User login
according to Duri Yun, MD, of the University of Chicago Medicine, and associates.
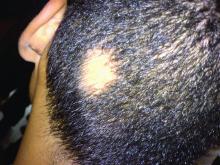
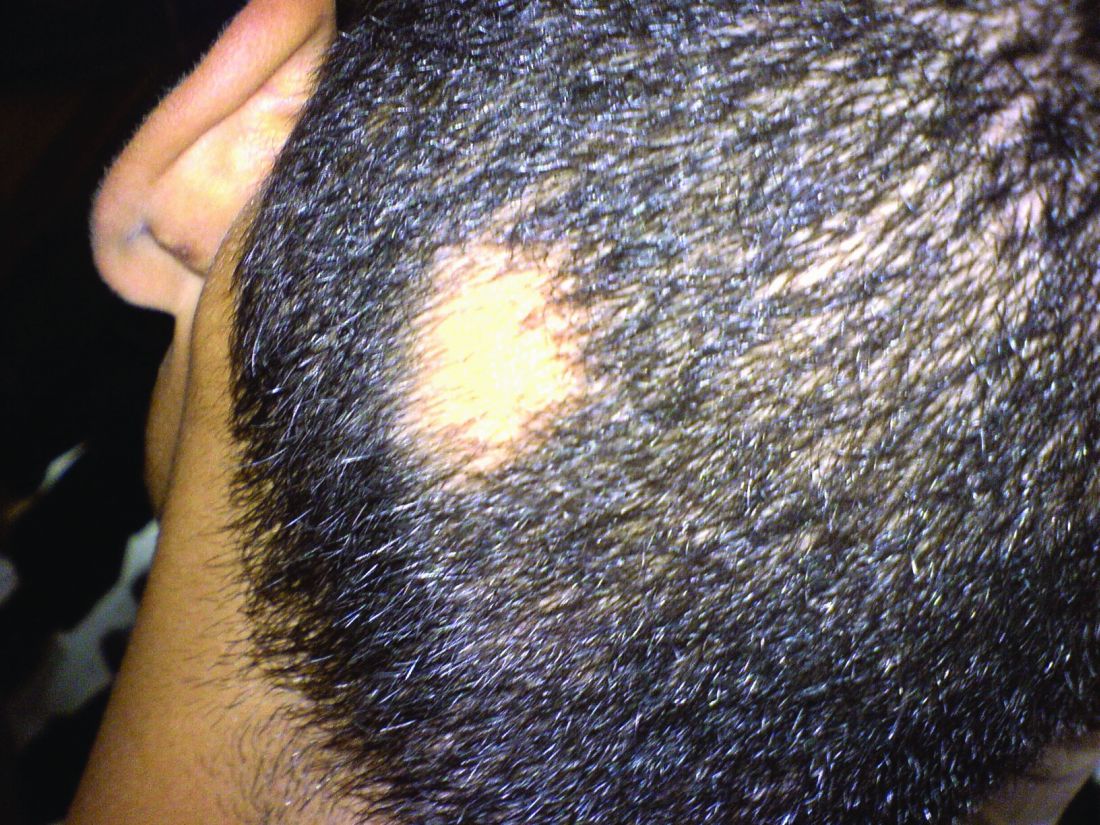
In a retrospective review published in Pediatric Dermatology, nine children aged 6-16 years with AA and diverse ethnicities were treated with hydroxychloroquine between July 1, 2013, and July 1, 2015; all had failed multiple previous treatment modalities. In patient 1, hydroxychloroquine therapy was initiated, fine hair regrowth occurred after 5 months of therapy and was maintained, with dosage tapered to 200 mg once daily after 1 year. After 2 years of therapy, hair had nearly completely regrown. Similar results occurred in patient 2, who had nearly complete hair loss within 2 weeks of initiating hydroxychloroquine. Steady regrowth continued to near-complete regrowth after 1 year of treatment, when dosage was tapered to 200 mg once daily.
Four patients (44%) had no evidence of regrowth after 4-6 months of hydroxychloroquine therapy so they discontinued therapy. The most common adverse events while taking hydroxychloroquine were abdominal pain in two patients (22%) and headache in two patients (22%).
“In the context of children with severe AA failing multiple first-line therapies, our findings suggest that there may be a subgroup that benefits from therapy with hydroxychloroquine,” the researchers concluded. “Determining which factors might predict response to various therapies will come from combined efforts to conduct well-controlled clinical trials of treatments for AA.”
SOURCE: Yun D et al., Pediatr Dermatol. 2018 Mar 25. doi: 10.1111/pde.13451.
according to Duri Yun, MD, of the University of Chicago Medicine, and associates.
In a retrospective review published in Pediatric Dermatology, nine children aged 6-16 years with AA and diverse ethnicities were treated with hydroxychloroquine between July 1, 2013, and July 1, 2015; all had failed multiple previous treatment modalities. In patient 1, hydroxychloroquine therapy was initiated, fine hair regrowth occurred after 5 months of therapy and was maintained, with dosage tapered to 200 mg once daily after 1 year. After 2 years of therapy, hair had nearly completely regrown. Similar results occurred in patient 2, who had nearly complete hair loss within 2 weeks of initiating hydroxychloroquine. Steady regrowth continued to near-complete regrowth after 1 year of treatment, when dosage was tapered to 200 mg once daily.
Four patients (44%) had no evidence of regrowth after 4-6 months of hydroxychloroquine therapy so they discontinued therapy. The most common adverse events while taking hydroxychloroquine were abdominal pain in two patients (22%) and headache in two patients (22%).
“In the context of children with severe AA failing multiple first-line therapies, our findings suggest that there may be a subgroup that benefits from therapy with hydroxychloroquine,” the researchers concluded. “Determining which factors might predict response to various therapies will come from combined efforts to conduct well-controlled clinical trials of treatments for AA.”
SOURCE: Yun D et al., Pediatr Dermatol. 2018 Mar 25. doi: 10.1111/pde.13451.
according to Duri Yun, MD, of the University of Chicago Medicine, and associates.
In a retrospective review published in Pediatric Dermatology, nine children aged 6-16 years with AA and diverse ethnicities were treated with hydroxychloroquine between July 1, 2013, and July 1, 2015; all had failed multiple previous treatment modalities. In patient 1, hydroxychloroquine therapy was initiated, fine hair regrowth occurred after 5 months of therapy and was maintained, with dosage tapered to 200 mg once daily after 1 year. After 2 years of therapy, hair had nearly completely regrown. Similar results occurred in patient 2, who had nearly complete hair loss within 2 weeks of initiating hydroxychloroquine. Steady regrowth continued to near-complete regrowth after 1 year of treatment, when dosage was tapered to 200 mg once daily.
Four patients (44%) had no evidence of regrowth after 4-6 months of hydroxychloroquine therapy so they discontinued therapy. The most common adverse events while taking hydroxychloroquine were abdominal pain in two patients (22%) and headache in two patients (22%).
“In the context of children with severe AA failing multiple first-line therapies, our findings suggest that there may be a subgroup that benefits from therapy with hydroxychloroquine,” the researchers concluded. “Determining which factors might predict response to various therapies will come from combined efforts to conduct well-controlled clinical trials of treatments for AA.”
SOURCE: Yun D et al., Pediatr Dermatol. 2018 Mar 25. doi: 10.1111/pde.13451.
FROM PEDIATRIC DERMATOLOGY