User login
Struggling not to nap: Causes of daytime sleepiness
Poor energy, hypersomnia, amotivation, irritability, and frustration can suggest depression or other psychiatric disorders to busy primary care physicians. As a result, psychiatrists often are referred patients with excessive daytime sleepiness (EDS) caused by undiagnosed primary sleep disorders.
Physicians may miss obstructive sleep apnea (OSA), restless legs syndrome, circadian rhythm disorders, or narcolepsy because:
- many have little training in sleep disorders and limited time to diagnose them1
- patients do not report sleepiness or recognize it as a legitimate medical concern
- definitive diagnostic tests are expensive and usually are not ordered.
Psychiatrists, therefore, need a clear understanding of the EDS differential diagnosis to determine whether a patient’s behavioral symptoms are a sleep or psychiatric issue.
How likely are you to doze off or fall asleep in the following situations, in contrast to feeling just tired? This refers to your usual way of life in recent times. Even if you have not done some of these things recently, try to work out how each situation would affect you now. Use the scale below to choose the most appropriate number for each situation:
0 no chance of dozing
1 slight chance of dozing
2 moderate chance of dozing
3 high chance of dozing
Chance of dozing Situation
Sitting and reading
Watching TV
Sitting inactive in a public place (such as in a theater or a meeting)
As a passenger in a car for an hour without a break
Lying down to rest in the afternoon when circumstances permit
Sitting and talking to someone
Sitting quietly after a lunch without alcohol
In a car, while stopped for a few minutes in traffic
Scoring key
1 to 6 Getting enough sleep
7 to 8 Average
>8 Seek a sleep specialist’s advice without delay
Assessing the sleepy patient
Sleepiness is an inability to stay awake at appropriate times. Fatigue, by comparison, does not involve sleepiness but very low energy associated with wakefulness. In general, sleepy patients get transient relief from napping, whereas fatigued patients report they cannot fall asleep.
Untreated EDS results in compromised quality of life, reduced productivity, and public safety concerns such as falling asleep while driving.2 Sleep complaints fall into three major categories:
- EDS
- insomnia (marked by distress because of poor sleep, but usually with minimal EDS)
- unusual nocturnal behaviors (ranging from arm waving to violent behaviors.
When you evaluate a patient with sleep complaints, valuable sources of data include observation, questionnaires, and screening devices. The most important may be common sense.
Observation. Observe the patient in the waiting room or office before starting the interview. Did he or she nod off while waiting to see you? Pay attention to anyone who appears sleepy—even those who deny having trouble staying awake. Over time, sleepy patients can lose their perspective on alertness. Some have had EDS so long that they no longer recall what it is like to feel fully awake.
Collateral history often is important because family members probably have observed the sleeping patient. The bed partner can provide information about snoring, irregular breathing, leg kicks, unplanned naps, and strained interpersonal relationships because of EDS. For the patient without a bed partner, consider interviewing a travel companion.
Questionnaires. Few useful screening tests exist for sleepiness; most are neither reliable nor valid. One of the better questionnaires—the Epworth Sleepiness Scale (Box 1)—helps confirm the presence of sleepiness with a score >8, differentiating the inability to stay awake from fatigue. This brief questionnaire also provides a useful measure of sleepiness severity.3
The Epworth scale’s value is limited because its questions of specific time and context might not represent a patient’s experiences. Additional validated surveys include the Pittsburgh Sleep Quality Inventory and several for sleep apnea.4
Screening. Electroencephalographic (EEG) monitoring can accurately measure the patient’s degree of sleep disruption. This information is key to understanding if a patient’s EDS is caused by a physiologic condition that prevents quality nocturnal sleep.
None of the widely used screening devices that assess leg kicks indicate the presence of possible periodic limb movements.
Overnight pulse oximetry has been used to screen for sleep-disordered breathing5 but also has limitations:
- Most pulse oximeters do not provide information about sleep stage or body position.
- Patients with sleep-disordered breathing can lack adequate oxygen desaturations but have frequent EEG arousals related to sleep issues. Because EEG data are not collected during arousals, pulse oximetry would generate a false-negative result in this scenario, which occurs most often in female and thin patients.
- Oximetry provides only oxygen saturation data and possibly heart rate, whereas other physiologic processes such as body movement or sleep architecture can be disrupted repetitively during sleep.
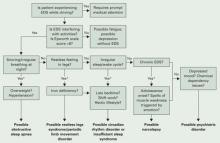
Common sense. The most productive tools for detecting sleep disorders are intuition and common sense. The Figure suggests sequential questions that might uncover specific sleep disorders. Then the decision whether to refer the patient to a sleep disorder center for diagnostic testing depends on the type of sleep disorder you detect.
Nasal continuous positive airway pressure
(CPAP) should be started in an observed setting so that the clinician can determine the optimal amount of positive pressure needed to keep the upper airway patent.
For some patients, CPAP is started in the second half of a “split-night” sleep study after a diagnosis of obstructive sleep apnea (OSA) is made. Other patients return a second night for a nasal CPAP trial. Those with severe OSA might notice improved sleep quality and reduced EDS after only a few hours of CPAP use. Some wish to start CPAP treatment immediately.
Advances in masks and equipment have improved patient adherence to CPAP. Innovations include auto-titrating machines, in which the pressure level can be varied depending on sleep state or body position. Many machines include a data microchip that allows the clinician to determine duration of usage, then use that information to counsel the patient about adherence, if necessary.
Patient education also can promote CPAP adherence. When patients are first told they might need to sleep each night wearing a nasal mask, they often voice well-founded concerns about comfort, claustrophobia, or sexual activity.
Obtaining the support of the bed partner by welcoming her or him to all appointments, including educational activities, is optimal. The bed partner’s concerns about the patient’s excessive snoring or apneas probably were the impetus for the appointment in the first place.
Medication. Some patients benefit from 1 to 2 weeks of a sleeping medication such as zolpidem or trazodone while they acclimate to using nasal CPAP.
Figure The sleepy patient: Possible medical and psychiatric explanations
* Supportive factors: Persuasive if present, but if absent do not exclude possible conditions
Obstructive sleep apnea
Because OSA affects at least 4% of men and 2% of women,6 you are virtually assured of seeing undiagnosed patients. OSA is caused by repeated collapse of the soft tissues surrounding the upper airway, decreasing airflow that is restored when the patient briefly awakens. Patients develop EDS because sleep is fragmented by frequent arousals.
Obese patients, because of their body habitus, are at higher risk for OSA than patients at normal weight. Carefully screen patients for OSA if they develop weight problems while taking psychotropics, such as antipsychotics.
Alcohol or sedatives used at bedtime can aggravate OSA. These substances promote muscle relaxation and increase the arousal threshold so that patients do not awaken readily when apneas occur.
Long-term complications of untreated OSA include sleepiness leading to accidents, hypertension, cerebrovascular disease, and progressive obesity. Data also associate OSA with cardiovascular complications such as arrhythmias, congestive heart failure, and myocardial infarction.7
Physical examination focuses on detecting:
- nasal obstruction (have patient sniff separately through each nostril)
- large neck
- crowded oropharynx (low-hanging palate, reddened uvula, enlarged tonsils, large tongue relative to oropharynx diameter)
- jaw structure (particularly a small, retrognathic mandible).
Sleep studies. Referral for nocturnal polysomnography might be the next step. A comprehensive sleep study collects data about respiratory, cardiovascular, and muscle activity at night, as well as the sounds the patient makes—such as snoring or coughing—when asleep. EEG monitoring also is performed. OSA may be diagnosed if repeated episodes of reduced airflow and oxygen desaturation (arousals) are observed as brief shifts in EEG frequency.
Treatment. First-line interventions for the patient with OSA include:
- no alcohol 1 to 2 hours before bedtime
- sleeping on the side instead of the back
- weight loss (ideally with exercise)
- nasal sprays for allergies.
If first-line treatments are ineffective, nasal continuous positive airway pressure (CPAP) works well for most patients who adhere to the regimen.8 CPAP requires the patient to wear a nasal mask that delivers room air, splinting open the nasopharynx and upper airway (Box 2).
Surgical options. The most common surgeries for OSA are uvulopalatopharyngoplasty and laser-assisted uvulopalatoplasty. Others include tongue reduction and mandibular advancement.
The response rate to surgery averages 50%, depending on patient characteristics and procedure.9 Positive outcomes are most likely for thin patients with obvious upper airway obstruction, including deviated nasal septum, large tonsils, low-hanging palate, and large uvula. Postsurgical complications include nasal regurgitation, voice change, pain, bleeding, infection, tongue numbness, and snoring without apnea (silent apnea).
Oral appliances open the oropharynx by moving the mandible and tongue out of way. Patients with mild to moderate OSA accept these devices well. Evidence suggests that oral appliances improve sleep and reduce EDS more effectively than nasal CPAP and are preferred by patients.10
Oral devices have drawbacks, however. In most settings, their effectiveness cannot be observed during a “split-night” laboratory sleep study because the patient has not yet purchased the device. Also, multiple visits sometimes are required to custom-fit the appliance; this can pose a hardship for patients who live a distance from the provider.
Restless legs syndrome
Patients with restless legs syndrome (RLS) typically report a restless, painful feeling in the limbs that occurs in the evening and at night, disrupting sleep. This condition—which affects 10% of the population—is associated with aging, blood loss, anemia, peripheral neuropathies, and pregnancy.11 Onset can occur in childhood, and in some cases there is a familial tendency.
Most patients with RLS have periodic limb movements (repetitive leg jerks or twitches). The clinical significance of periodic limb movements with no subjective disagreeable feelings in the limbs is controversial, and these cases usually are not treated.
The history usually confirms RLS. Order sleep studies only if you suspect a coexisting sleep problem or the diagnosis is unclear.
A suspected mechanism of restless legs is dopamine deficiency. Low serum ferritin levels have been associated with RLS—presumably because iron is a cofactor necessary for dopamine synthesis12—and may be diagnostically helpful.
The most common technique is to ask the patient to establish a consistent awakening time and a regular bedtime. Initially this could be unconventional by societal standards—such as bedtime at 5 AM and arising at 2 PM. After this pattern is in place, the patient gradually shifts the timing by 1 hour per day. Most patients find it easier to delay rather than advance the bedtime until it conforms to the desired time.
Reinforce this new sleep pattern with a structured daytime schedule that includes predictable mealtimes, regular exercise, social activities, and possibly bright light exposure. Provide reinforcement in the morning for patients with delayed sleep phase disorder and in the evening for advanced sleep phase disorder. These interventions take time and discipline.
Another approach is for the patient to skip sleep one night and, in a sleep-deprived state, establish a new bedtime at the desired time. Use the same modalities listed above to reinforce (“entrain”) this schedule; otherwise the patient will slip back into the previous abnormal sleep-wake rhythm.
Treatment can include iron repletion when indicated. Medications include dopaminergic agents, most notably pramipexole and levodopa/carbidopa. Other options include gabapentin, benzodiazepines, and narcotics.
Antidepressants have been suspected to worsen restless legs syndrome, but definitive studies are lacking.13
Circadian rhythm disorders
Instead of compromising the quality or quantity of sleep, circadian rhythm disorders cause sleep to occur at inappropriate times. These disorders are most common in adolescents and young adults.
Delayed sleep phase disorder—a persistent pattern of staying up late and “sleeping in”—is most common. Careful assessment will reveal that the patient is getting adequate sleep but at a socially unacceptable time, sometimes to the extreme that his or her nights and days are reversed.
Patients’ reluctance to acknowledge the severity of this problem can lead to inaccurate sleep diaries and interviews. A portable wrist actigraph can provide data about limb movement and is more objective than self-reports.
Delayed sleep phase disorder is highly comorbid with depressive disorders.14 The cause of this syndrome is unclear, but light exposure, social patterns, psychological issues, and possibly a genetic substrate are known to contribute.
Advanced sleep phase disorder—a less common circadian rhythm disorder—also can cause EDS. Patients have an inappropriately early time of sleep onset and then are fully awake in the middle of the night. A large family with a severe form of this disorder was found to have an abnormality on chromosome 2.15
Treatment. Relatively few treatments are effective for circadian rhythm disorders. Some patients elect not to pursue therapy, instead fitting activities around their unconventional sleep schedules.
Individuals with delayed sleep phase who cannot arrange their lives around their sleep schedules are at risk for poor early morning performance because of sleepiness. Their internal circadian clocks can be gradually readjusted with phototherapy or gradual shifting of the major sleep period (Box 3). Stimulants usually are not used, but hypnotics can sometimes help these patients fall asleep earlier.
Insufficient sleep syndrome
People attempting to “burn the candle at both ends” are at risk for developing insufficient sleep syndrome.16 In our 24/7 society, people trying to make do with less than the required 7.5 hours sleep per night may adversely affect their health. The problem is compounded for shift workers because of the difficulty in obtaining sufficient quality sleep during daylight hours.
Many patients do not seek treatment for fatigue or sleepiness because they are aware of their lifestyle choices. Still, they might develop psychological symptoms such as irritability, mood swings, and strained interpersonal relationships. These symptoms can prompt patients to request treatment.
Take a careful history that includes discussing the patient’s daily and weekly schedule. Avoid psychostimulants; instead, address the nonnegotiable need to get adequate sleep and challenge the patient to prioritize his or her activities around a full night’s sleep.
When to consider narcolepsy
Narcolepsy is a CNS disease characterized by abnormal regulation of REM sleep. EDS—the cardinal symptom—is often associated with cataplexy (75%), sleep paralysis (50%), vivid dreams, and insomnia, all of which interfere with REM phenomena. Narcolepsy affects 0.05% of the U.S. population and can lead to severe occupational, educational, and family disruption.
When you obtain a history that suggests narcolepsy, use the history, a sleep diary, or wrist actigraphy to document whether the patient is getting adequate sleep, with a consistent sleep/wake cycle. Next, consider referring the patient for polysomnography, primarily to rule out other causes of EDS such as sleep-disordered breathing. In some cases, REM latency on the overnight sleep study will be <20 minutes after sleep onset, which supports the diagnosis of narcolepsy.
A multiple sleep latency test (MSLT)—a diagnostic session in which the patient takes 4 to 5 daytime naps—is performed the following day. Narcolepsy is confirmed if the patient has a mean initial sleep latency of <10 minutes during these naps plus at least two REM episodes within 15 minutes after sleep onset.
The 4 most appropriate indications for an urgent sleep evaluation are:
- difficulty staying alert while driving
- nocturnal cardiac arrhythmias
- frequent observed apneas
- excessive daytime sleepiness (EDS) leading to academic or occupational problems.
Insurance companies usually cover a specialty sleep evaluation, particularly if the referring physician documents a suspicion of sleep-disordered breathing or EDS that jeopardizes safe driving.
Most patients with narcolepsy and cataplexy have undetectable cerebrospinal fluid levels of a neuropeptide called hypocretin or orexin.17 Hypocretin/orexin replacement therapy is a theoretical possibility, but for now treatment includes a combination of optimal sleep hygiene, psychostimulants, antidepressants, and hypnotics.
Other causes of EDS
EDS can also be caused by unrecognized alcohol dependence, inappropriate or excessive medication use, and depressive disorders. Overnight sleep studies are seldom indicated unless patients endorse the symptoms in the Figure.
Before pursuing polysomnography or an MSLT (Box 4), eliminate medications that might confound the results, such as:
- antidepressants, which alter the timing and duration of REM sleep
- sedating medications, which modify initial sleep latency and sleep efficiency and potentially aggravate sleep disordered breathing.
Initial REM latency provides a potential biologic marker of major depression but is used more often in research than in clinical psychiatry.
Primary insomnia is the distressing inability to sleep at night or nap during the day. It suggests a hyperarousal state—the opposite of EDS.18 In rare cases, however, patients who cannot sleep at night also have EDS. When evaluated, they typically endorse at least one symptom in the Figure. Sleep studies occasionally reveal OSA or restless legs syndrome.
Treating a patient with chronic insomnia may require several trials of behavioral interventions or sedating medications before you make a referral to a sleep disorder center. Patients can struggle with unrecognized primary sleep disorders for years, and many are given empiric trials of stimulating antidepressants. Antidepressants are unlikely to cause harm, but they might complicate diagnostic testing.
When you confirm coexisting depression and a primary sleep disorder, treatments that separately target each condition provide optimal management of the sleepy patient.
Medications to enhance wakefulness
Wake-promoting agents are a treatment option when EDS is contributing to compromised functioning. These drugs are no substitute for thoughtful evaluation of hypersomnolence, however. When you diagnose OSA or restless legs syndrome, first try treatments that target these conditions. If residual sleepiness persists, then consider augmenting with stimulating medications.
Modafinil is FDA-approved for residual sleepiness in patients with OSA and for shift work sleep disorder, a condition of circadian misalignment from frequent schedule changes. Evidence does not support its use for other circadian rhythm disorders, such as delayed sleep phase disorder.
Low-dose modafinil (such as 100 to 200 mg/d) is well tolerated, but its therapeutic effect as augmentation is modest.19 Increasing the dosage to >200 mg usually does not increase alertness.
Caffeine. Some patients report benefit from caffeine used in moderation and only in the morning. This practice is acceptable as long as patients do not use excessive amounts or experience insomnia, exacerbation of anxiety, or tachycardia.
Psychostimulants such as methylphenidate and amphetamines are less well-studied than modafinil for treating EDS in patients without narcolepsy. Monitor carefully for insomnia, exacerbation of anxiety, tachycardia, or hypertension and to prevent overuse of these habituating agents.
Related resources
- National Sleep Foundation. www.sleepfoundation.org.
- American Academy of Sleep Medicine. www.aasmnet.org.
- American Sleep Apnea Association. www.sleepapnea.org.
- Restless Legs Syndrome Foundation. www.rls.org.
- Association for the Study of Light Therapy and Biological Rhythms. www.sltbr.org.
Drug brand names
- Carbidopa/levodopa • Sinemet
- Gabapentin • Neurontin
- Modafinil • Provigil
- Pramipexole • Mirapex
- Trazodone • Desyrel
- Zolpidem • Ambien
Disclosure
Dr. Krahn reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Acknowledgment
Dr. Krahn published the original version this article in the January 2002 issue of Current Psychiatry and has updated it for this issue.
1. Punjabi N, Haponik E. Ask about daytime sleepiness. J Amer Geriatr Soc 2000;48:228-9.
2. Ronald J, Delaive K, Roos L, et al. Health care utilization in the 10 years prior to diagnosis in obstructive sleep apnea syndrome patients. Sleep 1999;22(2):225-9.
3. Johns M. A new method for measuring daytime sleepiness: The Epworth Sleepiness Scale. Sleep 1991;14(6):540-5.
4. Rowley J, Aboussouan L, Badr M. The use of clinical prediction formulas in the evaluation of obstructive sleep apnea. Sleep 2000;23:929-38.
5. Yamashiro Y, Kryger M. Nocturnal oximetry: Is it a screening tool for sleep disorders? Sleep 1995;18:167-71.
6. Morrell M, Finn L, Kim H, et al. Sleep fragmentation, awake blood pressure, and sleep-disordered breathing in a population-based study. Am J Respir Critical Care Med 2000;162(6):2091-6.
7. Roux F, D’Ambrosio C, Mohsenin V. Sleep-related breathing disorders and cardiovascular disease. Am J Med. 2000;108:396-402.
8. Engleman H, Martin S, Deary I, Douglas N. Effect of continuous positive airway pressure treatment on daytime function in sleep apnoea/hypopnoea syndrome. Lancet 1994;343(8897):572-5.
9. Lojander J, Maasilta P, Partinen M, et al. Nasal-CPAP, surgery, and conservative management for treatment of obstructive sleep apnea syndrome. A randomized study. Chest. 1996;110(1):114-9.
10. Mehta A, Qian J, Petocz P, et al. A randomized, controlled study of a mandibular advancement splint for obstructive sleep apnea. Am J Respir Critical Care Med 2001;163(6):1457-61.
11. Chesson A, Wise M, Davila D, et al. Practice parameters for the treatment of restless legs syndrome and periodic limb movement disorder. An American Academy of Sleep Medicine Report. Standards of Practice Committee of the American Academy of Sleep Medicine. Sleep 1999;22(7):961-8.
12. Phillips B, Young T, Finn L, et al. Epidemiology of restless legs symptoms in adults. Arch Intern Med 2000;160(14):2137-41.
13. Thorpy M, Ehrenberg B, Hening W, et al. Restless legs syndrome: Detection and management in primary care. Amer Fam Phys 2000;62:108-14.
14. Regestein Q, Monk T. Delayed sleep phase syndrome: A review of its clinical aspects. Am J Psychiatry 1995;152:602-8.
15. Toh K, Jones C, He Y, et al. An hPer2 phosphorylation site mutation in familial advanced sleep phase syndrome. Science 2001;291(5506):1040-3.
16. Yoshikawa N, Suzuki S, Ishimoto T, et al. A case of insufficient sleep syndrome. Psychiatry Clin Neuro 1998;52(2):200-1.
17. Nishino S, Ripley B, Overeem S, et al. Hypocretin (orexin) deficiency in human narcolepsy. Lancet. 2000;355:39-40.
18. Hauri P, Esther M. Insomnia. Mayo Clin Proc 1990;65:869-82.
19. Schwartz JR. Modafinil: new indications for wake promotion. Expert Opin Pharmacother 2005;6(1):115-29.
Poor energy, hypersomnia, amotivation, irritability, and frustration can suggest depression or other psychiatric disorders to busy primary care physicians. As a result, psychiatrists often are referred patients with excessive daytime sleepiness (EDS) caused by undiagnosed primary sleep disorders.
Physicians may miss obstructive sleep apnea (OSA), restless legs syndrome, circadian rhythm disorders, or narcolepsy because:
- many have little training in sleep disorders and limited time to diagnose them1
- patients do not report sleepiness or recognize it as a legitimate medical concern
- definitive diagnostic tests are expensive and usually are not ordered.
Psychiatrists, therefore, need a clear understanding of the EDS differential diagnosis to determine whether a patient’s behavioral symptoms are a sleep or psychiatric issue.
How likely are you to doze off or fall asleep in the following situations, in contrast to feeling just tired? This refers to your usual way of life in recent times. Even if you have not done some of these things recently, try to work out how each situation would affect you now. Use the scale below to choose the most appropriate number for each situation:
0 no chance of dozing
1 slight chance of dozing
2 moderate chance of dozing
3 high chance of dozing
Chance of dozing Situation
Sitting and reading
Watching TV
Sitting inactive in a public place (such as in a theater or a meeting)
As a passenger in a car for an hour without a break
Lying down to rest in the afternoon when circumstances permit
Sitting and talking to someone
Sitting quietly after a lunch without alcohol
In a car, while stopped for a few minutes in traffic
Scoring key
1 to 6 Getting enough sleep
7 to 8 Average
>8 Seek a sleep specialist’s advice without delay
Assessing the sleepy patient
Sleepiness is an inability to stay awake at appropriate times. Fatigue, by comparison, does not involve sleepiness but very low energy associated with wakefulness. In general, sleepy patients get transient relief from napping, whereas fatigued patients report they cannot fall asleep.
Untreated EDS results in compromised quality of life, reduced productivity, and public safety concerns such as falling asleep while driving.2 Sleep complaints fall into three major categories:
- EDS
- insomnia (marked by distress because of poor sleep, but usually with minimal EDS)
- unusual nocturnal behaviors (ranging from arm waving to violent behaviors.
When you evaluate a patient with sleep complaints, valuable sources of data include observation, questionnaires, and screening devices. The most important may be common sense.
Observation. Observe the patient in the waiting room or office before starting the interview. Did he or she nod off while waiting to see you? Pay attention to anyone who appears sleepy—even those who deny having trouble staying awake. Over time, sleepy patients can lose their perspective on alertness. Some have had EDS so long that they no longer recall what it is like to feel fully awake.
Collateral history often is important because family members probably have observed the sleeping patient. The bed partner can provide information about snoring, irregular breathing, leg kicks, unplanned naps, and strained interpersonal relationships because of EDS. For the patient without a bed partner, consider interviewing a travel companion.
Questionnaires. Few useful screening tests exist for sleepiness; most are neither reliable nor valid. One of the better questionnaires—the Epworth Sleepiness Scale (Box 1)—helps confirm the presence of sleepiness with a score >8, differentiating the inability to stay awake from fatigue. This brief questionnaire also provides a useful measure of sleepiness severity.3
The Epworth scale’s value is limited because its questions of specific time and context might not represent a patient’s experiences. Additional validated surveys include the Pittsburgh Sleep Quality Inventory and several for sleep apnea.4
Screening. Electroencephalographic (EEG) monitoring can accurately measure the patient’s degree of sleep disruption. This information is key to understanding if a patient’s EDS is caused by a physiologic condition that prevents quality nocturnal sleep.
None of the widely used screening devices that assess leg kicks indicate the presence of possible periodic limb movements.
Overnight pulse oximetry has been used to screen for sleep-disordered breathing5 but also has limitations:
- Most pulse oximeters do not provide information about sleep stage or body position.
- Patients with sleep-disordered breathing can lack adequate oxygen desaturations but have frequent EEG arousals related to sleep issues. Because EEG data are not collected during arousals, pulse oximetry would generate a false-negative result in this scenario, which occurs most often in female and thin patients.
- Oximetry provides only oxygen saturation data and possibly heart rate, whereas other physiologic processes such as body movement or sleep architecture can be disrupted repetitively during sleep.
Common sense. The most productive tools for detecting sleep disorders are intuition and common sense. The Figure suggests sequential questions that might uncover specific sleep disorders. Then the decision whether to refer the patient to a sleep disorder center for diagnostic testing depends on the type of sleep disorder you detect.
Nasal continuous positive airway pressure
(CPAP) should be started in an observed setting so that the clinician can determine the optimal amount of positive pressure needed to keep the upper airway patent.
For some patients, CPAP is started in the second half of a “split-night” sleep study after a diagnosis of obstructive sleep apnea (OSA) is made. Other patients return a second night for a nasal CPAP trial. Those with severe OSA might notice improved sleep quality and reduced EDS after only a few hours of CPAP use. Some wish to start CPAP treatment immediately.
Advances in masks and equipment have improved patient adherence to CPAP. Innovations include auto-titrating machines, in which the pressure level can be varied depending on sleep state or body position. Many machines include a data microchip that allows the clinician to determine duration of usage, then use that information to counsel the patient about adherence, if necessary.
Patient education also can promote CPAP adherence. When patients are first told they might need to sleep each night wearing a nasal mask, they often voice well-founded concerns about comfort, claustrophobia, or sexual activity.
Obtaining the support of the bed partner by welcoming her or him to all appointments, including educational activities, is optimal. The bed partner’s concerns about the patient’s excessive snoring or apneas probably were the impetus for the appointment in the first place.
Medication. Some patients benefit from 1 to 2 weeks of a sleeping medication such as zolpidem or trazodone while they acclimate to using nasal CPAP.
Figure The sleepy patient: Possible medical and psychiatric explanations
* Supportive factors: Persuasive if present, but if absent do not exclude possible conditions
Obstructive sleep apnea
Because OSA affects at least 4% of men and 2% of women,6 you are virtually assured of seeing undiagnosed patients. OSA is caused by repeated collapse of the soft tissues surrounding the upper airway, decreasing airflow that is restored when the patient briefly awakens. Patients develop EDS because sleep is fragmented by frequent arousals.
Obese patients, because of their body habitus, are at higher risk for OSA than patients at normal weight. Carefully screen patients for OSA if they develop weight problems while taking psychotropics, such as antipsychotics.
Alcohol or sedatives used at bedtime can aggravate OSA. These substances promote muscle relaxation and increase the arousal threshold so that patients do not awaken readily when apneas occur.
Long-term complications of untreated OSA include sleepiness leading to accidents, hypertension, cerebrovascular disease, and progressive obesity. Data also associate OSA with cardiovascular complications such as arrhythmias, congestive heart failure, and myocardial infarction.7
Physical examination focuses on detecting:
- nasal obstruction (have patient sniff separately through each nostril)
- large neck
- crowded oropharynx (low-hanging palate, reddened uvula, enlarged tonsils, large tongue relative to oropharynx diameter)
- jaw structure (particularly a small, retrognathic mandible).
Sleep studies. Referral for nocturnal polysomnography might be the next step. A comprehensive sleep study collects data about respiratory, cardiovascular, and muscle activity at night, as well as the sounds the patient makes—such as snoring or coughing—when asleep. EEG monitoring also is performed. OSA may be diagnosed if repeated episodes of reduced airflow and oxygen desaturation (arousals) are observed as brief shifts in EEG frequency.
Treatment. First-line interventions for the patient with OSA include:
- no alcohol 1 to 2 hours before bedtime
- sleeping on the side instead of the back
- weight loss (ideally with exercise)
- nasal sprays for allergies.
If first-line treatments are ineffective, nasal continuous positive airway pressure (CPAP) works well for most patients who adhere to the regimen.8 CPAP requires the patient to wear a nasal mask that delivers room air, splinting open the nasopharynx and upper airway (Box 2).
Surgical options. The most common surgeries for OSA are uvulopalatopharyngoplasty and laser-assisted uvulopalatoplasty. Others include tongue reduction and mandibular advancement.
The response rate to surgery averages 50%, depending on patient characteristics and procedure.9 Positive outcomes are most likely for thin patients with obvious upper airway obstruction, including deviated nasal septum, large tonsils, low-hanging palate, and large uvula. Postsurgical complications include nasal regurgitation, voice change, pain, bleeding, infection, tongue numbness, and snoring without apnea (silent apnea).
Oral appliances open the oropharynx by moving the mandible and tongue out of way. Patients with mild to moderate OSA accept these devices well. Evidence suggests that oral appliances improve sleep and reduce EDS more effectively than nasal CPAP and are preferred by patients.10
Oral devices have drawbacks, however. In most settings, their effectiveness cannot be observed during a “split-night” laboratory sleep study because the patient has not yet purchased the device. Also, multiple visits sometimes are required to custom-fit the appliance; this can pose a hardship for patients who live a distance from the provider.
Restless legs syndrome
Patients with restless legs syndrome (RLS) typically report a restless, painful feeling in the limbs that occurs in the evening and at night, disrupting sleep. This condition—which affects 10% of the population—is associated with aging, blood loss, anemia, peripheral neuropathies, and pregnancy.11 Onset can occur in childhood, and in some cases there is a familial tendency.
Most patients with RLS have periodic limb movements (repetitive leg jerks or twitches). The clinical significance of periodic limb movements with no subjective disagreeable feelings in the limbs is controversial, and these cases usually are not treated.
The history usually confirms RLS. Order sleep studies only if you suspect a coexisting sleep problem or the diagnosis is unclear.
A suspected mechanism of restless legs is dopamine deficiency. Low serum ferritin levels have been associated with RLS—presumably because iron is a cofactor necessary for dopamine synthesis12—and may be diagnostically helpful.
The most common technique is to ask the patient to establish a consistent awakening time and a regular bedtime. Initially this could be unconventional by societal standards—such as bedtime at 5 AM and arising at 2 PM. After this pattern is in place, the patient gradually shifts the timing by 1 hour per day. Most patients find it easier to delay rather than advance the bedtime until it conforms to the desired time.
Reinforce this new sleep pattern with a structured daytime schedule that includes predictable mealtimes, regular exercise, social activities, and possibly bright light exposure. Provide reinforcement in the morning for patients with delayed sleep phase disorder and in the evening for advanced sleep phase disorder. These interventions take time and discipline.
Another approach is for the patient to skip sleep one night and, in a sleep-deprived state, establish a new bedtime at the desired time. Use the same modalities listed above to reinforce (“entrain”) this schedule; otherwise the patient will slip back into the previous abnormal sleep-wake rhythm.
Treatment can include iron repletion when indicated. Medications include dopaminergic agents, most notably pramipexole and levodopa/carbidopa. Other options include gabapentin, benzodiazepines, and narcotics.
Antidepressants have been suspected to worsen restless legs syndrome, but definitive studies are lacking.13
Circadian rhythm disorders
Instead of compromising the quality or quantity of sleep, circadian rhythm disorders cause sleep to occur at inappropriate times. These disorders are most common in adolescents and young adults.
Delayed sleep phase disorder—a persistent pattern of staying up late and “sleeping in”—is most common. Careful assessment will reveal that the patient is getting adequate sleep but at a socially unacceptable time, sometimes to the extreme that his or her nights and days are reversed.
Patients’ reluctance to acknowledge the severity of this problem can lead to inaccurate sleep diaries and interviews. A portable wrist actigraph can provide data about limb movement and is more objective than self-reports.
Delayed sleep phase disorder is highly comorbid with depressive disorders.14 The cause of this syndrome is unclear, but light exposure, social patterns, psychological issues, and possibly a genetic substrate are known to contribute.
Advanced sleep phase disorder—a less common circadian rhythm disorder—also can cause EDS. Patients have an inappropriately early time of sleep onset and then are fully awake in the middle of the night. A large family with a severe form of this disorder was found to have an abnormality on chromosome 2.15
Treatment. Relatively few treatments are effective for circadian rhythm disorders. Some patients elect not to pursue therapy, instead fitting activities around their unconventional sleep schedules.
Individuals with delayed sleep phase who cannot arrange their lives around their sleep schedules are at risk for poor early morning performance because of sleepiness. Their internal circadian clocks can be gradually readjusted with phototherapy or gradual shifting of the major sleep period (Box 3). Stimulants usually are not used, but hypnotics can sometimes help these patients fall asleep earlier.
Insufficient sleep syndrome
People attempting to “burn the candle at both ends” are at risk for developing insufficient sleep syndrome.16 In our 24/7 society, people trying to make do with less than the required 7.5 hours sleep per night may adversely affect their health. The problem is compounded for shift workers because of the difficulty in obtaining sufficient quality sleep during daylight hours.
Many patients do not seek treatment for fatigue or sleepiness because they are aware of their lifestyle choices. Still, they might develop psychological symptoms such as irritability, mood swings, and strained interpersonal relationships. These symptoms can prompt patients to request treatment.
Take a careful history that includes discussing the patient’s daily and weekly schedule. Avoid psychostimulants; instead, address the nonnegotiable need to get adequate sleep and challenge the patient to prioritize his or her activities around a full night’s sleep.
When to consider narcolepsy
Narcolepsy is a CNS disease characterized by abnormal regulation of REM sleep. EDS—the cardinal symptom—is often associated with cataplexy (75%), sleep paralysis (50%), vivid dreams, and insomnia, all of which interfere with REM phenomena. Narcolepsy affects 0.05% of the U.S. population and can lead to severe occupational, educational, and family disruption.
When you obtain a history that suggests narcolepsy, use the history, a sleep diary, or wrist actigraphy to document whether the patient is getting adequate sleep, with a consistent sleep/wake cycle. Next, consider referring the patient for polysomnography, primarily to rule out other causes of EDS such as sleep-disordered breathing. In some cases, REM latency on the overnight sleep study will be <20 minutes after sleep onset, which supports the diagnosis of narcolepsy.
A multiple sleep latency test (MSLT)—a diagnostic session in which the patient takes 4 to 5 daytime naps—is performed the following day. Narcolepsy is confirmed if the patient has a mean initial sleep latency of <10 minutes during these naps plus at least two REM episodes within 15 minutes after sleep onset.
The 4 most appropriate indications for an urgent sleep evaluation are:
- difficulty staying alert while driving
- nocturnal cardiac arrhythmias
- frequent observed apneas
- excessive daytime sleepiness (EDS) leading to academic or occupational problems.
Insurance companies usually cover a specialty sleep evaluation, particularly if the referring physician documents a suspicion of sleep-disordered breathing or EDS that jeopardizes safe driving.
Most patients with narcolepsy and cataplexy have undetectable cerebrospinal fluid levels of a neuropeptide called hypocretin or orexin.17 Hypocretin/orexin replacement therapy is a theoretical possibility, but for now treatment includes a combination of optimal sleep hygiene, psychostimulants, antidepressants, and hypnotics.
Other causes of EDS
EDS can also be caused by unrecognized alcohol dependence, inappropriate or excessive medication use, and depressive disorders. Overnight sleep studies are seldom indicated unless patients endorse the symptoms in the Figure.
Before pursuing polysomnography or an MSLT (Box 4), eliminate medications that might confound the results, such as:
- antidepressants, which alter the timing and duration of REM sleep
- sedating medications, which modify initial sleep latency and sleep efficiency and potentially aggravate sleep disordered breathing.
Initial REM latency provides a potential biologic marker of major depression but is used more often in research than in clinical psychiatry.
Primary insomnia is the distressing inability to sleep at night or nap during the day. It suggests a hyperarousal state—the opposite of EDS.18 In rare cases, however, patients who cannot sleep at night also have EDS. When evaluated, they typically endorse at least one symptom in the Figure. Sleep studies occasionally reveal OSA or restless legs syndrome.
Treating a patient with chronic insomnia may require several trials of behavioral interventions or sedating medications before you make a referral to a sleep disorder center. Patients can struggle with unrecognized primary sleep disorders for years, and many are given empiric trials of stimulating antidepressants. Antidepressants are unlikely to cause harm, but they might complicate diagnostic testing.
When you confirm coexisting depression and a primary sleep disorder, treatments that separately target each condition provide optimal management of the sleepy patient.
Medications to enhance wakefulness
Wake-promoting agents are a treatment option when EDS is contributing to compromised functioning. These drugs are no substitute for thoughtful evaluation of hypersomnolence, however. When you diagnose OSA or restless legs syndrome, first try treatments that target these conditions. If residual sleepiness persists, then consider augmenting with stimulating medications.
Modafinil is FDA-approved for residual sleepiness in patients with OSA and for shift work sleep disorder, a condition of circadian misalignment from frequent schedule changes. Evidence does not support its use for other circadian rhythm disorders, such as delayed sleep phase disorder.
Low-dose modafinil (such as 100 to 200 mg/d) is well tolerated, but its therapeutic effect as augmentation is modest.19 Increasing the dosage to >200 mg usually does not increase alertness.
Caffeine. Some patients report benefit from caffeine used in moderation and only in the morning. This practice is acceptable as long as patients do not use excessive amounts or experience insomnia, exacerbation of anxiety, or tachycardia.
Psychostimulants such as methylphenidate and amphetamines are less well-studied than modafinil for treating EDS in patients without narcolepsy. Monitor carefully for insomnia, exacerbation of anxiety, tachycardia, or hypertension and to prevent overuse of these habituating agents.
Related resources
- National Sleep Foundation. www.sleepfoundation.org.
- American Academy of Sleep Medicine. www.aasmnet.org.
- American Sleep Apnea Association. www.sleepapnea.org.
- Restless Legs Syndrome Foundation. www.rls.org.
- Association for the Study of Light Therapy and Biological Rhythms. www.sltbr.org.
Drug brand names
- Carbidopa/levodopa • Sinemet
- Gabapentin • Neurontin
- Modafinil • Provigil
- Pramipexole • Mirapex
- Trazodone • Desyrel
- Zolpidem • Ambien
Disclosure
Dr. Krahn reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Acknowledgment
Dr. Krahn published the original version this article in the January 2002 issue of Current Psychiatry and has updated it for this issue.
Poor energy, hypersomnia, amotivation, irritability, and frustration can suggest depression or other psychiatric disorders to busy primary care physicians. As a result, psychiatrists often are referred patients with excessive daytime sleepiness (EDS) caused by undiagnosed primary sleep disorders.
Physicians may miss obstructive sleep apnea (OSA), restless legs syndrome, circadian rhythm disorders, or narcolepsy because:
- many have little training in sleep disorders and limited time to diagnose them1
- patients do not report sleepiness or recognize it as a legitimate medical concern
- definitive diagnostic tests are expensive and usually are not ordered.
Psychiatrists, therefore, need a clear understanding of the EDS differential diagnosis to determine whether a patient’s behavioral symptoms are a sleep or psychiatric issue.
How likely are you to doze off or fall asleep in the following situations, in contrast to feeling just tired? This refers to your usual way of life in recent times. Even if you have not done some of these things recently, try to work out how each situation would affect you now. Use the scale below to choose the most appropriate number for each situation:
0 no chance of dozing
1 slight chance of dozing
2 moderate chance of dozing
3 high chance of dozing
Chance of dozing Situation
Sitting and reading
Watching TV
Sitting inactive in a public place (such as in a theater or a meeting)
As a passenger in a car for an hour without a break
Lying down to rest in the afternoon when circumstances permit
Sitting and talking to someone
Sitting quietly after a lunch without alcohol
In a car, while stopped for a few minutes in traffic
Scoring key
1 to 6 Getting enough sleep
7 to 8 Average
>8 Seek a sleep specialist’s advice without delay
Assessing the sleepy patient
Sleepiness is an inability to stay awake at appropriate times. Fatigue, by comparison, does not involve sleepiness but very low energy associated with wakefulness. In general, sleepy patients get transient relief from napping, whereas fatigued patients report they cannot fall asleep.
Untreated EDS results in compromised quality of life, reduced productivity, and public safety concerns such as falling asleep while driving.2 Sleep complaints fall into three major categories:
- EDS
- insomnia (marked by distress because of poor sleep, but usually with minimal EDS)
- unusual nocturnal behaviors (ranging from arm waving to violent behaviors.
When you evaluate a patient with sleep complaints, valuable sources of data include observation, questionnaires, and screening devices. The most important may be common sense.
Observation. Observe the patient in the waiting room or office before starting the interview. Did he or she nod off while waiting to see you? Pay attention to anyone who appears sleepy—even those who deny having trouble staying awake. Over time, sleepy patients can lose their perspective on alertness. Some have had EDS so long that they no longer recall what it is like to feel fully awake.
Collateral history often is important because family members probably have observed the sleeping patient. The bed partner can provide information about snoring, irregular breathing, leg kicks, unplanned naps, and strained interpersonal relationships because of EDS. For the patient without a bed partner, consider interviewing a travel companion.
Questionnaires. Few useful screening tests exist for sleepiness; most are neither reliable nor valid. One of the better questionnaires—the Epworth Sleepiness Scale (Box 1)—helps confirm the presence of sleepiness with a score >8, differentiating the inability to stay awake from fatigue. This brief questionnaire also provides a useful measure of sleepiness severity.3
The Epworth scale’s value is limited because its questions of specific time and context might not represent a patient’s experiences. Additional validated surveys include the Pittsburgh Sleep Quality Inventory and several for sleep apnea.4
Screening. Electroencephalographic (EEG) monitoring can accurately measure the patient’s degree of sleep disruption. This information is key to understanding if a patient’s EDS is caused by a physiologic condition that prevents quality nocturnal sleep.
None of the widely used screening devices that assess leg kicks indicate the presence of possible periodic limb movements.
Overnight pulse oximetry has been used to screen for sleep-disordered breathing5 but also has limitations:
- Most pulse oximeters do not provide information about sleep stage or body position.
- Patients with sleep-disordered breathing can lack adequate oxygen desaturations but have frequent EEG arousals related to sleep issues. Because EEG data are not collected during arousals, pulse oximetry would generate a false-negative result in this scenario, which occurs most often in female and thin patients.
- Oximetry provides only oxygen saturation data and possibly heart rate, whereas other physiologic processes such as body movement or sleep architecture can be disrupted repetitively during sleep.
Common sense. The most productive tools for detecting sleep disorders are intuition and common sense. The Figure suggests sequential questions that might uncover specific sleep disorders. Then the decision whether to refer the patient to a sleep disorder center for diagnostic testing depends on the type of sleep disorder you detect.
Nasal continuous positive airway pressure
(CPAP) should be started in an observed setting so that the clinician can determine the optimal amount of positive pressure needed to keep the upper airway patent.
For some patients, CPAP is started in the second half of a “split-night” sleep study after a diagnosis of obstructive sleep apnea (OSA) is made. Other patients return a second night for a nasal CPAP trial. Those with severe OSA might notice improved sleep quality and reduced EDS after only a few hours of CPAP use. Some wish to start CPAP treatment immediately.
Advances in masks and equipment have improved patient adherence to CPAP. Innovations include auto-titrating machines, in which the pressure level can be varied depending on sleep state or body position. Many machines include a data microchip that allows the clinician to determine duration of usage, then use that information to counsel the patient about adherence, if necessary.
Patient education also can promote CPAP adherence. When patients are first told they might need to sleep each night wearing a nasal mask, they often voice well-founded concerns about comfort, claustrophobia, or sexual activity.
Obtaining the support of the bed partner by welcoming her or him to all appointments, including educational activities, is optimal. The bed partner’s concerns about the patient’s excessive snoring or apneas probably were the impetus for the appointment in the first place.
Medication. Some patients benefit from 1 to 2 weeks of a sleeping medication such as zolpidem or trazodone while they acclimate to using nasal CPAP.
Figure The sleepy patient: Possible medical and psychiatric explanations
* Supportive factors: Persuasive if present, but if absent do not exclude possible conditions
Obstructive sleep apnea
Because OSA affects at least 4% of men and 2% of women,6 you are virtually assured of seeing undiagnosed patients. OSA is caused by repeated collapse of the soft tissues surrounding the upper airway, decreasing airflow that is restored when the patient briefly awakens. Patients develop EDS because sleep is fragmented by frequent arousals.
Obese patients, because of their body habitus, are at higher risk for OSA than patients at normal weight. Carefully screen patients for OSA if they develop weight problems while taking psychotropics, such as antipsychotics.
Alcohol or sedatives used at bedtime can aggravate OSA. These substances promote muscle relaxation and increase the arousal threshold so that patients do not awaken readily when apneas occur.
Long-term complications of untreated OSA include sleepiness leading to accidents, hypertension, cerebrovascular disease, and progressive obesity. Data also associate OSA with cardiovascular complications such as arrhythmias, congestive heart failure, and myocardial infarction.7
Physical examination focuses on detecting:
- nasal obstruction (have patient sniff separately through each nostril)
- large neck
- crowded oropharynx (low-hanging palate, reddened uvula, enlarged tonsils, large tongue relative to oropharynx diameter)
- jaw structure (particularly a small, retrognathic mandible).
Sleep studies. Referral for nocturnal polysomnography might be the next step. A comprehensive sleep study collects data about respiratory, cardiovascular, and muscle activity at night, as well as the sounds the patient makes—such as snoring or coughing—when asleep. EEG monitoring also is performed. OSA may be diagnosed if repeated episodes of reduced airflow and oxygen desaturation (arousals) are observed as brief shifts in EEG frequency.
Treatment. First-line interventions for the patient with OSA include:
- no alcohol 1 to 2 hours before bedtime
- sleeping on the side instead of the back
- weight loss (ideally with exercise)
- nasal sprays for allergies.
If first-line treatments are ineffective, nasal continuous positive airway pressure (CPAP) works well for most patients who adhere to the regimen.8 CPAP requires the patient to wear a nasal mask that delivers room air, splinting open the nasopharynx and upper airway (Box 2).
Surgical options. The most common surgeries for OSA are uvulopalatopharyngoplasty and laser-assisted uvulopalatoplasty. Others include tongue reduction and mandibular advancement.
The response rate to surgery averages 50%, depending on patient characteristics and procedure.9 Positive outcomes are most likely for thin patients with obvious upper airway obstruction, including deviated nasal septum, large tonsils, low-hanging palate, and large uvula. Postsurgical complications include nasal regurgitation, voice change, pain, bleeding, infection, tongue numbness, and snoring without apnea (silent apnea).
Oral appliances open the oropharynx by moving the mandible and tongue out of way. Patients with mild to moderate OSA accept these devices well. Evidence suggests that oral appliances improve sleep and reduce EDS more effectively than nasal CPAP and are preferred by patients.10
Oral devices have drawbacks, however. In most settings, their effectiveness cannot be observed during a “split-night” laboratory sleep study because the patient has not yet purchased the device. Also, multiple visits sometimes are required to custom-fit the appliance; this can pose a hardship for patients who live a distance from the provider.
Restless legs syndrome
Patients with restless legs syndrome (RLS) typically report a restless, painful feeling in the limbs that occurs in the evening and at night, disrupting sleep. This condition—which affects 10% of the population—is associated with aging, blood loss, anemia, peripheral neuropathies, and pregnancy.11 Onset can occur in childhood, and in some cases there is a familial tendency.
Most patients with RLS have periodic limb movements (repetitive leg jerks or twitches). The clinical significance of periodic limb movements with no subjective disagreeable feelings in the limbs is controversial, and these cases usually are not treated.
The history usually confirms RLS. Order sleep studies only if you suspect a coexisting sleep problem or the diagnosis is unclear.
A suspected mechanism of restless legs is dopamine deficiency. Low serum ferritin levels have been associated with RLS—presumably because iron is a cofactor necessary for dopamine synthesis12—and may be diagnostically helpful.
The most common technique is to ask the patient to establish a consistent awakening time and a regular bedtime. Initially this could be unconventional by societal standards—such as bedtime at 5 AM and arising at 2 PM. After this pattern is in place, the patient gradually shifts the timing by 1 hour per day. Most patients find it easier to delay rather than advance the bedtime until it conforms to the desired time.
Reinforce this new sleep pattern with a structured daytime schedule that includes predictable mealtimes, regular exercise, social activities, and possibly bright light exposure. Provide reinforcement in the morning for patients with delayed sleep phase disorder and in the evening for advanced sleep phase disorder. These interventions take time and discipline.
Another approach is for the patient to skip sleep one night and, in a sleep-deprived state, establish a new bedtime at the desired time. Use the same modalities listed above to reinforce (“entrain”) this schedule; otherwise the patient will slip back into the previous abnormal sleep-wake rhythm.
Treatment can include iron repletion when indicated. Medications include dopaminergic agents, most notably pramipexole and levodopa/carbidopa. Other options include gabapentin, benzodiazepines, and narcotics.
Antidepressants have been suspected to worsen restless legs syndrome, but definitive studies are lacking.13
Circadian rhythm disorders
Instead of compromising the quality or quantity of sleep, circadian rhythm disorders cause sleep to occur at inappropriate times. These disorders are most common in adolescents and young adults.
Delayed sleep phase disorder—a persistent pattern of staying up late and “sleeping in”—is most common. Careful assessment will reveal that the patient is getting adequate sleep but at a socially unacceptable time, sometimes to the extreme that his or her nights and days are reversed.
Patients’ reluctance to acknowledge the severity of this problem can lead to inaccurate sleep diaries and interviews. A portable wrist actigraph can provide data about limb movement and is more objective than self-reports.
Delayed sleep phase disorder is highly comorbid with depressive disorders.14 The cause of this syndrome is unclear, but light exposure, social patterns, psychological issues, and possibly a genetic substrate are known to contribute.
Advanced sleep phase disorder—a less common circadian rhythm disorder—also can cause EDS. Patients have an inappropriately early time of sleep onset and then are fully awake in the middle of the night. A large family with a severe form of this disorder was found to have an abnormality on chromosome 2.15
Treatment. Relatively few treatments are effective for circadian rhythm disorders. Some patients elect not to pursue therapy, instead fitting activities around their unconventional sleep schedules.
Individuals with delayed sleep phase who cannot arrange their lives around their sleep schedules are at risk for poor early morning performance because of sleepiness. Their internal circadian clocks can be gradually readjusted with phototherapy or gradual shifting of the major sleep period (Box 3). Stimulants usually are not used, but hypnotics can sometimes help these patients fall asleep earlier.
Insufficient sleep syndrome
People attempting to “burn the candle at both ends” are at risk for developing insufficient sleep syndrome.16 In our 24/7 society, people trying to make do with less than the required 7.5 hours sleep per night may adversely affect their health. The problem is compounded for shift workers because of the difficulty in obtaining sufficient quality sleep during daylight hours.
Many patients do not seek treatment for fatigue or sleepiness because they are aware of their lifestyle choices. Still, they might develop psychological symptoms such as irritability, mood swings, and strained interpersonal relationships. These symptoms can prompt patients to request treatment.
Take a careful history that includes discussing the patient’s daily and weekly schedule. Avoid psychostimulants; instead, address the nonnegotiable need to get adequate sleep and challenge the patient to prioritize his or her activities around a full night’s sleep.
When to consider narcolepsy
Narcolepsy is a CNS disease characterized by abnormal regulation of REM sleep. EDS—the cardinal symptom—is often associated with cataplexy (75%), sleep paralysis (50%), vivid dreams, and insomnia, all of which interfere with REM phenomena. Narcolepsy affects 0.05% of the U.S. population and can lead to severe occupational, educational, and family disruption.
When you obtain a history that suggests narcolepsy, use the history, a sleep diary, or wrist actigraphy to document whether the patient is getting adequate sleep, with a consistent sleep/wake cycle. Next, consider referring the patient for polysomnography, primarily to rule out other causes of EDS such as sleep-disordered breathing. In some cases, REM latency on the overnight sleep study will be <20 minutes after sleep onset, which supports the diagnosis of narcolepsy.
A multiple sleep latency test (MSLT)—a diagnostic session in which the patient takes 4 to 5 daytime naps—is performed the following day. Narcolepsy is confirmed if the patient has a mean initial sleep latency of <10 minutes during these naps plus at least two REM episodes within 15 minutes after sleep onset.
The 4 most appropriate indications for an urgent sleep evaluation are:
- difficulty staying alert while driving
- nocturnal cardiac arrhythmias
- frequent observed apneas
- excessive daytime sleepiness (EDS) leading to academic or occupational problems.
Insurance companies usually cover a specialty sleep evaluation, particularly if the referring physician documents a suspicion of sleep-disordered breathing or EDS that jeopardizes safe driving.
Most patients with narcolepsy and cataplexy have undetectable cerebrospinal fluid levels of a neuropeptide called hypocretin or orexin.17 Hypocretin/orexin replacement therapy is a theoretical possibility, but for now treatment includes a combination of optimal sleep hygiene, psychostimulants, antidepressants, and hypnotics.
Other causes of EDS
EDS can also be caused by unrecognized alcohol dependence, inappropriate or excessive medication use, and depressive disorders. Overnight sleep studies are seldom indicated unless patients endorse the symptoms in the Figure.
Before pursuing polysomnography or an MSLT (Box 4), eliminate medications that might confound the results, such as:
- antidepressants, which alter the timing and duration of REM sleep
- sedating medications, which modify initial sleep latency and sleep efficiency and potentially aggravate sleep disordered breathing.
Initial REM latency provides a potential biologic marker of major depression but is used more often in research than in clinical psychiatry.
Primary insomnia is the distressing inability to sleep at night or nap during the day. It suggests a hyperarousal state—the opposite of EDS.18 In rare cases, however, patients who cannot sleep at night also have EDS. When evaluated, they typically endorse at least one symptom in the Figure. Sleep studies occasionally reveal OSA or restless legs syndrome.
Treating a patient with chronic insomnia may require several trials of behavioral interventions or sedating medications before you make a referral to a sleep disorder center. Patients can struggle with unrecognized primary sleep disorders for years, and many are given empiric trials of stimulating antidepressants. Antidepressants are unlikely to cause harm, but they might complicate diagnostic testing.
When you confirm coexisting depression and a primary sleep disorder, treatments that separately target each condition provide optimal management of the sleepy patient.
Medications to enhance wakefulness
Wake-promoting agents are a treatment option when EDS is contributing to compromised functioning. These drugs are no substitute for thoughtful evaluation of hypersomnolence, however. When you diagnose OSA or restless legs syndrome, first try treatments that target these conditions. If residual sleepiness persists, then consider augmenting with stimulating medications.
Modafinil is FDA-approved for residual sleepiness in patients with OSA and for shift work sleep disorder, a condition of circadian misalignment from frequent schedule changes. Evidence does not support its use for other circadian rhythm disorders, such as delayed sleep phase disorder.
Low-dose modafinil (such as 100 to 200 mg/d) is well tolerated, but its therapeutic effect as augmentation is modest.19 Increasing the dosage to >200 mg usually does not increase alertness.
Caffeine. Some patients report benefit from caffeine used in moderation and only in the morning. This practice is acceptable as long as patients do not use excessive amounts or experience insomnia, exacerbation of anxiety, or tachycardia.
Psychostimulants such as methylphenidate and amphetamines are less well-studied than modafinil for treating EDS in patients without narcolepsy. Monitor carefully for insomnia, exacerbation of anxiety, tachycardia, or hypertension and to prevent overuse of these habituating agents.
Related resources
- National Sleep Foundation. www.sleepfoundation.org.
- American Academy of Sleep Medicine. www.aasmnet.org.
- American Sleep Apnea Association. www.sleepapnea.org.
- Restless Legs Syndrome Foundation. www.rls.org.
- Association for the Study of Light Therapy and Biological Rhythms. www.sltbr.org.
Drug brand names
- Carbidopa/levodopa • Sinemet
- Gabapentin • Neurontin
- Modafinil • Provigil
- Pramipexole • Mirapex
- Trazodone • Desyrel
- Zolpidem • Ambien
Disclosure
Dr. Krahn reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Acknowledgment
Dr. Krahn published the original version this article in the January 2002 issue of Current Psychiatry and has updated it for this issue.
1. Punjabi N, Haponik E. Ask about daytime sleepiness. J Amer Geriatr Soc 2000;48:228-9.
2. Ronald J, Delaive K, Roos L, et al. Health care utilization in the 10 years prior to diagnosis in obstructive sleep apnea syndrome patients. Sleep 1999;22(2):225-9.
3. Johns M. A new method for measuring daytime sleepiness: The Epworth Sleepiness Scale. Sleep 1991;14(6):540-5.
4. Rowley J, Aboussouan L, Badr M. The use of clinical prediction formulas in the evaluation of obstructive sleep apnea. Sleep 2000;23:929-38.
5. Yamashiro Y, Kryger M. Nocturnal oximetry: Is it a screening tool for sleep disorders? Sleep 1995;18:167-71.
6. Morrell M, Finn L, Kim H, et al. Sleep fragmentation, awake blood pressure, and sleep-disordered breathing in a population-based study. Am J Respir Critical Care Med 2000;162(6):2091-6.
7. Roux F, D’Ambrosio C, Mohsenin V. Sleep-related breathing disorders and cardiovascular disease. Am J Med. 2000;108:396-402.
8. Engleman H, Martin S, Deary I, Douglas N. Effect of continuous positive airway pressure treatment on daytime function in sleep apnoea/hypopnoea syndrome. Lancet 1994;343(8897):572-5.
9. Lojander J, Maasilta P, Partinen M, et al. Nasal-CPAP, surgery, and conservative management for treatment of obstructive sleep apnea syndrome. A randomized study. Chest. 1996;110(1):114-9.
10. Mehta A, Qian J, Petocz P, et al. A randomized, controlled study of a mandibular advancement splint for obstructive sleep apnea. Am J Respir Critical Care Med 2001;163(6):1457-61.
11. Chesson A, Wise M, Davila D, et al. Practice parameters for the treatment of restless legs syndrome and periodic limb movement disorder. An American Academy of Sleep Medicine Report. Standards of Practice Committee of the American Academy of Sleep Medicine. Sleep 1999;22(7):961-8.
12. Phillips B, Young T, Finn L, et al. Epidemiology of restless legs symptoms in adults. Arch Intern Med 2000;160(14):2137-41.
13. Thorpy M, Ehrenberg B, Hening W, et al. Restless legs syndrome: Detection and management in primary care. Amer Fam Phys 2000;62:108-14.
14. Regestein Q, Monk T. Delayed sleep phase syndrome: A review of its clinical aspects. Am J Psychiatry 1995;152:602-8.
15. Toh K, Jones C, He Y, et al. An hPer2 phosphorylation site mutation in familial advanced sleep phase syndrome. Science 2001;291(5506):1040-3.
16. Yoshikawa N, Suzuki S, Ishimoto T, et al. A case of insufficient sleep syndrome. Psychiatry Clin Neuro 1998;52(2):200-1.
17. Nishino S, Ripley B, Overeem S, et al. Hypocretin (orexin) deficiency in human narcolepsy. Lancet. 2000;355:39-40.
18. Hauri P, Esther M. Insomnia. Mayo Clin Proc 1990;65:869-82.
19. Schwartz JR. Modafinil: new indications for wake promotion. Expert Opin Pharmacother 2005;6(1):115-29.
1. Punjabi N, Haponik E. Ask about daytime sleepiness. J Amer Geriatr Soc 2000;48:228-9.
2. Ronald J, Delaive K, Roos L, et al. Health care utilization in the 10 years prior to diagnosis in obstructive sleep apnea syndrome patients. Sleep 1999;22(2):225-9.
3. Johns M. A new method for measuring daytime sleepiness: The Epworth Sleepiness Scale. Sleep 1991;14(6):540-5.
4. Rowley J, Aboussouan L, Badr M. The use of clinical prediction formulas in the evaluation of obstructive sleep apnea. Sleep 2000;23:929-38.
5. Yamashiro Y, Kryger M. Nocturnal oximetry: Is it a screening tool for sleep disorders? Sleep 1995;18:167-71.
6. Morrell M, Finn L, Kim H, et al. Sleep fragmentation, awake blood pressure, and sleep-disordered breathing in a population-based study. Am J Respir Critical Care Med 2000;162(6):2091-6.
7. Roux F, D’Ambrosio C, Mohsenin V. Sleep-related breathing disorders and cardiovascular disease. Am J Med. 2000;108:396-402.
8. Engleman H, Martin S, Deary I, Douglas N. Effect of continuous positive airway pressure treatment on daytime function in sleep apnoea/hypopnoea syndrome. Lancet 1994;343(8897):572-5.
9. Lojander J, Maasilta P, Partinen M, et al. Nasal-CPAP, surgery, and conservative management for treatment of obstructive sleep apnea syndrome. A randomized study. Chest. 1996;110(1):114-9.
10. Mehta A, Qian J, Petocz P, et al. A randomized, controlled study of a mandibular advancement splint for obstructive sleep apnea. Am J Respir Critical Care Med 2001;163(6):1457-61.
11. Chesson A, Wise M, Davila D, et al. Practice parameters for the treatment of restless legs syndrome and periodic limb movement disorder. An American Academy of Sleep Medicine Report. Standards of Practice Committee of the American Academy of Sleep Medicine. Sleep 1999;22(7):961-8.
12. Phillips B, Young T, Finn L, et al. Epidemiology of restless legs symptoms in adults. Arch Intern Med 2000;160(14):2137-41.
13. Thorpy M, Ehrenberg B, Hening W, et al. Restless legs syndrome: Detection and management in primary care. Amer Fam Phys 2000;62:108-14.
14. Regestein Q, Monk T. Delayed sleep phase syndrome: A review of its clinical aspects. Am J Psychiatry 1995;152:602-8.
15. Toh K, Jones C, He Y, et al. An hPer2 phosphorylation site mutation in familial advanced sleep phase syndrome. Science 2001;291(5506):1040-3.
16. Yoshikawa N, Suzuki S, Ishimoto T, et al. A case of insufficient sleep syndrome. Psychiatry Clin Neuro 1998;52(2):200-1.
17. Nishino S, Ripley B, Overeem S, et al. Hypocretin (orexin) deficiency in human narcolepsy. Lancet. 2000;355:39-40.
18. Hauri P, Esther M. Insomnia. Mayo Clin Proc 1990;65:869-82.
19. Schwartz JR. Modafinil: new indications for wake promotion. Expert Opin Pharmacother 2005;6(1):115-29.
Perimenopausal depression? Ask how she’s sleeping
Trying to treat depression or anxiety in a midlife woman without asking how she’s sleeping may doom your treatment plan. Asking about sleep addresses issues that affect her quality of life and can provide valuable insight into effective interventions.
Psychiatric, psychosocial, and medical problems can disturb sleep during perimenopause.1 To help you diagnose and treat both mood disorders and insomnia, this article:
- describes how irregular hormone levels and psychosocial changes are linked to perimenopausal mood and sleep disorders
- offers evidence-based strategies for hormone replacement therapy (HRT), antidepressants, hypnotics, and psychotherapy.
DEPRESSION AND INSOMNIA AT MIDLIFE
Sixty-five percent of women seeking outpatient treatment for depression report disturbed sleep.2 Even mild anxiety and depression can undermine sleep quality, whereas insomnia can precede other symptoms of an evolving major depression.
Depressive disorders affect up to 29% of perimenopausal women (depending on the assessment tool used), compared with 8% to 12% of premenopausal women. Menopausal symptoms—hot flashes, poor sleep, memory problems—and not using HRT are associated with depression.3
Causes of midlife depression. Gonadal hormone changes have been implicated as a cause of increased depression in midlife women; declines in serum estradiol and testosterone are inversely associated with depression.4 The natural menopause transition (perimenopause) begins during the mid-40s, persists to the early 50s, and lasts an average 2 to 9 years. Estradiol produced by the ovary becomes erratic then decreases. Follicle-stimulating hormone (FSH) and luteinizing hormone (LH) serum levels increase, then plateau and serve as laboratory markers of menopause.5
Sociodemographic factors also may contribute to depression, anxiety, and insomnia. A midlife woman may experience role transitions—such as children leaving home and aging parents needing care. She may be adapting to her or her spouse’s retirement or to the loss of her partner by divorce or death. She may be grappling with her own aging and questions about mortality and life purpose.
In the workup, consider medical factors that may worsen sleep problems, such as hot flashes, sleep apnea, thyroid disease, urinary frequency, chronic pain, restless leg syndrome, caffeine use, sedentary lifestyle, and primary insomnia. Some women lose sleep from a bed partner’s snoring or movement (“spousal arousal”). Stimulating drugs such as theophylline can also play a role.
SLEEP CHANGES AT PERIMENOPAUSE
Sleep changes are among the most common physical and psychological experiences healthy women describe during perimenopause:
- 100 consecutive women surveyed at a menopause clinic reported fatigue (91%), hot flashes (80%), insomnia and early awakenings (77%), and depression (65%).6
- Sleep problems were reported by >50% of 203 women interviewed for the Decisions at Menopause Study (DAMES).7
- Difficulty sleeping across 2 weeks was reported by 38% of a multiethnic population of 12,603 women ages 40 to 55.8
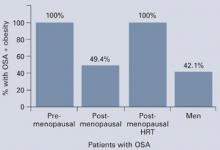
Sleep problems occur more often during perimenopause than earlier in life. In a clinic sample of 521 women, Owens et al1 found insomnia in 33% to 36% of those in premenopause and in 44% to 61% of women during perimenopause. In the total sample of healthy middle-aged women, 42% had sleep complaints, including:
- initial insomnia: 49%
- middle insomnia: 92%
- early morning awakening: 59%.
No association? Individuals experience sleep quality subjectively, and these assessments may not match those obtained objectively. The Wisconsin Sleep Cohort Study,9 for example, found no association between menopause and diminished sleep quality in polysomnographic studies of 589 community-dwelling women. Even so, the peri- and postmenopausal women in the study reported less sleep satisfaction than premenopausal women did.
Most clinicians agree that a woman’s subjective experience of sleep is clinically relevant. Thus, rule out underlying sleep disorders before you attribute a midlife woman’s depressive signs and symptoms primarily to menopause.10
Treatment. Combination therapy may be useful, depending on the patient’s psychiatric and medical comorbidities (Algorithm).
TREATING PERIMENOPAUSAL DEPRESSION
HRT. Before the Women’s Health Initiative (WHI),10 guidelines recommended HRT for a first depressive episode during perimenopause and antidepressants for severe depressive symptoms and for women with a history of depression.11 This practice changed when the WHI found risks of thromboembolism, breast cancer, stroke, and coronary artery disease that increased over time with HRT.
HRT remains a short-term treatment option but is no longer considered the first or only approach to mood symptoms at perimenopause. Discuss with your patient potential benefits of short-term HRT for a first episode of depression—especially if she has vasomotor symptoms—versus potential risks.
Antidepressants can improve perimenopausal depression, but few studies have tested these agents’ effects on sleep. To reduce treatment-associated insomnia:
- select a relatively sedating antidepressant such as mirtazapine
- accept some insomnia for 3 to 4 weeks, until a stimulating antidepressant has had a full ffect on mood and its associated side effects would be expected to resolve
- or augment the antidepressant with a hypnotic such as zolpidem, zaleplon, eszopiclone, or trazodone.
When choosing therapy, consider patient factors and insomnia severity. For example, mirtazapine is typically associated with weight gain, so consider other options for overweight patients. Those with severe insomnia may prefer not to wait 3 to 4 weeks for improved sleep. With hypnotics, consider cost, any coexisting chemical dependency, and potential for morning hangover.
Psychotherapy can help perimenopausal patients accept aging, evaluate relationships, and examine their roles in the lives of more-dependent parents and less-dependent children.
HOT FLASHES AND INSOMNIA
Persistent hot flashes that disturb sleep may cause depression.12 They can wake a perimenopausal woman repeatedly (Figure 1). The awakenings may be brief—90% last <3 minutes—but a severely affected woman can lose an hour of sleep in a night.13 Even after a hot flash resolves, other factors such as anxiety may keep her awake.
Up to 85% of perimenopausal women experience hot flashes, especially during the first year after menses cease. Hot flashes persist for 5 years after menopause in 25% of women and indefinitely in a minority (Box ).14
Estrogen deficiency is thought to cause hot flashes via decreased serotonin synthesis and up-regulated 5HT2A receptors—the mediators of heat loss. As a result, a woman’s thermoregulatory zone narrows during perimenopause, reducing her tolerance for core body temperature changes. The thermoregulatory nucleus resides in the medial preoptic area of the anterior hypothalamus.
A hot flash begins with facial warmth when core temperatures exceed the thermoregulatory line. Heat spreads to the chest, often accompanied by flushing, diaphoresis, and headache. A woman may feel agitated, irritable, and distressed.
CNS noradrenergic activity may initiate hot flashes. Freedman et al13 compared the effects of IV clonidine (an alpha2 adrenergic agonist) plus yohimbine (an alpha2 adrenergic antagonist) or placebo in menopausal women with or without vasomotor symptoms. Among 9 symptomatic women, 6 experienced hot flashes when given yohimbine, and none did with placebo. No hot flashes occurred in asymptomatic women. Clonidine increased the duration of peripheral heating needed to trigger a hot flash and reduced the number of hot flashes in symptomatic women, compared with baseline.
Risk factors for nocturnal hot flashes include surgical menopause, Caucasian versus Asian ethnicity, lack of exercise, and nicotine use.8 Women suffering anxiety and stress also are at increased risk.15
HOT-FLASH THERAPIES
Placebo-controlled trials of hot flash therapies have found efficacies from 85% for HRT to 25% for placebo, vitamin E, black cohosh, soy, and behavioral therapy (Figure 2).16 Most trials were not designed to test the link between hot flashes and sleep, and many enrolled cancer patients not experiencing natural menopause. With the 25% placeboresponse rate, some therapies’ efficacy is unclear.
HRT can reduce nocturnal hot flash frequency. In a polysomnographic study,17 21 postmenopausal women received 6 months of conjugated estrogens, 0.625 mg/d, with medroxyprogesterone, 5 mg/d, or micronized progesterone, 200 mg/d. Sleep efficiency improved by 8% in women receiving micronized progesterone but was unchanged with medroxyprogesterone. Even so, both groups reported improved sleep quality and duration, with decreased awakenings.
The Wisconsin Sleep Cohort Study9 found that HRT was not associated with improved sleep, as measured by polysomnography. Even so, the women in that study noted subjective sleep improvement with HRT.
Antidepressants. Venlafaxine, 75 mg/d, and fluoxetine, 20 mg/d, have shown benefit in reducing hot flashes,18 presumably by increasing CNS serotonin. As mentioned, however, many antidepressants can cause insomnia, and few studies have examined this problem.
Gabapentin has been effective for patients with hot flashes.19 This agent, which increases GABA levels and may modestly increase slow-wave sleep—can improve conditions that disrupt sleep, including restless legs syndrome and chronic pain. It is well-tolerated, even at 900 mg/d, and is more-sedating than most serotonergic antidepressants.
Hypnotics. Surprisingly little evidence addresses hypnotics’ role in managing insomnia caused by hot flashes. No data have been published on the role of benzodiazepines or the benzodiazepine receptor agonists (zolpidem, zaleplon, and eszopiclone). In my experience, benzodiazepine receptor agonists improve sleep quality compromised by multiple factors, including hot flashes.
Soy and black cohosh. Isoflavones in soy may be estrogen receptor modulators. Twelve randomized, controlled trials of soy or soy extracts have shown a modest benefit for hot flashes.20
Black cohosh extracts, 8 mg/d, were given to 80 postmenopausal women in a randomized, double-blind, placebo-controlled trial (RCT). Hot flashes in those receiving black cohosh decreased from 4.9 to 0.7 daily, compared with reductions of 5.2 to 3.2 in women receiving estrogen and 5.1 to 3.1 in those receiving placebo.21 As a result, the National Institutes of Health is funding a 12-month, RCT to determine whether black cohosh reduces hot flash frequency and intensity.
Alternative agents are widely used and warrant study. Those shown to be safe can be used alone or with other therapies, but advise the patient that these agents may not be effective. Relaxation and exercise may decrease hot flashes,22 although some outcomes have been similar to a placebo response.
SLEEP APNEA AT PERIMENOPAUSE
Obstructive sleep apnea (OSA), although more common in men than women, appears to increase during perimenopause. Women with untreated OSA are twice as likely as men to be treated for depression, less likely to report excessive daytime sleepiness and snoring, and more likely to present with depression, anxiety, and morning headache.
Bixler et al23 interviewed 12,219 women and 4,364 men ages 20 to 100 and conducted 1-night sleep studies in 1,000 women and 741 men. OSA rates were 3.9% in men, 0.6% in premenopausal women, 2.7% in postmenopausal women not taking HRT, and 0.5% in postmenopausal women taking HRT.
The risk of sleep-disordered breathing is lower during early menopause and peaks at approximately age 65. Declining hormones likely play a role; progesterone increases ventilatory drive, and estrogen increases ventilatory centers’ sensitivity to progesterone’s stimulant effect. In small studies, exogenous progesterone has shown a slight effect in improving OSA.24
OSA’s transient, repetitive upper airway collapse increases inspiratory effort and may cause hypoxemia. Repeated arousals can lead to prolonged awakenings and unrefreshing sleep. Snoring and increased body mass index are strongly associated factors, although the Wisconsin Sleep Cohort Study10 showed an increase in sleep apnea in perimenopausal women that was unrelated to increased body mass index.
Obesity may not explain the increase in obstructive sleep apnea at perimenopause (Figure 3),28 although body fat distribution does change with aging. Women at perimenopause are likely to develop abdominal weight distribution.
Figure 3 Increased OSA in postmenopausal women is unrelated to obesity (BMI >32)
Obstructive sleep apnea (OSA) in 1,000 women and 741 men was associated exclusively with obesity in premenopausal women and postmenopausal women using HRT, but nearly one-half of the postmenopausal women with OSA were not obese.
Source: Adapted from reference 23.Treatment. In the Sleep Heart Health Study25 of 2,852 women age 50 or older, HRT users had one-half the apnea prevalence of nonusers (6% vs 14%). HRT users were less likely to awaken at night and to get inadequate sleep. Snoring rates were similar (25% for HRT users, 23% for nonusers).
Nasal continuous positive airway pressure (CPAP) is the mainstay of apnea treatment, although some women appear to have difficulty accepting CPAP.26 Weight loss and moderate exercise can help manage weight and improve sleep quality by increasing slow-wave sleep. Regular exercise also may improve depressed mood.
Related resources
- American College of Obstetricians and Gynecologists. www.acog.org
- The North American Menopause Society. www.menopause.org
Drug brand names
- Conjugated estrogens • Premarin
- Eszopiclone • Lunesta
- Fluoxetine • Prozac
- Gabapentin • Neurontin
- Medroxyprogesterone • Provera
- Micronized progesterone • Prometrium
- Mirtazapine • Remeron
- Trazodone • Desyrel
- Venlafaxine • Effexor
- Zaleplon • Sonata
- Zolpidem • Ambien
Disclosures
Dr. Krahn reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Owens JF, Matthews KA. Sleep disturbance in healthy middle-aged women. Maturitas 1998;30:41-50.
2. Perlis ML, Giles DE, Buysse DJ, et al. Self-reported sleep disturbance as a prodromal symptom in recurrent depression. J Affect Disord 1997;42(2-3):209-12.
3. Bromberger JT, Assmann SF, Avis NE, et al. Persistent mood symptoms in a multiethnic community cohort of pre- and perimenopausal women. Am J Epidemiol 2003;158:347-56.
4. Sherwin BB. Changes in sexual behavior as a function of plasma sex steroid levels in post-menopausal women. Maturitas 1985;7(3):225-33.
5. Brizendine L. Minding menopause. Psychotropics vs estrogen: What you need to know now. Current Psychiatry 2003;2(10):12-31.
6. Anderson E, Hamburger S, Liu JH, Rebar RW. Characteristics of menopausal women seeking assistance. Am J Obstet Gynecol 1987;156(2):428-33.
7. Obermeyer CM, Reynolds RF, Price K, Abraham A. Therapeutic decisions for menopause: results of the DAMES project in central Massachusetts. Menopause 2004;11(4):456-65.
8. Kravitz HM, Ganz PA, Bromberger J, et al. Sleep difficulty in women at midlife: a community survey of sleep and the menopausal transition. Menopause 2003;10(1):19-28.
9. Young T, Rabago D, Zgierska A, et al. Objective and subjective sleep quality in premenopausal, perimenopausal, and postmenopausal women in the Wisconsin Sleep Cohort Study. Sleep 2003;26(6):667-72.
10. Wassertheil-Smoller S, Shumaker S, Ockene J, et al. Depression and cardiovascular sequelae in postmenopausal women. Arch Intern Med 2004;164:289-98.
11. Altshuler LL, Cohen LS, Moline ML, et al. The expert consensus guideline series. Treatment of depression in women. Postgrad Med 2001;March:1-107.
12. Krystal AD. Insomnia in women. Clin Cornerstone 2003;5(3):41-50.
13. Freedman RR. Physiology of hot flashes. Am J Hum Biol 2001;13(4):453-64.
14. Freedman RR, Woodward S, Sabharwal SC. A2-adrenergic mechanism in menopausal hot flushes. Obstet Gynecol 1990;76(4):573-8.
15. Miller AG, Li RM. Measuring hot flashes: summary of a NIH workshop. Mayo Clin Proc 2004;79:777-81.
16. Joffe H, Soares CN, Cohen LS. Assessment and treatment of hot flushes and menopausal mood disturbance. Psychiatr Clin North Am 2003 Sep;26(3):563-80.
17. Montplaisir J, Lorrain J, Denesle R, Petit D. Sleep in menopause: differential effects of two forms of hormone replacement therapy. Menopause 2001;8(1):10-16.
18. Loprinzi CL, Sloan JA, Perez EA, et al. Phase III evaluation of fluoxetine for treatment of hot flashes. J Clin Oncol 2002;20:1578-83.
19. Guttoso T, Jr, Kurlan R, McDermott MP, Kieburtz K. Gabapentin’s effects on hot flashes in postmenopausal women: a randomized controlled trial. Obstet Gynecol 2003;101:337-45.
20. Kessel B, Kronenberg F. The role of complementary and alternative medicine in management of menopausal symptoms. Endocrinol Metab Clin North Am 2004;33:717-39.
21. National Institutes of Health. National Center for Complementary and Alternative Medicine. Office of Dietary Supplements. Questions and answers about black cohosh and the symptoms of menopause. Available at: http://ods.od.nih.gov/factsheets/blackcohosh.asp. Accessed May 9, 2005.
22. Ivarsson T, Spetz AC, Hammar M. Physical exercise and vasomotor symptoms in postmenopausal women. Mauritas 1998;29:139-46.
23. Bixler EO, Vgontzas AN, Lin HM, et al. Prevalence of sleep-disordered breathing in women: effects of gender. Am J Respir Crit Care Med 2001;163:608-13.
24. Block AJ, Wynne JW, Boysen PG, et al. Menopause, medroxyprogesterone and breathing during sleep. Am J Med 1981;70:506-10.
25. Shahar E, Redline S, Young T, et al. Hormone replacement therapy and sleep-disordered breathing. Am J Respir Crit Care Med 2003;167:1186-92.
26. McArdle N, Devereux G, Heidarnejad H, et al. Long-term use of CPAP therapy for sleep apnea/hypopnea syndrome. Am J Respir Crit. Care Med 1999;159:1108-14.
Trying to treat depression or anxiety in a midlife woman without asking how she’s sleeping may doom your treatment plan. Asking about sleep addresses issues that affect her quality of life and can provide valuable insight into effective interventions.
Psychiatric, psychosocial, and medical problems can disturb sleep during perimenopause.1 To help you diagnose and treat both mood disorders and insomnia, this article:
- describes how irregular hormone levels and psychosocial changes are linked to perimenopausal mood and sleep disorders
- offers evidence-based strategies for hormone replacement therapy (HRT), antidepressants, hypnotics, and psychotherapy.
DEPRESSION AND INSOMNIA AT MIDLIFE
Sixty-five percent of women seeking outpatient treatment for depression report disturbed sleep.2 Even mild anxiety and depression can undermine sleep quality, whereas insomnia can precede other symptoms of an evolving major depression.
Depressive disorders affect up to 29% of perimenopausal women (depending on the assessment tool used), compared with 8% to 12% of premenopausal women. Menopausal symptoms—hot flashes, poor sleep, memory problems—and not using HRT are associated with depression.3
Causes of midlife depression. Gonadal hormone changes have been implicated as a cause of increased depression in midlife women; declines in serum estradiol and testosterone are inversely associated with depression.4 The natural menopause transition (perimenopause) begins during the mid-40s, persists to the early 50s, and lasts an average 2 to 9 years. Estradiol produced by the ovary becomes erratic then decreases. Follicle-stimulating hormone (FSH) and luteinizing hormone (LH) serum levels increase, then plateau and serve as laboratory markers of menopause.5
Sociodemographic factors also may contribute to depression, anxiety, and insomnia. A midlife woman may experience role transitions—such as children leaving home and aging parents needing care. She may be adapting to her or her spouse’s retirement or to the loss of her partner by divorce or death. She may be grappling with her own aging and questions about mortality and life purpose.
In the workup, consider medical factors that may worsen sleep problems, such as hot flashes, sleep apnea, thyroid disease, urinary frequency, chronic pain, restless leg syndrome, caffeine use, sedentary lifestyle, and primary insomnia. Some women lose sleep from a bed partner’s snoring or movement (“spousal arousal”). Stimulating drugs such as theophylline can also play a role.
SLEEP CHANGES AT PERIMENOPAUSE
Sleep changes are among the most common physical and psychological experiences healthy women describe during perimenopause:
- 100 consecutive women surveyed at a menopause clinic reported fatigue (91%), hot flashes (80%), insomnia and early awakenings (77%), and depression (65%).6
- Sleep problems were reported by >50% of 203 women interviewed for the Decisions at Menopause Study (DAMES).7
- Difficulty sleeping across 2 weeks was reported by 38% of a multiethnic population of 12,603 women ages 40 to 55.8
Sleep problems occur more often during perimenopause than earlier in life. In a clinic sample of 521 women, Owens et al1 found insomnia in 33% to 36% of those in premenopause and in 44% to 61% of women during perimenopause. In the total sample of healthy middle-aged women, 42% had sleep complaints, including:
- initial insomnia: 49%
- middle insomnia: 92%
- early morning awakening: 59%.
No association? Individuals experience sleep quality subjectively, and these assessments may not match those obtained objectively. The Wisconsin Sleep Cohort Study,9 for example, found no association between menopause and diminished sleep quality in polysomnographic studies of 589 community-dwelling women. Even so, the peri- and postmenopausal women in the study reported less sleep satisfaction than premenopausal women did.
Most clinicians agree that a woman’s subjective experience of sleep is clinically relevant. Thus, rule out underlying sleep disorders before you attribute a midlife woman’s depressive signs and symptoms primarily to menopause.10
Treatment. Combination therapy may be useful, depending on the patient’s psychiatric and medical comorbidities (Algorithm).
TREATING PERIMENOPAUSAL DEPRESSION
HRT. Before the Women’s Health Initiative (WHI),10 guidelines recommended HRT for a first depressive episode during perimenopause and antidepressants for severe depressive symptoms and for women with a history of depression.11 This practice changed when the WHI found risks of thromboembolism, breast cancer, stroke, and coronary artery disease that increased over time with HRT.
HRT remains a short-term treatment option but is no longer considered the first or only approach to mood symptoms at perimenopause. Discuss with your patient potential benefits of short-term HRT for a first episode of depression—especially if she has vasomotor symptoms—versus potential risks.
Antidepressants can improve perimenopausal depression, but few studies have tested these agents’ effects on sleep. To reduce treatment-associated insomnia:
- select a relatively sedating antidepressant such as mirtazapine
- accept some insomnia for 3 to 4 weeks, until a stimulating antidepressant has had a full ffect on mood and its associated side effects would be expected to resolve
- or augment the antidepressant with a hypnotic such as zolpidem, zaleplon, eszopiclone, or trazodone.
When choosing therapy, consider patient factors and insomnia severity. For example, mirtazapine is typically associated with weight gain, so consider other options for overweight patients. Those with severe insomnia may prefer not to wait 3 to 4 weeks for improved sleep. With hypnotics, consider cost, any coexisting chemical dependency, and potential for morning hangover.
Psychotherapy can help perimenopausal patients accept aging, evaluate relationships, and examine their roles in the lives of more-dependent parents and less-dependent children.
HOT FLASHES AND INSOMNIA
Persistent hot flashes that disturb sleep may cause depression.12 They can wake a perimenopausal woman repeatedly (Figure 1). The awakenings may be brief—90% last <3 minutes—but a severely affected woman can lose an hour of sleep in a night.13 Even after a hot flash resolves, other factors such as anxiety may keep her awake.
Up to 85% of perimenopausal women experience hot flashes, especially during the first year after menses cease. Hot flashes persist for 5 years after menopause in 25% of women and indefinitely in a minority (Box ).14
Estrogen deficiency is thought to cause hot flashes via decreased serotonin synthesis and up-regulated 5HT2A receptors—the mediators of heat loss. As a result, a woman’s thermoregulatory zone narrows during perimenopause, reducing her tolerance for core body temperature changes. The thermoregulatory nucleus resides in the medial preoptic area of the anterior hypothalamus.
A hot flash begins with facial warmth when core temperatures exceed the thermoregulatory line. Heat spreads to the chest, often accompanied by flushing, diaphoresis, and headache. A woman may feel agitated, irritable, and distressed.
CNS noradrenergic activity may initiate hot flashes. Freedman et al13 compared the effects of IV clonidine (an alpha2 adrenergic agonist) plus yohimbine (an alpha2 adrenergic antagonist) or placebo in menopausal women with or without vasomotor symptoms. Among 9 symptomatic women, 6 experienced hot flashes when given yohimbine, and none did with placebo. No hot flashes occurred in asymptomatic women. Clonidine increased the duration of peripheral heating needed to trigger a hot flash and reduced the number of hot flashes in symptomatic women, compared with baseline.
Risk factors for nocturnal hot flashes include surgical menopause, Caucasian versus Asian ethnicity, lack of exercise, and nicotine use.8 Women suffering anxiety and stress also are at increased risk.15
HOT-FLASH THERAPIES
Placebo-controlled trials of hot flash therapies have found efficacies from 85% for HRT to 25% for placebo, vitamin E, black cohosh, soy, and behavioral therapy (Figure 2).16 Most trials were not designed to test the link between hot flashes and sleep, and many enrolled cancer patients not experiencing natural menopause. With the 25% placeboresponse rate, some therapies’ efficacy is unclear.
HRT can reduce nocturnal hot flash frequency. In a polysomnographic study,17 21 postmenopausal women received 6 months of conjugated estrogens, 0.625 mg/d, with medroxyprogesterone, 5 mg/d, or micronized progesterone, 200 mg/d. Sleep efficiency improved by 8% in women receiving micronized progesterone but was unchanged with medroxyprogesterone. Even so, both groups reported improved sleep quality and duration, with decreased awakenings.
The Wisconsin Sleep Cohort Study9 found that HRT was not associated with improved sleep, as measured by polysomnography. Even so, the women in that study noted subjective sleep improvement with HRT.
Antidepressants. Venlafaxine, 75 mg/d, and fluoxetine, 20 mg/d, have shown benefit in reducing hot flashes,18 presumably by increasing CNS serotonin. As mentioned, however, many antidepressants can cause insomnia, and few studies have examined this problem.
Gabapentin has been effective for patients with hot flashes.19 This agent, which increases GABA levels and may modestly increase slow-wave sleep—can improve conditions that disrupt sleep, including restless legs syndrome and chronic pain. It is well-tolerated, even at 900 mg/d, and is more-sedating than most serotonergic antidepressants.
Hypnotics. Surprisingly little evidence addresses hypnotics’ role in managing insomnia caused by hot flashes. No data have been published on the role of benzodiazepines or the benzodiazepine receptor agonists (zolpidem, zaleplon, and eszopiclone). In my experience, benzodiazepine receptor agonists improve sleep quality compromised by multiple factors, including hot flashes.
Soy and black cohosh. Isoflavones in soy may be estrogen receptor modulators. Twelve randomized, controlled trials of soy or soy extracts have shown a modest benefit for hot flashes.20
Black cohosh extracts, 8 mg/d, were given to 80 postmenopausal women in a randomized, double-blind, placebo-controlled trial (RCT). Hot flashes in those receiving black cohosh decreased from 4.9 to 0.7 daily, compared with reductions of 5.2 to 3.2 in women receiving estrogen and 5.1 to 3.1 in those receiving placebo.21 As a result, the National Institutes of Health is funding a 12-month, RCT to determine whether black cohosh reduces hot flash frequency and intensity.
Alternative agents are widely used and warrant study. Those shown to be safe can be used alone or with other therapies, but advise the patient that these agents may not be effective. Relaxation and exercise may decrease hot flashes,22 although some outcomes have been similar to a placebo response.
SLEEP APNEA AT PERIMENOPAUSE
Obstructive sleep apnea (OSA), although more common in men than women, appears to increase during perimenopause. Women with untreated OSA are twice as likely as men to be treated for depression, less likely to report excessive daytime sleepiness and snoring, and more likely to present with depression, anxiety, and morning headache.
Bixler et al23 interviewed 12,219 women and 4,364 men ages 20 to 100 and conducted 1-night sleep studies in 1,000 women and 741 men. OSA rates were 3.9% in men, 0.6% in premenopausal women, 2.7% in postmenopausal women not taking HRT, and 0.5% in postmenopausal women taking HRT.
The risk of sleep-disordered breathing is lower during early menopause and peaks at approximately age 65. Declining hormones likely play a role; progesterone increases ventilatory drive, and estrogen increases ventilatory centers’ sensitivity to progesterone’s stimulant effect. In small studies, exogenous progesterone has shown a slight effect in improving OSA.24
OSA’s transient, repetitive upper airway collapse increases inspiratory effort and may cause hypoxemia. Repeated arousals can lead to prolonged awakenings and unrefreshing sleep. Snoring and increased body mass index are strongly associated factors, although the Wisconsin Sleep Cohort Study10 showed an increase in sleep apnea in perimenopausal women that was unrelated to increased body mass index.
Obesity may not explain the increase in obstructive sleep apnea at perimenopause (Figure 3),28 although body fat distribution does change with aging. Women at perimenopause are likely to develop abdominal weight distribution.
Figure 3 Increased OSA in postmenopausal women is unrelated to obesity (BMI >32)
Obstructive sleep apnea (OSA) in 1,000 women and 741 men was associated exclusively with obesity in premenopausal women and postmenopausal women using HRT, but nearly one-half of the postmenopausal women with OSA were not obese.
Source: Adapted from reference 23.Treatment. In the Sleep Heart Health Study25 of 2,852 women age 50 or older, HRT users had one-half the apnea prevalence of nonusers (6% vs 14%). HRT users were less likely to awaken at night and to get inadequate sleep. Snoring rates were similar (25% for HRT users, 23% for nonusers).
Nasal continuous positive airway pressure (CPAP) is the mainstay of apnea treatment, although some women appear to have difficulty accepting CPAP.26 Weight loss and moderate exercise can help manage weight and improve sleep quality by increasing slow-wave sleep. Regular exercise also may improve depressed mood.
Related resources
- American College of Obstetricians and Gynecologists. www.acog.org
- The North American Menopause Society. www.menopause.org
Drug brand names
- Conjugated estrogens • Premarin
- Eszopiclone • Lunesta
- Fluoxetine • Prozac
- Gabapentin • Neurontin
- Medroxyprogesterone • Provera
- Micronized progesterone • Prometrium
- Mirtazapine • Remeron
- Trazodone • Desyrel
- Venlafaxine • Effexor
- Zaleplon • Sonata
- Zolpidem • Ambien
Disclosures
Dr. Krahn reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Trying to treat depression or anxiety in a midlife woman without asking how she’s sleeping may doom your treatment plan. Asking about sleep addresses issues that affect her quality of life and can provide valuable insight into effective interventions.
Psychiatric, psychosocial, and medical problems can disturb sleep during perimenopause.1 To help you diagnose and treat both mood disorders and insomnia, this article:
- describes how irregular hormone levels and psychosocial changes are linked to perimenopausal mood and sleep disorders
- offers evidence-based strategies for hormone replacement therapy (HRT), antidepressants, hypnotics, and psychotherapy.
DEPRESSION AND INSOMNIA AT MIDLIFE
Sixty-five percent of women seeking outpatient treatment for depression report disturbed sleep.2 Even mild anxiety and depression can undermine sleep quality, whereas insomnia can precede other symptoms of an evolving major depression.
Depressive disorders affect up to 29% of perimenopausal women (depending on the assessment tool used), compared with 8% to 12% of premenopausal women. Menopausal symptoms—hot flashes, poor sleep, memory problems—and not using HRT are associated with depression.3
Causes of midlife depression. Gonadal hormone changes have been implicated as a cause of increased depression in midlife women; declines in serum estradiol and testosterone are inversely associated with depression.4 The natural menopause transition (perimenopause) begins during the mid-40s, persists to the early 50s, and lasts an average 2 to 9 years. Estradiol produced by the ovary becomes erratic then decreases. Follicle-stimulating hormone (FSH) and luteinizing hormone (LH) serum levels increase, then plateau and serve as laboratory markers of menopause.5
Sociodemographic factors also may contribute to depression, anxiety, and insomnia. A midlife woman may experience role transitions—such as children leaving home and aging parents needing care. She may be adapting to her or her spouse’s retirement or to the loss of her partner by divorce or death. She may be grappling with her own aging and questions about mortality and life purpose.
In the workup, consider medical factors that may worsen sleep problems, such as hot flashes, sleep apnea, thyroid disease, urinary frequency, chronic pain, restless leg syndrome, caffeine use, sedentary lifestyle, and primary insomnia. Some women lose sleep from a bed partner’s snoring or movement (“spousal arousal”). Stimulating drugs such as theophylline can also play a role.
SLEEP CHANGES AT PERIMENOPAUSE
Sleep changes are among the most common physical and psychological experiences healthy women describe during perimenopause:
- 100 consecutive women surveyed at a menopause clinic reported fatigue (91%), hot flashes (80%), insomnia and early awakenings (77%), and depression (65%).6
- Sleep problems were reported by >50% of 203 women interviewed for the Decisions at Menopause Study (DAMES).7
- Difficulty sleeping across 2 weeks was reported by 38% of a multiethnic population of 12,603 women ages 40 to 55.8
Sleep problems occur more often during perimenopause than earlier in life. In a clinic sample of 521 women, Owens et al1 found insomnia in 33% to 36% of those in premenopause and in 44% to 61% of women during perimenopause. In the total sample of healthy middle-aged women, 42% had sleep complaints, including:
- initial insomnia: 49%
- middle insomnia: 92%
- early morning awakening: 59%.
No association? Individuals experience sleep quality subjectively, and these assessments may not match those obtained objectively. The Wisconsin Sleep Cohort Study,9 for example, found no association between menopause and diminished sleep quality in polysomnographic studies of 589 community-dwelling women. Even so, the peri- and postmenopausal women in the study reported less sleep satisfaction than premenopausal women did.
Most clinicians agree that a woman’s subjective experience of sleep is clinically relevant. Thus, rule out underlying sleep disorders before you attribute a midlife woman’s depressive signs and symptoms primarily to menopause.10
Treatment. Combination therapy may be useful, depending on the patient’s psychiatric and medical comorbidities (Algorithm).
TREATING PERIMENOPAUSAL DEPRESSION
HRT. Before the Women’s Health Initiative (WHI),10 guidelines recommended HRT for a first depressive episode during perimenopause and antidepressants for severe depressive symptoms and for women with a history of depression.11 This practice changed when the WHI found risks of thromboembolism, breast cancer, stroke, and coronary artery disease that increased over time with HRT.
HRT remains a short-term treatment option but is no longer considered the first or only approach to mood symptoms at perimenopause. Discuss with your patient potential benefits of short-term HRT for a first episode of depression—especially if she has vasomotor symptoms—versus potential risks.
Antidepressants can improve perimenopausal depression, but few studies have tested these agents’ effects on sleep. To reduce treatment-associated insomnia:
- select a relatively sedating antidepressant such as mirtazapine
- accept some insomnia for 3 to 4 weeks, until a stimulating antidepressant has had a full ffect on mood and its associated side effects would be expected to resolve
- or augment the antidepressant with a hypnotic such as zolpidem, zaleplon, eszopiclone, or trazodone.
When choosing therapy, consider patient factors and insomnia severity. For example, mirtazapine is typically associated with weight gain, so consider other options for overweight patients. Those with severe insomnia may prefer not to wait 3 to 4 weeks for improved sleep. With hypnotics, consider cost, any coexisting chemical dependency, and potential for morning hangover.
Psychotherapy can help perimenopausal patients accept aging, evaluate relationships, and examine their roles in the lives of more-dependent parents and less-dependent children.
HOT FLASHES AND INSOMNIA
Persistent hot flashes that disturb sleep may cause depression.12 They can wake a perimenopausal woman repeatedly (Figure 1). The awakenings may be brief—90% last <3 minutes—but a severely affected woman can lose an hour of sleep in a night.13 Even after a hot flash resolves, other factors such as anxiety may keep her awake.
Up to 85% of perimenopausal women experience hot flashes, especially during the first year after menses cease. Hot flashes persist for 5 years after menopause in 25% of women and indefinitely in a minority (Box ).14
Estrogen deficiency is thought to cause hot flashes via decreased serotonin synthesis and up-regulated 5HT2A receptors—the mediators of heat loss. As a result, a woman’s thermoregulatory zone narrows during perimenopause, reducing her tolerance for core body temperature changes. The thermoregulatory nucleus resides in the medial preoptic area of the anterior hypothalamus.
A hot flash begins with facial warmth when core temperatures exceed the thermoregulatory line. Heat spreads to the chest, often accompanied by flushing, diaphoresis, and headache. A woman may feel agitated, irritable, and distressed.
CNS noradrenergic activity may initiate hot flashes. Freedman et al13 compared the effects of IV clonidine (an alpha2 adrenergic agonist) plus yohimbine (an alpha2 adrenergic antagonist) or placebo in menopausal women with or without vasomotor symptoms. Among 9 symptomatic women, 6 experienced hot flashes when given yohimbine, and none did with placebo. No hot flashes occurred in asymptomatic women. Clonidine increased the duration of peripheral heating needed to trigger a hot flash and reduced the number of hot flashes in symptomatic women, compared with baseline.
Risk factors for nocturnal hot flashes include surgical menopause, Caucasian versus Asian ethnicity, lack of exercise, and nicotine use.8 Women suffering anxiety and stress also are at increased risk.15
HOT-FLASH THERAPIES
Placebo-controlled trials of hot flash therapies have found efficacies from 85% for HRT to 25% for placebo, vitamin E, black cohosh, soy, and behavioral therapy (Figure 2).16 Most trials were not designed to test the link between hot flashes and sleep, and many enrolled cancer patients not experiencing natural menopause. With the 25% placeboresponse rate, some therapies’ efficacy is unclear.
HRT can reduce nocturnal hot flash frequency. In a polysomnographic study,17 21 postmenopausal women received 6 months of conjugated estrogens, 0.625 mg/d, with medroxyprogesterone, 5 mg/d, or micronized progesterone, 200 mg/d. Sleep efficiency improved by 8% in women receiving micronized progesterone but was unchanged with medroxyprogesterone. Even so, both groups reported improved sleep quality and duration, with decreased awakenings.
The Wisconsin Sleep Cohort Study9 found that HRT was not associated with improved sleep, as measured by polysomnography. Even so, the women in that study noted subjective sleep improvement with HRT.
Antidepressants. Venlafaxine, 75 mg/d, and fluoxetine, 20 mg/d, have shown benefit in reducing hot flashes,18 presumably by increasing CNS serotonin. As mentioned, however, many antidepressants can cause insomnia, and few studies have examined this problem.
Gabapentin has been effective for patients with hot flashes.19 This agent, which increases GABA levels and may modestly increase slow-wave sleep—can improve conditions that disrupt sleep, including restless legs syndrome and chronic pain. It is well-tolerated, even at 900 mg/d, and is more-sedating than most serotonergic antidepressants.
Hypnotics. Surprisingly little evidence addresses hypnotics’ role in managing insomnia caused by hot flashes. No data have been published on the role of benzodiazepines or the benzodiazepine receptor agonists (zolpidem, zaleplon, and eszopiclone). In my experience, benzodiazepine receptor agonists improve sleep quality compromised by multiple factors, including hot flashes.
Soy and black cohosh. Isoflavones in soy may be estrogen receptor modulators. Twelve randomized, controlled trials of soy or soy extracts have shown a modest benefit for hot flashes.20
Black cohosh extracts, 8 mg/d, were given to 80 postmenopausal women in a randomized, double-blind, placebo-controlled trial (RCT). Hot flashes in those receiving black cohosh decreased from 4.9 to 0.7 daily, compared with reductions of 5.2 to 3.2 in women receiving estrogen and 5.1 to 3.1 in those receiving placebo.21 As a result, the National Institutes of Health is funding a 12-month, RCT to determine whether black cohosh reduces hot flash frequency and intensity.
Alternative agents are widely used and warrant study. Those shown to be safe can be used alone or with other therapies, but advise the patient that these agents may not be effective. Relaxation and exercise may decrease hot flashes,22 although some outcomes have been similar to a placebo response.
SLEEP APNEA AT PERIMENOPAUSE
Obstructive sleep apnea (OSA), although more common in men than women, appears to increase during perimenopause. Women with untreated OSA are twice as likely as men to be treated for depression, less likely to report excessive daytime sleepiness and snoring, and more likely to present with depression, anxiety, and morning headache.
Bixler et al23 interviewed 12,219 women and 4,364 men ages 20 to 100 and conducted 1-night sleep studies in 1,000 women and 741 men. OSA rates were 3.9% in men, 0.6% in premenopausal women, 2.7% in postmenopausal women not taking HRT, and 0.5% in postmenopausal women taking HRT.
The risk of sleep-disordered breathing is lower during early menopause and peaks at approximately age 65. Declining hormones likely play a role; progesterone increases ventilatory drive, and estrogen increases ventilatory centers’ sensitivity to progesterone’s stimulant effect. In small studies, exogenous progesterone has shown a slight effect in improving OSA.24
OSA’s transient, repetitive upper airway collapse increases inspiratory effort and may cause hypoxemia. Repeated arousals can lead to prolonged awakenings and unrefreshing sleep. Snoring and increased body mass index are strongly associated factors, although the Wisconsin Sleep Cohort Study10 showed an increase in sleep apnea in perimenopausal women that was unrelated to increased body mass index.
Obesity may not explain the increase in obstructive sleep apnea at perimenopause (Figure 3),28 although body fat distribution does change with aging. Women at perimenopause are likely to develop abdominal weight distribution.
Figure 3 Increased OSA in postmenopausal women is unrelated to obesity (BMI >32)
Obstructive sleep apnea (OSA) in 1,000 women and 741 men was associated exclusively with obesity in premenopausal women and postmenopausal women using HRT, but nearly one-half of the postmenopausal women with OSA were not obese.
Source: Adapted from reference 23.Treatment. In the Sleep Heart Health Study25 of 2,852 women age 50 or older, HRT users had one-half the apnea prevalence of nonusers (6% vs 14%). HRT users were less likely to awaken at night and to get inadequate sleep. Snoring rates were similar (25% for HRT users, 23% for nonusers).
Nasal continuous positive airway pressure (CPAP) is the mainstay of apnea treatment, although some women appear to have difficulty accepting CPAP.26 Weight loss and moderate exercise can help manage weight and improve sleep quality by increasing slow-wave sleep. Regular exercise also may improve depressed mood.
Related resources
- American College of Obstetricians and Gynecologists. www.acog.org
- The North American Menopause Society. www.menopause.org
Drug brand names
- Conjugated estrogens • Premarin
- Eszopiclone • Lunesta
- Fluoxetine • Prozac
- Gabapentin • Neurontin
- Medroxyprogesterone • Provera
- Micronized progesterone • Prometrium
- Mirtazapine • Remeron
- Trazodone • Desyrel
- Venlafaxine • Effexor
- Zaleplon • Sonata
- Zolpidem • Ambien
Disclosures
Dr. Krahn reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Owens JF, Matthews KA. Sleep disturbance in healthy middle-aged women. Maturitas 1998;30:41-50.
2. Perlis ML, Giles DE, Buysse DJ, et al. Self-reported sleep disturbance as a prodromal symptom in recurrent depression. J Affect Disord 1997;42(2-3):209-12.
3. Bromberger JT, Assmann SF, Avis NE, et al. Persistent mood symptoms in a multiethnic community cohort of pre- and perimenopausal women. Am J Epidemiol 2003;158:347-56.
4. Sherwin BB. Changes in sexual behavior as a function of plasma sex steroid levels in post-menopausal women. Maturitas 1985;7(3):225-33.
5. Brizendine L. Minding menopause. Psychotropics vs estrogen: What you need to know now. Current Psychiatry 2003;2(10):12-31.
6. Anderson E, Hamburger S, Liu JH, Rebar RW. Characteristics of menopausal women seeking assistance. Am J Obstet Gynecol 1987;156(2):428-33.
7. Obermeyer CM, Reynolds RF, Price K, Abraham A. Therapeutic decisions for menopause: results of the DAMES project in central Massachusetts. Menopause 2004;11(4):456-65.
8. Kravitz HM, Ganz PA, Bromberger J, et al. Sleep difficulty in women at midlife: a community survey of sleep and the menopausal transition. Menopause 2003;10(1):19-28.
9. Young T, Rabago D, Zgierska A, et al. Objective and subjective sleep quality in premenopausal, perimenopausal, and postmenopausal women in the Wisconsin Sleep Cohort Study. Sleep 2003;26(6):667-72.
10. Wassertheil-Smoller S, Shumaker S, Ockene J, et al. Depression and cardiovascular sequelae in postmenopausal women. Arch Intern Med 2004;164:289-98.
11. Altshuler LL, Cohen LS, Moline ML, et al. The expert consensus guideline series. Treatment of depression in women. Postgrad Med 2001;March:1-107.
12. Krystal AD. Insomnia in women. Clin Cornerstone 2003;5(3):41-50.
13. Freedman RR. Physiology of hot flashes. Am J Hum Biol 2001;13(4):453-64.
14. Freedman RR, Woodward S, Sabharwal SC. A2-adrenergic mechanism in menopausal hot flushes. Obstet Gynecol 1990;76(4):573-8.
15. Miller AG, Li RM. Measuring hot flashes: summary of a NIH workshop. Mayo Clin Proc 2004;79:777-81.
16. Joffe H, Soares CN, Cohen LS. Assessment and treatment of hot flushes and menopausal mood disturbance. Psychiatr Clin North Am 2003 Sep;26(3):563-80.
17. Montplaisir J, Lorrain J, Denesle R, Petit D. Sleep in menopause: differential effects of two forms of hormone replacement therapy. Menopause 2001;8(1):10-16.
18. Loprinzi CL, Sloan JA, Perez EA, et al. Phase III evaluation of fluoxetine for treatment of hot flashes. J Clin Oncol 2002;20:1578-83.
19. Guttoso T, Jr, Kurlan R, McDermott MP, Kieburtz K. Gabapentin’s effects on hot flashes in postmenopausal women: a randomized controlled trial. Obstet Gynecol 2003;101:337-45.
20. Kessel B, Kronenberg F. The role of complementary and alternative medicine in management of menopausal symptoms. Endocrinol Metab Clin North Am 2004;33:717-39.
21. National Institutes of Health. National Center for Complementary and Alternative Medicine. Office of Dietary Supplements. Questions and answers about black cohosh and the symptoms of menopause. Available at: http://ods.od.nih.gov/factsheets/blackcohosh.asp. Accessed May 9, 2005.
22. Ivarsson T, Spetz AC, Hammar M. Physical exercise and vasomotor symptoms in postmenopausal women. Mauritas 1998;29:139-46.
23. Bixler EO, Vgontzas AN, Lin HM, et al. Prevalence of sleep-disordered breathing in women: effects of gender. Am J Respir Crit Care Med 2001;163:608-13.
24. Block AJ, Wynne JW, Boysen PG, et al. Menopause, medroxyprogesterone and breathing during sleep. Am J Med 1981;70:506-10.
25. Shahar E, Redline S, Young T, et al. Hormone replacement therapy and sleep-disordered breathing. Am J Respir Crit Care Med 2003;167:1186-92.
26. McArdle N, Devereux G, Heidarnejad H, et al. Long-term use of CPAP therapy for sleep apnea/hypopnea syndrome. Am J Respir Crit. Care Med 1999;159:1108-14.
1. Owens JF, Matthews KA. Sleep disturbance in healthy middle-aged women. Maturitas 1998;30:41-50.
2. Perlis ML, Giles DE, Buysse DJ, et al. Self-reported sleep disturbance as a prodromal symptom in recurrent depression. J Affect Disord 1997;42(2-3):209-12.
3. Bromberger JT, Assmann SF, Avis NE, et al. Persistent mood symptoms in a multiethnic community cohort of pre- and perimenopausal women. Am J Epidemiol 2003;158:347-56.
4. Sherwin BB. Changes in sexual behavior as a function of plasma sex steroid levels in post-menopausal women. Maturitas 1985;7(3):225-33.
5. Brizendine L. Minding menopause. Psychotropics vs estrogen: What you need to know now. Current Psychiatry 2003;2(10):12-31.
6. Anderson E, Hamburger S, Liu JH, Rebar RW. Characteristics of menopausal women seeking assistance. Am J Obstet Gynecol 1987;156(2):428-33.
7. Obermeyer CM, Reynolds RF, Price K, Abraham A. Therapeutic decisions for menopause: results of the DAMES project in central Massachusetts. Menopause 2004;11(4):456-65.
8. Kravitz HM, Ganz PA, Bromberger J, et al. Sleep difficulty in women at midlife: a community survey of sleep and the menopausal transition. Menopause 2003;10(1):19-28.
9. Young T, Rabago D, Zgierska A, et al. Objective and subjective sleep quality in premenopausal, perimenopausal, and postmenopausal women in the Wisconsin Sleep Cohort Study. Sleep 2003;26(6):667-72.
10. Wassertheil-Smoller S, Shumaker S, Ockene J, et al. Depression and cardiovascular sequelae in postmenopausal women. Arch Intern Med 2004;164:289-98.
11. Altshuler LL, Cohen LS, Moline ML, et al. The expert consensus guideline series. Treatment of depression in women. Postgrad Med 2001;March:1-107.
12. Krystal AD. Insomnia in women. Clin Cornerstone 2003;5(3):41-50.
13. Freedman RR. Physiology of hot flashes. Am J Hum Biol 2001;13(4):453-64.
14. Freedman RR, Woodward S, Sabharwal SC. A2-adrenergic mechanism in menopausal hot flushes. Obstet Gynecol 1990;76(4):573-8.
15. Miller AG, Li RM. Measuring hot flashes: summary of a NIH workshop. Mayo Clin Proc 2004;79:777-81.
16. Joffe H, Soares CN, Cohen LS. Assessment and treatment of hot flushes and menopausal mood disturbance. Psychiatr Clin North Am 2003 Sep;26(3):563-80.
17. Montplaisir J, Lorrain J, Denesle R, Petit D. Sleep in menopause: differential effects of two forms of hormone replacement therapy. Menopause 2001;8(1):10-16.
18. Loprinzi CL, Sloan JA, Perez EA, et al. Phase III evaluation of fluoxetine for treatment of hot flashes. J Clin Oncol 2002;20:1578-83.
19. Guttoso T, Jr, Kurlan R, McDermott MP, Kieburtz K. Gabapentin’s effects on hot flashes in postmenopausal women: a randomized controlled trial. Obstet Gynecol 2003;101:337-45.
20. Kessel B, Kronenberg F. The role of complementary and alternative medicine in management of menopausal symptoms. Endocrinol Metab Clin North Am 2004;33:717-39.
21. National Institutes of Health. National Center for Complementary and Alternative Medicine. Office of Dietary Supplements. Questions and answers about black cohosh and the symptoms of menopause. Available at: http://ods.od.nih.gov/factsheets/blackcohosh.asp. Accessed May 9, 2005.
22. Ivarsson T, Spetz AC, Hammar M. Physical exercise and vasomotor symptoms in postmenopausal women. Mauritas 1998;29:139-46.
23. Bixler EO, Vgontzas AN, Lin HM, et al. Prevalence of sleep-disordered breathing in women: effects of gender. Am J Respir Crit Care Med 2001;163:608-13.
24. Block AJ, Wynne JW, Boysen PG, et al. Menopause, medroxyprogesterone and breathing during sleep. Am J Med 1981;70:506-10.
25. Shahar E, Redline S, Young T, et al. Hormone replacement therapy and sleep-disordered breathing. Am J Respir Crit Care Med 2003;167:1186-92.
26. McArdle N, Devereux G, Heidarnejad H, et al. Long-term use of CPAP therapy for sleep apnea/hypopnea syndrome. Am J Respir Crit. Care Med 1999;159:1108-14.
Eszopiclone: Targeting chronic insomnia
Nonbenzodiazepine hypnotics have become mainstays in insomnia treatment. These agents do not interfere with cognitive function upon awakening, compared with benzodiazepines and other agents used off-label as hypnotics.1
Eszopiclone has shown efficacy in clinical trials for treating short-term and long-term (lasting ≥3 weeks) insomnia. By contrast, zaleplon and zolpidem are indicated for short-term insomnia treatment.
HOW IT WORKS
Eszopiclone, a cyclopyrrolone, is the racemic form of zopiclone, an agent used worldwide to treat insomnia but not available in the United States. The racemic zopiclone has a high affinity for benzodiazepine binding sites in the cerebral cortex, hippocampus, and cerebellum.
As with the selective benzodiazepine receptor agonists zaleplon and zolpidem, information on eszopiclone’s receptor binding profile is limited. It is unclear if the agent binds directly to the benzodiazepine receptor or to a related site on the GABA receptor complex.
Table
Eszopiclone: Fast facts
| Brand name: |
| Lunesta |
| Class |
| Novel cyclopyrrolone, nonbenzodiazepine hypnotic |
| FDA-approved indication: |
| Insomnia |
| Approval date: |
| Dec. 15, 2004 |
| Manufacturer: |
| Sepracor |
| Dosing form: |
| 1-, 2-, and 3-mg tablets |
| Recommended dosage: |
| 2 to 3 mg HS (at bedtime) for adults age ≤65 |
| 1 to 2 mg HS for adults age >65 |
PHARMACOKINETICS
Preliminary studies suggest eszopiclone is rapidly absorbed from the GI tract, mostly within 1 hour of taking it.2,3 The agent reaches peak concentration within 30 minutes to 4 hours in healthy persons. A high-fat or heavy meal may delay hypnotic onset by approximately 1 hour.
Eszopiclone is metabolized mostly through the 3A4 isoenzyme of the cytochrome P(CYP)-450 system, although the CYP 2E1 isoenzyme also plays a minor role. About 75% of the dose is excreted in urine.4 Because its elimination half-life is approximately 6 hours, eszopiclone leaves no residual effects when patients awaken after about 6 hours of sleep.1
Because they take weeks to eliminate, some older sleep-promoting medications can cause increasing daytime sedation when used daily. By contrast, eszopiclone can be taken once daily with no risk of drug accumulation.
EFFICACY
Although relatively few clinical studies of eszopiclone have been published, the new-drug application submitted to the FDA summarized 24 clinical trials totaling more than 2,700 subjects.
Zammit et al5 gave 308 patients eszopiclone, 2 or 3 mg HS (at bedtime), or placebo for 6 weeks. Eszopiclone decreased time to falling asleep, increased total sleep time, improved continuity of sleep, and increased overall sleep quality throughout the night. After 6 weeks, patients in the treatment group showed:
- no residual morning sedation based on repeated polysomnography and morning questionnaire measures
- no residual daytime sedation based on results of the Digit Symbol Substitution Test, which gauges psychomotor impairment.
Patients taking 3 mg showed reduced wakefulness at night on objective and subjective measures compared with the placebo group.
A randomized, double-blind, multicenter, placebo-controlled study (N=788)6,7 assessed eszopiclone’s safety and efficacy across 6 months in patients with chronic insomnia. Before enrollment, patients slept
SAFETY AND TOLERABILITY
Eszopiclone was well tolerated in preclinical and clinical trials. The most common adverse event was a bitter taste reported by 34% of participants; this prompted 1.7% of patients in one study4 to discontinue eszopiclone, compared with 0.5% of patients taking placebo. Other common adverse effects included:
- daytime somnolence, (8% prevalence, 2.2% dropout rate
- depression (1% dropout rate).4
Krystal et al found no clinically significant changes in vital signs, ECG results, laboratory values, and physical examination findings between the eszopiclone and placebo groups.6,7
Few significant interactions between eszopiclone and other drugs have been reported. However:
- Increased sedation and decreased psychomotor functioning were observed with eszopiclone, 3 mg, and olanzapine, 10 mg.
- Drugs that inhibit (eg, ketoconazole) or induce (eg, rifampicin) the CYP 3A4 isoenzyme may alter eszopiclone levels.8
- A possible drug-drug interaction between eszopiclone and alcohol, 0.7 g/kg, decreased psychomotor performance for up to 4 hours after alcohol use.8
No significant drug-drug interactions were reported between eszopiclone and paroxetine or lorazepam.4
In another case, the parent compound zopiclone given concomitantly with trimipramine decreased both drugs’ bioavailability but did not noticeably change either drug’s clinical effect.9 As eszopiclone and zopiclone are chemically similar, be careful when giving eszopiclone to patients taking trimipramine or similar medications, such as tricyclic antidepressants.
DOSING
Start eszopiclone at 2 mg HS for adults and titrate to 3 mg as needed. For many patients, 3 mg may suffice as maintenance therapy. The risks and benefits of dosing eszopiclone at >3 mg are not known.
Lower doses are recommended for patients age >65 because of the risk of decreased motor and/or cognitive performance. Give 2 mg for maintenance and 1 mg for difficulty falling asleep. There are no other known contraindications to eszopiclone use.
As with other hypnotics, supplement eszopiclone with sleep hygiene education and relaxation techniques.
CLINICAL IMPLICATIONS
Eszopiclone has shown efficacy for >2 weeks in primary insomnia, suggesting the agent may help treat chronic insomnia.
As with other nonbenzodiazepine hypnotics, off-label use of eszopiclone with antidepressants may help treat insomnia secondary to depressive or anxiety disorders. Research is needed to gauge the drug’s effectiveness for this use.
Related resources
- American Academy of Sleep Medicine. www.aasmnet.org
- Lunesta Web site. www.lunesta.com
Drug brand names
- Eszopiclone • Lunesta
- Ketoconazole • Nizoral
- Lorazepam • Ativan
- Olanzapine • Zyprexa
- Paroxetine • Paxil
- Trimipramine • Surmontil
- Zaleplon • Sonata
- Zolpidem • Ambien
Disclosure
Dr. Krahn reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Terzano MG, Rossi M, Palomba V, et al. New drugs for insomnia: comparative tolerability of zopiclone, zolpidem, and zaleplon. Drug Safety 2003;26:261-82.
2. Fernandez C, Martin C, Giminez F, Farinotti R. Clinical pharmacokinetics of zopiclone. Clin Pharmacokinet 1995;29:431-41.
3. Leese P, Maier G. Eszopiclone: Pharmacokinetic (PK) and pharmacodynamic effects of a novel sedative anti-insomnia agent after daytime administration in healthy subjects. Sleep 2002;25 (suppl):A45.-
4. Lunesta (eszopiclone) prescribing information. Available at: http://www.lunesta.com. Accessed Jan. 6, 2005.
5. Zammit GK, Gillin JC, McNabb L, et al. Eszopiclone, a novel non-benzodiazepine anti-insomnia agent: a six-week efficacy and safety study in adult patients with chronic insomnia. Sleep 2003;26(suppl):A297.-
6. Krystal A, Walsh J, Roth T, et al. The sustained efficacy and safety of eszopiclone over six months of nightly treatment: a placebo controlled study in patients with chronic insomnia. Sleep 2003;26(suppl):0779.-
7. Krystal A, Walsh J, Laska E, et al. Sustained efficacy of eszopiclone over six months of nightly treatment: results of a randomized, double-blind, placebo-controlled study in adults with chronic insomnia. Sleep 2003;26:793-9.
8. Hesse LM, von Moltke LL, Greenblatt DJ. Clinically important drug interactions with zopiclone, zolpidem, and zaleplon. CNS Drugs 2003;17:513-32.
9. Caille G, du Souich P, Spenard J, et al. Pharmacokinetic and clinical parameters of zopiclone and trimipramine when administered simultaneously to volunteers. Biopharm Drug Dispos 1984;5:117-25.
Nonbenzodiazepine hypnotics have become mainstays in insomnia treatment. These agents do not interfere with cognitive function upon awakening, compared with benzodiazepines and other agents used off-label as hypnotics.1
Eszopiclone has shown efficacy in clinical trials for treating short-term and long-term (lasting ≥3 weeks) insomnia. By contrast, zaleplon and zolpidem are indicated for short-term insomnia treatment.
HOW IT WORKS
Eszopiclone, a cyclopyrrolone, is the racemic form of zopiclone, an agent used worldwide to treat insomnia but not available in the United States. The racemic zopiclone has a high affinity for benzodiazepine binding sites in the cerebral cortex, hippocampus, and cerebellum.
As with the selective benzodiazepine receptor agonists zaleplon and zolpidem, information on eszopiclone’s receptor binding profile is limited. It is unclear if the agent binds directly to the benzodiazepine receptor or to a related site on the GABA receptor complex.
Table
Eszopiclone: Fast facts
| Brand name: |
| Lunesta |
| Class |
| Novel cyclopyrrolone, nonbenzodiazepine hypnotic |
| FDA-approved indication: |
| Insomnia |
| Approval date: |
| Dec. 15, 2004 |
| Manufacturer: |
| Sepracor |
| Dosing form: |
| 1-, 2-, and 3-mg tablets |
| Recommended dosage: |
| 2 to 3 mg HS (at bedtime) for adults age ≤65 |
| 1 to 2 mg HS for adults age >65 |
PHARMACOKINETICS
Preliminary studies suggest eszopiclone is rapidly absorbed from the GI tract, mostly within 1 hour of taking it.2,3 The agent reaches peak concentration within 30 minutes to 4 hours in healthy persons. A high-fat or heavy meal may delay hypnotic onset by approximately 1 hour.
Eszopiclone is metabolized mostly through the 3A4 isoenzyme of the cytochrome P(CYP)-450 system, although the CYP 2E1 isoenzyme also plays a minor role. About 75% of the dose is excreted in urine.4 Because its elimination half-life is approximately 6 hours, eszopiclone leaves no residual effects when patients awaken after about 6 hours of sleep.1
Because they take weeks to eliminate, some older sleep-promoting medications can cause increasing daytime sedation when used daily. By contrast, eszopiclone can be taken once daily with no risk of drug accumulation.
EFFICACY
Although relatively few clinical studies of eszopiclone have been published, the new-drug application submitted to the FDA summarized 24 clinical trials totaling more than 2,700 subjects.
Zammit et al5 gave 308 patients eszopiclone, 2 or 3 mg HS (at bedtime), or placebo for 6 weeks. Eszopiclone decreased time to falling asleep, increased total sleep time, improved continuity of sleep, and increased overall sleep quality throughout the night. After 6 weeks, patients in the treatment group showed:
- no residual morning sedation based on repeated polysomnography and morning questionnaire measures
- no residual daytime sedation based on results of the Digit Symbol Substitution Test, which gauges psychomotor impairment.
Patients taking 3 mg showed reduced wakefulness at night on objective and subjective measures compared with the placebo group.
A randomized, double-blind, multicenter, placebo-controlled study (N=788)6,7 assessed eszopiclone’s safety and efficacy across 6 months in patients with chronic insomnia. Before enrollment, patients slept
SAFETY AND TOLERABILITY
Eszopiclone was well tolerated in preclinical and clinical trials. The most common adverse event was a bitter taste reported by 34% of participants; this prompted 1.7% of patients in one study4 to discontinue eszopiclone, compared with 0.5% of patients taking placebo. Other common adverse effects included:
- daytime somnolence, (8% prevalence, 2.2% dropout rate
- depression (1% dropout rate).4
Krystal et al found no clinically significant changes in vital signs, ECG results, laboratory values, and physical examination findings between the eszopiclone and placebo groups.6,7
Few significant interactions between eszopiclone and other drugs have been reported. However:
- Increased sedation and decreased psychomotor functioning were observed with eszopiclone, 3 mg, and olanzapine, 10 mg.
- Drugs that inhibit (eg, ketoconazole) or induce (eg, rifampicin) the CYP 3A4 isoenzyme may alter eszopiclone levels.8
- A possible drug-drug interaction between eszopiclone and alcohol, 0.7 g/kg, decreased psychomotor performance for up to 4 hours after alcohol use.8
No significant drug-drug interactions were reported between eszopiclone and paroxetine or lorazepam.4
In another case, the parent compound zopiclone given concomitantly with trimipramine decreased both drugs’ bioavailability but did not noticeably change either drug’s clinical effect.9 As eszopiclone and zopiclone are chemically similar, be careful when giving eszopiclone to patients taking trimipramine or similar medications, such as tricyclic antidepressants.
DOSING
Start eszopiclone at 2 mg HS for adults and titrate to 3 mg as needed. For many patients, 3 mg may suffice as maintenance therapy. The risks and benefits of dosing eszopiclone at >3 mg are not known.
Lower doses are recommended for patients age >65 because of the risk of decreased motor and/or cognitive performance. Give 2 mg for maintenance and 1 mg for difficulty falling asleep. There are no other known contraindications to eszopiclone use.
As with other hypnotics, supplement eszopiclone with sleep hygiene education and relaxation techniques.
CLINICAL IMPLICATIONS
Eszopiclone has shown efficacy for >2 weeks in primary insomnia, suggesting the agent may help treat chronic insomnia.
As with other nonbenzodiazepine hypnotics, off-label use of eszopiclone with antidepressants may help treat insomnia secondary to depressive or anxiety disorders. Research is needed to gauge the drug’s effectiveness for this use.
Related resources
- American Academy of Sleep Medicine. www.aasmnet.org
- Lunesta Web site. www.lunesta.com
Drug brand names
- Eszopiclone • Lunesta
- Ketoconazole • Nizoral
- Lorazepam • Ativan
- Olanzapine • Zyprexa
- Paroxetine • Paxil
- Trimipramine • Surmontil
- Zaleplon • Sonata
- Zolpidem • Ambien
Disclosure
Dr. Krahn reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Nonbenzodiazepine hypnotics have become mainstays in insomnia treatment. These agents do not interfere with cognitive function upon awakening, compared with benzodiazepines and other agents used off-label as hypnotics.1
Eszopiclone has shown efficacy in clinical trials for treating short-term and long-term (lasting ≥3 weeks) insomnia. By contrast, zaleplon and zolpidem are indicated for short-term insomnia treatment.
HOW IT WORKS
Eszopiclone, a cyclopyrrolone, is the racemic form of zopiclone, an agent used worldwide to treat insomnia but not available in the United States. The racemic zopiclone has a high affinity for benzodiazepine binding sites in the cerebral cortex, hippocampus, and cerebellum.
As with the selective benzodiazepine receptor agonists zaleplon and zolpidem, information on eszopiclone’s receptor binding profile is limited. It is unclear if the agent binds directly to the benzodiazepine receptor or to a related site on the GABA receptor complex.
Table
Eszopiclone: Fast facts
| Brand name: |
| Lunesta |
| Class |
| Novel cyclopyrrolone, nonbenzodiazepine hypnotic |
| FDA-approved indication: |
| Insomnia |
| Approval date: |
| Dec. 15, 2004 |
| Manufacturer: |
| Sepracor |
| Dosing form: |
| 1-, 2-, and 3-mg tablets |
| Recommended dosage: |
| 2 to 3 mg HS (at bedtime) for adults age ≤65 |
| 1 to 2 mg HS for adults age >65 |
PHARMACOKINETICS
Preliminary studies suggest eszopiclone is rapidly absorbed from the GI tract, mostly within 1 hour of taking it.2,3 The agent reaches peak concentration within 30 minutes to 4 hours in healthy persons. A high-fat or heavy meal may delay hypnotic onset by approximately 1 hour.
Eszopiclone is metabolized mostly through the 3A4 isoenzyme of the cytochrome P(CYP)-450 system, although the CYP 2E1 isoenzyme also plays a minor role. About 75% of the dose is excreted in urine.4 Because its elimination half-life is approximately 6 hours, eszopiclone leaves no residual effects when patients awaken after about 6 hours of sleep.1
Because they take weeks to eliminate, some older sleep-promoting medications can cause increasing daytime sedation when used daily. By contrast, eszopiclone can be taken once daily with no risk of drug accumulation.
EFFICACY
Although relatively few clinical studies of eszopiclone have been published, the new-drug application submitted to the FDA summarized 24 clinical trials totaling more than 2,700 subjects.
Zammit et al5 gave 308 patients eszopiclone, 2 or 3 mg HS (at bedtime), or placebo for 6 weeks. Eszopiclone decreased time to falling asleep, increased total sleep time, improved continuity of sleep, and increased overall sleep quality throughout the night. After 6 weeks, patients in the treatment group showed:
- no residual morning sedation based on repeated polysomnography and morning questionnaire measures
- no residual daytime sedation based on results of the Digit Symbol Substitution Test, which gauges psychomotor impairment.
Patients taking 3 mg showed reduced wakefulness at night on objective and subjective measures compared with the placebo group.
A randomized, double-blind, multicenter, placebo-controlled study (N=788)6,7 assessed eszopiclone’s safety and efficacy across 6 months in patients with chronic insomnia. Before enrollment, patients slept
SAFETY AND TOLERABILITY
Eszopiclone was well tolerated in preclinical and clinical trials. The most common adverse event was a bitter taste reported by 34% of participants; this prompted 1.7% of patients in one study4 to discontinue eszopiclone, compared with 0.5% of patients taking placebo. Other common adverse effects included:
- daytime somnolence, (8% prevalence, 2.2% dropout rate
- depression (1% dropout rate).4
Krystal et al found no clinically significant changes in vital signs, ECG results, laboratory values, and physical examination findings between the eszopiclone and placebo groups.6,7
Few significant interactions between eszopiclone and other drugs have been reported. However:
- Increased sedation and decreased psychomotor functioning were observed with eszopiclone, 3 mg, and olanzapine, 10 mg.
- Drugs that inhibit (eg, ketoconazole) or induce (eg, rifampicin) the CYP 3A4 isoenzyme may alter eszopiclone levels.8
- A possible drug-drug interaction between eszopiclone and alcohol, 0.7 g/kg, decreased psychomotor performance for up to 4 hours after alcohol use.8
No significant drug-drug interactions were reported between eszopiclone and paroxetine or lorazepam.4
In another case, the parent compound zopiclone given concomitantly with trimipramine decreased both drugs’ bioavailability but did not noticeably change either drug’s clinical effect.9 As eszopiclone and zopiclone are chemically similar, be careful when giving eszopiclone to patients taking trimipramine or similar medications, such as tricyclic antidepressants.
DOSING
Start eszopiclone at 2 mg HS for adults and titrate to 3 mg as needed. For many patients, 3 mg may suffice as maintenance therapy. The risks and benefits of dosing eszopiclone at >3 mg are not known.
Lower doses are recommended for patients age >65 because of the risk of decreased motor and/or cognitive performance. Give 2 mg for maintenance and 1 mg for difficulty falling asleep. There are no other known contraindications to eszopiclone use.
As with other hypnotics, supplement eszopiclone with sleep hygiene education and relaxation techniques.
CLINICAL IMPLICATIONS
Eszopiclone has shown efficacy for >2 weeks in primary insomnia, suggesting the agent may help treat chronic insomnia.
As with other nonbenzodiazepine hypnotics, off-label use of eszopiclone with antidepressants may help treat insomnia secondary to depressive or anxiety disorders. Research is needed to gauge the drug’s effectiveness for this use.
Related resources
- American Academy of Sleep Medicine. www.aasmnet.org
- Lunesta Web site. www.lunesta.com
Drug brand names
- Eszopiclone • Lunesta
- Ketoconazole • Nizoral
- Lorazepam • Ativan
- Olanzapine • Zyprexa
- Paroxetine • Paxil
- Trimipramine • Surmontil
- Zaleplon • Sonata
- Zolpidem • Ambien
Disclosure
Dr. Krahn reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Terzano MG, Rossi M, Palomba V, et al. New drugs for insomnia: comparative tolerability of zopiclone, zolpidem, and zaleplon. Drug Safety 2003;26:261-82.
2. Fernandez C, Martin C, Giminez F, Farinotti R. Clinical pharmacokinetics of zopiclone. Clin Pharmacokinet 1995;29:431-41.
3. Leese P, Maier G. Eszopiclone: Pharmacokinetic (PK) and pharmacodynamic effects of a novel sedative anti-insomnia agent after daytime administration in healthy subjects. Sleep 2002;25 (suppl):A45.-
4. Lunesta (eszopiclone) prescribing information. Available at: http://www.lunesta.com. Accessed Jan. 6, 2005.
5. Zammit GK, Gillin JC, McNabb L, et al. Eszopiclone, a novel non-benzodiazepine anti-insomnia agent: a six-week efficacy and safety study in adult patients with chronic insomnia. Sleep 2003;26(suppl):A297.-
6. Krystal A, Walsh J, Roth T, et al. The sustained efficacy and safety of eszopiclone over six months of nightly treatment: a placebo controlled study in patients with chronic insomnia. Sleep 2003;26(suppl):0779.-
7. Krystal A, Walsh J, Laska E, et al. Sustained efficacy of eszopiclone over six months of nightly treatment: results of a randomized, double-blind, placebo-controlled study in adults with chronic insomnia. Sleep 2003;26:793-9.
8. Hesse LM, von Moltke LL, Greenblatt DJ. Clinically important drug interactions with zopiclone, zolpidem, and zaleplon. CNS Drugs 2003;17:513-32.
9. Caille G, du Souich P, Spenard J, et al. Pharmacokinetic and clinical parameters of zopiclone and trimipramine when administered simultaneously to volunteers. Biopharm Drug Dispos 1984;5:117-25.
1. Terzano MG, Rossi M, Palomba V, et al. New drugs for insomnia: comparative tolerability of zopiclone, zolpidem, and zaleplon. Drug Safety 2003;26:261-82.
2. Fernandez C, Martin C, Giminez F, Farinotti R. Clinical pharmacokinetics of zopiclone. Clin Pharmacokinet 1995;29:431-41.
3. Leese P, Maier G. Eszopiclone: Pharmacokinetic (PK) and pharmacodynamic effects of a novel sedative anti-insomnia agent after daytime administration in healthy subjects. Sleep 2002;25 (suppl):A45.-
4. Lunesta (eszopiclone) prescribing information. Available at: http://www.lunesta.com. Accessed Jan. 6, 2005.
5. Zammit GK, Gillin JC, McNabb L, et al. Eszopiclone, a novel non-benzodiazepine anti-insomnia agent: a six-week efficacy and safety study in adult patients with chronic insomnia. Sleep 2003;26(suppl):A297.-
6. Krystal A, Walsh J, Roth T, et al. The sustained efficacy and safety of eszopiclone over six months of nightly treatment: a placebo controlled study in patients with chronic insomnia. Sleep 2003;26(suppl):0779.-
7. Krystal A, Walsh J, Laska E, et al. Sustained efficacy of eszopiclone over six months of nightly treatment: results of a randomized, double-blind, placebo-controlled study in adults with chronic insomnia. Sleep 2003;26:793-9.
8. Hesse LM, von Moltke LL, Greenblatt DJ. Clinically important drug interactions with zopiclone, zolpidem, and zaleplon. CNS Drugs 2003;17:513-32.
9. Caille G, du Souich P, Spenard J, et al. Pharmacokinetic and clinical parameters of zopiclone and trimipramine when administered simultaneously to volunteers. Biopharm Drug Dispos 1984;5:117-25.
Treating affective illness in patients with chronic pain
Ms. A, age 44, fell from a 3-foot stool while reaching for a high kitchen shelf and suffered severe neck flexion. Her initial pain persisted for weeks and then months, resulting in chronic neck pain aggravated by movement.
Over the past year, her doctor has prescribed numerous analgesics and muscle relaxants, including tramadol, hydrocodone, oxycodone, tizanidine, and nonsteroidal anti-inflammatory drugs (NSAIDs). Treatments at a pain clinic have included triggerpoint injections, cervical epidural corticosteroid injection, left-sided cervical medial branch blocks, transcutaneous electrical nerve stimulation, and physical therapy. None provided sustained relief.
During a pain clinic visit, Ms. A wept and said she was tired of living with pain. She acknowledged depression and agreed to psychiatric consultation.
As in Ms. A’s case, physicians often refer patients with chronic pain and affective symptoms for psychiatric evaluation. These patients are often fearful, angry, and suspicious of any suggestion that their physical discomfort has a psychiatric component. They typically believe their pain had a clear onset and therefore should have an end point. Many have experienced unproductive specialty evaluations and failed treatments.
To help you overcome these obstacles when treating patients with chronic pain and depression, we discuss:
- strategies to gain patients’ trust and build a therapeutic alliance
- how to assess their pain, depression, and suicide risk
- the role of psychotherapy in treating chronic pain
- and evidence for choosing effective, nonaddicting medications.
Psychiatric evaluation
Depression and pain are linked psychologically and biochemically, sharing neurotransmitters involved in both nociceptive pathways and mood, especially serotonin and norepinephrine.1,2 One-third to one-half of patients with chronic pain report comorbid depression,3 and more than one-half of depressed patients presenting to primary care physicians report only somatic symptoms—various pain complaints among the most common.4,5
Primary care doctors tend to refer chronic pain and depression cases to psychiatrists when:
- patients are preoccupied with medication, have not followed treatment recommendations, or do not respond to treatment as expected
- extensive medical evaluations reveal few or equivocal findings
- somatic complaints are vague and diffuse, or there is marked disparity between pain complaints/disability and objective findings.6,7
Assessing pain. In the initial assessment, validate the patient’s pain experience by asking about the location, quality, and severity of pain. The visual analogue scale (VAS) is commonly used to measure pain severity. The patient marks a spot on a line from “no pain” to “worst possible pain,” or—on a numbered VAS—from 0 (no pain) to 10 (extreme pain). The least and most severe pain over the preceding month can be ranked as baseline values.8
Be sensitive to the patient’s fear that you will attribute the pain to psychosocial issues or imply that “the pain is in your head.” Emphasize that you intend to evaluate the “whole person,” not just the part that hurts. Focus on how the pain affects the patient’s lifestyle—rather than its cause—and explore medication use patterns.
Assessing depression. The word “depression” is emotionally charged for chronic pain patients, who view affective symptoms—if they acknowledge them at all—as secondary to pain. They may strongly resist treatment for anything but pain. One way to defuse this defensiveness is to avoid attributing the pain to stress or depression.
Begin by assessing vegetative symptoms, which overlap in chronic pain and depression. The Beck Depression Inventory-II (Beck-II) may be a useful screening tool in a busy practice; the short form (13 questions) takes about 5 minutes to complete.9
Explore cognitive and behavioral symptoms such as concentration, pleasure and interest level, activity, and self-esteem. Review the chronology of pain onset, mood changes, and stressors (proximate, remote, and cumulative).
Seek clues to endogenous factors by asking about past affective episodes, response to antidepressants, and family history of psychopathology. Substances that may induce depression include reserpine, interferon, and antiparkinsonian agents. Screen for potential organic mood disorders, such as depression secondary to hypothyroidism, corticosteroid use, Parkinson’s disease, lupus, HIV infection, or cerebrovascular disease. Where appropriate, obtain collateral information from family or friends.
Assessing suicide risk. Chronic pain patients may be at greater risk of suicide than the general population. Besides pain, other risk factors for suicide—such as major depression, anxiety disorders, alcohol/substance abuse, sleep disturbances, male gender, diminished social support, and recent loss—are common among these patients.10,11
Screen chronic pain patients with suicidal ideation for these risk factors. Interventions include:
- aggressively treat associated depression, anxiety, or insomnia
- elicit support from family or other caregivers
- pay close attention to talk about suicide
- hospitalize when necessary
- and, of course, treat pain.
Case continued: No stranger to depression
Ms. A’s psychiatric assessments revealed a pain severity ranking of 9 on a 1-to-10 scale, frequent crying, hopelessness, disrupted sleep, low energy, limited ability to concentrate, and fleeting suicidal thoughts. Her history included counseling during her first marriage and severe depression after separation from her second husband 3 years ago. An 8-week trial of fluoxetine, 20 mg/d, did not improve her depression then.
On examination, she displayed obvious pain behavior, constantly shifting her neck position and moving about the room. Her affect was tearful and her mood depressed. She was taking the NSAID celecoxib, 100 mg bid, and the skeletal muscle relaxant tizanidine, 4 mg tid. She was no longer using opioids and had no history of alcohol or illicit drug abuse.
Based on this assessment, the psychiatrist diagnosed Ms. A as having pain disorder with medical and psychological features, including symptom amplification and depression.
Table 1
4 treatment goals for patients with chronic pain and depression
|
Educating the patient
As part of your assessment, explain the reciprocal effects of depression and pain. Acknowledge that:
- chronic pain is different from acute pain, although the patient’s pain experience is the same
- treatment often becomes part of the problem in chronic pain.
Doctors tend to apply acute pain treatments chronically, risking long-term effects of polypharmacy to achieve short-term relief. Depressed patients may be more likely than nondepressed patients to receive opioids for chronic pain,12 and opioids and benzodiazepines may have depressive effects, as reflected by DSM-IV-TR’s inclusion of criteria for “opioid-induced mood disorder” and “sedative-, hypnotic-, or anxiolytic-induced mood disorder.”
To reduce patients’ resistance to antidepressants, reiterate any history of cumulative stressors and affective episodes unrelated to pain. Try using an analogy, such as “stress and pain are like waves on a rock” that eventually damage mood and coping mechanisms, or depression complicating pain is like having “too much on one’s plate.”
Finally, help patients understand that chronic pain is managed, not cured. Encourage them to set treatment goals beyond reducing pain (Table 1) and to make the transition from “patient with pain” to “client managing pain.”
Table 2
Dosing antidepressants and anticonvulsants
for chronic pain and depression
| Drug | Starting (mg/d) | Target (mg/d) | Administration tips |
|---|---|---|---|
| TCAs | Check serum levels for dosages ≥150 mg/d (nortriptyline 100 mg/d) to assess rapid metabolism, adherence, or toxic levels | ||
| Amitriptyline | 10 to 25 | 75 to 300 | |
| Clomipramine | 10 to 25 | 75 to 250 | |
| Desipramine | 10 to 25 | 75 to 200 | |
| Doxepin | 10 to 25 | 75 to 300 | |
| Imipramine | 10 to 25 | 75 to 300 | |
| Nortriptyline | 10 to 25 | 40 to 200 | |
| SNRI | |||
| Venlafaxine | 37.5 to 75 | 75 to 375 | Use XR form to minimize side effects and for once-daily dosing |
| SSRIs | |||
| Citalopram | 10 to 20 | 40 to 60 | |
| Fluoxetine | 10 to 20 | 20 to 80 | May increase carbamazepine, TCA blood levels and inhibit efficacy of codeine, dihydrocodeine, and hydrocodone |
| Paroxetine | 10 to 20 | 20 to 60 | Same as fluoxetine |
| Anticonvulsants | |||
| Carbamazepine | 200 | 800 to 1,200 | Check blood levels; may increase clomipramine levels, reduce acetaminophen, contraceptive levels |
| Clonazepam | 0.5 | 1 to 2 | Habituating potential with chronic use |
| Gabapentin | 300 to 900 | 3,600 to 4,800 | Blood monitoring not necessary |
| Valproate | 250 | 750 to 2,500 (maximum dosage 60 mg/kg/d) | Check blood levels (trough plasma level 50 to 100 μg/mL) |
| TCA: tricyclic antidepressant | |||
| SNRI: serotonin-norepinephrine reuptake inhibitor | |||
| SSRI: selective serotonin reuptake inhibitor | |||
Prescribing principles
Before adding any new pain medications, consider reducing dosages or discontinuing opioids or benzodiazepines and other substances the patient may be taking. Opioid use is associated with risks of dependence, addiction, and side effects including somnolence, cognitive impairment, and reduced activity that amplify depressive symptoms.
Benzodiazepines can generally be tapered by 10% per day, although you may need to extend the final taper over 3 to 4 days or longer, depending upon chronicity of use. Opioids may be tapered by 20% over 5 to 7 days. Breakthrough doses may be needed for marked withdrawal symptoms. Converting to longer half-life agents—such as clonazepam for benzodiazepines or methadone for opioids—often aids tapering, although other agents and strategies exist.13
To gauge patient attempts at self-medication, monitor use of alcohol or illicit drugs with urine screening. For patients with a substantial history of substance abuse or positive toxicology screens, monitor randomly every 2 to 4 weeks.
On the other hand, undertreated pain also may impair mood and function.1 If pain and mood improve and problematic drug-related behaviors resolve with increased opioid analgesia, consider maintaining opioids with regular re-evaluation of mood, coping, and medication adherence.11 Transfer from immediate-release to controlled-release opioids to reduce dosing frequency, clockwatching, and the likelihood of inter-dose pain escalation. In general, maintain and optimize the dosage of nonaddictive analgesics such as NSAIDs, anticonvulsants, or antidepressants.
Case continued: Switching medication
The psychiatrist started Ms. A on nortriptyline, 25 mg at bedtime, to be increased after 3 nights to 50 mg at bedtime. Tizanidine, which had been ineffective, was discontinued to reduce the risk of xerostomia and oversedation in combination with nortriptyline. If tolerated, nortriptyline was to be further increased by 25 mg every 3 days to an initial target dosage of 100 mg at bedtime. The psychiatrist explained to Ms. A that it might take 4 to 6 weeks to gauge the medication’s efficacy.
Psychoeducation addressed the importance of stress reduction, prioritizing commitments, and setting limits on other people’s expectations. The door was left open to future psychotherapeutic exploration of past cumulative stressors.
Because antidepressants may provide an analgesic effect,6,14 they are often used to treat affective symptoms in chronic pain. Headache and neuralgia tend to respond to antidepressants more robustly than do arthritis and low-back pain. Although some patients respond to low-dose antidepressants, a definitive trial requires full doses for 6 to 8 weeks (Table 2).
Matching a patient’s symptoms with medication side effects is useful when choosing antidepressants (Table 3). So-called “adverse” effects may have a corresponding benefit, depending on the clinical presentation. For example, a moreactivating antidepressant—such as the selective serotonin reuptake inhibitor (SSRI) fluoxetine—may help a patient with fatigue, whereas a moresedating agent—such as a tricyclic antidepressant (TCA) or mirtazapine—may improve sleep for a patient with insomnia.
Psychosocial therapies such as cognitive-behavioral therapy (CBT) or relaxation training (Table 4) may help patients with chronic pain to:
- process covert emotions such as fear and anger as well as guilt, loss, and disability
- reduce somatic preoccupation that is aggravating the pain
- adhere to treatment.
Evidence strongly supports using relaxation techniques to reduce chronic pain in many medical conditions and hypnosis to ameliorate cancer pain. CBT and biofeedback appear moderately effective in relieving chronic pain.15 CBT is significantly more effective than waiting list control conditions for relieving chronic nonheadache pain in measures of pain experience, mood/affect, cognitive coping and appraisal, pain behavior and activity level, and social role functioning.16
Pain and opioid medications can impair concentration and affective processing, so initial psychotherapy may need to be supportive while you provide other treatments and simplify medication regimens. Eventually the patient may be ready to address underlying issues that may be contributing to the pain syndrome, such as a history of abuse. However, it is important to address this potentially destabilizing subject only after carefully gauging a patient’s defenses and readiness.
Case continued: A bump in the road
The psychiatrist saw Ms. A 18 months later. Interim history revealed that her pain and mood improved on nortriptyline, 100 mg at bedtime. When she stopped taking nortriptyline 5 months earlier, her neck pain increased and she experienced a “deep blue mood.” Her physician restarted the nortriptyline.
At follow up, Ms. A reported no depressive symptoms and very little neck pain. The psychiatrist discussed with her depression’s relapse rate and the importance of continuing antidepressant therapy. As Ms. A was feeling much better and functioning normally, the psychiatrist decided additional psychotherapeutic intervention was not necessary.
Antidepressant options
TCAs provide analgesia via descending regulatory pathways by inhibiting serotonin and norepinephrine reuptake.17 When using TCAs for chronic pain, start with 10 to 25 mg at bedtime and increase by 10 to 25 mg every 3 to 7 days as tolerated. Increase incrementally until the pain responds or to the full antidepressant dosage (Table 2). Drug levels (when available) can help you provide an appropriate trial and monitor the patient’s adherence.
If the pain does not respond after 6 to 8 weeks, consider switching to another dual-action agent such as venlafaxine or to an SSRI.
SNRIs. Venlafaxine is a serotonin and norepineph-rine reuptake inhibitor (SNRI) with less-troublesome side effects than TCAs. It is structurally similar to tramadol18 and has combined serotonin and norepinephrine inhibition at dosages >75 mg/d. Although venlafaxine is not indicated for chronic pain, some studies have suggested possible benefits, including long-term analgesia, reduced polyneuropathic pain, and migraine prophylaxis.19-21 Venlafaxine may be a reasonable first or second choice for treating depression in patients with chronic pain, especially headache.14
Duloxetine—another SNRI—awaits FDA approval. Some studies have suggested that duloxetine improves painful physical symptoms as well as mood and functioning in major depression.22
SSRIs may be effective for certain types of pain, but the evidence is conflicting. Results of 41controlled trials support TCAs’ analgesic efficacy for neuropathic pain, headache, and central and post-stroke pain, whereas SSRIs’ analgesic efficacy varies from study to study. Comparisons of TCAs and SSRIs as analgesics uniformly show TCAs to be more effective, with the SSRIs often showing no analgesic effect.
Of three controlled trials of SSRIs for diabetic neuropathy, one showed fluoxetine similar to placebo, and two smaller studies showed paroxetine and citalopram more effective than placebo. Fluoxetine has shown analgesic effect for fibromyalgia in one study, but no effect in another. Citalopram showed no analgesic effect for fibromyalgia in another study.23
A prospective, double-blind study comparing fluoxetine, sertraline, paroxetine, and venlafaxine for migraines reported moderate to significant improvement in less than one-half of SSRI-treated patients vs two-thirds of venlafaxine-treated patients.21 SSRIs are no longer recognized by the International Headache Society as primary preventative medications for migraine.
Fluoxetine may help chronic daily headache, and paroxetine and citalopram may be useful for diabetic neuropathy. However, one cannot generalize that all SSRIs are similarly effective as analgesics.14
SSRIs have fewer side effects than TCAs and are less dangerous in overdose. In general, however, SSRIs are a second-line treatment for pain, to be used when dual-action agents pose disadvantageous side effects (Table 3) or have been poorly tolerated or ineffective.
Table 3
Antidepressant side effects:
Limitations and potential benefits in chronic pain
| Side effects/agents | Problems | Conditions potentially benefited | Possible alternatives |
|---|---|---|---|
| Anticholinergic TCAs | Xerostomia, constipation, urinary slowing (esp. when combined with opioids) | Diarrhea-predominant irritable bowel syndrome | SSRIs, nefazodone, venlafaxine |
| Sedation TCAs, mirtazapine, nefazodone, trazodone | Excessive sedation, cognitive impairment, driving risk (esp. when combined with opioids, benzodiazepines) | Pain with insomnia | SSRIs, venlafaxine, bupropion |
| Insomnia SSRIs, venlafaxine | Pain with pre-existing insomnia; equivocal analgesic effects | Excess sedation related to depression, polypharmacy for pain | TCAs, mirtazapine, trazodone, nefazodone |
| Orthostasis TCAs (esp. with methadone), nefazodone | Falls, especially in elderly patients | —— | Nortriptyline, SSRIs bupropion, venlafaxine |
| Weight gain TCAs, mirtazapine | Pain patients are often sedentary, get limited exercise | Pain and depression with weight loss | Bupropion, fluoxetine |
| Hypertension Bupropion, venlafaxine | Pre-existing hypertension | ? Hypotensive state | Citalopram (hypertensive side effects infrequent) |
| Cardiac TCAs | ECG abnormalities, conduction delays, arrhythmias aggravate pre-existing cardiac abnormalities; avoid if recent MI | ——— | SSRIs, bupropion |
| Overdose lethality TCAs | Prominent suicidal ideation | —— | SSRIs, venlafaxine |
| Seizures Esp. maprotiline, clomipramine, bupropion | Lower seizure threshold, aggravation of seizure disorders | ——- | SSRIs |
| Sexual dysfunction SSRIs | Pre-existing sexual dysfunction secondary to pain, medications, stress; equivocal analgesic effects | ——- | Bupropion, nefazodone, mirtazapine |
Table 4
How psychosocial therapies can help treat chronic pain and depression
| Therapies | Purpose/benefits |
|---|---|
| Behavioral therapy | Increase activity and learn to balance activity with limitations Reduce pain behaviors and analgesic use Decrease dependency and secondary gain |
| Cognitive-behavioral therapy | Identify automatic thoughts Challenge negative cognitions, catastrophizing Substitute and rehearse positive thoughts, capabilities Transition from patient role to self-care |
| Couples’ therapy | Assist adaptation to role changes Diminish spousal solicitousness or excessive caretaking Increase communication |
| Biofeedback, relaxation, imagery | Adjunctive role in pain management Reduce tension, comorbid anxiety |
| Hypnosis | Dissociate awareness of pain Substitute, displace, reinterpret pain sensations |
| Vocational rehabilitation | Increase activity, ability to distract Regain sense of control, identity, and productivity Increase socialization |
| Pain management program | Multiple treatment effects Useful for complex pain with affective states |
Monoamine oxidase inhibitors (MAOIs) may have some efficacy for neuropathy and headache, but the need for a tyramine-free diet and potential for drug-drug interactions limit their usefulness. Co-administering an MAOI and meperidine is always contraindicated, as this combination can produce fever, delirium, seizures, circulatory collapse, and death. Similarly, avoid using an MAOI with any other antidepressant.
Others. Evidence is very limited on using other antidepressants such as trazodone, nefazodone, bupropion, and mirtazapine in chronic pain:
- Trazodone may help pediatric migraine, but it is not a consistent analgesic and may not be well-tolerated.
- Case reports suggest bupropion may help with headaches and chronic low-back pain.14
- Mirtazapine and trazodone may be useful adjuncts for treating insomnia in depressed patients with chronic pain.
Other options
Anticonvulsants appear useful for neuropathic pain and are appropriate for chronic pain patients who cannot tolerate TCAs.24 Like TCAs, anticonvulsants are not addictive. Unlike TCAs, anticonvulsants may help stabilize other affective illnesses that may coexist with chronic pain, including bipolar disorder, schizoaffective disorder, and impulsivity/aggression related to dementia or personality disorder.6 If the starting dosage is not effective within 1 week, increase gradually every 2 to 3 days to target dosages comparable to those for anticonvulsant efficacy.
Carbamazepine and gabapentin are recommended first-line medications for neuropathy. Carbamazepine is indicated for treating trigeminal neuralgia, although its cytochrome P-450 3A3/4 isoenzyme induction may reduce serum levels of acetaminophen, opioids, and oral contraceptives. Gabapentin, although not clearly beneficial for bipolar disorder, has anxiolytic effects and a benign side-effect profile, which may help patients with chronic pain.
Valproate can help prevent migraines. Clonazepam can help reduce anxiety and restless legs syndrome but may be habituating. Anticonvulsants’ common adverse effects include sedation, GI upset, dizziness, and fatigue.
Lithium has known efficacy for mood stabilization in bipolar disorder and can ameliorate cluster headaches.
Antipsychotics. Evidence is sparse on whether antipsychotics have analgesic activity. Their side effects generally limit their usefulness to treating pain in patients with psychosis or delirium.6
Stimulants such as dextroamphetamine and methylphenidate can be helpful adjuncts for treating depression, especially for medical inpatients who require a rapid therapeutic response. Stimulants may reduce fatigue or excessive sedation and improve concentration in patients receiving opioids for chronic pain. They also may have analgesic effects when combined with opioids. Potential adverse effects include appetite suppression, anxiety or agitation, confusion, tics, and addiction.6
Precautions. The muscle relaxant carisoprodol is associated with potential dependence and withdrawal. Cyclobenzaprine, another muscle relaxant, has a TCA-like structure and can be lethal in overdose. Baclofen can be useful for chronic pain related to spasticity, although psychotic depression and mania have been reported with abrupt withdrawal.6
Related resources
- American Academy of Pain Medicine. www.painmed.org/
- American Academy of Pain Management. www.aapainmanage.org
- Pain.com: continuing medical education for anesthesiology professionals. www.pain.com/index.cfm
- Kerns RD, Turk DC, Rudy TE. The West Haven-Yale Multidimensional Pain Inventory (WHYMPI). Pain 1985;13:345-56
Drug brand names
- Amitriptyline • Elavil
- Baclofen • Lioresal
- Bupropion • Wellbutrin
- Carbamazepine • Tegretol
- Carisoprodol • Soma
- Celecoxib • Celebrex
- Citalopram • Celexa
- Clomipramine • Anafranil
- Clonazepam • Klonopin
- Cyclobenzaprine • Flexeril
- Desipramine • Norpramin
- Dihydrocodeine • Synalgos-DC
- Doxepin • Sinequan
- Duloxetine • Cymbalta
- Fluoxetine • Prozac
- Gabapentin • Neurontin
- Hydrocodone • Vicodin, Lortab
- Imipramine • Tofranil
- Lithium • Eskalith CR, Lithobid
- Maprotiline • Ludiomil
- Meperidine • Demerol
- Methadone • Dolophine
- Mirtazapine • Remeron
- Nefazodone • Serzone
- Nortriptyline • Pamelor
- Oxycodone • OxyContin
- Paroxetine • Paxil
- Sertraline • Zoloft
- Tizanidine • Zanaflex
- Tramadol • Ultram
- Trazodone • Desyrel
- Valproate • Depakote
- Venlafaxine • Effexor, Effexor XR
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Campbell LC, Clauw DJ, Keefe FJ. Persistent pain and depression: a biopsychosocial perspective. Biol Psychiatry 2003;54:399-409.
2. Fishbain DA, Cutler R, Rosomoff HL, et al. Chronic painassociated depression: antecedent or consequence of chronic pain? A review. Clin J Pain 1997;13(2):116-37.
3. Banks SM, Kerns RD. Explaining high rates of depression in chronic pain: a diathesis-stress framework. Psychol Bull 1996;119:95-110.
4. Simon GE, VonKorff M, Piccinelli M, et al. An international study of the relation between somatic symptoms and depression. N Engl J Med 1999;341(18):1329-35.
5. Kroenke K, Price RK. Symptoms in the community. Prevalence, classification, and psychiatric comorbidity. Arch Intern Med. 1993;153:2474-80.
6. Leo RJ. Concise guide to pain management for psychiatrists. Arlington, Va: American Psychiatric Publishing, Inc., 2003.
7. Sullivan MD, Turner JA, Romano J. Chronic pain in primary care. Identification and management of psychosocial factors. J Fam Pract 1991;32(2):193-9.
8. Holmgren A, Wise MG, Bouckoms AJ. Pain management. In: Wise MG, Rundell JR (eds). Psychiatry in the medically ill (2nd ed). Washington, DC: American Psychiatric Publishing Inc., 2002;989-1013.
9. Naifeh KH. Psychometric testing in functional GI disorders in: Olden K (ed). Handbook of functional GI disorders. New York: Marcel Dekker, 1996;79-126.
10. Fishbain DA. The association of chronic pain and suicide. Semin Clin Neuropsychiatry 1999;4(3):221-7.
11. Fishbain DA. Medico-legal rounds: medical-legal issues and breaches of “standards of medical care” in opioid tapering for alleged opioid addiction. Pain Med 2002;3(2):135-42.
12. Doan BD, Wadden NP. Relationships between depressive symptoms and descriptions of chronic pain. Pain 1989;36:75-84.
13. Franklin JE, Leamon MH, Frances RJ. Substance-related disorders. In: Wise MG, Rundell JR (eds). Psychiatry in the medically ill (2nd ed). Washington DC: American Psychiatric Publishing, 2002;417-53.
14. Ansari A. The efficacy of newer antidepressants in the treatment of chronic pain: a review of current literature. Harv Rev Psychiatry 2000;7(5):257-77.
15. NIH Technology Assessment Panel. Integration of behavioral relaxation approaches into the treatment of chronic pain and insomnia. JAMA 1996;276(4):313-18.
16. Morley S, Eccleston C, Williams A. Systematic review and metaanalysis of randomized controlled trials of cognitive behaviour therapy and behaviour therapy for chronic pain in adults, excluding headache. Pain 1999;80:1-13
17. Magni G. On the relationship between chronic pain and depression when there is no organic lesion. Pain 1987;31:1-21.
18. Markowitz JS, Patrick KS. Venlafaxine-tramadol similarities. Med Hypotheses 1998;5:167-8
19. Bradley RH, Barkin RL, Jerome J, et al. Efficacy of venlafaxine for the long-term treatment of chronic pain with associated major depressive disorder. Am J Ther 2003;10(5):318-23.
20. Sindrup SH, Bach FW, Madsen C, et al. Venlafaxine vs. imipramine in painful polyneuropathy—a randomized controlled trial. Neurology 2003;60:1284-9.
21. Kathpal GS. Role of SSRIs in the management of migraine. Headache Quarterly 1998;9:265-6.
22. Mallinckrodt CH, Goldstein DJ, Detke MJ, et al. Duloxetine: a new treatment for the emotional and physical symptoms of depression. Primary Care Companion J Clin Psychiatry 2003;5(1):19-28.
23. Lynch ME. Antidepressants as analgesics: a review of randomized control trials. J Psychiatry Neurosci 2001;26-36.
24. Swerdlow M. Anticonvulsant drugs and chronic pain. Clin Neuropharmacol 1984;7(1):51-82.
Ms. A, age 44, fell from a 3-foot stool while reaching for a high kitchen shelf and suffered severe neck flexion. Her initial pain persisted for weeks and then months, resulting in chronic neck pain aggravated by movement.
Over the past year, her doctor has prescribed numerous analgesics and muscle relaxants, including tramadol, hydrocodone, oxycodone, tizanidine, and nonsteroidal anti-inflammatory drugs (NSAIDs). Treatments at a pain clinic have included triggerpoint injections, cervical epidural corticosteroid injection, left-sided cervical medial branch blocks, transcutaneous electrical nerve stimulation, and physical therapy. None provided sustained relief.
During a pain clinic visit, Ms. A wept and said she was tired of living with pain. She acknowledged depression and agreed to psychiatric consultation.
As in Ms. A’s case, physicians often refer patients with chronic pain and affective symptoms for psychiatric evaluation. These patients are often fearful, angry, and suspicious of any suggestion that their physical discomfort has a psychiatric component. They typically believe their pain had a clear onset and therefore should have an end point. Many have experienced unproductive specialty evaluations and failed treatments.
To help you overcome these obstacles when treating patients with chronic pain and depression, we discuss:
- strategies to gain patients’ trust and build a therapeutic alliance
- how to assess their pain, depression, and suicide risk
- the role of psychotherapy in treating chronic pain
- and evidence for choosing effective, nonaddicting medications.
Psychiatric evaluation
Depression and pain are linked psychologically and biochemically, sharing neurotransmitters involved in both nociceptive pathways and mood, especially serotonin and norepinephrine.1,2 One-third to one-half of patients with chronic pain report comorbid depression,3 and more than one-half of depressed patients presenting to primary care physicians report only somatic symptoms—various pain complaints among the most common.4,5
Primary care doctors tend to refer chronic pain and depression cases to psychiatrists when:
- patients are preoccupied with medication, have not followed treatment recommendations, or do not respond to treatment as expected
- extensive medical evaluations reveal few or equivocal findings
- somatic complaints are vague and diffuse, or there is marked disparity between pain complaints/disability and objective findings.6,7
Assessing pain. In the initial assessment, validate the patient’s pain experience by asking about the location, quality, and severity of pain. The visual analogue scale (VAS) is commonly used to measure pain severity. The patient marks a spot on a line from “no pain” to “worst possible pain,” or—on a numbered VAS—from 0 (no pain) to 10 (extreme pain). The least and most severe pain over the preceding month can be ranked as baseline values.8
Be sensitive to the patient’s fear that you will attribute the pain to psychosocial issues or imply that “the pain is in your head.” Emphasize that you intend to evaluate the “whole person,” not just the part that hurts. Focus on how the pain affects the patient’s lifestyle—rather than its cause—and explore medication use patterns.
Assessing depression. The word “depression” is emotionally charged for chronic pain patients, who view affective symptoms—if they acknowledge them at all—as secondary to pain. They may strongly resist treatment for anything but pain. One way to defuse this defensiveness is to avoid attributing the pain to stress or depression.
Begin by assessing vegetative symptoms, which overlap in chronic pain and depression. The Beck Depression Inventory-II (Beck-II) may be a useful screening tool in a busy practice; the short form (13 questions) takes about 5 minutes to complete.9
Explore cognitive and behavioral symptoms such as concentration, pleasure and interest level, activity, and self-esteem. Review the chronology of pain onset, mood changes, and stressors (proximate, remote, and cumulative).
Seek clues to endogenous factors by asking about past affective episodes, response to antidepressants, and family history of psychopathology. Substances that may induce depression include reserpine, interferon, and antiparkinsonian agents. Screen for potential organic mood disorders, such as depression secondary to hypothyroidism, corticosteroid use, Parkinson’s disease, lupus, HIV infection, or cerebrovascular disease. Where appropriate, obtain collateral information from family or friends.
Assessing suicide risk. Chronic pain patients may be at greater risk of suicide than the general population. Besides pain, other risk factors for suicide—such as major depression, anxiety disorders, alcohol/substance abuse, sleep disturbances, male gender, diminished social support, and recent loss—are common among these patients.10,11
Screen chronic pain patients with suicidal ideation for these risk factors. Interventions include:
- aggressively treat associated depression, anxiety, or insomnia
- elicit support from family or other caregivers
- pay close attention to talk about suicide
- hospitalize when necessary
- and, of course, treat pain.
Case continued: No stranger to depression
Ms. A’s psychiatric assessments revealed a pain severity ranking of 9 on a 1-to-10 scale, frequent crying, hopelessness, disrupted sleep, low energy, limited ability to concentrate, and fleeting suicidal thoughts. Her history included counseling during her first marriage and severe depression after separation from her second husband 3 years ago. An 8-week trial of fluoxetine, 20 mg/d, did not improve her depression then.
On examination, she displayed obvious pain behavior, constantly shifting her neck position and moving about the room. Her affect was tearful and her mood depressed. She was taking the NSAID celecoxib, 100 mg bid, and the skeletal muscle relaxant tizanidine, 4 mg tid. She was no longer using opioids and had no history of alcohol or illicit drug abuse.
Based on this assessment, the psychiatrist diagnosed Ms. A as having pain disorder with medical and psychological features, including symptom amplification and depression.
Table 1
4 treatment goals for patients with chronic pain and depression
|
Educating the patient
As part of your assessment, explain the reciprocal effects of depression and pain. Acknowledge that:
- chronic pain is different from acute pain, although the patient’s pain experience is the same
- treatment often becomes part of the problem in chronic pain.
Doctors tend to apply acute pain treatments chronically, risking long-term effects of polypharmacy to achieve short-term relief. Depressed patients may be more likely than nondepressed patients to receive opioids for chronic pain,12 and opioids and benzodiazepines may have depressive effects, as reflected by DSM-IV-TR’s inclusion of criteria for “opioid-induced mood disorder” and “sedative-, hypnotic-, or anxiolytic-induced mood disorder.”
To reduce patients’ resistance to antidepressants, reiterate any history of cumulative stressors and affective episodes unrelated to pain. Try using an analogy, such as “stress and pain are like waves on a rock” that eventually damage mood and coping mechanisms, or depression complicating pain is like having “too much on one’s plate.”
Finally, help patients understand that chronic pain is managed, not cured. Encourage them to set treatment goals beyond reducing pain (Table 1) and to make the transition from “patient with pain” to “client managing pain.”
Table 2
Dosing antidepressants and anticonvulsants
for chronic pain and depression
| Drug | Starting (mg/d) | Target (mg/d) | Administration tips |
|---|---|---|---|
| TCAs | Check serum levels for dosages ≥150 mg/d (nortriptyline 100 mg/d) to assess rapid metabolism, adherence, or toxic levels | ||
| Amitriptyline | 10 to 25 | 75 to 300 | |
| Clomipramine | 10 to 25 | 75 to 250 | |
| Desipramine | 10 to 25 | 75 to 200 | |
| Doxepin | 10 to 25 | 75 to 300 | |
| Imipramine | 10 to 25 | 75 to 300 | |
| Nortriptyline | 10 to 25 | 40 to 200 | |
| SNRI | |||
| Venlafaxine | 37.5 to 75 | 75 to 375 | Use XR form to minimize side effects and for once-daily dosing |
| SSRIs | |||
| Citalopram | 10 to 20 | 40 to 60 | |
| Fluoxetine | 10 to 20 | 20 to 80 | May increase carbamazepine, TCA blood levels and inhibit efficacy of codeine, dihydrocodeine, and hydrocodone |
| Paroxetine | 10 to 20 | 20 to 60 | Same as fluoxetine |
| Anticonvulsants | |||
| Carbamazepine | 200 | 800 to 1,200 | Check blood levels; may increase clomipramine levels, reduce acetaminophen, contraceptive levels |
| Clonazepam | 0.5 | 1 to 2 | Habituating potential with chronic use |
| Gabapentin | 300 to 900 | 3,600 to 4,800 | Blood monitoring not necessary |
| Valproate | 250 | 750 to 2,500 (maximum dosage 60 mg/kg/d) | Check blood levels (trough plasma level 50 to 100 μg/mL) |
| TCA: tricyclic antidepressant | |||
| SNRI: serotonin-norepinephrine reuptake inhibitor | |||
| SSRI: selective serotonin reuptake inhibitor | |||
Prescribing principles
Before adding any new pain medications, consider reducing dosages or discontinuing opioids or benzodiazepines and other substances the patient may be taking. Opioid use is associated with risks of dependence, addiction, and side effects including somnolence, cognitive impairment, and reduced activity that amplify depressive symptoms.
Benzodiazepines can generally be tapered by 10% per day, although you may need to extend the final taper over 3 to 4 days or longer, depending upon chronicity of use. Opioids may be tapered by 20% over 5 to 7 days. Breakthrough doses may be needed for marked withdrawal symptoms. Converting to longer half-life agents—such as clonazepam for benzodiazepines or methadone for opioids—often aids tapering, although other agents and strategies exist.13
To gauge patient attempts at self-medication, monitor use of alcohol or illicit drugs with urine screening. For patients with a substantial history of substance abuse or positive toxicology screens, monitor randomly every 2 to 4 weeks.
On the other hand, undertreated pain also may impair mood and function.1 If pain and mood improve and problematic drug-related behaviors resolve with increased opioid analgesia, consider maintaining opioids with regular re-evaluation of mood, coping, and medication adherence.11 Transfer from immediate-release to controlled-release opioids to reduce dosing frequency, clockwatching, and the likelihood of inter-dose pain escalation. In general, maintain and optimize the dosage of nonaddictive analgesics such as NSAIDs, anticonvulsants, or antidepressants.
Case continued: Switching medication
The psychiatrist started Ms. A on nortriptyline, 25 mg at bedtime, to be increased after 3 nights to 50 mg at bedtime. Tizanidine, which had been ineffective, was discontinued to reduce the risk of xerostomia and oversedation in combination with nortriptyline. If tolerated, nortriptyline was to be further increased by 25 mg every 3 days to an initial target dosage of 100 mg at bedtime. The psychiatrist explained to Ms. A that it might take 4 to 6 weeks to gauge the medication’s efficacy.
Psychoeducation addressed the importance of stress reduction, prioritizing commitments, and setting limits on other people’s expectations. The door was left open to future psychotherapeutic exploration of past cumulative stressors.
Because antidepressants may provide an analgesic effect,6,14 they are often used to treat affective symptoms in chronic pain. Headache and neuralgia tend to respond to antidepressants more robustly than do arthritis and low-back pain. Although some patients respond to low-dose antidepressants, a definitive trial requires full doses for 6 to 8 weeks (Table 2).
Matching a patient’s symptoms with medication side effects is useful when choosing antidepressants (Table 3). So-called “adverse” effects may have a corresponding benefit, depending on the clinical presentation. For example, a moreactivating antidepressant—such as the selective serotonin reuptake inhibitor (SSRI) fluoxetine—may help a patient with fatigue, whereas a moresedating agent—such as a tricyclic antidepressant (TCA) or mirtazapine—may improve sleep for a patient with insomnia.
Psychosocial therapies such as cognitive-behavioral therapy (CBT) or relaxation training (Table 4) may help patients with chronic pain to:
- process covert emotions such as fear and anger as well as guilt, loss, and disability
- reduce somatic preoccupation that is aggravating the pain
- adhere to treatment.
Evidence strongly supports using relaxation techniques to reduce chronic pain in many medical conditions and hypnosis to ameliorate cancer pain. CBT and biofeedback appear moderately effective in relieving chronic pain.15 CBT is significantly more effective than waiting list control conditions for relieving chronic nonheadache pain in measures of pain experience, mood/affect, cognitive coping and appraisal, pain behavior and activity level, and social role functioning.16
Pain and opioid medications can impair concentration and affective processing, so initial psychotherapy may need to be supportive while you provide other treatments and simplify medication regimens. Eventually the patient may be ready to address underlying issues that may be contributing to the pain syndrome, such as a history of abuse. However, it is important to address this potentially destabilizing subject only after carefully gauging a patient’s defenses and readiness.
Case continued: A bump in the road
The psychiatrist saw Ms. A 18 months later. Interim history revealed that her pain and mood improved on nortriptyline, 100 mg at bedtime. When she stopped taking nortriptyline 5 months earlier, her neck pain increased and she experienced a “deep blue mood.” Her physician restarted the nortriptyline.
At follow up, Ms. A reported no depressive symptoms and very little neck pain. The psychiatrist discussed with her depression’s relapse rate and the importance of continuing antidepressant therapy. As Ms. A was feeling much better and functioning normally, the psychiatrist decided additional psychotherapeutic intervention was not necessary.
Antidepressant options
TCAs provide analgesia via descending regulatory pathways by inhibiting serotonin and norepinephrine reuptake.17 When using TCAs for chronic pain, start with 10 to 25 mg at bedtime and increase by 10 to 25 mg every 3 to 7 days as tolerated. Increase incrementally until the pain responds or to the full antidepressant dosage (Table 2). Drug levels (when available) can help you provide an appropriate trial and monitor the patient’s adherence.
If the pain does not respond after 6 to 8 weeks, consider switching to another dual-action agent such as venlafaxine or to an SSRI.
SNRIs. Venlafaxine is a serotonin and norepineph-rine reuptake inhibitor (SNRI) with less-troublesome side effects than TCAs. It is structurally similar to tramadol18 and has combined serotonin and norepinephrine inhibition at dosages >75 mg/d. Although venlafaxine is not indicated for chronic pain, some studies have suggested possible benefits, including long-term analgesia, reduced polyneuropathic pain, and migraine prophylaxis.19-21 Venlafaxine may be a reasonable first or second choice for treating depression in patients with chronic pain, especially headache.14
Duloxetine—another SNRI—awaits FDA approval. Some studies have suggested that duloxetine improves painful physical symptoms as well as mood and functioning in major depression.22
SSRIs may be effective for certain types of pain, but the evidence is conflicting. Results of 41controlled trials support TCAs’ analgesic efficacy for neuropathic pain, headache, and central and post-stroke pain, whereas SSRIs’ analgesic efficacy varies from study to study. Comparisons of TCAs and SSRIs as analgesics uniformly show TCAs to be more effective, with the SSRIs often showing no analgesic effect.
Of three controlled trials of SSRIs for diabetic neuropathy, one showed fluoxetine similar to placebo, and two smaller studies showed paroxetine and citalopram more effective than placebo. Fluoxetine has shown analgesic effect for fibromyalgia in one study, but no effect in another. Citalopram showed no analgesic effect for fibromyalgia in another study.23
A prospective, double-blind study comparing fluoxetine, sertraline, paroxetine, and venlafaxine for migraines reported moderate to significant improvement in less than one-half of SSRI-treated patients vs two-thirds of venlafaxine-treated patients.21 SSRIs are no longer recognized by the International Headache Society as primary preventative medications for migraine.
Fluoxetine may help chronic daily headache, and paroxetine and citalopram may be useful for diabetic neuropathy. However, one cannot generalize that all SSRIs are similarly effective as analgesics.14
SSRIs have fewer side effects than TCAs and are less dangerous in overdose. In general, however, SSRIs are a second-line treatment for pain, to be used when dual-action agents pose disadvantageous side effects (Table 3) or have been poorly tolerated or ineffective.
Table 3
Antidepressant side effects:
Limitations and potential benefits in chronic pain
| Side effects/agents | Problems | Conditions potentially benefited | Possible alternatives |
|---|---|---|---|
| Anticholinergic TCAs | Xerostomia, constipation, urinary slowing (esp. when combined with opioids) | Diarrhea-predominant irritable bowel syndrome | SSRIs, nefazodone, venlafaxine |
| Sedation TCAs, mirtazapine, nefazodone, trazodone | Excessive sedation, cognitive impairment, driving risk (esp. when combined with opioids, benzodiazepines) | Pain with insomnia | SSRIs, venlafaxine, bupropion |
| Insomnia SSRIs, venlafaxine | Pain with pre-existing insomnia; equivocal analgesic effects | Excess sedation related to depression, polypharmacy for pain | TCAs, mirtazapine, trazodone, nefazodone |
| Orthostasis TCAs (esp. with methadone), nefazodone | Falls, especially in elderly patients | —— | Nortriptyline, SSRIs bupropion, venlafaxine |
| Weight gain TCAs, mirtazapine | Pain patients are often sedentary, get limited exercise | Pain and depression with weight loss | Bupropion, fluoxetine |
| Hypertension Bupropion, venlafaxine | Pre-existing hypertension | ? Hypotensive state | Citalopram (hypertensive side effects infrequent) |
| Cardiac TCAs | ECG abnormalities, conduction delays, arrhythmias aggravate pre-existing cardiac abnormalities; avoid if recent MI | ——— | SSRIs, bupropion |
| Overdose lethality TCAs | Prominent suicidal ideation | —— | SSRIs, venlafaxine |
| Seizures Esp. maprotiline, clomipramine, bupropion | Lower seizure threshold, aggravation of seizure disorders | ——- | SSRIs |
| Sexual dysfunction SSRIs | Pre-existing sexual dysfunction secondary to pain, medications, stress; equivocal analgesic effects | ——- | Bupropion, nefazodone, mirtazapine |
Table 4
How psychosocial therapies can help treat chronic pain and depression
| Therapies | Purpose/benefits |
|---|---|
| Behavioral therapy | Increase activity and learn to balance activity with limitations Reduce pain behaviors and analgesic use Decrease dependency and secondary gain |
| Cognitive-behavioral therapy | Identify automatic thoughts Challenge negative cognitions, catastrophizing Substitute and rehearse positive thoughts, capabilities Transition from patient role to self-care |
| Couples’ therapy | Assist adaptation to role changes Diminish spousal solicitousness or excessive caretaking Increase communication |
| Biofeedback, relaxation, imagery | Adjunctive role in pain management Reduce tension, comorbid anxiety |
| Hypnosis | Dissociate awareness of pain Substitute, displace, reinterpret pain sensations |
| Vocational rehabilitation | Increase activity, ability to distract Regain sense of control, identity, and productivity Increase socialization |
| Pain management program | Multiple treatment effects Useful for complex pain with affective states |
Monoamine oxidase inhibitors (MAOIs) may have some efficacy for neuropathy and headache, but the need for a tyramine-free diet and potential for drug-drug interactions limit their usefulness. Co-administering an MAOI and meperidine is always contraindicated, as this combination can produce fever, delirium, seizures, circulatory collapse, and death. Similarly, avoid using an MAOI with any other antidepressant.
Others. Evidence is very limited on using other antidepressants such as trazodone, nefazodone, bupropion, and mirtazapine in chronic pain:
- Trazodone may help pediatric migraine, but it is not a consistent analgesic and may not be well-tolerated.
- Case reports suggest bupropion may help with headaches and chronic low-back pain.14
- Mirtazapine and trazodone may be useful adjuncts for treating insomnia in depressed patients with chronic pain.
Other options
Anticonvulsants appear useful for neuropathic pain and are appropriate for chronic pain patients who cannot tolerate TCAs.24 Like TCAs, anticonvulsants are not addictive. Unlike TCAs, anticonvulsants may help stabilize other affective illnesses that may coexist with chronic pain, including bipolar disorder, schizoaffective disorder, and impulsivity/aggression related to dementia or personality disorder.6 If the starting dosage is not effective within 1 week, increase gradually every 2 to 3 days to target dosages comparable to those for anticonvulsant efficacy.
Carbamazepine and gabapentin are recommended first-line medications for neuropathy. Carbamazepine is indicated for treating trigeminal neuralgia, although its cytochrome P-450 3A3/4 isoenzyme induction may reduce serum levels of acetaminophen, opioids, and oral contraceptives. Gabapentin, although not clearly beneficial for bipolar disorder, has anxiolytic effects and a benign side-effect profile, which may help patients with chronic pain.
Valproate can help prevent migraines. Clonazepam can help reduce anxiety and restless legs syndrome but may be habituating. Anticonvulsants’ common adverse effects include sedation, GI upset, dizziness, and fatigue.
Lithium has known efficacy for mood stabilization in bipolar disorder and can ameliorate cluster headaches.
Antipsychotics. Evidence is sparse on whether antipsychotics have analgesic activity. Their side effects generally limit their usefulness to treating pain in patients with psychosis or delirium.6
Stimulants such as dextroamphetamine and methylphenidate can be helpful adjuncts for treating depression, especially for medical inpatients who require a rapid therapeutic response. Stimulants may reduce fatigue or excessive sedation and improve concentration in patients receiving opioids for chronic pain. They also may have analgesic effects when combined with opioids. Potential adverse effects include appetite suppression, anxiety or agitation, confusion, tics, and addiction.6
Precautions. The muscle relaxant carisoprodol is associated with potential dependence and withdrawal. Cyclobenzaprine, another muscle relaxant, has a TCA-like structure and can be lethal in overdose. Baclofen can be useful for chronic pain related to spasticity, although psychotic depression and mania have been reported with abrupt withdrawal.6
Related resources
- American Academy of Pain Medicine. www.painmed.org/
- American Academy of Pain Management. www.aapainmanage.org
- Pain.com: continuing medical education for anesthesiology professionals. www.pain.com/index.cfm
- Kerns RD, Turk DC, Rudy TE. The West Haven-Yale Multidimensional Pain Inventory (WHYMPI). Pain 1985;13:345-56
Drug brand names
- Amitriptyline • Elavil
- Baclofen • Lioresal
- Bupropion • Wellbutrin
- Carbamazepine • Tegretol
- Carisoprodol • Soma
- Celecoxib • Celebrex
- Citalopram • Celexa
- Clomipramine • Anafranil
- Clonazepam • Klonopin
- Cyclobenzaprine • Flexeril
- Desipramine • Norpramin
- Dihydrocodeine • Synalgos-DC
- Doxepin • Sinequan
- Duloxetine • Cymbalta
- Fluoxetine • Prozac
- Gabapentin • Neurontin
- Hydrocodone • Vicodin, Lortab
- Imipramine • Tofranil
- Lithium • Eskalith CR, Lithobid
- Maprotiline • Ludiomil
- Meperidine • Demerol
- Methadone • Dolophine
- Mirtazapine • Remeron
- Nefazodone • Serzone
- Nortriptyline • Pamelor
- Oxycodone • OxyContin
- Paroxetine • Paxil
- Sertraline • Zoloft
- Tizanidine • Zanaflex
- Tramadol • Ultram
- Trazodone • Desyrel
- Valproate • Depakote
- Venlafaxine • Effexor, Effexor XR
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Ms. A, age 44, fell from a 3-foot stool while reaching for a high kitchen shelf and suffered severe neck flexion. Her initial pain persisted for weeks and then months, resulting in chronic neck pain aggravated by movement.
Over the past year, her doctor has prescribed numerous analgesics and muscle relaxants, including tramadol, hydrocodone, oxycodone, tizanidine, and nonsteroidal anti-inflammatory drugs (NSAIDs). Treatments at a pain clinic have included triggerpoint injections, cervical epidural corticosteroid injection, left-sided cervical medial branch blocks, transcutaneous electrical nerve stimulation, and physical therapy. None provided sustained relief.
During a pain clinic visit, Ms. A wept and said she was tired of living with pain. She acknowledged depression and agreed to psychiatric consultation.
As in Ms. A’s case, physicians often refer patients with chronic pain and affective symptoms for psychiatric evaluation. These patients are often fearful, angry, and suspicious of any suggestion that their physical discomfort has a psychiatric component. They typically believe their pain had a clear onset and therefore should have an end point. Many have experienced unproductive specialty evaluations and failed treatments.
To help you overcome these obstacles when treating patients with chronic pain and depression, we discuss:
- strategies to gain patients’ trust and build a therapeutic alliance
- how to assess their pain, depression, and suicide risk
- the role of psychotherapy in treating chronic pain
- and evidence for choosing effective, nonaddicting medications.
Psychiatric evaluation
Depression and pain are linked psychologically and biochemically, sharing neurotransmitters involved in both nociceptive pathways and mood, especially serotonin and norepinephrine.1,2 One-third to one-half of patients with chronic pain report comorbid depression,3 and more than one-half of depressed patients presenting to primary care physicians report only somatic symptoms—various pain complaints among the most common.4,5
Primary care doctors tend to refer chronic pain and depression cases to psychiatrists when:
- patients are preoccupied with medication, have not followed treatment recommendations, or do not respond to treatment as expected
- extensive medical evaluations reveal few or equivocal findings
- somatic complaints are vague and diffuse, or there is marked disparity between pain complaints/disability and objective findings.6,7
Assessing pain. In the initial assessment, validate the patient’s pain experience by asking about the location, quality, and severity of pain. The visual analogue scale (VAS) is commonly used to measure pain severity. The patient marks a spot on a line from “no pain” to “worst possible pain,” or—on a numbered VAS—from 0 (no pain) to 10 (extreme pain). The least and most severe pain over the preceding month can be ranked as baseline values.8
Be sensitive to the patient’s fear that you will attribute the pain to psychosocial issues or imply that “the pain is in your head.” Emphasize that you intend to evaluate the “whole person,” not just the part that hurts. Focus on how the pain affects the patient’s lifestyle—rather than its cause—and explore medication use patterns.
Assessing depression. The word “depression” is emotionally charged for chronic pain patients, who view affective symptoms—if they acknowledge them at all—as secondary to pain. They may strongly resist treatment for anything but pain. One way to defuse this defensiveness is to avoid attributing the pain to stress or depression.
Begin by assessing vegetative symptoms, which overlap in chronic pain and depression. The Beck Depression Inventory-II (Beck-II) may be a useful screening tool in a busy practice; the short form (13 questions) takes about 5 minutes to complete.9
Explore cognitive and behavioral symptoms such as concentration, pleasure and interest level, activity, and self-esteem. Review the chronology of pain onset, mood changes, and stressors (proximate, remote, and cumulative).
Seek clues to endogenous factors by asking about past affective episodes, response to antidepressants, and family history of psychopathology. Substances that may induce depression include reserpine, interferon, and antiparkinsonian agents. Screen for potential organic mood disorders, such as depression secondary to hypothyroidism, corticosteroid use, Parkinson’s disease, lupus, HIV infection, or cerebrovascular disease. Where appropriate, obtain collateral information from family or friends.
Assessing suicide risk. Chronic pain patients may be at greater risk of suicide than the general population. Besides pain, other risk factors for suicide—such as major depression, anxiety disorders, alcohol/substance abuse, sleep disturbances, male gender, diminished social support, and recent loss—are common among these patients.10,11
Screen chronic pain patients with suicidal ideation for these risk factors. Interventions include:
- aggressively treat associated depression, anxiety, or insomnia
- elicit support from family or other caregivers
- pay close attention to talk about suicide
- hospitalize when necessary
- and, of course, treat pain.
Case continued: No stranger to depression
Ms. A’s psychiatric assessments revealed a pain severity ranking of 9 on a 1-to-10 scale, frequent crying, hopelessness, disrupted sleep, low energy, limited ability to concentrate, and fleeting suicidal thoughts. Her history included counseling during her first marriage and severe depression after separation from her second husband 3 years ago. An 8-week trial of fluoxetine, 20 mg/d, did not improve her depression then.
On examination, she displayed obvious pain behavior, constantly shifting her neck position and moving about the room. Her affect was tearful and her mood depressed. She was taking the NSAID celecoxib, 100 mg bid, and the skeletal muscle relaxant tizanidine, 4 mg tid. She was no longer using opioids and had no history of alcohol or illicit drug abuse.
Based on this assessment, the psychiatrist diagnosed Ms. A as having pain disorder with medical and psychological features, including symptom amplification and depression.
Table 1
4 treatment goals for patients with chronic pain and depression
|
Educating the patient
As part of your assessment, explain the reciprocal effects of depression and pain. Acknowledge that:
- chronic pain is different from acute pain, although the patient’s pain experience is the same
- treatment often becomes part of the problem in chronic pain.
Doctors tend to apply acute pain treatments chronically, risking long-term effects of polypharmacy to achieve short-term relief. Depressed patients may be more likely than nondepressed patients to receive opioids for chronic pain,12 and opioids and benzodiazepines may have depressive effects, as reflected by DSM-IV-TR’s inclusion of criteria for “opioid-induced mood disorder” and “sedative-, hypnotic-, or anxiolytic-induced mood disorder.”
To reduce patients’ resistance to antidepressants, reiterate any history of cumulative stressors and affective episodes unrelated to pain. Try using an analogy, such as “stress and pain are like waves on a rock” that eventually damage mood and coping mechanisms, or depression complicating pain is like having “too much on one’s plate.”
Finally, help patients understand that chronic pain is managed, not cured. Encourage them to set treatment goals beyond reducing pain (Table 1) and to make the transition from “patient with pain” to “client managing pain.”
Table 2
Dosing antidepressants and anticonvulsants
for chronic pain and depression
| Drug | Starting (mg/d) | Target (mg/d) | Administration tips |
|---|---|---|---|
| TCAs | Check serum levels for dosages ≥150 mg/d (nortriptyline 100 mg/d) to assess rapid metabolism, adherence, or toxic levels | ||
| Amitriptyline | 10 to 25 | 75 to 300 | |
| Clomipramine | 10 to 25 | 75 to 250 | |
| Desipramine | 10 to 25 | 75 to 200 | |
| Doxepin | 10 to 25 | 75 to 300 | |
| Imipramine | 10 to 25 | 75 to 300 | |
| Nortriptyline | 10 to 25 | 40 to 200 | |
| SNRI | |||
| Venlafaxine | 37.5 to 75 | 75 to 375 | Use XR form to minimize side effects and for once-daily dosing |
| SSRIs | |||
| Citalopram | 10 to 20 | 40 to 60 | |
| Fluoxetine | 10 to 20 | 20 to 80 | May increase carbamazepine, TCA blood levels and inhibit efficacy of codeine, dihydrocodeine, and hydrocodone |
| Paroxetine | 10 to 20 | 20 to 60 | Same as fluoxetine |
| Anticonvulsants | |||
| Carbamazepine | 200 | 800 to 1,200 | Check blood levels; may increase clomipramine levels, reduce acetaminophen, contraceptive levels |
| Clonazepam | 0.5 | 1 to 2 | Habituating potential with chronic use |
| Gabapentin | 300 to 900 | 3,600 to 4,800 | Blood monitoring not necessary |
| Valproate | 250 | 750 to 2,500 (maximum dosage 60 mg/kg/d) | Check blood levels (trough plasma level 50 to 100 μg/mL) |
| TCA: tricyclic antidepressant | |||
| SNRI: serotonin-norepinephrine reuptake inhibitor | |||
| SSRI: selective serotonin reuptake inhibitor | |||
Prescribing principles
Before adding any new pain medications, consider reducing dosages or discontinuing opioids or benzodiazepines and other substances the patient may be taking. Opioid use is associated with risks of dependence, addiction, and side effects including somnolence, cognitive impairment, and reduced activity that amplify depressive symptoms.
Benzodiazepines can generally be tapered by 10% per day, although you may need to extend the final taper over 3 to 4 days or longer, depending upon chronicity of use. Opioids may be tapered by 20% over 5 to 7 days. Breakthrough doses may be needed for marked withdrawal symptoms. Converting to longer half-life agents—such as clonazepam for benzodiazepines or methadone for opioids—often aids tapering, although other agents and strategies exist.13
To gauge patient attempts at self-medication, monitor use of alcohol or illicit drugs with urine screening. For patients with a substantial history of substance abuse or positive toxicology screens, monitor randomly every 2 to 4 weeks.
On the other hand, undertreated pain also may impair mood and function.1 If pain and mood improve and problematic drug-related behaviors resolve with increased opioid analgesia, consider maintaining opioids with regular re-evaluation of mood, coping, and medication adherence.11 Transfer from immediate-release to controlled-release opioids to reduce dosing frequency, clockwatching, and the likelihood of inter-dose pain escalation. In general, maintain and optimize the dosage of nonaddictive analgesics such as NSAIDs, anticonvulsants, or antidepressants.
Case continued: Switching medication
The psychiatrist started Ms. A on nortriptyline, 25 mg at bedtime, to be increased after 3 nights to 50 mg at bedtime. Tizanidine, which had been ineffective, was discontinued to reduce the risk of xerostomia and oversedation in combination with nortriptyline. If tolerated, nortriptyline was to be further increased by 25 mg every 3 days to an initial target dosage of 100 mg at bedtime. The psychiatrist explained to Ms. A that it might take 4 to 6 weeks to gauge the medication’s efficacy.
Psychoeducation addressed the importance of stress reduction, prioritizing commitments, and setting limits on other people’s expectations. The door was left open to future psychotherapeutic exploration of past cumulative stressors.
Because antidepressants may provide an analgesic effect,6,14 they are often used to treat affective symptoms in chronic pain. Headache and neuralgia tend to respond to antidepressants more robustly than do arthritis and low-back pain. Although some patients respond to low-dose antidepressants, a definitive trial requires full doses for 6 to 8 weeks (Table 2).
Matching a patient’s symptoms with medication side effects is useful when choosing antidepressants (Table 3). So-called “adverse” effects may have a corresponding benefit, depending on the clinical presentation. For example, a moreactivating antidepressant—such as the selective serotonin reuptake inhibitor (SSRI) fluoxetine—may help a patient with fatigue, whereas a moresedating agent—such as a tricyclic antidepressant (TCA) or mirtazapine—may improve sleep for a patient with insomnia.
Psychosocial therapies such as cognitive-behavioral therapy (CBT) or relaxation training (Table 4) may help patients with chronic pain to:
- process covert emotions such as fear and anger as well as guilt, loss, and disability
- reduce somatic preoccupation that is aggravating the pain
- adhere to treatment.
Evidence strongly supports using relaxation techniques to reduce chronic pain in many medical conditions and hypnosis to ameliorate cancer pain. CBT and biofeedback appear moderately effective in relieving chronic pain.15 CBT is significantly more effective than waiting list control conditions for relieving chronic nonheadache pain in measures of pain experience, mood/affect, cognitive coping and appraisal, pain behavior and activity level, and social role functioning.16
Pain and opioid medications can impair concentration and affective processing, so initial psychotherapy may need to be supportive while you provide other treatments and simplify medication regimens. Eventually the patient may be ready to address underlying issues that may be contributing to the pain syndrome, such as a history of abuse. However, it is important to address this potentially destabilizing subject only after carefully gauging a patient’s defenses and readiness.
Case continued: A bump in the road
The psychiatrist saw Ms. A 18 months later. Interim history revealed that her pain and mood improved on nortriptyline, 100 mg at bedtime. When she stopped taking nortriptyline 5 months earlier, her neck pain increased and she experienced a “deep blue mood.” Her physician restarted the nortriptyline.
At follow up, Ms. A reported no depressive symptoms and very little neck pain. The psychiatrist discussed with her depression’s relapse rate and the importance of continuing antidepressant therapy. As Ms. A was feeling much better and functioning normally, the psychiatrist decided additional psychotherapeutic intervention was not necessary.
Antidepressant options
TCAs provide analgesia via descending regulatory pathways by inhibiting serotonin and norepinephrine reuptake.17 When using TCAs for chronic pain, start with 10 to 25 mg at bedtime and increase by 10 to 25 mg every 3 to 7 days as tolerated. Increase incrementally until the pain responds or to the full antidepressant dosage (Table 2). Drug levels (when available) can help you provide an appropriate trial and monitor the patient’s adherence.
If the pain does not respond after 6 to 8 weeks, consider switching to another dual-action agent such as venlafaxine or to an SSRI.
SNRIs. Venlafaxine is a serotonin and norepineph-rine reuptake inhibitor (SNRI) with less-troublesome side effects than TCAs. It is structurally similar to tramadol18 and has combined serotonin and norepinephrine inhibition at dosages >75 mg/d. Although venlafaxine is not indicated for chronic pain, some studies have suggested possible benefits, including long-term analgesia, reduced polyneuropathic pain, and migraine prophylaxis.19-21 Venlafaxine may be a reasonable first or second choice for treating depression in patients with chronic pain, especially headache.14
Duloxetine—another SNRI—awaits FDA approval. Some studies have suggested that duloxetine improves painful physical symptoms as well as mood and functioning in major depression.22
SSRIs may be effective for certain types of pain, but the evidence is conflicting. Results of 41controlled trials support TCAs’ analgesic efficacy for neuropathic pain, headache, and central and post-stroke pain, whereas SSRIs’ analgesic efficacy varies from study to study. Comparisons of TCAs and SSRIs as analgesics uniformly show TCAs to be more effective, with the SSRIs often showing no analgesic effect.
Of three controlled trials of SSRIs for diabetic neuropathy, one showed fluoxetine similar to placebo, and two smaller studies showed paroxetine and citalopram more effective than placebo. Fluoxetine has shown analgesic effect for fibromyalgia in one study, but no effect in another. Citalopram showed no analgesic effect for fibromyalgia in another study.23
A prospective, double-blind study comparing fluoxetine, sertraline, paroxetine, and venlafaxine for migraines reported moderate to significant improvement in less than one-half of SSRI-treated patients vs two-thirds of venlafaxine-treated patients.21 SSRIs are no longer recognized by the International Headache Society as primary preventative medications for migraine.
Fluoxetine may help chronic daily headache, and paroxetine and citalopram may be useful for diabetic neuropathy. However, one cannot generalize that all SSRIs are similarly effective as analgesics.14
SSRIs have fewer side effects than TCAs and are less dangerous in overdose. In general, however, SSRIs are a second-line treatment for pain, to be used when dual-action agents pose disadvantageous side effects (Table 3) or have been poorly tolerated or ineffective.
Table 3
Antidepressant side effects:
Limitations and potential benefits in chronic pain
| Side effects/agents | Problems | Conditions potentially benefited | Possible alternatives |
|---|---|---|---|
| Anticholinergic TCAs | Xerostomia, constipation, urinary slowing (esp. when combined with opioids) | Diarrhea-predominant irritable bowel syndrome | SSRIs, nefazodone, venlafaxine |
| Sedation TCAs, mirtazapine, nefazodone, trazodone | Excessive sedation, cognitive impairment, driving risk (esp. when combined with opioids, benzodiazepines) | Pain with insomnia | SSRIs, venlafaxine, bupropion |
| Insomnia SSRIs, venlafaxine | Pain with pre-existing insomnia; equivocal analgesic effects | Excess sedation related to depression, polypharmacy for pain | TCAs, mirtazapine, trazodone, nefazodone |
| Orthostasis TCAs (esp. with methadone), nefazodone | Falls, especially in elderly patients | —— | Nortriptyline, SSRIs bupropion, venlafaxine |
| Weight gain TCAs, mirtazapine | Pain patients are often sedentary, get limited exercise | Pain and depression with weight loss | Bupropion, fluoxetine |
| Hypertension Bupropion, venlafaxine | Pre-existing hypertension | ? Hypotensive state | Citalopram (hypertensive side effects infrequent) |
| Cardiac TCAs | ECG abnormalities, conduction delays, arrhythmias aggravate pre-existing cardiac abnormalities; avoid if recent MI | ——— | SSRIs, bupropion |
| Overdose lethality TCAs | Prominent suicidal ideation | —— | SSRIs, venlafaxine |
| Seizures Esp. maprotiline, clomipramine, bupropion | Lower seizure threshold, aggravation of seizure disorders | ——- | SSRIs |
| Sexual dysfunction SSRIs | Pre-existing sexual dysfunction secondary to pain, medications, stress; equivocal analgesic effects | ——- | Bupropion, nefazodone, mirtazapine |
Table 4
How psychosocial therapies can help treat chronic pain and depression
| Therapies | Purpose/benefits |
|---|---|
| Behavioral therapy | Increase activity and learn to balance activity with limitations Reduce pain behaviors and analgesic use Decrease dependency and secondary gain |
| Cognitive-behavioral therapy | Identify automatic thoughts Challenge negative cognitions, catastrophizing Substitute and rehearse positive thoughts, capabilities Transition from patient role to self-care |
| Couples’ therapy | Assist adaptation to role changes Diminish spousal solicitousness or excessive caretaking Increase communication |
| Biofeedback, relaxation, imagery | Adjunctive role in pain management Reduce tension, comorbid anxiety |
| Hypnosis | Dissociate awareness of pain Substitute, displace, reinterpret pain sensations |
| Vocational rehabilitation | Increase activity, ability to distract Regain sense of control, identity, and productivity Increase socialization |
| Pain management program | Multiple treatment effects Useful for complex pain with affective states |
Monoamine oxidase inhibitors (MAOIs) may have some efficacy for neuropathy and headache, but the need for a tyramine-free diet and potential for drug-drug interactions limit their usefulness. Co-administering an MAOI and meperidine is always contraindicated, as this combination can produce fever, delirium, seizures, circulatory collapse, and death. Similarly, avoid using an MAOI with any other antidepressant.
Others. Evidence is very limited on using other antidepressants such as trazodone, nefazodone, bupropion, and mirtazapine in chronic pain:
- Trazodone may help pediatric migraine, but it is not a consistent analgesic and may not be well-tolerated.
- Case reports suggest bupropion may help with headaches and chronic low-back pain.14
- Mirtazapine and trazodone may be useful adjuncts for treating insomnia in depressed patients with chronic pain.
Other options
Anticonvulsants appear useful for neuropathic pain and are appropriate for chronic pain patients who cannot tolerate TCAs.24 Like TCAs, anticonvulsants are not addictive. Unlike TCAs, anticonvulsants may help stabilize other affective illnesses that may coexist with chronic pain, including bipolar disorder, schizoaffective disorder, and impulsivity/aggression related to dementia or personality disorder.6 If the starting dosage is not effective within 1 week, increase gradually every 2 to 3 days to target dosages comparable to those for anticonvulsant efficacy.
Carbamazepine and gabapentin are recommended first-line medications for neuropathy. Carbamazepine is indicated for treating trigeminal neuralgia, although its cytochrome P-450 3A3/4 isoenzyme induction may reduce serum levels of acetaminophen, opioids, and oral contraceptives. Gabapentin, although not clearly beneficial for bipolar disorder, has anxiolytic effects and a benign side-effect profile, which may help patients with chronic pain.
Valproate can help prevent migraines. Clonazepam can help reduce anxiety and restless legs syndrome but may be habituating. Anticonvulsants’ common adverse effects include sedation, GI upset, dizziness, and fatigue.
Lithium has known efficacy for mood stabilization in bipolar disorder and can ameliorate cluster headaches.
Antipsychotics. Evidence is sparse on whether antipsychotics have analgesic activity. Their side effects generally limit their usefulness to treating pain in patients with psychosis or delirium.6
Stimulants such as dextroamphetamine and methylphenidate can be helpful adjuncts for treating depression, especially for medical inpatients who require a rapid therapeutic response. Stimulants may reduce fatigue or excessive sedation and improve concentration in patients receiving opioids for chronic pain. They also may have analgesic effects when combined with opioids. Potential adverse effects include appetite suppression, anxiety or agitation, confusion, tics, and addiction.6
Precautions. The muscle relaxant carisoprodol is associated with potential dependence and withdrawal. Cyclobenzaprine, another muscle relaxant, has a TCA-like structure and can be lethal in overdose. Baclofen can be useful for chronic pain related to spasticity, although psychotic depression and mania have been reported with abrupt withdrawal.6
Related resources
- American Academy of Pain Medicine. www.painmed.org/
- American Academy of Pain Management. www.aapainmanage.org
- Pain.com: continuing medical education for anesthesiology professionals. www.pain.com/index.cfm
- Kerns RD, Turk DC, Rudy TE. The West Haven-Yale Multidimensional Pain Inventory (WHYMPI). Pain 1985;13:345-56
Drug brand names
- Amitriptyline • Elavil
- Baclofen • Lioresal
- Bupropion • Wellbutrin
- Carbamazepine • Tegretol
- Carisoprodol • Soma
- Celecoxib • Celebrex
- Citalopram • Celexa
- Clomipramine • Anafranil
- Clonazepam • Klonopin
- Cyclobenzaprine • Flexeril
- Desipramine • Norpramin
- Dihydrocodeine • Synalgos-DC
- Doxepin • Sinequan
- Duloxetine • Cymbalta
- Fluoxetine • Prozac
- Gabapentin • Neurontin
- Hydrocodone • Vicodin, Lortab
- Imipramine • Tofranil
- Lithium • Eskalith CR, Lithobid
- Maprotiline • Ludiomil
- Meperidine • Demerol
- Methadone • Dolophine
- Mirtazapine • Remeron
- Nefazodone • Serzone
- Nortriptyline • Pamelor
- Oxycodone • OxyContin
- Paroxetine • Paxil
- Sertraline • Zoloft
- Tizanidine • Zanaflex
- Tramadol • Ultram
- Trazodone • Desyrel
- Valproate • Depakote
- Venlafaxine • Effexor, Effexor XR
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Campbell LC, Clauw DJ, Keefe FJ. Persistent pain and depression: a biopsychosocial perspective. Biol Psychiatry 2003;54:399-409.
2. Fishbain DA, Cutler R, Rosomoff HL, et al. Chronic painassociated depression: antecedent or consequence of chronic pain? A review. Clin J Pain 1997;13(2):116-37.
3. Banks SM, Kerns RD. Explaining high rates of depression in chronic pain: a diathesis-stress framework. Psychol Bull 1996;119:95-110.
4. Simon GE, VonKorff M, Piccinelli M, et al. An international study of the relation between somatic symptoms and depression. N Engl J Med 1999;341(18):1329-35.
5. Kroenke K, Price RK. Symptoms in the community. Prevalence, classification, and psychiatric comorbidity. Arch Intern Med. 1993;153:2474-80.
6. Leo RJ. Concise guide to pain management for psychiatrists. Arlington, Va: American Psychiatric Publishing, Inc., 2003.
7. Sullivan MD, Turner JA, Romano J. Chronic pain in primary care. Identification and management of psychosocial factors. J Fam Pract 1991;32(2):193-9.
8. Holmgren A, Wise MG, Bouckoms AJ. Pain management. In: Wise MG, Rundell JR (eds). Psychiatry in the medically ill (2nd ed). Washington, DC: American Psychiatric Publishing Inc., 2002;989-1013.
9. Naifeh KH. Psychometric testing in functional GI disorders in: Olden K (ed). Handbook of functional GI disorders. New York: Marcel Dekker, 1996;79-126.
10. Fishbain DA. The association of chronic pain and suicide. Semin Clin Neuropsychiatry 1999;4(3):221-7.
11. Fishbain DA. Medico-legal rounds: medical-legal issues and breaches of “standards of medical care” in opioid tapering for alleged opioid addiction. Pain Med 2002;3(2):135-42.
12. Doan BD, Wadden NP. Relationships between depressive symptoms and descriptions of chronic pain. Pain 1989;36:75-84.
13. Franklin JE, Leamon MH, Frances RJ. Substance-related disorders. In: Wise MG, Rundell JR (eds). Psychiatry in the medically ill (2nd ed). Washington DC: American Psychiatric Publishing, 2002;417-53.
14. Ansari A. The efficacy of newer antidepressants in the treatment of chronic pain: a review of current literature. Harv Rev Psychiatry 2000;7(5):257-77.
15. NIH Technology Assessment Panel. Integration of behavioral relaxation approaches into the treatment of chronic pain and insomnia. JAMA 1996;276(4):313-18.
16. Morley S, Eccleston C, Williams A. Systematic review and metaanalysis of randomized controlled trials of cognitive behaviour therapy and behaviour therapy for chronic pain in adults, excluding headache. Pain 1999;80:1-13
17. Magni G. On the relationship between chronic pain and depression when there is no organic lesion. Pain 1987;31:1-21.
18. Markowitz JS, Patrick KS. Venlafaxine-tramadol similarities. Med Hypotheses 1998;5:167-8
19. Bradley RH, Barkin RL, Jerome J, et al. Efficacy of venlafaxine for the long-term treatment of chronic pain with associated major depressive disorder. Am J Ther 2003;10(5):318-23.
20. Sindrup SH, Bach FW, Madsen C, et al. Venlafaxine vs. imipramine in painful polyneuropathy—a randomized controlled trial. Neurology 2003;60:1284-9.
21. Kathpal GS. Role of SSRIs in the management of migraine. Headache Quarterly 1998;9:265-6.
22. Mallinckrodt CH, Goldstein DJ, Detke MJ, et al. Duloxetine: a new treatment for the emotional and physical symptoms of depression. Primary Care Companion J Clin Psychiatry 2003;5(1):19-28.
23. Lynch ME. Antidepressants as analgesics: a review of randomized control trials. J Psychiatry Neurosci 2001;26-36.
24. Swerdlow M. Anticonvulsant drugs and chronic pain. Clin Neuropharmacol 1984;7(1):51-82.
1. Campbell LC, Clauw DJ, Keefe FJ. Persistent pain and depression: a biopsychosocial perspective. Biol Psychiatry 2003;54:399-409.
2. Fishbain DA, Cutler R, Rosomoff HL, et al. Chronic painassociated depression: antecedent or consequence of chronic pain? A review. Clin J Pain 1997;13(2):116-37.
3. Banks SM, Kerns RD. Explaining high rates of depression in chronic pain: a diathesis-stress framework. Psychol Bull 1996;119:95-110.
4. Simon GE, VonKorff M, Piccinelli M, et al. An international study of the relation between somatic symptoms and depression. N Engl J Med 1999;341(18):1329-35.
5. Kroenke K, Price RK. Symptoms in the community. Prevalence, classification, and psychiatric comorbidity. Arch Intern Med. 1993;153:2474-80.
6. Leo RJ. Concise guide to pain management for psychiatrists. Arlington, Va: American Psychiatric Publishing, Inc., 2003.
7. Sullivan MD, Turner JA, Romano J. Chronic pain in primary care. Identification and management of psychosocial factors. J Fam Pract 1991;32(2):193-9.
8. Holmgren A, Wise MG, Bouckoms AJ. Pain management. In: Wise MG, Rundell JR (eds). Psychiatry in the medically ill (2nd ed). Washington, DC: American Psychiatric Publishing Inc., 2002;989-1013.
9. Naifeh KH. Psychometric testing in functional GI disorders in: Olden K (ed). Handbook of functional GI disorders. New York: Marcel Dekker, 1996;79-126.
10. Fishbain DA. The association of chronic pain and suicide. Semin Clin Neuropsychiatry 1999;4(3):221-7.
11. Fishbain DA. Medico-legal rounds: medical-legal issues and breaches of “standards of medical care” in opioid tapering for alleged opioid addiction. Pain Med 2002;3(2):135-42.
12. Doan BD, Wadden NP. Relationships between depressive symptoms and descriptions of chronic pain. Pain 1989;36:75-84.
13. Franklin JE, Leamon MH, Frances RJ. Substance-related disorders. In: Wise MG, Rundell JR (eds). Psychiatry in the medically ill (2nd ed). Washington DC: American Psychiatric Publishing, 2002;417-53.
14. Ansari A. The efficacy of newer antidepressants in the treatment of chronic pain: a review of current literature. Harv Rev Psychiatry 2000;7(5):257-77.
15. NIH Technology Assessment Panel. Integration of behavioral relaxation approaches into the treatment of chronic pain and insomnia. JAMA 1996;276(4):313-18.
16. Morley S, Eccleston C, Williams A. Systematic review and metaanalysis of randomized controlled trials of cognitive behaviour therapy and behaviour therapy for chronic pain in adults, excluding headache. Pain 1999;80:1-13
17. Magni G. On the relationship between chronic pain and depression when there is no organic lesion. Pain 1987;31:1-21.
18. Markowitz JS, Patrick KS. Venlafaxine-tramadol similarities. Med Hypotheses 1998;5:167-8
19. Bradley RH, Barkin RL, Jerome J, et al. Efficacy of venlafaxine for the long-term treatment of chronic pain with associated major depressive disorder. Am J Ther 2003;10(5):318-23.
20. Sindrup SH, Bach FW, Madsen C, et al. Venlafaxine vs. imipramine in painful polyneuropathy—a randomized controlled trial. Neurology 2003;60:1284-9.
21. Kathpal GS. Role of SSRIs in the management of migraine. Headache Quarterly 1998;9:265-6.
22. Mallinckrodt CH, Goldstein DJ, Detke MJ, et al. Duloxetine: a new treatment for the emotional and physical symptoms of depression. Primary Care Companion J Clin Psychiatry 2003;5(1):19-28.
23. Lynch ME. Antidepressants as analgesics: a review of randomized control trials. J Psychiatry Neurosci 2001;26-36.
24. Swerdlow M. Anticonvulsant drugs and chronic pain. Clin Neuropharmacol 1984;7(1):51-82.
Sodium oxybate: A new way to treat narcolepsy
Existing drug treatments for narcolepsy enhance daytime alertness, and most improve cataplexy, sleep paralysis, and hypnagogic/hypnopompic hallucinations. None of these agents, however, target the nocturnal sleep deficits that lead to daytime symptoms.
Sodium oxybate, one of the most controversial medications to receive FDA approval in recent years (Table 1), has been found to reduce daytime sleepiness and cataplexy by improving nighttime sleep in patients with narcolepsy.
ABOUT SODIUM OXYBATE
Sodium oxybate is also known as gamma-hydroxybutyrate (GHB). An illegal form of GHB—the so-called “date rape drug”—is produced and used illicitly, typically at parties and nightclubs. Some users hide the fast-acting, sedating drug in a cocktail, rendering victims unable to defend against an assault or to recall details leading to the assault.1
Some athletes believe GHB enhances on-field performance by increasing production of growth hormone. Enhanced growth hormone release has no known clinical significance or effect on athletic performance, however.
Table 1
Sodium oxybate: Fast facts
| Drug brand name: Xyrem |
| Class: CNS depressant |
| FDA-approved indications: Treatment of cataplexy |
| Approval date: July 17, 2002 |
| Manufacturer: Orphan Medical |
| Dosing forms: 180 mL oral solution at a concentration of 0.5 grams/mL |
| Recommended dosage: Start at 2.25 grams at bedtime; repeat dose overnight (4.5 grams/d total). Dosage can be increased to 9 grams/d (4.5 grams per dose) by increments of 0.75 grams per dose every 2 weeks. A dropper is supplied to facilitate measurement. |
The U.S. Drug Enforcement Agency (DEA) considers GHB a Schedule 1 (illegal) drug. DEA considers the prescription version a Schedule 3 drug, meaning it can be prescribed with refills as long as a DEA number is listed on the prescription. To prevent misuse, a central pharmacy dispenses sodium oxybate and mandates use of a specific prescription form to verify the physician’s familiarity with the medication. Psychiatrists can call (866) 997-3688 to obtain the form.
Table 2
Sodium oxybate dosing recommendations for patients
|
Sodium oxybate is the only agent FDA-approved for treating cataplexy—muscle weakness common among patients with narcolepsy.
HOW IT WORKS
Developed as an anesthetic, sodium oxybate induces deep sleep and at higher doses causes amnesia.
Derived from gamma-aminobutyric acid (GABA), sodium oxybate’s mechanism of action is unknown. Some believe it binds to the GABA B receptor and partially inhibits the NMDA and AMPA receptor-mediated excitatory neurons in the hippocampus.2
Food alters its bioavailability, so sodium oxybate should be taken several hours after meals to prevent delays in absorption and effect. Patients taking it should not eat at bedtime.
The agent’s pharmacokinetics are nonlinear, meaning that if the dose is doubled, the medication effect is tripled or quadrupled. For this reason, dosage increases must be small (no more than 0.75 grams for each dose) and gradual (at intervals of at least 2 weeks). The medication reaches peak plasma concentration within 30 to 75 minutes, so patients should not take the medication until they are in bed. Its 1-hour half-life explains its brief duration of action and need for repeat dosing overnight (Table 2).
Sodium oxybate does not modify the activity of any cytochrome P-450 enzymes. The medication is high in sodium (0.5 grams in a 3-gram dose) and has a salty taste. Use caution when considering the agent for patients with hypertension or on low-sodium diets.
Sodium oxybate’s safety has not been adequately tested in patients younger than 18 or older than 65 or in those with dementia and other disease processes. Because the drug is metabolized by the liver, the manufacturer recommends prescribing one-half the starting dosage to patients with significant hepatic impairment.
EFFICACY
Sodium oxybate has been shown to indirectly reduce frequency of cataplexy by improving nocturnal sleep:
- In a placebo-controlled, 4-week trial, 136 patients received either placebo or sodium oxybate at bedtime and again overnight in two equally divided doses of 3, 6, or 9 grams each. Patients who received the medication experienced less-frequent cataplexy, reduced daytime sleepiness, and fewer unplanned daytime naps and nocturnal awakenings.3
- A placebo-controlled trial that followed 55 patients for more than 3 years demonstrated long-term efficacy based on the patients’ cataplexy diaries (mean duration of treatment 21 months). Cataplexy returned after abrupt discontinuation.4
Unlike patients with most other disorders, those with narcolepsy generally are willing to repeat a medication overnight. They awaken easily at night—often without an alarm. Patients taking the medication report that they fall asleep again more readily and experience dramatically improved sleep quality and duration.
TOLERABILITY
Sodium oxybate has been well tolerated in relatively small clinical trials.
In the 4-week, placebo-controlled trial,3 nausea, headache, dizziness, and enuresis were most frequently reported. Out of 136 participants, 1 withdrew because of acute confusion and 9 others left because of mild to moderate adverse events. Twelve others experienced one episode of enuresis—probably because they did not fully awaken from deep sleep when developing urinary urgency. Advise patients taking sodium oxybate to urinate before going to bed.
The medication’s propensity to increase slow-wave sleep may cause sleepwalking. Sleepwalking was reported in 32% of patients in one long-term, uncontrolled study.5 If a patient with a history of sleepwalking needs sodium oxybate, advise against sleeping in upper bunks and other dangerous settings, and recommend precautions such as locking doors.
Because of sodium oxybate’s sedating properties, concomitant use of alcohol, barbiturates, and benzodiazepines should be discouraged.
ABUSE POTENTIAL
As discussed, GHB has a high abuse potential with effects such as euphoria, relaxation, and heightened sexual feelings.
Tolerance and dependence has not been reported with sodium oxybate when used as prescribed. A withdrawal state—similar to alcohol and sedative/hypnotic withdrawal and marked by anxiety, tremor, agitation, and delirium—has been reported with GHB abuse (although other chemicals often are used simultaneously in such cases). Narcolepsy patients in clinical trials have abruptly discontinued sodium oxybate after months of use without significant withdrawal.4
Related resources
- Narcolepsy Network Inc. www.narcolepsynetwork.org
Disclosure
Dr. Krahn reports no financial relationship with Orphan Medical or with manufacturers of competing products.
1. Galloway GP, Frederick SL, Staggers FE, Jr, et al. Gamma-hydroxybutyrate: an emerging drug of abuse that causes physical dependence. Addiction 1997;92(1):89-96.
2. Cammalleri M, Brancucci A, Berton F, et al. Gamma-hydroxybutyrate reduces GABA(A)-mediated inhibitory postsynaptic potentials in the CA1 region of hippocampus. Neuropsychopharmacology. 2002;27(9):960-9.
3. U.S. Xyrem Multicenter Study Group. A randomized, double blind, placebo-controlled multicenter trial comparing the effects of three doses of orally administered sodium oxybate with placebo for the treatment of narcolepsy. Sleep 2002;25(1):42-9.
4. U.S. Xyrem Multi-Center Study Group. The abrupt cessation of therapeutically administered sodium oxybate (GHB) does not cause withdrawal symptoms. J Toxicol Clin Toxicol 2003;41:131-5.
5. Physicians’ Desk Reference (57th ed). Montvale, NJ: Thomson Healthcare, 2003.
6. Mitler MM, Hayduk R. Benefits and risks of pharmacotherapy for narcolepsy. Drug Saf. 2002;25(11):791-809.
Existing drug treatments for narcolepsy enhance daytime alertness, and most improve cataplexy, sleep paralysis, and hypnagogic/hypnopompic hallucinations. None of these agents, however, target the nocturnal sleep deficits that lead to daytime symptoms.
Sodium oxybate, one of the most controversial medications to receive FDA approval in recent years (Table 1), has been found to reduce daytime sleepiness and cataplexy by improving nighttime sleep in patients with narcolepsy.
ABOUT SODIUM OXYBATE
Sodium oxybate is also known as gamma-hydroxybutyrate (GHB). An illegal form of GHB—the so-called “date rape drug”—is produced and used illicitly, typically at parties and nightclubs. Some users hide the fast-acting, sedating drug in a cocktail, rendering victims unable to defend against an assault or to recall details leading to the assault.1
Some athletes believe GHB enhances on-field performance by increasing production of growth hormone. Enhanced growth hormone release has no known clinical significance or effect on athletic performance, however.
Table 1
Sodium oxybate: Fast facts
| Drug brand name: Xyrem |
| Class: CNS depressant |
| FDA-approved indications: Treatment of cataplexy |
| Approval date: July 17, 2002 |
| Manufacturer: Orphan Medical |
| Dosing forms: 180 mL oral solution at a concentration of 0.5 grams/mL |
| Recommended dosage: Start at 2.25 grams at bedtime; repeat dose overnight (4.5 grams/d total). Dosage can be increased to 9 grams/d (4.5 grams per dose) by increments of 0.75 grams per dose every 2 weeks. A dropper is supplied to facilitate measurement. |
The U.S. Drug Enforcement Agency (DEA) considers GHB a Schedule 1 (illegal) drug. DEA considers the prescription version a Schedule 3 drug, meaning it can be prescribed with refills as long as a DEA number is listed on the prescription. To prevent misuse, a central pharmacy dispenses sodium oxybate and mandates use of a specific prescription form to verify the physician’s familiarity with the medication. Psychiatrists can call (866) 997-3688 to obtain the form.
Table 2
Sodium oxybate dosing recommendations for patients
|
Sodium oxybate is the only agent FDA-approved for treating cataplexy—muscle weakness common among patients with narcolepsy.
HOW IT WORKS
Developed as an anesthetic, sodium oxybate induces deep sleep and at higher doses causes amnesia.
Derived from gamma-aminobutyric acid (GABA), sodium oxybate’s mechanism of action is unknown. Some believe it binds to the GABA B receptor and partially inhibits the NMDA and AMPA receptor-mediated excitatory neurons in the hippocampus.2
Food alters its bioavailability, so sodium oxybate should be taken several hours after meals to prevent delays in absorption and effect. Patients taking it should not eat at bedtime.
The agent’s pharmacokinetics are nonlinear, meaning that if the dose is doubled, the medication effect is tripled or quadrupled. For this reason, dosage increases must be small (no more than 0.75 grams for each dose) and gradual (at intervals of at least 2 weeks). The medication reaches peak plasma concentration within 30 to 75 minutes, so patients should not take the medication until they are in bed. Its 1-hour half-life explains its brief duration of action and need for repeat dosing overnight (Table 2).
Sodium oxybate does not modify the activity of any cytochrome P-450 enzymes. The medication is high in sodium (0.5 grams in a 3-gram dose) and has a salty taste. Use caution when considering the agent for patients with hypertension or on low-sodium diets.
Sodium oxybate’s safety has not been adequately tested in patients younger than 18 or older than 65 or in those with dementia and other disease processes. Because the drug is metabolized by the liver, the manufacturer recommends prescribing one-half the starting dosage to patients with significant hepatic impairment.
EFFICACY
Sodium oxybate has been shown to indirectly reduce frequency of cataplexy by improving nocturnal sleep:
- In a placebo-controlled, 4-week trial, 136 patients received either placebo or sodium oxybate at bedtime and again overnight in two equally divided doses of 3, 6, or 9 grams each. Patients who received the medication experienced less-frequent cataplexy, reduced daytime sleepiness, and fewer unplanned daytime naps and nocturnal awakenings.3
- A placebo-controlled trial that followed 55 patients for more than 3 years demonstrated long-term efficacy based on the patients’ cataplexy diaries (mean duration of treatment 21 months). Cataplexy returned after abrupt discontinuation.4
Unlike patients with most other disorders, those with narcolepsy generally are willing to repeat a medication overnight. They awaken easily at night—often without an alarm. Patients taking the medication report that they fall asleep again more readily and experience dramatically improved sleep quality and duration.
TOLERABILITY
Sodium oxybate has been well tolerated in relatively small clinical trials.
In the 4-week, placebo-controlled trial,3 nausea, headache, dizziness, and enuresis were most frequently reported. Out of 136 participants, 1 withdrew because of acute confusion and 9 others left because of mild to moderate adverse events. Twelve others experienced one episode of enuresis—probably because they did not fully awaken from deep sleep when developing urinary urgency. Advise patients taking sodium oxybate to urinate before going to bed.
The medication’s propensity to increase slow-wave sleep may cause sleepwalking. Sleepwalking was reported in 32% of patients in one long-term, uncontrolled study.5 If a patient with a history of sleepwalking needs sodium oxybate, advise against sleeping in upper bunks and other dangerous settings, and recommend precautions such as locking doors.
Because of sodium oxybate’s sedating properties, concomitant use of alcohol, barbiturates, and benzodiazepines should be discouraged.
ABUSE POTENTIAL
As discussed, GHB has a high abuse potential with effects such as euphoria, relaxation, and heightened sexual feelings.
Tolerance and dependence has not been reported with sodium oxybate when used as prescribed. A withdrawal state—similar to alcohol and sedative/hypnotic withdrawal and marked by anxiety, tremor, agitation, and delirium—has been reported with GHB abuse (although other chemicals often are used simultaneously in such cases). Narcolepsy patients in clinical trials have abruptly discontinued sodium oxybate after months of use without significant withdrawal.4
Related resources
- Narcolepsy Network Inc. www.narcolepsynetwork.org
Disclosure
Dr. Krahn reports no financial relationship with Orphan Medical or with manufacturers of competing products.
Existing drug treatments for narcolepsy enhance daytime alertness, and most improve cataplexy, sleep paralysis, and hypnagogic/hypnopompic hallucinations. None of these agents, however, target the nocturnal sleep deficits that lead to daytime symptoms.
Sodium oxybate, one of the most controversial medications to receive FDA approval in recent years (Table 1), has been found to reduce daytime sleepiness and cataplexy by improving nighttime sleep in patients with narcolepsy.
ABOUT SODIUM OXYBATE
Sodium oxybate is also known as gamma-hydroxybutyrate (GHB). An illegal form of GHB—the so-called “date rape drug”—is produced and used illicitly, typically at parties and nightclubs. Some users hide the fast-acting, sedating drug in a cocktail, rendering victims unable to defend against an assault or to recall details leading to the assault.1
Some athletes believe GHB enhances on-field performance by increasing production of growth hormone. Enhanced growth hormone release has no known clinical significance or effect on athletic performance, however.
Table 1
Sodium oxybate: Fast facts
| Drug brand name: Xyrem |
| Class: CNS depressant |
| FDA-approved indications: Treatment of cataplexy |
| Approval date: July 17, 2002 |
| Manufacturer: Orphan Medical |
| Dosing forms: 180 mL oral solution at a concentration of 0.5 grams/mL |
| Recommended dosage: Start at 2.25 grams at bedtime; repeat dose overnight (4.5 grams/d total). Dosage can be increased to 9 grams/d (4.5 grams per dose) by increments of 0.75 grams per dose every 2 weeks. A dropper is supplied to facilitate measurement. |
The U.S. Drug Enforcement Agency (DEA) considers GHB a Schedule 1 (illegal) drug. DEA considers the prescription version a Schedule 3 drug, meaning it can be prescribed with refills as long as a DEA number is listed on the prescription. To prevent misuse, a central pharmacy dispenses sodium oxybate and mandates use of a specific prescription form to verify the physician’s familiarity with the medication. Psychiatrists can call (866) 997-3688 to obtain the form.
Table 2
Sodium oxybate dosing recommendations for patients
|
Sodium oxybate is the only agent FDA-approved for treating cataplexy—muscle weakness common among patients with narcolepsy.
HOW IT WORKS
Developed as an anesthetic, sodium oxybate induces deep sleep and at higher doses causes amnesia.
Derived from gamma-aminobutyric acid (GABA), sodium oxybate’s mechanism of action is unknown. Some believe it binds to the GABA B receptor and partially inhibits the NMDA and AMPA receptor-mediated excitatory neurons in the hippocampus.2
Food alters its bioavailability, so sodium oxybate should be taken several hours after meals to prevent delays in absorption and effect. Patients taking it should not eat at bedtime.
The agent’s pharmacokinetics are nonlinear, meaning that if the dose is doubled, the medication effect is tripled or quadrupled. For this reason, dosage increases must be small (no more than 0.75 grams for each dose) and gradual (at intervals of at least 2 weeks). The medication reaches peak plasma concentration within 30 to 75 minutes, so patients should not take the medication until they are in bed. Its 1-hour half-life explains its brief duration of action and need for repeat dosing overnight (Table 2).
Sodium oxybate does not modify the activity of any cytochrome P-450 enzymes. The medication is high in sodium (0.5 grams in a 3-gram dose) and has a salty taste. Use caution when considering the agent for patients with hypertension or on low-sodium diets.
Sodium oxybate’s safety has not been adequately tested in patients younger than 18 or older than 65 or in those with dementia and other disease processes. Because the drug is metabolized by the liver, the manufacturer recommends prescribing one-half the starting dosage to patients with significant hepatic impairment.
EFFICACY
Sodium oxybate has been shown to indirectly reduce frequency of cataplexy by improving nocturnal sleep:
- In a placebo-controlled, 4-week trial, 136 patients received either placebo or sodium oxybate at bedtime and again overnight in two equally divided doses of 3, 6, or 9 grams each. Patients who received the medication experienced less-frequent cataplexy, reduced daytime sleepiness, and fewer unplanned daytime naps and nocturnal awakenings.3
- A placebo-controlled trial that followed 55 patients for more than 3 years demonstrated long-term efficacy based on the patients’ cataplexy diaries (mean duration of treatment 21 months). Cataplexy returned after abrupt discontinuation.4
Unlike patients with most other disorders, those with narcolepsy generally are willing to repeat a medication overnight. They awaken easily at night—often without an alarm. Patients taking the medication report that they fall asleep again more readily and experience dramatically improved sleep quality and duration.
TOLERABILITY
Sodium oxybate has been well tolerated in relatively small clinical trials.
In the 4-week, placebo-controlled trial,3 nausea, headache, dizziness, and enuresis were most frequently reported. Out of 136 participants, 1 withdrew because of acute confusion and 9 others left because of mild to moderate adverse events. Twelve others experienced one episode of enuresis—probably because they did not fully awaken from deep sleep when developing urinary urgency. Advise patients taking sodium oxybate to urinate before going to bed.
The medication’s propensity to increase slow-wave sleep may cause sleepwalking. Sleepwalking was reported in 32% of patients in one long-term, uncontrolled study.5 If a patient with a history of sleepwalking needs sodium oxybate, advise against sleeping in upper bunks and other dangerous settings, and recommend precautions such as locking doors.
Because of sodium oxybate’s sedating properties, concomitant use of alcohol, barbiturates, and benzodiazepines should be discouraged.
ABUSE POTENTIAL
As discussed, GHB has a high abuse potential with effects such as euphoria, relaxation, and heightened sexual feelings.
Tolerance and dependence has not been reported with sodium oxybate when used as prescribed. A withdrawal state—similar to alcohol and sedative/hypnotic withdrawal and marked by anxiety, tremor, agitation, and delirium—has been reported with GHB abuse (although other chemicals often are used simultaneously in such cases). Narcolepsy patients in clinical trials have abruptly discontinued sodium oxybate after months of use without significant withdrawal.4
Related resources
- Narcolepsy Network Inc. www.narcolepsynetwork.org
Disclosure
Dr. Krahn reports no financial relationship with Orphan Medical or with manufacturers of competing products.
1. Galloway GP, Frederick SL, Staggers FE, Jr, et al. Gamma-hydroxybutyrate: an emerging drug of abuse that causes physical dependence. Addiction 1997;92(1):89-96.
2. Cammalleri M, Brancucci A, Berton F, et al. Gamma-hydroxybutyrate reduces GABA(A)-mediated inhibitory postsynaptic potentials in the CA1 region of hippocampus. Neuropsychopharmacology. 2002;27(9):960-9.
3. U.S. Xyrem Multicenter Study Group. A randomized, double blind, placebo-controlled multicenter trial comparing the effects of three doses of orally administered sodium oxybate with placebo for the treatment of narcolepsy. Sleep 2002;25(1):42-9.
4. U.S. Xyrem Multi-Center Study Group. The abrupt cessation of therapeutically administered sodium oxybate (GHB) does not cause withdrawal symptoms. J Toxicol Clin Toxicol 2003;41:131-5.
5. Physicians’ Desk Reference (57th ed). Montvale, NJ: Thomson Healthcare, 2003.
6. Mitler MM, Hayduk R. Benefits and risks of pharmacotherapy for narcolepsy. Drug Saf. 2002;25(11):791-809.
1. Galloway GP, Frederick SL, Staggers FE, Jr, et al. Gamma-hydroxybutyrate: an emerging drug of abuse that causes physical dependence. Addiction 1997;92(1):89-96.
2. Cammalleri M, Brancucci A, Berton F, et al. Gamma-hydroxybutyrate reduces GABA(A)-mediated inhibitory postsynaptic potentials in the CA1 region of hippocampus. Neuropsychopharmacology. 2002;27(9):960-9.
3. U.S. Xyrem Multicenter Study Group. A randomized, double blind, placebo-controlled multicenter trial comparing the effects of three doses of orally administered sodium oxybate with placebo for the treatment of narcolepsy. Sleep 2002;25(1):42-9.
4. U.S. Xyrem Multi-Center Study Group. The abrupt cessation of therapeutically administered sodium oxybate (GHB) does not cause withdrawal symptoms. J Toxicol Clin Toxicol 2003;41:131-5.
5. Physicians’ Desk Reference (57th ed). Montvale, NJ: Thomson Healthcare, 2003.
6. Mitler MM, Hayduk R. Benefits and risks of pharmacotherapy for narcolepsy. Drug Saf. 2002;25(11):791-809.
Factitious disorder: What to do when someone plays sick
An orthopedic surgeon treating a patient, age 29, at a tertiary medical center asks a staff psychiatrist for advice. The patient—who has chronic bilateral knee infections—lives 350 miles away; her treatment-resistant disease has stymied and frustrated her local physicians. Her infections have persisted despite multiple courses of antibiotics and numerous surgical procedures.
Because of damage to the right knee joint, she cannot bear weight or walk. A registered nurse, she has been unable to work or care for her school-aged children for 2 years. The surgeon tells the psychiatrist that the patient denies psychiatric complaints beyond sadness over her inability to fulfill her responsibilities. She expresses a wish to recover and adamantly denies that she manipulates her wound or does anything to interfere with its healing. The medical/surgical team has noticed that while she is away from home receiving orthopedic care, her husband never visits or calls.
Cases such as the one described above are rare, but psychiatrists occasionally encounter patients with these baffling characteristics. When the patient’s disease fails to respond to treatment as expected—or progresses—members of the medical/psychiatric team need to ask themselves these questions:
- Are we dealing with a drug-resistant infection?
- Is the patient adhering fully with treatment?
- Does the patient do anything to perpetuate this disease process and wish to stay ill?
Asking this last question is difficult but necessary in certain situations. Most of us cannot imagine why a person would wish to remain sick. Why would someone be willing to endure pain and multiple hospital stays, remain isolated from family, and risk a permanent disability? Yet, an unknown number of people strive to appear unwell so that they can receive ongoing medical care.
What are factitious disorders?
Factitious disorders are psychiatric conditions in which patients deliberately portray themselves as ill. They may present with physical or psychological symptoms or both. Their objective is to assume the sick role—not to procure shelter, obtain financial assistance, avoid prison, etc., which would fall into other diagnoses such as malingering.
Table 1
DSM-IV DIAGNOSTIC CRITERIA FOR FACTITIOUS DISORDER
|
| Types |
|
| Source: DSM-IV-TR |
DSM-IV criteria are straightforward and inclusive (Table 1).1 They do not specify:
- the presence of medical and/or psychiatric disorders, which do not preclude the diagnosis
- reasons why a person may wish to assume the sick role.
The medical literature on factitious disorder includes many compelling case reports. However, the secretive nature of most patients with factitious complaints has made it difficult to conduct carefully designed community-based studies, prospective studies, or controlled randomized trials. Because research is scarce, much is unknown about who gets factitious disorder, what causes it, and how to treat it.
Differential diagnosis
Factitious disorder varies in severity. Among subtypes proposed by Folks et al (Table 2),2 patients in categories 3, 4, and 5—who produce physical illness—can potentially be identified by diagnostic testing.3 Patients in categories 1 and 2—who exaggerate physical symptoms and provide a false medical history—may be more difficult to detect.
In cases where patients exaggerate symptoms or fabricate histories, little objective information is typically available to the treating physicians. Medical records revealing multiple admissions or emergency room visits may be obtained from other institutions only if the patient gives permission. However, the patient often does not consent or the materials cannot be located.
Third-party payers’ pre-authorization procedures and utilization reviews may speak volumes about a patient’s search for health care. However, patients who are unemployed or estranged from spouses may lose insurance coverage over time. Government assistance programs such as Medicare and Medicaid provide care to many patients with these chronic problems and do not perform the same degree of utilization review.
Munchausen disorder—a variant of factitious disorder—is not recognized by DSM-IV. The term—while still used primarily by nonpsychiatrists—is generally viewed as outdated. The term is reserved for patients with the most severe and chronic form of factitious disorder.4 The few studies done of patients with this variant have not adequately examined the specificity and sensitivity of their core symptoms or other characteristics, such as production of a misleading medical condition, travel to multiple medical centers (peregrination), and the telling of tall tales (pseudologia fantastica).
Somatoform disorder. If physicians suspect that a patient’s illness is taking an unusual course, they may suspect a somatoform rather than factitious disorder. Patients with somatoform disorder do not intentionally produce their symptoms, whereas patients with factitious disorder deliberately try to appear ill. In both disorders, the underlying cause is unconscious.
Hypochondriasis. Patients with hypochondriasis are obsessed with concerns that they have an illness. Their worries may compel them to seek out examinations and diagnostic tests. Unlike patients with factitious disorder, these patients do not deliberately provide information or manufacture symptoms to create the appearance of a medical disorder.
Malingering. Patients who malinger may engage in deceitful behaviors that can include creating a misleading impression about a medical or psychiatric illness. Being a patient, however, is not their objective. They may be seeking disability payments, insurance settlements, shelter, or food.
Patient evaluation
Patients suspected of factitious disorder merit a thorough medical and psychiatric evaluation, guided by their presenting symptoms. They commonly have comorbid psychiatric disorders (Table 3), which medical/surgical team members and the psychiatrist need to identify before considering a diagnosis of factitious disorder.
Because invasive tests such as angiography, colonoscopy, biopsies, or exploratory surgery are required to exclude some underlying medical processes, the treatment team must take care not to cause harm. The expected benefits of diagnostic testing must be balanced against the risks of an iatrogenic event.
Table 2
FIVE PROPOSED SUBTYPES OF FACTITIOUS DISORDER
| Characteristic | Examples |
|---|---|
| May be most difficult to detect | |
| 1. Exaggerates physical symptoms 2. Provides a false medical history | An epileptic patient has a seizure while EEG is normal Describes a fictitious history of cancer |
| Can potentially be identified by diagnostic testing | |
| 3. Simulates physical symptoms 4. Modifies physiology to create physical signs 5. Induces physical illness | Puts gravel into urine sample Exerts oneself before vital signs test to elevate blood pressure Injects foreign material into a surgical wound to slow healing |
| Source: Adapted from Folks et al.2 | |
Relatively little is known about how to diagnose a factitious process coexisting with a genuine medical disorder. For example, a patient with well-documented chronic inflammatory disease may easily exaggerate pain and diarrhea to facilitate hospital admission.
To confront or not to confront?
Some patients may relish the patient role for a time—such as while being evaluated for a presumed opportunistic infection—but may not consent to more definitive tests—such as HIV testing. They may demand discharge while they still may be harming themselves, such as by injecting foreign material. The patient may plan to find another health care provider and continue the maladaptive behavior.
If you suspected that our case patient was playing a role in perpetuating her chronic knee infections, would you confront her with the evidence? The answer is unclear, but some experts argue against confrontation.5 Once a patient believes that the medical team suspects a factitious process, he or she may no longer wish to cooperate, even if the diagnostic evaluation is incomplete. Patients often become more guarded about what they reveal after they are confronted. They may become more careful to hide evidence of wound tampering (e.g., syringes) and hesitant to discuss emotional issues (e.g., estranged relationships, feeling overwhelmed by work and home duties).
Case reports suggest that patients who simulate symptoms, modify their physiology, or induce physical illness are at high risk of morbidity and mortality. For example, one report described a patient who underwent two cardiopulmonary resuscitations because of torsades de pointes triggered by hypokalemia related to covert laxative use.6 Physicians must manage these cases carefully to reduce patient risk. In rare cases where a patient’s behavior becomes life-threatening, admission to a psychiatric unit—even involuntarily—may be necessary.
Collaborating with the patient
A comprehensive treatment approach is optimal for patients with factitious disorder. All the patient’s objective medical disorders should be addressed in systematically and with empathy. Treating a co-existing medical disorder may help the physician gain the patient’s trust, which in turn can help keep treatment options open.
Some patients have been known to exaggerate their physical symptoms because they feel they have a serious, undiagnosed medical problem. They feel that their assessment has been cursory and that they need to compel the physician to do a more thorough evaluation in order to identify the true underlying problem. Although no research supports this observation, these patients may be reassured when their physicians carefully evaluate their medical problems.
Eisendrath5 recommends that the treatment team take time to get to know the patient and convey that this attention is devoted to the person, not just the medical illness. This approach may increase the likelihood of learning about psychosocial issues the person may be trying to resolve by taking the patient role. Patients also may be more willing to complete the evaluation and adhere to recommended treatment, although these outcomes are not guaranteed.
Table 3
DISORDERS KNOWN TO CO-EXIST WITH FACTITIOUS DISORDER
| Disorder | Possible issue |
|---|---|
| Medical | Coexisting medical disease |
| Delusional | Somatic delusions |
| Depressive | Somatic complaints, dependency on staff |
| Chemical dependency | Prescription drug abuse |
| Eating disorders | Persistent vomiting, weight loss |
| Obsessive-compulsive disorder | Somatic obsessions |
| Hypochondriasis | Conviction one is unwell |
| Pain disorders | Pain complaints |
| Malingering | Seeking shelter in hospital |
| Source: Adapted from Folks et al. Somatoform disorders, factitious disorders, and malingering. | |
| In: Stoudemire A, Fogel B, Greenberg D, eds. Psychiatric care of the medical patient (2nd ed). New York: Oxford University Press, 2000:458-75. | |
Case report
For the patient with chronic knee infections, the staff psychiatrist recommended that the orthopedist develop a collaborative relationship with her. Eventually the surgeon told her that she needed psychiatric care, and the patient agreed to psychiatric hospitalization.
In this setting, she was initially observed with a 24-hour monitor and received appropriate wound care. The staff encouraged her to talk about the emotional distress related to having a chronic disease. She never admitted to perpetuating the infections in her knees, although she was suspected of injecting herself with infected material. Psychiatric evaluation revealed a history of multiple strained relationships that suggested a severe personality disorder.
Her wounds slowly began to improve, and she was discharged after 2 weeks. Throughout her stay, she remained reluctant to discuss her relationship with her husband or examine other possible sources of stress in her life. Thus, factitious behavior will probably recur unless she tackles her unconscious motivations for adopting a patient role.
If patients’ emotional needs are being met, they may reveal the mechanism of their disease. Unfortunately, experience suggests that very few confess the false nature of their medical illness, fewer accept psychiatric treatment, and even fewer complete the recommended course of treatment.
Comorbid psychiatric disorders provide an opportunity to intervene with selected medications and psychotherapy to reduce patient distress. Chemical dependency treatment in particular can help stabilize a patient with a factitious disorder so that he or she no longer seeks pain medications or sedatives. Patients with an obsessive-compulsive disorder or hypochondriasis may require specifically targeted cognitive-behavioral therapy or pharmacotherapy.
Few references regarding treatment of factitious disorder exist; the only known review of cognitive-behavioral therapy’s role in treating this disorder awaits publication.
Related resources
- Sutherland AJ, Rodin GM. Factitious disorders in a general hospital setting: clinical features and a review of the literature. Psychosomatics 1990;31(4):392-9.
- Reich P, Gottfried LA. Factitious disorders in a teaching hospital. Ann Intern Med 1983;99(2):240-7.
1. American Psychiatric Association Diagnostic and statistical manual of mental disorders (4th ed). Washington DC: American Psychiatric Association, 1994-886.
2. Folks D, Feldman M, Ford C. Somatoform disorders, factitious disorders, and malingering. In: Stoudemire A, Fogel B, Greenberg D, eds. Psychiatric care of the medical patient (2nd ed). New York: Oxford University Press, 2000;458-75.
3. Wallach J. Laboratory diagnosis of factitious disorders. Arch Intern Med 1994;154:1690-6.
4. Asher R. Munchausen’s syndrome. Lancet 1951;1:339-41.
5. Eisendrath S. Factitious physical disorders treatment without confrontation. Psychosomatics 1990;31:357-8.
6. Krahn L, Lee J, Martin MJ, Richardson J, O’Connor M. Hypokalemia leading to torsades de pointes: Munchausen’s syndrome versus bulimia nervosa. Gen Hosp Psychiatry 1997;19:370-7.
An orthopedic surgeon treating a patient, age 29, at a tertiary medical center asks a staff psychiatrist for advice. The patient—who has chronic bilateral knee infections—lives 350 miles away; her treatment-resistant disease has stymied and frustrated her local physicians. Her infections have persisted despite multiple courses of antibiotics and numerous surgical procedures.
Because of damage to the right knee joint, she cannot bear weight or walk. A registered nurse, she has been unable to work or care for her school-aged children for 2 years. The surgeon tells the psychiatrist that the patient denies psychiatric complaints beyond sadness over her inability to fulfill her responsibilities. She expresses a wish to recover and adamantly denies that she manipulates her wound or does anything to interfere with its healing. The medical/surgical team has noticed that while she is away from home receiving orthopedic care, her husband never visits or calls.
Cases such as the one described above are rare, but psychiatrists occasionally encounter patients with these baffling characteristics. When the patient’s disease fails to respond to treatment as expected—or progresses—members of the medical/psychiatric team need to ask themselves these questions:
- Are we dealing with a drug-resistant infection?
- Is the patient adhering fully with treatment?
- Does the patient do anything to perpetuate this disease process and wish to stay ill?
Asking this last question is difficult but necessary in certain situations. Most of us cannot imagine why a person would wish to remain sick. Why would someone be willing to endure pain and multiple hospital stays, remain isolated from family, and risk a permanent disability? Yet, an unknown number of people strive to appear unwell so that they can receive ongoing medical care.
What are factitious disorders?
Factitious disorders are psychiatric conditions in which patients deliberately portray themselves as ill. They may present with physical or psychological symptoms or both. Their objective is to assume the sick role—not to procure shelter, obtain financial assistance, avoid prison, etc., which would fall into other diagnoses such as malingering.
Table 1
DSM-IV DIAGNOSTIC CRITERIA FOR FACTITIOUS DISORDER
|
| Types |
|
| Source: DSM-IV-TR |
DSM-IV criteria are straightforward and inclusive (Table 1).1 They do not specify:
- the presence of medical and/or psychiatric disorders, which do not preclude the diagnosis
- reasons why a person may wish to assume the sick role.
The medical literature on factitious disorder includes many compelling case reports. However, the secretive nature of most patients with factitious complaints has made it difficult to conduct carefully designed community-based studies, prospective studies, or controlled randomized trials. Because research is scarce, much is unknown about who gets factitious disorder, what causes it, and how to treat it.
Differential diagnosis
Factitious disorder varies in severity. Among subtypes proposed by Folks et al (Table 2),2 patients in categories 3, 4, and 5—who produce physical illness—can potentially be identified by diagnostic testing.3 Patients in categories 1 and 2—who exaggerate physical symptoms and provide a false medical history—may be more difficult to detect.
In cases where patients exaggerate symptoms or fabricate histories, little objective information is typically available to the treating physicians. Medical records revealing multiple admissions or emergency room visits may be obtained from other institutions only if the patient gives permission. However, the patient often does not consent or the materials cannot be located.
Third-party payers’ pre-authorization procedures and utilization reviews may speak volumes about a patient’s search for health care. However, patients who are unemployed or estranged from spouses may lose insurance coverage over time. Government assistance programs such as Medicare and Medicaid provide care to many patients with these chronic problems and do not perform the same degree of utilization review.
Munchausen disorder—a variant of factitious disorder—is not recognized by DSM-IV. The term—while still used primarily by nonpsychiatrists—is generally viewed as outdated. The term is reserved for patients with the most severe and chronic form of factitious disorder.4 The few studies done of patients with this variant have not adequately examined the specificity and sensitivity of their core symptoms or other characteristics, such as production of a misleading medical condition, travel to multiple medical centers (peregrination), and the telling of tall tales (pseudologia fantastica).
Somatoform disorder. If physicians suspect that a patient’s illness is taking an unusual course, they may suspect a somatoform rather than factitious disorder. Patients with somatoform disorder do not intentionally produce their symptoms, whereas patients with factitious disorder deliberately try to appear ill. In both disorders, the underlying cause is unconscious.
Hypochondriasis. Patients with hypochondriasis are obsessed with concerns that they have an illness. Their worries may compel them to seek out examinations and diagnostic tests. Unlike patients with factitious disorder, these patients do not deliberately provide information or manufacture symptoms to create the appearance of a medical disorder.
Malingering. Patients who malinger may engage in deceitful behaviors that can include creating a misleading impression about a medical or psychiatric illness. Being a patient, however, is not their objective. They may be seeking disability payments, insurance settlements, shelter, or food.
Patient evaluation
Patients suspected of factitious disorder merit a thorough medical and psychiatric evaluation, guided by their presenting symptoms. They commonly have comorbid psychiatric disorders (Table 3), which medical/surgical team members and the psychiatrist need to identify before considering a diagnosis of factitious disorder.
Because invasive tests such as angiography, colonoscopy, biopsies, or exploratory surgery are required to exclude some underlying medical processes, the treatment team must take care not to cause harm. The expected benefits of diagnostic testing must be balanced against the risks of an iatrogenic event.
Table 2
FIVE PROPOSED SUBTYPES OF FACTITIOUS DISORDER
| Characteristic | Examples |
|---|---|
| May be most difficult to detect | |
| 1. Exaggerates physical symptoms 2. Provides a false medical history | An epileptic patient has a seizure while EEG is normal Describes a fictitious history of cancer |
| Can potentially be identified by diagnostic testing | |
| 3. Simulates physical symptoms 4. Modifies physiology to create physical signs 5. Induces physical illness | Puts gravel into urine sample Exerts oneself before vital signs test to elevate blood pressure Injects foreign material into a surgical wound to slow healing |
| Source: Adapted from Folks et al.2 | |
Relatively little is known about how to diagnose a factitious process coexisting with a genuine medical disorder. For example, a patient with well-documented chronic inflammatory disease may easily exaggerate pain and diarrhea to facilitate hospital admission.
To confront or not to confront?
Some patients may relish the patient role for a time—such as while being evaluated for a presumed opportunistic infection—but may not consent to more definitive tests—such as HIV testing. They may demand discharge while they still may be harming themselves, such as by injecting foreign material. The patient may plan to find another health care provider and continue the maladaptive behavior.
If you suspected that our case patient was playing a role in perpetuating her chronic knee infections, would you confront her with the evidence? The answer is unclear, but some experts argue against confrontation.5 Once a patient believes that the medical team suspects a factitious process, he or she may no longer wish to cooperate, even if the diagnostic evaluation is incomplete. Patients often become more guarded about what they reveal after they are confronted. They may become more careful to hide evidence of wound tampering (e.g., syringes) and hesitant to discuss emotional issues (e.g., estranged relationships, feeling overwhelmed by work and home duties).
Case reports suggest that patients who simulate symptoms, modify their physiology, or induce physical illness are at high risk of morbidity and mortality. For example, one report described a patient who underwent two cardiopulmonary resuscitations because of torsades de pointes triggered by hypokalemia related to covert laxative use.6 Physicians must manage these cases carefully to reduce patient risk. In rare cases where a patient’s behavior becomes life-threatening, admission to a psychiatric unit—even involuntarily—may be necessary.
Collaborating with the patient
A comprehensive treatment approach is optimal for patients with factitious disorder. All the patient’s objective medical disorders should be addressed in systematically and with empathy. Treating a co-existing medical disorder may help the physician gain the patient’s trust, which in turn can help keep treatment options open.
Some patients have been known to exaggerate their physical symptoms because they feel they have a serious, undiagnosed medical problem. They feel that their assessment has been cursory and that they need to compel the physician to do a more thorough evaluation in order to identify the true underlying problem. Although no research supports this observation, these patients may be reassured when their physicians carefully evaluate their medical problems.
Eisendrath5 recommends that the treatment team take time to get to know the patient and convey that this attention is devoted to the person, not just the medical illness. This approach may increase the likelihood of learning about psychosocial issues the person may be trying to resolve by taking the patient role. Patients also may be more willing to complete the evaluation and adhere to recommended treatment, although these outcomes are not guaranteed.
Table 3
DISORDERS KNOWN TO CO-EXIST WITH FACTITIOUS DISORDER
| Disorder | Possible issue |
|---|---|
| Medical | Coexisting medical disease |
| Delusional | Somatic delusions |
| Depressive | Somatic complaints, dependency on staff |
| Chemical dependency | Prescription drug abuse |
| Eating disorders | Persistent vomiting, weight loss |
| Obsessive-compulsive disorder | Somatic obsessions |
| Hypochondriasis | Conviction one is unwell |
| Pain disorders | Pain complaints |
| Malingering | Seeking shelter in hospital |
| Source: Adapted from Folks et al. Somatoform disorders, factitious disorders, and malingering. | |
| In: Stoudemire A, Fogel B, Greenberg D, eds. Psychiatric care of the medical patient (2nd ed). New York: Oxford University Press, 2000:458-75. | |
Case report
For the patient with chronic knee infections, the staff psychiatrist recommended that the orthopedist develop a collaborative relationship with her. Eventually the surgeon told her that she needed psychiatric care, and the patient agreed to psychiatric hospitalization.
In this setting, she was initially observed with a 24-hour monitor and received appropriate wound care. The staff encouraged her to talk about the emotional distress related to having a chronic disease. She never admitted to perpetuating the infections in her knees, although she was suspected of injecting herself with infected material. Psychiatric evaluation revealed a history of multiple strained relationships that suggested a severe personality disorder.
Her wounds slowly began to improve, and she was discharged after 2 weeks. Throughout her stay, she remained reluctant to discuss her relationship with her husband or examine other possible sources of stress in her life. Thus, factitious behavior will probably recur unless she tackles her unconscious motivations for adopting a patient role.
If patients’ emotional needs are being met, they may reveal the mechanism of their disease. Unfortunately, experience suggests that very few confess the false nature of their medical illness, fewer accept psychiatric treatment, and even fewer complete the recommended course of treatment.
Comorbid psychiatric disorders provide an opportunity to intervene with selected medications and psychotherapy to reduce patient distress. Chemical dependency treatment in particular can help stabilize a patient with a factitious disorder so that he or she no longer seeks pain medications or sedatives. Patients with an obsessive-compulsive disorder or hypochondriasis may require specifically targeted cognitive-behavioral therapy or pharmacotherapy.
Few references regarding treatment of factitious disorder exist; the only known review of cognitive-behavioral therapy’s role in treating this disorder awaits publication.
Related resources
- Sutherland AJ, Rodin GM. Factitious disorders in a general hospital setting: clinical features and a review of the literature. Psychosomatics 1990;31(4):392-9.
- Reich P, Gottfried LA. Factitious disorders in a teaching hospital. Ann Intern Med 1983;99(2):240-7.
An orthopedic surgeon treating a patient, age 29, at a tertiary medical center asks a staff psychiatrist for advice. The patient—who has chronic bilateral knee infections—lives 350 miles away; her treatment-resistant disease has stymied and frustrated her local physicians. Her infections have persisted despite multiple courses of antibiotics and numerous surgical procedures.
Because of damage to the right knee joint, she cannot bear weight or walk. A registered nurse, she has been unable to work or care for her school-aged children for 2 years. The surgeon tells the psychiatrist that the patient denies psychiatric complaints beyond sadness over her inability to fulfill her responsibilities. She expresses a wish to recover and adamantly denies that she manipulates her wound or does anything to interfere with its healing. The medical/surgical team has noticed that while she is away from home receiving orthopedic care, her husband never visits or calls.
Cases such as the one described above are rare, but psychiatrists occasionally encounter patients with these baffling characteristics. When the patient’s disease fails to respond to treatment as expected—or progresses—members of the medical/psychiatric team need to ask themselves these questions:
- Are we dealing with a drug-resistant infection?
- Is the patient adhering fully with treatment?
- Does the patient do anything to perpetuate this disease process and wish to stay ill?
Asking this last question is difficult but necessary in certain situations. Most of us cannot imagine why a person would wish to remain sick. Why would someone be willing to endure pain and multiple hospital stays, remain isolated from family, and risk a permanent disability? Yet, an unknown number of people strive to appear unwell so that they can receive ongoing medical care.
What are factitious disorders?
Factitious disorders are psychiatric conditions in which patients deliberately portray themselves as ill. They may present with physical or psychological symptoms or both. Their objective is to assume the sick role—not to procure shelter, obtain financial assistance, avoid prison, etc., which would fall into other diagnoses such as malingering.
Table 1
DSM-IV DIAGNOSTIC CRITERIA FOR FACTITIOUS DISORDER
|
| Types |
|
| Source: DSM-IV-TR |
DSM-IV criteria are straightforward and inclusive (Table 1).1 They do not specify:
- the presence of medical and/or psychiatric disorders, which do not preclude the diagnosis
- reasons why a person may wish to assume the sick role.
The medical literature on factitious disorder includes many compelling case reports. However, the secretive nature of most patients with factitious complaints has made it difficult to conduct carefully designed community-based studies, prospective studies, or controlled randomized trials. Because research is scarce, much is unknown about who gets factitious disorder, what causes it, and how to treat it.
Differential diagnosis
Factitious disorder varies in severity. Among subtypes proposed by Folks et al (Table 2),2 patients in categories 3, 4, and 5—who produce physical illness—can potentially be identified by diagnostic testing.3 Patients in categories 1 and 2—who exaggerate physical symptoms and provide a false medical history—may be more difficult to detect.
In cases where patients exaggerate symptoms or fabricate histories, little objective information is typically available to the treating physicians. Medical records revealing multiple admissions or emergency room visits may be obtained from other institutions only if the patient gives permission. However, the patient often does not consent or the materials cannot be located.
Third-party payers’ pre-authorization procedures and utilization reviews may speak volumes about a patient’s search for health care. However, patients who are unemployed or estranged from spouses may lose insurance coverage over time. Government assistance programs such as Medicare and Medicaid provide care to many patients with these chronic problems and do not perform the same degree of utilization review.
Munchausen disorder—a variant of factitious disorder—is not recognized by DSM-IV. The term—while still used primarily by nonpsychiatrists—is generally viewed as outdated. The term is reserved for patients with the most severe and chronic form of factitious disorder.4 The few studies done of patients with this variant have not adequately examined the specificity and sensitivity of their core symptoms or other characteristics, such as production of a misleading medical condition, travel to multiple medical centers (peregrination), and the telling of tall tales (pseudologia fantastica).
Somatoform disorder. If physicians suspect that a patient’s illness is taking an unusual course, they may suspect a somatoform rather than factitious disorder. Patients with somatoform disorder do not intentionally produce their symptoms, whereas patients with factitious disorder deliberately try to appear ill. In both disorders, the underlying cause is unconscious.
Hypochondriasis. Patients with hypochondriasis are obsessed with concerns that they have an illness. Their worries may compel them to seek out examinations and diagnostic tests. Unlike patients with factitious disorder, these patients do not deliberately provide information or manufacture symptoms to create the appearance of a medical disorder.
Malingering. Patients who malinger may engage in deceitful behaviors that can include creating a misleading impression about a medical or psychiatric illness. Being a patient, however, is not their objective. They may be seeking disability payments, insurance settlements, shelter, or food.
Patient evaluation
Patients suspected of factitious disorder merit a thorough medical and psychiatric evaluation, guided by their presenting symptoms. They commonly have comorbid psychiatric disorders (Table 3), which medical/surgical team members and the psychiatrist need to identify before considering a diagnosis of factitious disorder.
Because invasive tests such as angiography, colonoscopy, biopsies, or exploratory surgery are required to exclude some underlying medical processes, the treatment team must take care not to cause harm. The expected benefits of diagnostic testing must be balanced against the risks of an iatrogenic event.
Table 2
FIVE PROPOSED SUBTYPES OF FACTITIOUS DISORDER
| Characteristic | Examples |
|---|---|
| May be most difficult to detect | |
| 1. Exaggerates physical symptoms 2. Provides a false medical history | An epileptic patient has a seizure while EEG is normal Describes a fictitious history of cancer |
| Can potentially be identified by diagnostic testing | |
| 3. Simulates physical symptoms 4. Modifies physiology to create physical signs 5. Induces physical illness | Puts gravel into urine sample Exerts oneself before vital signs test to elevate blood pressure Injects foreign material into a surgical wound to slow healing |
| Source: Adapted from Folks et al.2 | |
Relatively little is known about how to diagnose a factitious process coexisting with a genuine medical disorder. For example, a patient with well-documented chronic inflammatory disease may easily exaggerate pain and diarrhea to facilitate hospital admission.
To confront or not to confront?
Some patients may relish the patient role for a time—such as while being evaluated for a presumed opportunistic infection—but may not consent to more definitive tests—such as HIV testing. They may demand discharge while they still may be harming themselves, such as by injecting foreign material. The patient may plan to find another health care provider and continue the maladaptive behavior.
If you suspected that our case patient was playing a role in perpetuating her chronic knee infections, would you confront her with the evidence? The answer is unclear, but some experts argue against confrontation.5 Once a patient believes that the medical team suspects a factitious process, he or she may no longer wish to cooperate, even if the diagnostic evaluation is incomplete. Patients often become more guarded about what they reveal after they are confronted. They may become more careful to hide evidence of wound tampering (e.g., syringes) and hesitant to discuss emotional issues (e.g., estranged relationships, feeling overwhelmed by work and home duties).
Case reports suggest that patients who simulate symptoms, modify their physiology, or induce physical illness are at high risk of morbidity and mortality. For example, one report described a patient who underwent two cardiopulmonary resuscitations because of torsades de pointes triggered by hypokalemia related to covert laxative use.6 Physicians must manage these cases carefully to reduce patient risk. In rare cases where a patient’s behavior becomes life-threatening, admission to a psychiatric unit—even involuntarily—may be necessary.
Collaborating with the patient
A comprehensive treatment approach is optimal for patients with factitious disorder. All the patient’s objective medical disorders should be addressed in systematically and with empathy. Treating a co-existing medical disorder may help the physician gain the patient’s trust, which in turn can help keep treatment options open.
Some patients have been known to exaggerate their physical symptoms because they feel they have a serious, undiagnosed medical problem. They feel that their assessment has been cursory and that they need to compel the physician to do a more thorough evaluation in order to identify the true underlying problem. Although no research supports this observation, these patients may be reassured when their physicians carefully evaluate their medical problems.
Eisendrath5 recommends that the treatment team take time to get to know the patient and convey that this attention is devoted to the person, not just the medical illness. This approach may increase the likelihood of learning about psychosocial issues the person may be trying to resolve by taking the patient role. Patients also may be more willing to complete the evaluation and adhere to recommended treatment, although these outcomes are not guaranteed.
Table 3
DISORDERS KNOWN TO CO-EXIST WITH FACTITIOUS DISORDER
| Disorder | Possible issue |
|---|---|
| Medical | Coexisting medical disease |
| Delusional | Somatic delusions |
| Depressive | Somatic complaints, dependency on staff |
| Chemical dependency | Prescription drug abuse |
| Eating disorders | Persistent vomiting, weight loss |
| Obsessive-compulsive disorder | Somatic obsessions |
| Hypochondriasis | Conviction one is unwell |
| Pain disorders | Pain complaints |
| Malingering | Seeking shelter in hospital |
| Source: Adapted from Folks et al. Somatoform disorders, factitious disorders, and malingering. | |
| In: Stoudemire A, Fogel B, Greenberg D, eds. Psychiatric care of the medical patient (2nd ed). New York: Oxford University Press, 2000:458-75. | |
Case report
For the patient with chronic knee infections, the staff psychiatrist recommended that the orthopedist develop a collaborative relationship with her. Eventually the surgeon told her that she needed psychiatric care, and the patient agreed to psychiatric hospitalization.
In this setting, she was initially observed with a 24-hour monitor and received appropriate wound care. The staff encouraged her to talk about the emotional distress related to having a chronic disease. She never admitted to perpetuating the infections in her knees, although she was suspected of injecting herself with infected material. Psychiatric evaluation revealed a history of multiple strained relationships that suggested a severe personality disorder.
Her wounds slowly began to improve, and she was discharged after 2 weeks. Throughout her stay, she remained reluctant to discuss her relationship with her husband or examine other possible sources of stress in her life. Thus, factitious behavior will probably recur unless she tackles her unconscious motivations for adopting a patient role.
If patients’ emotional needs are being met, they may reveal the mechanism of their disease. Unfortunately, experience suggests that very few confess the false nature of their medical illness, fewer accept psychiatric treatment, and even fewer complete the recommended course of treatment.
Comorbid psychiatric disorders provide an opportunity to intervene with selected medications and psychotherapy to reduce patient distress. Chemical dependency treatment in particular can help stabilize a patient with a factitious disorder so that he or she no longer seeks pain medications or sedatives. Patients with an obsessive-compulsive disorder or hypochondriasis may require specifically targeted cognitive-behavioral therapy or pharmacotherapy.
Few references regarding treatment of factitious disorder exist; the only known review of cognitive-behavioral therapy’s role in treating this disorder awaits publication.
Related resources
- Sutherland AJ, Rodin GM. Factitious disorders in a general hospital setting: clinical features and a review of the literature. Psychosomatics 1990;31(4):392-9.
- Reich P, Gottfried LA. Factitious disorders in a teaching hospital. Ann Intern Med 1983;99(2):240-7.
1. American Psychiatric Association Diagnostic and statistical manual of mental disorders (4th ed). Washington DC: American Psychiatric Association, 1994-886.
2. Folks D, Feldman M, Ford C. Somatoform disorders, factitious disorders, and malingering. In: Stoudemire A, Fogel B, Greenberg D, eds. Psychiatric care of the medical patient (2nd ed). New York: Oxford University Press, 2000;458-75.
3. Wallach J. Laboratory diagnosis of factitious disorders. Arch Intern Med 1994;154:1690-6.
4. Asher R. Munchausen’s syndrome. Lancet 1951;1:339-41.
5. Eisendrath S. Factitious physical disorders treatment without confrontation. Psychosomatics 1990;31:357-8.
6. Krahn L, Lee J, Martin MJ, Richardson J, O’Connor M. Hypokalemia leading to torsades de pointes: Munchausen’s syndrome versus bulimia nervosa. Gen Hosp Psychiatry 1997;19:370-7.
1. American Psychiatric Association Diagnostic and statistical manual of mental disorders (4th ed). Washington DC: American Psychiatric Association, 1994-886.
2. Folks D, Feldman M, Ford C. Somatoform disorders, factitious disorders, and malingering. In: Stoudemire A, Fogel B, Greenberg D, eds. Psychiatric care of the medical patient (2nd ed). New York: Oxford University Press, 2000;458-75.
3. Wallach J. Laboratory diagnosis of factitious disorders. Arch Intern Med 1994;154:1690-6.
4. Asher R. Munchausen’s syndrome. Lancet 1951;1:339-41.
5. Eisendrath S. Factitious physical disorders treatment without confrontation. Psychosomatics 1990;31:357-8.
6. Krahn L, Lee J, Martin MJ, Richardson J, O’Connor M. Hypokalemia leading to torsades de pointes: Munchausen’s syndrome versus bulimia nervosa. Gen Hosp Psychiatry 1997;19:370-7.
Factitious disorder: What to do when someone plays sick
An orthopedic surgeon treating a patient, age 29, at a tertiary medical center asks a staff psychiatrist for advice. The patient—who has chronic bilateral knee infections—lives 350 miles away; her treatment-resistant disease has stymied and frustrated her local physicians. Her infections have persisted despite multiple courses of antibiotics and numerous surgical procedures.
Because of damage to the right knee joint, she cannot bear weight or walk. A registered nurse, she has been unable to work or care for her school-aged children for 2 years. The surgeon tells the psychiatrist that the patient denies psychiatric complaints beyond sadness over her inability to fulfill her responsibilities. She expresses a wish to recover and adamantly denies that she manipulates her wound or does anything to interfere with its healing. The medical/surgical team has noticed that while she is away from home receiving orthopedic care, her husband never visits or calls.
Cases such as the one described above are rare, but psychiatrists occasionally encounter patients with these baffling characteristics. When the patient’s disease fails to respond to treatment as expected—or progresses—members of the medical/psychiatric team need to ask themselves these questions:
- Are we dealing with a drug-resistant infection?
- Is the patient adhering fully with treatment?
- Does the patient do anything to perpetuate this disease process and wish to stay ill?
Asking this last question is difficult but necessary in certain situations. Most of us cannot imagine why a person would wish to remain sick. Why would someone be willing to endure pain and multiple hospital stays, remain isolated from family, and risk a permanent disability? Yet, an unknown number of people strive to appear unwell so that they can receive ongoing medical care.
What are factitious disorders?
Factitious disorders are psychiatric conditions in which patients deliberately portray themselves as ill. They may present with physical or psychological symptoms or both. Their objective is to assume the sick role—not to procure shelter, obtain financial assistance, avoid prison, etc., which would fall into other diagnoses such as malingering.
Table 1
DSM-IV DIAGNOSTIC CRITERIA FOR FACTITIOUS DISORDER
|
| Types |
|
| Source: DSM-IV-TR |
DSM-IV criteria are straightforward and inclusive (Table 1).1 They do not specify:
- the presence of medical and/or psychiatric disorders, which do not preclude the diagnosis
- reasons why a person may wish to assume the sick role.
The medical literature on factitious disorder includes many compelling case reports. However, the secretive nature of most patients with factitious complaints has made it difficult to conduct carefully designed community-based studies, prospective studies, or controlled randomized trials. Because research is scarce, much is unknown about who gets factitious disorder, what causes it, and how to treat it.
Differential diagnosis
Factitious disorder varies in severity. Among subtypes proposed by Folks et al (Table 2),2 patients in categories 3, 4, and 5—who produce physical illness—can potentially be identified by diagnostic testing.3 Patients in categories 1 and 2—who exaggerate physical symptoms and provide a false medical history—may be more difficult to detect.
In cases where patients exaggerate symptoms or fabricate histories, little objective information is typically available to the treating physicians. Medical records revealing multiple admissions or emergency room visits may be obtained from other institutions only if the patient gives permission. However, the patient often does not consent or the materials cannot be located.
Third-party payers’ pre-authorization procedures and utilization reviews may speak volumes about a patient’s search for health care. However, patients who are unemployed or estranged from spouses may lose insurance coverage over time. Government assistance programs such as Medicare and Medicaid provide care to many patients with these chronic problems and do not perform the same degree of utilization review.
Munchausen disorder—a variant of factitious disorder—is not recognized by DSM-IV. The term—while still used primarily by nonpsychiatrists—is generally viewed as outdated. The term is reserved for patients with the most severe and chronic form of factitious disorder.4 The few studies done of patients with this variant have not adequately examined the specificity and sensitivity of their core symptoms or other characteristics, such as production of a misleading medical condition, travel to multiple medical centers (peregrination), and the telling of tall tales (pseudologia fantastica).
Somatoform disorder. If physicians suspect that a patient’s illness is taking an unusual course, they may suspect a somatoform rather than factitious disorder. Patients with somatoform disorder do not intentionally produce their symptoms, whereas patients with factitious disorder deliberately try to appear ill. In both disorders, the underlying cause is unconscious.
Hypochondriasis. Patients with hypochondriasis are obsessed with concerns that they have an illness. Their worries may compel them to seek out examinations and diagnostic tests. Unlike patients with factitious disorder, these patients do not deliberately provide information or manufacture symptoms to create the appearance of a medical disorder.
Malingering. Patients who malinger may engage in deceitful behaviors that can include creating a misleading impression about a medical or psychiatric illness. Being a patient, however, is not their objective. They may be seeking disability payments, insurance settlements, shelter, or food.
Patient evaluation
Patients suspected of factitious disorder merit a thorough medical and psychiatric evaluation, guided by their presenting symptoms. They commonly have comorbid psychiatric disorders (Table 3), which medical/surgical team members and the psychiatrist need to identify before considering a diagnosis of factitious disorder.
Because invasive tests such as angiography, colonoscopy, biopsies, or exploratory surgery are required to exclude some underlying medical processes, the treatment team must take care not to cause harm. The expected benefits of diagnostic testing must be balanced against the risks of an iatrogenic event.
Table 2
FIVE PROPOSED SUBTYPES OF FACTITIOUS DISORDER
| Characteristic | Examples |
|---|---|
| May be most difficult to detect | |
| 1. Exaggerates physical symptoms 2. Provides a false medical history | An epileptic patient has a seizure while EEG is normal Describes a fictitious history of cancer |
| Can potentially be identified by diagnostic testing | |
| 3. Simulates physical symptoms 4. Modifies physiology to create physical signs 5. Induces physical illness | Puts gravel into urine sample Exerts oneself before vital signs test to elevate blood pressure Injects foreign material into a surgical wound to slow healing |
| Source: Adapted from Folks et al.2 | |
Relatively little is known about how to diagnose a factitious process coexisting with a genuine medical disorder. For example, a patient with well-documented chronic inflammatory disease may easily exaggerate pain and diarrhea to facilitate hospital admission.
To confront or not to confront?
Some patients may relish the patient role for a time—such as while being evaluated for a presumed opportunistic infection—but may not consent to more definitive tests—such as HIV testing. They may demand discharge while they still may be harming themselves, such as by injecting foreign material. The patient may plan to find another health care provider and continue the maladaptive behavior.
If you suspected that our case patient was playing a role in perpetuating her chronic knee infections, would you confront her with the evidence? The answer is unclear, but some experts argue against confrontation.5 Once a patient believes that the medical team suspects a factitious process, he or she may no longer wish to cooperate, even if the diagnostic evaluation is incomplete. Patients often become more guarded about what they reveal after they are confronted. They may become more careful to hide evidence of wound tampering (e.g., syringes) and hesitant to discuss emotional issues (e.g., estranged relationships, feeling overwhelmed by work and home duties).
Case reports suggest that patients who simulate symptoms, modify their physiology, or induce physical illness are at high risk of morbidity and mortality. For example, one report described a patient who underwent two cardiopulmonary resuscitations because of torsades de pointes triggered by hypokalemia related to covert laxative use.6 Physicians must manage these cases carefully to reduce patient risk. In rare cases where a patient’s behavior becomes life-threatening, admission to a psychiatric unit—even involuntarily—may be necessary.
Collaborating with the patient
A comprehensive treatment approach is optimal for patients with factitious disorder. All the patient’s objective medical disorders should be addressed in systematically and with empathy. Treating a co-existing medical disorder may help the physician gain the patient’s trust, which in turn can help keep treatment options open.
Some patients have been known to exaggerate their physical symptoms because they feel they have a serious, undiagnosed medical problem. They feel that their assessment has been cursory and that they need to compel the physician to do a more thorough evaluation in order to identify the true underlying problem. Although no research supports this observation, these patients may be reassured when their physicians carefully evaluate their medical problems.
Eisendrath5 recommends that the treatment team take time to get to know the patient and convey that this attention is devoted to the person, not just the medical illness. This approach may increase the likelihood of learning about psychosocial issues the person may be trying to resolve by taking the patient role. Patients also may be more willing to complete the evaluation and adhere to recommended treatment, although these outcomes are not guaranteed.
Table 3
DISORDERS KNOWN TO CO-EXIST WITH FACTITIOUS DISORDER
| Disorder | Possible issue |
|---|---|
| Medical | Coexisting medical disease |
| Delusional | Somatic delusions |
| Depressive | Somatic complaints, dependency on staff |
| Chemical dependency | Prescription drug abuse |
| Eating disorders | Persistent vomiting, weight loss |
| Obsessive-compulsive disorder | Somatic obsessions |
| Hypochondriasis | Conviction one is unwell |
| Pain disorders | Pain complaints |
| Malingering | Seeking shelter in hospital |
| Source: Adapted from Folks et al. Somatoform disorders, factitious disorders, and malingering. | |
| In: Stoudemire A, Fogel B, Greenberg D, eds. Psychiatric care of the medical patient (2nd ed). New York: Oxford University Press, 2000:458-75. | |
Case report
For the patient with chronic knee infections, the staff psychiatrist recommended that the orthopedist develop a collaborative relationship with her. Eventually the surgeon told her that she needed psychiatric care, and the patient agreed to psychiatric hospitalization.
In this setting, she was initially observed with a 24-hour monitor and received appropriate wound care. The staff encouraged her to talk about the emotional distress related to having a chronic disease. She never admitted to perpetuating the infections in her knees, although she was suspected of injecting herself with infected material. Psychiatric evaluation revealed a history of multiple strained relationships that suggested a severe personality disorder.
Her wounds slowly began to improve, and she was discharged after 2 weeks. Throughout her stay, she remained reluctant to discuss her relationship with her husband or examine other possible sources of stress in her life. Thus, factitious behavior will probably recur unless she tackles her unconscious motivations for adopting a patient role.
If patients’ emotional needs are being met, they may reveal the mechanism of their disease. Unfortunately, experience suggests that very few confess the false nature of their medical illness, fewer accept psychiatric treatment, and even fewer complete the recommended course of treatment.
Comorbid psychiatric disorders provide an opportunity to intervene with selected medications and psychotherapy to reduce patient distress. Chemical dependency treatment in particular can help stabilize a patient with a factitious disorder so that he or she no longer seeks pain medications or sedatives. Patients with an obsessive-compulsive disorder or hypochondriasis may require specifically targeted cognitive-behavioral therapy or pharmacotherapy.
Few references regarding treatment of factitious disorder exist; the only known review of cognitive-behavioral therapy’s role in treating this disorder awaits publication.
Related resources
- Sutherland AJ, Rodin GM. Factitious disorders in a general hospital setting: clinical features and a review of the literature. Psychosomatics 1990;31(4):392-9.
- Reich P, Gottfried LA. Factitious disorders in a teaching hospital. Ann Intern Med 1983;99(2):240-7.
1. American Psychiatric Association Diagnostic and statistical manual of mental disorders (4th ed). Washington DC: American Psychiatric Association, 1994-886.
2. Folks D, Feldman M, Ford C. Somatoform disorders, factitious disorders, and malingering. In: Stoudemire A, Fogel B, Greenberg D, eds. Psychiatric care of the medical patient (2nd ed). New York: Oxford University Press, 2000;458-75.
3. Wallach J. Laboratory diagnosis of factitious disorders. Arch Intern Med 1994;154:1690-6.
4. Asher R. Munchausen’s syndrome. Lancet 1951;1:339-41.
5. Eisendrath S. Factitious physical disorders treatment without confrontation. Psychosomatics 1990;31:357-8.
6. Krahn L, Lee J, Martin MJ, Richardson J, O’Connor M. Hypokalemia leading to torsades de pointes: Munchausen’s syndrome versus bulimia nervosa. Gen Hosp Psychiatry 1997;19:370-7.
An orthopedic surgeon treating a patient, age 29, at a tertiary medical center asks a staff psychiatrist for advice. The patient—who has chronic bilateral knee infections—lives 350 miles away; her treatment-resistant disease has stymied and frustrated her local physicians. Her infections have persisted despite multiple courses of antibiotics and numerous surgical procedures.
Because of damage to the right knee joint, she cannot bear weight or walk. A registered nurse, she has been unable to work or care for her school-aged children for 2 years. The surgeon tells the psychiatrist that the patient denies psychiatric complaints beyond sadness over her inability to fulfill her responsibilities. She expresses a wish to recover and adamantly denies that she manipulates her wound or does anything to interfere with its healing. The medical/surgical team has noticed that while she is away from home receiving orthopedic care, her husband never visits or calls.
Cases such as the one described above are rare, but psychiatrists occasionally encounter patients with these baffling characteristics. When the patient’s disease fails to respond to treatment as expected—or progresses—members of the medical/psychiatric team need to ask themselves these questions:
- Are we dealing with a drug-resistant infection?
- Is the patient adhering fully with treatment?
- Does the patient do anything to perpetuate this disease process and wish to stay ill?
Asking this last question is difficult but necessary in certain situations. Most of us cannot imagine why a person would wish to remain sick. Why would someone be willing to endure pain and multiple hospital stays, remain isolated from family, and risk a permanent disability? Yet, an unknown number of people strive to appear unwell so that they can receive ongoing medical care.
What are factitious disorders?
Factitious disorders are psychiatric conditions in which patients deliberately portray themselves as ill. They may present with physical or psychological symptoms or both. Their objective is to assume the sick role—not to procure shelter, obtain financial assistance, avoid prison, etc., which would fall into other diagnoses such as malingering.
Table 1
DSM-IV DIAGNOSTIC CRITERIA FOR FACTITIOUS DISORDER
|
| Types |
|
| Source: DSM-IV-TR |
DSM-IV criteria are straightforward and inclusive (Table 1).1 They do not specify:
- the presence of medical and/or psychiatric disorders, which do not preclude the diagnosis
- reasons why a person may wish to assume the sick role.
The medical literature on factitious disorder includes many compelling case reports. However, the secretive nature of most patients with factitious complaints has made it difficult to conduct carefully designed community-based studies, prospective studies, or controlled randomized trials. Because research is scarce, much is unknown about who gets factitious disorder, what causes it, and how to treat it.
Differential diagnosis
Factitious disorder varies in severity. Among subtypes proposed by Folks et al (Table 2),2 patients in categories 3, 4, and 5—who produce physical illness—can potentially be identified by diagnostic testing.3 Patients in categories 1 and 2—who exaggerate physical symptoms and provide a false medical history—may be more difficult to detect.
In cases where patients exaggerate symptoms or fabricate histories, little objective information is typically available to the treating physicians. Medical records revealing multiple admissions or emergency room visits may be obtained from other institutions only if the patient gives permission. However, the patient often does not consent or the materials cannot be located.
Third-party payers’ pre-authorization procedures and utilization reviews may speak volumes about a patient’s search for health care. However, patients who are unemployed or estranged from spouses may lose insurance coverage over time. Government assistance programs such as Medicare and Medicaid provide care to many patients with these chronic problems and do not perform the same degree of utilization review.
Munchausen disorder—a variant of factitious disorder—is not recognized by DSM-IV. The term—while still used primarily by nonpsychiatrists—is generally viewed as outdated. The term is reserved for patients with the most severe and chronic form of factitious disorder.4 The few studies done of patients with this variant have not adequately examined the specificity and sensitivity of their core symptoms or other characteristics, such as production of a misleading medical condition, travel to multiple medical centers (peregrination), and the telling of tall tales (pseudologia fantastica).
Somatoform disorder. If physicians suspect that a patient’s illness is taking an unusual course, they may suspect a somatoform rather than factitious disorder. Patients with somatoform disorder do not intentionally produce their symptoms, whereas patients with factitious disorder deliberately try to appear ill. In both disorders, the underlying cause is unconscious.
Hypochondriasis. Patients with hypochondriasis are obsessed with concerns that they have an illness. Their worries may compel them to seek out examinations and diagnostic tests. Unlike patients with factitious disorder, these patients do not deliberately provide information or manufacture symptoms to create the appearance of a medical disorder.
Malingering. Patients who malinger may engage in deceitful behaviors that can include creating a misleading impression about a medical or psychiatric illness. Being a patient, however, is not their objective. They may be seeking disability payments, insurance settlements, shelter, or food.
Patient evaluation
Patients suspected of factitious disorder merit a thorough medical and psychiatric evaluation, guided by their presenting symptoms. They commonly have comorbid psychiatric disorders (Table 3), which medical/surgical team members and the psychiatrist need to identify before considering a diagnosis of factitious disorder.
Because invasive tests such as angiography, colonoscopy, biopsies, or exploratory surgery are required to exclude some underlying medical processes, the treatment team must take care not to cause harm. The expected benefits of diagnostic testing must be balanced against the risks of an iatrogenic event.
Table 2
FIVE PROPOSED SUBTYPES OF FACTITIOUS DISORDER
| Characteristic | Examples |
|---|---|
| May be most difficult to detect | |
| 1. Exaggerates physical symptoms 2. Provides a false medical history | An epileptic patient has a seizure while EEG is normal Describes a fictitious history of cancer |
| Can potentially be identified by diagnostic testing | |
| 3. Simulates physical symptoms 4. Modifies physiology to create physical signs 5. Induces physical illness | Puts gravel into urine sample Exerts oneself before vital signs test to elevate blood pressure Injects foreign material into a surgical wound to slow healing |
| Source: Adapted from Folks et al.2 | |
Relatively little is known about how to diagnose a factitious process coexisting with a genuine medical disorder. For example, a patient with well-documented chronic inflammatory disease may easily exaggerate pain and diarrhea to facilitate hospital admission.
To confront or not to confront?
Some patients may relish the patient role for a time—such as while being evaluated for a presumed opportunistic infection—but may not consent to more definitive tests—such as HIV testing. They may demand discharge while they still may be harming themselves, such as by injecting foreign material. The patient may plan to find another health care provider and continue the maladaptive behavior.
If you suspected that our case patient was playing a role in perpetuating her chronic knee infections, would you confront her with the evidence? The answer is unclear, but some experts argue against confrontation.5 Once a patient believes that the medical team suspects a factitious process, he or she may no longer wish to cooperate, even if the diagnostic evaluation is incomplete. Patients often become more guarded about what they reveal after they are confronted. They may become more careful to hide evidence of wound tampering (e.g., syringes) and hesitant to discuss emotional issues (e.g., estranged relationships, feeling overwhelmed by work and home duties).
Case reports suggest that patients who simulate symptoms, modify their physiology, or induce physical illness are at high risk of morbidity and mortality. For example, one report described a patient who underwent two cardiopulmonary resuscitations because of torsades de pointes triggered by hypokalemia related to covert laxative use.6 Physicians must manage these cases carefully to reduce patient risk. In rare cases where a patient’s behavior becomes life-threatening, admission to a psychiatric unit—even involuntarily—may be necessary.
Collaborating with the patient
A comprehensive treatment approach is optimal for patients with factitious disorder. All the patient’s objective medical disorders should be addressed in systematically and with empathy. Treating a co-existing medical disorder may help the physician gain the patient’s trust, which in turn can help keep treatment options open.
Some patients have been known to exaggerate their physical symptoms because they feel they have a serious, undiagnosed medical problem. They feel that their assessment has been cursory and that they need to compel the physician to do a more thorough evaluation in order to identify the true underlying problem. Although no research supports this observation, these patients may be reassured when their physicians carefully evaluate their medical problems.
Eisendrath5 recommends that the treatment team take time to get to know the patient and convey that this attention is devoted to the person, not just the medical illness. This approach may increase the likelihood of learning about psychosocial issues the person may be trying to resolve by taking the patient role. Patients also may be more willing to complete the evaluation and adhere to recommended treatment, although these outcomes are not guaranteed.
Table 3
DISORDERS KNOWN TO CO-EXIST WITH FACTITIOUS DISORDER
| Disorder | Possible issue |
|---|---|
| Medical | Coexisting medical disease |
| Delusional | Somatic delusions |
| Depressive | Somatic complaints, dependency on staff |
| Chemical dependency | Prescription drug abuse |
| Eating disorders | Persistent vomiting, weight loss |
| Obsessive-compulsive disorder | Somatic obsessions |
| Hypochondriasis | Conviction one is unwell |
| Pain disorders | Pain complaints |
| Malingering | Seeking shelter in hospital |
| Source: Adapted from Folks et al. Somatoform disorders, factitious disorders, and malingering. | |
| In: Stoudemire A, Fogel B, Greenberg D, eds. Psychiatric care of the medical patient (2nd ed). New York: Oxford University Press, 2000:458-75. | |
Case report
For the patient with chronic knee infections, the staff psychiatrist recommended that the orthopedist develop a collaborative relationship with her. Eventually the surgeon told her that she needed psychiatric care, and the patient agreed to psychiatric hospitalization.
In this setting, she was initially observed with a 24-hour monitor and received appropriate wound care. The staff encouraged her to talk about the emotional distress related to having a chronic disease. She never admitted to perpetuating the infections in her knees, although she was suspected of injecting herself with infected material. Psychiatric evaluation revealed a history of multiple strained relationships that suggested a severe personality disorder.
Her wounds slowly began to improve, and she was discharged after 2 weeks. Throughout her stay, she remained reluctant to discuss her relationship with her husband or examine other possible sources of stress in her life. Thus, factitious behavior will probably recur unless she tackles her unconscious motivations for adopting a patient role.
If patients’ emotional needs are being met, they may reveal the mechanism of their disease. Unfortunately, experience suggests that very few confess the false nature of their medical illness, fewer accept psychiatric treatment, and even fewer complete the recommended course of treatment.
Comorbid psychiatric disorders provide an opportunity to intervene with selected medications and psychotherapy to reduce patient distress. Chemical dependency treatment in particular can help stabilize a patient with a factitious disorder so that he or she no longer seeks pain medications or sedatives. Patients with an obsessive-compulsive disorder or hypochondriasis may require specifically targeted cognitive-behavioral therapy or pharmacotherapy.
Few references regarding treatment of factitious disorder exist; the only known review of cognitive-behavioral therapy’s role in treating this disorder awaits publication.
Related resources
- Sutherland AJ, Rodin GM. Factitious disorders in a general hospital setting: clinical features and a review of the literature. Psychosomatics 1990;31(4):392-9.
- Reich P, Gottfried LA. Factitious disorders in a teaching hospital. Ann Intern Med 1983;99(2):240-7.
An orthopedic surgeon treating a patient, age 29, at a tertiary medical center asks a staff psychiatrist for advice. The patient—who has chronic bilateral knee infections—lives 350 miles away; her treatment-resistant disease has stymied and frustrated her local physicians. Her infections have persisted despite multiple courses of antibiotics and numerous surgical procedures.
Because of damage to the right knee joint, she cannot bear weight or walk. A registered nurse, she has been unable to work or care for her school-aged children for 2 years. The surgeon tells the psychiatrist that the patient denies psychiatric complaints beyond sadness over her inability to fulfill her responsibilities. She expresses a wish to recover and adamantly denies that she manipulates her wound or does anything to interfere with its healing. The medical/surgical team has noticed that while she is away from home receiving orthopedic care, her husband never visits or calls.
Cases such as the one described above are rare, but psychiatrists occasionally encounter patients with these baffling characteristics. When the patient’s disease fails to respond to treatment as expected—or progresses—members of the medical/psychiatric team need to ask themselves these questions:
- Are we dealing with a drug-resistant infection?
- Is the patient adhering fully with treatment?
- Does the patient do anything to perpetuate this disease process and wish to stay ill?
Asking this last question is difficult but necessary in certain situations. Most of us cannot imagine why a person would wish to remain sick. Why would someone be willing to endure pain and multiple hospital stays, remain isolated from family, and risk a permanent disability? Yet, an unknown number of people strive to appear unwell so that they can receive ongoing medical care.
What are factitious disorders?
Factitious disorders are psychiatric conditions in which patients deliberately portray themselves as ill. They may present with physical or psychological symptoms or both. Their objective is to assume the sick role—not to procure shelter, obtain financial assistance, avoid prison, etc., which would fall into other diagnoses such as malingering.
Table 1
DSM-IV DIAGNOSTIC CRITERIA FOR FACTITIOUS DISORDER
|
| Types |
|
| Source: DSM-IV-TR |
DSM-IV criteria are straightforward and inclusive (Table 1).1 They do not specify:
- the presence of medical and/or psychiatric disorders, which do not preclude the diagnosis
- reasons why a person may wish to assume the sick role.
The medical literature on factitious disorder includes many compelling case reports. However, the secretive nature of most patients with factitious complaints has made it difficult to conduct carefully designed community-based studies, prospective studies, or controlled randomized trials. Because research is scarce, much is unknown about who gets factitious disorder, what causes it, and how to treat it.
Differential diagnosis
Factitious disorder varies in severity. Among subtypes proposed by Folks et al (Table 2),2 patients in categories 3, 4, and 5—who produce physical illness—can potentially be identified by diagnostic testing.3 Patients in categories 1 and 2—who exaggerate physical symptoms and provide a false medical history—may be more difficult to detect.
In cases where patients exaggerate symptoms or fabricate histories, little objective information is typically available to the treating physicians. Medical records revealing multiple admissions or emergency room visits may be obtained from other institutions only if the patient gives permission. However, the patient often does not consent or the materials cannot be located.
Third-party payers’ pre-authorization procedures and utilization reviews may speak volumes about a patient’s search for health care. However, patients who are unemployed or estranged from spouses may lose insurance coverage over time. Government assistance programs such as Medicare and Medicaid provide care to many patients with these chronic problems and do not perform the same degree of utilization review.
Munchausen disorder—a variant of factitious disorder—is not recognized by DSM-IV. The term—while still used primarily by nonpsychiatrists—is generally viewed as outdated. The term is reserved for patients with the most severe and chronic form of factitious disorder.4 The few studies done of patients with this variant have not adequately examined the specificity and sensitivity of their core symptoms or other characteristics, such as production of a misleading medical condition, travel to multiple medical centers (peregrination), and the telling of tall tales (pseudologia fantastica).
Somatoform disorder. If physicians suspect that a patient’s illness is taking an unusual course, they may suspect a somatoform rather than factitious disorder. Patients with somatoform disorder do not intentionally produce their symptoms, whereas patients with factitious disorder deliberately try to appear ill. In both disorders, the underlying cause is unconscious.
Hypochondriasis. Patients with hypochondriasis are obsessed with concerns that they have an illness. Their worries may compel them to seek out examinations and diagnostic tests. Unlike patients with factitious disorder, these patients do not deliberately provide information or manufacture symptoms to create the appearance of a medical disorder.
Malingering. Patients who malinger may engage in deceitful behaviors that can include creating a misleading impression about a medical or psychiatric illness. Being a patient, however, is not their objective. They may be seeking disability payments, insurance settlements, shelter, or food.
Patient evaluation
Patients suspected of factitious disorder merit a thorough medical and psychiatric evaluation, guided by their presenting symptoms. They commonly have comorbid psychiatric disorders (Table 3), which medical/surgical team members and the psychiatrist need to identify before considering a diagnosis of factitious disorder.
Because invasive tests such as angiography, colonoscopy, biopsies, or exploratory surgery are required to exclude some underlying medical processes, the treatment team must take care not to cause harm. The expected benefits of diagnostic testing must be balanced against the risks of an iatrogenic event.
Table 2
FIVE PROPOSED SUBTYPES OF FACTITIOUS DISORDER
| Characteristic | Examples |
|---|---|
| May be most difficult to detect | |
| 1. Exaggerates physical symptoms 2. Provides a false medical history | An epileptic patient has a seizure while EEG is normal Describes a fictitious history of cancer |
| Can potentially be identified by diagnostic testing | |
| 3. Simulates physical symptoms 4. Modifies physiology to create physical signs 5. Induces physical illness | Puts gravel into urine sample Exerts oneself before vital signs test to elevate blood pressure Injects foreign material into a surgical wound to slow healing |
| Source: Adapted from Folks et al.2 | |
Relatively little is known about how to diagnose a factitious process coexisting with a genuine medical disorder. For example, a patient with well-documented chronic inflammatory disease may easily exaggerate pain and diarrhea to facilitate hospital admission.
To confront or not to confront?
Some patients may relish the patient role for a time—such as while being evaluated for a presumed opportunistic infection—but may not consent to more definitive tests—such as HIV testing. They may demand discharge while they still may be harming themselves, such as by injecting foreign material. The patient may plan to find another health care provider and continue the maladaptive behavior.
If you suspected that our case patient was playing a role in perpetuating her chronic knee infections, would you confront her with the evidence? The answer is unclear, but some experts argue against confrontation.5 Once a patient believes that the medical team suspects a factitious process, he or she may no longer wish to cooperate, even if the diagnostic evaluation is incomplete. Patients often become more guarded about what they reveal after they are confronted. They may become more careful to hide evidence of wound tampering (e.g., syringes) and hesitant to discuss emotional issues (e.g., estranged relationships, feeling overwhelmed by work and home duties).
Case reports suggest that patients who simulate symptoms, modify their physiology, or induce physical illness are at high risk of morbidity and mortality. For example, one report described a patient who underwent two cardiopulmonary resuscitations because of torsades de pointes triggered by hypokalemia related to covert laxative use.6 Physicians must manage these cases carefully to reduce patient risk. In rare cases where a patient’s behavior becomes life-threatening, admission to a psychiatric unit—even involuntarily—may be necessary.
Collaborating with the patient
A comprehensive treatment approach is optimal for patients with factitious disorder. All the patient’s objective medical disorders should be addressed in systematically and with empathy. Treating a co-existing medical disorder may help the physician gain the patient’s trust, which in turn can help keep treatment options open.
Some patients have been known to exaggerate their physical symptoms because they feel they have a serious, undiagnosed medical problem. They feel that their assessment has been cursory and that they need to compel the physician to do a more thorough evaluation in order to identify the true underlying problem. Although no research supports this observation, these patients may be reassured when their physicians carefully evaluate their medical problems.
Eisendrath5 recommends that the treatment team take time to get to know the patient and convey that this attention is devoted to the person, not just the medical illness. This approach may increase the likelihood of learning about psychosocial issues the person may be trying to resolve by taking the patient role. Patients also may be more willing to complete the evaluation and adhere to recommended treatment, although these outcomes are not guaranteed.
Table 3
DISORDERS KNOWN TO CO-EXIST WITH FACTITIOUS DISORDER
| Disorder | Possible issue |
|---|---|
| Medical | Coexisting medical disease |
| Delusional | Somatic delusions |
| Depressive | Somatic complaints, dependency on staff |
| Chemical dependency | Prescription drug abuse |
| Eating disorders | Persistent vomiting, weight loss |
| Obsessive-compulsive disorder | Somatic obsessions |
| Hypochondriasis | Conviction one is unwell |
| Pain disorders | Pain complaints |
| Malingering | Seeking shelter in hospital |
| Source: Adapted from Folks et al. Somatoform disorders, factitious disorders, and malingering. | |
| In: Stoudemire A, Fogel B, Greenberg D, eds. Psychiatric care of the medical patient (2nd ed). New York: Oxford University Press, 2000:458-75. | |
Case report
For the patient with chronic knee infections, the staff psychiatrist recommended that the orthopedist develop a collaborative relationship with her. Eventually the surgeon told her that she needed psychiatric care, and the patient agreed to psychiatric hospitalization.
In this setting, she was initially observed with a 24-hour monitor and received appropriate wound care. The staff encouraged her to talk about the emotional distress related to having a chronic disease. She never admitted to perpetuating the infections in her knees, although she was suspected of injecting herself with infected material. Psychiatric evaluation revealed a history of multiple strained relationships that suggested a severe personality disorder.
Her wounds slowly began to improve, and she was discharged after 2 weeks. Throughout her stay, she remained reluctant to discuss her relationship with her husband or examine other possible sources of stress in her life. Thus, factitious behavior will probably recur unless she tackles her unconscious motivations for adopting a patient role.
If patients’ emotional needs are being met, they may reveal the mechanism of their disease. Unfortunately, experience suggests that very few confess the false nature of their medical illness, fewer accept psychiatric treatment, and even fewer complete the recommended course of treatment.
Comorbid psychiatric disorders provide an opportunity to intervene with selected medications and psychotherapy to reduce patient distress. Chemical dependency treatment in particular can help stabilize a patient with a factitious disorder so that he or she no longer seeks pain medications or sedatives. Patients with an obsessive-compulsive disorder or hypochondriasis may require specifically targeted cognitive-behavioral therapy or pharmacotherapy.
Few references regarding treatment of factitious disorder exist; the only known review of cognitive-behavioral therapy’s role in treating this disorder awaits publication.
Related resources
- Sutherland AJ, Rodin GM. Factitious disorders in a general hospital setting: clinical features and a review of the literature. Psychosomatics 1990;31(4):392-9.
- Reich P, Gottfried LA. Factitious disorders in a teaching hospital. Ann Intern Med 1983;99(2):240-7.
1. American Psychiatric Association Diagnostic and statistical manual of mental disorders (4th ed). Washington DC: American Psychiatric Association, 1994-886.
2. Folks D, Feldman M, Ford C. Somatoform disorders, factitious disorders, and malingering. In: Stoudemire A, Fogel B, Greenberg D, eds. Psychiatric care of the medical patient (2nd ed). New York: Oxford University Press, 2000;458-75.
3. Wallach J. Laboratory diagnosis of factitious disorders. Arch Intern Med 1994;154:1690-6.
4. Asher R. Munchausen’s syndrome. Lancet 1951;1:339-41.
5. Eisendrath S. Factitious physical disorders treatment without confrontation. Psychosomatics 1990;31:357-8.
6. Krahn L, Lee J, Martin MJ, Richardson J, O’Connor M. Hypokalemia leading to torsades de pointes: Munchausen’s syndrome versus bulimia nervosa. Gen Hosp Psychiatry 1997;19:370-7.
1. American Psychiatric Association Diagnostic and statistical manual of mental disorders (4th ed). Washington DC: American Psychiatric Association, 1994-886.
2. Folks D, Feldman M, Ford C. Somatoform disorders, factitious disorders, and malingering. In: Stoudemire A, Fogel B, Greenberg D, eds. Psychiatric care of the medical patient (2nd ed). New York: Oxford University Press, 2000;458-75.
3. Wallach J. Laboratory diagnosis of factitious disorders. Arch Intern Med 1994;154:1690-6.
4. Asher R. Munchausen’s syndrome. Lancet 1951;1:339-41.
5. Eisendrath S. Factitious physical disorders treatment without confrontation. Psychosomatics 1990;31:357-8.
6. Krahn L, Lee J, Martin MJ, Richardson J, O’Connor M. Hypokalemia leading to torsades de pointes: Munchausen’s syndrome versus bulimia nervosa. Gen Hosp Psychiatry 1997;19:370-7.
Excessive daytime sleepiness: Diagnosing the causes
Untreated excessive daytime sleepiness (EDS) results in compromised quality of life, reduced productivity, and public safety concerns.1 Obstructive sleep apnea (OSA), restless legs syndrome, circadian rhythm disorders, and narcolepsy are frequently underdiagnosed sleep disorders that can cause EDS. These conditions commonly go undetected and untreated for several reasons:
- Patients may not recognize sleepiness as a legitimate medical concern.
- Physicians, with few exceptions, typically have little training in sleep disorders and limited time to diagnose them.2 Screening questions regarding sleep are typically absent.
- Definitive diagnostic tests are costly.
As a result, many patients go without appropriate sleep evaluations. Instead a depressive or other psychiatric disorder may be suspected because of the sleepy patient’s poor energy, hypersomnia, amotivation, irritability, and frustration. Because of ongoing behavioral symptoms, patients with an undiagnosed primary sleep disorder are often referred to psychiatrists. Thus, a clear understanding of the differential diagnosis of EDS is crucial.
Patients with sleep issues fall into three major categories:
- Patients with EDS.
- Individuals with insomnia, another large group often seen by psychiatrists. Generally, these patients are less hesitant than patients with EDS to seek help because of the marked distress they suffer nightly when trying to sleep. Insomniacs typically experience minimal EDS.
- Patients with unusual behaviors at night that range from arm waving to violent behaviors.
Assessing the sleepy patient
When evaluating a patient with sleep complaints, several valuable sources of data come into play.
Initially, observe the patient in the waiting room or office before starting the interview. Did the patient nod off while waiting for his or her appointment? Pay attention to any patient who appears sleepy—even if he or she denies having trouble staying awake. Over time, sleepy patients may have lost their perspective on alertness. Some patients have had EDS for so many years that they no longer recall what it is like to feel fully awake.
Collateral history is often important because family members generally observe the sleeping patient. The bed partner often provides valuable information about snoring, irregular breathing leg kicks, unplanned naps, and strained interpersonal relationships due to EDS. For the patient who does not have a bed partner, ask his or her travel companion, with whom the patient may have shared accommodations.
Unfortunately, few useful screening tests exist. Most questionnaires about sleepiness are neither very reliable nor valid. One of the better questionnaires, the Epworth Sleepiness Scale, helps confirm the presence of sleepiness with a score <8, differentiating the inability to stay awake from fatigue. (Box 1 can be cut out, copied, and handed to patients). This brief questionnaire also provides a useful measure of severity.3
The value of the Epworth scale is limited, however, because patient answers often are based on a specific time and context that may not be representative. Additional validated surveys include the Pittsburgh Sleep Quality Inventory and several that focus on OSA.4
How likely are you to doze off or fall asleep in the following situations, in contrast to feeling just tired? Even if you have not done some of these things recently, try to work out how each situation would affect you now. Use the scale below to choose the most appropriate number for each situation:
- 0 no chance of dozing
- 1 slight chance of dozing
- 2 moderate chance of dozing
- 3 high chance of dozing
| Chance of dozing | Situation |
|---|---|
| ○ | Sitting and reading |
| ○ | Watching TV |
| ○ | Sitting inactive in a public place (e.g., a theater or a meeting) |
| ○ | Sitting as a passenger in a car for an hour without a break |
| ○ | Lying down to rest in the afternoon when circumstances permit |
| ○ | Sitting and talking to someone |
| ○ | Sitting quietly after a lunch without alcohol |
| ○ | In a car, while stopped for a few minutes in traffic |
| Johns, M. Sleep 14:540-545, 1991. | |
Electroencephalographic (EEG) monitoring can accurately measure the patient’s degree of sleep disruption. This information is critical in understanding if a patient’s EDS is caused by a physiologic condition that prevents quality nocturnal sleep. At this time, however, no portable devices that employ EEG technology are used in clinical settings.
Additionally, none of the widely used screening devices that assess leg kicks indicate the presence of possible periodic limb movements.
Even though overnight pulse oximetry has been used to screen for sleep-disordered breathing,5 the technology has limitations. For one, most pulse oximeters do not provide information about sleep stage or body position. Some patients with significant sleep-disordered breathing lack adequate oxygen desaturations but have frequent EEG arousals due to sleep issues. In this case, pulse oximetry would generate a false negative result because EEG data is not collected. The inadequate sensitivity is most likely to occur with females and thin patients.
Oximetry provides only one or two types of data (oxygen saturation plus possibly heart rate), while other physiologic processes, e.g., body movement or sleep architecture, can repetitively be disrupted during sleep.
The most critical steps in detecting sleep disorders do not require technology or specialized expertise, but rather intuition and common sense. The psychiatrist should consider the possibility of a sleep disorder and incorporate pertinent questions into the clinical interview. Figure 1 lists sequential questions that might uncover specific sleep disorders. Once suspected, the decision whether to refer the patient to a sleep disorder center for diagnostic testing depends on the type of sleep disorder detected.
Diagnosing and treating OSA
Recent epidemiologic studies show that OSA affects at least 4% of men and 2% of women in the United States.6 Psychiatrists are virtually assured of seeing patients with undiagnosed OSA. The condition is caused by repeated collapse of the soft tissues surrounding the upper airway, decreasing airflow that is restored when the patient briefly awakens. Patients develop EDS because of sleep fragmented by frequent arousals.
Figure 1 THE SLEEPY PATIENT: Possible medical and psychiatric explanations
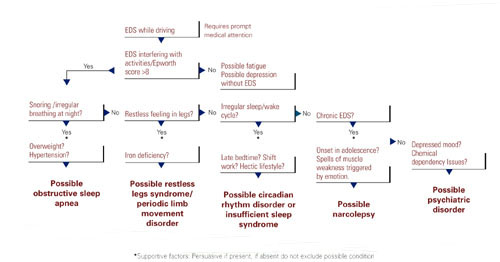
Obese patients are at higher risk than are patients at normal weight because of their body habitus. Alcohol or sedative medication use close to bedtime can aggravate OSA. These substances promote muscle relaxation and increase the arousal threshold, meaning that patients do not wake readily when apneas occur.
Long-term complications of untreated OSA include sleepiness leading to accidents, hypertension, cerebrovascular disease, and progressive obesity. New data associate OSA with multiple potential cardiovascular complications (arrhythmias, congestive heart failure, and myocardial infarction).7 Therefore, recognition and treatment are paramount.
The physical examination should focus on detecting nasal obstruction (having the patient sniff separately through each nostril can be helpful), big neck, crowded oropharynx (a low-hanging palate, reddened uvula, enlarged tonsils, large tongue size relative to oropharynx diameter) and jaw structure (particularly a small retrognathic mandible).
Referral for nocturnal polysomnography might be the next step. During a comprehensive sleep study, data is collected about respiratory, cardiovascular, and muscle activity at night, as well as the sounds the patient makes (e.g., snoring, coughing) when asleep. EEG monitoring also is performed. OSA may be diagnosed if repeated episodes of reduced airflow and oxygen desaturation are observed; these typically result in brief shifts in EEG frequency called arousals.
First-line interventions for OSA include avoidance of alcohol within 1 to 2 hours of bedtime, sleeping on the side instead of the back, weight loss (ideally with a regular exercise program), and nasal sprays for allergies.
If the first-line treatments for OSA are ineffective, nasal continuous positive airway pressure (CPAP) works well for almost all patients who adhere to the regimen.8 CPAP requires the patient to wear a nasal mask that delivers room air, splinting open the nasopharynx and the upper airway (Box 2). Some patients benefit from a brief trial of a sleeping medication, e.g., zolpidem or trazodone, for the first 1 to 2 weeks of nasal CPAP usage.
Nasal continuous positive airway pressure (CPAP) must be started in an observed setting so that the clinician can determine the optimal amount of positive pressure needed to keep the upper airway patent. CPAP can be started during the second half of a “split-night” sleep study after obstructive sleep apnea (OSA) has been diagnosed. Alternatively, the sleep laboratory might ask the patient to return for a second night for a trial of nasal CPAP.
Patients with severe OSA might notice improved sleep quality and reduced EDS, even after only a few hours of use. Such patients sometimes wish to start CPAP treatment immediately.
Overall, advances in masks and equipment have improved patient adherence to CPAP. Such innovations include auto-titrating machines, in which the pressure level can be varied depending on sleep state or body position. Many newer machines also have a data microchip that allows the clinician to determine the duration of usage, then use that information to counsel the patient about adherence if necessary.
Patient education also can promote CPAP adherence. Upon being first told they might need to sleep each night wearing a nasal mask, patients often voice well-founded concerns about comfort, claustrophobia, or sexual activity.
As part of a comprehensive approach at the Mayo Sleep Disorders Center, patients watch an educational videotape, tour the sleep laboratory bedrooms before the sleep study, and are carefully fitted for masks. Ideally, the technologists interact with the patient during the sleep study to adjust the headgear and fine-tune other aspects of the equipment. The sleep specialist meets with the patient to compare the baseline diagnostic study results with changes in breathing patterns after a trial of nasal CPAP.
Other useful patient compliance tools include a CPAP informational handout, telephone access to nursing staff, and a 30-day follow-up visit.
Obtaining the support of the bed partner by welcoming her or him to all appointments, including educational activities, is optimal. The bed partner was likely the impetus for the appointment in the first place because of concerns about excessive snoring or apneas.
Image reprinted from Oct. 2001 Mayo Clinic Health Letter with permission of Mayo Foundation for Medical Education and Research, Rochester, MN 55905
Surgical options exist for OSA. The most common procedures are uvulopalatopharyngoplasty (UPPP) and laser-assisted uvulopalatoplasty (LAUP). Other procedures in use include tongue reduction and mandibular advancement.
The response rate to OSA surgery averages around 50% but varies on the patient’s characteristics and procedure selected.9 Positive outcomes are most likely for thin patients with obvious upper airway obstruction, including a deviated nasal septum, large tonsils, a low-hanging palate, and large uvula. Potential complications include nasal regurgitation, voice change, postoperative pain, bleeding, infection, tongue numbness, and snoring without apnea (silent apnea).
Oral appliances have a vital niche in OSA treatment. Multiple devices have been developed that open the oropharynx by moving the mandible and tongue out of way. A growing body of data shows that oral appliances improve sleep and reduce EDS and promote patient satisfaction more effectively than nasal CPAP.10 Several studies also show that patients with mild to moderate OSA accept these devices well.
Oral devices do have drawbacks, however. In most settings, effectiveness cannot be observed during a “split-night” laboratory sleep study because the patient has not yet purchased the device. Also, multiple visits sometimes are required to custom fit the oral appliance; this can pose a hardship to patients who live a distance from the provider.
Restless legs syndrome, periodic limb movement disorder
The patient with restless legs syndrome typically reports a restless painful feeling in the limbs that occurs in the evening and at night, disrupting sleep. This condition, which affects 10% of the population, is associated with aging, blood loss, anemia, peripheral neuropathies, and pregnancy.11 Patients can have childhood onset and in some cases there is a familial tendency.
Most patients with restless legs syndrome have periodic limb movements (repetitive leg jerks or twitches). The clinical significance of periodic limb movements with no subjective disagreeable feelings in the limbs is controversial. Typically, treatment is not instituted in these cases.
The history usually confirms the diagnosis without a sleep study. Sleep studies are used only if a co-existing sleep problem is suspected or if the diagnosis is not clear-cut.
One suspected mechanism of restless legs syndrome is a dopamine-deficient state. A serum ferritin level can help detect a relative iron deficiency, iron being a cofactor for dopamine synthesis.12
Treatment can include iron repletion when indicated. Medications include dopaminergic agents, most notably pramipexole and levodopa/carbidopa. Other options include gabapentin, benzodiazepines, and narcotics. Antidepressants have been suspected to worsen this condition but definitive studies are lacking.13
Identifying, correcting circadian rhythm disorders
Instead of compromising the quality or quantity of sleep, circadian rhythm disorders cause sleep to occur at inappropriate times. Adolescents or young adults are most likely to confront these disorders.
The delayed sleep phase disorder—that is, a persistent pattern of staying up late and “sleeping in” the next morning—is the most common example. A careful assessment will reveal that the patient is getting a satisfactory amount of sleep that occurs at a socially unacceptable time, sometimes to the extreme that his or her nights and days are reversed.
Patients can be reluctant to acknowledge the severity of their problem, which can lead to both inaccurate sleep diaries and interviews. A portable device called a wrist actigraph provides data about limb movement, thus more objectively measuring the patient’s sleep schedule.
Psychiatrists frequently encounter patients with delayed sleep phase disorder because of a high degree of comorbidity with depressive disorders.14 The cause of this syndrome is unclear, but environmental factors including light exposure, social patterns, psychological issues, and possibly a genetic substrate, are known to contribute.
A less common circadian rhythm disorder, advanced sleep phase disorder, can also cause EDS. Patients have an inappropriately early time of sleep onset and then are fully awake in the middle of the night. A recent report describes a large family with a severe form of this disorder that is linked to an abnormality on chromosome two.15
Relatively few effective treatments have been identified for circadian rhythm disorders. Some patients elect not to pursue therapy, instead selecting activities that fit around their unconventional sleep schedules. Sometimes individuals with delayed sleep phase cannot arrange their education or work hours around their atypical sleep schedules. These patients experience poor early morning academic or work performance due to sleepiness.
The internal circadian clock can be gradually readjusted with either phototherapy or gradual shifting of the major sleep period (Box 4). Stimulant or hypnotic medications generally are not utilized.
Insufficient sleep syndrome
Studies indicate that more people are attempting to burn the candle at both ends and are consequently developing a newly identified condition, insufficient sleep syndrome.16 In our 24-hour society, people often are trying to make do with less than the required 7-1/2 hours sleep per day. This may have adverse consequences to their health. When people are required to perform shift work, the problem is compounded because of the difficulty in obtaining sufficient quality sleep during daylight hours.
Many patients do not seek out treatment for fatigue or sleepiness because they are aware of the lifestyle choices that they have made. Still, they might develop psychologic symptoms like irritability, mood swings, and strained interpersonal relationships. These symptoms often will prompt patients to request treatment.
The most common technique is to ask the patient to establish a consistent awakening time and subsequently a regular bedtime. Initially this could be unconventional by societal standards, i.e., bedtime at 5 a.m. and arising at 2 p.m. Once this pattern is in place, the patient gradually shifts the timing by an hour a day. For most patients it is easier to delay rather than advance the bedtime until it conforms to the desired time.
Reinforce this new sleep pattern with a structured daytime schedule that includes predictable mealtimes, regular exercise, social activities, and possibly bright light exposure. This reinforcement should occur in the morning for delayed sleep phase and in the evening for advanced sleep phase disorder. These interventions take time and discipline.
Another approach is for the patient to completely skip sleep one night and, in a sleep-deprived state, establish a new bedtime at the desired time. The same modalities listed above must be used to reinforce (or “entrain”) this schedule or the patient will gradually slip back into the previous abnormal sleep-wake rhythm.
Major medical centers and North American metropolitan areas are increasingly developing sleep disorder treatment centers. Insurance companies generally cover a specialty sleep evaluation, particularly if the referring physician documents a suspicion of sleep-disordered breathing or excessive daytime sleepiness (EDS)that jeopardizes safe driving.
The most appropriate conditions for an urgent sleep evaluation are:
- Difficulty staying alert while driving, nocturnal cardiac arrhythmias;
- Frequent observed apneas;
- EDS leading to academic or occupational problems.
Psychiatrists should take a careful history that includes a discussion of the patient’s daily and weekly schedule. Avoid psychostimulant medications. Instead, address the non-negotiable need to get adequate sleep and challenge the patients to prioritize his or her activities around a full night’s sleep.
When to consider narcolepsy
Narcolepsy, a less common sleep disorder, can lead to severe occupational, educational, and family disruption. Narcolepsy, which affects 0.05% of the population, is a potentially debilitating disease of the central nervous system that involves abnormal regulation of REM sleep. EDS is the cardinal symptom, often associated with cataplexy (75%), sleep paralysis (50%), vivid dreams, and insomnia, all of which can represent inappropriate intrusion of REM phenomena.
After obtaining a history suggestive of narcolepsy, the psychiatrist should employ either the history, a sleep diary, or wrist actigraphy to document whether the patient is getting adequate sleep with a consistent sleep/wake cycle. Next, consider referring the patient for polysomnography, primarily to rule out other causes of EDS like sleep disorder breathing. In some cases, the REM latency on the overnight sleep study will be less than 20 minutes after sleep onset, which supports the diagnosis of narcolepsy. A multiple sleep latency test (MSLT), a diagnostic test that consists of the patient taking four to five daytime naps, is performed the following day.
Narcolepsy is confirmed if the patient has a mean initial sleep latency of less than 10 minutes during these naps plus at least two REM episodes occurring within 15 minutes after sleep onset.
Recent research shows that most patients who have narcolepsy with cataplexy have undetectable levels of a specific neuropeptide (which is called either hypocretin or orexin) in the cerebrospinal fluid.17 Hypocretin/orexin replacement therapy is a theoretical future possibility, but for now treatment includes a combination of optimal sleep hygiene, psychostimulants, antidepressants, and hypnotics.
Other causes of EDS
Other causes of EDS include unrecognized alcohol dependence, inappropriate or excessive medication use, and depressive disorders. Overnight sleep studies are seldom indicated unless patients endorse the symptoms in Figure 1.
Before pursuing sleep studies (polysomnography or an MSLT), eliminate medications that might confound the results. Such agents include antidepressants, which alter the timing and duration of REM sleep, and sedating medications, which modify initial sleep latency and sleep efficiency and potentially aggravate sleep disordered breathing. Although initial REM latency provides a potential biologic marker of major depression, this measurement is more often used in research studies than in clinical psychiatry.
Primary insomnia is a distressing inability to sleep at night or nap during the day. This suggests a hyperarousal state in several ways, and is the opposite of EDS.18 In rare cases, however, patients who cannot sleep at night also do have EDS. When evaluated, these patients typically endorse at least one of the symptoms in Figure 1. Overnight sleep studies occasionally demonstrate that the insomnia is a symptom of another underlying specific sleep disorder, such as OSA or restless legs syndrome.
Psychiatrists treating a patient with chronic insomnia may appropriately undertake several trials of behavioral interventions or sedating medications before making a referral to a sleep disorder center. Patients can struggle with unrecognized primary sleep disorders for years, and many are evaluated by psychiatrists who institute an empiric trial of stimulating antidepressant medications. Use of antidepressants in these situations is unlikely to cause harm, but they might complicate diagnostic testing. When coexisting depression and a primary sleep disorder are confirmed, management of the sleepy patient optimally entails specific treatments that separately target each condition.
Related resources
- National Sleep Foundation www.sleepfoundation.org
- American Academy of Sleep Medicine www.asda.org
- American Sleep Apnea Association www.sleepapnea.org
- Restless Legs Syndrome Foundation www.rls.org
- Association for the Study of Light Therapy and Biological Rhythms www.sltbr.org
Disclosure
The author reports no affiliation or financial arrangements with any of the companies whose products are mentioned in this article.
Drug brand names
- Carbidopa/levodopa • Sinemet
- Gabapentin • Neurontin
- Pramipexole • Mirapex
- Trazodone • Desyrel
- Zolpidem • Ambien
1. Ronald J, Delaive K, et al. Health care utilization in the 10 years prior to diagnosis in obstructive sleep apnea syndrome patients. Sleep. 1999;22(2):225-29.
2. Punjabi N, Haponik E. Ask about daytime sleepiness. J Amer Geriatr Soc. 2000;48:228-29.
3. Johns M. A new method for measuring daytime sleepiness: The Epworth Sleepiness Scale. Sleep. 1991;14(6):540-45.
4. Rowley J, Aboussouan L, Badr M. The use of clinical prediction formulas in the evaluation of obstructive sleep apnea. Sleep. 2000;23:929-38.
5. Yamashiro Y, Kryger M. Nocturnal oximetry: Is it a screening tool for sleep disorders? Sleep. 1995;18:167-71.
6. Morrell M, Finn L, Kim H, Peppard P, Badr M, Young T. Sleep fragmentation, awake blood pressure, and sleep-disordered breathing in a population-based study. Am J Respir Critical Care Med. 2000;162(6):2091-96.
7. Roux F, D’Ambrosio C, Mohsenin V. Sleep-related breathing disorders and cardiovascular disease. Am J Med. 2000;108:396-402.
8. Engleman H, Martin S, Deary I, Douglas N. Effect of continuous positive airway pressure treatment on daytime function in sleep apnoea/hypopnoea syndrome. Lancet. 1994;343(8897):572-75.
9. Lojander J, Maasilta P, Partinen M, Brander P, Salmi T, Lehtonen H. Nasal-CPAP, surgery, and conservative management for treatment of obstructive sleep apnea syndrome. A randomized study. Chest. 1996;110(1):114-19.
10. Mehta A, Qian J, Petocz P, Darendeliler M, Cistulli P. A randomized, controlled study of a mandibular advancement splint for obstructive sleep apnea. Am J Respir Critical Care Med. 2001;163(6):1457-61.
11. Chesson A, Jr, Wise M, et al. Practice parameters for the treatment of restless legs syndrome and periodic limb movement disorder. Sleep. 1999;22(7):961-68.
12. Phillips B, Young T, Finn L, Asher K, Hening W, Purvis C. Epidemiology of restless legs symptoms in adults. Arch Intern Med. 2000;160(14):2137-41.
13. Thorpy M, Ehrenberg B, Hening W, et al. Restless legs syndrome: Detection and management in primary care. Amer Fam Phys. 2000;62:108-14.
14. Regestein Q, Monk T. Delayed sleep phase syndrome: A review of its clinical aspects. Am J Psychiatry. 1995;152:602-08.
15. Toh K, Jones C, et al. An hPer2 phosphorylation site mutation in familial advanced sleep phase syndrome. Science. 2001;291(5506):1040-43.
16. Yoshikawa N, Suzuki S, Ishimoto T, Matsumoto M, Miyagishi T. A case of insufficient sleep syndrome. Psychiatry Clin Neuro. 1998;52(2):200-01.
17. Nishino S, Ripley B, Overeem S, Lammers G, Mignot E. Hypocretin (orexin) deficiency in human narcolepsy. Lancet. 2000;355:39-40.
18. Hauri P, Esther M. Insomnia. Mayo Clin Proc. 1990;65:869-82.
Untreated excessive daytime sleepiness (EDS) results in compromised quality of life, reduced productivity, and public safety concerns.1 Obstructive sleep apnea (OSA), restless legs syndrome, circadian rhythm disorders, and narcolepsy are frequently underdiagnosed sleep disorders that can cause EDS. These conditions commonly go undetected and untreated for several reasons:
- Patients may not recognize sleepiness as a legitimate medical concern.
- Physicians, with few exceptions, typically have little training in sleep disorders and limited time to diagnose them.2 Screening questions regarding sleep are typically absent.
- Definitive diagnostic tests are costly.
As a result, many patients go without appropriate sleep evaluations. Instead a depressive or other psychiatric disorder may be suspected because of the sleepy patient’s poor energy, hypersomnia, amotivation, irritability, and frustration. Because of ongoing behavioral symptoms, patients with an undiagnosed primary sleep disorder are often referred to psychiatrists. Thus, a clear understanding of the differential diagnosis of EDS is crucial.
Patients with sleep issues fall into three major categories:
- Patients with EDS.
- Individuals with insomnia, another large group often seen by psychiatrists. Generally, these patients are less hesitant than patients with EDS to seek help because of the marked distress they suffer nightly when trying to sleep. Insomniacs typically experience minimal EDS.
- Patients with unusual behaviors at night that range from arm waving to violent behaviors.
Assessing the sleepy patient
When evaluating a patient with sleep complaints, several valuable sources of data come into play.
Initially, observe the patient in the waiting room or office before starting the interview. Did the patient nod off while waiting for his or her appointment? Pay attention to any patient who appears sleepy—even if he or she denies having trouble staying awake. Over time, sleepy patients may have lost their perspective on alertness. Some patients have had EDS for so many years that they no longer recall what it is like to feel fully awake.
Collateral history is often important because family members generally observe the sleeping patient. The bed partner often provides valuable information about snoring, irregular breathing leg kicks, unplanned naps, and strained interpersonal relationships due to EDS. For the patient who does not have a bed partner, ask his or her travel companion, with whom the patient may have shared accommodations.
Unfortunately, few useful screening tests exist. Most questionnaires about sleepiness are neither very reliable nor valid. One of the better questionnaires, the Epworth Sleepiness Scale, helps confirm the presence of sleepiness with a score <8, differentiating the inability to stay awake from fatigue. (Box 1 can be cut out, copied, and handed to patients). This brief questionnaire also provides a useful measure of severity.3
The value of the Epworth scale is limited, however, because patient answers often are based on a specific time and context that may not be representative. Additional validated surveys include the Pittsburgh Sleep Quality Inventory and several that focus on OSA.4
How likely are you to doze off or fall asleep in the following situations, in contrast to feeling just tired? Even if you have not done some of these things recently, try to work out how each situation would affect you now. Use the scale below to choose the most appropriate number for each situation:
- 0 no chance of dozing
- 1 slight chance of dozing
- 2 moderate chance of dozing
- 3 high chance of dozing
| Chance of dozing | Situation |
|---|---|
| ○ | Sitting and reading |
| ○ | Watching TV |
| ○ | Sitting inactive in a public place (e.g., a theater or a meeting) |
| ○ | Sitting as a passenger in a car for an hour without a break |
| ○ | Lying down to rest in the afternoon when circumstances permit |
| ○ | Sitting and talking to someone |
| ○ | Sitting quietly after a lunch without alcohol |
| ○ | In a car, while stopped for a few minutes in traffic |
| Johns, M. Sleep 14:540-545, 1991. | |
Electroencephalographic (EEG) monitoring can accurately measure the patient’s degree of sleep disruption. This information is critical in understanding if a patient’s EDS is caused by a physiologic condition that prevents quality nocturnal sleep. At this time, however, no portable devices that employ EEG technology are used in clinical settings.
Additionally, none of the widely used screening devices that assess leg kicks indicate the presence of possible periodic limb movements.
Even though overnight pulse oximetry has been used to screen for sleep-disordered breathing,5 the technology has limitations. For one, most pulse oximeters do not provide information about sleep stage or body position. Some patients with significant sleep-disordered breathing lack adequate oxygen desaturations but have frequent EEG arousals due to sleep issues. In this case, pulse oximetry would generate a false negative result because EEG data is not collected. The inadequate sensitivity is most likely to occur with females and thin patients.
Oximetry provides only one or two types of data (oxygen saturation plus possibly heart rate), while other physiologic processes, e.g., body movement or sleep architecture, can repetitively be disrupted during sleep.
The most critical steps in detecting sleep disorders do not require technology or specialized expertise, but rather intuition and common sense. The psychiatrist should consider the possibility of a sleep disorder and incorporate pertinent questions into the clinical interview. Figure 1 lists sequential questions that might uncover specific sleep disorders. Once suspected, the decision whether to refer the patient to a sleep disorder center for diagnostic testing depends on the type of sleep disorder detected.
Diagnosing and treating OSA
Recent epidemiologic studies show that OSA affects at least 4% of men and 2% of women in the United States.6 Psychiatrists are virtually assured of seeing patients with undiagnosed OSA. The condition is caused by repeated collapse of the soft tissues surrounding the upper airway, decreasing airflow that is restored when the patient briefly awakens. Patients develop EDS because of sleep fragmented by frequent arousals.
Figure 1 THE SLEEPY PATIENT: Possible medical and psychiatric explanations

Obese patients are at higher risk than are patients at normal weight because of their body habitus. Alcohol or sedative medication use close to bedtime can aggravate OSA. These substances promote muscle relaxation and increase the arousal threshold, meaning that patients do not wake readily when apneas occur.
Long-term complications of untreated OSA include sleepiness leading to accidents, hypertension, cerebrovascular disease, and progressive obesity. New data associate OSA with multiple potential cardiovascular complications (arrhythmias, congestive heart failure, and myocardial infarction).7 Therefore, recognition and treatment are paramount.
The physical examination should focus on detecting nasal obstruction (having the patient sniff separately through each nostril can be helpful), big neck, crowded oropharynx (a low-hanging palate, reddened uvula, enlarged tonsils, large tongue size relative to oropharynx diameter) and jaw structure (particularly a small retrognathic mandible).
Referral for nocturnal polysomnography might be the next step. During a comprehensive sleep study, data is collected about respiratory, cardiovascular, and muscle activity at night, as well as the sounds the patient makes (e.g., snoring, coughing) when asleep. EEG monitoring also is performed. OSA may be diagnosed if repeated episodes of reduced airflow and oxygen desaturation are observed; these typically result in brief shifts in EEG frequency called arousals.
First-line interventions for OSA include avoidance of alcohol within 1 to 2 hours of bedtime, sleeping on the side instead of the back, weight loss (ideally with a regular exercise program), and nasal sprays for allergies.
If the first-line treatments for OSA are ineffective, nasal continuous positive airway pressure (CPAP) works well for almost all patients who adhere to the regimen.8 CPAP requires the patient to wear a nasal mask that delivers room air, splinting open the nasopharynx and the upper airway (Box 2). Some patients benefit from a brief trial of a sleeping medication, e.g., zolpidem or trazodone, for the first 1 to 2 weeks of nasal CPAP usage.

Nasal continuous positive airway pressure (CPAP) must be started in an observed setting so that the clinician can determine the optimal amount of positive pressure needed to keep the upper airway patent. CPAP can be started during the second half of a “split-night” sleep study after obstructive sleep apnea (OSA) has been diagnosed. Alternatively, the sleep laboratory might ask the patient to return for a second night for a trial of nasal CPAP.
Patients with severe OSA might notice improved sleep quality and reduced EDS, even after only a few hours of use. Such patients sometimes wish to start CPAP treatment immediately.
Overall, advances in masks and equipment have improved patient adherence to CPAP. Such innovations include auto-titrating machines, in which the pressure level can be varied depending on sleep state or body position. Many newer machines also have a data microchip that allows the clinician to determine the duration of usage, then use that information to counsel the patient about adherence if necessary.
Patient education also can promote CPAP adherence. Upon being first told they might need to sleep each night wearing a nasal mask, patients often voice well-founded concerns about comfort, claustrophobia, or sexual activity.
As part of a comprehensive approach at the Mayo Sleep Disorders Center, patients watch an educational videotape, tour the sleep laboratory bedrooms before the sleep study, and are carefully fitted for masks. Ideally, the technologists interact with the patient during the sleep study to adjust the headgear and fine-tune other aspects of the equipment. The sleep specialist meets with the patient to compare the baseline diagnostic study results with changes in breathing patterns after a trial of nasal CPAP.
Other useful patient compliance tools include a CPAP informational handout, telephone access to nursing staff, and a 30-day follow-up visit.
Obtaining the support of the bed partner by welcoming her or him to all appointments, including educational activities, is optimal. The bed partner was likely the impetus for the appointment in the first place because of concerns about excessive snoring or apneas.
Image reprinted from Oct. 2001 Mayo Clinic Health Letter with permission of Mayo Foundation for Medical Education and Research, Rochester, MN 55905
Surgical options exist for OSA. The most common procedures are uvulopalatopharyngoplasty (UPPP) and laser-assisted uvulopalatoplasty (LAUP). Other procedures in use include tongue reduction and mandibular advancement.
The response rate to OSA surgery averages around 50% but varies on the patient’s characteristics and procedure selected.9 Positive outcomes are most likely for thin patients with obvious upper airway obstruction, including a deviated nasal septum, large tonsils, a low-hanging palate, and large uvula. Potential complications include nasal regurgitation, voice change, postoperative pain, bleeding, infection, tongue numbness, and snoring without apnea (silent apnea).
Oral appliances have a vital niche in OSA treatment. Multiple devices have been developed that open the oropharynx by moving the mandible and tongue out of way. A growing body of data shows that oral appliances improve sleep and reduce EDS and promote patient satisfaction more effectively than nasal CPAP.10 Several studies also show that patients with mild to moderate OSA accept these devices well.
Oral devices do have drawbacks, however. In most settings, effectiveness cannot be observed during a “split-night” laboratory sleep study because the patient has not yet purchased the device. Also, multiple visits sometimes are required to custom fit the oral appliance; this can pose a hardship to patients who live a distance from the provider.
Restless legs syndrome, periodic limb movement disorder
The patient with restless legs syndrome typically reports a restless painful feeling in the limbs that occurs in the evening and at night, disrupting sleep. This condition, which affects 10% of the population, is associated with aging, blood loss, anemia, peripheral neuropathies, and pregnancy.11 Patients can have childhood onset and in some cases there is a familial tendency.
Most patients with restless legs syndrome have periodic limb movements (repetitive leg jerks or twitches). The clinical significance of periodic limb movements with no subjective disagreeable feelings in the limbs is controversial. Typically, treatment is not instituted in these cases.
The history usually confirms the diagnosis without a sleep study. Sleep studies are used only if a co-existing sleep problem is suspected or if the diagnosis is not clear-cut.
One suspected mechanism of restless legs syndrome is a dopamine-deficient state. A serum ferritin level can help detect a relative iron deficiency, iron being a cofactor for dopamine synthesis.12
Treatment can include iron repletion when indicated. Medications include dopaminergic agents, most notably pramipexole and levodopa/carbidopa. Other options include gabapentin, benzodiazepines, and narcotics. Antidepressants have been suspected to worsen this condition but definitive studies are lacking.13
Identifying, correcting circadian rhythm disorders
Instead of compromising the quality or quantity of sleep, circadian rhythm disorders cause sleep to occur at inappropriate times. Adolescents or young adults are most likely to confront these disorders.
The delayed sleep phase disorder—that is, a persistent pattern of staying up late and “sleeping in” the next morning—is the most common example. A careful assessment will reveal that the patient is getting a satisfactory amount of sleep that occurs at a socially unacceptable time, sometimes to the extreme that his or her nights and days are reversed.
Patients can be reluctant to acknowledge the severity of their problem, which can lead to both inaccurate sleep diaries and interviews. A portable device called a wrist actigraph provides data about limb movement, thus more objectively measuring the patient’s sleep schedule.
Psychiatrists frequently encounter patients with delayed sleep phase disorder because of a high degree of comorbidity with depressive disorders.14 The cause of this syndrome is unclear, but environmental factors including light exposure, social patterns, psychological issues, and possibly a genetic substrate, are known to contribute.
A less common circadian rhythm disorder, advanced sleep phase disorder, can also cause EDS. Patients have an inappropriately early time of sleep onset and then are fully awake in the middle of the night. A recent report describes a large family with a severe form of this disorder that is linked to an abnormality on chromosome two.15
Relatively few effective treatments have been identified for circadian rhythm disorders. Some patients elect not to pursue therapy, instead selecting activities that fit around their unconventional sleep schedules. Sometimes individuals with delayed sleep phase cannot arrange their education or work hours around their atypical sleep schedules. These patients experience poor early morning academic or work performance due to sleepiness.
The internal circadian clock can be gradually readjusted with either phototherapy or gradual shifting of the major sleep period (Box 4). Stimulant or hypnotic medications generally are not utilized.
Insufficient sleep syndrome
Studies indicate that more people are attempting to burn the candle at both ends and are consequently developing a newly identified condition, insufficient sleep syndrome.16 In our 24-hour society, people often are trying to make do with less than the required 7-1/2 hours sleep per day. This may have adverse consequences to their health. When people are required to perform shift work, the problem is compounded because of the difficulty in obtaining sufficient quality sleep during daylight hours.
Many patients do not seek out treatment for fatigue or sleepiness because they are aware of the lifestyle choices that they have made. Still, they might develop psychologic symptoms like irritability, mood swings, and strained interpersonal relationships. These symptoms often will prompt patients to request treatment.
The most common technique is to ask the patient to establish a consistent awakening time and subsequently a regular bedtime. Initially this could be unconventional by societal standards, i.e., bedtime at 5 a.m. and arising at 2 p.m. Once this pattern is in place, the patient gradually shifts the timing by an hour a day. For most patients it is easier to delay rather than advance the bedtime until it conforms to the desired time.
Reinforce this new sleep pattern with a structured daytime schedule that includes predictable mealtimes, regular exercise, social activities, and possibly bright light exposure. This reinforcement should occur in the morning for delayed sleep phase and in the evening for advanced sleep phase disorder. These interventions take time and discipline.
Another approach is for the patient to completely skip sleep one night and, in a sleep-deprived state, establish a new bedtime at the desired time. The same modalities listed above must be used to reinforce (or “entrain”) this schedule or the patient will gradually slip back into the previous abnormal sleep-wake rhythm.
Major medical centers and North American metropolitan areas are increasingly developing sleep disorder treatment centers. Insurance companies generally cover a specialty sleep evaluation, particularly if the referring physician documents a suspicion of sleep-disordered breathing or excessive daytime sleepiness (EDS)that jeopardizes safe driving.
The most appropriate conditions for an urgent sleep evaluation are:
- Difficulty staying alert while driving, nocturnal cardiac arrhythmias;
- Frequent observed apneas;
- EDS leading to academic or occupational problems.
Psychiatrists should take a careful history that includes a discussion of the patient’s daily and weekly schedule. Avoid psychostimulant medications. Instead, address the non-negotiable need to get adequate sleep and challenge the patients to prioritize his or her activities around a full night’s sleep.
When to consider narcolepsy
Narcolepsy, a less common sleep disorder, can lead to severe occupational, educational, and family disruption. Narcolepsy, which affects 0.05% of the population, is a potentially debilitating disease of the central nervous system that involves abnormal regulation of REM sleep. EDS is the cardinal symptom, often associated with cataplexy (75%), sleep paralysis (50%), vivid dreams, and insomnia, all of which can represent inappropriate intrusion of REM phenomena.
After obtaining a history suggestive of narcolepsy, the psychiatrist should employ either the history, a sleep diary, or wrist actigraphy to document whether the patient is getting adequate sleep with a consistent sleep/wake cycle. Next, consider referring the patient for polysomnography, primarily to rule out other causes of EDS like sleep disorder breathing. In some cases, the REM latency on the overnight sleep study will be less than 20 minutes after sleep onset, which supports the diagnosis of narcolepsy. A multiple sleep latency test (MSLT), a diagnostic test that consists of the patient taking four to five daytime naps, is performed the following day.
Narcolepsy is confirmed if the patient has a mean initial sleep latency of less than 10 minutes during these naps plus at least two REM episodes occurring within 15 minutes after sleep onset.
Recent research shows that most patients who have narcolepsy with cataplexy have undetectable levels of a specific neuropeptide (which is called either hypocretin or orexin) in the cerebrospinal fluid.17 Hypocretin/orexin replacement therapy is a theoretical future possibility, but for now treatment includes a combination of optimal sleep hygiene, psychostimulants, antidepressants, and hypnotics.
Other causes of EDS
Other causes of EDS include unrecognized alcohol dependence, inappropriate or excessive medication use, and depressive disorders. Overnight sleep studies are seldom indicated unless patients endorse the symptoms in Figure 1.
Before pursuing sleep studies (polysomnography or an MSLT), eliminate medications that might confound the results. Such agents include antidepressants, which alter the timing and duration of REM sleep, and sedating medications, which modify initial sleep latency and sleep efficiency and potentially aggravate sleep disordered breathing. Although initial REM latency provides a potential biologic marker of major depression, this measurement is more often used in research studies than in clinical psychiatry.
Primary insomnia is a distressing inability to sleep at night or nap during the day. This suggests a hyperarousal state in several ways, and is the opposite of EDS.18 In rare cases, however, patients who cannot sleep at night also do have EDS. When evaluated, these patients typically endorse at least one of the symptoms in Figure 1. Overnight sleep studies occasionally demonstrate that the insomnia is a symptom of another underlying specific sleep disorder, such as OSA or restless legs syndrome.
Psychiatrists treating a patient with chronic insomnia may appropriately undertake several trials of behavioral interventions or sedating medications before making a referral to a sleep disorder center. Patients can struggle with unrecognized primary sleep disorders for years, and many are evaluated by psychiatrists who institute an empiric trial of stimulating antidepressant medications. Use of antidepressants in these situations is unlikely to cause harm, but they might complicate diagnostic testing. When coexisting depression and a primary sleep disorder are confirmed, management of the sleepy patient optimally entails specific treatments that separately target each condition.
Related resources
- National Sleep Foundation www.sleepfoundation.org
- American Academy of Sleep Medicine www.asda.org
- American Sleep Apnea Association www.sleepapnea.org
- Restless Legs Syndrome Foundation www.rls.org
- Association for the Study of Light Therapy and Biological Rhythms www.sltbr.org
Disclosure
The author reports no affiliation or financial arrangements with any of the companies whose products are mentioned in this article.
Drug brand names
- Carbidopa/levodopa • Sinemet
- Gabapentin • Neurontin
- Pramipexole • Mirapex
- Trazodone • Desyrel
- Zolpidem • Ambien
Untreated excessive daytime sleepiness (EDS) results in compromised quality of life, reduced productivity, and public safety concerns.1 Obstructive sleep apnea (OSA), restless legs syndrome, circadian rhythm disorders, and narcolepsy are frequently underdiagnosed sleep disorders that can cause EDS. These conditions commonly go undetected and untreated for several reasons:
- Patients may not recognize sleepiness as a legitimate medical concern.
- Physicians, with few exceptions, typically have little training in sleep disorders and limited time to diagnose them.2 Screening questions regarding sleep are typically absent.
- Definitive diagnostic tests are costly.
As a result, many patients go without appropriate sleep evaluations. Instead a depressive or other psychiatric disorder may be suspected because of the sleepy patient’s poor energy, hypersomnia, amotivation, irritability, and frustration. Because of ongoing behavioral symptoms, patients with an undiagnosed primary sleep disorder are often referred to psychiatrists. Thus, a clear understanding of the differential diagnosis of EDS is crucial.
Patients with sleep issues fall into three major categories:
- Patients with EDS.
- Individuals with insomnia, another large group often seen by psychiatrists. Generally, these patients are less hesitant than patients with EDS to seek help because of the marked distress they suffer nightly when trying to sleep. Insomniacs typically experience minimal EDS.
- Patients with unusual behaviors at night that range from arm waving to violent behaviors.
Assessing the sleepy patient
When evaluating a patient with sleep complaints, several valuable sources of data come into play.
Initially, observe the patient in the waiting room or office before starting the interview. Did the patient nod off while waiting for his or her appointment? Pay attention to any patient who appears sleepy—even if he or she denies having trouble staying awake. Over time, sleepy patients may have lost their perspective on alertness. Some patients have had EDS for so many years that they no longer recall what it is like to feel fully awake.
Collateral history is often important because family members generally observe the sleeping patient. The bed partner often provides valuable information about snoring, irregular breathing leg kicks, unplanned naps, and strained interpersonal relationships due to EDS. For the patient who does not have a bed partner, ask his or her travel companion, with whom the patient may have shared accommodations.
Unfortunately, few useful screening tests exist. Most questionnaires about sleepiness are neither very reliable nor valid. One of the better questionnaires, the Epworth Sleepiness Scale, helps confirm the presence of sleepiness with a score <8, differentiating the inability to stay awake from fatigue. (Box 1 can be cut out, copied, and handed to patients). This brief questionnaire also provides a useful measure of severity.3
The value of the Epworth scale is limited, however, because patient answers often are based on a specific time and context that may not be representative. Additional validated surveys include the Pittsburgh Sleep Quality Inventory and several that focus on OSA.4
How likely are you to doze off or fall asleep in the following situations, in contrast to feeling just tired? Even if you have not done some of these things recently, try to work out how each situation would affect you now. Use the scale below to choose the most appropriate number for each situation:
- 0 no chance of dozing
- 1 slight chance of dozing
- 2 moderate chance of dozing
- 3 high chance of dozing
| Chance of dozing | Situation |
|---|---|
| ○ | Sitting and reading |
| ○ | Watching TV |
| ○ | Sitting inactive in a public place (e.g., a theater or a meeting) |
| ○ | Sitting as a passenger in a car for an hour without a break |
| ○ | Lying down to rest in the afternoon when circumstances permit |
| ○ | Sitting and talking to someone |
| ○ | Sitting quietly after a lunch without alcohol |
| ○ | In a car, while stopped for a few minutes in traffic |
| Johns, M. Sleep 14:540-545, 1991. | |
Electroencephalographic (EEG) monitoring can accurately measure the patient’s degree of sleep disruption. This information is critical in understanding if a patient’s EDS is caused by a physiologic condition that prevents quality nocturnal sleep. At this time, however, no portable devices that employ EEG technology are used in clinical settings.
Additionally, none of the widely used screening devices that assess leg kicks indicate the presence of possible periodic limb movements.
Even though overnight pulse oximetry has been used to screen for sleep-disordered breathing,5 the technology has limitations. For one, most pulse oximeters do not provide information about sleep stage or body position. Some patients with significant sleep-disordered breathing lack adequate oxygen desaturations but have frequent EEG arousals due to sleep issues. In this case, pulse oximetry would generate a false negative result because EEG data is not collected. The inadequate sensitivity is most likely to occur with females and thin patients.
Oximetry provides only one or two types of data (oxygen saturation plus possibly heart rate), while other physiologic processes, e.g., body movement or sleep architecture, can repetitively be disrupted during sleep.
The most critical steps in detecting sleep disorders do not require technology or specialized expertise, but rather intuition and common sense. The psychiatrist should consider the possibility of a sleep disorder and incorporate pertinent questions into the clinical interview. Figure 1 lists sequential questions that might uncover specific sleep disorders. Once suspected, the decision whether to refer the patient to a sleep disorder center for diagnostic testing depends on the type of sleep disorder detected.
Diagnosing and treating OSA
Recent epidemiologic studies show that OSA affects at least 4% of men and 2% of women in the United States.6 Psychiatrists are virtually assured of seeing patients with undiagnosed OSA. The condition is caused by repeated collapse of the soft tissues surrounding the upper airway, decreasing airflow that is restored when the patient briefly awakens. Patients develop EDS because of sleep fragmented by frequent arousals.
Figure 1 THE SLEEPY PATIENT: Possible medical and psychiatric explanations

Obese patients are at higher risk than are patients at normal weight because of their body habitus. Alcohol or sedative medication use close to bedtime can aggravate OSA. These substances promote muscle relaxation and increase the arousal threshold, meaning that patients do not wake readily when apneas occur.
Long-term complications of untreated OSA include sleepiness leading to accidents, hypertension, cerebrovascular disease, and progressive obesity. New data associate OSA with multiple potential cardiovascular complications (arrhythmias, congestive heart failure, and myocardial infarction).7 Therefore, recognition and treatment are paramount.
The physical examination should focus on detecting nasal obstruction (having the patient sniff separately through each nostril can be helpful), big neck, crowded oropharynx (a low-hanging palate, reddened uvula, enlarged tonsils, large tongue size relative to oropharynx diameter) and jaw structure (particularly a small retrognathic mandible).
Referral for nocturnal polysomnography might be the next step. During a comprehensive sleep study, data is collected about respiratory, cardiovascular, and muscle activity at night, as well as the sounds the patient makes (e.g., snoring, coughing) when asleep. EEG monitoring also is performed. OSA may be diagnosed if repeated episodes of reduced airflow and oxygen desaturation are observed; these typically result in brief shifts in EEG frequency called arousals.
First-line interventions for OSA include avoidance of alcohol within 1 to 2 hours of bedtime, sleeping on the side instead of the back, weight loss (ideally with a regular exercise program), and nasal sprays for allergies.
If the first-line treatments for OSA are ineffective, nasal continuous positive airway pressure (CPAP) works well for almost all patients who adhere to the regimen.8 CPAP requires the patient to wear a nasal mask that delivers room air, splinting open the nasopharynx and the upper airway (Box 2). Some patients benefit from a brief trial of a sleeping medication, e.g., zolpidem or trazodone, for the first 1 to 2 weeks of nasal CPAP usage.

Nasal continuous positive airway pressure (CPAP) must be started in an observed setting so that the clinician can determine the optimal amount of positive pressure needed to keep the upper airway patent. CPAP can be started during the second half of a “split-night” sleep study after obstructive sleep apnea (OSA) has been diagnosed. Alternatively, the sleep laboratory might ask the patient to return for a second night for a trial of nasal CPAP.
Patients with severe OSA might notice improved sleep quality and reduced EDS, even after only a few hours of use. Such patients sometimes wish to start CPAP treatment immediately.
Overall, advances in masks and equipment have improved patient adherence to CPAP. Such innovations include auto-titrating machines, in which the pressure level can be varied depending on sleep state or body position. Many newer machines also have a data microchip that allows the clinician to determine the duration of usage, then use that information to counsel the patient about adherence if necessary.
Patient education also can promote CPAP adherence. Upon being first told they might need to sleep each night wearing a nasal mask, patients often voice well-founded concerns about comfort, claustrophobia, or sexual activity.
As part of a comprehensive approach at the Mayo Sleep Disorders Center, patients watch an educational videotape, tour the sleep laboratory bedrooms before the sleep study, and are carefully fitted for masks. Ideally, the technologists interact with the patient during the sleep study to adjust the headgear and fine-tune other aspects of the equipment. The sleep specialist meets with the patient to compare the baseline diagnostic study results with changes in breathing patterns after a trial of nasal CPAP.
Other useful patient compliance tools include a CPAP informational handout, telephone access to nursing staff, and a 30-day follow-up visit.
Obtaining the support of the bed partner by welcoming her or him to all appointments, including educational activities, is optimal. The bed partner was likely the impetus for the appointment in the first place because of concerns about excessive snoring or apneas.
Image reprinted from Oct. 2001 Mayo Clinic Health Letter with permission of Mayo Foundation for Medical Education and Research, Rochester, MN 55905
Surgical options exist for OSA. The most common procedures are uvulopalatopharyngoplasty (UPPP) and laser-assisted uvulopalatoplasty (LAUP). Other procedures in use include tongue reduction and mandibular advancement.
The response rate to OSA surgery averages around 50% but varies on the patient’s characteristics and procedure selected.9 Positive outcomes are most likely for thin patients with obvious upper airway obstruction, including a deviated nasal septum, large tonsils, a low-hanging palate, and large uvula. Potential complications include nasal regurgitation, voice change, postoperative pain, bleeding, infection, tongue numbness, and snoring without apnea (silent apnea).
Oral appliances have a vital niche in OSA treatment. Multiple devices have been developed that open the oropharynx by moving the mandible and tongue out of way. A growing body of data shows that oral appliances improve sleep and reduce EDS and promote patient satisfaction more effectively than nasal CPAP.10 Several studies also show that patients with mild to moderate OSA accept these devices well.
Oral devices do have drawbacks, however. In most settings, effectiveness cannot be observed during a “split-night” laboratory sleep study because the patient has not yet purchased the device. Also, multiple visits sometimes are required to custom fit the oral appliance; this can pose a hardship to patients who live a distance from the provider.
Restless legs syndrome, periodic limb movement disorder
The patient with restless legs syndrome typically reports a restless painful feeling in the limbs that occurs in the evening and at night, disrupting sleep. This condition, which affects 10% of the population, is associated with aging, blood loss, anemia, peripheral neuropathies, and pregnancy.11 Patients can have childhood onset and in some cases there is a familial tendency.
Most patients with restless legs syndrome have periodic limb movements (repetitive leg jerks or twitches). The clinical significance of periodic limb movements with no subjective disagreeable feelings in the limbs is controversial. Typically, treatment is not instituted in these cases.
The history usually confirms the diagnosis without a sleep study. Sleep studies are used only if a co-existing sleep problem is suspected or if the diagnosis is not clear-cut.
One suspected mechanism of restless legs syndrome is a dopamine-deficient state. A serum ferritin level can help detect a relative iron deficiency, iron being a cofactor for dopamine synthesis.12
Treatment can include iron repletion when indicated. Medications include dopaminergic agents, most notably pramipexole and levodopa/carbidopa. Other options include gabapentin, benzodiazepines, and narcotics. Antidepressants have been suspected to worsen this condition but definitive studies are lacking.13
Identifying, correcting circadian rhythm disorders
Instead of compromising the quality or quantity of sleep, circadian rhythm disorders cause sleep to occur at inappropriate times. Adolescents or young adults are most likely to confront these disorders.
The delayed sleep phase disorder—that is, a persistent pattern of staying up late and “sleeping in” the next morning—is the most common example. A careful assessment will reveal that the patient is getting a satisfactory amount of sleep that occurs at a socially unacceptable time, sometimes to the extreme that his or her nights and days are reversed.
Patients can be reluctant to acknowledge the severity of their problem, which can lead to both inaccurate sleep diaries and interviews. A portable device called a wrist actigraph provides data about limb movement, thus more objectively measuring the patient’s sleep schedule.
Psychiatrists frequently encounter patients with delayed sleep phase disorder because of a high degree of comorbidity with depressive disorders.14 The cause of this syndrome is unclear, but environmental factors including light exposure, social patterns, psychological issues, and possibly a genetic substrate, are known to contribute.
A less common circadian rhythm disorder, advanced sleep phase disorder, can also cause EDS. Patients have an inappropriately early time of sleep onset and then are fully awake in the middle of the night. A recent report describes a large family with a severe form of this disorder that is linked to an abnormality on chromosome two.15
Relatively few effective treatments have been identified for circadian rhythm disorders. Some patients elect not to pursue therapy, instead selecting activities that fit around their unconventional sleep schedules. Sometimes individuals with delayed sleep phase cannot arrange their education or work hours around their atypical sleep schedules. These patients experience poor early morning academic or work performance due to sleepiness.
The internal circadian clock can be gradually readjusted with either phototherapy or gradual shifting of the major sleep period (Box 4). Stimulant or hypnotic medications generally are not utilized.
Insufficient sleep syndrome
Studies indicate that more people are attempting to burn the candle at both ends and are consequently developing a newly identified condition, insufficient sleep syndrome.16 In our 24-hour society, people often are trying to make do with less than the required 7-1/2 hours sleep per day. This may have adverse consequences to their health. When people are required to perform shift work, the problem is compounded because of the difficulty in obtaining sufficient quality sleep during daylight hours.
Many patients do not seek out treatment for fatigue or sleepiness because they are aware of the lifestyle choices that they have made. Still, they might develop psychologic symptoms like irritability, mood swings, and strained interpersonal relationships. These symptoms often will prompt patients to request treatment.
The most common technique is to ask the patient to establish a consistent awakening time and subsequently a regular bedtime. Initially this could be unconventional by societal standards, i.e., bedtime at 5 a.m. and arising at 2 p.m. Once this pattern is in place, the patient gradually shifts the timing by an hour a day. For most patients it is easier to delay rather than advance the bedtime until it conforms to the desired time.
Reinforce this new sleep pattern with a structured daytime schedule that includes predictable mealtimes, regular exercise, social activities, and possibly bright light exposure. This reinforcement should occur in the morning for delayed sleep phase and in the evening for advanced sleep phase disorder. These interventions take time and discipline.
Another approach is for the patient to completely skip sleep one night and, in a sleep-deprived state, establish a new bedtime at the desired time. The same modalities listed above must be used to reinforce (or “entrain”) this schedule or the patient will gradually slip back into the previous abnormal sleep-wake rhythm.
Major medical centers and North American metropolitan areas are increasingly developing sleep disorder treatment centers. Insurance companies generally cover a specialty sleep evaluation, particularly if the referring physician documents a suspicion of sleep-disordered breathing or excessive daytime sleepiness (EDS)that jeopardizes safe driving.
The most appropriate conditions for an urgent sleep evaluation are:
- Difficulty staying alert while driving, nocturnal cardiac arrhythmias;
- Frequent observed apneas;
- EDS leading to academic or occupational problems.
Psychiatrists should take a careful history that includes a discussion of the patient’s daily and weekly schedule. Avoid psychostimulant medications. Instead, address the non-negotiable need to get adequate sleep and challenge the patients to prioritize his or her activities around a full night’s sleep.
When to consider narcolepsy
Narcolepsy, a less common sleep disorder, can lead to severe occupational, educational, and family disruption. Narcolepsy, which affects 0.05% of the population, is a potentially debilitating disease of the central nervous system that involves abnormal regulation of REM sleep. EDS is the cardinal symptom, often associated with cataplexy (75%), sleep paralysis (50%), vivid dreams, and insomnia, all of which can represent inappropriate intrusion of REM phenomena.
After obtaining a history suggestive of narcolepsy, the psychiatrist should employ either the history, a sleep diary, or wrist actigraphy to document whether the patient is getting adequate sleep with a consistent sleep/wake cycle. Next, consider referring the patient for polysomnography, primarily to rule out other causes of EDS like sleep disorder breathing. In some cases, the REM latency on the overnight sleep study will be less than 20 minutes after sleep onset, which supports the diagnosis of narcolepsy. A multiple sleep latency test (MSLT), a diagnostic test that consists of the patient taking four to five daytime naps, is performed the following day.
Narcolepsy is confirmed if the patient has a mean initial sleep latency of less than 10 minutes during these naps plus at least two REM episodes occurring within 15 minutes after sleep onset.
Recent research shows that most patients who have narcolepsy with cataplexy have undetectable levels of a specific neuropeptide (which is called either hypocretin or orexin) in the cerebrospinal fluid.17 Hypocretin/orexin replacement therapy is a theoretical future possibility, but for now treatment includes a combination of optimal sleep hygiene, psychostimulants, antidepressants, and hypnotics.
Other causes of EDS
Other causes of EDS include unrecognized alcohol dependence, inappropriate or excessive medication use, and depressive disorders. Overnight sleep studies are seldom indicated unless patients endorse the symptoms in Figure 1.
Before pursuing sleep studies (polysomnography or an MSLT), eliminate medications that might confound the results. Such agents include antidepressants, which alter the timing and duration of REM sleep, and sedating medications, which modify initial sleep latency and sleep efficiency and potentially aggravate sleep disordered breathing. Although initial REM latency provides a potential biologic marker of major depression, this measurement is more often used in research studies than in clinical psychiatry.
Primary insomnia is a distressing inability to sleep at night or nap during the day. This suggests a hyperarousal state in several ways, and is the opposite of EDS.18 In rare cases, however, patients who cannot sleep at night also do have EDS. When evaluated, these patients typically endorse at least one of the symptoms in Figure 1. Overnight sleep studies occasionally demonstrate that the insomnia is a symptom of another underlying specific sleep disorder, such as OSA or restless legs syndrome.
Psychiatrists treating a patient with chronic insomnia may appropriately undertake several trials of behavioral interventions or sedating medications before making a referral to a sleep disorder center. Patients can struggle with unrecognized primary sleep disorders for years, and many are evaluated by psychiatrists who institute an empiric trial of stimulating antidepressant medications. Use of antidepressants in these situations is unlikely to cause harm, but they might complicate diagnostic testing. When coexisting depression and a primary sleep disorder are confirmed, management of the sleepy patient optimally entails specific treatments that separately target each condition.
Related resources
- National Sleep Foundation www.sleepfoundation.org
- American Academy of Sleep Medicine www.asda.org
- American Sleep Apnea Association www.sleepapnea.org
- Restless Legs Syndrome Foundation www.rls.org
- Association for the Study of Light Therapy and Biological Rhythms www.sltbr.org
Disclosure
The author reports no affiliation or financial arrangements with any of the companies whose products are mentioned in this article.
Drug brand names
- Carbidopa/levodopa • Sinemet
- Gabapentin • Neurontin
- Pramipexole • Mirapex
- Trazodone • Desyrel
- Zolpidem • Ambien
1. Ronald J, Delaive K, et al. Health care utilization in the 10 years prior to diagnosis in obstructive sleep apnea syndrome patients. Sleep. 1999;22(2):225-29.
2. Punjabi N, Haponik E. Ask about daytime sleepiness. J Amer Geriatr Soc. 2000;48:228-29.
3. Johns M. A new method for measuring daytime sleepiness: The Epworth Sleepiness Scale. Sleep. 1991;14(6):540-45.
4. Rowley J, Aboussouan L, Badr M. The use of clinical prediction formulas in the evaluation of obstructive sleep apnea. Sleep. 2000;23:929-38.
5. Yamashiro Y, Kryger M. Nocturnal oximetry: Is it a screening tool for sleep disorders? Sleep. 1995;18:167-71.
6. Morrell M, Finn L, Kim H, Peppard P, Badr M, Young T. Sleep fragmentation, awake blood pressure, and sleep-disordered breathing in a population-based study. Am J Respir Critical Care Med. 2000;162(6):2091-96.
7. Roux F, D’Ambrosio C, Mohsenin V. Sleep-related breathing disorders and cardiovascular disease. Am J Med. 2000;108:396-402.
8. Engleman H, Martin S, Deary I, Douglas N. Effect of continuous positive airway pressure treatment on daytime function in sleep apnoea/hypopnoea syndrome. Lancet. 1994;343(8897):572-75.
9. Lojander J, Maasilta P, Partinen M, Brander P, Salmi T, Lehtonen H. Nasal-CPAP, surgery, and conservative management for treatment of obstructive sleep apnea syndrome. A randomized study. Chest. 1996;110(1):114-19.
10. Mehta A, Qian J, Petocz P, Darendeliler M, Cistulli P. A randomized, controlled study of a mandibular advancement splint for obstructive sleep apnea. Am J Respir Critical Care Med. 2001;163(6):1457-61.
11. Chesson A, Jr, Wise M, et al. Practice parameters for the treatment of restless legs syndrome and periodic limb movement disorder. Sleep. 1999;22(7):961-68.
12. Phillips B, Young T, Finn L, Asher K, Hening W, Purvis C. Epidemiology of restless legs symptoms in adults. Arch Intern Med. 2000;160(14):2137-41.
13. Thorpy M, Ehrenberg B, Hening W, et al. Restless legs syndrome: Detection and management in primary care. Amer Fam Phys. 2000;62:108-14.
14. Regestein Q, Monk T. Delayed sleep phase syndrome: A review of its clinical aspects. Am J Psychiatry. 1995;152:602-08.
15. Toh K, Jones C, et al. An hPer2 phosphorylation site mutation in familial advanced sleep phase syndrome. Science. 2001;291(5506):1040-43.
16. Yoshikawa N, Suzuki S, Ishimoto T, Matsumoto M, Miyagishi T. A case of insufficient sleep syndrome. Psychiatry Clin Neuro. 1998;52(2):200-01.
17. Nishino S, Ripley B, Overeem S, Lammers G, Mignot E. Hypocretin (orexin) deficiency in human narcolepsy. Lancet. 2000;355:39-40.
18. Hauri P, Esther M. Insomnia. Mayo Clin Proc. 1990;65:869-82.
1. Ronald J, Delaive K, et al. Health care utilization in the 10 years prior to diagnosis in obstructive sleep apnea syndrome patients. Sleep. 1999;22(2):225-29.
2. Punjabi N, Haponik E. Ask about daytime sleepiness. J Amer Geriatr Soc. 2000;48:228-29.
3. Johns M. A new method for measuring daytime sleepiness: The Epworth Sleepiness Scale. Sleep. 1991;14(6):540-45.
4. Rowley J, Aboussouan L, Badr M. The use of clinical prediction formulas in the evaluation of obstructive sleep apnea. Sleep. 2000;23:929-38.
5. Yamashiro Y, Kryger M. Nocturnal oximetry: Is it a screening tool for sleep disorders? Sleep. 1995;18:167-71.
6. Morrell M, Finn L, Kim H, Peppard P, Badr M, Young T. Sleep fragmentation, awake blood pressure, and sleep-disordered breathing in a population-based study. Am J Respir Critical Care Med. 2000;162(6):2091-96.
7. Roux F, D’Ambrosio C, Mohsenin V. Sleep-related breathing disorders and cardiovascular disease. Am J Med. 2000;108:396-402.
8. Engleman H, Martin S, Deary I, Douglas N. Effect of continuous positive airway pressure treatment on daytime function in sleep apnoea/hypopnoea syndrome. Lancet. 1994;343(8897):572-75.
9. Lojander J, Maasilta P, Partinen M, Brander P, Salmi T, Lehtonen H. Nasal-CPAP, surgery, and conservative management for treatment of obstructive sleep apnea syndrome. A randomized study. Chest. 1996;110(1):114-19.
10. Mehta A, Qian J, Petocz P, Darendeliler M, Cistulli P. A randomized, controlled study of a mandibular advancement splint for obstructive sleep apnea. Am J Respir Critical Care Med. 2001;163(6):1457-61.
11. Chesson A, Jr, Wise M, et al. Practice parameters for the treatment of restless legs syndrome and periodic limb movement disorder. Sleep. 1999;22(7):961-68.
12. Phillips B, Young T, Finn L, Asher K, Hening W, Purvis C. Epidemiology of restless legs symptoms in adults. Arch Intern Med. 2000;160(14):2137-41.
13. Thorpy M, Ehrenberg B, Hening W, et al. Restless legs syndrome: Detection and management in primary care. Amer Fam Phys. 2000;62:108-14.
14. Regestein Q, Monk T. Delayed sleep phase syndrome: A review of its clinical aspects. Am J Psychiatry. 1995;152:602-08.
15. Toh K, Jones C, et al. An hPer2 phosphorylation site mutation in familial advanced sleep phase syndrome. Science. 2001;291(5506):1040-43.
16. Yoshikawa N, Suzuki S, Ishimoto T, Matsumoto M, Miyagishi T. A case of insufficient sleep syndrome. Psychiatry Clin Neuro. 1998;52(2):200-01.
17. Nishino S, Ripley B, Overeem S, Lammers G, Mignot E. Hypocretin (orexin) deficiency in human narcolepsy. Lancet. 2000;355:39-40.
18. Hauri P, Esther M. Insomnia. Mayo Clin Proc. 1990;65:869-82.