User login
In the first part of this 2-part series (Cutis. 2021;108:271-275), we discussed topical medicament allergic contact dermatitis (ACD) from acne and rosacea medications, antimicrobials, antihistamines, and topical pain preparations. In part 2 of this series, we focus on topical corticosteroids, immunomodulators, and anesthetics.
Corticosteroids
Given their anti-inflammatory and immune-modulating effects, topical corticosteroids are utilized for the treatment of contact dermatitis and yet also are frequent culprits of ACD. The North American Contact Dermatitis Group (NACDG) demonstrated a 4% frequency of positive patch tests to at least one corticosteroid from 2007 to 2014; the relevant allergens were tixocortol pivalate (TP)(2.3%), budesonide (0.9%), hydrocortisone-17-butyrate (0.4%), clobetasol-17-propionate (0.3%), and desoximetasone (0.2%).1 Corticosteroid contact allergy can be difficult to recognize and may present as a flare of the underlying condition being treated. Clinically, these rashes may demonstrate an edge effect, characterized by pronounced dermatitis adjacent to and surrounding the treatment area due to concentrated anti-inflammatory effects in the center.
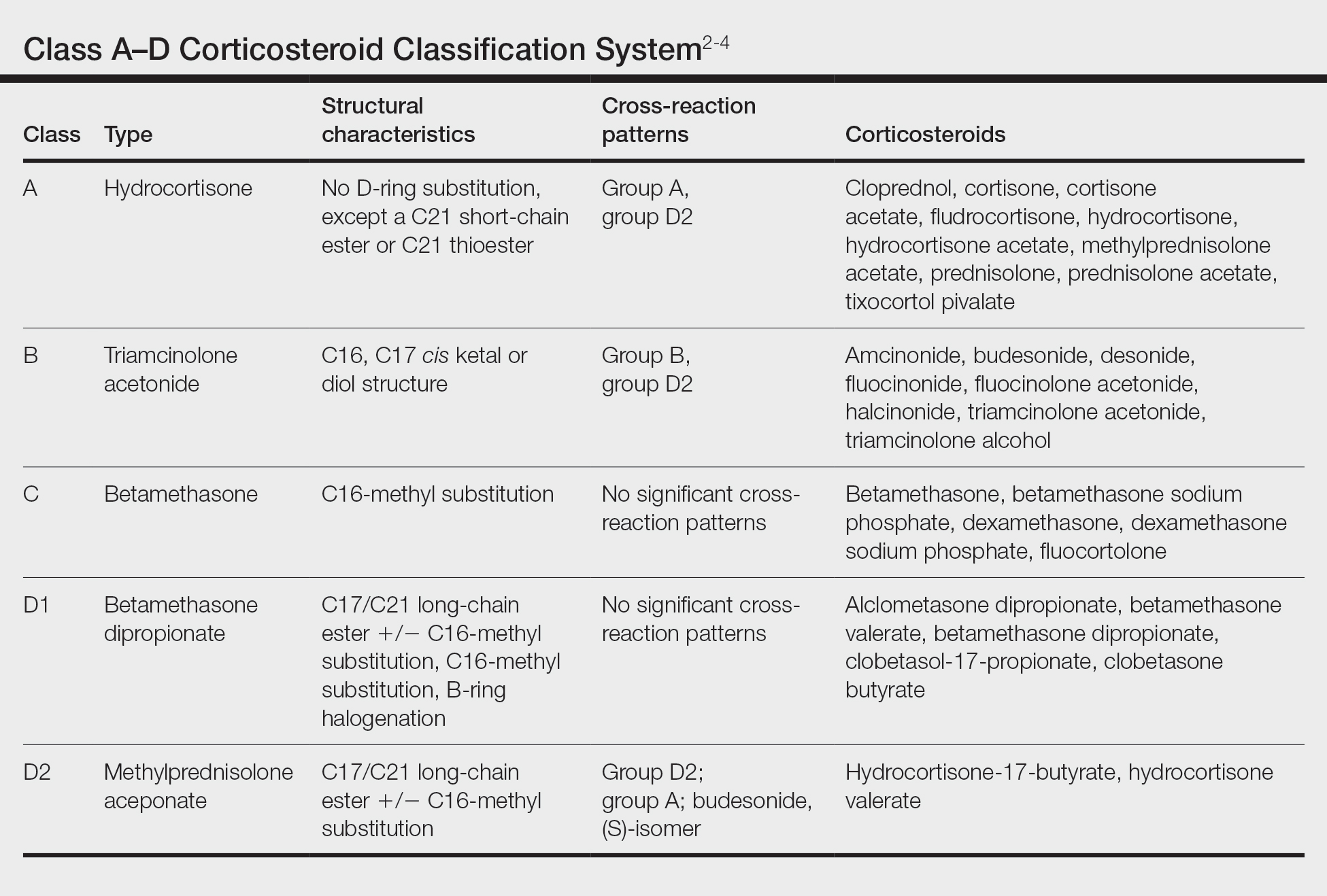
Traditionally, corticosteroids are divided into 4 basic structural groups—classes A, B, C, and D—based on the Coopman et al2 classification (Table). The class D corticosteroids were further subdivided into classes D1, defined by C16-methyl substitution and halogenation of the B ring, and D2, which lacks the aforementioned substitutions.4 However, more recently Baeck et al5 simplified this classification into 3 main groups of steroids based on molecular modeling in combination with patch test results. Group 1 combines the nonmethylated and (mostly) nonhalogenated class A and D2 molecules plus budesonide; group 2 accounts for some halogenated class B molecules with the C16, C17 cis ketal or diol structure; and group 3 includes halogenated and C16-methylated molecules from classes C and D1.4 For the purposes of this review, discussion of classes A through D refers to the Coopman et al2 classification, and groups 1 through 3 refers to Baeck et al.5
Tixocortol pivalate is used as a surrogate marker for hydrocortisone allergy and other class A corticosteroids and is part of the group 1 steroid classification. Interestingly, patients with TP-positive patch tests may not exhibit signs or symptoms of ACD from the use of hydrocortisone products. Repeat open application testing (ROAT) or provocative use testing may elicit a positive response in these patients, especially with the use of hydrocortisone cream (vs ointment), likely due to greater transepidermal penetration.6 There is little consensus on the optimal concentration of TP for patch testing. Although TP 1% often is recommended, studies have shown mixed findings of notable differences between high (1% petrolatum) and low (0.1% petrolatum) concentrations of TP.7,8
Budesonide also is part of group 1 and is a marker for contact allergy to class B corticosteroids, such as triamcinolone and fluocinonide. Cross-reactions between budesonide and other corticosteroids traditionally classified as group B may be explained by structural similarities, whereas cross-reactions with certain class D corticosteroids, such as hydrocortisone-17-butyrate, may be better explained by the diastereomer composition of budesonide.9,10 In a European study, budesonide 0.01% and TP 0.1% included in the European Baseline Series detected 85% (23/27) of cases of corticosteroid allergies.11 Use of inhaled budesonide can provoke recall dermatitis and therefore should be avoided in allergic patients.12
Testing for ACD to topical steroids is complex, as the potent anti-inflammatory properties of these medications can complicate results. Selecting the appropriate test, vehicle, and concentration can help avoid false negatives. Although intradermal testing previously was thought to be superior to patch testing in detecting topical corticosteroid contact allergy, newer data have demonstrated strong concordance between the two methods.13,14 The risk for skin atrophy, particularly with the use of suspensions, limits the use of intradermal testing.14 An ethanol vehicle is recommended for patch testing, except when testing with TP or budesonide when petrolatum provides greater corticosteroid stability.14-16 An irritant pattern or a rim effect on patch testing often is considered positive when testing corticosteroids, as the effect of the steroid itself can diminish a positive reaction. As a result, 0.1% dilutions sometimes are favored over 1% test concentrations.14,15,17 Late readings (>7 days) may be necessary to detect positive reactions in both adults and children.18,19
The authors (M.R., A.R.A.) find these varied classifications of steroids daunting (and somewhat confusing!). In general, when ACD to topical steroids is suspected, in addition to standard patch testing with a corticosteroid series, ROAT of the suspected steroid may be necessary, as the rules of steroid classification may not be reproducible in the real world. For patients with only corticosteroid allergy, calcineurin inhibitors are a safe alternative.
Immunomodulators
Calcipotriol is a vitamin D analogue commonly used to treat psoriasis. Although it is a well-known irritant, ACD to topical calcipotriol rarely has been reported.20-23 Topical calcipotriol does not seem to cross-react with other vitamin D analogues, including tacalcitol and calcitriol.21,24 Based on the literature and the nonirritant reactive thresholds described by Fullerton et al,25 recommended patch test concentrations of calcipotriol in isopropanol are 2 to 10 µg/mL. Given its immunomodulating effects, calcipotriol may suppress contact hypersensitization from other allergens, similar to the effects seen with UV radiation.26
Calcineurin inhibitors act on the nuclear factor of activated T cells signaling pathway, resulting in downstream suppression of proinflammatory cytokines. Contact allergy to these topical medications is rare and mainly has involved pimecrolimus.27-30 In one case, a patient with a previously documented topical tacrolimus contact allergy demonstrated cross-reactivity with pimecrolimus on a double-blinded, right-vs-left ROAT, as well as by patch testing with pimecrolimus cream 1%, which was only weakly positive (+).27 Patch test concentrations of 2.5% or higher may be required to elicit positive reactions to tacrolimus, as shown in one case where this was attributed to high molecular weight and poor extrafacial skin absorption of tacrolimus.30 In an unusual case, a patient reacted positively to patch testing and ROAT using pimecrolimus cream 1% but not pimecrolimus 1% to 5% in petrolatum or alcohol nor the individual excipients, illustrating the importance of testing with both active and inactive ingredients.29
Anesthetics
Local anesthetics can be separated into 2 main groups—amides and esters—based on their chemical structures. From 2001 to 2004, the NACDG patch tested 10,061 patients and found 344 (3.4%) with a positive reaction to at least one topical anesthetic.31 We will discuss some of the allergic cutaneous reactions associated with topical benzocaine (an ester) and lidocaine and prilocaine (amides).
According to the NACDG, the estimated prevalence of topical benzocaine allergy from 2001 to 2018 was roughly 3%.32 Allergic contact dermatitis has been reported in patients who used topical benzocaine to treat localized pain disorders, including herpes zoster and dental pain.33,34 Benzocaine may be used in the anogenital region in the form of antihemorrhoidal creams and in condoms and is a considerably more common allergen in those with anogenital dermatitis compared to those without.35-38 Although cross-reactions within the same anesthetic group are common, clinicians also should be aware of the potential for concomitant sensitivity between unrelated local anesthetics.39-41
From 2001 to 2018, the prevalence of ACD to topical lidocaine was estimated to be 7.9%, according to the NACDG.32 A topical anesthetic containing both lidocaine and prilocaine often is used preprocedurally and can be a source of ACD. Interestingly, several cases of ACD to combination lidocaine/prilocaine cream demonstrated positive patch tests to prilocaine but not lidocaine, despite their structural similarities.42-44 One case report described simultaneous positive reactions to both prilocaine 5% and lidocaine 1%.45
There are a few key points to consider when working up contact allergy to local anesthetics. Patients who develop positive patch test reactions to a local anesthetic should undergo further testing to better understand alternatives and future use. As previously mentioned, ACD to one anesthetic does not necessarily preclude the use of other related anesthetics. Intradermal testing may help differentiate immediate and delayed-type allergic reactions to local anesthetics and should therefore follow positive patch tests.46 Importantly, a delayed reading (ie, after day 6 or 7) also should be performed as part of intradermal testing. Patients with positive patch tests but negative intradermal test results may be able to tolerate systemic anesthetic use.47
Patch Testing for Potential Medicament ACD
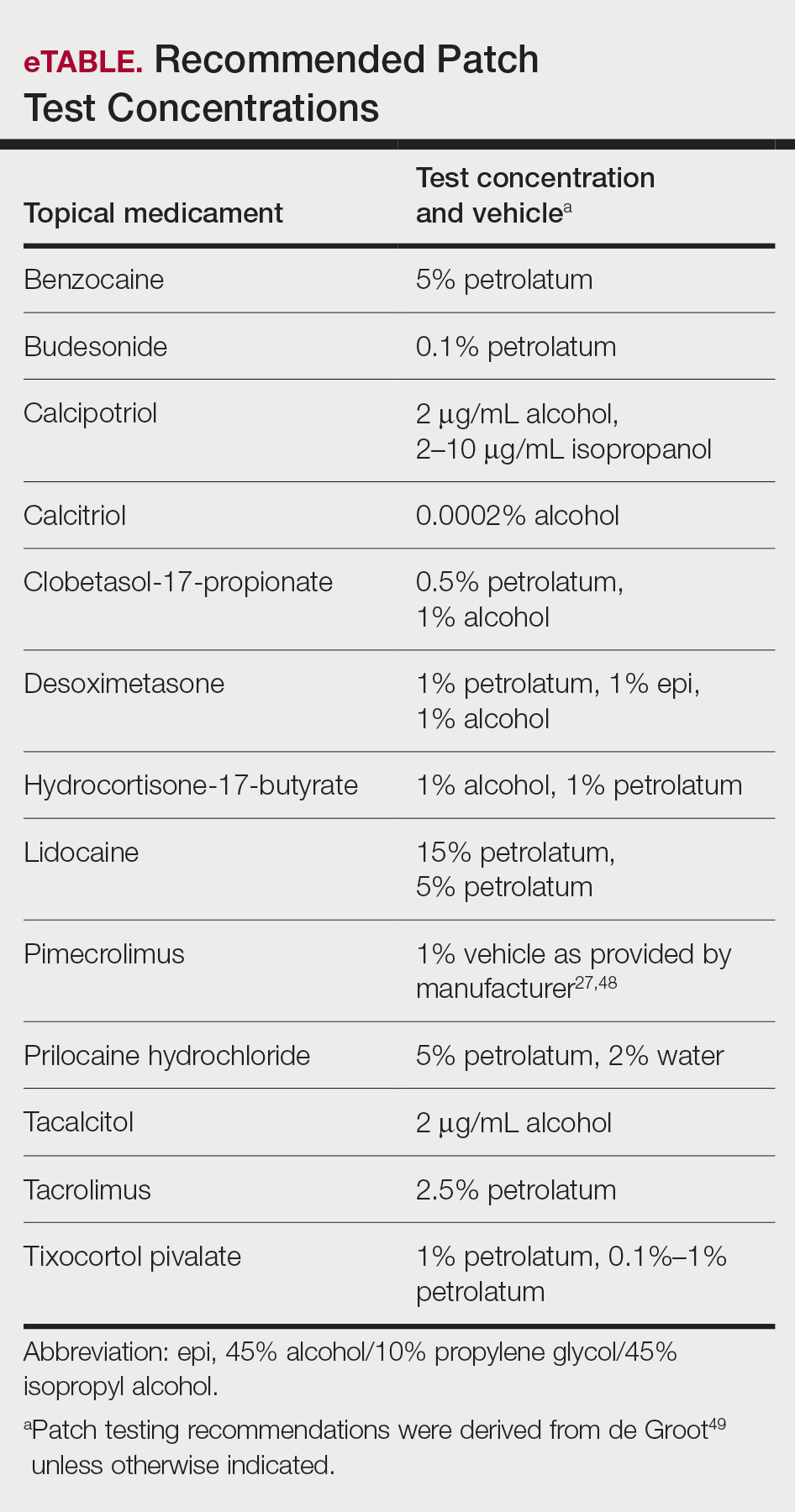
In this article, we touched on several topical medications that have nuanced patch testing specifications given their immunomodulating effects. A simplified outline of recommended patch test concentrations is provided in the eTable, and we encourage you to revisit these useful resources as needed. In many cases, referral to a specialized patch test clinic may be necessary. Although they are not reviewed in this article, always consider inactive ingredients such as preservatives, softening agents, and emulsifiers in the setting of medicament dermatitis, as they also may be culprits of ACD.
Final Interpretation
In this 2-part series, we covered ACD to several common topical drugs with a focus on active ingredients as the source of allergy, and yet this is just the tip of the iceberg. Topical medicaments are prevalent in the field of dermatology, and associated cases of ACD have been reported proportionately. Consider ACD when topical medication efficacy plateaus, triggers new-onset dermatitis, or seems to exacerbate an underlying dermatitis.
- Pratt MD, Mufti A, Lipson J, et al. Patch test reactions to corticosteroids: retrospective analysis from the North American Contact Dermatitis Group 2007-2014. Dermatitis. 2017;28:58-63. doi:10.1097/DER.0000000000000251
- Coopman S, Degreef H, Dooms-Goossens A. Identification of cross-reaction patterns in allergic contact dermatitis from topical corticosteroids. Br J Dermatol. 1989;121:27-34. doi:10.1111/j.1365-2133.1989.tb01396.x
- Jacob SE, Steele T. Corticosteroid classes: a quick reference guide including patch test substances and cross-reactivity. J Am Acad Dermatol. 2006;54:723-727. doi:10.1016/j.jaad.2005.12.028
- Matura M, Goossens A. Contact allergy to corticosteroids. Allergy. 2000;55:698-704. doi:10.1034/j.1398-9995.2000.00121.x
- Baeck M, Chemelle JA, Goossens A, et al. Corticosteroid cross-reactivity: clinical and molecular modelling tools. Allergy. 2011;66:1367-1374. doi:10.1111/j.1398-9995.2011.02666.x
- Shaw DW, Maibach HI. Clinical relevance of tixocortol pivalate-positive patch tests and questionable bioequivalence of different hydrocortisone preparations. Contact Dermatitis. 2013;68:369-375. doi:10.1111/cod.12066
- Kalavala M, Statham BN, Green CM, et al. Tixocortol pivalate: what is the right concentration? Contact Dermatitis. 2007;57:44-46. doi:10.1111/j.1600-0536.2007.01136.x
- Chowdhury MM, Statham BN, Sansom JE, et al. Patch testing for corticosteroid allergy with low and high concentrations of tixocortol pivalate and budesonide. Contact Dermatitis. 2002;46:311-312. doi:10.1034/j.1600-0536.2002.460519.x
- Isaksson M, Bruze M, Lepoittevin JP, et al. Patch testing with serial dilutions of budesonide, its R and S diastereomers, and potentially cross-reacting substances. Am J Contact Dermat. 2001;12:170-176.
- Ferguson AD, Emerson RM, English JS. Cross-reactivity patterns to budesonide. Contact Dermatitis. 2002;47:337-340. doi:10.1034/j.1600-0536.2002.470604.x
- Kot M, Bogaczewicz J, Kre˛cisz B, et al. Contact allergy in the population of patients with chronic inflammatory dermatoses and contact hypersensitivity to corticosteroids. Postepy Dermatol Alergol. 2017;34:253-259. doi:10.5114/ada.2017.67848
- Isaksson M, Bruze M. Allergic contact dermatitis in response to budesonide reactivated by inhalation of the allergen. J Am Acad Dermatol. 2002;46:880-885. doi:10.1067/mjd.2002.120464
- Mimesh S, Pratt M. Allergic contact dermatitis from corticosteroids: reproducibility of patch testing and correlation with intradermal testing. Dermatitis. 2006;17:137-142. doi:10.2310/6620.2006.05048
- Soria A, Baeck M, Goossens A, et al. Patch, prick or intradermal tests to detect delayed hypersensitivity to corticosteroids?. Contact Dermatitis. 2011;64:313-324. doi:10.1111/j.1600-0536.2011.01888.x
- Wilkinson SM, Beck MH. Corticosteroid contact hypersensitivity: what vehicle and concentration? Contact Dermatitis. 1996;34:305-308. doi:10.1111/j.1600-0536.1996.tb02212.x
- Isaksson M, Beck MH, Wilkinson SM. Comparative testing with budesonide in petrolatum and ethanol in a standard series. Contact Dermatitis. 2002;47:123-124. doi:10.1034/j.1600-0536.2002.470210_16.x
- Baeck M, Goossens A. Immediate and delayed allergic hypersensitivity to corticosteroids: practical guidelines. Contact Dermatitis. 2012;66:38-45. doi:10.1111/j.1600-0536.2011.01967.x
- Isaksson M. Corticosteroid contact allergy—the importance of late readings and testing with corticosteroids used by the patients. Contact Dermatitis. 2007;56:56-57. doi:10.1111/j.1600-0536.2007.00959.x
- Tam I, Yu J. Delayed patch test reaction to budesonide in an 8-year-old. Pediatr Dermatol. 2020;37:690-691. doi:10.1111/pde.14168
- Garcia-Bravo B, Camacho F. Two cases of contact dermatitis caused by calcipotriol cream. Am J Contact Dermat. 1996;7:118-119.
- Zollner TM, Ochsendorf FR, Hensel O, et al. Delayed-type reactivity to calcipotriol without cross-sensitization to tacalcitol. Contact Dermatitis. 1997;37:251. doi:10.1111/j.1600-0536.1997.tb02457.x
- Frosch PJ, Rustemeyer T. Contact allergy to calcipotriol does exist. report of an unequivocal case and review of the literature. Contact Dermatitis. 1999;40:66-71. doi:10.1111/j.1600-0536.1999.tb05993.x
- Gilissen L, Huygens S, Goossens A. Allergic contact dermatitis caused by calcipotriol. Contact Dermatitis. 2018;78:139-142. doi:10.1111/cod.12910
- Foti C, Carnimeo L, Bonamonte D, et al. Tolerance to calcitriol and tacalcitol in three patients with allergic contact dermatitis to calcipotriol. J Drugs Dermatol. 2005;4:756-759.
- Fullerton A, Benfeldt E, Petersen JR, et al. The calcipotriol dose-irritation relationship: 48-hour occlusive testing in healthy volunteers using Finn Chambers. Br J Dermatol. 1998;138:259-265. doi:10.1046/j.1365-2133.1998.02071.x
- Hanneman KK, Scull HM, Cooper KD, et al. Effect of topical vitamin D analogue on in vivo contact sensitization. Arch Dermatol. 2006;142:1332-1334. doi:10.1001/archderm.142.10.1332
- Shaw DW, Maibach HI, Eichenfield LF. Allergic contact dermatitis from pimecrolimus in a patient with tacrolimus allergy. J Am Acad Dermatol. 2007;56:342-345. doi:10.1016/j.jaad.2006.09.033
- Saitta P, Brancaccio R. Allergic contact dermatitis to pimecrolimus. Contact Dermatitis. 2007;56:43-44. doi:10.1111/j.1600-0536.2007.00822.x
- Neczyporenko F, Blondeel A. Allergic contact dermatitis to Elidel cream itself? Contact Dermatitis. 2010;63:171-172. doi:10.1111/j.1600-0536.2010.01764.x
- Shaw DW, Eichenfield LF, Shainhouse T, et al. Allergic contact dermatitis from tacrolimus. J Am Acad Dermatol. 2004;50:962-965. doi:10.1016/j.jaad.2003.09.013
- Warshaw EM, Schram SE, Belsito DV, et al. Patch-test reactions to topical anesthetics: retrospective analysis of cross-sectional data, 2001 to 2004. Dermatitis. 2008;19:81-85.
- Warshaw EM, Shaver RL, DeKoven JG, et al. Patch test reactions associated with topical medications: a retrospective analysis of the North American Contact Dermatitis Group data (2001-2018)[published online September 1, 2021]. Dermatitis. doi:10.1097/DER.0000000000000777
- Roos TC, Merk HF. Allergic contact dermatitis from benzocaine ointment during treatment of herpes zoster. Contact Dermatitis. 2001;44:104. doi:10.1034/j.1600-0536.2001.4402097.x
- González-Rodríguez AJ, Gutiérrez-Paredes EM, Revert Fernández Á, et al. Allergic contact dermatitis to benzocaine: the importance of concomitant positive patch test results. Actas Dermosifiliogr. 2013;104:156-158. doi:10.1016/j.ad.2011.07.023
- Muratore L, Calogiuri G, Foti C, et al. Contact allergy to benzocaine in a condom. Contact Dermatitis. 2008;59:173-174. doi:10.1111/j.1600-0536.2008.01359.x
- Sharma A, Agarwal S, Garg G, et al. Desire for lasting long in bed led to contact allergic dermatitis and subsequent superficial penile gangrene: a dreadful complication of benzocaine-containing extended-pleasure condom [published online September 27, 2018]. BMJ Case Rep. 2018;2018:bcr2018227351. doi:10.1136/bcr-2018-227351
- Bauer A, Geier J, Elsner P. Allergic contact dermatitis in patients with anogenital complaints. J Reprod Med. 2000;45:649-654.
- Warshaw EM, Kimyon RS, Silverberg JI, et al. Evaluation of patch test findings in patients with anogenital dermatitis. JAMA Dermatol. 2020;156:85-91. doi:10.1001/jamadermatol.2019.3844
- Weightman W, Turner T. Allergic contact dermatitis from lignocaine: report of 29 cases and review of the literature. Contact Dermatitis. 1998;39:265-266. doi:10.1111/j.1600-0536.1998.tb05928.x
- Jovanovic´ M, Karadaglic´ D, Brkic´ S. Contact urticaria and allergic contact dermatitis to lidocaine in a patient sensitive to benzocaine and propolis. Contact Dermatitis. 2006;54:124-126. doi:10.1111/j.0105-1873.2006.0560f.x
- Carazo JL, Morera BS, Colom LP, et al. Allergic contact dermatitis from ethyl chloride and benzocaine. Dermatitis. 2009;20:E13-E15.
- le Coz CJ, Cribier BJ, Heid E. Patch testing in suspected allergic contact dermatitis due to EMLA cream in haemodialyzed patients. Contact Dermatitis. 1996;35:316-317. doi:10.1111/j.1600-0536.1996.tb02407.x
- Ismail F, Goldsmith PC. EMLA cream-induced allergic contact dermatitis in a child with thalassaemia major. Contact Dermatitis. 2005;52:111. doi:10.1111/j.0105-1873.2005.00498e.x
- Pérez-Pérez LC, Fernández-Redondo V, Ginarte-Val M, et al. Allergic contact dermatitis from EMLA cream in a hemodialyzed patient. Dermatitis. 2006;17:85-87.
- Timmermans MW, Bruynzeel DP, Rustemeyer T. Allergic contact dermatitis from EMLA cream: concomitant sensitization to both local anesthetics lidocaine and prilocaine. J Dtsch Dermatol Ges. 2009;7:237-238. doi:10.1111/j.1610-0387.2008.06932.x
- Fuzier R, Lapeyre-Mestre M, Mertes PM, et al. Immediate- and delayed-type allergic reactions to amide local anesthetics: clinical features and skin testing. Pharmacoepidemiol Drug Saf. 2009;18:595-601. doi:10.1002/pds.1758
- Ruzicka T, Gerstmeier M, Przybilla B, et al. Allergy to local anesthetics: comparison of patch test with prick and intradermal test results. J Am Acad Dermatol. 1987;16:1202-1208. doi:10.1016/s0190-9622(87)70158-3
- Fowler JF Jr, Fowler L, Douglas JL, et al. Skin reactions to pimecrolimus cream 1% in patients allergic to propylene glycol: a double-blind randomized study. Dermatitis. 2007;18:134-139. doi:10.2310/6620.2007.06028
- de Groot A. Patch Testing. 3rd ed. acdegroot publishing; 2008.
In the first part of this 2-part series (Cutis. 2021;108:271-275), we discussed topical medicament allergic contact dermatitis (ACD) from acne and rosacea medications, antimicrobials, antihistamines, and topical pain preparations. In part 2 of this series, we focus on topical corticosteroids, immunomodulators, and anesthetics.
Corticosteroids
Given their anti-inflammatory and immune-modulating effects, topical corticosteroids are utilized for the treatment of contact dermatitis and yet also are frequent culprits of ACD. The North American Contact Dermatitis Group (NACDG) demonstrated a 4% frequency of positive patch tests to at least one corticosteroid from 2007 to 2014; the relevant allergens were tixocortol pivalate (TP)(2.3%), budesonide (0.9%), hydrocortisone-17-butyrate (0.4%), clobetasol-17-propionate (0.3%), and desoximetasone (0.2%).1 Corticosteroid contact allergy can be difficult to recognize and may present as a flare of the underlying condition being treated. Clinically, these rashes may demonstrate an edge effect, characterized by pronounced dermatitis adjacent to and surrounding the treatment area due to concentrated anti-inflammatory effects in the center.
Traditionally, corticosteroids are divided into 4 basic structural groups—classes A, B, C, and D—based on the Coopman et al2 classification (Table). The class D corticosteroids were further subdivided into classes D1, defined by C16-methyl substitution and halogenation of the B ring, and D2, which lacks the aforementioned substitutions.4 However, more recently Baeck et al5 simplified this classification into 3 main groups of steroids based on molecular modeling in combination with patch test results. Group 1 combines the nonmethylated and (mostly) nonhalogenated class A and D2 molecules plus budesonide; group 2 accounts for some halogenated class B molecules with the C16, C17 cis ketal or diol structure; and group 3 includes halogenated and C16-methylated molecules from classes C and D1.4 For the purposes of this review, discussion of classes A through D refers to the Coopman et al2 classification, and groups 1 through 3 refers to Baeck et al.5
Tixocortol pivalate is used as a surrogate marker for hydrocortisone allergy and other class A corticosteroids and is part of the group 1 steroid classification. Interestingly, patients with TP-positive patch tests may not exhibit signs or symptoms of ACD from the use of hydrocortisone products. Repeat open application testing (ROAT) or provocative use testing may elicit a positive response in these patients, especially with the use of hydrocortisone cream (vs ointment), likely due to greater transepidermal penetration.6 There is little consensus on the optimal concentration of TP for patch testing. Although TP 1% often is recommended, studies have shown mixed findings of notable differences between high (1% petrolatum) and low (0.1% petrolatum) concentrations of TP.7,8
Budesonide also is part of group 1 and is a marker for contact allergy to class B corticosteroids, such as triamcinolone and fluocinonide. Cross-reactions between budesonide and other corticosteroids traditionally classified as group B may be explained by structural similarities, whereas cross-reactions with certain class D corticosteroids, such as hydrocortisone-17-butyrate, may be better explained by the diastereomer composition of budesonide.9,10 In a European study, budesonide 0.01% and TP 0.1% included in the European Baseline Series detected 85% (23/27) of cases of corticosteroid allergies.11 Use of inhaled budesonide can provoke recall dermatitis and therefore should be avoided in allergic patients.12
Testing for ACD to topical steroids is complex, as the potent anti-inflammatory properties of these medications can complicate results. Selecting the appropriate test, vehicle, and concentration can help avoid false negatives. Although intradermal testing previously was thought to be superior to patch testing in detecting topical corticosteroid contact allergy, newer data have demonstrated strong concordance between the two methods.13,14 The risk for skin atrophy, particularly with the use of suspensions, limits the use of intradermal testing.14 An ethanol vehicle is recommended for patch testing, except when testing with TP or budesonide when petrolatum provides greater corticosteroid stability.14-16 An irritant pattern or a rim effect on patch testing often is considered positive when testing corticosteroids, as the effect of the steroid itself can diminish a positive reaction. As a result, 0.1% dilutions sometimes are favored over 1% test concentrations.14,15,17 Late readings (>7 days) may be necessary to detect positive reactions in both adults and children.18,19
The authors (M.R., A.R.A.) find these varied classifications of steroids daunting (and somewhat confusing!). In general, when ACD to topical steroids is suspected, in addition to standard patch testing with a corticosteroid series, ROAT of the suspected steroid may be necessary, as the rules of steroid classification may not be reproducible in the real world. For patients with only corticosteroid allergy, calcineurin inhibitors are a safe alternative.
Immunomodulators
Calcipotriol is a vitamin D analogue commonly used to treat psoriasis. Although it is a well-known irritant, ACD to topical calcipotriol rarely has been reported.20-23 Topical calcipotriol does not seem to cross-react with other vitamin D analogues, including tacalcitol and calcitriol.21,24 Based on the literature and the nonirritant reactive thresholds described by Fullerton et al,25 recommended patch test concentrations of calcipotriol in isopropanol are 2 to 10 µg/mL. Given its immunomodulating effects, calcipotriol may suppress contact hypersensitization from other allergens, similar to the effects seen with UV radiation.26
Calcineurin inhibitors act on the nuclear factor of activated T cells signaling pathway, resulting in downstream suppression of proinflammatory cytokines. Contact allergy to these topical medications is rare and mainly has involved pimecrolimus.27-30 In one case, a patient with a previously documented topical tacrolimus contact allergy demonstrated cross-reactivity with pimecrolimus on a double-blinded, right-vs-left ROAT, as well as by patch testing with pimecrolimus cream 1%, which was only weakly positive (+).27 Patch test concentrations of 2.5% or higher may be required to elicit positive reactions to tacrolimus, as shown in one case where this was attributed to high molecular weight and poor extrafacial skin absorption of tacrolimus.30 In an unusual case, a patient reacted positively to patch testing and ROAT using pimecrolimus cream 1% but not pimecrolimus 1% to 5% in petrolatum or alcohol nor the individual excipients, illustrating the importance of testing with both active and inactive ingredients.29
Anesthetics
Local anesthetics can be separated into 2 main groups—amides and esters—based on their chemical structures. From 2001 to 2004, the NACDG patch tested 10,061 patients and found 344 (3.4%) with a positive reaction to at least one topical anesthetic.31 We will discuss some of the allergic cutaneous reactions associated with topical benzocaine (an ester) and lidocaine and prilocaine (amides).
According to the NACDG, the estimated prevalence of topical benzocaine allergy from 2001 to 2018 was roughly 3%.32 Allergic contact dermatitis has been reported in patients who used topical benzocaine to treat localized pain disorders, including herpes zoster and dental pain.33,34 Benzocaine may be used in the anogenital region in the form of antihemorrhoidal creams and in condoms and is a considerably more common allergen in those with anogenital dermatitis compared to those without.35-38 Although cross-reactions within the same anesthetic group are common, clinicians also should be aware of the potential for concomitant sensitivity between unrelated local anesthetics.39-41
From 2001 to 2018, the prevalence of ACD to topical lidocaine was estimated to be 7.9%, according to the NACDG.32 A topical anesthetic containing both lidocaine and prilocaine often is used preprocedurally and can be a source of ACD. Interestingly, several cases of ACD to combination lidocaine/prilocaine cream demonstrated positive patch tests to prilocaine but not lidocaine, despite their structural similarities.42-44 One case report described simultaneous positive reactions to both prilocaine 5% and lidocaine 1%.45
There are a few key points to consider when working up contact allergy to local anesthetics. Patients who develop positive patch test reactions to a local anesthetic should undergo further testing to better understand alternatives and future use. As previously mentioned, ACD to one anesthetic does not necessarily preclude the use of other related anesthetics. Intradermal testing may help differentiate immediate and delayed-type allergic reactions to local anesthetics and should therefore follow positive patch tests.46 Importantly, a delayed reading (ie, after day 6 or 7) also should be performed as part of intradermal testing. Patients with positive patch tests but negative intradermal test results may be able to tolerate systemic anesthetic use.47
Patch Testing for Potential Medicament ACD
In this article, we touched on several topical medications that have nuanced patch testing specifications given their immunomodulating effects. A simplified outline of recommended patch test concentrations is provided in the eTable, and we encourage you to revisit these useful resources as needed. In many cases, referral to a specialized patch test clinic may be necessary. Although they are not reviewed in this article, always consider inactive ingredients such as preservatives, softening agents, and emulsifiers in the setting of medicament dermatitis, as they also may be culprits of ACD.
Final Interpretation
In this 2-part series, we covered ACD to several common topical drugs with a focus on active ingredients as the source of allergy, and yet this is just the tip of the iceberg. Topical medicaments are prevalent in the field of dermatology, and associated cases of ACD have been reported proportionately. Consider ACD when topical medication efficacy plateaus, triggers new-onset dermatitis, or seems to exacerbate an underlying dermatitis.
In the first part of this 2-part series (Cutis. 2021;108:271-275), we discussed topical medicament allergic contact dermatitis (ACD) from acne and rosacea medications, antimicrobials, antihistamines, and topical pain preparations. In part 2 of this series, we focus on topical corticosteroids, immunomodulators, and anesthetics.
Corticosteroids
Given their anti-inflammatory and immune-modulating effects, topical corticosteroids are utilized for the treatment of contact dermatitis and yet also are frequent culprits of ACD. The North American Contact Dermatitis Group (NACDG) demonstrated a 4% frequency of positive patch tests to at least one corticosteroid from 2007 to 2014; the relevant allergens were tixocortol pivalate (TP)(2.3%), budesonide (0.9%), hydrocortisone-17-butyrate (0.4%), clobetasol-17-propionate (0.3%), and desoximetasone (0.2%).1 Corticosteroid contact allergy can be difficult to recognize and may present as a flare of the underlying condition being treated. Clinically, these rashes may demonstrate an edge effect, characterized by pronounced dermatitis adjacent to and surrounding the treatment area due to concentrated anti-inflammatory effects in the center.
Traditionally, corticosteroids are divided into 4 basic structural groups—classes A, B, C, and D—based on the Coopman et al2 classification (Table). The class D corticosteroids were further subdivided into classes D1, defined by C16-methyl substitution and halogenation of the B ring, and D2, which lacks the aforementioned substitutions.4 However, more recently Baeck et al5 simplified this classification into 3 main groups of steroids based on molecular modeling in combination with patch test results. Group 1 combines the nonmethylated and (mostly) nonhalogenated class A and D2 molecules plus budesonide; group 2 accounts for some halogenated class B molecules with the C16, C17 cis ketal or diol structure; and group 3 includes halogenated and C16-methylated molecules from classes C and D1.4 For the purposes of this review, discussion of classes A through D refers to the Coopman et al2 classification, and groups 1 through 3 refers to Baeck et al.5
Tixocortol pivalate is used as a surrogate marker for hydrocortisone allergy and other class A corticosteroids and is part of the group 1 steroid classification. Interestingly, patients with TP-positive patch tests may not exhibit signs or symptoms of ACD from the use of hydrocortisone products. Repeat open application testing (ROAT) or provocative use testing may elicit a positive response in these patients, especially with the use of hydrocortisone cream (vs ointment), likely due to greater transepidermal penetration.6 There is little consensus on the optimal concentration of TP for patch testing. Although TP 1% often is recommended, studies have shown mixed findings of notable differences between high (1% petrolatum) and low (0.1% petrolatum) concentrations of TP.7,8
Budesonide also is part of group 1 and is a marker for contact allergy to class B corticosteroids, such as triamcinolone and fluocinonide. Cross-reactions between budesonide and other corticosteroids traditionally classified as group B may be explained by structural similarities, whereas cross-reactions with certain class D corticosteroids, such as hydrocortisone-17-butyrate, may be better explained by the diastereomer composition of budesonide.9,10 In a European study, budesonide 0.01% and TP 0.1% included in the European Baseline Series detected 85% (23/27) of cases of corticosteroid allergies.11 Use of inhaled budesonide can provoke recall dermatitis and therefore should be avoided in allergic patients.12
Testing for ACD to topical steroids is complex, as the potent anti-inflammatory properties of these medications can complicate results. Selecting the appropriate test, vehicle, and concentration can help avoid false negatives. Although intradermal testing previously was thought to be superior to patch testing in detecting topical corticosteroid contact allergy, newer data have demonstrated strong concordance between the two methods.13,14 The risk for skin atrophy, particularly with the use of suspensions, limits the use of intradermal testing.14 An ethanol vehicle is recommended for patch testing, except when testing with TP or budesonide when petrolatum provides greater corticosteroid stability.14-16 An irritant pattern or a rim effect on patch testing often is considered positive when testing corticosteroids, as the effect of the steroid itself can diminish a positive reaction. As a result, 0.1% dilutions sometimes are favored over 1% test concentrations.14,15,17 Late readings (>7 days) may be necessary to detect positive reactions in both adults and children.18,19
The authors (M.R., A.R.A.) find these varied classifications of steroids daunting (and somewhat confusing!). In general, when ACD to topical steroids is suspected, in addition to standard patch testing with a corticosteroid series, ROAT of the suspected steroid may be necessary, as the rules of steroid classification may not be reproducible in the real world. For patients with only corticosteroid allergy, calcineurin inhibitors are a safe alternative.
Immunomodulators
Calcipotriol is a vitamin D analogue commonly used to treat psoriasis. Although it is a well-known irritant, ACD to topical calcipotriol rarely has been reported.20-23 Topical calcipotriol does not seem to cross-react with other vitamin D analogues, including tacalcitol and calcitriol.21,24 Based on the literature and the nonirritant reactive thresholds described by Fullerton et al,25 recommended patch test concentrations of calcipotriol in isopropanol are 2 to 10 µg/mL. Given its immunomodulating effects, calcipotriol may suppress contact hypersensitization from other allergens, similar to the effects seen with UV radiation.26
Calcineurin inhibitors act on the nuclear factor of activated T cells signaling pathway, resulting in downstream suppression of proinflammatory cytokines. Contact allergy to these topical medications is rare and mainly has involved pimecrolimus.27-30 In one case, a patient with a previously documented topical tacrolimus contact allergy demonstrated cross-reactivity with pimecrolimus on a double-blinded, right-vs-left ROAT, as well as by patch testing with pimecrolimus cream 1%, which was only weakly positive (+).27 Patch test concentrations of 2.5% or higher may be required to elicit positive reactions to tacrolimus, as shown in one case where this was attributed to high molecular weight and poor extrafacial skin absorption of tacrolimus.30 In an unusual case, a patient reacted positively to patch testing and ROAT using pimecrolimus cream 1% but not pimecrolimus 1% to 5% in petrolatum or alcohol nor the individual excipients, illustrating the importance of testing with both active and inactive ingredients.29
Anesthetics
Local anesthetics can be separated into 2 main groups—amides and esters—based on their chemical structures. From 2001 to 2004, the NACDG patch tested 10,061 patients and found 344 (3.4%) with a positive reaction to at least one topical anesthetic.31 We will discuss some of the allergic cutaneous reactions associated with topical benzocaine (an ester) and lidocaine and prilocaine (amides).
According to the NACDG, the estimated prevalence of topical benzocaine allergy from 2001 to 2018 was roughly 3%.32 Allergic contact dermatitis has been reported in patients who used topical benzocaine to treat localized pain disorders, including herpes zoster and dental pain.33,34 Benzocaine may be used in the anogenital region in the form of antihemorrhoidal creams and in condoms and is a considerably more common allergen in those with anogenital dermatitis compared to those without.35-38 Although cross-reactions within the same anesthetic group are common, clinicians also should be aware of the potential for concomitant sensitivity between unrelated local anesthetics.39-41
From 2001 to 2018, the prevalence of ACD to topical lidocaine was estimated to be 7.9%, according to the NACDG.32 A topical anesthetic containing both lidocaine and prilocaine often is used preprocedurally and can be a source of ACD. Interestingly, several cases of ACD to combination lidocaine/prilocaine cream demonstrated positive patch tests to prilocaine but not lidocaine, despite their structural similarities.42-44 One case report described simultaneous positive reactions to both prilocaine 5% and lidocaine 1%.45
There are a few key points to consider when working up contact allergy to local anesthetics. Patients who develop positive patch test reactions to a local anesthetic should undergo further testing to better understand alternatives and future use. As previously mentioned, ACD to one anesthetic does not necessarily preclude the use of other related anesthetics. Intradermal testing may help differentiate immediate and delayed-type allergic reactions to local anesthetics and should therefore follow positive patch tests.46 Importantly, a delayed reading (ie, after day 6 or 7) also should be performed as part of intradermal testing. Patients with positive patch tests but negative intradermal test results may be able to tolerate systemic anesthetic use.47
Patch Testing for Potential Medicament ACD
In this article, we touched on several topical medications that have nuanced patch testing specifications given their immunomodulating effects. A simplified outline of recommended patch test concentrations is provided in the eTable, and we encourage you to revisit these useful resources as needed. In many cases, referral to a specialized patch test clinic may be necessary. Although they are not reviewed in this article, always consider inactive ingredients such as preservatives, softening agents, and emulsifiers in the setting of medicament dermatitis, as they also may be culprits of ACD.
Final Interpretation
In this 2-part series, we covered ACD to several common topical drugs with a focus on active ingredients as the source of allergy, and yet this is just the tip of the iceberg. Topical medicaments are prevalent in the field of dermatology, and associated cases of ACD have been reported proportionately. Consider ACD when topical medication efficacy plateaus, triggers new-onset dermatitis, or seems to exacerbate an underlying dermatitis.
- Pratt MD, Mufti A, Lipson J, et al. Patch test reactions to corticosteroids: retrospective analysis from the North American Contact Dermatitis Group 2007-2014. Dermatitis. 2017;28:58-63. doi:10.1097/DER.0000000000000251
- Coopman S, Degreef H, Dooms-Goossens A. Identification of cross-reaction patterns in allergic contact dermatitis from topical corticosteroids. Br J Dermatol. 1989;121:27-34. doi:10.1111/j.1365-2133.1989.tb01396.x
- Jacob SE, Steele T. Corticosteroid classes: a quick reference guide including patch test substances and cross-reactivity. J Am Acad Dermatol. 2006;54:723-727. doi:10.1016/j.jaad.2005.12.028
- Matura M, Goossens A. Contact allergy to corticosteroids. Allergy. 2000;55:698-704. doi:10.1034/j.1398-9995.2000.00121.x
- Baeck M, Chemelle JA, Goossens A, et al. Corticosteroid cross-reactivity: clinical and molecular modelling tools. Allergy. 2011;66:1367-1374. doi:10.1111/j.1398-9995.2011.02666.x
- Shaw DW, Maibach HI. Clinical relevance of tixocortol pivalate-positive patch tests and questionable bioequivalence of different hydrocortisone preparations. Contact Dermatitis. 2013;68:369-375. doi:10.1111/cod.12066
- Kalavala M, Statham BN, Green CM, et al. Tixocortol pivalate: what is the right concentration? Contact Dermatitis. 2007;57:44-46. doi:10.1111/j.1600-0536.2007.01136.x
- Chowdhury MM, Statham BN, Sansom JE, et al. Patch testing for corticosteroid allergy with low and high concentrations of tixocortol pivalate and budesonide. Contact Dermatitis. 2002;46:311-312. doi:10.1034/j.1600-0536.2002.460519.x
- Isaksson M, Bruze M, Lepoittevin JP, et al. Patch testing with serial dilutions of budesonide, its R and S diastereomers, and potentially cross-reacting substances. Am J Contact Dermat. 2001;12:170-176.
- Ferguson AD, Emerson RM, English JS. Cross-reactivity patterns to budesonide. Contact Dermatitis. 2002;47:337-340. doi:10.1034/j.1600-0536.2002.470604.x
- Kot M, Bogaczewicz J, Kre˛cisz B, et al. Contact allergy in the population of patients with chronic inflammatory dermatoses and contact hypersensitivity to corticosteroids. Postepy Dermatol Alergol. 2017;34:253-259. doi:10.5114/ada.2017.67848
- Isaksson M, Bruze M. Allergic contact dermatitis in response to budesonide reactivated by inhalation of the allergen. J Am Acad Dermatol. 2002;46:880-885. doi:10.1067/mjd.2002.120464
- Mimesh S, Pratt M. Allergic contact dermatitis from corticosteroids: reproducibility of patch testing and correlation with intradermal testing. Dermatitis. 2006;17:137-142. doi:10.2310/6620.2006.05048
- Soria A, Baeck M, Goossens A, et al. Patch, prick or intradermal tests to detect delayed hypersensitivity to corticosteroids?. Contact Dermatitis. 2011;64:313-324. doi:10.1111/j.1600-0536.2011.01888.x
- Wilkinson SM, Beck MH. Corticosteroid contact hypersensitivity: what vehicle and concentration? Contact Dermatitis. 1996;34:305-308. doi:10.1111/j.1600-0536.1996.tb02212.x
- Isaksson M, Beck MH, Wilkinson SM. Comparative testing with budesonide in petrolatum and ethanol in a standard series. Contact Dermatitis. 2002;47:123-124. doi:10.1034/j.1600-0536.2002.470210_16.x
- Baeck M, Goossens A. Immediate and delayed allergic hypersensitivity to corticosteroids: practical guidelines. Contact Dermatitis. 2012;66:38-45. doi:10.1111/j.1600-0536.2011.01967.x
- Isaksson M. Corticosteroid contact allergy—the importance of late readings and testing with corticosteroids used by the patients. Contact Dermatitis. 2007;56:56-57. doi:10.1111/j.1600-0536.2007.00959.x
- Tam I, Yu J. Delayed patch test reaction to budesonide in an 8-year-old. Pediatr Dermatol. 2020;37:690-691. doi:10.1111/pde.14168
- Garcia-Bravo B, Camacho F. Two cases of contact dermatitis caused by calcipotriol cream. Am J Contact Dermat. 1996;7:118-119.
- Zollner TM, Ochsendorf FR, Hensel O, et al. Delayed-type reactivity to calcipotriol without cross-sensitization to tacalcitol. Contact Dermatitis. 1997;37:251. doi:10.1111/j.1600-0536.1997.tb02457.x
- Frosch PJ, Rustemeyer T. Contact allergy to calcipotriol does exist. report of an unequivocal case and review of the literature. Contact Dermatitis. 1999;40:66-71. doi:10.1111/j.1600-0536.1999.tb05993.x
- Gilissen L, Huygens S, Goossens A. Allergic contact dermatitis caused by calcipotriol. Contact Dermatitis. 2018;78:139-142. doi:10.1111/cod.12910
- Foti C, Carnimeo L, Bonamonte D, et al. Tolerance to calcitriol and tacalcitol in three patients with allergic contact dermatitis to calcipotriol. J Drugs Dermatol. 2005;4:756-759.
- Fullerton A, Benfeldt E, Petersen JR, et al. The calcipotriol dose-irritation relationship: 48-hour occlusive testing in healthy volunteers using Finn Chambers. Br J Dermatol. 1998;138:259-265. doi:10.1046/j.1365-2133.1998.02071.x
- Hanneman KK, Scull HM, Cooper KD, et al. Effect of topical vitamin D analogue on in vivo contact sensitization. Arch Dermatol. 2006;142:1332-1334. doi:10.1001/archderm.142.10.1332
- Shaw DW, Maibach HI, Eichenfield LF. Allergic contact dermatitis from pimecrolimus in a patient with tacrolimus allergy. J Am Acad Dermatol. 2007;56:342-345. doi:10.1016/j.jaad.2006.09.033
- Saitta P, Brancaccio R. Allergic contact dermatitis to pimecrolimus. Contact Dermatitis. 2007;56:43-44. doi:10.1111/j.1600-0536.2007.00822.x
- Neczyporenko F, Blondeel A. Allergic contact dermatitis to Elidel cream itself? Contact Dermatitis. 2010;63:171-172. doi:10.1111/j.1600-0536.2010.01764.x
- Shaw DW, Eichenfield LF, Shainhouse T, et al. Allergic contact dermatitis from tacrolimus. J Am Acad Dermatol. 2004;50:962-965. doi:10.1016/j.jaad.2003.09.013
- Warshaw EM, Schram SE, Belsito DV, et al. Patch-test reactions to topical anesthetics: retrospective analysis of cross-sectional data, 2001 to 2004. Dermatitis. 2008;19:81-85.
- Warshaw EM, Shaver RL, DeKoven JG, et al. Patch test reactions associated with topical medications: a retrospective analysis of the North American Contact Dermatitis Group data (2001-2018)[published online September 1, 2021]. Dermatitis. doi:10.1097/DER.0000000000000777
- Roos TC, Merk HF. Allergic contact dermatitis from benzocaine ointment during treatment of herpes zoster. Contact Dermatitis. 2001;44:104. doi:10.1034/j.1600-0536.2001.4402097.x
- González-Rodríguez AJ, Gutiérrez-Paredes EM, Revert Fernández Á, et al. Allergic contact dermatitis to benzocaine: the importance of concomitant positive patch test results. Actas Dermosifiliogr. 2013;104:156-158. doi:10.1016/j.ad.2011.07.023
- Muratore L, Calogiuri G, Foti C, et al. Contact allergy to benzocaine in a condom. Contact Dermatitis. 2008;59:173-174. doi:10.1111/j.1600-0536.2008.01359.x
- Sharma A, Agarwal S, Garg G, et al. Desire for lasting long in bed led to contact allergic dermatitis and subsequent superficial penile gangrene: a dreadful complication of benzocaine-containing extended-pleasure condom [published online September 27, 2018]. BMJ Case Rep. 2018;2018:bcr2018227351. doi:10.1136/bcr-2018-227351
- Bauer A, Geier J, Elsner P. Allergic contact dermatitis in patients with anogenital complaints. J Reprod Med. 2000;45:649-654.
- Warshaw EM, Kimyon RS, Silverberg JI, et al. Evaluation of patch test findings in patients with anogenital dermatitis. JAMA Dermatol. 2020;156:85-91. doi:10.1001/jamadermatol.2019.3844
- Weightman W, Turner T. Allergic contact dermatitis from lignocaine: report of 29 cases and review of the literature. Contact Dermatitis. 1998;39:265-266. doi:10.1111/j.1600-0536.1998.tb05928.x
- Jovanovic´ M, Karadaglic´ D, Brkic´ S. Contact urticaria and allergic contact dermatitis to lidocaine in a patient sensitive to benzocaine and propolis. Contact Dermatitis. 2006;54:124-126. doi:10.1111/j.0105-1873.2006.0560f.x
- Carazo JL, Morera BS, Colom LP, et al. Allergic contact dermatitis from ethyl chloride and benzocaine. Dermatitis. 2009;20:E13-E15.
- le Coz CJ, Cribier BJ, Heid E. Patch testing in suspected allergic contact dermatitis due to EMLA cream in haemodialyzed patients. Contact Dermatitis. 1996;35:316-317. doi:10.1111/j.1600-0536.1996.tb02407.x
- Ismail F, Goldsmith PC. EMLA cream-induced allergic contact dermatitis in a child with thalassaemia major. Contact Dermatitis. 2005;52:111. doi:10.1111/j.0105-1873.2005.00498e.x
- Pérez-Pérez LC, Fernández-Redondo V, Ginarte-Val M, et al. Allergic contact dermatitis from EMLA cream in a hemodialyzed patient. Dermatitis. 2006;17:85-87.
- Timmermans MW, Bruynzeel DP, Rustemeyer T. Allergic contact dermatitis from EMLA cream: concomitant sensitization to both local anesthetics lidocaine and prilocaine. J Dtsch Dermatol Ges. 2009;7:237-238. doi:10.1111/j.1610-0387.2008.06932.x
- Fuzier R, Lapeyre-Mestre M, Mertes PM, et al. Immediate- and delayed-type allergic reactions to amide local anesthetics: clinical features and skin testing. Pharmacoepidemiol Drug Saf. 2009;18:595-601. doi:10.1002/pds.1758
- Ruzicka T, Gerstmeier M, Przybilla B, et al. Allergy to local anesthetics: comparison of patch test with prick and intradermal test results. J Am Acad Dermatol. 1987;16:1202-1208. doi:10.1016/s0190-9622(87)70158-3
- Fowler JF Jr, Fowler L, Douglas JL, et al. Skin reactions to pimecrolimus cream 1% in patients allergic to propylene glycol: a double-blind randomized study. Dermatitis. 2007;18:134-139. doi:10.2310/6620.2007.06028
- de Groot A. Patch Testing. 3rd ed. acdegroot publishing; 2008.
- Pratt MD, Mufti A, Lipson J, et al. Patch test reactions to corticosteroids: retrospective analysis from the North American Contact Dermatitis Group 2007-2014. Dermatitis. 2017;28:58-63. doi:10.1097/DER.0000000000000251
- Coopman S, Degreef H, Dooms-Goossens A. Identification of cross-reaction patterns in allergic contact dermatitis from topical corticosteroids. Br J Dermatol. 1989;121:27-34. doi:10.1111/j.1365-2133.1989.tb01396.x
- Jacob SE, Steele T. Corticosteroid classes: a quick reference guide including patch test substances and cross-reactivity. J Am Acad Dermatol. 2006;54:723-727. doi:10.1016/j.jaad.2005.12.028
- Matura M, Goossens A. Contact allergy to corticosteroids. Allergy. 2000;55:698-704. doi:10.1034/j.1398-9995.2000.00121.x
- Baeck M, Chemelle JA, Goossens A, et al. Corticosteroid cross-reactivity: clinical and molecular modelling tools. Allergy. 2011;66:1367-1374. doi:10.1111/j.1398-9995.2011.02666.x
- Shaw DW, Maibach HI. Clinical relevance of tixocortol pivalate-positive patch tests and questionable bioequivalence of different hydrocortisone preparations. Contact Dermatitis. 2013;68:369-375. doi:10.1111/cod.12066
- Kalavala M, Statham BN, Green CM, et al. Tixocortol pivalate: what is the right concentration? Contact Dermatitis. 2007;57:44-46. doi:10.1111/j.1600-0536.2007.01136.x
- Chowdhury MM, Statham BN, Sansom JE, et al. Patch testing for corticosteroid allergy with low and high concentrations of tixocortol pivalate and budesonide. Contact Dermatitis. 2002;46:311-312. doi:10.1034/j.1600-0536.2002.460519.x
- Isaksson M, Bruze M, Lepoittevin JP, et al. Patch testing with serial dilutions of budesonide, its R and S diastereomers, and potentially cross-reacting substances. Am J Contact Dermat. 2001;12:170-176.
- Ferguson AD, Emerson RM, English JS. Cross-reactivity patterns to budesonide. Contact Dermatitis. 2002;47:337-340. doi:10.1034/j.1600-0536.2002.470604.x
- Kot M, Bogaczewicz J, Kre˛cisz B, et al. Contact allergy in the population of patients with chronic inflammatory dermatoses and contact hypersensitivity to corticosteroids. Postepy Dermatol Alergol. 2017;34:253-259. doi:10.5114/ada.2017.67848
- Isaksson M, Bruze M. Allergic contact dermatitis in response to budesonide reactivated by inhalation of the allergen. J Am Acad Dermatol. 2002;46:880-885. doi:10.1067/mjd.2002.120464
- Mimesh S, Pratt M. Allergic contact dermatitis from corticosteroids: reproducibility of patch testing and correlation with intradermal testing. Dermatitis. 2006;17:137-142. doi:10.2310/6620.2006.05048
- Soria A, Baeck M, Goossens A, et al. Patch, prick or intradermal tests to detect delayed hypersensitivity to corticosteroids?. Contact Dermatitis. 2011;64:313-324. doi:10.1111/j.1600-0536.2011.01888.x
- Wilkinson SM, Beck MH. Corticosteroid contact hypersensitivity: what vehicle and concentration? Contact Dermatitis. 1996;34:305-308. doi:10.1111/j.1600-0536.1996.tb02212.x
- Isaksson M, Beck MH, Wilkinson SM. Comparative testing with budesonide in petrolatum and ethanol in a standard series. Contact Dermatitis. 2002;47:123-124. doi:10.1034/j.1600-0536.2002.470210_16.x
- Baeck M, Goossens A. Immediate and delayed allergic hypersensitivity to corticosteroids: practical guidelines. Contact Dermatitis. 2012;66:38-45. doi:10.1111/j.1600-0536.2011.01967.x
- Isaksson M. Corticosteroid contact allergy—the importance of late readings and testing with corticosteroids used by the patients. Contact Dermatitis. 2007;56:56-57. doi:10.1111/j.1600-0536.2007.00959.x
- Tam I, Yu J. Delayed patch test reaction to budesonide in an 8-year-old. Pediatr Dermatol. 2020;37:690-691. doi:10.1111/pde.14168
- Garcia-Bravo B, Camacho F. Two cases of contact dermatitis caused by calcipotriol cream. Am J Contact Dermat. 1996;7:118-119.
- Zollner TM, Ochsendorf FR, Hensel O, et al. Delayed-type reactivity to calcipotriol without cross-sensitization to tacalcitol. Contact Dermatitis. 1997;37:251. doi:10.1111/j.1600-0536.1997.tb02457.x
- Frosch PJ, Rustemeyer T. Contact allergy to calcipotriol does exist. report of an unequivocal case and review of the literature. Contact Dermatitis. 1999;40:66-71. doi:10.1111/j.1600-0536.1999.tb05993.x
- Gilissen L, Huygens S, Goossens A. Allergic contact dermatitis caused by calcipotriol. Contact Dermatitis. 2018;78:139-142. doi:10.1111/cod.12910
- Foti C, Carnimeo L, Bonamonte D, et al. Tolerance to calcitriol and tacalcitol in three patients with allergic contact dermatitis to calcipotriol. J Drugs Dermatol. 2005;4:756-759.
- Fullerton A, Benfeldt E, Petersen JR, et al. The calcipotriol dose-irritation relationship: 48-hour occlusive testing in healthy volunteers using Finn Chambers. Br J Dermatol. 1998;138:259-265. doi:10.1046/j.1365-2133.1998.02071.x
- Hanneman KK, Scull HM, Cooper KD, et al. Effect of topical vitamin D analogue on in vivo contact sensitization. Arch Dermatol. 2006;142:1332-1334. doi:10.1001/archderm.142.10.1332
- Shaw DW, Maibach HI, Eichenfield LF. Allergic contact dermatitis from pimecrolimus in a patient with tacrolimus allergy. J Am Acad Dermatol. 2007;56:342-345. doi:10.1016/j.jaad.2006.09.033
- Saitta P, Brancaccio R. Allergic contact dermatitis to pimecrolimus. Contact Dermatitis. 2007;56:43-44. doi:10.1111/j.1600-0536.2007.00822.x
- Neczyporenko F, Blondeel A. Allergic contact dermatitis to Elidel cream itself? Contact Dermatitis. 2010;63:171-172. doi:10.1111/j.1600-0536.2010.01764.x
- Shaw DW, Eichenfield LF, Shainhouse T, et al. Allergic contact dermatitis from tacrolimus. J Am Acad Dermatol. 2004;50:962-965. doi:10.1016/j.jaad.2003.09.013
- Warshaw EM, Schram SE, Belsito DV, et al. Patch-test reactions to topical anesthetics: retrospective analysis of cross-sectional data, 2001 to 2004. Dermatitis. 2008;19:81-85.
- Warshaw EM, Shaver RL, DeKoven JG, et al. Patch test reactions associated with topical medications: a retrospective analysis of the North American Contact Dermatitis Group data (2001-2018)[published online September 1, 2021]. Dermatitis. doi:10.1097/DER.0000000000000777
- Roos TC, Merk HF. Allergic contact dermatitis from benzocaine ointment during treatment of herpes zoster. Contact Dermatitis. 2001;44:104. doi:10.1034/j.1600-0536.2001.4402097.x
- González-Rodríguez AJ, Gutiérrez-Paredes EM, Revert Fernández Á, et al. Allergic contact dermatitis to benzocaine: the importance of concomitant positive patch test results. Actas Dermosifiliogr. 2013;104:156-158. doi:10.1016/j.ad.2011.07.023
- Muratore L, Calogiuri G, Foti C, et al. Contact allergy to benzocaine in a condom. Contact Dermatitis. 2008;59:173-174. doi:10.1111/j.1600-0536.2008.01359.x
- Sharma A, Agarwal S, Garg G, et al. Desire for lasting long in bed led to contact allergic dermatitis and subsequent superficial penile gangrene: a dreadful complication of benzocaine-containing extended-pleasure condom [published online September 27, 2018]. BMJ Case Rep. 2018;2018:bcr2018227351. doi:10.1136/bcr-2018-227351
- Bauer A, Geier J, Elsner P. Allergic contact dermatitis in patients with anogenital complaints. J Reprod Med. 2000;45:649-654.
- Warshaw EM, Kimyon RS, Silverberg JI, et al. Evaluation of patch test findings in patients with anogenital dermatitis. JAMA Dermatol. 2020;156:85-91. doi:10.1001/jamadermatol.2019.3844
- Weightman W, Turner T. Allergic contact dermatitis from lignocaine: report of 29 cases and review of the literature. Contact Dermatitis. 1998;39:265-266. doi:10.1111/j.1600-0536.1998.tb05928.x
- Jovanovic´ M, Karadaglic´ D, Brkic´ S. Contact urticaria and allergic contact dermatitis to lidocaine in a patient sensitive to benzocaine and propolis. Contact Dermatitis. 2006;54:124-126. doi:10.1111/j.0105-1873.2006.0560f.x
- Carazo JL, Morera BS, Colom LP, et al. Allergic contact dermatitis from ethyl chloride and benzocaine. Dermatitis. 2009;20:E13-E15.
- le Coz CJ, Cribier BJ, Heid E. Patch testing in suspected allergic contact dermatitis due to EMLA cream in haemodialyzed patients. Contact Dermatitis. 1996;35:316-317. doi:10.1111/j.1600-0536.1996.tb02407.x
- Ismail F, Goldsmith PC. EMLA cream-induced allergic contact dermatitis in a child with thalassaemia major. Contact Dermatitis. 2005;52:111. doi:10.1111/j.0105-1873.2005.00498e.x
- Pérez-Pérez LC, Fernández-Redondo V, Ginarte-Val M, et al. Allergic contact dermatitis from EMLA cream in a hemodialyzed patient. Dermatitis. 2006;17:85-87.
- Timmermans MW, Bruynzeel DP, Rustemeyer T. Allergic contact dermatitis from EMLA cream: concomitant sensitization to both local anesthetics lidocaine and prilocaine. J Dtsch Dermatol Ges. 2009;7:237-238. doi:10.1111/j.1610-0387.2008.06932.x
- Fuzier R, Lapeyre-Mestre M, Mertes PM, et al. Immediate- and delayed-type allergic reactions to amide local anesthetics: clinical features and skin testing. Pharmacoepidemiol Drug Saf. 2009;18:595-601. doi:10.1002/pds.1758
- Ruzicka T, Gerstmeier M, Przybilla B, et al. Allergy to local anesthetics: comparison of patch test with prick and intradermal test results. J Am Acad Dermatol. 1987;16:1202-1208. doi:10.1016/s0190-9622(87)70158-3
- Fowler JF Jr, Fowler L, Douglas JL, et al. Skin reactions to pimecrolimus cream 1% in patients allergic to propylene glycol: a double-blind randomized study. Dermatitis. 2007;18:134-139. doi:10.2310/6620.2007.06028
- de Groot A. Patch Testing. 3rd ed. acdegroot publishing; 2008.
Practice Points
- Allergic contact dermatitis (ACD) should be suspected in patients with persistent or worsening dermatitis after use of topical medications.
- Cross-reactions commonly occur between structurally similar compounds and occasionally between molecules from different drug classes.
- Some cases of topical medicament ACD remain elusive after patch testing, particularly drugs with potent immunomodulating effects.