisagenlecleucel (Kymriah) in the inpatient setting for fiscal year 2019, which began in October 2018.
In April 2018, the CMS announced payment rates for outpatient administration of the two drugs, settling on $395,380 for axicabtagene ciloleucel and $500,839 for tisagenlecleucel. The two medications have list prices of $373,000 and $475,000, respectively.
In February 2019, the CMS also proposed to cover CAR T-cell therapy for cancer patients participating in clinical trials that study the treatment’s effectiveness. A final decision on the proposal is expected in May 2019.
In the current proposal, the CMS acknowledged requests calling for the agency to create a new MS-DRG for procedures involving CAR T-cell therapies to improve payment in the inpatient setting. However, the agency declined to create a new MS-DRG for CAR T-cell cases, writing that the move is premature given the relative newness of CAR T-cell therapy and the agency’s proposal to continue new technology add-on payments for fiscal 2020 for Kymriah and Yescarta.
However, the agency is requesting public comments on whether, in light of additional experience with billing and payment for cases involving CAR T-cell therapies to Medicare patients, the CMS should consider using a specific cost-to-charge ratio for ICD-10-PCS procedure codes used to report the performance of procedures involving CAR T-cell therapies.
Comments on the proposed rule will be accepted until June 24.
Hospitals could get a payment bump for administering chimeric antigen receptor (CAR) T-cell therapies under a proposed rule issued by the Centers for Medicare & Medicaid Services.
The proposal calls for raising the new technology add-on payment (NTAP) associated with the therapies from 50% of the technology to 65%, an increase from $186,500 to $242,450.
Beginning with discharges on Oct. 1, 2019, if discharge costs involving a new medical service or technology exceed the full Medicare Severity Diagnosis-Related Group (DRG) payment, Medicare would make an add-on payment of either 65% of the cost of the new medical service or technology, or 65% of the amount by which the costs of the case exceed the standard DRG payment, whichever is less.
Roy Silverstein, MD, president of the American Society of Hematology, said the group was pleased that the CMS is examining its existing payment policies to identify more realistic ways to account for the costs of administering CAR T-cell therapies.
“While ASH had originally suggested a higher [new technology] payment, any increase is an improvement,” Dr. Silverstein said in a statement. “While the proposal from CMS is promising, it is not a one-stop solution for making CAR T more accessible to patients. Just as these therapies are innovative, it is going to take some innovation on the part of CMS to develop a plan that equitably compensates providers and institutions so that offering the therapy is sustainable.”
The agency’s proposal follows an August 2018 final rule by the CMS that set a new payment scheme for inpatient administration of two CAR T-cell therapies. The rule categorized CAR T-cell therapies under the umbrella of the renamed Medicare Severity–Diagnosis Related Groups (MS-DRG) 016 – Autologous Bone Marrow Transplant with CC/MCC or T-cell Immunotherapy – and assigned ICD-10 PCS procedure codes XW033C3 and XW043C3 to the use of axicabtagene ciloleucel (Yescarta) and tisagenlecleucel (Kymriah) in the inpatient setting for fiscal year 2019, which began in October 2018.
In April 2018, the CMS announced payment rates for outpatient administration of the two drugs, settling on $395,380 for axicabtagene ciloleucel and $500,839 for tisagenlecleucel. The two medications have list prices of $373,000 and $475,000, respectively.
In February 2019, the CMS also proposed to cover CAR T-cell therapy for cancer patients participating in clinical trials that study the treatment’s effectiveness. A final decision on the proposal is expected in May 2019.
In the current proposal, the CMS acknowledged requests calling for the agency to create a new MS-DRG for procedures involving CAR T-cell therapies to improve payment in the inpatient setting. However, the agency declined to create a new MS-DRG for CAR T-cell cases, writing that the move is premature given the relative newness of CAR T-cell therapy and the agency’s proposal to continue new technology add-on payments for fiscal 2020 for Kymriah and Yescarta.
However, the agency is requesting public comments on whether, in light of additional experience with billing and payment for cases involving CAR T-cell therapies to Medicare patients, the CMS should consider using a specific cost-to-charge ratio for ICD-10-PCS procedure codes used to report the performance of procedures involving CAR T-cell therapies.
Comments on the proposed rule will be accepted until June 24.
Hospitals could get a payment bump for administering chimeric antigen receptor (CAR) T-cell therapies under a proposed rule issued by the Centers for Medicare & Medicaid Services.
The proposal calls for raising the new technology add-on payment (NTAP) associated with the therapies from 50% of the technology to 65%, an increase from $186,500 to $242,450.
Beginning with discharges on Oct. 1, 2019, if discharge costs involving a new medical service or technology exceed the full Medicare Severity Diagnosis-Related Group (DRG) payment, Medicare would make an add-on payment of either 65% of the cost of the new medical service or technology, or 65% of the amount by which the costs of the case exceed the standard DRG payment, whichever is less.
Roy Silverstein, MD, president of the American Society of Hematology, said the group was pleased that the CMS is examining its existing payment policies to identify more realistic ways to account for the costs of administering CAR T-cell therapies.
“While ASH had originally suggested a higher [new technology] payment, any increase is an improvement,” Dr. Silverstein said in a statement. “While the proposal from CMS is promising, it is not a one-stop solution for making CAR T more accessible to patients. Just as these therapies are innovative, it is going to take some innovation on the part of CMS to develop a plan that equitably compensates providers and institutions so that offering the therapy is sustainable.”
The agency’s proposal follows an August 2018 final rule by the CMS that set a new payment scheme for inpatient administration of two CAR T-cell therapies. The rule categorized CAR T-cell therapies under the umbrella of the renamed Medicare Severity–Diagnosis Related Groups (MS-DRG) 016 – Autologous Bone Marrow Transplant with CC/MCC or T-cell Immunotherapy – and assigned ICD-10 PCS procedure codes XW033C3 and XW043C3 to the use of axicabtagene ciloleucel (Yescarta) and tisagenlecleucel (Kymriah) in the inpatient setting for fiscal year 2019, which began in October 2018.
In April 2018, the CMS announced payment rates for outpatient administration of the two drugs, settling on $395,380 for axicabtagene ciloleucel and $500,839 for tisagenlecleucel. The two medications have list prices of $373,000 and $475,000, respectively.
In February 2019, the CMS also proposed to cover CAR T-cell therapy for cancer patients participating in clinical trials that study the treatment’s effectiveness. A final decision on the proposal is expected in May 2019.
In the current proposal, the CMS acknowledged requests calling for the agency to create a new MS-DRG for procedures involving CAR T-cell therapies to improve payment in the inpatient setting. However, the agency declined to create a new MS-DRG for CAR T-cell cases, writing that the move is premature given the relative newness of CAR T-cell therapy and the agency’s proposal to continue new technology add-on payments for fiscal 2020 for Kymriah and Yescarta.
However, the agency is requesting public comments on whether, in light of additional experience with billing and payment for cases involving CAR T-cell therapies to Medicare patients, the CMS should consider using a specific cost-to-charge ratio for ICD-10-PCS procedure codes used to report the performance of procedures involving CAR T-cell therapies.
Comments on the proposed rule will be accepted until June 24.