User login
New research supports continued caution in prescribing a phosphodiesterase-5 inhibitor (PDE5i) to treat erectile dysfunction (ED) in men with heart disease using nitrate medications.
In a large Swedish population study of men with stable coronary artery disease (CAD), the combined use of a PDE5i and nitrates was associated with a higher risk for cardiovascular (CV) morbidity and mortality.
“According to current recommendations, PDE5i are contraindicated in patients taking organic nitrates; however, in clinical practice, both are commonly prescribed, and concomitant use has increased,” first author Ylva Trolle Lagerros, MD, PhD, with Karolinska Institutet, Stockholm, Sweden, told this news organization.
and weigh the benefits of the medication against the possible increased risk for cardiovascular morbidity and mortality given by this combination,” Dr. Lagerros said.
The study was published online in the Journal of the American College of Cardiology (JACC).
The researchers used the Swedish Patient Register and the Prescribed Drug Register to assess the association between PDE5i treatment and CV outcomes in men with stable CAD treated with nitrate medication.
Among 55,777 men with a history of previous myocardial infarction (MI) or coronary revascularization who had filled at least two nitrate prescriptions (sublingual, oral, or both), 5710 also had at least two filled prescriptions of a PDE5i.
In multivariate-adjusted analysis, the combined use of PDE5i treatment with nitrates was associated with an increased relative risk for all studied outcomes, including all-cause mortality, CV and non-CV mortality, MI, heart failure, cardiac revascularization (hazard ratio), and major adverse cardiovascular events.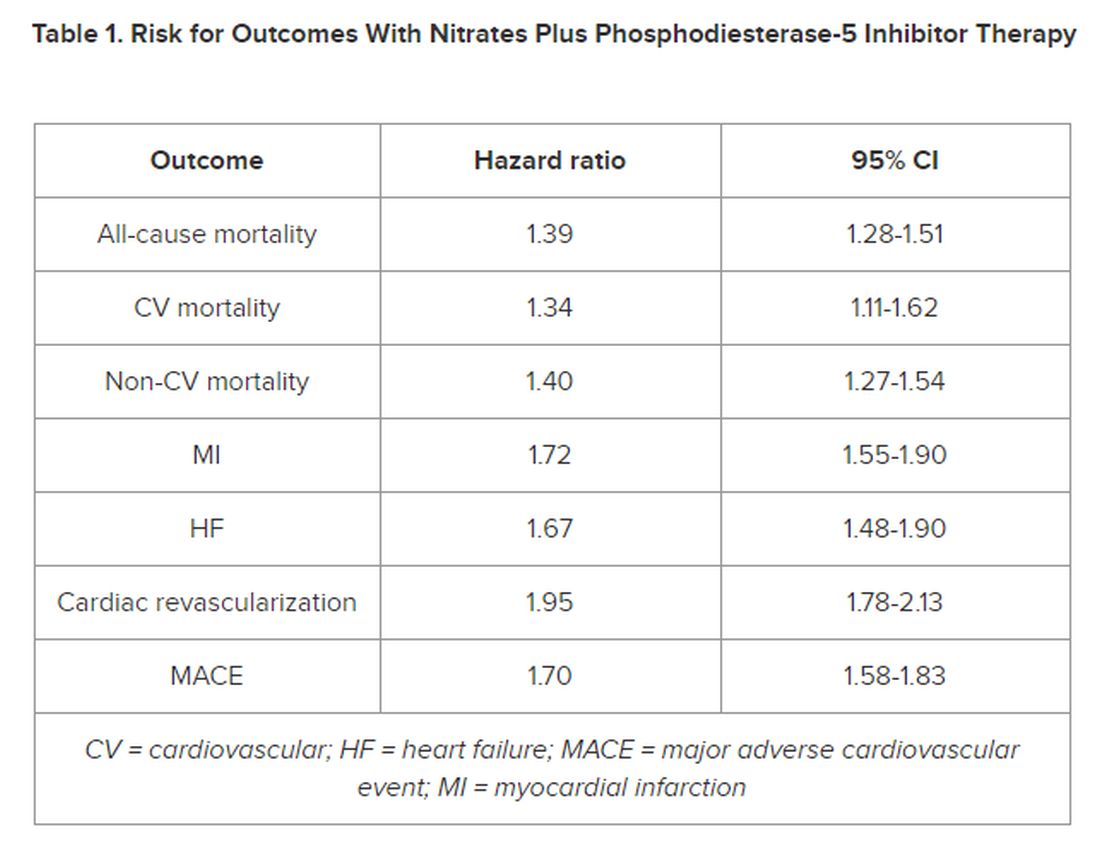
However, the number of events 28 days following a PDE5i prescription fill was “few, with lower incidence rates than in subjects taking nitrates only, indicating a low immediate risk for any event,” the authors noted in their article.
‘Common Bedfellows’
In a JACC editorial, Glenn N. Levine, MD, with Baylor College of Medicine, Houston, Texas, noted that, “ED and CAD are unfortunate, and all too common, bedfellows. But, as with most relationships, assuming proper precautions and care, they can coexist together for many years, perhaps even a lifetime.”
Dr. Levine noted that PDE5is are “reasonably safe” in most patients with stable CAD and only mild angina if not on chronic nitrate therapy. For those on chronic oral nitrate therapy, the use of PDE5is should continue to be regarded as “ill-advised at best and generally contraindicated.”
In some patients on oral nitrate therapy who want to use a PDE5i, particularly those who have undergone revascularization and have minimal or no angina, Dr. Levine said it may be reasonable to initiate a several-week trial of the nitrate therapy (or on a different class of antianginal therapy) and assess if the patient remains relatively angina-free.
In those patients with just rare exertional angina at generally higher levels of activity or those prescribed sublingual nitroglycerin “just in case,” it may be reasonable to prescribe PDE5i after a “clear and detailed” discussion with the patient of the risks for temporarily combining PDE5i and sublingual nitroglycerin.
Dr. Levine said these patients should be instructed not to take nitroglycerin within 24 hours of using a shorter-acting PDE5i and within 48 hours of using the longer-acting PDE5i tadalafil.
They should also be told to call 9-1-1 if angina develops during sexual intercourse and does not resolve upon cessation of such sexual activity, as well as to make medical personnel aware that they have recently used a PDE5i.
The study was funded by Region Stockholm, the Center for Innovative Medicine, and Karolinska Institutet. The researchers and editorial writer had declared no relevant conflicts of interest.
A version of this article appeared on Medscape.com.
New research supports continued caution in prescribing a phosphodiesterase-5 inhibitor (PDE5i) to treat erectile dysfunction (ED) in men with heart disease using nitrate medications.
In a large Swedish population study of men with stable coronary artery disease (CAD), the combined use of a PDE5i and nitrates was associated with a higher risk for cardiovascular (CV) morbidity and mortality.
“According to current recommendations, PDE5i are contraindicated in patients taking organic nitrates; however, in clinical practice, both are commonly prescribed, and concomitant use has increased,” first author Ylva Trolle Lagerros, MD, PhD, with Karolinska Institutet, Stockholm, Sweden, told this news organization.
and weigh the benefits of the medication against the possible increased risk for cardiovascular morbidity and mortality given by this combination,” Dr. Lagerros said.
The study was published online in the Journal of the American College of Cardiology (JACC).
The researchers used the Swedish Patient Register and the Prescribed Drug Register to assess the association between PDE5i treatment and CV outcomes in men with stable CAD treated with nitrate medication.
Among 55,777 men with a history of previous myocardial infarction (MI) or coronary revascularization who had filled at least two nitrate prescriptions (sublingual, oral, or both), 5710 also had at least two filled prescriptions of a PDE5i.
In multivariate-adjusted analysis, the combined use of PDE5i treatment with nitrates was associated with an increased relative risk for all studied outcomes, including all-cause mortality, CV and non-CV mortality, MI, heart failure, cardiac revascularization (hazard ratio), and major adverse cardiovascular events.
However, the number of events 28 days following a PDE5i prescription fill was “few, with lower incidence rates than in subjects taking nitrates only, indicating a low immediate risk for any event,” the authors noted in their article.
‘Common Bedfellows’
In a JACC editorial, Glenn N. Levine, MD, with Baylor College of Medicine, Houston, Texas, noted that, “ED and CAD are unfortunate, and all too common, bedfellows. But, as with most relationships, assuming proper precautions and care, they can coexist together for many years, perhaps even a lifetime.”
Dr. Levine noted that PDE5is are “reasonably safe” in most patients with stable CAD and only mild angina if not on chronic nitrate therapy. For those on chronic oral nitrate therapy, the use of PDE5is should continue to be regarded as “ill-advised at best and generally contraindicated.”
In some patients on oral nitrate therapy who want to use a PDE5i, particularly those who have undergone revascularization and have minimal or no angina, Dr. Levine said it may be reasonable to initiate a several-week trial of the nitrate therapy (or on a different class of antianginal therapy) and assess if the patient remains relatively angina-free.
In those patients with just rare exertional angina at generally higher levels of activity or those prescribed sublingual nitroglycerin “just in case,” it may be reasonable to prescribe PDE5i after a “clear and detailed” discussion with the patient of the risks for temporarily combining PDE5i and sublingual nitroglycerin.
Dr. Levine said these patients should be instructed not to take nitroglycerin within 24 hours of using a shorter-acting PDE5i and within 48 hours of using the longer-acting PDE5i tadalafil.
They should also be told to call 9-1-1 if angina develops during sexual intercourse and does not resolve upon cessation of such sexual activity, as well as to make medical personnel aware that they have recently used a PDE5i.
The study was funded by Region Stockholm, the Center for Innovative Medicine, and Karolinska Institutet. The researchers and editorial writer had declared no relevant conflicts of interest.
A version of this article appeared on Medscape.com.
New research supports continued caution in prescribing a phosphodiesterase-5 inhibitor (PDE5i) to treat erectile dysfunction (ED) in men with heart disease using nitrate medications.
In a large Swedish population study of men with stable coronary artery disease (CAD), the combined use of a PDE5i and nitrates was associated with a higher risk for cardiovascular (CV) morbidity and mortality.
“According to current recommendations, PDE5i are contraindicated in patients taking organic nitrates; however, in clinical practice, both are commonly prescribed, and concomitant use has increased,” first author Ylva Trolle Lagerros, MD, PhD, with Karolinska Institutet, Stockholm, Sweden, told this news organization.
and weigh the benefits of the medication against the possible increased risk for cardiovascular morbidity and mortality given by this combination,” Dr. Lagerros said.
The study was published online in the Journal of the American College of Cardiology (JACC).
The researchers used the Swedish Patient Register and the Prescribed Drug Register to assess the association between PDE5i treatment and CV outcomes in men with stable CAD treated with nitrate medication.
Among 55,777 men with a history of previous myocardial infarction (MI) or coronary revascularization who had filled at least two nitrate prescriptions (sublingual, oral, or both), 5710 also had at least two filled prescriptions of a PDE5i.
In multivariate-adjusted analysis, the combined use of PDE5i treatment with nitrates was associated with an increased relative risk for all studied outcomes, including all-cause mortality, CV and non-CV mortality, MI, heart failure, cardiac revascularization (hazard ratio), and major adverse cardiovascular events.
However, the number of events 28 days following a PDE5i prescription fill was “few, with lower incidence rates than in subjects taking nitrates only, indicating a low immediate risk for any event,” the authors noted in their article.
‘Common Bedfellows’
In a JACC editorial, Glenn N. Levine, MD, with Baylor College of Medicine, Houston, Texas, noted that, “ED and CAD are unfortunate, and all too common, bedfellows. But, as with most relationships, assuming proper precautions and care, they can coexist together for many years, perhaps even a lifetime.”
Dr. Levine noted that PDE5is are “reasonably safe” in most patients with stable CAD and only mild angina if not on chronic nitrate therapy. For those on chronic oral nitrate therapy, the use of PDE5is should continue to be regarded as “ill-advised at best and generally contraindicated.”
In some patients on oral nitrate therapy who want to use a PDE5i, particularly those who have undergone revascularization and have minimal or no angina, Dr. Levine said it may be reasonable to initiate a several-week trial of the nitrate therapy (or on a different class of antianginal therapy) and assess if the patient remains relatively angina-free.
In those patients with just rare exertional angina at generally higher levels of activity or those prescribed sublingual nitroglycerin “just in case,” it may be reasonable to prescribe PDE5i after a “clear and detailed” discussion with the patient of the risks for temporarily combining PDE5i and sublingual nitroglycerin.
Dr. Levine said these patients should be instructed not to take nitroglycerin within 24 hours of using a shorter-acting PDE5i and within 48 hours of using the longer-acting PDE5i tadalafil.
They should also be told to call 9-1-1 if angina develops during sexual intercourse and does not resolve upon cessation of such sexual activity, as well as to make medical personnel aware that they have recently used a PDE5i.
The study was funded by Region Stockholm, the Center for Innovative Medicine, and Karolinska Institutet. The researchers and editorial writer had declared no relevant conflicts of interest.
A version of this article appeared on Medscape.com.