User login
A 42‐year‐old woman with a history of mild asthma presented to the emergency department (ED) following 1 week of headache. She had been in her usual state of good health until 1 week prior to her presentation, when she noticed intermittent frontal headaches without neck stiffness or other neurologic symptoms. She then developed diffuse myalgias, fatigue, subjective fevers, and rigors for the 24 hours prior to presentation. On the morning of presentation, chest tightness, palpitations, and shortness of breath occurred. She used her albuterol metered‐dose inhaler without relief and went to the hospital.
Many of these features can be explained by a viral syndrome exacerbating underlying asthma or by a psychiatric condition such as anxiety or depression, but they may also be a harbinger of a systemic process, including infection, malignancy, or autoimmunity. Because the onset of headache is temporally distant from the other symptoms, I am more inclined to believe that it represents a primary intracranial process than I would if it were coincident with the onset of the other acute symptoms. If the fevers and rigors are verified, infection would be the initial concern. Failure to respond to her inhalers may either signify a severe asthma exacerbation or a nonbronchospastic cause of dyspnea.
She reported mild nausea, but denied photophobia, vomiting, abdominal pain, diarrhea, melena, or hematochezia. She did not have recent ill contacts, animal bites, or travel. Her medical history included asthma, diverticulitis, chronic right ankle pain, and obesity. She reported an allergic rash to amoxicillin. Her medications were sulindac and fluticasone/salmeterol, and albuterol metered‐dose inhalers. She worked as a preschool teacher and was married with 2 children. She denied any tobacco use and seldom drank alcoholic beverages. On exam, temperature was 36.7C, pulse was 107 beats per minute, blood pressure was 129/91 mm Hg, respiratory rate was 19 breaths per minute, and oxygen saturation was 98% while breathing ambient air. Her face and anterior neck were flushed and diaphoretic, and her sclerae were icteric. There was no nuchal rigidity. Her cardiac rhythm was regular without murmurs, lungs were clear to auscultation, and the abdomen was mildly tender to palpation in the epigastrium and right upper quadrant. The white blood cell (WBC) count was 9200/L, with 84% neutrophils, 3% lymphocytes, 6% monocytes, and 7% eosinophils. The hemoglobin was 14.8 g/dL and the platelet count was 166,000/L. Total serum bilirubin was 4.6 mg/dL, aspartate aminotransferase (AST) was 459 U/L (normal range, 8‐31), alanine aminotransferase (ALT) was 667 U/L (normal range, 7‐31), and alkaline phosphatase was 146 U/L (normal range, 39‐117). Serum electrolytes, creatinine, lactate, lipase, thyrotropin, coagulation studies, and cardiac enzymes were all normal. Urinalysis showed trace leukocyte esterase and bilirubin, as well as 3 WBCs and 2 red cells per high‐power field. Chest radiography and an electrocardiogram demonstrated no abnormalities.
The major findingwhich is critical to focusing problem‐solving in the face of a broad range of symptomsis her hepatitis. The common etiologies for hepatitis of this degree include viruses (hepatitis A and cytomegalovirus [CMV] should be considered given her work in preschool), toxins, autoimmunity, and vascular events. Liver disease in association with flushing raises the possibility of carcinoid syndrome with liver metastases. The lack of wheezing makes the bronchospasm of asthma or carcinoid less suitable explanations for her shortness of breath. Her eosinophilia is mild but probably is not accounted for alone by well‐controlled asthma in a person with no history of atopic disease. I would also ask her about any alternative and over‐the‐counter remedies. The paucity of lymphocytes raises the possibility of human immunodeficiency virus (HIV), Hodgkin's disease, or systemic lupus erythematosus. Although she does not have a documented fever or leukocytosis, she reported fevers and chills and is diaphoretic and tachycardic, so exclusion of biliary obstruction and cholangitis is the highest priority.
An abdominal ultrasound demonstrated hepatomegaly with moderate fatty infiltration and a normal gallbladder without pericholecystic fluid. The intrahepatic and extrahepatic biliary ducts were normal and the hepatic and portal veins were patent. Computed tomography of the abdomen showed slight thickening of the sigmoid colon wall. Ciprofloxacin and metronidazole were administered for possible diverticulitis. Over the first 48 hours of hospitalization her symptoms improved markedly. Her flushing resolved and she had no recorded fevers in the hospital. Serologies were negative for hepatitis A immunoglobulin M (IgM), hepatitis B surface antibody, hepatitis B surface antigen, and hepatitis C antibody. A monospot test was negative and the erythrocyte sedimentation rate was 11 mm/hour. Blood and urine cultures were negative. On the second hospital day the absolute eosinophil count rose to 855/L (15% of 5700 WBCs). On the fourth hospital day, the absolute eosinophil count was 1092/L, the total bilirubin was 1.9 mg/dL, and the AST and ALT were 174 U/L and 476 U/L, respectively. Antibiotics were stopped and she was discharged home.
Her prompt improvement suggests either a self‐limited condition or a response to the antibiotics. The rapid but incomplete resolution of her hepatitis is in keeping with a withdrawal of a toxin, relief of biliary obstruction, or a transient vascular event, and is less consistent with a viral hepatitis or an infiltrative process. With normal biliary system imaging, sterile blood cultures, and the absence of fever or leukocytosis, cholangitis is unlikely. Likewise, there is no suggestion of a vascular event, either obstructive or hemodynamic, that is impairing the liver.
A common cause of eosinophilia in hospitalized patients is medications, so it would be useful to monitor that count after the new antibiotics. At this point, I also wonder if the eosinophils are a feature of the underlying illness, as they were present to a modest degree on admission before any new medications were administered. The overlap of eosinophilia and hepatitis brings to mind a medication reaction (eg, to sulindac) or a hepatobiliary parasite, such as ascaris or clonorchis, for which she lacks a known exposure. Many patients experience flushing in the setting of fever or stress, but sustained flushing may suggest a systemic illness characterized by the release of vasoactive mediators such as carcinoid syndrome or mastocytosis. The latter might be considered more strongly if the eosinophilia is deemed to be primary (rather than reactive) after a thorough evaluation.
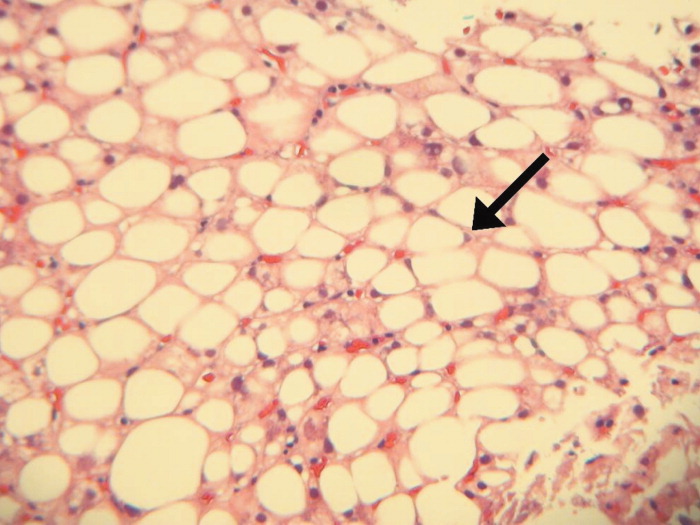
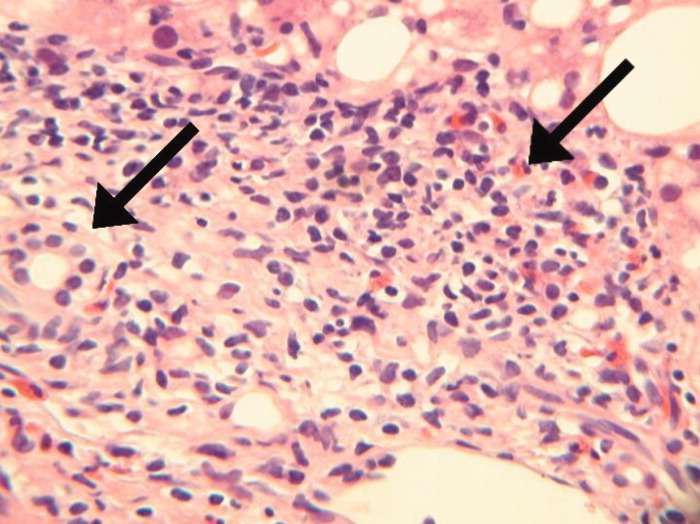
After 2 days at home, the patient had recurrence of subjective fevers, with chest, back, and abdominal pain, fatigue, loose stools, and rigors. She returned to the ED, where she was noted to have facial erythema and injected sclerae, but the remainder of her physical exam was normal. The total serum bilirubin was 1.1 mg/dL, AST was 156 U/L, ALT was 214 U/L, and alkaline phosphatase was 240 U/L. Serum lipase was normal. WBC count was 14,000/L, with 94% neutrophils, 3% lymphocytes, 2% monocytes, and 1% eosinophils. She was again treated empirically with ciprofloxacin and metronidazole. Endoscopic ultrasound was normal, with no evidence of gallbladder sludge or microlithiasis. Stool cultures, assay for Clostridium difficile, and examination for ova and parasites were negative. The 24‐hour urine demonstrated no elevation in 5‐hydroxyindoleacetic acid. An adrenocorticotropic hormone (ACTH) stimulation test was normal. HIV antibody was negative. Her symptoms improved within 2 days. The eosinophil count rose and peaked at 1541/L by the third hospital day, while the transaminase elevations resolved. Antibiotics were discontinued. A liver biopsy showed mixed macrovesicular and microvesicular fatty metamorphosis and steatohepatitis with eosinophils (Figures 1 and 2). She was discharged home on the sixth hospital day.

Her illness can now be characterized as relapsing inflammation, which given the frequency (over days) suggests either an indolent infectious focus that periodically causes systemic inflammation or reexposure to a toxic substance. The 2 most notable laboratory abnormalities, the hepatitis and the eosinophilia, persist but have differing trajectories. While the liver function tests have progressively normalized despite clinical relapses, the eosinophils have had a more fluctuating course characterized by increases during the hospitalization and higher levels during the second hospitalization. The absence of an infection, recurrent systemic inflammation, and eosinophilic hepatitis suggest a hypersensitivity reaction to a medication or other substance. She is most likely being reexposed at home, where her symptoms occur, and not in the hospital, where her symptoms resolve. Sulindac is a leading candidate, because nonsteroidal antiinflammatory drugs (NSAIDs) cause a number of hypersensitivity reactions and are frequently stopped when sick patients enter the hospital.
Seven days after discharge she developed acute onset of subjective fever, nausea, diffuse myalgias, and flushing, identical to the 2 prior episodes, and she again returned to the ED. Her temperature was 39.1C, heart rate was 120 beats per minute, blood pressure was 87/50 mm Hg, respiratory rate was 18 breaths per minute, and the oxygen saturation was 96% while breathing room air. She had diffuse flushing from her neck over her torso and was diaphoretic with injected sclera and conjunctiva. The WBC count was 11,400/L with 97% neutrophils and 3% lymphocytes. Total bilirubin was 0.8 mg/dL, AST was 134 U/L, ALT was 140 U/L, and alkaline phosphatase was 144 U/L. She was readmitted to the hospital. Following admission, she had no fevers, the flushing resolved, and AST and ALT levels decreased. The only treatment the patient received in the ED and during her hospital stay was acetaminophen as needed for pain or fever. The eosinophil count peaked at 1404/L by hospital day 4. Blood and urine cultures were negative. IgM antibodies to Epstein‐Barr virus were not detected, CMV DNA was not detected, and a rapid plasma reagin (RPR) test was nonreactive. Ferritin, ceruloplasmin, alpha‐1‐antitrypsin, and tryptase levels were normal. Antimitochondrial, antismooth muscle, antineutrophil cytoplasmic, and antinuclear antibodies were negative. There was no monoclonal band on serum protein electrophoresis. A blood smear for Borrelia detected no spirochetes.
A complete picture of the uncommon but classic flushing disorders, namely carcinoid, mastocytosis, and pheochromocytoma, has not emerged. The constellation of inflammation, mucosal and hepatic involvement, and eosinophilia are most consistent with a drug hypersensitivity reaction. Additionally, the recurrent inflammation is becoming more severe, as manifest by the fever and hemodynamic derangements, which suggests an increasing sensitization to the offending agent. I would review every drug she has received in the hospital, but given the recurrences after discharge her home medications are the most likely explanation. Of these, sulindac is the most likely culprit.
On further questioning, it was learned that the patient began taking sulindac 200 mg twice daily to treat her chronic ankle pain 6 weeks before the first admission. The medication had been stopped on each admission. She was instructed to discontinue sulindac. She has had no recurrences of symptoms and her hepatitis and eosinophilia have resolved.
DISCUSSION
This patient presented with recurrent skin findings, eosinophilia, hepatitis, and constitutional symptoms caused by hypersensitivity to sulindac. This drug‐induced hypersensitivity syndrome was originally described with anticonvulsant drugs (carbamazepine, phenytoin, and phenobarbitone) and named anticonvulsant hypersensitivity syndrome,1, 2 but has been observed with many other medications, including allopurinol, dapsone, minocycline, and nevirapine. The term drug rash with eosinophilia and systemic symptoms (DRESS) syndrome has been recently adopted to convey the cardinal features that characterize this disorder.3
DRESS syndrome is defined by rash, fever, and internal organ involvement.4 Also included in the diagnostic criteria are hematologic abnormalities (eosinophilia 1.5 109/mm3 or the presence of atypical lymphocytes) and lymphadenopathy.3 The multiorgan involvement distinguishes DRESS from other cutaneous drug eruptions. In a review of the French Pharmacovigilance Database for all cases of DRESS over a 15‐year period, 73% to 100% of patients were reported to have dermatologic abnormalities, most frequently a maculopapular rash or erythroderma. Less common skin findings include vesicles, bullae, pustules, erythroderma, and purpuric lesions. Liver abnormalities were observed in more than 60% of patients and were the most frequent systemic finding.5 Eosinophilia was the most common hematologic abnormality, present in more than 50% of cases. As this case underscores, DRESS syndrome typically begins 3 to 8 weeks after initiation of the drug because it is a delayed type IV hypersensitivity reaction.6 Fever can occur within hours on rechallenge because of the presence of memory T cells.
The main treatment for DRESS syndrome is withdrawal of the offending drug. Systemic corticosteroids have been recommended in cases with life‐threatening pulmonary or cardiac involvement, but have not been shown to be helpful in reversing renal or hepatic disease. Mortality, usually from end‐organ damage, occurs in about 10% of cases. The most common drugs are phenobarbital, carbamazepine, and phenytoin, with incidences of 1 in 5000 to 1 in 10,000. NSAIDs and antibiotics also have been implicated frequently. Human herpesvirus type 6 coinfection and genetically inherited slow acetylation have been associated with DRESS, although causal links have yet to be established.7, 8
The initial challenge in caring for a patient with multiple symptoms, exam findings, and test abnormalities is the coherent framing of the key clinical features that require explanation. This process, called problem representation, allows clinicians to search among a bounded list of possible diagnoses (or solutions) rather than invoking a differential diagnosis for every single abnormality. In searching for the proper diagnosis, this patient's clinical course required frequent reframing as more data became available.
Initially, the problem was framed as a 42‐year‐old woman with hepatitis. As the flushing and eosinophilia, which initially appeared to be transient and possibly nonspecific, became more prominent, the problem representation was revised to a 42‐year‐old woman with hepatitis, eosinophilia, and flushing. Since this triad did not immediately invoke a single diagnosis for the treating clinicians or the discussant, the differential diagnosis of hepatitis and eosinophilia and the differential diagnosis of flushing were considered in parallel.
Hepatitis and eosinophilia can occur coincidentally in the setting of parasitic infections, particularly helminths (ascaris, strongyloidiasis, and toxocaris) and liver flukes (opisthorchis and clonorchis), which invade the hepatobiliary system and induce a reactive eosinophilia. Some neoplasms, such as lymphomas and leukemias, and myeloproliferative disorders, including hypereosinophilic syndrome and mastocytosis, may have neoplastic cellular invasion of the liver and induce eosinophilia. Systemic drug hypersensitivity reactions are typically characterized by eosinophilia, transaminase elevations,9 and hepatitis on histology.10 As with this patient, liver biopsy in drug‐induced hepatitis shows a mixed microvesicular and macrovesicular steatosis, often with eosinophils.10
The flushing prompted a thorough but negative workup for the classic flushing disorders. The discussant's attempts to unify the flushing with the other clinical features illustrate how framing can affect our reasoning. Hepatitis, eosinophilia, and flushing defied an obvious single explanation and led the discussant down parallel diagnostic reasoning pathways. Although flushing is 1 of the well‐described dermatologic manifestations in drug‐hypersensitivity reactions, framing the central features as hepatitis, eosinophilia, and rash would have more readily suggested a drug reaction as a unifying diagnosis.11
The tempo and periodicity of this patient's illness provided the final formulation of a 42‐year‐old woman with hepatitis, eosinophilia, and flushing (or rash) that occurs every few days at home and resolves in the hospital. This formulation and the increasingly severe presentation, suggesting sensitization, were highly suggestive of an exogenous cause of her illness.
This case highlights how easily medication side effects can be overlooked during an extensive evaluation and how vigilant medication reconciliation coupled with an increased understanding of the spectrum of drug reactions can lead to early detection and prevention of potentially serious effects. In the case of DRESS, recognizing an association between a rash and organ involvement is central to making the diagnosis. Eosinophilia that accompanies a rash can further aid in narrowing the differential diagnosis.
The case also serves as a reminder of how framing with the slightest imprecision (eg, flushing instead of rash) can derail or delay the diagnostic process, yet is indispensable in tackling a complicated case. Finally, a time‐honored lesson in diagnosis is highlighted yet again: the diagnosis can usually be flushed out from the history.1214
Key Teaching Points
-
The constellation of skin findings, eosinophilia, organ involvement (particularly hepatitis), and constitutional symptoms should prompt consideration of DRESS syndrome and a hunt for a culprit drug.
-
Symptoms that resolve during hospitalization and repeatedly recur after discharge should prompt consideration of an exposure unique to the home, which may be environmental or pharmacologic.
-
Problem representation is critical in solving a complicated case, but adopting an inaccurate frame (representation) can derail or delay the diagnostic process.
The approach to clinical conundrums by an expert clinician is revealed through the presentation of an actual patient's case in an approach typical of a morning report. Similarly to patient care, sequential pieces of information are provided to the clinician, who is unfamiliar with the case. The focus is on the thought processes of both the clinical team caring for the patient and the discussant.
- ,.Anticonvulsant hypersensitivity syndrome. In vitro assessment of risk.J Clin Invest.1988;82:1826–1832.
- ,,.Anticonvulsant hypersensitivity syndrome: incidence, prevention, and management.Drug Saf.1999;21:489–501.
- ,,,.Drug rash with eosinophilia and systemic symptoms vs toxic epidermal necrolysis: the dilemma of classification.Clin Dermatol.2005;23:311–314.
- ,.Management of drug rash with eosinophilia and systemic symptoms (DRESS syndrome): an update.Dermatology.2003;206:353–356.
- ,,,,,Blayac J‐P; Network of the French Pharmacovigilance Centers. Variability in the clinical pattern of cutaneous side‐effects of drugs with systemic symptoms: does a DRESS syndrome really exist?Br J Dermatol.2006;155:422–428.
- ,.The immunological and clinical spectrum of delayed drug‐induced exanthems.Curr Opin Allergy Clin Immunol.2004;4:411–419.
- ,,.Association between anticonvulsant hypersensitivity syndrome and human herpesvirus 6 reactivation and hypogammaglobulinemia.Arch Dermatol.2004;140:183–188.
- ,.Treatment of severe drug reactions: Stevens‐Johnson Syndrome, toxic epidermal necrolysis and hypersensitivity syndrome.Dermatol Online J.2002;8:5.
- ,.Drug reactions. In: Bolognia JL, Jorizzo JL, Rapini RP, eds.Dermatology.2nd ed.Spain:Mosby Elsevier;2008:310–311.
- Pathology Outlines. Liver and intrahepatic bile ducts—non tumor. Hepatitis (non‐infectious). Drug/toxin‐induced hepatitis. Available at: http://www.pathologyoutlines.com/liver.html#drugtoxin. Accessed August2009.
- ,,.The flushing patient: differential diagnosis, workup and treatment.J Am Acad Dermatol.2006;55(2):193–208.
- ,,,,.Relative contributions of history‐taking, physical examination, and laboratory investigation to diagnosis and management of medical outpatients.Br Med J.1975;2(5969):486–489.
- ,,,,.Contributions to the history, physical examination, and laboratory investigation in making medical diagnosis.West J Med.1992;156(2):163–165.
- ,.A study on relative contributions of the history, physical examination and investigations in making medical diagnosis.J Assoc Physicians India.2000;48(8):771–775.
A 42‐year‐old woman with a history of mild asthma presented to the emergency department (ED) following 1 week of headache. She had been in her usual state of good health until 1 week prior to her presentation, when she noticed intermittent frontal headaches without neck stiffness or other neurologic symptoms. She then developed diffuse myalgias, fatigue, subjective fevers, and rigors for the 24 hours prior to presentation. On the morning of presentation, chest tightness, palpitations, and shortness of breath occurred. She used her albuterol metered‐dose inhaler without relief and went to the hospital.
Many of these features can be explained by a viral syndrome exacerbating underlying asthma or by a psychiatric condition such as anxiety or depression, but they may also be a harbinger of a systemic process, including infection, malignancy, or autoimmunity. Because the onset of headache is temporally distant from the other symptoms, I am more inclined to believe that it represents a primary intracranial process than I would if it were coincident with the onset of the other acute symptoms. If the fevers and rigors are verified, infection would be the initial concern. Failure to respond to her inhalers may either signify a severe asthma exacerbation or a nonbronchospastic cause of dyspnea.
She reported mild nausea, but denied photophobia, vomiting, abdominal pain, diarrhea, melena, or hematochezia. She did not have recent ill contacts, animal bites, or travel. Her medical history included asthma, diverticulitis, chronic right ankle pain, and obesity. She reported an allergic rash to amoxicillin. Her medications were sulindac and fluticasone/salmeterol, and albuterol metered‐dose inhalers. She worked as a preschool teacher and was married with 2 children. She denied any tobacco use and seldom drank alcoholic beverages. On exam, temperature was 36.7C, pulse was 107 beats per minute, blood pressure was 129/91 mm Hg, respiratory rate was 19 breaths per minute, and oxygen saturation was 98% while breathing ambient air. Her face and anterior neck were flushed and diaphoretic, and her sclerae were icteric. There was no nuchal rigidity. Her cardiac rhythm was regular without murmurs, lungs were clear to auscultation, and the abdomen was mildly tender to palpation in the epigastrium and right upper quadrant. The white blood cell (WBC) count was 9200/L, with 84% neutrophils, 3% lymphocytes, 6% monocytes, and 7% eosinophils. The hemoglobin was 14.8 g/dL and the platelet count was 166,000/L. Total serum bilirubin was 4.6 mg/dL, aspartate aminotransferase (AST) was 459 U/L (normal range, 8‐31), alanine aminotransferase (ALT) was 667 U/L (normal range, 7‐31), and alkaline phosphatase was 146 U/L (normal range, 39‐117). Serum electrolytes, creatinine, lactate, lipase, thyrotropin, coagulation studies, and cardiac enzymes were all normal. Urinalysis showed trace leukocyte esterase and bilirubin, as well as 3 WBCs and 2 red cells per high‐power field. Chest radiography and an electrocardiogram demonstrated no abnormalities.
The major findingwhich is critical to focusing problem‐solving in the face of a broad range of symptomsis her hepatitis. The common etiologies for hepatitis of this degree include viruses (hepatitis A and cytomegalovirus [CMV] should be considered given her work in preschool), toxins, autoimmunity, and vascular events. Liver disease in association with flushing raises the possibility of carcinoid syndrome with liver metastases. The lack of wheezing makes the bronchospasm of asthma or carcinoid less suitable explanations for her shortness of breath. Her eosinophilia is mild but probably is not accounted for alone by well‐controlled asthma in a person with no history of atopic disease. I would also ask her about any alternative and over‐the‐counter remedies. The paucity of lymphocytes raises the possibility of human immunodeficiency virus (HIV), Hodgkin's disease, or systemic lupus erythematosus. Although she does not have a documented fever or leukocytosis, she reported fevers and chills and is diaphoretic and tachycardic, so exclusion of biliary obstruction and cholangitis is the highest priority.
An abdominal ultrasound demonstrated hepatomegaly with moderate fatty infiltration and a normal gallbladder without pericholecystic fluid. The intrahepatic and extrahepatic biliary ducts were normal and the hepatic and portal veins were patent. Computed tomography of the abdomen showed slight thickening of the sigmoid colon wall. Ciprofloxacin and metronidazole were administered for possible diverticulitis. Over the first 48 hours of hospitalization her symptoms improved markedly. Her flushing resolved and she had no recorded fevers in the hospital. Serologies were negative for hepatitis A immunoglobulin M (IgM), hepatitis B surface antibody, hepatitis B surface antigen, and hepatitis C antibody. A monospot test was negative and the erythrocyte sedimentation rate was 11 mm/hour. Blood and urine cultures were negative. On the second hospital day the absolute eosinophil count rose to 855/L (15% of 5700 WBCs). On the fourth hospital day, the absolute eosinophil count was 1092/L, the total bilirubin was 1.9 mg/dL, and the AST and ALT were 174 U/L and 476 U/L, respectively. Antibiotics were stopped and she was discharged home.
Her prompt improvement suggests either a self‐limited condition or a response to the antibiotics. The rapid but incomplete resolution of her hepatitis is in keeping with a withdrawal of a toxin, relief of biliary obstruction, or a transient vascular event, and is less consistent with a viral hepatitis or an infiltrative process. With normal biliary system imaging, sterile blood cultures, and the absence of fever or leukocytosis, cholangitis is unlikely. Likewise, there is no suggestion of a vascular event, either obstructive or hemodynamic, that is impairing the liver.
A common cause of eosinophilia in hospitalized patients is medications, so it would be useful to monitor that count after the new antibiotics. At this point, I also wonder if the eosinophils are a feature of the underlying illness, as they were present to a modest degree on admission before any new medications were administered. The overlap of eosinophilia and hepatitis brings to mind a medication reaction (eg, to sulindac) or a hepatobiliary parasite, such as ascaris or clonorchis, for which she lacks a known exposure. Many patients experience flushing in the setting of fever or stress, but sustained flushing may suggest a systemic illness characterized by the release of vasoactive mediators such as carcinoid syndrome or mastocytosis. The latter might be considered more strongly if the eosinophilia is deemed to be primary (rather than reactive) after a thorough evaluation.
After 2 days at home, the patient had recurrence of subjective fevers, with chest, back, and abdominal pain, fatigue, loose stools, and rigors. She returned to the ED, where she was noted to have facial erythema and injected sclerae, but the remainder of her physical exam was normal. The total serum bilirubin was 1.1 mg/dL, AST was 156 U/L, ALT was 214 U/L, and alkaline phosphatase was 240 U/L. Serum lipase was normal. WBC count was 14,000/L, with 94% neutrophils, 3% lymphocytes, 2% monocytes, and 1% eosinophils. She was again treated empirically with ciprofloxacin and metronidazole. Endoscopic ultrasound was normal, with no evidence of gallbladder sludge or microlithiasis. Stool cultures, assay for Clostridium difficile, and examination for ova and parasites were negative. The 24‐hour urine demonstrated no elevation in 5‐hydroxyindoleacetic acid. An adrenocorticotropic hormone (ACTH) stimulation test was normal. HIV antibody was negative. Her symptoms improved within 2 days. The eosinophil count rose and peaked at 1541/L by the third hospital day, while the transaminase elevations resolved. Antibiotics were discontinued. A liver biopsy showed mixed macrovesicular and microvesicular fatty metamorphosis and steatohepatitis with eosinophils (Figures 1 and 2). She was discharged home on the sixth hospital day.


Her illness can now be characterized as relapsing inflammation, which given the frequency (over days) suggests either an indolent infectious focus that periodically causes systemic inflammation or reexposure to a toxic substance. The 2 most notable laboratory abnormalities, the hepatitis and the eosinophilia, persist but have differing trajectories. While the liver function tests have progressively normalized despite clinical relapses, the eosinophils have had a more fluctuating course characterized by increases during the hospitalization and higher levels during the second hospitalization. The absence of an infection, recurrent systemic inflammation, and eosinophilic hepatitis suggest a hypersensitivity reaction to a medication or other substance. She is most likely being reexposed at home, where her symptoms occur, and not in the hospital, where her symptoms resolve. Sulindac is a leading candidate, because nonsteroidal antiinflammatory drugs (NSAIDs) cause a number of hypersensitivity reactions and are frequently stopped when sick patients enter the hospital.
Seven days after discharge she developed acute onset of subjective fever, nausea, diffuse myalgias, and flushing, identical to the 2 prior episodes, and she again returned to the ED. Her temperature was 39.1C, heart rate was 120 beats per minute, blood pressure was 87/50 mm Hg, respiratory rate was 18 breaths per minute, and the oxygen saturation was 96% while breathing room air. She had diffuse flushing from her neck over her torso and was diaphoretic with injected sclera and conjunctiva. The WBC count was 11,400/L with 97% neutrophils and 3% lymphocytes. Total bilirubin was 0.8 mg/dL, AST was 134 U/L, ALT was 140 U/L, and alkaline phosphatase was 144 U/L. She was readmitted to the hospital. Following admission, she had no fevers, the flushing resolved, and AST and ALT levels decreased. The only treatment the patient received in the ED and during her hospital stay was acetaminophen as needed for pain or fever. The eosinophil count peaked at 1404/L by hospital day 4. Blood and urine cultures were negative. IgM antibodies to Epstein‐Barr virus were not detected, CMV DNA was not detected, and a rapid plasma reagin (RPR) test was nonreactive. Ferritin, ceruloplasmin, alpha‐1‐antitrypsin, and tryptase levels were normal. Antimitochondrial, antismooth muscle, antineutrophil cytoplasmic, and antinuclear antibodies were negative. There was no monoclonal band on serum protein electrophoresis. A blood smear for Borrelia detected no spirochetes.
A complete picture of the uncommon but classic flushing disorders, namely carcinoid, mastocytosis, and pheochromocytoma, has not emerged. The constellation of inflammation, mucosal and hepatic involvement, and eosinophilia are most consistent with a drug hypersensitivity reaction. Additionally, the recurrent inflammation is becoming more severe, as manifest by the fever and hemodynamic derangements, which suggests an increasing sensitization to the offending agent. I would review every drug she has received in the hospital, but given the recurrences after discharge her home medications are the most likely explanation. Of these, sulindac is the most likely culprit.
On further questioning, it was learned that the patient began taking sulindac 200 mg twice daily to treat her chronic ankle pain 6 weeks before the first admission. The medication had been stopped on each admission. She was instructed to discontinue sulindac. She has had no recurrences of symptoms and her hepatitis and eosinophilia have resolved.
DISCUSSION
This patient presented with recurrent skin findings, eosinophilia, hepatitis, and constitutional symptoms caused by hypersensitivity to sulindac. This drug‐induced hypersensitivity syndrome was originally described with anticonvulsant drugs (carbamazepine, phenytoin, and phenobarbitone) and named anticonvulsant hypersensitivity syndrome,1, 2 but has been observed with many other medications, including allopurinol, dapsone, minocycline, and nevirapine. The term drug rash with eosinophilia and systemic symptoms (DRESS) syndrome has been recently adopted to convey the cardinal features that characterize this disorder.3
DRESS syndrome is defined by rash, fever, and internal organ involvement.4 Also included in the diagnostic criteria are hematologic abnormalities (eosinophilia 1.5 109/mm3 or the presence of atypical lymphocytes) and lymphadenopathy.3 The multiorgan involvement distinguishes DRESS from other cutaneous drug eruptions. In a review of the French Pharmacovigilance Database for all cases of DRESS over a 15‐year period, 73% to 100% of patients were reported to have dermatologic abnormalities, most frequently a maculopapular rash or erythroderma. Less common skin findings include vesicles, bullae, pustules, erythroderma, and purpuric lesions. Liver abnormalities were observed in more than 60% of patients and were the most frequent systemic finding.5 Eosinophilia was the most common hematologic abnormality, present in more than 50% of cases. As this case underscores, DRESS syndrome typically begins 3 to 8 weeks after initiation of the drug because it is a delayed type IV hypersensitivity reaction.6 Fever can occur within hours on rechallenge because of the presence of memory T cells.
The main treatment for DRESS syndrome is withdrawal of the offending drug. Systemic corticosteroids have been recommended in cases with life‐threatening pulmonary or cardiac involvement, but have not been shown to be helpful in reversing renal or hepatic disease. Mortality, usually from end‐organ damage, occurs in about 10% of cases. The most common drugs are phenobarbital, carbamazepine, and phenytoin, with incidences of 1 in 5000 to 1 in 10,000. NSAIDs and antibiotics also have been implicated frequently. Human herpesvirus type 6 coinfection and genetically inherited slow acetylation have been associated with DRESS, although causal links have yet to be established.7, 8
The initial challenge in caring for a patient with multiple symptoms, exam findings, and test abnormalities is the coherent framing of the key clinical features that require explanation. This process, called problem representation, allows clinicians to search among a bounded list of possible diagnoses (or solutions) rather than invoking a differential diagnosis for every single abnormality. In searching for the proper diagnosis, this patient's clinical course required frequent reframing as more data became available.
Initially, the problem was framed as a 42‐year‐old woman with hepatitis. As the flushing and eosinophilia, which initially appeared to be transient and possibly nonspecific, became more prominent, the problem representation was revised to a 42‐year‐old woman with hepatitis, eosinophilia, and flushing. Since this triad did not immediately invoke a single diagnosis for the treating clinicians or the discussant, the differential diagnosis of hepatitis and eosinophilia and the differential diagnosis of flushing were considered in parallel.
Hepatitis and eosinophilia can occur coincidentally in the setting of parasitic infections, particularly helminths (ascaris, strongyloidiasis, and toxocaris) and liver flukes (opisthorchis and clonorchis), which invade the hepatobiliary system and induce a reactive eosinophilia. Some neoplasms, such as lymphomas and leukemias, and myeloproliferative disorders, including hypereosinophilic syndrome and mastocytosis, may have neoplastic cellular invasion of the liver and induce eosinophilia. Systemic drug hypersensitivity reactions are typically characterized by eosinophilia, transaminase elevations,9 and hepatitis on histology.10 As with this patient, liver biopsy in drug‐induced hepatitis shows a mixed microvesicular and macrovesicular steatosis, often with eosinophils.10
The flushing prompted a thorough but negative workup for the classic flushing disorders. The discussant's attempts to unify the flushing with the other clinical features illustrate how framing can affect our reasoning. Hepatitis, eosinophilia, and flushing defied an obvious single explanation and led the discussant down parallel diagnostic reasoning pathways. Although flushing is 1 of the well‐described dermatologic manifestations in drug‐hypersensitivity reactions, framing the central features as hepatitis, eosinophilia, and rash would have more readily suggested a drug reaction as a unifying diagnosis.11
The tempo and periodicity of this patient's illness provided the final formulation of a 42‐year‐old woman with hepatitis, eosinophilia, and flushing (or rash) that occurs every few days at home and resolves in the hospital. This formulation and the increasingly severe presentation, suggesting sensitization, were highly suggestive of an exogenous cause of her illness.
This case highlights how easily medication side effects can be overlooked during an extensive evaluation and how vigilant medication reconciliation coupled with an increased understanding of the spectrum of drug reactions can lead to early detection and prevention of potentially serious effects. In the case of DRESS, recognizing an association between a rash and organ involvement is central to making the diagnosis. Eosinophilia that accompanies a rash can further aid in narrowing the differential diagnosis.
The case also serves as a reminder of how framing with the slightest imprecision (eg, flushing instead of rash) can derail or delay the diagnostic process, yet is indispensable in tackling a complicated case. Finally, a time‐honored lesson in diagnosis is highlighted yet again: the diagnosis can usually be flushed out from the history.1214
Key Teaching Points
-
The constellation of skin findings, eosinophilia, organ involvement (particularly hepatitis), and constitutional symptoms should prompt consideration of DRESS syndrome and a hunt for a culprit drug.
-
Symptoms that resolve during hospitalization and repeatedly recur after discharge should prompt consideration of an exposure unique to the home, which may be environmental or pharmacologic.
-
Problem representation is critical in solving a complicated case, but adopting an inaccurate frame (representation) can derail or delay the diagnostic process.
The approach to clinical conundrums by an expert clinician is revealed through the presentation of an actual patient's case in an approach typical of a morning report. Similarly to patient care, sequential pieces of information are provided to the clinician, who is unfamiliar with the case. The focus is on the thought processes of both the clinical team caring for the patient and the discussant.
A 42‐year‐old woman with a history of mild asthma presented to the emergency department (ED) following 1 week of headache. She had been in her usual state of good health until 1 week prior to her presentation, when she noticed intermittent frontal headaches without neck stiffness or other neurologic symptoms. She then developed diffuse myalgias, fatigue, subjective fevers, and rigors for the 24 hours prior to presentation. On the morning of presentation, chest tightness, palpitations, and shortness of breath occurred. She used her albuterol metered‐dose inhaler without relief and went to the hospital.
Many of these features can be explained by a viral syndrome exacerbating underlying asthma or by a psychiatric condition such as anxiety or depression, but they may also be a harbinger of a systemic process, including infection, malignancy, or autoimmunity. Because the onset of headache is temporally distant from the other symptoms, I am more inclined to believe that it represents a primary intracranial process than I would if it were coincident with the onset of the other acute symptoms. If the fevers and rigors are verified, infection would be the initial concern. Failure to respond to her inhalers may either signify a severe asthma exacerbation or a nonbronchospastic cause of dyspnea.
She reported mild nausea, but denied photophobia, vomiting, abdominal pain, diarrhea, melena, or hematochezia. She did not have recent ill contacts, animal bites, or travel. Her medical history included asthma, diverticulitis, chronic right ankle pain, and obesity. She reported an allergic rash to amoxicillin. Her medications were sulindac and fluticasone/salmeterol, and albuterol metered‐dose inhalers. She worked as a preschool teacher and was married with 2 children. She denied any tobacco use and seldom drank alcoholic beverages. On exam, temperature was 36.7C, pulse was 107 beats per minute, blood pressure was 129/91 mm Hg, respiratory rate was 19 breaths per minute, and oxygen saturation was 98% while breathing ambient air. Her face and anterior neck were flushed and diaphoretic, and her sclerae were icteric. There was no nuchal rigidity. Her cardiac rhythm was regular without murmurs, lungs were clear to auscultation, and the abdomen was mildly tender to palpation in the epigastrium and right upper quadrant. The white blood cell (WBC) count was 9200/L, with 84% neutrophils, 3% lymphocytes, 6% monocytes, and 7% eosinophils. The hemoglobin was 14.8 g/dL and the platelet count was 166,000/L. Total serum bilirubin was 4.6 mg/dL, aspartate aminotransferase (AST) was 459 U/L (normal range, 8‐31), alanine aminotransferase (ALT) was 667 U/L (normal range, 7‐31), and alkaline phosphatase was 146 U/L (normal range, 39‐117). Serum electrolytes, creatinine, lactate, lipase, thyrotropin, coagulation studies, and cardiac enzymes were all normal. Urinalysis showed trace leukocyte esterase and bilirubin, as well as 3 WBCs and 2 red cells per high‐power field. Chest radiography and an electrocardiogram demonstrated no abnormalities.
The major findingwhich is critical to focusing problem‐solving in the face of a broad range of symptomsis her hepatitis. The common etiologies for hepatitis of this degree include viruses (hepatitis A and cytomegalovirus [CMV] should be considered given her work in preschool), toxins, autoimmunity, and vascular events. Liver disease in association with flushing raises the possibility of carcinoid syndrome with liver metastases. The lack of wheezing makes the bronchospasm of asthma or carcinoid less suitable explanations for her shortness of breath. Her eosinophilia is mild but probably is not accounted for alone by well‐controlled asthma in a person with no history of atopic disease. I would also ask her about any alternative and over‐the‐counter remedies. The paucity of lymphocytes raises the possibility of human immunodeficiency virus (HIV), Hodgkin's disease, or systemic lupus erythematosus. Although she does not have a documented fever or leukocytosis, she reported fevers and chills and is diaphoretic and tachycardic, so exclusion of biliary obstruction and cholangitis is the highest priority.
An abdominal ultrasound demonstrated hepatomegaly with moderate fatty infiltration and a normal gallbladder without pericholecystic fluid. The intrahepatic and extrahepatic biliary ducts were normal and the hepatic and portal veins were patent. Computed tomography of the abdomen showed slight thickening of the sigmoid colon wall. Ciprofloxacin and metronidazole were administered for possible diverticulitis. Over the first 48 hours of hospitalization her symptoms improved markedly. Her flushing resolved and she had no recorded fevers in the hospital. Serologies were negative for hepatitis A immunoglobulin M (IgM), hepatitis B surface antibody, hepatitis B surface antigen, and hepatitis C antibody. A monospot test was negative and the erythrocyte sedimentation rate was 11 mm/hour. Blood and urine cultures were negative. On the second hospital day the absolute eosinophil count rose to 855/L (15% of 5700 WBCs). On the fourth hospital day, the absolute eosinophil count was 1092/L, the total bilirubin was 1.9 mg/dL, and the AST and ALT were 174 U/L and 476 U/L, respectively. Antibiotics were stopped and she was discharged home.
Her prompt improvement suggests either a self‐limited condition or a response to the antibiotics. The rapid but incomplete resolution of her hepatitis is in keeping with a withdrawal of a toxin, relief of biliary obstruction, or a transient vascular event, and is less consistent with a viral hepatitis or an infiltrative process. With normal biliary system imaging, sterile blood cultures, and the absence of fever or leukocytosis, cholangitis is unlikely. Likewise, there is no suggestion of a vascular event, either obstructive or hemodynamic, that is impairing the liver.
A common cause of eosinophilia in hospitalized patients is medications, so it would be useful to monitor that count after the new antibiotics. At this point, I also wonder if the eosinophils are a feature of the underlying illness, as they were present to a modest degree on admission before any new medications were administered. The overlap of eosinophilia and hepatitis brings to mind a medication reaction (eg, to sulindac) or a hepatobiliary parasite, such as ascaris or clonorchis, for which she lacks a known exposure. Many patients experience flushing in the setting of fever or stress, but sustained flushing may suggest a systemic illness characterized by the release of vasoactive mediators such as carcinoid syndrome or mastocytosis. The latter might be considered more strongly if the eosinophilia is deemed to be primary (rather than reactive) after a thorough evaluation.
After 2 days at home, the patient had recurrence of subjective fevers, with chest, back, and abdominal pain, fatigue, loose stools, and rigors. She returned to the ED, where she was noted to have facial erythema and injected sclerae, but the remainder of her physical exam was normal. The total serum bilirubin was 1.1 mg/dL, AST was 156 U/L, ALT was 214 U/L, and alkaline phosphatase was 240 U/L. Serum lipase was normal. WBC count was 14,000/L, with 94% neutrophils, 3% lymphocytes, 2% monocytes, and 1% eosinophils. She was again treated empirically with ciprofloxacin and metronidazole. Endoscopic ultrasound was normal, with no evidence of gallbladder sludge or microlithiasis. Stool cultures, assay for Clostridium difficile, and examination for ova and parasites were negative. The 24‐hour urine demonstrated no elevation in 5‐hydroxyindoleacetic acid. An adrenocorticotropic hormone (ACTH) stimulation test was normal. HIV antibody was negative. Her symptoms improved within 2 days. The eosinophil count rose and peaked at 1541/L by the third hospital day, while the transaminase elevations resolved. Antibiotics were discontinued. A liver biopsy showed mixed macrovesicular and microvesicular fatty metamorphosis and steatohepatitis with eosinophils (Figures 1 and 2). She was discharged home on the sixth hospital day.


Her illness can now be characterized as relapsing inflammation, which given the frequency (over days) suggests either an indolent infectious focus that periodically causes systemic inflammation or reexposure to a toxic substance. The 2 most notable laboratory abnormalities, the hepatitis and the eosinophilia, persist but have differing trajectories. While the liver function tests have progressively normalized despite clinical relapses, the eosinophils have had a more fluctuating course characterized by increases during the hospitalization and higher levels during the second hospitalization. The absence of an infection, recurrent systemic inflammation, and eosinophilic hepatitis suggest a hypersensitivity reaction to a medication or other substance. She is most likely being reexposed at home, where her symptoms occur, and not in the hospital, where her symptoms resolve. Sulindac is a leading candidate, because nonsteroidal antiinflammatory drugs (NSAIDs) cause a number of hypersensitivity reactions and are frequently stopped when sick patients enter the hospital.
Seven days after discharge she developed acute onset of subjective fever, nausea, diffuse myalgias, and flushing, identical to the 2 prior episodes, and she again returned to the ED. Her temperature was 39.1C, heart rate was 120 beats per minute, blood pressure was 87/50 mm Hg, respiratory rate was 18 breaths per minute, and the oxygen saturation was 96% while breathing room air. She had diffuse flushing from her neck over her torso and was diaphoretic with injected sclera and conjunctiva. The WBC count was 11,400/L with 97% neutrophils and 3% lymphocytes. Total bilirubin was 0.8 mg/dL, AST was 134 U/L, ALT was 140 U/L, and alkaline phosphatase was 144 U/L. She was readmitted to the hospital. Following admission, she had no fevers, the flushing resolved, and AST and ALT levels decreased. The only treatment the patient received in the ED and during her hospital stay was acetaminophen as needed for pain or fever. The eosinophil count peaked at 1404/L by hospital day 4. Blood and urine cultures were negative. IgM antibodies to Epstein‐Barr virus were not detected, CMV DNA was not detected, and a rapid plasma reagin (RPR) test was nonreactive. Ferritin, ceruloplasmin, alpha‐1‐antitrypsin, and tryptase levels were normal. Antimitochondrial, antismooth muscle, antineutrophil cytoplasmic, and antinuclear antibodies were negative. There was no monoclonal band on serum protein electrophoresis. A blood smear for Borrelia detected no spirochetes.
A complete picture of the uncommon but classic flushing disorders, namely carcinoid, mastocytosis, and pheochromocytoma, has not emerged. The constellation of inflammation, mucosal and hepatic involvement, and eosinophilia are most consistent with a drug hypersensitivity reaction. Additionally, the recurrent inflammation is becoming more severe, as manifest by the fever and hemodynamic derangements, which suggests an increasing sensitization to the offending agent. I would review every drug she has received in the hospital, but given the recurrences after discharge her home medications are the most likely explanation. Of these, sulindac is the most likely culprit.
On further questioning, it was learned that the patient began taking sulindac 200 mg twice daily to treat her chronic ankle pain 6 weeks before the first admission. The medication had been stopped on each admission. She was instructed to discontinue sulindac. She has had no recurrences of symptoms and her hepatitis and eosinophilia have resolved.
DISCUSSION
This patient presented with recurrent skin findings, eosinophilia, hepatitis, and constitutional symptoms caused by hypersensitivity to sulindac. This drug‐induced hypersensitivity syndrome was originally described with anticonvulsant drugs (carbamazepine, phenytoin, and phenobarbitone) and named anticonvulsant hypersensitivity syndrome,1, 2 but has been observed with many other medications, including allopurinol, dapsone, minocycline, and nevirapine. The term drug rash with eosinophilia and systemic symptoms (DRESS) syndrome has been recently adopted to convey the cardinal features that characterize this disorder.3
DRESS syndrome is defined by rash, fever, and internal organ involvement.4 Also included in the diagnostic criteria are hematologic abnormalities (eosinophilia 1.5 109/mm3 or the presence of atypical lymphocytes) and lymphadenopathy.3 The multiorgan involvement distinguishes DRESS from other cutaneous drug eruptions. In a review of the French Pharmacovigilance Database for all cases of DRESS over a 15‐year period, 73% to 100% of patients were reported to have dermatologic abnormalities, most frequently a maculopapular rash or erythroderma. Less common skin findings include vesicles, bullae, pustules, erythroderma, and purpuric lesions. Liver abnormalities were observed in more than 60% of patients and were the most frequent systemic finding.5 Eosinophilia was the most common hematologic abnormality, present in more than 50% of cases. As this case underscores, DRESS syndrome typically begins 3 to 8 weeks after initiation of the drug because it is a delayed type IV hypersensitivity reaction.6 Fever can occur within hours on rechallenge because of the presence of memory T cells.
The main treatment for DRESS syndrome is withdrawal of the offending drug. Systemic corticosteroids have been recommended in cases with life‐threatening pulmonary or cardiac involvement, but have not been shown to be helpful in reversing renal or hepatic disease. Mortality, usually from end‐organ damage, occurs in about 10% of cases. The most common drugs are phenobarbital, carbamazepine, and phenytoin, with incidences of 1 in 5000 to 1 in 10,000. NSAIDs and antibiotics also have been implicated frequently. Human herpesvirus type 6 coinfection and genetically inherited slow acetylation have been associated with DRESS, although causal links have yet to be established.7, 8
The initial challenge in caring for a patient with multiple symptoms, exam findings, and test abnormalities is the coherent framing of the key clinical features that require explanation. This process, called problem representation, allows clinicians to search among a bounded list of possible diagnoses (or solutions) rather than invoking a differential diagnosis for every single abnormality. In searching for the proper diagnosis, this patient's clinical course required frequent reframing as more data became available.
Initially, the problem was framed as a 42‐year‐old woman with hepatitis. As the flushing and eosinophilia, which initially appeared to be transient and possibly nonspecific, became more prominent, the problem representation was revised to a 42‐year‐old woman with hepatitis, eosinophilia, and flushing. Since this triad did not immediately invoke a single diagnosis for the treating clinicians or the discussant, the differential diagnosis of hepatitis and eosinophilia and the differential diagnosis of flushing were considered in parallel.
Hepatitis and eosinophilia can occur coincidentally in the setting of parasitic infections, particularly helminths (ascaris, strongyloidiasis, and toxocaris) and liver flukes (opisthorchis and clonorchis), which invade the hepatobiliary system and induce a reactive eosinophilia. Some neoplasms, such as lymphomas and leukemias, and myeloproliferative disorders, including hypereosinophilic syndrome and mastocytosis, may have neoplastic cellular invasion of the liver and induce eosinophilia. Systemic drug hypersensitivity reactions are typically characterized by eosinophilia, transaminase elevations,9 and hepatitis on histology.10 As with this patient, liver biopsy in drug‐induced hepatitis shows a mixed microvesicular and macrovesicular steatosis, often with eosinophils.10
The flushing prompted a thorough but negative workup for the classic flushing disorders. The discussant's attempts to unify the flushing with the other clinical features illustrate how framing can affect our reasoning. Hepatitis, eosinophilia, and flushing defied an obvious single explanation and led the discussant down parallel diagnostic reasoning pathways. Although flushing is 1 of the well‐described dermatologic manifestations in drug‐hypersensitivity reactions, framing the central features as hepatitis, eosinophilia, and rash would have more readily suggested a drug reaction as a unifying diagnosis.11
The tempo and periodicity of this patient's illness provided the final formulation of a 42‐year‐old woman with hepatitis, eosinophilia, and flushing (or rash) that occurs every few days at home and resolves in the hospital. This formulation and the increasingly severe presentation, suggesting sensitization, were highly suggestive of an exogenous cause of her illness.
This case highlights how easily medication side effects can be overlooked during an extensive evaluation and how vigilant medication reconciliation coupled with an increased understanding of the spectrum of drug reactions can lead to early detection and prevention of potentially serious effects. In the case of DRESS, recognizing an association between a rash and organ involvement is central to making the diagnosis. Eosinophilia that accompanies a rash can further aid in narrowing the differential diagnosis.
The case also serves as a reminder of how framing with the slightest imprecision (eg, flushing instead of rash) can derail or delay the diagnostic process, yet is indispensable in tackling a complicated case. Finally, a time‐honored lesson in diagnosis is highlighted yet again: the diagnosis can usually be flushed out from the history.1214
Key Teaching Points
-
The constellation of skin findings, eosinophilia, organ involvement (particularly hepatitis), and constitutional symptoms should prompt consideration of DRESS syndrome and a hunt for a culprit drug.
-
Symptoms that resolve during hospitalization and repeatedly recur after discharge should prompt consideration of an exposure unique to the home, which may be environmental or pharmacologic.
-
Problem representation is critical in solving a complicated case, but adopting an inaccurate frame (representation) can derail or delay the diagnostic process.
The approach to clinical conundrums by an expert clinician is revealed through the presentation of an actual patient's case in an approach typical of a morning report. Similarly to patient care, sequential pieces of information are provided to the clinician, who is unfamiliar with the case. The focus is on the thought processes of both the clinical team caring for the patient and the discussant.
- ,.Anticonvulsant hypersensitivity syndrome. In vitro assessment of risk.J Clin Invest.1988;82:1826–1832.
- ,,.Anticonvulsant hypersensitivity syndrome: incidence, prevention, and management.Drug Saf.1999;21:489–501.
- ,,,.Drug rash with eosinophilia and systemic symptoms vs toxic epidermal necrolysis: the dilemma of classification.Clin Dermatol.2005;23:311–314.
- ,.Management of drug rash with eosinophilia and systemic symptoms (DRESS syndrome): an update.Dermatology.2003;206:353–356.
- ,,,,,Blayac J‐P; Network of the French Pharmacovigilance Centers. Variability in the clinical pattern of cutaneous side‐effects of drugs with systemic symptoms: does a DRESS syndrome really exist?Br J Dermatol.2006;155:422–428.
- ,.The immunological and clinical spectrum of delayed drug‐induced exanthems.Curr Opin Allergy Clin Immunol.2004;4:411–419.
- ,,.Association between anticonvulsant hypersensitivity syndrome and human herpesvirus 6 reactivation and hypogammaglobulinemia.Arch Dermatol.2004;140:183–188.
- ,.Treatment of severe drug reactions: Stevens‐Johnson Syndrome, toxic epidermal necrolysis and hypersensitivity syndrome.Dermatol Online J.2002;8:5.
- ,.Drug reactions. In: Bolognia JL, Jorizzo JL, Rapini RP, eds.Dermatology.2nd ed.Spain:Mosby Elsevier;2008:310–311.
- Pathology Outlines. Liver and intrahepatic bile ducts—non tumor. Hepatitis (non‐infectious). Drug/toxin‐induced hepatitis. Available at: http://www.pathologyoutlines.com/liver.html#drugtoxin. Accessed August2009.
- ,,.The flushing patient: differential diagnosis, workup and treatment.J Am Acad Dermatol.2006;55(2):193–208.
- ,,,,.Relative contributions of history‐taking, physical examination, and laboratory investigation to diagnosis and management of medical outpatients.Br Med J.1975;2(5969):486–489.
- ,,,,.Contributions to the history, physical examination, and laboratory investigation in making medical diagnosis.West J Med.1992;156(2):163–165.
- ,.A study on relative contributions of the history, physical examination and investigations in making medical diagnosis.J Assoc Physicians India.2000;48(8):771–775.
- ,.Anticonvulsant hypersensitivity syndrome. In vitro assessment of risk.J Clin Invest.1988;82:1826–1832.
- ,,.Anticonvulsant hypersensitivity syndrome: incidence, prevention, and management.Drug Saf.1999;21:489–501.
- ,,,.Drug rash with eosinophilia and systemic symptoms vs toxic epidermal necrolysis: the dilemma of classification.Clin Dermatol.2005;23:311–314.
- ,.Management of drug rash with eosinophilia and systemic symptoms (DRESS syndrome): an update.Dermatology.2003;206:353–356.
- ,,,,,Blayac J‐P; Network of the French Pharmacovigilance Centers. Variability in the clinical pattern of cutaneous side‐effects of drugs with systemic symptoms: does a DRESS syndrome really exist?Br J Dermatol.2006;155:422–428.
- ,.The immunological and clinical spectrum of delayed drug‐induced exanthems.Curr Opin Allergy Clin Immunol.2004;4:411–419.
- ,,.Association between anticonvulsant hypersensitivity syndrome and human herpesvirus 6 reactivation and hypogammaglobulinemia.Arch Dermatol.2004;140:183–188.
- ,.Treatment of severe drug reactions: Stevens‐Johnson Syndrome, toxic epidermal necrolysis and hypersensitivity syndrome.Dermatol Online J.2002;8:5.
- ,.Drug reactions. In: Bolognia JL, Jorizzo JL, Rapini RP, eds.Dermatology.2nd ed.Spain:Mosby Elsevier;2008:310–311.
- Pathology Outlines. Liver and intrahepatic bile ducts—non tumor. Hepatitis (non‐infectious). Drug/toxin‐induced hepatitis. Available at: http://www.pathologyoutlines.com/liver.html#drugtoxin. Accessed August2009.
- ,,.The flushing patient: differential diagnosis, workup and treatment.J Am Acad Dermatol.2006;55(2):193–208.
- ,,,,.Relative contributions of history‐taking, physical examination, and laboratory investigation to diagnosis and management of medical outpatients.Br Med J.1975;2(5969):486–489.
- ,,,,.Contributions to the history, physical examination, and laboratory investigation in making medical diagnosis.West J Med.1992;156(2):163–165.
- ,.A study on relative contributions of the history, physical examination and investigations in making medical diagnosis.J Assoc Physicians India.2000;48(8):771–775.