User login
Endoscopic ultrasonography (EUS) is a minimally invasive test that provides high-resolution imaging of the pancreas.1,2 As such, it is proving useful.
Accurate diagnosis and timely intervention are essential in managing acute and chronic pancreatitis, which are often encountered in the clinic and the hospital. However, the cause of acute pancreatitis is not always easy to determine. Furthermore, recurrent bouts can progress to chronic pancreatitis if the cause is not identified and eliminated. EUS has been studied extensively in the evaluation of both acute and chronic pancreatitis, as it can identify obstructive and biliary causes of acute pancreatitis and early structural features of chronic pancreatitis.
This article will review the indications and evidence for EUS in the evaluation of acute and chronic pancreatitis.
SPECIALIZED TRAINING REQUIRED
EUS involves passage of a specialized endoscope through the esophagus and stomach and into the duodenum. The scope has a very small ultrasound probe at the tip, allowing detailed imaging of the upper gastrointestinal tract and surrounding organs.
There are two types of EUS endoscope: radial and linear. A radial scope provides a 360° range of view perpendicular to the long axis of the scope. A linear scope provides a 150° view parallel to the long axis of the scope. Many endosonographers favor linear EUS for imaging the pancreas because it permits fine-needle aspiration biopsy of masses, cysts, and lymph nodes.
Specialized training beyond the gastroenterology fellowship is usually required to become proficient in performing EUS, in recognizing the anatomy it reveals, and in performing fine-needle aspiration biopsy.
ENDOSCOPIC ULTRASONOGRAPHY IN ACUTE PANCREATITIS
Finding the cause of acute pancreatitis can be challenging in patients who do not have typical risk factors, eg, those who do not drink substantial amounts of alcohol and in whom transabdominal ultrasonography fails to reveal gallstones.
Several studies have evaluated the role of EUS in recurrent “idiopathic” pancreatitis.3–5 Causes of acute pancreatitis detectable with EUS included gallbladder and bile duct microlithiasis (stones smaller than 3 mm), cysts, intraductal papillary mucinous neoplasms, ampullary neoplasms, pancreas divisum, and pancreatic masses.
Stones, sludge. Transabdominal ultrasonography is often performed in the workup of acute pancreatitis to rule out gallbladder stones and biliary dilation. Unfortunately, it does a poor job of imaging the distal common bile duct, where culprit stones may reside.
EUS provides a high-quality view of the bile duct from the ampulla of Vater to the region of the hepatic hilum and is safer than endoscopic retrograde cholangiopancreatography (ERCP). The available evidence supports the use of EUS as a diagnostic test for bile duct stones.3–7 In fact, using ERCP as the reference standard, EUS has been found to be more sensitive than transabdominal ultrasonography for bile duct stones.4
The yield of EUS for finding biliary sludge and stones may be high in patients with unexplained pancreatitis. EUS detected sludge, microlithiasis, or both in 33 of 35 patients with idiopathic acute pancreatitis who underwent transabdominal ultrasonography with negative results.8 Furthermore, most were symptom-free at an average of 10 months after cholecystectomy, suggesting that microlithiasis was the cause of the “idiopathic” pancreatitis.
EUS can also decrease the number of unnecessary ERCP procedures in patients with suspected biliary pancreatitis. In these patients, EUS can be performed as an initial diagnostic test to exclude retained biliary stones. If a stone is present, the endoscopist can proceed to ERCP for sphincterotomy and stone removal during the same endoscopic session. If EUS is negative, the endoscopy can be concluded without cannulating the bile duct and putting the patient at risk of acute pancreatitis. In one report, this approach eliminated the need for ERCP in five of six patients with suspected biliary pancreatitis.6
Tumors and other causes of bile duct obstruction can also cause recurrent acute pancreatitis and may be difficult to detect with cross-sectional imaging. EUS, on the other hand, can detect small pancreatic masses (< 2 cm), which may be missed by conventional computed tomography. Also, a linear EUS scope, with its forward oblique view, can image the duodenum and ampulla, where obstructing inflammation, tumors, and polyps may be found. One should strongly suspect occult malignancy in elderly patients with unexplained acute pancreatitis. In those patients, repeat imaging with high-resolution dual-phase computed tomography or with EUS should be considered after a few weeks once the acute inflammation resolves.
Pancreas divisum is a relatively common congenital abnormality in which the dorsal and ventral pancreatic ducts do not properly fuse during embryonic development. To rule out pancreas divisum, the endosonographer must carefully trace the pancreatic duct from the dorsal pancreas into the ventral pancreas, where it connects with the bile duct at the duodenal wall.
In summary, EUS appears to be safe and accurate for diagnosing bile duct stones and other structural causes of idiopathic acute pancreatitis.
ENDOSCOPIC ULTRASONOGRAPHY IN CHRONIC PANCREATITIS
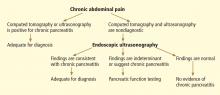
Chronic pancreatitis, a relatively common and sometimes debilitating cause of chronic upper abdominal pain, may be difficult to diagnose using noninvasive imaging tests. Minimal-change chronic pancreatitis is defined as a syndrome of pancreatic abdominal pain with no or slight structural changes detected on imaging but with histologic inflammation and fibrosis diagnostic of chronic pancreatitis.9
A clinical rationale for trying to detect chronic pancreatitis early in its course is that interventions can be started earlier. These include abstinence from alcohol, giving exogenous pancreatic enzymes, and advanced interventions such as celiac plexus blocks for pain control. Some patients may even benefit from resection of the pancreas if pain is severe and resistant to conservative measures.
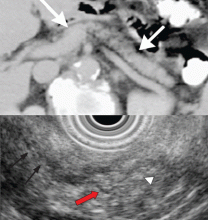
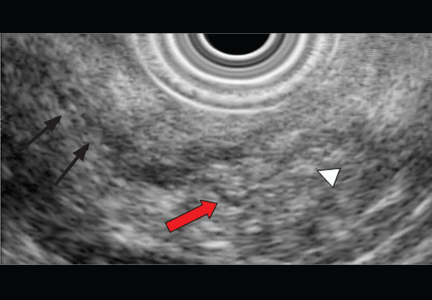
EUS can detect both parenchymal and ductal changes that correlate with histologic fibrosis.10 Parenchymal changes include hyperechoic foci, hyperechoic strands, lobularity, cysts, and shadowing calcifications. Ductal changes include dilation of the main pancreatic duct, irregularity, hyperechoic duct margins, and visible side branches.
Several studies have evaluated the ability of EUS to diagnose early chronic pancreatitis.9,11–15 Reference standards used to determine the accuracy of EUS have included histology,10,16–18 pancreatic function testing,19–22 and ERCP.11,15,23,24
The best diagnostic test may be pancreatic histology. However, biopsy of the pancreas is impractical and exposes patients to high risk. In addition, the patchy and focal distribution of histologic changes may decrease its reliability. Fortunately, the histologic findings of fibrosis have been shown to correlate with EUS criteria in patients undergoing EUS before surgical resection in three recent studies.16–18 A threshold of four or more criteria out of a possible nine was found to provide the optimal sensitivity and specificity for histologic pancreatic fibrosis.16,17 The criteria used were four parenchymal features (hyperechoic foci, strands, hypoechoic lobules, cysts) and five ductal features (irregularity of the main pancreatic duct, dilation, hyperechoic duct walls, visible side branches, and calcifications or stones).
EUS is sensitive for chronic pancreatitis, but ‘true’ accuracy is impossible to know
Unfortunately, greater sensitivity may come at the expense of worse specificity. Certain demographic variables may alter the EUS appearance of the pancreas. A multivariate analysis25 found several variables that predicted abnormalities on EUS even in the absence of clinically evident pancreatitis; the strongest were heavy ethanol use (odds ratio [OR] 5.1, 95% confidence interval [CI] 3.1–8.5), male sex (OR 1.8, 95% CI 1.3–2.55), clinical suspicion of pancreatic disease (OR 1.7, 95% CI 1.2–2.3), and heavy smoking (OR 1.7, 95% CI 1.2–2.4). More prospective studies are needed to further differentiate true disease from false-positive findings of chronic pancreatitis.
Also, traditional EUS scoring symptoms have counted features in an unweighted fashion and assigned an arbitrary cut point (eg, four or more features) for diagnosis. This approach fails to account for the greater importance of some features (eg, calcifications) compared with others.
Interobserver variability is another important limitation of EUS in diagnosing chronic pancreatitis.26,27 In one multicenter study of EUS interpretation, the overall kappa (agreement beyond chance) was only 0.45 for overall chronic pancreatitis diagnosis and worse for many individual criteria for chronic pancreatitis. The endosonographers disagreed most about hyperechoic strands and foci, main pancreatic duct irregularity, and visible side branches (kappa < 0.4).
The Rosemont classification
These limitations led a group of experts to meet in Chicago, IL, to develop a consensus-based and weighted EUS scoring system for the diagnosis of chronic pancreatitis, termed the Rosemont classification.
In this system, the previous parenchymal and ductal features are assigned stricter definitions and reclassified as major and minor criteria. Based on the presence of major and minor features, EUS results are stratified as “normal,” “indeterminate for chronic pancreatitis,” “suggestive of chronic pancreatitis,” or “most consistent with chronic pancreatitis.”15,28
Further validation of this scoring system is needed before it can be used widely.
ENDOSCOPIC ULTRASONOGRAPHY PLUS PANCREATIC FUNCTION TESTING
The best way to diagnose minimal-change chronic pancreatitis may be a combination of sensitive structural and functional testing. Although clinically apparent steatorrhea typically occurs late in the course of chronic pancreatitis, mild exocrine insufficiency may occur early and is detectable with hormone-stimulated pancreatic function testing. Therefore, pancreatic function tests are considered sensitive for diagnosing chronic pancreatitis.20,21,29
Endoscopic pancreatic function testing involves injecting secretin intravenously and then collecting duodenal aspirates through the endoscope. The duodenal fluid is analyzed for bicarbonate concentration as a measure of exocrine function.29
We have studied combined EUS and endoscopic pancreatic function testing in the diagnosis of chronic pancreatitis.16 The combination gives a simultaneous structural and functional assessment of the pancreas and may optimize sensitivity for detecting minimal-change chronic pancreatitis. In a small study, we found the combination had 100% sensitivity for noncalcific chronic pancreatitis compared with a histologic reference standard.16
- Sivak MV, Kaufman A. Endoscopic ultrasonography in the differential diagnosis of pancreatic disease. A preliminary report. Scand J Gastroenterol Suppl 1986; 123:130–134.
- Hisanaga K, Hisanaga A, Nagata K, Ichie Y. High speed rotating scanner for transgastric sonography. AJR Am J Roentgenol 1980; 135:627–629.
- Frossard JL, Sosa-Valencia L, Amouyal G, Marty O, Hadengue A, Amouyal P. Usefulness of endoscopic ultrasonography in patients with “idiopathic” acute pancreatitis. Am J Med 2000; 109:196–200.
- Sugiyama M, Wada N, Atomi Y, Kuroda A, Muto T. Diagnosis of acute pancreatitis: value of endoscopic sonography. AJR Am J Roentgenol 1995; 165:867–872.
- Tandon M, Topazian M. Endoscopic ultrasound in idiopathic acute pancreatitis. Am J Gastroenterol 2001; 96:705–709.
- Kotwal V, Talukdar R, Levy M, Vege SS. Role of endoscopic ultrasound during hospitalization for acute pancreatitis. World J Gastroenterol 2010; 16:4888–4891.
- Liu CL, Lo CM, Chan JK, et al. Detection of choledocholithiasis by EUS in acute pancreatitis: a prospective evaluation in 100 consecutive patients. Gastrointest Endosc 2001; 54:325–330.
- Mirbagheri SA, Mohamadnejad M, Nasiri J, Vahid AA, Ghadimi R, Malekzadeh R. Prospective evaluation of endoscopic ultrasonography in the diagnosis of biliary microlithiasis in patients with normal transabdominal ultrasonography. J Gastrointest Surg 2005; 9:961–964.
- Walsh TN, Rode J, Theis BA, Russell RC. Minimal change chronic pancreatitis. Gut 1992; 33:1566–1571.
- Bhutani MJ, Arantes VN, Verma D, et al. Histopathologic correlation of endoscopic ultrasound findings of chronic pancreatitis in human autopsies. Pancreas 2009; 38:820–824.
- Wiersema MJ, Hawes RH, Lehman GA, Kochman ML, Sherman S, Kopecky KK. Prospective evaluation of endoscopic ultrasonography and endoscopic retrograde cholangiopancreatography in patients with chronic abdominal pain of suspected pancreatic origin. Endoscopy 1993; 25:555–564.
- Kahl S, Glasbrenner B, Leodolter A, Pross M, Schulz HU, Malfertheiner P. EUS in the diagnosis of early chronic pancreatitis: a prospective follow-up study. Gastrointest Endosc 2002; 55:507–511.
- Jones SN, Lees WR, Frost RA. Diagnosis and grading of chronic pancreatitis by morphological criteria derived by ultrasound and pancreatography. Clin Radiol 1988; 39:43–48.
- Lees WR. Endoscopic ultrasonography of chronic pancreatitis and pancreatic pseudocysts. Scand J Gastroenterol Suppl 1986; 123:123–129.
- Sahai AV, Zimmerman M, Aabakken L, et al. Prospective assessment of the ability of endoscopic ultrasound to diagnose, exclude, or establish the severity of chronic pancreatitis found by endoscopic retrograde cholangiopancreatography. Gastrointest Endosc 1998; 48:18–25.
- Albashir S, Bronner MP, Parsi MA, Walsh RM, Stevens T. Endoscopic ultrasound, secretin endoscopic pancreatic function test, and histology: correlation in chronic pancreatitis. Am J Gastroenterol 2010; 105:2498–2503.
- Varadarajulu S, Eltoum I, Tamhane A, Eloubeidi MA. Histopathologic correlates of noncalcific chronic pancreatitis by EUS: a prospective tissue characterization study. Gastrointest Endosc 2007; 66:501–509.
- Chong AK, Hawes RH, Hoffman BJ, Adams DB, Lewin DN, Romagnuolo J. Diagnostic performance of EUS for chronic pancreatitis: a comparison with histopathology. Gastrointest Endosc 2007; 65:808–814.
- Chowdhury R, Bhutani MS, Mishra G, Toskes PP, Forsmark CE. Comparative analysis of direct pancreatic function testing versus morphological assessment by endoscopic ultrasonography for the evaluation of chronic unexplained abdominal pain of presumed pancreatic origin. Pancreas 2005; 31:63–68.
- Conwell DL, Zuccaro G, Purich E, et al. Comparison of endoscopic ultrasound chronic pancreatitis criteria to the endoscopic secretinstimulated pancreatic function test. Dig Dis Sci 2007; 52:1206–1210.
- Stevens T, Conwell DL, Zuccaro G, Vargo JJ, Dumot JA, Lopez R. Comparison of endoscopic ultrasound and endoscopic retrograde pancreatography for the prediction of pancreatic exocrine insufficiency. Dig Dis Sci 2008; 53:1146–1151.
- Stevens T, Dumot JA, Parsi MA, Zuccaro G, Vargo JJ. Combined endoscopic ultrasound and secretin endoscopic pancreatic function test in patients evaluated for chronic pancreatitis. Dig Dis Sci 2010; 55:2681–2687.
- Catalano MF, Lahoti S, Geenen JE, Hogan WJ. Prospective evaluation of endoscopic ultrasonography, endoscopic retrograde pancreatography, and secretin test in the diagnosis of chronic pancreatitis. Gastrointest Endosc 1998; 48:11–17.
- Irisawa A, Katakura K, Ohira H, et al. Usefulness of endoscopic ultrasound to diagnose the severity of chronic pancreatitis. J Gastroenterol 2007; 42(suppl 17):90–94.
- Yusoff IF, Sahai AV. A prospective, quantitative assessment of the effect of ethanol and other variables on the endosonographic appearance of the pancreas. Clin Gastroenterol Hepatol 2004; 2:405–409.
- Stevens T, Lopez R, Adler DG, et al. Multicenter comparison of the interobserver agreement of standard EUS scoring and Rosemont classification scoring for diagnosis of chronic pancreatitis. Gastrointest Endosc 2010; 71:519–526.
- Wallace MB, Hawes RH, Durkalski V, et al. The reliability of EUS for the diagnosis of chronic pancreatitis: interobserver agreement among experienced endosonographers. Gastrointest Endosc 2001; 53:294–299.
- Catalano MF, Sahai A, Levy M, et al. EUS-based criteria for the diagnosis of chronic pancreatitis: the Rosemont classification. Gastrointest Endosc 2009; 69:1251–1261.
- Stevens T, Conwell DL, Zuccaro G, et al. A prospective crossover study comparing secretin-stimulated endoscopic and Dreiling tube pancreatic function testing in patients evaluated for chronic pancreatitis. Gastrointest Endosc 2008; 67:458–466.
Endoscopic ultrasonography (EUS) is a minimally invasive test that provides high-resolution imaging of the pancreas.1,2 As such, it is proving useful.
Accurate diagnosis and timely intervention are essential in managing acute and chronic pancreatitis, which are often encountered in the clinic and the hospital. However, the cause of acute pancreatitis is not always easy to determine. Furthermore, recurrent bouts can progress to chronic pancreatitis if the cause is not identified and eliminated. EUS has been studied extensively in the evaluation of both acute and chronic pancreatitis, as it can identify obstructive and biliary causes of acute pancreatitis and early structural features of chronic pancreatitis.
This article will review the indications and evidence for EUS in the evaluation of acute and chronic pancreatitis.
SPECIALIZED TRAINING REQUIRED
EUS involves passage of a specialized endoscope through the esophagus and stomach and into the duodenum. The scope has a very small ultrasound probe at the tip, allowing detailed imaging of the upper gastrointestinal tract and surrounding organs.
There are two types of EUS endoscope: radial and linear. A radial scope provides a 360° range of view perpendicular to the long axis of the scope. A linear scope provides a 150° view parallel to the long axis of the scope. Many endosonographers favor linear EUS for imaging the pancreas because it permits fine-needle aspiration biopsy of masses, cysts, and lymph nodes.
Specialized training beyond the gastroenterology fellowship is usually required to become proficient in performing EUS, in recognizing the anatomy it reveals, and in performing fine-needle aspiration biopsy.
ENDOSCOPIC ULTRASONOGRAPHY IN ACUTE PANCREATITIS
Finding the cause of acute pancreatitis can be challenging in patients who do not have typical risk factors, eg, those who do not drink substantial amounts of alcohol and in whom transabdominal ultrasonography fails to reveal gallstones.
Several studies have evaluated the role of EUS in recurrent “idiopathic” pancreatitis.3–5 Causes of acute pancreatitis detectable with EUS included gallbladder and bile duct microlithiasis (stones smaller than 3 mm), cysts, intraductal papillary mucinous neoplasms, ampullary neoplasms, pancreas divisum, and pancreatic masses.
Stones, sludge. Transabdominal ultrasonography is often performed in the workup of acute pancreatitis to rule out gallbladder stones and biliary dilation. Unfortunately, it does a poor job of imaging the distal common bile duct, where culprit stones may reside.
EUS provides a high-quality view of the bile duct from the ampulla of Vater to the region of the hepatic hilum and is safer than endoscopic retrograde cholangiopancreatography (ERCP). The available evidence supports the use of EUS as a diagnostic test for bile duct stones.3–7 In fact, using ERCP as the reference standard, EUS has been found to be more sensitive than transabdominal ultrasonography for bile duct stones.4
The yield of EUS for finding biliary sludge and stones may be high in patients with unexplained pancreatitis. EUS detected sludge, microlithiasis, or both in 33 of 35 patients with idiopathic acute pancreatitis who underwent transabdominal ultrasonography with negative results.8 Furthermore, most were symptom-free at an average of 10 months after cholecystectomy, suggesting that microlithiasis was the cause of the “idiopathic” pancreatitis.
EUS can also decrease the number of unnecessary ERCP procedures in patients with suspected biliary pancreatitis. In these patients, EUS can be performed as an initial diagnostic test to exclude retained biliary stones. If a stone is present, the endoscopist can proceed to ERCP for sphincterotomy and stone removal during the same endoscopic session. If EUS is negative, the endoscopy can be concluded without cannulating the bile duct and putting the patient at risk of acute pancreatitis. In one report, this approach eliminated the need for ERCP in five of six patients with suspected biliary pancreatitis.6
Tumors and other causes of bile duct obstruction can also cause recurrent acute pancreatitis and may be difficult to detect with cross-sectional imaging. EUS, on the other hand, can detect small pancreatic masses (< 2 cm), which may be missed by conventional computed tomography. Also, a linear EUS scope, with its forward oblique view, can image the duodenum and ampulla, where obstructing inflammation, tumors, and polyps may be found. One should strongly suspect occult malignancy in elderly patients with unexplained acute pancreatitis. In those patients, repeat imaging with high-resolution dual-phase computed tomography or with EUS should be considered after a few weeks once the acute inflammation resolves.
Pancreas divisum is a relatively common congenital abnormality in which the dorsal and ventral pancreatic ducts do not properly fuse during embryonic development. To rule out pancreas divisum, the endosonographer must carefully trace the pancreatic duct from the dorsal pancreas into the ventral pancreas, where it connects with the bile duct at the duodenal wall.
In summary, EUS appears to be safe and accurate for diagnosing bile duct stones and other structural causes of idiopathic acute pancreatitis.
ENDOSCOPIC ULTRASONOGRAPHY IN CHRONIC PANCREATITIS
Chronic pancreatitis, a relatively common and sometimes debilitating cause of chronic upper abdominal pain, may be difficult to diagnose using noninvasive imaging tests. Minimal-change chronic pancreatitis is defined as a syndrome of pancreatic abdominal pain with no or slight structural changes detected on imaging but with histologic inflammation and fibrosis diagnostic of chronic pancreatitis.9
A clinical rationale for trying to detect chronic pancreatitis early in its course is that interventions can be started earlier. These include abstinence from alcohol, giving exogenous pancreatic enzymes, and advanced interventions such as celiac plexus blocks for pain control. Some patients may even benefit from resection of the pancreas if pain is severe and resistant to conservative measures.
EUS can detect both parenchymal and ductal changes that correlate with histologic fibrosis.10 Parenchymal changes include hyperechoic foci, hyperechoic strands, lobularity, cysts, and shadowing calcifications. Ductal changes include dilation of the main pancreatic duct, irregularity, hyperechoic duct margins, and visible side branches.
Several studies have evaluated the ability of EUS to diagnose early chronic pancreatitis.9,11–15 Reference standards used to determine the accuracy of EUS have included histology,10,16–18 pancreatic function testing,19–22 and ERCP.11,15,23,24
The best diagnostic test may be pancreatic histology. However, biopsy of the pancreas is impractical and exposes patients to high risk. In addition, the patchy and focal distribution of histologic changes may decrease its reliability. Fortunately, the histologic findings of fibrosis have been shown to correlate with EUS criteria in patients undergoing EUS before surgical resection in three recent studies.16–18 A threshold of four or more criteria out of a possible nine was found to provide the optimal sensitivity and specificity for histologic pancreatic fibrosis.16,17 The criteria used were four parenchymal features (hyperechoic foci, strands, hypoechoic lobules, cysts) and five ductal features (irregularity of the main pancreatic duct, dilation, hyperechoic duct walls, visible side branches, and calcifications or stones).
EUS is sensitive for chronic pancreatitis, but ‘true’ accuracy is impossible to know
Unfortunately, greater sensitivity may come at the expense of worse specificity. Certain demographic variables may alter the EUS appearance of the pancreas. A multivariate analysis25 found several variables that predicted abnormalities on EUS even in the absence of clinically evident pancreatitis; the strongest were heavy ethanol use (odds ratio [OR] 5.1, 95% confidence interval [CI] 3.1–8.5), male sex (OR 1.8, 95% CI 1.3–2.55), clinical suspicion of pancreatic disease (OR 1.7, 95% CI 1.2–2.3), and heavy smoking (OR 1.7, 95% CI 1.2–2.4). More prospective studies are needed to further differentiate true disease from false-positive findings of chronic pancreatitis.
Also, traditional EUS scoring symptoms have counted features in an unweighted fashion and assigned an arbitrary cut point (eg, four or more features) for diagnosis. This approach fails to account for the greater importance of some features (eg, calcifications) compared with others.
Interobserver variability is another important limitation of EUS in diagnosing chronic pancreatitis.26,27 In one multicenter study of EUS interpretation, the overall kappa (agreement beyond chance) was only 0.45 for overall chronic pancreatitis diagnosis and worse for many individual criteria for chronic pancreatitis. The endosonographers disagreed most about hyperechoic strands and foci, main pancreatic duct irregularity, and visible side branches (kappa < 0.4).
The Rosemont classification
These limitations led a group of experts to meet in Chicago, IL, to develop a consensus-based and weighted EUS scoring system for the diagnosis of chronic pancreatitis, termed the Rosemont classification.
In this system, the previous parenchymal and ductal features are assigned stricter definitions and reclassified as major and minor criteria. Based on the presence of major and minor features, EUS results are stratified as “normal,” “indeterminate for chronic pancreatitis,” “suggestive of chronic pancreatitis,” or “most consistent with chronic pancreatitis.”15,28
Further validation of this scoring system is needed before it can be used widely.
ENDOSCOPIC ULTRASONOGRAPHY PLUS PANCREATIC FUNCTION TESTING
The best way to diagnose minimal-change chronic pancreatitis may be a combination of sensitive structural and functional testing. Although clinically apparent steatorrhea typically occurs late in the course of chronic pancreatitis, mild exocrine insufficiency may occur early and is detectable with hormone-stimulated pancreatic function testing. Therefore, pancreatic function tests are considered sensitive for diagnosing chronic pancreatitis.20,21,29
Endoscopic pancreatic function testing involves injecting secretin intravenously and then collecting duodenal aspirates through the endoscope. The duodenal fluid is analyzed for bicarbonate concentration as a measure of exocrine function.29
We have studied combined EUS and endoscopic pancreatic function testing in the diagnosis of chronic pancreatitis.16 The combination gives a simultaneous structural and functional assessment of the pancreas and may optimize sensitivity for detecting minimal-change chronic pancreatitis. In a small study, we found the combination had 100% sensitivity for noncalcific chronic pancreatitis compared with a histologic reference standard.16
Endoscopic ultrasonography (EUS) is a minimally invasive test that provides high-resolution imaging of the pancreas.1,2 As such, it is proving useful.
Accurate diagnosis and timely intervention are essential in managing acute and chronic pancreatitis, which are often encountered in the clinic and the hospital. However, the cause of acute pancreatitis is not always easy to determine. Furthermore, recurrent bouts can progress to chronic pancreatitis if the cause is not identified and eliminated. EUS has been studied extensively in the evaluation of both acute and chronic pancreatitis, as it can identify obstructive and biliary causes of acute pancreatitis and early structural features of chronic pancreatitis.
This article will review the indications and evidence for EUS in the evaluation of acute and chronic pancreatitis.
SPECIALIZED TRAINING REQUIRED
EUS involves passage of a specialized endoscope through the esophagus and stomach and into the duodenum. The scope has a very small ultrasound probe at the tip, allowing detailed imaging of the upper gastrointestinal tract and surrounding organs.
There are two types of EUS endoscope: radial and linear. A radial scope provides a 360° range of view perpendicular to the long axis of the scope. A linear scope provides a 150° view parallel to the long axis of the scope. Many endosonographers favor linear EUS for imaging the pancreas because it permits fine-needle aspiration biopsy of masses, cysts, and lymph nodes.
Specialized training beyond the gastroenterology fellowship is usually required to become proficient in performing EUS, in recognizing the anatomy it reveals, and in performing fine-needle aspiration biopsy.
ENDOSCOPIC ULTRASONOGRAPHY IN ACUTE PANCREATITIS
Finding the cause of acute pancreatitis can be challenging in patients who do not have typical risk factors, eg, those who do not drink substantial amounts of alcohol and in whom transabdominal ultrasonography fails to reveal gallstones.
Several studies have evaluated the role of EUS in recurrent “idiopathic” pancreatitis.3–5 Causes of acute pancreatitis detectable with EUS included gallbladder and bile duct microlithiasis (stones smaller than 3 mm), cysts, intraductal papillary mucinous neoplasms, ampullary neoplasms, pancreas divisum, and pancreatic masses.
Stones, sludge. Transabdominal ultrasonography is often performed in the workup of acute pancreatitis to rule out gallbladder stones and biliary dilation. Unfortunately, it does a poor job of imaging the distal common bile duct, where culprit stones may reside.
EUS provides a high-quality view of the bile duct from the ampulla of Vater to the region of the hepatic hilum and is safer than endoscopic retrograde cholangiopancreatography (ERCP). The available evidence supports the use of EUS as a diagnostic test for bile duct stones.3–7 In fact, using ERCP as the reference standard, EUS has been found to be more sensitive than transabdominal ultrasonography for bile duct stones.4
The yield of EUS for finding biliary sludge and stones may be high in patients with unexplained pancreatitis. EUS detected sludge, microlithiasis, or both in 33 of 35 patients with idiopathic acute pancreatitis who underwent transabdominal ultrasonography with negative results.8 Furthermore, most were symptom-free at an average of 10 months after cholecystectomy, suggesting that microlithiasis was the cause of the “idiopathic” pancreatitis.
EUS can also decrease the number of unnecessary ERCP procedures in patients with suspected biliary pancreatitis. In these patients, EUS can be performed as an initial diagnostic test to exclude retained biliary stones. If a stone is present, the endoscopist can proceed to ERCP for sphincterotomy and stone removal during the same endoscopic session. If EUS is negative, the endoscopy can be concluded without cannulating the bile duct and putting the patient at risk of acute pancreatitis. In one report, this approach eliminated the need for ERCP in five of six patients with suspected biliary pancreatitis.6
Tumors and other causes of bile duct obstruction can also cause recurrent acute pancreatitis and may be difficult to detect with cross-sectional imaging. EUS, on the other hand, can detect small pancreatic masses (< 2 cm), which may be missed by conventional computed tomography. Also, a linear EUS scope, with its forward oblique view, can image the duodenum and ampulla, where obstructing inflammation, tumors, and polyps may be found. One should strongly suspect occult malignancy in elderly patients with unexplained acute pancreatitis. In those patients, repeat imaging with high-resolution dual-phase computed tomography or with EUS should be considered after a few weeks once the acute inflammation resolves.
Pancreas divisum is a relatively common congenital abnormality in which the dorsal and ventral pancreatic ducts do not properly fuse during embryonic development. To rule out pancreas divisum, the endosonographer must carefully trace the pancreatic duct from the dorsal pancreas into the ventral pancreas, where it connects with the bile duct at the duodenal wall.
In summary, EUS appears to be safe and accurate for diagnosing bile duct stones and other structural causes of idiopathic acute pancreatitis.
ENDOSCOPIC ULTRASONOGRAPHY IN CHRONIC PANCREATITIS
Chronic pancreatitis, a relatively common and sometimes debilitating cause of chronic upper abdominal pain, may be difficult to diagnose using noninvasive imaging tests. Minimal-change chronic pancreatitis is defined as a syndrome of pancreatic abdominal pain with no or slight structural changes detected on imaging but with histologic inflammation and fibrosis diagnostic of chronic pancreatitis.9
A clinical rationale for trying to detect chronic pancreatitis early in its course is that interventions can be started earlier. These include abstinence from alcohol, giving exogenous pancreatic enzymes, and advanced interventions such as celiac plexus blocks for pain control. Some patients may even benefit from resection of the pancreas if pain is severe and resistant to conservative measures.
EUS can detect both parenchymal and ductal changes that correlate with histologic fibrosis.10 Parenchymal changes include hyperechoic foci, hyperechoic strands, lobularity, cysts, and shadowing calcifications. Ductal changes include dilation of the main pancreatic duct, irregularity, hyperechoic duct margins, and visible side branches.
Several studies have evaluated the ability of EUS to diagnose early chronic pancreatitis.9,11–15 Reference standards used to determine the accuracy of EUS have included histology,10,16–18 pancreatic function testing,19–22 and ERCP.11,15,23,24
The best diagnostic test may be pancreatic histology. However, biopsy of the pancreas is impractical and exposes patients to high risk. In addition, the patchy and focal distribution of histologic changes may decrease its reliability. Fortunately, the histologic findings of fibrosis have been shown to correlate with EUS criteria in patients undergoing EUS before surgical resection in three recent studies.16–18 A threshold of four or more criteria out of a possible nine was found to provide the optimal sensitivity and specificity for histologic pancreatic fibrosis.16,17 The criteria used were four parenchymal features (hyperechoic foci, strands, hypoechoic lobules, cysts) and five ductal features (irregularity of the main pancreatic duct, dilation, hyperechoic duct walls, visible side branches, and calcifications or stones).
EUS is sensitive for chronic pancreatitis, but ‘true’ accuracy is impossible to know
Unfortunately, greater sensitivity may come at the expense of worse specificity. Certain demographic variables may alter the EUS appearance of the pancreas. A multivariate analysis25 found several variables that predicted abnormalities on EUS even in the absence of clinically evident pancreatitis; the strongest were heavy ethanol use (odds ratio [OR] 5.1, 95% confidence interval [CI] 3.1–8.5), male sex (OR 1.8, 95% CI 1.3–2.55), clinical suspicion of pancreatic disease (OR 1.7, 95% CI 1.2–2.3), and heavy smoking (OR 1.7, 95% CI 1.2–2.4). More prospective studies are needed to further differentiate true disease from false-positive findings of chronic pancreatitis.
Also, traditional EUS scoring symptoms have counted features in an unweighted fashion and assigned an arbitrary cut point (eg, four or more features) for diagnosis. This approach fails to account for the greater importance of some features (eg, calcifications) compared with others.
Interobserver variability is another important limitation of EUS in diagnosing chronic pancreatitis.26,27 In one multicenter study of EUS interpretation, the overall kappa (agreement beyond chance) was only 0.45 for overall chronic pancreatitis diagnosis and worse for many individual criteria for chronic pancreatitis. The endosonographers disagreed most about hyperechoic strands and foci, main pancreatic duct irregularity, and visible side branches (kappa < 0.4).
The Rosemont classification
These limitations led a group of experts to meet in Chicago, IL, to develop a consensus-based and weighted EUS scoring system for the diagnosis of chronic pancreatitis, termed the Rosemont classification.
In this system, the previous parenchymal and ductal features are assigned stricter definitions and reclassified as major and minor criteria. Based on the presence of major and minor features, EUS results are stratified as “normal,” “indeterminate for chronic pancreatitis,” “suggestive of chronic pancreatitis,” or “most consistent with chronic pancreatitis.”15,28
Further validation of this scoring system is needed before it can be used widely.
ENDOSCOPIC ULTRASONOGRAPHY PLUS PANCREATIC FUNCTION TESTING
The best way to diagnose minimal-change chronic pancreatitis may be a combination of sensitive structural and functional testing. Although clinically apparent steatorrhea typically occurs late in the course of chronic pancreatitis, mild exocrine insufficiency may occur early and is detectable with hormone-stimulated pancreatic function testing. Therefore, pancreatic function tests are considered sensitive for diagnosing chronic pancreatitis.20,21,29
Endoscopic pancreatic function testing involves injecting secretin intravenously and then collecting duodenal aspirates through the endoscope. The duodenal fluid is analyzed for bicarbonate concentration as a measure of exocrine function.29
We have studied combined EUS and endoscopic pancreatic function testing in the diagnosis of chronic pancreatitis.16 The combination gives a simultaneous structural and functional assessment of the pancreas and may optimize sensitivity for detecting minimal-change chronic pancreatitis. In a small study, we found the combination had 100% sensitivity for noncalcific chronic pancreatitis compared with a histologic reference standard.16
- Sivak MV, Kaufman A. Endoscopic ultrasonography in the differential diagnosis of pancreatic disease. A preliminary report. Scand J Gastroenterol Suppl 1986; 123:130–134.
- Hisanaga K, Hisanaga A, Nagata K, Ichie Y. High speed rotating scanner for transgastric sonography. AJR Am J Roentgenol 1980; 135:627–629.
- Frossard JL, Sosa-Valencia L, Amouyal G, Marty O, Hadengue A, Amouyal P. Usefulness of endoscopic ultrasonography in patients with “idiopathic” acute pancreatitis. Am J Med 2000; 109:196–200.
- Sugiyama M, Wada N, Atomi Y, Kuroda A, Muto T. Diagnosis of acute pancreatitis: value of endoscopic sonography. AJR Am J Roentgenol 1995; 165:867–872.
- Tandon M, Topazian M. Endoscopic ultrasound in idiopathic acute pancreatitis. Am J Gastroenterol 2001; 96:705–709.
- Kotwal V, Talukdar R, Levy M, Vege SS. Role of endoscopic ultrasound during hospitalization for acute pancreatitis. World J Gastroenterol 2010; 16:4888–4891.
- Liu CL, Lo CM, Chan JK, et al. Detection of choledocholithiasis by EUS in acute pancreatitis: a prospective evaluation in 100 consecutive patients. Gastrointest Endosc 2001; 54:325–330.
- Mirbagheri SA, Mohamadnejad M, Nasiri J, Vahid AA, Ghadimi R, Malekzadeh R. Prospective evaluation of endoscopic ultrasonography in the diagnosis of biliary microlithiasis in patients with normal transabdominal ultrasonography. J Gastrointest Surg 2005; 9:961–964.
- Walsh TN, Rode J, Theis BA, Russell RC. Minimal change chronic pancreatitis. Gut 1992; 33:1566–1571.
- Bhutani MJ, Arantes VN, Verma D, et al. Histopathologic correlation of endoscopic ultrasound findings of chronic pancreatitis in human autopsies. Pancreas 2009; 38:820–824.
- Wiersema MJ, Hawes RH, Lehman GA, Kochman ML, Sherman S, Kopecky KK. Prospective evaluation of endoscopic ultrasonography and endoscopic retrograde cholangiopancreatography in patients with chronic abdominal pain of suspected pancreatic origin. Endoscopy 1993; 25:555–564.
- Kahl S, Glasbrenner B, Leodolter A, Pross M, Schulz HU, Malfertheiner P. EUS in the diagnosis of early chronic pancreatitis: a prospective follow-up study. Gastrointest Endosc 2002; 55:507–511.
- Jones SN, Lees WR, Frost RA. Diagnosis and grading of chronic pancreatitis by morphological criteria derived by ultrasound and pancreatography. Clin Radiol 1988; 39:43–48.
- Lees WR. Endoscopic ultrasonography of chronic pancreatitis and pancreatic pseudocysts. Scand J Gastroenterol Suppl 1986; 123:123–129.
- Sahai AV, Zimmerman M, Aabakken L, et al. Prospective assessment of the ability of endoscopic ultrasound to diagnose, exclude, or establish the severity of chronic pancreatitis found by endoscopic retrograde cholangiopancreatography. Gastrointest Endosc 1998; 48:18–25.
- Albashir S, Bronner MP, Parsi MA, Walsh RM, Stevens T. Endoscopic ultrasound, secretin endoscopic pancreatic function test, and histology: correlation in chronic pancreatitis. Am J Gastroenterol 2010; 105:2498–2503.
- Varadarajulu S, Eltoum I, Tamhane A, Eloubeidi MA. Histopathologic correlates of noncalcific chronic pancreatitis by EUS: a prospective tissue characterization study. Gastrointest Endosc 2007; 66:501–509.
- Chong AK, Hawes RH, Hoffman BJ, Adams DB, Lewin DN, Romagnuolo J. Diagnostic performance of EUS for chronic pancreatitis: a comparison with histopathology. Gastrointest Endosc 2007; 65:808–814.
- Chowdhury R, Bhutani MS, Mishra G, Toskes PP, Forsmark CE. Comparative analysis of direct pancreatic function testing versus morphological assessment by endoscopic ultrasonography for the evaluation of chronic unexplained abdominal pain of presumed pancreatic origin. Pancreas 2005; 31:63–68.
- Conwell DL, Zuccaro G, Purich E, et al. Comparison of endoscopic ultrasound chronic pancreatitis criteria to the endoscopic secretinstimulated pancreatic function test. Dig Dis Sci 2007; 52:1206–1210.
- Stevens T, Conwell DL, Zuccaro G, Vargo JJ, Dumot JA, Lopez R. Comparison of endoscopic ultrasound and endoscopic retrograde pancreatography for the prediction of pancreatic exocrine insufficiency. Dig Dis Sci 2008; 53:1146–1151.
- Stevens T, Dumot JA, Parsi MA, Zuccaro G, Vargo JJ. Combined endoscopic ultrasound and secretin endoscopic pancreatic function test in patients evaluated for chronic pancreatitis. Dig Dis Sci 2010; 55:2681–2687.
- Catalano MF, Lahoti S, Geenen JE, Hogan WJ. Prospective evaluation of endoscopic ultrasonography, endoscopic retrograde pancreatography, and secretin test in the diagnosis of chronic pancreatitis. Gastrointest Endosc 1998; 48:11–17.
- Irisawa A, Katakura K, Ohira H, et al. Usefulness of endoscopic ultrasound to diagnose the severity of chronic pancreatitis. J Gastroenterol 2007; 42(suppl 17):90–94.
- Yusoff IF, Sahai AV. A prospective, quantitative assessment of the effect of ethanol and other variables on the endosonographic appearance of the pancreas. Clin Gastroenterol Hepatol 2004; 2:405–409.
- Stevens T, Lopez R, Adler DG, et al. Multicenter comparison of the interobserver agreement of standard EUS scoring and Rosemont classification scoring for diagnosis of chronic pancreatitis. Gastrointest Endosc 2010; 71:519–526.
- Wallace MB, Hawes RH, Durkalski V, et al. The reliability of EUS for the diagnosis of chronic pancreatitis: interobserver agreement among experienced endosonographers. Gastrointest Endosc 2001; 53:294–299.
- Catalano MF, Sahai A, Levy M, et al. EUS-based criteria for the diagnosis of chronic pancreatitis: the Rosemont classification. Gastrointest Endosc 2009; 69:1251–1261.
- Stevens T, Conwell DL, Zuccaro G, et al. A prospective crossover study comparing secretin-stimulated endoscopic and Dreiling tube pancreatic function testing in patients evaluated for chronic pancreatitis. Gastrointest Endosc 2008; 67:458–466.
- Sivak MV, Kaufman A. Endoscopic ultrasonography in the differential diagnosis of pancreatic disease. A preliminary report. Scand J Gastroenterol Suppl 1986; 123:130–134.
- Hisanaga K, Hisanaga A, Nagata K, Ichie Y. High speed rotating scanner for transgastric sonography. AJR Am J Roentgenol 1980; 135:627–629.
- Frossard JL, Sosa-Valencia L, Amouyal G, Marty O, Hadengue A, Amouyal P. Usefulness of endoscopic ultrasonography in patients with “idiopathic” acute pancreatitis. Am J Med 2000; 109:196–200.
- Sugiyama M, Wada N, Atomi Y, Kuroda A, Muto T. Diagnosis of acute pancreatitis: value of endoscopic sonography. AJR Am J Roentgenol 1995; 165:867–872.
- Tandon M, Topazian M. Endoscopic ultrasound in idiopathic acute pancreatitis. Am J Gastroenterol 2001; 96:705–709.
- Kotwal V, Talukdar R, Levy M, Vege SS. Role of endoscopic ultrasound during hospitalization for acute pancreatitis. World J Gastroenterol 2010; 16:4888–4891.
- Liu CL, Lo CM, Chan JK, et al. Detection of choledocholithiasis by EUS in acute pancreatitis: a prospective evaluation in 100 consecutive patients. Gastrointest Endosc 2001; 54:325–330.
- Mirbagheri SA, Mohamadnejad M, Nasiri J, Vahid AA, Ghadimi R, Malekzadeh R. Prospective evaluation of endoscopic ultrasonography in the diagnosis of biliary microlithiasis in patients with normal transabdominal ultrasonography. J Gastrointest Surg 2005; 9:961–964.
- Walsh TN, Rode J, Theis BA, Russell RC. Minimal change chronic pancreatitis. Gut 1992; 33:1566–1571.
- Bhutani MJ, Arantes VN, Verma D, et al. Histopathologic correlation of endoscopic ultrasound findings of chronic pancreatitis in human autopsies. Pancreas 2009; 38:820–824.
- Wiersema MJ, Hawes RH, Lehman GA, Kochman ML, Sherman S, Kopecky KK. Prospective evaluation of endoscopic ultrasonography and endoscopic retrograde cholangiopancreatography in patients with chronic abdominal pain of suspected pancreatic origin. Endoscopy 1993; 25:555–564.
- Kahl S, Glasbrenner B, Leodolter A, Pross M, Schulz HU, Malfertheiner P. EUS in the diagnosis of early chronic pancreatitis: a prospective follow-up study. Gastrointest Endosc 2002; 55:507–511.
- Jones SN, Lees WR, Frost RA. Diagnosis and grading of chronic pancreatitis by morphological criteria derived by ultrasound and pancreatography. Clin Radiol 1988; 39:43–48.
- Lees WR. Endoscopic ultrasonography of chronic pancreatitis and pancreatic pseudocysts. Scand J Gastroenterol Suppl 1986; 123:123–129.
- Sahai AV, Zimmerman M, Aabakken L, et al. Prospective assessment of the ability of endoscopic ultrasound to diagnose, exclude, or establish the severity of chronic pancreatitis found by endoscopic retrograde cholangiopancreatography. Gastrointest Endosc 1998; 48:18–25.
- Albashir S, Bronner MP, Parsi MA, Walsh RM, Stevens T. Endoscopic ultrasound, secretin endoscopic pancreatic function test, and histology: correlation in chronic pancreatitis. Am J Gastroenterol 2010; 105:2498–2503.
- Varadarajulu S, Eltoum I, Tamhane A, Eloubeidi MA. Histopathologic correlates of noncalcific chronic pancreatitis by EUS: a prospective tissue characterization study. Gastrointest Endosc 2007; 66:501–509.
- Chong AK, Hawes RH, Hoffman BJ, Adams DB, Lewin DN, Romagnuolo J. Diagnostic performance of EUS for chronic pancreatitis: a comparison with histopathology. Gastrointest Endosc 2007; 65:808–814.
- Chowdhury R, Bhutani MS, Mishra G, Toskes PP, Forsmark CE. Comparative analysis of direct pancreatic function testing versus morphological assessment by endoscopic ultrasonography for the evaluation of chronic unexplained abdominal pain of presumed pancreatic origin. Pancreas 2005; 31:63–68.
- Conwell DL, Zuccaro G, Purich E, et al. Comparison of endoscopic ultrasound chronic pancreatitis criteria to the endoscopic secretinstimulated pancreatic function test. Dig Dis Sci 2007; 52:1206–1210.
- Stevens T, Conwell DL, Zuccaro G, Vargo JJ, Dumot JA, Lopez R. Comparison of endoscopic ultrasound and endoscopic retrograde pancreatography for the prediction of pancreatic exocrine insufficiency. Dig Dis Sci 2008; 53:1146–1151.
- Stevens T, Dumot JA, Parsi MA, Zuccaro G, Vargo JJ. Combined endoscopic ultrasound and secretin endoscopic pancreatic function test in patients evaluated for chronic pancreatitis. Dig Dis Sci 2010; 55:2681–2687.
- Catalano MF, Lahoti S, Geenen JE, Hogan WJ. Prospective evaluation of endoscopic ultrasonography, endoscopic retrograde pancreatography, and secretin test in the diagnosis of chronic pancreatitis. Gastrointest Endosc 1998; 48:11–17.
- Irisawa A, Katakura K, Ohira H, et al. Usefulness of endoscopic ultrasound to diagnose the severity of chronic pancreatitis. J Gastroenterol 2007; 42(suppl 17):90–94.
- Yusoff IF, Sahai AV. A prospective, quantitative assessment of the effect of ethanol and other variables on the endosonographic appearance of the pancreas. Clin Gastroenterol Hepatol 2004; 2:405–409.
- Stevens T, Lopez R, Adler DG, et al. Multicenter comparison of the interobserver agreement of standard EUS scoring and Rosemont classification scoring for diagnosis of chronic pancreatitis. Gastrointest Endosc 2010; 71:519–526.
- Wallace MB, Hawes RH, Durkalski V, et al. The reliability of EUS for the diagnosis of chronic pancreatitis: interobserver agreement among experienced endosonographers. Gastrointest Endosc 2001; 53:294–299.
- Catalano MF, Sahai A, Levy M, et al. EUS-based criteria for the diagnosis of chronic pancreatitis: the Rosemont classification. Gastrointest Endosc 2009; 69:1251–1261.
- Stevens T, Conwell DL, Zuccaro G, et al. A prospective crossover study comparing secretin-stimulated endoscopic and Dreiling tube pancreatic function testing in patients evaluated for chronic pancreatitis. Gastrointest Endosc 2008; 67:458–466.
KEY POINTS
- EUS can identify the cause of acute pancreatitis when other imaging tests (computed tomography, transabdominal ultrasonography) are unrevealing.
- EUS can safely and accurately detect bile duct stones and other causes of recurrent acute pancreatitis. It can also detect mild and severe structural features of chronic pancreatitis.
- An endoscopic pancreatic function test may be a useful adjunct to EUS to detect mild exocrine insufficiency in early chronic pancreatitis.