User login
Despite decades of intensive research and improvements in medical care, severe sepsis affects an estimated 751,000 patients in the United States every year, killing 215,000 of them at an annual cost of 16.7 billion dollars.1 Because the elderly experience a 100‐fold increase in incidence, as compared with children, and a nearly 4‐fold increase in mortality (38.4% of those more than 85 years old), this burden is expected to increase with the aging population.1 Patients with severe sepsis have prolonged ICU14 and hospital stays and incur substantially increased costs compared with other patients.36
New research continues to explore the complex pathophysiology of sepsis,7 and clinicians, who once relied primarily on clinical experience and expert opinion to guide therapy, now have an increasing array of evidenced‐based sepsis therapies to employ. Recent meta‐analyses have evaluated several major treatments for severe sepsis,810 and recommendations (the Surviving Sepsis Campaign guidelines) for the treatment of severe sepsis were recently endorsed by 11 international critical care and infectious disease organizations.11 This article summarizes the current definitions of sepsis syndromes, the trials supporting the specific therapies for sepsis that are currently recommended, ongoing controversies and research, and implications for hospitalists, with a focus on early, effective antibiotics, activated protein C, early goal‐directed therapy, stress dose steroids, and intensive insulin therapy. For space considerations, readers are directed elsewhere for data supporting prophylaxis for deep venous thrombosis (DVT)12 and stress ulcer bleeding13 and for therapies less often directed by hospitalists, such as lung protective ventilation.14
DEFINITIONS
Systemic inflammatory response syndrome (SIRS), sepsis, severe sepsis, and septic shock were defined in 1992 to standardize the terminology of sepsis.15 These definitions have recently been reviewed and supported by a variety of American and European intensive care societies.16
SIRS is defined by the presence of at least 2 of the following:
-
Temperature > 38C or < 36C;
-
Heart rate > 90 beats/min;
-
Respiratory rate > 20 breaths/min or PaCO2 < 32 mm Hg;
-
WBC >12,000 or < 4000 cells/mm3, or >10% immature (band) forms.
Sepsis is SIRS due to documented or strongly suspected infection.
Severe sepsis is sepsis with organ dysfunction (such as lactic acidosis, oliguria, thrombocytopenia, or delirium), hypoperfusion, or hypotension (< 90 mm Hg systolic or more than 40 mm Hg below baseline).
Septic shock is severe sepsis complicated by hypotension or pressor dependence despite adequate (20‐30 mL/kg; 1.5‐3 liters in most patients) fluid resuscitation.
Sepsis terminology must be applied carefully. Many hospitalized patients meet criteria for SIRS, yet it is inaccurate to say a patient who has acute leukemia with leukocytosis, anemia‐induced tachycardia, and thrombocytopenia has severe sepsis if those abnormalities are not a result of inflammation or infection. Accurate documentation of sepsis syndromes can improve professional and institutional reimbursement and provide prognostic information: the in‐hospital mortality rates for severe sepsis and septic shock are approximately 30% and 50%, respectively.17 More importantly, thoughtful application of these definitions can help a hospitalist identify septic patients who qualify for one of the proven therapies for severe sepsis.
EARLY, EFFECTIVE ANTIBIOTICS
For obvious ethical reasons, randomized, controlled trials to study the impact of inappropriate or delayed antibiotic therapy for serious infections are not possible. However, the evidence supporting early, effective antibiotic therapy is still compelling, and because many hospitalists often initiate treatment with antibiotics before transferring a patient to intensive care, this may represent the most important intervention hospitalists can provide to patients with serious infections. Several studies have estimated the impact of early, effective antibiotics on outcomes.
Houck et al. retrospectively reviewed 13,771 cases of community‐acquired pneumonia among elderly Medicare patients. They found that 39.1% of the patients waited more than 4 hours for antibiotics and 7.6% waited more than 12 hours; three quarters of these delays resulted from delayed ordering of antibiotics.18 Further, 21.2% received an antibiotic selection incompatible with recent professional guidelines. Receiving antibiotics within 4 hours reduced in‐hospital and 30‐day mortality by 15% and length of stay by 0.4 days.18 Similar conclusions were reported by 3 of 4 previous analyses.1922 Extending these findings to critically ill patients, Iregui et al. found that delayed treatment with appropriate antibiotics (odds ratio, 7.68) was a greater predictor of mortality for 107 patients with ventilator‐acquired pneumonia than were APACHE II scores and malignancy; 31% failed to receive appropriate antibiotics within 24 hours, and again, three quarters of these delays resulted from delays in writing antibiotic orders.23
Not surprisingly, antibiotic therapy must be effective as well as timely. MacArthur et al. studied the impact of adequate (ie, active against cultured organisms, if isolated) antibiotics on the outcomes of 2634 septic patients enrolled in a randomized trial of an anti‐TNF antibody. Nearly 91% received appropriate antibiotics; their mortality rate was 33%, 10% lower than that of the patients whose initial antibiotics were inadequate (P < .001).24 Leibovici et al. reported similar findings in a prospective study of patients with bacteremia. Only 63% of 3413 subjects received an antibiotic active against the infecting pathogen, and their mortality was 20%, 14% lower than that in the group that received ineffective antibiotics (P = .0001).25 Other authors have reported even worse outcomes with ineffective therapy: 62% mortality among inadequately treated bacteremic or fungemic ICU patients, compared with 28.4% among those who were adequately treated26 and an odds ratio of dying of 8.14 for the 46 of 270 septic ICU patients who received inadequate initial antibiotics,27 making inadequate antibiotic therapy the strongest risk factor for death. Finally, Kollef et al. reported that 26% of 655 infected ICU patients received inadequate antibiotics and suffered an infection‐related mortality rate of 40.2%, more than twice the 17.7% rate among adequately treated patients (P < .001). Inadequate antimicrobial therapy was a greater risk factor for death than early respiratory failure or sepsis‐related organ failure assessment scores.28
Guidelines for anti‐infective care now recommend obtaining appropriate cultures and administering broad‐spectrum antibiotics (appropriate for suspected infections, local susceptibility patterns, and any relevant prior culture data from individual patients) within 1 hour of presentation.11 In addition, any removable focus of infection must be identified and managed (eg, an abscess, infected catheter, tampon, or infection requiring surgery).
ACTIVATED PROTEIN C
Recombinant human activated protein C (APC) is a protein with anticoagulant and anti‐inflammatory properties that is relatively deficient in approximately 87% of septic patients.29 Although numerous trials of other anticoagulants (antithrombin III and tissue factor pathway inhibitor) and immunosuppressives (tumor necrosis factor inhibitors, high‐dose steroids, interleukin‐1 receptor antagonists, and others) have failed to show any benefit,7 in 2001 APC became the first proven therapy specifically for sepsis. The PROWESS trial, which established its efficacy, randomized 1690 patients who met 3 SIRS criteria and dysfunction of at least 1 organ system to APC (24 g/kg IV per hour for 96 hours interrupted for bleeding or urgent procedures) or placebo. APC reduced 28‐day mortality from 30.8% to 24.7%, yielding an absolute risk reduction of 6.1% and a corresponding number needed to treat (NNT) of 16.4. This benefit was seen across all subgroups including those with normal baseline APC levels.29
Not surprisingly, APC increases the risk of serious bleeding. Although this effect was of borderline significance in PROWESS (3.5% vs. 2% in the placebo group, P = .06),29 it was confirmed in subsequent trials (3.9% vs. 2.2%, P = .01)30 and may be larger still in open‐label use, at 6.5%.17, 31 Intracerebral hemorrhage (ICH), a particularly devastating complication, occurred in 0.2% of the PROWESS patients and 0.5% of patients in 2 subsequent studies30, 32; in both major trials, there was a single extra event in the APC arm.29, 30 Like serious bleeding in general, ICH was more common in open‐label use, occurring in 1.5% of patients.31, 33 Therefore, it is vital to have strict adherence to exclusion criteria and familiarity with the risk factors for serious bleeding. In the PROWESS trial, after randomization, risk factors for serious bleeding included procedures and injury to vascular organs, an activated partial‐thromboplastin time of more than 120 seconds, an international normalized ratio greater than 3, gastrointestinal ulceration, and development of severe thrombocytopenia (< 30,000/mm3)29; in a 2002 study of 2786 APC recipients, ICH was largely confined to patients with meningitis or a platelet count less than 30,000/mm3.32
APC therapy has several other limitations and drawbacks. Multiple contraindications, including predisposition to bleeding, a recent history of bleeding, anticoagulant use, immunosuppression, liver disease, dialysis dependence, and hypercoagulable states, restrict its use. APC appears to work best when administered early, within 24 hours of the onset of organ dysfunction.31 In addition, APC is indicated only in adults with Acute Physiology and Chronic Health Evaluation (APACHE II) scores greater than 24 and multiorgan failure. Post hoc analysis of the PROWESS data showed that although the relative risk (RR) of death for those with APACHE II scores of 25 or more was .71 and statistically significant, the RR for those with scores below 25 was a nonsignificant .99.34 A subsequent study, ADDRESS, confirmed there was no benefit to septic patients with a low risk of death.30 In the ADDRESS study 2613 patients with severe sepsis and either an APACHE II score less than 25 or single organ failure were randomized to APC or placebo. No differences were found in 28‐day and in‐hospital mortality; among patients who had undergone surgery in the previous 30 days, those receiving APC had a significantly increased risk of death (20.7% vs. 14.1%, P = .03).
An additional drawback of APC therapy is its cost, approximately $6800 per infusion, although the cost per year of life gained, $24,484, or $52,360 per life saved (NNT $6800), is reasonable for those with APACHE II scores greater than 24.34 Concerns have also been raised about the PROWESS trial itself: the production of the study drug and some exclusion criteria were changed midtrial, after which the effectiveness of APC improved. APACHE II scores had not been validated for selection of patients for therapies and may have varied with time or by observer. The original PROWESS study population may have been skewed away from chronically ill patients.35 Experts differ on the significance of these concerns and even whether APC therapy should be considered the standard of care pending further research.32, 35 The ADDRESS trial also failed to demonstrate a benefit in a subgroup of patients with APACHE II scores above 24, although it was underpowered to do so, and according to enrollment criteria, none of those patients had multiorgan failure.30 However, in the subgroup of PROWESS patients with APACHE II scores greater than 24, the absolute reduction in mortality was a full 13%,17 with a corresponding NNT of 7.7, and although the PROWESS findings have not been duplicated in a second randomized trial, a single‐arm, open‐label study of APC (ENHANCE) showed a nearly identical mortality rate.31 Pending confirmatory trials, APC remains a recommended therapy for selected patients sick enough to benefit and without excessive bleeding risk.11
EARLY GOAL‐DIRECTED THERAPY
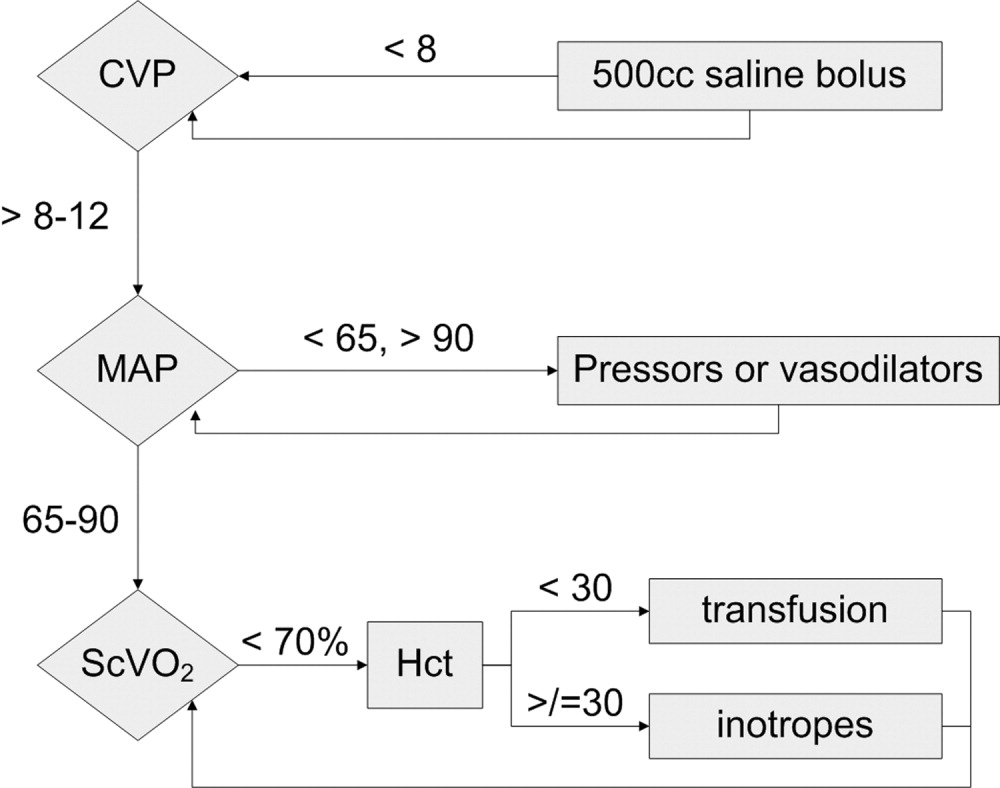
Because physician‐directed resuscitation for sepsis may normalize vital signs, central venous pressures (CVP), and urine output without correcting hypoperfusion, Rivers et al. tested a resuscitation protocol that incorporated a central line that continuously monitored mixed‐venous oxygen saturation as a surrogate for cardiac output.36 They randomized 263 patients with septic shock (defined as hypotension < 90 mm Hg after a 20‐30 mL/kg bolus, or lactate > 4 mmol/L, which is associated with at least a 3‐fold increase in the mortality of emergency department patients with suspected infection37) to either standard care or early goal‐directed therapy (EGDT) for the initial 6 hours of hospital care. Patients with acute coronary ischemia, pulmonary edema, stroke, asthma, overdose, trauma, dysrhythmia, immunosuppression, uncontrolled cancer, or a need for urgent procedures were excluded. Standard care was directed by physiologic parameters such as vital signs, urine output, and CVP. EGDT used sequential therapies designed to support organ perfusion: 500 mL of normal saline was given every half hour until the CVP was at least 8‐12 mm Hg. Pressors were given until the mean arterial pressure was 65‐90 mm Hg (norepinephrine36 or dopamine were preferred agents, and vasopressin [0.01‐0.04 units/min] was an option for shock refractory to first‐line pressors)11, 38 Transfusion (to a hematocrit goal of 30) and dobutamine were given until mixed‐venous oxygenation saturation was 70% or better (Fig. 1). Lastly, patients who did not achieve this goal were sedated and mechanically ventilated.
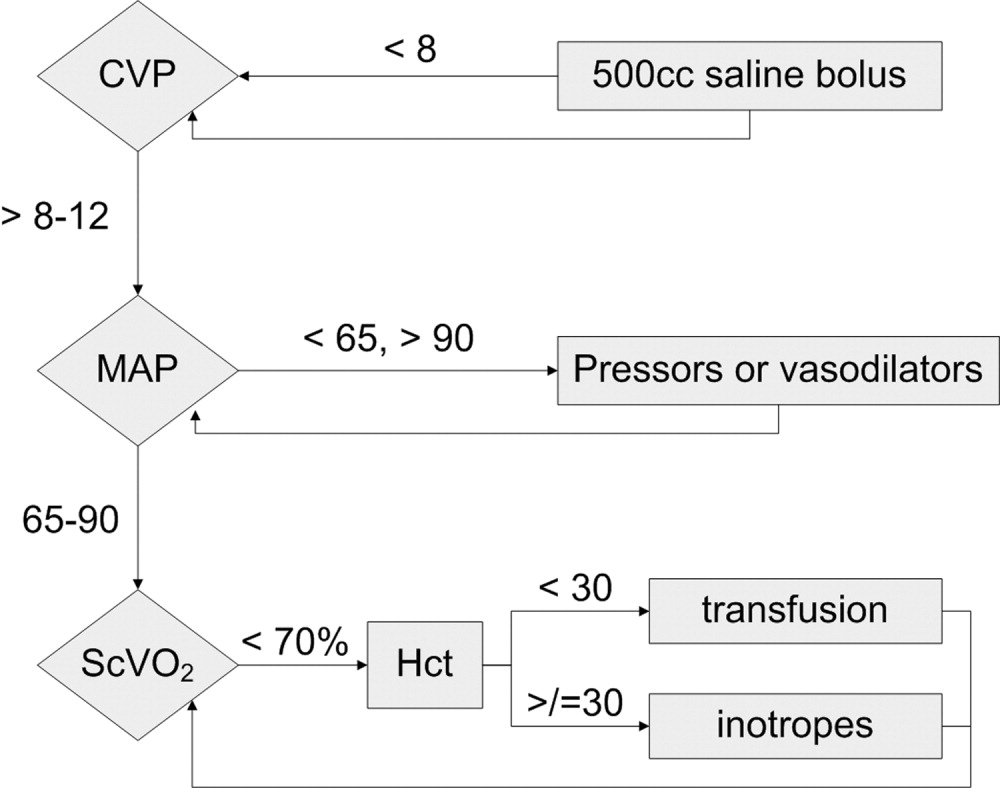
Results were dramatic: mortality was reduced from 46.5% to 30.5%, with an ARR of 16% and an NNT of 6.25. Study patients received similar amounts of crystalloid, but received it earlier than the standard care patients and received more transfusions and inotropes. Substantially more patients in the EGDT group than the standard care group achieved a mixed venous oxygen saturation of 70%; 13.7% of the EGDT patients had occult hypoperfusion (low mixed‐venous oxygenation that responded to inotropes despite satisfactory vital signs). EGDT improved length of stay (4 days shorter among survivors) and duration of intubation, as well as APACHE scores and several physiologic parameters.36
Critics of this trial note the impossibility of adequate blinding and the high mortality in the placebo group. Further, because the trial tested the EGDT protocol as a whole, there was no way to know if each step was optimal. For example, a different CVP goal could have been used or adjustments made for mechanical ventilation, which can falsely elevate a low CVP into the desired range (the Surviving Sepsis Campaign guidelines recommend a CVP goal of 12‐15 mm Hg in mechanically ventilated patients11). Also, the selection of pressor, the use of inotropes, and the transfusion threshold were chosen on the basis of physiologic rationales, but all of these are arguable.39 This was also a single trial, and earlier goal‐directed protocols for ICU patients actually showed harm,40, 41 although those trials targeted supranormal physiologic goals in more established critical illness.42 Finally, on a practical level, hospitals and particularly emergency departments must commit resources to train physicians and staff, purchase the appropriate central venous catheters, and convince eligible patients to undergo an invasive procedure. In a survey of 30 attending physicians in academic referral hospitals, only 7% reported standard use of EGDT. Barriers included the requirement for specialty monitoring equipment and other resources, and central venous cannulation.43
Despite these concerns, the striking reduction in mortality associated with EGDT led to its endorsement by the Surviving Sepsis Campaign guidelines and underscores the principle of aggressive early resuscitation for patients who do not meet eligibility criteria but appear at risk for worsening sepsis. As yet, however, no strong evidence mandates a specific approach to the septic patient without shock.
STRESS DOSE STEROIDS
Because of the importance of the inflammatory cascade in severe sepsis, a potential role for steroids in the management of sepsis has been repeatedly studied. More than 50 studies have been performed since the 1950s, generally with pharmacologic doses of steroid; a meta‐analysis showed that such a practice was ineffective.44, 45 However, data accumulated that relative adrenal insufficiency during severe sepsis was common and associated with an increased risk of death and that physiologic doses of steroids could reverse refractory hypotension.46 To define the role of a physiologic course of steroids in septic shock, Annane et al. randomized 299 critically ill adults to either 7 days of stress dose hydrocortisone (50 mg IV every 6 hours) and fludrocortisone (50 g NG every 24 hours) or matched placebos. Enrolled patients were severely ill; the placebo group had a 63% mortality, and patients had to have septic shock, oliguria or hypoxia, hypotension despite low‐dose dopamine, and lactate greater than 2 mmol/L and require mechanical ventilation. Pregnant women, those with myocardial infarction or pulmonary embolus, advanced malignancies, or immunodeficiency, and those with clear indications or contraindications to steroids were excluded.47 Enrollment criteria were modified midstudy; changes included the exclusion of patients who had recently received etomidate, which inhibits 11‐‐hydroxylase and has been identified as a risk factor for adrenal insufficiency in intensive care patients.48 All patients received a 250‐g cosyntropin stimulation test. The authors considered patients nonresponders to consyntropin if serum cortisol failed to increase to 9 g/dL or more.
Steroids reduced the duration that a vasopressor was required and reduced mortality from 63% to 53% among nonresponders, giving an NNT of only 10 to prevent 1 death at 28 days. Although the authors described no evidence of adverse effects, among the subset of 70 patients who responded appropriately to cosyntropin, there was a nonsignificant trend toward increased mortality, and rates of hyperglycemia were not provided.47 The authors concluded that physicians should test appropriate patients for adrenal reserve, give the studied steroid regimen while results are pending, and discontinue treatment if a patient retains adrenal reserve.
The literature on steroids and critical illness is complex, with more than 1300 articles on steroids and sepsis published since 1988, and several concerns were raised about the Annane study. For example, did much of the benefit for those patients enrolled before the protocol amendment come from reducing an adverse effect of etomidate?49 Does the high‐dose, 250‐g cosyntropin stimulation test overcome (and conceal) partial ACTH resistance that might benefit from treatment?50 Might not a robust baseline cortisol suggest sufficient adrenal function regardless of the incremental response to cosyntropin?51 Partial answers were provided by 2 subsequent meta‐analyses. Both found that more recent studies gave lower doses of steroids in longer, 5‐ to 7‐day courses to sicker patients and demonstrated improvement in mortality and shock reversal, with relative risk reductions of 14%‐22%; the NNT ranged from 8 to 11. One analysis found no difference in outcomes between adrenally sufficient and adrenally insufficient patients, and those authors advised considering treatment for all patients regardless of their adrenal function test results.8 The other analysis concluded that the data on steroids for those with adrenal reserve was too limited to recommend treating adrenally sufficient patients.9
Disputes about certain details, such as whether patients should be treated without regard to adrenal reserve, continue in the literature.45, 52 An ongoing randomized, controlled trial, CORTICUS, is expected to provide additional guidance on the use of low‐dose steroids in sepsis; in the meantime, the literature clearly supports a longer course of low‐dose steroid therapy for patients with pressor‐dependent septic shock with inadequate adrenal reserve by cosyntropin testing, and guidelines allow discretion about whether patients with adequate adrenal reserve should also be treated.11 Hospitalists may also want to treat septic shock with equivalent doses of dexamethasone (approximately 2 mg IV every 6 hours) if adrenal evaluation may be delayed, as this agent will not confound cosyntropin stimulation test results, and they may want to avoid etomidate in septic patients53, 54 for whom they perform or supervise intubations.
INTENSIVE INSULIN THERAPY
Mounting evidence supports the short‐term role of hyperglycemia in morbidity and mortality, especially in critical illness. Hyperglycemia impairs neutrophil and endothelial cell function as well as protective responses to cardiac and neuronal ischemia,55 whereas insulin has anti‐inflammatory and antiapoptotic effects,7, 56 suggesting that intensive insulin might improve the outcomes of critically ill patients. To test this theory, van den Berghe and colleagues randomized 1548 mostly surgical ICU patients to insulin infusions titrated for glucose goals of either 80‐110 or 180‐200 mg/dL, followed by subcutaneous insulin after ICU discharge. Although blinding was impossible, in both cases glucose management was performed by a separate research team. Multiple benefits were noted: ICU and total in‐hospital deaths were reduced, mostly among patients with an ICU stay of more than 5 days, whose risk of death fell from 20.2% to 10.6%. Intensive insulin also reduced septicemia, renal impairment, critical illness polyneuropathy, and duration of intensive care.57
Subsequently, a meta‐analysis of 35 trials suggested that insulin reduced the mortality of critically ill patients by 15%.10 Van den Berghe et al.'s results were also duplicated in a broad, medical‐surgical ICU population, although the reductions in morbidity and mortality were measured against historical controls.58 However, whether the results of the influential surgical ICU study could be applied to medical patients was not known until 2006, when the van den Berghe group reported the effects of similar insulin protocols on 1200 patients in the medical ICU who were expected to need intensive care for at least 3 days.59 In this study, intensive insulin failed to reduce overall mortality (40% and 37.3%, P = .33). However, intensive insulin did reduce mortality among the 64% of patients who stayed in the ICU 3 or more days from 52.5% to 43% (NNT 10.5, P = .009). This benefit was offset by an increased number of deaths in the intensive insulin group among patients with ICU stays of less than 3 days (P = .05‐.35 depending on the method used).59 Intensive insulin did reduce newly acquired kidney injury, duration of mechanical ventilation, and lengths of ICU and hospital stays, and the reduction in morbidity increased with the duration of intensive insulin therapy. Hypoglycemia (mean 32 mg/dL) occurred in 25% of patients with prolonged stays6.4 times as often as in the usual care group.60 Liver and renal failure were associated with hypoglycemia.59
Critics of the surgical ICU trial noted the high mortality among the usual care patients (5.1%), a robust 34% mortality reduction for a relatively small 50 mg/dL reduction in morning glucose levels, and the aggressive use of parenteral nutrition, raising the question of whether intensive insulin merely attenuated the side effects of intravenous glucose.61, 62 Also, the ideal blood glucose target is not known with certainty. Retrospective studies suggested the upper limit for target blood glucoses could be 145 mg/dL63 and found differing thresholds at which hyperglycemia increased mortality in nondiabetics (144 mg/dL) and diabetics (200 mg/dL).64 However, in the surgical ICU trial, there was no threshold below which there was no further reduction in risk; patients whose mean blood glucose was below 110 mg/dL had lower mortality than those whose levels were between 110 and 150 mg/dL (P = .026).65 Finally, the effects of hyperglycemia and intensive insulin may vary by population: retrospective studies found that ICU hyperglycemia was more strongly associated with mortality among nondiabetics,64, 66 and van den Berghe et al. noted no benefit from intensive insulin in a small subgroup of diabetics.59
In summary, large, well‐designed trials have demonstrated that intensive insulin reduced mortality in critically ill patients after a delay of 3‐5 days, but this benefit did not extend to all patients in the medical ICU.57, 59 Some authors have suggested deferring intensive insulin for 3 days,67 but because early therapy probably contributes to the delayed mortality benefit, this approach may deprive patients of the observed benefits. Ongoing clinical trials (NICE‐SUGAR) are likely to provide useful information about how hyperglycemia should be managed in different populations, including septic ICU patients.61 In the meantime, institutions can select the intensity of their insulin therapy by weighing morbidity and long‐term mortality benefits against possible short‐term harms and ensuring that hospital staff members are sufficiently trained to control hyperglycemia safely. For example, in critical illness, intravenous insulin is preferable to subcutaneous insulin, and the frequent measurement of whole‐blood glucose instead of finger‐stick glucose helps to avoid errors.55, 68 And although researchers were unable to prospectively identify patients with long ICU stays,59 severely septic patients have long ICU stays (generally 7.5‐16.6 days),14 and individual ICUs might observe enough stays of more than 2 days in their patient population to justify intensive insulin for this subgroup. And finally, although no conclusive evidence mandates a specific approach to hyperglycemia outside the ICU, the ICU data provide a physiologic rationale for cautious but tight control of glucose in more moderately ill patients. Guidelines for the management of inpatient hyperglycemia were published previously.55
SEPSIS AND THE HOSPITALIST
Hospitalists who provide critical care may make frequent decisions about the inclusion and exclusion criteria for the major trials of sepsis, weigh their relative benefits against risks and costs, contemplate gray areas such as adrenal testing in shock, and employ evidence‐based therapies for severe sepsis. However, hospitalists may also see patients who qualify for these therapies when they are called to see septic patients in the emergency department, when severe sepsis develops in patients on the medicine ward, or when they provide consultation services in an ICU. Sepsis care must be implemented urgently; patients in the pivotal trial of steroids had to be randomized within 3 hours of shock onset,47 data suggest that the window for optimal antibiotic therapy may be no greater than 4 hours from diagnosis,18 whereas guidelines suggest therapy within 1 hour,11 and early goal‐directed therapy was studied only for the first 6 or more hours of hospitalization.36 Thus, hospitalists who do not provide ICU care should be able to identify patients with severe sepsis and either deliver initial care or recognize the need for immediate consultation. Specifically, hospitalists can:
-
Recognize that both absolute (< 90 mm Hg) and relative hypotension (> 40 mm Hg below baseline) indicate septic shock;
-
Identify normotensive candidates for EGDT (severe sepsis with serum lactate > 4 mmol/L) by requesting a serum lactate in addition to prompt appropriate cultures for severe acute infection69;
-
Recognize atypical presentations of sepsis (tachypnea, tachycardia, confusion, etc.) and maintain a high suspicion for sepsis in patients who may be predisposed to infection and to atypical presentation because of age, immunosuppression, neutropenia, diabetes, or other conditions;
-
Initiate effective antibiotics and EGDT promptly for individual patients or by coordinating efforts to improve sepsis care at an institutional level, for example, as a component of medical emergency team services70, 71;
-
Rapidly identify and manage removable foci of infection such as abscesses, empyemas, necrotizing fasciitis, or infected vascular catheters; and
-
Competently educate hospital staff, residents, and medical students about sepsis care.
Hospitalists are busy physicians, and the task of reviewing sepsis literature and implementing recommendations is daunting. However, hospitalists can turn to resources such as the Surviving Sepsis Campaign Guidelines, a series of recommendations for managing severe sepsis that were endorsed by 11 international critical care and infectious disease societies and published in Critical Care Medicine in 2004.11 The Institute for Healthcare Improvement has also published a series of online severe sepsis bundles, or groups of proven interventions, complete with implementation tips and supporting literature, available at
CONCLUSION: DEADLY YET TREATABLE
The death toll from severe sepsis in the United States exceeds that of lung, breast, and colon cancer combined and equals that of myocardial infarction (MI),1 a condition that appropriately triggers a series of emergency interventions. Physicians now have an arsenal of therapies for severe sepsis analogous to those employed for MI, and a comparison between the 2 conditions underscores the high mortality rate of severe sepsis and the enormous impact on patient outcomes provided by evidence‐based sepsis therapy. Figure 2 compares the 9.5%‐16% ARR for death associated with APC in patients with APACHE 2 scores greater than 24 and multiorgan failure,29 EGDT,36 stress dose steroids in shock complicated by adrenal insufficiency,47 and intensive insulin in patients with medical ICU stays longer than 3 days,59 with the benefits of thrombolysis for ST‐elevation MI (2%‐3%)73 or antiplatelet therapy for acute MI (2.3%).74 Figure 3 compares the corresponding NNT values to save 1 life; according to the available data, a hospitalist is 5‐8 times more likely to save a life with EGDT than with fibrinolysis.
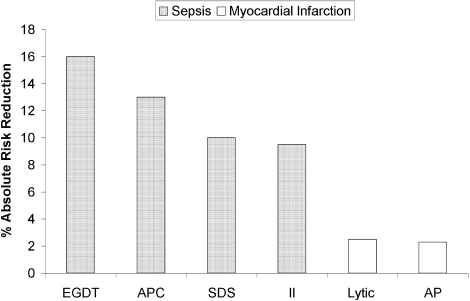
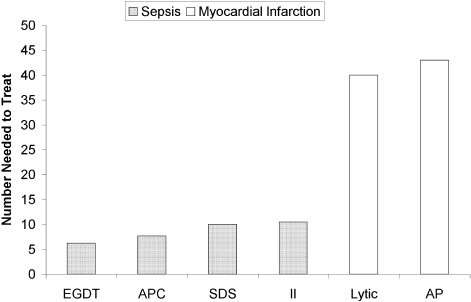
Because the literature supporting several major sepsis therapies have been limited to retrospective studies1828 and single randomized, controlled trials29, 36 and because key trials are still underway (CORTICUS, NICE‐SUGAR), the benefits of sepsis therapies are less certain than are those for the treatment of MI. This was underscored by the finding that the benefit in reduced mortality of intensive insulin in the surgical ICU57 did not extend to all patients in the medical ICU.59 However, the potentially marked survival benefit of early effective antibiotics, APC, EGDT, stress dose steroids, and intensive insulin and the urgency with which they must be applied demand that all hospitalists become or remain familiar with the evolving sepsis literature.
- ,,,,,.Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care.Crit Care Med.2001;29:1303–1310.
- ,,,.Prevalence and incidence of severe sepsis in Dutch intensive care units.Crit Care.2004;8:R153–R162.
- ,, et al.Direct costs of severe sepsis in three German intensive care units based on retrospective electronic patient record analysis of resource use.Intensive Care Med.2002;28:1440–1446.
- ,,,,.Effects of severity of illness on resource use by survivors and nonsurvivors of severe sepsis at intensive care unit admission.Crit Care Med.2002;30:2413–2419.
- ,,, et al.Resource utilization among patients with sepsis syndrome.Infect Control Hosp Epidemiol.2003;24:62–70.
- ,,,,.The costs of septic syndromes in the intensive care unit and influence of hospital‐acquired sepsis.Intensive Care Med,2003;29:1464–1471.
- ,.The pathophysiology and treatment of sepsis.N Engl J Med.2003;348:138–150.
- ,,,,.Meta‐analysis: the effect of steroids on survival and shock during sepsis depends on the dose.Ann Intern Med.2004;141:47–56.
- ,,,,,.Corticosteroids for severe sepsis and septic shock: a systematic review and meta‐analysis.Brit Med J.2004;329:480–488.
- ,,.Insulin therapy for critically ill hospitalized patients: a meta‐analysis of randomized controlled trials.Arch Intern Med.2004;164:2005–2011.
- ,,, et al.Surviving Sepsis Campaign guidelines for management of severe sepsis and septic shock.Crit Care Med.2004;32:858–873.
- ,,,,.Prevention of deep vein thrombosis in medical patients by low‐dose heparin.Scott Med J.1981;26:115–117.
- ,,, et al.A comparison of sucralfate and ranitidine for the prevention of upper gastrointestinal bleeding in patients requiring mechanical ventilation. Canadian Critical Care Trials Group.N Engl J Med.1998;338:791–797.
- The Acute Respiratory Distress Syndrome Network.Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome.N Engl J Med.2000;342:1301–1308.
- American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference: definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis.Crit Care Med.1992;20:864–874.
- ;;, et al. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference.Crit Care Med.2003;31:1250–1256.
- .Severe sepsis and therapy with activated protein C.New Engl J Med.2005;353:1398–1399.
- ,,,,.Timing of antibiotic administration and outcomes for Medicare patients hospitalized with community‐acquired pneumonia.Arch Intern Med.2004;164:637–644.
- ,,, et al.Measuring quality of care with explicit process criteria before and after implementation of the DRG‐based prospective payment system.JAMA.1990;264:1969–1973.
- ,.Pneumonia mortality reduction and quality improvement in a community hospital.Qual Rev Bull.1993;19:124–130.
- ,,, et al.Quality of care, process and outcomes in elderly patients with pneumonia.JAMA.1997;278:2080–2084.
- ,,,,.Processes of care, illness severity, and outcomes in the management of community‐acquired pneumonia at academic hospitals.Arch Intern Med.2001;161:2099–2104.
- ,,,,.Clinical importance of delays in the initiation of appropriate antibiotic treatment for ventilator‐associated pneumonia.Chest.2002;122:262–268.
- ,,, et al.Adequacy of early empiric antibiotic treatment and survival in severe sepsis: experience from the MONARCS Trial.Clin Infect Dis.2004;38:284–288.
- ,,,,,.The benefit of appropriate empirical antibiotic treatment in patients with bloodstream infection.J Intern Med.1998;244:379–386.
- ,,,,.The influence of inadequate antimicrobial treatment of bloodstream infections on patient outcomes in the ICU setting.Chest.2000;118:146–155.
- ,,,,,.Impact of adequate empirical antibiotic therapy on the outcome of patients admitted to the intensive care unit with sepsis.Crit Care Med.2003;31:2742–2751.
- ,,, et al.Inadequate antimicrobial treatment of infections: a risk factor for hospital mortality among critically ill patients.Chest.1999;115:462–474.
- ,,, et al.Efficacy and safety of recombinant human activated protein C for severe sepsis.N Engl J Med.2001;344:699–709.
- ,,, et al.Drotecogin alfa (activated) for adults with severe sepsis and a low risk of death.New Eng J Med.2005;353:1332–1341.
- ,,, et al.Drotecogin alfa (activated) treatment in severe sepsis from the global open label trial ENHANCE.Crit Care Med.2005;10:2266–2277.
- ,,.Activated protein C for severe sepsis.N Engl J Med.2002;347;1035–1036.
- .Assessing the use of activated protein C in the treatment of severe sepsis.N Engl J Med.2002;347:1030–1034.
- ,,,,.An economic evaluation of activated protein C treatment for severe sepsis.N Engl J Med.2002;347:993–1000.
- ,,,.Risks and benefits of activated protein C treatment for severe sepsis.N Engl J Med.2002;347:1027–1030.
- ,,, et al.Early goal‐directed therapy in the treatment of severe sepsis and septic shock.N Engl J Med.2001;345:1368–1377.
- ,,, et al.Serum lactate as a predictor of mortality in emergency department patients with infection.Ann Emerg Med.2005;45:524–528.
- ,,,.Vasopressor and inotropic support in septic shock: an evidence‐based review.Crit Care Med.2004;32(11 Suppl):S455–S465.
- ,,, et al.Goal‐directed therapy for severe sepsis [letter].N Engl J Med.2002;346:1025–1026.
- ,,,,,.Elevation of systemic oxygen delivery in the treatment of critically ill patients.New Engl J Med.1994;330:1717–1722.
- ,, et al.A trial of goal‐oriented hemodynamic therapy in critically ill patients.N Engl J Med.1995;333:1025–1032.
- .Hemodynamic and metabolic therapy in critically ill patients.New Engl J Med.2001;345:1417–1418.
- ,.Use of goal directed therapy for severe sepsis and septic shock in academic emergency departments.Crit Care Med.2005;33:1888–1889.
- ,,, et al.Corticosteroid treatment for sepsis: a critical appraisal and meta‐analysis of the literature.Crit Care Med.1995;23:1430–1439.
- .Physicians should administer low‐dose corticosteroids selectively to septic patients until an ongoing trial is completed.Ann Intern Med.2004;141:70–72.
- ,.Corticosteroids and septic shock.JAMA.2002;288:886–887.
- ,,, et al.Effect of treatment with low doses of hydrocortisone and fludrocortisone on mortality in patients with septic shock.JAMA.2002;288:862–871.
- ,,, et al.Risk factors of relative adrenocortical deficiency in intensive care patients needing mechanical ventilation.Intensive Care Med.2005;31:388–392.
- ,.Editorial III: Corticosteroids for septic shock—a standard of care?Br J Anaesth.2004;93:178–180.
- ,,.Corticosteroids for patients with septic shock [letter].JAMA.2003;289:42.
- ,,,,.Corticosteroids for patients with septic shock [letter].JAMA.2003;289:42.
- ,,,,.Corticosteroids for septic shock [letter].Ann Intern Med.2004;141:742–743.
- .Should we use etomidate as an induction agent for endotracheal intubation in patients with septic shock?: a critical appraisal.Chest.2005;127:1031–1038.
- .ICU physicians should abandon the use of etomidate!Intensive Care Med.2005;31:325–326.
- ,,, et al.Management of diabetes and hyperglycemia in hospitals.Diabetes Care.2004;27:553–591.
- ,,,,.Intensive insulin therapy exerts anti‐inflammatory effects in critically ill patients and counteracts the adverse effects of low mannose binding lectin levels.J Clin Endocrinol Metab.2003;88:1082–1088.
- ,,, et al.Intensive insulin therapy in critically ill patients.N Engl J Med.2001;345:1359–1367.
- .Effect of an intensive glucose management protocol on the mortality of critically ill adult patients.Mayo Clin Proc.2004;79:992–1000. Published erratum appears in Mayo Clin Proc.year="2005"2005;80:1101
- ,,, et al.Intensive insulin therapy in the medical ICU.N Engl J Med.2006;354:449–461.
- Supplement to:,,, et al.Intensive insulin therapy in the medical ICU.N Eng J Med.2006;354:449–461. Available at: http://content.nejm.org/cgi/data/354/5/449/DC1/1.
- .Glycemic control in the intensive care unit: why we should wait for NICE‐SUGAR.Mayo Clin Proc.2005;80:1546–1548.
- ,,.Intensive insulin therapy in critically ill patients [letter].New Engl J Med.2002;346:1586–1588.
- ,,,.Glucose control and mortality in critically ill patients.JAMA.2003;290:2041–2047.
- ,,,,.Influence of individual characteristics on outcome of glycemic control in intensive care unit patients with or without diabetes mellitus.Mayo Clin Proc.2005;80:1558–1567.
- ,,, et al.Outcome benefit of intensive insulin therapy in the critically ill: Insulin dose versus glycemic control.Crit Care Med.2003;31:634–635.
- ,,,,.Impact of admission hyperglycemia on hospital mortality in various intensive care unit populations.Crit Care Med.2005;33:2772–2777.
- .Intensive insulin in intensive care.New Engl J Med.2006;354:516–518.
- ,,,,.Fingerstick glucose determination in shock.Ann Intern Med.1991;114:1020–1024.
- ,,.A blueprint for a sepsis protocol.Acad Emerg Med.2005;12:352–359.
- ,,, et al.A prospective before‐and‐after trial of a medical emergency team.Med J Aust.2003;179:283–287.
- ,,, et al.A multidisciplinary community hospital program for early and rapid resuscitation of shock in nontrauma patients.Chest.2005;127:1729–1743.
- ,,, et al.Sepsis change bundles: converting guidelines into meaningful change in behavior and clinical outcome.Crit Care Med.2004;32:S595–S597.
- Fibrinolytic Therapy Trialists' Collaborative Group.Indications for fibrinolytic therapy in suspected acute myocardial infarction: collaborative overview of early mortality and major morbidity results from all randomised trials of more than 1000 patients.Lancet.1994;343:311–322.
- Antithrombotic Trialists' Collaboration.Collaborative meta‐analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients.Brit Med J.2002;324:71–86.
Despite decades of intensive research and improvements in medical care, severe sepsis affects an estimated 751,000 patients in the United States every year, killing 215,000 of them at an annual cost of 16.7 billion dollars.1 Because the elderly experience a 100‐fold increase in incidence, as compared with children, and a nearly 4‐fold increase in mortality (38.4% of those more than 85 years old), this burden is expected to increase with the aging population.1 Patients with severe sepsis have prolonged ICU14 and hospital stays and incur substantially increased costs compared with other patients.36
New research continues to explore the complex pathophysiology of sepsis,7 and clinicians, who once relied primarily on clinical experience and expert opinion to guide therapy, now have an increasing array of evidenced‐based sepsis therapies to employ. Recent meta‐analyses have evaluated several major treatments for severe sepsis,810 and recommendations (the Surviving Sepsis Campaign guidelines) for the treatment of severe sepsis were recently endorsed by 11 international critical care and infectious disease organizations.11 This article summarizes the current definitions of sepsis syndromes, the trials supporting the specific therapies for sepsis that are currently recommended, ongoing controversies and research, and implications for hospitalists, with a focus on early, effective antibiotics, activated protein C, early goal‐directed therapy, stress dose steroids, and intensive insulin therapy. For space considerations, readers are directed elsewhere for data supporting prophylaxis for deep venous thrombosis (DVT)12 and stress ulcer bleeding13 and for therapies less often directed by hospitalists, such as lung protective ventilation.14
DEFINITIONS
Systemic inflammatory response syndrome (SIRS), sepsis, severe sepsis, and septic shock were defined in 1992 to standardize the terminology of sepsis.15 These definitions have recently been reviewed and supported by a variety of American and European intensive care societies.16
SIRS is defined by the presence of at least 2 of the following:
-
Temperature > 38C or < 36C;
-
Heart rate > 90 beats/min;
-
Respiratory rate > 20 breaths/min or PaCO2 < 32 mm Hg;
-
WBC >12,000 or < 4000 cells/mm3, or >10% immature (band) forms.
Sepsis is SIRS due to documented or strongly suspected infection.
Severe sepsis is sepsis with organ dysfunction (such as lactic acidosis, oliguria, thrombocytopenia, or delirium), hypoperfusion, or hypotension (< 90 mm Hg systolic or more than 40 mm Hg below baseline).
Septic shock is severe sepsis complicated by hypotension or pressor dependence despite adequate (20‐30 mL/kg; 1.5‐3 liters in most patients) fluid resuscitation.
Sepsis terminology must be applied carefully. Many hospitalized patients meet criteria for SIRS, yet it is inaccurate to say a patient who has acute leukemia with leukocytosis, anemia‐induced tachycardia, and thrombocytopenia has severe sepsis if those abnormalities are not a result of inflammation or infection. Accurate documentation of sepsis syndromes can improve professional and institutional reimbursement and provide prognostic information: the in‐hospital mortality rates for severe sepsis and septic shock are approximately 30% and 50%, respectively.17 More importantly, thoughtful application of these definitions can help a hospitalist identify septic patients who qualify for one of the proven therapies for severe sepsis.
EARLY, EFFECTIVE ANTIBIOTICS
For obvious ethical reasons, randomized, controlled trials to study the impact of inappropriate or delayed antibiotic therapy for serious infections are not possible. However, the evidence supporting early, effective antibiotic therapy is still compelling, and because many hospitalists often initiate treatment with antibiotics before transferring a patient to intensive care, this may represent the most important intervention hospitalists can provide to patients with serious infections. Several studies have estimated the impact of early, effective antibiotics on outcomes.
Houck et al. retrospectively reviewed 13,771 cases of community‐acquired pneumonia among elderly Medicare patients. They found that 39.1% of the patients waited more than 4 hours for antibiotics and 7.6% waited more than 12 hours; three quarters of these delays resulted from delayed ordering of antibiotics.18 Further, 21.2% received an antibiotic selection incompatible with recent professional guidelines. Receiving antibiotics within 4 hours reduced in‐hospital and 30‐day mortality by 15% and length of stay by 0.4 days.18 Similar conclusions were reported by 3 of 4 previous analyses.1922 Extending these findings to critically ill patients, Iregui et al. found that delayed treatment with appropriate antibiotics (odds ratio, 7.68) was a greater predictor of mortality for 107 patients with ventilator‐acquired pneumonia than were APACHE II scores and malignancy; 31% failed to receive appropriate antibiotics within 24 hours, and again, three quarters of these delays resulted from delays in writing antibiotic orders.23
Not surprisingly, antibiotic therapy must be effective as well as timely. MacArthur et al. studied the impact of adequate (ie, active against cultured organisms, if isolated) antibiotics on the outcomes of 2634 septic patients enrolled in a randomized trial of an anti‐TNF antibody. Nearly 91% received appropriate antibiotics; their mortality rate was 33%, 10% lower than that of the patients whose initial antibiotics were inadequate (P < .001).24 Leibovici et al. reported similar findings in a prospective study of patients with bacteremia. Only 63% of 3413 subjects received an antibiotic active against the infecting pathogen, and their mortality was 20%, 14% lower than that in the group that received ineffective antibiotics (P = .0001).25 Other authors have reported even worse outcomes with ineffective therapy: 62% mortality among inadequately treated bacteremic or fungemic ICU patients, compared with 28.4% among those who were adequately treated26 and an odds ratio of dying of 8.14 for the 46 of 270 septic ICU patients who received inadequate initial antibiotics,27 making inadequate antibiotic therapy the strongest risk factor for death. Finally, Kollef et al. reported that 26% of 655 infected ICU patients received inadequate antibiotics and suffered an infection‐related mortality rate of 40.2%, more than twice the 17.7% rate among adequately treated patients (P < .001). Inadequate antimicrobial therapy was a greater risk factor for death than early respiratory failure or sepsis‐related organ failure assessment scores.28
Guidelines for anti‐infective care now recommend obtaining appropriate cultures and administering broad‐spectrum antibiotics (appropriate for suspected infections, local susceptibility patterns, and any relevant prior culture data from individual patients) within 1 hour of presentation.11 In addition, any removable focus of infection must be identified and managed (eg, an abscess, infected catheter, tampon, or infection requiring surgery).
ACTIVATED PROTEIN C
Recombinant human activated protein C (APC) is a protein with anticoagulant and anti‐inflammatory properties that is relatively deficient in approximately 87% of septic patients.29 Although numerous trials of other anticoagulants (antithrombin III and tissue factor pathway inhibitor) and immunosuppressives (tumor necrosis factor inhibitors, high‐dose steroids, interleukin‐1 receptor antagonists, and others) have failed to show any benefit,7 in 2001 APC became the first proven therapy specifically for sepsis. The PROWESS trial, which established its efficacy, randomized 1690 patients who met 3 SIRS criteria and dysfunction of at least 1 organ system to APC (24 g/kg IV per hour for 96 hours interrupted for bleeding or urgent procedures) or placebo. APC reduced 28‐day mortality from 30.8% to 24.7%, yielding an absolute risk reduction of 6.1% and a corresponding number needed to treat (NNT) of 16.4. This benefit was seen across all subgroups including those with normal baseline APC levels.29
Not surprisingly, APC increases the risk of serious bleeding. Although this effect was of borderline significance in PROWESS (3.5% vs. 2% in the placebo group, P = .06),29 it was confirmed in subsequent trials (3.9% vs. 2.2%, P = .01)30 and may be larger still in open‐label use, at 6.5%.17, 31 Intracerebral hemorrhage (ICH), a particularly devastating complication, occurred in 0.2% of the PROWESS patients and 0.5% of patients in 2 subsequent studies30, 32; in both major trials, there was a single extra event in the APC arm.29, 30 Like serious bleeding in general, ICH was more common in open‐label use, occurring in 1.5% of patients.31, 33 Therefore, it is vital to have strict adherence to exclusion criteria and familiarity with the risk factors for serious bleeding. In the PROWESS trial, after randomization, risk factors for serious bleeding included procedures and injury to vascular organs, an activated partial‐thromboplastin time of more than 120 seconds, an international normalized ratio greater than 3, gastrointestinal ulceration, and development of severe thrombocytopenia (< 30,000/mm3)29; in a 2002 study of 2786 APC recipients, ICH was largely confined to patients with meningitis or a platelet count less than 30,000/mm3.32
APC therapy has several other limitations and drawbacks. Multiple contraindications, including predisposition to bleeding, a recent history of bleeding, anticoagulant use, immunosuppression, liver disease, dialysis dependence, and hypercoagulable states, restrict its use. APC appears to work best when administered early, within 24 hours of the onset of organ dysfunction.31 In addition, APC is indicated only in adults with Acute Physiology and Chronic Health Evaluation (APACHE II) scores greater than 24 and multiorgan failure. Post hoc analysis of the PROWESS data showed that although the relative risk (RR) of death for those with APACHE II scores of 25 or more was .71 and statistically significant, the RR for those with scores below 25 was a nonsignificant .99.34 A subsequent study, ADDRESS, confirmed there was no benefit to septic patients with a low risk of death.30 In the ADDRESS study 2613 patients with severe sepsis and either an APACHE II score less than 25 or single organ failure were randomized to APC or placebo. No differences were found in 28‐day and in‐hospital mortality; among patients who had undergone surgery in the previous 30 days, those receiving APC had a significantly increased risk of death (20.7% vs. 14.1%, P = .03).
An additional drawback of APC therapy is its cost, approximately $6800 per infusion, although the cost per year of life gained, $24,484, or $52,360 per life saved (NNT $6800), is reasonable for those with APACHE II scores greater than 24.34 Concerns have also been raised about the PROWESS trial itself: the production of the study drug and some exclusion criteria were changed midtrial, after which the effectiveness of APC improved. APACHE II scores had not been validated for selection of patients for therapies and may have varied with time or by observer. The original PROWESS study population may have been skewed away from chronically ill patients.35 Experts differ on the significance of these concerns and even whether APC therapy should be considered the standard of care pending further research.32, 35 The ADDRESS trial also failed to demonstrate a benefit in a subgroup of patients with APACHE II scores above 24, although it was underpowered to do so, and according to enrollment criteria, none of those patients had multiorgan failure.30 However, in the subgroup of PROWESS patients with APACHE II scores greater than 24, the absolute reduction in mortality was a full 13%,17 with a corresponding NNT of 7.7, and although the PROWESS findings have not been duplicated in a second randomized trial, a single‐arm, open‐label study of APC (ENHANCE) showed a nearly identical mortality rate.31 Pending confirmatory trials, APC remains a recommended therapy for selected patients sick enough to benefit and without excessive bleeding risk.11
EARLY GOAL‐DIRECTED THERAPY
Because physician‐directed resuscitation for sepsis may normalize vital signs, central venous pressures (CVP), and urine output without correcting hypoperfusion, Rivers et al. tested a resuscitation protocol that incorporated a central line that continuously monitored mixed‐venous oxygen saturation as a surrogate for cardiac output.36 They randomized 263 patients with septic shock (defined as hypotension < 90 mm Hg after a 20‐30 mL/kg bolus, or lactate > 4 mmol/L, which is associated with at least a 3‐fold increase in the mortality of emergency department patients with suspected infection37) to either standard care or early goal‐directed therapy (EGDT) for the initial 6 hours of hospital care. Patients with acute coronary ischemia, pulmonary edema, stroke, asthma, overdose, trauma, dysrhythmia, immunosuppression, uncontrolled cancer, or a need for urgent procedures were excluded. Standard care was directed by physiologic parameters such as vital signs, urine output, and CVP. EGDT used sequential therapies designed to support organ perfusion: 500 mL of normal saline was given every half hour until the CVP was at least 8‐12 mm Hg. Pressors were given until the mean arterial pressure was 65‐90 mm Hg (norepinephrine36 or dopamine were preferred agents, and vasopressin [0.01‐0.04 units/min] was an option for shock refractory to first‐line pressors)11, 38 Transfusion (to a hematocrit goal of 30) and dobutamine were given until mixed‐venous oxygenation saturation was 70% or better (Fig. 1). Lastly, patients who did not achieve this goal were sedated and mechanically ventilated.

Results were dramatic: mortality was reduced from 46.5% to 30.5%, with an ARR of 16% and an NNT of 6.25. Study patients received similar amounts of crystalloid, but received it earlier than the standard care patients and received more transfusions and inotropes. Substantially more patients in the EGDT group than the standard care group achieved a mixed venous oxygen saturation of 70%; 13.7% of the EGDT patients had occult hypoperfusion (low mixed‐venous oxygenation that responded to inotropes despite satisfactory vital signs). EGDT improved length of stay (4 days shorter among survivors) and duration of intubation, as well as APACHE scores and several physiologic parameters.36
Critics of this trial note the impossibility of adequate blinding and the high mortality in the placebo group. Further, because the trial tested the EGDT protocol as a whole, there was no way to know if each step was optimal. For example, a different CVP goal could have been used or adjustments made for mechanical ventilation, which can falsely elevate a low CVP into the desired range (the Surviving Sepsis Campaign guidelines recommend a CVP goal of 12‐15 mm Hg in mechanically ventilated patients11). Also, the selection of pressor, the use of inotropes, and the transfusion threshold were chosen on the basis of physiologic rationales, but all of these are arguable.39 This was also a single trial, and earlier goal‐directed protocols for ICU patients actually showed harm,40, 41 although those trials targeted supranormal physiologic goals in more established critical illness.42 Finally, on a practical level, hospitals and particularly emergency departments must commit resources to train physicians and staff, purchase the appropriate central venous catheters, and convince eligible patients to undergo an invasive procedure. In a survey of 30 attending physicians in academic referral hospitals, only 7% reported standard use of EGDT. Barriers included the requirement for specialty monitoring equipment and other resources, and central venous cannulation.43
Despite these concerns, the striking reduction in mortality associated with EGDT led to its endorsement by the Surviving Sepsis Campaign guidelines and underscores the principle of aggressive early resuscitation for patients who do not meet eligibility criteria but appear at risk for worsening sepsis. As yet, however, no strong evidence mandates a specific approach to the septic patient without shock.
STRESS DOSE STEROIDS
Because of the importance of the inflammatory cascade in severe sepsis, a potential role for steroids in the management of sepsis has been repeatedly studied. More than 50 studies have been performed since the 1950s, generally with pharmacologic doses of steroid; a meta‐analysis showed that such a practice was ineffective.44, 45 However, data accumulated that relative adrenal insufficiency during severe sepsis was common and associated with an increased risk of death and that physiologic doses of steroids could reverse refractory hypotension.46 To define the role of a physiologic course of steroids in septic shock, Annane et al. randomized 299 critically ill adults to either 7 days of stress dose hydrocortisone (50 mg IV every 6 hours) and fludrocortisone (50 g NG every 24 hours) or matched placebos. Enrolled patients were severely ill; the placebo group had a 63% mortality, and patients had to have septic shock, oliguria or hypoxia, hypotension despite low‐dose dopamine, and lactate greater than 2 mmol/L and require mechanical ventilation. Pregnant women, those with myocardial infarction or pulmonary embolus, advanced malignancies, or immunodeficiency, and those with clear indications or contraindications to steroids were excluded.47 Enrollment criteria were modified midstudy; changes included the exclusion of patients who had recently received etomidate, which inhibits 11‐‐hydroxylase and has been identified as a risk factor for adrenal insufficiency in intensive care patients.48 All patients received a 250‐g cosyntropin stimulation test. The authors considered patients nonresponders to consyntropin if serum cortisol failed to increase to 9 g/dL or more.
Steroids reduced the duration that a vasopressor was required and reduced mortality from 63% to 53% among nonresponders, giving an NNT of only 10 to prevent 1 death at 28 days. Although the authors described no evidence of adverse effects, among the subset of 70 patients who responded appropriately to cosyntropin, there was a nonsignificant trend toward increased mortality, and rates of hyperglycemia were not provided.47 The authors concluded that physicians should test appropriate patients for adrenal reserve, give the studied steroid regimen while results are pending, and discontinue treatment if a patient retains adrenal reserve.
The literature on steroids and critical illness is complex, with more than 1300 articles on steroids and sepsis published since 1988, and several concerns were raised about the Annane study. For example, did much of the benefit for those patients enrolled before the protocol amendment come from reducing an adverse effect of etomidate?49 Does the high‐dose, 250‐g cosyntropin stimulation test overcome (and conceal) partial ACTH resistance that might benefit from treatment?50 Might not a robust baseline cortisol suggest sufficient adrenal function regardless of the incremental response to cosyntropin?51 Partial answers were provided by 2 subsequent meta‐analyses. Both found that more recent studies gave lower doses of steroids in longer, 5‐ to 7‐day courses to sicker patients and demonstrated improvement in mortality and shock reversal, with relative risk reductions of 14%‐22%; the NNT ranged from 8 to 11. One analysis found no difference in outcomes between adrenally sufficient and adrenally insufficient patients, and those authors advised considering treatment for all patients regardless of their adrenal function test results.8 The other analysis concluded that the data on steroids for those with adrenal reserve was too limited to recommend treating adrenally sufficient patients.9
Disputes about certain details, such as whether patients should be treated without regard to adrenal reserve, continue in the literature.45, 52 An ongoing randomized, controlled trial, CORTICUS, is expected to provide additional guidance on the use of low‐dose steroids in sepsis; in the meantime, the literature clearly supports a longer course of low‐dose steroid therapy for patients with pressor‐dependent septic shock with inadequate adrenal reserve by cosyntropin testing, and guidelines allow discretion about whether patients with adequate adrenal reserve should also be treated.11 Hospitalists may also want to treat septic shock with equivalent doses of dexamethasone (approximately 2 mg IV every 6 hours) if adrenal evaluation may be delayed, as this agent will not confound cosyntropin stimulation test results, and they may want to avoid etomidate in septic patients53, 54 for whom they perform or supervise intubations.
INTENSIVE INSULIN THERAPY
Mounting evidence supports the short‐term role of hyperglycemia in morbidity and mortality, especially in critical illness. Hyperglycemia impairs neutrophil and endothelial cell function as well as protective responses to cardiac and neuronal ischemia,55 whereas insulin has anti‐inflammatory and antiapoptotic effects,7, 56 suggesting that intensive insulin might improve the outcomes of critically ill patients. To test this theory, van den Berghe and colleagues randomized 1548 mostly surgical ICU patients to insulin infusions titrated for glucose goals of either 80‐110 or 180‐200 mg/dL, followed by subcutaneous insulin after ICU discharge. Although blinding was impossible, in both cases glucose management was performed by a separate research team. Multiple benefits were noted: ICU and total in‐hospital deaths were reduced, mostly among patients with an ICU stay of more than 5 days, whose risk of death fell from 20.2% to 10.6%. Intensive insulin also reduced septicemia, renal impairment, critical illness polyneuropathy, and duration of intensive care.57
Subsequently, a meta‐analysis of 35 trials suggested that insulin reduced the mortality of critically ill patients by 15%.10 Van den Berghe et al.'s results were also duplicated in a broad, medical‐surgical ICU population, although the reductions in morbidity and mortality were measured against historical controls.58 However, whether the results of the influential surgical ICU study could be applied to medical patients was not known until 2006, when the van den Berghe group reported the effects of similar insulin protocols on 1200 patients in the medical ICU who were expected to need intensive care for at least 3 days.59 In this study, intensive insulin failed to reduce overall mortality (40% and 37.3%, P = .33). However, intensive insulin did reduce mortality among the 64% of patients who stayed in the ICU 3 or more days from 52.5% to 43% (NNT 10.5, P = .009). This benefit was offset by an increased number of deaths in the intensive insulin group among patients with ICU stays of less than 3 days (P = .05‐.35 depending on the method used).59 Intensive insulin did reduce newly acquired kidney injury, duration of mechanical ventilation, and lengths of ICU and hospital stays, and the reduction in morbidity increased with the duration of intensive insulin therapy. Hypoglycemia (mean 32 mg/dL) occurred in 25% of patients with prolonged stays6.4 times as often as in the usual care group.60 Liver and renal failure were associated with hypoglycemia.59
Critics of the surgical ICU trial noted the high mortality among the usual care patients (5.1%), a robust 34% mortality reduction for a relatively small 50 mg/dL reduction in morning glucose levels, and the aggressive use of parenteral nutrition, raising the question of whether intensive insulin merely attenuated the side effects of intravenous glucose.61, 62 Also, the ideal blood glucose target is not known with certainty. Retrospective studies suggested the upper limit for target blood glucoses could be 145 mg/dL63 and found differing thresholds at which hyperglycemia increased mortality in nondiabetics (144 mg/dL) and diabetics (200 mg/dL).64 However, in the surgical ICU trial, there was no threshold below which there was no further reduction in risk; patients whose mean blood glucose was below 110 mg/dL had lower mortality than those whose levels were between 110 and 150 mg/dL (P = .026).65 Finally, the effects of hyperglycemia and intensive insulin may vary by population: retrospective studies found that ICU hyperglycemia was more strongly associated with mortality among nondiabetics,64, 66 and van den Berghe et al. noted no benefit from intensive insulin in a small subgroup of diabetics.59
In summary, large, well‐designed trials have demonstrated that intensive insulin reduced mortality in critically ill patients after a delay of 3‐5 days, but this benefit did not extend to all patients in the medical ICU.57, 59 Some authors have suggested deferring intensive insulin for 3 days,67 but because early therapy probably contributes to the delayed mortality benefit, this approach may deprive patients of the observed benefits. Ongoing clinical trials (NICE‐SUGAR) are likely to provide useful information about how hyperglycemia should be managed in different populations, including septic ICU patients.61 In the meantime, institutions can select the intensity of their insulin therapy by weighing morbidity and long‐term mortality benefits against possible short‐term harms and ensuring that hospital staff members are sufficiently trained to control hyperglycemia safely. For example, in critical illness, intravenous insulin is preferable to subcutaneous insulin, and the frequent measurement of whole‐blood glucose instead of finger‐stick glucose helps to avoid errors.55, 68 And although researchers were unable to prospectively identify patients with long ICU stays,59 severely septic patients have long ICU stays (generally 7.5‐16.6 days),14 and individual ICUs might observe enough stays of more than 2 days in their patient population to justify intensive insulin for this subgroup. And finally, although no conclusive evidence mandates a specific approach to hyperglycemia outside the ICU, the ICU data provide a physiologic rationale for cautious but tight control of glucose in more moderately ill patients. Guidelines for the management of inpatient hyperglycemia were published previously.55
SEPSIS AND THE HOSPITALIST
Hospitalists who provide critical care may make frequent decisions about the inclusion and exclusion criteria for the major trials of sepsis, weigh their relative benefits against risks and costs, contemplate gray areas such as adrenal testing in shock, and employ evidence‐based therapies for severe sepsis. However, hospitalists may also see patients who qualify for these therapies when they are called to see septic patients in the emergency department, when severe sepsis develops in patients on the medicine ward, or when they provide consultation services in an ICU. Sepsis care must be implemented urgently; patients in the pivotal trial of steroids had to be randomized within 3 hours of shock onset,47 data suggest that the window for optimal antibiotic therapy may be no greater than 4 hours from diagnosis,18 whereas guidelines suggest therapy within 1 hour,11 and early goal‐directed therapy was studied only for the first 6 or more hours of hospitalization.36 Thus, hospitalists who do not provide ICU care should be able to identify patients with severe sepsis and either deliver initial care or recognize the need for immediate consultation. Specifically, hospitalists can:
-
Recognize that both absolute (< 90 mm Hg) and relative hypotension (> 40 mm Hg below baseline) indicate septic shock;
-
Identify normotensive candidates for EGDT (severe sepsis with serum lactate > 4 mmol/L) by requesting a serum lactate in addition to prompt appropriate cultures for severe acute infection69;
-
Recognize atypical presentations of sepsis (tachypnea, tachycardia, confusion, etc.) and maintain a high suspicion for sepsis in patients who may be predisposed to infection and to atypical presentation because of age, immunosuppression, neutropenia, diabetes, or other conditions;
-
Initiate effective antibiotics and EGDT promptly for individual patients or by coordinating efforts to improve sepsis care at an institutional level, for example, as a component of medical emergency team services70, 71;
-
Rapidly identify and manage removable foci of infection such as abscesses, empyemas, necrotizing fasciitis, or infected vascular catheters; and
-
Competently educate hospital staff, residents, and medical students about sepsis care.
Hospitalists are busy physicians, and the task of reviewing sepsis literature and implementing recommendations is daunting. However, hospitalists can turn to resources such as the Surviving Sepsis Campaign Guidelines, a series of recommendations for managing severe sepsis that were endorsed by 11 international critical care and infectious disease societies and published in Critical Care Medicine in 2004.11 The Institute for Healthcare Improvement has also published a series of online severe sepsis bundles, or groups of proven interventions, complete with implementation tips and supporting literature, available at
CONCLUSION: DEADLY YET TREATABLE
The death toll from severe sepsis in the United States exceeds that of lung, breast, and colon cancer combined and equals that of myocardial infarction (MI),1 a condition that appropriately triggers a series of emergency interventions. Physicians now have an arsenal of therapies for severe sepsis analogous to those employed for MI, and a comparison between the 2 conditions underscores the high mortality rate of severe sepsis and the enormous impact on patient outcomes provided by evidence‐based sepsis therapy. Figure 2 compares the 9.5%‐16% ARR for death associated with APC in patients with APACHE 2 scores greater than 24 and multiorgan failure,29 EGDT,36 stress dose steroids in shock complicated by adrenal insufficiency,47 and intensive insulin in patients with medical ICU stays longer than 3 days,59 with the benefits of thrombolysis for ST‐elevation MI (2%‐3%)73 or antiplatelet therapy for acute MI (2.3%).74 Figure 3 compares the corresponding NNT values to save 1 life; according to the available data, a hospitalist is 5‐8 times more likely to save a life with EGDT than with fibrinolysis.


Because the literature supporting several major sepsis therapies have been limited to retrospective studies1828 and single randomized, controlled trials29, 36 and because key trials are still underway (CORTICUS, NICE‐SUGAR), the benefits of sepsis therapies are less certain than are those for the treatment of MI. This was underscored by the finding that the benefit in reduced mortality of intensive insulin in the surgical ICU57 did not extend to all patients in the medical ICU.59 However, the potentially marked survival benefit of early effective antibiotics, APC, EGDT, stress dose steroids, and intensive insulin and the urgency with which they must be applied demand that all hospitalists become or remain familiar with the evolving sepsis literature.
Despite decades of intensive research and improvements in medical care, severe sepsis affects an estimated 751,000 patients in the United States every year, killing 215,000 of them at an annual cost of 16.7 billion dollars.1 Because the elderly experience a 100‐fold increase in incidence, as compared with children, and a nearly 4‐fold increase in mortality (38.4% of those more than 85 years old), this burden is expected to increase with the aging population.1 Patients with severe sepsis have prolonged ICU14 and hospital stays and incur substantially increased costs compared with other patients.36
New research continues to explore the complex pathophysiology of sepsis,7 and clinicians, who once relied primarily on clinical experience and expert opinion to guide therapy, now have an increasing array of evidenced‐based sepsis therapies to employ. Recent meta‐analyses have evaluated several major treatments for severe sepsis,810 and recommendations (the Surviving Sepsis Campaign guidelines) for the treatment of severe sepsis were recently endorsed by 11 international critical care and infectious disease organizations.11 This article summarizes the current definitions of sepsis syndromes, the trials supporting the specific therapies for sepsis that are currently recommended, ongoing controversies and research, and implications for hospitalists, with a focus on early, effective antibiotics, activated protein C, early goal‐directed therapy, stress dose steroids, and intensive insulin therapy. For space considerations, readers are directed elsewhere for data supporting prophylaxis for deep venous thrombosis (DVT)12 and stress ulcer bleeding13 and for therapies less often directed by hospitalists, such as lung protective ventilation.14
DEFINITIONS
Systemic inflammatory response syndrome (SIRS), sepsis, severe sepsis, and septic shock were defined in 1992 to standardize the terminology of sepsis.15 These definitions have recently been reviewed and supported by a variety of American and European intensive care societies.16
SIRS is defined by the presence of at least 2 of the following:
-
Temperature > 38C or < 36C;
-
Heart rate > 90 beats/min;
-
Respiratory rate > 20 breaths/min or PaCO2 < 32 mm Hg;
-
WBC >12,000 or < 4000 cells/mm3, or >10% immature (band) forms.
Sepsis is SIRS due to documented or strongly suspected infection.
Severe sepsis is sepsis with organ dysfunction (such as lactic acidosis, oliguria, thrombocytopenia, or delirium), hypoperfusion, or hypotension (< 90 mm Hg systolic or more than 40 mm Hg below baseline).
Septic shock is severe sepsis complicated by hypotension or pressor dependence despite adequate (20‐30 mL/kg; 1.5‐3 liters in most patients) fluid resuscitation.
Sepsis terminology must be applied carefully. Many hospitalized patients meet criteria for SIRS, yet it is inaccurate to say a patient who has acute leukemia with leukocytosis, anemia‐induced tachycardia, and thrombocytopenia has severe sepsis if those abnormalities are not a result of inflammation or infection. Accurate documentation of sepsis syndromes can improve professional and institutional reimbursement and provide prognostic information: the in‐hospital mortality rates for severe sepsis and septic shock are approximately 30% and 50%, respectively.17 More importantly, thoughtful application of these definitions can help a hospitalist identify septic patients who qualify for one of the proven therapies for severe sepsis.
EARLY, EFFECTIVE ANTIBIOTICS
For obvious ethical reasons, randomized, controlled trials to study the impact of inappropriate or delayed antibiotic therapy for serious infections are not possible. However, the evidence supporting early, effective antibiotic therapy is still compelling, and because many hospitalists often initiate treatment with antibiotics before transferring a patient to intensive care, this may represent the most important intervention hospitalists can provide to patients with serious infections. Several studies have estimated the impact of early, effective antibiotics on outcomes.
Houck et al. retrospectively reviewed 13,771 cases of community‐acquired pneumonia among elderly Medicare patients. They found that 39.1% of the patients waited more than 4 hours for antibiotics and 7.6% waited more than 12 hours; three quarters of these delays resulted from delayed ordering of antibiotics.18 Further, 21.2% received an antibiotic selection incompatible with recent professional guidelines. Receiving antibiotics within 4 hours reduced in‐hospital and 30‐day mortality by 15% and length of stay by 0.4 days.18 Similar conclusions were reported by 3 of 4 previous analyses.1922 Extending these findings to critically ill patients, Iregui et al. found that delayed treatment with appropriate antibiotics (odds ratio, 7.68) was a greater predictor of mortality for 107 patients with ventilator‐acquired pneumonia than were APACHE II scores and malignancy; 31% failed to receive appropriate antibiotics within 24 hours, and again, three quarters of these delays resulted from delays in writing antibiotic orders.23
Not surprisingly, antibiotic therapy must be effective as well as timely. MacArthur et al. studied the impact of adequate (ie, active against cultured organisms, if isolated) antibiotics on the outcomes of 2634 septic patients enrolled in a randomized trial of an anti‐TNF antibody. Nearly 91% received appropriate antibiotics; their mortality rate was 33%, 10% lower than that of the patients whose initial antibiotics were inadequate (P < .001).24 Leibovici et al. reported similar findings in a prospective study of patients with bacteremia. Only 63% of 3413 subjects received an antibiotic active against the infecting pathogen, and their mortality was 20%, 14% lower than that in the group that received ineffective antibiotics (P = .0001).25 Other authors have reported even worse outcomes with ineffective therapy: 62% mortality among inadequately treated bacteremic or fungemic ICU patients, compared with 28.4% among those who were adequately treated26 and an odds ratio of dying of 8.14 for the 46 of 270 septic ICU patients who received inadequate initial antibiotics,27 making inadequate antibiotic therapy the strongest risk factor for death. Finally, Kollef et al. reported that 26% of 655 infected ICU patients received inadequate antibiotics and suffered an infection‐related mortality rate of 40.2%, more than twice the 17.7% rate among adequately treated patients (P < .001). Inadequate antimicrobial therapy was a greater risk factor for death than early respiratory failure or sepsis‐related organ failure assessment scores.28
Guidelines for anti‐infective care now recommend obtaining appropriate cultures and administering broad‐spectrum antibiotics (appropriate for suspected infections, local susceptibility patterns, and any relevant prior culture data from individual patients) within 1 hour of presentation.11 In addition, any removable focus of infection must be identified and managed (eg, an abscess, infected catheter, tampon, or infection requiring surgery).
ACTIVATED PROTEIN C
Recombinant human activated protein C (APC) is a protein with anticoagulant and anti‐inflammatory properties that is relatively deficient in approximately 87% of septic patients.29 Although numerous trials of other anticoagulants (antithrombin III and tissue factor pathway inhibitor) and immunosuppressives (tumor necrosis factor inhibitors, high‐dose steroids, interleukin‐1 receptor antagonists, and others) have failed to show any benefit,7 in 2001 APC became the first proven therapy specifically for sepsis. The PROWESS trial, which established its efficacy, randomized 1690 patients who met 3 SIRS criteria and dysfunction of at least 1 organ system to APC (24 g/kg IV per hour for 96 hours interrupted for bleeding or urgent procedures) or placebo. APC reduced 28‐day mortality from 30.8% to 24.7%, yielding an absolute risk reduction of 6.1% and a corresponding number needed to treat (NNT) of 16.4. This benefit was seen across all subgroups including those with normal baseline APC levels.29
Not surprisingly, APC increases the risk of serious bleeding. Although this effect was of borderline significance in PROWESS (3.5% vs. 2% in the placebo group, P = .06),29 it was confirmed in subsequent trials (3.9% vs. 2.2%, P = .01)30 and may be larger still in open‐label use, at 6.5%.17, 31 Intracerebral hemorrhage (ICH), a particularly devastating complication, occurred in 0.2% of the PROWESS patients and 0.5% of patients in 2 subsequent studies30, 32; in both major trials, there was a single extra event in the APC arm.29, 30 Like serious bleeding in general, ICH was more common in open‐label use, occurring in 1.5% of patients.31, 33 Therefore, it is vital to have strict adherence to exclusion criteria and familiarity with the risk factors for serious bleeding. In the PROWESS trial, after randomization, risk factors for serious bleeding included procedures and injury to vascular organs, an activated partial‐thromboplastin time of more than 120 seconds, an international normalized ratio greater than 3, gastrointestinal ulceration, and development of severe thrombocytopenia (< 30,000/mm3)29; in a 2002 study of 2786 APC recipients, ICH was largely confined to patients with meningitis or a platelet count less than 30,000/mm3.32
APC therapy has several other limitations and drawbacks. Multiple contraindications, including predisposition to bleeding, a recent history of bleeding, anticoagulant use, immunosuppression, liver disease, dialysis dependence, and hypercoagulable states, restrict its use. APC appears to work best when administered early, within 24 hours of the onset of organ dysfunction.31 In addition, APC is indicated only in adults with Acute Physiology and Chronic Health Evaluation (APACHE II) scores greater than 24 and multiorgan failure. Post hoc analysis of the PROWESS data showed that although the relative risk (RR) of death for those with APACHE II scores of 25 or more was .71 and statistically significant, the RR for those with scores below 25 was a nonsignificant .99.34 A subsequent study, ADDRESS, confirmed there was no benefit to septic patients with a low risk of death.30 In the ADDRESS study 2613 patients with severe sepsis and either an APACHE II score less than 25 or single organ failure were randomized to APC or placebo. No differences were found in 28‐day and in‐hospital mortality; among patients who had undergone surgery in the previous 30 days, those receiving APC had a significantly increased risk of death (20.7% vs. 14.1%, P = .03).
An additional drawback of APC therapy is its cost, approximately $6800 per infusion, although the cost per year of life gained, $24,484, or $52,360 per life saved (NNT $6800), is reasonable for those with APACHE II scores greater than 24.34 Concerns have also been raised about the PROWESS trial itself: the production of the study drug and some exclusion criteria were changed midtrial, after which the effectiveness of APC improved. APACHE II scores had not been validated for selection of patients for therapies and may have varied with time or by observer. The original PROWESS study population may have been skewed away from chronically ill patients.35 Experts differ on the significance of these concerns and even whether APC therapy should be considered the standard of care pending further research.32, 35 The ADDRESS trial also failed to demonstrate a benefit in a subgroup of patients with APACHE II scores above 24, although it was underpowered to do so, and according to enrollment criteria, none of those patients had multiorgan failure.30 However, in the subgroup of PROWESS patients with APACHE II scores greater than 24, the absolute reduction in mortality was a full 13%,17 with a corresponding NNT of 7.7, and although the PROWESS findings have not been duplicated in a second randomized trial, a single‐arm, open‐label study of APC (ENHANCE) showed a nearly identical mortality rate.31 Pending confirmatory trials, APC remains a recommended therapy for selected patients sick enough to benefit and without excessive bleeding risk.11
EARLY GOAL‐DIRECTED THERAPY
Because physician‐directed resuscitation for sepsis may normalize vital signs, central venous pressures (CVP), and urine output without correcting hypoperfusion, Rivers et al. tested a resuscitation protocol that incorporated a central line that continuously monitored mixed‐venous oxygen saturation as a surrogate for cardiac output.36 They randomized 263 patients with septic shock (defined as hypotension < 90 mm Hg after a 20‐30 mL/kg bolus, or lactate > 4 mmol/L, which is associated with at least a 3‐fold increase in the mortality of emergency department patients with suspected infection37) to either standard care or early goal‐directed therapy (EGDT) for the initial 6 hours of hospital care. Patients with acute coronary ischemia, pulmonary edema, stroke, asthma, overdose, trauma, dysrhythmia, immunosuppression, uncontrolled cancer, or a need for urgent procedures were excluded. Standard care was directed by physiologic parameters such as vital signs, urine output, and CVP. EGDT used sequential therapies designed to support organ perfusion: 500 mL of normal saline was given every half hour until the CVP was at least 8‐12 mm Hg. Pressors were given until the mean arterial pressure was 65‐90 mm Hg (norepinephrine36 or dopamine were preferred agents, and vasopressin [0.01‐0.04 units/min] was an option for shock refractory to first‐line pressors)11, 38 Transfusion (to a hematocrit goal of 30) and dobutamine were given until mixed‐venous oxygenation saturation was 70% or better (Fig. 1). Lastly, patients who did not achieve this goal were sedated and mechanically ventilated.

Results were dramatic: mortality was reduced from 46.5% to 30.5%, with an ARR of 16% and an NNT of 6.25. Study patients received similar amounts of crystalloid, but received it earlier than the standard care patients and received more transfusions and inotropes. Substantially more patients in the EGDT group than the standard care group achieved a mixed venous oxygen saturation of 70%; 13.7% of the EGDT patients had occult hypoperfusion (low mixed‐venous oxygenation that responded to inotropes despite satisfactory vital signs). EGDT improved length of stay (4 days shorter among survivors) and duration of intubation, as well as APACHE scores and several physiologic parameters.36
Critics of this trial note the impossibility of adequate blinding and the high mortality in the placebo group. Further, because the trial tested the EGDT protocol as a whole, there was no way to know if each step was optimal. For example, a different CVP goal could have been used or adjustments made for mechanical ventilation, which can falsely elevate a low CVP into the desired range (the Surviving Sepsis Campaign guidelines recommend a CVP goal of 12‐15 mm Hg in mechanically ventilated patients11). Also, the selection of pressor, the use of inotropes, and the transfusion threshold were chosen on the basis of physiologic rationales, but all of these are arguable.39 This was also a single trial, and earlier goal‐directed protocols for ICU patients actually showed harm,40, 41 although those trials targeted supranormal physiologic goals in more established critical illness.42 Finally, on a practical level, hospitals and particularly emergency departments must commit resources to train physicians and staff, purchase the appropriate central venous catheters, and convince eligible patients to undergo an invasive procedure. In a survey of 30 attending physicians in academic referral hospitals, only 7% reported standard use of EGDT. Barriers included the requirement for specialty monitoring equipment and other resources, and central venous cannulation.43
Despite these concerns, the striking reduction in mortality associated with EGDT led to its endorsement by the Surviving Sepsis Campaign guidelines and underscores the principle of aggressive early resuscitation for patients who do not meet eligibility criteria but appear at risk for worsening sepsis. As yet, however, no strong evidence mandates a specific approach to the septic patient without shock.
STRESS DOSE STEROIDS
Because of the importance of the inflammatory cascade in severe sepsis, a potential role for steroids in the management of sepsis has been repeatedly studied. More than 50 studies have been performed since the 1950s, generally with pharmacologic doses of steroid; a meta‐analysis showed that such a practice was ineffective.44, 45 However, data accumulated that relative adrenal insufficiency during severe sepsis was common and associated with an increased risk of death and that physiologic doses of steroids could reverse refractory hypotension.46 To define the role of a physiologic course of steroids in septic shock, Annane et al. randomized 299 critically ill adults to either 7 days of stress dose hydrocortisone (50 mg IV every 6 hours) and fludrocortisone (50 g NG every 24 hours) or matched placebos. Enrolled patients were severely ill; the placebo group had a 63% mortality, and patients had to have septic shock, oliguria or hypoxia, hypotension despite low‐dose dopamine, and lactate greater than 2 mmol/L and require mechanical ventilation. Pregnant women, those with myocardial infarction or pulmonary embolus, advanced malignancies, or immunodeficiency, and those with clear indications or contraindications to steroids were excluded.47 Enrollment criteria were modified midstudy; changes included the exclusion of patients who had recently received etomidate, which inhibits 11‐‐hydroxylase and has been identified as a risk factor for adrenal insufficiency in intensive care patients.48 All patients received a 250‐g cosyntropin stimulation test. The authors considered patients nonresponders to consyntropin if serum cortisol failed to increase to 9 g/dL or more.
Steroids reduced the duration that a vasopressor was required and reduced mortality from 63% to 53% among nonresponders, giving an NNT of only 10 to prevent 1 death at 28 days. Although the authors described no evidence of adverse effects, among the subset of 70 patients who responded appropriately to cosyntropin, there was a nonsignificant trend toward increased mortality, and rates of hyperglycemia were not provided.47 The authors concluded that physicians should test appropriate patients for adrenal reserve, give the studied steroid regimen while results are pending, and discontinue treatment if a patient retains adrenal reserve.
The literature on steroids and critical illness is complex, with more than 1300 articles on steroids and sepsis published since 1988, and several concerns were raised about the Annane study. For example, did much of the benefit for those patients enrolled before the protocol amendment come from reducing an adverse effect of etomidate?49 Does the high‐dose, 250‐g cosyntropin stimulation test overcome (and conceal) partial ACTH resistance that might benefit from treatment?50 Might not a robust baseline cortisol suggest sufficient adrenal function regardless of the incremental response to cosyntropin?51 Partial answers were provided by 2 subsequent meta‐analyses. Both found that more recent studies gave lower doses of steroids in longer, 5‐ to 7‐day courses to sicker patients and demonstrated improvement in mortality and shock reversal, with relative risk reductions of 14%‐22%; the NNT ranged from 8 to 11. One analysis found no difference in outcomes between adrenally sufficient and adrenally insufficient patients, and those authors advised considering treatment for all patients regardless of their adrenal function test results.8 The other analysis concluded that the data on steroids for those with adrenal reserve was too limited to recommend treating adrenally sufficient patients.9
Disputes about certain details, such as whether patients should be treated without regard to adrenal reserve, continue in the literature.45, 52 An ongoing randomized, controlled trial, CORTICUS, is expected to provide additional guidance on the use of low‐dose steroids in sepsis; in the meantime, the literature clearly supports a longer course of low‐dose steroid therapy for patients with pressor‐dependent septic shock with inadequate adrenal reserve by cosyntropin testing, and guidelines allow discretion about whether patients with adequate adrenal reserve should also be treated.11 Hospitalists may also want to treat septic shock with equivalent doses of dexamethasone (approximately 2 mg IV every 6 hours) if adrenal evaluation may be delayed, as this agent will not confound cosyntropin stimulation test results, and they may want to avoid etomidate in septic patients53, 54 for whom they perform or supervise intubations.
INTENSIVE INSULIN THERAPY
Mounting evidence supports the short‐term role of hyperglycemia in morbidity and mortality, especially in critical illness. Hyperglycemia impairs neutrophil and endothelial cell function as well as protective responses to cardiac and neuronal ischemia,55 whereas insulin has anti‐inflammatory and antiapoptotic effects,7, 56 suggesting that intensive insulin might improve the outcomes of critically ill patients. To test this theory, van den Berghe and colleagues randomized 1548 mostly surgical ICU patients to insulin infusions titrated for glucose goals of either 80‐110 or 180‐200 mg/dL, followed by subcutaneous insulin after ICU discharge. Although blinding was impossible, in both cases glucose management was performed by a separate research team. Multiple benefits were noted: ICU and total in‐hospital deaths were reduced, mostly among patients with an ICU stay of more than 5 days, whose risk of death fell from 20.2% to 10.6%. Intensive insulin also reduced septicemia, renal impairment, critical illness polyneuropathy, and duration of intensive care.57
Subsequently, a meta‐analysis of 35 trials suggested that insulin reduced the mortality of critically ill patients by 15%.10 Van den Berghe et al.'s results were also duplicated in a broad, medical‐surgical ICU population, although the reductions in morbidity and mortality were measured against historical controls.58 However, whether the results of the influential surgical ICU study could be applied to medical patients was not known until 2006, when the van den Berghe group reported the effects of similar insulin protocols on 1200 patients in the medical ICU who were expected to need intensive care for at least 3 days.59 In this study, intensive insulin failed to reduce overall mortality (40% and 37.3%, P = .33). However, intensive insulin did reduce mortality among the 64% of patients who stayed in the ICU 3 or more days from 52.5% to 43% (NNT 10.5, P = .009). This benefit was offset by an increased number of deaths in the intensive insulin group among patients with ICU stays of less than 3 days (P = .05‐.35 depending on the method used).59 Intensive insulin did reduce newly acquired kidney injury, duration of mechanical ventilation, and lengths of ICU and hospital stays, and the reduction in morbidity increased with the duration of intensive insulin therapy. Hypoglycemia (mean 32 mg/dL) occurred in 25% of patients with prolonged stays6.4 times as often as in the usual care group.60 Liver and renal failure were associated with hypoglycemia.59
Critics of the surgical ICU trial noted the high mortality among the usual care patients (5.1%), a robust 34% mortality reduction for a relatively small 50 mg/dL reduction in morning glucose levels, and the aggressive use of parenteral nutrition, raising the question of whether intensive insulin merely attenuated the side effects of intravenous glucose.61, 62 Also, the ideal blood glucose target is not known with certainty. Retrospective studies suggested the upper limit for target blood glucoses could be 145 mg/dL63 and found differing thresholds at which hyperglycemia increased mortality in nondiabetics (144 mg/dL) and diabetics (200 mg/dL).64 However, in the surgical ICU trial, there was no threshold below which there was no further reduction in risk; patients whose mean blood glucose was below 110 mg/dL had lower mortality than those whose levels were between 110 and 150 mg/dL (P = .026).65 Finally, the effects of hyperglycemia and intensive insulin may vary by population: retrospective studies found that ICU hyperglycemia was more strongly associated with mortality among nondiabetics,64, 66 and van den Berghe et al. noted no benefit from intensive insulin in a small subgroup of diabetics.59
In summary, large, well‐designed trials have demonstrated that intensive insulin reduced mortality in critically ill patients after a delay of 3‐5 days, but this benefit did not extend to all patients in the medical ICU.57, 59 Some authors have suggested deferring intensive insulin for 3 days,67 but because early therapy probably contributes to the delayed mortality benefit, this approach may deprive patients of the observed benefits. Ongoing clinical trials (NICE‐SUGAR) are likely to provide useful information about how hyperglycemia should be managed in different populations, including septic ICU patients.61 In the meantime, institutions can select the intensity of their insulin therapy by weighing morbidity and long‐term mortality benefits against possible short‐term harms and ensuring that hospital staff members are sufficiently trained to control hyperglycemia safely. For example, in critical illness, intravenous insulin is preferable to subcutaneous insulin, and the frequent measurement of whole‐blood glucose instead of finger‐stick glucose helps to avoid errors.55, 68 And although researchers were unable to prospectively identify patients with long ICU stays,59 severely septic patients have long ICU stays (generally 7.5‐16.6 days),14 and individual ICUs might observe enough stays of more than 2 days in their patient population to justify intensive insulin for this subgroup. And finally, although no conclusive evidence mandates a specific approach to hyperglycemia outside the ICU, the ICU data provide a physiologic rationale for cautious but tight control of glucose in more moderately ill patients. Guidelines for the management of inpatient hyperglycemia were published previously.55
SEPSIS AND THE HOSPITALIST
Hospitalists who provide critical care may make frequent decisions about the inclusion and exclusion criteria for the major trials of sepsis, weigh their relative benefits against risks and costs, contemplate gray areas such as adrenal testing in shock, and employ evidence‐based therapies for severe sepsis. However, hospitalists may also see patients who qualify for these therapies when they are called to see septic patients in the emergency department, when severe sepsis develops in patients on the medicine ward, or when they provide consultation services in an ICU. Sepsis care must be implemented urgently; patients in the pivotal trial of steroids had to be randomized within 3 hours of shock onset,47 data suggest that the window for optimal antibiotic therapy may be no greater than 4 hours from diagnosis,18 whereas guidelines suggest therapy within 1 hour,11 and early goal‐directed therapy was studied only for the first 6 or more hours of hospitalization.36 Thus, hospitalists who do not provide ICU care should be able to identify patients with severe sepsis and either deliver initial care or recognize the need for immediate consultation. Specifically, hospitalists can:
-
Recognize that both absolute (< 90 mm Hg) and relative hypotension (> 40 mm Hg below baseline) indicate septic shock;
-
Identify normotensive candidates for EGDT (severe sepsis with serum lactate > 4 mmol/L) by requesting a serum lactate in addition to prompt appropriate cultures for severe acute infection69;
-
Recognize atypical presentations of sepsis (tachypnea, tachycardia, confusion, etc.) and maintain a high suspicion for sepsis in patients who may be predisposed to infection and to atypical presentation because of age, immunosuppression, neutropenia, diabetes, or other conditions;
-
Initiate effective antibiotics and EGDT promptly for individual patients or by coordinating efforts to improve sepsis care at an institutional level, for example, as a component of medical emergency team services70, 71;
-
Rapidly identify and manage removable foci of infection such as abscesses, empyemas, necrotizing fasciitis, or infected vascular catheters; and
-
Competently educate hospital staff, residents, and medical students about sepsis care.
Hospitalists are busy physicians, and the task of reviewing sepsis literature and implementing recommendations is daunting. However, hospitalists can turn to resources such as the Surviving Sepsis Campaign Guidelines, a series of recommendations for managing severe sepsis that were endorsed by 11 international critical care and infectious disease societies and published in Critical Care Medicine in 2004.11 The Institute for Healthcare Improvement has also published a series of online severe sepsis bundles, or groups of proven interventions, complete with implementation tips and supporting literature, available at
CONCLUSION: DEADLY YET TREATABLE
The death toll from severe sepsis in the United States exceeds that of lung, breast, and colon cancer combined and equals that of myocardial infarction (MI),1 a condition that appropriately triggers a series of emergency interventions. Physicians now have an arsenal of therapies for severe sepsis analogous to those employed for MI, and a comparison between the 2 conditions underscores the high mortality rate of severe sepsis and the enormous impact on patient outcomes provided by evidence‐based sepsis therapy. Figure 2 compares the 9.5%‐16% ARR for death associated with APC in patients with APACHE 2 scores greater than 24 and multiorgan failure,29 EGDT,36 stress dose steroids in shock complicated by adrenal insufficiency,47 and intensive insulin in patients with medical ICU stays longer than 3 days,59 with the benefits of thrombolysis for ST‐elevation MI (2%‐3%)73 or antiplatelet therapy for acute MI (2.3%).74 Figure 3 compares the corresponding NNT values to save 1 life; according to the available data, a hospitalist is 5‐8 times more likely to save a life with EGDT than with fibrinolysis.


Because the literature supporting several major sepsis therapies have been limited to retrospective studies1828 and single randomized, controlled trials29, 36 and because key trials are still underway (CORTICUS, NICE‐SUGAR), the benefits of sepsis therapies are less certain than are those for the treatment of MI. This was underscored by the finding that the benefit in reduced mortality of intensive insulin in the surgical ICU57 did not extend to all patients in the medical ICU.59 However, the potentially marked survival benefit of early effective antibiotics, APC, EGDT, stress dose steroids, and intensive insulin and the urgency with which they must be applied demand that all hospitalists become or remain familiar with the evolving sepsis literature.
- ,,,,,.Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care.Crit Care Med.2001;29:1303–1310.
- ,,,.Prevalence and incidence of severe sepsis in Dutch intensive care units.Crit Care.2004;8:R153–R162.
- ,, et al.Direct costs of severe sepsis in three German intensive care units based on retrospective electronic patient record analysis of resource use.Intensive Care Med.2002;28:1440–1446.
- ,,,,.Effects of severity of illness on resource use by survivors and nonsurvivors of severe sepsis at intensive care unit admission.Crit Care Med.2002;30:2413–2419.
- ,,, et al.Resource utilization among patients with sepsis syndrome.Infect Control Hosp Epidemiol.2003;24:62–70.
- ,,,,.The costs of septic syndromes in the intensive care unit and influence of hospital‐acquired sepsis.Intensive Care Med,2003;29:1464–1471.
- ,.The pathophysiology and treatment of sepsis.N Engl J Med.2003;348:138–150.
- ,,,,.Meta‐analysis: the effect of steroids on survival and shock during sepsis depends on the dose.Ann Intern Med.2004;141:47–56.
- ,,,,,.Corticosteroids for severe sepsis and septic shock: a systematic review and meta‐analysis.Brit Med J.2004;329:480–488.
- ,,.Insulin therapy for critically ill hospitalized patients: a meta‐analysis of randomized controlled trials.Arch Intern Med.2004;164:2005–2011.
- ,,, et al.Surviving Sepsis Campaign guidelines for management of severe sepsis and septic shock.Crit Care Med.2004;32:858–873.
- ,,,,.Prevention of deep vein thrombosis in medical patients by low‐dose heparin.Scott Med J.1981;26:115–117.
- ,,, et al.A comparison of sucralfate and ranitidine for the prevention of upper gastrointestinal bleeding in patients requiring mechanical ventilation. Canadian Critical Care Trials Group.N Engl J Med.1998;338:791–797.
- The Acute Respiratory Distress Syndrome Network.Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome.N Engl J Med.2000;342:1301–1308.
- American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference: definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis.Crit Care Med.1992;20:864–874.
- ;;, et al. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference.Crit Care Med.2003;31:1250–1256.
- .Severe sepsis and therapy with activated protein C.New Engl J Med.2005;353:1398–1399.
- ,,,,.Timing of antibiotic administration and outcomes for Medicare patients hospitalized with community‐acquired pneumonia.Arch Intern Med.2004;164:637–644.
- ,,, et al.Measuring quality of care with explicit process criteria before and after implementation of the DRG‐based prospective payment system.JAMA.1990;264:1969–1973.
- ,.Pneumonia mortality reduction and quality improvement in a community hospital.Qual Rev Bull.1993;19:124–130.
- ,,, et al.Quality of care, process and outcomes in elderly patients with pneumonia.JAMA.1997;278:2080–2084.
- ,,,,.Processes of care, illness severity, and outcomes in the management of community‐acquired pneumonia at academic hospitals.Arch Intern Med.2001;161:2099–2104.
- ,,,,.Clinical importance of delays in the initiation of appropriate antibiotic treatment for ventilator‐associated pneumonia.Chest.2002;122:262–268.
- ,,, et al.Adequacy of early empiric antibiotic treatment and survival in severe sepsis: experience from the MONARCS Trial.Clin Infect Dis.2004;38:284–288.
- ,,,,,.The benefit of appropriate empirical antibiotic treatment in patients with bloodstream infection.J Intern Med.1998;244:379–386.
- ,,,,.The influence of inadequate antimicrobial treatment of bloodstream infections on patient outcomes in the ICU setting.Chest.2000;118:146–155.
- ,,,,,.Impact of adequate empirical antibiotic therapy on the outcome of patients admitted to the intensive care unit with sepsis.Crit Care Med.2003;31:2742–2751.
- ,,, et al.Inadequate antimicrobial treatment of infections: a risk factor for hospital mortality among critically ill patients.Chest.1999;115:462–474.
- ,,, et al.Efficacy and safety of recombinant human activated protein C for severe sepsis.N Engl J Med.2001;344:699–709.
- ,,, et al.Drotecogin alfa (activated) for adults with severe sepsis and a low risk of death.New Eng J Med.2005;353:1332–1341.
- ,,, et al.Drotecogin alfa (activated) treatment in severe sepsis from the global open label trial ENHANCE.Crit Care Med.2005;10:2266–2277.
- ,,.Activated protein C for severe sepsis.N Engl J Med.2002;347;1035–1036.
- .Assessing the use of activated protein C in the treatment of severe sepsis.N Engl J Med.2002;347:1030–1034.
- ,,,,.An economic evaluation of activated protein C treatment for severe sepsis.N Engl J Med.2002;347:993–1000.
- ,,,.Risks and benefits of activated protein C treatment for severe sepsis.N Engl J Med.2002;347:1027–1030.
- ,,, et al.Early goal‐directed therapy in the treatment of severe sepsis and septic shock.N Engl J Med.2001;345:1368–1377.
- ,,, et al.Serum lactate as a predictor of mortality in emergency department patients with infection.Ann Emerg Med.2005;45:524–528.
- ,,,.Vasopressor and inotropic support in septic shock: an evidence‐based review.Crit Care Med.2004;32(11 Suppl):S455–S465.
- ,,, et al.Goal‐directed therapy for severe sepsis [letter].N Engl J Med.2002;346:1025–1026.
- ,,,,,.Elevation of systemic oxygen delivery in the treatment of critically ill patients.New Engl J Med.1994;330:1717–1722.
- ,, et al.A trial of goal‐oriented hemodynamic therapy in critically ill patients.N Engl J Med.1995;333:1025–1032.
- .Hemodynamic and metabolic therapy in critically ill patients.New Engl J Med.2001;345:1417–1418.
- ,.Use of goal directed therapy for severe sepsis and septic shock in academic emergency departments.Crit Care Med.2005;33:1888–1889.
- ,,, et al.Corticosteroid treatment for sepsis: a critical appraisal and meta‐analysis of the literature.Crit Care Med.1995;23:1430–1439.
- .Physicians should administer low‐dose corticosteroids selectively to septic patients until an ongoing trial is completed.Ann Intern Med.2004;141:70–72.
- ,.Corticosteroids and septic shock.JAMA.2002;288:886–887.
- ,,, et al.Effect of treatment with low doses of hydrocortisone and fludrocortisone on mortality in patients with septic shock.JAMA.2002;288:862–871.
- ,,, et al.Risk factors of relative adrenocortical deficiency in intensive care patients needing mechanical ventilation.Intensive Care Med.2005;31:388–392.
- ,.Editorial III: Corticosteroids for septic shock—a standard of care?Br J Anaesth.2004;93:178–180.
- ,,.Corticosteroids for patients with septic shock [letter].JAMA.2003;289:42.
- ,,,,.Corticosteroids for patients with septic shock [letter].JAMA.2003;289:42.
- ,,,,.Corticosteroids for septic shock [letter].Ann Intern Med.2004;141:742–743.
- .Should we use etomidate as an induction agent for endotracheal intubation in patients with septic shock?: a critical appraisal.Chest.2005;127:1031–1038.
- .ICU physicians should abandon the use of etomidate!Intensive Care Med.2005;31:325–326.
- ,,, et al.Management of diabetes and hyperglycemia in hospitals.Diabetes Care.2004;27:553–591.
- ,,,,.Intensive insulin therapy exerts anti‐inflammatory effects in critically ill patients and counteracts the adverse effects of low mannose binding lectin levels.J Clin Endocrinol Metab.2003;88:1082–1088.
- ,,, et al.Intensive insulin therapy in critically ill patients.N Engl J Med.2001;345:1359–1367.
- .Effect of an intensive glucose management protocol on the mortality of critically ill adult patients.Mayo Clin Proc.2004;79:992–1000. Published erratum appears in Mayo Clin Proc.year="2005"2005;80:1101
- ,,, et al.Intensive insulin therapy in the medical ICU.N Engl J Med.2006;354:449–461.
- Supplement to:,,, et al.Intensive insulin therapy in the medical ICU.N Eng J Med.2006;354:449–461. Available at: http://content.nejm.org/cgi/data/354/5/449/DC1/1.
- .Glycemic control in the intensive care unit: why we should wait for NICE‐SUGAR.Mayo Clin Proc.2005;80:1546–1548.
- ,,.Intensive insulin therapy in critically ill patients [letter].New Engl J Med.2002;346:1586–1588.
- ,,,.Glucose control and mortality in critically ill patients.JAMA.2003;290:2041–2047.
- ,,,,.Influence of individual characteristics on outcome of glycemic control in intensive care unit patients with or without diabetes mellitus.Mayo Clin Proc.2005;80:1558–1567.
- ,,, et al.Outcome benefit of intensive insulin therapy in the critically ill: Insulin dose versus glycemic control.Crit Care Med.2003;31:634–635.
- ,,,,.Impact of admission hyperglycemia on hospital mortality in various intensive care unit populations.Crit Care Med.2005;33:2772–2777.
- .Intensive insulin in intensive care.New Engl J Med.2006;354:516–518.
- ,,,,.Fingerstick glucose determination in shock.Ann Intern Med.1991;114:1020–1024.
- ,,.A blueprint for a sepsis protocol.Acad Emerg Med.2005;12:352–359.
- ,,, et al.A prospective before‐and‐after trial of a medical emergency team.Med J Aust.2003;179:283–287.
- ,,, et al.A multidisciplinary community hospital program for early and rapid resuscitation of shock in nontrauma patients.Chest.2005;127:1729–1743.
- ,,, et al.Sepsis change bundles: converting guidelines into meaningful change in behavior and clinical outcome.Crit Care Med.2004;32:S595–S597.
- Fibrinolytic Therapy Trialists' Collaborative Group.Indications for fibrinolytic therapy in suspected acute myocardial infarction: collaborative overview of early mortality and major morbidity results from all randomised trials of more than 1000 patients.Lancet.1994;343:311–322.
- Antithrombotic Trialists' Collaboration.Collaborative meta‐analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients.Brit Med J.2002;324:71–86.
- ,,,,,.Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care.Crit Care Med.2001;29:1303–1310.
- ,,,.Prevalence and incidence of severe sepsis in Dutch intensive care units.Crit Care.2004;8:R153–R162.
- ,, et al.Direct costs of severe sepsis in three German intensive care units based on retrospective electronic patient record analysis of resource use.Intensive Care Med.2002;28:1440–1446.
- ,,,,.Effects of severity of illness on resource use by survivors and nonsurvivors of severe sepsis at intensive care unit admission.Crit Care Med.2002;30:2413–2419.
- ,,, et al.Resource utilization among patients with sepsis syndrome.Infect Control Hosp Epidemiol.2003;24:62–70.
- ,,,,.The costs of septic syndromes in the intensive care unit and influence of hospital‐acquired sepsis.Intensive Care Med,2003;29:1464–1471.
- ,.The pathophysiology and treatment of sepsis.N Engl J Med.2003;348:138–150.
- ,,,,.Meta‐analysis: the effect of steroids on survival and shock during sepsis depends on the dose.Ann Intern Med.2004;141:47–56.
- ,,,,,.Corticosteroids for severe sepsis and septic shock: a systematic review and meta‐analysis.Brit Med J.2004;329:480–488.
- ,,.Insulin therapy for critically ill hospitalized patients: a meta‐analysis of randomized controlled trials.Arch Intern Med.2004;164:2005–2011.
- ,,, et al.Surviving Sepsis Campaign guidelines for management of severe sepsis and septic shock.Crit Care Med.2004;32:858–873.
- ,,,,.Prevention of deep vein thrombosis in medical patients by low‐dose heparin.Scott Med J.1981;26:115–117.
- ,,, et al.A comparison of sucralfate and ranitidine for the prevention of upper gastrointestinal bleeding in patients requiring mechanical ventilation. Canadian Critical Care Trials Group.N Engl J Med.1998;338:791–797.
- The Acute Respiratory Distress Syndrome Network.Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome.N Engl J Med.2000;342:1301–1308.
- American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference: definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis.Crit Care Med.1992;20:864–874.
- ;;, et al. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference.Crit Care Med.2003;31:1250–1256.
- .Severe sepsis and therapy with activated protein C.New Engl J Med.2005;353:1398–1399.
- ,,,,.Timing of antibiotic administration and outcomes for Medicare patients hospitalized with community‐acquired pneumonia.Arch Intern Med.2004;164:637–644.
- ,,, et al.Measuring quality of care with explicit process criteria before and after implementation of the DRG‐based prospective payment system.JAMA.1990;264:1969–1973.
- ,.Pneumonia mortality reduction and quality improvement in a community hospital.Qual Rev Bull.1993;19:124–130.
- ,,, et al.Quality of care, process and outcomes in elderly patients with pneumonia.JAMA.1997;278:2080–2084.
- ,,,,.Processes of care, illness severity, and outcomes in the management of community‐acquired pneumonia at academic hospitals.Arch Intern Med.2001;161:2099–2104.
- ,,,,.Clinical importance of delays in the initiation of appropriate antibiotic treatment for ventilator‐associated pneumonia.Chest.2002;122:262–268.
- ,,, et al.Adequacy of early empiric antibiotic treatment and survival in severe sepsis: experience from the MONARCS Trial.Clin Infect Dis.2004;38:284–288.
- ,,,,,.The benefit of appropriate empirical antibiotic treatment in patients with bloodstream infection.J Intern Med.1998;244:379–386.
- ,,,,.The influence of inadequate antimicrobial treatment of bloodstream infections on patient outcomes in the ICU setting.Chest.2000;118:146–155.
- ,,,,,.Impact of adequate empirical antibiotic therapy on the outcome of patients admitted to the intensive care unit with sepsis.Crit Care Med.2003;31:2742–2751.
- ,,, et al.Inadequate antimicrobial treatment of infections: a risk factor for hospital mortality among critically ill patients.Chest.1999;115:462–474.
- ,,, et al.Efficacy and safety of recombinant human activated protein C for severe sepsis.N Engl J Med.2001;344:699–709.
- ,,, et al.Drotecogin alfa (activated) for adults with severe sepsis and a low risk of death.New Eng J Med.2005;353:1332–1341.
- ,,, et al.Drotecogin alfa (activated) treatment in severe sepsis from the global open label trial ENHANCE.Crit Care Med.2005;10:2266–2277.
- ,,.Activated protein C for severe sepsis.N Engl J Med.2002;347;1035–1036.
- .Assessing the use of activated protein C in the treatment of severe sepsis.N Engl J Med.2002;347:1030–1034.
- ,,,,.An economic evaluation of activated protein C treatment for severe sepsis.N Engl J Med.2002;347:993–1000.
- ,,,.Risks and benefits of activated protein C treatment for severe sepsis.N Engl J Med.2002;347:1027–1030.
- ,,, et al.Early goal‐directed therapy in the treatment of severe sepsis and septic shock.N Engl J Med.2001;345:1368–1377.
- ,,, et al.Serum lactate as a predictor of mortality in emergency department patients with infection.Ann Emerg Med.2005;45:524–528.
- ,,,.Vasopressor and inotropic support in septic shock: an evidence‐based review.Crit Care Med.2004;32(11 Suppl):S455–S465.
- ,,, et al.Goal‐directed therapy for severe sepsis [letter].N Engl J Med.2002;346:1025–1026.
- ,,,,,.Elevation of systemic oxygen delivery in the treatment of critically ill patients.New Engl J Med.1994;330:1717–1722.
- ,, et al.A trial of goal‐oriented hemodynamic therapy in critically ill patients.N Engl J Med.1995;333:1025–1032.
- .Hemodynamic and metabolic therapy in critically ill patients.New Engl J Med.2001;345:1417–1418.
- ,.Use of goal directed therapy for severe sepsis and septic shock in academic emergency departments.Crit Care Med.2005;33:1888–1889.
- ,,, et al.Corticosteroid treatment for sepsis: a critical appraisal and meta‐analysis of the literature.Crit Care Med.1995;23:1430–1439.
- .Physicians should administer low‐dose corticosteroids selectively to septic patients until an ongoing trial is completed.Ann Intern Med.2004;141:70–72.
- ,.Corticosteroids and septic shock.JAMA.2002;288:886–887.
- ,,, et al.Effect of treatment with low doses of hydrocortisone and fludrocortisone on mortality in patients with septic shock.JAMA.2002;288:862–871.
- ,,, et al.Risk factors of relative adrenocortical deficiency in intensive care patients needing mechanical ventilation.Intensive Care Med.2005;31:388–392.
- ,.Editorial III: Corticosteroids for septic shock—a standard of care?Br J Anaesth.2004;93:178–180.
- ,,.Corticosteroids for patients with septic shock [letter].JAMA.2003;289:42.
- ,,,,.Corticosteroids for patients with septic shock [letter].JAMA.2003;289:42.
- ,,,,.Corticosteroids for septic shock [letter].Ann Intern Med.2004;141:742–743.
- .Should we use etomidate as an induction agent for endotracheal intubation in patients with septic shock?: a critical appraisal.Chest.2005;127:1031–1038.
- .ICU physicians should abandon the use of etomidate!Intensive Care Med.2005;31:325–326.
- ,,, et al.Management of diabetes and hyperglycemia in hospitals.Diabetes Care.2004;27:553–591.
- ,,,,.Intensive insulin therapy exerts anti‐inflammatory effects in critically ill patients and counteracts the adverse effects of low mannose binding lectin levels.J Clin Endocrinol Metab.2003;88:1082–1088.
- ,,, et al.Intensive insulin therapy in critically ill patients.N Engl J Med.2001;345:1359–1367.
- .Effect of an intensive glucose management protocol on the mortality of critically ill adult patients.Mayo Clin Proc.2004;79:992–1000. Published erratum appears in Mayo Clin Proc.year="2005"2005;80:1101
- ,,, et al.Intensive insulin therapy in the medical ICU.N Engl J Med.2006;354:449–461.
- Supplement to:,,, et al.Intensive insulin therapy in the medical ICU.N Eng J Med.2006;354:449–461. Available at: http://content.nejm.org/cgi/data/354/5/449/DC1/1.
- .Glycemic control in the intensive care unit: why we should wait for NICE‐SUGAR.Mayo Clin Proc.2005;80:1546–1548.
- ,,.Intensive insulin therapy in critically ill patients [letter].New Engl J Med.2002;346:1586–1588.
- ,,,.Glucose control and mortality in critically ill patients.JAMA.2003;290:2041–2047.
- ,,,,.Influence of individual characteristics on outcome of glycemic control in intensive care unit patients with or without diabetes mellitus.Mayo Clin Proc.2005;80:1558–1567.
- ,,, et al.Outcome benefit of intensive insulin therapy in the critically ill: Insulin dose versus glycemic control.Crit Care Med.2003;31:634–635.
- ,,,,.Impact of admission hyperglycemia on hospital mortality in various intensive care unit populations.Crit Care Med.2005;33:2772–2777.
- .Intensive insulin in intensive care.New Engl J Med.2006;354:516–518.
- ,,,,.Fingerstick glucose determination in shock.Ann Intern Med.1991;114:1020–1024.
- ,,.A blueprint for a sepsis protocol.Acad Emerg Med.2005;12:352–359.
- ,,, et al.A prospective before‐and‐after trial of a medical emergency team.Med J Aust.2003;179:283–287.
- ,,, et al.A multidisciplinary community hospital program for early and rapid resuscitation of shock in nontrauma patients.Chest.2005;127:1729–1743.
- ,,, et al.Sepsis change bundles: converting guidelines into meaningful change in behavior and clinical outcome.Crit Care Med.2004;32:S595–S597.
- Fibrinolytic Therapy Trialists' Collaborative Group.Indications for fibrinolytic therapy in suspected acute myocardial infarction: collaborative overview of early mortality and major morbidity results from all randomised trials of more than 1000 patients.Lancet.1994;343:311–322.
- Antithrombotic Trialists' Collaboration.Collaborative meta‐analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients.Brit Med J.2002;324:71–86.