User login
Dabigatran etexilate (Pradaxa) is a new oral anticoagulant that has distinct advantages over warfarin (Coumadin) in terms of its ease of administration, efficacy, and safety.
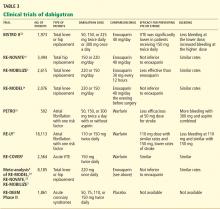
In the Randomized Evaluation of Long-Term Anticoagulation Therapy (RE-LY trial),1 in patients with nonvalvular atrial fibrillation, dabigatran 110 mg twice a day was found to be as good as warfarin in preventing systemic embolization and stroke (the primary outcome of the study), and at 150 mg twice a day it was superior.1 It has also shown efficacy in treating acute deep vein thrombosis and pulmonary embolism and in preventing these complications in orthopedic surgical patients.2–4
Dabigatran has been approved in 75 countries. It carries the trade name Pradaxa in Europe and the United States and Pradax in Canada. In October 2010, the US Food and Drug Administration (FDA) Cardiovascular and Renal Drugs Advisory Committee endorsed two twice-daily doses (75 mg and 150 mg) of dabigatran for the prevention of systemic embolization and stroke in patients with nonvalvular atrial fibrillation.
However, dabigatran is relatively expensive, and its current high cost might be a barrier to its wider use.
MANY PATIENTS NEED ANTICOAGULATION
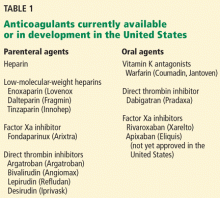
Anticoagulation plays a vital role in the primary and secondary prevention of stroke in patients with atrial fibrillation and of pulmonary embolism in patients with venous thromboembolism. It is also used during cardiothoracic and vascular surgery, endovascular procedures, and dialysis and in patients with mechanical heart valves and hypercoagulable conditions.
Atrial fibrillation affects 3.03 million people in the United States (2005 figures), and this number is predicted to be as high as 7.56 million by 2050.5 More than 10% of people over the age of 80 years have it, and the lifetime risk of developing it is approximately 25%.6,7 Its most serious complication is ischemic stroke (the risk of which increases with age) and systemic embolization.5,8
Until the recent introduction of dabigatran, the only oral anticoagulant available in the United States for treating patients with atrial fibrillation was warfarin. Although warfarin has a number of disadvantages (see below), it is actually very effective for preventing ischemic stroke, reducing the incidence by as much as 65%.9,10
Venous thromboembolism is the third most common cardiovascular disorder after myocardial infarction and stroke.11 Although its exact incidence is unknown, nearly 1 million cases of it (incident or recurrent, fatal and nonfatal events) occur in the United States each year.12 Many patients with venous thromboembolism need oral anticoagulation long-term, and currently warfarin remains the only option for them as well.
NEEDED: A BETTER ANTICOAGULANT
Warfarin has been the most commonly prescribed oral anticoagulant in the United States for more than 60 years. As of 2004, more than 30 million outpatient prescriptions for it were filled annually in this country alone.13 However, warfarin has several important limitations.
Warfarin has a narrow therapeutic index. Patients taking it require monitoring of their international normalized ratio (INR) and frequent dose adjustments, and this is time-consuming and inconvenient. The target INR for patients with venous thromboembolism and atrial fibrillation is 2.0 to 3.0, whereas patients with a mechanical heart valve need a higher INR (2.5 to 3.5). If the INR is below these ranges, warfarin is less effective, with a risk of new thrombosis. On the other hand, if the INR is too high, there is a risk of bleeding.14 In fact, the most important side effect of warfarin is the risk of major and minor bleeding.13 However, even in well-designed clinical trials in which patients are closely managed, only 55% to 60% of patients regularly achieve their therapeutic target INR.1,2,14,15
Warfarin also interacts with many drugs and with some foods. Compliance is difficult. It has a slow onset of action. Genetic variations require dose adjustments. When switching from a parenteral anticoagulant, overlapping is required. Skin necrosis is a possible side effect. And warfarin is teratogenic.
Despite these limitations, the American College of Chest Physicians endorses warfarin to prevent or treat venous thromboembolism, and to prevent stroke in patients with atrial fibrillation.16
DABIGATRAN, A THROMBIN INHIBITOR
A prodrug, dabigatran is rapidly absorbed and converted to its active form. Its plasma concentration reaches a peak 1.5 to 3 hours after an oral dose, and it has an elimination half-life of 12 to 14 hours. About 80% of its excretion is by the kidneys and the remaining 20% is through bile.
Dabigatran is not metabolized by cytochrome P450 isoenzymes, and therefore it has few major interactions with other drugs. An exception is rifampin, a P-glycoprotein inducer that blocks dabigatran’s absorption in the gut, so this combination should be avoided. Another is quinidine, a strong P-glycoprotein inhibitor that is contraindicated for use with dabigatran. Also, amiodarone (Cordarone), another P-glycoprotein inhibitor, increases blood levels of dabigatran, and therefore a lower dose of dabigatran is recommended if these drugs are given together.18–20
DOES DABIGATRAN NEED MONITORING? CAN IT EVEN BE MONITORED?
Dabigatran has a predictable pharmacodynamic effect, and current data indicate it does not need regular monitoring.18–20 However, one may need to be able to measure the drug’s activity in certain situations, such as suspected overdose, bleeding, need for emergency surgery, impaired renal function, pregnancy, and obesity, and in children.20
Dabigatran has little effect on the prothrombin time or the INR, even at therapeutic concentrations.19 Further, its effect on the activated partial thromboplastin time (aPTT) is neither linear nor dose-dependent, and the aPTT reaches a plateau and becomes less sensitive at very high concentrations. Therefore, the aPTT does not appear to be an appropriate test to monitor dabigatran’s therapeutic anticoagulant effect, although it does provide a qualitative indication of anticoagulant activity.18,19
The thrombin time is a very sensitive method for determining if dabigatran is present, but the test lacks standardization; the ecarin clotting time provides better evidence of the dose but is not readily available at most institutions.18,19,21
EVALUATED IN CLINICAL TRIALS
DABIGATRAN IS EXPENSIVE BUT MAY BE COST-EFFECTIVE
The estimated price of dabigatran 150 mg twice a day in the United States is about $6.75 to $8.00 per day.26,27
Warfarin, in contrast, costs as little as $50 per year.28 However, this low price does not include the cost of monitoring the INR (office visits and laboratory testing), and these combined expenses are much higher than the price of the warfarin itself.29 In addition, warfarin requires time-consuming management when bridging to a parenteral anticoagulant (for reversal of its anticoagulant action) before routine health maintenance procedures such as dental work and colonoscopy and interventional procedures and surgery. Any bleeding complication will also add to its cost and will be associated with a decrease in the patient’s perceived health and quality of life, but this is true for both drugs.30
In today’s health care environment, controlling costs is a universal priority, but it may be unfair to compare the cost of dabigatran with that of warfarin alone. The expense and morbidity associated with stroke and intracranial bleeding are high, and if patients on dabigatran have fewer strokes (as seen in the RE-LY trial with dabigatran 150 mg twice a day) and no added expense of monitoring, then dabigatran may be cost-effective.
Freeman et al31 analyzed the cost-effectiveness of dabigatran, using an estimated cost of $13.70 per day and data from the RE-LY trial. They concluded that dabigatran may be a cost-effective alternative to warfarin in preventing ischemic stroke in patients considered at higher risk for ischemic stroke or intracranial hemorrhage, ie, those with a CHADS2 score of 1 or higher or equivalent. (The CHADS2 score is calculated as 1 point each for congestive heart failure, hypertension, age 75 or older, and diabetes mellitus; 2 points for prior stroke or transient ischemic attack.)
As more new-generation oral anticoagulants become available (see below), the price of dabigatran will undoubtedly decrease. Until then, warfarin will remain a cost-effective and cost-saving drug that cannot yet be considered obsolete.
WHO SHOULD RECEIVE DABIGATRAN?
The ideal patient for dabigatran treatment is not yet defined. The decision to convert a patient’s treatment from warfarin to dabigatran will likely depend on several factors, including the patient’s response to warfarin and the physician’s comfort with this new drug.
Many patients do extremely well with warfarin, requiring infrequent monitoring to maintain a therapeutic INR and having no bleeding complications. For them, it would be more practical to continue warfarin. Another reason for staying with warfarin would be if twice-a-day dosing would pose a problem.
Dabigatran would be a reasonable choice for a patient whose INR is erratic, who requires more frequent monitoring, for whom cost is not an issue, and for whom there is concern about dietary or drug interactions.
Another consideration is whether the patient has access to a health care facility for warfarin monitoring: this is difficult for those who cannot drive, who depend on others for transportation, and who live in rural areas.
Additionally, dabigatran may be a cost-effective alternative to warfarin for a patient with a high CHADS2 score who is considered at a higher risk for stroke.31
In all cases, the option should be considered only after an open discussion with the patient about the risks and benefits of this new drug.
WHO SHOULD NOT RECEIVE IT?
Dabigatran is a twice-daily drug with a short half-life. No patient with a history of poor compliance will be a good candidate for dabigatran. Since there are no practical laboratory tests for monitoring compliance, one will have to reinforce at every visit the importance of taking this medication according to instructions.
Patients with underlying kidney disease will need close monitoring of their creatinine clearance, with dose adjustment if renal function deteriorates.
Additionally, one should use caution when prescribing dabigatran to obese patients, pregnant women, or children until more is known about its use in these populations.
ADVANTAGES AND DISADVANTAGES OF DABIGATRAN
A reason may be that patients with atrial fibrillation and poor INR control have higher rates of death, stroke, myocardial infarction, and major bleeding.14 In most clinical trials, only 55% to 60% of patients achieve a therapeutic INR on warfarin, leaving them at risk of thrombosis or, conversely, bleeding.1,2,15,32 Dabigatran has predictable pharmacokinetics, and its twice-daily dosing allows for less variability in its anticoagulant effect, making it more consistently therapeutic with less potential for bleeding or thrombosis.1
The Canadian Cardiovascular Society included dabigatran in its 2010 guidelines on atrial fibrillation, recommending it or warfarin.33 The American College of Cardiology, the American Heart Association, and the Heart Rhythm Society now give dabigatran a class I B recommendation (benefit greater than risk, but limited populations studied) in secondary stroke prevention.34
On the other hand, major concerns are the lack of an antidote for dabigatran and a lack of experience in treating bleeding complications. Since dabigatran is not monitored, physicians may be uncertain if we are overdosing or undertreating. As we gain experience, we will learn how to treat bleeding complications. Until then, it will be important to anticipate this problem and to develop an algorithm based on the best available evidence in managing this complication.
Although the overall rates of bleeding in the RE-LY trial were lower with dabigatran than with warfarin, there were more gastrointestinal bleeding events with the 150-mg dose of dabigatran, which was not readily explained.
Further, the rate of dyspepsia was almost twice as high with dabigatran than with warfarin, regardless of the dose of dabigatran. There were also more dropouts in the 2nd year of follow-up in the dabigatran groups, with gastrointestinal intolerance being one of the major reasons. Therefore, dyspepsia may cause intolerance and noncompliance.1
Dabigatran must be taken twice a day and has a relatively short half-life. For a noncompliant patient, missing one or two doses will cause a reversal of its anticoagulation effect, leaving the patient susceptible to thrombosis. In comparison, warfarin has a longer half-life and is taken once a day, so missing a dose is less likely to result in a similar reversal of its anticoagulant effect.
SPECIAL CONDITIONS
Switching from other anticoagulants to dabigatran
When making the transition from a subcutaneously administered anticoagulant, ie, a low-molecular-weight heparin or the anti-Xa inhibitor fondaparinux (Arixtra), dabigatran should be started 0 to 2 hours before the next subcutaneous dose of the parenteral anticoagulant was to be given.21,35
When switching from unfractionated heparin given by continuous intravenous infusion, the first dose of dabigatran should be given at the time the infusion is stopped.
When switching from warfarin, dabigatran should be started once the patient’s INR is less than 2.0.
Switching from dabigatran to a parenteral anticoagulant
When switching from dabigatran back to a parenteral anticoagulant, allow 12 to 24 hours after the last dabigatran dose before starting the parenteral agent.21,35
Elective surgery or invasive procedures
The manufacturer recommends stopping dabigatran 1 to 2 days before elective surgery for patients who have normal renal function and a low risk of bleeding, or 3 to 5 days before surgery for patients who have a creatinine clearance of 50 mL/min or less. Before major surgery or placement of a spinal or epidural catheter, the manufacturer recommends that dabigatran be held even longer.35
If emergency surgery is needed
If emergency surgery is needed, the clinician must use his or her judgment as to the risks of bleeding vs those of postponing the surgery.21,35
Overdose or bleeding
No antidote for dabigatran is currently available. It has a short half-life (12–14 hours), and the treatment for overdose or bleeding is to discontinue it immediately, maintain adequate diuresis, and transfuse fresh-frozen plasma or red blood cells as indicated.
The role of activated charcoal given orally to reduce absorption is under evaluation, but the charcoal must be given within 1 to 2 hours after the overdose is taken.21
Dabigatran does not bind very much to plasma proteins and hence is dialyzable—an approach that may be necessary in cases of persistent or life-threatening bleeding.
Recombinant activated factor VII or prothrombin complex concentrates may be additional options in cases of severe bleeding.18,21
TOPICS OF FUTURE RESEARCH
A limitation of the dabigatran trials was that they did not enroll patients who had renal or liver impairment, cancer, or other comorbidities; pregnant women; or children. Other topics of future research include its use in patients weighing less than 48 kg or more than 110 kg, its efficacy in patients with thrombophilia, in patients with mechanical heart valves, and in long-term follow-up and the use of thrombolytics in patients with acute stroke who are on dabigatran.
WILL DABIGATRAN CHANGE CLINICAL PRACTICE?
Despite some of the challenges listed above, we believe that dabigatran is likely to change medical practice in patients requiring anticoagulation.
Dabigatran’s biggest use will most likely be in patients with atrial fibrillation, mainly because this is the largest group of people receiving anticoagulation. In addition, the incidence of atrial fibrillation rises with age, the US population is living longer, and patients generally require life-long anticoagulation once this condition develops.
Dabigatran may be approved for additional indications in the near future. It has already shown efficacy in primary and secondary prevention of venous thromboembolism. Other important areas to be studied include its use in patients with mechanical heart valves and thrombophilia.
Whether dabigatran will be a worthy substitute for the parenteral anticoagulants (heparin, low-molecular-weight heparins, or factor Xa inhibitors) is not yet known, but it will have an enormous impact on anticoagulation management if proved efficacious.
If dabigatran becomes a major substitute for warfarin, it will affect the anticoagulation clinics, with their well-trained staff, that are currently monitoring millions of patients in the United States. These clinics would no longer be needed, and laboratory and technical costs could be saved. A downside is that patients on dabigatran will not be as closely supervised and reminded to take their medication as patients on warfarin are now at these clinics. Instead, they will likely be supervised by their own physician (or assistants), who will need to become familiar with this anticoagulant. This may affect compliance with dabigatran.
OTHER NEW ORAL ANTICOAGULANTS ARE ON THE WAY
Other oral anticoagulants, including rivaroxaban (Xarelto) and apixaban (Eliquis), have been under study and show promise in preventing both thrombotic stroke and venous thromboembolism. They will likely compete with dabigatran once they are approved.
Rivaroxaban, an oral direct factor Xa inhibitor, is being investigated for stroke prevention in patients with atrial fibrillation. It has also been shown to be not inferior to (and to be less expensive than) enoxaparin in treating and preventing venous thromboembolism in patients undergoing hip or knee arthroplasty.32,36,37 Rivaroxaban has recently been approved by the FDA for this indication.
Apixaban, another direct factor Xa inhibitor, is also being studied for the prevention of stroke and systemic embolism in patients with nonvalvular atrial fibrillation. To date, there are no head-to-head trials comparing dabigatran with either of these new oral anticoagulants.
- Connolly SJ, Ezekowitz MD, Yusuf S, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med 2009; 361:1139–1151.
- Schulman S, Kearon C, Kakkar AK, et al. Dabigatran versus warfarin in the treatment of acute venous thromboembolism. N Engl J Med 2009; 361:2342–2352.
- RE-MOBILIZE Writing Committee; Ginsberg JS, Davidson BL, Comp PC, et al. Oral thrombin inhibitor dabigatran etexilate vs North American enoxaparin regimen for prevention of venous thromboembolism after knee arthroplasty surgery. J Arthroplasty 2009; 24:1–9.
- Wolowacz SE, Roskell NS, Plumb JM, Caprini JA, Eriksson BI. Efficacy and safety of dabigatran etexilate for the prevention of venous thromboembolism following total hip or knee arthroplasty. A meta-analysis. Thromb Haemost 2009; 101:77–85.
- Naccarelli GV, Varker H, Lin J, Schulman KL. Increasing prevalence of atrial fibrillation and flutter in the United States. Am J Cardiol 2009; 104:1534–1539.
- Krahn AD, Manfreda J, Tate RB, Mathewson FA, Cuddy TE. The natural history of atrial fibrillation: incidence, risk factors, and prognosis in the Manitoba follow-up study. Am J Med 1995; 98:476–484.
- Lloyd-Jones DM, Wang TJ, Leip EP, et al. Lifetime risk for development of atrial fibrillation: the Framingham Heart Study. Circulation 2004; 110:1042–1046.
- Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham study. Stroke 1991; 22:983–988.
- Go AS, Hylek EM, Chang Y, et al. Anticoagulation therapy for stroke prevention in atrial fibrillation: how well do randomized trials translate into clinical practice? JAMA 2003; 290:2685–2692.
- Singer DE, Chang Y, Fang MC, et al. The net clinical benefit of warfarin anticoagulation in atrial fibrillation. Ann Intern Med 2009; 151:297–305.
- Goldhaber SZ. Pulmonary embolism thrombolysis: a clarion call for international collaboration. J Am Coll Cardiol 1992; 19:246–247.
- Heit JA. The epidemiology of venous thromboembolism in the community. Arterioscler Thromb Vasc Biol 2008; 28:370–372.
- Wysowski DK, Nourjah P, Swartz L. Bleeding complications with warfarin use: a prevalent adverse effect resulting in regulatory action. Arch Intern Med 2007; 167:1414–1419.
- White HD, Gruber M, Feyzi J, et al. Comparison of outcomes among patients randomized to warfarin therapy according to anticoagulant control: results from SPORTIF III and V. Arch Intern Med 2007; 167:239–245.
- ACTIVE Writing Group of the ACTIVE Investigators; Connolly S, Pogue J, Hart R, et al. Clopidogrel plus aspirin versus oral anticoagulation for atrial fibrillation in the Atrial Fibrillation Clopidogrel Trial with Irbesartan for prevention of Vascular Events (ACTIVE W): a randomised controlled trial. Lancet 2006; 367:1903–1912.
- Geerts WH, Bergqvist D, Pineo GF, et al. Prevention of venous thromboembolism: American College of Chest Physicians evidence-based clinical practice guidelines (8th edition). Chest 2008; 133(6 suppl):381S–453S.
- Mungall D. BIBR-1048 Boehringer Ingelheim. Curr Opin Investig Drugs 2002; 3:905–907.
- Stangier J, Clemens A. Pharmacology, pharmacokinetics, and pharmacodynamics of dabigatran etexilate, an oral direct thrombin inhibitor. Clin Appl Thromb Hemost 2009; 15(suppl 1):9S–16S.
- Eisert WG, Hauel N, Stangier J, Wienen W, Clemens A, van Ryn J. Dabigatran: an oral novel potent reversible nonpeptide inhibitor of thrombin. Arterioscler Thromb Vasc Biol 2010; 30:1885–1889.
- Bounameaux H, Reber G. New oral antithrombotics: a need for laboratory monitoring. Against. J Thromb Haemost 2010; 8:627–630.
- van Ryn J, Stangier J, Haertter S, et al. Dabigatran etexilate—a novel, reversible, oral direct thrombin inhibitor: interpretation of coagulation assays and reversal of anticoagulant activity. Thromb Haemost 2010; 103:1116–1127.
- Eriksson BI, Dahl OE, Buller HR, et al. A new oral direct thrombin inhibitor, dabigatran etexilate, compared with enoxaparin for prevention of thromboembolic events following total hip or knee replacement: the BISTRO II randomized trial. J Thromb Haemost 2005; 3:103–111.
- Eriksson BI, Dahl OE, Rosencher N, et al. Dabigatran etexilate versus enoxaparin for prevention of venous thromboembolism after total hip replacement: a randomised, double-blind, non-inferiority trial. Lancet 2007; 370:949–956.
- Eriksson BI, Dahl OE, Rosencher N, et al. Oral dabigatran etexilate vs. subcutaneous enoxaparin for the prevention of venous thromboembolism after total knee replacement: the RE-MODEL randomized trial. J Thromb Haemost 2007; 5:2178–2185.
- Ezekowitz MD, Reilly PA, Nehmiz G, et al. Dabigatran with or without concomitant aspirin compared with warfarin alone in patients with nonvalvular atrial fibrillation (PETRO study). Am J Cardiol 2007; 100:1419–1426.
- Burger L. Bayer rival Boehringer prices blood pill at $6.75. Reuters, October 26, 2010. Available at http://www.reuters.com. Accessed September 12, 2011.
- Drugstore.com. Pradaxa. http://www.drugstore.com/pradaxa/bottle-60-150mg-capsules/qxn00597013554. Accessed September 10, 2011.
- Wal-Mart Stores, Inc. Retail Prescription Program Drug List. http://i.walmartimages.com/i/if/hmp/fusion/customer_list.pdf. Accessed September 10, 2011.
- Teachey DT. Dabigatran versus warfarin for venous thromboembolism (letter). N Engl J Med 2010; 362:1050; author reply1050–1051.
- Lancaster TR, Singer DE, Sheehan MA, et al. The impact of long-term warfarin therapy on quality of life. Evidence from a randomized trial. Boston Area Anticoagulation Trial for Atrial Fibrillation Investigators. Arch Intern Med 1991; 151:1944–1949.
- Freeman JV, Zhu RP, Owens DK, et al. Cost-effectiveness of dabigatran compared with warfarin for stroke prevention in atrial fibrillation. Ann Intern Med 2011; 154:1–11.
- EINSTEIN Investigators; Bauersachs R, Berkowitz SD, Brenner B, et al. Oral rivaroxaban for symptomatic venous thromboembolism. N Engl J Med 2010; 363:2499–2510.
- Cairns JA, Connolly S, McMurtry S, Stephenson M, Talajic M; CCS Atrial Fibrillation Guidelines Committee. Canadian Cardiovascular Society atrial fibrillation guidelines 2010: prevention of stroke and systemic embolization in atrial fibrillation and flutter. Can J Cardiol 2011; 27:74–90.
- Wann LS, Curtis AB, January CT, et al. 2011 ACCF/AHA/HRS focused update on the management of patients with atrial fibrillation (update on dabigatran): a Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 2011; 123:1144–1150.
- Boehringer Ingelheim. Pradaxa prescribing information. http://www.pradaxa.com. Accessed September 8, 2011.
- Huisman MV, Quinlan DJ, Dahl OE, Schulman S. Enoxaparin versus dabigatran or rivaroxaban for thromboprophylaxis after hip or knee arthroplasty: results of separate pooled analyses of phase III multicenter randomized trials. Circ Cardiovasc Qual Outcomes 2010; 3:652–660.
- McCullagh L, Tilson L, Walsh C, Barry M. A cost-effectiveness model comparing rivaroxaban and dabigatran etexilate with enoxaparin sodium as thromboprophylaxis after total hip and total knee replacement in the Irish healthcare setting. Pharmacoeconomics 2009; 27:829–846.
Dabigatran etexilate (Pradaxa) is a new oral anticoagulant that has distinct advantages over warfarin (Coumadin) in terms of its ease of administration, efficacy, and safety.
In the Randomized Evaluation of Long-Term Anticoagulation Therapy (RE-LY trial),1 in patients with nonvalvular atrial fibrillation, dabigatran 110 mg twice a day was found to be as good as warfarin in preventing systemic embolization and stroke (the primary outcome of the study), and at 150 mg twice a day it was superior.1 It has also shown efficacy in treating acute deep vein thrombosis and pulmonary embolism and in preventing these complications in orthopedic surgical patients.2–4
Dabigatran has been approved in 75 countries. It carries the trade name Pradaxa in Europe and the United States and Pradax in Canada. In October 2010, the US Food and Drug Administration (FDA) Cardiovascular and Renal Drugs Advisory Committee endorsed two twice-daily doses (75 mg and 150 mg) of dabigatran for the prevention of systemic embolization and stroke in patients with nonvalvular atrial fibrillation.
However, dabigatran is relatively expensive, and its current high cost might be a barrier to its wider use.
MANY PATIENTS NEED ANTICOAGULATION
Anticoagulation plays a vital role in the primary and secondary prevention of stroke in patients with atrial fibrillation and of pulmonary embolism in patients with venous thromboembolism. It is also used during cardiothoracic and vascular surgery, endovascular procedures, and dialysis and in patients with mechanical heart valves and hypercoagulable conditions.
Atrial fibrillation affects 3.03 million people in the United States (2005 figures), and this number is predicted to be as high as 7.56 million by 2050.5 More than 10% of people over the age of 80 years have it, and the lifetime risk of developing it is approximately 25%.6,7 Its most serious complication is ischemic stroke (the risk of which increases with age) and systemic embolization.5,8
Until the recent introduction of dabigatran, the only oral anticoagulant available in the United States for treating patients with atrial fibrillation was warfarin. Although warfarin has a number of disadvantages (see below), it is actually very effective for preventing ischemic stroke, reducing the incidence by as much as 65%.9,10
Venous thromboembolism is the third most common cardiovascular disorder after myocardial infarction and stroke.11 Although its exact incidence is unknown, nearly 1 million cases of it (incident or recurrent, fatal and nonfatal events) occur in the United States each year.12 Many patients with venous thromboembolism need oral anticoagulation long-term, and currently warfarin remains the only option for them as well.
NEEDED: A BETTER ANTICOAGULANT
Warfarin has been the most commonly prescribed oral anticoagulant in the United States for more than 60 years. As of 2004, more than 30 million outpatient prescriptions for it were filled annually in this country alone.13 However, warfarin has several important limitations.
Warfarin has a narrow therapeutic index. Patients taking it require monitoring of their international normalized ratio (INR) and frequent dose adjustments, and this is time-consuming and inconvenient. The target INR for patients with venous thromboembolism and atrial fibrillation is 2.0 to 3.0, whereas patients with a mechanical heart valve need a higher INR (2.5 to 3.5). If the INR is below these ranges, warfarin is less effective, with a risk of new thrombosis. On the other hand, if the INR is too high, there is a risk of bleeding.14 In fact, the most important side effect of warfarin is the risk of major and minor bleeding.13 However, even in well-designed clinical trials in which patients are closely managed, only 55% to 60% of patients regularly achieve their therapeutic target INR.1,2,14,15
Warfarin also interacts with many drugs and with some foods. Compliance is difficult. It has a slow onset of action. Genetic variations require dose adjustments. When switching from a parenteral anticoagulant, overlapping is required. Skin necrosis is a possible side effect. And warfarin is teratogenic.
Despite these limitations, the American College of Chest Physicians endorses warfarin to prevent or treat venous thromboembolism, and to prevent stroke in patients with atrial fibrillation.16
DABIGATRAN, A THROMBIN INHIBITOR
A prodrug, dabigatran is rapidly absorbed and converted to its active form. Its plasma concentration reaches a peak 1.5 to 3 hours after an oral dose, and it has an elimination half-life of 12 to 14 hours. About 80% of its excretion is by the kidneys and the remaining 20% is through bile.
Dabigatran is not metabolized by cytochrome P450 isoenzymes, and therefore it has few major interactions with other drugs. An exception is rifampin, a P-glycoprotein inducer that blocks dabigatran’s absorption in the gut, so this combination should be avoided. Another is quinidine, a strong P-glycoprotein inhibitor that is contraindicated for use with dabigatran. Also, amiodarone (Cordarone), another P-glycoprotein inhibitor, increases blood levels of dabigatran, and therefore a lower dose of dabigatran is recommended if these drugs are given together.18–20
DOES DABIGATRAN NEED MONITORING? CAN IT EVEN BE MONITORED?
Dabigatran has a predictable pharmacodynamic effect, and current data indicate it does not need regular monitoring.18–20 However, one may need to be able to measure the drug’s activity in certain situations, such as suspected overdose, bleeding, need for emergency surgery, impaired renal function, pregnancy, and obesity, and in children.20
Dabigatran has little effect on the prothrombin time or the INR, even at therapeutic concentrations.19 Further, its effect on the activated partial thromboplastin time (aPTT) is neither linear nor dose-dependent, and the aPTT reaches a plateau and becomes less sensitive at very high concentrations. Therefore, the aPTT does not appear to be an appropriate test to monitor dabigatran’s therapeutic anticoagulant effect, although it does provide a qualitative indication of anticoagulant activity.18,19
The thrombin time is a very sensitive method for determining if dabigatran is present, but the test lacks standardization; the ecarin clotting time provides better evidence of the dose but is not readily available at most institutions.18,19,21
EVALUATED IN CLINICAL TRIALS
DABIGATRAN IS EXPENSIVE BUT MAY BE COST-EFFECTIVE
The estimated price of dabigatran 150 mg twice a day in the United States is about $6.75 to $8.00 per day.26,27
Warfarin, in contrast, costs as little as $50 per year.28 However, this low price does not include the cost of monitoring the INR (office visits and laboratory testing), and these combined expenses are much higher than the price of the warfarin itself.29 In addition, warfarin requires time-consuming management when bridging to a parenteral anticoagulant (for reversal of its anticoagulant action) before routine health maintenance procedures such as dental work and colonoscopy and interventional procedures and surgery. Any bleeding complication will also add to its cost and will be associated with a decrease in the patient’s perceived health and quality of life, but this is true for both drugs.30
In today’s health care environment, controlling costs is a universal priority, but it may be unfair to compare the cost of dabigatran with that of warfarin alone. The expense and morbidity associated with stroke and intracranial bleeding are high, and if patients on dabigatran have fewer strokes (as seen in the RE-LY trial with dabigatran 150 mg twice a day) and no added expense of monitoring, then dabigatran may be cost-effective.
Freeman et al31 analyzed the cost-effectiveness of dabigatran, using an estimated cost of $13.70 per day and data from the RE-LY trial. They concluded that dabigatran may be a cost-effective alternative to warfarin in preventing ischemic stroke in patients considered at higher risk for ischemic stroke or intracranial hemorrhage, ie, those with a CHADS2 score of 1 or higher or equivalent. (The CHADS2 score is calculated as 1 point each for congestive heart failure, hypertension, age 75 or older, and diabetes mellitus; 2 points for prior stroke or transient ischemic attack.)
As more new-generation oral anticoagulants become available (see below), the price of dabigatran will undoubtedly decrease. Until then, warfarin will remain a cost-effective and cost-saving drug that cannot yet be considered obsolete.
WHO SHOULD RECEIVE DABIGATRAN?
The ideal patient for dabigatran treatment is not yet defined. The decision to convert a patient’s treatment from warfarin to dabigatran will likely depend on several factors, including the patient’s response to warfarin and the physician’s comfort with this new drug.
Many patients do extremely well with warfarin, requiring infrequent monitoring to maintain a therapeutic INR and having no bleeding complications. For them, it would be more practical to continue warfarin. Another reason for staying with warfarin would be if twice-a-day dosing would pose a problem.
Dabigatran would be a reasonable choice for a patient whose INR is erratic, who requires more frequent monitoring, for whom cost is not an issue, and for whom there is concern about dietary or drug interactions.
Another consideration is whether the patient has access to a health care facility for warfarin monitoring: this is difficult for those who cannot drive, who depend on others for transportation, and who live in rural areas.
Additionally, dabigatran may be a cost-effective alternative to warfarin for a patient with a high CHADS2 score who is considered at a higher risk for stroke.31
In all cases, the option should be considered only after an open discussion with the patient about the risks and benefits of this new drug.
WHO SHOULD NOT RECEIVE IT?
Dabigatran is a twice-daily drug with a short half-life. No patient with a history of poor compliance will be a good candidate for dabigatran. Since there are no practical laboratory tests for monitoring compliance, one will have to reinforce at every visit the importance of taking this medication according to instructions.
Patients with underlying kidney disease will need close monitoring of their creatinine clearance, with dose adjustment if renal function deteriorates.
Additionally, one should use caution when prescribing dabigatran to obese patients, pregnant women, or children until more is known about its use in these populations.
ADVANTAGES AND DISADVANTAGES OF DABIGATRAN
A reason may be that patients with atrial fibrillation and poor INR control have higher rates of death, stroke, myocardial infarction, and major bleeding.14 In most clinical trials, only 55% to 60% of patients achieve a therapeutic INR on warfarin, leaving them at risk of thrombosis or, conversely, bleeding.1,2,15,32 Dabigatran has predictable pharmacokinetics, and its twice-daily dosing allows for less variability in its anticoagulant effect, making it more consistently therapeutic with less potential for bleeding or thrombosis.1
The Canadian Cardiovascular Society included dabigatran in its 2010 guidelines on atrial fibrillation, recommending it or warfarin.33 The American College of Cardiology, the American Heart Association, and the Heart Rhythm Society now give dabigatran a class I B recommendation (benefit greater than risk, but limited populations studied) in secondary stroke prevention.34
On the other hand, major concerns are the lack of an antidote for dabigatran and a lack of experience in treating bleeding complications. Since dabigatran is not monitored, physicians may be uncertain if we are overdosing or undertreating. As we gain experience, we will learn how to treat bleeding complications. Until then, it will be important to anticipate this problem and to develop an algorithm based on the best available evidence in managing this complication.
Although the overall rates of bleeding in the RE-LY trial were lower with dabigatran than with warfarin, there were more gastrointestinal bleeding events with the 150-mg dose of dabigatran, which was not readily explained.
Further, the rate of dyspepsia was almost twice as high with dabigatran than with warfarin, regardless of the dose of dabigatran. There were also more dropouts in the 2nd year of follow-up in the dabigatran groups, with gastrointestinal intolerance being one of the major reasons. Therefore, dyspepsia may cause intolerance and noncompliance.1
Dabigatran must be taken twice a day and has a relatively short half-life. For a noncompliant patient, missing one or two doses will cause a reversal of its anticoagulation effect, leaving the patient susceptible to thrombosis. In comparison, warfarin has a longer half-life and is taken once a day, so missing a dose is less likely to result in a similar reversal of its anticoagulant effect.
SPECIAL CONDITIONS
Switching from other anticoagulants to dabigatran
When making the transition from a subcutaneously administered anticoagulant, ie, a low-molecular-weight heparin or the anti-Xa inhibitor fondaparinux (Arixtra), dabigatran should be started 0 to 2 hours before the next subcutaneous dose of the parenteral anticoagulant was to be given.21,35
When switching from unfractionated heparin given by continuous intravenous infusion, the first dose of dabigatran should be given at the time the infusion is stopped.
When switching from warfarin, dabigatran should be started once the patient’s INR is less than 2.0.
Switching from dabigatran to a parenteral anticoagulant
When switching from dabigatran back to a parenteral anticoagulant, allow 12 to 24 hours after the last dabigatran dose before starting the parenteral agent.21,35
Elective surgery or invasive procedures
The manufacturer recommends stopping dabigatran 1 to 2 days before elective surgery for patients who have normal renal function and a low risk of bleeding, or 3 to 5 days before surgery for patients who have a creatinine clearance of 50 mL/min or less. Before major surgery or placement of a spinal or epidural catheter, the manufacturer recommends that dabigatran be held even longer.35
If emergency surgery is needed
If emergency surgery is needed, the clinician must use his or her judgment as to the risks of bleeding vs those of postponing the surgery.21,35
Overdose or bleeding
No antidote for dabigatran is currently available. It has a short half-life (12–14 hours), and the treatment for overdose or bleeding is to discontinue it immediately, maintain adequate diuresis, and transfuse fresh-frozen plasma or red blood cells as indicated.
The role of activated charcoal given orally to reduce absorption is under evaluation, but the charcoal must be given within 1 to 2 hours after the overdose is taken.21
Dabigatran does not bind very much to plasma proteins and hence is dialyzable—an approach that may be necessary in cases of persistent or life-threatening bleeding.
Recombinant activated factor VII or prothrombin complex concentrates may be additional options in cases of severe bleeding.18,21
TOPICS OF FUTURE RESEARCH
A limitation of the dabigatran trials was that they did not enroll patients who had renal or liver impairment, cancer, or other comorbidities; pregnant women; or children. Other topics of future research include its use in patients weighing less than 48 kg or more than 110 kg, its efficacy in patients with thrombophilia, in patients with mechanical heart valves, and in long-term follow-up and the use of thrombolytics in patients with acute stroke who are on dabigatran.
WILL DABIGATRAN CHANGE CLINICAL PRACTICE?
Despite some of the challenges listed above, we believe that dabigatran is likely to change medical practice in patients requiring anticoagulation.
Dabigatran’s biggest use will most likely be in patients with atrial fibrillation, mainly because this is the largest group of people receiving anticoagulation. In addition, the incidence of atrial fibrillation rises with age, the US population is living longer, and patients generally require life-long anticoagulation once this condition develops.
Dabigatran may be approved for additional indications in the near future. It has already shown efficacy in primary and secondary prevention of venous thromboembolism. Other important areas to be studied include its use in patients with mechanical heart valves and thrombophilia.
Whether dabigatran will be a worthy substitute for the parenteral anticoagulants (heparin, low-molecular-weight heparins, or factor Xa inhibitors) is not yet known, but it will have an enormous impact on anticoagulation management if proved efficacious.
If dabigatran becomes a major substitute for warfarin, it will affect the anticoagulation clinics, with their well-trained staff, that are currently monitoring millions of patients in the United States. These clinics would no longer be needed, and laboratory and technical costs could be saved. A downside is that patients on dabigatran will not be as closely supervised and reminded to take their medication as patients on warfarin are now at these clinics. Instead, they will likely be supervised by their own physician (or assistants), who will need to become familiar with this anticoagulant. This may affect compliance with dabigatran.
OTHER NEW ORAL ANTICOAGULANTS ARE ON THE WAY
Other oral anticoagulants, including rivaroxaban (Xarelto) and apixaban (Eliquis), have been under study and show promise in preventing both thrombotic stroke and venous thromboembolism. They will likely compete with dabigatran once they are approved.
Rivaroxaban, an oral direct factor Xa inhibitor, is being investigated for stroke prevention in patients with atrial fibrillation. It has also been shown to be not inferior to (and to be less expensive than) enoxaparin in treating and preventing venous thromboembolism in patients undergoing hip or knee arthroplasty.32,36,37 Rivaroxaban has recently been approved by the FDA for this indication.
Apixaban, another direct factor Xa inhibitor, is also being studied for the prevention of stroke and systemic embolism in patients with nonvalvular atrial fibrillation. To date, there are no head-to-head trials comparing dabigatran with either of these new oral anticoagulants.
Dabigatran etexilate (Pradaxa) is a new oral anticoagulant that has distinct advantages over warfarin (Coumadin) in terms of its ease of administration, efficacy, and safety.
In the Randomized Evaluation of Long-Term Anticoagulation Therapy (RE-LY trial),1 in patients with nonvalvular atrial fibrillation, dabigatran 110 mg twice a day was found to be as good as warfarin in preventing systemic embolization and stroke (the primary outcome of the study), and at 150 mg twice a day it was superior.1 It has also shown efficacy in treating acute deep vein thrombosis and pulmonary embolism and in preventing these complications in orthopedic surgical patients.2–4
Dabigatran has been approved in 75 countries. It carries the trade name Pradaxa in Europe and the United States and Pradax in Canada. In October 2010, the US Food and Drug Administration (FDA) Cardiovascular and Renal Drugs Advisory Committee endorsed two twice-daily doses (75 mg and 150 mg) of dabigatran for the prevention of systemic embolization and stroke in patients with nonvalvular atrial fibrillation.
However, dabigatran is relatively expensive, and its current high cost might be a barrier to its wider use.
MANY PATIENTS NEED ANTICOAGULATION
Anticoagulation plays a vital role in the primary and secondary prevention of stroke in patients with atrial fibrillation and of pulmonary embolism in patients with venous thromboembolism. It is also used during cardiothoracic and vascular surgery, endovascular procedures, and dialysis and in patients with mechanical heart valves and hypercoagulable conditions.
Atrial fibrillation affects 3.03 million people in the United States (2005 figures), and this number is predicted to be as high as 7.56 million by 2050.5 More than 10% of people over the age of 80 years have it, and the lifetime risk of developing it is approximately 25%.6,7 Its most serious complication is ischemic stroke (the risk of which increases with age) and systemic embolization.5,8
Until the recent introduction of dabigatran, the only oral anticoagulant available in the United States for treating patients with atrial fibrillation was warfarin. Although warfarin has a number of disadvantages (see below), it is actually very effective for preventing ischemic stroke, reducing the incidence by as much as 65%.9,10
Venous thromboembolism is the third most common cardiovascular disorder after myocardial infarction and stroke.11 Although its exact incidence is unknown, nearly 1 million cases of it (incident or recurrent, fatal and nonfatal events) occur in the United States each year.12 Many patients with venous thromboembolism need oral anticoagulation long-term, and currently warfarin remains the only option for them as well.
NEEDED: A BETTER ANTICOAGULANT
Warfarin has been the most commonly prescribed oral anticoagulant in the United States for more than 60 years. As of 2004, more than 30 million outpatient prescriptions for it were filled annually in this country alone.13 However, warfarin has several important limitations.
Warfarin has a narrow therapeutic index. Patients taking it require monitoring of their international normalized ratio (INR) and frequent dose adjustments, and this is time-consuming and inconvenient. The target INR for patients with venous thromboembolism and atrial fibrillation is 2.0 to 3.0, whereas patients with a mechanical heart valve need a higher INR (2.5 to 3.5). If the INR is below these ranges, warfarin is less effective, with a risk of new thrombosis. On the other hand, if the INR is too high, there is a risk of bleeding.14 In fact, the most important side effect of warfarin is the risk of major and minor bleeding.13 However, even in well-designed clinical trials in which patients are closely managed, only 55% to 60% of patients regularly achieve their therapeutic target INR.1,2,14,15
Warfarin also interacts with many drugs and with some foods. Compliance is difficult. It has a slow onset of action. Genetic variations require dose adjustments. When switching from a parenteral anticoagulant, overlapping is required. Skin necrosis is a possible side effect. And warfarin is teratogenic.
Despite these limitations, the American College of Chest Physicians endorses warfarin to prevent or treat venous thromboembolism, and to prevent stroke in patients with atrial fibrillation.16
DABIGATRAN, A THROMBIN INHIBITOR
A prodrug, dabigatran is rapidly absorbed and converted to its active form. Its plasma concentration reaches a peak 1.5 to 3 hours after an oral dose, and it has an elimination half-life of 12 to 14 hours. About 80% of its excretion is by the kidneys and the remaining 20% is through bile.
Dabigatran is not metabolized by cytochrome P450 isoenzymes, and therefore it has few major interactions with other drugs. An exception is rifampin, a P-glycoprotein inducer that blocks dabigatran’s absorption in the gut, so this combination should be avoided. Another is quinidine, a strong P-glycoprotein inhibitor that is contraindicated for use with dabigatran. Also, amiodarone (Cordarone), another P-glycoprotein inhibitor, increases blood levels of dabigatran, and therefore a lower dose of dabigatran is recommended if these drugs are given together.18–20
DOES DABIGATRAN NEED MONITORING? CAN IT EVEN BE MONITORED?
Dabigatran has a predictable pharmacodynamic effect, and current data indicate it does not need regular monitoring.18–20 However, one may need to be able to measure the drug’s activity in certain situations, such as suspected overdose, bleeding, need for emergency surgery, impaired renal function, pregnancy, and obesity, and in children.20
Dabigatran has little effect on the prothrombin time or the INR, even at therapeutic concentrations.19 Further, its effect on the activated partial thromboplastin time (aPTT) is neither linear nor dose-dependent, and the aPTT reaches a plateau and becomes less sensitive at very high concentrations. Therefore, the aPTT does not appear to be an appropriate test to monitor dabigatran’s therapeutic anticoagulant effect, although it does provide a qualitative indication of anticoagulant activity.18,19
The thrombin time is a very sensitive method for determining if dabigatran is present, but the test lacks standardization; the ecarin clotting time provides better evidence of the dose but is not readily available at most institutions.18,19,21
EVALUATED IN CLINICAL TRIALS
DABIGATRAN IS EXPENSIVE BUT MAY BE COST-EFFECTIVE
The estimated price of dabigatran 150 mg twice a day in the United States is about $6.75 to $8.00 per day.26,27
Warfarin, in contrast, costs as little as $50 per year.28 However, this low price does not include the cost of monitoring the INR (office visits and laboratory testing), and these combined expenses are much higher than the price of the warfarin itself.29 In addition, warfarin requires time-consuming management when bridging to a parenteral anticoagulant (for reversal of its anticoagulant action) before routine health maintenance procedures such as dental work and colonoscopy and interventional procedures and surgery. Any bleeding complication will also add to its cost and will be associated with a decrease in the patient’s perceived health and quality of life, but this is true for both drugs.30
In today’s health care environment, controlling costs is a universal priority, but it may be unfair to compare the cost of dabigatran with that of warfarin alone. The expense and morbidity associated with stroke and intracranial bleeding are high, and if patients on dabigatran have fewer strokes (as seen in the RE-LY trial with dabigatran 150 mg twice a day) and no added expense of monitoring, then dabigatran may be cost-effective.
Freeman et al31 analyzed the cost-effectiveness of dabigatran, using an estimated cost of $13.70 per day and data from the RE-LY trial. They concluded that dabigatran may be a cost-effective alternative to warfarin in preventing ischemic stroke in patients considered at higher risk for ischemic stroke or intracranial hemorrhage, ie, those with a CHADS2 score of 1 or higher or equivalent. (The CHADS2 score is calculated as 1 point each for congestive heart failure, hypertension, age 75 or older, and diabetes mellitus; 2 points for prior stroke or transient ischemic attack.)
As more new-generation oral anticoagulants become available (see below), the price of dabigatran will undoubtedly decrease. Until then, warfarin will remain a cost-effective and cost-saving drug that cannot yet be considered obsolete.
WHO SHOULD RECEIVE DABIGATRAN?
The ideal patient for dabigatran treatment is not yet defined. The decision to convert a patient’s treatment from warfarin to dabigatran will likely depend on several factors, including the patient’s response to warfarin and the physician’s comfort with this new drug.
Many patients do extremely well with warfarin, requiring infrequent monitoring to maintain a therapeutic INR and having no bleeding complications. For them, it would be more practical to continue warfarin. Another reason for staying with warfarin would be if twice-a-day dosing would pose a problem.
Dabigatran would be a reasonable choice for a patient whose INR is erratic, who requires more frequent monitoring, for whom cost is not an issue, and for whom there is concern about dietary or drug interactions.
Another consideration is whether the patient has access to a health care facility for warfarin monitoring: this is difficult for those who cannot drive, who depend on others for transportation, and who live in rural areas.
Additionally, dabigatran may be a cost-effective alternative to warfarin for a patient with a high CHADS2 score who is considered at a higher risk for stroke.31
In all cases, the option should be considered only after an open discussion with the patient about the risks and benefits of this new drug.
WHO SHOULD NOT RECEIVE IT?
Dabigatran is a twice-daily drug with a short half-life. No patient with a history of poor compliance will be a good candidate for dabigatran. Since there are no practical laboratory tests for monitoring compliance, one will have to reinforce at every visit the importance of taking this medication according to instructions.
Patients with underlying kidney disease will need close monitoring of their creatinine clearance, with dose adjustment if renal function deteriorates.
Additionally, one should use caution when prescribing dabigatran to obese patients, pregnant women, or children until more is known about its use in these populations.
ADVANTAGES AND DISADVANTAGES OF DABIGATRAN
A reason may be that patients with atrial fibrillation and poor INR control have higher rates of death, stroke, myocardial infarction, and major bleeding.14 In most clinical trials, only 55% to 60% of patients achieve a therapeutic INR on warfarin, leaving them at risk of thrombosis or, conversely, bleeding.1,2,15,32 Dabigatran has predictable pharmacokinetics, and its twice-daily dosing allows for less variability in its anticoagulant effect, making it more consistently therapeutic with less potential for bleeding or thrombosis.1
The Canadian Cardiovascular Society included dabigatran in its 2010 guidelines on atrial fibrillation, recommending it or warfarin.33 The American College of Cardiology, the American Heart Association, and the Heart Rhythm Society now give dabigatran a class I B recommendation (benefit greater than risk, but limited populations studied) in secondary stroke prevention.34
On the other hand, major concerns are the lack of an antidote for dabigatran and a lack of experience in treating bleeding complications. Since dabigatran is not monitored, physicians may be uncertain if we are overdosing or undertreating. As we gain experience, we will learn how to treat bleeding complications. Until then, it will be important to anticipate this problem and to develop an algorithm based on the best available evidence in managing this complication.
Although the overall rates of bleeding in the RE-LY trial were lower with dabigatran than with warfarin, there were more gastrointestinal bleeding events with the 150-mg dose of dabigatran, which was not readily explained.
Further, the rate of dyspepsia was almost twice as high with dabigatran than with warfarin, regardless of the dose of dabigatran. There were also more dropouts in the 2nd year of follow-up in the dabigatran groups, with gastrointestinal intolerance being one of the major reasons. Therefore, dyspepsia may cause intolerance and noncompliance.1
Dabigatran must be taken twice a day and has a relatively short half-life. For a noncompliant patient, missing one or two doses will cause a reversal of its anticoagulation effect, leaving the patient susceptible to thrombosis. In comparison, warfarin has a longer half-life and is taken once a day, so missing a dose is less likely to result in a similar reversal of its anticoagulant effect.
SPECIAL CONDITIONS
Switching from other anticoagulants to dabigatran
When making the transition from a subcutaneously administered anticoagulant, ie, a low-molecular-weight heparin or the anti-Xa inhibitor fondaparinux (Arixtra), dabigatran should be started 0 to 2 hours before the next subcutaneous dose of the parenteral anticoagulant was to be given.21,35
When switching from unfractionated heparin given by continuous intravenous infusion, the first dose of dabigatran should be given at the time the infusion is stopped.
When switching from warfarin, dabigatran should be started once the patient’s INR is less than 2.0.
Switching from dabigatran to a parenteral anticoagulant
When switching from dabigatran back to a parenteral anticoagulant, allow 12 to 24 hours after the last dabigatran dose before starting the parenteral agent.21,35
Elective surgery or invasive procedures
The manufacturer recommends stopping dabigatran 1 to 2 days before elective surgery for patients who have normal renal function and a low risk of bleeding, or 3 to 5 days before surgery for patients who have a creatinine clearance of 50 mL/min or less. Before major surgery or placement of a spinal or epidural catheter, the manufacturer recommends that dabigatran be held even longer.35
If emergency surgery is needed
If emergency surgery is needed, the clinician must use his or her judgment as to the risks of bleeding vs those of postponing the surgery.21,35
Overdose or bleeding
No antidote for dabigatran is currently available. It has a short half-life (12–14 hours), and the treatment for overdose or bleeding is to discontinue it immediately, maintain adequate diuresis, and transfuse fresh-frozen plasma or red blood cells as indicated.
The role of activated charcoal given orally to reduce absorption is under evaluation, but the charcoal must be given within 1 to 2 hours after the overdose is taken.21
Dabigatran does not bind very much to plasma proteins and hence is dialyzable—an approach that may be necessary in cases of persistent or life-threatening bleeding.
Recombinant activated factor VII or prothrombin complex concentrates may be additional options in cases of severe bleeding.18,21
TOPICS OF FUTURE RESEARCH
A limitation of the dabigatran trials was that they did not enroll patients who had renal or liver impairment, cancer, or other comorbidities; pregnant women; or children. Other topics of future research include its use in patients weighing less than 48 kg or more than 110 kg, its efficacy in patients with thrombophilia, in patients with mechanical heart valves, and in long-term follow-up and the use of thrombolytics in patients with acute stroke who are on dabigatran.
WILL DABIGATRAN CHANGE CLINICAL PRACTICE?
Despite some of the challenges listed above, we believe that dabigatran is likely to change medical practice in patients requiring anticoagulation.
Dabigatran’s biggest use will most likely be in patients with atrial fibrillation, mainly because this is the largest group of people receiving anticoagulation. In addition, the incidence of atrial fibrillation rises with age, the US population is living longer, and patients generally require life-long anticoagulation once this condition develops.
Dabigatran may be approved for additional indications in the near future. It has already shown efficacy in primary and secondary prevention of venous thromboembolism. Other important areas to be studied include its use in patients with mechanical heart valves and thrombophilia.
Whether dabigatran will be a worthy substitute for the parenteral anticoagulants (heparin, low-molecular-weight heparins, or factor Xa inhibitors) is not yet known, but it will have an enormous impact on anticoagulation management if proved efficacious.
If dabigatran becomes a major substitute for warfarin, it will affect the anticoagulation clinics, with their well-trained staff, that are currently monitoring millions of patients in the United States. These clinics would no longer be needed, and laboratory and technical costs could be saved. A downside is that patients on dabigatran will not be as closely supervised and reminded to take their medication as patients on warfarin are now at these clinics. Instead, they will likely be supervised by their own physician (or assistants), who will need to become familiar with this anticoagulant. This may affect compliance with dabigatran.
OTHER NEW ORAL ANTICOAGULANTS ARE ON THE WAY
Other oral anticoagulants, including rivaroxaban (Xarelto) and apixaban (Eliquis), have been under study and show promise in preventing both thrombotic stroke and venous thromboembolism. They will likely compete with dabigatran once they are approved.
Rivaroxaban, an oral direct factor Xa inhibitor, is being investigated for stroke prevention in patients with atrial fibrillation. It has also been shown to be not inferior to (and to be less expensive than) enoxaparin in treating and preventing venous thromboembolism in patients undergoing hip or knee arthroplasty.32,36,37 Rivaroxaban has recently been approved by the FDA for this indication.
Apixaban, another direct factor Xa inhibitor, is also being studied for the prevention of stroke and systemic embolism in patients with nonvalvular atrial fibrillation. To date, there are no head-to-head trials comparing dabigatran with either of these new oral anticoagulants.
- Connolly SJ, Ezekowitz MD, Yusuf S, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med 2009; 361:1139–1151.
- Schulman S, Kearon C, Kakkar AK, et al. Dabigatran versus warfarin in the treatment of acute venous thromboembolism. N Engl J Med 2009; 361:2342–2352.
- RE-MOBILIZE Writing Committee; Ginsberg JS, Davidson BL, Comp PC, et al. Oral thrombin inhibitor dabigatran etexilate vs North American enoxaparin regimen for prevention of venous thromboembolism after knee arthroplasty surgery. J Arthroplasty 2009; 24:1–9.
- Wolowacz SE, Roskell NS, Plumb JM, Caprini JA, Eriksson BI. Efficacy and safety of dabigatran etexilate for the prevention of venous thromboembolism following total hip or knee arthroplasty. A meta-analysis. Thromb Haemost 2009; 101:77–85.
- Naccarelli GV, Varker H, Lin J, Schulman KL. Increasing prevalence of atrial fibrillation and flutter in the United States. Am J Cardiol 2009; 104:1534–1539.
- Krahn AD, Manfreda J, Tate RB, Mathewson FA, Cuddy TE. The natural history of atrial fibrillation: incidence, risk factors, and prognosis in the Manitoba follow-up study. Am J Med 1995; 98:476–484.
- Lloyd-Jones DM, Wang TJ, Leip EP, et al. Lifetime risk for development of atrial fibrillation: the Framingham Heart Study. Circulation 2004; 110:1042–1046.
- Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham study. Stroke 1991; 22:983–988.
- Go AS, Hylek EM, Chang Y, et al. Anticoagulation therapy for stroke prevention in atrial fibrillation: how well do randomized trials translate into clinical practice? JAMA 2003; 290:2685–2692.
- Singer DE, Chang Y, Fang MC, et al. The net clinical benefit of warfarin anticoagulation in atrial fibrillation. Ann Intern Med 2009; 151:297–305.
- Goldhaber SZ. Pulmonary embolism thrombolysis: a clarion call for international collaboration. J Am Coll Cardiol 1992; 19:246–247.
- Heit JA. The epidemiology of venous thromboembolism in the community. Arterioscler Thromb Vasc Biol 2008; 28:370–372.
- Wysowski DK, Nourjah P, Swartz L. Bleeding complications with warfarin use: a prevalent adverse effect resulting in regulatory action. Arch Intern Med 2007; 167:1414–1419.
- White HD, Gruber M, Feyzi J, et al. Comparison of outcomes among patients randomized to warfarin therapy according to anticoagulant control: results from SPORTIF III and V. Arch Intern Med 2007; 167:239–245.
- ACTIVE Writing Group of the ACTIVE Investigators; Connolly S, Pogue J, Hart R, et al. Clopidogrel plus aspirin versus oral anticoagulation for atrial fibrillation in the Atrial Fibrillation Clopidogrel Trial with Irbesartan for prevention of Vascular Events (ACTIVE W): a randomised controlled trial. Lancet 2006; 367:1903–1912.
- Geerts WH, Bergqvist D, Pineo GF, et al. Prevention of venous thromboembolism: American College of Chest Physicians evidence-based clinical practice guidelines (8th edition). Chest 2008; 133(6 suppl):381S–453S.
- Mungall D. BIBR-1048 Boehringer Ingelheim. Curr Opin Investig Drugs 2002; 3:905–907.
- Stangier J, Clemens A. Pharmacology, pharmacokinetics, and pharmacodynamics of dabigatran etexilate, an oral direct thrombin inhibitor. Clin Appl Thromb Hemost 2009; 15(suppl 1):9S–16S.
- Eisert WG, Hauel N, Stangier J, Wienen W, Clemens A, van Ryn J. Dabigatran: an oral novel potent reversible nonpeptide inhibitor of thrombin. Arterioscler Thromb Vasc Biol 2010; 30:1885–1889.
- Bounameaux H, Reber G. New oral antithrombotics: a need for laboratory monitoring. Against. J Thromb Haemost 2010; 8:627–630.
- van Ryn J, Stangier J, Haertter S, et al. Dabigatran etexilate—a novel, reversible, oral direct thrombin inhibitor: interpretation of coagulation assays and reversal of anticoagulant activity. Thromb Haemost 2010; 103:1116–1127.
- Eriksson BI, Dahl OE, Buller HR, et al. A new oral direct thrombin inhibitor, dabigatran etexilate, compared with enoxaparin for prevention of thromboembolic events following total hip or knee replacement: the BISTRO II randomized trial. J Thromb Haemost 2005; 3:103–111.
- Eriksson BI, Dahl OE, Rosencher N, et al. Dabigatran etexilate versus enoxaparin for prevention of venous thromboembolism after total hip replacement: a randomised, double-blind, non-inferiority trial. Lancet 2007; 370:949–956.
- Eriksson BI, Dahl OE, Rosencher N, et al. Oral dabigatran etexilate vs. subcutaneous enoxaparin for the prevention of venous thromboembolism after total knee replacement: the RE-MODEL randomized trial. J Thromb Haemost 2007; 5:2178–2185.
- Ezekowitz MD, Reilly PA, Nehmiz G, et al. Dabigatran with or without concomitant aspirin compared with warfarin alone in patients with nonvalvular atrial fibrillation (PETRO study). Am J Cardiol 2007; 100:1419–1426.
- Burger L. Bayer rival Boehringer prices blood pill at $6.75. Reuters, October 26, 2010. Available at http://www.reuters.com. Accessed September 12, 2011.
- Drugstore.com. Pradaxa. http://www.drugstore.com/pradaxa/bottle-60-150mg-capsules/qxn00597013554. Accessed September 10, 2011.
- Wal-Mart Stores, Inc. Retail Prescription Program Drug List. http://i.walmartimages.com/i/if/hmp/fusion/customer_list.pdf. Accessed September 10, 2011.
- Teachey DT. Dabigatran versus warfarin for venous thromboembolism (letter). N Engl J Med 2010; 362:1050; author reply1050–1051.
- Lancaster TR, Singer DE, Sheehan MA, et al. The impact of long-term warfarin therapy on quality of life. Evidence from a randomized trial. Boston Area Anticoagulation Trial for Atrial Fibrillation Investigators. Arch Intern Med 1991; 151:1944–1949.
- Freeman JV, Zhu RP, Owens DK, et al. Cost-effectiveness of dabigatran compared with warfarin for stroke prevention in atrial fibrillation. Ann Intern Med 2011; 154:1–11.
- EINSTEIN Investigators; Bauersachs R, Berkowitz SD, Brenner B, et al. Oral rivaroxaban for symptomatic venous thromboembolism. N Engl J Med 2010; 363:2499–2510.
- Cairns JA, Connolly S, McMurtry S, Stephenson M, Talajic M; CCS Atrial Fibrillation Guidelines Committee. Canadian Cardiovascular Society atrial fibrillation guidelines 2010: prevention of stroke and systemic embolization in atrial fibrillation and flutter. Can J Cardiol 2011; 27:74–90.
- Wann LS, Curtis AB, January CT, et al. 2011 ACCF/AHA/HRS focused update on the management of patients with atrial fibrillation (update on dabigatran): a Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 2011; 123:1144–1150.
- Boehringer Ingelheim. Pradaxa prescribing information. http://www.pradaxa.com. Accessed September 8, 2011.
- Huisman MV, Quinlan DJ, Dahl OE, Schulman S. Enoxaparin versus dabigatran or rivaroxaban for thromboprophylaxis after hip or knee arthroplasty: results of separate pooled analyses of phase III multicenter randomized trials. Circ Cardiovasc Qual Outcomes 2010; 3:652–660.
- McCullagh L, Tilson L, Walsh C, Barry M. A cost-effectiveness model comparing rivaroxaban and dabigatran etexilate with enoxaparin sodium as thromboprophylaxis after total hip and total knee replacement in the Irish healthcare setting. Pharmacoeconomics 2009; 27:829–846.
- Connolly SJ, Ezekowitz MD, Yusuf S, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med 2009; 361:1139–1151.
- Schulman S, Kearon C, Kakkar AK, et al. Dabigatran versus warfarin in the treatment of acute venous thromboembolism. N Engl J Med 2009; 361:2342–2352.
- RE-MOBILIZE Writing Committee; Ginsberg JS, Davidson BL, Comp PC, et al. Oral thrombin inhibitor dabigatran etexilate vs North American enoxaparin regimen for prevention of venous thromboembolism after knee arthroplasty surgery. J Arthroplasty 2009; 24:1–9.
- Wolowacz SE, Roskell NS, Plumb JM, Caprini JA, Eriksson BI. Efficacy and safety of dabigatran etexilate for the prevention of venous thromboembolism following total hip or knee arthroplasty. A meta-analysis. Thromb Haemost 2009; 101:77–85.
- Naccarelli GV, Varker H, Lin J, Schulman KL. Increasing prevalence of atrial fibrillation and flutter in the United States. Am J Cardiol 2009; 104:1534–1539.
- Krahn AD, Manfreda J, Tate RB, Mathewson FA, Cuddy TE. The natural history of atrial fibrillation: incidence, risk factors, and prognosis in the Manitoba follow-up study. Am J Med 1995; 98:476–484.
- Lloyd-Jones DM, Wang TJ, Leip EP, et al. Lifetime risk for development of atrial fibrillation: the Framingham Heart Study. Circulation 2004; 110:1042–1046.
- Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham study. Stroke 1991; 22:983–988.
- Go AS, Hylek EM, Chang Y, et al. Anticoagulation therapy for stroke prevention in atrial fibrillation: how well do randomized trials translate into clinical practice? JAMA 2003; 290:2685–2692.
- Singer DE, Chang Y, Fang MC, et al. The net clinical benefit of warfarin anticoagulation in atrial fibrillation. Ann Intern Med 2009; 151:297–305.
- Goldhaber SZ. Pulmonary embolism thrombolysis: a clarion call for international collaboration. J Am Coll Cardiol 1992; 19:246–247.
- Heit JA. The epidemiology of venous thromboembolism in the community. Arterioscler Thromb Vasc Biol 2008; 28:370–372.
- Wysowski DK, Nourjah P, Swartz L. Bleeding complications with warfarin use: a prevalent adverse effect resulting in regulatory action. Arch Intern Med 2007; 167:1414–1419.
- White HD, Gruber M, Feyzi J, et al. Comparison of outcomes among patients randomized to warfarin therapy according to anticoagulant control: results from SPORTIF III and V. Arch Intern Med 2007; 167:239–245.
- ACTIVE Writing Group of the ACTIVE Investigators; Connolly S, Pogue J, Hart R, et al. Clopidogrel plus aspirin versus oral anticoagulation for atrial fibrillation in the Atrial Fibrillation Clopidogrel Trial with Irbesartan for prevention of Vascular Events (ACTIVE W): a randomised controlled trial. Lancet 2006; 367:1903–1912.
- Geerts WH, Bergqvist D, Pineo GF, et al. Prevention of venous thromboembolism: American College of Chest Physicians evidence-based clinical practice guidelines (8th edition). Chest 2008; 133(6 suppl):381S–453S.
- Mungall D. BIBR-1048 Boehringer Ingelheim. Curr Opin Investig Drugs 2002; 3:905–907.
- Stangier J, Clemens A. Pharmacology, pharmacokinetics, and pharmacodynamics of dabigatran etexilate, an oral direct thrombin inhibitor. Clin Appl Thromb Hemost 2009; 15(suppl 1):9S–16S.
- Eisert WG, Hauel N, Stangier J, Wienen W, Clemens A, van Ryn J. Dabigatran: an oral novel potent reversible nonpeptide inhibitor of thrombin. Arterioscler Thromb Vasc Biol 2010; 30:1885–1889.
- Bounameaux H, Reber G. New oral antithrombotics: a need for laboratory monitoring. Against. J Thromb Haemost 2010; 8:627–630.
- van Ryn J, Stangier J, Haertter S, et al. Dabigatran etexilate—a novel, reversible, oral direct thrombin inhibitor: interpretation of coagulation assays and reversal of anticoagulant activity. Thromb Haemost 2010; 103:1116–1127.
- Eriksson BI, Dahl OE, Buller HR, et al. A new oral direct thrombin inhibitor, dabigatran etexilate, compared with enoxaparin for prevention of thromboembolic events following total hip or knee replacement: the BISTRO II randomized trial. J Thromb Haemost 2005; 3:103–111.
- Eriksson BI, Dahl OE, Rosencher N, et al. Dabigatran etexilate versus enoxaparin for prevention of venous thromboembolism after total hip replacement: a randomised, double-blind, non-inferiority trial. Lancet 2007; 370:949–956.
- Eriksson BI, Dahl OE, Rosencher N, et al. Oral dabigatran etexilate vs. subcutaneous enoxaparin for the prevention of venous thromboembolism after total knee replacement: the RE-MODEL randomized trial. J Thromb Haemost 2007; 5:2178–2185.
- Ezekowitz MD, Reilly PA, Nehmiz G, et al. Dabigatran with or without concomitant aspirin compared with warfarin alone in patients with nonvalvular atrial fibrillation (PETRO study). Am J Cardiol 2007; 100:1419–1426.
- Burger L. Bayer rival Boehringer prices blood pill at $6.75. Reuters, October 26, 2010. Available at http://www.reuters.com. Accessed September 12, 2011.
- Drugstore.com. Pradaxa. http://www.drugstore.com/pradaxa/bottle-60-150mg-capsules/qxn00597013554. Accessed September 10, 2011.
- Wal-Mart Stores, Inc. Retail Prescription Program Drug List. http://i.walmartimages.com/i/if/hmp/fusion/customer_list.pdf. Accessed September 10, 2011.
- Teachey DT. Dabigatran versus warfarin for venous thromboembolism (letter). N Engl J Med 2010; 362:1050; author reply1050–1051.
- Lancaster TR, Singer DE, Sheehan MA, et al. The impact of long-term warfarin therapy on quality of life. Evidence from a randomized trial. Boston Area Anticoagulation Trial for Atrial Fibrillation Investigators. Arch Intern Med 1991; 151:1944–1949.
- Freeman JV, Zhu RP, Owens DK, et al. Cost-effectiveness of dabigatran compared with warfarin for stroke prevention in atrial fibrillation. Ann Intern Med 2011; 154:1–11.
- EINSTEIN Investigators; Bauersachs R, Berkowitz SD, Brenner B, et al. Oral rivaroxaban for symptomatic venous thromboembolism. N Engl J Med 2010; 363:2499–2510.
- Cairns JA, Connolly S, McMurtry S, Stephenson M, Talajic M; CCS Atrial Fibrillation Guidelines Committee. Canadian Cardiovascular Society atrial fibrillation guidelines 2010: prevention of stroke and systemic embolization in atrial fibrillation and flutter. Can J Cardiol 2011; 27:74–90.
- Wann LS, Curtis AB, January CT, et al. 2011 ACCF/AHA/HRS focused update on the management of patients with atrial fibrillation (update on dabigatran): a Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 2011; 123:1144–1150.
- Boehringer Ingelheim. Pradaxa prescribing information. http://www.pradaxa.com. Accessed September 8, 2011.
- Huisman MV, Quinlan DJ, Dahl OE, Schulman S. Enoxaparin versus dabigatran or rivaroxaban for thromboprophylaxis after hip or knee arthroplasty: results of separate pooled analyses of phase III multicenter randomized trials. Circ Cardiovasc Qual Outcomes 2010; 3:652–660.
- McCullagh L, Tilson L, Walsh C, Barry M. A cost-effectiveness model comparing rivaroxaban and dabigatran etexilate with enoxaparin sodium as thromboprophylaxis after total hip and total knee replacement in the Irish healthcare setting. Pharmacoeconomics 2009; 27:829–846.
KEY POINTS
- Dabigatran is a potent, reversible, direct thrombin inhibitor. Available only in oral form, it has a rapid onset of action, a predictable anticoagulant response, and few major interactions.
- Dabigatran does not require dose adjustments (except for renal insufficiency) or monitoring of its effect during treatment.
- In trials in patients with nonvalvular atrial fibrillation, two different doses of dabigatran were compared with warfarin. Less bleeding occurred with the lower dose than with warfarin, while the higher dose was more effective than warfarin in preventing stroke and systemic embolization.
- The American College of Cardiology, the American Heart Association, and the Heart Rhythm Society have given dabigatran a class I B recommendation for secondary stroke prevention in patients with nonvalvular atrial fibrillation.