User login
You never know what will happen when you look into a mouse’s eyes.
Twelve years ago, Dr. Lee Goldstein was investigating reactive oxygen species’ effect on the brain of a young Alzheimer’s model mouse. Holding the tiny creature in his hand, he carefully inserted a miniscule microdialysis probe through its skull. As he did, he happened to look right into the mouse’s face. And since he was doing some unrelated work on cataracts at the time, something unusual caught his very practiced gaze.
"The mouse had a cataract. I looked at the other eye, and there was a cataract there, too. That’s very unusual – really not ever seen – in a mouse this age."
Then he looked at all the other mice he was using in that experiment, all of which were older. They all had bilateral cataracts. My first thought was, "This can’t be related to Alzheimer’s disease."
But in fact, he said, it appeared to be. He and his lab soon showed that the cataracts contained a large concentration of aggregated beta-amyloid (Abeta) in the same fraction that’s measured in today’s cerebrospinal fluid Alzheimer’s biomarker tests.
That first observation has birthed two investigational noninvasive amyloid eye tests, which Dr. Goldstein envisions could some day be part of everyone’s annual physical exam.
In people destined to develop Alzheimer’s, some research suggests that Abeta proteins may begin to accumulate in the lens long before they build up to dangerous levels in the brain. If this turns out to be a reliable marker of risk, it could be a sign that would trigger early, presymptomatic Alzheimer’s treatment.
That’s in the future, though, because right now, there is no such treatment. But at this time, Dr. Goldstein said, an amyloid eye test could prove invaluable in reaching that goal. One reason that symptomatic patients don’t respond to investigational drugs could be that by the time the patients are treated, irreversible brain damage has already occurred.
"Once you have cognitive symptoms, the horse is not only out of the barn, it’s run out of the state," said Dr. Goldstein, director of the molecular aging and development laboratory at Boston University. "I hate the term ‘mild cognitive impairment,’ because by the time you have that, there’s nothing mild about it."
Researchers now almost universally agree that the best way to get a true picture of any drug’s potential effectiveness in Alzheimer’s will be to implement treatment before symptoms set in. In addition, Dr. Goldstein said, "Research pools are polluted. Control groups contain subjects who would develop Alzheimer’s if they live long enough," which could be skewing study results. Lens amyloid measurements might help stratify groups in drug studies, and even be a way to track very early effect on amyloid.
But that is a future yet to be determined. In the meantime, researchers still need definitive proof that supranuclear amyloid cataracts are inextricably linked to the amyloid brain plaques of Alzheimer’s.
Initial findings
In 2003, Dr. Goldstein, then at Harvard Medical School, published his original proof of concept study. It comprised postmortem eye and brain specimens from nine subjects with Alzheimer’s and eight controls without the disease, and samples of primary aqueous humor from three people without the disorder who were undergoing cataract surgery (Lancet 2003;361:1258-65).
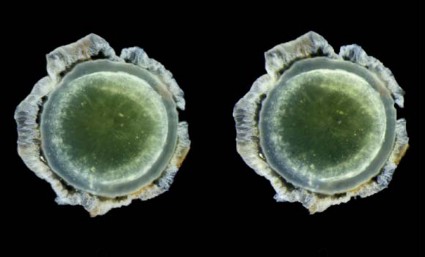
Abeta-40 and Abeta-42 were present in all of the lenses, in amounts similar to those seen in the corresponding brains. But in patients with Alzheimer’s, the protein aggregated into clumps within the lens fiber cells, forming unusual supranuclear cataracts at the equatorial periphery that appeared to be different from common age-related cataracts and that weren’t present in the control subjects.
The cataract location is an important clue to how long the Abeta has been accumulating, Dr. Goldstein said. Lens fiber cells are particularly long lived, remaining alive for as long as a person lives or until the lens is removed during cataract surgery. The lens starts to form in very early fetal life, with more and more lens cells forming in an outward direction, creating a virtual map of a person’s lifetime, "like the rings of a tree," he said.
Dr. Goldstein and his team discovered that these distinctive cataracts develop in some patients with Alzheimer’s. They appear toward the outer edge of the lens and are composed of the same toxic Abeta protein that builds up in the brain. "The history of amyloid in the body is time stamped in the lens," he said.
As lens fiber cells age, they lose most of their organelles and become transparent – just the right state for a device meant to focus light. "They also make tons of Abeta," Dr. Goldstein said, and it appears to have a very specific function in the eye, one Dr. Rudolph Tanzi and his colleagues at Massachusetts General Hospital, Boston, identified in collaboration with Dr. Goldstein’s team (PLoS ONE 2010;5:e9505).
"It turns out to be a very potent antimicrobial peptide," one of several the eye and brain produce to defend themselves. Amyloid’s sticky nature causes foreign invaders to clump together, so they’re more easily destroyed. This finding also suggests that Abeta could have a similar function in the brain, supporting some theories that Alzheimer’s might be at least partially triggered by a hyperinflammatory response toward an invading pathogen or another immunoreactive incident.
Testing lenses in Down syndrome patients
Interesting as all of that is, it doesn’t prove the theory that the lens amyloid record somehow tracks Alzheimer’s development. But other studies do explore that concept, including one Dr. Goldstein published in 2010. In this study, Dr. Goldstein and his colleagues examined lens amyloid in people with Down syndrome, a group predestined to develop Alzheimer’s (PLoS ONE 2010;5:e10659). The genetic mutation that causes the syndrome also increases production of the amyloid precursor protein (APP), Abeta’s antecedent.
The lenses from subjects with Down syndrome, aged 2-69 years, were compared with lenses from control subjects and people with both familial and late-onset Alzheimer’s. "The 2-year-old with Down syndrome in this study actually had more lens amyloid than the adults with familial Alzheimer’s," Dr. Goldstein said. In unpublished data, he added, the protein has even been observed in Down syndrome fetal lenses.
He expanded on this work in a poster presented at the 2013 Alzheimer’s Association International Conference. Dr. Goldstein and his team have developed and validated a laser eye scanning instrument that noninvasively measures how light is reflected from the tiniest particles – in this case clumps of Abeta protein – within the lens of living human subjects.
"We hypothesize that due to the trisomy of chromosome 21 in Down syndrome (and triplication of the APP gene), which results in increased expression of Abeta in the lens, the intensity of scattered light in Down syndrome patients will be higher than [in] age-matched controls," he noted in the poster.
Not everyone agrees with this idea, however. It has stirred controversy since he first introduced the idea, when, he said, "mainstream Alzheimer’s research simply didn’t believe it." In fact, at least two other researchers’ studies have come to quite different conclusions.
Dr. Charles Eberhart, a pathologist at Johns Hopkins University, Baltimore, published his data in the journal Brain Pathology (2013 June 28 [doi:10.1111/bpa.12070]). The study examined retinas, lenses, and brains from 11 patients with Alzheimer’s, 6 with Parkinson’s, and 6 age-matched controls. Eight eyes (five from Alzheimer’s patients and three from controls) did have cataracts. Dr. Eberhart and his colleagues used immunohistochemistry and Congo red staining to look for amyloid, phosphorylated tau, and alpha-synuclein.
"The short answer is – we didn’t find any amyloid deposits in the lens, or any abnormal tau accumulations," he said in an interview.
The study has two possible interpretations, he said: Either Abeta, tau, and synuclein don’t accumulate in Alzheimer’s eyes as they do in Alzheimer’s brains, or they are there, but simply not detected by his methods. "It certainly might be there. All we can say is that with this method, which is the accepted way of determining amyloid in brain tissue, we didn’t see it in eyes," he said.
The second study, conducted by Dr. Ralph Michael of the Universitat Autònoma de Barcelona and his colleagues, came to a similar conclusion (Exp. Eye Res. 2013;106:5-13). It involved 39 lenses and brains from 21 Alzheimer’s patients, and 15 lenses from age-matched controls. Six of the Alzheimer’s lenses and seven control lenses had cataracts. These investigators used staining methods similar to those in the Hopkins study.
"Beta-amyloid immunohistochemistry was positive in the brain tissues but not in the cornea sample," they wrote. "Lenses from control and AD [Alzheimer’s disease] donors were, without exception, negative after Congo red, thioflavin, and beta-amyloid immunohistochemical staining. ... The absence of staining in AD and control lenses with the techniques employed lead us to conclude that there is no beta-amyloid in lenses from donors with AD or in control cortical cataracts."
Dr. Goldstein said he doesn’t doubt these findings. Congo red staining yields a difficult-to-interpret sign, he said. Amyloid appears red under standard light spectroscopy, but takes on a very characteristic shade, called apple green under polarized light. "This is an old staining method that’s not very sensitive nor is it specific for Abeta – it’s also highly variable."
Technique is critical, he added. "It took us years to perfect our technique for the lens. It’s very difficult to work with lens, harder to work with old lens, and extremely hard to work with old, sick lens."
Instead of relying solely on Congo red or other staining techniques, Dr. Goldstein’s team confirmed their findings using a combination of biochemical analyses, immunogold electron microscopy, and two different types of mass spectrometry – methods he said are irrefutably accurate. "You can’t argue with this unless you are willing to argue with the very concept of mass spectrometry. It’s the gold standard," he said.
Confirmation in transgenic mice and Down syndrome patients strengthens the hypothesis, he said, as do the conclusions of his most recent paper. It looked at data from 1,249 people included in the Framingham Eye Study, and found a genetic link between a specific type of midlife cataracts (consistent with those previously found in Alzheimer’s) and later cognitive and brain structural changes associated with Alzheimer’s (PLoS ONE 2012;7:e43728) .
The culprit appeared to be a mutation of a gene that codes for delta-catenin, which Dr. Goldstein postulated may normally help suppress Abeta production. The altered form, however, appears to affect neuronal structure and is instead associated with an increase in Abeta-42 production in cell culture. The malformed delta-catenin protein was also found throughout the lenses of study subjects with Alzheimer’s, but not in control lenses.
Screening patients in the future?
Dr. Goldstein said he envisions a future in which annual lens exams might guide risk assessment and treatment initiation. But physicians who might someday screen patients certainly won’t have a mass spectrometer in the back room.
He has invented two devices, he said, that will fill that need. The most recent is a laser scanning ophthalmoscope that uses dynamic light scattering to detect the tiniest amyloid particles in the lens – particles less than 30 nm. This is the device he’s using in the ongoing Boston University/Boston Children’s Hospital study of lens amyloid in children with Down syndrome.
The second device combines optical imaging with aftobetin, a fluorescent amyloid ligand. Dr. Goldstein holds a patent on this device, which he invented in partnership with Cognoptix (formerly Neuroptix), a company he cofounded in 2001, although he is no longer operationally affiliated with it.
Cognoptix has developed the SAPPHIRE II system, a combination drug/device that detects amyloid in the lens using aftobetin. The company licensed aftobetin from the University of California, San Diego. It’s formulated into an ophthalmic ointment administered prior to scanning with the SAPPHIRE II system. The procedure uses fluorescent ligand scanning to detect amyloid aggregates in the lens, said Paul Hartung, president and chief executive officer of the Acton, Mass., company.
"We use an eye-safe laser tuned to pick up the fluorescence. It doesn’t require dilation of the pupil, and it has the capability of actually registering itself in the correct location in the eye," he said in an interview.
SAPPHIRE II has had a busy year, including a proof of concept study published in May and reported at the Alzheimer’s Association International Conference. In this study, the system successfully differentiated five Alzheimer’s patients from five controls (Front. Neurol. 2013 May 27 [doi:10.3389/fneur.2013.00062]).
Cognoptix has begun a second study testing the system against PET amyloid brain imaging in 20 patients with probable Alzheimer’s and 20 controls, Mr. Hartung said.
A third planned study is a pivotal phase III trial that will enroll 400 subjects, all of whom will undergo both the eye exam and PET amyloid imaging. It’s designed to support premarketing approval, Mr. Hartung said. Currently SAPPHIRE II has an investigational device exemption from the Food and Drug Administration’s Center for Devices and Radiological Health.
"Our end goal is to get this into the general practitioner’s office, where about 40% of Alzheimer’s drug prescriptions are written by general practitioners who really have no data on hand. Right now, based on cognitive assessments, they have only a 50-50 chance of getting the right diagnosis," Mr. Hartung said.
Dr. Goldstein and Mr. Hartung hold financial interests in devices to measure lens amyloid. Dr. Ralph Michael listed no financial disclosures. Dr. Charles Eberhart said he had no relevant financial disclosures.
On Twitter @Alz_Gal
You never know what will happen when you look into a mouse’s eyes.
Twelve years ago, Dr. Lee Goldstein was investigating reactive oxygen species’ effect on the brain of a young Alzheimer’s model mouse. Holding the tiny creature in his hand, he carefully inserted a miniscule microdialysis probe through its skull. As he did, he happened to look right into the mouse’s face. And since he was doing some unrelated work on cataracts at the time, something unusual caught his very practiced gaze.
"The mouse had a cataract. I looked at the other eye, and there was a cataract there, too. That’s very unusual – really not ever seen – in a mouse this age."
Then he looked at all the other mice he was using in that experiment, all of which were older. They all had bilateral cataracts. My first thought was, "This can’t be related to Alzheimer’s disease."
But in fact, he said, it appeared to be. He and his lab soon showed that the cataracts contained a large concentration of aggregated beta-amyloid (Abeta) in the same fraction that’s measured in today’s cerebrospinal fluid Alzheimer’s biomarker tests.
That first observation has birthed two investigational noninvasive amyloid eye tests, which Dr. Goldstein envisions could some day be part of everyone’s annual physical exam.
In people destined to develop Alzheimer’s, some research suggests that Abeta proteins may begin to accumulate in the lens long before they build up to dangerous levels in the brain. If this turns out to be a reliable marker of risk, it could be a sign that would trigger early, presymptomatic Alzheimer’s treatment.
That’s in the future, though, because right now, there is no such treatment. But at this time, Dr. Goldstein said, an amyloid eye test could prove invaluable in reaching that goal. One reason that symptomatic patients don’t respond to investigational drugs could be that by the time the patients are treated, irreversible brain damage has already occurred.
"Once you have cognitive symptoms, the horse is not only out of the barn, it’s run out of the state," said Dr. Goldstein, director of the molecular aging and development laboratory at Boston University. "I hate the term ‘mild cognitive impairment,’ because by the time you have that, there’s nothing mild about it."
Researchers now almost universally agree that the best way to get a true picture of any drug’s potential effectiveness in Alzheimer’s will be to implement treatment before symptoms set in. In addition, Dr. Goldstein said, "Research pools are polluted. Control groups contain subjects who would develop Alzheimer’s if they live long enough," which could be skewing study results. Lens amyloid measurements might help stratify groups in drug studies, and even be a way to track very early effect on amyloid.
But that is a future yet to be determined. In the meantime, researchers still need definitive proof that supranuclear amyloid cataracts are inextricably linked to the amyloid brain plaques of Alzheimer’s.
Initial findings
In 2003, Dr. Goldstein, then at Harvard Medical School, published his original proof of concept study. It comprised postmortem eye and brain specimens from nine subjects with Alzheimer’s and eight controls without the disease, and samples of primary aqueous humor from three people without the disorder who were undergoing cataract surgery (Lancet 2003;361:1258-65).
Abeta-40 and Abeta-42 were present in all of the lenses, in amounts similar to those seen in the corresponding brains. But in patients with Alzheimer’s, the protein aggregated into clumps within the lens fiber cells, forming unusual supranuclear cataracts at the equatorial periphery that appeared to be different from common age-related cataracts and that weren’t present in the control subjects.
The cataract location is an important clue to how long the Abeta has been accumulating, Dr. Goldstein said. Lens fiber cells are particularly long lived, remaining alive for as long as a person lives or until the lens is removed during cataract surgery. The lens starts to form in very early fetal life, with more and more lens cells forming in an outward direction, creating a virtual map of a person’s lifetime, "like the rings of a tree," he said.
Dr. Goldstein and his team discovered that these distinctive cataracts develop in some patients with Alzheimer’s. They appear toward the outer edge of the lens and are composed of the same toxic Abeta protein that builds up in the brain. "The history of amyloid in the body is time stamped in the lens," he said.
As lens fiber cells age, they lose most of their organelles and become transparent – just the right state for a device meant to focus light. "They also make tons of Abeta," Dr. Goldstein said, and it appears to have a very specific function in the eye, one Dr. Rudolph Tanzi and his colleagues at Massachusetts General Hospital, Boston, identified in collaboration with Dr. Goldstein’s team (PLoS ONE 2010;5:e9505).
"It turns out to be a very potent antimicrobial peptide," one of several the eye and brain produce to defend themselves. Amyloid’s sticky nature causes foreign invaders to clump together, so they’re more easily destroyed. This finding also suggests that Abeta could have a similar function in the brain, supporting some theories that Alzheimer’s might be at least partially triggered by a hyperinflammatory response toward an invading pathogen or another immunoreactive incident.
Testing lenses in Down syndrome patients
Interesting as all of that is, it doesn’t prove the theory that the lens amyloid record somehow tracks Alzheimer’s development. But other studies do explore that concept, including one Dr. Goldstein published in 2010. In this study, Dr. Goldstein and his colleagues examined lens amyloid in people with Down syndrome, a group predestined to develop Alzheimer’s (PLoS ONE 2010;5:e10659). The genetic mutation that causes the syndrome also increases production of the amyloid precursor protein (APP), Abeta’s antecedent.
The lenses from subjects with Down syndrome, aged 2-69 years, were compared with lenses from control subjects and people with both familial and late-onset Alzheimer’s. "The 2-year-old with Down syndrome in this study actually had more lens amyloid than the adults with familial Alzheimer’s," Dr. Goldstein said. In unpublished data, he added, the protein has even been observed in Down syndrome fetal lenses.
He expanded on this work in a poster presented at the 2013 Alzheimer’s Association International Conference. Dr. Goldstein and his team have developed and validated a laser eye scanning instrument that noninvasively measures how light is reflected from the tiniest particles – in this case clumps of Abeta protein – within the lens of living human subjects.
"We hypothesize that due to the trisomy of chromosome 21 in Down syndrome (and triplication of the APP gene), which results in increased expression of Abeta in the lens, the intensity of scattered light in Down syndrome patients will be higher than [in] age-matched controls," he noted in the poster.
Not everyone agrees with this idea, however. It has stirred controversy since he first introduced the idea, when, he said, "mainstream Alzheimer’s research simply didn’t believe it." In fact, at least two other researchers’ studies have come to quite different conclusions.
Dr. Charles Eberhart, a pathologist at Johns Hopkins University, Baltimore, published his data in the journal Brain Pathology (2013 June 28 [doi:10.1111/bpa.12070]). The study examined retinas, lenses, and brains from 11 patients with Alzheimer’s, 6 with Parkinson’s, and 6 age-matched controls. Eight eyes (five from Alzheimer’s patients and three from controls) did have cataracts. Dr. Eberhart and his colleagues used immunohistochemistry and Congo red staining to look for amyloid, phosphorylated tau, and alpha-synuclein.
"The short answer is – we didn’t find any amyloid deposits in the lens, or any abnormal tau accumulations," he said in an interview.
The study has two possible interpretations, he said: Either Abeta, tau, and synuclein don’t accumulate in Alzheimer’s eyes as they do in Alzheimer’s brains, or they are there, but simply not detected by his methods. "It certainly might be there. All we can say is that with this method, which is the accepted way of determining amyloid in brain tissue, we didn’t see it in eyes," he said.
The second study, conducted by Dr. Ralph Michael of the Universitat Autònoma de Barcelona and his colleagues, came to a similar conclusion (Exp. Eye Res. 2013;106:5-13). It involved 39 lenses and brains from 21 Alzheimer’s patients, and 15 lenses from age-matched controls. Six of the Alzheimer’s lenses and seven control lenses had cataracts. These investigators used staining methods similar to those in the Hopkins study.
"Beta-amyloid immunohistochemistry was positive in the brain tissues but not in the cornea sample," they wrote. "Lenses from control and AD [Alzheimer’s disease] donors were, without exception, negative after Congo red, thioflavin, and beta-amyloid immunohistochemical staining. ... The absence of staining in AD and control lenses with the techniques employed lead us to conclude that there is no beta-amyloid in lenses from donors with AD or in control cortical cataracts."
Dr. Goldstein said he doesn’t doubt these findings. Congo red staining yields a difficult-to-interpret sign, he said. Amyloid appears red under standard light spectroscopy, but takes on a very characteristic shade, called apple green under polarized light. "This is an old staining method that’s not very sensitive nor is it specific for Abeta – it’s also highly variable."
Technique is critical, he added. "It took us years to perfect our technique for the lens. It’s very difficult to work with lens, harder to work with old lens, and extremely hard to work with old, sick lens."
Instead of relying solely on Congo red or other staining techniques, Dr. Goldstein’s team confirmed their findings using a combination of biochemical analyses, immunogold electron microscopy, and two different types of mass spectrometry – methods he said are irrefutably accurate. "You can’t argue with this unless you are willing to argue with the very concept of mass spectrometry. It’s the gold standard," he said.
Confirmation in transgenic mice and Down syndrome patients strengthens the hypothesis, he said, as do the conclusions of his most recent paper. It looked at data from 1,249 people included in the Framingham Eye Study, and found a genetic link between a specific type of midlife cataracts (consistent with those previously found in Alzheimer’s) and later cognitive and brain structural changes associated with Alzheimer’s (PLoS ONE 2012;7:e43728) .
The culprit appeared to be a mutation of a gene that codes for delta-catenin, which Dr. Goldstein postulated may normally help suppress Abeta production. The altered form, however, appears to affect neuronal structure and is instead associated with an increase in Abeta-42 production in cell culture. The malformed delta-catenin protein was also found throughout the lenses of study subjects with Alzheimer’s, but not in control lenses.
Screening patients in the future?
Dr. Goldstein said he envisions a future in which annual lens exams might guide risk assessment and treatment initiation. But physicians who might someday screen patients certainly won’t have a mass spectrometer in the back room.
He has invented two devices, he said, that will fill that need. The most recent is a laser scanning ophthalmoscope that uses dynamic light scattering to detect the tiniest amyloid particles in the lens – particles less than 30 nm. This is the device he’s using in the ongoing Boston University/Boston Children’s Hospital study of lens amyloid in children with Down syndrome.
The second device combines optical imaging with aftobetin, a fluorescent amyloid ligand. Dr. Goldstein holds a patent on this device, which he invented in partnership with Cognoptix (formerly Neuroptix), a company he cofounded in 2001, although he is no longer operationally affiliated with it.
Cognoptix has developed the SAPPHIRE II system, a combination drug/device that detects amyloid in the lens using aftobetin. The company licensed aftobetin from the University of California, San Diego. It’s formulated into an ophthalmic ointment administered prior to scanning with the SAPPHIRE II system. The procedure uses fluorescent ligand scanning to detect amyloid aggregates in the lens, said Paul Hartung, president and chief executive officer of the Acton, Mass., company.
"We use an eye-safe laser tuned to pick up the fluorescence. It doesn’t require dilation of the pupil, and it has the capability of actually registering itself in the correct location in the eye," he said in an interview.
SAPPHIRE II has had a busy year, including a proof of concept study published in May and reported at the Alzheimer’s Association International Conference. In this study, the system successfully differentiated five Alzheimer’s patients from five controls (Front. Neurol. 2013 May 27 [doi:10.3389/fneur.2013.00062]).
Cognoptix has begun a second study testing the system against PET amyloid brain imaging in 20 patients with probable Alzheimer’s and 20 controls, Mr. Hartung said.
A third planned study is a pivotal phase III trial that will enroll 400 subjects, all of whom will undergo both the eye exam and PET amyloid imaging. It’s designed to support premarketing approval, Mr. Hartung said. Currently SAPPHIRE II has an investigational device exemption from the Food and Drug Administration’s Center for Devices and Radiological Health.
"Our end goal is to get this into the general practitioner’s office, where about 40% of Alzheimer’s drug prescriptions are written by general practitioners who really have no data on hand. Right now, based on cognitive assessments, they have only a 50-50 chance of getting the right diagnosis," Mr. Hartung said.
Dr. Goldstein and Mr. Hartung hold financial interests in devices to measure lens amyloid. Dr. Ralph Michael listed no financial disclosures. Dr. Charles Eberhart said he had no relevant financial disclosures.
On Twitter @Alz_Gal
You never know what will happen when you look into a mouse’s eyes.
Twelve years ago, Dr. Lee Goldstein was investigating reactive oxygen species’ effect on the brain of a young Alzheimer’s model mouse. Holding the tiny creature in his hand, he carefully inserted a miniscule microdialysis probe through its skull. As he did, he happened to look right into the mouse’s face. And since he was doing some unrelated work on cataracts at the time, something unusual caught his very practiced gaze.
"The mouse had a cataract. I looked at the other eye, and there was a cataract there, too. That’s very unusual – really not ever seen – in a mouse this age."
Then he looked at all the other mice he was using in that experiment, all of which were older. They all had bilateral cataracts. My first thought was, "This can’t be related to Alzheimer’s disease."
But in fact, he said, it appeared to be. He and his lab soon showed that the cataracts contained a large concentration of aggregated beta-amyloid (Abeta) in the same fraction that’s measured in today’s cerebrospinal fluid Alzheimer’s biomarker tests.
That first observation has birthed two investigational noninvasive amyloid eye tests, which Dr. Goldstein envisions could some day be part of everyone’s annual physical exam.
In people destined to develop Alzheimer’s, some research suggests that Abeta proteins may begin to accumulate in the lens long before they build up to dangerous levels in the brain. If this turns out to be a reliable marker of risk, it could be a sign that would trigger early, presymptomatic Alzheimer’s treatment.
That’s in the future, though, because right now, there is no such treatment. But at this time, Dr. Goldstein said, an amyloid eye test could prove invaluable in reaching that goal. One reason that symptomatic patients don’t respond to investigational drugs could be that by the time the patients are treated, irreversible brain damage has already occurred.
"Once you have cognitive symptoms, the horse is not only out of the barn, it’s run out of the state," said Dr. Goldstein, director of the molecular aging and development laboratory at Boston University. "I hate the term ‘mild cognitive impairment,’ because by the time you have that, there’s nothing mild about it."
Researchers now almost universally agree that the best way to get a true picture of any drug’s potential effectiveness in Alzheimer’s will be to implement treatment before symptoms set in. In addition, Dr. Goldstein said, "Research pools are polluted. Control groups contain subjects who would develop Alzheimer’s if they live long enough," which could be skewing study results. Lens amyloid measurements might help stratify groups in drug studies, and even be a way to track very early effect on amyloid.
But that is a future yet to be determined. In the meantime, researchers still need definitive proof that supranuclear amyloid cataracts are inextricably linked to the amyloid brain plaques of Alzheimer’s.
Initial findings
In 2003, Dr. Goldstein, then at Harvard Medical School, published his original proof of concept study. It comprised postmortem eye and brain specimens from nine subjects with Alzheimer’s and eight controls without the disease, and samples of primary aqueous humor from three people without the disorder who were undergoing cataract surgery (Lancet 2003;361:1258-65).
Abeta-40 and Abeta-42 were present in all of the lenses, in amounts similar to those seen in the corresponding brains. But in patients with Alzheimer’s, the protein aggregated into clumps within the lens fiber cells, forming unusual supranuclear cataracts at the equatorial periphery that appeared to be different from common age-related cataracts and that weren’t present in the control subjects.
The cataract location is an important clue to how long the Abeta has been accumulating, Dr. Goldstein said. Lens fiber cells are particularly long lived, remaining alive for as long as a person lives or until the lens is removed during cataract surgery. The lens starts to form in very early fetal life, with more and more lens cells forming in an outward direction, creating a virtual map of a person’s lifetime, "like the rings of a tree," he said.
Dr. Goldstein and his team discovered that these distinctive cataracts develop in some patients with Alzheimer’s. They appear toward the outer edge of the lens and are composed of the same toxic Abeta protein that builds up in the brain. "The history of amyloid in the body is time stamped in the lens," he said.
As lens fiber cells age, they lose most of their organelles and become transparent – just the right state for a device meant to focus light. "They also make tons of Abeta," Dr. Goldstein said, and it appears to have a very specific function in the eye, one Dr. Rudolph Tanzi and his colleagues at Massachusetts General Hospital, Boston, identified in collaboration with Dr. Goldstein’s team (PLoS ONE 2010;5:e9505).
"It turns out to be a very potent antimicrobial peptide," one of several the eye and brain produce to defend themselves. Amyloid’s sticky nature causes foreign invaders to clump together, so they’re more easily destroyed. This finding also suggests that Abeta could have a similar function in the brain, supporting some theories that Alzheimer’s might be at least partially triggered by a hyperinflammatory response toward an invading pathogen or another immunoreactive incident.
Testing lenses in Down syndrome patients
Interesting as all of that is, it doesn’t prove the theory that the lens amyloid record somehow tracks Alzheimer’s development. But other studies do explore that concept, including one Dr. Goldstein published in 2010. In this study, Dr. Goldstein and his colleagues examined lens amyloid in people with Down syndrome, a group predestined to develop Alzheimer’s (PLoS ONE 2010;5:e10659). The genetic mutation that causes the syndrome also increases production of the amyloid precursor protein (APP), Abeta’s antecedent.
The lenses from subjects with Down syndrome, aged 2-69 years, were compared with lenses from control subjects and people with both familial and late-onset Alzheimer’s. "The 2-year-old with Down syndrome in this study actually had more lens amyloid than the adults with familial Alzheimer’s," Dr. Goldstein said. In unpublished data, he added, the protein has even been observed in Down syndrome fetal lenses.
He expanded on this work in a poster presented at the 2013 Alzheimer’s Association International Conference. Dr. Goldstein and his team have developed and validated a laser eye scanning instrument that noninvasively measures how light is reflected from the tiniest particles – in this case clumps of Abeta protein – within the lens of living human subjects.
"We hypothesize that due to the trisomy of chromosome 21 in Down syndrome (and triplication of the APP gene), which results in increased expression of Abeta in the lens, the intensity of scattered light in Down syndrome patients will be higher than [in] age-matched controls," he noted in the poster.
Not everyone agrees with this idea, however. It has stirred controversy since he first introduced the idea, when, he said, "mainstream Alzheimer’s research simply didn’t believe it." In fact, at least two other researchers’ studies have come to quite different conclusions.
Dr. Charles Eberhart, a pathologist at Johns Hopkins University, Baltimore, published his data in the journal Brain Pathology (2013 June 28 [doi:10.1111/bpa.12070]). The study examined retinas, lenses, and brains from 11 patients with Alzheimer’s, 6 with Parkinson’s, and 6 age-matched controls. Eight eyes (five from Alzheimer’s patients and three from controls) did have cataracts. Dr. Eberhart and his colleagues used immunohistochemistry and Congo red staining to look for amyloid, phosphorylated tau, and alpha-synuclein.
"The short answer is – we didn’t find any amyloid deposits in the lens, or any abnormal tau accumulations," he said in an interview.
The study has two possible interpretations, he said: Either Abeta, tau, and synuclein don’t accumulate in Alzheimer’s eyes as they do in Alzheimer’s brains, or they are there, but simply not detected by his methods. "It certainly might be there. All we can say is that with this method, which is the accepted way of determining amyloid in brain tissue, we didn’t see it in eyes," he said.
The second study, conducted by Dr. Ralph Michael of the Universitat Autònoma de Barcelona and his colleagues, came to a similar conclusion (Exp. Eye Res. 2013;106:5-13). It involved 39 lenses and brains from 21 Alzheimer’s patients, and 15 lenses from age-matched controls. Six of the Alzheimer’s lenses and seven control lenses had cataracts. These investigators used staining methods similar to those in the Hopkins study.
"Beta-amyloid immunohistochemistry was positive in the brain tissues but not in the cornea sample," they wrote. "Lenses from control and AD [Alzheimer’s disease] donors were, without exception, negative after Congo red, thioflavin, and beta-amyloid immunohistochemical staining. ... The absence of staining in AD and control lenses with the techniques employed lead us to conclude that there is no beta-amyloid in lenses from donors with AD or in control cortical cataracts."
Dr. Goldstein said he doesn’t doubt these findings. Congo red staining yields a difficult-to-interpret sign, he said. Amyloid appears red under standard light spectroscopy, but takes on a very characteristic shade, called apple green under polarized light. "This is an old staining method that’s not very sensitive nor is it specific for Abeta – it’s also highly variable."
Technique is critical, he added. "It took us years to perfect our technique for the lens. It’s very difficult to work with lens, harder to work with old lens, and extremely hard to work with old, sick lens."
Instead of relying solely on Congo red or other staining techniques, Dr. Goldstein’s team confirmed their findings using a combination of biochemical analyses, immunogold electron microscopy, and two different types of mass spectrometry – methods he said are irrefutably accurate. "You can’t argue with this unless you are willing to argue with the very concept of mass spectrometry. It’s the gold standard," he said.
Confirmation in transgenic mice and Down syndrome patients strengthens the hypothesis, he said, as do the conclusions of his most recent paper. It looked at data from 1,249 people included in the Framingham Eye Study, and found a genetic link between a specific type of midlife cataracts (consistent with those previously found in Alzheimer’s) and later cognitive and brain structural changes associated with Alzheimer’s (PLoS ONE 2012;7:e43728) .
The culprit appeared to be a mutation of a gene that codes for delta-catenin, which Dr. Goldstein postulated may normally help suppress Abeta production. The altered form, however, appears to affect neuronal structure and is instead associated with an increase in Abeta-42 production in cell culture. The malformed delta-catenin protein was also found throughout the lenses of study subjects with Alzheimer’s, but not in control lenses.
Screening patients in the future?
Dr. Goldstein said he envisions a future in which annual lens exams might guide risk assessment and treatment initiation. But physicians who might someday screen patients certainly won’t have a mass spectrometer in the back room.
He has invented two devices, he said, that will fill that need. The most recent is a laser scanning ophthalmoscope that uses dynamic light scattering to detect the tiniest amyloid particles in the lens – particles less than 30 nm. This is the device he’s using in the ongoing Boston University/Boston Children’s Hospital study of lens amyloid in children with Down syndrome.
The second device combines optical imaging with aftobetin, a fluorescent amyloid ligand. Dr. Goldstein holds a patent on this device, which he invented in partnership with Cognoptix (formerly Neuroptix), a company he cofounded in 2001, although he is no longer operationally affiliated with it.
Cognoptix has developed the SAPPHIRE II system, a combination drug/device that detects amyloid in the lens using aftobetin. The company licensed aftobetin from the University of California, San Diego. It’s formulated into an ophthalmic ointment administered prior to scanning with the SAPPHIRE II system. The procedure uses fluorescent ligand scanning to detect amyloid aggregates in the lens, said Paul Hartung, president and chief executive officer of the Acton, Mass., company.
"We use an eye-safe laser tuned to pick up the fluorescence. It doesn’t require dilation of the pupil, and it has the capability of actually registering itself in the correct location in the eye," he said in an interview.
SAPPHIRE II has had a busy year, including a proof of concept study published in May and reported at the Alzheimer’s Association International Conference. In this study, the system successfully differentiated five Alzheimer’s patients from five controls (Front. Neurol. 2013 May 27 [doi:10.3389/fneur.2013.00062]).
Cognoptix has begun a second study testing the system against PET amyloid brain imaging in 20 patients with probable Alzheimer’s and 20 controls, Mr. Hartung said.
A third planned study is a pivotal phase III trial that will enroll 400 subjects, all of whom will undergo both the eye exam and PET amyloid imaging. It’s designed to support premarketing approval, Mr. Hartung said. Currently SAPPHIRE II has an investigational device exemption from the Food and Drug Administration’s Center for Devices and Radiological Health.
"Our end goal is to get this into the general practitioner’s office, where about 40% of Alzheimer’s drug prescriptions are written by general practitioners who really have no data on hand. Right now, based on cognitive assessments, they have only a 50-50 chance of getting the right diagnosis," Mr. Hartung said.
Dr. Goldstein and Mr. Hartung hold financial interests in devices to measure lens amyloid. Dr. Ralph Michael listed no financial disclosures. Dr. Charles Eberhart said he had no relevant financial disclosures.
On Twitter @Alz_Gal