User login
Surgical aortic valve replacement (SAVR) started in the 1960s with a porcine aortic valve sutured to a stainless steel frame. The first human transcatheter aortic valve replacement (TAVR) procedure in the United States was in 2002. In the past 15 years, technological advances in heart valve design have made TAVR the preferred alternative in patients at high risk for surgical complications. This article outlines studies comparing balloon-expandable TAVR vs SAVR for patients at extreme, high, and intermediate surgical risk, and presents evidence that supports the expanded use of TAVR in patients at lower surgical risk.
TAVR: THE PREFERRED ALTERNATIVE TO SURGERY
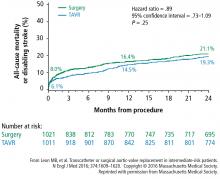
Investigators next established TAVR outcomes as being noninferior to SAVR in high surgical risk patients (PARTNER trial cohort A) at 1 year.2 A midterm follow-up of this study published in 2015 reported comparable rates of all-cause mortality at 5 years in high-risk patients undergoing TAVR vs SAVR, thus confirming the noninferiority of TAVR vs a surgical approach in high-risk patients for the longest duration of follow-up currently available.3
For patients, if the results of 2 different procedures are similar, they are typically going to choose the less invasive option. As a result, use of TAVR has increased: nearly 300,000 procedures have been performed worldwide, and approximately 75,000 were completed in 2016 alone. These numbers are projected to increase fourfold in the next 10 years. In the United States, almost one-third of Medicare-reported aortic valve replacements in 2015 were performed using TAVR.4
These data show that TAVR has become the preferred alternative to SAVR in inoperable and high-risk patients.
TAVR IN INTERMEDIATE-RISK PATIENTS
The US Food and Drug Administration (FDA) initially approved TAVR for patients judged to be ineligible for open-chest valve replacement cardiac surgery or at high risk for SAVR. This represents a small percentage of the total patient population needing aortic valve replacement. The Society of Thoracic Surgeons database of aortic valve disease cases during 2002 to 2010 (N = 141,905) shows that just 6.2% were ranked as high risk (ie, population eligible for TAVR in 2016). Most patients (79.9%) were low risk, and 13.9% were intermediate risk.5
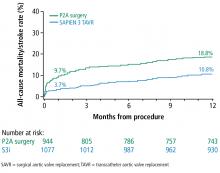
A subanalysis of the transfemoral-access cohort provided additional support for TAVR. It showed that the rate of death and stroke in this cohort began to trend more favorably for TAVR. At 24 months, the difference in the primary end point was statistically significant in favor of TAVR (16.3% vs 20.0% for surgery; P = .04).1
Based on these data, in August 2016, the FDA approved the Sapien valves for use in patients with aortic valve stenosis who are at intermediate risk of death or complications associated with open-heart surgery. If the differences in outcomes reported during the PARTNER S3i trial are extrapolated to the total number of valve replacement surgeries performed worldwide, the potential number of patients who may benefit from TAVR is substantial.
DOWNSIDE OF TAVR
Although results with TAVR appear promising, there are important issues to address before it can be adopted in a wider patient population (ie, low-risk patients). These primarily focus on the following:
- Stroke
- Paravalvular leak
- Need for pacemaker replacement
- Valve durability
- Leaflet immobility or valve thrombosis.
Stroke
The incidence of stroke associated with TAVR is a concern, but it has decreased with the introduction of the Sapien 3 valve. In the PARTNER 2 trial, the 30-day stroke rate in intermediate-risk patients who received the Sapien 3 valve was 2.6%.1 This compares with a 5.6% overall rate in the PARTNER 1A trials using the first Sapien valve.2 The rate of stroke events is expected to decrease further as TAVR is expanded into healthier populations with better vasculature.
Paravalvular leak
Rates of moderate or severe paravalvular leak at 30 days have also decreased with the Sapien 3 valve and were 4.2% overall in the PARTNER S3i trial.6 These rates have ranged from 11.5% overall in the PARTNER 1A trial2 to 4.2% in the PARTNER 2B trial1 that used the Sapien XT valve for transfemoral-access TAVR.
New pacemakers
The percentage of TAVR procedures that result in a new requirement for a pacemaker increased to about 11% in 2014, up from 6.8% in 2012 to 2013.8 The requirement for a new pacemaker within 30 days following TAVR appeared to decrease again in the PARTER 2 trial, to 8.5%.1
Durability
Evidence is emerging showing the limited durability of bioprosthetic aortic valve. Multiple studies have reportedly shown this, and this is true for all tissue valves, including those surgically inserted. A study assessing data from 357 patients showed that structural valve degeneration begins at 7 years postoperatively. By 10 years, only about 86% of valves were free from degeneration. At 12 years, that dropped to 69%.9
A study comparing TAVR vs SAVR showed that under identical loading conditions and with identical leaflet tissue properties, leaflets of valves placed via TAVR sustained higher stresses, strains, and fatigue damage.10
Overall, these results provide the possibility that TAVR valves may have reduced valve life compared with SAVR valves. Unknown durability may be an issue to consider when evaluating TAVR for implantation in intermediate- and low-risk patients.
Leaflet immobility and valve thrombosis
In the past 2 years, the problem of potential subclinical valve leaflet thrombosis, on both surgically inserted and TAVR valves, has emerged.11 The FDA is monitoring these complications because of their potential impact on the safety and efficacy of these valves.
This complication was first reported as an unexpected finding of reduced leaflet motion on 4-dimensional computed tomography, a sign suspicious for valve thrombosis, in a subgroup of patients evaluated 30 days after implantation.12 A study from Denmark found a 7% incidence of valve thrombosis in TAVR valves. They reported that warfarin could prevent thrombosis.13
At the Heart Hospital Baylor Plano, our TAVR team has identified approximately 50 cases of thrombosis that caused partial valve occlusion. Administering warfarin for 3 months resolved the thrombosis in virtually all cases. In 1 case, a thrombosed valve was surgically explanted with good patient outcome. Pathological analysis confirmed that reduced leaflet motion seen on 4-dimensional CT was valve thrombosis, as suspected by imaging specialists.14
IS TAVR APPROPRIATE FOR INTERMEDIATE-RISK PATIENTS?
Although there are ample data supporting the use of TAVR in intermediate-risk patients, SAVR remains the most effective option in certain clinical situations:
- Younger patients who will need valve replacement later in life
- Bicuspid valves with eccentric bulky calcification
- Aortopathy (aortic disease above the valve)
- Small calcified roots
- Severe calcification of left ventricular outflow tract
- Low-lying coronary arteries (typically, ≤ 6 mm from the aortic annulus)
- Severe septal bulging
- Severe mitral regurgitation and/or tricuspid regurgitation
- Conduction system disease that puts the patient at high risk for pacemaker implantation
- Valve replacement in valves with a diameter 20 mm or smaller.
Nevertheless, outcomes seem to support TAVR in intermediate-risk patients. At the Heart Hospital Baylor Plano, 30-day outcomes with the Sapien 3 valve have shown all-cause mortality of 1.1% and all-stroke mortality of 2.6% (1.0% for disabling stroke). Large registries of the Sapien 3 valve have reported similar outcomes at 30 days: mortality 1%, disabling stroke 2%, major vascular complications 2%, and moderate to severe paravalvular leak 2%.15
Overall, the rates of major vascular complications and of life-threatening bleeding are 2%, and the need for new pacemakers is 4%. Results from several trials support TAVR as an alternative to surgery in intermediate-risk patients. In patients who are candidates for transfemoral access, TAVR may provide additional clinical advantages. However, questions about long-term durability and new requirements for pacemakers are issues for TAVR use in intermediate- and low-risk patients. More data are needed to answer these questions.
At the Heart Hospital Baylor Plano, the number of TAVR procedures from 2012 to 2015 increased from 49 cases to 215, while the number of SAVR procedures remained constant (166 in 2012 and 162 in 2015). During that time, outcomes improved dramatically: in-hospital mortality rates dropped from 2% to 0% and 30-day mortality dropped from 3% to 0%. There have been 227 consecutive SAVR patients with no in-hospital or 30-day mortality and 261 consecutive TAVR patients with no mortality.
These results support initiating clinical trials of TAVR in low-risk patients. In 2016, the FDA approved TAVR valves for 2 clinical trials in patients with aortic stenosis who are at low risk of surgical mortality. These large clinical trials, each with about 1,200 patients, are expected to provide data that will help determine whether TAVR is a safe and effective option for low-risk patients.
- Leon MB, Smith CR, Mack MJ, et al; for the PARTNER 2 Investigators. Transcatheter or surgical aortic-valve replacement in intermediate-risk patients. N Engl J Med 2016; 374:1609–1620.
- Smith CR, Leon MB, Mack MJ, et al; for the PARTNER Trial Investigators. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med 2011; 364:2187–2198.
- Mack MJ, Leon MB, Smith CR, et al; for the PARTNER 1 trial investigators. 5-year outcomes of transcatheter aortic valve replacement or surgical aortic valve replacement for high surgical risk patients with aortic stenosis (PARTNER 1): a randomised controlled trial. Lancet 2015; 385:2477–2484.
- Nazif T. Where we are and where we are going. Presented at Transcatheter Cardiovascular Therapeutics 2016 Annual Meeting; October 2016; Washington, DC.
- Thourani VH, Suri RM, Gunter RL, et al. Contemporary real-world outcomes of surgical aortic valve replacement in 141,905 low-risk, intermediate-risk, and high-risk patients. Ann Thorac Surg 2015; 99:55–61.
- Thourani VH, Kodali S, Makkar RR, et al. Transcatheter aortic valve replacement versus surgical valve replacement in intermediate-risk patients: a propensity score analysis. Lancet 2016; 387:2218–2225.
- Thourani VH on behalf of the PARTNER Trial Investigators. SAPIEN 3 transcatheter aortic valve replacement compared with surgery in intermediate-risk patients: a propensity score analysis. Presented at: American College of Cardiology 65th Annual Meeting; April 2016; Chicago, IL.
- Holmes DR Jr, Nishimura RA, Grover FL, et al; for the STS/ACC TVT Registry. Annual outcomes with transcatheter valve therapy: from the STS/ACC TVT Registry. J Am Coll Cardiol 2015; 66:2813–2823.
- David TE, Feindel CM, Bos J, Ivanov J, Armstrong S. Aortic valve replacement with Toronto SPV bioprosthesis: optimal patient survival but suboptimal valve durability. J Thorac Cardiovasc Surg 2008; 135:19–24.
- Martin C, Sun W. Comparison of transcatheter aortic valve and surgical bioprosthetic valve durability: a fatigue simulation study. J Biomech 2015; 48:3026–3034.
- Laschinger JC, Wu C, Ibrahim NG, Shuren JE. Reduced leaflet motion in bioprosthetic aortic valves—the FDA perspective. N Engl J Med 2015; 373:1996–1998.
- Makkar RR, Fontana G, Jilaihawi H, et al. Possible subclinical leaflet thrombosis in bioprosthetic aortic valves. N Engl J Med 2015; 373:2015–2024.
- Hansson NC, Grove EL, Andersen HR, et al. Transcatheter aortic valve thrombosis: incidence, predisposing factors, and clinical implications. J Am Coll Cardiol 2016; 68:2059–2069.
- Gopal A, Ribeiro N, Squiers JJ, et al. Pathologic confirmation of valve thrombosis detected by four-dimensional computed tomography following valve-in-valve transcatheter aortic valve replacement. Glob Cardiol Sci Prac 2017. In press.
- Kodali S, Thourani VH, White J, et al. Early clinical and echocardiographic outcomes after SAPIEN 3 transcatheter aortic valve replacement in inoperable, high-risk, and intermediate-risk patients with aortic stenosis. Eur Heart J 2016; 37:2252–2262.
Surgical aortic valve replacement (SAVR) started in the 1960s with a porcine aortic valve sutured to a stainless steel frame. The first human transcatheter aortic valve replacement (TAVR) procedure in the United States was in 2002. In the past 15 years, technological advances in heart valve design have made TAVR the preferred alternative in patients at high risk for surgical complications. This article outlines studies comparing balloon-expandable TAVR vs SAVR for patients at extreme, high, and intermediate surgical risk, and presents evidence that supports the expanded use of TAVR in patients at lower surgical risk.
TAVR: THE PREFERRED ALTERNATIVE TO SURGERY
Investigators next established TAVR outcomes as being noninferior to SAVR in high surgical risk patients (PARTNER trial cohort A) at 1 year.2 A midterm follow-up of this study published in 2015 reported comparable rates of all-cause mortality at 5 years in high-risk patients undergoing TAVR vs SAVR, thus confirming the noninferiority of TAVR vs a surgical approach in high-risk patients for the longest duration of follow-up currently available.3
For patients, if the results of 2 different procedures are similar, they are typically going to choose the less invasive option. As a result, use of TAVR has increased: nearly 300,000 procedures have been performed worldwide, and approximately 75,000 were completed in 2016 alone. These numbers are projected to increase fourfold in the next 10 years. In the United States, almost one-third of Medicare-reported aortic valve replacements in 2015 were performed using TAVR.4
These data show that TAVR has become the preferred alternative to SAVR in inoperable and high-risk patients.
TAVR IN INTERMEDIATE-RISK PATIENTS
The US Food and Drug Administration (FDA) initially approved TAVR for patients judged to be ineligible for open-chest valve replacement cardiac surgery or at high risk for SAVR. This represents a small percentage of the total patient population needing aortic valve replacement. The Society of Thoracic Surgeons database of aortic valve disease cases during 2002 to 2010 (N = 141,905) shows that just 6.2% were ranked as high risk (ie, population eligible for TAVR in 2016). Most patients (79.9%) were low risk, and 13.9% were intermediate risk.5
A subanalysis of the transfemoral-access cohort provided additional support for TAVR. It showed that the rate of death and stroke in this cohort began to trend more favorably for TAVR. At 24 months, the difference in the primary end point was statistically significant in favor of TAVR (16.3% vs 20.0% for surgery; P = .04).1
Based on these data, in August 2016, the FDA approved the Sapien valves for use in patients with aortic valve stenosis who are at intermediate risk of death or complications associated with open-heart surgery. If the differences in outcomes reported during the PARTNER S3i trial are extrapolated to the total number of valve replacement surgeries performed worldwide, the potential number of patients who may benefit from TAVR is substantial.
DOWNSIDE OF TAVR
Although results with TAVR appear promising, there are important issues to address before it can be adopted in a wider patient population (ie, low-risk patients). These primarily focus on the following:
- Stroke
- Paravalvular leak
- Need for pacemaker replacement
- Valve durability
- Leaflet immobility or valve thrombosis.
Stroke
The incidence of stroke associated with TAVR is a concern, but it has decreased with the introduction of the Sapien 3 valve. In the PARTNER 2 trial, the 30-day stroke rate in intermediate-risk patients who received the Sapien 3 valve was 2.6%.1 This compares with a 5.6% overall rate in the PARTNER 1A trials using the first Sapien valve.2 The rate of stroke events is expected to decrease further as TAVR is expanded into healthier populations with better vasculature.
Paravalvular leak
Rates of moderate or severe paravalvular leak at 30 days have also decreased with the Sapien 3 valve and were 4.2% overall in the PARTNER S3i trial.6 These rates have ranged from 11.5% overall in the PARTNER 1A trial2 to 4.2% in the PARTNER 2B trial1 that used the Sapien XT valve for transfemoral-access TAVR.
New pacemakers
The percentage of TAVR procedures that result in a new requirement for a pacemaker increased to about 11% in 2014, up from 6.8% in 2012 to 2013.8 The requirement for a new pacemaker within 30 days following TAVR appeared to decrease again in the PARTER 2 trial, to 8.5%.1
Durability
Evidence is emerging showing the limited durability of bioprosthetic aortic valve. Multiple studies have reportedly shown this, and this is true for all tissue valves, including those surgically inserted. A study assessing data from 357 patients showed that structural valve degeneration begins at 7 years postoperatively. By 10 years, only about 86% of valves were free from degeneration. At 12 years, that dropped to 69%.9
A study comparing TAVR vs SAVR showed that under identical loading conditions and with identical leaflet tissue properties, leaflets of valves placed via TAVR sustained higher stresses, strains, and fatigue damage.10
Overall, these results provide the possibility that TAVR valves may have reduced valve life compared with SAVR valves. Unknown durability may be an issue to consider when evaluating TAVR for implantation in intermediate- and low-risk patients.
Leaflet immobility and valve thrombosis
In the past 2 years, the problem of potential subclinical valve leaflet thrombosis, on both surgically inserted and TAVR valves, has emerged.11 The FDA is monitoring these complications because of their potential impact on the safety and efficacy of these valves.
This complication was first reported as an unexpected finding of reduced leaflet motion on 4-dimensional computed tomography, a sign suspicious for valve thrombosis, in a subgroup of patients evaluated 30 days after implantation.12 A study from Denmark found a 7% incidence of valve thrombosis in TAVR valves. They reported that warfarin could prevent thrombosis.13
At the Heart Hospital Baylor Plano, our TAVR team has identified approximately 50 cases of thrombosis that caused partial valve occlusion. Administering warfarin for 3 months resolved the thrombosis in virtually all cases. In 1 case, a thrombosed valve was surgically explanted with good patient outcome. Pathological analysis confirmed that reduced leaflet motion seen on 4-dimensional CT was valve thrombosis, as suspected by imaging specialists.14
IS TAVR APPROPRIATE FOR INTERMEDIATE-RISK PATIENTS?
Although there are ample data supporting the use of TAVR in intermediate-risk patients, SAVR remains the most effective option in certain clinical situations:
- Younger patients who will need valve replacement later in life
- Bicuspid valves with eccentric bulky calcification
- Aortopathy (aortic disease above the valve)
- Small calcified roots
- Severe calcification of left ventricular outflow tract
- Low-lying coronary arteries (typically, ≤ 6 mm from the aortic annulus)
- Severe septal bulging
- Severe mitral regurgitation and/or tricuspid regurgitation
- Conduction system disease that puts the patient at high risk for pacemaker implantation
- Valve replacement in valves with a diameter 20 mm or smaller.
Nevertheless, outcomes seem to support TAVR in intermediate-risk patients. At the Heart Hospital Baylor Plano, 30-day outcomes with the Sapien 3 valve have shown all-cause mortality of 1.1% and all-stroke mortality of 2.6% (1.0% for disabling stroke). Large registries of the Sapien 3 valve have reported similar outcomes at 30 days: mortality 1%, disabling stroke 2%, major vascular complications 2%, and moderate to severe paravalvular leak 2%.15
Overall, the rates of major vascular complications and of life-threatening bleeding are 2%, and the need for new pacemakers is 4%. Results from several trials support TAVR as an alternative to surgery in intermediate-risk patients. In patients who are candidates for transfemoral access, TAVR may provide additional clinical advantages. However, questions about long-term durability and new requirements for pacemakers are issues for TAVR use in intermediate- and low-risk patients. More data are needed to answer these questions.
At the Heart Hospital Baylor Plano, the number of TAVR procedures from 2012 to 2015 increased from 49 cases to 215, while the number of SAVR procedures remained constant (166 in 2012 and 162 in 2015). During that time, outcomes improved dramatically: in-hospital mortality rates dropped from 2% to 0% and 30-day mortality dropped from 3% to 0%. There have been 227 consecutive SAVR patients with no in-hospital or 30-day mortality and 261 consecutive TAVR patients with no mortality.
These results support initiating clinical trials of TAVR in low-risk patients. In 2016, the FDA approved TAVR valves for 2 clinical trials in patients with aortic stenosis who are at low risk of surgical mortality. These large clinical trials, each with about 1,200 patients, are expected to provide data that will help determine whether TAVR is a safe and effective option for low-risk patients.
Surgical aortic valve replacement (SAVR) started in the 1960s with a porcine aortic valve sutured to a stainless steel frame. The first human transcatheter aortic valve replacement (TAVR) procedure in the United States was in 2002. In the past 15 years, technological advances in heart valve design have made TAVR the preferred alternative in patients at high risk for surgical complications. This article outlines studies comparing balloon-expandable TAVR vs SAVR for patients at extreme, high, and intermediate surgical risk, and presents evidence that supports the expanded use of TAVR in patients at lower surgical risk.
TAVR: THE PREFERRED ALTERNATIVE TO SURGERY
Investigators next established TAVR outcomes as being noninferior to SAVR in high surgical risk patients (PARTNER trial cohort A) at 1 year.2 A midterm follow-up of this study published in 2015 reported comparable rates of all-cause mortality at 5 years in high-risk patients undergoing TAVR vs SAVR, thus confirming the noninferiority of TAVR vs a surgical approach in high-risk patients for the longest duration of follow-up currently available.3
For patients, if the results of 2 different procedures are similar, they are typically going to choose the less invasive option. As a result, use of TAVR has increased: nearly 300,000 procedures have been performed worldwide, and approximately 75,000 were completed in 2016 alone. These numbers are projected to increase fourfold in the next 10 years. In the United States, almost one-third of Medicare-reported aortic valve replacements in 2015 were performed using TAVR.4
These data show that TAVR has become the preferred alternative to SAVR in inoperable and high-risk patients.
TAVR IN INTERMEDIATE-RISK PATIENTS
The US Food and Drug Administration (FDA) initially approved TAVR for patients judged to be ineligible for open-chest valve replacement cardiac surgery or at high risk for SAVR. This represents a small percentage of the total patient population needing aortic valve replacement. The Society of Thoracic Surgeons database of aortic valve disease cases during 2002 to 2010 (N = 141,905) shows that just 6.2% were ranked as high risk (ie, population eligible for TAVR in 2016). Most patients (79.9%) were low risk, and 13.9% were intermediate risk.5
A subanalysis of the transfemoral-access cohort provided additional support for TAVR. It showed that the rate of death and stroke in this cohort began to trend more favorably for TAVR. At 24 months, the difference in the primary end point was statistically significant in favor of TAVR (16.3% vs 20.0% for surgery; P = .04).1
Based on these data, in August 2016, the FDA approved the Sapien valves for use in patients with aortic valve stenosis who are at intermediate risk of death or complications associated with open-heart surgery. If the differences in outcomes reported during the PARTNER S3i trial are extrapolated to the total number of valve replacement surgeries performed worldwide, the potential number of patients who may benefit from TAVR is substantial.
DOWNSIDE OF TAVR
Although results with TAVR appear promising, there are important issues to address before it can be adopted in a wider patient population (ie, low-risk patients). These primarily focus on the following:
- Stroke
- Paravalvular leak
- Need for pacemaker replacement
- Valve durability
- Leaflet immobility or valve thrombosis.
Stroke
The incidence of stroke associated with TAVR is a concern, but it has decreased with the introduction of the Sapien 3 valve. In the PARTNER 2 trial, the 30-day stroke rate in intermediate-risk patients who received the Sapien 3 valve was 2.6%.1 This compares with a 5.6% overall rate in the PARTNER 1A trials using the first Sapien valve.2 The rate of stroke events is expected to decrease further as TAVR is expanded into healthier populations with better vasculature.
Paravalvular leak
Rates of moderate or severe paravalvular leak at 30 days have also decreased with the Sapien 3 valve and were 4.2% overall in the PARTNER S3i trial.6 These rates have ranged from 11.5% overall in the PARTNER 1A trial2 to 4.2% in the PARTNER 2B trial1 that used the Sapien XT valve for transfemoral-access TAVR.
New pacemakers
The percentage of TAVR procedures that result in a new requirement for a pacemaker increased to about 11% in 2014, up from 6.8% in 2012 to 2013.8 The requirement for a new pacemaker within 30 days following TAVR appeared to decrease again in the PARTER 2 trial, to 8.5%.1
Durability
Evidence is emerging showing the limited durability of bioprosthetic aortic valve. Multiple studies have reportedly shown this, and this is true for all tissue valves, including those surgically inserted. A study assessing data from 357 patients showed that structural valve degeneration begins at 7 years postoperatively. By 10 years, only about 86% of valves were free from degeneration. At 12 years, that dropped to 69%.9
A study comparing TAVR vs SAVR showed that under identical loading conditions and with identical leaflet tissue properties, leaflets of valves placed via TAVR sustained higher stresses, strains, and fatigue damage.10
Overall, these results provide the possibility that TAVR valves may have reduced valve life compared with SAVR valves. Unknown durability may be an issue to consider when evaluating TAVR for implantation in intermediate- and low-risk patients.
Leaflet immobility and valve thrombosis
In the past 2 years, the problem of potential subclinical valve leaflet thrombosis, on both surgically inserted and TAVR valves, has emerged.11 The FDA is monitoring these complications because of their potential impact on the safety and efficacy of these valves.
This complication was first reported as an unexpected finding of reduced leaflet motion on 4-dimensional computed tomography, a sign suspicious for valve thrombosis, in a subgroup of patients evaluated 30 days after implantation.12 A study from Denmark found a 7% incidence of valve thrombosis in TAVR valves. They reported that warfarin could prevent thrombosis.13
At the Heart Hospital Baylor Plano, our TAVR team has identified approximately 50 cases of thrombosis that caused partial valve occlusion. Administering warfarin for 3 months resolved the thrombosis in virtually all cases. In 1 case, a thrombosed valve was surgically explanted with good patient outcome. Pathological analysis confirmed that reduced leaflet motion seen on 4-dimensional CT was valve thrombosis, as suspected by imaging specialists.14
IS TAVR APPROPRIATE FOR INTERMEDIATE-RISK PATIENTS?
Although there are ample data supporting the use of TAVR in intermediate-risk patients, SAVR remains the most effective option in certain clinical situations:
- Younger patients who will need valve replacement later in life
- Bicuspid valves with eccentric bulky calcification
- Aortopathy (aortic disease above the valve)
- Small calcified roots
- Severe calcification of left ventricular outflow tract
- Low-lying coronary arteries (typically, ≤ 6 mm from the aortic annulus)
- Severe septal bulging
- Severe mitral regurgitation and/or tricuspid regurgitation
- Conduction system disease that puts the patient at high risk for pacemaker implantation
- Valve replacement in valves with a diameter 20 mm or smaller.
Nevertheless, outcomes seem to support TAVR in intermediate-risk patients. At the Heart Hospital Baylor Plano, 30-day outcomes with the Sapien 3 valve have shown all-cause mortality of 1.1% and all-stroke mortality of 2.6% (1.0% for disabling stroke). Large registries of the Sapien 3 valve have reported similar outcomes at 30 days: mortality 1%, disabling stroke 2%, major vascular complications 2%, and moderate to severe paravalvular leak 2%.15
Overall, the rates of major vascular complications and of life-threatening bleeding are 2%, and the need for new pacemakers is 4%. Results from several trials support TAVR as an alternative to surgery in intermediate-risk patients. In patients who are candidates for transfemoral access, TAVR may provide additional clinical advantages. However, questions about long-term durability and new requirements for pacemakers are issues for TAVR use in intermediate- and low-risk patients. More data are needed to answer these questions.
At the Heart Hospital Baylor Plano, the number of TAVR procedures from 2012 to 2015 increased from 49 cases to 215, while the number of SAVR procedures remained constant (166 in 2012 and 162 in 2015). During that time, outcomes improved dramatically: in-hospital mortality rates dropped from 2% to 0% and 30-day mortality dropped from 3% to 0%. There have been 227 consecutive SAVR patients with no in-hospital or 30-day mortality and 261 consecutive TAVR patients with no mortality.
These results support initiating clinical trials of TAVR in low-risk patients. In 2016, the FDA approved TAVR valves for 2 clinical trials in patients with aortic stenosis who are at low risk of surgical mortality. These large clinical trials, each with about 1,200 patients, are expected to provide data that will help determine whether TAVR is a safe and effective option for low-risk patients.
- Leon MB, Smith CR, Mack MJ, et al; for the PARTNER 2 Investigators. Transcatheter or surgical aortic-valve replacement in intermediate-risk patients. N Engl J Med 2016; 374:1609–1620.
- Smith CR, Leon MB, Mack MJ, et al; for the PARTNER Trial Investigators. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med 2011; 364:2187–2198.
- Mack MJ, Leon MB, Smith CR, et al; for the PARTNER 1 trial investigators. 5-year outcomes of transcatheter aortic valve replacement or surgical aortic valve replacement for high surgical risk patients with aortic stenosis (PARTNER 1): a randomised controlled trial. Lancet 2015; 385:2477–2484.
- Nazif T. Where we are and where we are going. Presented at Transcatheter Cardiovascular Therapeutics 2016 Annual Meeting; October 2016; Washington, DC.
- Thourani VH, Suri RM, Gunter RL, et al. Contemporary real-world outcomes of surgical aortic valve replacement in 141,905 low-risk, intermediate-risk, and high-risk patients. Ann Thorac Surg 2015; 99:55–61.
- Thourani VH, Kodali S, Makkar RR, et al. Transcatheter aortic valve replacement versus surgical valve replacement in intermediate-risk patients: a propensity score analysis. Lancet 2016; 387:2218–2225.
- Thourani VH on behalf of the PARTNER Trial Investigators. SAPIEN 3 transcatheter aortic valve replacement compared with surgery in intermediate-risk patients: a propensity score analysis. Presented at: American College of Cardiology 65th Annual Meeting; April 2016; Chicago, IL.
- Holmes DR Jr, Nishimura RA, Grover FL, et al; for the STS/ACC TVT Registry. Annual outcomes with transcatheter valve therapy: from the STS/ACC TVT Registry. J Am Coll Cardiol 2015; 66:2813–2823.
- David TE, Feindel CM, Bos J, Ivanov J, Armstrong S. Aortic valve replacement with Toronto SPV bioprosthesis: optimal patient survival but suboptimal valve durability. J Thorac Cardiovasc Surg 2008; 135:19–24.
- Martin C, Sun W. Comparison of transcatheter aortic valve and surgical bioprosthetic valve durability: a fatigue simulation study. J Biomech 2015; 48:3026–3034.
- Laschinger JC, Wu C, Ibrahim NG, Shuren JE. Reduced leaflet motion in bioprosthetic aortic valves—the FDA perspective. N Engl J Med 2015; 373:1996–1998.
- Makkar RR, Fontana G, Jilaihawi H, et al. Possible subclinical leaflet thrombosis in bioprosthetic aortic valves. N Engl J Med 2015; 373:2015–2024.
- Hansson NC, Grove EL, Andersen HR, et al. Transcatheter aortic valve thrombosis: incidence, predisposing factors, and clinical implications. J Am Coll Cardiol 2016; 68:2059–2069.
- Gopal A, Ribeiro N, Squiers JJ, et al. Pathologic confirmation of valve thrombosis detected by four-dimensional computed tomography following valve-in-valve transcatheter aortic valve replacement. Glob Cardiol Sci Prac 2017. In press.
- Kodali S, Thourani VH, White J, et al. Early clinical and echocardiographic outcomes after SAPIEN 3 transcatheter aortic valve replacement in inoperable, high-risk, and intermediate-risk patients with aortic stenosis. Eur Heart J 2016; 37:2252–2262.
- Leon MB, Smith CR, Mack MJ, et al; for the PARTNER 2 Investigators. Transcatheter or surgical aortic-valve replacement in intermediate-risk patients. N Engl J Med 2016; 374:1609–1620.
- Smith CR, Leon MB, Mack MJ, et al; for the PARTNER Trial Investigators. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med 2011; 364:2187–2198.
- Mack MJ, Leon MB, Smith CR, et al; for the PARTNER 1 trial investigators. 5-year outcomes of transcatheter aortic valve replacement or surgical aortic valve replacement for high surgical risk patients with aortic stenosis (PARTNER 1): a randomised controlled trial. Lancet 2015; 385:2477–2484.
- Nazif T. Where we are and where we are going. Presented at Transcatheter Cardiovascular Therapeutics 2016 Annual Meeting; October 2016; Washington, DC.
- Thourani VH, Suri RM, Gunter RL, et al. Contemporary real-world outcomes of surgical aortic valve replacement in 141,905 low-risk, intermediate-risk, and high-risk patients. Ann Thorac Surg 2015; 99:55–61.
- Thourani VH, Kodali S, Makkar RR, et al. Transcatheter aortic valve replacement versus surgical valve replacement in intermediate-risk patients: a propensity score analysis. Lancet 2016; 387:2218–2225.
- Thourani VH on behalf of the PARTNER Trial Investigators. SAPIEN 3 transcatheter aortic valve replacement compared with surgery in intermediate-risk patients: a propensity score analysis. Presented at: American College of Cardiology 65th Annual Meeting; April 2016; Chicago, IL.
- Holmes DR Jr, Nishimura RA, Grover FL, et al; for the STS/ACC TVT Registry. Annual outcomes with transcatheter valve therapy: from the STS/ACC TVT Registry. J Am Coll Cardiol 2015; 66:2813–2823.
- David TE, Feindel CM, Bos J, Ivanov J, Armstrong S. Aortic valve replacement with Toronto SPV bioprosthesis: optimal patient survival but suboptimal valve durability. J Thorac Cardiovasc Surg 2008; 135:19–24.
- Martin C, Sun W. Comparison of transcatheter aortic valve and surgical bioprosthetic valve durability: a fatigue simulation study. J Biomech 2015; 48:3026–3034.
- Laschinger JC, Wu C, Ibrahim NG, Shuren JE. Reduced leaflet motion in bioprosthetic aortic valves—the FDA perspective. N Engl J Med 2015; 373:1996–1998.
- Makkar RR, Fontana G, Jilaihawi H, et al. Possible subclinical leaflet thrombosis in bioprosthetic aortic valves. N Engl J Med 2015; 373:2015–2024.
- Hansson NC, Grove EL, Andersen HR, et al. Transcatheter aortic valve thrombosis: incidence, predisposing factors, and clinical implications. J Am Coll Cardiol 2016; 68:2059–2069.
- Gopal A, Ribeiro N, Squiers JJ, et al. Pathologic confirmation of valve thrombosis detected by four-dimensional computed tomography following valve-in-valve transcatheter aortic valve replacement. Glob Cardiol Sci Prac 2017. In press.
- Kodali S, Thourani VH, White J, et al. Early clinical and echocardiographic outcomes after SAPIEN 3 transcatheter aortic valve replacement in inoperable, high-risk, and intermediate-risk patients with aortic stenosis. Eur Heart J 2016; 37:2252–2262.
KEY POINTS
- TAVR has become the preferred alternative to SAVR in inoperable and high-risk patients.
- The US Food and Drug Administration has approved TAVR with open-heart surgery.
- Initial outcomes support expanding TAVR to intermediate-risk patients, including mortality and stroke data, but concerns exist related to valve durability, valve thrombosis, and rates of permanent pacemaker implantation.