User login
Poor energy, hypersomnia, amotivation, irritability, and frustration can suggest depression or other psychiatric disorders to busy primary care physicians. As a result, psychiatrists often are referred patients with excessive daytime sleepiness (EDS) caused by undiagnosed primary sleep disorders.
Physicians may miss obstructive sleep apnea (OSA), restless legs syndrome, circadian rhythm disorders, or narcolepsy because:
- many have little training in sleep disorders and limited time to diagnose them1
- patients do not report sleepiness or recognize it as a legitimate medical concern
- definitive diagnostic tests are expensive and usually are not ordered.
Psychiatrists, therefore, need a clear understanding of the EDS differential diagnosis to determine whether a patient’s behavioral symptoms are a sleep or psychiatric issue.
How likely are you to doze off or fall asleep in the following situations, in contrast to feeling just tired? This refers to your usual way of life in recent times. Even if you have not done some of these things recently, try to work out how each situation would affect you now. Use the scale below to choose the most appropriate number for each situation:
0 no chance of dozing
1 slight chance of dozing
2 moderate chance of dozing
3 high chance of dozing
Chance of dozing Situation
Sitting and reading
Watching TV
Sitting inactive in a public place (such as in a theater or a meeting)
As a passenger in a car for an hour without a break
Lying down to rest in the afternoon when circumstances permit
Sitting and talking to someone
Sitting quietly after a lunch without alcohol
In a car, while stopped for a few minutes in traffic
Scoring key
1 to 6 Getting enough sleep
7 to 8 Average
>8 Seek a sleep specialist’s advice without delay
Assessing the sleepy patient
Sleepiness is an inability to stay awake at appropriate times. Fatigue, by comparison, does not involve sleepiness but very low energy associated with wakefulness. In general, sleepy patients get transient relief from napping, whereas fatigued patients report they cannot fall asleep.
Untreated EDS results in compromised quality of life, reduced productivity, and public safety concerns such as falling asleep while driving.2 Sleep complaints fall into three major categories:
- EDS
- insomnia (marked by distress because of poor sleep, but usually with minimal EDS)
- unusual nocturnal behaviors (ranging from arm waving to violent behaviors.
When you evaluate a patient with sleep complaints, valuable sources of data include observation, questionnaires, and screening devices. The most important may be common sense.
Observation. Observe the patient in the waiting room or office before starting the interview. Did he or she nod off while waiting to see you? Pay attention to anyone who appears sleepy—even those who deny having trouble staying awake. Over time, sleepy patients can lose their perspective on alertness. Some have had EDS so long that they no longer recall what it is like to feel fully awake.
Collateral history often is important because family members probably have observed the sleeping patient. The bed partner can provide information about snoring, irregular breathing, leg kicks, unplanned naps, and strained interpersonal relationships because of EDS. For the patient without a bed partner, consider interviewing a travel companion.
Questionnaires. Few useful screening tests exist for sleepiness; most are neither reliable nor valid. One of the better questionnaires—the Epworth Sleepiness Scale (Box 1)—helps confirm the presence of sleepiness with a score >8, differentiating the inability to stay awake from fatigue. This brief questionnaire also provides a useful measure of sleepiness severity.3
The Epworth scale’s value is limited because its questions of specific time and context might not represent a patient’s experiences. Additional validated surveys include the Pittsburgh Sleep Quality Inventory and several for sleep apnea.4
Screening. Electroencephalographic (EEG) monitoring can accurately measure the patient’s degree of sleep disruption. This information is key to understanding if a patient’s EDS is caused by a physiologic condition that prevents quality nocturnal sleep.
None of the widely used screening devices that assess leg kicks indicate the presence of possible periodic limb movements.
Overnight pulse oximetry has been used to screen for sleep-disordered breathing5 but also has limitations:
- Most pulse oximeters do not provide information about sleep stage or body position.
- Patients with sleep-disordered breathing can lack adequate oxygen desaturations but have frequent EEG arousals related to sleep issues. Because EEG data are not collected during arousals, pulse oximetry would generate a false-negative result in this scenario, which occurs most often in female and thin patients.
- Oximetry provides only oxygen saturation data and possibly heart rate, whereas other physiologic processes such as body movement or sleep architecture can be disrupted repetitively during sleep.
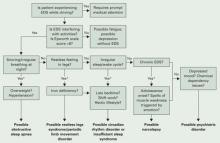
Common sense. The most productive tools for detecting sleep disorders are intuition and common sense. The Figure suggests sequential questions that might uncover specific sleep disorders. Then the decision whether to refer the patient to a sleep disorder center for diagnostic testing depends on the type of sleep disorder you detect.
Nasal continuous positive airway pressure
(CPAP) should be started in an observed setting so that the clinician can determine the optimal amount of positive pressure needed to keep the upper airway patent.
For some patients, CPAP is started in the second half of a “split-night” sleep study after a diagnosis of obstructive sleep apnea (OSA) is made. Other patients return a second night for a nasal CPAP trial. Those with severe OSA might notice improved sleep quality and reduced EDS after only a few hours of CPAP use. Some wish to start CPAP treatment immediately.
Advances in masks and equipment have improved patient adherence to CPAP. Innovations include auto-titrating machines, in which the pressure level can be varied depending on sleep state or body position. Many machines include a data microchip that allows the clinician to determine duration of usage, then use that information to counsel the patient about adherence, if necessary.
Patient education also can promote CPAP adherence. When patients are first told they might need to sleep each night wearing a nasal mask, they often voice well-founded concerns about comfort, claustrophobia, or sexual activity.
Obtaining the support of the bed partner by welcoming her or him to all appointments, including educational activities, is optimal. The bed partner’s concerns about the patient’s excessive snoring or apneas probably were the impetus for the appointment in the first place.
Medication. Some patients benefit from 1 to 2 weeks of a sleeping medication such as zolpidem or trazodone while they acclimate to using nasal CPAP.
Figure The sleepy patient: Possible medical and psychiatric explanations
* Supportive factors: Persuasive if present, but if absent do not exclude possible conditions
Obstructive sleep apnea
Because OSA affects at least 4% of men and 2% of women,6 you are virtually assured of seeing undiagnosed patients. OSA is caused by repeated collapse of the soft tissues surrounding the upper airway, decreasing airflow that is restored when the patient briefly awakens. Patients develop EDS because sleep is fragmented by frequent arousals.
Obese patients, because of their body habitus, are at higher risk for OSA than patients at normal weight. Carefully screen patients for OSA if they develop weight problems while taking psychotropics, such as antipsychotics.
Alcohol or sedatives used at bedtime can aggravate OSA. These substances promote muscle relaxation and increase the arousal threshold so that patients do not awaken readily when apneas occur.
Long-term complications of untreated OSA include sleepiness leading to accidents, hypertension, cerebrovascular disease, and progressive obesity. Data also associate OSA with cardiovascular complications such as arrhythmias, congestive heart failure, and myocardial infarction.7
Physical examination focuses on detecting:
- nasal obstruction (have patient sniff separately through each nostril)
- large neck
- crowded oropharynx (low-hanging palate, reddened uvula, enlarged tonsils, large tongue relative to oropharynx diameter)
- jaw structure (particularly a small, retrognathic mandible).
Sleep studies. Referral for nocturnal polysomnography might be the next step. A comprehensive sleep study collects data about respiratory, cardiovascular, and muscle activity at night, as well as the sounds the patient makes—such as snoring or coughing—when asleep. EEG monitoring also is performed. OSA may be diagnosed if repeated episodes of reduced airflow and oxygen desaturation (arousals) are observed as brief shifts in EEG frequency.
Treatment. First-line interventions for the patient with OSA include:
- no alcohol 1 to 2 hours before bedtime
- sleeping on the side instead of the back
- weight loss (ideally with exercise)
- nasal sprays for allergies.
If first-line treatments are ineffective, nasal continuous positive airway pressure (CPAP) works well for most patients who adhere to the regimen.8 CPAP requires the patient to wear a nasal mask that delivers room air, splinting open the nasopharynx and upper airway (Box 2).
Surgical options. The most common surgeries for OSA are uvulopalatopharyngoplasty and laser-assisted uvulopalatoplasty. Others include tongue reduction and mandibular advancement.
The response rate to surgery averages 50%, depending on patient characteristics and procedure.9 Positive outcomes are most likely for thin patients with obvious upper airway obstruction, including deviated nasal septum, large tonsils, low-hanging palate, and large uvula. Postsurgical complications include nasal regurgitation, voice change, pain, bleeding, infection, tongue numbness, and snoring without apnea (silent apnea).
Oral appliances open the oropharynx by moving the mandible and tongue out of way. Patients with mild to moderate OSA accept these devices well. Evidence suggests that oral appliances improve sleep and reduce EDS more effectively than nasal CPAP and are preferred by patients.10
Oral devices have drawbacks, however. In most settings, their effectiveness cannot be observed during a “split-night” laboratory sleep study because the patient has not yet purchased the device. Also, multiple visits sometimes are required to custom-fit the appliance; this can pose a hardship for patients who live a distance from the provider.
Restless legs syndrome
Patients with restless legs syndrome (RLS) typically report a restless, painful feeling in the limbs that occurs in the evening and at night, disrupting sleep. This condition—which affects 10% of the population—is associated with aging, blood loss, anemia, peripheral neuropathies, and pregnancy.11 Onset can occur in childhood, and in some cases there is a familial tendency.
Most patients with RLS have periodic limb movements (repetitive leg jerks or twitches). The clinical significance of periodic limb movements with no subjective disagreeable feelings in the limbs is controversial, and these cases usually are not treated.
The history usually confirms RLS. Order sleep studies only if you suspect a coexisting sleep problem or the diagnosis is unclear.
A suspected mechanism of restless legs is dopamine deficiency. Low serum ferritin levels have been associated with RLS—presumably because iron is a cofactor necessary for dopamine synthesis12—and may be diagnostically helpful.
The most common technique is to ask the patient to establish a consistent awakening time and a regular bedtime. Initially this could be unconventional by societal standards—such as bedtime at 5 AM and arising at 2 PM. After this pattern is in place, the patient gradually shifts the timing by 1 hour per day. Most patients find it easier to delay rather than advance the bedtime until it conforms to the desired time.
Reinforce this new sleep pattern with a structured daytime schedule that includes predictable mealtimes, regular exercise, social activities, and possibly bright light exposure. Provide reinforcement in the morning for patients with delayed sleep phase disorder and in the evening for advanced sleep phase disorder. These interventions take time and discipline.
Another approach is for the patient to skip sleep one night and, in a sleep-deprived state, establish a new bedtime at the desired time. Use the same modalities listed above to reinforce (“entrain”) this schedule; otherwise the patient will slip back into the previous abnormal sleep-wake rhythm.
Treatment can include iron repletion when indicated. Medications include dopaminergic agents, most notably pramipexole and levodopa/carbidopa. Other options include gabapentin, benzodiazepines, and narcotics.
Antidepressants have been suspected to worsen restless legs syndrome, but definitive studies are lacking.13
Circadian rhythm disorders
Instead of compromising the quality or quantity of sleep, circadian rhythm disorders cause sleep to occur at inappropriate times. These disorders are most common in adolescents and young adults.
Delayed sleep phase disorder—a persistent pattern of staying up late and “sleeping in”—is most common. Careful assessment will reveal that the patient is getting adequate sleep but at a socially unacceptable time, sometimes to the extreme that his or her nights and days are reversed.
Patients’ reluctance to acknowledge the severity of this problem can lead to inaccurate sleep diaries and interviews. A portable wrist actigraph can provide data about limb movement and is more objective than self-reports.
Delayed sleep phase disorder is highly comorbid with depressive disorders.14 The cause of this syndrome is unclear, but light exposure, social patterns, psychological issues, and possibly a genetic substrate are known to contribute.
Advanced sleep phase disorder—a less common circadian rhythm disorder—also can cause EDS. Patients have an inappropriately early time of sleep onset and then are fully awake in the middle of the night. A large family with a severe form of this disorder was found to have an abnormality on chromosome 2.15
Treatment. Relatively few treatments are effective for circadian rhythm disorders. Some patients elect not to pursue therapy, instead fitting activities around their unconventional sleep schedules.
Individuals with delayed sleep phase who cannot arrange their lives around their sleep schedules are at risk for poor early morning performance because of sleepiness. Their internal circadian clocks can be gradually readjusted with phototherapy or gradual shifting of the major sleep period (Box 3). Stimulants usually are not used, but hypnotics can sometimes help these patients fall asleep earlier.
Insufficient sleep syndrome
People attempting to “burn the candle at both ends” are at risk for developing insufficient sleep syndrome.16 In our 24/7 society, people trying to make do with less than the required 7.5 hours sleep per night may adversely affect their health. The problem is compounded for shift workers because of the difficulty in obtaining sufficient quality sleep during daylight hours.
Many patients do not seek treatment for fatigue or sleepiness because they are aware of their lifestyle choices. Still, they might develop psychological symptoms such as irritability, mood swings, and strained interpersonal relationships. These symptoms can prompt patients to request treatment.
Take a careful history that includes discussing the patient’s daily and weekly schedule. Avoid psychostimulants; instead, address the nonnegotiable need to get adequate sleep and challenge the patient to prioritize his or her activities around a full night’s sleep.
When to consider narcolepsy
Narcolepsy is a CNS disease characterized by abnormal regulation of REM sleep. EDS—the cardinal symptom—is often associated with cataplexy (75%), sleep paralysis (50%), vivid dreams, and insomnia, all of which interfere with REM phenomena. Narcolepsy affects 0.05% of the U.S. population and can lead to severe occupational, educational, and family disruption.
When you obtain a history that suggests narcolepsy, use the history, a sleep diary, or wrist actigraphy to document whether the patient is getting adequate sleep, with a consistent sleep/wake cycle. Next, consider referring the patient for polysomnography, primarily to rule out other causes of EDS such as sleep-disordered breathing. In some cases, REM latency on the overnight sleep study will be <20 minutes after sleep onset, which supports the diagnosis of narcolepsy.
A multiple sleep latency test (MSLT)—a diagnostic session in which the patient takes 4 to 5 daytime naps—is performed the following day. Narcolepsy is confirmed if the patient has a mean initial sleep latency of <10 minutes during these naps plus at least two REM episodes within 15 minutes after sleep onset.
The 4 most appropriate indications for an urgent sleep evaluation are:
- difficulty staying alert while driving
- nocturnal cardiac arrhythmias
- frequent observed apneas
- excessive daytime sleepiness (EDS) leading to academic or occupational problems.
Insurance companies usually cover a specialty sleep evaluation, particularly if the referring physician documents a suspicion of sleep-disordered breathing or EDS that jeopardizes safe driving.
Most patients with narcolepsy and cataplexy have undetectable cerebrospinal fluid levels of a neuropeptide called hypocretin or orexin.17 Hypocretin/orexin replacement therapy is a theoretical possibility, but for now treatment includes a combination of optimal sleep hygiene, psychostimulants, antidepressants, and hypnotics.
Other causes of EDS
EDS can also be caused by unrecognized alcohol dependence, inappropriate or excessive medication use, and depressive disorders. Overnight sleep studies are seldom indicated unless patients endorse the symptoms in the Figure.
Before pursuing polysomnography or an MSLT (Box 4), eliminate medications that might confound the results, such as:
- antidepressants, which alter the timing and duration of REM sleep
- sedating medications, which modify initial sleep latency and sleep efficiency and potentially aggravate sleep disordered breathing.
Initial REM latency provides a potential biologic marker of major depression but is used more often in research than in clinical psychiatry.
Primary insomnia is the distressing inability to sleep at night or nap during the day. It suggests a hyperarousal state—the opposite of EDS.18 In rare cases, however, patients who cannot sleep at night also have EDS. When evaluated, they typically endorse at least one symptom in the Figure. Sleep studies occasionally reveal OSA or restless legs syndrome.
Treating a patient with chronic insomnia may require several trials of behavioral interventions or sedating medications before you make a referral to a sleep disorder center. Patients can struggle with unrecognized primary sleep disorders for years, and many are given empiric trials of stimulating antidepressants. Antidepressants are unlikely to cause harm, but they might complicate diagnostic testing.
When you confirm coexisting depression and a primary sleep disorder, treatments that separately target each condition provide optimal management of the sleepy patient.
Medications to enhance wakefulness
Wake-promoting agents are a treatment option when EDS is contributing to compromised functioning. These drugs are no substitute for thoughtful evaluation of hypersomnolence, however. When you diagnose OSA or restless legs syndrome, first try treatments that target these conditions. If residual sleepiness persists, then consider augmenting with stimulating medications.
Modafinil is FDA-approved for residual sleepiness in patients with OSA and for shift work sleep disorder, a condition of circadian misalignment from frequent schedule changes. Evidence does not support its use for other circadian rhythm disorders, such as delayed sleep phase disorder.
Low-dose modafinil (such as 100 to 200 mg/d) is well tolerated, but its therapeutic effect as augmentation is modest.19 Increasing the dosage to >200 mg usually does not increase alertness.
Caffeine. Some patients report benefit from caffeine used in moderation and only in the morning. This practice is acceptable as long as patients do not use excessive amounts or experience insomnia, exacerbation of anxiety, or tachycardia.
Psychostimulants such as methylphenidate and amphetamines are less well-studied than modafinil for treating EDS in patients without narcolepsy. Monitor carefully for insomnia, exacerbation of anxiety, tachycardia, or hypertension and to prevent overuse of these habituating agents.
Related resources
- National Sleep Foundation. www.sleepfoundation.org.
- American Academy of Sleep Medicine. www.aasmnet.org.
- American Sleep Apnea Association. www.sleepapnea.org.
- Restless Legs Syndrome Foundation. www.rls.org.
- Association for the Study of Light Therapy and Biological Rhythms. www.sltbr.org.
Drug brand names
- Carbidopa/levodopa • Sinemet
- Gabapentin • Neurontin
- Modafinil • Provigil
- Pramipexole • Mirapex
- Trazodone • Desyrel
- Zolpidem • Ambien
Disclosure
Dr. Krahn reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Acknowledgment
Dr. Krahn published the original version this article in the January 2002 issue of Current Psychiatry and has updated it for this issue.
1. Punjabi N, Haponik E. Ask about daytime sleepiness. J Amer Geriatr Soc 2000;48:228-9.
2. Ronald J, Delaive K, Roos L, et al. Health care utilization in the 10 years prior to diagnosis in obstructive sleep apnea syndrome patients. Sleep 1999;22(2):225-9.
3. Johns M. A new method for measuring daytime sleepiness: The Epworth Sleepiness Scale. Sleep 1991;14(6):540-5.
4. Rowley J, Aboussouan L, Badr M. The use of clinical prediction formulas in the evaluation of obstructive sleep apnea. Sleep 2000;23:929-38.
5. Yamashiro Y, Kryger M. Nocturnal oximetry: Is it a screening tool for sleep disorders? Sleep 1995;18:167-71.
6. Morrell M, Finn L, Kim H, et al. Sleep fragmentation, awake blood pressure, and sleep-disordered breathing in a population-based study. Am J Respir Critical Care Med 2000;162(6):2091-6.
7. Roux F, D’Ambrosio C, Mohsenin V. Sleep-related breathing disorders and cardiovascular disease. Am J Med. 2000;108:396-402.
8. Engleman H, Martin S, Deary I, Douglas N. Effect of continuous positive airway pressure treatment on daytime function in sleep apnoea/hypopnoea syndrome. Lancet 1994;343(8897):572-5.
9. Lojander J, Maasilta P, Partinen M, et al. Nasal-CPAP, surgery, and conservative management for treatment of obstructive sleep apnea syndrome. A randomized study. Chest. 1996;110(1):114-9.
10. Mehta A, Qian J, Petocz P, et al. A randomized, controlled study of a mandibular advancement splint for obstructive sleep apnea. Am J Respir Critical Care Med 2001;163(6):1457-61.
11. Chesson A, Wise M, Davila D, et al. Practice parameters for the treatment of restless legs syndrome and periodic limb movement disorder. An American Academy of Sleep Medicine Report. Standards of Practice Committee of the American Academy of Sleep Medicine. Sleep 1999;22(7):961-8.
12. Phillips B, Young T, Finn L, et al. Epidemiology of restless legs symptoms in adults. Arch Intern Med 2000;160(14):2137-41.
13. Thorpy M, Ehrenberg B, Hening W, et al. Restless legs syndrome: Detection and management in primary care. Amer Fam Phys 2000;62:108-14.
14. Regestein Q, Monk T. Delayed sleep phase syndrome: A review of its clinical aspects. Am J Psychiatry 1995;152:602-8.
15. Toh K, Jones C, He Y, et al. An hPer2 phosphorylation site mutation in familial advanced sleep phase syndrome. Science 2001;291(5506):1040-3.
16. Yoshikawa N, Suzuki S, Ishimoto T, et al. A case of insufficient sleep syndrome. Psychiatry Clin Neuro 1998;52(2):200-1.
17. Nishino S, Ripley B, Overeem S, et al. Hypocretin (orexin) deficiency in human narcolepsy. Lancet. 2000;355:39-40.
18. Hauri P, Esther M. Insomnia. Mayo Clin Proc 1990;65:869-82.
19. Schwartz JR. Modafinil: new indications for wake promotion. Expert Opin Pharmacother 2005;6(1):115-29.
Poor energy, hypersomnia, amotivation, irritability, and frustration can suggest depression or other psychiatric disorders to busy primary care physicians. As a result, psychiatrists often are referred patients with excessive daytime sleepiness (EDS) caused by undiagnosed primary sleep disorders.
Physicians may miss obstructive sleep apnea (OSA), restless legs syndrome, circadian rhythm disorders, or narcolepsy because:
- many have little training in sleep disorders and limited time to diagnose them1
- patients do not report sleepiness or recognize it as a legitimate medical concern
- definitive diagnostic tests are expensive and usually are not ordered.
Psychiatrists, therefore, need a clear understanding of the EDS differential diagnosis to determine whether a patient’s behavioral symptoms are a sleep or psychiatric issue.
How likely are you to doze off or fall asleep in the following situations, in contrast to feeling just tired? This refers to your usual way of life in recent times. Even if you have not done some of these things recently, try to work out how each situation would affect you now. Use the scale below to choose the most appropriate number for each situation:
0 no chance of dozing
1 slight chance of dozing
2 moderate chance of dozing
3 high chance of dozing
Chance of dozing Situation
Sitting and reading
Watching TV
Sitting inactive in a public place (such as in a theater or a meeting)
As a passenger in a car for an hour without a break
Lying down to rest in the afternoon when circumstances permit
Sitting and talking to someone
Sitting quietly after a lunch without alcohol
In a car, while stopped for a few minutes in traffic
Scoring key
1 to 6 Getting enough sleep
7 to 8 Average
>8 Seek a sleep specialist’s advice without delay
Assessing the sleepy patient
Sleepiness is an inability to stay awake at appropriate times. Fatigue, by comparison, does not involve sleepiness but very low energy associated with wakefulness. In general, sleepy patients get transient relief from napping, whereas fatigued patients report they cannot fall asleep.
Untreated EDS results in compromised quality of life, reduced productivity, and public safety concerns such as falling asleep while driving.2 Sleep complaints fall into three major categories:
- EDS
- insomnia (marked by distress because of poor sleep, but usually with minimal EDS)
- unusual nocturnal behaviors (ranging from arm waving to violent behaviors.
When you evaluate a patient with sleep complaints, valuable sources of data include observation, questionnaires, and screening devices. The most important may be common sense.
Observation. Observe the patient in the waiting room or office before starting the interview. Did he or she nod off while waiting to see you? Pay attention to anyone who appears sleepy—even those who deny having trouble staying awake. Over time, sleepy patients can lose their perspective on alertness. Some have had EDS so long that they no longer recall what it is like to feel fully awake.
Collateral history often is important because family members probably have observed the sleeping patient. The bed partner can provide information about snoring, irregular breathing, leg kicks, unplanned naps, and strained interpersonal relationships because of EDS. For the patient without a bed partner, consider interviewing a travel companion.
Questionnaires. Few useful screening tests exist for sleepiness; most are neither reliable nor valid. One of the better questionnaires—the Epworth Sleepiness Scale (Box 1)—helps confirm the presence of sleepiness with a score >8, differentiating the inability to stay awake from fatigue. This brief questionnaire also provides a useful measure of sleepiness severity.3
The Epworth scale’s value is limited because its questions of specific time and context might not represent a patient’s experiences. Additional validated surveys include the Pittsburgh Sleep Quality Inventory and several for sleep apnea.4
Screening. Electroencephalographic (EEG) monitoring can accurately measure the patient’s degree of sleep disruption. This information is key to understanding if a patient’s EDS is caused by a physiologic condition that prevents quality nocturnal sleep.
None of the widely used screening devices that assess leg kicks indicate the presence of possible periodic limb movements.
Overnight pulse oximetry has been used to screen for sleep-disordered breathing5 but also has limitations:
- Most pulse oximeters do not provide information about sleep stage or body position.
- Patients with sleep-disordered breathing can lack adequate oxygen desaturations but have frequent EEG arousals related to sleep issues. Because EEG data are not collected during arousals, pulse oximetry would generate a false-negative result in this scenario, which occurs most often in female and thin patients.
- Oximetry provides only oxygen saturation data and possibly heart rate, whereas other physiologic processes such as body movement or sleep architecture can be disrupted repetitively during sleep.
Common sense. The most productive tools for detecting sleep disorders are intuition and common sense. The Figure suggests sequential questions that might uncover specific sleep disorders. Then the decision whether to refer the patient to a sleep disorder center for diagnostic testing depends on the type of sleep disorder you detect.
Nasal continuous positive airway pressure
(CPAP) should be started in an observed setting so that the clinician can determine the optimal amount of positive pressure needed to keep the upper airway patent.
For some patients, CPAP is started in the second half of a “split-night” sleep study after a diagnosis of obstructive sleep apnea (OSA) is made. Other patients return a second night for a nasal CPAP trial. Those with severe OSA might notice improved sleep quality and reduced EDS after only a few hours of CPAP use. Some wish to start CPAP treatment immediately.
Advances in masks and equipment have improved patient adherence to CPAP. Innovations include auto-titrating machines, in which the pressure level can be varied depending on sleep state or body position. Many machines include a data microchip that allows the clinician to determine duration of usage, then use that information to counsel the patient about adherence, if necessary.
Patient education also can promote CPAP adherence. When patients are first told they might need to sleep each night wearing a nasal mask, they often voice well-founded concerns about comfort, claustrophobia, or sexual activity.
Obtaining the support of the bed partner by welcoming her or him to all appointments, including educational activities, is optimal. The bed partner’s concerns about the patient’s excessive snoring or apneas probably were the impetus for the appointment in the first place.
Medication. Some patients benefit from 1 to 2 weeks of a sleeping medication such as zolpidem or trazodone while they acclimate to using nasal CPAP.
Figure The sleepy patient: Possible medical and psychiatric explanations
* Supportive factors: Persuasive if present, but if absent do not exclude possible conditions
Obstructive sleep apnea
Because OSA affects at least 4% of men and 2% of women,6 you are virtually assured of seeing undiagnosed patients. OSA is caused by repeated collapse of the soft tissues surrounding the upper airway, decreasing airflow that is restored when the patient briefly awakens. Patients develop EDS because sleep is fragmented by frequent arousals.
Obese patients, because of their body habitus, are at higher risk for OSA than patients at normal weight. Carefully screen patients for OSA if they develop weight problems while taking psychotropics, such as antipsychotics.
Alcohol or sedatives used at bedtime can aggravate OSA. These substances promote muscle relaxation and increase the arousal threshold so that patients do not awaken readily when apneas occur.
Long-term complications of untreated OSA include sleepiness leading to accidents, hypertension, cerebrovascular disease, and progressive obesity. Data also associate OSA with cardiovascular complications such as arrhythmias, congestive heart failure, and myocardial infarction.7
Physical examination focuses on detecting:
- nasal obstruction (have patient sniff separately through each nostril)
- large neck
- crowded oropharynx (low-hanging palate, reddened uvula, enlarged tonsils, large tongue relative to oropharynx diameter)
- jaw structure (particularly a small, retrognathic mandible).
Sleep studies. Referral for nocturnal polysomnography might be the next step. A comprehensive sleep study collects data about respiratory, cardiovascular, and muscle activity at night, as well as the sounds the patient makes—such as snoring or coughing—when asleep. EEG monitoring also is performed. OSA may be diagnosed if repeated episodes of reduced airflow and oxygen desaturation (arousals) are observed as brief shifts in EEG frequency.
Treatment. First-line interventions for the patient with OSA include:
- no alcohol 1 to 2 hours before bedtime
- sleeping on the side instead of the back
- weight loss (ideally with exercise)
- nasal sprays for allergies.
If first-line treatments are ineffective, nasal continuous positive airway pressure (CPAP) works well for most patients who adhere to the regimen.8 CPAP requires the patient to wear a nasal mask that delivers room air, splinting open the nasopharynx and upper airway (Box 2).
Surgical options. The most common surgeries for OSA are uvulopalatopharyngoplasty and laser-assisted uvulopalatoplasty. Others include tongue reduction and mandibular advancement.
The response rate to surgery averages 50%, depending on patient characteristics and procedure.9 Positive outcomes are most likely for thin patients with obvious upper airway obstruction, including deviated nasal septum, large tonsils, low-hanging palate, and large uvula. Postsurgical complications include nasal regurgitation, voice change, pain, bleeding, infection, tongue numbness, and snoring without apnea (silent apnea).
Oral appliances open the oropharynx by moving the mandible and tongue out of way. Patients with mild to moderate OSA accept these devices well. Evidence suggests that oral appliances improve sleep and reduce EDS more effectively than nasal CPAP and are preferred by patients.10
Oral devices have drawbacks, however. In most settings, their effectiveness cannot be observed during a “split-night” laboratory sleep study because the patient has not yet purchased the device. Also, multiple visits sometimes are required to custom-fit the appliance; this can pose a hardship for patients who live a distance from the provider.
Restless legs syndrome
Patients with restless legs syndrome (RLS) typically report a restless, painful feeling in the limbs that occurs in the evening and at night, disrupting sleep. This condition—which affects 10% of the population—is associated with aging, blood loss, anemia, peripheral neuropathies, and pregnancy.11 Onset can occur in childhood, and in some cases there is a familial tendency.
Most patients with RLS have periodic limb movements (repetitive leg jerks or twitches). The clinical significance of periodic limb movements with no subjective disagreeable feelings in the limbs is controversial, and these cases usually are not treated.
The history usually confirms RLS. Order sleep studies only if you suspect a coexisting sleep problem or the diagnosis is unclear.
A suspected mechanism of restless legs is dopamine deficiency. Low serum ferritin levels have been associated with RLS—presumably because iron is a cofactor necessary for dopamine synthesis12—and may be diagnostically helpful.
The most common technique is to ask the patient to establish a consistent awakening time and a regular bedtime. Initially this could be unconventional by societal standards—such as bedtime at 5 AM and arising at 2 PM. After this pattern is in place, the patient gradually shifts the timing by 1 hour per day. Most patients find it easier to delay rather than advance the bedtime until it conforms to the desired time.
Reinforce this new sleep pattern with a structured daytime schedule that includes predictable mealtimes, regular exercise, social activities, and possibly bright light exposure. Provide reinforcement in the morning for patients with delayed sleep phase disorder and in the evening for advanced sleep phase disorder. These interventions take time and discipline.
Another approach is for the patient to skip sleep one night and, in a sleep-deprived state, establish a new bedtime at the desired time. Use the same modalities listed above to reinforce (“entrain”) this schedule; otherwise the patient will slip back into the previous abnormal sleep-wake rhythm.
Treatment can include iron repletion when indicated. Medications include dopaminergic agents, most notably pramipexole and levodopa/carbidopa. Other options include gabapentin, benzodiazepines, and narcotics.
Antidepressants have been suspected to worsen restless legs syndrome, but definitive studies are lacking.13
Circadian rhythm disorders
Instead of compromising the quality or quantity of sleep, circadian rhythm disorders cause sleep to occur at inappropriate times. These disorders are most common in adolescents and young adults.
Delayed sleep phase disorder—a persistent pattern of staying up late and “sleeping in”—is most common. Careful assessment will reveal that the patient is getting adequate sleep but at a socially unacceptable time, sometimes to the extreme that his or her nights and days are reversed.
Patients’ reluctance to acknowledge the severity of this problem can lead to inaccurate sleep diaries and interviews. A portable wrist actigraph can provide data about limb movement and is more objective than self-reports.
Delayed sleep phase disorder is highly comorbid with depressive disorders.14 The cause of this syndrome is unclear, but light exposure, social patterns, psychological issues, and possibly a genetic substrate are known to contribute.
Advanced sleep phase disorder—a less common circadian rhythm disorder—also can cause EDS. Patients have an inappropriately early time of sleep onset and then are fully awake in the middle of the night. A large family with a severe form of this disorder was found to have an abnormality on chromosome 2.15
Treatment. Relatively few treatments are effective for circadian rhythm disorders. Some patients elect not to pursue therapy, instead fitting activities around their unconventional sleep schedules.
Individuals with delayed sleep phase who cannot arrange their lives around their sleep schedules are at risk for poor early morning performance because of sleepiness. Their internal circadian clocks can be gradually readjusted with phototherapy or gradual shifting of the major sleep period (Box 3). Stimulants usually are not used, but hypnotics can sometimes help these patients fall asleep earlier.
Insufficient sleep syndrome
People attempting to “burn the candle at both ends” are at risk for developing insufficient sleep syndrome.16 In our 24/7 society, people trying to make do with less than the required 7.5 hours sleep per night may adversely affect their health. The problem is compounded for shift workers because of the difficulty in obtaining sufficient quality sleep during daylight hours.
Many patients do not seek treatment for fatigue or sleepiness because they are aware of their lifestyle choices. Still, they might develop psychological symptoms such as irritability, mood swings, and strained interpersonal relationships. These symptoms can prompt patients to request treatment.
Take a careful history that includes discussing the patient’s daily and weekly schedule. Avoid psychostimulants; instead, address the nonnegotiable need to get adequate sleep and challenge the patient to prioritize his or her activities around a full night’s sleep.
When to consider narcolepsy
Narcolepsy is a CNS disease characterized by abnormal regulation of REM sleep. EDS—the cardinal symptom—is often associated with cataplexy (75%), sleep paralysis (50%), vivid dreams, and insomnia, all of which interfere with REM phenomena. Narcolepsy affects 0.05% of the U.S. population and can lead to severe occupational, educational, and family disruption.
When you obtain a history that suggests narcolepsy, use the history, a sleep diary, or wrist actigraphy to document whether the patient is getting adequate sleep, with a consistent sleep/wake cycle. Next, consider referring the patient for polysomnography, primarily to rule out other causes of EDS such as sleep-disordered breathing. In some cases, REM latency on the overnight sleep study will be <20 minutes after sleep onset, which supports the diagnosis of narcolepsy.
A multiple sleep latency test (MSLT)—a diagnostic session in which the patient takes 4 to 5 daytime naps—is performed the following day. Narcolepsy is confirmed if the patient has a mean initial sleep latency of <10 minutes during these naps plus at least two REM episodes within 15 minutes after sleep onset.
The 4 most appropriate indications for an urgent sleep evaluation are:
- difficulty staying alert while driving
- nocturnal cardiac arrhythmias
- frequent observed apneas
- excessive daytime sleepiness (EDS) leading to academic or occupational problems.
Insurance companies usually cover a specialty sleep evaluation, particularly if the referring physician documents a suspicion of sleep-disordered breathing or EDS that jeopardizes safe driving.
Most patients with narcolepsy and cataplexy have undetectable cerebrospinal fluid levels of a neuropeptide called hypocretin or orexin.17 Hypocretin/orexin replacement therapy is a theoretical possibility, but for now treatment includes a combination of optimal sleep hygiene, psychostimulants, antidepressants, and hypnotics.
Other causes of EDS
EDS can also be caused by unrecognized alcohol dependence, inappropriate or excessive medication use, and depressive disorders. Overnight sleep studies are seldom indicated unless patients endorse the symptoms in the Figure.
Before pursuing polysomnography or an MSLT (Box 4), eliminate medications that might confound the results, such as:
- antidepressants, which alter the timing and duration of REM sleep
- sedating medications, which modify initial sleep latency and sleep efficiency and potentially aggravate sleep disordered breathing.
Initial REM latency provides a potential biologic marker of major depression but is used more often in research than in clinical psychiatry.
Primary insomnia is the distressing inability to sleep at night or nap during the day. It suggests a hyperarousal state—the opposite of EDS.18 In rare cases, however, patients who cannot sleep at night also have EDS. When evaluated, they typically endorse at least one symptom in the Figure. Sleep studies occasionally reveal OSA or restless legs syndrome.
Treating a patient with chronic insomnia may require several trials of behavioral interventions or sedating medications before you make a referral to a sleep disorder center. Patients can struggle with unrecognized primary sleep disorders for years, and many are given empiric trials of stimulating antidepressants. Antidepressants are unlikely to cause harm, but they might complicate diagnostic testing.
When you confirm coexisting depression and a primary sleep disorder, treatments that separately target each condition provide optimal management of the sleepy patient.
Medications to enhance wakefulness
Wake-promoting agents are a treatment option when EDS is contributing to compromised functioning. These drugs are no substitute for thoughtful evaluation of hypersomnolence, however. When you diagnose OSA or restless legs syndrome, first try treatments that target these conditions. If residual sleepiness persists, then consider augmenting with stimulating medications.
Modafinil is FDA-approved for residual sleepiness in patients with OSA and for shift work sleep disorder, a condition of circadian misalignment from frequent schedule changes. Evidence does not support its use for other circadian rhythm disorders, such as delayed sleep phase disorder.
Low-dose modafinil (such as 100 to 200 mg/d) is well tolerated, but its therapeutic effect as augmentation is modest.19 Increasing the dosage to >200 mg usually does not increase alertness.
Caffeine. Some patients report benefit from caffeine used in moderation and only in the morning. This practice is acceptable as long as patients do not use excessive amounts or experience insomnia, exacerbation of anxiety, or tachycardia.
Psychostimulants such as methylphenidate and amphetamines are less well-studied than modafinil for treating EDS in patients without narcolepsy. Monitor carefully for insomnia, exacerbation of anxiety, tachycardia, or hypertension and to prevent overuse of these habituating agents.
Related resources
- National Sleep Foundation. www.sleepfoundation.org.
- American Academy of Sleep Medicine. www.aasmnet.org.
- American Sleep Apnea Association. www.sleepapnea.org.
- Restless Legs Syndrome Foundation. www.rls.org.
- Association for the Study of Light Therapy and Biological Rhythms. www.sltbr.org.
Drug brand names
- Carbidopa/levodopa • Sinemet
- Gabapentin • Neurontin
- Modafinil • Provigil
- Pramipexole • Mirapex
- Trazodone • Desyrel
- Zolpidem • Ambien
Disclosure
Dr. Krahn reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Acknowledgment
Dr. Krahn published the original version this article in the January 2002 issue of Current Psychiatry and has updated it for this issue.
Poor energy, hypersomnia, amotivation, irritability, and frustration can suggest depression or other psychiatric disorders to busy primary care physicians. As a result, psychiatrists often are referred patients with excessive daytime sleepiness (EDS) caused by undiagnosed primary sleep disorders.
Physicians may miss obstructive sleep apnea (OSA), restless legs syndrome, circadian rhythm disorders, or narcolepsy because:
- many have little training in sleep disorders and limited time to diagnose them1
- patients do not report sleepiness or recognize it as a legitimate medical concern
- definitive diagnostic tests are expensive and usually are not ordered.
Psychiatrists, therefore, need a clear understanding of the EDS differential diagnosis to determine whether a patient’s behavioral symptoms are a sleep or psychiatric issue.
How likely are you to doze off or fall asleep in the following situations, in contrast to feeling just tired? This refers to your usual way of life in recent times. Even if you have not done some of these things recently, try to work out how each situation would affect you now. Use the scale below to choose the most appropriate number for each situation:
0 no chance of dozing
1 slight chance of dozing
2 moderate chance of dozing
3 high chance of dozing
Chance of dozing Situation
Sitting and reading
Watching TV
Sitting inactive in a public place (such as in a theater or a meeting)
As a passenger in a car for an hour without a break
Lying down to rest in the afternoon when circumstances permit
Sitting and talking to someone
Sitting quietly after a lunch without alcohol
In a car, while stopped for a few minutes in traffic
Scoring key
1 to 6 Getting enough sleep
7 to 8 Average
>8 Seek a sleep specialist’s advice without delay
Assessing the sleepy patient
Sleepiness is an inability to stay awake at appropriate times. Fatigue, by comparison, does not involve sleepiness but very low energy associated with wakefulness. In general, sleepy patients get transient relief from napping, whereas fatigued patients report they cannot fall asleep.
Untreated EDS results in compromised quality of life, reduced productivity, and public safety concerns such as falling asleep while driving.2 Sleep complaints fall into three major categories:
- EDS
- insomnia (marked by distress because of poor sleep, but usually with minimal EDS)
- unusual nocturnal behaviors (ranging from arm waving to violent behaviors.
When you evaluate a patient with sleep complaints, valuable sources of data include observation, questionnaires, and screening devices. The most important may be common sense.
Observation. Observe the patient in the waiting room or office before starting the interview. Did he or she nod off while waiting to see you? Pay attention to anyone who appears sleepy—even those who deny having trouble staying awake. Over time, sleepy patients can lose their perspective on alertness. Some have had EDS so long that they no longer recall what it is like to feel fully awake.
Collateral history often is important because family members probably have observed the sleeping patient. The bed partner can provide information about snoring, irregular breathing, leg kicks, unplanned naps, and strained interpersonal relationships because of EDS. For the patient without a bed partner, consider interviewing a travel companion.
Questionnaires. Few useful screening tests exist for sleepiness; most are neither reliable nor valid. One of the better questionnaires—the Epworth Sleepiness Scale (Box 1)—helps confirm the presence of sleepiness with a score >8, differentiating the inability to stay awake from fatigue. This brief questionnaire also provides a useful measure of sleepiness severity.3
The Epworth scale’s value is limited because its questions of specific time and context might not represent a patient’s experiences. Additional validated surveys include the Pittsburgh Sleep Quality Inventory and several for sleep apnea.4
Screening. Electroencephalographic (EEG) monitoring can accurately measure the patient’s degree of sleep disruption. This information is key to understanding if a patient’s EDS is caused by a physiologic condition that prevents quality nocturnal sleep.
None of the widely used screening devices that assess leg kicks indicate the presence of possible periodic limb movements.
Overnight pulse oximetry has been used to screen for sleep-disordered breathing5 but also has limitations:
- Most pulse oximeters do not provide information about sleep stage or body position.
- Patients with sleep-disordered breathing can lack adequate oxygen desaturations but have frequent EEG arousals related to sleep issues. Because EEG data are not collected during arousals, pulse oximetry would generate a false-negative result in this scenario, which occurs most often in female and thin patients.
- Oximetry provides only oxygen saturation data and possibly heart rate, whereas other physiologic processes such as body movement or sleep architecture can be disrupted repetitively during sleep.
Common sense. The most productive tools for detecting sleep disorders are intuition and common sense. The Figure suggests sequential questions that might uncover specific sleep disorders. Then the decision whether to refer the patient to a sleep disorder center for diagnostic testing depends on the type of sleep disorder you detect.
Nasal continuous positive airway pressure
(CPAP) should be started in an observed setting so that the clinician can determine the optimal amount of positive pressure needed to keep the upper airway patent.
For some patients, CPAP is started in the second half of a “split-night” sleep study after a diagnosis of obstructive sleep apnea (OSA) is made. Other patients return a second night for a nasal CPAP trial. Those with severe OSA might notice improved sleep quality and reduced EDS after only a few hours of CPAP use. Some wish to start CPAP treatment immediately.
Advances in masks and equipment have improved patient adherence to CPAP. Innovations include auto-titrating machines, in which the pressure level can be varied depending on sleep state or body position. Many machines include a data microchip that allows the clinician to determine duration of usage, then use that information to counsel the patient about adherence, if necessary.
Patient education also can promote CPAP adherence. When patients are first told they might need to sleep each night wearing a nasal mask, they often voice well-founded concerns about comfort, claustrophobia, or sexual activity.
Obtaining the support of the bed partner by welcoming her or him to all appointments, including educational activities, is optimal. The bed partner’s concerns about the patient’s excessive snoring or apneas probably were the impetus for the appointment in the first place.
Medication. Some patients benefit from 1 to 2 weeks of a sleeping medication such as zolpidem or trazodone while they acclimate to using nasal CPAP.
Figure The sleepy patient: Possible medical and psychiatric explanations
* Supportive factors: Persuasive if present, but if absent do not exclude possible conditions
Obstructive sleep apnea
Because OSA affects at least 4% of men and 2% of women,6 you are virtually assured of seeing undiagnosed patients. OSA is caused by repeated collapse of the soft tissues surrounding the upper airway, decreasing airflow that is restored when the patient briefly awakens. Patients develop EDS because sleep is fragmented by frequent arousals.
Obese patients, because of their body habitus, are at higher risk for OSA than patients at normal weight. Carefully screen patients for OSA if they develop weight problems while taking psychotropics, such as antipsychotics.
Alcohol or sedatives used at bedtime can aggravate OSA. These substances promote muscle relaxation and increase the arousal threshold so that patients do not awaken readily when apneas occur.
Long-term complications of untreated OSA include sleepiness leading to accidents, hypertension, cerebrovascular disease, and progressive obesity. Data also associate OSA with cardiovascular complications such as arrhythmias, congestive heart failure, and myocardial infarction.7
Physical examination focuses on detecting:
- nasal obstruction (have patient sniff separately through each nostril)
- large neck
- crowded oropharynx (low-hanging palate, reddened uvula, enlarged tonsils, large tongue relative to oropharynx diameter)
- jaw structure (particularly a small, retrognathic mandible).
Sleep studies. Referral for nocturnal polysomnography might be the next step. A comprehensive sleep study collects data about respiratory, cardiovascular, and muscle activity at night, as well as the sounds the patient makes—such as snoring or coughing—when asleep. EEG monitoring also is performed. OSA may be diagnosed if repeated episodes of reduced airflow and oxygen desaturation (arousals) are observed as brief shifts in EEG frequency.
Treatment. First-line interventions for the patient with OSA include:
- no alcohol 1 to 2 hours before bedtime
- sleeping on the side instead of the back
- weight loss (ideally with exercise)
- nasal sprays for allergies.
If first-line treatments are ineffective, nasal continuous positive airway pressure (CPAP) works well for most patients who adhere to the regimen.8 CPAP requires the patient to wear a nasal mask that delivers room air, splinting open the nasopharynx and upper airway (Box 2).
Surgical options. The most common surgeries for OSA are uvulopalatopharyngoplasty and laser-assisted uvulopalatoplasty. Others include tongue reduction and mandibular advancement.
The response rate to surgery averages 50%, depending on patient characteristics and procedure.9 Positive outcomes are most likely for thin patients with obvious upper airway obstruction, including deviated nasal septum, large tonsils, low-hanging palate, and large uvula. Postsurgical complications include nasal regurgitation, voice change, pain, bleeding, infection, tongue numbness, and snoring without apnea (silent apnea).
Oral appliances open the oropharynx by moving the mandible and tongue out of way. Patients with mild to moderate OSA accept these devices well. Evidence suggests that oral appliances improve sleep and reduce EDS more effectively than nasal CPAP and are preferred by patients.10
Oral devices have drawbacks, however. In most settings, their effectiveness cannot be observed during a “split-night” laboratory sleep study because the patient has not yet purchased the device. Also, multiple visits sometimes are required to custom-fit the appliance; this can pose a hardship for patients who live a distance from the provider.
Restless legs syndrome
Patients with restless legs syndrome (RLS) typically report a restless, painful feeling in the limbs that occurs in the evening and at night, disrupting sleep. This condition—which affects 10% of the population—is associated with aging, blood loss, anemia, peripheral neuropathies, and pregnancy.11 Onset can occur in childhood, and in some cases there is a familial tendency.
Most patients with RLS have periodic limb movements (repetitive leg jerks or twitches). The clinical significance of periodic limb movements with no subjective disagreeable feelings in the limbs is controversial, and these cases usually are not treated.
The history usually confirms RLS. Order sleep studies only if you suspect a coexisting sleep problem or the diagnosis is unclear.
A suspected mechanism of restless legs is dopamine deficiency. Low serum ferritin levels have been associated with RLS—presumably because iron is a cofactor necessary for dopamine synthesis12—and may be diagnostically helpful.
The most common technique is to ask the patient to establish a consistent awakening time and a regular bedtime. Initially this could be unconventional by societal standards—such as bedtime at 5 AM and arising at 2 PM. After this pattern is in place, the patient gradually shifts the timing by 1 hour per day. Most patients find it easier to delay rather than advance the bedtime until it conforms to the desired time.
Reinforce this new sleep pattern with a structured daytime schedule that includes predictable mealtimes, regular exercise, social activities, and possibly bright light exposure. Provide reinforcement in the morning for patients with delayed sleep phase disorder and in the evening for advanced sleep phase disorder. These interventions take time and discipline.
Another approach is for the patient to skip sleep one night and, in a sleep-deprived state, establish a new bedtime at the desired time. Use the same modalities listed above to reinforce (“entrain”) this schedule; otherwise the patient will slip back into the previous abnormal sleep-wake rhythm.
Treatment can include iron repletion when indicated. Medications include dopaminergic agents, most notably pramipexole and levodopa/carbidopa. Other options include gabapentin, benzodiazepines, and narcotics.
Antidepressants have been suspected to worsen restless legs syndrome, but definitive studies are lacking.13
Circadian rhythm disorders
Instead of compromising the quality or quantity of sleep, circadian rhythm disorders cause sleep to occur at inappropriate times. These disorders are most common in adolescents and young adults.
Delayed sleep phase disorder—a persistent pattern of staying up late and “sleeping in”—is most common. Careful assessment will reveal that the patient is getting adequate sleep but at a socially unacceptable time, sometimes to the extreme that his or her nights and days are reversed.
Patients’ reluctance to acknowledge the severity of this problem can lead to inaccurate sleep diaries and interviews. A portable wrist actigraph can provide data about limb movement and is more objective than self-reports.
Delayed sleep phase disorder is highly comorbid with depressive disorders.14 The cause of this syndrome is unclear, but light exposure, social patterns, psychological issues, and possibly a genetic substrate are known to contribute.
Advanced sleep phase disorder—a less common circadian rhythm disorder—also can cause EDS. Patients have an inappropriately early time of sleep onset and then are fully awake in the middle of the night. A large family with a severe form of this disorder was found to have an abnormality on chromosome 2.15
Treatment. Relatively few treatments are effective for circadian rhythm disorders. Some patients elect not to pursue therapy, instead fitting activities around their unconventional sleep schedules.
Individuals with delayed sleep phase who cannot arrange their lives around their sleep schedules are at risk for poor early morning performance because of sleepiness. Their internal circadian clocks can be gradually readjusted with phototherapy or gradual shifting of the major sleep period (Box 3). Stimulants usually are not used, but hypnotics can sometimes help these patients fall asleep earlier.
Insufficient sleep syndrome
People attempting to “burn the candle at both ends” are at risk for developing insufficient sleep syndrome.16 In our 24/7 society, people trying to make do with less than the required 7.5 hours sleep per night may adversely affect their health. The problem is compounded for shift workers because of the difficulty in obtaining sufficient quality sleep during daylight hours.
Many patients do not seek treatment for fatigue or sleepiness because they are aware of their lifestyle choices. Still, they might develop psychological symptoms such as irritability, mood swings, and strained interpersonal relationships. These symptoms can prompt patients to request treatment.
Take a careful history that includes discussing the patient’s daily and weekly schedule. Avoid psychostimulants; instead, address the nonnegotiable need to get adequate sleep and challenge the patient to prioritize his or her activities around a full night’s sleep.
When to consider narcolepsy
Narcolepsy is a CNS disease characterized by abnormal regulation of REM sleep. EDS—the cardinal symptom—is often associated with cataplexy (75%), sleep paralysis (50%), vivid dreams, and insomnia, all of which interfere with REM phenomena. Narcolepsy affects 0.05% of the U.S. population and can lead to severe occupational, educational, and family disruption.
When you obtain a history that suggests narcolepsy, use the history, a sleep diary, or wrist actigraphy to document whether the patient is getting adequate sleep, with a consistent sleep/wake cycle. Next, consider referring the patient for polysomnography, primarily to rule out other causes of EDS such as sleep-disordered breathing. In some cases, REM latency on the overnight sleep study will be <20 minutes after sleep onset, which supports the diagnosis of narcolepsy.
A multiple sleep latency test (MSLT)—a diagnostic session in which the patient takes 4 to 5 daytime naps—is performed the following day. Narcolepsy is confirmed if the patient has a mean initial sleep latency of <10 minutes during these naps plus at least two REM episodes within 15 minutes after sleep onset.
The 4 most appropriate indications for an urgent sleep evaluation are:
- difficulty staying alert while driving
- nocturnal cardiac arrhythmias
- frequent observed apneas
- excessive daytime sleepiness (EDS) leading to academic or occupational problems.
Insurance companies usually cover a specialty sleep evaluation, particularly if the referring physician documents a suspicion of sleep-disordered breathing or EDS that jeopardizes safe driving.
Most patients with narcolepsy and cataplexy have undetectable cerebrospinal fluid levels of a neuropeptide called hypocretin or orexin.17 Hypocretin/orexin replacement therapy is a theoretical possibility, but for now treatment includes a combination of optimal sleep hygiene, psychostimulants, antidepressants, and hypnotics.
Other causes of EDS
EDS can also be caused by unrecognized alcohol dependence, inappropriate or excessive medication use, and depressive disorders. Overnight sleep studies are seldom indicated unless patients endorse the symptoms in the Figure.
Before pursuing polysomnography or an MSLT (Box 4), eliminate medications that might confound the results, such as:
- antidepressants, which alter the timing and duration of REM sleep
- sedating medications, which modify initial sleep latency and sleep efficiency and potentially aggravate sleep disordered breathing.
Initial REM latency provides a potential biologic marker of major depression but is used more often in research than in clinical psychiatry.
Primary insomnia is the distressing inability to sleep at night or nap during the day. It suggests a hyperarousal state—the opposite of EDS.18 In rare cases, however, patients who cannot sleep at night also have EDS. When evaluated, they typically endorse at least one symptom in the Figure. Sleep studies occasionally reveal OSA or restless legs syndrome.
Treating a patient with chronic insomnia may require several trials of behavioral interventions or sedating medications before you make a referral to a sleep disorder center. Patients can struggle with unrecognized primary sleep disorders for years, and many are given empiric trials of stimulating antidepressants. Antidepressants are unlikely to cause harm, but they might complicate diagnostic testing.
When you confirm coexisting depression and a primary sleep disorder, treatments that separately target each condition provide optimal management of the sleepy patient.
Medications to enhance wakefulness
Wake-promoting agents are a treatment option when EDS is contributing to compromised functioning. These drugs are no substitute for thoughtful evaluation of hypersomnolence, however. When you diagnose OSA or restless legs syndrome, first try treatments that target these conditions. If residual sleepiness persists, then consider augmenting with stimulating medications.
Modafinil is FDA-approved for residual sleepiness in patients with OSA and for shift work sleep disorder, a condition of circadian misalignment from frequent schedule changes. Evidence does not support its use for other circadian rhythm disorders, such as delayed sleep phase disorder.
Low-dose modafinil (such as 100 to 200 mg/d) is well tolerated, but its therapeutic effect as augmentation is modest.19 Increasing the dosage to >200 mg usually does not increase alertness.
Caffeine. Some patients report benefit from caffeine used in moderation and only in the morning. This practice is acceptable as long as patients do not use excessive amounts or experience insomnia, exacerbation of anxiety, or tachycardia.
Psychostimulants such as methylphenidate and amphetamines are less well-studied than modafinil for treating EDS in patients without narcolepsy. Monitor carefully for insomnia, exacerbation of anxiety, tachycardia, or hypertension and to prevent overuse of these habituating agents.
Related resources
- National Sleep Foundation. www.sleepfoundation.org.
- American Academy of Sleep Medicine. www.aasmnet.org.
- American Sleep Apnea Association. www.sleepapnea.org.
- Restless Legs Syndrome Foundation. www.rls.org.
- Association for the Study of Light Therapy and Biological Rhythms. www.sltbr.org.
Drug brand names
- Carbidopa/levodopa • Sinemet
- Gabapentin • Neurontin
- Modafinil • Provigil
- Pramipexole • Mirapex
- Trazodone • Desyrel
- Zolpidem • Ambien
Disclosure
Dr. Krahn reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Acknowledgment
Dr. Krahn published the original version this article in the January 2002 issue of Current Psychiatry and has updated it for this issue.
1. Punjabi N, Haponik E. Ask about daytime sleepiness. J Amer Geriatr Soc 2000;48:228-9.
2. Ronald J, Delaive K, Roos L, et al. Health care utilization in the 10 years prior to diagnosis in obstructive sleep apnea syndrome patients. Sleep 1999;22(2):225-9.
3. Johns M. A new method for measuring daytime sleepiness: The Epworth Sleepiness Scale. Sleep 1991;14(6):540-5.
4. Rowley J, Aboussouan L, Badr M. The use of clinical prediction formulas in the evaluation of obstructive sleep apnea. Sleep 2000;23:929-38.
5. Yamashiro Y, Kryger M. Nocturnal oximetry: Is it a screening tool for sleep disorders? Sleep 1995;18:167-71.
6. Morrell M, Finn L, Kim H, et al. Sleep fragmentation, awake blood pressure, and sleep-disordered breathing in a population-based study. Am J Respir Critical Care Med 2000;162(6):2091-6.
7. Roux F, D’Ambrosio C, Mohsenin V. Sleep-related breathing disorders and cardiovascular disease. Am J Med. 2000;108:396-402.
8. Engleman H, Martin S, Deary I, Douglas N. Effect of continuous positive airway pressure treatment on daytime function in sleep apnoea/hypopnoea syndrome. Lancet 1994;343(8897):572-5.
9. Lojander J, Maasilta P, Partinen M, et al. Nasal-CPAP, surgery, and conservative management for treatment of obstructive sleep apnea syndrome. A randomized study. Chest. 1996;110(1):114-9.
10. Mehta A, Qian J, Petocz P, et al. A randomized, controlled study of a mandibular advancement splint for obstructive sleep apnea. Am J Respir Critical Care Med 2001;163(6):1457-61.
11. Chesson A, Wise M, Davila D, et al. Practice parameters for the treatment of restless legs syndrome and periodic limb movement disorder. An American Academy of Sleep Medicine Report. Standards of Practice Committee of the American Academy of Sleep Medicine. Sleep 1999;22(7):961-8.
12. Phillips B, Young T, Finn L, et al. Epidemiology of restless legs symptoms in adults. Arch Intern Med 2000;160(14):2137-41.
13. Thorpy M, Ehrenberg B, Hening W, et al. Restless legs syndrome: Detection and management in primary care. Amer Fam Phys 2000;62:108-14.
14. Regestein Q, Monk T. Delayed sleep phase syndrome: A review of its clinical aspects. Am J Psychiatry 1995;152:602-8.
15. Toh K, Jones C, He Y, et al. An hPer2 phosphorylation site mutation in familial advanced sleep phase syndrome. Science 2001;291(5506):1040-3.
16. Yoshikawa N, Suzuki S, Ishimoto T, et al. A case of insufficient sleep syndrome. Psychiatry Clin Neuro 1998;52(2):200-1.
17. Nishino S, Ripley B, Overeem S, et al. Hypocretin (orexin) deficiency in human narcolepsy. Lancet. 2000;355:39-40.
18. Hauri P, Esther M. Insomnia. Mayo Clin Proc 1990;65:869-82.
19. Schwartz JR. Modafinil: new indications for wake promotion. Expert Opin Pharmacother 2005;6(1):115-29.
1. Punjabi N, Haponik E. Ask about daytime sleepiness. J Amer Geriatr Soc 2000;48:228-9.
2. Ronald J, Delaive K, Roos L, et al. Health care utilization in the 10 years prior to diagnosis in obstructive sleep apnea syndrome patients. Sleep 1999;22(2):225-9.
3. Johns M. A new method for measuring daytime sleepiness: The Epworth Sleepiness Scale. Sleep 1991;14(6):540-5.
4. Rowley J, Aboussouan L, Badr M. The use of clinical prediction formulas in the evaluation of obstructive sleep apnea. Sleep 2000;23:929-38.
5. Yamashiro Y, Kryger M. Nocturnal oximetry: Is it a screening tool for sleep disorders? Sleep 1995;18:167-71.
6. Morrell M, Finn L, Kim H, et al. Sleep fragmentation, awake blood pressure, and sleep-disordered breathing in a population-based study. Am J Respir Critical Care Med 2000;162(6):2091-6.
7. Roux F, D’Ambrosio C, Mohsenin V. Sleep-related breathing disorders and cardiovascular disease. Am J Med. 2000;108:396-402.
8. Engleman H, Martin S, Deary I, Douglas N. Effect of continuous positive airway pressure treatment on daytime function in sleep apnoea/hypopnoea syndrome. Lancet 1994;343(8897):572-5.
9. Lojander J, Maasilta P, Partinen M, et al. Nasal-CPAP, surgery, and conservative management for treatment of obstructive sleep apnea syndrome. A randomized study. Chest. 1996;110(1):114-9.
10. Mehta A, Qian J, Petocz P, et al. A randomized, controlled study of a mandibular advancement splint for obstructive sleep apnea. Am J Respir Critical Care Med 2001;163(6):1457-61.
11. Chesson A, Wise M, Davila D, et al. Practice parameters for the treatment of restless legs syndrome and periodic limb movement disorder. An American Academy of Sleep Medicine Report. Standards of Practice Committee of the American Academy of Sleep Medicine. Sleep 1999;22(7):961-8.
12. Phillips B, Young T, Finn L, et al. Epidemiology of restless legs symptoms in adults. Arch Intern Med 2000;160(14):2137-41.
13. Thorpy M, Ehrenberg B, Hening W, et al. Restless legs syndrome: Detection and management in primary care. Amer Fam Phys 2000;62:108-14.
14. Regestein Q, Monk T. Delayed sleep phase syndrome: A review of its clinical aspects. Am J Psychiatry 1995;152:602-8.
15. Toh K, Jones C, He Y, et al. An hPer2 phosphorylation site mutation in familial advanced sleep phase syndrome. Science 2001;291(5506):1040-3.
16. Yoshikawa N, Suzuki S, Ishimoto T, et al. A case of insufficient sleep syndrome. Psychiatry Clin Neuro 1998;52(2):200-1.
17. Nishino S, Ripley B, Overeem S, et al. Hypocretin (orexin) deficiency in human narcolepsy. Lancet. 2000;355:39-40.
18. Hauri P, Esther M. Insomnia. Mayo Clin Proc 1990;65:869-82.
19. Schwartz JR. Modafinil: new indications for wake promotion. Expert Opin Pharmacother 2005;6(1):115-29.