User login
Dronedarone (Multaq), approved by the US Food and Drug Administration in July 2009, is a congener of the antiarrhythmic drug amiodarone (Cordarone). Designed in the hope that it would be safer than amiodarone, its official indication is to lower the risk of hospitalization in patients with paroxysmal or persistent atrial fibrillation or atrial flutter. However, its precise role in the management of atrial fibrillation is yet to be defined. If dronedarone remains well tolerated, it may permit clinicians to pursue a rhythm control strategy more often. In this article, we present a progress report on this new agent.
BETTER ANTIARRHYTHMIC DRUGS ARE NEEDED
Atrial fibrillation increases the risk of stroke fivefold and accounts for 15% to 20% of all strokes.1 It also increases the risk of heart failure. Drugs are the mainstay of therapy, but many antiarrhythmic drugs are not very effective and cause cardiac and extracardiac toxicity. Thus, the need for safe and effective new drugs.2
Much effort is going into the development of drugs that target specific ion channels or proteins expressed predominantly in atrial myocardium. The rationale is to avoid the unwanted effects of ionic currents on the ventricle and thus avoid ventricular proarrhythmic effects. At the same time, alternatives to the multiple channel blocker amiodarone, the mainstay of heart rhythm control therapy in atrial fibrillation, are being developed to retain the electrophysiologic efficacy of the mother compound but avoid its extracardiac toxicity.
RATE CONTROL VS RHYTHM CONTROL
In the acute care setting, heart rate control with atrioventricular nodal agents (beta-blockers, calcium channel blockers, and digitalis) is the preferred initial strategy in most hemodynamically stable patients presenting with new-onset atrial fibrillation.3
Since we lack an effective method for maintaining sinus rhythm without incurring significant adverse effects, rate control is also often chosen for chronic management of atrial fibrillation. This is particularly true for patients who have no symptoms or only minimal symptoms and in whom adequate rate control is easily attained. Indeed, results of large clinical trials suggest that rate control is satisfactory for many patients.
The main purpose of rate control is to control symptoms as opposed to merely lowering the ventricular rate. Effective rate control often prevents hemodynamic instability in patients with underlying heart disease who present acutely with atrial fibrillation. In patients with permanent atrial fibrillation, the RACE II study4 (Rate Control Efficacy in Permanent Atrial Fibrillation: a Comparison between Lenient Versus Strict Rate Control II), during a 3-year follow-up, showed that lenient rate control (resting heart rate < 110 beats per minute) is not inferior to strict rate control (resting heart rate < 80 beats per minute) in preventing major cardiovascular events (heart failure, stroke) or arrhythmic events such as syncope and sustained ventricular tachycardia.4
As a long-term strategy, rate control also prevents tachycardia-induced cardiomyopathy, reduces the risk of worsening of underlying heart failure, and can improve symptoms and quality of life.
Although maintenance of sinus rhythm is most likely associated with a survival benefit, heart rhythm control with antiarrhythmic drugs has not shown an advantage over rate control in overall or cardiovascular death rates, thromboembolic complications, or impact on heart failure. Indeed, a rhythm control strategy has been associated only with better exercise tolerance and, although less clear, with better quality of life.5
One possible explanation as to why a rhythm control strategy has not been shown to be superior to a rate control strategy is the side effects of the presently available drugs for rhythm control.
In a subgroup analysis of the Atrial Fibrillation Follow-up Investigation of Rhythm Management (AFFIRM) trial,6 antiarrhythmic therapy was associated with a 49% increase in the mortality rate that offset the benefits of conversion and maintenance of sinus rhythm, which was associated with a 53% reduction in mortality rates.
The hope is that newer drugs with less toxicity may produce better outcomes for patients treated with rhythm control.
AN ANALOGUE OF AMIODARONE, WITHOUT THE IODINE
Dronedarone is a structurally modified version of amiodarone, the antiarrhythmic drug that has shown the greatest efficacy at maintaining sinus rhythm in patients with paroxysmal atrial fibrillation. Although historically amiodarone has been effective in maintaining sinus rhythm and has been used safely in patients with advanced heart failure, its use has been limited by cumulative and often irreversible extracardiac organ toxicity.
Dronedarone was designed to match amiodarone’s efficacy but with a better safety profile. An iodine radical makes up more than one-third of amiodarone’s molecular weight. The omission of iodine in dronedarone was intended to reduce the likelihood of toxic side effects.
Dronedarone is a benzofuran derivative pharmacologically related to amiodarone, with the addition of a methylsulfonamide group. This reduces lipophilicity and the propensity to cross the blood-brain barrier; over a 2-year period this drug has not been shown to have neurotoxic effects.7
Dronedarone has proved efficacious without toxic or proarrhythmic effects and has minimal side effects, but concerns remain regarding its use in advanced heart failure. To date, its adverse-event profile appears comparable to that of placebo. However, whether its efficacy and incidence of adverse effects are comparable to what has been reported in the literature may take time to assess.
DRONEDARONE’S PHARMACOLOGY
Dronedarone, like amiodarone, blocks multiple sodium and potassium ion channels. It also exerts an antiadrenergic effect by noncompetitive binding to beta-adrenergic receptors as well as by inhibiting an agonist-induced increase in adenylate cyclase activity.8 Compared with amiodarone, dronedarone is a more potent blocker of peak sodium current.
Dronedarone is largely metabolized by the hepatic enzyme cytochrome P450 3A4 isoform (CYP3A4). Only 6% of dronedarone is excreted renally; however, no trial has yet assessed dronedarone’s safety in patients with marked kidney dysfunction.89
Dronedarone’s steady-state terminal elimination half-life is approximately 30 hours. When taken twice a day, it achieves steady-state concentrations in 5 to 7 days.
Dronedarone is available only for oral administration at 400 mg twice daily. Dose adjustment or titration is not recommended.
CLINICAL TRIALS OF DRONEDARONE
Dronedarone vs placebo
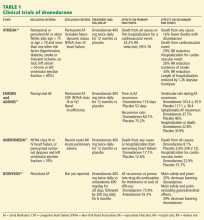
ATHENA (A Placebo-Controlled, Double-Blind, Parallel Arm Trial to Assess the Efficacy of Dronedarone 400 mg bid for the Prevention of Cardiovascular Hospitalization or Death From any Cause in Patients With Atrial Fibrillation/Atrial Flutter)10 was a prospective, double-blind study to assess morbidity and death rates in 4,628 patients with atrial fibrillation or atrial flutter and at least one other cardiovascular risk factor.
EURIDIS and ADONIS. Two trials,11 EURIDIS (European Trial in Atrial Fibrillation or Flutter Patients Receiving Dronedarone for the Maintenance of Sinus Rhythm) and ADONIS (American-Australian Trial With Dronedarone in Atrial Fibrillation or Flutter Patients for the Maintenance of Sinus Rhythm), enrolled a total of more than 1,200 patients and showed that dronedarone 400 mg twice a day produced a significantly lower rate of recurrence of atrial fibrillation after electrical cardioversion compared with placebo.
Overall, treatment with dronedarone significantly reduced the risk of a first recurrence of atrial fibrillation by 22% (ADONIS) and 27.5% (EURIDIS) (Table 1).
ERATO (Efficacy and Safety of Dronedarone for the Control of Ventricular Rate During Atrial Fibrillation),12 an additional phase III study, showed that dronedarone controlled the heart rate in patients with persistently accelerated ventricular rates despite concomitant standard therapy with a beta-blocker, digitalis, or a calcium-channel blocker. Dronedarone reduced the mean 24-hour heart rate by 11.7 beats per minute and the maximal exercise ventricular rate by 24.5 beats per minute at the 14th day.
ANDROMEDA (Anti-arrhythmic Trial With Dronedarone in Moderate to Severe CHF Evaluating Morbidity Decrease)13 was a study not of patients with atrial fibrillation but rather of patients with symptomatic congestive heart failure, a left ventricular ejection fraction of 35% or less, and recent hospitalization with new or worsening heart failure. The study was terminated early because of a higher rate of death with dronedarone13 (Table 1).
Dronedarone vs amiodarone
DIONYSOS (Efficacy and Safety of Dronedarone Versus Amiodarone for the Maintenance of Sinus Rhythm in Patients With Atrial Fibrillation)14 was a randomized double-blind trial. It evaluated the efficacy and safety of dronedarone (400 mg twice daily) or amiodarone (600 mg daily for 28 days, then 200 mg daily thereafter) for at least 6 months for the maintenance of sinus rhythm in patients with atrial fibrillation. It enrolled 504 patients with persistent atrial fibrillation; patients had not previously taken amiodarone. Dronedarone was less effective than amiodarone in maintaining sinus rhythm: the rate of recurrent atrial fibrillation was 63% with dronedarone and 42% with amiodarone. But dronedarone was associated with fewer adverse effects and less need for premature discontinuation of drug treatment at a mean follow-up of 7 months (Table 1).
WHERE DOES DRONEDARONE FIT IN ATRIAL FIBRILLATION MANAGEMENT?
Dronedarone is indicated in persistent or paroxysmal atrial fibrillation, based on the observed reduction of the rate of hospitalization. It is indicated for the maintenance of sinus rhythm and may be used in patients with persistent or paroxysmal atrial fibrillation and flutter who are in sinus rhythm or will be undergoing cardioversion soon after starting the drug. Dronedarone has no role in the acute management of atrial fibrillation, such as in cardioversion to sinus rhythm in the emergency department.
We do not have substantial evidence of the efficacy of dronedarone in patients with resistant atrial fibrillation, in whom multiple antiarrhythmics have failed to maintain sinus rhythm, and no published trial has used the inclusion criterion of treatment failure with other antiarrhythmic drugs.
The role of dronedarone in heart failure with preserved systolic function is unclear. Patients taking dronedarone are twice as likely as those taking amiodarone to have a recurrence of atrial fibrillation.
The main advantage of dronedarone is its lower adverse effect profile. However, this statement is based on only a few years of observation. If the patient has developed adverse effects with amiodarone, or if the clinician is concerned about the risk of serious adverse effects, dronedarone presents an alternative for those patients without heart failure or significant left ventricular dysfunction. One such group may be younger patients, because of concerns about the cumulative effects of amiodarone taken over a lifetime.
Dronedarone may represent an acceptable alternative to many of the current antiarrhythmic drugs. Based on the results of the Cardiac Arrhythmia Suppression Trial (CAST),15 class IC antiarrhythmics such as flecainide (Tambocor) are generally avoided in patients with prior myocardial infarction or with known or even suspected coronary artery disease. Similarly, sotalol (Betapace) is generally avoided in patients with marked left ventricular hypertrophy because of adverse effects.16 Dofetilide (Tikosyn) and often sotalol require hospitalization with telemetric monitoring for QTc prolongation and the risk of proarrhythmia with torsades de pointes. Dronedarone, however, generally can be safely started in the outpatient setting.
As when considering prescribing any antiarrhythmic, the clinician must assess the patient’s thromboembolic risk, since this risk persists with a rhythm control strategy.
There is substantial evidence from the ATHENA trial,10 in which 30% of the patients had coronary artery disease, that dronedarone is safe and effective in patients with coronary artery disease. Its use in patients who have undergone coronary artery bypass surgery remains to be defined.
WHEN SHOULD WE SWITCH PATIENTS TO DRONEDARONE?
Preliminary experience suggests that dronedarone, unlike most antiarrhythmic drugs, can be safely started about 48 hours after amiodarone is discontinued. Cumulative toxicity has not been noted with dronedarone. Caution should be exercised when switching if the patient has baseline bradycardia or QT interval prolongation. No algorithm has been developed for switching from other antiarrhythmic drugs to dronedarone.
CONTRAINDICATIONS TO DRONEDARONE
Dronedarone is contraindicated in:
- Patients with New York Heart Association (NYHA) class IV heart failure or NYHA class II or III heart failure with recent decompensation requiring hospitalization or referral to a specialized heart failure clinic
- Patients with second- or third-degree atrioventricular block or sick sinus syndrome (except when used in conjunction with a functioning pacemaker) or bradycardia (a heart rate < 50 beats per minute)
- Patients with a QTc interval of 500 ms or longer
- Patients with severe hepatic impairment
- Women who are pregnant, are attempting to become pregnant, or are breast-feeding
- Patients taking potent CYP3A inhibitors—antifungals like ketoconazole (Nizoral), itraconazole (Sporanox), or voriconazole (VFEND); macrolide antibiotics like telithromycin (Ketek) or clarithromycin (Biaxin); protease inhibitors; or other drugs that prolong the QT interval.
In patients with new or worsening heart failure, one should consider suspending or stopping dronedarone therapy.
DRONEDARONE’S ADVERSE EFFECTS
In trials to date, dronedarone has not shown evidence of proarrhythmia (tachyarrhythmia or bradyarrythmia), torsades de pointes, or amiodarone-like organ toxicity affecting the thyroid or the lungs. Recently, rare cases of severe hepatic injury were associated with dronedarone; therefore, periodic liver function testing is advised for patients taking dronedarone, especially during the first 6 months of therapy.
Dronedarone has been associated with higher rates of diarrhea, nausea, bradycardia, QT interval prolongation, and cutaneous rash compared with placebo. In DAFNE (Dronedarone Atrial Fibrillation Study After Electrical Cardioversion),17 10.8% of patients taking dronedarone had to stop taking it because of adverse events. With 800 mg daily, the discontinuation rate was only 3.9%. The most common cause of drug discontinuation was gastrointestinal effects. Anecdotal reports suggest that the gastrointestinal side effects may be self-limited and may not always require discontinuation of the drug.
Serum creatinine levels increase by about 0.1 mg/dL after the start of treatment. This elevation occurs after 1 to 2 days, reaches a plateau after 7 days, and is reversible. The mechanism is thought to be that dronedarone partially inhibits tubular organic cation transporters, which in turn reduces renal creatinine clearance by about 18%, but with no evidence of an effect on glomerular filtration, renal plasma flow, or electrolyte exchanges.18 A limited increase in serum creatinine is, therefore, expected with dronedarone treatment, but this does not mean there is a decline in renal function.
DRONEDARONE AND POTENTIAL DRUG INTERACTIONS
Warfarin. Dronedarone does not increase the international normalized ratio when used with warfarin (Coumadin).
Verapamil, diltiazem. Dose reduction is required to avoid bradyarrhythmias with co-administration of moderate CYP3A4 inhibitors such as verapamil (Calan, Verelan) and diltiazem (Cardizem).
Simvastatin. Dronedarone increases levels of simvastatin (Zocor), a CYP3A4 substrate, two to four times, thus increasing the risk of statin-induced myopathy.
Digoxin. Dronedarone increases the serum digoxin concentration about 2.5 times, and this necessitates monitoring the digoxin level and possibly reducing the digoxin dose.13
Diuretics. Hypokalemia and hypomagnesemia may occur with concomitant administration of potassium-depleting diuretics. Potassium levels should be maintained in the normal range before and during administration of dronedarone.
Tacrolimus, sirolimus. Dronedarone may increase levels of tacrolimus (Prograf) or sirolimus (Rapamune) in posttransplantation patients. This requires dose monitoring and adjustment in concomitant therapy with these agents.
COST VARIES
The cost of dronedarone varies based on factors that include location. Dronedarone’s retail cost ranges from $3.20 to $4.00 per pill (approximately $7.20 per day). It is not available in generic form. It is presently covered by many health plans as a tier 2 drug, representing a $15 to $40 monthly copay.
MORE DATA NEEDED
Dronedarone represents the first in what may well be a number of new antiarrhythmic drugs for the treatment of patients with paroxysmal atrial fibrillation. Although less efficacious then amiodarone, dronedarone appears to be better tolerated and have less serious side effects. It is contraindicated in patients with severe systolic dysfunction and in those with recent heart failure decompensation. It appears safe in coronary artery disease and marked left ventricular hypertrophy, unlike flecainide, propafenone (Rythmol), and sotalol.
To further understand how dronedarone will fare against other antiarrhythmic drugs, more studies with longer follow-up are needed. These studies need to demonstrate superior tolerability of dronedarone, acceptable quality of life without unacceptable loss of efficacy, or a decrease in morbidity or mortality rates compared with amiodarone.
Dronedarone can be safely started in most patients on an outpatient basis. The risk of proarrhythmia with dronedarone appears to be very low.
- Mathew ST, Patel J, Joseph S. Atrial fibrillation: mechanistic insights and treatment options. Eur J Intern Med 2009; 20:672–681.
- Schmitt J, Ehrlich JR, Hohnloser SH. New antiarrhythmic drugs for the treatment of atrial fibrillation. Herz 2008; 33:562–567.
- Wyse DG, Waldo AL, DiMarco JP, et al; Atrial Fibrillation Follow-up Investigation of Rhythm Management (AFFIRM) Investigators. A comparison of rate control and rhythm control in patients with atrial fibrillation. N Engl J Med 2002; 347:1825–1833.
- Van Gelder IC, Groenveld HF, Crijns HJ, et al; RACE II Investigators. Lenient versus strict rate control in patients with atrial fibrillation. N Engl J Med 2010; 362:1363–1373.
- Singh BN, Singh SN, Reda DJ, et al; Sotalol Amiodarone Atrial Fibrillation Efficacy Trial (SAFE-T) Investigators. Amiodarone versus sotalol for atrial fibrillation. N Engl J Med 2005; 352:1861–1872.
- The AFFIRM Investigators. Relationships between sinus rhythm, treatment, and survival in the Atrial Fibrillation Follow-Up Investigation of Rhythm Management (AFFIRM) study. Circulation 2004; 109:1509–1513.
- Van Beeren HC, Jong WM, Kaptein E, Visser TJ, Bakker O, Wiersinga WM. Dronerarone [sic] acts as a selective inhibitor of 3,5,3′-triiodothyronine binding to thyroid hormone receptor-alpha1: in vitro and in vivo evidence. Endocrinology 2003; 144:552–558.
- Patel C, Yan GX, Kowey PR. Dronedarone. Circulation 2009; 120:636–644.
- Dale KM, White CM. Dronedarone: an amiodarone analog for the treatment of atrial fibrillation and atrial flutter. Ann Pharmacother 2007; 41:599–605.
- Hohnloser SH, Crijns HJ, van Eickels M; ATHENA Investigators. Effect of dronedarone on cardiovascular events in atrial fibrillation. N Engl J Med 2009; 360:668–678.
- Singh BN, Connolly SJ, Crijns HJ, et al; EURIDIS and ADONIS Investigators. Dronedarone for maintenance of sinus rhythm in atrial fibrillation or flutter. N Engl J Med 2007; 357:987–999.
- Davy JM, Herold M, Hoglund CERATO Study Investigators. Dronedarone for the control of ventricular rate in permanent atrial fibrillation: the Efficacy and safety of dRonedArone for the cOntrol of ventricular rate during atrial fibrillation (ERATO) study. Am Heart J 2008; 156:527.e1–e9.
- Køber L, Torp-Pedersen C, McMurray JJ; Dronedarone Study Group. Increased mortality after dronedarone therapy for severe heart failure. N Engl J Med 2008; 358:2678–2687.
- Le Heuzey JY, De Ferrari GM, Radzik D, Santini M, Zhu J, Davy JM. A short-term, randomized, double-blind, parallel-group study to evaluate the efficacy and safety of dronedarone versus amiodarone in patients with persistent atrial fibrillation: the DIONYSOS study. J Cardiovasc Electrophysiol 2010; 21:597–605.
- The Cardiac Arrhythmia Suppression Trial (CAST) Investigators. Preliminary report: effect of encainide and flecainide on mortality in a randomized trial of arrhythmia suppression after myocardial infarction. N Engl J Med 1989; 321:406–412.
- Pratt CM. Clinical implications of the Survival With Oral D-sotalol (SWORD) trial: an investigation of patients with left ventricular dysfunction after myocardial infarction. Card Electrophysiol Rev 1998; 2:28–29.
- Touboul P, Brugada J, Capucci A, Crijns HJ, Edvardsson N, Hohnloser SH. Dronedarone for prevention of atrial fibrillation: a dose-ranging study. Eur Heart J 2003; 24:1481–1487.
- Tschuppert Y, Buclin T, Rothuizen LE, et al. Effect of dronedarone on renal function in healthy subjects. Br J Clin Pharmacol 2007; 64:785–791.
Dronedarone (Multaq), approved by the US Food and Drug Administration in July 2009, is a congener of the antiarrhythmic drug amiodarone (Cordarone). Designed in the hope that it would be safer than amiodarone, its official indication is to lower the risk of hospitalization in patients with paroxysmal or persistent atrial fibrillation or atrial flutter. However, its precise role in the management of atrial fibrillation is yet to be defined. If dronedarone remains well tolerated, it may permit clinicians to pursue a rhythm control strategy more often. In this article, we present a progress report on this new agent.
BETTER ANTIARRHYTHMIC DRUGS ARE NEEDED
Atrial fibrillation increases the risk of stroke fivefold and accounts for 15% to 20% of all strokes.1 It also increases the risk of heart failure. Drugs are the mainstay of therapy, but many antiarrhythmic drugs are not very effective and cause cardiac and extracardiac toxicity. Thus, the need for safe and effective new drugs.2
Much effort is going into the development of drugs that target specific ion channels or proteins expressed predominantly in atrial myocardium. The rationale is to avoid the unwanted effects of ionic currents on the ventricle and thus avoid ventricular proarrhythmic effects. At the same time, alternatives to the multiple channel blocker amiodarone, the mainstay of heart rhythm control therapy in atrial fibrillation, are being developed to retain the electrophysiologic efficacy of the mother compound but avoid its extracardiac toxicity.
RATE CONTROL VS RHYTHM CONTROL
In the acute care setting, heart rate control with atrioventricular nodal agents (beta-blockers, calcium channel blockers, and digitalis) is the preferred initial strategy in most hemodynamically stable patients presenting with new-onset atrial fibrillation.3
Since we lack an effective method for maintaining sinus rhythm without incurring significant adverse effects, rate control is also often chosen for chronic management of atrial fibrillation. This is particularly true for patients who have no symptoms or only minimal symptoms and in whom adequate rate control is easily attained. Indeed, results of large clinical trials suggest that rate control is satisfactory for many patients.
The main purpose of rate control is to control symptoms as opposed to merely lowering the ventricular rate. Effective rate control often prevents hemodynamic instability in patients with underlying heart disease who present acutely with atrial fibrillation. In patients with permanent atrial fibrillation, the RACE II study4 (Rate Control Efficacy in Permanent Atrial Fibrillation: a Comparison between Lenient Versus Strict Rate Control II), during a 3-year follow-up, showed that lenient rate control (resting heart rate < 110 beats per minute) is not inferior to strict rate control (resting heart rate < 80 beats per minute) in preventing major cardiovascular events (heart failure, stroke) or arrhythmic events such as syncope and sustained ventricular tachycardia.4
As a long-term strategy, rate control also prevents tachycardia-induced cardiomyopathy, reduces the risk of worsening of underlying heart failure, and can improve symptoms and quality of life.
Although maintenance of sinus rhythm is most likely associated with a survival benefit, heart rhythm control with antiarrhythmic drugs has not shown an advantage over rate control in overall or cardiovascular death rates, thromboembolic complications, or impact on heart failure. Indeed, a rhythm control strategy has been associated only with better exercise tolerance and, although less clear, with better quality of life.5
One possible explanation as to why a rhythm control strategy has not been shown to be superior to a rate control strategy is the side effects of the presently available drugs for rhythm control.
In a subgroup analysis of the Atrial Fibrillation Follow-up Investigation of Rhythm Management (AFFIRM) trial,6 antiarrhythmic therapy was associated with a 49% increase in the mortality rate that offset the benefits of conversion and maintenance of sinus rhythm, which was associated with a 53% reduction in mortality rates.
The hope is that newer drugs with less toxicity may produce better outcomes for patients treated with rhythm control.
AN ANALOGUE OF AMIODARONE, WITHOUT THE IODINE
Dronedarone is a structurally modified version of amiodarone, the antiarrhythmic drug that has shown the greatest efficacy at maintaining sinus rhythm in patients with paroxysmal atrial fibrillation. Although historically amiodarone has been effective in maintaining sinus rhythm and has been used safely in patients with advanced heart failure, its use has been limited by cumulative and often irreversible extracardiac organ toxicity.
Dronedarone was designed to match amiodarone’s efficacy but with a better safety profile. An iodine radical makes up more than one-third of amiodarone’s molecular weight. The omission of iodine in dronedarone was intended to reduce the likelihood of toxic side effects.
Dronedarone is a benzofuran derivative pharmacologically related to amiodarone, with the addition of a methylsulfonamide group. This reduces lipophilicity and the propensity to cross the blood-brain barrier; over a 2-year period this drug has not been shown to have neurotoxic effects.7
Dronedarone has proved efficacious without toxic or proarrhythmic effects and has minimal side effects, but concerns remain regarding its use in advanced heart failure. To date, its adverse-event profile appears comparable to that of placebo. However, whether its efficacy and incidence of adverse effects are comparable to what has been reported in the literature may take time to assess.
DRONEDARONE’S PHARMACOLOGY
Dronedarone, like amiodarone, blocks multiple sodium and potassium ion channels. It also exerts an antiadrenergic effect by noncompetitive binding to beta-adrenergic receptors as well as by inhibiting an agonist-induced increase in adenylate cyclase activity.8 Compared with amiodarone, dronedarone is a more potent blocker of peak sodium current.
Dronedarone is largely metabolized by the hepatic enzyme cytochrome P450 3A4 isoform (CYP3A4). Only 6% of dronedarone is excreted renally; however, no trial has yet assessed dronedarone’s safety in patients with marked kidney dysfunction.89
Dronedarone’s steady-state terminal elimination half-life is approximately 30 hours. When taken twice a day, it achieves steady-state concentrations in 5 to 7 days.
Dronedarone is available only for oral administration at 400 mg twice daily. Dose adjustment or titration is not recommended.
CLINICAL TRIALS OF DRONEDARONE
Dronedarone vs placebo
ATHENA (A Placebo-Controlled, Double-Blind, Parallel Arm Trial to Assess the Efficacy of Dronedarone 400 mg bid for the Prevention of Cardiovascular Hospitalization or Death From any Cause in Patients With Atrial Fibrillation/Atrial Flutter)10 was a prospective, double-blind study to assess morbidity and death rates in 4,628 patients with atrial fibrillation or atrial flutter and at least one other cardiovascular risk factor.
EURIDIS and ADONIS. Two trials,11 EURIDIS (European Trial in Atrial Fibrillation or Flutter Patients Receiving Dronedarone for the Maintenance of Sinus Rhythm) and ADONIS (American-Australian Trial With Dronedarone in Atrial Fibrillation or Flutter Patients for the Maintenance of Sinus Rhythm), enrolled a total of more than 1,200 patients and showed that dronedarone 400 mg twice a day produced a significantly lower rate of recurrence of atrial fibrillation after electrical cardioversion compared with placebo.
Overall, treatment with dronedarone significantly reduced the risk of a first recurrence of atrial fibrillation by 22% (ADONIS) and 27.5% (EURIDIS) (Table 1).
ERATO (Efficacy and Safety of Dronedarone for the Control of Ventricular Rate During Atrial Fibrillation),12 an additional phase III study, showed that dronedarone controlled the heart rate in patients with persistently accelerated ventricular rates despite concomitant standard therapy with a beta-blocker, digitalis, or a calcium-channel blocker. Dronedarone reduced the mean 24-hour heart rate by 11.7 beats per minute and the maximal exercise ventricular rate by 24.5 beats per minute at the 14th day.
ANDROMEDA (Anti-arrhythmic Trial With Dronedarone in Moderate to Severe CHF Evaluating Morbidity Decrease)13 was a study not of patients with atrial fibrillation but rather of patients with symptomatic congestive heart failure, a left ventricular ejection fraction of 35% or less, and recent hospitalization with new or worsening heart failure. The study was terminated early because of a higher rate of death with dronedarone13 (Table 1).
Dronedarone vs amiodarone
DIONYSOS (Efficacy and Safety of Dronedarone Versus Amiodarone for the Maintenance of Sinus Rhythm in Patients With Atrial Fibrillation)14 was a randomized double-blind trial. It evaluated the efficacy and safety of dronedarone (400 mg twice daily) or amiodarone (600 mg daily for 28 days, then 200 mg daily thereafter) for at least 6 months for the maintenance of sinus rhythm in patients with atrial fibrillation. It enrolled 504 patients with persistent atrial fibrillation; patients had not previously taken amiodarone. Dronedarone was less effective than amiodarone in maintaining sinus rhythm: the rate of recurrent atrial fibrillation was 63% with dronedarone and 42% with amiodarone. But dronedarone was associated with fewer adverse effects and less need for premature discontinuation of drug treatment at a mean follow-up of 7 months (Table 1).
WHERE DOES DRONEDARONE FIT IN ATRIAL FIBRILLATION MANAGEMENT?
Dronedarone is indicated in persistent or paroxysmal atrial fibrillation, based on the observed reduction of the rate of hospitalization. It is indicated for the maintenance of sinus rhythm and may be used in patients with persistent or paroxysmal atrial fibrillation and flutter who are in sinus rhythm or will be undergoing cardioversion soon after starting the drug. Dronedarone has no role in the acute management of atrial fibrillation, such as in cardioversion to sinus rhythm in the emergency department.
We do not have substantial evidence of the efficacy of dronedarone in patients with resistant atrial fibrillation, in whom multiple antiarrhythmics have failed to maintain sinus rhythm, and no published trial has used the inclusion criterion of treatment failure with other antiarrhythmic drugs.
The role of dronedarone in heart failure with preserved systolic function is unclear. Patients taking dronedarone are twice as likely as those taking amiodarone to have a recurrence of atrial fibrillation.
The main advantage of dronedarone is its lower adverse effect profile. However, this statement is based on only a few years of observation. If the patient has developed adverse effects with amiodarone, or if the clinician is concerned about the risk of serious adverse effects, dronedarone presents an alternative for those patients without heart failure or significant left ventricular dysfunction. One such group may be younger patients, because of concerns about the cumulative effects of amiodarone taken over a lifetime.
Dronedarone may represent an acceptable alternative to many of the current antiarrhythmic drugs. Based on the results of the Cardiac Arrhythmia Suppression Trial (CAST),15 class IC antiarrhythmics such as flecainide (Tambocor) are generally avoided in patients with prior myocardial infarction or with known or even suspected coronary artery disease. Similarly, sotalol (Betapace) is generally avoided in patients with marked left ventricular hypertrophy because of adverse effects.16 Dofetilide (Tikosyn) and often sotalol require hospitalization with telemetric monitoring for QTc prolongation and the risk of proarrhythmia with torsades de pointes. Dronedarone, however, generally can be safely started in the outpatient setting.
As when considering prescribing any antiarrhythmic, the clinician must assess the patient’s thromboembolic risk, since this risk persists with a rhythm control strategy.
There is substantial evidence from the ATHENA trial,10 in which 30% of the patients had coronary artery disease, that dronedarone is safe and effective in patients with coronary artery disease. Its use in patients who have undergone coronary artery bypass surgery remains to be defined.
WHEN SHOULD WE SWITCH PATIENTS TO DRONEDARONE?
Preliminary experience suggests that dronedarone, unlike most antiarrhythmic drugs, can be safely started about 48 hours after amiodarone is discontinued. Cumulative toxicity has not been noted with dronedarone. Caution should be exercised when switching if the patient has baseline bradycardia or QT interval prolongation. No algorithm has been developed for switching from other antiarrhythmic drugs to dronedarone.
CONTRAINDICATIONS TO DRONEDARONE
Dronedarone is contraindicated in:
- Patients with New York Heart Association (NYHA) class IV heart failure or NYHA class II or III heart failure with recent decompensation requiring hospitalization or referral to a specialized heart failure clinic
- Patients with second- or third-degree atrioventricular block or sick sinus syndrome (except when used in conjunction with a functioning pacemaker) or bradycardia (a heart rate < 50 beats per minute)
- Patients with a QTc interval of 500 ms or longer
- Patients with severe hepatic impairment
- Women who are pregnant, are attempting to become pregnant, or are breast-feeding
- Patients taking potent CYP3A inhibitors—antifungals like ketoconazole (Nizoral), itraconazole (Sporanox), or voriconazole (VFEND); macrolide antibiotics like telithromycin (Ketek) or clarithromycin (Biaxin); protease inhibitors; or other drugs that prolong the QT interval.
In patients with new or worsening heart failure, one should consider suspending or stopping dronedarone therapy.
DRONEDARONE’S ADVERSE EFFECTS
In trials to date, dronedarone has not shown evidence of proarrhythmia (tachyarrhythmia or bradyarrythmia), torsades de pointes, or amiodarone-like organ toxicity affecting the thyroid or the lungs. Recently, rare cases of severe hepatic injury were associated with dronedarone; therefore, periodic liver function testing is advised for patients taking dronedarone, especially during the first 6 months of therapy.
Dronedarone has been associated with higher rates of diarrhea, nausea, bradycardia, QT interval prolongation, and cutaneous rash compared with placebo. In DAFNE (Dronedarone Atrial Fibrillation Study After Electrical Cardioversion),17 10.8% of patients taking dronedarone had to stop taking it because of adverse events. With 800 mg daily, the discontinuation rate was only 3.9%. The most common cause of drug discontinuation was gastrointestinal effects. Anecdotal reports suggest that the gastrointestinal side effects may be self-limited and may not always require discontinuation of the drug.
Serum creatinine levels increase by about 0.1 mg/dL after the start of treatment. This elevation occurs after 1 to 2 days, reaches a plateau after 7 days, and is reversible. The mechanism is thought to be that dronedarone partially inhibits tubular organic cation transporters, which in turn reduces renal creatinine clearance by about 18%, but with no evidence of an effect on glomerular filtration, renal plasma flow, or electrolyte exchanges.18 A limited increase in serum creatinine is, therefore, expected with dronedarone treatment, but this does not mean there is a decline in renal function.
DRONEDARONE AND POTENTIAL DRUG INTERACTIONS
Warfarin. Dronedarone does not increase the international normalized ratio when used with warfarin (Coumadin).
Verapamil, diltiazem. Dose reduction is required to avoid bradyarrhythmias with co-administration of moderate CYP3A4 inhibitors such as verapamil (Calan, Verelan) and diltiazem (Cardizem).
Simvastatin. Dronedarone increases levels of simvastatin (Zocor), a CYP3A4 substrate, two to four times, thus increasing the risk of statin-induced myopathy.
Digoxin. Dronedarone increases the serum digoxin concentration about 2.5 times, and this necessitates monitoring the digoxin level and possibly reducing the digoxin dose.13
Diuretics. Hypokalemia and hypomagnesemia may occur with concomitant administration of potassium-depleting diuretics. Potassium levels should be maintained in the normal range before and during administration of dronedarone.
Tacrolimus, sirolimus. Dronedarone may increase levels of tacrolimus (Prograf) or sirolimus (Rapamune) in posttransplantation patients. This requires dose monitoring and adjustment in concomitant therapy with these agents.
COST VARIES
The cost of dronedarone varies based on factors that include location. Dronedarone’s retail cost ranges from $3.20 to $4.00 per pill (approximately $7.20 per day). It is not available in generic form. It is presently covered by many health plans as a tier 2 drug, representing a $15 to $40 monthly copay.
MORE DATA NEEDED
Dronedarone represents the first in what may well be a number of new antiarrhythmic drugs for the treatment of patients with paroxysmal atrial fibrillation. Although less efficacious then amiodarone, dronedarone appears to be better tolerated and have less serious side effects. It is contraindicated in patients with severe systolic dysfunction and in those with recent heart failure decompensation. It appears safe in coronary artery disease and marked left ventricular hypertrophy, unlike flecainide, propafenone (Rythmol), and sotalol.
To further understand how dronedarone will fare against other antiarrhythmic drugs, more studies with longer follow-up are needed. These studies need to demonstrate superior tolerability of dronedarone, acceptable quality of life without unacceptable loss of efficacy, or a decrease in morbidity or mortality rates compared with amiodarone.
Dronedarone can be safely started in most patients on an outpatient basis. The risk of proarrhythmia with dronedarone appears to be very low.
Dronedarone (Multaq), approved by the US Food and Drug Administration in July 2009, is a congener of the antiarrhythmic drug amiodarone (Cordarone). Designed in the hope that it would be safer than amiodarone, its official indication is to lower the risk of hospitalization in patients with paroxysmal or persistent atrial fibrillation or atrial flutter. However, its precise role in the management of atrial fibrillation is yet to be defined. If dronedarone remains well tolerated, it may permit clinicians to pursue a rhythm control strategy more often. In this article, we present a progress report on this new agent.
BETTER ANTIARRHYTHMIC DRUGS ARE NEEDED
Atrial fibrillation increases the risk of stroke fivefold and accounts for 15% to 20% of all strokes.1 It also increases the risk of heart failure. Drugs are the mainstay of therapy, but many antiarrhythmic drugs are not very effective and cause cardiac and extracardiac toxicity. Thus, the need for safe and effective new drugs.2
Much effort is going into the development of drugs that target specific ion channels or proteins expressed predominantly in atrial myocardium. The rationale is to avoid the unwanted effects of ionic currents on the ventricle and thus avoid ventricular proarrhythmic effects. At the same time, alternatives to the multiple channel blocker amiodarone, the mainstay of heart rhythm control therapy in atrial fibrillation, are being developed to retain the electrophysiologic efficacy of the mother compound but avoid its extracardiac toxicity.
RATE CONTROL VS RHYTHM CONTROL
In the acute care setting, heart rate control with atrioventricular nodal agents (beta-blockers, calcium channel blockers, and digitalis) is the preferred initial strategy in most hemodynamically stable patients presenting with new-onset atrial fibrillation.3
Since we lack an effective method for maintaining sinus rhythm without incurring significant adverse effects, rate control is also often chosen for chronic management of atrial fibrillation. This is particularly true for patients who have no symptoms or only minimal symptoms and in whom adequate rate control is easily attained. Indeed, results of large clinical trials suggest that rate control is satisfactory for many patients.
The main purpose of rate control is to control symptoms as opposed to merely lowering the ventricular rate. Effective rate control often prevents hemodynamic instability in patients with underlying heart disease who present acutely with atrial fibrillation. In patients with permanent atrial fibrillation, the RACE II study4 (Rate Control Efficacy in Permanent Atrial Fibrillation: a Comparison between Lenient Versus Strict Rate Control II), during a 3-year follow-up, showed that lenient rate control (resting heart rate < 110 beats per minute) is not inferior to strict rate control (resting heart rate < 80 beats per minute) in preventing major cardiovascular events (heart failure, stroke) or arrhythmic events such as syncope and sustained ventricular tachycardia.4
As a long-term strategy, rate control also prevents tachycardia-induced cardiomyopathy, reduces the risk of worsening of underlying heart failure, and can improve symptoms and quality of life.
Although maintenance of sinus rhythm is most likely associated with a survival benefit, heart rhythm control with antiarrhythmic drugs has not shown an advantage over rate control in overall or cardiovascular death rates, thromboembolic complications, or impact on heart failure. Indeed, a rhythm control strategy has been associated only with better exercise tolerance and, although less clear, with better quality of life.5
One possible explanation as to why a rhythm control strategy has not been shown to be superior to a rate control strategy is the side effects of the presently available drugs for rhythm control.
In a subgroup analysis of the Atrial Fibrillation Follow-up Investigation of Rhythm Management (AFFIRM) trial,6 antiarrhythmic therapy was associated with a 49% increase in the mortality rate that offset the benefits of conversion and maintenance of sinus rhythm, which was associated with a 53% reduction in mortality rates.
The hope is that newer drugs with less toxicity may produce better outcomes for patients treated with rhythm control.
AN ANALOGUE OF AMIODARONE, WITHOUT THE IODINE
Dronedarone is a structurally modified version of amiodarone, the antiarrhythmic drug that has shown the greatest efficacy at maintaining sinus rhythm in patients with paroxysmal atrial fibrillation. Although historically amiodarone has been effective in maintaining sinus rhythm and has been used safely in patients with advanced heart failure, its use has been limited by cumulative and often irreversible extracardiac organ toxicity.
Dronedarone was designed to match amiodarone’s efficacy but with a better safety profile. An iodine radical makes up more than one-third of amiodarone’s molecular weight. The omission of iodine in dronedarone was intended to reduce the likelihood of toxic side effects.
Dronedarone is a benzofuran derivative pharmacologically related to amiodarone, with the addition of a methylsulfonamide group. This reduces lipophilicity and the propensity to cross the blood-brain barrier; over a 2-year period this drug has not been shown to have neurotoxic effects.7
Dronedarone has proved efficacious without toxic or proarrhythmic effects and has minimal side effects, but concerns remain regarding its use in advanced heart failure. To date, its adverse-event profile appears comparable to that of placebo. However, whether its efficacy and incidence of adverse effects are comparable to what has been reported in the literature may take time to assess.
DRONEDARONE’S PHARMACOLOGY
Dronedarone, like amiodarone, blocks multiple sodium and potassium ion channels. It also exerts an antiadrenergic effect by noncompetitive binding to beta-adrenergic receptors as well as by inhibiting an agonist-induced increase in adenylate cyclase activity.8 Compared with amiodarone, dronedarone is a more potent blocker of peak sodium current.
Dronedarone is largely metabolized by the hepatic enzyme cytochrome P450 3A4 isoform (CYP3A4). Only 6% of dronedarone is excreted renally; however, no trial has yet assessed dronedarone’s safety in patients with marked kidney dysfunction.89
Dronedarone’s steady-state terminal elimination half-life is approximately 30 hours. When taken twice a day, it achieves steady-state concentrations in 5 to 7 days.
Dronedarone is available only for oral administration at 400 mg twice daily. Dose adjustment or titration is not recommended.
CLINICAL TRIALS OF DRONEDARONE
Dronedarone vs placebo
ATHENA (A Placebo-Controlled, Double-Blind, Parallel Arm Trial to Assess the Efficacy of Dronedarone 400 mg bid for the Prevention of Cardiovascular Hospitalization or Death From any Cause in Patients With Atrial Fibrillation/Atrial Flutter)10 was a prospective, double-blind study to assess morbidity and death rates in 4,628 patients with atrial fibrillation or atrial flutter and at least one other cardiovascular risk factor.
EURIDIS and ADONIS. Two trials,11 EURIDIS (European Trial in Atrial Fibrillation or Flutter Patients Receiving Dronedarone for the Maintenance of Sinus Rhythm) and ADONIS (American-Australian Trial With Dronedarone in Atrial Fibrillation or Flutter Patients for the Maintenance of Sinus Rhythm), enrolled a total of more than 1,200 patients and showed that dronedarone 400 mg twice a day produced a significantly lower rate of recurrence of atrial fibrillation after electrical cardioversion compared with placebo.
Overall, treatment with dronedarone significantly reduced the risk of a first recurrence of atrial fibrillation by 22% (ADONIS) and 27.5% (EURIDIS) (Table 1).
ERATO (Efficacy and Safety of Dronedarone for the Control of Ventricular Rate During Atrial Fibrillation),12 an additional phase III study, showed that dronedarone controlled the heart rate in patients with persistently accelerated ventricular rates despite concomitant standard therapy with a beta-blocker, digitalis, or a calcium-channel blocker. Dronedarone reduced the mean 24-hour heart rate by 11.7 beats per minute and the maximal exercise ventricular rate by 24.5 beats per minute at the 14th day.
ANDROMEDA (Anti-arrhythmic Trial With Dronedarone in Moderate to Severe CHF Evaluating Morbidity Decrease)13 was a study not of patients with atrial fibrillation but rather of patients with symptomatic congestive heart failure, a left ventricular ejection fraction of 35% or less, and recent hospitalization with new or worsening heart failure. The study was terminated early because of a higher rate of death with dronedarone13 (Table 1).
Dronedarone vs amiodarone
DIONYSOS (Efficacy and Safety of Dronedarone Versus Amiodarone for the Maintenance of Sinus Rhythm in Patients With Atrial Fibrillation)14 was a randomized double-blind trial. It evaluated the efficacy and safety of dronedarone (400 mg twice daily) or amiodarone (600 mg daily for 28 days, then 200 mg daily thereafter) for at least 6 months for the maintenance of sinus rhythm in patients with atrial fibrillation. It enrolled 504 patients with persistent atrial fibrillation; patients had not previously taken amiodarone. Dronedarone was less effective than amiodarone in maintaining sinus rhythm: the rate of recurrent atrial fibrillation was 63% with dronedarone and 42% with amiodarone. But dronedarone was associated with fewer adverse effects and less need for premature discontinuation of drug treatment at a mean follow-up of 7 months (Table 1).
WHERE DOES DRONEDARONE FIT IN ATRIAL FIBRILLATION MANAGEMENT?
Dronedarone is indicated in persistent or paroxysmal atrial fibrillation, based on the observed reduction of the rate of hospitalization. It is indicated for the maintenance of sinus rhythm and may be used in patients with persistent or paroxysmal atrial fibrillation and flutter who are in sinus rhythm or will be undergoing cardioversion soon after starting the drug. Dronedarone has no role in the acute management of atrial fibrillation, such as in cardioversion to sinus rhythm in the emergency department.
We do not have substantial evidence of the efficacy of dronedarone in patients with resistant atrial fibrillation, in whom multiple antiarrhythmics have failed to maintain sinus rhythm, and no published trial has used the inclusion criterion of treatment failure with other antiarrhythmic drugs.
The role of dronedarone in heart failure with preserved systolic function is unclear. Patients taking dronedarone are twice as likely as those taking amiodarone to have a recurrence of atrial fibrillation.
The main advantage of dronedarone is its lower adverse effect profile. However, this statement is based on only a few years of observation. If the patient has developed adverse effects with amiodarone, or if the clinician is concerned about the risk of serious adverse effects, dronedarone presents an alternative for those patients without heart failure or significant left ventricular dysfunction. One such group may be younger patients, because of concerns about the cumulative effects of amiodarone taken over a lifetime.
Dronedarone may represent an acceptable alternative to many of the current antiarrhythmic drugs. Based on the results of the Cardiac Arrhythmia Suppression Trial (CAST),15 class IC antiarrhythmics such as flecainide (Tambocor) are generally avoided in patients with prior myocardial infarction or with known or even suspected coronary artery disease. Similarly, sotalol (Betapace) is generally avoided in patients with marked left ventricular hypertrophy because of adverse effects.16 Dofetilide (Tikosyn) and often sotalol require hospitalization with telemetric monitoring for QTc prolongation and the risk of proarrhythmia with torsades de pointes. Dronedarone, however, generally can be safely started in the outpatient setting.
As when considering prescribing any antiarrhythmic, the clinician must assess the patient’s thromboembolic risk, since this risk persists with a rhythm control strategy.
There is substantial evidence from the ATHENA trial,10 in which 30% of the patients had coronary artery disease, that dronedarone is safe and effective in patients with coronary artery disease. Its use in patients who have undergone coronary artery bypass surgery remains to be defined.
WHEN SHOULD WE SWITCH PATIENTS TO DRONEDARONE?
Preliminary experience suggests that dronedarone, unlike most antiarrhythmic drugs, can be safely started about 48 hours after amiodarone is discontinued. Cumulative toxicity has not been noted with dronedarone. Caution should be exercised when switching if the patient has baseline bradycardia or QT interval prolongation. No algorithm has been developed for switching from other antiarrhythmic drugs to dronedarone.
CONTRAINDICATIONS TO DRONEDARONE
Dronedarone is contraindicated in:
- Patients with New York Heart Association (NYHA) class IV heart failure or NYHA class II or III heart failure with recent decompensation requiring hospitalization or referral to a specialized heart failure clinic
- Patients with second- or third-degree atrioventricular block or sick sinus syndrome (except when used in conjunction with a functioning pacemaker) or bradycardia (a heart rate < 50 beats per minute)
- Patients with a QTc interval of 500 ms or longer
- Patients with severe hepatic impairment
- Women who are pregnant, are attempting to become pregnant, or are breast-feeding
- Patients taking potent CYP3A inhibitors—antifungals like ketoconazole (Nizoral), itraconazole (Sporanox), or voriconazole (VFEND); macrolide antibiotics like telithromycin (Ketek) or clarithromycin (Biaxin); protease inhibitors; or other drugs that prolong the QT interval.
In patients with new or worsening heart failure, one should consider suspending or stopping dronedarone therapy.
DRONEDARONE’S ADVERSE EFFECTS
In trials to date, dronedarone has not shown evidence of proarrhythmia (tachyarrhythmia or bradyarrythmia), torsades de pointes, or amiodarone-like organ toxicity affecting the thyroid or the lungs. Recently, rare cases of severe hepatic injury were associated with dronedarone; therefore, periodic liver function testing is advised for patients taking dronedarone, especially during the first 6 months of therapy.
Dronedarone has been associated with higher rates of diarrhea, nausea, bradycardia, QT interval prolongation, and cutaneous rash compared with placebo. In DAFNE (Dronedarone Atrial Fibrillation Study After Electrical Cardioversion),17 10.8% of patients taking dronedarone had to stop taking it because of adverse events. With 800 mg daily, the discontinuation rate was only 3.9%. The most common cause of drug discontinuation was gastrointestinal effects. Anecdotal reports suggest that the gastrointestinal side effects may be self-limited and may not always require discontinuation of the drug.
Serum creatinine levels increase by about 0.1 mg/dL after the start of treatment. This elevation occurs after 1 to 2 days, reaches a plateau after 7 days, and is reversible. The mechanism is thought to be that dronedarone partially inhibits tubular organic cation transporters, which in turn reduces renal creatinine clearance by about 18%, but with no evidence of an effect on glomerular filtration, renal plasma flow, or electrolyte exchanges.18 A limited increase in serum creatinine is, therefore, expected with dronedarone treatment, but this does not mean there is a decline in renal function.
DRONEDARONE AND POTENTIAL DRUG INTERACTIONS
Warfarin. Dronedarone does not increase the international normalized ratio when used with warfarin (Coumadin).
Verapamil, diltiazem. Dose reduction is required to avoid bradyarrhythmias with co-administration of moderate CYP3A4 inhibitors such as verapamil (Calan, Verelan) and diltiazem (Cardizem).
Simvastatin. Dronedarone increases levels of simvastatin (Zocor), a CYP3A4 substrate, two to four times, thus increasing the risk of statin-induced myopathy.
Digoxin. Dronedarone increases the serum digoxin concentration about 2.5 times, and this necessitates monitoring the digoxin level and possibly reducing the digoxin dose.13
Diuretics. Hypokalemia and hypomagnesemia may occur with concomitant administration of potassium-depleting diuretics. Potassium levels should be maintained in the normal range before and during administration of dronedarone.
Tacrolimus, sirolimus. Dronedarone may increase levels of tacrolimus (Prograf) or sirolimus (Rapamune) in posttransplantation patients. This requires dose monitoring and adjustment in concomitant therapy with these agents.
COST VARIES
The cost of dronedarone varies based on factors that include location. Dronedarone’s retail cost ranges from $3.20 to $4.00 per pill (approximately $7.20 per day). It is not available in generic form. It is presently covered by many health plans as a tier 2 drug, representing a $15 to $40 monthly copay.
MORE DATA NEEDED
Dronedarone represents the first in what may well be a number of new antiarrhythmic drugs for the treatment of patients with paroxysmal atrial fibrillation. Although less efficacious then amiodarone, dronedarone appears to be better tolerated and have less serious side effects. It is contraindicated in patients with severe systolic dysfunction and in those with recent heart failure decompensation. It appears safe in coronary artery disease and marked left ventricular hypertrophy, unlike flecainide, propafenone (Rythmol), and sotalol.
To further understand how dronedarone will fare against other antiarrhythmic drugs, more studies with longer follow-up are needed. These studies need to demonstrate superior tolerability of dronedarone, acceptable quality of life without unacceptable loss of efficacy, or a decrease in morbidity or mortality rates compared with amiodarone.
Dronedarone can be safely started in most patients on an outpatient basis. The risk of proarrhythmia with dronedarone appears to be very low.
- Mathew ST, Patel J, Joseph S. Atrial fibrillation: mechanistic insights and treatment options. Eur J Intern Med 2009; 20:672–681.
- Schmitt J, Ehrlich JR, Hohnloser SH. New antiarrhythmic drugs for the treatment of atrial fibrillation. Herz 2008; 33:562–567.
- Wyse DG, Waldo AL, DiMarco JP, et al; Atrial Fibrillation Follow-up Investigation of Rhythm Management (AFFIRM) Investigators. A comparison of rate control and rhythm control in patients with atrial fibrillation. N Engl J Med 2002; 347:1825–1833.
- Van Gelder IC, Groenveld HF, Crijns HJ, et al; RACE II Investigators. Lenient versus strict rate control in patients with atrial fibrillation. N Engl J Med 2010; 362:1363–1373.
- Singh BN, Singh SN, Reda DJ, et al; Sotalol Amiodarone Atrial Fibrillation Efficacy Trial (SAFE-T) Investigators. Amiodarone versus sotalol for atrial fibrillation. N Engl J Med 2005; 352:1861–1872.
- The AFFIRM Investigators. Relationships between sinus rhythm, treatment, and survival in the Atrial Fibrillation Follow-Up Investigation of Rhythm Management (AFFIRM) study. Circulation 2004; 109:1509–1513.
- Van Beeren HC, Jong WM, Kaptein E, Visser TJ, Bakker O, Wiersinga WM. Dronerarone [sic] acts as a selective inhibitor of 3,5,3′-triiodothyronine binding to thyroid hormone receptor-alpha1: in vitro and in vivo evidence. Endocrinology 2003; 144:552–558.
- Patel C, Yan GX, Kowey PR. Dronedarone. Circulation 2009; 120:636–644.
- Dale KM, White CM. Dronedarone: an amiodarone analog for the treatment of atrial fibrillation and atrial flutter. Ann Pharmacother 2007; 41:599–605.
- Hohnloser SH, Crijns HJ, van Eickels M; ATHENA Investigators. Effect of dronedarone on cardiovascular events in atrial fibrillation. N Engl J Med 2009; 360:668–678.
- Singh BN, Connolly SJ, Crijns HJ, et al; EURIDIS and ADONIS Investigators. Dronedarone for maintenance of sinus rhythm in atrial fibrillation or flutter. N Engl J Med 2007; 357:987–999.
- Davy JM, Herold M, Hoglund CERATO Study Investigators. Dronedarone for the control of ventricular rate in permanent atrial fibrillation: the Efficacy and safety of dRonedArone for the cOntrol of ventricular rate during atrial fibrillation (ERATO) study. Am Heart J 2008; 156:527.e1–e9.
- Køber L, Torp-Pedersen C, McMurray JJ; Dronedarone Study Group. Increased mortality after dronedarone therapy for severe heart failure. N Engl J Med 2008; 358:2678–2687.
- Le Heuzey JY, De Ferrari GM, Radzik D, Santini M, Zhu J, Davy JM. A short-term, randomized, double-blind, parallel-group study to evaluate the efficacy and safety of dronedarone versus amiodarone in patients with persistent atrial fibrillation: the DIONYSOS study. J Cardiovasc Electrophysiol 2010; 21:597–605.
- The Cardiac Arrhythmia Suppression Trial (CAST) Investigators. Preliminary report: effect of encainide and flecainide on mortality in a randomized trial of arrhythmia suppression after myocardial infarction. N Engl J Med 1989; 321:406–412.
- Pratt CM. Clinical implications of the Survival With Oral D-sotalol (SWORD) trial: an investigation of patients with left ventricular dysfunction after myocardial infarction. Card Electrophysiol Rev 1998; 2:28–29.
- Touboul P, Brugada J, Capucci A, Crijns HJ, Edvardsson N, Hohnloser SH. Dronedarone for prevention of atrial fibrillation: a dose-ranging study. Eur Heart J 2003; 24:1481–1487.
- Tschuppert Y, Buclin T, Rothuizen LE, et al. Effect of dronedarone on renal function in healthy subjects. Br J Clin Pharmacol 2007; 64:785–791.
- Mathew ST, Patel J, Joseph S. Atrial fibrillation: mechanistic insights and treatment options. Eur J Intern Med 2009; 20:672–681.
- Schmitt J, Ehrlich JR, Hohnloser SH. New antiarrhythmic drugs for the treatment of atrial fibrillation. Herz 2008; 33:562–567.
- Wyse DG, Waldo AL, DiMarco JP, et al; Atrial Fibrillation Follow-up Investigation of Rhythm Management (AFFIRM) Investigators. A comparison of rate control and rhythm control in patients with atrial fibrillation. N Engl J Med 2002; 347:1825–1833.
- Van Gelder IC, Groenveld HF, Crijns HJ, et al; RACE II Investigators. Lenient versus strict rate control in patients with atrial fibrillation. N Engl J Med 2010; 362:1363–1373.
- Singh BN, Singh SN, Reda DJ, et al; Sotalol Amiodarone Atrial Fibrillation Efficacy Trial (SAFE-T) Investigators. Amiodarone versus sotalol for atrial fibrillation. N Engl J Med 2005; 352:1861–1872.
- The AFFIRM Investigators. Relationships between sinus rhythm, treatment, and survival in the Atrial Fibrillation Follow-Up Investigation of Rhythm Management (AFFIRM) study. Circulation 2004; 109:1509–1513.
- Van Beeren HC, Jong WM, Kaptein E, Visser TJ, Bakker O, Wiersinga WM. Dronerarone [sic] acts as a selective inhibitor of 3,5,3′-triiodothyronine binding to thyroid hormone receptor-alpha1: in vitro and in vivo evidence. Endocrinology 2003; 144:552–558.
- Patel C, Yan GX, Kowey PR. Dronedarone. Circulation 2009; 120:636–644.
- Dale KM, White CM. Dronedarone: an amiodarone analog for the treatment of atrial fibrillation and atrial flutter. Ann Pharmacother 2007; 41:599–605.
- Hohnloser SH, Crijns HJ, van Eickels M; ATHENA Investigators. Effect of dronedarone on cardiovascular events in atrial fibrillation. N Engl J Med 2009; 360:668–678.
- Singh BN, Connolly SJ, Crijns HJ, et al; EURIDIS and ADONIS Investigators. Dronedarone for maintenance of sinus rhythm in atrial fibrillation or flutter. N Engl J Med 2007; 357:987–999.
- Davy JM, Herold M, Hoglund CERATO Study Investigators. Dronedarone for the control of ventricular rate in permanent atrial fibrillation: the Efficacy and safety of dRonedArone for the cOntrol of ventricular rate during atrial fibrillation (ERATO) study. Am Heart J 2008; 156:527.e1–e9.
- Køber L, Torp-Pedersen C, McMurray JJ; Dronedarone Study Group. Increased mortality after dronedarone therapy for severe heart failure. N Engl J Med 2008; 358:2678–2687.
- Le Heuzey JY, De Ferrari GM, Radzik D, Santini M, Zhu J, Davy JM. A short-term, randomized, double-blind, parallel-group study to evaluate the efficacy and safety of dronedarone versus amiodarone in patients with persistent atrial fibrillation: the DIONYSOS study. J Cardiovasc Electrophysiol 2010; 21:597–605.
- The Cardiac Arrhythmia Suppression Trial (CAST) Investigators. Preliminary report: effect of encainide and flecainide on mortality in a randomized trial of arrhythmia suppression after myocardial infarction. N Engl J Med 1989; 321:406–412.
- Pratt CM. Clinical implications of the Survival With Oral D-sotalol (SWORD) trial: an investigation of patients with left ventricular dysfunction after myocardial infarction. Card Electrophysiol Rev 1998; 2:28–29.
- Touboul P, Brugada J, Capucci A, Crijns HJ, Edvardsson N, Hohnloser SH. Dronedarone for prevention of atrial fibrillation: a dose-ranging study. Eur Heart J 2003; 24:1481–1487.
- Tschuppert Y, Buclin T, Rothuizen LE, et al. Effect of dronedarone on renal function in healthy subjects. Br J Clin Pharmacol 2007; 64:785–791.
KEY POINTS
- Patients with persistent or paroxysmal atrial fibrillation are candidates for dronedarone therapy if they are in sinus rhythm or will be cardioverted soon after starting. This drug is not indicated for the acute management of atrial fibrillation, for example, in the emergency department.
- Dronedarone is an option if a patient cannot tolerate amiodarone or has an underlying condition such as pulmonary or thyroid disease that is a contraindication to amiodarone.
- Dronedarone is contraindicated in patients with significant left ventricular dysfunction or heart failure with recent decompensation.
- The ultimate role for dronedarone is yet to be defined. Little evidence exists as to whether it will succeed when other drugs have failed.