User login
Patient complexity is associated with greater hospital readmission rates,1,2 poorer quality of care,3 and lower patient satisfaction.4 Improving outcomes for complex patients is a global priority,5 and local initiatives such as Ontario’s Health Links are being developed, yet evidence to inform care is lacking.6-8
The prevalence of patients living with multiple comorbidities is increasing as advances in medicine enable people to live and manage chronic diseases.9-11 However, these medical gains have resulted in an increased burden on both patients and healthcare systems. Socioeconomic status and co-occurring psychosocial challenges further complicate health and healthcare in marginalized populations.12,13
Human immunodeficiency virus/acquired immunodeficiency syndrome (HIV/AIDS) is one example of a disease that medicine has transformed. Individuals living with HIV today, on antiretroviral medications, may be able to manage their chronic illness for decades.14,15 However, in addition to social determinants of health that influence ongoing adherence and engagement in care, these medications do not completely eradicate the impact of HIV and, as a result, HIV-positive individuals are at a greater risk of developing additional comorbidities.15 People living with HIV may, therefore, represent an important patient population in which healthcare interventions and system improvements for complex patients should be explored.
Improving health systems and better supporting complex patients requires a broader understanding of the patient experience and the challenges encountered, especially during high-risk periods such as hospital discharge. Qualitative research approaches are designed to help us understand social phenomena in their “natural” settings,16 and thus suited to achieve this goal, providing critical insight to inform healthcare systems and policies.17,18 This study sought to answer the question, “What are the obstacles and challenges faced by complex patients during hospital discharge and post-discharge transition?” We approached patient complexity holistically, using a unified Complexity Framework6 that connects 5 health dimensions—social capital, mental health, demographics, health and social experiences, and physical health—identified as important to understanding complex patients and their interaction with healthcare. A longitudinal case study approach was used, with multiple sources of data, to understand the clinical context and discharge plans in relation to the lived experience of patients over time, exploring potential misalignment and areas for improvement.
METHODS
This community-based research study was conducted at Casey House, a 13-bed subacute care hospital in Toronto, Canada that provides in-patient and community programs to a complex patient group. All patients are HIV-positive. Inpatient hospital care is provided by an interdisciplinary team, including physicians, social workers, nurses, and healthcare aides. A harm reduction approach is taken to substance use. Twelve beds are for general admission. Patients may be transferred from acute-care hospitals or referred by community-based providers. One bed is reserved for scheduled 2-week respite stays.
The primary research team for this community-based project consisted of clinicians and community and academic researchers. The study was conducted in collaboration with housing, healthcare, and HIV service providers and was advised by 2 individuals with lived experience of discharge from Casey House. Community members with lived experience attended team meetings, provided feedback on all stages of the project (ie, interview guides, recruitment, analysis and dissemination), and helped facilitate community engagement sessions with other patients at the start and the end of the project.
Standard practice for discharge planning involves clinicians determining a tentative discharge date and identifying strategies to support the patient. Planning is informed by knowledge gathered by the interdisciplinary team throughout the admission, including social determinants of health (ie, housing, social support, food security). Patients are encouraged to invite an individual from their social support network to attend a discharge meeting, where the care team reviews goals for admission, course of treatment, referrals, and important follow-up dates.
We used a multi-case study approach to explore the discharge process and post-discharge period. A case was defined as the discharge and transition of a patient from hospital to community. Data were collected through serial interviews with patients (n = 4), medical chart abstraction, and review of discharge summaries. Serial interviews, although not frequently used in clinical research, have been proposed as a strong approach for exploring complex processes and to build trust between researcher and participant,19 both of which were relevant in this study. Patient interviews were conducted by the Master’s trained research coordinator (SM) using tailored semi-structured interview guides for 4 time points: before the discharge meeting (I1); after the discharge meeting but before discharge (I2); within a week of discharge (I3); and approximately 30 days after discharge (I4). Interviews were audio recorded and transcribed verbatim.
Cases were eligible if the patient had a general admission and a planned discharge to the community, and was able to communicate in English and direct his/her own care. Patient-initiated discharges and discharges to another healthcare facility were excluded. Casey House clinical staff approached consecutive potentially eligible patients for their willingness to speak with the researcher coordinator. The research coordinator met with patients to assess eligibility and obtain informed consent to participate. All participants provided informed written consent. The study was approved by the University of Toronto HIV Research Ethics Board.
Interview data, managed with MAXQDA software (VERBI GmbH, Berlin, Germany), were analyzed using a framework analysis approach.20,21 At least 3 authors read each transcript in its entirety. Priority questions/topics identified a priori by stakeholders as important to inform change in care and practices were used as the first draft of the coding framework. The framework was modified through team discussion in the analysis phase to integrate emerging themes. Participant demographic and clinical data were extracted using a structured data collection form.
Preliminary data analysis was completed for the separate data sources including inter- and intra-case comparisons: exploring how experiences and perceptions changed over time and themes that emerged across cases at the same time point. Data sources were combined to strengthen the understanding of the cases and identify relationships and discrepancies across sources.22 Audit trails, reflexive journaling, group coding and analysis meetings and member-checking, were used to enhance analytical rigor.
RESULTS
The results focus on the patient experience of the “discharge plan” and are presented in terms of 3 pre-identified categories: 1) social support; 2) discharge process and transition experience; and 3) post-discharge follow-up and referrals; and 1 emergent theme, patient priorities.
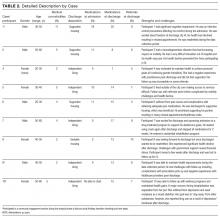
Participants experienced complex medical and psychosocial challenges (Table 1, participant characteristics). All participants were living with HIV plus a mean of 5 additional comorbidities, the most common being hepatitis C (n = 3), chronic obstructive pulmonary disease (n = 2), herpes (n = 2) and opportunistic infections (n = 2). Eight of 9 participants had a history of an Axis 1 diagnosis, most commonly mood disorder (n = 4). Substance use was identified in all participants. An overview of each case is presented in Table 2.
Three patients declined to be considered for the study. Informed consent was obtained for 10 cases. One participant withdrew after interview 1. Data are presented here for 9 cases, including 32 interviews, between October 2013 and June 2014. Interviews 1 (I1) and 2 (I2) were combined for 3 participants. Two participants were lost to follow-up for interview 4.
Social Support
For the purposes of this paper, we define “social support” as the emotional or instrumental assistance an individual perceives and experiences from people in his/her self-identified network (ie, family, friends). Participants’ discharge-related experience of social support did not align, in most cases, with the information from their medical charts or their expectations. At admission, 8 of 9 participants identified at least 1 person in their social support network, yet only 1 participant had someone attend the discharge meeting. One participant said she had expected “my daughter, my mother, my brother, somebody. At least somebody. But they never show up.” (P5, I2).
The complexity of her relationship with her family and her unmet needs for support continued after discharge:
I try and be as independent as possible. I don’t have to call them for nothing. Because, even the other day, I called my mom and I asked her, I said, “Mom, I’m going to give you $400 [to pay back a personal loan] and I’m going to give you an extra $100, you could buy me some food.” And she goes “Okay.” But, I didn’t give it to her yet. I don’t know, she seems money hungry right now, so I’m like no, I’ll wait. (P5, I4)
In the hospital, participants frequently spoke about discharge and transition planning that was inclusive of their social support networks. However, a sense of isolation and loneliness was common post-discharge. Often, friends and family members did not provide the support that participants anticipated, but instead were sources of anxiety and stress. One participant conveyed his experience with a friend he listed as a social support:
I gave him some money to get me some groceries, to make sure I had some food in the house when I got home. He didn’t do that. All of a sudden he was called away to [another city]. He told me his father had a heart attack. He told [others] his father had a slip. I still have yet to receive my money. (P7, I4)
Discharge Process and Transition Experience
While some participants were excited about the thought of freedom of being home, others were anxious about the burdens of returning to life outside of the hospital.
I kind of feel like, yeah, I want to go home, but then I think to myself what am I going to do when I get home. Am I just going to go back to what I’ve been doing? Am I going to really change? Am I going to forget to take my pill one day because I’m home and stuff like that. (P4, I1)
The discharge process was often perceived by participants to be rushed. Some participants found the discharge meetings helpful, while others did not feel the process empowered them to engage in a meaningful conversation with hospital staff.
There was no one there with me to even help me with my brain, to think. But it’s afterwards I’m like why didn’t I say that, like that’s what I meant to say. The brain just doesn’t function that way. (P8, I2).
This participant struggled with the transition. One week after discharge when she was asked how her health was she replied:
Terrible. I’ve got no energy. I haven’t eaten for 3 days. I haven’t drank for 3 days. I’ve got diarrhea galore […] Just no appetite whatsoever. I can’t even make it up the stairs without losing my breath. If I make it up the stairs, I have to sit for 15 or 20 minutes… (P8, I3)
The weight of maintaining activities of daily living was prominent in all post-discharge interviews, in many cases accentuated by declining health. The transition to home was more challenging than participants expected; the experience was strongly influenced by the stability of their health, their environment, and the complexity of their lives.
Follow-up and Referrals
Discharge summaries included a mean of 7 referrals. All participants were referred to a case coordinator, nurse, and family physician. Other referrals included pharmacist (n = 8); personal support worker (n = 6); housing (n = 5); and food-support programs (n = 5).
Several factors led to challenges accessing and receiving services. Participants identified: difficulty with requisite paperwork; mobility and financial constraints; personal and logistical challenges with home-care providers; and competing priorities, such as caring for family. These experiences were frequently accompanied by frustration and anxiety.
Because, if I’m in [city where girlfriend lives], I will not get the support that I get when I’m home. Like my nurse comes. [She] was supposed to come and see me twice and I missed that. I missed like 4 [appointments]. You understand? Certain things I’ve been missing. (P6, I4)
When one participant was asked if she had followed up with the food support program she had been referred to, she responded:
Oh, baby, no. I’ve been so confused. I’ve had ODSP [referring to Ontario Disability Support Program, a government disability program] on my case. I’ve got all the files all mixed up. My worker’s a real bitch. She hates me, big time. I was supposed to go bring in papers today, but I couldn’t get out of bed. I don’t know how much trouble I’m going to be in with ODSP now. (P8, I3)
Despite comprehensive discharge plans and referrals, all participants experienced delays and difficulties in accessing and receiving services. In most cases, there was no single contributing factor to these challenges; the unique experiences were a result of the complex interplay of multiple factors for each individual.
Patient Priorities
In the hospital, participants primarily identified goals of improving physical health and medication adherence. However, these goals often shifted to meeting basic living necessities and supporting others upon discharge. Barriers to adequate food and mobility were prominent themes.
One participant spoke about the challenges of supporting her son while struggling with her own health after discharge:
Well, I’ve been dying, I can’t even walk, and yet I’m the one that still has to go to WalMart, to grab milk and bread for my kid. It’s not like I need any of that stuff, because I don’t even eat. (P8, I3)
Participants were admitted on a mean of 6 medications and discharged with a mean of 14 (Table 1). In the hospital, medications are dispensed directly to patients; however, maintaining optimal adherence at home was complex. When 1 participant was asked about her medications after being home for a week, she said:
My meds, you know I have the cream that I’m supposed to put … and I can’t find it. I lost it yesterday. I used it yesterday morning and all day yesterday I’m looking, like, did it fall behind there? But, obviously, I can’t look over there [because of mobility challenges] … I don’t think I can get it covered [by insurance to replace it]. (P5, I3)
Participants found it difficult to follow a specific dosing schedule, ensure food intake corresponded to medication guidelines, and navigate the impact of substance use. Substance use for some was associated with nonadherence. A participant, explaining his quickly declining health, spoke about the impact of using crack cocaine:
Yeah, when I use I don’t think about medicating, taking my pills or anything like that. That’s not even on your mind. It doesn’t come across your mind. […] I guess, that’s part of the addictive personality. It wants to grab hold of you and say “no, focus on me, focus on me.” (P7, I4)
Others used marijuana as an appetite stimulant and a critical piece of their medication adherence routine.
DISCUSSION
This study followed complex patients through hospital discharge and transition back into the community. In the hospital, participants focused on medical goals, but following discharge basic living needs became the priority. Despite a comprehensive plan to provide support upon discharge, participants found executing and following up with referrals, services, and medication adherence was often overwhelming and not achieved in the month post-hospitalization.
Our study provides depth and context to support and understand the findings of reviews evaluating interventions to improve transitions in care.23,24 A systematic review of interventions to decrease 30-day readmission rates concluded that comprehensive support interventions (with many components) contributed to the greatest reduction in risk of readmission.16 Components that showed the greatest impact were those that were designed to improve patients’ capacity for self-care (including their ability to access and follow through with post-discharge care plans) and those that involved more individuals in the delivery of care.23
Our results also support and expand on other qualitative findings of complex patients. Kangovi et al.25 interviewed patients with low socioeconomic status at a single time point post-discharge to identify common experiences. They summarized their findings in 6 themes: powerlessness during hospitalization; incongruence of patient and clinical team goals; competing issues influencing prominence of health behaviors; socioeconomic constraints on patients’ ability to perform recommended behaviors; sense of abandonment after discharge; and loss of self-efficacy resulting from the “failure” to follow the discharge plan. Our findings tell a very similar story but provide the additional context and understanding of the lived experience over time. We found that the transition experience was most challenging when the home environment was unstable, resulting in a shift in priorities from those set during hospitalization.
While increased support may improve outcomes, there is a need to improve awareness, integration, and support for building capacity within complex patients.26 Capacity is defined here as the sum of resources and abilities that a patient can draw on, and includes physical and mental as well as social, financial, personal, and environmental capabilities and resources.27 This includes understanding the potential negative impact of developing a clinical plan which, in order to operationalize, requires resources in excess of the patient’s capacity at that time.27 Minimally disruptive medicine, a promising theoretical approach for improving the care of complex clients, embodies the awareness of capacity in achieving patient-centered care while “imposing the smallest possible treatment burden on patients’ lives.”28
This study, although not without its limitations, provides an in-depth exploration of the experiences of a small number of patients living with HIV, recruited from a single facility in Toronto, Canada after relatively long hospital stays. There are specific context issues related to HIV, such as stigma and severe consequences for suboptimal medication adherence. Furthermore, this study took place where many urban health resources exist; complex patients in rural settings or in environments less tailored to the needs associated with complex medical, psychiatric, and social conditions may experience greater barriers in the transition process. Although this study captured data from medical charts and documents relevant to the cases, further exploration of the clinician decision-making process in creating the discharge plans and additional sources of data on health outcomes post-discharge would be beneficial.
Despite its limitations, this study provides detail and depth to understand some of the most complex patients who suffer from significant challenges in the health system and who are amongst the highest-cost healthcare users. The case study approach, with serial interviews, is an important strength of this study, enabling meaningful insight into hospital discharge processes and challenges experienced by complex patients that can inform individual-level care practice and the development of new programs and interventions.
This study builds on recent research with complex patients in calling for a new approach to clinical care.6,29,30 In order to support complex patients through discharge, clinical goals and referrals must be made in light of a patient’s capacity in the community. Structural changes may be made to improve coordination and access to services, decreasing the burden and improving the healthcare experience. Albreht et al.31 highlight a number of promising programs across Europe (such as the Clinic for Multimorbidity and Polypharmacy in Denmark) designed to improve the health and healthcare for individuals living with multiple chronic conditions. Small-scale changes are also important such as increasing conversations about the capacity and limitations of individuals listed as social supports, and making appropriate and realistic referrals based on an understanding of a patient’s capacity and motivation for follow-up. Shippee et al.32 identify a list of approaches in line with minimally disruptive medicine that can be integrated into existing systems as part of a developing “toolkit” (eg, elicitation of transcendent patient goals, and integration of patient-reported outcome tracking of challenges and burdens associated with health and daily living). The findings of this study suggest that the elements of the toolkit may provide a foundation for future interventions and research to improve hospital care and discharge outcomes for complex patients.
Disclosures
This project was funded by a Canadian Institutes of Health Research (CIHR) HIV/AIDS Community-based Research Catalyst Grant (#126669). Dr. Brennan’s research is supported by an Ontario HIV Treatment Network (OHTN) Applied HIV Research Chair. Dr. Chan Carusone reports grants from Canadian Institutes of Health Research during the conduct of the study.
1. Allaudeen N, Vidyarthi A, Masselli J, Auerback A. Redefining readmission risk factors for general medicine patients. J Hosp Med. 2011;6:54-60. PubMed
2. Hu J, Gonsahn MD, Nerenz DR. Socioeconomic status and readmissions: evidence from an urban teaching hospital. Health Aff (Millwood). 2014;33:778-785. PubMed
3. Panagioti M, Stokes J, Esmail A, et al. Multimorbidity and patient safety incidents in primary care: a systematic review and meta-analysis. PLoS One. 2015;10:e0135947. PubMed
4. Paddison CA, Saunders CL, Abel GA, Payne RA, Campbell JL, Roland M. Why do patients with multimorbidity in England report worse experiences in primary care? Evidence from the General Practice Patient Survey. BMJ Open. 2015;5:e006172. PubMed
5. Barnett K, Mercer SW, Norbury M, Watt G, Wyke S, Guthrie B. Epidemiology of multimorbidity and implications for health care, research, and medical education: a cross-sectional study. Lancet. 2012;380:37-43. PubMed
6. Schaink AK, Kuluski K, Lyons RF, et al. A scoping review and thematic classification of patient complexity: offering a unifying framework. J Comorbidity. 2012;2:1-9.
7. Roland M, Paddison C. Better management of patients with multimorbidity. BMJ. 2013;346:f2510. PubMed
8. Smith SM, Soubhi H, Fortin M, Hudon C, O’Dowd T. Managing patients with multimorbidity: a systematic review of interventions in primary care and community settings. BMJ. 2012;345:e5205. PubMed
9. Afshar S, Roderick PJ, Kowal P, Dimitrov BD, Hill AG. Multimorbidity and the inequalities of global ageing: a cross-sectional study of 28 countries using the World Health Surveys. BMC Public Health. 2015;15:776. PubMed
10. Pefoyo AJK, Bronskill SE, Gruneir A, et al. The increasing burden and complexity of multimorbidity. BMC Public Health. 2015;15:415. PubMed
11. Ward BW, Schiller JS. Prevalence of multiple chronic conditions among US adults: estimates from the National Health Interview Survey, 2010. Prev Chronic Dis. 2013;10:E65. PubMed
12. World Health Organization. Commission on Social Determinants of Health Final Report: Closing the Gap in a Generation: Health Equity through Action on Social Determinants of Health. Geneva, Switzerland: World Health Organization, 2008.
13. Barnett K, Mercer SW, Norbury M, Watt G, Wyke S, Guthrie B.. Epidemiology of multimorbidity and implications for health care, research and medical education: a cross-sectional study. Lancet. 2012;380:37-43. PubMed
14. Samji H, Cescon A, Hogg RS, et al. Closing the gap: increases in life expectancy among treated HIV-positive individuals in the United States and Canada. PLoS One. 2013;8:e81355. PubMed
15. Deeks SG, Lewin SR, Havlir DV. The end of AIDS: HIV infection as a chronic disease. Lancet. 2013;382:1525-1533. PubMed
16. Mays N, Pope C. Qualitative research: rigour and qualitative research. BMJ. 1995;311:109-112. PubMed
17. Gilson L, Hanson K, Sheikh K, Agyepong IA, Ssengooba F, Bennett S. Building the field of health policy and systems research: social science matters. PLoS Med. 2011;8:e1001079. PubMed
18. Stoto MA, Nelson CD, Klaiman T. Getting from what to why: using qualitative research to conduct public health systems research. AcademyHealth; August 2013. http://www.academyhealth.org/files/publications/qmforph.pdf. Accessed May 24, 2016.
19. Murray SA, Kendall M, Carduff E, et al. Use of serial qualitative interviews to understand patients’ evolving experiences and needs. BMJ. 2009;339:b3702. PubMed
20. Pope C, Ziebland S, Mays N. Qualitative research in health care. Analysing qualitative data. BMJ. 2000;320:114-116. PubMed
21. Dixon-Woods M. Using framework-based synthesis for conducting reviews of qualitative studies. BMC Med. 2011;9:39. PubMed
22. Yin RK. Case Study Research: Design and Methods. 5th ed. Thousand Oaks, CA: Sage Publications, Inc.; 2014.
23. Leppin AL, Gionfriddo MR, Kessler M, et al. Preventing 30-day hospital readmissions: a systematic review and meta-analysis of randomized trials. JAMA Intern Med. 2014;174:1095-1107. PubMed
24. Kansagara D, Chiovaro JC, Kagen D, et al. So many options, where do we start? An overview of the care transitions literature. J Hosp Med. 2016;11:221-230. PubMed
25. Kangovi S, Barg FK, Carter T, et al. Challenges faced by patients with low socioeconomic status during the post-hospital transition. J Gen Intern Med. 2013;29:283-289. PubMed
26. Gill A, Kuluski K, Jaakimainen L, Naganathan G, Upshur R, Wodchis WP. “Where do we go from here?” Health system frustrations expressed by patients with multimorbidity, their caregivers and family physicians. Healthc Policy. 2014;9:73-89. PubMed
27. Shippee ND, Shah ND, May CR, Mair FS, Montori VM. Cumulative complexity: a functional, patient-centered model of patient complexity can improve research and practice. J Clin Epidemiol. 2012;65:1041-1051. PubMed
28. Leppin AL, Montori VM, Gionfriddo MR. Minimally disruptive medicine: a pragmatically comprehensive model for delivering care to patients with multiple chronic conditions. Healthcare (Basel). 2015;3:50-63. PubMed
29. Salisbury C. Multimorbidity: redesigning health care for people who use it. Lancet. 2012;380:7-9. PubMed
30. Upshur R, Tracy S. Chronicity and complexity: is what’s good for the diseases always good for the patients? Can Fam Physician. 2008;54:1655-1658. PubMed
31. Albreht A, Dyakova M, Schellevis FG, Van den Broucke S. Many diseases, one model of care? J Comorbidity. 2016;6:12-20.
32. Shippee ND, Allen SV, Leppin AL, May CR, Montori VM. Attaining minimally disruptive medicine: context, challenges and a roadmap for implementation. J R Coll Physicians Edinb. 2015;45:118-122. PubMed
Patient complexity is associated with greater hospital readmission rates,1,2 poorer quality of care,3 and lower patient satisfaction.4 Improving outcomes for complex patients is a global priority,5 and local initiatives such as Ontario’s Health Links are being developed, yet evidence to inform care is lacking.6-8
The prevalence of patients living with multiple comorbidities is increasing as advances in medicine enable people to live and manage chronic diseases.9-11 However, these medical gains have resulted in an increased burden on both patients and healthcare systems. Socioeconomic status and co-occurring psychosocial challenges further complicate health and healthcare in marginalized populations.12,13
Human immunodeficiency virus/acquired immunodeficiency syndrome (HIV/AIDS) is one example of a disease that medicine has transformed. Individuals living with HIV today, on antiretroviral medications, may be able to manage their chronic illness for decades.14,15 However, in addition to social determinants of health that influence ongoing adherence and engagement in care, these medications do not completely eradicate the impact of HIV and, as a result, HIV-positive individuals are at a greater risk of developing additional comorbidities.15 People living with HIV may, therefore, represent an important patient population in which healthcare interventions and system improvements for complex patients should be explored.
Improving health systems and better supporting complex patients requires a broader understanding of the patient experience and the challenges encountered, especially during high-risk periods such as hospital discharge. Qualitative research approaches are designed to help us understand social phenomena in their “natural” settings,16 and thus suited to achieve this goal, providing critical insight to inform healthcare systems and policies.17,18 This study sought to answer the question, “What are the obstacles and challenges faced by complex patients during hospital discharge and post-discharge transition?” We approached patient complexity holistically, using a unified Complexity Framework6 that connects 5 health dimensions—social capital, mental health, demographics, health and social experiences, and physical health—identified as important to understanding complex patients and their interaction with healthcare. A longitudinal case study approach was used, with multiple sources of data, to understand the clinical context and discharge plans in relation to the lived experience of patients over time, exploring potential misalignment and areas for improvement.
METHODS
This community-based research study was conducted at Casey House, a 13-bed subacute care hospital in Toronto, Canada that provides in-patient and community programs to a complex patient group. All patients are HIV-positive. Inpatient hospital care is provided by an interdisciplinary team, including physicians, social workers, nurses, and healthcare aides. A harm reduction approach is taken to substance use. Twelve beds are for general admission. Patients may be transferred from acute-care hospitals or referred by community-based providers. One bed is reserved for scheduled 2-week respite stays.
The primary research team for this community-based project consisted of clinicians and community and academic researchers. The study was conducted in collaboration with housing, healthcare, and HIV service providers and was advised by 2 individuals with lived experience of discharge from Casey House. Community members with lived experience attended team meetings, provided feedback on all stages of the project (ie, interview guides, recruitment, analysis and dissemination), and helped facilitate community engagement sessions with other patients at the start and the end of the project.
Standard practice for discharge planning involves clinicians determining a tentative discharge date and identifying strategies to support the patient. Planning is informed by knowledge gathered by the interdisciplinary team throughout the admission, including social determinants of health (ie, housing, social support, food security). Patients are encouraged to invite an individual from their social support network to attend a discharge meeting, where the care team reviews goals for admission, course of treatment, referrals, and important follow-up dates.
We used a multi-case study approach to explore the discharge process and post-discharge period. A case was defined as the discharge and transition of a patient from hospital to community. Data were collected through serial interviews with patients (n = 4), medical chart abstraction, and review of discharge summaries. Serial interviews, although not frequently used in clinical research, have been proposed as a strong approach for exploring complex processes and to build trust between researcher and participant,19 both of which were relevant in this study. Patient interviews were conducted by the Master’s trained research coordinator (SM) using tailored semi-structured interview guides for 4 time points: before the discharge meeting (I1); after the discharge meeting but before discharge (I2); within a week of discharge (I3); and approximately 30 days after discharge (I4). Interviews were audio recorded and transcribed verbatim.
Cases were eligible if the patient had a general admission and a planned discharge to the community, and was able to communicate in English and direct his/her own care. Patient-initiated discharges and discharges to another healthcare facility were excluded. Casey House clinical staff approached consecutive potentially eligible patients for their willingness to speak with the researcher coordinator. The research coordinator met with patients to assess eligibility and obtain informed consent to participate. All participants provided informed written consent. The study was approved by the University of Toronto HIV Research Ethics Board.
Interview data, managed with MAXQDA software (VERBI GmbH, Berlin, Germany), were analyzed using a framework analysis approach.20,21 At least 3 authors read each transcript in its entirety. Priority questions/topics identified a priori by stakeholders as important to inform change in care and practices were used as the first draft of the coding framework. The framework was modified through team discussion in the analysis phase to integrate emerging themes. Participant demographic and clinical data were extracted using a structured data collection form.
Preliminary data analysis was completed for the separate data sources including inter- and intra-case comparisons: exploring how experiences and perceptions changed over time and themes that emerged across cases at the same time point. Data sources were combined to strengthen the understanding of the cases and identify relationships and discrepancies across sources.22 Audit trails, reflexive journaling, group coding and analysis meetings and member-checking, were used to enhance analytical rigor.
RESULTS
The results focus on the patient experience of the “discharge plan” and are presented in terms of 3 pre-identified categories: 1) social support; 2) discharge process and transition experience; and 3) post-discharge follow-up and referrals; and 1 emergent theme, patient priorities.
Participants experienced complex medical and psychosocial challenges (Table 1, participant characteristics). All participants were living with HIV plus a mean of 5 additional comorbidities, the most common being hepatitis C (n = 3), chronic obstructive pulmonary disease (n = 2), herpes (n = 2) and opportunistic infections (n = 2). Eight of 9 participants had a history of an Axis 1 diagnosis, most commonly mood disorder (n = 4). Substance use was identified in all participants. An overview of each case is presented in Table 2.
Three patients declined to be considered for the study. Informed consent was obtained for 10 cases. One participant withdrew after interview 1. Data are presented here for 9 cases, including 32 interviews, between October 2013 and June 2014. Interviews 1 (I1) and 2 (I2) were combined for 3 participants. Two participants were lost to follow-up for interview 4.
Social Support
For the purposes of this paper, we define “social support” as the emotional or instrumental assistance an individual perceives and experiences from people in his/her self-identified network (ie, family, friends). Participants’ discharge-related experience of social support did not align, in most cases, with the information from their medical charts or their expectations. At admission, 8 of 9 participants identified at least 1 person in their social support network, yet only 1 participant had someone attend the discharge meeting. One participant said she had expected “my daughter, my mother, my brother, somebody. At least somebody. But they never show up.” (P5, I2).
The complexity of her relationship with her family and her unmet needs for support continued after discharge:
I try and be as independent as possible. I don’t have to call them for nothing. Because, even the other day, I called my mom and I asked her, I said, “Mom, I’m going to give you $400 [to pay back a personal loan] and I’m going to give you an extra $100, you could buy me some food.” And she goes “Okay.” But, I didn’t give it to her yet. I don’t know, she seems money hungry right now, so I’m like no, I’ll wait. (P5, I4)
In the hospital, participants frequently spoke about discharge and transition planning that was inclusive of their social support networks. However, a sense of isolation and loneliness was common post-discharge. Often, friends and family members did not provide the support that participants anticipated, but instead were sources of anxiety and stress. One participant conveyed his experience with a friend he listed as a social support:
I gave him some money to get me some groceries, to make sure I had some food in the house when I got home. He didn’t do that. All of a sudden he was called away to [another city]. He told me his father had a heart attack. He told [others] his father had a slip. I still have yet to receive my money. (P7, I4)
Discharge Process and Transition Experience
While some participants were excited about the thought of freedom of being home, others were anxious about the burdens of returning to life outside of the hospital.
I kind of feel like, yeah, I want to go home, but then I think to myself what am I going to do when I get home. Am I just going to go back to what I’ve been doing? Am I going to really change? Am I going to forget to take my pill one day because I’m home and stuff like that. (P4, I1)
The discharge process was often perceived by participants to be rushed. Some participants found the discharge meetings helpful, while others did not feel the process empowered them to engage in a meaningful conversation with hospital staff.
There was no one there with me to even help me with my brain, to think. But it’s afterwards I’m like why didn’t I say that, like that’s what I meant to say. The brain just doesn’t function that way. (P8, I2).
This participant struggled with the transition. One week after discharge when she was asked how her health was she replied:
Terrible. I’ve got no energy. I haven’t eaten for 3 days. I haven’t drank for 3 days. I’ve got diarrhea galore […] Just no appetite whatsoever. I can’t even make it up the stairs without losing my breath. If I make it up the stairs, I have to sit for 15 or 20 minutes… (P8, I3)
The weight of maintaining activities of daily living was prominent in all post-discharge interviews, in many cases accentuated by declining health. The transition to home was more challenging than participants expected; the experience was strongly influenced by the stability of their health, their environment, and the complexity of their lives.
Follow-up and Referrals
Discharge summaries included a mean of 7 referrals. All participants were referred to a case coordinator, nurse, and family physician. Other referrals included pharmacist (n = 8); personal support worker (n = 6); housing (n = 5); and food-support programs (n = 5).
Several factors led to challenges accessing and receiving services. Participants identified: difficulty with requisite paperwork; mobility and financial constraints; personal and logistical challenges with home-care providers; and competing priorities, such as caring for family. These experiences were frequently accompanied by frustration and anxiety.
Because, if I’m in [city where girlfriend lives], I will not get the support that I get when I’m home. Like my nurse comes. [She] was supposed to come and see me twice and I missed that. I missed like 4 [appointments]. You understand? Certain things I’ve been missing. (P6, I4)
When one participant was asked if she had followed up with the food support program she had been referred to, she responded:
Oh, baby, no. I’ve been so confused. I’ve had ODSP [referring to Ontario Disability Support Program, a government disability program] on my case. I’ve got all the files all mixed up. My worker’s a real bitch. She hates me, big time. I was supposed to go bring in papers today, but I couldn’t get out of bed. I don’t know how much trouble I’m going to be in with ODSP now. (P8, I3)
Despite comprehensive discharge plans and referrals, all participants experienced delays and difficulties in accessing and receiving services. In most cases, there was no single contributing factor to these challenges; the unique experiences were a result of the complex interplay of multiple factors for each individual.
Patient Priorities
In the hospital, participants primarily identified goals of improving physical health and medication adherence. However, these goals often shifted to meeting basic living necessities and supporting others upon discharge. Barriers to adequate food and mobility were prominent themes.
One participant spoke about the challenges of supporting her son while struggling with her own health after discharge:
Well, I’ve been dying, I can’t even walk, and yet I’m the one that still has to go to WalMart, to grab milk and bread for my kid. It’s not like I need any of that stuff, because I don’t even eat. (P8, I3)
Participants were admitted on a mean of 6 medications and discharged with a mean of 14 (Table 1). In the hospital, medications are dispensed directly to patients; however, maintaining optimal adherence at home was complex. When 1 participant was asked about her medications after being home for a week, she said:
My meds, you know I have the cream that I’m supposed to put … and I can’t find it. I lost it yesterday. I used it yesterday morning and all day yesterday I’m looking, like, did it fall behind there? But, obviously, I can’t look over there [because of mobility challenges] … I don’t think I can get it covered [by insurance to replace it]. (P5, I3)
Participants found it difficult to follow a specific dosing schedule, ensure food intake corresponded to medication guidelines, and navigate the impact of substance use. Substance use for some was associated with nonadherence. A participant, explaining his quickly declining health, spoke about the impact of using crack cocaine:
Yeah, when I use I don’t think about medicating, taking my pills or anything like that. That’s not even on your mind. It doesn’t come across your mind. […] I guess, that’s part of the addictive personality. It wants to grab hold of you and say “no, focus on me, focus on me.” (P7, I4)
Others used marijuana as an appetite stimulant and a critical piece of their medication adherence routine.
DISCUSSION
This study followed complex patients through hospital discharge and transition back into the community. In the hospital, participants focused on medical goals, but following discharge basic living needs became the priority. Despite a comprehensive plan to provide support upon discharge, participants found executing and following up with referrals, services, and medication adherence was often overwhelming and not achieved in the month post-hospitalization.
Our study provides depth and context to support and understand the findings of reviews evaluating interventions to improve transitions in care.23,24 A systematic review of interventions to decrease 30-day readmission rates concluded that comprehensive support interventions (with many components) contributed to the greatest reduction in risk of readmission.16 Components that showed the greatest impact were those that were designed to improve patients’ capacity for self-care (including their ability to access and follow through with post-discharge care plans) and those that involved more individuals in the delivery of care.23
Our results also support and expand on other qualitative findings of complex patients. Kangovi et al.25 interviewed patients with low socioeconomic status at a single time point post-discharge to identify common experiences. They summarized their findings in 6 themes: powerlessness during hospitalization; incongruence of patient and clinical team goals; competing issues influencing prominence of health behaviors; socioeconomic constraints on patients’ ability to perform recommended behaviors; sense of abandonment after discharge; and loss of self-efficacy resulting from the “failure” to follow the discharge plan. Our findings tell a very similar story but provide the additional context and understanding of the lived experience over time. We found that the transition experience was most challenging when the home environment was unstable, resulting in a shift in priorities from those set during hospitalization.
While increased support may improve outcomes, there is a need to improve awareness, integration, and support for building capacity within complex patients.26 Capacity is defined here as the sum of resources and abilities that a patient can draw on, and includes physical and mental as well as social, financial, personal, and environmental capabilities and resources.27 This includes understanding the potential negative impact of developing a clinical plan which, in order to operationalize, requires resources in excess of the patient’s capacity at that time.27 Minimally disruptive medicine, a promising theoretical approach for improving the care of complex clients, embodies the awareness of capacity in achieving patient-centered care while “imposing the smallest possible treatment burden on patients’ lives.”28
This study, although not without its limitations, provides an in-depth exploration of the experiences of a small number of patients living with HIV, recruited from a single facility in Toronto, Canada after relatively long hospital stays. There are specific context issues related to HIV, such as stigma and severe consequences for suboptimal medication adherence. Furthermore, this study took place where many urban health resources exist; complex patients in rural settings or in environments less tailored to the needs associated with complex medical, psychiatric, and social conditions may experience greater barriers in the transition process. Although this study captured data from medical charts and documents relevant to the cases, further exploration of the clinician decision-making process in creating the discharge plans and additional sources of data on health outcomes post-discharge would be beneficial.
Despite its limitations, this study provides detail and depth to understand some of the most complex patients who suffer from significant challenges in the health system and who are amongst the highest-cost healthcare users. The case study approach, with serial interviews, is an important strength of this study, enabling meaningful insight into hospital discharge processes and challenges experienced by complex patients that can inform individual-level care practice and the development of new programs and interventions.
This study builds on recent research with complex patients in calling for a new approach to clinical care.6,29,30 In order to support complex patients through discharge, clinical goals and referrals must be made in light of a patient’s capacity in the community. Structural changes may be made to improve coordination and access to services, decreasing the burden and improving the healthcare experience. Albreht et al.31 highlight a number of promising programs across Europe (such as the Clinic for Multimorbidity and Polypharmacy in Denmark) designed to improve the health and healthcare for individuals living with multiple chronic conditions. Small-scale changes are also important such as increasing conversations about the capacity and limitations of individuals listed as social supports, and making appropriate and realistic referrals based on an understanding of a patient’s capacity and motivation for follow-up. Shippee et al.32 identify a list of approaches in line with minimally disruptive medicine that can be integrated into existing systems as part of a developing “toolkit” (eg, elicitation of transcendent patient goals, and integration of patient-reported outcome tracking of challenges and burdens associated with health and daily living). The findings of this study suggest that the elements of the toolkit may provide a foundation for future interventions and research to improve hospital care and discharge outcomes for complex patients.
Disclosures
This project was funded by a Canadian Institutes of Health Research (CIHR) HIV/AIDS Community-based Research Catalyst Grant (#126669). Dr. Brennan’s research is supported by an Ontario HIV Treatment Network (OHTN) Applied HIV Research Chair. Dr. Chan Carusone reports grants from Canadian Institutes of Health Research during the conduct of the study.
Patient complexity is associated with greater hospital readmission rates,1,2 poorer quality of care,3 and lower patient satisfaction.4 Improving outcomes for complex patients is a global priority,5 and local initiatives such as Ontario’s Health Links are being developed, yet evidence to inform care is lacking.6-8
The prevalence of patients living with multiple comorbidities is increasing as advances in medicine enable people to live and manage chronic diseases.9-11 However, these medical gains have resulted in an increased burden on both patients and healthcare systems. Socioeconomic status and co-occurring psychosocial challenges further complicate health and healthcare in marginalized populations.12,13
Human immunodeficiency virus/acquired immunodeficiency syndrome (HIV/AIDS) is one example of a disease that medicine has transformed. Individuals living with HIV today, on antiretroviral medications, may be able to manage their chronic illness for decades.14,15 However, in addition to social determinants of health that influence ongoing adherence and engagement in care, these medications do not completely eradicate the impact of HIV and, as a result, HIV-positive individuals are at a greater risk of developing additional comorbidities.15 People living with HIV may, therefore, represent an important patient population in which healthcare interventions and system improvements for complex patients should be explored.
Improving health systems and better supporting complex patients requires a broader understanding of the patient experience and the challenges encountered, especially during high-risk periods such as hospital discharge. Qualitative research approaches are designed to help us understand social phenomena in their “natural” settings,16 and thus suited to achieve this goal, providing critical insight to inform healthcare systems and policies.17,18 This study sought to answer the question, “What are the obstacles and challenges faced by complex patients during hospital discharge and post-discharge transition?” We approached patient complexity holistically, using a unified Complexity Framework6 that connects 5 health dimensions—social capital, mental health, demographics, health and social experiences, and physical health—identified as important to understanding complex patients and their interaction with healthcare. A longitudinal case study approach was used, with multiple sources of data, to understand the clinical context and discharge plans in relation to the lived experience of patients over time, exploring potential misalignment and areas for improvement.
METHODS
This community-based research study was conducted at Casey House, a 13-bed subacute care hospital in Toronto, Canada that provides in-patient and community programs to a complex patient group. All patients are HIV-positive. Inpatient hospital care is provided by an interdisciplinary team, including physicians, social workers, nurses, and healthcare aides. A harm reduction approach is taken to substance use. Twelve beds are for general admission. Patients may be transferred from acute-care hospitals or referred by community-based providers. One bed is reserved for scheduled 2-week respite stays.
The primary research team for this community-based project consisted of clinicians and community and academic researchers. The study was conducted in collaboration with housing, healthcare, and HIV service providers and was advised by 2 individuals with lived experience of discharge from Casey House. Community members with lived experience attended team meetings, provided feedback on all stages of the project (ie, interview guides, recruitment, analysis and dissemination), and helped facilitate community engagement sessions with other patients at the start and the end of the project.
Standard practice for discharge planning involves clinicians determining a tentative discharge date and identifying strategies to support the patient. Planning is informed by knowledge gathered by the interdisciplinary team throughout the admission, including social determinants of health (ie, housing, social support, food security). Patients are encouraged to invite an individual from their social support network to attend a discharge meeting, where the care team reviews goals for admission, course of treatment, referrals, and important follow-up dates.
We used a multi-case study approach to explore the discharge process and post-discharge period. A case was defined as the discharge and transition of a patient from hospital to community. Data were collected through serial interviews with patients (n = 4), medical chart abstraction, and review of discharge summaries. Serial interviews, although not frequently used in clinical research, have been proposed as a strong approach for exploring complex processes and to build trust between researcher and participant,19 both of which were relevant in this study. Patient interviews were conducted by the Master’s trained research coordinator (SM) using tailored semi-structured interview guides for 4 time points: before the discharge meeting (I1); after the discharge meeting but before discharge (I2); within a week of discharge (I3); and approximately 30 days after discharge (I4). Interviews were audio recorded and transcribed verbatim.
Cases were eligible if the patient had a general admission and a planned discharge to the community, and was able to communicate in English and direct his/her own care. Patient-initiated discharges and discharges to another healthcare facility were excluded. Casey House clinical staff approached consecutive potentially eligible patients for their willingness to speak with the researcher coordinator. The research coordinator met with patients to assess eligibility and obtain informed consent to participate. All participants provided informed written consent. The study was approved by the University of Toronto HIV Research Ethics Board.
Interview data, managed with MAXQDA software (VERBI GmbH, Berlin, Germany), were analyzed using a framework analysis approach.20,21 At least 3 authors read each transcript in its entirety. Priority questions/topics identified a priori by stakeholders as important to inform change in care and practices were used as the first draft of the coding framework. The framework was modified through team discussion in the analysis phase to integrate emerging themes. Participant demographic and clinical data were extracted using a structured data collection form.
Preliminary data analysis was completed for the separate data sources including inter- and intra-case comparisons: exploring how experiences and perceptions changed over time and themes that emerged across cases at the same time point. Data sources were combined to strengthen the understanding of the cases and identify relationships and discrepancies across sources.22 Audit trails, reflexive journaling, group coding and analysis meetings and member-checking, were used to enhance analytical rigor.
RESULTS
The results focus on the patient experience of the “discharge plan” and are presented in terms of 3 pre-identified categories: 1) social support; 2) discharge process and transition experience; and 3) post-discharge follow-up and referrals; and 1 emergent theme, patient priorities.
Participants experienced complex medical and psychosocial challenges (Table 1, participant characteristics). All participants were living with HIV plus a mean of 5 additional comorbidities, the most common being hepatitis C (n = 3), chronic obstructive pulmonary disease (n = 2), herpes (n = 2) and opportunistic infections (n = 2). Eight of 9 participants had a history of an Axis 1 diagnosis, most commonly mood disorder (n = 4). Substance use was identified in all participants. An overview of each case is presented in Table 2.
Three patients declined to be considered for the study. Informed consent was obtained for 10 cases. One participant withdrew after interview 1. Data are presented here for 9 cases, including 32 interviews, between October 2013 and June 2014. Interviews 1 (I1) and 2 (I2) were combined for 3 participants. Two participants were lost to follow-up for interview 4.
Social Support
For the purposes of this paper, we define “social support” as the emotional or instrumental assistance an individual perceives and experiences from people in his/her self-identified network (ie, family, friends). Participants’ discharge-related experience of social support did not align, in most cases, with the information from their medical charts or their expectations. At admission, 8 of 9 participants identified at least 1 person in their social support network, yet only 1 participant had someone attend the discharge meeting. One participant said she had expected “my daughter, my mother, my brother, somebody. At least somebody. But they never show up.” (P5, I2).
The complexity of her relationship with her family and her unmet needs for support continued after discharge:
I try and be as independent as possible. I don’t have to call them for nothing. Because, even the other day, I called my mom and I asked her, I said, “Mom, I’m going to give you $400 [to pay back a personal loan] and I’m going to give you an extra $100, you could buy me some food.” And she goes “Okay.” But, I didn’t give it to her yet. I don’t know, she seems money hungry right now, so I’m like no, I’ll wait. (P5, I4)
In the hospital, participants frequently spoke about discharge and transition planning that was inclusive of their social support networks. However, a sense of isolation and loneliness was common post-discharge. Often, friends and family members did not provide the support that participants anticipated, but instead were sources of anxiety and stress. One participant conveyed his experience with a friend he listed as a social support:
I gave him some money to get me some groceries, to make sure I had some food in the house when I got home. He didn’t do that. All of a sudden he was called away to [another city]. He told me his father had a heart attack. He told [others] his father had a slip. I still have yet to receive my money. (P7, I4)
Discharge Process and Transition Experience
While some participants were excited about the thought of freedom of being home, others were anxious about the burdens of returning to life outside of the hospital.
I kind of feel like, yeah, I want to go home, but then I think to myself what am I going to do when I get home. Am I just going to go back to what I’ve been doing? Am I going to really change? Am I going to forget to take my pill one day because I’m home and stuff like that. (P4, I1)
The discharge process was often perceived by participants to be rushed. Some participants found the discharge meetings helpful, while others did not feel the process empowered them to engage in a meaningful conversation with hospital staff.
There was no one there with me to even help me with my brain, to think. But it’s afterwards I’m like why didn’t I say that, like that’s what I meant to say. The brain just doesn’t function that way. (P8, I2).
This participant struggled with the transition. One week after discharge when she was asked how her health was she replied:
Terrible. I’ve got no energy. I haven’t eaten for 3 days. I haven’t drank for 3 days. I’ve got diarrhea galore […] Just no appetite whatsoever. I can’t even make it up the stairs without losing my breath. If I make it up the stairs, I have to sit for 15 or 20 minutes… (P8, I3)
The weight of maintaining activities of daily living was prominent in all post-discharge interviews, in many cases accentuated by declining health. The transition to home was more challenging than participants expected; the experience was strongly influenced by the stability of their health, their environment, and the complexity of their lives.
Follow-up and Referrals
Discharge summaries included a mean of 7 referrals. All participants were referred to a case coordinator, nurse, and family physician. Other referrals included pharmacist (n = 8); personal support worker (n = 6); housing (n = 5); and food-support programs (n = 5).
Several factors led to challenges accessing and receiving services. Participants identified: difficulty with requisite paperwork; mobility and financial constraints; personal and logistical challenges with home-care providers; and competing priorities, such as caring for family. These experiences were frequently accompanied by frustration and anxiety.
Because, if I’m in [city where girlfriend lives], I will not get the support that I get when I’m home. Like my nurse comes. [She] was supposed to come and see me twice and I missed that. I missed like 4 [appointments]. You understand? Certain things I’ve been missing. (P6, I4)
When one participant was asked if she had followed up with the food support program she had been referred to, she responded:
Oh, baby, no. I’ve been so confused. I’ve had ODSP [referring to Ontario Disability Support Program, a government disability program] on my case. I’ve got all the files all mixed up. My worker’s a real bitch. She hates me, big time. I was supposed to go bring in papers today, but I couldn’t get out of bed. I don’t know how much trouble I’m going to be in with ODSP now. (P8, I3)
Despite comprehensive discharge plans and referrals, all participants experienced delays and difficulties in accessing and receiving services. In most cases, there was no single contributing factor to these challenges; the unique experiences were a result of the complex interplay of multiple factors for each individual.
Patient Priorities
In the hospital, participants primarily identified goals of improving physical health and medication adherence. However, these goals often shifted to meeting basic living necessities and supporting others upon discharge. Barriers to adequate food and mobility were prominent themes.
One participant spoke about the challenges of supporting her son while struggling with her own health after discharge:
Well, I’ve been dying, I can’t even walk, and yet I’m the one that still has to go to WalMart, to grab milk and bread for my kid. It’s not like I need any of that stuff, because I don’t even eat. (P8, I3)
Participants were admitted on a mean of 6 medications and discharged with a mean of 14 (Table 1). In the hospital, medications are dispensed directly to patients; however, maintaining optimal adherence at home was complex. When 1 participant was asked about her medications after being home for a week, she said:
My meds, you know I have the cream that I’m supposed to put … and I can’t find it. I lost it yesterday. I used it yesterday morning and all day yesterday I’m looking, like, did it fall behind there? But, obviously, I can’t look over there [because of mobility challenges] … I don’t think I can get it covered [by insurance to replace it]. (P5, I3)
Participants found it difficult to follow a specific dosing schedule, ensure food intake corresponded to medication guidelines, and navigate the impact of substance use. Substance use for some was associated with nonadherence. A participant, explaining his quickly declining health, spoke about the impact of using crack cocaine:
Yeah, when I use I don’t think about medicating, taking my pills or anything like that. That’s not even on your mind. It doesn’t come across your mind. […] I guess, that’s part of the addictive personality. It wants to grab hold of you and say “no, focus on me, focus on me.” (P7, I4)
Others used marijuana as an appetite stimulant and a critical piece of their medication adherence routine.
DISCUSSION
This study followed complex patients through hospital discharge and transition back into the community. In the hospital, participants focused on medical goals, but following discharge basic living needs became the priority. Despite a comprehensive plan to provide support upon discharge, participants found executing and following up with referrals, services, and medication adherence was often overwhelming and not achieved in the month post-hospitalization.
Our study provides depth and context to support and understand the findings of reviews evaluating interventions to improve transitions in care.23,24 A systematic review of interventions to decrease 30-day readmission rates concluded that comprehensive support interventions (with many components) contributed to the greatest reduction in risk of readmission.16 Components that showed the greatest impact were those that were designed to improve patients’ capacity for self-care (including their ability to access and follow through with post-discharge care plans) and those that involved more individuals in the delivery of care.23
Our results also support and expand on other qualitative findings of complex patients. Kangovi et al.25 interviewed patients with low socioeconomic status at a single time point post-discharge to identify common experiences. They summarized their findings in 6 themes: powerlessness during hospitalization; incongruence of patient and clinical team goals; competing issues influencing prominence of health behaviors; socioeconomic constraints on patients’ ability to perform recommended behaviors; sense of abandonment after discharge; and loss of self-efficacy resulting from the “failure” to follow the discharge plan. Our findings tell a very similar story but provide the additional context and understanding of the lived experience over time. We found that the transition experience was most challenging when the home environment was unstable, resulting in a shift in priorities from those set during hospitalization.
While increased support may improve outcomes, there is a need to improve awareness, integration, and support for building capacity within complex patients.26 Capacity is defined here as the sum of resources and abilities that a patient can draw on, and includes physical and mental as well as social, financial, personal, and environmental capabilities and resources.27 This includes understanding the potential negative impact of developing a clinical plan which, in order to operationalize, requires resources in excess of the patient’s capacity at that time.27 Minimally disruptive medicine, a promising theoretical approach for improving the care of complex clients, embodies the awareness of capacity in achieving patient-centered care while “imposing the smallest possible treatment burden on patients’ lives.”28
This study, although not without its limitations, provides an in-depth exploration of the experiences of a small number of patients living with HIV, recruited from a single facility in Toronto, Canada after relatively long hospital stays. There are specific context issues related to HIV, such as stigma and severe consequences for suboptimal medication adherence. Furthermore, this study took place where many urban health resources exist; complex patients in rural settings or in environments less tailored to the needs associated with complex medical, psychiatric, and social conditions may experience greater barriers in the transition process. Although this study captured data from medical charts and documents relevant to the cases, further exploration of the clinician decision-making process in creating the discharge plans and additional sources of data on health outcomes post-discharge would be beneficial.
Despite its limitations, this study provides detail and depth to understand some of the most complex patients who suffer from significant challenges in the health system and who are amongst the highest-cost healthcare users. The case study approach, with serial interviews, is an important strength of this study, enabling meaningful insight into hospital discharge processes and challenges experienced by complex patients that can inform individual-level care practice and the development of new programs and interventions.
This study builds on recent research with complex patients in calling for a new approach to clinical care.6,29,30 In order to support complex patients through discharge, clinical goals and referrals must be made in light of a patient’s capacity in the community. Structural changes may be made to improve coordination and access to services, decreasing the burden and improving the healthcare experience. Albreht et al.31 highlight a number of promising programs across Europe (such as the Clinic for Multimorbidity and Polypharmacy in Denmark) designed to improve the health and healthcare for individuals living with multiple chronic conditions. Small-scale changes are also important such as increasing conversations about the capacity and limitations of individuals listed as social supports, and making appropriate and realistic referrals based on an understanding of a patient’s capacity and motivation for follow-up. Shippee et al.32 identify a list of approaches in line with minimally disruptive medicine that can be integrated into existing systems as part of a developing “toolkit” (eg, elicitation of transcendent patient goals, and integration of patient-reported outcome tracking of challenges and burdens associated with health and daily living). The findings of this study suggest that the elements of the toolkit may provide a foundation for future interventions and research to improve hospital care and discharge outcomes for complex patients.
Disclosures
This project was funded by a Canadian Institutes of Health Research (CIHR) HIV/AIDS Community-based Research Catalyst Grant (#126669). Dr. Brennan’s research is supported by an Ontario HIV Treatment Network (OHTN) Applied HIV Research Chair. Dr. Chan Carusone reports grants from Canadian Institutes of Health Research during the conduct of the study.
1. Allaudeen N, Vidyarthi A, Masselli J, Auerback A. Redefining readmission risk factors for general medicine patients. J Hosp Med. 2011;6:54-60. PubMed
2. Hu J, Gonsahn MD, Nerenz DR. Socioeconomic status and readmissions: evidence from an urban teaching hospital. Health Aff (Millwood). 2014;33:778-785. PubMed
3. Panagioti M, Stokes J, Esmail A, et al. Multimorbidity and patient safety incidents in primary care: a systematic review and meta-analysis. PLoS One. 2015;10:e0135947. PubMed
4. Paddison CA, Saunders CL, Abel GA, Payne RA, Campbell JL, Roland M. Why do patients with multimorbidity in England report worse experiences in primary care? Evidence from the General Practice Patient Survey. BMJ Open. 2015;5:e006172. PubMed
5. Barnett K, Mercer SW, Norbury M, Watt G, Wyke S, Guthrie B. Epidemiology of multimorbidity and implications for health care, research, and medical education: a cross-sectional study. Lancet. 2012;380:37-43. PubMed
6. Schaink AK, Kuluski K, Lyons RF, et al. A scoping review and thematic classification of patient complexity: offering a unifying framework. J Comorbidity. 2012;2:1-9.
7. Roland M, Paddison C. Better management of patients with multimorbidity. BMJ. 2013;346:f2510. PubMed
8. Smith SM, Soubhi H, Fortin M, Hudon C, O’Dowd T. Managing patients with multimorbidity: a systematic review of interventions in primary care and community settings. BMJ. 2012;345:e5205. PubMed
9. Afshar S, Roderick PJ, Kowal P, Dimitrov BD, Hill AG. Multimorbidity and the inequalities of global ageing: a cross-sectional study of 28 countries using the World Health Surveys. BMC Public Health. 2015;15:776. PubMed
10. Pefoyo AJK, Bronskill SE, Gruneir A, et al. The increasing burden and complexity of multimorbidity. BMC Public Health. 2015;15:415. PubMed
11. Ward BW, Schiller JS. Prevalence of multiple chronic conditions among US adults: estimates from the National Health Interview Survey, 2010. Prev Chronic Dis. 2013;10:E65. PubMed
12. World Health Organization. Commission on Social Determinants of Health Final Report: Closing the Gap in a Generation: Health Equity through Action on Social Determinants of Health. Geneva, Switzerland: World Health Organization, 2008.
13. Barnett K, Mercer SW, Norbury M, Watt G, Wyke S, Guthrie B.. Epidemiology of multimorbidity and implications for health care, research and medical education: a cross-sectional study. Lancet. 2012;380:37-43. PubMed
14. Samji H, Cescon A, Hogg RS, et al. Closing the gap: increases in life expectancy among treated HIV-positive individuals in the United States and Canada. PLoS One. 2013;8:e81355. PubMed
15. Deeks SG, Lewin SR, Havlir DV. The end of AIDS: HIV infection as a chronic disease. Lancet. 2013;382:1525-1533. PubMed
16. Mays N, Pope C. Qualitative research: rigour and qualitative research. BMJ. 1995;311:109-112. PubMed
17. Gilson L, Hanson K, Sheikh K, Agyepong IA, Ssengooba F, Bennett S. Building the field of health policy and systems research: social science matters. PLoS Med. 2011;8:e1001079. PubMed
18. Stoto MA, Nelson CD, Klaiman T. Getting from what to why: using qualitative research to conduct public health systems research. AcademyHealth; August 2013. http://www.academyhealth.org/files/publications/qmforph.pdf. Accessed May 24, 2016.
19. Murray SA, Kendall M, Carduff E, et al. Use of serial qualitative interviews to understand patients’ evolving experiences and needs. BMJ. 2009;339:b3702. PubMed
20. Pope C, Ziebland S, Mays N. Qualitative research in health care. Analysing qualitative data. BMJ. 2000;320:114-116. PubMed
21. Dixon-Woods M. Using framework-based synthesis for conducting reviews of qualitative studies. BMC Med. 2011;9:39. PubMed
22. Yin RK. Case Study Research: Design and Methods. 5th ed. Thousand Oaks, CA: Sage Publications, Inc.; 2014.
23. Leppin AL, Gionfriddo MR, Kessler M, et al. Preventing 30-day hospital readmissions: a systematic review and meta-analysis of randomized trials. JAMA Intern Med. 2014;174:1095-1107. PubMed
24. Kansagara D, Chiovaro JC, Kagen D, et al. So many options, where do we start? An overview of the care transitions literature. J Hosp Med. 2016;11:221-230. PubMed
25. Kangovi S, Barg FK, Carter T, et al. Challenges faced by patients with low socioeconomic status during the post-hospital transition. J Gen Intern Med. 2013;29:283-289. PubMed
26. Gill A, Kuluski K, Jaakimainen L, Naganathan G, Upshur R, Wodchis WP. “Where do we go from here?” Health system frustrations expressed by patients with multimorbidity, their caregivers and family physicians. Healthc Policy. 2014;9:73-89. PubMed
27. Shippee ND, Shah ND, May CR, Mair FS, Montori VM. Cumulative complexity: a functional, patient-centered model of patient complexity can improve research and practice. J Clin Epidemiol. 2012;65:1041-1051. PubMed
28. Leppin AL, Montori VM, Gionfriddo MR. Minimally disruptive medicine: a pragmatically comprehensive model for delivering care to patients with multiple chronic conditions. Healthcare (Basel). 2015;3:50-63. PubMed
29. Salisbury C. Multimorbidity: redesigning health care for people who use it. Lancet. 2012;380:7-9. PubMed
30. Upshur R, Tracy S. Chronicity and complexity: is what’s good for the diseases always good for the patients? Can Fam Physician. 2008;54:1655-1658. PubMed
31. Albreht A, Dyakova M, Schellevis FG, Van den Broucke S. Many diseases, one model of care? J Comorbidity. 2016;6:12-20.
32. Shippee ND, Allen SV, Leppin AL, May CR, Montori VM. Attaining minimally disruptive medicine: context, challenges and a roadmap for implementation. J R Coll Physicians Edinb. 2015;45:118-122. PubMed
1. Allaudeen N, Vidyarthi A, Masselli J, Auerback A. Redefining readmission risk factors for general medicine patients. J Hosp Med. 2011;6:54-60. PubMed
2. Hu J, Gonsahn MD, Nerenz DR. Socioeconomic status and readmissions: evidence from an urban teaching hospital. Health Aff (Millwood). 2014;33:778-785. PubMed
3. Panagioti M, Stokes J, Esmail A, et al. Multimorbidity and patient safety incidents in primary care: a systematic review and meta-analysis. PLoS One. 2015;10:e0135947. PubMed
4. Paddison CA, Saunders CL, Abel GA, Payne RA, Campbell JL, Roland M. Why do patients with multimorbidity in England report worse experiences in primary care? Evidence from the General Practice Patient Survey. BMJ Open. 2015;5:e006172. PubMed
5. Barnett K, Mercer SW, Norbury M, Watt G, Wyke S, Guthrie B. Epidemiology of multimorbidity and implications for health care, research, and medical education: a cross-sectional study. Lancet. 2012;380:37-43. PubMed
6. Schaink AK, Kuluski K, Lyons RF, et al. A scoping review and thematic classification of patient complexity: offering a unifying framework. J Comorbidity. 2012;2:1-9.
7. Roland M, Paddison C. Better management of patients with multimorbidity. BMJ. 2013;346:f2510. PubMed
8. Smith SM, Soubhi H, Fortin M, Hudon C, O’Dowd T. Managing patients with multimorbidity: a systematic review of interventions in primary care and community settings. BMJ. 2012;345:e5205. PubMed
9. Afshar S, Roderick PJ, Kowal P, Dimitrov BD, Hill AG. Multimorbidity and the inequalities of global ageing: a cross-sectional study of 28 countries using the World Health Surveys. BMC Public Health. 2015;15:776. PubMed
10. Pefoyo AJK, Bronskill SE, Gruneir A, et al. The increasing burden and complexity of multimorbidity. BMC Public Health. 2015;15:415. PubMed
11. Ward BW, Schiller JS. Prevalence of multiple chronic conditions among US adults: estimates from the National Health Interview Survey, 2010. Prev Chronic Dis. 2013;10:E65. PubMed
12. World Health Organization. Commission on Social Determinants of Health Final Report: Closing the Gap in a Generation: Health Equity through Action on Social Determinants of Health. Geneva, Switzerland: World Health Organization, 2008.
13. Barnett K, Mercer SW, Norbury M, Watt G, Wyke S, Guthrie B.. Epidemiology of multimorbidity and implications for health care, research and medical education: a cross-sectional study. Lancet. 2012;380:37-43. PubMed
14. Samji H, Cescon A, Hogg RS, et al. Closing the gap: increases in life expectancy among treated HIV-positive individuals in the United States and Canada. PLoS One. 2013;8:e81355. PubMed
15. Deeks SG, Lewin SR, Havlir DV. The end of AIDS: HIV infection as a chronic disease. Lancet. 2013;382:1525-1533. PubMed
16. Mays N, Pope C. Qualitative research: rigour and qualitative research. BMJ. 1995;311:109-112. PubMed
17. Gilson L, Hanson K, Sheikh K, Agyepong IA, Ssengooba F, Bennett S. Building the field of health policy and systems research: social science matters. PLoS Med. 2011;8:e1001079. PubMed
18. Stoto MA, Nelson CD, Klaiman T. Getting from what to why: using qualitative research to conduct public health systems research. AcademyHealth; August 2013. http://www.academyhealth.org/files/publications/qmforph.pdf. Accessed May 24, 2016.
19. Murray SA, Kendall M, Carduff E, et al. Use of serial qualitative interviews to understand patients’ evolving experiences and needs. BMJ. 2009;339:b3702. PubMed
20. Pope C, Ziebland S, Mays N. Qualitative research in health care. Analysing qualitative data. BMJ. 2000;320:114-116. PubMed
21. Dixon-Woods M. Using framework-based synthesis for conducting reviews of qualitative studies. BMC Med. 2011;9:39. PubMed
22. Yin RK. Case Study Research: Design and Methods. 5th ed. Thousand Oaks, CA: Sage Publications, Inc.; 2014.
23. Leppin AL, Gionfriddo MR, Kessler M, et al. Preventing 30-day hospital readmissions: a systematic review and meta-analysis of randomized trials. JAMA Intern Med. 2014;174:1095-1107. PubMed
24. Kansagara D, Chiovaro JC, Kagen D, et al. So many options, where do we start? An overview of the care transitions literature. J Hosp Med. 2016;11:221-230. PubMed
25. Kangovi S, Barg FK, Carter T, et al. Challenges faced by patients with low socioeconomic status during the post-hospital transition. J Gen Intern Med. 2013;29:283-289. PubMed
26. Gill A, Kuluski K, Jaakimainen L, Naganathan G, Upshur R, Wodchis WP. “Where do we go from here?” Health system frustrations expressed by patients with multimorbidity, their caregivers and family physicians. Healthc Policy. 2014;9:73-89. PubMed
27. Shippee ND, Shah ND, May CR, Mair FS, Montori VM. Cumulative complexity: a functional, patient-centered model of patient complexity can improve research and practice. J Clin Epidemiol. 2012;65:1041-1051. PubMed
28. Leppin AL, Montori VM, Gionfriddo MR. Minimally disruptive medicine: a pragmatically comprehensive model for delivering care to patients with multiple chronic conditions. Healthcare (Basel). 2015;3:50-63. PubMed
29. Salisbury C. Multimorbidity: redesigning health care for people who use it. Lancet. 2012;380:7-9. PubMed
30. Upshur R, Tracy S. Chronicity and complexity: is what’s good for the diseases always good for the patients? Can Fam Physician. 2008;54:1655-1658. PubMed
31. Albreht A, Dyakova M, Schellevis FG, Van den Broucke S. Many diseases, one model of care? J Comorbidity. 2016;6:12-20.
32. Shippee ND, Allen SV, Leppin AL, May CR, Montori VM. Attaining minimally disruptive medicine: context, challenges and a roadmap for implementation. J R Coll Physicians Edinb. 2015;45:118-122. PubMed
© 2017 Society of Hospital Medicine