User login
Alzheimer's Association International Conference 2013 (AAIC)
Diagnosing Alzheimer’s: The eyes may have it
You never know what will happen when you look into a mouse’s eyes.
Twelve years ago, Dr. Lee Goldstein was investigating reactive oxygen species’ effect on the brain of a young Alzheimer’s model mouse. Holding the tiny creature in his hand, he carefully inserted a miniscule microdialysis probe through its skull. As he did, he happened to look right into the mouse’s face. And since he was doing some unrelated work on cataracts at the time, something unusual caught his very practiced gaze.
"The mouse had a cataract. I looked at the other eye, and there was a cataract there, too. That’s very unusual – really not ever seen – in a mouse this age."
Then he looked at all the other mice he was using in that experiment, all of which were older. They all had bilateral cataracts. My first thought was, "This can’t be related to Alzheimer’s disease."
But in fact, he said, it appeared to be. He and his lab soon showed that the cataracts contained a large concentration of aggregated beta-amyloid (Abeta) in the same fraction that’s measured in today’s cerebrospinal fluid Alzheimer’s biomarker tests.
That first observation has birthed two investigational noninvasive amyloid eye tests, which Dr. Goldstein envisions could some day be part of everyone’s annual physical exam.
In people destined to develop Alzheimer’s, some research suggests that Abeta proteins may begin to accumulate in the lens long before they build up to dangerous levels in the brain. If this turns out to be a reliable marker of risk, it could be a sign that would trigger early, presymptomatic Alzheimer’s treatment.
That’s in the future, though, because right now, there is no such treatment. But at this time, Dr. Goldstein said, an amyloid eye test could prove invaluable in reaching that goal. One reason that symptomatic patients don’t respond to investigational drugs could be that by the time the patients are treated, irreversible brain damage has already occurred.
"Once you have cognitive symptoms, the horse is not only out of the barn, it’s run out of the state," said Dr. Goldstein, director of the molecular aging and development laboratory at Boston University. "I hate the term ‘mild cognitive impairment,’ because by the time you have that, there’s nothing mild about it."
Researchers now almost universally agree that the best way to get a true picture of any drug’s potential effectiveness in Alzheimer’s will be to implement treatment before symptoms set in. In addition, Dr. Goldstein said, "Research pools are polluted. Control groups contain subjects who would develop Alzheimer’s if they live long enough," which could be skewing study results. Lens amyloid measurements might help stratify groups in drug studies, and even be a way to track very early effect on amyloid.
But that is a future yet to be determined. In the meantime, researchers still need definitive proof that supranuclear amyloid cataracts are inextricably linked to the amyloid brain plaques of Alzheimer’s.
Initial findings
In 2003, Dr. Goldstein, then at Harvard Medical School, published his original proof of concept study. It comprised postmortem eye and brain specimens from nine subjects with Alzheimer’s and eight controls without the disease, and samples of primary aqueous humor from three people without the disorder who were undergoing cataract surgery (Lancet 2003;361:1258-65).
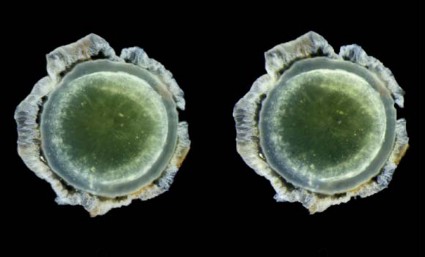
Abeta-40 and Abeta-42 were present in all of the lenses, in amounts similar to those seen in the corresponding brains. But in patients with Alzheimer’s, the protein aggregated into clumps within the lens fiber cells, forming unusual supranuclear cataracts at the equatorial periphery that appeared to be different from common age-related cataracts and that weren’t present in the control subjects.
The cataract location is an important clue to how long the Abeta has been accumulating, Dr. Goldstein said. Lens fiber cells are particularly long lived, remaining alive for as long as a person lives or until the lens is removed during cataract surgery. The lens starts to form in very early fetal life, with more and more lens cells forming in an outward direction, creating a virtual map of a person’s lifetime, "like the rings of a tree," he said.
Dr. Goldstein and his team discovered that these distinctive cataracts develop in some patients with Alzheimer’s. They appear toward the outer edge of the lens and are composed of the same toxic Abeta protein that builds up in the brain. "The history of amyloid in the body is time stamped in the lens," he said.
As lens fiber cells age, they lose most of their organelles and become transparent – just the right state for a device meant to focus light. "They also make tons of Abeta," Dr. Goldstein said, and it appears to have a very specific function in the eye, one Dr. Rudolph Tanzi and his colleagues at Massachusetts General Hospital, Boston, identified in collaboration with Dr. Goldstein’s team (PLoS ONE 2010;5:e9505).
"It turns out to be a very potent antimicrobial peptide," one of several the eye and brain produce to defend themselves. Amyloid’s sticky nature causes foreign invaders to clump together, so they’re more easily destroyed. This finding also suggests that Abeta could have a similar function in the brain, supporting some theories that Alzheimer’s might be at least partially triggered by a hyperinflammatory response toward an invading pathogen or another immunoreactive incident.
Testing lenses in Down syndrome patients
Interesting as all of that is, it doesn’t prove the theory that the lens amyloid record somehow tracks Alzheimer’s development. But other studies do explore that concept, including one Dr. Goldstein published in 2010. In this study, Dr. Goldstein and his colleagues examined lens amyloid in people with Down syndrome, a group predestined to develop Alzheimer’s (PLoS ONE 2010;5:e10659). The genetic mutation that causes the syndrome also increases production of the amyloid precursor protein (APP), Abeta’s antecedent.
The lenses from subjects with Down syndrome, aged 2-69 years, were compared with lenses from control subjects and people with both familial and late-onset Alzheimer’s. "The 2-year-old with Down syndrome in this study actually had more lens amyloid than the adults with familial Alzheimer’s," Dr. Goldstein said. In unpublished data, he added, the protein has even been observed in Down syndrome fetal lenses.
He expanded on this work in a poster presented at the 2013 Alzheimer’s Association International Conference. Dr. Goldstein and his team have developed and validated a laser eye scanning instrument that noninvasively measures how light is reflected from the tiniest particles – in this case clumps of Abeta protein – within the lens of living human subjects.
"We hypothesize that due to the trisomy of chromosome 21 in Down syndrome (and triplication of the APP gene), which results in increased expression of Abeta in the lens, the intensity of scattered light in Down syndrome patients will be higher than [in] age-matched controls," he noted in the poster.
Not everyone agrees with this idea, however. It has stirred controversy since he first introduced the idea, when, he said, "mainstream Alzheimer’s research simply didn’t believe it." In fact, at least two other researchers’ studies have come to quite different conclusions.
Dr. Charles Eberhart, a pathologist at Johns Hopkins University, Baltimore, published his data in the journal Brain Pathology (2013 June 28 [doi:10.1111/bpa.12070]). The study examined retinas, lenses, and brains from 11 patients with Alzheimer’s, 6 with Parkinson’s, and 6 age-matched controls. Eight eyes (five from Alzheimer’s patients and three from controls) did have cataracts. Dr. Eberhart and his colleagues used immunohistochemistry and Congo red staining to look for amyloid, phosphorylated tau, and alpha-synuclein.
"The short answer is – we didn’t find any amyloid deposits in the lens, or any abnormal tau accumulations," he said in an interview.
The study has two possible interpretations, he said: Either Abeta, tau, and synuclein don’t accumulate in Alzheimer’s eyes as they do in Alzheimer’s brains, or they are there, but simply not detected by his methods. "It certainly might be there. All we can say is that with this method, which is the accepted way of determining amyloid in brain tissue, we didn’t see it in eyes," he said.
The second study, conducted by Dr. Ralph Michael of the Universitat Autònoma de Barcelona and his colleagues, came to a similar conclusion (Exp. Eye Res. 2013;106:5-13). It involved 39 lenses and brains from 21 Alzheimer’s patients, and 15 lenses from age-matched controls. Six of the Alzheimer’s lenses and seven control lenses had cataracts. These investigators used staining methods similar to those in the Hopkins study.
"Beta-amyloid immunohistochemistry was positive in the brain tissues but not in the cornea sample," they wrote. "Lenses from control and AD [Alzheimer’s disease] donors were, without exception, negative after Congo red, thioflavin, and beta-amyloid immunohistochemical staining. ... The absence of staining in AD and control lenses with the techniques employed lead us to conclude that there is no beta-amyloid in lenses from donors with AD or in control cortical cataracts."
Dr. Goldstein said he doesn’t doubt these findings. Congo red staining yields a difficult-to-interpret sign, he said. Amyloid appears red under standard light spectroscopy, but takes on a very characteristic shade, called apple green under polarized light. "This is an old staining method that’s not very sensitive nor is it specific for Abeta – it’s also highly variable."
Technique is critical, he added. "It took us years to perfect our technique for the lens. It’s very difficult to work with lens, harder to work with old lens, and extremely hard to work with old, sick lens."
Instead of relying solely on Congo red or other staining techniques, Dr. Goldstein’s team confirmed their findings using a combination of biochemical analyses, immunogold electron microscopy, and two different types of mass spectrometry – methods he said are irrefutably accurate. "You can’t argue with this unless you are willing to argue with the very concept of mass spectrometry. It’s the gold standard," he said.
Confirmation in transgenic mice and Down syndrome patients strengthens the hypothesis, he said, as do the conclusions of his most recent paper. It looked at data from 1,249 people included in the Framingham Eye Study, and found a genetic link between a specific type of midlife cataracts (consistent with those previously found in Alzheimer’s) and later cognitive and brain structural changes associated with Alzheimer’s (PLoS ONE 2012;7:e43728) .
The culprit appeared to be a mutation of a gene that codes for delta-catenin, which Dr. Goldstein postulated may normally help suppress Abeta production. The altered form, however, appears to affect neuronal structure and is instead associated with an increase in Abeta-42 production in cell culture. The malformed delta-catenin protein was also found throughout the lenses of study subjects with Alzheimer’s, but not in control lenses.
Screening patients in the future?
Dr. Goldstein said he envisions a future in which annual lens exams might guide risk assessment and treatment initiation. But physicians who might someday screen patients certainly won’t have a mass spectrometer in the back room.
He has invented two devices, he said, that will fill that need. The most recent is a laser scanning ophthalmoscope that uses dynamic light scattering to detect the tiniest amyloid particles in the lens – particles less than 30 nm. This is the device he’s using in the ongoing Boston University/Boston Children’s Hospital study of lens amyloid in children with Down syndrome.
The second device combines optical imaging with aftobetin, a fluorescent amyloid ligand. Dr. Goldstein holds a patent on this device, which he invented in partnership with Cognoptix (formerly Neuroptix), a company he cofounded in 2001, although he is no longer operationally affiliated with it.
Cognoptix has developed the SAPPHIRE II system, a combination drug/device that detects amyloid in the lens using aftobetin. The company licensed aftobetin from the University of California, San Diego. It’s formulated into an ophthalmic ointment administered prior to scanning with the SAPPHIRE II system. The procedure uses fluorescent ligand scanning to detect amyloid aggregates in the lens, said Paul Hartung, president and chief executive officer of the Acton, Mass., company.
"We use an eye-safe laser tuned to pick up the fluorescence. It doesn’t require dilation of the pupil, and it has the capability of actually registering itself in the correct location in the eye," he said in an interview.
SAPPHIRE II has had a busy year, including a proof of concept study published in May and reported at the Alzheimer’s Association International Conference. In this study, the system successfully differentiated five Alzheimer’s patients from five controls (Front. Neurol. 2013 May 27 [doi:10.3389/fneur.2013.00062]).
Cognoptix has begun a second study testing the system against PET amyloid brain imaging in 20 patients with probable Alzheimer’s and 20 controls, Mr. Hartung said.
A third planned study is a pivotal phase III trial that will enroll 400 subjects, all of whom will undergo both the eye exam and PET amyloid imaging. It’s designed to support premarketing approval, Mr. Hartung said. Currently SAPPHIRE II has an investigational device exemption from the Food and Drug Administration’s Center for Devices and Radiological Health.
"Our end goal is to get this into the general practitioner’s office, where about 40% of Alzheimer’s drug prescriptions are written by general practitioners who really have no data on hand. Right now, based on cognitive assessments, they have only a 50-50 chance of getting the right diagnosis," Mr. Hartung said.
Dr. Goldstein and Mr. Hartung hold financial interests in devices to measure lens amyloid. Dr. Ralph Michael listed no financial disclosures. Dr. Charles Eberhart said he had no relevant financial disclosures.
On Twitter @Alz_Gal
You never know what will happen when you look into a mouse’s eyes.
Twelve years ago, Dr. Lee Goldstein was investigating reactive oxygen species’ effect on the brain of a young Alzheimer’s model mouse. Holding the tiny creature in his hand, he carefully inserted a miniscule microdialysis probe through its skull. As he did, he happened to look right into the mouse’s face. And since he was doing some unrelated work on cataracts at the time, something unusual caught his very practiced gaze.
"The mouse had a cataract. I looked at the other eye, and there was a cataract there, too. That’s very unusual – really not ever seen – in a mouse this age."
Then he looked at all the other mice he was using in that experiment, all of which were older. They all had bilateral cataracts. My first thought was, "This can’t be related to Alzheimer’s disease."
But in fact, he said, it appeared to be. He and his lab soon showed that the cataracts contained a large concentration of aggregated beta-amyloid (Abeta) in the same fraction that’s measured in today’s cerebrospinal fluid Alzheimer’s biomarker tests.
That first observation has birthed two investigational noninvasive amyloid eye tests, which Dr. Goldstein envisions could some day be part of everyone’s annual physical exam.
In people destined to develop Alzheimer’s, some research suggests that Abeta proteins may begin to accumulate in the lens long before they build up to dangerous levels in the brain. If this turns out to be a reliable marker of risk, it could be a sign that would trigger early, presymptomatic Alzheimer’s treatment.
That’s in the future, though, because right now, there is no such treatment. But at this time, Dr. Goldstein said, an amyloid eye test could prove invaluable in reaching that goal. One reason that symptomatic patients don’t respond to investigational drugs could be that by the time the patients are treated, irreversible brain damage has already occurred.
"Once you have cognitive symptoms, the horse is not only out of the barn, it’s run out of the state," said Dr. Goldstein, director of the molecular aging and development laboratory at Boston University. "I hate the term ‘mild cognitive impairment,’ because by the time you have that, there’s nothing mild about it."
Researchers now almost universally agree that the best way to get a true picture of any drug’s potential effectiveness in Alzheimer’s will be to implement treatment before symptoms set in. In addition, Dr. Goldstein said, "Research pools are polluted. Control groups contain subjects who would develop Alzheimer’s if they live long enough," which could be skewing study results. Lens amyloid measurements might help stratify groups in drug studies, and even be a way to track very early effect on amyloid.
But that is a future yet to be determined. In the meantime, researchers still need definitive proof that supranuclear amyloid cataracts are inextricably linked to the amyloid brain plaques of Alzheimer’s.
Initial findings
In 2003, Dr. Goldstein, then at Harvard Medical School, published his original proof of concept study. It comprised postmortem eye and brain specimens from nine subjects with Alzheimer’s and eight controls without the disease, and samples of primary aqueous humor from three people without the disorder who were undergoing cataract surgery (Lancet 2003;361:1258-65).
Abeta-40 and Abeta-42 were present in all of the lenses, in amounts similar to those seen in the corresponding brains. But in patients with Alzheimer’s, the protein aggregated into clumps within the lens fiber cells, forming unusual supranuclear cataracts at the equatorial periphery that appeared to be different from common age-related cataracts and that weren’t present in the control subjects.
The cataract location is an important clue to how long the Abeta has been accumulating, Dr. Goldstein said. Lens fiber cells are particularly long lived, remaining alive for as long as a person lives or until the lens is removed during cataract surgery. The lens starts to form in very early fetal life, with more and more lens cells forming in an outward direction, creating a virtual map of a person’s lifetime, "like the rings of a tree," he said.
Dr. Goldstein and his team discovered that these distinctive cataracts develop in some patients with Alzheimer’s. They appear toward the outer edge of the lens and are composed of the same toxic Abeta protein that builds up in the brain. "The history of amyloid in the body is time stamped in the lens," he said.
As lens fiber cells age, they lose most of their organelles and become transparent – just the right state for a device meant to focus light. "They also make tons of Abeta," Dr. Goldstein said, and it appears to have a very specific function in the eye, one Dr. Rudolph Tanzi and his colleagues at Massachusetts General Hospital, Boston, identified in collaboration with Dr. Goldstein’s team (PLoS ONE 2010;5:e9505).
"It turns out to be a very potent antimicrobial peptide," one of several the eye and brain produce to defend themselves. Amyloid’s sticky nature causes foreign invaders to clump together, so they’re more easily destroyed. This finding also suggests that Abeta could have a similar function in the brain, supporting some theories that Alzheimer’s might be at least partially triggered by a hyperinflammatory response toward an invading pathogen or another immunoreactive incident.
Testing lenses in Down syndrome patients
Interesting as all of that is, it doesn’t prove the theory that the lens amyloid record somehow tracks Alzheimer’s development. But other studies do explore that concept, including one Dr. Goldstein published in 2010. In this study, Dr. Goldstein and his colleagues examined lens amyloid in people with Down syndrome, a group predestined to develop Alzheimer’s (PLoS ONE 2010;5:e10659). The genetic mutation that causes the syndrome also increases production of the amyloid precursor protein (APP), Abeta’s antecedent.
The lenses from subjects with Down syndrome, aged 2-69 years, were compared with lenses from control subjects and people with both familial and late-onset Alzheimer’s. "The 2-year-old with Down syndrome in this study actually had more lens amyloid than the adults with familial Alzheimer’s," Dr. Goldstein said. In unpublished data, he added, the protein has even been observed in Down syndrome fetal lenses.
He expanded on this work in a poster presented at the 2013 Alzheimer’s Association International Conference. Dr. Goldstein and his team have developed and validated a laser eye scanning instrument that noninvasively measures how light is reflected from the tiniest particles – in this case clumps of Abeta protein – within the lens of living human subjects.
"We hypothesize that due to the trisomy of chromosome 21 in Down syndrome (and triplication of the APP gene), which results in increased expression of Abeta in the lens, the intensity of scattered light in Down syndrome patients will be higher than [in] age-matched controls," he noted in the poster.
Not everyone agrees with this idea, however. It has stirred controversy since he first introduced the idea, when, he said, "mainstream Alzheimer’s research simply didn’t believe it." In fact, at least two other researchers’ studies have come to quite different conclusions.
Dr. Charles Eberhart, a pathologist at Johns Hopkins University, Baltimore, published his data in the journal Brain Pathology (2013 June 28 [doi:10.1111/bpa.12070]). The study examined retinas, lenses, and brains from 11 patients with Alzheimer’s, 6 with Parkinson’s, and 6 age-matched controls. Eight eyes (five from Alzheimer’s patients and three from controls) did have cataracts. Dr. Eberhart and his colleagues used immunohistochemistry and Congo red staining to look for amyloid, phosphorylated tau, and alpha-synuclein.
"The short answer is – we didn’t find any amyloid deposits in the lens, or any abnormal tau accumulations," he said in an interview.
The study has two possible interpretations, he said: Either Abeta, tau, and synuclein don’t accumulate in Alzheimer’s eyes as they do in Alzheimer’s brains, or they are there, but simply not detected by his methods. "It certainly might be there. All we can say is that with this method, which is the accepted way of determining amyloid in brain tissue, we didn’t see it in eyes," he said.
The second study, conducted by Dr. Ralph Michael of the Universitat Autònoma de Barcelona and his colleagues, came to a similar conclusion (Exp. Eye Res. 2013;106:5-13). It involved 39 lenses and brains from 21 Alzheimer’s patients, and 15 lenses from age-matched controls. Six of the Alzheimer’s lenses and seven control lenses had cataracts. These investigators used staining methods similar to those in the Hopkins study.
"Beta-amyloid immunohistochemistry was positive in the brain tissues but not in the cornea sample," they wrote. "Lenses from control and AD [Alzheimer’s disease] donors were, without exception, negative after Congo red, thioflavin, and beta-amyloid immunohistochemical staining. ... The absence of staining in AD and control lenses with the techniques employed lead us to conclude that there is no beta-amyloid in lenses from donors with AD or in control cortical cataracts."
Dr. Goldstein said he doesn’t doubt these findings. Congo red staining yields a difficult-to-interpret sign, he said. Amyloid appears red under standard light spectroscopy, but takes on a very characteristic shade, called apple green under polarized light. "This is an old staining method that’s not very sensitive nor is it specific for Abeta – it’s also highly variable."
Technique is critical, he added. "It took us years to perfect our technique for the lens. It’s very difficult to work with lens, harder to work with old lens, and extremely hard to work with old, sick lens."
Instead of relying solely on Congo red or other staining techniques, Dr. Goldstein’s team confirmed their findings using a combination of biochemical analyses, immunogold electron microscopy, and two different types of mass spectrometry – methods he said are irrefutably accurate. "You can’t argue with this unless you are willing to argue with the very concept of mass spectrometry. It’s the gold standard," he said.
Confirmation in transgenic mice and Down syndrome patients strengthens the hypothesis, he said, as do the conclusions of his most recent paper. It looked at data from 1,249 people included in the Framingham Eye Study, and found a genetic link between a specific type of midlife cataracts (consistent with those previously found in Alzheimer’s) and later cognitive and brain structural changes associated with Alzheimer’s (PLoS ONE 2012;7:e43728) .
The culprit appeared to be a mutation of a gene that codes for delta-catenin, which Dr. Goldstein postulated may normally help suppress Abeta production. The altered form, however, appears to affect neuronal structure and is instead associated with an increase in Abeta-42 production in cell culture. The malformed delta-catenin protein was also found throughout the lenses of study subjects with Alzheimer’s, but not in control lenses.
Screening patients in the future?
Dr. Goldstein said he envisions a future in which annual lens exams might guide risk assessment and treatment initiation. But physicians who might someday screen patients certainly won’t have a mass spectrometer in the back room.
He has invented two devices, he said, that will fill that need. The most recent is a laser scanning ophthalmoscope that uses dynamic light scattering to detect the tiniest amyloid particles in the lens – particles less than 30 nm. This is the device he’s using in the ongoing Boston University/Boston Children’s Hospital study of lens amyloid in children with Down syndrome.
The second device combines optical imaging with aftobetin, a fluorescent amyloid ligand. Dr. Goldstein holds a patent on this device, which he invented in partnership with Cognoptix (formerly Neuroptix), a company he cofounded in 2001, although he is no longer operationally affiliated with it.
Cognoptix has developed the SAPPHIRE II system, a combination drug/device that detects amyloid in the lens using aftobetin. The company licensed aftobetin from the University of California, San Diego. It’s formulated into an ophthalmic ointment administered prior to scanning with the SAPPHIRE II system. The procedure uses fluorescent ligand scanning to detect amyloid aggregates in the lens, said Paul Hartung, president and chief executive officer of the Acton, Mass., company.
"We use an eye-safe laser tuned to pick up the fluorescence. It doesn’t require dilation of the pupil, and it has the capability of actually registering itself in the correct location in the eye," he said in an interview.
SAPPHIRE II has had a busy year, including a proof of concept study published in May and reported at the Alzheimer’s Association International Conference. In this study, the system successfully differentiated five Alzheimer’s patients from five controls (Front. Neurol. 2013 May 27 [doi:10.3389/fneur.2013.00062]).
Cognoptix has begun a second study testing the system against PET amyloid brain imaging in 20 patients with probable Alzheimer’s and 20 controls, Mr. Hartung said.
A third planned study is a pivotal phase III trial that will enroll 400 subjects, all of whom will undergo both the eye exam and PET amyloid imaging. It’s designed to support premarketing approval, Mr. Hartung said. Currently SAPPHIRE II has an investigational device exemption from the Food and Drug Administration’s Center for Devices and Radiological Health.
"Our end goal is to get this into the general practitioner’s office, where about 40% of Alzheimer’s drug prescriptions are written by general practitioners who really have no data on hand. Right now, based on cognitive assessments, they have only a 50-50 chance of getting the right diagnosis," Mr. Hartung said.
Dr. Goldstein and Mr. Hartung hold financial interests in devices to measure lens amyloid. Dr. Ralph Michael listed no financial disclosures. Dr. Charles Eberhart said he had no relevant financial disclosures.
On Twitter @Alz_Gal
You never know what will happen when you look into a mouse’s eyes.
Twelve years ago, Dr. Lee Goldstein was investigating reactive oxygen species’ effect on the brain of a young Alzheimer’s model mouse. Holding the tiny creature in his hand, he carefully inserted a miniscule microdialysis probe through its skull. As he did, he happened to look right into the mouse’s face. And since he was doing some unrelated work on cataracts at the time, something unusual caught his very practiced gaze.
"The mouse had a cataract. I looked at the other eye, and there was a cataract there, too. That’s very unusual – really not ever seen – in a mouse this age."
Then he looked at all the other mice he was using in that experiment, all of which were older. They all had bilateral cataracts. My first thought was, "This can’t be related to Alzheimer’s disease."
But in fact, he said, it appeared to be. He and his lab soon showed that the cataracts contained a large concentration of aggregated beta-amyloid (Abeta) in the same fraction that’s measured in today’s cerebrospinal fluid Alzheimer’s biomarker tests.
That first observation has birthed two investigational noninvasive amyloid eye tests, which Dr. Goldstein envisions could some day be part of everyone’s annual physical exam.
In people destined to develop Alzheimer’s, some research suggests that Abeta proteins may begin to accumulate in the lens long before they build up to dangerous levels in the brain. If this turns out to be a reliable marker of risk, it could be a sign that would trigger early, presymptomatic Alzheimer’s treatment.
That’s in the future, though, because right now, there is no such treatment. But at this time, Dr. Goldstein said, an amyloid eye test could prove invaluable in reaching that goal. One reason that symptomatic patients don’t respond to investigational drugs could be that by the time the patients are treated, irreversible brain damage has already occurred.
"Once you have cognitive symptoms, the horse is not only out of the barn, it’s run out of the state," said Dr. Goldstein, director of the molecular aging and development laboratory at Boston University. "I hate the term ‘mild cognitive impairment,’ because by the time you have that, there’s nothing mild about it."
Researchers now almost universally agree that the best way to get a true picture of any drug’s potential effectiveness in Alzheimer’s will be to implement treatment before symptoms set in. In addition, Dr. Goldstein said, "Research pools are polluted. Control groups contain subjects who would develop Alzheimer’s if they live long enough," which could be skewing study results. Lens amyloid measurements might help stratify groups in drug studies, and even be a way to track very early effect on amyloid.
But that is a future yet to be determined. In the meantime, researchers still need definitive proof that supranuclear amyloid cataracts are inextricably linked to the amyloid brain plaques of Alzheimer’s.
Initial findings
In 2003, Dr. Goldstein, then at Harvard Medical School, published his original proof of concept study. It comprised postmortem eye and brain specimens from nine subjects with Alzheimer’s and eight controls without the disease, and samples of primary aqueous humor from three people without the disorder who were undergoing cataract surgery (Lancet 2003;361:1258-65).
Abeta-40 and Abeta-42 were present in all of the lenses, in amounts similar to those seen in the corresponding brains. But in patients with Alzheimer’s, the protein aggregated into clumps within the lens fiber cells, forming unusual supranuclear cataracts at the equatorial periphery that appeared to be different from common age-related cataracts and that weren’t present in the control subjects.
The cataract location is an important clue to how long the Abeta has been accumulating, Dr. Goldstein said. Lens fiber cells are particularly long lived, remaining alive for as long as a person lives or until the lens is removed during cataract surgery. The lens starts to form in very early fetal life, with more and more lens cells forming in an outward direction, creating a virtual map of a person’s lifetime, "like the rings of a tree," he said.
Dr. Goldstein and his team discovered that these distinctive cataracts develop in some patients with Alzheimer’s. They appear toward the outer edge of the lens and are composed of the same toxic Abeta protein that builds up in the brain. "The history of amyloid in the body is time stamped in the lens," he said.
As lens fiber cells age, they lose most of their organelles and become transparent – just the right state for a device meant to focus light. "They also make tons of Abeta," Dr. Goldstein said, and it appears to have a very specific function in the eye, one Dr. Rudolph Tanzi and his colleagues at Massachusetts General Hospital, Boston, identified in collaboration with Dr. Goldstein’s team (PLoS ONE 2010;5:e9505).
"It turns out to be a very potent antimicrobial peptide," one of several the eye and brain produce to defend themselves. Amyloid’s sticky nature causes foreign invaders to clump together, so they’re more easily destroyed. This finding also suggests that Abeta could have a similar function in the brain, supporting some theories that Alzheimer’s might be at least partially triggered by a hyperinflammatory response toward an invading pathogen or another immunoreactive incident.
Testing lenses in Down syndrome patients
Interesting as all of that is, it doesn’t prove the theory that the lens amyloid record somehow tracks Alzheimer’s development. But other studies do explore that concept, including one Dr. Goldstein published in 2010. In this study, Dr. Goldstein and his colleagues examined lens amyloid in people with Down syndrome, a group predestined to develop Alzheimer’s (PLoS ONE 2010;5:e10659). The genetic mutation that causes the syndrome also increases production of the amyloid precursor protein (APP), Abeta’s antecedent.
The lenses from subjects with Down syndrome, aged 2-69 years, were compared with lenses from control subjects and people with both familial and late-onset Alzheimer’s. "The 2-year-old with Down syndrome in this study actually had more lens amyloid than the adults with familial Alzheimer’s," Dr. Goldstein said. In unpublished data, he added, the protein has even been observed in Down syndrome fetal lenses.
He expanded on this work in a poster presented at the 2013 Alzheimer’s Association International Conference. Dr. Goldstein and his team have developed and validated a laser eye scanning instrument that noninvasively measures how light is reflected from the tiniest particles – in this case clumps of Abeta protein – within the lens of living human subjects.
"We hypothesize that due to the trisomy of chromosome 21 in Down syndrome (and triplication of the APP gene), which results in increased expression of Abeta in the lens, the intensity of scattered light in Down syndrome patients will be higher than [in] age-matched controls," he noted in the poster.
Not everyone agrees with this idea, however. It has stirred controversy since he first introduced the idea, when, he said, "mainstream Alzheimer’s research simply didn’t believe it." In fact, at least two other researchers’ studies have come to quite different conclusions.
Dr. Charles Eberhart, a pathologist at Johns Hopkins University, Baltimore, published his data in the journal Brain Pathology (2013 June 28 [doi:10.1111/bpa.12070]). The study examined retinas, lenses, and brains from 11 patients with Alzheimer’s, 6 with Parkinson’s, and 6 age-matched controls. Eight eyes (five from Alzheimer’s patients and three from controls) did have cataracts. Dr. Eberhart and his colleagues used immunohistochemistry and Congo red staining to look for amyloid, phosphorylated tau, and alpha-synuclein.
"The short answer is – we didn’t find any amyloid deposits in the lens, or any abnormal tau accumulations," he said in an interview.
The study has two possible interpretations, he said: Either Abeta, tau, and synuclein don’t accumulate in Alzheimer’s eyes as they do in Alzheimer’s brains, or they are there, but simply not detected by his methods. "It certainly might be there. All we can say is that with this method, which is the accepted way of determining amyloid in brain tissue, we didn’t see it in eyes," he said.
The second study, conducted by Dr. Ralph Michael of the Universitat Autònoma de Barcelona and his colleagues, came to a similar conclusion (Exp. Eye Res. 2013;106:5-13). It involved 39 lenses and brains from 21 Alzheimer’s patients, and 15 lenses from age-matched controls. Six of the Alzheimer’s lenses and seven control lenses had cataracts. These investigators used staining methods similar to those in the Hopkins study.
"Beta-amyloid immunohistochemistry was positive in the brain tissues but not in the cornea sample," they wrote. "Lenses from control and AD [Alzheimer’s disease] donors were, without exception, negative after Congo red, thioflavin, and beta-amyloid immunohistochemical staining. ... The absence of staining in AD and control lenses with the techniques employed lead us to conclude that there is no beta-amyloid in lenses from donors with AD or in control cortical cataracts."
Dr. Goldstein said he doesn’t doubt these findings. Congo red staining yields a difficult-to-interpret sign, he said. Amyloid appears red under standard light spectroscopy, but takes on a very characteristic shade, called apple green under polarized light. "This is an old staining method that’s not very sensitive nor is it specific for Abeta – it’s also highly variable."
Technique is critical, he added. "It took us years to perfect our technique for the lens. It’s very difficult to work with lens, harder to work with old lens, and extremely hard to work with old, sick lens."
Instead of relying solely on Congo red or other staining techniques, Dr. Goldstein’s team confirmed their findings using a combination of biochemical analyses, immunogold electron microscopy, and two different types of mass spectrometry – methods he said are irrefutably accurate. "You can’t argue with this unless you are willing to argue with the very concept of mass spectrometry. It’s the gold standard," he said.
Confirmation in transgenic mice and Down syndrome patients strengthens the hypothesis, he said, as do the conclusions of his most recent paper. It looked at data from 1,249 people included in the Framingham Eye Study, and found a genetic link between a specific type of midlife cataracts (consistent with those previously found in Alzheimer’s) and later cognitive and brain structural changes associated with Alzheimer’s (PLoS ONE 2012;7:e43728) .
The culprit appeared to be a mutation of a gene that codes for delta-catenin, which Dr. Goldstein postulated may normally help suppress Abeta production. The altered form, however, appears to affect neuronal structure and is instead associated with an increase in Abeta-42 production in cell culture. The malformed delta-catenin protein was also found throughout the lenses of study subjects with Alzheimer’s, but not in control lenses.
Screening patients in the future?
Dr. Goldstein said he envisions a future in which annual lens exams might guide risk assessment and treatment initiation. But physicians who might someday screen patients certainly won’t have a mass spectrometer in the back room.
He has invented two devices, he said, that will fill that need. The most recent is a laser scanning ophthalmoscope that uses dynamic light scattering to detect the tiniest amyloid particles in the lens – particles less than 30 nm. This is the device he’s using in the ongoing Boston University/Boston Children’s Hospital study of lens amyloid in children with Down syndrome.
The second device combines optical imaging with aftobetin, a fluorescent amyloid ligand. Dr. Goldstein holds a patent on this device, which he invented in partnership with Cognoptix (formerly Neuroptix), a company he cofounded in 2001, although he is no longer operationally affiliated with it.
Cognoptix has developed the SAPPHIRE II system, a combination drug/device that detects amyloid in the lens using aftobetin. The company licensed aftobetin from the University of California, San Diego. It’s formulated into an ophthalmic ointment administered prior to scanning with the SAPPHIRE II system. The procedure uses fluorescent ligand scanning to detect amyloid aggregates in the lens, said Paul Hartung, president and chief executive officer of the Acton, Mass., company.
"We use an eye-safe laser tuned to pick up the fluorescence. It doesn’t require dilation of the pupil, and it has the capability of actually registering itself in the correct location in the eye," he said in an interview.
SAPPHIRE II has had a busy year, including a proof of concept study published in May and reported at the Alzheimer’s Association International Conference. In this study, the system successfully differentiated five Alzheimer’s patients from five controls (Front. Neurol. 2013 May 27 [doi:10.3389/fneur.2013.00062]).
Cognoptix has begun a second study testing the system against PET amyloid brain imaging in 20 patients with probable Alzheimer’s and 20 controls, Mr. Hartung said.
A third planned study is a pivotal phase III trial that will enroll 400 subjects, all of whom will undergo both the eye exam and PET amyloid imaging. It’s designed to support premarketing approval, Mr. Hartung said. Currently SAPPHIRE II has an investigational device exemption from the Food and Drug Administration’s Center for Devices and Radiological Health.
"Our end goal is to get this into the general practitioner’s office, where about 40% of Alzheimer’s drug prescriptions are written by general practitioners who really have no data on hand. Right now, based on cognitive assessments, they have only a 50-50 chance of getting the right diagnosis," Mr. Hartung said.
Dr. Goldstein and Mr. Hartung hold financial interests in devices to measure lens amyloid. Dr. Ralph Michael listed no financial disclosures. Dr. Charles Eberhart said he had no relevant financial disclosures.
On Twitter @Alz_Gal
Caregiver support program decreases dementia emergency visits
BOSTON – A pilot program that supports the caregivers of Alzheimer’s disease patients decreased emergency admissions for those patients in San Francisco by more than 40% in 6 months, Elizabeth Edgerly, Ph.D., reported at the Alzheimer’s Association International Conference 2013.
The preliminary analysis also found that caregivers reported significant improvements in 7 out of 10 quality of life and quality of care measures, according to Dr. Edgerly, chief program officer of the Alzheimer’s Association Northern California and Northern Nevada Chapter.
The early results paint an encouraging picture of the future, she said in an interview.
"We are very excited about the emergency utilization data. We’re always confident about our capacity to impact efficacy, but affecting service utilization is a tough nut to crack. The beauty of this is, if we can improve quality of care while reducing utilization costs, it may actually be cost neutral to have this kind of a dementia support program."
The association created its Excellence in Dementia Care program in conjunction with Kaiser Permanente Northern California, the city and county of San Francisco, and the University of California, San Francisco. The Administration on Aging also provided funding.
San Francisco was the perfect city for the pilot project, Dr. Edgerly said. "It’s the only city in the United States with a strategic plan related to dementia. It also has an elderly, diverse population and many of the residents live alone."
The pilot program is part of the city plan’s goal of partnering with other institutions to improve quality of life and quality of care for people with dementia. It was designed to improve dementia care by both enhancing services and educating caregivers.
"Kaiser hired a full-time social worker for just dementia support and who only worked with the caregivers," Dr. Edgerly said. The social worker conducted initial evaluations and assessments and created an individualized dementia care program that was uploaded into the electronic medical record of each patient, making the individualized program available to everyone on the patient’s care team. The social worker also called caregivers proactively to make sure the caregivers’ needs were being met and that they could access community services.
The Alzheimer’s Association provided dementia care support experts manning a 24-hour help line, a MedicAlert + Alzheimer’s Association Safe Return bracelet for the patient, respite grants for day or evening supplemental care to give caregivers a break, and a support group where caregivers could meet to discusses their challenges.
The association also administered the educational portion of the program. The first component provided a primer on Alzheimer’s stage-by-stage effects on thinking, emotions, and behavior, and noted resources that were available for help. Caregivers also were informed about legal and financial planning and ways to keep a positive, safe, and compassionate home environment for as long as the patient could stay at home.
In surveys at baseline and after 6 months, caregivers rated their feelings about their abilities in 10 different areas: handling current patient problems in memory and behavior, handling future patient problems, dealing with their own frustrations, keeping the patient independent, caring for the patient as independently as possible, getting answers to patient problems, finding community organizations that provide answers, finding community organizations that provide services, getting answers to questions about services, independently arranging for services, and paying for services.
Overall, 92 of the 105 patient-caregiver dyads have completed the 6-month assessments, Dr. Edgerly said. The surveys indicated significant improvements on seven of the caregiver measures. Caregivers said they felt better able to handle concerns, to get information, and to obtain and pay for services.
Most importantly, the program led to about a 40% decrease in emergency department visits – a significant change, Dr. Edgerly said. There were also nonsignificant decreases in hospital length of stay, physician visits, and days spent in post–acute or long-term care facilities.
"Even the outcomes that didn’t improve significantly were all going in the right direction," Dr. Edgerly said, adding that she’s hoping for even better results when the data are analyzed in their entirety.
The program’s positive impact on health care resources could be enough to attract the interest of other insurers, she said. "If this intervention reduced expenses associated with hospital or emergency department visits, an insurer might see that as good business sense."
The project was funded by the Alzheimer’s Association of Northern California and Northern Nevada, Kaiser Permanente Northern California, and the National Institute on Aging.
On Twitter @Alz_Gal
BOSTON – A pilot program that supports the caregivers of Alzheimer’s disease patients decreased emergency admissions for those patients in San Francisco by more than 40% in 6 months, Elizabeth Edgerly, Ph.D., reported at the Alzheimer’s Association International Conference 2013.
The preliminary analysis also found that caregivers reported significant improvements in 7 out of 10 quality of life and quality of care measures, according to Dr. Edgerly, chief program officer of the Alzheimer’s Association Northern California and Northern Nevada Chapter.
The early results paint an encouraging picture of the future, she said in an interview.
"We are very excited about the emergency utilization data. We’re always confident about our capacity to impact efficacy, but affecting service utilization is a tough nut to crack. The beauty of this is, if we can improve quality of care while reducing utilization costs, it may actually be cost neutral to have this kind of a dementia support program."
The association created its Excellence in Dementia Care program in conjunction with Kaiser Permanente Northern California, the city and county of San Francisco, and the University of California, San Francisco. The Administration on Aging also provided funding.
San Francisco was the perfect city for the pilot project, Dr. Edgerly said. "It’s the only city in the United States with a strategic plan related to dementia. It also has an elderly, diverse population and many of the residents live alone."
The pilot program is part of the city plan’s goal of partnering with other institutions to improve quality of life and quality of care for people with dementia. It was designed to improve dementia care by both enhancing services and educating caregivers.
"Kaiser hired a full-time social worker for just dementia support and who only worked with the caregivers," Dr. Edgerly said. The social worker conducted initial evaluations and assessments and created an individualized dementia care program that was uploaded into the electronic medical record of each patient, making the individualized program available to everyone on the patient’s care team. The social worker also called caregivers proactively to make sure the caregivers’ needs were being met and that they could access community services.
The Alzheimer’s Association provided dementia care support experts manning a 24-hour help line, a MedicAlert + Alzheimer’s Association Safe Return bracelet for the patient, respite grants for day or evening supplemental care to give caregivers a break, and a support group where caregivers could meet to discusses their challenges.
The association also administered the educational portion of the program. The first component provided a primer on Alzheimer’s stage-by-stage effects on thinking, emotions, and behavior, and noted resources that were available for help. Caregivers also were informed about legal and financial planning and ways to keep a positive, safe, and compassionate home environment for as long as the patient could stay at home.
In surveys at baseline and after 6 months, caregivers rated their feelings about their abilities in 10 different areas: handling current patient problems in memory and behavior, handling future patient problems, dealing with their own frustrations, keeping the patient independent, caring for the patient as independently as possible, getting answers to patient problems, finding community organizations that provide answers, finding community organizations that provide services, getting answers to questions about services, independently arranging for services, and paying for services.
Overall, 92 of the 105 patient-caregiver dyads have completed the 6-month assessments, Dr. Edgerly said. The surveys indicated significant improvements on seven of the caregiver measures. Caregivers said they felt better able to handle concerns, to get information, and to obtain and pay for services.
Most importantly, the program led to about a 40% decrease in emergency department visits – a significant change, Dr. Edgerly said. There were also nonsignificant decreases in hospital length of stay, physician visits, and days spent in post–acute or long-term care facilities.
"Even the outcomes that didn’t improve significantly were all going in the right direction," Dr. Edgerly said, adding that she’s hoping for even better results when the data are analyzed in their entirety.
The program’s positive impact on health care resources could be enough to attract the interest of other insurers, she said. "If this intervention reduced expenses associated with hospital or emergency department visits, an insurer might see that as good business sense."
The project was funded by the Alzheimer’s Association of Northern California and Northern Nevada, Kaiser Permanente Northern California, and the National Institute on Aging.
On Twitter @Alz_Gal
BOSTON – A pilot program that supports the caregivers of Alzheimer’s disease patients decreased emergency admissions for those patients in San Francisco by more than 40% in 6 months, Elizabeth Edgerly, Ph.D., reported at the Alzheimer’s Association International Conference 2013.
The preliminary analysis also found that caregivers reported significant improvements in 7 out of 10 quality of life and quality of care measures, according to Dr. Edgerly, chief program officer of the Alzheimer’s Association Northern California and Northern Nevada Chapter.
The early results paint an encouraging picture of the future, she said in an interview.
"We are very excited about the emergency utilization data. We’re always confident about our capacity to impact efficacy, but affecting service utilization is a tough nut to crack. The beauty of this is, if we can improve quality of care while reducing utilization costs, it may actually be cost neutral to have this kind of a dementia support program."
The association created its Excellence in Dementia Care program in conjunction with Kaiser Permanente Northern California, the city and county of San Francisco, and the University of California, San Francisco. The Administration on Aging also provided funding.
San Francisco was the perfect city for the pilot project, Dr. Edgerly said. "It’s the only city in the United States with a strategic plan related to dementia. It also has an elderly, diverse population and many of the residents live alone."
The pilot program is part of the city plan’s goal of partnering with other institutions to improve quality of life and quality of care for people with dementia. It was designed to improve dementia care by both enhancing services and educating caregivers.
"Kaiser hired a full-time social worker for just dementia support and who only worked with the caregivers," Dr. Edgerly said. The social worker conducted initial evaluations and assessments and created an individualized dementia care program that was uploaded into the electronic medical record of each patient, making the individualized program available to everyone on the patient’s care team. The social worker also called caregivers proactively to make sure the caregivers’ needs were being met and that they could access community services.
The Alzheimer’s Association provided dementia care support experts manning a 24-hour help line, a MedicAlert + Alzheimer’s Association Safe Return bracelet for the patient, respite grants for day or evening supplemental care to give caregivers a break, and a support group where caregivers could meet to discusses their challenges.
The association also administered the educational portion of the program. The first component provided a primer on Alzheimer’s stage-by-stage effects on thinking, emotions, and behavior, and noted resources that were available for help. Caregivers also were informed about legal and financial planning and ways to keep a positive, safe, and compassionate home environment for as long as the patient could stay at home.
In surveys at baseline and after 6 months, caregivers rated their feelings about their abilities in 10 different areas: handling current patient problems in memory and behavior, handling future patient problems, dealing with their own frustrations, keeping the patient independent, caring for the patient as independently as possible, getting answers to patient problems, finding community organizations that provide answers, finding community organizations that provide services, getting answers to questions about services, independently arranging for services, and paying for services.
Overall, 92 of the 105 patient-caregiver dyads have completed the 6-month assessments, Dr. Edgerly said. The surveys indicated significant improvements on seven of the caregiver measures. Caregivers said they felt better able to handle concerns, to get information, and to obtain and pay for services.
Most importantly, the program led to about a 40% decrease in emergency department visits – a significant change, Dr. Edgerly said. There were also nonsignificant decreases in hospital length of stay, physician visits, and days spent in post–acute or long-term care facilities.
"Even the outcomes that didn’t improve significantly were all going in the right direction," Dr. Edgerly said, adding that she’s hoping for even better results when the data are analyzed in their entirety.
The program’s positive impact on health care resources could be enough to attract the interest of other insurers, she said. "If this intervention reduced expenses associated with hospital or emergency department visits, an insurer might see that as good business sense."
The project was funded by the Alzheimer’s Association of Northern California and Northern Nevada, Kaiser Permanente Northern California, and the National Institute on Aging.
On Twitter @Alz_Gal
AT AAIC 2013
Major finding: A program designed to support the caregivers of Alzheimer’s disease patients decreased emergency room visits by 40% over a 6-month period.
Data source: A program that integrated medical and social care and enrolled 105 caregiver-patient pairs.
Disclosures: The project was funded by the Alzheimer’s Association of Northern California and Northern Nevada, Kaiser Permanente Northern California, and the National Institute on Aging.
Studies speak volumes about brain changes and cognition in women
BOSTON – Women who complain about cognitive problems after menopause might have increases rather than decreases in volume of certain brain regions, reported investigators at the Alzheimer’s Association International Conference 2013.
Brain imaging studies of 48 women in the early years of menopause showed that those with subjective cognitive complaints had significantly greater volumes in certain regions of the brain than did noncomplainers, including the right posterior cingulate gyrus (P less than .04), the right transverse temporal cortex (P less than .03), The left caudal middle frontal gyrus, and the right paracentral gyrus (P less than .05 for both), said Lilia Zurkovsky, Ph.D., a postdoctoral fellow from the Center for Cognitive Medicine at Vanderbilt University Medical Center in Nashville, Tenn.
The findings appear to run counter to those of another study (Neurology 2006;12:67: 834-42) showing that older adults with cognitive complaints had smaller volumes in temporal and frontal areas, compared with healthy age-matched controls, Dr. Zurkovsky acknowledged. She noted, however, that her group studied postmenopausal women in their 50s and 60s, whereas the earlier study included men. In addition, the mean age for each group in that study was above 70 years.
Combined with recent findings pointing to subjective cognitive and/or memory complaints as possible early signs of Alzheimer’s disease, the studies hint at a possible pattern of change as women age.
"If we look at all the data together, it would seem as though in early middle age, 50- to 60-year old individuals with complaints may be having some sort of compensatory mechanism. That’s speculation; all we know is that they have this increase in volume before they have a decrease in volume as shown by other papers," Dr. Zurkovsky said.
She and her colleagues scanned the participants with T1-weight magnetic resonance imaging with a 3 Tesla magnet, and measured cognitive complaints with the Cognitive Complaint Index (CCI) and five surveys with 120 total questions about the individual’s perceptions of cognitive abilities since menopause.
Brain changes in healthy postmenopausal women were the focus of a second, unrelated study also reported at AAIC2013.
Australian investigators followed participants in the Womens Healthy Ageing Project, a longitudinal study of the menopausal transition among cognitively normal women. The investigators looked at MRI brain scans conducted in 2002 and 2012, and brain-amyloid imaging studies with 18-F florbetaben positron emission tomography (FBB-PET). Florbetaben is an investigational amyloid imaging agent, similar to florbetapir (Amyvid).
They found that among the 26 women who had both FBB-PET and the two MRI scans spaced a decade apart, a significant decline was found over time in hippocampal volume, but not in gray matter volume. There was also a nonsignificant trend correlating higher levels of FBB uptake, as measured by a standardized uptake value ratio (SUVR) with greater declines in hippocampal volume.
"Those who were in the higher tertile of florbetaben SUVR have had an 18%-19% decrement in hippocampal volume over that 10-year period," said lead author Paul Yates, Ph.D., a neuroscientist and imaging research fellow at Austin Health in Heidelberg, Australia.
Although these findings are preliminary and the changes observed were at the trend level only, they suggest that people with intermediate levels of uptake of an amyloid imaging agent might be at increased risk for Alzheimer’s disease, he said.
Dr. Zurkovsky’s study is supported by funding from the National Institute on Aging, Vanderbilt University, and the University of Vermont, Burlington. Dr. Yates’s study is supported by the National Ageing Research Institute at the University of Melbourne, with additional support from Bayer and Piramal Imaging. Neither Dr. Zurkovsky nor Dr. Yates reported having financial disclosures.
BOSTON – Women who complain about cognitive problems after menopause might have increases rather than decreases in volume of certain brain regions, reported investigators at the Alzheimer’s Association International Conference 2013.
Brain imaging studies of 48 women in the early years of menopause showed that those with subjective cognitive complaints had significantly greater volumes in certain regions of the brain than did noncomplainers, including the right posterior cingulate gyrus (P less than .04), the right transverse temporal cortex (P less than .03), The left caudal middle frontal gyrus, and the right paracentral gyrus (P less than .05 for both), said Lilia Zurkovsky, Ph.D., a postdoctoral fellow from the Center for Cognitive Medicine at Vanderbilt University Medical Center in Nashville, Tenn.
The findings appear to run counter to those of another study (Neurology 2006;12:67: 834-42) showing that older adults with cognitive complaints had smaller volumes in temporal and frontal areas, compared with healthy age-matched controls, Dr. Zurkovsky acknowledged. She noted, however, that her group studied postmenopausal women in their 50s and 60s, whereas the earlier study included men. In addition, the mean age for each group in that study was above 70 years.
Combined with recent findings pointing to subjective cognitive and/or memory complaints as possible early signs of Alzheimer’s disease, the studies hint at a possible pattern of change as women age.
"If we look at all the data together, it would seem as though in early middle age, 50- to 60-year old individuals with complaints may be having some sort of compensatory mechanism. That’s speculation; all we know is that they have this increase in volume before they have a decrease in volume as shown by other papers," Dr. Zurkovsky said.
She and her colleagues scanned the participants with T1-weight magnetic resonance imaging with a 3 Tesla magnet, and measured cognitive complaints with the Cognitive Complaint Index (CCI) and five surveys with 120 total questions about the individual’s perceptions of cognitive abilities since menopause.
Brain changes in healthy postmenopausal women were the focus of a second, unrelated study also reported at AAIC2013.
Australian investigators followed participants in the Womens Healthy Ageing Project, a longitudinal study of the menopausal transition among cognitively normal women. The investigators looked at MRI brain scans conducted in 2002 and 2012, and brain-amyloid imaging studies with 18-F florbetaben positron emission tomography (FBB-PET). Florbetaben is an investigational amyloid imaging agent, similar to florbetapir (Amyvid).
They found that among the 26 women who had both FBB-PET and the two MRI scans spaced a decade apart, a significant decline was found over time in hippocampal volume, but not in gray matter volume. There was also a nonsignificant trend correlating higher levels of FBB uptake, as measured by a standardized uptake value ratio (SUVR) with greater declines in hippocampal volume.
"Those who were in the higher tertile of florbetaben SUVR have had an 18%-19% decrement in hippocampal volume over that 10-year period," said lead author Paul Yates, Ph.D., a neuroscientist and imaging research fellow at Austin Health in Heidelberg, Australia.
Although these findings are preliminary and the changes observed were at the trend level only, they suggest that people with intermediate levels of uptake of an amyloid imaging agent might be at increased risk for Alzheimer’s disease, he said.
Dr. Zurkovsky’s study is supported by funding from the National Institute on Aging, Vanderbilt University, and the University of Vermont, Burlington. Dr. Yates’s study is supported by the National Ageing Research Institute at the University of Melbourne, with additional support from Bayer and Piramal Imaging. Neither Dr. Zurkovsky nor Dr. Yates reported having financial disclosures.
BOSTON – Women who complain about cognitive problems after menopause might have increases rather than decreases in volume of certain brain regions, reported investigators at the Alzheimer’s Association International Conference 2013.
Brain imaging studies of 48 women in the early years of menopause showed that those with subjective cognitive complaints had significantly greater volumes in certain regions of the brain than did noncomplainers, including the right posterior cingulate gyrus (P less than .04), the right transverse temporal cortex (P less than .03), The left caudal middle frontal gyrus, and the right paracentral gyrus (P less than .05 for both), said Lilia Zurkovsky, Ph.D., a postdoctoral fellow from the Center for Cognitive Medicine at Vanderbilt University Medical Center in Nashville, Tenn.
The findings appear to run counter to those of another study (Neurology 2006;12:67: 834-42) showing that older adults with cognitive complaints had smaller volumes in temporal and frontal areas, compared with healthy age-matched controls, Dr. Zurkovsky acknowledged. She noted, however, that her group studied postmenopausal women in their 50s and 60s, whereas the earlier study included men. In addition, the mean age for each group in that study was above 70 years.
Combined with recent findings pointing to subjective cognitive and/or memory complaints as possible early signs of Alzheimer’s disease, the studies hint at a possible pattern of change as women age.
"If we look at all the data together, it would seem as though in early middle age, 50- to 60-year old individuals with complaints may be having some sort of compensatory mechanism. That’s speculation; all we know is that they have this increase in volume before they have a decrease in volume as shown by other papers," Dr. Zurkovsky said.
She and her colleagues scanned the participants with T1-weight magnetic resonance imaging with a 3 Tesla magnet, and measured cognitive complaints with the Cognitive Complaint Index (CCI) and five surveys with 120 total questions about the individual’s perceptions of cognitive abilities since menopause.
Brain changes in healthy postmenopausal women were the focus of a second, unrelated study also reported at AAIC2013.
Australian investigators followed participants in the Womens Healthy Ageing Project, a longitudinal study of the menopausal transition among cognitively normal women. The investigators looked at MRI brain scans conducted in 2002 and 2012, and brain-amyloid imaging studies with 18-F florbetaben positron emission tomography (FBB-PET). Florbetaben is an investigational amyloid imaging agent, similar to florbetapir (Amyvid).
They found that among the 26 women who had both FBB-PET and the two MRI scans spaced a decade apart, a significant decline was found over time in hippocampal volume, but not in gray matter volume. There was also a nonsignificant trend correlating higher levels of FBB uptake, as measured by a standardized uptake value ratio (SUVR) with greater declines in hippocampal volume.
"Those who were in the higher tertile of florbetaben SUVR have had an 18%-19% decrement in hippocampal volume over that 10-year period," said lead author Paul Yates, Ph.D., a neuroscientist and imaging research fellow at Austin Health in Heidelberg, Australia.
Although these findings are preliminary and the changes observed were at the trend level only, they suggest that people with intermediate levels of uptake of an amyloid imaging agent might be at increased risk for Alzheimer’s disease, he said.
Dr. Zurkovsky’s study is supported by funding from the National Institute on Aging, Vanderbilt University, and the University of Vermont, Burlington. Dr. Yates’s study is supported by the National Ageing Research Institute at the University of Melbourne, with additional support from Bayer and Piramal Imaging. Neither Dr. Zurkovsky nor Dr. Yates reported having financial disclosures.
AT AAIC2013
Major finding: Postmenopausal women with subjective cognitive complaints had significantly larger volumes of some brain regions, compared with women without subjective cognitive complaints.
Data source: Prospective study of 48 postmenopausal women in their 50s and 60s; and a prospective, longitudinal study of healthy postmenopausal women, 26 of whom had MRI brain scans a decade apart, and brain amyloid imaging.
Disclosures: Dr. Zurkovsky’s study is supported by funding from the National Institute on Aging, Vanderbilt University, and the University of Vermont, Burlington. Dr. Yates’s study is supported by the National Ageing Research Institute at the University of Melbourne, with additional support from Bayer and Piramal Imaging. Neither Dr. Zurkovsky nor Dr. Yates reported having financial disclosures.
Alzheimer’s biomarkers have limited use in diagnosing frontotemporal dementia
BOSTON – The same biomarkers that successfully discriminate frontotemporal dementia from Alzheimer’s disease don’t appear very helpful in differentiating patients with frontotemporal dementia from those with subjective memory problems.
Low cerebrospinal fluid levels of amyloid beta 42 (Abeta42) and high levels of tau are now seen as important diagnostic hallmarks for Alzheimer’s. The median levels of those biomarkers in patients with FTD are significantly different from what is measured in individuals with Alzheimer’s disease, but levels of those markers are too similar between FTD patients and those with subjective memory problems to be useful in differential diagnosis, Dr. Yolande Pijnenburg said at the Alzheimer’s Association International Conference 2013.
Even if there were a significant correlation, its clinical specificity might be doubtful at this point, said Dr. Pijnenburg of the VU University Medical Center, Amsterdam. "Frontotemporal dementia is so pathologically heterogeneous that it’s almost unthinkable that we would find one specific biomarker."
The findings of her new study were a bit of a disappointment, failing to confirm her earlier work (Clin. Chem. Lab. Med. 2011;49:353-66), which suggested that both tau and phosphorylated tau (P-tau) might be diagnostically useful.
She examined biomarker levels in a cohort of 363 patients recruited from the Amsterdam Dementia Cohort and an associated memory clinic. Of these, 121 had Alzheimer’s disease, 121 had FTD, and 121 had subjective memory complaints that were not pathologic.
The FTD group illustrated Dr. Pijnenburg’s comment about diverse pathology: 91 had behavioral variant FTD and 30 met the Gorno-Tempini criteria for semantic dementia (temporal variant FTD), but 30 also met clinical criteria for Alzheimer’s.
All subjects had cerebrospinal fluid drawn for Abeta42, tau, and P-tau levels. The groups were well matched for age (mean, 62 years). The mean disease duration was about 3 years. The mean Mini Mental State Examination score was 24 in the FTD group, 21 in the Alzheimer’s group, and 28 in the controls with memory complaints.
Abeta42 was lowest in the Alzheimer’s patients at a median of 488 pg/mL. This was significantly lower than the level in either the FTD patients (848 pg/mL) or the normal controls (935 pg/mL). But between the FTD and control groups, neither the median levels nor the ranges were significantly different. The diagnostic accuracy was 89% for discriminating FTD from Alzheimer’s and 57% for discriminating FTD from controls.
The picture was similar for total tau. The level was highest in Alzheimer’s patients (median, 662 pg/mL), followed by the FTD group (345 pg/mL) and the control group (245 pg/mL). But again, neither those levels nor their ranges were significantly different from each other. The diagnostic accuracy was 81% for discriminating FTD from Alzheimer’s and 69% for discriminating FTD from controls.
P-tau was similarly elevated in Alzheimer’s patients (median, 86 pg/mL) and nearly identical in both FTD (42 pg/mL) and controls (45 pg/mL). The diagnostic accuracy was 87% for discriminating FTD from Alzheimer’s and 53% for discriminating FTD from controls.
The tau/Abeta42 ratio had a diagnostic accuracy of 91% for discriminating FTD from Alzheimer’s and 72% for discriminating FTD from the controls.
Dr. Pijnenburg expressed some hope for biomarker utility in the future, despite the rather low accuracy levels for the FTD/control discrimination in this study. "There was very little relevance for measuring Abeta42 or P-tau, but total tau and the tau/Abeta42ratio both do have some diagnostic interest," she said.
During discussion, she addressed several questions concerning unexpectedly low tau levels in the FTD cohort. Patients with FTD have a more rapid disease progression and more brain atrophy than do Alzheimer’s patients. Tau is also directly related to neurodegeneration. So why, she was asked, was there not more tau in the cerebrospinal fluid?
"My thought is that it could be related to the focal nature of FTD," she said. "Alzheimer’s is more diffuse."
Dr. Pijnenburg had no financial disclosures.
msullivan@frontlinemedcom.com
On Twitter @Alz_Gal
BOSTON – The same biomarkers that successfully discriminate frontotemporal dementia from Alzheimer’s disease don’t appear very helpful in differentiating patients with frontotemporal dementia from those with subjective memory problems.
Low cerebrospinal fluid levels of amyloid beta 42 (Abeta42) and high levels of tau are now seen as important diagnostic hallmarks for Alzheimer’s. The median levels of those biomarkers in patients with FTD are significantly different from what is measured in individuals with Alzheimer’s disease, but levels of those markers are too similar between FTD patients and those with subjective memory problems to be useful in differential diagnosis, Dr. Yolande Pijnenburg said at the Alzheimer’s Association International Conference 2013.
Even if there were a significant correlation, its clinical specificity might be doubtful at this point, said Dr. Pijnenburg of the VU University Medical Center, Amsterdam. "Frontotemporal dementia is so pathologically heterogeneous that it’s almost unthinkable that we would find one specific biomarker."
The findings of her new study were a bit of a disappointment, failing to confirm her earlier work (Clin. Chem. Lab. Med. 2011;49:353-66), which suggested that both tau and phosphorylated tau (P-tau) might be diagnostically useful.
She examined biomarker levels in a cohort of 363 patients recruited from the Amsterdam Dementia Cohort and an associated memory clinic. Of these, 121 had Alzheimer’s disease, 121 had FTD, and 121 had subjective memory complaints that were not pathologic.
The FTD group illustrated Dr. Pijnenburg’s comment about diverse pathology: 91 had behavioral variant FTD and 30 met the Gorno-Tempini criteria for semantic dementia (temporal variant FTD), but 30 also met clinical criteria for Alzheimer’s.
All subjects had cerebrospinal fluid drawn for Abeta42, tau, and P-tau levels. The groups were well matched for age (mean, 62 years). The mean disease duration was about 3 years. The mean Mini Mental State Examination score was 24 in the FTD group, 21 in the Alzheimer’s group, and 28 in the controls with memory complaints.
Abeta42 was lowest in the Alzheimer’s patients at a median of 488 pg/mL. This was significantly lower than the level in either the FTD patients (848 pg/mL) or the normal controls (935 pg/mL). But between the FTD and control groups, neither the median levels nor the ranges were significantly different. The diagnostic accuracy was 89% for discriminating FTD from Alzheimer’s and 57% for discriminating FTD from controls.
The picture was similar for total tau. The level was highest in Alzheimer’s patients (median, 662 pg/mL), followed by the FTD group (345 pg/mL) and the control group (245 pg/mL). But again, neither those levels nor their ranges were significantly different from each other. The diagnostic accuracy was 81% for discriminating FTD from Alzheimer’s and 69% for discriminating FTD from controls.
P-tau was similarly elevated in Alzheimer’s patients (median, 86 pg/mL) and nearly identical in both FTD (42 pg/mL) and controls (45 pg/mL). The diagnostic accuracy was 87% for discriminating FTD from Alzheimer’s and 53% for discriminating FTD from controls.
The tau/Abeta42 ratio had a diagnostic accuracy of 91% for discriminating FTD from Alzheimer’s and 72% for discriminating FTD from the controls.
Dr. Pijnenburg expressed some hope for biomarker utility in the future, despite the rather low accuracy levels for the FTD/control discrimination in this study. "There was very little relevance for measuring Abeta42 or P-tau, but total tau and the tau/Abeta42ratio both do have some diagnostic interest," she said.
During discussion, she addressed several questions concerning unexpectedly low tau levels in the FTD cohort. Patients with FTD have a more rapid disease progression and more brain atrophy than do Alzheimer’s patients. Tau is also directly related to neurodegeneration. So why, she was asked, was there not more tau in the cerebrospinal fluid?
"My thought is that it could be related to the focal nature of FTD," she said. "Alzheimer’s is more diffuse."
Dr. Pijnenburg had no financial disclosures.
msullivan@frontlinemedcom.com
On Twitter @Alz_Gal
BOSTON – The same biomarkers that successfully discriminate frontotemporal dementia from Alzheimer’s disease don’t appear very helpful in differentiating patients with frontotemporal dementia from those with subjective memory problems.
Low cerebrospinal fluid levels of amyloid beta 42 (Abeta42) and high levels of tau are now seen as important diagnostic hallmarks for Alzheimer’s. The median levels of those biomarkers in patients with FTD are significantly different from what is measured in individuals with Alzheimer’s disease, but levels of those markers are too similar between FTD patients and those with subjective memory problems to be useful in differential diagnosis, Dr. Yolande Pijnenburg said at the Alzheimer’s Association International Conference 2013.
Even if there were a significant correlation, its clinical specificity might be doubtful at this point, said Dr. Pijnenburg of the VU University Medical Center, Amsterdam. "Frontotemporal dementia is so pathologically heterogeneous that it’s almost unthinkable that we would find one specific biomarker."
The findings of her new study were a bit of a disappointment, failing to confirm her earlier work (Clin. Chem. Lab. Med. 2011;49:353-66), which suggested that both tau and phosphorylated tau (P-tau) might be diagnostically useful.
She examined biomarker levels in a cohort of 363 patients recruited from the Amsterdam Dementia Cohort and an associated memory clinic. Of these, 121 had Alzheimer’s disease, 121 had FTD, and 121 had subjective memory complaints that were not pathologic.
The FTD group illustrated Dr. Pijnenburg’s comment about diverse pathology: 91 had behavioral variant FTD and 30 met the Gorno-Tempini criteria for semantic dementia (temporal variant FTD), but 30 also met clinical criteria for Alzheimer’s.
All subjects had cerebrospinal fluid drawn for Abeta42, tau, and P-tau levels. The groups were well matched for age (mean, 62 years). The mean disease duration was about 3 years. The mean Mini Mental State Examination score was 24 in the FTD group, 21 in the Alzheimer’s group, and 28 in the controls with memory complaints.
Abeta42 was lowest in the Alzheimer’s patients at a median of 488 pg/mL. This was significantly lower than the level in either the FTD patients (848 pg/mL) or the normal controls (935 pg/mL). But between the FTD and control groups, neither the median levels nor the ranges were significantly different. The diagnostic accuracy was 89% for discriminating FTD from Alzheimer’s and 57% for discriminating FTD from controls.
The picture was similar for total tau. The level was highest in Alzheimer’s patients (median, 662 pg/mL), followed by the FTD group (345 pg/mL) and the control group (245 pg/mL). But again, neither those levels nor their ranges were significantly different from each other. The diagnostic accuracy was 81% for discriminating FTD from Alzheimer’s and 69% for discriminating FTD from controls.
P-tau was similarly elevated in Alzheimer’s patients (median, 86 pg/mL) and nearly identical in both FTD (42 pg/mL) and controls (45 pg/mL). The diagnostic accuracy was 87% for discriminating FTD from Alzheimer’s and 53% for discriminating FTD from controls.
The tau/Abeta42 ratio had a diagnostic accuracy of 91% for discriminating FTD from Alzheimer’s and 72% for discriminating FTD from the controls.
Dr. Pijnenburg expressed some hope for biomarker utility in the future, despite the rather low accuracy levels for the FTD/control discrimination in this study. "There was very little relevance for measuring Abeta42 or P-tau, but total tau and the tau/Abeta42ratio both do have some diagnostic interest," she said.
During discussion, she addressed several questions concerning unexpectedly low tau levels in the FTD cohort. Patients with FTD have a more rapid disease progression and more brain atrophy than do Alzheimer’s patients. Tau is also directly related to neurodegeneration. So why, she was asked, was there not more tau in the cerebrospinal fluid?
"My thought is that it could be related to the focal nature of FTD," she said. "Alzheimer’s is more diffuse."
Dr. Pijnenburg had no financial disclosures.
msullivan@frontlinemedcom.com
On Twitter @Alz_Gal
AT AAIC 2013
Major finding: The tau/Abeta42 ratio had a diagnostic accuracy of 91% for discriminating frontotemporal dementia from Alzheimer’s and 72% for discriminating FTD from the controls.
Data source: The study cohort comprised 121 patients with FTD, 121 with AD, and 121 with subjective, nonpathologic memory complaints.
Disclosures: Dr. Pijnenburg had no financial disclosures.
New drug matched donepezil’s efficacy in mild to moderate Alzheimer’s
BOSTON – An investigational nicotinic receptor agonist demonstrated cognitive benefits similar to those seen with donepezil in a phase II trial of patients with mild to moderate Alzheimer’s disease.
Based on positive results of the study, research on the drug, ABT-126, will continue, Dr. Laura Gault said at the Alzheimer’s Association International Conference 2013.
"ABT-126 showed evidence of a treatment effect on cognition, in a dose- and exposure-response fashion," said Dr. Gault of AbbVie Pharmaceuticals, Abbott’s newly created pharmaceuticals research and development arm.
ABT-126 potentiates the action of the alpha-7 nicotinic receptor, which is expressed both pre- and postsynaptically. The hippocampus and prefrontal cortex are especially rich in nicotinic receptors, which influence the release of acetylcholine, dopamine, and glutamate.
Researchers have postulated that nicotinic receptor agonists could have similar benefits to acetylcholinesterase inhibitors, but with a better side effect profile.
In animal models of Alzheimer’s, ABT-126 boosted cognition and memory. It has also demonstrated safety and good tolerability in several phase I studies totaling 249 subjects; the groups included Alzheimer’s and schizophrenia patients, as well as healthy elderly controls.
The 12-week, phase II study randomized 274 subjects to either placebo, 10 mg donepezil, or 5 or 10 mg ABT-126. The 5-mg dose was chosen to match plasma levels obtained in Alzheimer’s model studies, Dr. Gault said. The 10-mg dose was the highest dose supported by the extant safety and tolerability data. Since then, she said, additional studies on ABT-126 have supported a larger dose, which is currently being investigated.
The primary endpoint was the total change from baseline on an 11-item version of the Alzheimer’s Disease Assessment Scale-Cognition domain (ADAS-Cog 11). The study was powered to detect a 30% change on this measure over that which has been demonstrated with donepezil.
Secondary endpoints included the change from baseline on a 13-item version of the ADAS-Cog, the Mini Mental State Examination, the Clinician’s Interview-Based Impression of Change, the Neuropsychiatric Inventory, and the Alzheimer’s Disease Cooperative Study-Activities of Daily Living (ADCS-ADL).
The patients were a mean of 74 years old; 51% tested positive for the apolipoprotein E epsilon-4 allele. Half had been on prior pharmacologic treatment, but since the study was a monotherapy trial, no additional drugs were allowed for its duration. The mean baseline score on the ADAS-Cog was 26 and the mean MMSE was 19.
On the ADAS-Cog 11 total score, donepezil performed the best, with a statistically significant 1.4-point increase. The 10-mg ABT-126 group also improved over baseline, with a statistically significant 1.2-point change. The 5-mg dose did not separate from placebo.
On the memory subscale of the ADAS-Cog 11, both the 10-mg dose of ABT-126 and donepezil showed significant improvements. Scores on the praxis subscale were significantly higher for patients who took donepezil, compared with those who took ABT-126. Neither drug significantly affected scores in the language subscale.
Both the 10-mg dose of ABT-126 and donepezil significantly improved total scores on the ADAS-Cog 13.
A linear model showed a relationship between exposure to the study drug and cognitive improvement, with no plateau over 12 weeks. "This suggests that a higher dose might lead to improved efficacy," Dr. Gault said.
There were 17 dropouts in the study. The rates were highest in the 10-mg ABT-126 and donepezil groups (6 each). Nine were due to adverse events, including five with donepezil, two with placebo, one with 10-mg ABT-126 and one with 5-mg ABT-126.
The most common adverse events were nausea, diarrhea, and vomiting, all of which were most frequent in the donepezil group.
AbbVie’s next steps with the drug are two 24-week, placebo-controlled phase II trials, both of which are expected to enroll about 400 patients with mild-moderate disease. One will test the drug as monotherapy, and the other as an add-on to rivastigmine and donepezil.
The company is also investigating the drug’s possible effect on cognition in patients with schizophrenia.
AbbVie sponsored the trial.
On Twitter @Alz_Gal
BOSTON – An investigational nicotinic receptor agonist demonstrated cognitive benefits similar to those seen with donepezil in a phase II trial of patients with mild to moderate Alzheimer’s disease.
Based on positive results of the study, research on the drug, ABT-126, will continue, Dr. Laura Gault said at the Alzheimer’s Association International Conference 2013.
"ABT-126 showed evidence of a treatment effect on cognition, in a dose- and exposure-response fashion," said Dr. Gault of AbbVie Pharmaceuticals, Abbott’s newly created pharmaceuticals research and development arm.
ABT-126 potentiates the action of the alpha-7 nicotinic receptor, which is expressed both pre- and postsynaptically. The hippocampus and prefrontal cortex are especially rich in nicotinic receptors, which influence the release of acetylcholine, dopamine, and glutamate.
Researchers have postulated that nicotinic receptor agonists could have similar benefits to acetylcholinesterase inhibitors, but with a better side effect profile.
In animal models of Alzheimer’s, ABT-126 boosted cognition and memory. It has also demonstrated safety and good tolerability in several phase I studies totaling 249 subjects; the groups included Alzheimer’s and schizophrenia patients, as well as healthy elderly controls.
The 12-week, phase II study randomized 274 subjects to either placebo, 10 mg donepezil, or 5 or 10 mg ABT-126. The 5-mg dose was chosen to match plasma levels obtained in Alzheimer’s model studies, Dr. Gault said. The 10-mg dose was the highest dose supported by the extant safety and tolerability data. Since then, she said, additional studies on ABT-126 have supported a larger dose, which is currently being investigated.
The primary endpoint was the total change from baseline on an 11-item version of the Alzheimer’s Disease Assessment Scale-Cognition domain (ADAS-Cog 11). The study was powered to detect a 30% change on this measure over that which has been demonstrated with donepezil.
Secondary endpoints included the change from baseline on a 13-item version of the ADAS-Cog, the Mini Mental State Examination, the Clinician’s Interview-Based Impression of Change, the Neuropsychiatric Inventory, and the Alzheimer’s Disease Cooperative Study-Activities of Daily Living (ADCS-ADL).
The patients were a mean of 74 years old; 51% tested positive for the apolipoprotein E epsilon-4 allele. Half had been on prior pharmacologic treatment, but since the study was a monotherapy trial, no additional drugs were allowed for its duration. The mean baseline score on the ADAS-Cog was 26 and the mean MMSE was 19.
On the ADAS-Cog 11 total score, donepezil performed the best, with a statistically significant 1.4-point increase. The 10-mg ABT-126 group also improved over baseline, with a statistically significant 1.2-point change. The 5-mg dose did not separate from placebo.
On the memory subscale of the ADAS-Cog 11, both the 10-mg dose of ABT-126 and donepezil showed significant improvements. Scores on the praxis subscale were significantly higher for patients who took donepezil, compared with those who took ABT-126. Neither drug significantly affected scores in the language subscale.
Both the 10-mg dose of ABT-126 and donepezil significantly improved total scores on the ADAS-Cog 13.
A linear model showed a relationship between exposure to the study drug and cognitive improvement, with no plateau over 12 weeks. "This suggests that a higher dose might lead to improved efficacy," Dr. Gault said.
There were 17 dropouts in the study. The rates were highest in the 10-mg ABT-126 and donepezil groups (6 each). Nine were due to adverse events, including five with donepezil, two with placebo, one with 10-mg ABT-126 and one with 5-mg ABT-126.
The most common adverse events were nausea, diarrhea, and vomiting, all of which were most frequent in the donepezil group.
AbbVie’s next steps with the drug are two 24-week, placebo-controlled phase II trials, both of which are expected to enroll about 400 patients with mild-moderate disease. One will test the drug as monotherapy, and the other as an add-on to rivastigmine and donepezil.
The company is also investigating the drug’s possible effect on cognition in patients with schizophrenia.
AbbVie sponsored the trial.
On Twitter @Alz_Gal
BOSTON – An investigational nicotinic receptor agonist demonstrated cognitive benefits similar to those seen with donepezil in a phase II trial of patients with mild to moderate Alzheimer’s disease.
Based on positive results of the study, research on the drug, ABT-126, will continue, Dr. Laura Gault said at the Alzheimer’s Association International Conference 2013.
"ABT-126 showed evidence of a treatment effect on cognition, in a dose- and exposure-response fashion," said Dr. Gault of AbbVie Pharmaceuticals, Abbott’s newly created pharmaceuticals research and development arm.
ABT-126 potentiates the action of the alpha-7 nicotinic receptor, which is expressed both pre- and postsynaptically. The hippocampus and prefrontal cortex are especially rich in nicotinic receptors, which influence the release of acetylcholine, dopamine, and glutamate.
Researchers have postulated that nicotinic receptor agonists could have similar benefits to acetylcholinesterase inhibitors, but with a better side effect profile.
In animal models of Alzheimer’s, ABT-126 boosted cognition and memory. It has also demonstrated safety and good tolerability in several phase I studies totaling 249 subjects; the groups included Alzheimer’s and schizophrenia patients, as well as healthy elderly controls.
The 12-week, phase II study randomized 274 subjects to either placebo, 10 mg donepezil, or 5 or 10 mg ABT-126. The 5-mg dose was chosen to match plasma levels obtained in Alzheimer’s model studies, Dr. Gault said. The 10-mg dose was the highest dose supported by the extant safety and tolerability data. Since then, she said, additional studies on ABT-126 have supported a larger dose, which is currently being investigated.
The primary endpoint was the total change from baseline on an 11-item version of the Alzheimer’s Disease Assessment Scale-Cognition domain (ADAS-Cog 11). The study was powered to detect a 30% change on this measure over that which has been demonstrated with donepezil.
Secondary endpoints included the change from baseline on a 13-item version of the ADAS-Cog, the Mini Mental State Examination, the Clinician’s Interview-Based Impression of Change, the Neuropsychiatric Inventory, and the Alzheimer’s Disease Cooperative Study-Activities of Daily Living (ADCS-ADL).
The patients were a mean of 74 years old; 51% tested positive for the apolipoprotein E epsilon-4 allele. Half had been on prior pharmacologic treatment, but since the study was a monotherapy trial, no additional drugs were allowed for its duration. The mean baseline score on the ADAS-Cog was 26 and the mean MMSE was 19.
On the ADAS-Cog 11 total score, donepezil performed the best, with a statistically significant 1.4-point increase. The 10-mg ABT-126 group also improved over baseline, with a statistically significant 1.2-point change. The 5-mg dose did not separate from placebo.
On the memory subscale of the ADAS-Cog 11, both the 10-mg dose of ABT-126 and donepezil showed significant improvements. Scores on the praxis subscale were significantly higher for patients who took donepezil, compared with those who took ABT-126. Neither drug significantly affected scores in the language subscale.
Both the 10-mg dose of ABT-126 and donepezil significantly improved total scores on the ADAS-Cog 13.
A linear model showed a relationship between exposure to the study drug and cognitive improvement, with no plateau over 12 weeks. "This suggests that a higher dose might lead to improved efficacy," Dr. Gault said.
There were 17 dropouts in the study. The rates were highest in the 10-mg ABT-126 and donepezil groups (6 each). Nine were due to adverse events, including five with donepezil, two with placebo, one with 10-mg ABT-126 and one with 5-mg ABT-126.
The most common adverse events were nausea, diarrhea, and vomiting, all of which were most frequent in the donepezil group.
AbbVie’s next steps with the drug are two 24-week, placebo-controlled phase II trials, both of which are expected to enroll about 400 patients with mild-moderate disease. One will test the drug as monotherapy, and the other as an add-on to rivastigmine and donepezil.
The company is also investigating the drug’s possible effect on cognition in patients with schizophrenia.
AbbVie sponsored the trial.
On Twitter @Alz_Gal
AT AAIC 2013
Major finding: Donepezil users performed the best on the 11-item ADAS-Cog total score, with a statistically significant 1.4-point increase. The 10-mg ABT-126 group also improved over baseline, with a statistically significant 1.2-point change.
Data source: The phase II study randomized 274 patients to placebo, 10 mg donepezil, or 5- or 10-mg ABT-126 for 12 weeks.
Disclosures: AbbVie sponsored the trial. Dr. Laura Gault is the company’s medical director.
Learning genetic risk for Alzheimer’s may not be so distressing
BOSTON – Cognitively normal adults who learn that they are at high risk for developing Alzheimer’s disease do not, as some clinicians fear, spiral downward into depression, anxiety, or distress, investigators reported at the Alzheimer’s Association International Conference 2013.
An analysis of data from three randomized trials testing the effects of genetic testing disclosure found that that cognitively normal adults who learned that they were homozygous for the high-risk apolipoprotein E epsilon-4 allele (APOE epsilon-4) had a spike in test-specific distress score until about 6 months after learning the results but returned to levels similar to those of heterozygous carriers, reported Dr. Jason Karlawish, professor of medicine at the University of Pennsylvania, Philadelphia, and his colleagues.
However, both homozygous and heterozygous APOE epsilon-4 carriers had more test-specific distress than did carriers of other APOE alleles, noted coauthor Leo B. Waterston of Harvard Medical School, Boston.
"While there is no question that learning that you’re at higher risk causes some test-specific distress, this further validates that there is no long-term, sustained psychological distress," Dr. Karlawish said in an interview.
People who learned that they were at high risk for developing Alzheimer’s disease (AD) were significantly more like to adopt putative AD risk-reducing behaviors, such as dietary changes, exercise, and medication or vitamin supplementation, he said.
The authors looked at participants in three Risk Evaluation and Education for Alzheimer’s Disease (REVEAL) trial cohorts. The REVEAL study is a series of multicenter trials looking at the effects of APOE genotyping.
They pooled data from three randomized REVEAL trials on a total of 648 patients, identifying 399 (62%) participants who were negative for the epsilon-4 allele, 221 (34%) who were heterozygous carriers of the epsilon-4 allele, and 28 (4%) who were epsilon-4 homozygotes.
They found that epsilon-4 homozygotes and heterozygotes had significantly higher mean test-specific distress levels, compared with noncarriers (P less than .0001), but there were no significant differences between the two epsilon-4–positive groups.
Asked at 1-year follow-up how they perceived their risk for AD, 64% of heterozygous carriers and 72% of homozygous carriers rated their risk as high or very high, a difference that was not significant. In contrast, about half of all noncarriers rated their risk as average and about 15% rated it as high, but none rated their risk as very high.
There were no significant differences in mean depression scores on the Center for Epidemiologic Studies Depression Scale.
Nearly two-thirds (61%) of homozygous carriers reported dietary changes at 12 months, compared with 34% of heterozygous carriers and 27% of noncarriers. Homozygotes also were more likely to report increases in exercise (58% vs. 39% and 28%, respectively), and to take medication and/or vitamins to try to stave off AD (58% vs. 38% and 27%, P less than .001 for all comparisons).
Dr. Karlawish noted that, although there were only 28 participants who carried both copies of the epsilon-4 allele, analysis of individual participants suggested that the data across this group were uniform and not skewed by outliers. He also noted that the distribution of APOE alleles in the study mirrored that of the population at large.
Mr. Waterston said that they are currently mailing follow-up surveys to participants and hope to continue with long-term follow-up.
The research was supported by grants from the National Institutes of Health. Dr. Karlawish and Mr. Waterston reported having no financial disclosures.
BOSTON – Cognitively normal adults who learn that they are at high risk for developing Alzheimer’s disease do not, as some clinicians fear, spiral downward into depression, anxiety, or distress, investigators reported at the Alzheimer’s Association International Conference 2013.
An analysis of data from three randomized trials testing the effects of genetic testing disclosure found that that cognitively normal adults who learned that they were homozygous for the high-risk apolipoprotein E epsilon-4 allele (APOE epsilon-4) had a spike in test-specific distress score until about 6 months after learning the results but returned to levels similar to those of heterozygous carriers, reported Dr. Jason Karlawish, professor of medicine at the University of Pennsylvania, Philadelphia, and his colleagues.
However, both homozygous and heterozygous APOE epsilon-4 carriers had more test-specific distress than did carriers of other APOE alleles, noted coauthor Leo B. Waterston of Harvard Medical School, Boston.
"While there is no question that learning that you’re at higher risk causes some test-specific distress, this further validates that there is no long-term, sustained psychological distress," Dr. Karlawish said in an interview.
People who learned that they were at high risk for developing Alzheimer’s disease (AD) were significantly more like to adopt putative AD risk-reducing behaviors, such as dietary changes, exercise, and medication or vitamin supplementation, he said.
The authors looked at participants in three Risk Evaluation and Education for Alzheimer’s Disease (REVEAL) trial cohorts. The REVEAL study is a series of multicenter trials looking at the effects of APOE genotyping.
They pooled data from three randomized REVEAL trials on a total of 648 patients, identifying 399 (62%) participants who were negative for the epsilon-4 allele, 221 (34%) who were heterozygous carriers of the epsilon-4 allele, and 28 (4%) who were epsilon-4 homozygotes.
They found that epsilon-4 homozygotes and heterozygotes had significantly higher mean test-specific distress levels, compared with noncarriers (P less than .0001), but there were no significant differences between the two epsilon-4–positive groups.
Asked at 1-year follow-up how they perceived their risk for AD, 64% of heterozygous carriers and 72% of homozygous carriers rated their risk as high or very high, a difference that was not significant. In contrast, about half of all noncarriers rated their risk as average and about 15% rated it as high, but none rated their risk as very high.
There were no significant differences in mean depression scores on the Center for Epidemiologic Studies Depression Scale.
Nearly two-thirds (61%) of homozygous carriers reported dietary changes at 12 months, compared with 34% of heterozygous carriers and 27% of noncarriers. Homozygotes also were more likely to report increases in exercise (58% vs. 39% and 28%, respectively), and to take medication and/or vitamins to try to stave off AD (58% vs. 38% and 27%, P less than .001 for all comparisons).
Dr. Karlawish noted that, although there were only 28 participants who carried both copies of the epsilon-4 allele, analysis of individual participants suggested that the data across this group were uniform and not skewed by outliers. He also noted that the distribution of APOE alleles in the study mirrored that of the population at large.
Mr. Waterston said that they are currently mailing follow-up surveys to participants and hope to continue with long-term follow-up.
The research was supported by grants from the National Institutes of Health. Dr. Karlawish and Mr. Waterston reported having no financial disclosures.
BOSTON – Cognitively normal adults who learn that they are at high risk for developing Alzheimer’s disease do not, as some clinicians fear, spiral downward into depression, anxiety, or distress, investigators reported at the Alzheimer’s Association International Conference 2013.
An analysis of data from three randomized trials testing the effects of genetic testing disclosure found that that cognitively normal adults who learned that they were homozygous for the high-risk apolipoprotein E epsilon-4 allele (APOE epsilon-4) had a spike in test-specific distress score until about 6 months after learning the results but returned to levels similar to those of heterozygous carriers, reported Dr. Jason Karlawish, professor of medicine at the University of Pennsylvania, Philadelphia, and his colleagues.
However, both homozygous and heterozygous APOE epsilon-4 carriers had more test-specific distress than did carriers of other APOE alleles, noted coauthor Leo B. Waterston of Harvard Medical School, Boston.
"While there is no question that learning that you’re at higher risk causes some test-specific distress, this further validates that there is no long-term, sustained psychological distress," Dr. Karlawish said in an interview.
People who learned that they were at high risk for developing Alzheimer’s disease (AD) were significantly more like to adopt putative AD risk-reducing behaviors, such as dietary changes, exercise, and medication or vitamin supplementation, he said.
The authors looked at participants in three Risk Evaluation and Education for Alzheimer’s Disease (REVEAL) trial cohorts. The REVEAL study is a series of multicenter trials looking at the effects of APOE genotyping.
They pooled data from three randomized REVEAL trials on a total of 648 patients, identifying 399 (62%) participants who were negative for the epsilon-4 allele, 221 (34%) who were heterozygous carriers of the epsilon-4 allele, and 28 (4%) who were epsilon-4 homozygotes.
They found that epsilon-4 homozygotes and heterozygotes had significantly higher mean test-specific distress levels, compared with noncarriers (P less than .0001), but there were no significant differences between the two epsilon-4–positive groups.
Asked at 1-year follow-up how they perceived their risk for AD, 64% of heterozygous carriers and 72% of homozygous carriers rated their risk as high or very high, a difference that was not significant. In contrast, about half of all noncarriers rated their risk as average and about 15% rated it as high, but none rated their risk as very high.
There were no significant differences in mean depression scores on the Center for Epidemiologic Studies Depression Scale.
Nearly two-thirds (61%) of homozygous carriers reported dietary changes at 12 months, compared with 34% of heterozygous carriers and 27% of noncarriers. Homozygotes also were more likely to report increases in exercise (58% vs. 39% and 28%, respectively), and to take medication and/or vitamins to try to stave off AD (58% vs. 38% and 27%, P less than .001 for all comparisons).
Dr. Karlawish noted that, although there were only 28 participants who carried both copies of the epsilon-4 allele, analysis of individual participants suggested that the data across this group were uniform and not skewed by outliers. He also noted that the distribution of APOE alleles in the study mirrored that of the population at large.
Mr. Waterston said that they are currently mailing follow-up surveys to participants and hope to continue with long-term follow-up.
The research was supported by grants from the National Institutes of Health. Dr. Karlawish and Mr. Waterston reported having no financial disclosures.
AT AAIC 2013
Major finding: Patients who were homozygous for the apolipoprotein E epsilon-4 allele more likely than heterozygotes and noncarriers to report increases in exercise (58% vs. 39% and 28%, respectively), and to take medication and/or vitamins to try to stave off AD (58% vs. 38% and 27%, P less than .001 for all comparisons)
Data source: Analysis of pooled data on 648 patients from three randomized clinical trials examining the effects of apolipoprotein E status.
Disclosures: The research was supported by grants from the National Institutes of Health. Dr. Karlawish and Mr. Waterston reported having no financial disclosures.
Aerobic exercise boosts brain power in mild cognitive impairment
BOSTON – Combined results from two 6-month, randomized trials indicate that aerobic exercise improves executive function to a significantly greater degree than do stretching and tone exercises in patients with mild cognitive impairment or mild insulin resistance, Laura Baker, Ph.D., reported at the Alzheimer’s Association International Conference 2013.
The benefits were greatest in carriers of the apo E epsilon 4 allele, which confers a higher risk for developing Alzheimer’s disease.
But the "how" behind this brain boost remains something of a mystery, said Dr. Baker of Wake Forest University, Winston-Salem, N.C. "In apo E epsilon 4–negative participants, we think the mechanism is probably improved insulin sensitivity. But in the apo E epsilon 4–positive patients, it seems to be some other mechanism that we don’t understand."
Exercise modulates the risk of cognitive decline in a number of ways, she said. "It has a diversified portfolio of targets. Exercise exerts benefits on inflammation, improves the integrity of both macro- and microvessels, has a beneficial effect on physical and psychological stress, and modulates inflammation – all of which have been shown to have potent effects on amyloid burden."
These findings over the years have "set the stage nicely for clinical trials. But what’s the right exposure? Who are the best responders? And what is the clinical significance of the changes? Do they really translate into any improvement in quality of life?"
To try and answer some of these questions, she and her colleagues conducted two randomized physical activity trials. They recruited a total of 67 participants: 33 in a study of exercise in adults with mild cognitive impairment (MCI) and 34 with impaired insulin sensitivity who were in an identical study.
The two studies took a look at different aspects of the relationship between cognitive and metabolic health, Dr. Baker said at the meeting. "Mild metabolic disease leads to vascular dysfunction and poor glucose tolerance, which puts a person at an increased risk of mild cognitive impairment and Alzheimer’s dementia."
Both studies were 6 months long and randomized participants to 45-minute sessions of either a stretching/toning program or aerobic exercise, conducted four times a week. Both were held at local YMCAs and supervised by trained staff.
For the duration of the stretching program, the target heart rate was less than 35% of the maximum; the exercises also included balance and gentle yoga. The aerobic program aimed for a heart rate of 70%-80% of maximum. It consisted of ever-increasing workouts on treadmill, stationary bike, or elliptical trainer. Adherence was very good, Dr. Baker said, with 93% of the subjects in each study completing the protocol.
Each trial had the same endpoints: tests of executive function (a composite of trail-making, word fluency, symbol-digit, and visual working memory) and short-term memory (story recall, list learning, and delayed matching).
The participants were a mean of 68.5 years old; altogether, 46 were randomized to the aerobic program and 21 to the stretching program. They were out of shape, Dr. Baker said, with a mean VO2 peak of 22 mL/kg per minute – considered below average for that age. They were not an obese group overall, but they had a high body fat percentage of around 38%.
In the MCI study, the mean Mini-Mental State Exam score was 27. In the metabolic dysfunction study, the mean fasting glucose was 100 pg/dL and the mean fasting insulin, 11.47 mU/mL.
At the studies’ ends, aerobic exercise groups had made significant gains in their cardiorespiratory fitness, as measured by improvements in the VO2 peak, the treadmill incline, and the duration they could exercise. These measures were unchanged from baseline in the stretching group. The VO2 peak changes were not significantly associated with executive function.
The exercise groups in both studies also experienced significant gains on the composite measure of executive function, Dr. Baker said. They had an increase of about 2 points from baseline, compared with a decline of about 3 points from baseline in the stretching group. The gains were similar whether the subjects had MCI or insulin resistance.
All of the subjects in both studies also underwent oral glucose tolerance testing at baseline and at their last visit to determine any changes in insulin resistance. Measures of fasting glucose, fasting insulin, insulin resistance, and glucose disposal during metabolic clamp improved significantly in the exercise groups, with no difference by patient group or by apo E epsilon 4 status.
A subanalysis that controlled for insulin sensitivity examined exercise’s contribution to the changes in executive function among the 52 individuals who were apo E epsilon 4 negative and the 15 who were apo E epsilon 4 positive. "With the change in insulin sensitivity out of the picture, the exercise-related cognitive benefit was greater for apo E epsilon 4–positive adults," she said.
Improvements in insulin sensitivity have been associated with cognitive improvement, Dr. Baker said. However, it’s not clear how this effect is mediated by apo E epsilon 4 status. "These adults with the high-risk allele show cognitive improvement that’s probably related to other mechanisms."
The study has prompted a new trial, set to take place next year. The large, multicenter, randomized study will enroll 300 subjects with amnestic MCI to 18 months of moderate- to high-intensity aerobic exercise or the stretching/toning program. Exercises will again occur four times weekly.
The future trial will measure outcomes with the Alzheimer’s Disease Assessment Scale-cognitive domain and the Clinical Dementia Rating-sum of boxes, and performance on computerized tests of memory.
This trial will also include MRI and cerebrospinal fluid biomarker data obtained at baseline and study’s end.
Dr. Baker had no financial disclosures. The studies were funded by the Alzheimer’s Association, the American Diabetes Association, and the National Institute on Aging.
msullivan@frontlinemedcom.com
On Twitter @Alz_Gal
BOSTON – Combined results from two 6-month, randomized trials indicate that aerobic exercise improves executive function to a significantly greater degree than do stretching and tone exercises in patients with mild cognitive impairment or mild insulin resistance, Laura Baker, Ph.D., reported at the Alzheimer’s Association International Conference 2013.
The benefits were greatest in carriers of the apo E epsilon 4 allele, which confers a higher risk for developing Alzheimer’s disease.
But the "how" behind this brain boost remains something of a mystery, said Dr. Baker of Wake Forest University, Winston-Salem, N.C. "In apo E epsilon 4–negative participants, we think the mechanism is probably improved insulin sensitivity. But in the apo E epsilon 4–positive patients, it seems to be some other mechanism that we don’t understand."
Exercise modulates the risk of cognitive decline in a number of ways, she said. "It has a diversified portfolio of targets. Exercise exerts benefits on inflammation, improves the integrity of both macro- and microvessels, has a beneficial effect on physical and psychological stress, and modulates inflammation – all of which have been shown to have potent effects on amyloid burden."
These findings over the years have "set the stage nicely for clinical trials. But what’s the right exposure? Who are the best responders? And what is the clinical significance of the changes? Do they really translate into any improvement in quality of life?"
To try and answer some of these questions, she and her colleagues conducted two randomized physical activity trials. They recruited a total of 67 participants: 33 in a study of exercise in adults with mild cognitive impairment (MCI) and 34 with impaired insulin sensitivity who were in an identical study.
The two studies took a look at different aspects of the relationship between cognitive and metabolic health, Dr. Baker said at the meeting. "Mild metabolic disease leads to vascular dysfunction and poor glucose tolerance, which puts a person at an increased risk of mild cognitive impairment and Alzheimer’s dementia."
Both studies were 6 months long and randomized participants to 45-minute sessions of either a stretching/toning program or aerobic exercise, conducted four times a week. Both were held at local YMCAs and supervised by trained staff.
For the duration of the stretching program, the target heart rate was less than 35% of the maximum; the exercises also included balance and gentle yoga. The aerobic program aimed for a heart rate of 70%-80% of maximum. It consisted of ever-increasing workouts on treadmill, stationary bike, or elliptical trainer. Adherence was very good, Dr. Baker said, with 93% of the subjects in each study completing the protocol.
Each trial had the same endpoints: tests of executive function (a composite of trail-making, word fluency, symbol-digit, and visual working memory) and short-term memory (story recall, list learning, and delayed matching).
The participants were a mean of 68.5 years old; altogether, 46 were randomized to the aerobic program and 21 to the stretching program. They were out of shape, Dr. Baker said, with a mean VO2 peak of 22 mL/kg per minute – considered below average for that age. They were not an obese group overall, but they had a high body fat percentage of around 38%.
In the MCI study, the mean Mini-Mental State Exam score was 27. In the metabolic dysfunction study, the mean fasting glucose was 100 pg/dL and the mean fasting insulin, 11.47 mU/mL.
At the studies’ ends, aerobic exercise groups had made significant gains in their cardiorespiratory fitness, as measured by improvements in the VO2 peak, the treadmill incline, and the duration they could exercise. These measures were unchanged from baseline in the stretching group. The VO2 peak changes were not significantly associated with executive function.
The exercise groups in both studies also experienced significant gains on the composite measure of executive function, Dr. Baker said. They had an increase of about 2 points from baseline, compared with a decline of about 3 points from baseline in the stretching group. The gains were similar whether the subjects had MCI or insulin resistance.
All of the subjects in both studies also underwent oral glucose tolerance testing at baseline and at their last visit to determine any changes in insulin resistance. Measures of fasting glucose, fasting insulin, insulin resistance, and glucose disposal during metabolic clamp improved significantly in the exercise groups, with no difference by patient group or by apo E epsilon 4 status.
A subanalysis that controlled for insulin sensitivity examined exercise’s contribution to the changes in executive function among the 52 individuals who were apo E epsilon 4 negative and the 15 who were apo E epsilon 4 positive. "With the change in insulin sensitivity out of the picture, the exercise-related cognitive benefit was greater for apo E epsilon 4–positive adults," she said.
Improvements in insulin sensitivity have been associated with cognitive improvement, Dr. Baker said. However, it’s not clear how this effect is mediated by apo E epsilon 4 status. "These adults with the high-risk allele show cognitive improvement that’s probably related to other mechanisms."
The study has prompted a new trial, set to take place next year. The large, multicenter, randomized study will enroll 300 subjects with amnestic MCI to 18 months of moderate- to high-intensity aerobic exercise or the stretching/toning program. Exercises will again occur four times weekly.
The future trial will measure outcomes with the Alzheimer’s Disease Assessment Scale-cognitive domain and the Clinical Dementia Rating-sum of boxes, and performance on computerized tests of memory.
This trial will also include MRI and cerebrospinal fluid biomarker data obtained at baseline and study’s end.
Dr. Baker had no financial disclosures. The studies were funded by the Alzheimer’s Association, the American Diabetes Association, and the National Institute on Aging.
msullivan@frontlinemedcom.com
On Twitter @Alz_Gal
BOSTON – Combined results from two 6-month, randomized trials indicate that aerobic exercise improves executive function to a significantly greater degree than do stretching and tone exercises in patients with mild cognitive impairment or mild insulin resistance, Laura Baker, Ph.D., reported at the Alzheimer’s Association International Conference 2013.
The benefits were greatest in carriers of the apo E epsilon 4 allele, which confers a higher risk for developing Alzheimer’s disease.
But the "how" behind this brain boost remains something of a mystery, said Dr. Baker of Wake Forest University, Winston-Salem, N.C. "In apo E epsilon 4–negative participants, we think the mechanism is probably improved insulin sensitivity. But in the apo E epsilon 4–positive patients, it seems to be some other mechanism that we don’t understand."
Exercise modulates the risk of cognitive decline in a number of ways, she said. "It has a diversified portfolio of targets. Exercise exerts benefits on inflammation, improves the integrity of both macro- and microvessels, has a beneficial effect on physical and psychological stress, and modulates inflammation – all of which have been shown to have potent effects on amyloid burden."
These findings over the years have "set the stage nicely for clinical trials. But what’s the right exposure? Who are the best responders? And what is the clinical significance of the changes? Do they really translate into any improvement in quality of life?"
To try and answer some of these questions, she and her colleagues conducted two randomized physical activity trials. They recruited a total of 67 participants: 33 in a study of exercise in adults with mild cognitive impairment (MCI) and 34 with impaired insulin sensitivity who were in an identical study.
The two studies took a look at different aspects of the relationship between cognitive and metabolic health, Dr. Baker said at the meeting. "Mild metabolic disease leads to vascular dysfunction and poor glucose tolerance, which puts a person at an increased risk of mild cognitive impairment and Alzheimer’s dementia."
Both studies were 6 months long and randomized participants to 45-minute sessions of either a stretching/toning program or aerobic exercise, conducted four times a week. Both were held at local YMCAs and supervised by trained staff.
For the duration of the stretching program, the target heart rate was less than 35% of the maximum; the exercises also included balance and gentle yoga. The aerobic program aimed for a heart rate of 70%-80% of maximum. It consisted of ever-increasing workouts on treadmill, stationary bike, or elliptical trainer. Adherence was very good, Dr. Baker said, with 93% of the subjects in each study completing the protocol.
Each trial had the same endpoints: tests of executive function (a composite of trail-making, word fluency, symbol-digit, and visual working memory) and short-term memory (story recall, list learning, and delayed matching).
The participants were a mean of 68.5 years old; altogether, 46 were randomized to the aerobic program and 21 to the stretching program. They were out of shape, Dr. Baker said, with a mean VO2 peak of 22 mL/kg per minute – considered below average for that age. They were not an obese group overall, but they had a high body fat percentage of around 38%.
In the MCI study, the mean Mini-Mental State Exam score was 27. In the metabolic dysfunction study, the mean fasting glucose was 100 pg/dL and the mean fasting insulin, 11.47 mU/mL.
At the studies’ ends, aerobic exercise groups had made significant gains in their cardiorespiratory fitness, as measured by improvements in the VO2 peak, the treadmill incline, and the duration they could exercise. These measures were unchanged from baseline in the stretching group. The VO2 peak changes were not significantly associated with executive function.
The exercise groups in both studies also experienced significant gains on the composite measure of executive function, Dr. Baker said. They had an increase of about 2 points from baseline, compared with a decline of about 3 points from baseline in the stretching group. The gains were similar whether the subjects had MCI or insulin resistance.
All of the subjects in both studies also underwent oral glucose tolerance testing at baseline and at their last visit to determine any changes in insulin resistance. Measures of fasting glucose, fasting insulin, insulin resistance, and glucose disposal during metabolic clamp improved significantly in the exercise groups, with no difference by patient group or by apo E epsilon 4 status.
A subanalysis that controlled for insulin sensitivity examined exercise’s contribution to the changes in executive function among the 52 individuals who were apo E epsilon 4 negative and the 15 who were apo E epsilon 4 positive. "With the change in insulin sensitivity out of the picture, the exercise-related cognitive benefit was greater for apo E epsilon 4–positive adults," she said.
Improvements in insulin sensitivity have been associated with cognitive improvement, Dr. Baker said. However, it’s not clear how this effect is mediated by apo E epsilon 4 status. "These adults with the high-risk allele show cognitive improvement that’s probably related to other mechanisms."
The study has prompted a new trial, set to take place next year. The large, multicenter, randomized study will enroll 300 subjects with amnestic MCI to 18 months of moderate- to high-intensity aerobic exercise or the stretching/toning program. Exercises will again occur four times weekly.
The future trial will measure outcomes with the Alzheimer’s Disease Assessment Scale-cognitive domain and the Clinical Dementia Rating-sum of boxes, and performance on computerized tests of memory.
This trial will also include MRI and cerebrospinal fluid biomarker data obtained at baseline and study’s end.
Dr. Baker had no financial disclosures. The studies were funded by the Alzheimer’s Association, the American Diabetes Association, and the National Institute on Aging.
msullivan@frontlinemedcom.com
On Twitter @Alz_Gal
AT AAIC2013
Major finding: Individuals who performed aerobic exercises had a significant increase of about 2 points from baseline on a composite measure of executive function, compared with a decline of about 3 points from baseline in those who performed stretching and toning exercises, regardless of whether the subjects had MCI or insulin resistance.
Data source: Two 6-month studies that randomized a total of 67 patients to either aerobic exercise or stretching and toning exercises.
Disclosures: Dr. Baker had no financial disclosures. The studies were funded by the Alzheimer’s Association, the America Diabetes Association, and the National Institute on Aging.
Metformin bests other antidiabetics in protection from dementia
BOSTON – Older adults with type 2 diabetes who were taking metformin had a significantly lower 5-year risk for dementia than did peers who were taking other oral antidiabetic agents, investigators reported at the Alzheimer’s Association International Conference 2013.
A retrospective study of nearly 15,000 adults aged 55 years and older who started on single-agent drug therapy for type 2 diabetes between 1999 and 2001 showed that 5 years after starting therapy, patients who started on metformin had a 23% lower 5-year risk for dementia compared with patients who started on a thiazolidinedione (TZD) such as rosiglitazone (Avandia), reported Rachel Whitmer, Ph.D., an investigator in the research division of Kaiser Permanente Northern California, Oakland.
Metformin users also had a 20% lower risk for any dementia compared with those on a sulfonylurea such as glyburide. Among patients with a diabetes duration of less than 5 years, metformin users had a 40% lower dementia risk than did sulfonylurea users, and among those with a diabetes duration of at least 10 years, the risk for metformin users was 19% lower.
It is estimated that 12%-25% of people over age 65 have type 2 diabetes, putting them at about a 50% increase in risk for dementia, compared with nondiabetic people of the same age. - Dr. Rachel Whitmer
The differences between the drug types held up after adjustment for race, sex, education, and hemoglobin A1c (HbA1c) levels, Dr. Whitmer said.
It is estimated that 12%-25% of people over age 65 have type 2 diabetes, putting them at about a 50% increase in risk for dementia, compared with nondiabetic people of the same age. Nearly all elderly patients with type 2 diabetes will be started on some antidiabetic agent at some point, she noted.
"A lot of work out there [indicates] that insulin, and specifically insulin resistance, may play a role in Alzheimer’s disease, and there has been some nice work from animal models and cell-culture studies looking at metformin specifically," Dr. Whitmer said.
Metformin appears to lessen neuronal insulin resistance and is associated with neurogenesis. In addition, because it is associated with reduced risk of myocardial infarction and stroke, metformin may protect against vascular dementias, she added.
Dr. Whitmer and her colleagues delved into the Kaiser Permanente database to identify 14,891 patients aged 55 years and up with no history of dementia who were started on new diabetes pharmacotherapy between October 1999 and November 2001.
In all, 9.9% of patients in the sample were diagnosed with dementia during 5 years of follow-up, including 9.5% of 8,528 patients on metformin, 19.9% of 3,383 patients on a sulfonylurea, 9.8% of 2,095 patients on a TZD, and 11.1% of 905 patients on insulin.
In multivariate models adjusting for age, race, education, and diabetes duration, metformin was associated with a hazard ratio (HR) for any dementia of 0.77, compared with a TZD. Neither sulfonylureas nor insulin was associated with a significant reduction in risk. When glycemic control (HbA1c) was added to the model, metformin remained significantly better (HR, 0.84) at protecting against dementia, whereas the other agents were not significantly better than a TZD.
Similarly, in a model with sulfonylureas as the referent, metformin was significantly better, with adjusted HRs of 0.79 and 0.80 in models without and with HbA1c, respectively.
An analysis of effect by dementia subtype also showed significant benefit for metformin vs. sulfonylurea for protection against Alzheimer’s disease (HR, 0.70) and vascular dementia (HR, 0.75).
The investigators plan to examine relationships between dose and dementia, and to look at the effects of combination therapies.
The study was supported by the National Institute of Diabetes and Digestive and Kidney Diseases, and by Kaiser Permanente. Dr. Whitmer is employed by Kaiser Permanente Northern California.
BOSTON – Older adults with type 2 diabetes who were taking metformin had a significantly lower 5-year risk for dementia than did peers who were taking other oral antidiabetic agents, investigators reported at the Alzheimer’s Association International Conference 2013.
A retrospective study of nearly 15,000 adults aged 55 years and older who started on single-agent drug therapy for type 2 diabetes between 1999 and 2001 showed that 5 years after starting therapy, patients who started on metformin had a 23% lower 5-year risk for dementia compared with patients who started on a thiazolidinedione (TZD) such as rosiglitazone (Avandia), reported Rachel Whitmer, Ph.D., an investigator in the research division of Kaiser Permanente Northern California, Oakland.
Metformin users also had a 20% lower risk for any dementia compared with those on a sulfonylurea such as glyburide. Among patients with a diabetes duration of less than 5 years, metformin users had a 40% lower dementia risk than did sulfonylurea users, and among those with a diabetes duration of at least 10 years, the risk for metformin users was 19% lower.
It is estimated that 12%-25% of people over age 65 have type 2 diabetes, putting them at about a 50% increase in risk for dementia, compared with nondiabetic people of the same age. - Dr. Rachel Whitmer
The differences between the drug types held up after adjustment for race, sex, education, and hemoglobin A1c (HbA1c) levels, Dr. Whitmer said.
It is estimated that 12%-25% of people over age 65 have type 2 diabetes, putting them at about a 50% increase in risk for dementia, compared with nondiabetic people of the same age. Nearly all elderly patients with type 2 diabetes will be started on some antidiabetic agent at some point, she noted.
"A lot of work out there [indicates] that insulin, and specifically insulin resistance, may play a role in Alzheimer’s disease, and there has been some nice work from animal models and cell-culture studies looking at metformin specifically," Dr. Whitmer said.
Metformin appears to lessen neuronal insulin resistance and is associated with neurogenesis. In addition, because it is associated with reduced risk of myocardial infarction and stroke, metformin may protect against vascular dementias, she added.
Dr. Whitmer and her colleagues delved into the Kaiser Permanente database to identify 14,891 patients aged 55 years and up with no history of dementia who were started on new diabetes pharmacotherapy between October 1999 and November 2001.
In all, 9.9% of patients in the sample were diagnosed with dementia during 5 years of follow-up, including 9.5% of 8,528 patients on metformin, 19.9% of 3,383 patients on a sulfonylurea, 9.8% of 2,095 patients on a TZD, and 11.1% of 905 patients on insulin.
In multivariate models adjusting for age, race, education, and diabetes duration, metformin was associated with a hazard ratio (HR) for any dementia of 0.77, compared with a TZD. Neither sulfonylureas nor insulin was associated with a significant reduction in risk. When glycemic control (HbA1c) was added to the model, metformin remained significantly better (HR, 0.84) at protecting against dementia, whereas the other agents were not significantly better than a TZD.
Similarly, in a model with sulfonylureas as the referent, metformin was significantly better, with adjusted HRs of 0.79 and 0.80 in models without and with HbA1c, respectively.
An analysis of effect by dementia subtype also showed significant benefit for metformin vs. sulfonylurea for protection against Alzheimer’s disease (HR, 0.70) and vascular dementia (HR, 0.75).
The investigators plan to examine relationships between dose and dementia, and to look at the effects of combination therapies.
The study was supported by the National Institute of Diabetes and Digestive and Kidney Diseases, and by Kaiser Permanente. Dr. Whitmer is employed by Kaiser Permanente Northern California.
BOSTON – Older adults with type 2 diabetes who were taking metformin had a significantly lower 5-year risk for dementia than did peers who were taking other oral antidiabetic agents, investigators reported at the Alzheimer’s Association International Conference 2013.
A retrospective study of nearly 15,000 adults aged 55 years and older who started on single-agent drug therapy for type 2 diabetes between 1999 and 2001 showed that 5 years after starting therapy, patients who started on metformin had a 23% lower 5-year risk for dementia compared with patients who started on a thiazolidinedione (TZD) such as rosiglitazone (Avandia), reported Rachel Whitmer, Ph.D., an investigator in the research division of Kaiser Permanente Northern California, Oakland.
Metformin users also had a 20% lower risk for any dementia compared with those on a sulfonylurea such as glyburide. Among patients with a diabetes duration of less than 5 years, metformin users had a 40% lower dementia risk than did sulfonylurea users, and among those with a diabetes duration of at least 10 years, the risk for metformin users was 19% lower.
It is estimated that 12%-25% of people over age 65 have type 2 diabetes, putting them at about a 50% increase in risk for dementia, compared with nondiabetic people of the same age. - Dr. Rachel Whitmer
The differences between the drug types held up after adjustment for race, sex, education, and hemoglobin A1c (HbA1c) levels, Dr. Whitmer said.
It is estimated that 12%-25% of people over age 65 have type 2 diabetes, putting them at about a 50% increase in risk for dementia, compared with nondiabetic people of the same age. Nearly all elderly patients with type 2 diabetes will be started on some antidiabetic agent at some point, she noted.
"A lot of work out there [indicates] that insulin, and specifically insulin resistance, may play a role in Alzheimer’s disease, and there has been some nice work from animal models and cell-culture studies looking at metformin specifically," Dr. Whitmer said.
Metformin appears to lessen neuronal insulin resistance and is associated with neurogenesis. In addition, because it is associated with reduced risk of myocardial infarction and stroke, metformin may protect against vascular dementias, she added.
Dr. Whitmer and her colleagues delved into the Kaiser Permanente database to identify 14,891 patients aged 55 years and up with no history of dementia who were started on new diabetes pharmacotherapy between October 1999 and November 2001.
In all, 9.9% of patients in the sample were diagnosed with dementia during 5 years of follow-up, including 9.5% of 8,528 patients on metformin, 19.9% of 3,383 patients on a sulfonylurea, 9.8% of 2,095 patients on a TZD, and 11.1% of 905 patients on insulin.
In multivariate models adjusting for age, race, education, and diabetes duration, metformin was associated with a hazard ratio (HR) for any dementia of 0.77, compared with a TZD. Neither sulfonylureas nor insulin was associated with a significant reduction in risk. When glycemic control (HbA1c) was added to the model, metformin remained significantly better (HR, 0.84) at protecting against dementia, whereas the other agents were not significantly better than a TZD.
Similarly, in a model with sulfonylureas as the referent, metformin was significantly better, with adjusted HRs of 0.79 and 0.80 in models without and with HbA1c, respectively.
An analysis of effect by dementia subtype also showed significant benefit for metformin vs. sulfonylurea for protection against Alzheimer’s disease (HR, 0.70) and vascular dementia (HR, 0.75).
The investigators plan to examine relationships between dose and dementia, and to look at the effects of combination therapies.
The study was supported by the National Institute of Diabetes and Digestive and Kidney Diseases, and by Kaiser Permanente. Dr. Whitmer is employed by Kaiser Permanente Northern California.
AT AAIC2013
Major finding: Metformin was associated with a 23% reduction in dementia risk, compared with a thiazolidinedione, and a 21% reduction vs. a sulfonylurea.
Data source: A retrospective study of 14,891 adults aged 55 years and older after initiation of pharmacotherapy for type 2 diabetes.
Disclosures: The study was supported by the National Institute of Diabetes and Digestive and Kidney Diseases, and by Kaiser Permanente. Dr. Whitmer is employed by Kaiser Permanente Northern California.
Pimavanserin reduced Parkinson’s psychosis without motor worsening
BOSTON – A novel 5-HT2A receptor inverse agonist significantly improved psychotic symptoms in Parkinson’s disease patients without the worsening of motor symptoms that usually occurs with antipsychotic treatment, according to the results of a randomized, placebo-controlled phase III trial.*
In addition to benefiting patients, the drug eased caregiver burden, Dr. Clive Ballard said at the Alzheimer’s Association International Conference 2013.
"By 4 weeks into the study, the benefit for caregivers began to appear, and it continued to increase" until the study ended at 6 weeks, said Dr. Ballard, professor of age-related diseases at King’s College London.
Parkinson’s psychosis is a common manifestation of the disease, and about 50% of patients will experience it, he said. "It’s not just frequent. It’s impactful. It’s distressing for patients and for those caring for them. It’s also associated with more impairment, with mortality, and it’s the leading cause of nursing home placement."
Sadly, he noted that there are not many medical options for the problem. Most antipsychotics produce extrapyramidal symptoms, which worsen parkinsonism. Atypical antipsychotics may be well tolerated, but are not very effective against the delusions and hallucinations that can occur in Parkinson’s patients.
"The situation in Parkinson’s is even worse than it is in Alzheimer’s. At least we do have some options for Alzheimer’s psychosis, even if they’re not great," Dr. Ballard said.
The atypical antipsychotics quetiapine and clozapine are the only well-tolerated options, he said. "Quetiapine is ineffective, though, leaving only clozapine. It’s well tolerated from a motor point of view, but it has other safety and tolerability issues that limit its use in clinical practice."
As a selective 5-HT2A inverse agonist, pimavanserin is in a unique drug class. According to Acadia Pharmaceuticals, the company developing the drug, it has the benefits of a 5HT2A agonist but doesn’t affect the dopaminergic, histaminergic, adrenergic, or muscarinic systems.
"Previous studies suggested that it was well tolerated and had some signal of potential benefit in psychosis," Dr. Ballard said. But those trials posted an unusually large placebo effect as well, making the results "difficult to interpret."
Investigators in the pimavanserin phase III study made a number of study design changes to compensate for the placebo response. In order to qualify for the trial, for example, patients had to have high baseline minimum scores on the Neuropsychiatric Inventory and the Scale for the Assessment of Positive Symptoms (SAPS).
They also took part in a brief psychosocial intervention before randomization. "In this intervention, the caregiver spends about 10 minutes each day with the patient, enabling the patient to do an activity they enjoy." This reduces the placebo response rate when the study medication commences, Dr. Ballard said.
The study also centralized any robust key findings, which were interpreted by independent, blinded raters.
The primary endpoint was antipsychotic efficacy as measured using the SAPS-PD, a nine-item scale adapted from the hallucinations and delusions domains of SAPS.
Secondary endpoints included the entire 20-item SAPS, the SAPS hallucinations and delusions subscores, the Clinical Global Impression (CGI) score, Unified Parkinson’s Disease Rating Scale (UPDRS) domains I and II, and a measure of caregiver burden.
The patients were randomized to 40 mg of pimavanserin daily (n = 105) or to placebo (n = 94) for 6 weeks. They were assessed at baseline and at 2, 4, and 6 weeks. After the randomization period, they could join a long-term, open-label extension study at the 40-mg/day dose. Most of the patients completed the trial protocol (87 in the placebo group and 89 in the pimavanserin group). Seven placebo patients dropped out, including two because of an adverse event. Of 15 patients in the pimavanserin group who dropped out, 10 did so because of adverse events. One patient randomized to pimavanserin did not take the medication.
At 6 weeks, patients in the active treatment group had significantly greater improvement on the SAPS-PD, the primary endpoint. By 15 days, both groups had experienced a significant improvement from baseline. Thereafter, the curves separated, with the placebo group becoming stable; Dr. Ballard said this indicated that the study design compensated for a large placebo response. The pimavanserin group, however, continued to improve. By the study’s end, there was a mean SAPS-PD decrease of 5.79 points in the active group, significantly better than the mean 2.73-point decrease in the placebo group. This translated to a 37% improvement for the study drug, compared with a 14% improvement for placebo.
The scores also translated into a meaningful clinical benefit, Dr. Ballard added. By the SAPS-PD measurement, response rates were 42% and 65%, respectively – a significant difference.
Changes on the CGI subscores were also significantly different. Patients in the placebo group experienced a mean decrease of less than half a point on the improvement subscale, compared with a decrease of about 1 point in the pimavanserin group. On the severity subscale, the placebo group stayed close to baseline, while the pimavanserin group decreased by about 1 point. About 27% of those taking placebo and 49% of those taking pimavanserin were considered responders.
Caregiver burden also significantly improved with the study drug.
In an exploratory analysis examining pimavanserin’s effect on sleep, the drug was associated with improvements in both nighttime sleep and daytime wakefulness on the Scale for Outcomes in Parkinson’s Disease sleep measure, Dr. Ballard said.
There were three deaths during the study – one in the placebo group (sudden cardiac death) and two in the active group (sepsis and septic shock). There were also four urinary tract infections – one in the placebo group and three in the active group. Other adverse events reported during the trial included peripheral edema (7% active vs. 3% placebo), falls (11% vs. 6%), confusion (6% vs. 3%), headache (1% vs. 5%), and hallucination (7% vs. 4%).
Based on the favorable results, Acadia announced that it had discontinued its work on a planned confirmatory phase III study. A New Drug Application for pimavanserin is in preparation, Dr. Ballard said.*
Acadia is also planning to study the drug’s effect in Alzheimer’s-associated psychosis in a phase II feasibility trial later this year, according to the company’s website.
"Similar to Parkinson’s disease psychosis, there is no therapy approved to treat Alzheimer’s psychosis in the U.S.," the company said in a written statement. "As symptoms progress and become more severe, physicians often resort to off-label use of antipsychotic medications in these patients. ... Antipsychotic drugs may exacerbate the cognitive disturbances associated with Alzheimer’s disease and also have a black box warning for use in elderly patients with dementia-related psychosis due to increased risks of mortality and morbidity."
The trial was funded by Acadia. Dr. Ballard disclosed that he has received honoraria and consulting fees from the company.
msullivan@fronlinemedcom.com
On Twitter @Alz_Gal
* Correction 7/30/13: An earlier version of this article misstated pimavanserin's mechanism of action and the status of its New Drug Application.
BOSTON – A novel 5-HT2A receptor inverse agonist significantly improved psychotic symptoms in Parkinson’s disease patients without the worsening of motor symptoms that usually occurs with antipsychotic treatment, according to the results of a randomized, placebo-controlled phase III trial.*
In addition to benefiting patients, the drug eased caregiver burden, Dr. Clive Ballard said at the Alzheimer’s Association International Conference 2013.
"By 4 weeks into the study, the benefit for caregivers began to appear, and it continued to increase" until the study ended at 6 weeks, said Dr. Ballard, professor of age-related diseases at King’s College London.
Parkinson’s psychosis is a common manifestation of the disease, and about 50% of patients will experience it, he said. "It’s not just frequent. It’s impactful. It’s distressing for patients and for those caring for them. It’s also associated with more impairment, with mortality, and it’s the leading cause of nursing home placement."
Sadly, he noted that there are not many medical options for the problem. Most antipsychotics produce extrapyramidal symptoms, which worsen parkinsonism. Atypical antipsychotics may be well tolerated, but are not very effective against the delusions and hallucinations that can occur in Parkinson’s patients.
"The situation in Parkinson’s is even worse than it is in Alzheimer’s. At least we do have some options for Alzheimer’s psychosis, even if they’re not great," Dr. Ballard said.
The atypical antipsychotics quetiapine and clozapine are the only well-tolerated options, he said. "Quetiapine is ineffective, though, leaving only clozapine. It’s well tolerated from a motor point of view, but it has other safety and tolerability issues that limit its use in clinical practice."
As a selective 5-HT2A inverse agonist, pimavanserin is in a unique drug class. According to Acadia Pharmaceuticals, the company developing the drug, it has the benefits of a 5HT2A agonist but doesn’t affect the dopaminergic, histaminergic, adrenergic, or muscarinic systems.
"Previous studies suggested that it was well tolerated and had some signal of potential benefit in psychosis," Dr. Ballard said. But those trials posted an unusually large placebo effect as well, making the results "difficult to interpret."
Investigators in the pimavanserin phase III study made a number of study design changes to compensate for the placebo response. In order to qualify for the trial, for example, patients had to have high baseline minimum scores on the Neuropsychiatric Inventory and the Scale for the Assessment of Positive Symptoms (SAPS).
They also took part in a brief psychosocial intervention before randomization. "In this intervention, the caregiver spends about 10 minutes each day with the patient, enabling the patient to do an activity they enjoy." This reduces the placebo response rate when the study medication commences, Dr. Ballard said.
The study also centralized any robust key findings, which were interpreted by independent, blinded raters.
The primary endpoint was antipsychotic efficacy as measured using the SAPS-PD, a nine-item scale adapted from the hallucinations and delusions domains of SAPS.
Secondary endpoints included the entire 20-item SAPS, the SAPS hallucinations and delusions subscores, the Clinical Global Impression (CGI) score, Unified Parkinson’s Disease Rating Scale (UPDRS) domains I and II, and a measure of caregiver burden.
The patients were randomized to 40 mg of pimavanserin daily (n = 105) or to placebo (n = 94) for 6 weeks. They were assessed at baseline and at 2, 4, and 6 weeks. After the randomization period, they could join a long-term, open-label extension study at the 40-mg/day dose. Most of the patients completed the trial protocol (87 in the placebo group and 89 in the pimavanserin group). Seven placebo patients dropped out, including two because of an adverse event. Of 15 patients in the pimavanserin group who dropped out, 10 did so because of adverse events. One patient randomized to pimavanserin did not take the medication.
At 6 weeks, patients in the active treatment group had significantly greater improvement on the SAPS-PD, the primary endpoint. By 15 days, both groups had experienced a significant improvement from baseline. Thereafter, the curves separated, with the placebo group becoming stable; Dr. Ballard said this indicated that the study design compensated for a large placebo response. The pimavanserin group, however, continued to improve. By the study’s end, there was a mean SAPS-PD decrease of 5.79 points in the active group, significantly better than the mean 2.73-point decrease in the placebo group. This translated to a 37% improvement for the study drug, compared with a 14% improvement for placebo.
The scores also translated into a meaningful clinical benefit, Dr. Ballard added. By the SAPS-PD measurement, response rates were 42% and 65%, respectively – a significant difference.
Changes on the CGI subscores were also significantly different. Patients in the placebo group experienced a mean decrease of less than half a point on the improvement subscale, compared with a decrease of about 1 point in the pimavanserin group. On the severity subscale, the placebo group stayed close to baseline, while the pimavanserin group decreased by about 1 point. About 27% of those taking placebo and 49% of those taking pimavanserin were considered responders.
Caregiver burden also significantly improved with the study drug.
In an exploratory analysis examining pimavanserin’s effect on sleep, the drug was associated with improvements in both nighttime sleep and daytime wakefulness on the Scale for Outcomes in Parkinson’s Disease sleep measure, Dr. Ballard said.
There were three deaths during the study – one in the placebo group (sudden cardiac death) and two in the active group (sepsis and septic shock). There were also four urinary tract infections – one in the placebo group and three in the active group. Other adverse events reported during the trial included peripheral edema (7% active vs. 3% placebo), falls (11% vs. 6%), confusion (6% vs. 3%), headache (1% vs. 5%), and hallucination (7% vs. 4%).
Based on the favorable results, Acadia announced that it had discontinued its work on a planned confirmatory phase III study. A New Drug Application for pimavanserin is in preparation, Dr. Ballard said.*
Acadia is also planning to study the drug’s effect in Alzheimer’s-associated psychosis in a phase II feasibility trial later this year, according to the company’s website.
"Similar to Parkinson’s disease psychosis, there is no therapy approved to treat Alzheimer’s psychosis in the U.S.," the company said in a written statement. "As symptoms progress and become more severe, physicians often resort to off-label use of antipsychotic medications in these patients. ... Antipsychotic drugs may exacerbate the cognitive disturbances associated with Alzheimer’s disease and also have a black box warning for use in elderly patients with dementia-related psychosis due to increased risks of mortality and morbidity."
The trial was funded by Acadia. Dr. Ballard disclosed that he has received honoraria and consulting fees from the company.
msullivan@fronlinemedcom.com
On Twitter @Alz_Gal
* Correction 7/30/13: An earlier version of this article misstated pimavanserin's mechanism of action and the status of its New Drug Application.
BOSTON – A novel 5-HT2A receptor inverse agonist significantly improved psychotic symptoms in Parkinson’s disease patients without the worsening of motor symptoms that usually occurs with antipsychotic treatment, according to the results of a randomized, placebo-controlled phase III trial.*
In addition to benefiting patients, the drug eased caregiver burden, Dr. Clive Ballard said at the Alzheimer’s Association International Conference 2013.
"By 4 weeks into the study, the benefit for caregivers began to appear, and it continued to increase" until the study ended at 6 weeks, said Dr. Ballard, professor of age-related diseases at King’s College London.
Parkinson’s psychosis is a common manifestation of the disease, and about 50% of patients will experience it, he said. "It’s not just frequent. It’s impactful. It’s distressing for patients and for those caring for them. It’s also associated with more impairment, with mortality, and it’s the leading cause of nursing home placement."
Sadly, he noted that there are not many medical options for the problem. Most antipsychotics produce extrapyramidal symptoms, which worsen parkinsonism. Atypical antipsychotics may be well tolerated, but are not very effective against the delusions and hallucinations that can occur in Parkinson’s patients.
"The situation in Parkinson’s is even worse than it is in Alzheimer’s. At least we do have some options for Alzheimer’s psychosis, even if they’re not great," Dr. Ballard said.
The atypical antipsychotics quetiapine and clozapine are the only well-tolerated options, he said. "Quetiapine is ineffective, though, leaving only clozapine. It’s well tolerated from a motor point of view, but it has other safety and tolerability issues that limit its use in clinical practice."
As a selective 5-HT2A inverse agonist, pimavanserin is in a unique drug class. According to Acadia Pharmaceuticals, the company developing the drug, it has the benefits of a 5HT2A agonist but doesn’t affect the dopaminergic, histaminergic, adrenergic, or muscarinic systems.
"Previous studies suggested that it was well tolerated and had some signal of potential benefit in psychosis," Dr. Ballard said. But those trials posted an unusually large placebo effect as well, making the results "difficult to interpret."
Investigators in the pimavanserin phase III study made a number of study design changes to compensate for the placebo response. In order to qualify for the trial, for example, patients had to have high baseline minimum scores on the Neuropsychiatric Inventory and the Scale for the Assessment of Positive Symptoms (SAPS).
They also took part in a brief psychosocial intervention before randomization. "In this intervention, the caregiver spends about 10 minutes each day with the patient, enabling the patient to do an activity they enjoy." This reduces the placebo response rate when the study medication commences, Dr. Ballard said.
The study also centralized any robust key findings, which were interpreted by independent, blinded raters.
The primary endpoint was antipsychotic efficacy as measured using the SAPS-PD, a nine-item scale adapted from the hallucinations and delusions domains of SAPS.
Secondary endpoints included the entire 20-item SAPS, the SAPS hallucinations and delusions subscores, the Clinical Global Impression (CGI) score, Unified Parkinson’s Disease Rating Scale (UPDRS) domains I and II, and a measure of caregiver burden.
The patients were randomized to 40 mg of pimavanserin daily (n = 105) or to placebo (n = 94) for 6 weeks. They were assessed at baseline and at 2, 4, and 6 weeks. After the randomization period, they could join a long-term, open-label extension study at the 40-mg/day dose. Most of the patients completed the trial protocol (87 in the placebo group and 89 in the pimavanserin group). Seven placebo patients dropped out, including two because of an adverse event. Of 15 patients in the pimavanserin group who dropped out, 10 did so because of adverse events. One patient randomized to pimavanserin did not take the medication.
At 6 weeks, patients in the active treatment group had significantly greater improvement on the SAPS-PD, the primary endpoint. By 15 days, both groups had experienced a significant improvement from baseline. Thereafter, the curves separated, with the placebo group becoming stable; Dr. Ballard said this indicated that the study design compensated for a large placebo response. The pimavanserin group, however, continued to improve. By the study’s end, there was a mean SAPS-PD decrease of 5.79 points in the active group, significantly better than the mean 2.73-point decrease in the placebo group. This translated to a 37% improvement for the study drug, compared with a 14% improvement for placebo.
The scores also translated into a meaningful clinical benefit, Dr. Ballard added. By the SAPS-PD measurement, response rates were 42% and 65%, respectively – a significant difference.
Changes on the CGI subscores were also significantly different. Patients in the placebo group experienced a mean decrease of less than half a point on the improvement subscale, compared with a decrease of about 1 point in the pimavanserin group. On the severity subscale, the placebo group stayed close to baseline, while the pimavanserin group decreased by about 1 point. About 27% of those taking placebo and 49% of those taking pimavanserin were considered responders.
Caregiver burden also significantly improved with the study drug.
In an exploratory analysis examining pimavanserin’s effect on sleep, the drug was associated with improvements in both nighttime sleep and daytime wakefulness on the Scale for Outcomes in Parkinson’s Disease sleep measure, Dr. Ballard said.
There were three deaths during the study – one in the placebo group (sudden cardiac death) and two in the active group (sepsis and septic shock). There were also four urinary tract infections – one in the placebo group and three in the active group. Other adverse events reported during the trial included peripheral edema (7% active vs. 3% placebo), falls (11% vs. 6%), confusion (6% vs. 3%), headache (1% vs. 5%), and hallucination (7% vs. 4%).
Based on the favorable results, Acadia announced that it had discontinued its work on a planned confirmatory phase III study. A New Drug Application for pimavanserin is in preparation, Dr. Ballard said.*
Acadia is also planning to study the drug’s effect in Alzheimer’s-associated psychosis in a phase II feasibility trial later this year, according to the company’s website.
"Similar to Parkinson’s disease psychosis, there is no therapy approved to treat Alzheimer’s psychosis in the U.S.," the company said in a written statement. "As symptoms progress and become more severe, physicians often resort to off-label use of antipsychotic medications in these patients. ... Antipsychotic drugs may exacerbate the cognitive disturbances associated with Alzheimer’s disease and also have a black box warning for use in elderly patients with dementia-related psychosis due to increased risks of mortality and morbidity."
The trial was funded by Acadia. Dr. Ballard disclosed that he has received honoraria and consulting fees from the company.
msullivan@fronlinemedcom.com
On Twitter @Alz_Gal
* Correction 7/30/13: An earlier version of this article misstated pimavanserin's mechanism of action and the status of its New Drug Application.
AT AAIC2013
Major finding: Pimavanserin improved symptoms of Parkinson’s disease psychosis by 37%, compared with a 14% improvement for placebo, without inducing extrapyramidal symptoms.
Data source: The phase III study randomized 199 patients to either placebo or pimavanserin 40 mg/day for 6 weeks.
Disclosures: Acadia Pharmaceuticals funded the study. Dr. Ballard disclosed that he has received honoraria and consulting fees from the company.
Brain atrophy rate may predict later cognitive decline
BOSTON – For people with mild cognitive impairment, the rate of brain atrophy now may predict the level of cognitive decline years from now.
Every 1% increase in the rate of whole brain atrophy during a single year translated into 1.7-point decline on the Mini Mental State Examination (MMSE) score at 3 years, Kelvin Leung, Ph.D., said at the Alzheimer’s Association International Conference 2013.
"Many studies have shown associations between concurrent brain atrophy rates and cognitive decline, when compared and measured over the same time period," said Dr. Leung of the University College, London. "However, fewer have looked at the association between brain atrophy rates and future cognitive decline."
To examine that question, Dr. Leung used cognitive and imaging data from the Alzheimer’s Disease Neuroimaging Initiative. His study cohort included 471 patients (295 with mild cognitive impairment and 176 normal controls). The primary outcome was 36-month change on the MMSE. Secondary measures included changes in auditory verbal learning, immediate recall, category fluency, trail making, and backward digit span.
He used a magnetic resonance imaging measurement called boundary shift integral to calculate brain volume changes from baseline to 12 months. The boundary shift integral takes into account whole brain shrinkage, hippocampus shrinkage, and ventricle expansion to calculate a percentage difference from one time point to another.
When looking at the raw scores, every 1% increase in the 1-year brain atrophy rate was associated with a statistically significant 1.7-point decrease on the MMSE.
That atrophy rate also was significantly associated with a 2-point decline on the audio verbal learning test, a 2.8-point decline on the category fluency test, and an 11-point decline on the Trail Making Test A. The control subjects showed no significant cognitive changes.
Dr. Leung saw similar changes when he looked only at the change in hippocampal volume. For every 1% increase in the 1-year rate of hippocampal atrophy, patients with mild cognitive impairment experienced significant declines in the MMSE, audio verbal learning test, immediate memory, category fluency, and trail making. Again, control patients showed no changes.
There was a nearly identical correlation between ventricular volume expansion and the cognitive measures, he said.
The next step will be to investigate which specific cognitive changes are most associated with specific brain region changes.
Dr. Leung made no financial disclosures.
BOSTON – For people with mild cognitive impairment, the rate of brain atrophy now may predict the level of cognitive decline years from now.
Every 1% increase in the rate of whole brain atrophy during a single year translated into 1.7-point decline on the Mini Mental State Examination (MMSE) score at 3 years, Kelvin Leung, Ph.D., said at the Alzheimer’s Association International Conference 2013.
"Many studies have shown associations between concurrent brain atrophy rates and cognitive decline, when compared and measured over the same time period," said Dr. Leung of the University College, London. "However, fewer have looked at the association between brain atrophy rates and future cognitive decline."
To examine that question, Dr. Leung used cognitive and imaging data from the Alzheimer’s Disease Neuroimaging Initiative. His study cohort included 471 patients (295 with mild cognitive impairment and 176 normal controls). The primary outcome was 36-month change on the MMSE. Secondary measures included changes in auditory verbal learning, immediate recall, category fluency, trail making, and backward digit span.
He used a magnetic resonance imaging measurement called boundary shift integral to calculate brain volume changes from baseline to 12 months. The boundary shift integral takes into account whole brain shrinkage, hippocampus shrinkage, and ventricle expansion to calculate a percentage difference from one time point to another.
When looking at the raw scores, every 1% increase in the 1-year brain atrophy rate was associated with a statistically significant 1.7-point decrease on the MMSE.
That atrophy rate also was significantly associated with a 2-point decline on the audio verbal learning test, a 2.8-point decline on the category fluency test, and an 11-point decline on the Trail Making Test A. The control subjects showed no significant cognitive changes.
Dr. Leung saw similar changes when he looked only at the change in hippocampal volume. For every 1% increase in the 1-year rate of hippocampal atrophy, patients with mild cognitive impairment experienced significant declines in the MMSE, audio verbal learning test, immediate memory, category fluency, and trail making. Again, control patients showed no changes.
There was a nearly identical correlation between ventricular volume expansion and the cognitive measures, he said.
The next step will be to investigate which specific cognitive changes are most associated with specific brain region changes.
Dr. Leung made no financial disclosures.
BOSTON – For people with mild cognitive impairment, the rate of brain atrophy now may predict the level of cognitive decline years from now.
Every 1% increase in the rate of whole brain atrophy during a single year translated into 1.7-point decline on the Mini Mental State Examination (MMSE) score at 3 years, Kelvin Leung, Ph.D., said at the Alzheimer’s Association International Conference 2013.
"Many studies have shown associations between concurrent brain atrophy rates and cognitive decline, when compared and measured over the same time period," said Dr. Leung of the University College, London. "However, fewer have looked at the association between brain atrophy rates and future cognitive decline."
To examine that question, Dr. Leung used cognitive and imaging data from the Alzheimer’s Disease Neuroimaging Initiative. His study cohort included 471 patients (295 with mild cognitive impairment and 176 normal controls). The primary outcome was 36-month change on the MMSE. Secondary measures included changes in auditory verbal learning, immediate recall, category fluency, trail making, and backward digit span.
He used a magnetic resonance imaging measurement called boundary shift integral to calculate brain volume changes from baseline to 12 months. The boundary shift integral takes into account whole brain shrinkage, hippocampus shrinkage, and ventricle expansion to calculate a percentage difference from one time point to another.
When looking at the raw scores, every 1% increase in the 1-year brain atrophy rate was associated with a statistically significant 1.7-point decrease on the MMSE.
That atrophy rate also was significantly associated with a 2-point decline on the audio verbal learning test, a 2.8-point decline on the category fluency test, and an 11-point decline on the Trail Making Test A. The control subjects showed no significant cognitive changes.
Dr. Leung saw similar changes when he looked only at the change in hippocampal volume. For every 1% increase in the 1-year rate of hippocampal atrophy, patients with mild cognitive impairment experienced significant declines in the MMSE, audio verbal learning test, immediate memory, category fluency, and trail making. Again, control patients showed no changes.
There was a nearly identical correlation between ventricular volume expansion and the cognitive measures, he said.
The next step will be to investigate which specific cognitive changes are most associated with specific brain region changes.
Dr. Leung made no financial disclosures.
AT AAIC2013
Major finding: For patients with mild cognitive impairment, every 1% increase in the rate of whole brain atrophy during a single year was associated with a 1.7-point MMSE score decrease 3 years later.
Data source: Retrospective study of 471 patients from the Alzheimer’s Disease Neuroimaging Initiative.
Disclosures: Dr. Leung had no financial disclosures.