User login
After Rena Barrow-Wells, an African American mother, fought mightily to prevent a repeat of her experience of two decades earlier when her first child’s cystic fibrosis (CF) took 4 years to diagnose, her story became the subject of a New York Times feature covering disparities in diagnostic CF screening. The article highlighted not only her struggles, but also the utter transformation of the CF landscape since the introduction of small molecule mutation-specific drugs. These drugs restore function to defective CF transmembrane conductance regulator (CFTR) proteins. By the time Ms. Barrow-Wells’ young son was treated, lung and pancreatic scarring were already significant. So when the 39-mutation variant screening test available in Ms. Barrow-Wells’ Lawrenceville, Georgia, clinic turned out negative for CF, her pediatrician told her to stop worrying despite her new son’s inherent genetic risk, telltale salty skin, foul-smelling diapers, and her pleas to test for sweat chloride. It still took 3 months for a confirmed diagnosis and the initiation of treatment.
Current genetic tests, based largely on older clinical trials that enrolled mostly white children, are highly accurate for identifying CF in white babies (95%), but often fail to identify substantial percentages of mutations originating in Africa, Asia, and Latin America. They miss CF in Asian (44%), Black (22%), and Hispanic, Native American and Alaskan Native babies (14%), the Times article stated. In the United States, the number of CF variants tested for falls into a wide range: from the one variant found mostly in White populations in Mississippi (with a 38% Black populace) to 689 variants in Wisconsin.
Not too far back, CF was thought of as an inherited childhood disease leading often to childhood or adolescent mortality.
Today’s CF challenges
Beyond refinements in screening instruments and policies that broaden access leading to the earliest possible diagnoses, ongoing research needs include finding treatments for other variants, and caring for adult populations living with treated CF and the disease’s multisystem manifestations. “As people with CF live longer, we need to be very focused on optimized adult medical care for this population,” Marc A. Sala, MD, assistant professor of medicine, Adult CF Program, Northwestern University Feinberg School of Medicine, Chicago, said in an interview. “For example, we need higher vigilance for liver, microvascular, coronary artery disease, and various cancer screenings. We do not know exactly how these will manifest differently from the way they do in non-CF populations, so this is where more work needs to be done.”
Emphasis on monitoring
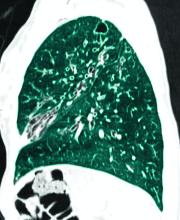
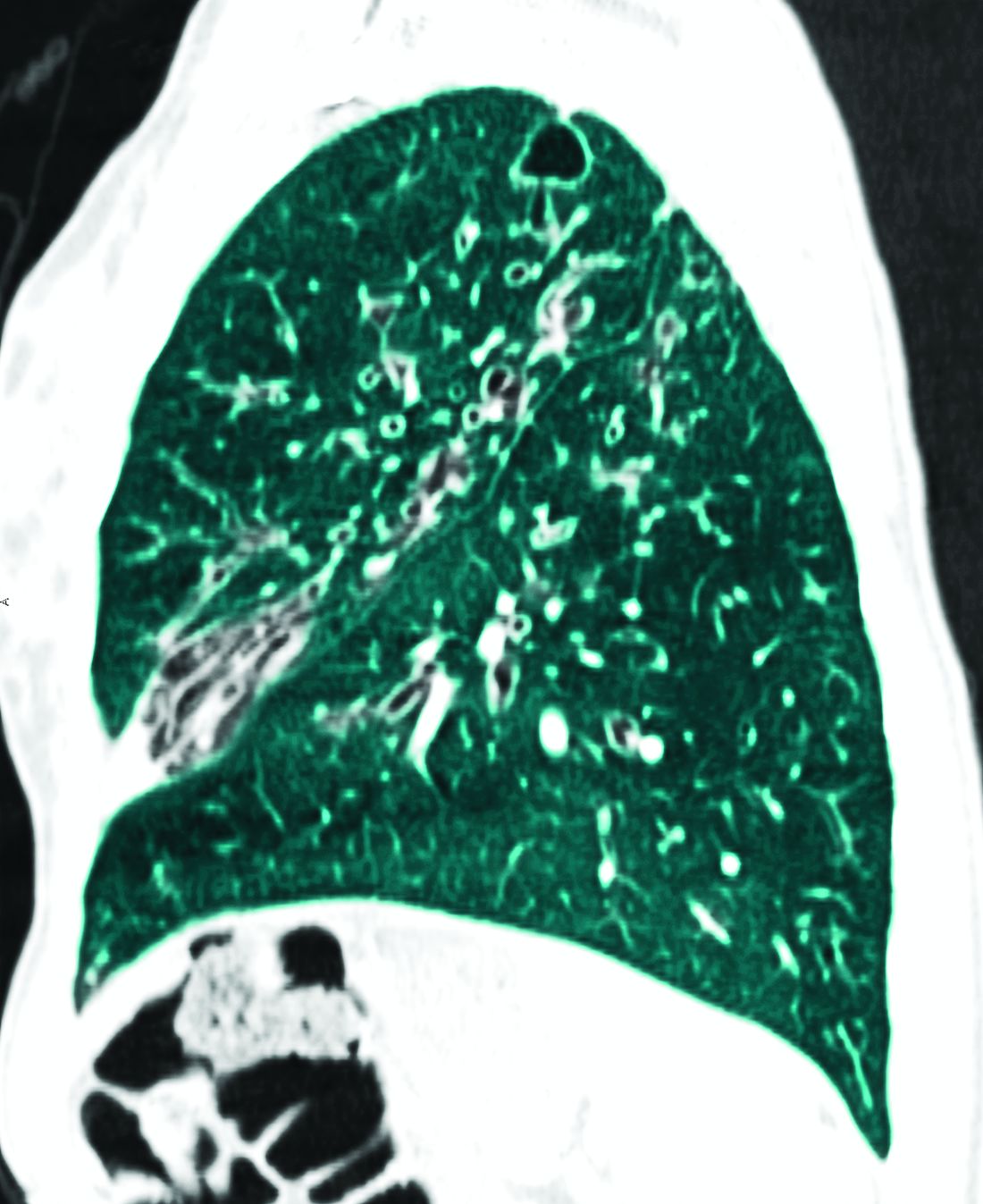
The authors of “Future therapies for cystic fibrosis” (Allen et al. Nature Communications, 2023 Feb 8), after citing the ongoing transformative change for people with CF since the introduction of CFTR drugs, gave voice to important cautions. “Disease will progress, albeit more slowly, and will be more challenging to monitor. Effective CFTR modulators will likely slow or, at best, halt disease progression, but will not reverse a disease that has already become fixed.” They cited pancreatic destruction in the majority, bronchiectasis, and absence of the vas deferens, with still recurring (although less frequently) pulmonary exacerbations along with chronic infections and persistent airway inflammation. “It is essential that we do not become complacent about disease progression in this population,” the researchers stated. They cautioned also that effective surveillance for infection is critical in asymptomatic patients, emphasizing that it underpins the management of young healthy children with CF who demonstrate disease progression despite a lack of symptoms.
Among the ~90% for whom Trikafta is suitable and approved (those with least one copy of F508del or specific other responsive mutations), improvements include increased percent predicted FEV1 by 10%-15% or more, decreased exacerbations, and improved quality of life,” Dr. Sala said. “Subsequent ‘real world’ experience shows dramatic reductions in sputum production and decreased frequency of lung transplant.”
Mutation agnostic therapy
Unfortunately, CF mutants, outside the population eligible for Trikafta, are prodigious in number and do not fall into just a few major groups. “Furthermore, although CF is a monogenic disease, it has variable phenotypes even for two individuals with the same mutations,” Dr. Sala said. “Current CFTR modulators act on the dysfunctional CFTR protein (either as channel gating potentiators or molecular chaperones to improve misfolding). That leaves about 10% of the CF population, those with little to no protein production (such as in nonsense mutations) ineligible for treatment with CFTR modulators. “The ideal for efficacy and equity, given that some CFTR mutations only exist in a handful of people, would be to develop a ‘mutation agnostic’ strategy — such as with mRNA or gene delivery. Here you could imagine that regardless of the type of mutation, a patient would then be able to receive the technology to increase CFTR channel function,” Dr. Sala said. Many modifiable factors, including host immunity and non-CFTR genes that impact CFTR indirectly, may underlie the fact that one person has a worse trajectory than another. “New therapies may also be found in this area of research,” Dr. Sala said.
Strategies in testing phases
“For patients with class I (nonsense) mutations there is hope that small molecules will be identified that can facilitate premature truncation codon (PTC) read-through and/or impede mRNA decay allowing for clinically relevant levels of functional CFTR,” the researchers noted. While the most extensively developed, ataluren, an oxadiazole, failed in phase 3 trials after initial promise, other ribosomal read-through drugs are in preclinical and early phase clinical trials. Also, early encouraging results support an alternative strategy, engineered transfer RNAs (tRNAs) that introduce an amino acid to an elongating peptide in place of the termination codon.
While these will address specific mutations, DNA or mRNA replacement strategies would be “mutation agnostic,” the researchers stated. The major challenge: delivery to the respiratory epithelium. Approaches currently in early testing include an inhaled aerosolized, lipid-based nanoparticle carrier for mRNA delivery, viral and non-viral DNA transfer, lipid-mediated CFTR gene transfer, pseudotyped lentiviral vector and adeno-associated vector transfer of CFTR DNA.
Adult CF care
“Adult CF care in general is a completely new frontier,” Meilinh Thi, DO, director of the adult cystic fibrosis program and assistant professor at University of Texas Health at San Antonio, said in an interview. “It’s fairly new to have separate pediatric and adult CF centers. There’s been a shift,” she said. “We’re encountering diseases in CF that we have not in the past had to deal with: diabetes that has features of both type 1 and type 2, increased colon cancer risk, bone disease, and mental health issues. Also, while pregnancy was previously discouraged for women with CF because of lung disease, now many are giving birth without complications and living normal lives,” Dr. Thi said.
“We do encourage our patients to talk to us before becoming pregnant so we can discuss the risk of passing on the gene. And, we do encourage their significant others to get testing. Some patients and their others, however, do decline to get tested,” she added.
The lifetime health issues conferred by CF, Dr. Thi noted, include lung disease with chronic inflammation, infection, respiratory failure (still the most common cause of death), gastrointestinal disorders (including of the pancreas) , colon obstruction and colon cancer, sinus disease, and reproductive system effects. Their permanence, she said, depends on how far their disease has progressed. “So the earlier you can provide these newer therapies — the modulators, for example, or the gene therapy whenever that comes out, then the less damage these organ systems will have, and the patients, we hope, will then do better.”
After Rena Barrow-Wells, an African American mother, fought mightily to prevent a repeat of her experience of two decades earlier when her first child’s cystic fibrosis (CF) took 4 years to diagnose, her story became the subject of a New York Times feature covering disparities in diagnostic CF screening. The article highlighted not only her struggles, but also the utter transformation of the CF landscape since the introduction of small molecule mutation-specific drugs. These drugs restore function to defective CF transmembrane conductance regulator (CFTR) proteins. By the time Ms. Barrow-Wells’ young son was treated, lung and pancreatic scarring were already significant. So when the 39-mutation variant screening test available in Ms. Barrow-Wells’ Lawrenceville, Georgia, clinic turned out negative for CF, her pediatrician told her to stop worrying despite her new son’s inherent genetic risk, telltale salty skin, foul-smelling diapers, and her pleas to test for sweat chloride. It still took 3 months for a confirmed diagnosis and the initiation of treatment.
Current genetic tests, based largely on older clinical trials that enrolled mostly white children, are highly accurate for identifying CF in white babies (95%), but often fail to identify substantial percentages of mutations originating in Africa, Asia, and Latin America. They miss CF in Asian (44%), Black (22%), and Hispanic, Native American and Alaskan Native babies (14%), the Times article stated. In the United States, the number of CF variants tested for falls into a wide range: from the one variant found mostly in White populations in Mississippi (with a 38% Black populace) to 689 variants in Wisconsin.
Not too far back, CF was thought of as an inherited childhood disease leading often to childhood or adolescent mortality.
Today’s CF challenges
Beyond refinements in screening instruments and policies that broaden access leading to the earliest possible diagnoses, ongoing research needs include finding treatments for other variants, and caring for adult populations living with treated CF and the disease’s multisystem manifestations. “As people with CF live longer, we need to be very focused on optimized adult medical care for this population,” Marc A. Sala, MD, assistant professor of medicine, Adult CF Program, Northwestern University Feinberg School of Medicine, Chicago, said in an interview. “For example, we need higher vigilance for liver, microvascular, coronary artery disease, and various cancer screenings. We do not know exactly how these will manifest differently from the way they do in non-CF populations, so this is where more work needs to be done.”
Emphasis on monitoring
The authors of “Future therapies for cystic fibrosis” (Allen et al. Nature Communications, 2023 Feb 8), after citing the ongoing transformative change for people with CF since the introduction of CFTR drugs, gave voice to important cautions. “Disease will progress, albeit more slowly, and will be more challenging to monitor. Effective CFTR modulators will likely slow or, at best, halt disease progression, but will not reverse a disease that has already become fixed.” They cited pancreatic destruction in the majority, bronchiectasis, and absence of the vas deferens, with still recurring (although less frequently) pulmonary exacerbations along with chronic infections and persistent airway inflammation. “It is essential that we do not become complacent about disease progression in this population,” the researchers stated. They cautioned also that effective surveillance for infection is critical in asymptomatic patients, emphasizing that it underpins the management of young healthy children with CF who demonstrate disease progression despite a lack of symptoms.
Among the ~90% for whom Trikafta is suitable and approved (those with least one copy of F508del or specific other responsive mutations), improvements include increased percent predicted FEV1 by 10%-15% or more, decreased exacerbations, and improved quality of life,” Dr. Sala said. “Subsequent ‘real world’ experience shows dramatic reductions in sputum production and decreased frequency of lung transplant.”
Mutation agnostic therapy
Unfortunately, CF mutants, outside the population eligible for Trikafta, are prodigious in number and do not fall into just a few major groups. “Furthermore, although CF is a monogenic disease, it has variable phenotypes even for two individuals with the same mutations,” Dr. Sala said. “Current CFTR modulators act on the dysfunctional CFTR protein (either as channel gating potentiators or molecular chaperones to improve misfolding). That leaves about 10% of the CF population, those with little to no protein production (such as in nonsense mutations) ineligible for treatment with CFTR modulators. “The ideal for efficacy and equity, given that some CFTR mutations only exist in a handful of people, would be to develop a ‘mutation agnostic’ strategy — such as with mRNA or gene delivery. Here you could imagine that regardless of the type of mutation, a patient would then be able to receive the technology to increase CFTR channel function,” Dr. Sala said. Many modifiable factors, including host immunity and non-CFTR genes that impact CFTR indirectly, may underlie the fact that one person has a worse trajectory than another. “New therapies may also be found in this area of research,” Dr. Sala said.
Strategies in testing phases
“For patients with class I (nonsense) mutations there is hope that small molecules will be identified that can facilitate premature truncation codon (PTC) read-through and/or impede mRNA decay allowing for clinically relevant levels of functional CFTR,” the researchers noted. While the most extensively developed, ataluren, an oxadiazole, failed in phase 3 trials after initial promise, other ribosomal read-through drugs are in preclinical and early phase clinical trials. Also, early encouraging results support an alternative strategy, engineered transfer RNAs (tRNAs) that introduce an amino acid to an elongating peptide in place of the termination codon.
While these will address specific mutations, DNA or mRNA replacement strategies would be “mutation agnostic,” the researchers stated. The major challenge: delivery to the respiratory epithelium. Approaches currently in early testing include an inhaled aerosolized, lipid-based nanoparticle carrier for mRNA delivery, viral and non-viral DNA transfer, lipid-mediated CFTR gene transfer, pseudotyped lentiviral vector and adeno-associated vector transfer of CFTR DNA.
Adult CF care
“Adult CF care in general is a completely new frontier,” Meilinh Thi, DO, director of the adult cystic fibrosis program and assistant professor at University of Texas Health at San Antonio, said in an interview. “It’s fairly new to have separate pediatric and adult CF centers. There’s been a shift,” she said. “We’re encountering diseases in CF that we have not in the past had to deal with: diabetes that has features of both type 1 and type 2, increased colon cancer risk, bone disease, and mental health issues. Also, while pregnancy was previously discouraged for women with CF because of lung disease, now many are giving birth without complications and living normal lives,” Dr. Thi said.
“We do encourage our patients to talk to us before becoming pregnant so we can discuss the risk of passing on the gene. And, we do encourage their significant others to get testing. Some patients and their others, however, do decline to get tested,” she added.
The lifetime health issues conferred by CF, Dr. Thi noted, include lung disease with chronic inflammation, infection, respiratory failure (still the most common cause of death), gastrointestinal disorders (including of the pancreas) , colon obstruction and colon cancer, sinus disease, and reproductive system effects. Their permanence, she said, depends on how far their disease has progressed. “So the earlier you can provide these newer therapies — the modulators, for example, or the gene therapy whenever that comes out, then the less damage these organ systems will have, and the patients, we hope, will then do better.”
After Rena Barrow-Wells, an African American mother, fought mightily to prevent a repeat of her experience of two decades earlier when her first child’s cystic fibrosis (CF) took 4 years to diagnose, her story became the subject of a New York Times feature covering disparities in diagnostic CF screening. The article highlighted not only her struggles, but also the utter transformation of the CF landscape since the introduction of small molecule mutation-specific drugs. These drugs restore function to defective CF transmembrane conductance regulator (CFTR) proteins. By the time Ms. Barrow-Wells’ young son was treated, lung and pancreatic scarring were already significant. So when the 39-mutation variant screening test available in Ms. Barrow-Wells’ Lawrenceville, Georgia, clinic turned out negative for CF, her pediatrician told her to stop worrying despite her new son’s inherent genetic risk, telltale salty skin, foul-smelling diapers, and her pleas to test for sweat chloride. It still took 3 months for a confirmed diagnosis and the initiation of treatment.
Current genetic tests, based largely on older clinical trials that enrolled mostly white children, are highly accurate for identifying CF in white babies (95%), but often fail to identify substantial percentages of mutations originating in Africa, Asia, and Latin America. They miss CF in Asian (44%), Black (22%), and Hispanic, Native American and Alaskan Native babies (14%), the Times article stated. In the United States, the number of CF variants tested for falls into a wide range: from the one variant found mostly in White populations in Mississippi (with a 38% Black populace) to 689 variants in Wisconsin.
Not too far back, CF was thought of as an inherited childhood disease leading often to childhood or adolescent mortality.
Today’s CF challenges
Beyond refinements in screening instruments and policies that broaden access leading to the earliest possible diagnoses, ongoing research needs include finding treatments for other variants, and caring for adult populations living with treated CF and the disease’s multisystem manifestations. “As people with CF live longer, we need to be very focused on optimized adult medical care for this population,” Marc A. Sala, MD, assistant professor of medicine, Adult CF Program, Northwestern University Feinberg School of Medicine, Chicago, said in an interview. “For example, we need higher vigilance for liver, microvascular, coronary artery disease, and various cancer screenings. We do not know exactly how these will manifest differently from the way they do in non-CF populations, so this is where more work needs to be done.”
Emphasis on monitoring
The authors of “Future therapies for cystic fibrosis” (Allen et al. Nature Communications, 2023 Feb 8), after citing the ongoing transformative change for people with CF since the introduction of CFTR drugs, gave voice to important cautions. “Disease will progress, albeit more slowly, and will be more challenging to monitor. Effective CFTR modulators will likely slow or, at best, halt disease progression, but will not reverse a disease that has already become fixed.” They cited pancreatic destruction in the majority, bronchiectasis, and absence of the vas deferens, with still recurring (although less frequently) pulmonary exacerbations along with chronic infections and persistent airway inflammation. “It is essential that we do not become complacent about disease progression in this population,” the researchers stated. They cautioned also that effective surveillance for infection is critical in asymptomatic patients, emphasizing that it underpins the management of young healthy children with CF who demonstrate disease progression despite a lack of symptoms.
Among the ~90% for whom Trikafta is suitable and approved (those with least one copy of F508del or specific other responsive mutations), improvements include increased percent predicted FEV1 by 10%-15% or more, decreased exacerbations, and improved quality of life,” Dr. Sala said. “Subsequent ‘real world’ experience shows dramatic reductions in sputum production and decreased frequency of lung transplant.”
Mutation agnostic therapy
Unfortunately, CF mutants, outside the population eligible for Trikafta, are prodigious in number and do not fall into just a few major groups. “Furthermore, although CF is a monogenic disease, it has variable phenotypes even for two individuals with the same mutations,” Dr. Sala said. “Current CFTR modulators act on the dysfunctional CFTR protein (either as channel gating potentiators or molecular chaperones to improve misfolding). That leaves about 10% of the CF population, those with little to no protein production (such as in nonsense mutations) ineligible for treatment with CFTR modulators. “The ideal for efficacy and equity, given that some CFTR mutations only exist in a handful of people, would be to develop a ‘mutation agnostic’ strategy — such as with mRNA or gene delivery. Here you could imagine that regardless of the type of mutation, a patient would then be able to receive the technology to increase CFTR channel function,” Dr. Sala said. Many modifiable factors, including host immunity and non-CFTR genes that impact CFTR indirectly, may underlie the fact that one person has a worse trajectory than another. “New therapies may also be found in this area of research,” Dr. Sala said.
Strategies in testing phases
“For patients with class I (nonsense) mutations there is hope that small molecules will be identified that can facilitate premature truncation codon (PTC) read-through and/or impede mRNA decay allowing for clinically relevant levels of functional CFTR,” the researchers noted. While the most extensively developed, ataluren, an oxadiazole, failed in phase 3 trials after initial promise, other ribosomal read-through drugs are in preclinical and early phase clinical trials. Also, early encouraging results support an alternative strategy, engineered transfer RNAs (tRNAs) that introduce an amino acid to an elongating peptide in place of the termination codon.
While these will address specific mutations, DNA or mRNA replacement strategies would be “mutation agnostic,” the researchers stated. The major challenge: delivery to the respiratory epithelium. Approaches currently in early testing include an inhaled aerosolized, lipid-based nanoparticle carrier for mRNA delivery, viral and non-viral DNA transfer, lipid-mediated CFTR gene transfer, pseudotyped lentiviral vector and adeno-associated vector transfer of CFTR DNA.
Adult CF care
“Adult CF care in general is a completely new frontier,” Meilinh Thi, DO, director of the adult cystic fibrosis program and assistant professor at University of Texas Health at San Antonio, said in an interview. “It’s fairly new to have separate pediatric and adult CF centers. There’s been a shift,” she said. “We’re encountering diseases in CF that we have not in the past had to deal with: diabetes that has features of both type 1 and type 2, increased colon cancer risk, bone disease, and mental health issues. Also, while pregnancy was previously discouraged for women with CF because of lung disease, now many are giving birth without complications and living normal lives,” Dr. Thi said.
“We do encourage our patients to talk to us before becoming pregnant so we can discuss the risk of passing on the gene. And, we do encourage their significant others to get testing. Some patients and their others, however, do decline to get tested,” she added.
The lifetime health issues conferred by CF, Dr. Thi noted, include lung disease with chronic inflammation, infection, respiratory failure (still the most common cause of death), gastrointestinal disorders (including of the pancreas) , colon obstruction and colon cancer, sinus disease, and reproductive system effects. Their permanence, she said, depends on how far their disease has progressed. “So the earlier you can provide these newer therapies — the modulators, for example, or the gene therapy whenever that comes out, then the less damage these organ systems will have, and the patients, we hope, will then do better.”