User login
The Diagnosis: Spindle Cell Malignant Melanoma With Perineural Invasion
The incidence of melanoma has steadily increased in the United States since the 1930s when the incidence was reported at 1.0 per 100,000.1 In 1973 melanoma incidence was 6.8 per 100,000, and by 2007 the rate increased to 20.1 per 100,000.2 The American Cancer Society projects 73,870 new cases of melanoma in 2015, with melanoma as the fifth most common cancer in males and the seventh most common in females.3 Melanoma-related deaths are projected to be 9940. The lifetime probability of developing melanoma is 1 in 34 for males and 1 in 53 for females. Twice as many males are estimated to have melanoma-related deaths compared to females. The 5-year relative survival rate is 93% for white individuals and 75% for black individuals.3
Spindle cell melanoma is a rare variant of melanoma that was originally described by Conley et al4 in 1971. The lesion represents 2% to 4% of all melanomas and presents in older patients on sun-exposed skin as a pink or variably pigmented nodule measuring an average of 2 cm.5 Males are affected more than females, and prominent neural invasion is present in 30% to 100% of cases.6-10 Neural invasion can result in nerve palsies and/or dysesthesia. Because half of these lesions are amelanotic, they are often clinically misdiagnosed prior to biopsy.11
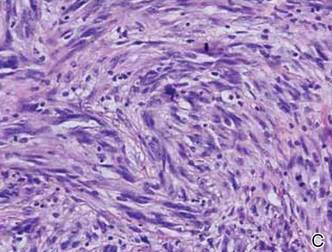
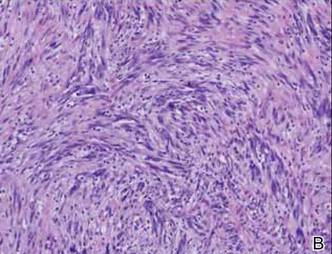
Histopathologically, these lesions can be quite challenging. Spindle cell melanoma histologically is an intradermal lesion composed of spindle cells distributed in bundles, fascicles, or nests, or singly between collagen fibers of the dermis. Other spindle tumors such as spindle cell squamous cell carcinoma, atypical fibroxanthoma, dermatofibrosarcoma protuberans, angiosarcoma, and leiomyosarcoma have a similar presentation with hematoxylin and eosin stain. The diagnostic process for spindle cell tumors is greatly aided by immunohistologic analysis. Spindle cell melanoma usually shows immunoreactivity with S-100. Human melanoma black 45, CD57, and neuron-specific enolase usually do not stain, and CD68 has been demonstrated in a minority of cases.
The initial biopsy specimen in our case displayed a dense dermal atypical spindle cell proliferation with hematoxylin and eosin stain. The differential diagnosis of the proliferation included desmoplastic melanoma, spindle cell squamous cell carcinoma, leiomyosarcoma, angiosarcoma, and atypical fibroxanthoma. Immunostains were used to further study the lesional biopsy. Cytokeratin 34bE12, CK5/6, cerium ammonium molybdate 5.2, CK7, epithelial membrane antigen, CK18, high-molecular-weight cytokeratin, S-100, Melan-A, human melanoma black 45, smooth muscle actin, desmin, CD68, CD34, CD10, and p63 were studied. The atypical dermal spindle cells were positive for S-100. S-100 and Melan-A highlighted an increased number of single melanocytes at the dermoepidermal junction. Other markers were negative.
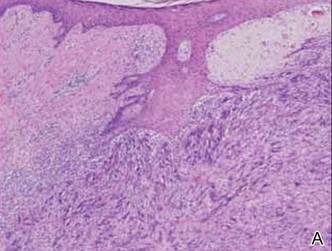
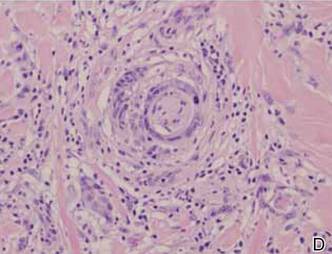
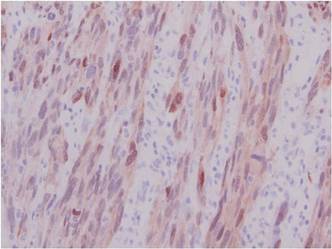
A 1.0-cm wide excision to fascia was performed. Routine hematoxylin and eosin stain showed atypical dermal spindle cells with perineural invasion (Figure 1). The malignant spindle cells were S-100 positive (Figure 2). The spindle cells were negative for high-molecular-weight cytokeratin, CK5/6, p63, Melan-A, A103, microphthalmia, and tyrosinase. These findings confirm melanoma of the spindle cell type.
|
The lesion was a Clark level V melanoma with a Breslow thickness of at least 12 mm. Perineural invasion was noted and the mitotic index was 7 cells/mm2. Vascular and lymphatic invasion was negative and ulceration was present. Tumor-infiltrating lymphocytes were brisk, resulting in a pathologic staging of pT4NXMX.
On initial histologic study with hematoxylin and eosin stain, spindle cell melanoma lesions tend to be generally quite thick. Manganoni et al12 reported in their series a Breslow thickness ranging from 2.1 to 12 mm with a mean of 5.8 mm.
Treatment in our case involved a second wide incision to fascia with an additional 2-cm margin. The role of sentinel lymph node biopsy (SLNB) remains undefined. Patients with spindle cell melanoma have a lower frequency of positive sentinel lymph nodes than nondesmoplastic melanomas.13 As such, the need to perform SLNB has not been determined.13-15 Our patient had a history of breast cancer. The lesion appeared on the left side of the back and she pre-viously had a complete axillary lymphadenectomy on the left axillae. She declined SLNB. A systematic workup by the oncology service was negative. Continued follow-up has revealed no recurrent disease and recent workup was negative for metastatic disease.
Many studies report the increased incidence of local recurrence after excision for spindle cell melanoma as compared to non–spindle cell melanoma,16-18 which is likely related to perineural invasion as in our patient.
Spindle cell melanoma is a rare tumor that is often amelanotic and difficult to diagnose clinically. Routine hematoxylin and eosin staining shows a dermal spindle cell tumor. Immunohistochemical study is of great aid in defining the tumor. The clinician and pathologist must work together to correctly diagnose and treat this lesion.
1. Mikkilineni R, Weinstock MA. Epidemiology. In: Sober AJ, Haluska FG, eds. Atlas of Clinical Oncology: Skin Cancer. London, England: BC Decker; 2001:1-15.
2. Rigel D. Epidemiology of melanoma. Semin Cutan Med Surg. 2010;29:204-209.
3. American Cancer Society. Cancer Facts & Figures 2015. Atlanta, GA: American Cancer Society; 2015. http://www.cancer.org/acs/groups/content/@editorial/documents/document/acspc-044552.pdf. Accessed February 10, 2015.
4. Conley J, Lattes R, Orr W. Desmoplastic malignant melanoma (a rare variant of malignant melanoma. Cancer. 1971;28:914-936.
5. Repertinger SK, Teruya B, Sarma DP. Common spindle cell malignant neoplasms of the skin: differential diagnosis and review of the literature. Internet J Dermatol. 2009;7. https://ispub.com/IJD/7/2/11747. Accessed February 10, 2015.
6. Chang PC, Fischbein NJ, McCalmont TH, et al. Perineural spread of malignant melanoma of the head and neck: clinical and imaging features. AJNR Am J Neuroradial. 2004;25:5-11.
7. Cruz J, Reis-Filho JS, Lopes JM. Malignant peripheral nerve sheath tumor-like primary cutaneous melanoma. J Clin Pathol. 2004;57:218-220.
8. Tsao H, Sober AJ, Barnhill RL. Desmoplastic neurotropic melanoma. Semin Cutan Med Surg. 1997;16:45-47.
9. Kossard S, Doherty E, Murray E. Neurotropic melanoma. a variant of desmoplastic melanoma. Arch Dermatol. 1997;7:907-912.
10. Bruijn JA, Salasche S, Sober AJ, et al. Desmoplastic melanoma: clinicopathologic aspects of six cases. Dermatology. 1992;185:3-8.
11. Jesitus J. Desmoplastic melanoma. Dermatology Times. March 2009:1-2.
12. Manganoni AM, Farisoglio C, Bassissi S, et al. Desmoplastic melanoma: report of 5 cases. Dermatol Res Pract. 2009;2009:679010.
13. Gyorki DE, Busam K, Panageas K, et al. Sentinel lymph node biopsy for patients with desmoplastic melanoma. Ann Surg Oncol. 2003;10:403-407.
14. Livestro DP, Muzikansky A, Kaine EM, et al. of desmoplastic melanoma: a case-control comparison with other melanomas. J Clin Oncol. 2005;23:6739-6746.
15. Pawlik TM, Ross MI, Prieto VG, et al. Assessment of the role of sentinel lymph node biopsy for primary cutaneous desmoplastic melanoma. Cancer. 2006;106:900-906.
16. Smithers HM, McLeod GR, Little JH. Desmoplastic melanoma: patterns of recurrence. World J Surg. 1992;16:186-190.
17. McCarthy SW, Scolyer RA, Palmer AA. Desmoplastic melanoma: a diagnostic trap for the unwary. Pathology. 2004;36:445-451.
18. Bruijn JA, Mihm MC Jr, Barnhill RL. Desmoplastic melanoma. Histopathology. 1992;20:197-205.
The Diagnosis: Spindle Cell Malignant Melanoma With Perineural Invasion
The incidence of melanoma has steadily increased in the United States since the 1930s when the incidence was reported at 1.0 per 100,000.1 In 1973 melanoma incidence was 6.8 per 100,000, and by 2007 the rate increased to 20.1 per 100,000.2 The American Cancer Society projects 73,870 new cases of melanoma in 2015, with melanoma as the fifth most common cancer in males and the seventh most common in females.3 Melanoma-related deaths are projected to be 9940. The lifetime probability of developing melanoma is 1 in 34 for males and 1 in 53 for females. Twice as many males are estimated to have melanoma-related deaths compared to females. The 5-year relative survival rate is 93% for white individuals and 75% for black individuals.3
Spindle cell melanoma is a rare variant of melanoma that was originally described by Conley et al4 in 1971. The lesion represents 2% to 4% of all melanomas and presents in older patients on sun-exposed skin as a pink or variably pigmented nodule measuring an average of 2 cm.5 Males are affected more than females, and prominent neural invasion is present in 30% to 100% of cases.6-10 Neural invasion can result in nerve palsies and/or dysesthesia. Because half of these lesions are amelanotic, they are often clinically misdiagnosed prior to biopsy.11
Histopathologically, these lesions can be quite challenging. Spindle cell melanoma histologically is an intradermal lesion composed of spindle cells distributed in bundles, fascicles, or nests, or singly between collagen fibers of the dermis. Other spindle tumors such as spindle cell squamous cell carcinoma, atypical fibroxanthoma, dermatofibrosarcoma protuberans, angiosarcoma, and leiomyosarcoma have a similar presentation with hematoxylin and eosin stain. The diagnostic process for spindle cell tumors is greatly aided by immunohistologic analysis. Spindle cell melanoma usually shows immunoreactivity with S-100. Human melanoma black 45, CD57, and neuron-specific enolase usually do not stain, and CD68 has been demonstrated in a minority of cases.
The initial biopsy specimen in our case displayed a dense dermal atypical spindle cell proliferation with hematoxylin and eosin stain. The differential diagnosis of the proliferation included desmoplastic melanoma, spindle cell squamous cell carcinoma, leiomyosarcoma, angiosarcoma, and atypical fibroxanthoma. Immunostains were used to further study the lesional biopsy. Cytokeratin 34bE12, CK5/6, cerium ammonium molybdate 5.2, CK7, epithelial membrane antigen, CK18, high-molecular-weight cytokeratin, S-100, Melan-A, human melanoma black 45, smooth muscle actin, desmin, CD68, CD34, CD10, and p63 were studied. The atypical dermal spindle cells were positive for S-100. S-100 and Melan-A highlighted an increased number of single melanocytes at the dermoepidermal junction. Other markers were negative.
A 1.0-cm wide excision to fascia was performed. Routine hematoxylin and eosin stain showed atypical dermal spindle cells with perineural invasion (Figure 1). The malignant spindle cells were S-100 positive (Figure 2). The spindle cells were negative for high-molecular-weight cytokeratin, CK5/6, p63, Melan-A, A103, microphthalmia, and tyrosinase. These findings confirm melanoma of the spindle cell type.
|
The lesion was a Clark level V melanoma with a Breslow thickness of at least 12 mm. Perineural invasion was noted and the mitotic index was 7 cells/mm2. Vascular and lymphatic invasion was negative and ulceration was present. Tumor-infiltrating lymphocytes were brisk, resulting in a pathologic staging of pT4NXMX.
On initial histologic study with hematoxylin and eosin stain, spindle cell melanoma lesions tend to be generally quite thick. Manganoni et al12 reported in their series a Breslow thickness ranging from 2.1 to 12 mm with a mean of 5.8 mm.
Treatment in our case involved a second wide incision to fascia with an additional 2-cm margin. The role of sentinel lymph node biopsy (SLNB) remains undefined. Patients with spindle cell melanoma have a lower frequency of positive sentinel lymph nodes than nondesmoplastic melanomas.13 As such, the need to perform SLNB has not been determined.13-15 Our patient had a history of breast cancer. The lesion appeared on the left side of the back and she pre-viously had a complete axillary lymphadenectomy on the left axillae. She declined SLNB. A systematic workup by the oncology service was negative. Continued follow-up has revealed no recurrent disease and recent workup was negative for metastatic disease.
Many studies report the increased incidence of local recurrence after excision for spindle cell melanoma as compared to non–spindle cell melanoma,16-18 which is likely related to perineural invasion as in our patient.
Spindle cell melanoma is a rare tumor that is often amelanotic and difficult to diagnose clinically. Routine hematoxylin and eosin staining shows a dermal spindle cell tumor. Immunohistochemical study is of great aid in defining the tumor. The clinician and pathologist must work together to correctly diagnose and treat this lesion.
The Diagnosis: Spindle Cell Malignant Melanoma With Perineural Invasion
The incidence of melanoma has steadily increased in the United States since the 1930s when the incidence was reported at 1.0 per 100,000.1 In 1973 melanoma incidence was 6.8 per 100,000, and by 2007 the rate increased to 20.1 per 100,000.2 The American Cancer Society projects 73,870 new cases of melanoma in 2015, with melanoma as the fifth most common cancer in males and the seventh most common in females.3 Melanoma-related deaths are projected to be 9940. The lifetime probability of developing melanoma is 1 in 34 for males and 1 in 53 for females. Twice as many males are estimated to have melanoma-related deaths compared to females. The 5-year relative survival rate is 93% for white individuals and 75% for black individuals.3
Spindle cell melanoma is a rare variant of melanoma that was originally described by Conley et al4 in 1971. The lesion represents 2% to 4% of all melanomas and presents in older patients on sun-exposed skin as a pink or variably pigmented nodule measuring an average of 2 cm.5 Males are affected more than females, and prominent neural invasion is present in 30% to 100% of cases.6-10 Neural invasion can result in nerve palsies and/or dysesthesia. Because half of these lesions are amelanotic, they are often clinically misdiagnosed prior to biopsy.11
Histopathologically, these lesions can be quite challenging. Spindle cell melanoma histologically is an intradermal lesion composed of spindle cells distributed in bundles, fascicles, or nests, or singly between collagen fibers of the dermis. Other spindle tumors such as spindle cell squamous cell carcinoma, atypical fibroxanthoma, dermatofibrosarcoma protuberans, angiosarcoma, and leiomyosarcoma have a similar presentation with hematoxylin and eosin stain. The diagnostic process for spindle cell tumors is greatly aided by immunohistologic analysis. Spindle cell melanoma usually shows immunoreactivity with S-100. Human melanoma black 45, CD57, and neuron-specific enolase usually do not stain, and CD68 has been demonstrated in a minority of cases.
The initial biopsy specimen in our case displayed a dense dermal atypical spindle cell proliferation with hematoxylin and eosin stain. The differential diagnosis of the proliferation included desmoplastic melanoma, spindle cell squamous cell carcinoma, leiomyosarcoma, angiosarcoma, and atypical fibroxanthoma. Immunostains were used to further study the lesional biopsy. Cytokeratin 34bE12, CK5/6, cerium ammonium molybdate 5.2, CK7, epithelial membrane antigen, CK18, high-molecular-weight cytokeratin, S-100, Melan-A, human melanoma black 45, smooth muscle actin, desmin, CD68, CD34, CD10, and p63 were studied. The atypical dermal spindle cells were positive for S-100. S-100 and Melan-A highlighted an increased number of single melanocytes at the dermoepidermal junction. Other markers were negative.
A 1.0-cm wide excision to fascia was performed. Routine hematoxylin and eosin stain showed atypical dermal spindle cells with perineural invasion (Figure 1). The malignant spindle cells were S-100 positive (Figure 2). The spindle cells were negative for high-molecular-weight cytokeratin, CK5/6, p63, Melan-A, A103, microphthalmia, and tyrosinase. These findings confirm melanoma of the spindle cell type.
|
The lesion was a Clark level V melanoma with a Breslow thickness of at least 12 mm. Perineural invasion was noted and the mitotic index was 7 cells/mm2. Vascular and lymphatic invasion was negative and ulceration was present. Tumor-infiltrating lymphocytes were brisk, resulting in a pathologic staging of pT4NXMX.
On initial histologic study with hematoxylin and eosin stain, spindle cell melanoma lesions tend to be generally quite thick. Manganoni et al12 reported in their series a Breslow thickness ranging from 2.1 to 12 mm with a mean of 5.8 mm.
Treatment in our case involved a second wide incision to fascia with an additional 2-cm margin. The role of sentinel lymph node biopsy (SLNB) remains undefined. Patients with spindle cell melanoma have a lower frequency of positive sentinel lymph nodes than nondesmoplastic melanomas.13 As such, the need to perform SLNB has not been determined.13-15 Our patient had a history of breast cancer. The lesion appeared on the left side of the back and she pre-viously had a complete axillary lymphadenectomy on the left axillae. She declined SLNB. A systematic workup by the oncology service was negative. Continued follow-up has revealed no recurrent disease and recent workup was negative for metastatic disease.
Many studies report the increased incidence of local recurrence after excision for spindle cell melanoma as compared to non–spindle cell melanoma,16-18 which is likely related to perineural invasion as in our patient.
Spindle cell melanoma is a rare tumor that is often amelanotic and difficult to diagnose clinically. Routine hematoxylin and eosin staining shows a dermal spindle cell tumor. Immunohistochemical study is of great aid in defining the tumor. The clinician and pathologist must work together to correctly diagnose and treat this lesion.
1. Mikkilineni R, Weinstock MA. Epidemiology. In: Sober AJ, Haluska FG, eds. Atlas of Clinical Oncology: Skin Cancer. London, England: BC Decker; 2001:1-15.
2. Rigel D. Epidemiology of melanoma. Semin Cutan Med Surg. 2010;29:204-209.
3. American Cancer Society. Cancer Facts & Figures 2015. Atlanta, GA: American Cancer Society; 2015. http://www.cancer.org/acs/groups/content/@editorial/documents/document/acspc-044552.pdf. Accessed February 10, 2015.
4. Conley J, Lattes R, Orr W. Desmoplastic malignant melanoma (a rare variant of malignant melanoma. Cancer. 1971;28:914-936.
5. Repertinger SK, Teruya B, Sarma DP. Common spindle cell malignant neoplasms of the skin: differential diagnosis and review of the literature. Internet J Dermatol. 2009;7. https://ispub.com/IJD/7/2/11747. Accessed February 10, 2015.
6. Chang PC, Fischbein NJ, McCalmont TH, et al. Perineural spread of malignant melanoma of the head and neck: clinical and imaging features. AJNR Am J Neuroradial. 2004;25:5-11.
7. Cruz J, Reis-Filho JS, Lopes JM. Malignant peripheral nerve sheath tumor-like primary cutaneous melanoma. J Clin Pathol. 2004;57:218-220.
8. Tsao H, Sober AJ, Barnhill RL. Desmoplastic neurotropic melanoma. Semin Cutan Med Surg. 1997;16:45-47.
9. Kossard S, Doherty E, Murray E. Neurotropic melanoma. a variant of desmoplastic melanoma. Arch Dermatol. 1997;7:907-912.
10. Bruijn JA, Salasche S, Sober AJ, et al. Desmoplastic melanoma: clinicopathologic aspects of six cases. Dermatology. 1992;185:3-8.
11. Jesitus J. Desmoplastic melanoma. Dermatology Times. March 2009:1-2.
12. Manganoni AM, Farisoglio C, Bassissi S, et al. Desmoplastic melanoma: report of 5 cases. Dermatol Res Pract. 2009;2009:679010.
13. Gyorki DE, Busam K, Panageas K, et al. Sentinel lymph node biopsy for patients with desmoplastic melanoma. Ann Surg Oncol. 2003;10:403-407.
14. Livestro DP, Muzikansky A, Kaine EM, et al. of desmoplastic melanoma: a case-control comparison with other melanomas. J Clin Oncol. 2005;23:6739-6746.
15. Pawlik TM, Ross MI, Prieto VG, et al. Assessment of the role of sentinel lymph node biopsy for primary cutaneous desmoplastic melanoma. Cancer. 2006;106:900-906.
16. Smithers HM, McLeod GR, Little JH. Desmoplastic melanoma: patterns of recurrence. World J Surg. 1992;16:186-190.
17. McCarthy SW, Scolyer RA, Palmer AA. Desmoplastic melanoma: a diagnostic trap for the unwary. Pathology. 2004;36:445-451.
18. Bruijn JA, Mihm MC Jr, Barnhill RL. Desmoplastic melanoma. Histopathology. 1992;20:197-205.
1. Mikkilineni R, Weinstock MA. Epidemiology. In: Sober AJ, Haluska FG, eds. Atlas of Clinical Oncology: Skin Cancer. London, England: BC Decker; 2001:1-15.
2. Rigel D. Epidemiology of melanoma. Semin Cutan Med Surg. 2010;29:204-209.
3. American Cancer Society. Cancer Facts & Figures 2015. Atlanta, GA: American Cancer Society; 2015. http://www.cancer.org/acs/groups/content/@editorial/documents/document/acspc-044552.pdf. Accessed February 10, 2015.
4. Conley J, Lattes R, Orr W. Desmoplastic malignant melanoma (a rare variant of malignant melanoma. Cancer. 1971;28:914-936.
5. Repertinger SK, Teruya B, Sarma DP. Common spindle cell malignant neoplasms of the skin: differential diagnosis and review of the literature. Internet J Dermatol. 2009;7. https://ispub.com/IJD/7/2/11747. Accessed February 10, 2015.
6. Chang PC, Fischbein NJ, McCalmont TH, et al. Perineural spread of malignant melanoma of the head and neck: clinical and imaging features. AJNR Am J Neuroradial. 2004;25:5-11.
7. Cruz J, Reis-Filho JS, Lopes JM. Malignant peripheral nerve sheath tumor-like primary cutaneous melanoma. J Clin Pathol. 2004;57:218-220.
8. Tsao H, Sober AJ, Barnhill RL. Desmoplastic neurotropic melanoma. Semin Cutan Med Surg. 1997;16:45-47.
9. Kossard S, Doherty E, Murray E. Neurotropic melanoma. a variant of desmoplastic melanoma. Arch Dermatol. 1997;7:907-912.
10. Bruijn JA, Salasche S, Sober AJ, et al. Desmoplastic melanoma: clinicopathologic aspects of six cases. Dermatology. 1992;185:3-8.
11. Jesitus J. Desmoplastic melanoma. Dermatology Times. March 2009:1-2.
12. Manganoni AM, Farisoglio C, Bassissi S, et al. Desmoplastic melanoma: report of 5 cases. Dermatol Res Pract. 2009;2009:679010.
13. Gyorki DE, Busam K, Panageas K, et al. Sentinel lymph node biopsy for patients with desmoplastic melanoma. Ann Surg Oncol. 2003;10:403-407.
14. Livestro DP, Muzikansky A, Kaine EM, et al. of desmoplastic melanoma: a case-control comparison with other melanomas. J Clin Oncol. 2005;23:6739-6746.
15. Pawlik TM, Ross MI, Prieto VG, et al. Assessment of the role of sentinel lymph node biopsy for primary cutaneous desmoplastic melanoma. Cancer. 2006;106:900-906.
16. Smithers HM, McLeod GR, Little JH. Desmoplastic melanoma: patterns of recurrence. World J Surg. 1992;16:186-190.
17. McCarthy SW, Scolyer RA, Palmer AA. Desmoplastic melanoma: a diagnostic trap for the unwary. Pathology. 2004;36:445-451.
18. Bruijn JA, Mihm MC Jr, Barnhill RL. Desmoplastic melanoma. Histopathology. 1992;20:197-205.
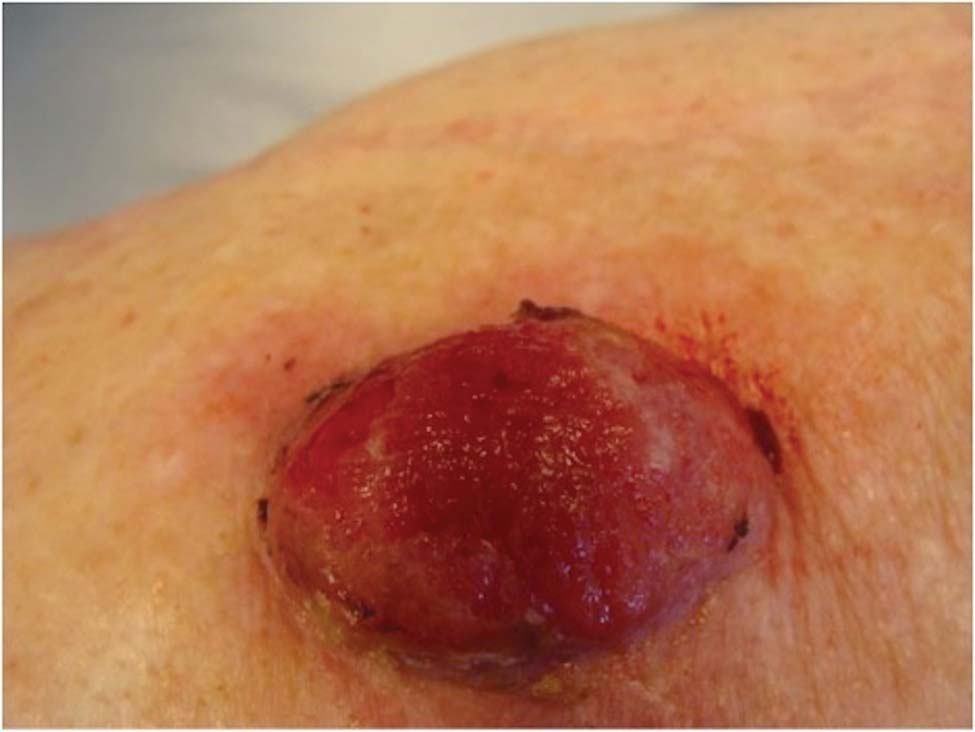
A 78-year-old woman presented with a large friable, sharply demarcated nodule of 3 months’ duration on the left side of the back. The lesion occasionally bled but was otherwise asymptomatic. There was no perilesional paresthesia. The patient’s medical history included hypertension, depression, chronic obstructive pulmonary disease, breast cancer, osteoporosis, and aortic valve disease.