User login
Epithelioid sarcoma (ES) is a rare malignant soft tissue neoplasm that is most often encountered on the distal extremities of young adults.1 Epithelioid sarcoma is notorious for its tendency to mimic palisading granulomatous processes such as granuloma annulare. We report a case of ES on the right hand of a 23-year-old man that resembled a benign fibrous histiocytoma (dermatofibroma) on incisional biopsy. The typical histopathologic features of ES were identified after amputation of the hand and evaluation of the deeper regions of the tumor. The tendency for ES to mimic granulomatous processes is a common diagnostic pitfall, but the potential for its close resemblance to benign fibrous histiocytoma is less recognized.
|
Case Report
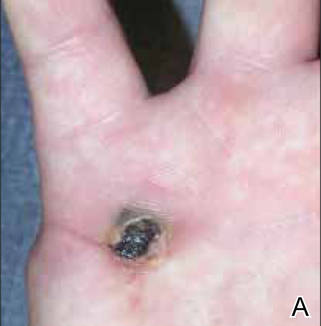
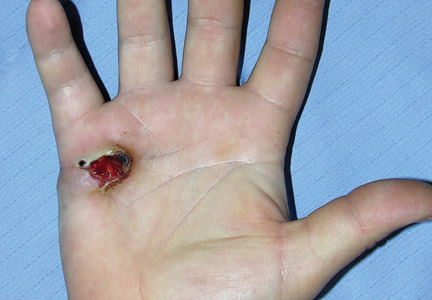
A 23-year-old man presented with a nonhealing lesion on the right palm. His medical history was remarkable for a giant cell tumor of the tendon sheath involving the right fifth finger that had been treated via excision at an outside institution 2 years prior. Clinical examination revealed a 0.8×0.6-cm painful, firm, ulcerated dermal nodule with a hemorrhagic crust on the palmar surface of the right hand (Figure 1A). The clinical differential diagnosis included melanoma, traumatized verruca vulgaris, thrombosed pyogenic granuloma, and foreign body. A shave biopsy demonstrated verrucous epidermal hyperplasia, but the specimen did not include the dermis. Cultures of the lesion were positive for Staphylococcus aureus, and antibiotic therapy was initiated. In light of the clinical findings and the patient’s history of a giant cell tumor, imaging studies were performed. Magnetic resonance angiography showed abnormal masslike infiltrative enhancement throughout the soft tissues surrounding the right fifth metacarpal bone. The differential included a recurrent giant cell tumor, fibromatosis, and other soft tissue neoplasms.
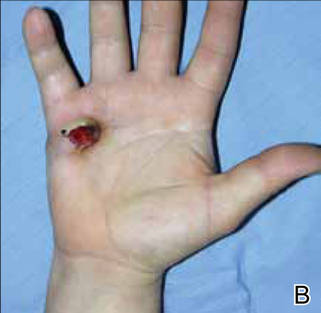
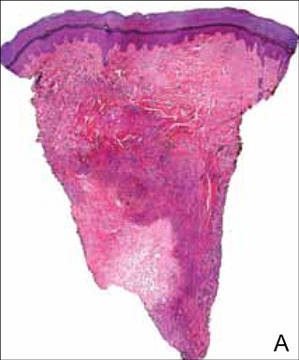
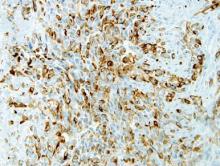
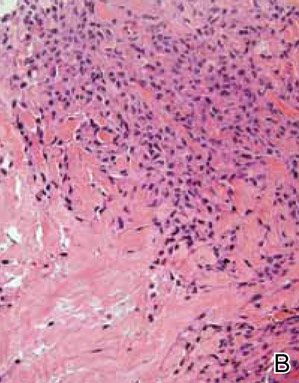
After several missed appointments and surgery cancellations, the patient returned 4 months later for an incisional biopsy. Physical examination revealed a persistent palmar ulcer that had grown to 1.4×1 cm in size, along with an indurated purple plaque wrapping around the ulnar aspect of the right hand (Figure 1B). The biopsy demonstrated a proliferation of spindled and ovoid cells with scant cytoplasm that surrounded sclerotic collagen bundles resembling a dermatofibroma (Figure 2A). Cytologic atypia and mitotic activity were absent (Figure 2B). Glass slides of the original biopsy, which ultimately led to the diagnosis of the giant cell tumor of the tendon sheath more than 2 years earlier, were obtained and showed similar features. The proliferating cells were strongly and diffusely immunoreactive for vimentin, CD34, and cancer antigen 125 (CA 125). Scattered tumor cells strongly expressed cytokeratins (CKs) AE1/AE3 and cell adhesion molecule 5.2 (Figure 3). Staining for CD99 and epithelial membrane antigen was diffuse but weak. Factor XIIIa, S-100, CK7, smooth muscle actin, muscle-specific actin (HHF35), CD31, CD68, and B-cell lymphoma 2 were negative within the proliferating cells. Based on the clinical examination and results of the immunohistochemical staining, a diagnosis of ES was favored.
|
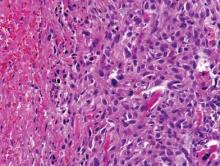
After a negative metastatic workup, amputation of the right hand was performed. The amputation specimen showed a tumor that extended through the entire hand with encasement of large vessels and tendons. Although the more superficial regions were cytologically bland, deep-seated regions of the tumor exhibited greater cellularity, nuclear pleomorphism, and mitotic activity (Figure 4). There was no bone involvement. Right axillary sentinel lymph nodes were negative for metastasis. Eighteen months later the patient developed chest and back pain with dyspnea. Thorascopic surgery was performed for a left pleural effusion and metastases to the left parietal pleura and adjacent soft tissue were identified. The patient was subsequently lost to follow-up.
Comment
First described by Enzinger1 in 1970, ES is a rare malignant soft tissue neoplasm that most frequently arises on the hands, forearms, and pretibial soft tissues of young adults.1-3 It is an aggressive tumor characterized by frequent recurrences and a high metastatic rate, with lung and regional lymph nodes being favored metastatic sites.1-5 Periods of several months or even years often pass between the initial presentation and establishment of a correct diagnosis, as ES frequently is mistaken for other benign conditions. The tendency for ES to mimic granulomatous processes is a common diagnostic pitfall, but the potential for its close resemblance to benign fibrous histiocytoma is less recognized.6,7 In his original series of 62 cases, Enzinger1 noted that 17 patients were referred for treatment with a diagnosis of a benign fibrohistiocytic neoplasm, and other reports have described a resemblance to fibrous and fibrohistiocytic neoplasms.8-11 Mirra et al10 designated these tumors as fibromalike variants of ES. Additional subtypes of ES have subsequently been recognized, including those described as angiomatoid or angiosarcomalike, reflecting the potential of ES to resemble vascular tumors.12 A proximal type of ES also has been described. This lesion presents as a deep-seated tumor on the proximal limbs and is associated with more aggressive behavior. It lacks the granulomalike pattern and has more prominent epithelioid and rhabdoid histological presentation.13-15
Epithelioid sarcoma is a mesenchymal tumor that can display multidirectional differentiation that is primarily epithelial.16 The precise histogenesis of ES remains unclear, but studies have demonstrated a spectrum of differentiation that ranges from primitive myofibroblast or fibrohistiocytelike cells to those with well-developed epithelial properties.16,17 Epithelioid sarcoma characteristically coexpresses vimentin and low-molecular-weight CKs such as cell adhesion molecule 5.2. The tumor cells often are immunoreactive for epithelial membrane antigen and more than 50% of cases exhibit remarkable CD34 positivity.16 More recent studies have further refined the immunophenotype, demonstrating frequent expression of CK8 and CK19 but less commonly CK7, CK20, CK34bE12, and CK5/6.18-20 Additional studies reported that in 10 of 11 cases, ES was positive for CA 125 on immunohistochemical staining, and 3 of 5 patients also had elevated serum CA 125 levels.21,22 More recently, Hoshino et al23 showed elevated serum CA 125 levels in 5 of 7 patients with ES. Cancer antigen 125 is a high-molecular-weight glycoprotein commonly used in the identification of epithelial ovarian carcinomas; however, it also has been described in a number of other neoplasms including carcinomas of the breast, lungs, and colon and lymphoma.24-27 Although it appears that the addition of CA 125 to a panel of other immunohistochemical stains may be helpful in differentiating ES from other soft tissue sarcomas and serum CA 125 levels may help determine tumor burden, currently the number of cases studied is too small to definitively make that conclusion.21,23 In our case, the tumor cells were strongly and diffusely positive for CA 125. Serum CA 125 levels were not available.
Cytogenetic studies have failed to identify a consistent chromosomal abnormality in ES.5 Some analyses performed by comparative genomic hybridization on isolated cases and small case series indicate that the most frequent alterations involve 8q, 18q11, and 22q11.13,28,29 The tumor suppressor gene SMARCB1/INI1 (SWI/SNF related, matrix associated, actin dependent regulator of chromatin, subfamily B, member 1/integrase interactor 1) has been mapped to 22q11, and ES commonly shows absence of nuclear staining for this protein, indicating inactivation.13-15
Conclusion
Benign fibrohistiocytic proliferations should be included in the differential of histological mimickers of ES. Deep biopsies are essential to differentiate these benign tumors from fibrous histiocytomalike or fibromalike lesions of ES because superficial portions of ES may be well differentiated.
1. Enzinger FM. Epitheloid sarcoma. a sarcoma simulating a granuloma or a carcinoma. Cancer. 1970;26:1029-1041.
2. Spillane AJ, Thomas JM, Fisher C. Epithelioid sarcoma: the clinicopathological complexities of this rare soft tissue sarcoma. Ann Surg Oncol. 2000;7:218-225.
3. Chase DR, Enzinger FM. Epithelioid sarcoma. diagnosis, prognostic indicators, and treatment. Am J Surg Pathol. 1985;9:241-263.
4. Fisher C. Epithelioid sarcoma of Enzinger. Adv Anat Pathol. 2006;13:114-121.
5. Evans HL, Baer SC. Epithelioid sarcoma: a clinicopathologic and prognostic study of 26 cases. Semin Diagn Pathol. 1993;10:286-291.
6. Heenan PJ, Quirk CJ, Papadimitriou JM. Epithelioid sarcoma. a diagnostic problem. Am J Dermatopathol. 1986;8:95-104.
7. DiCaudo DJ, McCalmont TH, Wick MR. Selected diagnostic problems in neoplastic dermatopathology. Arch Pathol Lab Med. 2007;131:434-439.
8. Ormsby AH, Liou LS, Oriba HA, et al. Epithelioid sarcoma of the penis: report of an unusual case and review of the literature. Ann Diagn Pathol. 2000;4:88-94.
9. Lowentritt B, Parsons JK, Argani P, et al. Pediatric epithelioid sarcoma of the penis. J Urol. 2004;172:296-297.
10. Mirra JM, Kessler S, Bhuta S, et al. The fibroma-like variant of epithelioid sarcoma. a fibrohistiocytic/myoid cell lesion often confused with benign and malignant spindle cell tumors. Cancer. 1992;69:1382-1395.
11. Tan SH, Ong BH. Spindle cell variant of epithelioid sarcoma: an easily misdiagnosed tumour. Australas J Dermatol. 2001;42:139-141.
12. von Hochstetter AR, Grant JW, Meyer VE, et al. Angiomatoid variant of epithelioid sarcoma. the value of immunohistochemistry in the differential diagnosis. Chir Organi Mov. 1990;75(suppl 1):158-162.
13. Modena P, Lualdi E, Facchinetti F, et al. SMARCB1/INI1 tumor suppressor gene is frequently inactivated in epithelioid sarcomas. Cancer Res. 2005;65:4012-4019.
14. Lualdi E, Modena P, Debiec-Rychter M, et al. Molecular cytogenetic characterization of proximal-type epithelioid sarcoma. Genes Chromosomes Cancer. 2004;41:283-290.
15. Kosemehmetoglu K, Kaygusuz G, Bahrami A, et al. Intra-articular epithelioid sarcoma showing mixed classic and proximal-type features: report of 2 cases, with immunohistochemical and molecular cytogenetic INI-1 study. Am J Surg Pathol. 2011;35:891-897.
16. Armah HB, Parwani AV. Epithelioid sarcoma. Arch Pathol Lab Med. 2009;133:814-819.
17. Fisher C. Epithelioid sarcoma: the spectrum of ultrastructural differentiation in seven immunohistochemically defined cases. Hum Pathol. 1988;19:265-275.
18. Miettinen M, Fanburg-Smith JC, Virolainen M, et al. Epithelioid sarcoma: an immunohistochemical analysis of 112 classical and variant cases and a discussion of the differential diagnosis. Hum Pathol. 1999;30:934-942.
19. Humble SD, Prieto VG, Horenstein MG. Cytokeratin 7 and 20 expression in epithelioid sarcoma. J Cutan Pathol. 2003;30:242-246.
20. Lin L, Skacel M, Sigel JE, et al. Epithelioid sarcoma: an immunohistochemical analysis evaluating the utility of cytokeratin 5/6 in distinguishing superficial epithelioid sarcoma from spindled squamous cell carcinoma. J Cutan Pathol. 2003;30:114-117.
21. Kato H, Hatori M, Kokubun S, et al. CA125 expression in epithelioid sarcoma. Jpn J Clin Oncol. 2004;34:149-154.
22. Kato H, Hatori M, Watanabe M, et al. Epithelioid sarcomas with elevated serum CA125: report of two cases. Jpn J Clin Oncol. 2003;33:141-144.
23. Hoshino M, Kawashima H, Ogose A, et al. Serum CA 125 expression as a tumor marker for the diagnosis and monitoring the clinical course of epithelioid sarcoma [published online ahead of print September 16, 2009]. J Cancer Res Clin Oncol. 2010;136:457-464.
24. Lee AH, Paish EC, Marchio C, et al. The expression of Wilm’s tumour-1 and CA125 in invasive micropapillary carcinoma of the breast. Histopathology. 2007;51:824-828.
25. Homma S, Satoh H, Kagohashi K, et al. Production of CA125 by human lung cancer cell lines. Clin Exp Med. 2004;4:139-141.
26. Streppel MM, Vincent A, Mukherjee R, et al. Mucin 16 (cancer antigen 125) expression in human tissues and cell lines and correlation with clinical outcome in adenocarcinomas of the pancreas, esophagus, stomach, and colon. Hum Pathol. 2012;42:1755-1763.
27. Wei G, Yuping Z, Jun W, et al. CA125 expression in patients with non-Hodgkin’s lymphoma. Leuk Lymphoma. 2006; 47:1322-1326.
28. Feely MG, Fidler ME, Nelson M, et al. Cytogenetic findings in a case of epithelioid sarcoma and a review of the literature. Cancer Genet Cytogenet. 2000;119:155-157.
29. Lushnikova T, Knuutila S, Miettinen M. DNA copy number changes in epithelioid sarcoma and its variants: a comparative genomic hybridization study. Mod Pathol. 2000;13:1092-1096.
Epithelioid sarcoma (ES) is a rare malignant soft tissue neoplasm that is most often encountered on the distal extremities of young adults.1 Epithelioid sarcoma is notorious for its tendency to mimic palisading granulomatous processes such as granuloma annulare. We report a case of ES on the right hand of a 23-year-old man that resembled a benign fibrous histiocytoma (dermatofibroma) on incisional biopsy. The typical histopathologic features of ES were identified after amputation of the hand and evaluation of the deeper regions of the tumor. The tendency for ES to mimic granulomatous processes is a common diagnostic pitfall, but the potential for its close resemblance to benign fibrous histiocytoma is less recognized.
|
Case Report
A 23-year-old man presented with a nonhealing lesion on the right palm. His medical history was remarkable for a giant cell tumor of the tendon sheath involving the right fifth finger that had been treated via excision at an outside institution 2 years prior. Clinical examination revealed a 0.8×0.6-cm painful, firm, ulcerated dermal nodule with a hemorrhagic crust on the palmar surface of the right hand (Figure 1A). The clinical differential diagnosis included melanoma, traumatized verruca vulgaris, thrombosed pyogenic granuloma, and foreign body. A shave biopsy demonstrated verrucous epidermal hyperplasia, but the specimen did not include the dermis. Cultures of the lesion were positive for Staphylococcus aureus, and antibiotic therapy was initiated. In light of the clinical findings and the patient’s history of a giant cell tumor, imaging studies were performed. Magnetic resonance angiography showed abnormal masslike infiltrative enhancement throughout the soft tissues surrounding the right fifth metacarpal bone. The differential included a recurrent giant cell tumor, fibromatosis, and other soft tissue neoplasms.
After several missed appointments and surgery cancellations, the patient returned 4 months later for an incisional biopsy. Physical examination revealed a persistent palmar ulcer that had grown to 1.4×1 cm in size, along with an indurated purple plaque wrapping around the ulnar aspect of the right hand (Figure 1B). The biopsy demonstrated a proliferation of spindled and ovoid cells with scant cytoplasm that surrounded sclerotic collagen bundles resembling a dermatofibroma (Figure 2A). Cytologic atypia and mitotic activity were absent (Figure 2B). Glass slides of the original biopsy, which ultimately led to the diagnosis of the giant cell tumor of the tendon sheath more than 2 years earlier, were obtained and showed similar features. The proliferating cells were strongly and diffusely immunoreactive for vimentin, CD34, and cancer antigen 125 (CA 125). Scattered tumor cells strongly expressed cytokeratins (CKs) AE1/AE3 and cell adhesion molecule 5.2 (Figure 3). Staining for CD99 and epithelial membrane antigen was diffuse but weak. Factor XIIIa, S-100, CK7, smooth muscle actin, muscle-specific actin (HHF35), CD31, CD68, and B-cell lymphoma 2 were negative within the proliferating cells. Based on the clinical examination and results of the immunohistochemical staining, a diagnosis of ES was favored.

|
After a negative metastatic workup, amputation of the right hand was performed. The amputation specimen showed a tumor that extended through the entire hand with encasement of large vessels and tendons. Although the more superficial regions were cytologically bland, deep-seated regions of the tumor exhibited greater cellularity, nuclear pleomorphism, and mitotic activity (Figure 4). There was no bone involvement. Right axillary sentinel lymph nodes were negative for metastasis. Eighteen months later the patient developed chest and back pain with dyspnea. Thorascopic surgery was performed for a left pleural effusion and metastases to the left parietal pleura and adjacent soft tissue were identified. The patient was subsequently lost to follow-up.
Comment
First described by Enzinger1 in 1970, ES is a rare malignant soft tissue neoplasm that most frequently arises on the hands, forearms, and pretibial soft tissues of young adults.1-3 It is an aggressive tumor characterized by frequent recurrences and a high metastatic rate, with lung and regional lymph nodes being favored metastatic sites.1-5 Periods of several months or even years often pass between the initial presentation and establishment of a correct diagnosis, as ES frequently is mistaken for other benign conditions. The tendency for ES to mimic granulomatous processes is a common diagnostic pitfall, but the potential for its close resemblance to benign fibrous histiocytoma is less recognized.6,7 In his original series of 62 cases, Enzinger1 noted that 17 patients were referred for treatment with a diagnosis of a benign fibrohistiocytic neoplasm, and other reports have described a resemblance to fibrous and fibrohistiocytic neoplasms.8-11 Mirra et al10 designated these tumors as fibromalike variants of ES. Additional subtypes of ES have subsequently been recognized, including those described as angiomatoid or angiosarcomalike, reflecting the potential of ES to resemble vascular tumors.12 A proximal type of ES also has been described. This lesion presents as a deep-seated tumor on the proximal limbs and is associated with more aggressive behavior. It lacks the granulomalike pattern and has more prominent epithelioid and rhabdoid histological presentation.13-15
Epithelioid sarcoma is a mesenchymal tumor that can display multidirectional differentiation that is primarily epithelial.16 The precise histogenesis of ES remains unclear, but studies have demonstrated a spectrum of differentiation that ranges from primitive myofibroblast or fibrohistiocytelike cells to those with well-developed epithelial properties.16,17 Epithelioid sarcoma characteristically coexpresses vimentin and low-molecular-weight CKs such as cell adhesion molecule 5.2. The tumor cells often are immunoreactive for epithelial membrane antigen and more than 50% of cases exhibit remarkable CD34 positivity.16 More recent studies have further refined the immunophenotype, demonstrating frequent expression of CK8 and CK19 but less commonly CK7, CK20, CK34bE12, and CK5/6.18-20 Additional studies reported that in 10 of 11 cases, ES was positive for CA 125 on immunohistochemical staining, and 3 of 5 patients also had elevated serum CA 125 levels.21,22 More recently, Hoshino et al23 showed elevated serum CA 125 levels in 5 of 7 patients with ES. Cancer antigen 125 is a high-molecular-weight glycoprotein commonly used in the identification of epithelial ovarian carcinomas; however, it also has been described in a number of other neoplasms including carcinomas of the breast, lungs, and colon and lymphoma.24-27 Although it appears that the addition of CA 125 to a panel of other immunohistochemical stains may be helpful in differentiating ES from other soft tissue sarcomas and serum CA 125 levels may help determine tumor burden, currently the number of cases studied is too small to definitively make that conclusion.21,23 In our case, the tumor cells were strongly and diffusely positive for CA 125. Serum CA 125 levels were not available.
Cytogenetic studies have failed to identify a consistent chromosomal abnormality in ES.5 Some analyses performed by comparative genomic hybridization on isolated cases and small case series indicate that the most frequent alterations involve 8q, 18q11, and 22q11.13,28,29 The tumor suppressor gene SMARCB1/INI1 (SWI/SNF related, matrix associated, actin dependent regulator of chromatin, subfamily B, member 1/integrase interactor 1) has been mapped to 22q11, and ES commonly shows absence of nuclear staining for this protein, indicating inactivation.13-15
Conclusion
Benign fibrohistiocytic proliferations should be included in the differential of histological mimickers of ES. Deep biopsies are essential to differentiate these benign tumors from fibrous histiocytomalike or fibromalike lesions of ES because superficial portions of ES may be well differentiated.
Epithelioid sarcoma (ES) is a rare malignant soft tissue neoplasm that is most often encountered on the distal extremities of young adults.1 Epithelioid sarcoma is notorious for its tendency to mimic palisading granulomatous processes such as granuloma annulare. We report a case of ES on the right hand of a 23-year-old man that resembled a benign fibrous histiocytoma (dermatofibroma) on incisional biopsy. The typical histopathologic features of ES were identified after amputation of the hand and evaluation of the deeper regions of the tumor. The tendency for ES to mimic granulomatous processes is a common diagnostic pitfall, but the potential for its close resemblance to benign fibrous histiocytoma is less recognized.
|
Case Report
A 23-year-old man presented with a nonhealing lesion on the right palm. His medical history was remarkable for a giant cell tumor of the tendon sheath involving the right fifth finger that had been treated via excision at an outside institution 2 years prior. Clinical examination revealed a 0.8×0.6-cm painful, firm, ulcerated dermal nodule with a hemorrhagic crust on the palmar surface of the right hand (Figure 1A). The clinical differential diagnosis included melanoma, traumatized verruca vulgaris, thrombosed pyogenic granuloma, and foreign body. A shave biopsy demonstrated verrucous epidermal hyperplasia, but the specimen did not include the dermis. Cultures of the lesion were positive for Staphylococcus aureus, and antibiotic therapy was initiated. In light of the clinical findings and the patient’s history of a giant cell tumor, imaging studies were performed. Magnetic resonance angiography showed abnormal masslike infiltrative enhancement throughout the soft tissues surrounding the right fifth metacarpal bone. The differential included a recurrent giant cell tumor, fibromatosis, and other soft tissue neoplasms.
After several missed appointments and surgery cancellations, the patient returned 4 months later for an incisional biopsy. Physical examination revealed a persistent palmar ulcer that had grown to 1.4×1 cm in size, along with an indurated purple plaque wrapping around the ulnar aspect of the right hand (Figure 1B). The biopsy demonstrated a proliferation of spindled and ovoid cells with scant cytoplasm that surrounded sclerotic collagen bundles resembling a dermatofibroma (Figure 2A). Cytologic atypia and mitotic activity were absent (Figure 2B). Glass slides of the original biopsy, which ultimately led to the diagnosis of the giant cell tumor of the tendon sheath more than 2 years earlier, were obtained and showed similar features. The proliferating cells were strongly and diffusely immunoreactive for vimentin, CD34, and cancer antigen 125 (CA 125). Scattered tumor cells strongly expressed cytokeratins (CKs) AE1/AE3 and cell adhesion molecule 5.2 (Figure 3). Staining for CD99 and epithelial membrane antigen was diffuse but weak. Factor XIIIa, S-100, CK7, smooth muscle actin, muscle-specific actin (HHF35), CD31, CD68, and B-cell lymphoma 2 were negative within the proliferating cells. Based on the clinical examination and results of the immunohistochemical staining, a diagnosis of ES was favored.

|
After a negative metastatic workup, amputation of the right hand was performed. The amputation specimen showed a tumor that extended through the entire hand with encasement of large vessels and tendons. Although the more superficial regions were cytologically bland, deep-seated regions of the tumor exhibited greater cellularity, nuclear pleomorphism, and mitotic activity (Figure 4). There was no bone involvement. Right axillary sentinel lymph nodes were negative for metastasis. Eighteen months later the patient developed chest and back pain with dyspnea. Thorascopic surgery was performed for a left pleural effusion and metastases to the left parietal pleura and adjacent soft tissue were identified. The patient was subsequently lost to follow-up.
Comment
First described by Enzinger1 in 1970, ES is a rare malignant soft tissue neoplasm that most frequently arises on the hands, forearms, and pretibial soft tissues of young adults.1-3 It is an aggressive tumor characterized by frequent recurrences and a high metastatic rate, with lung and regional lymph nodes being favored metastatic sites.1-5 Periods of several months or even years often pass between the initial presentation and establishment of a correct diagnosis, as ES frequently is mistaken for other benign conditions. The tendency for ES to mimic granulomatous processes is a common diagnostic pitfall, but the potential for its close resemblance to benign fibrous histiocytoma is less recognized.6,7 In his original series of 62 cases, Enzinger1 noted that 17 patients were referred for treatment with a diagnosis of a benign fibrohistiocytic neoplasm, and other reports have described a resemblance to fibrous and fibrohistiocytic neoplasms.8-11 Mirra et al10 designated these tumors as fibromalike variants of ES. Additional subtypes of ES have subsequently been recognized, including those described as angiomatoid or angiosarcomalike, reflecting the potential of ES to resemble vascular tumors.12 A proximal type of ES also has been described. This lesion presents as a deep-seated tumor on the proximal limbs and is associated with more aggressive behavior. It lacks the granulomalike pattern and has more prominent epithelioid and rhabdoid histological presentation.13-15
Epithelioid sarcoma is a mesenchymal tumor that can display multidirectional differentiation that is primarily epithelial.16 The precise histogenesis of ES remains unclear, but studies have demonstrated a spectrum of differentiation that ranges from primitive myofibroblast or fibrohistiocytelike cells to those with well-developed epithelial properties.16,17 Epithelioid sarcoma characteristically coexpresses vimentin and low-molecular-weight CKs such as cell adhesion molecule 5.2. The tumor cells often are immunoreactive for epithelial membrane antigen and more than 50% of cases exhibit remarkable CD34 positivity.16 More recent studies have further refined the immunophenotype, demonstrating frequent expression of CK8 and CK19 but less commonly CK7, CK20, CK34bE12, and CK5/6.18-20 Additional studies reported that in 10 of 11 cases, ES was positive for CA 125 on immunohistochemical staining, and 3 of 5 patients also had elevated serum CA 125 levels.21,22 More recently, Hoshino et al23 showed elevated serum CA 125 levels in 5 of 7 patients with ES. Cancer antigen 125 is a high-molecular-weight glycoprotein commonly used in the identification of epithelial ovarian carcinomas; however, it also has been described in a number of other neoplasms including carcinomas of the breast, lungs, and colon and lymphoma.24-27 Although it appears that the addition of CA 125 to a panel of other immunohistochemical stains may be helpful in differentiating ES from other soft tissue sarcomas and serum CA 125 levels may help determine tumor burden, currently the number of cases studied is too small to definitively make that conclusion.21,23 In our case, the tumor cells were strongly and diffusely positive for CA 125. Serum CA 125 levels were not available.
Cytogenetic studies have failed to identify a consistent chromosomal abnormality in ES.5 Some analyses performed by comparative genomic hybridization on isolated cases and small case series indicate that the most frequent alterations involve 8q, 18q11, and 22q11.13,28,29 The tumor suppressor gene SMARCB1/INI1 (SWI/SNF related, matrix associated, actin dependent regulator of chromatin, subfamily B, member 1/integrase interactor 1) has been mapped to 22q11, and ES commonly shows absence of nuclear staining for this protein, indicating inactivation.13-15
Conclusion
Benign fibrohistiocytic proliferations should be included in the differential of histological mimickers of ES. Deep biopsies are essential to differentiate these benign tumors from fibrous histiocytomalike or fibromalike lesions of ES because superficial portions of ES may be well differentiated.
1. Enzinger FM. Epitheloid sarcoma. a sarcoma simulating a granuloma or a carcinoma. Cancer. 1970;26:1029-1041.
2. Spillane AJ, Thomas JM, Fisher C. Epithelioid sarcoma: the clinicopathological complexities of this rare soft tissue sarcoma. Ann Surg Oncol. 2000;7:218-225.
3. Chase DR, Enzinger FM. Epithelioid sarcoma. diagnosis, prognostic indicators, and treatment. Am J Surg Pathol. 1985;9:241-263.
4. Fisher C. Epithelioid sarcoma of Enzinger. Adv Anat Pathol. 2006;13:114-121.
5. Evans HL, Baer SC. Epithelioid sarcoma: a clinicopathologic and prognostic study of 26 cases. Semin Diagn Pathol. 1993;10:286-291.
6. Heenan PJ, Quirk CJ, Papadimitriou JM. Epithelioid sarcoma. a diagnostic problem. Am J Dermatopathol. 1986;8:95-104.
7. DiCaudo DJ, McCalmont TH, Wick MR. Selected diagnostic problems in neoplastic dermatopathology. Arch Pathol Lab Med. 2007;131:434-439.
8. Ormsby AH, Liou LS, Oriba HA, et al. Epithelioid sarcoma of the penis: report of an unusual case and review of the literature. Ann Diagn Pathol. 2000;4:88-94.
9. Lowentritt B, Parsons JK, Argani P, et al. Pediatric epithelioid sarcoma of the penis. J Urol. 2004;172:296-297.
10. Mirra JM, Kessler S, Bhuta S, et al. The fibroma-like variant of epithelioid sarcoma. a fibrohistiocytic/myoid cell lesion often confused with benign and malignant spindle cell tumors. Cancer. 1992;69:1382-1395.
11. Tan SH, Ong BH. Spindle cell variant of epithelioid sarcoma: an easily misdiagnosed tumour. Australas J Dermatol. 2001;42:139-141.
12. von Hochstetter AR, Grant JW, Meyer VE, et al. Angiomatoid variant of epithelioid sarcoma. the value of immunohistochemistry in the differential diagnosis. Chir Organi Mov. 1990;75(suppl 1):158-162.
13. Modena P, Lualdi E, Facchinetti F, et al. SMARCB1/INI1 tumor suppressor gene is frequently inactivated in epithelioid sarcomas. Cancer Res. 2005;65:4012-4019.
14. Lualdi E, Modena P, Debiec-Rychter M, et al. Molecular cytogenetic characterization of proximal-type epithelioid sarcoma. Genes Chromosomes Cancer. 2004;41:283-290.
15. Kosemehmetoglu K, Kaygusuz G, Bahrami A, et al. Intra-articular epithelioid sarcoma showing mixed classic and proximal-type features: report of 2 cases, with immunohistochemical and molecular cytogenetic INI-1 study. Am J Surg Pathol. 2011;35:891-897.
16. Armah HB, Parwani AV. Epithelioid sarcoma. Arch Pathol Lab Med. 2009;133:814-819.
17. Fisher C. Epithelioid sarcoma: the spectrum of ultrastructural differentiation in seven immunohistochemically defined cases. Hum Pathol. 1988;19:265-275.
18. Miettinen M, Fanburg-Smith JC, Virolainen M, et al. Epithelioid sarcoma: an immunohistochemical analysis of 112 classical and variant cases and a discussion of the differential diagnosis. Hum Pathol. 1999;30:934-942.
19. Humble SD, Prieto VG, Horenstein MG. Cytokeratin 7 and 20 expression in epithelioid sarcoma. J Cutan Pathol. 2003;30:242-246.
20. Lin L, Skacel M, Sigel JE, et al. Epithelioid sarcoma: an immunohistochemical analysis evaluating the utility of cytokeratin 5/6 in distinguishing superficial epithelioid sarcoma from spindled squamous cell carcinoma. J Cutan Pathol. 2003;30:114-117.
21. Kato H, Hatori M, Kokubun S, et al. CA125 expression in epithelioid sarcoma. Jpn J Clin Oncol. 2004;34:149-154.
22. Kato H, Hatori M, Watanabe M, et al. Epithelioid sarcomas with elevated serum CA125: report of two cases. Jpn J Clin Oncol. 2003;33:141-144.
23. Hoshino M, Kawashima H, Ogose A, et al. Serum CA 125 expression as a tumor marker for the diagnosis and monitoring the clinical course of epithelioid sarcoma [published online ahead of print September 16, 2009]. J Cancer Res Clin Oncol. 2010;136:457-464.
24. Lee AH, Paish EC, Marchio C, et al. The expression of Wilm’s tumour-1 and CA125 in invasive micropapillary carcinoma of the breast. Histopathology. 2007;51:824-828.
25. Homma S, Satoh H, Kagohashi K, et al. Production of CA125 by human lung cancer cell lines. Clin Exp Med. 2004;4:139-141.
26. Streppel MM, Vincent A, Mukherjee R, et al. Mucin 16 (cancer antigen 125) expression in human tissues and cell lines and correlation with clinical outcome in adenocarcinomas of the pancreas, esophagus, stomach, and colon. Hum Pathol. 2012;42:1755-1763.
27. Wei G, Yuping Z, Jun W, et al. CA125 expression in patients with non-Hodgkin’s lymphoma. Leuk Lymphoma. 2006; 47:1322-1326.
28. Feely MG, Fidler ME, Nelson M, et al. Cytogenetic findings in a case of epithelioid sarcoma and a review of the literature. Cancer Genet Cytogenet. 2000;119:155-157.
29. Lushnikova T, Knuutila S, Miettinen M. DNA copy number changes in epithelioid sarcoma and its variants: a comparative genomic hybridization study. Mod Pathol. 2000;13:1092-1096.
1. Enzinger FM. Epitheloid sarcoma. a sarcoma simulating a granuloma or a carcinoma. Cancer. 1970;26:1029-1041.
2. Spillane AJ, Thomas JM, Fisher C. Epithelioid sarcoma: the clinicopathological complexities of this rare soft tissue sarcoma. Ann Surg Oncol. 2000;7:218-225.
3. Chase DR, Enzinger FM. Epithelioid sarcoma. diagnosis, prognostic indicators, and treatment. Am J Surg Pathol. 1985;9:241-263.
4. Fisher C. Epithelioid sarcoma of Enzinger. Adv Anat Pathol. 2006;13:114-121.
5. Evans HL, Baer SC. Epithelioid sarcoma: a clinicopathologic and prognostic study of 26 cases. Semin Diagn Pathol. 1993;10:286-291.
6. Heenan PJ, Quirk CJ, Papadimitriou JM. Epithelioid sarcoma. a diagnostic problem. Am J Dermatopathol. 1986;8:95-104.
7. DiCaudo DJ, McCalmont TH, Wick MR. Selected diagnostic problems in neoplastic dermatopathology. Arch Pathol Lab Med. 2007;131:434-439.
8. Ormsby AH, Liou LS, Oriba HA, et al. Epithelioid sarcoma of the penis: report of an unusual case and review of the literature. Ann Diagn Pathol. 2000;4:88-94.
9. Lowentritt B, Parsons JK, Argani P, et al. Pediatric epithelioid sarcoma of the penis. J Urol. 2004;172:296-297.
10. Mirra JM, Kessler S, Bhuta S, et al. The fibroma-like variant of epithelioid sarcoma. a fibrohistiocytic/myoid cell lesion often confused with benign and malignant spindle cell tumors. Cancer. 1992;69:1382-1395.
11. Tan SH, Ong BH. Spindle cell variant of epithelioid sarcoma: an easily misdiagnosed tumour. Australas J Dermatol. 2001;42:139-141.
12. von Hochstetter AR, Grant JW, Meyer VE, et al. Angiomatoid variant of epithelioid sarcoma. the value of immunohistochemistry in the differential diagnosis. Chir Organi Mov. 1990;75(suppl 1):158-162.
13. Modena P, Lualdi E, Facchinetti F, et al. SMARCB1/INI1 tumor suppressor gene is frequently inactivated in epithelioid sarcomas. Cancer Res. 2005;65:4012-4019.
14. Lualdi E, Modena P, Debiec-Rychter M, et al. Molecular cytogenetic characterization of proximal-type epithelioid sarcoma. Genes Chromosomes Cancer. 2004;41:283-290.
15. Kosemehmetoglu K, Kaygusuz G, Bahrami A, et al. Intra-articular epithelioid sarcoma showing mixed classic and proximal-type features: report of 2 cases, with immunohistochemical and molecular cytogenetic INI-1 study. Am J Surg Pathol. 2011;35:891-897.
16. Armah HB, Parwani AV. Epithelioid sarcoma. Arch Pathol Lab Med. 2009;133:814-819.
17. Fisher C. Epithelioid sarcoma: the spectrum of ultrastructural differentiation in seven immunohistochemically defined cases. Hum Pathol. 1988;19:265-275.
18. Miettinen M, Fanburg-Smith JC, Virolainen M, et al. Epithelioid sarcoma: an immunohistochemical analysis of 112 classical and variant cases and a discussion of the differential diagnosis. Hum Pathol. 1999;30:934-942.
19. Humble SD, Prieto VG, Horenstein MG. Cytokeratin 7 and 20 expression in epithelioid sarcoma. J Cutan Pathol. 2003;30:242-246.
20. Lin L, Skacel M, Sigel JE, et al. Epithelioid sarcoma: an immunohistochemical analysis evaluating the utility of cytokeratin 5/6 in distinguishing superficial epithelioid sarcoma from spindled squamous cell carcinoma. J Cutan Pathol. 2003;30:114-117.
21. Kato H, Hatori M, Kokubun S, et al. CA125 expression in epithelioid sarcoma. Jpn J Clin Oncol. 2004;34:149-154.
22. Kato H, Hatori M, Watanabe M, et al. Epithelioid sarcomas with elevated serum CA125: report of two cases. Jpn J Clin Oncol. 2003;33:141-144.
23. Hoshino M, Kawashima H, Ogose A, et al. Serum CA 125 expression as a tumor marker for the diagnosis and monitoring the clinical course of epithelioid sarcoma [published online ahead of print September 16, 2009]. J Cancer Res Clin Oncol. 2010;136:457-464.
24. Lee AH, Paish EC, Marchio C, et al. The expression of Wilm’s tumour-1 and CA125 in invasive micropapillary carcinoma of the breast. Histopathology. 2007;51:824-828.
25. Homma S, Satoh H, Kagohashi K, et al. Production of CA125 by human lung cancer cell lines. Clin Exp Med. 2004;4:139-141.
26. Streppel MM, Vincent A, Mukherjee R, et al. Mucin 16 (cancer antigen 125) expression in human tissues and cell lines and correlation with clinical outcome in adenocarcinomas of the pancreas, esophagus, stomach, and colon. Hum Pathol. 2012;42:1755-1763.
27. Wei G, Yuping Z, Jun W, et al. CA125 expression in patients with non-Hodgkin’s lymphoma. Leuk Lymphoma. 2006; 47:1322-1326.
28. Feely MG, Fidler ME, Nelson M, et al. Cytogenetic findings in a case of epithelioid sarcoma and a review of the literature. Cancer Genet Cytogenet. 2000;119:155-157.
29. Lushnikova T, Knuutila S, Miettinen M. DNA copy number changes in epithelioid sarcoma and its variants: a comparative genomic hybridization study. Mod Pathol. 2000;13:1092-1096.
Practice Points
- Epithelioid sarcoma should be considered in the clinical differential diagnosis of nonhealing recurrent lesions of the distal extremities in a young adult.
- Histological presentation of epithelioid sarcoma can mimic a number of benign granulomatous and fibrohistiocytic processes, including benign fibrous histiocytoma.
- Deeper biopsies may be needed to demonstrate the overtly malignant morphology characteristic of epithelioid sarcoma.
- Inactivation of SMARCB1/INI1 is a common molecular aberration identified in epithelioid sarcoma and can be demonstrated immunohistochemically by absence of nuclear staining in tumor cells.