User login
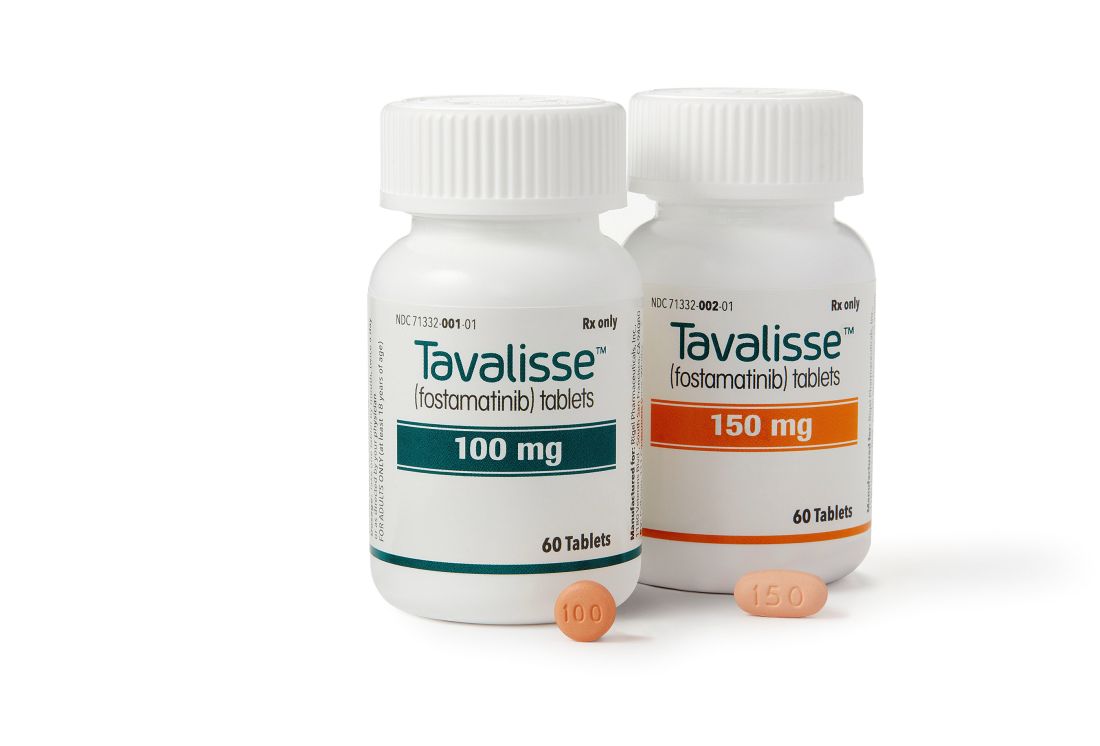
with chronic immune thrombocytopenia (ITP).
The Food and Drug Administration approved the oral spleen tyrosine kinase (SYK) inhibitor, which works by impeding platelet destruction, on April 17. Its approval was based on data from FIT clinical program, including two randomized placebo-controlled phase 3 trials.
Physicians are advised to monitor blood pressure and liver function with the drug. Fostamatinib may interact with CYP3A4 inhibitors and inducers. Concomitant use of fostamatinib with CYP3A4 inhibitors increases exposure to R406 – the drug’s major metabolite – and may increase the risk of adverse reactions. Use with strong CYP3A4 inducers is not recommended because it reduces exposure to R406.
Women of reproductive potential should be advised to use appropriate contraception while using fostamatinib and for a month after stopping the drug; pregnant women should be told of potential risk to the fetus. Advise women not to breastfeed during treatment and for at least 1 month after the last dose, according to a press statement.
Rigel Pharmaceuticals plans to release the drug in the United States in late May 2018.
with chronic immune thrombocytopenia (ITP).
The Food and Drug Administration approved the oral spleen tyrosine kinase (SYK) inhibitor, which works by impeding platelet destruction, on April 17. Its approval was based on data from FIT clinical program, including two randomized placebo-controlled phase 3 trials.
Physicians are advised to monitor blood pressure and liver function with the drug. Fostamatinib may interact with CYP3A4 inhibitors and inducers. Concomitant use of fostamatinib with CYP3A4 inhibitors increases exposure to R406 – the drug’s major metabolite – and may increase the risk of adverse reactions. Use with strong CYP3A4 inducers is not recommended because it reduces exposure to R406.
Women of reproductive potential should be advised to use appropriate contraception while using fostamatinib and for a month after stopping the drug; pregnant women should be told of potential risk to the fetus. Advise women not to breastfeed during treatment and for at least 1 month after the last dose, according to a press statement.
Rigel Pharmaceuticals plans to release the drug in the United States in late May 2018.
with chronic immune thrombocytopenia (ITP).
The Food and Drug Administration approved the oral spleen tyrosine kinase (SYK) inhibitor, which works by impeding platelet destruction, on April 17. Its approval was based on data from FIT clinical program, including two randomized placebo-controlled phase 3 trials.
Physicians are advised to monitor blood pressure and liver function with the drug. Fostamatinib may interact with CYP3A4 inhibitors and inducers. Concomitant use of fostamatinib with CYP3A4 inhibitors increases exposure to R406 – the drug’s major metabolite – and may increase the risk of adverse reactions. Use with strong CYP3A4 inducers is not recommended because it reduces exposure to R406.
Women of reproductive potential should be advised to use appropriate contraception while using fostamatinib and for a month after stopping the drug; pregnant women should be told of potential risk to the fetus. Advise women not to breastfeed during treatment and for at least 1 month after the last dose, according to a press statement.
Rigel Pharmaceuticals plans to release the drug in the United States in late May 2018.