User login
To the Editor:
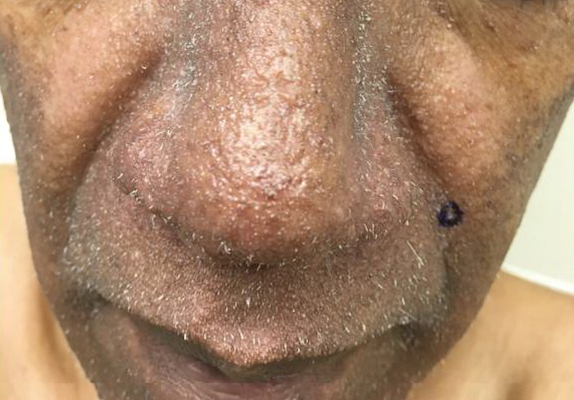
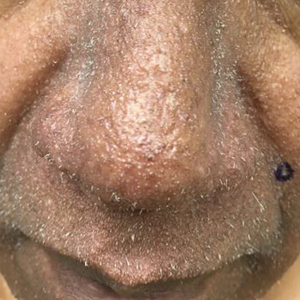
A 57-year-old man with hypertension, dyslipidemia, and congestive heart failure presented with a disfiguring eruption comprised of asymptomatic papules on the face that appeared 12 months post–heart transplantation. Immunosuppressive medications included mycophenolic acid and tacrolimus ointment (FK506). The pinpoint papules spread from the central face to the ears, arms, and legs. Physical examination revealed multiple 0.5- to 1-mm flesh-colored papules over the glabella, nose, nasolabial folds, philtrum, chin, ears, arms, and legs sparing the trunk. The initial appearance of the facial rash resembled the surface of a nutmeg grater with central white spiny excrescences overlying fine papules (spinulosism)(Figure 1). In addition, eyebrow alopecia was present.
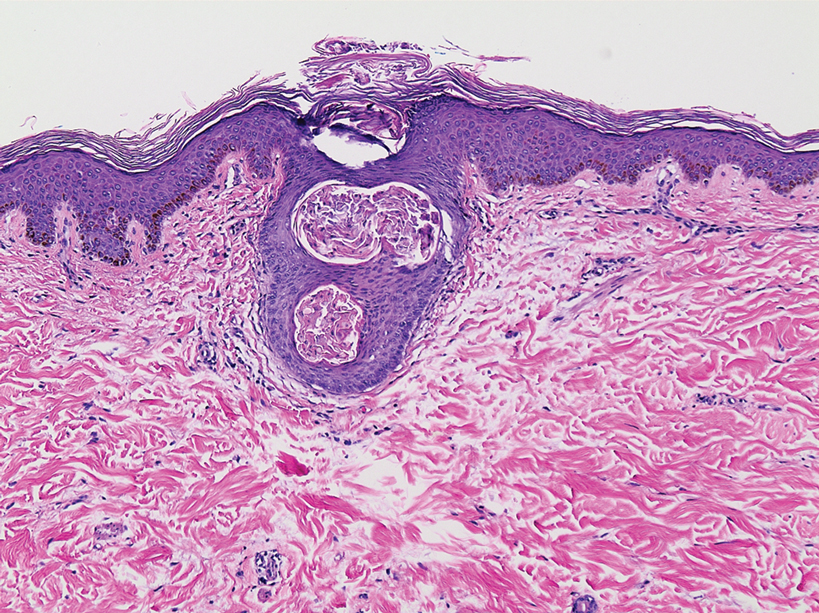
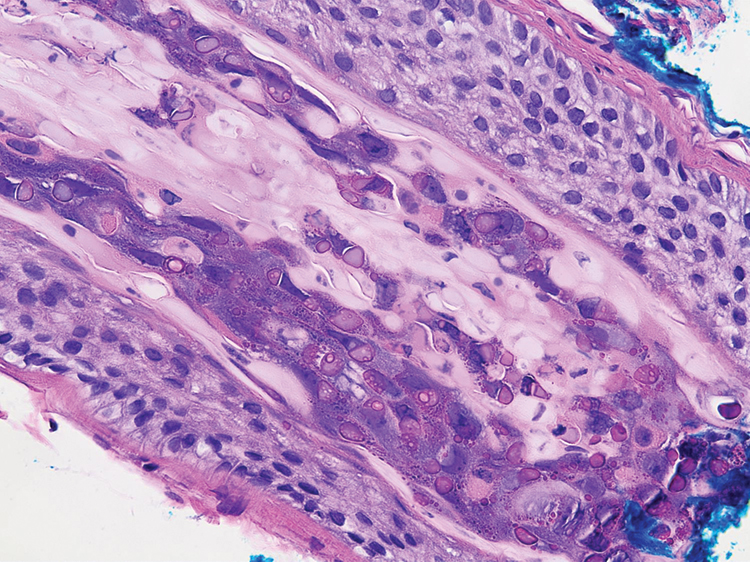
A 3-mm punch biopsy of a papule with a central spine was performed on the left thigh. Microscopic examination revealed marked dilatation of anagen hair follicles with a proliferation of haphazard inner root sheath cells replacing the follicular lumen. Hair shafts were absent, and plugged infundibula were observed (Figure 2). The inner root sheath keratinocytes were enlarged and dystrophic with deeply eosinophilic trichohyalin granules (Figure 3). The epidermis, outer root sheath epithelium, and eccrine structures were unremarkable.
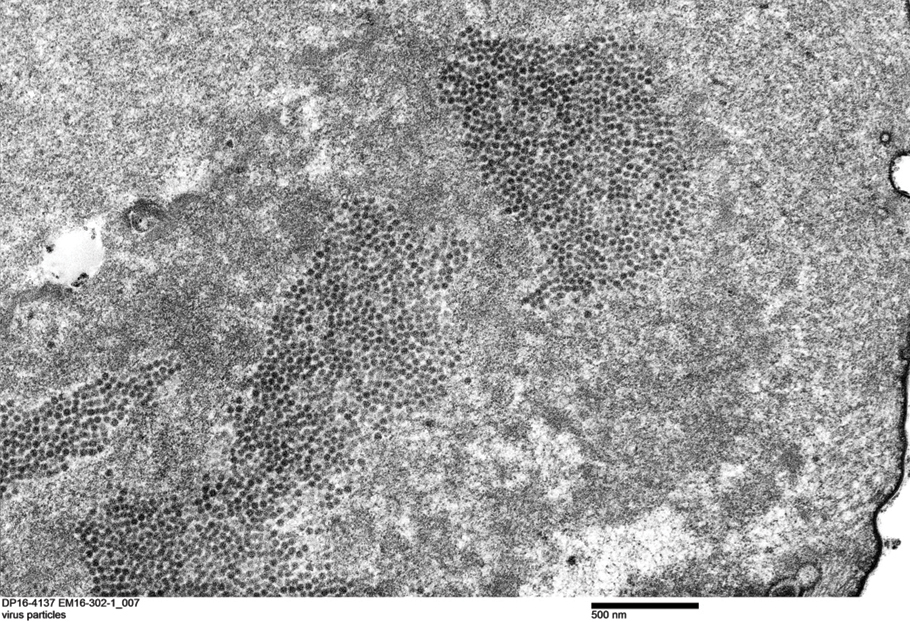
Transmission electron microscopy (TEM) confirmed the presence of intranuclear viral inclusions within affected inner root sheath keratinocytes composed of nonenveloped icosahedral viral particles measuring 33 to 38 nm in diameter (Figure 4). These findings morphologically were consistent with a polyomavirus. No intracytoplasmic or extracellular viral particles were identified. The clinical history, physical examination, histopathology, and electron microscopy features strongly supported the diagnosis of trichodysplasia spinulosa (TS) despite insufficient material being retrieved for polymerase chain reaction identification.
Trichodysplasia spinulosa was first described by Haycox et al1 in 1999. The authors suggested a viral etiology. Eleven years later, TS-associated polyomavirus (TSPyV) was identified by van der Meijden et al.2 Follicular keratinocytes are the specific target for TSPyV.3 Evidence has been presented suggesting that TS is caused by a primary infection or reactivation of TSPyV in the setting of immunosuppression.4,5
Patients with TS present with papular eruptions that appear on the central face with spiny excrescences and various degrees of alopecia involving the eyebrows or eyelashes. Histopathologic features include distended hair follicles with expansion of inner root sheath cells, eosinophilic trichohyalin granules, and the absence of hair shafts. The viral protein can be verified through immunohistochemistry TSPyV VP1 staining that demonstrates co-localization with trichohyalin. Viral particles also can be visualized as 35- to 38-nm intranuclear particles with an organized crystalloid morphology on TEM.6,7 The negative polymerase chain reaction in our patient could be the result of suboptimal template DNA concentration extracted from the limited amount of tissue remaining in the block after hematoxylin and eosin staining.
The clinical differential diagnosis of central facial spinulosism includes the follicular spicules of multiple myeloma (FSMM). In fact, FSMM and TS can only be differentiated after obtaining a blood profile and bone marrow biopsy that excludes the diagnosis of FSMM. A history of immunosuppression typically suggests TS. Histopathology often is equivocal in FSMM8; however, TEM reveals viral particles (TSPyV) in TS. Transmission electron microscopy in FSMM demonstrates fibrillary structures arranged in a paracrystalline configuration with unknown significance instead of viral particles. Despite the absence of viral particles on TEM, a low mean copy number of Merkel cell polyomavirus was isolated from a patient with FSMM who responded dramatically to treatment with topical cidofovir gel 1%.8 In addition to treating the underlying multiple myeloma in FSMM, topical cidofovir gel 1% also may have a role in treatment of these patients, suggesting a possible viral rather than simply paraneoplastic etiology of FSMM. Therefore, polyomavirus infection should be considered in the initial workup of any patient with fine facial follicular spicules.
The most effective management of TS in transplant recipients is to reduce immunosuppression to the lowest level possible without jeopardizing the transplanted organ.9 In our case, reduction of immunosuppressive drugs was not possible. In fact, immunosuppression in our patient was increased following evidence of early rejection of the heart transplant. Although manual extraction of the keratin spicules resulted in considerable improvement in a similar facial eruption in a patient with pediatric pre–B-cell acute lymphoblastic leukemia developing TS,10 it is impossible to apply this approach to patients such as ours who have thousands of tiny lesions. Fortunately, custom-compounded cidofovir gel 1% applied twice daily to the patient’s face and ears for 4 weeks led to near-complete clearance at follow-up (Figure 5). Due to the high cost of the medication (approaching $700 for one tube), our patient applied this medication to the face only several times weekly with excellent improvement. Thus, it appears that it is possible to suppress this virus with topical medication alone.
Polyomavirus infection should be considered in patients presenting with fine follicular spiny papules, especially those who are immunosuppressed. The possibility of coexisting multiple myeloma should be excluded.
Acknowledgment—We sincerely thank Glenn A. Hoskins (Jackson, Mississippi), the electron microscopy technologist, for the detection of viral particles and the electron microscope photographs.
- Haycox CL, Kim S, Fleckman P, et al. Trichodysplasia spinulosa: a newly described folliculocentric viral infection in an immunocompromised host. J Investig Dermatol Symp Proc. 1999;4:268-271.
- van der Meijden E, Janssens RWA, Lauber C, et al. Discovery of a new human polyomavirus associated with trichodysplasia spinulosa in an immunocompromized patient. PLoS Pathog. 2010;6:E1001024.
- Rouanet J, Aubin F, Gaboriaud P, et al. Trichodysplasia spinulosa: a polyomavirus infection specifically targeting follicular keratinocytes in immunocompromised patients. Br J Dermatol. 2016;174:629-632.
- van der Meijden E, Kazem S, Burgers MM, et al. Seroprevalence of trichodysplasia spinulosa-associated polyomavirus. Emerg Infect Dis. 2011;17:1355-1363.
- van der Meijden E, Horváth B, Nijland M, et al. Primary polyomavirus infection, not reactivation, as the cause of trichodysplasia spinulosa in immunocompromised patients. J Infect Dis. 2017;215:1080-1084.
- Fischer MK, Kao GF, Nguyen HP, et al. Specific detection of trichodysplasia spinulosa-associated polyomavirus DNA in skin and renal allograft tissues in a patient with trichodysplasia spinulosa. Arch Dermatol. 2012;148:726-733.
- Kazem S, van der Meijden E, Feltkamp MC. The trichodysplasia spinulosa-associated polyomavirus: virological background and clinical implications. APMIS. 2013;121:770-782.
- van Boheemen S, Jones T, Muhlemann B, et al. Cidofovir gel as treatment of follicular spicules in multiple myeloma. JAMA Dermatol. 2015;151:82-84.
- DeCrescenzo AJ, Philips RC, Wilkerson MG. Trichodysplasia spinulosa: a rare complication of immunosuppression. JAAD Case Rep. 2016;2:307-309.
- Barton M, Lockhart S, Sidbury R, et al. Trichodysplasia spinulosa in a 7-year-old boy managed using physical extraction of keratin spicules. Pediatr Dermatol. 2017;34:E74-E76.
To the Editor:
A 57-year-old man with hypertension, dyslipidemia, and congestive heart failure presented with a disfiguring eruption comprised of asymptomatic papules on the face that appeared 12 months post–heart transplantation. Immunosuppressive medications included mycophenolic acid and tacrolimus ointment (FK506). The pinpoint papules spread from the central face to the ears, arms, and legs. Physical examination revealed multiple 0.5- to 1-mm flesh-colored papules over the glabella, nose, nasolabial folds, philtrum, chin, ears, arms, and legs sparing the trunk. The initial appearance of the facial rash resembled the surface of a nutmeg grater with central white spiny excrescences overlying fine papules (spinulosism)(Figure 1). In addition, eyebrow alopecia was present.
A 3-mm punch biopsy of a papule with a central spine was performed on the left thigh. Microscopic examination revealed marked dilatation of anagen hair follicles with a proliferation of haphazard inner root sheath cells replacing the follicular lumen. Hair shafts were absent, and plugged infundibula were observed (Figure 2). The inner root sheath keratinocytes were enlarged and dystrophic with deeply eosinophilic trichohyalin granules (Figure 3). The epidermis, outer root sheath epithelium, and eccrine structures were unremarkable.
Transmission electron microscopy (TEM) confirmed the presence of intranuclear viral inclusions within affected inner root sheath keratinocytes composed of nonenveloped icosahedral viral particles measuring 33 to 38 nm in diameter (Figure 4). These findings morphologically were consistent with a polyomavirus. No intracytoplasmic or extracellular viral particles were identified. The clinical history, physical examination, histopathology, and electron microscopy features strongly supported the diagnosis of trichodysplasia spinulosa (TS) despite insufficient material being retrieved for polymerase chain reaction identification.
Trichodysplasia spinulosa was first described by Haycox et al1 in 1999. The authors suggested a viral etiology. Eleven years later, TS-associated polyomavirus (TSPyV) was identified by van der Meijden et al.2 Follicular keratinocytes are the specific target for TSPyV.3 Evidence has been presented suggesting that TS is caused by a primary infection or reactivation of TSPyV in the setting of immunosuppression.4,5
Patients with TS present with papular eruptions that appear on the central face with spiny excrescences and various degrees of alopecia involving the eyebrows or eyelashes. Histopathologic features include distended hair follicles with expansion of inner root sheath cells, eosinophilic trichohyalin granules, and the absence of hair shafts. The viral protein can be verified through immunohistochemistry TSPyV VP1 staining that demonstrates co-localization with trichohyalin. Viral particles also can be visualized as 35- to 38-nm intranuclear particles with an organized crystalloid morphology on TEM.6,7 The negative polymerase chain reaction in our patient could be the result of suboptimal template DNA concentration extracted from the limited amount of tissue remaining in the block after hematoxylin and eosin staining.
The clinical differential diagnosis of central facial spinulosism includes the follicular spicules of multiple myeloma (FSMM). In fact, FSMM and TS can only be differentiated after obtaining a blood profile and bone marrow biopsy that excludes the diagnosis of FSMM. A history of immunosuppression typically suggests TS. Histopathology often is equivocal in FSMM8; however, TEM reveals viral particles (TSPyV) in TS. Transmission electron microscopy in FSMM demonstrates fibrillary structures arranged in a paracrystalline configuration with unknown significance instead of viral particles. Despite the absence of viral particles on TEM, a low mean copy number of Merkel cell polyomavirus was isolated from a patient with FSMM who responded dramatically to treatment with topical cidofovir gel 1%.8 In addition to treating the underlying multiple myeloma in FSMM, topical cidofovir gel 1% also may have a role in treatment of these patients, suggesting a possible viral rather than simply paraneoplastic etiology of FSMM. Therefore, polyomavirus infection should be considered in the initial workup of any patient with fine facial follicular spicules.
The most effective management of TS in transplant recipients is to reduce immunosuppression to the lowest level possible without jeopardizing the transplanted organ.9 In our case, reduction of immunosuppressive drugs was not possible. In fact, immunosuppression in our patient was increased following evidence of early rejection of the heart transplant. Although manual extraction of the keratin spicules resulted in considerable improvement in a similar facial eruption in a patient with pediatric pre–B-cell acute lymphoblastic leukemia developing TS,10 it is impossible to apply this approach to patients such as ours who have thousands of tiny lesions. Fortunately, custom-compounded cidofovir gel 1% applied twice daily to the patient’s face and ears for 4 weeks led to near-complete clearance at follow-up (Figure 5). Due to the high cost of the medication (approaching $700 for one tube), our patient applied this medication to the face only several times weekly with excellent improvement. Thus, it appears that it is possible to suppress this virus with topical medication alone.
Polyomavirus infection should be considered in patients presenting with fine follicular spiny papules, especially those who are immunosuppressed. The possibility of coexisting multiple myeloma should be excluded.
Acknowledgment—We sincerely thank Glenn A. Hoskins (Jackson, Mississippi), the electron microscopy technologist, for the detection of viral particles and the electron microscope photographs.
To the Editor:
A 57-year-old man with hypertension, dyslipidemia, and congestive heart failure presented with a disfiguring eruption comprised of asymptomatic papules on the face that appeared 12 months post–heart transplantation. Immunosuppressive medications included mycophenolic acid and tacrolimus ointment (FK506). The pinpoint papules spread from the central face to the ears, arms, and legs. Physical examination revealed multiple 0.5- to 1-mm flesh-colored papules over the glabella, nose, nasolabial folds, philtrum, chin, ears, arms, and legs sparing the trunk. The initial appearance of the facial rash resembled the surface of a nutmeg grater with central white spiny excrescences overlying fine papules (spinulosism)(Figure 1). In addition, eyebrow alopecia was present.
A 3-mm punch biopsy of a papule with a central spine was performed on the left thigh. Microscopic examination revealed marked dilatation of anagen hair follicles with a proliferation of haphazard inner root sheath cells replacing the follicular lumen. Hair shafts were absent, and plugged infundibula were observed (Figure 2). The inner root sheath keratinocytes were enlarged and dystrophic with deeply eosinophilic trichohyalin granules (Figure 3). The epidermis, outer root sheath epithelium, and eccrine structures were unremarkable.
Transmission electron microscopy (TEM) confirmed the presence of intranuclear viral inclusions within affected inner root sheath keratinocytes composed of nonenveloped icosahedral viral particles measuring 33 to 38 nm in diameter (Figure 4). These findings morphologically were consistent with a polyomavirus. No intracytoplasmic or extracellular viral particles were identified. The clinical history, physical examination, histopathology, and electron microscopy features strongly supported the diagnosis of trichodysplasia spinulosa (TS) despite insufficient material being retrieved for polymerase chain reaction identification.
Trichodysplasia spinulosa was first described by Haycox et al1 in 1999. The authors suggested a viral etiology. Eleven years later, TS-associated polyomavirus (TSPyV) was identified by van der Meijden et al.2 Follicular keratinocytes are the specific target for TSPyV.3 Evidence has been presented suggesting that TS is caused by a primary infection or reactivation of TSPyV in the setting of immunosuppression.4,5
Patients with TS present with papular eruptions that appear on the central face with spiny excrescences and various degrees of alopecia involving the eyebrows or eyelashes. Histopathologic features include distended hair follicles with expansion of inner root sheath cells, eosinophilic trichohyalin granules, and the absence of hair shafts. The viral protein can be verified through immunohistochemistry TSPyV VP1 staining that demonstrates co-localization with trichohyalin. Viral particles also can be visualized as 35- to 38-nm intranuclear particles with an organized crystalloid morphology on TEM.6,7 The negative polymerase chain reaction in our patient could be the result of suboptimal template DNA concentration extracted from the limited amount of tissue remaining in the block after hematoxylin and eosin staining.
The clinical differential diagnosis of central facial spinulosism includes the follicular spicules of multiple myeloma (FSMM). In fact, FSMM and TS can only be differentiated after obtaining a blood profile and bone marrow biopsy that excludes the diagnosis of FSMM. A history of immunosuppression typically suggests TS. Histopathology often is equivocal in FSMM8; however, TEM reveals viral particles (TSPyV) in TS. Transmission electron microscopy in FSMM demonstrates fibrillary structures arranged in a paracrystalline configuration with unknown significance instead of viral particles. Despite the absence of viral particles on TEM, a low mean copy number of Merkel cell polyomavirus was isolated from a patient with FSMM who responded dramatically to treatment with topical cidofovir gel 1%.8 In addition to treating the underlying multiple myeloma in FSMM, topical cidofovir gel 1% also may have a role in treatment of these patients, suggesting a possible viral rather than simply paraneoplastic etiology of FSMM. Therefore, polyomavirus infection should be considered in the initial workup of any patient with fine facial follicular spicules.
The most effective management of TS in transplant recipients is to reduce immunosuppression to the lowest level possible without jeopardizing the transplanted organ.9 In our case, reduction of immunosuppressive drugs was not possible. In fact, immunosuppression in our patient was increased following evidence of early rejection of the heart transplant. Although manual extraction of the keratin spicules resulted in considerable improvement in a similar facial eruption in a patient with pediatric pre–B-cell acute lymphoblastic leukemia developing TS,10 it is impossible to apply this approach to patients such as ours who have thousands of tiny lesions. Fortunately, custom-compounded cidofovir gel 1% applied twice daily to the patient’s face and ears for 4 weeks led to near-complete clearance at follow-up (Figure 5). Due to the high cost of the medication (approaching $700 for one tube), our patient applied this medication to the face only several times weekly with excellent improvement. Thus, it appears that it is possible to suppress this virus with topical medication alone.
Polyomavirus infection should be considered in patients presenting with fine follicular spiny papules, especially those who are immunosuppressed. The possibility of coexisting multiple myeloma should be excluded.
Acknowledgment—We sincerely thank Glenn A. Hoskins (Jackson, Mississippi), the electron microscopy technologist, for the detection of viral particles and the electron microscope photographs.
- Haycox CL, Kim S, Fleckman P, et al. Trichodysplasia spinulosa: a newly described folliculocentric viral infection in an immunocompromised host. J Investig Dermatol Symp Proc. 1999;4:268-271.
- van der Meijden E, Janssens RWA, Lauber C, et al. Discovery of a new human polyomavirus associated with trichodysplasia spinulosa in an immunocompromized patient. PLoS Pathog. 2010;6:E1001024.
- Rouanet J, Aubin F, Gaboriaud P, et al. Trichodysplasia spinulosa: a polyomavirus infection specifically targeting follicular keratinocytes in immunocompromised patients. Br J Dermatol. 2016;174:629-632.
- van der Meijden E, Kazem S, Burgers MM, et al. Seroprevalence of trichodysplasia spinulosa-associated polyomavirus. Emerg Infect Dis. 2011;17:1355-1363.
- van der Meijden E, Horváth B, Nijland M, et al. Primary polyomavirus infection, not reactivation, as the cause of trichodysplasia spinulosa in immunocompromised patients. J Infect Dis. 2017;215:1080-1084.
- Fischer MK, Kao GF, Nguyen HP, et al. Specific detection of trichodysplasia spinulosa-associated polyomavirus DNA in skin and renal allograft tissues in a patient with trichodysplasia spinulosa. Arch Dermatol. 2012;148:726-733.
- Kazem S, van der Meijden E, Feltkamp MC. The trichodysplasia spinulosa-associated polyomavirus: virological background and clinical implications. APMIS. 2013;121:770-782.
- van Boheemen S, Jones T, Muhlemann B, et al. Cidofovir gel as treatment of follicular spicules in multiple myeloma. JAMA Dermatol. 2015;151:82-84.
- DeCrescenzo AJ, Philips RC, Wilkerson MG. Trichodysplasia spinulosa: a rare complication of immunosuppression. JAAD Case Rep. 2016;2:307-309.
- Barton M, Lockhart S, Sidbury R, et al. Trichodysplasia spinulosa in a 7-year-old boy managed using physical extraction of keratin spicules. Pediatr Dermatol. 2017;34:E74-E76.
- Haycox CL, Kim S, Fleckman P, et al. Trichodysplasia spinulosa: a newly described folliculocentric viral infection in an immunocompromised host. J Investig Dermatol Symp Proc. 1999;4:268-271.
- van der Meijden E, Janssens RWA, Lauber C, et al. Discovery of a new human polyomavirus associated with trichodysplasia spinulosa in an immunocompromized patient. PLoS Pathog. 2010;6:E1001024.
- Rouanet J, Aubin F, Gaboriaud P, et al. Trichodysplasia spinulosa: a polyomavirus infection specifically targeting follicular keratinocytes in immunocompromised patients. Br J Dermatol. 2016;174:629-632.
- van der Meijden E, Kazem S, Burgers MM, et al. Seroprevalence of trichodysplasia spinulosa-associated polyomavirus. Emerg Infect Dis. 2011;17:1355-1363.
- van der Meijden E, Horváth B, Nijland M, et al. Primary polyomavirus infection, not reactivation, as the cause of trichodysplasia spinulosa in immunocompromised patients. J Infect Dis. 2017;215:1080-1084.
- Fischer MK, Kao GF, Nguyen HP, et al. Specific detection of trichodysplasia spinulosa-associated polyomavirus DNA in skin and renal allograft tissues in a patient with trichodysplasia spinulosa. Arch Dermatol. 2012;148:726-733.
- Kazem S, van der Meijden E, Feltkamp MC. The trichodysplasia spinulosa-associated polyomavirus: virological background and clinical implications. APMIS. 2013;121:770-782.
- van Boheemen S, Jones T, Muhlemann B, et al. Cidofovir gel as treatment of follicular spicules in multiple myeloma. JAMA Dermatol. 2015;151:82-84.
- DeCrescenzo AJ, Philips RC, Wilkerson MG. Trichodysplasia spinulosa: a rare complication of immunosuppression. JAAD Case Rep. 2016;2:307-309.
- Barton M, Lockhart S, Sidbury R, et al. Trichodysplasia spinulosa in a 7-year-old boy managed using physical extraction of keratin spicules. Pediatr Dermatol. 2017;34:E74-E76.
Practice Points
- Trichodysplasia spinulosa (TS) is a rare skin disease caused by primary TS-associated polyomavirus (TSPyV) infecting follicular keratinocytes in immunocompromised patients.
- Trichodysplasia spinulosa typically presents with papular eruptions that appear on the central face with spiny excrescences and various degrees of alopecia involving the eyebrows or eyelashes.
- The viral protein can be verified through immunohistochemistry TSPyV major capsid protein VP1 staining or can be visualized on transmission electron microscopy.
- Follicular spicules of multiple myeloma should be ruled out before initiating treatment with cidofovir gel 1% for TS.