User login
Pneumonia is associated with as many as 2 million annual deaths among children globally and 19% of all deaths in children less than 5 years of age (1). It is one of the most common diagnoses made in the acutely ill child, with an annual incidence of 34 to 40 cases per 1,000 children in Europe and North America.
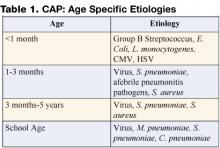
In the past, viral pathogens were estimated to cause as many as 80% of cases. Streptococcus pneumoniae was generally regarded as the most frequent bacterial cause of community-acquired pneumonia (CAP), especially in cases with complicated parapneumonic effusions. Infectious etiologies are age specific, with bacterial etiologies predominating in the very young infant and viral pathogens in the older infant and adult (Table 1). Knowledge of the most likely pathogen, the prevailing susceptibilities of these infecting pathogens, and the severity of the illness will help guide antibiotic and other treatment decision making.
Most children do not require hospital admission, and mildly ill children who likely have a viral illness do not need antibiotics. The following guideline will attempt to help the practitioner identify those who do require hospitalization and provide an approach to management of those with complicated infection.
Recognition of the Patient with CAP
The first obstacle is to identify the patient with pneumonia. In managing the child with CAP, it is important to distinguish those with other underlying pathology, including asthma, RSV, or other confirmed viral etiology. It is important to remember that pathogens in the compromised host, cystic fibrosis patient, or patient with other chronic pulmonary pathology are different from typical CAP pathogens and include a wide differential. Most patients with CAP have an acute illness associated with fever (>38°C), cough, and evidence of lower respiratory tract symptoms/signs. Chest radiograph typically shows pulmonary infiltrate. Whether this is patchy infiltrate or lobar in appearance can assist the practitioner in treatment decision making in that the latter is much more likely to be associated with a bacterial etiology.
Once the diagnosis is considered, further assessment should focus on hydration status, hemodynamic parameters, and oxygenation. A careful assessment should identify other associated foci (i.e., meningitis or bacteremia) on examination and laboratory evaluation.
Identification of the Patient Requiring Hospitalization
Consider hospital admission for the toxic patient, those with altered mental status, significant dehydration, hypoxemia, dyspnea, grunting respirations, or retractions, and any patient with hemodynamic instability. Chest radiograph showing a significant pleural effusion should also be considered an indicator for hospital admission.
Bacterial pathogens are more likely in the severely ill patient, patients with a rapidly progressive process, and those with radiographic evidence of lobar consolidation or pleural effusion. Some children with viral processes may require admission for supportive care.
Prompt Recognition of the Patient with Empyema
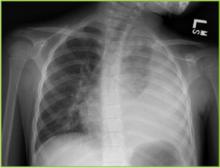
For the patient with pneumonia and parapneumonic effusion, distinction between a free-flowing effusion and pleural empyema is critical. Standard plain film can identify pulmonary infiltrate and often effusion and lateral decubitus films can help identify free flowing effusion (Figure 1). While CT scan more effectively identifies pleural fibrinous adhesions that may entrap lung, ultrasound most effectively identifies complex fluid collections with loculation and septation, and it can be utilized to guide thoracentesis.
Empyema is defined as pus in the pleural space and is estimated to occur in 10–40% of patients with pneumonia. Empyema may also result from causes other than a complication of bacterial pneumonia, such as thoracic trauma or postsurgical complication, rupture of lung abscess, esophageal tear, or complication of indwelling catheter. It generally occurs in stages including acute (early-cloudy fluid), fibrino-purulent (thicker, multiloculated fluid), and organized (late with thick pleural peel and entrapment of lung).
Pleural fluid evaluation is important both in diagnosis and in guiding treatment in such cases. Pleural fluid collections are defined as transudative or exudative based on biochemical evaluation. Evaluation includes cell type and differential, pH, glucose, protein and LDH. Gram-stained smear needs to be performed on all specimens at the time of culture. Empyema is exudative, typically with low glucose and high LDH (Table 2 on page 64) (2).
Changing Epidemiology and Antibiotic Decision Making
Data presented by Finland and Barnes in 1978 confirmed that S. pneumoniae, group A streptococcus (GAS), and Staphylococcus aureus were the most commonly identified pathogens in empyema cases in 1935, with S. aureus emerging in the 1950s (3). Most literature from the 1960–1980s detailing etiology of pneumonia with pleural empyema continued to emphasize the role of S. aureus in such cases. In all reviews, staphylococcal pneumonia is noted primarily to be a disease of infants. In 1 review of 100 cases of staphylococcal pneumonia, the median age was 5 months, 78 patients being below 1 year of age (4). Chartrand and McCracken analyzed 79 cases of staphylococcal pneumonia and noted that in about 75% of cases, staphylococcal pneumonia was a primary pneumonia in infants with a median age of 6 months. In this study, older children were more likely to have pulmonary involvement as a secondary finding in the setting of disseminated staphylococcal disease. A pleural effusion was found in 80% of infants with primary pneumonia and in 61% of those with secondary disease, thus providing the tip-off of a more serious process to the clinician (5). A high index of suspicion for S. aureus in the young infant with pneumonia is important, as physicians need to expect a rapidly progressive clinical course. Those infants frequently require ventilatory support, alteration in antibiotic choice, and the prompt recognition of pleural complications including pneumothoraces and pneumatoceles.
Data in the 1990s emphasized the role of multidrug resistant pneumococcus as a pathogen in empyema. In a recent review of cases in the postpneumococcal conjugate disease era, pneumococcus remained the most commonly confirmed etiologic agent, with other gram-positive pathogens, including GAS and S. aureus, also documented (6). Despite widespread implementation of pneumococcal conjugate vaccine (PCV), and a population based surveillance study in the US that suggested that adding PCV to the childhood immunization schedule was associated with a 10-fold greater reduction in pneumonia (7), serious pneumonia caused by S. pneumoniae continued to be reported. The prevalence of serotype 1 and 3 as the etiologies of such infections may limit the utility of the current vaccine. One study from Greece demonstrated that the most common serotypes causing bacteremic pneumonia were 14, 6B, 1 and 19F (8). Childhood empyema in the UK is noted to be increasing, and a recent study of 47 empyema cases confirmed pneumococcus as the major pathogen, with over half caused by serotype 1 (9).
More recent data suggest yet another change to the epidemiology of empyema. Schultz et al. from Houston, TX, reviewed a decade of experience from 1993–2002, and while they identified a decrease in total cases of empyema, the emergence of methicillin-resistant S. aureus (MRSA) infection was noted (10).
While MRSA has long been considered an important pathogen in the etiology of healthcare-associated infection, experience in our institution also confirms the appearance of an increasing number of cases of community-acquired MRSA disease. Vancomycin is clearly part of the treatment regimen in the child at risk for staphylococcal pneumonia, though many have utilized clindamycin for the non–critically ill patient. The increase in such cases clearly has important implications for treatment decisions, as MRSA with inducible clindamycin resistance is not yet recognized in every facility. Data are not available to confirm the utility of trimethoprim-sulfamethoxazole in serious community-aquired MRSA infections, and the role for newer antibiotics, such as linezolid, has not been clearly defined.
Management: Antibiotics and the Role of Pleural Drainage Procedures
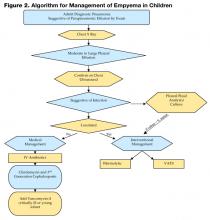
Figure 2 on page 58 shows an algorithm that guides clinical management of the empyema patient. Once a diagnosis is made, attention should be directed to fluid and electrolyte correction, hemodynamic stabilization, and respiratory support (i.e., oxygenation and ventilation). Antibiotics should be initiated and the choice is based on severity of illness and age of the child.
Drainage of the pleural pus has long been recognized as integral to the success in treatment of pneumonia with empyema. Recently, there has been much debate concerning which modality to use and when.
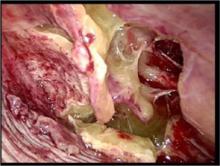
Intrapleural fibrinolytic therapy has been shown in multiple studies to decrease length of stay without increased risk. Data compiled in the Cochrane database comparing fibrinolytic therapy vs. more conservative management suggests that intrapleural fibrinolytic therapy confers significant benefit when compared with normal saline control; however, a definitive statement was not made, given that the trial numbers were too small (11). More recent data from the Cochrane database and a systematic review suggest that video-assisted thoracostomy (VATS) performed early in the disease course is associated with better outcome than chest tube drainage with streptokinase with regard to duration of chest tube placement and hospital stay. However there are questions about validity, and this study is also too small to draw conclusions (12,13). Figure 3 shows the typical findings encountered at VATS in a child with empyema.
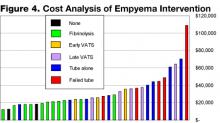
A retrospective chart review from our institution from December 2000 to March 2004, excluding immunocompromised hosts, found 96 cases of radiographic pneumonia with pleural effusion. Thirty-four met criteria for empyema, including ultrasound and/or chest CT showing pleural fluid loculation and septation, or purulent fluid/positive culture. Average age was 5 years, and pathogens were defined in 38% of patients. Length of stay averaged 9 days, with a range of 5–23 days. Two had no intervention and had a stay of 8 days, 14 had tube thoracostomy and had an average stay of 11.5 days with 6 failures, 10 had thoracostomy and fibrinolytic therapy with an average stay of 7 days, 3 had early VATS with an average stay of 7 days, and 5 had late VATS with an average stay of 10.4 days. In our institution, among invasive interventions, tube thoracostomy alone had longer LOS and more failures. Early VATS and intrapleural fibrinolysis have shorter stays and are on the lower end of the cost scale: $25,549 vs. $21,062 respectively (Figure 4).
The decision for interventional management of empyema will likely be institutionally variable in the absence of large randomized controlled studies. Institutions with aggressive interventional radiologists may favor thoracostomy tube with fibrinolysis. Those with surgeons skilled in video-scopic surgery may provide early VATS. Data on interventions clearly show benefit beyond that provided by routine chest tube placement. The key becomes prompt diagnosis of empyema with early use of ultrasound, knowledge of local antibiotic susceptibilities, and clear guidelines set up by each institution to guide interventional management.
The Future
Increasing the accuracy of diagnostic testing for children with CAP would likely lead to decreased morbidity, mortality, and total cost of care. The use of PCR is becoming more widespread and could be utilized to more rapidly confirm the diagnosis of both chlamydophila pneumoniae, mycoplasma pneumoniae, and Influenza A virus (14). Influenza A is well known to cause serious morbidity and mortality and may be the most common virus causing CAP, with a comparable clinical burden to viruses such as respiratory syneytial virus. This is further evidence supporting universal childhood influenza immunization. Expansion of the serotypes included in pneumococcal conjugate vaccines (PCV) is to include serotypes 1 and 3, both currently non-PCV strains in the U.S. vaccine, is underway.
As the epidemiology of CAP continues to evolve, practitioners need to be aware of the prevalent pathogens in their region. In the age of continuing antimicrobial resistance of bacterial pathogens, it is important to know the local antimicrobial susceptibility patterns to appropriately choose empiric therapy when a bacterial process is suspected. Local laboratories can commonly provide this data.
Whatever the future holds, we continue to need the collaboration and expertise of the inpatient practitioner, the infectious disease specialist, and the surgeon/interventionalist. All are necessary to ensure the prompt recognition of empyema and the need for timely medical and surgical intervention for these patients.
References
- Bryce J, Boschi-Pinto C, Shibuya K, Black RE; WHO Child Health Epidemiology Reference Group. WHO estimates of the causes of death in children. Lancet. 2005;365:1147-52.
- Wheeler JG, Jacobs RF. Pleural effusions and empyema. In: Feigin RD, Cherry JD, Demmler GJ, Kaplan SL, eds. Textbook of Pediatric Infectious Diseases. 5th ed. Philadelphia, Pa: Saunders;2004:320-30.
- Finland M, Barnes MW. Changing ecology of acute bacterial empyema: occurrence and mortality at Boston City Hospital during 12 selected years from 1935 to 1972. J Infect Dis. 1978;137:274-91.
- Goel A, Bamford L, Hanslo D, Hussey G. Primary staphylococcal pneumonia in young children: a review of 100 cases. J Trop Pediatr. 1999;45:233-6.
- Chartrand SA, McCracken GH Jr. Staphylococcal pneumonia in infants and children Pediatr Infect Dis. 1982;1:19-23.
- Buckingham SC, King MD, Miller ML. Incidence and etiologies of complicated parapneumonic effusions in children, 1996 to 2001. Pediatr Infect Dis J. 2003;22:499-504.
- Poehling KA, Lafleur BJ, Szilagyi PG, et al. Populationbased impact of pneumococcal conjugate vaccine in young children. Pediatrics. 2004;114:755-61.
- Syriopoulou V, Daikos GL, Soulis K, et al. Epidemiology of invasive childhood pneumococcal infections in Greece. Acta Paediatr Suppl. 2000;89:30-4.
- Eastham KM, Freeman R, Kearns AM, et al. Clinical features, aetiology and outcome of empyema in children in the north east of England. Thorax. 2004;59:522-5.
- Schultz KD, Fan LL, Pinsky J, et al. The changing face of pleural empyemas in children: epidemiology and management. Pediatrics. 2004;113:1735-40.
- Cameron R, Davies HR. Intra-pleural fibrinolytic therapy versus conservative management in the treatment of para-pneumonic effusions and empyema. Cochrane Database Syst Rev. 2004:CD002312. Review.
- Coote N. Surgical versus non-surgical management of pleural empyema. Cochrane Database Syst Rev. 2002:CD001956. Review.
- Gates RL, Caniano DA, Hayes JR, Arca MJ. Does VATS provide optimal treatment of empyema in children? A systematic review. J Pediatr Surg. 2004;39:381-6. Review.
- Laundy M, Ajayi-Obe E, Hawrami K, Aitken C, Breuer J, Booy R. Influenza A community-acquired pneumonia in East London infants and young children. Pediatr Infect Dis J. 2003;22(Suppl):S223-7.
Pneumonia is associated with as many as 2 million annual deaths among children globally and 19% of all deaths in children less than 5 years of age (1). It is one of the most common diagnoses made in the acutely ill child, with an annual incidence of 34 to 40 cases per 1,000 children in Europe and North America.
In the past, viral pathogens were estimated to cause as many as 80% of cases. Streptococcus pneumoniae was generally regarded as the most frequent bacterial cause of community-acquired pneumonia (CAP), especially in cases with complicated parapneumonic effusions. Infectious etiologies are age specific, with bacterial etiologies predominating in the very young infant and viral pathogens in the older infant and adult (Table 1). Knowledge of the most likely pathogen, the prevailing susceptibilities of these infecting pathogens, and the severity of the illness will help guide antibiotic and other treatment decision making.
Most children do not require hospital admission, and mildly ill children who likely have a viral illness do not need antibiotics. The following guideline will attempt to help the practitioner identify those who do require hospitalization and provide an approach to management of those with complicated infection.
Recognition of the Patient with CAP
The first obstacle is to identify the patient with pneumonia. In managing the child with CAP, it is important to distinguish those with other underlying pathology, including asthma, RSV, or other confirmed viral etiology. It is important to remember that pathogens in the compromised host, cystic fibrosis patient, or patient with other chronic pulmonary pathology are different from typical CAP pathogens and include a wide differential. Most patients with CAP have an acute illness associated with fever (>38°C), cough, and evidence of lower respiratory tract symptoms/signs. Chest radiograph typically shows pulmonary infiltrate. Whether this is patchy infiltrate or lobar in appearance can assist the practitioner in treatment decision making in that the latter is much more likely to be associated with a bacterial etiology.
Once the diagnosis is considered, further assessment should focus on hydration status, hemodynamic parameters, and oxygenation. A careful assessment should identify other associated foci (i.e., meningitis or bacteremia) on examination and laboratory evaluation.
Identification of the Patient Requiring Hospitalization
Consider hospital admission for the toxic patient, those with altered mental status, significant dehydration, hypoxemia, dyspnea, grunting respirations, or retractions, and any patient with hemodynamic instability. Chest radiograph showing a significant pleural effusion should also be considered an indicator for hospital admission.
Bacterial pathogens are more likely in the severely ill patient, patients with a rapidly progressive process, and those with radiographic evidence of lobar consolidation or pleural effusion. Some children with viral processes may require admission for supportive care.
Prompt Recognition of the Patient with Empyema
For the patient with pneumonia and parapneumonic effusion, distinction between a free-flowing effusion and pleural empyema is critical. Standard plain film can identify pulmonary infiltrate and often effusion and lateral decubitus films can help identify free flowing effusion (Figure 1). While CT scan more effectively identifies pleural fibrinous adhesions that may entrap lung, ultrasound most effectively identifies complex fluid collections with loculation and septation, and it can be utilized to guide thoracentesis.
Empyema is defined as pus in the pleural space and is estimated to occur in 10–40% of patients with pneumonia. Empyema may also result from causes other than a complication of bacterial pneumonia, such as thoracic trauma or postsurgical complication, rupture of lung abscess, esophageal tear, or complication of indwelling catheter. It generally occurs in stages including acute (early-cloudy fluid), fibrino-purulent (thicker, multiloculated fluid), and organized (late with thick pleural peel and entrapment of lung).
Pleural fluid evaluation is important both in diagnosis and in guiding treatment in such cases. Pleural fluid collections are defined as transudative or exudative based on biochemical evaluation. Evaluation includes cell type and differential, pH, glucose, protein and LDH. Gram-stained smear needs to be performed on all specimens at the time of culture. Empyema is exudative, typically with low glucose and high LDH (Table 2 on page 64) (2).
Changing Epidemiology and Antibiotic Decision Making
Data presented by Finland and Barnes in 1978 confirmed that S. pneumoniae, group A streptococcus (GAS), and Staphylococcus aureus were the most commonly identified pathogens in empyema cases in 1935, with S. aureus emerging in the 1950s (3). Most literature from the 1960–1980s detailing etiology of pneumonia with pleural empyema continued to emphasize the role of S. aureus in such cases. In all reviews, staphylococcal pneumonia is noted primarily to be a disease of infants. In 1 review of 100 cases of staphylococcal pneumonia, the median age was 5 months, 78 patients being below 1 year of age (4). Chartrand and McCracken analyzed 79 cases of staphylococcal pneumonia and noted that in about 75% of cases, staphylococcal pneumonia was a primary pneumonia in infants with a median age of 6 months. In this study, older children were more likely to have pulmonary involvement as a secondary finding in the setting of disseminated staphylococcal disease. A pleural effusion was found in 80% of infants with primary pneumonia and in 61% of those with secondary disease, thus providing the tip-off of a more serious process to the clinician (5). A high index of suspicion for S. aureus in the young infant with pneumonia is important, as physicians need to expect a rapidly progressive clinical course. Those infants frequently require ventilatory support, alteration in antibiotic choice, and the prompt recognition of pleural complications including pneumothoraces and pneumatoceles.
Data in the 1990s emphasized the role of multidrug resistant pneumococcus as a pathogen in empyema. In a recent review of cases in the postpneumococcal conjugate disease era, pneumococcus remained the most commonly confirmed etiologic agent, with other gram-positive pathogens, including GAS and S. aureus, also documented (6). Despite widespread implementation of pneumococcal conjugate vaccine (PCV), and a population based surveillance study in the US that suggested that adding PCV to the childhood immunization schedule was associated with a 10-fold greater reduction in pneumonia (7), serious pneumonia caused by S. pneumoniae continued to be reported. The prevalence of serotype 1 and 3 as the etiologies of such infections may limit the utility of the current vaccine. One study from Greece demonstrated that the most common serotypes causing bacteremic pneumonia were 14, 6B, 1 and 19F (8). Childhood empyema in the UK is noted to be increasing, and a recent study of 47 empyema cases confirmed pneumococcus as the major pathogen, with over half caused by serotype 1 (9).
More recent data suggest yet another change to the epidemiology of empyema. Schultz et al. from Houston, TX, reviewed a decade of experience from 1993–2002, and while they identified a decrease in total cases of empyema, the emergence of methicillin-resistant S. aureus (MRSA) infection was noted (10).
While MRSA has long been considered an important pathogen in the etiology of healthcare-associated infection, experience in our institution also confirms the appearance of an increasing number of cases of community-acquired MRSA disease. Vancomycin is clearly part of the treatment regimen in the child at risk for staphylococcal pneumonia, though many have utilized clindamycin for the non–critically ill patient. The increase in such cases clearly has important implications for treatment decisions, as MRSA with inducible clindamycin resistance is not yet recognized in every facility. Data are not available to confirm the utility of trimethoprim-sulfamethoxazole in serious community-aquired MRSA infections, and the role for newer antibiotics, such as linezolid, has not been clearly defined.
Management: Antibiotics and the Role of Pleural Drainage Procedures
Figure 2 on page 58 shows an algorithm that guides clinical management of the empyema patient. Once a diagnosis is made, attention should be directed to fluid and electrolyte correction, hemodynamic stabilization, and respiratory support (i.e., oxygenation and ventilation). Antibiotics should be initiated and the choice is based on severity of illness and age of the child.
Drainage of the pleural pus has long been recognized as integral to the success in treatment of pneumonia with empyema. Recently, there has been much debate concerning which modality to use and when.
Intrapleural fibrinolytic therapy has been shown in multiple studies to decrease length of stay without increased risk. Data compiled in the Cochrane database comparing fibrinolytic therapy vs. more conservative management suggests that intrapleural fibrinolytic therapy confers significant benefit when compared with normal saline control; however, a definitive statement was not made, given that the trial numbers were too small (11). More recent data from the Cochrane database and a systematic review suggest that video-assisted thoracostomy (VATS) performed early in the disease course is associated with better outcome than chest tube drainage with streptokinase with regard to duration of chest tube placement and hospital stay. However there are questions about validity, and this study is also too small to draw conclusions (12,13). Figure 3 shows the typical findings encountered at VATS in a child with empyema.
A retrospective chart review from our institution from December 2000 to March 2004, excluding immunocompromised hosts, found 96 cases of radiographic pneumonia with pleural effusion. Thirty-four met criteria for empyema, including ultrasound and/or chest CT showing pleural fluid loculation and septation, or purulent fluid/positive culture. Average age was 5 years, and pathogens were defined in 38% of patients. Length of stay averaged 9 days, with a range of 5–23 days. Two had no intervention and had a stay of 8 days, 14 had tube thoracostomy and had an average stay of 11.5 days with 6 failures, 10 had thoracostomy and fibrinolytic therapy with an average stay of 7 days, 3 had early VATS with an average stay of 7 days, and 5 had late VATS with an average stay of 10.4 days. In our institution, among invasive interventions, tube thoracostomy alone had longer LOS and more failures. Early VATS and intrapleural fibrinolysis have shorter stays and are on the lower end of the cost scale: $25,549 vs. $21,062 respectively (Figure 4).
The decision for interventional management of empyema will likely be institutionally variable in the absence of large randomized controlled studies. Institutions with aggressive interventional radiologists may favor thoracostomy tube with fibrinolysis. Those with surgeons skilled in video-scopic surgery may provide early VATS. Data on interventions clearly show benefit beyond that provided by routine chest tube placement. The key becomes prompt diagnosis of empyema with early use of ultrasound, knowledge of local antibiotic susceptibilities, and clear guidelines set up by each institution to guide interventional management.
The Future
Increasing the accuracy of diagnostic testing for children with CAP would likely lead to decreased morbidity, mortality, and total cost of care. The use of PCR is becoming more widespread and could be utilized to more rapidly confirm the diagnosis of both chlamydophila pneumoniae, mycoplasma pneumoniae, and Influenza A virus (14). Influenza A is well known to cause serious morbidity and mortality and may be the most common virus causing CAP, with a comparable clinical burden to viruses such as respiratory syneytial virus. This is further evidence supporting universal childhood influenza immunization. Expansion of the serotypes included in pneumococcal conjugate vaccines (PCV) is to include serotypes 1 and 3, both currently non-PCV strains in the U.S. vaccine, is underway.
As the epidemiology of CAP continues to evolve, practitioners need to be aware of the prevalent pathogens in their region. In the age of continuing antimicrobial resistance of bacterial pathogens, it is important to know the local antimicrobial susceptibility patterns to appropriately choose empiric therapy when a bacterial process is suspected. Local laboratories can commonly provide this data.
Whatever the future holds, we continue to need the collaboration and expertise of the inpatient practitioner, the infectious disease specialist, and the surgeon/interventionalist. All are necessary to ensure the prompt recognition of empyema and the need for timely medical and surgical intervention for these patients.
References
- Bryce J, Boschi-Pinto C, Shibuya K, Black RE; WHO Child Health Epidemiology Reference Group. WHO estimates of the causes of death in children. Lancet. 2005;365:1147-52.
- Wheeler JG, Jacobs RF. Pleural effusions and empyema. In: Feigin RD, Cherry JD, Demmler GJ, Kaplan SL, eds. Textbook of Pediatric Infectious Diseases. 5th ed. Philadelphia, Pa: Saunders;2004:320-30.
- Finland M, Barnes MW. Changing ecology of acute bacterial empyema: occurrence and mortality at Boston City Hospital during 12 selected years from 1935 to 1972. J Infect Dis. 1978;137:274-91.
- Goel A, Bamford L, Hanslo D, Hussey G. Primary staphylococcal pneumonia in young children: a review of 100 cases. J Trop Pediatr. 1999;45:233-6.
- Chartrand SA, McCracken GH Jr. Staphylococcal pneumonia in infants and children Pediatr Infect Dis. 1982;1:19-23.
- Buckingham SC, King MD, Miller ML. Incidence and etiologies of complicated parapneumonic effusions in children, 1996 to 2001. Pediatr Infect Dis J. 2003;22:499-504.
- Poehling KA, Lafleur BJ, Szilagyi PG, et al. Populationbased impact of pneumococcal conjugate vaccine in young children. Pediatrics. 2004;114:755-61.
- Syriopoulou V, Daikos GL, Soulis K, et al. Epidemiology of invasive childhood pneumococcal infections in Greece. Acta Paediatr Suppl. 2000;89:30-4.
- Eastham KM, Freeman R, Kearns AM, et al. Clinical features, aetiology and outcome of empyema in children in the north east of England. Thorax. 2004;59:522-5.
- Schultz KD, Fan LL, Pinsky J, et al. The changing face of pleural empyemas in children: epidemiology and management. Pediatrics. 2004;113:1735-40.
- Cameron R, Davies HR. Intra-pleural fibrinolytic therapy versus conservative management in the treatment of para-pneumonic effusions and empyema. Cochrane Database Syst Rev. 2004:CD002312. Review.
- Coote N. Surgical versus non-surgical management of pleural empyema. Cochrane Database Syst Rev. 2002:CD001956. Review.
- Gates RL, Caniano DA, Hayes JR, Arca MJ. Does VATS provide optimal treatment of empyema in children? A systematic review. J Pediatr Surg. 2004;39:381-6. Review.
- Laundy M, Ajayi-Obe E, Hawrami K, Aitken C, Breuer J, Booy R. Influenza A community-acquired pneumonia in East London infants and young children. Pediatr Infect Dis J. 2003;22(Suppl):S223-7.
Pneumonia is associated with as many as 2 million annual deaths among children globally and 19% of all deaths in children less than 5 years of age (1). It is one of the most common diagnoses made in the acutely ill child, with an annual incidence of 34 to 40 cases per 1,000 children in Europe and North America.
In the past, viral pathogens were estimated to cause as many as 80% of cases. Streptococcus pneumoniae was generally regarded as the most frequent bacterial cause of community-acquired pneumonia (CAP), especially in cases with complicated parapneumonic effusions. Infectious etiologies are age specific, with bacterial etiologies predominating in the very young infant and viral pathogens in the older infant and adult (Table 1). Knowledge of the most likely pathogen, the prevailing susceptibilities of these infecting pathogens, and the severity of the illness will help guide antibiotic and other treatment decision making.
Most children do not require hospital admission, and mildly ill children who likely have a viral illness do not need antibiotics. The following guideline will attempt to help the practitioner identify those who do require hospitalization and provide an approach to management of those with complicated infection.
Recognition of the Patient with CAP
The first obstacle is to identify the patient with pneumonia. In managing the child with CAP, it is important to distinguish those with other underlying pathology, including asthma, RSV, or other confirmed viral etiology. It is important to remember that pathogens in the compromised host, cystic fibrosis patient, or patient with other chronic pulmonary pathology are different from typical CAP pathogens and include a wide differential. Most patients with CAP have an acute illness associated with fever (>38°C), cough, and evidence of lower respiratory tract symptoms/signs. Chest radiograph typically shows pulmonary infiltrate. Whether this is patchy infiltrate or lobar in appearance can assist the practitioner in treatment decision making in that the latter is much more likely to be associated with a bacterial etiology.
Once the diagnosis is considered, further assessment should focus on hydration status, hemodynamic parameters, and oxygenation. A careful assessment should identify other associated foci (i.e., meningitis or bacteremia) on examination and laboratory evaluation.
Identification of the Patient Requiring Hospitalization
Consider hospital admission for the toxic patient, those with altered mental status, significant dehydration, hypoxemia, dyspnea, grunting respirations, or retractions, and any patient with hemodynamic instability. Chest radiograph showing a significant pleural effusion should also be considered an indicator for hospital admission.
Bacterial pathogens are more likely in the severely ill patient, patients with a rapidly progressive process, and those with radiographic evidence of lobar consolidation or pleural effusion. Some children with viral processes may require admission for supportive care.
Prompt Recognition of the Patient with Empyema
For the patient with pneumonia and parapneumonic effusion, distinction between a free-flowing effusion and pleural empyema is critical. Standard plain film can identify pulmonary infiltrate and often effusion and lateral decubitus films can help identify free flowing effusion (Figure 1). While CT scan more effectively identifies pleural fibrinous adhesions that may entrap lung, ultrasound most effectively identifies complex fluid collections with loculation and septation, and it can be utilized to guide thoracentesis.
Empyema is defined as pus in the pleural space and is estimated to occur in 10–40% of patients with pneumonia. Empyema may also result from causes other than a complication of bacterial pneumonia, such as thoracic trauma or postsurgical complication, rupture of lung abscess, esophageal tear, or complication of indwelling catheter. It generally occurs in stages including acute (early-cloudy fluid), fibrino-purulent (thicker, multiloculated fluid), and organized (late with thick pleural peel and entrapment of lung).
Pleural fluid evaluation is important both in diagnosis and in guiding treatment in such cases. Pleural fluid collections are defined as transudative or exudative based on biochemical evaluation. Evaluation includes cell type and differential, pH, glucose, protein and LDH. Gram-stained smear needs to be performed on all specimens at the time of culture. Empyema is exudative, typically with low glucose and high LDH (Table 2 on page 64) (2).
Changing Epidemiology and Antibiotic Decision Making
Data presented by Finland and Barnes in 1978 confirmed that S. pneumoniae, group A streptococcus (GAS), and Staphylococcus aureus were the most commonly identified pathogens in empyema cases in 1935, with S. aureus emerging in the 1950s (3). Most literature from the 1960–1980s detailing etiology of pneumonia with pleural empyema continued to emphasize the role of S. aureus in such cases. In all reviews, staphylococcal pneumonia is noted primarily to be a disease of infants. In 1 review of 100 cases of staphylococcal pneumonia, the median age was 5 months, 78 patients being below 1 year of age (4). Chartrand and McCracken analyzed 79 cases of staphylococcal pneumonia and noted that in about 75% of cases, staphylococcal pneumonia was a primary pneumonia in infants with a median age of 6 months. In this study, older children were more likely to have pulmonary involvement as a secondary finding in the setting of disseminated staphylococcal disease. A pleural effusion was found in 80% of infants with primary pneumonia and in 61% of those with secondary disease, thus providing the tip-off of a more serious process to the clinician (5). A high index of suspicion for S. aureus in the young infant with pneumonia is important, as physicians need to expect a rapidly progressive clinical course. Those infants frequently require ventilatory support, alteration in antibiotic choice, and the prompt recognition of pleural complications including pneumothoraces and pneumatoceles.
Data in the 1990s emphasized the role of multidrug resistant pneumococcus as a pathogen in empyema. In a recent review of cases in the postpneumococcal conjugate disease era, pneumococcus remained the most commonly confirmed etiologic agent, with other gram-positive pathogens, including GAS and S. aureus, also documented (6). Despite widespread implementation of pneumococcal conjugate vaccine (PCV), and a population based surveillance study in the US that suggested that adding PCV to the childhood immunization schedule was associated with a 10-fold greater reduction in pneumonia (7), serious pneumonia caused by S. pneumoniae continued to be reported. The prevalence of serotype 1 and 3 as the etiologies of such infections may limit the utility of the current vaccine. One study from Greece demonstrated that the most common serotypes causing bacteremic pneumonia were 14, 6B, 1 and 19F (8). Childhood empyema in the UK is noted to be increasing, and a recent study of 47 empyema cases confirmed pneumococcus as the major pathogen, with over half caused by serotype 1 (9).
More recent data suggest yet another change to the epidemiology of empyema. Schultz et al. from Houston, TX, reviewed a decade of experience from 1993–2002, and while they identified a decrease in total cases of empyema, the emergence of methicillin-resistant S. aureus (MRSA) infection was noted (10).
While MRSA has long been considered an important pathogen in the etiology of healthcare-associated infection, experience in our institution also confirms the appearance of an increasing number of cases of community-acquired MRSA disease. Vancomycin is clearly part of the treatment regimen in the child at risk for staphylococcal pneumonia, though many have utilized clindamycin for the non–critically ill patient. The increase in such cases clearly has important implications for treatment decisions, as MRSA with inducible clindamycin resistance is not yet recognized in every facility. Data are not available to confirm the utility of trimethoprim-sulfamethoxazole in serious community-aquired MRSA infections, and the role for newer antibiotics, such as linezolid, has not been clearly defined.
Management: Antibiotics and the Role of Pleural Drainage Procedures
Figure 2 on page 58 shows an algorithm that guides clinical management of the empyema patient. Once a diagnosis is made, attention should be directed to fluid and electrolyte correction, hemodynamic stabilization, and respiratory support (i.e., oxygenation and ventilation). Antibiotics should be initiated and the choice is based on severity of illness and age of the child.
Drainage of the pleural pus has long been recognized as integral to the success in treatment of pneumonia with empyema. Recently, there has been much debate concerning which modality to use and when.
Intrapleural fibrinolytic therapy has been shown in multiple studies to decrease length of stay without increased risk. Data compiled in the Cochrane database comparing fibrinolytic therapy vs. more conservative management suggests that intrapleural fibrinolytic therapy confers significant benefit when compared with normal saline control; however, a definitive statement was not made, given that the trial numbers were too small (11). More recent data from the Cochrane database and a systematic review suggest that video-assisted thoracostomy (VATS) performed early in the disease course is associated with better outcome than chest tube drainage with streptokinase with regard to duration of chest tube placement and hospital stay. However there are questions about validity, and this study is also too small to draw conclusions (12,13). Figure 3 shows the typical findings encountered at VATS in a child with empyema.
A retrospective chart review from our institution from December 2000 to March 2004, excluding immunocompromised hosts, found 96 cases of radiographic pneumonia with pleural effusion. Thirty-four met criteria for empyema, including ultrasound and/or chest CT showing pleural fluid loculation and septation, or purulent fluid/positive culture. Average age was 5 years, and pathogens were defined in 38% of patients. Length of stay averaged 9 days, with a range of 5–23 days. Two had no intervention and had a stay of 8 days, 14 had tube thoracostomy and had an average stay of 11.5 days with 6 failures, 10 had thoracostomy and fibrinolytic therapy with an average stay of 7 days, 3 had early VATS with an average stay of 7 days, and 5 had late VATS with an average stay of 10.4 days. In our institution, among invasive interventions, tube thoracostomy alone had longer LOS and more failures. Early VATS and intrapleural fibrinolysis have shorter stays and are on the lower end of the cost scale: $25,549 vs. $21,062 respectively (Figure 4).
The decision for interventional management of empyema will likely be institutionally variable in the absence of large randomized controlled studies. Institutions with aggressive interventional radiologists may favor thoracostomy tube with fibrinolysis. Those with surgeons skilled in video-scopic surgery may provide early VATS. Data on interventions clearly show benefit beyond that provided by routine chest tube placement. The key becomes prompt diagnosis of empyema with early use of ultrasound, knowledge of local antibiotic susceptibilities, and clear guidelines set up by each institution to guide interventional management.
The Future
Increasing the accuracy of diagnostic testing for children with CAP would likely lead to decreased morbidity, mortality, and total cost of care. The use of PCR is becoming more widespread and could be utilized to more rapidly confirm the diagnosis of both chlamydophila pneumoniae, mycoplasma pneumoniae, and Influenza A virus (14). Influenza A is well known to cause serious morbidity and mortality and may be the most common virus causing CAP, with a comparable clinical burden to viruses such as respiratory syneytial virus. This is further evidence supporting universal childhood influenza immunization. Expansion of the serotypes included in pneumococcal conjugate vaccines (PCV) is to include serotypes 1 and 3, both currently non-PCV strains in the U.S. vaccine, is underway.
As the epidemiology of CAP continues to evolve, practitioners need to be aware of the prevalent pathogens in their region. In the age of continuing antimicrobial resistance of bacterial pathogens, it is important to know the local antimicrobial susceptibility patterns to appropriately choose empiric therapy when a bacterial process is suspected. Local laboratories can commonly provide this data.
Whatever the future holds, we continue to need the collaboration and expertise of the inpatient practitioner, the infectious disease specialist, and the surgeon/interventionalist. All are necessary to ensure the prompt recognition of empyema and the need for timely medical and surgical intervention for these patients.
References
- Bryce J, Boschi-Pinto C, Shibuya K, Black RE; WHO Child Health Epidemiology Reference Group. WHO estimates of the causes of death in children. Lancet. 2005;365:1147-52.
- Wheeler JG, Jacobs RF. Pleural effusions and empyema. In: Feigin RD, Cherry JD, Demmler GJ, Kaplan SL, eds. Textbook of Pediatric Infectious Diseases. 5th ed. Philadelphia, Pa: Saunders;2004:320-30.
- Finland M, Barnes MW. Changing ecology of acute bacterial empyema: occurrence and mortality at Boston City Hospital during 12 selected years from 1935 to 1972. J Infect Dis. 1978;137:274-91.
- Goel A, Bamford L, Hanslo D, Hussey G. Primary staphylococcal pneumonia in young children: a review of 100 cases. J Trop Pediatr. 1999;45:233-6.
- Chartrand SA, McCracken GH Jr. Staphylococcal pneumonia in infants and children Pediatr Infect Dis. 1982;1:19-23.
- Buckingham SC, King MD, Miller ML. Incidence and etiologies of complicated parapneumonic effusions in children, 1996 to 2001. Pediatr Infect Dis J. 2003;22:499-504.
- Poehling KA, Lafleur BJ, Szilagyi PG, et al. Populationbased impact of pneumococcal conjugate vaccine in young children. Pediatrics. 2004;114:755-61.
- Syriopoulou V, Daikos GL, Soulis K, et al. Epidemiology of invasive childhood pneumococcal infections in Greece. Acta Paediatr Suppl. 2000;89:30-4.
- Eastham KM, Freeman R, Kearns AM, et al. Clinical features, aetiology and outcome of empyema in children in the north east of England. Thorax. 2004;59:522-5.
- Schultz KD, Fan LL, Pinsky J, et al. The changing face of pleural empyemas in children: epidemiology and management. Pediatrics. 2004;113:1735-40.
- Cameron R, Davies HR. Intra-pleural fibrinolytic therapy versus conservative management in the treatment of para-pneumonic effusions and empyema. Cochrane Database Syst Rev. 2004:CD002312. Review.
- Coote N. Surgical versus non-surgical management of pleural empyema. Cochrane Database Syst Rev. 2002:CD001956. Review.
- Gates RL, Caniano DA, Hayes JR, Arca MJ. Does VATS provide optimal treatment of empyema in children? A systematic review. J Pediatr Surg. 2004;39:381-6. Review.
- Laundy M, Ajayi-Obe E, Hawrami K, Aitken C, Breuer J, Booy R. Influenza A community-acquired pneumonia in East London infants and young children. Pediatr Infect Dis J. 2003;22(Suppl):S223-7.