User login
Endoscopic ultrasonography to evaluate pancreatitis
Endoscopic ultrasonography (EUS) is a minimally invasive test that provides high-resolution imaging of the pancreas.1,2 As such, it is proving useful.
Accurate diagnosis and timely intervention are essential in managing acute and chronic pancreatitis, which are often encountered in the clinic and the hospital. However, the cause of acute pancreatitis is not always easy to determine. Furthermore, recurrent bouts can progress to chronic pancreatitis if the cause is not identified and eliminated. EUS has been studied extensively in the evaluation of both acute and chronic pancreatitis, as it can identify obstructive and biliary causes of acute pancreatitis and early structural features of chronic pancreatitis.
This article will review the indications and evidence for EUS in the evaluation of acute and chronic pancreatitis.
SPECIALIZED TRAINING REQUIRED
EUS involves passage of a specialized endoscope through the esophagus and stomach and into the duodenum. The scope has a very small ultrasound probe at the tip, allowing detailed imaging of the upper gastrointestinal tract and surrounding organs.
There are two types of EUS endoscope: radial and linear. A radial scope provides a 360° range of view perpendicular to the long axis of the scope. A linear scope provides a 150° view parallel to the long axis of the scope. Many endosonographers favor linear EUS for imaging the pancreas because it permits fine-needle aspiration biopsy of masses, cysts, and lymph nodes.
Specialized training beyond the gastroenterology fellowship is usually required to become proficient in performing EUS, in recognizing the anatomy it reveals, and in performing fine-needle aspiration biopsy.
ENDOSCOPIC ULTRASONOGRAPHY IN ACUTE PANCREATITIS
Finding the cause of acute pancreatitis can be challenging in patients who do not have typical risk factors, eg, those who do not drink substantial amounts of alcohol and in whom transabdominal ultrasonography fails to reveal gallstones.
Several studies have evaluated the role of EUS in recurrent “idiopathic” pancreatitis.3–5 Causes of acute pancreatitis detectable with EUS included gallbladder and bile duct microlithiasis (stones smaller than 3 mm), cysts, intraductal papillary mucinous neoplasms, ampullary neoplasms, pancreas divisum, and pancreatic masses.
Stones, sludge. Transabdominal ultrasonography is often performed in the workup of acute pancreatitis to rule out gallbladder stones and biliary dilation. Unfortunately, it does a poor job of imaging the distal common bile duct, where culprit stones may reside.
EUS provides a high-quality view of the bile duct from the ampulla of Vater to the region of the hepatic hilum and is safer than endoscopic retrograde cholangiopancreatography (ERCP). The available evidence supports the use of EUS as a diagnostic test for bile duct stones.3–7 In fact, using ERCP as the reference standard, EUS has been found to be more sensitive than transabdominal ultrasonography for bile duct stones.4
The yield of EUS for finding biliary sludge and stones may be high in patients with unexplained pancreatitis. EUS detected sludge, microlithiasis, or both in 33 of 35 patients with idiopathic acute pancreatitis who underwent transabdominal ultrasonography with negative results.8 Furthermore, most were symptom-free at an average of 10 months after cholecystectomy, suggesting that microlithiasis was the cause of the “idiopathic” pancreatitis.
EUS can also decrease the number of unnecessary ERCP procedures in patients with suspected biliary pancreatitis. In these patients, EUS can be performed as an initial diagnostic test to exclude retained biliary stones. If a stone is present, the endoscopist can proceed to ERCP for sphincterotomy and stone removal during the same endoscopic session. If EUS is negative, the endoscopy can be concluded without cannulating the bile duct and putting the patient at risk of acute pancreatitis. In one report, this approach eliminated the need for ERCP in five of six patients with suspected biliary pancreatitis.6
Tumors and other causes of bile duct obstruction can also cause recurrent acute pancreatitis and may be difficult to detect with cross-sectional imaging. EUS, on the other hand, can detect small pancreatic masses (< 2 cm), which may be missed by conventional computed tomography. Also, a linear EUS scope, with its forward oblique view, can image the duodenum and ampulla, where obstructing inflammation, tumors, and polyps may be found. One should strongly suspect occult malignancy in elderly patients with unexplained acute pancreatitis. In those patients, repeat imaging with high-resolution dual-phase computed tomography or with EUS should be considered after a few weeks once the acute inflammation resolves.
Pancreas divisum is a relatively common congenital abnormality in which the dorsal and ventral pancreatic ducts do not properly fuse during embryonic development. To rule out pancreas divisum, the endosonographer must carefully trace the pancreatic duct from the dorsal pancreas into the ventral pancreas, where it connects with the bile duct at the duodenal wall.
In summary, EUS appears to be safe and accurate for diagnosing bile duct stones and other structural causes of idiopathic acute pancreatitis.
ENDOSCOPIC ULTRASONOGRAPHY IN CHRONIC PANCREATITIS
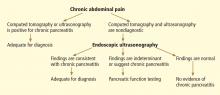
Chronic pancreatitis, a relatively common and sometimes debilitating cause of chronic upper abdominal pain, may be difficult to diagnose using noninvasive imaging tests. Minimal-change chronic pancreatitis is defined as a syndrome of pancreatic abdominal pain with no or slight structural changes detected on imaging but with histologic inflammation and fibrosis diagnostic of chronic pancreatitis.9
A clinical rationale for trying to detect chronic pancreatitis early in its course is that interventions can be started earlier. These include abstinence from alcohol, giving exogenous pancreatic enzymes, and advanced interventions such as celiac plexus blocks for pain control. Some patients may even benefit from resection of the pancreas if pain is severe and resistant to conservative measures.
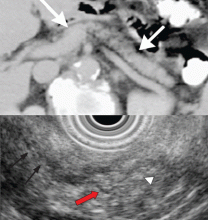
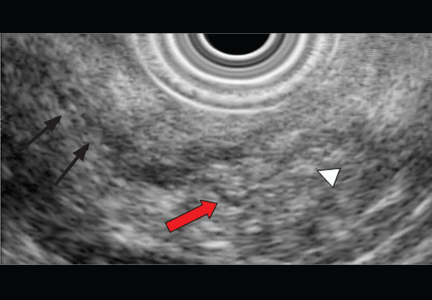
EUS can detect both parenchymal and ductal changes that correlate with histologic fibrosis.10 Parenchymal changes include hyperechoic foci, hyperechoic strands, lobularity, cysts, and shadowing calcifications. Ductal changes include dilation of the main pancreatic duct, irregularity, hyperechoic duct margins, and visible side branches.
Several studies have evaluated the ability of EUS to diagnose early chronic pancreatitis.9,11–15 Reference standards used to determine the accuracy of EUS have included histology,10,16–18 pancreatic function testing,19–22 and ERCP.11,15,23,24
The best diagnostic test may be pancreatic histology. However, biopsy of the pancreas is impractical and exposes patients to high risk. In addition, the patchy and focal distribution of histologic changes may decrease its reliability. Fortunately, the histologic findings of fibrosis have been shown to correlate with EUS criteria in patients undergoing EUS before surgical resection in three recent studies.16–18 A threshold of four or more criteria out of a possible nine was found to provide the optimal sensitivity and specificity for histologic pancreatic fibrosis.16,17 The criteria used were four parenchymal features (hyperechoic foci, strands, hypoechoic lobules, cysts) and five ductal features (irregularity of the main pancreatic duct, dilation, hyperechoic duct walls, visible side branches, and calcifications or stones).
EUS is sensitive for chronic pancreatitis, but ‘true’ accuracy is impossible to know
Unfortunately, greater sensitivity may come at the expense of worse specificity. Certain demographic variables may alter the EUS appearance of the pancreas. A multivariate analysis25 found several variables that predicted abnormalities on EUS even in the absence of clinically evident pancreatitis; the strongest were heavy ethanol use (odds ratio [OR] 5.1, 95% confidence interval [CI] 3.1–8.5), male sex (OR 1.8, 95% CI 1.3–2.55), clinical suspicion of pancreatic disease (OR 1.7, 95% CI 1.2–2.3), and heavy smoking (OR 1.7, 95% CI 1.2–2.4). More prospective studies are needed to further differentiate true disease from false-positive findings of chronic pancreatitis.
Also, traditional EUS scoring symptoms have counted features in an unweighted fashion and assigned an arbitrary cut point (eg, four or more features) for diagnosis. This approach fails to account for the greater importance of some features (eg, calcifications) compared with others.
Interobserver variability is another important limitation of EUS in diagnosing chronic pancreatitis.26,27 In one multicenter study of EUS interpretation, the overall kappa (agreement beyond chance) was only 0.45 for overall chronic pancreatitis diagnosis and worse for many individual criteria for chronic pancreatitis. The endosonographers disagreed most about hyperechoic strands and foci, main pancreatic duct irregularity, and visible side branches (kappa < 0.4).
The Rosemont classification
These limitations led a group of experts to meet in Chicago, IL, to develop a consensus-based and weighted EUS scoring system for the diagnosis of chronic pancreatitis, termed the Rosemont classification.
In this system, the previous parenchymal and ductal features are assigned stricter definitions and reclassified as major and minor criteria. Based on the presence of major and minor features, EUS results are stratified as “normal,” “indeterminate for chronic pancreatitis,” “suggestive of chronic pancreatitis,” or “most consistent with chronic pancreatitis.”15,28
Further validation of this scoring system is needed before it can be used widely.
ENDOSCOPIC ULTRASONOGRAPHY PLUS PANCREATIC FUNCTION TESTING
The best way to diagnose minimal-change chronic pancreatitis may be a combination of sensitive structural and functional testing. Although clinically apparent steatorrhea typically occurs late in the course of chronic pancreatitis, mild exocrine insufficiency may occur early and is detectable with hormone-stimulated pancreatic function testing. Therefore, pancreatic function tests are considered sensitive for diagnosing chronic pancreatitis.20,21,29
Endoscopic pancreatic function testing involves injecting secretin intravenously and then collecting duodenal aspirates through the endoscope. The duodenal fluid is analyzed for bicarbonate concentration as a measure of exocrine function.29
We have studied combined EUS and endoscopic pancreatic function testing in the diagnosis of chronic pancreatitis.16 The combination gives a simultaneous structural and functional assessment of the pancreas and may optimize sensitivity for detecting minimal-change chronic pancreatitis. In a small study, we found the combination had 100% sensitivity for noncalcific chronic pancreatitis compared with a histologic reference standard.16
- Sivak MV, Kaufman A. Endoscopic ultrasonography in the differential diagnosis of pancreatic disease. A preliminary report. Scand J Gastroenterol Suppl 1986; 123:130–134.
- Hisanaga K, Hisanaga A, Nagata K, Ichie Y. High speed rotating scanner for transgastric sonography. AJR Am J Roentgenol 1980; 135:627–629.
- Frossard JL, Sosa-Valencia L, Amouyal G, Marty O, Hadengue A, Amouyal P. Usefulness of endoscopic ultrasonography in patients with “idiopathic” acute pancreatitis. Am J Med 2000; 109:196–200.
- Sugiyama M, Wada N, Atomi Y, Kuroda A, Muto T. Diagnosis of acute pancreatitis: value of endoscopic sonography. AJR Am J Roentgenol 1995; 165:867–872.
- Tandon M, Topazian M. Endoscopic ultrasound in idiopathic acute pancreatitis. Am J Gastroenterol 2001; 96:705–709.
- Kotwal V, Talukdar R, Levy M, Vege SS. Role of endoscopic ultrasound during hospitalization for acute pancreatitis. World J Gastroenterol 2010; 16:4888–4891.
- Liu CL, Lo CM, Chan JK, et al. Detection of choledocholithiasis by EUS in acute pancreatitis: a prospective evaluation in 100 consecutive patients. Gastrointest Endosc 2001; 54:325–330.
- Mirbagheri SA, Mohamadnejad M, Nasiri J, Vahid AA, Ghadimi R, Malekzadeh R. Prospective evaluation of endoscopic ultrasonography in the diagnosis of biliary microlithiasis in patients with normal transabdominal ultrasonography. J Gastrointest Surg 2005; 9:961–964.
- Walsh TN, Rode J, Theis BA, Russell RC. Minimal change chronic pancreatitis. Gut 1992; 33:1566–1571.
- Bhutani MJ, Arantes VN, Verma D, et al. Histopathologic correlation of endoscopic ultrasound findings of chronic pancreatitis in human autopsies. Pancreas 2009; 38:820–824.
- Wiersema MJ, Hawes RH, Lehman GA, Kochman ML, Sherman S, Kopecky KK. Prospective evaluation of endoscopic ultrasonography and endoscopic retrograde cholangiopancreatography in patients with chronic abdominal pain of suspected pancreatic origin. Endoscopy 1993; 25:555–564.
- Kahl S, Glasbrenner B, Leodolter A, Pross M, Schulz HU, Malfertheiner P. EUS in the diagnosis of early chronic pancreatitis: a prospective follow-up study. Gastrointest Endosc 2002; 55:507–511.
- Jones SN, Lees WR, Frost RA. Diagnosis and grading of chronic pancreatitis by morphological criteria derived by ultrasound and pancreatography. Clin Radiol 1988; 39:43–48.
- Lees WR. Endoscopic ultrasonography of chronic pancreatitis and pancreatic pseudocysts. Scand J Gastroenterol Suppl 1986; 123:123–129.
- Sahai AV, Zimmerman M, Aabakken L, et al. Prospective assessment of the ability of endoscopic ultrasound to diagnose, exclude, or establish the severity of chronic pancreatitis found by endoscopic retrograde cholangiopancreatography. Gastrointest Endosc 1998; 48:18–25.
- Albashir S, Bronner MP, Parsi MA, Walsh RM, Stevens T. Endoscopic ultrasound, secretin endoscopic pancreatic function test, and histology: correlation in chronic pancreatitis. Am J Gastroenterol 2010; 105:2498–2503.
- Varadarajulu S, Eltoum I, Tamhane A, Eloubeidi MA. Histopathologic correlates of noncalcific chronic pancreatitis by EUS: a prospective tissue characterization study. Gastrointest Endosc 2007; 66:501–509.
- Chong AK, Hawes RH, Hoffman BJ, Adams DB, Lewin DN, Romagnuolo J. Diagnostic performance of EUS for chronic pancreatitis: a comparison with histopathology. Gastrointest Endosc 2007; 65:808–814.
- Chowdhury R, Bhutani MS, Mishra G, Toskes PP, Forsmark CE. Comparative analysis of direct pancreatic function testing versus morphological assessment by endoscopic ultrasonography for the evaluation of chronic unexplained abdominal pain of presumed pancreatic origin. Pancreas 2005; 31:63–68.
- Conwell DL, Zuccaro G, Purich E, et al. Comparison of endoscopic ultrasound chronic pancreatitis criteria to the endoscopic secretinstimulated pancreatic function test. Dig Dis Sci 2007; 52:1206–1210.
- Stevens T, Conwell DL, Zuccaro G, Vargo JJ, Dumot JA, Lopez R. Comparison of endoscopic ultrasound and endoscopic retrograde pancreatography for the prediction of pancreatic exocrine insufficiency. Dig Dis Sci 2008; 53:1146–1151.
- Stevens T, Dumot JA, Parsi MA, Zuccaro G, Vargo JJ. Combined endoscopic ultrasound and secretin endoscopic pancreatic function test in patients evaluated for chronic pancreatitis. Dig Dis Sci 2010; 55:2681–2687.
- Catalano MF, Lahoti S, Geenen JE, Hogan WJ. Prospective evaluation of endoscopic ultrasonography, endoscopic retrograde pancreatography, and secretin test in the diagnosis of chronic pancreatitis. Gastrointest Endosc 1998; 48:11–17.
- Irisawa A, Katakura K, Ohira H, et al. Usefulness of endoscopic ultrasound to diagnose the severity of chronic pancreatitis. J Gastroenterol 2007; 42(suppl 17):90–94.
- Yusoff IF, Sahai AV. A prospective, quantitative assessment of the effect of ethanol and other variables on the endosonographic appearance of the pancreas. Clin Gastroenterol Hepatol 2004; 2:405–409.
- Stevens T, Lopez R, Adler DG, et al. Multicenter comparison of the interobserver agreement of standard EUS scoring and Rosemont classification scoring for diagnosis of chronic pancreatitis. Gastrointest Endosc 2010; 71:519–526.
- Wallace MB, Hawes RH, Durkalski V, et al. The reliability of EUS for the diagnosis of chronic pancreatitis: interobserver agreement among experienced endosonographers. Gastrointest Endosc 2001; 53:294–299.
- Catalano MF, Sahai A, Levy M, et al. EUS-based criteria for the diagnosis of chronic pancreatitis: the Rosemont classification. Gastrointest Endosc 2009; 69:1251–1261.
- Stevens T, Conwell DL, Zuccaro G, et al. A prospective crossover study comparing secretin-stimulated endoscopic and Dreiling tube pancreatic function testing in patients evaluated for chronic pancreatitis. Gastrointest Endosc 2008; 67:458–466.
Endoscopic ultrasonography (EUS) is a minimally invasive test that provides high-resolution imaging of the pancreas.1,2 As such, it is proving useful.
Accurate diagnosis and timely intervention are essential in managing acute and chronic pancreatitis, which are often encountered in the clinic and the hospital. However, the cause of acute pancreatitis is not always easy to determine. Furthermore, recurrent bouts can progress to chronic pancreatitis if the cause is not identified and eliminated. EUS has been studied extensively in the evaluation of both acute and chronic pancreatitis, as it can identify obstructive and biliary causes of acute pancreatitis and early structural features of chronic pancreatitis.
This article will review the indications and evidence for EUS in the evaluation of acute and chronic pancreatitis.
SPECIALIZED TRAINING REQUIRED
EUS involves passage of a specialized endoscope through the esophagus and stomach and into the duodenum. The scope has a very small ultrasound probe at the tip, allowing detailed imaging of the upper gastrointestinal tract and surrounding organs.
There are two types of EUS endoscope: radial and linear. A radial scope provides a 360° range of view perpendicular to the long axis of the scope. A linear scope provides a 150° view parallel to the long axis of the scope. Many endosonographers favor linear EUS for imaging the pancreas because it permits fine-needle aspiration biopsy of masses, cysts, and lymph nodes.
Specialized training beyond the gastroenterology fellowship is usually required to become proficient in performing EUS, in recognizing the anatomy it reveals, and in performing fine-needle aspiration biopsy.
ENDOSCOPIC ULTRASONOGRAPHY IN ACUTE PANCREATITIS
Finding the cause of acute pancreatitis can be challenging in patients who do not have typical risk factors, eg, those who do not drink substantial amounts of alcohol and in whom transabdominal ultrasonography fails to reveal gallstones.
Several studies have evaluated the role of EUS in recurrent “idiopathic” pancreatitis.3–5 Causes of acute pancreatitis detectable with EUS included gallbladder and bile duct microlithiasis (stones smaller than 3 mm), cysts, intraductal papillary mucinous neoplasms, ampullary neoplasms, pancreas divisum, and pancreatic masses.
Stones, sludge. Transabdominal ultrasonography is often performed in the workup of acute pancreatitis to rule out gallbladder stones and biliary dilation. Unfortunately, it does a poor job of imaging the distal common bile duct, where culprit stones may reside.
EUS provides a high-quality view of the bile duct from the ampulla of Vater to the region of the hepatic hilum and is safer than endoscopic retrograde cholangiopancreatography (ERCP). The available evidence supports the use of EUS as a diagnostic test for bile duct stones.3–7 In fact, using ERCP as the reference standard, EUS has been found to be more sensitive than transabdominal ultrasonography for bile duct stones.4
The yield of EUS for finding biliary sludge and stones may be high in patients with unexplained pancreatitis. EUS detected sludge, microlithiasis, or both in 33 of 35 patients with idiopathic acute pancreatitis who underwent transabdominal ultrasonography with negative results.8 Furthermore, most were symptom-free at an average of 10 months after cholecystectomy, suggesting that microlithiasis was the cause of the “idiopathic” pancreatitis.
EUS can also decrease the number of unnecessary ERCP procedures in patients with suspected biliary pancreatitis. In these patients, EUS can be performed as an initial diagnostic test to exclude retained biliary stones. If a stone is present, the endoscopist can proceed to ERCP for sphincterotomy and stone removal during the same endoscopic session. If EUS is negative, the endoscopy can be concluded without cannulating the bile duct and putting the patient at risk of acute pancreatitis. In one report, this approach eliminated the need for ERCP in five of six patients with suspected biliary pancreatitis.6
Tumors and other causes of bile duct obstruction can also cause recurrent acute pancreatitis and may be difficult to detect with cross-sectional imaging. EUS, on the other hand, can detect small pancreatic masses (< 2 cm), which may be missed by conventional computed tomography. Also, a linear EUS scope, with its forward oblique view, can image the duodenum and ampulla, where obstructing inflammation, tumors, and polyps may be found. One should strongly suspect occult malignancy in elderly patients with unexplained acute pancreatitis. In those patients, repeat imaging with high-resolution dual-phase computed tomography or with EUS should be considered after a few weeks once the acute inflammation resolves.
Pancreas divisum is a relatively common congenital abnormality in which the dorsal and ventral pancreatic ducts do not properly fuse during embryonic development. To rule out pancreas divisum, the endosonographer must carefully trace the pancreatic duct from the dorsal pancreas into the ventral pancreas, where it connects with the bile duct at the duodenal wall.
In summary, EUS appears to be safe and accurate for diagnosing bile duct stones and other structural causes of idiopathic acute pancreatitis.
ENDOSCOPIC ULTRASONOGRAPHY IN CHRONIC PANCREATITIS
Chronic pancreatitis, a relatively common and sometimes debilitating cause of chronic upper abdominal pain, may be difficult to diagnose using noninvasive imaging tests. Minimal-change chronic pancreatitis is defined as a syndrome of pancreatic abdominal pain with no or slight structural changes detected on imaging but with histologic inflammation and fibrosis diagnostic of chronic pancreatitis.9
A clinical rationale for trying to detect chronic pancreatitis early in its course is that interventions can be started earlier. These include abstinence from alcohol, giving exogenous pancreatic enzymes, and advanced interventions such as celiac plexus blocks for pain control. Some patients may even benefit from resection of the pancreas if pain is severe and resistant to conservative measures.
EUS can detect both parenchymal and ductal changes that correlate with histologic fibrosis.10 Parenchymal changes include hyperechoic foci, hyperechoic strands, lobularity, cysts, and shadowing calcifications. Ductal changes include dilation of the main pancreatic duct, irregularity, hyperechoic duct margins, and visible side branches.
Several studies have evaluated the ability of EUS to diagnose early chronic pancreatitis.9,11–15 Reference standards used to determine the accuracy of EUS have included histology,10,16–18 pancreatic function testing,19–22 and ERCP.11,15,23,24
The best diagnostic test may be pancreatic histology. However, biopsy of the pancreas is impractical and exposes patients to high risk. In addition, the patchy and focal distribution of histologic changes may decrease its reliability. Fortunately, the histologic findings of fibrosis have been shown to correlate with EUS criteria in patients undergoing EUS before surgical resection in three recent studies.16–18 A threshold of four or more criteria out of a possible nine was found to provide the optimal sensitivity and specificity for histologic pancreatic fibrosis.16,17 The criteria used were four parenchymal features (hyperechoic foci, strands, hypoechoic lobules, cysts) and five ductal features (irregularity of the main pancreatic duct, dilation, hyperechoic duct walls, visible side branches, and calcifications or stones).
EUS is sensitive for chronic pancreatitis, but ‘true’ accuracy is impossible to know
Unfortunately, greater sensitivity may come at the expense of worse specificity. Certain demographic variables may alter the EUS appearance of the pancreas. A multivariate analysis25 found several variables that predicted abnormalities on EUS even in the absence of clinically evident pancreatitis; the strongest were heavy ethanol use (odds ratio [OR] 5.1, 95% confidence interval [CI] 3.1–8.5), male sex (OR 1.8, 95% CI 1.3–2.55), clinical suspicion of pancreatic disease (OR 1.7, 95% CI 1.2–2.3), and heavy smoking (OR 1.7, 95% CI 1.2–2.4). More prospective studies are needed to further differentiate true disease from false-positive findings of chronic pancreatitis.
Also, traditional EUS scoring symptoms have counted features in an unweighted fashion and assigned an arbitrary cut point (eg, four or more features) for diagnosis. This approach fails to account for the greater importance of some features (eg, calcifications) compared with others.
Interobserver variability is another important limitation of EUS in diagnosing chronic pancreatitis.26,27 In one multicenter study of EUS interpretation, the overall kappa (agreement beyond chance) was only 0.45 for overall chronic pancreatitis diagnosis and worse for many individual criteria for chronic pancreatitis. The endosonographers disagreed most about hyperechoic strands and foci, main pancreatic duct irregularity, and visible side branches (kappa < 0.4).
The Rosemont classification
These limitations led a group of experts to meet in Chicago, IL, to develop a consensus-based and weighted EUS scoring system for the diagnosis of chronic pancreatitis, termed the Rosemont classification.
In this system, the previous parenchymal and ductal features are assigned stricter definitions and reclassified as major and minor criteria. Based on the presence of major and minor features, EUS results are stratified as “normal,” “indeterminate for chronic pancreatitis,” “suggestive of chronic pancreatitis,” or “most consistent with chronic pancreatitis.”15,28
Further validation of this scoring system is needed before it can be used widely.
ENDOSCOPIC ULTRASONOGRAPHY PLUS PANCREATIC FUNCTION TESTING
The best way to diagnose minimal-change chronic pancreatitis may be a combination of sensitive structural and functional testing. Although clinically apparent steatorrhea typically occurs late in the course of chronic pancreatitis, mild exocrine insufficiency may occur early and is detectable with hormone-stimulated pancreatic function testing. Therefore, pancreatic function tests are considered sensitive for diagnosing chronic pancreatitis.20,21,29
Endoscopic pancreatic function testing involves injecting secretin intravenously and then collecting duodenal aspirates through the endoscope. The duodenal fluid is analyzed for bicarbonate concentration as a measure of exocrine function.29
We have studied combined EUS and endoscopic pancreatic function testing in the diagnosis of chronic pancreatitis.16 The combination gives a simultaneous structural and functional assessment of the pancreas and may optimize sensitivity for detecting minimal-change chronic pancreatitis. In a small study, we found the combination had 100% sensitivity for noncalcific chronic pancreatitis compared with a histologic reference standard.16
Endoscopic ultrasonography (EUS) is a minimally invasive test that provides high-resolution imaging of the pancreas.1,2 As such, it is proving useful.
Accurate diagnosis and timely intervention are essential in managing acute and chronic pancreatitis, which are often encountered in the clinic and the hospital. However, the cause of acute pancreatitis is not always easy to determine. Furthermore, recurrent bouts can progress to chronic pancreatitis if the cause is not identified and eliminated. EUS has been studied extensively in the evaluation of both acute and chronic pancreatitis, as it can identify obstructive and biliary causes of acute pancreatitis and early structural features of chronic pancreatitis.
This article will review the indications and evidence for EUS in the evaluation of acute and chronic pancreatitis.
SPECIALIZED TRAINING REQUIRED
EUS involves passage of a specialized endoscope through the esophagus and stomach and into the duodenum. The scope has a very small ultrasound probe at the tip, allowing detailed imaging of the upper gastrointestinal tract and surrounding organs.
There are two types of EUS endoscope: radial and linear. A radial scope provides a 360° range of view perpendicular to the long axis of the scope. A linear scope provides a 150° view parallel to the long axis of the scope. Many endosonographers favor linear EUS for imaging the pancreas because it permits fine-needle aspiration biopsy of masses, cysts, and lymph nodes.
Specialized training beyond the gastroenterology fellowship is usually required to become proficient in performing EUS, in recognizing the anatomy it reveals, and in performing fine-needle aspiration biopsy.
ENDOSCOPIC ULTRASONOGRAPHY IN ACUTE PANCREATITIS
Finding the cause of acute pancreatitis can be challenging in patients who do not have typical risk factors, eg, those who do not drink substantial amounts of alcohol and in whom transabdominal ultrasonography fails to reveal gallstones.
Several studies have evaluated the role of EUS in recurrent “idiopathic” pancreatitis.3–5 Causes of acute pancreatitis detectable with EUS included gallbladder and bile duct microlithiasis (stones smaller than 3 mm), cysts, intraductal papillary mucinous neoplasms, ampullary neoplasms, pancreas divisum, and pancreatic masses.
Stones, sludge. Transabdominal ultrasonography is often performed in the workup of acute pancreatitis to rule out gallbladder stones and biliary dilation. Unfortunately, it does a poor job of imaging the distal common bile duct, where culprit stones may reside.
EUS provides a high-quality view of the bile duct from the ampulla of Vater to the region of the hepatic hilum and is safer than endoscopic retrograde cholangiopancreatography (ERCP). The available evidence supports the use of EUS as a diagnostic test for bile duct stones.3–7 In fact, using ERCP as the reference standard, EUS has been found to be more sensitive than transabdominal ultrasonography for bile duct stones.4
The yield of EUS for finding biliary sludge and stones may be high in patients with unexplained pancreatitis. EUS detected sludge, microlithiasis, or both in 33 of 35 patients with idiopathic acute pancreatitis who underwent transabdominal ultrasonography with negative results.8 Furthermore, most were symptom-free at an average of 10 months after cholecystectomy, suggesting that microlithiasis was the cause of the “idiopathic” pancreatitis.
EUS can also decrease the number of unnecessary ERCP procedures in patients with suspected biliary pancreatitis. In these patients, EUS can be performed as an initial diagnostic test to exclude retained biliary stones. If a stone is present, the endoscopist can proceed to ERCP for sphincterotomy and stone removal during the same endoscopic session. If EUS is negative, the endoscopy can be concluded without cannulating the bile duct and putting the patient at risk of acute pancreatitis. In one report, this approach eliminated the need for ERCP in five of six patients with suspected biliary pancreatitis.6
Tumors and other causes of bile duct obstruction can also cause recurrent acute pancreatitis and may be difficult to detect with cross-sectional imaging. EUS, on the other hand, can detect small pancreatic masses (< 2 cm), which may be missed by conventional computed tomography. Also, a linear EUS scope, with its forward oblique view, can image the duodenum and ampulla, where obstructing inflammation, tumors, and polyps may be found. One should strongly suspect occult malignancy in elderly patients with unexplained acute pancreatitis. In those patients, repeat imaging with high-resolution dual-phase computed tomography or with EUS should be considered after a few weeks once the acute inflammation resolves.
Pancreas divisum is a relatively common congenital abnormality in which the dorsal and ventral pancreatic ducts do not properly fuse during embryonic development. To rule out pancreas divisum, the endosonographer must carefully trace the pancreatic duct from the dorsal pancreas into the ventral pancreas, where it connects with the bile duct at the duodenal wall.
In summary, EUS appears to be safe and accurate for diagnosing bile duct stones and other structural causes of idiopathic acute pancreatitis.
ENDOSCOPIC ULTRASONOGRAPHY IN CHRONIC PANCREATITIS
Chronic pancreatitis, a relatively common and sometimes debilitating cause of chronic upper abdominal pain, may be difficult to diagnose using noninvasive imaging tests. Minimal-change chronic pancreatitis is defined as a syndrome of pancreatic abdominal pain with no or slight structural changes detected on imaging but with histologic inflammation and fibrosis diagnostic of chronic pancreatitis.9
A clinical rationale for trying to detect chronic pancreatitis early in its course is that interventions can be started earlier. These include abstinence from alcohol, giving exogenous pancreatic enzymes, and advanced interventions such as celiac plexus blocks for pain control. Some patients may even benefit from resection of the pancreas if pain is severe and resistant to conservative measures.
EUS can detect both parenchymal and ductal changes that correlate with histologic fibrosis.10 Parenchymal changes include hyperechoic foci, hyperechoic strands, lobularity, cysts, and shadowing calcifications. Ductal changes include dilation of the main pancreatic duct, irregularity, hyperechoic duct margins, and visible side branches.
Several studies have evaluated the ability of EUS to diagnose early chronic pancreatitis.9,11–15 Reference standards used to determine the accuracy of EUS have included histology,10,16–18 pancreatic function testing,19–22 and ERCP.11,15,23,24
The best diagnostic test may be pancreatic histology. However, biopsy of the pancreas is impractical and exposes patients to high risk. In addition, the patchy and focal distribution of histologic changes may decrease its reliability. Fortunately, the histologic findings of fibrosis have been shown to correlate with EUS criteria in patients undergoing EUS before surgical resection in three recent studies.16–18 A threshold of four or more criteria out of a possible nine was found to provide the optimal sensitivity and specificity for histologic pancreatic fibrosis.16,17 The criteria used were four parenchymal features (hyperechoic foci, strands, hypoechoic lobules, cysts) and five ductal features (irregularity of the main pancreatic duct, dilation, hyperechoic duct walls, visible side branches, and calcifications or stones).
EUS is sensitive for chronic pancreatitis, but ‘true’ accuracy is impossible to know
Unfortunately, greater sensitivity may come at the expense of worse specificity. Certain demographic variables may alter the EUS appearance of the pancreas. A multivariate analysis25 found several variables that predicted abnormalities on EUS even in the absence of clinically evident pancreatitis; the strongest were heavy ethanol use (odds ratio [OR] 5.1, 95% confidence interval [CI] 3.1–8.5), male sex (OR 1.8, 95% CI 1.3–2.55), clinical suspicion of pancreatic disease (OR 1.7, 95% CI 1.2–2.3), and heavy smoking (OR 1.7, 95% CI 1.2–2.4). More prospective studies are needed to further differentiate true disease from false-positive findings of chronic pancreatitis.
Also, traditional EUS scoring symptoms have counted features in an unweighted fashion and assigned an arbitrary cut point (eg, four or more features) for diagnosis. This approach fails to account for the greater importance of some features (eg, calcifications) compared with others.
Interobserver variability is another important limitation of EUS in diagnosing chronic pancreatitis.26,27 In one multicenter study of EUS interpretation, the overall kappa (agreement beyond chance) was only 0.45 for overall chronic pancreatitis diagnosis and worse for many individual criteria for chronic pancreatitis. The endosonographers disagreed most about hyperechoic strands and foci, main pancreatic duct irregularity, and visible side branches (kappa < 0.4).
The Rosemont classification
These limitations led a group of experts to meet in Chicago, IL, to develop a consensus-based and weighted EUS scoring system for the diagnosis of chronic pancreatitis, termed the Rosemont classification.
In this system, the previous parenchymal and ductal features are assigned stricter definitions and reclassified as major and minor criteria. Based on the presence of major and minor features, EUS results are stratified as “normal,” “indeterminate for chronic pancreatitis,” “suggestive of chronic pancreatitis,” or “most consistent with chronic pancreatitis.”15,28
Further validation of this scoring system is needed before it can be used widely.
ENDOSCOPIC ULTRASONOGRAPHY PLUS PANCREATIC FUNCTION TESTING
The best way to diagnose minimal-change chronic pancreatitis may be a combination of sensitive structural and functional testing. Although clinically apparent steatorrhea typically occurs late in the course of chronic pancreatitis, mild exocrine insufficiency may occur early and is detectable with hormone-stimulated pancreatic function testing. Therefore, pancreatic function tests are considered sensitive for diagnosing chronic pancreatitis.20,21,29
Endoscopic pancreatic function testing involves injecting secretin intravenously and then collecting duodenal aspirates through the endoscope. The duodenal fluid is analyzed for bicarbonate concentration as a measure of exocrine function.29
We have studied combined EUS and endoscopic pancreatic function testing in the diagnosis of chronic pancreatitis.16 The combination gives a simultaneous structural and functional assessment of the pancreas and may optimize sensitivity for detecting minimal-change chronic pancreatitis. In a small study, we found the combination had 100% sensitivity for noncalcific chronic pancreatitis compared with a histologic reference standard.16
- Sivak MV, Kaufman A. Endoscopic ultrasonography in the differential diagnosis of pancreatic disease. A preliminary report. Scand J Gastroenterol Suppl 1986; 123:130–134.
- Hisanaga K, Hisanaga A, Nagata K, Ichie Y. High speed rotating scanner for transgastric sonography. AJR Am J Roentgenol 1980; 135:627–629.
- Frossard JL, Sosa-Valencia L, Amouyal G, Marty O, Hadengue A, Amouyal P. Usefulness of endoscopic ultrasonography in patients with “idiopathic” acute pancreatitis. Am J Med 2000; 109:196–200.
- Sugiyama M, Wada N, Atomi Y, Kuroda A, Muto T. Diagnosis of acute pancreatitis: value of endoscopic sonography. AJR Am J Roentgenol 1995; 165:867–872.
- Tandon M, Topazian M. Endoscopic ultrasound in idiopathic acute pancreatitis. Am J Gastroenterol 2001; 96:705–709.
- Kotwal V, Talukdar R, Levy M, Vege SS. Role of endoscopic ultrasound during hospitalization for acute pancreatitis. World J Gastroenterol 2010; 16:4888–4891.
- Liu CL, Lo CM, Chan JK, et al. Detection of choledocholithiasis by EUS in acute pancreatitis: a prospective evaluation in 100 consecutive patients. Gastrointest Endosc 2001; 54:325–330.
- Mirbagheri SA, Mohamadnejad M, Nasiri J, Vahid AA, Ghadimi R, Malekzadeh R. Prospective evaluation of endoscopic ultrasonography in the diagnosis of biliary microlithiasis in patients with normal transabdominal ultrasonography. J Gastrointest Surg 2005; 9:961–964.
- Walsh TN, Rode J, Theis BA, Russell RC. Minimal change chronic pancreatitis. Gut 1992; 33:1566–1571.
- Bhutani MJ, Arantes VN, Verma D, et al. Histopathologic correlation of endoscopic ultrasound findings of chronic pancreatitis in human autopsies. Pancreas 2009; 38:820–824.
- Wiersema MJ, Hawes RH, Lehman GA, Kochman ML, Sherman S, Kopecky KK. Prospective evaluation of endoscopic ultrasonography and endoscopic retrograde cholangiopancreatography in patients with chronic abdominal pain of suspected pancreatic origin. Endoscopy 1993; 25:555–564.
- Kahl S, Glasbrenner B, Leodolter A, Pross M, Schulz HU, Malfertheiner P. EUS in the diagnosis of early chronic pancreatitis: a prospective follow-up study. Gastrointest Endosc 2002; 55:507–511.
- Jones SN, Lees WR, Frost RA. Diagnosis and grading of chronic pancreatitis by morphological criteria derived by ultrasound and pancreatography. Clin Radiol 1988; 39:43–48.
- Lees WR. Endoscopic ultrasonography of chronic pancreatitis and pancreatic pseudocysts. Scand J Gastroenterol Suppl 1986; 123:123–129.
- Sahai AV, Zimmerman M, Aabakken L, et al. Prospective assessment of the ability of endoscopic ultrasound to diagnose, exclude, or establish the severity of chronic pancreatitis found by endoscopic retrograde cholangiopancreatography. Gastrointest Endosc 1998; 48:18–25.
- Albashir S, Bronner MP, Parsi MA, Walsh RM, Stevens T. Endoscopic ultrasound, secretin endoscopic pancreatic function test, and histology: correlation in chronic pancreatitis. Am J Gastroenterol 2010; 105:2498–2503.
- Varadarajulu S, Eltoum I, Tamhane A, Eloubeidi MA. Histopathologic correlates of noncalcific chronic pancreatitis by EUS: a prospective tissue characterization study. Gastrointest Endosc 2007; 66:501–509.
- Chong AK, Hawes RH, Hoffman BJ, Adams DB, Lewin DN, Romagnuolo J. Diagnostic performance of EUS for chronic pancreatitis: a comparison with histopathology. Gastrointest Endosc 2007; 65:808–814.
- Chowdhury R, Bhutani MS, Mishra G, Toskes PP, Forsmark CE. Comparative analysis of direct pancreatic function testing versus morphological assessment by endoscopic ultrasonography for the evaluation of chronic unexplained abdominal pain of presumed pancreatic origin. Pancreas 2005; 31:63–68.
- Conwell DL, Zuccaro G, Purich E, et al. Comparison of endoscopic ultrasound chronic pancreatitis criteria to the endoscopic secretinstimulated pancreatic function test. Dig Dis Sci 2007; 52:1206–1210.
- Stevens T, Conwell DL, Zuccaro G, Vargo JJ, Dumot JA, Lopez R. Comparison of endoscopic ultrasound and endoscopic retrograde pancreatography for the prediction of pancreatic exocrine insufficiency. Dig Dis Sci 2008; 53:1146–1151.
- Stevens T, Dumot JA, Parsi MA, Zuccaro G, Vargo JJ. Combined endoscopic ultrasound and secretin endoscopic pancreatic function test in patients evaluated for chronic pancreatitis. Dig Dis Sci 2010; 55:2681–2687.
- Catalano MF, Lahoti S, Geenen JE, Hogan WJ. Prospective evaluation of endoscopic ultrasonography, endoscopic retrograde pancreatography, and secretin test in the diagnosis of chronic pancreatitis. Gastrointest Endosc 1998; 48:11–17.
- Irisawa A, Katakura K, Ohira H, et al. Usefulness of endoscopic ultrasound to diagnose the severity of chronic pancreatitis. J Gastroenterol 2007; 42(suppl 17):90–94.
- Yusoff IF, Sahai AV. A prospective, quantitative assessment of the effect of ethanol and other variables on the endosonographic appearance of the pancreas. Clin Gastroenterol Hepatol 2004; 2:405–409.
- Stevens T, Lopez R, Adler DG, et al. Multicenter comparison of the interobserver agreement of standard EUS scoring and Rosemont classification scoring for diagnosis of chronic pancreatitis. Gastrointest Endosc 2010; 71:519–526.
- Wallace MB, Hawes RH, Durkalski V, et al. The reliability of EUS for the diagnosis of chronic pancreatitis: interobserver agreement among experienced endosonographers. Gastrointest Endosc 2001; 53:294–299.
- Catalano MF, Sahai A, Levy M, et al. EUS-based criteria for the diagnosis of chronic pancreatitis: the Rosemont classification. Gastrointest Endosc 2009; 69:1251–1261.
- Stevens T, Conwell DL, Zuccaro G, et al. A prospective crossover study comparing secretin-stimulated endoscopic and Dreiling tube pancreatic function testing in patients evaluated for chronic pancreatitis. Gastrointest Endosc 2008; 67:458–466.
- Sivak MV, Kaufman A. Endoscopic ultrasonography in the differential diagnosis of pancreatic disease. A preliminary report. Scand J Gastroenterol Suppl 1986; 123:130–134.
- Hisanaga K, Hisanaga A, Nagata K, Ichie Y. High speed rotating scanner for transgastric sonography. AJR Am J Roentgenol 1980; 135:627–629.
- Frossard JL, Sosa-Valencia L, Amouyal G, Marty O, Hadengue A, Amouyal P. Usefulness of endoscopic ultrasonography in patients with “idiopathic” acute pancreatitis. Am J Med 2000; 109:196–200.
- Sugiyama M, Wada N, Atomi Y, Kuroda A, Muto T. Diagnosis of acute pancreatitis: value of endoscopic sonography. AJR Am J Roentgenol 1995; 165:867–872.
- Tandon M, Topazian M. Endoscopic ultrasound in idiopathic acute pancreatitis. Am J Gastroenterol 2001; 96:705–709.
- Kotwal V, Talukdar R, Levy M, Vege SS. Role of endoscopic ultrasound during hospitalization for acute pancreatitis. World J Gastroenterol 2010; 16:4888–4891.
- Liu CL, Lo CM, Chan JK, et al. Detection of choledocholithiasis by EUS in acute pancreatitis: a prospective evaluation in 100 consecutive patients. Gastrointest Endosc 2001; 54:325–330.
- Mirbagheri SA, Mohamadnejad M, Nasiri J, Vahid AA, Ghadimi R, Malekzadeh R. Prospective evaluation of endoscopic ultrasonography in the diagnosis of biliary microlithiasis in patients with normal transabdominal ultrasonography. J Gastrointest Surg 2005; 9:961–964.
- Walsh TN, Rode J, Theis BA, Russell RC. Minimal change chronic pancreatitis. Gut 1992; 33:1566–1571.
- Bhutani MJ, Arantes VN, Verma D, et al. Histopathologic correlation of endoscopic ultrasound findings of chronic pancreatitis in human autopsies. Pancreas 2009; 38:820–824.
- Wiersema MJ, Hawes RH, Lehman GA, Kochman ML, Sherman S, Kopecky KK. Prospective evaluation of endoscopic ultrasonography and endoscopic retrograde cholangiopancreatography in patients with chronic abdominal pain of suspected pancreatic origin. Endoscopy 1993; 25:555–564.
- Kahl S, Glasbrenner B, Leodolter A, Pross M, Schulz HU, Malfertheiner P. EUS in the diagnosis of early chronic pancreatitis: a prospective follow-up study. Gastrointest Endosc 2002; 55:507–511.
- Jones SN, Lees WR, Frost RA. Diagnosis and grading of chronic pancreatitis by morphological criteria derived by ultrasound and pancreatography. Clin Radiol 1988; 39:43–48.
- Lees WR. Endoscopic ultrasonography of chronic pancreatitis and pancreatic pseudocysts. Scand J Gastroenterol Suppl 1986; 123:123–129.
- Sahai AV, Zimmerman M, Aabakken L, et al. Prospective assessment of the ability of endoscopic ultrasound to diagnose, exclude, or establish the severity of chronic pancreatitis found by endoscopic retrograde cholangiopancreatography. Gastrointest Endosc 1998; 48:18–25.
- Albashir S, Bronner MP, Parsi MA, Walsh RM, Stevens T. Endoscopic ultrasound, secretin endoscopic pancreatic function test, and histology: correlation in chronic pancreatitis. Am J Gastroenterol 2010; 105:2498–2503.
- Varadarajulu S, Eltoum I, Tamhane A, Eloubeidi MA. Histopathologic correlates of noncalcific chronic pancreatitis by EUS: a prospective tissue characterization study. Gastrointest Endosc 2007; 66:501–509.
- Chong AK, Hawes RH, Hoffman BJ, Adams DB, Lewin DN, Romagnuolo J. Diagnostic performance of EUS for chronic pancreatitis: a comparison with histopathology. Gastrointest Endosc 2007; 65:808–814.
- Chowdhury R, Bhutani MS, Mishra G, Toskes PP, Forsmark CE. Comparative analysis of direct pancreatic function testing versus morphological assessment by endoscopic ultrasonography for the evaluation of chronic unexplained abdominal pain of presumed pancreatic origin. Pancreas 2005; 31:63–68.
- Conwell DL, Zuccaro G, Purich E, et al. Comparison of endoscopic ultrasound chronic pancreatitis criteria to the endoscopic secretinstimulated pancreatic function test. Dig Dis Sci 2007; 52:1206–1210.
- Stevens T, Conwell DL, Zuccaro G, Vargo JJ, Dumot JA, Lopez R. Comparison of endoscopic ultrasound and endoscopic retrograde pancreatography for the prediction of pancreatic exocrine insufficiency. Dig Dis Sci 2008; 53:1146–1151.
- Stevens T, Dumot JA, Parsi MA, Zuccaro G, Vargo JJ. Combined endoscopic ultrasound and secretin endoscopic pancreatic function test in patients evaluated for chronic pancreatitis. Dig Dis Sci 2010; 55:2681–2687.
- Catalano MF, Lahoti S, Geenen JE, Hogan WJ. Prospective evaluation of endoscopic ultrasonography, endoscopic retrograde pancreatography, and secretin test in the diagnosis of chronic pancreatitis. Gastrointest Endosc 1998; 48:11–17.
- Irisawa A, Katakura K, Ohira H, et al. Usefulness of endoscopic ultrasound to diagnose the severity of chronic pancreatitis. J Gastroenterol 2007; 42(suppl 17):90–94.
- Yusoff IF, Sahai AV. A prospective, quantitative assessment of the effect of ethanol and other variables on the endosonographic appearance of the pancreas. Clin Gastroenterol Hepatol 2004; 2:405–409.
- Stevens T, Lopez R, Adler DG, et al. Multicenter comparison of the interobserver agreement of standard EUS scoring and Rosemont classification scoring for diagnosis of chronic pancreatitis. Gastrointest Endosc 2010; 71:519–526.
- Wallace MB, Hawes RH, Durkalski V, et al. The reliability of EUS for the diagnosis of chronic pancreatitis: interobserver agreement among experienced endosonographers. Gastrointest Endosc 2001; 53:294–299.
- Catalano MF, Sahai A, Levy M, et al. EUS-based criteria for the diagnosis of chronic pancreatitis: the Rosemont classification. Gastrointest Endosc 2009; 69:1251–1261.
- Stevens T, Conwell DL, Zuccaro G, et al. A prospective crossover study comparing secretin-stimulated endoscopic and Dreiling tube pancreatic function testing in patients evaluated for chronic pancreatitis. Gastrointest Endosc 2008; 67:458–466.
KEY POINTS
- EUS can identify the cause of acute pancreatitis when other imaging tests (computed tomography, transabdominal ultrasonography) are unrevealing.
- EUS can safely and accurately detect bile duct stones and other causes of recurrent acute pancreatitis. It can also detect mild and severe structural features of chronic pancreatitis.
- An endoscopic pancreatic function test may be a useful adjunct to EUS to detect mild exocrine insufficiency in early chronic pancreatitis.
Progressive muscle weakness: More there than meets the eye
Our patient, a 56-year-old woman, presents with proximal muscle weakness in all four limbs. It started a few months ago and has gradually become severe, so that she now has difficulty rising from a seated position and has trouble opening jars. She has fallen several times. She says she has no muscle pain, difficulty swallowing, or difficulty breathing.
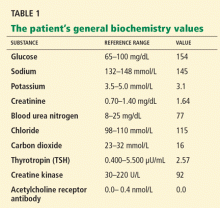
She sought medical attention at another hospital and was found to be hypothyroid, with a thyrotropin (thyroid-stimulating hormone [TSH]) level of 38 μU/mL (reference range 0.4–5.5), for which she was started on levothyroxine (Synthroid) 100 μg daily. She also had a low serum potassium level, for which potassium supplements and spironolactone (Aldactone) were started. She was taking furosemide (Lasix) 20 mg/day at the time.
Despite the thyroid replacement therapy, she continued to become weaker and had more falls. She also noticed a new, nonpainful rash on her lower abdomen.
Review of systems
- Night sweats
- Leg swelling
- Puffiness and discoloration around the eyes, with easy bruisability.
Medical history
- Diabetes mellitus
- Seizures in the 1970s
- Resection of a thymic tumor in 2003 (the exact pathology is unknown)
- Cirrhosis of unknown etiology
- No known history of hypertension
- No history of alcohol or intravenous drug use
- Quit smoking many years ago
- Coronary artery bypass surgery in 2003
- One sibling with myasthenia gravis.
Medications
- Levothyroxine
- Rosuvastatin (Crestor)
- Omeprazole (Prilosec)
- Spironolactone
- Furosemide
- Potassium chloride
- Metoprolol tartrate (Lopressor)
- Metformin (Glucophage)
- Ramipril (Altace).
Physical examination
She is hemodynamically stable and is not hypertensive. Her thyroid is not enlarged. Her lungs are clear to auscultation. Her heart sounds are normal, except for a nonradiating pansystolic murmur most audible at the apex.
Her abdomen is soft and is not distended. Her abdominal rash has a dermatomal distribution consistent with an L1 distribution, with vesicles over an erythematous base. Purpuric lesions are noted over her lower extremities.
Her leg strength is 3 on a scale of 5 on both sides; her arm strength is normal. Ankle and knee reflexes are absent bilaterally.
Initial laboratory analysis
PROGRESSIVE MUSCLE WEAKNESS
1. What are possible causes of her muscle weakness?
- Myasthenia gravis
- Hypothyroidism
- Dermatomyositis-polymyositis
- Drug-induced myopathy
- Cushing syndrome
- All of the above
All of these are potential causes of muscle weakness.
Myasthenia gravis
Myasthenia gravis, an autoimmune disease, can affect people of all ages and either sex. It presents with muscle weakness and fatigability, which characteristically fluctuate during the day. Some patients present in crisis with respiratory failure, which may require ventilatory support.1,2
Myasthenia gravis is characterized by auto-antibodies against the postsynaptic membrane of the neuromuscular junction. Most patients have antibodies to the extracellular portion of the acetylcholine receptor; a small number of patients have antibodies against a muscle-specific tyrosine kinase that interacts with this receptor.
About 15% of patients with myasthenia gravis have a thymoma thought to be involved in the pathogenesis of the disease. Treatments include immune suppressive therapy and thymectomy.
Our patient has a history of thymic lesion resection, but her antibody workup for myasthenia gravis was negative.
Hypothyroidism
Hypothyroidism, the most common disorder of the thyroid gland, is especially prevalent in women.3 Its common symptoms include fatigue, exercise intolerance, muscle weakness, cramps, and stiffness.
Both the TSH and the free thyroxine (T4) level must be measured to diagnose hypothyroidism. This information can also help differentiate primary hypothyroidism (ie, due to a defect in the thyroid gland) from secondary hypothyroidism (ie, due to a defect in the pituitary gland). Elevated TSH with low free T4 levels indicates primary thyroid failure, whereas the combination of a normal or low TSH and a low free T4 usually indicates pituitary failure. Subclinical hypothyroidism is characterized by mildly to moderately elevated TSH, but total T4 and free T4 values are still within the reference range. Replacement therapy is with levothyroxine.3–6
Our patient has a history of hypothyroidism, which could explain her muscle weakness, but she is currently on replacement therapy, and her TSH level on admission was normal.
Dermatomyositis-polymyositis
Dermatomyositis-polymyositis is characterized by proximal muscle weakness, creatine kinase elevation, erythema on sun-exposed skin, heliotrope rash, and Gottron papules. It occurs mostly in women after the second decade of life. Some medications have been implicated in its pathogenesis, such as statins, fibrates, hydroxyurea, penicillamine, and omeprazole (Prilosec).7
In a middle-aged patient, this diagnosis should prompt a search for cancer, especially of the gastrointestinal system, breast, and lung.8 Cancer can arise up to 3 years after the diagnosis of dermatomyositis or polymyositis.
Antisynthetase antibody syndrome is suspected if the patient is positive for antisynthetase antibody and has the following manifestations: acute onset of disease, constitutional symptoms, interstitial lung disease, inflammatory arthritis, mechanic’s hands (thickened, cracked skin on the palmar aspect of the thumb and index finger), and Raynaud phenomenon.4,8,9
The diagnosis is made by a thorough clinical evaluation. Electromyography can show an inflammatory pattern of myopathy. The gold standard test for this diagnosis is muscle biopsy.
Our patient has a normal creatine kinase level, which excludes the diagnosis of dermatomyositis-polymyositis.
Statin-induced myopathy
Up to 10% of patients taking statins develop myalgia. Rhabdomyolysis, the extreme form of myopathy, is rare.
The exact mechanism of statin-induced myopathy remains unclear; mitochondrial dysfunction, cholesterol composition of cell membranes, and coenzyme Q10 deficiency have been proposed.
Risk factors for statin-induced myopathy include female sex, older age, higher doses of statins, a family history of statin-induced myopathy, and hypothyroidism. Drugs that increase the risk include fibric acid derivatives, macrolides, and amiodarone (Cordarone). If a statin and any of the above drugs are both required, certain statins—ie, pravastatin (Pravachol) and rosuvastatin—are recommended, since they are the statins least likely to cause rhabdomyolysis.5,7,10–12
The combination of fluvastatin (Lescol) and gemfibrozil (Lopid) has also been found to be safe.13 In a crossover study in 17 patients, no significant difference was seen in the area under the curve for plasma concentration over time, in the maximum plasma concentration, or in the time to maximum concentration with the combination vs with each drug alone.13
Our patient is taking a statin and has hypothyroidism, which increases the risk of statin-induced myopathy. However, her creatine kinase level is normal.
Cushing syndrome
Cushing syndrome (hypercortisolism) is one of the most challenging endocrine diseases to diagnose. Most of its clinical features overlap with those of common diseases, and some patients have an atypical clinical presentation with only isolated symptoms. Further, its presentation can be subtle, with weight gain, amenorrhea, muscle weakness, and easy bruisability. Acne, moon facies, plethora, abdominal striae, and purpura are other common signs. It is three to 10 times more common in women than in men.
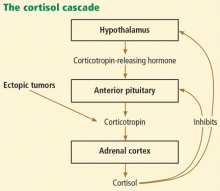
Cushing syndrome can be classified according to whether or not the excess cortisol secretion depends on corticotropin (formerly called adrenocorticotropic hormone or ACTH) (Figure 1). In corticotropin-dependent cases, the most common cause is pituitary adenoma. (When Cushing syndrome is due to excessive pituitary secretion of corticotropin, which in turn stimulates the adrenal glands to secrete excessive amounts of cortisol, it is called Cushing disease). Other causes of corticotropin-dependent Cushing syndrome are ectopic corticotropin-producing tumors such as carcinoid tumors or medullary thyroid cancers. Corticotropin-independent Cushing syndrome can be caused by adrenal adenomas, adrenal carcinoma, and bilateral primary micronodular or macronodular adrenocortical hyperplasia.14–17
However, the most common cause of Cushing syndrome is glucocorticoid therapy.
BACK TO OUR PATIENT: HER CONDITION DETERIORATES
Our patient’s physical condition deteriorates, she develops respiratory distress, and she is admitted to the medical intensive care unit. Her mental status also deteriorates, and she becomes lethargic and unresponsive.
She is intubated to protect her airway. After this, she develops hypotension that does not respond to fluid resuscitation and that requires vasopressors. Her condition continues to worsen as she develops acute kidney injury and disseminated intravascular coagulation. Her vesicular rash becomes more widespread, involving the entire trunk.
A workup for sepsis is initiated, but her initial blood and urine cultures are negative. Chest radiography does not reveal any infiltrates. No other source of an infection is found.
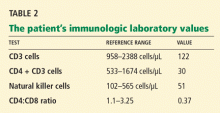
Varicella zoster is isolated on viral culture of a specimen obtained from the rash, and a polymerase chain reaction test of her blood shows cytomegalovirus DNA (64,092 copies per mL). Immune suppression is suspected, so a CD4 count is ordered (Table 2). Serologic tests for human immunodeficiency virus are negative.
What could have caused our patient to have muscle weakness in addition to disseminated zoster with cytomegalovirus viremia?
The diagnosis here is Cushing syndrome.
HOW TO TEST FOR CUSHING SYNDROME
2. In any practice, you may meet many perimenopausal women who have complaints of weight gain, amenorrhea, and acne. How can you determine if this is Cushing syndrome? What are the screening tests?
- 24-Hour urinary cortisol excretion
- A late-night salivary cortisol level
- A low-dose dexamethasone suppression test
- All of the above
- None of the above
Any of the tests listed here can be used to determine whether this is truly Cushing syndrome.
24-Hour urinary cortisol excretion has a reference range of 20 to 100 μg/24 hours. However, results may be falsely high in patients who are depressed or who abuse alcohol.
The late-night salivary cortisol level is another useful test.14,16,18 Patients with Cushing syndrome are found to have high late-night salivary cortisol levels as compared with normal people, indicating the loss of natural circadian rhythm.14,16,18
The low-dose dexamethasone suppression test, as first described by Liddle in 1960,19 involved giving dexamethasone 0.5 mg by mouth every 6 hours for 48 hours and measuring the serum cortisol level 6 hours after the last dose. In healthy people, this low dose of dexamethasone suppresses the production of corticotropin by the pituitary gland and in turn the production of cortisol, but in patients with Cushing syndrome the cortisol level remains high. An alternative is the overnight 1-mg dexamethasone suppression test—ie, giving 1 mg of dexamethasone at 11:00 pm and measuring the serum cortisol level early the next morning. Failure of the cortisol level to drop to less than 1.8 μg/dL suggests Cushing syndrome and warrants a complete evaluation for it.
Confirmatory testing is sometimes needed if patients have mild abnormalities in their screening tests. A combination low-dose dexamethasone suppression test and corticotropin-releasing hormone test can be used to differentiate Cushing syndrome from pseudo-Cushing syndrome. This is performed by giving dexamethasone orally 0.5 mg every 6 hours for 48 hours and then giving ovine-sequence corticotropin-releasing hormone 1 μg/kg intravenously 2 hours after the last dose of dexamethasone. The plasma cortisol value 15 minutes after the dose of corticotropin-releasing hormone is greater than 1.4 μg/dL (38 nmol/L) in patients with Cushing syndrome but remains low in patients with pseudo-Cushing syndrome.
Is this corticotropin-dependent or corticotropin-independent?
Once Cushing syndrome is diagnosed by one of the screening methods described above, the source of the excess glucocorticoids needs to be determined. Measuring the serum corticotropin level early in the morning would be the next step.
A low corticotropin level (< 10 pg/mL) indicates a corticotropin-independent source, most likely in the adrenal glands. Hence, computed tomography or magnetic resonance imaging (MRI) of the adrenal glands is warranted. Of note: adrenal incidentalomas are quite common, present in 5% of the general population, and a lesion on the adrenal gland does not prove that the patient has primary adrenal disease.16,20
IS THE EXCESS CORTICOTROPIN FROM A PITUITARY OR AN ECTOPIC SOURCE?
3. If the corticotropin level is elevated, how can you determine if it is from the pituitary or from an ectopic source?
- MRI of the pituitary gland
- High-dose dexamethasone suppression test
- Corticotropin-releasing hormone stimulation test
- Bilateral inferior petrosal sinus sampling
If the corticotropin level is high (> 10 pg/mL), it is of paramount importance to determine whether the corticotropin comes from the pituitary gland or from an ectopic source.
MRI of the pituitary gland should be done in patients with suspected corticotropin-dependent Cushing syndrome. However, MRI may be negative in 50% of patients with Cushing disease, and it should therefore not be used for screening. In addition, 10% of the population may have pituitary incidentalomas on MRI.
Most cases of corticotropin-dependent Cushing syndrome are caused by microadenomas (smaller than 1 cm), while a few cases are caused by macroadenomas (larger than 1 cm). If a microadenoma is found on MRI, further testing with bilateral inferior petrosal sinus sampling is recommended (described below); if a macroadenoma is found, then no further testing is required.21,22 In fact, patients who have biochemical findings compatible with Cushing disease (ie, due to an overactive pituitary) and who have an adenoma larger than 6 mm do not require further evaluation.23
A high-dose dexamethasone suppression test involves giving 8 mg of dexamethasone in the evening and measuring the cortisol level the next morning. If the cortisol level declines to 50% of the baseline level after this dose, this suggests a pituitary cause.
Corticotropin-releasing hormone stimulation testing. In most cases of pituitary tumors and a few cases of ectopic corticotropin-secreting tumors, giving corticotropin-releasing hormone leads to an increase in serum corticotropin and cortisol levels. In contrast, these levels do not respond to corticotropin-releasing hormone stimulation if the problem is in the adrenal gland. The test is performed by giving 1 μg/kg or 100 μg synthetic or human corticotropin-releasing hormone. A 35% to 50% increase above baseline in corticotropin suggests a pituitary cause.23
Bilateral inferior petrosal sinus sampling can be used to confirm a pituitary source, as it is the gold standard for differentiating ectopic from pituitary corticotropin production. Once this is confirmed, a neurosurgical consult is warranted.16,18
This procedure is usually done by advancing a sheath from the femoral vein to reach the inferior petrosal sinuses. Blood samples are obtained from both the inferior petrosal sinuses and from a peripheral vein to measure corticotropin levels before and after giving corticotropin-releasing hormone (1 μg/kg). Before corticotropin-releasing hormone is given, a gradient of central-peripheral corticotropin levels of 2.0 or greater indicates a pituitary source. With ectopic corticotropin production, the corticotropin gradient is usually less than 1.5. Corticotropin-releasing hormone is given to increase the sensitivity: after it is given, a gradient of 3.0 or greater is considered indicative of Cushing disease.24
If the corticotropin level is elevated and the above tests indicate ectopic production, the source should be sought. The most common site of ectopic corticotropin production is the chest. Common causes are bronchial, thymic, and pancreatic carcinoid tumors. Other causes are small-cell lung cancer, medullary cell cancer, and pheochromocytoma.15,18,25
BACK TO OUR PATIENT
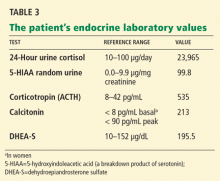
Our patient’s further laboratory results are listed in Table 3.
She has elevated 24-hour urinary cortisol excretion, consistent with Cushing syndrome. Her corticotropin level is elevated, which rules out an adrenal cause. Her 5-HIAA (a serotonin breakdown product) and calcitonin levels are also elevated, suggesting either medullary thyroid cancer or a carcinoid tumor. She also has a mild elevation of dehydroepiandrosterone sulfate, which is consistent with corticotropin-dependent Cushing syndrome.
Our patient’s elevated levels of cortisol were the cause of her muscle weakness and severe immune deficiency, which in turn led to cytomegalovirus viremia and sepsis. Cushing syndrome usually causes hypertension, especially in cases of ectopic corticotropin production. However, our patient was normotensive on admission and then developed cytomegalovirus sepsis, which led to hypotension and shock.
Immune suppression is a well-known effect of glucocorticoids.26–28 Kronfol et al28 found that CD4 and CD8 counts and the CD4-to-CD8 ratio were low in patients with Cushing syndrome, and natural killer cell activity was suppressed. Opportunistic infections have been described in patients with Cushing syndrome.26,27,29
MANAGEMENT OF CUSHING SYNDROME
Management of Cushing syndrome should be tailored after determining its source.
A neurosurgical consultation is warranted in cases of pituitary adenoma, with surgical resection of the adrenal source or ectopic tumor if feasible.25
Medical management is recommended if surgical resection is not possible.30,31 Several drugs can be used to inhibit cortisol synthesis in this situation.30,32
Adrenal-acting agents
Aminoglutethimide (Cytadren) acts by blocking the conversion of cholesterol to pregnenolone, a precursor of cortisol. The dosage is 250 mg twice or three times a day. This drug is no longer available in the United States.
Ketoconazole (Nizoral) inhibits side-chain cleavage, 11-beta hydroxylase, and 17-alpha hydroxylase, thus inhibiting cortisol synthesis; it also inhibits corticotropin secretion. The dosage is 200 to 400 mg three times a day.
Metyrapone (Metopirone) blocks 11-beta-hydroxylation of deoxycortisol, the reaction that produces cortisol. The dosage is 500 to 750 mg three times a day. This drug can be obtained only from the manufacturer and only on a named-patient basis.
Etomidate (Amidate), an anesthetic drug, also blocks 11-beta-hydroxylation of deoxycortisol. It is given intravenously at a rate of 0.3 mg/kg per hour.
Centrally acting agents
Cabergoline (Dostinex). It is believed that corticotropin-producing pituitary tumors express D2 receptors. Cabergoline is a dopamine agonist that has been used in patients with Cushing disease. The dosage is 0.5 to 7 mg/week.
Pasireotide is still investigational. It is a somatostatin receptor agonist given subcutaneously for 15 consecutive days to patients with Cushing disease.
Glucocorticoid receptor antagonist
Mifepristone (Mifeprex) is a progesterone receptor and glucocorticoid II receptor antagonist that is being investigated in the treatment of persistent or recurrent Cushing disease. It is not yet approved by the US Food and Drug Administration for this indication.
BACK TO OUR PATIENT
The patient was too ill to undergo additional imaging, including octreotide scanning to identify an ectopic corticotropin-secreting tumor. She was medically treated with intravenous etomidate to reduce her cortisol level.30,31
Unfortunately, our patient died of multiorgan failure. The exact site of her ectopic corticotropin-producing tumor was never identified, and no autopsy was done.
- Meriggioli MN. Myasthenia gravis with anti-acetylcholine receptor antibodies. Front Neurol Neurosci 2009; 26:94–108.
- Gilhus NE. Autoimmune myasthenia gravis. Expert Rev Neurother 2009; 9:351–358.
- Heitman B, Irizarry A. Hypothyroidism: common complaints, perplexing diagnosis. Nurse Pract 1995; 20:54–60.
- Brick JE, Brick JF, Elnicki DM. Musculoskeletal disorders. When are they caused by hormone imbalance? Postgrad Med 1991; 90:129–132,135–136.
- Bar SL, Holmes DT, Frohlich J. Asymptomatic hypothyroidism and statin-induced myopathy. Can Fam Physician 2007; 53:428–431.
- McDermott MT. In the clinic. Hypothyroidism. Ann Intern Med 2009; 151:ITC61.
- Klopstock T. Drug-induced myopathies. Curr Opin Neurol 2008; 21:590–595.
- Dimachkie MM, Barohn RJ. Idiopathic inflammatory myopathies. Front Neurol Neurosci 2009; 26:126–146.
- Joseph A, Brasington R, Kahl L, Ranganathan P, Cheng TP, Atkinson J. Immunologic rheumatic disorders. J Allergy Clin Immunol 2010; 125(suppl 2):S204–S215.
- Joy TR, Hegele RA. Narrative review: statin-related myopathy. Ann Intern Med 2009; 150:858–868.
- Kiernan TJ, Rochford M, McDermott JH. Simvastatin induced rhabdomyolysis and an important clinical link with hypothyroidism. Int J Cardiol 2007; 119:374–376.
- Thompson PD, Clarkson P, Karas RH. Statin-associated myopathy. JAMA 2003; 289:1681–1690.
- Spence JD, Munoz CE, Hendricks L, Latchinian L, Khouri HE. Pharmacokinetics of the combination of fluvastatin and gemfibrozil. Am J Cardiol 1995; 76:80A–83A.
- Boscaro M, Arnaldi G. Approach to the patient with possible Cushing’s syndrome. J Clin Endocrinol Metab 2009; 94:3121–3131.
- Ilias I, Torpy DJ, Pacak K, Mullen N, Wesley RA, Nieman LK. Cushing’s syndrome due to ectopic corticotropin secretion: twenty years’ experience at the National Institutes of Health. J Clin Endocrinol Metab 2005; 90:4955–4962.
- Pecori Giraldi F. Recent challenges in the diagnosis of Cushing’s syndrome. Horm Res 2009; 71(suppl 1):123–127.
- von Mach MA, Kann P, Piepkorn B, Bruder S, Beyer J. [Cushing’s syndrome caused by paraneoplastic ACTH secretion 11 years after occurrence of a medullary thyroid carcinoma]. Dtsch Med Wochenschr 2002; 127:850–852.
- Beauregard C, Dickstein G, Lacroix A. Classic and recent etiologies of Cushing’s syndrome: diagnosis and therapy. Treat Endocrinol 2002; 1:79–94.
- Liddle GW. Tests of pituitary-adrenal suppressibility in the diagnosis of Cushing’s syndrome. J Clin Endocrinol Metab 1960; 20:1539–1560.
- Louiset E, Gobet F, Libé R, et al. ACTH-independent Cushing’s syndrome with bilateral micronodular adrenal hyperplasia and ectopic adrenocortical adenoma. J Clin Endocrinol Metab 2010; 95:18–24.
- Andrioli M, Pecori Giraldi F, De Martin M, Cattaneo A, Carzaniga C, Cavagnini F. Differential diagnosis of ACTH-dependent hypercortisolism: imaging versus laboratory. Pituitary 2009; 12:294–296.
- Sahdev A, Reznek RH, Evanson J, Grossman AB. Imaging in Cushing’s syndrome. Arq Bras Endocrinol Metabol 2007; 51:1319–1328.
- Arnaldi G, Angeli A, Atkinson AB, et al. Diagnosis and complications of Cushing’s syndrome: a consensus statement. J Clin Endocrinol Metab 2003; 88:5593–5602.
- Lad SP, Patil CG, Laws ER, Katznelson L. The role of inferior petrosal sinus sampling in the diagnostic localization of Cushing’s disease. Neurosurg Focus 2007; 23:E2.
- Bhansali A, Walia R, Rana SS, et al. Ectopic Cushing’s syndrome: experience from a tertiary care centre. Indian J Med Res 2009; 129:33–41.
- Arlt A, Harbeck B, Anlauf M, et al. Fatal Pneumocystis jirovecii pneumonia in a case of ectopic Cushing’s syndrome due to neuroendocrine carcinoma of the kidney. Exp Clin Endocrinol Diabetes 2008; 116:515–519.
- Graham BS, Tucker WS. Opportunistic infections in endogenous Cushing’s syndrome. Ann Intern Med 1984; 101:334–338.
- Kronfol Z, Starkman M, Schteingart DE, Singh V, Zhang Q, Hill E. Immune regulation in Cushing’s syndrome: relationship to hypothalamic-pituitary-adrenal axis hormones. Psychoneuroendocrinology 1996; 21:599–608.
- Sepkowitz KA. Opportunistic infections in patients with and patients without acquired immunodeficiency syndrome. Clin Infect Dis 2002; 34:1098–1107.
- Schteingart DE. Drugs in the medical treatment of Cushing’s syndrome. Expert Opin Emerg Drugs 2009; 14:661–671.
- Shalet S, Mukherjee A. Pharmacological treatment of hypercortisolism. Curr Opin Endocrinol Diabetes Obes 2008; 15:234–238.
- Arnaldi G, Boscaro M. Pasireotide for the treatment of Cushing’s disease. Expert Opin Investig Drugs 2010; 19:889–898.
Our patient, a 56-year-old woman, presents with proximal muscle weakness in all four limbs. It started a few months ago and has gradually become severe, so that she now has difficulty rising from a seated position and has trouble opening jars. She has fallen several times. She says she has no muscle pain, difficulty swallowing, or difficulty breathing.
She sought medical attention at another hospital and was found to be hypothyroid, with a thyrotropin (thyroid-stimulating hormone [TSH]) level of 38 μU/mL (reference range 0.4–5.5), for which she was started on levothyroxine (Synthroid) 100 μg daily. She also had a low serum potassium level, for which potassium supplements and spironolactone (Aldactone) were started. She was taking furosemide (Lasix) 20 mg/day at the time.
Despite the thyroid replacement therapy, she continued to become weaker and had more falls. She also noticed a new, nonpainful rash on her lower abdomen.
Review of systems
- Night sweats
- Leg swelling
- Puffiness and discoloration around the eyes, with easy bruisability.
Medical history
- Diabetes mellitus
- Seizures in the 1970s
- Resection of a thymic tumor in 2003 (the exact pathology is unknown)
- Cirrhosis of unknown etiology
- No known history of hypertension
- No history of alcohol or intravenous drug use
- Quit smoking many years ago
- Coronary artery bypass surgery in 2003
- One sibling with myasthenia gravis.
Medications
- Levothyroxine
- Rosuvastatin (Crestor)
- Omeprazole (Prilosec)
- Spironolactone
- Furosemide
- Potassium chloride
- Metoprolol tartrate (Lopressor)
- Metformin (Glucophage)
- Ramipril (Altace).
Physical examination
She is hemodynamically stable and is not hypertensive. Her thyroid is not enlarged. Her lungs are clear to auscultation. Her heart sounds are normal, except for a nonradiating pansystolic murmur most audible at the apex.
Her abdomen is soft and is not distended. Her abdominal rash has a dermatomal distribution consistent with an L1 distribution, with vesicles over an erythematous base. Purpuric lesions are noted over her lower extremities.
Her leg strength is 3 on a scale of 5 on both sides; her arm strength is normal. Ankle and knee reflexes are absent bilaterally.
Initial laboratory analysis
PROGRESSIVE MUSCLE WEAKNESS
1. What are possible causes of her muscle weakness?
- Myasthenia gravis
- Hypothyroidism
- Dermatomyositis-polymyositis
- Drug-induced myopathy
- Cushing syndrome
- All of the above
All of these are potential causes of muscle weakness.
Myasthenia gravis
Myasthenia gravis, an autoimmune disease, can affect people of all ages and either sex. It presents with muscle weakness and fatigability, which characteristically fluctuate during the day. Some patients present in crisis with respiratory failure, which may require ventilatory support.1,2
Myasthenia gravis is characterized by auto-antibodies against the postsynaptic membrane of the neuromuscular junction. Most patients have antibodies to the extracellular portion of the acetylcholine receptor; a small number of patients have antibodies against a muscle-specific tyrosine kinase that interacts with this receptor.
About 15% of patients with myasthenia gravis have a thymoma thought to be involved in the pathogenesis of the disease. Treatments include immune suppressive therapy and thymectomy.
Our patient has a history of thymic lesion resection, but her antibody workup for myasthenia gravis was negative.
Hypothyroidism
Hypothyroidism, the most common disorder of the thyroid gland, is especially prevalent in women.3 Its common symptoms include fatigue, exercise intolerance, muscle weakness, cramps, and stiffness.
Both the TSH and the free thyroxine (T4) level must be measured to diagnose hypothyroidism. This information can also help differentiate primary hypothyroidism (ie, due to a defect in the thyroid gland) from secondary hypothyroidism (ie, due to a defect in the pituitary gland). Elevated TSH with low free T4 levels indicates primary thyroid failure, whereas the combination of a normal or low TSH and a low free T4 usually indicates pituitary failure. Subclinical hypothyroidism is characterized by mildly to moderately elevated TSH, but total T4 and free T4 values are still within the reference range. Replacement therapy is with levothyroxine.3–6
Our patient has a history of hypothyroidism, which could explain her muscle weakness, but she is currently on replacement therapy, and her TSH level on admission was normal.
Dermatomyositis-polymyositis
Dermatomyositis-polymyositis is characterized by proximal muscle weakness, creatine kinase elevation, erythema on sun-exposed skin, heliotrope rash, and Gottron papules. It occurs mostly in women after the second decade of life. Some medications have been implicated in its pathogenesis, such as statins, fibrates, hydroxyurea, penicillamine, and omeprazole (Prilosec).7
In a middle-aged patient, this diagnosis should prompt a search for cancer, especially of the gastrointestinal system, breast, and lung.8 Cancer can arise up to 3 years after the diagnosis of dermatomyositis or polymyositis.
Antisynthetase antibody syndrome is suspected if the patient is positive for antisynthetase antibody and has the following manifestations: acute onset of disease, constitutional symptoms, interstitial lung disease, inflammatory arthritis, mechanic’s hands (thickened, cracked skin on the palmar aspect of the thumb and index finger), and Raynaud phenomenon.4,8,9
The diagnosis is made by a thorough clinical evaluation. Electromyography can show an inflammatory pattern of myopathy. The gold standard test for this diagnosis is muscle biopsy.
Our patient has a normal creatine kinase level, which excludes the diagnosis of dermatomyositis-polymyositis.
Statin-induced myopathy
Up to 10% of patients taking statins develop myalgia. Rhabdomyolysis, the extreme form of myopathy, is rare.
The exact mechanism of statin-induced myopathy remains unclear; mitochondrial dysfunction, cholesterol composition of cell membranes, and coenzyme Q10 deficiency have been proposed.
Risk factors for statin-induced myopathy include female sex, older age, higher doses of statins, a family history of statin-induced myopathy, and hypothyroidism. Drugs that increase the risk include fibric acid derivatives, macrolides, and amiodarone (Cordarone). If a statin and any of the above drugs are both required, certain statins—ie, pravastatin (Pravachol) and rosuvastatin—are recommended, since they are the statins least likely to cause rhabdomyolysis.5,7,10–12
The combination of fluvastatin (Lescol) and gemfibrozil (Lopid) has also been found to be safe.13 In a crossover study in 17 patients, no significant difference was seen in the area under the curve for plasma concentration over time, in the maximum plasma concentration, or in the time to maximum concentration with the combination vs with each drug alone.13
Our patient is taking a statin and has hypothyroidism, which increases the risk of statin-induced myopathy. However, her creatine kinase level is normal.
Cushing syndrome
Cushing syndrome (hypercortisolism) is one of the most challenging endocrine diseases to diagnose. Most of its clinical features overlap with those of common diseases, and some patients have an atypical clinical presentation with only isolated symptoms. Further, its presentation can be subtle, with weight gain, amenorrhea, muscle weakness, and easy bruisability. Acne, moon facies, plethora, abdominal striae, and purpura are other common signs. It is three to 10 times more common in women than in men.
Cushing syndrome can be classified according to whether or not the excess cortisol secretion depends on corticotropin (formerly called adrenocorticotropic hormone or ACTH) (Figure 1). In corticotropin-dependent cases, the most common cause is pituitary adenoma. (When Cushing syndrome is due to excessive pituitary secretion of corticotropin, which in turn stimulates the adrenal glands to secrete excessive amounts of cortisol, it is called Cushing disease). Other causes of corticotropin-dependent Cushing syndrome are ectopic corticotropin-producing tumors such as carcinoid tumors or medullary thyroid cancers. Corticotropin-independent Cushing syndrome can be caused by adrenal adenomas, adrenal carcinoma, and bilateral primary micronodular or macronodular adrenocortical hyperplasia.14–17
However, the most common cause of Cushing syndrome is glucocorticoid therapy.
BACK TO OUR PATIENT: HER CONDITION DETERIORATES
Our patient’s physical condition deteriorates, she develops respiratory distress, and she is admitted to the medical intensive care unit. Her mental status also deteriorates, and she becomes lethargic and unresponsive.
She is intubated to protect her airway. After this, she develops hypotension that does not respond to fluid resuscitation and that requires vasopressors. Her condition continues to worsen as she develops acute kidney injury and disseminated intravascular coagulation. Her vesicular rash becomes more widespread, involving the entire trunk.
A workup for sepsis is initiated, but her initial blood and urine cultures are negative. Chest radiography does not reveal any infiltrates. No other source of an infection is found.
Varicella zoster is isolated on viral culture of a specimen obtained from the rash, and a polymerase chain reaction test of her blood shows cytomegalovirus DNA (64,092 copies per mL). Immune suppression is suspected, so a CD4 count is ordered (Table 2). Serologic tests for human immunodeficiency virus are negative.
What could have caused our patient to have muscle weakness in addition to disseminated zoster with cytomegalovirus viremia?
The diagnosis here is Cushing syndrome.
HOW TO TEST FOR CUSHING SYNDROME
2. In any practice, you may meet many perimenopausal women who have complaints of weight gain, amenorrhea, and acne. How can you determine if this is Cushing syndrome? What are the screening tests?
- 24-Hour urinary cortisol excretion
- A late-night salivary cortisol level
- A low-dose dexamethasone suppression test
- All of the above
- None of the above
Any of the tests listed here can be used to determine whether this is truly Cushing syndrome.
24-Hour urinary cortisol excretion has a reference range of 20 to 100 μg/24 hours. However, results may be falsely high in patients who are depressed or who abuse alcohol.
The late-night salivary cortisol level is another useful test.14,16,18 Patients with Cushing syndrome are found to have high late-night salivary cortisol levels as compared with normal people, indicating the loss of natural circadian rhythm.14,16,18
The low-dose dexamethasone suppression test, as first described by Liddle in 1960,19 involved giving dexamethasone 0.5 mg by mouth every 6 hours for 48 hours and measuring the serum cortisol level 6 hours after the last dose. In healthy people, this low dose of dexamethasone suppresses the production of corticotropin by the pituitary gland and in turn the production of cortisol, but in patients with Cushing syndrome the cortisol level remains high. An alternative is the overnight 1-mg dexamethasone suppression test—ie, giving 1 mg of dexamethasone at 11:00 pm and measuring the serum cortisol level early the next morning. Failure of the cortisol level to drop to less than 1.8 μg/dL suggests Cushing syndrome and warrants a complete evaluation for it.
Confirmatory testing is sometimes needed if patients have mild abnormalities in their screening tests. A combination low-dose dexamethasone suppression test and corticotropin-releasing hormone test can be used to differentiate Cushing syndrome from pseudo-Cushing syndrome. This is performed by giving dexamethasone orally 0.5 mg every 6 hours for 48 hours and then giving ovine-sequence corticotropin-releasing hormone 1 μg/kg intravenously 2 hours after the last dose of dexamethasone. The plasma cortisol value 15 minutes after the dose of corticotropin-releasing hormone is greater than 1.4 μg/dL (38 nmol/L) in patients with Cushing syndrome but remains low in patients with pseudo-Cushing syndrome.
Is this corticotropin-dependent or corticotropin-independent?
Once Cushing syndrome is diagnosed by one of the screening methods described above, the source of the excess glucocorticoids needs to be determined. Measuring the serum corticotropin level early in the morning would be the next step.
A low corticotropin level (< 10 pg/mL) indicates a corticotropin-independent source, most likely in the adrenal glands. Hence, computed tomography or magnetic resonance imaging (MRI) of the adrenal glands is warranted. Of note: adrenal incidentalomas are quite common, present in 5% of the general population, and a lesion on the adrenal gland does not prove that the patient has primary adrenal disease.16,20
IS THE EXCESS CORTICOTROPIN FROM A PITUITARY OR AN ECTOPIC SOURCE?
3. If the corticotropin level is elevated, how can you determine if it is from the pituitary or from an ectopic source?
- MRI of the pituitary gland
- High-dose dexamethasone suppression test
- Corticotropin-releasing hormone stimulation test
- Bilateral inferior petrosal sinus sampling
If the corticotropin level is high (> 10 pg/mL), it is of paramount importance to determine whether the corticotropin comes from the pituitary gland or from an ectopic source.
MRI of the pituitary gland should be done in patients with suspected corticotropin-dependent Cushing syndrome. However, MRI may be negative in 50% of patients with Cushing disease, and it should therefore not be used for screening. In addition, 10% of the population may have pituitary incidentalomas on MRI.
Most cases of corticotropin-dependent Cushing syndrome are caused by microadenomas (smaller than 1 cm), while a few cases are caused by macroadenomas (larger than 1 cm). If a microadenoma is found on MRI, further testing with bilateral inferior petrosal sinus sampling is recommended (described below); if a macroadenoma is found, then no further testing is required.21,22 In fact, patients who have biochemical findings compatible with Cushing disease (ie, due to an overactive pituitary) and who have an adenoma larger than 6 mm do not require further evaluation.23
A high-dose dexamethasone suppression test involves giving 8 mg of dexamethasone in the evening and measuring the cortisol level the next morning. If the cortisol level declines to 50% of the baseline level after this dose, this suggests a pituitary cause.
Corticotropin-releasing hormone stimulation testing. In most cases of pituitary tumors and a few cases of ectopic corticotropin-secreting tumors, giving corticotropin-releasing hormone leads to an increase in serum corticotropin and cortisol levels. In contrast, these levels do not respond to corticotropin-releasing hormone stimulation if the problem is in the adrenal gland. The test is performed by giving 1 μg/kg or 100 μg synthetic or human corticotropin-releasing hormone. A 35% to 50% increase above baseline in corticotropin suggests a pituitary cause.23
Bilateral inferior petrosal sinus sampling can be used to confirm a pituitary source, as it is the gold standard for differentiating ectopic from pituitary corticotropin production. Once this is confirmed, a neurosurgical consult is warranted.16,18
This procedure is usually done by advancing a sheath from the femoral vein to reach the inferior petrosal sinuses. Blood samples are obtained from both the inferior petrosal sinuses and from a peripheral vein to measure corticotropin levels before and after giving corticotropin-releasing hormone (1 μg/kg). Before corticotropin-releasing hormone is given, a gradient of central-peripheral corticotropin levels of 2.0 or greater indicates a pituitary source. With ectopic corticotropin production, the corticotropin gradient is usually less than 1.5. Corticotropin-releasing hormone is given to increase the sensitivity: after it is given, a gradient of 3.0 or greater is considered indicative of Cushing disease.24
If the corticotropin level is elevated and the above tests indicate ectopic production, the source should be sought. The most common site of ectopic corticotropin production is the chest. Common causes are bronchial, thymic, and pancreatic carcinoid tumors. Other causes are small-cell lung cancer, medullary cell cancer, and pheochromocytoma.15,18,25
BACK TO OUR PATIENT
Our patient’s further laboratory results are listed in Table 3.
She has elevated 24-hour urinary cortisol excretion, consistent with Cushing syndrome. Her corticotropin level is elevated, which rules out an adrenal cause. Her 5-HIAA (a serotonin breakdown product) and calcitonin levels are also elevated, suggesting either medullary thyroid cancer or a carcinoid tumor. She also has a mild elevation of dehydroepiandrosterone sulfate, which is consistent with corticotropin-dependent Cushing syndrome.
Our patient’s elevated levels of cortisol were the cause of her muscle weakness and severe immune deficiency, which in turn led to cytomegalovirus viremia and sepsis. Cushing syndrome usually causes hypertension, especially in cases of ectopic corticotropin production. However, our patient was normotensive on admission and then developed cytomegalovirus sepsis, which led to hypotension and shock.
Immune suppression is a well-known effect of glucocorticoids.26–28 Kronfol et al28 found that CD4 and CD8 counts and the CD4-to-CD8 ratio were low in patients with Cushing syndrome, and natural killer cell activity was suppressed. Opportunistic infections have been described in patients with Cushing syndrome.26,27,29
MANAGEMENT OF CUSHING SYNDROME
Management of Cushing syndrome should be tailored after determining its source.
A neurosurgical consultation is warranted in cases of pituitary adenoma, with surgical resection of the adrenal source or ectopic tumor if feasible.25
Medical management is recommended if surgical resection is not possible.30,31 Several drugs can be used to inhibit cortisol synthesis in this situation.30,32
Adrenal-acting agents
Aminoglutethimide (Cytadren) acts by blocking the conversion of cholesterol to pregnenolone, a precursor of cortisol. The dosage is 250 mg twice or three times a day. This drug is no longer available in the United States.
Ketoconazole (Nizoral) inhibits side-chain cleavage, 11-beta hydroxylase, and 17-alpha hydroxylase, thus inhibiting cortisol synthesis; it also inhibits corticotropin secretion. The dosage is 200 to 400 mg three times a day.
Metyrapone (Metopirone) blocks 11-beta-hydroxylation of deoxycortisol, the reaction that produces cortisol. The dosage is 500 to 750 mg three times a day. This drug can be obtained only from the manufacturer and only on a named-patient basis.
Etomidate (Amidate), an anesthetic drug, also blocks 11-beta-hydroxylation of deoxycortisol. It is given intravenously at a rate of 0.3 mg/kg per hour.
Centrally acting agents
Cabergoline (Dostinex). It is believed that corticotropin-producing pituitary tumors express D2 receptors. Cabergoline is a dopamine agonist that has been used in patients with Cushing disease. The dosage is 0.5 to 7 mg/week.
Pasireotide is still investigational. It is a somatostatin receptor agonist given subcutaneously for 15 consecutive days to patients with Cushing disease.
Glucocorticoid receptor antagonist
Mifepristone (Mifeprex) is a progesterone receptor and glucocorticoid II receptor antagonist that is being investigated in the treatment of persistent or recurrent Cushing disease. It is not yet approved by the US Food and Drug Administration for this indication.
BACK TO OUR PATIENT
The patient was too ill to undergo additional imaging, including octreotide scanning to identify an ectopic corticotropin-secreting tumor. She was medically treated with intravenous etomidate to reduce her cortisol level.30,31
Unfortunately, our patient died of multiorgan failure. The exact site of her ectopic corticotropin-producing tumor was never identified, and no autopsy was done.
Our patient, a 56-year-old woman, presents with proximal muscle weakness in all four limbs. It started a few months ago and has gradually become severe, so that she now has difficulty rising from a seated position and has trouble opening jars. She has fallen several times. She says she has no muscle pain, difficulty swallowing, or difficulty breathing.
She sought medical attention at another hospital and was found to be hypothyroid, with a thyrotropin (thyroid-stimulating hormone [TSH]) level of 38 μU/mL (reference range 0.4–5.5), for which she was started on levothyroxine (Synthroid) 100 μg daily. She also had a low serum potassium level, for which potassium supplements and spironolactone (Aldactone) were started. She was taking furosemide (Lasix) 20 mg/day at the time.
Despite the thyroid replacement therapy, she continued to become weaker and had more falls. She also noticed a new, nonpainful rash on her lower abdomen.
Review of systems
- Night sweats
- Leg swelling
- Puffiness and discoloration around the eyes, with easy bruisability.
Medical history
- Diabetes mellitus
- Seizures in the 1970s
- Resection of a thymic tumor in 2003 (the exact pathology is unknown)
- Cirrhosis of unknown etiology
- No known history of hypertension
- No history of alcohol or intravenous drug use
- Quit smoking many years ago
- Coronary artery bypass surgery in 2003
- One sibling with myasthenia gravis.
Medications
- Levothyroxine
- Rosuvastatin (Crestor)
- Omeprazole (Prilosec)
- Spironolactone
- Furosemide
- Potassium chloride
- Metoprolol tartrate (Lopressor)
- Metformin (Glucophage)
- Ramipril (Altace).
Physical examination
She is hemodynamically stable and is not hypertensive. Her thyroid is not enlarged. Her lungs are clear to auscultation. Her heart sounds are normal, except for a nonradiating pansystolic murmur most audible at the apex.
Her abdomen is soft and is not distended. Her abdominal rash has a dermatomal distribution consistent with an L1 distribution, with vesicles over an erythematous base. Purpuric lesions are noted over her lower extremities.
Her leg strength is 3 on a scale of 5 on both sides; her arm strength is normal. Ankle and knee reflexes are absent bilaterally.
Initial laboratory analysis
PROGRESSIVE MUSCLE WEAKNESS
1. What are possible causes of her muscle weakness?
- Myasthenia gravis
- Hypothyroidism
- Dermatomyositis-polymyositis
- Drug-induced myopathy
- Cushing syndrome
- All of the above
All of these are potential causes of muscle weakness.
Myasthenia gravis
Myasthenia gravis, an autoimmune disease, can affect people of all ages and either sex. It presents with muscle weakness and fatigability, which characteristically fluctuate during the day. Some patients present in crisis with respiratory failure, which may require ventilatory support.1,2
Myasthenia gravis is characterized by auto-antibodies against the postsynaptic membrane of the neuromuscular junction. Most patients have antibodies to the extracellular portion of the acetylcholine receptor; a small number of patients have antibodies against a muscle-specific tyrosine kinase that interacts with this receptor.
About 15% of patients with myasthenia gravis have a thymoma thought to be involved in the pathogenesis of the disease. Treatments include immune suppressive therapy and thymectomy.
Our patient has a history of thymic lesion resection, but her antibody workup for myasthenia gravis was negative.
Hypothyroidism
Hypothyroidism, the most common disorder of the thyroid gland, is especially prevalent in women.3 Its common symptoms include fatigue, exercise intolerance, muscle weakness, cramps, and stiffness.
Both the TSH and the free thyroxine (T4) level must be measured to diagnose hypothyroidism. This information can also help differentiate primary hypothyroidism (ie, due to a defect in the thyroid gland) from secondary hypothyroidism (ie, due to a defect in the pituitary gland). Elevated TSH with low free T4 levels indicates primary thyroid failure, whereas the combination of a normal or low TSH and a low free T4 usually indicates pituitary failure. Subclinical hypothyroidism is characterized by mildly to moderately elevated TSH, but total T4 and free T4 values are still within the reference range. Replacement therapy is with levothyroxine.3–6
Our patient has a history of hypothyroidism, which could explain her muscle weakness, but she is currently on replacement therapy, and her TSH level on admission was normal.
Dermatomyositis-polymyositis
Dermatomyositis-polymyositis is characterized by proximal muscle weakness, creatine kinase elevation, erythema on sun-exposed skin, heliotrope rash, and Gottron papules. It occurs mostly in women after the second decade of life. Some medications have been implicated in its pathogenesis, such as statins, fibrates, hydroxyurea, penicillamine, and omeprazole (Prilosec).7
In a middle-aged patient, this diagnosis should prompt a search for cancer, especially of the gastrointestinal system, breast, and lung.8 Cancer can arise up to 3 years after the diagnosis of dermatomyositis or polymyositis.
Antisynthetase antibody syndrome is suspected if the patient is positive for antisynthetase antibody and has the following manifestations: acute onset of disease, constitutional symptoms, interstitial lung disease, inflammatory arthritis, mechanic’s hands (thickened, cracked skin on the palmar aspect of the thumb and index finger), and Raynaud phenomenon.4,8,9
The diagnosis is made by a thorough clinical evaluation. Electromyography can show an inflammatory pattern of myopathy. The gold standard test for this diagnosis is muscle biopsy.
Our patient has a normal creatine kinase level, which excludes the diagnosis of dermatomyositis-polymyositis.
Statin-induced myopathy
Up to 10% of patients taking statins develop myalgia. Rhabdomyolysis, the extreme form of myopathy, is rare.
The exact mechanism of statin-induced myopathy remains unclear; mitochondrial dysfunction, cholesterol composition of cell membranes, and coenzyme Q10 deficiency have been proposed.
Risk factors for statin-induced myopathy include female sex, older age, higher doses of statins, a family history of statin-induced myopathy, and hypothyroidism. Drugs that increase the risk include fibric acid derivatives, macrolides, and amiodarone (Cordarone). If a statin and any of the above drugs are both required, certain statins—ie, pravastatin (Pravachol) and rosuvastatin—are recommended, since they are the statins least likely to cause rhabdomyolysis.5,7,10–12
The combination of fluvastatin (Lescol) and gemfibrozil (Lopid) has also been found to be safe.13 In a crossover study in 17 patients, no significant difference was seen in the area under the curve for plasma concentration over time, in the maximum plasma concentration, or in the time to maximum concentration with the combination vs with each drug alone.13
Our patient is taking a statin and has hypothyroidism, which increases the risk of statin-induced myopathy. However, her creatine kinase level is normal.
Cushing syndrome
Cushing syndrome (hypercortisolism) is one of the most challenging endocrine diseases to diagnose. Most of its clinical features overlap with those of common diseases, and some patients have an atypical clinical presentation with only isolated symptoms. Further, its presentation can be subtle, with weight gain, amenorrhea, muscle weakness, and easy bruisability. Acne, moon facies, plethora, abdominal striae, and purpura are other common signs. It is three to 10 times more common in women than in men.
Cushing syndrome can be classified according to whether or not the excess cortisol secretion depends on corticotropin (formerly called adrenocorticotropic hormone or ACTH) (Figure 1). In corticotropin-dependent cases, the most common cause is pituitary adenoma. (When Cushing syndrome is due to excessive pituitary secretion of corticotropin, which in turn stimulates the adrenal glands to secrete excessive amounts of cortisol, it is called Cushing disease). Other causes of corticotropin-dependent Cushing syndrome are ectopic corticotropin-producing tumors such as carcinoid tumors or medullary thyroid cancers. Corticotropin-independent Cushing syndrome can be caused by adrenal adenomas, adrenal carcinoma, and bilateral primary micronodular or macronodular adrenocortical hyperplasia.14–17
However, the most common cause of Cushing syndrome is glucocorticoid therapy.
BACK TO OUR PATIENT: HER CONDITION DETERIORATES
Our patient’s physical condition deteriorates, she develops respiratory distress, and she is admitted to the medical intensive care unit. Her mental status also deteriorates, and she becomes lethargic and unresponsive.
She is intubated to protect her airway. After this, she develops hypotension that does not respond to fluid resuscitation and that requires vasopressors. Her condition continues to worsen as she develops acute kidney injury and disseminated intravascular coagulation. Her vesicular rash becomes more widespread, involving the entire trunk.
A workup for sepsis is initiated, but her initial blood and urine cultures are negative. Chest radiography does not reveal any infiltrates. No other source of an infection is found.
Varicella zoster is isolated on viral culture of a specimen obtained from the rash, and a polymerase chain reaction test of her blood shows cytomegalovirus DNA (64,092 copies per mL). Immune suppression is suspected, so a CD4 count is ordered (Table 2). Serologic tests for human immunodeficiency virus are negative.
What could have caused our patient to have muscle weakness in addition to disseminated zoster with cytomegalovirus viremia?
The diagnosis here is Cushing syndrome.
HOW TO TEST FOR CUSHING SYNDROME
2. In any practice, you may meet many perimenopausal women who have complaints of weight gain, amenorrhea, and acne. How can you determine if this is Cushing syndrome? What are the screening tests?
- 24-Hour urinary cortisol excretion
- A late-night salivary cortisol level
- A low-dose dexamethasone suppression test
- All of the above
- None of the above
Any of the tests listed here can be used to determine whether this is truly Cushing syndrome.
24-Hour urinary cortisol excretion has a reference range of 20 to 100 μg/24 hours. However, results may be falsely high in patients who are depressed or who abuse alcohol.
The late-night salivary cortisol level is another useful test.14,16,18 Patients with Cushing syndrome are found to have high late-night salivary cortisol levels as compared with normal people, indicating the loss of natural circadian rhythm.14,16,18
The low-dose dexamethasone suppression test, as first described by Liddle in 1960,19 involved giving dexamethasone 0.5 mg by mouth every 6 hours for 48 hours and measuring the serum cortisol level 6 hours after the last dose. In healthy people, this low dose of dexamethasone suppresses the production of corticotropin by the pituitary gland and in turn the production of cortisol, but in patients with Cushing syndrome the cortisol level remains high. An alternative is the overnight 1-mg dexamethasone suppression test—ie, giving 1 mg of dexamethasone at 11:00 pm and measuring the serum cortisol level early the next morning. Failure of the cortisol level to drop to less than 1.8 μg/dL suggests Cushing syndrome and warrants a complete evaluation for it.
Confirmatory testing is sometimes needed if patients have mild abnormalities in their screening tests. A combination low-dose dexamethasone suppression test and corticotropin-releasing hormone test can be used to differentiate Cushing syndrome from pseudo-Cushing syndrome. This is performed by giving dexamethasone orally 0.5 mg every 6 hours for 48 hours and then giving ovine-sequence corticotropin-releasing hormone 1 μg/kg intravenously 2 hours after the last dose of dexamethasone. The plasma cortisol value 15 minutes after the dose of corticotropin-releasing hormone is greater than 1.4 μg/dL (38 nmol/L) in patients with Cushing syndrome but remains low in patients with pseudo-Cushing syndrome.
Is this corticotropin-dependent or corticotropin-independent?
Once Cushing syndrome is diagnosed by one of the screening methods described above, the source of the excess glucocorticoids needs to be determined. Measuring the serum corticotropin level early in the morning would be the next step.
A low corticotropin level (< 10 pg/mL) indicates a corticotropin-independent source, most likely in the adrenal glands. Hence, computed tomography or magnetic resonance imaging (MRI) of the adrenal glands is warranted. Of note: adrenal incidentalomas are quite common, present in 5% of the general population, and a lesion on the adrenal gland does not prove that the patient has primary adrenal disease.16,20
IS THE EXCESS CORTICOTROPIN FROM A PITUITARY OR AN ECTOPIC SOURCE?
3. If the corticotropin level is elevated, how can you determine if it is from the pituitary or from an ectopic source?
- MRI of the pituitary gland
- High-dose dexamethasone suppression test
- Corticotropin-releasing hormone stimulation test
- Bilateral inferior petrosal sinus sampling
If the corticotropin level is high (> 10 pg/mL), it is of paramount importance to determine whether the corticotropin comes from the pituitary gland or from an ectopic source.
MRI of the pituitary gland should be done in patients with suspected corticotropin-dependent Cushing syndrome. However, MRI may be negative in 50% of patients with Cushing disease, and it should therefore not be used for screening. In addition, 10% of the population may have pituitary incidentalomas on MRI.
Most cases of corticotropin-dependent Cushing syndrome are caused by microadenomas (smaller than 1 cm), while a few cases are caused by macroadenomas (larger than 1 cm). If a microadenoma is found on MRI, further testing with bilateral inferior petrosal sinus sampling is recommended (described below); if a macroadenoma is found, then no further testing is required.21,22 In fact, patients who have biochemical findings compatible with Cushing disease (ie, due to an overactive pituitary) and who have an adenoma larger than 6 mm do not require further evaluation.23
A high-dose dexamethasone suppression test involves giving 8 mg of dexamethasone in the evening and measuring the cortisol level the next morning. If the cortisol level declines to 50% of the baseline level after this dose, this suggests a pituitary cause.
Corticotropin-releasing hormone stimulation testing. In most cases of pituitary tumors and a few cases of ectopic corticotropin-secreting tumors, giving corticotropin-releasing hormone leads to an increase in serum corticotropin and cortisol levels. In contrast, these levels do not respond to corticotropin-releasing hormone stimulation if the problem is in the adrenal gland. The test is performed by giving 1 μg/kg or 100 μg synthetic or human corticotropin-releasing hormone. A 35% to 50% increase above baseline in corticotropin suggests a pituitary cause.23
Bilateral inferior petrosal sinus sampling can be used to confirm a pituitary source, as it is the gold standard for differentiating ectopic from pituitary corticotropin production. Once this is confirmed, a neurosurgical consult is warranted.16,18
This procedure is usually done by advancing a sheath from the femoral vein to reach the inferior petrosal sinuses. Blood samples are obtained from both the inferior petrosal sinuses and from a peripheral vein to measure corticotropin levels before and after giving corticotropin-releasing hormone (1 μg/kg). Before corticotropin-releasing hormone is given, a gradient of central-peripheral corticotropin levels of 2.0 or greater indicates a pituitary source. With ectopic corticotropin production, the corticotropin gradient is usually less than 1.5. Corticotropin-releasing hormone is given to increase the sensitivity: after it is given, a gradient of 3.0 or greater is considered indicative of Cushing disease.24
If the corticotropin level is elevated and the above tests indicate ectopic production, the source should be sought. The most common site of ectopic corticotropin production is the chest. Common causes are bronchial, thymic, and pancreatic carcinoid tumors. Other causes are small-cell lung cancer, medullary cell cancer, and pheochromocytoma.15,18,25
BACK TO OUR PATIENT
Our patient’s further laboratory results are listed in Table 3.
She has elevated 24-hour urinary cortisol excretion, consistent with Cushing syndrome. Her corticotropin level is elevated, which rules out an adrenal cause. Her 5-HIAA (a serotonin breakdown product) and calcitonin levels are also elevated, suggesting either medullary thyroid cancer or a carcinoid tumor. She also has a mild elevation of dehydroepiandrosterone sulfate, which is consistent with corticotropin-dependent Cushing syndrome.
Our patient’s elevated levels of cortisol were the cause of her muscle weakness and severe immune deficiency, which in turn led to cytomegalovirus viremia and sepsis. Cushing syndrome usually causes hypertension, especially in cases of ectopic corticotropin production. However, our patient was normotensive on admission and then developed cytomegalovirus sepsis, which led to hypotension and shock.
Immune suppression is a well-known effect of glucocorticoids.26–28 Kronfol et al28 found that CD4 and CD8 counts and the CD4-to-CD8 ratio were low in patients with Cushing syndrome, and natural killer cell activity was suppressed. Opportunistic infections have been described in patients with Cushing syndrome.26,27,29
MANAGEMENT OF CUSHING SYNDROME
Management of Cushing syndrome should be tailored after determining its source.
A neurosurgical consultation is warranted in cases of pituitary adenoma, with surgical resection of the adrenal source or ectopic tumor if feasible.25
Medical management is recommended if surgical resection is not possible.30,31 Several drugs can be used to inhibit cortisol synthesis in this situation.30,32
Adrenal-acting agents
Aminoglutethimide (Cytadren) acts by blocking the conversion of cholesterol to pregnenolone, a precursor of cortisol. The dosage is 250 mg twice or three times a day. This drug is no longer available in the United States.
Ketoconazole (Nizoral) inhibits side-chain cleavage, 11-beta hydroxylase, and 17-alpha hydroxylase, thus inhibiting cortisol synthesis; it also inhibits corticotropin secretion. The dosage is 200 to 400 mg three times a day.
Metyrapone (Metopirone) blocks 11-beta-hydroxylation of deoxycortisol, the reaction that produces cortisol. The dosage is 500 to 750 mg three times a day. This drug can be obtained only from the manufacturer and only on a named-patient basis.
Etomidate (Amidate), an anesthetic drug, also blocks 11-beta-hydroxylation of deoxycortisol. It is given intravenously at a rate of 0.3 mg/kg per hour.
Centrally acting agents
Cabergoline (Dostinex). It is believed that corticotropin-producing pituitary tumors express D2 receptors. Cabergoline is a dopamine agonist that has been used in patients with Cushing disease. The dosage is 0.5 to 7 mg/week.
Pasireotide is still investigational. It is a somatostatin receptor agonist given subcutaneously for 15 consecutive days to patients with Cushing disease.
Glucocorticoid receptor antagonist
Mifepristone (Mifeprex) is a progesterone receptor and glucocorticoid II receptor antagonist that is being investigated in the treatment of persistent or recurrent Cushing disease. It is not yet approved by the US Food and Drug Administration for this indication.
BACK TO OUR PATIENT
The patient was too ill to undergo additional imaging, including octreotide scanning to identify an ectopic corticotropin-secreting tumor. She was medically treated with intravenous etomidate to reduce her cortisol level.30,31
Unfortunately, our patient died of multiorgan failure. The exact site of her ectopic corticotropin-producing tumor was never identified, and no autopsy was done.
- Meriggioli MN. Myasthenia gravis with anti-acetylcholine receptor antibodies. Front Neurol Neurosci 2009; 26:94–108.
- Gilhus NE. Autoimmune myasthenia gravis. Expert Rev Neurother 2009; 9:351–358.
- Heitman B, Irizarry A. Hypothyroidism: common complaints, perplexing diagnosis. Nurse Pract 1995; 20:54–60.
- Brick JE, Brick JF, Elnicki DM. Musculoskeletal disorders. When are they caused by hormone imbalance? Postgrad Med 1991; 90:129–132,135–136.
- Bar SL, Holmes DT, Frohlich J. Asymptomatic hypothyroidism and statin-induced myopathy. Can Fam Physician 2007; 53:428–431.
- McDermott MT. In the clinic. Hypothyroidism. Ann Intern Med 2009; 151:ITC61.
- Klopstock T. Drug-induced myopathies. Curr Opin Neurol 2008; 21:590–595.
- Dimachkie MM, Barohn RJ. Idiopathic inflammatory myopathies. Front Neurol Neurosci 2009; 26:126–146.
- Joseph A, Brasington R, Kahl L, Ranganathan P, Cheng TP, Atkinson J. Immunologic rheumatic disorders. J Allergy Clin Immunol 2010; 125(suppl 2):S204–S215.
- Joy TR, Hegele RA. Narrative review: statin-related myopathy. Ann Intern Med 2009; 150:858–868.
- Kiernan TJ, Rochford M, McDermott JH. Simvastatin induced rhabdomyolysis and an important clinical link with hypothyroidism. Int J Cardiol 2007; 119:374–376.
- Thompson PD, Clarkson P, Karas RH. Statin-associated myopathy. JAMA 2003; 289:1681–1690.
- Spence JD, Munoz CE, Hendricks L, Latchinian L, Khouri HE. Pharmacokinetics of the combination of fluvastatin and gemfibrozil. Am J Cardiol 1995; 76:80A–83A.
- Boscaro M, Arnaldi G. Approach to the patient with possible Cushing’s syndrome. J Clin Endocrinol Metab 2009; 94:3121–3131.
- Ilias I, Torpy DJ, Pacak K, Mullen N, Wesley RA, Nieman LK. Cushing’s syndrome due to ectopic corticotropin secretion: twenty years’ experience at the National Institutes of Health. J Clin Endocrinol Metab 2005; 90:4955–4962.
- Pecori Giraldi F. Recent challenges in the diagnosis of Cushing’s syndrome. Horm Res 2009; 71(suppl 1):123–127.
- von Mach MA, Kann P, Piepkorn B, Bruder S, Beyer J. [Cushing’s syndrome caused by paraneoplastic ACTH secretion 11 years after occurrence of a medullary thyroid carcinoma]. Dtsch Med Wochenschr 2002; 127:850–852.
- Beauregard C, Dickstein G, Lacroix A. Classic and recent etiologies of Cushing’s syndrome: diagnosis and therapy. Treat Endocrinol 2002; 1:79–94.
- Liddle GW. Tests of pituitary-adrenal suppressibility in the diagnosis of Cushing’s syndrome. J Clin Endocrinol Metab 1960; 20:1539–1560.
- Louiset E, Gobet F, Libé R, et al. ACTH-independent Cushing’s syndrome with bilateral micronodular adrenal hyperplasia and ectopic adrenocortical adenoma. J Clin Endocrinol Metab 2010; 95:18–24.
- Andrioli M, Pecori Giraldi F, De Martin M, Cattaneo A, Carzaniga C, Cavagnini F. Differential diagnosis of ACTH-dependent hypercortisolism: imaging versus laboratory. Pituitary 2009; 12:294–296.
- Sahdev A, Reznek RH, Evanson J, Grossman AB. Imaging in Cushing’s syndrome. Arq Bras Endocrinol Metabol 2007; 51:1319–1328.
- Arnaldi G, Angeli A, Atkinson AB, et al. Diagnosis and complications of Cushing’s syndrome: a consensus statement. J Clin Endocrinol Metab 2003; 88:5593–5602.
- Lad SP, Patil CG, Laws ER, Katznelson L. The role of inferior petrosal sinus sampling in the diagnostic localization of Cushing’s disease. Neurosurg Focus 2007; 23:E2.
- Bhansali A, Walia R, Rana SS, et al. Ectopic Cushing’s syndrome: experience from a tertiary care centre. Indian J Med Res 2009; 129:33–41.
- Arlt A, Harbeck B, Anlauf M, et al. Fatal Pneumocystis jirovecii pneumonia in a case of ectopic Cushing’s syndrome due to neuroendocrine carcinoma of the kidney. Exp Clin Endocrinol Diabetes 2008; 116:515–519.
- Graham BS, Tucker WS. Opportunistic infections in endogenous Cushing’s syndrome. Ann Intern Med 1984; 101:334–338.
- Kronfol Z, Starkman M, Schteingart DE, Singh V, Zhang Q, Hill E. Immune regulation in Cushing’s syndrome: relationship to hypothalamic-pituitary-adrenal axis hormones. Psychoneuroendocrinology 1996; 21:599–608.
- Sepkowitz KA. Opportunistic infections in patients with and patients without acquired immunodeficiency syndrome. Clin Infect Dis 2002; 34:1098–1107.
- Schteingart DE. Drugs in the medical treatment of Cushing’s syndrome. Expert Opin Emerg Drugs 2009; 14:661–671.
- Shalet S, Mukherjee A. Pharmacological treatment of hypercortisolism. Curr Opin Endocrinol Diabetes Obes 2008; 15:234–238.
- Arnaldi G, Boscaro M. Pasireotide for the treatment of Cushing’s disease. Expert Opin Investig Drugs 2010; 19:889–898.
- Meriggioli MN. Myasthenia gravis with anti-acetylcholine receptor antibodies. Front Neurol Neurosci 2009; 26:94–108.
- Gilhus NE. Autoimmune myasthenia gravis. Expert Rev Neurother 2009; 9:351–358.
- Heitman B, Irizarry A. Hypothyroidism: common complaints, perplexing diagnosis. Nurse Pract 1995; 20:54–60.
- Brick JE, Brick JF, Elnicki DM. Musculoskeletal disorders. When are they caused by hormone imbalance? Postgrad Med 1991; 90:129–132,135–136.
- Bar SL, Holmes DT, Frohlich J. Asymptomatic hypothyroidism and statin-induced myopathy. Can Fam Physician 2007; 53:428–431.
- McDermott MT. In the clinic. Hypothyroidism. Ann Intern Med 2009; 151:ITC61.
- Klopstock T. Drug-induced myopathies. Curr Opin Neurol 2008; 21:590–595.
- Dimachkie MM, Barohn RJ. Idiopathic inflammatory myopathies. Front Neurol Neurosci 2009; 26:126–146.
- Joseph A, Brasington R, Kahl L, Ranganathan P, Cheng TP, Atkinson J. Immunologic rheumatic disorders. J Allergy Clin Immunol 2010; 125(suppl 2):S204–S215.
- Joy TR, Hegele RA. Narrative review: statin-related myopathy. Ann Intern Med 2009; 150:858–868.
- Kiernan TJ, Rochford M, McDermott JH. Simvastatin induced rhabdomyolysis and an important clinical link with hypothyroidism. Int J Cardiol 2007; 119:374–376.
- Thompson PD, Clarkson P, Karas RH. Statin-associated myopathy. JAMA 2003; 289:1681–1690.
- Spence JD, Munoz CE, Hendricks L, Latchinian L, Khouri HE. Pharmacokinetics of the combination of fluvastatin and gemfibrozil. Am J Cardiol 1995; 76:80A–83A.
- Boscaro M, Arnaldi G. Approach to the patient with possible Cushing’s syndrome. J Clin Endocrinol Metab 2009; 94:3121–3131.
- Ilias I, Torpy DJ, Pacak K, Mullen N, Wesley RA, Nieman LK. Cushing’s syndrome due to ectopic corticotropin secretion: twenty years’ experience at the National Institutes of Health. J Clin Endocrinol Metab 2005; 90:4955–4962.
- Pecori Giraldi F. Recent challenges in the diagnosis of Cushing’s syndrome. Horm Res 2009; 71(suppl 1):123–127.
- von Mach MA, Kann P, Piepkorn B, Bruder S, Beyer J. [Cushing’s syndrome caused by paraneoplastic ACTH secretion 11 years after occurrence of a medullary thyroid carcinoma]. Dtsch Med Wochenschr 2002; 127:850–852.
- Beauregard C, Dickstein G, Lacroix A. Classic and recent etiologies of Cushing’s syndrome: diagnosis and therapy. Treat Endocrinol 2002; 1:79–94.
- Liddle GW. Tests of pituitary-adrenal suppressibility in the diagnosis of Cushing’s syndrome. J Clin Endocrinol Metab 1960; 20:1539–1560.
- Louiset E, Gobet F, Libé R, et al. ACTH-independent Cushing’s syndrome with bilateral micronodular adrenal hyperplasia and ectopic adrenocortical adenoma. J Clin Endocrinol Metab 2010; 95:18–24.
- Andrioli M, Pecori Giraldi F, De Martin M, Cattaneo A, Carzaniga C, Cavagnini F. Differential diagnosis of ACTH-dependent hypercortisolism: imaging versus laboratory. Pituitary 2009; 12:294–296.
- Sahdev A, Reznek RH, Evanson J, Grossman AB. Imaging in Cushing’s syndrome. Arq Bras Endocrinol Metabol 2007; 51:1319–1328.
- Arnaldi G, Angeli A, Atkinson AB, et al. Diagnosis and complications of Cushing’s syndrome: a consensus statement. J Clin Endocrinol Metab 2003; 88:5593–5602.
- Lad SP, Patil CG, Laws ER, Katznelson L. The role of inferior petrosal sinus sampling in the diagnostic localization of Cushing’s disease. Neurosurg Focus 2007; 23:E2.
- Bhansali A, Walia R, Rana SS, et al. Ectopic Cushing’s syndrome: experience from a tertiary care centre. Indian J Med Res 2009; 129:33–41.
- Arlt A, Harbeck B, Anlauf M, et al. Fatal Pneumocystis jirovecii pneumonia in a case of ectopic Cushing’s syndrome due to neuroendocrine carcinoma of the kidney. Exp Clin Endocrinol Diabetes 2008; 116:515–519.
- Graham BS, Tucker WS. Opportunistic infections in endogenous Cushing’s syndrome. Ann Intern Med 1984; 101:334–338.
- Kronfol Z, Starkman M, Schteingart DE, Singh V, Zhang Q, Hill E. Immune regulation in Cushing’s syndrome: relationship to hypothalamic-pituitary-adrenal axis hormones. Psychoneuroendocrinology 1996; 21:599–608.
- Sepkowitz KA. Opportunistic infections in patients with and patients without acquired immunodeficiency syndrome. Clin Infect Dis 2002; 34:1098–1107.
- Schteingart DE. Drugs in the medical treatment of Cushing’s syndrome. Expert Opin Emerg Drugs 2009; 14:661–671.
- Shalet S, Mukherjee A. Pharmacological treatment of hypercortisolism. Curr Opin Endocrinol Diabetes Obes 2008; 15:234–238.
- Arnaldi G, Boscaro M. Pasireotide for the treatment of Cushing’s disease. Expert Opin Investig Drugs 2010; 19:889–898.