User login
As of 2015, an estimated 30.2 million adults in the United States—12.2% of the population— had diabetes mellitus (DM). During that year, approximately 1.5 million new cases (6.7 cases for every 1000 people) were diagnosed in adults (≥ 18 years of age).1
As the number of people with DM increases, so will the number of cases of diabetic retinopathy, the main cause of new cases of blindness in adults in the United States2 and the leading cause of blindness among US working-age (20 to 74 years) adults.3 It is estimated that 4.1 million Americans have diabetic retinopathy3; it is projected that prevalence will reach 6 million this year.4
Blindness related to DM costs the United States approximately $500 million each year,5 including health care utilization: physician office visits, diagnostic testing, medication and other treatments, and hospitalization.6 Impairment of vision also results in social isolation, dependence on others to perform daily functions, and a decline in physical activity.
Several professional organizations, including the American Diabetes Association and the American Academy of Ophthalmology, have developed practice guidelines for diabetic retinopathy screening. Guidelines notwithstanding, only about 55% of people with DM in the United States receive the recommended dilated eye examination at established intervals.2,3 In addition to screening by an ophthalmologist or optometrist, adherence to clinical guidelines for risk assessment, prevention, and early referral helps reduce the incidence and severity of retinopathy.5
This article describes how to assess the risk of diabetic retinopathy in your patients, details the crucial role that you, the primary care physician, can play in prevention, and emphasizes the importance of referral to an eye specialist for screening, evaluation, treatment (when indicated), and follow-up.
Pathophysiology and classification
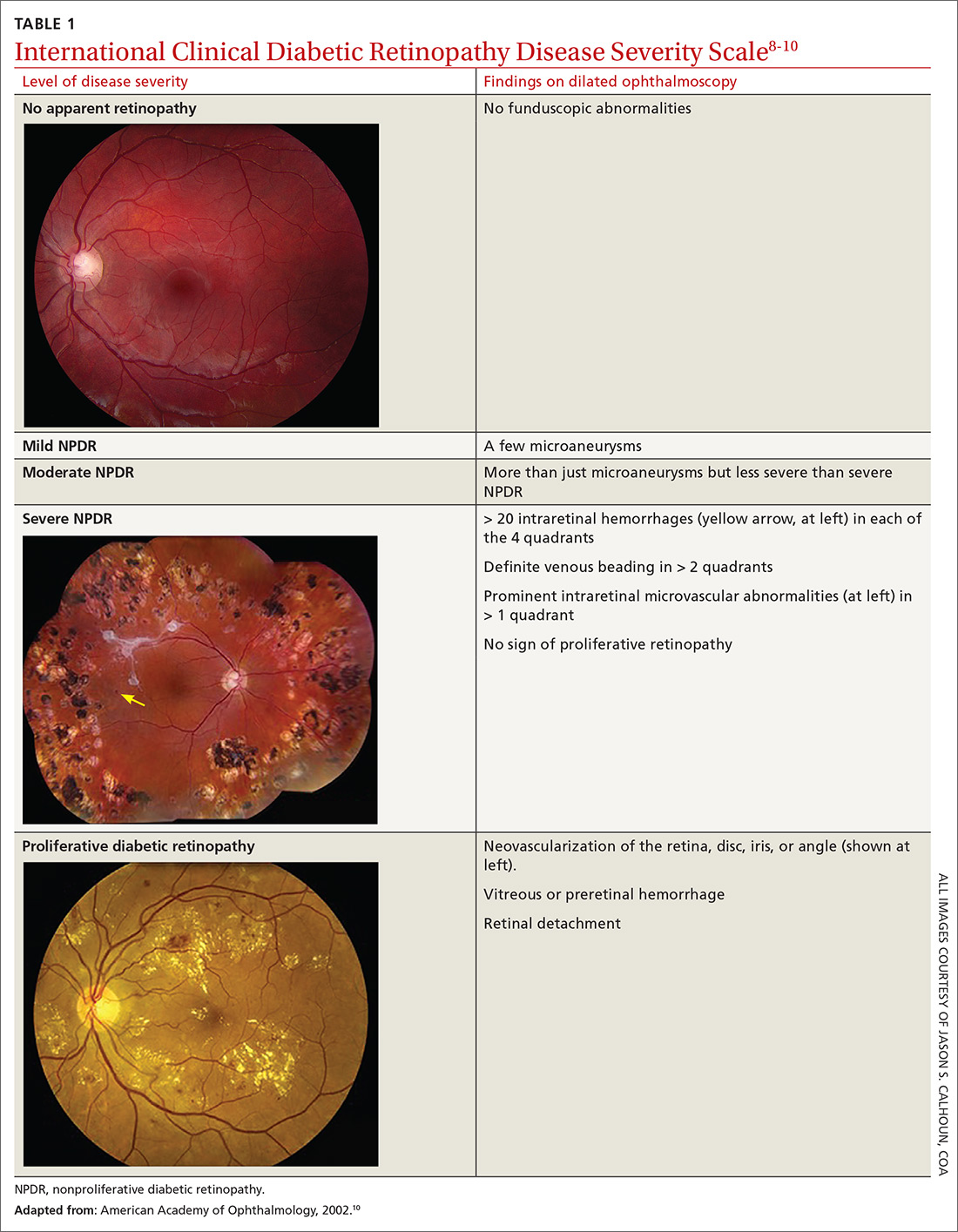
Diabetic retinopathy, the result of progressive blood vessel damage to the retina, has 2 major forms: nonproliferative and proliferative. Those forms are distinguished by the absence or presence of new growth of blood vessels (retinal neovascularization).3,7 To improve communication and coordination among physicians who care for patients with DM worldwide, the International Clinical Diabetic Retinopathy Disease Severity Scale for diabetic retinopathy was developed,8-10 comprising 5 levels of severity that are based on findings on dilated ophthalmoscopy (Table 18-10):
- Level 1. No apparent retinopathy. Funduscopic abnormalities are absent.
- Level 2. Mild nonproliferative diabetic retinopathy (NPDR). Only a few microaneurysms are seen.
- Level 3: Moderate NPDR. Characterized by microaneurysms and by intraretinal hemorrhage and venous beading, but less severe than what is seen in Level 4.
- Level 4. Severe NPDR. More than 20 intraretinal hemorrhages in each quadrant of the retina, definite venous beading in > 2 quadrants, intraretinal microvascular abnormalities in > 1 quadrant, or any combination of these findings.
- Level 5. Proliferative diabetic retinopathy. Characterized by neovascularization of the disc, retina, iris, or angle; vitreous hemorrhage; retinal detachment; or any combination of these findings. Further classified as “mild,” “moderate,” or “severe” if macular edema is present; severity is dependent on the distance of thickening and exudates from the center of the macula.9
Be attentive to risk factors
There are several risk factors for diabetic retinopathy, including duration of disease, type 1 DM, male gender, black race (non-Hispanic), elevated hemoglobin A1C(HbA1C) level, elevated systolic and diastolic blood pressure (BP), and insulin therapy. 4,5,11,12
Continue to: Time since diagnosis
Time since diagnosis. The Wisconsin Epidemiologic Study of Diabetic Retinopathy found that the prevalence of diabetic retinopathy varied from 28.8% in people who had DM for < 5 years to 77.8% in people who had DM for ≥ 15 years. The rate of proliferative diabetic retinopathy was 2% in people who had DM for < 5 years and 15.5% in those who had DM for ≥ 15 years.11
Demographic variables. The prevalence of diabetic retinopathy is higher in men, non-Hispanic blacks (38.8%), and people with type 1 DM.4,5,11-13 The Veterans Affairs Diabetes Trial found a higher prevalence of moderate-to-severe diabetic retinopathy in Hispanics (36%) and African Americans (29%) than in non-Hispanic whites (22%).14
Among people with DM who have diabetic retinopathy, systolic and diastolic BP and the HbA1C level tend to be higher. They are more likely to use insulin to control disease.4,5,13 In a recent cross-sectional analysis, the prevalence of vision-threatening retinopathy was higher among people ≥ 65 years of age (1%; 95% confidence interval [CI], 0.7%-1.5%) than among people 40 to 64 years of age (0.4%; 95% CI, 0.3%-0.7%) (P = .009).5
Does pregnancy exacerbate retinopathy? Controversy surrounds the role of pregnancy in the development and progression of diabetic retinopathy. The Diabetes Control and Complications Trial found a short-term increase in the level of retinopathy during pregnancy that persisted into the first postpartum year. A 1.63-fold greater risk of any deterioration of retinopathy was observed in women who received intensive DM treatment from before to during pregnancy (P < .05); pregnant women who received conventional treatment had a 2.48-fold greater risk than nonpregnant women with DM who received conventional treatment (P < .001).
Deterioration of retinopathy during pregnancy had no long-term consequences, however, regardless of type of treatment.15 More importantly, in most cases, changes in the level of retinopathy revert to the pre-pregnancy level after 1 year or longer, and pregnancy does not appear to affect long-term progression of retinopathy.15
Continue to: Proven primary prevention strategies
Proven primary prevention strategies
Glycemic control. Optimal glycemic control is an essential component of prevention of diabetic retinopathy. From 1983 to 1993, the Diabetes Control and Complications Trial randomized 1441 patients with type 1 DM to receive intensive therapy (median HbA1C level, 7.2%) or conventional therapy (median HbA1C level, 9.1%). During a mean of 6 years of follow-up, intensive therapy reduced the adjusted mean risk of retinopathy by 76% (95% CI, 62%-85%).16,17 A 2007 systematic review of 44 studies of the treatment of diabetic retinopathy found that strict glycemic control was beneficial in reducing the incidence and progression of retinopathy.17
The American Diabetes Association’s Standards of Medical Care in Diabetes—2019 Abridged for Primary Care Providers recommends that most nonpregnant adults maintain an HbA1Clevel < 7%. For patients with a history of hypoglycemia, limited life expectancy, advanced microvascular or macrovascular disease, other significant comorbid conditions, or longstanding DM in which it is difficult to achieve the optimal goal, a higher HbA1clevel (< 8%) might be appropriate.18
Control of BP. Strict control of BP is a major modifier of the incidence and progression of diabetic retinopathy.17,19 In the United Kingdom Prospective Diabetes Study, 1148 patients with type 2 DM and a mean BP of 160/94 mm Hg at the onset of the study were randomly assigned to either (1) a “tight” blood pressure group (< 150/85 mm Hg) or (2) a “less-tight” group (< 180/105 mm Hg). The primary therapy for controlling BP was captopril or atenolol. After 9 years of follow-up, the tight-control group had a 34% mean reduction in risk in the percentage of patients with deterioration of retinopathy (99% CI, 11%-50%; P = .0004) and a 47% reduction in risk (99% CI, 7%-70%; P = .004) of deterioration in visual acuity.20
Most patients with DM and hypertension should be treated to maintain a BP < 140/90 mm Hg. Although there is insufficient evidence to recommend a specific antihypertensive agent for preventing diabetic retinopathy, therapy should include agents from drug classes that have a demonstrated reduction in cardiovascular events in patients with DM. These include angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, thiazide diuretics, and dihydropyridine calcium channel blockers.18
Lipid management. The benefit of targeted therapy for lowering lipids for the prevention of diabetic retinopathy is not well established.17 In the Collaborative Atorvastatin Diabetes Study, 2838 patients with type 2 DM were randomized to atorvastatin (10 mg) or placebo; microvascular endpoint analysis demonstrated that patients taking atorvastatin needed less laser therapy (P = .14); however, progression of diabetic retinopathy was not reduced.21 Similarly, in the Action to Control Cardiovascular Risk in Diabetes Eye Study, slowing of progression to retinopathy was observed in patients with type 2 DM who were treated with fenofibrate (ie, progression in 6.5%, compared with progression in 10.2% of untreated subjects [odds ratio = 0.60 (95% CI, 0.42-0.87); P = .0056]).22
Continue to: Despite limited data on...
Despite limited data on the impact of lipid-lowering agents on patients with diabetic retinopathy, those with type 2 DM (especially) and those who have, or are at risk of, atherosclerotic cardiovascular disease should receive statin therapy.18
Aspirin therapy. Aspirin has not been found to be beneficial for slowing progression of diabetic retinopathy. However, aspirin did not cause further deterioration of disease, specifically in patients with vitreous hemorrhages4; patients with diabetic retinopathy who require aspirin therapy for other medical reasons can therefore continue to take it without increasing the risk of damage to the retina.4,18
When should you refer patients for screening?
Screening for diabetic retinopathy is important because affected patients can be asymptomatic but have significant disease. Early detection also helps determine which patients need treatment when it is most beneficial: early in its course.4
Type 1 DM. Retinopathy can become apparent as early as 6 or 7 years after the onset of disease, and is rare in children prior to puberty.4,11 As a result, patients with type 1 DM should first be screened with a comprehensive eye examination by an ophthalmologist or optometrist within 5 years of DM onset.4,18
Type 2 DM. Because of the insidious onset of type 2 DM, patients who are given a diagnosis of DM after 30 years of age might already have high-risk features of retinopathy.9 In patients with type 2 DM, therefore, initial screening for diabetic retinopathy should begin at the time of diagnosis and include a comprehensive eye examination by an ophthalmologist or optometrist.4,18,23
Continue to: Components of the exam
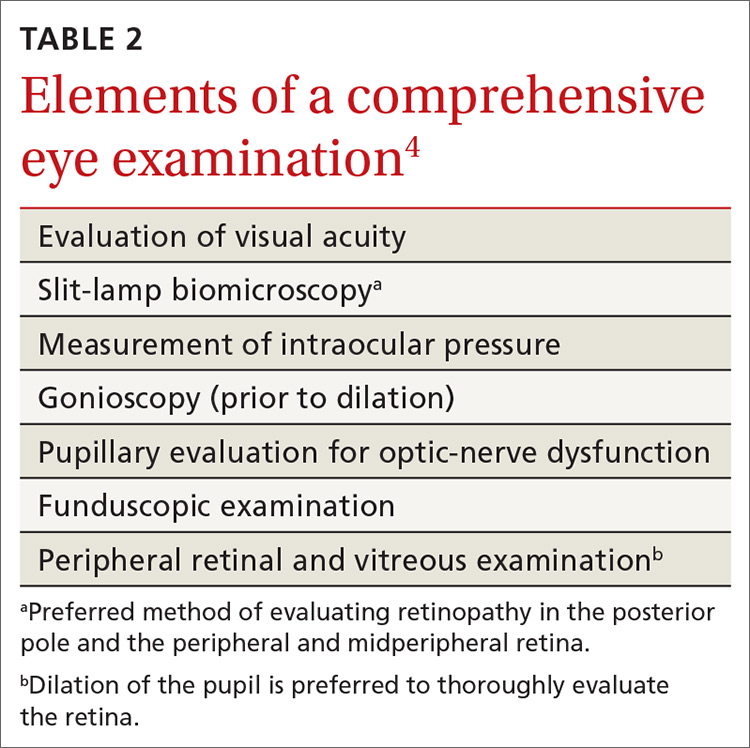
Components of the exam. Initial evaluation by the ophthalmologist or optometrist should include a detailed history and comprehensive eye exam with pupil dilation. Table 24 lists elements of the initial physical exam, which should assess for features that often lead to visual impairment. These features include macular edema, retinal hemorrhage, venous beading, neovascularization, and vitreous hemorrhage.4
Frequency of follow-up. The interval between subsequent examinations should be individualized, based on the findings of the initial assessment. Consider that:
- Screening should occur every 1 or 2 years in patients without evidence of retinopathy and with adequate glycemic control.4,18,23
- Screening every 1 or 2 years appears to be cost-effective in patients who have had 1 or more normal eye exams.
- A 3-year screening interval does not appear to present a risk in well-controlled patients with type 2 DM.24
- Women with type 1 or type 2 DM who are planning pregnancy or who are pregnant should have an eye exam prior to pregnancy or early in the first trimester.4,18,23 They should then be monitored each trimester and at the end of the first postpartum year, depending on the severity of retinopathy.18
Alternative screening modalities
Seven-field stereoscopic fundus photography is an alternative screening tool that compares favorably to ophthalmoscopy when performed by an experienced ophthalmologist, optometrist, or ophthalmologic technician.25 Nonmydriatic digital stereoscopic retinal imaging has been shown to be a cost-effective method of screening patients for diabetic retinopathy.26 In a study that compared digital imaging with dilated funduscopic examination, investigators reported that, of 311 eyes evaluated, there was agreement between the methods in 86% of cases. Disagreement was mostly related to the greater frequency of finding mild-to-moderate NPDR when using digital imaging.27
Screening in primary care
Programs that use telemedicine-based fundus photography to screen for diabetic retinopathy during primary care visits, followed by remote interpretation by an ophthalmologist, have been shown to increase the rate of retinal screening by offering an option other than direct referral to an ophthalmologist or optometrist.28 However, telemedicine-based retinal photography can be successful as a screening tool for retinopathy only if timely referral to an eye specialist is arranged when indicated by findings.18
SIDEBAR
Key points in the progression of diabetic retinopathy care
Duration of diabetes, poor glycemic control, and uncontrolled hypertension are major risk factors for diabetic retinopathy.
To reduce the risk of diabetic retinopathy, patients with diabetes mellitus should:
- sustain good glycemic control (hemoglobin A1C level, < 7%)
- maintain blood pressure < 140/90 mm Hg
- undergo periodic routine screening eye examination.
Early detection of diabetic retinopathy by dilated eye examination or fundus photography can lead to early therapeutic intervention, which can reduce the risk of visual impairment and vision loss.
Treatment is based on severity of disease and can include anti-vascular-endothelial growth factor therapy, photocoagulation, or surgery.
What therapy will your referred patients receive?
Patients found to have signs of diabetic retinopathy should be referred to an ophthalmologist who is knowledgeable and experienced in the management of diabetic retinopathy. Care will be managed according to the severity of the patient’s diabetic retinopathy.
Continue to: Patients with mild-to-moderate NPDR but without macular edema
Patients with mild-to-moderate NPDR but without macular edema. Treatment is generally not recommended. Patients should be reevaluated every 6 to 12 months because they have an increased risk of progression.5
Patients with mild-to-moderate NPDR and clinically significant macular edema (CSME). It is important for the eye specialist to assess for edema at the center of the macula because the risk of vision loss and need for treatment is greater when the center is involved. Vascular–endothelial growth factor (VEGF) is an important mediator of neovascularization and macular edema in diabetic retinopathy. For patients with center-involving CSME, intravitreous injection of an anti-VEGF agent provides significant benefit and is first-line treatment in these cases.4,29
The Early Treatment for Diabetic Retinopathy Study evaluated the efficacy of focal photocoagulation, a painless laser therapy, for CSME and demonstrated that this modality reduces the risk of moderate visual loss; increases the likelihood of improvement in vision; and decreases the frequency of persistent macular edema.30 Focal photocoagulation has been found effective in both non-center-involving CSME and center-involving CSME.5
Patients with severe NPDR. The recommendation is to initiate full panretinal photocoagulation prior to progression to proliferative diabetic retinopathy PDR. Researchers noted a 50% reduction in vision loss and vitrectomy when patients with type 2 DM were treated with panretinal photocoagulation early, compared with those in whom treatment was deferred until PDR developed.4,31 The role of anti-VEGF treatment of severe NPDR is under investigation.4
Patients with high-risk and severe PDR. Panretinal photocoagulation is the recommended treatment for patients with high-risk and severe PDR, and usually induces regression of retinal neovascularization. In patients with CSME and high-risk PDR, the combination of anti-VEGF therapy and panretinal photocoagulation should be considered. Vitrectomy should be considered for patients who have failed panretinal photocoagulation or are not amenable to photocoagulation.4
CORRESPONDENCE
Bryan Farford, DO, Mayo Clinic, 4500 San Pablo Road, Jacksonville, FL 32224; Farford.Bryan@mayo.edu.
1. National Diabetes Statistic Report 2020: Estimates of Diabetes and Its Burden in the United States. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention. www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf. Accessed March 20, 2020.
2. Fitch K, Weisman T, Engel T, et al. Longitudinal commercial claims-based cost analysis of diabetic retinopathy screening patterns. Am Health Drug Benefits. 2015;8:300-308.
3. Centers for Disease Control and Prevention. Common eye disorders. September 29, 2015. www.cdc.gov/visionhealth/basics/ced/index.html. Accessed March 20, 2020.
4. American Academy of Ophthalmology PPP Retina/Vitreous Committee, Hoskins Center for Quality Eye Care. Diabetic Retinopathy PPP 2019. San Francisco, CA: American Academy of Ophthalmology. October 2019. https://www.aao.org/preferred-practice-pattern/diabetic-retinopathy-ppp. Accessed March 20, 2020.
5. Zhang X, Saaddine JB, Chou C-F, et al. Prevalence of diabetic retinopathy in the United States, 2005-2008. JAMA. 2010;304:649-656.
6. Stewart MW. Socioeconomic cost of diabetic retinopathy and therapy. In: Diabetic Retinopathy. Singapore: Adis; 2017:257-268.
7. Tarr JM, Kaul K, Chopra M, et al. Pathophysiology of diabetic retinopathy. ISRN Ophthalmol. 2013;2013:343560.
8. Wilkinson CP, Ferris FL 3rd, Klein RE, et al. Proposed International Clinical Diabetic Retinopathy and Diabetic Macular Edema Disease Severity Scales. Ophthalmology. 2003;110:1677-1682.
9. Wu L, Fernandez-Loaiza P, Sauma J, et al. Classification of diabetic retinopathy and diabetic macular edema. World J Diabetes. 2013;4:290-294.
10. American Academy of Ophthalmology. International Clinical Diabetic Retinopathy Disease Severity Scale detailed table. October 2002. http://www.icoph.org/downloads/Diabetic-Retinopathy-Detail.pdf. Accessed March 20, 2020.
11. Klein R, Klein BE, Moss SE, et al. The Wisconsin Epidemiologic Study of Diabetic Retinopathy. Arch Ophthalmol. 1984;102:527-532.
12. Klein R, Klein BE, Moss SE, et al. The Wisconsin Epidemiologic Study of Diabetic Retinopathy. Ten-year incidence and progression of diabetic retinopathy. Arch Ophthalmol. 1994;112:1217-1228.
13. Klein R, Knudtson MD, Lee KE, et al. The Wisconsin Epidemiologic Study of Diabetic Retinopathy XXII. The twenty-five-year progression of retinopathy in persons with type 1 diabetes. Ophthalmology. 2008;115:1859-1868.
14. Emanuele N, Sacks J, Klein R, et al. Ethnicity, race, and baseline retinopathy correlates in the Veterans Affairs Diabetes Trial. Diabetes Care. 2005;28:1954-1958.
15. Effect of pregnancy on microvascular complications in the diabetes control and complications trial. The Diabetes Control and Complications Trial Research Group. Diabetes Care. 2000;23:1084-1091.
16. , , The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977-986.
17. Mohamed Q, Gillies MC, Wong TY. Management of diabetic retinopathy: a systematic review. JAMA. 2007;298:902-916.
18. American Diabetes Association. Standards of Medical Care in Diabetes—2019 abridged for primary care providers. Clin Diabetes. 2019;37:11-34.
19. Do DV, Wang X, Vedula SS, et al. Blood pressure control for diabetic retinopathy. Cochrane Database Syst Rev. 2015;(1):CD006127.
20. UK Prospective Diabetes Study Group. Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. UK Prospective Diabetes Study Group. BMJ. 1998;317:703-713.
21. Colhoun HM, Betteridge DJ, Durrington PN; CARDS Investigators. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomised placebo-controlled trial. Lancet. 2004;364:685-696.
22. Chew EY, Davis MD, Danis RP, et al. Action to Control Cardiovascular Risk in Diabetes Eye Study Research Group. The effects of medical management on the progression of diabetic retinopathy in persons with type 2 diabetes: The Action to Control Cardiovascular Risk in Diabetes (ACCORD) Eye Study. Ophthalmology. 2014;121:2443-2451.
23. Fong DS, Aiello L, Gardner TW, et al American Diabetes Association. Retinopathy in diabetes. Diabetes Care. 2004;27(suppl 1):S84-S87.
24. 11. Microvascular complications and foot care: Standards of Medical Care in Diabetes—2019. Diabetes Care. 2019;42(suppl 1):S124-S138.
25. Moss SE, Klein R, Kessler SD, et al. Comparison between ophthalmoscopy and fundus photography in determining severity of diabetic retinopathy. Ophthalmology. 1985;92:62-67.
26. Kirkizlar E, Serban N, Sisson JA, et al. Evaluation of telemedicine for screening of diabetic Retinopathy in the Veterans Health Administration. Ophthalmology. 2013;120:2604-2610.
27. Ahmed J, Ward TP, Bursell S-E, et al. The sensitivity and specificity of nonmydriatic digital stereoscopic retinal imaging in detecting diabetic retinopathy. Diabetes Care. 2006;29:2205-2209.
28. Taylor CR, Merin LM, Salunga AM, et al. Improving diabetic retinopathy screening ratios using telemedicine-based digital retinal imaging technology: the Vine Hill study. Diabetes Care. 2007;30:574.
29. , , , Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema. N Engl J Med. 2015;372:1193-1203.
30. Early photocoagulation for diabetic retinopathy. ETDRS Report Number 9. Early Treatment Diabetic Retinopathy Study Research Group. Ophthalmology. 1991;98(5 suppl):766-785.
31. Ferris F. Early photocoagulation in patients with either type I or type II diabetes. Trans Am Ophthalmol Soc. 1996;94:505-537.
As of 2015, an estimated 30.2 million adults in the United States—12.2% of the population— had diabetes mellitus (DM). During that year, approximately 1.5 million new cases (6.7 cases for every 1000 people) were diagnosed in adults (≥ 18 years of age).1
As the number of people with DM increases, so will the number of cases of diabetic retinopathy, the main cause of new cases of blindness in adults in the United States2 and the leading cause of blindness among US working-age (20 to 74 years) adults.3 It is estimated that 4.1 million Americans have diabetic retinopathy3; it is projected that prevalence will reach 6 million this year.4
Blindness related to DM costs the United States approximately $500 million each year,5 including health care utilization: physician office visits, diagnostic testing, medication and other treatments, and hospitalization.6 Impairment of vision also results in social isolation, dependence on others to perform daily functions, and a decline in physical activity.
Several professional organizations, including the American Diabetes Association and the American Academy of Ophthalmology, have developed practice guidelines for diabetic retinopathy screening. Guidelines notwithstanding, only about 55% of people with DM in the United States receive the recommended dilated eye examination at established intervals.2,3 In addition to screening by an ophthalmologist or optometrist, adherence to clinical guidelines for risk assessment, prevention, and early referral helps reduce the incidence and severity of retinopathy.5
This article describes how to assess the risk of diabetic retinopathy in your patients, details the crucial role that you, the primary care physician, can play in prevention, and emphasizes the importance of referral to an eye specialist for screening, evaluation, treatment (when indicated), and follow-up.
Pathophysiology and classification
Diabetic retinopathy, the result of progressive blood vessel damage to the retina, has 2 major forms: nonproliferative and proliferative. Those forms are distinguished by the absence or presence of new growth of blood vessels (retinal neovascularization).3,7 To improve communication and coordination among physicians who care for patients with DM worldwide, the International Clinical Diabetic Retinopathy Disease Severity Scale for diabetic retinopathy was developed,8-10 comprising 5 levels of severity that are based on findings on dilated ophthalmoscopy (Table 18-10):
- Level 1. No apparent retinopathy. Funduscopic abnormalities are absent.
- Level 2. Mild nonproliferative diabetic retinopathy (NPDR). Only a few microaneurysms are seen.
- Level 3: Moderate NPDR. Characterized by microaneurysms and by intraretinal hemorrhage and venous beading, but less severe than what is seen in Level 4.
- Level 4. Severe NPDR. More than 20 intraretinal hemorrhages in each quadrant of the retina, definite venous beading in > 2 quadrants, intraretinal microvascular abnormalities in > 1 quadrant, or any combination of these findings.
- Level 5. Proliferative diabetic retinopathy. Characterized by neovascularization of the disc, retina, iris, or angle; vitreous hemorrhage; retinal detachment; or any combination of these findings. Further classified as “mild,” “moderate,” or “severe” if macular edema is present; severity is dependent on the distance of thickening and exudates from the center of the macula.9
Be attentive to risk factors
There are several risk factors for diabetic retinopathy, including duration of disease, type 1 DM, male gender, black race (non-Hispanic), elevated hemoglobin A1C(HbA1C) level, elevated systolic and diastolic blood pressure (BP), and insulin therapy. 4,5,11,12
Continue to: Time since diagnosis
Time since diagnosis. The Wisconsin Epidemiologic Study of Diabetic Retinopathy found that the prevalence of diabetic retinopathy varied from 28.8% in people who had DM for < 5 years to 77.8% in people who had DM for ≥ 15 years. The rate of proliferative diabetic retinopathy was 2% in people who had DM for < 5 years and 15.5% in those who had DM for ≥ 15 years.11
Demographic variables. The prevalence of diabetic retinopathy is higher in men, non-Hispanic blacks (38.8%), and people with type 1 DM.4,5,11-13 The Veterans Affairs Diabetes Trial found a higher prevalence of moderate-to-severe diabetic retinopathy in Hispanics (36%) and African Americans (29%) than in non-Hispanic whites (22%).14
Among people with DM who have diabetic retinopathy, systolic and diastolic BP and the HbA1C level tend to be higher. They are more likely to use insulin to control disease.4,5,13 In a recent cross-sectional analysis, the prevalence of vision-threatening retinopathy was higher among people ≥ 65 years of age (1%; 95% confidence interval [CI], 0.7%-1.5%) than among people 40 to 64 years of age (0.4%; 95% CI, 0.3%-0.7%) (P = .009).5
Does pregnancy exacerbate retinopathy? Controversy surrounds the role of pregnancy in the development and progression of diabetic retinopathy. The Diabetes Control and Complications Trial found a short-term increase in the level of retinopathy during pregnancy that persisted into the first postpartum year. A 1.63-fold greater risk of any deterioration of retinopathy was observed in women who received intensive DM treatment from before to during pregnancy (P < .05); pregnant women who received conventional treatment had a 2.48-fold greater risk than nonpregnant women with DM who received conventional treatment (P < .001).
Deterioration of retinopathy during pregnancy had no long-term consequences, however, regardless of type of treatment.15 More importantly, in most cases, changes in the level of retinopathy revert to the pre-pregnancy level after 1 year or longer, and pregnancy does not appear to affect long-term progression of retinopathy.15
Continue to: Proven primary prevention strategies
Proven primary prevention strategies
Glycemic control. Optimal glycemic control is an essential component of prevention of diabetic retinopathy. From 1983 to 1993, the Diabetes Control and Complications Trial randomized 1441 patients with type 1 DM to receive intensive therapy (median HbA1C level, 7.2%) or conventional therapy (median HbA1C level, 9.1%). During a mean of 6 years of follow-up, intensive therapy reduced the adjusted mean risk of retinopathy by 76% (95% CI, 62%-85%).16,17 A 2007 systematic review of 44 studies of the treatment of diabetic retinopathy found that strict glycemic control was beneficial in reducing the incidence and progression of retinopathy.17
The American Diabetes Association’s Standards of Medical Care in Diabetes—2019 Abridged for Primary Care Providers recommends that most nonpregnant adults maintain an HbA1Clevel < 7%. For patients with a history of hypoglycemia, limited life expectancy, advanced microvascular or macrovascular disease, other significant comorbid conditions, or longstanding DM in which it is difficult to achieve the optimal goal, a higher HbA1clevel (< 8%) might be appropriate.18
Control of BP. Strict control of BP is a major modifier of the incidence and progression of diabetic retinopathy.17,19 In the United Kingdom Prospective Diabetes Study, 1148 patients with type 2 DM and a mean BP of 160/94 mm Hg at the onset of the study were randomly assigned to either (1) a “tight” blood pressure group (< 150/85 mm Hg) or (2) a “less-tight” group (< 180/105 mm Hg). The primary therapy for controlling BP was captopril or atenolol. After 9 years of follow-up, the tight-control group had a 34% mean reduction in risk in the percentage of patients with deterioration of retinopathy (99% CI, 11%-50%; P = .0004) and a 47% reduction in risk (99% CI, 7%-70%; P = .004) of deterioration in visual acuity.20
Most patients with DM and hypertension should be treated to maintain a BP < 140/90 mm Hg. Although there is insufficient evidence to recommend a specific antihypertensive agent for preventing diabetic retinopathy, therapy should include agents from drug classes that have a demonstrated reduction in cardiovascular events in patients with DM. These include angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, thiazide diuretics, and dihydropyridine calcium channel blockers.18
Lipid management. The benefit of targeted therapy for lowering lipids for the prevention of diabetic retinopathy is not well established.17 In the Collaborative Atorvastatin Diabetes Study, 2838 patients with type 2 DM were randomized to atorvastatin (10 mg) or placebo; microvascular endpoint analysis demonstrated that patients taking atorvastatin needed less laser therapy (P = .14); however, progression of diabetic retinopathy was not reduced.21 Similarly, in the Action to Control Cardiovascular Risk in Diabetes Eye Study, slowing of progression to retinopathy was observed in patients with type 2 DM who were treated with fenofibrate (ie, progression in 6.5%, compared with progression in 10.2% of untreated subjects [odds ratio = 0.60 (95% CI, 0.42-0.87); P = .0056]).22
Continue to: Despite limited data on...
Despite limited data on the impact of lipid-lowering agents on patients with diabetic retinopathy, those with type 2 DM (especially) and those who have, or are at risk of, atherosclerotic cardiovascular disease should receive statin therapy.18
Aspirin therapy. Aspirin has not been found to be beneficial for slowing progression of diabetic retinopathy. However, aspirin did not cause further deterioration of disease, specifically in patients with vitreous hemorrhages4; patients with diabetic retinopathy who require aspirin therapy for other medical reasons can therefore continue to take it without increasing the risk of damage to the retina.4,18
When should you refer patients for screening?
Screening for diabetic retinopathy is important because affected patients can be asymptomatic but have significant disease. Early detection also helps determine which patients need treatment when it is most beneficial: early in its course.4
Type 1 DM. Retinopathy can become apparent as early as 6 or 7 years after the onset of disease, and is rare in children prior to puberty.4,11 As a result, patients with type 1 DM should first be screened with a comprehensive eye examination by an ophthalmologist or optometrist within 5 years of DM onset.4,18
Type 2 DM. Because of the insidious onset of type 2 DM, patients who are given a diagnosis of DM after 30 years of age might already have high-risk features of retinopathy.9 In patients with type 2 DM, therefore, initial screening for diabetic retinopathy should begin at the time of diagnosis and include a comprehensive eye examination by an ophthalmologist or optometrist.4,18,23
Continue to: Components of the exam
Components of the exam. Initial evaluation by the ophthalmologist or optometrist should include a detailed history and comprehensive eye exam with pupil dilation. Table 24 lists elements of the initial physical exam, which should assess for features that often lead to visual impairment. These features include macular edema, retinal hemorrhage, venous beading, neovascularization, and vitreous hemorrhage.4
Frequency of follow-up. The interval between subsequent examinations should be individualized, based on the findings of the initial assessment. Consider that:
- Screening should occur every 1 or 2 years in patients without evidence of retinopathy and with adequate glycemic control.4,18,23
- Screening every 1 or 2 years appears to be cost-effective in patients who have had 1 or more normal eye exams.
- A 3-year screening interval does not appear to present a risk in well-controlled patients with type 2 DM.24
- Women with type 1 or type 2 DM who are planning pregnancy or who are pregnant should have an eye exam prior to pregnancy or early in the first trimester.4,18,23 They should then be monitored each trimester and at the end of the first postpartum year, depending on the severity of retinopathy.18
Alternative screening modalities
Seven-field stereoscopic fundus photography is an alternative screening tool that compares favorably to ophthalmoscopy when performed by an experienced ophthalmologist, optometrist, or ophthalmologic technician.25 Nonmydriatic digital stereoscopic retinal imaging has been shown to be a cost-effective method of screening patients for diabetic retinopathy.26 In a study that compared digital imaging with dilated funduscopic examination, investigators reported that, of 311 eyes evaluated, there was agreement between the methods in 86% of cases. Disagreement was mostly related to the greater frequency of finding mild-to-moderate NPDR when using digital imaging.27
Screening in primary care
Programs that use telemedicine-based fundus photography to screen for diabetic retinopathy during primary care visits, followed by remote interpretation by an ophthalmologist, have been shown to increase the rate of retinal screening by offering an option other than direct referral to an ophthalmologist or optometrist.28 However, telemedicine-based retinal photography can be successful as a screening tool for retinopathy only if timely referral to an eye specialist is arranged when indicated by findings.18
SIDEBAR
Key points in the progression of diabetic retinopathy care
Duration of diabetes, poor glycemic control, and uncontrolled hypertension are major risk factors for diabetic retinopathy.
To reduce the risk of diabetic retinopathy, patients with diabetes mellitus should:
- sustain good glycemic control (hemoglobin A1C level, < 7%)
- maintain blood pressure < 140/90 mm Hg
- undergo periodic routine screening eye examination.
Early detection of diabetic retinopathy by dilated eye examination or fundus photography can lead to early therapeutic intervention, which can reduce the risk of visual impairment and vision loss.
Treatment is based on severity of disease and can include anti-vascular-endothelial growth factor therapy, photocoagulation, or surgery.
What therapy will your referred patients receive?
Patients found to have signs of diabetic retinopathy should be referred to an ophthalmologist who is knowledgeable and experienced in the management of diabetic retinopathy. Care will be managed according to the severity of the patient’s diabetic retinopathy.
Continue to: Patients with mild-to-moderate NPDR but without macular edema
Patients with mild-to-moderate NPDR but without macular edema. Treatment is generally not recommended. Patients should be reevaluated every 6 to 12 months because they have an increased risk of progression.5
Patients with mild-to-moderate NPDR and clinically significant macular edema (CSME). It is important for the eye specialist to assess for edema at the center of the macula because the risk of vision loss and need for treatment is greater when the center is involved. Vascular–endothelial growth factor (VEGF) is an important mediator of neovascularization and macular edema in diabetic retinopathy. For patients with center-involving CSME, intravitreous injection of an anti-VEGF agent provides significant benefit and is first-line treatment in these cases.4,29
The Early Treatment for Diabetic Retinopathy Study evaluated the efficacy of focal photocoagulation, a painless laser therapy, for CSME and demonstrated that this modality reduces the risk of moderate visual loss; increases the likelihood of improvement in vision; and decreases the frequency of persistent macular edema.30 Focal photocoagulation has been found effective in both non-center-involving CSME and center-involving CSME.5
Patients with severe NPDR. The recommendation is to initiate full panretinal photocoagulation prior to progression to proliferative diabetic retinopathy PDR. Researchers noted a 50% reduction in vision loss and vitrectomy when patients with type 2 DM were treated with panretinal photocoagulation early, compared with those in whom treatment was deferred until PDR developed.4,31 The role of anti-VEGF treatment of severe NPDR is under investigation.4
Patients with high-risk and severe PDR. Panretinal photocoagulation is the recommended treatment for patients with high-risk and severe PDR, and usually induces regression of retinal neovascularization. In patients with CSME and high-risk PDR, the combination of anti-VEGF therapy and panretinal photocoagulation should be considered. Vitrectomy should be considered for patients who have failed panretinal photocoagulation or are not amenable to photocoagulation.4
CORRESPONDENCE
Bryan Farford, DO, Mayo Clinic, 4500 San Pablo Road, Jacksonville, FL 32224; Farford.Bryan@mayo.edu.
As of 2015, an estimated 30.2 million adults in the United States—12.2% of the population— had diabetes mellitus (DM). During that year, approximately 1.5 million new cases (6.7 cases for every 1000 people) were diagnosed in adults (≥ 18 years of age).1
As the number of people with DM increases, so will the number of cases of diabetic retinopathy, the main cause of new cases of blindness in adults in the United States2 and the leading cause of blindness among US working-age (20 to 74 years) adults.3 It is estimated that 4.1 million Americans have diabetic retinopathy3; it is projected that prevalence will reach 6 million this year.4
Blindness related to DM costs the United States approximately $500 million each year,5 including health care utilization: physician office visits, diagnostic testing, medication and other treatments, and hospitalization.6 Impairment of vision also results in social isolation, dependence on others to perform daily functions, and a decline in physical activity.
Several professional organizations, including the American Diabetes Association and the American Academy of Ophthalmology, have developed practice guidelines for diabetic retinopathy screening. Guidelines notwithstanding, only about 55% of people with DM in the United States receive the recommended dilated eye examination at established intervals.2,3 In addition to screening by an ophthalmologist or optometrist, adherence to clinical guidelines for risk assessment, prevention, and early referral helps reduce the incidence and severity of retinopathy.5
This article describes how to assess the risk of diabetic retinopathy in your patients, details the crucial role that you, the primary care physician, can play in prevention, and emphasizes the importance of referral to an eye specialist for screening, evaluation, treatment (when indicated), and follow-up.
Pathophysiology and classification
Diabetic retinopathy, the result of progressive blood vessel damage to the retina, has 2 major forms: nonproliferative and proliferative. Those forms are distinguished by the absence or presence of new growth of blood vessels (retinal neovascularization).3,7 To improve communication and coordination among physicians who care for patients with DM worldwide, the International Clinical Diabetic Retinopathy Disease Severity Scale for diabetic retinopathy was developed,8-10 comprising 5 levels of severity that are based on findings on dilated ophthalmoscopy (Table 18-10):
- Level 1. No apparent retinopathy. Funduscopic abnormalities are absent.
- Level 2. Mild nonproliferative diabetic retinopathy (NPDR). Only a few microaneurysms are seen.
- Level 3: Moderate NPDR. Characterized by microaneurysms and by intraretinal hemorrhage and venous beading, but less severe than what is seen in Level 4.
- Level 4. Severe NPDR. More than 20 intraretinal hemorrhages in each quadrant of the retina, definite venous beading in > 2 quadrants, intraretinal microvascular abnormalities in > 1 quadrant, or any combination of these findings.
- Level 5. Proliferative diabetic retinopathy. Characterized by neovascularization of the disc, retina, iris, or angle; vitreous hemorrhage; retinal detachment; or any combination of these findings. Further classified as “mild,” “moderate,” or “severe” if macular edema is present; severity is dependent on the distance of thickening and exudates from the center of the macula.9
Be attentive to risk factors
There are several risk factors for diabetic retinopathy, including duration of disease, type 1 DM, male gender, black race (non-Hispanic), elevated hemoglobin A1C(HbA1C) level, elevated systolic and diastolic blood pressure (BP), and insulin therapy. 4,5,11,12
Continue to: Time since diagnosis
Time since diagnosis. The Wisconsin Epidemiologic Study of Diabetic Retinopathy found that the prevalence of diabetic retinopathy varied from 28.8% in people who had DM for < 5 years to 77.8% in people who had DM for ≥ 15 years. The rate of proliferative diabetic retinopathy was 2% in people who had DM for < 5 years and 15.5% in those who had DM for ≥ 15 years.11
Demographic variables. The prevalence of diabetic retinopathy is higher in men, non-Hispanic blacks (38.8%), and people with type 1 DM.4,5,11-13 The Veterans Affairs Diabetes Trial found a higher prevalence of moderate-to-severe diabetic retinopathy in Hispanics (36%) and African Americans (29%) than in non-Hispanic whites (22%).14
Among people with DM who have diabetic retinopathy, systolic and diastolic BP and the HbA1C level tend to be higher. They are more likely to use insulin to control disease.4,5,13 In a recent cross-sectional analysis, the prevalence of vision-threatening retinopathy was higher among people ≥ 65 years of age (1%; 95% confidence interval [CI], 0.7%-1.5%) than among people 40 to 64 years of age (0.4%; 95% CI, 0.3%-0.7%) (P = .009).5
Does pregnancy exacerbate retinopathy? Controversy surrounds the role of pregnancy in the development and progression of diabetic retinopathy. The Diabetes Control and Complications Trial found a short-term increase in the level of retinopathy during pregnancy that persisted into the first postpartum year. A 1.63-fold greater risk of any deterioration of retinopathy was observed in women who received intensive DM treatment from before to during pregnancy (P < .05); pregnant women who received conventional treatment had a 2.48-fold greater risk than nonpregnant women with DM who received conventional treatment (P < .001).
Deterioration of retinopathy during pregnancy had no long-term consequences, however, regardless of type of treatment.15 More importantly, in most cases, changes in the level of retinopathy revert to the pre-pregnancy level after 1 year or longer, and pregnancy does not appear to affect long-term progression of retinopathy.15
Continue to: Proven primary prevention strategies
Proven primary prevention strategies
Glycemic control. Optimal glycemic control is an essential component of prevention of diabetic retinopathy. From 1983 to 1993, the Diabetes Control and Complications Trial randomized 1441 patients with type 1 DM to receive intensive therapy (median HbA1C level, 7.2%) or conventional therapy (median HbA1C level, 9.1%). During a mean of 6 years of follow-up, intensive therapy reduced the adjusted mean risk of retinopathy by 76% (95% CI, 62%-85%).16,17 A 2007 systematic review of 44 studies of the treatment of diabetic retinopathy found that strict glycemic control was beneficial in reducing the incidence and progression of retinopathy.17
The American Diabetes Association’s Standards of Medical Care in Diabetes—2019 Abridged for Primary Care Providers recommends that most nonpregnant adults maintain an HbA1Clevel < 7%. For patients with a history of hypoglycemia, limited life expectancy, advanced microvascular or macrovascular disease, other significant comorbid conditions, or longstanding DM in which it is difficult to achieve the optimal goal, a higher HbA1clevel (< 8%) might be appropriate.18
Control of BP. Strict control of BP is a major modifier of the incidence and progression of diabetic retinopathy.17,19 In the United Kingdom Prospective Diabetes Study, 1148 patients with type 2 DM and a mean BP of 160/94 mm Hg at the onset of the study were randomly assigned to either (1) a “tight” blood pressure group (< 150/85 mm Hg) or (2) a “less-tight” group (< 180/105 mm Hg). The primary therapy for controlling BP was captopril or atenolol. After 9 years of follow-up, the tight-control group had a 34% mean reduction in risk in the percentage of patients with deterioration of retinopathy (99% CI, 11%-50%; P = .0004) and a 47% reduction in risk (99% CI, 7%-70%; P = .004) of deterioration in visual acuity.20
Most patients with DM and hypertension should be treated to maintain a BP < 140/90 mm Hg. Although there is insufficient evidence to recommend a specific antihypertensive agent for preventing diabetic retinopathy, therapy should include agents from drug classes that have a demonstrated reduction in cardiovascular events in patients with DM. These include angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, thiazide diuretics, and dihydropyridine calcium channel blockers.18
Lipid management. The benefit of targeted therapy for lowering lipids for the prevention of diabetic retinopathy is not well established.17 In the Collaborative Atorvastatin Diabetes Study, 2838 patients with type 2 DM were randomized to atorvastatin (10 mg) or placebo; microvascular endpoint analysis demonstrated that patients taking atorvastatin needed less laser therapy (P = .14); however, progression of diabetic retinopathy was not reduced.21 Similarly, in the Action to Control Cardiovascular Risk in Diabetes Eye Study, slowing of progression to retinopathy was observed in patients with type 2 DM who were treated with fenofibrate (ie, progression in 6.5%, compared with progression in 10.2% of untreated subjects [odds ratio = 0.60 (95% CI, 0.42-0.87); P = .0056]).22
Continue to: Despite limited data on...
Despite limited data on the impact of lipid-lowering agents on patients with diabetic retinopathy, those with type 2 DM (especially) and those who have, or are at risk of, atherosclerotic cardiovascular disease should receive statin therapy.18
Aspirin therapy. Aspirin has not been found to be beneficial for slowing progression of diabetic retinopathy. However, aspirin did not cause further deterioration of disease, specifically in patients with vitreous hemorrhages4; patients with diabetic retinopathy who require aspirin therapy for other medical reasons can therefore continue to take it without increasing the risk of damage to the retina.4,18
When should you refer patients for screening?
Screening for diabetic retinopathy is important because affected patients can be asymptomatic but have significant disease. Early detection also helps determine which patients need treatment when it is most beneficial: early in its course.4
Type 1 DM. Retinopathy can become apparent as early as 6 or 7 years after the onset of disease, and is rare in children prior to puberty.4,11 As a result, patients with type 1 DM should first be screened with a comprehensive eye examination by an ophthalmologist or optometrist within 5 years of DM onset.4,18
Type 2 DM. Because of the insidious onset of type 2 DM, patients who are given a diagnosis of DM after 30 years of age might already have high-risk features of retinopathy.9 In patients with type 2 DM, therefore, initial screening for diabetic retinopathy should begin at the time of diagnosis and include a comprehensive eye examination by an ophthalmologist or optometrist.4,18,23
Continue to: Components of the exam
Components of the exam. Initial evaluation by the ophthalmologist or optometrist should include a detailed history and comprehensive eye exam with pupil dilation. Table 24 lists elements of the initial physical exam, which should assess for features that often lead to visual impairment. These features include macular edema, retinal hemorrhage, venous beading, neovascularization, and vitreous hemorrhage.4
Frequency of follow-up. The interval between subsequent examinations should be individualized, based on the findings of the initial assessment. Consider that:
- Screening should occur every 1 or 2 years in patients without evidence of retinopathy and with adequate glycemic control.4,18,23
- Screening every 1 or 2 years appears to be cost-effective in patients who have had 1 or more normal eye exams.
- A 3-year screening interval does not appear to present a risk in well-controlled patients with type 2 DM.24
- Women with type 1 or type 2 DM who are planning pregnancy or who are pregnant should have an eye exam prior to pregnancy or early in the first trimester.4,18,23 They should then be monitored each trimester and at the end of the first postpartum year, depending on the severity of retinopathy.18
Alternative screening modalities
Seven-field stereoscopic fundus photography is an alternative screening tool that compares favorably to ophthalmoscopy when performed by an experienced ophthalmologist, optometrist, or ophthalmologic technician.25 Nonmydriatic digital stereoscopic retinal imaging has been shown to be a cost-effective method of screening patients for diabetic retinopathy.26 In a study that compared digital imaging with dilated funduscopic examination, investigators reported that, of 311 eyes evaluated, there was agreement between the methods in 86% of cases. Disagreement was mostly related to the greater frequency of finding mild-to-moderate NPDR when using digital imaging.27
Screening in primary care
Programs that use telemedicine-based fundus photography to screen for diabetic retinopathy during primary care visits, followed by remote interpretation by an ophthalmologist, have been shown to increase the rate of retinal screening by offering an option other than direct referral to an ophthalmologist or optometrist.28 However, telemedicine-based retinal photography can be successful as a screening tool for retinopathy only if timely referral to an eye specialist is arranged when indicated by findings.18
SIDEBAR
Key points in the progression of diabetic retinopathy care
Duration of diabetes, poor glycemic control, and uncontrolled hypertension are major risk factors for diabetic retinopathy.
To reduce the risk of diabetic retinopathy, patients with diabetes mellitus should:
- sustain good glycemic control (hemoglobin A1C level, < 7%)
- maintain blood pressure < 140/90 mm Hg
- undergo periodic routine screening eye examination.
Early detection of diabetic retinopathy by dilated eye examination or fundus photography can lead to early therapeutic intervention, which can reduce the risk of visual impairment and vision loss.
Treatment is based on severity of disease and can include anti-vascular-endothelial growth factor therapy, photocoagulation, or surgery.
What therapy will your referred patients receive?
Patients found to have signs of diabetic retinopathy should be referred to an ophthalmologist who is knowledgeable and experienced in the management of diabetic retinopathy. Care will be managed according to the severity of the patient’s diabetic retinopathy.
Continue to: Patients with mild-to-moderate NPDR but without macular edema
Patients with mild-to-moderate NPDR but without macular edema. Treatment is generally not recommended. Patients should be reevaluated every 6 to 12 months because they have an increased risk of progression.5
Patients with mild-to-moderate NPDR and clinically significant macular edema (CSME). It is important for the eye specialist to assess for edema at the center of the macula because the risk of vision loss and need for treatment is greater when the center is involved. Vascular–endothelial growth factor (VEGF) is an important mediator of neovascularization and macular edema in diabetic retinopathy. For patients with center-involving CSME, intravitreous injection of an anti-VEGF agent provides significant benefit and is first-line treatment in these cases.4,29
The Early Treatment for Diabetic Retinopathy Study evaluated the efficacy of focal photocoagulation, a painless laser therapy, for CSME and demonstrated that this modality reduces the risk of moderate visual loss; increases the likelihood of improvement in vision; and decreases the frequency of persistent macular edema.30 Focal photocoagulation has been found effective in both non-center-involving CSME and center-involving CSME.5
Patients with severe NPDR. The recommendation is to initiate full panretinal photocoagulation prior to progression to proliferative diabetic retinopathy PDR. Researchers noted a 50% reduction in vision loss and vitrectomy when patients with type 2 DM were treated with panretinal photocoagulation early, compared with those in whom treatment was deferred until PDR developed.4,31 The role of anti-VEGF treatment of severe NPDR is under investigation.4
Patients with high-risk and severe PDR. Panretinal photocoagulation is the recommended treatment for patients with high-risk and severe PDR, and usually induces regression of retinal neovascularization. In patients with CSME and high-risk PDR, the combination of anti-VEGF therapy and panretinal photocoagulation should be considered. Vitrectomy should be considered for patients who have failed panretinal photocoagulation or are not amenable to photocoagulation.4
CORRESPONDENCE
Bryan Farford, DO, Mayo Clinic, 4500 San Pablo Road, Jacksonville, FL 32224; Farford.Bryan@mayo.edu.
1. National Diabetes Statistic Report 2020: Estimates of Diabetes and Its Burden in the United States. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention. www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf. Accessed March 20, 2020.
2. Fitch K, Weisman T, Engel T, et al. Longitudinal commercial claims-based cost analysis of diabetic retinopathy screening patterns. Am Health Drug Benefits. 2015;8:300-308.
3. Centers for Disease Control and Prevention. Common eye disorders. September 29, 2015. www.cdc.gov/visionhealth/basics/ced/index.html. Accessed March 20, 2020.
4. American Academy of Ophthalmology PPP Retina/Vitreous Committee, Hoskins Center for Quality Eye Care. Diabetic Retinopathy PPP 2019. San Francisco, CA: American Academy of Ophthalmology. October 2019. https://www.aao.org/preferred-practice-pattern/diabetic-retinopathy-ppp. Accessed March 20, 2020.
5. Zhang X, Saaddine JB, Chou C-F, et al. Prevalence of diabetic retinopathy in the United States, 2005-2008. JAMA. 2010;304:649-656.
6. Stewart MW. Socioeconomic cost of diabetic retinopathy and therapy. In: Diabetic Retinopathy. Singapore: Adis; 2017:257-268.
7. Tarr JM, Kaul K, Chopra M, et al. Pathophysiology of diabetic retinopathy. ISRN Ophthalmol. 2013;2013:343560.
8. Wilkinson CP, Ferris FL 3rd, Klein RE, et al. Proposed International Clinical Diabetic Retinopathy and Diabetic Macular Edema Disease Severity Scales. Ophthalmology. 2003;110:1677-1682.
9. Wu L, Fernandez-Loaiza P, Sauma J, et al. Classification of diabetic retinopathy and diabetic macular edema. World J Diabetes. 2013;4:290-294.
10. American Academy of Ophthalmology. International Clinical Diabetic Retinopathy Disease Severity Scale detailed table. October 2002. http://www.icoph.org/downloads/Diabetic-Retinopathy-Detail.pdf. Accessed March 20, 2020.
11. Klein R, Klein BE, Moss SE, et al. The Wisconsin Epidemiologic Study of Diabetic Retinopathy. Arch Ophthalmol. 1984;102:527-532.
12. Klein R, Klein BE, Moss SE, et al. The Wisconsin Epidemiologic Study of Diabetic Retinopathy. Ten-year incidence and progression of diabetic retinopathy. Arch Ophthalmol. 1994;112:1217-1228.
13. Klein R, Knudtson MD, Lee KE, et al. The Wisconsin Epidemiologic Study of Diabetic Retinopathy XXII. The twenty-five-year progression of retinopathy in persons with type 1 diabetes. Ophthalmology. 2008;115:1859-1868.
14. Emanuele N, Sacks J, Klein R, et al. Ethnicity, race, and baseline retinopathy correlates in the Veterans Affairs Diabetes Trial. Diabetes Care. 2005;28:1954-1958.
15. Effect of pregnancy on microvascular complications in the diabetes control and complications trial. The Diabetes Control and Complications Trial Research Group. Diabetes Care. 2000;23:1084-1091.
16. , , The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977-986.
17. Mohamed Q, Gillies MC, Wong TY. Management of diabetic retinopathy: a systematic review. JAMA. 2007;298:902-916.
18. American Diabetes Association. Standards of Medical Care in Diabetes—2019 abridged for primary care providers. Clin Diabetes. 2019;37:11-34.
19. Do DV, Wang X, Vedula SS, et al. Blood pressure control for diabetic retinopathy. Cochrane Database Syst Rev. 2015;(1):CD006127.
20. UK Prospective Diabetes Study Group. Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. UK Prospective Diabetes Study Group. BMJ. 1998;317:703-713.
21. Colhoun HM, Betteridge DJ, Durrington PN; CARDS Investigators. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomised placebo-controlled trial. Lancet. 2004;364:685-696.
22. Chew EY, Davis MD, Danis RP, et al. Action to Control Cardiovascular Risk in Diabetes Eye Study Research Group. The effects of medical management on the progression of diabetic retinopathy in persons with type 2 diabetes: The Action to Control Cardiovascular Risk in Diabetes (ACCORD) Eye Study. Ophthalmology. 2014;121:2443-2451.
23. Fong DS, Aiello L, Gardner TW, et al American Diabetes Association. Retinopathy in diabetes. Diabetes Care. 2004;27(suppl 1):S84-S87.
24. 11. Microvascular complications and foot care: Standards of Medical Care in Diabetes—2019. Diabetes Care. 2019;42(suppl 1):S124-S138.
25. Moss SE, Klein R, Kessler SD, et al. Comparison between ophthalmoscopy and fundus photography in determining severity of diabetic retinopathy. Ophthalmology. 1985;92:62-67.
26. Kirkizlar E, Serban N, Sisson JA, et al. Evaluation of telemedicine for screening of diabetic Retinopathy in the Veterans Health Administration. Ophthalmology. 2013;120:2604-2610.
27. Ahmed J, Ward TP, Bursell S-E, et al. The sensitivity and specificity of nonmydriatic digital stereoscopic retinal imaging in detecting diabetic retinopathy. Diabetes Care. 2006;29:2205-2209.
28. Taylor CR, Merin LM, Salunga AM, et al. Improving diabetic retinopathy screening ratios using telemedicine-based digital retinal imaging technology: the Vine Hill study. Diabetes Care. 2007;30:574.
29. , , , Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema. N Engl J Med. 2015;372:1193-1203.
30. Early photocoagulation for diabetic retinopathy. ETDRS Report Number 9. Early Treatment Diabetic Retinopathy Study Research Group. Ophthalmology. 1991;98(5 suppl):766-785.
31. Ferris F. Early photocoagulation in patients with either type I or type II diabetes. Trans Am Ophthalmol Soc. 1996;94:505-537.
1. National Diabetes Statistic Report 2020: Estimates of Diabetes and Its Burden in the United States. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention. www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf. Accessed March 20, 2020.
2. Fitch K, Weisman T, Engel T, et al. Longitudinal commercial claims-based cost analysis of diabetic retinopathy screening patterns. Am Health Drug Benefits. 2015;8:300-308.
3. Centers for Disease Control and Prevention. Common eye disorders. September 29, 2015. www.cdc.gov/visionhealth/basics/ced/index.html. Accessed March 20, 2020.
4. American Academy of Ophthalmology PPP Retina/Vitreous Committee, Hoskins Center for Quality Eye Care. Diabetic Retinopathy PPP 2019. San Francisco, CA: American Academy of Ophthalmology. October 2019. https://www.aao.org/preferred-practice-pattern/diabetic-retinopathy-ppp. Accessed March 20, 2020.
5. Zhang X, Saaddine JB, Chou C-F, et al. Prevalence of diabetic retinopathy in the United States, 2005-2008. JAMA. 2010;304:649-656.
6. Stewart MW. Socioeconomic cost of diabetic retinopathy and therapy. In: Diabetic Retinopathy. Singapore: Adis; 2017:257-268.
7. Tarr JM, Kaul K, Chopra M, et al. Pathophysiology of diabetic retinopathy. ISRN Ophthalmol. 2013;2013:343560.
8. Wilkinson CP, Ferris FL 3rd, Klein RE, et al. Proposed International Clinical Diabetic Retinopathy and Diabetic Macular Edema Disease Severity Scales. Ophthalmology. 2003;110:1677-1682.
9. Wu L, Fernandez-Loaiza P, Sauma J, et al. Classification of diabetic retinopathy and diabetic macular edema. World J Diabetes. 2013;4:290-294.
10. American Academy of Ophthalmology. International Clinical Diabetic Retinopathy Disease Severity Scale detailed table. October 2002. http://www.icoph.org/downloads/Diabetic-Retinopathy-Detail.pdf. Accessed March 20, 2020.
11. Klein R, Klein BE, Moss SE, et al. The Wisconsin Epidemiologic Study of Diabetic Retinopathy. Arch Ophthalmol. 1984;102:527-532.
12. Klein R, Klein BE, Moss SE, et al. The Wisconsin Epidemiologic Study of Diabetic Retinopathy. Ten-year incidence and progression of diabetic retinopathy. Arch Ophthalmol. 1994;112:1217-1228.
13. Klein R, Knudtson MD, Lee KE, et al. The Wisconsin Epidemiologic Study of Diabetic Retinopathy XXII. The twenty-five-year progression of retinopathy in persons with type 1 diabetes. Ophthalmology. 2008;115:1859-1868.
14. Emanuele N, Sacks J, Klein R, et al. Ethnicity, race, and baseline retinopathy correlates in the Veterans Affairs Diabetes Trial. Diabetes Care. 2005;28:1954-1958.
15. Effect of pregnancy on microvascular complications in the diabetes control and complications trial. The Diabetes Control and Complications Trial Research Group. Diabetes Care. 2000;23:1084-1091.
16. , , The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977-986.
17. Mohamed Q, Gillies MC, Wong TY. Management of diabetic retinopathy: a systematic review. JAMA. 2007;298:902-916.
18. American Diabetes Association. Standards of Medical Care in Diabetes—2019 abridged for primary care providers. Clin Diabetes. 2019;37:11-34.
19. Do DV, Wang X, Vedula SS, et al. Blood pressure control for diabetic retinopathy. Cochrane Database Syst Rev. 2015;(1):CD006127.
20. UK Prospective Diabetes Study Group. Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. UK Prospective Diabetes Study Group. BMJ. 1998;317:703-713.
21. Colhoun HM, Betteridge DJ, Durrington PN; CARDS Investigators. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomised placebo-controlled trial. Lancet. 2004;364:685-696.
22. Chew EY, Davis MD, Danis RP, et al. Action to Control Cardiovascular Risk in Diabetes Eye Study Research Group. The effects of medical management on the progression of diabetic retinopathy in persons with type 2 diabetes: The Action to Control Cardiovascular Risk in Diabetes (ACCORD) Eye Study. Ophthalmology. 2014;121:2443-2451.
23. Fong DS, Aiello L, Gardner TW, et al American Diabetes Association. Retinopathy in diabetes. Diabetes Care. 2004;27(suppl 1):S84-S87.
24. 11. Microvascular complications and foot care: Standards of Medical Care in Diabetes—2019. Diabetes Care. 2019;42(suppl 1):S124-S138.
25. Moss SE, Klein R, Kessler SD, et al. Comparison between ophthalmoscopy and fundus photography in determining severity of diabetic retinopathy. Ophthalmology. 1985;92:62-67.
26. Kirkizlar E, Serban N, Sisson JA, et al. Evaluation of telemedicine for screening of diabetic Retinopathy in the Veterans Health Administration. Ophthalmology. 2013;120:2604-2610.
27. Ahmed J, Ward TP, Bursell S-E, et al. The sensitivity and specificity of nonmydriatic digital stereoscopic retinal imaging in detecting diabetic retinopathy. Diabetes Care. 2006;29:2205-2209.
28. Taylor CR, Merin LM, Salunga AM, et al. Improving diabetic retinopathy screening ratios using telemedicine-based digital retinal imaging technology: the Vine Hill study. Diabetes Care. 2007;30:574.
29. , , , Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema. N Engl J Med. 2015;372:1193-1203.
30. Early photocoagulation for diabetic retinopathy. ETDRS Report Number 9. Early Treatment Diabetic Retinopathy Study Research Group. Ophthalmology. 1991;98(5 suppl):766-785.
31. Ferris F. Early photocoagulation in patients with either type I or type II diabetes. Trans Am Ophthalmol Soc. 1996;94:505-537.
PRACTICE RECOMMENDATIONS
› Refer patients with type 1 diabetes mellitus (DM) to an ophthalmologist or optometrist for a dilated and comprehensive eye examination within 5 years of disease onset. B
› Refer patients with type 2 DM to an ophthalmologist or optometrist for an initial dilated and comprehensive eye examination at time of diagnosis. B
› Control blood pressure—ideally, < 140/90 mm Hg—in patients with DM to reduce the risk of diabetic retinopathy. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series