User login
Pediatric oncology patients can present with various skin lesions related to both their primary disease and immunosuppressive treatments. In the majority of cases, cutaneous findings are associated with the use of chemotherapeutic agents. The toxic effects of chemotherapeutic agents, which generally are associated with treatment of solid organ malignancies (eg, liver, kidneys), can be detected by oncologists using clinical signs and laboratory tests.1-3 However, it also is important for dermatologists to recognize and evaluate cutaneous side effects associated with chemotherapeutic agents. Reports in the literature of cutaneous side effects of chemotherapy in pediatric patients generally are limited to case studies. This study aimed to evaluate the characteristics of cutaneous side effects of chemotherapy in pediatric oncology patients.
Materials and Methods
The study was performed through the collaboration of the departments of dermatology and venereology and pediatric oncology in the Faculty of Medicine at Ege University, Izmir, Turkey. Sixty-five pediatric oncology patients who were scheduled to undergo chemotherapy from May 2011 to May 2013 were included in the study. Clinical examination of dermatologic findings was conducted at baseline (prior to beginning chemotherapy) and at months 1, 3, and 6 of treatment. Patients were examined a total of 4 times during the study. Patients with a history of skin disease prior to diagnosis of their malignancy were excluded, as the study aimed to evaluate cutaneous side effects of chemotherapy. Patients who developed cutaneous side effects during the study period were photographed. Skin biopsy was performed to confirm clinical diagnosis. Patients were split into 5 groups according to oncological diagnoses, including hematological malignancies, solid organ tumors, bone and soft tissue tumors, central nervous system tumors, and Langerhans cell histiocytosis. Data regarding age, gender, treatments administered (ie, chemotherapeutics, antibiotics, antifungals, antivirals), and dermatologic signs were recorded. Mucocutaneous findings were classified as infectious (viral, bacterial, fungal) lesions, bullous lesions, inflammatory dermatoses (eg, diaper dermatitis, asteatotic eczema, contact dermatitis, seborrheic dermatitis), xeroderma, petechiae/ecchymoses, nail signs, alopecia, mucositis, cheilitis, oral aphthae, drug reactions confirmed by histopathology, cushingoid signs (eg, striae, acneform eruption, hypertrichosis), and cutaneous hyperpigmentation.
Statistical analysis was performed using SPSS version 15.0 and χ2 test was applied to the analysis.
Results
Of 65 patients, 62 completed the study and were included in the analysis. Three patients were excluded from the results, as 2 patients died during treatment and 1 patient withdrew from the study prior to completion. Twenty-seven (43.5%) patients were female and 35 (56.5%) were male ranging in age from 1 to 17 years (mean age, 8.14 years; median age [standard deviation], 7.25 [5.42] years). There were 31 (50%) patients in the hematological malignancies group, 11 (17.7%) in the solid organ tumors group, 10 (16.1%) in the bone and soft tissue tumors group, and 9 (14.5%) in the central nervous system tumors group; Langerhans cell histiocytosis was diagnosed in 1 (1.6%) patient. Hodgkin lymphoma made up 29.0% (n=9) of hematological malignancies. Other hematological malignancies included acute myeloblastic leukemia (n=7 [22.5%]), acute lymphoblastic leukemia (n=7 [22.5%]), T-cell lymphoma (n=5 [16.1%]), non-Hodgkin lym-phoma (n=1 [3.2%]), anaplastic giant cell lymphoma (n=1 [3.2%]), and diffuse giant cell lymphoma (n=1 [3.2%]).
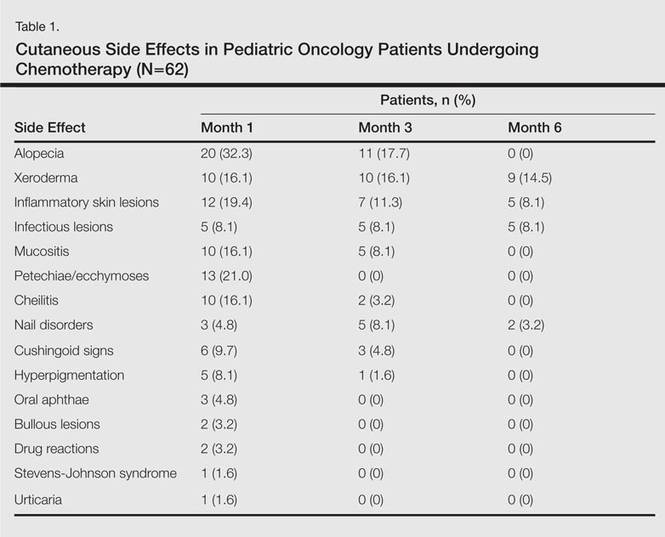
In addition to chemotherapeutic agents, 7 (11.3%) patients in this study also received antibiotics and 3 (4.8%) received antivirals. The most frequently employed chemotherapeutic agents were vincristine, methotrexate, cytarabine, etoposide, and dexamethasone. Cyclophosphamide, doxorubicin, ifosfamide, asparaginase, carboplatin, procarbazine, daunorubicin, actinomycin D, vinblastine, cisplatin, bleomycin, idarubicin, 6-mercaptopurine, temozolamide, and cyclosporine also were administered. The most commonly encountered dermatological side effects were alopecia, xeroderma, inflammatory skin lesions, infectious lesions, and mucositis, respectively (Table 1). Cutaneous side effects were frequently seen at months 1 and 3 of treatment.
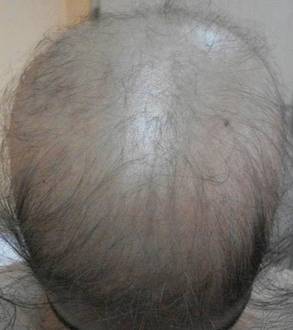
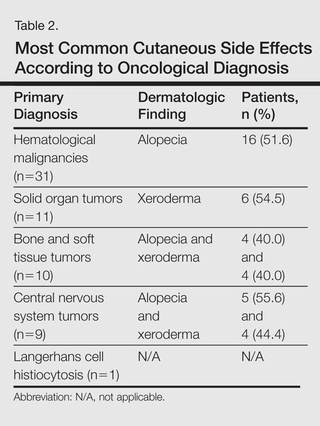
The most commonly encountered dermatologic side effect was alopecia (31/62 [50%]). Anagen effluvium (Figure 1) was detected in half of the cases, while complete scalp hair loss was noted in the rest. Alopecia was encountered more commonly in cases with central nervous system tumors (5/9 [55.6%]) and hematological malignancies (16/31 [51.6%])(Table 2).
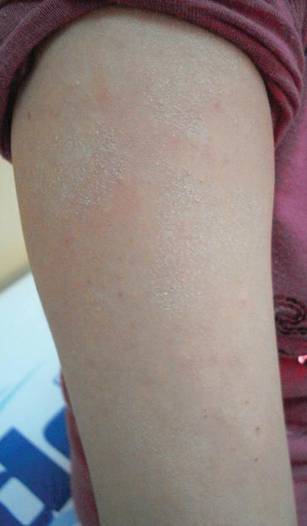
The second most commonly encountered side effect was xeroderma (29/62 [46.8%])(Figure 2). This side effect was most commonly encountered in patients with solid organ tumors (6/11 [54.5%]) and central nervous system tumors (4/9 [44.4%]), and occurred less frequently with bone and soft tissue tumors (4/10 [40.0%]).
Findings of eczema accounted for the majority of inflammatory lesions, which were the third most commonly encountered side effects. Among 24 cases of inflammatory skin lesions, 8 patients (33.3%) had diaper dermatitis, 7 (29.2%) had asteatotic eczema, 6 (25.0%) had contact dermatitis, and 3 (12.5%) had seborrheic dermatitis. Although inflammatory skin lesions were commonly encountered in patients with hematological malignancies (14/31 [45.2%]), the difference was not statistically significant.
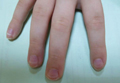
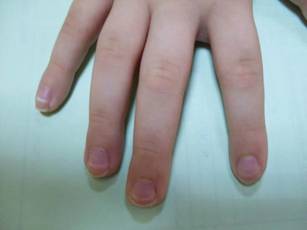
Mucositis and oral aphthous lesions were observed in 15 (24.2%) and 3 (4.8%) patients, respectively. Nail signs were noted in 10 (16.1%) patients; 4 patients had transverse streaks on the nail plates, 3 had linear streaks, 2 had nail plate fragility, and 1 had increased pigmentation at the nail bed and periungual area. Figure 3 shows linear streaks on the nail plate. These side effects were most commonly encountered in patients with solid organ tumors (5/11 [45.5%]); however, the difference was not statistically significant when compared with the other diagnostic groups.
Dermatologic signs with infectious origins were detected in 15 (24.2%) patients; 2 patients had herpes labialis, 2 had verruca vulgaris, 3 had bacterial folliculitis, 1 had acute paronychia, 1 had soft tissue infection, 2 had tinea versicolor, and 4 had mucocutaneous candidiasis. Dermatologic side effects due to infectious causes were more commonly encountered in patients with bone and soft tissue tumors (4/11 [36.4%]), and the difference was statistically significant when compared with the other diagnostic groups (P=.04).
Petechiae and ecchymotic lesions were present in 13 (21.0%) patients. These side effects occurred mainly in the first month of chemotherapy, namely when patients were in the pancytopenic phase.
Comment
Variability among the oncological diagnosis and drugs used in treatment as well as increased numbers of chemotherapeutic agents available have led to many side effects and complications in pediatric oncology patients undergoing chemotherapy.1,2 Comprehensive studies regarding the cutaneous side effects of chemotherapeuticagents in cancer treatment have been conducted in adult patients. Side effects in pediatric patients have only been documented in case reports in the literature. In our study of pediatric oncology patients undergoing treatment with chemotherapy, the most commonly observed dermatologic side effect was alopecia, followed by xeroderma, inflammatory lesions, infectious lesions, mucositis, petechiae/ecchymoses, cheilitis, nail disorders, cushingoid signs, oral aphthae, bullous lesions, and drug reactions confirmed histopathologically (Table 1).
Because the common effects of chemotherapeutic agents used in cancer treatment are greatest in areas of rapidly dividing cells, the skin and skin appendages frequently are affected by these drugs.1-3 Cutaneous signs are frequently observed, especially in regions with increased mitotic activity such as the hair, mucosa, and nails.
Kamil et al1 reported that the incidence of alopecia was 64.3% (74/115) in a study of adult cancer patients who underwent chemotherapy. Chemotherapeutic agents that have commonly caused alopecia are vincristine, daunorubicin, doxorubicin, cyclophosphamide, etoposide, cytarabine, and carboplatin.1,2 In our study, alopecia was noted in 31 (50.0%) patients, especially with the use of vincristine (7/31 [22.6%]), daunorubicin (8/31 [25.8%]), doxorubicin (6/31 [19.4%]), and cyclophosphamide (10/31 [32.3%]).
Darkening of the skin and paleness accompanied the majority of cases of xeroderma in our study. Skin dryness was in an ichthyosiform appearance and was severe in 1 patient who was diagnosed with osteosarcoma. Asteatotic eczema and cheilitis were related to skin dryness. It has been reported that acquired paraneoplastic ichthyosis can develop in hematological malignancies, primarily in patients with Hodgkin lymphoma.4
The incidence of mucositis has been related to the doses of chemotherapeutic agents. Although it is a commonly encountered side effect, there is no standard treatment of mucositis; therefore, preventive care in patients undergoing chemotherapy is important. It has been reported that practicing good oral hygiene before the treatment period can decrease the incidence of mucositis.5-9 The lower incidence of mucositis in our study compared to the literature (55.6%)5 can be attributed to the lower doses of chemotherapy drugs administered to children due to their weights; they also had active oral mucosa care during chemotherapy.
Another common complication observed in our study was nail disorders. Transverse streaks commonly are encountered due to damage in the nail matrix. Other signs are increased linear streaks, longitudinal melanonychia, nail plate fragility, and onycholysis.10
Cancer patients acquire infections more frequently because of immunosuppression from chemotherapy and malignancy.11,12 In our study, cutaneous side effects with infectious causes were noted in 15 patients. Steroids, which are included in the majority of chemotherapeutic protocols, can cause cushingoid changes. Striae from rapid weight gain, acneform eruptions, hypertrichosis, and atrophy of the skin also have been observed among secondary changes to chemotherapy.1,11
Other skin signs observed in the study were acute urticaria in 1 patient (1.6%) following administration of intrathecal methotrexate; Stevens-Johnson syndrome related to voriconazole was noted in 1 (1.6%) patient.
Hyperpigmentation is a common side effect observed in oncology patients.13-15 It can be observed locally in the skin as well as the mucosa, teeth, hair, and nails, and it generally develops secondary to alkylating agents.16 Moreover, hyperpigmentation may develop in regions with occlusions (eg, electrocardiogram pads, adhesion sites of plasters), and commonly is associated with ifosfamide, etoposide, carboplatin, and cyclosporine. Although the development mechanism of hyperpigmentation related to chemotherapy drugs is not clearly known, it is thought to be due to direct toxicity, melanocyte stimulation, or postinflammatory changes.1,6,17 In our study, xeroderma was noted in some patients with hyperpigmentation; all of them had received cyclosporine and systemic steroid treatments. The other chemotherapeutics were defined as etoposide, cytarabine, dacarbazine, and ifosfamide.1 Our patients with hyperpigmentation were not taking these therapies.
Increased skin malignancies have been reported in adult cases with hematological malignancies.18 None of the patients in our study had a secondary skin malignancy, likely because we evaluated a pediatric population and the follow-up period (6 months) was too short for the development of a secondary malignancy.
Conclusion
A wide range of cutaneous side effects can be observed in pediatric oncology patients undergoing chemotherapy based on oncological diagnosis and treatment protocol. Although these side effects are not fatal, they may negatively affect morbidity and can lead to emotional distress. Knowing the possible cutaneous side effects of chemotherapy in pediatric patients and their causes is important for early diagnosis and minimal treatment.
- Kamil N, Kamil S, Ahmed SP, et al. Toxic effects of multiple anticancer drugs on skin. Pak J Pharm Sci. 2010;23:7-14.
- Alley E, Green R, Schuchter L. Cutaneous toxicities of cancer therapy. Curr Opin Oncol. 2002;14:212-216.
- Ozkan A, Apak H, Celkan T, et al. Toxic epidermal necrolysis after the use of high-dose cytosine arabinoside. Pediatr Dermatol. 2001;18:38-40.
- Rizos E, Milionis HJ, Pavlidis N, et al. Acquired ichthyosis: a paraneoplastic skin manifestation of Hodgkin’s disease. Lancet Oncol. 2002;3:727.
- Otmani N, Alami R, Hessissen L, et al. Determinants of severe oral mucositis in pediatric cancer patients: a prospective study. Int J Pediatr Dent. 2011;21:210-216.
- Mateus C, Robert C. New drugs in oncology and skin toxicity [in French]. Rev Med Interne. 2009;30:401-410.
- Manji A, Tomlinson D, Ethier MC, et al. Psychometric properties of the Oral Mucositis Daily Questionnaire for child self-report and importance of mucositis in children treated with chemotherapy. Support Care Cancer. 2012;20:1251-1258.
- Keefe DM. Mucositis management in patients with cancer. Support Cancer Ther. 2006;3:154-157.
- Raber-Durlacher JE, Elad S, Barasch A. Oral mucositis. Oral Oncol. 2010;46:452-456.
- Utas S, Kulluk P. A case of hydroxyurea-induced longitudinal melanonychia. Int J Dermatol. 2010;49:466-474.
- Ott H, Höger PH. Dermatologic manifestations of infections in pediatric cancer patients [in German]. Klin Padiatr. 2005;217(suppl 1):110-119.
- Ramphal R, Grant RM, Dzolganovski B, et al. Herpes simplex virus in febrile neutropenic children undergoing chemotherapy for cancer: a prospective cohort study. Pediatr Infect Dis J. 2007;26:700-704.
- Yaris N, Cakir M, Kalyoncu M, et al. Bleomycin induced hyperpigmentation with yolk sac tumor. Indian J Pediatr. 2007;74:505-506.
- Kleynberg RL, Sofi AA, Chaudhary RT. Hand-foot hyperpigmentation skin lesions associated with combination gemcitabine-carboplatin (GemCarbo) therapy. Am J Ther. 2011;18:261-263.
- Blaya M, Saba N. Chemotherapy-induced hyperpigmentation of the tongue. N Engl J Med. 2011;365:e20.
- Anandajeya WV, Corrêa ZM, Augsburger JJ. Primary acquired melanosis with atypia treated with mitomycin C. Int Ophthalmol. 2009;29:285-288.
- Torres C, Wong L, Welsh O, et al. Skin manifestations associated with chemotherapy in children with hematologic malignancies. Pediatr Dermatol. 2011;2:123-147.
- Mays SR, Cohen PR. Emerging dermatologic issues in the oncology patient. Semin Cutan Med Surg. 2006;25:179-189.
Pediatric oncology patients can present with various skin lesions related to both their primary disease and immunosuppressive treatments. In the majority of cases, cutaneous findings are associated with the use of chemotherapeutic agents. The toxic effects of chemotherapeutic agents, which generally are associated with treatment of solid organ malignancies (eg, liver, kidneys), can be detected by oncologists using clinical signs and laboratory tests.1-3 However, it also is important for dermatologists to recognize and evaluate cutaneous side effects associated with chemotherapeutic agents. Reports in the literature of cutaneous side effects of chemotherapy in pediatric patients generally are limited to case studies. This study aimed to evaluate the characteristics of cutaneous side effects of chemotherapy in pediatric oncology patients.
Materials and Methods
The study was performed through the collaboration of the departments of dermatology and venereology and pediatric oncology in the Faculty of Medicine at Ege University, Izmir, Turkey. Sixty-five pediatric oncology patients who were scheduled to undergo chemotherapy from May 2011 to May 2013 were included in the study. Clinical examination of dermatologic findings was conducted at baseline (prior to beginning chemotherapy) and at months 1, 3, and 6 of treatment. Patients were examined a total of 4 times during the study. Patients with a history of skin disease prior to diagnosis of their malignancy were excluded, as the study aimed to evaluate cutaneous side effects of chemotherapy. Patients who developed cutaneous side effects during the study period were photographed. Skin biopsy was performed to confirm clinical diagnosis. Patients were split into 5 groups according to oncological diagnoses, including hematological malignancies, solid organ tumors, bone and soft tissue tumors, central nervous system tumors, and Langerhans cell histiocytosis. Data regarding age, gender, treatments administered (ie, chemotherapeutics, antibiotics, antifungals, antivirals), and dermatologic signs were recorded. Mucocutaneous findings were classified as infectious (viral, bacterial, fungal) lesions, bullous lesions, inflammatory dermatoses (eg, diaper dermatitis, asteatotic eczema, contact dermatitis, seborrheic dermatitis), xeroderma, petechiae/ecchymoses, nail signs, alopecia, mucositis, cheilitis, oral aphthae, drug reactions confirmed by histopathology, cushingoid signs (eg, striae, acneform eruption, hypertrichosis), and cutaneous hyperpigmentation.
Statistical analysis was performed using SPSS version 15.0 and χ2 test was applied to the analysis.
Results
Of 65 patients, 62 completed the study and were included in the analysis. Three patients were excluded from the results, as 2 patients died during treatment and 1 patient withdrew from the study prior to completion. Twenty-seven (43.5%) patients were female and 35 (56.5%) were male ranging in age from 1 to 17 years (mean age, 8.14 years; median age [standard deviation], 7.25 [5.42] years). There were 31 (50%) patients in the hematological malignancies group, 11 (17.7%) in the solid organ tumors group, 10 (16.1%) in the bone and soft tissue tumors group, and 9 (14.5%) in the central nervous system tumors group; Langerhans cell histiocytosis was diagnosed in 1 (1.6%) patient. Hodgkin lymphoma made up 29.0% (n=9) of hematological malignancies. Other hematological malignancies included acute myeloblastic leukemia (n=7 [22.5%]), acute lymphoblastic leukemia (n=7 [22.5%]), T-cell lymphoma (n=5 [16.1%]), non-Hodgkin lym-phoma (n=1 [3.2%]), anaplastic giant cell lymphoma (n=1 [3.2%]), and diffuse giant cell lymphoma (n=1 [3.2%]).
In addition to chemotherapeutic agents, 7 (11.3%) patients in this study also received antibiotics and 3 (4.8%) received antivirals. The most frequently employed chemotherapeutic agents were vincristine, methotrexate, cytarabine, etoposide, and dexamethasone. Cyclophosphamide, doxorubicin, ifosfamide, asparaginase, carboplatin, procarbazine, daunorubicin, actinomycin D, vinblastine, cisplatin, bleomycin, idarubicin, 6-mercaptopurine, temozolamide, and cyclosporine also were administered. The most commonly encountered dermatological side effects were alopecia, xeroderma, inflammatory skin lesions, infectious lesions, and mucositis, respectively (Table 1). Cutaneous side effects were frequently seen at months 1 and 3 of treatment.

The most commonly encountered dermatologic side effect was alopecia (31/62 [50%]). Anagen effluvium (Figure 1) was detected in half of the cases, while complete scalp hair loss was noted in the rest. Alopecia was encountered more commonly in cases with central nervous system tumors (5/9 [55.6%]) and hematological malignancies (16/31 [51.6%])(Table 2).


The second most commonly encountered side effect was xeroderma (29/62 [46.8%])(Figure 2). This side effect was most commonly encountered in patients with solid organ tumors (6/11 [54.5%]) and central nervous system tumors (4/9 [44.4%]), and occurred less frequently with bone and soft tissue tumors (4/10 [40.0%]).

Findings of eczema accounted for the majority of inflammatory lesions, which were the third most commonly encountered side effects. Among 24 cases of inflammatory skin lesions, 8 patients (33.3%) had diaper dermatitis, 7 (29.2%) had asteatotic eczema, 6 (25.0%) had contact dermatitis, and 3 (12.5%) had seborrheic dermatitis. Although inflammatory skin lesions were commonly encountered in patients with hematological malignancies (14/31 [45.2%]), the difference was not statistically significant.
Mucositis and oral aphthous lesions were observed in 15 (24.2%) and 3 (4.8%) patients, respectively. Nail signs were noted in 10 (16.1%) patients; 4 patients had transverse streaks on the nail plates, 3 had linear streaks, 2 had nail plate fragility, and 1 had increased pigmentation at the nail bed and periungual area. Figure 3 shows linear streaks on the nail plate. These side effects were most commonly encountered in patients with solid organ tumors (5/11 [45.5%]); however, the difference was not statistically significant when compared with the other diagnostic groups.

Dermatologic signs with infectious origins were detected in 15 (24.2%) patients; 2 patients had herpes labialis, 2 had verruca vulgaris, 3 had bacterial folliculitis, 1 had acute paronychia, 1 had soft tissue infection, 2 had tinea versicolor, and 4 had mucocutaneous candidiasis. Dermatologic side effects due to infectious causes were more commonly encountered in patients with bone and soft tissue tumors (4/11 [36.4%]), and the difference was statistically significant when compared with the other diagnostic groups (P=.04).
Petechiae and ecchymotic lesions were present in 13 (21.0%) patients. These side effects occurred mainly in the first month of chemotherapy, namely when patients were in the pancytopenic phase.
Comment
Variability among the oncological diagnosis and drugs used in treatment as well as increased numbers of chemotherapeutic agents available have led to many side effects and complications in pediatric oncology patients undergoing chemotherapy.1,2 Comprehensive studies regarding the cutaneous side effects of chemotherapeuticagents in cancer treatment have been conducted in adult patients. Side effects in pediatric patients have only been documented in case reports in the literature. In our study of pediatric oncology patients undergoing treatment with chemotherapy, the most commonly observed dermatologic side effect was alopecia, followed by xeroderma, inflammatory lesions, infectious lesions, mucositis, petechiae/ecchymoses, cheilitis, nail disorders, cushingoid signs, oral aphthae, bullous lesions, and drug reactions confirmed histopathologically (Table 1).
Because the common effects of chemotherapeutic agents used in cancer treatment are greatest in areas of rapidly dividing cells, the skin and skin appendages frequently are affected by these drugs.1-3 Cutaneous signs are frequently observed, especially in regions with increased mitotic activity such as the hair, mucosa, and nails.
Kamil et al1 reported that the incidence of alopecia was 64.3% (74/115) in a study of adult cancer patients who underwent chemotherapy. Chemotherapeutic agents that have commonly caused alopecia are vincristine, daunorubicin, doxorubicin, cyclophosphamide, etoposide, cytarabine, and carboplatin.1,2 In our study, alopecia was noted in 31 (50.0%) patients, especially with the use of vincristine (7/31 [22.6%]), daunorubicin (8/31 [25.8%]), doxorubicin (6/31 [19.4%]), and cyclophosphamide (10/31 [32.3%]).
Darkening of the skin and paleness accompanied the majority of cases of xeroderma in our study. Skin dryness was in an ichthyosiform appearance and was severe in 1 patient who was diagnosed with osteosarcoma. Asteatotic eczema and cheilitis were related to skin dryness. It has been reported that acquired paraneoplastic ichthyosis can develop in hematological malignancies, primarily in patients with Hodgkin lymphoma.4
The incidence of mucositis has been related to the doses of chemotherapeutic agents. Although it is a commonly encountered side effect, there is no standard treatment of mucositis; therefore, preventive care in patients undergoing chemotherapy is important. It has been reported that practicing good oral hygiene before the treatment period can decrease the incidence of mucositis.5-9 The lower incidence of mucositis in our study compared to the literature (55.6%)5 can be attributed to the lower doses of chemotherapy drugs administered to children due to their weights; they also had active oral mucosa care during chemotherapy.
Another common complication observed in our study was nail disorders. Transverse streaks commonly are encountered due to damage in the nail matrix. Other signs are increased linear streaks, longitudinal melanonychia, nail plate fragility, and onycholysis.10
Cancer patients acquire infections more frequently because of immunosuppression from chemotherapy and malignancy.11,12 In our study, cutaneous side effects with infectious causes were noted in 15 patients. Steroids, which are included in the majority of chemotherapeutic protocols, can cause cushingoid changes. Striae from rapid weight gain, acneform eruptions, hypertrichosis, and atrophy of the skin also have been observed among secondary changes to chemotherapy.1,11
Other skin signs observed in the study were acute urticaria in 1 patient (1.6%) following administration of intrathecal methotrexate; Stevens-Johnson syndrome related to voriconazole was noted in 1 (1.6%) patient.
Hyperpigmentation is a common side effect observed in oncology patients.13-15 It can be observed locally in the skin as well as the mucosa, teeth, hair, and nails, and it generally develops secondary to alkylating agents.16 Moreover, hyperpigmentation may develop in regions with occlusions (eg, electrocardiogram pads, adhesion sites of plasters), and commonly is associated with ifosfamide, etoposide, carboplatin, and cyclosporine. Although the development mechanism of hyperpigmentation related to chemotherapy drugs is not clearly known, it is thought to be due to direct toxicity, melanocyte stimulation, or postinflammatory changes.1,6,17 In our study, xeroderma was noted in some patients with hyperpigmentation; all of them had received cyclosporine and systemic steroid treatments. The other chemotherapeutics were defined as etoposide, cytarabine, dacarbazine, and ifosfamide.1 Our patients with hyperpigmentation were not taking these therapies.
Increased skin malignancies have been reported in adult cases with hematological malignancies.18 None of the patients in our study had a secondary skin malignancy, likely because we evaluated a pediatric population and the follow-up period (6 months) was too short for the development of a secondary malignancy.
Conclusion
A wide range of cutaneous side effects can be observed in pediatric oncology patients undergoing chemotherapy based on oncological diagnosis and treatment protocol. Although these side effects are not fatal, they may negatively affect morbidity and can lead to emotional distress. Knowing the possible cutaneous side effects of chemotherapy in pediatric patients and their causes is important for early diagnosis and minimal treatment.
Pediatric oncology patients can present with various skin lesions related to both their primary disease and immunosuppressive treatments. In the majority of cases, cutaneous findings are associated with the use of chemotherapeutic agents. The toxic effects of chemotherapeutic agents, which generally are associated with treatment of solid organ malignancies (eg, liver, kidneys), can be detected by oncologists using clinical signs and laboratory tests.1-3 However, it also is important for dermatologists to recognize and evaluate cutaneous side effects associated with chemotherapeutic agents. Reports in the literature of cutaneous side effects of chemotherapy in pediatric patients generally are limited to case studies. This study aimed to evaluate the characteristics of cutaneous side effects of chemotherapy in pediatric oncology patients.
Materials and Methods
The study was performed through the collaboration of the departments of dermatology and venereology and pediatric oncology in the Faculty of Medicine at Ege University, Izmir, Turkey. Sixty-five pediatric oncology patients who were scheduled to undergo chemotherapy from May 2011 to May 2013 were included in the study. Clinical examination of dermatologic findings was conducted at baseline (prior to beginning chemotherapy) and at months 1, 3, and 6 of treatment. Patients were examined a total of 4 times during the study. Patients with a history of skin disease prior to diagnosis of their malignancy were excluded, as the study aimed to evaluate cutaneous side effects of chemotherapy. Patients who developed cutaneous side effects during the study period were photographed. Skin biopsy was performed to confirm clinical diagnosis. Patients were split into 5 groups according to oncological diagnoses, including hematological malignancies, solid organ tumors, bone and soft tissue tumors, central nervous system tumors, and Langerhans cell histiocytosis. Data regarding age, gender, treatments administered (ie, chemotherapeutics, antibiotics, antifungals, antivirals), and dermatologic signs were recorded. Mucocutaneous findings were classified as infectious (viral, bacterial, fungal) lesions, bullous lesions, inflammatory dermatoses (eg, diaper dermatitis, asteatotic eczema, contact dermatitis, seborrheic dermatitis), xeroderma, petechiae/ecchymoses, nail signs, alopecia, mucositis, cheilitis, oral aphthae, drug reactions confirmed by histopathology, cushingoid signs (eg, striae, acneform eruption, hypertrichosis), and cutaneous hyperpigmentation.
Statistical analysis was performed using SPSS version 15.0 and χ2 test was applied to the analysis.
Results
Of 65 patients, 62 completed the study and were included in the analysis. Three patients were excluded from the results, as 2 patients died during treatment and 1 patient withdrew from the study prior to completion. Twenty-seven (43.5%) patients were female and 35 (56.5%) were male ranging in age from 1 to 17 years (mean age, 8.14 years; median age [standard deviation], 7.25 [5.42] years). There were 31 (50%) patients in the hematological malignancies group, 11 (17.7%) in the solid organ tumors group, 10 (16.1%) in the bone and soft tissue tumors group, and 9 (14.5%) in the central nervous system tumors group; Langerhans cell histiocytosis was diagnosed in 1 (1.6%) patient. Hodgkin lymphoma made up 29.0% (n=9) of hematological malignancies. Other hematological malignancies included acute myeloblastic leukemia (n=7 [22.5%]), acute lymphoblastic leukemia (n=7 [22.5%]), T-cell lymphoma (n=5 [16.1%]), non-Hodgkin lym-phoma (n=1 [3.2%]), anaplastic giant cell lymphoma (n=1 [3.2%]), and diffuse giant cell lymphoma (n=1 [3.2%]).
In addition to chemotherapeutic agents, 7 (11.3%) patients in this study also received antibiotics and 3 (4.8%) received antivirals. The most frequently employed chemotherapeutic agents were vincristine, methotrexate, cytarabine, etoposide, and dexamethasone. Cyclophosphamide, doxorubicin, ifosfamide, asparaginase, carboplatin, procarbazine, daunorubicin, actinomycin D, vinblastine, cisplatin, bleomycin, idarubicin, 6-mercaptopurine, temozolamide, and cyclosporine also were administered. The most commonly encountered dermatological side effects were alopecia, xeroderma, inflammatory skin lesions, infectious lesions, and mucositis, respectively (Table 1). Cutaneous side effects were frequently seen at months 1 and 3 of treatment.

The most commonly encountered dermatologic side effect was alopecia (31/62 [50%]). Anagen effluvium (Figure 1) was detected in half of the cases, while complete scalp hair loss was noted in the rest. Alopecia was encountered more commonly in cases with central nervous system tumors (5/9 [55.6%]) and hematological malignancies (16/31 [51.6%])(Table 2).


The second most commonly encountered side effect was xeroderma (29/62 [46.8%])(Figure 2). This side effect was most commonly encountered in patients with solid organ tumors (6/11 [54.5%]) and central nervous system tumors (4/9 [44.4%]), and occurred less frequently with bone and soft tissue tumors (4/10 [40.0%]).

Findings of eczema accounted for the majority of inflammatory lesions, which were the third most commonly encountered side effects. Among 24 cases of inflammatory skin lesions, 8 patients (33.3%) had diaper dermatitis, 7 (29.2%) had asteatotic eczema, 6 (25.0%) had contact dermatitis, and 3 (12.5%) had seborrheic dermatitis. Although inflammatory skin lesions were commonly encountered in patients with hematological malignancies (14/31 [45.2%]), the difference was not statistically significant.
Mucositis and oral aphthous lesions were observed in 15 (24.2%) and 3 (4.8%) patients, respectively. Nail signs were noted in 10 (16.1%) patients; 4 patients had transverse streaks on the nail plates, 3 had linear streaks, 2 had nail plate fragility, and 1 had increased pigmentation at the nail bed and periungual area. Figure 3 shows linear streaks on the nail plate. These side effects were most commonly encountered in patients with solid organ tumors (5/11 [45.5%]); however, the difference was not statistically significant when compared with the other diagnostic groups.

Dermatologic signs with infectious origins were detected in 15 (24.2%) patients; 2 patients had herpes labialis, 2 had verruca vulgaris, 3 had bacterial folliculitis, 1 had acute paronychia, 1 had soft tissue infection, 2 had tinea versicolor, and 4 had mucocutaneous candidiasis. Dermatologic side effects due to infectious causes were more commonly encountered in patients with bone and soft tissue tumors (4/11 [36.4%]), and the difference was statistically significant when compared with the other diagnostic groups (P=.04).
Petechiae and ecchymotic lesions were present in 13 (21.0%) patients. These side effects occurred mainly in the first month of chemotherapy, namely when patients were in the pancytopenic phase.
Comment
Variability among the oncological diagnosis and drugs used in treatment as well as increased numbers of chemotherapeutic agents available have led to many side effects and complications in pediatric oncology patients undergoing chemotherapy.1,2 Comprehensive studies regarding the cutaneous side effects of chemotherapeuticagents in cancer treatment have been conducted in adult patients. Side effects in pediatric patients have only been documented in case reports in the literature. In our study of pediatric oncology patients undergoing treatment with chemotherapy, the most commonly observed dermatologic side effect was alopecia, followed by xeroderma, inflammatory lesions, infectious lesions, mucositis, petechiae/ecchymoses, cheilitis, nail disorders, cushingoid signs, oral aphthae, bullous lesions, and drug reactions confirmed histopathologically (Table 1).
Because the common effects of chemotherapeutic agents used in cancer treatment are greatest in areas of rapidly dividing cells, the skin and skin appendages frequently are affected by these drugs.1-3 Cutaneous signs are frequently observed, especially in regions with increased mitotic activity such as the hair, mucosa, and nails.
Kamil et al1 reported that the incidence of alopecia was 64.3% (74/115) in a study of adult cancer patients who underwent chemotherapy. Chemotherapeutic agents that have commonly caused alopecia are vincristine, daunorubicin, doxorubicin, cyclophosphamide, etoposide, cytarabine, and carboplatin.1,2 In our study, alopecia was noted in 31 (50.0%) patients, especially with the use of vincristine (7/31 [22.6%]), daunorubicin (8/31 [25.8%]), doxorubicin (6/31 [19.4%]), and cyclophosphamide (10/31 [32.3%]).
Darkening of the skin and paleness accompanied the majority of cases of xeroderma in our study. Skin dryness was in an ichthyosiform appearance and was severe in 1 patient who was diagnosed with osteosarcoma. Asteatotic eczema and cheilitis were related to skin dryness. It has been reported that acquired paraneoplastic ichthyosis can develop in hematological malignancies, primarily in patients with Hodgkin lymphoma.4
The incidence of mucositis has been related to the doses of chemotherapeutic agents. Although it is a commonly encountered side effect, there is no standard treatment of mucositis; therefore, preventive care in patients undergoing chemotherapy is important. It has been reported that practicing good oral hygiene before the treatment period can decrease the incidence of mucositis.5-9 The lower incidence of mucositis in our study compared to the literature (55.6%)5 can be attributed to the lower doses of chemotherapy drugs administered to children due to their weights; they also had active oral mucosa care during chemotherapy.
Another common complication observed in our study was nail disorders. Transverse streaks commonly are encountered due to damage in the nail matrix. Other signs are increased linear streaks, longitudinal melanonychia, nail plate fragility, and onycholysis.10
Cancer patients acquire infections more frequently because of immunosuppression from chemotherapy and malignancy.11,12 In our study, cutaneous side effects with infectious causes were noted in 15 patients. Steroids, which are included in the majority of chemotherapeutic protocols, can cause cushingoid changes. Striae from rapid weight gain, acneform eruptions, hypertrichosis, and atrophy of the skin also have been observed among secondary changes to chemotherapy.1,11
Other skin signs observed in the study were acute urticaria in 1 patient (1.6%) following administration of intrathecal methotrexate; Stevens-Johnson syndrome related to voriconazole was noted in 1 (1.6%) patient.
Hyperpigmentation is a common side effect observed in oncology patients.13-15 It can be observed locally in the skin as well as the mucosa, teeth, hair, and nails, and it generally develops secondary to alkylating agents.16 Moreover, hyperpigmentation may develop in regions with occlusions (eg, electrocardiogram pads, adhesion sites of plasters), and commonly is associated with ifosfamide, etoposide, carboplatin, and cyclosporine. Although the development mechanism of hyperpigmentation related to chemotherapy drugs is not clearly known, it is thought to be due to direct toxicity, melanocyte stimulation, or postinflammatory changes.1,6,17 In our study, xeroderma was noted in some patients with hyperpigmentation; all of them had received cyclosporine and systemic steroid treatments. The other chemotherapeutics were defined as etoposide, cytarabine, dacarbazine, and ifosfamide.1 Our patients with hyperpigmentation were not taking these therapies.
Increased skin malignancies have been reported in adult cases with hematological malignancies.18 None of the patients in our study had a secondary skin malignancy, likely because we evaluated a pediatric population and the follow-up period (6 months) was too short for the development of a secondary malignancy.
Conclusion
A wide range of cutaneous side effects can be observed in pediatric oncology patients undergoing chemotherapy based on oncological diagnosis and treatment protocol. Although these side effects are not fatal, they may negatively affect morbidity and can lead to emotional distress. Knowing the possible cutaneous side effects of chemotherapy in pediatric patients and their causes is important for early diagnosis and minimal treatment.
- Kamil N, Kamil S, Ahmed SP, et al. Toxic effects of multiple anticancer drugs on skin. Pak J Pharm Sci. 2010;23:7-14.
- Alley E, Green R, Schuchter L. Cutaneous toxicities of cancer therapy. Curr Opin Oncol. 2002;14:212-216.
- Ozkan A, Apak H, Celkan T, et al. Toxic epidermal necrolysis after the use of high-dose cytosine arabinoside. Pediatr Dermatol. 2001;18:38-40.
- Rizos E, Milionis HJ, Pavlidis N, et al. Acquired ichthyosis: a paraneoplastic skin manifestation of Hodgkin’s disease. Lancet Oncol. 2002;3:727.
- Otmani N, Alami R, Hessissen L, et al. Determinants of severe oral mucositis in pediatric cancer patients: a prospective study. Int J Pediatr Dent. 2011;21:210-216.
- Mateus C, Robert C. New drugs in oncology and skin toxicity [in French]. Rev Med Interne. 2009;30:401-410.
- Manji A, Tomlinson D, Ethier MC, et al. Psychometric properties of the Oral Mucositis Daily Questionnaire for child self-report and importance of mucositis in children treated with chemotherapy. Support Care Cancer. 2012;20:1251-1258.
- Keefe DM. Mucositis management in patients with cancer. Support Cancer Ther. 2006;3:154-157.
- Raber-Durlacher JE, Elad S, Barasch A. Oral mucositis. Oral Oncol. 2010;46:452-456.
- Utas S, Kulluk P. A case of hydroxyurea-induced longitudinal melanonychia. Int J Dermatol. 2010;49:466-474.
- Ott H, Höger PH. Dermatologic manifestations of infections in pediatric cancer patients [in German]. Klin Padiatr. 2005;217(suppl 1):110-119.
- Ramphal R, Grant RM, Dzolganovski B, et al. Herpes simplex virus in febrile neutropenic children undergoing chemotherapy for cancer: a prospective cohort study. Pediatr Infect Dis J. 2007;26:700-704.
- Yaris N, Cakir M, Kalyoncu M, et al. Bleomycin induced hyperpigmentation with yolk sac tumor. Indian J Pediatr. 2007;74:505-506.
- Kleynberg RL, Sofi AA, Chaudhary RT. Hand-foot hyperpigmentation skin lesions associated with combination gemcitabine-carboplatin (GemCarbo) therapy. Am J Ther. 2011;18:261-263.
- Blaya M, Saba N. Chemotherapy-induced hyperpigmentation of the tongue. N Engl J Med. 2011;365:e20.
- Anandajeya WV, Corrêa ZM, Augsburger JJ. Primary acquired melanosis with atypia treated with mitomycin C. Int Ophthalmol. 2009;29:285-288.
- Torres C, Wong L, Welsh O, et al. Skin manifestations associated with chemotherapy in children with hematologic malignancies. Pediatr Dermatol. 2011;2:123-147.
- Mays SR, Cohen PR. Emerging dermatologic issues in the oncology patient. Semin Cutan Med Surg. 2006;25:179-189.
- Kamil N, Kamil S, Ahmed SP, et al. Toxic effects of multiple anticancer drugs on skin. Pak J Pharm Sci. 2010;23:7-14.
- Alley E, Green R, Schuchter L. Cutaneous toxicities of cancer therapy. Curr Opin Oncol. 2002;14:212-216.
- Ozkan A, Apak H, Celkan T, et al. Toxic epidermal necrolysis after the use of high-dose cytosine arabinoside. Pediatr Dermatol. 2001;18:38-40.
- Rizos E, Milionis HJ, Pavlidis N, et al. Acquired ichthyosis: a paraneoplastic skin manifestation of Hodgkin’s disease. Lancet Oncol. 2002;3:727.
- Otmani N, Alami R, Hessissen L, et al. Determinants of severe oral mucositis in pediatric cancer patients: a prospective study. Int J Pediatr Dent. 2011;21:210-216.
- Mateus C, Robert C. New drugs in oncology and skin toxicity [in French]. Rev Med Interne. 2009;30:401-410.
- Manji A, Tomlinson D, Ethier MC, et al. Psychometric properties of the Oral Mucositis Daily Questionnaire for child self-report and importance of mucositis in children treated with chemotherapy. Support Care Cancer. 2012;20:1251-1258.
- Keefe DM. Mucositis management in patients with cancer. Support Cancer Ther. 2006;3:154-157.
- Raber-Durlacher JE, Elad S, Barasch A. Oral mucositis. Oral Oncol. 2010;46:452-456.
- Utas S, Kulluk P. A case of hydroxyurea-induced longitudinal melanonychia. Int J Dermatol. 2010;49:466-474.
- Ott H, Höger PH. Dermatologic manifestations of infections in pediatric cancer patients [in German]. Klin Padiatr. 2005;217(suppl 1):110-119.
- Ramphal R, Grant RM, Dzolganovski B, et al. Herpes simplex virus in febrile neutropenic children undergoing chemotherapy for cancer: a prospective cohort study. Pediatr Infect Dis J. 2007;26:700-704.
- Yaris N, Cakir M, Kalyoncu M, et al. Bleomycin induced hyperpigmentation with yolk sac tumor. Indian J Pediatr. 2007;74:505-506.
- Kleynberg RL, Sofi AA, Chaudhary RT. Hand-foot hyperpigmentation skin lesions associated with combination gemcitabine-carboplatin (GemCarbo) therapy. Am J Ther. 2011;18:261-263.
- Blaya M, Saba N. Chemotherapy-induced hyperpigmentation of the tongue. N Engl J Med. 2011;365:e20.
- Anandajeya WV, Corrêa ZM, Augsburger JJ. Primary acquired melanosis with atypia treated with mitomycin C. Int Ophthalmol. 2009;29:285-288.
- Torres C, Wong L, Welsh O, et al. Skin manifestations associated with chemotherapy in children with hematologic malignancies. Pediatr Dermatol. 2011;2:123-147.
- Mays SR, Cohen PR. Emerging dermatologic issues in the oncology patient. Semin Cutan Med Surg. 2006;25:179-189.
Practice Points
- Chemotherapeutic agents can cause a variety of cutaneous side effects.
- Pediatric oncology patients should be examined regularly for cutaneous side effects of chemotherapeutics.