User login
Untreated excessive daytime sleepiness (EDS) results in compromised quality of life, reduced productivity, and public safety concerns.1 Obstructive sleep apnea (OSA), restless legs syndrome, circadian rhythm disorders, and narcolepsy are frequently underdiagnosed sleep disorders that can cause EDS. These conditions commonly go undetected and untreated for several reasons:
- Patients may not recognize sleepiness as a legitimate medical concern.
- Physicians, with few exceptions, typically have little training in sleep disorders and limited time to diagnose them.2 Screening questions regarding sleep are typically absent.
- Definitive diagnostic tests are costly.
As a result, many patients go without appropriate sleep evaluations. Instead a depressive or other psychiatric disorder may be suspected because of the sleepy patient’s poor energy, hypersomnia, amotivation, irritability, and frustration. Because of ongoing behavioral symptoms, patients with an undiagnosed primary sleep disorder are often referred to psychiatrists. Thus, a clear understanding of the differential diagnosis of EDS is crucial.
Patients with sleep issues fall into three major categories:
- Patients with EDS.
- Individuals with insomnia, another large group often seen by psychiatrists. Generally, these patients are less hesitant than patients with EDS to seek help because of the marked distress they suffer nightly when trying to sleep. Insomniacs typically experience minimal EDS.
- Patients with unusual behaviors at night that range from arm waving to violent behaviors.
Assessing the sleepy patient
When evaluating a patient with sleep complaints, several valuable sources of data come into play.
Initially, observe the patient in the waiting room or office before starting the interview. Did the patient nod off while waiting for his or her appointment? Pay attention to any patient who appears sleepy—even if he or she denies having trouble staying awake. Over time, sleepy patients may have lost their perspective on alertness. Some patients have had EDS for so many years that they no longer recall what it is like to feel fully awake.
Collateral history is often important because family members generally observe the sleeping patient. The bed partner often provides valuable information about snoring, irregular breathing leg kicks, unplanned naps, and strained interpersonal relationships due to EDS. For the patient who does not have a bed partner, ask his or her travel companion, with whom the patient may have shared accommodations.
Unfortunately, few useful screening tests exist. Most questionnaires about sleepiness are neither very reliable nor valid. One of the better questionnaires, the Epworth Sleepiness Scale, helps confirm the presence of sleepiness with a score <8, differentiating the inability to stay awake from fatigue. (Box 1 can be cut out, copied, and handed to patients). This brief questionnaire also provides a useful measure of severity.3
The value of the Epworth scale is limited, however, because patient answers often are based on a specific time and context that may not be representative. Additional validated surveys include the Pittsburgh Sleep Quality Inventory and several that focus on OSA.4
How likely are you to doze off or fall asleep in the following situations, in contrast to feeling just tired? Even if you have not done some of these things recently, try to work out how each situation would affect you now. Use the scale below to choose the most appropriate number for each situation:
- 0 no chance of dozing
- 1 slight chance of dozing
- 2 moderate chance of dozing
- 3 high chance of dozing
| Chance of dozing | Situation |
|---|---|
| ○ | Sitting and reading |
| ○ | Watching TV |
| ○ | Sitting inactive in a public place (e.g., a theater or a meeting) |
| ○ | Sitting as a passenger in a car for an hour without a break |
| ○ | Lying down to rest in the afternoon when circumstances permit |
| ○ | Sitting and talking to someone |
| ○ | Sitting quietly after a lunch without alcohol |
| ○ | In a car, while stopped for a few minutes in traffic |
| Johns, M. Sleep 14:540-545, 1991. | |
Electroencephalographic (EEG) monitoring can accurately measure the patient’s degree of sleep disruption. This information is critical in understanding if a patient’s EDS is caused by a physiologic condition that prevents quality nocturnal sleep. At this time, however, no portable devices that employ EEG technology are used in clinical settings.
Additionally, none of the widely used screening devices that assess leg kicks indicate the presence of possible periodic limb movements.
Even though overnight pulse oximetry has been used to screen for sleep-disordered breathing,5 the technology has limitations. For one, most pulse oximeters do not provide information about sleep stage or body position. Some patients with significant sleep-disordered breathing lack adequate oxygen desaturations but have frequent EEG arousals due to sleep issues. In this case, pulse oximetry would generate a false negative result because EEG data is not collected. The inadequate sensitivity is most likely to occur with females and thin patients.
Oximetry provides only one or two types of data (oxygen saturation plus possibly heart rate), while other physiologic processes, e.g., body movement or sleep architecture, can repetitively be disrupted during sleep.
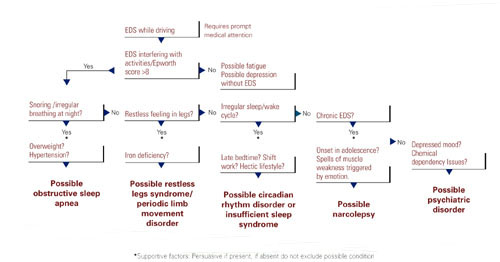
The most critical steps in detecting sleep disorders do not require technology or specialized expertise, but rather intuition and common sense. The psychiatrist should consider the possibility of a sleep disorder and incorporate pertinent questions into the clinical interview. Figure 1 lists sequential questions that might uncover specific sleep disorders. Once suspected, the decision whether to refer the patient to a sleep disorder center for diagnostic testing depends on the type of sleep disorder detected.
Diagnosing and treating OSA
Recent epidemiologic studies show that OSA affects at least 4% of men and 2% of women in the United States.6 Psychiatrists are virtually assured of seeing patients with undiagnosed OSA. The condition is caused by repeated collapse of the soft tissues surrounding the upper airway, decreasing airflow that is restored when the patient briefly awakens. Patients develop EDS because of sleep fragmented by frequent arousals.
Figure 1 THE SLEEPY PATIENT: Possible medical and psychiatric explanations
Obese patients are at higher risk than are patients at normal weight because of their body habitus. Alcohol or sedative medication use close to bedtime can aggravate OSA. These substances promote muscle relaxation and increase the arousal threshold, meaning that patients do not wake readily when apneas occur.
Long-term complications of untreated OSA include sleepiness leading to accidents, hypertension, cerebrovascular disease, and progressive obesity. New data associate OSA with multiple potential cardiovascular complications (arrhythmias, congestive heart failure, and myocardial infarction).7 Therefore, recognition and treatment are paramount.
The physical examination should focus on detecting nasal obstruction (having the patient sniff separately through each nostril can be helpful), big neck, crowded oropharynx (a low-hanging palate, reddened uvula, enlarged tonsils, large tongue size relative to oropharynx diameter) and jaw structure (particularly a small retrognathic mandible).
Referral for nocturnal polysomnography might be the next step. During a comprehensive sleep study, data is collected about respiratory, cardiovascular, and muscle activity at night, as well as the sounds the patient makes (e.g., snoring, coughing) when asleep. EEG monitoring also is performed. OSA may be diagnosed if repeated episodes of reduced airflow and oxygen desaturation are observed; these typically result in brief shifts in EEG frequency called arousals.
First-line interventions for OSA include avoidance of alcohol within 1 to 2 hours of bedtime, sleeping on the side instead of the back, weight loss (ideally with a regular exercise program), and nasal sprays for allergies.
If the first-line treatments for OSA are ineffective, nasal continuous positive airway pressure (CPAP) works well for almost all patients who adhere to the regimen.8 CPAP requires the patient to wear a nasal mask that delivers room air, splinting open the nasopharynx and the upper airway (Box 2). Some patients benefit from a brief trial of a sleeping medication, e.g., zolpidem or trazodone, for the first 1 to 2 weeks of nasal CPAP usage.
Nasal continuous positive airway pressure (CPAP) must be started in an observed setting so that the clinician can determine the optimal amount of positive pressure needed to keep the upper airway patent. CPAP can be started during the second half of a “split-night” sleep study after obstructive sleep apnea (OSA) has been diagnosed. Alternatively, the sleep laboratory might ask the patient to return for a second night for a trial of nasal CPAP.
Patients with severe OSA might notice improved sleep quality and reduced EDS, even after only a few hours of use. Such patients sometimes wish to start CPAP treatment immediately.
Overall, advances in masks and equipment have improved patient adherence to CPAP. Such innovations include auto-titrating machines, in which the pressure level can be varied depending on sleep state or body position. Many newer machines also have a data microchip that allows the clinician to determine the duration of usage, then use that information to counsel the patient about adherence if necessary.
Patient education also can promote CPAP adherence. Upon being first told they might need to sleep each night wearing a nasal mask, patients often voice well-founded concerns about comfort, claustrophobia, or sexual activity.
As part of a comprehensive approach at the Mayo Sleep Disorders Center, patients watch an educational videotape, tour the sleep laboratory bedrooms before the sleep study, and are carefully fitted for masks. Ideally, the technologists interact with the patient during the sleep study to adjust the headgear and fine-tune other aspects of the equipment. The sleep specialist meets with the patient to compare the baseline diagnostic study results with changes in breathing patterns after a trial of nasal CPAP.
Other useful patient compliance tools include a CPAP informational handout, telephone access to nursing staff, and a 30-day follow-up visit.
Obtaining the support of the bed partner by welcoming her or him to all appointments, including educational activities, is optimal. The bed partner was likely the impetus for the appointment in the first place because of concerns about excessive snoring or apneas.
Image reprinted from Oct. 2001 Mayo Clinic Health Letter with permission of Mayo Foundation for Medical Education and Research, Rochester, MN 55905
Surgical options exist for OSA. The most common procedures are uvulopalatopharyngoplasty (UPPP) and laser-assisted uvulopalatoplasty (LAUP). Other procedures in use include tongue reduction and mandibular advancement.
The response rate to OSA surgery averages around 50% but varies on the patient’s characteristics and procedure selected.9 Positive outcomes are most likely for thin patients with obvious upper airway obstruction, including a deviated nasal septum, large tonsils, a low-hanging palate, and large uvula. Potential complications include nasal regurgitation, voice change, postoperative pain, bleeding, infection, tongue numbness, and snoring without apnea (silent apnea).
Oral appliances have a vital niche in OSA treatment. Multiple devices have been developed that open the oropharynx by moving the mandible and tongue out of way. A growing body of data shows that oral appliances improve sleep and reduce EDS and promote patient satisfaction more effectively than nasal CPAP.10 Several studies also show that patients with mild to moderate OSA accept these devices well.
Oral devices do have drawbacks, however. In most settings, effectiveness cannot be observed during a “split-night” laboratory sleep study because the patient has not yet purchased the device. Also, multiple visits sometimes are required to custom fit the oral appliance; this can pose a hardship to patients who live a distance from the provider.
Restless legs syndrome, periodic limb movement disorder
The patient with restless legs syndrome typically reports a restless painful feeling in the limbs that occurs in the evening and at night, disrupting sleep. This condition, which affects 10% of the population, is associated with aging, blood loss, anemia, peripheral neuropathies, and pregnancy.11 Patients can have childhood onset and in some cases there is a familial tendency.
Most patients with restless legs syndrome have periodic limb movements (repetitive leg jerks or twitches). The clinical significance of periodic limb movements with no subjective disagreeable feelings in the limbs is controversial. Typically, treatment is not instituted in these cases.
The history usually confirms the diagnosis without a sleep study. Sleep studies are used only if a co-existing sleep problem is suspected or if the diagnosis is not clear-cut.
One suspected mechanism of restless legs syndrome is a dopamine-deficient state. A serum ferritin level can help detect a relative iron deficiency, iron being a cofactor for dopamine synthesis.12
Treatment can include iron repletion when indicated. Medications include dopaminergic agents, most notably pramipexole and levodopa/carbidopa. Other options include gabapentin, benzodiazepines, and narcotics. Antidepressants have been suspected to worsen this condition but definitive studies are lacking.13
Identifying, correcting circadian rhythm disorders
Instead of compromising the quality or quantity of sleep, circadian rhythm disorders cause sleep to occur at inappropriate times. Adolescents or young adults are most likely to confront these disorders.
The delayed sleep phase disorder—that is, a persistent pattern of staying up late and “sleeping in” the next morning—is the most common example. A careful assessment will reveal that the patient is getting a satisfactory amount of sleep that occurs at a socially unacceptable time, sometimes to the extreme that his or her nights and days are reversed.
Patients can be reluctant to acknowledge the severity of their problem, which can lead to both inaccurate sleep diaries and interviews. A portable device called a wrist actigraph provides data about limb movement, thus more objectively measuring the patient’s sleep schedule.
Psychiatrists frequently encounter patients with delayed sleep phase disorder because of a high degree of comorbidity with depressive disorders.14 The cause of this syndrome is unclear, but environmental factors including light exposure, social patterns, psychological issues, and possibly a genetic substrate, are known to contribute.
A less common circadian rhythm disorder, advanced sleep phase disorder, can also cause EDS. Patients have an inappropriately early time of sleep onset and then are fully awake in the middle of the night. A recent report describes a large family with a severe form of this disorder that is linked to an abnormality on chromosome two.15
Relatively few effective treatments have been identified for circadian rhythm disorders. Some patients elect not to pursue therapy, instead selecting activities that fit around their unconventional sleep schedules. Sometimes individuals with delayed sleep phase cannot arrange their education or work hours around their atypical sleep schedules. These patients experience poor early morning academic or work performance due to sleepiness.
The internal circadian clock can be gradually readjusted with either phototherapy or gradual shifting of the major sleep period (Box 4). Stimulant or hypnotic medications generally are not utilized.
Insufficient sleep syndrome
Studies indicate that more people are attempting to burn the candle at both ends and are consequently developing a newly identified condition, insufficient sleep syndrome.16 In our 24-hour society, people often are trying to make do with less than the required 7-1/2 hours sleep per day. This may have adverse consequences to their health. When people are required to perform shift work, the problem is compounded because of the difficulty in obtaining sufficient quality sleep during daylight hours.
Many patients do not seek out treatment for fatigue or sleepiness because they are aware of the lifestyle choices that they have made. Still, they might develop psychologic symptoms like irritability, mood swings, and strained interpersonal relationships. These symptoms often will prompt patients to request treatment.
The most common technique is to ask the patient to establish a consistent awakening time and subsequently a regular bedtime. Initially this could be unconventional by societal standards, i.e., bedtime at 5 a.m. and arising at 2 p.m. Once this pattern is in place, the patient gradually shifts the timing by an hour a day. For most patients it is easier to delay rather than advance the bedtime until it conforms to the desired time.
Reinforce this new sleep pattern with a structured daytime schedule that includes predictable mealtimes, regular exercise, social activities, and possibly bright light exposure. This reinforcement should occur in the morning for delayed sleep phase and in the evening for advanced sleep phase disorder. These interventions take time and discipline.
Another approach is for the patient to completely skip sleep one night and, in a sleep-deprived state, establish a new bedtime at the desired time. The same modalities listed above must be used to reinforce (or “entrain”) this schedule or the patient will gradually slip back into the previous abnormal sleep-wake rhythm.
Major medical centers and North American metropolitan areas are increasingly developing sleep disorder treatment centers. Insurance companies generally cover a specialty sleep evaluation, particularly if the referring physician documents a suspicion of sleep-disordered breathing or excessive daytime sleepiness (EDS)that jeopardizes safe driving.
The most appropriate conditions for an urgent sleep evaluation are:
- Difficulty staying alert while driving, nocturnal cardiac arrhythmias;
- Frequent observed apneas;
- EDS leading to academic or occupational problems.
Psychiatrists should take a careful history that includes a discussion of the patient’s daily and weekly schedule. Avoid psychostimulant medications. Instead, address the non-negotiable need to get adequate sleep and challenge the patients to prioritize his or her activities around a full night’s sleep.
When to consider narcolepsy
Narcolepsy, a less common sleep disorder, can lead to severe occupational, educational, and family disruption. Narcolepsy, which affects 0.05% of the population, is a potentially debilitating disease of the central nervous system that involves abnormal regulation of REM sleep. EDS is the cardinal symptom, often associated with cataplexy (75%), sleep paralysis (50%), vivid dreams, and insomnia, all of which can represent inappropriate intrusion of REM phenomena.
After obtaining a history suggestive of narcolepsy, the psychiatrist should employ either the history, a sleep diary, or wrist actigraphy to document whether the patient is getting adequate sleep with a consistent sleep/wake cycle. Next, consider referring the patient for polysomnography, primarily to rule out other causes of EDS like sleep disorder breathing. In some cases, the REM latency on the overnight sleep study will be less than 20 minutes after sleep onset, which supports the diagnosis of narcolepsy. A multiple sleep latency test (MSLT), a diagnostic test that consists of the patient taking four to five daytime naps, is performed the following day.
Narcolepsy is confirmed if the patient has a mean initial sleep latency of less than 10 minutes during these naps plus at least two REM episodes occurring within 15 minutes after sleep onset.
Recent research shows that most patients who have narcolepsy with cataplexy have undetectable levels of a specific neuropeptide (which is called either hypocretin or orexin) in the cerebrospinal fluid.17 Hypocretin/orexin replacement therapy is a theoretical future possibility, but for now treatment includes a combination of optimal sleep hygiene, psychostimulants, antidepressants, and hypnotics.
Other causes of EDS
Other causes of EDS include unrecognized alcohol dependence, inappropriate or excessive medication use, and depressive disorders. Overnight sleep studies are seldom indicated unless patients endorse the symptoms in Figure 1.
Before pursuing sleep studies (polysomnography or an MSLT), eliminate medications that might confound the results. Such agents include antidepressants, which alter the timing and duration of REM sleep, and sedating medications, which modify initial sleep latency and sleep efficiency and potentially aggravate sleep disordered breathing. Although initial REM latency provides a potential biologic marker of major depression, this measurement is more often used in research studies than in clinical psychiatry.
Primary insomnia is a distressing inability to sleep at night or nap during the day. This suggests a hyperarousal state in several ways, and is the opposite of EDS.18 In rare cases, however, patients who cannot sleep at night also do have EDS. When evaluated, these patients typically endorse at least one of the symptoms in Figure 1. Overnight sleep studies occasionally demonstrate that the insomnia is a symptom of another underlying specific sleep disorder, such as OSA or restless legs syndrome.
Psychiatrists treating a patient with chronic insomnia may appropriately undertake several trials of behavioral interventions or sedating medications before making a referral to a sleep disorder center. Patients can struggle with unrecognized primary sleep disorders for years, and many are evaluated by psychiatrists who institute an empiric trial of stimulating antidepressant medications. Use of antidepressants in these situations is unlikely to cause harm, but they might complicate diagnostic testing. When coexisting depression and a primary sleep disorder are confirmed, management of the sleepy patient optimally entails specific treatments that separately target each condition.
Related resources
- National Sleep Foundation www.sleepfoundation.org
- American Academy of Sleep Medicine www.asda.org
- American Sleep Apnea Association www.sleepapnea.org
- Restless Legs Syndrome Foundation www.rls.org
- Association for the Study of Light Therapy and Biological Rhythms www.sltbr.org
Disclosure
The author reports no affiliation or financial arrangements with any of the companies whose products are mentioned in this article.
Drug brand names
- Carbidopa/levodopa • Sinemet
- Gabapentin • Neurontin
- Pramipexole • Mirapex
- Trazodone • Desyrel
- Zolpidem • Ambien
1. Ronald J, Delaive K, et al. Health care utilization in the 10 years prior to diagnosis in obstructive sleep apnea syndrome patients. Sleep. 1999;22(2):225-29.
2. Punjabi N, Haponik E. Ask about daytime sleepiness. J Amer Geriatr Soc. 2000;48:228-29.
3. Johns M. A new method for measuring daytime sleepiness: The Epworth Sleepiness Scale. Sleep. 1991;14(6):540-45.
4. Rowley J, Aboussouan L, Badr M. The use of clinical prediction formulas in the evaluation of obstructive sleep apnea. Sleep. 2000;23:929-38.
5. Yamashiro Y, Kryger M. Nocturnal oximetry: Is it a screening tool for sleep disorders? Sleep. 1995;18:167-71.
6. Morrell M, Finn L, Kim H, Peppard P, Badr M, Young T. Sleep fragmentation, awake blood pressure, and sleep-disordered breathing in a population-based study. Am J Respir Critical Care Med. 2000;162(6):2091-96.
7. Roux F, D’Ambrosio C, Mohsenin V. Sleep-related breathing disorders and cardiovascular disease. Am J Med. 2000;108:396-402.
8. Engleman H, Martin S, Deary I, Douglas N. Effect of continuous positive airway pressure treatment on daytime function in sleep apnoea/hypopnoea syndrome. Lancet. 1994;343(8897):572-75.
9. Lojander J, Maasilta P, Partinen M, Brander P, Salmi T, Lehtonen H. Nasal-CPAP, surgery, and conservative management for treatment of obstructive sleep apnea syndrome. A randomized study. Chest. 1996;110(1):114-19.
10. Mehta A, Qian J, Petocz P, Darendeliler M, Cistulli P. A randomized, controlled study of a mandibular advancement splint for obstructive sleep apnea. Am J Respir Critical Care Med. 2001;163(6):1457-61.
11. Chesson A, Jr, Wise M, et al. Practice parameters for the treatment of restless legs syndrome and periodic limb movement disorder. Sleep. 1999;22(7):961-68.
12. Phillips B, Young T, Finn L, Asher K, Hening W, Purvis C. Epidemiology of restless legs symptoms in adults. Arch Intern Med. 2000;160(14):2137-41.
13. Thorpy M, Ehrenberg B, Hening W, et al. Restless legs syndrome: Detection and management in primary care. Amer Fam Phys. 2000;62:108-14.
14. Regestein Q, Monk T. Delayed sleep phase syndrome: A review of its clinical aspects. Am J Psychiatry. 1995;152:602-08.
15. Toh K, Jones C, et al. An hPer2 phosphorylation site mutation in familial advanced sleep phase syndrome. Science. 2001;291(5506):1040-43.
16. Yoshikawa N, Suzuki S, Ishimoto T, Matsumoto M, Miyagishi T. A case of insufficient sleep syndrome. Psychiatry Clin Neuro. 1998;52(2):200-01.
17. Nishino S, Ripley B, Overeem S, Lammers G, Mignot E. Hypocretin (orexin) deficiency in human narcolepsy. Lancet. 2000;355:39-40.
18. Hauri P, Esther M. Insomnia. Mayo Clin Proc. 1990;65:869-82.
Untreated excessive daytime sleepiness (EDS) results in compromised quality of life, reduced productivity, and public safety concerns.1 Obstructive sleep apnea (OSA), restless legs syndrome, circadian rhythm disorders, and narcolepsy are frequently underdiagnosed sleep disorders that can cause EDS. These conditions commonly go undetected and untreated for several reasons:
- Patients may not recognize sleepiness as a legitimate medical concern.
- Physicians, with few exceptions, typically have little training in sleep disorders and limited time to diagnose them.2 Screening questions regarding sleep are typically absent.
- Definitive diagnostic tests are costly.
As a result, many patients go without appropriate sleep evaluations. Instead a depressive or other psychiatric disorder may be suspected because of the sleepy patient’s poor energy, hypersomnia, amotivation, irritability, and frustration. Because of ongoing behavioral symptoms, patients with an undiagnosed primary sleep disorder are often referred to psychiatrists. Thus, a clear understanding of the differential diagnosis of EDS is crucial.
Patients with sleep issues fall into three major categories:
- Patients with EDS.
- Individuals with insomnia, another large group often seen by psychiatrists. Generally, these patients are less hesitant than patients with EDS to seek help because of the marked distress they suffer nightly when trying to sleep. Insomniacs typically experience minimal EDS.
- Patients with unusual behaviors at night that range from arm waving to violent behaviors.
Assessing the sleepy patient
When evaluating a patient with sleep complaints, several valuable sources of data come into play.
Initially, observe the patient in the waiting room or office before starting the interview. Did the patient nod off while waiting for his or her appointment? Pay attention to any patient who appears sleepy—even if he or she denies having trouble staying awake. Over time, sleepy patients may have lost their perspective on alertness. Some patients have had EDS for so many years that they no longer recall what it is like to feel fully awake.
Collateral history is often important because family members generally observe the sleeping patient. The bed partner often provides valuable information about snoring, irregular breathing leg kicks, unplanned naps, and strained interpersonal relationships due to EDS. For the patient who does not have a bed partner, ask his or her travel companion, with whom the patient may have shared accommodations.
Unfortunately, few useful screening tests exist. Most questionnaires about sleepiness are neither very reliable nor valid. One of the better questionnaires, the Epworth Sleepiness Scale, helps confirm the presence of sleepiness with a score <8, differentiating the inability to stay awake from fatigue. (Box 1 can be cut out, copied, and handed to patients). This brief questionnaire also provides a useful measure of severity.3
The value of the Epworth scale is limited, however, because patient answers often are based on a specific time and context that may not be representative. Additional validated surveys include the Pittsburgh Sleep Quality Inventory and several that focus on OSA.4
How likely are you to doze off or fall asleep in the following situations, in contrast to feeling just tired? Even if you have not done some of these things recently, try to work out how each situation would affect you now. Use the scale below to choose the most appropriate number for each situation:
- 0 no chance of dozing
- 1 slight chance of dozing
- 2 moderate chance of dozing
- 3 high chance of dozing
| Chance of dozing | Situation |
|---|---|
| ○ | Sitting and reading |
| ○ | Watching TV |
| ○ | Sitting inactive in a public place (e.g., a theater or a meeting) |
| ○ | Sitting as a passenger in a car for an hour without a break |
| ○ | Lying down to rest in the afternoon when circumstances permit |
| ○ | Sitting and talking to someone |
| ○ | Sitting quietly after a lunch without alcohol |
| ○ | In a car, while stopped for a few minutes in traffic |
| Johns, M. Sleep 14:540-545, 1991. | |
Electroencephalographic (EEG) monitoring can accurately measure the patient’s degree of sleep disruption. This information is critical in understanding if a patient’s EDS is caused by a physiologic condition that prevents quality nocturnal sleep. At this time, however, no portable devices that employ EEG technology are used in clinical settings.
Additionally, none of the widely used screening devices that assess leg kicks indicate the presence of possible periodic limb movements.
Even though overnight pulse oximetry has been used to screen for sleep-disordered breathing,5 the technology has limitations. For one, most pulse oximeters do not provide information about sleep stage or body position. Some patients with significant sleep-disordered breathing lack adequate oxygen desaturations but have frequent EEG arousals due to sleep issues. In this case, pulse oximetry would generate a false negative result because EEG data is not collected. The inadequate sensitivity is most likely to occur with females and thin patients.
Oximetry provides only one or two types of data (oxygen saturation plus possibly heart rate), while other physiologic processes, e.g., body movement or sleep architecture, can repetitively be disrupted during sleep.
The most critical steps in detecting sleep disorders do not require technology or specialized expertise, but rather intuition and common sense. The psychiatrist should consider the possibility of a sleep disorder and incorporate pertinent questions into the clinical interview. Figure 1 lists sequential questions that might uncover specific sleep disorders. Once suspected, the decision whether to refer the patient to a sleep disorder center for diagnostic testing depends on the type of sleep disorder detected.
Diagnosing and treating OSA
Recent epidemiologic studies show that OSA affects at least 4% of men and 2% of women in the United States.6 Psychiatrists are virtually assured of seeing patients with undiagnosed OSA. The condition is caused by repeated collapse of the soft tissues surrounding the upper airway, decreasing airflow that is restored when the patient briefly awakens. Patients develop EDS because of sleep fragmented by frequent arousals.
Figure 1 THE SLEEPY PATIENT: Possible medical and psychiatric explanations

Obese patients are at higher risk than are patients at normal weight because of their body habitus. Alcohol or sedative medication use close to bedtime can aggravate OSA. These substances promote muscle relaxation and increase the arousal threshold, meaning that patients do not wake readily when apneas occur.
Long-term complications of untreated OSA include sleepiness leading to accidents, hypertension, cerebrovascular disease, and progressive obesity. New data associate OSA with multiple potential cardiovascular complications (arrhythmias, congestive heart failure, and myocardial infarction).7 Therefore, recognition and treatment are paramount.
The physical examination should focus on detecting nasal obstruction (having the patient sniff separately through each nostril can be helpful), big neck, crowded oropharynx (a low-hanging palate, reddened uvula, enlarged tonsils, large tongue size relative to oropharynx diameter) and jaw structure (particularly a small retrognathic mandible).
Referral for nocturnal polysomnography might be the next step. During a comprehensive sleep study, data is collected about respiratory, cardiovascular, and muscle activity at night, as well as the sounds the patient makes (e.g., snoring, coughing) when asleep. EEG monitoring also is performed. OSA may be diagnosed if repeated episodes of reduced airflow and oxygen desaturation are observed; these typically result in brief shifts in EEG frequency called arousals.
First-line interventions for OSA include avoidance of alcohol within 1 to 2 hours of bedtime, sleeping on the side instead of the back, weight loss (ideally with a regular exercise program), and nasal sprays for allergies.
If the first-line treatments for OSA are ineffective, nasal continuous positive airway pressure (CPAP) works well for almost all patients who adhere to the regimen.8 CPAP requires the patient to wear a nasal mask that delivers room air, splinting open the nasopharynx and the upper airway (Box 2). Some patients benefit from a brief trial of a sleeping medication, e.g., zolpidem or trazodone, for the first 1 to 2 weeks of nasal CPAP usage.

Nasal continuous positive airway pressure (CPAP) must be started in an observed setting so that the clinician can determine the optimal amount of positive pressure needed to keep the upper airway patent. CPAP can be started during the second half of a “split-night” sleep study after obstructive sleep apnea (OSA) has been diagnosed. Alternatively, the sleep laboratory might ask the patient to return for a second night for a trial of nasal CPAP.
Patients with severe OSA might notice improved sleep quality and reduced EDS, even after only a few hours of use. Such patients sometimes wish to start CPAP treatment immediately.
Overall, advances in masks and equipment have improved patient adherence to CPAP. Such innovations include auto-titrating machines, in which the pressure level can be varied depending on sleep state or body position. Many newer machines also have a data microchip that allows the clinician to determine the duration of usage, then use that information to counsel the patient about adherence if necessary.
Patient education also can promote CPAP adherence. Upon being first told they might need to sleep each night wearing a nasal mask, patients often voice well-founded concerns about comfort, claustrophobia, or sexual activity.
As part of a comprehensive approach at the Mayo Sleep Disorders Center, patients watch an educational videotape, tour the sleep laboratory bedrooms before the sleep study, and are carefully fitted for masks. Ideally, the technologists interact with the patient during the sleep study to adjust the headgear and fine-tune other aspects of the equipment. The sleep specialist meets with the patient to compare the baseline diagnostic study results with changes in breathing patterns after a trial of nasal CPAP.
Other useful patient compliance tools include a CPAP informational handout, telephone access to nursing staff, and a 30-day follow-up visit.
Obtaining the support of the bed partner by welcoming her or him to all appointments, including educational activities, is optimal. The bed partner was likely the impetus for the appointment in the first place because of concerns about excessive snoring or apneas.
Image reprinted from Oct. 2001 Mayo Clinic Health Letter with permission of Mayo Foundation for Medical Education and Research, Rochester, MN 55905
Surgical options exist for OSA. The most common procedures are uvulopalatopharyngoplasty (UPPP) and laser-assisted uvulopalatoplasty (LAUP). Other procedures in use include tongue reduction and mandibular advancement.
The response rate to OSA surgery averages around 50% but varies on the patient’s characteristics and procedure selected.9 Positive outcomes are most likely for thin patients with obvious upper airway obstruction, including a deviated nasal septum, large tonsils, a low-hanging palate, and large uvula. Potential complications include nasal regurgitation, voice change, postoperative pain, bleeding, infection, tongue numbness, and snoring without apnea (silent apnea).
Oral appliances have a vital niche in OSA treatment. Multiple devices have been developed that open the oropharynx by moving the mandible and tongue out of way. A growing body of data shows that oral appliances improve sleep and reduce EDS and promote patient satisfaction more effectively than nasal CPAP.10 Several studies also show that patients with mild to moderate OSA accept these devices well.
Oral devices do have drawbacks, however. In most settings, effectiveness cannot be observed during a “split-night” laboratory sleep study because the patient has not yet purchased the device. Also, multiple visits sometimes are required to custom fit the oral appliance; this can pose a hardship to patients who live a distance from the provider.
Restless legs syndrome, periodic limb movement disorder
The patient with restless legs syndrome typically reports a restless painful feeling in the limbs that occurs in the evening and at night, disrupting sleep. This condition, which affects 10% of the population, is associated with aging, blood loss, anemia, peripheral neuropathies, and pregnancy.11 Patients can have childhood onset and in some cases there is a familial tendency.
Most patients with restless legs syndrome have periodic limb movements (repetitive leg jerks or twitches). The clinical significance of periodic limb movements with no subjective disagreeable feelings in the limbs is controversial. Typically, treatment is not instituted in these cases.
The history usually confirms the diagnosis without a sleep study. Sleep studies are used only if a co-existing sleep problem is suspected or if the diagnosis is not clear-cut.
One suspected mechanism of restless legs syndrome is a dopamine-deficient state. A serum ferritin level can help detect a relative iron deficiency, iron being a cofactor for dopamine synthesis.12
Treatment can include iron repletion when indicated. Medications include dopaminergic agents, most notably pramipexole and levodopa/carbidopa. Other options include gabapentin, benzodiazepines, and narcotics. Antidepressants have been suspected to worsen this condition but definitive studies are lacking.13
Identifying, correcting circadian rhythm disorders
Instead of compromising the quality or quantity of sleep, circadian rhythm disorders cause sleep to occur at inappropriate times. Adolescents or young adults are most likely to confront these disorders.
The delayed sleep phase disorder—that is, a persistent pattern of staying up late and “sleeping in” the next morning—is the most common example. A careful assessment will reveal that the patient is getting a satisfactory amount of sleep that occurs at a socially unacceptable time, sometimes to the extreme that his or her nights and days are reversed.
Patients can be reluctant to acknowledge the severity of their problem, which can lead to both inaccurate sleep diaries and interviews. A portable device called a wrist actigraph provides data about limb movement, thus more objectively measuring the patient’s sleep schedule.
Psychiatrists frequently encounter patients with delayed sleep phase disorder because of a high degree of comorbidity with depressive disorders.14 The cause of this syndrome is unclear, but environmental factors including light exposure, social patterns, psychological issues, and possibly a genetic substrate, are known to contribute.
A less common circadian rhythm disorder, advanced sleep phase disorder, can also cause EDS. Patients have an inappropriately early time of sleep onset and then are fully awake in the middle of the night. A recent report describes a large family with a severe form of this disorder that is linked to an abnormality on chromosome two.15
Relatively few effective treatments have been identified for circadian rhythm disorders. Some patients elect not to pursue therapy, instead selecting activities that fit around their unconventional sleep schedules. Sometimes individuals with delayed sleep phase cannot arrange their education or work hours around their atypical sleep schedules. These patients experience poor early morning academic or work performance due to sleepiness.
The internal circadian clock can be gradually readjusted with either phototherapy or gradual shifting of the major sleep period (Box 4). Stimulant or hypnotic medications generally are not utilized.
Insufficient sleep syndrome
Studies indicate that more people are attempting to burn the candle at both ends and are consequently developing a newly identified condition, insufficient sleep syndrome.16 In our 24-hour society, people often are trying to make do with less than the required 7-1/2 hours sleep per day. This may have adverse consequences to their health. When people are required to perform shift work, the problem is compounded because of the difficulty in obtaining sufficient quality sleep during daylight hours.
Many patients do not seek out treatment for fatigue or sleepiness because they are aware of the lifestyle choices that they have made. Still, they might develop psychologic symptoms like irritability, mood swings, and strained interpersonal relationships. These symptoms often will prompt patients to request treatment.
The most common technique is to ask the patient to establish a consistent awakening time and subsequently a regular bedtime. Initially this could be unconventional by societal standards, i.e., bedtime at 5 a.m. and arising at 2 p.m. Once this pattern is in place, the patient gradually shifts the timing by an hour a day. For most patients it is easier to delay rather than advance the bedtime until it conforms to the desired time.
Reinforce this new sleep pattern with a structured daytime schedule that includes predictable mealtimes, regular exercise, social activities, and possibly bright light exposure. This reinforcement should occur in the morning for delayed sleep phase and in the evening for advanced sleep phase disorder. These interventions take time and discipline.
Another approach is for the patient to completely skip sleep one night and, in a sleep-deprived state, establish a new bedtime at the desired time. The same modalities listed above must be used to reinforce (or “entrain”) this schedule or the patient will gradually slip back into the previous abnormal sleep-wake rhythm.
Major medical centers and North American metropolitan areas are increasingly developing sleep disorder treatment centers. Insurance companies generally cover a specialty sleep evaluation, particularly if the referring physician documents a suspicion of sleep-disordered breathing or excessive daytime sleepiness (EDS)that jeopardizes safe driving.
The most appropriate conditions for an urgent sleep evaluation are:
- Difficulty staying alert while driving, nocturnal cardiac arrhythmias;
- Frequent observed apneas;
- EDS leading to academic or occupational problems.
Psychiatrists should take a careful history that includes a discussion of the patient’s daily and weekly schedule. Avoid psychostimulant medications. Instead, address the non-negotiable need to get adequate sleep and challenge the patients to prioritize his or her activities around a full night’s sleep.
When to consider narcolepsy
Narcolepsy, a less common sleep disorder, can lead to severe occupational, educational, and family disruption. Narcolepsy, which affects 0.05% of the population, is a potentially debilitating disease of the central nervous system that involves abnormal regulation of REM sleep. EDS is the cardinal symptom, often associated with cataplexy (75%), sleep paralysis (50%), vivid dreams, and insomnia, all of which can represent inappropriate intrusion of REM phenomena.
After obtaining a history suggestive of narcolepsy, the psychiatrist should employ either the history, a sleep diary, or wrist actigraphy to document whether the patient is getting adequate sleep with a consistent sleep/wake cycle. Next, consider referring the patient for polysomnography, primarily to rule out other causes of EDS like sleep disorder breathing. In some cases, the REM latency on the overnight sleep study will be less than 20 minutes after sleep onset, which supports the diagnosis of narcolepsy. A multiple sleep latency test (MSLT), a diagnostic test that consists of the patient taking four to five daytime naps, is performed the following day.
Narcolepsy is confirmed if the patient has a mean initial sleep latency of less than 10 minutes during these naps plus at least two REM episodes occurring within 15 minutes after sleep onset.
Recent research shows that most patients who have narcolepsy with cataplexy have undetectable levels of a specific neuropeptide (which is called either hypocretin or orexin) in the cerebrospinal fluid.17 Hypocretin/orexin replacement therapy is a theoretical future possibility, but for now treatment includes a combination of optimal sleep hygiene, psychostimulants, antidepressants, and hypnotics.
Other causes of EDS
Other causes of EDS include unrecognized alcohol dependence, inappropriate or excessive medication use, and depressive disorders. Overnight sleep studies are seldom indicated unless patients endorse the symptoms in Figure 1.
Before pursuing sleep studies (polysomnography or an MSLT), eliminate medications that might confound the results. Such agents include antidepressants, which alter the timing and duration of REM sleep, and sedating medications, which modify initial sleep latency and sleep efficiency and potentially aggravate sleep disordered breathing. Although initial REM latency provides a potential biologic marker of major depression, this measurement is more often used in research studies than in clinical psychiatry.
Primary insomnia is a distressing inability to sleep at night or nap during the day. This suggests a hyperarousal state in several ways, and is the opposite of EDS.18 In rare cases, however, patients who cannot sleep at night also do have EDS. When evaluated, these patients typically endorse at least one of the symptoms in Figure 1. Overnight sleep studies occasionally demonstrate that the insomnia is a symptom of another underlying specific sleep disorder, such as OSA or restless legs syndrome.
Psychiatrists treating a patient with chronic insomnia may appropriately undertake several trials of behavioral interventions or sedating medications before making a referral to a sleep disorder center. Patients can struggle with unrecognized primary sleep disorders for years, and many are evaluated by psychiatrists who institute an empiric trial of stimulating antidepressant medications. Use of antidepressants in these situations is unlikely to cause harm, but they might complicate diagnostic testing. When coexisting depression and a primary sleep disorder are confirmed, management of the sleepy patient optimally entails specific treatments that separately target each condition.
Related resources
- National Sleep Foundation www.sleepfoundation.org
- American Academy of Sleep Medicine www.asda.org
- American Sleep Apnea Association www.sleepapnea.org
- Restless Legs Syndrome Foundation www.rls.org
- Association for the Study of Light Therapy and Biological Rhythms www.sltbr.org
Disclosure
The author reports no affiliation or financial arrangements with any of the companies whose products are mentioned in this article.
Drug brand names
- Carbidopa/levodopa • Sinemet
- Gabapentin • Neurontin
- Pramipexole • Mirapex
- Trazodone • Desyrel
- Zolpidem • Ambien
Untreated excessive daytime sleepiness (EDS) results in compromised quality of life, reduced productivity, and public safety concerns.1 Obstructive sleep apnea (OSA), restless legs syndrome, circadian rhythm disorders, and narcolepsy are frequently underdiagnosed sleep disorders that can cause EDS. These conditions commonly go undetected and untreated for several reasons:
- Patients may not recognize sleepiness as a legitimate medical concern.
- Physicians, with few exceptions, typically have little training in sleep disorders and limited time to diagnose them.2 Screening questions regarding sleep are typically absent.
- Definitive diagnostic tests are costly.
As a result, many patients go without appropriate sleep evaluations. Instead a depressive or other psychiatric disorder may be suspected because of the sleepy patient’s poor energy, hypersomnia, amotivation, irritability, and frustration. Because of ongoing behavioral symptoms, patients with an undiagnosed primary sleep disorder are often referred to psychiatrists. Thus, a clear understanding of the differential diagnosis of EDS is crucial.
Patients with sleep issues fall into three major categories:
- Patients with EDS.
- Individuals with insomnia, another large group often seen by psychiatrists. Generally, these patients are less hesitant than patients with EDS to seek help because of the marked distress they suffer nightly when trying to sleep. Insomniacs typically experience minimal EDS.
- Patients with unusual behaviors at night that range from arm waving to violent behaviors.
Assessing the sleepy patient
When evaluating a patient with sleep complaints, several valuable sources of data come into play.
Initially, observe the patient in the waiting room or office before starting the interview. Did the patient nod off while waiting for his or her appointment? Pay attention to any patient who appears sleepy—even if he or she denies having trouble staying awake. Over time, sleepy patients may have lost their perspective on alertness. Some patients have had EDS for so many years that they no longer recall what it is like to feel fully awake.
Collateral history is often important because family members generally observe the sleeping patient. The bed partner often provides valuable information about snoring, irregular breathing leg kicks, unplanned naps, and strained interpersonal relationships due to EDS. For the patient who does not have a bed partner, ask his or her travel companion, with whom the patient may have shared accommodations.
Unfortunately, few useful screening tests exist. Most questionnaires about sleepiness are neither very reliable nor valid. One of the better questionnaires, the Epworth Sleepiness Scale, helps confirm the presence of sleepiness with a score <8, differentiating the inability to stay awake from fatigue. (Box 1 can be cut out, copied, and handed to patients). This brief questionnaire also provides a useful measure of severity.3
The value of the Epworth scale is limited, however, because patient answers often are based on a specific time and context that may not be representative. Additional validated surveys include the Pittsburgh Sleep Quality Inventory and several that focus on OSA.4
How likely are you to doze off or fall asleep in the following situations, in contrast to feeling just tired? Even if you have not done some of these things recently, try to work out how each situation would affect you now. Use the scale below to choose the most appropriate number for each situation:
- 0 no chance of dozing
- 1 slight chance of dozing
- 2 moderate chance of dozing
- 3 high chance of dozing
| Chance of dozing | Situation |
|---|---|
| ○ | Sitting and reading |
| ○ | Watching TV |
| ○ | Sitting inactive in a public place (e.g., a theater or a meeting) |
| ○ | Sitting as a passenger in a car for an hour without a break |
| ○ | Lying down to rest in the afternoon when circumstances permit |
| ○ | Sitting and talking to someone |
| ○ | Sitting quietly after a lunch without alcohol |
| ○ | In a car, while stopped for a few minutes in traffic |
| Johns, M. Sleep 14:540-545, 1991. | |
Electroencephalographic (EEG) monitoring can accurately measure the patient’s degree of sleep disruption. This information is critical in understanding if a patient’s EDS is caused by a physiologic condition that prevents quality nocturnal sleep. At this time, however, no portable devices that employ EEG technology are used in clinical settings.
Additionally, none of the widely used screening devices that assess leg kicks indicate the presence of possible periodic limb movements.
Even though overnight pulse oximetry has been used to screen for sleep-disordered breathing,5 the technology has limitations. For one, most pulse oximeters do not provide information about sleep stage or body position. Some patients with significant sleep-disordered breathing lack adequate oxygen desaturations but have frequent EEG arousals due to sleep issues. In this case, pulse oximetry would generate a false negative result because EEG data is not collected. The inadequate sensitivity is most likely to occur with females and thin patients.
Oximetry provides only one or two types of data (oxygen saturation plus possibly heart rate), while other physiologic processes, e.g., body movement or sleep architecture, can repetitively be disrupted during sleep.
The most critical steps in detecting sleep disorders do not require technology or specialized expertise, but rather intuition and common sense. The psychiatrist should consider the possibility of a sleep disorder and incorporate pertinent questions into the clinical interview. Figure 1 lists sequential questions that might uncover specific sleep disorders. Once suspected, the decision whether to refer the patient to a sleep disorder center for diagnostic testing depends on the type of sleep disorder detected.
Diagnosing and treating OSA
Recent epidemiologic studies show that OSA affects at least 4% of men and 2% of women in the United States.6 Psychiatrists are virtually assured of seeing patients with undiagnosed OSA. The condition is caused by repeated collapse of the soft tissues surrounding the upper airway, decreasing airflow that is restored when the patient briefly awakens. Patients develop EDS because of sleep fragmented by frequent arousals.
Figure 1 THE SLEEPY PATIENT: Possible medical and psychiatric explanations

Obese patients are at higher risk than are patients at normal weight because of their body habitus. Alcohol or sedative medication use close to bedtime can aggravate OSA. These substances promote muscle relaxation and increase the arousal threshold, meaning that patients do not wake readily when apneas occur.
Long-term complications of untreated OSA include sleepiness leading to accidents, hypertension, cerebrovascular disease, and progressive obesity. New data associate OSA with multiple potential cardiovascular complications (arrhythmias, congestive heart failure, and myocardial infarction).7 Therefore, recognition and treatment are paramount.
The physical examination should focus on detecting nasal obstruction (having the patient sniff separately through each nostril can be helpful), big neck, crowded oropharynx (a low-hanging palate, reddened uvula, enlarged tonsils, large tongue size relative to oropharynx diameter) and jaw structure (particularly a small retrognathic mandible).
Referral for nocturnal polysomnography might be the next step. During a comprehensive sleep study, data is collected about respiratory, cardiovascular, and muscle activity at night, as well as the sounds the patient makes (e.g., snoring, coughing) when asleep. EEG monitoring also is performed. OSA may be diagnosed if repeated episodes of reduced airflow and oxygen desaturation are observed; these typically result in brief shifts in EEG frequency called arousals.
First-line interventions for OSA include avoidance of alcohol within 1 to 2 hours of bedtime, sleeping on the side instead of the back, weight loss (ideally with a regular exercise program), and nasal sprays for allergies.
If the first-line treatments for OSA are ineffective, nasal continuous positive airway pressure (CPAP) works well for almost all patients who adhere to the regimen.8 CPAP requires the patient to wear a nasal mask that delivers room air, splinting open the nasopharynx and the upper airway (Box 2). Some patients benefit from a brief trial of a sleeping medication, e.g., zolpidem or trazodone, for the first 1 to 2 weeks of nasal CPAP usage.

Nasal continuous positive airway pressure (CPAP) must be started in an observed setting so that the clinician can determine the optimal amount of positive pressure needed to keep the upper airway patent. CPAP can be started during the second half of a “split-night” sleep study after obstructive sleep apnea (OSA) has been diagnosed. Alternatively, the sleep laboratory might ask the patient to return for a second night for a trial of nasal CPAP.
Patients with severe OSA might notice improved sleep quality and reduced EDS, even after only a few hours of use. Such patients sometimes wish to start CPAP treatment immediately.
Overall, advances in masks and equipment have improved patient adherence to CPAP. Such innovations include auto-titrating machines, in which the pressure level can be varied depending on sleep state or body position. Many newer machines also have a data microchip that allows the clinician to determine the duration of usage, then use that information to counsel the patient about adherence if necessary.
Patient education also can promote CPAP adherence. Upon being first told they might need to sleep each night wearing a nasal mask, patients often voice well-founded concerns about comfort, claustrophobia, or sexual activity.
As part of a comprehensive approach at the Mayo Sleep Disorders Center, patients watch an educational videotape, tour the sleep laboratory bedrooms before the sleep study, and are carefully fitted for masks. Ideally, the technologists interact with the patient during the sleep study to adjust the headgear and fine-tune other aspects of the equipment. The sleep specialist meets with the patient to compare the baseline diagnostic study results with changes in breathing patterns after a trial of nasal CPAP.
Other useful patient compliance tools include a CPAP informational handout, telephone access to nursing staff, and a 30-day follow-up visit.
Obtaining the support of the bed partner by welcoming her or him to all appointments, including educational activities, is optimal. The bed partner was likely the impetus for the appointment in the first place because of concerns about excessive snoring or apneas.
Image reprinted from Oct. 2001 Mayo Clinic Health Letter with permission of Mayo Foundation for Medical Education and Research, Rochester, MN 55905
Surgical options exist for OSA. The most common procedures are uvulopalatopharyngoplasty (UPPP) and laser-assisted uvulopalatoplasty (LAUP). Other procedures in use include tongue reduction and mandibular advancement.
The response rate to OSA surgery averages around 50% but varies on the patient’s characteristics and procedure selected.9 Positive outcomes are most likely for thin patients with obvious upper airway obstruction, including a deviated nasal septum, large tonsils, a low-hanging palate, and large uvula. Potential complications include nasal regurgitation, voice change, postoperative pain, bleeding, infection, tongue numbness, and snoring without apnea (silent apnea).
Oral appliances have a vital niche in OSA treatment. Multiple devices have been developed that open the oropharynx by moving the mandible and tongue out of way. A growing body of data shows that oral appliances improve sleep and reduce EDS and promote patient satisfaction more effectively than nasal CPAP.10 Several studies also show that patients with mild to moderate OSA accept these devices well.
Oral devices do have drawbacks, however. In most settings, effectiveness cannot be observed during a “split-night” laboratory sleep study because the patient has not yet purchased the device. Also, multiple visits sometimes are required to custom fit the oral appliance; this can pose a hardship to patients who live a distance from the provider.
Restless legs syndrome, periodic limb movement disorder
The patient with restless legs syndrome typically reports a restless painful feeling in the limbs that occurs in the evening and at night, disrupting sleep. This condition, which affects 10% of the population, is associated with aging, blood loss, anemia, peripheral neuropathies, and pregnancy.11 Patients can have childhood onset and in some cases there is a familial tendency.
Most patients with restless legs syndrome have periodic limb movements (repetitive leg jerks or twitches). The clinical significance of periodic limb movements with no subjective disagreeable feelings in the limbs is controversial. Typically, treatment is not instituted in these cases.
The history usually confirms the diagnosis without a sleep study. Sleep studies are used only if a co-existing sleep problem is suspected or if the diagnosis is not clear-cut.
One suspected mechanism of restless legs syndrome is a dopamine-deficient state. A serum ferritin level can help detect a relative iron deficiency, iron being a cofactor for dopamine synthesis.12
Treatment can include iron repletion when indicated. Medications include dopaminergic agents, most notably pramipexole and levodopa/carbidopa. Other options include gabapentin, benzodiazepines, and narcotics. Antidepressants have been suspected to worsen this condition but definitive studies are lacking.13
Identifying, correcting circadian rhythm disorders
Instead of compromising the quality or quantity of sleep, circadian rhythm disorders cause sleep to occur at inappropriate times. Adolescents or young adults are most likely to confront these disorders.
The delayed sleep phase disorder—that is, a persistent pattern of staying up late and “sleeping in” the next morning—is the most common example. A careful assessment will reveal that the patient is getting a satisfactory amount of sleep that occurs at a socially unacceptable time, sometimes to the extreme that his or her nights and days are reversed.
Patients can be reluctant to acknowledge the severity of their problem, which can lead to both inaccurate sleep diaries and interviews. A portable device called a wrist actigraph provides data about limb movement, thus more objectively measuring the patient’s sleep schedule.
Psychiatrists frequently encounter patients with delayed sleep phase disorder because of a high degree of comorbidity with depressive disorders.14 The cause of this syndrome is unclear, but environmental factors including light exposure, social patterns, psychological issues, and possibly a genetic substrate, are known to contribute.
A less common circadian rhythm disorder, advanced sleep phase disorder, can also cause EDS. Patients have an inappropriately early time of sleep onset and then are fully awake in the middle of the night. A recent report describes a large family with a severe form of this disorder that is linked to an abnormality on chromosome two.15
Relatively few effective treatments have been identified for circadian rhythm disorders. Some patients elect not to pursue therapy, instead selecting activities that fit around their unconventional sleep schedules. Sometimes individuals with delayed sleep phase cannot arrange their education or work hours around their atypical sleep schedules. These patients experience poor early morning academic or work performance due to sleepiness.
The internal circadian clock can be gradually readjusted with either phototherapy or gradual shifting of the major sleep period (Box 4). Stimulant or hypnotic medications generally are not utilized.
Insufficient sleep syndrome
Studies indicate that more people are attempting to burn the candle at both ends and are consequently developing a newly identified condition, insufficient sleep syndrome.16 In our 24-hour society, people often are trying to make do with less than the required 7-1/2 hours sleep per day. This may have adverse consequences to their health. When people are required to perform shift work, the problem is compounded because of the difficulty in obtaining sufficient quality sleep during daylight hours.
Many patients do not seek out treatment for fatigue or sleepiness because they are aware of the lifestyle choices that they have made. Still, they might develop psychologic symptoms like irritability, mood swings, and strained interpersonal relationships. These symptoms often will prompt patients to request treatment.
The most common technique is to ask the patient to establish a consistent awakening time and subsequently a regular bedtime. Initially this could be unconventional by societal standards, i.e., bedtime at 5 a.m. and arising at 2 p.m. Once this pattern is in place, the patient gradually shifts the timing by an hour a day. For most patients it is easier to delay rather than advance the bedtime until it conforms to the desired time.
Reinforce this new sleep pattern with a structured daytime schedule that includes predictable mealtimes, regular exercise, social activities, and possibly bright light exposure. This reinforcement should occur in the morning for delayed sleep phase and in the evening for advanced sleep phase disorder. These interventions take time and discipline.
Another approach is for the patient to completely skip sleep one night and, in a sleep-deprived state, establish a new bedtime at the desired time. The same modalities listed above must be used to reinforce (or “entrain”) this schedule or the patient will gradually slip back into the previous abnormal sleep-wake rhythm.
Major medical centers and North American metropolitan areas are increasingly developing sleep disorder treatment centers. Insurance companies generally cover a specialty sleep evaluation, particularly if the referring physician documents a suspicion of sleep-disordered breathing or excessive daytime sleepiness (EDS)that jeopardizes safe driving.
The most appropriate conditions for an urgent sleep evaluation are:
- Difficulty staying alert while driving, nocturnal cardiac arrhythmias;
- Frequent observed apneas;
- EDS leading to academic or occupational problems.
Psychiatrists should take a careful history that includes a discussion of the patient’s daily and weekly schedule. Avoid psychostimulant medications. Instead, address the non-negotiable need to get adequate sleep and challenge the patients to prioritize his or her activities around a full night’s sleep.
When to consider narcolepsy
Narcolepsy, a less common sleep disorder, can lead to severe occupational, educational, and family disruption. Narcolepsy, which affects 0.05% of the population, is a potentially debilitating disease of the central nervous system that involves abnormal regulation of REM sleep. EDS is the cardinal symptom, often associated with cataplexy (75%), sleep paralysis (50%), vivid dreams, and insomnia, all of which can represent inappropriate intrusion of REM phenomena.
After obtaining a history suggestive of narcolepsy, the psychiatrist should employ either the history, a sleep diary, or wrist actigraphy to document whether the patient is getting adequate sleep with a consistent sleep/wake cycle. Next, consider referring the patient for polysomnography, primarily to rule out other causes of EDS like sleep disorder breathing. In some cases, the REM latency on the overnight sleep study will be less than 20 minutes after sleep onset, which supports the diagnosis of narcolepsy. A multiple sleep latency test (MSLT), a diagnostic test that consists of the patient taking four to five daytime naps, is performed the following day.
Narcolepsy is confirmed if the patient has a mean initial sleep latency of less than 10 minutes during these naps plus at least two REM episodes occurring within 15 minutes after sleep onset.
Recent research shows that most patients who have narcolepsy with cataplexy have undetectable levels of a specific neuropeptide (which is called either hypocretin or orexin) in the cerebrospinal fluid.17 Hypocretin/orexin replacement therapy is a theoretical future possibility, but for now treatment includes a combination of optimal sleep hygiene, psychostimulants, antidepressants, and hypnotics.
Other causes of EDS
Other causes of EDS include unrecognized alcohol dependence, inappropriate or excessive medication use, and depressive disorders. Overnight sleep studies are seldom indicated unless patients endorse the symptoms in Figure 1.
Before pursuing sleep studies (polysomnography or an MSLT), eliminate medications that might confound the results. Such agents include antidepressants, which alter the timing and duration of REM sleep, and sedating medications, which modify initial sleep latency and sleep efficiency and potentially aggravate sleep disordered breathing. Although initial REM latency provides a potential biologic marker of major depression, this measurement is more often used in research studies than in clinical psychiatry.
Primary insomnia is a distressing inability to sleep at night or nap during the day. This suggests a hyperarousal state in several ways, and is the opposite of EDS.18 In rare cases, however, patients who cannot sleep at night also do have EDS. When evaluated, these patients typically endorse at least one of the symptoms in Figure 1. Overnight sleep studies occasionally demonstrate that the insomnia is a symptom of another underlying specific sleep disorder, such as OSA or restless legs syndrome.
Psychiatrists treating a patient with chronic insomnia may appropriately undertake several trials of behavioral interventions or sedating medications before making a referral to a sleep disorder center. Patients can struggle with unrecognized primary sleep disorders for years, and many are evaluated by psychiatrists who institute an empiric trial of stimulating antidepressant medications. Use of antidepressants in these situations is unlikely to cause harm, but they might complicate diagnostic testing. When coexisting depression and a primary sleep disorder are confirmed, management of the sleepy patient optimally entails specific treatments that separately target each condition.
Related resources
- National Sleep Foundation www.sleepfoundation.org
- American Academy of Sleep Medicine www.asda.org
- American Sleep Apnea Association www.sleepapnea.org
- Restless Legs Syndrome Foundation www.rls.org
- Association for the Study of Light Therapy and Biological Rhythms www.sltbr.org
Disclosure
The author reports no affiliation or financial arrangements with any of the companies whose products are mentioned in this article.
Drug brand names
- Carbidopa/levodopa • Sinemet
- Gabapentin • Neurontin
- Pramipexole • Mirapex
- Trazodone • Desyrel
- Zolpidem • Ambien
1. Ronald J, Delaive K, et al. Health care utilization in the 10 years prior to diagnosis in obstructive sleep apnea syndrome patients. Sleep. 1999;22(2):225-29.
2. Punjabi N, Haponik E. Ask about daytime sleepiness. J Amer Geriatr Soc. 2000;48:228-29.
3. Johns M. A new method for measuring daytime sleepiness: The Epworth Sleepiness Scale. Sleep. 1991;14(6):540-45.
4. Rowley J, Aboussouan L, Badr M. The use of clinical prediction formulas in the evaluation of obstructive sleep apnea. Sleep. 2000;23:929-38.
5. Yamashiro Y, Kryger M. Nocturnal oximetry: Is it a screening tool for sleep disorders? Sleep. 1995;18:167-71.
6. Morrell M, Finn L, Kim H, Peppard P, Badr M, Young T. Sleep fragmentation, awake blood pressure, and sleep-disordered breathing in a population-based study. Am J Respir Critical Care Med. 2000;162(6):2091-96.
7. Roux F, D’Ambrosio C, Mohsenin V. Sleep-related breathing disorders and cardiovascular disease. Am J Med. 2000;108:396-402.
8. Engleman H, Martin S, Deary I, Douglas N. Effect of continuous positive airway pressure treatment on daytime function in sleep apnoea/hypopnoea syndrome. Lancet. 1994;343(8897):572-75.
9. Lojander J, Maasilta P, Partinen M, Brander P, Salmi T, Lehtonen H. Nasal-CPAP, surgery, and conservative management for treatment of obstructive sleep apnea syndrome. A randomized study. Chest. 1996;110(1):114-19.
10. Mehta A, Qian J, Petocz P, Darendeliler M, Cistulli P. A randomized, controlled study of a mandibular advancement splint for obstructive sleep apnea. Am J Respir Critical Care Med. 2001;163(6):1457-61.
11. Chesson A, Jr, Wise M, et al. Practice parameters for the treatment of restless legs syndrome and periodic limb movement disorder. Sleep. 1999;22(7):961-68.
12. Phillips B, Young T, Finn L, Asher K, Hening W, Purvis C. Epidemiology of restless legs symptoms in adults. Arch Intern Med. 2000;160(14):2137-41.
13. Thorpy M, Ehrenberg B, Hening W, et al. Restless legs syndrome: Detection and management in primary care. Amer Fam Phys. 2000;62:108-14.
14. Regestein Q, Monk T. Delayed sleep phase syndrome: A review of its clinical aspects. Am J Psychiatry. 1995;152:602-08.
15. Toh K, Jones C, et al. An hPer2 phosphorylation site mutation in familial advanced sleep phase syndrome. Science. 2001;291(5506):1040-43.
16. Yoshikawa N, Suzuki S, Ishimoto T, Matsumoto M, Miyagishi T. A case of insufficient sleep syndrome. Psychiatry Clin Neuro. 1998;52(2):200-01.
17. Nishino S, Ripley B, Overeem S, Lammers G, Mignot E. Hypocretin (orexin) deficiency in human narcolepsy. Lancet. 2000;355:39-40.
18. Hauri P, Esther M. Insomnia. Mayo Clin Proc. 1990;65:869-82.
1. Ronald J, Delaive K, et al. Health care utilization in the 10 years prior to diagnosis in obstructive sleep apnea syndrome patients. Sleep. 1999;22(2):225-29.
2. Punjabi N, Haponik E. Ask about daytime sleepiness. J Amer Geriatr Soc. 2000;48:228-29.
3. Johns M. A new method for measuring daytime sleepiness: The Epworth Sleepiness Scale. Sleep. 1991;14(6):540-45.
4. Rowley J, Aboussouan L, Badr M. The use of clinical prediction formulas in the evaluation of obstructive sleep apnea. Sleep. 2000;23:929-38.
5. Yamashiro Y, Kryger M. Nocturnal oximetry: Is it a screening tool for sleep disorders? Sleep. 1995;18:167-71.
6. Morrell M, Finn L, Kim H, Peppard P, Badr M, Young T. Sleep fragmentation, awake blood pressure, and sleep-disordered breathing in a population-based study. Am J Respir Critical Care Med. 2000;162(6):2091-96.
7. Roux F, D’Ambrosio C, Mohsenin V. Sleep-related breathing disorders and cardiovascular disease. Am J Med. 2000;108:396-402.
8. Engleman H, Martin S, Deary I, Douglas N. Effect of continuous positive airway pressure treatment on daytime function in sleep apnoea/hypopnoea syndrome. Lancet. 1994;343(8897):572-75.
9. Lojander J, Maasilta P, Partinen M, Brander P, Salmi T, Lehtonen H. Nasal-CPAP, surgery, and conservative management for treatment of obstructive sleep apnea syndrome. A randomized study. Chest. 1996;110(1):114-19.
10. Mehta A, Qian J, Petocz P, Darendeliler M, Cistulli P. A randomized, controlled study of a mandibular advancement splint for obstructive sleep apnea. Am J Respir Critical Care Med. 2001;163(6):1457-61.
11. Chesson A, Jr, Wise M, et al. Practice parameters for the treatment of restless legs syndrome and periodic limb movement disorder. Sleep. 1999;22(7):961-68.
12. Phillips B, Young T, Finn L, Asher K, Hening W, Purvis C. Epidemiology of restless legs symptoms in adults. Arch Intern Med. 2000;160(14):2137-41.
13. Thorpy M, Ehrenberg B, Hening W, et al. Restless legs syndrome: Detection and management in primary care. Amer Fam Phys. 2000;62:108-14.
14. Regestein Q, Monk T. Delayed sleep phase syndrome: A review of its clinical aspects. Am J Psychiatry. 1995;152:602-08.
15. Toh K, Jones C, et al. An hPer2 phosphorylation site mutation in familial advanced sleep phase syndrome. Science. 2001;291(5506):1040-43.
16. Yoshikawa N, Suzuki S, Ishimoto T, Matsumoto M, Miyagishi T. A case of insufficient sleep syndrome. Psychiatry Clin Neuro. 1998;52(2):200-01.
17. Nishino S, Ripley B, Overeem S, Lammers G, Mignot E. Hypocretin (orexin) deficiency in human narcolepsy. Lancet. 2000;355:39-40.
18. Hauri P, Esther M. Insomnia. Mayo Clin Proc. 1990;65:869-82.