User login
- Heartburn on 2 or more days a week warrants medical attention, as patients are likely to suffer from gastroesophageal reflux disease (GERD).Chronic GERD can lead to the development of complications including erosive esophagitis, stricture formation, and Barrett’s esophagus, which increases the risk of esophageal adenocarcinoma.
- A trial with a proton pump inhibitor (PPI) is the quickest and most cost-effective way to diagnose GERD, and is at least as sensitive as 24-hour intraesophageal pH monitoring.
- As PPIs only bind to actively secreting proton pumps, they should be dosed 30 to 60 minutes before a meal.Despite these recommendations, a recent survey of over 1000 US primary care physicians found that 36% instructed their patients to take a PPI with or after a meal or did not specify the timing of dosing.
- The patients who will have the best response to surgical therapy for GERD are those who had clearly documented acid reflux with typical symptoms, and who have responded to PPI treatment. Unfortunately, the same survey found that most physicians recommend antireflux surgery for patients in whom medical therapy has failed.
Gastroesophageal reflux disease (GERD) is a common, multifactorial condition that often results in decreased quality of life with interruptions of sleep, work, and social activities. Patients have reported that GERD affects emotional well-being to a greater degree than diabetes or hypertension.1,2 GERD is also associated with well-established complications, including Barrett’s esophagus. The role of reflux in carcinogenesis is controversial; the possibility of an association, however, implies that GERD should be treated aggressively and early.3
Symptoms of gerd
The typical symptoms of GERD are heartburn and regurgitation. Heartburn is best defined as a burning retrosternal discomfort starting in the epigastrium or lower chest and moving upwards towards the neck. Regurgitation is the effortless movement of gastric contents up into the esophagus or pharynx.
Most patients with GERD do not have endoscopically visible lesions; a careful analysis of symptoms generally forms the basis of a preliminary diagnosis.
The occurrence of heartburn on 2 or more days a week has been suggested as a basis for further investigation for GERD.4 However, symptoms vary greatly. Patients may be asymptomatic or experience symptoms that more closely resemble gastric disorders, infectious and motor disorders of the esophagus, biliary tract disease, or even coronary artery disease.
Extraesophageal manifestations
Adding to the complexity of diagnosis, GERD has been shown to have extraesophageal manifestations, including chronic cough, asthma, recurrent aspiration, chronic sore throat, reflux laryngitis, and paroxysmal laryngospasm or voice changes.
Although the relationship between asthma and GERD remains unclear, it has been estimated that 24% to 98% of patients with asthma also have GERD.5 Some patients with asthma have been shown to have excess acid reflux into the esophagus. Reflux-like symptoms may precede episodes of asthma that occur after meals or when lying down.6 8
Additionally, GERD has been noted in 10% to 50% of patients with non-cardiac chest pain.9,10
Diagnostic strategies
Trial of treatment
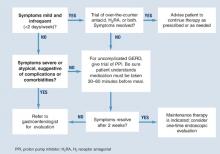
Diagnosis is usually based on typical symptoms—heartburn or regurgitation—in the clinical history. (The Figure shows a treatment algorithm for both severe and mild symptoms.)
A 2-week trial of treatment with a proton pump inhibitor (PPI) provides the quickest and most cost-effective confirmation of diagnosis and is recommended for the patient whose history suggests uncomplicated GERD. A positive response to PPI treatment in a patient with symptoms suggestive of GERD is at least as sensitive and specific as 24-hour intraesophageal pH monitoring, which is still often considered the “gold standard” for the diagnosis of GERD. Furthermore, complete lack of improvement in response to PPI treatment is highly predictive that the patient does not have GERD and indicates the need for further evaluation and a possible revision of diagnosis.11,12
H2 receptor antagonists (H2RAs) have also been investigated in empirical trials for usefulness in diagnosing GERD. H2RAs are less effective than PPIs.13,14
FIGURE Medical management of suspected GERD
Endoscopy
No data support routine endoscopy for patients with the recent onset of uncomplicated heartburn who respond to medical therapy. Endoscopy is recommended, however, for patients with severe or atypical GERD symptoms, when other diseases may be present, or when a treatment trial with a PPI is ineffective.15 Endoscopy is useful for diagnosing complications of GERD, such as Barrett’s esophagus, esophagitis, and strictures. Fewer than 50% of patients with GERD symptoms have evidence of esophagitis on endoscopy.16
The American Society for Gastrointestinal Endoscopy recommends endoscopy when there are clinical suggestions of severe reflux or other disease.17 The American College of Gastroenterology recommends further testing
- when empiric therapy has failed
- when symptoms of complicated disease exist
- when there is dysphagia, bleeding, weight loss, choking, chest pain, or long-standing symptoms
- when continuous therapy is required
- to screen for Barrett’s esophagus.18
The Canadian Consensus Conference recommends endoscopy in the presence of
- dysphagia
- odynophagia
- bleeding
- weight loss
- noncardiac chest pain
- failure to respond to 4 to 8 weeks of pharmacologic therapy.19
It also recommends a single test if maintenance therapy is required.
Other diagnostic tests
Other diagnostic tools may be of use in some settings.
A barium esophagram can document reflux, and Bernstein testing (esophageal acid infusion test) can identify esophageal hypersensitivity to acid, although neither establishes a diagnosis of GERD. Ambulatory 24-hour intraesophageal pH monitoring can help to establish the presence of GERD by documenting the proportion of time during which the intraesophageal pH is acidic (<4) and can also establish the degree of association between patients’ symptoms and episodes of esophageal acidification.
Esophageal manometry is not recommended as a routine diagnostic test for GERD. It is important in selected patients to exclude an esophageal motility disorder and may be necessary as part of the pre-operative evaluation for patients in whom a surgical operation for GERD is being considered.
Management of gerd
GERD commonly requires long-term management that includes dietary, lifestyle, and pharmacological interventions. Surgery may be considered for the long-term management of the condition in carefully selected patients.
Diet and lifestyle
Dietary modifications. Patients should not consume large meals and should avoid lying down for 3 to 4 hours after eating. Caffeinated products, peppermint, fatty foods, chocolate, spicy foods, citrus fruits and juices, tomato-based products, and alcohol may contribute to episodes of GERD.18,21 Lozenges of any kind are able to stimulate salivary secretion, help clear refluxed acid, and hence, help relieve symptoms.
Lifestyle modifications. Changes in lifestyle may include such seemingly sensible interventions as sleeping with the head elevated, stopping smoking, and losing weight. There is little or no established evidence for the efficacy of these and other lifestyle modifications in the management of GERD. However, in1 trial of 63 patients, elevating the head of the bed with 6-inch blocks resulted in 1 less episode of heartburn or acid regurgitation per night when compared with lying flat.22 In another trial of 71 patients with esophagitis, elevating the bed was nearly as effective as ranitidine for reducing symptoms and producing endoscopically verifiable healing.23
Arecent survey20 of 1046 primary care physicians found that:
- 36% instructed patients to take PPIs during or after a meal or did not specify a time of dosing
- 75% referred patients for surgical antireflux therapy and 20% referred patients directly to a surgeon without gastrointestinal consultation
- 15% reported that a trial with a H2 receptor antagonist was required by their healthsystem or insurance company prior to using a PPI.
Drug interventions
Pharmacological interventions include over-the-counter remedies such as antacids and H2RAs (Table 1), as well as prescription-only doses of H2RAs and PPIs. At the time of writing, no PPI was available in an over-the-counter preparation in the United States, although over-the-counter omeprazole may soon be approved. Many authorities believe an incremental approach to the management of GERD is appropriate, beginning with lifestyle modifications and over-the-counter preparations, continuing with H2 blockers, and reserving PPIs for nonresponders. While this approach may have appeal from a cost perspective, we believe another approach (as illustrated in the Figure) is clinically superior.
Antacids. Over-the-counter antacids rapidly increase the pH of the intraesophageal contents and also neutralize acidic gastric contents that might be refluxed. They are frequently used to treat heartburn. However, few clinical trials have evaluated the efficacy of antacids. Published trials24-26 are limited by small sample sizes and a lack of intention-to-treat analysis. Only 1 showed positive evidence for antacid efficacy.25
The utility of antacids is limited by the need for frequent dosing and possible interactions with such drugs as fluoroquinolones, tetracycline, and ferrous sulfate.27 Alginate/antacids have shown statistically significant benefit compared with placebo for relief of mild-to-moderate GERD symptoms and healing of esophagitis.24,28-34
H 2 receptor antagonists. H2RAs have shown positive effects on symptoms in some studies, although symptomatic response rates observed were only around 60% to 70%. Additionally, most of the trials to date have been for 2 to 6 weeks in duration.35 43 An issue worthy of consideration with the H2RAs is the development of tolerance with continuous use.44
An H2RA-antacid combination was recently evaluated in a trial that compared it with monotherapy using either agent. Of the patients receiving combination therapy, 81% reported an excellent or good symptom response. Those receiving famotidine or atacid alone reported a 72% excellent or good symptom response.3
Proton pump inhibitors. PPIs potently reduce gastric acid secretion by inhibiting the H+-K+adenosine triphosphatase pump of the parietal cell. As a result, they suppress gastric acid secretion for a longer period than H2RAs.45 Evidence from randomized, controlled trials has demonstrated the superiority of PPIs over any other class of drugs for the relief of GERD symptoms, for healing esophagitis, and for maintaining patients in remission. Standard doses of omeprazole, lansoprazole, panto-prazole, esomeprazole, and rabeprazole have, for the most part, shown comparable rates of healing and remission in erosive esophagitis.46-52
PPIs are best absorbed in the absence of food. Ingestion of food after a PPI stimulates parietal cell activity when blood levels of the PPI are increasing; this promotes uptake of the PPI by the parietal cells. Therefore, patients should be advised to take their PPI between 30 and 60 minutes before eating. For patients on a once-daily PPI, the best time to take it is about 30 to 60 minutes before breakfast. Despite these recommendations, a recent survey of over 1000 US primary care physicians found that 36% instructed their patients to take their PPI with or after a meal or did not specify the timing of dosing.53
Clinical evidence indicates that a trial with a PPI provides the quickest and most cost-effective method for diagnosing GERD. Despite this, many physicians use a trial of H2 receptor antagonists prior to initiation of PPI therapy.
- Clinicians should clearly instruct their patients regarding optimal timing of the dose, since this can have a significant effect on the success of therapy.
- Patients for whom antireflux surgery is being considered should first be referred for consultation with a gastroenterologist to assist in patient selection, to ensure that appropriate preoperative evaluation has been performed and to help exclude other possible causes of their symptoms.21,54
PPI therapy can be tailored to control GERD symptoms. Treatment can start with the most effective dosage and then be stepped down, or start with a minimum dosage and then be stepped up (Table 2). Patients with predominantly daytime symptoms should take PPIs before breakfast. Concerns that were once expressed about the long-term use of PPIs, such as predisposing patients to stomach cancer, have been refuted by extensive clinical experience and intensive monitoring (Table 3).3
TABLE 1
Over-the-counter therapy for GERD
|
| Adapted from Peterson, WL.GERD:Evidence-based therapeutic strategies. |
| Bethesda, Md.:American Gastroenterological Association;2002. |
TABLE 2
Step-down and step-up treatments: advantages and disadvantages
| Regimen | Advantages | Disadvantages |
|---|---|---|
| Step-down therapy (high-dose initial therapy) | Rapid symptom relief | Potential overtreatment |
| Efficient for physician | Higher initial drug cost | |
| Avoids overinvestigation and associated costs | ||
| Step-up therapy (minimum-dose initial therapy) | Avoids overtreatment | Patient may continue with symptoms unnecessarily |
| Lower initial drug cost | Inefficient for physician | |
| May lead to overinvestigation | ||
| Uncertain end point (partial symptom relief) | ||
| Adapted from Dent J, et al. Management of gastro-oesophageal reflux disease in general practice.BMJ 2001;322:344-347. | ||
TABLE 3
Potential concerns associated with the use of proton pump inhibitors
| Potential concern | Level of Evidence* | Grade † | Comments |
|---|---|---|---|
| Long-term PPI treatment may lead to reduced serum cobalamin levels | 2b | B | This is most likely to occur in individuals with atrophic gastritis |
| Increased acid output has been seen after stopping a PPI | 2b | B | Effects of PPI treatment on corpus glandular atrophy in H pylori-infected individuals are difficult to interpret due to possible sampling error and short study duration |
| PPI treatment may predispose to bacterial enteric infection | 3 | B | Only shown in a single case control study |
| *Level of evidence:1, Evidence for and/or general agreement that treatment is useful and effective;1a, systematic review with homogeneity of randomized controlled trials (RCTs);1b, individual RCTs (with narrow confidence interval);2, conflicting evidence and/or divergent opinion about efficacy and use;2a, evidence or opinion is in favor of treatment;2b, use and efficacy is less well established by evidence or opinion;3, evidence and/or general agreement that treatment is not useful or effective and may be harmful in some cases. | |||
| †Quality grading:A, well-designed, clinical trials;B, well-designed cohort or case-control studies;C, case reports, flawed trials;D, personal clinical experience;E, insufficient evidence to form opinion. | |||
| Adapted from Peterson, WL.GERD:Evidence-based therapeutic strategies. Bethesda, Md:American Gastroenterological Association, 2002. | |||
Surgery
Surgical antireflux therapy is an option in carefully selected patients. Those who respond best to surgical therapy will have had clearly documented acid reflux, typical symptoms, and symptomatic improvement while on PPI treatment.54
Unfortunately, a recent survey suggests that physicians tend to recommend surgery for patients in whom medical therapy has failed.53 However, patients who failed to respond to PPI therapy are unlikely to have GERD and, therefore, are highly unlikely to have a good outcome from antireflux surgery. Recent studies suggest that up to 62% of patients who have had open surgery for GERD continue to require medical treatment afterward. Although some studies demonstrate that surgery has greater efficacy over medical therapy initially, long-term follow-up has shown that surgically treated patients often need further medical therapy for persistent GERD symptoms.55 Community-based studies of antireflux surgery indicate that many patients develop new symptoms that they did not have before surgery and that these substantially diminish quality of life.
New endoscopic therapies, including radiofrequency energy delivery to the region of the lower esophageal sphincter and endoscopic suturing, have recently been approved for use by the FDA. This approval was based largely on safety rather than efficacy data. Clinical evidence is limited to uncontrolled studies in patients with no or mild esophagitis.3 These techniques should not be used in preference to established medical treatment unless and until data from randomized, controlled trials become available that demonstrate safety and efficacy.56
1. Dimenas E. Methodological aspects of evaluation of quality of life in upper gastrointestinal diseases. Scand J Gastroenterol Suppl 1993;199:18-21.
2. Revicki DA, Wood M, Maton PN, Sorensen S. The impact of gastroesophageal reflux disease on health-related quality of life. Am J Med 1998;104:252-8.
3. Peterson WL. GERD: Evidence-based therapeutic strategies. Bethesda, Md: American Gastroenterological Association; 2002.
4. Dent J, Jones R, Kahrilas P, Talley NJ. Management of gastro-oesophageal reflux disease in general practice. BMJ 2001;322:344-7.
5. Harding SM. Nocturnal asthma: role of gastroesophageal reflux. Chronobiol Int 1999;16:641-2.
6. Kjellen G, Wranne B. The prevalence of asymptomatic gastro-oesophageal relfux in adult patients with asthma. Eur J Respir Dis 1984;65:233.-
7. Schnatz PF, Castell JA, Castell DO. Pulmonary symptoms associated with gastroesophageal reflux: use of ambulatory pH monitoring to diagnose and to direct therapy. Am J Gastroenterol 1996;91:1715-8.
8. Sontag SJ, Schnell TG, Miller TQ, Khandelwal S, O’Connell S, Chejfec G, et al. Prevalence of oesophagitis in asthmatics. Gut 1992;33:872-6.
9. Schofield PM, Bennett DH, Whorwell PJ, Brooks NH, Bray CL, Ward C, et al. Exertional gastro-oesophageal reflux: a mechanism for symptoms in patients with angina pectoris and normal coronary angiograms. Br Med J (Clin Res Ed) 1987;294:1459-61.
10. Hewson EG, Sinclair JW, Dalton CB, Richter JE. Twenty-four-hour esophageal pH monitoring: the most useful test for evaluating noncardiac chest pain. Am J Med 1991;90:576-83.
11. Goyal RK. Diseases of the esophagus. In: Braunwald E, ed. Harrison’s principles of internal medicine. 15th ed. New York: McGraw-Hill; 2001;1642-9.
12. Schenk BE, Kuipers EJ, Klinkenberg-Knol EC, Festen HP, Jansen EH, Tuynman HA, et al. Omeprazole as a diagnostic tool in gastroesophageal reflux disease. Am J Gastroenterol 1997;92:1997-2000.
13. van Pinxteren B, Numans ME, Bonis PA, Lau J. Short-term treatment with proton pump inhibitors, H2-receptor antagonists and prokinetics for gastrooesophageal reflux disease-like symptoms and endoscopy negative reflux disease. Cochrane Database Syst Rev 2001;(4):CD002095.-
14. Brun J, Sorngard H. High dose proton pump inhibitor response as an initial strategy for a clinical diagnosis of gastro-oesophageal reflux disease (GERD).Swedish multi-centre group in primary health care. Fam Pract 2000;17:401-4.
15. Lundell L. Anti-reflux surgery in the laparoscopic era. Baillieres Best Pract Res Clin Gastroenterol 2000;14:793-810.
16. Chen MY, Ott DJ, Sinclair JW, Wu WC, Gelfand DW. Gastroesophageal reflux disease: correlation of esophageal pH testing and radiographic findings. Radiology 1992;185:483-6.
17. The role of endoscopy in the management of GERD: guidelines for clinical application. From the ASGE. American Society for Gastrointestinal Endoscopy. Gastrointest Endosc 1999;49:834-5.
18. DeVault KR, Castell DO. Updated guidelines for the diagnosis and treatment of gastroesophageal reflux disease. The Practice Parameters Committee of the American College of Gastroenterology. Am J Gastroenterol 1999;94:1434-42.
19. Beck IT, Champion MC, Lemire S, Thomson AB, Anvari M, Armstrong D, et al. The Second Canadian Consensus Conference on the Management of Patients with Gastroesophageal Reflux Disease. Can J Gastroenterol 1997;11(suppl B):7B-20B.
20. Chey WD, Inadomi JM, Booher AK, Fendrick AM. Primary care physicians’ perceptions and practices of the management of GERD: results of a national survey. Abstract presented at: DDW 2003; May 17-23, 2003; Orlando, Fla.
21. Fennerty MB, Castell D, Fendrick AM, Halpern M, Johnson D, Kahrilas PJ, et al. The diagnosis and treatment of gastroesophageal reflux disease in a managed care environment, Suggested disease management guidelines. Arch Intern Med 1996;156:477-84.
22. Stanciu C, Bennett JR. Effects of posture on gastro-oesophageal reflux. Digestion 1977;15:104-9.
23. Harvey RF, Gordon PC, Hadley N, Long DE, Gill TR, Macpherson RI, et al. Effects of sleeping with the bed-head raised and of ranitidine in patients with severe peptic oesophagitis. Lancet 1987;2:1200-3.
24. Graham DY, Patterson DJ. Double-blind comparison of liquid antacid and placebo in the treatment of symptomatic reflux esophagitis. Dig Dis Sci 1983;28:559-63.
25. Grove O, Bekker C, Jeppe-Hansen MG, Karstoft E, Sanchez G, Axelsson CK, et al. Ranitidine and high-dose antacid in reflux oesophagitis. A randomized, placebo-controlled trial. Scand J Gastroenterol 1985;20:457-61.
26. Weberg R, Berstad A. Symptomatic effect of a low-dose antacid regimen in reflux oesophagitis. Scand J Gastroenterol 1989;24:401-6.
27. Welage LS, Berardi RR. Evaluation of omeprazole, lansoprazole, pantopra-zole, and rabeprazole in the treatment of acid-related diseases. J Am Pharm Assoc (Wash) 2000;40:52-62.
28. Barnardo DE, Lancaster-Smith M, Strickland ID, Wright JT. A double-blind controlled trial of ‘Gaviscon’ in patients with symptomatic gastro-oesophageal reflux. Curr Med Res Opin 1975;3:388-91.
29. Beeley M, Warner JO. Medical treatment of symptomatic hiatus hernia with low-density compounds. Curr Med Res Opin 1972;1:63-9.
30. Chevrel B. A comparative crossover study on the treatment of heartburn and epigastric pain: liquid Gaviscon and a magnesium-aluminium antacid gel. J Int Med Res. 1980;8:300-2.
31. Laitinen S, Stahlberg M, Kairaluoma MI, Kiviniemi H, Paakkonen M, Lahtinen J, et al. Sucralfate and algi-nate/antacid in reflux esophagitis. Scand J Gastroenterol 1985;20:229-32.
32. Lanza FL, Smith V, Page-Castell JA, Castell DO. Effectiveness of foaming antacid in relieving induced heartburn. South Med J 1986;79:327-30.
33. McHardy G. A multicentric, randomized clinical trial of Gaviscon in reflux esophagitis. South Med J 1978;71(suppl 1):16-21.
34. Stanciu C, Bennett JR. Alginate-antacid in the reduction of gastro-oesophageal reflux. Lancet 1974;1:109-11.
35. Paul K, Redman CM, Chen M. Effectiveness and safety of nizatidine, 75 mg, for the relief of episodic heartburn. Aliment Pharmacol Ther 2001;15:1571-7.
36. Spiegel JE, Thoden WR, Pappas K, Fratarcangelo P, Furey SA. A double-blind, placebo-controlled study of the effectiveness and safety of nizatidine in the prevention of postprandial heartburn. Arch Intern Med 1997;157:1594-9.
37. Pappa KA, Buaron K, Payne JE, Sirgo MA, Giefer EE. An evaluation of increasing doses of ranitidine for treatment of heartburn. Aliment Pharmacol Ther 1999;13:475-81.
38. Pappa KA, Gooch WM, Buaron K, Payne JE, Giefer EE, Sirgo MA, et al. Low-dose ranitidine for the relief of heartburn. Aliment Pharmacol Ther 1999;13:459-65.
39. Pappa KA, Williams BO, Payne JE, Buaron KS, Mussari KL, Ciociola AA. A double-blind, placebo-controlled study of the efficacy and safety of non-pre-scription ranitidine 75 mg in the prevention of meal-induced heartburn. Aliment Pharmacol Ther 1999;13:467-73.
40. Ciociola AA, Pappa KA, Sirgo MA. Nonprescription doses of ranitidine are effective in the relief of episodic heartburn. Am J Ther 2001;8:399-408.
41. Gottlieb S, Decktor DL, Eckert JM, Simon TJ, Stauffer L, Ciccone PE. Efficacy and tolerability of famotidine in preventing heartburn and related symptoms of upper gastrointestinal discomfort. Am J Ther 1995;2:314-9.
42. Simon TJ, Berlin RG, Gardner AH, Stauffer LA, Gould AL, Getson AJ. Self-directed treatment of intermittent heartburn: a randomized, multicenter, double-blind, placebo-controlled evaluation of antacid and low doses of an H(2)-receptor antagonist (famotidine). Am J Ther 1995;2:304-13.
43. Galmiche JP, Shi G, Simon B, Casset-Semanza F, Slama A. On-demand treatment of gastro-oesophageal reflux symptoms: a comparison of ranitidine 75 mg with cimetidine 200 mg or place-bo. Aliment Pharmacol Ther 1998;12:909-17.
44. Qvigstad G, Arnestad JS, Brenna E, Waldum HL. Treatment with proton pump inhibitors induces tolerance to histamine-2 receptor antagonists in Helicobacter pylori-negative patients. Scand J Gastroenterol 1998;33:1244-8.
45. Howden CW. Optimizing the pharmacology of acid control in acid-related disorders. Am J Gastroenterol 1997;92(suppl):17S-21S.
46. Sharma VK, Leontiadis GI, Howden CW. Meta-analysis of randomized controlled trials comparing standard clinical doses of omeprazole and lansopra-zole in erosive oesophagitis. Aliment Pharmacol Ther 2001;15:227-31.
47. Edwards SJ, Lind T, Lundell L. Systematic review of proton pump inhibitors for the acute treatment of reflux oesophagitis. Aliment Pharmacol Ther 2001;15:1729-36.
48. Dupas JL, Houcke P, Samoyeau R. Pantoprazole versus lansoprazole in French patients with reflux esophagi-tis. Gastroenterol Clin Biol 2001;25:245-50.
49. Castell DO, Kahrilas PJ, Richter JE, Vakil NB, Johnson DA, Zuckerman S, et al. Esomeprazole (40 mg) compared with lansoprazole (30 mg) in the treatment of erosive esophagitis. Am J Gastroenterol 2002;97:575-83.
50. Howden CW, Ballard ED, Robison W. Evidence for therapeutic equivalence of lansoprazole 30 mg and esomeprazole 40 mg in the treatment of erosive oesophagitis. Clin Drug Invest 2002;22:99-109.
51. Thjodleifsson B, Beker JA, Dekkers C, Bjaaland T, Finnegan V, Humphries TJ. Rabeprazole versus omeprazole in preventing relapse of erosive or ulcerative gastroesophageal reflux disease: a dou-ble-blind, multicenter, European trial. The European Rabeprazole Study Group. Dig Dis Sci 2000;45:845-53.
52. Carling L, Axelsson CK, Forssell H, Stubberod A, Kraglund K, Bonnevie O, et al. Lansoprazole and omeprazole in the prevention of relapse of reflux oesophagitis: a long-term comparative study. Aliment Pharmacol Ther 1998;12:985-90.
53. Chey WD, Inadmoni JM, Boojer AK, Fendrick AM. What do primary care physicians think about Barrett’s esoph-agus, the relationship between GERD and H. pylori, and treatment of nocturnal heartburn? Abstract presented at: DDW 2003; May 17-23, 2003; Orlando, Fla.
54. Sampliner RE and The Practice Parameters Committee of the American College of Gastroenterology. Updated guidelines for the diagnosis, surveillance, and therapy of Barrett’s esophagus. Am J Gastroenterol 2002;97:1888-95.
55. Spechler SJ, Lee E, Ahnen D, Goyal RK, Hirano I, Ramirez F, et al. Long-term outcome of medical and surgical therapies for gastroesophageal reflux disease: follow-up of a randomized controlled trial. JAMA 2001;285:2331-8.
56. Katz PO. Gastroesophageal reflux disease: new treatments. Rev Gastroenterol Disord 2002;2:66-74.
- Heartburn on 2 or more days a week warrants medical attention, as patients are likely to suffer from gastroesophageal reflux disease (GERD).Chronic GERD can lead to the development of complications including erosive esophagitis, stricture formation, and Barrett’s esophagus, which increases the risk of esophageal adenocarcinoma.
- A trial with a proton pump inhibitor (PPI) is the quickest and most cost-effective way to diagnose GERD, and is at least as sensitive as 24-hour intraesophageal pH monitoring.
- As PPIs only bind to actively secreting proton pumps, they should be dosed 30 to 60 minutes before a meal.Despite these recommendations, a recent survey of over 1000 US primary care physicians found that 36% instructed their patients to take a PPI with or after a meal or did not specify the timing of dosing.
- The patients who will have the best response to surgical therapy for GERD are those who had clearly documented acid reflux with typical symptoms, and who have responded to PPI treatment. Unfortunately, the same survey found that most physicians recommend antireflux surgery for patients in whom medical therapy has failed.
Gastroesophageal reflux disease (GERD) is a common, multifactorial condition that often results in decreased quality of life with interruptions of sleep, work, and social activities. Patients have reported that GERD affects emotional well-being to a greater degree than diabetes or hypertension.1,2 GERD is also associated with well-established complications, including Barrett’s esophagus. The role of reflux in carcinogenesis is controversial; the possibility of an association, however, implies that GERD should be treated aggressively and early.3
Symptoms of gerd
The typical symptoms of GERD are heartburn and regurgitation. Heartburn is best defined as a burning retrosternal discomfort starting in the epigastrium or lower chest and moving upwards towards the neck. Regurgitation is the effortless movement of gastric contents up into the esophagus or pharynx.
Most patients with GERD do not have endoscopically visible lesions; a careful analysis of symptoms generally forms the basis of a preliminary diagnosis.
The occurrence of heartburn on 2 or more days a week has been suggested as a basis for further investigation for GERD.4 However, symptoms vary greatly. Patients may be asymptomatic or experience symptoms that more closely resemble gastric disorders, infectious and motor disorders of the esophagus, biliary tract disease, or even coronary artery disease.
Extraesophageal manifestations
Adding to the complexity of diagnosis, GERD has been shown to have extraesophageal manifestations, including chronic cough, asthma, recurrent aspiration, chronic sore throat, reflux laryngitis, and paroxysmal laryngospasm or voice changes.
Although the relationship between asthma and GERD remains unclear, it has been estimated that 24% to 98% of patients with asthma also have GERD.5 Some patients with asthma have been shown to have excess acid reflux into the esophagus. Reflux-like symptoms may precede episodes of asthma that occur after meals or when lying down.6 8
Additionally, GERD has been noted in 10% to 50% of patients with non-cardiac chest pain.9,10
Diagnostic strategies
Trial of treatment
Diagnosis is usually based on typical symptoms—heartburn or regurgitation—in the clinical history. (The Figure shows a treatment algorithm for both severe and mild symptoms.)
A 2-week trial of treatment with a proton pump inhibitor (PPI) provides the quickest and most cost-effective confirmation of diagnosis and is recommended for the patient whose history suggests uncomplicated GERD. A positive response to PPI treatment in a patient with symptoms suggestive of GERD is at least as sensitive and specific as 24-hour intraesophageal pH monitoring, which is still often considered the “gold standard” for the diagnosis of GERD. Furthermore, complete lack of improvement in response to PPI treatment is highly predictive that the patient does not have GERD and indicates the need for further evaluation and a possible revision of diagnosis.11,12
H2 receptor antagonists (H2RAs) have also been investigated in empirical trials for usefulness in diagnosing GERD. H2RAs are less effective than PPIs.13,14
FIGURE Medical management of suspected GERD
Endoscopy
No data support routine endoscopy for patients with the recent onset of uncomplicated heartburn who respond to medical therapy. Endoscopy is recommended, however, for patients with severe or atypical GERD symptoms, when other diseases may be present, or when a treatment trial with a PPI is ineffective.15 Endoscopy is useful for diagnosing complications of GERD, such as Barrett’s esophagus, esophagitis, and strictures. Fewer than 50% of patients with GERD symptoms have evidence of esophagitis on endoscopy.16
The American Society for Gastrointestinal Endoscopy recommends endoscopy when there are clinical suggestions of severe reflux or other disease.17 The American College of Gastroenterology recommends further testing
- when empiric therapy has failed
- when symptoms of complicated disease exist
- when there is dysphagia, bleeding, weight loss, choking, chest pain, or long-standing symptoms
- when continuous therapy is required
- to screen for Barrett’s esophagus.18
The Canadian Consensus Conference recommends endoscopy in the presence of
- dysphagia
- odynophagia
- bleeding
- weight loss
- noncardiac chest pain
- failure to respond to 4 to 8 weeks of pharmacologic therapy.19
It also recommends a single test if maintenance therapy is required.
Other diagnostic tests
Other diagnostic tools may be of use in some settings.
A barium esophagram can document reflux, and Bernstein testing (esophageal acid infusion test) can identify esophageal hypersensitivity to acid, although neither establishes a diagnosis of GERD. Ambulatory 24-hour intraesophageal pH monitoring can help to establish the presence of GERD by documenting the proportion of time during which the intraesophageal pH is acidic (<4) and can also establish the degree of association between patients’ symptoms and episodes of esophageal acidification.
Esophageal manometry is not recommended as a routine diagnostic test for GERD. It is important in selected patients to exclude an esophageal motility disorder and may be necessary as part of the pre-operative evaluation for patients in whom a surgical operation for GERD is being considered.
Management of gerd
GERD commonly requires long-term management that includes dietary, lifestyle, and pharmacological interventions. Surgery may be considered for the long-term management of the condition in carefully selected patients.
Diet and lifestyle
Dietary modifications. Patients should not consume large meals and should avoid lying down for 3 to 4 hours after eating. Caffeinated products, peppermint, fatty foods, chocolate, spicy foods, citrus fruits and juices, tomato-based products, and alcohol may contribute to episodes of GERD.18,21 Lozenges of any kind are able to stimulate salivary secretion, help clear refluxed acid, and hence, help relieve symptoms.
Lifestyle modifications. Changes in lifestyle may include such seemingly sensible interventions as sleeping with the head elevated, stopping smoking, and losing weight. There is little or no established evidence for the efficacy of these and other lifestyle modifications in the management of GERD. However, in1 trial of 63 patients, elevating the head of the bed with 6-inch blocks resulted in 1 less episode of heartburn or acid regurgitation per night when compared with lying flat.22 In another trial of 71 patients with esophagitis, elevating the bed was nearly as effective as ranitidine for reducing symptoms and producing endoscopically verifiable healing.23
Arecent survey20 of 1046 primary care physicians found that:
- 36% instructed patients to take PPIs during or after a meal or did not specify a time of dosing
- 75% referred patients for surgical antireflux therapy and 20% referred patients directly to a surgeon without gastrointestinal consultation
- 15% reported that a trial with a H2 receptor antagonist was required by their healthsystem or insurance company prior to using a PPI.
Drug interventions
Pharmacological interventions include over-the-counter remedies such as antacids and H2RAs (Table 1), as well as prescription-only doses of H2RAs and PPIs. At the time of writing, no PPI was available in an over-the-counter preparation in the United States, although over-the-counter omeprazole may soon be approved. Many authorities believe an incremental approach to the management of GERD is appropriate, beginning with lifestyle modifications and over-the-counter preparations, continuing with H2 blockers, and reserving PPIs for nonresponders. While this approach may have appeal from a cost perspective, we believe another approach (as illustrated in the Figure) is clinically superior.
Antacids. Over-the-counter antacids rapidly increase the pH of the intraesophageal contents and also neutralize acidic gastric contents that might be refluxed. They are frequently used to treat heartburn. However, few clinical trials have evaluated the efficacy of antacids. Published trials24-26 are limited by small sample sizes and a lack of intention-to-treat analysis. Only 1 showed positive evidence for antacid efficacy.25
The utility of antacids is limited by the need for frequent dosing and possible interactions with such drugs as fluoroquinolones, tetracycline, and ferrous sulfate.27 Alginate/antacids have shown statistically significant benefit compared with placebo for relief of mild-to-moderate GERD symptoms and healing of esophagitis.24,28-34
H 2 receptor antagonists. H2RAs have shown positive effects on symptoms in some studies, although symptomatic response rates observed were only around 60% to 70%. Additionally, most of the trials to date have been for 2 to 6 weeks in duration.35 43 An issue worthy of consideration with the H2RAs is the development of tolerance with continuous use.44
An H2RA-antacid combination was recently evaluated in a trial that compared it with monotherapy using either agent. Of the patients receiving combination therapy, 81% reported an excellent or good symptom response. Those receiving famotidine or atacid alone reported a 72% excellent or good symptom response.3
Proton pump inhibitors. PPIs potently reduce gastric acid secretion by inhibiting the H+-K+adenosine triphosphatase pump of the parietal cell. As a result, they suppress gastric acid secretion for a longer period than H2RAs.45 Evidence from randomized, controlled trials has demonstrated the superiority of PPIs over any other class of drugs for the relief of GERD symptoms, for healing esophagitis, and for maintaining patients in remission. Standard doses of omeprazole, lansoprazole, panto-prazole, esomeprazole, and rabeprazole have, for the most part, shown comparable rates of healing and remission in erosive esophagitis.46-52
PPIs are best absorbed in the absence of food. Ingestion of food after a PPI stimulates parietal cell activity when blood levels of the PPI are increasing; this promotes uptake of the PPI by the parietal cells. Therefore, patients should be advised to take their PPI between 30 and 60 minutes before eating. For patients on a once-daily PPI, the best time to take it is about 30 to 60 minutes before breakfast. Despite these recommendations, a recent survey of over 1000 US primary care physicians found that 36% instructed their patients to take their PPI with or after a meal or did not specify the timing of dosing.53
Clinical evidence indicates that a trial with a PPI provides the quickest and most cost-effective method for diagnosing GERD. Despite this, many physicians use a trial of H2 receptor antagonists prior to initiation of PPI therapy.
- Clinicians should clearly instruct their patients regarding optimal timing of the dose, since this can have a significant effect on the success of therapy.
- Patients for whom antireflux surgery is being considered should first be referred for consultation with a gastroenterologist to assist in patient selection, to ensure that appropriate preoperative evaluation has been performed and to help exclude other possible causes of their symptoms.21,54
PPI therapy can be tailored to control GERD symptoms. Treatment can start with the most effective dosage and then be stepped down, or start with a minimum dosage and then be stepped up (Table 2). Patients with predominantly daytime symptoms should take PPIs before breakfast. Concerns that were once expressed about the long-term use of PPIs, such as predisposing patients to stomach cancer, have been refuted by extensive clinical experience and intensive monitoring (Table 3).3
TABLE 1
Over-the-counter therapy for GERD
|
| Adapted from Peterson, WL.GERD:Evidence-based therapeutic strategies. |
| Bethesda, Md.:American Gastroenterological Association;2002. |
TABLE 2
Step-down and step-up treatments: advantages and disadvantages
| Regimen | Advantages | Disadvantages |
|---|---|---|
| Step-down therapy (high-dose initial therapy) | Rapid symptom relief | Potential overtreatment |
| Efficient for physician | Higher initial drug cost | |
| Avoids overinvestigation and associated costs | ||
| Step-up therapy (minimum-dose initial therapy) | Avoids overtreatment | Patient may continue with symptoms unnecessarily |
| Lower initial drug cost | Inefficient for physician | |
| May lead to overinvestigation | ||
| Uncertain end point (partial symptom relief) | ||
| Adapted from Dent J, et al. Management of gastro-oesophageal reflux disease in general practice.BMJ 2001;322:344-347. | ||
TABLE 3
Potential concerns associated with the use of proton pump inhibitors
| Potential concern | Level of Evidence* | Grade † | Comments |
|---|---|---|---|
| Long-term PPI treatment may lead to reduced serum cobalamin levels | 2b | B | This is most likely to occur in individuals with atrophic gastritis |
| Increased acid output has been seen after stopping a PPI | 2b | B | Effects of PPI treatment on corpus glandular atrophy in H pylori-infected individuals are difficult to interpret due to possible sampling error and short study duration |
| PPI treatment may predispose to bacterial enteric infection | 3 | B | Only shown in a single case control study |
| *Level of evidence:1, Evidence for and/or general agreement that treatment is useful and effective;1a, systematic review with homogeneity of randomized controlled trials (RCTs);1b, individual RCTs (with narrow confidence interval);2, conflicting evidence and/or divergent opinion about efficacy and use;2a, evidence or opinion is in favor of treatment;2b, use and efficacy is less well established by evidence or opinion;3, evidence and/or general agreement that treatment is not useful or effective and may be harmful in some cases. | |||
| †Quality grading:A, well-designed, clinical trials;B, well-designed cohort or case-control studies;C, case reports, flawed trials;D, personal clinical experience;E, insufficient evidence to form opinion. | |||
| Adapted from Peterson, WL.GERD:Evidence-based therapeutic strategies. Bethesda, Md:American Gastroenterological Association, 2002. | |||
Surgery
Surgical antireflux therapy is an option in carefully selected patients. Those who respond best to surgical therapy will have had clearly documented acid reflux, typical symptoms, and symptomatic improvement while on PPI treatment.54
Unfortunately, a recent survey suggests that physicians tend to recommend surgery for patients in whom medical therapy has failed.53 However, patients who failed to respond to PPI therapy are unlikely to have GERD and, therefore, are highly unlikely to have a good outcome from antireflux surgery. Recent studies suggest that up to 62% of patients who have had open surgery for GERD continue to require medical treatment afterward. Although some studies demonstrate that surgery has greater efficacy over medical therapy initially, long-term follow-up has shown that surgically treated patients often need further medical therapy for persistent GERD symptoms.55 Community-based studies of antireflux surgery indicate that many patients develop new symptoms that they did not have before surgery and that these substantially diminish quality of life.
New endoscopic therapies, including radiofrequency energy delivery to the region of the lower esophageal sphincter and endoscopic suturing, have recently been approved for use by the FDA. This approval was based largely on safety rather than efficacy data. Clinical evidence is limited to uncontrolled studies in patients with no or mild esophagitis.3 These techniques should not be used in preference to established medical treatment unless and until data from randomized, controlled trials become available that demonstrate safety and efficacy.56
- Heartburn on 2 or more days a week warrants medical attention, as patients are likely to suffer from gastroesophageal reflux disease (GERD).Chronic GERD can lead to the development of complications including erosive esophagitis, stricture formation, and Barrett’s esophagus, which increases the risk of esophageal adenocarcinoma.
- A trial with a proton pump inhibitor (PPI) is the quickest and most cost-effective way to diagnose GERD, and is at least as sensitive as 24-hour intraesophageal pH monitoring.
- As PPIs only bind to actively secreting proton pumps, they should be dosed 30 to 60 minutes before a meal.Despite these recommendations, a recent survey of over 1000 US primary care physicians found that 36% instructed their patients to take a PPI with or after a meal or did not specify the timing of dosing.
- The patients who will have the best response to surgical therapy for GERD are those who had clearly documented acid reflux with typical symptoms, and who have responded to PPI treatment. Unfortunately, the same survey found that most physicians recommend antireflux surgery for patients in whom medical therapy has failed.
Gastroesophageal reflux disease (GERD) is a common, multifactorial condition that often results in decreased quality of life with interruptions of sleep, work, and social activities. Patients have reported that GERD affects emotional well-being to a greater degree than diabetes or hypertension.1,2 GERD is also associated with well-established complications, including Barrett’s esophagus. The role of reflux in carcinogenesis is controversial; the possibility of an association, however, implies that GERD should be treated aggressively and early.3
Symptoms of gerd
The typical symptoms of GERD are heartburn and regurgitation. Heartburn is best defined as a burning retrosternal discomfort starting in the epigastrium or lower chest and moving upwards towards the neck. Regurgitation is the effortless movement of gastric contents up into the esophagus or pharynx.
Most patients with GERD do not have endoscopically visible lesions; a careful analysis of symptoms generally forms the basis of a preliminary diagnosis.
The occurrence of heartburn on 2 or more days a week has been suggested as a basis for further investigation for GERD.4 However, symptoms vary greatly. Patients may be asymptomatic or experience symptoms that more closely resemble gastric disorders, infectious and motor disorders of the esophagus, biliary tract disease, or even coronary artery disease.
Extraesophageal manifestations
Adding to the complexity of diagnosis, GERD has been shown to have extraesophageal manifestations, including chronic cough, asthma, recurrent aspiration, chronic sore throat, reflux laryngitis, and paroxysmal laryngospasm or voice changes.
Although the relationship between asthma and GERD remains unclear, it has been estimated that 24% to 98% of patients with asthma also have GERD.5 Some patients with asthma have been shown to have excess acid reflux into the esophagus. Reflux-like symptoms may precede episodes of asthma that occur after meals or when lying down.6 8
Additionally, GERD has been noted in 10% to 50% of patients with non-cardiac chest pain.9,10
Diagnostic strategies
Trial of treatment
Diagnosis is usually based on typical symptoms—heartburn or regurgitation—in the clinical history. (The Figure shows a treatment algorithm for both severe and mild symptoms.)
A 2-week trial of treatment with a proton pump inhibitor (PPI) provides the quickest and most cost-effective confirmation of diagnosis and is recommended for the patient whose history suggests uncomplicated GERD. A positive response to PPI treatment in a patient with symptoms suggestive of GERD is at least as sensitive and specific as 24-hour intraesophageal pH monitoring, which is still often considered the “gold standard” for the diagnosis of GERD. Furthermore, complete lack of improvement in response to PPI treatment is highly predictive that the patient does not have GERD and indicates the need for further evaluation and a possible revision of diagnosis.11,12
H2 receptor antagonists (H2RAs) have also been investigated in empirical trials for usefulness in diagnosing GERD. H2RAs are less effective than PPIs.13,14
FIGURE Medical management of suspected GERD
Endoscopy
No data support routine endoscopy for patients with the recent onset of uncomplicated heartburn who respond to medical therapy. Endoscopy is recommended, however, for patients with severe or atypical GERD symptoms, when other diseases may be present, or when a treatment trial with a PPI is ineffective.15 Endoscopy is useful for diagnosing complications of GERD, such as Barrett’s esophagus, esophagitis, and strictures. Fewer than 50% of patients with GERD symptoms have evidence of esophagitis on endoscopy.16
The American Society for Gastrointestinal Endoscopy recommends endoscopy when there are clinical suggestions of severe reflux or other disease.17 The American College of Gastroenterology recommends further testing
- when empiric therapy has failed
- when symptoms of complicated disease exist
- when there is dysphagia, bleeding, weight loss, choking, chest pain, or long-standing symptoms
- when continuous therapy is required
- to screen for Barrett’s esophagus.18
The Canadian Consensus Conference recommends endoscopy in the presence of
- dysphagia
- odynophagia
- bleeding
- weight loss
- noncardiac chest pain
- failure to respond to 4 to 8 weeks of pharmacologic therapy.19
It also recommends a single test if maintenance therapy is required.
Other diagnostic tests
Other diagnostic tools may be of use in some settings.
A barium esophagram can document reflux, and Bernstein testing (esophageal acid infusion test) can identify esophageal hypersensitivity to acid, although neither establishes a diagnosis of GERD. Ambulatory 24-hour intraesophageal pH monitoring can help to establish the presence of GERD by documenting the proportion of time during which the intraesophageal pH is acidic (<4) and can also establish the degree of association between patients’ symptoms and episodes of esophageal acidification.
Esophageal manometry is not recommended as a routine diagnostic test for GERD. It is important in selected patients to exclude an esophageal motility disorder and may be necessary as part of the pre-operative evaluation for patients in whom a surgical operation for GERD is being considered.
Management of gerd
GERD commonly requires long-term management that includes dietary, lifestyle, and pharmacological interventions. Surgery may be considered for the long-term management of the condition in carefully selected patients.
Diet and lifestyle
Dietary modifications. Patients should not consume large meals and should avoid lying down for 3 to 4 hours after eating. Caffeinated products, peppermint, fatty foods, chocolate, spicy foods, citrus fruits and juices, tomato-based products, and alcohol may contribute to episodes of GERD.18,21 Lozenges of any kind are able to stimulate salivary secretion, help clear refluxed acid, and hence, help relieve symptoms.
Lifestyle modifications. Changes in lifestyle may include such seemingly sensible interventions as sleeping with the head elevated, stopping smoking, and losing weight. There is little or no established evidence for the efficacy of these and other lifestyle modifications in the management of GERD. However, in1 trial of 63 patients, elevating the head of the bed with 6-inch blocks resulted in 1 less episode of heartburn or acid regurgitation per night when compared with lying flat.22 In another trial of 71 patients with esophagitis, elevating the bed was nearly as effective as ranitidine for reducing symptoms and producing endoscopically verifiable healing.23
Arecent survey20 of 1046 primary care physicians found that:
- 36% instructed patients to take PPIs during or after a meal or did not specify a time of dosing
- 75% referred patients for surgical antireflux therapy and 20% referred patients directly to a surgeon without gastrointestinal consultation
- 15% reported that a trial with a H2 receptor antagonist was required by their healthsystem or insurance company prior to using a PPI.
Drug interventions
Pharmacological interventions include over-the-counter remedies such as antacids and H2RAs (Table 1), as well as prescription-only doses of H2RAs and PPIs. At the time of writing, no PPI was available in an over-the-counter preparation in the United States, although over-the-counter omeprazole may soon be approved. Many authorities believe an incremental approach to the management of GERD is appropriate, beginning with lifestyle modifications and over-the-counter preparations, continuing with H2 blockers, and reserving PPIs for nonresponders. While this approach may have appeal from a cost perspective, we believe another approach (as illustrated in the Figure) is clinically superior.
Antacids. Over-the-counter antacids rapidly increase the pH of the intraesophageal contents and also neutralize acidic gastric contents that might be refluxed. They are frequently used to treat heartburn. However, few clinical trials have evaluated the efficacy of antacids. Published trials24-26 are limited by small sample sizes and a lack of intention-to-treat analysis. Only 1 showed positive evidence for antacid efficacy.25
The utility of antacids is limited by the need for frequent dosing and possible interactions with such drugs as fluoroquinolones, tetracycline, and ferrous sulfate.27 Alginate/antacids have shown statistically significant benefit compared with placebo for relief of mild-to-moderate GERD symptoms and healing of esophagitis.24,28-34
H 2 receptor antagonists. H2RAs have shown positive effects on symptoms in some studies, although symptomatic response rates observed were only around 60% to 70%. Additionally, most of the trials to date have been for 2 to 6 weeks in duration.35 43 An issue worthy of consideration with the H2RAs is the development of tolerance with continuous use.44
An H2RA-antacid combination was recently evaluated in a trial that compared it with monotherapy using either agent. Of the patients receiving combination therapy, 81% reported an excellent or good symptom response. Those receiving famotidine or atacid alone reported a 72% excellent or good symptom response.3
Proton pump inhibitors. PPIs potently reduce gastric acid secretion by inhibiting the H+-K+adenosine triphosphatase pump of the parietal cell. As a result, they suppress gastric acid secretion for a longer period than H2RAs.45 Evidence from randomized, controlled trials has demonstrated the superiority of PPIs over any other class of drugs for the relief of GERD symptoms, for healing esophagitis, and for maintaining patients in remission. Standard doses of omeprazole, lansoprazole, panto-prazole, esomeprazole, and rabeprazole have, for the most part, shown comparable rates of healing and remission in erosive esophagitis.46-52
PPIs are best absorbed in the absence of food. Ingestion of food after a PPI stimulates parietal cell activity when blood levels of the PPI are increasing; this promotes uptake of the PPI by the parietal cells. Therefore, patients should be advised to take their PPI between 30 and 60 minutes before eating. For patients on a once-daily PPI, the best time to take it is about 30 to 60 minutes before breakfast. Despite these recommendations, a recent survey of over 1000 US primary care physicians found that 36% instructed their patients to take their PPI with or after a meal or did not specify the timing of dosing.53
Clinical evidence indicates that a trial with a PPI provides the quickest and most cost-effective method for diagnosing GERD. Despite this, many physicians use a trial of H2 receptor antagonists prior to initiation of PPI therapy.
- Clinicians should clearly instruct their patients regarding optimal timing of the dose, since this can have a significant effect on the success of therapy.
- Patients for whom antireflux surgery is being considered should first be referred for consultation with a gastroenterologist to assist in patient selection, to ensure that appropriate preoperative evaluation has been performed and to help exclude other possible causes of their symptoms.21,54
PPI therapy can be tailored to control GERD symptoms. Treatment can start with the most effective dosage and then be stepped down, or start with a minimum dosage and then be stepped up (Table 2). Patients with predominantly daytime symptoms should take PPIs before breakfast. Concerns that were once expressed about the long-term use of PPIs, such as predisposing patients to stomach cancer, have been refuted by extensive clinical experience and intensive monitoring (Table 3).3
TABLE 1
Over-the-counter therapy for GERD
|
| Adapted from Peterson, WL.GERD:Evidence-based therapeutic strategies. |
| Bethesda, Md.:American Gastroenterological Association;2002. |
TABLE 2
Step-down and step-up treatments: advantages and disadvantages
| Regimen | Advantages | Disadvantages |
|---|---|---|
| Step-down therapy (high-dose initial therapy) | Rapid symptom relief | Potential overtreatment |
| Efficient for physician | Higher initial drug cost | |
| Avoids overinvestigation and associated costs | ||
| Step-up therapy (minimum-dose initial therapy) | Avoids overtreatment | Patient may continue with symptoms unnecessarily |
| Lower initial drug cost | Inefficient for physician | |
| May lead to overinvestigation | ||
| Uncertain end point (partial symptom relief) | ||
| Adapted from Dent J, et al. Management of gastro-oesophageal reflux disease in general practice.BMJ 2001;322:344-347. | ||
TABLE 3
Potential concerns associated with the use of proton pump inhibitors
| Potential concern | Level of Evidence* | Grade † | Comments |
|---|---|---|---|
| Long-term PPI treatment may lead to reduced serum cobalamin levels | 2b | B | This is most likely to occur in individuals with atrophic gastritis |
| Increased acid output has been seen after stopping a PPI | 2b | B | Effects of PPI treatment on corpus glandular atrophy in H pylori-infected individuals are difficult to interpret due to possible sampling error and short study duration |
| PPI treatment may predispose to bacterial enteric infection | 3 | B | Only shown in a single case control study |
| *Level of evidence:1, Evidence for and/or general agreement that treatment is useful and effective;1a, systematic review with homogeneity of randomized controlled trials (RCTs);1b, individual RCTs (with narrow confidence interval);2, conflicting evidence and/or divergent opinion about efficacy and use;2a, evidence or opinion is in favor of treatment;2b, use and efficacy is less well established by evidence or opinion;3, evidence and/or general agreement that treatment is not useful or effective and may be harmful in some cases. | |||
| †Quality grading:A, well-designed, clinical trials;B, well-designed cohort or case-control studies;C, case reports, flawed trials;D, personal clinical experience;E, insufficient evidence to form opinion. | |||
| Adapted from Peterson, WL.GERD:Evidence-based therapeutic strategies. Bethesda, Md:American Gastroenterological Association, 2002. | |||
Surgery
Surgical antireflux therapy is an option in carefully selected patients. Those who respond best to surgical therapy will have had clearly documented acid reflux, typical symptoms, and symptomatic improvement while on PPI treatment.54
Unfortunately, a recent survey suggests that physicians tend to recommend surgery for patients in whom medical therapy has failed.53 However, patients who failed to respond to PPI therapy are unlikely to have GERD and, therefore, are highly unlikely to have a good outcome from antireflux surgery. Recent studies suggest that up to 62% of patients who have had open surgery for GERD continue to require medical treatment afterward. Although some studies demonstrate that surgery has greater efficacy over medical therapy initially, long-term follow-up has shown that surgically treated patients often need further medical therapy for persistent GERD symptoms.55 Community-based studies of antireflux surgery indicate that many patients develop new symptoms that they did not have before surgery and that these substantially diminish quality of life.
New endoscopic therapies, including radiofrequency energy delivery to the region of the lower esophageal sphincter and endoscopic suturing, have recently been approved for use by the FDA. This approval was based largely on safety rather than efficacy data. Clinical evidence is limited to uncontrolled studies in patients with no or mild esophagitis.3 These techniques should not be used in preference to established medical treatment unless and until data from randomized, controlled trials become available that demonstrate safety and efficacy.56
1. Dimenas E. Methodological aspects of evaluation of quality of life in upper gastrointestinal diseases. Scand J Gastroenterol Suppl 1993;199:18-21.
2. Revicki DA, Wood M, Maton PN, Sorensen S. The impact of gastroesophageal reflux disease on health-related quality of life. Am J Med 1998;104:252-8.
3. Peterson WL. GERD: Evidence-based therapeutic strategies. Bethesda, Md: American Gastroenterological Association; 2002.
4. Dent J, Jones R, Kahrilas P, Talley NJ. Management of gastro-oesophageal reflux disease in general practice. BMJ 2001;322:344-7.
5. Harding SM. Nocturnal asthma: role of gastroesophageal reflux. Chronobiol Int 1999;16:641-2.
6. Kjellen G, Wranne B. The prevalence of asymptomatic gastro-oesophageal relfux in adult patients with asthma. Eur J Respir Dis 1984;65:233.-
7. Schnatz PF, Castell JA, Castell DO. Pulmonary symptoms associated with gastroesophageal reflux: use of ambulatory pH monitoring to diagnose and to direct therapy. Am J Gastroenterol 1996;91:1715-8.
8. Sontag SJ, Schnell TG, Miller TQ, Khandelwal S, O’Connell S, Chejfec G, et al. Prevalence of oesophagitis in asthmatics. Gut 1992;33:872-6.
9. Schofield PM, Bennett DH, Whorwell PJ, Brooks NH, Bray CL, Ward C, et al. Exertional gastro-oesophageal reflux: a mechanism for symptoms in patients with angina pectoris and normal coronary angiograms. Br Med J (Clin Res Ed) 1987;294:1459-61.
10. Hewson EG, Sinclair JW, Dalton CB, Richter JE. Twenty-four-hour esophageal pH monitoring: the most useful test for evaluating noncardiac chest pain. Am J Med 1991;90:576-83.
11. Goyal RK. Diseases of the esophagus. In: Braunwald E, ed. Harrison’s principles of internal medicine. 15th ed. New York: McGraw-Hill; 2001;1642-9.
12. Schenk BE, Kuipers EJ, Klinkenberg-Knol EC, Festen HP, Jansen EH, Tuynman HA, et al. Omeprazole as a diagnostic tool in gastroesophageal reflux disease. Am J Gastroenterol 1997;92:1997-2000.
13. van Pinxteren B, Numans ME, Bonis PA, Lau J. Short-term treatment with proton pump inhibitors, H2-receptor antagonists and prokinetics for gastrooesophageal reflux disease-like symptoms and endoscopy negative reflux disease. Cochrane Database Syst Rev 2001;(4):CD002095.-
14. Brun J, Sorngard H. High dose proton pump inhibitor response as an initial strategy for a clinical diagnosis of gastro-oesophageal reflux disease (GERD).Swedish multi-centre group in primary health care. Fam Pract 2000;17:401-4.
15. Lundell L. Anti-reflux surgery in the laparoscopic era. Baillieres Best Pract Res Clin Gastroenterol 2000;14:793-810.
16. Chen MY, Ott DJ, Sinclair JW, Wu WC, Gelfand DW. Gastroesophageal reflux disease: correlation of esophageal pH testing and radiographic findings. Radiology 1992;185:483-6.
17. The role of endoscopy in the management of GERD: guidelines for clinical application. From the ASGE. American Society for Gastrointestinal Endoscopy. Gastrointest Endosc 1999;49:834-5.
18. DeVault KR, Castell DO. Updated guidelines for the diagnosis and treatment of gastroesophageal reflux disease. The Practice Parameters Committee of the American College of Gastroenterology. Am J Gastroenterol 1999;94:1434-42.
19. Beck IT, Champion MC, Lemire S, Thomson AB, Anvari M, Armstrong D, et al. The Second Canadian Consensus Conference on the Management of Patients with Gastroesophageal Reflux Disease. Can J Gastroenterol 1997;11(suppl B):7B-20B.
20. Chey WD, Inadomi JM, Booher AK, Fendrick AM. Primary care physicians’ perceptions and practices of the management of GERD: results of a national survey. Abstract presented at: DDW 2003; May 17-23, 2003; Orlando, Fla.
21. Fennerty MB, Castell D, Fendrick AM, Halpern M, Johnson D, Kahrilas PJ, et al. The diagnosis and treatment of gastroesophageal reflux disease in a managed care environment, Suggested disease management guidelines. Arch Intern Med 1996;156:477-84.
22. Stanciu C, Bennett JR. Effects of posture on gastro-oesophageal reflux. Digestion 1977;15:104-9.
23. Harvey RF, Gordon PC, Hadley N, Long DE, Gill TR, Macpherson RI, et al. Effects of sleeping with the bed-head raised and of ranitidine in patients with severe peptic oesophagitis. Lancet 1987;2:1200-3.
24. Graham DY, Patterson DJ. Double-blind comparison of liquid antacid and placebo in the treatment of symptomatic reflux esophagitis. Dig Dis Sci 1983;28:559-63.
25. Grove O, Bekker C, Jeppe-Hansen MG, Karstoft E, Sanchez G, Axelsson CK, et al. Ranitidine and high-dose antacid in reflux oesophagitis. A randomized, placebo-controlled trial. Scand J Gastroenterol 1985;20:457-61.
26. Weberg R, Berstad A. Symptomatic effect of a low-dose antacid regimen in reflux oesophagitis. Scand J Gastroenterol 1989;24:401-6.
27. Welage LS, Berardi RR. Evaluation of omeprazole, lansoprazole, pantopra-zole, and rabeprazole in the treatment of acid-related diseases. J Am Pharm Assoc (Wash) 2000;40:52-62.
28. Barnardo DE, Lancaster-Smith M, Strickland ID, Wright JT. A double-blind controlled trial of ‘Gaviscon’ in patients with symptomatic gastro-oesophageal reflux. Curr Med Res Opin 1975;3:388-91.
29. Beeley M, Warner JO. Medical treatment of symptomatic hiatus hernia with low-density compounds. Curr Med Res Opin 1972;1:63-9.
30. Chevrel B. A comparative crossover study on the treatment of heartburn and epigastric pain: liquid Gaviscon and a magnesium-aluminium antacid gel. J Int Med Res. 1980;8:300-2.
31. Laitinen S, Stahlberg M, Kairaluoma MI, Kiviniemi H, Paakkonen M, Lahtinen J, et al. Sucralfate and algi-nate/antacid in reflux esophagitis. Scand J Gastroenterol 1985;20:229-32.
32. Lanza FL, Smith V, Page-Castell JA, Castell DO. Effectiveness of foaming antacid in relieving induced heartburn. South Med J 1986;79:327-30.
33. McHardy G. A multicentric, randomized clinical trial of Gaviscon in reflux esophagitis. South Med J 1978;71(suppl 1):16-21.
34. Stanciu C, Bennett JR. Alginate-antacid in the reduction of gastro-oesophageal reflux. Lancet 1974;1:109-11.
35. Paul K, Redman CM, Chen M. Effectiveness and safety of nizatidine, 75 mg, for the relief of episodic heartburn. Aliment Pharmacol Ther 2001;15:1571-7.
36. Spiegel JE, Thoden WR, Pappas K, Fratarcangelo P, Furey SA. A double-blind, placebo-controlled study of the effectiveness and safety of nizatidine in the prevention of postprandial heartburn. Arch Intern Med 1997;157:1594-9.
37. Pappa KA, Buaron K, Payne JE, Sirgo MA, Giefer EE. An evaluation of increasing doses of ranitidine for treatment of heartburn. Aliment Pharmacol Ther 1999;13:475-81.
38. Pappa KA, Gooch WM, Buaron K, Payne JE, Giefer EE, Sirgo MA, et al. Low-dose ranitidine for the relief of heartburn. Aliment Pharmacol Ther 1999;13:459-65.
39. Pappa KA, Williams BO, Payne JE, Buaron KS, Mussari KL, Ciociola AA. A double-blind, placebo-controlled study of the efficacy and safety of non-pre-scription ranitidine 75 mg in the prevention of meal-induced heartburn. Aliment Pharmacol Ther 1999;13:467-73.
40. Ciociola AA, Pappa KA, Sirgo MA. Nonprescription doses of ranitidine are effective in the relief of episodic heartburn. Am J Ther 2001;8:399-408.
41. Gottlieb S, Decktor DL, Eckert JM, Simon TJ, Stauffer L, Ciccone PE. Efficacy and tolerability of famotidine in preventing heartburn and related symptoms of upper gastrointestinal discomfort. Am J Ther 1995;2:314-9.
42. Simon TJ, Berlin RG, Gardner AH, Stauffer LA, Gould AL, Getson AJ. Self-directed treatment of intermittent heartburn: a randomized, multicenter, double-blind, placebo-controlled evaluation of antacid and low doses of an H(2)-receptor antagonist (famotidine). Am J Ther 1995;2:304-13.
43. Galmiche JP, Shi G, Simon B, Casset-Semanza F, Slama A. On-demand treatment of gastro-oesophageal reflux symptoms: a comparison of ranitidine 75 mg with cimetidine 200 mg or place-bo. Aliment Pharmacol Ther 1998;12:909-17.
44. Qvigstad G, Arnestad JS, Brenna E, Waldum HL. Treatment with proton pump inhibitors induces tolerance to histamine-2 receptor antagonists in Helicobacter pylori-negative patients. Scand J Gastroenterol 1998;33:1244-8.
45. Howden CW. Optimizing the pharmacology of acid control in acid-related disorders. Am J Gastroenterol 1997;92(suppl):17S-21S.
46. Sharma VK, Leontiadis GI, Howden CW. Meta-analysis of randomized controlled trials comparing standard clinical doses of omeprazole and lansopra-zole in erosive oesophagitis. Aliment Pharmacol Ther 2001;15:227-31.
47. Edwards SJ, Lind T, Lundell L. Systematic review of proton pump inhibitors for the acute treatment of reflux oesophagitis. Aliment Pharmacol Ther 2001;15:1729-36.
48. Dupas JL, Houcke P, Samoyeau R. Pantoprazole versus lansoprazole in French patients with reflux esophagi-tis. Gastroenterol Clin Biol 2001;25:245-50.
49. Castell DO, Kahrilas PJ, Richter JE, Vakil NB, Johnson DA, Zuckerman S, et al. Esomeprazole (40 mg) compared with lansoprazole (30 mg) in the treatment of erosive esophagitis. Am J Gastroenterol 2002;97:575-83.
50. Howden CW, Ballard ED, Robison W. Evidence for therapeutic equivalence of lansoprazole 30 mg and esomeprazole 40 mg in the treatment of erosive oesophagitis. Clin Drug Invest 2002;22:99-109.
51. Thjodleifsson B, Beker JA, Dekkers C, Bjaaland T, Finnegan V, Humphries TJ. Rabeprazole versus omeprazole in preventing relapse of erosive or ulcerative gastroesophageal reflux disease: a dou-ble-blind, multicenter, European trial. The European Rabeprazole Study Group. Dig Dis Sci 2000;45:845-53.
52. Carling L, Axelsson CK, Forssell H, Stubberod A, Kraglund K, Bonnevie O, et al. Lansoprazole and omeprazole in the prevention of relapse of reflux oesophagitis: a long-term comparative study. Aliment Pharmacol Ther 1998;12:985-90.
53. Chey WD, Inadmoni JM, Boojer AK, Fendrick AM. What do primary care physicians think about Barrett’s esoph-agus, the relationship between GERD and H. pylori, and treatment of nocturnal heartburn? Abstract presented at: DDW 2003; May 17-23, 2003; Orlando, Fla.
54. Sampliner RE and The Practice Parameters Committee of the American College of Gastroenterology. Updated guidelines for the diagnosis, surveillance, and therapy of Barrett’s esophagus. Am J Gastroenterol 2002;97:1888-95.
55. Spechler SJ, Lee E, Ahnen D, Goyal RK, Hirano I, Ramirez F, et al. Long-term outcome of medical and surgical therapies for gastroesophageal reflux disease: follow-up of a randomized controlled trial. JAMA 2001;285:2331-8.
56. Katz PO. Gastroesophageal reflux disease: new treatments. Rev Gastroenterol Disord 2002;2:66-74.
1. Dimenas E. Methodological aspects of evaluation of quality of life in upper gastrointestinal diseases. Scand J Gastroenterol Suppl 1993;199:18-21.
2. Revicki DA, Wood M, Maton PN, Sorensen S. The impact of gastroesophageal reflux disease on health-related quality of life. Am J Med 1998;104:252-8.
3. Peterson WL. GERD: Evidence-based therapeutic strategies. Bethesda, Md: American Gastroenterological Association; 2002.
4. Dent J, Jones R, Kahrilas P, Talley NJ. Management of gastro-oesophageal reflux disease in general practice. BMJ 2001;322:344-7.
5. Harding SM. Nocturnal asthma: role of gastroesophageal reflux. Chronobiol Int 1999;16:641-2.
6. Kjellen G, Wranne B. The prevalence of asymptomatic gastro-oesophageal relfux in adult patients with asthma. Eur J Respir Dis 1984;65:233.-
7. Schnatz PF, Castell JA, Castell DO. Pulmonary symptoms associated with gastroesophageal reflux: use of ambulatory pH monitoring to diagnose and to direct therapy. Am J Gastroenterol 1996;91:1715-8.
8. Sontag SJ, Schnell TG, Miller TQ, Khandelwal S, O’Connell S, Chejfec G, et al. Prevalence of oesophagitis in asthmatics. Gut 1992;33:872-6.
9. Schofield PM, Bennett DH, Whorwell PJ, Brooks NH, Bray CL, Ward C, et al. Exertional gastro-oesophageal reflux: a mechanism for symptoms in patients with angina pectoris and normal coronary angiograms. Br Med J (Clin Res Ed) 1987;294:1459-61.
10. Hewson EG, Sinclair JW, Dalton CB, Richter JE. Twenty-four-hour esophageal pH monitoring: the most useful test for evaluating noncardiac chest pain. Am J Med 1991;90:576-83.
11. Goyal RK. Diseases of the esophagus. In: Braunwald E, ed. Harrison’s principles of internal medicine. 15th ed. New York: McGraw-Hill; 2001;1642-9.
12. Schenk BE, Kuipers EJ, Klinkenberg-Knol EC, Festen HP, Jansen EH, Tuynman HA, et al. Omeprazole as a diagnostic tool in gastroesophageal reflux disease. Am J Gastroenterol 1997;92:1997-2000.
13. van Pinxteren B, Numans ME, Bonis PA, Lau J. Short-term treatment with proton pump inhibitors, H2-receptor antagonists and prokinetics for gastrooesophageal reflux disease-like symptoms and endoscopy negative reflux disease. Cochrane Database Syst Rev 2001;(4):CD002095.-
14. Brun J, Sorngard H. High dose proton pump inhibitor response as an initial strategy for a clinical diagnosis of gastro-oesophageal reflux disease (GERD).Swedish multi-centre group in primary health care. Fam Pract 2000;17:401-4.
15. Lundell L. Anti-reflux surgery in the laparoscopic era. Baillieres Best Pract Res Clin Gastroenterol 2000;14:793-810.
16. Chen MY, Ott DJ, Sinclair JW, Wu WC, Gelfand DW. Gastroesophageal reflux disease: correlation of esophageal pH testing and radiographic findings. Radiology 1992;185:483-6.
17. The role of endoscopy in the management of GERD: guidelines for clinical application. From the ASGE. American Society for Gastrointestinal Endoscopy. Gastrointest Endosc 1999;49:834-5.
18. DeVault KR, Castell DO. Updated guidelines for the diagnosis and treatment of gastroesophageal reflux disease. The Practice Parameters Committee of the American College of Gastroenterology. Am J Gastroenterol 1999;94:1434-42.
19. Beck IT, Champion MC, Lemire S, Thomson AB, Anvari M, Armstrong D, et al. The Second Canadian Consensus Conference on the Management of Patients with Gastroesophageal Reflux Disease. Can J Gastroenterol 1997;11(suppl B):7B-20B.
20. Chey WD, Inadomi JM, Booher AK, Fendrick AM. Primary care physicians’ perceptions and practices of the management of GERD: results of a national survey. Abstract presented at: DDW 2003; May 17-23, 2003; Orlando, Fla.
21. Fennerty MB, Castell D, Fendrick AM, Halpern M, Johnson D, Kahrilas PJ, et al. The diagnosis and treatment of gastroesophageal reflux disease in a managed care environment, Suggested disease management guidelines. Arch Intern Med 1996;156:477-84.
22. Stanciu C, Bennett JR. Effects of posture on gastro-oesophageal reflux. Digestion 1977;15:104-9.
23. Harvey RF, Gordon PC, Hadley N, Long DE, Gill TR, Macpherson RI, et al. Effects of sleeping with the bed-head raised and of ranitidine in patients with severe peptic oesophagitis. Lancet 1987;2:1200-3.
24. Graham DY, Patterson DJ. Double-blind comparison of liquid antacid and placebo in the treatment of symptomatic reflux esophagitis. Dig Dis Sci 1983;28:559-63.
25. Grove O, Bekker C, Jeppe-Hansen MG, Karstoft E, Sanchez G, Axelsson CK, et al. Ranitidine and high-dose antacid in reflux oesophagitis. A randomized, placebo-controlled trial. Scand J Gastroenterol 1985;20:457-61.
26. Weberg R, Berstad A. Symptomatic effect of a low-dose antacid regimen in reflux oesophagitis. Scand J Gastroenterol 1989;24:401-6.
27. Welage LS, Berardi RR. Evaluation of omeprazole, lansoprazole, pantopra-zole, and rabeprazole in the treatment of acid-related diseases. J Am Pharm Assoc (Wash) 2000;40:52-62.
28. Barnardo DE, Lancaster-Smith M, Strickland ID, Wright JT. A double-blind controlled trial of ‘Gaviscon’ in patients with symptomatic gastro-oesophageal reflux. Curr Med Res Opin 1975;3:388-91.
29. Beeley M, Warner JO. Medical treatment of symptomatic hiatus hernia with low-density compounds. Curr Med Res Opin 1972;1:63-9.
30. Chevrel B. A comparative crossover study on the treatment of heartburn and epigastric pain: liquid Gaviscon and a magnesium-aluminium antacid gel. J Int Med Res. 1980;8:300-2.
31. Laitinen S, Stahlberg M, Kairaluoma MI, Kiviniemi H, Paakkonen M, Lahtinen J, et al. Sucralfate and algi-nate/antacid in reflux esophagitis. Scand J Gastroenterol 1985;20:229-32.
32. Lanza FL, Smith V, Page-Castell JA, Castell DO. Effectiveness of foaming antacid in relieving induced heartburn. South Med J 1986;79:327-30.
33. McHardy G. A multicentric, randomized clinical trial of Gaviscon in reflux esophagitis. South Med J 1978;71(suppl 1):16-21.
34. Stanciu C, Bennett JR. Alginate-antacid in the reduction of gastro-oesophageal reflux. Lancet 1974;1:109-11.
35. Paul K, Redman CM, Chen M. Effectiveness and safety of nizatidine, 75 mg, for the relief of episodic heartburn. Aliment Pharmacol Ther 2001;15:1571-7.
36. Spiegel JE, Thoden WR, Pappas K, Fratarcangelo P, Furey SA. A double-blind, placebo-controlled study of the effectiveness and safety of nizatidine in the prevention of postprandial heartburn. Arch Intern Med 1997;157:1594-9.
37. Pappa KA, Buaron K, Payne JE, Sirgo MA, Giefer EE. An evaluation of increasing doses of ranitidine for treatment of heartburn. Aliment Pharmacol Ther 1999;13:475-81.
38. Pappa KA, Gooch WM, Buaron K, Payne JE, Giefer EE, Sirgo MA, et al. Low-dose ranitidine for the relief of heartburn. Aliment Pharmacol Ther 1999;13:459-65.
39. Pappa KA, Williams BO, Payne JE, Buaron KS, Mussari KL, Ciociola AA. A double-blind, placebo-controlled study of the efficacy and safety of non-pre-scription ranitidine 75 mg in the prevention of meal-induced heartburn. Aliment Pharmacol Ther 1999;13:467-73.
40. Ciociola AA, Pappa KA, Sirgo MA. Nonprescription doses of ranitidine are effective in the relief of episodic heartburn. Am J Ther 2001;8:399-408.
41. Gottlieb S, Decktor DL, Eckert JM, Simon TJ, Stauffer L, Ciccone PE. Efficacy and tolerability of famotidine in preventing heartburn and related symptoms of upper gastrointestinal discomfort. Am J Ther 1995;2:314-9.
42. Simon TJ, Berlin RG, Gardner AH, Stauffer LA, Gould AL, Getson AJ. Self-directed treatment of intermittent heartburn: a randomized, multicenter, double-blind, placebo-controlled evaluation of antacid and low doses of an H(2)-receptor antagonist (famotidine). Am J Ther 1995;2:304-13.
43. Galmiche JP, Shi G, Simon B, Casset-Semanza F, Slama A. On-demand treatment of gastro-oesophageal reflux symptoms: a comparison of ranitidine 75 mg with cimetidine 200 mg or place-bo. Aliment Pharmacol Ther 1998;12:909-17.
44. Qvigstad G, Arnestad JS, Brenna E, Waldum HL. Treatment with proton pump inhibitors induces tolerance to histamine-2 receptor antagonists in Helicobacter pylori-negative patients. Scand J Gastroenterol 1998;33:1244-8.
45. Howden CW. Optimizing the pharmacology of acid control in acid-related disorders. Am J Gastroenterol 1997;92(suppl):17S-21S.
46. Sharma VK, Leontiadis GI, Howden CW. Meta-analysis of randomized controlled trials comparing standard clinical doses of omeprazole and lansopra-zole in erosive oesophagitis. Aliment Pharmacol Ther 2001;15:227-31.
47. Edwards SJ, Lind T, Lundell L. Systematic review of proton pump inhibitors for the acute treatment of reflux oesophagitis. Aliment Pharmacol Ther 2001;15:1729-36.
48. Dupas JL, Houcke P, Samoyeau R. Pantoprazole versus lansoprazole in French patients with reflux esophagi-tis. Gastroenterol Clin Biol 2001;25:245-50.
49. Castell DO, Kahrilas PJ, Richter JE, Vakil NB, Johnson DA, Zuckerman S, et al. Esomeprazole (40 mg) compared with lansoprazole (30 mg) in the treatment of erosive esophagitis. Am J Gastroenterol 2002;97:575-83.
50. Howden CW, Ballard ED, Robison W. Evidence for therapeutic equivalence of lansoprazole 30 mg and esomeprazole 40 mg in the treatment of erosive oesophagitis. Clin Drug Invest 2002;22:99-109.
51. Thjodleifsson B, Beker JA, Dekkers C, Bjaaland T, Finnegan V, Humphries TJ. Rabeprazole versus omeprazole in preventing relapse of erosive or ulcerative gastroesophageal reflux disease: a dou-ble-blind, multicenter, European trial. The European Rabeprazole Study Group. Dig Dis Sci 2000;45:845-53.
52. Carling L, Axelsson CK, Forssell H, Stubberod A, Kraglund K, Bonnevie O, et al. Lansoprazole and omeprazole in the prevention of relapse of reflux oesophagitis: a long-term comparative study. Aliment Pharmacol Ther 1998;12:985-90.
53. Chey WD, Inadmoni JM, Boojer AK, Fendrick AM. What do primary care physicians think about Barrett’s esoph-agus, the relationship between GERD and H. pylori, and treatment of nocturnal heartburn? Abstract presented at: DDW 2003; May 17-23, 2003; Orlando, Fla.
54. Sampliner RE and The Practice Parameters Committee of the American College of Gastroenterology. Updated guidelines for the diagnosis, surveillance, and therapy of Barrett’s esophagus. Am J Gastroenterol 2002;97:1888-95.
55. Spechler SJ, Lee E, Ahnen D, Goyal RK, Hirano I, Ramirez F, et al. Long-term outcome of medical and surgical therapies for gastroesophageal reflux disease: follow-up of a randomized controlled trial. JAMA 2001;285:2331-8.
56. Katz PO. Gastroesophageal reflux disease: new treatments. Rev Gastroenterol Disord 2002;2:66-74.